 The inner morphology of the petrosal bone of the endemic elephant of Tilos Island, Greece
The inner morphology of the petrosal bone of the endemic elephant of Tilos Island, Greece
Article number: 24.2.a23
https://doi.org/10.26879/1034
Copyright Society for Vertebrate Paleontology, June 2021
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 24 September 2019. Acceptance: 7 June 2021.
ABSTRACT
The bony labyrinth, as part of the inner structure of the petrosal bone, contains the sensory organs of balance and hearing. The semicircular canals, as part of the vestibular apparatus of the inner ear, are involved in the detection of angular motion of the head for maintaining balance and guiding locomotor behavior. While the overall structure of the bony labyrinth is inaccessible embedded in the petrosal bone, high resolution computed tomography makes the study of these structures possible. The purpose of this study is to visualize and precisely quantify the complex inner ear structures of the insular mammal Palaeoloxodon tiliensis and comment on the relationship of these morphologies to the agility and hearing frequency ranges. This study focuses on imaging the shape of the bony labyrinth as well as the semicircular canals, of three petrosal bones, using micro-computed tomography (micro-CT). Shape and size analysis of the cochlea allow for an assessment of morphological differences between species. Specifically, measuring the dimensions of inner ear components as well as the angular distances can express the variation in their balancing abilities and the frequencies of their auditory perception. The morphological characteristics obtained through micro-CT lead to the conclusion that P. tiliensis retained similar conditions to that of its larger relatives, and it was an animal that had hearing in the low frequency ranges.
Dionysia E. Liakopoulou. National and Kapodistrian University of Athens, Faculty of Geology and Geoenvironment, Panepistimiopolis, 15784 Athens, Greece, dliakopoulou@geol.uoa.gr
George E. Theodorou. National and Kapodistrian University of Athens, Faculty of Geology and Geoenvironment, Panepistimiopolis, 15784 Athens, Greece, gtheodor@geol.uoa.gr
Anneke H. van Heteren. Zoologische Staatssammlung München - Staatliche Naturwissenschaftliche Sammlungen Bayerns, Münchhausenstraße 21, 81247 München, Germany; Ludwig-Maximilians-Universität München, GeoBio-Center, Richard-Wagner-Straße 10, 80333 Munich, Germany; Ludwig-Maximilians-Universität München, Department Biologie II, Großhaderner Straße 2, 82152 Planegg-Martinsried, Germany, vanHeteren@snsb.de
Keywords: petrosal; micro-CT; bony labyrinth; endemism; Palaeoloxodon tiliensis
Final citation: Liakopoulou, Dionysia E., Theodorou, George E., and van Heteren, Anneke H. 2021. The inner morphology of the petrosal bone of the endemic elephant of Tilos Island, Greece. Palaeontologia Electronica, 24(2):a23. https://doi.org/10.26879/1034
palaeo-electronica.org/content/2021/3394-petrosal-bone-morphology
Copyright: June 2021 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Petrosal bones carry important phylogenetic information, since their morphology can vary between taxa, making them ideal study objects for phylogenetic analyses. Some aspects of the petrosal bone also carry important functional information. The labyrinth, which includes the cochlea anteriorly and the vestibular apparatus with the semicircular canals posteriorly, has been shown to vary in terms of size and shape between species (e.g., Spoor et al., 2007; Kirk and Gosselin-Ildari, 2009; Ekdale and Rowe, 2011; Gunz et al., 2012). Differences in certain morphological features are thought to be related to important information about the coordination of head movements, the balance of the individual, and the range of hearing frequencies (Rook et al., 2004; Alberti, 2006; Spoor et al., 2007; Ladevèze et al., 2010; Gunz et al., 2012; Pfaff et al., 2015). The size of the cochlea is correlated with overall body mass, so larger species are expected to have larger labyrinths (Rook et al., 2004; Nummela and Sánchez-Villagra, 2006; Kirk and Gosselin-Ildari, 2009; Ekdale, 2013).
Comparative studies have shown varying hearing abilities in mammals (Heffner and Heffner, 1982; Heffner, 2004). Several factors are responsible for the range of frequencies an animal can hear, the most important being the morphology of the inner ear, since this apparatus determines the mechanical response of the animal to air-born sounds and vibrations. Studies assessing the inner ear structures are becoming more and more part of the scientific literature, due to advances in micro-computed tomography (micro-CT) that make it possible to represent these structures digitally in three dimensions without destroying the specimen.
The present study concerns three petrosal bones of the dwarf elephants, Palaeoloxodon tiliensis that lived on Tilos Island in Greece during the Late Quaternary (Theodorou et al., 2007). Since there is little information on the morphology of the ear region of proboscideans (Blair, 1710; Court, 1992, 1994; Ekdale and Rowe, 2011; Benoit et al, 2013a, 2013b; Ekdale, 2013; Schmitt and Gheerbrant, 2016) and none of insular endemic species, this study will contribute to the available knowledge on Proboscidean inner ears. The work presented here will also contribute to our understanding of insular evolution and is part of a series of various research papers on proboscideans, providing comparison of their inner ears and commenting on their agility. The advantages of applying micro-CT scanning to the study of the inner ear of Palaeoloxodon tiliensis include generating new data on its morphology and phylogenetic characters as well as the non-destructive nature of the methodology.
