 Spider crabs (Decapoda: Brachyura: Majoidea) from the upper Eocene of south Pyrenees (Huesca, Spain)
Spider crabs (Decapoda: Brachyura: Majoidea) from the upper Eocene of south Pyrenees (Huesca, Spain)
Article number: 26.2.a27
https://doi.org/10.26879/1270
Copyright Palaeontological Association, July 2023
Proceedings of the 8th Symposium on Fossil Decapod Crustaceans
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 16 January 2023. Acceptance: 7 July 2023.
ABSTRACT
Majoidea are one of the most plesiomorphic clades of Eubrachyura and display a huge diversity in modern ecosystems. We describe one new fossil genus and three new species including Eoparanaxia eocenica n. gen. n. sp., Planobranchia elongata n. sp., Spinirostrimaia echinata n. sp.; and one indeterminate species tentatively assigned to Macrocheira sp. from the Pamplona Marls Formation (upper Eocene, southern Pyrenees, Spain). All of them are new or first reported from the Iberian Peninsula. This shows a highly diversified fauna associated with prodelta clays that favoured preservation of decapods and other invertebrates. Cluster analyses based on Jaccard and Raup-Crick coefficients of Eocene Majidae suggest close affinities of Iberia with other European basins.
Fernando A. Ferratges. Departamento de Ciencias de la Tierra-IUCA, Universidad de Zaragoza, Zaragoza E-50009, Spain. fer.afk87@gmail.es
orcid.org/0000-0002-9532-6972
Josep Lluis Domínguez. Padre Manjón, 12. 50010 Zaragoza, Spain. jl.domin@hotmail.com
Àlex Ossó. Llorenç de Villalonga, 17B, 1-1 43007 Tarragona, Catalonia. aosso@tinet.cat
orcid.org/0000-0003-2528-9915
Samuel Zamora. Instituto Geológico y Minero de España (IGME-CSIC), Residencia CSIC, Campus Aula Dei, Av. Montañana 1005, 50059 Zaragoza, Spain. s.zamora@igme.es
orcid.org/0000-0002-3917-4628
Keywords: benthic; biodiversity; crustaceans; new genus; new species; marine; taxonomy; palaeoecology
Final citation: Ferratges, Fernando A., Domínguez, Josep Lluis, Ossó, Àlex, and Zamora, Samuel. 2023. Spider crabs (Decapoda: Brachyura: Majoidea) from the upper Eocene of south Pyrenees (Huesca, Spain). Palaeontologia Electronica, 26(2):a27.
https://doi.org/10.26879/1270
palaeo-electronica.org/content/2023/3901-eocene-spider-crabs-from-spain
Copyright: July 2023 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/37475BBF-A769-47C0-8A84-39C14264C2EB
INTRODUCTION
The superfamily Majoidea Samouelle, 1819, is considered as monophyletic according to molecular studies (i.e., Spears et al., 1992; Porter et al., 2005; Hultgren and Stachowicz, 2008; Tsang et al., 2014), larval development (i.e., Rice, 1980, 1983; Clark and Ng, 2004), and morphological studies (Brösing et al. 2007; Ng et al., 2008; Guinot and Wicksten, 2015). This group is generally represented by species with a characteristic morphology, including elongated and pyriform carapace and bifid front, and hooked setae (i.e., Davie et al., 2015). Majoidea comprises about 1000 species and more than 200 genera (i.e., Ng et al., 2008; De Grave et al., 2009). From a molecular point of view, this clade is considered one of the most plesiomorphic crabs within Eubrachyura de Saint Laurent, 1980 with an origin in the Jurassic (Porter et al., 2005, Crandall et al., 2009; Guinot, 2019 and references therein; Wolfe et al., 2019, 2022).
The fossil record does not match molecular estimates and the oldest considered fossil Majoidea correspond to species from the mid-Cretaceous of Europe (Breton, 2009; Klompmaker, 2013) and Mexico (Vega et al., 2019). There are more than 125 majoid species known in the fossil record (De Grave et al., 2009; Schweitzer et al., 2010), from which 47 have been found in the Eocene (see Table 1). Based on fossils some authors suggested that spider crabs diversified in the Miocene (Klompmaker et al., 2015). The aim of this paper is to describe a high diversified assemblage of spider crabs coming from a single formation in the late Eocene of the Pyrenees. This will serve as a basis to analyse the diversity of spider crabs in the Eocene and possible paleobiogeographic relationships of Iberia with other areas.
GEOLOGICAL SETTING
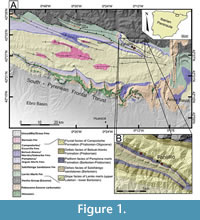 The studied material was collected from the Pamplona Marls Formation (Puigdefàbregas, 1975) in the Jaca-Pamplona Basin. This basin corresponds to an elongated basin from east to west in the south-central Pyrenean Zone (Figure 1) and was formed as a result of the southward propagation of the tectonic structures during the Paleogene (Millán et al., 1994; Muñoz et al., 1994; Castelltort et al., 2003; Huyghe et al., 2009).
The studied material was collected from the Pamplona Marls Formation (Puigdefàbregas, 1975) in the Jaca-Pamplona Basin. This basin corresponds to an elongated basin from east to west in the south-central Pyrenean Zone (Figure 1) and was formed as a result of the southward propagation of the tectonic structures during the Paleogene (Millán et al., 1994; Muñoz et al., 1994; Castelltort et al., 2003; Huyghe et al., 2009).
The propagation fold and thrust belt of the southern Pyrenean resulted in the formation of a coeval relief, acting as a sediment source area for deltaic complexes (e.g., Dreyer et al., 1999). These deltaic complexes prograded progressively westwards, covering the turbiditic systems of the lower and middle Eocene of the Hecho Group (i.e., Mutti et al., 1985; Remacha et al., 2005). These middle and upper Eocene units form a c. 2 km thick succession, in which diverse environments were developed, including shallow-marine limestones of the Guara Formation, prodelta/outer ramp marls/clays of the Pamplona Marls Formation, deltaic Belsue-Atares Formation and the fluvial Campodarbe Formation (Puigdefàbregas, 1975; Silva-Casal et al., 2019).
Decapod crustaceans studied in the present study come from lower Priabonian strata and were collected near the village of Fanlillo (Figure 1), in the municipality of Yebra de Basa (province of Huesca, Spain). This outcrop shows a great abundance and diversity of small benthic invertebrates including gastropods, bivalves, bryozoans, foraminifera, and decapod crustaceans (Artal et al., 2013; Ossó et al., 2014; Domínguez and Ossó, 2016; Ossó and Domínguez, 2017; Ossó et al., 2020; Ferratges et al., 2023).
MATERIALS AND METHODS
The data presented here is based on the analysis of fossil specimens collected from the outcrop exposed in the road cut N-260 near the village of Fanlillo (42º28'30"N, 0º13'35"W, see Figure 1). Additionally, two specimens are included from a laterally equivalent outcrop (42º29'02"N, 0º15'13"W, see Figure 1), located west of Fanlillo (MPZ 2023/3 and MPZ 2023/4). The studied material comprises 22 specimens, represented by isolated carapaces and one specimen with one cheliped and partial thoracic sternum preserved. These specimens belong to four genera, represented by four species, from which three are new. Specimens are preserved in marls and are extremely delicate; this prevents the use of mechanical tools. For this reason, material was prepared manually with a needle, under a binocular magnifying microscope and, eventually with the help of a wet brush. During the process it was necessary to apply a 15 percent solution of Paraloid B-72 and acetone.
The specimens were then photographed dry and coated with ammonium chloride sublimated to enhance anatomical details and ornamentation of the cuticle. Detailed photography of the carapace surfaces was made using a Nikon d7100 camera (Nikon, Tokyo, Japan) with a 60 mm macro lens. Specimens were legally sampled under permit EXP: 032/2018 from the Servicio de Prevención, Protección e Investigación del Patrimonio Cultural (Gobierno de Aragón) and are deposited in the palaeontological collection of the Museo de Ciencias Naturales de la Universidad de Zaragoza under the acronym MPZ.
To analyse the degree of similarity between the different areas that have provided Eocene spider crabs (Majoidea), the Jaccard and Raup-Crick Coefficients have been analysed (following Cascales-Miñana, 2010). This probabilistic statistic shows the confidence level associated with a one-sided randomization test for each pair of time units (Maridet et al., 2007). For detailed absolute ages and information about the time units see Table 2 (absolute ages have been taken from Cohen et al., 2013). The presence/absence of each genus in each area/period is summarized in Table 3. The presence and absence of the different genera is summarized in Table 4 and Table 5.
Time units showing a significant similarity of taxonomic composition are characterized by a high Raup-Crick index (i.e., RC > 0.95); on the contrary, a low Raup-Crick index value (i.e., RC < 0.05) suggests a significant difference between taxonomic composition, which can be interpreted as a measure of robustness (see Cascales-Miñana, 2010, and references).
