Putative Ordovician green alga Krejciella reinterpreted as enteropneust hemichordate tube (Czech Republic)
Article number: 25.2.a25
https://doi.org/10.26879/1185
Copyright Paleontological Society, August 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 September 2021. Acceptance: 28 June 2022.
ABSTRACT
Fossil and extant representatives of Enteropneusta play an important role in the interpretation of early evolution of animals, such as echinoderms, hemichordates and chordates. However, remains of fossil Enteropneusta are rare. Re-examination of available specimens of organic tubes of the Ordovician putative green alga Krejciella putzkeri Obrhel 1968 does not show any morphological difference from the Cambrian Margaretia dorus Walcott, 1931. The latter species has been recently interpreted as an organic tube produced and inhabited by the worm-like enteropneust hemichordate Oesia disjuncta Walcott, 1911. However, the absence of the subterranean lateral extension in Ordovician specimens excludes the synonymy of Krejciella and Margaretia. Geographic distribution of Cambrian organic tubes classified as Margaretia Walcott, 1931 indicates a possible latitudinal control, as all occurrences are apparently restricted to tropical and subtropical belts when plotted in Cambrian palaeogeographic maps. In comparison, the occurrence of the herein studied specimens of Krejciella is restricted to cold-water localities of West Gondwana. The micropalaeontological analysis of a rock sample bearing one specimen of Krejciella shows the presence of moderately preserved chitinozoans, including the zonal species Linochitina pissotensis. This taxon is, for the first time, documented from the Prague Basin and determines the Middle/Late Ordovician boundary interval of the analysed sample. The herein studied specimens of Krejciella extend the record of organic tubes produced by enteropneust hemichordates both stratigraphically and palaeogeographically to the Middle/Late Ordovician cold-water area.
Oldřich Fatka. Charles University, Institute of Geology and Palaeontology, Albertov 6, CZ-128 43, Prague 2, Czech Republic. https://orcid.org/0000-0003-1898-3319. fatka@natur.cuni.cz
Jakub Vodička. Charles University, Institute of Geology and Palaeontology, Albertov 6, CZ-128 43, Prague 2, Czech Republic. https://orcid.org/0000-0003-0136-1804. psoqvl@gmail.com
Keywords: Ordovician; hemichordates; Enteropneusta, stratigraphy; chitinozoans; palaeogeography
Final citation: Fatka, Oldřich and Vodička, Jakub. 2022. Putative Ordovician green alga Krejciella reinterpreted as enteropneust hemichordate tube (Czech Republic). Palaeontologia Electronica, 25(2):a25. https://doi.org/10.26879/1185
palaeo-electronica.org/content/2022/3659-krejciella-reinterpreted
Copyright: August 2022 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
There are numerous poorly substantiated reports of algal macrofossils from the Lower Paleozoic. The recent research has shown that some of these putative algae are related to animal groups (e.g., Kenrick et al., 1999; Steiner and Maletz, 2012; Maletz and Steiner, 2016; LoDuca et al., 2017), but the affinities of others remain controversial (e.g., Handle and Powell, 2012; LoDuca et al., 2015).
Recently, the interpretation of two of the well-known Cambrian fossils, particularly of Margaretia dorus Walcott, 1931 from the Burgess Shale, formerly assigned to algae and Aldanophyton antiquissimum Krishtofovich, 1953 from the Sinsk Formation, formerly assigned to ?Lycophyta or algae; see Krishtofovich (1953), Obrhel (1968) and Krassilov (1982, p. 28) has been questioned by Nanglu et al. (2016, p. 1).
The occurrence of numerous specimens of the vermiform enteropneust hemichordate Oesia disjuncta Walcott, 1911 preserved inside tubes classified as Margaretia dorus, has been recently published from the Kootenay National Park of British Columbia and interpreted as an original association (Nanglu et al., 2016). Consequently, the earlier classification of Margaretia Walcott, 1931 as a putative green alga changed, and M. dorus has been re-interpreted as an organic tube produced and inhabited by an enteropneust hemichordate (Nanglu et al., 2016). Nanglu et al. (2016) also followed the synonymization of the Early Cambrian genus Aldanophyton Krishtofovich, 1953 from Yakutia (Siberian Platform) with Margaretia proposed by Conway Morris and Robison (1988, p. 6).
The rare tubiculous fossil genus Krejciella Obrhel, 1968 (type species Krejciella putzkeri Obrhel 1968) from the Middle-Upper Ordovician Dobrotivá Formation of the Prague Basin (Czech Republic) is morphologically very similar to the Cambrian genus Margaretia. Krejciella was originally interpreted to represent an early land plant of Lycophyta by Obrhel (1968); later it was regarded as similar to Margaretia and classified as a non-calcareous alga of Chlorophyta by Havlíček et al. (1993).
The aim of this contribution is to provide new data on the putative alga Krejciella, to analyse its morphology and to specify its stratigraphic occurrence. Based on morphological comparison with specimens classified as Margaretia and Aldanophyton, the genus Krejciella is re-interpreted as an enteropneust hemichordate. Earlier opinions on systematic position of Margaretia, Aldanophyton and Krejciella are summarised. Stratigraphic range, taphonomy and palaeogeographical distribution of all known Cambrian and Ordovician organic tubes produced by enteropneust hemichordates are discussed. The assemblage of chitinozoans obtained from the rock-sample containing on its surface remain of Krejciella is analysed to refine the stratigraphic position of the rock-sample.
GEOLOGICAL SETTING
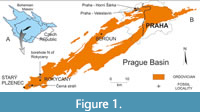 The Prague Basin comprises Lower Ordovician to Middle Devonian sediments and volcanites preserved in a Variscan-developed syncline (see Havlíček, 1998; Vacek and Žák, 2017) in the central part of the Bohemian Massif, Czech Republic (Figure 1A). The Ordovician is dominated by siliciclastic sediments with local volcanites (Figure 1B). The first lithostratigraphic subdivision was proposed by Barrande (1846). Havlíček and Marek (1973) summarized all earlier data and distinguished 12 formations, including the Dobrotivá Formation.
The Prague Basin comprises Lower Ordovician to Middle Devonian sediments and volcanites preserved in a Variscan-developed syncline (see Havlíček, 1998; Vacek and Žák, 2017) in the central part of the Bohemian Massif, Czech Republic (Figure 1A). The Ordovician is dominated by siliciclastic sediments with local volcanites (Figure 1B). The first lithostratigraphic subdivision was proposed by Barrande (1846). Havlíček and Marek (1973) summarized all earlier data and distinguished 12 formations, including the Dobrotivá Formation.
Dobrotivá Formation
The Dobrotivá Formation was established as a separate unit by Kettner and Kodym (1919). General overviews on stratigraphy and depositional setting are available in Kukal (1957), Havlíček and Vaněk (1966), Havlíček (1982, 1998), Havlíček and Fatka (1992) and Servais et al. (2008).
In the Dobrotivá Formation, two main lithofacies have been distinguished: quartzose sandstones of the Skalka Member are developed in the shallow water environment, while fine-grained Dobrotivá Shales are characteristic for the deeper water setting (Havlíček and Vaněk, 1966). The abrupt onset of the quartzose sandstones of the Skalka Member led Havlíček and Marek (1973) to the proposal of the Dobrotivian Series in the Prague Basin.
Traditionally, the Dobrotivá Formation was considered isochronous with the British Llandeilo Series (more broadly, the British-Avalonian Regional Series) by Havlíček and Vaněk (1966) and Fortey et al. (2000). The Dobrotivá Series of Havlíček and Marek (1973) has been accepted as the Dobrotivian Stage (Fatka et al., 1995) in the Ibero-Bohemian regional chronostratigraphical scale and is correlated with the latest Darriwillian to the earliest Sandbian (Gutiérrez-Marco et al., 2017).
Fossils of the Dobrotivá Formation. The Dobrotivá Formation contains locally common, highly diverse skeletal fauna (e.g., Havlíček and Vaněk, 1966, 1990; Fatka and Mergl, 2009). Diverse trilobites, brachiopods, gastropods, echinoderms, phyllocarids, ostracods, bivalves, agnostids, hyoliths, cephalopods and conulariids associated with graptolites and ichnofossils have been studied for more than 150 years (see Havlíček and Vaněk, 1966). Detailed lists of fauna established in the Dobrotivá Formation were compiled by Havlíček and Vaněk (1966, 1990); they were later supplemented by Mikuláš (1991), Havlíček et al. (1993), Vaněk (1995, 1999), Mergl (2002), Mergl et al. (2007, 2008), Manda (2008), Peršín and Budil (2009), David and Budil (2015) and Aubrechtová and Turek (2018).
