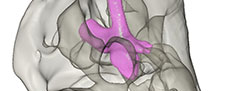
 Estimating the endocranial volume and body mass of Anteosaurus, Jonkeria, and Moschops (Dinocephalia, Therapsida) using 3D sculpting
Estimating the endocranial volume and body mass of Anteosaurus, Jonkeria, and Moschops (Dinocephalia, Therapsida) using 3D sculpting
Article number: 27.2.a39
https://doi.org/10.26879/1377
Copyright Palaeontological Association, August 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 22 February 2024. Acceptance: 26 July 2024.
ABSTRACT
Dinocephalians represent a highly successful early radiation of mid-Permian therapsids. Despite the abundance of their fossils, data on their paleoneurology is challenging to acquire and remain scarce. The current study proposes to overcome these challenges by using digital 3D sculpting. Based on previous works, realistic digital models of brain endocasts and live reconstructions of three taxa sampling the major families of Dinocephalia (Anteosauridae, Titanosuchidae, and Tapinocephalidae) were generated, their volumes measured, and endocranial volume and body mass were estimated. Encephalization quotients were calculated. The results suggest that the dinocephalians evolved a surprisingly high degree of encephalization for such early tetrapods. Some dinocephalians are hypothesized to have practiced head-butting, a complex social behavior, which would have favored the enlargement of the brain. Alternatively, the enlarged endocast may have been filled mostly by protective tissue to protect the central nervous system when the head was used as a weapon. This is supported by the apparent absence of direct imprints of the soft tissue of the brain on the endocast.
Julien Benoit. Evolutionary Studies Institute, University of the Witwatersrand, Johannesburg, South Africa. (corresponding author) julien.benoit@wits.ac.za
A.J. Midzuk. Evolutionary Studies Institute and School of Geosciences, University of the Witwatersrand, Johannesburg, South Africa. adamjaymid@gmail.com
Keywords: brain; endocast; paleoneurology; encephalization; head-butting
Final citation: Benoit, Julien and Midzuk, A.J. 2024. Estimating the endocranial volume and body mass of Anteosaurus, Jonkeria, and Moschops (Dinocephalia, Therapsida) using 3D sculpting. Palaeontologia Electronica, 27(2):a39.
https://doi.org/10.26879/1377
palaeo-electronica.org/content/2024/5296-brain-size-in-dinocephalia
Copyright: August 2024 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Dinocephalians were among the most conspicuous tetrapods in high latitude terrestrial ecosystems of the Guadalupian. They had a worldwide distribution, and their diversity encompassed large bone-crushing predators, small sectorial-toothed carnivores, and medium-sized to gigantic herbivores (Kammerer, 2011; Angielczyk and Kammerer, 2018). Alongside the pareiasaurs, they were among the first terrestrial vertebrates to come close to the 1000 kg threshold (Romano et al., 2021; Romano and Rubidge, 2021; Van den Brandt et al., 2024). This success and their abundance in the fossil record contrast sharply with the admittedly poor state of our knowledge about the evolution of their brain and behavior.
The paleoneurology of non-mammalian therapsids, particularly cynodonts, is a highly active field of research, with many dozens of endocasts described and measured, and many others being described each year (Jerison, 1973; Edinger, 1975; Rowe et al., 2011; de Simão-Oliveira et al., 2020; Kerber et al., 2021, 2023; Gigliotti et al., 2023; Benoit, 2023). In comparison, the literature on the behavior and paleoneurology of dinocephalians is scarce, most acutely when it comes to addressing their brain evolution using endocranial casts (Brink, 1958; Boonstra, 1968; Barghusen, 1975; Benoit et al., 2017a, b, 2021) as data is notoriously difficult to obtain.
Dinocephalian fossils are large, very heavy (due to pachyostosis), difficult to transport, and often preserved in compact sandstone that is difficult to prepare or X-ray scan. Well-preserved cranial material is rare, often broken, weathered, and unprepared. The dinocephalian braincase is often hidden beneath a thick pachyostosed skull that makes the endocast difficult to observe non-destructively. Moreover, dinocephalian paleoneurology has been the object of less research interest because, compared to cynodonts, they are less closely related to mammals (Hopson, 1979). So far, only two papers describe dinocephalian endocasts: Boonstra (1968) using sectioned and serially sectioned skulls, and Benoit et al. (2017a) using synchrotron scanning. The volume of the endocast has been estimated in only two species: Moschognathus whaitsi (AM 4950) and Struthiocephalus whaitsi (SAM-PK-12049), the former using the digitally segmented volume of the endocast and the second using graphic double integration on Boonstra’s figure of SAM-PK-12049. The small sample size and fact that they both belong to the tapinocephalid family makes tracing general trends in dinocephalian brain evolution impossible. Additionally, the endocranial volume of AM4950 is based on a subadult individual, and that of SAM-PK-12049 is based on an estimate made using graphic double integration, a technique whose accuracy has been criticized (Hurlburt, 1999; Hurlburt et al., 2013). More data is desperately needed to better understand dinocephalian evolution, their adaptation to head butting, and their possible gregariousness (Barghusen, 1975; Benoit et al., 2023a).
