 Teganium (Porifera, Hexactinellida) from the Middle Ordovician Castle Bank fauna of Avalonia (Wales, UK)
Teganium (Porifera, Hexactinellida) from the Middle Ordovician Castle Bank fauna of Avalonia (Wales, UK)
Article number: 26.2.21
https://doi.org/10.26879/1247
Copyright Paleontological Society, June 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 16 October 2022. Acceptance: 22 May 2023.
ABSTRACT
The hexactinellid sponges Teganium and Teganiella are widespread in Ordovician, Devonian and Carboniferous strata in Laurentia, but have not previously been reported outside that palaeocontinent; some other members of the family Teganiidae are also restricted to Laurentia. The genus Teganiella in particular is considered to be a Laurentian endemic, with all species specialised for equatorial, shallow-water conditions. However, it is now clear that the diagnostic separation of Teganiella from Teganium was based on misunderstanding of the latter, and Teganiella should be considered a junior synonym. Based on this prior distribution, the discovery of two new species of Teganium (T. avalonensis sp. nov. and Teganium sp.) from the Middle Ordovician (Didymograptus murchisoni Biozone) of Castle Bank, Wales, is unexpected. These species are the oldest known and indicate diversification of the genus within a temperate microcontinent, prior to its appearance in Laurentia. The exceptional preservation of the new sponges also reveals detail of a complex body wall, supporting a more derived phylogenetic position within Hexactinellida (possibly affiliated to the Lyssacinosida) than previously recognised. The palaeobiogeography may suggest a relatively deep-water origin for the group (as for modern hexactinellids), which then became secondarily specialised for shallow-water environments within a Laurentian diversification.
Joseph P. Botting. Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, 39 East Beijing Road, Nanjing 210008, China and Department of Natural Sciences, Amgueddfa Cymru - National Museum Wales, Cathays Park, Cardiff CF10 3NP, UK. acutipuerilis@yahoo.co.uk
ORCID: 0000-0003-0388-8677
Lucy A. Muir. Department of Natural Sciences, Amgueddfa Cymru - National Museum Wales, Cathays Park, Cardiff CF10 3NP, UK. lucy@asoldasthehills.org
ORCID: 0000-0001-6324-2259
Junye Ma. Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, 39 East Beijing Road, Nanjing 210008, China. jyma@nigpas.ac.cn
ORCID: 0000-0002-0798-0529
Keywords: Porifera; new species; Hexactinellida; Castle Bank; Ordovician; Darriwilian
Final citation: Botting, Joseph P., Muir, Lucy A., and Ma, Junye. 2023. Teganium (Porifera, Hexactinellida) from the Middle Ordovician Castle Bank fauna of Avalonia (Wales, UK). Palaeontologia Electronica, 26(2):a21.
https://doi.org/10.26879/1247
palaeo-electronica.org/content/2023/3857-teganium-from-wales
Copyright: June 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/B66F3F99-3211-416F-BDFD-8583DF0DC844
INTRODUCTION
The potential usefulness of palaeobiogeographic studies on Palaeozoic sponges is strongly dependent on the groups in question, but where it can be understood, they provide substantial insights into both palaeogeography and the ecology of the groups in question. Such distribution patterns can reveal palaeoceanographic circulation patterns, unsuspected palaeoenvironmental links and simultaenously constrain the ecological requirements of the taxa involved. Reef-building sponges such as lithistids (Carrera and Rigby, 1999) and stromatoporoids (Nestor and Webby, 2013; Jeon et al., 2022) show informative distribution patterns within carbonate facies, but are tied to these conditions, primarily in the equatorial latitudes. Non-lithistid sponges are generally more limited in their potential, because of their low preservation potential and lack of study (Muir et al., 2013). Preservation of the more delicate sponges that lack fused skeletons requires abrupt burial or some other form of exceptional preservation, and this normally occurs in offshore environments influenced by intermittent rapid deposition. In the Cambrian Burgess Shale-type faunas, where sponges are generally diverse and abundant (Rigby and Collins, 2004; Wu et al., 2014; Botting and Peel, 2016; Botting and Muir, 2019) sponge genera were often widely distributed, but the Ordovician communities were much more strongly endemic (Botting and Muir, 2018). Even within a small area with high levels of exceptional sponge preservation such as the Builth Inlier of Wales (Muir and Botting, 2015), the majority of species and even genera are known from single occurrences.
In post-Cambrian offshore sponge faunas, very few genera are known from more than one or two areas, and the majority of these are holdovers from the widely distributed Cambrian lineages (Botting and Muir, 2019). The most widespread Ordovician and Silurian genus in these environments was probably Cyathophycus, which is known from several palaeocontinents around the margins of the Iapetus Ocean (Muir et al., 2013). The genus is, however, poorly defined, in that it consists of a very generalised, orthogonal reticulate skeleton with overlap of the skeletal rays. The range of taxa assigned to Cyathophycus is very wide (Botting, 2004), including variation in seemingly critical characters such as the existence and nature of an inner skeletal wall. In some species, preservation of fine details such as anomalous (hexagonal) axial canal symmetry suggests a position close to the base of Silicea, and perhaps in the demosponge stem group (Botting and Muir, 2013, 2018). As the various species are found in a variety of palaeoenvironments (with each species being recorded from a single site in many cases), and may be taxonomically disparate, such interpretative limitations also impact the reliability of assessments of palaeobiogeography based on these taxa.
Another complex of Palaeozoic hexactinellid-like taxa with multiple described species is composed of Teganium and Teganiella (summarised by Finks and Rigby, 2004), which show an environmental shift from offshore to sheltered inshore environments of Laurentia between the Ordovician and Carboniferous. In this study, we describe new material of Teganiella from Middle Ordovician strata of Wales, UK. This genus was previously known only from Upper Ordovician to Carboniferous strata of Laurentia; thus, the Welsh material represents a substantial range increase for the taxon. Based on an assessment of the supposed differences between the two genera, we show that they are effectively identical and synonymize them into the senior name, Teganium. We demonstrate that the evolution of this genus involved a transition into shallower water through time.
