 Feeding in marine mammals: An integration of evolution and ecology through time
Feeding in marine mammals: An integration of evolution and ecology through time
Article number: 23(2):a40
https://doi.org/10.26879/951
Copyright Society for Vertebrate Paleontology, August 2020
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 December 2018. Acceptance: 11 August 2020.
ABSTRACT
Marine mammals are key components of aquatic ecosystems. Feeding strategies identified in extant cetaceans, pinnipeds, sirenians, marine otters, and polar bears are associated with anatomical specializations of the head (rostrum, palate, temporomandibular joint, teeth/baleen, mandible). Genetic and ontogenetic evidence of skull and tooth morphology provide the mechanisms that underlie patterns of feeding diversity. Based on a comprehensive diversity data set derived from the Paleobiology Database, we considered feeding strategies (suction, biting, filter feeding, grazing), prey type (squid, fish, benthic invertebrates, zooplankton, tetrapods, sea grasses), tooth pattern and cusp shape (homodont, heterodont, pointed, rounded, edentulous), and habitat (marine, riverine, estuarine) in fossil and extant marine mammals. These variables were then tested for correlation and their changes through time examined in relation to productivity and climate variables.
We provide an integrated analysis of the evolution of feeding and trophic structure in marine mammals and explore the origin and timing of particular feeding strategies over the last 50 million years. In agreement with earlier reports, updated generic counts reveal that the greatest diversity of pinnipedimorphs and cetaceans occurred during the late Miocene (Tortonian), following the Mid-Miocene Climatic Optimum. These historical data are used as a framework to inform past and present structure and trophic interactions and enable predictions about future marine ecosystems.The drivers of diet and feeding patterns are both environmental (sea level fluctuations, climate change) and biotic (anatomical specializations, competition, predator-prey). The influence of these processes on paleodiversity varies depending on taxonomic group, timing, and geographic scale.
Annalisa Berta. Department of Biology, San Diego State University, San Diego, California 92182, USA. aberta@sdsu.edu
Agnese Lanzetti. Department of Biology, San Diego State University, San Diego, California 92182, USA. agnese.lanzetti@gmail.com
Keywords: marine mammals; diversity curves; feeding strategies; biotic and environmental drivers
Berta, Annalisa and Lanzetti, Agnese. 2020. Feeding in marine mammals: An integration of evolution and ecology through time. Palaeontologia Electronica, 23(2):a40. https://doi.org/10.26879/951
palaeo-electronica.org/content/2020/3136-feeding-in-marine-mammals
Copyright: August 2020 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Marine mammals comprise a diverse assemblage of at least seven distinct evolutionary lineages that independently returned to the sea and spend the majority of their time in water. They occupy aquatic ecosystems ranging from the poles to the equator and riverine, estuarine, and a variety of marine environments from shallow coastal to deep sea. Across this diverse range of habitats they occupy almost every trophic level from top predators to primary consumers. In addition to the three major extant lineages that include pinnipeds (seals, sea lions, and walruses), cetaceans, odontocetes [toothed whales], mysticetes [baleen whales] and sirenians (manatees and dugongs), other marine mammals include sea otters and polar bears plus several entirely extinct lineages: desmostylians, aquatic sloths, and an aquatic bear (Berta, 2017).
Today marine mammals include 130 living species (Committee on Taxonomy, 2017). Marine mammals have been on Earth for a little more than 50 million years, and their past diversity includes more than 450 genera (Paleobiology Database, see Materials and Methods). All of their ancestors had well-developed limbs and feet and lived in terrestrial environments. The transition from land to sea involved the acquisition of many diverse adaptations including the ability to capture and consume prey in the marine environment. Environmental pressures involved in foraging in water, such as increased pressure, density, and viscosity, have resulted in morphological specializations related to locomotion, prey capture, and food processing.
The objective of this study is to provide an integrative analysis of the anatomy, evolution, and ecology of feeding in marine mammals. We begin by examining feeding strategies in extant and extinct marine mammals by associating these strategies with anatomical specializations of the head. We next consider genetic and ontogenetic evidence of skull and tooth morphology, which underlies the evolution of feeding types. Finally, using key morphological features, we performed a comprehensive analysis of the evolution of feeding diversity and trophic structure in marine mammals. Also incorporated in this framework is evidence from diatoms diversity, stable isotopes (e.g., δ18O, δ13C), and sea level changes which track changes in productivity, diet, and the environment over geologic time.
Feeding Strategies and Prey
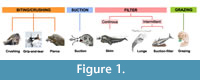 Marine mammals have evolved multiple feeding strategies to capture and consume food or prey underwater. We have adopted the three feeding categories of extant aquatic predators identified by Kienle et al. (2017): biting, suction, and filter feeding with the addition of a fourth category, grazing to include sirenians (Figure 1). It should be noted that, like most animals, marine mammals are opportunistic feeders and capture prey as efficiently as possible, which may require them to switch between prey types depending on their availability, and they also employ combinations of these strategies (Hocking et al., 2013, 2017a, b; Kienle et al., 2017). Furthermore, many species show variation in foraging strategy that can be related to age, sex, season, and prey type (Kienle and Berta, 2016).
Marine mammals have evolved multiple feeding strategies to capture and consume food or prey underwater. We have adopted the three feeding categories of extant aquatic predators identified by Kienle et al. (2017): biting, suction, and filter feeding with the addition of a fourth category, grazing to include sirenians (Figure 1). It should be noted that, like most animals, marine mammals are opportunistic feeders and capture prey as efficiently as possible, which may require them to switch between prey types depending on their availability, and they also employ combinations of these strategies (Hocking et al., 2013, 2017a, b; Kienle et al., 2017). Furthermore, many species show variation in foraging strategy that can be related to age, sex, season, and prey type (Kienle and Berta, 2016).
Biting. Biting (raptorial) feeding, a common marine mammal feeding strategy, can be divided into three subcategories: crushing, grip-and-tear, and pierce feeding (Kienle et al., 2017). Crushing is performed when prey is fragmented by the teeth during ingestion and intraoral processing. This strategy is exemplified by sea otters (Enhydra lutris) using the molars to break down hard shelled prey (e.g., sea urchins, crustaceans, and molluscs). Grip-and-tear feeding defines animals that hold prey with the jaws/forelimbs, shake prey and/or rip off smaller pieces during ingestion. This strategy has also been documented in some pinnipeds (e.g., leopard seals Hydrurga leptonyx), some odontocetes (e.g., killer whales Orcinus orca), polar bears (Ursus maritimus), and sea otters (Adam and Berta, 2002; Kienle and Berta, 2016). In pierce feeding, animals bite prey during ingestion, often swallowing prey whole with little manipulation or external processing. Suction can be used in combination with both pierce and grip-and-tear feeding to pull prey inside the mouth. Pierce and suction feeding are seen in most pinnipeds (e.g., Weddell seal Leptonychotes weddellii, Hawaiian monk seal Neomonachus schauinslandi), and delphinid odontocetes (Stenella, Delphinus, and Tursiops spp.) (Werth, 2006a; Kienle et al., 2015).
Suction. Suction feeding is the most common feeding strategy seen in aquatic vertebrates including fish, sea birds, turtles, and some marine mammals (Werth, 2000; Kienle et al., 2017). Suction feeders generate negative pressure within the oral cavity, which draws water and prey into the mouth (e.g., Marshall et al., 2008; Kane and Marshall, 2009). Most marine mammals, including some pinnipeds (e.g., bearded seal Erignathus barbatus, hooded seal Cystophoca cristata, elephant seal Mirounga spp.) and some odontocetes (e.g., beaked and sperm whales, ziphiids and physeteroids, and globicephaline delphinids) use some form of suction to get prey in the mouth (Kienle and Berta, 2016). Beaked and sperm whales are proficient squid feeders (Heyning and Mead, 1996; Werth, 2006a). Bearded and hooded seals employ suction feeding to prey on shrimp, and the diet of elephant seals is primarily composed of squid (Marshall et al., 2008; Churchill and Clementz, 2015; Kienle and Berta, 2016). Suction feeding is also used by highly specialized feeders. The modern walrus, Odobenus rosmarus, employs this strategy to feed on benthic mollusks (Kastelein et al., 1997).
Filter. Two main subcategories of filter feeding have been defined behaviorally: continuous and intermittent (Hocking et al., 2017a; Kienle et al., 2017). Continuous filter feeders (or skim feeders) swim slowly through dense patches of prey with open mouths, and prey passively enter the oral cavity (Werth and Potvin, 2016). This strategy is best exemplified by balaenid whales (bowhead and right whales). Intermittent filter feeders actively engulf a single mouthful of prey and remove water using various filtering structures during a distinct water removal stage. Intermittent filter feeding can be further subdivided into lunge and suction filter feeding based on the method of ingestion (Hocking et al., 2017a; Kienle et al., 2017). Lunge filter feeding (also called intermittent ram filter feeding, gulping, and ram gulping) is best exemplified by balaenopterid whales (e.g., minke, fin, humpback, blue, and sei whales also known as rorquals) that swim rapidly at a prey patch while opening their mouths to draw in prey (Goldbogen et al., 2010; Werth and Ito, 2017). In suction filter feeding, animals such as some phocid seals (e.g., crabeater seal Lobodon carcinophaga, and leopard seal Hydrurga leptonyx) use suction to pull prey from the water (e.g., Adam and Berta, 2002; Hocking et al., 2013; Kienle and Berta, 2016; Kienle et al., 2017). Lateral suction filter feeding (also called benthic suction feeding) is a specialized strategy seen in the gray whale (Eschrichtius robustus). It involves gray whales rolling laterally onto their right sides and sucking in prey, primarily amphipods, expelling water and sediment through the baleen (El Adli et al., 2014; Kienle et al., 2017).
Grazing. Sirenians, the only extant herbivorous marine mammals, graze on marine grasses and algae. Manatees feed on the blades, leaves, and stems of a variety of aquatic and terrestrial plants, including grasses (e.g., water hyacinth and waterthyme Hydrilla), sedges, forbs, herbs, and mangroves. Dugongs graze on offshore and intertidal seagrass beds, plowing through mudflats to dislodge anchored vegetation or stripping leaves from taller stems, targeting the rhizomes of sea grasses (Werth, 2000; Domning and Beatty, 2007).
Anatomy of Feeding
Feeding strategies involve a number of specializations of the head including both osteological and soft tissue structures. Major osteological features associated with feeding strategies in various extant and fossil marine mammal lineages include size and shape of the head, palate structure, as well as tooth number, shape, and size. Quantitative examination of morphological features in both extant and fossil marine mammals has been limited (e.g., Jones and Goswami, 2010; Jones et al., 2013; Churchill and Clementz, 2015, 2016; Kienle and Berta, 2016, 2019). We describe below anatomical features associated with feeding strategies in both extant and fossil marine mammals.
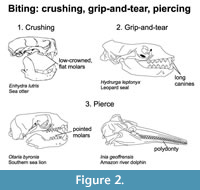 Biting. Pierce, grip-and-tear, and crushing biting strategies are associated with slightly different cranial and dental morphologies (Figure 2). Prior workers have described pierce feeders as having elongated orbits, enlarged pterygoid bones, and unequal but limited tooth spacing (Adam and Berta, 2002; Churchill and Clementz, 2015; Kienle and Berta, 2016). Grip-and-tear feeders have long skulls with wide bases and elongated orbits, auditory bullae, palate, and parietals, and additionally, they sometimes have enlarged canines and long, sharp postcanine tooth cusps (Adam and Berta, 2002; Kienle and Berta, 2016). In contrast to most biting marine mammals, polar bears exhibit sharp, shearing carnassials, characteristic of terrestrial carnivorans.
Biting. Pierce, grip-and-tear, and crushing biting strategies are associated with slightly different cranial and dental morphologies (Figure 2). Prior workers have described pierce feeders as having elongated orbits, enlarged pterygoid bones, and unequal but limited tooth spacing (Adam and Berta, 2002; Churchill and Clementz, 2015; Kienle and Berta, 2016). Grip-and-tear feeders have long skulls with wide bases and elongated orbits, auditory bullae, palate, and parietals, and additionally, they sometimes have enlarged canines and long, sharp postcanine tooth cusps (Adam and Berta, 2002; Kienle and Berta, 2016). In contrast to most biting marine mammals, polar bears exhibit sharp, shearing carnassials, characteristic of terrestrial carnivorans.
In addition, marine mammals that employ pierce and grip-and-tear biting strategies typically possess numerous slender pointed teeth to retain and grasp prey (Heyning and Mead, 1996; Werth, 2000; Adam and Berta, 2002; Johnston and Berta, 2011). Prey is swallowed whole if small or torn apart. Polydonty, significant increase in tooth number, and homodonty, presence of simple, conical, tooth crowns, are characteristics of modern cetaceans and pinnipeds and differ from the ancestral mammalian dental pattern of heterodonty (Armfield et al., 2013; Churchill and Clementz, 2015). Extreme cases of polydonty and homodonty are documented in oceanic dolphins, which can have more than 130 conical teeth (Jefferson et al., 2015). Long mandibles and toothrows have been associated with raptorial snap feeding characteristic of river dolphins (e.g., Inia, Pontoporia), which developed convergently to gavials (Werth, 2006a; McCurry et al., 2017).
The sea otter is the only extant marine mammal that employs a crushing biting strategy. In the past, the durophagous niche was occupied by the phocid Hadrokirus (Amson and de Muizon, 2014) and aquatic bear/pinnipedimorph Kolponomos (Tseng et al., 2016; Paterson et al., 2020). Marine mammals that employ a crushing biting strategy typically possess massive skulls and broad, low crowned flat molars. The canines are often rounded in cross section and the shovel-like incisors are inclined anteriorly to extract soft invertebrate prey from their shells. In comparison to suction feeders, cetaceans that employ biting strategies rely more heavily on lingual shape changes for prey capture, ingestion, and transport (Werth, 2007).
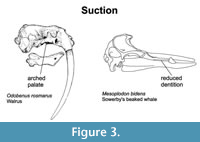 Suction Feeding. Many suction feeders have wide skulls, reduced dentition, and large, arched palates (e.g., beaked and sperm whales and the extinct bizarre cetacean Odobenocetops (Adam and Berta, 2002; Churchill and Clementz, 2015; Kienle and Berta, 2016; de Muizon and Domning, 2002) (Figure 3). Experiments, simulations, and biomechanical models have shown that a blunt head and a short, wide rostrum facilitates the formation of a small, circular oral opening characteristic of suction feeders (Werth, 2006a, 2006b). An arched or vaulted palate has been associated with suction feeding as it provides the tongue with an extended space in which it can be rapidly depressed and protracted prior to being retracted to produce suction (Kastelein and Gerrits, 1990). Palatal rugosities (ribbing), which function to hold slippery prey such as cephalopods, have also been associated with suction feeding (Heyning and Mead, 1996; Werth, 2000). Another palatal structure - large pterygoid hamuli - has also been associated with suction feeding in the walrus (Adam and Berta, 2002). This enlargement has been correlated with an expansion of the m. velum palatini in the walrus, which is presumably related to reinforcing the soft palate for large intraoral pressure changes during suction feeding (Kastelein et al., 1991). Similarly, the enlarged pterygoid hamuli on the crania of ziphiids have been associated with suction feeding and tentatively correlated with deep diving (Heyning and Mead, 1996; Ramassamy et al., 2018).
Suction Feeding. Many suction feeders have wide skulls, reduced dentition, and large, arched palates (e.g., beaked and sperm whales and the extinct bizarre cetacean Odobenocetops (Adam and Berta, 2002; Churchill and Clementz, 2015; Kienle and Berta, 2016; de Muizon and Domning, 2002) (Figure 3). Experiments, simulations, and biomechanical models have shown that a blunt head and a short, wide rostrum facilitates the formation of a small, circular oral opening characteristic of suction feeders (Werth, 2006a, 2006b). An arched or vaulted palate has been associated with suction feeding as it provides the tongue with an extended space in which it can be rapidly depressed and protracted prior to being retracted to produce suction (Kastelein and Gerrits, 1990). Palatal rugosities (ribbing), which function to hold slippery prey such as cephalopods, have also been associated with suction feeding (Heyning and Mead, 1996; Werth, 2000). Another palatal structure - large pterygoid hamuli - has also been associated with suction feeding in the walrus (Adam and Berta, 2002). This enlargement has been correlated with an expansion of the m. velum palatini in the walrus, which is presumably related to reinforcing the soft palate for large intraoral pressure changes during suction feeding (Kastelein et al., 1991). Similarly, the enlarged pterygoid hamuli on the crania of ziphiids have been associated with suction feeding and tentatively correlated with deep diving (Heyning and Mead, 1996; Ramassamy et al., 2018).
Suction feeders are also characterized by reduced dentition and complete tooth loss in some lineages. The walrus and bearded seal have a reduced number of postcanine teeth lacking cusps (Churchill and Clementz, 2015). Sperm whales and most beaked whales exhibit a reduced tooth count associated with their typical teuthophagous habit. For example, most beaked whale species undergo eruptions of only one or two pairs of teeth in males, and these are likely involved in sexual selection and species recognition rather than feeding (Dalebout et al., 2008).
A fused mandibular symphysis seen in the walrus has been identified as a characteristic feature of suction feeders (Adam and Berta, 2002). By providing a solid foundation, it contributes to an overall increase in mandible robustness, which would allow little or no mandibular deformation accompanying tongue retraction during feeding. Blunt mandibles have been correlated with dental reduction and associated with suction feeding in many odontocete lineages (e.g., some ziphiids, some physeterids, and globicephaline delphinids) (Werth, 2006a, 2006b; Bloodworth and Marshall, 2007).
Soft tissue structures including the tongue, hyoid apparatus, and associated musculature are specialized in various ways given their different uses in feeding. Enlarged hyoid apparatus and musculature, providing more surface area for tongue muscle attachments, are associated with suction feeding (Reidenberg and Laitman, 1994; Heyning and Mead, 1996; Werth, 2006a, 2006b, 2007; Bloodworth and Marshall, 2007; Johnston and Berta, 2011).