Similarly, body mass estimates, which are necessary to calculate the Encephalization Quotient (EQ) to compare brain size across taxa, are also difficult to obtain for dinocephalians. Equations based on individual limb elements fail to capture realistic body weight estimates in therapsids, in part because of their unusual body proportions (large head and relatively smaller body) and posture (Romano and Manucci, 2019; Romano and Rubidge, 2021). As a result, body mass estimates of dinocephalians are rare. Bakker (1975) proposed body weights that were rough visual estimates of taxa designated as Anteosaurus, moschopid, struthiocephalid, tapinocephalid, and Titanosuchus. Benoit et al (2017a, b) calculated the body mass of AM4950 and SAM-PK-12049 based on their skull length but admitted the equations used were not well-adapted to large specimens. Romano and Rubidge (2021) estimated the body mass of Tapinocaninus pamelae based on a 3D life reconstruction and argued this is the most accurate way to approximate a realistic body weight. This last method requires that complete skeletal reconstructions are available. Body mass can strongly affect EQ results, so accurate estimates are essential to compare endocranial size across taxa. Over the last 10 years, many independent teams have shown that, to estimate body mass of extinct taxa, volumetric methods are experimentally better performing than classical regression formulas based on long bones or cranial dimensions, which have often given unlikely results in many tetrapod groups (Sellers et al., 2012; Bates et al., 2015; Brassey et al., 2015; Romano and Manucci, 2019; Romano et al., 2021, 2022; 2023).
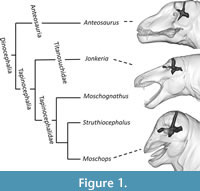 Innovative and original approaches are necessary to cope with the issues presented above and enrich the therapsid dataset with more data on dinocephalian brain and body sizes. This study proposes to use Boonstra’s (1968) figures to digitally recreate the endocasts of three dinocephalians in 3D: the Anteosauridae Anteosaurus, the Titanosuchidae Jonkeria, and the Tapinocephalidae Moschops, thus expending the phylogenetic coverage to two new families of dinocephalians (Figure 1). Following the method of Romano and Rubidge (2021), digital 3D sculpturing is used to provide accurate estimates of the body mass of these taxa. These data are then used to discuss the evolution of the EQ in dinocephalians.
Innovative and original approaches are necessary to cope with the issues presented above and enrich the therapsid dataset with more data on dinocephalian brain and body sizes. This study proposes to use Boonstra’s (1968) figures to digitally recreate the endocasts of three dinocephalians in 3D: the Anteosauridae Anteosaurus, the Titanosuchidae Jonkeria, and the Tapinocephalidae Moschops, thus expending the phylogenetic coverage to two new families of dinocephalians (Figure 1). Following the method of Romano and Rubidge (2021), digital 3D sculpturing is used to provide accurate estimates of the body mass of these taxa. These data are then used to discuss the evolution of the EQ in dinocephalians.
MATERIAL AND METHODS
To be included in this study, dinocephalian taxa had to fulfil two conditions: firstly, a complete skeletal reconstruction of, at least, a very close relative had to be available in the scientific literature, and secondly the endocast had to be figured by Boonstra (1968). Only three genera matched this description, Anteosaurus, Jonkeria, and Moschops. Measurements of volume were made using Avizo (Thermo Fisher Scientific, Hillsborough, U.S.A.).
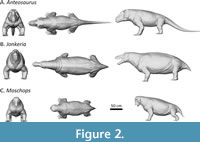 The life reconstructions of Anteosaurus, Jonkeria, and Moschops (Figure 2) were created in the open-source 3D software Blender (versions 3.8 and 4.8). Simple low-detail “base meshes” were generated from scratch in Blender’s Edit Mode and then converted into high detail models using a Multiresolution Modifier and Blender’s Sculpt Mode Tools (Midzuk, 2020). The life reconstructions of Moschops and Jonkeria were based on complete skeletal reconstructions of Moschops capensis (Gregory, 1926) and Jonkeria truculenta (Broom, 1929; Boonstra, 1969) and digital cranial reconstructions (Midzuk, 2020). The reconstruction of Anteosaurus is based on the more complete postcranial remains of the related taxon Titanophoneus (Orlov, 1958; Kemp, 2005) but incorporates a more robust torso, longer limbs and hypertrophied neck musculature needed to support the proportionally more robust and heavier skull. Life reconstructions based on figures in the existing literature is ultimately an interpretive process since i) different views of figured material do not always align perfectly, so slight proportional stretching is needed to reach realistic proportions and ii) these models represent the best interpretation of the data displayed by previous authors and not direct observation.
