 Biostratigraphy and biochronology of late Cenozoic North American rodent assemblages
Biostratigraphy and biochronology of late Cenozoic North American rodent assemblages
Article number: 26.2.a29
https://doi.org/10.26879/1303
Copyright Paleontological Society, August 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 30 April 2023. Acceptance: 9 July 2023.
ABSTRACT
Late Cenozoic rodent assemblages from western North America were added to a previous database limited to the central and eastern United States to develop a continental correlational system for the past 5 million years. Three hundred and twelve species representing 124 rodent assemblages were included, from which 12 Cenozoic Mammal Zones (CMZs) were constructed. Replacement chronologies for rodent assemblages based upon superposition, radiometric dates, paleomagnetic profiles, and evolutionary stages of dentitions from various depositional basins formed the skeleton for the chronological ordering of assemblages, and those lacking one or more information sources were sequenced based upon greatest concordance with available data. Arvicoline cricetids provided the most useful information for sequencing assemblages, followed by neotomine and sigmodontine cricetids and geomyids. The appearance of modern-sized cotton rats in CMZ 4, followed by the immigration of Allophaiomys across Beringia in CMZ 3, heralds the shift to the dominance of cotton rats and arhizodont voles in North American grassland ecosystems. No rapid bouts of significant turnover are associated with the beginning of the Pleistocene at 2.58 Ma (million years ago), but a pronounced turnover event was observed in the Meade Basin of southwestern Kansas immediately following the Huckleberry Ridge ash-fall at 2.07 Ma. Preliminary observations suggest two categories of rodent turnover; low-level background rotation determined by stochastic short-term regional and long-term global environmental change, and short-term turnover spikes mediated by catastrophic events such as volcanic eruptions.
Robert A. Martin. Department of Biological Sciences, Murray State University, Murray, Kentucky 42071, USA. rmartin@murraystate.edu
Thomas S. Kelly. Research Associate, Department of Vertebrate Paleontology, Natural History Museum of Los Angeles County, Los Angeles, California 90007, USA. tom@tskelly.gardnerville.nv.us
Keywords: North America, Neogene, Quaternary, mammal, rodent, biochronology
Final citation: Martin, Robert A. and Kelly, Thomas S. 2023. Biostratigraphy and biochronology of late Cenozoic North American rodent assemblages. Palaeontologia Electronica, 26(2):a29.
https://doi.org/10.26879/1303
palaeo-electronica.org/content/2023/3903-cenozoic-rodent-assemblages
Copyright: August 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
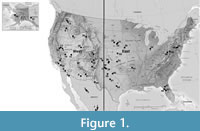 This study extends an earlier treatment of North American Late Neogene and Quaternary (~ last 5.0 My) fossil rodent assemblages from the eastern and central United States (Martin, 2019), adding regions of the continental United States, Canada and Mexico west of a line drawn roughly from western Texas north to western North Dakota (black line in Figure 1).
This study extends an earlier treatment of North American Late Neogene and Quaternary (~ last 5.0 My) fossil rodent assemblages from the eastern and central United States (Martin, 2019), adding regions of the continental United States, Canada and Mexico west of a line drawn roughly from western Texas north to western North Dakota (black line in Figure 1).
We recognize that in the future, paleontologists might choose to include other small or large mammal groups, and for that reason on the continental scale we have designated our categories as Cenozoic Mammal Zones (CMZs). Replacement chronologies for rodent assemblages based upon superposition, radiometric dates, paleomagnetic profiles, and evolutionary stages of dentitions from various depositional basins formed the skeleton for the chronological ordering of assemblages (Figure 2), and those lacking one or more information sources were sequenced based upon greatest concordance with available data. The use of many independently determined data sources minimized the amount of diachronism commonly associated with correlational unit boundaries (e.g., Ezquerro et al., 2022; Dam et al., 2023). Figure 1 provides locations of select pre-Rancholabrean rodent assemblages from the Supplementary Material table (color coding explained below). The primary purpose in developing a continental species-level rodent correlational scheme is to introduce into North American mammalian paleontology a system for the past five million years with at least the same refinement and utility as the MN/MQ Zones of Europe (Mein, 1975, 1976). A detailed chronology will provide a system within which mammalian assemblages without radiometric or paleomagnetic data may be tentatively sequenced and will consequently allow more accurate evaluation of mammal dispersal and evolutionary patterns.
 Martin (2019) reviewed the history, benefits, and limitations of previous mammalian biochronologic/biostratigraphic systems in North America, including North American Land Mammal Ages (NALMAs) and various regional and taxonomic systems (Schultz et al., 1977, 1978; Lundelius et al., 1987; Martin, 1979, 2003; Repenning, 1987; Bell, 2000; Woodburne, 2004a, b; Bell et al., 2004), and these studies can be consulted for comparison. The most complete depositional sequence in North America for the later Cenozoic is the Meade Basin system of southwestern Kansas (Figure 2), and that sequence is used as a primary base reference for North American continental correlations. While the stratigraphic positions of the Meade Basin fossil localities have been resolved, because of a lack of radiometric dates beneath the Huckleberry Ridge ash at 2.07 Ma (Singer, 2014) and minimal paleomagnetic refinement beneath the Blancan Bender site, ages of assemblages from older Pliocene sites have been determined primarily by biostratigraphic correlation. A particularly difficult issue has been the placement of a series of sites on the north bank of the Cimarron River, including Wiens, Vasquez/Newt, XIT1B, Raptor IC, and Ripley. The stratigraphic relationships of these localities are clear (Honey et al., 2005), but their placement in time is uncertain because all the sites and associated sediments display reversed paleomagnetic signatures. An argument based on biostratigraphy and field mapping suggested that the sites noted above could be allocated to C3n.2r of the Gilbert chron, between 4.48-4.30 Ma (Marcolini et al., 2008; Martin and Peláez-Campomanes, 2014). Recently, Lukens et al. (2019) plotted this set of sites at a younger date, in C2ar, between 4.30-3.60 Ma. Consequently, it is imperative to also consult other systems noted in Supplementary Material and Figure 2 that may have older levels of the Meade Basin better constrained.
Martin (2019) reviewed the history, benefits, and limitations of previous mammalian biochronologic/biostratigraphic systems in North America, including North American Land Mammal Ages (NALMAs) and various regional and taxonomic systems (Schultz et al., 1977, 1978; Lundelius et al., 1987; Martin, 1979, 2003; Repenning, 1987; Bell, 2000; Woodburne, 2004a, b; Bell et al., 2004), and these studies can be consulted for comparison. The most complete depositional sequence in North America for the later Cenozoic is the Meade Basin system of southwestern Kansas (Figure 2), and that sequence is used as a primary base reference for North American continental correlations. While the stratigraphic positions of the Meade Basin fossil localities have been resolved, because of a lack of radiometric dates beneath the Huckleberry Ridge ash at 2.07 Ma (Singer, 2014) and minimal paleomagnetic refinement beneath the Blancan Bender site, ages of assemblages from older Pliocene sites have been determined primarily by biostratigraphic correlation. A particularly difficult issue has been the placement of a series of sites on the north bank of the Cimarron River, including Wiens, Vasquez/Newt, XIT1B, Raptor IC, and Ripley. The stratigraphic relationships of these localities are clear (Honey et al., 2005), but their placement in time is uncertain because all the sites and associated sediments display reversed paleomagnetic signatures. An argument based on biostratigraphy and field mapping suggested that the sites noted above could be allocated to C3n.2r of the Gilbert chron, between 4.48-4.30 Ma (Marcolini et al., 2008; Martin and Peláez-Campomanes, 2014). Recently, Lukens et al. (2019) plotted this set of sites at a younger date, in C2ar, between 4.30-3.60 Ma. Consequently, it is imperative to also consult other systems noted in Supplementary Material and Figure 2 that may have older levels of the Meade Basin better constrained.
Despite the relatively complete Meade Basin and other Central Great Plains records, evidence is accumulating for a more complicated set of rodent dispersals with northern origins that may not be completely represented in the Meade Basin system and may be better represented in northwestern localities (e.g., Barnosky and Bell, 2004; Withnell et al., 2021). Also, because dispersal was generally from west to east, species appearances and durations in the western states differed from those in the Meade Basin. As in the case of Florida Pleistocene localities, there may have also been considerable regional endemism. Consequently, it will continue to be helpful to construct replacement chronologies from the depositional basin scale through continental geographic scales. An example application of a North American regional depositional system was a rodent replacement chronology developed for Florida (Martin, 2005) based in part on the work of Morgan and Hulbert (1995) and Morgan and White (1995). The recently published rodent chronology for the late Neogene of Spain (Dam et al., 2023) provides a blueprint for the application of many data sources to establishment of a regional biochronologic system.
MATERIALS AND METHODS
General Considerations
The difficulties associated with identifying CMZ boundaries primarily include problems with determining accurate depositional times for localities without radiometric dates and separated by considerable distance, sampling bias, taxonomic inaccuracies, and diachronic evolution (mosaic evolution) and dispersal. Attempts have been made to mitigate error by first establishing species replacement patterns in depositional sequences with radiometric dates and/or extensive paleomagnetic profiles (Figure 2). Dated immigration events provided further refinement, such as the Promimomys immigration event between 5.8-5.3 Ma (Martin, 2010; J. Martin et al., 2018) and the Allophaiomys immigration event at about 2.0 Ma (Martin et al., 2008). Entire species communities were consulted rather than relying on single species for correlational purposes; consequently, each CMZ is characterized by more than one taxon.
Following Tedford (1970), Woodburne (2004a, p. xiii) defines a “local fauna” as “An aggregate of fossil vertebrate species from a limited distribution in time from a number of closely grouped localities in a limited geographic area.... A local fauna could be based on taxa from a single locality.” Woodburne (2004a) also indicates that a “fauna” is often composed of a number of local faunas. We provide these definitions because there is considerable variation in the stratigraphic occurrence of species in Supplementary Material depending on collecting approaches. In the Meade Basin record, each named level represents a single quarry, generally no more than 1 m in depth, from which all identified species were collected. On the other hand, species in Supplementary Material from the Glenns Ferry Formation were collected from hundreds of localities along the Snake River in Idaho. We assume the resulting faunal associations can be roughly considered as communities extant during their respective intervals, and thus useful for biochronologic and biological comparisons because they are relatively consistent in composition, but some ambiguity must remain because of vertical dispersion of collecting localities and specimens collected as float. For reviews of paleontological work on the Glenns Ferry Formation, see Zakrzewski (1969), Neville et al. (1979), Conrad (1980), Repenning et al. (1995), Hart and Brueseke (1999), Sankey (1996), Hearst (1999), Ruez (2007, 2009), Walkup et al. (2016, 2021), and Prassack et al. (2021). In this paper the term “assemblage” is considered synonymous with both local fauna and locality, as the names of the assemblages are the same as the locality designations.
Species in Supplementary Material were taken from the literature, updated to modern taxonomic usage. In some cases, we used our personal experience to modify or question species determinations. We are preparing a study describing the Microtodon sp. noted in the Supplementary Material table for Hoye Canyon, Nevada. Supplementary Material includes full rodent assemblage records for select localities from North America, and the assemblage axis (columns) is color coded as follows: light green = west U.S., yellow = east U.S. NALMA boundaries, dated ashes and paleomagnetic events are identified in clear boxes, and significant faunal changes are identified by light tan boxes. Taxa from each assemblage are provided on the row axis, color-coded as follows: light green = west U.S., yellow = east U.S., and clear = recorded from both or unidentified to species. The column axis in Supplementary Material for the west U.S. begins with McKay Reservoir of Oregon and Hoye Canyon of Nevada, localities in the western group recording the Promimomys and Microtodon immigration events (Shotwell, 1956; Martin, 2010; J. Martin et al., 2018). Supplementary Material is meant to include enough assemblages to delimit broad CMZs to be used for correlational purposes, not to be exhaustive in locality or species coverage. We have not included the majority of late Pleistocene (Rancholabrean) assemblages, some of which undoubtedly include extant and possibly extinct species unrecorded in Supplementary Material (e.g., Wilson, 1967; Domning, 1969; Saunders, 1977; Graham et al., 1987; Jefferson, 1991; Jefferson et al., 1994, 2004; Gillette et al., 1999; Smith and Cifelli, 2000; Hill, 2001; Harington, 2009, 2011; Ferrusquía-Villafranca et al., 2010; Harris, 2014; Morgan and Harris, 2015). Many late Pleistocene cavern and sinkhole localities have produced rich rodent assemblages (e.g., Reddick [Gut and Ray, 1963] and other sites from Florida), but without radiometric dates, paleomagnetic profiles, or superpositional relationships with other sites it is unclear where they fit in the Late Pleistocene, and consequently they were omitted from Supplementary Material. Counting archeological sites, more than 100 late Pleistocene mammalian assemblages with extinct species are known from the Great Basin alone (Grayson, 2006). This treasure of data should be developed into an independent chronologically refined late Pleistocene database.
The Supplementary Material also includes the Little Sioux/Wright assemblages associated with the Lava Creek B (LCB) ash in Iowa. The latter was not covered in Martin (2019) and is included because it provides a rare glimpse of rodent diversity both above and beneath the same ash. The rodent records from Leisey Shell Pit, Florida (Morgan and White, 1995) and the Gilliland assemblage of Texas (Dalquest and Carpenter, 1988) were also added. Thomomys orientalis was added to the Coleman 2A, Florida, assemblage following Wilkins (1985).
