 Middle Eocene cartilaginous fishes (Vertebrata: Chondrichthyes) of the Dnieper–Donets Basin, northern Ukraine
Middle Eocene cartilaginous fishes (Vertebrata: Chondrichthyes) of the Dnieper–Donets Basin, northern Ukraine
Article number: 26.2.a32
https://doi.org/10.26879/1283
Copyright Paleontological Society, August 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 15 March 2023. Acceptance: 28 July 2023.
ABSTRACT
Marine basins that existed in present-day Ukraine during the Eocene harboured various groups of cartilaginous and bony fishes, reptiles, aquatic birds, and marine mammals. Fish remains from Paleogene deposits of Kyiv and its vicinities were first collected and described by O.S. Rogovich in the mid-19th century. Here we have carried out a re-examination of chondrichthyan fossils from Rogovich’s collection and evaluated several later records, all of which were recovered from middle Eocene deposits. In total, 88 specimens represented by teeth, vertebrae, and other skeletal elements were analysed and described. As a result, the sample revealed to a single chimaeriform species (Edaphodon bucklandi), and 12 shark and two ray taxa, respectively. Ten sharks were identified to species level, whereas the rays could be identified only at higher systematic ranks (Myliobatidae, Myliobatiformes). Several nomenclatural changes are proposed here, including the synonimisation of Carcharodon megalotis, C. lanceolatus, C. productus, Lamna cuspidata, L. denticulata, L. compressa, L. (Odontaspis) hispida, L. lata, Oxyrhina brevidens, and O. biflena with more recently proposed taxa. Seven species erected by Rogovich (Galeocerdo paradoxus, Otodus microtus, Lamna elegans, Oxyrhina falcata, Oxyrhina leptodon, Chomatodus dubius, and Hybodus helophorus) are suggested to most likely be nomina dubia. Many identified taxa represent the epi- and mesopelagic fishes and only a relatively small number of them belong to benthopelagic, demersal, and bathydemersal forms. The studied sample is of important historical and scientific value and substantially contribute to the understanding of the palaeodiversity of Eocene marine ecosystems that existed in present-day Ukraine and generally in Eastern Europe.
Oleksandr Kovalchuk. Department of Palaeontology, National Museum of Natural History, National Academy of Sciences of Ukraine, Bohdan Khmelnytskyi 15, Kyiv 01054 Ukraine. biologiest@ukr.net and Department of Palaeozoology, Faculty of Biological Sciences, University of Wrocław, Sienkiewicza 21, Wrocław 50-335, Poland. Corresponding author
Jürgen Kriwet. Department of Palaeontology, Faculty of Earth Sciences, Geography and Astronomy, University of Vienna, Josef-Holaubek-Platz 2, Vienna 1190, Austria. juergen.kriwet@univie.ac.at and Vienna Doctoral School of Ecology and Evolution (VDSEE), University of Vienna, Josef-Holaubek-Platz 2, Vienna 1190, Austria
Kenshu Shimada. Department of Environmental Science and Studies and Department of Biological Sciences, DePaul University, Chicago, IL, USA. kshimada@depaul.edu and Sternberg Museum of Natural History, Fort Hays State University, Hays, KS, USA
Tamara Ryabokon. Institute of Geological Sciences, National Academy of Sciences of Ukraine, O. Honchara 55b, Kyiv 01054, Ukraine. tamararyabokon@gmail.com
Zoltán Barkaszi. Department of Palaeontology, National Museum of Natural History, National Academy of Sciences of Ukraine, Bohdan Khmelnytskyi 15, Kyiv 01054 Ukraine. zlbarkasi@ukr.net
Anastasiia Dubikovska. Department of Biology and Biology Teaching Methodology, Faculty of Natural Sciences and Geography, A.S. Makarenko Sumy State Pedagogical University, Romenska 87, Sumy 40002, Ukraine. oakovska@gmail.com
Galina Anfimova. Department of Geology, National Museum of Natural History, National Academy of Sciences of Ukraine, Bohdan Khmelnytskyi 15, Kyiv 01054 Ukraine. anfimova77@ukr.net
Svitozar Davydenko. Schmalhausen Institute of Zoology, National Academy of Sciences of Ukraine, Bohdan Khmelnytskyi 15, 01054, Kyiv, Ukraine. yurgenvorona@ukr.net
Keywords: chimaeras; sharks; rays; taxonomy; diversity; Europe
Final citation: Kovalchuk, Oleksandr, Kriwet, Jürgen, Shimada, Kenshu, Ryabokon, Tamara, Barkaszi, Zoltán, Dubikovska, Anastasiia, Anfimova, Galina, and Davydenko, Svitozar. 2023. Middle Eocene cartilaginous fishes (Vertebrata: Chondrichthyes) of the Dnieper–Donets Basin, northern Ukraine. Palaeontologia Electronica, 26(2):a32.
https://doi.org/10.26879/1283
palaeo-electronica.org/content/2023/3925-eocene-chondrichthyans-of-kyiv
Copyright: August 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
The Paleogene was a period of major global changes in both marine environments and biota. It was characterised by the continuous break-up of the supercontinent of Laurasia and opening the Norway-Greenland Sea of the North Atlantic and its connection with the Paleoarctic ocean. At that time, the Neo-Tethys has been closed because of collision of the African and Indian plates with the Eurasian plate, Australia separated from Antarctica, and the Circum-Antarctic current emerged. Warmhouse climate changed to global coolhouse climate disrupted by short time hyperthermal and cooling/glaciation events (e.g., Zachos et al., 2001; Prothero, 2021; Speijer et al., 2020). The K-Pg boundary extinction event left the deep-sea realm seemingly unaffected while causing a significant biodiversity loss among pelagic marine vertebrates and durophagous demersal feeders on the continental shelf (Patterson, 1993; Kriwet and Benton, 2004; Sibert and Norris, 2015; Adnet et al., 2020; Guinot and Condamine, 2023).
As a result, new ecosystems with new ecological niches, favouring new faunistic compositions within food webs, appeared, leading to exceptional radiation and diversification among vertebrates, including fishes. At the Paleocene-Eocene boundary, volcanism and orogeny triggered an enhanced supply of greenhouse gases into the atmosphere leading to the rise of sea surface temperatures and sea level, ocean deoxygenation, and changes in Earth’s carbon cycle (Jenkyns, 2003; Zachos et al., 2008; Miller et al., 2009; Sluijs et al., 2014; Hessler et al., 2017; Dykan et al., 2018). The organic flux into the deep sea collapsed, but a subsequent major biochemical recovery may have contributed to the creation of new evolutionary opportunities for a wide range of marine organisms (D’Hondt, 2005). The Paleocene-Eocene Tethyan realms, similarly to reef ecosystems, harboured biodiversity hotspots of vertebrates in the warm and shallow marine environments (Guinot and Cavin, 2016).
The marine basin of the Dnieper-Donets depression that existed in the territory of present-day northern Ukraine during most of the Eocene, namely in the early Ypresian, Lutetian, Bartonian, and Priabonian stages, connected the basins located to the east (Turan and Siberian Seas) and west (North Sea) (Ronov and Khain, 1961; Savytska, 1996; Beniamovski, 2005, 2007; Akhmetiev, 2010; Vasilieva, 2018). This created favourable conditions for the development of diverse vertebrate assemblages (Bosboom et al., 2017 and references therein).
A large number of Eocene taxa of various vertebrate groups have been reported from this basin, including elasmobranchs (Glickman, 1964; Udovichenko and Nessov, 1987; Udovichenko, 2006, 2009), bony fishes (Bannikov, 2010; Bratishko, 2011, 2013), sea turtles (Averianov, 2002; Danilov et al., 2011; Zvonok, 2011; Zvonok et al., 2013; Zvonok and Danilov, 2017), crocodiles (Zvonok and Skutschas, 2011; Kuzmin and Zvonok, 2021), snakes (Snetkov and Bannikov, 2010; Zvonok and Snetkov, 2012), aquatic birds (Averianov et al., 1990; Mayr and Zvonok, 2011, 2012; Zvonok et al., 2015; Zvonok and Gorobets, 2016; Dobrovolsky, 2023; Dobrovolsky and Gorobets, 2023), and marine mammals (Gol’din et al., 2012; Gol’din and Zvonok, 2013; Gol’din et al., 2014; Averianov and Zvonok, 2021; Davydenko et al., 2021).
Fish remains from Paleogene deposits of Kyiv and its vicinities were first described by Afanasii [Opanas] Semenovych Rogovich (1813-1878), who was a professor at Saint Volodymyr Imperial University (now Taras Shevchenko National University of Kyiv, Ukraine). Rogovich assembled a large collection of fossils from different regions of present-day Ukraine and described most of them in a series of papers (Rogovich, 1861, 1870, 1875a, 1875b). The taxonomic identity of Jurassic and Cretaceous fish specimens from this collection were previously revised (Kovalchuk and Anfimova, 2020; Kovalchuk et al., 2022). However, remains of middle Eocene sharks, rays, and chimaeras from the so-called ‘blue brick clays’ in the vicinity of Vyshhorod near Kyiv, which were initially reported by Rogovich (1861), have remained largely ignored for more than 150 years despite major taxonomic revisions of many of the taxa.
The aim of this study, therefore, is to carry out a thorough examination of chondrichtyan fossils from O.S. Rogovich’s collection and to describe several later records from coeval deposits to clarify the taxonomic composition of the chondrichthyan assemblage that existed in the Dnieper-Donets Basin of northern Ukraine during the middle Eocene (Kyiv time).
NOTES ON the HISTORY OF O.S. ROGOVICH’S FOSSIL COLLECTION
During the Paleogene, most of the area of present-day Ukraine was covered by sea. For many decades, our knowledge on Eocene chondrichtyans that existed in the Dnieper-Donets Basin was based on materials described by Rogovich from his fossil collection. According to the minutes of meeting of the Council of the Physics and Mathematics Faculty at Saint Volodymyr Imperial University dated to 22 November 1869, this collection was acquired for the mineralogical cabinet in the same year for a significant amount of money (2000 silver rubles; about 18 grams of silver in 1 ruble; O. Kozlov, pers. comm., 2021). Professor Kostiantyn Matviyovych Feofilaktov (1818-1901), head of the mineralogical cabinet, especially singled out the collection of fish remains, which included 440 specimens, ‘both because of its completeness and rarity, and the excellent preservation of specimens’ (Archive of the Department of Geology at the National Museum of Natural History (NMNHU-G), National Academy of Sciences of Ukraine). Feofilaktov considered the amount of money to be paid for the collection ‘very moderate’ and noted that ‘through the acquired collection, the palaeontological department of the mineralogical cabinet will obtain not only a significant local collection of fossils, but also a unique one among those of both Russian and foreign cabinets’ (Archive NMNHU-G). Unfortunately, the collection was subsequently divided into several parts and transferred to different institutions in Kyiv, Moscow, and Saint Petersburg (see Zvonok and Averianov, 2017 for more details). The part of the collection that remained in Kyiv was first relocated to the Academy of Sciences and was housed at the Institute of Geological Sciences (Zvonok and Danilov, 2017). Later it was transferred to the Institute of Zoology and subsequently it became part of the scientific collections of the NMNHU-G, National Academy of Sciences of Ukraine. The nomenclature of chondrichthyan taxa described by Rogovich was revised by Capetta (2006) and Pollerspöck and Straube (2022), but the specimens have not been re-examined in detail.
MATERIAL AND METHODS
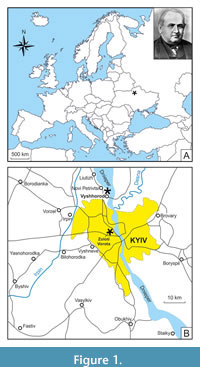 In total, 88 remains of sharks, rays, and chimaeras represented by isolated teeth, dental plates, and vertebrae were studied. Of them, 83 specimens are part of Rogovich’s collection, and the other five specimens represented by isolated vertebrae were collected later, in the 1960s, from coeval deposits at the construction site of the Zoloti Vorota station of the Kyiv Metro (Figure 1). Isolated teeth are the most numerous remains (62 specimens), followed by vertebrae (n = 21), four chimaeroid tooth plate fragments and a single caudal spine of a ray. The specimens from Rogovich’s collection come from middle Eocene deposits of Vyshhorod and are housed in NMNHU-G, whereas shark vertebrae of the same age from Zoloti Vorota are deposited in the Department of Palaeontology at the National Museum of Natural History, National Academy of Sciences of Ukraine, Kyiv (NMNHU-P, collection PI). The fossils were identified using diagnostic features based on descriptions mostly taken from literature sources (e.g., Cappetta, 2012) and on information provided by the shark reference database compiled by Pollerspöck and Straube (2022). The taxonomic hierarchy and descriptive terminology follow Stahl (1999), Stahl and Parris (2004), and Cicimurri and Ebersole (2015) for chimaeroids, and Cappetta (2012) for sharks and rays. X-ray images of shark vertebrae were taken with VATEL Alpha 1 in the veterinary clinic Vetmedservice (National University of Life and Environmental Sciences of Ukraine, Kyiv).
In total, 88 remains of sharks, rays, and chimaeras represented by isolated teeth, dental plates, and vertebrae were studied. Of them, 83 specimens are part of Rogovich’s collection, and the other five specimens represented by isolated vertebrae were collected later, in the 1960s, from coeval deposits at the construction site of the Zoloti Vorota station of the Kyiv Metro (Figure 1). Isolated teeth are the most numerous remains (62 specimens), followed by vertebrae (n = 21), four chimaeroid tooth plate fragments and a single caudal spine of a ray. The specimens from Rogovich’s collection come from middle Eocene deposits of Vyshhorod and are housed in NMNHU-G, whereas shark vertebrae of the same age from Zoloti Vorota are deposited in the Department of Palaeontology at the National Museum of Natural History, National Academy of Sciences of Ukraine, Kyiv (NMNHU-P, collection PI). The fossils were identified using diagnostic features based on descriptions mostly taken from literature sources (e.g., Cappetta, 2012) and on information provided by the shark reference database compiled by Pollerspöck and Straube (2022). The taxonomic hierarchy and descriptive terminology follow Stahl (1999), Stahl and Parris (2004), and Cicimurri and Ebersole (2015) for chimaeroids, and Cappetta (2012) for sharks and rays. X-ray images of shark vertebrae were taken with VATEL Alpha 1 in the veterinary clinic Vetmedservice (National University of Life and Environmental Sciences of Ukraine, Kyiv).
GEOLOGICAL SETTING
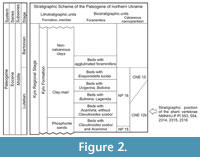 All the material described herein was derived from the Kyiv Formation, the type formation of the middle Eocene Kyiv regional stage (upper Lutetian-Bartonian) of northern Ukraine. The deposits of the Kyiv regional stage are exposed in the western and south-western parts of the East European Platform. In the northern part of Ukraine, the sediments representing this stage are widespread within the Dnieper-Donets Depression, the Pripyat Depression, the Ukrainian Shield, on adjacent slopes of the Voronezh Anticline, and outskirts of the Donets Basin (Figure 2). In older reports (from the late nineteenth century), the Kyiv Formation was usually described as the ‘Kiev Stage’ (see table 1 in Zosimovich and Shevchenko, 2014 for more details).
All the material described herein was derived from the Kyiv Formation, the type formation of the middle Eocene Kyiv regional stage (upper Lutetian-Bartonian) of northern Ukraine. The deposits of the Kyiv regional stage are exposed in the western and south-western parts of the East European Platform. In the northern part of Ukraine, the sediments representing this stage are widespread within the Dnieper-Donets Depression, the Pripyat Depression, the Ukrainian Shield, on adjacent slopes of the Voronezh Anticline, and outskirts of the Donets Basin (Figure 2). In older reports (from the late nineteenth century), the Kyiv Formation was usually described as the ‘Kiev Stage’ (see table 1 in Zosimovich and Shevchenko, 2014 for more details).
The Kyiv Formation in the northern part of Ukraine is comprised of (in ascending order) a horizon of yellowish-greenish-grey coarse and fine-grained phosphorite sands, a clay-marl member, and a member of non-carbonate clays (Ryabokon, 2002; Zosimovich and Shevchenko, 2014, 2015). The Kyiv Formation is now considered to be of middle Eocene (upper Lutetian-Bartonian) age (Figure 2): the horizon of phosphorite sands and the clay-marl member represent the late Lutetian stage, whereas the member of non-carbonate clays is dated to the Bartonian (Zosimovich and Shevchenko, 2015).
Nowadays, fish remains, mainly shark teeth, in the vicinities of Kyiv are known from two stratigraphic levels: the first of them is confined to the interval from the upper part of the horizon of phosphorite sands up to the lowest part of the clay-marl member, and the second one is from the ‘phosphorite plate’ (the upper part of the Kyiv Formation) of local distribution between the marls and non-calcareous silty clays in Pyrohiv quarry (Udovichenko and Nessov, 1987; Bratishko, 2011). Most of the fish specimens were recovered by O.S. Rogovich from the first stratigraphic level - marls and clays in the former quarry of the Eismann’s brickworks near Vyshhorod (50°35ʹ N, 30°29ʹ E; Figure 1).