Biostratigraphy of the Dobrotivá Formation. The Dobrotivá Formation has been divided into two graptolite biozones, i.e., Hustedograptus teretiusculus and Cryptograptus tricornis (see Havlíček and Vaněk, 1966, 1990). However, graptolites are generally rare, and no graptolite specimen is known to occur together with herein studied tubiculous fossils. The chitinozoan record of the Dobrotivá Formation is rather poor. Eisenack (1948) studied chitinozoans from 11 nodules collected at six outcrops, most probably representing several stratigraphic levels. However, the research of Eisenack (1948) did not contribute to the biostratigraphy of the Dobrotivá Formation. Fatka et al. (1997) reported the co-occurrence of chitinozoans Laufeldochitina clavata (Jenkins, 1967) and Linochitina aff. pissotensis (Paris, 1981) associated with the Baltic graptolite Gymnograptus linarssoni (Moberg) in one core sample (Cekov HJ 1 drill core) from the Dobrotivá Formation. These specimens were, however, not figured.
Localities with Krejciella
Specimens of Krejciella have been collected at four different fossil sites in the Prague Basin, (1) at the Praha - Horní Šárka locality (Pod libockým hřbitovem), (2) at the Praha - Veleslavín locality, (3) in an unnumbered borehole north of Rokycany, (4) at the Černá stráň slope locality near Starý Plzenec (Figure 1B). The herein examined material comprises three originals of Obrhel (1968) and seven other specimens (Table 1).
Praha - Horní Šárka locality. Type specimens of Obrhel come from loose siliceous nodules collected at this locality (see Table 1). Early diagenetic siliceous nodules originate from distinct levels of the sedimentary sequence and such individual levels could contain fauna of various fossil associations (Loi and Dabard, 2002). During the weathering, these nodules were freed and mixed in the Quaternary soil. Consequently, loose nodules collected at one locality could belong to several fossil associations. Generally, several fossil species co-occur in one nodule, in such a case the original fossil association can be determined. However, the herein studied tubiculous fossils are preserved in two small nodules that do not contain other fossil remnants.
Praha - Veleslavín locality. Specimens reported by Havlíček et al. (1993) and part of other herein studied specimens (see Table 1) were collected from excavations of a water pipe and sewerage system at Praha - Veleslavín (details see Peršín and Budil, 2009, p. 32, locality number 19). At this outcrop, specimens of Krejciella were collected together with articulated exoskeletons of the cyclopygid trilobite Emmrichops are associated with Cyclopyge, Placoparia, diverse echinoderms (e.g., Aristocystites), linguliformean brachiopods and the gastropod Grandostoma (Peršín and Budil, 2009, p. 32).
Černá stráň locality near Starý Plzenec. From this large outcrop (locality no. 57 of Mergl, 2002, p. 8) a small fragment of Krejciella (specimen OMR 11794; see Table 1) preserved in dark mudstone was reported by Mergl and Vohradský (2000, p. 108). The associated skeletal fauna is dominated by brachiopods (Paterula and Benignites) and trilobites (Cyclopyge, Degamella, Zeliszkella, Dindymene, Ormathops) associated with echinoderms (e.g., Mitrocystella) and conulariids. The level containing this fossil association was assigned to the middle through upper levels of the Dobrotivá Formation by Mergl (2002, p. 8).
The unnumbered Borehole N of Rokycany. One specimen (OMR 19316, collected by P. Kraft; see Table 1) comes from an unnumbered borehole N of Rokycany (see Mergl, 2002, p. 8, locality no. 41). No other fossil is preserved on the small slab of the dark shale originating from this borehole.
MATERIAL AND METHODS
Studied Material
The studied material is housed in the National Museum Prague (NML D), in the Czech Geological Survey, Prague (ČGS) and in the Museum of Dr. B. Horák in Rokycany (OMR).
 Type specimens of Krejciella. Original specimens described by Obrhel (1968) were collected by an amateur collector F. Putzker in loose siliceous nodules; such loose siliceous nodules containing Middle Ordovician fossils are widely distributed in the Prague Basin and are known at numerous outcrops of Šárka and Dobrotivá formations (see Lajblová and Kraft, 2014). Type specimens of Obrhel (1968) are comparatively small fragments of three-dimensionally preserved tube-shaped fossils. The holotype represents both internal and external moulds (NML D497a, b, Figure 2A-B), while the paratype is only an external mould (NML D498, Figure 2C).
Type specimens of Krejciella. Original specimens described by Obrhel (1968) were collected by an amateur collector F. Putzker in loose siliceous nodules; such loose siliceous nodules containing Middle Ordovician fossils are widely distributed in the Prague Basin and are known at numerous outcrops of Šárka and Dobrotivá formations (see Lajblová and Kraft, 2014). Type specimens of Obrhel (1968) are comparatively small fragments of three-dimensionally preserved tube-shaped fossils. The holotype represents both internal and external moulds (NML D497a, b, Figure 2A-B), while the paratype is only an external mould (NML D498, Figure 2C).
 Other material of Krejciella. The material described by Havlíček et al. (1993) and the newly studied specimens, all from the Praha - Veleslavín locality, are preserved as incomplete, strongly flattened fragments of internal and external moulds in dark mudstones (specimens ČGS JP 2015a, b; ČGS JP 2016a, b, c; ČGS JP 2017; ČGS JP 2018a, b; see Table 1; Figure 3).
Other material of Krejciella. The material described by Havlíček et al. (1993) and the newly studied specimens, all from the Praha - Veleslavín locality, are preserved as incomplete, strongly flattened fragments of internal and external moulds in dark mudstones (specimens ČGS JP 2015a, b; ČGS JP 2016a, b, c; ČGS JP 2017; ČGS JP 2018a, b; see Table 1; Figure 3).
Small fragments of Krejciella were ascertained also at two localities in the western part of the Prague Basin (Figure 1B). One specimen (OMR 19316, collected by P. Kraft) comes from an unnumbered borehole N of Rokycany (see Mergl, 2002, p. 8, locality no. 41). The second specimen (OMR 11794) was reported by Mergl and Vohradský (2000, p. 108) from the locality Černá stráň slope near Starý Plzenec (see Mergl, 2002, p. 8, locality no. 57).
Methods
Study of macrofossils. Construction, width, length, fragility and disposition of pores on the tube surface and on the internal mould of the herein studied material are used for morphological comparison of remains of Krejciella and Margaretia.
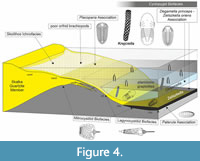 Fossils associated with Krejciella are summarized and interpreted in respect to bathymetry of skeletal fauna associations (Figure 4), taphonomy and stratigraphy.
Fossils associated with Krejciella are summarized and interpreted in respect to bathymetry of skeletal fauna associations (Figure 4), taphonomy and stratigraphy.
Study of microfossils. A standard palynological method based on the HCl and HF treating (e.g., Paris, 2006) was applied to a piece of 300 g dark mudstone from the sample ČGS JP2016a with the aim to study its micropalaeontological content. The organic residuum was examined using a stereomicroscope, individual microfossils were picked and documented using the optical microscope Carl Zeiss Jena and scanning electron microscope JEOL-JSM 6380; morphometric data were obtained from digital images.
RESULTS
Specimens of Krejciella
Description. The type material, internal and external moulds of short fragments is preserved in siliceous nodules (Figure 2). All more recently collected specimens originate from dark mudstones and are preserved as strongly flattened moulds. Internal moulds are slightly convex, and they measure less than 1 mm in thickness (Figure 3A-B, E); external moulds are either flat or very subtly concave (Figure 3C-E). Gentle mounds are usually seen on the surface of the internal mould (Figure 3B) while depressions prevail on the external mould. Mounds and depressions show a regularly spiral arrangement. The shape of wall perforations varies from a slightly opened elliptical slit through rhombic to circular. Some slits may be almost closed, while others are opened, and their width is equal to one-fourth of the width of the flattened tube (Figure 3A). The number of perforations ranges around eight to nine per revolution. In well-preserved parts of the tube wall, fine longitudinal fibres are discernible (white arrows in Figure 3D).
Dimensions. The three-dimensional holotype (NML D497a, b) is around 20 mm in length and is up to 6 mm wide; similarly, the preserved paratype (NML D498) is 17.5 mm long and reaches 7.4 mm in width. Other studied specimens are strongly flattened with the length ranging from 35.8 to 124 mm, and their width ranges from 11.1 to 17.2 mm (see Table 1).
DISCUSSION
Comparison of Morphology of Krejciella with Margaretia and Aldanophyton
Specimens classified as Margaretia, Aldanophyton and Krejciella have traditionally been interpreted as green algae or as early land plants (e.g., Walcott, 1931; Krishtofovich, 1953; Obrhel, 1968; Conway Morris and Robison, 1988). Recent re-examination of Cambrian material resulted in synonymization of Margaretia and Aldanophyton and re-interpretation of these tubiculous fossils as organic tubes produced and inhabited by a worm-like enteropneust hemichordate (Nanglu et al., 2016). However, the Ordovician specimens of Krejciella were not considered. Therefore, the morphology of specimens of Krejciella, Margaretia including Aldanophyton is compared in detail.
