Early Eocene plant macrofossils from the Booval Basin, Dinmore, near Brisbane, Queensland
Early Eocene plant macrofossils from the Booval Basin, Dinmore, near Brisbane, Queensland
Article number: 22.3.60
https://doi.org/10.26879/922
Copyright Paleontological Society, October 2019
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 31 August 2018. Acceptance: 17 September 2019.
ABSTRACT
Early Eocene plant macrofossils are documented from Dinmore in the Redbank Plains Formation within the Booval Basin, west of Brisbane, Australia. It is one of the few Cenozoic Australian fossil macrofloras occurring outside the south-eastern corner. Angiosperms dominate the Dinmore assemblage, with 20 taxa having a mixture of simple and compound leaves, and which are almost equally distributed across the microphyll and notophyll size classes. Although cuticle is absent, gross leaf morphology and architecture suggests the taxa include: Proteaceae (probable Lomatia, Parafatsia), Myrtaceae (including Eucalyptus s.l. i.e, the clade including Angophora, Corymbia, and Eucalyptus), Smilacaceae, and probable Lauraceae. Conifers are rare with only two taxa, a multi-veined leaf consistent with Agathis (Araucariaceae) or Nageia (Podocarpaceae) and another possible Podocarpaceae. The Mean Annual Temperature predicted by a variety of leaf physiognomic methods ranges from 8-19 °C. However, average leaf size suggests 18-19 °C, and this warmer end is considered more likely. Even this may be underestimated as moisture was likely at least seasonally limited and would act to decrease leaf size. Dinmore was at a much higher latitude in the early Eocene (c. 46 °S), and the estimate of a much higher temperature is consistent with other proxies for globally warmer conditions at the time.
The presence of Eucalyptus s.l. is curious, and this additional early record of its occurrence in a vegetation community that would otherwise likely be considered ‘rainforest’ hints that its origin might not involve fire, but lie within a vegetation type no longer extant.
Mike Pole. Queensland Herbarium, Brisbane Botanic Gardens Mt Coot-tha, Mt Coot-tha Rd, Toowong, Australia. murihiku@yahoo.com
Keywords: paleoclimate; Eucalyptus; Lauraceae; Proteaceae; foliar physiognomy
Pole, Mike. 2019. Early Eocene plant macrofossils from the Booval Basin, Dinmore, near Brisbane, Queensland. Palaeontologia Electronica 22.3.60 1-33. https://doi.org/10.26879/922
palaeo-electronica.org/content/2019/2760-dinmore-plant-macrofossil
Copyright: October 2019 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Understanding of the Cenozoic vegetation history of Australia, and of the climate and other factors which drove it, has increased dramatically over the past few decades (e.g., Hill, 1984). However, with the exception of a few far-flung studies (e.g., central Australia, Christophel et al., 1992; Carpenter and Pole, 1995; Carpenter et al., 2011; Western Australia, McLoughlin and Guppy, 1993; northernmost Australia, Pole, 1998), most of the macrofossil knowledge still comes from fossil assemblages close to Australia’s south-east corner (e.g., Hill, 1988, 2004; Hill et al., 1999). Cenozoic plant macrofossil localities in Queensland are rare, and leaf material is often of relatively poor quality (although fruits can be very well preserved). However, several studies have added information to this poorly-known area (e.g., Rozefelds, 1988, 1991, 1992; Rozefelds and Christophel, 1996, 2000; Clifford and Dettman, 2002; Conran and Rozefelds, 2003; Guerin and Hill, 2006; Rozefelds et al., 2014, 2017).
Plant fossils from several localities in the Oxley Basin were described by Ettingshausen (1895, who regarded the age as Cretaceous). He identified 62 species in 41 genera of angiosperms and gymnosperms, although by modern standards, most of the generic identifications are dubious. A conifer macrofossil, Dacrycarpus, was recorded by Selling (1950). Hill et al. (1970) illustrated a ?Eucalyptus sp. and “eucalypt-like” fruits were described by Rozefelds (1996). Two ferns are known; namely, Lygodium (Churchill, 1969; Hill et al., 1970; Rozefelds et al., 1992) and a new genus of water fern, Tecaropteris (Rozefelds et al., 2015).
The Booval Basin has produced a wide range of vertebrates and insects (Riek, 1952; Rix, 1991, 1992; Knezour, 1992) and through the efforts of several collectors over many years a small exposure in a clay pit near Dinmore has provided a large collection of leaf impressions. The aim of this paper is to describe the leaf macrofossils from this Dinmore locality, and investigate their climatic implications.
GEOLOGICAL SETTING
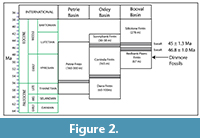
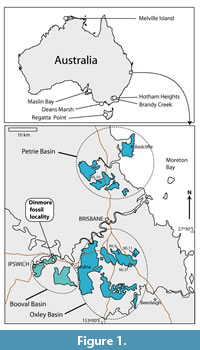 Cenozoic sediments in south east Queensland are restricted to several small basins (in the order of 10-20 km maximum dimensions) representing foci of fluvial-lacustrine activity along with some volcanicity. In the vicinity of Brisbane there are three, namely, the Oxley, Booval, and Petrie Basins (Figure 1). Sediments in the Oxley Basin are divided into the Darra, Corinda, and Sunnybank Formations (Houston, 1967). The mainly lacustrine sediments of the Booval Basin are divided into the lower Redbank Plains Formation and the upper Silkstone Formation (Staines, 1960; Day et al., 1982). Jones (1926) provided a summary of the early geological investigations of the area and concluded that the basins were Cenozoic. Basalt near the base of the Silkstone Formation (Figure 2) was isotopically dated as 45 Ma (middle Eocene) and provides an upper limit for the age of the Redbank Plains Formation (Green and Stevens, 1975). Another basalt, which probably belongs to the Silkstone Formation, was dated as 46.3 Ma. A Paleocene age was considered by Day et al. (1982), although Stephens (pers. comm. to Kemp, 1991) suggested Eocene. In the nearby Corinda Formation the Archerfield Basalt and Eight Mile Plains Basalt were isotopically dated by Green and Stevens (1975) as 54-55 Ma and 45.7 Ma, respectively. On this basis, they concluded that the Corinda Formation (maximum thickness of 165 m) was deposited in about eight million years. If this rate of deposition is broadly applicable to the Redbank Plains Formation, which has an average thickness of 67 m (Houston, 1967; Cranfield, 1976), the base of the formation is not likely to be more than about 52 Ma. The most likely age of the Dinmore fossil leaves is therefore early Eocene.
Cenozoic sediments in south east Queensland are restricted to several small basins (in the order of 10-20 km maximum dimensions) representing foci of fluvial-lacustrine activity along with some volcanicity. In the vicinity of Brisbane there are three, namely, the Oxley, Booval, and Petrie Basins (Figure 1). Sediments in the Oxley Basin are divided into the Darra, Corinda, and Sunnybank Formations (Houston, 1967). The mainly lacustrine sediments of the Booval Basin are divided into the lower Redbank Plains Formation and the upper Silkstone Formation (Staines, 1960; Day et al., 1982). Jones (1926) provided a summary of the early geological investigations of the area and concluded that the basins were Cenozoic. Basalt near the base of the Silkstone Formation (Figure 2) was isotopically dated as 45 Ma (middle Eocene) and provides an upper limit for the age of the Redbank Plains Formation (Green and Stevens, 1975). Another basalt, which probably belongs to the Silkstone Formation, was dated as 46.3 Ma. A Paleocene age was considered by Day et al. (1982), although Stephens (pers. comm. to Kemp, 1991) suggested Eocene. In the nearby Corinda Formation the Archerfield Basalt and Eight Mile Plains Basalt were isotopically dated by Green and Stevens (1975) as 54-55 Ma and 45.7 Ma, respectively. On this basis, they concluded that the Corinda Formation (maximum thickness of 165 m) was deposited in about eight million years. If this rate of deposition is broadly applicable to the Redbank Plains Formation, which has an average thickness of 67 m (Houston, 1967; Cranfield, 1976), the base of the formation is not likely to be more than about 52 Ma. The most likely age of the Dinmore fossil leaves is therefore early Eocene.
Most of the surficial Cenozoic sediments in the Brisbane region have been too deeply weathered for preservation of pollen. Although no productive palynological samples are known from the Booval Basin, Harris (1965) reported on productive bore-hole samples from all three formations of the Oxley Basin. The oldest sample from the Darra Formation was dominated by Myrcipites harrisii pollen (often considered to be Casuarinaceae) and had rare Nothofagus (trace amounts). However, there is also a similarity with the New Zealand palynological zonation in which Zone MH1 of the Myrcipites harrisi i Assemblage is “characterised by the abundance, and frequent dominance of M. harrisii (Raine, 1984, p. 24), and in which Nothofagus is typically very rare or absent. Zone MH1 extends from about the start of the early Eocene until the earliest middle Eocene and thus broadly correlates with the early Eocene Climatic Optimum.
