Application of shell spiral deviation methodology to fossil brachiopods: Implications for obtaining specimen ontogenetic ages
Application of shell spiral deviation methodology to fossil brachiopods: Implications for obtaining specimen ontogenetic ages
Article number: 18.3.54A
https://doi.org/10.26879/577
Copyright Palaeontological Association, December 2015
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 4 June 2015. Acceptance: 4 November 2015
{flike id=1189}
ABSTRACT
Knowledge of specimen ontogenetic ages of fossil brachiopods has great utility in paleoecological and paleoclimatic studies, but there is currently no efficient means of obtaining this information. Previous studies have shown that shell spiral deviations correspond to ontogenetic ages and seasonal growth in modern brachiopods, but this approach has not been tested on fossil specimens. Here, we analyze the application of this methodology using four species of fossil brachiopods, including Laqueus rubellus, Terebratula terebratula, Platystrophia ponderosa, and Pseudoatrypa sp., each of which possesses different shell outlines and surface ornamentation patterns. The computer programs Vextractor and R were used for digitizing and morphometric analyses, respectively. Our results indicate that smooth biconvex shells produce spiral deviation graphs similar to those generated from modern brachiopods that yield plausible ontogenetic ages, but without providing by themselves information on seasonal growth and factors controlling shell growth.
Joanna V. Clark. Department of Geological Sciences, University of Alabama, 2003 Bevill Building, Tuscaloosa, Alabama, 35487, United States. jvclark@crimson.ua.edu
Anthony E. Aldridge. PO Box 19576, Woolston, Christchurch, 8241, New Zealand. tony@southnet.co.nz
Matías Reolid. Departamento de Geología, Universidad de Jaen, Campus Las Lagunillas s/n, 23071 Jaén, Spain. mreolid@ujaen.es
Kazuyoshi Endo. Department of Earth and Planetary Sciences, The University of Tokyo, Tokyo 113-003, Japan. endo@eps.s.u-tokyo.ac.jp
Alberto Pérez-Huerta. Department of Geological Sciences, The University of Alabama, Tuscaloosa, Alabama, 35487, United States. aphuerta@ua.edu
Keywords: spiral deviations; fossil brachiopods; ontogenetic age; digitizing; R code
Final citation: Clark, Joanna V., Aldridge, Anthony E., Reolid, Matías, Endo, Kazuyoshi, and Pérez-Huerta, Alberto. 2015. Application of shell spiral deviation methodology to fossil brachiopods: Implications for obtaining specimen ontogenetic age. Palaeontologia Electronica 18.3.54A: 1-39. https://doi.org/10.26879/577
palaeo-electronica.org/content/2015/1189-brachiopod-spiral-deviations
INTRODUCTION
Knowledge of ontogenetic ages of fossil specimens is essential to many studies focused on phylogeny, life histories, and population dynamics. Within marine invertebrates, brachiopods have been intensely studied because they have a long geologic history, their shells are resistant to diagenesis, they are geographically widespread, and they have lived in a variety of marine habitats throughout the Phanerozoic (e.g., Brand et al., 2011; Ivany, 2012; Pérez-Huerta et al., 2014). Brachiopod shells also demonstrate morphologies reflecting ontogeny throughout their lives, as do other invertebrates secreting a shell, and thus, it is important to consider ontogenetic ages and variations during ontogeny when making taxonomic descriptions because of the implications on phylogeny and evolutionary patterns (e.g., Beecher, 1893; Balthasar, 2004; Pérez-Huerta, 2004). Furthermore, life history of an organism is another area of research that requires the knowledge of specimen ontogenetic age, and many of these studies investigate how organisms change their life habits throughout ontogeny (e.g., Tomašových et al., 2008). Finally, population dynamics require the knowledge of specimen ontogenetic age; for example, brachiopod ontogenetic age has been used to determine the effect of current-sorting on the ontogenetic age frequencies in fossil assemblages (e.g., Boucot, 1953; Boucot et al., 1958).