More than 1,000 specimens are known in Cambrian Margaretia including Aldanophyton; in comparison about 15 Ordovician specimens of Krejciella are known (Table 1). The Czech material is, however, sufficient to allow comparison of a number of key details.
Morphological details. Specimens classified to all three genera show a common and very characteristic morphology. Cylindrical tubes with walls composed of longitudinal fibres bear spirally arranged rhombic to circular pores (= mounds on the internal mould and depressions on the external mould of the tube wall; compare white arrows in Figure 3D with Nanglu et al., 2016, fig. 4f, g). Along individual tubes, the width is consistent in both Cambrian and Ordovician specimens. In addition, the tube width is also comparable. In Cambrian specimens it varies from 4 to 20 mm whilst in Ordovician specimens it ranges from 4.6 to 7.4 mm in 3D preserved material and from 11.1 to 17.2 mm in flattened specimens. A slight difference is also seen in the number of pores. Nanglu et al. (2016) reported 10 pores per revolution, while in specimens of Krejciella eight to nine pores per revolution seem to be present. Because of the apparent elasticity of wall fibres, it is not possible to measure exact dimensions of pores. The shape of pores in Krejciella is identical to Margaretia; the pore diameters reach about one-third of the tube width in both taxa.
Tubes are usually preserved as short fragments; longer (up to 544 mm long) or even complete tubes and branched tubes are rarely observed in the Cambrian material (Nanglu et al., 2016, p. 5). The absence of tubes longer than 124 mm as well as missing bifurcations in specimens of Krejciella can be explained by the limited material.
Taphonomy. The state of preservation as longer or shorter fragments combined with the possibly high fragility of the studied Ordovician material would suggest that tubes were probably quickly buried or transported on a short distance and represent parautochthonous to autochthonous material. All studied fragments of tubes are empty, lacking remains of the supposed enteropneust hemichordate producer. However, the fossil record of enteropneust hemichordates is extremely poor due to the high fragility of their body and the absence of preservable hard parts (Mauviel et al., 1987; Cameron, 2005; Maletz, 2014, 2019; Nanglu et al., 2015). Soft tissue preservation of the enteropneust animal is restricted to several lagerstätten like the Cambrian Burgess Shale (Conway Morris, 1979; Caron et al., 2013; Nanglu et al., 2016), the Carboniferous Mazon Creek Biota (Bardack, 1997; Cameron, 2016) or the Jurassic La Voulte-sur-Rhône (Alessandrello et al., 2004) but even here, these organisms appear to be uncommon. Maletz (2014, p. 21) stressed that body fossils of enteropneust can be preserved only under very specific conditions in the marine environment, i.e., in Lagerstätten.
Palaeolatitudinal distribution. The Cambrian Margaretia and the Ordovician Krejciella show a distinctive difference in their palaeolatitudinal distribution.
Margaretia: Specimens classified as Margaretia have been documented from five biotas in Laurentia (Canada and U.S.A.) and in one biota in East Gondwana (China).
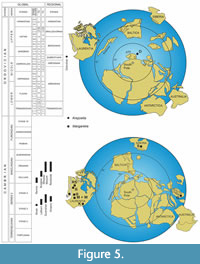 The occurrence in Laurentia: (1) Burgess Lagerstätte (see Walcott, 1931; Nanglu et al., 2016; B in Figure 5C). (2) Kinzers Formation of Pennsylvania (see Resser and Howell 1938, p. 211; K in Figure 5C). Margaretia ramosa Resser and Howell 1938 and M. stosei Resser and Howell 1938. Both taxa were revised and considered synonymous with the type species by Conway Morris and Robison (1988, p. 6). (3) Rennie Formation of Idaho (see Resser, 1938; R in Figure 5C). M. angustata Resser 1938 was accepted as a separate species by Conway Morris and Robison (1988, p. 6) and Nanglu et al. (2016, table 1). (4) Ravens Throat River Lagerstätte, Rockslide Formation of the Mackenzie Mountains, Northwest Territories, Canada contains rare specimens classified as M. angustata (see Kimming and Pratt, 2015a, b; RS in Figure 5C). (5) The early Cambrian Latham Formation (Bristolia suzone of the Bonnia - Olenellus Zone, Dyeran Stage of the Waucoban Series) in Marble Mountains of California contains a separate species M. chamblessi (see Waggoner and Hagadorn, 2004; L in Figure 5C).
The occurrence in Laurentia: (1) Burgess Lagerstätte (see Walcott, 1931; Nanglu et al., 2016; B in Figure 5C). (2) Kinzers Formation of Pennsylvania (see Resser and Howell 1938, p. 211; K in Figure 5C). Margaretia ramosa Resser and Howell 1938 and M. stosei Resser and Howell 1938. Both taxa were revised and considered synonymous with the type species by Conway Morris and Robison (1988, p. 6). (3) Rennie Formation of Idaho (see Resser, 1938; R in Figure 5C). M. angustata Resser 1938 was accepted as a separate species by Conway Morris and Robison (1988, p. 6) and Nanglu et al. (2016, table 1). (4) Ravens Throat River Lagerstätte, Rockslide Formation of the Mackenzie Mountains, Northwest Territories, Canada contains rare specimens classified as M. angustata (see Kimming and Pratt, 2015a, b; RS in Figure 5C). (5) The early Cambrian Latham Formation (Bristolia suzone of the Bonnia - Olenellus Zone, Dyeran Stage of the Waucoban Series) in Marble Mountains of California contains a separate species M. chamblessi (see Waggoner and Hagadorn, 2004; L in Figure 5C).
The occurrence in East Gondwana: Hu et al. (2010, figures 54, 55, p. 203; 2013, p. 1771, 1772) reported the occurrence of Margaretia sp. In the Guanshan Biota, Wulongqing Formation, Eastern Yunnan of China (G in Figure 5C).
Aldanophyton (= Margaretia): Krishtofovich (1953) and Ivantsov et al. (2005a, p. 10, 21) reported Aldanophyton from the outcrop called Tuoydakh (or Tuoydakhskoe) and wrote that Aldanophyton is usually abundant in numerous sections of Sinsk and Inikan formations (S in Figure 5C). Krassilov (2005, p. 41) reported the occurrence of this species in the Inikan Formation, middle Maya River, Yakutia.
The Sinsk biota of Ivantsov et al. (2005a, p. 70) belongs to the Bergeroniellus gurarii Trilobite Zone of the early Cambrian Botomian Regional Stage (see Rozanov et al. 2008). Krassilov (2005, p. 41) reported the occurrence in the Inikan Formation, middle Maya River, Yakutia) - Amganian Regional Stage (equals to upper Stage 4 to lower Drumian, after Peng et al., 2012).
Krejciella: Havlíček et al. (1993) described remains of several larger pieces of Krejciella collected by the late J. Vaněk from black shales from an extensive temporary excavation at Praha - Veleslavín (Figure 1B). Remains of Krejciella have been reported solely from the Dobrotivá Formation of the Prague Basin (Figure 5).
Palaeoenvironment. The preservation of the Burgess Shale-type biota has been apparently related to oxygen deficient environments (e.g., Gaines, 2014). Deposition under dysoxic to anoxic conditions was proposed for the Burgess Shale Formation by Powell et al. (2003). Ivantsov et al. (2005b, p. 73) supposed a dysoxic environment for the Algal Lens of the Sinsk Lagerstäte. In the Wheeler and Marjum lagerstäten, dysoxic to anoxic bottom waters and dysoxic bottom conditions were responsible for the exceptionally preserved fossils, including the tubiculous fossils (Gaines and Droser, 2010, p. 656, 659). After Kimming and Pratt (2015b, p. 14) the lack of bioturbation in the lime mudstone interbeds likely reflects rapid sedimentation rates or possible dysoxic conditions in the Ravens Throat River Lagerstäte of the Rockslide Formation, where dysoxic to anoxic conditions are supported also by the trace-element composition (Kimming and Pratt, 2015b, p. 154). The above mentioned papers show that all known occurrences of Margaretia are apparently restricted to the Cambrian Burgess-type Lagerstätten (see also Nanglu and Caron 2016 and Nanglu et al. 2020).
At the Praha - Veleslavín and the Černá stráň localities, the Dobrotivá Formation is developed as a monotonous, dark-coloured shale without event layers, which indicates deposition below the Storm Wave Base. Dysoxic/anoxic bottom conditions are demonstrated by the absence of bioturbation in rock samples bearing tubiculous fossils of Krejciella. At both localities the associated fauna is dominated by cyclopygid trilobites indicating the Cyclopygid Biofacies of Fortey (1985) and Fatka and Mergl (2009); compare Figure 4.