Similarity coefficients were clustered using the UPGMA algorithm, because this method shows the best cophenetic correlation values regardless of the similarity measures used (Table 6). The results are illustrated using dendrograms. The grouping was stratigraphically restricted (Ypresian-Lutetian and Bartonian-Priabonian). Although this might impose a default pattern on the output, bootstrapping reveals that a stratigraphically constrained cluster analysis provides a stronger reflection of the data structure than an unrestricted cluster. Analyses were performed using the PAST software package (Hammer et al., 2001).
SYSTEMATIC PALAEONTOLOGY
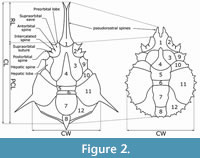 Relationships between families of Majoidea are strongly discussed in recent years (see Guinot and Van Bakel, 2020). Higher ranks systematic placement follows Guinot (2019), Guinot and Van Bakel (2020), and Guinot et al. (2022). Morphological terminology of Majidae follows Griffin, (1966), Griffin and Tranter (1986), and Davie et al. (2015). Carapace measurements are given as carapace width (CW), carapace length (CL), and postrostral carapace length (PCL) in millimetres. The carapace width was measured in the widest part of the branchial region, carapace length was measured from the rostral apices to the posterior margin of the carapace, and postrostral carapace length was measured from the base of the rostral spines to the posterior margin of the carapace (Figure 2).
Relationships between families of Majoidea are strongly discussed in recent years (see Guinot and Van Bakel, 2020). Higher ranks systematic placement follows Guinot (2019), Guinot and Van Bakel (2020), and Guinot et al. (2022). Morphological terminology of Majidae follows Griffin, (1966), Griffin and Tranter (1986), and Davie et al. (2015). Carapace measurements are given as carapace width (CW), carapace length (CL), and postrostral carapace length (PCL) in millimetres. The carapace width was measured in the widest part of the branchial region, carapace length was measured from the rostral apices to the posterior margin of the carapace, and postrostral carapace length was measured from the base of the rostral spines to the posterior margin of the carapace (Figure 2).
Superfamily MAJOIDEA Samouelle, 1819
Family EPIALTIDAE MacLeay, 1838
Subfamily PISINAE Dana, 1851
Genus EOPARANAXIA n. gen.
Figure 3
zoobank.org/07B9F785-4E4A-4018-9D7B-50B3576421F4
Type species. Eoparanaxia eocenica n. gen. n. sp. by monotypy and present designation. Gender feminine.
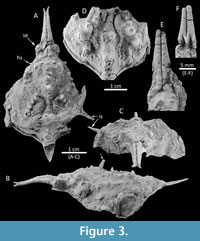 Diagnosis. Carapace pyriform, longer than wide; rostrum axially sulcate, with two fused, long pseudorostral spines, length 0.53 times CW. Hepatic region with small spine, directed anterolaterally. Intestinal tubercle strong, protruding beyond posterior carapace margin; carapace regions well defined with metagastric and urogastric regions narrower than mesogastric and cardiac regions; mesobranchial region with posteriorly directed large spine. Dorsal regions armed with long spines.
Diagnosis. Carapace pyriform, longer than wide; rostrum axially sulcate, with two fused, long pseudorostral spines, length 0.53 times CW. Hepatic region with small spine, directed anterolaterally. Intestinal tubercle strong, protruding beyond posterior carapace margin; carapace regions well defined with metagastric and urogastric regions narrower than mesogastric and cardiac regions; mesobranchial region with posteriorly directed large spine. Dorsal regions armed with long spines.
Etymology. The generic name derives from the prefix Eo- (from ɳꞷς (gr.)= aurora), to generically indicate an ancestral form, in arbitrary combination with the generic name Paranaxia Rathbun, 1924, to refer to its morphological affinities with the new genus.
Remarks. The material herein described is referred to the subfamily Pisinae Dana, 1851. Species of this subfamily possess elongated carapaces; elongated pseudorostral spines; orbits always with postorbital spine or lobe, usually cupped, sometimes with antorbital spine; carapace triangular, sometimes with posterior spine (see Schweitzer et al., 2020). The new genus possesses all these characteristics and is therefore referred to this subfamily.
The new genus Eoparanaxia has similarities with Paranaxia, in the general outline of the carapace, showing a very elongated pseudorostral spines, parallel and distally bifid, slightly differentiated dorsal regions, with aligned spines in the dorsal part of the mesobranchial region, and well developed mesobranchial and intestinal spines (i.e., Windsor and Felder, 2014), sometimes beyond posterior carapace margin (see Rathbun, 1924, and Hosie and Hara, 2016, p. 128, figure 2; p. 129, figure 3).
However, Paranaxia presents the pseudorostral spines separated from their base, a supraorbital eave with a pronounced spine at the preorbital lobe, postorbital angle with a spine separated from the anterior lobe by a notch. In addition, Eoparanaxia n. gen. exhibits a postorbital spine between the gastric region and the hepatic region, which is not present in Paranaxia.
The studied material has similar characteristics with the modern genus Sphenocarcinus A. Milne-Edwards, 1875, Oxypleurodon Miers, 1885, and Rhinocarcinus de Forges and Ng, 2009, including the shape of the rostrum with two long and coalescent cylindrical spines with slightly diverging sharp tips, and the shape and location of the orbits. However, Eoparanaxia n. gen. shows a different distribution of dorsal regions, with shallower dorsal grooves than in Sphenocarcinus, Oxypleurodon, and Rhinocacinus; intestinal region with a prominent conical spine unlike in Sphenocarcinus, Oxypleurodon, and Rhinocacinus, which lack such conical expansion; sinuous posterior margin; longitudinal antennal pits, parallel to the axis of the body unlike in the other three genera, which are oblique.
The modern genera Pisa Leach, 1815, Leptopisa Stimpson, 1871 (both included in Pisinae Dana, 1851), and Oregonia Dana, 1851 (Oregoniidae Garth, 1958) also show similarities with the new genus in the shape of the rostrum and orbits (i.e., Zariquiey-Álvarez, 1968; p.449, figure 151; Carmona-Suárez and Poupin, 2016, p. 369, figure 5). However, the shape and distribution of dorsal regions, posterior margin, dorsal surface with elevated regions, without spines, are clearly different in the modern genus. Furthermore, Eoparanaxia n. gen. presents the pseudorostrum fused throughout its length, only diverging at the tip, (while in the three mentioned taxa it separates from the base); presents a strong and long intestinal spine, a long branchial spines and spiny ridges on the dorsal surface (of which are absent in the three taxa). In addition, Pisa has anterolateral margins generally straight or slightly concave, with fewer or without spines and lacks the strong and prominent spine in the intestinal region that the new genus possesses.
Other modern genera, Rochinia A. Milne-Edwards, 1875, Scyramathia A. Milne-Edwards, 1880, Minyorhyncha Tavares and Santana, 2018, and Anamathia Smith, 1885 share with the new genus the general outline of the carapace, distribution of spines in the posterolateral and posterior margins, especially in juvenile stages (see Tavares and Santana, 2018; p. 206-214, figures 1-9). However, all these genera have clearly separated and divergent pseudorostral spines unlike in Eoparanaxia n. gen., which presents parallel and fused spines. Some species of Doclea Leach, 1815, show similarities with the new genus, for instance a fused bilobed pseudorostrum only diverging at the tip, a long and acute intestinal spine, a row of axial spines, oblique carinae, paralleling anterolateral margin, and also in the branchial regions. However, the modern genus Doclea differs from Eoparanaxia n. gen. in having a shorter pseudorostrum, a notch in the supraorbital eave, and mostly rounded or less elongated outline.
The material studied here also shows a certain resemblance to some representatives of Inachoididae Dana, 1851, in view of the general outline of the carapace (see Santana, 2008; Lima et al., 2022). However, Inachoididae have usually a shorter pseudorostral spine, strongly fused, and generally ended in a single tip, concave posterior margin, and less spinose/tuberculate dorsal surface.
Eoparanaxia eocenica n. sp.
Figure 3 and Figure 4
zoobank.org/1AAD44D5-0D9C-4642-8FC2-85193465EFDF
Type material. Holotype (MPZ 2023/1), a near-complete carapace, partially decorticated. Paratypes (MPZ 2023/2 and MPZ 2023/3) that correspond to one rostrum and half of a posterior carapace.
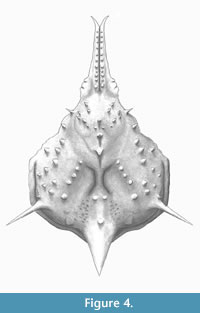 Diagnosis. As for the genus, by monotypy.
Diagnosis. As for the genus, by monotypy.