MATERIAL AND METHODS
 Most of the plant fossils described here were collected by R. Knezour, A. Rozefelds, and S. McLoughlin. They are preserved as impressions (no pollen or cuticle is preserved) on the bedding surfaces of clay-rich sediment. Preparation involved further excavation of selected specimens using a vibrating chisel. Most investigation used a binocular microscope, as surface details which might have been visible using a scanning electron microscope are absent. Specimens were photographed using low angle light. Specimens were drawn with a camera lucida at about five to seven times full size, reduced to two times, then traced onto architectural paper using Indian ink. All material is catalogued and stored in the Queensland Museum. Leaf architecture terminology mostly follows Hickey (1973) and Dilcher (1974) and Ellis et al. (2009), but with the modifications of Pole (1991).
Most of the plant fossils described here were collected by R. Knezour, A. Rozefelds, and S. McLoughlin. They are preserved as impressions (no pollen or cuticle is preserved) on the bedding surfaces of clay-rich sediment. Preparation involved further excavation of selected specimens using a vibrating chisel. Most investigation used a binocular microscope, as surface details which might have been visible using a scanning electron microscope are absent. Specimens were photographed using low angle light. Specimens were drawn with a camera lucida at about five to seven times full size, reduced to two times, then traced onto architectural paper using Indian ink. All material is catalogued and stored in the Queensland Museum. Leaf architecture terminology mostly follows Hickey (1973) and Dilcher (1974) and Ellis et al. (2009), but with the modifications of Pole (1991).
The leaves are described as parataxa (an arbitrary string of three letters prefixed with ‘DINM’) and a key (Table 1) is provided to help clarify the distinctions.
Leaf physiognomic techniques, including univariate (based on Wolfe, 1979), and the multivariate ‘CLAMP’ (Wolfe, 1990; Yang et al., 2011) are used to estimate the mean annual temperature. Following normal practise, leaflets of compound leaves are treated as leaves.
DESCRIPTIONS
Multi-veined conifer
Figure 3
 Reference specimen. QMF34435A,B.
Reference specimen. QMF34435A,B.
Description. Isolated leaf flattened, scale-like, multi-veined, almost elliptical, expanding from a false petiole (about 3.0 mm long, 1.0 mm wide, slightly expanded at the base) to a maximum width of 19 mm, and around 38 veins, then contracting. The apex is missing and the preserved length is 54 mm.
Remarks. The expanding, multi-veined blade is consistent with Agathis and Nageia (Offler, 1984; Jin et al., 2010)
?Podocarpaceae gen. indet.
Figure 3
Reference specimen. QMF14925.
Description. Shoot: flattened into two dimensions, leaves single-veined, bilaterally flattened, not constricting towards the base, length 8 mm, width 1 mm, straight to slightly sinusoidal.
Remarks. This is probably the same taxon are that described by Selling (1950) from the same or nearby locality, as Podocarpus (sect. Dacrycarpus) sp.
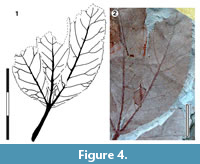
Leaves palmately trifoliate and toothed
Parataxon: DINM-JJA
Figure 4
Referred specimens. QMF34306, QMF34332, QMF34333.
Description. Organisation: palmately trifoliate. Leaflet size: length 50 mm, width 18 mm, microphyll. attachment sessile. Leaflet shape: elliptic, unlobed, apex unknown, base convex. Margin serrate, teeth simple, irregularly-spaced, close to distant, several teeth per lateral, sinuses sharp. Venation externodromous (sensu Pole, 1991), development normal. Lateral veins well-spaced, not decurrent on midrib, forking where externals branch-off.
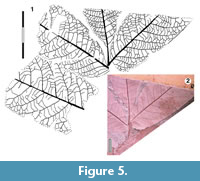
Leaves pinnately compound with a terminal leaflet and toothed
Parataxon: DINM-JJB
Figure 5
Referred specimen. QMF34342A,B.
Description. Organisation: pinnate with a terminal leaflet. Size: length probably c. 100 mm, width c. 40 mm, microphyll-notophyll. Leaflet shape: not clear, unlobed. Lateral leaflet attachment sessile, terminal leaflet with petiolule. Apex unknown, base possibly concave and asymmetrical. Margin serrate, teeth simple, several teeth per lateral, sinuses sharp. Venation externodromous, development normal. Lateral veins closely-spaced, not decurrent on midrib, gradually losing distinction towards the margins, no clear lateral loops. Finer venation prominently percurrent
Parataxon: DINM-JBD
Figure 6
Reference specimen. QMF35317.
Description. Organisation: pinnate, unclear whether with a terminal leaflet or not. Size: leaflets length 55-85 mm, width 11-19 mm, microphyll. Shape: ovate-lanceolate, unlobed. Leaflet attachment with petiolule. Apex acute. Base cuneate-decurrent, asymmetrical. Margin serrate, teeth simple, distantly-spaced, sinuses smooth. Venation craspedodromous or cemicraspedodromous, development normal. Lateral veins well-spaced, not decurrent on midrib. First order lateral veins running direct to apices of teeth or becoming tangential with the margin, losing distinction towards the margins, no clear lateral loops. Finer venation indistinct.
Remarks. Identification: Compares with “Lomatia” preferruginea (Proteaceae) from the early Eocene of the Laguna del Hunco flora, Patagonia (Wilf et al., 2003; Gonzalez et al., 2007).
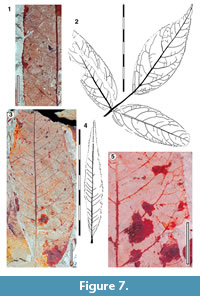
Leaves pinately trifoliate
Parataxon: DINM-JJC
Figure 7
Referred specimens. QMF34326, QMF35280, QMF35285, QMF35290, QMF35296, QMF35310, QMF35324, QMF35338, QMF35341, QMF35348, QMF35350, QMF35351, QMF35352, QMF34355.
Description. Organisation: pinnately trifoliate. Size: length 55-150 mm, width 11-43 mm, microphyll-notophyll. Shape elliptic, unlobed. Leaflet attachment lateral leaflets sessile, terminal leaflet with petiolule. Apex acute, base cuneate, rounded-acute. Margin serrate, teeth simple, closely-spaced, several teeth per lateral, sinuses sharp. Venation externodromous. Development normal. Lateral veins well-spaced, not decurrent on midrib, strongly arched, gradually loosing coherence towards the margins, as more external veins branch off, no clear lateral loops.
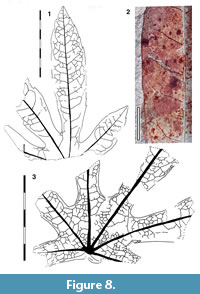
Leaves simple, palmately lobed, and toothed, no domatia
Parataxon: DINM-JJD
Figure 8
Referred specimens. QMF34366, QMF49484.
Description. Organisation: simple. Size: lobe length c. 130-170 mm, width c. 26-34 mm, mesophyll. Shape: palmately lobed, five to six lobes, lobes broadest in the middle (elliptical), apex acute. Whole leaf base cordate. Margin serrate, teeth simple, distantly-spaced, irregular, probably about one lateral vein per tooth, a vein runs from the lateral loop to the sinus or apical margin of the tooth. Venation externodromous. Development normal. Lateral veins well-spaced, not decurrent on midrib, lateral loops fine but distinct, looping zone narrow. Domatia absent.
Remarks. This leaf is probably conspecific with Ettingshausen’s (1895) Aralia subformosa, and similar, if not identical to Parafatsia subpeltata, which was initially described from the middle Eocene of Maslin Bay, South Australia by Blackburn (1981) as Araliaceae. Subsequent reinvestigation of this species and its cuticular anatomy by Carpenter et al. (2006) resulted in its reassignment to the Proteaceae. This may also apply to the similar ‘cf. Cochlospermum ’ recorded from central Australia by Greenwood (1996). A more broadly similar form from the early Eocene Laguna del Hunco flora of Argentina was placed in Cochlospermum by Berry (1935). More illustrations of this taxon and a wider comparison would be welcome.
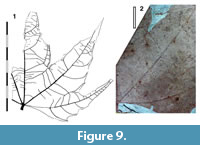
Leaves simple, palmately lobed, not toothed, no domatia
Parataxon: DINM-JJE
Figure 9
Description. Organisation: simple. Size: length c. 88 mm, width c. 138 mm, mesophyll. Shape: palmately lobed, probably five lobes, tapering to a point (acute-triangular), apex straight, base truncate-cordate. Margin serrate, teeth simple, distantly-spaced, irregular, probably about one lateral vein per tooth. Venation externodromous with the veins becoming tangential to the margin, or perhaps also craspedodromous. Lateral veins well-spaced, not decurrent on midrib. Domatia scattered along the primary and first-order laterals at the junction of the tertiary veins.
Leaves simple, trilobed, and entire
Parataxon: DINM-JJG
Figure 10
Referred specimens. QMF35311, QMF35343.
Description. Organisation: simple. Size: length c. 95 mm, width c. 100 mm, mesophyll. Shape: palmately lobed, three-five lobes, lobes narrowing to a rounded apex, base unknown, probably acute. Margin entire. Venation externodromous. Basal lateral veins prominent, probably paired or nearly so, near the base, with prominent external and thinner counter-external veins. Lateral veins well-spaced, not decurrent on midrib. Second order venation markedly percurrent.
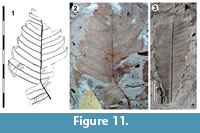
Leaves simple, with toothed margin, not lobed
Parataxon: DINM-JJH
Figure 11
Referred specimens. QMF35303.
