 Glossary of fossil tetrapod tracks
Glossary of fossil tetrapod tracks
Article number: 28.1.a8
https://doi.org/10.26879/1389
Copyright Society of Vertebrate Paleontology, February 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 8 March 2024. Acceptance: 26 December 2024.
ABSTRACT
The terminology used in fossil tetrapod track research has expanded and evolved considerably in recent decades. The main drivers of this change are conceptual and methodological advances, the emergence of distinct subfields such as hominin track research, and increased interdisciplinarity. This growing lexicon has led to confusion and conflict, as long-standing usage of terms has been questioned (e.g., the term “preservation”) or conflicts with the terminology of related fields such as invertebrate ichnology and biomechanics (e.g., the terms “elite track/trace” and “pace gait”). In addition, the definition of a number of key terms, including the term “track” itself, has remained vague. The present glossary provides a comprehensive review of the terminology used in tetrapod track research. In addition to documenting past usage of terms, we aim to provide a standard terminology that is 1) precisely defined, unambiguous, and consistent; 2) compatible with terminology used in related fields; 3) reflects current knowledge and is not misleading, and 4) is easily understood and follows traditional usage as much as possible. In addition to terminology, we also aim to briefly explain and discuss the concepts and methods behind each term, and, where appropriate, refer the reader to the relevant literature.
Jens N. Lallensack. Departamento de Ciência da Computação, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil. jens.lallensack@posteo.de
Giuseppe Leonardi. Istituto Cavanis, Dorsoduro 898, 30123 Venezia, Italy and Universidade Federal do Rio de Janeiro, CCMN, Instituto de Geociências - Geology Department, Cidade Universitária, Ilha do Fundão, 21949-900 Rio de Janeiro, RJ, Brazil. leonardigiuseppe879@gmail.com
Peter L. Falkingham. School of Biological and Environmental Sciences, John Moores University
Liverpool, Merseyside, UK. P.L.Falkingham@ljmu.ac.uk
Keywords: terminology; ichnology; fossil footprints; trackways; definitions; palaeontology
Final citation: Lallensack, Jens N., Leonardi, Giuseppe, and Falkingham, Peter L. 2025. Glossary of fossil tetrapod tracks. Palaeontologia Electronica, 28(1):a8.
https://doi.org/10.26879/1389
palaeo-electronica.org/content/2025/5439-glossary-of-tetrapod-tracks
Copyright: February 2025 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
The field of fossil tetrapod track research has grown steadily over the last few decades. Increased knowledge and methodological advances have led to an ever-increasing pool of terminology. Distinct sub-fields have emerged, such as the study of dinosaur tracks or the study of fossil hominin tracks. Each of these subfields uses slightly different approaches and conventions, especially in ichnotaxonomy, and has developed specialised and sometimes conflicting terminology. More importantly, tetrapod track research has become increasingly interdisciplinary, with integrative approaches involving body fossils, modern animals, sedimentology, biomechanics, soil mechanics, tectonics, statistics, experiments and computer simulations, and, in the case of fossil hominin tracks, even forensics. These developments also bring challenges, as specialised terminology is no longer widely understood across the broader field of ichnology. The increasing interdisciplinarity has also led to collisions between traditional ichnological terminology and modern terminology “borrowed” from outside of the field. This can include identical words that convey different meanings, as is, for example, the case with the terminology of quadrupedal gaits.
Traditional ichnological terminology was greatly influenced by the “Glossary and Manual of Tetrapod Footprint Palaeoichnology” (Leonardi et al., 1987), which contained a comprehensive list of terms in eight languages as well as a discussion of terms and methods. Given the ever-growing pool of terminology, Leonardi et al. (1987) discussed only a fraction of the terms currently in use, and some of their definitions are becoming a growing source of confusion due to conflicting definitions from related fields of study – an inevitable consequence of the increasing interdisciplinarity. A few attempts at updated glossaries have since been made (Vintaned and Liñan, 1996; Marty et al., 2016), although the only systematic attempt in English (Marty et al., 2016) was limited to dinosaurs and focused on a few central terms rather than attempting to be exhaustive. As a result, no comprehensive glossary is available that adequately reflects current terminology. The purpose of our present contribution is therefore three-fold:
First and foremost, we aim to provide a comprehensive review of the existing terminology used in tetrapod track research. We focus on terms that are specific to this field of study or that take on new meanings, including subtle differences in definitions between fields that have not yet been formally documented. We also discuss terms that are important in the context of tetrapod ichnology for other reasons but are not strictly ichnological terms. We aim to include all such terms, including those specific to particular subfields, and only omit terms that have not yet found application beyond the work of the author who first introduced them. We do not include names of ichnotaxa. Unlike Leonardi et al. (1987), we restrict ourselves to the English language, as publications in other languages are much rarer than they were three decades ago. However, we would welcome future translations of this work, either formally or informally.
Second, we aim to provide a standard terminology that is ideally 1) precise, unambiguous, and consistent; 2) compatible with terminology used in related fields; 3) reflects current knowledge while not being misleading, and 4) follows traditional usage of terms as closely as possible. We will give preference to the most used terms in the literature that meet these criteria and will avoid introducing new terms whenever possible. To arrive at such a standard terminology, we will 1) point out any ambiguities and logical problems that may be associated with certain terms, and 2) make recommendations as to which terms should be used. While we do not seek to abandon redundant terminology that may have become an integral part of ichnological history, we hope that these discussions will at least raise awareness of the many pitfalls and urge authors to be clear about the meaning of the terms they use.
Third, following the tradition established by Leonardi et al. (1987), we aim to provide not only precise definitions of terms, but also concise introductions to key concepts and methods, including some general suggestions for the study of tracks. Where appropriate, we will also refer the reader to the most relevant literature.
The terms discussed are organised into a total of 277 numbered entries. Each of these entries represents a distinct concept and may discuss a single term or a related group of terms. For example, the entry “track” covers not only the term “track” and its synonyms (e.g., “footprint”, “ichnite”), but also similar but distinct concepts such as “track volume” and “maximum zone of deformation”. Terms that, in the judgment of the authors, are particularly important and/or distinct are bolded for quick access, but such bolding is used sparingly to maximise practical value.
The entries themselves are divided into ten separate sections. Each section may contain subtopics to group similar terms and is accompanied by overviews that briefly introduce broad topics. The glossary includes a Table of Contents, which lists all the terms discussed in alphabetical order and synonyms that may be useful. There is also a number indicating the entry in which a given term is primarily discussed.
As these files are electronic, readers can always use CTRL F or COMMAND F to find any topic.
TABLE OF CONTENTS
The Table of Contents lists the terms and synonyms included in this glossary in alphabetical order, indicating the number of the glossary entry in which the term is primarily discussed. To avoid redundancy, this index excludes synonyms and spelling variations if they are similar to and in the same alphabetical position as the main entry (e.g., “trotting gait” and “trotter” vs. “trot”). Alphabetical terminology in the Table of Contents is linked to the numbered terminology below.
BASIC TERMINOLOGY
This glossary deals with tetrapod tracks and associated impressions of the body. Tracks are generally the most common type of tetrapod trace fossil. Although most of our knowledge of fossil tetrapods is based on body fossils, tracks are an important complementary source of information. Tracks record the activity of the trackmakers at a particular point in their life, and therefore record behaviour and posture (Lockley, 1991). Tracks are common in the fossil record (an individual may leave many tracks during its life but only a single skeleton) and typically record the entire surface of the undersides of the feet. In contrast, complete pedal skeletons are rare, and soft tissues are preserved only in exceptional cases. Finally, because of their different preservation potential, tracks often occur in rock units devoid of bones, filling important gaps in the fossil record. Tracks can also help to date stratigraphic units. At the same time, the use of tracks as a source of information can be limited by 1) difficulties in distinguishing anatomical information from the effects of substrate properties, foot kinematics (foot movements), and post-formational alteration; 2) difficulties in trackmaker identification; and 3) time averaging of tracksites. It is both a major challenge and an opportunity for future studies to improve on these issues.
1 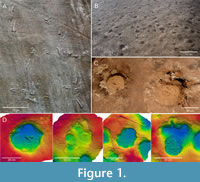 Recognising tetrapod tracks. Distinguishing genuine tetrapod tracks from other structures is not always unequivocal (see Lallensack et al., 2022c, and references therein). Most commonly, tetrapod tracks have been confused with arthropod trackways, especially those of limulids (horseshoe crabs; Figure 1A); fish feeding traces (or nests; Figure 1B); and weathering pits (Figure 1C-D) (Lucas, 2015; Breithaupt et al., 2021; Lallensack et al., 2022c). The most reliable criterion is a regular trackway morphology with predictable track positions. Tetrapod trackways also show alternating foot placement (rather than side-by-side placement as in arthropod trackways), and, in quadrupeds, a differentiation into pes and manus tracks based on size and/or shape (Lucas, 2015; Lallensack et al., 2022c). Track morphology can provide strong evidence if it is consistent across multiple tracks and matches the known anatomy of the trackmaker (Lucas, 2015). Evidence can also be provided by deformation structures, such as asymmetric displacement rims and downward deflection of subsurface layers seen in cross section; the latter distinguish tracks from erosional features or human carvings (Lallensack et al., 2022c). For criteria to distinguish tracks in cross-section from similar soft-sediment deformation structures such as load casts and ball-and-pillow structures, see, e.g., Jackson et al. (2009) and Carvalho et al. (2022). Finally, the stratigraphic, temporal, and environmental context must be considered.
Recognising tetrapod tracks. Distinguishing genuine tetrapod tracks from other structures is not always unequivocal (see Lallensack et al., 2022c, and references therein). Most commonly, tetrapod tracks have been confused with arthropod trackways, especially those of limulids (horseshoe crabs; Figure 1A); fish feeding traces (or nests; Figure 1B); and weathering pits (Figure 1C-D) (Lucas, 2015; Breithaupt et al., 2021; Lallensack et al., 2022c). The most reliable criterion is a regular trackway morphology with predictable track positions. Tetrapod trackways also show alternating foot placement (rather than side-by-side placement as in arthropod trackways), and, in quadrupeds, a differentiation into pes and manus tracks based on size and/or shape (Lucas, 2015; Lallensack et al., 2022c). Track morphology can provide strong evidence if it is consistent across multiple tracks and matches the known anatomy of the trackmaker (Lucas, 2015). Evidence can also be provided by deformation structures, such as asymmetric displacement rims and downward deflection of subsurface layers seen in cross section; the latter distinguish tracks from erosional features or human carvings (Lallensack et al., 2022c). For criteria to distinguish tracks in cross-section from similar soft-sediment deformation structures such as load casts and ball-and-pillow structures, see, e.g., Jackson et al. (2009) and Carvalho et al. (2022). Finally, the stratigraphic, temporal, and environmental context must be considered.
2 Trace fossil (synonym: ichnofossil). The fossilised (i.e., pre-Holocene) result of activity an organism left behind by modifying a substrate (cf. Bertling et al., 2006). More inclusive terms that are not restricted to ancient traces are “ichnospecimen”, “Lebensspur” (German for “life trace”), and “biogenic structure”. The ICZN defines the term “work of an animal” (International Commission on Zoological Nomenclature, 1999). The term “biogenic sedimentary structure” refers to fossil and modern traces produced in unconsolidated sediments (Frey 1973).
Common types of tetrapod trace fossils include tracks; skin impressions; coprolites (fossil faeces) and other material from the digestive tract (see Hunt and Lucas, 2012); urolites (or “uroliths”), i.e., nonliquid urinary excrements as well as sediment structures formed by the impact and flow of liquid urine (see Fernandes et al., 2004); bite marks; burrows; and nests (but not eggs, e.g., Frey, 1973). “Body fossils” (sometimes termed “somatofossils”), in contrast, are remains of the organism itself, such as bones and teeth, or impressions thereof. The term “trace fossil” also does not apply to tool marks (e.g., role marks left by shells) and other structures that do not directly record organism activity (Frey, 1973).
3 Ichnology. The scientific study of traces of biological activity. Researchers who study traces are known as “ichnologists”. Palaeoichnology (also “paleoichnology” or “palichnology”) is the study of trace fossils, as opposed to neoichnology, the study of modern traces. A distinction is also made between invertebrate ichnology and vertebrate ichnology (or “tetrapod ichnology”), as these two fields differ in their aims and approaches (e.g., Lockley, 2007; Minter et al., 2007). Vertebrate ichnology includes traces of tetrapods (a taxon that includes all amphibians and amniotes) as well as fish and fish-like animals such as placoderms, and is therefore more inclusive than “tetrapod ichnology”. Another distinction is made between “marine ichnology” and continental ichnology, which encompasses freshwater and terrestrial settings (e.g., Hasiotis, 2002). The field of ichnology, and neoichnology in particular, is poorly defined outside the geosciences, and traces are often studied by workers of related fields who may not consider themselves ichnologists (e.g., biologists, archaeologists, or forensic scientists) (Baucon et al., 2012).
4 Impression. Generic term for an indentation left by an object in a substrate. A common synonym is “imprint”. When used as a modifier, “mark” is sometimes used interchangeably with “impression”, although this depends on the term in question: “pes impression” and “skin impression” are common terms, whereas “pes mark” or “skin mark” are not in use. Conversely, “claw mark” and “drag mark” are more common than the alternative terms “claw impression” and “drag impression”. In general, we observe that “impression” is usually connotated with a more or less vertical movement of the object, while “mark” is most often used for relatively small accessory traces (e.g., “claw mark”, “probe mark”) and/or when there is substantial horizontal movement involved (e.g., “tool mark”, “drag mark”, “slide mark”). The term “trace” can be used interchangeably with both “impression” and “mark” but is usually restricted to structures actively created by organisms (e.g., the combination “tool trace” as an alternative to “tool mark” is not in use). The term “trace” is more commonly used in invertebrate ichnology than in tetrapod ichnology.
5 Mark. The use of this term differs between invertebrate and vertebrate ichnology. In invertebrate ichnology, the use of the term “mark” is often restricted to non-biogenic structures resulting from physical processes (e.g., “tool mark”, “scour mark”) and is thus opposed to the term “trace”, which describes structures resulting from biological activity (Ekdale et al., 1984). In vertebrate ichnology and some other fields of science, “mark” also refers to structures produced by organism activity (e.g., “tail drag mark”, “tooth mark”, “claw mark”). This more inclusive use of the term “mark” has been repeatedly criticised as being “inconsistent”, “colloquial”, or “incorrect” (e.g., Ekdale et al., 1984; Jacobsen and Bromley, 2009; Vallon et al., 2015). This criticism was countered by Zonneveld et al. (2022), who pointed out that combinations such as “tooth mark” or “claw mark” are long-standing terms that are more common than alternative combinations such as “tooth impression” or “claw trace”; are consistent with usage in related fields such as anthropology and zoology; and are unambiguous in their meaning.
6 Track. 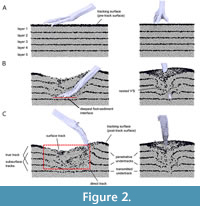 The result of an interaction of the foot, or parts of it, with a substrate. A track can be viewed as a deformation of the substrate, or, at a finer scale, as a redistribution of sedimentary grains (Gatesy, 2003). A track may extend vertically to be visible on separate sedimentary layers, and may include a true track, overtracks, and/or penetrative or transmitted undertracks (Figure 2). “Track” is also used to refer to a particular track surface, usually the one that is visible (e.g., an undertrack on the exposed bedding surface). “Track” can also refer to just the impression of the foot. The term track volume has been used when discussing the entire vertical extent of a track (Margetts et al., 2006) (but see “negative volume” and “fossil volume” for different meanings of “volume”). A related concept is the maximum zone of deformation, which is defined as the full extent of the track, including all folds and faults surrounding the shaft (Manning, 2004). “Track”, “track volume”, and “maximum zone of deformation” are essentially synonymous. The term “track” is also commonly applied to natural casts and overtracks, and although these are not technically tracks as they are formed after the actual track is formed, we do not see strong reasons for discouraging such usage. In common usage, and occasionally in technical literature, “track” is also used as a synonym for trackway; such usage is misleading and should be avoided.
The result of an interaction of the foot, or parts of it, with a substrate. A track can be viewed as a deformation of the substrate, or, at a finer scale, as a redistribution of sedimentary grains (Gatesy, 2003). A track may extend vertically to be visible on separate sedimentary layers, and may include a true track, overtracks, and/or penetrative or transmitted undertracks (Figure 2). “Track” is also used to refer to a particular track surface, usually the one that is visible (e.g., an undertrack on the exposed bedding surface). “Track” can also refer to just the impression of the foot. The term track volume has been used when discussing the entire vertical extent of a track (Margetts et al., 2006) (but see “negative volume” and “fossil volume” for different meanings of “volume”). A related concept is the maximum zone of deformation, which is defined as the full extent of the track, including all folds and faults surrounding the shaft (Manning, 2004). “Track”, “track volume”, and “maximum zone of deformation” are essentially synonymous. The term “track” is also commonly applied to natural casts and overtracks, and although these are not technically tracks as they are formed after the actual track is formed, we do not see strong reasons for discouraging such usage. In common usage, and occasionally in technical literature, “track” is also used as a synonym for trackway; such usage is misleading and should be avoided.
Footprint is commonly treated as a synonym of “track” (Leonardi et al., 1987; Marty et al., 2016), but is generally used in a narrower sense: the actual “print of a foot”. For example, “footprint” is rarely used to describe swimming tracks or the full vertical and horizontal extent of a track (i.e., the maximum zone of deformation). Thulborn (2012, p. 3) defines the term “footprint” or “footprint sensu stricto” as “the area of the substrate impressed directly by the undersurface of a track-maker’s foot”, while explicitly excluding “any surrounding or subsurface feature” (p. 4) such as track walls, displacement rims, undertracks, and natural casts. Under this definition, “footprint” is restricted to a single track surface rather than encompassing the entire track volume, and is synonymous with “true track sensu stricto”. This definition also matches the meaning of “footprint” in common language (Thulborn, 2012). However, in practice, fossil tracks can often not easily be demonstrated to be footprints sensu stricto, and consequently, the more general term “track” may be preferable in many cases (Gatesy and Falkingham, 2017).
Several synonyms of “track” and “footprint” have been used. Although combinations such as “manus footprints” are used to specify the autopodium involved, the more common term in such situations is “print”, which allows combinations such as “manus prints” and “pes prints” (we advocate the use of “manus track” and “pes track” instead). “Footstep” is a synonym that is rarely used in technical papers, while “footmark” has been proposed for cases where the trackmaker is buoyant (see discussion in entry “swimming track”). “Trample mark” is sometimes used as a synonym of “track”, a usage that should be avoided because “trample mark” more commonly refers to a taphonomic feature of bones (Fiorillo, 1984). For mammals, terms such as “paw mark”, “paw print” or “paw track” have been used. Flipper tracks are left by aquatic animals with flippers, such as sea turtles and pinnipeds (synonyms: “flipper traces”, “flipper prints”, “flipper impressions”, “paddle traces”, “paddle prints”). Ichnite is a common technical synonym of “track”. However, the definition of “ichnite” is not self-evident and is easily confused with the broader term ichnofossil.
Marchetti et al. (2019a), following the definition of “trace fossil” proposed by Bertling et al. (2006), defined “vertebrate footprints” as “morphologically recurrent biogenic structures resulting from the locomotion of an individually limbed vertebrate modifying the substrate” (p. 110). However, we argue that a track that is not recurrent would still be a track. Marchetti et al. (2019a) clarify their definition by explicitly excluding behaviours other than terrestrial limbed locomotion, such as crawling traces (e.g., snake traces), resting traces, nests, burrows, and swim traces. We agree that nests and burrows are not tracks, and have never been considered as such. Such structures do not fall under our definition of “track” because a nest or burrow is formed by repeated retraction of the limb rather than by a single interaction of the foot with the substrate. Although snakes do not have feet, their traces have sometimes been referred to as “tracks”, especially the discontinuous locomotion traces of sidewinders, and we do not see strong reasons for discouraging the latter use. However, we argue that the notion of a track should be independent of the presumed behaviour. Consequently, impressions of the feet in swimming and resting traces must be considered tracks, and are widely recognised as such in the literature.
7 Morphology. The form of a track or trackway, which is the result of trackmaker anatomy, trackmaker behaviour and foot movements, substrate properties, and post-formational alteration (Gatesy and Falkingham, 2017; Marchetti et al., 2019a, p. 110). “Morphology” is also used in a narrower sense to refer only to the anatomical information conveyed by a track (e.g., Marchetti et al., 2019a). This usage is reflected in terms such as “extramorphology”, which literally means “outside of the morphology” and is applied to features that do not reflect anatomy. Gatesy and Falkingham (2017) have criticised this usage, arguing that tracks cannot simply be considered as imperfect copies of the trackmaker’s feet. In invertebrate ichnology, “morphology” has been defined as the “inventory of parts, form, and size of organism-produced ethologic structures” (Miller, 2007, p. 461).
Synonyms of the term “morphology” (in its broader sense) include “shape”, “form”, “relief”, “topography”, “topology”, “microtopography”, and “texture”, although these are often used in slightly different contexts. “Shape” refers to the outline or 3D-shape of a track but not to its size, whereas “form” specifically includes size. “Topography”, “relief”, and “topology” refer to the spatial variation in surface elevation. “Texture” (or “microtopography”) is used to refer to fine-scale relief features such as skin impressions. Note that “texture” also refers to the microstructure of rocks, as well as to colour information in a 3D model.
8 Tracking. Sometimes used to describe the discovery, documentation, and interpretation of tracks conducted by an ichnologist (e.g., Lockley, 1991). The person doing the tracking is the tracker. The term “tracking” is borrowed from, and most appropriate to, the practical investigation of modern tracks (e.g., a hunter “tracks” an animal). In this context, tracking is not limited to the tracks themselves, but also involves any hints on the presence of the animal or human that is being “tracked” (Liebenberg et al., 2010; Baucon et al., 2012). Such hints are termed spoor or signs. Note that, in the term tracking surface, “tracking” refers to the activity of the trackmaker.
9 Trackmaker (also “track maker”). The animal that makes the tracks. The term can refer to an individual animal or to the biological taxon that made the track. The latter meaning can be specified with more precise terms such as “trackmaker taxon” and “trackmaker species”. Tracemaker (also “trace maker”) is a more general term that is not restricted to tracks (e.g., “the tracemaker of the coprolite”). A rarer synonym is “print maker” (= printmaker).
10 Trackmaker identification (also “track-trackmaker correlation”). The identification of the trackmaker taxon is a major challenge in ichnology. It is complicated by the possible occurrence of convergent evolution (i.e., a similar foot morphology evolves independently in unrelated taxa) and by morphological conservatism (i.e., the ancestral foot morphology is retained). The three most common approaches, according to Carrano and Wilson (2001), are:
phenetic correlation, based on the total of characteristics shared by fossil tracks/trackways and the inferred trackmaker. These characteristics may include anatomy/body shape, posture, locomotion, and behaviour. Phenetic correlation can also be based on comparisons with other tracks for which the trackmaker is known (e.g., Buckley et al., 2015).
coincidence correlation, based on the geographic and stratigraphic position of tracks and their presumed trackmakers. This is an extension of phenetic correlation and is to be used in conjunction with the latter (Carrano and Wilson, 2001). For example, a very large theropod track from the Maastrichtian of New Mexico has been attributed to Tyrannosaurus rex because the latter is the only known large theropod from this time and region (Lockley and Hunt, 1994).
synapomorphy-based correlation (also “synapomorphy-based approach”), based on skeletal synapomorphies (shared derived characters) identified in the tracks. While this is the most rigorous approach to trackmaker identification (Olsen, 1995), it can only be applied if relevant skeletal synapomorphies are available to start with.
11 Behaviour. The responses of an organism to internal or external stimuli. In ichnology and elsewhere in palaeontology, “behaviour” has often been applied more broadly, and more vaguely, to various related aspects such as posture, foot kinematics, and biotic interactions (Plotnick, 2012). In the context of invertebrate trace fossils, Seilacher (1986, p. 62) defined behaviour as “the rules, or programs, underlying animal activities”, but, as Plotnick (2012) pointed out, the view of behaviours as the result of underlying fixed “programs” is outdated. Ethology (or palaeoethology) is the study of animal behaviour, but note that in biology, the synonyms “behavioural biology” and “animal behaviour” are now more common (Bolhuis et al., 2022).
Seilacher developed an ethological classification of ichnofossils that includes the categories “repichnia” (locomotion traces), “pascichnia” (grazing traces), “agrichnia” (farming traces), “fodinichnia” (feeding traces), “domichnia” (dwelling structures), and “cubichnia” (resting traces) (e.g., Seilacher, 1953, 2007, p. 93). The vast majority of tetrapod tracks are repichnia. Müller (1962) proposed several sub-categories of repichnia (“movichnia” in his usage), including “natichnia” (produced during swimming), “cursichnia” (produced during limbed locomotion, i.e., tracks and trackways), and “volichnia” (produced during flight, e.g., wing impressions) (Vallon et al., 2016).
Note that these ethological categories are commonly used only in invertebrate ichnology, and are based on interpretation and are not descriptive. Miller (2007) and Plotnick (2012) argued that these categories should be abandoned as they do not represent actual behaviours and often cannot be applied to traces that show multiple behaviours, but see Vallon et al. (2016) for a defence and an updated classification.
12 Polarity. The variation in a feature that results in two notably and visibly different end-members. An example is trackway gauge in sauropod dinosaurs, where narrow-gauged and wide-gauged trackways form the end-members, and a polarity between narrow- and wide gauged trackways may be pointed out (e.g., Lockley et al., 2023). Note that the term “polarity” makes no assumptions about the frequency distribution of the feature: Intermediate conditions (e.g., “medium gauge”) can occur and may even be more common than the end-members; it is also possible that one end-member is more common than the other (e.g., narrow-gauged sauropod trackways are much more common than wide-gauged ones). The frequency distribution of the character is therefore often unimodal rather than bimodal. We note that the term “polarity” in this sense is not precisely defined, as both the definition of the end-members and the notion of what constitutes a “notable” or “visible” difference between these end-members is partly subjective and/or arbitrary. We also note that there is a potential of confusion with the more widespread use of the term in cladistics, where “polarity” is the direction between two character states (e.g., character state A may be the basal condition, and character state B the derived condition, implying that B evolved from A, not vice versa) (Brower and Schuh, 2021).
13 Three-dimensional (3D) model. D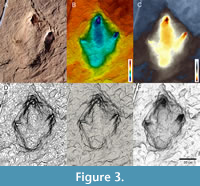 igital three-dimensional replica of a track or tracksite (Figure 3). Although 3D models sensu stricto can be obtained by casting (e.g., plaster casting), they are rarely if ever referred to in this way, and the term is generally restricted to the digital domain. Photogrammetry is a method for creating 3D models based on multiple photographs of an object (Figure 4E) (Matthews and Breithaupt, 2001; Matthews et al., 2016). A more specific term is structure from motion, which refers to photogrammetry when camera and object positions are unknown. The generation and publication of 3D models greatly facilitates data collection, archiving and distribution, and has been established as part of a standard protocol for the study of tracks (Falkingham et al., 2018). A 3D model can consist of a point cloud (a set of x, y, and z coordinates) or a mesh (a surface of connected polygons called faces). Colour information can be stored with a 3D model, either as vertex colours (where each connection point between faces is assigned a colour value) or as a texture (which maps a 2D image containing the colour information onto the 3D shape). Three-dimensional models of tracks should be aligned in the horizontal plane to allow accurate top views, and should be presented in orthographic (camera-parallel) projection to avoid perspective distortion.
igital three-dimensional replica of a track or tracksite (Figure 3). Although 3D models sensu stricto can be obtained by casting (e.g., plaster casting), they are rarely if ever referred to in this way, and the term is generally restricted to the digital domain. Photogrammetry is a method for creating 3D models based on multiple photographs of an object (Figure 4E) (Matthews and Breithaupt, 2001; Matthews et al., 2016). A more specific term is structure from motion, which refers to photogrammetry when camera and object positions are unknown. The generation and publication of 3D models greatly facilitates data collection, archiving and distribution, and has been established as part of a standard protocol for the study of tracks (Falkingham et al., 2018). A 3D model can consist of a point cloud (a set of x, y, and z coordinates) or a mesh (a surface of connected polygons called faces). Colour information can be stored with a 3D model, either as vertex colours (where each connection point between faces is assigned a colour value) or as a texture (which maps a 2D image containing the colour information onto the 3D shape). Three-dimensional models of tracks should be aligned in the horizontal plane to allow accurate top views, and should be presented in orthographic (camera-parallel) projection to avoid perspective distortion.
Three-dimensional models can be visualised in two dimensions using different methods that can enhance different details (Figure 3) (Falkingham et al., 2018; Lallensack et al., 2022a). Elevation maps (Figure 3B, C; synonyms: depth map, colour relief, height map, depth-colour map, digital elevation model, DEM, false-colour map) enhance the topography of a model surface by coding different elevations with different colours. Contour plots (Figure 3D) describe the model topography using contour lines. Shaded reliefs (synonym: hillshading) use an artificial low angle light source to enhance subtle surface details. Inclination maps (Figure 3E) assign colour values to each face depending on its inclination. In ambient occlusion (Figure 3F), each point is shaded according to its exposure to ambient light. Orthophotos (Figure 3A) are photographs that have been rectified to produce an orthographic projection and can be used as maps. Orthophotos can be derived directly from textured and aligned 3D models.
can be visualised in two dimensions using different methods that can enhance different details (Figure 3) (Falkingham et al., 2018; Lallensack et al., 2022a). Elevation maps (Figure 3B, C; synonyms: depth map, colour relief, height map, depth-colour map, digital elevation model, DEM, false-colour map) enhance the topography of a model surface by coding different elevations with different colours. Contour plots (Figure 3D) describe the model topography using contour lines. Shaded reliefs (synonym: hillshading) use an artificial low angle light source to enhance subtle surface details. Inclination maps (Figure 3E) assign colour values to each face depending on its inclination. In ambient occlusion (Figure 3F), each point is shaded according to its exposure to ambient light. Orthophotos (Figure 3A) are photographs that have been rectified to produce an orthographic projection and can be used as maps. Orthophotos can be derived directly from textured and aligned 3D models.
14 Artificial casts and moulds are usually made from silicon rubber, latex, or plaster of Paris (gypsum plaster). Latex must be peeled from the rock after application, when the product is termed a “latex peel” (Figure 4B) (Thulborn, 1990). Silicone rubber and latex replicas are deformable and are therefore often supported or replaced by a rigid fibreglass jacket. Physical replicas can also be made by using 3D printers to print 3D models.
TRACK FORMATION AND ALTERATION
While tracks were traditionally considered to be exact copies of the palmar/plantar surface of the foot (Hitchcock, 1858), it has since been shown that a fresh track is rarely a mould of the foot, but always the result of three factors: the anatomy of the trackmaker, the properties of the substrate, and the movement of the trackmaker (Minter et al., 2007; Falkingham, 2014). Many studies of fossil tetrapod tracks are concerned with ichnotaxonomy, which is based on features that are thought to convey information about the anatomy of the trackmaker - as opposed to extramorphological features that do not convey such information. The information about substrate properties (and hence the environment) and kinematics (and hence locomotion and behaviour) that can potentially be derived from tracks has its own merits and is an important aspect of tetrapod track ichnology.
15 Track formation. The interaction of the foot with the substrate that creates a track (e.g., Leonardi et al., 1987; Thulborn, 1990; Bennett and Morse, 2014; Falkingham and Gatesy, 2014). More precisely, track formation includes all movements of substrate particles induced by the moving foot; such movements may continue after the foot is removed, for example as the sediment collapses back to a stable state (Gatesy and Falkingham, 2017). “Track registration” is a synonym used across disciplines (e.g., Halfpenny, 1986). Several less common synonyms and variants have been used, often ad hoc, such as “footprint creation” or “track genesis”. Processes that occur simultaneously or slightly later than the formation of a track (or the deposition of a layer) can be described as penecontemporaneous.
Marchetti et al. (2019a, 2020) argued that the term “registration” is more appropriate than “formation” because the latter suggests “an active action of the biogenic structure” (Marchetti et al., 2019a, p. 110). However, “formation” does not necessarily imply such an active action, as shown by, e.g., similar uses in geology such as “formation of ripple marks” or “impact crater formation”. Falkingham and Gatesy (2020) argued that “registration” can be misleading because it implies a simple, two-dimensional process, whereas a track is a complex volumetric structure. We prefer the term “formation” because it is consistent with usage in other fields of ichnology (e.g., Brown, 1911) - for example, the combination “trace formation” has many precedents in the literature, while the alternative “trace registration” has only been used by Marchetti et al. (2019a). Furthermore, the term “track formation” is consistent with geological terminology that refers to similar sedimentological structures (e.g., “impact crater formation”). Note that, in some contexts, care should be taken to avoid confusion with the term “geological formation”.
16 Sources of variation (or “causes of variation”). The factors that lead to the morphology of a fossil track in its current form (Díaz-Martínez et al., 2009). It is generally accepted that not only the trackmaker anatomy, but also substrate properties, foot movements, and post-formational alteration play a key role (Minter et al., 2007; Falkingham, 2014). Gatesy and Falkingham (2017) proposed a comprehensive framework that distinguishes between seven hierarchical levels at which variation can occur, which are (from highest to lowest):
Interspecific variation - the differences between trackmaker species. This variation is the objective of ichnotaxonomy.
Intraspecific variation - the differences between individuals of the same species. This includes differences between populations as well as differences between individuals due to age, sex, health, and other factors.
Appendage variation - the differences between the feet of the same individual. These are most obvious between pes and manus due to their different anatomy.
Behavioural variation - the differences in tracks resulting from trackmaker behaviour, such as mode of locomotion (walking, swimming); gait; and acceleration, deceleration, and turning. Variation at this level (and all lower levels) can occur between tracks left by the same foot.
Formational variation - the differences produced by the interplay between foot kinematics (movements) and the substrate. Differences in substrate properties are widely recognised as an important source of variation (e.g., Razzolini et al., 2014).
Intravolumetric variation - the differences between different track surfaces within the track volume. For example, a true track will differ from a transmitted undertrack, even if both are part of the same track volume, and hence the same foot-sediment interaction.
Post-formational alteration (also 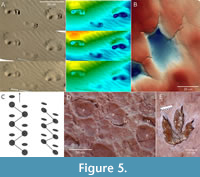 “post-formational modification”) - any modifications of the track morphology that has occurred after formation, including (but not limited to) deformation by other traces, ancient and recent erosion, diagenesis, and even “enhancement” for public display (Figure 5). The processes leading to such alteration have sometimes been summarised as post-formation processes (=post-registration processes).
“post-formational modification”) - any modifications of the track morphology that has occurred after formation, including (but not limited to) deformation by other traces, ancient and recent erosion, diagenesis, and even “enhancement” for public display (Figure 5). The processes leading to such alteration have sometimes been summarised as post-formation processes (=post-registration processes).
A closely related term is intratrackway variability (also “intra-trackway variability” or “intra-trackway variation”), which describes the variation of tracks within the same trackway, where the anatomy of the trackmaker can be assumed to be constant (e.g., Razzolini et al., 2014; Lallensack et al., 2016).
17 Anatomical fidelity (synonyms: morphological quality, anatomical informativeness, anatomy-consistent morphological features). The degree of anatomical information conveyed by a track; its fidelity to the shape of the foot. A high-fidelity track is anatomically fidelitous, while a low-fidelity track lacks anatomical fidelity. The terms “well preserved” and “poorly preserved” are commonly used as synonyms of “high fidelity” and “low fidelity”, respectively, but note that this usage is inconsistent with the general usage of the term “preservation” (see below for further discussion and related terms). The term “morphological quality” is also used as a synonym for “anatomical fidelity” (Belvedere and Farlow, 2017; Marchetti et al., 2019a), with “high-quality tracks” referring to those that are anatomically fidelitous, and “poor-quality tracks” referring to those that are not. Furthermore, the terms “well defined” and “poorly defined” are used, but do not always refer to anatomical fidelity.
Note that Plotnick (2012) proposed the term “behavioural fidelity” (the degree to which trace fossils inform about trackmaker behaviour) and Savrda (2007) proposed the term “ichnologic fidelity” (the degree to which a trace fossil assemblage or ichnofabric reflects the complete range of trackmaker activities). Both terms have, to our knowledge, not been applied to tetrapod tracks.
18 Preservation. This term, as used in the study of tetrapod tracks, conveys two conflicting meanings:
The degree of alteration of a track after its formation. In this sense, a “well-preserved track” is one that has not been significantly affected by post-formational alteration. Thus, a freshly formed track in dry sand is “well-preserved” even if it records little information about the anatomy of the trackmaker (Gatesy and Falkingham, 2017). This definition is prevalent in the ichnological literature not concerned with ichnotaxonomy (e.g., Bennett and Morse, 2014); for example, swimming tracks are regularly described as “well-preserved” but tend to reveal little information about the anatomy of the trackmaker’s foot. This definition is also consistent with terminology used elsewhere in palaeontology, biology, geology, and modern tracking (e.g., Halfpenny, 1986; Elbroch, 2003), and is consistent with dictionary definitions.
The anatomical fidelity of a track. In this sense, a track is “poorly-preserved” when it is not anatomically fidelitous, even if it is freshly formed. This definition is prevalent when ichnotaxonomy, and therefore anatomical fidelity, is the primary focus. These conflicting definitions and the resulting problems have led to much debate. Gatesy and Falkingham (2017) and Falkingham and Gatesy (2020) argued that the use of “preservation” as a synonym of “anatomical fidelity” needs to be abandoned as it is confusing and illogical, as one cannot preserve anatomical details that never existed in the track. Marchetti et al. (2019a; 2020) have defended this use of the term, arguing that it is the foot of the trackmaker that is preserved in the track. We acknowledge that the meaning of “preservation” in the second sense is longstanding and widespread, at least in ichnotaxonomy. Nevertheless, we agree with Gatesy and Falkingham (2017) that an ideal state that could possibly be “preserved” in a track does not actually exist, and that such conceptual problems are a cause for concern.