Most of the rodent assemblages included in Supplementary Material are not bracketed by radiometric dates, although a few are found in proximity to a dated volcanic level. The majority of the assemblages are sequenced based on a few radiometric anchoring points, paleomagnetic profiles, evolutionary stages represented by rodent dentitions, and, where present, stratigraphic superposition. Paleomagnetic data are absent or limited for some sites, and the latter are sequenced exclusively on the rodent species represented in the larger context of continental data. Consequently, the order of assemblages listed in Supplementary Material within a given CMZ, unless including two or more assemblages with stratigraphic data (e.g., 111 Ranch [lower]; 111 Ranch [upper] or any of the Meade Basin sites) should not be considered as a confirmed chronological sequence. The LSDs (lowest stratigraphic datums) and HSDs (highest stratigraphic datums) in Figure 2 represent depositional basin records, not North American limits, although that may be the case as well. Unless determined from a radiometric date or coinciding with a paleomagnetic boundary, CMZ and boundary dates are approximations.
Many of the late Pleistocene assemblages derive from isolated sites, predominantly caves, sinkholes, and rock shelters. If open for long time periods and structurally complex, depositional patterns can be difficult to decipher. For example, only recently has it been determined that deposition at Natural Trap Cave, Wyoming, was discontinuous, with an almost 50 Ky (thousand year) hiatus between depositional regimes (Lovelace et al., 2022). Thus, Williams’ (2009) mammal species lists for levels 1-3 in Supplementary Material are now considered together as late Wisconsinan (< 25 Ka [thousand years ago]), and level 6 is considered pre - Wisconsinan and > 132 Ka. The species lists for levels 4 and 5 were lumped by Williams (2009) and omitted from Supplementary Material because they consist of a mixture of depositional periods. Additionally, radiocarbon dates may be limited in number and, if done many years ago, less accurate than more recent dating based on bone collagen. Consequently, the dates provided in Supplementary Material, taken from the literature, must be accepted with some caution. They have also been rounded to the nearest Ka or 0.1 Ka in Supplementary Material depending on precision of the original report. Likewise, the rodent species list compiled for San Josecito Cave from Nuevo Leon, Mexico, by Jakway (1958), has been refined based on new excavations revealing multiple temporal levels (Arroyo-Cabrales et al., 2021).
Index taxa are provided for each CMZ, but the list for a given zone may not be inclusive of all species in Supplementary Material limited to that zone. The taxonomy of certain clades, such as the sciurids and heteromyids, has not been revised lately, and we do not feel comfortable using many of these species to define rodent zones; likewise with some species of better-studied clades, such as Peromyscus within the cricetids. CMZ index taxa represent the most taxonomically stable and phylogenetically determined taxa in Supplementary Material with chronological value. Index taxa fall into one of two categories: 1) species limited to an CMZ (no parenthetical abbreviations) and 2) either LSD or HSD species for that zone. The combination must be unique to that CMZ. Due to the vagaries of sampling and chronological range, some CMZs may include more index taxa than others and, as noted above, not all LSD or HSD species in Supplementary Material are listed as index species.
The data in Table 1 were generated from Supplementary Material within the following guidelines: 1) generic records not identified to species (genus sp.) were counted in species tallies only if the record was the sole representative of its genus, and 2) species with chronomorphs (representing phyletic evolution) were identified by their species taxonomy when determining presence or absence in a given geographic region (e.g., Sigmodon bakeri /libitinus and S. bakeri /bakeri were counted once in the eastern USA).
Taxonomic Considerations
The effectiveness of a biostratigraphic/biochronologic system is dependent to a great extent on the taxonomic accuracy of the species identifications in its constituent database. The authors of this study have personally studied much of the material that forms the basis for the database, but we have not made comprehensive continental-wide comparisons among all rodent groups. In most cases, we relied on published regional analyses, our personal observations, and opinions of experts in certain rodent clades to construct Supplementary Material. Because arvicoline rodents figure prominently in late Neogene biochronology, it is important to explain our taxonomic approach and philosophy regarding this group to some extent.
The database includes the use of Martin’s (1993) chronomorphs, roughly equivalent to the lineage segments of Krishtalka and Stucky (1985), to identify intermediates in presumed phyletic sequences. The philosophy behind this usage and its comparison with the subspecies concept can be found in Martin (1993, 1995, 2019). Unlike lineage segments, chronomorphs may overlap in time, representing intraspecific mosaic evolution, as is commonly encountered in arvicolines, both fossil and Recent (Barnosky, 1993; Gordon, 1999; Marcolini and Martin, 2008). We treat phyletic change as evolution, and no matter how much morphological or size change is recorded, we recognize only a single species lineage. Speciation is considered a branching process, and in order for speciation to be identified both an ancestral and descendant species must be recognized as sympatric or at least synchronic. Nevertheless, in some cases, related allochronic species are maintained because the evidence is not sufficient to conclude they represent a phyletic series or there is considerable time between them. Species recognition, always dependent ultimately on expert recognition of synapomorphies, is based on published knowledge of dental morphology showing there is considerable local as well as geographic shape and size variation, especially in first lower molars (e.g., Guthrie, 1965; Guilday, 1982; Davis, 1987; Nadachowski, 1990; Martin, 1993; Rekovets and Nadachowski, 1995; Martin and O’Bryan, 2014). We base species recognition on the presence of synapomorphies, not percentages of molar morphotypes. For example, the dominant m1 morphotype in the Trout Cave No. 2 population of Pedomys is similar to the advanced m1 morphotype of the Java species, but more than 25% of the Trout Cave No. 2 m1s display the advanced morphotype characteristic of P. ochrogaster (Pfaff, 1990). Consequently, we view the Trout Cave population as an archaic population of P. ochrogaster recognized as P. o. /llanensis.
In a series of papers ending with his review of Mimomys (Repenning, 2003), the late Charles Repenning developed a taxonomic framework for North American arvicolines that allocated all extinct arvicolines with rhizodont molars and an occlusal pattern similar to that of Old World Mimomys to the latter genus. Repenning’s concept of Mimomys included taxa previously named as distinct genera, such as Cosomys, Ophiomys, and Ogmodontomys, plus two early Pleistocene species considered today to be correctly allocated to Mimomys, M. virginianus, and M. dakotaensis. Repenning (2003) reviewed the publication history of these taxa, and that will not be repeated here. We accept Repenning’s argument that generic identity should not be determined on continental allopatry alone. We also agree with Repenning (2003) that the occlusal molar morphology of the above North American taxa bear similarity to that of Old World Mimomys. If one were to place inordinate weight on the generality of that pattern, it is not difficult to see why Repenning considered them to be congeneric. However, detailed analyses of the microscopic enamel banding pattern of North American archaic arvicoline molars, the schmelzmuster of von Koenigswald (1980), demonstrate differences between the North American taxa and Old World Mimomys (Mou, 1998; von Koenigswald and Martin, 1984; Martin et al., 2006). Cosomys has three layers on both leading and trailing triangle edges of lower molars (opposite on uppers), with a lamellar enamel layer on the entire leading edge, showing that it developed the lamellar layer independently of Old World Mimomys that have only two layers on the leading edges when lamellar enamel is present (von Koenigswald, 1980). Ophiomys and Ogmodontomys never developed the full lamellar layer on the leading triangle edges, expressing only occasional lamellar patches (Mou, 1998; Martin et al., 2009). In addition to a well-developed lamellar layer on the leading edges of lower triangles and a tangential layer on the trailing edges, almost all Mimomys molars express negative enamel differentiation, with the trailing edges on the lowers (and leading edges on the uppers), thicker than their leading edges and, with the exception only of the most primitive recognized Mimomys, M. davakosi (= M. vandermeuleni; Hordijk and de Bruijn, 2009), added cementum in the reentrant folds. Cosomys, Ogmodontomys, and Ophiomys only have undifferentiated enamel and never express cementum. Additionally, in all Ophiomys, Cosomys, and Ogmodontomys atolls on M3 are rare or disappear with early wear, whereas in the Old World Mimomys that resemble North American archaic genera, such as Mimomys davakosi, one or often two atolls remain in all wear stages except the most senile specimens (van de Weerd, 1979; Hordijk and de Bruijn, 2009). Mimomys davakosi is the only Mimomys without cementum in the reentrant folds, and it is so similar in all dental features to Promimomys cor that Hordijk and de Bruijn (2009) noted that separating some specimens of each species found in the Ptolemais lignitic sequence of Greece was difficult. Despite the general resemblance in occlusal morphology of archaic North American arvicoline molars to those of Mimomys, we conclude there is compelling evidence that Ophiomys, Cosomys, and Ogmodontomys represent a separate New World clade of arvicolines, originating from one or two ancestral species such as Ophiomys mcknighti (Gustafson, 1978) or O. panacaensis (= Mimomys panacaensis, Mou, 1997). There remain legitimate different interpretations of the taxonomy of North American archaic arvicoline populations (e.g., how many Ophiomys species are present in North America; what is the correct assignment of the archaic arvicoline from the Upper Alturas locality of California [Repenning, 2003], etc.), but resolution of these problems will require further study.
Although only one species, Microtus deceitensis, is recorded from the Ft. Selkirk locality in Supplementary Material, specimens were recovered from beneath a dated ash, and thus provide evidence for an early appearance of a vole with arhizodont molars and a Microtus -like dental pattern. Microtus deceitensis and a sample of molars from Hamilton Cave in West Virginia were referred by Repenning and Grady (1988) and Repenning (1992) to Lasiopodomys deceitensis because of a similarity of m1 patterns, but this taxonomic assessment has been challenged by Storer (2003) and Alexeeva et al. (2015). We accept the position of extant arvicolines as a subfamily of the Cricetidae following Steppan and Schenk (2017) and Galewski et al. (2021).
In their review of fossil woodrats and relatives, Martin and Zakrzewski (2019) suggested that an extinct woodrat (Tribe Neotomini, Subtribe Neotomina) from the Hemphillian Rancho el Ocote assemblage of Mexico referred by Carranza-Castañeda and Walton (1992) to Neotoma (Paraneotoma) sp. might represent an early species of the extant Xenomys. As noted by Carranza-Castañeda and Walton (1992), the m1s available were from juveniles, and the m3 of this woodrat was not preserved. After further examination, without going into detail, we now consider the Xenomys attribution uncertain, and in Supplementary Material we refer to this taxon as Neotomina sp.
RESULTS
CMZ 12 (5.8 - 5.3 Ma)
Index taxa. Promimomys mimus, Microtodon n. sp., Protorepomys mckayi, Marmota korthi, Perognathus sargenti, Prodipodomys griggsorum, P. idahoensis (LSD), Onychomys (LSD), mylagaulids (HSD), Parapliosaccomys oregonensis (HSD), Repomys (LSD).
Characteristic assemblages. McKay Reservoir, Hoye Canyon, Mailbox, Zwiebel Channel.
Remarks. Arvicoline rodents first entered North America between 5.8-5.3 Ma (J. Martin et al., 2018) as Promimomys mimus (= Prosomys mimus) and dispersed from the west coast to the northern Great Plains in Nebraska (Voorhies, 1990). An arvicoline-like cricetid, Microtodon, also appears at this time. Both were quickly supplanted by the arvicoline tribe Pliophenacomyini, including Protopliophenacomys, Pliophenacomys, Guildayomys, and Pliolemmus (L. Martin, 1979; Zakrzewski, 1984; Martin, 2008). J. Martin et al. (2018) recently resurrected Shotwell’s (1956) Prosomys, and the taxonomic status of the McKay arvicoline needs to be reconsidered if, as Martin (2008) proposed, it is ancestral to the Pliophenacomyini. This radiation primarily took place on the Great Plains, as these genera are rare in the western states (Supplementary Material). No extant cricetid genera are recognized from this zone.
CMZ 11 (5.3 - 5.0 Ma)
Index taxa. Protopliophenacomys parkeri, Paenemarmota mexicana (LSD), Parapliosaccomys (HSD), Postcopemys (LSD); Pliogeomys carranzai, Prosigmodon oroscoi (LSD), P. chihuahuensis (LSD), Bensonomys elachys (LSD), B. baskini (LSD), Baiomys kolbi (LSD), Neotoma cf sawrockensis (LSD).
Characteristic assemblages. Santee, Yepómera.
Remarks. CMZ 11 is characterized by the first appearance of hypsodont, rhizodont geomyids (Pliogeomys), Protopliophenacomys parkeri, and a southwestern radiation of the extinct neotomine Bensonomys (Lindsay and Jacobs, 1985; Voorhies, 1990; Carranza-Casteñeda and Walton, 1992). The giant ground squirrel Paenemarmota mexicana first appears during this interval (McDonald et al., 2022).
Although Yepómera and Concha have a number of species in common, their respective localities are chronologically separated. Localities at Yepómera span most all the Thvera subchron (= C3n.4n) of the Gilbert chron with one locality (Y39) extending into an overlying reversed interval (= C3n.3r), just above the boundary between C3n.4n/C3n.3r, a total range of about 5.2-4.95 Ma (Lindsay et al 2006). Localities at Concha span the Sidufjall subchron (= C3n.3n) of the Gilbert chron from just below its boundary with C3n.3r to just above its boundary with C3n.3r, a total range of about 4.9 - 4.8 Ma (Lindsay et al. 2006). Notably, arvicolines are absent from Yepómera, which can in part be explained by its southern position, but also by the likelihood that the Pliophenacomyini had not extensively radiated by this time.