At the Černá stráň locality near Starý Plzenec the associated fauna includes also elements of the Paterula Association (Havlíček and Vaněk, 1990; Fatka and Mergl, 2009) and rarely also echinoderms of the Mitrocystitid Biofacies (Lefebvre, 2007); compare Figure 4. Such an oxygen depleted, for benthos unsuitable environment in combination with the fragmentary preservation of Krejciella specimens could be explained by a short transport from a more oxygenated benthic habitat. The occurrence of elements of the Cyclopygid and Mitrocystitid biofacies and Paterula Associations combined with the absence of bioturbation at these two localities is comparable with the palaeoenvironment typical for the Burgess Shales-type preservation.
Systematic Position
In the following text, the earlier opinions on systematic position of Ordovician specimens of Krejciella and Cambrian organic tubes produced and inhabited by worm-like enteropneust hemichordates are reviewed and discussed.
Margaretia. The type specimen classified as Margaretia dorus was described in the posthumously published contribution assembled by Charles E. Resser (see prefatory statement in Walcott, 1931) from the “thick” Stephen Shale (Fossil Ridge Yoho National Park, British Columbia, Canada). Walcott (1931, p. 2) considered M. dorus as an alcyonarian coral, but he compared it also with living algae (see Handle and Powell, 2012, p. 306). In the original description of Margaretia ramosa Resser and Howell, 1938 and M. stosei Resser and Howell, 1938, both from the Kinzers Formation, Resser and Howell (1938) considered this taxon to be a possible coelenterate. Satterthwaite (1976), Conway Morris and Robison (1988) and Waggoner and Hagadorn (2004) classified Margaretia as a chlorophyte and compared it with an unspecified species of the extant, polymorphic genus Caulerpa. Zhuravlev and Wood (1996, p. 313) and Garcia-Bellido and Conway Morris (1999, p. 396) consider Margaretia as a chlorophyte caulerpacean. Burzin et al. (2001, p. 217) classified Margaretia as carbonaceous algae.
Aldanophyton (= Margaretia). The type species Aldanophyton antiquissimum Krishtofovich 1953 was originally described from the Sinsk Formation, Yakutia (Lena River near the mouths of Achchagyy-Tuoydakh and Ulakhan-Tuoydakh rivers). Originally, Krishtofovich (1953) supposed that Aldanophyton belongs to an early land plant of Lycopodiaceae. Satterthwaite (1976) and Conway Morris and Robison (1988, p. 6) considered Aldanophyton as synonymous with Margaretia and classified both as a chlorophyte; this view was followed by Rozanov and Zhuravlev (1992, p. 259, 261). The synonymy of both genera was accepted also by Ivantsov and Wrona (2004, p. 3) by mentioning Margaretia antiquissima as a caulerpacean chlorophyte in the list of fossils. Similarly, Ivantsov et al. (2005b, p. 80) reported Margaretia as a green siphonous algae. In comparison, Krassilov (2005, p. 41) provided a new diagnosis of Aldanophyton antiquissimum and prefers to treat it as a taxon of chlorophyta. After re-examination of the original material of Krishtofovich in St. Petersburg, Lemoigne (1975, p. 869) preferred, for the first time, an animal origin of Aldanophyton. More recently, Luchinina (2013, p. 993) classified Aldanophyton as an organic walled “macroalgae”.
Krejciella. The type material described by Obrhel (1968) is 3D preserved in siliceous nodules. In agreement with the original classification of Aldanophyton by Krishtofovich (1953), also Obrhel (1968) assigned Krejciella to ?Lycophyta. However, Blazer (1975, p. 16) and Dijikstra and van Ameron (1994, p. 280) questioned the lycopod origin of both genera. Krassilov (1982, p. 28) classified Aldanophyton and Krejciella as poorly studied taxa showing features of Lycophyta. Havlíček et al. (1993) compared specimens of Krejciella with the Cambrian material of Margaretia from Laurentia. In agreement with Conway-Morris and Robison (1988), they assigned both taxa to non-calcareous algae and also briefly discussed their possible palaeoecology. Kvaček and Kraft (2015, p. 9) interpreted the Cambrian Aldanophyton and the Ordovician Krejciella as unspecified algae.
The fossil remain determined by Vokáč and Grigar (2010, p. 161) as Krejciella putzkeri from the area between Tymákov and Letkov municipalities west of Rokycany has been re-studied and is newly interpreted as an ichnofossil.
Summary. Conway Morris and Robison (1988, p. 6) synomized the Early Cambrian genus Aldanophyton with Margaretia. Nanglu et al. (2016) followed this synonymization and interpreted Margaretia as remains of organic tubes produced by enteropneusts. Because of the above discussed morphological similarities of Cambrian specimens classified as Margaretia, including Aldanophyton, and the herein studied Ordovician specimens of Krejciella, we newly interpret Krejciella the remains of an organic tube produced by an enteropneust. The unknown morphology of the subterranean anchoring part (= prostrate rhizome of Conway Morris and Robison, 1988 or the subterranean lateral extension of Nanglu et al., 2016) of the tube in Ordovician specimens excludes the synonymy of Krejciella and Margaretia (see discussion in Havlíček et al. 1993, p. 82).
Stratigraphy and Palaeogeography
Stratigraphy. Up to now, specimens classified as Margaretia have been documented from the early Cambrian Stage 3 (Sinsk and Latham Lagerstäten), through the early Cambrian Stage 4 (Latham, Guanshan and Kinzers Lagerstäten) to the middle Cambrian Wuliuan (Rennie and Burgess Lagerstäten) and Drumian stages (Wheeler, Marjum and Rockslide Lagerstäten; Figure 5A). The herein studied specimens of Krejciella extend the stratigraphic range of organic tubes produced by enteropneust hemichordates to the Middle/Late Ordovician (Figure 5A).
Palaeogeography. All above-mentioned occurrences of specimens classified as Margaretia and/or Aldanophyton are described from tropical and subtropical localities in Laurentia, Siberia and East Gondwana (Figure 5C). In comparison, the Middle/Late Ordovician record of specimens of Krejciella is from the cold-water subpolar area of European peri-Gondwana (see Havlíček et al., 1994; Fatka and Mergl, 2009; Figure 5B).
Palaeobiological Significance of the Krejciella Interpreted as an Enteropneust Hemichordate Tube
Enteropneusts, known also as acorn worms, represent a group of crucial importance for elucidating the early evolution of hemichordates and deuterostomates (e.g., Simakov et al., 2015; Nanglu et al., 2016). Due to a limited fossil record of this group (see Maletz, 2014, 2019, 2020) the enteropneust research relied mainly on extant morphological and phylogenetic data (see Cameron, 2005; Maletz et al., 2014; Simakov et al., 2015; Li et al. 2018 and references therein). Only the recent findings of soft-bodied Cambrian acorn worms Spartobranchus tenuis (see Caron et al., 2013), Oesia disjuncta (see Nanglu et al., 2016) and Spartobranchus -like enteropneust (Nanglu and Caron, 2021) from the Burgess shale Konservat-Lagerstätte allowed to supplement the genetic data with fossils. These Cambrian taxa are associated with an external organic tube. Caron et al. (2013) proposed that the organic fibrous tube of S. tenuis could be a precursor of graptolite tubaria. Similar interpretation was proposed also for organic tubes formerly classified as Margaretia associated with O. disjuncta by Nanglu et al. (2016). These proposals were not unanimously accepted and have provoked a discussion (e.g., Caron et al., 2013, Halanych, 2013, Nanglu et al., 2015, 2016, Cameron, 2018). In the review of acorn worm classification, Cameron (2018) established the “Cambrian stem-group” containing S. tenuis and O. disjuncta. Maletz (2019, p. 62) expressed some doubts on the interpretation of O. disjuncta as an enteropneust. However, Maletz (2019, p. 59) noted that “...early hemichordates may already have had tube building capacities before the differentiation of the Enteropneusta and the Pterobranchia, or the tube building evolved independently in both groups. It would have considerable influence on the interpretation of the early evolution of the Hemichordata as a group.” Finally, Nanglu and Caron (2021) suggested the ability for tube building as a plesiomorphic trait in Hemichordata.
There is no “soft-bodied” remnant of the supposed acorn worm inhabitant inside of the Krejciella tubiculous fossil. Yet, the presence of Krejciella in the Middle/Late Ordovician of the Prague Basin significantly extends the stratigraphic range and palaeogeographic distribution of the enteropneust “Cambrian stem-group” fossils (sensu Cameron, 2018). Krejciella occurs in anoxic to dysoxic facies of the cold-temperate zone, i.e., in a different environment and some 53 million years after the previous youngest known record of enteropneust (Nanglu et al., 2016).
