Body mass divergence in sympatric deer species of Pleistocene Crete (Greece)
Article number: 25.2.a23
https://doi.org/10.26879/1221
Copyright Paleontological Society, July 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Appendix
Submission: 4 April 2022. Acceptance: 4 July 2022.
ABSTRACT
Adaptive radiations play a crucial role in macroevolutionary theory. Insular adaptive radiations of mammals are, however, rare and often insufficiently understood. We here investigate the disparity in body mass in an insular deer genus (Candiacervus), represented with eight species of the Pleistocene of Crete (Aegean Sea, Greece). Our results, taking derived body proportions into account, show the following mass distribution: 27.8 kg (C. ropalophorus), 41.5 kg (C. listeri, C. devosi, C. reumeri), 74.7 kg (C. cretensis), 105.9 kg (C. rethymnensis), 170.1 (C. dorothensis) and 245.4 kg (C. major). The reconstructed body mass range accounts for nearly one quarter of the total range of living and fossil Cervidae. The largest species of Cretan deer (C. major) is approximately eight times heavier than the smallest species (C. ropalophorus). This remarkable degree of body mass divergence, which is unique among Cervidae, apparently evolved under ecological release on a terrestrial predator-free island with limited inter-specific competition.
Eva Besiou. Faculty of Geology and Geoenvironment, Department of Historical Geology-Palaeontology, National and Kapodistrian University of Athens, 15784 Zografos, Greece (corresponding author). evabesiou@geol.uoa.gr
Maria Nefeli Choupa. Department of History and Ethnology, Laboratory of Physical Anthropology, Democritus University of Thrace, 69100 Komotini, Greece. nefelihoupa@gmail.com
George Lyras. Faculty of Geology and Geoenvironment, Department of Historical Geology-Palaeontology, National and Kapodistrian University of Athens, 15784 Zografos, Greece. glyras@geol.uoa.g
Alexandra van der Geer. Vertebrate Evolution, Developm ent and Ecology, Naturalis Biodiversity Center, 2300 RA Leiden, the Netherlands. alexandra.vandergeer@naturalis.nl
Keywords: Candiacervus; cladogenesis; Cervidae; body size evolution; island gigantism; adaptive radiation
Final citation: Besiou, Eva, Choupa, Maria Nefeli, Lyras, George, and van der Geer, Alexandra. 2022. Body mass divergence in sympatric deer species of Pleistocene Crete (Greece) Palaeontologia Electronica, 25(2):a23. https://doi.org/10.26879/1221
palaeo-electronica.org/content/2022/3663-body-mass-of-candiacervus
Copyright: July 2022 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Understanding the factors that explain speciation patterns in natural populations is a central theme in macroevolutionary studies, and long-term studies are a crucial element. Especially islands may offer new ecological opportunities as prerequisite to speciation to the successful colonizers provided the right circumstances, such as large island size, large variation in habitats, and lack of competitors and predators, are guaranteed for a sufficiently long time period (Lomolino et al., 2017). Under such conditions primary colonizers can even diversify in terms of morphology, behaviour, physiology and ecology beyond the degree seen on the mainland. This ecological and evolutionary diversification from an ancestral species into a multitude of new forms, or adaptive radiation (Losos et al., 1998; Schluter, 2000; Glor, 2010), can take place rapidly. Textbook examples of island adaptive radiation include Darwin’s finches, Hawaiian honeycreepers (Navalón et al., 2020), and Hawaiian lobeliads (Givnish et al., 2008). Cases of insular adaptive radiation have been described for vertebrates, invertebrates, and plants (Lomolino et al., 2017). Examples are rarer for mammals and include the murids of Luzon (Jansa et al., 2006), tenrecs (Poux et al., 2008), Malagasy mice (Terray et al., 2022), and lemurs of Madagascar (Martin, 1972; Tattersall, 1982; see, however, Herrera, 2017 in case the requirement of an ‘early burst’ of phenotypic evolution is included in the definition). The range of morphological disparity that can evolve is, amongst others, phylogenetically and spatio-temporally constrained. This paper discusses and tests this maximal range of size disparity in a deer lineage that was isolated for less than 250 Ky on a Mediterranean island during the Pleistocene.
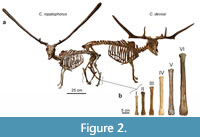
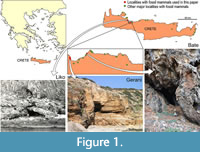 During the late Middle and Late Pleistocene the island of Crete (Figure 1) harbored a depauperate, yet highly endemic, insular mammalian fauna consisting only of deer, elephants, mice, and otters (van der Geer et al., 2021; Lyras et al., 2022) on a maximal surface of c. 10,000 km2 (van der Geer et al., 2016). The deer (Candiacervus) has been proposed to constitute a case of insular adaptive radiation (de Vos and van der Geer, 2002). In total, eight nominal species of Cretan deer have been named (van der Geer, 2018a). Because fossils of deer species of different sizes and/or with different antler types are frequently found together (de Vos, 2000; van der Geer, 2018a), their contemporary existence is best explained as the result of an evolutionary radiation to occupy different ecological niches (de Vos and van der Geer, 2002). The most conspicuous results of this radiation are divergent body sizes ranging from dwarf, medium to giant forms (Figure 2). Body mass is one of the most important biological properties as it is strongly correlated with numerous anatomical, physiological, ecological, and life-history characteristics (e.g., Damuth, 1981; Gillooly et al., 2001, 2002). Moreover, the body mass in ungulates plays an important role in the spatial range of individuals (Grüner et al., 2016), resource partitioning, and coexistence with other species (Demment and van Soest, 1985).