Description. Organisation: simple. Size: length c. 56 mm-unclear, width c. 18-44 mm, microphyll-notophyll. Shape: probably elliptical, unlobed, apex unknown, base acute. Margin dentate-serrate to mucronate, teeth simple, closely-spaced, mostly one tooth per two laterals, convex on both margins, sinuses sharp. Venation craspedodromous. Development normal. Lateral veins well-spaced, regular, not decurrent on midrib. First order lateral veins alternately running to sinuses and tooth apices.
Remarks. Identification: Possibly Proteaceae. The alternation of veins running to teeth and sinuses is found in extant Banksia and in the fossil taxa Banksieaephyllum and Banksieaeformis (Cookson and Duigan, 1950; Hill and Christophel, 1988). Some of these fossil taxa are clearly Proteaceae, but Carpenter et al. (2016) have pointed out that some do not have epidermal details typical of Proteaceae, and are therefore unlikely to belong in the family. Of relevance here is a taxon that was first described as Banksia fastigata (Deane, 1925), which is smaller, but has a similar venation and margin as the Dinmore material. This was placed in Banksieaephyllum by Cookson and Duigan (1950), a genus accepted as Proteaceae by Hill and Christophel (1988). Carpenter et al. (2016) reconsidered the material and concluded that the stomata “do not appear brachyparacytic,” and both the form and location (restricted to epidermal cell junctions) of the trichome bases were not typical of the Proteaceae. They moved the species into a new genus, Pseudobanksia, of unknown affinity. The absence of brachyparacytic stomata does not clearly rule out identification as Proteaceae, as extant Bellendena does not have them either (Carpenter et al., 2005). But the combination of stomatal and trichome details are a compelling argument that the Dinmore material should not automatically be considered Proteaceae.
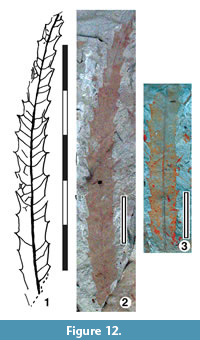
Parataxon: DINM-JJI
Figure 12
Referred specimens. QMF35362.
Description. Organisation: simple. Size: length c. 75 mm, width c. 7 mm, microphyll. Shape: lanceolate, unlobed, straight to slightly curved, apex probably acute, base unknown, assumed acute. Margin serrate-spinose, teeth simple, sinuses smooth. Venation craspedodromous. Development normal. Lateral veins well-spaced, not decurrent on midrib. First order lateral vein termination alternately running to sinuses and tooth apices.
Parataxon: DINM-JBB
Figure 13
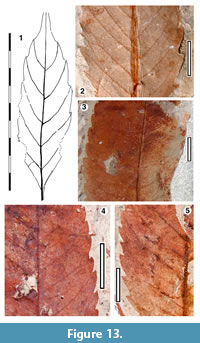 Reference specimen. QMF35373A,B.
Reference specimen. QMF35373A,B.
Referred specimens. QMF22638, QMF34292, QMF34302, QMF34305, QMF34311, QMF34344, QMF34349, QMF34357, QMF34358, QMF34361, QMF34365, QMF34369, QMF34370, QMF34374, QMF34376, QMF34377, QMF34378, QMF35282, QMF35297A,B, QMF35320, QMF35322, QMF35323, QMF35333, QMF35364, QMF49459, QMF49467A,B, QMF49472A,B, QMF55781A,B, QMF55786, QMF55791, QMF55792, QMF55801, QMF55815.
Description. Organisation: simple. Size: length 46-102 mm, width 16-45 mm, microphyll-notophyll. Shape: elliptic, unlobed, apex attenuate, base acute-cuneate. Margin serrate to slightly crenate, teeth simple, closely to distantly-spaced, mostly one tooth per lateral, sinuses mostly sharp. Venation craspedodromous. Development normal. Lateral veins well-spaced, not decurrent on midrib, sometimes forking, running to tooth apex.
Remarks. Without cuticle information this remains unclear, but is probably conspecific with Ettingshausen’s (1895) Myrica.
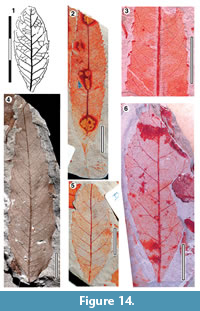
Parataxon JBJ
Figure 14
Referred specimens. QMF34309, QMF34315, QMF34318, QMF34337, QMF34359, QMF49458A,B, QMF49461A,B, QMF49494A,B, QMF55788, QMF55799A,B, QMF55798.
Description. Organisation: simple. Size: length 41-73 mm, width 12-42 mm, microphyll. Shape: elliptic, unlobed, apex unclear, probably acute but one specimen appears rounded, base acute-cuneate. Margin serrate, teeth simple, closely to distantly spaced, sometimes glandular-tipped, mostly one or two teeth per lateral, sinuses sharp. Venation externodromous. Development normal. Lateral veins well-spaced, not decurrent on midrib, course irregular, straight, or curving towards the margin, frequently forking where external veins branch off, running to sinus or most apical margin of tooth.
Remarks. The elliptical shape with a relatively rounded apex and acute-cuneate base, in combination with frequently forking lateral veins, suggest Cunoniaceae or Elaeocarpaceae (compare with a range of figures in Hyland et al., 2003).
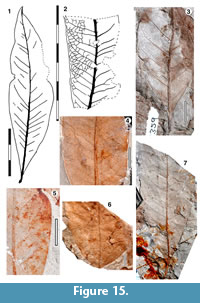
Leaves simple, with entire margin, not lobed
Parataxon JBH
Figure 15
Referred specimens. QMF34293, QMF34298A,B, QMF34308, QMF34372, QMF34381, QMF35299, QMF35330, QMF35331, QMF35336, QMF35337, QMF35359, QMF35340, QMF49465, QMF49475A,B, QMF49476, QMF49478.
Description. Organisation: simple. Size: length 80-110 mm, width 21-45 (perhaps >60) mm, microphyll-notophyll. Shape: elliptical-obovate, unlobed, apex acute, base acute. Margin entire. Venation externodromous. Development normal. Lateral veins well-spaced, not decurrent on midrib, losing coherence towards the margins, lateral loops poorly distinct.
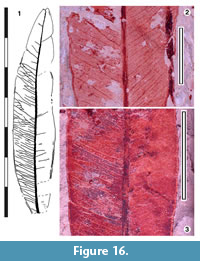
Parataxon: DINM-JAB
Figure 16
Referred specimens. QMF34373, QMF35329.
Description. Organisation: simple. Size: length 70-c. 110 mm, width 13-24 mm, microphyll. Shape: elliptic, unlobed, apex acute, base assumed acute. Margin entire. Venation with intramarginal vein. Development normal. Lateral veins closely-spaced, not decurrent on midrib, running either direct to the intramarginal vein, or branching first. Oil glands numerous in inter-venal areas.
Remarks. Identification: Eucalyptus s.l., on the basis of the intramarginal vein and laminar oil glands. It is the wider of two Eucalyptus taxa at Dinmore. These will be conspecific with some of Ettingshausen’s (1895) five species of Eucalyptus.
Parataxon: DINM-JAH
Figure 17
Referred specimens. QMF35287, QMF35283, QMF35293, QMF35298, QMF35313, QMF34334, QMF49456, QMF49492, QMF55800.
Description. Organisation: simple. Size: length 45-67 mm, width 7.5-8 mm, microphyll. Shape: narrow elliptic to narrow ovate, unlobed, apex acute, base decurrent, acute. Margin entire. Venation with intramarginal vein, no looping zone. Development normal. Lateral veins closely-spaced, not decurrent on midrib. Oil glands scattered over lamina.
Remarks. Identification: DINM-JAH is regarded as Eucalyptus s.l. (i.e. falling within the clade which includes Eucalyptus, Corymbia and Angophora, e.g., Hill and Johnson, 1995; Ladiges et al., 1995; Brooker, 2000) on the basis of the narrow and sometimes falcate shape and the presence of an intramarginal vein, or more specifically, a longitudinal vein. Pole (1991), drawing on Carr et al., 1986) discussed how a longitudinal vein differs from the typically broad usage of an intramarginal vein. A longitudinal vein appears as a single entity, thicker than other veins, which may anastomose with it. The term ‘intramarginal’ vein does not distinguish between an individual vein and one which has formed from the loops of succeeding lateral (or secondary) veins. The later can be identified by close observation. Longitudinal veins (see Carr et al., 1986) are a feature of Eucalyptus s.l. leaves, distinguishing them from leaves of Eucalyptus ‘look-alikes’, such as Syzygium eucalyptoides, which have an intramarginal vein, formed of thickened loops. DINM-JAH is the narrower of two Eucalyptus taxa at Dinmore. Similar forms were recorded by Ettingshausen (1895) as Eucalyptus from the Oxley Basin.
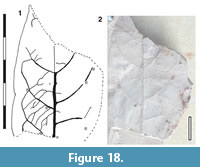
Parataxon: DINM-JAC
Figure 18
Description. Organisation: simple. Size: length unknown, width c. 36 mm, probably notophyll. Shape: not clear. Apex and base unknown. Margin entire. Venation externodromous. Development normal. Lateral veins well-spaced, not decurrent on midrib. Domatia apparently present along lateral veins.