Filter feeding. Most pinniped suction filter feeders have morphological adaptations for this strategy that include a long narrow skull, a narrow palate, an elongated toothrow, thick mandibular symphysis, and proportionally large postcanine teeth (Adam and Berta, 2002; Jones et al., 2013; Churchill and Clementz, 2015; Kienle and Berta, 2016, 2019). The crabeater seal uses its highly specialized, multi-cusped teeth as a sieve to filter krill from the water, and prey become trapped by the interdigitating teeth (Adam and Berta, 2002) (Figure 4).
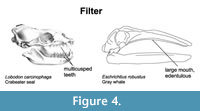 Large heads and mouths have been associated with lunge filter feeding in balaenopterids (e.g., Goldbogen et al., 2010). All modern baleen whales lack teeth and employ baleen, a series of keratin laminae arranged transversely in two racks that erupt from the gums on either side of the upper jaw, as a sieve for filter feeding (Figure 4). The number, length, and texture of baleen plates of modern mysticetes vary according to feeding strategy and diet (e.g., Williamson, 1973; Werth, 2000; Young et al., 2015; Jensen et al., 2017). The high rostral arch of balaenids accommodates their exceptionally long, fine baleen laminae (250 to 350 laminae, some exceeding 3 m in length) that are used to filter tiny copepod prey in skim feeding. The baleen of balaenopterids that feed mostly on fish and krill range from 181 to 402 laminae for engulfment feeding. In contrast to balaenids and balaenopterids, gray whales possess fewer baleen laminae (130-180) that are shorter and coarser, for suction feeding on benthic dwelling amphipods and mysid shrimp.
Large heads and mouths have been associated with lunge filter feeding in balaenopterids (e.g., Goldbogen et al., 2010). All modern baleen whales lack teeth and employ baleen, a series of keratin laminae arranged transversely in two racks that erupt from the gums on either side of the upper jaw, as a sieve for filter feeding (Figure 4). The number, length, and texture of baleen plates of modern mysticetes vary according to feeding strategy and diet (e.g., Williamson, 1973; Werth, 2000; Young et al., 2015; Jensen et al., 2017). The high rostral arch of balaenids accommodates their exceptionally long, fine baleen laminae (250 to 350 laminae, some exceeding 3 m in length) that are used to filter tiny copepod prey in skim feeding. The baleen of balaenopterids that feed mostly on fish and krill range from 181 to 402 laminae for engulfment feeding. In contrast to balaenids and balaenopterids, gray whales possess fewer baleen laminae (130-180) that are shorter and coarser, for suction feeding on benthic dwelling amphipods and mysid shrimp.
Several features of the lower jaw facilitate expansion of the oral cavity during bulk filter feeding. The unfused, ligamentous lower jaw symphysis exhibited by modern baleen whales permits independent rotation of the jaws (Lambertsen et al., 2005). The mandibular symphysis of rorquals possesses a gel-like cavity hypothesized as a sensory organ that coordinates lunge feeding (Pyenson et al., 2012). The mandibles of rorquals also exhibit a prominent, laterally deflected coronoid process, which serves as an attachment point for the m. temporalis, an important muscle in lateral rotation of the jaw during engulfment feeding (Brodie, 1993; Lambertsen, 1983). Several other morphological features of the mandible are associated with engulfment feeding in balaenopterids. Laterally bowed mandibles contribute to the expansive gape of balaenopterids (Lambertsen et al., 1995). A fibrocartilaginous temporomandibular joint (TMJ) was found to facilitate alpha (longitudinal), omega (transverse), and delta (vertical) rotation of the mandible (Lambertsen et al., 1995). By contrast, balaenids possess a synovial joint typical of mammals. The TMJ of the gray whale represents an intermediate condition, modified from the balaenopterid condition, with a vestigial joint cavity lacking a fibrous capsule, synovial membrane, and articular disk. In gray whales, this results in an increased importance of alpha rotation of the mandible while limiting the degree of delta rotation (angle of gape) of the lower jaw, an important factor in suction feeding (Johnston et al., 2010; El Adli et al., 2014).
The flaccid and floppy tongue of lunge filter feeding rorquals permits ingested prey and water to extend the throat pouch. This sac-like structure holds the water engulfed during a feeding event (Lambertsen, 1983; Goldbogen et al., 2011; Werth and Ito, 2017). By contrast, the muscular free-moving tongue of the gray whale (Kienle et al., 2015) likely plays an important role in generating the suction forces used in the unique lateral suction feeding employed by this species. Bulk lunge filter feeding rorquals expand the gular cavity by numerous longitudinal throat grooves (70-80) on the ventral throat pouch (cavum ventrale), extending to the umbilicus in some species. Ventral groove blubber consisting of tough ridges separated by deep channels of elastic tissue permits the extraordinary extensibility of the engulfment apparatus (Goldbogen et al., 2010; Pyenson et al., 2012). In the mouths of rorquals, a tendinous part of the m. temporalis, the frontomandibular stay, provides a continuous mechanical linkage between the skull and serves to optimize the gape of the mouth during engulfment filter feeding (Lambertsen et al., 1995). Suction feeding marine mammals typically exhibit smaller throat grooves that aid in tongue retraction. The gray whale possesses two to three throat grooves confined to the gular region (Berta et al., 2015), and both sperm and beaked whales also possess gular throat grooves (Heyning and Mead, 1996).
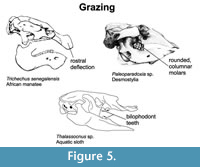 Grazing. Grazing marine mammals like sirenians possess cranial adaptations allowing them to feed at various depths in the water column. The degree of rostral deflection is correlated with degree of specialization for bottom feeding (Figure 5). Dugongs exhibit extreme rostral deflection (70 degrees) associated with their bottom feeding habit, consuming mostly sea grasses. Manatees exhibit less rostral deflection (26-30 degrees) and feed on aquatic plants throughout the water column (Domning, 2001; Vélez-Juarbe et al., 2012). Similar to this last group, stem sirenians (e.g., prorastomids) possessed undeflected rostra, indicating that they likely fed on aquatic plants. Enlarged blade-like, self-sharpening tusks developed in some later diverging dugongines (e.g., Bharatisiren, Rytiodus, Corystosiren, Domniniga, Kutchisiren, Xenosiren, and Dioplotherium) were likely employed for digging up rhizomes of larger sea grasses (Domning and Beatty, 2007).
Grazing. Grazing marine mammals like sirenians possess cranial adaptations allowing them to feed at various depths in the water column. The degree of rostral deflection is correlated with degree of specialization for bottom feeding (Figure 5). Dugongs exhibit extreme rostral deflection (70 degrees) associated with their bottom feeding habit, consuming mostly sea grasses. Manatees exhibit less rostral deflection (26-30 degrees) and feed on aquatic plants throughout the water column (Domning, 2001; Vélez-Juarbe et al., 2012). Similar to this last group, stem sirenians (e.g., prorastomids) possessed undeflected rostra, indicating that they likely fed on aquatic plants. Enlarged blade-like, self-sharpening tusks developed in some later diverging dugongines (e.g., Bharatisiren, Rytiodus, Corystosiren, Domniniga, Kutchisiren, Xenosiren, and Dioplotherium) were likely employed for digging up rhizomes of larger sea grasses (Domning and Beatty, 2007).
The cheek teeth of most grazing marine mammals are typically bilophodont, arranged in two long ridges, with rounded cusps and thick enamel. The teeth of extinct desmostylians exhibit a distinctive morphology consisting of closely bundled columnar cusps. Both extinct desmostylians and the aquatic sloth Thalassocnus possessed anteriorly extended, wide premaxillae and wide mandibular symphyses, indicating the development of strong lips that presumably facilitated grasping marine vegetation (de Muizon et al., 2004). Although sirenians lack expanded premaxillae, their wide upper lips and horny pads on the maxillae function similarly. In addition to grazing, the vaulted palate of desmostylians has been suggested to enable suction feeding (Beatty, 2004; Uno et al., 2008). The elongate jaws and expanded facial region of some later diverging desmostylians (e.g., Ounalashkastylus) likely facilitated extensive development of hyoid, facial, and adductor musculature, which supports this feeding method (Chiba et al., 2016).
Ontogeny and Growth of the Skull and Teeth
Skull growth. The feeding apparatus of marine mammals is driven by developmental changes in the morphology and growth of the skull and teeth. Skull growth in pinnipeds revealed that ontogenetic shape changes in otariids are greater, due to dimorphism, than in phocids although sample sizes were limited (Jones and Goswami, 2010). Brunner et al. (2004) found that the rostral and palatal regions in otariids required significantly longer periods to complete growth than the neurocranium (Brunner et al., 2004; Tarnawski et al., 2013). The primary importance of the postcanine teeth in feeding is reflected by their more rapid development after weaning compared to growth of the canines in otariids (Brunner et al., 2004). Skull ontogenetic studies in pinnipeds suggest that life history variables (e.g., gestation time, weaning time, growth rate) are more influential than environmental variables (e.g., latitude, temperature, productivity, and seasonality) in phocid cranial evolution (Jones and Goswami, 2010; Jones et al., 2013; Randau et al., 2019). Future studies are needed, comparing feeding components of the skull (e.g., tooth size and shape, postcanine tooth length) across larger samples of phocids, otariids, and odobenids, to elucidate growth patterns as well as the factors driving their development.
Little is known of the ontogeny of cetacean cranial morphology during fetal growth (Klima, 1995; Armfield et al., 2011; Lanzetti et al., 2020). Investigations of skull growth changes suggest that, compared to odontocetes, mysticetes begin developing larger mouths in the early fetal stages, presumably related to their filter feeding strategy (Armfield et al., 2011; Lanzetti et al., 2020). However, while the rostrum approximates the adult shape and curvature only at late stages of gestation, the mandible seems to reach its definitive proportions and curvature earlier (Lanzetti et al., 2020).
Tooth development. The developmental controls of tooth differentiation and tooth number have been little studied in marine mammals. Four conserved signaling pathways, including the bone morphogenetic proteins (Bmp), fibroblast growth factors (Fgf), sonic hedgehog (Shh), and wingless-related (Wnt) pathways, play important roles in tooth development in mammals. Recent studies show that these signaling pathways interact through positive and negative feedback loops to regulate not only morphogenesis of individual teeth but also tooth number, shape, and spatial pattern (Bei, 2009; Jernvall and Thesleff, 2012; Lan et al., 2014).
Armfield et al. (2013) investigated the genetic pathways that cause characteristic homodont, pointed tooth shape in dolphins. They discovered that, in the early developmental stages, a caudal extension of the expression of a specific growth factor (Bmp4), normally expressed in other mammals only in the anterior portion of the jaw, is responsible for the observed adult tooth shape. Modern mysticetes instead lack teeth postnatally, but they still develop tooth germs that are resorbed before birth (e.g., Ridewood, 1923; Karlsen, 1962; Fudge et al., 2009; Thewissen et al., 2017). Several molecular studies revealed that only some of the putative enamel and tooth genes are inactivated or deleted in mysticetes (Deméré et al., 2008; Meredith et al., 2009, 2011, 2013; Springer et al., 2016). Thewissen et al. (2017) found evidence for the presence of Fgf4, known to be implicated in tooth growth, during baleen growth. This suggests a partially shared genetic pathway for both teeth and baleen formation.
Among marine mammals and most other mammals, tooth development in modern manatees is unique. The cheek teeth are replaced horizontally; the new tooth erupts from the rear of the tooth row, pushing the anterior tooth out of the tooth row (Domning and Hayek, 1984; Beatty et al., 2012). Tooth replacement is similar in elephants although the latter have a limited number of teeth (Domning and Hayek, 1984). Little is known about the molecular mechanisms behind this mode of tooth replacement. It is possible that in the future a mouse model could be used to study this process, as the formation of supernumerary molars might employ a regulatory pathway similar to the one responsible for the ever-growing incisors of rodents (Jernvall and Thesleff, 2012). A better understanding of tooth development in various marine mammal lineages will provide important data that can then be correlated with major shifts in diet and food processing.
MATERIALS AND METHODS
Data Compilation
Diversity through time. Raw taxon counts for 61 extant and 374 fossil marine mammal genera recorded at the level of geological stage were downloaded from the Paleobiology Database (PBDB, www.paleobiodb.org). The search was performed separately for the following groups: “Archaeoceti, Cetacea”, “Pinnipedimorpha”, “Sirenia”, “Desmostylia”, “Thalassocnus”, “Ursus maritimus, Kolponomos”, “Aonychini, Aonyxini, Enhydra, Enhydrini, Enhydriodontini, Lontra, Lutra, Pteronura, Siamogale” (sea otters). Occurrences for each group were downloaded for each time bin (geological stage), starting from the Ypresian (early Eocene), using the “major (default)” time rule. Only occurrences with certain generic assignment were considered (“genus_certain”). The data include all occurrences entered up to 12/31/2017. Since geological stages in the Pleistocene and Holocene result in time bins that are of much shorter duration than any of the other stages, creating few occurrences in each of these time intervals, we followed Peredo and Uhen (2016) in binning the Pleistocene stages along with the Holocene series in the Quaternary, which at 2.6 Ma, is comparable in length to earlier time bins. Accessory data were also downloaded for these records, such as depositional environment and published sources, to allow verification of each occurrence individually and to be able to use the published references as a starting point to assign genera to the different categories. Diversity plots were obtained by counting the valid genera for each time bin in each group or family after the categorization was completed.
Feeding strategies and ecological adaptations through time. To interpret patterns in the evolution of feeding adaptations through time in marine mammals, we categorized each taxon using four parameters: prey capture, tooth and cusp shape, diet, and habitat. We first defined the categories for each parameter in extant genera and then applied them to the fossils. It is possible that fossil taxa presented completely different adaptations not present in any modern genera. By using broad categories, we aim to characterize trends and possible climatic drivers in the evolutionary history of these lineages that would not be obliterated by the incorrect categorization of single taxa. Categories were assigned to valid taxa only. The categories assigned to each taxon, along with its taxonomic placement, justification for the exclusion of invalid taxa, and all literature sources used are reported in the Supplementary Information that can be found in the Dryad repository subdivided by group (Cetacea, Pinnipedimorpha, Sirenia, Desmostylia, and Thalassoconus, Other Carnivores).
We started by assigning categories to extant marine mammals to then use this information to better categorize extinct taxa. We divided marine mammals into four prey capture categories: biting/crushing, filter, suction, and grazing, as described above. Prey capture types for extant taxa were compiled from primary and secondary sources (e.g., Adam and Berta, 2002). In an effort to further categorize prey selection accurately in the fossils, we examined tooth pattern and cusp shape (tooth presence/absence, homodonty, heterodonty; pointed, rounded, or bilophodont cusps). Type of dentition and cusp shape in extant taxa was determined based on photographs or personal observations of the authors. Diet was characterized using the five distinct dietary categories of Pauly et al. (1998): benthic invertebrates, fish, squid/soft-bodied pelagic invertebrates, zooplankton, and tetrapods. We included an additional dietary category for multicellular primary producers (sea grasses and algae) to characterize herbivorous taxa. Diet of carnivorous living species was categorized based on data from Pauly et al. (1998) and additional literature sources for taxa that were not included in this study. If two categories were indicated by Pauly et al. (1998) for at least one species, both were considered to categorize the genus if neither made up more than 80 % of the diet, but only one was chosen if it accounted for more than 80 % of the diet alone. If three or more categories were indicated by Pauly et al. (1998) in a least one species, the first two or three prey items that made up at least 80 % of the diet were used. Finally, we considered the environment(s) occupied by marine mammals. Six habitat categories were based on environments used by extant genera: marine pelagic, marine coastal, marine benthic, riverine, estuarine, and lacustrine, and were compiled by relevant secondary literature (e.g., Jefferson et al., 2015).
Based on prey capture categories, tooth shape, diet, and habitat data for extant marine mammals, we inferred relevant groupings for fossil taxa. When any hypotheses about feeding and paleoenvironment for a taxon were reported in the literature, these were used to determine the category assignment. When these data were not available, we based our assessment on a variety of factors. Soft tissue feeding structures (i.e., lips, tongue, and hyoid muscles) typically do not fossilize. However, it is possible to infer feeding strategy in fossil marine mammals from the presence of osteological correlates. For example, the presence of lateral palatal nutrient sulci and foramina in some toothed mysticetes (Aetiocetidae) has been interpreted as bony correlates for the presence of baleen (Deméré and Berta, 2008; but see Peredo et al., 2017). These structures have also been observed in fossil stem baleen whales, although they still retained a full dentition (Deméré and Berta, 2008). It remains unclear whether these vessels are the same that feed the developing teeth and are later co-opted for baleen, and if they can be used to assess the presence of baleen in fossils (Deméré and Berta, 2008; Ekdale et al., 2015; Peredo et al., 2017). Observations on in situ fossilized stomach contents of associated skeletons and bite marks on bones provide a direct and accurate method for predicting diet (e.g., Boessenecker and Perry, 2011; Collareta et al., 2015). Another method is to catalog potential prey that occur in the same geologic horizon and locality as the predator (e.g., Lambert et al., 2015) although care must be taken to ensure that the carcasses of predator or prey have not been transported prior to fossilization (Adam and Berta, 2002; Lambert et al., 2015). In this case, further confirmation came from fish remains that were actually found in the whale. Diet and habitat can also be assessed using isotope analysis of fossil tooth enamel (e.g., Clementz et al., 2006, 2014). Whereas analyses of tooth microwear (Thewissen et al., 2011; Fahlke et al., 2013) provide clues about prey types that have been consumed, enamel microstructure (Loch et al., 2016) reveal information about tooth behavior (i.e., resistance). By far the most commonly used method for inferring the diet of fossil taxa employs functional morphology and analogy with living forms. By examining the morphology associated with a particular method of capturing and handling prey in living species, both in terms of skull morphology and tooth and cusps shape, inferences of feeding behavior and diet can often be drawn for fossil taxa with similar morphologies (e.g., Kolponomos and sea otters - Tseng et al., 2016). Similarly, habitat preference in fossil was assessed based on modern taxa displaying similar adaptations in skull, limb, and tooth morphology. Depositional environment of the fossil was considered but not used exclusively to determine the habitat of the taxon, as sedimentation, sedimentary outcrop availability, and preservation bias potentially make this parameter unreliable (Uhen and Pyenson, 2007; Marx, 2009).