During the late Middle and Late Pleistocene the island of Crete (Figure 1) harbored a depauperate, yet highly endemic, insular mammalian fauna consisting only of deer, elephants, mice, and otters (van der Geer et al., 2021; Lyras et al., 2022) on a maximal surface of c. 10,000 km2 (van der Geer et al., 2016). The deer (Candiacervus) has been proposed to constitute a case of insular adaptive radiation (de Vos and van der Geer, 2002). In total, eight nominal species of Cretan deer have been named (van der Geer, 2018a). Because fossils of deer species of different sizes and/or with different antler types are frequently found together (de Vos, 2000; van der Geer, 2018a), their contemporary existence is best explained as the result of an evolutionary radiation to occupy different ecological niches (de Vos and van der Geer, 2002). The most conspicuous results of this radiation are divergent body sizes ranging from dwarf, medium to giant forms (Figure 2). Body mass is one of the most important biological properties as it is strongly correlated with numerous anatomical, physiological, ecological, and life-history characteristics (e.g., Damuth, 1981; Gillooly et al., 2001, 2002). Moreover, the body mass in ungulates plays an important role in the spatial range of individuals (Grüner et al., 2016), resource partitioning, and coexistence with other species (Demment and van Soest, 1985).
 The Cretan deer offers a unique opportunity to test the range of body mass divergence that evolved within the context of adaptive radiation in insular megafauna in a temperate climate zone on a predator-free, medium-sized island, and evaluate this divergence in a phylogenetic context. To our knowledge, despite the numerous studies on the morphology of Candiacervus, only two works include estimations of its body mass (Palombo et al., 2008; van der Geer et al., 2013). Palombo et al. (2008) focused on the smallest species, while van der Geer et al. (2013) only provided a size range for the genus as a whole, varying between 22 kg and 316 kg.
The Cretan deer offers a unique opportunity to test the range of body mass divergence that evolved within the context of adaptive radiation in insular megafauna in a temperate climate zone on a predator-free, medium-sized island, and evaluate this divergence in a phylogenetic context. To our knowledge, despite the numerous studies on the morphology of Candiacervus, only two works include estimations of its body mass (Palombo et al., 2008; van der Geer et al., 2013). Palombo et al. (2008) focused on the smallest species, while van der Geer et al. (2013) only provided a size range for the genus as a whole, varying between 22 kg and 316 kg.
The aim of this contribution is to reconstruct the body mass of the individual species. Future studies can then investigate various aspects of the ecology of these species, in particular the nature of their interaction and the evolution thereof.
MATERIALS AND METHODS
Taxonomy of the Cretan Deer
The Cretan deer have been grouped by de Vos (1979) into six size classes (size classes I till VI; Figure 2). This grouping was based on a biometrical study of postcranial materials from several sites. Inferred on shared morphological features, the size classes are best explained as the result of a single colonisation, followed by an evolutionary radiation or cladogenesis (de Vos and Dermitzakis, 1986; de Vos, 2000; de Vos and van der Geer, 2002). As no cranial or antler material is known from the largest forms, this evolutionary scheme is based on the morphology of postcranial elements alone (van der Geer et al., 2013). Accordingly, all species are classified into the genus Candiacervus, as follows: C. ropalophorus (size class I), C. devosi, C. listeri, and C. reumeri (size class II), C. cretensis (size class III), C. rethymnensis (size class IV), C. dorothensis (size class V), and C. major (size class VI). The three species of size II can be further distinguished based on antler morphology (van der Geer, 2018a).
Fossil Localities
There are over a hundred localities with fossils of insular mammals on Crete (Lyras et al., 2021). The fossil materials used in this study have been collected from four sites: Gerani 2, Gerani 4, Liko, and Bate caves (Figure 1). Gerani 2 and 4 preserve mostly the smallest deer, whereas Liko cave preserves mostly size class II (de Vos, 1984). Bate cave is the only locality where complete elements of the two largest size classes have been found.
Gerani and Liko caves were excavated in the 1970s by a team led by the late Paul Sondaar (1934-2003) of the University of Utrecht, the Netherlands (de Vos, 1984). Bate cave was excavated in 1975 by a team led by the late Alberto Malatesta (1915-2007) of the University of Rome, Italy (Kotsakis et al., 1976). The fossil deer specimens collected by these two teams are currently curated at the Museum of Palaeontology and Geology, National and Kapodistrian University of Athens, Greece (AMPG), Naturalis Biodiversity Center, Leiden, the Netherlands (RGM), and the Museum of Palaeontology, University of Rome La Sapienza, Italy (MPUR).
Fossil Material
The materials used here for estimating the body sizes of the Cretan deer have been described in detail by de Vos (1979; 1984), Capasso Barbato and Petronio (1986), and Capasso Barbato (1992).