The shark vertebrae found during earthworks at the construction site of the Zoloti Vorota metro station in Kyiv (50°26ʹ N, 30°30ʹ E; Figure 1) come from a depth of 110 m. Using standard approaches (Bugrova, 2005), the following foraminifera were identified in the rock sample from the cavities in these vertebrae: Acarinina medizzai (Toumarkin et Bolli, 1975), A. aff. A. rohri (Brönnimann et Bermudez, 1953), A. rugosoaculeata Subbotina, 1953, Bolivina pussilla Schwager, 1866, Bulimina aksuatica Morozova, 1936, Cibicides karpaticus Mjatliuk, 1950, C. tenellus (Reuss, 1865), Dentalina approximata Reuss, 1866, Epistominella vitrea Parker, 1953, Globocassidulina globosa (Hantken, 1875), Guttulina problema d’Orbigny, 1846, Gyroidinoides octocameratus (Cushman et Hanna, 1927), Gyroidinoides soldanii (d’Orbigny, 1826), Heterolepa eocaena (Gümbel, 1870), Pseudohastigerina micra (Cole, 1927), Siphonodosaria annulifera (Cushman et Bermudez, 1936), Spiroplectammina carinatiformis Morozova, 1939, Spiroplectammina guembeli Hagn, 1956, Quinqueloculina ludwigi Reuss, 1866, Turrilina alsatica Andreae, 1884, and Trifarina budensis (Hantken, 1875).
The assemblage of small benthic foraminifera extracted from the sample is characteristic for the lower, carbonated part of the Kyiv Formation. The assemblage of planktonic foraminifera–A. medizzai, A. rugosoaculeata, A. aff. A. rohri, and Pseudohastigerina micra–indicates that the sample containing Acarinina without Clavulinoides szaboi comes from layers of the lower part of the clay-marl member of the Kyiv Formation, just above the horizon of phosphorite sands (Ryabokon, 2002). Based on the planktonic foraminifera, these layers are of late Lutetian age and correspond to the chronostratigraphic level of zones E10-E11 of the zonal planktonic foraminiferal scale (Speijer et al., 2020). According to Solyanik (2009), Musatov (2020), and Musatov and Ryabokon (2017), these layers belong to the time from the first common (abundant) appearance of Discoaster bifax and Blackites gladius, accompanied with Nannotetrina fulgens, to the abundant and large Reticulofenestra umbilica (≥14 μm), representing the upper part of zone CNE12b (Agnini et al., 2014; Ogg et al., 2016) or the lowest part of Zone NP16. In terms of geological history, the deposition of the lower part of Kyiv Formation broadly corresponds to the climatic (hyperthermal) ‘C19r event’ dated at 41.5 Ma, representing the late Lutetian Thermal Maximum (LLTM) (Westerhold et al., 2018; Ryabokon, 2021). It was a short period of warming by approximately 2°C of deep-sea waters of the South Atlantic, which is of interest due to its coincidence with the highest means of insolation for the past 45 million years. Consequently, the accumulation of these layers (i.e., the rocks comprising shark vertebrae from Zoloti Vorota) directly preceded the LLTM (Speijer et al., 2020).
SYSTEMATIC PALAEONTOLOGY
Class CHONDRICHTHYES Huxley, 1880
Subclass HOLOCEPHALI Bonaparte, 1832
Order CHIMAERIFORMES Obruchev, 1953
Family CALLORHINCHIDAE Garman, 1901
Genus EDAPHODON Buckland, 1838
Edaphodon bucklandi Agassiz, 1843
Figure 3
1843 Edaphodon bucklandi; Agassiz, p. 351-352, pl. 40d, figs. 1-4, 9-12, 19-24.
1843 Edaphodon eurygnathus; Agassiz, p. 352.
1848 Edaphodon Bucklandii [sic] Ag.; Giebel, p. 378.
1848 Edaphodon eurygnathus Ag.; Giebel, p. 379.
1850 Edaphodon eurygnathus; Dixon, p. 111, pl. 10, figs. 18, 19, 22; pl. 12, fig. 5.
1850 Edaphodon Bucklandi [sic]; Dixon, p. 111, pl. 10, figs. 20, 21.
1861 Edaphodon Bucklandii [sic] Ag.; Rogovich, p. 60, pl. I, figs. 5, 6.
1861 Edaphodon eurygnathus Ag.; Rogovich, p. 61, pl. VIII, figs. 18, 19.
1891 Edaphodon bucklandi, Agassiz; Woodward, p. 80-81.
1901 Edaphodon Bucklandi [sic] Agassiz; Priem, p. 485.
1902 Edaphodon Bucklandi [sic] Agassiz, 1843; Leriche, p. 35, pl. I, fig. 51.
1905 Edaphodon Bucklandi [sic] Agassiz, 1843; Leriche, p. 137-140, figs. 18, 19.
2012 Edaphodon bucklandi Agassiz, 1843; Diedrich, p. 19, fig. 14.19.
Material. Three right palatinal fragments, NMNHU-G 391/114, 391/138/1-2; one left mandibular fragment, NMNHU-G 391/115, Vyshhorod.
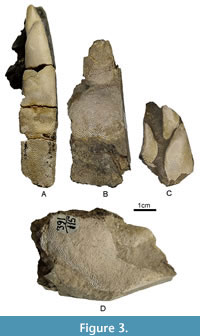 Description. Palatine tooth plates (Figure 3A-C) are robust and broad, with a stepped oral surface. NMNHU-G 391/138/1 bears three tritors, of which the posterior inner one is the largest and broadest (albeit partly destroyed in its distal part), reaching 12.6 mm in width. It is separated from the anterior inner tritor with a narrow groove and smoothly tapers anteriorly. The outer tritor is elongated and well separated from both inner ones with a deep valley, and its anterior end lies at the level of the middle part of the anterior inner tritor. Two inner tritors are preserved in NMNHU-G 391/114, which is more than twice as large as in NMNHU-G 391/138/1. The length of the anterior and posterior inner tritors is 21.7 and 26.6 mm, and their width is 14.3 and 23.6 mm, respectively. The mandibular tooth plate (Figure 3D) with a broad symphyseal surface is also robust. The median tritor occupies more than two-thirds of the lingual surface, has irregular oval shape, and is separated from the posterior border of the symphysis with a narrow band. The anterior outer tritor is triangular with rounded edges, and it is much smaller and lies below the median tritor. Numerous vertical tubules are visible at the cross section of both tritors.
Description. Palatine tooth plates (Figure 3A-C) are robust and broad, with a stepped oral surface. NMNHU-G 391/138/1 bears three tritors, of which the posterior inner one is the largest and broadest (albeit partly destroyed in its distal part), reaching 12.6 mm in width. It is separated from the anterior inner tritor with a narrow groove and smoothly tapers anteriorly. The outer tritor is elongated and well separated from both inner ones with a deep valley, and its anterior end lies at the level of the middle part of the anterior inner tritor. Two inner tritors are preserved in NMNHU-G 391/114, which is more than twice as large as in NMNHU-G 391/138/1. The length of the anterior and posterior inner tritors is 21.7 and 26.6 mm, and their width is 14.3 and 23.6 mm, respectively. The mandibular tooth plate (Figure 3D) with a broad symphyseal surface is also robust. The median tritor occupies more than two-thirds of the lingual surface, has irregular oval shape, and is separated from the posterior border of the symphysis with a narrow band. The anterior outer tritor is triangular with rounded edges, and it is much smaller and lies below the median tritor. Numerous vertical tubules are visible at the cross section of both tritors.
Remarks. The specimens from Vyshhorod were assigned to the genus Edaphodon based on the number of tritors on palatine tooth plates (Woodward, 1891; Stahl, 1999; Stahl and Parris, 2004; Cicimurri and Ebersole, 2015). Albeit being partly broken, they are similar in gross morphology (shape, size, and orientation of tritors, morphology of the labial margin) to those of E. bucklandi erected by Agassiz (1843) from the Eocene of England. Rogovich (1861) identified two of the specimens described (NMNHU-G 391/114, 391/115) as E. bucklandi, and two others he assigned to another species, E. eurygnathus Agassiz, 1843. The latter is no longer valid because it was synonymised with E. bucklandi by Woodward (1891:80) as the ‘differences between the palatine teeth of this species and those of the so-called E. eurygnathus are solely due to the imperfect state of preservation of the type specimens of the latter.’ Apart from England, E. bucklandi is recorded from the Paleocene and Eocene of Belgium (Leriche, 1902, 1905; Casier, 1943; Dobbels, 1994), as well as from the Eocene of France (Priem, 1901, 1908; Leriche, 1906), Germany (Giebel, 1848; Diedrich, 2012) and Morocco (Case and Herman, 1973). An Edaphodon fossil, represented by a left mandibular fragment with morphological traits similar to those in E. bucklandi, was also reported from the late Eocene of North America (Parmley and Cicimurri, 2005). It is similar to NMNHU-G 391/115 in having a broad, flat symphyseal surface, a large medium tritor, and an anterior outer tritor of similar shape and punctuate surface pattern.
Subclass ELASMOBRANCHII Bonaparte, 1838
Cohort NEOSELACHII Compagno, 1970
Order HEXANCHIFORMES Buen, 1926
Family HEXANCHIDAE Gray, 1851
Genus HEXANCHUS Rafinesque, 1810
Hexanchus agassizi Cappetta, 1976
Figure 4A-C
1861 Notidanus microdon Ag.; Rogovich, p. 29, pl. III, figs. 25, 26.
1886 Notidanus serratissimus Ag.; Woodward, p. 216, pl. VI, figs. 24, 26.
1899 Notidanus serratissimus Ag.; Woodward, p. 6, pl. I, fig. 7.
1928 Notidanus serratissimus Ag.; Menner, p. 294, pl. X, fig. 1.
1964 Notidanus serratissimus Ag.; Glickman, p. 157, pl. VI, fig. 5-5a; pl. XXVI, fig. 22.
1966 Notidanus serratissimus Ag.; Casier, p. 44, pl. 1, figs. 10-12.
1967 “Nodidanus” serratissimus Ag.; Pledge, p. 140, pl. 1, fig. 2.
1976 Hexanchus agassizi nov. sp.; Cappetta, p. 553-554, pl. 1, figs. 5-8.
1979 Hexanchus agassizi Cappetta, 1976; Ward, p. 114-115, pl. 2, figs. 1, 2.
2005 Hexanchus agassizi Cappetta, 1976; Mustafa et al., p. 405-406, figs. 3-5.
2006 Hexanchus agassizi Cappetta; Malyshkina, pl. 12, fig. 4.
2006 Hexanchus cf. agassizi Cappetta, 1976; Udovichenko, p. 203, pl. I, fig. 1.
2012 Hexanchus agassizi Cappetta, 1976; Cappetta, p. 92-93, fig. 82.
2012 Hexanchus agassizi Cappetta, 1976; Zalmout et al., p. 74, fig. 3A.
2013 Hexanchus agassizi Cappetta, 1976; Otero et al., fig. 5.1-6.
2013 Hexanchus agassizi Cappetta, 1976; Schultz, p. 23-24, pl. 4, figs. 4a, b, 5.
2016 Hexanchus agassizi Cappetta, 1976; Szabó and Kocsis, p. 38-40, figs. 14-17.
2017 Hexanchus agassizi Cappetta, 1976; Zalat et al., p. 204, pl. 1, fig. 15.
2021 Hexanchus agassizi Cappetta, 1976; Adnet et al., p. 44, fig. 12.1-12.2.
Material. One upper left anterolateral tooth, NMNHU-G 391/21/2; two lower (left and right) lateral teeth, NMNHU-G 391/21/3, 391/21/4, Vyshhorod.
 Description. The upper anterolateral tooth (Figure 4A) is 8.7 mm long mesiodistally and 8.3 mm high. The root is robust and wide, it has a striated ornamentation on its lingual face, and a shallow longitudinal groove on the smooth labial face. There is a long and sharp central cusp oriented posteriorly to the tooth base. A small lateral cusplet is separated from the central cusp with a deep arcuate notch. Edges of the central cusp and the cusplet are laterally compressed.
Description. The upper anterolateral tooth (Figure 4A) is 8.7 mm long mesiodistally and 8.3 mm high. The root is robust and wide, it has a striated ornamentation on its lingual face, and a shallow longitudinal groove on the smooth labial face. There is a long and sharp central cusp oriented posteriorly to the tooth base. A small lateral cusplet is separated from the central cusp with a deep arcuate notch. Edges of the central cusp and the cusplet are laterally compressed.
The lower lateral teeth (Figure 4B-C) are also small (11.9 and 11.3 mm wide), about two times wider than high. The root is compressed labiolingually with a concave mesial edge and bears numerous small foramina on both the labial and lingual faces. There are eight to nine cones (including the acrocone) decreasing in height distally. The acrocone is slightly more developed than the first accessory cone; its mesial edge is serrated near the base in one of the specimens, whereas it is smooth in the second tooth. The L2/L3 ratio (see Adnet, 2006a for details) is 0.8 and 0.9, that is, L2<L3 in both cases. The number of cusps per tooth width equals 7.3 and 7.5.
Remarks. The teeth are morphologically identical to and metrically close to those in Hexanchus agassizi Cappetta, 1976. The specimens described are somewhat similar to those in Notorynchus primigenius (Agassiz, 1843) but differ by their smaller size and having more numerous accessory cones. The estimated length of the body (based on lower lateral teeth), following the equations in Compagno (1984) and Adnet (2006a), could have reached 129 and 136 cm. Hexanchus agassizi had a worldwide distribution during the Eocene (Cappetta, 2012). The presence of this species (also under the name Notidanus serratissimus Agassiz, 1843) is documented in the Eocene fossil record of Europe (Woodward, 1886, 1899; Casier, 1966; Cappetta, 1976; Ward, 1979; Dutheil, 1991; Adnet, 2006a; Adnet et al., 2008), Asia (Menner, 1928; Glickman, 1964; Case et al., 1996; Zhelezko and Kozlov, 1999; Mustafa et al., 2005; Malyshkina, 2006), Africa (Dartevelle and Casier, 1943; Zalmout et al., 2012; Zalat et al., 2017), South and North America (Case, 1981; Otero et al., 2013), and Australia (Pledge, 1967; Kemp, 1978). Adnet (2006a) considered H. agassizi to be the only lower-middle Eocene species of Hexanchus, whereas other taxa described from coeval deposits (Hexanchus collinsonae Ward, 1979 and Hexanchus hookeri Ward, 1979) may represent different ontogenetic stages of this species.
Genus NOTORYNCHUS Ayres, 1855
Notorynchus kempi Ward, 1979
Figure 4D-E
1861 Notidanus serratissimus Ag.; Rogovich, p. 29, pl. III, figs. 23, 24.
1912 Notidanus primigenius Ag.; Savtchenko, p. 168-170, pl. XIII, figs. 11, 12.
1979 Notorynchus kempi sp. nov.; Ward, p. 121-122, pl. 3, figs. 4-7.
1999 Notorhynchus kempi; Zhelezko and Kozlov, pl. 32, figs. 4a-9b, pl. 34, figs. 1-6.
2006 Notorhynchus kempi Ward, 1979; Udovichenko, p. 204, pl. I, fig. 5.
Material. One upper left anterolateral tooth, NMNHU-G 391/20; one lower left lateral tooth, NMNHU-G 391/21/1, Vyshhorod.
Description. The upper anterolateral tooth (Figure 4D) is quite large, 15.6 mm wide and 18.0 mm high, with an erect, convex crown inclined distally and possessing a complete cutting edge. The root is wide, compressed labiolingually, and wedge-shaped in anterior view (the lingual surface is steeply inclined towards the root base); its mesial and apical edges form a right angle. There is a single weakly serrated mesial cusplet and two separated distal cusplets, the first of which is almost two times shorter than the main cusp.
The lower lateral tooth (Figure 4E) is 24.8 mm wide and 12.3 mm high. Its crown is labiolingually compressed and mesiodistally extended with an acrocone followed by six gradually decreasing lateral cusplets angled posteriorly. There are eight or nine small denticles increasing in size apically on the mesial edge of the main cusp.
Remarks. The specimens described were assigned to the genus Notorynchus based on the presence of denticles on the mesial edge of the main cusp of lower lateral teeth (Applegate, 1965; Kocsis, 2007) and a characteristic shape of the upper anterolateral tooth (Cappetta, 2012). These teeth resemble those in Notorynchus kempi Ward, 1979, in overall morphology and size. The teeth of N. serratissimus (Agassiz, 1843) are considerably smaller, have relatively coarser mesial cusplets as well as less numerous, larger, and less curved distal cusplets (Ward, 1979). As compared to N. kempi Ward, 1979, N. primigenius is characterised by larger tooth size, coarser mesial cusplets, relatively larger, more pointed, and upright main cusp and distal cusplets (Ward, 1979). The specimens considered differ from N. cepedianus (Péron, 1807) in having a smaller overall tooth size and more erect cusplets. Notorynchus kempi ranged from the middle to late Eocene, being restricted to Zones NP 15-17 (Ward, 1979; Udovichenko, 2006). This species originally was described from the Bartonian of England (Ward, 1979), and later its remains were also found in Kazakhstan (Zhelezko and Kozlov, 1999) and Ukraine (Udovichenko, 2006).
Order HETERODONTIFORMES Berg, 1937
Family HETERODONTIDAE Gray, 1851
Genus HETERODONTUS Blainville, 1816
Heterodontus sp.
Figure 4F
1861 Acrodus kioviensis n. sp.; Rogovich, p. 17, pl. II, figs. 4-10.
Material. Two lateral teeth, NMNHU-G 391/6, 391/7, Vyshhorod.
Description. The teeth are asymmetrical, equal in size, reaching 2 cm in length. The crown is low (up to 5 mm), with one cutting edge being straight, while the others are slightly convex and wrinkled. The occlusal surface is smooth due to abrasion. There are poorly developed radial folds that converge in the central part of the crown. The basal surface of the root is flat.
Remarks. Seven isolated teeth were collected and described by Rogovich (1861) from the Eocene deposits of Vyshhorod. A new species–Acrodus kioviensis–was erected based on these specimens, only two of which are now present in the collection of NMNHU-G. Nevertheless, the teeth share some diagnostic characters of heterodontiform sharks (in particular, the genus Heterodontus) albeit resemble those in acrodontids due to a dental convergence (Cappetta, 2012). Representatives of the family Acrodontidae existed in Mesozoic seas and went extinct no later (if not earlier) than the Paleocene (Cook and Ramsdell, 1991). We refrain from assigning the specimens considered to a particular species, although we assume that this should be Heterodontus vincenti (Leriche, 1905) or another morphologically similar Eocene representative of this genus.