CMZ 10 (5.0 - 4.75 Ma)
Index taxa. Ophiomys panacaensis, O. mcknighti, Pliophenacomys wilsoni, Prosigmodon ferrusquiai, P. tecolotum, P. oroscoi (HSD), P. chihuahuaensis (HSD), Repomys gustelyi, Bensonomys winklerorum, Ammospermophilus hanfordi, Paenemarmota mexicana (HSD), Prothomomys warrensis, Baiomys kolbi (HSD), Jacobsomys dailyi (LSD), Geomys minor (LSD), Peromyscus (LSD).
Characteristic assemblages. Panaca, White Bluffs, Rancho el Ocote, Concha, Warren.
Remarks. CMZ 10 spans the Hemphillian - Blancan boundary at about 4.9 Ma (Lindsay et al., 2002) and is best characterized north of Mexico by the LSD for Ophiomys panacaensis from the Panaca Formation of Nevada (Mou, 2011) and O. mcknighti from the Ringold Formation of Washington state (Gustafson, 1978). These records, the first for Ophiomys in North America, are likely near the time of the Ophiomys immigration event from the Old World, and thus provide data points for our understanding of arvicoline evolution and dispersal. As observed by Repenning (2003), the first lower molar of Mimomys antiquus (Zazhigin, 1980) from Peshniovo 3, western Siberia, bears a strong resemblance to that of early Ophiomys, and according to Vasilyn et al. (2017) is assigned a date of about 4.9 Ma.
CMZ 10 is constrained in the Mexican sections that produced the Concha and Rancho el Ocote assemblages. Based on radiometric dates and paleomagnetic profiles, both the latter sections span almost the entirety of the Sidufjall subchron of the Gilbert chron (Lindsay et al., 2006) and extend to reversely magnetized sediments above. An advanced Pliophenacomys, P. wilsoni, is found at Concha, associated with many of the rodent species from the nearby, earlier Yepómera section. At Concha we see the last association of a Pliogeomys with early Geomys.
A new species of Prosigmodon, P. tecolotum, was recently described from the upper level of sediments in the Jalteco 26 section from the Tecolotlán Basin of Jalisco, central Mexico (Pacheco-Castro et al., 2019; Ronez et al., 2021). Very similar to other Prosigmodon species, it differs primarily in retaining a mesoloph on M1-M2, a plesiomorphic condition. This species was recovered from a lens in the section directly above an ash that was dated at 4.89 ± 0.16 Ma.
CMZ 9 (4.75 - 4.5 Ma)
Index taxa. Paenemarmota barbouri (LSD), Ogmodontomys sawrockensis, O. pipecreekensis, O. poaphagus (LSD), Pliophenacomys koenigswaldi, Bensonomys hershkovitzi, B. stirtoni, B. elachys (HSD), Symmetrodontomys daamsi, ?Ictidomys pipecreekensis, Otospermophilus rexroadensis (LSD).
Characteristic assemblages. Saw Rock Canyon, Pipe Creek Sinkhole, Fox Canyon.
Remarks. Ophiomys, Ogmodontomys, and Cosomys comprise the Ogmodontomyini (Martin, 2008; Marcolini et al., 2008), and both Ogmodontomys and Cosomys likely originated from Ophiomys as the latter genus spread eastward, eventually reaching the Central Great Plains. CMZ 9 represents the initial appearance of Ogmodontomys in Kansas and Indiana and further eastward dispersal and dental development in Pliophenacomys, expressed by P. koenigswaldi. In the Meade Basin, CMZ 9 also includes Fallen Angel B and Argonaut, both with Ogmodontomys sawrockensis, that lie above Saw Rock Canyon and beneath Fox Canyon (Martin and Peláez-Campomanes, 2014). Fallen Angel B and Argonaut have produced meager remains of a primitive sigmodontine that Peláez-Campomanes and Martin (2005) referred to Sigmodon sp., and Martin et al. (2021) referred to S. minor, but could also represent Prosigmodon. The earliest confirmed record of S. minor is in CMZ 8. Fox Canyon includes the first appearances of some common Pliocene rodents in the Meade Basin plus a dentally derived Ogmodontomys referable to O. poaphagus. Fox Canyon, like Saw Rock Canyon, lacks cotton rats.
CMZ 8 (4.5 - 3.9 Ma)
Index taxa. Sigmodon minor (LSD), S. holocuspis, Jacobsomys verdensis, Geomys anzensis, Perognathus strigipes, Postcopemys repenningi, Neotoma vaughani, Ellesmereomys haringtoni.
Characteristic assemblages. Ripley B, Verde (House Mountain Loc. 318), Raptor 1C, Wiens/Vasquez-Newt, Layer Cake, Strathcona.
Remarks. This zone includes the earliest North American appearances of Sigmodon minor, a characteristic Pliocene rodent, and a modern-sized cotton rat, Sigmodon holocuspis. Sigmodon cf minor is first encountered in the Meade Basin at Ripley B, lying above Fox Canyon and just above the Bishop Gravel, a characteristic early Pliocene marker bed. Sigmodon holocuspis was described from House Mountain Loc. 318 in the Verde Formation of Arizona, in magnetically reversed sediments referred to the Gilbert chron, between 4.48-4.29 Ma (Czaplewski, 1990). For reasons noted in Marcolini and Martin (2008), we tentatively conclude that House Mountain Loc. 318 and Raptor 1C were deposited towards the end of this reversal. S. holocuspis has only been recovered in the Meade Basin from Raptor 1C and a contemporaneous locality, Keefe Canyon (Peláez-Campomanes and Martin, 2005), and the evidence appears to indicate S. holocuspis had a brief geological lifespan in North America, providing a useful chronological anchor for part of the early Blancan.
Ruez and Gensler (2008) reported an unexpected discovery of Mictomys vetus, an extinct lemming, from a level in the Glenns Ferry Formation dated to about 3.93 Ma. Tesakov and Bondarev (2021) pointed out how unlikely this record was to be valid when compared to the evolutionary history of lemming dental features. Consequently, we do not include this record as an LSD for CMZ 8, awaiting further collections for corroboration.
The presence of Ellesmereomys haringtoni at Strathcona, on Ellesmere Island in the Canadian Arctic, documents the second North American record of a primitive arvicoline-like cricetid, bounded above by a cosmogenic nuclide date of 3.9 + 1.5/ - 0.5 Ma (Fletcher et al., 2019; Martin and Zakrzewski, 2022).
CMZ 7 (3.9 - 3.1 Ma)
Index taxa. Ophiomys taylori (LSD), Ondatra zibethicus /minor, Procastoroides intermedius, P. idahoensis, P. sweeti (LSD), Oregonomys magnus.
Characteristic assemblages. Hagerman, Sand Point, Taunton, Beck Ranch, Benson.
Remarks. Hagerman and Sand Point represent lower outcrops of a long Pliocene-Pleistocene rodent record in the Glenns Ferry Formation of Idaho (Figure 2) running from about 3.93 Ma to about 1.6 Ma. These sections crop out along the Snake River at various points (Zakrzewski, 1967; Repenning et al., 1995). Ophiomys taylori and Cosomys primus evolved from the earlier O. panacaensis/O. mcknighti evolutionary grade, and the earliest muskrats (Ondatra) and giant beavers (Procastoroides) appear during this interval. The last continental record of a geomyid with rooted molars (Pliogeomys) occurs at Hagerman. The LSD for Cosomys is in the Hagerman section, occurring beneath an ash bed of ca. 3.84 Ma (Figure 2; Hart and Brueseke, 1999; Ruez, 2009).
CMZ 6 (3.1 - 2.8 Ma)
Index taxa. Paenemarmota barbouri (HSD), Ondatra zibethicus /meadensis, Procastoroides sweeti (HSD), Ophiomys taylori (HSD), O. parvus (LSD), Nebraskomys rexroadensis, Pliophenacomys primaevus, Pliolemmus antiquus (LSD), Hibbardomys skinneri, Geomys quinni (LSD).
Characteristic assemblages. Rexroad Loc. 3, Sand Draw, Deer Park, Birch Creek, Boyle Ditch, Mesa del Sol, Blanco.
Remarks. Nebraskomys (ancestral to the Pleistocene Atopomys), Pliolemmus, and Hibbardomys appear during CMZ 6. The relationships of Hibbardomys are obscure, but there is some resemblance of the Hibbardomys dentition to that of Phenacomys, and Martin (2008) included both in the arvicoline tribe Phenacomyini. Symmetrodontomys, Pliolemmus, and Ogmodontomys become extinct before CMZ 5. Ondatra zibethicus /meadensis is the characteristic muskrat chronomorph of CMZ 6. Ophiomys parvus originates from O. taylori in CMZ 6.
In their description of the new Zwiebel Channel Hemphillian locality from the Sand Draw area of Nebraska, Martin et al. (2017) suggested that localities with Ophiomys (e.g., O. magilli from UM Loc. 3-67) occurred higher in the stratigraphic section of the Sand Draw area than sites with Ogmodontomys poaphagus. Although there may be some stratigraphic differences in species occurrences, we now consider O. magilli a slightly advanced form of O. taylori, and the rodents from all localities listed by Hibbard (1972) from the Sand Draw region of Nebraska to be roughly equivalent in age to other localities with Pliolemmus, Nebraskomys, and Ogmodontomys poaphagus, such as Deer Park, Kansas.
CMZ 5 (2.8 - 2.2 Ma)
Index taxa. Thomomys carsonensis, Ondatra zibethicus /idahoensis, Ophiomys parvus (LSD), O. meadensis (LSD), Pliophenacomys osborni (LSD), Repomys arizonensis, Geomys floralindae (LSD).
Characteristic assemblages. Flatiron Butte, Grand View, 111 Ranch, Arroyo Seco, Sanders.
Remarks. This interval is characterized by the first appearance of Ondatra zibethicus /idahoensis, a chronomorph of the North American muskrat larger in size than O. z. /meadensis and with the first evidence of cementum in reentrant folds. There is also an increase in dental complexity in Ophiomys, leading from the last record of O. taylori at Flatiron Butte, ID to O. parvus at the Grand View/Ninefoot Rapids level (Conrad, 1980). A slightly more advanced Ophiomys currently recognized as O. meadensis appears during CMZ 5 on the Great Plains in Texas and Kansas.
CMZ 5 also includes the cold period that begins the Pleistocene epoch at 2.58 Ma. Although rodent species turnover and some evolutionary changes are observed, this does not seem to be a particularly dramatic period of flux as compared with, for example, change seen on the Central Great Plains in the Meade Basin following the Huckleberry Ridge ash-fall at 2.07 Ma (see Discussion).
CMZ 4 (2.2 - 2.0 Ma)
Index taxa. Geomys floralindae (HSD), Sigmodon hudspethensis, S. curtisi (LSD), Ophiomys meadensis (HSD), Plioctomys rinkeri, Hibbardomys fayae, H. voorhiesi, Reynoldsomys timoteoensis, Plioctomys rinkeri, Pliolemmus antiquus (HSD), Zapus sandersi (HSD).
Characteristic assemblages. Hudspeth, Cita Canyon, San Timoteo, Curtis Ranch, Borchers, Froman Ferry, Inglis 1A.
Remarks. CMZ 4 is characterized by the appearance of modern-sized cotton rats with advanced occlusal patterns, S. hudspethensis and S. curtisi (Strain, 1966; Akersten, 1970). Almost all the holdover “Pliocene fauna” of Great Plains rodents becomes extinct during this interval, mostly near its end, following the Huckleberry Ridge ash-fall at 2.07 Ma. With the exception of the immigration of Mimomys in CMZ 3, this interval represents the end of dominance in North America of arvicoline rodents with rooted molars. Myodes and Ondatra are the only extant rhizodont arvicolines, the former restricted to forest ecosystems and the latter to aquatic habitats.
CMZ 3 (2.0 - 1.29 Ma)
Index taxa. Ondatra zibethicus /annectens (LSD), Allophaiomys sp., Pedomys n. sp., Tyrranomys harkseni, Javazapus weeksi, Mimomys virginianus, M. dakotaensis, Atopomys texensis, Microtus (LSD).
Characteristic assemblages. Java, Short Haul, Aries A, Hamilton Cave, SAM Cave, Fyllan Cave, Sappa.
Remarks. This interval is well represented and constrained in the Meade Basin by several localities, radiometric dates, paleomagnetism, and biostratigraphy. The Allophaiomys immigration event across Beringia is considered to have occurred around 2.0 Ma, and defines the base of the Irvingtonian NALMA (Martin et al., 2008). This event represents the first wave of dispersal from Asia of arhizodont arvicoline rodents destined to become the dominant small grassland herbivores, later represented by various species of Microtus, Pedomys and Pitymys. A single m3 of an arhizodont arvicoline was recovered from a geologic core just above the Huckleberry Ridge ash (2.07 Ma) at Hansen Bluff, Colorado (Rogers et al. 1992). The age was estimated at about 1.89 Ma. While at this period of time the most likely arhizodont arvicoline with the m3 morphology illustrated by Rogers et al. (1992; fig. 9) would be Allophaiomys, identification cannot be made solely on the morphology of m3. Until records of Allophaiomys from Little Dell Dam, Utah (Gillette et al., 1999) and SAM Cave, Colorado (Rogers et al., 2000) can be confirmed with additional specimens or illustrations, Allophaiomys is otherwise absent from western sites prior to its appearance at Porcupine Cave in CMZ 2. This absence could be considered sampling bias, except that Allophaiomys has not been recovered from the San Timoteo, Glenns Ferry, Vallecito-Fish Creek, or San Pedro Valley lengthy sequences, and thus appears have first dispersed eastward through North America by a northern route, bypassing both the Rocky Mountain chain and arid western ecosystems. Later, perhaps during glacial advances, Allophaiomys moved briefly into some western states, mostly at high elevation. Atopomys texensis, an arvicoline descended from Nebraskomys, appears during this interval, associated with Allophaiomys at Fyllan Cave, Texas (Winkler and Grady, 1990; Winkler and Gose, 2003). The new species of Pedomys from Java listed in Supplementary Material includes many m1s with simple Allophaiomys morphotypes and thus likely represents a common ancestor for later North American Pedomys species.