Candiacervus ropalophorus (size class I). The postcranial material used in this study consists of five metacarpals, four humeri, three radii, two tibiae, three femora and six metatarsals. The fossils have been collected from Gerani caves and are curated at AMPG and RGM.
Candiacervus devosi, C. reumeri, and C. listeri (size class II). The three deer species of this size class are generally referred to as Candiacervus sp. II or C. sp. 2. As such this is a heterogeneous group consisting of three distinct species, Candiacervus devosi, C. reumeri, and C. listeri, which differ in antler and skull morphology but cannot be distinguished based on postcranial elements (van der Geer, 2018). The material used in this study consists of 57 metacarpals, 22 humeri, 57 radii, 39 tibiae, 38 femora, and 50 metatarsals. The male skull belongs to the species C. listeri. The fossils have been collected from Liko cave and are curated at AMPG.
Candiacervus cretensis (size class III). The material used here consists of one metacarpal, one humerus, one radius and one metatarsal. The fossils have been collected from Liko cave and are curated at AMPG. The humerus and radius were found in association and likely belong to one individual. The fossils have been collected from Liko cave and are curated at AMPG.
Candiacervus rethymnensis (size class IV). The material used here consists of one humerus, one tibia, one metacarpal, one metatarsa,l and one partial femur. The fossils have been collected from Liko cave and are curated at AMPG.
Candiacervus dorothensis (size class V). The material used here consists of three metacarpals, two radii, one tibia, twofemora, and two metatarsals. The fossils were collected from Bate cave and are curated at MPUR.
Candiacervus major (size class VI). The material used here consists of one incomplete humerus, one radius-ulna, one tibia, and two metatarsals. All fossils were collected from Bate cave and are stored at MPUR. The (nearly) complete bones likely belong to a single individual as suggested by their dimensions (Palombo and Zerda, 2021).
In addition to the postcranial elements, we used craniodental measurements for the two smallest size classes (five skulls from size I and one skull from size II) from de Vos (1984).
Body Mass Estimations
We applied two different allometric based methods for estimating the body mass of the different Candiacervus species.
The first method is based on postcranial elements and predicting equations developed by Scott (1990). Although Scott (1990) provides equations that use lengths and transverse diameters, we limit our data set to measurements of articular surfaces and transverse sections of long bones only. This was done because bone width dimensions have been shown to correlate better with body mass than bone length dimensions (Weston and Lister, 2003; Gordon et al., 2008). For this study we used three humeral (H3, H4, H5), four tibial (T2, T3, T4, T5), three femoral (F2, F3, F5), four radial (R2, R3, R4, R5), four metatarsal (Mt2, Mt3, Mt4, Mt5), and four metacarpal (Mc2, Mc3, Mc4, Mc5) measurements. See Scott (1983, 1990) for definitions of measurements and predictive equations.
The second method is based on allometric equations developed by Janis (1990) for estimating the body mass of Cervidae using craniodental measurements. For this study we use the total skull length (TSL), the length of the upper second molar (SUML), and the total length of the lower molar row (TLML).
Deer Phylogeny and Body Mass Evolution
Candiacervus has been suggested to have evolved from a fallow deer (van der Made, 2005a), probably Dama mesopotamica (van der Geer, 2018a). There are several phylogenetic analyses of living and fossil deer, including total evidence approaches (e.g., Groves and Grubb, 1987; Lister et al., 2005; Groves, 2014; Heckeberg, 2020). Despite these efforts, the phylogenetic relationships of many fossil taxa remain unresolved. Here we use the cervid branch of a phylogenetic tree published by Carotenuto et al. (2015). Since Carotenuto et al. (2015) did not include Candiacervus in their phylogeny, we inserted Candiacervus manually. Following the phylogenetic scheme of van der Geer (2018a), we added it as a sister clade to Dama mesopotamica. Since the precise phylogenetic relationships among Candiacervus species are unknown, we placed the Cretan deer species as a polytomy. This scheme is analogous to the evolutionary adaptive radiation (model C) of Dermitzakis and de Vos (1987). Although de Vos (1996, 2000) considers this model as the most likely mode of evolution for the Cretan deer, we are aware that simultaneous speciation of all Candiacervus species is one of the many possible phylogenies. Nevertheless, our analysis is not influenced by this assumption, as our intention is not to quantitatively examine the phylogenetic distances within Candiacervus.
We further added two fossil and two living taxa to the tree: Praemegaceros cazioti (Sardinian dwarf deer), Alces gallicus (Villafranchian elk), Rusa marianna (Philippine sambar), and Cervus elaphus corsicanus (Corsican red deer). Carotenuto et al. (2015) did not resolve the phylogeny of Rusa and Cervus, therefore, we manually added R. marianna and C. e. corsicanus to the polytomy. We similarly added Praemegaceros cazioti as sister taxon to P. solilhacus, following van der Made (2005b) and van der Made and Palombo (2006). We follow Heintz and Poplin (1980), Lister (1987), Croitor (2014), and van der Made et al. (2014) in including the fossil elk species under the genus Alces. We also adapted the elk branch of the tree following van der Made et al. (2014).