Order LAMNIFORMES Berg, 1937
Family MITSUKURINIDAE Jordan, 1898
Genus STRIATOLAMIA Glickman, 1964
Striatolamia macrota (Agassiz, 1843)
Figure 4G-M
1843 Otodus macrotus n. sp.; Agassiz, p. 273, pl. 32, figs. 29, 30.
1843 Lamna elegans n. sp.; Agassiz, p. 289, pl. 35, figs. 1-7; pl. 37a, figs. 58, 59.
1861 Otodus macrotus Ag.; Rogovich, p. 43, pl. V, figs. 16-22.
1861 Lamna compressa Ag.; Rogovich, p. 46, pl. V, figs. 29-30a.
1861 Oxyrhina brevidens; Rogovich, p. 57, pl. VIII, figs. 11, 11a.
1874 Otodus striatus; Winkler, p. 8, pl. 1, figs. 7-9.
1895 Odontaspis macrota var. rossica; Jaekel, p. 11, pl. 1, figs. 8-17; pl. 2, figs. 8-10.
1901 Odontaspis macrota; Eastman, p. 105, pl. 14, fig. 4.
1901 Lamna striata n. sp.; Priem, p. 484, pl. 11, figs. 29, 30.
1905 Odontaspis macrota; Leriche, p. 75.
1912 Otodus macrotus Ag.; Savtchenko, p. 173-174, pl. XIII, figs. 7-10.
1912 Oxyrhina brevidens Rog.; Savtchenko, p. 181.
1928 Odontaspis macrota Ag.; Menner, p. 301-302.
1942 Odontaspis macrota striata var. semistriata; Leriche, p. 13-14, pl. 1, figs. 6-8.
1964 Striatolamia macrota (Agassiz, 1843); Glickman, p. 120-121, 124-126, 143-144, pl. XIII, figs. 1-3, 5, 7-9, 14; pl. XXIII, figs. 12, 13, 15, 16.
1964 Striatolamia rossica prima; Glickman, p. 124, 128, 172, 182, pl. XXIV, figs. 1-9.
1964 Striatolamia rossica usakensis; Glickman, p. 124, 126, 148-149, 172, 177-178, pl. VII, figs. 3, 4; pl. XII, figs. 9-13, 16-18; pl. XIII, figs. 2-6, 11, 13.
1964 Striatolamia rossica rossica; Glickman, p. 121, 124, 126, 172, 178, pl. XI, figs. 9-13; pl. XII, figs. 3-8, 14-15.
1968 Striatolamia macrota; Applegate, p. 32-36, pls. 1-3.
1985 Striatolamia macrota (Agassiz, 1843); Bor, p. 92, pl. 2, figs. 9-11.
1987 Striatolamia macrota (Agassiz); Cappetta, p. 90, fig. 80A-E.
1988 Odontaspis (Synodontaspis) macrota (L. Agassiz, 1843); Bauzá and Gómez Pallerola, p. 123-126, fig. 2.14-2.21.
1988 Striatolamia macrota (Agassiz); Nolf, p. 110, pl. 27.
1999 Striatolamia macrota (Agassiz, 1843); Zhelezko and Kozlov, p. 131-135, pl. 9, figs. 1-3; pl. 10; pl. 11, figs. 1-4; pl. 57; pl. 58, figs. 1-5, 7-10; pl. 59; pl. 60, figs. 7, 9.
2000 Striatolamia macrota (Agassiz); Cunningham, pl. 2, fig. 2; pl. 5, figs. 2, 4; pl. 6, figs. 2, 4; pl. 7, fig. 2; pl. 8, fig. 2; pl. 9, fig. 2; pl. 10, fig. 2; pl. 11, fig. 2; pl. 12, fig. 2; pl. 13, fig. 2; pl. 14, fig. 2; pl. 15, fig. 2; pl. 16, fig. 2; pl. 17, fig. 2.
2006 Striatolamia macrota (Agassiz); Malyshkina, pl. 6, fig. 1; pl. 10, figs. 1-4.
2006 Striatolamia macrota (Agassiz); Udovichenko, p. 202, pl. I, fig. 13.
2012 Striatolamia macrota (Agassiz, 1838); Cappetta, p. 189-190, fig. 178.
2012 Striatolamia macrota (Agassiz, 1843); Diedrich, p. 16, fig. 12.
2013 Striatolamia macrota (Agassiz, 1843); Malyshkina et al., pl. 13, figs. 2, 3.
2013 Striatolamia macrota (Agassiz, 1843); Otero et al., fig. 4.19-4.26.
2014 Striatolamia macrota (Agassiz, 1843); Carlsen and Cuny, p. 43-45, fig. 3G-L.
2019 Striatolamia macrota (Agassiz, 1843); Ebersole et al., p. 32-37, fig. 11.
2019 Striatolamia macrota (Agassiz, 1843); Trif et al., p. 8-10, figs. 5.5-7’, 10-11.
2021 Striatolamia macrota (Agassiz, 1843); Adnet et al., p. 28-29, fig. 2.2-2.4.
2022 Striatolamia macrota (Agassiz, 1843); Trif et al., fig. 5G-O.
Material. Two upper lateral teeth, NMNHU-G 391/54, 391/105; one lower lateral tooth, NMNHU-G 391/55; one upper posterior tooth, NMNHU-G 391/92, Vyshhorod.
Description. Lateral teeth (Figure 4G-L) with triangular labiolingually flattened crowns are quite large or moderate-sized, and their height varies from 12.6 to 21.5 mm and width from 14.2 to 20.2 mm. Both upper and lower teeth are recognised in the series according to the curvature of the labial side of their crowns (Cunningham, 2000). The labial surface of the upper teeth is nearly straight from the base of the enameloid to the crown tip, whereas that of the lower teeth is slightly convex. There is a main cusp with a distal inclination and two shovel-shaped cusplets on the crown of each lateral tooth. The lingual face is ornamented with weak parallel striations reaching the middle of the crown. The striations are better pronounced in upper lateral teeth and less developed or absent in the lower lateral teeth described herein. The blade-like cutting edges are complete in all specimens regardless of their position in the jaw.
The upper posterior tooth (Figure 4M) has a very low and wide crown, and its total height is 3.4 mm and maximum width is 6.4 mm. The labial side of the crown is straight. There is a semi-circular ridge with numerous thin vertical striations on the lingual side of the crown. The distal lateral denticle retains its definition from the crown, but the mesial lateral denticle is shoulder-like (sensu Cunningham, 2000:10). The root is notched labially, whereas its lingual side is swollen and is divided in the middle by a deep vertical furrow. The shallow interspace between the root lobes is V-shaped. The enameloid on the lingual surface is strongly striated.
Remarks. The lateral teeth described are similar in morphology and size to those in Striatolamia macrota (Agassiz, 1843). This species was widely distributed in Europe and Asia during the Ypresian, Lutetian, and Bartonian (Cappetta, 2012). The presence of S. macrota in the fossil record of Ukraine was documented by Zhelezko and Kozlov (1999) and Udovichenko (2006). Eight species of the genus Striatolamia are now considered to be valid (Pollerspöck and Straube, 2022), including two Paleocene taxa (Striatolamia ex gr. S. whitei (Arambourg, 1952), S. striata (Winkler, 1874)) and two Eocene species from the territory of Ukraine (Zhelezko and Kozlov, 1999). Striatolamia sibirica Zhelezko in Zhelezko and Kozlov (1999) was represented in the Dnieper-Donets Basin during the Bartonian. The specimens considered differ from those of S. sibirica in having larger roots, thicker crowns, and cusplets more isolated from the main cusp (Zhelezko and Kozlov, 1999). According to Ebersole et al. (2019), there are no appreciable differences between the teeth of S. macrota and S. striata, and these taxa therefore could be part of a species complex that cannot be differentiated morphologically. As compared to the specimens considered here, the teeth of the Bartonian/Ypresian species Striatolamia tchelkarnurensis are much larger and more robust, and have a less pronounced lingual ornamentation (Malyshkina, 2021).
Rogovich (1861) described and figured a new species of the genus Oxyrhina–O. brevidens–from ‘blue clay near Kyiv.’ The specimen considered is part of the type series (syntype), while the other ‘few teeth’ mentioned in the original description were subsequently lost. Savtchenko (1912), when analysing shark remains from the Eocene of Mangyshlak, used the material from Rogovich’s collection for comparison. He concluded that one specimen from his own collection was almost identical morphologically to that of Oxyrhina brevidens. This species is represented in the database by Pollerspöck and Straube (2022) but placed in the genus Striatolamia, probably because of the presence of characteristic (albeit weak) striation on the lingual side of the crown. We suggest that the specimen initially described by Rogovich (1861) as O. brevidens in fact represents the upper left posterior tooth of S. macrota. It is very similar to the one figured by Cunningham (2000: pl. 10, fig. 2, upper row, second from the left).
Family ODONTASPIDIDAE Müller and Henle, 1838
Genus BRACHYCARCHARIAS Cappetta and Nolf, 2005
Brachycarcharias lerichei (Casier, 1946)
Figure 4N-O
1861 Otodus macrotus Ag. (partim); Rogovich, p. 43, pl. V, fig. 23.
1946 Lamna lerichei n. sp.; Casier, p. 80, pl. 2, fig. 7a-b.
1988 Lamna lerichei Casier; Nolf, pl. 30, figs. 2-11.
1990 Lamna lerichei Casier; Kemp et al., p. 9, pl. 3, figs. 9, 10.
2005 Brachycarcharias lerichei (Casier, 1946); Cappetta and Nolf, p. 241-242, pl. 2.
2012 Brachycarcharias lerichei (Casier, 1946); Diedrich, p. 19, fig. 14.1-14.6.
2013 Brachycarcharias lerichei (Casier, 1946); Clayton et al., fig. 2L.
2016 Brachycarcharias lerichei (Casier, 1946); Cappetta and Case, pl. 3, figs. 8-22.
2018 Brachycarcharias lerichei (Casier, 1946); Marramà et al., p. 291, fig. 4.
2019 Brachycarcharias lerichei (Casier, 1946); Ebersole et al., p. 39-41, fig. 13.
2022 Brachycrcharias lerichei; Perez, p. 635, fig. 4A-B.
Material. One lower lateral tooth, NMNHU-G 391/52, Vyshhorod.
Description. The lateral tooth has a triangular crown; its height reaches 14.0 mm, and its width is 14.3 mm. The main cusp is slightly inclined distally and has an accentuated apex. There are two pairs of triangular lateral cusplets, the first of which is considerably larger than the second one. The crown surface is smooth and devoid of ornamentation. The root is holaulacorhize with a V-shaped interlobe area. There is a shallow nutritive groove on the lingual root protuberance.
Remarks. The specimen described here is identical in morphology and size to teeth of Brachycarcharias lerichei (Casier, 1946). It differs from the respective teeth of other species of this genus in their smaller overall size, narrower main cusp, and smaller lateral cusplets (see Ebersole et al., 2019, for more details). In addition, the specimen considered differs from those of the coeval Brachycarcharias atlasi (Arambourg, 1952) in the absence of faint striations at the lingual crown base. The lateral teeth of Hypotodus have smaller and less divergent cusplets compared to B. lerichei. The latter species is characterised by less hooked lateral teeth with larger lateral cusplets than those in Jaekelotodus (Ebersole et al., 2019). In addition, the surface of the lateral teeth of Striatolamia macrota is ornamented, their lateral cusplets are blunt, and the root lobes are slightly wider. Brachycarcharias lerichei was distributed across the Northern Hemisphere during the early Paleogene (Marramà et al., 2018; Ebersole et al., 2019).
Genus JAEKELOTODUS Menner, 1928
Jaekelotodus trigonalis (Jaekel, 1895)
Figure 5A-F
1861 Lamna cuspidata Ag.; Rogovich, p. 46, pl. VI, figs. 7-14b.
1861 Lamna denticulata Ag.; Rogovich, p. 47, pl. VI, figs. 15-23.
1895 Hypotodus trigonalis n. sp.; Jaekel, pl. 1, figs. 6, 7.
1912 Lamna (Odont.) crassidens Ag. 1843; Savtchenko, p. 177-178, pl. XIII, figs. 20-23.
1928 Iekelotodus trigonalis (Iek.); Menner, p. 315.
1964 Jaekelotodus trigonalis (Jaekel) minor Gluckman; Glickman, p. 137, pl. XVIII, figs. 32, 33, 40-42, 44, 45; pl. XIX, figs. 1-3.
1964 Jaekelotodus trigonalis (Jaekel) medius Gluckman; Glickman, p. 137, pl. XIX, figs. 4, 5, 7, 8, 11-13, 18.
1964 Jaekelotodus trigonalis trigonalis (Jaekel); Glickman, pl. XIX, figs. 15-17, 20-22.
1988 Hypotodus trigonalis (Jaekel); Nolf, p. 114, pl. 29, figs. 1, 2.
1994 Jaekelotodus trigonalis (Jaekel, 1895); Zhelezko and Kozlov, p. 110-112, pl. 1, figs. 1-2; pl. 2, figs. 1-3; pls. 37-39.
2005 Jaekelotodus trigonalis (Jaekel, 1895); Cappetta and Nolf, p. 247, pl. 6, figs. 1-4.
2006 Jaekelotodus trigonalis (Jaekel); Malyshkina, p. 99, pl. 9, figs. 3-5.
2006 Jaekelotodus trigonalis (Jaekel); Udovichenko, p. 202, pl. I, fig. 10.
2012 Jaekelotodus trigonalis (Jaekel, 1895); Cappetta, p. 200, fig. 189.
2012 Jaeckelotodus trigonalis (Jaeckel, 1895); Diedrich, p. 15, fig. 11.11-11.15.
2022 Jaekelotodus trigonalis; Perez, p. 635, fig. 4C.
Material. Six lateral teeth, NMNHU-G 391/62, 391/139/1-5, Vyshhorod.
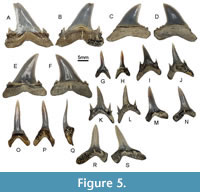 Description. The teeth (Figure 5A-F) measure from 16.9 to 20.0 mm apicobasally (mean 19.0 mm) and from 19.5 to 21.5 mm mesiodistally (mean 20.2 mm). The main cusp is broad, with a curved tip, and has a concave, V-shaped cavity on its labial surface. Cutting edges are complete and progress into broad triangular primary cusplets. Secondary cusplets are also present, but they are smaller. Both pairs of cusplets are turned towards the main cusp, which has irregular and variably strong serrations near the base. On some of the specimens considered here, there are accessory denticles between the main cusp and lateral cusplets. The roots are massive and thickened labiolingually. The labial root surface is convex while the lingual is concave.
Description. The teeth (Figure 5A-F) measure from 16.9 to 20.0 mm apicobasally (mean 19.0 mm) and from 19.5 to 21.5 mm mesiodistally (mean 20.2 mm). The main cusp is broad, with a curved tip, and has a concave, V-shaped cavity on its labial surface. Cutting edges are complete and progress into broad triangular primary cusplets. Secondary cusplets are also present, but they are smaller. Both pairs of cusplets are turned towards the main cusp, which has irregular and variably strong serrations near the base. On some of the specimens considered here, there are accessory denticles between the main cusp and lateral cusplets. The roots are massive and thickened labiolingually. The labial root surface is convex while the lingual is concave.
Remarks. The specimens described resemble those of Jaekelotodus trigonalis in overall morphology, although they are somewhat smaller. The teeth of Ypresian species (J. londonensis Zhelezko, 1994 and J. borystenicus Glickman, 1964) have larger, narrower, and more erect crowns. Jaekelotodus trigonalis differs from J. robustus (Leriche, 1921) in having higher, sharper, and more lateral cusplets, as well as a less pronounced furrow at the top of the root (Cappetta and Nolf, 2005). It differs from the teeth of Hypotodus verticalis (Agassiz, 1843) by having complete cutting edges of the main cusp, which extend to the lateral cusplets. We need to stress that our identification is somewhat tentative: these specimens may also belong to or include similar odontaspidid taxa, such as Mennerotodus, Tethylamna, or Brachycarcharias.
Genus MENNEROTODUS Zhelezko, 1994
Mennerotodus cf. M. parmleyi Cicimurri, Ebersole and Martin, 2020
Figure 5G-H
1861 Lamna (Odontaspis) Hoppei [sic] Ag.; Rogovich, p. 49, pl. VII, figs. 3-10.
Material. One lower anterior tooth, NMNHU-G 391/71; one anterolateral tooth, NMNHU-G 391/88, Vyshhorod.
Description. The anterior tooth (Figure 5G-H) is quite small; it reaches 13.0 mm apicobasally and 7.4 mm mesiodistally. The main cusp is narrow, triangular, and slightly inclined distally. Cutting edges are sharp, smooth, and do not reach the base of the main cusp. There is a single pair of short, conical cusplets at the crown base. The labial face of the main cusp is smooth and flat while the lingual face is convex. Root lobes are elongated and almost of equal length.
The lateral tooth is much smaller, 6.5 mm high and 5.3 mm wide. It is morphologically similar to the anterior tooth, albeit has a shorter and relatively broader main cusp, cutting edges extending to the crown base, and root lobes are shorter but wider and more widely separated.
Remarks. The teeth resemble those of Mennerotodus Zhelezko, 1994, and their morphological characteristics fit well into the emended diagnosis of this genus provided by Cicimurri et al. (2020). The specimens from Vyshhorod are close in overall morphology to teeth assigned to Mennerotodus parmleyi from the middle Eocene of the USA, but we only tentatively assign them to this species considering the absence of denticles between the cutting edges and lateral cusplets (which are characteristic for this species according to Cicimurri et al., 2020) and the small size of the studied sample. Mennerotodus parmleyi differs from M. glueckmani Zhelezko, 1994 from the middle Eocene of Kazakhstan in having much smaller teeth and the presence of a single pair of lateral cusplets on lateral teeth (Cicimurri et al., 2020). It further differs from the Paleocene (Danian) M. mackayi Cicimurri et al., 2020 in having a more conspicuous and extensively developed denticulation and a greater crown-root height ratio (Cicimurri et al., 2020).