GEOLOGICAL BACKGROUND
Castle Bank is a recently discovered Burgess Shale-type Konservat-Lagerstätte close to Llandrindod, central Wales, within the Ordovician Builth–Llandrindod Inlier (Botting, 2021; Botting and Ma, 2022; Pates et al., 2022; Botting et al., 2023). Precise locality information is housed with the specimens. There are numerous sponge faunas in the inlier, from a range of palaeoenvironments (Muir and Botting, 2015), with Castle Bank representing an intermediate water depth interpreted as being close to storm wave base, on the basis of sedimentology and associated faunas. In particular, the sedimentary succession consists of slowly deposited, laminated graptolitic siltstone interspersed with rapidly deposited event beds that contain exceptionally preserved fossils (Botting et al., 2023), the combination characteristic of conditions around storm wave base. The fauna is a mixture of shallow-water and (primarily) offshore taxa, with echinoderms in particular being composed only of asterozoans and rare stylophorans that are unique to the site (Botting et al., 2023). The lack of both crinoids (shallow-water) and the typical offshore stylophorans is characteristic of an ecological gap in echinoderm faunas that occur around storm wave base, as described by Botting et al. (2013). Algae are present but rare and transported with the exceptionally preserved fauna. Exact water depth cannot be assessed, but given the palaeoceanic context (a semi-isolated back-arc basin on the margin of the Iapetus Ocean) and proximity to a photic-zone region, is estimated at around 50–100 m.
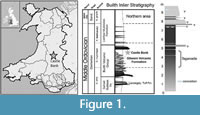 The age of the site (Figure 1) is constrained by abundant graptolites to the Didymograptus murchisoni Biozone, and lithostratigrapically to the Gilwern Volcanic Formation of the Builth Volcanic Group (Schofield et al., 2004). The Castle Bank quarry exposes approximately 10 metres of siltstone and volcanic ash beds, representing a very small part of the formation (which is several hundred metres thick) and a short time interval.
The age of the site (Figure 1) is constrained by abundant graptolites to the Didymograptus murchisoni Biozone, and lithostratigrapically to the Gilwern Volcanic Formation of the Builth Volcanic Group (Schofield et al., 2004). The Castle Bank quarry exposes approximately 10 metres of siltstone and volcanic ash beds, representing a very small part of the formation (which is several hundred metres thick) and a short time interval.
Sponge remains are preserved at multiple levels, but especially within a two-metre interval in the centre of the quarry, where they are usually fully articulated with preserved soft tissue, and co-occur with other exceptionally preserved taxa. The new species is widely distributed within this interval, and is one of the most abundant sponges in the assemblage. Outside that interval, sponge remains are mostly disarticulated, and the presence of the species cannot be assessed.
MATERIALS AND METHODS
Type and figured specimens are deposited in the Amgueddfa Cymru–National Museum Wales (NMW); comparative material will also be placed in the Nanjing Institute of Geology and Palaeontology, China. Specimens were illustrated primarily using Leica S8APO and M125C (with camera lucida) microscopes (Leica, Wetzlar, Germany), with cross-polarizing ring lights (Leica LED 3000 RL) and a HiChrome MET-AF digital camera (GX Microscopes, Wickhambrook, Suffolk, UK). Some high-magnification images were obtained with a Maozua digital USB microscope. Images were compiled and processed (contrast/brightness) using the OpenOffice software.
SYSTEMATIC PALAEONTOLOGY
Phylum PORIFERA Grant, 1836
Class HEXACTINELLIDA Schmidt, 1870
Remarks. Although any sponges with hexactins are traditionally assigned to the Hexactinellida, palaeontological evidence implies that these spicules pre-date the class (Botting and Muir, 2018). The majority of early hexactin-bearing taxa have simple, often single-layered skeletons in at least a semi-regular array, and are referred to generally as reticulosans; however, this is probably a paraphyletic grouping of early siliceans, at least. As a result, assignment of fossils to the hexactinellid crown or total group can only be confident when based on specific structures of the skeleton, or the existence of known microscleres (almost never preserved in situ). Crown-group lyssacine (lacking a fused skeleton) hexactinellids differ from most reticulosans in the complexity of that skeleton, with multiple distinct skeletal layers composed of different spicules in different arrangements. The complex wall structure of Teganiella (as seen with additional detail in the new material) supports at least a total-group hexactinellid assignment, and plausibly a crown-group affinity. Specific characters implying a relationship to particular hexactinellid groups are discussed below.
Family TEGANIIDAE de Laubenfels, 1955
Remarks. Finks and Rigby (2004) included the following taxa within the family Teganiidae: Teganium Walcott, 1879 (the type genus); Bulbospongia Rigby and Mehl, 1994; Echidnina Bengtson, 1986; Rhombodictyon Whitfield, 1886; Rufuspongia Rigby and Mehl, 1994; Taleolaspongia Rigby and Mehl, 1994; and Teganiella Rigby, 1986. Some of these taxa (Bulbospongia, Rufuspongia and Taleolaspongia) are known only from Devonian strata of Laurentia (Rigby and Mehl, 1994; Finks and Rigby, 2004). Teganiella, as stated elsewhere in this manuscript, is previously known from Devonian and Carboniferous rocks of the USA and Teganium is an Ordovician genus. Rhombodictyon is known from Ordovician strata of Laurentia (Whitfield, 1886; James, 1891; the Devonian stratigraphic range given by Finks and Rigby, 2004, is clearly a typographical error), plus one record from Carboniferous rocks of Poland (Hurcewicz and Czarniecki, 1985). However, we note that Rhombodictyon was considered to be an inorganic structure by Ruedemann (1925a). Resolving the affinity of this taxon would require restudy of the type material and is beyond the scope of this study. Echidnina consists of tiny (210–300 µm diameter) globular spicular bodies (Bengtson, 1986) and is generally considered to be a radiolarian (e.g., Zhang et al., 2021). Thus, the family is primarily Devonian, with some examples from Ordovician and Carboniferous rocks.
Genus TEGANIUM Rauff, 1894
Diagnosis. (Emended after diagnosis of Teganiella, Finks and Rigby, 2004.) Sphaeroidal reticulosans with thin wall composed of minute hexactin-based spicules in mostly continuous but irregularly oriented reticulate array, and choanosomal body wall with small, circular internal cavities; circular osculum is in some species lined by dense array of fine marginalia.
Type species. Cyathophycus subsphaericus Walcott, 1879, by original designation.
Other species. Teganiella heathi Rigby, 1986; Tl. ovata Rigby and Mehl, 1994; Teganium avalonensis sp. nov.; questionably also Tl. finksi Mouro et al., 2019.
Remarks. Separation of Teganiella from Teganium is highly problematic and is here considered invalid. The structure of the skeleton and soft tissue in the new species is remarkably similar to that described for Teganium (Walcott, 1879; Rauff, 1894; Finks and Rigby, 2004), only lacking the presence of abundant prostalia (Hall and Clarke, 1899). The absence of an osculum was stated by Finks and Rigby (2004) to be a defining feature of the genus Teganium. However, the original description of Cyathophycus subsphaericus Walcott, 1879 (the type species of Teganium), not only states that there is an osculum in many specimens but also gives measurements (9 mm diameter for a 30 mm-diameter sponge). Hall and Clarke (1899) illustrated several specimens that all apparently lack oscules, but the drawings alone are inconclusive. Finks and Rigby (2004) confirmed the non-existence of an osculum based on only the holotype, and it is likely that in this specimen it is concealed by an awkward angle of flattening. In particular, if the holotype is vertically compressed and viewed from below, this could have both obscured the osculum and potentially also given the appearance of abundant lateral prostalia due to flattening of the radial array of basalia. The original description of Walcott (1879) makes it clear that the type species possesses an osculum and lacks prominent lateral prostalia.