MATERIALS AND METHODS
Material
The endemic elephants of Charkadio cave (Tilos Island, Dodecanese, Greece), Palaeoloxodon tiliensis (Theodorou et al., 2007), represent the last European elephant species, that lived from 45,000y to 3,500y BP (Bachmayer et al., 1976; Bachmayer et al., 1984). In the present study specimens are referred to as Palaeoloxodon tiliensis according to the prevailing opinion (Shoshani and Tassy, 2005; Shoshani et al., 2007; Ferretti, 2008; Herridge, 2010; Mitsopoulou et al., 2015), though one co- author of this study (G. Theodorou) disagrees with this perspective, considering that a more detailed analysis is required for the definite conclusion relating to the ancestry of the taxon, and considers that the taxon should be referred to as Elephas tiliensis (Theodorou et al., 2007).
The remains of Palaeoloxodon tiliensis are restricted to the upper layers of the cave and include many skeletal elements including petrosal bones. The remains from Charkadio cave represent different ontogenetic stages of P. tiliensis, based on dental and postcranial material (Theodorou and Symeonides, 2001; Theodorou et al., 2007; Mitsopoulou et al., 2015). Additionally, the petrosals studied here could also come from individuals of different individual ages, since they were not found in association with the rest of the skull or skeleton. The material is stored in the Paleontological and Geological Museum of the University of Athens. For this study, three petrosal bones were at our disposal (specimens T137, T2406 and T2506), two isolated and one attached to the tympanic part of the temporal bone. All three petrosals come from the right side of the skull. The preservational state was not equal between all specimens. The poorest preservation was observed in specimen T2406.
Micro-CT Acquisition
High resolution micro-CT was used to visualize the auditory structures, both external and internal. The micro-CT scans of the three petrosals were performed at the Steinmann Institute of the University of Bonn in Germany. The visualization of the structures was performed using image stacks (approximately 1000 per sample) and with voxel sizes of 0.048 mm for specimen T2406, 0.056 mm for specimen T137, and 0.106 mm for specimen T2506.
Avizo 8.1 was used to complete the visualization, segmentation, and 3D rendering of the petrosals and their bony labyrinths. The regions of interest, the cavities corresponding to the labyrinths, were selected through threshold methods that create a segmentation mask. In some cases, (e.g., specimen T2406) manual selection was imperative due to partially fragmented cavities that lacked some parts of the structures. The 3D surface was calculated, leading to the reconstructed models.
Measurements
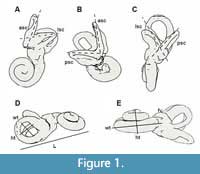 Measurements follow Ekdale (2013) and Spoor et al. (2007), while nomenclature and orientation of petrosals follow the work of O’Leary (2010). The 3D models of the bony labyrinth were rotated in such a position that two of the semicircular canals were in a perpendicular view to the observing plane, in order for angles to be measured. Angles were taken between the planes of all semicircular canals (Figure 1). Measurements on the specimens were performed using Avizo 8.1 (angular and linear measurements) and Rhino 5.0 (linear and measurements of radius).
Measurements follow Ekdale (2013) and Spoor et al. (2007), while nomenclature and orientation of petrosals follow the work of O’Leary (2010). The 3D models of the bony labyrinth were rotated in such a position that two of the semicircular canals were in a perpendicular view to the observing plane, in order for angles to be measured. Angles were taken between the planes of all semicircular canals (Figure 1). Measurements on the specimens were performed using Avizo 8.1 (angular and linear measurements) and Rhino 5.0 (linear and measurements of radius).
Moreover, we took linear measurements of the size of the cochlea to calculate its aspect ratio, which is defined by the division of its width by its height (Figure 1). The width of the cochlea was measured as the greatest distance of the two edges of the basal whorl, and its height from the top of the spiral to the level of the dorsal edge of the fenestra cochlea, perpendicular to the width. A low aspect ratio is considered to be below 0.55 and indicates a "flattened" cochlea while a higher ratio refers to "sharp-pointed" ones (Gray, 1907, 1908). A further measurement was the overall length of the labyrinth, along with the cochlea.
The radius of the curvature of the arc of the semicircular canals was also an important measurement. The radius of curvature of each canal arc was calculated by taking half the average of the height and width measurements (0.5[h + w]/2). The height of each canal was the distance from the wall of the apical point of the canal to the center of it, and the width was the perpendicular distance to the respective heights of the two opposing limbs of each canal. The size of the semicircular canals influences the afferent sensitivity of the canals (Yang and Hullar, 2007; Ekdale, 2013). The stapedial ratio is expressed by the quotient of the length and width of the fenestra vestibuli. The greater the value the more oval is the fenestra. This ratio is also used to describe the shape of the stapedial footplates (Benoit et al., 2013b).