The life reconstructions of Anteosaurus, Jonkeria, and Moschops (Figure 2) were created in the open-source 3D software Blender (versions 3.8 and 4.8). Simple low-detail “base meshes” were generated from scratch in Blender’s Edit Mode and then converted into high detail models using a Multiresolution Modifier and Blender’s Sculpt Mode Tools (Midzuk, 2020). The life reconstructions of Moschops and Jonkeria were based on complete skeletal reconstructions of Moschops capensis (Gregory, 1926) and Jonkeria truculenta (Broom, 1929; Boonstra, 1969) and digital cranial reconstructions (Midzuk, 2020). The reconstruction of Anteosaurus is based on the more complete postcranial remains of the related taxon Titanophoneus (Orlov, 1958; Kemp, 2005) but incorporates a more robust torso, longer limbs and hypertrophied neck musculature needed to support the proportionally more robust and heavier skull. Life reconstructions based on figures in the existing literature is ultimately an interpretive process since i) different views of figured material do not always align perfectly, so slight proportional stretching is needed to reach realistic proportions and ii) these models represent the best interpretation of the data displayed by previous authors and not direct observation.
Body models were scaled to Boonstra’s specimens, and their volume measured. Body volumes were then converted into body weight by multiplying them with average body density (Table 1). For the alleged semi-aquatic taxa Moschops and Jonkeria (Bhat et al., 2022), the body density of aquatic mammals was preferred (i.e., 1.05; Larramendi, 2016; Larramendi et al., 2021). For Anteosaurus, a terrestrial predator (Benoit et al., 2021; Bhat et al., 2022), the average body density of terrestrial tetrapods was preferred (i.e., 1.00; Larramendi, 2016; Larramendi et al., 2021).
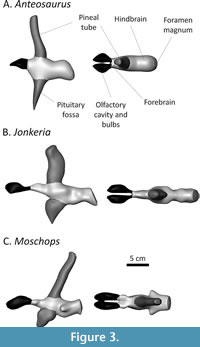 The endocast models (Figure 3) are based on the figured endocranial casts in Boonstra (1968). The 3D models of the endocranial casts of Anteosaurus magnificus (SAM-PK-12082), Jonkeria truculenta (SAM-PK-11574), and Moschops sp. (SAM-PK-11972) were digitally sculpted in Blender using blender’s sculpt mode and remeshing options. As a dorsal view of the endocranial cast of Anteosaurus is not supplied, so shape information in the coronal plane is inferred from the Jonkeria specimen. The olfactory cavities were included in the models, though these were not included in the volumetric analysis (see below). Blood vessels, the cochlea, and other smaller structures were excluded from the sculpt.
The endocast models (Figure 3) are based on the figured endocranial casts in Boonstra (1968). The 3D models of the endocranial casts of Anteosaurus magnificus (SAM-PK-12082), Jonkeria truculenta (SAM-PK-11574), and Moschops sp. (SAM-PK-11972) were digitally sculpted in Blender using blender’s sculpt mode and remeshing options. As a dorsal view of the endocranial cast of Anteosaurus is not supplied, so shape information in the coronal plane is inferred from the Jonkeria specimen. The olfactory cavities were included in the models, though these were not included in the volumetric analysis (see below). Blood vessels, the cochlea, and other smaller structures were excluded from the sculpt.
The endocast models were scaled to Boonstra’s figures (Boonstra, 1968, figures 6, 7, 22, 24, 32, 37, 46, 49) in Avizo and their volume measured directly. The cast of the pineal tube and pituitary fossa are enlarged in dinocephalians, so measurements were made including and excluding these parts of the endocast (Table 1). In Boonstra’s endocast drawings, part of the nasal chamber is mistakenly included with the olfactory bulbs (Benoit et al., 2017a). These are colored in black in Figure 3 and were not considered for measurements. Olfactory bulbs are usually removed from measure of endocranial volumes in the literature (Jerison, 1973; Benoit et al 2017a, b; Rodrigues et al., 2018) so this does not alter the interpretation of the results presented here. Rather than converting endocranial volume into brain mass, raw endocranial volumes were used here to compute the EQ (see Discussion section).
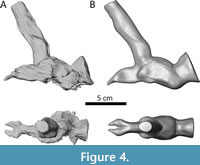 As a validation step, a braincase model of the Moschognathus specimen AM4950 was digitally sculpted based on figure 3 in Benoit et al. (2017a) in order to compare its volume to that of the actual endocast (Figure 4). The volume of the actual endocast, excluding the pituitary fossa, is 61.1398 cm3 and that of the 3D sculpture is 60.9171 cm3, which indicates the volume measured from the sculpted model is 99.6% accurate. As such, it can be safely assumed that the volume of the 3D sculpted models provides a reasonable approximation for the volume of the corresponding original endocast, likely because 3D sculpted models average deformation artifacts and reconstruction errors resulting in a similar volume.