Genus ODONTASPIS Agassiz, 1838
Odontaspis winkleri Leriche, 1905
Figure 5I-L
1861 Lamna (Odontaspis) hispida Rog.; Rogovich, p. 50, pl. VII, figs. 11, 12.
1905 Odontaspis winkleri sp. nov.; Leriche, p. 74, p. 117, pl. 6, fig. 1-12.
1912 Odontaspis winkleri [sic] Ler. 1904; Savtchenko, p. 178-179.
1928 Oxyrhina hopei Ag.; Menner, p. 303, pl. X, figs. 7, 8.
1946 Odontaspis (Synodontaspis) winkleri Leriche, 1905; Casier, p. 72, pl. 2, fig. 6.
1966 Odontaspis (Synodontaspis) winkleri Leriche, 1905; Casier, p. 72, pl. 5, figs. 1-4.
1985 Odontaspis winkleri Leriche, 1905; Bor, p. 91, pl. 2, fig. 8.
1987 Odontaspis winkleri Leriche; Cappetta, p. 89, fig. 79A-D.
1988 Odontaspis winkleri Leriche; Nolf, pl. 26, figs. 1-12.
2005 Odontaspis winkleri Leriche, 1905; Cappetta and Nolf, p. 248.
2006 Odontaspis winkleri (Leriche); Udovichenko, p. 202, pl. I, figs. 11, 12.
2012 Odontaspis winkleri Leriche, 1905; Cappetta, p. 204, fig. 192A-D.
2012 Otodus winkleri Lériche, 1905; Diedrich, p. 19, fig. 14.11.
2014 Odontaspis cf. winkleri Leriche, 1905; Carlsen and Cuny, p. 54, fig. 9G-L.
2016 Odontaspis winkleri Leriche, 1905; Cappetta and Case, p. 49, pl. 2, fig. 6.
2016 Odontaspis winkleri Leriche, 1905; Malyshkina and Ward, p. 53, fig. 3N, O.
2019 Odontaspis winkleri Leriche, 1905; Ebersole et al., p. 52-53, fig. 18.
Material. Two anterior teeth, NMNHU-G 391/83; one anterolateral tooth, NMNHU-G 391/74; three lateral teeth, NMNHU-G 391/76, 391/79, 391/82, Vyshhorod.
Description. The teeth (Figure 5I-L) vary in size from 10.9 to 17.3 mm apicobasally and from 9.1 to 12.5 mm mesiodistally. The crown is slender and has a smooth surface. In addition to a narrow and pointed main cusp, there are two pairs of very sharp cusplets, which are fused by their bases and differ in size: the inner ones are high (up to 4 mm) and circular at the base while the outer cusplets are vestigial. Cutting edges are not serrated and do not reach the crown base. The root is massive, usually arcuate, and has two relatively long lobes with rounded tips. Its labial face is concave, whereas the lingual face is convex and bears a deep nutritive groove.
Remarks. The specimens considered are similar in morphology and size to those in Odontaspis winkleri originally described by Leriche (1905). They differ from those of other odontaspidids by high and cylindrical lateral cusplets, incomplete cutting edges in anterior teeth, and well-pronounced folds at the base of the labial side in lateral teeth (Ebersole et al., 2019). Odontaspis winkleri is well represented in the Eocene fossil record of Europe (Leriche, 1905; Casier, 1946, 1966; Bor, 1985; Nolf, 1988; Adnet 2006b; Dutheil et al., 2006; Udovichenko, 2006; Eeckhaut and De Schutter, 2009; Rayner et al., 2009; Cappetta, 2012; Diedrich, 2012; Carlsen and Cuny, 2014), Asia (Savtshenko, 1912; Menner, 1928; Malyshkina and Ward, 2016), and North America (Cappetta and Case, 2016; Ebersole et al., 2019).
Family LAMNIDAE Bonaparte, 1835
Genus ISUROLAMNA Cappetta, 1976
Isurolamna affinis (Casier, 1946)
Figure 5M-S
1861 Oxyrhina biflena n. sp.; Rogovich, p. 55, pl. VIII, figs. 4, 5.
1912 Oxyrhina biflexa Rog.; Savtchenko, p. 180, pl. XIII, figs. 28, 33, 39.
1928 Oxyrhina biflexa Rog.; Menner, p. 306.
1946 Odontaspis hopei var. affinis; Casier, p. 65, pl. 2, fig. 11b-c.
1950 Lamna affinis (Casier); Casier, p. 17.
1966 Lamna affinis (Casier); Casier, p. 80, pl. 5, figs. 7-14.
1976 Isurolamna affinis (Casier, 1946); Cappetta, p. 555-556, pl. 2, figs. 1-8.
2012 Isurolamna affinis (Casier, 1946); Cappetta, p. 217, fig. 203A-G.
2012 Isurolamna affinis (Casier, 1946); Diedrich, p. 19, fig. 14.10.
2014 Isurolamna affinis (Casier, 1946); Carlsen and Cuny, p. 46, fig. 4A-G.
2021 Isurolamna affinis (Casier, 1946); Adnet et al., p. 31-33, fig. 4.5-4.6.
Material. Three anterior teeth, NMNHU-G 391/94, 391/99, 391/100; one anterolateral tooth, NMNHU-G 391/97; three lateral teeth, NMNHU-G 391/95, 391/140/1-2, Vyshhorod.
Description. The anterior teeth (Figure 5O-Q) measure from 16 to 17 mm apicobasally. The crown is slender and moderately inclined lingually. The lingual face is strongly convex, and the labial face is almost flat with a median depression near the crown-root junction. The enameloid is smooth on both faces, and the cutting edges reach the base of the crown. There is only a main cusp without cusplets, and both mesial and distal sides of the crown are smooth. The root with an arcuate basal edge shows two rounded lobes, which are broken and therefore it is not possible to measure the angle between them. There is a round foramen on the lingual protuberance.
The anterolateral tooth (Figure 5M-N) is similar in morphology and size to the anterior ones (measuring 16.5 mm apicobasally and 11.1 mm mesiodistally), although it has a wider main cusp. The root consists of two lobes differing in size and the degree of roundness.
The lateral teeth (Figure 5R-S) measure from 10.5 to 18.4 mm apicobasally and from 10.8 to 16.0 mm mesiodistally. The crown is triangular, inclined distally, and flattened labiolingually. There are two doubled triangular cusplets that are well separated from the main cusp in lingual view, although they are absent in one specimen (as in the case of anterior and anterolateral teeth). One of the root lobes is shorter and rectangular, whereas the other one is longer and has a rounded edge. The basal root edge is arcuate, and the angle between the root lobes equals 100°.
Remarks. The genus Isurolamna has characteristic heterodonty: anterior and anterolateral teeth of its representatives are of isuroid morphology, whereas the lateral teeth are morphologically similar to those in certain lamnids (Cappetta, 1976). Of the three species known so far from the Eocene of Europe and Asia (Adnet et al., 2021), the specimens described in this study are the most similar to those of I. affinis (Casier, 1946). These teeth differ from I. inflata Leriche, 1905 in lacking lateral cusplets, and they are much smaller than those of I. bajarunasi Glickman and Zhelezko, 1985 from the middle Eocene of Kazakhstan (see Adnet et al., 2021 for details). Udovichenko (2006) noted the presence of the latter species in the middle Eocene of Hradyzk (Ukraine). We assume that the specimen depicted there should rather be assigned to I. affinis. It is, however, possible that teeth of taxa such as Anomotodon may be mixed in this sample.
Genus MACRORHIZODUS Cappetta, 1976
Macrorhizodus praecursor (Leriche, 1905)
Figure 6A-H
1861 Lamna lata Rog.; Rogovich, p. 49, pl. VII, figs. 1-2a.
1861 Oxyrhina Desorii [sic] Ag.; Rogovich, p. 54, pl. VIII, figs. 1-3.
1905 Oxyrhina desori praecursor; Leriche, p. 128.
1928 Oxyrhina desori Sismonda; Menner, p. 304, pl. X, figs. 28-31.
1928 Oxyrhina desori mut. praecursor Leriche; Menner, p. 305.
1942 Oxyrhina praecursor americana; Leriche, p. 45, pl. 3, figs. 6-13.
2002 Cosmopolitodus praecursor; Mustafa and Zalmout, p. 82, pl. 1, figs. 7-11.
2005 Cosmopolitodus praecursor (Leriche, 1905); Mustafa et al., p. 408-409, figs. 14-20.
2006 Isurus praecursor (Leriche); Udovichenko, p. 202, pl. I, fig. 16.
2011 Macrorhizodus praecursor (Leriche, 1905); Underwood et al., p. 54, fig. 4C, D.
2012 Macrorhizodus praecursor (Leriche, 1905); Cappetta, p. 221-222, fig. 207.
2012 Isurus praecursor (Leriche, 1905); Diedrich, p. 15, fig. 11.1-11.10.
2012 Macrorhizodus praecursor (Leriche, 1905); Zalmout et al., p. 76, fig. 4A-V.
2013 Macrorhizodus praecursor (Leriche, 1905); Otero et al., fig. 3.28-3.34.
2013 Macrorhizodus praecursor (Leriche, 1905); Malyshkina et al., pl. 13, fig. 7.
2017 Macrorhizodus praecursor (Leriche, 1905); Zalat et al., p. 207; pl. 1, fig. 5.
2019 Macrorhizodus praecursor (Leriche, 1905); Ebersole et al., p. 56-58, fig. 20.
2019 Macrorhizodus praecursor (Leriche, 1905); Trif et al., p. 8, fig. 5.1-5.4.
2021 Macrorhizodus praecursor (Leriche, 1905); Adnet et al., p. 31, fig. 4.1-4.4.
2021 Macrorhizodus praecursor (Leriche, 1905); Zouhri et al., p. 125, fig. 2H-K.
2022 Macrorhizodus praecursor (Leriche, 1905); Perez, p. 635, fig. 4L, M.
Material. One anterior tooth, NMNHU-G 391/101; one anterolateral tooth, NMNHU-G 391/103, Vyshhorod; one additional anterior tooth, NMNHU-G 391/68, and one additional anterolateral tooth, NMNHU-G 391/69, also from Vyshhorod, tentatively identified to this taxon.
 Description. NMNHU-G 391/101 (Figure 6A-B) is quite large, although the crown is broken at the tip; therefore, it is not possible to measure its apicobasal height. The main cusp is stout, has a lingual inclination in profile, and is not accompanied by any cusplets. Both the labial and lingual faces of the crown are smooth and convex. The cutting edges extend from the apex to the crown base. The robust root ends with lanceolate lobes, one of which is partly broken.
Description. NMNHU-G 391/101 (Figure 6A-B) is quite large, although the crown is broken at the tip; therefore, it is not possible to measure its apicobasal height. The main cusp is stout, has a lingual inclination in profile, and is not accompanied by any cusplets. Both the labial and lingual faces of the crown are smooth and convex. The cutting edges extend from the apex to the crown base. The robust root ends with lanceolate lobes, one of which is partly broken.
NMNHU-G 391/68 (Figure 6C-D) represents a crown broken at the base near its junction with the root. The crown is triangular and reaches 19.4 mm mesiodistally. Its labial face is almost flat, whereas the lingual face is convex. The cutting edges extend to the crown base.
NMNHU-G 391/103 (Figure 6E-F) measures 23.8 mm apicobasally and 17.6 mm mesiodistally. The triangular cusp is shorter than that in the anterior tooth. The root lobes with slightly pointed tips are unequal in length. There is a weak lingual protuberance on the root.
NMNHU-G 391/69 (Figure 6G-H) measures 22.8 mm apicobasally and ca. 20.5 mm mesiodistally. It is preserved better, albeit its root is broken. The cusp is asymmetrical and lateral cusplets are absent. Both the mesial and distal cutting edges are convex.
Remarks. The specimens described are morphologically similar to those in Macrorhizodus praecursor and fit well into the diagnosis of this species. Macrorhizodus praecursor had a worldwide distribution during the middle and late Eocene (Cappetta, 2012; Adnet et al., 2021). However, the taxonomic identification of NMNHU-G 391/68 and NMNHU-G 391/69 is tentative because of their poor state of preservation, particularly due to the absence of roots.
Family OTODONTIDAE Glickman, 1964
Genus OTODUS Agassiz, 1838
Otodus (Carcharocles) sp.
Figure 6I-P, Figure 7A-M
1861 Carcharodon megalotis Ag.; Rogovich, p. 36, pl. IV, figs. 14-16b.
1861 Carcharodon lanceolatus Ag.; Rogovich, p. 37, pl. IV, fig. 17; pl. 9, figs. 58, 58a.
1861 Carcharodon productus Ag.?; Rogovich, p. 37, pl. IV, figs. 18, 19.
Material. One upper lateral tooth, NMNHU-G 391/32; three lower anterolateral teeth, NMNHU-G 391/26, 391/29, 391/35, Vyshhorod; five isolated vertebrae, NMNHU-P PI 553, PI 554, PI 2314, PI 2315, PI 2316, Zoloti Vorota.
Description. The anterolateral and lateral teeth (Figure 6I-P) are large and wide, triangular or lanceolate in shape; all the specimens are broken near the crown-root junction. The total height of the crown is in the range of 39.1-46.8 mm with the maximum width of about 30 mm. Both crown faces are smooth; the lingual face is convex and the labial one is flat. The cutting edges are irregularly serrated with saw-like serrations from the rounded crown apex to the base.
 The vertebrae specimens (Figure 7A-M) are represented by robust, well-calcified, disk-shaped centra, where the smallest one (NMNHU-P PI 554) measures 64 mm in diameter and 29 mm in anteroposterior length; the largest one (NMNHU-P PI 2314) measures 88 mm in diameter and 32 mm in anteroposterior length. They are characterised as ‘lamnoid vertebrae’ (Applegate, 1967) by exhibiting many radiating calcified lamellae (asterospondylic) connecting the two primary cones of unperforated amphicoelous calcification (corpora calcarea) (for terminology, see Ridewood, 1921; Newbrey et al., 2015). The walls of the pairs of circular to oval foramina for the basidorsal and basiventral cartilages (Welton and Farish, 1993) make direct contact with the corpora calcarea. Both articular surfaces exhibit many concentric growth bands.
The vertebrae specimens (Figure 7A-M) are represented by robust, well-calcified, disk-shaped centra, where the smallest one (NMNHU-P PI 554) measures 64 mm in diameter and 29 mm in anteroposterior length; the largest one (NMNHU-P PI 2314) measures 88 mm in diameter and 32 mm in anteroposterior length. They are characterised as ‘lamnoid vertebrae’ (Applegate, 1967) by exhibiting many radiating calcified lamellae (asterospondylic) connecting the two primary cones of unperforated amphicoelous calcification (corpora calcarea) (for terminology, see Ridewood, 1921; Newbrey et al., 2015). The walls of the pairs of circular to oval foramina for the basidorsal and basiventral cartilages (Welton and Farish, 1993) make direct contact with the corpora calcarea. Both articular surfaces exhibit many concentric growth bands.
Remarks. The tooth specimens from Vyshhorod are identical in morphology and size to those of Otodus (Carcharocles) auriculatus from the Eocene of Europe, Asia, and North America (e.g., Savtchenko, 1912; Glickman, 1964; Nolf, 1988; Zhelezko and Kozlov, 1999; Cappetta, 2012; Carlsen and Cuny, 2014; Maisch et al., 2015; Adnet et al., 2021). Zhelezko and Kozlov (1999) reported on the presence of a specific subspecies–Otodus auriculatus auriculatus (Blainville, 1818)–occurring in the Lutetian of Ukraine, Kazakhstan, and Central Asia. It differs from Otodus auriculatus disauris (Agassiz, 1843) in the presence of regular serrations on the cutting edges. While the state of preservation of the material described in this study is insufficient for identifying it closer than to genus level, the merit of such subspecies concept for the genus Otodus is uncertain. The specimens considered are represented by broken crowns only, therefore we do not speciate them. The five isolated vertebrae likely represent the same taxon due to their similar morphology and belong to a lamniform based on their ‘lamnoid’ type (see above). The tentative identification is based on their large vertebral sizes that precludes to be any other known lamniform taxa from the Eocene, and the fact that their morphology does not contradict with that of previously described vertebrae of O. auriculatus (Ehret and Ebersole, 2014).
Lamniformes indet.
Figure 8A-M
1861 Shark vertebrae; Rogovich, p. 63, pl. IX, figs. 6-6b, 11-16.
Material. Seven vertebrae, NMNHU-G 391/118, 391/119, 391/120, 391/121, 391/122, 391/136, 391/137, Vyshhorod.
 Description. NMNHU-G 391/118, 391/119, 391/120, 391/121, and 391/122 represent five of the six vertebral centra that are considered to have come from a single vertebral column, which were found near Vyshhorod (Rogovich, 1861), whereas NMNHU-G 391/136 and 391/137 represent isolated vertebrae. All the vertebrae are represented by gracile but well-calcified, unperforated, amphicoelous centra with several thin radiating calcified lamellae. Their articular surfaces are circular ranging up to 44.1 mm in diameter and 19.4 mm in anteroposterior length (based on NMNHU-G 391/136). The walls of the pairs of robust circular to oval foramina for the basidorsal and basiventral cartilages make direct contact with the corpora calcarea. Many faint concentric growth bands are present on both articular surfaces of the vertebrae considered.