The body masses of living and fossil deer (other than Candiacervus spp., P. cazioti, M. giganteus and Alces spp.) are from Carotenuto et al. (2015). The body mass of P. cazioti is taken from Lomolino et al. (2013). The body masses of Megaloceros giganteus, Alces acles, and A. latifrons are from Saarinen et al. (2016). We estimated the body mass of Alces gallicus using the length of the metacarpals from Sénèze (measurements from van der Made et al., 2014). The visualizations of body mass onto phylogeny were created using Phytools, a package in R developed by Revell (2012).
Previous Works
Palombo et al. (2008) estimated the body mass of small-sized Candiacervus (C. ropalophorus and C. sp. II in their analysis) from Simonelli and Bate caves. They used the width of the occipital condyles and an equation developed by Giovinazzo et al. (2006). The body masses estimated by Palombo et al. (2008) range from 46.36 to 57.45 kg (average 50.4 kg) for the males of Simonelli cave, 33.02 to 58.02 kg and 55.09 to 72.79 for the females and males from Bate cave, respectively. Palombo et al. (2008) assigned their specimens into size classes using cranial measurements. According to them, a male of size II from Simonelli (their specimen n. 4) weighs 57.45 kg, while the weight of males of size class I from Simonelli ranges from 46.36 to 51.45 kg. The weight of the males of size II from Bate cave had a range of 64.04 to 72.79 kg, and those of size I had a range of 55.09 to 55.19. One female of size II was estimated to weigh 58.02 kg, and one female of size I was estimated to weigh 33.02 kg (Palombo et al., 2008).
RESULTS
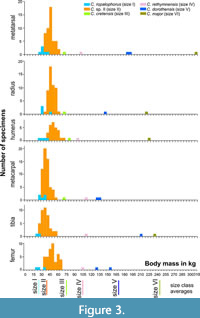 Table 1 shows body mass estimations based on postcranial elements (see Appendix 1 for measurements). Figure 3 presents histograms of the calculated body sizes for each postcranial element. We binned the body masses into 5 kg increments. There is an overlap in the estimated body masses of the two smaller size classes, however, they can be separated as Gerani 2 and 4 yielded mainly size I specimens, while Liko cave yielded no size I specimens.
Table 1 shows body mass estimations based on postcranial elements (see Appendix 1 for measurements). Figure 3 presents histograms of the calculated body sizes for each postcranial element. We binned the body masses into 5 kg increments. There is an overlap in the estimated body masses of the two smaller size classes, however, they can be separated as Gerani 2 and 4 yielded mainly size I specimens, while Liko cave yielded no size I specimens.
Table 2 shows body mass estimations based on cranial and dental elements. These results are comparable to those based on postcranials. It should be noted here that the cranial material is very limited.
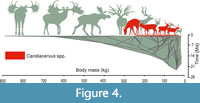
We projected the cervid tree into a space defined by time on the horizontal axis and body mass on the vertical axis (Figure 4). The Cretan deer are indicated in red, while all other taxa are indicated in green. The size variation of Candiacervus equals approximately one quarter of the maximum size variation of the entire family. Furthermore, the Cretan deer lineage achieved this variation in a relatively short time.
 We examined the body size diversity of Cervidae in the following way. The size ratio is calculated based on the mass of the largest and the smallest species (Figure 5). The body mass of the genus is estimated as the average body mass of the species within that genus that are present in our dataset.
We examined the body size diversity of Cervidae in the following way. The size ratio is calculated based on the mass of the largest and the smallest species (Figure 5). The body mass of the genus is estimated as the average body mass of the species within that genus that are present in our dataset.
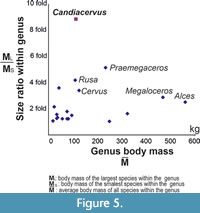 The genera with the largest size range are Alces, Megaloceros, Praemegaceros and Candiacervus, followed by Megaceroides, Rusa, and Cervus. The average body size of all Candiacervus size classes is 111 kg. The largest species of Cretan deer (C. major) is approximately eight times heavier than the smaller species (C. ropalophorus). No other cervid genus exhibits such a high ratio (Figure 5). The other taxa in our dataset have significantly lower size ratios.
The genera with the largest size range are Alces, Megaloceros, Praemegaceros and Candiacervus, followed by Megaceroides, Rusa, and Cervus. The average body size of all Candiacervus size classes is 111 kg. The largest species of Cretan deer (C. major) is approximately eight times heavier than the smaller species (C. ropalophorus). No other cervid genus exhibits such a high ratio (Figure 5). The other taxa in our dataset have significantly lower size ratios.
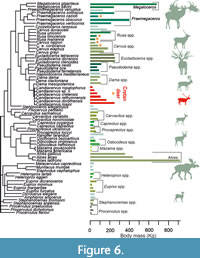 In order to pictorially examine the influence of phylogeny in our analysis, we plotted the body mass of each species onto the cervid phylogenetic tree (Figure 6). Each species is represented by a bar. Congeneric species are represented by bars with the same shade. The size range of Megaloceros spp. and Alces spp. is broader than that of Candiacervus. However, both in Megaloceros and Alces the ratios between the largest and smallest species are small. To our knowledge, the size ratio in Candiacervus is larger than in any other living or fossil cervid genus (Figure 5).