As a validation step, a braincase model of the Moschognathus specimen AM4950 was digitally sculpted based on figure 3 in Benoit et al. (2017a) in order to compare its volume to that of the actual endocast (Figure 4). The volume of the actual endocast, excluding the pituitary fossa, is 61.1398 cm3 and that of the 3D sculpture is 60.9171 cm3, which indicates the volume measured from the sculpted model is 99.6% accurate. As such, it can be safely assumed that the volume of the 3D sculpted models provides a reasonable approximation for the volume of the corresponding original endocast, likely because 3D sculpted models average deformation artifacts and reconstruction errors resulting in a similar volume.
Relative endocranial volumes were compared using the synapsid adjusted Encephalization Quotient (SEQ, Benoit et al., 2023a). It is expressed as follows: SEQ = EV/10^((0.669 x logBM)-1.8188), where EV is the endocranial volume and BM the body mass. An SEQ of one indicates an animal with an average relative endocast size for a synapsid of the Paleozoic or Mesozoic. An SEQ above one indicates that the relative size of the endocast is larger than expected for a synapsid of similar body mass, whereas an SEQ below one indicates that the relative endocast size is smaller than expected. The resulting body mass, endocranial volumes, and encephalization quotients are summarized in Table 1.
Abbreviations. SAM-PK: Iziko Museum of Natural History, Cape Town, South Africa; AM: Albany Museum, Grahamstown (Makhanda), South Africa.
RESULTS
The body weight of the Jonkeria specimen approaches a ton (989 kg), which is close to the estimate given by Bakker (1975). The estimated body weights of Anteosaurus and Moschops are about 400 kg, which is below what Bakker (1975) estimated. The endocranial volumes are all comprised between 150 and 200 cm3, and between 80 and 130 cm3 when excluding the pineal tube and pituitary fossa (Table 1). The pineal tube in the dinocephalians studied here occupies 15% (Anteosaurus) to 25% (Jonkeria) of the volume of the braincase. The pituitary fossa occupies 13% of the endocranial volume in Anteosaurus, 15% in Jonkeria, and 20% in Moschops (Table 1). These are a lot more than measured in Moschognathus (less than 3%, Benoit et al., 2017a), although the latter is not a fully grown individual. That the tapinocephalids have proportionally larger pituitary fossa would support Nopcsa’s (1926) and Boonstra’s (1968) hypotheses that pachyostosis in this clade was linked to hyperpituitarism.
The SEQ (excluding the pineal tube and pituitary fossa) is slightly above one in Anteosaurus and equals one in Moschops; consistent with that previously calculated in Moschognathus (Benoit et al 2017a). Jonkeria has the smallest SEQ. The SEQs calculated based on complete endocasts all exceed one (Figure 4). Anteosaurus even reaches a SEQ close to two (Table 1). Noticeably, Boonstra’s figure of the endocast of Anteosaurus failed to capture the long and slender floccular fossa later described by Benoit et al. (2021). Although the volume difference may be close to negligible, an even higher EQ for this genus may be expected.
DISCUSSION
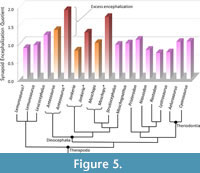 Previous attempts at calculating the EQ and SEQ in tapinocephalids (i.e., Moschognathus and Struthiocephalus) found that they were surprisingly encephalized for such large, Paleozoic animals (Benoit et al., 2017a, 2023). The data presented here bolster these previous results (Table 1). Dinocephalian SEQs approaching one supports that their brain was not small given their body size, except Jonkeria, which has an SEQ (excluding the pineal tube and pituitary fossa) well below one. The condition in Jonkeria is consistent with most non-theriodont synapsids (Figure 5) and may be the ancestral condition for the clade. This would imply that anteosaurids and tapinocephalids independently evolved a larger endocast (Figure 5). Alternatively, an SEQ of one could be the ancestral condition, and the increase in body mass in titanosuchids would be responsible for the low SEQ in Jonkeria. Although the first hypothesis seems more likely given the current knowledge, the sampling of non-theriodont synapsids is still too small to properly address these hypotheses.
Previous attempts at calculating the EQ and SEQ in tapinocephalids (i.e., Moschognathus and Struthiocephalus) found that they were surprisingly encephalized for such large, Paleozoic animals (Benoit et al., 2017a, 2023). The data presented here bolster these previous results (Table 1). Dinocephalian SEQs approaching one supports that their brain was not small given their body size, except Jonkeria, which has an SEQ (excluding the pineal tube and pituitary fossa) well below one. The condition in Jonkeria is consistent with most non-theriodont synapsids (Figure 5) and may be the ancestral condition for the clade. This would imply that anteosaurids and tapinocephalids independently evolved a larger endocast (Figure 5). Alternatively, an SEQ of one could be the ancestral condition, and the increase in body mass in titanosuchids would be responsible for the low SEQ in Jonkeria. Although the first hypothesis seems more likely given the current knowledge, the sampling of non-theriodont synapsids is still too small to properly address these hypotheses.