Brachiopods have also been particularly useful in reconstructing past ecosystems and climatic conditions because they precipitate calcite shells in oxygen isotopic equilibrium with ambient seawater, and these shells are very resistant to diagenesis (e.g., Carpenter and Lohmann, 1995; Geldern et al., 2006; Suan et al., 2008; Yamamoto et al., 2010; Brand et al., 2013). Thus, studies have been conducted to investigate the effectiveness of brachiopod shells as paleoclimate indicators at different stages during their ontogeny (e.g., Buening and Spero, 1996; Yamamoto et al., 2010; Kercher and Carlson, 2012). Additionally, studies that relate shell morphology to seasonal growth and specimen ontogenetic age have the potential to provide a record of seasonality throughout geologic history (Pérez-Huerta et al., 2014; Butler et al., 2015).
Despite numerous studies using brachiopods in paleoecology and as bioarchives for paleoclimate reconstructions, obtaining an accurate and efficient method of determining specimen ontogenetic age in fossil specimens is elusive. Modern brachiopod ontogenetic ages and growth rates have been studied based on in situ shell measurements from living brachiopods (Terebratalia transversa and Terebratulina retusa), although these studies are sparse (e.g., Thayer, 1977; Curry, 1982). Approaches using a combination of shell chemistry and growth lines have been used to determine growth rates of modern specimens, (Barbin and Gaspard, 1995; Yamamoto et al., 2010), but both of these techniques have associated problems (e.g., interpretation of growth lines) that limit their effectiveness (see Pérez-Huerta et al., 2014 and references therein).
Most recently, shell spiral deviations have been used to determine ontogenetic ages of modern brachiopods, where deviations of the shell outline from a perfect logarithmic spiral represent changes in growth (Aldridge and Gaspard, 2011 and references therein). A perfect logarithmic spiral is a shape that maintains the same rate of vertical to anterior growth and has been shown to resemble the growth patterns of organisms that grow their shells by accretion (see Aldridge, 1998 and references therein). Thus, logarithmic spirals have been used to precisely describe the shape of shells with accretionary growth (e.g., Aldridge, 1998, Pérez-Huerta et al., 2014). Although spiral deviations have been observed in modern brachiopods and linked to ontogenetic age and seasonal growth, it has not yet been shown to be fully effective in the study of fossil specimens (Angiolini et al., 2012). In this study, fossil brachiopods, ranging in age from the Ordovician to the Pleistocene, are analyzed to determine whether shell spiral deviations are similar to those observed in modern brachiopods and are potentially useful in specimen ontogenetic age determination. The main focus of this study is the R-based spiral deviation methodology for determining ontogenetic ages in fossil brachiopods. Discussion of problems inherent to this methodology and related to different shell morphologies of fossil specimens is included.
MATERIALS AND METHODS
Sample Information
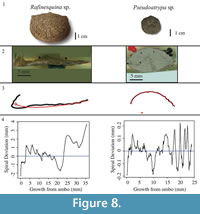
 The studied specimens are well-preserved shells of four species of fossil brachiopods, Laqueus rubellus (Family Laqueidae), Terebratula terebratula (Family Terebratulidae), Platystrophia ponderosa (Family Plectorthidae), and Pseudoatrypa sp. (Family Atrypidae) (Figure 1). Shell parameters (length, width, and thickness) and spiral deviations (Table 1) were determined for three specimens of each species, with larger specimens selectively chosen to maximize the shell growth record (see Pérez-Huerta et al., 2014). All four species possessed biconvex shells without ornamentations, such as spines, but shells with ribs and growth lamellae are included (Figure 1). In addition to the four species analyzed, the ventral valve of a strophomenid brachiopod, Rafinesquina sp., was analyzed for its spiral deviations to determine if they could be detected in shells with flatter convexities.
The studied specimens are well-preserved shells of four species of fossil brachiopods, Laqueus rubellus (Family Laqueidae), Terebratula terebratula (Family Terebratulidae), Platystrophia ponderosa (Family Plectorthidae), and Pseudoatrypa sp. (Family Atrypidae) (Figure 1). Shell parameters (length, width, and thickness) and spiral deviations (Table 1) were determined for three specimens of each species, with larger specimens selectively chosen to maximize the shell growth record (see Pérez-Huerta et al., 2014). All four species possessed biconvex shells without ornamentations, such as spines, but shells with ribs and growth lamellae are included (Figure 1). In addition to the four species analyzed, the ventral valve of a strophomenid brachiopod, Rafinesquina sp., was analyzed for its spiral deviations to determine if they could be detected in shells with flatter convexities.