Statistical Analyses for Environmental Drivers
Using this dataset, we performed statistical analyses to explore the correlations and changes through time of patterns of generic diversity and category distribution. We also examined the correlations between this dataset and productivity and climatic factors that have been used to explain diversity patterns in previous work. Specifically, we used δ18O values that reflect both temperature and global ice volume and δ13C values that serve as a proxy for changes in the global carbon cycle (Zachos et al., 2001). Values of δ18O and δ13C were taken from Prokoph et al. (2008). In particular, data originally published by Zachos et al. (2001) from benthic foraminifera and deep sea locations were used, as they represented the most complete record for the time interval considered. The values are reported using absolute age; we selected the ones belonging to each time bin and averaged them to get a single value representing that geological stage and creating a comparable dataset to the taxa occurrences. In addition, previous studies suggested that an increase in diatom species diversity, which suggests an increase in primary productivity, has driven cetacean diversity (Marx and Uhen, 2010), and therefore we also included this measure in the analyses. The diatom species data were downloaded from the Neptune Database on 03/05/2018 (NSB, www.nsb-mfn-berlin.de). The species present in each time bin were counted to determine the diversity in that interval. We also considered how changes in sea level might have affected diet and diversity of marine mammals. Estimates of sea level were taken from Miller et al. (2005) and the mean value for each stage was calculated as it was done for the isotope values. Correlation between diversity and category distribution with climatic variables for each group was tested using linear regression analysis. Best model including one to four climatic variables was chosen based on Akaike’s information criterion (AIC) score if a significant correlation was found (p < 0.05). Correlation between Sirenia and Desmostylia diversity and climate variables was tested using Pearson’s correlation test due to their low sample size. All statistical analyses were conducted in XLSTATS (2017). Raw data used for the analyses can be found in the Supplementary Information in the Dryad repository (https://doi.org/10.6086/d14671).
Ancestral State Reconstructions
To expand on the evolutionary significance of these data, pinnipedimorph and cetacean phylogenetic trees were assembled using previously published phylogenies and ancestral state reconstructions (ASR) were performed by coding the taxa according to the categories described. The topologies are assembled for the most recent work on each group in order to include the highest number of confidently assigned taxa in the analyses and represent all major groups. The phylogenetic position of some taxa is currently debated in the literature, and different phylogenetic placement might alter the results of the ASR analyses. However, it is beyond the scope of this paper to weigh in on these debates, and therefore we chose to use recent and comprehensive topologies that are best suited for ancestral state reconstruction. Combined topologies and sources used to assemble the trees are reported in Appendix 1. All analyses were conducted in Mesquite v3.5 (Maddison and Maddison, 2018) using maximum likelihood. The matrices used for the ASR analyses are available in the Supplementary Information on Dryad (https://doi.org/10.6086/d14671).
RESULTS AND DISCUSSION
Diversity through Time
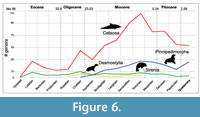 Updated generic plots of marine mammal paleodiversity reveal that cetaceans and pinnipedimorphs achieved greatest diversity during the late Miocene (Tortonian) (Figure 6). Generally, our results confirm earlier reports of diversity changes through time (e.g., Marx and Uhen, 2010; Peredo and Uhen, 2016; Boessenecker and Churchill, 2018). The herbivorous taxa (Desmostylia and Sirenia) appear to maintain a low but constant diversity throughout their distribution, while Cetacea and Pinnipedimorpha present wider fluctuations. We confirm previous observations (Peredo and Uhen, 2016) of large drops in marine mammal diversity at the end of the Oligocene, Miocene, and Pliocene (Figure 6). The late Oligocene diversity drop has been explained as the result of poor sampling (Marx and Fordyce, 2015) but a rapidly improving record from this time interval may require revision of this observation (e.g., Fordyce and Marx, 2018; Peredo and Pyenson, 2018; Tsai and Fordyce, 2018). The late Miocene drop in diversity was influenced by the Messinian Salinity Crisis, a geologic event during which the Mediterranean Sea went into a cycle of desiccation resulting in deep, dry basins (Krijgsman et al., 1999). Phocids, cetaceans, and sirenians in the Mediterranean were particularly affected beginning about 6 Ma (Bianucci, 2013). The Pliocene drop in marine mammal diversity has been associated with the onset of Northern Hemisphere glaciation (Marx and Fordyce, 2015) and loss of habitat by sea level fluctuations (Pimiento et al., 2017). Whether these fluctuations in diversity represent real biological signals will require integrated comparative studies of potential biases of the record (Kelley and Pyenson, 2015).
Updated generic plots of marine mammal paleodiversity reveal that cetaceans and pinnipedimorphs achieved greatest diversity during the late Miocene (Tortonian) (Figure 6). Generally, our results confirm earlier reports of diversity changes through time (e.g., Marx and Uhen, 2010; Peredo and Uhen, 2016; Boessenecker and Churchill, 2018). The herbivorous taxa (Desmostylia and Sirenia) appear to maintain a low but constant diversity throughout their distribution, while Cetacea and Pinnipedimorpha present wider fluctuations. We confirm previous observations (Peredo and Uhen, 2016) of large drops in marine mammal diversity at the end of the Oligocene, Miocene, and Pliocene (Figure 6). The late Oligocene diversity drop has been explained as the result of poor sampling (Marx and Fordyce, 2015) but a rapidly improving record from this time interval may require revision of this observation (e.g., Fordyce and Marx, 2018; Peredo and Pyenson, 2018; Tsai and Fordyce, 2018). The late Miocene drop in diversity was influenced by the Messinian Salinity Crisis, a geologic event during which the Mediterranean Sea went into a cycle of desiccation resulting in deep, dry basins (Krijgsman et al., 1999). Phocids, cetaceans, and sirenians in the Mediterranean were particularly affected beginning about 6 Ma (Bianucci, 2013). The Pliocene drop in marine mammal diversity has been associated with the onset of Northern Hemisphere glaciation (Marx and Fordyce, 2015) and loss of habitat by sea level fluctuations (Pimiento et al., 2017). Whether these fluctuations in diversity represent real biological signals will require integrated comparative studies of potential biases of the record (Kelley and Pyenson, 2015).
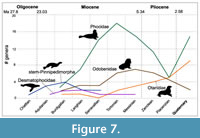 Pinnipedimorpha. Pinnipedimorpha is a monophyletic group within Carnivora (i.e., dogs, felids, bears, otters, etc.) that originated 30.6-23 Ma (Berta et al., 2018). Pinnipedimorpha includes several stem taxa (e.g., Enaliarctos, Pteronarctos, Pacificotaria, and Pinnarctidion) in addition to crown pinnipeds composed of the extinct Desmatophocidae and three extant clades: Otariidae (fur seals and sea lions), Odobenidae (walruses), and Phocidae (seals). With the exception of phocids, the pattern of pinnipedimorph diversity documented by Boessenecker and Churchill (2018) for the North Pacific is in agreement with our results. Early diverging stem-Pinnipedimorpha, also known as ‘enaliarctines’, represent the earliest diversification of pinnipeds, originating in the late Chattian, peaking in the Burdigalian, and becoming extinct by the end of the Serravallian (Figure 7). Desmatophocidae appear during the Aquitanian and gradually increase in diversity during the Burdigalian before declining in diversity and apparently becoming extinct in the Messinian (Figure 7). Otariids appear in the Burdigalian (but lack a Serravallian record); they diversify in the Messinian and Pliocene and mostly maintain that diversity until the Holocene (Figure 7). Walruses (Odobenidae) were somewhat diverse in the Burdigalian and remained at low diversity through to the Serravallian, gradually increasing until their peak in the Messinian, with their diversity decreasing sharply in the Pliocene and Pleistocene (Figure 7). Phocids appear in the Burdigalian and quickly diversify, reaching peak diversity during the Tortonian, and continue to be the dominant living pinniped group, even after a reduction in diversity in the Pliocene (Figure 7).
Pinnipedimorpha. Pinnipedimorpha is a monophyletic group within Carnivora (i.e., dogs, felids, bears, otters, etc.) that originated 30.6-23 Ma (Berta et al., 2018). Pinnipedimorpha includes several stem taxa (e.g., Enaliarctos, Pteronarctos, Pacificotaria, and Pinnarctidion) in addition to crown pinnipeds composed of the extinct Desmatophocidae and three extant clades: Otariidae (fur seals and sea lions), Odobenidae (walruses), and Phocidae (seals). With the exception of phocids, the pattern of pinnipedimorph diversity documented by Boessenecker and Churchill (2018) for the North Pacific is in agreement with our results. Early diverging stem-Pinnipedimorpha, also known as ‘enaliarctines’, represent the earliest diversification of pinnipeds, originating in the late Chattian, peaking in the Burdigalian, and becoming extinct by the end of the Serravallian (Figure 7). Desmatophocidae appear during the Aquitanian and gradually increase in diversity during the Burdigalian before declining in diversity and apparently becoming extinct in the Messinian (Figure 7). Otariids appear in the Burdigalian (but lack a Serravallian record); they diversify in the Messinian and Pliocene and mostly maintain that diversity until the Holocene (Figure 7). Walruses (Odobenidae) were somewhat diverse in the Burdigalian and remained at low diversity through to the Serravallian, gradually increasing until their peak in the Messinian, with their diversity decreasing sharply in the Pliocene and Pleistocene (Figure 7). Phocids appear in the Burdigalian and quickly diversify, reaching peak diversity during the Tortonian, and continue to be the dominant living pinniped group, even after a reduction in diversity in the Pliocene (Figure 7).
Several ecological explanations have been given for these diversity changes. Vélez-Juarbe (2017) suggested possible competitive displacement of desmatophocids by walruses and Boessenecker and Churchill (2018) noted that the extinction of desmatophocids appears to be followed by an increasing diversity of otariids, perhaps as the result of niche filling. The suggestion by these latter authors that several Tortonian odobenids (e.g., Imagotaria, Pontolis, and Gomphotaria) lacking benthic feeding specializations may have exploited similar prey (i.e., fish) by employing a generalized biting strategy like desmatophocids is in agreement with results of our study.
The effects of climate and environmental changes on pinnipedimorph diversity allow the following observations. The peak in phocid diversity and increase in odobenids and otariids during the Tortonian (11 Ma) is probably a result of the mid Miocene Climatic Optimum (MMCO) that occurred in the Langhian (~15 Ma) and is followed by decreases in diversity along with colder temperatures (Figure 7). Previous work suggested that sea level changes could affect pinniped haul-out areas, which provide breeding areas. A rise in sea level may have been involved in the Mio-Pliocene extinction of phocids in South America, resulting in the decrease of sandy beach haul-out areas favored by phocids. Beginning in the Plio-Pleistocene and continuing at present, rocky shore habitats predominated in South America and elsewhere in the Southern Hemisphere (e.g., Africa, Oceania), areas exclusively occupied by otariids (Valenzuela-Toro et al., 2013, 2016). This hypothesis of ecological replacement of phocids by otariids is difficult to confirm at the present time since marine mammal fossils in South America are relatively poorly known and not well sampled during this time. Examination of habitat preferences in several extant otariids and phocids off the coast of Baja California, Mexico, revealed overlap in habitat although phocids (harbor and elephant seals) preferred sandy beaches and cobblestone beaches, which may be associated with their postcranial morphology (i.e., inability of rotating hind limbs forward) and terrestrial locomotion (Arias-del Raza and Heckel, 2016).
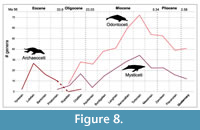 Cetacea. Cetacea are a monophyletic group nested within Artiodactyla (i.e., pigs, deer, camels, hippos, etc.). Stem cetaceans, or archaeocetes, the paraphyletic group ancestral to all other cetaceans, evolved during the Eocene (Ypresian) approximately 53.5 Ma. Stem cetaceans evolved in fresh water and later in the Eocene transitioned to marine environments, which is supported by dental stable isotope values (Roe et al., 1998; Clementz et al., 2006). Crown Cetacea (or Neoceti), defined by the common ancestor of all living mysticete and odontocete cetaceans, originated approximately 36.4 Ma at the end of the Eocene (Gatesy et al., 2013; Pyenson, 2017). Absolute divergence dates calculated using molecular data are mostly in line with evidence from the fossil record (Figure 8).
Cetacea. Cetacea are a monophyletic group nested within Artiodactyla (i.e., pigs, deer, camels, hippos, etc.). Stem cetaceans, or archaeocetes, the paraphyletic group ancestral to all other cetaceans, evolved during the Eocene (Ypresian) approximately 53.5 Ma. Stem cetaceans evolved in fresh water and later in the Eocene transitioned to marine environments, which is supported by dental stable isotope values (Roe et al., 1998; Clementz et al., 2006). Crown Cetacea (or Neoceti), defined by the common ancestor of all living mysticete and odontocete cetaceans, originated approximately 36.4 Ma at the end of the Eocene (Gatesy et al., 2013; Pyenson, 2017). Absolute divergence dates calculated using molecular data are mostly in line with evidence from the fossil record (Figure 8).
The origin and diversification of crown cetaceans near the Eocene-Oligocene boundary has long been explained as the result of establishment of the Antarctic Circumpolar Current and large-scale changes that accompanied it. Global cooling fed Antarctic ice expansion and Southern Ocean upwelling that resulted in an increase in productivity (e.g., Fordyce, 1980; Steeman et al., 2009). The role of this process in the evolution of Cetacea has been debated given that the origin of crown cetaceans in the late Eocene, recovered both in the fossil record and in phylogenetic analysis using molecular data, actually precedes the estimated onset of the current, which likely occurred right at the epoch boundary at 33.5 Ma (Hassold et al., 2009). As previously noted, testable models that explain the ecological context of cetacean evolution are lacking (Pyenson, 2017).
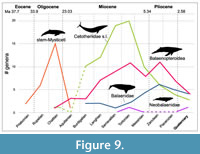 Late Miocene diversity of mysticetes (Figure 8) appears to be driven dominantly by an increase in diversity of extinct Cetotheriidae (Figure 9 - Marx and Uhen, 2010; Marx and Fordyce, 2015; Slater et al., 2017). Balaenopteridae and Balaenidae instead reach their peak diversity in the Zanclean before sharply declining (Figure 9). Odontoceti also increase in diversity in the late Miocene (Figure 8). This distribution is driven by a simultaneous peak in diversity of four of the five extant groups of toothed whales (Figure 10). The four living or recently extinct river dolphin taxa, Platanistidae (Asian river dolphins), Pontoporiidae (La Plata river/coastal dolphins), Lipotidae (Yangtze river dolphin), and Iniidae (Amazon river dolphins), belong to two distinct groups that appear to have transitioned from marine to freshwater independently at least twice, in the Aquitanian (Platanistidae) and Serravallian (Iniidae/Pontoporiidae/Lipotidae)
Late Miocene diversity of mysticetes (Figure 8) appears to be driven dominantly by an increase in diversity of extinct Cetotheriidae (Figure 9 - Marx and Uhen, 2010; Marx and Fordyce, 2015; Slater et al., 2017). Balaenopteridae and Balaenidae instead reach their peak diversity in the Zanclean before sharply declining (Figure 9). Odontoceti also increase in diversity in the late Miocene (Figure 8). This distribution is driven by a simultaneous peak in diversity of four of the five extant groups of toothed whales (Figure 10). The four living or recently extinct river dolphin taxa, Platanistidae (Asian river dolphins), Pontoporiidae (La Plata river/coastal dolphins), Lipotidae (Yangtze river dolphin), and Iniidae (Amazon river dolphins), belong to two distinct groups that appear to have transitioned from marine to freshwater independently at least twice, in the Aquitanian (Platanistidae) and Serravallian (Iniidae/Pontoporiidae/Lipotidae)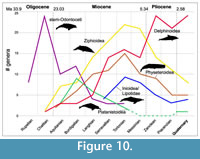 (Figure 10 - Cassens et al., 2000; Geisler et al., 2011). The explosive radiation of delphinids during the last 3.5 Ma (Figure 10) has been explained as a response to global climate cooling and the beginning of the Northern Hemisphere glaciation and related changes in sea level (e.g., Whitmore, 1994; Fordyce and de Muizon, 2001; McGowen et al., 2009; Steeman et al., 2009; Amaral et al., 2018). This increased diversity of the late Pliocene Mediterranean marine mammal assemblages (i.e., cetaceans, pinnipedimorphs, and sirenians) was hypothesized as possibly due to rapid colonization of this region after the Messinian salinity crisis (Bianucci, 2005). More specifically, the diversification of delphinine dolphins (e.g., Stenella spp., Delphinus spp., Lagenorynchus spp., and Tursiops spp.) in the tropical Atlantic and Indian Oceans has been related to pulses of diatom productivity during the late Pliocene and early Pleistocene (Mannocci et al., 2015).
(Figure 10 - Cassens et al., 2000; Geisler et al., 2011). The explosive radiation of delphinids during the last 3.5 Ma (Figure 10) has been explained as a response to global climate cooling and the beginning of the Northern Hemisphere glaciation and related changes in sea level (e.g., Whitmore, 1994; Fordyce and de Muizon, 2001; McGowen et al., 2009; Steeman et al., 2009; Amaral et al., 2018). This increased diversity of the late Pliocene Mediterranean marine mammal assemblages (i.e., cetaceans, pinnipedimorphs, and sirenians) was hypothesized as possibly due to rapid colonization of this region after the Messinian salinity crisis (Bianucci, 2005). More specifically, the diversification of delphinine dolphins (e.g., Stenella spp., Delphinus spp., Lagenorynchus spp., and Tursiops spp.) in the tropical Atlantic and Indian Oceans has been related to pulses of diatom productivity during the late Pliocene and early Pleistocene (Mannocci et al., 2015).