Whether the enlarged endocast of anteosaurids and tapinocephalids accommodated an equally large brain warrants some discussion. On the one hand, tapinocephalids most likely engaged in head-butting combat for mates and territory, a behavior that implies complex social interactions and hierarchy, which suggests they were likely gregarious tetrapods (Benoit et al., 2017a, 2023). A larger brain would have been advantageous to navigate this social space, which in turn would have favored brain enlargement (Dunbar, 2009). On the other hand, it must be noted that in cynodonts and early mammaliaforms, the enlarged olfactory bulbs, cerebellar vermis, and brain hemispheres leaves marks on the endocast (Kielan-Jaworowska, 2004; Rowe et al., 2011; Benoit et al., 2016). Similar observations were made on the endocast of the highly encephalized dicynodont Kawingasaurus (Laaß and Kaestner, 2017), which suggest that if the skull of dinocephalians was accommodating an enlarged brain, their braincase would likely have preserved imprints of the brain tissue like in their closest relatives. No such marks are present on the endocast of dinocephalians (Boonstra, 1968; Benoit et al., 2017a), which suggests that the central nervous system did not fill up the braincase, as in modern reptiles (Jerison, 1973).
When the complete endocast is considered (including the pineal tube and pituitary fossa), the SEQ of dinocephalians is much higher (Table 1, Figure 5). Reasoning on the principle that a hypothetical typical therapsid SEQ should be around one, the excess encephalization in dinocephalians could be interpreted as reflecting additional non-brain matter inside the endocast (Figure 5). It becomes possible to roughly estimate the relative amount of non-neural tissue in the endocast of dinocephalians. Some 48% of the endocast would be filled with non-neural tissue in Anteosaurus, 22% in Jonkeria, and 42% in Moschops. The same applies to the subadult Moschognathus, which, using published data (Benoit et al., 2017a), gives a value of 35%. These values are probably underestimated given that i) in modern reptiles, non-neural tissue is classically considered to account for 50% of the volume of the endocranial cavity (Jerison, 1973; Hurlburt et al., 2013); and ii) it is not known how completely the brain would have filled the endocranial space in non-mammalian therapsids (Benoit et al., 2017b). This non-neural tissue could be a mix of meninges, blood vessels, and protective adnexa (Bauchot and Stephan, 1967), although the values presented above far exceed the amount of non-neural tissue measured in the endocast of modern mammals (which averages 11% according to Benoit, 2015). Noticeably, the taxa displaying the most pachyostosis (i.e., Moschops, Moschognathus, and Anteosaurus) are also those that would have had the most non-neural tissue, which would be consistent with the use of the head as a weapon (Boonstra, 1968; Benoit et al., 2017a, but see Benoit et al., 2021 about Anteosaurus). In modern mammals, meningeal thickness is directly proportional to absolute brain size (Benoit, 2015), but species that practice high-energy head-butting (e.g., bighorn sheep) have thicker meninges that cushion the part of their brain that is the most exposed to impacts (Ackermans et al., 2022).
Following the interpretation that the excess encephalization is the result of thickened intracranial protective tissue, it is remarkable that tapinocephalid dinocephalians would display all the most prominent adaptations to head-butting observed in modern high-energy head-butting animals, i.e. braincase re-orientation, enlarged absolute cranial size (to diffuse impact-induced stress), and thickened meninges (Ackermans et al., 2021; 2022). In modern bovids, complete protection against head-butting induced brain damage is rarely achieved as genes can be passed through to the next generation despite the accumulation of brain trauma (Ackermans et al., 2022). The more extreme adaptations observed in tapinocephalids, as exemplified by their grotesque cranial pachyostosis, suggest that they must have practiced intense head butting from a young age for these traits to be selected. Play-fighting in young tapinocephalids was recently supported by the description of an intracranial injury in the sub-adult specimen of Moschognathus and would imply complex intra-specific social communication to convey the idea of combat without aggression (Benoit et al., 2024). However, it must be reminded that besides paleoneurology, independent fossil evidence supporting the “social tapinocephalids” hypothesis remains ambiguous. Although dinocephalian skeletons can be found in small groups, the rich record of footprints has failed to produce parallel trackways that would demonstrate gregariousness (Benoit et al., 2023 a, b).
CONCLUSIONS
The approach proposed here based on 3D sculpturing provides the first reasonably accurate body mass estimates for three dinocephalian taxa. The 3D sculpted endocasts compensate for the absence of more direct data on the dinocephalian brain. Their unexpectedly high volume is puzzling to interpret but remains consistent with adaptations to head-butting. It is more parsimonious to hypothesize that the endocast was filled with protective tissues rather than by an enlarged brain, but with all the caveats possibly linked to correlating brain size with intelligence (Manger, 2006, 2013; Healy and Rowe, 2007; Manger et al., 2013), the possibility that dinocephalians were smarter than usually assumed nevertheless remains open. The future capabilities to CT and synchrotron study large and heavy dinocephalian skulls will hopefully enable to test the accuracy of the above-presented results and hypotheses and bring new data to compliment the current study.