Laqueus rubellus (Sowerby, 1846).Shells of Laqueus (Figure 1) were collected from the Jizodo Formation (0.39 Ma) and the Yabu Formation (0.31 Ma) of the Shimosa Group in central Japan. The length of these shells ranges from 23.2 to 29.3 mm. The depositional environment of specimen collection is representative of a shallow embayment, known as the paleo-Tokyo Bay. More specifically, the Jizodo Formation contains facies associated to littoral and sub-littoral zones (Endo, 1987). Original bilateral symmetry in these specimens is preserved, suggesting that these units are not tectonically deformed (Figure 1). Additionally, previous studies of this unit do not suggest significant tectonic deformation (Endo, 1987). Fossil specimens of this species were selected as a “control group” because equivalent modern individuals can be collected from the Sagami Bay at water depths between 70 to 90 m and have been used to determine shell spiral deviations (Pérez-Huerta et al., 2014).
Terebratula terebratula (Linnaeus, 1758). Shells ranging from 38.7 to 47.3 mm in length (Figure 1) were collected from Tortonian (Upper Miocene) deposits of the Guadix Basin, a Neogene intra-mountain basin located in southern Spain. They correspond to accumulations in prodelta facies with an inferred depth around 30 m (see more details in Reolid et al., 2012). Original bilateral symmetry in these specimens is preserved, suggesting that these units are not tectonically deformed (Figure 1). Additionally, tectonic deformation was not reported in previous descriptions of this unit (Reolid et al., 2012).
Pseudoatrypa sp. Shells ranging from 19.0 to 21.0 mm in length (Figure 1) are Early Devonian in age and were sampled from an active quarry in Parson, Tennessee. Samples were collected from the Ross Formation, which has been interpreted to represent a shallow marine shelf depositional environment, below normal wave base but above storm wave base (Rigby and Clement, 1995). Original bilateral symmetry in these specimens is preserved, suggesting that these units are not tectonically deformed (Figure 1). Additionally, the literature reference for this unit does not suggest significant tectonic deformation.
Platystrophia ponderosa (King, 1850). Shells ranging from 23.9 to 35.5 mm in length (Figure 1) are Late Ordovician in age and were collected from the Arnheim Formation in southwestern Ohio. This formation is representative of a shallow sub-tidal depositional environment (Holland, 2013). Original bilateral symmetry in these specimens is preserved, suggesting that these units are not tectonically deformed (Figure 1). Additionally, the literature reference for this unit does not suggest significant tectonic deformation.
Rafinesquina sp. Specimen is Late Ordovician in age and was sampled from the Leipers Formation of the Maysville Group in south-central Tennessee. The lower Leipers Formation has been interpreted as a lagoon depositional environment (Wahlman, 1992). Original bilateral symmetry in these specimens is preserved, suggesting that these units are not tectonically deformed. Additionally, the literature reference for this unit does not suggest significant tectonic deformation.
 Sample Preparation
Sample Preparation
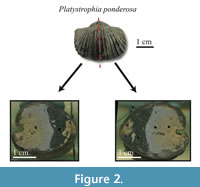
Samples were brushed off with Kim-Wipes to ensure that there was minimal unconsolidated material adhered onto the outside of the shells. Any non-shell material on the shell surface can make digitizing the shell outline difficult because it becomes unclear where the edge of the shell is located. Shells were then embedded in epoxy resin and cut with a Buehler Isomet 1000 Precision Saw along the plane of symmetry, producing two identical halves (Figure 2). The shell surface of each half was ground and polished with aluminum oxide (grit size = 1.0 and 0.3 μm) until the surface was flat and free of scratches.
Digitizing
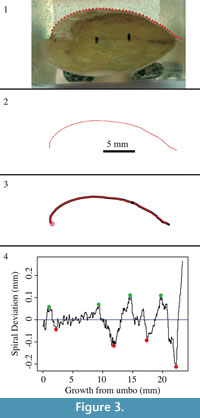 A 5 mm scale was drawn onto the non-shell portion of the cross section of each shell (Figure 3). Both halves of each shell were then scanned into a computer, producing high-resolution TIFF images of shell cross-sections. These images were edited in Adobe Photoshop, with dorsal valves on top and curved in a counter-clockwise direction from the umbo. The edited images were then imported into the image digitization software called Vextractor (Version 6.31; VextraSoft, Vladivostok, Russia; available at www.vextrasoft.com).