In an attempt to provide a more precise terminology, Marchetti et al. (2019a) introduced the terms “M-preservation” (“morphological preservation”; the morphological quality of footprints, or, in our usage, anatomical fidelity) and “P-preservation” (“physical preservation”; or, in our usage, post-formational alteration). In addition to “morphological preservation” and “physical preservation”, Marchetti et al. (2019a) also introduce the terms “taphonomic preservation”, “diagenetic preservation”, “ichnostratinomic preservation”, “biostratinomic preservation”, and “registrational preservation”, all of which are grouped under the umbrella term “morphological preservation”. A complementary set of terms has been grouped outside of “morphological preservation”: the terms “taphonomy”, “diagenesis”, and “biostratinomy” fall under “physical preservation”, while “ichnostratinomy” and “ichnotaphonomy” fall under both “physical preservation” and “registration”. We are concerned that many of these terms and their distinctions are not intuitive or even misleading (Falkingham and Gatesy, 2020), and are not consistent with previous usage.
19 Fidelity scale. Numerical scale for assessing the anatomical fidelity of a track (Belvedere and Farlow, 2017; Marchetti et al., 2019a). This scale is commonly referred to as the “preservation scale”, with “preservation” referring to the second meaning of the term as a synonym of “anatomical fidelity”. The scale proposed by Belvedere and Farlow (2017) consists of four grades (grade 0 to 3), with grade “0” used for tracks of low fidelity, and grade “3” for tracks of exceptional fidelity. Marchetti et al. (2019a) suggested that intermediate values could be used, resulting in a continuous scale.
20 Extramorphological feature (or “extramorphology”). A feature of a track that does not inform about trackmaker anatomy (cf. Peabody, 1948). The most important criterion for identifying an extramorphology is its lack of consistency: variations in foot movement and substrate properties typically result in seemingly random variation from one step to the next (Thulborn, 1990). In contrast, features that are consistent within a trackway, or sample of tracks, are more likely to reflect the anatomy of the trackmaker. However, this criterion is far from infallible, as extramorphologies can be consistent as well (e.g., digit impressions along a trackway can be consistently widened by sediment collapse). We urge caution in applying the term ‘extramorphological’ to any feature of a track.
21 Direct feature and indirect feature. Direct features are formed by sediment grains that directly contacted the foot, whereas indirect features are formed by grains that did not contact the foot (Gatesy, 2003). For example, an exit trace is a direct feature, whereas a displacement rim is an indirect feature. The direct track is the sum of the direct features of a track (Figure 2C). These terms were introduced by Gatesy (2003).
A similar term is pressure-release structure (also: “pressure release structure”, “pressure releases”), which includes any feature formed by the interaction of the foot with the substrate, except for features that directly record the shape or behaviour of the trackmaker (cf. Martin et al., 2012, 2014). Following this usage, displacement rims, pull-up features, and radial fractures are considered pressure-release structures, whereas digit impressions, drag marks, or skin impressions are not. The term was coined by Brown (1999), who defined it as “disturbances in the soil in and around the track” (Brown, 1999, p. 40). We note, however, that most of these structures would be formed during the application of pressure rather than during its release; the term is therefore potentially misleading.
22 Ontogeny. The term has been used in two contexts:
An ontogenetic series of tracks (or “growth series”) describes tracks of different sizes that are thought to represent different ontogenetic stages of the same trackmaker species. Such series have been proposed by, e.g., Peabody (1948), Olsen (1980), and Avanzini and Lockley (2002). In invertebrate ichnology, a similar concept, “ichnogeny”, has been proposed for boring and burrowing traces (Belaústegui et al., 2016). Here, a continuum of successive stages, or “ichnogenetic stages”, is defined to describe the ontogenetic development of the trace.
Track ontogeny describes the development of track characteristics during track formation, analogous to the development of traits during the life history of an organism (Falkingham and Gatesy, 2014). Note that “track ontogeny” describes the formation of a single track (e.g., from touch-down to lift-off), and does not refer to the actual ontogenetic stages of the trackmaker.
23 Ichnotaphonomy (or simply “taphonomy”). The study of the processes that affect a trace after its formation (e.g., Cohen et al.,1991; Bromley, 1996; Savrda, 2007; Bennett and Morse, 2014). We note that, originally, “taphonomy” was defined as “the study of the transition (in all its details) of animal remains from the biosphere into the lithosphere”, and described as “the science of the laws of embedding” (Efremov, 1940, pp. 85, 93). Trace fossils, however, are sedimentary structures, not organic remains that could be embedded. Indeed, trace fossils and taphonomy were treated as separate fields of study from the beginning (Richter, 1928; Efremov, 1940), and trace fossils are generally not considered in the taphonomical literature (e.g., Behrensmeyer and Kidwell, 1985; Lyman, 2010).
Marchetti et al. (2019a; 2020) proposed a new definition of the term “ichnotaphonomy” that encompasses both the formation and post-formational alteration of a trace, while defining “taphonomy” and “taphonomic preservation” as separate concepts that exclude trace formation. We argue that such terminology is unnecessarily complicated and, in the case of “ichnotaphonomy”, counterintuitive, as “taphonomy”, if it is to be applied to trace fossils, most naturally translates to “after track formation”, as defined above.
24 Ichnostratinomy. The study of the processes that affect a trace fossil after its formation and before its final burial. The term was first used by Savrda (2007) for invertebrate traces, in reference to the term “biostratinomy” in general palaeontology, which is the study of the processes that occur between death and burial. Marchetti et al. (2019a) proposed to re-define “ichnostratinomy” to include all processes “from the beginning of the trace registration until its final burial” (p. 111-112), and treated “biostratinomy” and “biostratinomic preservation” as separate concepts (see also discussion in the entry “preservation”). We consider these terms, when applied to trace fossils, to be synonymous with “ichnostratinomy”.
25 Exit trace. Feature of a track formed as the foot withdraws from the substrate. In tridactyl tracks of birds and dinosaurs, the digits typically collapse (are pulled together) to facilitate withdrawal. Because of this collapse, exit traces in these groups may be much smaller than the track as a whole (for examples, see Turner et al., 2020; Oussou et al., 2023). Exit traces may also appear as large overturned mounds at the front of the track, as seen in some sauropod trackways.
26 Natural mould  (or “natural mold”) and natural cast (Figure 6). When a track is buried, sediment will fill the concave impression, or natural mould, forming a natural cast of the footprint. Natural moulds will therefore appear on the upper surface of a layer, while natural casts will appear on the lower surface of the overlying layer. Natural casts are often found on the underside of overhangs in cliffs after the less resistant layer containing the moulds has been eroded away (Figure 6A); such casts can sometimes fall down and accumulate as loose blocks ex situ (Figure 6B) (e.g., Lockley, 1991). Marty et al. (2009) suggested restricting the term “natural cast” to the common case of homogeneous sedimentary infill. If the infill consists of separate thin layers, these individual layers are referred to as overtracks. The terms “footprint filling”, “infilling”, “shaft fill”, and “plug” are sometimes used as synonyms of “natural cast”, typically when the infill is still present in the mould (e.g., Thulborn, 1990; Allen, 1997; Engelmann and Hasiotis, 1999). Often the terms “natural mould” and “natural cast” are abbreviated to simply “mould” and “cast”, a practice that should be avoided given the frequent confusion with artificial moulds and casts.
(or “natural mold”) and natural cast (Figure 6). When a track is buried, sediment will fill the concave impression, or natural mould, forming a natural cast of the footprint. Natural moulds will therefore appear on the upper surface of a layer, while natural casts will appear on the lower surface of the overlying layer. Natural casts are often found on the underside of overhangs in cliffs after the less resistant layer containing the moulds has been eroded away (Figure 6A); such casts can sometimes fall down and accumulate as loose blocks ex situ (Figure 6B) (e.g., Lockley, 1991). Marty et al. (2009) suggested restricting the term “natural cast” to the common case of homogeneous sedimentary infill. If the infill consists of separate thin layers, these individual layers are referred to as overtracks. The terms “footprint filling”, “infilling”, “shaft fill”, and “plug” are sometimes used as synonyms of “natural cast”, typically when the infill is still present in the mould (e.g., Thulborn, 1990; Allen, 1997; Engelmann and Hasiotis, 1999). Often the terms “natural mould” and “natural cast” are abbreviated to simply “mould” and “cast”, a practice that should be avoided given the frequent confusion with artificial moulds and casts.
27 Infill (synonyms: plug, infilling, sediment filling). Any sediment present in the natural mould of a surface track (Figure 6D). This may be the natural cast, an overtrack, or parts thereof that remain in the mould after separation of layers. Such infill can protect the mould from erosion, but may need to be removed to examine the track. Infill may be more resistant to erosion than the surrounding sediment, which may result in positive relief of the track.
The above definitions assume that the tracks described are surface tracks. In the case of undertracks (transmitted or penetrative) or overtracks, the more general and descriptive terms concave epirelief (instead of “mould”) and convex hyporelief (instead of “cast”) are preferable. Alternatively, the terms epichnia and hypichnia refer to traces on the upper and lower surfaces of a stratum, respectively. The more specific term “undertrack cast” is rarely used, and should be avoided. Sometimes, the terms positive and negative are used, with “positive” often but incorrectly applied to the mould and “negative” to the cast. However, these terms are ambiguous and, in fact, the mould must be regarded as the negative of the foot and the cast as the positive copy of the mould (Leonardi et al., 1987). In invertebrate ichnology, the classification of traces based on their mode of occurrence (e.g., as epirelief or hyporelief) and the nature of the relief (e.g., concave or convex) is known as toponomy (Frey, 1973).
An ex situ slab containing only the concave epireliefs is referred to as the main slab, while the corresponding slab containing the convex hyporeliefs is referred to as the counter slab (or “counterslab”). Note, however, that the split into main slab and counter slab does not always occur cleanly across a track surface, so that material belonging to the natural cast may sometimes be found as infill in the main slab (Thulborn, 1990, p 26).
28  Overhang and undercut. In a mould, the track walls may protrude into the shaft, forming overhangs that, in top view, hide parts of the track floor from view (Figure 6C). In a cast, such morphologies result in undercuts; i.e., the track walls appear to be excavated (Figure 6B, Figure 7A) (e.g., Milner and Lockley, 2016). Overhangs/undercuts do often occur at the distal ends of digit impressions, either because the digit was pushed forwards into the sediment (and was then withdrawn backward), or because the sediment collapsed over the descending digit. In moulds, extreme overhangs can result in “toe tunnels” (Figure 6C) (Farlow et al., 2012b), while in casts, extreme undercuts can result in “free” digit impressions that protrude below the bedding plane.
Overhang and undercut. In a mould, the track walls may protrude into the shaft, forming overhangs that, in top view, hide parts of the track floor from view (Figure 6C). In a cast, such morphologies result in undercuts; i.e., the track walls appear to be excavated (Figure 6B, Figure 7A) (e.g., Milner and Lockley, 2016). Overhangs/undercuts do often occur at the distal ends of digit impressions, either because the digit was pushed forwards into the sediment (and was then withdrawn backward), or because the sediment collapsed over the descending digit. In moulds, extreme overhangs can result in “toe tunnels” (Figure 6C) (Farlow et al., 2012b), while in casts, extreme undercuts can result in “free” digit impressions that protrude below the bedding plane.
29 Counter-relief. A track on the upper surface of a sedimentary layer that has positive relief (i.e., is elevated relative to the surrounding surface) (cf. Courel and Demathieu, 1984). This can occur due to differential erosion, where the compacted sediment beneath the surface track is more resistant to erosion than the surrounding less compacted sediment, leading to inversion of the relief as erosion progresses (Thulborn, 1990; for examples, see Kuban, 1989a; Manning et al., 2008). In laminated sediments, the stack of down-bent laminae beneath the surface track may result in an “onion-ring pattern” when exposed by differential erosion (Thulborn, 2012). Counter-reliefs can also form due to suction or adhesion as sediment is pulled upwards as the foot lifts (Leonardi and Carvalho, 2021a).
30 Elite tracks (synonym: elite footprints). The term has two conflicting meanings:
The “clearest and most distinct” tracks on a trampled surface that overprint previously formed tracks (Figure 5D) (Lockley, 1993, p. 340). Lockley (1993) derived the term from the term elite trace fossil of Bromley (1990), which refers to invertebrate trace fossils that “totally dominate the fabric” (p. 154). Such dominance may be the result of obliteration of other traces that formed earlier, or even diagenetic enhancement. Elite tracks are often, but not necessarily, of higher anatomical fidelity than the tracks they overprint. Conversely, the occurrence of indistinct and often incomplete tracks on a trampled surface can be described as background trampling (Figure 5D; “background bioturbation” is a more general term used in invertebrate ichnology).
Tracks of high anatomical fidelity in general (Gatesy, 2003). This definition is now the dominant usage in the literature (e.g., Marty et al., 2016; Marchetti et al., 2019a), but is not consistent with the meaning of “elite trace fossil” and can therefore be a source of confusion. Synonyms include “high-fidelity tracks” and “stamps” (Pérez-Lorente, 2015, p. 41). In modern tracking, tracks with exceptionally high anatomical fidelity have sometimes been referred to as “perfect tracks” (e.g., Murie, 1982, p. 65; Brown et al., 2022, p. 43).
31 Foot-sediment interface (also: skin-sediment interface; Figure 2B). The contact surface between the foot and the sediment. This surface changes during the time the foot is in ground contact (Gatesy and Falkingham, 2020).
32 Tracking surface (synonyms: “tracked surface”, “original ground surface”). The upper surface of the substrate at the time of track formation (Figure 2). The term was introduced by Fornós et al. (2002). The tracking surface can be at either the sediment-air interface (or surface/boundary) or the sediment-water interface (or surface/boundary); these terms describe the boundary between the sediment below and the air (or water) above, depending on whether the track is submerged or not. Layers below (and not exposed at) the tracking surface are referred to as subsurface layers (or levels; Figure 2C). Gatesy (2003) distinguished between the pre-track surface and the post-track surface: the former includes all grains exposed before track formation, while the latter includes those grains exposed after track formation (Figure 2). Note that the tracking surface is not always the surface on which the animal walks. As the trackmaker sinks deeper into soft sediment, the body weight may only be supported at a subsurface level. There may be a continuum from supportive surface to fluid (e.g., in submerged mud), and therefore no discrete surface on which the animal walks.
The individual layers of a track volume (e.g., surface track and undertracks) may also be referred to as track surfaces. The term ichnosurface can be used to refer to the surface of the bed (layer) containing the tracks as it is seen today.
33 Negative volume. The volume of the space between the track floor and the level of the tracking surface (i.e., excluding displacement rims). Note that the terms “track volume” and “fossil volume” have been used to refer to different concepts.
34 True track (also: “true trace”). The portion of a track down to the final (deepest) foot-sediment interface (Figure 2C) (sensu Gatesy, 2003). The true track thus includes the surface track as well as any possible subsurface tracks that were in direct contact with the foot. This definition excludes overtracks and transmitted undertracks, but includes penetrative surface- and undertracks as well as collapsed tracks. Originally, “true track” was defined as tracks formed in the tracking surface (sensu Sarjeant, 1990, p. 303; Lockley, 1991, p. 25), but this layer-based definition is not applicable to deep tracks that may extend through multiple layers (Gatesy, 2003). The term “true track” has often been used to imply fidelity to the anatomy of the foot even though many true tracks are not anatomically fidelitous.
“True track” has alternatively been defined to include just the track floor of the final foot-sediment interface (Marty et al., 2016). Synonyms of “true track” in this narrow sense are “true track sensu stricto” (Marty et al., 2009) and “footprint sensu stricto” (Thulborn, 2012). This narrow usage of “true track” follows Brown (1999), who used the term as a synonym of “track floor”, as opposed to the “overall track” (or “entire track”) that also comprises the track walls (Brown, 1999, p. 51). However, Brown, who was concerned with modern tracking, did not discuss the vertical extent of tracks into the subsurface. Marty et al. (2009) proposed the term “modified true track” for true tracks that have been affected by post-formational alteration to such an extent that “fine details of the anatomy of the foot” are no longer preserved. We note, however, that anatomical detail may be absent from the outset (e.g., when the track is formed in very soft or coarse substrate), which is often difficult to distinguish from post-formational alteration. The usefulness of the term “modified true track” may therefore be limited.
35 Deep track. A track formed by deep sinking of the foot into soft sediment (cf. Gatesy et al., 1999). This may be a penetrative track, in which the path of the foot is sealed (Figure 2), or an open track where the track walls have not collapsed (Figure 7G). The latter is often preserved as a natural cast (e.g., Engelmann and Hasiotis, 1999). Deep tracks generally record more of the foot movement but less of the trackmaker anatomy than shallow tracks (Figure 7G) (Gatesy, 2003). Deep and shallow tracks can be seen as end members of a continuum (Gatesy, 2003).
36 Surface track. A track formed and exposed at the same sediment-air or sediment-water interface on which the animal walked (Figure 2C) (cf. Goldring and Seilacher, 1971). This includes the uppermost track surface of penetrative tracks and collapsed tracks. In other words, if an observer were to walk behind the trackmaker looking down, the observed tracks would be “surface tracks”.
37 Undertrack. A track formed in a subsurface layer, as opposed to a surface track that is exposed at the tracking surface (Figure 2C) (cf. Goldring and Seilacher, 1971). The history of the term “undertrack” has been reviewed by Gatesy and Falkingham (2020). The term was originally proposed by Goldring and Seilacher (1971) for limulid traces where the legs penetrated the surface layers to leave tracks at different depths. The term was then adopted in tetrapod track ichnology but with a different meaning: A track formed in a subsurface layer by transmission, without direct contact with the foot. Seilacher (2007) proposed the terms “penetrative undertrack” and “compressive undertrack” to distinguish between the two concepts. Gatesy and Falkingham (2020) adopted the concept but proposed the modified terms penetrative track and transmitted undertrack, the former adopted from Rainforth (2005). These are discussed separately below.
38 Penetrative track (synonym: sealed track). A track in which the path of the foot is sealed by substrate flowing around the descending foot or by collapse of the track walls (Figure 2C). Layers or laminae dragged down by the descending digit or foot are V-shaped in cross section, a feature for which the term nested ‘V’s has been coined (Figure 2B) (Gatesy and Falkingham, 2020). When below the tracking surface, the track visible in each of these downfolded layers can be termed a penetrative undertrack (Gatesy and Falkingham, 2020). The nested ‘V’s can break off when the layers are separated and remain within the impression. This creates a flat bottom of the impression, which has been termed a false bottom because the track extends below this apparent bottom surface (Gatesy and Falkingham, 2020). Because the downfolded laminae that seal the track will be sub-vertically oriented, the “false bottom” may have a series of subparallel striations, or ridges. Erosion may also produce edges of such downfolded laminae around the track. Such structures have sometimes been referred to as “wrinkle structures”. Penetrative tracks generally do not reflect the anatomy of the trackmaker and have been a major source of misinterpretation (e.g., Lallensack et al., 2022b).
Thulborn (1990) used the term “underprint” for cases where only the lower (deepest) parts of a true track are visible because the rock was split into slab and counterslab at a subsurface level rather than at the tracking surface. Marty et al. (2009, 2016), following this usage, consequently proposed restricting the term undertrack to transmitted undertracks. However, “underprint” has often been, and sometimes still is, used as a synonym of “undertrack” and in these cases refers to transmitted undertracks. The term “subtrack” has been used for a concept similar to that of a penetrative track (e.g., Pérez-Lorente, 2015) but has also been applied to transmitted undertracks (e.g., Leonardi, 1996). Romano and Whyte (2003) suggested the term collapsed track (in their use, “collapsed print”, p. 197), which by their definition would be synonymous with “penetrative track”. However, we argue that sediment flowing around the foot does not represent collapse, and suggest restricting “collapsed track” to cases where the track walls have collapsed under gravity (usually after the foot has been removed).
39 Wrinkle structures (also: “wrinkled structures”, “wrinkle marks”, “wrinkles”, “crinkle marks”). Grooves and creases in or outside of a track. Several different mechanisms can lead to the formation of these structures, including microbial mats, erosion, and impressions of the integument. A common type of wrinkle structure, consisting of sub-parallel grooves in and around the track, caused some confusion. Lockley et al. (2018) interpreted such structures on the underside of natural casts left by hadrosaurs as “small extensional horst- and graben-like ridges” (p. 397). Xing et al. (2021) described similar structures, noting that they could potentially represent the broken downfolded layers of penetrative tracks. Martin et al. (2012, 2014) used the term “pressure-release structures” to describe extensive wrinkle structures in and around tracks. Very similar features to those observed by Martin et al. (2014) have been described by Hadri and Pérez-Lorente (2012), who interpreted them as the edges of laminae that were broken by the descending foot, and Carvalho (2004), who interpreted them as fluidisation structures caused by “dinostatic pressure”. We argue that, in most of these cases, the described “wrinkle marks” are indeed the broken downfolded layers of penetrative tracks.
40 Transmitted undertrack. An undertrack formed by transmission of force into the sediment volume. Transmitted undertracks are formed indirectly, without direct contact with the foot, and should be distinguished from penetrative tracks, where the foot penetrates the layers (Allen, 1989). In tetrapod track ichnology, transmitted undertracks have long been known simply as undertracks or underprints (note that “underprint” has also been used as a synonym of penetrative undertrack). Synonyms include “compressive undertrack”, “ghost track”, ”transmitted relief“, “subtrace”, “undertrace”, and “cleavage relief“, but note that the latter four terms do not necessarily imply the involvement of limbs. See also Gatesy and Falkingham (2020) for a review of terms.
41 Squelch mark. A track of little definite shape formed in semi-liquid substrates (cf. Tucker and Burchette, 1977). Squelch marks can be penetrative tracks or tracks confined to a thinner semi-liquid surface layer with a solid layer beneath.
42 Axial downfold or central downfold. The basin-shaped structure below the true track caused by downfolded layers, as observed in cross section (cf. Allen, 1997). The axial downfold includes all transmitted undertracks present in a track.
43 Leptodactylous and pachydactylous. Terms introduced by Hitchcock (1836) to denote very slender-toed and broad-toed tracks, respectively. Hitchcock’s leptodactylous tracks are now interpreted as penetrative tracks, i.e., the digit impressions are narrow because of sediment flow or collapse, not because the trackmaker had narrow digits (Gatesy and Falkingham, 2020).
44 Overtrack. A track in a sediment layer above the true track (Langston Jr, 1986). An instructive example has been figured by Farlow (Farlow et al., 2006, fig. 23). Multiple overtracks per track can occur in laminated sediments. Overtracks can form if the track is buried during multiple sedimentation events, or due to the growth of microbial mats that can trap sediment particles (Marty et al., 2009). Synonyms include “overprint”, “overtrace”, “supertrace”, and “ghost track” (also used as a synonym for transmitted undertrack). In the terminology of Brown (1999) and Marty et al. (2009), an “internal overtrack” covers only the footprint sensu stricto, not the entire overall track.
45  Displacement rim (synonyms: raised rim, bourrelet, lip, marginal ridge, extrusion rim, pressure ridge, displacement field, mud rim). An elevated bulge surrounding the shaft of the track that forms when sediment is displaced by the sinking foot (Figure 8E, Figure 9B). The development of a displacement rim is caused by the expulsion of sediment as the foot sinks and depends on the properties of the substrate: A compressible substrate will result in small or absent displacement rims, while an incompressible substrate will produce displacement rims with a volume equal to the negative volume of the track. In the mammalian track literature, the synonym “marginal ridge” is more commonly used; other synonyms include “raised rim”, “bourrelet”, and “lip”. Allen (1997) distinguished the marginal ridge from the “marginal upfold” (or “marginal fold”), which is in the subsurface below the marginal ridge and lateral to the shaft. Where the sediment is more brittle, the marginal upfold may be accompanied or replaced by marginal thrusts (Allen, 1997). Well-developed and asymmetric displacement rims can be used to distinguish tracks from similar non-biogenic sedimentary structures (Falkingham et al., 2021).
Displacement rim (synonyms: raised rim, bourrelet, lip, marginal ridge, extrusion rim, pressure ridge, displacement field, mud rim). An elevated bulge surrounding the shaft of the track that forms when sediment is displaced by the sinking foot (Figure 8E, Figure 9B). The development of a displacement rim is caused by the expulsion of sediment as the foot sinks and depends on the properties of the substrate: A compressible substrate will result in small or absent displacement rims, while an incompressible substrate will produce displacement rims with a volume equal to the negative volume of the track. In the mammalian track literature, the synonym “marginal ridge” is more commonly used; other synonyms include “raised rim”, “bourrelet”, and “lip”. Allen (1997) distinguished the marginal ridge from the “marginal upfold” (or “marginal fold”), which is in the subsurface below the marginal ridge and lateral to the shaft. Where the sediment is more brittle, the marginal upfold may be accompanied or replaced by marginal thrusts (Allen, 1997). Well-developed and asymmetric displacement rims can be used to distinguish tracks from similar non-biogenic sedimentary structures (Falkingham et al., 2021).
46 Withdrawal rim.  Similar to a displacement rim but formed by uplift of sediment as the foot was withdrawn (cf. Jackson et al., 2009, 2010).
Similar to a displacement rim but formed by uplift of sediment as the foot was withdrawn (cf. Jackson et al., 2009, 2010).
47 Sediment mound. A mound of sediment on the tracking surface that was piled up by the movement of the foot. Sediment mounds most commonly occur behind the digits as they were pushing backwards, when they are also referred to as “push-back structures” (Figure 10E) (Xing et al., 2016). A synonym is “mud mound” but note that this feature has a different meaning in sedimentology. Sediment mounds can be considered direct features, as they consist of sediment that was at least partially in direct contact with the foot, whereas displacement rims are indirect features. Sediment mounds are commonly observed in swimming tracks (Milner and Lockley, 2016).
48 Concretionary track. Natu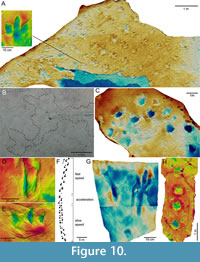 ral cast in the form of sideritic or calcareous concretions which lie on top of a fine-grained deposit (cf. Therrien et al., 2015). The term was suggested by Therrien et al. (2015). The rarity of such tracks may be partly explained by their transient nature, as they tend to disintegrate rapidly once the surrounding sediment is removed by modern erosion (Therrien et al., 2015).
ral cast in the form of sideritic or calcareous concretions which lie on top of a fine-grained deposit (cf. Therrien et al., 2015). The term was suggested by Therrien et al. (2015). The rarity of such tracks may be partly explained by their transient nature, as they tend to disintegrate rapidly once the surrounding sediment is removed by modern erosion (Therrien et al., 2015).
49 Dry-sand track (or “dry sand track”). Tracks left in dry, cohesionless sand may be easily eroded and difficult to detect because the track walls collapse immediately when the foot is withdrawn (Figure 11D). However, dry-sand tracks may be common on the slip faces of dunes where feet have sunk deep into grainflows (avalanches on the lee face of the dune): erosion by subsequent grainflows would only truncate the tracks but leave the deeper parts intact. Dry-sand tracks on dune slip faces are often diachronic, i.e., subsequent tracks of a trackway may be formed in different layers as the animal steps on a newly formed grainflow triggered by its previous step (Loope, 2006).
(or “dry sand track”). Tracks left in dry, cohesionless sand may be easily eroded and difficult to detect because the track walls collapse immediately when the foot is withdrawn (Figure 11D). However, dry-sand tracks may be common on the slip faces of dunes where feet have sunk deep into grainflows (avalanches on the lee face of the dune): erosion by subsequent grainflows would only truncate the tracks but leave the deeper parts intact. Dry-sand tracks on dune slip faces are often diachronic, i.e., subsequent tracks of a trackway may be formed in different layers as the animal steps on a newly formed grainflow triggered by its previous step (Loope, 2006).
50 Microbial mat.  A sheet of microorganisms that is layered and up to a centimetre in thickness (cf. Rich and Maier, 2015). Microbial mats typically grow subaqueously on the sediment surface, where they trap and incorporate sedimentary particles to avoid becoming buried by sedimentation (Callefo et al., 2021). The resulting sedimentary structures are known as “microbially induced sedimentary structures” (MISS), which are a form of microbialite (Figure 9A, Figure 12A-B). Different morphologies of MISS on bedding surfaces can be distinguished, such as reticulate textures (“elephant skin”); warty structures (Figure 9A); and subparallel and sinuous wrinkles (Figure 12A). The latter are often referred to as “wrinkle structures” or “wrinkle marks”; note that these terms have also been used to refer to other structures associated with tracks. Recently, some of these types were given ichnotaxonomic names (Figure 9A, Figure 12A) (Stimson et al., 2017; Simpson et al., 2022). The terminology for the morphological description of MISS has been reviewed by Bouougri et al. (2007). Microbial mats can play an important role in the formation and preservation of tracks (Marty et al., 2009; Carvalho et al., 2013).
A sheet of microorganisms that is layered and up to a centimetre in thickness (cf. Rich and Maier, 2015). Microbial mats typically grow subaqueously on the sediment surface, where they trap and incorporate sedimentary particles to avoid becoming buried by sedimentation (Callefo et al., 2021). The resulting sedimentary structures are known as “microbially induced sedimentary structures” (MISS), which are a form of microbialite (Figure 9A, Figure 12A-B). Different morphologies of MISS on bedding surfaces can be distinguished, such as reticulate textures (“elephant skin”); warty structures (Figure 9A); and subparallel and sinuous wrinkles (Figure 12A). The latter are often referred to as “wrinkle structures” or “wrinkle marks”; note that these terms have also been used to refer to other structures associated with tracks. Recently, some of these types were given ichnotaxonomic names (Figure 9A, Figure 12A) (Stimson et al., 2017; Simpson et al., 2022). The terminology for the morphological description of MISS has been reviewed by Bouougri et al. (2007). Microbial mats can play an important role in the formation and preservation of tracks (Marty et al., 2009; Carvalho et al., 2013).
51 Pressure bulb. The distribution of stress (and therefore displacement) beneath the indenter (i.e., the foot). Described in detail by Manning (2004) as resulting in transmitted undertracks that are wider and longer than the true track.
52 Displacement bulb (synonyms: dead zone, detached undertrack). A relatively undeformed volume of sediment pressed down into the sediment by the foot (cf. Jackson et al., 2009).
53 Deformation (synonym: distortion). A track is the result of sediment deformation by the foot. Such deformation may be elastic-plastic or viscous-plastic (e.g., the formation of displacement rims), or brittle (e.g., the formation of fractures and thrusts) (Allen, 1997), or a combination of these. Deformation may also occur in the fleshy pads of the trackmaker’s feet when interacting with a firmer substrate, adding to the variability of the tracks; such deformation has been termed pad deformation (Gatesy, 2001). Existing tracks can be deformed by other tracks created nearby later (Figure 5D). During diagenesis, plastic deformation can lead to a reduction in track relief due to compaction (Lallensack et al., 2022b), but can also introduce shearing that can skew tracks and trackways (Figure 5C) (Schulp and Brokx, 1999; Schulp, 2002).
Tracks of low anatomical fidelity, such as penetrative tracks formed in soft mud, have often been characterised as being “deformed” or “distorted”. This usage has been criticised by Gatesy and Falkingham (2017), as it implies that the tracks were initially copies of the feet that were deformed, when in fact the tracks were never foot-like.
54 Compaction. The reduction in sediment volume due to overburden. Mud and peat can have high levels of compaction that can significantly reduce the relief of the track, which must be taken into account when interpreting fossil tracks (Lallensack et al., 2022b). In coarser sediments such as sand, compaction tends to be insignificant. Compaction is also caused directly by the trackmaker, and the sediment below the direct track tends to be more strongly compacted than outside the track.
Lockley and Xing (2015) introduced the term “flattened track” for several examples of natural track casts composed of sandstone and associated with very thin mudstone and siltstone intervals, which appear to be both flattened (reduced in depth) and widened (resulting in more rounded outlines) (Lockley and Xing, 2015). We note that, in general, compaction causes shortening of fossils but not widening (Lallensack et al., 2021); “flattened tracks” may therefore represent a special case and, as such, are rare (Lockley and Xing, 2015).
55 Tectonics. The small-scale structural deformations of the sediment that occur during track formation are analogous to the large-scale structures caused by plate tectonics and can therefore be described using the terminology of structural geology (Graversen et al., 2007). Such terms include “microfold”, “microfault”, “detachment fault”, and “lateral ramp”, amongst many others (Allen, 1997; Manning, 2004; Graversen et al., 2007; Jackson et al., 2010).
56 Pressure pad. A semicircular body of sediment around the shaft that is slightly rotated out of position and sharply demarcated by microfaults (Figure 11A-C) (Fornós et al., 2002). Pressure pads may form posterior to the shaft as the foot pushes backwards before withdrawing. On sloping surfaces, larger pressure pads typically face downhill. There may be one or more pressure pads per track. A similar term is sand crescent, which has been applied to semi-circular structures found in trackways on the slipfaces of dunes (Leonardi, 1977, 1980). Fornós et al. (2002) argued that sand crescents are avalanche structures (i.e., dry sand flowing down a surface due to gravity after being triggered by the impact of the foot; Figure 11D). However, Loope (2006) suggested that many structures described as “sand crescents” were formed in moist sand and must be considered pressure pads rather than avalanche structures (Loope, 2006).
57 Radial fractures (synonym: “radial cracks”). Surface fractures on the displacement rim that originate from the shaft in a radial manner (Lockley et al., 1989; Allen, 1997; Schanz et al., 2016). Concentric fractures (synonym: “concentric cracks”) can also occur (Leonardi, 1979; Allen, 1997). Hwang et al. (2008) described unusual dinosaur tracks with radial internal ridges, which these authors suggested represent “molds of radial cracks” formed in transmitted undertracks and therefore represent a different formational mechanism.
58 Ejecta. Sediment that is forcefully ejected from beneath the descending foot onto the sediment surface in front of or beside the track (Allen, 1997). Such ejection can occur through the spaces between digits.
59 Substrate strength (also: substrate consistency or substrate competency). Tracks can only form in sediments that are neither too soft nor too firm, a phenomenon termed the Goldilocks effect by Falkingham et al. (2011a). A soft substrate might also be described as compliant, or having a low yield strength or bearing capacity, if it deforms easily (= high deformability) (Falkingham et al., 2014; Schanz et al., 2016). Conversely, a substrate that is resistant to deformation would have a high yield strength/bearing capacity, and be described as firm, stiff, or competent.
60 Cohesion/Adhesion. The degree to which particles or grains are attracted to each other and to indenters (e.g., feet). This cohesion is usually due to the presence of water (although too much water will reduce cohesion) but can also take the form of electrostatic attraction in very small particles (clays and powders). Both stiff and soft substrates can be cohesive (or not). Sediment that sticks (adheres) to the foot can produce curvilinear to concentric ridges or spikes on the floor of the track as the foot is withdrawn, which have been termed adhesion traces (synonyms: adhesion spikes Allen, 1997; adhesion ridges, Carey et al., 2011).
61 Elasticity. The degree to which deformed sediment may rebound to its original state. A highly elastic soft substrate will behave very differently from a low elastic soft substrate. For example, uncompacted volcanic ash would be described as soft but would lead to very different tracks to a springy surface. High levels of elasticity can occur in peat, where tracks can be obliterated by the rebounding peat if they are not filled with sediment quickly enough (Parker and Rowley Jr, 1989).
62 Suction (also: suction effects). During foot withdrawal, a vacuum can form between the foot and the sediment, which can cause sediment to be drawn upwards or the track walls to be drawn inwards (e.g., Thulborn, 2004; Bennett and Morse, 2014). An elevation of the track floor interpreted as caused by suction has been termed a pull-up feature (or “pull-up structure”) (e.g., Kvale et al., 2001).
63 Liquefaction (also: fluidisation). Sediments with a high-water content can lose their strength and behave like liquids when subjected to stress, which can be caused by the foot during track formation. Reversible liquefaction is known as thixotropy, i.e., the liquefied sediment regains its strength after a short period of time (Leeder, 1982). Liquefaction increases sediment softness locally and can lead to substantial sinking depths (Jackson et al., 2010). Deep dinosaur tracks in the Wucaiwan area of China have yielded numerous skeletons of smaller non-avian dinosaurs that possibly got trapped due to liquefaction that took place during track formation (Eberth et al., 2010).
64 Weathering and erosion. The degradation or destruction of tracks by physical, chemical, and/or biological processes (Figure 5A, B) (e.g., Henderson, 2006a; Ledoux et al., 2021). Weathering and erosion can take place both before burial (ancient weathering/erosion) and after the fossil tracks have been exposed (modern or recent weathering/erosion). Erosion of the tracking surface creates an erosional surface that may still preserve the lower parts of the tracks (Allen, 1997). Distinct erosional features are generally aligned with the flow of water (Figure 5B) or wind (Figure 5A) and may be mistaken for tracks or modify existing tracks. Such features have been termed erosion marks or scour marks.
65 Fossil volume. Term coined by Hitchcock (1858) for a famous specimen (ACM-ICH 27/4) consisting of five split slabs that expose the same dinosaur track at different depths. The slabs were mounted together and displayed in a book-like fashion. The fossil volume specimen has long been regarded as the prime example of transmitted undertracks, but Gatesy and Falkingham (2020) have shown that it and similar fossils instead represent penetrative tracks. This and similar specimens allow for reconstructing the trajectory of the foot through the substrate (Falkingham et al. 2020; Turner et al., 2020). The term is useful to refer to any single track exposed on multiple surfaces.
TRACKS AND FEET - ANATOMY AND MEASURES
The terminology used to describe the anatomy of the pes and manus and corresponding features found in tracks is extensive, reflecting the biodiversity involved. Adding to this complexity, the terminology used by hominin track workers can differ drastically from that used in other subfields of tetrapod ichnology.