Description. Carapace pyriform, longer than wide, with spines and conical tubercles; pseudorostral spines parallel and fused, distally bifid, 0.53 times CW, with a row of aligned granules on each spine; orbits not well preserved, small, rounded, obliquely directed. Regions well defined by shallow grooves and deep branchiocardiac groove, with spaced and large spines at the top of the dorsal regions. Gastric region prominent, slightly higher than other regions, with pointed tubercles distributed in anterior gastric region, becoming long gastric spines on the axis of the carapace; urogastric region more depressed. Hepatic region slightly swollen, with one acute stout spine directed anterolaterally. Branchial regions inflated; epibranchial region inflated; mesobranchial region crossed by oblique ridge with five tubercles/spines. Cardiac region elevated, with two tubercles in the anterior part and one in the posterior margin. Intestinal tubercle large, protruding beyond posterior margin of carapace, conical, apex pointed.
Anterolateral margin slightly sinuous. Posterolateral margin convex, with a long spine laterally directed obliquely. Posterolateral and posterior margin rimmed.
Etymology. The specific name refers to the Eocene.
Remarks. Some species of the genus Paranaxia like P. keesingi Hosie and Hara, 2016, and P. serpulifera (Guérin, 1832, in Guérin-Méneville 1829-1837) show similarities with the material studied here. Nevertheless, these taxa have unfused pseudorostral spines, clearly divergent. The new taxon bears some similarities with Macrocheira longirostra Schweitzer and Feldmann, 1999, with a similar outline of the carapace, bifid rostrum, very long pseudorostral spines fused at the base, and divergent at the tip. However, M. longirostra show some differences: 1) lacks spines at the posterolateral margins; 2) has a posterior margin almost straight without intestinal spine; and 3) a dorsal surface much more tuberculated than the new taxon.
Genus PLANOBRANCHIA Schweitzer and Feldmann, 2010
Type species. Micromaia laevis Lőrenthey, 1909, by original designation of Schweitzer and Feldmann (2010, p. 407).
Fossil included species. ? Planobranchia egyptensis Feldmann, Schweitzer, Bennett, Franţescu, Resar, and Trudeau, 2011; P. elongata n. sp. (herein); P. laevis (Lőrenthey, 1909); P. palmuelleri Artal, Van Bakel, and Onetti, 2014; P. simplex (Remy in Gorodiski and Remy, 1959).
Emended diagnosis. Carapace pyriform, widest at midlength of branchial region; moderately vaulted transversely and longitudinally, front produced, singular, sulcate longitudinally. Orbits small, laterally situated, with strong and subtriangular orbital spines. Gastric regions only weakly differentiated; defined laterally by prominent V-shaped Groove converging from anterior margin of orbits to urogastric region, the narrowest part of axial regions. Cardiac region nearly as wide as widest part of gastric regions, hexagonal to ovoid in outline; bearing two nodes on medial transverse ridge. Intestinal region well defined, long, approximately as wide as urogastric region. Epibranchial and mesobranchial regions strongly inflated, separated from one another by subtle arcuate attachment scar expressed on mold of the interior of the carapace; widest part of these regions converge as angular projections toward urogastric region. Metabranchial region extends from widest part of cardiac region posterolaterally around posterior margin of metabranchial region and clearly defined axially by posterior margin of cardiac region and intestinal region; depressed below other regions. Surface of carapace weakly ornamented by fine granules or pits; lacking strong tubercles, posterior margin convex, rimmed (new additions to the original diagnosis of Schweitzer and Feldmann, 2010).
Remarks. The studied specimen can be assigned to Planobranchia Schweitzer and Feldmann, 2010, because it shares the diagnostic characteristics of the genus (see Schweitzer and Feldmann, 2010) like: 1) the moderately vaulted transversely and longitudinally pyriform carapace; 2) weakly differentiated gastric regions, defined laterally by prominent V-shaped groove converging from anterior margin of orbits to urogastric region; 3) hexagonal to ovoid cardiac region, bearing two nodes on medial transverse ridge; 4) strongly inflated epibranchial and mesobranchial regions, separated from one another by subtle arcuate attachment scar; 4) weakly ornamented surface of the carapace by fine pits, lacking strong tubercles.
Some authors assigned Planobranchia to the subfamily Majinae (Schweitzer and Feldmann, 2010; Feldmann et al., 2011; and Schweitzer et al., 2020), and justify this inclusion by the supraorbital margin with an “eave orbital” and a postorbital spine. Subsequently, Artal et al. (2014) proposed to include the genus Planobranchia in Inachidae, justifying its inclusion by similarities in the frontal and orbital construction. Nevertheless, due to the bad preservation, specifically of the pseudorostrum and part of the supraorbital margin, Artal et al. (2014) have misinterpreted the fronto-orbital conformation and the anterolateral spines. Due to the lack of the anterior part of the pseudorostrum spines, these authors have suggested that Planobranchia could have a short pseudorostrum like many inachids, placing the orbits laterally on the sides of the pseudorostrum. Also, the antorbital spine has been interpreted as the postorbital spine, and the two following spines (intercalated spine and postorbital spine) as anterolateral spines. Instead, Planobranchia has a rather elongated rostrum and an orbital construction consisting of three spines.
The characteristics of the new material and the reanalysis of previously known taxa suggest that this genus has more affinity with the subfamily Pisinae, so its inclusion in this group is suggested here. Placement in Pisinae is supported by the morphology of the carapace outline, the distribution and shape of the dorsal regions, supraorbital margin formed by a prominent antorbital spine, a small intercalated spine, and a well-developed postorbital spine; axial regions separated from the periphery by deep grooves; hepatic lobe marked by a lump or spine; highly developed branchial regions; thickened cardiac region; mesogastric region in which a prominent lump stands out (see Griffin and Tranter, 1986, Schweitzer et al., 2020).
Planobranchia elongata n. sp.
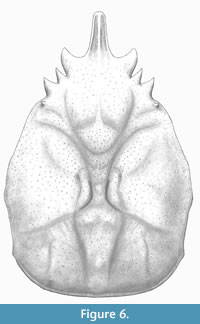
Figure 5 A-B and Figure 6
zoobank.org/8FE98FE3-0E49-4C45-A30A-73324B9512B3
Type material. One partial specimen, partially decorticated: MPZ 2023/4.
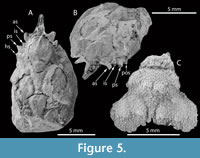 Diagnosis. Carapace pyriform, longer than wide, maximum width in mesobranchial region; front singular, sulcate longitudinally, pseudorostral spines short, fused; orbits small, laterally situated, with strong and subtriangular orbital spines; dorsal regions swollen, distinct, bounded by grooves; mesogastric region inflated and smooth, narrow and elongated anteriorly, bounded by two elongated ridges; metagastric region narrow, U-shaped; branchial regions differentiated; mesobranchial region arched; posterior margin broad, rimmed.
Diagnosis. Carapace pyriform, longer than wide, maximum width in mesobranchial region; front singular, sulcate longitudinally, pseudorostral spines short, fused; orbits small, laterally situated, with strong and subtriangular orbital spines; dorsal regions swollen, distinct, bounded by grooves; mesogastric region inflated and smooth, narrow and elongated anteriorly, bounded by two elongated ridges; metagastric region narrow, U-shaped; branchial regions differentiated; mesobranchial region arched; posterior margin broad, rimmed.
Description. Carapace pyriform in outline, longer than wide; dorsal surface covered by small pits, convex in both directions; maximum width in mesobranchial region; front produced, straight, directed forwards, with two fused spines, with longitudinal ridges; orbits anterolaterally directed, supraorbital margin with strong spines: antorbital spine is the largest, triangular in shape and slightly forward; intercalated spine shortest, conical, separated by supraorbital sutures; postorbital spine triangular, medium sized, facing out. Lateral margins smooth, arched; hepatic region slightly inflated, defined by shallow groves; notable subtriangular spine in the postorbital lobe; mesogastric region inflated, u-shaped, apparently smooth and anterior portion ridged; protogastric region weakly defined; urogastric region bounded by shallow grooves; branchial regions delimited from axial regions by grooves; cardiac region triangular; epibranchial region inflated, oblique, arched posteriorly; mesobranchial region broadly inflated; metabranchial region depressed; intestinal region not preserved; posterior margin not preserved, appears broad.
 Etymology. The specific name refers to its elongated morphology.
Etymology. The specific name refers to its elongated morphology.
Remarks. The type species, Planobranchia laevis, from the Lutetian of Egypt, shows clear affinity with P. elongata n. sp., but has some differences: 1) distinct hepatic region, with smaller hepatic spine than the new species; 2) gastric regions less pronounced and more elongated and dorsal surface ornated with very small granules, instead of the smooth surface with setal pits that the new species has. Planobranchia palmuelleri from the Lutetian of Catalonia is clearly different from P. elongata n. sp. in some aspects like: 1) its carapace outline, being much wider and shorter than the new species; and 2) by its shorter rostrum. Planobranchia simplex from the Lutetian of Senegal, shows certain similarities with the new species, such as an arched margin, defined by a thin rim that does not appear to be separated by strong depressions on the branchial regions, however; P. simplex clearly differs from the new species in: 1) the outline of the carapace, much wider than the new species and 2) the deeper axial grooves. The new taxon differs from ?P. egyptensis from the Lutetian of Egypt, in having a slimmer outline and more inflated dorsal regions.
