RESULTS
General Results
A total of 40 arenaceous and 53 calcareous taxa were recorded from the sponge fragment fraction. Two of the foraminiferal species are new: Placopsilina spongiphila n. sp. and Ramulina siphonifera n. sp.; they are described in the
Appendix. The specimens of the "sponge fraction" fall into three broad categories: loose, trapped and attached. The loose specimens are those that fall off when sponge fragments are tapped on, or that can be picked out with a wet brush. Such individuals may be there accidentally (postmortem transport, sample processing) or may have crawled in. They are small compared to the meshwork cells. The trapped specimens became caught in the sponge meshwork after crawling into it and then growing to the point of being tightly trapped between sponge spicules, occasionally overflowing their silica prison. Many specimens are slightly loose but still cannot be extracted from the meshwork. The determination of whether a specimen is trapped or loose is subjective, because the distinction is not always sharp, particularly for specimens situated deep inside the meshwork and impossible to reach without breaking through many sponge rods.
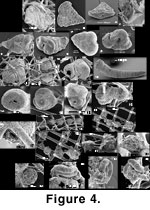 Among the specimens that are attached to the silica mesh, some are typically attached forms (Lobatula, Tritaxis) that happen to have settled on or in the sponge meshwork. These specimens are often contorted due to adaptation to the surface of the meshwork. There are also forms that are normally free-living but that here managed to weakly attach themselves, particularly the trochamminids. Others form a category all by themselves: they visually appear to have been impaled on sponge spicules, actually growing as if the meshwork was not there and engulfing it while deviating only slightly from their normal growth pattern (ex.:
Figure 4.4-4.5, 4.14-4.15). Both attached and impaled individuals may occur within a given species. Many impaled forms are normally free-living forms which at some point in their development engulfed the meshwork and are therefore attached to it by their late chambers (ex.:
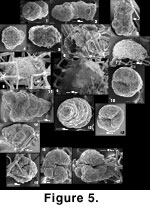
Figure 5.14-5.15).
Among the specimens that are attached to the silica mesh, some are typically attached forms (Lobatula, Tritaxis) that happen to have settled on or in the sponge meshwork. These specimens are often contorted due to adaptation to the surface of the meshwork. There are also forms that are normally free-living but that here managed to weakly attach themselves, particularly the trochamminids. Others form a category all by themselves: they visually appear to have been impaled on sponge spicules, actually growing as if the meshwork was not there and engulfing it while deviating only slightly from their normal growth pattern (ex.:
Figure 4.4-4.5, 4.14-4.15). Both attached and impaled individuals may occur within a given species. Many impaled forms are normally free-living forms which at some point in their development engulfed the meshwork and are therefore attached to it by their late chambers (ex.:
Figure 5.14-5.15).
Table 3 gives the list of the species encountered while doing the counts; species found separately in non-quantitative searches are not included. The mode of occurrence, loose, attached, etc., is also given. Despite the limited representation of the counts, the table gives the reader an idea of the frequency with which a given species was encountered, and its most common mode of occurrence.
 Species not accompanied by a heavy checkmark in
Table 3—i.e., which are represented mostly by loose specimens—are numerous but represented by only a few specimens each. The only common forms among these are Trochammina sp. 5 and Chilostomella oolina. The loose fauna resembles the <1 mm fraction and the trapped mud fauna, being dominated by the same calcareous species: Epistominella vitrea, Bolivina decussata, Eponides pusillus, Seabrookia earlandi, Angulogerina spp., Lobatula spp., Cassidulina reniforme and Astrononion gallowayi.
Species not accompanied by a heavy checkmark in
Table 3—i.e., which are represented mostly by loose specimens—are numerous but represented by only a few specimens each. The only common forms among these are Trochammina sp. 5 and Chilostomella oolina. The loose fauna resembles the <1 mm fraction and the trapped mud fauna, being dominated by the same calcareous species: Epistominella vitrea, Bolivina decussata, Eponides pusillus, Seabrookia earlandi, Angulogerina spp., Lobatula spp., Cassidulina reniforme and Astrononion gallowayi.
Attached, Trapped and Impaled Species
The attached-trapped-impaled assemblage of the sponge fraction contains a greater abundance and diversity of arenaceous
foraminifera than the assemblages of the surrounding sediment (i.e., trapped by
the sponges). The grab samples we examined elsewhere on the British Columbia shelf show a strong dominance of calcareous species.
Among the attached forms (including impaled forms), the most common are Crithionina sp., Placopsilina spongiphila, Karreriella bradyi, cf. Tritaxis fusca, Trochammina spp., Lobatula lobatula, Lobatula mckannai and Ramulina siphonifera. The most frequent trapped forms are Gaudryina spp., Karreriella bradyi, Trochammina spp., Chilostomella oolina and Islandiella californica. This also includes Martinottiella pallida, Ammobaculinus recurvus, Dorothia aff. bradyana and Reophax scorpiurus, which are few in number but proportionately more frequent than in the surrounding mud. Rhabdammina is absent while Ammodiscus arenaceus, Psammosphaera and Saccammina atlantica are few; however, these forms are common in the >1 mm fraction elsewhere in the material. Species most commonly impaled are Crithionina, Karreriella bradyi, Tritaxis fusca, Lobatula spp. and Ramulina siphonifera.