66 Adjectives and nouns. When two nouns are combined, the first usually becomes an adjective, as in “digital pad” (not “digit pad”) and “manual phalanx” (not “manus phalanx”). However, when referring to the trace of a body part, the first noun remains unchanged, as in “skin impression” (not “dermal impression”), “manus track” (not “manual track”), “pes track” (not “pedal track”), or “hallux impression” (not “hallucal impression”). We follow this common usage here but note that some ichnologists prefer to use the adjectival forms even in the latter cases.
Terms of Location
67 Upper and lower. When describing tracks, the terms “up/above” and “down/below” are always understood relative to the orientation of the sediment layers, which does not necessarily coincide with the vertical.
68 Outer and median. 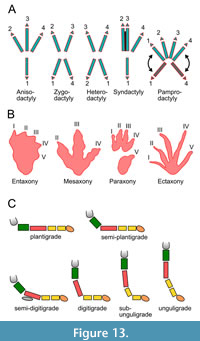 Generally, “outer” refers to the most lateral and medial digits, e.g., digits I and V in a pentadactyl foot (Leonardi et al., 1987). Conversely, “median” refers to an inner digit, which is often digit III or, in a pentadactyl foot, digits II to IV (Leonardi et al., 1987). These terms are typically restricted to the functional digits that are facing anteriorly and are regularly impressed in a track (i.e., excluding dewclaws and retroverted digits). For example, in anisodactyl bird tracks (Figure 13A), the outer digits are digits II and IV (Halfpenny, 2019). Note that, in statistics, “median” is a measure of average.
Generally, “outer” refers to the most lateral and medial digits, e.g., digits I and V in a pentadactyl foot (Leonardi et al., 1987). Conversely, “median” refers to an inner digit, which is often digit III or, in a pentadactyl foot, digits II to IV (Leonardi et al., 1987). These terms are typically restricted to the functional digits that are facing anteriorly and are regularly impressed in a track (i.e., excluding dewclaws and retroverted digits). For example, in anisodactyl bird tracks (Figure 13A), the outer digits are digits II and IV (Halfpenny, 2019). Note that, in statistics, “median” is a measure of average.
69 Axial and abaxial. “Close to the central axis” and “away from the central axis”, respectively (e.g., Leach, 1993). The central axis can be that of a digit, foot, or body. The respective directional terms are “axially” and “abaxially".
70 Left track and right track. A track formed by a left foot and a right foot, respectively. In a cast, left and right are reversed, so that the left track appears as the right, and vice versa. However, these terms have also been used in a general sense to describe positions as visible in the specimen, regardless of preservation as a cast or mould (Haubold, 1971). In sitemaps or interpretative outline drawings, casts are often intentionally mirrored to match the mould. Interpretative outline drawings of tracks are also sometimes mirrored so that they always correspond to the track of the right (or left) foot for ease of comparison.
71 Anatomical planes. Constructed planes that transect a body and are orthogonal or parallel to each other. The sagittal plane (synonym: longitudinal plane) divides the body into left and right halves; the coronal plane (synonym: frontal plane) divides the body into dorsal and ventral halves; and the transverse plane (synonyms: horizontal plane, axial plane) divides the body into cranial and caudal halves. Note that these planes are defined relative to the axis of the body - for example, the coronal plane divides the body into upper and lower halves if the axis is horizontal (e.g., in a dog) but into front and back halves if the axis is vertical (e.g., in humans). A parasagittal plane is any plane parallel to the sagittal plane.
72 Anterior/posterior (synonyms: cranial/caudal and frontwards/rearwards) and medial/lateral. The meaning of these terms depends on whether they are used to describe features of a trackway or features of an individual track:
For trackways, these terms are defined relative to the trackway midline, which approximates the sagittal plane of the body: “medial” means “towards the trackway midline” and “lateral” means “away from the trackway midline”. “Anterior” means “towards the direction of travel” and “posterior” means “opposite to the direction of travel”.
For tracks, they are defined relative to the long axis of the track in question: “medial” here refers to the radial/tibial side, i.e., the side where digit I would be located if present, while “lateral” refers to the ulnar/fibular side, i.e., the side where digit V would be located if present. “Anterior” means “towards the front of the track”, and “posterior” means “towards the rear of the track”.
However, these terms can be ambiguous in some contexts. Firstly, the two usages (referring to a trackway or an individual track) are only equivalent when track rotation is 0°. For example, when a track is rotated outwards by 90°, the front margin of the track is the “anterior” side if the trackway midline is the reference, and the “medial” side if the long axis of the track is the reference. As both definitions are used when describing tracks and trackways, they can conflict and potentially lead to ambiguity. Secondly, they do not reflect the plesiomorphic (ancestral) state of tetrapods, in which the digits are directed laterally (away from the body midline). In fact, in human anatomy these terms are often defined according to embryological terminology: “Anterior” here means “towards the thumb/great toe”, and “posterior” means “towards the little finger/small toe”) (Biesecker et al., 2009). Less ambiguous terms are available, such as “preaxial” or “radial”/“tibial” instead of “medial” and “postaxial” or “ulnar”/“fibular” instead of “lateral” (Romer, 1933). However, to our knowledge, such terms have not yet been applied to tracks.
73 Proximal and distal. In a limb, “proximal” refers to “towards the hip (or shoulder) joint” and “distal” refers to “towards the digit tips”. These terms are defined independently of posture - for example, an ungual is always distal to the penultimate phalanx of the same digit. When describing tracks, “proximal” and “distal” are often equivalent to “posterior” (or “rearwards”) and “anterior” (or “frontwards”), respectively. However, when describing the tracks of unguligrade animals (i.e., animals that walk on the most distal bone of the foot), the terms “proximal” and “distal” are used as synonyms for “upper” and “lower”, respectively (e.g., in a cow track, the track floor is distal to the lip) (Fornós et al., 2002). Another special case is knuckle walkers, where the digits point rearwards, in which case the rear of the track is distal while the front is proximal. When describing trackways, “proximal” means “towards the beginning of the trackway” and “distal” means “towards the end of the trackway” (as determined by the direction of travel of the trackmaker). Farlow (1987) instead used the terms “uptrail” (towards the end of the trackway) and “downtrail” (towards the beginning of the trackway).
74 Plantar/palmar. In most cases these terms refer to the surface of the pes (plantar) or manus (palmar) that is in regular contact with the ground during locomotion, i.e., the “palmar/plantar surface”. The latter terms can also refer directly to the corresponding impression in a track. Plantar/palmar can mean either “relating to” or, when used as a directional term, “towards” the palmar/plantar surface. The opposite direction is dorsal. However, “plantar” and “palmar” have also been used to refer only to the sole (in the pes) or palm (in the manus) to the exclusion of the digits.
Studies of ungulate anatomy often use “dorsal” to describe the anterior aspect and “plantar/palmar” to describe the posterior aspect of unguligrade feet, while the terms “distal” or “solar” are used to refer to the surface that is in regular contact with the ground (i.e., the underside of the hoof). Note that the term “sole” has a different meaning in ungulate anatomy (namely the subunguis), and the use of the term “solar” is therefore restricted to this group. Another special case are knuckle walkers, which walk on the dorsal surfaces of the manual phalanges, with the palmar surface facing upwards and therefore not in regular contact with the substrate.
75 Ipsilateral, contralateral, and diagonal. “On the same side of the body”, “laterally on the opposite side of the body”, and “diagonally on the opposite side of the body”, respectively. For example, the right pes and right manus are ipsilateral, the right and left pes are contralateral, and the right pes and left manus are diagonal.
76 Cross section. If the sediment layer containing the track is truncated, the track can be seen in cross section. Allen (1997) introduced terminology to indicate the view in which a track may be exposed in section: Sections can be “vertical” (perpendicular to the strata), “transverse” (parallel to the strata), or “oblique” (between vertical and transverse). Vertical sections can be “axial” (intersect the central axis of the shaft), “internal-vertical” (intersecting the shaft but not the central shaft axis), or “external-vertical” (intersecting the deformed zone around the shaft but not the shaft itself). Fornós et al. (2002) extended this terminology by defining longitudinal sections that coincide with the sagittal plane of the trackmaker, and lateral sections that are perpendicular to this plane. We propose to redefine longitudinal sections to be parallel to the long axis of the track (rather than the sagittal plane of the body), and lateral sections to be parallel to the transverse axis of the track, as otherwise the sections would not be comparable if track rotation varies (see discussion in medial/lateral).
Qualitative Description of Tracks and Feet
77 Autopodium (also: autopod; plural: autopodia). The distal part of the limb, i.e., the pes or the manus. The autopodium is one of three parts of the limb; the more proximal parts are the stylopodium (also: stylopod; synonym: propodium), which comprises the humerus or the femur, and the zeugopodium (also: zeugopod; synonyms: epipodium, antebrachium, forearm), which comprises the radius and ulna/tibia and fibula. The autopodium itself is made up of three parts: the basipodium (tarsals and carpals), the metapodium (metatarsals and metacarpals, or, collectively, metapodials), and the acropodium (the phalanges that form the digits).
78 Manus (plural: manus). The anterior autopodium, which may be a hand or, when used for locomotion, a forefoot (also: front foot) or, in mammals, a front paw. Note that in human anatomy, “forefoot” instead refers to the front part of the pes. The term “hand” is also used as a synonym for “manus” in both bipedal and quadrupedal animals. To refer to the track left by a manus, the combinations “manus track”, “manus print”, and “manus impression” are most common. The adjectival form is “manual”.
79 Pes (plural: pedes). The posterior autopodium. Less technical synonyms are “hind foot” (also “hindfoot”), “rear foot”, and, in mammals, “rear paw”. The term foot itself refers to any autopodium used for locomotion (Leonardi et al., 1987) but is also used as a synonym for “pes” (e.g., in human anatomy, “hind foot” refers to the rear part of the pes). To indicate the track of a pes, the combinations “pes track”, “pes print”, and “pes impression” are common. The adjectival form is “pedal”.
80 Foot. An autopodium used for terrestrial locomotion (i.e., bipeds have two feet, quadrupeds have four feet). “Paw” is a synonym that applies only to mammals. The term “foot” usually refers to a functional unit distinct from the lower leg. If individual tarsals or carpals instead form a functional unit with the lower leg (as is the case with, e.g., the calcaneus and astragalus in dinosaurs), they may not be considered part of the foot proper (Brusatte, 2012, p. 53).
81 Heel. The term has two meanings:
The tarsal region of the pes. In plantigrade trackmakers, the impression of the heel is called the “heel impression” or “heel pad”.
The rear of a track. In this usage, “heel” does not necessarily refer to the anatomical heel. The term is commonly used to describe the rear of pes tracks but has also been used for manus tracks (e.g., Halfpenny, 2019). In hoofed mammals such as cows and horses, the “heel” is the posteroventral edge of the foot just posterior to the hoof. This use of the term has been a frequent source of confusion, and we suggest to either avoid the non-anatomical meaning of “heel” (i.e., the rear of tridactyl dinosaur tracks should not be referred to as the “heel” unless the full metatarsus is impressed), or to specify the intended meaning. Some authors put “heel” in quotation marks to indicate that their use of the term does not refer to the anatomical heel.
82 Pternion. The most posterior point, or apex, of the heel. In contrast, the acropodion (original German spelling: Akropodion; sometimes misspelled “acropodian” or “akropodian”; not to be confused with acropodium) is the most anterior point of the foot (i.e., the tip of the most protruding digit). The terms apply to both tracks and feet. They originate from anthropology (Martin, 1914) and have so far only been applied to hominin feet and tracks but are potentially useful in other disciplines. In order to make the term “pternion” applicable to non-plantigrade tracks (which do not impress the anatomical heel), we here alternatively define it as the most posterior point of the plantar/palmar surface (i.e., the most posterior point of a fully impressed track).
83 Digits. The parts of the autopodium distal to the metapodials, consisting of phalanges. The digits of the pes are called “pedal digits” and those of the manus are called “manual digits”. The non-technical words “finger” and “toe” are generally treated as synonyms of “digit”. The digits of a pes or manus are collectively known as the acropodium. Individual digits are identified by Roman numerals from medial to lateral (e.g., digit I of the manus is the pollex or thumb, while digit I of the pes is the hallux or big toe). The writer should be aware that the adjectival form digital (used in, e.g., “digital pad”), while correct and recommended, can be ambiguous in some contexts, as it can alternatively be understood as “in form of a 3D model”. The term ray refers to a digit plus its corresponding metapodial.
The trace of a digit is called a digit impression (synonyms: “digit trace”, as well as the combinations toe/finger impression/mark/trace). Digit impressions may be “straight” (also: “rectlinear”) or “bent” (also: “curved”). The term “hooked” (also: “crooked”) may be applied to digit impressions that are straight in their proximal part but bent in their distal part (Leonardi et al., 1987). If a claw mark differs in orientation from its digit impression, it is “deflected” (laterally or medially).
84 Phalanges (singular: phalanx). The individual bones that make up the digits. Individual phalanges are identified by numerals from proximal to distal (e.g., “pedal phalanx II-2” is the second phalanx of the second digit of the pes). The number of phalanges in each digit can be expressed as a phalangeal formula. Such a formula consists of five numbers representing digits I to V, separated by hyphens. For example, the theropod pes typically has the formula 0-3-4-5-0, meaning that digits I and V are absent (0 phalanges), while the second digit has 3, the third 4, and the fourth 5 phalanges (Thulborn, 1990).
85 Unguals or ungual phalanges. The terminal phalanges, which are often modified to support a keratinous covering such as a claw, nail, or hoof. Note that the keratinous covering is not part of the ungual, which refers to the bony part only (i.e., the terms “claw mark”, “nail mark”, or “hoof mark” are more accurate than “ungual mark” or “ungual impression” when such a keratinous covering is present).
86 Pollex. The thumb, or digit I, of the manus. The adjectival form is “pollical” (as in “pollical phalanx”).
87 Hallux. The “big toe”, or digit I, of the pes. In birds, the hallux is often retroverted (pointing backwards). The adjectival form is “hallucal” (as in “hallucal phalanx”).
88 Dewclaws (also “dew claws”). Reduced, accessory digits in the forefoot or hind foot. Dewclaws often do not touch the ground, but those of artiodactyls (digits II and V) regularly leave a pair of dewclaw impressions. In artiodactyls, dewclaws are also known as “false hooves” and their impressions as “external pits” (Allen, 1997).
89 Dactyly. The condition of having a certain number of digits in an autopodium or track. For example, a monodactyl track has one digit impression, while didactyl, tridactyl, tetradactyl and pentadactyl tracks have two, three, four, and five digit impressions, respectively. A track with more than five digits, as found in some basal tetrapods, is polydactyl. Note here that in more derived tetrapods, “polydactyl” refers to the pathological condition of having supernumerary, or “extra”, digits, the opposite of which is “oligodactyl” (the condition of having fewer digits). Also note that these adjectives can also be formed with the “-ous” suffix (didactylous, tridactylous, tetradactylous, pentadactylous, polydactylous, oligodactylous). The corresponding nouns are monodactyly, didactyly, tridactyly, tetradactyly, pentadactyly, and polydactyly/oligodactyly. When referring to a track, these terms indicate the number of visible digit impressions, which does not necessarily reflect the actual number of digits in the autopodium. For example, in most non-avian theropods, only digits II to IV are used for locomotion and regularly touch the ground, while digit I (and, ancestrally, digit V) is a dewclaw. Although these trackmakers leave tridactyl tracks, their pedes are tetradactyl or even pentadactyl, and only functionally tridactyl.
In birds, specialised terms are used to describe the arrangement of the four digits of the foot (Figure 13A) (Proctor and Lynch, 1993). The most common is anisodactyly, where digit I points posteriorly and digits II-IV point anteriorly (Figure 7D). The second most common is zygodactyly, where digits II and III point anteriorly and digits I and IV point posteriorly (i.e., digit IV is reversed to facilitate perching). Heterodactyly is similar to zygodactyly, but with digit II reversed instead of IV; this configuration is only found in trogons. Syndactyly is similar to anisodactyly, but digits II and III are mostly fused together. Note that outside birds, “syndactyly” refers to any occurrence of fused digits, not just the fusion of digits II and III. In pamprodactyly, digits I and IV are mobile and therefore can point backwards or forwards. The corresponding adjectives are anisodactyl, zygodactyl, heterodactyl, syndactyl, and pamprodactyl (Proctor and Lynch, 1993).
90 Principal digit. The most important digit of a foot or, correspondingly, the most important digit impression of a track (cf. Thulborn, 1990). If two digits seem equally important (as, e.g., digits III and IV in artiodactyls), both can be considered as principal digits. The principal digit(s) forms the axis of the foot or track and is used to determine its axony (note that “axis” is not identical to the long axis of the track; Leonardi et al., 1987).
There is disagreement as to what should be considered the principal digit (Romano et al., 2020). Leonardi et al. (1987) suggested that the principal digit is the most important digit for supporting the body weight of the trackmaker; this is generally considered to be the most deeply impressed digit. We follow this functional definition here. Other authors have used a geometric definition based on the most developed or relatively longest digit (see Romano et al., 2020). We urge authors to define which definition is being followed. Note that this terminology is also used outside the field of ichnology in zoology and palaeontology, where similar conflicting definitions exist (Romano et al., 2020).
The position of the axis in a foot or track is termed axony (Figure 13B). An entaxonic foot or track has its axis medially, as in the human foot where digit I is the principal digit. If the principal digit is the central one, the foot or track is mesaxonic; this is the case in many tridactyl archosaurs (Leonardi et al., 1987). If the foot is either didactyl or tetradactyl, and digits III and IV are equally important, the foot or track is paraxonic, a condition commonly found in artiodactyls (Leonardi et al., 1987). If the principal digit is located laterally, the foot or track is ectaxonic; this condition is found in lepidosaurs (Leonardi et al., 1987). The corresponding nouns are “entaxony”, “mesaxony”, “paraxony”, and “ectaxony”. The term “mesaxony” has also been used as a synonym for digit III projection, with a “strongly mesaxonic” track having a high digit III projection and a “weakly mesaxonic” track having a low digit III projection. Note, however, that such usage is restricted to tetrapod track ichnology.
91 Relative depth pattern. The depth of impression of different parts of a track relative to each other. As defined by Mjual et al. (2020), the relative depth pattern describes trackmaker anatomy to the exclusion of extramorphological features and therefore requires anatomical fidelity; it therefore must be consistent within a trackway or ichnotaxon. The term functional prevalence describes whether the lateral, median, or medial part of a track is deepest. Mujal et al. (2020) also used the terms “medial-median functionality”, “median functionality”, and “median-lateral functionality” for these three cases, respectively. See discussion in the entry “foot pressure” for caveats on functional interpretations.
92 Grady. A set of terms describing which part of the foot is in contact with solid ground when standing (Figure 13C) (cf. Leonardi et al., 1987; Carrano, 1997). Possible postures lie between the two extremes of unguligrady and plantigrady (Carrano, 1997), and different conditions and definitions will be reviewed below. The respective adjectives (e.g., unguligrade and plantigrade) can be applied to the foot posture (e.g., a plantigrade foot posture), to the tracks (e.g., a plantigrade track), or to the trackmakers (e.g., a plantigrade trackmaker).
Michilsens et al. (2009) introduced the terms “unguliportal”, “digitiportal”, and “plantiportal”, which indicate the part of the foot that carries the body weight rather than the part that is in ground contact when standing. These terms therefore describe dynamic foot postures rather than static foot postures (Michilsens et al., 2009). In most animals, the dynamic foot postures are equivalent to the respective static foot postures. Exceptions include the hippopotamus and the tapir, which are unguligrade but digitiportal, and the elephant, which is digitigrade but plantiportal (Michilsens et al., 2009).
93 Unguligrady. In an unguligrade foot, only the unguals are in contact with the ground (e.g., in a horse), with the phalanges and metatarsals (or metacarpals) being sub-vertical (Figure 13C). A subunguligrade (sub-unguligrade) foot has both the ungual and the penultimate phalanx in contact with the ground (Figure 13C).
94 Digitigrady. In a broad sense, the term has been used for a range of postures between unguligrady and plantigrady (Figure 13C) (Carrano, 1997). In the strict sense, a digitigrade foot has all the phalanges of the weight-bearing digits in full contact with the ground, including the metatarso-phalangeal joints where the primarily flexure occurs (Carrano, 1997). In a sub-digitigrade (=subdigitigrade) foot, most of the phalanges are in contact with the ground, but the metatarso-phalangeal joints are elevated above the ground. In a semi-digitigrade (=semidigitigrade; synonym: mid-digitigrade) foot, the metapodium is partly in contact with the ground or is supported by a metapodial pad that transfers weight from the metapodium directly to the ground (Figure 13C). Note that semi-digitigrady is often confused with semi-plantigrady.
95 Plantigrady. A plantigrade foot has both digits and the anatomical heel in contact with the ground, and the phalanges and metatarsals/metacarpals are approximately horizontal (Figure 13C). Because “plantigrade” refers to the plantar surface of the pes, the corresponding term for the manus, palmigrade, can be used when describing forefeet. In a semi-plantigrade (or semi-palmigrade; also semiplantigrade/semipalmigrade) foot, the basipodium is elevated above the ground, as is the case in primates (except African apes and humans, which are fully plantigrade) (Figure 13C) (Gebo, 1992). To reflect the functional meaning of these terms, we argue that the term “semi-plantigrade” should not be used in cases where the tarsals or carpals form a functional unit with the long bones of the lower leg, because in such cases they are not part of the foot proper. Note that the term “semi-plantigrade” is often incorrectly used as a synonym for semi-digitigrade.
Kuban (1989) defined the term “quasi-plantigrade” to describe hypothetical crouching postures in tridactyl dinosaurs, where the metatarsus is held low to the ground but not fully horizontal. In such a case, the metatarsals would only make contact with the ground if the substrate is compliant so that the foot sinks in to some extent; it is therefore actually a digitigrade posture and the term may therefore be misleading (Lallensack et al., 2022b).
96 Calcigrade. Condition where the (anatomical) heel is most deeply impressed, which can occur when the trackmaker is standing still (Leonardi et al., 1987). This term does not necessarily refer to foot posture or anatomy and is used to describe the morphology of tracks.
97 Pads. Cushion-like swellings on the undersides of the feet and hands. Pads often act as shock absorbers during locomotion. The corresponding traces are termed “pad impressions”.
Pads on the digits are digital pads. Common synonyms include “phalangeal pads”, “nodes”, “phalangeal/digital nodes”, and “toe pads” (=”toepads”) and “finger pads” for the pes and manus, respectively. In zoology, medicine, and veterinary science, the digital pads are also known as “digital pulps”, “tori digitales” (singular: torus digitalis) or “pulvini” (singular: pulvinus). The term “volar pad” is sometimes used to refer to the digital and palmar pads of the manus in mammals. Individual pads may be separated by interpad grooves, which in the mesarthral condition may be flexion creases. In birds, the individual digital pads are often not directly adjacent but separated by an interpad space (Lucas and Stettenheim, 1972). In human footprints, the connections between the rounded toe pad impressions and the ball area have been termed toe stems (Robbins, 1985).
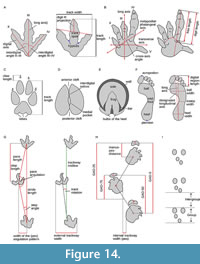 A pad beneath a metapodial-phalangeal joint is termed a “metapodial-phalangeal pad”. More common are the corresponding terms for the pes and manus: metatarsophalangeal pad (or metatarso-phalangeal pad) and metacarpophalangeal pad (or metacarpo-phalangeal pad). In mammals, these pads are termed “plantar pads”, “interdigital pads”, or “intermediate pads”; the most medial of these, the “hallucal pad” (or, in the manus, “pollical pad”), is often separated from the other pads (Halfpenny, 1986; Ewer, 1998). In carnivorans, the “plantar pads” are often fused into a single large pad, which may have notches on its posterior margin forming lobes (Figure 14C).
A pad beneath a metapodial-phalangeal joint is termed a “metapodial-phalangeal pad”. More common are the corresponding terms for the pes and manus: metatarsophalangeal pad (or metatarso-phalangeal pad) and metacarpophalangeal pad (or metacarpo-phalangeal pad). In mammals, these pads are termed “plantar pads”, “interdigital pads”, or “intermediate pads”; the most medial of these, the “hallucal pad” (or, in the manus, “pollical pad”), is often separated from the other pads (Halfpenny, 1986; Ewer, 1998). In carnivorans, the “plantar pads” are often fused into a single large pad, which may have notches on its posterior margin forming lobes (Figure 14C).
A pad beneath the metatarsal or metacarpal region is termed a “metapodial pad”; this is often a single, larger pad. Again, the corresponding terms for the pes and manus are more common: metatarsal pad and metacarpal pad. A pad beneath the tarsal region is termed a heel pad; humans are an example. Note that in dinosaurs, “heel pad” usually refers to a larger pad impression posterior to the digit impressions that does not correspond to the anatomical heel, and is often actually a metatarsophalangeal pad. More general terms for a pad posterior to the digits are plantar pad (or “sole pad”) for the pes and palmar pad (or “palm pad”) for the manus. Very large pads that extend below much of the foot have also been referred to as cushions (e.g., Lucas and Hunt, 2007); examples include elephants, camels, and sauropods.
98 Basal pad. A markedly enlarged pad at or posterior to the proximal end of a digit impression. For example, the basal pad of the seymouriamorph track Amphisauropus is associated with digit I and corresponds to the carpal (and tarsal) areas (Marchetti et al., 2017), while the basal pad of chirothere tracks is the metatarsophalangeal pad of digit V (Figure 8F, Figure 12B) (Peabody, 1948).
99 Arthral and mesarthral. Terms referring to the position of the digital pads. Pads that enclose an interphalangeal joint are “arthral”, while those that enclose the phalanx itself are “mesarthral”. In the mesarthral condition, the interpad grooves mark the position of the interphalangeal joints.
100 Hypex (plural: “hypexes” or “hypices”). The most proximal point of the empty space between two digits; “the apex of the reentrant angle between digits” (Figure 14A) (Peabody, 1948, p. 299; Leonardi et al., 1987). In contrast, the distal end of a digit impression has been referred to as the “digit tip”; “claw tip”; “toetip”/“fingertip”; or the “apex” or “terminus” (of a digit impression). These terms are used to describe tracks as well as hands and feet.
101 Sole impression and palm impression. The impression of the sole of the pes and the palm of the manus, respectively. Leonardi (1987) defined the terms “sole” and “palm” as the area posterior to the metapodial-phalangeal axis (i.e., excluding digits), a recommendation we follow here. By this definition, the terms “sole” and “palm” are not synonymous with the term plantar/palmar surface, which refers to the entire underside of the foot that is in regular contact with the ground. It should also be noted that the sole and palm impressions seen in a track do not always correspond exactly to the phalangeal and metapodial parts of the hand or foot (Farlow and Britton, 2000). Note that in ungulate anatomy, “sole” instead refers to the subunguis of the underside of the hoof (Figure 14E).
102 Track wall (synonym: shaft wall). The inclined to vertical margin of a track that surrounds the track floor (cf. Allen, 1997).
103 Shaft. The three-dimensional space between the track walls (cf. Allen, 1997). Distinct shafts may be absent in shallow tracks. Fornós et al. (2002) suggested using the term “axis” instead of “shaft” because the latter term is already used to describe open vertical burrows in invertebrate ichnology. However, we argue that “shaft” is unambiguous when used in the context of tetrapod tracks and note that “axis” conveys other meanings as well.
104 Track floor (synonym: track bottom). The base of a track that is bounded by the track walls.
105 Lip. The upper edge of the shaft, i.e., the margin between the track walls and the surrounding sediment surface (cf. Allen, 1997). The lip is often not well defined, especially where the transition between the track and the surrounding sediment is smooth and/or the track is shallow. Note that “lip” has occasionally been used as a synonym for displacement rim, a usage that we recommend avoiding.
106 Claws, hooves, and nails. The keratinous covering of the unguals. Claws generally have a pointed tip and are round or oval in cross section, whereas hooves (singular: hoof; alternative plural: “hoofs”) are broadly rounded and often have a flat underside (Thulborn, 1990). Nails lie between these extremes. The term semiclaw has been introduced to describe claws that are pointed but have a flat underside, as in most pre-Cretaceous ornithopods (Coombs, 1980). This term is rarely used and is currently restricted to dinosaurs. The claws of birds of prey are known as talons (e.g., Csermely and Rossi, 2006). The corresponding traces are termed “claw marks” (Figure 7B-F, M; synonyms: claw traces, claw impressions, claw prints), “hoof marks” (synonyms: hoof impressions, hoof prints), and “nail marks” (synonyms: nail impressions, nail prints). Claws, hooves, and nails are composed of the unguis, the harder upper layer, and the subunguis, the softer lower layer.
The hooves of hoofed mammals have their own terminology. The unguis is known as the wall and forms the outer edge of the foot in plantar/palmar view. The subunguis on the inside of the wall is known as the sole. Horses have a unique keratinous structure known as the frog (e.g., Vincelette et al., 2023); this structure is V-shaped and occupies the central and posterior areas of the plantar/palmar surface of the hoof. In horses, the posterior part of the wall is folded inwards in a V-shape; the two halves of these inward folds are known as the bars. Posterior to the frog and bars, and usually elevated above the ground, is a pair of bulbous structures known as the heel bulbs.
107 Interdigital space. General term for the space between digits or digit impressions. For example, this may be the space between the diverging digit impressions of a tridactyl track, or the space between the paired hooves of an ungulate.
For ungulate tracks, Halfpenny (1986) uses the term “interhoof distance” to refer to the width of the interdigital space. In camels, the two weight-bearing digits (digits III and IV) are only partially separated by a groove between the digital pads; this groove is referred to as the “interdigital sulcus” (Figure 14D) (e.g., Lucas and Hunt, 2007). In a track preserved as a natural mould, this sulcus appears as a ridge separating the digit impressions. Anterior and posterior to the interdigital sulcus are notches formed by the diverging digits; these are known as the “anterior cleft” (synonym: “interclavular gap”) and the “posterior cleft” (synonyms: “posterior gap”, “proximal gap”), respectively (Figure 14D) (e.g., Lucas and Hunt, 2007). The sulcus may extend into a “medial pocket” (Figure 14D) (Sarjeant and Reynolds, 1999).
108 Ball. In hominin track terminology, the ball is the area proximal to the digital pads and below the metatarsal heads (i.e., the distal ends of the metatarsals) (Figure 14F) (Bennett and Morse, 2014). Ball width is often measured perpendicular to the long axis of the track.
109 Midfoot (=mid-foot). In human track terminology, the midfoot is the area between the ball and the heel (Figure 14F) (Bennett and Morse, 2014). The human foot is arched both anteroposteriorly and mediolaterally, resulting in a concave sole (see Singh, 2014, for a general overview). There are two longitudinal arches, the medial and lateral longitudinal arches, as well as a series of transversal arches. The most pronounced arch, the medial longitudinal arch, is formed by the calcaneus, talus, navicular, three cuneiform bones, and metatarsals I-III (Singh, 2014). The condition of a low medial longitudinal arch is known as a “flat foot” (pes planus), while the opposite is a “high arched foot” (pes cavus). The lateral longitudinal arch is much lower than the medial longitudinal arch, and formed by the calcaneus, cuboid, and metatarsals IV and V. The anterior transverse arch is formed by the metatarsal heads, while the posterior transverse arch is formed by the remaining part of the metatarsus and tarsus (Singh, 2014). Hatala et al. (2023, 2024) defined the "arch volume" to quantify arch morphology in hominin tracks and feet. Because the midfoot is arched, it forms an isthmus (constricted area) in shallow tracks.
110 Midtarsal break (=mid-tarsal break). The lifting of the heel independently of the rest of the foot in non-human primates (Elftman and Manter, 1935). In humans, which lack a midtarsal break, the heel and midfoot are lifted off the ground simultaneously during walking, with dorsiflexion occurring only at the metatarsophalangeal joints. Other primates show greater foot mobility, and heel lifting occurs before midfoot lifting (DeSilva, 2010). A synonym is “two-stage heel lift” (Kidd et al., 1996).
Measures
Quantitative analysis of tracks relies on reference points (landmarks) that can be used to define linear and angular measures and to align (superimpose) outlines or 3D models of different tracks. Such landmarks can be defined on the outline of the track. For example, track length is measured between the posteriormost and the anteriormost point of the outline. Problematically, a clearly defined outline often does not exist in a track, and its definition is partly subjective and can vary significantly between observers (Falkingham, 2016; Lallensack, 2019). Landmarks can also be defined based on the centres of outlines (typically of pads); such landmarks are generally less affected by the uncertain definition of the outline but are not always available. In forensics, a measurement scheme based on the centres of circles placed on the pads of the human foot is known as the “optical centre method” (e.g., Mukhra et al., 2018). Machine learning is a promising method that allows quantitative analysis while avoiding measurements altogether, potentially overcoming the limitations of traditional quantitative approaches (Lallensack et al., 2022d).
Here, we review the terminology of the most common measures used to quantify track shape. Many additional combinations that are commonly defined ad hoc and are relatively unambiguous (such as “digit impression width”) are omitted here.
111 Track outline (also “outline”, “footprint outline”). The margin of the track. This margin is not precisely defined, especially where the track wall gradually fades with the surrounding sediment and can be ambiguous where the track walls are complex. Tracings of the outline as interpreted by the ichnologist are called “interpretative outline drawings” (or “interpretive outline drawings”) (Figure 4C). Track outlines are often traced along the steepest slope of the track wall (e.g., Olsen and Baird, 1986). Outlines can alternatively be traced around the track floor while excluding the track walls, in which case they are called internal track outlines (also “internal outlines”, “inner outlines”, “minimum outlines”), or at the intersection of the track wall with the original sediment surface, when they are called external track outlines (also “external outlines”, “outer outlines”, “maximum outlines”). Internal outlines are generally assumed to reflect the original shape of the foot more accurately than external outlines, and where an interpretative outline drawing includes both, the internal outline is often indicated by a solid line and the external outline by a dashed line (e.g., Marty, 2008). Interpretative outline drawings may be idealised to varying degrees (e.g., including or excluding extramorphological features such as cracks), and may include additional features inside and outside of the track walls. A non-idealised outline that precisely reflects the track at a given depth is called a contour.
Interpretative outline drawings have been the primary means of conveying track morphology in the literature and form a basis for measurements and landmarks. However, their two-dimensional nature is an oversimplification of a complex three-dimensional morphology. In addition, the subjectivity involved in defining the track margins affects the resulting measurements, compromising quantitative analysis (Falkingham, 2016). A number of approaches have been proposed to define track margins and measures more objectively, including the use of both internal and external outlines (Falkingham, 2016); contours sampled from a consistent depth (Razzolini et al., 2016); centroids of features such as digital pads (Kennedy et al., 2005); and objective outlines generated using computer algorithms (Lallensack, 2019). None of these approaches are universally applicable. It is strongly recommended that 3D models, and 2D visualisations of them, are provided alongside outline drawings to allow for independent assessment (Falkingham et al., 2018).
112 Composite track. A hypothetical shape derived from two or more superimposed tracks. Traditionally, composite outlines were drawn by ichnologists by manual superimposition and interpolation. The concept was introduced by Baird (1952) as a novel method to “minimize the errors which may arise when a single set of impressions rather than the entire trackway is used as a basis for description” (p. 833). Thanks to computational advances, outlines can now be superimposed (aligned) based on landmarks using Procrustes superimposition, median or mean outlines can be computed, and statistical differences between samples can be calculated. This concept has also been applied to 3D models (e.g., Crompton et al., 2011; McClymont and Crompton, 2021) and sometimes referred to as “whole-track analysis” (Bennett and Morse, 2014). The resulting 3D composite tracks have been termed stat-tracks, and stat-tracks derived from type specimens have been termed mediotypes (Belvedere et al., 2018).
A “composite manus-pes set“ combines the most fidelitous manus track with the most fidelitous pes track of a sample. Note that the term “composite track” has also been used to refer to actual tracks that overlap each other (amalgam in our usage).
113 Reference point (also “landmark”). A point that can be consistently identified across multiple tracks of a sample, such as the tips of digit impressions or the centroids of phalangeal pads. Mathematically, reference points can be expressed as 2D or 3D coordinates. Landmarks form the basis for linear measurements and can be subjected to geometric morphometric analysis. Note that in tracks, landmarks do not precisely capture homologous anatomical features (e.g., differences in the relative positions of the claw marks are often the result of variations in foot kinematics and behaviour rather than anatomy).
Trackway parameters such as pace and stride length require a reference point that is consistently identifiable in all measurable pes (or manus) tracks of the trackway. In amphibians and reptiles, this is conventionally the base of digit impression III. In tridactyl tracks, the tip of digit impression III is commonly chosen, while in sauropod tracks and tracks lacking anatomical detail, the centroid of the track may be used (Leonardi et al., 1987). In hominin tracks, the reference point is often the pternion of the heel (e.g., Bennett and Morse, 2014). Note that a distal reference point (such as the tip of digit impression III) will be more affected by variations in foot rotation.
114 Digital axis (also “digit axis”). The midline, and generally the axis of symmetry, of a digit impression (Figure 14A) (Leonardi et al., 1987). The digital axis can be difficult to define, particularly if the digit impression is curved. If only the distal part of the digit impression is curved, the digital axis is defined based on the proximal part (Leonardi et al., 1987). Digital axes form the basis for measures such as digit impression width and interdigital angle.
115 Track long axis (synonyms: longitudinal axis, central axis, footprint long axis, foot axis). Axis indicating the orientation of a track (Figure 14A). Per convention, the long axis corresponds with the digital axis of digit impression III. If digit III is absent, the long axis is instead the axis of symmetry of the track (Leonardi et al., 1987). The long axis forms the basis for measures such as track rotation, track length, and track width. In fossil hominin tracks, the track long axis is termed the central axis and often defined between the pternion and the tip of digit impression II (Figure 14F) (Bennett and Morse, 2014). Alternatively, the “designated longitudinal axis” is defined between the pternion and the most lateral point of the toe pad of digit I (Figure 14F) (Robbins, 1985).