A 5 mm scale was drawn onto the non-shell portion of the cross section of each shell (Figure 3). Both halves of each shell were then scanned into a computer, producing high-resolution TIFF images of shell cross-sections. These images were edited in Adobe Photoshop, with dorsal valves on top and curved in a counter-clockwise direction from the umbo. The edited images were then imported into the image digitization software called Vextractor (Version 6.31; VextraSoft, Vladivostok, Russia; available at www.vextrasoft.com).
 It is important to digitize only one valve at a time so that each valve has its own set of (x,y) coordinates that can be analyzed separately for spiral deviations. Before digitizing, the scale that was drawn on the cross-section was used to create reference coordinates on the digital image (Figure 3). The shell was digitized with a polyline tool by clicking the edge of the shell surface, at < 0.1 mm intervals, from the umbo to anterior shell regions. After the valve was digitized, the outline was selected and the vector was saved in ASCII format as a xyz file. The xyz file was then opened and saved as a Microsoft Excel file for future use.
It is important to digitize only one valve at a time so that each valve has its own set of (x,y) coordinates that can be analyzed separately for spiral deviations. Before digitizing, the scale that was drawn on the cross-section was used to create reference coordinates on the digital image (Figure 3). The shell was digitized with a polyline tool by clicking the edge of the shell surface, at < 0.1 mm intervals, from the umbo to anterior shell regions. After the valve was digitized, the outline was selected and the vector was saved in ASCII format as a xyz file. The xyz file was then opened and saved as a Microsoft Excel file for future use.
Spiral Deviations
The coordinates for each specimen were imported into a free, downloadable computer program called R (Version 3.0.2; R Core Team, 2013), which is oftentimes used in biology and statistics (e.g., Zuur et al., 2009; Aldridge and Gaspard, 2011). These coordinates were used with R code previously developed (see Aldridge and Gaspard, 2011; Appendix 1, Appendix 2, Appendix 3, and Appendix 4) to produce biological outlines as well as spiral deviation graphs (Figure 3).
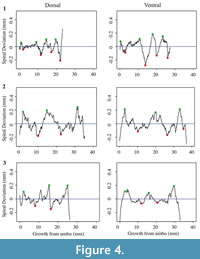
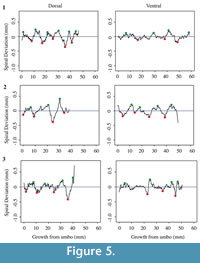
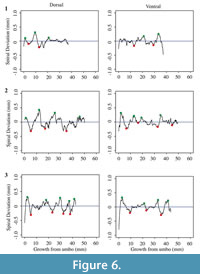
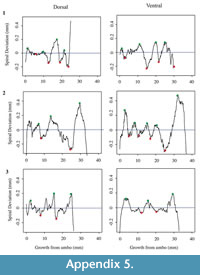
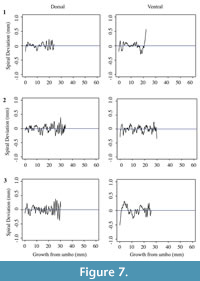
Each shell has a spiral deviation graph for both the dorsal and ventral valves of each half (Figure 4, Figure 5, Figure 6, and Figure 7; Appendix 5). It is important that the coordinates, saved in Microsoft Excel files, be imported and analyzed separately. The basic procedure of creating spiral deviation graphs is as follows, and it is described in more detail in the appendices. The x and y coordinates are defined as objects in R (Appendix 1). A biological outline of a valve is created (Appendix 2). The spiral deviation code is entered into R (Appendix 3). A spiral axis location is manually chosen. R estimates the best spiral axis location based on the initial location that is chosen manually (Appendix 4). Code is used to plot spiral deviations versus growth from umbo (Appendix 4).
RESULTS
Specimens of Laqueus rubellus, Terebratula terebratula, and Platystrophia ponderosa produced spiral deviations patterns that were wide and large in magnitude (Figure 4, Figure 5, and Figure 6), while specimens of Pseudoatrypa sp. produced spiral deviations patterns that were large in amplitude, and frequent or very narrow in width (Figure 7). Shells of Laqueus rubellus produced between two and four spiral maxima and minima per valve (Figure 4 and Table 1), between three and six spiral maxima and minima per valve for shells of Terebratula terebratula (Figure 5 and Table 1), and between two and five spiral maxima and minima per valve for shells of Platystrophia ponderosa (Figure 6 and Table 1). In contrast to these species, specimens of Pseudoatrypa sp. produced many spiral deviations, resulting in numerous potential maxima and minima for both valves. Finally, it was impossible to fit a perfect spiral to the ventral valve of one specimen of Rafinesquina sp. because of its flat shape.