Foraminifera can attach themselves to various sponge species. Many of the illustrated specimens are attached to Farrea occa giving the impression they prefer that sponge. Because of its rectangular skeleton and of the fact it tends to break into thin chips, F. occa is an ideal substrate on which to find attached foraminiferal tests that can be easily photographed. On other sponges,
foraminifera are often situated deep inside the chip where it is difficult to extract them without damage—unless a considerable amount of time is spent doing so.
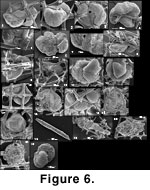 Some "trapped" specimens may have become so accidentally. This interpretation is implied by the rare presence in the meshwork of trapped sand grains (Figure 6.16-6.17). However, the existence of many specimens that grew to the point of bulging beyond their lattice cage, demonstrates the existence of this type of growth. So does the fact that many trapped, normally vagile calcareous species (Islandiella californica for example) commonly have their surface etched, the most clearly trapped being usually the most etched (Figure 7.18-7.22). This suggests that they underwent a distinct mode of postmortem preservation in comparison with well-preserved, loose specimens.
Some "trapped" specimens may have become so accidentally. This interpretation is implied by the rare presence in the meshwork of trapped sand grains (Figure 6.16-6.17). However, the existence of many specimens that grew to the point of bulging beyond their lattice cage, demonstrates the existence of this type of growth. So does the fact that many trapped, normally vagile calcareous species (Islandiella californica for example) commonly have their surface etched, the most clearly trapped being usually the most etched (Figure 7.18-7.22). This suggests that they underwent a distinct mode of postmortem preservation in comparison with well-preserved, loose specimens.
 Sponge fragments vary in preservation from clean and pristine, to slightly stained, to heavily encrusted with oxides. The surfaces of exposed biogenic silica (e.g., siliceous sponge skeletons) are quickly coated and enriched in Fe and Al (Dixit and van Capellen 2002;
Michalopoulos and Aller 2004). The present sponge material has been analyzed by inductively coupled plasma spectroscopy and X-ray fluorescence and the crusts found to consist of a mixture of oxides, iron oxide being the most abundant. The coating with oxide crusts gives a "dirty" appearance to the skeletons.
In general, a clean sponge meshwork holds fewer attached, trapped or impaled foraminifera than a "dirty" sponge. On a clean meshwork, one will commonly find calcareous forms, mostly Lobatula spp. and Ramulina siphonifera. On dirty meshwork, arenaceous forms are more common, the dominant taxon being Placopsilina spongiphila.
Sponge fragments vary in preservation from clean and pristine, to slightly stained, to heavily encrusted with oxides. The surfaces of exposed biogenic silica (e.g., siliceous sponge skeletons) are quickly coated and enriched in Fe and Al (Dixit and van Capellen 2002;
Michalopoulos and Aller 2004). The present sponge material has been analyzed by inductively coupled plasma spectroscopy and X-ray fluorescence and the crusts found to consist of a mixture of oxides, iron oxide being the most abundant. The coating with oxide crusts gives a "dirty" appearance to the skeletons.
In general, a clean sponge meshwork holds fewer attached, trapped or impaled foraminifera than a "dirty" sponge. On a clean meshwork, one will commonly find calcareous forms, mostly Lobatula spp. and Ramulina siphonifera. On dirty meshwork, arenaceous forms are more common, the dominant taxon being Placopsilina spongiphila.
Stained Foraminifera
In the sponge meshwork fractions, only a very small number of specimens were stained with Rose Bengal, and they were found in only five of the samples. One was a Shipek sample, one was a sponge fragment lying at the surface of an IKU scoop and the rest were the three slurp gun samples. The stained sponge fragment foraminifera (and stained foraminifera from other fraction too) were thus collected preferentially at or near the very surface of the sediment, which is normal in an area of low sedimentation rate. A total of 18 taxa were found stained in the sponge fractions (Table 4). They tend to belong to the more commonly occurring taxa in the fraction. The only form that is present in larger proportions is Crithionina; its stained/unstained ratio is also quite high, probably because it disintegrates rapidly after its death.
Close Examination of Pertinent Species
Modern foraminiferal taxa are examined here, either because of their abundance, because their relationship to the sponge meshwork is unusual, or because similar forms are known from Jurassic sponge reefs. Trapped specimens are often deformed and difficult to photograph. Because of this difficulty, for many species, representative pictures of specimens picked outside of the meshwork (<1 mm and >1 mm fractions) have been added so the reader may have an idea of their undeformed appearance.
