 Redescription and phylogenetic placement of the Cretaceous wasp Parviformosus wohlrabeae (Hymenoptera: Proctotrupomorpha)
Redescription and phylogenetic placement of the Cretaceous wasp Parviformosus wohlrabeae (Hymenoptera: Proctotrupomorpha)
Article number: 23(1):a05
https://doi.org/10.26879/1031
Copyright Paleontological Society, February 2020
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 18 September 2019. Acceptance: 14 February 2020.
ABSTRACT
The Early Cretaceous fossil Parviformosus wohlrabeae Barling et al., 2013, has been described as the oldest known member of the chalcidoid family Pteromalidae. However, its phylogenetic placement has raised doubts, highlighting the necessity for a proper revision. A redescription as well as a re-interpretation of morphological characters is presented here and differences to the original description of Barling et al. (2013) are discussed. Phylogenetic placement of P. wohlrabeae is critically examined. We did not find any synapomorphies shared with Chalcidoidea nor any other superfamily and therefore place the fossil as Proctotrupomorpha incertae sedis. Despite its unclear phylogenetic position, P. wohlrabeae remains a significant fossil as it provides a very rare example of a member of Proctotrupomorpha from the Lower Cretaceous Crato Formation of Brazil.
Michael Haas, Department of Entomology, State Museum of Natural History Stuttgart, 70191 Stuttgart, Germany. Michael.Haas@smns-bw.de
Roger A. Burks, Department of Entomology, University of California, Riverside, California, 92521, USA. Roger.Burks@gmail.com
Petr Janšta, Department of Zoology, Charles University, CZ-128 44 Prague, Czech Republic. Petr.Jansta@natur.cuni.cz
Lars Krogmann, Department of Entomology, State Museum of Natural History Stuttgart, 70191 Stuttgart, Germany. University of Hohenheim, Institute of Biology, Systematic Entomology (190n), 70593 Stuttgart, Germany. Lars.Krogmann@smns-bw.de
Keywords: systematic entomology; Crato formation; parasitoid wasp; Chalcidoidea; Pteromalidae
Final citation: Haas, Michael, Burks, Roger A., Janšta, Petr, and Krogmann, Lars. 2020. Redescription and phylogenetic placement of the Cretaceous wasp Parviformosus wohlrabeae (Hymenoptera: Proctotrupomorpha). Palaeontologia Electronica, 23(1):a05. https://doi.org/10.26879/1031
palaeo-electronica.org/content/2020/2933-redescription-of-parviformosus
INTRODUCTION
Parasitoid wasps are extremely important in our terrestrial ecosystems and significantly influence their host populations (Quicke, 1997). They constitute over three-fourths of all known parasitoid insect species and include some of the most diverse insect groups (Eggleton and Belshaw, 1992; Heraty, 2009). The origin of hymenopteran parasitoids is dated to be between the Permian-Triassic boundary, about 247 m.y.a. (Peters et al., 2017) and the Triassic-Jurassic boundary, 210 m.y.a. (Grimaldi and Engel, 2005), followed by an unprecedented megaradiation leading to the species richness we observe today (Huber, 2009). One of these groups are the Proctotrupomorpha (sensu Rasnitsyn, 1988), which include species-rich but minute parasitoid taxa. The oldest proctotrupomorphan fossils date back 165 m.y.a., excavated from deposits in China (Shih et al., 2011), but most of the fossil diversity appeared more recent as documented by Cretaceous and Palaeogene deposits (Grimaldi and Engel, 2005; Penney, 2010; Rasnitsyn and Kühnle, 2019). With an estimated age of approximately 110 m.y., the limestone compression fossil Parviformosus wohlrabeae Barling et al., 2013, is currently one of the oldest representatives of the proctotrupomorphan superfamily Chalcidoidea. Besides P. wohlrabeae, few other chalcidoid wasps have been recorded or even described from that time (Grimaldi and Engel, 2005; Kaddumi, 2005; Penney, 2010; Poinar and Huber, 2011; Gumovsky et al., 2018; Haas et al., 2018).