ACKNOWLEDGEMENTS
We acknowledge the financial support of the DSI-NRF African Origins Platform (AOP210218587003; UID: 136505).
REFERENCES
Ackermans, N.L., Varghese, M., Williams, T.M., Grimaldi, N., Selmanovic, E., Alipour, A., Balchandani, P., Reidenberg, J.S., and Hof, P.R. 2022. Evidence of traumatic brain injury in headbutting bovids. Acta Neuropathologica, 144(1):5-26.
https://doi.org/10.1007/s00401-022-02427-2
Ackermans, N., Hof, P., and Reidenberg, J. 2021. Does Headbutting Cause Traumatic Brain Injury? The Case of Combative Bovids. The FASEB Journal, 35:1.
https://doi.org/10.1096/fasebj.2021.35.S1.03638
Angielczyk, K.D. and Kammerer, C.F. 2018. 5. Non-Mammalian synapsids: the deep roots of the mammalian family tree, p. 117-198. In Zachos, F. and Asher, R. (eds.), Mammalian Evolution, Diversity and Systematics. De Gruyter, Berlin, Boston.
https://doi.org/10.1515/9783110341553-005
Bakker, R.T. 1975. Dinosaur renaissance. Scientific American, 232:58-78.
Barghusen, H.R. 1975. A review of fighting adaptations in dinocephalians (Reptilia, Therapsida). Paleobiology, 1:295-311.
https://doi.org/10.1017/S0094837300002542
Bates, K.T., Falkingham, P.L., Macaulay, S., Brassey, C., and Maidment, S.C. 2015. Downsizing a giant: re-evaluating Dreadnoughtus body mass. Biology Letters, 11: 20150215.
https://doi.org/10.1098/rsbl.2015.0215
Bauchot, R. and Stephan, H. 1967. Encéphales et moulages endocrâniens de quelques insectivores et primates actuels. Colloque International du CNRS, 163:575-587.
Benoit, J. 2015. A new method of estimating brain mass through cranial capacity in extinct proboscideans to account for the non-neural tissues surrounding their brain. Journal of Vertebrate Paleontology, 35(6):e991021.
https://doi.org/10.1080/02724634.2014.991021
Benoit, J. 2023. Synchrotron scanning reveals the deep evolutionary root of the mammalian brain: the surprisingly advanced endocast morphology of Lumkuia fuzzi (Cynodontia: Probainognathia). Palaeontologia Africana, 56:103-110.
Benoit, J., Manger, P.R., and Rubidge, B.S. 2016. Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Scientific Reports, 6:25604.
https://doi.org/10.1038/srep25604
Benoit, J., Manger, P.R., Norton, L., Fernandez, V., and Rubidge, B.S. 2017a. Synchrotron scanning reveals the palaeoneurology of the head-butting Moschops capensis (Therapsida, Dinocephalia). PeerJ, 5:e3496.
https://doi.org/10.7717/peerj.3496
Benoit, J., Fernandez, V., Manger, P.R., and Rubidge, B.S. 2017b. Endocranial Casts of Pre-Mammalian Therapsids Reveal an Unexpected Neurological Diversity at the Deep Evolutionary Root of Mammals. Brain, Behavior and Evolution, 90:311-333.
https://doi.org/10.1159/000481525
Benoit, J., Kruger, A., Jirah, S., Fernandez, V., and Rubidge, B. 2021. Palaeoneurology and palaeobiology of the dinocephalian Anteosaurus magnificus. Acta Palaeontologia Polonica, 66:29-39.
https://doi.org/10.4202/app.00800.2020
Benoit, J., Dollman, K.N., Smith, R.M.H., and Manger, P.R. 2023a. At the root of the mammalian mind: The sensory organs, brain and behavior of pre-mammalian synapsids. Progress in Brain Research, 275:25-72. https://doi.org/10.1016/bs.pbr.2022.10.001
Benoit J., Norton L.A., and Jirah S. 2023b. The maxillary canal of the titanosuchid Jonkeria (Synapsida, Dinocephalia). The Science of Nature, 110:27.
https://doi.org/10.1007/s00114-023-01853-w
Benoit, J., Araujo, R., Lund, E.S., Bolton, A., Lafferty, T., Macungo, Z., and Fernandez, V. 2024. Early synapsids neurosensory diversity revealed by CT and synchrotron scanning. Anatomical Record (online only).
https://doi.org/10.1002/ar.25445
Bhat, M.S., Shelton, C.D., and Chinsamy, A. 2022. Bone histology of dinocephalians (Therapsida, Dinocephalia): palaeobiological and palaeoecological inferences. Papers in Palaeontology, 8: e1411.
https://doi.org/10.1002/spp2.1411
Boonstra, L.D. 1968. The braincase, basicranial axis and median septum in the Dinocephalia. Annals of the South African Museum, 50:195-273.