DISCUSSION
Which Shells Produce Annual Spiral Deviations?
Biconvex shells without ornamentation, such as ribs and spines, as in the case of Laqueus rubellus and Terebratula terebratula, produced shell spiral deviations that were wide and large in magnitude, and similar to those described for modern brachiopod shells with biological significance (Aldridge and Gaspard, 2011; Pérez-Huerta et al., 2014). Pseudoatypa sp. displayed numerous shell spiral deviations that were narrow and large in magnitude, but these were caused by the combination of lateral ribs and growth lamellae, which produce noises masking any signals representing ontogenetic growth patterns (Figure 7 and Figure 8). Platystrophia ponderosa produced shell spiral deviations that were wide and large in magnitude, despite the presence of prominent ribs aligned along the anterior-posterior direction. When using shells that possess ribs aligned along the anterior-posterior direction or shells with a fold and sulcus, it is important to section the shell straight along the plane of symmetry. Cutting off the plane of symmetry or through ribs can produce spiral deviations that appear to have no biological significance. Because spiral deviations can be on the order of 0.1 mm in magnitude, the use of shells with a fold and sulcus or ribs can result in spiral deviations that are not due to ontogenetic growth if sample preparation is not precise. Therefore, shells without any ornamentation present the best potential for determining specimen ontogenetic age based on spiral deviations.
In addition to the work in selected species, a spiral deviations graph was generated from the ventral valve of a strophomenid brachiopod (Rafinesquina sp.) to determine if spiral deviations could be detected on shells with minimal ornamentation but flatter valves (Figure 8).
It was impossible to fit a spiral to this shell because of its flat shape, and therefore it could not be used to determine a specimen ontogenetic age. Overall, biconvex shells are the best target of study for ontogenetic age determination using the present methodology.
Is It Necessary to Fit More Than One Spiral?
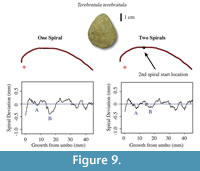
In some shells, the rate of anterior to vertical growth changes throughout the individual’s lifetime. A valve may be very rounded towards the umbo but flatter towards the anterior region. Abrupt changes in valve convexity have been observed in modern brachiopod species including Laqueus rubellus, where valve convexity changes from circular to ovular at maturity (Endo, 1987). Therefore, a spiral that fits the posterior region of the shell may not fit the anterior region of the shell. When one spiral is fit to an outline that experiences an abrupt change in growth, there will be a defined “M” shape in the spiral deviation graph that is due to a poor spiral fitting rather than to seasonal growth episodes (Figure 9). To solve the problem of poor spiral fitting, two or more spirals have to be fitted to the shell using the R code that we designed (Appendix 4). In this analysis, fitting two spirals to the shells instead of one resulted in a reduction in spiral fitting error (Figure 9), meaning that the spirals more closely fitted the outlines and any resulting deviations were more likely to have biological significance (Table 2, Table 3, Table 4, and Table 5).
Selecting a location where the second spiral starts is a user-involved process. The shell outline is composed of coordinates from the digitizing process, and every set of (x,y) coordinates has a point number. The point number “1” is the point closest to the umbo and higher numbers are closer to the anterior region of the shell. To find a spiral change location, the point number of the location where the shell shape changes has to be determined. Once an approximate location is determined, a spiral deviation graph is generated using this point number as the second spiral start location. If the “M” shape has been reduced, the spiral matches the biological outline better. Thus, the spiral fitting error has been reduced and the deviations are more likely to have biological significance (Figure 9).
 What Are Valid Criteria to Select Annual Spiral Deviations?
What Are Valid Criteria to Select Annual Spiral Deviations?