In order to pictorially examine the influence of phylogeny in our analysis, we plotted the body mass of each species onto the cervid phylogenetic tree (Figure 6). Each species is represented by a bar. Congeneric species are represented by bars with the same shade. The size range of Megaloceros spp. and Alces spp. is broader than that of Candiacervus. However, both in Megaloceros and Alces the ratios between the largest and smallest species are small. To our knowledge, the size ratio in Candiacervus is larger than in any other living or fossil cervid genus (Figure 5).
DISCUSSION
Our estimates are based on predictive allometric equations developed by Scott (1990) and Janis (1990), which were developed using modern deer taxa with known weight. Extant deer have a rather broad spectrum of body size, and therefore these equations can be used for predicting the body mass of fossil deer of various sizes without having to extrapolate beyond the range of modern species. A more challenging issue is the different body proportions exhibited by the smallest and largest Cretan species. Of all Cretan deer, only C. rethymnensis retained mainland limb proportions. The smaller species have proportionally shorter limbs, and the largest species have proportionally longer limbs (de Vos, 1979; Capasso Barbato and Petronio, 1986; van der Geer, 2018a). Therefore, in order to avoid this issue, we used only width dimensions, which have been shown to correlate better with body mass than bone length measurements (Gordon et al., 2008; Weston and Lister, 2003).
Our estimates for size classes I and II are lower than those provided by Palombo et al. (2008; see under Materials and methods), based on cranial measurements. Using only cranial measurements, our estimate for C. ropalophorus (size class I) is 30.1 kg for the males and 28.6 kg for the females and 41 kg for male C. listeri (size class II). These estimations are in line with our results based on postcranial elements.
Red deer (Cervus elaphus) is known for its broad size range as it occupies a broad geographical range across different latitudes and altitudes. Yet, van der Geer (2014) noticed that the size range of metatarsals in Candiacervus exceeds that of the extant red deer. Our results confirm that observation. Surprisingly, the body size range of Candiacervus accounts for nearly one quarter of the total body range of living and fossil Cervidae (Figure 4). The size range of Candiacervus is not an exceptional one. The large-bodied lineages, Alces, Megaloceros and Praemegaceros, have a broader body mass range between the smallest and largest chronospecies. What is exceptional in Candiacervus is the size ratio between the largest and smallest species within the genus. The genus Candiacervus contains species with body masses ranging from 27.8 kg (which is comparable to that of a muntjac) to 245.4 kg (which is comparable to that of large-sized sambar). This means that the largest species is more than eight times heavier than the smallest species (Figure 5). That is not the case in any other deer genus, including Megaloceros and Alces. Usually, the body sizes of congeneric mammalian species are roughly similar (Smith et al., 2004). Deer follow this general pattern as well (Figure 5 and Figure 6). Candiacervus thus presents a remarkable exception. It appears that ecological release in the absence of competition and predation eventually led to a significantly increased body mass divergence, including dwarf species as well as giant species. It should be stressed here that this large size variation of Candiacervus was achieved within the rather limited geographic space of a single island. Similar cases of insular body size divergence in ruminants including giant and dwarf forms have been reported from the Late Miocene of Gargano (van der Geer, 2014) and Scontrone (Mazza et al., 2015).
Remarkable is the speed under which this body mass range was achieved. Deer colonized Crete during the late Middle Pleistocene (van der Geer, 2018a). This means that within a relatively short time period, the Cretan deer evolved a remarkable body size diversity. Body size shifts, either towards dwarfing or gigantism, were shown to occur rapidly in other island mammals as well (e.g., Heaney, 1978; Lister, 1989, 1996; Millien, 2006; van der Geer, 2018b; van der Geer et al., 2018). It appears similarly rapid size changes occur, not just in anagenetic evolution, but in evolutionary radiations as well.
ACKNOWLEDGMENTS
This work was made possible thanks to the fossil material collected in the 1970s by Dutch and Italian teams. We further thank J. de Vos for the discussions we had throughout the years on the taxonomy and evolution of the Cretan deer. We are much indebted to H. Brinkerink for his invaluable help in the field. We thank M.R. Palombo (MPUR), J. de Vos and N. den Ouden (RGM) for providing access to specimens under their care. We thank the reviewers, J. van der Made, K. Stefaniak, and an anonymous reviewer for their constructive comments which improved the manuscript.
REFERENCES
Capasso Barbato, L. 1992. Nuova specie di cervide del Pleistocene di Creta. Atti Accademia Nazionale dei Lincei, Memorie Lincee, Scienze Fisiche e Naturali (Serie 9), 1:183-220.
Capasso Barbato, L. and Petronio, C. 1986. Cervus major n. sp. of Bate Cave (Rethymnon, Crete). Atti Accademia Nazionale dei Lincei, Memorie Lincee, Scienze Fisiche, Matematiche e Naturali (Serie 8, 2a), 18:59-100.