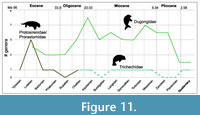 Sirenia and Desmostylia. Sirenians are members of Afrotheria (i.e., elephants, hyraxes, tenrecs, etc.) and include several stem taxa (i.e., Prorastomidae and Protosirenidae) and one crown clade encompassing Trichechidae (manatees) and Dugongidae (dugongs). The stem sirenians, known from the early to middle Eocene (late Ypresian to early Lutetian) of Africa and Central America (Benoit et al., 2013), reached their peak in diversity during the Lutetian (Figure 11). Appearing at about this same time were dugongids, the most successful and diverse sirenians, that typically inhabited warm, nearshore waters, with a worldwide distribution. A cold-water adapted lineage, first known in the middle Oligocene in the eastern Pacific, culminated in Steller’s sea cow (Hydrodamalis gigas) and evolved large body size as an adaptation to cool climates beginning in the Miocene (Pyenson and Vermeij, 2016). Dugongid diversity dramatically decreased in the late Pliocene and at present they are represented by a single species found in the Indo-Pacific region (Figure 11). The evolution of manatees (Trichechidae) began in South America during the Miocene and is tied to Caribbean paleobiogeography, notably uplift of the Andes, which created erosion and runoff into river systems that resulted in an abundance of abrasive grasses (Domning, 1982). Trichechids adapted to this new food resource by evolving thickened tooth enamel and smaller, more numerous teeth that were continuously replaced. The particular set of adaptations that characterize this lineage might have contributed to their low but stable diversity (Figure 11).
Sirenia and Desmostylia. Sirenians are members of Afrotheria (i.e., elephants, hyraxes, tenrecs, etc.) and include several stem taxa (i.e., Prorastomidae and Protosirenidae) and one crown clade encompassing Trichechidae (manatees) and Dugongidae (dugongs). The stem sirenians, known from the early to middle Eocene (late Ypresian to early Lutetian) of Africa and Central America (Benoit et al., 2013), reached their peak in diversity during the Lutetian (Figure 11). Appearing at about this same time were dugongids, the most successful and diverse sirenians, that typically inhabited warm, nearshore waters, with a worldwide distribution. A cold-water adapted lineage, first known in the middle Oligocene in the eastern Pacific, culminated in Steller’s sea cow (Hydrodamalis gigas) and evolved large body size as an adaptation to cool climates beginning in the Miocene (Pyenson and Vermeij, 2016). Dugongid diversity dramatically decreased in the late Pliocene and at present they are represented by a single species found in the Indo-Pacific region (Figure 11). The evolution of manatees (Trichechidae) began in South America during the Miocene and is tied to Caribbean paleobiogeography, notably uplift of the Andes, which created erosion and runoff into river systems that resulted in an abundance of abrasive grasses (Domning, 1982). Trichechids adapted to this new food resource by evolving thickened tooth enamel and smaller, more numerous teeth that were continuously replaced. The particular set of adaptations that characterize this lineage might have contributed to their low but stable diversity (Figure 11).
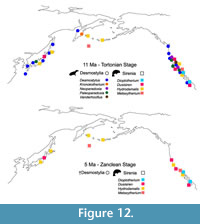 Sirenians appear to have replaced desmostylians during the late Miocene (Tortonian) as the result of competition for the same food resource: sea grasses (Vélez-Juarbe et al., 2012). Desmostylia are a completely extinct, enigmatic group of herbivorous aquatic mammals, exclusively distributed in the North Pacific, likely related to either Afrotheria, Perissodactyla, or Paenungulatomorpha (Matsui and Tsuihiji, 2019). Evidence for their replacement by sirenians is provided by the decrease in diversity of desmostylians after the Tortonian followed by their disappearance in the Messinian, which coincides with the appearance of Hydrodamalis and other dugongids in the same localities and habitats they occupied (Figure 12). Additional data on the paleoecology of these lineages and climatic changes of the region need to be conducted in order to test this hypothesis.
Sirenians appear to have replaced desmostylians during the late Miocene (Tortonian) as the result of competition for the same food resource: sea grasses (Vélez-Juarbe et al., 2012). Desmostylia are a completely extinct, enigmatic group of herbivorous aquatic mammals, exclusively distributed in the North Pacific, likely related to either Afrotheria, Perissodactyla, or Paenungulatomorpha (Matsui and Tsuihiji, 2019). Evidence for their replacement by sirenians is provided by the decrease in diversity of desmostylians after the Tortonian followed by their disappearance in the Messinian, which coincides with the appearance of Hydrodamalis and other dugongids in the same localities and habitats they occupied (Figure 12). Additional data on the paleoecology of these lineages and climatic changes of the region need to be conducted in order to test this hypothesis.
Other marine mammals. In the most recent combined phylogeny of otters, the extant sea otter Enhydra lutris and its sister taxon Enhydriodon spp. are embedded within the river otter (Lutrinae) clade (Wang et al., 2018). Although this same phylogenetic analysis placed Enhydritherium with other lutrine otters and distant from the sea otter we included it in our study since recent fossil evidence suggested that Enhydritherium occupied freshwater environments in addition to estuarine and coastal environments (Tseng et al., 2017). The oldest record of Enhydra sp. from the Pacific is Middle Pleistocene in age although an earlier representative of this lineage (Enhydra reevei) arose in the North Atlantic during the early Pleistocene about 2.2-1.7 Ma and presumably dispersed into the Pacific though the Bering Strait (Boessenecker, 2016). The extinct sea mink Neovison macrodon known from the early to Late Pleistocene in marine and estuarine habitats along the coastal islands of the Gulf of Maine was hunted to extinction, a victim of the fur trade in the late 1800s (Mead et al., 2000).
The polar bear Ursus maritimus diverged from brown bears probably less than 500,000 years ago (Liu et al., 2014). Polar bears have a fossil record limited to the Late Pleistocene. The oldest remains of a polar bear are dated at 110-13 ka (Ingólfsson and Wiig, 2008). Stable isotope analyses of fossil polar bear teeth indicate that by at least 110 ka they had acquired a feeding strategy quite different than that of brown bears, having adapted to feeding on fish and marine mammals (Lindquist et al., 2010).
Aquatic sloths (Thalassocnus spp.) evolved in the Southeastern Pacific during the Miocene (e.g., de Muizon et al., 2004; Amson et al., 2015). There is some evidence to suggest that they occupied the sea cow niche (de Muizon et al., 2004; Amson et al., 2015). The similarity of these marine sloths to extinct desmostylians in the North Pacific in terms of diet raises the possibility that they may be the ecological homologues of desmostylians in the South Pacific, a hypothesis that requires more rigorous testing.
Biotic and Environmental Drivers of Feeding Diversification
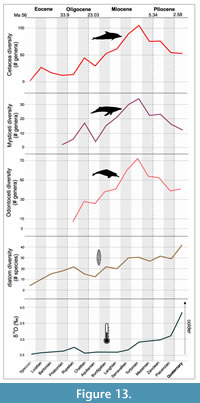 Pinnipedimorphs. Pinnipedimorph diversity was most strongly associated with all four climatic variables considered - δ18O, δ13C, diatom species diversity, and sea level changes (Table 1, Appendix 2). The link between specific climatic indicators and pinnipedimorph diversity has not been previously indicated, although research on modern ecosystems has associated these variables (Hirons et al., 2001; Fréon et al., 2009). In particular, when the three modern families of Pinnipedimorpha are examined separately, Otariidae (fur seals and sea lions) appear to be driving the correlation, with the diversity of this group being strongly associated with all four climatic variables (Table 1; Figure 13). This might be the result of habitat and ecological differences. For example, extant otariids are confined to regions of high upwelling and cool water temperature usually less than 24°C (Deméré et al., 2003), which confirms the importance of climate and temperature on their distribution. By contrast, phocids are found in a wider variety of environments including tropical, oligotrophic waters (Churchill et al., 2014). Odobenidae (walrus) diversity was also found to be correlated with three variables: δ18O, δ13C, and sea level changes. This fits with a Messinian peak diversity of fossil walruses and the evolution of more specialized feeding systems (i.e., tusks and molluscivory) (Magallanes et al., 2018). The drop in walrus diversity beginning at the end of the Pliocene is associated with the onset of Northern Hemisphere glaciation, large-scale sea level oscillations, and other oceanographic alterations (e.g., changes in productivity and ocean circulation). By the end the Pleistocene, the loss of shallow foraging areas utilized by molluscivorous odobenines was an important driver of extinction for walruses and other megafauna (Pimiento et al., 2017).
Pinnipedimorphs. Pinnipedimorph diversity was most strongly associated with all four climatic variables considered - δ18O, δ13C, diatom species diversity, and sea level changes (Table 1, Appendix 2). The link between specific climatic indicators and pinnipedimorph diversity has not been previously indicated, although research on modern ecosystems has associated these variables (Hirons et al., 2001; Fréon et al., 2009). In particular, when the three modern families of Pinnipedimorpha are examined separately, Otariidae (fur seals and sea lions) appear to be driving the correlation, with the diversity of this group being strongly associated with all four climatic variables (Table 1; Figure 13). This might be the result of habitat and ecological differences. For example, extant otariids are confined to regions of high upwelling and cool water temperature usually less than 24°C (Deméré et al., 2003), which confirms the importance of climate and temperature on their distribution. By contrast, phocids are found in a wider variety of environments including tropical, oligotrophic waters (Churchill et al., 2014). Odobenidae (walrus) diversity was also found to be correlated with three variables: δ18O, δ13C, and sea level changes. This fits with a Messinian peak diversity of fossil walruses and the evolution of more specialized feeding systems (i.e., tusks and molluscivory) (Magallanes et al., 2018). The drop in walrus diversity beginning at the end of the Pliocene is associated with the onset of Northern Hemisphere glaciation, large-scale sea level oscillations, and other oceanographic alterations (e.g., changes in productivity and ocean circulation). By the end the Pleistocene, the loss of shallow foraging areas utilized by molluscivorous odobenines was an important driver of extinction for walruses and other megafauna (Pimiento et al., 2017).
We also examined whether habitat selection and prey type changes in this group were independently associated with climate and primary productivity variables. The distribution through time of the three predominant marine habitats for pinnipedimorphs (pelagic, coastal, and benthic) as well as the three main prey types they feed on (fish, squid, and benthic invertebrates) were each correlated with at least two of the climatic variables (Table 2, Table 3). This confirms the role of climate change and primary productivity on habitat and prey choice in pinnipedimorph evolution and suggests that the diversification of this group should be considered in the context of global environmental changes.
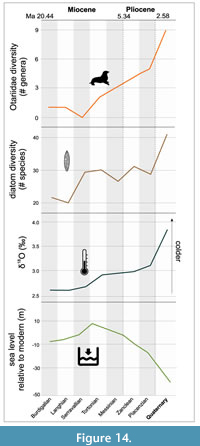 Cetaceans. Diversity of cetaceans as a whole, and both odontocetes and mysticetes separately, is correlated with diatom species diversity and δ18O, a proxy for global ocean temperature (Table 4; Figure 14). These results for Cetacea confirm the findings of some previous studies that linked cetacean and mysticete evolution to climate change (Marx and Uhen, 2010; Marx and Fordyce, 2015). Recent fossil discoveries show that the timing of divergence of both mysticetes and odontocetes is earlier than previously reported, thus raising questions on the role of the establishment of the Antarctic Circumpolar Current in the evolution of these two groups (Pyenson, 2017). For Cetacea, we analyzed the possible correlation between habitat and diet changes through time, as well as prey capture strategy given the high variability in this group (Table 5, Table 6, Table 7). For the habitats (Table 5), riverine taxa diversity appears to be correlated with δ18O and sea level changes, while we did not find any significant correlations between estuarine habitat preference and changes in climate or primary productivity. These correlations might be influenced by the low counts of fossil occurrences with adaptations compatible with these habitats, but more research needs to be conducted on the causes of re-invasion of freshwater and estuarine habitats by river dolphins. All marine habitats (pelagic, coastal, benthic) instead were correlated with δ18O and diatom diversity, with marine benthic being correlated also with δ13C. This is expected since benthic invertebrates like mollusks are reliant on dissolved carbon for their shells (Byrne, 2011). The correlations of the habitat preference to climate are confirmed by the strong associations between these same variables and prey type. The most common prey types (fish, soft-bodied free-swimming invertebrates/squid, and zooplankton) are correlated with primary productivity (diatom diversity) and ocean temperature changes (δ18O), with free-swimming invertebrates again showing significant correlation with carbon isotopes fluctuations (Table 6). Benthic invertebrates were most strongly correlated with δ13C and diatom diversity, again showing the importance of carbon fluctuations and primary productivity in the distribution of this prey type. Tetrapods are the only prey type that does not present a significant correlation with climatic variables. This might be due to low counts, but also by the possible opportunistic nature of this prey type choice and other constraints on this prey type (size, dentition). Finally, the three main prey capture strategies (biting/crushing, suction, and filter) present throughout cetacean evolution appear to be associated as well with diatom diversity and δ18O, with suction also being associated with δ13C (Table 7). This is due to the fact that the preferred prey type by suction feeding extant taxa are free-swimming invertebrates (e.g., squid), and this prey type is in turn associated with all three climatic variables.
Cetaceans. Diversity of cetaceans as a whole, and both odontocetes and mysticetes separately, is correlated with diatom species diversity and δ18O, a proxy for global ocean temperature (Table 4; Figure 14). These results for Cetacea confirm the findings of some previous studies that linked cetacean and mysticete evolution to climate change (Marx and Uhen, 2010; Marx and Fordyce, 2015). Recent fossil discoveries show that the timing of divergence of both mysticetes and odontocetes is earlier than previously reported, thus raising questions on the role of the establishment of the Antarctic Circumpolar Current in the evolution of these two groups (Pyenson, 2017). For Cetacea, we analyzed the possible correlation between habitat and diet changes through time, as well as prey capture strategy given the high variability in this group (Table 5, Table 6, Table 7). For the habitats (Table 5), riverine taxa diversity appears to be correlated with δ18O and sea level changes, while we did not find any significant correlations between estuarine habitat preference and changes in climate or primary productivity. These correlations might be influenced by the low counts of fossil occurrences with adaptations compatible with these habitats, but more research needs to be conducted on the causes of re-invasion of freshwater and estuarine habitats by river dolphins. All marine habitats (pelagic, coastal, benthic) instead were correlated with δ18O and diatom diversity, with marine benthic being correlated also with δ13C. This is expected since benthic invertebrates like mollusks are reliant on dissolved carbon for their shells (Byrne, 2011). The correlations of the habitat preference to climate are confirmed by the strong associations between these same variables and prey type. The most common prey types (fish, soft-bodied free-swimming invertebrates/squid, and zooplankton) are correlated with primary productivity (diatom diversity) and ocean temperature changes (δ18O), with free-swimming invertebrates again showing significant correlation with carbon isotopes fluctuations (Table 6). Benthic invertebrates were most strongly correlated with δ13C and diatom diversity, again showing the importance of carbon fluctuations and primary productivity in the distribution of this prey type. Tetrapods are the only prey type that does not present a significant correlation with climatic variables. This might be due to low counts, but also by the possible opportunistic nature of this prey type choice and other constraints on this prey type (size, dentition). Finally, the three main prey capture strategies (biting/crushing, suction, and filter) present throughout cetacean evolution appear to be associated as well with diatom diversity and δ18O, with suction also being associated with δ13C (Table 7). This is due to the fact that the preferred prey type by suction feeding extant taxa are free-swimming invertebrates (e.g., squid), and this prey type is in turn associated with all three climatic variables.
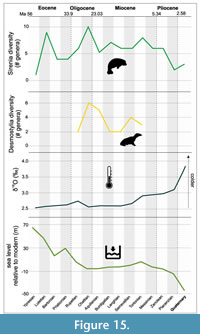 Sirenia and Desmostylia. When Sirenia and Desmostylia taxa are analyzed together, to account for the overall low taxonomic diversity of these groups, we found that their diversity is correlated with changes in global ocean temperature (δ18O) and sea level changes (Table 8; Figure 15). This also implies that their prey type (sea grasses and algae) and grazing habits are correlated with these variables. This is consistent with previous work showing that sirenians (with the exception of cold adapted hydrodamalines) and desmostylians are primarily subtropical and tropical and associated with seagrasses (Vélez-Juarbe, 2014). When performing a Pearson’s correlation test to account for the low counts and check intergroup relationships, we found that, while no single climatic variable is correlated with the diversity of these taxa, the temporal distributions of these two groups are correlated with each other (Table 9). These correlations support the hypotheses of the extinction of Desmostylia being mostly driven by the colonization of the North Pacific by Sirenia. By looking at distribution maps of the main genera for these groups in this area, it appears clear that the appearance of the genus Hydrodamalis in the Tortonian (~11 Ma) coincides with the decline of desmostylians, which went completely extinct by the end of the Tortonian (7.2 Ma) (Figure 12). While other variables connected with changes in climate and primary productivity (e.g., δ15N) might be correlated with the diversity of herbivorous groups, their relatively low and stable numbers point to the fact that they occupy a specific ecological niche and are particularly vulnerable to competition.
Sirenia and Desmostylia. When Sirenia and Desmostylia taxa are analyzed together, to account for the overall low taxonomic diversity of these groups, we found that their diversity is correlated with changes in global ocean temperature (δ18O) and sea level changes (Table 8; Figure 15). This also implies that their prey type (sea grasses and algae) and grazing habits are correlated with these variables. This is consistent with previous work showing that sirenians (with the exception of cold adapted hydrodamalines) and desmostylians are primarily subtropical and tropical and associated with seagrasses (Vélez-Juarbe, 2014). When performing a Pearson’s correlation test to account for the low counts and check intergroup relationships, we found that, while no single climatic variable is correlated with the diversity of these taxa, the temporal distributions of these two groups are correlated with each other (Table 9). These correlations support the hypotheses of the extinction of Desmostylia being mostly driven by the colonization of the North Pacific by Sirenia. By looking at distribution maps of the main genera for these groups in this area, it appears clear that the appearance of the genus Hydrodamalis in the Tortonian (~11 Ma) coincides with the decline of desmostylians, which went completely extinct by the end of the Tortonian (7.2 Ma) (Figure 12). While other variables connected with changes in climate and primary productivity (e.g., δ15N) might be correlated with the diversity of herbivorous groups, their relatively low and stable numbers point to the fact that they occupy a specific ecological niche and are particularly vulnerable to competition.