Description. NMNHU-G 391/118, 391/119, 391/120, 391/121, and 391/122 represent five of the six vertebral centra that are considered to have come from a single vertebral column, which were found near Vyshhorod (Rogovich, 1861), whereas NMNHU-G 391/136 and 391/137 represent isolated vertebrae. All the vertebrae are represented by gracile but well-calcified, unperforated, amphicoelous centra with several thin radiating calcified lamellae. Their articular surfaces are circular ranging up to 44.1 mm in diameter and 19.4 mm in anteroposterior length (based on NMNHU-G 391/136). The walls of the pairs of robust circular to oval foramina for the basidorsal and basiventral cartilages make direct contact with the corpora calcarea. Many faint concentric growth bands are present on both articular surfaces of the vertebrae considered.
Remarks. The seven vertebrae described here exhibit a generally similar morphology, but whether all of them are conspecific cannot be ascertained. Similarly to the vertebrae of Otodus (Carcharocles) sp. (see above), the entire surface of the intermedialia (i.e., the body of each centrum between both sides of corpora calcarea) is rough because of the terminal edges of the radiating calcified lamellae, indicating that they are of ‘lamnoid type’ (see above). They differ from all other elasmobranch vertebrae described below by the surface of the intermedialia being either largely smooth or massive in appearance (except for the oval foramina for the basidorsal and basiventral cartilages) or having laterally oblong (non-circular) articular surfaces. However, because they are isolated finds with no associated teeth, their exact taxonomic identity beyond ‘Lamniformes indet.’ is uncertain.
Order CARCHARHINIFORMES Compagno, 1973
Family CARCHARHINIDAE Jordan and Evermann, 1896
Genus PHYSOGALEUS Cappetta, 1980
Physogaleus secundus (Winkler, 1876)
Figure 9
1861 Galeocerdo minor Ag.; Rogovich, p. 30, pl. IV, figs. 1-7.
1876 Trigonodus secundus n. sp.; Winkler, p. 16-48, pl. 2, figs. A-F.
1905 Physodon secundus (Winkler); Leriche, p. 189, pl. 8, figs. 6, 17, 18.
1912 Galeocerdo minor Ag.; Savtchenko, p. 170-171, pl. XIII, figs. 13-15.
1980 Physogaleus secundus (Winkler, 1876); Cappetta, p. 37, pl. 5.
1985 Striatolamia macrota (Agassiz, 1843); Bor, p. 95, pl. 3, figs. 3-8.
2002 Physogaleus secundus (Winkler, 1874); Dutheil et al., p. 758, fig. 4F, G.
2006 Physogaleus secundus (Winkler); Malyshkina, pl. 7, figs. 7-8.
2012 Physogaleus secundus (Winkler, 1876); Cappetta, p. 313-315, fig. 297.
2014 Physogaleus cf. secundus Winkler, 1876; Carlsen and Cuny, p. 61-62, fig. 14.
2019 Physogaleus secundus (Winkler, 1876); Ebersole et al., p. 95-98, fig. 34.
2019 Physogaleus secundus Winkler, 1876; Trif et al., p. 7-8, fig. 4.5-4.11.
Material. Three upper lateral teeth NMNHU-G 391/24/2-4, one lower anterolateral tooth NMNHU-G 391/24/1, one lower lateral tooth, NMNHU-G 391/24/5, Vyshhorod.
 Description. The anterolateral tooth (Figure 9B) is quite large measuring 9.4 mm mesiodistally, 9.0 mm apicobasally, and 4.1 mm labiolingually. It has a slender and sigmoidal main cusp. The lingual face of the crown is convex, and the labial face is almost flat. The mesial cutting edge is long and faintly serrated near the base, whereas the distal edge is shorter and bears two rounded cusplets. The root has a lingual protuberance and deep central furrow.
Description. The anterolateral tooth (Figure 9B) is quite large measuring 9.4 mm mesiodistally, 9.0 mm apicobasally, and 4.1 mm labiolingually. It has a slender and sigmoidal main cusp. The lingual face of the crown is convex, and the labial face is almost flat. The mesial cutting edge is long and faintly serrated near the base, whereas the distal edge is shorter and bears two rounded cusplets. The root has a lingual protuberance and deep central furrow.
The lateral teeth (Figure 9A, C) range in size from 7.8 to 9.9 mm mesiodistally (mean 8.6 mm), from 5.2 to 5.9 mm apicobasally (mean 5.6 mm), and from 2.2 to 2.5 mm labiolingually (mean 2.3 mm). The lower lateral tooth (Figure 9C) is slightly larger than the upper lateral ones. The sigmoidal crowns are angled distally. The mesial and distal edges of the main cusp are smooth. The base of the mesial cutting edge is slightly serrated, and there are four coarse triangular cusplets at the base of the distal cutting edge. The lingual and labial tooth surfaces are convex and the labial one overhangs the root. The root has a large lingual protuberance with a deep nutritive groove. The rectilinear basal root surface is nearly flat or slightly concave.
Remarks. The teeth are identical in morphology and size to those of Physogaleus secundus. The latter differs from other species of the genus in tooth size, cusp width, development of cusplets, and morphology of the cutting edge (Carlsen and Cuny, 2014; Ebersole et al., 2019; Trif et al., 2019). In particular, the teeth of P. secundus can be differentiated from those in the coeval species Physogaleus alabamensis (Leriche, 1942) by the number and strength of the mesial and distal denticles (Ebersole et al., 2019). The remains of P. secundus are known from the Eocene of Europe (Leriche, 1905; Bor, 1985; Dutheil et al., 2002; Cappetta, 2012; Carlsen and Cuny, 2014; Trif et al., 2019), Asia (Malyshkina, 2006), and North America (Maisch et al., 2015; Ebersole et al., 2019). It should be emphasised that Rogovich (1861) described a series of carcharinid teeth from Vyshhorod that he identified as Galeocerdo minor. This taxon originally was erected and figured by Agassiz (1835; Agassiz, 1833-1843, vol. 3, p. 232, pl. 26a, figs. 64-66, pl. 26, figs. 15-21) under the name Galeus minor. Woodward (1889) considered Galeocerdo minor to be valid but questioned its generic attribution. Although the locality and age of the original sample were unknown, Agassiz (1843) assumed that it probably came from Tertiary deposits of the Swiss Molasse Basin. Galeocerdo minor was reported from the Eocene of Belgium, England, the USA, and from the Miocene of France (Woodward, 1889). In addition, Savtchenko (1912) documented the remains of this taxon in the Eocene of Mangyshlak (Kazakhstan). It is noteworthy that the latter was the last published reference to Galeocerdo minor in the literature. It is figured in the database compiled by Pollerspöck and Straube (2022) as Physogaleus minor (Agassiz, 1835). We cautiously assume that P. minor and P. secundus could be conspecific, although such a revision is beyond the scope of our present study.
Order MYLIOBATIFORMES Compagno, 1973
Family MYLIOBATIDAE Bonaparte, 1838
Myliobatidae gen. et sp. indet.
Figure 10A-F
1861 Myliobates toliapicus Ag.; Rogovich, p. 12, pl. 2, figs. 14, 22.
Material. Nineteen teeth, NMNHU-G 391/3, Vyshhorod.
Description. The teeth (Figure 10A-F ) are hexagonal and mesiodistally elongated. The root consists of thin, uniformly narrow lobes, fused at the tip, and oriented perpendicular to the crown in the form of a comb. The crown overhangs the root, and it is separated from it by a sharp transverse ridge on the labial side and has a respective recess on the lingual side. It consists of a thin enameloid layer, the surface of which is either straight or convex. There are faint longitudinal ridges and numerous nutritive foramina on the transverse edges of the crown.
) are hexagonal and mesiodistally elongated. The root consists of thin, uniformly narrow lobes, fused at the tip, and oriented perpendicular to the crown in the form of a comb. The crown overhangs the root, and it is separated from it by a sharp transverse ridge on the labial side and has a respective recess on the lingual side. It consists of a thin enameloid layer, the surface of which is either straight or convex. There are faint longitudinal ridges and numerous nutritive foramina on the transverse edges of the crown.
Remarks. The series of teeth described above was assigned to the family Myliobatidae based on several characters, including hexagonal shape, mesiodistal elongation, and the presence of multiple nutritive grooves (Cappetta, 2012). We refrain from assigning these fragmentary specimens to a particular genus due to the poor state of their preservation. In addition, the recent molecular divergence estimated by Villalobos-Segura and Underwood (2020) indicates that myliobatid genera did not diverge until the Neogene. Thus, Paleogene forms seemingly represent stem members of their respective lineages and cannot be assigned to any extant genera.
Myliobatiformes indet.
Figure 10G-I
1843 Myliobates Owenii [sic]; Agassiz, p. 331, pl. 45, figs. 11-13.
1861 Myliobates Owenii [sic] Ag.; Rogovich, p. 11, pl. I, fig. 7.
1912 Myliobates Owenii [sic] Ag.; Savtchenko, p. 167, pl. XIII, fig. 4.
1912 Myliobates sp.; Savtchenko, p. 167-168, pl. XIII, figs. 1-3.
2019 Myliobatinae indet. 2; Trif et al., p. 13-14, fig. 7.13-7.15.
2021 Myliobatiformes indet.; Szabó et al., p. 391, pl. IX, figs. J’-M’.
2022 Myliobatiformes indet.; Trif et al., fig. 9A-C.
Material. One broken caudal spine, NMNHU-G 391/2, Vyshhorod.
Description. The flattened caudal spine (Figure 10G-I) is large with a preserved length of 14.6 cm and maximum width of 2.0 cm. It is broken into three fragments of different sizes. The spine gradually tapers towards the distal tip. Both lateral edges at a distance of about 4 cm from the base bear short sawtooth barbs that are proximally curved and increase in size to the tip. Both dorsal and ventral surfaces of the spine are striated and covered by irregular grooves parallel to each other. In addition, there are two longitudinal ridges on the ventral side of the spine.
Remarks. Rogovich (1861) initially identified this specimen as Myliobates Owenii [sic], and, in fact, it looks identical to those figured by Agassiz (1843, pl. 45, figs. 11-13). However, M. owenii is now recognised a nomen dubium because it was erected based on the caudal spine and not on dentition (Pollerspöck and Straube, 2022). According to Hovestadt and Hovestadt-Euler (2013) and Trif et al. (2022), caudal spines of Myliobatiformes have little diagnostic value and therefore we leave the specimen considered here in open nomenclature.
Elasmobranchii indet.
Figure 11
1861 Lamna (vertebrae); Rogovich, p. 62, pl. IX, figs. 2, 3, 5.
1861 Shark vertebrae; Rogovich, p. 63, pl. IX, figs. 7, 8.
Material. Nine vertebrae, NMNHU-G 391/125, 391/126, 391/127, 391/129, 391/130, 391/131, 391/133, 391/134, 391/135, Vyshhorod.
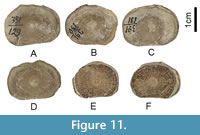 Description. The vertebrae are represented by well-calcified, unperforated, amphicoelous centra with a variable range of sizes but quite similar in overall morphology.
Description. The vertebrae are represented by well-calcified, unperforated, amphicoelous centra with a variable range of sizes but quite similar in overall morphology.
Remarks. Because of the wide range of size and morphological variations observed among the nine vertebrae, they most certainly represent multiple taxa. For example, NMNHU-G 391/126, 391/129, 391/130, 391/131, 391/133, 391/134, and 391/135 may belong to the same taxon because they exhibit laterally oblong articular surfaces, but the articular surfaces are circular in NMNHU-G 391/125 and 391/127. However, they cannot be interpreted as Lamniformes because their intermedialia does not exhibit any noticeable radiating calcified lamellae. The slight dorsoventral compression observed in these vertebrae may indicate that they belong to a batoid, but we conservatively describe them here as Elasmobranchii indet. They may belong to one or more of the aforementioned taxa in this study, and their exact taxonomic identifications are difficult because they are not accompanied by any teeth, which have higher diagnostic value.
DISCUSSION
Palaeogeographic Features of the Dnieper-Donets Basin
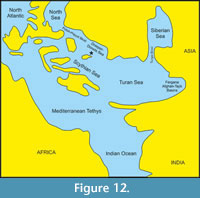 The Paleogene marine basin that existed in the area of present-day Ukraine was the successor of the Cretaceous Ocean. During the Cretaceous, the southern and western regions of the East European Platform, as well as the Ukrainian Shield, subsided, sea-level rose, and by the Turonian-Coniacian only a few islands remained above the water (Kraeva et al., 1960; Ivanik et al., 2013; Kyselevych and Kovalchuk, 2021; Kovalchuk et al., 2022; Amadori et al., 2023). Following the uplift of the north-western and part of the southern regions of the East European Platform during the Cretaceous-Paleogene boundary, these and adjacent regions began to submerge again in the Paleogene. Palaeoceanographic changes, the opening and closing of seaways, have had a major impact on faunal exchange between different marine basins. Rögl (1998) considered these factors as major driving forces in the evolution of marine ecosystems. During the Eocene, marine waters filled the Pripyat Fault and the Dnieper-Donets depression, and the area of present-day Ukraine from the middle Lutetian was covered by sea, with only a few islands within the Ukrainian Shield and Near-Azov Massif remaining above the water (Ronov and Khain, 1961). During the middle Eocene, the Dnieper-Donets Basin was part of the Trans-European sub-latitudal marine corridor between the North Sea and marine basins in the Northern Near-Black Sea region, the Crimea-Caucasus region, and the Scythian Plate (Figure 12). These basins were also connected with the Turan Sea of the north-eastern Peri-Tethys and with the Siberian Sea through the Turgai Strait in the east, as well as with Tethyan basins in the south (e.g., see Ronov and Khain, 1961; Savytska, 1996; Beniamovski, 2005, 2007; Akhmetiev, 2010; Vasilieva, 2018).
The Paleogene marine basin that existed in the area of present-day Ukraine was the successor of the Cretaceous Ocean. During the Cretaceous, the southern and western regions of the East European Platform, as well as the Ukrainian Shield, subsided, sea-level rose, and by the Turonian-Coniacian only a few islands remained above the water (Kraeva et al., 1960; Ivanik et al., 2013; Kyselevych and Kovalchuk, 2021; Kovalchuk et al., 2022; Amadori et al., 2023). Following the uplift of the north-western and part of the southern regions of the East European Platform during the Cretaceous-Paleogene boundary, these and adjacent regions began to submerge again in the Paleogene. Palaeoceanographic changes, the opening and closing of seaways, have had a major impact on faunal exchange between different marine basins. Rögl (1998) considered these factors as major driving forces in the evolution of marine ecosystems. During the Eocene, marine waters filled the Pripyat Fault and the Dnieper-Donets depression, and the area of present-day Ukraine from the middle Lutetian was covered by sea, with only a few islands within the Ukrainian Shield and Near-Azov Massif remaining above the water (Ronov and Khain, 1961). During the middle Eocene, the Dnieper-Donets Basin was part of the Trans-European sub-latitudal marine corridor between the North Sea and marine basins in the Northern Near-Black Sea region, the Crimea-Caucasus region, and the Scythian Plate (Figure 12). These basins were also connected with the Turan Sea of the north-eastern Peri-Tethys and with the Siberian Sea through the Turgai Strait in the east, as well as with Tethyan basins in the south (e.g., see Ronov and Khain, 1961; Savytska, 1996; Beniamovski, 2005, 2007; Akhmetiev, 2010; Vasilieva, 2018).
Such an extensive network of interconnected bodies of water facilitated the dispersal of marine organisms, including sharks, rays, and chimaeras. At the time of the accumulation of phosphorite sands, the shallow-marine, neritic Dnieper-Donets Basin in the Kyiv region was well-aerated and had normal salinity (Sokolov, 1986; Savytska, 1996; Shevchenko, 2000, 2002; Ryabokon and Shevchenko, 2001). Shallow-dwelling taxa prevailed among the calcareous nannoplankton, including equatorial-tropical and subtropical forms, which suggests a high surface water temperature (Savytska, 1996). The rich benthic fauna was represented by solitary and colonial corals, bryozoans, and stenohaline and stenothermic molluscs (Sokolov, 1986).
The progressive transgression led to the accumulation of a marl-clayey series in a deep marine, middle-outer neritic basin as suggested by the presence of calcareous nanoplankton characteristic of a deep-sea shelf (Savytska, 1996). This is also indicated by the ratio of phytoplankton groups, and many oceanic dinocysts (Shevchenko, 2000, 2002).
Middle Eocene chondrichthyans of the Dnieper-Donets Basin
Our knowledge on the taxonomic composition of cartilaginous fishes in the middle Eocene marine ecosystem of the Dnieper-Donets Basin has long been based on the faunal list published by Rogovich (1861). Based on the fossils collected from the Vyshhorod locality, O. S. Rogovich identified 29 chondrichthyan taxa, including two species of chimaeras, 24 sharks, and three rays. As mentioned above, some specimens described by him were subsequently lost or (less likely) transferred to other institutions; those are remains of eight species (either valid or synonymised), mostly of lamniform sharks, but also Myliobatis striatus Buckland, 1836 (Table 1). This material is not available and thus cannot be considered in further discussion.
The revision of the remaining part of the sample has resulted in a consolidated faunal list comprising a single chimaeriform species (Edaphodon bucklandi), 12 shark and one or two ray taxa. Of the latter two groups, 10 sharks are identified to species level, whereas rays are only identified to order and family ranks (Myliobatiformes, Myliobatidae). Shark remains belong to representatives of the orders Hybodontiformes, Hexanchiformes, Lamniformes, and Carchariniformes. Because of the poor preservation and/or lack of reliable diagnostic characters, vertebral centra from Rogovich’s collection and those recovered from Zoloti Vorota are all described in open nomenclature (Lamniformes indet. and Elasmobranchii indet., repectively).