Determining quantitative criteria to define spiral deviations is problematic because growth rates are affected by many factors other than inter-species variation. Even within the same species, growth rates can vary because of individual genetics and health, degree of attachment, among others. Although growth rates vary from specimen to specimen, brachiopod growth rates tend to decrease with increasing ontogenetic age (e.g., Thayer, 1977; Endo, 1987). This trend has been demonstrated in many species of modern brachiopods (e.g., Terebratalia transversa), and has been observed in the spiral deviation graphs of some, but not all, of the specimens analyzed in this study (e.g., Figure 4.1 and Figure 6.3). In this study, quantitative criteria were set as to what defined spiral maxima and minima, but it is important to remember that there is a qualitative component to spiral deviation analysis because growth rates vary within the same species and tend to decrease with increasing ontogenetic age, as shown by the analysis of the
dorsal and ventral valves of three specimens of each taxon (Figure 4, Figure 5, Figure 6, and Figure 7).
 Previous work showed that spiral deviations greater than 0.025 mm in magnitude represented seasonal growth on the dorsal valves of fully matured, modern specimens of Laqueus rubellus (see Pérez-Huerta et al., 2014). In this study, deviations were interpreted as growth episodes if they were 0.05 mm above or below zero. These criteria yielded ontogenetic ages between three and four years (young adult specimens), and possessed length and width measurements that agree with growth rate analyses by Yamamoto et al. (2010). Specimens two and three had similar maximum lengths, and spiral deviation analyses showed that both specimens were approximately three years old at death (Table 1). These specimens were approximately one to two years younger than predicted by Yamamoto et al. (2010), based on their maximum lengths. Specimen one had a slightly smaller maximum length and yielded an ontogenetic age of four years, which strongly agreed with the growth rate analysis by Yamamoto et al. (2010). These slight differences are most likely the result of differing growth rates of individuals within the same species, reinforcing the idea that there is not a unique growth rate for a given brachiopod taxon (Pérez-Huerta et al., 2014).
Previous work showed that spiral deviations greater than 0.025 mm in magnitude represented seasonal growth on the dorsal valves of fully matured, modern specimens of Laqueus rubellus (see Pérez-Huerta et al., 2014). In this study, deviations were interpreted as growth episodes if they were 0.05 mm above or below zero. These criteria yielded ontogenetic ages between three and four years (young adult specimens), and possessed length and width measurements that agree with growth rate analyses by Yamamoto et al. (2010). Specimens two and three had similar maximum lengths, and spiral deviation analyses showed that both specimens were approximately three years old at death (Table 1). These specimens were approximately one to two years younger than predicted by Yamamoto et al. (2010), based on their maximum lengths. Specimen one had a slightly smaller maximum length and yielded an ontogenetic age of four years, which strongly agreed with the growth rate analysis by Yamamoto et al. (2010). These slight differences are most likely the result of differing growth rates of individuals within the same species, reinforcing the idea that there is not a unique growth rate for a given brachiopod taxon (Pérez-Huerta et al., 2014).
Thayer (1977) studied growth rates of the extant brachiopod Terebratalia transversa throughout ontogeny. His study found that the average growth rate for individuals less than 10 mm in length was approximately 3.6 mm/year and the average growth rate for individuals greater than 10 mm was approximately 1.5 mm/year.
 This study, as well as the spiral deviation study by Pérez-Huerta et al. (2014), was taken into account when choosing spiral maxima and minima for the species Terebratula terebratula, because there are no growth rate studies for this extinct species. Spiral deviations had to be 0.1 mm above or below zero to be considered a seasonal growth episode. Resulting ontogenetic ages ranged from three to approximately five years (Table 1), which appears to be reasonable because the abundance of specimens over the age of five is less than 1 % in other species of modern terebratulid brachiopods (e.g., Curry, 1982).
This study, as well as the spiral deviation study by Pérez-Huerta et al. (2014), was taken into account when choosing spiral maxima and minima for the species Terebratula terebratula, because there are no growth rate studies for this extinct species. Spiral deviations had to be 0.1 mm above or below zero to be considered a seasonal growth episode. Resulting ontogenetic ages ranged from three to approximately five years (Table 1), which appears to be reasonable because the abundance of specimens over the age of five is less than 1 % in other species of modern terebratulid brachiopods (e.g., Curry, 1982).
Ontogenetic studies of modern rhynchonellid brachiopods, which are phylogenetically related to the extinct species Platystrophia ponderosa, are less abundant and conclusive than for terebratulid taxa. Therefore, the same criteria that were used to define spiral deviations for Terebratula terebratula were used for P. ponderosa because of their similar sizes. The three specimens of P. ponderosa produced ontogenetic ages between 3 and 5 years, which appear plausible based on their relative sizes (Table 1).