An interesting combination of arvicolines is found at Hamilton Cave, West Virginia (Repenning and Grady, 1988; Repenning, 1992). The arhizodont component may include two or three species, one of which may be an early Pitymys retaining some Allophaiomys m1 morphotypes. If this identification is confirmed, the Hamilton Cave Pitymys could represent an ancestral grade for both later extinct and extant Pitymys.
The North American muskrat increases in size and becomes recognized as Ondatra zibethicus /annectens, extending through CMZ 2. The earliest record of the modern Great Plains Pocket Gopher, Geomys bursarius, is from this interval in the Meade Basin of Kansas (Martin et al., 2011; Martin, 2016). CMZ 3 is bounded on the young side by the Mesa Falls ash-fall from the Yellowstone caldera (Lanphere et al., 2002), correlative with the Coleridge ash of Nebraska (Boellstorff, 1978), directly overlying the Sappa local fauna of Kansas.
CMZ 2 (1.29 - 0.30 Ma)
Index taxa. Ondatra zibethicus /annectens (HSD), Microtus meadensis (HSD), M. paroperarius (HSD), M. morlani, Pedomys guildayi, P. australis, P. ochrogaster /llanensis (HSD), P. o. /ochrogaster (LSD), Pitymys aratai, P. cumberlandensis.
Characteristic assemblages. Hansen Bluff, Haile 16A, Cumberland Cave, Thistle Creek, Cudahy, Vera, Conard Fissure, Port Kennedy, Coleman 2A.
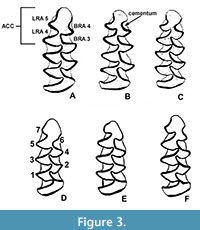 Remarks. This interval is characterized by an almost bewildering array of small arhizodont arvicolines, representing a combination of autochthonous radiations and continued dispersals across Beringia. Allophaiomys splits into the Pedomys complex, beginning with P. guildayi, P. australis, and P. ochrogaster, and the characteristic middle Pleistocene species Microtus paroperarius and M. meadensis first appear (Hibbard, 1944; Paulson, 1961; Martin, 1995). Microtus deceitensis and those earliest Microtus m1 specimens reported in the literature as displaying either the paroperarius or Lasiopodomys m1 morphotype (as at Hamilton Cave; Repenning and Grady [1988]), are variants of the pattern seen in the extant Alexandromys oeconomus, in which the anteroconid complex is asymmetrical, T 4-5 are alternate, LRA 4 is well-developed, BRA 3 is often shallow, and BRA 4 ranges from absent to modestly developed but usually without cementum when it is present (Figure 3). A. oeconomus (= Microtus operarius; hence M. paroperarius for the extinct species) is a tundra-steppe species, and fossil Microtus-like m1s with the paroperarius m1 morphotype tend to be found in the far north (Fort Selkirk, Yukon; Cape Deceit, Alaska), in mountain chain cavern localities (Hamilton Cave, WV; Porcupine Cave, CO), or on the Central Great Plains (Cudahy, KS; Vera, TX, etc.) only during glacial intervals, suggesting these species represent a distinct clade with northern origins, perhaps Alexandromys, separate from other Microtus (such as M. pennsylvanicus) that may also have had a northern origin, but at a later time, and are more temperate-adapted. It is instructive that cotton rats, genus Sigmodon, are absent from fossil localities with the M. paroperarius-like m1 morphotype throughout the continent, further supporting the hypothesis that species with an M. poaroperarius -like dental morphology represent a clade with northern environmental preferences.
Remarks. This interval is characterized by an almost bewildering array of small arhizodont arvicolines, representing a combination of autochthonous radiations and continued dispersals across Beringia. Allophaiomys splits into the Pedomys complex, beginning with P. guildayi, P. australis, and P. ochrogaster, and the characteristic middle Pleistocene species Microtus paroperarius and M. meadensis first appear (Hibbard, 1944; Paulson, 1961; Martin, 1995). Microtus deceitensis and those earliest Microtus m1 specimens reported in the literature as displaying either the paroperarius or Lasiopodomys m1 morphotype (as at Hamilton Cave; Repenning and Grady [1988]), are variants of the pattern seen in the extant Alexandromys oeconomus, in which the anteroconid complex is asymmetrical, T 4-5 are alternate, LRA 4 is well-developed, BRA 3 is often shallow, and BRA 4 ranges from absent to modestly developed but usually without cementum when it is present (Figure 3). A. oeconomus (= Microtus operarius; hence M. paroperarius for the extinct species) is a tundra-steppe species, and fossil Microtus-like m1s with the paroperarius m1 morphotype tend to be found in the far north (Fort Selkirk, Yukon; Cape Deceit, Alaska), in mountain chain cavern localities (Hamilton Cave, WV; Porcupine Cave, CO), or on the Central Great Plains (Cudahy, KS; Vera, TX, etc.) only during glacial intervals, suggesting these species represent a distinct clade with northern origins, perhaps Alexandromys, separate from other Microtus (such as M. pennsylvanicus) that may also have had a northern origin, but at a later time, and are more temperate-adapted. It is instructive that cotton rats, genus Sigmodon, are absent from fossil localities with the M. paroperarius-like m1 morphotype throughout the continent, further supporting the hypothesis that species with an M. poaroperarius -like dental morphology represent a clade with northern environmental preferences.
In North America the earliest record of a Microtus species not referable to either the paroperarius group or M. meadensis is from Porcupine Cave, Colorado. Illustrations of this taxon, identified as Microtus sp., were provided by Bell and Barnosky (2000), and are similar to Microtus pennsylvanicus m1s with five triangles. Numerous molars from this species were recovered. The vast majority of Microtus sp. specimens (104/128 = 81%) were recovered from levels 1-2 in the Pit controlled excavations. Microtus meadensis specimens were also excavated from the same levels. A few M. paroperarius m1s were scattered relatively evenly through the first two levels. Allophaiomys pliocaenicus molars were found only in levels 4-6. Barnosky (2004) recognized this difference in relative abundance, and identified two depositional zones. He estimated the age of the Microtus sp. zone to at >0.60 - < 0.85 Ma and the Allophaiomys zone >0.78 - 1.0 Ma. Other records of this association, such as at Little Dell Dam locality in Utah (Gillette et al., 1999), SAM Cave, Colorado (Rogers et al., 2000), Hamilton Cave in West Virginia (Repenning and Grady, 1988) or Cathedral Cave, Nevada (Jass and Bell, 2011) cannot be confirmed based on published information.
The oldest association of Microtus paroperarius and M. meadensis may be from Hansen Bluff in Colorado, from sediments directly above the Bishop ash at 0.76 Ma (Rogers et al., 1985, 1992; Sarna-Wojcicki et. al., 2000). However, M. meadensis was recovered lower in the sections than M. paroperarius, and there could be as much as 50 thousand years between the levels. On the Central Great Plains these species are found together in a number of localities directly underneath the Lava Creek B ash at 0.64 Ma (Lanphere et al., 2002; Schultz, 2010). In none has a more advanced Microtus been securely documented; thus it appears Microtus pennsylvanicus, the dominant extant Microtus on the Great Plains, had not reached that region by 0.64 Ma, further suggesting that fossil Microtus morphologically referable to modern species from Porcupine Cave and elsewhere in the western United States are not likely much older than 0.64 Ma. The youngest records of Microtus paroperarius and M. meadensis may be from Salamander Cave in the Black Hills of South Dakota (Mead et al., 1993). Uranium series dates suggest a range from 0.15-0.45 Ma, and three of the four authors concluded they preferred a depositional date for the majority of the fossils at 0.25 Ma based on a Th/Pa ratio from an Equus phalanx from the main bone bed, while one of the authors (C.A. Repenning) preferred a date closer to 0.60 Ma based on previously known biochronology of the Salamander Cave arvicolines.
Continuing with the arvicolines, the earliest Pitymys, P. cumberlandensis, appears at Cumberland Cave, Maryland (van der Meulen, 1978). New U-series Electron Spin resonance dates from Cumberland Cave provided dates of 722 and 790 Ka for the deposits (Withnell et al., 2020). A middle Pleistocene Pitymys radiation apparently followed, culminating with the late Pleistocene P. aratai (Martin, 1974) and modern P. pinetorum and Mexican species such as P. quasiater (Martin, 1995). It is not likely P. cumberlandensis was ancestral to later pine vole species (Martin, 2014), but the Hamilton Cave populations mentioned in CMZ 3 could represent that evolutionary grade.
CMZ 1 (0.30 - 0.001 Ma)
Index taxa. Microtus pennsylvanicus (LSD), Pedomys parmaleei, dominance of modern species.
Characteristic assemblages. Kennewick, San Josecito Cave, Papago Springs Cave, Jinglebob, Cragin Quarry, Dry Cave, Natural Trap Cave, Jones.
Remarks. Microtus referable to modern species do not appear until the late Pleistocene, and because the timing of the modern Microtus radiation apparently began between the Lava Creek B ash-fall and the first radiocarbon-dated assemblages about 50 Ka, we have no trustworthy dates for this interval. A search for species of Pleistocene Microtus in the Neotoma (FAUNMAP) database reveals no published records prior to a flurry of specimens from extant species reported from localities younger than the 0.64 Ma Lava Creek B ash. Microtus, Pedomys, and Pitymys species become common components of North American late Pleistocene faunas, particularly during the Rancholabrean NALMA, and in the central and eastern states the most common species are the extant Microtus pennsylvanicus, Pedomys ochrogaster, and Pitymys pinetorum and the extinct Pedomys parmaleei. As noted by Murray et al. (2011) in the western states there are a number of Microtus species with overlapping dental morphology, and thus identification to species is often difficult. Nevertheless, extant species dominate Rancholabrean sites. From these observations we can generalize that the radiation of continental North American Microtus (not including Pitymys and Pedomys) into extant species likely began no earlier than the middle Pleistocene about 0.70 Ma, but the details of this radiation remain unknown.
DISCUSSION
General Patterns
On the Central Great Plains a regional rodent zone can be distinguished at 0.64 Ma, coincident with Lava Creek B ash deposition, because a number of rodent assemblages in Kansas and Texas with comparable rodent communities have been recovered from directly beneath this ash (Martin, 2019). None contain Allophaiomys, and it seems clear Allophaiomys had evolved into early Pedomys by this time, as the assemblages include Pedomys ochrogaster/llanensis (Supplementary Material). Extant populations and late Pleistocene samples of Pedomys ochrogaster occasionally display Allophaiomys m1 morphotypes (Harris, 1988; Martin, 1991). As noted above, Allophaiomys is rare in western North America, and its chronology there is not well constrained. Although deposits of Lava Creek B ash are found commonly in the western states, no rodent assemblages have been reported associated with them, and there is no record of extant or extinct Pedomys in western states prior to the late Pleistocene. These observations demonstrate the value of both regional and continental rodent databases in determining distributional and evolutionary patterns.
Table 1 provides a rough geographic breakdown of the 312 species in Supplementary Material. The list does not include many late Pleistocene sites and therefore many extant species. Nevertheless, the “pull of the Recent” probably produces a certain degree of bias. The totals, therefore, represent only a crude estimate of regional species richness over the last 5 million years, and should be examined chronologically to illuminate both regional and continental diversity dynamics. Western states appear to have had a greater richness of cricetids and heteromyids, and the absence of heteromyids from eastern states even during periods of aridity in which there was likely at least a patchy, arid corridor connecting Texas with the Gulf states (e.g., the late Irvingtonian Coleman 2A assemblage from Florida with Thomomys, Lepus, Conepatus, Felis onca, and Hydrochoerus; Martin, 1974; Wilkins, 1985), suggests heteromyids were limited throughout the late Cenozoic to the Great Plains and points west. Neotominin cricetid diversity (e.g., Neotoma, Repomys) has been consistently higher in western than central or eastern North America.
As noted above under CMZ 5, this interval spans the currently recognized beginning of the Pleistocene epoch at 2.58 Ma, also the base of the Matuyama chron. A logical question is to what extent the beginning of the Pleistocene affected rodent biogeography and evolution. The answer is probably best addressed through regional depositional basins where the base of the Matuyama paleomagnetic chron is recognized or assumed to be present because this boundary is roughly contemporaneous with the beginning of the Pleistocene. In North America basins spanning that time with superposed rodent assemblages are limited, but those available do not demonstrate enhanced rates of rodent species turnover at the advent of the Pleistocene, though changes in other mammal groups may provide additional information. In the extensive Vallecito-Fish Creek (VFC) sequence of southern California (Cassiliano, 1999), change in the rodent contingent above the onset of the Pleistocene is limited only to the appearance of a large cotton rat, Sigmodon lindsayi and, at the top of the sequence, a Microtus near M. deceitensis (with a likely northern origin). In the VFC sequence there are some notable early Pleistocene shifts in other mammal groups, including extinction of ancient lagomorphs such as Hypolagus and Nekrolagus and the appearance of Sylvilagus. The sloths, Megalonyx, Nothrotheriops, and Glossotherium first appear almost coeval with the base of the Matuyama. However, these are not new South American immigrants, as Megalonyx is likely a descendant of the much earlier immigrant Pliometanastes (Morgan, 2005), and Glossotherium was present in Mexico 4.6-4.8 Ma (Carranza-Casteñeda and Miller, 2004). Nothrotheriops is an Irvingtonian immigrant, appearing in North America about 1.3 Ma at Leisey 1A in Florida (McDonald, 1995).