The superfamily Chalcidoidea, with about 22,000 described and 500,000 estimated species (Heraty, 2009; Noyes, 2019), is probably the largest proctotrupomorphan superfamily. Despite numerous morphological and molecular phylogenetic studies (Gibson, 1986; Gibson et al., 1999; Munro et al., 2011; Heraty et al., 2013; Peters et al., 2018), the phylogenetic relationships of Chalcidoidea are not satisfactorily resolved. Fossils often offer valuable information for such phylogenetic studies, by providing a minimum age for their divergence and the possibility to trace back morphological or even biological shifts, which are necessary to understand evolutionary patterns. Appropriate comprehensive description and conclusive phylogenetic placement of those few fossils is therefore imperative for untangling chalcidoid evolution. Parviformosus wohlrabeae has been described as the oldest member of the family Pteromalidae (Chalcidoidea), which would make it a key fossil of this group. As Pteromalidae is a polyphyletic assemblage of unrelated chalcidoid subfamilies (Munro et al., 2011; Heraty et al., 2013), any phylogenetic placement of fossils in “Pteromalidae” needs to consider its various subfamilies.
The possibility of a precisely identified early Cretaceous fossil of Pteromalidae would be highly intriguing for the purposes of determining the timing of chalcidoid diversification relative to the diversification of flowering plants and potential insect host lineages. However, the description and phylogenetic placement of Parviformosus wohlrabeae has been followed by some doubts, even in the original study. Barling et al. (2013) already mentioned in their description that “Familial placement for P. wohlrabeae is extremely difficult due to the lack of three key taxonomic structures; the legs, wings and antennae.” Moreover, Farache et al. (2016) were also skeptical about the phylogenetic positioning of P. wohlrabeae and even questioned whether it should be placed in Chalcidoidea. Even Barling (2018) questioned the validity of the placement of P. wohlrabeae. Here we present a redescription of P. wohlrabeae and revise its phylogenetic position.
MATERIAL AND METHODS
Specimen
As described by Barling et al. (2013), the female holotype of Parviformosus wohlrabeae is preserved in fine-grained laminated limestone, originating from the Crato Formation of the Chapada do Araripe tableland in Brazil, probably from an area of active quarrying between Santana do Cariri, Nova Olinda and Tatajuba, with its quarry of origin unknown. It is dated to the Early Cretaceous (Aptian) period. The specimen is preserved within a circular slab of limestone, which was etched in 10% hydrochloric acid and subsequently sputtered with carbon and gold (Barling et al., 2013). Barling et al. (2013) mentioned that the specimen was damaged, so that parts of the head are missing from its original conservational state (Barling et al., 2013, figure 3B). The specimen is deposited in the Stuttgart State Museum of Natural History (SMNS) under the accession number “SMNS 700902”.
Imaging
New scanning electron microscopic images were taken using a Zeiss EVO LS 15 microscope. Measurements and high resolution images for the digital drawing were taken using a Keyence VHX 5000 digital microscope. Adobe Illustrator CS4 (Version: 14.0.0) was used to create the drawing based on digital images and to assemble the figure plates.
Terminology
Terminology and abbreviations follow Gibson et al. (1997) and the Hymenoptera Anatomy Ontology (HAO) (Yoder et al., 2010).
SYSTEMATIC PALAEONTOLOGY
Order HYMENOPTERA Linnaeus, 1758
Suborder APOCRITA Gerstaecker, 1867
Infraorder PROCTOTRUPOMORPHA sensu Rasnitsyn, 1988, incertae sedis
Type. Parviformosus wohlrabeae Barling et al. 2013 (Figure 1, Figure 2, Figure 3), holotype female
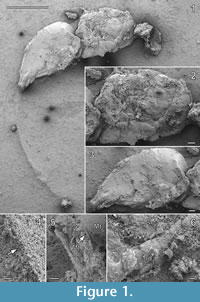 Diagnosis. Pronotum large, almost as long as half of mesoscutum (Figure 1.1-2). Metanotum medially long, about half as long as propodeum (Figure 1.1-2) and submedially overlapping propodeum (Figure 2.2). Propodeum smooth with distinct median carina ending posteriorly in transverse adpetiolar strip (Figure 2.2). Propodeal spiracle large, kidney-shaped with distinct rim (Figure 2.1). Petiole small, transverse. Metasoma with vaguely indicated segmentation, metasoma length subequal to mesosoma (Figure 1.1, 1.3). Ovipositor elongate and ventrally curved (Figure 1.1).