Low-frequency Estimation
A method to estimate the low-frequency limit of hearing in extinct mammals has been proposed by Manoussaki et al. (2008) that takes into consideration the morphology of the cochlear canal. The equation f = f0*exp[-β(ρ-1)] estimates the low-frequency limit at 60 dB of the sampled taxa, where f0 = 1.507 ± 241 Hz and β = 0.578 ± 0.167. The radii ratio (ρ) is the ratio between the radius of the basal turn and the radius of the apical turn of the cochlear canal in apical view. This ratio correlates strongly with the low-frequency limit of a mammal: the higher ρ, the lower the low-frequency limit of hearing (Manoussaki et al., 2008).
Abbreviations in figures. aa, anterior ampulla; aF, aqueductus Fallopii; ant, anterior direction; asc, anterior semicircular canal; av, bony channel for vestibular aqueduct; ci, crista intefenestralis; co, cochlea; cr, common crus; dor, dorsal direction; ew, epitympanic wing; fv, fenestra vestibuli; hF, hiatus Fallopii; ht, height; im, internal auditory meatus; L, length of the labyrinth including the cochlea; la, lateral ampulla; lat, lateral direction; lsc, lateral semicircular canal; mr, mastoid region; pa, posterior ampulla; psc, posterior semicircular canal; pf, perilymphatic foramen; pr, promontorium; sp, sulcus for perilymphatic duct; stmf, stapedial muscle fossa; sr, spherical recess; th, tympanohyal; wt, width.
RESULTS
External Surface of the Petrosal
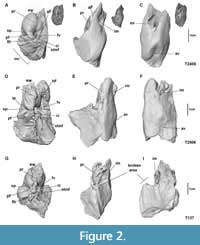 The three studied petrosal bones are quite similar. The specimens T137 and T2406 concern two isolated petrosal bones and the third one T2506 is attached to the tympanic part of the temporal bone and therefore the depiction of the tympanic external surface of this petrosal is not complete (Figure 2).
The three studied petrosal bones are quite similar. The specimens T137 and T2406 concern two isolated petrosal bones and the third one T2506 is attached to the tympanic part of the temporal bone and therefore the depiction of the tympanic external surface of this petrosal is not complete (Figure 2).
On the tympanic surface (Figure 2A, 2D, 2G) the epitympanic wing is observed, positioned anteromedially to the promontorium. The promontorium is located in the anterior portion of the tympanic surface and internally houses the cochlea. It appears to be bulbous, and it is irregularly shaped; especially in specimen T2406 it appears rather bulbous and spherical (Figure 2A). At the posterior part of the promontorium is a broad crista interfenestralis that divides two apertures. The first opening inclined towards the tympanic cavity is the fenestra vestibuli (oval foramen) with an elliptical shape (stapedial ratio = 1.9) that received the stapes. The poor preservation of the specimen T2406 artefactly gives the fenestra vestibule a wide size. The second aperture positioned posteromedially to the promontorium is the perilymphatic foramen. There is no evidence of a separate opening for the canaliculus cochleae that drained the perilymphatic fluid in life, therefore it shares the same undivided foramen with the fenestra cochleae. The portion that leads to a depression medially corresponds to the perilymphatic duct. Lateral to the promontorium is the aqueductus Fallopii: for the passage of the greater petrosal nerve. In the two isolated petrosals examined (Figure 2A, 2G) the hiatus Fallopii appears incomplete, whereas in specimen T2506 (Figure 2D) it appears fully enclosed.
Posteromedial to the crista interfenestralis lies a shallow depression corresponding to the stapedial muscle fossa, in the vicinity of the oval foramen. The crista intrerfenestralis connects posteriorly to the tympanohyal, creating a passage - foramen possibly for the stapedial muscle.
In cerebellar view, lying within the part of the petrosal facing the cranial cavity, is a deep depression interpreted as the internal acoustic meatus. It is widely open, and oriented towards the anterior apex of the petrosal, with an opening quite round and parallel to the long axis of the bone. It is the point where the vestibulocochlear nerves enter the cranium. The subarcuate fossa, which in most mammals is located dorsally on the canalicular part of the petrosal, is absent in these specimens. At the posterior end of the petrosal bone, the cleft-like opening for the aqueduct vestibuli is situated (Figure 2C, 2F, 2I).
Bony Labyrinth
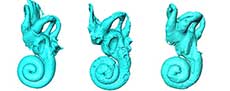
 The morphology of the bony labyrinth of Palaeoloxodon tiliensis is based on three specimens (T137, T2406, T2506, Figure 3), which were micro-CT scanned and reconstructed in 3D. The cochlea has a degree of coiling approximately of 752° (2 to 2.25 full turns). It forms a fairly planispiral shape, as it is illustrated in Figure 3. It appears to be tightly coiled, with a large and massive basal whorl. As measured in specimens the diameter of the basal whorl (11.4 mm) is approximately twice as large as the height (5.2 mm) of the cochlea and almost twice the diameter of the second whorl (6.7 mm). The aspect ratio as measured in the samples is 0.46, indicating flattened cochleae. A secondary bony lamina seems not to be developed. The cochlea contributes 36% of the total volume of the bony labyrinth.