In their species Teganiella finksi, Mouro et al. (2019) noted that although no osculum was visible in the moderately poor material, some specimens have a flattened summit that may indicate its presence and position; this is entirely normal for a laterally compressed reticulosan. Given the requirement for at least one exhalent opening in order for the sponge to function, this apparent lack of any terminal aperture is probably due to inconvenient orientation of compression in a globose sponge with a strongly narrowed osculum, to the extent that it is not clearly visible. It, therefore, appears that Teganium and Teganiella are identical, and given that the latter is well defined and the former (which would be the senior synonym) is more problematic in (according to some authors only) lacking an osculum, the genera should be considered identical and are here formally synonymized.
An assessment of previously known species of Teganiella (here transferred to the senior synonym Teganium) was provided by Mouro et al. (2019), who erected the species Tl. finksi. The generic definition is revised here, due to some confusion in Finks and Rigby (2004), in which the familial diagnosis contradicts the generic diagnosis in mistakenly requiring the absence of an osculum. The first two named species of Teganiella (Tl. heathi Rigby, 1986 and Tl. ovata Rigby and Mehl, 1994), both show a clear osculum, in at least the latter surrounded by marginalia. The current species is closest to Tl. ovata, which differs in some ways from the type species, but is close enough to support congeneric status.
Stratigraphic range. The genus is previously known from Upper Ordovician, Devonian and Carboniferous rocks of the USA (Laurentia); the new species extend this to the Middle Ordovician of Avalonia.
Teganium avalonensis sp. nov.
Figure 2, Figure 3, Figure 4, Figure 5
zoobank.org/ A5C8BA9B-C4D6-4404-8150-CC7C75F6BBDF
Name. After the geographic origin, the palaeocontinent Avalonia.
Diagnosis. Oval to rounded quadrate Teganium with pronounced, slightly inwardly-directed marginalia surrounding broad osculum, and fine skeleton composed of numerous very small hexactins and derivatives; outer (dermal or hypodermal) layer with a semi-regular reticulation and inner layer with numerous, densely-packed, rounded cavities.
Holotype. NMW.2021.3G.71, large complete specimen with marginalia and basalia, but generally lacking fine skeletal detail.
Paratypes. NMW.2021.3G.72, pair of sponges with well-preserved marginalia but little fine skeletal detail; NMW.2021.3G.73, large complete example; NMW.2021.3G.74, small partial specimen showing oscular margin; NMW.2021.3G.77, the largest complete specimen; NMW.2021.3G.78, three moderate-size specimens; NMW.2021.3G.79, one small and relatively elongate specimen; NMW.2021.3G.80, small complete specimen; NMW.2021.3G.81, cluster of four moderately large specimens; NMW.2021.3G.82, partial specimen with well-preserved fine detail of body wall.
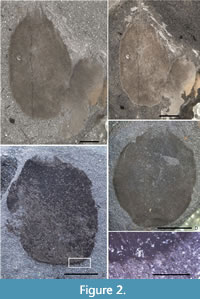 Description. Sponges are oval, slightly taller than wide, in some cases with somewhat squared outline showing a flattened base (Figure 2C). Specimens up to 14 mm wide and 16 mm tall (excluding marginalia). Osculum of holotype (Figure 2C) 4 mm wide, and proportionally similar in other specimens, being approximately one-third of maximum width; where basal margin is flattened, this covers approximately half of maximum sponge width. In most cases the specimens are preserved in a thick film of carbon and/or iron minerals, but in some specimens, body wall structure is preserved (Figure 3C–F). Wall thickness visible at the lateral margins in several specimens (including holotype, Figure 2C, and in Figure 3C–D), and is irregular but normally up to 0.5 mm thick.
Description. Sponges are oval, slightly taller than wide, in some cases with somewhat squared outline showing a flattened base (Figure 2C). Specimens up to 14 mm wide and 16 mm tall (excluding marginalia). Osculum of holotype (Figure 2C) 4 mm wide, and proportionally similar in other specimens, being approximately one-third of maximum width; where basal margin is flattened, this covers approximately half of maximum sponge width. In most cases the specimens are preserved in a thick film of carbon and/or iron minerals, but in some specimens, body wall structure is preserved (Figure 3C–F). Wall thickness visible at the lateral margins in several specimens (including holotype, Figure 2C, and in Figure 3C–D), and is irregular but normally up to 0.5 mm thick.
 Internal organisation of wall (preserved as denser brown material representing soft tissue; Figure 3C, F) with numerous rounded polygonal to circular cavities or exhalent canals (leading into central atrium) on similar scale to primary skeletal grid (c. 0.1–0.4 mm). In most areas (perhaps excluding base and extreme apex), tissue regions between spaces are narrower than the cavities, with a 0.1 mm-thick wall.
Internal organisation of wall (preserved as denser brown material representing soft tissue; Figure 3C, F) with numerous rounded polygonal to circular cavities or exhalent canals (leading into central atrium) on similar scale to primary skeletal grid (c. 0.1–0.4 mm). In most areas (perhaps excluding base and extreme apex), tissue regions between spaces are narrower than the cavities, with a 0.1 mm-thick wall.
 Skeleton faintly preserved due to delicate spiculation, but fine dimpled texture reflecting the primary external grid is visible over surface (Figure 3B); spicules are visible when wet, but specimens are prone to cracking in contact with water. Dimples, representing spaces between spicules, are 0.1–0.2 mm wide and often arranged in sinuous lines resulting from semi-regular spicule grid. Grid is dominantly diagonal, but irregular. Spicules (Figure 3D–E) are simple triaxons, presumed to be hexactins but with gastral and distal rays not confirmed. Ray length typically up to 0.2 mm and extremely fine. The appearance of longer spicules in low-angle light is a result of relief due to locally aligned skeletal grid. Finest spicules appear to be randomly arranged between the larger spicules, including over the spaces within the primary grid, and over cavities preserved in paratype, implying that these occupy an outer layer (probably hypodermal or dermal) overlying internal spaces.