Diagnosis. Pronotum large, almost as long as half of mesoscutum (Figure 1.1-2). Metanotum medially long, about half as long as propodeum (Figure 1.1-2) and submedially overlapping propodeum (Figure 2.2). Propodeum smooth with distinct median carina ending posteriorly in transverse adpetiolar strip (Figure 2.2). Propodeal spiracle large, kidney-shaped with distinct rim (Figure 2.1). Petiole small, transverse. Metasoma with vaguely indicated segmentation, metasoma length subequal to mesosoma (Figure 1.1, 1.3). Ovipositor elongate and ventrally curved (Figure 1.1).
Specimen condition. The antennae are missing. The left fore- and midleg are embedded under the specimen and are not visible. Other legs are preserved only in part (see description). Wings are poorly preserved, being at most represented by small remnants in their sockets, except for a large piece of wing membrane on the right side of the body. The specimen itself is heavily deformed from possible dorsolateral force effect, either through taphonomy or before fossilization. The head and ventral metasoma suffered severe breakage, with the head being only partly preserved and metasomal sterna not discernable. The ovipositor is embedded within a small limestone ridge, which has partly been broken, seemingly by scratches with a tool.
Redescription. Total body length excluding ovipositor 3.5 mm; ovipositor length about 2.8 mm.
Head: Height about 0.68 mm, severely damaged and distorted (Figure 1.1), at least right and frontal part of head missing. Left eye partly identifiable. Left mandible distinguishable, broad, with three teeth, ventral tooth broadest with ventral margin straight. Inner structure, possibly pharynx, visible. Antennal insertion as described in Barling et al. (2013) not recognizable. Left labial palp mentioned by Barling et al. (2013) visible, covered by setae, but identity not certain.
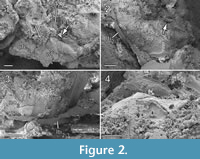 Mesosoma: Length 1.3 mm, without sculpture, appearing smooth, dorsolaterally depressed from right side (Figure 1.2). Pronotum long, with moderately long collar, posterolateral corner distinctly overlapping mesopleuron (Figure 1.6). Right procoxa partly visible at lateroventral pronotal margin. Mesoscutum with right notaulus faint, but straight and solid, reaching posterior mesoscutal margin. Axilla reaching transscutal articulation mesad of notaulus. Mesoscutellum deformed but convex. Metanotum about one-third the length of propodeum as indicated on the left side by straight suture (Figure 2.2-4), overlapping propodeum submedially (Figure 2.2). Metanotal area on right side submedially very strongly deformed and forced in. Metascutellum probably large. Propodeum about one-fourth the length of mesosoma, smooth, with distinct median carina ending posteriorly in transverse adpetiolar strip (Figure 2.2), partly cracked and distorted (Figure 1.2), original form hardly assessable, lateral and hind margin bordered by strong carina. Right spiracle situated close to anterior propodeal margin, kidney-shaped with distinct rim (Figure 2.1). Mesopleuron concave, smooth to lightly sculptured, dorsally marked by deep and broad groove, possibly mesopleural line. On both sides area adjacent to postalar process marked by deep mesoscutellar trough, delimitated posteriorly by broad posterior mesoscutellar arm (Figure 2.1). Only fore wing articulation most probably preserved, marked by small remnant of (probably) wing membrane attached to mesosoma (Figure 2.1, Figure 3, rfw?). Large piece of seemingly detached wing membrane located at left hindwing articulation, folded over right meso- and metapleuron, identity uncertain (Figure 2.1, Figure 3, wf). Remnant of left meso- or metatibia located between propodeum and metasoma (Figure 2.3, Figure 3, lti2/3). Right metafemur and trochanter bent forwards over metapleural area (Figure 2.1, Figure 3).