DETERMINATION OF STRATIGRAPHIC ORIGIN OF STUDIED SPECIMENS
The biostratigraphy of Middle to Late Ordovician is usually based on restricted stratigraphic ranges of conodonts, graptolites and chitinozoans (e.g., Webby et al., 2004). However, the biostratigraphic application of conodonts is strongly restricted in West Gondwana (Gutiérrez-Marco et al., 2017) and this group is not applicable in the Ordovician of the Prague Basin (Havlíček and Vaněk, 1996). The Dobrotivá Formation has been divided into two graptolite biozones, i.e., Hustedograptus teretiusculus and Cryptograptus tricornis (see Havlíček and Vaněk, 1966, 1990). However, graptolites are generally rare and no graptolite specimen is known to occur together with herein studied tubiculous fossils.
Chitinozoan biostratigraphy has been widely applied for correlation of Ordovician sequences (e.g., Paris, 1996; Paris et al., 2004). Paris (1990) established 22 chitinozoan biozones in the Ordovician of the Northern Gondwana Domain (= West Gondwana in actual terminology) and supposed a possible presence of the zonal species Linochitina pissotensis in the Dobrotivá Formation. Small dimensions of chitinozoan vesicles in combination with their abundant occurrence make it possible to obtain chitinozoa from small rock samples.
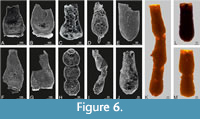 We utilized these attributes and analyzed chitinozoans from a piece of a rock separated from the sample ČGS JP2016. Observed chitinozoans are assigned to Ancyrochitina Eisenack, Conochitina Eisenack, Desmochitina Eisenack, Linochitina Paris, Euconochitina Taugourdeau, incompletely preserved specimens most probably belong to Belonechitina Jansonius, Cyathochitina Eisenack, and Eremochitina Taugourdeau and de Jekhowsky (Figure 6; see Supplementary material). From observed chitinozoan taxa, only one species, Linochitina pissotensis, could be used to assess the stratigraphic position.
We utilized these attributes and analyzed chitinozoans from a piece of a rock separated from the sample ČGS JP2016. Observed chitinozoans are assigned to Ancyrochitina Eisenack, Conochitina Eisenack, Desmochitina Eisenack, Linochitina Paris, Euconochitina Taugourdeau, incompletely preserved specimens most probably belong to Belonechitina Jansonius, Cyathochitina Eisenack, and Eremochitina Taugourdeau and de Jekhowsky (Figure 6; see Supplementary material). From observed chitinozoan taxa, only one species, Linochitina pissotensis, could be used to assess the stratigraphic position.
Since the definition of the Linochitina pissotensis Biozone by Paris (1990), the zonal species has been recorded and figured from West Gondwana (Algerian Sahara, Oulebsir and Paris, 1995; Spain, Gutiérrez-Marco et al., 1996; Bohemia, Vodička and Fatka, 2017; Southeastern Turkey, Paris et al., 2007; north-western Saudi Arabia, Al-Hajri, 1995; central Saudi Arabia, Paris et al., 2000) and East Gondwana (Kuruktag area, Tarim, China, Tang et al., 2003).
In West Gondwana, the boundary between the Middle and the Late Ordovician was originally defined by the LAD of Linochitina pissotensis (Paris, 1990, 1996, 2006; Paris et al., 2004; Videt et al., 2010). However, Dabard et al. (2015, p. 105) suggested a possible occurrence of L. pissotensis in the lowermost Sandbian of western France. Similarly, the application of the correlation software CONOP9 shows that L. pissotensis extends into the Late Ordovician in Gondwana (Sales, 2015, fig. 6). In East Gondwana, the range of L. pissotensis is not precisely established; it seems to be present only in the Darriwillian (Tang et al., 2003). In Gondwana, it is apparent that the occurrence of L. pissotensis indicates the Middle/Late Ordovician boundary interval.
The presence of Linochitina pissotensis was only presumed in the Dobrotivá Formation by Paris (1990) and by Fatka et al. (1997) who reported the occurrence of L. aff. pissotensis from one sample from the Dobrotivá Formation (Cekov HJ 1 drill core). L. pissotensis was figured from the Kazín section from the overlying Sandbian Letná Formation (Vodička and Fatka, 2017).
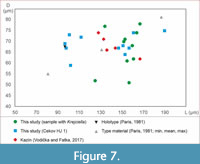 To prove the presence of Linochitina pissotensis in the Dobrotivá Formation, biometric parameters of original specimens of Fatka et al. (1997), Vodička and Fatka (2017) and the new herein studied specimens are compared with parameters of the type material of Paris (1981). Figure 7 shows that specimens from all three localities in the Prague Basin fall within the variability range of the original population of Paris (1981). Consequently, they all are classified as L. pissotensis and prove the L. pissotensis Biozone in the Prague Basin.
To prove the presence of Linochitina pissotensis in the Dobrotivá Formation, biometric parameters of original specimens of Fatka et al. (1997), Vodička and Fatka (2017) and the new herein studied specimens are compared with parameters of the type material of Paris (1981). Figure 7 shows that specimens from all three localities in the Prague Basin fall within the variability range of the original population of Paris (1981). Consequently, they all are classified as L. pissotensis and prove the L. pissotensis Biozone in the Prague Basin.
CONCLUSIONS
(1) The Ordovician tubiculous fossil Krejciella putzkeri as well as Cambrian Margaretia show a characteristic morphology combining the wall composed of longitudinal fibres and pore architecture. On the basis of the high morphological similarity of Krejciella with Margaretia, we re-interpret Krejciella as remnants of organic tube produced by an enteropneust.
(2) The presence of the zonal chitinozoan species Linochitina pissotensis in organic residuum from the rock sample bearing on its surface a specimen of Krejciella putzkeri documents the Linochitina pissotensis Biozone in the Prague Basin for the first time.
(3) The occurrence of tubiculous fossils gives evidence of the Burgess Shale-type preservation in the Middle/Upper Ordovician Dobrotivá Formation of the Prague Basin.
(4) The occurrence of specimens of Krejciella in the Dobrotivá Formation extends both palaeogeographic and stratigraphic records of tubiculous enteropneusts to the Middle/Late Ordovician cold-water West Gondwana.
(5) The Ordovician tubulous fossil Krejciella putzkeri is the youngest occurrence of organic tubes produced by enteropneust hemichordates.
(6) Diverse skeletal fauna associated with Krejciella putzkeri can be assigned to the Cyclopygid Biofacies with elements of the Placoparia and Paterula associations.
ACKNOWLEDGEMENTS
Late J. Peršín (Prague, Czech Republic) provided material from his private collection (currently housed in the Czech Geological Survey Prague, Czech Republic) and consulted the associated fauna at the Praha - Veleslavín locality. P. Budil and R. Šarič (both Czech Geological Survey Prague) are acknowledged for consultation of diverse aspects of associated fauna, stratigraphy and ecology. P. Kraft (Charles University Prague) drew our attention to specimens housed in the Museum of West Bohemia in Pilsen; these samples were kindly made available by M. Korandová (Museum of West Bohemia, Rokycany). M. Mergl (University of West Bohemia Pilsen) consulted the associated fauna at the Černá stráň slope locality near Starý Plzenec. This research was supported by the Grant Agency of the Czech Republic Project No.18-14575S and the program Cooperatio GEOL. Reviews by three anonymous referees significantly improved our contribution.
REFERENCES
Alessandrello, A., Bracchi, G., and Riou, B. 2004. Polychaete, sipunculan and enteropneust worms from the Lower Callovian (Middle Jurassic) of La Voulte-sur-Rhône (Ardèche, France). Memorie della Società` Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano (Fascicolo I), 32:1-16.
Al-Hajri, S. 1995. Biostratigraphy of the Ordovician chitinozoa of northwestern Saudi Arabia. Review of Palaeobotany and Palynology, 89:27-48. https://doi.org/10.1016/0034-6667(95)00040-5
Aubrechtová, M. and Turek, V. 2018. Lituitid nautiloids from the Middle Ordovician of Bohemia and their paleobiogeographic affinities. Bulletin of Geosciences, 93:401-417. https://doi.org/10.3140/bull.geosci.1707
Bardack, D. 1997. Wormlike animals: Enteropeusta, p. 89-92. In Shabica, C.W. and Hay, A.A. (eds.), Richardson's Guide to the fossil fauna of Mazon Creek. Northeastern Illinois University, Chicago.
Barrande, J. 1846. Notice Préliminaire sur le Systême Silurien et les Trilobites de Bohême. C. L. Hirschfeld, Leipzig.
Blazer, A.M. 1975. Index of generic names of fossil plants, 1966-1973. Geological Survey Bulletin, 1396:1-67.
Burzin, M.B., Debrenne, F., and Zhuravlev, A.Yu. 2001. Evolution of Shallow-Water Level-Bottom Communities. In Zhuravlev, A.Y. and Riding, R. (eds.), The Ecology of the Cambrian Radiation, 217-237. Columbia University Press, New York.