Family MACROCHEIRIDAE Dana, 1851
Genus ?Macrocheira de Haan, 1839
Type species. Maja kaempferi Temminck, 1836
Fossil included species. Macrocheira longirostra Schweitzer and Feldmann 1999 (Eocene), M. teglandi Rathbun, 1926 (Oligocene), M. ginzaensis Imaizumi, 1965 (Miocene), and M. yabei (Imaizumi, 1957 as Parotymolus yabei) (Miocene).
? Macrocheira sp.
Figure 5C
Material. One incomplete carapace with cuticle not well preserved: MPZ 2023/5.
Description. Carapace pyriform, moderately convex, slightly more swollen in gastric and epibranchial portions. Frontal, posterior, and anterolateral margins not preserved; lateral margins sinuous, with strong incision between hepatic and branchial margins; mesobranchial margin strongly rounded. Dorsal surface densely granulated. Branchiocardiac grooves very close to each other; hepatic region inflated; Protogastric region inflated, with two large tubercles; mesogastric region narrow, moderately inflated; urogastric region extremely narrow, not well defined. Mesobranchial region large, inflated and rounded, separated from gastric regions by the cervical groove, with two tubercles separated from each other. Cardiac, intestinal, and metabranchial regions not preserved.
Remarks. Species of Macrocheira are characterized by a pyriform outline of the carapace, hepatic spine, bifurcate rostrum (two spines), poorly developed supraorbital eave, small ant- and postorbital spines, and well-developed carapace regions (see Miers, 1886; Rathbun, 1926; Sakai, 1976; Schweitzer and Feldmann, 1999). Moreover, Macrocheira usually have inflated protogastric and mesogastric regions; depressed metagastric region; a urogastric region poorly developed; epi- and mesobranchial regions inflated, and depressed metabranchial region; cardiac region oblong and bounded by wide grooves with parallel crenulations positioned oblique to the axis of the grooves.
The different fossil species of the genus Macrocheira are differentiated by the degree of inflation and shape of carapace regions, ornamentation on dorsal regions, length of the rostrum and size of the orbital and hepatic spines (see Schweitzer and Feldmann, 1999). The genus is only represented by one modern species, Macrocheira kaempferi, from Japan, reported from mud or sand bottoms at 50-300 m depth (Sakai, 1976).
Given the fragmentary condition of the specimen, the generic assignment is given tentatively and a proper specific assignation is not possible.
Family MAJIDAE Samouelle, 1819
Subfamily ?MAJINAE Samouelle, 1819
Genus SPINIROSTRIMAIA Beschin, De Angeli, Checchi, and Zarantonello, 2012
Type species. Micromaia margaritata Fabiani, 1910.
Fossil included species. Spinirostrimaia margaritata (Fabiani, 1910) (from Lutetian of Italy); S. echinate n. sp. (herein).
Remarks. The studied material can be assigned to Spinirostrimaia based on the carapace general outline elongate, distribution of dorsal regions, orbital position and shape of the orbits, and the long pseudorostral spines with three lateral spines on the outer margins (see diagnosis in Beschin et al., 2012).
Spinirostrimaia echinata n. sp.
Figure 7, Figure 8, and Figure 9
zoobank.org/B169EC2C-D88A-465D-A140-14B3A9395D48
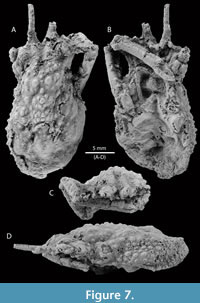 Type material. The holotype is a female specimen, near-complete carapace, partially decorticated, with one cheliped and thoracic sternum (MPZ 2023/6) (Figure 7). There are four paratypes (MPZ 2023/7-2023/10) (Figure 8).
Type material. The holotype is a female specimen, near-complete carapace, partially decorticated, with one cheliped and thoracic sternum (MPZ 2023/6) (Figure 7). There are four paratypes (MPZ 2023/7-2023/10) (Figure 8).
Additional material. Thirteen additional specimens composed by near-complete carapaces (MPZ 2023/11-2023/23).
Diagnosis. Carapace pyriform, longer than wide, convex; regions separated by shallow grooves; dorsal surface covered by small spines. Frontal margin narrow, sulcate, with two long, subparallel pseudorostral spines with spiny outer margin proximally situated; dorsal surface covered by spines (pearl-shaped tubercles if partially decorticated); orbits with prominent supraorbital eaves, without orbital spines; anterolateral margins elongated, interrupted with hepatic and branchial grooves; hepatic lobe with three lateral spines; mesobranchial margin strongly convex, with numerous spines (10-13); posterior margin with small spines.
Description. Carapace pyriform, twice as long as wide, (not counting the pseudorostrum), ovate; pseudorostrum long, bifid, composed of two spines which have three tiny spines on outer margin; frontal region with two longitudinal crests, tuberculate. Almost complete orbits, with prominent supraorbital eave, without antorbital spine, but marked preorbital lobe; intercalated spine triangular; postorbital spine elongated, slightly curved forward; hepatic lobe with three pointed spines, slightly curved forward. Lateral margins convex, notched by the cervical groove. Dorsal regions ornamented with sharp spines, with pearl-shaped tubercles appearance if partially decorticated; carapace regions well defined by relatively shallow grooves; axial regions elevate above other regions. Proto- and mesogastric regions inflated; meta- and urogastric regions narrower than mesogastric and cardiac regions; hepatic region inflated; branchial regions wide; epi- and mesobranchial regions inflated, poorly differentiated by a shallow groove; metabranchial region slightly depressed; cardiac region inflated, with two lateral subtriangular extensions defined by shallow grooves; intestinal region small, slightly depressed; posterior margin broad, rimmed, with small spines. Branchiocardiac grooves deep.
 Epistome wide, smooth, and rimmed. Female thoracic sternum strongly concave, with interrupted sutures (only preserved 1/2 to 5/6, see Figure 7B). Female chelipeds elongated and thin; merus elongated, with longitudinal depression on the ventral surface, surrounded by well-separated blunt spines; carpus slightly elongated, ornamented with small spines; palm slightly compressed, oval in cross section; fingers acute, relatively short, square in section, with longitudinal striae. Basal antennal article moderately wide, broader at the base than at its distal extremity.
Epistome wide, smooth, and rimmed. Female thoracic sternum strongly concave, with interrupted sutures (only preserved 1/2 to 5/6, see Figure 7B). Female chelipeds elongated and thin; merus elongated, with longitudinal depression on the ventral surface, surrounded by well-separated blunt spines; carpus slightly elongated, ornamented with small spines; palm slightly compressed, oval in cross section; fingers acute, relatively short, square in section, with longitudinal striae. Basal antennal article moderately wide, broader at the base than at its distal extremity.
Etymology. The specific epithet make reference to its spinose carapace.
Remarks. The new species shows similarities with the type species Spinirostrimaia margaritata in the general shape of the carapace, piriform and elongated, and long pseudorostral spines. However, the new species differs in some aspects: 1)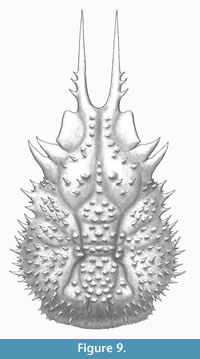 subparallel pseudorostral spines, not convergent as in S. margaritata; 2) slightly smaller postorbital spine, without ornamentation; 3) the dorsal ornamentation, more spinose in the new species (instead of pearl-shaped tubercles of S. margaritata); 4) prominent spine in the margin of the hepatic region, that is absent in S. margaritata; 5) small, sharp spines covering the dorsal surface, lacking pearl-shaped tubercles as in S. margaritata; 6) wider posterior margin than S. margaritata, and less convex and rimmed; and 7) more spiny margins in the carapace of the new species than S. margaritata (see Beschin et al., 2012, figures 41, 80; t. 6, figures 3, 6a, b, 7a, b).
subparallel pseudorostral spines, not convergent as in S. margaritata; 2) slightly smaller postorbital spine, without ornamentation; 3) the dorsal ornamentation, more spinose in the new species (instead of pearl-shaped tubercles of S. margaritata); 4) prominent spine in the margin of the hepatic region, that is absent in S. margaritata; 5) small, sharp spines covering the dorsal surface, lacking pearl-shaped tubercles as in S. margaritata; 6) wider posterior margin than S. margaritata, and less convex and rimmed; and 7) more spiny margins in the carapace of the new species than S. margaritata (see Beschin et al., 2012, figures 41, 80; t. 6, figures 3, 6a, b, 7a, b).