The spiral deviation methodology applied to fossil brachiopod taxa produces encouraging results to determining ontogenetic growth and accurate specimen ontogenetic age estimates based on a comparison of growth in modern species. However, in order to better define criteria for what are actually meaningful spiral maxima and minima, high-resolution chemical analyses should be done at locations of spiral deviations to determine a correspondence to periodical environmental parameters (e.g., seawater temperatures; Buening and Spero, 1996; Yamamoto et al., 2010; Pérez-Huerta et al., 2014; Butler et al., 2015).
CONCLUSIONS
The methodology of shell spiral deviation applied to fossil brachiopods has great potential for utility in determining specimen ontogenetic ages, which has significant implications for the use of brachiopods in paleoclimatic and paleoecological studies (i.e., determining paleoseasonality). Our findings show that spiral deviations can be obtained for fossil taxa (Laqueus rubellus, Terebratula terebratula, and Platystrophia ponderosa), yielding specimen ontogenetic ages that agree with previous growth rate studies in similar modern taxa and are plausible based on relative shell measurements. In contrast, results indicate the problems of using this methodology with shells that have complex ornamentation patterns (Pseudoatrypa sp.) or without biconvex shells (Rafinesquina sp.). Also, a refinement of the methodology as well as analyzing more shells within a wider ontogenetic sampling dataset is needed to define more objective criteria to detect “true” or “significant” maxima and minima deviations. Furthermore, the method can determine specimen ontogenetic ages assuming that the spiral deviations chosen are due to seasonal growth. Thus, conducting a chemical analysis at locations of spiral deviations on the shell outline would help confirm the criteria for deviations that are due to seasonal growth episodes.
ACKNOWLEDGMENTS
This research was partially funded by the University of Alabama Graduate Student Travel Fund, the DGS Hook’s Fund, and the Johnson Travel Fund. S. Ebersole (Alabama Geological Survey) is thanked for facilitating specimens of P. ponderosa and R. Keyes for providing specimens of Pseudoatrypa sp. and Rafinesquina sp.
REFERENCES
Aldridge, A.E. 1998. Brachiopod outline and the importance of the logarithmic spiral. Paleobiology, 24: 215-226.
Aldridge, A.E. and Gaspard, D. 2011. Brachiopod life histories from spiral deviations in shell shape and microstructural signature-preliminary report. Memoirs of the Association of Australian Palaeontologists, 41:257-268.
Angiolini, L., Stephenson, M., Leng, M.J., Jadoul, F., Millward, D., Aldridge, A., Andrews, J., Chenery, S., and Williams, G. 2012. Heterogeneity, cyclicity and diagenesis in a Mississippian brachiopod shell of palaeoequatorial Britain. Terra Nova, 24:16-26.
Balthasar, U. 2004. Shell structure, ontogeny and affinities of the Lower Cambrian bivalve problematic fossil Michwitzia muralensis Walcott, 1913. Lethaia, 27:381-400.
Barbin, V. and Gaspard, D. 1995. Cathodoluminescence of recent articulate brachiopod shells: Implications for growth stages and diagenesis evaluation. Geobios (Special Memoir), 18:39-45.
Beecher, C.E. 1893. Some correlation of ontogeny and phylogeny in the brachiopoda. The American Naturalist, 27:599-604.
Boucot, A.J. 1953. Life and death assemblages among fossils. American Journal of Science, 251:25-40.
Boucot, A.J., Brace, W., and Demar, R. 1958. Distribution of brachiopod of pelecypod shells by currents. Journal of Sedimentary Petrology, 28:321-332.
Brand, U., Azmy, K., Bitner, M.A., Logan, A., Zuschin, M., Came, R., and Ruggiero, E. 2013. Oxygen isotopes and MgCO3 in brachiopod calcite and a new paleotemperature equation. Chemical Geology, 359:23-31.
Brand, U., Logan, A., Bitner, M.A., Griesshaber, E., Azmy, K., and Buhl, D. 2011. What is the ideal proxy for Palaeozoic seawater chemistry? Memoirs of the Association of Australasian Palaeontologists , 41:9-24.
Buening, N. and Spero, H.J. 1996. Oxygen and carbon-isotope analyses of the articulate brachiopod Laqueus californianus : a recorder of environmental changes in the subeuphotic zone. Marine Biology, 127:105-114.