Ancestral Reconstructions of Feeding Strategies and Trophic Structure
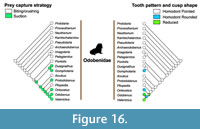 Pinnipedimorphs. Extant and fossil pinnipedimorphs use multiple strategies to capture and consume prey underwater. The ancestral feeding strategy of pinnipedimorphs was a biting strategy (Appendix 3) employing a heterodont dentition in agreement with previous studies based on skull and dental morphologies (e.g., Adam and Berta, 2002; Churchill and Clementz, 2015). Phocoids (phocids + desmatophocids) are biters with several taxa having evolved specialist strategies (Appendix 3). The extant phocid Hydrurga (leopard seal) employs biting and filter strategies, and this is predicted for the fossil seal (Homiphoca). Lobodon (crabeater seal) is the only extant pinnipedimorph that is a specialized filter feeder. Mirounga and Erignathus (bearded seal) employ a suction feeding strategy. Although we reconstructed the extinct phocid Hadrokirus as having a biting/crushing diet largely on the basis of robust, well-worn teeth, Amson and de Muizon (2014) described this taxon as having a more exclusive durophagous habit. Otariids uniformly employ a biting strategy with the exception of some species of Arctocephalus that are more generally classified as biting/suction feeders. Odobenids have been hypothesized as exhibiting a biting strategy ancestrally (Boessenecker and Churchill, 2013), and this pattern is also seen in our analysis (Figure 16). The characteristic suction feeding strategy of the modern walrus apparently evolved twice, once among dusignathine walruses (e.g., Dusignathus) and again among odobenine walruses (e.g., Protodobenus).
Pinnipedimorphs. Extant and fossil pinnipedimorphs use multiple strategies to capture and consume prey underwater. The ancestral feeding strategy of pinnipedimorphs was a biting strategy (Appendix 3) employing a heterodont dentition in agreement with previous studies based on skull and dental morphologies (e.g., Adam and Berta, 2002; Churchill and Clementz, 2015). Phocoids (phocids + desmatophocids) are biters with several taxa having evolved specialist strategies (Appendix 3). The extant phocid Hydrurga (leopard seal) employs biting and filter strategies, and this is predicted for the fossil seal (Homiphoca). Lobodon (crabeater seal) is the only extant pinnipedimorph that is a specialized filter feeder. Mirounga and Erignathus (bearded seal) employ a suction feeding strategy. Although we reconstructed the extinct phocid Hadrokirus as having a biting/crushing diet largely on the basis of robust, well-worn teeth, Amson and de Muizon (2014) described this taxon as having a more exclusive durophagous habit. Otariids uniformly employ a biting strategy with the exception of some species of Arctocephalus that are more generally classified as biting/suction feeders. Odobenids have been hypothesized as exhibiting a biting strategy ancestrally (Boessenecker and Churchill, 2013), and this pattern is also seen in our analysis (Figure 16). The characteristic suction feeding strategy of the modern walrus apparently evolved twice, once among dusignathine walruses (e.g., Dusignathus) and again among odobenine walruses (e.g., Protodobenus).
Reconstructions of tooth shape indicate that ancestrally the teeth of stem pinnipedimorphs are heterodont with pointed cusps (Appendix 4). Crown pinnipeds are characterized by the development of homodonty with stem taxa in all three major lineages exhibiting variable loss of cusps and tooth simplification (Boessenecker and Churchill, 2013). This pattern is best exemplified by Odobenidae, where in conjunction with the evolution of suction feeding, the teeth were not only simplified but also greatly reduced in number (Figure 16).
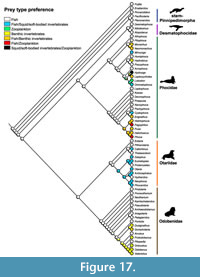 Ancestral state reconstruction analysis suggests that the ancestral prey type for pinnipedimorphs was fish (Figure 17). Among phocids several extant monachines (e.g., Monachus and Neomonachus) feed on fish and benthic invertebrates. Mirounga (elephant seal) is a specialized squid and fish feeder. The fossil seal Hadrokirus is hypothesized as a benthic invertebrate feeder based on mandibular and dental morphology (Amson and de Muizon, 2014). Cystophora (hooded seal) and Ommatophoca (Ross seal) are specialists on fish and squid, Lobodon (crabeater seal) feeds primarily on zooplankton and Hydrurga (leopard seal) consumes seals and penguins. A diet of fish and squid apparently evolved twice among otariids, once in Callorhinus (northern fur seal) and again in the common ancestor of sea lions and arctocephalines (southern fur seals). Ancestrally, odobenids fed on fish. A specialized diet of benthic invertebrates evolved twice among walruses, once in some dusignathines (e.g., Dusignathus) and again in odobenines, the lineage that includes the modern walrus.
Ancestral state reconstruction analysis suggests that the ancestral prey type for pinnipedimorphs was fish (Figure 17). Among phocids several extant monachines (e.g., Monachus and Neomonachus) feed on fish and benthic invertebrates. Mirounga (elephant seal) is a specialized squid and fish feeder. The fossil seal Hadrokirus is hypothesized as a benthic invertebrate feeder based on mandibular and dental morphology (Amson and de Muizon, 2014). Cystophora (hooded seal) and Ommatophoca (Ross seal) are specialists on fish and squid, Lobodon (crabeater seal) feeds primarily on zooplankton and Hydrurga (leopard seal) consumes seals and penguins. A diet of fish and squid apparently evolved twice among otariids, once in Callorhinus (northern fur seal) and again in the common ancestor of sea lions and arctocephalines (southern fur seals). Ancestrally, odobenids fed on fish. A specialized diet of benthic invertebrates evolved twice among walruses, once in some dusignathines (e.g., Dusignathus) and again in odobenines, the lineage that includes the modern walrus.
Pinnipedimorphs occupy diverse habitats, including both marine and freshwater environments. Stem pinnipedimorphs are mostly reconstructed as having had an ancestral coastal marine habitat as is characteristic of most extant taxa (Appendix 5). Since the fossil Puijila is now identified as a pinnipedimorph (Paterson et al., 2020) a lacustrine ancestral habitat is also indicated. The stem otariid Eotaria is reconstructed as having had a pelagic marine habitat. Benthic marine habitats appear to have been colonized several times, in the extinct seal Allodesmus, some monachine phocids (Monachus, Neomonachus, and Leptonychotes), and odobenine walruses (Protodobenus, Ontocetus, Valenictus, and Odobenus).
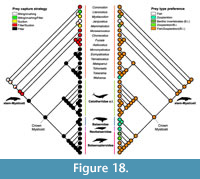 Cetaceans. Archaeocetes hunted and caught prey using their teeth (Appendix 6). This inference is supported by the large teeth with multiple cusps and fossilized stomach contents of basilosaurids (Uhen, 2004; Voss et al., 2019) and bite marks on small archaeocetes (Fahlke, 2012). The stem mysticete Coronodon was reconstructed as having been a toothed filter feeders indicating that the origin of the filter feeding niche characteristic of extant mysticetes had an earlier history than is generally reported (Geisler et al., 2017), although this reconstruction has been challenged (Hocking et al., 2017a; Fordyce and Marx, 2018; Peredo and Pyenson, 2018). Other stem toothed mysticetes (e.g., Mammalodon, Mystacodon) are reconstructed as having been suction feeders and still other stem taxa (e.g., aetiocetids) employed a filter/suction feeding strategy (Deméré and Berta, 2008, but see Marx et al., 2016 and Peredo et al., 2018 for alternate interpretations). Beginning 30 Ma, stem edentulous mysticetes (e.g., eomysticetids) exhibited bulk filter feeding that was retained by all crown mysticetes (Gatesy et al., 2013). Our analysis recovers this transition and also highlights that the switch to bulk filter feeding was likely mediated by intermediate forms that were capturing prey using a combination of filter and suction feeding, in line with previous hypotheses described above (Figure 18). The split between skim feeding balaenoids and more diverse generalized and specialized filter feeders, the thalassotherians, was completed by early Miocene (20-18 Ma) (Berta et al., 2016). Among thalassotherians, some cetotheriids (e.g., Herpetocetus - Gol’din and Startsev, 2014; El Adli et al., 2014) display a continuous suction feeding mode as early as 11-10 Ma (Berta et al., 2016). The specialized intermittent suction feeding that characterizes Eschrichtiidae, may have occurred as early as 5 Ma. The balaenopterid record extends into the late Miocene (12-10 Ma), which likely marks the origin of engulfment feeding, although the advanced lunge feeding behavior seen in living rorquals (i.e., characterized by greater degree of mandibular alpha rotation and lateral expansion of the mouth) developed later (Berta et al., 2016). The evolution of specialized filter feeding modes allowed mysticetes to feed on different prey types, as highlighted later in the analysis of prey preference.
Cetaceans. Archaeocetes hunted and caught prey using their teeth (Appendix 6). This inference is supported by the large teeth with multiple cusps and fossilized stomach contents of basilosaurids (Uhen, 2004; Voss et al., 2019) and bite marks on small archaeocetes (Fahlke, 2012). The stem mysticete Coronodon was reconstructed as having been a toothed filter feeders indicating that the origin of the filter feeding niche characteristic of extant mysticetes had an earlier history than is generally reported (Geisler et al., 2017), although this reconstruction has been challenged (Hocking et al., 2017a; Fordyce and Marx, 2018; Peredo and Pyenson, 2018). Other stem toothed mysticetes (e.g., Mammalodon, Mystacodon) are reconstructed as having been suction feeders and still other stem taxa (e.g., aetiocetids) employed a filter/suction feeding strategy (Deméré and Berta, 2008, but see Marx et al., 2016 and Peredo et al., 2018 for alternate interpretations). Beginning 30 Ma, stem edentulous mysticetes (e.g., eomysticetids) exhibited bulk filter feeding that was retained by all crown mysticetes (Gatesy et al., 2013). Our analysis recovers this transition and also highlights that the switch to bulk filter feeding was likely mediated by intermediate forms that were capturing prey using a combination of filter and suction feeding, in line with previous hypotheses described above (Figure 18). The split between skim feeding balaenoids and more diverse generalized and specialized filter feeders, the thalassotherians, was completed by early Miocene (20-18 Ma) (Berta et al., 2016). Among thalassotherians, some cetotheriids (e.g., Herpetocetus - Gol’din and Startsev, 2014; El Adli et al., 2014) display a continuous suction feeding mode as early as 11-10 Ma (Berta et al., 2016). The specialized intermittent suction feeding that characterizes Eschrichtiidae, may have occurred as early as 5 Ma. The balaenopterid record extends into the late Miocene (12-10 Ma), which likely marks the origin of engulfment feeding, although the advanced lunge feeding behavior seen in living rorquals (i.e., characterized by greater degree of mandibular alpha rotation and lateral expansion of the mouth) developed later (Berta et al., 2016). The evolution of specialized filter feeding modes allowed mysticetes to feed on different prey types, as highlighted later in the analysis of prey preference.
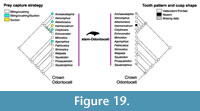 Odontocetes exhibit more diverse prey capture strategies. Ancestrally, stem odontocetes were similar to stem cetaceans in their employment of a biting/crushing strategy (Figure 19). Although some stem taxa (e.g., Archaeodelphis, Xenorophus, Cotylocara) are reconstructed as using a combination of biting/crushing and suction feeding. Crown odontocetes exhibit a greater diversity of feeding strategies and cranial morphologies (Figure 20). The ancestor of crown odontocetes was likely employing the ancestral biting/crushing prey capture mode. Living sperm whales (Physeteridae and Kogiidae) and beaked whales (Ziphiidae) have been suction feeders since their first appearance in the fossil record, although the teeth and skull morphology of some extinct genera closely related to the modern taxa (e.g., Acrophyseter, Livyatan, and Ninoziphius) support the hypothesis that ancestrally they were able to feed via biting and/or suction on different kinds of prey (Bianucci et al., 2016; Lambert et al., 2016). As characteristic of opportunistic feeding, ancestral Delphinoidea (Delphinidae, Monodontidae, and Phocoenidae) are reconstructed as having employed a biting/crushing/suction feeding strategy.
Odontocetes exhibit more diverse prey capture strategies. Ancestrally, stem odontocetes were similar to stem cetaceans in their employment of a biting/crushing strategy (Figure 19). Although some stem taxa (e.g., Archaeodelphis, Xenorophus, Cotylocara) are reconstructed as using a combination of biting/crushing and suction feeding. Crown odontocetes exhibit a greater diversity of feeding strategies and cranial morphologies (Figure 20). The ancestor of crown odontocetes was likely employing the ancestral biting/crushing prey capture mode. Living sperm whales (Physeteridae and Kogiidae) and beaked whales (Ziphiidae) have been suction feeders since their first appearance in the fossil record, although the teeth and skull morphology of some extinct genera closely related to the modern taxa (e.g., Acrophyseter, Livyatan, and Ninoziphius) support the hypothesis that ancestrally they were able to feed via biting and/or suction on different kinds of prey (Bianucci et al., 2016; Lambert et al., 2016). As characteristic of opportunistic feeding, ancestral Delphinoidea (Delphinidae, Monodontidae, and Phocoenidae) are reconstructed as having employed a biting/crushing/suction feeding strategy.
 Fossil stem mysticetes and odontocetes are similar to archaeocetes in exhibiting heterodonty (Figure 19; Appendix 7). Beginning 30 Ma, edentulous stem mysticetes (e.g., eomysticetids) are characterized by their lack of functional teeth and presumably possessed baleen (Appendix 7). A homodont dentition evolved among Physeteroidea (Kogiidae and Physeteridae) and is retained among all later odontocetes with the exception of ziphiids which feature a reduced dentition (Figure 20).
Fossil stem mysticetes and odontocetes are similar to archaeocetes in exhibiting heterodonty (Figure 19; Appendix 7). Beginning 30 Ma, edentulous stem mysticetes (e.g., eomysticetids) are characterized by their lack of functional teeth and presumably possessed baleen (Appendix 7). A homodont dentition evolved among Physeteroidea (Kogiidae and Physeteridae) and is retained among all later odontocetes with the exception of ziphiids which feature a reduced dentition (Figure 20).
Inferred diets are a direct result of these feeding adaptations and tooth morphologies. Ancestrally, cetaceans fed on fish (Appendix 8). Some archaeocetes (e.g., Basilosaurus) fed on fish and tetrapods. Most stem mysticetes (e.g., Coronodon and Llanocetus) likely fed on fish and zooplankton whereas several others (e.g., Mystacodon and Mammalodon) consumed benthic invertebrates. Stem taxa at the base of odontocete divergence are reconstructed as predominately having fed on fish. Ancestrally, a diet composed of fish/squid evolved among some stem odontocetes (e.g., Cotylocara, Archaeodelphis) and is characteristic of all crown odontocetes. A diet composed of fish and tetrapods evolved only a few times among crown odontocetes: in Orcinus and in some Physeteridae (e.g., Livyatan - not represented on the tree). In support of this result, diversity of delphinine dolphins (e.g., spinner dolphins, bottlenose dolphins, common dolphins, etc.) in the early Pliocene has been related to phytoplankton groups most abundant in the tropics, which in turn supports high fish abundances (Amaral et al., 2018).
With the exception of Pakicetus which occupied a riverine/estuarine habitat, the ancestral habitat for cetaceans is reconstructed as mixed estuarine/coastal marine environments (Appendix 9). There is isotopic evidence to suggest that the earliest cetaceans inhabited freshwater (Clementz et al., 2006). The protocetid-basilosaurid transition corresponds to the rise of the first fully aquatic cetaceans. Some of these later evolving stem cetaceans occupied more variable marine habitats including both pelagic and coastal environments. Most stem and crown mysticetes are characterized as having occupied these same environments. The extant gray whale Eschrichtius and its close fossil relative Eschrichtoides are reconstructed to inhabit marine coastal and pelagic as well as benthic habitats; the latter is a consequence of their suction feeding strategy. Additional isotopic analyses and other methods of investigation are needed to more narrowly define the habitat of extinct cetaceans, as the depositional environment of the fossils is not always a reliable indicator.
Other marine mammals. We did not perform ancestral state feeding reconstructions for sirenians, desmostylians, marine otters, aquatic sloths, and bears given their relatively low generic diversity and categorical data with little variation. Relevant feeding and habitat data collected for these taxa (Supplementary Information) allow a few general comments. Since their first appearance in the Eocene, sirenians have been identified as herbivores feeding on aquatic plants. Prorastomids possessed low crowned, rounded tooth cusps and were probably browsers that fed on floating aquatic plants and to a lesser extent sea grasses, the primary food of modern dugongs (Domning, 2001). Later dugongids evolved tusks and bilophodont postcanine teeth for feeding on sea grass rhizomes. In response to cooler climates, members of the hydrodamaline lineage are hypothesized to have fed on kelp which replaced (to a large extent) sea grasses (Pyenson and Vermeij, 2016). Since the oldest known sirenian locality in Africa consists of lake deposits, sirenians like cetaceans may have first evolved in freshwater (Clementz et al., 2006; Benoit et al., 2013). Based on isotope data, Clementz et al. (2003) concluded that desmostylians were aquatic herbivores that, like sirenians, foraged on sea grasses in estuarine and coastal freshwater systems. Coincident with the evolution of large sirenians in the North Pacific there is evidence to suggest that this region served as the origin of kelps (Laminariales) (Pyenson and Vermeij, 2016). Another herbivore, the aquatic sloth (Thalassocnus spp.), evolved in the South Atlantic (Peru and Chile) and fed on sea grasses in marine coastal habitats (de Muizon et al., 2004). The aquatic ursid/pinnipedimorph Kolponomos occupied a specialized crushing/suction feeding guild consuming various hard benthic invertebrates (e.g., sea urchins, clams, and abalones) during the Miocene in the North Pacific (Tseng et al., 2016). More recent occupants of this niche are the otters Enhydra and Enhydritherium and the sea mink Neovison. Paleoenvironmental evidence indicates that they occupied a broad range of habitats including freshwater as well as estuarine and coastal marine environments.
Evolution of Feeding Guilds
Results of this study allow preliminary inferences about the evolution of feeding guilds in marine mammals. Most fossil and extant marine mammals fall into one of four feeding guilds: biting/crushing, suction, filter, and grazing. By far the most common method of feeding for both cetaceans and pinnipedimorphs was a biting/crushing strategy using pointed teeth to consume fish. For pinnipedimorphs, this strategy evolved during the late Oligocene. Most stem taxa lived in coastal marine habitats, which is also the dominant habitat of extant pinnipeds. The distinctive suction feeding habit of the modern walrus feeding on mollusks in a benthic habitat evolved in later diverging walruses beginning in the late Pliocene (3 Ma). Earlier stem walruses were more generalist feeders consuming fish and employing a biting strategy. Extinct desmatophocid pinnipeds exhibited varied diets, with Allodesmus possessing adaptations associated with specialized suction feeding and Desmatophoca spp. identified as more generalized biting/crushing/suction feeder. The filter feeding niche is primarily occupied at present by a single pinniped species, the crabeater seal Lobodon carcinophagus, which employs multi-cusped teeth as a sieve to filter zooplankton from the water.