The fossil species Cromimaia meneguzzoi (Beschin, Busulini, De Angeli, and Tessier, 1985) bears some resemblance to the new species. However, S. echinata n. sp. differs in some aspects: 1) the urogastric region is narrower in the new species, with three tubercles forming a triangle, and not aligned as in C. meneguzzoi; 2) narrower cardiac region, better delimited by branchiocardiac grooves in the new species; 3) the pseudorostral spines are exceedingly shorter in C. meneguzzoi, in contrast to the long spines of the new species (see Beschin et al., 2012, figure 40; t. 6, figures 4a-c). The new species also shows similarities with Micromaia tuberculata Bittner, 1875, but has differences in the branchiocardiac groove and both the urogastric region and the beginning of the cardiac region are narrower; the intestinal region is slightly more swollen; and chiefly in having two long and parallel pseudorostral spines, whereas in M. tuberculata they are much shorter and flattened subtriangular (see Beschin et al., 2012, figure 38; t. 6, figure 2).
DISCUSSION
Spider crabs are found both in siliciclastic and reef environments, but apparently they show preferences by siliciclastic meadows. Modern small-sized majoids are usually associated with specific substrates. Specially, they show a clear preference for life on hard substrates (like cavities of corals, stones, rubble, and sponges) or associated with aquatic vegetation, at depths between 1 and 60 m (see Carmona-Suárez and Poupin, 2016; Bearham et al., 2022), and only occasionally associated with sand or mud. However, almost all published Eocene occurrences are from siliciclastic areas (≈72.5%) and only a small percentage of this group has preference for coral or bryozoan meadows (≈ 21.5%, see Table 1).
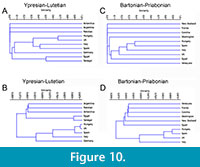 During the middle-upper Eocene, spider crabs were relatively common and diversified. They are found all over the world, and the maximum diversity is concentrated in Europe (Table 1). Jaccard index descriptive binary similarity measure (Figure 10A and C) and Raup-Crick probabilistic similarity measure (Figure 10B and D), provide evidence for the similarity between different areas with spider crabs during the Eocene.
During the middle-upper Eocene, spider crabs were relatively common and diversified. They are found all over the world, and the maximum diversity is concentrated in Europe (Table 1). Jaccard index descriptive binary similarity measure (Figure 10A and C) and Raup-Crick probabilistic similarity measure (Figure 10B and D), provide evidence for the similarity between different areas with spider crabs during the Eocene.
Based on the Jaccard Coefficients, similarity decreases non proportionally toward younger intervals (Figure 10A and C). The analyses carried out for the first interval (Ypresian-Lutetian) show that the Iberian basins have more affinity with Italy, and to a lesser extent with the UK and Hungary (<0.4), remaining very far (<0.05) from South America, Africa, and Middle East (Figure 10A). In the second interval (Bartonian-Priabonian) the results show that Iberia has higher differences with the rest of the areas, Hungary being the closest area (>0.4), followed by Italy (>0.3), and UK (>0.2), and there is a significant difference (<0.05) with America and New Zealand (Figure 10C).
Although in all cases the Raup-Crick Coefficient shows values higher than 0.05 (considered non-significant differences), the differences between the European basins and the rest of the basins can be observed. This coefficient provides better resolution, and UPGMA algorithm reveals major affinity between Spain, Hungary, UK (>0.9), Italy (>0.7), and Germany (0.535) during Ypresian-Lutetian interval, and only a value of 0.4 with more distant (not European) basins (Figure 10B). During Bartonian-Priabonian interval, the results show a slight increase in the difference between areas (Figure 10D). These results show the strongest affinity with Hungary (0.915), followed by UK (0.830) and Italy (0.585), and only of 0.345 with more distant areas.
According to the obtained results and even considering that the duration of the Ypresian-Lutetian interval (14.8 Ma) is twice as long than the Bartonian-Priabonian interval (7.3 Ma, see Table 2), differences are more significant in the late Eocene. This suggests more changes in distribution in the late Eocene that should be analysed including more crustacean groups.
CONCLUSIONS
The Eocene record of majoids includes 51 species described to date (Table 1), most records are from basins in the Mediterranean area (38 species). On the basis of sedimentological data, the Eocene majoid assemblage from Fanlillo corresponds to taxa associated with shallow-marine siliciclastic environments, probably developed in the euphotic zone, at depths that did not exceeded 20-30 m (see Ferratges et al., 2023).
The outcrop of Fanlillo includes a great abundance and diversity of small gastropods, and epiphytic bryozoans suggesting that some taxa were associated with a seagrass environment. This might support the coexistence of high diversities of crabs, especially of small sizes that found in such environment important areas for hiding against predators and enough food resources. The description of this assemblage increases our knowledge of majoids in the Eocene of Iberian Peninsula. They represent an interesting find in the prodelta marls of the Margas de Pamplona Formation and increase the spatial and temporal distribution of the genera Planobranchia, Macrocheira, and Spinirostrimaia to the central Pyrenees. The great diversity of this single group of decapod crustaceans, associated with other small decapods in same outcrop, also suggest a complex soft bottom ecosystem, under favourable condition, probably related to seagrass meadows under euphotic conditions. Other European Eocene localities have similar spider crabs diversity (e.g., Beschin et al.,1985, 2012; De Angeli and Garassino, 2006; De Angeli et al., 2019).
The obtained results with the different coefficients employed, show great similarity of the Iberian basins with other relatively near areas (especially Italy, UK, and Hungary), but the degree of similarity varies over time. The data does not reveal a large difference with respect to the clustered groups in the different time intervals and assemblages from Iberian basins appear clustered with other European areas. The abundance and diversity of spider crabs in the upper Eocene, suggest this group was diversified and specialized for inhabiting this type of environment during the Eocene.
ACKNOWLEDGEMENTS
The present work has been supported by project CGL2017-85038-P, subsidised by the Spanish Ministry of Science and Innovation, the European Regional Development Fund, and Project E18 Aragosaurus: Recursos Geológicos y Paleoambientes of the government of Aragón-FEDER. The research of F.A.F. was funded by a FPU Grant (Spanish Ministry of Science and Innovation). I. Pérez provided photographic assistance. We are also grateful to the reviewers B. van Bakel (Oertijdmuseum De Groene Poort, the Netherlands), an anonymous reviewer, and the Editor-in-Chief who greatly improved the resulting manuscript.
REFERENCES
Artal, P., Van Bakel, B.W.M., Fraaije, R.H.B., and Jagt, J.W.M. 2013. New retroplumid crabs (Crustacea, Brachyura, Retroplumidae Gill, 1894) from the Eocene of Huesca (Aragón, Spain). Zootaxa, 3652:343-352.
https://doi.org/10.11646/zootaxa.3652.3.3
Artal, P., Van Bakel, B.W.M., and Onetti, A. 2014. A new inachid crab (Brachyura, Majoidea) from the Middle Eocene of the provinces of Barcelona and Girona (Catalonia, Spain). In Fraaije, R.H.B., Hyžný, M., Jagt, J.W.M., Krobicki, M., and Van Bakel, B.W.M., (eds.), Proceedings of the 5th symposium on Mesozoic and Cenozoic decapod crustaceans, Krakow, Poland, 2013: a tribute to Pál Mihály Müller, Scripta Geologica, 147:153-161.
Bearham, D., Strzelecki, J., McLaughlin, J., Bryce, M., Fromont, J., Hara, A., Hosie, A., Huisman, J.H., Kirkendale, L., Liu, D., Moore, G., Morrison, S., Naughton, K., O’Hara, T.D., O’Loughlin, M., Richards, Z., Snedden, Z., Whisson, C., and Keesing, J.K. 2022. Habitats and benthic biodiversity across a tropical estuarine-marine gradient in the eastern Kimberley region of Australia. Regional Studies in Marine Science, 49:102039.
https://doi.org/10.1016/j.rsma.2021.102039
Bell, T. 1858. A Monograph of the Fossil Malacostracous Crustacea of Great Britain, Part I. Crustacea of the London Clay. Palaeontographical Society Monograph, 10:1-44.
Beschin, C., Busulini, A., De Angeli, A., and Tessier, G. 1985. Il genere Micromaia Bittner (Crustacea, Decapoda) nel Terziario dell’area dei Berici e dei Lessini, con descrizione di tre nuove specie. Lavori-Società Veneziana di Scienze Naturali, 10:97-119.
Beschin, C., Busulini, A., De Angeli, A., and Tessier, G. 1994. I crostacei eocenici della Cava “Boschetto” di Nogarole Vicentino (Vicenza - Italia settentrionale). Lavori-Societe Veneziana di Scienze Naturali, 19:159-215.
Beschin, C., Busulini, A., Fornaciari, E., Papazzoni, C.A., and Tessier, G. 2018. La fauna di crostacei associati a coralli dell’Eocene superiore di Campolongo di Val Liona (Monti Berici, Vicenza, Italia nordorientale). Bollettino del Museo di Storia Naturale di Venezia, 69:129-215.
Beschin, C., Busulini, A., Tessier, G., and Zorzin, R. 2016. I crostacei associati a coralli nell' Eocene inferiore dell' area di Bolca: (Verona e Vicenza, Italia nordorientale). Memorie del Museo Civico di Storia Naturale di Verona, Sezione Scienze della Terra, 9(2):13-168.