Skeleton faintly preserved due to delicate spiculation, but fine dimpled texture reflecting the primary external grid is visible over surface (Figure 3B); spicules are visible when wet, but specimens are prone to cracking in contact with water. Dimples, representing spaces between spicules, are 0.1–0.2 mm wide and often arranged in sinuous lines resulting from semi-regular spicule grid. Grid is dominantly diagonal, but irregular. Spicules (Figure 3D–E) are simple triaxons, presumed to be hexactins but with gastral and distal rays not confirmed. Ray length typically up to 0.2 mm and extremely fine. The appearance of longer spicules in low-angle light is a result of relief due to locally aligned skeletal grid. Finest spicules appear to be randomly arranged between the larger spicules, including over the spaces within the primary grid, and over cavities preserved in paratype, implying that these occupy an outer layer (probably hypodermal or dermal) overlying internal spaces.
Marginalia (Figure 2A, C–D and Figure 3A) consistently around 2 mm long (even in largest specimen, implying a maximum length reached early), fine (less than 0.1 mm wide), with bases embedded in body wall; they are either diactins or greatly extended vertical rays of hexactins. Rays are straight and inclined inwards at around 45–60°, forming a barrier around sides of slightly depressed osculum. No lateral prostalia or even projecting distal rays are visible, although the lateral margin of flattened sponges is slightly rough; the preservation is not detailed enough to demonstrate conclusively whether any of the smaller dermal/hypodermal spicules had any rays projecting.
Basalia (Figure 2C, E) preserved in holotype as bases of coarse spicules protruding into sediment. Spicules 0.1–0.2 mm wide, extending unknown distance into sediment, and are less well preserved with increasing distance from the sponge body (a documented feature of sponge preservation in, for example, the Anji Biota; see Botting et al., 2020). Basalia appear to have been numerous, extending into sediment from the entire basal part, equating approximately to the flattened region of the body.
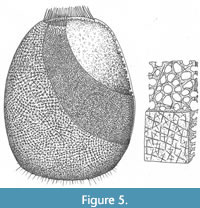 Remarks. A reconstruction of the sponge is shown in Figure 5. The new species differs from all others that have been assigned to the genus in the pronounced marginalia, and in the rather more quadrate shape, but in other respects most closely resembles Teganium ovata (Rigby and Mehl, 1994)–including the existence of a similar array of basalia. Teganium heathi (Rigby, 1986) appears to show no marginalia (a possible taphonomic artefact) and also possessed coarser body wall spicules, and meridional tracts of spicules or spicule rays that are not present in other species. The species described as T. finksi (Mouro et al., 2019) lacks any visible osculum or marginalia and includes much coarser spicules, in some cases up to 2 mm across.
Remarks. A reconstruction of the sponge is shown in Figure 5. The new species differs from all others that have been assigned to the genus in the pronounced marginalia, and in the rather more quadrate shape, but in other respects most closely resembles Teganium ovata (Rigby and Mehl, 1994)–including the existence of a similar array of basalia. Teganium heathi (Rigby, 1986) appears to show no marginalia (a possible taphonomic artefact) and also possessed coarser body wall spicules, and meridional tracts of spicules or spicule rays that are not present in other species. The species described as T. finksi (Mouro et al., 2019) lacks any visible osculum or marginalia and includes much coarser spicules, in some cases up to 2 mm across.
The dense association of multiple specimens on several slabs is typical of the genus (Rigby and Mehl, 1994) and appears to be a consistent ecological feature. In many (but not all) examples, there is little variation in size in such assemblages at Castle Bank, implying that the sponges were approximately the same age; they may therefore represent a colonisation event, as discussed for other sponges found as similar dense assemblages by Botting (2016) and Botting et al. (2020).
Occurrence. Known only from the Middle Ordovician (Didymograptus murchisoni Biozone) of Castle Bank, Llandrindod, UK; Gilwern Volcanic Formation.
Teganium sp.
Figure 6
Material. NMW.2021.3G.76, two adjacent specimens, partially overlying.
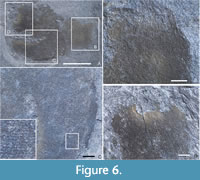 Description. Two specimens, effectively unweathered, adjacent and pointing in opposite directions (Figure 6A); one slightly overlies the other. Information is based primarily on the first (Figure 6B-C), which shows a near-complete outline, especially at the apex; the other has damage to apex and edges, but confirms observations based on the first.
Description. Two specimens, effectively unweathered, adjacent and pointing in opposite directions (Figure 6A); one slightly overlies the other. Information is based primarily on the first (Figure 6B-C), which shows a near-complete outline, especially at the apex; the other has damage to apex and edges, but confirms observations based on the first.
The better-preserved specimen is 24 mm tall, conical and slightly sinuous, with maximum width 7 mm, at or near apex, with upper half of approximately even width. Lower half tapers from 5 mm width at mid-height to rounded point. Second specimen also 7 mm wide at apex, total length approximately equal to first, but exact base invisible. Apex narrows in uppermost millimetre such that osculum occupies around 80% of full width of sponge, surrounded by dense array of marginalia, 2 mm long (0.02–0.07 mm thick), converging (and sometimes curving) somewhat inwards.
Wall thickness unclear and irregular, but indicated by dark line at edges (representing more massive preservation of soft tissues; Figure 6C), approximately 0.5–1.0 mm thick. Mineralised film over body wall shows irregular mottled patterning (Figure 6B–C) indicating relatively coarse internal cavernosity with cavities typically 0.5–1.0 mm across and separated by bands of more continuous tissue typically 0.5 mm wide. Finer cavities not visible, but may have been present originally.
Skeletal detail not preserved except for reticulation of dermal skeleton, seen in low-angle light and especially clear in mid-height area of better specimen (Figure 6C): a semi-regular grid, roughly longitudinal and transverse, with cell size approximately 0.1 mm or slightly less. Individual body wall spicules not preserved (invisible even when wet). No basalia visible.
Remarks. At present, these two specimens are distinguished from other members of the genus largely by the shape; although internal wall structure also appears to be coarser, it is poorly preserved. It shares the distinctive appearance of the finely reticulate surface with other species, and shares the convergent, dense fringe of marginalia with T. avalonensis sp. nov. The unweathered state of the material obscures many of the fine details of the skeleton, which cannot therefore be compared in detail with other species. The same is true for previously described species (Mouro et al., 2019), however, and the key characters are preserved well enough to allow an unambiguous assignment to the genus. The species is left in open nomenclature because the sample size is too small to reject the possibility of an extreme variation of shape within T. avalonensis sp. nov.