The biting/crushing feeding guild strategy is exhibited by stem whales (archaeocetes) approximately 50 Ma. Early whales inhabited riverine and estuarine environments and later dispersed to occupy marine coastal and pelagic habitats. One of the more interesting results of the ASR of prey capture in whales is that filter/suction feeding in whales appeared early in the evolution of mysticetes (e.g., Coronodon), at approximately 30 Ma. The shift toward bulk-based filter feeding as the sole feeding strategy began with eomysticetids that employed baleen to filter fish and zooplankton. Odontocetes are characterized by several feeding strategies including biting/crushing/suction, biting/crushing, and specialized suction feeding. Specialized suction feeding evolved multiple times in extinct stem odontocetes (e.g., Inermorostrum), physeteroids, ziphiids, and some delphinoid odontocetes (e.g., Monodon, Delphinapterus).
The specialized crushing/suction feeding niche was occupied by the extinct aquatic carnivore Kolponomos in the Miocene and the extant sea otter Enhydra and its fossil relatives in the Pliocene. Marine herbivores (desmostylians, sirenians, and aquatic sloths) filled the grazing feeding guild with stem sirenians appearing first in the late Eocene (50 Ma). There are strong geographical differences in the occupants of this niche. Aquatic sloths were restricted to the South Pacific while desmostylians occupied the North Pacific. Sirenians were more broadly distributed in the past, especially in the subtropical tropical western Atlantic and Caribbean regions, with the large bodied hydrodamaline lineage occupying temperate to sub-polar waters.
Several previous studies of feeding guild evolution in marine mammals have shown the importance of body size as an important variable (e.g., Churchill et al., 2015; Pyenson and Vermeij, 2016; Slater et al., 2017). The next step involves the incorporation of quantitative data (e.g., cranial morphology) with dietary and feeding mode data as has been done with other marine vertebrates (Friedman, 2012; Motani et al., 2015; Kelley and Motani, 2015; Pyenson and Vermeij, 2016). Only then will it be possible to identify patterns in skull and tooth morphology and better discriminate trophic groups among clades. It is also important to consider these patterns in the context of the geographic and temporal distributions of marine mammal faunas. Mapping these patterns onto phylogenies will enable testing of previous hypotheses of trophic convergence, niche diversity, and replacement.
The Future: Marine Mammals and Ecosystems
By analyzing the past and present feeding diversity of marine mammals, we can make predictions about the future. In addition to changes in global climate and ocean productivity, human interactions since the Late Pleistocene have strongly influenced marine mammals and their ecosystems. Several marine mammals, notably Steller’s sea cow (Hydrodamalis gigas) and the Caribbean monk seal (Neomonachus tropicalis) have been hunted to extinction (Turvey and Risley, 2006; Baisre, 2013). The extinction of other species such as the Yangtze river dolphin, Lipotes vexillifer, has been attributed to habitat degradation (Pyenson, 2009). Historical records document the extensive hunting of manatees, Trichechus spp., by early colonists in the Americas and dugongids by Aboriginal Australians (Estes et al., 2016). Anthropogenic climate change and resultant expansions and contractions of habitat are responsible for distributional shifts as well as changes in feeding ecology. For example, Pyenson and Lindberg (2011) showed that changes in Pleistocene sea level cycles resulted in fluctuations in available benthic feeding areas. These scientists suggested that gray whales survived Pleistocene glacial maxima by feeding outside of the Sea of Okhotsk and Bering region in the North Pole, which were ice covered, employing a more generalized feeding strategy. Given this change in available habitat, gray whales likely fed on fish during the Pleistocene, rather that benthic amphipods consumed by the living gray whale. Changing food web dynamics, such as the historic interactions between killer whales and sea otters or baleen whales (Estes et al., 1998) and Stellers’s sea cow-sea otters and kelp (Estes et al., 2016), would benefit from further exploration in the fossil record.
Another area of investigation with important implications for modern marine ecosystems is the evolution of fossil marine mammal communities, little studied thus far. A notable exception is identification of the co-occurrence of species, or sympatry, among dugongids evolving in separate time periods and geographic regions, the late Oligocene in Florida, the early Miocene in India, and the early Pliocene in Mexico. Resource partitioning among fossil sirenians was examined in these communities defined on the basis of dugongid body size and ecomorphological differences such as rostral deflection and tusk morphology (Vélez-Juarbe et al., 2012).
Also worthy of consideration is the decline of marine mammals that have been attributed to predators, such as the decline of Pliocene mysticetes that has been linked to the appearance and diversification of their likely predators, carcharodontid sharks (Lindberg and Pyenson, 2006).
These examples point up the importance of collecting fossil data as fossils provide critical, unique reference points for understanding changes in marine mammal communities and diversity through time.
CONCLUSIONS
Marine mammals have evolved four feeding strategies to capture prey underwater: biting, filter, suction, and grazing. Distinct skull and soft tissue morphologies have been identified and associated with each feeding strategy, and future studies would benefit from more comparative and quantitative approaches to the study of feeding structures, styles, and performance. By analyzing the evolution of feeding in marine mammals, we confirm that the earliest feeding guild in the late Eocene was occupied by both herbivores (grazing sirenians) and carnivores (biting/crushing archaeocetes). Morphological and paleoecological evidence indicate that a biting feeding mode was predominant among Miocene cetaceans and pinnipedimorphs. Other feeding styles, notably the specialized crushing of hard-shelled invertebrates, had various occupants in the past, including a phocid seal and an aquatic ursid, and this niche has been represented since the Pleistocene by sea otters. The filter feeding niche appears to have originated perhaps as early as the late Eocene although details of the tooth to baleen transition remain contentious as does the broader ecological context. Large marine herbivores (desmostylians and sirenians) were continual occupants of the grazing niche with the addition of aquatic sloths in the South Pacific.
Understanding how drivers of past diversity, both biotic and environmental, continue to operate today helps shape our efforts to conserve and manage these remarkable mammals of the sea. Major changes in productivity of the world’s oceans was a primary driver of feeding diversification in both pinnipedimorphs and cetaceans. The peak in diversity of major lineages of marine mammals occurred during the mid-Miocene and has been associated with the Mid-Miocene Climate Optimum. Later changes in diversity can be related to the Messinian salinity crisis, especially for those taxa occupying the Mediterranean, and to Pleistocene sea level changes that affected habitat and prey availability for coastal dwelling pinnipeds and cetaceans. Ecological replacements are more difficult to recognize in the absence of a good, well-sampled fossil record. Perhaps the best example is replacement of desmostylians by sirenians in the North Pacific that occurred in the late Miocene. Evidence for trophic convergence during this same time comes from sea grass feeding aquatic sloths in the South Pacific that appear to be the ecological homologue of desmostylians in the Pacific. Disentangling the effects of ecosystem changes requires the development of rigorous hypotheses that incorporate biotic as well as environmental drivers of ecosystem change. Additionally, further study of the prey of marine mammals would enable understanding of the co-evolution of predator-prey communities through time.
ACKNOWLEDGMENTS
We greatly appreciate the discussion of data and assistance of E. Ekdale and M. Uhen. Several anonymous reviewers and the editor A. Souron improved the quality of the manuscript. Thanks also to editor A. Bush. This is Paleobiology Database publication 380.
REFERENCES
Adam, P.J. and Berta, A. 2002. Evolution of prey capture strategies and diet in Pinnipedimorpha (Mammalia, Carnivora). Oryctos, 4:83-107.
Amaral, B.K. do, Amaral, A.R., Fordyce, R.E., and Moreno, I.B. 2018. Historical biogeography of Delphininae dolphins and related taxa (Artiodactyla: Delphinidae). Journal of Mammalian Evolution, 25:241-259. https://doi.org/10.1007/s10914-016-9376-3
Amson, E., Argot, C., McDonald, H.G., and de Muizon, C. 2015. Osteology and functional morphology of the forelimb of the marine sloth Thalassocnus (Mammalia, Tardigrada). Journal of Mammalian Evolution, 22: 169-242. https://doi.org/10.1007/s10914-014-9268-3
Amson, E. and de Muizon, C. 2014. A new durophagous phocid (Mammalia: Carnivora) from the late Neogene of Peru and considerations on monachine seals phylogeny. Journal of Systematic Palaeontology, 12:523-548. https://doi.org/10.1080/14772019.2013.799610
Arias-del Raza, A. and Heckel, G. 2016. Terrestrial habitat preferences and segregation of four pinniped species on the islands off the western coast of the Baja California Peninsula, Mexico. Marine Mammal Science, 32(4):1416-1432. https://doi.org/10.1111/mms.12339
Armfield, B.A., George, J.C., Vinyard, C.J., and Thewissen, J.G.M. 2011. Allometric patterns of fetal head growth in mysticetes and odontocetes: comparison of Balaena mysticetus and Stenella attenuata. Marine Mammal Science, 27:819-827. https://doi.org/10.1111/j.1748-7692.2010.00445.x
Armfield, B.A., Zheng, Z., Bajpai, S., Vinyard, C.J., and Thewissen, J.G.M. 2013. Development and evolution of the unique cetacean dentition. PeerJ, 1:e24. https://doi.org/10.7717/peerj.24
Baisre, J.A. 2013. Shifting baselines and the extinction of the Caribbean monk seal. Conservation Biology, 27(5):927-935. https://doi.org/10.1111/cobi.12107
Beatty, B.L. 2004. Evidence for suction feeding in the Desmostylidae (Desmostylia, Mammalia). ICVM‐7 abstracts, Journal of Morphology, 260:276-277. https://doi.org/10.1002/jmor.10223
Beatty, B.L., Vitkovski, T., Lambert, O., and Macrini, T.E. 2012. Osteological associations with unique tooth development in manatees (Trichechidae, Sirenia): a detailed look at modern Trichechus and a review of the fossil record. The Anatomical Record, 295:1504-1512. https://doi.org/10.1002/ar.22525
Bei, M. 2009. Molecular genetics of tooth development. Current Opinion in Genetics & Development, 9:504-510. https://doi.org/10.1016/j.gde.2009.09.002
Benoit, J., Adnet, S., El Mabrouk, E., Khayati, H., Ben Haj Ali, M., Marivaux, L., Merzeraud, G., Merigeaud, S., Vianey-Liaud, M., and Tabuce, R. 2013. Cranial remain from Tunisia provides new clues for the origin and evolution of Sirenia (Mammalia, Afrotheria) in Africa. PLoS ONE, 8(1):e54307. https://doi.org/10.1371/journal.pone.0054307
Berta, A. 2017. The Rise of Marine Mammals: 50 Million Years of Evolution. Johns Hopkins University Press, Baltimore, Maryland. https://doi.org/10.1353/book.56360
Berta, A., Churchill, M., and Boessenecker, R.W. 2018. The origin and evolutionary biology of pinnipeds: seals, sea lions, and walruses. Annual Reviews in Earth and Planetary Sciences, 46:203-228. https://doi.org/10.1146/annurev-earth-082517-010009
Berta, A., Ekdale, E.G., Zellmer, N.T., Deméré, T.A., Kienle, S.S., and Smallcomb, M. 2015. Eye, nose, and throat: external anatomy of the head of a neonate gray whale (Cetacea, Mysticeti, Eschrichtius robustus). The Anatomical Record, 298(4):648-659. https://doi.org/10.1002/ar.23112
Berta, A., Lanzetti, A., Ekdale, E.G., and Deméré, T.A. 2016. From teeth to baleen and raptorial to bulk filter feeding in Mysticete Cetaceans: The role of paleontological, genetic, and geochemical data in feeding evolution and ecology. Integrative and Comparative Biology, 56:1271-1284. https://doi.org/10.1093/icb/icw128
Bianucci, G. 2005. Arimidelphis sorbinii a new small killer whale-like dolphin from the Pliocene of Marecchia river (Central eastern Italy) and a phylogenetic analysis of the Orcininae (Cetacea: Odontoceti). Rivista Italiana di Paleontologia e Stratigrafia, 111:329-344. https://doi.org/10.13130/2039-4942/6324
Bianucci, G. 2013. Septidelphis morii, n. gen. et sp., from the Pliocene of Italy: new evidence of the explosive radiation of true dolphins (Odontoceti, Delphinidae). Journal of Vertebrate Paleontology, 33(3):722-740. https://doi.org/10.1080/02724634.2013.744757
Bianucci, G., Di Celma, C., Urbina, M., and Lambert, O. 2016. New beaked whales from the late Miocene of Peru and evidence for convergent evolution in stem and crown Ziphiidae (Cetacea, Odontoceti). PeerJ, 4:e2479. https://doi.org/10.7717/peerj.2479
Bloodworth, B.E. and Marshall, C.D. 2007. A functional comparison of the hyolingual complex in pygmy and dwarf sperm whales (Kogia breviceps and K. sima), and bottlenose dolphins (Tursiops truncatus). Journal of Anatomy, 211:78-91. https://doi.org/10.1111/j.1469-7580.2007.00755.x
Boessenecker, R.W. 2016. A middle Pleistocene sea otter from northern California and the antiquity of Enhydra in the Pacific Basin. Journal of Mammalian Evolution, 25:27-35. https://doi.org/10.1007/s10914-016-9373-6
Boessenecker, R.W. and Churchill, M. 2013. A reevaluation of the morphology, paleoecology, and phylogenetic relationships of the enigmatic walrus Pelagiarctos. PLoS ONE, 8(1):e54311. https://doi.org/10.1371/journal.pone.0054311
Boessenecker, R.W. and Churchill, M. 2018. The last of the desmatophocid seals: a new species of Allodesmus from the upper Miocene of Washington, USA, and a revision of the taxonomy of Desmatophocidae. Zoological Journal of the Linnean Society, 184:211-235. https://doi.org/10.1093/zoolinnean/zlx098
Boessenecker, R.W., Fraser, D., Churchill, M., and Geisler, J.H., 2017. A toothless dwarf dolphin (Odontoceti: Xenorophiidae) points to explosive feeding diversification of modern whales (Neoceti). Proceedings of the Royal Society B, 284(1861):20170531. https://doi.org/10.1098/rspb.2017.0531
Boessenecker, R.W. and Perry, F.A. 2011. Mammalian bite marks on juvenile fur seal bones from the late Neogene Purisima Formation of Central California. Palaios, 26:115-120. https://doi.org/10.2110/palo.2010.p10-088r
Brunner, S., Bryden, M., and Shaughnessy, P.D. 2004. Cranial ontogeny of otariid seals. Systematic Biodiversity, 2(1):83-110. https://doi.org/10.1017/s1477200004001367
Byrne, M. 2011. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean, p. 1-42. In Gibson, R., Atkinson, R., Gordon, J., Smith, I., and Hughes, D. (eds.), Oceanography and Marine Biology: An Annual Review, 49. Taylor & Francis, Milton Park, Abingdon, United Kindgom.