In the western and Great Plains states, many characteristic Pliocene species, such as Sigmodon minor, Bensonomys arizonae, Prodipodomys idahoensis, and Pliophenacomys osborni, continue through CMZ 5 into CMZ 4. Unfortunately, only the Sanders assemblage in the Meade Basin of Kansas lies at the approximate base of the Matuyama/earliest Pleistocene boundary and its rodent contingent is sparse. Notably present is the advanced arvicoline Ophiomys meadensis, replacing the earlier Ogmodontomys poaphagus, but there is an absence of fossil localities between Sanders and Margaret, the latter lying beneath Borchers and the Huckleberry Ridge ash (older than 2.07 Ma). In the Duncan Basin of Arizona, the 111 Ranch rodent sequence extends from the end of the Gauss through the early Matuyama chrons. Tomida (1987) conveniently broke the faunal list into lower (presumably prior to the Gauss-Matuyama boundary) and upper units. Other than the disappearance of Repomys arizonae and Pliophenacomys primaevus from the lower level, both representing global extinctions, there is little difference between the two assemblages. The lemming Mictomys vetus is limited to the lower beds, but it is a common element of younger continental deposits elsewhere, so it is difficult to ascribe any particular significance to its absence in the upper quarries.
Circumstantial evidence suggests there are at least two categories of rodent species turnover in North America. The first, which we can label background turnover, is a relatively low, constant rate driven primarily by Milankovich cycles and stochastic regional climate changes (Martin and Peláez-Campomanes, 2014). Second category events are short-term catastrophic environmental changes on either a global or regional scale, including massive floods and volcanic eruptions. In the Meade Basin rodent record over the past five million years, background turnover averages < 2 E/ or I/MSY (extinctions or immigrations/million species years; Martin and Peláez-Campomanes, 2016). Category 2 is represented by the Huckleberry Ridge and Lava Creek B ash-falls at 2.07 and 0.64 Ma. Between Borchers at 2.07 Ma and Aries A at ~2.0 Ma, 40% of the Meade Basin rodent community turned over. A small part of the change appears to represent cladogenesis, as both the modern Ictidomys tridecemlineatus and Geomys bursarius appear soon after the Huckleberry Ridge deposition (Martin et al., 2021). There is a depositional hiatus between about 1.35-0.64 Ma and a lack of dates for localities from 0.64 Ma to the first radiocarbon-dated sites in the Meade Basin, so we cannot precisely determine turnover rates during these periods, but it is clear the modern rodent community of the Meade Basin area was mostly established by the middle late Pleistocene (e.g., Kanopolis, Berends, Doby Springs; Supplementary Material), in perhaps a period of ~100 - 200 Ka following the Lava Creek B ash-fall. A research program designed to sample for rodent communities above and beneath dated ashes could test the ash-fall hypothesis and, concomitantly, provide better chronological control to the continental system.
Arvicoline Diversity
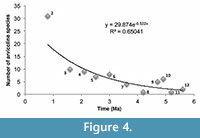 The database of Supplementary Material can be used to examine a number of evolutionary and distributional questions. We provide an example with a preliminary evaluation of North American arvicoline diversity from Supplementary Material over the past five million years. Figure 4 presents a graph of this diversity. Numbers of species on the ordinate were plotted against midpoints of CMZs in Ma on the abcissa. Because species numbers in CMZ 1-2 are affected so greatly by the number of Rancholabrean assemblages tallied, we omitted the total for CMZ 1; the tally for CMZ 2 should be considered only as a rough approximation, but an indication of a significant rise over CMZ 3. The data were best fit by an exponential function. Increase in numbers was modest through most of the Pliocene and early Pleistocene, comprised mostly of rhizodont extinct taxa such as Ophiomys, Ogmodontomys, Cosomys, Pliophenaomys, Hibbardomys, Nevadomys, Nebraskomys, and Plioctomys. As observed above, no elevated arvicoline turnover events characterize the advent of the Pleistocene at 2.58 Ma. All the archaic, mostly rhizodont arvicolines became extinct by the end of CMZ 3. CMZ 4, a brief interval between 2.2-2.0 Ma, includes the Huckleberry Ridge (HR) ash-fall at 2.07 Ma and the subsequent Allophaiomys immigration event documented in the Meade Basin of Kansas. Elsewhere in North America, roughly at the same time, the LSDs for Phenacomys and Mimomys are encountered. These observations are consistent with a glacial scenario bringing new arvicolines into North America via a northern route at a time when much of the central and western USA had been devastated by the HR ash-fall. However, only cotton rats have been recovered in the Meade Basin prior to and just following the HR event (Margaret/beneath, Borchers/immediately above; Martin et al., 2021), and it is therefore likely that the archaic arvicolines such as Hibbardomys, Pliophenacomys and Ophiomys, were extinct by that time. That is, although no punctuational turnover was observed at the beginning of the Pleistocene, early Pleistocene environmental change may have gradually hastened the demise of archaic arvicolines prior to the HR ash-fall.
The database of Supplementary Material can be used to examine a number of evolutionary and distributional questions. We provide an example with a preliminary evaluation of North American arvicoline diversity from Supplementary Material over the past five million years. Figure 4 presents a graph of this diversity. Numbers of species on the ordinate were plotted against midpoints of CMZs in Ma on the abcissa. Because species numbers in CMZ 1-2 are affected so greatly by the number of Rancholabrean assemblages tallied, we omitted the total for CMZ 1; the tally for CMZ 2 should be considered only as a rough approximation, but an indication of a significant rise over CMZ 3. The data were best fit by an exponential function. Increase in numbers was modest through most of the Pliocene and early Pleistocene, comprised mostly of rhizodont extinct taxa such as Ophiomys, Ogmodontomys, Cosomys, Pliophenaomys, Hibbardomys, Nevadomys, Nebraskomys, and Plioctomys. As observed above, no elevated arvicoline turnover events characterize the advent of the Pleistocene at 2.58 Ma. All the archaic, mostly rhizodont arvicolines became extinct by the end of CMZ 3. CMZ 4, a brief interval between 2.2-2.0 Ma, includes the Huckleberry Ridge (HR) ash-fall at 2.07 Ma and the subsequent Allophaiomys immigration event documented in the Meade Basin of Kansas. Elsewhere in North America, roughly at the same time, the LSDs for Phenacomys and Mimomys are encountered. These observations are consistent with a glacial scenario bringing new arvicolines into North America via a northern route at a time when much of the central and western USA had been devastated by the HR ash-fall. However, only cotton rats have been recovered in the Meade Basin prior to and just following the HR event (Margaret/beneath, Borchers/immediately above; Martin et al., 2021), and it is therefore likely that the archaic arvicolines such as Hibbardomys, Pliophenacomys and Ophiomys, were extinct by that time. That is, although no punctuational turnover was observed at the beginning of the Pleistocene, early Pleistocene environmental change may have gradually hastened the demise of archaic arvicolines prior to the HR ash-fall.
Although North American Allophaiomys may be ancestral to both Pedomys and Pitymys, arvicoline diversity increased little in CMZ 3. By CMZ 2, one or more dispersal events across Beringia introduced a number of new Microtus clades, including M. meadensis and a group of species related to M. paroperarius, the latter with northern affinities. New Pedomys and Pitymys species and modern lemming genera appeared. Extant species of Microtus are recorded from many North American sites in CMZ 1, probably representing a combination of late Pleistocene Beringian immigrants and phylogenetic radiation from CMZ 2 ancestors. Concomitantly, large cotton rats, genus Sigmodon, diversified and spread into austroriparian prairie habitats in southern North America and northern South America. From that time onward, arhizodont voles and cotton rats become the dominant small pastoral herbivores in global Holarctic ecosystems.
CONCLUSIONS
A database of rodent species communities for the past five million years is extended from the eastern and central United States (Martin, 2019) to include all of North America. This new correlational scheme, including 312 species from 124 communities, allows recognition of 12 Cenozoic Mammal Zones (CMZs), each defined on the basis of a series of index taxa. Turnover in the arvicoline cricetids, followed by neotomine and sigmodontine cricetids and geomyids, provided the most useful information. Further taxonomic study, particularly with the sciurids, heteromyids, and cricetids, will continue to refine the database and its conclusions. Preliminary examination of the data indicate no rapid bouts of rodent turnover at the Pliocene-Pleistocene boundary about 2.6 Ma, but a pronounced turnover event is recorded in the Meade Basin of Kansas following the Huckleberry Ridge ash-fall at 2.07 Ma. Arvicoline diversity is best modeled by an exponential curve, identifying a late Pleistocene surge in species diversity.
ACKNOWLEDGMENTS
This North American rodent database is the result of field and lab research by many colleagues, and we thank them all for their contributions. In particular, we are grateful for the pioneering work by E.H. Lindsay on fossil rodent phylogeny and the introduction of screen-washing to paleontology by C.W. Hibbard, whose concentrated fieldwork in the Meade Basin of Kansas, along with that of his students, established a biostratigraphic framework for the Central Great Plains. We also appreciate the comments by two anonymous reviewers.
REFERENCES
Akersten, W.A. 1970. Red Light local fauna (Blancan) of the Love Formation, southeastern Hudspeth County, Texas. Bulletin Texas Memorial Museum, 20:1-52.
Albright, L.B. III. 1999. Biostratigraphy and vertebrate paleontology of the San Timoteo Badlands, Southern California. University of California Publications, Geological Sciences, 144:1-121.
Alexeeva, N.V., Erbajeva, M.A., and Khenzykhenova, F.I. 2015. Lasiopodomys brandti in Pleistocene of Transbaikalia and adjacent territories: distribution area, evolutionary development in context of global and regional events. Quaternary International, 355:11-17.
https://doi.org/10.1016/j.quaint.2014.08.017
Arroyo-Cabrales, J., Johnson, E., and Cruz, J.A. 2021. San Josecito Cave and its paleoecological contributions for Quaternary studies in Mexico. Quaternary 4 (4):34.
https://doi.org/10.3390/quaty4040034
Barnosky, A.D. 1985. Late Blancan (Pliocene) microtine rodents from Jackson Hole, Wyoming: biostratigraphy and biogeography. Journal of Vertebrate Paleontology, 5:255-271.
https://doi.org/10.1080/02724634.1985.10011860
Barnosky, A.D. 1993. Mosaic evolution at the population level in Microtus pennsylvanicus; p. 25-59. In Martin, R.A. and Barnosky, A.D. (eds.), Morphological Change in Quaternary Mammals of North America. Cambridge University Press, New York, USA.
Barnosky, A.D. 2004. Faunal dynamics of small mammals through the Pit Sequence. p. 318-331. In Barnosky, A.D. (ed.), Biodiversity Response to Climatic Change in the Middle Pleistocene: The Porcupine Cave Fauna from Colorado. University of California Press, Berkeley, USA.
Barnosky, A.D. and Bell, C.J. 2004. Age and correlation of key fossil sites in Porcupine Cave, p. 64-73. In Barnosky, A.D. (ed.), Biodiversity response to climate change in the middle Pleistocene: the Porcupine Cave Fauna from Colorado. University of California Press, Berkeley, USA.
Bell, C.J. 2000. Biochronology of North American microtine rodents, p. 379-406. In Noller, J.S., Sowers, J.M., and Latti, W.R. (eds.), Quaternary geochronology: methods and applications. American Geophysical Union Reference Shelf 4. Washington DC, USA.
Bell, C.J. and Barnosky, A.D. 2000. The microtine rodents from the Pit Locality in Porcupine Cave, Park County, Colorado. Annals Carnegie Museum, 69:93-134.
Bell, C.J., Lundelius, E.L. Jr., Barnosky, A.D., Graham, R.W., Lindsay, E.H., Ruez, D.R. Jr., Semken H.A. Jr., Webb, S.D., and Zakrzewski, R.J. 2004. The Blancan, Irvingtonian and Rancholabrean mammal ages, p. 232-314. In Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic mammals of North America: biostratigraphy and geochronology. Columbia University Press, New York, USA.
Boellstorff, J. 1978. Chronology of some late Cenozoic deposits from the central United States and the ice ages. Transactions Nebraska Academy of Sciences, 331:35-49.
Carranza-Castañeda, O. and Miller, W.E. 2004. Late Tertiary terrestrial mammals from central Mexico and their relationship to South American immigrants. Revista Brasileira, 7:249-261.
Carranza-Castañeda, O. and Walton, A.H. 1992. Cricetid rodents from the Rancho el Ocote fauna, Late Hemphilllian (Pliocene) of Guanajuato, Mexico. Universidad National Autónoma de México, Instituto de Geologia. Revista, 10:71-93.
Cassiliano, M.L. 1999. Biostratigraphy of Blancan and Irvingtonian mammals in the Fish Creek-Vallecito Creek section, southern California, and a review of the Blancan-Irvingtonian boundary. Journal of Vertebrate Paleontology, 19:169-186.
https://doi.org/10.1080/02724634.1999.10011131
Conrad, G.S. 1980. The biostratigraphy and mammalian paleontology of the Glenns Ferry Formation from Hammett to Oreana, Idaho. PhD thesis. Idaho State University Pocatello, Idaho, USA.
Czaplewski, N.J. 1990. The Verde local fauna: small vertebrate fossils from the Verde Formation, Arizona. San Bernardino County Museum Association, 37:1-39.