Ammobaculinus cf. recurvus (Figure 5.1-5.6). Until now, this taxon has been reported only by
Saidova (1975) at three stations, two off southern Alaska and one off the Strait of Juan de Fuca. The only illustration available to our knowledge is the original of
Saidova (1975), which does not show the aperture. We sent pictures of our material to
Khadija Saidova (personal commun. 2003) who confirmed the generic identification; however, she could not confirm the species on the basis of only a few pictures. Our specimens range gradually from morphologies close to the type illustration of A. recurvus to extreme variants with multiple apertures, which Saidova
(personal commun. 2003) does not recall having seen. Because of the intergradation between all our specimens, we believe they all belong to a single species. Ammobaculinus cf. recurvus is a rather large arenaceous form. It is rarely found inside sponges, where it is mostly trapped (Figure 5.6); it is more common outside, particularly in slurp gun samples.
Crithionina sp. (Figure 5.7-5.9). Crithionina occurs either as a ball attached to the exterior of a sponge fragment (Figure 5.7) or to other objects, such as sand grains or tubes of Rhabdammina abyssorum. Free specimens are observed as well. It can also be found impaled inside the sponge framework (Figure 5.8-5.9). Crithionina granum from Sweden is known to be a predator attacking prey larger than itself (Cedhagen 1992,
and personal commun., 2003) while Crithionina delacai from Antarctica seems to prefer a diet of diatoms and possibly bacteria and detritus (Gooday et al. 1995). It is not clear why a predator would settle on the interior of a sponge fragment. Other
foraminifera do not seem affected by its presence though, which suggests that foraminifera are not part of its diet. However, most of the Crithionina we observed were attached on the outside of sponge fragments, to Rhabdammina tubes or to sand grains which is a behaviour more suggestive of a predator looking for the best spot to catch prey. It is possible that we have two species of Crithionina in our material, each with its own diet. Analysis of gene sequencing (Pawlowski et al. 2002; Cedhagen 1992,
and personal commun., 2003) has shown that what is commonly reported as Crithionina may include different genotypes that are distinct enough to include not only different species but different genera.
The agglutinated grains in Crithionina granum and C. delacai are held in place by fine reticulopodia and not by secreted adhesives; they can therefore change shape by moving agglutinated grains around their test (Cedhagen,
personal commun., 2003;
Gooday et al. 1995) to adapt themselves to their prey or to the substrate. A consequence of this is that the test is fragile and ephemeral. If our Crithionina is related to these two species, this would explain how it can wrap itself on the meshwork. This may be the case, as the test wall of our specimens disaggregates easily when repeatedly wetted and dried.
Dorothia aff. bradyana (Figure 5.10-5.17). This is probably the same as Dorothia aff. bradyana in
Todd and Low (1967). According to these authors, it differs from the type material in that the chambers are lower and more bulging between the incised sutures, and in a more nearly circular cross-section. It is an uncommon dweller of sponge fragments. It is often found loose but with an indentation on the last chamber that gives the impression it is triserial (Figure 5.13). However, we found a few specimens attached by engulfing some of the meshwork in the adult part of their test (Figure 5.14-5.15). The indentation of
Figure 5.13 thus appears to be the trace of the sponge meshwork from which the specimen fell off. On the completely entangled specimen of
Figure 5.16-5.17 it is possible to see, on the apertural face, the scars left by two broken off spicules. A roughly similar mode of attachment can be seen in Gaudryina and Martinottiella.
Gaudryina spp. (Figure 4.1-4.6). Gaudryina is a common genus in the sponge fragments. Three species are found: Gaudryina subglabrata, Gaudryina arenaria and Gaudryina accelerata. Gaudryina arenaria is a minor occurrence and is observed more often outside the sponge fragments. Gaudryina accelerata (Figure 4.1-4.3) may be loose or trapped. Some specimens have grown so that their test fits the surrounding sponge spicules. These are tightly trapped and can be considered as attached, although the overall shape of the test is not affected. Specimens grow in a single plane and do not bend around the rods (Figure 4.1). It seems to attach itself in later life, i.e., by its adult chambers. Some specimens have sponge spicules that penetrate them, but are not impaled throughout. Gaudryina subglabrata may be loose, trapped or impaled (Figure 4.4-4.6). It is the most common Gaudryina species inside sponge fragments. Even if not quite completely trapped, it may have notches due to the presence of the spicules. Its overall test shape may be more or less twisted in order to adapt to the meshwork around it.
Karreriella bradyi (Figure 4.7-4.11).
This is one of the most commonly trapped species in the material, and it tends to bulge beyond the bars of its silica trap more often than any other. Outside of the sponge reefs, K. bradyi is common in the bank areas where it constitutes, along with Islandiella californica, Islandiella limbata and some attached forms, the major portion of the very rich, mostly epifaunal assemblages that occurs there. These bank faunas can be found in Queen Charlotte Sound and further north, off southern Alaska (Bergen and O'Neil 1979). Karreriella bradyi is a rather large form, often exceeding 1 mm in length, and consequently it tends to become trapped when it grows.
