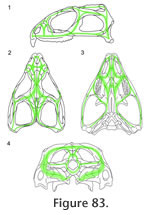
|
|
|
SKULL MECHANICS IN SPHENODONAlmost all of the cranial joints examined in Sphenodon remain patent through life, which suggests following the majority of skull growth they remain important to skull function. Using inferences similar to those employed by Herring (1972), Buckland-Wright (1978) and Taylor (1992), we combine our observations on cranial joints, with current understanding of the jaw muscles (e.g., Jones et al. 2009) and observed skull architecture to develop a series of hypotheses about skull biomechanics in Sphenodon. Forces Acting on the SkullForces acting on the skull in the living animal include gravity and occasional blows during fighting (Seligman et al. 2008; Jones and Lappin 2009) and prey acquisition, but most mechanical stress will be experienced during feeding: the temporal region and skull roof will be pulled downward as the jaw adductor muscles contract; the back of the cranium will be pushed up and to some extent anteriorly as the lower jaws are pulled against the quadrate condyles by the jaw adductor muscles; and the maxillae and other facial bones will be pushed dorsally as the jaws are brought against resistant food items. Therefore, in a very general way, we may expect the skull to act like a beam subject to three-point bending with compression along the dorsal surface and tension along the ventral margin (Taylor 1992; Russell and Thomason 1993; Weishampel 1993; Preuschoft and Witzel 2002; Henderson 2002; Rafferty et al. 2003). However, this simple model is complicated by several other considerations:
These additional considerations highlight the unique feeding apparatus of Sphenodon and make it unlikely that the skull will behave exactly as a beam. Others (e.g., Thomason et al. 2001) have suggested that a tube might be a better model, at least for mammal skulls, but they also acknowledged that forces in the skull can be highly localised, as shown by Herring and Teng (2000). Thus although bite force was recently obtained for adult Sphenodon (Jones and Lappin 2009; Herrel et al. 2010), the nature and magnitude of the stress generated during biting will vary depending on the exact location of the bite, gape and the amount of force used. Ridges and ThickeningsAlthough the dermatocranial bones of vertebrates essentially develop as thin flat sheets, the adult elements are more complex in their morphology and typically show variation in bone thickness resulting from remodelling during ontogeny. This remodelling is thought to be a response to varying levels of mechanical loading within the skull, leading to the concept of 'Benninghoff trajectories' (Benninghoff 1934; Lehman 1973a, 1973b) whereby the areas of thickening form a network of reinforcing bony ridges (Benninghoff 1934; Fox 1964; Herring 1972; Lehman 1973a, 1973b; Buckland-Wright 1978; Lanyon 1980; Reisz 1981; Taylor 1992; Lieberman 1997; Thomason et al. 2001). In experiments that simulated biting action in dried skulls, Buckland-Wright (1978) found that these thickenings ("continua") were associated with the alignment of surface strains.
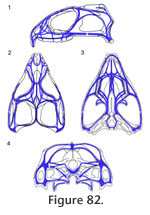
Overall the HCST predicts that compressive stress in Sphenodon is being resisted around the orbits, postfrontals, and quadratojugal foramina, and along the lower temporal bars, the upper margin of the upper temporal fenestrae, the margins and midline of the palate, and the margins of the quadrate-pterygoid wings.
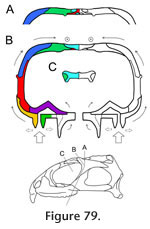
Preuschoft and Witzel (2002, text figures 4b, 5b, 5g, 12b) proposed a hypothesis of stress distribution during biting in Sphenodon, using lateral, dorsal, ventral and occipital views of the skull and depicting zones of expected tension and compression. It posits that the dorsal and occipital surfaces of the skull are held mainly in compression whereas the ventral part of the skull (including the lower temporal bar) is primarily in tension, with the postorbital bar and quadrate-pterygoid wings as "distance elements" within a neutral axis. In this scheme the skull is acting like a beam held posteriorly and loaded anteriorly. The fact that the skull roof of Sphenodon is composed of thicker bone than the palate lends some support to this interpretation but, as discussed in the previous section, a simple beam model may not be appropriate. The details of the skeletal architecture described here suggest that the lower temporal bars, postorbital bars, central part of the palate and edges of the palate at some point undergo compressive forces. Tensile forces are likely to be resisted by the fibrocellular sutures, tendons, fascia, skin and other soft tissues (Preuschoft and Witzel 2002) The Cranial JointsGiven the hypothesis of loading represented by the HCST, we would predict that key cranial joints would be those: between the premaxilla, maxilla and neighbouring bones in the rostrum; along the longitudinal axis of the skull roof; resisting the pull and torsional effects of the jaw adductor muscles; supporting the jaw joint around the suspensorium; and