Mesosoma: Length 1.3 mm, without sculpture, appearing smooth, dorsolaterally depressed from right side (Figure 1.2). Pronotum long, with moderately long collar, posterolateral corner distinctly overlapping mesopleuron (Figure 1.6). Right procoxa partly visible at lateroventral pronotal margin. Mesoscutum with right notaulus faint, but straight and solid, reaching posterior mesoscutal margin. Axilla reaching transscutal articulation mesad of notaulus. Mesoscutellum deformed but convex. Metanotum about one-third the length of propodeum as indicated on the left side by straight suture (Figure 2.2-4), overlapping propodeum submedially (Figure 2.2). Metanotal area on right side submedially very strongly deformed and forced in. Metascutellum probably large. Propodeum about one-fourth the length of mesosoma, smooth, with distinct median carina ending posteriorly in transverse adpetiolar strip (Figure 2.2), partly cracked and distorted (Figure 1.2), original form hardly assessable, lateral and hind margin bordered by strong carina. Right spiracle situated close to anterior propodeal margin, kidney-shaped with distinct rim (Figure 2.1). Mesopleuron concave, smooth to lightly sculptured, dorsally marked by deep and broad groove, possibly mesopleural line. On both sides area adjacent to postalar process marked by deep mesoscutellar trough, delimitated posteriorly by broad posterior mesoscutellar arm (Figure 2.1). Only fore wing articulation most probably preserved, marked by small remnant of (probably) wing membrane attached to mesosoma (Figure 2.1, Figure 3, rfw?). Large piece of seemingly detached wing membrane located at left hindwing articulation, folded over right meso- and metapleuron, identity uncertain (Figure 2.1, Figure 3, wf). Remnant of left meso- or metatibia located between propodeum and metasoma (Figure 2.3, Figure 3, lti2/3). Right metafemur and trochanter bent forwards over metapleural area (Figure 2.1, Figure 3).
Metasoma: Length 1.6 mm, without sculpture, smooth (Figure 1.3), with at least seven vaguely indicated separate terga, artificially dorsolaterally depressed from right side, tip broken. Petiole small, transverse. Mt2-4 (Figure 1.3, Figure 3) subequal in length, Mt5 occupying one-third of metasoma, Mt6 damaged posteriorly, Mt7 indicated by a visible layer of cuticle at the breakage point (Figure 1.5). All terga not emarginated medially. Ovipositor curved, partly broken, traceable on limestone and encompassed in limestone ridge (Figure 1.4), apex not clearly delimited. Piece of ovipositor mounted on a second SEM stub mentioned by Barling et al. (2013) not present.
DISCUSSION
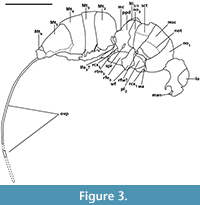 In the light of our new morphological interpretation, the original diagnosis of the genus Parviformosus by Barling et al. (2013) needs to be modified. Comparing our morphological interpretation with the interpretation of Barling et al. (2013), it is apparent, that many structures, especially those from the mesosoma, have to be re-interpreted.
In the light of our new morphological interpretation, the original diagnosis of the genus Parviformosus by Barling et al. (2013) needs to be modified. Comparing our morphological interpretation with the interpretation of Barling et al. (2013), it is apparent, that many structures, especially those from the mesosoma, have to be re-interpreted.
Species Diagnosis
The petiole is partly visible in between the propodeum and the first gastral tergite, contrasting Barling et al. (2013) who misinterpreted part of the propodeum as part of the petiole. The described dorsal “lip” on the fourth metasomal segment appears to result from severe deformation of the metasoma and is therefore considered as an artefact. Total length of ovipositor is difficult to assess, because the distal portion is missing from the holotype, but its length is indicated on the limestone slab by a diffuse marking. In addition to those characters mentioned by Barling et al. (2013), we found a number of additional diagnostic characters. These include a broad mandible with three distinct teeth; an unusually deep and long groove on the mesopleuron, probably representing the mesopleural sulcus; a rather broad metanotum, overlapping the propodeum and a smooth propodeum, which is laterally and posteriorly delimited by a broad shiny rim and bears a distinct median carina.