Martinottiella pallida (Figure 4.12-4.15). Like the preceding species, M. pallida is a fairly large arenaceous form. It is rare in the bank fauna but more common in and around sponges. However, it is much less common in the sponge fauna than K. bradyi. It is not usually trapped in the meshwork but rather impaled on it. Large and long specimens (Figure 4.12-4.13) are not found inside sponge fragments. Instead we find short specimens (Figure 4.14-4.15) that attach or impale themselves by the side or by the distal chambers of their test, implying that they started their life free and attached themselves later. Small specimens like this one have often not reached their uniserial stage and are differentiated from Dorothia, etc., by the fact they agglutinate almost only fine, pure white grains (essentially quartz according to electron microprobe analysis).
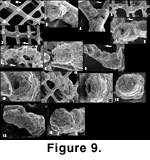
 Placopsilina spongiphila (Figure 8 and
Figure 9.1-9.12). This new species (Appendix) is the most frequent taxon associated with sponge fragments. Placopsilina spongiphila grows attached to rods of the meshwork, most commonly on oxide-covered sponge fragments, implying that the sponge had already decayed and the skeleton had been exposed to seawater for a while before the Placopsilina settled. Its diameter being small in comparison to the size of the meshwork's cells, it may grow without ever having to squeeze between silica rods. Its earliest chambers, however, may wind around a spicule.
Placopsilina spongiphila (Figure 8 and
Figure 9.1-9.12). This new species (Appendix) is the most frequent taxon associated with sponge fragments. Placopsilina spongiphila grows attached to rods of the meshwork, most commonly on oxide-covered sponge fragments, implying that the sponge had already decayed and the skeleton had been exposed to seawater for a while before the Placopsilina settled. Its diameter being small in comparison to the size of the meshwork's cells, it may grow without ever having to squeeze between silica rods. Its earliest chambers, however, may wind around a spicule.
 The genus Placopsilina is known to grow on hard surfaces such as hardgrounds or shell surfaces. It has been found in abundance on indurated sediment near hydrothermal vents on the Juan de Fuca Ridge, off British Columbia, by
Jonasson and Schröder-Adams (1996) along with other attached arenaceous forms, mostly Tolypammina, Tumidotubus and Subreophax. Resig and Glenn (1997) report Placopsilina from phosphatic hardgrounds in the oxygen minimum zone off Peru where its main companions are Ammodiscellites and Tholosina. They interpret the fact that Placopsilina never becomes erect as suggesting that it finds its food on the surface.
Gooday and Haynes (1983) have found it attached to empty Bathysiphon tubes in the abyssal Atlantic, where the main attached forms were Crithionina, ?Psammosphaera, Tumidotubus and Telammina and where dense populations coincide with iron and manganese coatings (both other papers report this too
(Jonasson
and Schröder-Adams, 1996, and
Resig and
Glenn, 1997, also report this phenomenon). There were also abundant attached calcareous microforaminifers, juvenile miliolids and indeterminate small hemispherical forms.
The genus Placopsilina is known to grow on hard surfaces such as hardgrounds or shell surfaces. It has been found in abundance on indurated sediment near hydrothermal vents on the Juan de Fuca Ridge, off British Columbia, by
Jonasson and Schröder-Adams (1996) along with other attached arenaceous forms, mostly Tolypammina, Tumidotubus and Subreophax. Resig and Glenn (1997) report Placopsilina from phosphatic hardgrounds in the oxygen minimum zone off Peru where its main companions are Ammodiscellites and Tholosina. They interpret the fact that Placopsilina never becomes erect as suggesting that it finds its food on the surface.
Gooday and Haynes (1983) have found it attached to empty Bathysiphon tubes in the abyssal Atlantic, where the main attached forms were Crithionina, ?Psammosphaera, Tumidotubus and Telammina and where dense populations coincide with iron and manganese coatings (both other papers report this too
(Jonasson
and Schröder-Adams, 1996, and
Resig and
Glenn, 1997, also report this phenomenon). There were also abundant attached calcareous microforaminifers, juvenile miliolids and indeterminate small hemispherical forms.
Placopsilina spp. (Figure 4.16-4.17 and
Figure 9.13-9.15). Some Placopsilina specimens could not be identified as P. spongiphila. Only a few were recorded, all in open nomenclature, one of which was attached to a sponge fragment from the Strait of Georgia (Figure 4.16-4.17), another attached to the test of an Ammobaculinus recurvus (Figure 9.13-9.15) and the rest, detached from their support.