When comparing the original and revised lists of taxa, it becomes clear that only two species were identified accurately in the original publication (Rogovich, 1861), whereas the other taxa have been either synonymised or re-assigned to other genera or even families. The explanation for the considerable number of erroneous taxonomic assignments by O.S. Rogovich is that Agassiz (1833-1843) was the only reference source he used in his study, and he had no comparative materials in his possession. These incorrect taxonomic identifications were later replicated without revision by other researchers, although nomenclatural changes of several taxa described by Rogovich were introduced by Capetta (2006), presumably based on the images of the corresponding specimens published in Rogovich’s monograph. Our findings confirm and amend these earlier revisions. In particular, ‘species’ sensu Rogovich (1861) such as Carcharodon megalotis, C. lanceolatus, and C. productus belong to a single taxon, Otodus (Carcharocles) sp. The specimens of ‘Lamna cuspidata’ and ‘L. denticulata’ were re-identified here as Jaekelotodus trigonalis. At the same time, we assume that the teeth assigned by Rogovich (1861) to Notidanus serratissimus represent two different species–Hexanchus agassizi and Notorynchus kempi. Another important issue is that characters used by O.S. Rogovich to describe new species (e.g., Acrodus kioviensis) are now considered not diagnostic enough due to their wide range of variation. In the light of our findings, such species names, including those created for the lost specimens, that is, Galeocerdo paradoxus (Rogovich, 1861, pl. IV, figs. 10, 11), Otodus microtus (Rogovich, 1861, pl. V, figs. 24-27), Lamna elegans (Rogovich, 1861, pl. IV, figs. 10, 11), Oxyrhina falcata (Rogovich, 1861, pl. VII, figs. 24, 25), Oxyrhina leptodon (Rogovich, 1861, pl. VIII, figs. 9, 10), Chomatodus dubius (Rogovich, 1861, pl. I, fig. 8), and Hybodus helophorus (Rogovich, 1861, pl. III, figs. 19, 20), are most likely nomina dubia. We conclude that Lamna compressa and Oxyrhina brevidens erected by Rogovich (1861) should indeed be synonymised with Striatolamia macrota. Another name, Lamna (Odontaspis) hispida, also should be regarded a synonym of Odontaspis winkleri. In addition, we synonymise Lamna lata and Oxyrhina biflena with Macrorhizodus praecursor and Isurolamna affinis, respectively. The latter conclusion is based on direct examination of the specimens by the authors, and it corroborates the assumption previously made by Cappetta (2006).
Certainly, the analysed sample does not fully reflect the taxonomic diversity of the middle Eocene chondrichthyan assemblage in this part of the Dnieper-Donets Basin, and thus palaeoecological reconstructions based only on these materials would substantially be biased. Little is known about the collecting approach, which is not described in detail by Rogovich (1861). Most likely, the teeth, vertebrae, and other skeletal elements (such as fragments of the caudal spine) were collected by hand and the bone-bearing rock was not sieved or screen-washed. The presence of individual small teeth in the collection is therefore considered rather accidental. Nonetheless, the studied sample of fishes is of important historical and scientific value and contributes to the understanding of the palaeodiversity of Eocene marine ecosystems that existed in present-day Ukraine and, more widely, in Eastern Europe.
Most taxa identified in the present study represent the epi- and mesopelagic assemblage and only a relatively small number of them represent benthopelagic (Edaphodon bucklandi, Myliobatidae gen. et sp. indet.) and demersal or bathydemersal forms (Hexanchus agassizi, Notorynchus kempi). These chondrichthyans inhabited shallow, warm waters (up to 200 m depth but most likely to 100 m) and were confined to the continental shelf. Their remains could have been transported by currents over some distance and deposited in a deeper marine environment. Further studies based on a more comprehensive sample are needed to shed more light on the structure of palaeocommunities in middle Eocene marine ecosystems of Ukraine.
ACKNOWLEDGMENTS
The research of OK and ZB was carried out as part of the research projects ‘Development of the biota in the late Cenozoic of Ukraine’ (0120U100451) and ‘The role of particular fish groups in the functioning of late Mesozoic and Cenozoic ecosystems of Eastern Europe’ (0123U102984) funded by the National Academy of Sciences of Ukraine. This research was funded in part by the Austrian Science Fund (FWF) [P 33820] to JK. The authors sincerely thank D. Cicimurri, J. Ebersole, and an anonymous reviewer for their valuable notes and suggestions to the early draft that significantly improved the manuscript. O. Kozlov (Armed Forces of Ukraine) is acknowledged for providing important historical information. We are thankful to P.L. Jambura (University of Vienna, Austria) and J. Pollerspöck (Zoologische Staatssammlung München, Germany) for their kind advisory help. We also wish to thank O. Guliakova (National University of Life and Environmental Sciences of Ukraine) for preparing X-ray images of shark vertebrae.
REFERENCES
Adnet, S. 2006a. Biometric analysis of the teeth of fossil and Recent hexanchid sharks and its taxonomic implications. Acta Palaeontologica Polonica, 51(3):477-488.
Adnet, S. 2006b. Nouvelles faune de sélaciens (Elasmobranchii, Neoselachii) de l’Eocéne Moyen des Landes (Sud-Ouest, France). Implication de la connaissance des communautés d’eaux profondes. Paleo Ichthyologica, 10:1-128.
Adnet, S., Cappetta, H., and Reynders, J. 2008. Contribution of Eocene sharks and rays from southern France to the history of deep-sea selachians. Acta Geologica Polonica, 58(2):257-260.
Adnet, S., Marivaux, L., Cappetta, H., Charruault, A.-L., Essid, E.M., Jiquel, S., Ammar, H.K., Marandat, B., Marzougui, W., Merzeraud, G., Temani, R., Vianey-Liaud, M., and Tabuce, R. 2020. Diversity and renewal of tropical elasmobranchs around the Middle Eocene Climatic Optimum (MECO) in North Africa: New data from the lagoonal deposits of Djebel el Kébar, Central Tunisia. Palaeontologia Electronica, 23(2):a38.
https://doi.org/10.26879/1085
Adnet, S., Feichtinger, I., Harzhauser, M., and Pollerspöck, J. 2021. A mesopelagic selachian fauna from the middle Eocene of St. Pankraz (Austria) reveals homogeneity in deep-marine environments during the warm period in Europe. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 301/1:25-63.
https://doi.org/10.1127/njgpa/2021/0996
Agassiz, L. 1833-1843. Recherches sur les poissons fossiles. Imprimerie de Petitpierre, Neuchâtel.
Agnini, C., Fornaciari, E., Raffi, I., Catanzariti, R., Pälike, H., Backmann, J., and Rio, D. 2014. Biozonation and biochronology of Paleogene calcareous nannofossils from low and middle latitudes. Newsletters on Stratigraphy, 47(2):131-181.
https://doi.org/10.1127/0078-0421/2014/0042
Akhmetiev, M.A. 2010. Paleocene and Eocene floristic and climatic change in Russia and Northern Kazakhstan. Bulletin of Geosciences, 85(1):17-34.
https://doi.org/10.3140/bull.geosci.1145
Amadori, M., Kovalchuk, O., Barkaszi, Z., Giusberti, L., Kindlimann, R., and Kriwet, J. 2023. A diverse assemblage of Ptychodus species (Elasmobranchii: Ptychodontidae) from the Upper Cretaceous of Ukraine, with comments on possible diversification drivers during the Cenomanian. Cretaceous Research, 151:105659.
https://doi.org/10.1016/j.cretres.2023.105659
Applegate, S.P. 1965. A confirmation of the validity of Notorhynchus pectinatus; the second record of this Upper Cretaceous cow shark. Bulletin of the Southern California Academy of Sciences, 64(3):122-126.
Applegate, S. P. 1967. A survey of shark hard parts, p. 37-67. In Gilbert, P.W., Mathewson, R.F., and Rall, D.P. (eds.), Sharks, Skates, and Rays. John Hopkins University Press, Baltimore.
Applegate, S.P. 1968. A large sand shark of the genus Odontaspis from Oregon. The Ore Bin, 30(2):32-36.
Arambourg, C. 1952. Les vertébrés fossiles des gisements de phosphates (Maroc-Algérie-Tunisie). Notes et Mémoires du Service Géologique du Maroc, 92:1-372.
Averianov, A.O. 2002. Review of Mesozoic and Cenozoic sea turtles from the former USSR. Russian Journal of Herpetology, 9(2):137-154.
Averianov, A.O. and Zvonok, E. 2021. First sirenian remains from the Palaeogene of Crimea. Historical Biology, 33(12):3166-3172.
https://doi.org/10.1080/08912963.2020.1852558
Averianov, A.O., Potapova, O.R., and Nessov, L.A. 1990. On the first native finds of the bones of ancient birds. Proceedings of the Zoological Institute of the Russian Academy of Sciences, 210:3-9. (In Russian)
Ayres, W.O. 1855. Shark of a new generic type: Notorynchus maculatus. Proceedings of the California Academy of Sciences, 1:72-73.
Bannikov, A.F. 2010. Fossil acanthopterygian fishes (Teleostei, Acanthopterygii), p. 1-244. In Parin, N.V. (ed.), Fossil vertebrates of Russia and adjacent countries. GEOS, Moscow. (In Russian)
Bauzá, J. and Gómez Pallerola, J.E. 1988. Contribución al conocimiento de la ictiología fósil de España. Bolletí de la Societat d'Història Natural de les Balears, 32:115-138.
Beniamovski, V.N. 2005. Late Cretaceous - Early Paleogene paleobiogeographic scripts for the northern periphery of the Tethys, p. 267-308. In Gladenkov, Yu.B. and Kuznetsova, K.I. (eds.), Transactions of Geological Institute. Vol. 516: Biosphere - ecosystem - biota in the Earth history: paleobiographic aspects. Nauka, Moscow. (In Russian)
Beniamovski, V.N. 2007. Paleogene meridional straits of the northern Eurasia, p. 80-118. In Baraboshkin, E.Yu. (ed.), Straits of the Northern Hemisphere in the Cretaceous and Paleogene. Moscow State University, Moscow. (In Russian)
Berg, L.S. 1937. A classification of fish-like vertebrates. Izvestiya Akademii Nauk USSR, Seriya Biologicheskaya, 4:1277-1280.
Bonaparte, C.L. 1832. Iconografia della fauna italica per le quattro classi degli animali vertebrati. Tomo III. Pesci. Roma.
Bonaparte, C.L. 1835. Iconografia della fauna italica per le quattro classi degli animali vertebrati. Tomo III. Pesci. Roma.
Bonaparte, C.L. 1838. Selachorum tabula analytica, Systema Ichthyologicum. Memoires de la Societe Neuchateloise des Sciences Naturelles, 2:1-16.
https://doi.org/10.5169/seals-100091
Bor, T.J. 1985. Elasmobranch teeth (Vertebrata, Pisces) from the Dongen Formation (Eocene) in The Nederlands. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie, 22(2):73-122.
Bosboom, R., Mandic, O., Dupont-Nivet, G., Proust, J.-N., Ormukov, C., and Aminov, J. 2017. Late Eocene palaeogeography of the proto-Paratethys Sea in Central Asia (NW China, southern Kyrgyzstan and SW Tajikistan), p. 565-588. In Brunet, M.-F., McCann, T., and Sobel, E.R. (eds.), Geological Evolution of Central Asian Basins and the Western Tien Shan Range. Geological Society, London, Special Publications, 427(1).
http://doi.org/10.1144/SP427.11
Bratishko, A.V. 2011. Otoliths and teeth of teleosts of Paleogene of Ukraine. The dissertation thesis for obtaining a degree of Candidate of Geological Sciences. Institute of Geological Sciences of the National Academy of Sciences of Ukraine, Kyiv. (In Ukrainian)
Bratishko, A.V. 2013. Fish otolith associations the Paleogene of Ukraine. Collection of scientific works of the Institute of Geological Sciences NAS of Ukraine, 6(1):123-127. (In Russian)
Buckland, W. 1838. On the discovery of fossil fishes in the Bagshot Sands at Goldworth Hill, 4 miles north of Guildford. Proceedings of the Geological Society of London, 2 (58):687-688.
Bugrova, E.M. (ed.). 2005. Guidebook of microfauna. Vol. 8. Cenozoic Foraminifera. VSEGEI, Saint Petersburg. (In Russian)
Cappetta, H. 1976. Sélaciens nouveaux du London Clay de l’Essex (Yprésien du Bassin de Londres). Geobios, 9:551-575.
Cappetta, H. 1980. Modification du statut générique de quelques espèces de sélaciens crétacés et tertiaires. Palaeovertebrata, 10 (1):29-42.
Cappetta, H. 1987. Chondrichthyes II. Mesozoic and Cenozoic Elasmobranchii, p. 1-193. In Schultze, H.-P. and Kuhn, O. (eds.), Handbook of Paleoichthyology 3B. Gustav Fischer Verlag, Stuttgart, New York.
Cappetta, H. 2006. Elasmobranchii Post-Triadici (Index specierum et generum). Backhuys Publishers, Leiden, The Netherlands.
Cappetta, H. 2012. Chondrichthyes (Mesozoic and Cenozoic Elasmobranchii: Teeth), p. 1-512. In Schultze, H.-P. (ed.), Handbook of Paleoichthyology 3E. Verlag Dr. Friedrich Pfeil, München, Germany.
Cappetta, H. and Nolf, D. 2005. Révision de quelques Odontaspididae (Neoselachii: Lamniformes) du Paléocène et de l'Eocène du Bassin de la Mer du Nord. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Science de la Terre, 75:237-266.
Cappetta, H. and Case, G.R. 2016. A Selachian Fauna from the Middle Eocene (Lutetian, Lisbon Formation) of Andalusia, Covington County, Alabama, USA. Palaeontographica Abteilung A, 307(1-6):43-103.
https://doi.org/10.1127/pala/307/2016/43
Carlsen, A.W. and Cuny, G. 2014. A study of the sharks and rays from the Lillebælt Clay (Early-Middle Eocene) of Denmark, and their palaeoecology. Bulletin of the Geological Society of Denmark, 62:39-88.
https://doi.org/10.37570/bgsd-2014-62-04
Case, G.R., Udovichenko, N.I., Nessov, L.A., Averianov, A.O., and Borodin, P.D. 1996. A Middle Eocene selachian fauna from the White Mountain Formation of the Kizylkum Desert, Uzbekistan, C.I.S. Palaeontographica A, 242:99-126.
Case, G.R. 1981. Late Eocene selachians from south Central Georgia. Palaeontographica A, 176:52-79.
Case, G.R. and Herman, J. 1973. A dorsal fin spine of the chimeroid fish, Edaphodon cf. bucklandi (Agassiz) from the Eocene of Morocco. Bulletin de la Société Belge de Géologie, de Paleontologie et d'Hydrologie, 82(3):445-449.
Casier, E. 1943. Contributions à l'étude des Poissons fossiles de la Belgique. IV. Observations sur la faune ichtyologique du Landénien. Bulletin du Musée Royal d'Histoire Naturelle de Belgique, 19(36):1-16.
Casier, E. 1946. La faune ichthyologique de l'Yprésien de la Belgique. Mémoires du Musée Royal d'Histoire Naturelle de Belgique, 104:1-267.
Casier, E. 1950. Contributions à l'étude des poissons fossiles de la Belgique. IX. La faune des formations dites “paniséliennes”. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, 26(42):1-52.
Casier, E. 1966. Faune ichthyologique du London Clay. Trustees of the British Museum, Londres.
Cicimurri, D.J. and Ebersole, J.A. 2015. Paleocene chimaeroid fishes (Chondrichthyes: Holocephali) from the eastern United States, including two new species of Callorhinchus. PaleoBios, 32:1-29.
https://doi.org/10.5070/P9321028055
Cicimurri, D.J., Ebersole, J.A., and Martin, G. 2020. Two new species of Mennerotodus Zhelezko, 1994 (Chondrichthyes: Lamniformes: Odontaspididae), from the Paleogene of the southeastern United States. Fossil Record, 23(2):117-140.
https://doi.org/10.5194/fr-23-117-2020
Ciobanu, R. 2002. Selacienii Paleogeni din România. Editura Universităţii Lucian Blaga, Sibiu.
Clayton, A.A., Ciampaglio, C.N.. and Cicimurri, D.J. 2013. An inquiry into the stratigraphic occurrence of a Claibornian (Eocene) vertebrate fauna from Covington County, Alabama. Bulletin of the Alabama Museum of Natural History, 31:60-73.
Compagno, L.J.V. 1970. Systematics of the genus Hemitriakis (Selachii: Carcharinidae), and related genera. Proceedings of the California Academy of Sciences, 38(4):63-98.
Compagno, L.J.V. 1973. Interrelationships of living elasmobranchs. Zoological Journal of the Linnean Society, 53 (Supplement 1):15-61.
Compagno, L.J.V. 1984. FAO species catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1. Hexanchiformes to Lamniformes. FAO Fisheries Synopsis, Rome.
Cook, J.J. and Ramsdell R.C. 1991. Macrofossils from the Vincentown Formation (Paleocene) of New Jersey. Bulletin of the New Jersey Academy of Science, 36(1):11-15.
Cunningham, S.B. 2000. A comparison of isolated teeth of early Eocene Striatolamia macrota (Chondrichthyes, Lamniformes), with those of a Recent sand shark, Carcharias taurus. Tertiary Research, 20(1-4):17-31.
Danilov, I.G., Zvonok, E.A., Syromyatnikova, E.V., and Udovichenko, N. I. 2011. A new species of soft-shelled turtle (Trionychidae) from the Middle Eocene of Ukraine. Proceedings of the Zoological Institute of the Russian Academy of Sciences, 315(4):399-411.
Dartevelle, E. and Casier, E. 1943. Les Poissons Fossiles du Bas-Congo et des régions (Première Partie). Annales du Musée Royal du Congo Belge, 3(2):1-200.