Family Placement
In the original description, Barling et al. (2013) argued that the posterior end of the metasoma as well as the shape and morphology of the ovipositor allow placement within the Pteromalidae. Principally, offering a solid and universally accepted morphological definition of Pteromalidae is not possible because the group is polyphyletic (Heraty et al., 2013) and therefore lacks synapomorphic characters (Graham, 1969; Bouček and Rasplus, 1991; Grissell and Schauff, 1997). Therefore, a phylogenetic placement of pteromalid lineages makes only sense on the subfamily level. Barling et al. (2013) claimed that because of 1) the “length and shape of the ovipositor”, 2) the “height relative to width of the gaster”, 3) the “short pronotum”, 4) the “shortened head” and 5) the “apically expanding ovipositor sheath” the specimen might belong to the pteromalid subfamily Sycophaginae, now placed in Agaonidae (Heraty et al., 2013). None of the abovementioned characters is diagnostic nor represents a synapomorphy for any pteromalid or chalcidoid subfamily. The two defining apomorphies of Sycophaginae, i.e., the presence of grooves framing the mesoscutellum and a deeply sinuated margin of the eighth metasomal tergite, leading to a thumbnail-like medial flap (Cruaud et al., 2011) are not visible on the specimen. Placement of Parviformus wohlrabeae in Sycophaginae would also have challenged the current and well-established view that the age of this subfamily and their association with figs (Ficus, Moraceae) are of post-Gondwanan origin (Cruaud et al., 2011) and therefore roughly 60 m.y. younger than the fossil. Based on Rønsted et al. (2005) the evolutionary root of figs might lay at the boundary of the Early to the Late Cretaceous, about 100 m.y.a., establishing a time frame of 60 - 100 m.y. for the first development of the mutualism between figs and their chalcidoid pollinators. More recently, the origin of this mutualism was narrowed down to roughly 75 m.y.a. (Cruaud et al. 2012). It is therefore highly unlikely, that P. wohlrabeae, with an age of about 110 m.y., had any association with figs.
Superfamily Placement
As Parviformosus wohlrabeae possesses a deeply incised wasp waist and discernable wing articulations with fragments of wings attached, it can therefore be classified as an apocritan Hymenoptera. Furthermore, possession of a long exserted ovipositor sheath eliminates the possibility of placement in Aculeata. Positioning of the metasoma excludes the parasitoid superfamilies Evanioidea and Stephanoidea. The superfamilies Trigonaloidea and Megalyroidea can also be dismissed by the structure of the pronotum, which is dorsally extremely shortened in the mentioned superfamilies. Additionally, the metasoma of these groups has more apparent sterna than that of P. wohlrabeae, where the observable part of the metasoma is largely covered by terga. Due to severe damage, the metasomal sterna of P. wohlrabeae are hardly discernable, let alone countable. Metasomal characters also contradict placement in the Ichneumonoidea, which possess a metasoma that is usually elongate and petiolate with spiracular openings on each tergum. The metasoma in Ceraphronoidea is also quite different with their first tergite covering most of its length. Moreover, the structure of the mesosoma in P. wohlrabeae, like the notauli and the pronotum, does not agree with the morphological features presented in Ceraphronoidea. The only remaining alternative is a placement within the infraorder Proctotrupomorpha, sensu Rasnitsyn (1988), which is comprised mostly of minute parasitoids, being morphologically highly diverse and species rich. Extant superfamilies included in Proctotrupomorpha are the Cynipoidea, Platygastroidea, Chalcidoidea, Mymarommatoidea, Diaprioidea, and Proctotrupoidea with addition of the fossil groups Serphitoidea, Cretacoformicidae incertae sedis, and the genus Koonwarrus incertae sedis. A more precise phylogenetic placement of P. wohlrabeae is highly speculative due to its preservational state. Reliable evidence would arise from detectable synapomorphic characters. Such characters, however, are not visible, missing, or subject to vague interpretation, because of distortion and damage.
As it was described as a member of Pteromalidae, Parviformosus wohlrabeae should exhibit the synapomorphies of its superordinate superfamily, the Chalcidoidea. Those include 1) possession of a free prepectus, separating the pronotum from the tegula, 2) the position of the mesothoracic spiracle (Gibson, 1985, 1999; Gibson et al., 1999), 3) morphologically unique multiporous plate sensillae on the flagellar segments (Barlin and Vinson, 1981; Gibson, 1986; Basibuyuk and Quicke, 1999). As Barling et al. (2013) state, the prepectus cannot be identified in the fossil (Figure 1.2, 1.6). Barling et al. (2013) further hypothesize that the prepectus “... is probably internalized, as in many pteromalid subfamilies.” Internalization of the prepectus is so far not reported in Pteromalidae, although miniaturization might occur, e.g., Macromesinae (Gibson, 1986). This miniaturization also appears in other chalcidoid families and is often linked to a small body size, sometimes leading to an almost fully reduced prepectus (Gibson, 1986). Only the chalcidoid family Rotoitidae was reported to completely lack a visible prepectus (Bouček and Noyes, 1987), but subsequent examination revealed a very slender, independent prepectus, which might be concealed by the pronotum if it is directly adjacent to the mesepisternum (Gibson and Huber, 2000). In P. wohlrabeae, the posterolateral corner of the pronotum is distinctly overlapping the mesopleuron, with no indication of a hidden prepectus underneath (Figure 1.6). Evaluating the position of the mesothoracic spiracle is not possible due to the poor conservational state of the specimen. Presence or absence of multiporous plate sensilla on the flagellar segments cannot be assessed as well, because the antennae are missing. Conclusively, none of the aforementioned synapomorphies of Chalcidoidea can be used for placing the fossil in this superfamily. The long ovipositor is the most obvious feature of the fossil and represents a character state that is common in various chalcidoid lineages but it does not unequivocally support placement in this superfamily, with many parasitoid groups including taxa with an exerted ovipositor.