116 Transverse axis. The horizontal axis perpendicular to the long axis (Figure 14B) (Leonardi et al., 1987). In Robbins (1985) terminology of human tracks, a line perpendicular to the designated longitudinal axis and tangent to the pternion is termed the base line.
117 Metapodial-phalangeal axis. The axis formed by the metapodial-phalangeal joints (Figure 14B). In the pes, this axis is also termed the “metatarso-phalangeal axis”, and in the manus, it is termed the “metacarpo-phalangeal axis”. In a track, this axis can be defined as a single straight line that coincides as closely as possible with the midpoints of the metapodial-phalangeal pads (Leonardi et al., 1987).
118 Cross-axis angle (cross axis angle). Angle between the metapodial-phalangeal axis and the long axis (Figure 14B). These two axes form four angles when they cross; the cross-axis angle is the anterolateral angle (Leonardi et al., 1987).
119 Track dimensions. The size of a track can be used as a proxy for the size of the trackmaker (Figure 14A). The most important measure is track length (=footprint length), typically measured between the track walls and parallel to the long axis from the most posterior to the most anterior point of the outline (Leonardi et al., 1987). In fossil hominin tracks, track length is often measured between the pternion and the tip of digit impression II (Bennett and Morse, 2014). Farlow et al. (2018a) used the term “field length” for quick measurements of track length that are not necessarily aligned with the long axis. The influence of substrate and speed on track length has been tested by Wiseman and De Groote (2022). Track width (=footprint width) is usually measured parallel to the transverse axis between the most medial and lateral points of the outline (Leonardi et al., 1987). Track span is an alternative measure of track width that is unaffected by the width of the individual digit impressions. Track depth (also “footprint depth”, “true track depth”, “surface track depth”, “thickness of the relief”) is measured between the track floor and the tracking surface (excluding displacement rims). “Relative track depth” is track depth relative to size (e.g., track depth / track length). Lockley et al. (2002) distinguished between true dimensions (or “true track dimensions”) that are measured based on the internal outline of the tracks, and apparent dimensions (or “apparent track dimensions”) that include the displacement rim.
Trackmaker size is usually estimated in terms of body height – depending on the trackmaker taxon, this may be hip height, height at the withers, or, in humans, total height or stature. In quadrupeds, trackmaker size can also be approximated by the apparent gleno-acetabular distance. Body mass has been estimated based on track area (e.g., Kubo, 2011). Estimating body mass and centre of mass based on track depth has been tried (Demathieu, 1987; Schanz et al., 2013) but remains difficult, as track depth is influenced by factors other than weight, such as the softness of the substrate and the shape of the foot (Falkingham et al., 2010).
120 Hip height (synonyms: skeletal hip height, acetabular height). The height of the hip joint above the ground during normal standing (Alexander and Jayes, 1983). Hip height is a central parameter in Alexander’s formula for estimating locomotion speed. Hip height can only be estimated based on assumptions about the anatomy of the trackmaker, and the error associated with such estimates is often problematic (Rainforth and Manzella, 2007). For dinosaurs, hip height is commonly approximated as “four times track length” (Henderson, 2003), although efforts have been made to refine this number for specific taxa (e.g., Thulborn, 1990). Thulborn (1982) also defined the “height of the hindlimb” and the “height of the forelimb” as the combined lengths of the long bones of the respective limb plus an extra 9% to account for the ankle and soft tissues.
A related concept is the effective limb length, which is the distance between the hip joint and the base of the foot (e.g., Henderson, 2003). Effective limb length depends on the degree of limb flexion and is equivalent to hip height in normal standing. Fieler and Jayne (1998) defined effective limb length as the distance between hip joint and the base of the foot at the time when the footfall occurs; this definition is equivalent to that of the “apparent limb length”.
121 Index of track size (=index of footprint size). A proxy for track area that combines track length and width as SI = (TL x TW)0.5, where SI is the index of track size, TL is track length, and TW is track width (Thulborn and Wade, 1984).
122 Length-to-width ratio (synonym: aspect ratio). Track length divided by track width as a measure of elongation. In hominin tracks, this ratio is known as the “foot index” (Bennett and Morse, 2014).
123 Centroid (synonyms: mid-point or midpoint). The centre of gravity of a shape (typically a track or pad impression). This coordinate can be calculated from a given number of landmarks but is more conveniently approximated by half the length and half the width of the shape. Centroid size is the square root of the sum of squared distances of a given number of landmarks from their centroid. Centroid size can be a more accurate size proxy than track length or the index of track size because the precise shape of the track can be considered. Centroid size is widely used in geometric morphometrics.
124 Interdigital angle. The angle between two digital axes of a track. Common synonyms are “divarication angle” (also: divarication of digits, digit divarication angle), “divergence angle”, “spread angle”, and, in human track terminology, “angle of declination” (Robbins, 1985). Interdigital angles can be measured between two adjacent digits, which has also been termed “partial divarication”, or between the outer digits (“outer digit angle”, also “outer toe angle” or “total divarication”). The term “opposed” has been used to characterise digit impressions that are widely divergent from the other digit impressions (Thulborn, 1990). Note that this does not necessarily imply that the digit is opposable (i.e., that it can be diametrically opposed to another digit, allowing for a grasping function).
125 Digit impression length (or “digit length”; Figure 14B) has been measured in various ways, including parallel to the digital axis or along the curve; with or without claw marks; and from the first phalangeal pad or from the metatarsophalangeal pad (e.g., Leonardi et al., 1987; Farlow et al., 2018a). The true length of the digit (also “length of the phalangeal portion of the digit”) approximates the actual digit length when the digit is completely impressed and is measured from the midpoint of the metapodial-phalangeal pad or from the flexure crease beneath the metapodial-phalangeal joint (Leonardi et al., 1987). The free length (also “free digit length”; synonym: “hypex length”) is the distance from the tip of a digit impression to the midpoint between the two adjacent hypexes (or to a single hypex when measuring an outer digit), measured parallel to the digital axis (Figure 14B). The digital region length (also: toe region length) is measured from the most posterior digital pad of the foot to the tip of the most projecting digit, and parallel to the track long axis (Figure 14F; e.g., Robbins, 1985).
126 Digit impression width (also “width of the digit imprints”). Typically measured perpendicular to the long axis of the digit impression (Leonardi et al., 1987). This measure must be used with great caution, as it often does not reflect the actual digit width of the trackmaker.
127 Palm length and sole length. The maximum length of the palm impression (or sole impression) measured parallel to the long axis of the track. Palm width (or sole width) is the maximum width of the palm (or sole) measured perpendicular to the long axis of the track (Leonardi et al., 1987).
128 Track span. The distance between the tips of the outer digit impressions (Figure 14A) (Thulborn, 1990). Synonyms are “toetip width” (Farlow et al., 2018a) and, in tridactyl tracks, the width of the anterior triangle (Weems, 1992).
129 Digit projection. The distal extension of a digit (or digit impression) beyond the other digits (Figure 14A). The term was introduced by Olsen (1980), who defined it as the “projection of digit III past digits II and IV” in tridactyl dinosaur tracks. Synonyms are “toe extension” and “toetip extension” (Farlow et al., 2018a). The projection of a digit is usually measured from a line connecting the tips of the two adjacent digits; in tridactyl tracks, this line corresponds to the track span. In tridactyl tracks, digit III projection is typically reported as the ratio to the rest of the track. This rest of the track (i.e., track length minus digit projection) has been referred to as backfoot length (Farlow et al., 2018a), “length of the rear of the phalangeal part” (Olsen et al., 1998) or “rear projection” (Abrahams et al., 2023). Olsen et al. (1998) defined the projection ratio (backfoot length/digit III projection) as well as the “corrected projection ratio” which accounts for differences in interdigital angles that would otherwise affect these measurements. Tridactyl tracks have also been characterised as showing “strong mesaxony” (high digit III projection) or “weak mesaxony” (low digit III projection) (see the entry “principal digit” for details). In tridactyl tracks, a strong digit III projection has been associated with increased cursoriality (Lallensack et al., 2020).
A closely related concept applicable to functionally tridactyl tracks is the anterior triangle, introduced by Weems (1992) and later adopted by Lockley (2009). The anterior triangle is spanned between the tips of digits II, III, and IV, while the posterior triangle (or “backfoot triangle”; Leonardi et al., 2024) is spanned between the tips of digits II and IV and the rear of the track. From these triangles, various ratios can be derived, the most common of which is the “anterior triangle length/width ratio” (Weems, 1992; Lockley, 2009).
130 Heel width. The maximum width of the (anatomical) heel of a track (Figure 14F). Synonyms in human track terminology include “calc width”, “calcaneus width”, and “breath at heel”.
131 Instep width. The minimum width of the midfoot in hominin track terminology (Figure 14F).
132 Arch volume. The volume of the raised area between the posterior and anterior of the sole impression of a plantigrade track, or the volume of the anatomical arch of the foot. So far, this measure has only been used for hominin tracks and feet. “Relative arch volume” is a dimensionless comparative value defined by a normalized prism delimited by three landmarks that are placed on the plantar surface at the heel and at the metatarsophalangeal joints of digits I and V (Hatala et al., 2023). The relative arch volume of a track has been shown to be more closely linked to walking kinematics, rather than anatomy (Hatala et al., 2023, 2024).
TRACKWAYS
Unlike the complex 3D shape of individual tracks, which can be difficult to quantify, the positions of tracks within a trackway can be described by simple coordinates or linear and angular measurements. Trackways do not only inform about body proportions and posture, but can also provide information about the behaviour of the trackmaker as they record a longer duration of time than a single track.
133 Trackway. A sequence of tracks left by the same individual during locomotion (Figure 8). This term is often restricted to cases with at least three consecutive tracks in bipeds and six consecutive tracks (three manus-pes sets) in quadrupeds, as this is the minimum number of tracks required to obtain a complete set of trackway parameters (Leonardi et al., 1987; Thulborn, 1990; Marty et al., 2016). In practice, however, shorter sequences (at least two tracks for bipeds and three for quadrupeds), as well as incomplete sequences such as manus-only trackways, are commonly referred to as trackways. “Trackway asymmetry” typically refers to differences between the left and right step lengths, which can be due to footedness, limping, or tectonic deformation (Schulp, 2002). The “trackway course” can be described as “straight” or “undulating” (also “sinuous”), and the spacing of tracks as “regular” or “irregular”. “Tortuosity” is the degree of convolution of a trackway.
A more general term, traceway, can be used to refer to a series of impressions made during locomotion that do not necessarily represent tracks. In tetrapod track ichnology, the term “traceway” has often been used to describe trackways made by buoyant trackmakers (McAllister, 1989), in which case it is considered here as a synonym of “trackway” (see also discussion in the entry “swimming tracks”). A closely related term is trail. The term movement path is even more general and does not necessarily imply the formation of traces. If a series of movements and stops is associated with a particular goal (e.g., feeding), the series is referred to as a “movement phase”. Both terms originate in behavioural biology and have been introduced into ichnology by Plotnick (2012) but have not yet found wide application in this field.
134 Trail. This term has several meanings:
A trace of locomotion in general. This may be a trackway or a trace produced by any form of limbless locomotion (e.g., “the trail of a snail”, “fish trails”).
In invertebrate ichnology, a trail is a continuous trace of locomotion on the substrate surface, while a trackway comprises multiple separate impressions (e.g., Buatois and Mángano, 2011).
A path that is repeatedly used by animals (e.g., “a network of hippo trails”).
135 Tortuosity. The degree of convolution of the movement path. The tortuosity is high if the animal made many turns (Figure 10B), and low if it followed a straight line. The tortuosity of trackways is usually measured in two dimensions, but the concept can be extended to three dimensions, for example when describing burrows (e.g., Hasiotis et al., 2007). A simple estimate of tortuosity is the “straightness index” of Batschelet (1981), which is the ratio between the “beeline distance“ (the length of the straight line connecting the first to the last track) and the path length (the sum of the stride lengths). The straightness index is appropriate if the movement was directional (i.e., when the animal moved towards a goal). If the movement was non-directional (milling), other methods such as the sinuosity index may be more appropriate (see Bovet and Benhamou, 1988; Benhamou, 2004), but to our knowledge, such parameters have not yet been applied to fossil trackways. Note that the term “sinuosity index” has also been used as a synonym of “straightness index” (Falk et al., 2017); we here recommend following the original definitions of these terms to avoid confusion.
136 Trackway parameters (synonym: trackway measures). Linear and angular measures to describe the relative positions and orientations of the tracks of a trackway (Figure 14G, H).
137 Trackway pattern. The sum of recurring features in a trackway, the general trackway characteristics. This includes the relative position of tracks, their orientation, and associated traces such as drag marks and tail traces, but not the morphology of the manus and pes tracks themselves (Leonardi et al., 1987). In the ichnological literature, “trackway pattern” has also been used as a synonym of both trackway configuration and trackway orientation pattern (see Marty, 2008, for a detailed discussion). Marty and Meyer (2006) restricted the term “trackway pattern” to the presence or absence of pes or manus tracks; following this usage, the trackway pattern can be quadrupedal or bipedal, pes or manus only, and pes or manus dominated.
138 Trackway configuration. The relative track positions and orientations that characterise a trackway (Marty, 2008). Trackway configuration is usually described by averages of the trackway parameters. By this definition, trackway configuration is less inclusive than the similar term trackway pattern, which may also include associated traces.
139 Stride length. The distance between two consecutive footfalls of the same foot (Figure 14G). This is equivalent to the distance travelled during one step cycle. The stride line is the imaginary line along which stride length is measured. Both “stride length” and “stride line” have often been referred to simply as “stride”, but this term also refers to the pendular movement of a limb as well as to a complete movement cycle (Alexander, 2003). When used in the latter sense, “stride length” is the distance travelled during one movement cycle, a definition that can be applied to both limbed and limbless animals (Alexander, 2003). Note that “stride” is not restricted to striding gaits and can be applied to asymmetrical gaits such as the gallop (“jump stride” in equestrian terminology; Leach, 1993). In hominin tracks, the stride line is also known as the “ipsilateral line of progression” (Wilkinson et al., 1995).
140 Trackway midline (synonym: line of progression, line of travel). The passage of the trackmaker’s centre of mass projected on the substrate. (Figure 14G). The trackway midline can be approximated by connecting the midpoints of successive pace lines (Voigt and Haubold, 2000). In quadrupeds, a single trackway midline can be defined for pes- and manus tracks. Defining the trackway midline separately for pes and manus tracks may provide additional insights in some cases, especially for turning trackways. For example, Leonardi (1981) found that in the ichnogenus Brasilichnium, the manus trackway midline is less regular than the pes trackway midline, indicating that the forelimbs were used for steering while the hind limbs were only following.
141 Pace length. The distance between two consecutive footfalls of a contralateral limb pair (Figure 14G). The pace line is the imaginary line along which pace length is measured. Both “pace length” and “pace line” are often referred to simply as “pace” but note that “pace” is also a synonym for “pace gait” and “speed”, which can be a source of confusion. Leonardi et al. (1987) used the term “oblique pace” to distinguish this measure from step length (“pace length” in their usage).
142 Step length. This term has three meanings:
The distance between two consecutive footfalls of a contralateral limb pair measured parallel to the trackway midline (Figure 14G). If the left and right step lengths are equal (i.e., the trackmaker is not limping), step length is generally equal to half the stride length. This definition is the predominant one in the ichnological literature, and the term is used here in this sense.
The distance travelled by the animal while a given foot is in ground contact (Gray, 1968). Following this definition, “step length” is equal to stride length multiplied by duty factor (Alexander et al., 1977). Consequently, the first and second definitions of “step length” are equivalent only when duty factor is 0.5.
The pace length.
143 Coupling value. The gleno-acetabular distance divided by the sum of the lengths of the forelimb and the hind limb (Peabody, 1959). Most trackmakers are “short-coupled” (i.e., have proportionally long limbs and short trunks), while “long-coupled” trackmakers (e.g., some lizards and salamanders) are rarer (Peabody, 1959).
144 Set (synonyms: manus-pes set, pes-manus set, couplet). Pair of manus and pes tracks formed by ipsilateral footfalls of the same step cycle in a symmetrical gait, with the pes footfall occurring after or, in the case of a perfect pace gait, at the same time as the manus footfall. According to this definition (sensu Peabody, 1959, and Leonardi et al., 1987), the distance between the two tracks of a set is the manus-pes distance. At higher coupling values, the actual set may be separated by footfalls of different step cycles, including “pseudo-sets” (Leonardi et al., 1987). The term “set” has also been used to denote a series of consecutive tracks that allow a complete set of trackway parameters to be measured (three tracks for a biped and six tracks for a quadruped) (Thulborn, 1990).
145 Group and intergroup. When a quadruped uses an asymmetrical gait with aerial phases, such as a bound or gallop, the tracks form a characteristic pattern of four, known as a “group” (Figure 14I). The groups of a trackway are separated by an empty space, the “intergroup” (Halfpenny, 1986). Group length and intergroup length (also: “group/intergroup distance”) are measured parallel to the trackway midline (Figure 14I).
146 Manus-pes distance (synonyms: pes-manus distance, interautopodial distance). The distance between the manus and pes of a set, measured parallel to the trackway midline (Figure 14H). If the trackmaker has overstepped, the manus-pes distance will be negative (Leonardi et al., 1987). In a perfect pace gait, the manus-pes distance is identical to the apparent gleno-acetabular distance.
147 Track phase. The position of a manus track relative to the positions of the previously and subsequently formed ipsilateral pes tracks, expressed as a value between 0 and 1 (Stevens et al., 2016). This value can be calculated as manus-pes distance divided by stride length (Polet and Hutchinson, 2022). For example, a manus track midway between two pes tracks corresponds to a track phase of 0.5.
148 Trackway width (synonyms: straddle, gauge width). The width of a trackway, measured separately for the manus tracks (manus trackway width) and pes tracks (pes trackway width) when applied to quadrupedal trackways (Figure 14G-H). Outside the field of ichnology, various alternative terms have been used for equivalent concepts, including “base of gait”, “stride width”, “dynamic base”, “walking base” (Low and Reed 1996), “step width”, and “base of support” (see Wilkinson et al., 1995). Trackway width has been measured in a number of ways:
Pace width (synonym: width of pace): the distance between the centroids of two consecutive tracks of a contralateral limb pair measured perpendicular to the trackway midline (Leonardi et al., 1987).
Width of the angulation pattern: roughly equivalent to the pace width as defined by Leonardi et al. (1987) but may be based on other reference points and is always measured between three consecutive tracks produced by the same contralateral limb pair (e.g., Marty, 2008). In a quadrupedal trackway, both the “width of the pes angulation pattern” and the “width of the manus angulation pattern” are measured.
External trackway width (synonyms: maximum trackway width, overall width): measured between the lateralmost points of the track outlines (i.e., the points furthest from the trackway midline) of two consecutive contralateral tracks and perpendicular to the trackway midline (Figure 14G).
Internal trackway width (synonyms: minimum trackway width): measured in the same way as the external trackway width, but between the medialmost points of the track outlines (Figure 14H). When measured for the pes tracks, this measure has been termed the “interpedes distance”, “interpes distance”, or “internal pes trackway width”, and when measured for the manus tracks, it has been termed the “intermanus distance” or the “internal manus trackway width” (e.g., Hasiotis et al., 2007).
Breadth between tracks: as internal trackway width but measured between two successive left and right sets (i.e., between those tracks of the sets that happen to be closest to the trackway midline). The “breath between tracks” has been defined by Leonardi et al. (1987), but its use remains limited.
An informative measure is the difference between the manus and pes trackway widths. This measure has been termed “trackway deviation” (e.g., Pérez-Lorente, 2015) but note that “trackway deviation” has also been used to describe tortuosity, i.e., the deviation of a trackway from a straight line (Lockley et al., 2021). The measure has also been described as the “difference between pedal and manual gauge width” (Buchwitz et al., 2021) and as the “midpoint manus midline distance−midpoint pes midline distance” (Farlow et al., 2018a). As defined by Farlow et al. (2018a), positive values indicate that the manus was farther from the trackway midline than the pes, and negative values indicate that the manus was closer to the trackway midline than the pes; this definition is followed herein. A closely related measure is the difference between the manus and pes pace angulations (e.g., Lallensack et al., 2019).
149 Gauge (or trackway gauge, synonym: gait width). The distance of the tracks from the trackway midline relative to the size of the trackmaker. Narrow-gauge trackways (also: trackways with a “narrow base”) are characterised by tracks close to or intersecting the trackway midline, and wide-gauge trackways (also: trackways with a “wide base”) are characterised by tracks away from the trackway midline (Farlow 1992). An intermediate category has been termed “medium gauge” (Meyer et al., 1994). This terminology is particularly popular when describing sauropod trackways but is increasingly being applied to other quadrupedal tetrapod trackways (e.g., Petti et al., 2009; Castanera et al., 2021).
Gauge is typically quantified as the ratio of trackway width to pes or manus length (or width). For sauropod trackways, Marty (2008) defined the width of the angulation pattern divided by track length or width. For the pes tracks, this is the WAP/PL ratio, i.e., the width of the pes angulation pattern divided by pes track length, and for the manus tracks, it is the WAM/MW ratio, i.e., the width of the manus angulation pattern divided by manus track width. According to Marty (2008), WAP/PL ratios below 1 can be considered “narrow gauge”; ratios between 1 and 1.2 as “medium gauge”, and ratios above 1.2 as “wide gauge”.
Romano et al. (2007) defined the “trackway ratio” for quadrupedal trackways as the ratio between “side width” (the maximum track width measured perpendicular to the trackway midline) and the “overall width” (external trackway width). The respective ratios for pes and manus tracks were termed the “pes trackway ratio” and the “manus trackway ratio”, respectively. According to Romano et al. (2007), trackway ratios above 50% can be classified as “narrow gauge”; ratios between 35% and 50% as “medium gauge”, and ratios lower than 35% as “wide gauge”. Marty (2008) criticised the trackway ratio as a measure, arguing that it is susceptible to variations in track rotation.
150 Pace angulation (synonym: pace angle). The angle between two consecutive pace lines (Figure 14G). The “pes pace angulation” and the “manus pace angulation” are measured between the pes and manus tracks, respectively; these terms are sometimes abbreviated to “pes angulation” and “manus angulation”. The pace angulation will be 180° if the feet are placed exactly in front of each other in a straight line, and will be lower the more pronounced the zigzag arrangement.
151 Step angle. The angle between the pace line and the stride line of the same track. Note that “step angle” has sometimes been used as a synonym of “pace angulation”. Step angle and pace angulation have similar implications and are usually redundant.
152 Gleno-acetabular distance 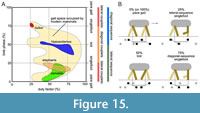 (synonyms: glenoid-acetabular distance, trunk length, intergirdle distance). The distance between the glenoid (shoulder joint) and the acetabulum (hip joint) (Figure 14H, Figure 15B). This distance can be projected onto a quadrupedal trackway, when it may be referred to as the apparent gleno-acetabular distance (also “apparent trunk length”), to distinguish it from the true (osteological) gleno-acetabular distance (in the ichnological literature, both are typically referred to simply as the gleno-acetabular distance). The concept of apparent gleno-acetabular distance was developed by Soergel (1925) (see also Lallensack et al., 2019).
(synonyms: glenoid-acetabular distance, trunk length, intergirdle distance). The distance between the glenoid (shoulder joint) and the acetabulum (hip joint) (Figure 14H, Figure 15B). This distance can be projected onto a quadrupedal trackway, when it may be referred to as the apparent gleno-acetabular distance (also “apparent trunk length”), to distinguish it from the true (osteological) gleno-acetabular distance (in the ichnological literature, both are typically referred to simply as the gleno-acetabular distance). The concept of apparent gleno-acetabular distance was developed by Soergel (1925) (see also Lallensack et al., 2019).
Apparent gleno-acetabular distance can be a precise approximation of the (horizontal) trunk length, and therefore body size. However, it depends on the limb phase used by the trackmaker (Figure 15B), and separate ways of measuring it have been defined, assuming limb phases of 0 (or 100%; pace gait); 25% (lateral-sequence singlefoot); 50% (trot), and 75% (diagonal-sequence singlefoot). In all cases, it is measured based on the expected position of the feet at the time when a footfall of a pes occurs. At 0% (or 100%) and 50% limb phase, four feet are simultaneously on the ground when the footfall of a pes occurs, and the apparent gleno-acetabular distance is measured between the midpoints of these pes- and manus pairs. At 25% and 75% limb phase, one of the manus will be in mid-swing at the moment the footfall of a pes occurs, and the apparent gleno-acetabular distance is measured to the inferred position of this swinging manus (based on the position of the contralateral manus footfall, the midpoint of the stride, or both) (Figure 15B).
When measured on the same trackway segment, these distances decrease linearly with increasing limb phase (i.e., the distance assuming 75% limb phase is the shortest and that assuming 0% or 100% is the longest) (Figure 15B). Because of this linearity, the apparent gleno-acetabular distance corresponding to all intermediate limb phases can be derived (Lallensack and Falkingham, 2022). Stevens et al. (2022) referred to such a continuous apparent gleno-acetabular distance as the “coupling length” (not to be confused with “coupling value”), which we here consider as a synonym of “apparent gleno-acetabular distance”. The variation of apparent gleno-acetabular distance with speed has been used to calculate limb phase, and hence gait (Lallensack and Falkingham, 2022; Stevens et al., 2022).
The relationship between limb phase (LP), gleno-acetabular distance (GAD), manus-pes distance (MPD), and stride length can be expressed as LP = 1 − (GAD-MPD) / stride length (Lallensack and Falkingham, 2022).
153 Track rotation (synonyms: footprint rotation, divarication from midline, foot placement angle). The horizontal orientation of a track relative to the direction of travel (Figure 14G). Track rotation can be measured as the angle between the track long axis and the previous stride line, the next stride line, the opposite stride line, or the average of all three stride lines. It can also be measured between the long axes of two consecutive left and right tracks. The terms “inward rotation” and “outward rotation” are used to indicate whether a track is rotated towards or away from the trackway midline, respectively. Per convention, an inward rotation is indicated by a negative value of the measured angle, while outward rotation is indicated by a positive value (Leonardi et al., 1987). Alternatively, the terms “negative rotation” (inward rotation) and “positive rotation” (outward rotation) are used (Leonardi et al., 1987); however, these terms are also used in the opposite sense and are therefore ambiguous (e.g., Thulborn, 1990). Rarer synonyms include “track/footprint orientation”, “pes angulation” (or “manus angulation”), “toeing in” and “toeing out”, and “yaw”; see Thulborn (1990) for a brief review. For human tracks, the terms “angle of gait”, “foot angle”, and “toe out angle” (or “toe in angle”) have been used (Wilkinson et al., 1995; Levine et al., 2012). Note that the terms “pes/manus angulation”, “divarication”, and “angle of gait” convey different meanings in general tetrapod palaeoichnology and are therefore ambiguous. Ellenberger (1972, 1974) used the medical terms “valgus” as a synonym of “outward rotation” and “varus” as a synonym of “inward rotation”, but because these terms are reserved for deformities, this usage should be avoided.
154 Heteropody and homopody. These terms have two meanings:
The difference in area between manus and pes tracks (e.g., Sarjeant and Stringer, 1978). In this sense, “strong heteropody” indicates a large discrepancy in area, while “weak heteropody” indicates a small discrepancy and “homopody” indicates no discrepancy. Heteropody is often quantified using the heteropody index, which is the ratio between the manus and pes track areas (Marty, 2008; Gonzalez Riga and Calvo, 2009).
More generally, the difference (or similarity) in both size and morphology between pes and manus tracks (Figure 11B) (Leonardi et al., 1987).
155 Manus-only trackway. Trackway of a quadruped consisting of manus tracks without pes tracks (Figure 9A). Conversely, the trackway of a quadruped that consists only of pes tracks is called a pes-only trackway. Bird (1944) famously interpreted a manus-only sauropod trackway from Texas as evidence for floating of the posterior trunk during swimming, which became known as the “punting hypothesis” (see Farlow et al., 2020). Trackways that show clear manus tracks but barely visible or often missing pes tracks have been described as “manus-dominated”, while the reverse condition has been termed “pes-dominated”.
Manus-only trackways are now generally thought to be the result of differential pressure (e.g., Falkingham et al., 2011b). The deformation of a firmer substrate remains small until its bearing capacity is reached and the substrate deforms, at which point the foot sinks in (Falkingham et al., 2011b). Consequently, if the peak pressure during stance phase is higher in the manus than in the pes, the manus may exceed the bearing capacity and form deeper tracks, whereas the pes does not exceed bearing capacity and consequently forms only a very shallow track (Falkingham et al., 2011b).
156 Overstep (also: overstepping). The condition in which the pes is placed anterior to the manus (Leonardi et al., 1987). The opposite condition, in which the pes is placed posterior to the manus, is termed an understep (also: understepping). Depending on the coupling value, the overstep can be primary, secondary, or tertiary if the overstepped manus was formed during earlier step cycles (Leonardi et al., 1987). Note that “overstep” has also been erroneously used as a synonym for “overprint”.
157 Overprint  (also: overprinting; synonyms: overlap, register). The condition in which the pes is placed on the manus track (Figure 7F, Figure 16A). An overprint can be total, partial, or marginal (Leonardi et al., 1987). The term has also been used in a more general sense for any overlapping tracks. The term amalgam (synonyms: compound track; composite track) can be used to describe the coincidental overlap of two or more tracks, typically from different individuals (Thulborn and Wade, 1984). Intersections of trackways are often called crosscuts.
(also: overprinting; synonyms: overlap, register). The condition in which the pes is placed on the manus track (Figure 7F, Figure 16A). An overprint can be total, partial, or marginal (Leonardi et al., 1987). The term has also been used in a more general sense for any overlapping tracks. The term amalgam (synonyms: compound track; composite track) can be used to describe the coincidental overlap of two or more tracks, typically from different individuals (Thulborn and Wade, 1984). Intersections of trackways are often called crosscuts.
158 Sequence of tracks. The numbering of the tracks along a trackway is usually unambiguous for bipeds. In quadrupeds, the numbers are often given according to the manus-pes sets (either the actual sets or pseudo-sets), starting with the first left (or right) manus-pes set (e.g., LP1, LM1) followed by the first right (or left) manus-pes set (e.g., RP1, RM1). Note here that such numbering generally does not reflect the actual timing of the footfalls. Sequence numbering of a trackway may change as additional tracks are discovered that extend the trackway proximally; some workers assign negative numbers in such cases.
A sequence graph is a diagram that visualises the changes in a trackway measure along a trackway (Leonardi, 1981; Leonardi and Carvalho, 2021a). The horizontal axis of the graph shows the footfalls of the trackways (numbered consecutively), while the vertical axis shows the measure of interest (e.g., the stride length). Plotting stride length in this way allows the variability, accelerations, and decelerations of a trackway to be visualised. For quadrupeds, sequence graphs can be plotted separately for pes and manus tracks. If pes and manus tracks are to be plotted as a single graph, their sequence should be defined according to the actual footfall timings rather than per set, as this is the only way the graph will accurately show the events over time (see Lallensack and Falkingham, 2022, for methods).
159 Turning trackway. Trackway that shows a marked change in the direction of travel over a relatively short trackway segment (Figure 10C) (Ishigaki and Matsumoto, 2009). In quadrupedal trackways, the manus trackway midline may deviate significantly from the pes trackway midline at the turn. This phenomenon is known as off-tracking, a term borrowed from automobile engineering (Ishigaki and Matsumoto, 2009). Based on comparisons with four-wheel vehicles, Ishigaki and Matsumoto (2009) argued that the more exterior (wider) midline is formed by the contralateral limb pair that steers - in sauropods, this would be the front limbs.
TRACKSITES
The most important information conveyed by a track is often not in the track itself, but in its context as part of a tracksite and palaeohabitat. Unlike bones, tracks are unlikely to be reworked and transported away from the site where they were made. An assemblage of tracks therefore often reflects the palaeocommunity of a particular habitat. Tracksites can inform about the behaviour of the trackmakers; for example, parallel trackways can sometimes be interpreted as evidence of herding. It is therefore often desirable to leave, and ideally protect, tracks in the field. As tracks are both fossils and sedimentary structures, the sedimentology of a tracksite is of paramount importance in understanding the formation, preservation, and morphology of the track, the sequence of events recorded by the tracksite, and the palaeoenvironmental setting. While important, such sedimentological analyses remain the sole preserve of the discipline of sedimentology, the terminology of which is beyond the scope of this glossary.
160 Uses of tracks in geology. Fossil tracks can inform about geology. They can help date deposits, show that a surface had emerged above sea level or, in the case of swimming tracks, even provide a constraint on the depth of water at the time the track was formed. They also serve as geopetal indicators (synonym: way-up criteria), features that indicate the original orientation of layers that may have been overturned. Tracks can help to detect and measure deformation and displacement in rocks (Thulborn, 1990; Schulp, 2002). Finally, they provide important clues to sedimentary properties at the time of track formation and in this function have been termed palaeopenetrometers (or “paleopenetrometers”; a penetrometer is a device used to measure the strength of a substrate) (Falkingham et al., 2010).
161 Substrate. The medium in which tracks form, typically sediments or soils. Note that in the biomechanics literature, “substrate” often refers to what an animal locomotes on, even if no tracks are formed (e.g., branches can be a substrate for primates).
162 Track horizon. The sedimentary horizon in which tracks occur (e.g., Salisbury et al., 2016). “Horizon” refers to a “particular level without thickness in a stratigraphic sequence”, but note that in lithostratigraphy, the term is also used to refer to a very thin bed (Rey and Galeotti, 2008). A more general term is ichnosurface, which can refer to any surface containing ichnofossils (e.g., Bennett and Reynolds, 2021). Alternatively, the term “palaeosurface” (or paleosurface) is commonly used, but in geology and geomorphology this term is restricted to large-scale topographic surfaces of regional significance (Widdowson, 1997).
163 Tracksite (synonym: footprint site). A more or less continuous exposure of one or more track horizons (cf. Lockley and Meyer, 2022). It is often possible to distinguish between transit sites (or “trackway sites”), which record the directional movement of individuals across the surface, and congregation sites, where individuals are concentrated in an area (milling behaviour; e.g., mammals around a waterhole), typically with few or no discernible trackways (Morse et al., 2010). If a single ichnotaxon is present, a tracksite or assemblage can be described as “monotypic”, and if more than one ichnotaxon is present, it can be described as “polytypic” (Thulborn, 1990). Ichnosite is a more general term that is not necessarily restricted to tracks (but note that “ichnosite” and “tracksite” have often been used interchangeably). Tracksites are often time averaged. In some cases, the trampling history (also: sequence of events) during the track-forming window can be reconstructed - e.g., when a track of one individual overprints the tracks of another individual, it may be inferred that the former individual traversed the surface after the latter individual (e.g., Citton et al., 2016; Lockley, 2022). The positions and orientations of the individual tracks of a tracksite are documented in a sitemap. Traditionally, mapping relied on transparent foil and/or grids that were placed over the surface (Figure 4A1-2). Three-dimensional models can be oriented to match the bedding plane and rendered in orthographic (distortion-free) projection and are then an accurate basis for sitemaps.
164 Megatracksite. A track horizon, or a sequence of track horizons, that is laterally extensive (Lockley and Hunt, 1995; Wagensommer et al., 2021; Lockley and Meyer, 2022). The term was introduced by Lockley and Pittman (1989). Megatracksites consist of multiple tracksites and, unlike the latter, are not continuously exposed. The term “megatracksite” is not strictly defined, but currently recognised megatracksites are at least several kilometres in lateral extent and may cover more than 1,000 km² (Lockley and Meyer, 2022). Lockley and Meyer (2022) proposed to distinguish between three types of megatracksites based on their vertical extent: “Type 1 megatracksites” consist of a single track horizon, “Type 2 megatracksites” consist of a vertically restricted “package” of track horizons, and “Type 3 megatracksites” are vertically extensive “track-rich zones” up to the level of a geological formation. Hunt and Lucas (2007, p. 64) proposed the terms “monostratal megatracksites” for Type 1 and “multistratal megatracksites” for Type 2 megatracksites. Lockley and Meyer (2022) adopted the term ichnofaunal province (in their usage “zoological ichnofaunal province”, but as the “zoological” is redundant because of the “faunal”, we suggest just “ichnofaunal province”). The term “ichnofaunal province” was first introduced by Breithaupt et al. (2003) to generally refer to track-rich regions that are more vertically extensive than a megatracksite and may comprise multiple geological formations.
165 Direction of travel (synonyms: “direction of movement”, “advancement direction”). The direction of progression of the trackmaker. A closely related term is trackway orientation, which usually refers to the cardinal direction of a trackway measured between the first and last track. The pattern of trackway orientations at a particular tracksite is termed the trackway orientation pattern (synonym: movement pattern). The trackway orientation pattern can appear to be more or less random (no preferred direction of travel among trackmakers); unidirectional (trackmakers moving in the same direction); or bidirectional (two preferred directions of travel) (Thulborn, 1990). The trackway orientation pattern is most relevant in transit sites and less so in congregation sites. It can be visualised using a rose diagram.
166 Milling. Non-directional movement of a trackmaker, characterised by a highly tortuous (see entry "tortuosity"), and often self-intersecting, movement path (Cohen et al., 1993). This behaviour is shown by, e.g., larger mammals congregating around a water hole, or herbivores feeding at a patch of vegetation. The result may be a congregation site.
167 Time averaging. The accumulation of tracks on the same surface over a period of time (Figure 9B). This period can be in the order of hours, days, months, or much longer (Cohen et al., 1993). Consequently, associated tracks do not necessarily indicate that the responsible trackmakers traversed the surface at approximately the same time. A time-averaged ichnosurface can also be described as “diachronous”, while the opposite condition - synchronous track formation - can be described as “isochronous” (e.g., Bennett and Reynolds, 2021).