DISCUSSION
Phylogenetic Affinity
Morphologically simple hexactin-bearing sponges are difficult to classify in relation to the hexactinellid crown or total group, due to poor knowledge of the sequence of character acquisition in hexactinellid (and deeper silicean) evolution, and limited information in the preserved fossils. The morphological information preserved in the new species clarifies some aspects of the skeleton of Teganium, together with the complexity of the body wall and internal cavity and canal organisation. These features allow a comparison with similarly aged fossil taxa that have a better-constrained phylogenetic position, as well as potentially allowing direct comparisons with modern taxa.
Extant Hexactinellida are a highly derived group, which itself includes some even more derived characteristics. In several lineages, fused choanosomal skeletons appear to have evolved independently (Dohrmann et al., 2017), and these lineages have in many cases reduced other features of their construction. Allowing for such secondary reduction, comparison of the extant unfused or partly-fused taxa in the Lyssacinosida and the Amphidiscophora show that a complex skeletal wall is shared across the class (Dohrmann et al., 2017). In particular, there are multiple skeletal layers (Leys et al., 2007) that were probably present in the last common ancestor (LCA) of extant hexactinellids: dermalia (on the outer surface), hypodermalia (within the surface 'skin' layer), choanosomalia and usually gastralia/atrialia (marking the inner margin of the body wall). These layers normally show clearly distinct spicule morphologies and distinct arrangements of those spicules (e.g., Leys et al., 2007). The choanosomal layer shows the most complex architecture, as it houses the choanocyte chambers (that are too small to fossilize commonly), which lead into exhalent canals that feed into the internal cavity of the sponge, prior to water being expelled through the osculum. These canals are reasonably large (e.g., Reiswig and Mehl, 1991) and easily fossilizable as gaps in the skeleton or soft tissues. Such canals and chambers also seem to be a universal feature of modern hexactinellids (except where secondarily lost), and were probably present in their LCA.
In contrast, many of the hexactin-bearing reticulosan sponges described from the Palaeozoic fossil record show a much simpler construction, and there are multiple lines of evidence indicating that hexactins pre-date the Hexactinellida and are likely plesiomorphic, being present even in the LCA of Porifera (Botting and Muir, 2018). The simplest reticulosans, such as Diagoniella and allies, show only a single layer of spicules, which may possibly be homologous with the hypodermalia of hexactinellids (although this is yet to be properly assessed). In any case, multiple skeletal layers are not developed, and neither is obvious cavernosity or canals within the body wall; it is likely that at least some of these sponges were asconoid in construction (Rigby, 1978), with the choanosomal surface covering the interior of the body wall, rather than being in specialised chambers. These sponges, without any complex architecture to the body wall, are almost certainly deeply nested within Porifera, prior to the crown group of Silicea (Botting and Muir, 2018). One early, hexactin-bearing sponge with calcite spicules that does not fall into modern crown-group classes indicates that choanocyte chambers developed reasonably early in sponge evolution, at least in one lineage (Nadhira et al., 2019).
The separation of hexactinellids from demosponges (together forming Silicea) appears to be most closely seen in more derived reticulosans such as Cyathophycus (Botting and Muir, 2013, 2018). An inner skeletal layer of smaller spicules in a separate arrangement had also developed, including visible internal cavities or canals within the thin body wall, showing that the basic structure of a differentiated wall had evolved by this point. Primitive characteristics were still present, however, in the thin wall, the regular and simple morphology of the spicules forming the primary skeletal layer, and their simple organisation into a square grid aligned to the body axes and with semi-regular quadruling. Furthermore, the spicule construction was shown to be anomalous (Botting and Muir, 2013), with thick organic layers within the silica; this is a primitive trait that was also found in Cambrian hexactins by Tang et al. (2019).
Based on these key points, the development of a full suite of skeletal layers, including increased modification of spicules and loss of simple regularity in the primary grid, combined with thickening of the body wall (in many taxa) and increased complexity of the internal canal architecture, must all have developed within the stem group of Hexactinellida. These features are recognisable in well-preserved fossils. The evolution of the two main groups of microscleres (amphidiscs and hexasters) must also have occurred either within the stem group, or independently within the two subclasses of the crown group, but these spicules are rarely preserved as fossils.
In Teganium, the presence of a dermal or hypodermal skeleton that overlies small internal cavities surrounded by a very fine hexactin-based choanosomal skeleton is a relatively derived feature, although superficially similar to Cyathophycus (Botting and Muir, 2013). Teganium differs from Cyathophycus, however, in the irregularity of the primary skeleton, which is not tied to the sponge axis, and does not show quadruling; this is much more similar to the dermal surface structure seen in many extant hexactinellids (e.g., Chonelasma; Shen et al., 2022). Among known Palaeozoic fossils, Teganium is in some ways similar to the Hirnantian stem-group rossellids Matteolaspongia Botting, Zhang and Muir, 2018 and Shouzhispongia Botting, Janussen, Zhang and Muir, 2020; the latter also possessed similarly minute spicules. Unlike those rossellids, there are no pentactine prostalia in Teganium, but the presence of marginalia is similar, and numerous long basalia are also common among the Rossellidae and related families (Tabachnick, 2002). There are still uncertainties and limitations on our understanding of Teganium’ s morphology, however, due to the minute size of the spicules. At this stage, a relatively derived position within the stem group or crown group of Hexactinellida is the most plausible interpretation, but the similarities to early rossellids may be convergent, and result from the limitations imposed by possession of such small spicules. Recognition of a relatively derived position for Teganium within hexactinellid evolution is useful, but a more detailed assignment will depend on better understanding of character evolution around the base of the hexactinellid crown group. It should also be noted that Teganiella was assigned by Mehl (1996) to her ‘Rossellimorpha’: a loosely-defined grouping of relatively complex, loose-spiculed sponges that may include taxa related to the Rossellidae, or may be dominated by earlier-branching forms, generating a similar ambiguity to our assessment here.
Palaeobiogeography
The fossil record of sponges with loose spicules is known to be extremely incomplete (Pisera, 2004, 2006; Muir et al., 2013), and much of the known record is based on a small number of faunas with some degree of exceptional preservation. Where multiple Ordovician faunas are known in a small area such as the Builth Inlier, there tends to be little taxonomic overlap, implying a very high total diversity, but localised occurrence of each taxon (Muir and Botting, 2015). This ecological disparity is a feature of Ordovician faunas, but not Cambrian assemblages (Botting and Muir, 2019), and cannot be due to the lack of Burgess Shale-type faunas; many sponge faunas are known from Ordovician rocks, with or without soft tissue preservation. Genera and species that occur in multiple Ordovician sites and regions can therefore be assumed to have been very common and widespread, even though the full extent of their geographical and stratigraphic ranges cannot be assessed with confidence. As these species are the most likely to be informative for palaeobiogeographic patterns, significant range extensions are of great interest, especially if they are accompanied by unexpected discrepancies in palaeoenvironment. This is the case here, with a range extension to a second palaeocontinent, an older age, and confirmation of the deeper-water environment indicated by the type species.