Cameron, C.B. 2005. A phylogeny of the hemichordates based on morphological characters. Canadian Journal of Zoology, 83:196-215. https://doi.org/10.1139/z04-190
Cameron, C.B. 2016. Saccoglossus testa f rom the Mazon Creek fauna (Pennsylvanian of Illinois) and the evolution of acorn worms (Enteropneusta: Hemichordata). Palaeontology, 59:329-336. https://doi.org/10.1111/pala.12235
Cameron, C.B. 2018. Treatise Online no. 109: Part V, Second Revision, Chapter 2: Class Enteropneusta: Introduction, morphology, life habits, systematic descriptions, and future research. Treatise Online. 109:1-22.
Caron, J.B., Conway Morris, S., and Cameron, C.B. 2013. Tubicolous enteropneusts from the Cambrian period. Nature, 495:503-506. https://doi.org/10.1038/nature12017
Cocks, L.R.M. and Torsvik, T.H. 2002. Earth geography from 500 to 400 million years ago: a faunal and palaeomagnetic review. Journal of the Geological Society, 159:631-644. https://doi.org/10.1144/0016-764901-118
Cocks, L.R.M. and Torsvik, T.H. 2011. The Palaeozoic geography of Laurentia and western Laurussia: A stable craton with mobile margins. Earth-Science Reviews, 106:1-51. https://doi.org/10.1016/j.earscirev.2011.01.007
Conway Morris, S. 1979. The Burgess Shale (Middle Cambrian) Fauna. Annual Review of Ecology, Evolution, and Systematics, 10:327-349.
Conway Morris, S. and Robison, R.A. 1988. More soft-bodied animals and algae from the Middle Cambrian of Utah and British Columbia. University of Kansas Paleontological Contributions, 122:1-48.
Dabard, M.P., Loi, A., Paris, F., Ghienne, J.F., Pistis, M., and Vidal, M. 2015. Sea-level curve for the Middle to early Late Ordovician in the Armorican Massif (western France): icehouse third-order glacio-eustatic cycles. Palaeogeography, Palaeoclimatology, Palaeoecology, 436:96-111. https://doi.org/10.1016/j.palaeo.2015.06.038
David, M. and Budil, P. 2015. Complementary description of the Middle Ordovician trilobite associations at Praha-Vokovice. Folia Musei rerum naturalium Bohemiae occidentalis. Geologica et Paleobiologica. The Journal of West Bohemian Museum in Pilsen, 49:1-7. https://doi.org/10.1515/fbgp-2015-0001
Dijikstra, S.J. and van Ameron, H.W.J. 1994. Lycopodiales XI, Supplement 2 und Nachtrag zum 2. Supplement. In van Ameron, H.W.J. (ed.), Fossilium Catalogus II: Plantae. Kugler Publications, Amsterdam.
Eisenack, A. 1948. Mikrofossilien aus Kieselknollen des böhmischen Ordoviziums. Senckenbergiana, 28:105-117.
Fatka, O., Kraft, J., Kraft, P., Mergl, M., Mikuláš, R., and Štorch, P. 1995. Ordovician of the Prague Basin: Stratigraphy and development, p. 241-244. In Cooper, J.D., Droser, M.L., and Finney, S.C. (eds.), Ordovician Odyssey: Short Papers for the 7th International Symposium on the Ordovician System. Las Vegas.
Fatka, O., Kraft, J., and Kraft, P. 1997. Occurrence of selected „Baltic“ graptolites in peri-Gondwana and associated microfossils. Geoscience Research Reports, 31:80-83. (In Czech)
Fatka, O. and Mergl, M. 2009. The ‘‘microcontinent’’ Perunica - status and story 15 years after its conception. Special Publications Geological Society, London, 325:65-101. https://doi.org/10.1144/SP325.4
Fatka, O., Budil, P., and David, M. 2015. Digestive structures in Ordovician trilobites Colpocoryphe and Flexicalymene from the Barrandian area of Czech Republic. Estonian Journal of Earth Sciences, 64:255-266. https://doi.org/10.3176/earth.2015.32
Fortey, R.A. 1985. Pelagic trilobites as an example of deducing the life habits of extinct arthropods. Transactions of the Royal Society of Edinburgh: Earth Sciences, 76:219-230. https://doi.org/10.1017/S0263593300010452
Fortey, R.A., Harper, D.A.T., Ingham, J.K., Owen, A.W., Parkes, M.A., Rushton, A.W.A., and Woodcock, N.H. 2000. A revised correlation of Ordovician rocks in the British Isles. Geological Society, London, Special Report, 24:1-83. https://doi.org/10.1144/SR24
Gaines, R.R. 2014. Burgess Shale-type preservation and its distribution in space and time. The Paleontological Society Papers, 20:123-146. https://doi.org/10.1017/S1089332600002837
Gaines, R.R. and Droser, M.L. 2010. The paleoredox setting of Burgess Shale-type deposits. Palaeogeography, Palaeoclimatology, Palaeoecology, 297:649-661. https://doi.org/10.1016/j.palaeo.2010.09.014
Garcia-Bellido, C.D. and Conway Morris, S. 1999. New fossil worms from the Lower Cambrian of the Kinzers Formation, Pennsylvania, with some comments on Burgess Shale-Type preservation. Journal of Paleontology, 73:394-402.
https://doi.org/10.1017/S0022336000027918
Gutiérrez-Marco, J.C. Albani, R., Aramburu, C., Arbizu, M., Babin, C., García-Ramos, J.C., Méndez-Bedia, I., Rábano, I., Truyols, J., Vannier, J., and Villas, E. 1996. Bioestratigrafía de la Formación Pizarras del Sueve (Ordovícico Medio) en el sector septentrional de la escama de Laviana-Sueve (Zona Cantábrica, Norte de España). Revista Española de Paleontología, 11:48-74.
Gutiérrez-Marco, J.C., Sá, A.A., García-Bellido, D.C., and Rábano, I. 2017. The Bohemo-Iberian regional chronostratigraphical scale for the Ordovician System and palaeontological correlations within South Gondwana. Lethaia, 50:258-295. https://doi.org/10.1111/let.12197
Halanych, K.M., Cannon, J.T., Mahon, A.R., Swalla, B.J., and Smith, C.R. 2013. Modern Antarctic acorn worms form tubes. Nature Communications, 4(1):1-4. https://doi.org/10.1038/ncomms3738
Handle, K.C. and Powell, W.G. 2012. Morphologically simple enigmatic fossils from the Wheeler Formation: A comparison with definitive algal fossils. Palaios, 27:304-316. https://doi.org/10.2307/41692708
Havlíček, V. 1982. Ordovician of Bohemia: development of the Prague Basin and its benthic communities. Sborník geologických věd, Geologie, 37:103-136.
Havlíček, V. 1998. Ordovician, p. 149-164. In Chlupáč, I., Havlíček, V., Kříž, J., Kukal, Z., and Štorch, P. (eds.), Palaeozoic of the Barrandian. Czech Geological Survey, Prague.
Havlíček, V. and Fatka, O. 1992. Ordovician of the Prague Basin (Barrandian area, Czechoslovakia), p. 461-472. In Webby, B.D. and Laurie, J.R. (eds.), Global perspectives on Ordovician geology. Balkema, Rotterdam.
Havlíček, V. and Marek, L. 1973. Bohemian Ordovician and its international correlation. Časopis pro mineralogii a geologii, 18:225-232.
Havlíček, V. and Vaněk, J. 1966. The Biostratigraphy of the Ordovician of Bohemia. Sborník geologických věd, Paleontologie, 8:7-69.
Havlíček, V. and Vaněk, J. 1990. Ordovician invertebrate communities in black-shale lithofacies (Prague Basin, Czechoslovakia). Věstník Ústředního ústavu geologického, 65:223-236.
Havlíček, V. and Vaněk, J. 1996. Dobrotivian/Berounian boundary interval in the Prague Basin with a special emphasis on the deepest part of the trough (Ordovician, Czech Republic). Věstník Českého geologického ústavu, 71:225-243.
Havlíček, V., Vaněk, J., and Fatka, O. 1993. Floating algae of the genus Krejciella as probable hosts of epiplanktic organisms (Dobrotiv Series, Ordovician: Prague Basin). Journal of the Czech Geological Society, 38:79-88.
Havlíček, V., Vaněk, J., and Fatka, O. 1994. Perunica microcontinent in the Ordovician (its position within Mediterranean Province, series division, benthic and pelagic association). Sborník geologických věd, Geologie, 46:23-56.