The morphology of the bony labyrinth of Palaeoloxodon tiliensis is based on three specimens (T137, T2406, T2506, Figure 3), which were micro-CT scanned and reconstructed in 3D. The cochlea has a degree of coiling approximately of 752° (2 to 2.25 full turns). It forms a fairly planispiral shape, as it is illustrated in Figure 3. It appears to be tightly coiled, with a large and massive basal whorl. As measured in specimens the diameter of the basal whorl (11.4 mm) is approximately twice as large as the height (5.2 mm) of the cochlea and almost twice the diameter of the second whorl (6.7 mm). The aspect ratio as measured in the samples is 0.46, indicating flattened cochleae. A secondary bony lamina seems not to be developed. The cochlea contributes 36% of the total volume of the bony labyrinth.
Between the cochlea and the semicircular canals is the vestibule, which is bulky and consists of a not well distinguished utricle and a saccule, with the latter being slightly bigger. The spherical recess, which houses the membranous saccule, is situated anteriorly and the utricle posteriorly. The fenestra vestibuli faces posteriorly and the perilymphatic foramen laterally (Figure 3A). The vestibular aqueduct originates from the vestibule at the base of the common crus, from which it extends detached and dorsally to the cerebellar surface of the petrosal bone (Figure 3B). Its entire aspect in all specimens could not be depicted, however, due to the preservation of the samples.
The lengths of the labyrinth components and the radius of the semicircular canals are presented in Table 1. The semicircular canals are connected to the vestibule through their ampulla at their anterior limbs (ends). The canals are broad and quite round in shape, and the ampullas at their base are bulbous. Both anterior and posterior canals are extended above the level of the common crus linking them. The lateral semicircular canal, more elliptical in shape than the other two, appears more planar and it opens in the vestibule separately from the other two. The lateral canal lies above the foramen vestibuli. The posterior end of the lateral semicircular canal begins anteriorly of the posterior semicircular canal. The angles between the planes of the canals were also measured, the most acute (values) angle is that between the anterior and lateral semicircular canal, and the higher value of angle is that between the lateral and posterior canal (Table 1). In terms of radius the lateral canal is the smallest and the posterior the largest. In terms of length (ampulla not included) the largest of all three is the anterior semicircular canal.
DISCUSSION
The petrosal bone of Proboscidea holds important information on locomotor behavior and auditory perception. In most cases, however, it is quite difficult to be examined, because it is fused inside the skull and the massive skull size prevents its study. Previous studies on petrosal bones of extinct and extant Proboscidea, even though limited, offer a comparison of the main characteristics of this bone and its internal structures and an interpretation of these features in terms of hearing capacities and locomotion.
The specimens CT-scanned and described in this study belong to the endemic species of Palaeoloxodon tiliensis, representing an interesting study to investigate character evolution within Proboscidea. These specimens could represent individuals of different ontogenetic stages based, also, on dental and postcranial material of the Charkadio cave that they were excavated from (Theodorou and Symeonides, 2001; Theodorou et al., 2007; Mitsopoulou et al., 2015). This parallels to the fact that adult morphologies and dimensions of the inner ear are generally already attained at the embryonic stage (Hoyte, 1961; Rinkwitz et al., 2001; Jeffery and Spoor, 2004; Ekdale, 2010; Richard et al., 2010; Costeur et al., 2017). Allometric changes only occur on the external surface of the petrosal bone and can be detected by a gradual increase of the bone externally (Hoyte, 1961; Ekdale, 2010, 2011). The incomplete nature of the aqueductus Falloppi, which is displayed in specimens T137 and T2406, in contrast with T2506 where it appears fully enclosed, is hypothesized to be a sign of an immature developmental stage (Ekdale, 2011). Internally, there are no major differences or variations observed between the labyrinths of the specimens studied.
As the petrosal bone is difficult to study, there is little known about the morphology of the petrosal bones of proboscideans, externally and internally. However, there are some studies that reveal phylogenetic relationships, as well as similarities and differences in their morphology. The first studies concern extant elephants, and they date back to as early as 1710 conducted by Blair (1710). Comparisons were made with the published data on two Eocene taxa Moeritherium (Court, 1994) and Numidotherium koholense (Court, 1992; Benoit et al., 2013b; Schmitt and Gheerbrant, 2016), the Pleistocene Elephantimorpha from Friesenhahn Cave (Ekdale, 2011) and extant elephants Elephas and Loxodonta (Spoor et al., 2007; Manoussaki et al., 2008; Ekdale, 2011; Benoit et al., 2013a, 2013b). The present paper increases the knowledge on Proboscidean petrosal morphology by describing specimens of an endemic taxa.