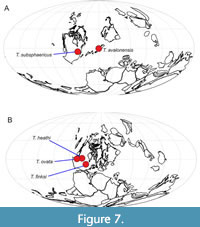 Previous records of taxa assigned to Teganiella have been limited to relatively shallow environments from the Devonian and Carboniferous of Laurentia, across several modern US states (Figure 7). Teganium heathi is from the Bashkirian (Carboniferous) of Montana (Rigby, 1986); the other Carboniferous occurrence is from the upper Moscovian of Indiana (Mouro et al., 2019). There has been one report of the genus (T. ovata) from the Middle Devonian of Nevada (Rigby and Mehl, 1994). Earlier members of the genus have been assigned to Teganium, originally described from the Upper Ordovician Utica Shale of New York State (Walcott, 1879). Other species of Teganium were named by Ruedemann (T. macrosclera Ruedemann, 1925b; T. rauffi Ruedemann, 1925b; T. minutum Ruedemann, 1934; and T. merino Ruedemann, 1942) from the Utica Shale and other Ordovician units in New York State. Ruedemann also named a Silurian species of Teganium, T. claviforme Ruedemann, 1934, from Silurian strata of Klakes Bay, Alaska. Unfortunately, it is not possible to confirm the assignment of any of the material of Ruedemann (1925b, 1934, 1942) to Teganium on the basis of his illustrations or descriptions, and so all of these records must be regarded as doubtful pending restudy of the type specimens of each species. To summarise, the only certain record of a species previously assigned to Teganium is the type species from the Utica Shale of New York State; most of the other records (which are doubtful) are also from Ordovician strata of the same state. This distribution, including the new species in offshore sediments of Avalonia, suggests an origin of the genus and family in deeper waters (either mid to deep shelf, or below), perhaps within the Iapetus Ocean region, before the lineage became specialised for inhabiting sheltered, shallow-water facies in Laurentia. The morphology is not particularly useful in interpreting such habitat preferences, but the small size, subspherical shape and very small spicules form a distinctive combination. The small size of spicules may be an adaptation to the limitation of dissolved silica in shallow environments, which is known to impact spicule form in modern taxa (Maldonado, 1999; Shetye et al., 2014), but no clear inferences can be made.
Previous records of taxa assigned to Teganiella have been limited to relatively shallow environments from the Devonian and Carboniferous of Laurentia, across several modern US states (Figure 7). Teganium heathi is from the Bashkirian (Carboniferous) of Montana (Rigby, 1986); the other Carboniferous occurrence is from the upper Moscovian of Indiana (Mouro et al., 2019). There has been one report of the genus (T. ovata) from the Middle Devonian of Nevada (Rigby and Mehl, 1994). Earlier members of the genus have been assigned to Teganium, originally described from the Upper Ordovician Utica Shale of New York State (Walcott, 1879). Other species of Teganium were named by Ruedemann (T. macrosclera Ruedemann, 1925b; T. rauffi Ruedemann, 1925b; T. minutum Ruedemann, 1934; and T. merino Ruedemann, 1942) from the Utica Shale and other Ordovician units in New York State. Ruedemann also named a Silurian species of Teganium, T. claviforme Ruedemann, 1934, from Silurian strata of Klakes Bay, Alaska. Unfortunately, it is not possible to confirm the assignment of any of the material of Ruedemann (1925b, 1934, 1942) to Teganium on the basis of his illustrations or descriptions, and so all of these records must be regarded as doubtful pending restudy of the type specimens of each species. To summarise, the only certain record of a species previously assigned to Teganium is the type species from the Utica Shale of New York State; most of the other records (which are doubtful) are also from Ordovician strata of the same state. This distribution, including the new species in offshore sediments of Avalonia, suggests an origin of the genus and family in deeper waters (either mid to deep shelf, or below), perhaps within the Iapetus Ocean region, before the lineage became specialised for inhabiting sheltered, shallow-water facies in Laurentia. The morphology is not particularly useful in interpreting such habitat preferences, but the small size, subspherical shape and very small spicules form a distinctive combination. The small size of spicules may be an adaptation to the limitation of dissolved silica in shallow environments, which is known to impact spicule form in modern taxa (Maldonado, 1999; Shetye et al., 2014), but no clear inferences can be made.
Given the number of occurrences of the Teganium complex in Laurentia (and now Avalonia), it is surprising that no records are known from other palaeocontinents, at least of the earlier, offshore representatives. The only similarly widely distributed genus of Ordovician and later reticulosan is Cyathophycus, which is known widely from the Iapetus region, across a range of palaeolatitudes (Muir et al., 2013). However, these records are often taxonomically uncertain outside of the Laurentia–Avalonia area (Muir et al., 2013), enhancing the similarity to the distribution of Teganium.
CONCLUSIONS
A new species of Teganium (which is here synonymized with Teganiella) from the Castle Bank Biota is the oldest known, the second from offshore environments, and the first from outside Laurentia; a second, unnamed species demonstrates diversification at this time. This discovery suggests that this successful later-Palaeozoic group of sponges originated in deeper-water environments, probably within the Iapetus region, before becoming specialised for sheltered inshore environments in Laurentia. New information on the detailed morphology of Teganium from Castle Bank (including fine-scale cavernosity and at least two skeletal layers) supports a hexactinellid affinity for the group, either in the later part of the stem group or possibly within the total-group of Lyssacinosida. However, in the absence of clear links to known hexactinellid taxa, no further detail of the specific classification is possible at this stage.
ACKNOWLEDGEMENTS
The support of the landowners is gratefully acknowledged, as are all contributors to a crowdfunding appeal (including a Holloway Bursary from the Warwickshire Geological Conservation Society) that allowed the purchase of photomicroscope equipment for use by JPB and LAM. JPB and LAM were funded by Chinese Academy of Sciences PIFI fellowships (2020VCB0013 and 2018VCB0014, respectively). We thank C. Howells, Amgueddfa Cymru for providing accession numbers for the Castle Bank specimens. This paper is a contribution to IGCP project 735 “Rocks and the Rise of Ordovician Life: Filling knowledge gaps in the Early Palaeozoic Biodiversification.” We thank Carolin Haug (the Executive Editor), Lukáš Laibl (Handling Editor), and two anonymous reviewers for their useful comments.