reinforcing the palate against bending. RostrumThe primary forces experienced by the rostrum will be those generated by loading on the anterior premaxillary chisel teeth and/or on the anterior maxillary teeth. The former may occur when the premaxillary teeth are used to impale relatively large prey (Gorniak et al. 1982, p. 337). This behaviour will generate dorsally directed forces within the premaxilla relative to the rest of the skull, resulting in dorsal shear in the premaxilla-nasal joints and ventral tension in the premaxilla-vomerine and premaxilla-maxilla joints (Taylor 1992; Preuschoft and Witzel 2002; Rafferty et al. 2003). The premaxilla-nasal joint will resist excessive posterodorsal slippage of the premaxilla (Figure 12, Figure 13) whereas the extensive soft tissue between the base of the premaxilla and its neighbouring bones (vomer, maxilla) may permit some limited separation. This location of flexibility may also be important during prooral jaw movement when the premaxillary chisel tooth is contacted by the lower jaw. The anterior maxilla will be loaded when Sphenodon uses its caniniform teeth (Figure 5.1), with compressive forces being directed up the anterior edge of the facial process (Figure 82.1) into the nasal and prefrontal. The rather box-like or tubular cross-section of the rostrum at this level is shaped to resist both bending and torsional stress (Preuschoft and Witzel 2002; Rafferty et al. 2003), aided by soft tissue in the large overlaps between the prefrontal, nasal and maxilla. The maxilla-nasal slot joint (Figure 17) may serve to support the maxilla as the lower jaw pushes gripped food forward against the maxillary teeth during prooral shearing. The Skull RoofThe skull roof may be subject to long axis bending because of upward force from the teeth and downward force from the adductor muscles (Taylor 1992; Russell and Thomason 1993; Rayfield 2004, 2005a, 2005b), aggravated by upward forces from the jaw joints and a posterior pull from the neck muscles. There will also be torsion across the orbital region, especially during unilateral biting, and mediolateral tension due to the pull of the adductor muscles. Longitudinal bending in the antorbital skull is resisted by the long overlapping nasal-frontal joint that resists significant separation while allowing small anteroposterior adjustive movements (as postulated for Allosaurus, Rayfield 2005b). The longitudinal ridges on the frontal and nasal facets (Figure 41, Figure 43) may help translate any small mediolateral movements (caused by torsion) into anteroposterior movement. Longitudinal bending of the parietal will be resisted by its dorsoventral expansion. The HCST suggests that compressive forces in the skull roof will converge between the orbits and in front of the upper temporal fenestrae (Figure 82). Significantly, this is where some of the most heavily interlocked joints are located (Figure 77, e.g., frontal-prefrontal, frontal-postfrontal, postorbital-postfrontal). The frontal-parietal joint is relatively narrow in Sphenodon but the interlocking structure maintains a rigid connection, resisting both dorsoventral bending forces and mediolateral torsional forces. It is reinforced laterally by the large spanning postfrontals (Figure 77.2). The interfrontal joint may act as a 'keystone' (Figure 79) to the arches of the orbits with forces travelling posteriorly up the postorbital bars (Figure 82) and being directed transversely against each other in another arch meeting around the postfrontal-parietal-frontal suture (Figure 82). As previously suggested (e.g., Arnold 1998), the complex joints between the parietal, frontal and postfrontals would prevent fronto-parietal hingeing ('mesokinesis'). Even in the hatchling Sphenodon where there is a large midline fontanelle, overlaps lateral to this make bending at this joint unlikely (Rieppel 1992). The Temporal Region and Adductor MusclesThe jaw adductor muscles originate from the sides of the parietal, from the proximal parts of the postorbital and post-temporal bars, and from fascia over the lower temporal fenestra (Gorniak et al. 1982; Jones et al. 2009). Contraction of these muscles during biting imposes powerful anteroventral, anterolateral and ventrolateral forces on the posterior skull roof and temporal region (e.g., Beherents et al. 1978; Herring and Teng 2000; Sun et al. 2004; Byron et al. 2004). At the same time, the region will be subject to a posterodorsal force from the maxilla anteriorly, and a strong upward force from the jaw joint posteriorly. Hence, there may be shear between the component parts, as well as torsion in the upper and lower temporal bars during unilateral biting. Tension across the interparietal joint is resisted by soft tissue spanning the deep contact surface. Anteriorly, as in other amniotes, the convex "arch" formed by the postorbital bars would resist the downward pull of the adductor muscles (Frazzetta 1968), aided by the tight fit of the postorbital-postfrontal joint and, further ventrally, by the tall medial process of the