The external petrosal surface of Palaeoloxodon tiliensis resembles other extinct and extant proboscideans with regards to several features. The first significant similarity is that of the fusion of the fenestra cochleae and canaliculus cochleae into a common perilymphatic foramen. This is a structure observed in proboscideans, at least from Eocene onwards (Court, 1994). Contrary to other studied Proboscidea, in Numidotherium they appear not to be merged into a common aperture (Court, 1992, 1994; Benoit et al., 2013b; Schmitt and Gheerbrant, 2016).
Characters, such as an undivided perilymphatic foramen, are indicative of an adaptation to hearing lower frequencies, because this feature reduces the containment of cochlear fluid, it reduces the force of stapedial impulse and augments low frequency acuity (Luo and Gingerich, 1999; Sánchez-Villagra et al., 2002; Ladevèze et al., 2008; Ladevèze et al., 2010). The middle and inner ear of elephants is adapted to receive infrasonic air-born sounds (Payne, et al., 1986; Poole et al., 1988), and it is well established that extant elephants have the ability to respond to low frequencies, which have long distance capacity (Payne, et al., 1986; Poole et al., 1988; Ruggero and Temchin, 2002).
Another feature observed in most mammals, but not in all the proboscideans studied so far, is the subarcuate fossa: a character that, when it is well developed, is associated with the agility of the animal (Jeffery and Spoor, 2006; Ekdale, 2011). It also plays a role in the coordination of head and eye movements (Alberti, 2001; Rook et al., 2004; Spoor et al., 2007; Ladevèze et al., 2010; Gunz et al., 2012; Pfaff et al., 2015). This feature is absent in Palaeoloxodon tiliensis, and it appears to be lost in Proboscidea at least by the Eocene (Court, 1994; Schmitt and Gheerbrant, 2016). Extant elephants are characterized by their low agility (Spoor et al., 2007), which is in accordance with the absence of the subarcuate fossa, and could possibly be the case also for P. tiliensis.
The internal structures of the petrosal bone and its morphology also hold valuable information on auditory frequencies. The bony labyrinth, as mentioned before, is subdivided into the cochlea, the vestibule, and the semicircular canals. A spiral-shaped cochlea is an evolutionary characteristic that is observed exclusively in mammals, enhancing the span of octaves that they can hear (Doran, 1879; Gray, 1951; Manoussaki et al., 2008). Middle ear characteristics and bone conduction play as much an important role as the cochlea for the low frequency octave limit. Animals with tightly coiled cochleae tend to have greater hearing ranges, but previous attempts to associate these auditory effects with the physical characteristics of the cochlea have proven unsatisfactory (Manoussaki et al., 2008).
The number of turns of the cochlea has been used in several analyses (Ekdale, 2013). A large number of turns and possessing a large basal whorl gives better perception of low frequencies (Manoussaki et al., 2008; Kirk and Gosselin-Ildari, 2009; Ekdale, 2013). There is at least one full turn in all studied Proboscidea. The cochlea of Palaeoloxodon tiliensis completes a little over two full coils to 2.25 coils, with a fairly planispiral shape. Elephas maximus and Loxodonta africana have a wide cochlea with at least two full turns (Court, 1992; Garstang, 2004), and so does Moeritherium (Court, 1994). Numidotherium displays the lowest number of turns as of only 1.5 turns in its cochlea (Court, 1992; Benoit et al., 2013b). However, Blair (1710) in his research mentions an unidentified extant elephant, whose cochlea completes a little more than three coils.
It has been suggested that the absence of secondary bony lamina provides further proof on the hypothesis of the adaptation to low frequencies hearing capabilities (Ekdale, 2011; Benoit et al., 2013b; Schmitt and Gheerbrant, 2016). The exact loss of a secondary bony lamina within proboscidean tree is ambiguous because the information is lacking for several taxa (e.g., Moeritherium). There is no evidence of a secondary bony lamina in Palaeoloxodon tiliensis, on the contrary primitively it can be observed in Numidotherium and Phosphatherium (Benoit et al., 2013b; Schmitt and Gheerbrant, 2016). Another primitive character is that of secondary common crus at the ampular limb of the posterior canal with the posterior limb of the lateral one, character that is evident in Numidotherium and Phospatherium but not to other Proboscidea (Benoit et al., 2013b; Schmitt and Gheerbrant, 2016).
Using the equation of Manoussaki et al. (2008) we were able to estimate the low frequency limit of Palaeoloxodon tiliensis. This equation uses the radii ratio, which is the quotient between the radius of the basal turn and that of the apical turn, and denotes that the higher the value of the ratio the lower the limit of the hearing frequencies. Herein, we calculated the radii ratio of Palaeoloxodon tiliensis to be 8.6 (Table 1), which predicts a low frequency limit of 18.6 Hz. This value is close to the calculations of Elephas 17 Hz at 60 dB (Manoussaki et al., 2008), hence it is most likely that Palaeoloxodon tiliensis was capable of low frequency hearing. On the contrary the same equation suggests that Numidotherium with values between 450.3 and 266.1 Hz (Benoit et al., 2013b) may have not been able to hear low frequencies in contrast to its later counterparts.