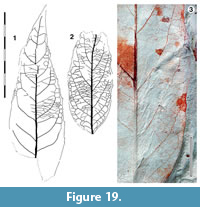
Parataxon: DINM-JAD
Figure 19
Referred specimen. QMF49485.
Description. Organisation: Size: length 85-c. 148 mm, width 35-45 mm, notophyll. Shape ovate, unlobed, apex acute, base possibly rounded. Margin irregularly serrate-entire, teeth simple, distantly-spaced, irregular, sometimes up to three teeth per lateral, Sinuses smooth. Venation externodromous. Development normal. Lateral veins well-spaced, furthest apart in mid lamina, decreasing towards apex and base, decurrent, or often decurrent, on midrib, losing coherence towards the margins as many external veins branch off.
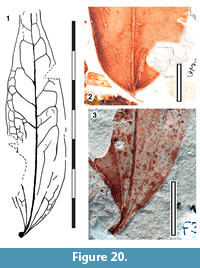
Parataxon: DINM-JAE
Figure 20
Referred specimens. QMF34325, QMF35294, QMF35306, QMF35369, QMF55801.
Description. Organisation: simple. Size: length 26-90 mm, width 22-25 mm, microphyll. Shape: ovate, unlobed, apex unknown, base cuneate-decurrent to rounded. Margin entire. Development acrodromous, basal lateral veins paired, entering the leaf base/petiole. Lateral veins well-spaced, not decurrent on midrib, well-defined lateral loops. Domatia absent.
Remarks. Identification: Lauraceae.
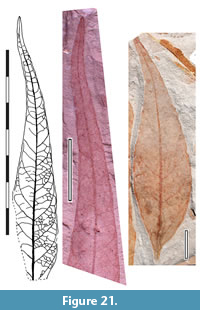
Parataxon: DINM-JAF
Figure 21
Referred specimens. QMF34294, QMF34320, QMF34321, QMF35325, QMF34379,, QMF35325, QMF35374, QMF49477.
Description. Organisation: probably simple. Size: length 100-c. 110 mm, width 14-c. 32 mm, microphyll or notophyll. Shape: ovate, unlobed, slightly flexuous, apex attenuate, base unknown. Margin entire. Venation externodromous, with an apparent intramarginal vein, at least near the base. Development normal. Lateral veins well-spaced, not decurrent on midrib, relatively thin, running towards and almost tangential with the margin, resulting in little or no looping zone. Higher order venation consists of a loose network broadly oriented at a higher angle to the midrib than the lateral veins. Laminar glands possibly present.
Remarks. Identification: possibly Myrtaceae.
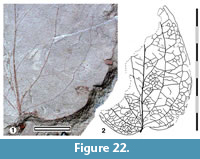
Parataxon: DINM-JAG
Figure 22
Description. Organisation: simple. Size: length c. 86 mm, width c. 60 mm, microphyll-notophyll. Shape: probably ovate, unlobed, apex unknown, base cordate. Margin entire. Lateral veins well-spaced, two to three sets of longitudinal veins (sensu Pole, 1991) on each side of the midrib, decurrent on midrib. Well-developed looping zone outside the outermost longitudinal veins.
Remarks. The venation pattern suggests Smilacaceae, although to no specific extant genus (Inamdar et al., 1983; Pole, 1993a, 1996; Carpenter et al., 2014).
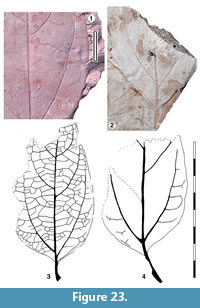
Parataxon: DINM-JAJ
Figure 23
Referred specimens. QMF34329, QMF49531.
Description. Organisation: simple. Size: length c. 28-c. 120 mm, width 24-45 mm, microphyll-notophyll. Shape: ovate, unlobed, apex unknown, base cuneate. Margin entire. Venation externodromous. Development acrodromous with the basal lateral veins almost paired, well above (c. 5-10 mm) the base of the leaf. Lateral veins well-spaced, slightly decurrent on midrib, strongly arched.
Remarks. Identification: probably Lauraceae. This taxon is probably conspecific with Ettingshausen’s (1885) Cinnamomum species. This genus was once applied widely to acrodromous leaves in the past but it occurs in several Lauraceae genera and even other families. Without cuticle details (e.g., Hill, 1986; Christophel and Rowett, 1996), a generic name is unwarranted, although the taxon exhibits basal lateral veins which are almost paired, but well above the leaf base, and a modest amount of development above the basal laterals ‒ a combination suggesting Cryptocarya (see the range of figures in Christophel and Rowett, 1996).
DISCUSSION
Floristics
Most of Ettingshausen’s (1895) identifications are either unlikely (e.g., Quercus) or are probably taxonomically close, though not warranted by the poor preservation (e.g., Cinnamomum), or on the basis of his figures, cannot reliably be related to the material here. However, his record of Eucalyptus s.l. leaves is accepted. It is supported by Rozefeld’s (1996) Myrtaceae fruits, which appear more similar to Eucalyptus than any other genus. The Dinmore specimens therefore seem to represent some of the oldest Eucalyptus known, although they probably post-date those from Laguna del Hunco in Patagonia (Gandolfo et al., 2011).
Climatic Interpretation
Foliar physiognomy is a well-established source of climatic information. However, the accuracy and most appropriate methods to extract it are subject to vigorous debate. Because Dinmore is distant from other floras of similar age, it provides a potentially useful window into the paleoclimate.
Leaf physiognomy. Of the 20 species described from Dinmore, eight (40 %) have entire margins. Based on a range of forest-inventory based univariate regression equations (Wolfe, 1979; Greenwood, 1992; Wing and Greenwood, 1993; Wilf, 1997; Greenwood et al., 2004; Gregory-Wodzicki, 2000; Traiser et al., 2005; Miller et al., 2006; Steart et al., 2010; Su et al., 2010; Kennedy et al., 2014) a MAT of around 8-14 °C is suggested.
The multivariate CLAMP method (Yang et al., 2011, using both Physg3brcAZ and PhysgAsia2 calibration sets) predicts a similar MAT to the leaf margin approach of around 12-15 °C, but with strong seasonality (a Cool Month Minima c. 0-3 °C). The CLAMP analysis predicts just 16-17 mm precipitation for the three driest months, and around 140-166 mm for the entire growing season. Realistic errors for both the univariate and CLAMP methods may be around ± 5°C (e.g., Royer, 2012), and the accuracy of CLAMP precipitation figures is probably far less than for temperature. But the broad indication is that seasonal dryness may have been a significant factor.
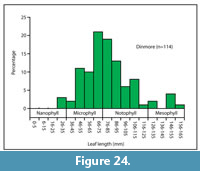 Leaf size. The average length of the Dinmore leaves is around 82 mm (Figure 24). Assuming an approximately elliptical shaped leaf where the area is two-thirds of the rectangular area of length times width (Cain et al., 1956; Webb, 1959) the average leaf size therefore lies close to the boundary of the microphyll-notophyll size classes (Webb, 1959). Average leaf size in Australian rainforest litter is closely correlated with MAT (Greenwood, 1992; Carpenter et al., 1994) and based on this correlation, the average Dinmore leaf size suggests a MAT of around 18-19 °C.
Leaf size. The average length of the Dinmore leaves is around 82 mm (Figure 24). Assuming an approximately elliptical shaped leaf where the area is two-thirds of the rectangular area of length times width (Cain et al., 1956; Webb, 1959) the average leaf size therefore lies close to the boundary of the microphyll-notophyll size classes (Webb, 1959). Average leaf size in Australian rainforest litter is closely correlated with MAT (Greenwood, 1992; Carpenter et al., 1994) and based on this correlation, the average Dinmore leaf size suggests a MAT of around 18-19 °C.
The overall range of MAT results, 8-19 °C, is remarkably wide, and the large difference between leaf physiognomy and leaf area results prompts some discussion. Wolfe (1979) suggested that leaf margin analysis requires at least 20 taxa to be reasonably accurate. At 20 taxa, the Dinmore assemblage is therefore marginal for the technique. Some entire margined taxa may have been grouped, and thus their overall proportion underestimated (entire-margined taxa are probably more-likely to be grouped than toothed ones). An entire-margined proportion of about 60% is needed for the leaf margin MAT predictions to match those of leaf area. In that case, a revision of the assemblage would need to describe an additional nine entire-margined taxa (and no more toothed ones). This is regarded as unlikely.
Average leaf area is specimen-based and avoids the potential problem of an incorrect taxonomy (Greenwood, 1992). However, despite the higher MAT indicated by average leaf size, this is likely to still be a minimum. Factors other than temperature are known to affect leaf size; a dry season and/or low nutrient soils will tend to decrease it (e.g., Webb, 1959; Adam, 1992) and thus result in a lower than predicted MAT.