Based on the limited morphological evidence, we propose to treat Parviformosus wohlrabeae as Proctotrupomorpha incertae sedis. Placement in Chalcidoidea is tempting, mostly due to the lack of satisfactory alternative options, but based on the poor preservation and the absence of any synapomorphies, a reliable superfamily placement of the fossil is not possible. Considering the morphological diversity observed in proctotrupomorphan wasps, P. wohlrabeae might as well belong to a yet unknown "morphotype", not bearing an exact resemblance to any known representative of this clade.
ACKNOWLEDGMENTS
We thank D.M. Martill, S.W. Heads, and N. Barling for donating the specimen to the collection of the SMNS. We also thank C. Gascó Martín for operating the SEM, as well as N. Barling and an anonymous reviewer for providing helpful comments on an earlier version of this paper.
REFERENCES
Barlin M.R. and Vinson B.S. 1981. Multiporous plate sensilla in antennae of the Chalcidoidea (Hymenoptera). International Journal of Insect Morphology and Embryology, 10:29-42. https://doi.org/10.1016/0020-7322(81)90011-8
Barling, N. 2018. The fidelity of preservation of insects from the Crato Formation (Lower Cretaceous) of Brazil. Unpublished PhD Thesis, University of Portsmouth, Portsmouth, United Kingdom.
Barling, N., Heads, S.W., and Martill, D.M. 2013. A new parasitoid wasp (Hymenoptera: Chalcidoidea) from the Lower Cretaceous Crato Formation of Brazil: The first Mesozoic Pteromalidae. Cretaceous Research, 45:258-264. https://doi.org/10.1016/j.cretres.2013.05.001
Basibuyuk, H.H. and Quicke D.L.J. 1999. Gross morphology of multiporous plate sensilla in the Hymenoptera (Insecta). Zoologica Scripta, 28:51-67. https://doi.org/10.1046/j.1463-6409.1999.00007.x
Bouček, Z. and Noyes, J.S. 1987. Rotoitidae, a curious new family of Chalcidoidea (Hymenoptera) from New Zealand. Systematic Entomology, 12:407-412. https://doi.org/10.1111/j.1365-3113.1987.tb00212.x
Bouček, Z. and Rasplus, J.-Y. 1991. Illustrated Key to West-Palearctic Genera of Pteromalidae. Institut National de la Recherche Agronomique, Paris.
Cruaud, A., Jabbour-Zahab, R., Genson, G., Kjellberg, F., Kobmoo, N., van Noort, S., Da-Rong, Y., Yan-Qiong, P., Ubaidillah, R., Hanson, P.E., Santos-Mattos, O., Farache, F.H.A., Pereira, R.A.S., Kerdelhué, C., and Rasplus, J.-Y. 2011. Phylogeny and evolution of life-history strategies in the Sycophaginae non-pollinating fig wasps (Hymenoptera, Chalcidoidea). BMC Evolutionary Biology, 11:178. https://doi.org/10.1186/1471-2148-11-178
Cruaud, A., Rønsted, N., Chantarasuwan, B., Chou, L.S., Clement, W.L., Couloux, A., Cousins, B., Genson, G., Harrison, R.D., Hanson, P.E., Hossaert-McKey, M., Jabbour-Zahab, R., Jousselin, E., Kerdelhué, C., Kjellberg, F., Lopez-Vaamonde, C., Peebles, J., Peng, Y.Q., Pereira, R.A., Schramm, T., Ubaidillah, R., van Noort, S., Weiblen, G.D., Yang, D.R., Yodpinyanee, A., Libeskind-Hadas, R., Cook, J.M., Rasplus, J.Y., and Savolainen, V. 2012. An extreme case of plant-insect codiversification: figs and fig-pollinating wasps. Systematic Biology, 61:1029-1047. https://doi.org/10.1093/sysbio/sys068
Eggleton, P. and Belshaw, R. 1992. Insect parasitoids: An evolutionary overview. Philosophical Transactions of the Royal Society of London Series B Biological Sciences, 337:1-20. https://doi.org/10.1098/rstb.1992.0079
Farache, F.H.A., Rasplus, J.-Y., Azar, D., Pereira, R.A.S., and Compton, S.G. 2016. First record of a non-pollinating fig wasp (Hymenoptera: Sycophaginae) from Dominican amber, with estimation of the size of its host figs. Journal of Natural History, 50:2237-2247. https://doi.org/10.1080/00222933.2016.1193646
Gerstaecker, A., 1867. Ueber die Gattung Oxybelus Latr. und die bei Berlin vorkommenden Arten derselben. Eduard Anton, Halle.