Butler, S., Bailey, T., Lear, C., Curry, G., Cherns, L., and McDonalad, I. 2015. The Mg/Ca-temperature relationship in brachiopod shells: calibrating a potential paleoseasonality proxy. Chemical Geology, 397:106-117.
Carpenter, S.J. and Lohmann, K.C. 1995. δ18O and δ13C values of modern brachiopod shells. Geochimica et Cosmochimica Acta, 59:3749-3764.
Curry, G. 1982. Ecology and population-structure of the recent brachiopod Terebratulina retusa from Scotland. Palaeontology, 25:227-246.
Endo, K. 1987. Life habit and relative growth of some Laqueid brachiopods from Japan. Transactions and Proceeding of the Palaeontological Society of Japan, 147:180-194.
Geldern. R., Joachimski, M.M., Day, J., Jansen, U., Alvarez, F., Yolkin, E.A., and Ma X. 2006. Carbon, oxygen and strontium isotope records of Devonian brachiopod shell calcite. Palaeogeography, Palaeoclimatology, Palaeoecology, 240:47-67.
Holland, S. 2013. Lithostratigraphic Glossary, retrieved March 2014. UGA Stratigraphy Lab. http://strata.uga.edu/cincy/strata/LithostratGlossary.html
Ivany, L. 2012. Reconstructing paleoseasonality from accretionary skeletal carbonates- challenges and opportunities. The Paleontological Society Papers, 18:133-165.
Kercher, P. and Carlson, S. 2012. Recent terebratulide brachiopods: Do they faithfully record oceanographic conditions throughout ontogeny?, Abstract #B21C-0376 presented at 2012 Fall Meeting, American Geophysical Union, 3-7 December, San Francisco (USA).
King, W. 1850. A monograph of the Permian fossils of England. Palaeontographical Society, London, England.
Linnaeus, C. 1758. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Lugduni Batavorum, Leiden.
Pérez-Huerta, A. 2004. New carboniferous brachiopods from the eastern great basin, Nevada, USA: implications for loop ontogeny and evolution in late Palaeozoic terebratuloids. Palaeontology, 47:1519-1537.
Pérez-Huerta, A., Aldridge, A.E., Endo, K., and Jeffries, T. E. 2014. Brachiopod shell spiral deviations (SSD): Implications for trace element proxies. Chemical Geology, 374-375:13-24.
R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org/.
Reolid, M., García-García, F., Tomašových, A., and Soria, J. 2012. Thick brachiopod shell concentrations from prodelta and siliciclastic ramp in a Tortonian Atlantic- Mediterranean strait (Miocene, Guadix Basin, southern Spain). Facies, 58:549-571.
Rigby, J.K. and Clement, C.R. 1995. Demosponges and hexactinellid sponges from the Lower Devonian Ross Formation of West-Central Tennessee. Journal of Paleontology, 69:211-232.
Sowerby, G.B. 1846. Description of Tertiary fossil shells from South America, p. 249-264. In Darwin, C.R. (ed.), Geological observations on South America. Smith, Elder and Co., Cornhill.
Suan, G., Mattioli, E., Pittet, B., and Lecuyer, C. 2008. Evidence for major environmental perturbation prior to and during the Toarcian (Early Jurassic) oceanic anoxic event from the Lusitanian Basin, Portugal. Paleoceanography, 23:PA1202.
Thayer, C. 1977. Recruitment, Growth, and Mortality of a Living Brachiopod, with Implications for the Interpretation of Survivorship Curves. Paleobiology, 3:98-109.
Tomašových, A., Carlson, S., and Labarbera, M. 2008. Ontogenetic niche shift in the brachiopod Terebratalia transversa : relationship between the loss of rotation ability and allometric growth. Palaeontology, 51:1471-1496.
Wahlman, G. 1992. Middle and upper Ordovician symmetrical univalved mollusks (Monoplacophora and Bellerophontina) of the Cincinnati Arch region. United State Government Printing Office, Washington.
Yamamoto, K., Asami, R., and Iryu, Y. 2010. Carbon and oxygen isotopic compositions of modern brachiopod shells from a warm-temperature shelf environment, Sagami Bay, central Japan. Palaeogeography, Palaeoclimatology, Palaeoecology, 291:348-359.
Zuur, A., Ieno, E., Walker, N., Saveliev, A., and Smith, G. 2009. Mixed effect models and extensions in ecology with R. Springer science and business media, New York.