It is unlikely that the Dinmore assemblage represents a type of vegetation that is currently extant, but not yet sampled by the various researchers of leaf physiognomy. The high proportion of teeth, but relatively large leaves, suggests a physiognomy, and perhaps a climate, that is not represented today. The presence of three prominently lobed taxa (15%) in a small flora is also curious, and appears to be outside the range for evergreen rainforest as represented in the various calibration datasets of CLAMP lodged online. In these datasets, comparable proportions of lobed species occur only in dry/open or deciduous vegetation (although it is noted that some of the South African datasets treat compound leaves, e.g., Acacia, as lobed). Other factors argue that Dinmore was likely either relatively dry or had a dry season. These include both the presence of elongate leaves (with Length: Width >4) and their identification of Eucalyptus, as well as the general absence of coal in the region and rarity of Nothofagus pollen (Harris, 1965, proposed a geographical control for this genus from southern to central Australia in the early Cenozoic). Dinmore was possibly a form of dry rainforest, and therefore with moisture at least seasonally limited, leaf size would under-represent MAT. The results reinforce a previous observation that MAT based on leaf-litter size and inventory-based margin proportions were slightly different (Pole, 2010). Note that this is a different issue too where Dinmore, dominated by microphyll and notophyll leaves, would likely be classified as a ‘notophyll’ forest if it grew in Australia today (Webb, 1959; Greenwood, 1992; Pole, 2014).
The study recognised 20 leaf taxa out of 169 fossil specimens, which were considered identifiable to taxa. On a number of specimens versus number of taxa graph, this plots on a moderately steep line (see Pole, 2014, fig. 11), and this diversity is consistent with the Dinmore assemblage accumulating under relatively warm conditions.
Currently the Dinmore area has a mean annual temperature (MAT) of c. 20 °C and lies at about 27 °S latitude. Thus the leaf area results suggest an early Eocene MAT at least as high as today. However, around 50 Ma Dinmore would have lain in significantly higher latitudes - about 46 °S (using GPlates with the Muller et al., 2008, rotation model). That latitude is higher than Tasmania today and is equivalent to southernmost New Zealand. Coastal MATs in both areas today are around 10 °C. The results are consistent with a wide range of other proxies for significantly elevated sea surface temperatures at mid-latitudes in the Southern Ocean early Eocene (e.g., Davies et al., 1989; Feary et al., 1991; Ivany et al., 2008; Creech et al., 2010; Contreras et al., 2013; Pancost et al., 2013) and globally (e.g., Greenwood and Wing, 1995; Huber, and Caballero, 2011). Hollis et al. (2009, fig. 2) modelled sea surface temperatures of 20-25 °C offshore the Brisbane region in the early Eocene. Given the inherent error, the Dinmore results compare favourably with the cooler end of this range. However, some predictions for mid-latitude temperatures at this time (e.g., Naafs et al., 2018) are higher still.
Comparison with Other Fossil Floras
The Dinmore assemblage, with just two conifer taxa, is highly distinct from the higher latitude early Eocene assemblage of Regatta Point, in Tasmania where they are abundant and diverse (Wells and Hill, 1989; Hill and Carpenter, 1991). Lower paleolatitude early Eocene assemblages from Victoria include Hotham Heights and Brandy Creek, and Deans Marsh (Greenwood et al., 2003; Carpenter et al., 2004). In those assemblages conifers appear to have been less diverse than Tasmania, but still much more prominent than Dinmore. This broad gradient is likely to be another reflection of drier conditions in Queensland (e.g., Brodribb and Hill, 1998).
Closer physiognomic comparison with Dinmore is difficult, as the Victorian and Tasmanian fossil assemblages tend to be known from dispersed cuticle rather than leaf impressions. Eucalyptus is not yet known from any of them.
The general physiognomy of the Dinmore assemblage is comparable to lower latitude fossil assemblages of Melville Island, in northernmost Australia (White, 1976; Pole and Bowman, 1996; Pole, 1998). The Melville assemblages are also angiosperm dominated with minor conifers (including Araucaria), also have prominent lobed, compound and very narrow leaves (Eucalyptus s.l. is potentially present, but venation details are lacking). They are of an unknown age but were suggested to be early Cenozoic by Pole (1998). The similarity with Dinmore is consistent with that conclusion. Eocene material in central Australia also includes some of these morphologies (Greenwood, 1996).
In New Zealand, broadly similar foliar physiognomies (lobed and narrow leaves) are seen in the early Eocene of Kakahu (Pole, 1997, 2010, work in progress has also recovered Eucalyptus s.l.) and the mid Eocene of Livingstone, New Zealand (Pole, 1994). Further afield, the early Eocene flora of Laguna del Hunco, Patagonia (Wilf et al., 2003, 2005; Carpenter et al., 2014) lay at approximately the same paleolatitude as Dinmore. It has a far higher diversity than Dinmore, and with more conifers, but has a more prominent microphyll component. Despite these differences, it has several angiosperm leaf forms similar to Dinmore, including Eucalyptus (Gandolfo et al., 2011).
The leaf assemblages at Dinmore and Melville Island in Australia, Livingstone in New Zealand, and Laguna del Hunco, in Patagonia suggest a remarkably similar flora over a what would have been the mid latitudes of the Southern Hemisphere. All floras hint at seasonal dryness, and perhaps all contain Eucalyptus s.l. To account for the light-demanding Eucalyptus at Lajuna del Hunco, Gandolfo et al. (2011) proposed a mosaic, with Eucalyptus dominating on disturbed areas related to volcanism, with “intact rainforest” adjacent on more stable areas. This is certainly possible, and the argument has been used (e.g., New Zealand: Pole, 1993b; Australia: Pole et al., 1993) to account for Eucalyptus fossils co-occurring with Nothofagus in situations interpreted in terms of modern-day fire and wet-rainforest interaction in Tasmania (Jackson, 1968). However, at least in an early Miocene New Zealand deposit (Pole, 1993b,c), Eucalyptus was prominent in one assemblage, while in associated assemblages representing unmixed rainforest, it appeared absent. Finding Eucalyptus -dominated assemblages as well as those where it was a minor component amongst rainforest taxa would be expected under a fire-disturbance model. But the repeated presence of fossil Eucalyptus s.l. leaves in diverse assemblages of an otherwise, ‘rainforest’ aspect in the early Cenozoic should give pause for thought. These might rather be taken at face-value, as indicating Eucalyptus s.l. as a ‘normal’ component of the vegetation. There is little doubt that the group of plants which gave rise to the Angophora-Corymbia-Eucalyptus clade (i.e., Arillastrum and the clade currently including Allosyncarpia, Stockwellia, and Eucalyptopsis, Macphail and Thornhill, 2016) were rainforest plants. However, the current paper suggests that even the ecology of early members of the Angophora-Corymbia-Eucalyptus clade may not have been fire-based. This conflicts with evidence that Eucalyptus s.l. origins were intimately connected with fire (see Crisp et al., 2011), but is more consistent with the later spread of fire (Scott, 2000; Belcher et al., 2013) triggering its expansion. Perhaps something about early Eocene conditions allowed early Eucalyptus s.l. to perpetuate in non-burning, rainforest and not be reliant on fire. A minority of extant taxa, e.g., Eucalyptus deglupta, may perpetuate such a lifestyle.
CONCLUSIONS
The early Eocene Dinmore leaf fossil assemblage is important as being one of the few of Cenozoic age located away from the south-eastern corner of Australia. As such it provides an important data point for what the vegetation and climate were doing in this more northerly region, in the early Eocene. It records an angiosperm-dominated flora, including one of the oldest records of Eucalyptus s.l. in the world (consistent with the results of Macphail and Thornhill, 2016), along with probable rainforest taxa, and a minor conifer component. Despite being at a significantly higher latitude at around 50 Ma, the MAT was probably much higher than at that latitude today. The assemblage has broad physiognomic and floristic similarity with the poorly-dated, most northern assemblages in Australia, on Melville Island, as well as with New Zealand and Patagonia.
ACKNOWLEDGEMENTS
A special thanks to those individuals who collected the bulk of the collection; S. McLoughlin, R. Knezour, A. Rix, A. Rozefelds, and J.T. Woods. Thanks also to the staff at the Queensland Museum, particularly K. Spring and A. Cook, who allowed me to work on the material, and provided space and facilities. A. Rozefelds and an anonymous reviewer are gratefully acknowledged for improving the paper.
REFERENCES
Adam, P. 1992. Australian Rainforests. Oxford University Press, Oxford.
Belcher, C.M., Collinson, M.E., and Scott, A.C. 2013. A 450-million-year history of fire, p. 229-249. In Belcher, C.M. (ed.), Fire Phenomena and the Earth System: An Interdisciplinary Guide to Fire Science. John Wiley & Sons, Oxford. https://doi.org/10.1002/9781118529539.ch12
Berry, A.K. 1935. A fossil Cochlospermum from northern Patagonia. Bulletin of the Torrey Botanical Club, 62:65-67. https://doi.org/10.2307/2480867
Blackburn, D.T. 1981. Tertiary megafossil flora of Maslin Bay, South Australia: numerical taxonomic study of selected leaves. Alcheringa, 5:9-28. https://doi.org/10.1080/03115518108565430
Brodribb, T. and Hill, R.S. 1998. The photosynthetic drought physiology of a diverse group of southern hemisphere conifer species is correlated with minimum seasonal rainfall. Functional Ecology, 12:465-471. https://doi.org/10.1046/j.1365-2435.1998.00213.x
Brooker, M.I.H. 2000. A new classification of the genus Eucalyptus L'Hér. (Myrtaceae). Australian Systematic Botany, 13:79-148. https://doi.org/10.1071/sb98008
Cain, S.A., de Okiviera Castro, G.M., Murca Peres, J., and da Silva, N.T. 1956. Applications of some phytosociological techniques to the Brazilian rain forest. American Journal of Botany, 43:911-941. https://doi.org/10.1002/j.1537-2197.1956.tb11184.x
Carpenter, R.J., Hill, R.S., and Jordan, G.J. 1994. Cenozoic vegetation in Tasmania. Macrofossil evidence, p. 276-298. In Hill, R.S. (ed.), History of the Australian Vegetation. Cretaceous to Recent. Cambridge University Press, Cambridge.