Gibson, G.A.P. 1985. Some pro- and mesothoracic structures important for phylogenetic analysis of Hymenoptera, with a review of terms used for the structures. The Canadian Entomologist, 117:1395-1443. https://doi.org/10.4039/ent1171395-11
Gibson, G.A.P. 1986. Evidence for monophyly and relationships of Chalcidoidea, Mymaridae, and Mymarommatidae (Hymenoptera: Terebrantes). The Canadian Entomologist, 118:205-240. https://doi.org/10.4039/ent118205-3
Gibson, G.A.P. 1999. Sister-group relationships of the Platygastroidea and Chalcidoidea (Hymenoptera) - an alternate hypothesis to Rasnitsyn (1988). Zoologica Scripta, 28:125-138. https://doi.org/10.1046/j.1463-6409.1999.00015.x
Gibson, G.A.P. and Huber, J.T. 2000. Review of the family Rotoitidae (Hymenoptera: Chalcidoidea), with description of a new genus and species from Chile. Journal of Natural History, 34:2293-2314. https://doi.org/10.1080/002229300750037901
Gibson, G.A.P., Huber, J.T., and Woolley, J.B. 1997. Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera). NRC Research Press, Canada.
Gibson, G.A.P., Heraty, J.M., and Woolley, J.B. 1999. Phylogenetics and classification of Chalcidoidea and Mymarommatoidea - a review of current concepts (Hymenoptera, Apocrita). Zoologica Scripta, 28:87-124. https://doi.org/10.1046/j.1463-6409.1999.00016.x
Graham, M.W. 1969. The Pteromalidae of North-Western Europe. Bulletin of the British Museum (Natural History) Entomology, Supplements, 16:1-909.
Grimaldi, D. and Engel, M.S. 2005. Evolution of the Insects. Cambridge University Press, Cambridge.
Grissell, E.E. and Schauff, M.E. 1997. A Handbook of the Families of Nearctic Chalcidoidea (Hymenoptera). Entomological Society of Washington, Washington (DC).
Gumovsky, A., Perkovsky, E., and Rasnitsyn, A. 2018. Laurasian ancestors and “Gondwanan” descendants of Rotoitidae (Hymenoptera: Chalcidoidea): What a review of Late Cretaceous Baeomorpha revealed. Cretaceous Research, 84:286-322. https://doi.org/10.1016/j.cretres.2017.10.027
Haas, M., Burks, R.A., and Krogmann, L. 2018. A new lineage of Cretaceous jewel wasps (Chalcidoidea: Diversinitidae). PeerJ, 6:e4633. https://doi.org/10.7717/peerj.4633
Heraty, J.M. 2009. Parasitoid biodiversity and insect pest management. In Foottit, B. and Adler, P. (eds.), Insect Biodiversity: Science and Society. Springer-Verlag, Hague, Netherlands.