The effects of time averaging present a major challenge to the interpretation of tracksites, particularly in behavioural and palaeoecological terms. Severe time averaging may occur when old strata are naturally exhumed and then reburied, a mechanism that has been suggested to be responsible for the association of presumed Holocene human tracks with tracks of Pleistocene megafauna (Bennett and Morse, 2014; Rachal et al., 2021). Severe time averaging can also occur in caves, where surfaces can remain exposed for long periods of time (Bennett and Morse, 2014).
168 Track-forming window. The time interval during which tracks can form. Tracks on a given surface may have formed during a single or multiple track-forming windows, such as when the substrate has repeatedly dried and rewetted (Rachal et al., 2021). The track-forming window may be different for different trackmakers, e.g., lighter animals may not leave tracks if a substrate is too firm to reach bearing capacity, and heavier animals may avoid a soft surface if there is a risk of becoming mired (Falkingham et al., 2011a, 2014). The term has also been used to refer to the likely interval of geologic time during which a given set of tracks was formed (Bennett and Budka, 2019).
169 Trace fossil assemblage (also “ichnoassemblage”, “ichnofossil assemblage”, or, more specifically, “track assemblage” or “footprint assemblage”). A group of trace fossils in a rock unit or facies, usually in a single bed (McIlroy, 2004, p. 18; Buatois and Mángano, 2011). The term does not imply contemporaneity of the tracemakers (Buatois and Mángano, 2011). For example, tracks in a single track horizon may be collectively called a track assemblage even when they accumulated over a long time period (time averaging) and were left by different biological communities. The term trace-fossil suite (or “ichnofossil suite”) can be used instead to imply that the assemblage is roughly contemporary. The similar term ichnoassociation (also “ichnofossil association” or “trace-fossil association”) is not precisely defined and has been used in different ways, and in some cases is a synonym of “ichnoassemblage” (Buatois and Mángano, 2011).
170 Ichnocoenosis (synonyms: ichnocoenose, ichnocommunity). A group of trace fossils produced by a biological community (Buatois and Mángano, 2011).
171 Ichnofacies (plural: ichnofacies). A type of trace fossil assemblage that is defined by key features related to environmental conditions and that recurs over long geological time periods (cf. Buatois and Mángano 2011, p. 58). Ichnofacies are conceptual constructs that have practical value, for example in interpreting depositional settings and as a standard for comparison (Buatois and Mángano, 2011). The ichnofacies paradigm was developed by Adolf Seilacher (e.g., Seilacher, 1964, 1967), who originally named five marine (Skolithos, Cruziana, Zoophyscus, Nereites, Glossifungites) and one continental (Scoyenia) ichnofacies. “True” ichnofacies as originally defined by Seilacher are known as “archetypal ichnofacies” or “Seilacherian ichnofacies”. In contrast, “medium-scale ichnofacies” or “ichnosubfacies” relate to a subenvironment and may be considered part of an archetypal ichnofacies (MacEachern et al., 2012). Ichnofacies are named after a characteristic ichnogenus (e.g., the Scoyenia ichnofacies); such names are sometimes considered to be proper names, in which case they are capitalised (e.g., the Scoyenia Ichnofacies) (MacEachern et al., 2012).
Lockley et al. (1994a) were the first to propose a system of vertebrate ichnofacies (or “tetrapod ichnofacies”). Their ichnofacies are, however, of limited temporal range and were therefore downgraded to ichnocoenoses by Hunt and Lucas (2007). The latter authors instead proposed five “archetypal vertebrate ichnofacies”: Chelichnus (aeolian strata), Batrachichnus (tidal flats and fluvial plains), Brontopodus (coastal plain and marine shorelines), Grallator (marginal lacustrine), and Characichnos (shallow lacustrine). Vertebrate ichnofacies are fundamentally different from invertebrate ichnofacies in that they are based on the taxonomy of the tracemakers rather than their behaviour (for background, see introduction to the section “Ichnotaxonomy”). They are therefore biotaxonichnofacies, while invertebrate ichnofacies are ethoichnofacies (Hunt and Lucas, 2007). Note, however, that vertebrate ichnofacies are not restricted to the range of their namesake ichnotaxon; for example, the Grallator ichnofacies encompasses tridactyl avian and non-avian dinosaurs from the Late Triassic to the present. The concept of vertebrate ichnofacies has repeatedly been questioned. Santi and Nicosia (2008) argued that such ichnofacies may not be specific for particular palaeoenvironments and are therefore of limited practical value, while MacEachern et al. (2012) considered them to be invalid as they are restricted to vertebrates rather than being taxonomically inclusive. Hunt and Lucas (2016) presented a defence of the concept.
172 Ichnofauna. General term used to refer to any group of trace fossils produced by animals, including ichnoassemblages, ichnocoenoses, and ichnofacies (Marty et al., 2016).
173 Ichnofaunal unit. Assemblage of trace fossils produced by animals that is considered a biochronological unit and can therefore be used in biostratigraphy (Conti et al., 1997; Avanzini et al., 2001). A more specific term is “land ichnofaunal unit”.
174 Ichnopopulation. Term sometimes used to describe a regional occurrence of tracks that are presumed to have been made by the same biological population of trackmakers (e.g., Gand et al., 2005).
175 Bioturbation. Displacements within sediments and soils caused by the activity of organisms (Richter, 1936; Flügel, 2004). Bioturbated substrates can also be described as “bioturbation fabrics” or ichnofabrics, which are defined as “all aspects of the texture and internal structure of a sediment that result from bioturbation” (Ekdale et al., 1984).
176 Trampling. A special case of bioturbation resulting from the repeated formation of tetrapod tracks. Note that in other areas of vertebrate palaeontology, “trampling” does not necessarily require the formation of tracks; for example, “trample marks” refer to subparallel gouges on bone surfaces resulting from the trampling of the bone (Fiorillo, 1984).
Trampling is a general term (e.g., Morse et al., 2013), while the specific term dinoturbation has been used when the trackmakers are exclusively dinosaurs. When coining the term, Dodson et al. (1980) restricted it to “large dinosaurs”, although the term has since been correctly used for dinosaur tracks of any size (e.g., Richter and Böhme, 2016). We argue that there is little reason to use a term that specifically excludes non-dinosaurs, and suggest that the more general term “trampling” should take precedence.
Inspired by the ichnofabric index of Droser and Bottjer (1986), Lockley and Conrad (1989) defined the dinoturbation index to quantify the density of tracks on a surface using a scale from 0% to 100% dinoturbation. Here 0-33% is classified as “light”, 33-67% as “moderate”, and 67-100% as “heavy” dinoturbation (Lockley and Conrad, 1989). As with “dinoturbation” itself, the term is restricted to dinosaurs even though the concept is also relevant for other tetrapod groups. Therefore, we suggest using the more general term trampling index.
177 Ichnostratigraphy. Biostratigraphy based on trace fossils. Tetrapod track biostratigraphy has a long tradition but is limited by the accuracy of ichnotaxonomy (see Lucas, 2007, for a review). Time intervals defined based on the temporal distribution of ichnotaxa are known as footprint biochrons (synonym: track biochrons) (e.g., Voigt and Lucas, 2018). An index ichnotaxon is an ichnotaxon that is characteristic and specific to a particular footprint biochron and can therefore be used to date a deposit. The term is derived from the more general term “index fossil”.
178 Ichnofossil-Lagerstätte (plural: Ichnofossil-Lagerstätten). Deposit containing exceptional amounts of ichnological information, either qualitatively or quantitatively (Seilacher et al., 1985; Savrda, 2007). The term “fossil Lagerstätte” was originally proposed for body fossil deposits and is derived from the German term “Lagerstätte”, which is used to describe deposits rich in natural resources (Seilacher et al., 1985). Conservation ichnofossil-Lagerstätten (or “Konservat-Lagerstätten”) are characterised by exceptional preservation, whereas concentration ichnofossil-Lagerstätten (or “Konzentrat-Lagerstätten”) are characterised by exceptional abundance of fossils (Savrda, 2007).
179 Geoheritage. Geological features of significant scientific, cultural, or educational value (see Brocx and Semeniuk, 2007 for a full definition). Geoheritage may include both in situ features (known as geosites) or ex situ features (e.g., museum collections). Geoconservation is the preservation of geoheritage features. The more specific term ichnoheritage has sometimes been used when ichnofossils are the primary feature (e.g., Baucon et al., 2012). Tetrapod tracksites can be an important target for geotourism.
LOCOMOTION AND POSTURE
The study of locomotion and posture from tracks is probably the most interdisciplinary area of tetrapod track research. More than in other areas, traditional ichnological terminology collides with that of biomechanics and zoology. In addition to ichnology-specific terms, we review a wide array of biomechanical and zoological terms that are relevant to tetrapod ichnology, with the aim of providing a standard terminology that is as consistent as possible with usage in these related fields.
180 Flexion and extension. “Flexion” is the bending, and “extension” the straightening, of a joint or body part. In the autopodia, flexion can occur in two directions, with dorsiflexion (or “dorsi-flexion”) pulling the digits upwards and towards the dorsal (upper) surface of the autopodium, and plantarflexion (or, in the manus, palmarflexion; also “plantar flexion” and “palmar flexion”) pulling the digits downwards and towards the plantar (lower) surface of the foot.
181 Protraction and retraction. Protraction is the anterior movement and retraction the posterior movement of a body part. For example, a limb is protracted during the swing phase and retracted during the stance phase.
182 Supination and pronation. Rotation of the forelimb so that the palm faces posteriorly (pronation) or anteriorly (supination) when the forelimb is fully extended and held vertically (cf. Bonnan, 2003). When standing on a straight forelimb, the digits point anteriorly in pronation and posteriorly in supination. The intermediate state, with the palm facing medially, is called semi-supination; this is the basal condition in tetrapods. Many mammals are able to actively pronate and supinate their hands by rotating the radius relative to the ulna, a movement lacking in most other tetrapods (Bonnan, 2003). Sprawling tetrapods have their manual digits pointing anteriorly or even medially due to the sprawling posture of their forelimbs (Bonnan, 2003).
183 Abduction and adduction. The movement of a body part away from the sagittal plane (abduction) and towards the sagittal plane (adduction). For example, when the femur of a sprawling tetrapod is brought into a more vertical (upright) orientation, it is being adducted. When applied to the foot, abduction rotates the foot outwards, and adduction rotates the foot inwards.
184 Inversion and eversion. Inversion is the movement of the foot so that the sole (or palm) faces medially with the body weight on its lateral edge, whereas eversion is the movement so that the sole (or palm) faces laterally with the body weight on its medial edge.
185 Pitch, roll, and yaw. Borrowed from aerodynamics, these terms are sometimes used to describe the rotation of a track about the mediolateral, anteroposterior, and vertical axis, respectively (e.g., Mossman et al., 2003). In other words, “pitch” is the anteroposterior tilt and “roll” is the mediolateral tilt of a track, while “yaw” is a synonym for track rotation. When applied to tracks, these terms have been used to interpret locomotion.
186 Sprawling and erect. In a sprawling posture (rarely: “transversal posture”) the limbs are spread away from the trunk (abducted) because of subhorizontal humeri and femora. This condition is found in basal tetrapods and many reptiles. In contrast, an erect posture (also: “parasagittal” posture) is characterised by adducted limbs positioned below the trunk. Erect limbs are often referred to as “parasagittal limbs”, and sprawling limbs are very rarely referred to as “transversal limbs”. Sprawling and erect postures form the end members of a continuum. An intermediate posture is called semi-erect. Many authors substitute “posture” with “stance” or “gait” (e.g., “sprawling stance”; “erect gait”), which we recommend to avoid because “stance” can be confused with “stance phase”.
Modern crocodylians can use both a sprawling and an erect gait. The sprawling gait can be a slow “sprawling walk” or a fast “belly slide” that is used to escape into the water (Grigg and Kirshner, 2015). The erect gait is termed the “high walk” and is the usual gait on land (Grigg and Kirshner, 2015).
187 Crouched and upright. In a crouched posture, the limbs are bent (flexed), with the distal limb angled against the proximal limb, whereas in an upright posture, the limbs are straight (extended). In hominids and apes, walking in a crouched posture is often referred to as bent-hip, bent-knee walking (e.g., Stern Jr. and Susman, 1983; Wang et al., 2003). “Upright” can also refer to the orientation of the body: Humans have an upright, or “orthograde”, body posture, while most other mammals are “pronograde”, with the long axis of the body parallel to the ground.
188 Stance phase. The period during which a foot interacts with the ground to support, decelerate, accelerate, and manoeuvre the trackmaker during locomotion (e.g., Turner et al., 2022). In contrast, the swing phase is when a foot is lifted to take a step forwards. A stance and swing phase of the same foot together make up a step cycle. While stance and swing phases are readily distinguishable when walking on firm surfaces, they form continua when sinking into deformable substrates (Turner et al., 2022). Mid-stance (=midstance) is the middle of the stance phase; in bipeds it is the point at which the centre of mass is directly above the stance foot. Conversely, mid-swing (=midswing) is the middle of the swing phase, when the foot has travelled half the distance from one footfall position to the next. Note that “stance” has sometimes been used to describe limb postures (e.g., “sprawling stance”). The term support phase is often used as a synonym of “stance phase”, but has also been defined as “the period of time that an entire organism has at least one foot in stance phase” (Struble and Gibb, 2022, p. 6).
In addition to the cross-disciplinary terms “stance phase” and “swing phase”, other terms and concepts have been proposed:
Contact phase - often synonymous with “stance phase”, but slightly different in definition: the phase during which a foot (or a part of it) is in contact with the ground.
Touch-down phase, weight-bearing phase, and kick-off phase - terms introduced by Thulborn and Wade (1989) that are widely used, especially in dinosaur track research. During the touch-down phase (or “T-phase”), the foot makes contact with the ground; during the weight-bearing phase (or “W-phase”), the centre of mass passes over the foot; and during the kick-off phase (or “K-phase”), final propulsion, possibly including slipping of digits, and lift-off occur. However, these phases are not precisely defined because of their continuous nature.
For striding bipeds on deformable substrates, Turner et al. (2022) proposed to subdivide the stance phase (equated to “contact phase”) into five sub-phases based on the interaction with the contralateral foot: time intervals between initial ground contact, maximum sinking depth, and the complete lift-off, for both the left and right foot. This interaction between contralateral feet might have a major impact on track formation (e.g., the foot may sink more quickly once the contralateral foot begins to withdraw). These phases are precisely defined even in cases of deep sinking.
In humans, stance phase may be subdivided into three phases: the heel-strike phase (also “heel-contact phase”, or simply “heel strike”), where only the heel is in ground contact; the mid-stance phase (also simply “mid stance”), where both the heel and the ball and toes are in ground contact, and the propulsion phase, where forward thrust is generated and only the ball and toes are in ground contact (e.g., Roca-Dols et al., 2018). These terms are roughly analogous to Thulborn’s touchdown, weight-bearing, and kick-off phases.
189 Supports (synonym: base). The limbs that are in stance phase at a given time during locomotion. In “single supports” (also “unipedal base”), only one limb is in stance phase, while in “double supports” (also “bipedal support” or “bipedal base”), two limbs are in stance phase. In “triple supports” (also “tripedal base”), three limbs are in stance, while four limbs are in stance in “quadruple supports” (also “quadripedal base”). In quadrupedal locomotion, “diagonal supports” (synonym: “diagonal bipedality”) are two diagonal limbs (e.g., right hind and left fore) in stance phase, while “lateral supports” (synonyms: “unilateral supports”, “unilateral bipedality”) are two ipsilateral limbs (e.g., right hind and right fore) in stance phase. A trot is characterised by diagonal supports during most of the step cycle, whereas a pace gait is characterised by lateral supports.
190 Support polygon (also: stability triangle). In quadrupedal locomotion, the minimum polygon bounding the supports at a given time during the step cycle (Cartmill et al., 2002). Support polygons have been used to estimate the static (instantaneous) stability of a given gait (Cartmill et al., 2002; Henderson, 2006b).
191 Step cycle (synonyms: gait cycle, stride, stride cycle). The limb movements that occur between two successive footfalls of the same hind limb (Hildebrand, 1976). Within a step cycle, each leg will have moved once, and the body will have covered a distance of one stride length. A step cycle includes all sequential limb movements until repetition starts (Hildebrand, 1976). A step cycle could be defined based on any repetitive events; however, the use of a footfall of a hind limb (often the left) as the starting point is convention (see Hildebrand, 1976, for discussion). The duration of one step cycle has been termed a “step cycle duration”, “stride period”, or “stride interval”. The term “step cycle” is also applied directly to tracks: In a biped, one step cycle consists of three consecutive tracks (two left and one right, or vice versa), whereas for a quadruped, a step cycle consists of five or six consecutive tracks. Note that the term stride is a synonym of “step cycle” and also applies to limbless animals (Alexander, 2003). In tetrapod ichnology, “stride” is generally used as a synonym of “stride length” or “stride line”. The term “movement cycle” refers to cyclical movement patterns in general and is not limited to limbed animals.
192 Striding. Term describing a bipedal or quadrupedal gait with alternating limb movements, as opposed to hopping or jumping, where the contralateral limbs move in unison (Hutchinson and Gatesy, 2001).
193 Duty factor (synonyms: duty cycle, stance percentage). The percentage of the step cycle duration that a foot is in stance phase. Duty factors below 50% are characterised by aerial phases in bipeds. The duty factors of the hind- and forelimbs can be different. This difference can be expressed by the duty factor index, which is defined as 100 * hindlimb duty factor / forelimb duty factor (Cartmill et al., 2002). Therefore, duty factor indices greater than 100 indicate that the duty factor of the hind limbs is greater than that of the forelimbs, and vice versa.
194 Step duration. The amount of time that a foot is in stance phase. If the duty factor is 50%, the step duration is approximately half the step cycle duration.
195 Bipedal and quadrupedal. Locomotion on two legs and four legs, respectively. Tetrapods that move bipedally are termed “bipeds”, while those that move quadrupedally are termed “quadrupeds”. The corresponding nouns are “bipedalism” and “quadrupedalism” (synonyms: “bipedality” and “quadrupedality”). In ichnology, a “bipedal trackway” (or a “quadrupedal trackway”) is a trackway left by a trackmaker that walked bipedally (or quadrupedally) (Leonardi et al., 1987). “Obligate bipeds” (or “obligate quadrupeds”) are tetrapods that move exclusively on two (or four) legs. “Facultative bipeds” (rarer: “facultative quadrupeds” or “semibipeds”) are tetrapods that can switch between bipedal and quadrupedal locomotion (Figure 10H) (e.g., Hutchinson and Gatesy, 2001). Note that “quadruped” is not synonymous with “tetrapod”, which is a taxon that includes all amphibians and amniotes regardless of the number of legs.
196 Apparent limb (synonym: apparent member). A straight line from the acetabulum (or glenoid) to the base of the foot, at the time when the acetabula (or glenoids) are midway between the contralateral footfalls and both contralateral feet are in stance (cf. Leonardi et al., 1987). The angle of gait (synonyms: angle of step, walking angle) is the angle between the contralateral apparent limbs when projected onto the sagittal plane (Leonardi et al., 1987). If the angle of gait can be estimated, these concepts allow for estimating limb length and hip height of the trackmaker (see also Thulborn, 1990). These concepts are only defined for symmetrical walking gaits.
197 Aerial phase. The portion of a step cycle in which all feet are in swing phase (and therefore not in ground contact). Synonyms include “airborne phase”, “air phase”, and “suspension phase”, but the latter can be misleading as the body is not suspended by anything when all feet are off the ground. If there is an aerial phase, the duty factor will be less than 50%.
198 Stride frequency (synonyms: cycle frequency, cadence, step rate). The number of step cycles per second (Struble and Gibb, 2022). The unit is hertz (Hz).
199 Speed (synonyms: speed of locomotion, velocity of gait, pace). The velocity of a locomoting animal, measured in unit distance/unit time, usually metres per second (m/s). This parameter has also been termed “absolute speed” to distinguish it from “relative speed”, which is the speed relative to the size of the trackmaker (Thulborn and Wade, 1984). Of interest may be the maximum speeds achieved within a trackway (or sample of trackways), or the average speeds (also: cruising speed, cruise speed, standard speed) that may reflect the preferred walking speed (e.g., Thulborn, 1990) and can be calculated based on the median of the stride lengths. An increase in speed is referred to as “acceleration”, and a decrease as “deceleration” (Figure 10F). A trackmaker can increase speed by increasing stride length, increasing stride frequency, or a combination of both (Granatosky and McElroy, 2022). The consequences of acceleration generally include longer strides and, for quadrupeds, shorter manus-pes distances (the reverse is true for deceleration).
Trackmakers may stop intermittently, which has been called “start-stop walking” by Falk et al. (2017). Weems (2021) used the terms “stopping points” or “pausing points” when referring to such instances of interrupted locomotion in trackways. In trackways, stopping can be evident when the left and right feet are placed side by side. However, modern birds can stop mid-stride without such side-by-side placement, and it may often not be possible to infer such stopping from trackways (Falk et al., 2017; Farlow et al., 2018a, pp. 187, 189).
200 Alexander’s formula. Equation for estimating the speed of locomotion of mammals and dinosaurs that was proposed by Robert McNeill Alexander (Alexander, 1976) based on the dynamic similarity hypothesis, which assumes a linear relationship between relative stride length and Froude number. The equation is
u = 0.25g0.5st1.67h-1.17
where u is the speed of locomotion, g the acceleration of the free fall (9.81 m/s2), st the stride length, and h the hip height. Similar methods have been proposed by Georges R. Demathieu (Demathieu, 1984, 1986).
Alexander’s formula can only be used as a rough approximation of speeds for comparative purposes, not as a precise estimate. We note that Alexander himself stated that “the method cannot claim to be accurate” (Alexander, 2006, p. 1850). It can provide reasonable estimates for quadrupedal mammals with erect limbs that are the size of a domestic cat or larger; it has also been shown to be roughly accurate for large bipeds such as humans and ostriches. Since dinosaurs are comparable in size and posture, it has been suggested that the formula is also applicable to this group (Alexander, 1976, 1989a; Alexander and Jayes, 1983). However, the method may be subject to large errors when applied to sprawling trackmakers, because these trackmakers 1) are not geometrically (and therefore dynamically) similar to mammals with erect legs and therefore follow different relationships (Alexander and Jayes, 1983), and 2) tend to increase speed by increasing stride frequency rather than stride length (Granatosky and McElroy, 2022). Even in erect mammals, a correlation between stride length and speed is not always warranted; for example, in humans, stride length does not increase above a dimensionless speed of 2 (Struble and Gibb, 2022).
201 Relative stride length  (synonym: standardized stride length). Stride length relative to body size, typically defined as stride length / hip height (Alexander and Jayes, 1983). Relative stride length approximates the speed of locomotion relative to body size (its “relative speed”). It is greater in mammals with flexed limbs than in cursorial mammals with straight limbs (Alexander and Jayes, 1983). Modern mammals tend to switch from a walk to a run (i.e., trot or gallop) at relative speeds of around 2.0 (Figure 17E) (Alexander, 1976).
(synonym: standardized stride length). Stride length relative to body size, typically defined as stride length / hip height (Alexander and Jayes, 1983). Relative stride length approximates the speed of locomotion relative to body size (its “relative speed”). It is greater in mammals with flexed limbs than in cursorial mammals with straight limbs (Alexander and Jayes, 1983). Modern mammals tend to switch from a walk to a run (i.e., trot or gallop) at relative speeds of around 2.0 (Figure 17E) (Alexander, 1976).
202 Dynamic similarity hypothesis. Different tetrapods tend to move in a dynamically similar way when walking or running with equal Froude numbers. In general, two systems (e.g., two dinosaurs with different hip heights) are dynamically similar if their movements can be made identical by multiplying “all linear dimensions by some constant factor; all time intervals by another constant factor; and all forces by a third constant factor” (Alexander and Jayes, 1983, p. 136). The dynamic similarity hypothesis allows comparisons between tetrapods of different sizes moving at similar relative speeds. For example, it predicts that mammals of different sizes will transition from a trot to a gallop at approximately the same Froude number (Alexander and Jayes, 1983), although this only applies to mammals that trot and gallop, which is not the case for, for example, elephants, hippos, or giraffes (Hutchinson, 2021). Dynamic similarity theory was first discussed in detail by Alexander and Jayes (1983) and Alexander (1989a).
203 Froude number. Dimensionless number that can be used as a measure of the relative speed of locomotion. When applied to mammals and dinosaurs, it can be defined as the ratio of the speed of locomotion (u) to hip height (h), taking into account gravity (g) (Alexander, 1976):
F = u2/gh
The square root of the Froude number is used as a measure of relative speed and has been referred to as dimensionless speed (Bishop et al., 2018; Struble and Gibb, 2022). According to Alexander (1976), mammals tend to switch from a walk to a run at Froude numbers of around 0.6.
204 Inverted pendulum model. Simplified model to describe walking gaits using vaulting mechanics, where the limb is abstracted as an inverted pendulum, with the hip or shoulder vaulted over the stance foot by a stiff leg (Hutchinson and Gatesy, 2001; Hutchinson, 2021). The hip (or shoulder) reaches its maximum height above the ground at mid-stance, after which the body falls forwards; this acceleration is used to lift the body on the next step, conserving energy (Hutchinson, 2001).
205 Spring-mass model. Simplified model to describe running and hopping gaits using bouncing mechanics, where the limb is compressed during the first half of the stance phase, storing elastic energy which is then released during the second half of the stance phase (Blickhan, 1989; Hutchinson and Gatesy, 2001). In contrast to a walking gait that can be described by an inverted pendulum model, the hip (or shoulder) reaches its minimum height above the ground in mid-stance (Hutchinson and Gatesy, 2001). A gait characterised by bouncing mechanics is termed a bouncing gait.
206 Compliant walk (synonyms: grounded walk, Groucho walk). A walking gait characterised by flexed (bent) limbs that allow for high duty factors (Alexander, 1977; Alexander and Jayes, 1978). Because of this high limb compliance, compliant walking does not follow the inverted pendulum model which assumes stiff limbs. Compliant walking is found in, for example, birds such as quails and non-human primates (Alexander and Jayes, 1978; Schmitt, 1999).
207 Compliant run (synonyms: grounded run, Groucho run, running walk). Running gait characterised by bouncing mechanics and the absence of aerial phases (e.g., Lee and Harris, 2018). This gait may be used during the transition between walking and running (e.g., Rubenson et al., 2004). Elephants use a compliant run at high speeds, in which the contralateral limb pair may lose ground contact, but at least one leg always remains in ground contact (Hutchinson et al., 2003). In horses, the compliant run is known as the “amble”, “tölt” (or “toelt”), or “rack” (Lee and Harris, 2018).
208 Plantar pressure. The pressure exerted by the pes on the substrate. “Palmar pressure” is the pressure exerted by the manus. The topography of a track is often used to reconstruct the pressures exerted by different parts of the foot; i.e., deeply impressed parts are thought to reflect high pressures. However, experiments and simulations on human tracks have shown that the correlation between foot pressures and track shape is not always strong, especially when tracks are deep (Bates et al., 2013). Studies have also equated foot pressures with weight distribution; for example, a deeper impressed medial part of a track would indicate that the medial edge of the foot carries more weight (see also terms and discussion in the entry “principal digit”). However, peak pressures are often applied during kick-off, so the deepest parts of a track may not necessarily reflect the actual weight distribution.
209 Ground reaction force (also: substrate reaction force). Force exerted on the limb during stance phase due to the resistance of the ground. It is equal and opposite to the force exerted by the limb (Hutchinson, 2021). When plotted against time (i.e., % of stance phase duration), the vertical component of the ground reaction force typically shows a biphasic (two-peaked) distribution in walks and a monophasic (single-peaked) distribution in runs (Clayton and Hobbs, 2019).
210 Footfall (also: “strike” of a foot). The instance where a foot touches the ground to end the swing phase during locomotion. Footfall positions are often identical to the corresponding track positions, but the term “footfall” also applies to cases where no tracks are formed.
211 Footfall pattern. The order and timing of footfalls during locomotion. Footfall patterns can be visualised using a footfall formula or, more commonly, a gait diagram. In a footfall formula, light circles often represent limbs in swing phase and dark circles represent limbs in support phase (Muybridge, 1899). Gait diagrams also provide information on the relative duration of the stance and swing phases of each limb (Hildebrand, 1976).
212 Terrestrial (noun: terrestriality). Terrestrial organisms are those that live primarily on the ground (e.g., Rose, 2006). In contrast, aquatic organisms live in the water (marine or freshwater), including natatorial (swimming) animals. Semi-aquatic animals are terrestrial animals with swimming adaptations (e.g., beavers). Arboreal organisms instead live in trees, while scansorial animals live both in trees and on the ground. Volant (or “aerial”) animals are those that fly; these are often also terrestrial, arboreal, or aquatic. Fossorial animals are those that dig burrows or show adaptations for efficient burrowing (Hildebrand, 1974). The term is sometimes reserved for just subterranean species that spend most of the time underground, but typically refers more generally to any terrestrial animal that burrows. Burrowing animals that do not spend most of the time underground are also termed “semi-fossorial” (Rose, 2006). When not referring to the organisms directly, the term “terrestrial” instead refers to any environment on land, as opposed to aquatic environments (e.g., terrestrial trace fossils vs. marine/freshwater trace fossils).
213 Scratch-digging - Digging with the use of claws or nails by extending and flexing the limbs (cf. Hildebrand, 1974). Other methods of digging in tetrapods include chisel-tooth digging (in some rodents), head-lift digging, hook-and-pull digging (in anteaters), humeral-rotation digging (e.g., in moles), and hind-feet-first digging (e.g., in frogs) (Hildebrand, 1985).
214 Cursorial (noun: cursoriality). Morphology adapted for running (Carrano, 1999). An animal with cursorial adaptations is called a “cursor”. The term has also been defined to refer to locomotory performance rather than morphology (see Carrano, 1999). However, tetrapods that can run fast or frequently do not necessarily have cursorial adaptations, especially smaller species (Carrano, 1999). Typical cursorial adaptations include a slender skeleton and elongated distal limb bones. In the foot, reduction or loss of non-central digits and overall shortening of the foot are often observed in cursorial taxa (Schaller et al., 2011). In tridactyl tracks, digit III projection might be correlated with cursoriality (Lallensack et al., 2020). Cursoriality and graviportality are end members of a continuum, and states are sometimes referred to as “subcursorial” (closer to the cursorial state) and “mediportal” (closer to the graviportal state).
215 Saltatorial. Morphology adapted for jumping or hopping (e.g., Rose, 2006). Saltatorial animals are known as “saltators”.
216 Graviportal (noun: graviportality). Morphology adapted to support large body masses. Graviportal adaptations may include columnar limb and foot structures (e.g., Moreno et al., 2007) and a more robust appendicular skeleton. As shown by Biewener (1989), limbs become increasingly straight to maintain constant stress up to a mass of 300 kg in modern mammals. Above this threshold, the shape of the bones (e.g., thickness) changes to maintain constant stress.
217 Gait. Repetitive pattern of body movements that advances the position of an animal (cf. Alexander, 1989b; Struble and Gibb, 2022). The term is most commonly applied to patterns of terrestrial locomotion on legs, but it has also been used to describe other types of repetitive locomotion patterns, such as swimming in cephalopods or flight (Alexander, 1989b; Struble and Gibb, 2022). In the ichnological literature, the meaning of “gait” is often vague and the term has also been used to refer to posture (e.g., “narrow-gauge gait” and “bipedal gait”). A more general term that does not necessarily involve repetitive body movements is mode of locomotion (synonyms: “locomotor mode”, “manner of locomotion”); for example, bipedal and quadrupedal animals have bipedal and quadrupedal modes of locomotion, respectively. In the following, we will discuss the terrestrial gaits of tetrapods, using the term “gait” to refer to the repetitive pattern of limb movement rather than posture.
Tetrapod gaits are often characterised as either symmetrical or asymmetrical, and as either walking or running. Gaits can also be classified according to their footfall pattern. The exact definition of “walking” and “running” is controversial, and gait terminology has been confusing and contradictory in many areas of biology (Biknevicius and Reilly, 2006), including the study of fossil tracks. In quadrupeds, gait terms such as “trot” and “pace gait” have historically been reserved for running gaits, especially when applied to mammals. We follow recent calls for a consistent terminology across disciplines, which suggest that gait terms such as “trot” and “pace gait” should be reserved for footfall patterns (i.e., limb phase) only (Biknevicius and Reilly, 2006). Consequently, slow walking gaits of the same limb phase, as typically observed in reptiles and amphibians, must be classified as “trots”. We generally follow the terminology of Hildebrand (1965, 1976, 1980, 1989), which has become a consensus across different fields (Biknevicius and Reilly, 2006; Pfau et al., 2011).
218 Symmetrical gaits. Gaits in which the footfalls of the left and right limbs are evenly spaced in time (i.e., half a step cycle out of phase). This is the condition seen in striding tetrapods. In asymmetrical gaits, the footfalls of the left and right limbs are not evenly spaced, such as in a gallop or in a limp (Hildebrand, 1989; Struble and Gibb, 2022). Symmetrical gaits are the most common gaits in tetrapods, while asymmetrical gaits are entirely absent in some extant groups, such as lizards, monotremes, and salamanders (McElroy and Granatosky, 2022). Note that Struble and Gibb (2022) use these terms in a different sense, with “symmetrical gaits” describing gaits in which the left and right limbs of a girdle move in synchrony (e.g., in hopping gaits), and “asymmetrical gaits” describing those in which the limbs move out of synchrony (e.g., in striding tetrapods). In ichnology, the term “symmetrical gait” has also been used to describe the “ladder-like” (Clack, 1997) trackway pattern of arthropods, where left and right tracks are placed next to each other rather than arranged in the zigzag pattern typical of striding tetrapods, which has conversely been termed an “asymmetrical gait” (Lucas, 2015).
219 Walking gait and running gait (synonyms: walk and run). These terms have been defined in various ways:
Traditionally, runs have been defined by the presence of an aerial phase. However, while all walks lack an aerial phase, there are some fast gaits such as Groucho runs that also lack an aerial phase.
More recently, walks and runs have been defined based on patterns of energetic fluctuations (see Hutchinson and Gatesy, 2001, for a brief introduction). Here, walks are defined as gaits that follow the inverted pendulum model, while runs are defined as gaits that follow the spring-mass model. However, for unclear reasons, sprawling tetrapods do not closely fit any of these models (Struble and Gibb, 2022).
Struble and Gibb (2022) proposed a comprehensive definition of the term “walk” based on a combination of several criteria, including, amongst others, the absence of aerial phases, the typical presence of inverted pendulum mechanics, and a dimensionless speed less than 1.
We note that none of the above definitions can be met using data from fossil trackways, and the use of “walking” and “running” when referring to the trackways of extinct taxa will necessarily be informal.
220 Limb phase (synonyms: relative phase, limb phasing, lateral advanced placement, diagonality, inter-paired-appendage phasing). In symmetrical gaits of quadrupeds, the percentage of the step cycle duration that the footfall of a forefoot follows that of the ipsilateral hind foot (Figure 15) (Hildebrand, 1965). The term therefore describes the relative timing of the footfalls of the fore- and hind feet. Note that limb phase could also be defined based on other leg pairs (e.g., a hind foot and the diagonal forefoot), but the above definition is convention (Hildebrand, 1976). Gaits in which two footfalls occur at the same time are also known as two-beat gaits (two beats per step cycle). This is the case in the trot (limb phase = 50%), where diagonal footfalls occur simultaneously, and in the pace gait (limb phase = 0% or 100%), where ipsilateral footfalls occur simultaneously. In all other gaits, four separate footfalls occur at different times, resulting in a four-beat gait. A four-beat gait in which all four footfalls are evenly spaced in time (i.e., at limb phases of 25% and 75%) is termed a singlefoot. Methods for calculating limb phase from trackways have recently been proposed (Lallensack and Falkingham, 2022; Polet and Hutchinson, 2022; Stevens et al., 2022).
Limb phase is the only measure that defines quadrupedal gaits such as trot, pace gait, and singlefoot (Figure 15A) (Biknevicius and Reilly, 2006). Following Cartmill et al. (2002), four ideal gaits can be defined based on limb phase: the pace gait (0% and 100%), the lateral-sequence singlefoot (25%), the trot (50%), and the diagonal-sequence singlefoot (75%). Gaits with limb phases less than 50% are termed lateral sequence gaits, while those with limb phases greater than 50% are termed diagonal sequence gaits. In a lateral sequence gait, the footfall of a hind foot is followed by that of the ipsilateral forefoot, whereas in a diagonal sequence gait, it is followed by the footfall of the diagonal forefoot. Furthermore, limb phases between 25% and 75% are termed diagonal couplet gaits because the diagonal footfalls are closer in time, and limb phases between 0% and 25% and between 75% and 100% are termed lateral couplet gaits because the ipsilateral footfalls are closer in time. It follows that four gait intervals can be distinguished (Cartmill et al., 2002) (Figure 15A): Lateral sequence lateral couplet gaits (or LSLC gaits; limb phases 0-25%); lateral sequence diagonal couplet gaits (or LSDC gaits; limb phases 25%-50%); diagonal sequence diagonal couplet gaits (or DSDC gaits; limb phases 50%-75%), and diagonal sequence lateral couplet gaits (or DSLC gaits; limb phases 75%-100%). Note that Hildebrand (1965) originally subdivided the possible limb phases (0-100%) equally into eight gaits that a trained observer could distinguish by eye, with the trot, for example, occupying limb phases between 43.75% and 56.25%. However, this categorisation is arbitrary, and the majority of studies follow the definitions of Cartmill et al. (2002) presented above. In the following, we review previous use of some of the gait terms mentioned.