Davydenko, S., Shevchenko, T., Ryabokon, T., Tretiakov, R., and Gol’din, P. 2021. A giant Eocene whale from Ukraine uncovers early cetacean adaptations to the fully aquatic life. Evolutionary Biology, 48:67-80.
https://doi.org/10.1007/s11692-020-09524-8
de Blainville, H.M.D. 1816. Prodrome d'une nouvelle distribution systématique du règne animal. Bulletin des Sciences, par la Société Philomatique de Paris, 8:105-124.
de Blainville, H.M.D. 1818. Sur les ichthyolites ou les poissons fossiles. Nouveau Dictionnaire d'Histoire Naturelle, 27:310-391.
de Buen, F. 1926. Catalogo ictiologico del Mediterraneo Español y de Marruecos, recopilando lo publicado sobrepeces de las costas mediterraneas y proximas del Atlantico (Mar de España). Resultados de las ampafias Realizadas por Acuerdos Internacionales. Instituto Español de Oceanografia, 2:1-221.
D’Hondt, S. 2005. Consequences of the Cretaceous/Paleogene mass extinction for marine ecosystems. Annual Review of Ecology, Evolution, and Systematics, 36:295-317.
https://doi.org/10.1146/annurev.ecolsys.35.021103.105715
Diedrich, C.G. 2012. Eocene (Lutetian) Shark-Rich Coastal Paleoenvironments of the Southern North Sea Basin in Europe: Biodiversity of the Marine Fürstenau Formation Including Early White and Megatooth Sharks. International Journal of Oceanography, 2012:1-22.
https://doi.org/10.1155/2012/565326
Dixon, F. 1850. The Geology and Fossils of the Tertiary and Cretaceous Formations of Sussex. Longman, Brown, Green, and Longmans, London.
Dobbels, L. 1994. Soortenlijst van fossiele vissen uit het Eoceen van België. Afzettingen WTKG, 15(4):6-12.
https://doi.org/10.1080/
https://doi.org/10.15407/
Dutheil, D.B. 1991. A Cheklist of Neoselachii (Pisces, Chondrichthyes) from the Paleogene of the Paris Basin (France). Tertiary Research, 13:27-36.
Dutheil, D.B., Moreau, F., and Delhaye-Prat, V. 2002. Cycle sédimentaire et vertébrés d’une formation peu connue du Bassin de Paris, l’unité des Sables de Bourguillemont (Oise, France) (Paléocène supérieur). Geodiversitas, 24(4):753-764.
Dutheil, D.B., Moreau, F. and De Plöeg, G. 2006. Les ichthyofaunes du gisement à ambre de Le Quesnoy (Paléocène et Éocène du bassin de Paris, France). Cossmanniana, 11(1-4):1-13.
Dykan, N., Kovalchuk, O., Dykan, K., Gurov, E., Dašková, J., and Přikryl, T. 2018. New data on Paleocene-Eocene fauna (gastropods, ostracods, fishes) and palynoflora of the Boltysh impact structure (Ukraine) with biostatigraphical and paleoecological inferences. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 287(2):213-239.
https://doi.org/10.1127/njgpa/2018/0714
Eastman, C.R. 1901. Pisces (of Eocene of Maryland). Maryland Geological Survey, 1:98-115.
Ebersole, J.A., Cicimurri, D.J., and Stringer, G.L. 2019. Taxonomy and biostratigraphy of the elasmobranchs and bony fishes (Chondrichthyes and Osteichthyes) of the lower-to-middle Eocene (Ypresian to Bartonian) Claiborne Group in Alabama, USA, including an analysis of otoliths. European Journal of Taxonomy, 585:1-274.
https://doi.org/10.5852/ejt.2019.585
Eeckhaut, G. and De Schutter, P. 2009. The elasmobranch fauna of the Lede Sand Formation at Oosterzele (Lutetian, Middle Eocene of Belgium). Palaeofocus, 1:1-57.
Ehret, D.J. and Ebersole, J. 2014. Occurrence of the megatoothed sharks (Lamniformes: Otodontidae) in Alabama, USA. PeerJ, 2:e625.
https://doi.org/10.7717/peerj.625
Garman, S. 1901. Genera and families of the chimaeroids. Proceedings of the New England Zoological Club, 2:75-77.
Giebel, C.G. 1848. Die Fische der Vorwelt, mit steter Berücksichtigung der lebenden Fische. Erster Band: Wirbelthiere. Dritte Abtheilung: Fische. Brockhaus, Leipzig.
Glickman, L.S. 1964. Akuly paleogena i ikh stratigraficheskoye znacheniye [Sharks of the Palaeogene and their stratigraphic significance]. Nauka, Moscow. (In Russian)
Glikman, L.S. and Zhelezko, V.I. 1985. Paleogene sharks of the Mangyshlak Peninsula and the Eocene/Oligocene boundary. Byulleten’ Moskovskogo obshchestva ispytateley prirody. Otdel geologicheskiy, 60(5):86-99. (In Russian)
Gol’din, P. and Zvonok, E. 2013. Basilotritus uheni, a new cetacean (Cetacea, Basilosauridae) from the late Middle Eocene of Eastern Europe. Journal of Palaeontology, 87:254-268.
https://doi.org/10.1666/12-080R.1
Gol’din, P., Zvonok, E., and Krakhmalnaya, T. 2012. New materials of “Eocetus” sp. (Mammalia: Cetacea) from the Eocene of Ukraine. Geolog Ukrainy, 39(3):104-113. (In Russian)
Gol’din, P., Zvonok, E., Rekovets, L., Kovalchuk, A., and Krakhmalnaya, T. 2014. Basilotritus (Cetacea: Pelagiceti) from the Eocene of Nagornoe (Ukraine): new data on anatomy, ontogeny and feeding of early basilosaurids. Comptes Rendus Palevol, 13:267-276.
http://doi.org/10.1016/j.crpv.2013.11.002
Gray, J.E. 1851. List of the specimens of fish in the collection of the British Museum. Part I. Chondropterygii. British Museum (Natural History), London, Great Britain.
Guinot, G. and Cavin, L. 2016. ‘Fish’ (Actinopterygii and Elasmobranchii) diversification patterns through deep time. Biological Reviews, 91(4):950-981.
https://doi.org/10.1111/brv.12203
Guinot, G. and Condamine, F.L. 2023. Global impact and selectivity of the Cretaceous-Paleogene mass extinction among sharks, skates, and rays. Science, 379:802.
https://doi.org/10.1126/science.abn.2080
Hessler, A.M., Zhang, J., Covault, J., and Ambrose, W. 2017. Continental weathering coupled to Paleogene climate changes in North America. Geology, 45(10):911-914.
https://doi.org/10.1130/G39245.1
Hovestadt, D.C. and Hovestadt-Euler, M. 2013. Generic assessment and reallocation of Cenozoic myliobatins based on new information of tooth, tooth plate and caudal spine morphology of extant taxa. Paleontos, 20:1-65.
Huxley, T.H. 1880. A Manual of the Anatomy of Vertebrated Animals. D. Appleton & Company, New York, USA.
Ivanik, M.M., Plotnikova, L.F., Leshchukh, R.I., Zhabina, N.M., Shevchuk, O.A., Anikeyeva, O.V., Prykhodko, M.G., Veklych, O.D., Tuziak, Ya.M., Yakushin, L.M., and Klymenko, Yu.V. 2013. Cretaceous system, p. 498-619. In Gozhyk, P.F. (ed.), Stratigraphy of Upper Proterozoic and Phanerozoic of Ukraine, in two volumes. Vol. 1. Stratigraphy of Upper Proterozoic, Paleozoic and Mesozoic of Ukraine. Logos, Kyiv. (In Ukrainian)
Jaekel, O. 1895. Unter-tertiäre Selachier aus Südrussland. Mémoirs du Comité géologique de St. Petersburg, 9(4):19-35.
Jenkyns, H.C. 2003. Evidence for rapid climate change in the Mesozoic-Palaeogene greenhouse world. Philosophical Transactions of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences, 361:1885-1916.
https://doi.org/10.1098/rsta.2003.1240
Jordan, D.S. 1898. Description of a species of fish (Mitsukurina owstoni) from Japan, the type of a distinct family of lamnoid sharks. Proceedings of the California Academy of Sciences, (Series 3), 1:199-202.
Jordan, D.S. and Evermann, B.W. 1896. The fishes of North and Middle America: a descriptive catalogue of the species of fish-like vertebrates found in the waters of North America, north of the Isthmus of Panama. Part I. Bulletin of the United States National Museum, 47:1-1240.
Kemp, N.R. 1978. Detailed comparisons of the dentitions of extant hexanchid sharks and Tertiary hexanchid teeth from South Australia and Victoria, Australia (Selachii: Hexanchidae). Memoirs of the Museum Victoria, 39:61-83.
Kovalchuk, O.M. and Anfimova, G.V. 2020. Lepisosteiform fish (Holostei) ganoid scales from the Middle Jurassic deposits of Ukraine. Zoodiversity, 54(1):35-42.
https://doi.org/10.15407/zoo2020.01.035
Kovalchuk, O., Barkaszi, Z., and Anfimova, G. 2022. Records of Enchodus (Teleostei, Aulopiformes) from the Cenomanian of Ukraine in the light of European distribution of enchodontid fishes. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 303(3):295-307.
https://doi.org/10.1127/njgpa/2022/1049
Kraeva, Ye.Ya., Mulina, A.M., and Pasternak, S.I. 1960. The Cretaceous period, late epoch, Cenomanian age, p. 49. In Bondarchuk, V.G. (ed.), Atlas of Palaeogeographic Maps of the Ukrainian and Moldavian SSRs. Academy of Sciences of the Ukrainian SSR, Kyiv. (In Ukrainian)
Kriwet, J. and Benton, M.J. 2004. Neoselachian (Chondrichthyes, Elasmobranchii) diversity across the Cretaceous-Tertiary boundary. Palaeogeography, Palaeoclimatology, Palaeoecology, 214(3):181-194.
https://doi.org/10.1016/j.palaeo.2004.02.049
Kyselevych, L.S. and Kovalchuk, O.M. 2021. Biostratigraphy and conditions of formation of Albian and Cenomanian deposits on the south-western slope of the Ukrainian Shield (Middle Dniester region). Geo&Bio, 21:95-114.
https://doi.org/10.15407/gb2110
Kuzmin, I.T. and Zvonok, E.A. 2021. Crocodylian assemblage from the middle Eocene Ikovo locality (Lugansk Province, Ukraine), with a discussion of the fossil record and geographic origins of crocodyliform fauna in the Paleogene of Europe. Geobios, 65:7-27.
https://doi.org/10.1016/j.geobios.2021.02.002
Leriche, M. 1902. Les poissons paléocènes de la Belgique. Mémoires du Musée Royal d'Histoire Naturelle de Belgique, 2(5):1-48.
Leriche, M. 1905. Les poissons éocènes de la Belgique. Mémoires du Musée Royal d'Histoire Naturelle de Belgique, 3(11):49-228.
Leriche, M. 1906. Contribution à lètude des poisons fossils du Nord de la France et des regions voisines. Mémoires de la Société géologique du Nord, 5:1-430.
Leriche, M. 1942. Contribution à l’étude des faunes ichthyologiques marines des terrains tertiaires de la Plaine côtière atlantique et du centre des Etats-Unis. Les synchronismes des formations tertiaires des deux côtés de l’Atlantique. Mémoires de la Société géologique de France, 45(2-4):1-110.
MacFadden, B.J., Labs-Hochstein, J., Quitmyer, I., and Jones, D.S. 2004. Incremental growth and diagenesis of skeletal parts of the lamnoid shark Otodus obliquus from the early Eocene (Ypresian) of Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology, 206:179-192.
https://doi.org/10.1016/j.palaeo.2004.01.002
Maisch IV, H.M., Becker, M.A., and Chamberlain Jr., J.A. 2015. Chondrichthyans from a lag deposit between the Shark River Formation (middle Eocene) and Kirkwood Formation (early Miocene), Monmouth County, New Jersey. Paludicola, 10(3):149-183.
Malyshkina, T.P. 2006. Elasmobranchs of the western margin of the West Siberian Paleogene basin. Institute of Geology and Geochemistry, Ural Branch of the Russian Academy of Sciences, Ekaterinburg. (In Russian)
Malyshkina, T.P. 2021. Striatolamia tchelkarnurensis Glickman (Elasmobranchii: Lamniformes), the Youngest Valid Striatolamia Species. Paleontological Journal, 55:193-204.
https://doi.org/10.1134/S0031030121020088
Malyshkina, T.P., González-Barba, G., and Bannikov, A.F. 2013. Records of Elasmobranchian Teeth in the Bartonian of the Northern Caucasus (Russia) and Crimea (Ukraine). Paleontological Journal, 47(1):98-103.
Malyshkina, T.P. and Ward, D.J. 2016. The Turanian Basin in the Eocene: the new data on the fossil sharks and rays from the Kyzylkum Desert (Uzbekistan). Proceedings of the Zoological Institute, Russian Academy of Sciences, 320(1):50-65.
Marramà, G., Carnevale, G., Engelbrecht, A., Claeson, K.M., and Zorzin, R. 2018. A synoptic review of the Eocene (Ypresian) cartilaginous fishes (Chondrichthyes: Holocephali, Elasmobranchii) of the Bolca Konservat-Lagerstätte, Italy. Paläontologische Zeitschrift, 92 (2):283-313.
https://doi.org/10.1007/s12542-017-0387-z
Mayr, G. and Zvonok, E. 2011. Middle Eocene Pelagornithidae and Gaviiformes (Aves) from the Ukrainian Paratethys. Palaeontology, 54:1347-1359.
https://doi.org/10.1111/j.1475-4983.2011.01109.x
Mayr, G. and Zvonok, E. 2012. A new genus and species of Pelagornithidae with well-preserved pseudodentition and further avian remains from the Middle Eocene of the Ukraine. Journal of Vertebrate Paleontology, 32:914-925.
https://doi.org/10.1080/02724634.2012.676114
Menner, V.V. 1928. Les sélaciens du Paléogène de Manghyschlak, d’Emba et du versant oriental d’Oural. Bulletin de la Société des Naturalistes de Moscou, Section Géologique, 6(3-4):292-338. (In Russian, with French summary)
Miller, K.G., Wright, J.D., Katz, M.E., Wade, B.S., Browning, J.V., Cramer, B.S., and Rosenthal, Y. 2009. Climate threshold at the Eocene-Oligocene transition: Antarctic ice sheet influence on ocean circulation, p. 169-178. In Koeberl, C. and Montanari, A. (eds.), The Late Eocene Earth: Hothouse, Icehouse, and Impacts. Volume 452. Geological Society of America Special Paper, USA.
https://doi.org/10.1130/2009.2452(11)
Musatov, V.A. 2020. Lutetian or Bartonian? Age of the Sergeevskaya Formation on nannoplankton in the Kantemirovka section of the Voronezh anteclise and correlation with the near regions. Volga and Precaspian regional resources, 101:4-26. (In Russian)
https://doi.org/10.24411/1997-8316-2020-11011
Musatov, V.A. and Ryabokon, T.S. 2017. Distribution of nannofossils and foraminifera in the key section of the Kyiv Formation the Khalepya village (the Kyiv Pridneprovya, Ukraine), p. 117-119. In Gozhyk, P.F. (ed.), 40 years of the Paleontological society of Ukraine: Materials of the 38th session of the Palaeontological society NAS of Ukraine (Kaniv, May 23-26, 2017). Kyiv. (In Russian)
Mustafa, H.A. and Zalmout, I.S. 2002. Elasmobranchs from the late Eocene Wadi Esh-Shallala Formation of Qa’Faydat and Dahikiya, east Jordan. Tertiary Research, 21(1-4):77-94.
Mustafa, H.A., Zalmout, I.S., Smadi, A.A., and Nazzal, J. 2005. Review of the Middle Eocene (Lutetian) selachian fauna of Jebal eth Thuleithuwat, east Jordan. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 237(3):399-422.
Müller, J. and Henle, F.G.J. 1838. Ueber die Gattungen der Plagiostomen. Archiv für Naturgeschichte, 4:83-85.
Natanson, L.J., Skomal, G.B., Hoffmann, S.L., Porter, M.E., Goldman, K.J., and Serra, D. 2018. Age and growth of sharks: do vertebral band pairs record age? Marine and Freshwater Research, 69(9):1440-1452.
https://doi.org/10.1071/MF17279
Newbrey, M.B., Siversson, M., Cook, T.D., Fotheringham, A.M., and Sanchez, R.L. 2015. Vertebral morphology, dentition, age, growth, and ecology of the large lamniform shark Cardabiodon ricki. Acta Palaeontologica Polonica, 60(4):877-897.
https://doi.org/10.4202/app.2012.0047
Nolf, D. 1988. Fossiles de Belgique. Dents de Requins et de Raies du Tertiaire de la Belgique. Institut royal des Sciences naturelles de Belgique, Bruxelles.
Obruchev, D.V. 1953. Izuchenie edestid i raboty A.P. Karpinskogo [Studies on edestids and the works of A.P. Karpinski]. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 45:1-86. (In Russian)
Ogg, J.G., Ogg, G.M., and Gradstein, F.M. 2016. 14-Paleogene. A Concise Geologic Time Scale, 2016:187-201.
https://doi.org/10.1016/B978-0-444-59467-9.00014-5
Otero, R.O., Oyarzún, J.L., Soto-Acuña, S., Yury-Yáñez, R.E., Gutierrez, N.M., Le Roux, J.P., Torres, T., and Hervé, F. 2013. Neoselachians and Chimaeriformes (Chondrichthyes) from the latest Cretaceous-Paleogene of Sierra Baguales, southernmost Chile. Chronostratigraphic, paleobiogeographic and paleoenvironmental implications. Journal of South American Earth Sciences, 48:13-30.
https://doi.org/10.1016/j.jsames.2013.07.013
Parmley, D. and Cicimurri, D.J. 2005. First Record of a Chimaeroid Fish from the Eocene of the Southeastern United States. Journal of Paleontology, 79(6):1219-1221.
https://doi.org/10.1666/0022-3360(2005)079[1219:FROACF]2.0.CO;2
Patterson, C. 1993. Osteichthyes: Teleostei, p. 621-656. In Benton, M.J. (ed.), The Fossil Record. Vol. 2. Springer, Germany.