Beschin, C., Busulini, A., Tessier, G., and Zorzin, R. 2019. La fauna di crostacei dell’ Eocene superiore di Parona di Verona (Italia Nordorientale): Nuovi ritrovamenti. Bollettino del Museo di Storia Naturale di Venezia, 70:71-142.
Beschin, C., De Angeli, A., Checchi, A., and Zarantonello, G. 2005. Crostaceieocenici di Grola presso Spagnago (Vicenza, Italia settentrionale). Studi e Ricerche, Associazione Amici del Museo, Museo Civico ‘G. Zannato’, Montecchio Maggiore (Vicenza), 12:5-35.
Beschin, C., De Angeli, A., Checchi, A., and Zarantonello, G. 2012. Crostacei del giacimento eocenico di Grola presso Spagnago di Cornedo Vicentino (Vicenza, Italia settentrionale) (Decapoda, Stomatopoda, Isopoda). Museo di archeologia e scienze naturali “G. Zannato”. Montecchio Maggiore, Vicenza.
Bittner, A. 1875. Die Brachyuren des Vicentinischen Tertiärgerbirges. Denkschriften der kaiserlichen Akademie der Wissenschaften in Wien, 34:63-106.
Blow, W.C. and Manning, R.B. 1996. Preliminary descriptions of 25 new decapod crustaceans from the middle Eocene of the Carolinas, USA. Tulane Studies in Geology and Paleontology, 29(1):1-26.
Breton, G. 2009. Description of Priscinachus elongatus n. gen., n. sp., and Priscinachidae n. fam. for the earliest spider crab (Crustacea, Decapoda, Majoidea), from the French Cretaceous (Cenomanian). Geodiversitas, 31:509-523.
https://doi.org/10.5252/g2009n3a2
Brösing, A., Richter, S., and Scholtz, G. 2007. Phylogenetic analysis of the Brachyura (Crustacea, Decapoda) based on characters of the foregut with establishment of a new taxon. Journal of Zoological Systematics and Evolutionary Research, 45(1):20-32.
https://doi.org/10.1111/j.1439-0469.2006.00367.x
Carmona-Suárez, C. and Poupin, J. 2016. Majoidea crabs from Guadeloupe Island, with a documented list of species for the Lesser Antilles (Crustacea, Decapoda, Brachyura, Majoidea). Zoosystema, 38(3):353-387.
https://doi.org/10.5252/z2016n3a5
Cascales-Miñana, B. 2010. Testing similarity coefficients for analysis of the fossil record using clustering methods: the Palaeozoic flora as a study case. Revista Española de Paleontología, 25(1):19-34.
Castelltort, S., Guillocheau, F., Robin, C., Rouby, D., Nalpas, T., Lafont, F., and Eschard, R. 2003. Fold control on the stratigraphic record: a quantified sequence stratigraphic study of the Pico del Aguila anticline in the south-western Pyrenees (Spain). Basin Research, 15:527-551.
https://doi.org/10.1111/j.1365-2117.2003.00218.x
Ceccon, L. and De Angeli, A. 2018. Lessiniamathia bolcense n. gen., n. sp., nuovo crostaceo Epialtidae dell’Eocene inferiore dei Monti Lessini orientali (Verona, Italia nordorientale). Lavori-Società Veneziana di Scienze Naturali, 43:147-154.
Clark, P.F. and Ng, P.K. 2004. Two zoeal stages and the megalop of Pilumnus sluiteri De Man, 1892 [Crustacea: Brachyura: Xanthoidea: Pilumnidae] described from laboratory-reared material. Invertebrate reproduction & development, 45(3):205-219.
https://doi.org/10.1080/07924259.2004.9652592
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J.X. 2013. The ICS International Chronostratigraphic Chart. Episodes, 36:199-204.
Crandall, K.A., Porter, M.L., and Pérez-Losada, M. 2009. Crabs, shrimps, and lobsters (Decapoda). In Hedges, S.B. and Kumar, S. (eds.), The Time Tree of Life. Oxford University Press, Oxford, UK.
Dana, J.D. 1851. On the classification of the maioid Crustacea or Oxyrhyncha. American Journal of Science and Arts, Second Series, 11(33):425-434.
Davie, P.J.F., Guinot, D., and Ng, P.K.L. 2015. Anatomy and Functional morphology of Brachyura. In Castro, P., Davie, P.J.F., Guinot, D., Schram, F.R., and Von Vaupel Klein, J.C. (eds.), Treatise on Zoology. Anatomy, Taxonomy, Biology. The Crustacea. Brill, Leiden and Boston.
https://doi.org/10.1163/9789004190832_004
De Angeli, A. and Ceccon, L. 2015. Nuovi crostacei Brachyura dell' Eocene di Monte Magrè (Vicenza, Italia settentrionale). Lavori Società veneziana di Scienze naturali, 40:119-138.
De Angeli, A. and Garassino, A. 2006. Catalogue and bibliography of fossil Stomatopoda and Decapoda from Italy. Memoriedella Società italiana di Scienze naturali e del Museo civico di Storia naturale di Milano, 35(1):3-96.
De Angeli, A, Garassino, A., and Pasini, G. 2019. Catalog and bibliography of fossil Stomatopoda and Decapoda from Italy (2007-2018). Memorie della Società Italiana di Scienze Naturali, 45:1-86.
de Forges, B.R. and Ng, P.K. 2009. On the majoid genera Oxypleurodon Miers, 1886, and Sphenocarcinus A. Milne-Edwards, 1875 (Crustacea: Brachyura: Epialtidae), with descriptions of two new genera and five new species. The Raffles Bulletin of Zoology, 20:247-266.
De Grave, S., Pentcheff, N.D., Ahyong, S.T., Chan, T-Y., Crandall, K.A., Dworschak, P.C., Felder, D.L., Feldmann, R.M., Fransen, C.H.J.M., Goulding, L.Y.D., Lemaitre, R., Low M.E.Y., Martin, J.W., Ng, P.K.L., Schweitzer, C.E., Tan, S.H., Tshudy, D., and Wetzer, R. 2009. A classification of living and fossil genera of decapods crustaceans. Raffles Bulletin of Zoology, 21(Supplement):1-109.
de Haan, W. 1833-1850. Crustacea. In Von Siebold, P.F. (ed.), Fauna Japonica sive descriptio animalium, quae in itinere per Japoniam, jussu et auspiciis superiorum, qui summum in India Batava imperium tenent, suscepto, annis 1823-1830 collegit, notis, observationibus et adumbrationibus illustravit. J. Müller et Co., Lugduni Batavorum [Leiden].
De Saint Laurent, M. 1980. Sur la classification et la phylogénie des crustacés décapodes brachyoures. I. Podotremata Guinot, 1977, et Eubrachyura sect. nov. Comptes Rendus hebdomadaires des Séances de l’Académie des Sciences, Paris, D290:1265-1268.
Desmarest, A.G. 1823. Malacostracés. In Dictionnaire des sciences naturelles, dans lequel on traite méthodiquement des différens êtres de la nature, considérés soit en eux-mêmes, d´après l´état actuel de nos connoissances, soit relativement à l´utilité qu´en peuvent retirer la médecine, l´agriculture, le commerce et les artes. Suivi d´une biographie des plus célèbres naturalistes. Strasbourg, Paris.
Domínguez, J.L. and Ossó, À. 2016. New decapod fauna at midway of the Tethys Sea and Atlantic Ocean; central Pyrenees of Huesca (Aragon, Spain), p. 23-24. In Charbonnier, S. (ed.), 6th Symposium on Mesozoic and Cenozoic decapod crustaceans. Villers-sur-Mer, Normandy, France.
Dreyer, T., Corregidor, J., Arbués, P., and Puigdefàbregas, C. 1999. Architecture of the tectonically influenced Sobrarbe deltaic complex in the Ainsa Basin, northern Spain. Sedimentary Geology, 127:127-169. https://doi.org/10.1016/S0037-0738(99)00056-1
Fabiani, R. 1910. I Crostacei terziari del Vicentino. Illustrazione di alcune specie e catalogo generale delle forme finora segnalate nella provincia. Bollettino del Museo Civico di Vicenza, 1:29-45.
Feldmann, R.M., Schweitzer, C.E., Bennett, O., Franescu, O.D., Resar, N., and Trudeau, A. 2011. New Eocene Brachyura (Crustacea: Decapoda) from Egypt. Neues Jahrbuch für Geologie und Palaontologie - Abhandlungen, 262(3):1-31.
https://doi.org/10.1127/0077-7749/2011/0202
Ferratges, F.A., Luque, J., Domínguez, J. L., Ossó, À., Aurell, M., and Zamora, S. 2023. The oldest dairoidid crab (Decapoda, Brachyura, Parthenopoidea) from the Eocene of Spain. Papers in Palaeontology, 9(3): e1494.
https://doi.org/10.1002/spp2.1494
Förster, R. and Mundlos, R. 1982. Krebse aus dem Alttertiär von Helmstedt und Handorf (Niedersachsen). Palaeontographica A, 179:148-184.