221 Trot (also: “trotting gait”). Symmetrical quadrupedal gait with a limb phase of around 50%, in which the diagonal limb pairs move approximately in synchrony (Figure 15B). A “trotting” animal is termed a “trotter”. In mammals, the trot is often used at faster speeds (intermediate between a walk and a gallop) and involves bouncing mechanics and is therefore also known as a running trot. In the past, the term “trot” was often reserved for the mammalian running trot. However, a walking trot does exist as well, and the term “trot” is now commonly used to refer only to the footfall pattern and therefore applies to both runs and walks (Biknevicius and Reilly, 2006). Leonardi et al. (1987) used the term “primitive alternate pace” instead of “walking trot”. More rarely, the terms “alternating gait”, “walk in diagonal sequence”, and “lateral sequence walking” have been used to describe walking trots. In horses, a slow and relaxed (running) trot is called a “jog” (Hildebrand, 1965). The “fox trot” in horses is a lateral sequence diagonal couplet gait, i.e. the diagonal limbs are not fully in synchrony (Clayton and Bradbury, 1995). Some carnivorous mammals may show a “side trot”, in which the manus are placed on one side of the trackway midline and the pedes on the other (Halfpenny, 1986).
222 Pace gait (also: “pacing gait”). Symmetrical quadrupedal gait with a limb phase of around 0% (or 100%), in which the ipsilateral limb pairs move approximately in synchrony (Figure 15B). In the literature, the pace gait is often referred to simply as the “pace”, but in the ichnological literature, this term is also a synonym for “pace length” and “pace line” (i.e., the distance/line between two consecutive left and right tracks). Also note that “pace” is also a synonym for “speed“ (e.g., “a steady walking pace”). A “pacing” animal is called a “pacer”. Leonardi et al. (1987) used the term “amble” instead of “pace gait”. In horses, “amble” describes different types of four-beat gaits, but its definition is vague (Hildebrand, 1965). Note also that the “primitive alternate pace” of Leonardi et al. (1987) refers to a walking trot. In modern animals the pace gait is relatively rare but can be found, for example, in camels (Hildebrand, 1976). In larger animals, pace gaits appear to be restricted to narrow-gauged trackmakers because they would cause lateral swaying of the body at wider gauges (Lallensack and Falkingham, 2022; but see Sellers et al., 2013).
223 Singlefoot (synonym: square gait). Symmetrical quadrupedal gait in which the time intervals between all four footfalls are approximately equal (Figure 15B). The singlefoot occurs at limb phases of around 25% and 75%. At 25%, it is termed the lateral-sequence singlefoot. In horses, this gait is known as a “walk” for slow gaits, and as a “running walk”, “rack”, “paso”, “amble”, or “tölt” (or “toelt”) for runs (which often lack aerial phases, see groucho run). The diagonal-sequence singlefoot, at around limb phase 75%, is rarely used by extant tetrapods; however limb phases between 60% and 75% are used by muntjacs (Muntiacus), duikers (Cephalophus), and many primates (Hildebrand, 1976; Cartmill et al., 2002).
224 Hildebrand diagram. A plot of limb phase versus duty factor, the two primary variables used to describe symmetrical gaits (Figure 15A). When the duty factor in fore and hind limbs is unequal, a third variable is required to fully characterise the gait (see also duty factor index), and modified versions of the diagram have been proposed to deal with this complexity (Hildebrand, 1976). The diagram is named after gait analysis pioneer Milton Hildebrand.
225 Leading foot. In asymmetrical gaits, the leading foot is the last of a contralateral pair to contact the ground. Conversely, the trailing foot is the first of a pair to contact the ground (Hildebrand, 1977). Additional terms are specific to quadrupedal gaits and are discussed in the following.
226 Gallop. Quadrupedal gait in which both the fore and hind limb pairs show a lead (Hildebrand, 1977); i.e., all four footfalls are unevenly spaced in time (McElroy and Granatosky, 2022). In equestrian terminology, the terms “canter” and “lope” describe slower variants of the gallop. A gallop in which the ipsilateral limbs have the same lead is termed a transverse gallop, while a gallop in which the diagonal limbs have the same lead is termed a rotary gallop (Hildebrand, 1977). Gallops can furthermore be subdivided into single suspension (one aerial phase per step cycle) and double suspension (two aerial phases per step cycle) (Struble and Gibb, 2022). Note that the fastest terrestrial gait of modern crocodylians is commonly referred to as a “gallop” (Zug, 1974), but is actually a bound (Grigg and Kirshner, 2015).
227 Bipedal hopping (synonyms: bipedal hop, ricochet, ricocheting, bipedal saltatory gait, in-phase hopping). Bipedal bouncing gait in which the footfalls of the hindfeet occur in unison, such as in kangaroos (“in-phase hopping”). Bipedal hopping trackways are rare in the fossil record (but see Lockley and Milner, 2014; Leonardi, 2021b).
228 Skipping (synonyms: out-of-phase hopping). Bipedal bouncing gait in which the footfalls are unevenly spaced in time (Figure 17F). Skipping is mechanically similar to the gallop of quadrupeds, and is found in, e.g., some birds such as crows and in children that are about 4.5 years old (Minetti, 1998). Skipping gaits are possibly recorded by some fossil synapsid trackways (Brasilichnium) of the Botucatu Formation (D’Orazi Porchetti et al., 2017).
229 Bound (synonyms: bounding gait, quadrupedal hop, quadrupedal hopping gait). Quadrupedal gait in which the footfalls of the two hind limbs occur simultaneously and the same is true for the forelimb footfalls, but with a time difference between the hind- and forelimb footfalls. Therefore, there is no lead in either the hind limbs or the forelimbs (Hildebrand, 1977). Examples of proposed fossil bounding trackways include those of frogs and rodents (e.g., Lockley and Milner, 2014).
230 Half-bound. Quadrupedal gait in which the forelimbs show a lead but the footfalls of the hind limbs are simultaneous (Hildebrand, 1977). The reverse condition (i.e., the lead is expressed only in the hind limbs) is termed a crutch walk (Hildebrand, 1977).
231 Pronk (synonym: stott). Quadrupedal gait in which all four footfalls occur at the same time (Hildebrand, 1977).
232 Bottom walk. Walking on the bottom of a body of water while fully or partially submerged (e.g., Grigg and Kirshner, 2015, p. 151). A related subaqueous mode of locomotion is the punt (synonym: half-swimming), which involves phases of suspension. In crocodylians, bottom walking and punting cannot be considered distinct gaits (Farlow et al., 2018b). Despite its slow speed, punting is not a true walking gait due to the presence of phases of suspension (Struble and Gibb, 2022). Bottom walks and punts might be relatively common in the fossil track record (Farlow et al., 2018b).
233 Limbless locomotion. Progression without the use of limbs. Several groups of amphibians and reptiles have strongly reduced or lost their limbs, including caecilians and several groups of salamanders and squamates, as an adaptation to either aquatic environments or environments that provide little space (Wake, 2001). “Serpentine progression” has been used as a synonym, but is discouraged here since “serpentine” refers specifically to snakes, not to limbless animals in general. Crawling traces are traces produced during limbless locomotion. We note that this term is also used for traces of invertebrates such as snails and arthropods, where it is not necessarily restricted to limbless locomotion. The mode of locomotion is termed “crawling” or a “crawl”. Modern crawling traces have also been referred to as “tracks” (e.g., “the track of a snake”), but we here suggest using the more general term “trail” instead (e.g., “the trail of a snake”).
Four main types of limbless locomotion are distinguished in modern long-bodied tetrapods (Gans, 1970; Wake, 2001):
Undulation (synonyms: “lateral undulation”, “serpentine crawling”) uses alternate mediolateral bending. The body bends around an irregularity (“contact point” or “anchor point”) of the substrate surface to generate a forward thrust. This bend passes down the body as the animal moves. Multiple simultaneous contact points create a single irregularly undulating path that the entire body follows. Undulation is plesiomorphic (inherited) in tetrapods and is found in all limbless members of this group (Gans, 1970; Wake, 2001). Undulatory progression within dry sand, as found in some reptiles, is known as sand swimming (e.g., Catena and Hembree, 2014).
Concertina locomotion (also: “concertina crawling”) is used when the substrate surface provides insufficient anchor points (surface irregularities) to generate forward thrust. Here, the anteriormost part of the body forms an S-curve to generate friction against which the animal can push itself forwards. This S-curve then travels down the body as the animal moves, and a new S-curve is formed behind the head as the previous S-shape at the end of the body dissolves (Gans, 1970; Wake, 2001). The principle of concertina locomotion resembles the two-anchor crawling (Alexander, 1982) described for non-tetrapods such as geometer moth caterpillars (Geometridae) and lungfish (Falkingham and Horner, 2016).
Rectilinear locomotion allows for steady forward progression in a straight line. Parts of the body contract to brace against the ground while other parts are being stretched. These contracted parts pass continuously down the body (Gans, 1970; Wake, 2001). In some invertebrates such as snails, this mode of locomotion has been termed “pedal locomotion” (Alexander, 2003).
Sidewinding is a rapid and efficient mode of locomotion found only in snakes. In sidewinding, the entire body is oriented almost 90° to the direction of travel. Only part of the body length contacts the ground, while the rest is arched. Where contact does occur, the body is oriented about 60° to the direction of travel, creating a very wide trace that increases the chances of encountering surface irregularities that the snake can press against to generate forward thrust. The trace is very distinctive, with a series of single, well-defined and straight impressions as long as the snake itself (Gans, 1970). The term “track” has been used to refer both to the series of impressions as a whole (“trail” in our use), and to the individual impressions themselves.
234 Galumphing. Term sometimes used to describe the slow undulatory progression of earless seals (Phocidae) on land that does not involve much use of the flippers (e.g., Fuiman et al., 2021).
235 Digital arcade. The upward arching of the digits during most of the stance phase, which is found in most synapsids, including modern mammals, but not in extant reptiles (Kümmell and Frey, 2012). This arching is due to flexion of the middle phalangeal joint. As a result, only the distal portions of the metapodials and digits are deeply impressed, while the middle portion of the digits is weakly impressed or not impressed at all. This feature is evident in many fossil tracks attributed to synapsids, such as Dimetropus (Kümmell and Frey, 2012).
236 High-stepping. Drawing the foot closer to the body than normal. This may be done to increase foot clearance while walking over obstacles or through soft sediment. The high-stepping (or “goose-stepping”) in modern plovers is a courtship behaviour in which the male approaches the female by taking very short steps with feet lifted high, which produces a characteristic trackway pattern (Elbroch and Marks, 2001, p. 103).
237 Knuckle-walking. Walking on flexed manual digits so that the dorsal surface of the digits is in ground contact. A “knuckle-walker” does not actually walk on the knuckles (i.e., phalangeal joints), but rather on the dorsal surfaces of the middle phalanges (Wunderlich, 2022). Knuckle-walking is best known from some extant and extinct xenarthrans (e.g., Toledo and Arregui, 2023), as well as from gorillas, bonobos, and chimpanzees, which in the past led to controversy over whether or not humans evolved from a knuckle-walking ancestor, an idea that has now been refuted (Kivell and Schmitt, 2009).
BEHAVIOUR, PALAEOECOLOGY, AND ASSOCIATED TRACES
238 Subaqueous track. Track that formed under water cover (Morales, 1987). In contrast, a subaerial track formed when the tracking surface was exposed to the air. Da Silva et al. (2008) categorised Late Triassic lacertoid footprints from Brazil as “underwater tracks”, “semi-aquatic tracks”, “semi-terrestrial tracks”, “wet-substrate tracks”, and “damp substrate tracks”.
239 Swimming tracks (also: swim tracks, swim traces, paddling traces, paddling tracks). General term for tracks made by punting trackmakers or, incidentally, by fully buoyant (suspended) trackmakers using other swimming gaits (Figure 10D-E). The latter include “paddling” (use of limbs for propulsion) and “undulation” (use of the tail or the whole body for propulsion) (e.g., Sadlok and Pawełczyk, 2021). McAllister (1989) proposed the term “footmark” for swimming tracks, with three or more footmarks forming a “traceway”. This terminology has been adopted by a number of ichnologists, but the general terms “track” and “trackway”, respectively, remain more common and are preferred here. McAllister (1989) and McAllister and Kirby (1998) proposed additional terms specific for swimming tracks. These include:
Reflectures: changes in the orientation of the digit traces in a track. Single reflectures document a single change in direction and can result in “V” or “C” shaped traces. Double reflectures indicate two changes of direction within the same track and can result in “S” or “Z” shapes. Such traces, sometimes referred to as “Z-traces” (McAllister and Kirby, 1998), can form when the foot is moving backwards, then forwards, and then backwards again (Thomson and Lovelace, 2014; Milner and Lockley, 2016).
Kick-off scours (or kickoff scours): irregular and indistinct grooves posterior to the track, apparently excavated by water eddies formed by the foot’s withdrawal from the sediment (McAllister and Kirby, 1998; Thomson and Lovelace, 2014).
240 Takeoff trace (=take-off trace). Trace recording the take-off of a flying animal such as a bird or pterosaur (Leonardi et al., 1987) (Figure 17F). In contrast, a landing trace records the landing of a flying animal (Leonardi et al., 1987). Such traces are very rare in the fossil record but see Genise et al. (2009) and Mazin et al. (2009) for examples. Falk et al. (2017) described takeoff and landing traces in modern chickens and observed that they were deeper than tracks left during walking and not always characterised by a side-by-side placement of the feet.
241 Integument (also: integumentary system). In tetrapods, the skin and its appendages such as scales, hair, and feathers. When not covered by such appendages, the skin is “bare” (or “naked”), directly exposing its outer layer, the epidermis. However, bare skin, especially of the plantar/palmar surfaces of the feet, may be keratinized (synonym: cornified) for robustness. Scales (or more precisely “epidermal scales”, as opposed to the dermal scales of teleost fish) are found in modern reptiles and birds. Hair, feathers, and similar structures are generally referred to as “filaments” (Campione et al., 2020). Feathers are complex filaments with a central shaft (the rachis) that gives rise to multiple branches (the barbs), which may themselves be branched (forming barbules). An extensive covering of feathers and/or filaments is known as “plumage” (Hendrickx et al., 2022), while an extensive covering of hair is known as “fur”. The feet of some birds (e.g., willow ptarmigan, see Höhn, 1977) and mammals are densely covered with feathers or hair, and feather and fur impressions may be present in tracks (e.g., Elbroch, 2003, pp. 41, 166). Possible feather and hair impressions have been reported from the fossil track record, but such identifications remain difficult to confirm (Retallack, 1996; Kundrát, 2004).
242 Skin impression (synonym: skin texture). Impression showing the texture of the bare skin or the scalation (scale bearing skin; also: “squamous skin”) (Figure 7M, Figure 16). Skin impressions may be left by the plantar (or palmar) surface of a foot, or by other parts of the body in contact with the ground, particularly in resting traces. Skin impressions can demonstrate that a particular track is a true track, but do not always correlate with clear track morphology, especially when tracks are very shallow (Figure 16D).
Impressions of bare skin can appear as folds, ridges, and grooves (Figure 16A, B). One of the most conspicuous are impressions of flexion creases. Flexion creases are found in areas where flexion occurs, such as beneath the metacarpo-phalangeal joints of the human hand. Depending on their location, digital flexion creases (or “interpad creases”) and plantar (or palmar) flexion creases can be distinguished. Flexion creases have been reported in fossil tracks (Figure 16B) (e.g., Calábková et al., 2023).
Scale impressions may document “imbricated” (also “imbricate”; overlapping) or non-imbricated scales. The shape of scales can be described as “irregular” (no obvious geometry) or “polygonal” (geometry of three or more sides) (Hendrickx et al., 2022). Separate types of scales are distinguished in the feet of dinosaurs and birds, which are described using avian terminology (Hendrickx et al., 2022). The plantar surface of birds and dinosaurs has small, non-imbricating, circular or polygonal scales known as reticulate scales. A skin impression of multiple reticulate scales has also been termed a “reticulate array” (Gatesy, 2001). The dorsal surfaces of the toes and the anterior surface of the tarsometatarsus are typically covered with large, sub-rectangular, and regularly arranged scales known as “scutate scales”, or “scutes”. Similar but smaller scales are known as “scutellate scales” or “scutella” (Lucas and Stettenheim, 1972; Stettenheim, 2000; Hendrickx et al., 2022). The specialised scale layer covering the feet of birds and at least some theropod dinosaurs (Cuesta et al., 2015) is known as the podotheca (Stettenheim, 2000).
243 Striations (synonyms: striae, striation marks, scratch lines). Narrow grooves in a track that are typically parallel to each other and record the movement of the foot while in contact with the substrate. Striations may be formed by scales or other features of the foot, or by sediment particles attached to or dragged by the foot. Striations formed by scales have also been termed “scale striations” or “scale scratch lines” (Milner and Lockley, 2016). Entry striations are those formed as the foot enters the substrate, whereas exit striations are formed as the foot withdraws (Gatesy, 2001). Striations are sometimes classified as a type of skin impression (Gatesy, 2001); however, they form by the movement of the skin against the substrate and can therefore be more aptly referred to as striation “marks” rather than striation “impressions”.
244 Ichnopathology. A pathology of the tracemaker inferred from its traces (McCrea et al., 2015). The term also refers to the study of such pathologies, as a subdiscipline of palaeopathology (the study of fossil pathologies). Pathologies have been inferred both from morphological features of individual tracks (especially when repetitive in a trackway) and from the trackway pattern. For example, track features interpreted as pathologies in dinosaurs include swellings, extreme curvature, and lack of particular digit impressions (McCrea et al., 2015). Such pathologies can be difficult to distinguish from features that are unrelated to foot shape - for example, trackways of modern birds may consistently lack one of the main digits under certain conditions (Figure 17E) (Richter and Böhme, 2016, fig. 17.7; Lallensack, pers. obs.). Pronounced differences in the right and left step lengths (Figure 17A) may possibly indicate a limp, with the longer step associated with an injured foot or leg (Lockley et al., 1994b). However, in most cases, such asymmetries are more likely the result of laterality, or footedness - the trackmaker’s preference of one foot over the other (Figure 17A). McCrea et al. (2015 p. 246) therefore proposed to use the general term irregular gait when there are “irregularities in the pace or stride values”, and the more specific term “limping gait” when the irregularity can be clearly associated with a pathology. Note that differences between the right and left step lengths may also be caused by tectonic deformation (Figure 5C) (Schulp, 2002).
245 Posterior mark. Any extension posterior to the expected impression of the foot (Lallensack et al., 2022b). Posterior marks include drag marks, retro-scratches, metatarsal marks, and traces of uncertain origin. Tridactyl dinosaur tracks with long metatarsal marks have been termed elongate tracks (Kuban, 1989b). Such tracks have been interpreted as evidence for facultative plantigrade locomotion (Kuban, 1989b), but in most cases are the result of deep penetration of the foot into soft sediment followed by sediment collapse (Lallensack et al., 2022b).
246 Drag mark (synonyms: drag trace, smear mark, smears). Trace that records the dragging of a body part across or through the substrate (Figure 7D, Figure 17B-D). Unlike scratch marks, drag marks are generally formed by dragging in the direction of travel. Common types include digit drag marks (or digit drags), tail drag marks (or tail drags), and belly drag marks (or belly drags). Drag marks of digits or of the whole foot can form both when entering and exiting the substrate and can extend from one track to the next. Similar, but less commonly used terms are “skim mark” and “skid mark”. Allen (1997) described “skim marks” as long traces left by “part of the bent foot before a descent into deep mud” (Allen, 1997, p. 484) and “skid marks” as slipping of the whole foot “in a soft but thin layer, before engaging with and descending into firmer sediment below” (Allen, 1997, p. 484). The exact distinction between these types is unclear, and, given their inconsistent use in the literature, we consider them as synonymous with “drag marks”. The term “scrape mark” has been used as a synonym for both “drag mark” and “scratch mark”. Note that in sedimentology, “drag mark” has also been defined as a type of tool mark (Dżułyński and Walton, 1965).
247 Scratch marks (synonyms: scratches, scratch traces, scrape marks). Grooves formed by the digit when the limb (or parts of it) is retracted, e.g., when scratch-digging, swimming, climbing, or kicking off the ground. “Scratch mark” is sometimes treated as a synonym of “drag mark” (e.g., Allen, 1997). Thulborn and Wade (1989) defined the term retro-scratches for scratches that extend beyond the rear wall of the track as the foot slipped backwards during kick-off. Many modern birds, particularly shorebirds, excavate nest scrapes (or simply “scrapes”), which are relatively simple depressions in the ground. In plovers, males construct multiple nest scrapes as a part of their courtship display. Lockley et al. (2016) described fossil scrapes associated with tracks of non-avian theropods, which might indicate similar nest scrape display behaviours in dinosaurs.
248 Slip mark (synonym: slide mark). Trace documenting the slipping of a foot on a wet surface (Figure 8C, Figure 11E). Typically found as a posterior mark merging into the track itself. Slip marks are often broad with an indistinct posterior margin, asymmetrically curved and sometimes angled against the long axis of the track, and may show striations aligned with the direction of sliding (Lallensack et al., 2022b).
249 Traction. The friction between the foot and the substrate. Modern tetrapods may have adaptations to increase traction to reduce slipping on wet substrates, such as furrows covering the keratinous pads of elephants (Figure 16A) or scales on the sole area of tortoises; such adaptations may potentially be preserved in fossil tracks (Hall et al., 2016).
250 Tail traces. Two types of tail traces can be distinguished: tail impressions and tail drag marks (or “tail drags”) (Platt and Hasiotis, 2008; Kim and Lockley, 2013). Tail drag marks document the dragging of the tail across the substrate, whereas tail impressions show no evidence of forward movement (e.g., in resting traces). Platt and Hasiotis (2008) proposed quantifying tail drag marks based on two metrics: the “percent interruption metric” (the amount of interruption of the tail trace) and “sinuosity” (the amount of curvature of the tail trace). These metrics approximate the vertical and lateral tail motion, respectively, and can therefore inform about trackmaker locomotion. Kim and Lockley (2013) preferred more general and qualitative descriptors to describe the “path” of the tail trace, which can be “straight”, “irregular”, or “sinuous”. Tail drag marks may show various ornamentations (repetitive markings within the trace), most conspicuously chevron patterns (or “herringbone striae”) which possibly form when sediment is dragged into the trace by the tail (Rainforth, 2002; Kim and Lockley, 2013).
251 Resting trace (synonyms: crouching trace, sitting trace, cubichnia). Trace documenting resting of the trackmaker, usually by crouching or lying down (Figure 10G). Resting traces are generally (but not necessarily) characterised by a side-by-side arrangement of the tracks rather than a zig-zag arrangement, and the impression of body parts that are not normally in ground contact, such as tail impressions, manus impressions (in bipeds), metatarsal impressions (in digitigrade trackmakers), or ischial callosity impressions (Lockley et al., 2003, p. 200; Milner et al., 2009).
252 Ischial callosity impression. The impression of the soft tissue bulging around the distal end of the ischium (Figure 10G). Ischial callosity impressions (sometimes simply referred to as “ischial callosities”) are sometimes found in dinosaur resting traces. Less commonly, the term “callosity impression” is used to refer to impressions of bulge-like soft tissues within a track (Lockley et al., 2003; Milner et al., 2009).
253 Feeding trace (synonym: foraging trace). Traces documenting the feeding activity of the trackmaker may be associated with tracks, particularly bird tracks. Bird feeding traces include peck marks, formed by pecking at the sediment surface, and probe marks, formed by straight insertion of the beak into the sediment (see Falk et al., 2010, and references therein).
254 Palmate track. Track in which the interdigital space (the area between the digit impressions) is depressed (Figure 7E). Palmate tracks may indicate the presence of extensive webbing, i.e., interdigital webs (membranes between the digits) that may have been used for swimming. However, palmate tracks can also form as a result of sediment failure, which can be difficult to distinguish from actual webbing in isolated tracks (Falkingham et al., 2009). A track showing evidence of a “webbed foot” is also termed a “webbed track”.
A distinction is made between “distal webs” which cover much of the interdigital space; “mesial webs” which reach only to the mid-lengths of the digit impressions; and “proximal webs” which are restricted to the area close to the hypexes (e.g., Halfpenny, 2019). Furthermore, the term semipalmate (=semi-palmate) is used when the web extends only between the proximal part of the digits (Proctor and Lynch, 1993). In birds, the terms “palmate” and “semi-palmate” are used when digits II, III, and IV, but not digit I, are connected by webs. In cases where an additional web is present between I and IV, the condition is termed totipalmate (=toti-palmate) (Proctor and Lynch, 1993). The condition where digits are widened by skin lobes (also “fringes”), but the lobes of adjacent digits are not connected, is known as lobate; such feet are found in grebes (Proctor and Lynch, 1993).
255 Gregariousness. The association of trackmakers in groups (Figure 8A). Thulborn (1990) referred to groups of herbivorous dinosaurs as herds and to groups of theropods as packs; groups of birds are often referred to as flocks (e.g., Yang et al., 1995). The occurrences of multiple sub-parallel trackways has been taken as evidence of gregariousness (Ostrom, 1972). However, such interpretations are rarely unambiguous, and similar trackway orientations could also be due to the presence of physical barriers (e.g., lake shores) combined with time averaging (Myers and Fiorillo, 2009; Getty et al., 2015). Features such as similar impression depths may indicate that the tracks were made at approximately the same time (Lockley, 1991), but cannot rule out possible intervals of time between the presence of independent trackmakers (Myers and Fiorillo, 2009). Additional lines of evidence for gregariousness may include side-by-side trackways that rarely cross (Currie, 1983) or show consistent intertrackway spacing (distance between parallel trackways). Interaction between trackmakers may also be evident, for example when trackways change course in unison (Currie, 1983). Cotton et al. (1998) also required that the speed of locomotion inferred from trackways must be consistent.
256 Stampede. Event of sudden escape of a larger group of trackmakers. The only stampede so far described from the fossil track record is Lark Quarry, Australia (Thulborn and Wade, 1984); this interpretation of the site has been controversial (Romilio and Salisbury, 2011, 2014; Romilio et al., 2013; Thulborn, 2013, 2017; Lallensack et al., 2022d).
257 Tool mark. A mark on the sediment surface left by an object (organic or inorganic) that has been passively moved by a current (Figure 12C). The object responsible is called a tool. Tool marks are a type of sole mark and erosional structure. In sedimentology, different types of tool marks have been defined, such as groove marks (long, straight, and through-like), chevron marks, prod marks, and roll marks, among others (Dżułyński and Walton, 1965). Reineck and Singh (1980) also classified impressions of stationary objects as well as obstacle marks (deposition or erosion of sediment behind an object due to deflection of the current flow) as a type of tool mark. Note that anthropic traces such as chisel marks associated with tetrapod tracks have also been described as “tool marks”.
258 Ichnodiversity. The ichnotaxonomic richness, i.e., the number of ichnotaxa (typically at the ichnogenus level) present in a sample (Figure 9A) (Buatois and Mángano, 2013). The number of individuals per ichnotaxon has been termed ichnoabundance (Knaust et al., 2014), and the systematic counting of individuals is called a census. A complementary concept is ichnodisparity, which assesses the diversity of gross morphological plans of the tracemakers and can therefore reflect diversity at broader scales than is possible with ichnodiversity (Buatois and Mángano, 2011). Note that the terms “ichnodiversity”, “ichnoabundance”, and “ichnodisparity” originate from invertebrate ichnology, and that only “ichnodiversity” has already found wider use in studies of fossil tetrapod tracks.
259 Census. The systematic counting of individuals per trackmaker taxon, ichnotaxon, or morphotype. In the case of transit sites that show directional movements, it may be assumed that one trackway represents one individual, with the caveat that separate trackways could theoretically have been left by the same individual trackmaker at different times (Lockley, 1997). This approach may not be feasible when the trackmakers might have been milling, in which case the individual tracks (rather than trackways) could be counted to estimate trackmaker abundance (Cohen et al., 1993). If the tracks are preserved on multiple small slabs, the slabs containing the respective taxon or morphotype could be counted (“track/slab method”; Marchetti et al., 2017). In modern wildlife monitoring, track densities are used to estimate population densities, and machine learning approaches are available to estimate the minimum number of individuals of particular species based on photographs of individual tracks (e.g., Funston et al., 2010; Moreira et al., 2018; Alibhai et al., 2023).
ICHNOTAXONOMY
Names are crucial to scientific communication. The formal naming and classification of trace fossils is known as ichnotaxonomy. However, the question of how such names should be defined has led to ongoing debate. In tetrapod track ichnotaxonomy, ichnotaxa are assumed to relate to the taxonomy of the trackmakers and have therefore been described as “proxies of biotaxa” (Hunt and Lucas, 2007). A tetrapod ichnotaxon ideally reflects some higher-level biological taxon, although near-identical tracks may be produced by trackmakers that are only distantly related (Farlow et al., 2012a). In invertebrate ichnotaxonomy, in contrast, ichnotaxa relate to the behaviour of the trackmakers, and, consequently, an ichnotaxon can be produced by different unrelated tracemaker taxa (Lockley, 2007; Hunt and Lucas, 2016). These different approaches have also been termed the “biotaxonomic approach” and the “ethological approach”, respectively (Hunt and Lucas, 2007).
Tetrapod ichnotaxonomy suffers from oversplitting (Lucas, 2007) - the erection of more ichnotaxa than can be reliably distinguished. This is particularly true of dinosaur track ichnotaxonomy, where new, poorly defined ichnotaxa continue to be named. Oversplitting has been attributed to the use of features that do not inform, or mislead, about the anatomy of the trackmaker (Lucas, 2007). However, a deeper cause may be at play as well: Fossils that remain unnamed are “bound to sink into oblivion sooner or later” (Bertling et al., 2022, p. 1). New names, in particular, create a higher impact, even if they are built on shaky foundations. While understandable, such a practise threatens the credibility of ichnotaxonomy as such (Marchetti et al., 2019a, p. 115), and makes it more difficult to achieve its ultimate goal: a better understanding of palaeobiology, palaeoecology, palaeogeography, and evolution.
260 Ichnotaxonomy. The classification, definition, and formal naming of groups of trace fossils based on shared features. These groups of trace fossils are known as ichnotaxa. Researchers who study ichnotaxonomy are called “ichnotaxonomists”. Ichnotaxonomy mirrors biological taxonomy but is entirely decoupled from it (i.e., an ichnotaxon applies only to the trace, not to the tracemaker). Therefore, ichnotaxonomy is a parataxonomy, i.e., a taxonomy that is parallel but independent from “biological taxonomy” (synonyms: “biotaxonomy”, “orthotaxonomy”), and ichnotaxa are “parataxa” (as opposed to “biological taxa”, “biotaxa”, or “orthotaxa”) (Bengtson, 1985). Note that outside of palaeontology, the term “parataxonomy” instead describes taxonomic identifications made by a junior taxonomist.
261 Nomenclature. The formalised rules for the erection and handling of taxonomic names. Nomenclature is governed by the International Code of Zoological Nomenclature (ICZN, or simply “the Code”) (International Commission on Zoological Nomenclature, 1999). The ICZN restricts ichnotaxonomy to fossil traces, while names for recent traces are not allowed unless published before 1931 (see Bertling et al., 2022, for an overview). Note that ichnological criteria (e.g., which ichnotaxobases are considered to be valid) are not the objective of nomenclature and are therefore not covered by the ICZN.
262 Open nomenclature. An approach to assigning ichnotaxonomic names to trace fossils without committing to a formal assignment. This can be achieved by using the abbreviation cf. (“compare”, e.g., “cf. Brontopodus”) to indicate uncertainty in the assignment, and the abbreviations aff. (“closely related to” or “affinity to”) to indicate that an ichnotaxon is similar but not identical to the described material (Bertling, 2007).
263 Ichnosystematics (also: systematic ichnology, systematic palaeoichnology). The study of the relationships between ichnotaxa (Bertling et al., 2006). Researchers concerned with systematics are known as “systematicists”.
According to the above definition, ichnosystematics is concerned with the structure of the classification system, and is therefore distinct from ichnotaxonomy (Bertling et al., 2006; Bertling, 2007). We note, however, that in biology there is no agreement on the precise definitions of “taxonomy” and “systematics”, and taxonomy is often considered a branch of systematics. This latter definition is reflected in the common “Systematic ichnology” sections in ichnological publications, which mostly deal with ichnotaxonomy. We also note that ichnologists rarely deal with ichnotaxa above the ichnogenus level, and consequently ichnosystematics in the narrow sense as defined by Bertling et al. (2006) remains a poorly developed branch of ichnology.
264 Ichnotaxon (plural: ichnotaxa). “A taxon based on the fossilized work of an organism, including fossilized trails, tracks or burrows (trace fossils) made by an animal” (International Commission on Zoological Nomenclature, 1999). An “ichnotaxonomic name” is the name of an ichnotaxon.
265 Ichnogenus (plural: ichnogenera; abbreviation: igen.). The primary rank in tetrapod ichnotaxonomy, analogous to the genus rank in biological taxonomy. An intermediate rank between ichnospecies and ichnogenus is the ichnosubgenus; this rank is recognised by the ICZN but rarely used (Bertling et al., 2006).
266 Ichnospecies (abbreviation: isp.). According to binominal nomenclature, at least one ichnospecies (the type ichnospecies) is erected with each new ichnogenus. An ichnogenus is “monotypic” if it contains only a single ichnospecies; this is the case for many, if not most, currently recognised ichnogenera in both invertebrate and vertebrate ichnology (Bertling, 2007). Additional ichnospecies may be named when variation within an ichnogenus allows. The extent of said variation is not formalised, however. Bertling (2007) argued that the diagnosis of an ichnogenus should always be sufficiently broader than that of an ichnospecies. Ichnosubspecies are recognised by the ICZN but rarely used (Bertling et al., 2006).
267 Ichnofamily (abbreviation: ifam.). Although recognised by the ICZN, this higher ichnotaxonomic rank should be (and is) used sparingly (Bertling, 2007). Ichnotaxa above the rank of an ichnofamily, such as ichnoorders, have been defined but are not recognised by the ICZN.
268 Characters. Observable characteristics of taxa. In ichnotaxonomy, characters are often continuous (e.g., length-to-width ratio) rather than discrete (e.g., the number of digit impressions). The expression of a character in a particular taxon or specimen (e.g., the presence of three digit impressions) is known as a trait or “character state”.
269 Ichnotaxobases. Characters suitable for defining ichnotaxa (cf. Bromley, 1996). For tetrapod tracks, only morphological characters that inform about the anatomy of the trackmaker taxon qualify as ichnotaxobases (Marchetti et al., 2019a). The mode of locomotion can be considered an ichnotaxobase if it is unlikely to merely reflect intraspecific differences in behaviour (Marchetti et al., 2019a). The use of size as an ichnotaxobase is controversial, as differences in size may result from different ontogenetic stages of the same trackmaker. Bertling et al. (2022) argued that size cannot be the sole ichnotaxobase unless recent analogues indicate that the observed size differences are indeed due to different trackmaker taxa. The more general term “taxobase” also applies to body fossils, although its use outside the field of ichnology is limited. “Ichnogeneric taxobases” apply to ichnogenera, while “ichnospecific taxobases” apply to ichnospecies.
270 Diagnosis (plural: diagnoses). The set of traits that define a taxon. A diagnosis should consist of either autapomorphies (unique traits), a unique combination of traits, or a combination of both. In tetrapod ichnology, however, diagnoses commonly include general traits that are neither unique nor form unique combinations. A diagnosis does not fulfil its purpose if the precise traits that distinguish the ichnotaxon from similar ichnotaxa remain unclear.
271 Holotype. The single representative specimen that defines a species (or ichnospecies). The set of specimens on which the original description of the taxon was based is termed the type series; any member of a type series (including the holotype) is known as a type specimen (Quicke, 2013). If the taxon was originally defined based on a set of specimens but no individual specimen was chosen as holotype, such a single specimen may subsequently be selected and is then referred to as the lectotype. The locality where the holotype was discovered is the type locality. If other specimens from the type locality belong to the same species, they are termed topotypes (Quicke, 2013). If the holotype of a taxon is lost, a new one may be defined, which is then termed the neotype. An artificial physical replica made from a holotype specimen is often termed a plastotype, although the ICZN does not consider replicas to be eligible types (Lucas and Harris, 2020). Note that the term “plastotype” is also used when the replica consists of materials other than plaster (Lucas and Harris, 2020). The term digitype refers to digital 3D models of holotype specimens (Mujal et al., 2020).
272 Synonyms. Two or more names applied to the same taxon. The first published synonym (the “senior synonym”) takes priority (precedence) over all later names (the “junior synonyms”) according to the rules of the ICZN. Synonyms may be objective (derived directly from ICZN rules or actions) or subjective (based on the judgement of an ichnologist). For example, if ichnogenus A is merged with ichnogenus B, and ichnogenus B was named earlier, ichnogenus A becomes a “junior subjective synonym” of ichnogenus B.
273 Invalid name. The name of an ichnotaxon that either does not conform to ICZN rules (and is therefore an “unavailable name”), is a junior synonym, or is not applicable to the taxon in question (International Commission on Zoological Nomenclature, 1999). Note that the term “invalid” is commonly used in a broader sense to refer to any doubtful name that should be abandoned. The technically correct term for such use cases is “nomen dubium”.
274 Nomen dubium (“dubious name”; plural: nomina dubia). “A name of unknown or doubtful application” (International Commission on Zoological Nomenclature, 1999). An ichnotaxon may be declared a nomen dubium if it is based on inadequate material (e.g., poor preservation or lack of anatomical fidelity), or if it cannot be adequately diagnosed (morphologically distinguished from similar ichnotaxa).
275 Phantom taxon (plural: phantom taxa). An ichnotaxon based on extramorphological features (Haubold, 1996). Phantom taxa are nomina dubia, as only morphological features that reflect the anatomy of the trackmaker are considered useful ichnotaxobases. Marchetti et al. (2019a) introduced the term “ichnotaphotaxon” as a synonym of “phantom taxon”, derived from the term “taphotaxon” proposed by Lucas (2001) for body fossils. However, in the literature, “taphonomy” typically refers to “after track formation” when applied to tracks, so the term “ichnotaphotaxon” is potentially misleading.
276 Morphotype (synonym: ichnomorph). A morphologically distinguishable category within a sample of tracks or trackways. The morphotype concept is informal and is often used before ichnotaxonomic assignments are made, or when such assignments are not possible or desirable.