Inner ear dimensions of different characters like the angles between the semicircular canals and their radius of curvature are related to auditory sensitivity as well as to locomotor behaviour (Heffner, 2004; Jeffery and Spoor, 2004; Rook et al., 2004; Ekdale and Rowe, 2011; Pfaff et al., 2015). Agile animals tend to have large canal radius relative to their body mass; slower movements of the head were detected in large animals with large radius of curvature of their semicircular canals (Spoor et al., 2007; Pfaff et al, 2015). In all specimens of the present study, the posterior semicircular canal has the largest radius and the lateral one the smallest. Also the angles as seen in Table 1 follow a pattern: the most acute angle is that between the anterior and lateral canals and the higher angle value between the lateral and posterior canals.
Concluding the results converge to the fact that Palaeoloxodon tiliensis retains the morphological characters that show an adaptation to lower frequencies. However, Palaeloxodon tiliensis derived from a Palaeoloxodon antiquus ancestor, as supported by morphological traits (Shoshani and Tassy, 2005; Shoshani et al., 2007; Ferretti, 2008; Herridge, 2010; Mitsopoulou et al., 2015), and therefore further comparisons could be useful determining evolutionary traits within Proboscidea.
CONCLUSIONS
The present study contributes the investigation of proboscidean inner ear evolution and morphology, given the fact that there is a poor representation of isolated proboscidean petrosals and even more so insular ones.
The morphology of the petrosal bone, with a common perilymphatic foramen, and the morphology of the cochlear canal, with no trace of secondary bony lamina, the large and massive basal whorl of the cochlea and in general the dimensions and shape of the cochlea supports the hypothesis of an animal that was able to hear low frequency sounds. The equation suggested by Manoussaki et al. (2008) used here also displays close values with that of extant elephants. Therefore, the petrosal bone and the bony labyrinth morphology of Palaeoloxodon tiliensis, adding information on the evolution of the ear region of elephants, display characters involved to an adaptation at lower frequencies capacities, and these characters closely resemble that of extant Proboscidea.
ACKNOWLEDGMENTS
We would like to thank all the reviewers for their insightful comments that improved the quality of this paper. The editors and Dr. A. Sharp deserve many thanks for their comments and patience. The Tilos project is financed by Greek national funds of Special Account of Research Grants of National and Kapodistrian University of Athens (70/3/10323) that provided funding for the CT scans to be collected at the University of Bonn Steinmann Institute, Germany. The Humbolt Foundation funded AHvH for the duration of this project.
REFERENCES
Alberti, P.W. 2006. The anatomy and physiology of the ear and hearing, p. 53-62. In Occupational Exposure to Noise: Evaluation, Prevention and Control. Technical Report, University of Toronto, Toronto, Canada.
Bachmayer, F., Symeonidis, N., Seemann, R., and Zapfe, H. 1976. Die Ausgrabungen in der Zwergelefantenhöhle “Charkadio” auf der Insel Tilos (Dodekanes, Griechenland) in den Jahren 1974 und 1975. Annalen des Naturhistorischen Museums in Wien, 80:113-144.
Bachmayer, F., Symeonidis, N., and Zapfe, H. 1984. Die Ausgrabungen in der Zwerkelefantenhφhle der Insel Tilos (Dodekanes, Griechenland) im Jahr 1983. Sitzungsberichte der Φsterreichischen Akademie der Wissenschaften - Mathematisch-Naturwissenschaftliche Klasse, 193:321-328.
Benoit, J., Adnet, S., El Mabrouk, E., Khayati, H., Ben Haj Ali, M., Marivaux, L., Merzeraud, G., Merigeaud, S., Vianey-Liaud, M., and Tabuce, R. 2013a. Cranial remain from Tunisia provides new clues for the origin and evolution of Sirenia (Mammalia, Afrotheria) in Africa. PLoS ONE, 8(1):e54307. https://doi.org/10.1371/journal.pone.0054307
Benoit, J., Merigeaud, S., and Tabuce, R. 2013b. Homoplasy in the ear region of Tethytheria and the systematic position of Embrithopoda (Mammalia, Afrotheria). Geobios, 46(5):357-370. https://doi.org/10.1016/j.geobios.2013.07.002
Blair, P. 1710. A description of the organ of hearing in the elephant, with the figures and situation of the ossicles, labyrinth and cochlea in the ear of that large animal. Philosophical Transactions of the Royal Society of London, 30:885-898. https://doi.org/10.1098/rstl.1717.0043
Costeur, L., Mennecart, B., Müller, B., and Schulz, G. 2017. Prenatal growth stages show the development of the ruminant bony labyrinth and petrosal bone. Journal of Anatomy, 230(2):347-353. https://doi.org/10.1111/joa.12549
Court, N. 1992. Cochlea anatomy of Numidotherium koholense: auditory acuity in the oldest known proboscidean. Lethaia, 25:211-215. https://doi.org/10.1111/j.1502-3931.1992.tb01385.x
Court, N. 1994. The periotic of Moeritherium (Mammalia, Proboscidea): homology or homoplasy in the ear region of Thethytheria McKenna, 1975? Zoological Journal of the Linnean Society, 112:13-28.