REFERENCES
Bengtson, S. 1986. Siliceous microfossils from the Upper Cambrian of Queensland. Alcheringa 10:195–216. https://doi.org/10.1080/03115518608619155
Botting, J.P. 2004. An exceptional Caradoc sponge fauna from the Llanfawr Quarries, central Wales and phylogenetic implications. Journal of Systematic Palaeontology, 2:31–63. https://doi.org/10.1017/S147720190300110X
Botting, J.P. 2016. Diversity and ecology of sponges in the Early Ordovician Fezouata Biota, Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology, 460:75–86. https://doi.org/10.1016/j.palaeo.2016.05.018
Botting, J.P. 2021. Hexactins in the ‘protomonaxonid’ sponge Choiaella and proposal of Ascospongiae (class nov.) as a formal replacement for the Protomonaxonida. Bulletin of Geosciences, 96:265–277. https://doi.org/10.3140/bull.geosci.1823
Botting, J.P. and Ma, J.-Y. 2022. A probable hyalonematid sponge (Hexactinellida: Amphidiscophora) from the Middle Ordovician of the Builth Inlier, Wales. Palaeoworld, 31:621–632. https://doi.org/10.1016/j.palwor.2022.01.011
Botting, J.P. and Muir, L.A. 2013. Spicule structure and affinities of the Late Ordovician hexactinellid‐like sponge Cyathophycus loydelli from the Llanfawr Mudstones Lagerstätte, Wales. Lethaia, 46:454–469. https://doi.org/10.1111/let.12022
Botting, J.P. and Muir, L.A. 2018. Early sponge evolution: a review and phylogenetic framework. Palaeoworld, 27:1–29. https://doi.org/10.1016/j.palwor.2017.07.001
Botting, J.P. and Muir, L.A. 2019. Dispersal and endemic diversification: differences in non-lithistid spiculate sponge faunas between the Cambrian Explosion and the GOBE. Palaeoworld, 28:24–36. https://doi.org/10.1016/j.palwor.2018.03.002
Botting, J.P. and Peel, J.S. 2016. Early Cambrian sponges of the Sirius Passet Biota, North Greenland. Papers in Palaeontology, 2:463–487. https://doi.org/10.1002/spp2.1048
Botting, J.P., Muir, L.A., and Lefebvre, B. 2013. Echinoderm diversity and environmental distribution in the Ordovician of the Builth Inlier, Wales. Palaios, 28:293–304. https://doi.org/10.2110/palo.2012.p12-118r
Botting, J.P., Janussen, D., Zhang, Y.D., and Muir, L.A. 2020. Exceptional preservation of two new early rossellid sponges: the dominant species in the Hirnantian (Late Ordovician) Anji Biota of China. Journal of the Geological Society, 177:1025–1038. https://doi.org/10.1144/jgs2020-002
Botting, J.P., Muir, L.A., Pates, S., McCobb, L.M.E., Wallet, E., Willman, S., Ma, J.Y., and Zhang, Y.D. 2023. A Middle Ordovician Burgess Shale-type fauna from Castle Bank, Wales (UK). Nature Ecology and Evolution, 7:666–674. https://doi.org/10.1038/s41559-023-02038-4
Carrera, M.G. and Rigby, J.K. 1999. Biogeography of Ordovician sponges. Journal of Paleontology, 73:26–37. https://doi.org/10.1017/S0022336000027517
de Laubenfels, M.W. 1955. Porifera, p. E21–E122. In Moore, R.C. (ed.), Treatise on Invertebrate Paleontology, Pt. E. Geological Society of America, Boulder, and University of Kansas Press, Lawrence, 122 pp.
Dohrmann, M., Kelley, C., Kelly, M., Pisera, A., Hooper, J.N., and Reiswig, H.M. 2017. An integrative systematic framework helps to reconstruct skeletal evolution of glass sponges (Porifera, Hexactinellida). Frontiers in Zoology, 14:1–31. https://doi.org/10.1186/s12983-017-0191-3
Finks, R.M. and Rigby, J.K. 2004. Paleozoic Hexactinellids, p. E319–E556. In Kaesler, R.L. (ed.), Treatise on Invertebrate Paleontology, Pt. E. Porifera (revised) 3. Geological Society of America, Boulder, and University of Kansas Press, Lawrence.
Grant, R.E. 1836. Animal kingdom, p. 107–118. In Todd, R.B. (ed.), The encyclopedia of anatomy and physiology, vol. 1. London: Sherwood, Gilbert and Piper.
Hall, J. and Clarke, J.M. 1899. A memoir on the Palaeozoic reticulate sponges constituting the family Dictyospongidae. Wynkoop Hallenbeck Crawford, New York.
Hurcewicz, H. and Czarniecki, S. 1985. Lyssakidae sponges from the Carboniferous Limestone and the Culm of southern Poland and their environmental differentiation. Annales Societatis Geologorum Poloniae, 55:333–354.
James, J.F. 1891. Manual of the Paleontology of the Cincinnati Group. Part I. Journal of the Cincinnati Society of Natural History, 14:45–72.
Jeon, J., Li, Q.J., Na, L., Liang, K., and Zhang, Y.D. 2022. Skeletal variation in the early clathrodictyid stromatoporoids of Upper Ordovician and its paleoecological and phylogenetic implications. Palaeoworld, 31:58–68. https://doi.org/10.1016/j.palwor.2021.01.005
Leys, S.P., Mackie, G.O., and Reiswig, H.M. 2007. The biology of glass sponges. Advances in Marine Biology, 52:1–145. https://doi.org/10.1016/S0065-2881(06)52001-2
Maldonado, M., Carmona, M.C., Uriz, M.J., and Cruzado, A. 1999. Decline in Mesozoic reef-building sponges explained by silicon limitation. Nature, 401:785–788. https://doi.org/10.1038/44560
Mehl, D. 1996. Phylogenie und Evolutionsökologie der Hexactinellida (Porifera) im Paläozoikum. Geologische und Paläontologische Mitteilungen der Universität Innsbruck , 4:1–55.