Cassens, I., Vicario, S., Waddell, V., Balchowsky, H., Van Belle, D., Ding, W., Fan, C., Lal Mohan, R.S., Simões-Lopes, P.C., Bastida, R., Meyer, A., Stanhope, M., and Milinkovitch, M., 2000. Independent adaptation to riverine habitats allowed survival of ancient cetacean lineages. Proceedings of the National Academy of Sciences, 97:11343-11347. https://doi.org/10.1073/pnas.97.21.11343
Chiba, K.A., Fiorillo, A.R., Jacobs, L.L., Kimura. Y., Kobayashi, Y., Kohno, N., Nishida, Y., Polcyn, M.J., and Tanaka, K. 2016. A new desmostylian mammal from Unalaska (USA) and the robust Sanjussen jaw from Hokkaido (Japan), with comments on feeding in derived desmostylids. Historical Biology, 28(1-2):289-303. https://doi.org/10.1080/08912963.2015.1046718
Churchill, M. and Clementz, M.T. 2015. Functional implications of variation in tooth spacing and crown size in Pinnipedimorpha (Mammalia: Carnivora). The Anatomical Record, 298:878-902. https://doi.org/10.1002/ar.23082
Churchill, M. and Clementz, M.T., 2016. The evolution of aquatic feeding in seals: insights from Enaliarctos (Carnivora: Pinnipedimorpha), the oldest known seal. Journal of Evolutionary Biology, 29(2):319-334. https://doi.org/10.1111/jeb.12783
Churchill, M., Boessenecker, R.W., and Clementz, M.T. 2014. Colonization of the Southern Hemisphere by fur seals and sea lions (Carnivora: Otariidae) revealed by combined evidence phylogenetic and Bayesian biogeographical analysis. Zoological Journal of the Linnean Society, 172:200-225. https://doi.org/10.1111/zoj12163
Churchill, M., Clementz, M.T., and Kohno, N. 2015. Cope’s rule and the evolution of body size in Pinnipedimorpha (Mammalia: Carnivora). Evolution, 69:201-215. https://doi.org/10.1111/evo.12560
Clementz, M.T., Fordyce, E., Peek, S.L., and Fox, D.L. 2014. Ancient marine isoscapes and isotopic evidence of bulk-feeding by Oligocene cetaceans. Palaeogeography, Palaeoclimatology, Palaeoecology, 400:28-40. https://doi.org/10.1016/j.palaeo.2012.09.009
Clementz, M.T., Goswami, A., Gingerich, P.D., and Koch, P.L. 2006. Isotopic records from early whales and sea cows: contrasting patterns of ecological transition. Journal of Vertebrate Paleontology, 26(2):355-370. https://doi.org/10.1671/0272-4634(2006)26[355:irfewa]2.0.co;2
Clementz, M.T., Hoppe, K.A., and Koch, P.L. 2003. A paleoecological paradox: the habitat and dietary preferences of the extinct tethythere Desmostylus, inferred from stable isotope analysis. Paleobiology, 29(4):506-519. https://doi.org/10.1666/0094-8373(2003)029%3C0506:apptha%3E2.0.co;2
Collareta, A., Landini, W., Lambert, O., Post, K., Tinelli, C., Di Celma, C., Panetta, D., Tripodi, M., Salvadori, P., Caramella, D., Marchi, D., Urbina, M., and Bianucci, G. 2015. Piscivory in a Miocene Cetotheriidae of Peru: first-record of fossilized stomach content for an extinct baleen-bearing whale. The Science of Nature, 102:70. https://doi.org/10.1007/s00114-015-1319-y
Committee on Taxonomy. 2017. List of marine mammal species and subspecies consulted on March 30, 2018. Society for Marine Mammalogy. https://marinemammalscience.org
Dalebout, M.L., Steel, D., and Baker, C.S. 2008. Phylogeny of the beaked whale genus Mesoplodon (Ziphiidae: Cetacea) revealed by nuclear introns: implications for the evolution of male tusks. Systematic Biology, 57:857-875. https://doi.org/10.1080/10635150802559257
Deméré, T.A. and Berta, A. 2008. Skull anatomy of the Oligocene toothed mysticete Aetiocetus weltoni (Mammalia: Cetacea): implications for mysticete evolution and functional anatomy. Zoological Journal of the Linnean Society, 154:308-352. https://doi.org/10.1111/j.1096-3642.2008.00414.x
Deméré, T.A., Berta, A., and Adam, P. 2003. Pinnipedimorph evolutionary biogeography. Bulletin of the American Museum of Natural History, 279:32-76. https://doi.org/10.1206/0003-0090(2003)279%3C0032:c%3E2.0.co;2
Deméré, T.A., McGowen, M.R., Berta, A., and Gatesy, J., 2008. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Systematic Biology, 57(1):15-37. https://doi.org/10.1080/10635150701884632
de Muizon, C. and Domning, D.P. 2002. The anatomy of Odobenocetops (Delphinoidea, Mammalia), the walrus-like dolphin from the Pliocene of Peru and its palaeobiological implications. Zoological Journal of the Linnean Society, 134:423-452. https://doi.org/10.1046/j.1096-3642.2002.00015.x
de Muizon, C., McDonald, H.G., Salas, R., and Urbina, M. 2004.The evolution of feeding adaptations of the aquatic sloth Thalassocnus. Journal of Vertebrate Paleontology, 24:398-410. https://doi.org/10.1671/2429b
Dewaele, L., Amson, E., Lambert, O., and Louwye, S. 2017. Reappraisal of the extinct seal “Phoca” vitulinoides from the Neogene of the North Sea Basin, with bearing on its geological age, phylogenetic affinities, and locomotion. PeerJ, 5:e3316. https://doi.org/10.7717/peerj.3316
Domning, D.P. 1982. The evolution of manatees: a speculative history. Journal of Paleontology, 56:599-619. https://jstor.org/stable/1304394
Domning, D.P. 2001. Sirenians, seagrasses, and Cenozoic ecological change in the Caribbean. Palaeogeography, Palaeoclimatology, Palaeoecology, 166:27-50. https://doi.org/10.1016/s0031-0182(00)00200-5
Domning, D.P. and Beatty, B.L. 2007. Use of tusks in feeding by dugongid sirenians: observations and tests of hypotheses. The Anatomical Record, 290:523-538. https://doi.org/10.1002/ar.20540
Domning, D.P. and Hayek, L.-A.C. 1984. Horizontal tooth replacement in the Amazonian manatee (Trichechus inunguis). Mammalia, 48(1):105-127. https://doi.org/10.1515/mamm.1984.48.1.105
Ekdale, E.G., Deméré, T.A., and Berta, A. 2015. Vascularization of the gray whale palate (Cetacea, Mysticeti, Eschrichtius robustus): soft tissue evidence for an alveolar source of blood to baleen. The Anatomical Record, 298:691-702. https://doi.org/10.1002/ar.23119
El Adli, J.J., Deméré, T.A., and Boessenecker, R.W. 2014. Herpetocetus morrowi (Cetacea: Mysticeti), a new species of diminutive baleen whale from the upper Pliocene (Piacenzian) of California, USA, with observations on the evolution and relationships of the Cetotheriidae. Zoological Journal of the Linnean Society, 170:400-466. https://doi.org/10.1111/zoj.12108
Estes, J.A., Burdin, A., and Doak, D.F. 2016. Sea otters, kelp forests, and the extinction of Steller’s sea cow. Proceedings of the National Academy of Sciences, 113(4):880-885. https://doi.org/10.1073/pnas.1502552112
Estes, J.A., Tinker, M.T., Williams, T.M., and Doak, D.F. 1998. Killer whale predation on sea otters linking coastal with oceanic ecosystems. Science, 282:473-476. https://doi.org/10.1126/science.282.5388.473
Fahlke, J.M. 2012. Bite marks revisited-evidence for middle-to-late Eocene Basilosaurus isis predation on Dorudon atrox (both Cetacea, Basilosauridae). Palaeontologia Electronica, 15.3.32A:1-16. https://doi.org/10.26879/341
Fahlke, J.M., Bastl, K.A., Semprebon, G.M., and Gingerich, P.D. 2013. Paleoecology of archaeocete whales throughout the Eocene: dietary adaptations revealed by microwear analysis. Palaeogeography, Palaeoclimatology, Palaeoecology, 386:690-701. https://doi.org/10.1016/j.palaeo.2013.06.032
Fordyce, R.E. 1980. Whale evolution and Oligocene Southern Ocean environments. Palaeogeography, Palaeoclimatology, Palaeoecology, 31:319-336. https://doi.org/10.1016/0031-0182(80)90024-3
Fordyce, R.E. and Marx, F.G. 2018. Gigantism precedes filter feeding in baleen whale evolution. Current Biology, 28:1670-1676. https://doi.org/10.1016/j.cub.2018.04.027
Fordyce, R.E. and Muizon, C. de 2001. Evolutionary history of cetaceans: a review, p. 169-233. In Mazin, J.M. and de Buffrenil, V. (eds.), Secondary Adaptation of Tetrapods to Life in Water. Verlag Dr Friedrich Pfeil, Munchen, Germany.
Fréon, P., Arístegui, J., Bertrand, A., Crawford, R.J., Field, J.C., Gibbons, M.J., and Ramdani, M. 2009. Functional group biodiversity in eastern boundary upwelling ecosystems questions the wasp-waist trophic structure. Progress in Oceanography, 83(1-4):97-106. https://doi.org/10.1016/j.pocean.2009.07.034
Friedman, M. 2012. Parallel evolutionary trajectories underlie the origin of giant suspension-feeding whales and bony fishes. Proceedings of the Royal Society B, 279:944-951. https://doi.org/10.1098/rspb.2011.1381
Fudge, D.S., Szewciw, L.J., and Schwalb, A.N. 2009. Morphology and development of blue whale baleen: an annotated translation of Tycho Tullberg’s classic 1883 paper. Aquatic Mammals, 35:226-252. https://doi.org/10.1578/am.35.2.2009.226
Gatesy, J., Geisler, J.H., Chang, J., Buell, C., Berta, A., Meredith, R.W., Springer, M.S., and McGowen, M.R. 2013. A phylogenetic blueprint for a modern whale. Molecular Phylogenetics and Evolution, 66:479-506. https://doi.org/10.1016/j.ympev.2012.10.012
Geisler, J.H., Boessenecker, R.W., Brown, M., and Beatty, B.L. 2017. The origin of filter feeding in whales. Current Biology, 27:2036-2042. https://doi.org/10.1016/j.cub.2017.06.003
Geisler, J.H., McGowen, M.R., Yang, G., and Gatesy, J. 2011. A supermatrix analysis of genomic, morphological and paleontological data from crown Cetacea. BMC Evolutionary Biology, 11:112. https://doi.org/10.1186/1471-2148-11-112
Goldbogen, J.A., Calambokidis, J., Oleson, E., Potvin, J., Pyenson, N.D., Schorr, G., and Shadwick, R.E. 2011. Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density. Journal of Experimental Biology, 214:131-146. https://doi.org/10.1242/jeb.054726
Goldbogen, J.A., Potvin, J., and Shadwick, R.E. 2010. Skull and buccal cavity allometry increase mass-specific engulfment capacity in fin whales. Proceedings of the Royal Society B, 277:861-868. https://doi.org/10.1098/rspb.2009.1680
Gol’din, P. and Startsev, D. 2014. A systematic review of cetothere baleen whales (Cetacea, Cetotheriidae) from the late Miocene of Crimea and Caucasus, with a new genus. Papers in Palaeontology, 3:49-68. https://doi.org/10.1002/spp2.1066
Hassold, N.J.C., Rea, D.K., van der Pluijm, B.A., and Parés, J.M. 2009. A physical record of the Antarctic Circumpolar Current: late Miocene to recent slowing of abyssal circulation. Palaeogeography, Palaeoclimatology, Palaeoecology, 275:28-36. https://doi.org/10.1016/j.palaeo.2009.01.011
Heyning, J.E. and Mead, J.G. 1996. Suction feeding in beaked whales: morphological and observational evidence. Contributions to Science, Natural History Museum of Los Angeles County, 464:1-12.
Hirons, A.C., Schell, D.M., and Finney, B.P. 2001. Temporal records of δ13C and δ15N in North Pacific pinnipeds: inferences regarding environmental change and diet. Oecologia, 129(4):591- 601. https://doi.org/10.1007/s004420100756
Hocking, D.P., Evans, A.R., and Fitzgerald, E.M.G. 2013. Leopard seals (Hydrurga leptonyx) use suction and filter feeding when hunting small prey underwater. Polar Biology, 36:211-222. https://doi.org/10.1007/s00300-012-1253-9
Hocking, D.P., Marx, F.G., Park, T., Fitzgerald, E.M.G., and Evans, A.R. 2017a. A behavioural framework for the evolution of feeding in predatory aquatic mammals. Proceedings of the Royal Society B, 284:20162750. https://doi.org/10.1098/rspb.2016.2750
Hocking, D.P., Marx, F.G., Park, T., Fitzgerald, E.M.G., and Evans, A.R. 2017b. Reply to comment by Kienle et al. 2017. Proceedings of the Royal Society B, 284:20171836. https://doi.org/10.1098/rspb.2017.1836
Ingólfsson, I.Ó. and Wiig, Ø. 2008. Late Pleistocene find in Svalbard: the oldest remains of a polar bear (Ursus maritimus Phipps, 1744) ever discovered. Polar Research, 28:455-462. https://doi.org/10.1111/j.1751-8369.2008.00087.x
Jefferson, T.A., Webber, M.A., and Pittman, R.L. 2015. Marine Mammals of the World: a Comprehensive Guide to Their Identification, 2nd Edition. Academic Press, London, United Kingdom.
Jensen, M.M., Saladrigas, A.H., and Goldbogen, J.A. 2017. Comparative three-dimensional morphology of baleen: cross-sectional profiles and volume measurements. The Anatomical Record, 300:1942-1952. https://doi.org/10.1002/ar.23648
Jernvall, J. and Thesleff, I. 2012. Tooth shape formation and tooth renewal: evolving with the same signals. Development, 139:3487-3497. https://doi.org/10.1242/dev.085084
Johnston, C.J. and Berta, A. 2011. Comparative anatomy and evolutionary history of suction feeding in cetaceans. Marine Mammal Science, 27:493-513. https://doi.org/10.1111/j.1748-7692.2010.00420.x
Johnston, C.J., Deméré, T.A., Berta, A., Yonas, J., and St. Leger, J. 2010. Observations on the musculoskeletal anatomy of the head of a neonate gray whale (Eschrichtius robustus). Marine Mammal Science, 26:186-194. https://doi.org/10.1111/j.1748-7692.2009.00305.x
Jones, K.E. and Goswami, A. 2010. Quantitative analysis of the influences of phylogeny and ecology on phocid and otariid pinniped (Mammalia; Carnivora) cranial morphology. Journal of Zoology, 280:297-308. https://doi.org/10.1111/j.1469-7998.2009.00662.x
Jones, K.E., Ruff, C.R., and Goswami, A. 2013. Morphology and biomechanics of the pinniped jaw: mandibular evolution without mastication. The Anatomical Record, 296:1049-1063. https://doi.org/10.1002/ar.22710
Kane, E.A. and Marshall, C.D. 2009. Comparative feeding kinematics and performance of odontocetes: belugas, Pacific white-sided dolphins and long-finned pilot whales. Journal of Experimental Biology, 212:3939-3950. https://doi.org/10.1242/jeb.034686
Karlsen, K. 1962. Development of tooth germs and adjacent structures in the whalebone whale ((Balaenoptera physalus) L.): with a contribution to the theories of the mammalian dentition. Hvalrådets Skrifter, 45:5-56.
Kastelein, R.A., Dubbldam, J.L., and De Bakker, M.A.G. 1997. The anatomy of the walrus head (Odobenus rosmarus). Part 5: The tongue and its function in walrus ecology. Aquatic Mammals, 23:29-47.
Kastelein, R.A. and Gerrits, N.M. 1990. The anatomy of the walrus head (Odobenus rosmarus). Part 1: The skull. Aquatic Mammals, 16:101-119.
Kastelein, R.A., Gerrits, N.M., and Dubbeldam, J.L. 1991. The anatomy of the walrus head (Odobenus rosmarus). Part 2: Description of the muscles and of their role in feeding and haul-out behavior. Aquatic Mammals, 17:156-180.
Kelley, N.P. and Motani, R. 2015. Trophic convergence drives morphological convergence in marine tetrapods. Biology Letters, 11:20140709. https://doi.org/10.1098/rsbl.2014.0709
Kelley, N.P. and Pyenson, N.D. 2015. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science, 348:aaa3716. https://doi.org/10.1126/science.aaa3716
Kienle, S.S. and Berta, A. 2016. The better to eat you with: the comparative feeding morphology of phocid seals (Pinnipedia, Phocidae). Journal of Anatomy, 228:396-413. https://doi.org/10.1111/joa.12410
Kienle, S.S. and Berta, A. 2019. The evolution of feeding strategies in phocid seals (Pinnipedia, Phocidae). Journal of Vertebrate Paleontology, 38:e1559172. https://doi.org/10.1080/02724634.2018.1559172
Kienle, S.S., Ekdale, E.G., Reidenberg, J.S., and Deméré T.A. 2015. Tongue and hyoid musculature and functional morphology of a neonate gray whale (Cetacea, Mysticeti, Eschrichtius robustus). The Anatomical Record, 298:660-674. https://doi.org/10.1002/ar.23107
Kienle, S.S., Law, C.J., Costa, D.P., Berta, A., and Mehta, R.S. 2017. Revisiting the behavioural framework of feeding in predatory aquatic mammals. Proceedings of the Royal Society B, 284:20171035. https://doi.org/10.1098/rspb.2017.1035
Klima, M. 1995. Cetacean phylogeny and systematics based on the morphogenesis of the nasal skull. Aquatic Mammals, 21:79-89.
Krijgsman, W., Hilgen, F.J., Raffi, I., Sierro, F.J., and Wilson, D.S. 1999. Chronology, causes and progression of the Messinian salinity crisis. Nature, 400:652-655. https://doi.org/10.1038/23231
Lambert, O., Bianucci, G., and Muizon, C. de. 2016. Macroraptorial sperm whales (Cetacea, Odontoceti, Physeteroidea) from the Miocene of Peru. Zoological Journal of the Linnean Society, 179(2):404-474. https://doi.org/10.1111/zoj.12456
Lambert, O., Collareta, A., Landini, W., Post, K., Ramassamy, B., Di Celma, C., Urbina, M., and Bianucci, G. 2015. No deep diving: evidence of predation on epipelagic fish for a stem beaked whale from the Late Miocene of Peru. Proceedings of the Royal Society B, 282:20151530. https://doi.org/10.1098/rspb.2015.1530
Lambertsen, R.H. 1983. Internal mechanism of rorqual feeding. Journal of Mammalogy, 64:76- 88. https://doi.org/10.2307/1380752
Lambertsen, R.H., Rasmussen, K.J., Lancaster, W.C., and Hintz, R.J. 2005. Functional morphology of the mouth of the bowhead whale and its implications for conservation. Journal of Mammalogy, 86:342-352. https://doi.org/10.1644/ber-123.1
Lambertsen, R.H., Ulrich, N., and Straley, J. 1995. Frontomandibular stay of Balaenopteridae: a mechanism for momentum recapture during feeding. Journal of Mammalogy, 76:877-899. https://doi.org/10.2307/1382758
Lan, Y., Jia, S., and Jiang, R. 2014. Molecular patterning of the mammalian dentition. Seminars in Cell & Developmental Biology, 25-26:61-70. https://doi.org/10.1016/j.semcdb.2013.12.003
Lanzetti, A., Berta, A., and Ekdale, E.G. 2020. Prenatal development of the humpback whale: growth rate, tooth loss and skull shape changes in an evolutionary framework. The Anatomical Record, 303:180-204. https://doi.org/10.1002/ar.23990
Lawlor, T. 1979. Handbook to the Order and Families of Living Mammals. Mad River Press, Eureka, California.