Garth, J.S. 1958. Brachyura of the Pacific coast of America. Oxyrhyncha. Allan Hancock Pacific Expedition, 21:1-854.
Gorodiski, A. and Remy, J.M. 1959. Sur les décapodes éocènes du Sénégal occidental. Bulletin de la Société géologique de France, 7(1):315-319.
Griffin, D.J.G. 1966. The genus Chlorinoides (Crustacea, Brachyura, Majidae), 1. A redescription of C. tenuirostris Haswell and the status of the genus Acanthophrys A. Milne Edwards. Records of the Australian Museum, 27(1):1-16.
https://doi.org/10.3853/j.0067-1975.27.1966.436
Griffin, D.J.G. and Tranter, H.A. 1986. The Decapoda Brachyura of the Siboga expedition. Part VIII: Majidae. Siboga Expéditie, 39:1-335.
Guérin-Méneville, F.E. 1829-1837. Crustacés. In Cuvier, G. (ed.), Iconographie du Règne animal de G. Cuvier, ou représentation d’après nature de l’une des espèces les plus remarquables et souvent non encore figurées de chaque genre d’animaux Avec une text descriptif mis au courant de la science. Ouvrage pouvant servir d’atlas à tous les traités de zoologie. J.B. Baillière, Paris and London.
Guinot, D. 2019. New hypotheses concerning the earliest brachyurans (Crustacea, Decapoda, Brachyura). Geodiversitas, 41(22):747-796.
https://doi.org/10.5252/geodiversitas2019v41a22
Guinot, D. and Van Bakel, B.W.M. 2020. Extraordinary majoid crabs: the genus Esopus A. Milne-Edwards, 1875 in the new subfamily Esopinae subfam. nov., and erection of Paulitinae subfam. nov. (Crustacea, Decapoda, Brachyura, Majoidea, Inachoididae Dana, 1851). Zootaxa, 4766(1):101-127.
https://doi.org/10.11646/zootaxa.4766.1.5
Guinot, D. and Wicksten, M.K. (eds.). 2015. Camouflage: carrying behaviour, decoration behaviour, and other modalities of concealment in Brachyura. In Treatise on Zoology-Anatomy, Taxonomy, Biology. The Crustacea, Volume 9 Part C (2 vols). Brill.
https://doi.org/10.1163/9789004190832_013
Guinot, D., Davie, P.J., Tsang, L.M., and Ng, P.K. 2022. Formal re-establishment of Macrocheiridae Dana, 1851 (Decapoda: Brachyura: Majoidea) for the giant spider crab Macrocheira kaempferi (Temminck, 1836) based on a reappraisal of morphological and genetic characters. Journal of Crustacean Biology, 42(2): ruac022.
https://doi.org/10.1093/jcbiol/ruac022
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica, 4:1-9.
Hosie, A.M. and Hara, A. 2016. Description of a new species of brooding spider crab in the genus Paranaxia Rathbun, 1924 (Brachyura: Majoidea), from northern Australia and Indonesia. Zootaxa, 4127(1):121-134.
https://doi.org/10.11646/zootaxa.4127.1.6
Hultgren, K.M. and Stachowicz, J.J. 2008. Molecular phylogeny of the brachyuran crab superfamily Majoidea indicates close congruence with trees based on larval morphology. Molecular Phylogenetics and Evolution, 48(3):986-996.
https://doi.org/10.1016/j.ympev.2008.05.004
Huyghe, D., Mouthereau, F., Castelltort, S., Filleaudeau, P.Y., and Emmanuel, L. 2009. Paleogene propagation of the southern Pyrenean thrust wedge revealed by finite strain analysis in frontal thrust sheets: implications for mountain building. Earth and Planetary Science Letters, 288:421-433.
https://doi.org/10.1016/j.epsl.2009.10.002
Imaizumi, R. 1957. A Miocene fossil crab Paratymolus yabei n. sp. from the Nagana Prefecture. Transactions and Proceedings of the Palaeontological Society of Japan, New Series, 25:26-30.
Imaizumi, R. 1965. Miocene Macrocheria from Japan. Researches on Crustacea, 2:27-36.
Klompmaker, A.A. 2013. Extreme diversity of decapod crustaceans from the mid-Cretaceous (late Albian) of Spain: implications for Cretaceous decapod paleoecology. Cretaceous Research, 41:150-185.
https://doi.org/10.1016/j.cretres.2012.12.003
Klompmaker, A.A., Portell, R.W., Klier, A.T., Prueter, V., and Tucker, A.L. 2015. Spider crabs of the Western Atlantic with special reference to fossil and some modern Mithracidae. PeerJ, 3:e1301.
https://doi.org/10.7717/peerj.1301
Larghi, C. 2002. Mithracia oppioni sp. nov. (Crustacea, Decapoda, Brachyura) from the Eocene of Chiampo (Vincenza, Italy). Bulletin of the Mizumami Fossil Museum, 29:61-68.
Leach, W.E. 1815. The zoological miscellany; being descriptions of new or interesting animals. Nodder and Son, London, UK.
Lima, D., Aguilera, O., and Tavares, M. 2022. The Inachoididae spider crabs (Crustacea, Brachyura) from the Neogene of the tropical Americas. Journal of Paleontology, 96(2): 334-354.
https://doi.org/10.1017/jpa.2021.91
Lőrenthey, E. 1909. Beiträge zur Tertiären Dekapodenfauna Sardiniens. Mathematische und Naturwissenschaftliche Berichte aus Ungarn, 24:202-259.
MacLeay, W.S. 1838. On the brachyurous decapod Crustacea. Brought from the Cape by Dr. Smith. In Smith, A. (ed.), Illustrations of the Zoology of South Africa; consisting chiefly of Figures and Descriptions of the Objects of Natural History Collected During an Expedition into the Interior of South Africa, in the Years 1834, 1835, and 1836; Fitted Out by ‘The Cape of Good Hope Association for Exploring Central Africa’. Published under the Authority of the Lords Commissioners of Her Majesty’s Treasury, London, UK.
Maridet, O., Escarguel, G., Costeur, L., Mein, P., Hugueney, M., and Legendre, S. 2007. Small mammal (rodents and lagomorphs) European biogeography from the Late Oligocene to the mid Pliocene. Global Ecology and Biogeography, 16:529-544.
Miers, E.J. 1876. Descriptions of some new species of Crustacea, chiefly from New Zealand. Annals and Magazine of Natural History, 4(17):218-229.
Miers, E.J. 1885. The Brachyura, p. 585-592. In Tizard, T.H., Moseley, H.N., Buchanan, J.Y., and Murray, J. (eds.), Narrative of the cruise of H.M.S. Challenger with a general account of the scientific results of the expedition. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873-1876 under the command of Captain George S. Nares, R.N., F.R.S. and the late Captain Frank Tourle Thomson, R.N. prepared under the Superintendence of the late Sir C. Wyville Thomson, Knt., F.R.S. &c. Regius Professor of Natural History in the University of Edinburgh Director of the civilian scientific staff on board and now of John Murray, one of the naturalists of the Expedition. Narrative 1(2). Published by Order of Her Majesty’s Government, London, Edinburgh and Her Majesty Stationery Office, Dublin, UK.
Miers, E.J. 1886. Report on the Brachyura collected by H. M. S. Challenger during the years 1873-1876. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873-76. Zoology, New York.
Millán, H., Aurell, M., and Meléndez, A. 1994. Synchronous detachment folds and coeval sedimentation in the Prepyrenean External Sierras (Spain): a case study for a tectonic origin of sequences and systems tracts. Sedimentology, 41(5):1001-1024.
Milne-Edwards, A. 1873-1881. Études sur les Xiphosures et les Crustacés podophthalmaires. Mission scientifique au Mexique et dans l'Amérique centrale. Recherches Zoologiques pour servir l'histoire de la faune de l'Amérique centrale et du Mexique, 5:1-368.
Müller, P. and Collins, J.S.H. 1991. Late Eocene coral-associated decapods (Crustacea) from Hungary. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie, 28(2/3):47-92.
Muñoz, J.A., McClay, K., and Poblet, J. 1994. Synchronous extension and contraction in frontal thrust sheets of the Spanish Pyrenees. Geology, 22:921-924.
Mutti, E., Remacha, E., Sgavetti, M., Rosell, J., Valloni, R., and Zamorano, M. 1985. Stratigraphy andfacies characteristics of the Eocene Hecho group turbidite systems, south−central Pyrenees. IAS 6th European regional meeting, Lleida. Field Trip Guidebook, 12:519-576.
Ng, P.K.L., Guinot, D., and Davie, P.J.F. 2008. Systema Brachyurorum: Part 1. An annotated checklist of extant brachyuran crabs of the world. Raffles Bulletin of Zoology, 17:1-286.