Czaplewski, N.J., Mead, J.I., Bell, C.J., Peachey, W.D., and Ku, T-L. 1999. Papago Springs Cave revisited, Part II: vertebrate paleofauna. Oklahoma Museum of Natural History, Occasional Papers, 5:1-41.
Dalquest, W.W. and Carpenter, R.M. 1988. Early Pleistocene (Irvingtonian) mammals from the Seymour Formation, Knox and Baylor Counties, Texas, exclusive of Camelidae. Occasional Papers Museum Texas Tech University, 124:1-28.
Davis, L.C. 1987. Late Pleistocene/Holocene environmental changes in the Central Great Plains of the United Sates: the mammalian record, pp. 88-143. In Graham, R.W., Semken, H.A. Jr., and Graham, M.A. (eds.), Late Quaternary Mammalian Biogeography and Environments of the Great Plains and Prairies. Scientific Papers Illinois State Museum, 22, Springfield, USA.
Domning, D.P. 1969. A list, bibliography, and index of the fossil vertebrates of Louisiana and Mississippi. Transactions Gulf Coast Association of Geological Societies, 19:385-422.
Ezquerro, L., Luzon, A., Simon, J. L., and Liesa, C. 2022. A review of the European Neogene mammal zones from integration of litho-, bio- and magnetostratigraphy in the Teruel Basin. Earth-Science Reviews, 234:104223.
https://doi.org/10.1016/j.earscirev.2022.104223
Feranec, R.S., Hadly, E.A., Blois, J.L., and Barnosky, A.D. 2007. Radiocarbon dates from the fossil deposits of Samwell Cave, Shasta County, California, USA. Radiocarbon, 49:117-121.
Ferrusquía-Villafranca, I., Arroyo-Cabrales, J., Martínez-Hernández, E., Gama-Castro, Ruiz-Gonzáles, J., Polaco, O.J., and Johnson, E. 2010. Pleistocene mammals of Mexico: a critical review of regional chronofaunas, climate change response and biogeographic provinciality. Quaternary International, 217:53-104.
https://doi.org/10.1016/j.quaint.2009.11.036
Fletcher, T.L., Warden, L., Sinninghe Damsté, J.S., Brown, K.J., Rybczynski, N., Gosse, J.C., and Ballantyne, A.P. 2019. Evidence for fire in the Pliocene Arctic in response to amplified temperature. Climate of the Past, 15:1063-1081.
https://doi.org/10.5194/cp-15-1063-2019
Galewski, T., Tilak, M.-k., Sanchez, S., Chevret, P., Paradis, E., and Douzery, E. J. P. 2021. The evolutionary radiation of Arvicolinae rodents (voles and lemmings): relative contribution of nuclear and mitochondrial DNA phylogenies. BMC Evolutionary Biology, 6:80.
https://doi.org/10.1186/1471-2148-6-80
Gillette, D.D. and Miller, W.E. 1999. Catalog of new Pleistocene sites and recovered fossils from Utah, p. 523-530. In Gillette, D.D. (ed.), Vertebrate paleontology in Utah. Utah Geological Survey, Miscellaneous Publication, 99-1.
Gillette, D.D., Bell, C.J., and Hayden, M.C. 1999. Preliminary report on the Little Dell Dam fauna, Salt Lake County, Utah (middle Pleistocene, Irvingtonian land mammal age), p. 495-500. In Gillette, D.D. (ed.), Vertebrate paleontology in Utah. Utah Geological Survey Miscellaneous Publication, 99-1.
Gordon, C.G. 1999. Morphological variation in the dentition of late Pleistocene meadow voles (Microtus pennsylvanicus) from Yarborugh Cave, Bartow County, Georgia. Paludicola, 2:207-231.
Graham, R.W., Semken Jr., H.A., and Graham, M.A. (eds.) 1987. Late Quaternary mammalian biogeography and environments of the Great Plains and prairies. Illinois State Museum, Scientific Papers, 22:1-491.
Grayson, D.K. 2006. The late Quaternary biogeographic histories of some Great Basin mammals (western USA). Quaternary Science Reviews, 25:2964-2991.
https://doi.org/10.1016/j.quascirev.2006.03.004
Guilday, J.E. 1982. Dental variation in Microtus xanthognathus, M. chrotorrhinus, and M. pennsylvanicus (Rodentia, Mammalia). Annals Carnegie Museum of Natural History, 51:211-230.
Gustafson, E.P. 1978. The vertebrate faunas of the Pliocene Ringold Formation, south-central Washington. Bulletin of the Museum of Natural History, University of Oregon, 23:1-62.
Gut, H.J. and Ray, C.E. 1963. The Pleistocene vertebrate fauna of Reddick, Florida. Quarterly Journal Florida Academy of Sciences, 26:315-328.
Guthrie, R.D. 1965. Variability in characters undergoing rapid evolution, an analysis of Microtus molars. Evolution, 19:214-233.
Hager, M.W. 1974. Late Pliocene and Pleistocene history of the Donnelly Ranch vertebrate sit, southwestern Colorado. University of Wyoming, Contributions to Geology, Special Paper, 2:1-62.
Harington, C.R. 2009. Quaternary cave faunas of Canada: a review of the vertebrate remains. Journal of Cave and Karst Studies, 73:162-180.
Harington, C.R. 2011. Pleistocene Vertebrates of the Yukon Territory. Quaternary Science Reviews, 30:2341-2354.
https://doi.org/10.1016/j.quascirev.2011.05.020
Harris, A.H. 1988. Late Pleistocene and Holocene Microtus (Pitymys) (Rodentia: Cricetidae) in New Mexico. Journal of Vertebrate Paleontology, 8:307-313.
https://doi.org/10.1080/02724634.1988.10011713
Harris, A.H. 2014. Pleistocene vertebrates of southwestern USA and northwestern Mexico. UTEP Biodiversity Collections, University of Texas at El Paso; NMSW-Paleo.
Harrison, J.A. 1978. Mammals of the Wolf Ranch local fauna, Pliocene of the San Pedro Valley, Arizona. Museum of Natural History, University of Kansas, Occasional Papers, 73:1-18.
Hart, W.K. and Brueseke, M.E. 1999. Analysis and dating of volcanic horizons from Hagerman Fossil Beds National Monument and a revised interpretation of eastern Glenns Ferry Formation chronostratigraphy. Hagerman Fossil Beds National Monument, Hagerman, ID. Report of work accomplished and scientific results, Order No. 1443-PX9608-97-003:1-37.
Hearst, J.M. 1999. The Mammalian Paleontology and Depositional Environments of the Birch Creek Local Fauna (Pliocene: Blancan), Owyhee County, Idaho. PhD dissertation, University of Kansas, Lawrence, Kansas, USA.
Hibbard, C.W. 1944. Stratigraphy and vertebrate paleontology of Pleistocene deposits of southwestern Kansas. Bulletin Geological Society of America, 55:707-754.
Hibbard, C.W. 1972. Class Mammalia, p. 77-130. In Skinner, M.F. and Hibbard, C.W. (eds.), Early Pleistocene pre-glacial and glacial rocks and faunas of north-central Nebraska. Bulletin American Museum of Natural History, vol. 148.
Hill, C.L. 2001. Pleistocene Mammals of Montana and Their Geologic Context, p. 127-143. In Hill, C.L. (ed.), Mesozoic and Cenozoic Vertebrate Paleontology in the Western Plains and Rocky Mountains, Guidebook for the Field Trips, Society of Vertebrate Paleontology, 61st Annual Meeting. Occasional Paper No. 3, Museum of the Rockies, Bozeman.
Hordijk, K. and de Bruijn, H. 2009. The succession of rodent faunas from the Mio/Pliocene lacustrine deposits of the Florina-Ptolemais-Servia Basin (Greece). Hellenic Journal of Geosciences, 44:21-103.
Jackson, L.E., Nelson, F.E., Huscroft, C.A., Villeneuve, M., Barendregt, R.W., Storer, J.E., and Ward, B.C. 2012. Pliocene and Pleistocene volcanic interaction with Cordilleran ice sheets, damming of the Yukon River and vertebrate paleontology, Fork Selkirk Volcanic Group, west-central Yukon, Canada. Quaternary International, 260:3-20.
https://doi.org/10.1016/j.quaint.2011.08.033
Jakway, G.E. 1958. Pleistocene Lagomorpha and Rodentia from the San Josecito Cave, Nueva Leon, Mexico. Transactions Kansas Academy of Science, 61:313-327.
Jass, C.N. and Bell, C.J. 2011. Arvicoline rodent fauna from the Room 2 excavations in Cathedral Cave, White Pine County, Nevada, and its biochronological significance. Journal of Vertebrate Paleontology, 31:684-699. https://doi.org/10.1080/02724634.2011.563764
Jefferson, G.T. 1991. A catalogue of late Quaternary vertebrates from California: Part 2, mammals. Natural History Museum of Los Angeles County Technical Report, 7:1-129.
Jefferson, G.T., McDonald, H.H., and Livingston, S.D. 2004. Catalogue of Late Quaternary and Holocene fossil vertebrates from Idaho, p. 157-192. In Akersten, W.A., Thompson, M.E., Meldrum, D.J., Rapp, R.A., and McDonald, H.G. (eds.), And Whereas...papers on the vertebrate paleontology of Idaho honoring John A. White, Vol. 2. Idaho Museum of Natural History, Occasional Paper 37.
Jefferson, G.T., Miller, W.E., Nelson, M.E., and Madsen, Jr., J.H. 1994. Catalog of late Quaternary vertebrates from Utah. Natural History Museum of Los Angeles County, Technical Report, 9:1-34.
Kelly, T.S. 1994. Two Pliocene (Blancan) vertebrate faunas from Douglas County, Nevada. PaleoBios, 16:1-23.
Kelly, T.S. 1997. Additional Late Cenozoic (latest Hemphillian to earliest Irvingtonian) mammals from Douglas County, Nevada. PaleoBios, 18:1-31.
Kelly, T.S. 2000. A new Hemphillian (late Miocene) mammalian fauna from Hoye Canyon, west central Nevada. Natural History Museum of Los Angeles County, Contributions in Science, 481:1-21.
Krishtalka, L. and Stucky, R.K. 1985. Revision of the Wind River faunas, early Eocene of central Wyoming, Part 7, revision of Diacodexis (Mammalia, Artiodactyla). Annals Carnegie Museum, 54:413-486.
Kurten, B. and Anderson, E. 1972. The sediments and fauna of Jaguar Cave: II–the fauna. Tebiwa 15:21-45.
Lanphere, M.A., Champion, D.E., Christiansen, R.L., Izett, G.A., and Obradovitch, J.D. 2002. Revised ages of tuffs for the Yellowstone Plateau volcanic field: assignment of the Huckleberry Ridge Tuff to a new geomagnetic polarity event. Bulletin Geological Society of America, 114:559-568.
Lindsay, E.H. and Jacobs, L.L. 1985. Pliocene small mammal fossils from Chihuahua, Mexico. Universidad Nacional Autonóma de México, Instituto de Geología, Paleontología Mexicana, 51:1-45.
Lindsay, E.H., Jacobs, L.L., and Tessman, N.D. 2006. Vertebrate fossils from Yepómera, Chihuahua, Mexico - the University of Arizona connection, pp. 19-32. In Carranza-Castañeda, O. and Lindsay, E.H. (eds.), Advances in Late Tertiary Vertebrate Paleontology in Mexico and the Great Biotic Interchange. Universidad Nacional Autonóma de México, Instituto de Geología, Publicación Especial No. 4.
Lindsay, E.H., Mou, Y., Downs W., Pederson, J., Kelly, T.S., Henry, C., and Trexler J. 2002. Recognition of the Hemphillian/Blancan boundary in Nevada. Journal of Vertebrate Paleontology, 22:429-442.
https://doi.org/10.1671/0272-4634(2002)022[0429:ROTHBB]2.0.CO;2
Lovelace, D.M., Redman, C.M., Minckley, T.A., Schubert, B.W., Mahan, S., Wood, J.R., McGuire, J.L., Laden, J., Bitterman, K., Heiniger, H., Fenderson, L., Cooper, A., Mitchell, K.J., and Meachen, J.A. 2023. An age-depth model and revised straigraphy of vertebrate-bearing units in Natural Trap Cave, Wyoming. Quaternary International, 647-648:4-21.
https://doi.org/10.1016/j.quaint.2022.02.008
Lundelius, E.L. Jr, Churcher, C.S., Downs, T., Harington, C.R., Lindsay, E.H., Schultz, G.E., Semken, H.A. Jr., Webb, S.D., and Zakrzewski R.J. 1987. The North American Quaternary sequence, p. 211-235. In Woodburne M.O. (ed.), Cenozoic mammals of North America: geochronology and biostratigraphy. University California Press, Berkeley, USA.
Mack, G.H., Dubar, N., and Foster, R. 2009. New sites of 3.1-Ma pumice beds in axial-fluvial strata of the Camp Rice and Palomas Formations, southern Rio Grande Rift. New Mexico Geology, 31:31-37.
Marcolini, F. and Martin, R.A. 2008. Mosaic evolution in first lower molars of Pliocene Ogmodontomys (Rodentia: Arvicolidae) from the Meade Basin of Southwestern Kansas (USA). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 249:313-332.
https://doi.org/10.1127/0077-7749/2008/0249-0313
Martin, J.E., Hargrave, J.E., and Ball, J.L. 2018. Refinements of the Late Miocene Fort Rock Formation in south-central Oregon, the McKay Formation in northern Oregon, and the timing of the Prosomys intercontinental dispersal event. Proceeding South Dakota Academy of Science, 97:165-179.