Heraty, J.M., Burks, R.A., Cruaud, A., Gibson, G.A.P., Liljeblad, J., Munro, J., Rasplus, J.Y., Delvare, G., Janšta, P., Gumovsky, A., Huber, J.T., Woolley, J.B., Krogmann, L., Heydon, S., Polaszek, A., Schmidt, S., Darling, D.C., Gates, M.W., Mottern, J., Murray, E., Dal Molin, A., Triapitsyn, S., Baur, H., Pinto, J.D., van Noort, S., George, J., and Yoder, M. 2013. A phylogenetic analysis of the megadiverse Chalcidoidea (Hymenoptera). Cladistics, 29:466-542. https://doi.org/10.1111/cla.12006
Huber, J.T. 2009. Biodiversity of Hymenoptera. In Foottit, B. and Adler, P. (eds.), Insect Biodiversity: Science and Society. Springer-Verlag, Hague, Netherlands.
Kaddumi, H.F. 2005. Amber of Jordan the oldest Prehistoric Insects in fossilized Resin. Publications of the Eternal River Museum of Natural History, Amman.
Linnaeus, C. 1758. Systema naturae per regna tria naturae secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Holmiae. (Salvius).
Munro, J.B., Heraty, J.M., Burks, R.A., Hawks, D., Mottern, J., Cruaud, A., Rasplus, J.-Y., and Janšta, P. 2011. A molecular phylogeny of the Chalcidoidea (Hymenoptera). PLoS One, 6:e27023. https://doi.org/10.1371/journal.pone.0027023
Noyes, J.S. 2019. Universal Chalcidoidea Database. Available from http://www.nhm.ac.uk/chalcidoids.
Penney, D. 2010. Biodiversity of Fossils in Amber from the Major World Deposits. Siri Scientific Press, Manchester.
Peters, R.S., Krogmann, L., Mayer, C., Donath, A., Gunkel, S., Meusemann, K., Kozlov, A., Podsiadlowski, L., Petersen, M., Lanfear, R., Diez, P.A., Heraty, J., Kjer, K.M., Klopfstein, S., Meier, R., Polidori, C., Schmitt, T., Liu, S., Zhou, X., Wappler, T., Rust, J., Misof, B., and Niehuis, O. 2017. Evolutionary history of the Hymenoptera. Current Biology, 27:1013-1018. https://doi.org/10.1016/j.cub.2017.01.027
Peters, R.S., Niehuis, O., Gunkel, S., Bläser, M., Mayer, C., Podsiadlowski, L., Kozlov, A., Donath, A., van Noort, S., Liu, S., Zhou, X., Misof, B., Heraty, J., and Krogmann, L. 2018. Transcriptome sequence-based phylogeny of chalcidoid wasps (Hymenoptera: Chalcidoidea) reveals a history of rapid radiations, convergence, and evolutionary success. Molecular Phylogenetics and Evolution, 120:286-296. https://doi.org/10.1016/j.ympev.2017.12.005
Poinar, G. and Huber, J.T. 2011. A new genus of fossil Mymaridae (Hymenoptera) from Cretaceous amber and key to Cretaceous mymarid genera. Zookeys, 130:461-472. https://doi.org/10.3897/zookeys.130.1241
Quicke, D.L.J. 1997. Parasitic Wasps. Springer-Verlag, Hague, Netherlands.
Rasnitsyn, A.P. 1988. An outline of evolution of the hymenopterous insects (order Vespida). Oriental Insects, 22:115-145. https://doi.org/10.1080/00305316.1988.11835485
Rasnitsyn, A.P. and Öhm-Kühnle, C. 2019. Revision of the Cretaceous Proctotrupomorpha (Insecta: Hymenoptera) of Australia. Cretaceous Research, 100:91-96. https://doi.org/10.1016/j.cretres.2019.03.017
Rønsted, N., Weiblen, G.D., Cook, J.M., Salamin, N., Machado, C.A., and Savolainen, V. 2005. 60 million years of co-divergence in the fig-wasp symbiosis. Proceedings of the Royal Society B, Biological Sciences, 272:2593-2599. https://doi.org/10.1098/rspb.2005.3249
Shih, C.K., Feng, H., and Ren, D. 2011. New fossil Heloridae and Mesoserphidae wasps (Insecta, Hymenoptera, Proctotrupoidea) from the Middle Jurassic of China. Annals of the Entomological Society of America, 104:1334-1348. https://doi.org/10.1603/an10194
Yoder, M.J., Mikó, I., Seltmann, K.C., Bertone, M.A., and Deans, A.R. 2010. A gross anatomy ontology for Hymenoptera. PLoS One, 5:e15991. https://doi.org/10.1371/journal.pone.0015991