Telammina fragilis (Figure 4.18-4.21). We found T. fragilis on a few sponge fragments only. It is characterized by a very thin and fragile stolon connecting the chambers. Andrew Gooday, co-author of T. fragilis, confirmed our identification. This genus would not be recognizable if it was not still attached to its substrate because the stolon would break apart immediately. Therefore, it is possible that some of the small arenaceous balls that we see elsewhere in our material are isolated T. fragilis chambers (Figure 4.22-4.23). Telammina fragilis is a deep-sea dweller, and this could be its shallowest record ever. The surface of the sediment around the sponges (slurp gun samples in particular) contains abundant Rhabdammina and large Ammodiscus, often stained. This, along with T. fragilis, gives a definitely deep-water, if not deep-sea, appearance to the assemblage as if the conditions, locally, mimicked those of the deep-sea.
Gooday and Haynes (1983) discovered T. fragilis in the abyssal North Atlantic growing inside the dead tests of Bathysiphon in assemblages that show some similarities with our own material (Table 5).
?Tolypammina sp. (Figure 4.24-4.26). We found only two sponge fragments holding a total of nine very small specimens of this unchambered and loosely tubular form. We are not sure of the generic determination because we could not observe the typical ovoid proloculus of Tolypammina. The diameter of the tubes is only 40-50 µm, and it is probably not Tolypammina vagans (Brady,
1879), whose tube usually has a diameter of 100 µm or more. Tolypammina schaudinni
Rhumbler (1904) may be closer to our material.
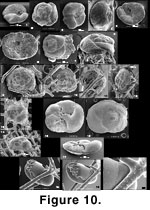 cf. Tritaxis fusca (Figure 10.1-10.12). This species is frequent and commonly attached by a cyst to sponge meshwork as well as to other hard substrate including Rhabdammina or Hyperammina tubes, other arenaceous foraminifera and sand grains. It can be both attached and impaled at the same time. The specimens in the sponge fraction, whether loose or attached, are often deformed or have an attachment cyst hiding apertural characteristics, therefore a few loose and undeformed specimens from other fractions are also illustrated (Figure 10.1-10.5). We leave cf. T. fusca in open nomenclature because 1) it usually has 3½ chambers in the last whorl, at times more, compared to T. fusca's less than 3 (typically 2½), and 2) the sutures on the umbilical side curve slightly backwards whereas in T. fusca they are straight. Our specimens are not quite like Trochamminella siphonifera either, because they have a deep umbilicus not found in this last species; it is even larger/deeper than that found on T. fusca. The distinction between Trochamminella and Tritaxis is made on whether the aperture is interio-areal (in Trochamminella) or interiomarginal (Brönniman and Whittaker 1984;
Loeblich and Tappan 1988). In our material, this is often not clear though the specimen of
Figure 10.1-10.3 seems closer to Tritaxis. Also, Trochamminella may show, in its attachment cyst, radial tunnels that open terminally; this feature is absent from our material.
cf. Tritaxis fusca (Figure 10.1-10.12). This species is frequent and commonly attached by a cyst to sponge meshwork as well as to other hard substrate including Rhabdammina or Hyperammina tubes, other arenaceous foraminifera and sand grains. It can be both attached and impaled at the same time. The specimens in the sponge fraction, whether loose or attached, are often deformed or have an attachment cyst hiding apertural characteristics, therefore a few loose and undeformed specimens from other fractions are also illustrated (Figure 10.1-10.5). We leave cf. T. fusca in open nomenclature because 1) it usually has 3½ chambers in the last whorl, at times more, compared to T. fusca's less than 3 (typically 2½), and 2) the sutures on the umbilical side curve slightly backwards whereas in T. fusca they are straight. Our specimens are not quite like Trochamminella siphonifera either, because they have a deep umbilicus not found in this last species; it is even larger/deeper than that found on T. fusca. The distinction between Trochamminella and Tritaxis is made on whether the aperture is interio-areal (in Trochamminella) or interiomarginal (Brönniman and Whittaker 1984;
Loeblich and Tappan 1988). In our material, this is often not clear though the specimen of
Figure 10.1-10.3 seems closer to Tritaxis. Also, Trochamminella may show, in its attachment cyst, radial tunnels that open terminally; this feature is absent from our material.
We find some trochospiral arenaceous forms with approximately five chambers in the last whorl whose later chambers, contrary to cf. T. fusca, overlap the preceding ones on the spiral side (Figure 10.13-10.14). With the data we have, it is not possible to say whether there is an intergradation between ?T. fusca and cf. T. fusca. Because of the attachment cyst, ?T. fusca is closer to Tritaxis than to Trochammina. Both forms add up to a total of 79 specimens; since we recognized this distinction late in the study, a complete recount would be necessary to find out how many of each are present (hence the count of "<79" for both forms in
Table 3).