Noetling, F. 1881. Ueber einige Brachyuren aus dem Senon von Mastricht und dem Tertiär Norddeutschlands. Zeitschrift der deutschen geologischen Gesellschaft, 33:357-371.
Ossó, À. and Domínguez, J.L. 2017. A new genus and new species of decapod crustacean (Decapoda: Brachyura: Panopeidae) from the early Priabonian (Late Eocene) of the central Pyrenees of Huesca (Aragón, Spain), with remarks on its habitat and ecology. Journal of Crustacean Biology, 37(5):602-607.
https://doi.org/10.1093/jcbiol/rux072
Ossó, À., Domínguez, J.L., and Artal, P. 2014. Pyreneplax basaensis new genus, new species (Decapoda, Brachyura, Vultocinidae) from the Priabonian (Late Eocene) of the Pyrenees of Huesca (Aragón, Spain), and remarks on the genus Lobonotus A. Milne-Edwards, 1863. Treballs del Museu de Geologia de Barcelona, 20:33-43.
https://doi.org/10.32800/tmgb.2014.20.0033
Ossó, À., Domínguez, J.L., De Angeli, A., and Ferratges, F.A 2020. First record of Dynomene (Brachyura, Dromioidea) from the Eocene of the Iberian Peninsula and remarks on the generic placement of Eoacantholobulus oscensis (Brachyura, Xanthoidea). Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen, 296:185-192.
https://doi.org/10.1127/njgpa/2020/0874
Porter, M.L., Pérez-Losada, M., and Crandall, K.A. 2005. Model-based multi-locus estimation of decapod phylogeny and divergence times. Molecular Phylogenetics and Evolution, 37:355--369.
https://doi.org/10.1016/j.ympev.2005.06.021
Puigdefàbregas, C. 1975. La sedimentación molásica en la cuenca de Jaca. Monografías del Instituto de Estudios Pirenaicos, Número extraordinario de Pirineos, 104:1-188.
Rathbun, M.J. 1924. Results of Dr. E. Mjöberg’s Swedish scientific expeditions to Australia 1910-1913. 37. Brachyura, Albuneidae and Porcellanidae. Arkiv för Zoologi, 16:1-33.
Rathbun, M.J. 1926. The fossil stalk-eyed Crustacea of the Pacific Slope of North America. United States National Museum Bulletin, 138:1-155.
Rathbun, M.J. 1935. Fossil Crustacea of the Atlantic and Gulf coastal plain. Geological Society of America Special Paper, 2:1-160.
Remacha, E., Fernández, L.P., and Maestro, E. 2005. The transition between sheet-like lobe and basin-plain turbidites in the Hecho Basin (South-Central Pyrenees, Spain). Journal of Sedimentary Research, 75(5):798-819.
https://doi.org/10.2110/jsr.2005.064
Rice, A.L. 1980. Crab zoeal morphology and its bearing on the classification of the Brachyura. Transactions of the Zoological Society of London 35:271-424.
https://doi.org/10.1111/j.1096-3642.1980.tb00060.x
Rice, A.L. 1983. Zoeal evidence for brachyuran phylogeny, p. 313-329. In Schram, F.R. (ed.), Crustacean Phylogeny. Crustacean Issues, 1.
Sakai, T. 1976. Crabs of Japan and the adjacent seas. Kodansha Ltd., Tokyo, Japan.
Samouelle, G. 1819. The entomologists’ useful compendium; or an introduction to the knowledge of British Insects, comprising the best means of obtaining and preserving them, and a description of the apparatus generally used; together with the genera of Linné, and modern methods of arranging the Classes Crustacea, Myriapoda, spiders, mites and insects, from their affinities and structure, according to the views of Dr. Leach. Also an explanation of the terms used in entomology; a calendar of the times of appearance and usual situations of near 3,000 species of British Insects; with instructions for collecting and fitting up objects for the microscope. Thomas Boys, London, UK.
Santana, W.R.A. 2008. Revisão taxonômica e relações filogenéticas em Inachoididae Dana, 1851 (Crustacea, Brachyura, Majoidea). PhD Thesis, University of São Paulo, São Paulo.
https://doi.org/10.11606/T.41.2008.tde-29012009-100852
Schweitzer, C.E. and Feldmann, R.M. 1999. Fossil decapod crustaceans of the late Oligocene to early Miocene Physt Formation and the late Eocene Quimper Sandstone, Olympic Peninsula, Washington. Annals of Carnegie Museum, 68:215-273.
Schweitzer, C.E. and Feldmann, R.M. 2010. New fossil decapod crustaceans from the Remy Collection, Muséum national d’Histoire naturelle, Paris. Geodiversitas, 32:399-415.
https://doi.org/10.5252/g2010n3a3
Schweitzer, C.E., Feldmann, R.M., Garassino, A., Karasawa, H., and Schweigert, G. 2010. Systematic list of fossil decapod crustacean species. Crustaceana Monographs, 10:1-222.
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. (eds.). 2020. Treatise Online, Part R, Revised. Volume 1, Chapter 8T11: Systematic descriptions: Superfamily Majoidea.
Silva-Casal, R., Aurell, M., Payros, A., Pueyo, E.L., and Serra-Kiel, J. 2019. Carbonate ramp drowning caused by flexural subsidence: the South Pyrenean middle Eocene foreland basin. Sedimentary Geology, 393:105538.
https://doi.org/10.1016/j.sedgeo.2019.105538
Smith, S.I. 1885. On some new or little known decapod Crustacea, from recent Fish Commission dredgings off the east coast of the United States. Proceedings of the United States National Museum, 7:493-511.
https://doi.org/10.5479/si.00963801.455.493
Spears, T., Abele, L.G., and Kim, W. 1992. The monophyly of brachyuran crabs: a phylogenetic study based on 18S rRNA. Systematic biology, 41:446-461.
Stimpson, W. 1871. Preliminary report on the Crustacea dredged in the Gulf Stream in the Straits of Florida by L. F. de Pourtalés, Assist. United States Coast Survey. Part I. Brachyura. Bulletin of the Museum of Comparative Zoology at Harvard College, 2:109-160.
Tavares, M. and Santana, W. 2018. Refining the genus Rochinia A. Milne-Edwards, 1875: reinstatement of Scyramathia A. Milne-Edwards, 1880 and Anamathia Smith, 1885, and a new genus for Amathia crassa A. Milne-Edwards, 1879, with notes on its ontogeny (Crustacea: Brachyura: Epialtidae). Zootaxa, 4418(3):201-227.
https://doi.org/10.11646/zootaxa.4418.3.1
Temminck, C.J. 1836. Coup-d’oeil sur la faune des Iles de la Sonde et de l’Empire du Japon. Discours préliminaire destiné a servir d’introduction a la Faune du Japon. Japan.
Tsang, L.M., Schubart, C.D., Ahyong, S.T., Lai, J.C., Au, E.Y., Chan, T.Y., Ng, P.K.L., and Chu, K.H. 2014. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Molecular Biology and Evolution, 31(5):1173-1187.
https://doi.org/10.1093/molbev/msu068
Vega, F.J., Gonzalez-Leon, O., and Moreno-Bedmar, J.A. 2019. Early Cretaceous (late Barremian) Crustacea from Puebla, Mexico. Journal of South American Earth Sciences, 96, 102330.
https://doi.org/10.1016/j.jsames.2019.102330
Via, L. 1959. Decápodos fósiles del Eoceno español. Boletín del Instituto geológico y minero española, 70:331-402.
Windsor, A.M. and Felder, D.L. 2014. Molecular phylogenetics and taxonomic reanalysis of the family Mithracidae MacLeay (Decapoda: Brachyura: Majoidea). Invertebrate Systematics, 28:145-173.
https://doi.org/10.1071/IS13011
Wolfe, J.M., Ballou, L., Luque, J., Watson-Zink, V.M., Ahyong, S.T., Barido-Sottani, J., Chan, T.Y., Chu, K.H., Crandall, K.A., Daniels, S.R., Felder, D.L., Mancke, H., Martin, J.W., Ng, P.K.L., Ortega-Hernández, J., Palacios-Theil, E., Pentcheff, N.D., Robles, R., Thoma, B.P., Tsang, L.M., Wetzer, R., Windsor, A.M., and Bracken-Grissom, H.D. 2022. Convergent adaptation of true crabs (Decapoda: Brachyura) to a gradient of terrestrial environments. bioRxiv.
https://doi.org/10.1101/2022.12.09.519815
Wolfe, J.M., Breinholt, J.W., Crandall, K.A., Lemmon, A.R., Moriarty-Lemmon, E., Timm, L.E., Siddall, M.E., and Bracken-Grissom, H.D. 2019. A phylogenomic framework, evolutionary timeline, and genomic resources for comparative studies of decapod crustaceans. Proceedings of the Royal Society B: Biological Sciences, 286:20190079.
https://doi.org/10.1098/rspb.2019.0079
Zariquiey-Álvarez, R. 1968. Crustáceas decápodos ibéricos. Investigación Pesquera, Barcelona, Spain.

