|
|
|
MATERIAL AND METHODSkeletal material of Sphenodon from a number of collections was examined:
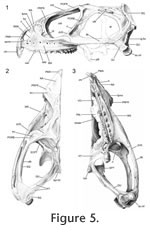
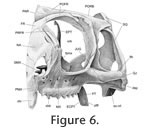
A All drawings were made using a Wild stereomicroscope with camera lucida. Sand was used to control and maintain orientation. In general, lighting was directed on to the specimens from the top left hand corner, although for some facets low angled lighting was used to examine and draw particularly subtle texture. As stated in some figure captions, drawings were occasionally made in a view perpendicular to the surface of a facet rather than of the bone as a whole. The sutures of adult Sphenodon were also manually segmented in two specimens using data from microCT. The first specimen, YPM 9194, was segmented using VG Studio MAX (Volume Graphics GmbH, Heidelberg, Germany) under the supervision of Dr Jessie Maisano at the High-Resolution X-ray Computed Tomography Facility, University of Texas, Austin, USA. The second specimen, LDUCZ x036, was segmented at UCL using AMIRA (Visualization Sciences Group, Burlington MA, USA) after micro-CT scanning at the University of Hull, UK.
Digital imaging software, 'Adobe Photoshop 2.0' (Adobe Systems, San Jose, California), was used to combine drawings of bones with the "ghosts" of overlying or underlying bones. Once all the sutures had been assessed in detail, summary diagrams were produced. These include outlines of the skulls in lateral, ventral, dorsal, and occipital view with the inferred areas of underlying bone superimposed (e.g., Bolt and Wassersug 1975; Busbey 1995; Clack 2002; Jenkins et al. 2002). In addition, diagrammatic cross-sections were produced. A consistent colour code is used throughout for each bone (Table 3). A small problem requiring consideration is the slight distortion of bone shape caused by dehydration (Tyler 1976, p. 6), particularly in long and thin bones or processes. Together with loss of soft tissue this is probably why bones in articulated dry skulls may separate slightly (e.g., maxillae in LDUCZ x343). This distortion can make rearticulation difficult. The anterior superior process of the squamosal in DGPC1, for example, has split, probably due to the loss of the organic component and subsequent dehydration. Another factor inhibiting accurate rearticulation is that in life soft tissue may push individual bones against each other or pull bones away from each other. In addition, small thin processes are delicate and liable to breakage, e.g., the triangular tips of the anterodorsal processes of the squamosal in DGPC1. Cleaning and disarticulation of the skull may also produce artificial surface texture. The full history of DGPC1 is unknown, including how the bulk of the soft tissue was removed and whether solvents were used to clean the surface. |
|