Carotenuto, F., Diniz-Filho, J.A.F., and Raia, P. 2015. Space and time: The two dimensions of Artiodactyla body mass evolution. Palaeogeography, Palaeoclimatology, Palaeoecology, 437:18-25. https://doi.org/10.1016/j.palaeo.2015.07.013
Croitor, R. 2014. Deer from Late Miocene to Pleistocene of Western Palearctic: matching fossil record and molecular phylogeny data. Zitteliana, B 32:115-153. https://doi.org/10.5282/ubm/epub.22391
Damuth, J. 1981. Population density and body size in mammals. Nature, 290:699-700. https://doi.org/10.1038/290699a0
Demment, M.W. and Van Soest, P.J. 1985. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. American Naturalist, 125:641-672.
Dermitzakis, M.D. and de Vos, J. 1987. Faunal succession and the evolution of mammals in Crete during the Pleistocene. Neues Jahrbuch Geologische und Paläontologische Abhandlungen, 173:377-408.
de Vos, J. 1979. The endemic Pleistocene deer of Crete. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen B, 82:59-90.
de Vos, J. 1984. The endemic Pleistocene deer of Crete. Verhandelingen der Koninklijke Akademie van Wetenschappen, afd. Natuurkunde, Eerste Reeks, 31:1-100
de Vos, J. 1996. Taxonomy, ancestry and speciation of the endemic Pleistocene deer of Crete compared with the taxonomy ancestry and speciation of Darwin’s finches, p. 111-124. In Reese, D. (ed.), Pleistocene and Holocene Fauna of Crete and Its First Settlers. Monographs in World Archaeology, 28. Prehistory Press, Madison.
de Vos, J. 2000. Pleistocene deer fauna in Crete: its adaptive radiation and extinction. Tropics, 10:125-134. https://doi.org/10.3759/TROPICS.10.125
de Vos, J. and Dermitzakis, M.D. 1986. Models of the development of Pleistocene deer on Crete (Greece). Modern Geology, 10:243-248.
de Vos, J. and van der Geer, A.A.E. 2002. Major patterns and processes in biodiversity: taxonomic diversity on islands explained in terms of sympatric speciation, p. 395-405. In Waldren, B. and Ensenyat, J.A. (eds.), World Islands in Prehistory, International Insular Investigations. BAR International Series, 1095. Archaeopress, Oxford.
Gillooly, J.F., Brown, J.H., West, G.B., Savage, V.M., and Charnov, E.L. 2001. Effects of size and temperature on metabolic rate. Science, 293:2248-2251. https://doi.org/10.1126/science.1061967
Gillooly, J.F., Charnov, E.L., West, G.B., Savage, V.M., and Brown, J.H. 2002. Effects of size and temperature on developmental time. Nature, 417:70-73. https://doi.org/10.1038/417070a
Giovinazzo, C., Altamura, S., and Palombo, M.R. 2006. Determinazione della massa corporea nei ruminanti di media e piccolo taglia: un nuovo approccio metodologico, p. 39. In Fonda, G., Melis, R., and Romano, R. (eds.), Abstracts Giornate Paleontologia 2006, Trieste 8-10 giugno.
Givnish, T.J., Millam, K.C., Mast, A.R., Paterson, T.B., Theim, T.J., Hipp, A.L., Henss, J.M., Smith, J.F., Wood, K.R., and Sytsma, K.J. 2009. Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae). Proceedings of the Royal Society B, 276:407-416. https://doi.org/10.1098/rspb.2008.1204
Glor, R.E. 2010. Phylogenetic insights on adaptive radiation. Annual Review of Ecology, Evolution, and Systematics, 41:251-270. https://doi.org/10.1146/annurev.ecolsys.39.110707.173447
Gordon, A.D., Green, D.J., and Richmond, B.G. 2008. Strong postcranial size dimorphism in Australopithecus afarensis: results from two new resampling methods for multivariate data sets with missing data. American Journal of Physical Anthropology, 135:311-328. https://doi.org/10.1002/ajpa.20745
Groves, C., and Grubb, P. 1987. Relationships of living deer, p. 21-59. In Wemmer, C.M. (ed.), Biology and Management of the Cervidae. Smithsonian Institution Press, Washington, D.C.
Groves, C. 2014. Current taxonomy and diversity of crown ruminants above the species level. Zitteliana B, 32:5-14. https://doi.org/10.5282/UBM/EPUB.17110
Grüner, O.E., Ivar, H., Johan, S.E., and Bernt-Erik, S. 2016. Home ranges, habitat and body mass: simple correlates of home range size in ungulates. Proceedings of the Royal Society B, 283:20161234. https://doi.org/10.1098/rspb.2016.1234
Heaney, L. 1978. Island area and body size of insular mammals: Evidence from the tri-colored squirrel (Callosciurus prevosti) of Southeast Asia. Evolution, 32(1):29-44. https://doi.org/10.2307/2407408
Heckeberg, N.S. 2020. The systematics of the Cervidae: a total evidence approach. PeerJ, 8: e8114. https://doi.org/10.7717/peerj.8114
Heintz, E. and Poplin, F. 1980. Alces carnutorum (Laugel, 1862) du Pléistocéne de Saint-Prest (France): Systematique et évolution des Alcinés (Cervidae, Mammalia). Quartarpalaontologie, 4: 106-122. https://doi.org/10.1515/9783112594124-008
Herrera, J.P. 2017. Testing the adaptive radiation hypothesis for the lemurs of Madagascar. Royal Society Open Science, 4:161014. https://doi.org/10.1098/rsos.161014
Janis, C.M. 1990. Correlation of cranial and dental variables with body size in ungulates and macropodoids, p. 255-300. In Damuth, J. and MacFadden, B.J. (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, New York.