jugal which is positioned to brace the postorbital (Figure 79). The long overlapping postorbital-squamosal joints in the upper temporal bars (Figure 62) should allow the small adjustive movements necessary to reduce torsion and shearing in this part of the skull. Similarly, as the plane of the jugal-postorbital joint is almost parallel to the orbital margin, the intrasutural collagen fibres should be orientated perpendicular to resist posterodorsally directed forces from the upper jaw (Figure 82). The parietal-squamosal joint alternatively resists the anteroventral pull of the m. adductor mandibulae externus and posterior pull of the m. depressor mandibulae and neck muscles (Gorniak et al. 1982; Al-Hassawi, 2007; Curtis et al. 2009; Jones et al. 2009). The slotted joint allows fibres to be arranged to resist both these movements. The Jaw Joint and SuspensoriumThe forces generated at the jaw joint may be greater than those from the upper tooth row as the jaw joint is closer to most of the muscles (Crompton and Parker 1978; Jones et al. 2009). The exact direction of these forces will also vary as gape and muscle activity changes over the course of a bite and swallowing cycle. Fusion of the quadrate and quadratojugal may reflect the need to further strengthen this part of the skull (Herring 2000). The jugal-squamosal and jugal-quadratojugal joints appear well-structured to prevent the posterior process of the jugal from rotating medially and/or the posteroventral corner of the skull from twisting laterally. The near vertical facet surfaces permit intersutural soft tissue to be orientated so as to prevent excessive shear or torsion between the lower temporal bar and squamosal-quadratojugal. The joint between the squamosal and quadrate-quadratojugal is roughly parallel to the jaw joint (Figure 77) and is probably important for transmitting forces from the jaw joint to neighbouring parts of the skull (Figure 82). The lateral part of the interlocking quadrate-squamosal joint appears already well established in hatchlings (Rieppel 1992) but the medial lappet that supports the quadrate in adults does not. The presence of a synovial cavity within the quadrate-squamosal joint (Rayfield 2005a; Jones 2006; Holliday and Witmer 2008) needs to be confirmed by direct observation or histological sections, but it could be important for dissipating compressive forces from both the jaw joint and jaw muscles. Medially, the quadrate-pterygoid joint may be strained as the pterygoid muscles pull the two bones down but the quadrate is pushed upward by the lower jaw. However, excessive movements of the quadrate on the pterygoid would be resisted by the strong overlap (Figure 77), present even in hatchlings, whereas the deep pterygoid-quadrate wings will resist dorsoventral bending while probably allowing for some lateral deflection during swallowing. The PalateDuring biting the palatal bones and joints in Sphenodon are subject to forces from the marginal and palatal tooth rows; from the lower jaws pushing against the pterygoid flanges (Taylor 1992); from the dentary teeth wedging between the maxillary and palatine tooth rows during prooral shear; and from the actions of the deeper adductor muscles pulling the braincase (e.g., m. adductor mandibular profundus) and jaws (e.g., m. pterygoideus) against the pterygoids (Haas 1973; Gorniak et al. 1982; Wu 2003; Curtis et al. 2009; Jones et al. 2009).
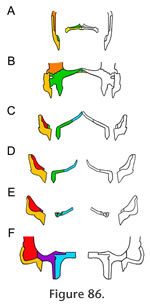
Together, the maxilla and jugal form much of the lateral wall of the skull as well as the margins of the palate. The maxilla-jugal joint is thus crucial to the overall stability of the skull, linking its anterior and posterior halves. The posterior end of the maxilla is dorsally and medially expanded, giving it an 'L' shape in cross-section (Figure 86). This shape strengthens the long axis of the bone against both dorsoventral bending and torsion, and braces the jugal medially. Medial movement of the jugal (caused by m. adductor mandibulae externus superficialis sensu stricto pulling on the lower temporal fascia and postorbital, Jones et al. [2009]) would also be resisted by the palatine and ectopterygoid.
The Metakinetic AxisMetakinesis is a movement of the whole braincase against the rest of the skull. It occurs in at least some lizards (e.g., Herrel et al. 1999, Evans 2008) but distribution within other amniotes remains uncertain. In Sphenodon, the joints between the braincase and the dermal skull do not appear to allow metakinetic movements comparable to those found in some lizards (contra Gardiner 1983, p. 50). Thus, the constrictor dorsalis muscles (Johnston 2010), important in lizard metakinesis (e.g., Evans 2008), are more likely to be important for proprioception and support in Sphenodon (Evans 2008; Johnston 2010). Nevertheless, the small movements that doubtless exist between the skull and braincase, particularly at the synovial basipterygoid joint, would help to transmit or dissipate stress between the braincase and the rest of the skull (Evans 2008; Johnston 2010). |
|