Mouro, L.D., Horodyski, R.S., Fernandes, A.C., Carvalho, M.A., Silva, M.S., Waichel, B.L., and Saldanha, J.P. 2019. Pennsylvanian sponge from the Mecca Quarry Shale, Carbondale Group (Indiana, USA) and the paleobiogeographic distribution of Teganiella in the paleoequatorial region of Laurentia. Journal of Paleontology, 93:827–838. https://doi.org/10.1017/jpa.2019.7
Muir, L.A. and Botting, J.P. 2015. An outline of the distribution and diversity of Porifera in the Ordovician Builth Inlier (Wales, UK). Palaeoworld, 24:176–190. https://doi.org/10.1016/j.palwor.2014.11.003
Muir, L.A., Botting, J.P., Carrera, M.G., and Beresi, M. 2013. Cambrian, Ordovician and Silurian non-stromatoporoid Porifera. In Harper D.A.T. and Servais T. (eds), Early Palaeozoic Palaeobiogeography and Palaeogeography. Geological Society, London, Memoirs, 38:81–95. https://doi.org/10.1144/M38.8
Müller, R.D., Cannon, J., Qin, X., Watson, R.J., Gurnis, M., Williams, S., Pfaffelmoser, T., Seton, M., Russell, S.H., and Zahirovic, S. 2018. GPlates: Building a virtual Earth through deep time. Geochemistry Geophysics Geosystems, 19:2243–2261. https://doi.org/10.1029/2018GC007584
Nadhira, A., Sutton, M.D., Botting, J.P., Muir, L.A., Gueriau, P., King, A., Briggs, D.E., Siveter, D.J., and Siveter, D.J. 2019. Three-dimensionally preserved soft tissues and calcareous hexactins in a Silurian sponge: implications for early sponge evolution. Royal Society Open Science, 6(190911):1–13. https://doi.org/10.1098/rsos.190911
Nestor, H. and Webby, B.D. 2013. Biogeography of the Ordovician and Silurian Stromatoporoidea. In Harper, D.A.T. and Servais, T. (eds.), Early Palaeozoic Palaeobiogeography and Palaeogeography. Geological Society, London, Memoirs, 38:67–79. https://doi.org/10.1144/M38.7
Pates, S., Botting, J.P., Muir, L.A., and Wolfe, J.M. 2022. Ordovician opabiniid-like animals and the role of the proboscis in euarthropod head evolution. Nature Communications, 13(6969):1–15. https://doi.org/10.1038/s41467-022-34204-w
Pisera, A. 2004. What can we learn about siliceous sponges from palaeontology. BMIB-Bollettino dei Musei e degli Istituti Biologici, 68:55–69.
Pisera, A. 2006. Palaeontology of sponges–a review. Canadian Journal of Zoology, 84:242–261. https://doi.org/10.1139/z05-169
Rauff, H. 1894. Palaeospongiologie, Erster oder allgemeiner Theil, und Zweiter Theil, erste Hälfte. Palaeontographica, 41:233–346, figs. 49–75, pls. 1–17.
Reiswig, H.M. and Mehl, D. 1991. Tissue organization of Farrea occa (Porifera, Hexactinellida). Zoomorphology, 110:301–311.
Rigby, J.K. 1978. Porifera of the Middle Cambrian Wheeler Shale, from the Wheeler Amphitheater, House Range, in western Utah. Journal of Paleontology, 52:1325–1345.
Rigby, J.K. 1986. The sponge fauna from the Mississippian Heath Formation of central Montana, p. 443–456, 5 figs., 2 pl. In Dutro, J.T., Jr. and Pfefferkorn, H.W. (eds.), Neuvième Congrès International de Stratigraphie et de Géologie du Carbonifère. Compte Rendu, vol. 5, Paleontology, Paleoecology, Paleogeography. Southern Illinois University Press, Carbondale & Edwardsville.
Rigby, J.K. and Collins, D. 2004. Sponges of the Middle Cambrian Burgess Shale and Stephen Formations, British Columbia. ROM Contributions in Science, 1:1–164.
Rigby, J.K. and Mehl, D. 1994. Middle Devonian sponges from the Northern Simpson Park Range, Nevada. Brigham Young University Geological Studies, 40:111–153.
Ruedemann, R. 1925a. The Utica and Lorraine Formations of New York. Part 1. Stratigraphy. New York State Museum Bulletin, 258:1–175.
Ruedemann, R. 1925b. The Utica and Lorraine Formations of New York. Part 2. Systematic Paleontology. No. 1. Plants, Sponges, Corals, Graptolites, Crinoids, Worms, Bryozoans, Brachiopods. New York State Museum Bulletin, 262:1–171.
Ruedemann, R. 1934. Paleozoic plankton of North America. Geological Society of America Memoir, 2:1–141. https://doi.org/10.1130/MEM2
Ruedemann, R. 1942. Cambrian and Ordovician fossils. New York State Museum Bulletin, 327:19–32.
Schmidt, O. 1870. Grundziige einer Spongien-fauna des Atlantischen Gebietes. Engelman, Leipzig.
Schofield, D.I., Davies, J.R., Waters, R.A., Wilby, P.R., Williams, M., and Wilson, D. 2004. Geology of the Builth Wells district: a brief explanation of the geological map sheet 196 Builth Wells. British Geological Survey, Keyworth, Nottingham.
Shen, C., Cheng, H., Zhang, D., and Wang, C. 2022. Two new species and one new genus of glass sponges (Hexactinellida: Euplectellidae and Euretidae), from a transect on a seamount in the Northwestern Pacific Ocean. Frontiers in Marine Science, 9(852498):1–12. https://doi.org/10.3389/fmars.2022.852498
Shetye, S., Mohan, R., Jafar, S.A., Nair, A., Patil, S., Jawak, S., Asthana, R., and Gazi, S. 2014. Diatom burst-driven silica depletion under the Antarctic sea ice: evidence from sponge spicules. Current Science, 107:273–277.
Tabachnick, K.R. 2002. Family Rossellidae, p. 1441-1505. In Hooper, J.N.A and van Soest, R.M.W. (eds.), Systema Porifera: a guide to the classification of sponges, volume 2. Kluwer Academic/Plenum Publishers, New York.
Tang, Q., Wan, B., Yuan, X., Muscente, A.D., and Xiao, S. 2019. Spiculogenesis and biomineralization in early sponge animals. Nature Communications, 10(3348):1–10. https://doi.org/10.1038/s41467-019-11297-4
Torsvik, T.H. and Cocks, L.R.M. 2016. Earth History and Palaeogeography. Cambridge University Press, Cambridge, UK. Data downloaded from http://www.earthdynamics.org/earthhistory/Data_Software.html, accessed 3 November 2019.
Walcott, C.D. 1879. Fossils of the Utica Slate. Transactions of the Albany Institute, 10:18–38.
Whitfield, R.P. 1886. Notice of a new fossil body, probably a sponge related to Dictyophyton. Bulletin of the American Museum of Natural History, 1:346–348
Wu, W., Zhu, M., and Steiner, M. 2014. Composition and tiering of the Cambrian sponge communities. Palaeogeography, Palaeoclimatology, Palaeoecology, 398:86–96. https://doi.org/10.1016/j.palaeo.2013.08.003
Zhang, Y., Feng, Q., Nakamura, Y., and Suzuki, N. 2021. Microfossils from the Liuchapo Formation: possible oldest radiolarians from deep-water chert and phylogenetic analysis. Precambrian Research, 362:106312. https://doi.org/10.1016/j.precamres.2021.106312