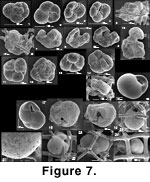
Trochamminids (Figure 7.1-7.15). We find various morphotypes of trochamminids in and around the sponges. We leave all of them in open nomenclature. Those from inside the sponges are harder to identify because of damage that occurs when trying to remove them from the meshwork to see their umbilical side. Trochammina sp. 2 is rather flat with five to seven chambers in the last whorl (Figure 7.7-7.10, specimens deformed by the presence of spicules). Trochammina sp. 5 (Figure 7.11-7.14) is thick and its chambers are inflated; it has about four chambers in the last whorl. Trochammina sp. 3 (Figure 7.1-7.3) is intermediate between the other two but clearly different. A few Portatrochammina bipolaris (Brönniman and Whittaker 1984) (Figure 7.4) are included under "Trochammina sp. 3" in
Table 3.
Trochamminids are mostly loose or trapped. Often they are too contorted to be identified even at the informal morphotype level (Figure 7.5-7.6). Some, like the Trochammina sp. 2 of
Figure 7.7-7.9, wrap themselves around sponge spicules but do not get impaled. Trochamminids at times may adhere only because of residual dry mud in which case we do not consider them attached. Attached trochamminids are not as firmly attached as Lobatula.
Chilostomella oolina, Globobulimina auriculata and Nonionella digitata (Figure 10.18-10.20). Streamlined, ovoid species are not rare in the meshwork. Their distribution is irregular, and one single sample accounts for most of the C. oolina reported in
Table 3. It is difficult to decide whether they are trapped or loose. Only once did we find a C. oolina with perforations possibly corresponding to sponge spicules. The N. digitata of
Figure 10.18-10.20, with its reaction boss is most likely trapped, but it is the only one of its kind. Specimens are commonly easy to dislodge but become damaged in the process because of the thinness of their test. These are typical deep sediment infaunal species and could have crawled easily into the meshwork especially if the sponge fragment was buried in the mud.
Hyrrokkin cf. sarcophaga
Cedhagen (1994) (Figure 10.15-10.17). We found only a few of these large (>1 mm) Rosalinidae. None of them were in or on the dead sponge fragments and thus the species is not listed in
Table 3. It is a parasite known to attack marine invertebrates, in particular sponges (Cedhagen 1994), and therefore it is logical to think that our specimens were living as parasites on the reef's sponges. It is probably not a coincidence that it occurs here but has been reported nowhere else on the west coast of North America (except for the similar form Vonkleinsmidia elizabethae reported by
McCulloch 1977, from off California). Tomas Cedhagen
(personal commun.,
2003) examined our specimens and found them to be not quite like Hyrrokkin sarcophaga and preferred to leave them in open nomenclature.
Islandiella californica, Islandiella limbata and Globocassidulina subglobosa (Figure 7.16-7.24). These three large cassidulinid species are the most important constituents of the bank fauna on the British Columbia shelf and southern Alaska but are rare or absent in the sediment infauna (Bergen and O'Neil 1979;
Echols and Armentrout 1980). In the sponge reef samples, they are frequently very close to the surface (particularly in slurp gun samples) where many are stained. Although many are large, they are often small enough to tread into the sponge meshwork. Islandiella californica may grow until it becomes tightly trapped inside the meshwork but will not tend to bulge as Karreriella bradyi does. The mark of the meshwork may remain imprinted in the test, which may be impaled, though this is rare (Figure 7.18-7.19). Specimens of subglobular cassidulinids, either I. californica or G. subglobosa, which are found trapped or somewhat loose, are often deeply etched (Figure 7.18-7.22), whereas loose individuals are well preserved and fresh. It could be that the trapped specimen died in their trap and then remained exposed to seawater above the sediment/water interface.
The most etched
specimens tend indeed to occur on the most oxide-covered sponge fragments, which
have probably been the longest exposed to seawater (see above about P.
spongiphila). Even though the British Columbia shelf lies far above the CCD, seawater is still undersaturated with respect to CaCO3 and slow dissolution remains possible. Specimens that are not associated with sponge fragments instead
probably become quickly buried and escape dissolution.
Lobatula spp. (Figure 6.1-6.15). Four forms of Lobatula were found: Lobatula lobatula, Lobatula fletcheri, Lobatula mckannai and Lobatula pseudoungeriana. Intergradations between L. lobatula and L. fletcheri can be seen, here and at other localities on the British Columbia shelf. Some specimens may be L. fletcheri-like in the first half of their last whorl and L. lobatula-like in the last half. As a consequence both are lumped as "Lobatula fletcheri + lobatula" in
Table 3. The L. fletcheri type may be observed in the loose fauna and in the 63-1000 µm fraction but only the L. lobatula type is present among the attached, trapped and impaled.
