|
|
|
INTRODUCTIONInference of behavior in extinct vertebrates often involves conflicting intuitions and approaches, especially when the animals possessed novel structures without clear extant analogs. The functional capability of osseous structures is testable biomechanically, with each result contributing to a reasonable continuum of behavioral possibilities. We apply finite element analysis to circumscribe inferences of head-butting combat in pachycephalosaurian dinosaurs, by testing whether forces of such collisions would fall within the limits of bone strength for two representative taxa. This approach does not answer whether pachycephalosaurs engaged in such intraspecific combat, but does ground the debate within a quantitative biomechanical framework. Dome-Based Combat in Pachycephalosaurs: Arguments from Analogy and StructurePachycephalosaurs have among the most distinctive head morphologies of any amniote clade, with a dorsally thickened cranium of comparatively solid bone, and uniquely shaped dome heads in some genera. The dome is suggestive that pachycephalosaurs exhibited head-butting behavior, analogous to that seen in mountain sheep and other ungulates, and hypothesized for dinocephalian synapsids (Barghusen 1975). Whereas we can only circumscribe possibilities of agonistic behavior in pachycephalosaurs, workers have considered copious evidence from dome functional morphology and extant analogs. Precise structural analogs for pachycephalosaurs are elusive. Head-butting ovids such as mountain sheep and musk oxen have hollow horns, unlike the osseous expansion seen in pachycephalosaurs. Giraffes engage in side-butting with dome-like projections on the head (suggested for chalicotheres with similar domes: Munthe and Coombs 1979), but these are hollow structures and proportionally small compared with pachycephalosaur domes. Male marine iguanas engage in head-butting and shoving matches at low speeds (Carpenter 1967). Bakker et al. (2006) identified male giant forest hogs (Hylochoerus meinertzhageni) as analogs for combat more popularly envisioned for pachycephalosaurs. The hogs engage in high-energy head-to-head combat without regard to damaging their integument (Estes 1991), and even fracture and induce pseudarthroses of the frontals and parietals. Such arguments from analogy elicit intriguing parallels and hypotheses, but will be unconvincing without specific tests derived from functional morphology. The primacy of biomechanics over analogy applies to function, evolution, and even ontogeny of agonistic behavior. Dome morphology within specific lineages of pachycephalosaurs, and their conformance or divergence with expectations of agonistic theory (Geist 1966), will be informative about the evolution of combat between these dinosaurs. Maryánska et al. (2004) and Fastovsky and Weishampel (2005) emphasize morphology in critical overviews of pachycephalosaur head-butting. In addition to the presence of a dome, evidence cited for intraspecific combat includes vertebral articulations providing spinal rigidity and the shape of the back of the skull indicative of strong neck musculature. Buckling through tongue-in-groove articulations between the presacral vertebrae (Maryánska and Osmólska 1974), and probable dense connective tissue within the endocranial cavity (Evans 2005), would have moderated deceleration on the brain (the most critical factor for surviving collisions). In an analysis of dome shape, Chapman et al. (1998) determined that neck-jarring "glancing blows" (Carpenter 1997) were unlikely even in highly-domed forms, indicating that self-correction (secondary, stabilizing impacts onto other parts of the skull: Barghusen 1975) was not essential for combat. As Sues (1978) noted, glancing blows are common in mountain sheep (Geist 1971), and their potential injurious effects are easily countered by the nuchal ligaments and neck muscles. Alexander (1997) determined that neck muscle with only a small percentage of head mass would absorb kinetic energy of a glancing blow in pachycephalosaurs, even under extreme conditions. Sues (1978) identified a long moment arm for the extensive insertion of dorsiflexor m. spinalis capitis (Maryánska and Osmólska 1974; m. transversospinalis capitis: Tsuihiji 2005), ideally sized and situated to counter and absorb such forces. Alexander (1989) further calculated instantaneous impact forces from head-on collisions, and concluded that axial musculature could absorb collision energy as the spinal column buckled behind the point of impact. Nevertheless, the dome would have to absorb initial stresses of the collision. Galton (1971) and Sues (1978) noted column-like trabeculae in Stegoceras that are perpendicular to the dome's external surface and potentially resisted compressive stresses. Plexiglas models (Sues 1978) supported this hypothesis, indicating that compressive stress trajectories would be coincident with the trabeculae. However, morphological contraindications of head-butting behavior have arisen from multiple sources. The lack of air spaces