Perez, V.J. 2022. The chondrichthyan fossil record of the Florida Platform (Eocene-Pleistocene). Paleobiology, 48(4):622-654.
https://doi.org/10.1017/pab.2021.47
Pledge, N.S. 1967. Fossil Elasmobranch teeth of South Australia and their stratigraphic distribution. Transactions of the Royal Society of South Australia, 91:135-160.
Pollerspöck, J. and Straube, N. 2022. www.shark-references.com, World Wide Web electronic publication, version 2022.
Priem, M.F. 1901. Sur les poissons de l’Eocène inférieur des environs de Reims. Bulletin de Société géologique de France (Serie 4), 1:477-504.
Priem, M.F. 1908. Etude des poissons fossiles du Bassin Parisien. Annales de Paléontologie:1-144.
Prothero, D.R. 2021. Paleogene, p. 62-68. In Alderton, D. and Eias, S.A. (eds.), Encyclopedia of Geology (Second Edition). Academic Press.
Rafinesque, C.S. 1810. Indice d'ittiologia siciliana ossia catalogo metodico dei nomi latini, italiani, e siciliani, e siciliani dei pesci, che si rinvengono in Sicilia disposti secondo un metodo naturale eseguito da un appendice che contiene la descrizione di alcuni nuovi pesci siciliani. Ill Opuscolo del signore C.S. Rafinesque Schmaltz. Giovanni del Nobolo, Messina.
Rayner, R., Mitchell, T., Rayner, M., and Clouter, F. 2009. London Clay fossils of Kent and Essex. Medway Fossil and Mineral Society, Rochester, Kent, UK.
Ridewood, W.G. 1921. On the calcification of the vertebral centra in sharks and rays. Philosophical Transactions of Royal Society of London, 210:311-407.
Rögl, F. 1998. Palaeogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene). Annalen des Naturhistorischen Museums in Wien, Serie A, 99:279-310.
Rogovich, A.S. 1861. On fossil fishes of provinces of the Kyiv educational district. Trudy komissii, vysochayshe utverzhdennoy pri Imperatorskom universitete Sv. Vladimira, dlya opisaniya guberniy Kievskogo uchebnogo okruga [Proceedings of the Commission, highly approved at the Imperial University of St. Vladimir, to describe the provinces of the Kyiv educational district], Natural history, 4(1):1-87. (In Russian)
Rogovich, A.S. 1870. Fossil fishes of the Kyiv Tertiary basin and adjacent formations, p. 19-54. In Tshurovskii, G.E. (ed.), Transactions of the Second congress of Russian naturalists on the Section of mineralogy, geology, and paleontology. Moscow. (In Russian)
Rogovich, A.S. 1875a. Note on the localities of fossil mammal animal bones in the southwestern Russia. Zapiski Kievskogo obshchestva estestvoispytateley, 4(3):33-45. (In Russian)
Rogovich, A.S. 1875b. Investigation of the brown coal formation in the Kyiv Province. Zapiski Kievskogo obshchestva estestvoispytateley, 4(3):46-49. (In Russian)
Ronov, A.V. and Khain, V.E. (eds.). 1961. Atlas of the lithological and paleogeographical maps of the Russian platform and its geocynclinal framing. Part II: Mesozoic and Cenozoic. Scale 1:5 000 000. Moscow, Leningrad. (In Russian)
Ryabokon, T.S. 2002. Biostratigraphy of Kyiv Suite type section (Middle Eocene) of Dnieper-Donets depression by data of studying foraminifera. Geology and Mineralogy Bulletin of Kryvyi Rih National University, 2(8):39-50. (In Russian)
Ryabokon, T.S. 2021. Position of the Paleogene stages boundaries in the sedimentary section of platform Ukraine: current stage, determination criteria. Collection of scientific works of the Institute of Geological sciences NAS of Ukraine, 14:72-99. (In Ukrainian)
https://doi.org/10.30836/igs.2522-9753.2021.228226
Ryabokon, T.S. and Shevchenko, T.V. 2001. Organic-walled microphytoplankton and foraminifera of the Kyiv Formation in the central region of the Ukrainian Shield, p. 34-41. In Gozhyk, P.F. (ed.), Aspects of geological science at the turn of the thousand: materials of the youth scientific conference. Institute of Geological Sciences of the National Academy of Sciences of Ukraine, Kyiv. (In Russian)
Savtchenko, A.S. 1912. Elasmobranchii de l’eocene de Manghuichlak. Mémoires de la Société des Naturalistes de Kieff, 22(2):149-186. (In Russian, with French summary)
Savytska, N.A. 1996. Nanoplankton and dinocysts of the middle-upper Eocene deposits of platform Ukraine (biostratigraphy and paleoecology). The dissertation thesis for obtaining a degree of Candidate of Geological Sciences. Institute of Geological Sciences of the National Academy of Sciences of Ukraine, Kyiv. (In Ukrainian)
Schultz, O. 2013. Pisces, p. 1-576. In Piller, W. (ed.), Catalogus Fossilium Austriae, 3. Verlag der Österreichischen Akademie der Wissenschaften, Wien.
Shevchenko, T.V. 2000. Changes in the composition of dinocysts at the turn of the middle and late Eocene of Northern Ukraine. Geological journal, 1:87-92. (In Russian)
Shevchenko, T.V. 2002. Microphytofossils (dinocysts) of the Late Paleogene of the Ukrainian Shield and their stratigraphic significance. The dissertation thesis for obtaining a degree of Candidate of Geological and Mineralogical Sciences. Institute of Geological Sciences of the National Academy of Sciences of Ukraine, Kyiv. (In Russian)
Sibert, E.C. and Norris, R.D. 2015. New age of fishes initiated by the Cretaceous−Paleogene mass extinction. Proceedings of the National Academy of Sciences, 112(28):8537-8542.
https://doi.org/10.1073/pnas.1504985112
Sluijs, A., Van Roij, L., Harrington, G.J., Schouten, S., Sessa, J.A., LeVay, L.J., Reichart, G.-J., and Slomp, C.P. 2014. Warming, euxinia and sea level rise during the Paleocene-Eocene Thermal Maximum on the Gulf Coastal Plain: implications for ocean oxygenation and nutrient cycling. Climate of the Past, 10(4):1421-1439.
https://doi.org/10.5194/cp-10-1421-2014
Snetkov, P.B. and Bannikov, A.F. 2010. Vertebrae of sea snakes from the Eocene of the Crimea. Palaeontological Journal, 44(6):698-700.
https://doi.org/10.1134/S0031030110060122
Sokolov, I.P. 1986. Biostratigraphy and conditions for the formation of Eocene deposits in the Middle Dnieper region. Dissertation for the degree of Candidate of Geological and Mineralogical Sciences [Manuscript]. Kyiv. (In Russian)
Solyanik E.A. 2009. Towards the recognition of standard nannozones in the Middle Eocene of platform Ukraine. p.243-254. In Gozhyk, P.F. (ed.), Fossil flora and fauna of Ukraine: paleoecological and stratigraphic aspects. Proceedings of the Institute of Geological Sciences of the NAS of Ukraine, Kyiv. (In Russian)
Speijer, R.P., Pälike, H., Hollis, C.J., Hooker, J.J., and Ogg, J.G. 2020. The Paleogene Period, p. 1087-1140. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. (eds.), Geologic Time Scale. Vol. 2. Elsevier.
https://doi.org/10.1016/B978-0-12-824360-2.00028-0
Stahl, B.J. 1999. Chondrichthyes III: Holocephali, p. 1-164. In Schultze, H.-P. (ed.), Handbook of Paleoichthyology, vol. 4. Verlag Dr. Friedrich Pfeil, München, Germany.
Stahl, B.J. and Parris, D.C. 2004. The complete dentition of Edaphodon murificus (Chondrichthyes: Holocephali) from a single individual. Journal of Paleontology, 78(2):388-392.
https://doi.org/10.1666/0022-3360(2004)078<0388:TCDOEM>2.0.CO;2
Szabó, M. and Kocsis, L. 2016. A preliminary report on the Early Oligocene (Rupelian, Kiscellian) selachians from the Kiscell Formation (Buda Mts, Hungary), with the re-discovery of Wilhelm Weiler’s shark teeth. Fragmenta Palaeontologica Hungarica, 151(4):363-410.
https://doi.org/10.17111/FragmPalHung.2016.33.31
Szabó, M., Kocsis, L., Bosnakoff, M., and Sebe, K. 2021. A diverse Miocene fish assemblage (Chondrichthyes and Osteichthyes) from the Pécs-Danitzpuszta sand pit (Mecsek Mts, Hungary). Földtani Közlöny, 33:31-64.
https://doi.org/10.17111/FragmPalHung.2016.33.31
Trif, N., Codrea, V., and Arghiuș, V. 2019. A fish fauna from the lowermost Bartonian of the Transylvanian Basin, Romania. Palaeontologia Electronica, 22.3.56:1-29.
https://doi.org/10.23928/foldt.kozl.2021.151.4.363
Trif, N., Arghiuș, V., Seitz, J.C., Codrea, V.A., Bălc, R., and Bindiu-Haitonic, R. 2022. Integrated palaeontological investigation of a new mid-late Bartonian fish fauna from Călata area, Transylvanian Basin, Romania. Historical Biology, 34(9):1788-1816.
https://doi.org/10.1080/08912963.2021.1980879
Udovichenko, N.I. 2006. Shark teeth from the sediments of the Kievian regional stage from the Gradizhsk area, p. 201-208. In Gozhyk, P.F. (ed.), Palaeontological and biostratigraphic problems of the Proterozoic and Phanerozoic of Ukraine. Proceedings of the Institute of Geological Sciences NAS of Ukraine, Kyiv. (In Russian)
Udovichenko, N.I. 2009. Ichthyofauna and age of the Paleogene sands of Osinovo area, Luhansk region, p. 255-261. In Gozhyk, P.F. (ed.), Fossil flora and fauna of Ukraine: paleoecological and stratigraphic aspects. Proceedings of the Institute of Geological Sciences of the NAS of Ukraine, Kyiv. (In Russian)
Udovichenko, N.I. and Nessov, L.A. 1987. Comparison of chondrichthyan and other vertebrate assemblages from the Paleogene of Tashkent Chuli (Maisk) and Ukraine (Pirogovo), p. 167-174. In Vyalov, O.S. (ed.), Biostratigrafiya, paleontologiya osadochnogo chekhla Ukrainy [Biostratigraphy and paleontology of the depositional cover of Ukraine]. Naukova dumka, Kyiv. (In Russian)
Underwood, C.J., Ward, D.J., King, C., Antar, S.M., Zalmout, I.S., and Gingerich, P.D. 2011. Shark and ray faunas in the Middle and Late Eocene of the Fayum Area, Egypt. Proceedings of the Geologists’ Association, 122:47-66.
https://doi.org/10.1016/j.pgeola.2010.09.004
Vasilieva, O.N. 2018. Dinocysts and biostratigraphy of the Paleogene of the Turgai trough, Trans-Urals and Pricaspian depression. The dissertation thesis for obtaining a degree of Doctor of Geological and Mineralogical Sciences. Novosibirsk. (In Russian)
Villalobos-Segura, E. and Underwood, C.J. 2020. Radiation and divergence times of Batoidea, Journal of Vertebrate Paleontology, 40 (3).
https://doi.org/10.1080/02724634.2020.1777147
Ward, D.J. 1979. Additions to the fish fauna of the English Paleogene. 3. A review of the Hexanchid sharks with a description of four new species. Tertiary Research, 2:111-129.
Welton, B.J. and Farish, R.F. 1993. The Collector’s Guide to Fossil Sharks and Rays from the Cretaceous of Texas. Before Time, Lewisville, Texas.
Westerhold, T., Röhl, U., Donner, B., Frederichs, T., Kordesch, W.E.C., Bohaty, S.M., Hodell, D.A., Laskar, J., and Zeebe, R.E. 2018. Late Lutetian Thermal Maximum - Crossing a thermal threshold in Earth’s climate system? Geochemistry, Geophysics, Geosystems, 19:1-10.
https://doi.org/10.1002/2017GC007240
Winkler T.C. 1874. Mémoire sur des dents de poissons du terrain bruxellien. Archives du Musée Teyler, 3(4):285-304.
Winkler, T. 1876. Deuxième mémoire sur des dents de poissons fossiles du terrain bruxellien. Archives du Musée Teyler, 4(1):16-48.
Woodward, A.S. 1886. On the paleontology of the selachian genus Notidanus, Cuvier. Geological Magazine, 3:205-217, 253-259.
Woodward, A.S. 1889. Catalogue of the fossil fishes in the British Museum. Part I, containing the Elasmobranchii. British Museum (Natural History), London.
Woodward, A.S. 1891. Catalogue of the fossil fishes in the British Museum (Natural History). Part II, containing the Elasmobranchii (Acanthodii). Holocephali, Ichthyodorulites, Ostracodermi, Dipnoi, and Teleostomi (Crossopterygii and chondrostean Actinopterygii). Taylor and Francis, London.
Woodward, A.S. 1899. Catalogue of the fossil fishes in the British Museum. London.
Zachos, J.C., Dickens, G.R., and Zeebe, R.E. 2008. An early Cenozoic perspective on greenhouse warming and Carbon-cycle dynamics. Nature, 451:279-283.
https://doi.org/10.1038/nature06588
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292:686-693.
https://doi.org/10.1126/science.1059412
Zalat, A.A., Khalil, H.M., Fathy, M.S., and Tarek, R.M. 2017. Taxonomy and morphological study on the vertebrate remains of shark and rays fauna from the Middle and Late Eocene succession, Fayoum Depression, Egypt. Delta Journal of Science, 38:202-217.
Zalmout, I.S.A., Antar, M.S.M., Abd-El Shafy, E., Metwally, M.H., Hatab, E.-B.E., and Gingerich, P.D. 2012. Priabonian sharks and rays (Late Eocene: Neoselachii) from Minquar Tabaghbagh in the western Qattara Depression, Egypt. Contributions from the Museum of Paleontology, University of Michigan, 32(6):71-90.
Zhelezko, V.I. 1994. Sharks of family Jaekelotodontidae of European and middle Asian paleobiogeographic provinces. Bulletin Moscow Society of Naturalists, 69(6):47-62.
Zhelezko, V.I. and Kozlov, V.A. 1999. Elasmobranhii i biostratigraphia paleogena Zauralia i Srednei Asii [Elasmobranchii and Palaeogene biostratigraphy of Transural and Central Asia]. Ural Branch of the Russian Academy of Sciences, Ekaterinburg. (In Russian)
Zosimovich, V.Yu. and Shevchenko, T.V. 2014. Development stages of Paleogene sedimentary basins of northern Ukraine. Collection of scientific works of the Institute of Geological sciences of the National Academy of Sciences of Ukraine, 7:83-100. (In Ukrainian)
Zosimovich, V.Yu. and Shevchenko, T.V. 2015. Paleogene deposits of the northern Ukraine paleosedimentary province. Collection of scientific works of the Institute of Geological sciences of the National Academy of Sciences of Ukraine, 8:68-121. (In Ukrainian)
https://doi.org/10.30836/igs.2522-9753.2015.146712
Zouhri, S., Gingerich, P., Khalloufi, B., Bourdon, E., Adnet, S., Jouve, S., Elboudali, N., Amane, A., Rage, J.-C., Tabuce, R., and Lapparent de Broin, F. 2021. Middle Eocene vertebrate fauna from the Aridal Formation, Sabkha of Gueran, southwestern Morocco. Geodiversitas, 43(5):121-150.
https://doi.org/10.5252/geodiversitas2021v43a5
Zvonok, E. 2011. New data on the localities and taxonomic diversity of the Eocene crocodiles and turtles of Ukraine. Paleontological collection, 43:107-120. (In Ukrainian)
Zvonok, E.A. and Danilov, I.G. 2017. A revision of fossil turtles from the Kiev clays (Ukraine, middle Eocene) with comments on the history of the collection of fossil vertebrates of A.S. Rogovich. Proceedings of the Zoological Institute of the Russian Academy of Sciences, 321(4):485-516.
Zvonok, E. and Gorobets, L. 2016. A record of a landbird (Telluraves) from the Eocene Ikovo locality (East Ukraine). Acta zoologica cracoviensia, 59(1):37-45.
https://doi.org/10.3409/azc.59_1.37
Zvonok, E.A. and Snetkov, P.B. 2012. New findings of snakes of the genus Palaeophis Owen, 1841 (Acrochordoidea: Palaeophiidae) from the Middle Eocene of Crimea. Proceedings of the Zoological Institute of the Russian Academy of Sciences, 316(4):392-400.
Zvonok, E.A. and Skutschas, P.P. 2011. On a tomistomine crocodile (Crocodylidae, Tomistominae) from the Middle Eocene of Ukraine. Paleontological Journal, 45(6):661-664.
https://doi.org/10.1134/S0031030111060165
Zvonok, E.A., Danilov, I.G., and Syromyatnikova, E.V. 2013. The first reliable record of fossil leatherback sea turtle (Dermochelyidae) in Northern Eurasia (Middle Eocene of Ukraine). Paleontological Journal, 47(2):199-202.
https://doi.org/10.1134/S0031030113020160
Zvonok, E., Mayr, G., and Gorobets, L. 2015. New material of the Eocene marine bird Kievornis Averianov et al., 1990 and a reassessment of the affinities of this taxon. Vertebrata PalAsiatica, 53(3):238-244.