Lindberg, D.R. and Pyenson, N.P. 2006. Evolutionary patterns in Cetacea: fishing up prey sizes through deep time, p. 68-82. In Estes, J.A., DeMaster, D.P., Doak, D.F., Williams, T.M., and Brownell, R.L. Jr. (eds.), Whales, Whaling and Ocean Ecosystems. University of California Press, Berkeley, California. https://doi.org/10.1525/california/9780520248847.003.0007
Lindqvist, C., Schuster, S.C., Sun, Y., Talbot, S.L., Qi, J., Ratan, A., Tomsco, L.P., Kasson, L., Zeyl, E., Aars, J., Miller, W., Ingólfsson, Ó., Bachmann, L., and Wiig, Ø., 2010. Complete mitochondrial genome of a Pleistocene jawbone unveils the origin of polar bear. Proceedings of the National Academy of Sciences, 107:5053-5057. https://doi.org/10.1073/pnas.0914266107
Liu, S., Lorenzen, E.D., Fumagalli, M., Li, B., Harris, K., Xiong, Z., Zhou, L., Korneliussen, T.S., Somel, M., Babbitt, C., Wray, G., Li, J., He, W., Wang, Z., Fu, W., Xiang, X., Morgan, C.C., Doherty, A., O’Connell, M.J., McInerney, J.O., Born, E.W., Dalén, L., Dietz, R., Orlando, L., Sonne, C., Zhang, G., Nielsen, R., Willerslev, E., and Wang, J. 2014. Population genomics reveal recent speciation and rapid evolutionary adaptation in polar bears. Cell, 157:785-794. https://doi.org/10.1016/j.cell.2014.03.054
Loch, C., Boessenecker, R.W., Churchill, M., and Kieser, J. 2016. Enamel ultrastructure of fossil and modern pinnipeds: evaluating hypotheses of feeding adaptations in the extinct walrus Pelagiarctos. The Science of Nature, 103:44. https://doi.org/10.1007/s00114-016-1366-z
Maddison, W.P. and Maddison, D.R. 2018. Mesquite: a modular system for evolutionary analysis. Version 3.5. http://mesquiteproject.org
Magallanes, I., Parham, J.F., Santos, G.-P., and Vélez-Juarbe, J. 2018. A new tuskless walrus from the Miocene of Orange County, California, with comments on the diversity and taxonomy of odobenids. PeerJ, 6:e5708. https://doi.org/10.7717/peerj.5708
Mannocci, L., Monestiez, P., Spitz, J., and Ridoux, V. 2015. Extrapolating cetacean densities beyond surveyed regions: habitat-based predictions in the circumtropical belt. Journal of Biogeography, 42:1267-1280. https://doi.org/10.1111/jbi.12530
Marshall, C.D., Kovacs, K.M., and Lydersen, C. 2008. Feeding kinematics, suction and hydraulic jetting capabilities in bearded seals (Erignathus barbatus). Journal of Experimental Biology, 211:699-708. https://doi.org/10.1242/jeb.009852
Marx, F.G. 2009. Marine mammals through time: when less is more in studying palaeodiversity. Proceedings of the Royal Society B, 276:887-892. https://doi.org/10.1098/rspb.2008.1473
Marx, F.G. and Fordyce, R.E. 2015. Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity. Royal Society Open Science, 2:140434. https://doi.org/10.1098/rsos.140434
Marx, F.G. and Uhen, M.D. 2010. Climate, critters, and cetaceans: Cenozoic drivers of the evolution of modern whales. Science, 327:993-996. https://doi.org/10.1126/science.1185581
Marx, F.G., Hocking, D.P., Park, T., Ziegler, T., Evans, A.R., and Fitzgerald, E.M.G. 2016. Suction feeding preceded filtering in baleen whale evolution. Memoirs of Museum Victoria, 75:71-82. https://doi.org/10.24199/j.mmv.2016.75.04
Matsui, K. and Tsuihiji, T. 2019. The phylogeny of desmostylians revisited: proposal of new clades based on robust phylogenetic hypotheses. PeerJ, 7:e7430. https://doi.org/10.7717/peerj.7430
McCurry, M.R., Fitzgerald, E.M.G., Evans, A.R., Adams, J.W., and McHenry, C.R. 2017. Skull shape reflects prey size niche in toothed whales. Biological Journal of the Linnean Society, 121:936-946. https://doi.org/10.1093/biolinnean/blx032
McGowen, M.R., Spaulding, M., and Gatesy, J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Molecular Phylogenetics and Evolution, 53:891-906. https://doi.org/10.1016/j.ympev.2009.08.018
Mead, J.I., Spiess, A.E., and Sobolik, K.D. 2000. Skeleton of extinct North American sea mink. Quaternary Research, 53:247-262. https://doi.org/10.1006/qres.1999.2109
Meredith, R.W., Gatesy, J., Cheng, J., and Springer, M.S. 2011. Pseudogenization of the tooth gene enamelysin (MMP20) in the common ancestor of extant baleen whales. Proceedings of the Royal Society B, 278:993-1002. https://doi.org/10.1098/rspb.2010.1280
Meredith, R.W., Gatesy, J., Murphy, W.J., Ryder, O.A., and Springer, M.S. 2009. Molecular decay of the tooth gene enamelin (ENAM) mirrors the loss of enamel in the fossil record of placental mammals. PLoS Genetics, 5:e1000634. https://doi.org/10.1371/journal.pgen.1000634
Meredith, R.W., Gatesy, J., and Springer, M.S. 2013. Molecular decay of enamel matrix protein genes in turtles and other edentulous amniotes. BMC Evolutionary Biology, 13:20. https://doi.org/10.1186/1471-2148-13-20
Miller, K.G., Kominz, M.A., Browning, J.V., Wright, J.D., Mountain, G.S., Katz, M.E., Sugarman, P.J., Cramer, B.S., Christie-Blick, N., and Pekar, S.F. 2005. The Phanerozoic record of global sea-level change. Science, 310(5752):1293-1298. https://doi.org/10.1126/science.1116412
Motani, R., Chen, X.-H., Jiang, D.-Y., Cheng, L., Tintori, A., and Rieppel, O. 2015. Lunge feeding in early marine reptiles and fast evolution of marine tetrapod feeding guilds. Scientific Reports, 5:8900. https://doi.org/10.1038/srep08900
Paterson, R.S., Rybczynski, N., Kohno, N., and Maddin, H. C. 2020. A total evidence phylogenetic analysis of pinniped phylogeny and the possibility of parallel evolution within a monophyletic framework. Frontiers in Ecology and Evolution, 7:457. https://doi.org/10.3389/fevo.2019.00457
Pauly, D., Trites, A.W., Capuli, E., and Christensen, V. 1998. Diet composition and trophic levels of marine mammals. ICES Journal of Marine Science, 55:467-481. https://doi.org/10.1006/jmsc.1997.0280
Peredo, C.M. and Pyenson, N.D. 2018. Salishicetus meadi, a new aetiocetid from the late Oligocene of Washington State and implications for feeding transitions in early mysticete evolution. Royal Society Open Science, 5:172336. https://doi.org/10.1098/rsos.172336
Peredo, C.M., Pyenson, N.D., Marshall, C.D., and Uhen, M.D. 2018. Tooth loss precedes the origin of baleen in whales. Current Biology, 28:3992-4000. https://doi.org/10.1016/j.cub.2018.10.047
Peredo, C.M., Pyenson, N.D., Uhen, M.D., and Marshall, C.D. 2017. Alveoli, teeth, and tooth loss: understanding the homology of internal mandibular structures in mysticete cetaceans. PLoS ONE, 12(5):e0178243. https://doi.org/10.1371/journal.pone.0178243
Peredo, C.M. and Uhen, M.D. 2016. Exploration of marine mammal paleogeography in the Northern Hemisphere over the Cenozoic using beta diversity. Palaeogeography, Palaeoclimatology, Palaeoecology, 449:227-235. https://doi.org/10.1016/j.palaeo.2016.02.034
Pimiento, C., Griffin, J.N., Clements, C.F., Silvestro, D., Varela, S., Uhen, M.D., and Jaramilllo, C. 2017. The Pliocene marine megafauna extinction and its impact on functional diversity. Nature, Ecology & Evolution, 1:1100-1106. https://doi.org/10.1038/s41559-017-0223-6
Prokoph, A., Shields, G.A., and Veizer, J. 2008. Compilation and time-series analysis of a marine carbonate δ18O, δ13C, 87Sr/86Sr and δ34S database through Earth history. Earth-Science Reviews, 87:113-133. https://doi.org/10.1016/j.earscirev.2007.12.003
Pyenson, N.D. 2009. Requiem for Lipotes: an evolutionary perspective on marine mammal extinction. Marine Mammal Science, 25:714-724. https://doi.org/10.1111/j.1748-7692.2008.00266.x
Pyenson, N.D. 2017. The ecological rise of whales chronicled by the fossil record. Current Biology, 27:R558-R564. https://doi.org/10.1016/j.cub.2017.05.001
Pyenson, N.D., Goldbogen, J.A., Vogl, A.W., Szathmary, G., Drake, R.L., and Shadwick, R.E. 2012. Discovery of a sensory organ that coordinates lunge feeding in rorqual whales. Nature, 485:498-501. https://doi.org/10.1038/nature11135
Pyenson, N.D. and Lindberg, D.R. 2011. What happened to gray whales during the Pleistocene? The ecological impact of sea-level change on benthic feeding areas in the North Pacific Ocean. PLoS ONE, 6(7):e21295. https://doi.org/10.1371/journal.pone.0021295
Pyenson, N.D. and Vermeij, G. 2016. The rise of ocean giants: maximum body size in Cenozoic marine mammals as an indicator for productivity in the Pacific and Atlantic Oceans. Biology Letters, 12:20160186. https://doi.org/10.1098/rsbl.2016.0186
Ramassamy, B., Lambert, O., Collareta, A., Urbina, M., and Bianucci, G. 2018. Description of the skeleton of the fossil beaked whale Messapicetus gregarius: searching potential proxies for deep-diving abilities. Fossil Record, 21:11-32. https://doi.org/10.5194/fr-21-11-2018
Randau, M., Sanfelice, D., and Goswami, A. 2019. Shifts in cranial integration associated with ecological specialization in pinnipeds (Mammalia, Carnivora). Royal Society Open Science, 6:190201. https://doi.org/10.1098/rsos.190201
Reidenberg, J.S. and Laitman, J.T. 1994. Anatomy of the hyoid apparatus in Odontoceti (toothed whales): specializations of their skeleton and musculature compared with those of terrestrial mammals. The Anatomical Record, 240:598-624. https://doi.org/10.1002/ar.1092400417
Ridewood, W.G. 1923. Observations on the skull in foetal specimens of whales of the genera Megaptera and Balaenoptera. Philosophical Transactions of the Royal Society of London B Biological Sciences, 211:209-272. https://doi.org/10.1098/rstb.1923.0005
Roe, L.J., Thewissen, J.G.M., Quade, J.R., O’Neil, S., Bajpai, S., Sahni, A., and Hussain, S.T. 1998. Isotopic approaches to understanding the terrestrial-to-marine transition of the earliest cetaceans, p. 399-422. In Thewissen, J.G.M (ed.), The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. Plenum Press, New York, New York. https://doi.org/10.1007/978-1-4899-0159-0_14
Rybczynski, N., Dawson, M.R., and Tedford, R.H. 2009. A semi-aquatic Arctic mammalian carnivore from the Miocene epoch and origin of Pinnipedia. Nature, 458:1021-1024. https://doi.org/10.1038/nature07985
Slater, G.J., Goldbogen, J.A., and Pyenson, N.D. 2017. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proceedings of the Royal Society B, 284:20170546. https://doi.org/10.1098/rspb.2017.0546
Springer, M.S., Starrett, J., Morin, P.A., Lanzetti, A., Hayashi, C., and Gatesy, J. 2016. Inactivation of C4orf26 in toothless placental mammals. Molecular Phylogenetics and Evolution, 95:34-45. https://doi.org/10.1016/j.ympev.2015.11.002
Steeman, M.E., Hebsgaard, M.B., Fordyce, R.E., Ho, S.Y.W., Rabosky, D.L., Nielsen, R., Rahbek, C., Glenner, H., Sørensen, M.V., and Willerslev, E. 2009. Radiation of extant cetaceans driven by restructuring of the oceans. Systematic Biology, 58:573-585. https://doi.org/10.1093/sysbio/syp060
Tarnawski, B.A., Cassini, G.H., and Flores, D.A. 2013. Allometry of the postnatal cranial ontogeny and sexual dimorphism in Otaria byronia (Otariidae). Acta Theriologica, 59:81-97. https://doi.org/10.1007/s13364-012-0124-7
Thewissen, J.G.M., Hieronymus, T.L., George, J.C., Suydam, R., Stimmelmayr, R., and McBurney, D. 2017. Evolutionary aspects of the development of teeth and baleen in the bowhead whale. Journal of Anatomy, 230:549-566. https://doi.org/10.1111/joa.12579
Thewissen, J.G.M., Sensor, J.D., Clementz, M.T., and Bajpai, S. 2011. Evolution of dental wear and diet during the origin of whales. Paleobiology, 37(4):655-669. https://doi.org/10.1666/10038.1
Tsai, C.-H. and Fordyce, R.E. 2018. A new archaic baleen whale Toipahautea waitaki (early Late Oligocene, New Zealand) and the origins of crown Mysticeti. Royal Society Open Science, 5:172453. https://doi.org/10.1098/rsos.172453
Tseng, Z.J., Grohé, C., and Flynn, J.J. 2016. A unique feeding strategy of the extinct marine mammal Kolponomos: convergence on sabretooths and sea otters. Proceedings of the Royal Society B, 283:20160044. https://doi.org/10.1098/rspb.2016.0044
Tseng, Z.J., Pacheco-Castro, A., Carranza-Castañeda, O., Aranda-Gómez, J.J., Wang, X., and Troncoso, H. 2017. Discovery of the fossil otter Enhydritherium terraenovae (Carnivora, Mammalia) in Mexico reconciles a palaeozoogeographic mystery. Biology Letters, 13:20170259. https://doi.org/10.1098/rsbl.2017.0259
Turvey, S.T. and Risley, C.L. 2006. Modelling the extinction of Steller’s sea cow. Biology Letters, 2:94-97. https://doi.org/10.1098/rsbl.2005.0415
Uhen, M.D. 2004. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): an archaeocete from the Middle to Late Eocene of Egypt. University of Michigan Papers in Paleontology, 34:1-222.
Uhen, M.D. and Pyenson, N.D. 2007. Diversity estimates, biases, and historiographic effects: resolving cetacean diversity in the Tertiary. Palaeontologia Electronica, 10.2.11A:1-22.
palaeo-electronica.org/2007_2/00123/index.html
Uno, H., Yoneda, M., Taru, H., and Kohno, N. 2008. Dietary preferences of desmostylians based on isotope, microwear and cranial morphology. Journal of Vertebrate Paleontology, 28(3):155A. https://jstor.org/stable/20491023
Valenzuela-Toro, A.M., Gutstein, C.S., Varas-Malca, R.M., Suárez, M.E., and Pyenson, N.D. 2013. Pinniped turnover in the South Pacific Ocean: new evidence from the Plio-Pleistocene of the Atacama Desert, Chile. Journal of Vertebrate Paleontology, 33:216-223. https://doi.org/10.1080/02724634.2012.710282
Valenzuela-Toro, A.M., Pyenson, N.D., Gutstein, C.S., and Suárez, M.E. 2016. A new dwarf seal from the late Neogene of South America and the evolution of pinnipeds in the southern hemisphere. Papers in Palaeontology, 2:101-115. https://doi.org/10.1002/spp2.1033
Vélez-Juarbe, J. 2014. Ghost of seagrasses past: using sirenians as a proxy for historical distribution of seagrasses. Palaeogeography, Palaeoclimatology, Palaeoecology, 400:41-49. https://doi.org/10.1016/j.palaeo.2013.05.012
Vélez-Juarbe, J. 2017. Eotaria citrica, sp. nov., a new stem otariid from the “Topanga” formation of Southern California. PeerJ, 5:e3022. https://doi.org/10.7717/peerj.3022
Vélez-Juarbe, J., Domning, D.P., and Pyenson, N.D. 2012. Iterative evolution of sympatric seacow (Dugongidae, Sirenia) assemblages during the past ∼26 million years. PLoS ONE, 7(2):e31294. https://doi.org/10.1371/journal.pone.0031294
Voss, M., Antar, M.S.M., Zalmout, I.S., and Gingerich, P.D. 2019. Stomach contents of the archaeocete Basilosaurus isis: apex predator in oceans of the late Eocene. PLoS ONE, 14(1):e0209021. https://doi.org/10.1371/journal.pone.0209021
Wang, X., Grohé, C., Su, D.F., White, S.C., Ji, X., Kelley, J., Jablonski, N.G., Deng, T., You, Y., and Yang, X. 2018. A new otter of giant size, Siamogale melilutra sp. nov. (Lutrinae: Mustelidae: Carnivora), from the latest Miocene Shuitangba site in north-eastern Yunnan, south-western China, and a total-evidence phylogeny of lutrines. Journal of Systematic Palaeontology, 16:39-65. https://doi.org/10.1080/14772019.2016.1267666
Werth, A.J. 2000. Feeding in marine mammals, p. 487-526. In Schwenk, K. (ed.), Feeding: Form, Function and Evolution in Tetrapods. Academic Press, San Diego, California.
Werth, A.J. 2006a. Mandibular and dental variation and the evolution of suction feeding in Odontoceti. Journal of Mammalogy, 87:579-588. https://doi.org/10.1644/05-mamm-a-279r1.1
Werth, A.J. 2006b. Odontocete suction feeding: Experimental analysis of water flow and head shape. Journal of Morphology, 267:1415-1428. https://doi.org/10.1002/jmor.10486
Werth, A.J. 2007. Adaptations of the cetacean hyolingual apparatus for aquatic feeding and thermoregulation. The Anatomical Record, 290:546-568. https://doi.org/10.1002/ar.20538
Werth, A.J. and Ito, H. 2017. Sling, scoop, and squirter: anatomical features facilitating prey transport, processing, and swallowing in rorqual whales (Mammalia: Balaenopteridae). The Anatomical Record, 300:2070-2086. https://doi.org/10.1002/ar.23606
Werth, A.J. and Potvin, J. 2016. Baleen hydrodynamics and morphology of cross-flow filtration in balaenid whale suspension feeding. PLoS ONE, 11(2):e0150106. https://doi.org/10.1371/journal.pone.0150106
Whitmore, F.C. 1994. Neogene climatic change and the emergence of the modern whale fauna of the North Atlantic Ocean. Proceedings of the San Diego Museum of Natural History, 29:223-227.
Williamson, G.R. 1973. Counting and measuring baleen and ventral grooves of whales. Scientific Reports of Whales Research Institute, 25:279-292.
XLSTAT. 2017. XLSTAT 2017: Data Analysis and Statistical Solution for Microsoft Excel. Addinsoft, Paris, France. https://xlstat.com
Young, S., Deméré, T.A., Ekdale, E.G., Berta, A., and Zellmer, N. 2015. Morphometrics and structure of complete baleen racks in gray whales (Eschrichtius robustus) from the eastern North Pacific Ocean. The Anatomical Record, 298:703-719. https://doi.org/10.1002/ar.23108
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292:686-693. https://doi.org/10.1126/science.1059412