Boonstra, L.D. 1969. The fauna of The tapinocephalus Zone (Beaufort beds of the Karoo). Annals of The South African Museum, 56:1-73.
Brassey, C.A., Maidment, S.C.R., and Barrett, P.M. 2015. Body mass estimates of an exceptionally complete Stegosaurus (Ornithischia: Thyreophora): comparing volumetric and linear bivariate mass estimation methods. Biology Letters, 11: 20140984.
https://doi.org/10.1098/rsbl.2014.0984
Brink, A.S. 1958. Struthiocephalus kitchingi sp. nov. Palaeontologia Africana, 5:39-56.
Broom, R. 1929. On the carnivorous mammal-like reptiles of the family Titanosuchidae. Annals of the Transvaal Museum, 13:9-36.
de Simão-Oliveira, D., Kerber, L., and Pinheiro, F. 2020. Endocranial morphology of the Brazilian Permian dicynodont Rastodon procurvidens (Therapsida: Anomodontia). Journal of Anatomy, 236:384-397.
https://doi.org/10.1111/joa.13107
Dunbar, R.I.M. 2009. The social brain hypothesis and its implications for social evolution. Annals of Human Biology, 36:562-572.
https://doi.org/10.1080/03014460902960289
Edinger, T. 1975. Paleoneurology 1804-1966: an annotated bibliography. Advances in Anatomy, Embryology and Cell Biology, 49:3-258.
Fraser-King, S.W., Benoit, J., Day, M.O., and Rubidge, B.S. 2019. Cranial morphology and phylogenetic relationship of the enigmatic dinocephalian Styracocephalus platyrhynchus from the Karoo Supergroup, South Africa. Palaeontologia Africana, 54: 14-29.
Gigliotti, A., Pusch, L.S., Kammerer, C.F., Benoit, J., and Fröbisch, J. 2023. Craniomandibular anatomy of the akidnognathid therocephalian Olivierosuchus parringtoni from the Early Triassic of South Africa. Palaeontologia Africana, 56: 142-170.
Gregory, W.K. 1926. The skeleton of Moschops capensis Broom, a dinocephalian reptile from the Permian of South Africa. Bulletin American Museum National History, 56:179-251.
Healy, S.D. and Rowe, C. 2007. A critique of comparative studies of brain size. Proceedings of the Royal Society of London, B, Biological Sciences, 274:453-464.
https://doi.org/10.1098/rspb.2006.3748
Hopson, J.A. 1979. Paleoneurology, p. 39-146. In Gans et al., (eds.), Biology of the Reptilia, Volume 9. Academic Press, London.
Hurlburt, G. 1999. Comparison of body mass estimation techniques, using Recent reptiles and the pelycosaur Edaphosaurus boanerges. Journal of Vertebrate Paleontology, 19:338-350.
Hurlburt, G.R., Ridgely, R.C., and Witmer, L.M. 2013. Relative size of brain and cerebrum in tyrannosaurid dinosaurs: An analysis using brain-endocast quantitative relationships in extant alligators, p. 135-155. In Parrish, J.M., Henderson, P.J., Currie, P.J., and Koppelhus, E. (eds.), Origin, Systematics, and Paleobiology of the Tyrannosauridae. Indiana University Press, Bloomington.
Jerison, H. 1973. Evolution of The Brain and Intelligence. Elsevier Science, Saint Louis.
Kammerer, C.F. 2011. Systematics of the Anteosauria (Therapsida: Dinocephalia). Journal of Systematic Palaeontology, 9:261-304.
https://doi.org/10.1080/14772019.2010.492645
Kemp, T.S. 2005. The origin and evolution of mammals. Oxford University Press, Oxford, New York.
Kerber, L., Ferreira, J.D., Fonseca, P.H.M., Franco, A., Martinelli, A.G., Soares, M.B., and Ribeiro, A.M. 2021. An additional brain endocast of the ictidosaur Riograndia guaibensis (Eucynodontia: Probainognathia): intraspecific variation of endocranial traits. Anais da Academia Brasileira de Ciências, 93:e20200084.
https://doi.org/10.1590/0001-3765202120200084
Kerber, L., Roese-Miron, L., Bubadué, J.M., and Martinelli, A.G. 2023. Endocranial anatomy of the early prozostrodonts (Eucynodontia: Probainognathia) and the neurosensory evolution in mammal forerunners. The Anatomical Record, 1-32.
https://doi.org/10.1002/ar.25215
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z.-X. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. Columbia University Press.