Doran, A.H.G. 1879. Morphology of the mammalian ossicula auditus. Transactions, Linnean Society of London (2nd Ser. Zool.), 1:371-497.
Ekdale, E.G. 2010. Ontogenetic variation in the bony labyrinth of Monodelphis domestica (Mammalia: Marsupialia) following ossification of the inner ear cavities. Anatomical Record, 293:1896-1912. https://doi.org/10.1002/ar.21234
Ekdale, E.G. 2011. Morphological variation in the ear region of Pleistocene Elephantimorpha (Mammalia, Proboscidea) from central Texas. Journal of Morphology, 272:452-464. https://doi.org/10.1002/jmor.10924
Ekdale, E.G. 2013. Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS ONE, 8:27-28. https://doi.org/10.1371/journal.pone.0066624
Ekdale, E.G. and Rowe, T. 2011. Morphology and variation within the bony labyrinth of zhelestids (Mammalia, Eutheria) and other therian mammals. Journal of Vertebrate Paleontology, 31:658-675. https://doi.org/10.1080/02724634.2011.557284
Ferretti, M.P. 2008. The dwarf elephant Palaeoloxodon mnaidriensis from Puntali Cave, Carini (Sicily; late Middle Pleistocene): anatomy, systematics and phylogenetic relationships. Quaternary International, 182:90-108.
Garstang, M. 2004. Long-distance, low-frequency elephant communication. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 190:791-805. https://doi.org/10.1007/s00359-004-0598-0
Gray, A.A. 1907. The Labyrinth of Animals. Volume 1. J. and A. Churchill, London.
Gray, A.A. 1908. The Labyrinth of Animals. Volume 2. J. and A. Churchill, London.
Gray, O. 1951. An introduction to the study of the comparative anatomy of the labyrinth. The Journal of Laryngology and Otology, 65:681-703.
Gunz, P., Ramsier, M., Kuhrig, M., Hublin, J.J., and Spoor, F. 2012. The mammalian bony labyrinth reconsidered, introducing a comprehensive geometric morphometric approach. Journal of Anatomy, 220:529-543. https://doi.org/10.1111/j.1469-7580.2012.01493.x
Heffner, R.S. 2004. Primate hearing from a mammalian perspective. Anatomical Record, 281A:1111-1122. https://doi.org/10.1002/ar.a.20117
Heffner, R.S. and Heffner, H.E. 1982. Hearing in the elephant (Elephas maximus): absolute sensitivity, frequency discrimination, and sound localization. Journal of Comparative and Physiological Psychology, 96:926-944. https://doi.org/10.1037/0735-7036.96.6.926
Herridge, V.L. 2010. Dwarf elephants on Mediterranean Islands: a Natural Experiment in Parallel Evolution. PhD thesis, University College, London.
Hoyte D. 1961. The postnatal growth of the ear capsule in the rabbit. American Journal of Anatomy, 108:1-16.
Jeffery, N. and Spoor, F. 2004. Prenatal growth and development of the modern human labyrinth. Journal of Anatomy, 204:71-92. https://doi.org/10.1111/j.1469-7580.2004.00250.x
Jeffery, N. and Spoor, F. 2006. The primate subarcuate fossa and its relationship to the semicircular canals. Part I. Prenatal growth. Journal of Human Evolution, 51:537-549.
Kirk, E.C. and Gosselin-Ildari, A.D. 2009. Cochlear labyrinth volume and hearing abilities in primates. Anatomical Record, 292:765-776. https://doi.org/10.1002/ar.20907
Ladevèze, S., Asher, R.J., and Sánchez-Villagra, M.R. 2008. Petrosal anatomy in the fossil mammal Necrolestes: Evidence for metatherian affinities and comparisons with the extant marsupial mole. Journal of Anatomy, 213:686-697. https://doi.org/10.1111/j.1469-7580.2008.00985.x
Ladevèze, S., de Muizon, C., Colbert, M., and Smith, T. 2010. 3D computational imaging of the petrosal of a new multituberculate mammal from the Late Cretaceous of China and its paleobiologic inferences. Comptes Rendus Palevol, 9:319-330. https://doi.org/10.1016/j.crpv.2010.07.008
Luo, Z. and Gingerich, P.D. 1999. Terrestrial Mesonychia to aquatic Cetacea: Transformation of the basicranium and evolution of hearling in whales. University of Michigan Papers on Paleontology, 31:i-viii, 1-98.