Martin, L.D. 1979. The biostratigraphy of arvicoline rodents in North America. Transactions Nebraska Academy of Science, 7:91-100.
Martin, L.D. and Schultz, C.B. 1985. Small mammals of the Seneca and Sappa local faunas (post-Ogallala of Nebraska), p. 163-179. In Dort, W. (ed.), Institute for Tertiary Quaternary Studies, TER-QUA symposium series, Vol. 1. Nebraska Academy of Sciences, Lincoln.
Martin, R.A. 1974. Fossil mammals from the Coleman IIA fauna, Sumter County, p. 35-99. In Webb, S.D. (ed.), Pleistocene Mammals of Florida. Gainesville, University of Florida Press, Florida, USA.
Martin, R.A. 1991. Evolutionary relationships and biogeography of late Pleistocene prairie voles from the eastern United States, p. 251-260. In Purdue, J.R., Klippel, W.E., and Styles, B.W. (eds.), Beamers, Bobwhites, and Blue-Points: Tributes to the Career of Paul W. Parmalee. Illinois State Museum Scientific Papers, No. 23, Springfield, USA.
Martin R.A. 1993. Patterns of variation and speciation in Quaternary rodents, p. 226-280. In Martin, R.A. and Barnosky, A.D. (eds.), Morphological change in Quaternary mammals of North America. Cambridge University Press, New York, USA.
Martin, R.A. 1995. A new middle Pleistocene species of Microtus (Pedomys) from the southern United States, with comments on the taxonomy and early evolution of Pedomys and Pitymys in North America. Journal of Vertebrate Paleontology, 15:171-186.
https://doi.org/10.1080/02724634.1995.10011216
Martin R.A. 2003. Biochronology of latest Miocene through Pleistocene arvicolid rodents from the central Great Plains of North America. Coloquios de Paleontologia Volumen Extraordinario, I:373-383.
Martin R.A. 2005. Preliminary correlation of Florida and Great Plains Pliocene and Pleistocene local faunas based on rodent biostratigraphy. Bulletin Florida State Museum, 45:363-367.
Martin R.A. 2008. Arvicolidae, p. 480-497. In Janis, C.M., Gunnell, G.G., and Jacobs, L. (eds.), Evolution of Tertiary Mammals of North America. Vol. II. Cambridge University Press, New York, USA.
Martin R.A. 2010. The North American Promimomys immigration event. Paludicola, 8:14-21.
Martin, R.A. 2014. Further notes on the Port Kennedy arvicolid rodents. Paludicola, 9:13-20.
Martin, R.A. 2016. Geomys tyrioni, a new species of dwarf pocket gopher from the Meade Basin of southwestern Kansas. Journal of Mammalogy, 97:949-959.
https://doi.org/10.1093/jmammal/gyw024
Martin, R.A. 2021. Correlation of Pliocene and Pleistocene fossil assemblages from the central and eastern United States: toward a continental rodent biochronology. Historical Biology, 33(6):880-896.
Martin, R.A. and Prince, R.H. 1990. Variation and evolutionary trends in the dentition of late Pleistocene Microtus pennsylvanicus from three levels in Bell Cave, Alabama. Historical Biology, 4:117-129.
Martin, R.A. and O’Bryan, K. 2014. Size and shape variation in late Pleistocene pine vole (Mammalia: Rodentia: Pitymys pinetorum) first lower molars from three caves in Kentucky and Georiga. Paludicola, 9:159-166.
Martin, R.A. and Peláez-Campomanes, P. 2014. Diversity dynamics of the late Cenozoic rodent community from south-western Kansas: the influences of historical processes on community structure. Journal of Quaternary Science, 29:221-231.
https://doi.org/10.1002/jqs.2695
Martin, R.A. and Peláez-Campomanes, P. 2016. Extinction rates of the Meade Basin rodents: application to current biodiversity losses. Lethaia, 50:217-221.
https://doi.org/10.1111/let.12195
Martin, R.A. and Zakrzewski, R.J. 2019. On the ancestry of woodrats: Journal of Mammalogy, 100:1564-1582.
Martin, R.A. and Zakrzewski, R.J. 2022. An unusual arvicoline-like cricetid rodent from Ellesmere Island in the Canadian Arctic. Journal of Vertebrate Paleontology, 42(3):e2167605.
https://doi.org/10.1080/02724634.2023.2167605
Martin, R.A., Crockett, C.P., and Marcolini, F. 2006. Variation of the schmelzmuster and other enamel characters in molars of the primitive Pliocene vole Ogmodontomys from Kansas. Journal of Mammalian Evolution, 13:223-241.
https://doi.org/10.1007/s10914-006-9018-2
Martin, R.A., Fox, D.L., Urevig, A., Dean, M.R.P., Rountrey, A.N., and Peláez-Campomanes P. 2021. Fluctuation in body mass of cotton rats and pocket gophers during the late Cenozoic in the Meade Basin of Kansas: possible effects of the Huckleberry Ridge ash-fall. Historical Biology, 33: 983-994.
https://doi.org/10.1080/08912963.2021.1959576
Martin, R.A., Peláez-Campomanes, P., Honey, J.G., Marcolini, F., and Akersten, W.A. 2011. Five million years of pocket gopher history in the Meade Basin of southwestern Kansas and northwestern Oklahoma. Journal of Vertebrate Paleontology, 31:866-884.
https://doi.org/10.1080/02724634.2011.576729
Martin, R.A., Peláez-Campomanes, P., Honey, J.G., Fox, D.L., Zakrzewski, R.J., Albright, L.B., Lindsay, E.H., Opdyke, N.D., and Goodwin, H.T. 2008. Rodent community change at the Pliocene-Pleistocene transition in southwestern Kansas and identification of the Microtus immigration event on the central Great Plains. Palaeogeography, Palaeoclimatology, Palaeoecology, 267:196-207.
https://doi.org/10.1016/j.palaeo.2008.06.017
Martin, R.A., Siefker, A., and Marcolini, F. 2009. Modelling the morphology and evolution of the linea sinuosa (crown-root junction) in arvicolid rodents; a test with Pliocene Ogmodontomys from Kansas, USA. Lethaia, 42(2):155-166.
https://doi.org/10.1111/j.1502-3931.2008.00139.x
May, S.R., Sarna-Wojcicki, A.M., Lindsay, E.H., Woodburne, M.O., Opdyke, N.D., Qan, E., Wahl, D.B., and Olson, H. 2014. Geochronology of the upper Alturas Formation, northern California: implications for the Hemphillian-Blancan North American Land Mammal Age boundary. Palaeontologia Electronica, 17.3.43A:1-13.
https://doi.org/10.26879/493
May, S.R., Woodburne, M.O., Lindsay, E.H., Albright, L.B., Sama-Wojcicki, A., Wan, E., and Wahl, D.B. 2011. Geology and mammalian paleontology of the Horned Toad Hills, Mojave Desert, California, USA. Palaeontologia Electronica, 14(23A):1-63.
https://palaeo-electronica.org/2011_3/11_may/index.html
McDonald, H.G. 1995. Gravigrade xenarthrans from the early Pleistocene Leisey Shell Pit 1A, Hillsborough County, Florida, p. 345-374. In Hulbert, Jr., R.C., Morgan, G.S., and Webb, S.D. (eds.), Paleontology and geology of the Leisey Shell Pits Early Pleistocene of Florida. Bulletin Florida State Museum, Gainesville, USA.
McDonald, H.G., Morgan, G.S., and White, R.S. Jr. 2022. The giant marmot Paenemarmota (Mammalia: Rodentia: Sciuridae) from the Pliocene (Blancan) of southwestern New Mexico, p. 107-121. In Morgan, G.S. Baskin, J.A., Czaplewski, N.J., Lucas, S.G., McDonald, H.G., Mead, J.I., White, R.S., Jr., and Lichtig, A.J. (eds.), Late Cenozoic vertebrates from the American southwest: a tribute to Arthur H. Harris. New Mexico Museum of Natural History and Science, Albuquerque, USA.
Mead, J.I., Manganaro, C., Repenning. C.A., and Agenbroad, L.D. 1993. Early Rancholabran mammals from Salamander Cave, Black Hills, South Dakota, p. 458-481. In Stewart, K.M. and Seymour, K.L. (eds), Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals; Tributes to the Career of C. S. (Rufus) Churcher. University of Toronto Press, Toronto, Canada.
Mein, P. 1975. Résultats du groupe de travail des vertébrés: Biozonation du Néogène méditerranéen à partir des mammifères, p. 78-91. In Senes, J. (ed.), Report on Activity of the RCMNS Working Groups (1971-1975), Bratislava, Slovakia.
Mein, P. 1976. Biozonation du Néogène méditerranéen à partir des mammifères. Proceedings of VI Congress RCMNS, Bratislava, September 4-7, 1976, vol. 2.
Morgan, G.S. 2005. The Great American Biotic Interchange in Florida. Bulletin of the Florida Museum of Natural History, 45:271-311.
Morgan, G.S. and Harris, A.H. 2015. Pliocene and Pleistocene vertebrates of New Mexico, p. 233-428. In Lucas, S.G. and Sullivan, R.M. (eds.), Fossil Vertebrates in New Mexico. New Mexico Museum of Natural History & Science, Bulletin No. 68.
Morgan, G.S. and Hulbert, Jr., R.C. 1995. Overview of the geology and paleontology of the Leisey Shell Pit local fauna, Hillsborough County, Florida, p. 1-92. In Hulbert, Jr., R.C., Morgan, G. S. and Webb, S.D. (eds.), Paleontology and geology of the Leisey Shell Pits Early Pleistocene of Florida. Bulletin Florida State Museum, Gainesville, USA.
Morgan, G.S. and White, J.A. 1995. Small mammals (Insectivora, Lagomorpha, and Rodentia) from the early Pleistocene (early Irvingtonian) Leisey Shell Pit local fauna, Hillsborough County, Florida, p. 345-375. In Hulbert, Jr., R.C., Morgan, G.S., and Webb, S.D. (eds.), Paleontology and geology of the Leisey Shell Pits Early Pleistocene of Florida. Bulletin Florida State Museum, Gainesville, USA.
Morgan, G.S. and White, R.S. Jr. 2005. Miocene and Pliocene vertebrates from Arizona, p. 115-135. In Heckert, A.B. and Lucas, S.G. (eds.), Vertebrate Paleontology in Arizona. New Mexico Museum of Natural History & Science, Bulletin No. 29.
Mou, Y. 1997. A new arvicoline species (Rodentia: Cricetidae) from the Pliocene Panaca Formation, Southeast Nevada. Journal of Vertebrate Paleontology, 17:376-383.
Mou, Y. 1998. Schmelzmuster of Mimomys panacaensis, p. 79-90. In Tomida, Y.Y., Flynn, L.J., and Jacobs, L.L. (eds.), Advances in Vertebrate Paleontology and Geochronology. National Science Museum Monographs No. 14. Japan, Tokyo.
Mou, Y. 2011. Cricetid rodents from the Pliocene Panaca Formation, southeastern Nevada, USA. Palaeontologia Electronica, 14:1-53.
https://palaeo-electronica.org/2011_3/19_mou/index.html
Murray, L.K., Ruez, D.R. Jr., and Bell, C.J. 2011. New perspectives on lagomorph and rodent biochronology in the Anza-Borrego Desert of southern California, USA. Palaeontologia Electronica, 14(36A):1-56.
https://palaeo-electronica.org/2011_3/12_murray/index.html
Nadachowaki, A. 1990. Comments on variation, evolution, and phylogeny of Chionomys (Arvicolidae), p. 353-368. In Fejfar, O. and Heinrich, W.D. (eds.), International Symposium on the evolution, phylogeny and biostratigraphy of arvicolids (Rodentia, Mammalia, Prague, Czachoslovakia.
Neville, C., Opdyke, N.D., Lindsay, E.H., and Johnson, N.M. 1979. Magnetic stratigraphy of Pliocene Deposits of the Glenns Ferry Formation, Idaho, and its implications for North American mammalian biostratigraphy. American Journal of Science, 279:503-526.
Pacheco-Castro, A., Carranza-Castañeda, O., and Jimenez-Hidalgo, E. 2019. A new species of Sigmodontinae (Rodentia) from the late Hemphillian of central Mexico, and comments on the possible radiation of this group. Revista Mexicana Ciencias Geológicas, 36(3):321-333.
https://doi.org/10.22201/cgeo.20072902e.2019.3.1162
Paulson, G.R. 1961. The mammals of the Cudahy fauna. Papers Michigan Academy Sciences, Arts and Letters, 46:127-153.
Pfaff, K.S. 1990. Irvingtonian Microtus, Pedomys, and Pitymys (Mammalia, Rodentia, Cricetidae) from Trout Cave No. 2, West Virginia. Annals Carnegie Museum, 59:105-134.
Prassack, T., Walkup, L.C, Hart, W., Wan, E., and Starratt, S.W. 2021. A horse, an otter, and a bear walk into a bar and get carded: refining ages for important fossil localities at Hagerman Fossil Beds National Mounument, Idaho, USA. Geol Soc Amer. Connects 2021, From the Afar Rift to Alaska area (and the Oregon Plateau in between), Honoring the career and contributions of William K. Hart, Session 67, Abstract and recorded presentation 67-8; Oct 10-13; Portland, Oregon, USA.
Rekovets, L. and Nadachowski, A. 1995. Pleistocene voles (Arvicolidae) of the Ukraine. Paleontologia a Evolutió, 28-29:145-245.