Jansa, S.A., Barker, F.K., and Heaney, L.R. 2006. The pattern and timing of diversification of Philippine endemic rodents: Evidence from mitochondrial and nuclear gene sequences. Systematic Biology, 55:73-88. https://doi.org/10.1080/10635150500431254
Kotsakis, T., Melentis, J., and Sirna, G. 1976. Seconda spedizione paleontologica Lincea nell’isola di Creta (1975). Accademia Nazionale dei Lincei, 223:3-10.
Lister, A.M. 1987. Diversity and evolution of antler form in Quaternary deer, p. 81-91. In Wemmer, C.M., (ed.), Biology and management of Cervidae. Washington, Smithsonian, 81-98.
Lister, A.M. 1989. Rapid dwarfing of red deer on Jersey in the Last Interglacial. Nature, 342:539-542. https://doi.org/10.1038/342539a0
Lister, A.M. 1996. Dwarfing in island elephants and deer: processes in relation to time of isolation. Symposia of the Zoological Society of London, 69:277-292.
Lister, A.M., Edwards, C.J., Nock, D.A.W., Bunce, D.M., Van Pijlen, I.A., Bradley, D.G., Thomas, M.G., and Barne, I. 2005. The phylogenetic position of the ‘giant deer’ Megaloceros giganteus. Nature, 438:850-853. https://doi.org/10.1038/nature04134
Lomolino, M.V., van der Geer, A.A.E., Lyras, G.A., Palombo, M.R., Sax, D., and Rozzi, R. 2013. Of mice and mammoths: generality and antiquity of the island rule. Journal of Biogeography, 40:1427-1439. https://doi.org/10.1111/jbi.12096
Lomolino, M.V., Riddle, B.R., and Whittaker, R.J. 2017. Biogeography: Biological Diversity Across Space and Time, 5th ed. Sinauer Associates, Sunderland, MA.
Losos, J.B., Jackman, T.R., Larson, A., de Queiroz, K., and Rodriguez-Schettino, L. 1998. Contingency and determinism in replicated adaptive radiations of island lizards. Science, 279: 2115-2118. https://doi.org/10.1126/science.279.5359.2115
Lyras, G.A., Athanassiou, A., and van der Geer, A.A.E. 2022. The fossil record of endemic mammals from Greece, p. 661-701. In Vlachos, E. (ed.), The Fossil Vertebrates of Greece: A Synopsis of the Fossil Record and Taxonomy, Vol. 2. Springer-Nature, Switzerland. https://doi.org/10.1007/978-3-030-68442-6_25
Martin, R.D. 1972. Adaptive radiation and behaviour of the Malagasy lemurs. Philosophical Transactions of the Royal Society London B, 264:295-352. https://doi.org/10.1098/rstb.1972.0013
Mazza, P.P.A., Rossi, M.A., and Agostini, S. 2015. Hoplitomerycidae (Late Miocene, Italy), and example of giantism in insular ruminants. Journal of Mammal Evolution, 22:271-277. https://doi.org/10.1007/s10914-014-9277-2
Millien, V. 2006 Morphological evolution is accelerated among island mammals. PLOS Biology, 4(10): e321. https://doi.org/10.1371/journal.pbio.0040321
Navalón, G., Marugán-Lobón, J., Bright, J.A. Cooney, C.R., and Rayfield, E.J. 2020. The consequences of craniofacial integration for the adaptive radiations of Darwin’s finches and Hawaiian honeycreepers. Nature Ecology and Evolution, 4:270-278. https://doi.org/10.1038/s41559-019-1092-y
Palombo, M.R., Köhler, M., Moyà-Solà, S., and Giovinazzo, C. 2008. Brain versus body mass in endemic ruminant artiodactyls: A case studied of Myotragus balearicus and smallest Candiacervus species from Mediterranean Islands. Quaternary International, 182:160-183. https://doi.org/10.1016/j.quaint.2007.08.037
Palombo, M.R. and Zedda, M. 2016. Surviving in a predator-free environment: Hints from a bone remodelling process in a dwarf Pleistocene deer from Crete. Comptes Rendus Palevol, 15:245-254. https://doi.org/10.1016/j.crpv.2015.03.003
Palombo, M.R. and Zedda, M. 2021. The intriguing giant deer from the Bate cave (Crete): could paleohistological evidence question its taxonomy and nomenclature? Integrative Zoology, 17:54-77. https://doi.org/10.1111/1749-4877.12533
Poux, C., Madsen, O., Glos, J., de Jong, W.W., and Vences, M. 2008. Molecular phylogeny and divergence times of Malagasy tenrecs: Influence of data partitioning and taxon sampling on dating analyses. BMC Evolutionary Biology, 8:102. https://doi.org/10.1186/1471-2148-8-102
Revell, L.J. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3:217-223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Saarinen, J., Eronen, J., Fortelius, M., Seppä, H., and Lister, A.M. 2016. Patterns of diet and body mass of large ungulates from the Pleistocene of Western Europe, and their relation to vegetation. Palaeontologia Electronica, 19(2):1-58. https://doi.org/10.26879/443
Schluter, D. 2000. The Ecology of Adaptive Radiation. Oxford University Press, Oxford, UK.
