|
|
|
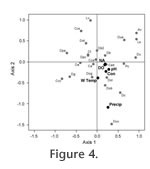
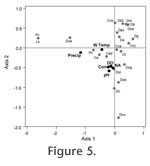
DiscussionSeasonal variations of the living (stained) thecamoebian populations and variations among the species composing the difflugiid population can be related to environmental changes (temperature and precipitation) (Table 1 and Table 2, Figure 3). The monthly consistency of the total assemblage composition in terms of difflugiids to centropyxids can be attributed to water chemistry, as the proportion of difflugiids (vs. centropyxids) did not change with changing environmental parameters (Table 1, Figure 3). Scott and Medioli (1980) observed similar results while conducting a seasonality study using foraminiferal populations. Their results indicated a highly variable living foraminiferal population with insignificant changes to the total assemblage (Scott and Medioli, 1980). The warmer spring and summer months of May to August harbored high numbers of C. tricuspis, D. oblong, D. amphora and D. globula (Figure 3). The substantial increase in D.globula in July appears to be an anomaly because D.globula is typically considered an indicator of cool to cold climates (Collins et al., 1990). C. tricuspis is a common taxon recorded in most freshwater environments, due in part to the unusual ecology of this species, which has a planktonic phase in its life cycle (Schonborn, 1984; Medioli et al., 1987). It is possible that September does not harbor a sufficient amount of algal food source to support its planktonic phase. The high presence of D. oblonga in the warmer summer months suggests that it can somewhat tolerate climate extremes (Collins et al., 1990; McCarthy et al., 1995), but it lives in higher numbers in temperatures above 10ºC (Table 1). During the cooler month of September a change in the dominant species composing the thecamoebian population is observed in relation to species tolerances. In response to a change in environmental parameters an increase in A. vulgaris, D. urceolata and P. compressa is observed. D. urceolata was also present during May, its absence in July and August, may be due to predation or because it prefers to live in lower temperatures (Collins et al., 1990; McCarthy et al., 1995) than those averaging 16ºC as were observed in the mid summer months. A. vulgaris is typically considered an indicator of a drop in water body pH (Patterson and Kumar, 2000) or consistently low pH conditions (Boudreau et al., 2005; Kumar and Patterson, 2000). In this study, however, the increase of A. vulgaris in September coincides with the highest pH values recorded (pH=8.31), which is unusual as Patterson and Kumar (2000) and Boudreau et al., (2005) suggest A. vulgaris is an indicator of a low pH environment. Typically P.compressa appears to tolerate climate extremes (McCarthy et al., 1995), and is often found in high numbers where other species, more sensitive to climate conditions, can't thrive. It is common in all ponds except those undergoing eutrophication (Collins et al., 1990). This may explain its significant increase in total and living numbers during September, when we observe the lowest proportion of C. tricuspis. Similar to the findings of Heal (1964), we observed maximum numbers of both the total (living + dead) and the living (stained) thecamoebians from spring to fall, after which time total numbers decreased as the organisms encysted in preparation for winter (Heal, 1964).
Water chemistry influences the distribution of the total (living + dead) populations, in terms of the proportions of difflugiids to centropyxids while environmental factors such as temperature and precipitation influence the living population. The relative abundance of living (stained) thecamoebians is positively correlated with temperature and precipitation (Table 1), although the impact of these climatic parameters on benthic protists is probably indirect, possibly related to bio-productivity and the availability of food (Burbidge and Schroder-Adams, 1998). Environmental factors also influence the dominant species within the thecamoebian population and cause monthly taxon shifts within the difflugiid population (Figure 3) even though the proportion of the difflugiid population in relation to the centropyxid population remains relatively consistent. This is consistent with the observation that difflugiid taxa are very sensitive to environmental parameters (Collins et al., 1990). Neither Heal (1964) nor Warner et al., (2007) attributed seasonal changes they observed in testate amoebae communities directly to seasonal changes in environmental conditions. Heal (1964) considered changes in light conditions affecting symbiotic zoochlorellae in organisms to be one of the main driving factors in seasonal taxon shifts. The literature suggests that D. amphora is typically found in eutrophic environments (Ellison, 1995), consistent with its increased presence during the summer months. D. amphora is not typically found in fossil records, probably because it has a low preservation potential. Typically when D. amphora is present it is observed as a stained (living) test. In May, July and August (the spring and summer months) we observe a higher frequency of stained in comparison to unstained tests, while in March D. amphora is completely absent both stained and unstained. This data strengthens the argument that D. amphora has a low preservation potential, as it is mainly observed as a living (stained) tests and very rarely as an empty unstained test.
In terms of strain level variability it does appear that some strains prefer certain conditions (Table 2). Among centropyxiids, it appears that Centropyxis aculeata "discoides" and Centropyxis constricta "aerophila" are the most opportunistic, more tolerant of climate extremes than other centropyxiids observed during the course of this study, as only the above mentioned were present in March. Difflugia urceolata "urceolata" appears to be more sensitive to cold in comparison to Difflugia urceolata "elongata" as D. urceolata "urceolata" was only present during the summer months when water temperatures were <17 C. D. urceolata "urceolata" was present in March but only as a member of the fossil record. |
|