277 Plexus (plural: plexuses). A group of similar ichnotaxa that form a morphological continuum, i.e., intermediate morphologies are common. An example is the Grallator-Anchisauripus-Eubrontes (GAE) plexus, where Anchisauripus is an intermediate form between the end members Grallator and Eubrontes (Lockley, 2009).
ACKNOWLEDGEMENTS
The authors like to thank J.O. Farlow for a pre-submission review and additional figures. M.G. Lockley, P.M. Sander, and A.R. Vincelette are thanked for discussions. We thank L. Xing, O. Wings, H. Klein, M. Avanzini, and G. Calábková for additional figures. H. Singleton, M. Ross, and J. Lively are thanked for access to specimens. JNL thanks M. Amzil, M. Oukassou, H. Saber, F. Pérez-Lorente, M. Buchwitz, D. Falk, and T-R Yang for fruitful fieldwork in Morocco, Spain, Germany, and the US that produced many of the figures. G.L. thanks Christian Meyer for fruitful fieldwork in Bolivia and Tony Thulborn for fruitful fieldwork in Austrialia. We also thank two anonymous reviewers for their comprehensive and constructive reviews that improved the manuscript. JNL is currently funded by the Alexander von Humboldt Foundation (Feodor-Lynen Research Fellowship). PLF was funded by UKRI Frontier Research Grant TRACKEVOL (selected by the ERC for a consolidator award). GL is funded by the Istituto Cavanis of Venice.
REFERENCES
Abrahams, M., Rampersadh, A., and Mpangala, L. 2023. Riches of the Roma valley: theropod and ornithischian tracks from the Early Jurassic southern Africa. Historical Biology, 1-13.
https://doi.org/10.1080/08912963.2023.2221306
Alexander, R.McN. 1976. Estimates of speeds of dinosaurs. Nature, 261:129-130.
https://doi.org/10.1038/261129a0
Alexander, R.McN. 1977. Mechanics and scaling of terrestrial locomotion, p. 93-110. In Pedley, T.J. (ed.), Scale Effects in Animal Locomotion. Academic Press, London.
Alexander, R.McN. 1982. Crawling, p. 114-125. Locomotion of Animals. Springer Netherlands, Dordrecht.
Alexander, R.McN. 1989a. Mechanics of fossil vertebrates. Journal of the Geological Society, 146:41-52.
https://doi.org/10.1144/gsjgs.146.1.0041
Alexander, R.McN. 1989b. Optimization and gaits in the locomotion of vertebrates. Physiological reviews, 69:1199-1227.
Alexander, R.McN. 2003. Principles of Animal Locomotion. Princeton University Press, Princeton, New Jersey.
Alexander, R.McN. 2006. Dinosaur biomechanics. Proceedings of the Royal Society B: Biological Sciences, 273:1849-1855.
Alexander, R.McN., Langman, V.A., and Jayes, A.S. 1977. Fast locomotion of some African ungulates. Journal of Zoology, 183:291-300.
Alexander, R.McN. and Jayes, A.S. 1978. Vertical movements in walking and running. Journal of Zoology, 185:27-40.
https://doi.org/10.1111/j.1469-7998.1978.tb03311.x
Alexander, R.McN. and Jayes, A.S. 1983. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. Journal of Zoology, 201:135-152.
https://doi.org/10.1111/j.1469-7998.1983.tb04266.x
Alibhai, S.K., Gu, J., Jewell, Z.C., Morgan, J., Liu, D., and Jiang, G. 2023. ‘I know the tiger by his paw’: A non-invasive footprint identification technique for monitoring individual Amur tigers (Panthera tigris altaica) in snow. Ecological Informatics, 73:101947.
https://doi.org/10.1016/j.ecoinf.2022.101947
Allen, J.R.L. 1989. Fossil vertebrate tracks and indenter mechanics. Journal of the Geological Society, 146:600-602.
https://doi.org/10.1144/gsjgs.146.4.0600
Allen, J.R.L. 1997. Subfossil mammalian tracks (Flandrian) in the Severn Estuary, SW Britain: mechanics of formation, preservation and distribution. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 352:481-518.
https://doi.org/10.1098/rstb.1997.0035
Amzil, M., Oukassou, M., Lallensack, J.N., Klein, H., Zafaty, O., Saber, H., Charrière, A., Meyer, C., and Gierliński, G. 2024. New records of dinosaur tracks from Middle Jurassic red beds in the Middle Atlas, Morocco: Application of photogrammetry to ichnology and conservation of geological heritage. Proceedings of the Geologist’s Association, 135:458-480.
Avanzini, M., Ceoloni, P., Conti, M.A., Leonardi, G., Manni, R., Mariotti, N., Mietto, P., Muraro, C., Nicosia, U., and Sacchi, E. 2001. Permian and Triassic tetrapod ichnofaunal units of Northern Italy: their potential contribution to continental biochronology, p. 89-107. Permian continental deposits of Europe and other areas. Regional reports and correlations. Museo Civico di Science Naturali di Brescia.
Avanzini, M. and Lockley, M.G. 2002. Middle Triassic archosaur population structure: interpretation based on Isochirotherium delicatum fossil footprints (Southern Alps, Italy). Palaeogeography, Palaeoclimatology, Palaeoecology, 185:391-402.
https://doi.org/10.1016/S0031-0182(02)00441-8
Baird, D. 1952. Revision of the Pennsylvanian and Permian footprints Limnopus, Allopus and Baropus. Journal of Paleontology, 26:832-840.
Barco, J.L., Canudo, J.I., and Ruiz-Omeñaca, J.I. 2006. New data on Therangospodus oncalensis from the Berriasian Fuentesalvo Tracksite (Villar del Río, Soria, Spain): An example of gregarious behaviour in theropod dinosaurs. Ichnos, 13:237-248.
https://doi.org/10.1080/10420940600843682
Bates, K.T., Savage, R., Pataky, T.C., Morse, S.A., Webster, E., Falkingham, P.L., Ren, L., Qian, Z., Collins, D., Bennett, M.R., McClymont, J., and Crompton, R.H. 2013. Does footprint depth correlate with foot motion and pressure? Journal of The Royal Society Interface, 10:20130009.
https://doi.org/10.1098/rsif.2013.0009
Batschelet, E. 1981. Circular Statistics in Biology. Academic Press, London.
Baucon, A., Bordy, E., Brustur, T., Buatois, L.A., Cunningham, T., De, C., Duffin, C., Felletti, F., Gaillard, C., Hu, B., Hu, L., Jensen, S., Knaust, D., Lockley, M.G., Lowe, P., Mayor, A., Mayoral, E., Mikuláš, R., Muttoni, G., Neto de Carvalho, C., Pemberton, S.G., Pollard, J., Rindsberg, A.K., Santos, A., Seike, K., Song, H., Turner, S., Uchman, A., Wang, Y., Yi-ming, G., Zhang, L., and Zhang, W. 2012. A History of Ideas in Ichnology, p. 64, 3-43. In Knaust, D. and Bromley, R.G. (eds.), Developments in Sedimentology. Trace Fossils as Indicators of Sedimentary Environments. Elsevier.
Behrensmeyer, A.K. and Kidwell, S.M. 1985. Taphonomy’s contributions to paleobiology. Paleobiology, 11:105-119.
https://doi.org/10.1017/S009483730001143X
Belaústegui, Z., Muñiz, F., Mángano, M.G., Buatois, L.A., Domènech, R., and Martinell, J. 2016. Lepeichnus giberti igen. nov. isp. nov. from the upper Miocene of Lepe (Huelva, SW Spain): Evidence for its origin and development with proposal of a new concept, ichnogeny. Palaeogeography, Palaeoclimatology, Palaeoecology, 452:80-89.
https://doi.org/10.1016/j.palaeo.2016.04.018
Belvedere, M., Bennett, M.R., Marty, D., Budka, M., Reynolds, S.C., and Bakirov, R. 2018. Stat-tracks and mediotypes: powerful tools for modern ichnology based on 3D models. PeerJ, 6:e4247.
https://doi.org/10.7717/peerj.4247
Belvedere, M. and Farlow, J.O. 2017. A numerical scale for qantifying the quality of preservation of vertebrate tracks, p. 92-98. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press.
Bengtson, S. 1985. Taxonomy of disarticulated fossils. Journal of Paleontology, 1350-1358.
Benhamou, S. 2004. How to reliably estimate the tortuosity of an animal’s path: straightness, sinuosity, or fractal dimension? Journal of Theoretical Biology, 229:209-220.
https://doi.org/10.1016/j.jtbi.2004.03.016
Bennett, M.R. and Budka, M. 2019. Vertebrate Ichnology: Issues and Case Studies, p. 189-219. Digital Technology for Forensic Footwear Analysis and Vertebrate Ichnology. Springer International Publishing, Cham.
https://doi.org/10.1007/978-3-319-93689-5_6
Bennett, M.R. and Morse, S.A. 2014. Human Footprints: Fossilised Locomotion? Springer, Cham.
Bennett, M.R. and Reynolds, S.C. 2021. Inferences from footprints: Archaeological best practice, p. 15-39. In Pastoors, A. and Lenssen-Erz, T. (eds.), Reading Prehistoric Human Tracks. Springer International Publishing, Cham.
https://doi.org/10.1007/978-3-030-60406-6_2
Bertling, M. 2007. What’s in a Name? Nomenclature, Systematics, Ichnotaxonomy, p. 81-91. In Miller, W. (ed.), Trace Fossils: Concepts, Problems, Prospects. Elsevier, Amsterdam.
Bertling, M., Braddy, S.J., Bromley, R.G., Demathieu, G.R., Genise, J., Mikuláš, R., Nielsen, J.K., Nielsen, K.S., Rindsberg, A.K., Schlirf, M., and Uchman, A. 2006. Names for trace fossils: a uniform approach. Lethaia, 39:265-286.
https://doi.org/10.1080/00241160600787890
Bertling, M., Buatois, L.A., Knaust, D., Laing, B., Mángano, M.G., Meyer, N., Mikuláš, R., Minter, N.J., Neumann, C., Rindsberg, A.K., Uchman, A., and Wisshak, M. 2022. Names for trace fossils 2.0: theory and practice in ichnotaxonomy. Lethaia, 55:1-19.
https://doi.org/10.18261/let.55.3.3
Biesecker, L.G., Aase, J.M., Clericuzio, C., Gurrieri, F., Temple, I.K., and Toriello, H. 2009. Elements of morphology: Standard terminology for the hands and feet. American Journal of Medical Genetics Part A, 149A:93-127.
https://doi.org/10.1002/ajmg.a.32596
Biewener, A.A. 1989. Mammalian terrestrial locomotion and size. Bioscience, 39:776-783.
https://doi.org/10.2307/1311183
Biknevicius, A.R. and Reilly, S.M. 2006. Correlation of symmetrical gaits and whole body mechanics: debunking myths in locomotor biodynamics. Journal of Experimental Zoology Part A: Comparative Experimental Biology, 305A:923-934.
https://doi.org/10.1002/jez.a.332
Bird, R.T. 1944. Did Brontosaurus ever walk on land? Natural History, 53:60-67.
Bishop, P.J., Graham, D.F., Lamas, L.P., Hutchinson, J.R., Rubenson, J., Hancock, J.A., Wilson, R.S., Hocknull, S.A., Barrett, R.S., Lloyd, D.G., and Clemente, C.J. 2018. The influence of speed and size on avian terrestrial locomotor biomechanics: Predicting locomotion in extinct theropod dinosaurs. PLoS ONE, 13:e0192172.
https://doi.org/10.1371/journal.pone.0192172
Blickhan, R. 1989. The spring-mass model for running and hopping. Journal of biomechanics, 22:1217-1227.
https://doi.org/10.1016/0021-9290(89)90224-8
Bolhuis, J.J., Giraldeau, L.-A., and Hogan, J.A. 2022. The study of animal behavior, p. 2nd ed., 1-11. In Bolhuis, J.J., Giraldeau, L.-A., and Hogan, J.A. (eds.), The Behavior of Animals: Mechanisms, Function, and Evolution. John Wiley & Sons.
Bonnan, M.F. 2003. The evolution of manus shape in sauropod dinosaurs: implications for functional morphology, forelimb orientation, and phylogeny. Journal of Vertebrate Paleontology, 23:595-613.
https://doi.org/10.1671/A1108
Bouougri, E., Gerdes, G., and Porada, H. 2007. Inherent problems of terminology: definition of terms frequently used in connection with microbial mats, p. 1st ed., 2, 145-151. In Schieber, J., Bose, P.K., Eriksson, P.G., Banerjee, S., Sarkar, S., Altermann, W., and Catuneanu, O. (eds.), Atlas of Microbial Mat Features Preserved within the Siliciclastic Rock Record. Elsevier.
Bovet, P. and Benhamou, S. 1988. Spatial analysis of animals’ movements using a correlated random walk model. Journal of Theoretical Biology, 131:419-433.
https://doi.org/10.1016/S0022-5193(88)80038-9
Breithaupt, B.H., Southwell, E.H., Adams, T.L., Shinn, J.P., and Matthews, N.A. 2003. Interpreting theropod community dynamics and dispelling the myths of the Sundance vertebrate ichnofaunal province: comparison of Bathonian dinosaur tracksites in the Bighorn Basin. Wyoming (abstract): Geological Society of America Abstracts with Programs, 35:499.
Breithaupt, B.H., Chan, M.A., Seiler, W.M., and Matthews, N.A. 2021. Weathering pits versus trample marks: a reinterpretation of the “dinosaur dance floor”: a Jurassic Navajo Sandstone surface in the Vermilion Cliffs National Monument, Arizona. PALAIOS, 36:331-338.
https://doi.org/10.2110/palo.2020.077
Brocx, M. and Semeniuk, V. 2007. Geoheritage and geoconservation-history, definition, scope and scale. Journal of the Royal Society of Western Australia, 90:53-87.
Bromley, R.G. 1990. Trace Fossils, Biology and Taphonomy. 1st ed. Chapman & Hall.
Bromley, R.G. 1996. Trace Fossils: Biology, Taphonomy and Applications. 2nd ed. Chapman & Hall.
Brower, A.V. and Schuh, R.T. 2021. Biological Systematics: Principles and Applications. Cornell University Press.
Brown, A.P. 1911. The formation of ripple-marks, tracks, and trails. Proceedings of the Academy of Natural Sciences of Philadelphia, 63:536-547.
Brown, R., Ferguson, J., Lawrence, M., and Lees, D. 2022. Tracks and Signs of the Birds of Britain and Europe. Bloomsbury Naturalist Ser. Bloomsbury Publishing Plc, London.
Brown, T. 1999. The Science and Art of Tracking: Nature’s Path to Spiritual Discovery. Berkley, New York City.
Brusatte, S.L. 2012. Dinosaur Paleobiology. Wiley-Blackwell, Hoboken, New Jersey.
Buatois, L.A. and Mángano, M.G. 2011. Ichnology: Organism-Substrate Interactions in Space and Time. Cambridge University Press, Cambridge, UK.
Buatois, L.A. and Mángano, M.G. 2013. Ichnodiversity and ichnodisparity: significance and caveats. Lethaia, 46:281-292.
Buchwitz, M., Jansen, M., Renaudie, J., Marchetti, L., and Voigt, S. 2021. Evolutionary change in locomotion close to the origin of amniotes inferred from trackway data in an ancestral state reconstruction approach. Frontiers in Ecology and Evolution, 9:266.
Buckley, L.G., McCrea, R.T., and Lockley, M.G. 2015. Birding by foot: a critical look at the synapomorphy-and phenetic-based approaches to trackmaker identification of enigmatic tridactyl Mesozoic traces. Ichnos, 22:192-207.
Calábková, G., Březina, J., Nosek, V., and Madzia, D. 2023. Synapsid tracks with skin impressions illuminate the terrestrial tetrapod diversity in the earliest Permian of equatorial Pangea. Scientific Reports, 13:1130.
https://doi.org/10.1038/s41598-023-27939-z
Callefo, F., Ricardi-Branco, F., Cataldo, R.A., and Noffke, N. 2021. Microbially induced sedimentary structures (MISS), p. 2nd ed., 545-554. In Alderton, D. and Elias, S.A. (eds.), Encyclopedia of Geology. Academic Press, Oxford.
Campione, N.E., Barrett, P.M., and Evans, D.C. 2020. On the ancestry of feathers in Mesozoic dinosaurs, p. 213-243. In Foth, C. and Rauhut, O.W.M. (eds.), The Evolution of Feathers: From Their Origin to the Present. Fascinating Life Sciences. Springer International Publishing, Cham.
Carey, S.P., Camens, A.B., Cupper, M.L., Grün, R., Hellstrom, J.C., McKnight, S.W., Mclennan, I., Pickering, D.A., Trusler, P., and Aubert, M. 2011. A diverse Pleistocene marsupial trackway assemblage from the Victorian Volcanic Plains, Australia. Quaternary Science Reviews, 30:591-610.
https://doi.org/10.1016/j.quascirev.2010.11.021
Carrano, M.T. 1997. Morphological indicators of foot posture in mammals: a statistical and biomechanical analysis. Zoological Journal of the Linnean Society, 121:77-104.
https://doi.org/10.1111/j.1096-3642.1997.tb00148.x
Carrano, M.T. 1999. What, if anything, is a cursor? Categories versus continua for determining locomotor habit in mammals and dinosaurs. Journal of Zoology, 247:29-42.
https://doi.org/10.1111/j.1469-7998.1999.tb00190.x
Carrano, M.T. and Wilson, J.A. 2001. Taxon distributions and the tetrapod track record. Paleobiology, 27:564-582.
https://doi.org/10.1666/0094-8373(2001)027<0564:TDATTT>2.0.CO;2
Cartmill, M., Lemelin, P., and Schmitt, D. 2002. Support polygons and symmetrical gaits in mammals. Zoological Journal of the Linnean Society, 136:401-420.
Carvalho, I.S. 2004. Dinosaur footprints from northeastern Brazil: taphonomy and environmental setting. Ichnos, 11:311-321.
https://doi.org/10.1080/10420940490442368
Carvalho, I.S., Borghi, L., and Leonardi, G. 2013. Preservation of dinosaur tracks induced by microbial mats in the Sousa Basin (Lower Cretaceous), Brazil. Cretaceous Research, 44:112-121.
https://doi.org/10.1016/j.cretres.2013.04.004
Carvalho, I.S., Cunha, P.P., and Figueiredo, S.M.D. 2022. Dinoturbation in Upper Jurassic siliciclastic levels at Cabo Mondego (Lusitanian Basin, Portugal): evidences in a fluvial-dominated deltaic succession. Palaeoworld, 31:455-477.
https://doi.org/10.1016/j.palwor.2021.09.001
Castanera, D., Pascual, C., Razzolini, N.L., Vila, B., Barco, J.L., and Canudo, J.I. 2013. Discriminating between medium-sized tridactyl trackmakers: Tracking ornithopod tracks in the base of the Cretaceous (Berriasian, Spain). PLoS ONE, 8:e81830.
https://doi.org/10.1371/journal.pone.0081830
Castanera, D., Pascual-Arribas, C., Canudo, J.I., and Puértolas-Pascual, E. 2021. A new look at Crocodylopodus meijidei: implications for crocodylomorph locomotion. Journal of Vertebrate Paleontology, 41:e2020803.
https://doi.org/10.1080/02724634.2021.2020803
Catena, A. and Hembree, D. 2014. Swimming through the substrate: the neoichnology of Chalcides ocellatus and biogenic structures of sand-swimming vertebrates. Palaeontologia Electronica, 17.3.37A; 1_19.
https://doi.org/10.26879/463
Citton, P., Nicolosi, I., Carluccio, R., and Nicosia, U. 2016. Unveiling trampling history through trackway interferences and track preservational features: a case study from the Bletterbach gorge (Redagno, Western Dolomites, Italy). Palaeontologia Electronica, 19.2.20A:1-20.
https://doi.org/10.26879/611
Clack, J.A. 1997. Devonian tetrapod trackways and trackmakers; a review of the fossils and footprints. Palaeogeography, Palaeoclimatology, Palaeoecology, 130:227-250.
https://doi.org/10.1016/S0031-0182(96)00142-3
Clayton, H.M. and Bradbury, J.W. 1995. Temporal characteristics of the fox trot, a symmetrical equine gait. Applied Animal Behaviour Science, 42:153-159.
https://doi.org/10.1016/0168-1591(94)00539-Q
Clayton, H.M. and Hobbs, S.J. 2019. A review of biomechanical gait classification with reference to collected trot, passage and piaffe in dressage horses. Animals, 9:763.
Cohen, A., Lockley, M.G., Halfpenny, J., and Michel, A.E. 1991. Modern vertebrate track taphonomy at Lake Manyara, Tanzania. PALAIOS, 371-389.
Cohen, A.S., Halfpenny, J., Lockley, M.G., and Michel, E. 1993. Modern vertebrate tracks from Lake Manyara, Tanzania and their paleobiological implications. Paleobiology, 19:433-458.
Conti, M.A., Mariotti, N., Nicosia, U., and Pittau, P. 1997. Succession of selected bioevents in the continental Permian of the Southern Alps (Italy): improvements in intrabasinal and interregional correlations, p. 51-65. In Dickins, J.M. (ed.), Late Palaeozoic and Early Mesozoic Circum-Pacific Events and their Global Correlation. Cambridge University Press, Cambridge.
Coombs, W.P. 1980. Swimming ability of carnivorous dinosaurs. Science, 207:1198-1200.
https://doi.org/10.1126/science.207.4436.1198
Cotton, W.D., Cotton, J.E., and Hunt, A.P. 1998. Evidence for social behavior in ornithopod dinosaurs from the Dakota group of northeastern New Mexico, U.S.A. Ichnos, 6:141-149.
https://doi.org/10.1080/10420949809386445
Courel, L. and Demathieu, G. 1984. Les inversions de relief dans les traces fossiles; leur signification, p. 1, 373-383. 109 Congrès National des Sociétés Savante, Dijon.
Crompton, R.H., Pataky, T.C., Savage, R., D’Août, K., Bennett, M.R., Day, M.H., Bates, K., Morse, S., and Sellers, W.I. 2011. Human-like external function of the foot, and fully upright gait, confirmed in the 3.66 million year old Laetoli hominin footprints by topographic statistics, experimental footprint-formation and computer simulation. Journal of The Royal Society Interface, 9:707-719.
https://doi.org/10.1098/rsif.2011.0258
Csermely, D. and Rossi, O. 2006. Bird claws and bird of prey talons: Where is the difference? Italian Journal of Zoology, 73:43-53.
https://doi.org/10.1080/11250000500502368
Cuesta, E., Díaz-Martínez, I., Ortega, F., and Sanz, J.L. 2015. Did all theropods have chicken-like feet? First evidence of a non-avian dinosaur podotheca. Cretaceous Research, 56:53-59.
https://doi.org/10.1016/j.cretres.2015.03.008
Currie, P.J. 1983. Hadrosaur trackways from the Lower Cretaceous of Canada. Acta Palaeontologica Polonica, 28.
Demathieu, G.R. 1984. Utilisation de lois de la mécanique pour l’Estimationde la vitesse de locomotion des Vertébrés tétrapodes du passé. Geobios, 17:439-446.
https://doi.org/10.1016/S0016-6995(84)80018-2
Demathieu, G.R. 1986. Nouvelles recherches sur la vitesse des vertébrés, auteurs de traces fossiles. Geobios, 19:327-333.
https://doi.org/10.1016/S0016-6995(86)80021-3
Demathieu, G.R. 1987. Thickness of the footprint-reliefs and its significance: Research on the distribution of the weights upon the autopodia, p. 61-62. In Leonardi, G. (ed.), Glossary and Manual of Tetrapod Footprint Palaeoichnology. Publicação do Departemento Nacional da Produção Mineral Brasil, Brasília, 117 pp.
DeSilva, J.M. 2010. Revisiting the “midtarsal break.” American Journal of Physical Anthropology, 141:245-258.
https://doi.org/10.1002/ajpa.21140
Díaz-Martínez, I., Pérez-Lorente, F., Canudo, J.I., and Pereda-Suberbiola, X. 2009. Causas de la variabilidad en icnitas de dinosaurios y su aplicación en icnotaxonomía. Actas de las IV Jornadas Internacionales sobre Paleontología de Dinosaurios y su Entorno. Salas de los Infantes, Burgos, 207-220.
Dodson, P., Behrensmeyer, A.K., Bakker, R.T., and McIntosh, J.S. 1980. Taphonomy and paleoecology of the dinosaur beds of the Jurassic Morrison Formation. Paleobiology, 6:208-232.
https://doi.org/10.1017/S009483730000676X
D’Orazi Porchetti, S., Bertini, R.J., and Langer, M.C. 2017. Walking, running, hopping. Palaeogeography, Palaeoclimatology, Palaeoecology, 465:14-29.
https://doi.org/10.1016/j.palaeo.2016.10.009
Droser, M.L. and Bottjer, D.J. 1986. A semiquantitative field classification of ichnofabric. Journal of Sedimentary Research, 56.
Dżułyński, S. and Walton, E.K. 1965. Sedimentary features of flysch and greywackes. Developments in Sedimentology, 7:1-274.
Eberth, D.A., Xing, X., and Clark, J.M. 2010. Dinosaur death pits from the Jurassic of China. PALAIOS, 25:112-125.
Efremov, J.A. 1940. Taphonomy: a new branch of paleontology. Pan-American Geologist, 74:81-93.
Ekdale, A.A., Bromley, R.G., and Pemberton, S.G. 1984. Ichnology: The Use of Trace Fossils in Sedimentology and Stratigraphy. SEPM Society for Sedimentary Geology, 15.
https://doi.org/10.2110/scn.84.15
Elbroch, M. and Marks, E. 2001. Bird Tracks & Sign: A Guide to North American Species. Stackpole Books, Mechanicsburg, Pennsylvania.
Elbroch, M. 2003. Mammal Tracks and Sign - A Guide to North American Species. 1st ed. Stackpole Books, Mechanicsburg.
Elftman, H. and Manter, J. 1935. Chimpanzee and human feet in bipedal walking. American Journal of Physical Anthropology, 20:69-79.
Ellenberger, P. 1972. Contribution à la classification des pistes de vertébrés du Trias: Les types du Stormberg d´Afrique du Sud (I). Palaeovertebrata, Memoire Extraordinaire, 1-152.
Ellenberger, P. 1974. Contribution à la classification des pistes de vertébrés du Trias: Les types du Stormberg d´Afrique du Sud (II éme partie: Le Stormberg Superieur - I. Le biome de la zona B/1 ou niveau de Moyeni: ses biocénoses). Palaeovertebrata, Memoire Extraordinaire, 1-143.
Engelmann, G.F. and Hasiotis, S.T. 1999. Deep dinosaur tracks in the Morrison Formation: Sole marks that are really sole marks, p. 179-183. In Gillette, D.D. (ed.), Vertebrate Paleontology in Utah. Miscellaneous Publication 99-1. Utah Geological Survey.
Ewer, R.F. 1998. The Carnivores. Cornell University Press, Ithaca, New York.
Falk, A.R., Hasiotis, S.T., and Martin, L.D. 2010. Feeding traces associated with bird tracks from the Lower Cretaceous Haman Formation, Republic of Korea. PALAIOS, 25:730-741.
https://doi.org/10.2110/palo.2010.p10-057r
Falk, A.R., Hasiotis, S.T., Gong, E., Lim, J.-D., and Brewer, E.D. 2017. A new experimental setup for studying avian neoichnology and the effects of grain size and moisture content on tracks: trials using the domestic chicken (Gallus gallus). PALAIOS, 32:689-707.
https://doi.org/10.2110/palo.2017.022
Falkingham, P.L. 2014. Interpreting ecology and behaviour from the vertebrate fossil track record. Journal of Zoology, 292:222-228.
https://doi.org/10.1111/jzo.12110
Falkingham, P.L. 2016. Applying objective methods to subjective track outlines, p. 72-81. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press, Bloomington.
Falkingham, P.L., Margetts, L., Smith, I.M., and Manning, P.L. 2009. Reinterpretation of palmate and semi-palmate (webbed) fossil tracks; insights from finite element modelling. Palaeogeography, Palaeoclimatology, Palaeoecology, 271:69-76.
https://doi.org/10.1016/j.palaeo.2008.09.011
Falkingham, P.L., Margetts, L., and Manning, P.L. 2010. Fossil vertebrate tracks as paleopenetrometers: confounding effects of foot morphology. PALAIOS, 25:356-360.
https://doi.org/10.2110/palo.2009.p09-164r
Falkingham, P.L., Bates, K.T., Margetts, L., and Manning, P.L. 2011a. The ‘Goldilocks’ effect: preservation bias in vertebrate track assemblages. Journal of the Royal Society Interface, 8:1142-1154.
https://doi.org/10.1098/rsos.140225
Falkingham, P.L., Bates, K.T., Margetts, L., and Manning, P.L. 2011b. Simulating sauropod manus-only trackway formation using finite-element analysis. Biology Letters, 7:142-145.
https://doi.org/10.1098/rsbl.2010.0403
Falkingham, P.L. and Gatesy, S.M. 2014. The birth of a dinosaur footprint: Subsurface 3D motion reconstruction and discrete element simulation reveal track ontogeny. Proceedings of the National Academy of Sciences, 111:18279-18284.
https://doi.org/10.1073/pnas.1416252111
Falkingham, P.L., Hage, J., and Bäker, M. 2014. Mitigating the Goldilocks effect: the effects of different substrate models on track formation potential. Royal Society Open Science, 1:140225.
https://doi.org/10.1098/rsos.140225
Falkingham, P.L. and Horner, A.M. 2016. Trackways produced by lungfish during terrestrial locomotion. Scientific Reports, 6:33734.
https://doi.org/10.1038/srep33734
Falkingham, P.L., Bates, K.T., Avanzini, M., Bennett, M., Bordy, E.M., Breithaupt, B.H., Castanera, D., Citton, P., Díaz‐Martínez, I., Farlow, J.O., Fiorillo, A.R., Gatesy, S.M., Getty, P., Hatala, K.G., Hornung, J.J., Hyatt, J.A., Klein, H., Lallensack, J.N., Martin, A.J., Marty, D., Matthews, N.A., Meyer, C.A., Milàn, J., Minter, N.J., Razzolini, N.L., Romilio, A., Salisbury, S.W., Sciscio, L., Tanaka, I., Wiseman, A.L.A., Xing, L.D., and Belvedere, M. 2018. A standard protocol for documenting modern and fossil ichnological data. Palaeontology, 61:469-480.
https://doi.org/10.1111/pala.12373
Falkingham, P.L. and Gatesy, S.M. 2020. Discussion: Defining the morphological quality of fossil footprints. Problems and principles of preservation in tetrapod ichnology with examples from the Palaeozoic to the present by Lorenzo Marchetti et al. Earth Science Reviews, 208:103320.
https://doi.org/10.1016/j.earscirev.2020.103320
Falkingham, P.L., Turner, M.L., and Gatesy, S.M. 2020. Constructing and testing hypotheses of dinosaur foot motions from fossil tracks using digitization and simulation. Palaeontology, 63:865---880.
https://doi.org/10.1111/pala.12502
Falkingham, P.L., Maidment, S.C.R., Lallensack, J.N., Martin, J.E., Suan, G., Cherns, L., Howells, C., and Barrett, P.M. 2021. Late Triassic dinosaur tracks from Penarth, south Wales. Geological Magazine, 1-12.
https://doi.org/10.1017/S0016756821001308
Farlow, J.O. 1987. A guide to Lower Cretaceous dinosaur footprints and tracksites of the Paluxy River Valley, Somervell County, Texas. Field Trip Guidebook, South-Central Section, Geological Society of America, Baylor University, Waco, Texas, 50.
Farlow, J.O. 1992. Sauropod tracks and trackmakers integrating the ichnological and skeletal records. Zubia, 89-138.
Farlow, J.O. and Britton, A. 2000. Size and body proportions in Alligator mississippiensis: implications for archosaurian ichnology. Paleontological Society of Korea Special Publications, 189-206.
Farlow, J.O., Langston Jr, W., Deschner, E.E., Solis, R., Ward, W., Kirkland, B.L., Hovorka, S., Reece, T.L., and Whitcraft, J. 2006. Texas giants: dinosaurs of the Heritage Museum of the Texas Hill Country. The Heritage Museum of the Texas Hill Country, Canyon Lake, Texas.
Farlow, J.O., Chapman, R.E., Breithaupt, B.H., and Matthews, N.A. 2012a. The Scientific Study of Dinosaur Footprints, p. 712-759. In Brett-Surman, M.K., Holtz, T.R., and Farlow, J.O. (eds.), The Complete Dinosaur. Indiana University Press.
Farlow, J.O., O’Brien, M., Kuban, G.J., Dattilo, B.F., Bates, K.T., Falkingham, P.L., and Piñuela, L. 2012b. Dinosaur Tracksites of the Paluxy River Valley (Glen Rose Formation, Lower Cretaceous), Dinosaur Valley State Park, Somervell County, Texas. Actas de V Jornadas Internacionales sobre Paleontología de Dinosaurios y su Entorno. Salas de los Infantes, Burgos, 41-69.
Farlow, J.O., Coroian, D., and Currie, P.J. 2018a. Noah’s Ravens: Interpreting the Makers of Tridactyl Dinosaur Footprints. Life of the Past. Indiana University Press, Bloomington, Indiana.
Farlow, J.O., Robinson, N.J., Turner, M.L., Black, J., and Gatesy, S.M. 2018b. Footfall pattern of a bottom-walking crocodile (Crocodylus acutus). PALAIOS, 33:406-413.
Farlow, J.O., Bakker, R.T., Dattilo, B.F., Everett Deschner, E., Falkingham, P.L., Harter, C., Solis, R., Temple, D., and Ward, W. 2020. Thunder lizard handstands: Manus-only sauropod trackways from the Glen Rose Formation (Lower Cretaceous, Kendall County, Texas). Ichnos, 27:167-199.
https://doi.org/10.1080/10420940.2019.1698424
Fernandes, M.A., Fernandes, L. dos R., and Souto, P. de F. 2004. Occurrence of urolites related to dinosaurs in the Lower Cretaceous of the Botucatu formation, Paraná Basin, São Paulo State, Brazil. Revista Brasileira de Paleontologia, 7:263-268
Fieler, C.L. and Jayne, B.C. 1998. Effects of speed on the hindlimb kinematics of the lizard Dipsosaurus dorsalis. The Journal of Experimental Biology, 201:609-622.
Fiorillo, A.R. 1984. An introduction to the identification of trample marks. Current Research in the Pleistocene, 1:47-48.
Flügel, E. 2004. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application. Springer-Verlag, Berlin Heidelberg.
Fornós, J.J., Bromley, R.G., Clemmensen, L.B., and Rodrıguez-Perea, A. 2002. Tracks and trackways of Myotragus balearicus Bate (Artiodactyla, Caprinae) in Pleistocene aeolianites from Mallorca (Balearic Islands, Western Mediterranean). Palaeogeography, Palaeoclimatology, Palaeoecology, 180:277-313.
https://doi.org/10.1016/S0031-0182(01)00431-X
Frey, R.W. 1973. Concepts in the study of biogenic sedimentary structures. Journal of Sedimentary Research, 43:6-19. Society for Sedimentary Geology.
Fuiman, L.A., Williams, T.M., and Davis, R.W. 2021. On the straight and narrow: directed movement by Weddell seals (Leptonychotes weddellii) during on-ice travel. Polar Biology, 44:601-606.
https://doi.org/10.1007/s00300-021-02811-w
Funston, P.J., Frank, L., Stephens, T., Davidson, Z., Loveridge, A., Macdonald, D.M., Durant, S., Packer, C., Mosser, A., and Ferreira, S.M. 2010. Substrate and species constraints on the use of track incidences to estimate African large carnivore abundance. Journal of Zoology, 281:56-65.
https://doi.org/10.1111/j.1469-7998.2009.00682.x
Gand, G., Demathieu, G., Grancier, M., and Sciau, J. 2005. Les traces dinosauroïdes du Trias supérieur français: discrimination, interprétation et comparaison. Bulletin de la Société géologique de France, 176:69-80.
Gans, C. 1970. How Snakes Move. Scientific American, 222:82-99.
Gatesy, S.M. 2001. Skin impressions of Triassic theropods as records of foot movement. Bulletin of the Museum of Comparative Zoology, 156:137-149.
Gatesy, S.M. 2003. Direct and indirect track features: what sediment did a dinosaur touch? Ichnos, 10:91-98.
https://doi.org/10.1080/10420940390255484
Gatesy, S.M., Middleton, K.M., Jenkins Jr, F.A., and Shubin, N.H. 1999. Three-dimensional preservation of foot movements in Triassic theropod dinosaurs. Nature, 399:141-144.
https://doi.org/10.1038/20167
Gatesy, S.M. and Falkingham, P.L. 2017. Neither bones nor feet: track morphological variation and ‘preservation quality.’ Journal of Vertebrate Paleontology, 37:e1314298.
https://doi.org/10.1080/02724634.2017.1314298
Gatesy, S.M. and Falkingham, P. 2020. Hitchcock’s Leptodactyli, penetrative tracks, and dinosaur footprint diversity. Journal of Vertebrate Paleontology, e1781142.
https://doi.org/10.1080/02724634.2020.1781142
Gebo, D.L. 1992. Plantigrady and foot adaptation in African apes: implications for hominid origins. American Journal of Physical Anthropology, 89:29-58.
Genise, J.F., Melchor, R.N., Archangelsky, M., Bala, L.O., Straneck, R., and De Valais, S. 2009. Application of neoichnological studies to behavioural and taphonomic interpretation of fossil bird-like tracks from lacustrine settings: The Late Triassic-Early Jurassic? Santo Domingo Formation, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology, 272:143-161.
https://doi.org/10.1016/j.palaeo.2008.08.014
Getty, P.R., Hardy, L., and Bush, A.M. 2015. Was the Eubrontes track maker gregarious? Testing the herding hypothesis at Powder Hill Dinosaur Park, Middlefield, Connecticut. Bulletin of the Peabody Museum of Natural History, 56:95-106.
https://doi.org/10.3374/014.056.0109
Goldring, R. and Seilacher, A. 1971. Limulid undertracks and their sedimentological implications. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 137:422-442.