Laaß, M. and Kaestner, A. 2017. Evidence for convergent evolution of a neocortex-like structure in a late Permian therapsid. Journal of Morphology, 278:1033-1057.
https://doi.org/10.1002/jmor.20712
Larramendi, A. 2016. Shoulder height, body mass and shape of proboscideans. Acta Palaeontologica Polonica, 61:537-574.
https://doi.org/10.4202/app.00136.2014
Larramendi, A., Paul, G.S., and Hsu, S. 2021. A review and reappraisal of the specific gravities of present and past multicellular organisms, with an emphasis on tetrapods. The Anatomical Record, 304:1833-1888.
Manger, P.R. 2006. An examination of cetacean brain structure with a novel hypothesis correlating thermogenesis to the evolution of a big brain. Biological Reviews, 81: 293-338.
https://doi.org/10.1017/S1464793106007019
Manger, P.R. 2013. Questioning the interpretations of behavioral observations of cetaceans: Is there really support for a special intellectual status for this mammalian order? Neuroscience, 250:664-696.
https://doi.org/10.1016/j.neuroscience.2013.07.041
Manger, P.R., Spocter, M.A., and Patzke, N. 2013. The Evolutions of Large Brain Size in Mammals: The ‘Over-700-Gram Club Quartet’. Brain Behavior and Evolution, 82: 68-78.
https://doi.org/10.1159/000352056
Midzuk, A.J. 2020. The 3d reconstruction of the skulls of some Dinocephalia (Therapsida) for usage in finite element study. Unpublished Honors Thesis, University of the Witwatersrand, Johannesburg, South Africa.
Neumann, S. 2020. Taxonomic revision of the short-snouted tapinocephalid Dinocephalia (Amniota- Therapsida)- The key to understanding Middle Permian tetrapod biodiversity. Unpublished Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa.
Nopcsa, F. 1926. Heredity and evolution. Proceedings of the Zoological Society of London, 96: 633-665.
Orlov, Y.A. 1958. The carnivorous dinocephalians of the Isheevo fauna (titanosuchians). Trudy Paleontologischeskoso Instituta, Akademyia Nauk SSSR, 71:1-114.
Rodrigues, P.G., Martinelli, A.G., Schultz, C.L., Corfe, I.J., Gill, P.G., Soares, M.B., and Rayfield, E.J. 2018. Digital cranial endocast of Riograndia guaibensis (Late Triassic, Brazil) sheds light on the evolution of the brain in non-mammalian cynodonts. Historical Biology, 31:1-18.
https://doi.org/10.1080/08912963.2018.1427742
Romano, M. and Manucci, F. 2019. Resizing Lisowicia bojani: volumetric body mass estimate and 3D reconstruction of the giant Late Triassic dicynodont. Historical Biology 33, 474-479.
https://doi.org/10.1080/08912963.2019.1631819
Romano, M., Manucci, F., Rubidge, B., and Van den Brandt, M.J. 2021. Volumetric body mass estimate and in vivo reconstruction of the Russian pareiasaur Scutosaurus karpinskii. Frontiers in Ecology and Evolution, 9:692035.
https://doi.org/10.3389/fevo.2021.692035
Romano, M. and Rubidge, B.S. 2021. First 3D reconstruction and volumetric body mass estimate of the tapinocephalid dinocephalian Tapinocaninus pamelae (Synapsida: Therapsida). Historical Biology, 33:498-505.
https://doi.org/10.1080/08912963.2019.1640219
Romano, M., Manucci, F., Antonelli, M., Rossi, M.A., Agostini, S., and Palombo, M.R. 2022. In vivo restoration and volumetric body mass estimate of Mammuthus meridionalis from Madonna della Strada (Scoppito, L'Aquila). Rivista Italiana di Paleontologia e Stratigrafia, 128:559-573.
Romano, M., Bellucci, L., Antonelli, M., Manucci, F., and Palombo, M.R. 2023. Body mass estimate of Anancus arvernensis (Croizet and Jobert 1828): comparison of the regression and volumetric methods. Journal of Quaternary Science, 38:1357-1381.
Rowe, T.B., Macrini, T.E., and Luo, Z.-X., 2011. Fossil Evidence on Origin of the Mammalian Brain. Science 332, 955-957.
https://doi.org/10.1126/science.1203117
Sellers, W.I., Hepworth-Bell, J., Falkingham, P.L., Bates, K.T., Brassey, C.A., Egerton, V.M., and Manning, P.L. 2012. Minimum convex hull mass estimations of complete mounted skeletons. Biology Letters, 8:842-845.
Van den Brandt, M.J., Day, M.O., Manucci, F., Viglietti, P.A., Angielczyk, K.D., and Romano, M. 2024. First volumetric body mass estimate and a new in vivo 3D reconstruction of the oldest Karoo pareiasaur Bradysaurus baini, and body size evolution in Pareiasauria. Historical Biology, 36:587-601.
https://doi.org/10.1080/08912963.2023.2175211