within the dome (as seen in the horns of head-butting ovids), and absence of surface pathologies, which would presumably be linked to head-butting, have been proffered as contrary evidence that at best indicates flank-butting combat (Fastovsky and Weishampel 2005). The diversity of dome shapes, and their association with boss and spike ornamentation, suggests that the domes were important for species recognition (Goodwin and Horner 2004). The taxon with the most spectacular ornamentation, Stygimoloch spinifer, had a relatively narrow dome cited as incompatible with head-butting (Goodwin et al. 1998). In a histological study of sectioned pachycephalosaur domes, Goodwin and Horner (2004) established that trabeculae hypothesized as resisting compression were only present in some specimens (see also Brown and Schlaikjer 1943: plate 43). The trabeculae constitute one zone (Zone II) of the dome, which diminished in thickness during ontogeny and was nearly obliterated by reworking in a large Pachycephalosaurus wyomingensis. Superficial to Zone II is a thick, nearly acellular cap of compact bone, which lacks traces of structures perpendicular to the dome's outer surface. This histology calls into question the facilities of the dome to resist compression, and for remodeling and repair after damaging collisions. Biomechanical Tests of Head-Butting Capability in PachycephalosaursCranial structure has been the starting point for debates about pachycephalosaur behavior. We therefore tested the structural capacity of pachycephalosaur domes to withstand forces of putative intraspecific combat, using finite element analysis (FEA) of representative low- and dome-headed taxa. Our criteria for contradicting the domes' suitability for head-butting are that von Mises (yield) stress exceeds that of bone strength (approximately 300 MPa), and the presence of high stress in bone encompassing the endocranial cavity. We constructed finite element models of the skulls dorsal to the braincase in the flat-headed Homalocephale colathoceros (GI SPS 100/51: Maryánska and Osmólska 1974) and the largest dome-headed species, Pachycephalosaurus wyomingensis (AMNH 1696: Brown and Schlaikjer 1943). Finite element modeling decomposes a continuous field into a mesh of multiple individual elements. In models of solid structures, the elements act analogously to a set of interconnected springs with collective physical properties of the original material. When calculated forces are applied to the finite element model, FEA yields distribution and magnitude of stress (force/area) and strain (proportional deformation) that would have occurred within the mechanically loaded structure. FEA of pachycephalosaur skulls requires modeling the shape and material properties of their thickened cranial dorsa and determining the impact forces of a simulated collision. Force would vary intertaxonomically and with the speed of an encounter, and material properties could vary histologically even within the skull of an individual. We therefore vary these parameters to encompass possible collision velocities and test for the effects of histological zonation within dome-headed forms.
Tissues of the integument that covered the dome would have been the first to absorb or transmit impact energy. The epidermis of pachycephalosaurs undoubtedly incorporated
Because putative combat behaviors would have been complex affairs, we term the magnitude of each collision velocity a "closing speed" that accommodates multiple combinations of approach velocities in the antagonists. The effects of a head-on closing speed of 5 m/s, for example, would be the same if both animals approached at 2.5 m/s, if one charged at 5 m/s as another held its ground, or if one approached even faster as the other retreated. (For simulations of head-on collisions, signs of velocity vectors would be opposite.) Additionally, by simulating forces of an impact to the side of a Pachycephalosaurus dome, we examined the possible effects on the dome if one animal collided obliquely with another. Whether evidence ultimately favors combat or display functions of pachycephalosaur domes, their features can be traced phylogenetically to explore the structure's evolution through the clade. We therefore sought to complement the character optimization of Maryánska et al. (2004) by running a phylogenetic analysis of their included taxa. If the dome lacked a mechanical function and was primarily for species recognition, we might expect it to diminish in size as other, visually stimulating ornamentation accrued through pachycephalosaur evolution. To test this hypothesis, we mapped dome features and other "species recognition" characters from Maryánska et al. (2004) onto the results of our phylogenetic analysis. |
|
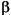 -keratin, similar to their extant avian-crocodilian bracket. Although keratin is not as stiff as the mineralized component of bone (
-keratin, similar to their extant avian-crocodilian bracket. Although keratin is not as stiff as the mineralized component of bone (