Manoussaki, D., Chadwick, R.S., Ketten, D.R., Arruda, J., Dimitriadis, E.K., and O’Malley, J.T. 2008. The influence of cochlear shape on low-frequency hearing. Proceedings of the National Academy of Sciences, 105:6162-6166. https://doi.org/10.1073/pnas.0710037105
Mitsopoulou, V., Michailidis, D., Theodorou, E., Isidorou, S., Roussiakis, S., Vasilopoulos, T., Polydoras, S., Kaisarlis, G., Spitas, V., Stathopoulou, E., Provatidis, C., and Theodorou, G. 2015. Digitizing, modelling and 3D printing of skeletal digital models of Palaeoloxodon tiliensis (Tilos, Dodecanese, Greece). Quaternary International, 379:4-13. https://doi.org/10.1016/j.quaint.2015.06.068
Nummela, S. and Sánchez-Villagra, M.R. 2006. Scaling of the marsupial middle ear and its functional significance. Journal of Zoology, 270:256-267. https://doi.org/10.1111/j.1469-7998.2006.00126.x
O’Leary, M.A. 2010. An anatomical and phylogenetic study of the osteology of the petrosal of extant and extinct Artiodactylans (Mammalia) and relatives. Bulletin of the American Museum of Natural History, 335:1-206. https://doi.org/10.1206/335.1
Payne, K.B., Langbauer, W.R., and Thomas, E.M. 1986. Infrasonic calls of the Asian elephant (Elephas maximus). Behavioral Ecology and Sociobiology, 18:297-301.
Pfaff, C., Martin, T., and Ruf, I. 2015. Bony labyrinth morphometry indicates locomotor adaptations in the squirrel-related clade (Rodentia, Mammalia). Proceedings of the Royal Society B: Biological Sciences, 282:20150744. https://doi.org/10.1098/rspb.2015.0744
Poole, J.H., Payne, K., Langbauer, W.R. Jr., and Moss, C.J. 1988. The social contexts of some very low-frequency calls of African elephants. Behavioral Ecology and Sociobiology, 22:385-392. https://doi.org/10.1007/BF00294975
Richard, C., Laroche, N., Malaval, L., Dumollard, J.M., Martin, C., Peoch, M., Vico, L., and Prades, J.M. 2010. New insight into the bony labyrinth: A microcomputed tomography study. Auris Nasus Larynx, 37:155-161. https://doi.org/10.1016/j.anl.2009.04.014
Rinkwitz, S., Bober, E., and Baker, R. 2001. Development of the vertebrate inner ear. Annals of the New York Academy of Sciences, 942:1-14.
Rook, L., Bondioli, L., Casali, F., Rossi, M., Köhler, M., Moyá Solá, S., and Macchiarelli, R. 2004. The bony labyrinth of Oreopithecus bambolii. Journal of Human Evolution, 46:347-354. https://doi.org/10.1016/j.jhevol.2004.01.001
Ruggero, M.A. and Temchin, A.N. 2002. The roles of the external, middle, and inner ears in determining the bandwidth of hearing. Proceedings of the National Academy of Sciences, 99:13206-13210. https://doi.org/10.1073/pnas.202492699
Sánchez-Villagra, M.R., Gemballa, S., Nummela, S., Smith, K.K., and Maier, W. 2002. Ontogenetic and phylogenetic transformations of the ear ossicles in marsupial mammals. Journal of Morphology, 251:219-238. https://doi.org/10.1002/jmor.1085
Schmitt, A. and Gheerbrant, E. 2016. The ear region of earliest known elephant relatives: New light on the ancestral morphotype of proboscideans and afrotherians. Journal of Anatomy, 228(1):137-152. https://doi.org/10.1111/joa.12396
Shoshani, J. and Tassy, P. 2005. Advances in proboscidean taxonomy & classification, anatomy & physiology, and ecology & behavior. Quaternary International, 126-128:5-20. https://doi.org/10.1016/j.quaint.2004.04.011
Shoshani, J., Ferretti, M.P., Listerd, A.M., Larry, D., Agenbroad, L.D., Saegusa, H., Mol, D., and Takahashi, K. 2007. Relationships within the Elephantinae using hyoid characters. Quaternary International, 169-170:174-185.
Spoor, F., Garland, T., Krovitz, G., Ryan, T.M., Silcox, M.T., and Walker, A. 2007. The primate semicircular canal system and locomotion. Proceedings of the National Academy of Sciences, 104:10808-10812. https://doi.org/10.1073/pnas.0704250104
Theodorou, G.E. and Symeonidis, N.K. 2001. The excavations of the last ten years at Charkadio cave on Tilos Island, Dodekanese, Greece, p. 514-518. 1st International Congress “The World of Elephants.”
Theodorou, G.E., Symeonidis, N.K., and Stathopoulou, E. 2007. Elephas tiliensis n. sp. from Tilos Island (Dodecanese, Greece). Hellenic Journal of Geosciences, 42:19-32.
Yang, A. and Hullar, T.E. 2007. Relationship of semicircular canal size to vestibular-nerve afferent sensitivity in mammals. Journal of Neurophysiology, 98:3197-3205. https://doi.org/10.1152/jn.00798.2007