Scott, K.M. 1983. Prediction of body weight of fossil Artiodactyla. Zoological Journal of the Linnean Society, 77:199-215. https://doi.org/10.1111/j.1096-3642.1983.tb00098.x
Scott, K.M. 1990. Postcranial dimensions of ungulates as predictors of body mass, p. 301-336. In Damuth, J. and MacFadden, B.J. (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, New York. https://doi.org/10.1111/j.1469-7998.2006.00094.x
Smith, F.A., Brown, J.H., Haskell, J.P., Lyons, S.K., Alroy, J., Charnov, E.L., Dayan, T., Enquist, B.J., Morgan Ernest, S.K., Hadly, E.A., Jones, K.E., Kaufman, D.M., Marquet, P.A., Maurer, B.A., Niklas, K.J., Porter, W.P., Tiffney, B., and Willig, M.R. 2004. Similarity of mammalian body size across the taxonomic hierarchy and across space and time. The American Naturalist, 163:672-691. https://doi.org/10.1086/382898
Tattersall, I. 1982. The Primates of Madagascar. Columbia University Press, New York. https://doi.org/10.1002/ajpa.1330590417
Terray, L., Denys, C., Goodman, S.M., Soarimalala, V., Lalis, A., and Cornette, R. 2022. Skull morphological evolution in Malagasy endemic Nesomyinae rodents. PLoS ONE, 17(2): e0263045. https://doi.org/10.1371/journal.pone.0263045
van der Geer, A.A.E. 2014. Systematic revision of the family Hoplitomerycidae Leinders, 1984 (Artiodactyla: Cervoidea), with the description of a new genus and four new species. Zootaxa, 3847:1-32. https://doi.org/10.11646/zootaxa.3847.1.1
van der Geer, A.A.E. 2018a. Uniformity in variety: Antler morphology and evolution in a predator-free environment. Palaeontologia Electronica, 21:1-31. https://doi.org/10.26879/834
van der Geer, A.A.E. 2018b. Changing invaders: trends of gigantism in insular introduced rats. Environmental Conservation, 45:203-211. https://doi.org/10.1017/S0376892918000085
van der Geer, A.A., Lyras, G., de Vos, J., and Hara, D. 2013. Morphology of articular surfaces can solve a phylogenetic issue: one instead of two ancestors for Candiacervus (Mammalia: Cervoidea). Zitteliana B, 31:33-34. https://doi.org/10.5282/ubm/epub.17156
van der Geer, A.A.E., Lyras, G.A., MacPhee, R.D.E., Lomolino, M.V., and Drinia, H. 2014. Mortality in a predator-free insular environment: the dwarf deer of Crete. American Museum Novitates, 3807:1-26. https://doi.org/10.1206/3807.1
van der Geer, A.A.E., van den Bergh, G., Lyras, G.A., Prasetyo, U.W., Due, R.A., Setiyabudi, E., and Drinia, H. 2016. The effect of area and isolation on insular dwarf proboscideans. Journal of Biogeography, 43:1656-1666. https://doi.org/10.1111/jbi.12743
van der Geer, A.A.E., Lomolino, M., and Lyras, G.A. 2018. On being the right size - do aliens follow the rules. Journal of Biogeography, 45:515-529. https://doi.org/10.1111/jbi.13159
van der Geer, A.A.E., Lyras, G., and de Vos, J. 2021. Evolution of Island Mammals: Adaptation and Extinction of Placental Mammals on Islands, 2nd ed. Wiley-Blackwell, Hoboken, NJ.
van der Made, J. 2005a. La fauna del Pleistoceno europeo, p. 394-432. In Carbonell, E. (ed.), Homínidos: las primeras ocupaciones de los continents. Ariel Historia.
van der Made, J. 2005b. The fossil endemic goat Nesogoral cenisae n.sp. from Campidano, Sardinia - cursorial adaptations in insular environment. p. 347-368. In Alcover, J.A. and Bover, P. (eds.), Proceedings of the International Symposium Insular Vertebrate Evolution: the Palaeontological Approach. Monografies de la Societat d’Història Natural de les Balears, 12:347-368.
van der Made, J. and Palombo, M.R. 2006. Megaloceros sardus n.sp., a large deer from the Pleistocene of Sardinia. Annales Géologiques des Pays Helléniques, 41:163-176.
van der Made, J., Stefaniak, K., and Marciszak, A. 2014. The Polish fossil record of the wolf Canis and the deer Alces, Capreolus, Megaloceros, Dama and Cervus in an evolutionary perspective. Quaternary International, 326-327:406-430.
https://doi.org/10.1016/j.quaint.2013.11.015
Weston, E.M. and Lister, A.M. 2009. Insular dwarfism in hippos and a model for brain size reduction in Homo floresiensis. Nature, 459:85-88. https://doi.org/10.1038/nature07922