Lobatula is a widespread genus in the sponge lattice where it can be attached but also impaled. Some are trapped (Table 3), being attached but at the same time bulging beyond the exiguous mesh cells. They are often considerably deformed having to grow in such a setting (Figure 6.8-6.10, 6-15). On the other hand, they may grow as if spicules were not there, engulfing them and (or) at times having their spiral face, normally attached to a continuous substrate, facing empty space (Figure 6.1, 6.8-6.10, 6.13). This suggests that when the individual was living, the attached face was actually lying on the surface of something which is not there anymore. That could have been sponge tissue. Jenö
Nagy of Oslo University (personal commun., 2003) once observed off Spitsbergen abundant Lobatula lobatula living attached to the surface of an ascidian. This is not sponge tissue, but it is nevertheless a soft substrate. Our Lobatula may thus have grown
on living sponges, but as they are firmly attached to the meshwork, we believe that they settled after the death of the sponge.
Lobatula, a suspension feeder, is usually found at of near the surface of sponge fragments. Infaunal forms on the other hand, may be seen deeper. It may be that the Lobatula in their larval stage, if they originate from outside the sponge fragment, find it easier to settle at the most immediately accessible place, but it may be also that the exterior of a fragment is a better place to catch drifting particles. Contrary to Islandiella spp., Lobatula spp. rarely if ever show traces of dissolution or etching. This does not agree with the notion that CaCO3 tests will etch more if exposed to seawater for a longer period of time.
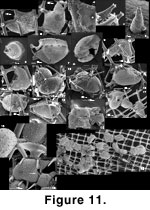 Ramulina siphonifera (Figure 11 and
Figure 12). This attached Ramulina is a new species (Appendix) and is the most distinctive taxon in the sponge fauna. It is widespread in our material and can be found wherever there are foraminifera in or on the sponge fragments. Even the Strait of Georgia sponge fragments, nearly devoid of foraminifera, have yielded four specimens. The genus has never been reported from the west coast of North America between Alaska to Oregon (Culver and Buzas 1985; other authors quoted in the present paper) but none of the workers in the region have specifically searched the content of sponge fragments. The presence of this species seems therefore linked to the existence of a particular habitat: dead sponge fragments. The fragility of the apertural siphon is such that it must live attached in a protected habitat. Sponge fragments supply this habitat but a specimen found attached to a sand grain (Figure 11.16), shows that this is not an absolute necessity. However, this is one of only two specimens of its kind. The sponge fragment may offer a base from which to catch drifting food although Polymorphinidae are not usually recognized as suspension feeders.
Ramulina siphonifera (Figure 11 and
Figure 12). This attached Ramulina is a new species (Appendix) and is the most distinctive taxon in the sponge fauna. It is widespread in our material and can be found wherever there are foraminifera in or on the sponge fragments. Even the Strait of Georgia sponge fragments, nearly devoid of foraminifera, have yielded four specimens. The genus has never been reported from the west coast of North America between Alaska to Oregon (Culver and Buzas 1985; other authors quoted in the present paper) but none of the workers in the region have specifically searched the content of sponge fragments. The presence of this species seems therefore linked to the existence of a particular habitat: dead sponge fragments. The fragility of the apertural siphon is such that it must live attached in a protected habitat. Sponge fragments supply this habitat but a specimen found attached to a sand grain (Figure 11.16), shows that this is not an absolute necessity. However, this is one of only two specimens of its kind. The sponge fragment may offer a base from which to catch drifting food although Polymorphinidae are not usually recognized as suspension feeders.
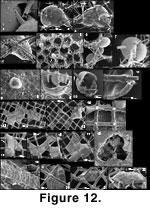 We have a few specimens which, like some Lobatula, are flat and non-spinose on one side as if they had been growing on "something" that is not there anymore (Figure 12.20-12.22). Even more than in Islandiella, it is common to find specimens of R. siphonifera that have been etched, possibly due to exposure to seawater for a long time postmortem.
We have a few specimens which, like some Lobatula, are flat and non-spinose on one side as if they had been growing on "something" that is not there anymore (Figure 12.20-12.22). Even more than in Islandiella, it is common to find specimens of R. siphonifera that have been etched, possibly due to exposure to seawater for a long time postmortem.
Two modern Ramulina species are described as attached: Ramulina grimaldii
Schlumberger
(1891a) and Ramulina vanandeli
Loeblich and Tappan
(1994); they have rarely been reported after their original publications. Modern reports of Ramulina are few, and almost nothing is known of its ecology. Hugh Grenfell and Brian Hayward (University of Auckland, personal commun.,
2005) record Ramulina occasionally from deeper waters, as broken fragments and very rarely as whole specimens, with no evidence of attachment.
Ramulina siphonifera engulfs silica rods by wrapping them completely and tightly with its wall so that the content of the lumen is completely insulated from the meshwork. An individual may thus appear completely pierced by the meshwork and still the protoplasm would have no contact with it (Figure 12.10-12.14). Thus, R. siphonifera may have two growth modes: it may creep between the rods of the meshwork, or it may engulf
them.