Carpenter, R.J., Hill, R.S., and Jordan, G.J. 2005. Leaf cuticular morphology links Platanaceae and Proteaceae. International Journal of Plant Sciences, 166:843-855. https://doi.org/10.1086/431806
Carpenter, R.J., Hill, R.S., and Scriven, L.J. 2006. Palmately lobed Proteaceae leaf fossils from the middle Eocene of South Australia. International Journal of Plant Science, 167:1049-1060. https://doi.org/10.1086/505537
Carpenter, R.J., Hill, R.S., Greenwood, D.R., Partridge, A.D., and Banks, M.A. 2004. No snow in the mountains: Early Eocene plant fossils from Hotham Heights, Victoria, Australia. Australian Journal of Botany, 52:685-718. https://doi.org/10.1071/bt04032
Carpenter, R.J. and Pole, M.S. 1995. Eocene plant fossils from the Lefroy and Cowan paleodrainages, western Australia. Australian Systematic Botany, 8:1107-1154. https://doi.org/10.1071/sb9951107
Carpenter, R.J., Goodwin, M.P., Hill, R.S., and Kanold, K. 2011. Silcrete plant fossils from Lightning Ridge, New South Wales: new evidence for climate change and monsoon elements in the Australian Cenozoic. Australian Journal of Botany, 59:399-342. https://doi.org/10.1071/bt11037
Carpenter, R.J., Jordan, G.J., and Hill, R.S. 2016. Fossil leaves of Banksia, Banksieae and pretenders: resolving the fossil genus Banksieaephyllum. Australian Systematic Botany, 29:126-141. https://doi.org/10.1071/sb16005
Carpenter, Raymond J., Wilf, P., Conran, J.G., and Cúneo, N. R. 2014. A Paleogene trans-Antarctic distribution for Ripogonum (Ripogonaceae: Liliales)? Palaeontologia Electronica 17.3.39A: 1-9. https://doi.org/10.26879/460
palaeo-electronica.org/content/2014/921-early-eocene-ripogonum
Christophel, D.C., Scriven, L.J., and Greenwood, D.R. 1992. An Eocene megafossil flora from Nelly Creek, South Australia. Transactions of the Royal Society of South Australia, 116:65-76.
Christophel, D.C. and Rowett, A.I. 1996. Leaf and cuticle atlas of Australian leafy Lauraceae. Flora of Australia Supplementary Series, Number 6. Australian Biological Resources Study, Canberra.
Churchill, D.M. 1969. The fossil occurrence of Lygodium in Australia. Neues Jahrbuch für Geologie und Paläontologie. Monatshefte, 5:257-265.
Clifford, H.T. and Dettman, M.E. 2002. A winged fruit from the Tertiary of Queensland. Memoirs of the Queensland Museum, 48:79-83.
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J.-X. 2013; updated. The ICS International Chronostratigraphic Chart. Episodes, 36:199-204
Conran, J.G. and Rozefelds, A.C. 2003. Palmoxylon queenslandicum: a permineralised Oligocene palm trunk from near Springsure, southeastern Queensland. Alcheringa, 27:125-134. https://doi.org/10.1080/03115510308619553
Contreras, L., Pross, J., Bijl, P.K., Koutsodendris, A., Raine, J.I., Schootbrugge, B. van der, and Brinkhuis, H. 2013. Early to middle Eocene vegetation dynamics at the Wilkes Land Margin (Antarctica). Review of Palaeobotany and Palynology, 197:119-142. https://doi.org/10.1016/j.revpalbo.2013.05.009
Cookson, I.C. and Duigan, S.L. 1950. Fossil Banksieae from Yallourn, Victoria, with notes on the morphology and anatomy of living species. Australian Journal of Scientific Research Series, B3:133-165. https://doi.org/10.1071/bi9500133
Cranfield, L.C., Schwarzbock, H., and Day, R.W. 1976. Geology of the Ipswich and Brisbane 1:250 000 sheet areas. Geological Survey of Queensland Report, 95:1-175.
Creech, J.B., Baker, J.A., Hollis, C.J., Morgans, H.E.G., and Smith, E.G.C. 2010. Eocene sea temperatures for the mid-latitude southwest Pacific from Mg/Ca ratios in planktonic and benthic foraminifera. Earth and Planetary Science Letters, 299:483-495. https://doi.org/10.1016/j.epsl.2010.09.039
Crisp, M.D., Burrows, G.E., Cook, L.G., Thornhill, A.H., and Bowman, D.M.J.S. 2011. Flammable biomes dominated by eucalypts originated at the Cretaceous-Paleogene boundary. Nature Communications, 193:2-8. https://doi.org/10.1038/ncomms1191
Day, R.W., Whitaker, W.G., Murray, C.G., Wilson, I.H., and Grimes, K.G. 1982. Queensland geology. A companion volume to the 1:250,000 scale geological map 1975. Geological Survey of Queensland, Publication, 383. Government Printer, Queensland.
Davies, P.J., Symonds, P.A., Feary, D.A., and Pigram, C.J. 1989.The evolution of the carbonate platforms of northeast Australia. Controls on carbonate platform and basin development. SEPM Special Publication, 44:233-258. https://doi.org/10.2110/pec.89.44.0233
Deane H. 1925. Fossil leaves from the open cut, State Brown Coal Mine, Morwell. Records of the Geological Survey of Victoria, 4:492-498.
Dilcher, D.L. 1974. Approaches to the identification of angiosperm leaf remains. The Botanical Review, 40:1-157. https://doi.org/10.1007/bf02860067
Ellis, B., Daly, D.C., Hickey, L.J., Mitchell, J.V., Johnson, K.R., Wilf, P., and Wing, S.L. 2009. Manual of Leaf Architecture. Cornell University Press, Ithaca.
Feary, D.A., Davies, P.J., Pigram, C.J., and Symonds, P.A. 1991. Climatic evolution and control on carbonate deposition in northeast Australia. Palaeogeography, Palaeoclimatology, Palaeoecology, 89:341-361. https://doi.org/10.1016/0921-8181(91)90116-e
Gandolfo, M.A., Hermsen, E.J., Zamaloa, M.C., Nixon, K.C., González, C.C., Wilf, P., Cúneo, N.R., and Johnson, K.R. 2011. Oldest known Eucalyptus macrofossils are from South America. PLoS ONE, 6:1-9 e21084. https://doi.org/10.1371/journal.pone.0021084
Gonzalez, C.C., Gandolfo, M.A., Zamaloa, M.C., Cúneo, N.R., Wilf, P., and Johnson, K.R. 2007. Revision of the Proteaceae macrofossil record from Patagonia, Argentina. The Botanical Review, 73:235-266. https://doi.org/10.1663/0006-8101(2007)73[235:rotpmr]2.0.co;2
Green, D.C. and Stevens, N.C. 1975. Age and stratigraphy of Tertiary volcanics and sedimentary rocks of the Ipswich district, southeast Queensland. Queensland Government Mining Journal, 76:148-150.
Greenwood, D.R. 1992. Taphonomic constraints on foliar physiognomic interpretations of Late Cretaceous and Tertiary palaeoclimates. Review of Palynology and Palaeobotany, 71:149-190. https://doi.org/10.1016/0034-6667(92)90161-9
Greenwood, D.R. 1996. Eocene monsoon forests in central Australia? Australian Systematic Botany, 9:95-112. https://doi.org/10.1071/sb9960095
Greenwood, D.R., Wilf, P., Wing, S.L., and Christophel, D.C. 2004. Paleotemperature estimation using leaf-margin analysis: is Australia different? Palaios, 19:129-142. https://doi.org/10.1669/0883-1351(2004)019<0129:peulai>2.0.co;2
Greenwood, D.R. and Wing, S.L. 1995. Eocene continental climates and latitudinal temperature gradients. Geology, 23:1044-1048. https://doi.org/10.1130/0091-7613(1995)023<1044:eccalt>2.3.co;2
Gregory-Wodzicki, K.M. 2000. Relationships between leaf morphology and climate, Bolivia: Implications for estimating paleoclimate from fossil floras. Paleobiology, 26:668-688. https://doi.org/10.1666/0094-8373(2000)026<0668:rblmac>2.0.co;2
Guerin, G.R. and Hill, R.S. 2006. Plant macrofossil evidence for the environment associated with the Riversleigh fauna. Australian Journal of Botany, 54:717-731. https://doi.org/10.1071/bt04220
Harris, W.K. 1965. Tertiary microfloras from Brisbane, Queensland. Geological Survey of Queensland Report, 10:1-7.