Hu, S.X., Zhu, M.Y., Luo, H.L., Steiner, M., Hui, L., Zhao, F.C., and Liu, Q. 2010. Biodiversity and taphonomy of the Early Cambrian Guanshan biota, eastern Yunnan. Science in China, 53:1765-1773. https://doi.org/10.1007/s11430-010-4086-9
Ivantsov, A.Yu. and Wrona, R. 2004. Articulated palaeoscolecid sclerite arrays from the Lower Cambrian of eastern Siberia. Acta Geologica Polonica, 54:1-222.
Ivantsov, A.Yu., Zhuravlev, A.Yu., Krassilov, V.A., Leguta, A.V., Melnikova, L.M., Urbanek, A., Ushatinskaya, G.T., and Malakhovskaya, Y.E. 2005a. Unique Sinsk localities of Early Cambrian organisms. Transactions of the Palaeontological Institute, 284:1-143. (In Russian)
Ivantsov, A.Yu., Zhuravlev, A.Yu., Leguta, A.V., Krassilov, V.A., Melnikova, L.M., and Ushatinskaya, G.T. 2005b. Palaeoecology of the Early Cambrian Sinsk Biota from the Siberian Platform. Palaeogeography, Palaeoclimatology, Palaeoecology, 220:69-88. https://doi.org/10.1016/j.palaeo.2004.01.022
Jenkins, W.A.M. 1967. Ordovician Chitinozoa from Shropshire. Palaeontology, 10:436-488.
Kenrick, P., Kvaček, Z., and Bengtson, S. 1999. Semblant land plants from the Middle Ordovician of the Prague Basin reinterpreted as animals. Palaeontology, 42:991-1002. https://doi.org/10.1111/1475-4983.00106
Kettner, R. and Kodym, O. 1919. New stratigraphy of the Barrandian. Časopis Musea Království českého, 93:47-55. (In Czech)
Kimming, J. and Pratt, B.R. 2015a. Soft-bodied biota from the middle Cambrian (Drumian) Rockslide Formation, Mackenzie Mountains, northwestern Canada. Journal of Paleontology, 89:51-71. https://doi.org/10.1017/jpa.2014.5
Kimming, J. and Pratt, B.R. 2015b. Taphonomy of the middle Cambrian (Drumian) Ravens Throat River Lagerstätte, Rockslide Formation, Mackenzie Mountains, Northwest Territories, Canada. Lethaia, 49:150-169. https://doi.org/10.1111/let.12135
Krassilov, V.A. 1982. New data on Orestovia and the problem of origin of higher plants. Komarovskie chtenia, 29:23-33. (In Russian)
Krassilov, V.A. 2005. Cyanobacteria and algae, p. 34-42. In Ivantsov, A.Y., Zhuravlev, A.Y., Krassilov, V.A., Leguta, A.V., Melnikova, L.M., Urbanek, A., Ushatinskaya, G.T., and Malakhovskaya, Y.E. (eds.), Unique Sinsk localities of Early Cambrian organisms. Transactions of the Palaeontological Institute 284.
Krishtofovich, A.N. 1953. Discovery of Lycopodiaceae in the Cambrian deposits of eastern Siberia. Doklady Akademii Nauk SSSR, 91:1377-1379.
Kukal, Z. 1957. A lithological investigation of the Skalka and Drabov beds of the Ordovician of tha Barrandian. Sborník Ústředního ústavu geologického, 23:215-295.
Kvaček, Z. and Kraft, P. 2015. The oldest plants on the territory of the Bohemian Massif. Botanika 3:8-9. (In Czech)
Lajblová, K. and Kraft, P. 2014. The earliest ostracods from the Ordovician of the Prague Basin, Czech Republic. Acta Geologica Polonica, 64:367-392. https://doi.org/10.2478/agp-2014-0021
Lefebvre, B. 2007. Early Palaeozoic palaeobiogeography and palaeoecology of stylophoran echinoderms. Palaeogeography, Palaeoclimatology, Palaeoecology, 245:156-199. https://doi.org/10.1016/j.palaeo.2006.02.021
Lemoigne, Y. 1975. Paléoflores et provinces paléofloristiques au cours des temps géologiques. Bulletin de la Société Géologique de France S7-XVII:867-877. https://doi.org/10.2113/gssgfbull.S7-XVII.5.867
Li, Y., Kocot, K.M., Tassia, M.G., Cannon, J.T., Bernt, M., and Halanych, K.M. 2019. Mitogenomics reveals a novel genetic code in Hemichordata. Genome biology and evolution, 11(1):29-40. https://doi.org/10.1093/gbe/evy254
LoDuca, S.T., Caron, J.B., Schiffbauer, J.D., Xiao, S., and Kramer, A. 2015. A reexamination of Yuknessia from the Cambrian of British Columbia and Utah. Journal of Paleontology, 89:82-95. https://doi.org/10.1017/jpa.2014.7
LoDuca, S.T., Bykova, N., Wu, M., Xiao, S., and Zhao, Y. 2017. Seaweed morphology and ecology during the great animal diversification events of the early Paleozoic: A tale of two floras. Geobiology, 15:588-616. https://doi.org/10.1111/gbi.12244
Loi, A. and Dabard, M.P. 2002. Controls of sea level fluctuations on the formation of Ordovician siliceous nodules in terrigenous offshore environments. Sedimentary Geology 153:65-84. https://doi.org/10.1016/S0037-0738(02)00102-1
Luchinina, V.A. 2013. Cambrian Algoflora: Association of various microorganism groups. Paleontological Journal, 47:989-996.
Maletz, J. 2014. Hemichordata (Pterobranchia, Enteropneusta) and the fossil record. Palaeogeography, Palaeoclimatology, Palaeoecology, 398:16-27. https://doi.org/10.1016/j.palaeo.2013.06.010
Maletz, J. 2019. Tracing the evolutionary origins of the Hemichordata (Enteropneusta and Pterobranchia). Palaeoworld, 28:58-72. https://doi.org/10.1016/j.palwor.2018.07.002
Maletz, J. 2020. Hemichordata (Enteropneusta & Pterobranchia, incl. Graptolithina): A review of their fossil preservation as organic material. Bulletin of Geosciences, 95(1):41-80. https://doi.org/10.3140/bull.geosci.1776
Maletz, J. and Steiner, M. 2016. Graptolite (Hemichordata, Pterobranchia) preservation and identification in the Cambrian Series 3. Palaeontology, 58:1073-1107. https://doi.org/10.1111/pala.12200
Manda, Š. 2008. Trocholites Conrad, 1838 (Nautiloidea, Tarphycerida) in the Middle Ordovician of the Prague Basin and its palaeobiogeographical significance. Bulletin of Geosciences, 83:327-334. https://doi.org/10.3140/bull.geosci.2008.03.327
Mauviel, A., Juniper, S.K., and Sibuet, M. 1987. Discovery of an enteropneust associated with a mound-burrows trace in the deep sea: ecological and geochemical implications. Deep-Sea Research, 34:329-335.
https://doi.org/10.1016/0198-0149(87)90141-5
Mergl, M. 2002. Linguliformean and craniiformean brachiopods of the Ordovician (Třenice to Dobrotivá formations) of the Barrandian, Bohemia. Acta Musei Nationalis Pragae, Series B, 58:1-82.
Mergl, M., Fatka, O., and Budil, P. 2007. Lower and early Middle Ordovician trilobite associations of the Prague Basin (Perunica, Czech Republic). Acta Palaeontologica Sinica, 46 (Suppl.):320-327.
Mergl, M., Fatka, O., and Budil, P. 2008. Lower and Middle Ordovician trilobite associations of Perunica: from shoreface endemicity to offshore uniformity (Prague Basin, Czech Republic), p. 275-282. In Rábano, I., Gozalo, R., and García-Bellido, D. (eds.), Advances in trilobite research. Cuadernos del Museo Geominero 9. Instituto Geológico y Minero de España.
Mergl, M. and Vohradský, O. 2000. Geological excursions to the Plzeň area. KOURA publishing. Mariánské Lázně. (In Czech)
Mikuláš, R. 1991. Trace fossils from siliceous concretions in the Šárka and Dobrotivá Formations (Ordovician, Central Bohemia). Journal of the Czech Geological Society, 36:29-38.
Nanglu, K. and Caron, J.B. 2021. Symbiosis in the Cambrian: enteropneust tubes from the Burgess Shale co-inhabited by commensal polychaetes. Proceedings of the Royal Society B 288.1951 (2021): 20210061. https://doi.org/10.1098/rspb.2021.0061
Nanglu, K., Caron, J.B., and Cameron, C.B. 2015. Using experimental decay of modern forms to reconstruct the early evolution and morphology of fossil enteropneusts. Paleobiology, 41:460-478. http://doi.org/10.1017/pab.2015.11
Nanglu, K., Caron, J.B., Conway Morris, S., and Cameron, C.B. 2016. Cambrian suspension-feeding tubicolous hemichordates. BMC Biology 14, 56. https://doi.org/10.1186/s12915-016-0271-4
Nanglu, K., Caron, J.B., and Gaines, R.R. 2020. The Burgess Shale paleocommunity with new insights from Marble Canyon, British Columbia. Paleobiology, 46:58-81. https://doi.org/10.1017/pab.2019.42
Obrhel, J. 1968. Neue Pflanzenfunde im mittelböhmischen Ordovizium. Věstník Ústředního ústavu geologického, 43:463-464.