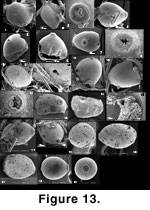 Aff. Oolina caudigera. Only 19 specimens of this form have been observed from the sponge meshwork. The free specimen of
Figure 13.22-13.23 is from the >1000 µm fraction. This taxon resembles O. caudigera except that most specimens were found attached to the meshwork, usually by the aboral end (Figure 13.1-13.2). The test tends to be symmetrical relative to an axis passing through the aperture, but among the attached specimens, it may be laterally compressed or deformed depending on its relationship to the meshwork. The basal spine or tube may be placed sideways depending on the deformation of the test (Figure 13.11-13.12, 13.21). The very finely porous, optically radial calcareous wall is deformed or completely interrupted at the contact with the sponge spicules to which the specimen is attached, leaving open scars in detached specimens (Figure 13.6). It is not possible to know whether or not the wall wraps completely around the silica rods as in Ramulina siphonifera because of the limited availability of material. The aperture is radiate (Figure 13.8, 13.14, 13.23) contrary to Oolina where it is rounded; hence it cannot be included in that genus. The only entosolenian tube we found was short but broken (Figure 13.16). Overgrowths or frills develop at the contact between wall and substrate as in Ramulina siphonifera (Figure 13.10).
Aff. Oolina caudigera. Only 19 specimens of this form have been observed from the sponge meshwork. The free specimen of
Figure 13.22-13.23 is from the >1000 µm fraction. This taxon resembles O. caudigera except that most specimens were found attached to the meshwork, usually by the aboral end (Figure 13.1-13.2). The test tends to be symmetrical relative to an axis passing through the aperture, but among the attached specimens, it may be laterally compressed or deformed depending on its relationship to the meshwork. The basal spine or tube may be placed sideways depending on the deformation of the test (Figure 13.11-13.12, 13.21). The very finely porous, optically radial calcareous wall is deformed or completely interrupted at the contact with the sponge spicules to which the specimen is attached, leaving open scars in detached specimens (Figure 13.6). It is not possible to know whether or not the wall wraps completely around the silica rods as in Ramulina siphonifera because of the limited availability of material. The aperture is radiate (Figure 13.8, 13.14, 13.23) contrary to Oolina where it is rounded; hence it cannot be included in that genus. The only entosolenian tube we found was short but broken (Figure 13.16). Overgrowths or frills develop at the contact between wall and substrate as in Ramulina siphonifera (Figure 13.10).
Oolina and other unilocular lagenids are never attached. For this reason, a new genus ought to be erected for these specimens. However, the existence of essentially identical, unattached and undeformed specimens shows that this is not a fixed feature of this form. The attachment may be seen as an adaptation to suspension feeding; however, this is unexpected in unilocular lagenids. Also, one may wonder why some specimens are not attached. The attachment in aff. O. caudigera is more of the impaled type, the specimen engulfing parts of the meshwork. There are other taxa in this material that become attached in this way, often late in their development (ex.: Gaudryina, Dorothia). A more plausible explanation would be that these specimens have grown inside the meshwork to the point of being trapped and that one reaction to this stress consists in engulfing part of the meshwork, because nothing else is possible (this explanation could be applied to most other taxa observed in an impaled position). Many specimens appear attached with their aperture pointing away from the meshwork, but this could be an illusion. Since all these are broken sponge fragments, one has to imagine what the position of the specimen was before fragmentation of the sponge.
Figure 13.18 shows a specimen whose aperture is resting against an already broken segment of meshwork; clearly, it was trapped in a very restricted space.

 Among the specimens that are attached to the silica mesh, some are typically attached forms (Lobatula, Tritaxis) that happen to have settled on or in the sponge meshwork. These specimens are often contorted due to adaptation to the surface of the meshwork. There are also forms that are normally free-living but that here managed to weakly attach themselves, particularly the trochamminids. Others form a category all by themselves: they visually appear to have been impaled on sponge spicules, actually growing as if the meshwork was not there and engulfing it while deviating only slightly from their normal growth pattern (ex.:
Figure 4.4-4.5, 4.14-4.15). Both attached and impaled individuals may occur within a given species. Many impaled forms are normally free-living forms which at some point in their development engulfed the meshwork and are therefore attached to it by their late chambers (ex.:
Figure 5.14-5.15).
Among the specimens that are attached to the silica mesh, some are typically attached forms (Lobatula, Tritaxis) that happen to have settled on or in the sponge meshwork. These specimens are often contorted due to adaptation to the surface of the meshwork. There are also forms that are normally free-living but that here managed to weakly attach themselves, particularly the trochamminids. Others form a category all by themselves: they visually appear to have been impaled on sponge spicules, actually growing as if the meshwork was not there and engulfing it while deviating only slightly from their normal growth pattern (ex.:
Figure 4.4-4.5, 4.14-4.15). Both attached and impaled individuals may occur within a given species. Many impaled forms are normally free-living forms which at some point in their development engulfed the meshwork and are therefore attached to it by their late chambers (ex.:
Figure 5.14-5.15).