Head, M.J., Aubry, M.-P., Walker, M., Miller, K.G., and Pratt, B.R. 2017. A case for formalizing subseries (subepochs) of the Cenozoic Era. Episodes, 40:22-27. https://doi.org/10.18814/epiiugs/2017/v40i1/017004
Hickey, L.J. 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany, 60:17-33. https://doi.org/10.2307/2441319
Hill, D., Playford, G., and Woods, J.T. 1970. Cainozoic Fossils of Queensland. Queensland Palaeontological Society, Brisbane.
Hill, K.D. and Johnson, L.A.S. 1995. Systematic studies in the Eucalypts 7. A revision of the bloodwoods, genus Corymbia (Myrtaceae). Telopea, 6:185-504. https://doi.org/10.7751/telopea19953017
Hill, R.S. 1986. Lauraceous leaves from the Eocene of Nerriga, New South Wales. Alcheringa, 10:327-351. https://doi.org/10.1080/03115518608619144
Hill, R.S. (ed.) 1984. History of the Australian Vegetation. Cretaceous to Recent. Cambridge University Press, Cambridge.
Hill, R.S. 1988. Australian Tertiary angiosperm and gymnosperm remains - an updated catalogue. Alcheringa, 12:207-219. https://doi.org/10.1080/03115518808619133
Hill, R.S. 2004. Origins of the southeastern Australian vegetation. Philosophical Transactions of the Royal Society of London B, 359: 1537-1549. https://doi.org/10.1098/rstb.2004.1526
Hill, R.S. and Carpenter, R. 1991. Evolution of Acmopyle and Dacrycarpus (Podocarpaceae) foliage as inferred from macrofossils in south-eastern Australia. Australian Systematic Botany, 4:449-479. https://doi.org/10.1071/sb9910449
Hill, R.S. and Christophel, D.S. 1988. Tertiary leaves of the tribe Banksieae (Proteaceae) from south-eastern Australia. Botanical Journal of the Linnean Society, 97: 205-227. https://doi.org/10.1111/j.1095-8339.1988.tb02462.x
Hill, R.S., Truswell, E.M., McLoughlin, S. and Dettmann, M.E. 1999. Evolution of the Australian flora: fossil evidence, p. 251-305. In Orchard, A.E. (ed.), Flora of Australia 1. Australian Biological Resources Study, Canberra.
Hollis, C.J., Handley, L., Crouch, E.M., Morgans, H.E.G., Baker, J.A., Creech, J., Collins, K.S., Gibbs, S.J., Huber, M., Schouten, S., Zachos, J.C., and Pancost, R.D. 2009. Tropical sea temperatures in the high-latitude South Pacific during the Eocene. Geology, 37:99-102. https://doi.org/10.1130/g25200a.1
Houston, B.R. 1967. Geology of the City of Brisbane. Part II. The post-Palaeozoic sediments and volcanics. Geological Survey of Queensland Publication, 324.
Huber, M. and Caballero, R. 2011. The early Eocene equable climate problem revisited. Climate of the Past, 7:603-633. https://doi.org/10.5194/cpd-7-241-2011
Hyland, B.P.M., Whiffin, T., Christophel, D.C., Gray, B., and Elick, R.W. 2003. Australian Tropical Rain Forest Plants: Trees Shrubs and Vines. CSIRO, Melbourne.
Inamdar, J.A., Shenoy, K.N., and Rao, N.V. 1983. Leaf architecture of some monocotyledons with reticulate venation. Annals of Botany, 52:725-735. https://doi.org/10.1093/oxfordjournals.aob.a086631
Ivany, L.C., Lohmann, K.C., Hasiuk, F., Blake, D.B., Glass, A., Aronson, R.B., and Moody, R.M. 2008. Eocene climate record of a high southern latitude continental shelf: Seymour Island, Antarctica. GSA Bulletin, 120:659-678. https://doi.org/10.1130/b26269.1
Jackson, W.D. 1968. Fire, air, water and earth - an elemental ecology of Tasmania. Proceedings of the Ecological Society of Australia, 3:9-16.
Jin, J., Jue Qiu, J., Zhu, Y., and Kodrul, T.M. 2010. First fossil record of the genus Nageia (Podocarpaceae) in south China and its phytogeographic implications. Plant Systematics and Evolution, 285:159-163 https://doi.org/10.1007/s00606-010-0267-4
Jones, O.A. 1926. The Tertiary deposits of the Moreton District, South-Eastern Queensland. Proceedings of the Royal Society of Queensland, 38:23-46.
Kemp A. 1991. Australian Mesozoic and Cenozoic lungfish, p. 465-496. In Vickers-Rich P., Monaghan, J.M., Baird, R.F., and Rich, T.H. (eds.), Vertebrate Palaeontology of Australasia. Pioneer Design Studio, Lilydale.
Kennedy, E.M., Arens, N.C., Reichgelt, T., Spicer, R.A., Spicer, T.E.V., Stranks, L., and Yang, J. 2014. Deriving temperature estimates from Southern Hemisphere leaves. Palaeogeography, Palaeoclimatology, Palaeoecology, 412:80-90. https://doi.org/10.1016/j.palaeo.2014.07.015
Knezour, R. 1992. Fossil collecting in Queensland. The Fossil Collector, January 1992: 31-35.
Ladiges, P.Y., Udovicic, F., and Drinnan, A.N. (1995). Eucalypt phylogeny - molecules and morphology. Australian Systematic Botany, 8:483-487. https://doi.org/10.1071/sb9950483
Luterbacher, H.P., Ali, J.R., Brinkhuis, H., Gradstein, F.M., Hooker, J.J., Monechi, S., Ogg, J.G., Powell, J., Röhl, U., Sanfilippo, A., and Schmotz, B., 2005. The Paleogene Period, p. 384-408. In Gradstein, F.M., Ogg, J.G., and Smith, A.G. (eds.), A Geologic Time Scale 2004. Cambridge, Cambridge, University Press.
Macphail, M. and Thornhill, A.H. 2016. How old are the eucalypts? A review of the microfossil and phylogenetic evidence. Australian Journal of Botany, 64:579-599. https://doi.org/10.1071/bt16124
McLoughlin, S., and Guppy, L. 1993. Western Australia's Tertiary floras. The Fossil Collector Bulletin, May:13-22.
Miller, I.M., Brandon, M.T., and Hickey, L.J. 2006. Using leaf margin analysis to estimate the mid-Cretaceous (Albian) paleolatitude of the Baja BC block. Earth and Planetary Science Letters, 245:95-114. https://doi.org/10.1016/j.epsl.2006.02.022
Müller, R.D., Sdrolias, M., Gaina, C., and Roest, W.R., 2008. Age, spreading rates and spreading asymmetry of the world’s ocean crust. Geochemistry, Geophysics, Geosystems, 9: Q04006. https://doi.org/10.1029/2007GC001743
Naafs, B.D.A., Rohrssen, M., Inglis, G.N., Lähteenoja, O., Feakins, S.J., Collinson, M.E., Kennedy, E.M., Singh, P.K., Singh, M.P., Lunt, D.J., and Pancost, R.D. 2018. High temperatures in the terrestrial mid-latitudes during the early Palaeogene. Nature Geoscience. https://doi.org/10.1038/s41561-018-0199-0
Offler, C.E. 1984. Extant and fossil coniferales of Australia and New Guinea, part 1: a study of the external morphology of the vegetative shoots of the extant species. Palaeontographica, B193:18-120.
Pancost, R.D., Taylor, K.W.R., Inglis, G.N., Kennedy, E.M., Handley, L., Hollis, C.J., Crouch, E.M., Pross, J., Huber, M., Pearson, P.N., Morgans, H.E.G., and Raine, J.I. 2013. Early Paleogene evolution of terrestrial climate in the SW Pacific, Southern New Zealand. Geochemistry, Geophysics, Geosystems, 14:5413-5429. https://doi.org/10.1002/2013gc004935
Pole, M.S. 1991. A modified terminology for angiosperm leaf architecture. Journal of the Royal Society of New Zealand, 21:297-312. https://doi.org/10.1080/03036758.1991.10420828
Pole, M. 1993a. Early Miocene flora of the Manuherikia Group, New Zealand. 5. Smilacaceae, Polygonaceae, Elaeocarpaceae. Journal of the Royal Society of New Zealand, 23:289-302. https://doi.org/10.1080/03036758.1993.10721227
Pole, M.S. 1993b. Early Miocene flora of the Manuherikia Group, New Zealand. 10. Paleoecology and stratigraphy. Journal of the Royal Society of New Zealand, 23:393-426. https://doi.org/10.1080/03036758.1993.10721232
Pole, M.S. 1993c. Early Miocene floras of the Manuherikia Group, New Zealand. 7. Myrtaceae, including Eucalyptus. Journal of the Royal Society of New Zealand, 23:313-328. https://doi.org/10.1080/03036758.1993.10721229
Pole, M.S. 1994. An Eocene macroflora from the Taratu Formation at Livingstone, North Otago, New Zealand. Australian Journal of Botany, 42:341-367. https://doi.org/10.1071/bt9940341
Pole, M. 1996. Plant macrofossils from the Foulden Hills Diatomite (Miocene), Central Otago, New Zealand. Journal of the Royal Society of New Zealand, 26:1-39 https://doi.org/10.1080/03014223.1996.9517503
Pole, M.S. 1997. Paleocene plant macrofossils from Kakahu, south Canterbury, New Zealand. Journal of the Royal Society of New Zealand, 27:371-400. https://doi.org/10.1080/03014223.1997.9517544
Pole, M. 1998. The fossil flora of Melville Island, northern Australia. The Beagle, Records of the Museums and Art Galleries of the Northern Territory, 14:1-28.