Oulebsir, L. and Paris, F. 1995. Chitinozoaires ordoviciens du Sahara algérien: biostratigraphie et affinités paléogéographiques. Review of Palaeobotany and Palynology, 86(1):49-68.
https://doi.org/10.1016/0034-6667(94)00098-5
Paris, F. 1981. Les Chitinozoaires dans le Paléozoique du Sud-Ouest de l’Europe (Cadre géologique Etude systématique. Biostratigraphie). Mémoires de la Société géologique et minéralogique de Bretagne, 26:1-496.
Paris, F. 1990. The Ordovician chitinozoan biozones of the Northern Gondwana Domain. Review of Palaeobotany and Palynology, 66:181-209. https://doi.org/10.1016/0034-6667(90)90038-K
Paris, F. 1996. Chitinozoan biostratigraphy and palaeoecology, p. 531-552. In Jansonius, J. and McGregor, D.C. (eds.), Palynology: principles and applications. American Association of Stratigraphic Palynologists Foundation, 2.
Paris, F. 2006. Chitinozoans a fascinating and mysterious microfossil group. 1-81. Rennes. https://doi.org/10.13140/RG.2.1.2462.7682
Paris, F., Achab, A., Asselin, E., Chen, X.H., Grahn, Y., Nõlvak, J., Obut, O., Samuelsson, J., Sennikov, N., Vecoli, M., Verniers, J., Wang, X.F., and Winchester-Seeto, T. 2004. Chapter 28: Chitinozoans, p. 294-311. In Webby, B.D., Paris, F., Droser, M.L., and Percival, I.G. (eds.), The Great Ordovician Biodiversification Event. Columbia University Press.
Paris, F., Le Hérissé, A., Monod, O., Kozlu, H., Ghienne, J.F., Dean, W.T., Vecoli, M., and Günay, Y. 2007. Ordovician chitinozoans and acritarchs from southern and southeastern Turkey. Revue de micropaléontologie, 50:81-107. https://doi.org/10.1016/j.revmic.2006.11.004
Paris, F., Verniers, J., and Al-Hajri, S. 2000. Ordovician Chitinozoans from Central Saudi Arabia. In Al-Hajri, S. and Owens, B. (eds.), Palaeozoic Palynostratigraphy of Saudi Arabia. GeoArabia, special publication, 81:42-56.
Peng, S., Babcock, L.E., and Cooper, R.A. 2012. Chapter 19. The Cambrian Period, p. 437-488. In Gradstein, F.M., Ogg, J.G., Schmitz, M., and Ogg, G. (eds.), The Geologic Time Scale. https://doi.org/10.1016/B978-0-444-59425-9.00019-6
Peršín, J. and Budil, P. 2009. New observations on Šárka and Dobrotivá Formation (Ordovician, Darriwilian Stage) in northwestern and northern surroundings of Prague. Český kras, 35:25-35. (In Czech with English summary)
Powell, W.G., Johnston, P.A., and Collom, C.J. 2003. Geochemical evidence for oxygenated bottom waters during deposition of fossiliferous strata of the Burgess Shale Formation. Palaeogeography, Palaeoclimatology, Palaeoecology, 201:249-268. https://doi.org/10.1016/S0031-0182(03)00612-6
Resser, C.E. 1938. Middle Cambrian fossils from Pend Oreille Lake, Idaho. Smithsonian Miscellaneous Collections, 97:1-12.
Resser, C.E. and Howell, B.F. 1938. Lower Cambrian Olenellus Zone of the Appalachians. Geological Society of America, Bulletin, 49:195-248. https://doi.org/10.1130/GSAB-49-195
Rozanov, A.Y., Parkhaev, P.Y., Shabanov, Y.Y., Pegel, T.V., Raevskaya, E.G., Zhuravlev, A.Yu., Gámez, V.J., and Ergaliev, G.K. 2008. The 13th international field conference of the Cambrian stage subdivision working group. Episodes, 31:440-441.
Rozanov, A.Yu. and Zhuravlev, A.Yu. 1992. The Lower Cambrian fossil record of the Soviet Union, p. 205-282. In Lipps, J.H. and Signor, P.W. (eds.), Origin and Early Evolution of the Metazoa. Plenum Press. New York. https://doi.org/10.1007/978-1-4899-2427-8_7
Sales, R. 2015. Chitinozoan Biodiversity in the Ordovician of Gondwana: An Interval-Free Approach Using the Quantitative Stratigraphic Correlation Program CONOP9. Honor theses, University of Dayton, Ohio.
Satterthwaite, D.F. 1976. Paleobiology and paleoecology of Middle Cambrian algae from western North America. Unpublished Ph.D. dissertation, University of California, Los Angeles, 121 pp.
Servais, T., Dzik, J., Fatka, O., Heuse, T., Vecoli, M., and Verniers, J. 2008. Ordovician, p. 203-248. In McCann, T. (ed.). Geology of Central Europe. Geological Society, London.
Simakov, O., Kawashima, T., Marlétaz, F., Jenkins, J., Koyanagi, R., Mitros, T., Hisata, K., Bredeson, J., Shoguchi, E., Gyoja, F., and Yue, J.X. 2015. Hemichordate genomes and deuterostome origins. Nature, 527(7579):459-465. https://doi.org/10.1038/nature16150
Steiner, M. and Maletz, J. 2012. Cambrian graptolites (Pterobranchia) and the origin of colonial organization in metazoans. Terra Nostra, 2012:174-175.
Tang, P., Geng, L.Y., and Zhang, S.B., 2003. Cold-water chitinozoan species from the Ordovician sediments in Tarim Basin, Xinjiang, China. Acta Palaeontologica Sinica, 42:104-110.
Torsvik, T.H. and Cocks, L.R.M. 2013. Gondwana from top to base in space and time. Gondwana Research, 24:999-1030. https://doi.org/10.1016/j.gr.2013.06.012
Vacek, F. and Žák, J. 2017. A lifetime of the Variscan orogenic plateau from uplift to collapse as recorded by the Prague Basin, Bohemian Massif. Geological Magazine. 156:1-25. https://doi.org/10.1017/S0016756817000875
Vaněk, J. 1995. New deeper water trilobites in the Ordovician of the Prague Basin Czech Republic. Palaeontologia Bohemiae, 1:1-12.
Vaněk, J. 1999. Ordovician in the easternmost part of the Prague Basin (Úvaly and Brandýs areas) and its comparison with the Rokycany area (westernmost part of the basin). Palaeontologia Bohemiae, 5:5-20.
Videt, B., Paris, F. Rubino, J.L., Boumendjel, K., Dabard, M.P., Loi, A., Ghienne, J. F., Marante, A., and Gorini, A. 2010. Biostratigraphical calibration of third order Ordovician sequences on the northern Gondwana Platform. Palaeogeography, Palaeoclimatology, Palaeoecology, 296:359-375. https://doi.org/10.1016/j.palaeo.2010.03.050
Vodička, J. and Fatka, O. 2017. Occurrences of chitinozoans in the Upper Ordovician Letná Formation of the Barrandian area (Czech Republic). Geoscience Research Reports, 50:235-239. (In Czech with English summary). https://doi.org/10.3140/zpravy.geol.2017.43
Vokáč, V. and Grigar, L. 2010. Occurence of the fossiliferous Ordovician Dobrotivá Formation (Upper Darriwilian to Lower Sandbian?) at Tymákov (western part of the Prague Basin, Barrandian area, Czech Republic). Erica, 17:159-163. (In Czech with English summary)
Waggoner, B.C. and Hagadorn, J.W. 2004. An unmineralized Alga from the Lower Cambrian of California, USA. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 231:67-83. https://doi.org/10.1127/njgpa/231/2004/67
Walcott, C.D. 1911. Middle Cambrian annelids. Smithsonian Miscellaneous Collections, 57:109-144.
Walcott, C.D. 1931. Addenda to descriptions of Burgess Shale fossils. Smithsonian Miscellaneous Collections, 85:1-46. https://doi.org/10.1086/623925
Webby, B.D., Droser, M.L., Paris, F., and Percival, I.G. (eds.) 2004. The Great Ordovician Biodiversification Event. Columbia University Press, New York.
Zhuravlev, A.Yu. and Wood, R.A. 1996. Anoxia as the cause of the mid-Early Cambrian (Botomian) extinction event. Geology, 24:311-314. https://doi.org/10.1130/0091-7613(1996)024<0311:AATCOT>2.3.CO;2