Rensberger, J.M. and Barnosky, A.D. 1993. Short-term fluctuations in small mammals of the late Pleistocene from eastern Washington, p. 299-342. In Martin, R.A. and Barnosky, A.D. (eds.), Morphological change in Quaternary mammals of North America. Cambridge University Press, New York, USA.
Repenning, C.A. 1987. Biochronology of the microtine rodents of the United States, p. 314-323. In Woodburne, M.O. (ed.), Cenozoic mammals of North America: geochronology and biostratigraphy. University California Press, Berkeley, USA.
Repenning, C.A. 1992. Allophiaomys and the age of the Olyor Suite, Krestovka Sections, Yakutia. U.S. Geological Survey Bulletin, 2037:1-98.
Repenning, C.A. 2003. Mimomys in North America, p. 469-512. In Flynn, L.J. (ed.), Vertebrate Fossils and Their Context: Contributions in Honor of Richard H. Tedford. Bulletin American Museum of Natural History, vol. 279.
Repenning, C.A. and Grady, F. 1988. The microtine rodents of the Cheetah Room Fauna, Hamilton cave, West Virginia, and the spontaneous origin of Synaptomys. U.S. Geological Survey Bulletin, 1853:1-32.
Repenning C.A., Weasma T.R., and Scott G.R. 1995. The early Pleistocene (latest Blancan-earliest Irvingtonian) Froman Ferry fauna and history of the Glenns Ferry Formation, southwestern Idaho. U.S. Geologic Survey Bulletin, 2105:1-86.
Rogers, K.L., Larson, E.E., Smith, G., Katzman, D., Smith, G.R., Cerling, T., Wang, Y., Baker, G., Lohmann, K.C., Repenning, C.A., Patterson, P., and Mackie, G. 1992. Pliocene and Pleistocene geologic and climatic evolution in the San Luis Valley of south-central Colorado. Palaeogeography, Palaeoclimatology, Palaeoecology, 94:55-86.
https://doi.org/10.1016/0031-0182(92)90113-J
Rogers, K.L., Repenning, C.A., Forester, R.M., Larson, E.E., Hall, S.A., Smith, G.R., Anderson, E., and Brown, T.J. 1985. Middle Pleistocene (Late Irvingtonian: Nebraskan) climatic change in south-central Colorado. National Geographic Research, 1:535-563.
Rogers, K.L., Repenning, C.A., and Luiszer, F.G. 2000. Geologic history, stratigraphy, and paleontology of SAM Cave, north-central New Mexico. New Mexico Geology, 22:89-116.
Ronez, C., Martin, R.A., Kelly, T.S., Barbiére, F., and Pardiñas, U.F.J. 2021. A brief critical review of sigmodontine rodent origins, with emphasis on paleontological data, p. 1-26. In Pardiñas, U.F.J. and Galliari, C. (eds.), Mastozoología Neotropical Sección Especial, El Último Naturalista Tipólogia: Contribuciones en honor a Elio Massola (1936-2001), 28.
Ruez, D.R. 2007. Effects of climate change on mammalian fauna composition and structure during the advent of North American continental glaciations in the Pliocene. PhD dissertation, University of Texas at Austin, USA.
Ruez, D.R. 2009. Revision of the Blancan (Pliocene) mammals from Hagerman Fossil Beds Monument, Idaho. Journal of the Idaho Academy of Science, 45:1-143.
Ruez, D.R. and Gensler, P.A. 2008. An unexpected early record of Mictomys vetus (Arvicolinae, Rodentia) from the Blancan (Pliocene) Glenns Ferry Formation, Hagerman Fossil Beds National Monument, Idaho. Journal of Vertebrate Paleontology, 82:638-642.
https://doi.org/10.1666/06-098.1
Sankey, J.T. 1996. Vertebrate paleontology and magnetostratigraphy of the upper Glenns Ferry (latest Pliocene) and lower Bruneau (Pliocene-Pleistocene) Formations, near Murphey, southwestern Idaho. Journal of Idaho Academy of Sciences, 32:71-88.
Saunders, J.J. 1977. Late Pleistocene vertebrates of the western Ozark Highlands, Missouri. Illinois State Museum, Reports of Investigations, 33:1-118.
Schultz, G.E. 2010. Pleistocene (Irvingtonian, Cudahyan) vertebrates from the Texas Panhandle, and their geographic and paleontologic significance. Quaternary International, 217:195-224.
https://doi.org/10.1016/j.quaint.2009.12.012
Schultz, C.B., Martin, L.D., Tanner, L.G., and Corner, R.G. 1977. Provincial land mammal ages for the North American Quaternary, p. 408. Abstracts of X INQUA Congress.
Schultz, C.B., Martin, L.D., Tanner, L.G., and Corner, R.G. 1978. Provincial land mammal ages for the North American Quaternary. Transactions Nebraska Academy of Sciences Affiliated Society, 5:59-64.
Sertich, J.J.W., Stucky, R.K., McDonald, H.G., Newton, C., Fisher, D.C., Scott, E., Demboski, J.R., Lucking, C., McHorse, B.K., and Davis, E.B. 2014. High elevation (Late Pleistocene) vertebrate faunas from the Ziegler Reservoir fossil site, Snowmass Vikkage, Colorado. Quaternary Research, 82:504-517.
https://doi.org/10.1016/j.yqres.2014.08.002
Shotwell, J.A. 1956. Hemphillian mammalian assemblage from northeastern Oregon. Bulletin Geological Society of America, 67:717-738.
Shotwell, J.A. 1967. Peromyscus of the late Tertiary in Oregon. Museum of Natural History, University of Oregon Bulletin, 5:1-35.
Singer, B.S. 2014. A Quaternary geomagnetic instability time scale. Quaternary Geochronology 21:29-52. https://doi.org/10.1016/j.quageo.2013.10.003
Smith, K.S. and Cifelli, R.L. 2000. A synopsis of the Pleistocene vertebrates of Oklahoma. Oklahoma Geological Survey Bulletin, 147:1-36.
Steppan, S.J. and Schenk, J.J. 2017. Muroid rodent phylogenetics: 900-species tree reveals increasing diversification rates. PLoS ONE, 12(8):e0183070.
https://doi.org/10.1371/journal.pone.0183070
Storer, J.E. 2003. The eastern Beringian vole Microtus deceitensis (Rodentia, Muridae, Arvicolinae) in late Pliocene and early Pleistocene faunas of Alaska and Yukon. Quaternary Research, 60:84-93.
https://doi.org/10.1016/S0033-5894(03)00067-X
Storer, J.E. 2004. A middle Pleistocene (late Irvingtonian) mammalian fauna from Thistle Creek, Klondike Goldfields region of Yukon Territory, Canada. Paludicola, 4:137-150.
Strain, W.S. 1966. Blancan mammalian fauna and Pleistocene formations, Hudspeth County, Texas. Bulletin Texas Memorial Museum, 10:1-55.
Tedford, R.H. 1970. Principles and practices of mammalian geochronology in North America. Proceedings of the North American Paleontological Convention, Part F:666-703.
Tesakov, A. and Bondarev, A. 2021. Down to the roots of lemmings: a new species of basal lemming from the upper Pliocene of west Siberia. Journal of Vertebrate Paleontology. 41(5):e2036173.
https://doi.org/10.1080/02724634.2021.2036173
Tomida, Y. 1987. Small mammal fossils and correlation of continental deposits, Safford and Duncan basins, Arizona, USA. National Science Museum, Tokyo, Japan.
van Dam, J. A., Mein, P., Garcés, M., van Balen, R.T., Furió, M., and Acalá, L. 2023. A new rodent chronology for the late Neogene of Spain. Geobios, 76:53-74.
https://doi.org/10.1016/j.geobios.2023.01.001
Van der Meulen, A.J. 1978. Microtus and Pitymys (Arvicolidae) from Cumberland Cave, Maryland, with a comparison of some New and Old World species. Annals Carnegie Museum, 47:101-145.
Van de Weerd, A. 1979. Early Ruscinian rodents and lagomorphs (Mammalia) from the lignites near Ptolemais (Macedonia, Greece). Koninklijke Nederlandse Akademie van Wetenschapen, Proceedings B, 82:127-180.
Vasilyn, D., Böhme, M., and Zazhigin, V. 2014. Neogene amphibians and reptiles (Anura, Gekkota, Lacertilia, and testudines) from the south of western Siberia, and northeastern Kazakhstan. PeerJ.
https://doi.org/10.7717/peerj.3025
von Koenigswald, W. 1980. Schmelzstruktur und morphologie in den molaren der Arvicolidae (Rodentia). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 539:1-129.
von Koenigswald, W. and Martin, L.D. 1984. The status of the genus Mimomys (Arvicolidae, Rodentia, Mamm.) in North America. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 168:108-124.
Voorhies, M.R. 1990. Vertebrate biostratigraphy of the Ogallala Group in Nebraska, p. 115-151. In Gustavson, T.C. (ed.), Geological Framework and Regional Hydrology: Upper Cenozoic Blackwater Draw and Ogallala Formations, Great Plains. Bureau of Economic Geology, University of Texas, Austin, USA.
Walkup L., Prassack, K.A., Hart, W.K., and Wan, E. 2016: Tephrochronology and stratigraphy of silicic and basaltic volcanic ash layers at Hagerman Fossil Beds National Monument, Idaho, USA [abstract]. Amer Geophysical Union Fall Meeting Abstracts V11C-2799; December 12-16, 2016. San Francisco, California, USA.
Walkup, L., Prassack, K., Hart, W., Wan, E., and Premo, W.R. 2021. New mapping and tephrochonrology at Hagerman Fossil Beds National Monument, Idaho, USA - a project decades in the making. Geol Soc Amer. Connects 2021, From the Afar Rift to Alaska area (and the Oregon Plateau in between), Honoring the career and contributions of William K. Hart, Session 67, Abstract and recorded presentation 67-8; Oct 10-13; Portland, Oregon, USA. https://gsa.confex.com/gsa/2021AM/webprogram/Paper364824.html
Westgate, J.A., Preece, S.J., Froese, D.J., Telka, A.M., Storer, J.E., Pearce, N.J.G., Enkin, R.J., Jackson Jr., L.E., LeBarge, W., and Perkins, W.T. 2009. Gold Run tephra: a Middle Pleistocene stratigraphic and paleoenvironmental across west-central Yukon Territory, Canada. Canadian Journal of Earth Sciences, 46:465-478.
Whistler, D.P. 1990. A late Pleistocene (Rancholabrean) fossil assemblage from the northwestern Mojave Desert, California. San Bernardino County Museum Association Quarterly, 37:3-17.
Wilkins, K.T. 1985. Pocket gophers of the genus Thomomys (Rodentia; Geomyidae) from the Pleistocene of Florida. Proceedings Biological Society of Washington, 98:761-767.
Williams, D.R. 2009. Small mammal faunal stasis in Natural Trap Cave (Pleistocene-Holocene), Bighorn Mountains, Wyoming. Unpublished PhD thesis, University of Kansas, Lawrence.
Wilson, R.L. 1967. The Pleistocene vertebrates of Michigan. Papers of the Michigan Academy of Science, Arts, and Letters, 52:197-234.
Winkler, A.J. and Gose, W. 2003. Mammalian fauna and paleomagnetics of the middle Irvingtonian (early Pleistocene) Fyllan Cave and Kirchen Door localities, Travis County, Texas, p. 215-261. In Shubert, B.J., Mead, J.I., and Graham, R.W. (eds.), Ice Age Cave Faunas of North America. Bloomington, Indiana University Press, USA.
Winkler, A.J., and Grady, F. 1990. The middle Pleistocene rodent Atopomys (Cricetidae: Arvicolinae) from the eastern and south-central United States. Journal of Vertebrate Paleontology, 10:484-490.
Withnell, C.B., Bell, C.J. and Jass, C.N. 2021. Extraordinary accumulation of arvicoline rodents from Little Dell Dam (Pleistocene), Utah. Southwestern Naturalist, 66:91-101.
Withnell, C.B, Joannes-Boyau, R., and Bell, C.J. 2020. A reassessment of the age of the fauna from Cumberland Bone Cave, Maryland (middle Pleistocene) using coupled U-series and electron spin resonance dating (ESR). Quaternary Research, 97:187-198.
https://doi.org/10.1017/qua.2020.30
Woodburne, M.O. 2004a. Definitions, p. xxi-xv. In Woodburne M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: biostratigraphy and geochronology. Columbia University Press, New York, USA.
Woodburne, M.O. 2004b. Principles and Procedures, p. 1-20. In Woodburne M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: biostratigraphy and geochronology. Columbia University Press, New York, USA.
Zakrzewski, R.J. 1967. The primitive vole, Ogmodontomys, from the late Cenozoic of Kansas and Nebraska. Papers of the Michigan Academy of Science, Arts, and Letters, 52:133-150.
Zakrzewski, R.J. 1969. The rodents from the Hagerman Local Fauna, upper Pliocene of Idaho. Contributions from the Museum of Paleontology, University of Michigan, 23:1-36.
Zakrzewski, R.J. 1984. New arvicolines (Mammalia: Rodentia) from the Blancan of Kansas and Nebraska, p. 200-217. In Genoways, H.H., and Dawson, M.R. (eds.), Contributions in quaternary vertebrate paleontology: a volume in memorial to John E. Guilday. Special Publication 8, Carnegie Museum Natural History, Pittsburgh.
Zazhigin, V.S. 1980. Late Pliocene and Anthropogene rodents of the south of western Siberia. Nauka, Moscow. (In Russian)