Pole, M. 2010. Ecology of Paleocene-Eocene vegetation at Kakahu, South Canterbury, New Zealand. Palaeontologia Electronica 13.2.14A: 1-29.
https://palaeo-electronica.org/2010_2/227/index.html
Pole, M. 2014. The Miocene climate in New Zealand: Estimates from paleobotanical data. Palaeontologia Electronica 17.2.27A:1-79. https://doi.org/10.26879/436
palaeo-electronica.org/content/2014/780-miocene-climate-of-new-zealand.
Pole, M.S. and Bowman, D.M.J.S. 1996. Tertiary plant fossils from Australia's ‘Top End’. Australian Systematic Botany, 9:113-126. https://doi.org/10.1071/sb9960113
Pole, M.S., Hill, R.S., Green, N., and Macphail, M.K. 1993. The Oligocene Berwick Quarry flora - rainforest in a drying environment. Australian Systematic Botany, 6:399-427. https://doi.org/10.1071/sb9930399
Raine, J.I. 1984. Outline of a palynological zonation of Cretaceous to Paleogene terrestrial sediments in West Coast region, South Island, New Zealand. New Zealand Geological Survey Report, 109:1-82.
Riek, E.F. 1952. The fossil insects of the Tertiary Redbank Plains Series, Part 1: An outline of the fossil assemblage with descriptions of the fossil insects of the orders Mecoptera and Neuroptera. University of Queensland Department of Geology Papers, 14:3-16.
Rix, A. 1991. A re-examination of the Redbank Plains Tertiary fossil Site, Queensland. The Fossil Collector, 32/33:31-36.
Rix, A. 1992. Fossil insect larvae from the Lower Tertiary Redbank Plains site, southeast Queensland. The Fossil Collector, 36:35-37.
Royer, D. 2012. Climate reconstruction from leaf size and shape, new developments and challenges. In Ivany, L.C. and Huber, B.T. (eds.), Reconstructing Earth’s deep-time climate--the state of the art in 2012. Paleontological Society Papers, 18:195-212.
Rozefelds, A.C. 1988. A mid-Tertiary rainforest flora from Capella, central Queensland, p. 123-136. In Douglas, J.G. and Christophel, D.C. (eds.), Proceedings of the Third International Organisation of Palaeobotany symposium, A-Z Publishers, Melbourne.
Rozefelds, A.C. 1991. Mid Tertiary Sarcopetalum (Menispermaceae) from Glencoe, mid-eastern Queensland. Alcheringa, 15:145-149. https://doi.org/10.1080/03115519108619013
Rozefelds, A.C. 1992. The subtribe Hicksbeachiinae (Proteaceae) in the Australian Tertiary. Memoirs of the Queensland Museum, 32:195-202.
Rozefelds, A.C. 1996. Eucalyptus phylogeny and history: a brief summary. Tasforests,8:15-26.
Rozefelds, A.C. and Christophel, D.C. 1996. Elaeocarpus (Elaeocarpaceae) endocarps from the Oligo-Miocene of eastern Australia. Papers and Proceedings of the Royal Society of Tasmania, 130:41-48.
Rozefelds, A.C. and Christophel, D.C. 2000. Cenozoic Elaeocarpus (Elaeocarpaceae) fruits from Australia. Alcheringa, 26:261-274. https://doi.org/10.1080/03115510208619256
Rozefelds, A.C., Christophel, D.C., and Alley, N.F. 1992. Tertiary occurrence of the fern Lygodium (Schizaeaceae) in Australia and New Zealand. Memoirs of the Queensland Museum, 32:203-222.
Rozefelds, A.C., Clifford, H.T., and Lewis, D. 2015. Macrofossil evidence of early sporophyte stages of a new genus of water fern Tecaropteris (Ceratopteridoideae: Pteridaceae) from the Paleogene Redbank Plains Formation, southeast Queensland, Australia. Alcheringa, 39:1-11. https://doi.org/10.1080/03115518.2015.1069460
Rozefelds, A.C., Dettmann, M.E., Clifford, H.T., and Ekins, M. 2017. An Australian origin for the candle nut (Aleurites, Crotonoideae, Euphorbiaceae) and the fossil record of the Euphorbiaceae and related families in Australia and New Zealand. Review of Palaeobotany and Palynology, 241:39-48. https://doi.org/10.1016/j.revpalbo.2017.01.006
Rozefelds, A., Dettmann, M., Clifford, T., Hocknull, S., Newman, N., Godthelp, H., Hand, S., and Archer, M. 2014. Traditional and computed tomographic (CT) techniques link modern and Cenozoic fruits of Pleiogynium (Anacardiaceae) from Australia. Alcheringa, 39:24-39. https://doi.org/10.1080/03115518.2014.951916
Scott, A.C. 2000. The Pre-Quaternary history of fire. Palaeogeography, Palaeoclimatology, Palaeoecology, 164:281-329. https://doi.org/10.1016/s0031-0182(00)00192-9
Selling, O.H. 1950. Some Tertiary plants from Australia. Svensk Botanisk Tidskrift, 44:551-561.
Staines, H.R.E. 1960. The Ipswich area. Journal of the Geological Society of Australia, 7:346-348.
Steart, D.C., Spicer, R.A., and Bamford, M.K. 2010. Is southern Africa different? An investigation of the relationship between leaf physiognomy and climate in southern African mesic vegetation. Review of Palaeobotany and Palynology, 162:607-620. https://doi.org/10.1016/j.revpalbo.2010.08.002
Su, T., Xing, Y., Liu, Y.S., Jacques, F.M.B., Chen, W., Huang, Y., and Zhou, Z. 2010. Leaf margin analysis: a new equation from the humid to mesic forests in China. Palaios, 25:234-238. https://doi.org/10.2110/palo.2009.p09-129r
Traiser, C., Klotz, S., Uhl, D., and Mosbrugger, V. 2005. Environmental signals from leaves - a physiognomic analysis of European vegetation. New Phytologis t, 166:465-484. https://doi.org/10.1111/j.1469-8137.2005.01316.x
Von Ettingshausen, C.F. 1895. Beiträge zur Kenntniss der Kreideflora Australiens. Denkschriften der Kaiserlichen Akademie der Wissenschaften (Mathematisch-naturwissenchaftliche), 62:1-56. https://doi.org/10.5962/bhl.title.7837
Webb, L.J. 1959. A physiognomic classification of Australian rainforests. Journal of Ecology, 47:551-570. https://doi.org/10.2307/2257290
Wells, P.M. and Hill, R.S. 1989. Fossil imbricate-leaved Podocarpaceae from Tertiary sediments in Tasmania. Australian Systematic Botany, 2:387-423. https://doi.org/10.1071/sb9890387
White, M.E. 1976. Tertiary plant fossils from Melville Island, N.T. Appendix 2 in Hughes, R.J. (ed.), Bathurst and Melville Island, Northern Territory. Sheets SC/52-15 and SC/52-16 International Index. 1:250 000 Geological Series - Explanatory Notes. Australian Government, Canberra.
Wilf, P. 1997. When are leaves good thermometers? A new case for Leaf Margin Analysis. Paleobiology, 23:373-390. https://doi.org/10.1017/s0094837300019746
Wilf, P., Cúneo, N.R., Johnson, K.R., Hicks, J.F., Wing, S.L., and Obradovich, J.D. 2003. High plant diversity in Eocene South America: evidence from Patagonia. Science, 300:122-125. https://doi.org/10.1126/science.1080475
Wilf, P., Johnson, K.R., Cuneo, N.R., Smith, M.E., Singer, B.S., and Gandolfo, M.A. 2005. Eocene Plant Diversity at Laguna del Hunco and Rio Pichileufu, Patagonia, Argentina. The American Naturalist, 165:634-650. https://doi.org/10.1086/430055
Wing, S.L. and Greenwood, D.R. 1993. Fossils and fossil climate: The case for equable continental interiors in the Eocene. Philosophical Transactions: Biological Sciences, 341:243-252. https://doi.org/10.1007/978-94-011-1254-3_5
Wolfe, J.A. 1979. Temperature parameters of humid to mesic forests of eastern Asia and their relation to forests of other regions of the northern hemisphere and Australasia. US Geological Survey Professional Paper, 1106:1-37. https://doi.org/10.3133/pp1106
Wolfe, J.A. 1990. Palaeobotanical evidence for a marked temperature increase following the Cretaceous/Tertiary boundary. Nature, 343:153-156. https://doi.org/10.1038/343153a0
Yang, J., Spicer, R.A., Spicer, T.E.V., and Li, C.-S. 2011. ‘CLAMP’ Online: a new web-based palaeoclimate tool and its application to the terrestrial Paleogene and Neogene of North America. Palaeobiodiversity and Palaeoenvironments, 91:163-183. https://doi.org/10.1007/s12549-011-0056-2