Gonzalez Riga, B.J. and Calvo, J.O. 2009. A new wide-gauge sauropod track site from the Late Cretaceous of Mendoza, Neuquén Basin, Argentina. Palaeontology, 52:631-640.
https://doi.org/10.1111/j.1475-4983.2009.00869.x
Granatosky, M.C. and McElroy, E.J. 2022. Stride frequency or length? A phylogenetic approach to understand how animals regulate locomotor speed. Journal of Experimental Biology, 225:1-9.
https://doi.org/10.1242/jeb.243231
Graversen, O., Milàn, J., and Loope, D.B. 2007. Dinosaur tectonics: a structural analysis of theropod undertracks with a reconstruction of theropod walking dynamics. The Journal of Geology, 115:641-654.
https://doi.org/10.1086/521608
Gray, S.J. 1968. Animal Locomotion. Littlehampton Book Services Ltd.
Grigg, G. and Kirshner, D. 2015. Biology and Evolution of Crocodylians. Csiro Publishing.
Hadri, M. and Pérez-Lorente, F. 2012. Historia de yacimientos con huellas de dinosaurio, desde su descubrimiento hasta su primer estudio: alrededores de El Mers (Marruecos). Zubia, 93.
Halfpenny, J. 2019. Scats and Tracks of the Great Plains: A Field Guide to the Signs of Seventy Wildlife Species. Scats and Tracks Series2nd ed. Globe Pequot Press, The, Blue Ridge Summit.
Halfpenny, J.C. 1986. A Field Guide to Mammal Tracking in North America. Big Earth Publishing.
Hall, L.E., Fragomeni, A.E., and Fowler, D.W. 2016. The flexion of sauropod pedal unguals and testing the substrate grip hypothesis using the trackway fossil record, p. 138-151. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press.
Hasiotis, S.T. 2002. Continental Ichnology: Using Terrestrial and Freshwater Trace Fossils for Environmental and Climatic Interpretations, p. 1-53. Continental Trace Fossils. SEPM Society for Sedimentary Geology.
https://doi.org/10.2110/scn.06.51.0001
Hasiotis, S.T., Platt, B.F., Hembree, D.I., and Everhart, M.J. 2007. The Trace-Fossil Record of Vertebrates, p. 196-218. In Miller, W. (ed.), Trace Fossils. Elsevier, Amsterdam.
Hatala, K.G., Gatesy, S.M., and Falkingham, P.L. 2023. Arched footprints preserve the motions of fossil hominin feet. Nature Ecology & Evolution, 7:32-41.
https://doi.org/10.1038/s41559-022-01929-2
Hatala, K.G., Roach, N.T., Behrensmeyer, A.K., Falkingham, P.L., Gatesy, S.M., Williams-Hatala, E.M., Feibel, C.S., Dalacha, I., Kirinya, M., Linga, E., Loki, R., Alkoro, A., Longaye, Longaye, M., Lonyericho, E., Loyapan, I., Nakudo, N., Nyete, C., and Leakey, L.N. 2024. Footprint evidence for locomotor diversity and shared habitats among early Pleistocene hominins. Science, 386:1004-1010.
https://doi.org/10.1126/science.ado5275
Haubold, H. 1971. Ichnia amphibiorum et reptiliorum fossilium: Handbuch der Paleoherpetologie18. Gustav Fischer Verlag, Stuttgart.
Haubold, H. 1996. Ichnotaxonomie und Klassifikation von Tetrapodenfährten aus dem Perm. Hallesches Jahrbuch für Geowissenschaften B, 18:23-88.
Henderson, D.M. 2003. Footprints, trackways, and hip heights of bipedal dinosaurs--testing hip height predictions with computer models. Ichnos, 10:99-114.
https://doi.org/10.1080/10420940390257914
Henderson, D.M. 2006a. Simulated weathering of dinosaur tracks and the implications for their characterization. Canadian Journal of Earth Sciences, 43:691-704.
https://doi.org/10.1139/e06-024
Henderson, D.M. 2006b. Burly gaits: centers of mass, stability, and the trackways of sauropod dinosaurs. Journal of Vertebrate Paleontology, 26:907-921.
https://doi.org/10.1671/0272-4634(2006)26[907:BGCOMS]2.0.CO;2
Hendrickx, C., Bell, P.R., Pittman, M., Milner, A.R.C., Cuesta, E., O’Connor, J., Loewen, M., Currie, P.J., Mateus, O., Kaye, T.G., and Delcourt, R. 2022. Morphology and distribution of scales, dermal ossifications, and other non-feather integumentary structures in non-avialan theropod dinosaurs. Biological Reviews, 97:960-1004.
https://doi.org/10.1111/brv.12829
Hildebrand, M. 1965. Symmetrical gaits of horses. Science, 150:701-708.
Hildebrand, M. 1974. Analysis of vertebrate structure. Wiley, New York.
Hildebrand, M. 1976. Analysis of Tetrapod Gaits: General Considerations and Symmetrical Gaits, p. 203-236. In Herman, R.M., Grillner, S., Stein, P.S.G., and Stuart, D.G. (eds.), Neural Control of Locomotion. Advances in Behavioral Biology. Springer US, Boston, MA.
Hildebrand, M. 1977. Analysis of asymmetrical gaits. Journal of Mammalogy, 58:131-156.
Hildebrand, M. 1980. The adaptive significance of tetrapod gait selection. Integrative and Comparative Biology, 20:255-267.
https://doi.org/10.1093/icb/20.1.255
Hildebrand, M. 1985. Digging of Quadrupeds, p. 89-109. In Hildebrand, M., Bramble, D.M., Liem, K.F., and Wake, D.B. (eds.), Functional Vertebrate Morphology. Harvard University Press.
https://doi.org/10.4159/harvard.9780674184404.c6
Hildebrand, M. 1989. The quadrupedal gaits of vertebrates. BioScience, 39:766.
Hitchcock, E. 1836. Ornithichnology. Description of the foot marks of birds, (Ornithichnites) on new Red Sandstone in Massachusetts. American Journal of Science and Arts, 29:307.
Hitchcock, E. 1858. Ichnology of New England: A Report on the Sandstone of the Connecticut Valley Especially Its Fossil Footmarks, Made to the Government of the Commonwealth of Massachusetts. William White, Boston.
Höhn, E.O. 1977. The “snowshoe effect” of the feathering on ptarmigan feet. The Condor, 79:380-382.
Hunt, A.P. and Lucas, S.G. 2007. Tetrapod ichnofacies: a new paradigm. Ichnos, 14:59-68.
https://doi.org/10.1080/10420940601006826
Hunt, A.P. and Lucas, S.G. 2012. Classification of vertebrate coprolites and related trace fossils. New Mexico Museum of Natural History and Science Bulletin, 57:137-146.
Hunt, A.P. and Lucas, S.G. 2016. The case for archetypal vertebrate ichnofacies. Ichnos, 23:237-247. Taylor & Francis.
https://doi.org/10.1080/10420940.2016.1164153
Hutchinson, J.R. 2001. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes). Zoological Journal of the Linnean Society, 131:123-168.
https://doi.org/10.1111/j.1096-3642.2001.tb01313.x
Hutchinson, J.R. 2021. The evolutionary biomechanics of locomotor function in giant land animals. Journal of Experimental Biology, 224:jeb217463.
https://doi.org/10.1242/jeb.217463
Hutchinson, J.R. and Gatesy, S.M. 2001. Bipedalism. Encyclopedia of Life Sciences.
https://doi.org/10.1038/npg.els.0001869
Hutchinson, J.R., Famini, D., Lair, R., and Kram, R. 2003. Are fast-moving elephants really running? Nature, 422:493-494.
https://doi.org/10.1038/422493a
Hwang, K.-G., Lockley, M.G., Huh, M., and Paik, I.S. 2008. A reinterpretation of dinosaur footprints with internal ridges from the Upper Cretaceous Uhangri Formation, Korea. Palaeogeography, Palaeoclimatology, Palaeoecology, 258:59-70.
https://doi.org/10.1016/j.palaeo.2007.10.029
International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature 4th ed. International Trust for Zoological Nomenclature, London.
Ishigaki, S. and Matsumoto, Y. 2009. “Off-tracking”-like phenomenon observed in the turning sauropod trackway from the Upper Jurassic of Morocco. Memoir of the Fukui Prefectural Dinosaur Museum, 8:1-10.
Jackson, S.J., Whyte, M.A., and Romano, M. 2009. Laboratory-controlled simulations of dinosaur footprints in sand: a key to understanding vertebrate track formation and preservation. PALAIOS, 24:222-238.
https://doi.org/10.2110/palo.2007.p07-070r
Jackson, S.J., Whyte, M.A., and Romano, M. 2010. Range of experimental dinosaur (Hypsilophodon foxii) footprints due to variation in sand consistency: How wet was the track? Ichnos, 17:197-214.
https://doi.org/10.1080/10420940.2010.510026
Jacobsen, A.R. and Bromley, R.G. 2009. New ichnotaxa based on tooth impressions on dinosaur and whale bones. Geological Quarterly, 53:373-382
Kennedy, R.B., Chen, S., Pressman, I.S., Yamashita, A.B., and Pressman, A.E. 2005. A large-scale statistical analysis of barefoot impressions. Journal of Forensic Science, 50:JFS2004277-10.
Kidd, R.S., O’Higgins, P., and Oxnard, C.E. 1996. The OH8 foot: a reappraisal of the functional morphology of the hindfoot utilizing a multivariate analysis. Journal of Human Evolution, 31:269-291.
https://doi.org/10.1006/jhev.1996.0061
Kim, J.Y. and Lockley, M.G. 2013. Review of dinosaur tail traces. Ichnos, 20:129-141.
https://doi.org/10.1080/10420940.2013.817405
Kivell, T.L. and Schmitt, D. 2009. Independent evolution of knuckle-walking in African apes shows that humans did not evolve from a knuckle-walking ancestor. Proceedings of the National Academy of Sciences, 106:14241-14246.
https://doi.org/10.1073/pnas.0901280106
Klein, H., Gierliński, G., Lallensack, J.N., Hamad, A.A., Al-Mashakbeh, H., Alhejoj, I., Konopka, M., and Błoński, M. 2020. First Upper Cretaceous dinosaur track assemblage from Jordan (Middle East) - preliminary results. Annales Societatis Geologorum Poloniae, 90:331-342,
https://doi.org/10.14241/asgp.2020.10
Kloss, C. and Goniva, C. 2011. LIGGGHTS - open source discrete element simulations of granular materials based on lammps, p. 2nd. Supplemental Proceedings: Materials Fabrication, Properties, Characterization, and Modeling. Wiley.
https://doi.org/10.1002/9781118062142
Knaust, D., Warchoł, M., and Kane, I.A. 2014. Ichnodiversity and ichnoabundance: Revealing depositional trends in a confined turbidite system. Sedimentology, 61:2218-2267.
https://doi.org/10.1111/sed.12134
Kuban, G.J. 1989a. Color distinctions and other curious features of dinosaur tracks near Glen Rose, Texas, p. 427-440. In Gillette, D.D. and Lockley, G.M. (eds.), Dinosaur Tracks and Traces. Cambridge University Press, Cambridge.
Kuban, G.J. 1989b. Elongate dinosaur tracks, p. 57-72. In Gillette, D.D. and Lockley, G.M. (eds.), Dinosaur Tracks and Traces. Cambridge University Press.
Kubo, T. 2011. Estimating body weight from footprints: application to pterosaurs. Palaeogeography, Palaeoclimatology, Palaeoecology, 299:197-199.
Kümmell, S.B. and Frey, E. 2012. Digital arcade in the autopodia of Synapsida: standard position of the digits and dorsoventral excursion angle of digital joints in the rays II-V. Palaeobiodiversity and Palaeoenvironments, 92:171-196.
https://doi.org/10.1007/s12549-012-0076-6
Kundrát, M. 2004. When did theropods become feathered?--evidence for pre-archaeopteryx feathery appendages. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 302B:355-364.
https://doi.org/10.1002/jez.b.20014
Kvale, E.P., Johnson, A.D., Mickelson, D.L., Keller, K., Furer, L.C., and Archer, A.W. 2001. Middle Jurassic (Bajocian and Bathonian) dinosaur megatracksites, Bighorn Basin, Wyoming, USA. PALAIOS, 16:233-254.
https://doi.org/10.1669/0883-1351(2001)016%3C0233:MJBABD%3E2.0.CO;2
Lallensack, J.N. 2019. Automatic generation of objective footprint outlines. PeerJ, 7:e7203.
https://doi.org/10.7717/peerj.7203
Lallensack, J.N., van Heteren, A.H., and Wings, O. 2016. Geometric morphometric analysis of intratrackway variability: a case study on theropod and ornithopod dinosaur trackways from Münchehagen (Lower Cretaceous, Germany). PeerJ, 4:e2059.
https://doi.org/10.7717/peerj.2059
Lallensack, J.N., Klein, H., Milàn, J., Wings, O., Mateus, O., and Clemmensen, L.B. 2017. Sauropodomorph dinosaur trackways from the Fleming Fjord Formation of East Greenland: evidence for Late Triassic sauropods. Acta Palaeontologica Polonica, 62:833-843.
https://doi.org/10.4202/app.00374.2017
Lallensack, J.N., Ishigaki, S., Lagnaoui, A., Buchwitz, M., and Wings, O. 2019. Forelimb orientation and locomotion of sauropod dinosaurs: insights from the Middle Jurassic Tafaytour Tracksite (Argana Basin, Morocco). Journal of Vertebrate Paleontology, 5:1-18.
https://doi.org/10.1080/02724634.2018.1512501
Lallensack, J.N., Engler, T., and Barthel, H.J. 2020. Shape variability in tridactyl dinosaur footprints: the significance of size and function. Palaeontology, 63:203-228.
https://doi.org/10.1111/pala.12449
Lallensack, J.N., Teschner, E., Pabst, B., and Sander, P.M. 2021. New skulls of the basal sauropodomorph Plateosaurus trossingensis from Frick, Switzerland: Is there more than one species? Acta Palaeontologica Polonica, 66:1-28.
https://doi.org/10.4202/app.00804.2020
Lallensack, J.N. and Falkingham, P.L. 2022. A new method to calculate limb phase from trackways reveals gaits of sauropod dinosaurs. Current Biology, 32:1635-1640.E4.
https://doi.org/10.1016/j.cub.2022.02.012
Lallensack, J.N., Buchwitz, M., and Romilio, A. 2022a. Photogrammetry in ichnology: 3D model generation, visualisation, and data extraction. Journal of Paleontological Techniques, 20:1-17.
Lallensack, J.N., Farlow, J.O., and Falkingham, P.L. 2022b. A new solution to an old riddle: elongate dinosaur tracks explained as deep penetration of the foot, not plantigrade locomotion. Palaeontology, 65:1-17.
https://doi.org/10.1111/pala.12584
Lallensack, J.N., Owais, A., Falkingham, P.L., Breithaupt, B.H., and Sander, P.M. 2022c. How to verify fossil tracks: the first record of dinosaurs from Palestine. Historical Biology, 35:924-934.
https://doi.org/10.1080/08912963.2022.2069020
Lallensack, J.N., Romilio, A., and Falkingham, P.L. 2022d. A machine learning approach for the discrimination of theropod and ornithischian dinosaur tracks. Journal of The Royal Society Interface, 19:20220588.
https://doi.org/10.1098/rsif.2022.0588
Langston Jr., W. 1986. Stacked dinosaur tracks from the Lower Cretaceous of Texas--a caution for ichnologists, p. Abstract with programs, First International Symposium on Dinosaur Tracks and Traces.
Ledoux, L., Berillon, G., Fourment, N., and Jaubert, J. 2021. Reproduce to Understand: Experimental Approach Based on Footprints in Cussac Cave (Southwestern France), p. 67-89. In Pastoors, A. and Lenssen-Erz, T. (eds.), Reading Prehistoric Human Tracks. Springer International Publishing, Cham.
https://doi.org/10.1007/978-3-030-60406-6_4
Leach, D. 1993. Recommended terminology for researchers in locomotion and biomechanics of quadrupedal animals. Acta Anatomica, 146:130-136.
Lee, D.V. and Harris, S.L. 2018. Linking gait dynamics to mechanical cost of legged locomotion. Frontiers in Robotics and AI, 5.
https://doi.org/10.3389/frobt.2018.00111
Leeder, M.R. 1982. Sedimentology - Process and Product. Chapman & Hall, London.
Leonardi, G. 1977. Two new ichnofaunas, vertebrates and invertebrates, in the eolian Cretaceous sandstones of the Caiuá Formation in northwest Paraná. Atas do I Simpósio de Geologia Regional, São Paulo, 112-128.
Leonardi, G. 1979. Nota preliminar sobre seis pistas de dinossauros Ornithischia da Bacia do Rio do Peixe (Cretáceo Inferior) em Sousa, Paraíba, Brasil. Anais Academia brasileira de Ciências, 51:501-516.
Leonardi, G. 1980. On the discovery of an abundant ichno-fauna (vertebrates and invertebrates) in the Botucatu Formation ss in Araraquara, São Paulo, Brazil.
Leonardi, G. 1981. Novo icnogênero de tetrápode Mesozóico da Formação Botucatu, Araraquara, SP. Anais da Academia Brasileira de Ciências, 53:793-805.
Leonardi, G. 1996. Le piste di dinosauri dei Lavini di Marco (Rovereto, TN, Italia) e alcune questioni generali sull’icnologia dei tetrapodi. Atti dell’Accademia Roveretana Degli Agiati, 246:65-104.
Leonardi, G., Casamiquela, R.M., Demathieu, G.R., Haubold, H., and Sarjeant, W.A.S. 1987. Glossary and Manual of Tetrapod Footprint Palaeoichnology. (G. Leonardi, Ed.). Publicação do Departemento Nacional da Produção Mineral Brasil, Brasília.
Leonardi, G. and Carvalho, I.S. 2021a. Dinosaur Tracks from Brazil: A Lost World of Gondwana. Indiana University Press, Bloomington, Indiana.
Leonardi, G. and Carvalho, I.S. 2021b. Review of the early Mammal Brasilichnium and Brasilichnium -like tracks from the Lower Cretaceous of South America. Journal of South American Earth Sciences, 106:102940.
https://doi.org/10.1016/j.jsames.2020.102940
Leonardi, G., Fernandes, M.A., Carvalho, I.S., Schutzer, J.B., and Silva, R.C. da. 2024. Farlowichnus rapidus new ichnogen., new ichnosp.: A speedy and small theropod in the Early Cretaceous Botucatu paleodesert (Paraná Basin), Brazil. Cretaceous Research, 153:105720.
https://doi.org/10.1016/j.cretres.2023.105720
Levine, D., Richards, J., and Whittle, M.W. 2012. Whittle’s gait analysis. 5th ed. Elsevier Health Sciences, London.
Liebenberg, L., Louw, A., and Elbroch, M. 2010. Practical Tracking: a Guide to Following Footprints and Finding Animals. Stackpole Books.
Lockley, M.G. 2022. The distribution of theropod-dominated ichnofaunas in the Moab Megatracksite area, Utah: implications for Late Jurassic palaeobiology along an arid coast. Historical Biology, 34:1717-1751.
https://doi.org/10.1080/08912963.2021.1975279
Lockley, M.G. 1991. Tracking Dinosaurs. Cambridge University Press, Cambridge, UK.
Lockley, M.G. 1993. Ichnotopia - the Paleontology Society short course on trace fossils. Ichnos, 2:337-342.
https://doi.org/10.1080/10420949309380107
Lockley, M.G. 1997. The paleoecological and paleoenvironmental utility of dinosaur tracks, p. 554-578. In Farlow, J.O. and Brett-Surman, M.K. (eds.), The Complete Dinosaur. Indiana University Press, Bloomington.
Lockley, M.G. 2007. A tale of two ichnologies: the different goals and potentials of invertebrate and vertebrate (tetrapod) ichnotaxonomy and how they relate to ichnofacies analysis. Ichnos, 14:39-57.
https://doi.org/10.1080/10420940601006818
Lockley, M.G. 2009. New perspectives on morphological variation in tridactyl footprints: clues to widespread convergence in developmental dynamics. Geological Quarterly, 53:415-432.
Lockley, M.G. and Conrad, K. 1989. The paleoenvironmental context, preservation and paleoecological significance of dinosaur tracksites in the western USA, p. 121-134. In Gillette, D.D. and Lockley, M.G. (eds.), Dinosaur tracks and traces. Cambridge University Press.
Lockley, M.G., Matsukawa, M., and Obata, I. 1989. Dinosaur tracks and radial cracks: unusual footprint features. Bulletin of the National Science Museum. Series C, 15:151-160.
Lockley, M.G. and Pittman, J.G. 1989. The megatracksite phenomenon: implications for paleoecology, evolution and stratigraphy. Journal of Vertebrate Paleontology, 9:30A.
Lockley, M.G. and Hunt, A.P. 1994. A track of the giant theropod dinosaur Tyrannosaurus from close to the Cretaceous/Tertiary boundary, northern New Mexico. Ichnos, 3:213-218.
https://doi.org/10.1080/10420949409386390
Lockley, M.G., Hunt, A.P., and Meyer, C.A. 1994a. Vertebrate tracks and the ichnofacies concept: implications for palaeoecology and palichno-stratigraphy, p. 241-268. In Donovan, S.K. (ed.), The Palaeobiology of Trace Fossils. Wiley and Sons, New York.
Lockley, M.G., Hunt, A.P., Moratalla, J., and Matsukawa, M. 1994b. Limping Dinosaurs? Trackway evidence for abnormal gaits. Ichnos, 3:193-202.
https://doi.org/10.1080/10420949409386388
Lockley, M.G. and Hunt, A.P. 1995. Dinosaur tracks and other fossil footprints of the western United States. Columbia University Press, New York.
Lockley, M.G., Schulp, A.S., Meyer, C.A., Leonardi, G., and Kerumba Mamani, D. 2002. Titanosaurid trackways from the Upper Cretaceous of Bolivia: Evidence for large manus, wide-gauge locomotion and gregarious behaviour. Cretaceous Research, 23:383-400.
https://doi.org/10.1006/cres.2002.1006
Lockley, M.G., Matsukawa, M., and Jianjun, L. 2003. Crouching theropods in taxonomic jungles: Ichnological and ichnotaxonomic investigations of footprints with metatarsal and uschial impressions. Ichnos, 10:169-177.
https://doi.org/10.1080/10420940390256249
Lockley, M.G., Gierliński, G.D., Houck, K., Lim, J.-D., Kim, K.S., Kim, D.Y., Kim, T.K., Kang, S.-H., Hunt Foster, R., and Li, R. 2014. New excavations at the Mill Canyon Dinosaur Track Site (Cedar Mountain Formation, Lower Cretaceous) of Eastern Utah. In Lockley, M.G. and Lucas, S.G. (eds.), Fossil footprints of western North America. New Mexico Museum of Natural History and Science Bulletin, 62:287-300.
Lockley, M.G. and Milner, A.R. 2014. The ichnotaxonomy of hopping vertebrate trackways from the Cenozoic. In Lockley, G.M. and Lucas, S.G. (eds.), Fossil footprints of western North America. New Mexico Museum of Natural History and Science Bulletin, 62:489-500.
Lockley, M.G. and Xing, L. 2015. Flattened fossil footprints: Implications for paleobiology. Palaeogeography, Palaeoclimatology, Palaeoecology, 426:85-94.
https://doi.org/10.1016/j.palaeo.2015.03.008
Lockley, M.G., McCrea, R.T., Buckley, L.G., Deock Lim, J., Matthews, N.A., Breithaupt, B.H., Houck, K.J., Gierliński, G.D., Surmik, D., Soo Kim, K., Xing, L., Yong Kong, D., Cart, K., Martin, J., and Hadden, G. 2016. Theropod courtship: large scale physical evidence of display arenas and avian-like scrape ceremony behaviour by Cretaceous dinosaurs. Scientific Reports, 6:18952.
https://doi.org/10.1038/srep18952
Lockley, M.G., Hirschfeld, S.E., and Simmons, B. 2018. A new dinosaur track locality in the Late Cretaceous (Maastrichtian) Laramie Formation of Colorado. Fossil Record 6. New Mexico Museum of Natural History & Science Bulletin, 79:395–406.
Lockley, M.G., Xing, L., Kim, K.S., and Meyer, C.A. 2021. Tortuous trackways: Evidence and implications of deviations, turns and changes in direction by dinosaurian trackmakers. Historical Biology, 33:3326-3339.
https://doi.org/10.1080/08912963.2020.1865945
Lockley, M.G. and Meyer, C.A. 2022. The megatracksite phenomenon: implications for tetrapod palaeobiology across terrestrial-shallow marine transitional zones. Geological Society, London, Special Publications, 522:285-324.
https://doi.org/10.1144/SP522-2021-164
Lockley, M.G., Lallensack, J.N., Sciscio, L., and Bordy, E.M. 2023. The early Mesozoic saurischian trackways Evazoum and Otozoum: Implications for ‘prosauropod’ (basal sauropodomorph) gaits. Historical Biology, 1-19.
https://doi.org/10.1080/08912963.2022.2163170
Loope, D.B. 2006. Dry-season tracks in dinosaur-triggered grainflows. PALAIOS, 21:132-142. https://doi.org/10.2110/palo.2005.p05-55
Low, J.L. and Reed, A. 1996. Basic Biomechanics Explained. Butterworth-Heinemann Medical.
Lucas, A.M. and Stettenheim, P.R. 1972. Avian Anatomy-Integument. Agricultural Handbook 362. US Department of Agriculture, Washington, D.C., 485-635.
Lucas, S.G. 2001. Taphotaxon. Lethaia, 34:30-30.
Lucas, S.G. 2007. Tetrapod footprint biostratigraphy and biochronology. Ichnos, 14:5-38.
https://doi.org/10.1080/10420940601006792
Lucas, S.G. 2015. Thinopus and a critical review of Devonian tetrapod footprints. Ichnos, 22:136-154.
https://doi.org/10.1080/10420940.2015.1063491
Lucas, S.G. and Harris, J.D. 2020. The “plastotype problem” in ichnological taxonomy. Ichnos, 27:107-110.
https://doi.org/10.1080/10420940.2019.1688802
Lucas, S.G. and Hunt, A.P. 2007. Ichnotaxonomy of camel footprints. Cenozoic Vertebrate Tracks and Traces. New Mexico of Natural History and Science Bulletin, 42:155-168.
Lyman, R.L. 2010. What taphonomy is, what it isn’t, and why taphonomists should care about the difference. Journal of Taphonomy, 8:1-16.
MacEachern, J.A., Bann, K.L., Gingras, M.K., Zonneveld, J.-P., Dashtgard, S.E., and Pemberton, S.G. 2012. Chapter 4 - The Ichnofacies Paradigm, p. 64, 103-138. In Knaust, D. and Bromley, R.G. (eds.), Developments in Sedimentology. Trace Fossils as Indicators of Sedimentary Environments. Elsevier.
https://doi.org/10.1016/B978-0-444-53813-0.00004-6
Manning, P.L. 2004. A new approach to the analysis and interpretation of tracks: examples from the Dinosauria. Geological Society, London, Special Publications, 228:93-123. In McIlroy, D. (ed.), The Application of lchnology to Palaeoenvironmental and Stratigraphic Analysis.
Manning, P.L., Ott, C., and Falkingham, P.L. 2008. A probable tyrannosaurid track from the Hell Creek Formation (Upper Cretaceous), Montana, United States. PALAIOS, 23:645-647.
https://doi.org/10.2110/palo.2008.p08-030r
Marchetti, L., Mujal, E., and Bernardi, M. 2017. An unusual Amphisauropus trackway and its implication for understanding seymouriamorph locomotion. Lethaia, 50:162-174.
https://doi.org/10.1111/let.12184
Marchetti, L., Tessarollo, A., Felletti, F., and Ronchi, A. 2017. Tetrapod footprint paleoecology: Behavior, taphonomy and ichnofauna disentangled. A case study from the Lower Permian of the southern Alps (Italy). PALAIOS, 32:506-527.
https://doi.org/10.2110/palo.2016.108
Marchetti, L., Belvedere, M., Voigt, S., Klein, H., Castanera, D., Díaz-Martínez, I., Marty, D., Xing, L., Feola, S., Melchor, R.N., and Farlow, J.O. 2019a. Defining the morphological quality of fossil footprints. Problems and principles of preservation in tetrapod ichnology with examples from the Palaeozoic to the present. Earth-Science Reviews, 193:109-145.
https://doi.org/10.1016/j.earscirev.2019.04.008
Marchetti, L., Belvedere, M., Voigt, S., Klein, H., Castanera, D., Díaz-Martínez, I., Marty, D., Xing, L., Feola, S., and Melchor, R.N. 2020. Reply to discussion of “Defining the morphological quality of fossil footprints. Problems and principles of preservation in tetrapod ichnology with examples from the Palaeozoic to the present” by Marchetti et al. (2019). Earth Science Reviews, 208:103319.
https://doi.org/10.1016/j.earscirev.2020.103319
Margetts, L., Leng, J., Smith, I.M., and Manning, P.L. 2006. Parallel three-dimensional finite element analysis of dinosaur trackway formation, p. 1st ed., 743-749. In Schweigert, H.F. (ed.), Numerical Methods in Geotechnical Engineering. CRC Press, London.
Martin, A.J., Rich, T.H., Hall, M., Vickers-Rich, P., and Vazquez-Prokopec, G. 2012. A polar dinosaur-track assemblage from the Eumeralla Formation (Albian), Victoria, Australia. Alcheringa: An Australasian Journal of Palaeontology, 36:171-188.
https://doi.org/10.1080/03115518.2011.597564
Martin, A.J., Vickers-Rich, P., Rich, T.H., and Hall, M. 2014. Oldest known avian footprints from Australia: Eumeralla Formation (Albian), Dinosaur Cove, Victoria. Palaeontology, 57:7-19.
https://doi.org/10.1111/pala.12082
Martin, R. 1914. Lehrbuch der Anthropologie in systematischer Darstellung. Verlag von Gustav Fischer, Jena.
https://doi.org/10.11588/DIGLIT.37612
Marty, D. 2008. Sedimentology, taphonomy, and ichnology of Late Jurassic dinosaur tracks from the Jura carbonate platform (Chevenez-Combe Ronde tracksite, NW Switzerland): insights into the tidal-flat palaeoenvironment and dinosaur diversity, locomotion, and palaeoecology. GeoFocus, 21:1-278.
Marty, D. and Meyer, C. 2006. Sauropod trackway patterns expression of special behaviour related to substrate consistency. An example from the Late Jurassic of northwestern Switzerland. Hantkeniana: Contributions of the Department of Palaeontology, Eötvös University, 5:38.
Marty, D., Strasser, A., and Meyer, C.A. 2009. Formation and taphonomy of human footprints in microbial mats of present-day tidal-flat environments: implications for the study of fossil footprints. Ichnos, 16:127-142.
https://doi.org/10.1080/10420940802471027
Marty, D., Falkingham, P.L., and Richter, A. 2016. Dinosaur track terminology: a Glossary of terms, p. 399-400. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press, Bloomington.
Matthews, N.A. and Breithaupt, B.H. 2001. Close-range Photogrammetric Experiments at Dinosaur Ridge. The Mountain Geologist, 38:147-153.
Matthews, N.A., Noble, T., and Breithaupt, B.H. 2016. Close-Range Photogrammetry for 3-D Ichnology: The Basics of Photogrammetric Ichnology, p. 28-55. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press, Bloomington, Indiana.
Mazin, J.-M., Billon-Bruyat, J.-P., and Padian, K. 2009. First record of a pterosaur landing trackway. Proceedings of the Royal Society B: Biological Sciences, 276:3881-3886.
https://doi.org/10.1098/rspb.2009.1161
McAllister, J.A. 1989. Subaqueous vertebrate footmarks from the upper Dakota Formation (Cretaceous) of Kansas, USA. Occasional Papers of the Museum of Natural History, the University of Kansas, 127:1-22.
McAllister, J.A. and Kirby, J. 1998. An occurrence of reptile subaqueous traces in the Moenkopi Formation (Triassic) of Capitol Reef National Park, south central Utah, USA. National Park Service, Technical Report, 98:45-49.
McClymont, J. and Crompton, R.H. 2021. Repetition without repetition: A comparison of the Laetoli G1, Ileret, Namibian Holocene and modern human footprints using pedobarographic statistical parametric mapping, p. 41-65. In Pastoors, A. and Lenssen-Erz, T. (eds.), Reading Prehistoric Human Tracks. Springer International Publishing, Cham.
https://doi.org/10.1007/978-3-030-60406-6_3
McCrea, R.T., Tanke, D.H., Buckley, L.G., Lockley, M.G., Farlow, J.O., Xing, L., Matthews, N.A., Helm, C.W., Pemberton, S.G., and Breithaupt, B.H. 2015. Vertebrate ichnopathology: pathologies inferred from dinosaur tracks and trackways from the Mesozoic. Ichnos, 22:235-260.
https://doi.org/10.1080/10420940.2015.1064408
McElroy, E.J. and Granatosky, M.C. 2022. The evolution of asymmetrical gaits in gnathostome vertebrates. Journal of Experimental Biology, 225:jeb243235.
https://doi.org/10.1242/jeb.243235.
McIlroy, D. 2004. Some ichnological concepts, methodologies, applications and frontiers. Geological Society, London, Special Publications, 228:3-27.
https://doi.org/10.1144/GSL.SP.2004.228.01.02
Meyer, C., Lockley, M.G., Robinson, J.W., and Dos Santos, V.F. 1994. A comparison of well-preserved sauropod tracks from the Late Jurassic of Portugal and the Western United States: evidence and implications. Gaia, 10:57-64.
Michilsens, F., Aerts, P., Van Damme, R., and D’Août, K. 2009. Scaling of plantar pressures in mammals. Journal of Zoology, 279:236-242.
https://doi.org/10.1111/j.1469-7998.2009.00611.x
Miller, W. 2007. Complex trace fossils, p. 458-465. In Miller, W. (ed.), Trace Fossils. Elsevier.
Milner, A.R., Harris, J.D., Lockley, M.G., Kirkland, J.I., and Matthews, N.A. 2009. Bird-like anatomy, posture, and behavior revealed by an Early Jurassic theropod dinosaur resting trace. PLoS ONE, 4:e4591.
Milner, A.R.C. and Lockley, G.M. 2016. Dinosaur swim track assemblages: characteristics, contexts, and ichnofacies implications, p. 152-180. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press.
Minetti, A.E. 1998. The biomechanics of skipping gaits: a third locomotion paradigm? Proceedings of the Royal Society of London. Series B: Biological Sciences, 265:1227-1233.
https://doi.org/10.1098/rspb.1998.0424
Minter, N.J., Braddy, S.J., and Davis, R.B. 2007. Between a rock and a hard place: arthropod trackways and ichnotaxonomy. Lethaia, 40:365-375.
Morales, M. 1987. Terrestrial fauna and flora from the Triassic Moenkopi Formation of the southwestern United States. Journal of the Arizona-Nevada Academy of Science, 22:1-19.
Moratalla, J.J. 1993. PhD Thesis, Universidad Autónoma de Madrid, Madrid.
Moreira, D.O., Alibhai, S.K., Jewell, Z.C., Da Cunha, C.J., Seibert, J.B., and Gatti, A. 2018. Determining the numbers of a landscape architect species (Tapirus terrestris), using footprints. PeerJ, 6:e4591.
https://doi.org/10.7717/peerj.4591
Moreno, K., Carrano, M.T., and Snyder, R. 2007. Morphological changes in pedal phalanges through ornithopod dinosaur evolution: A biomechanical approach. Journal of Morphology, 268:50-63.
https://doi.org/10.1002/jmor.10498
Morse, S.A., Bennett, M.R., Gonzalez, S., and Huddart, D. 2010. Techniques for verifying human footprints: reappraisal of pre-Clovis footprints in Central Mexico. Quaternary Science Reviews, Special Theme: Case Studies of Neodymium Isotopes in Paleoceanography 29:2571-2578.
https://doi.org/10.1016/j.quascirev.2010.03.012
Morse, S.A., Bennett, M.R., Liutkus-Pierce, C., Thackeray, F., McClymont, J., Savage, R., and Crompton, R.H. 2013. Holocene footprints in Namibia: The influence of substrate on footprint variability. American Journal of Physical Anthropology, 151:265-279.
https://doi.org/10.1002/ajpa.22276
Mossman, D.J., Bruning, R., and Powell, H.P. 2003. Anatomy of a Jurassic theropod trackway from Ardley, Oxfordshire, U.K. Ichnos, 10:195-207.
https://doi.org/10.1080/10420940390257941
Mujal, E., Marchetti, L., Schoch, R.R., and Fortuny, J. 2020. Upper Paleozoic to Lower Mesozoic tetrapod ichnology revisited: Photogrammetry and relative depth pattern inferences on functional prevalence of autopodia. Frontiers in Earth Science, 8.
https://doi.org/10.3389/feart.2020.00248
Mukhra, R., Krishan, K., and Kanchan, T. 2018. Bare footprint metric analysis methods for comparison and identification in forensic examinations: A review of literature. Journal of Forensic and Legal Medicine, 58:101-112.
https://doi.org/10.1016/j.jflm.2018.05.006
Müller, A.H. 1962. Zur Ichnologie, Taxiologie und Ökologie fossiler Tiere, Teil 1. Freiberger Forschungshefte, C 151:5-49.
Murie, O.J. 1982. A field guide to animal tracks. 2nd edition. Houghton Mifflin Company, Boston.
Muybridge, E. 1899. Animals in motion. Chapman and Hall, London.
Myers, T.S. and Fiorillo, A.R. 2009. Evidence for gregarious behavior and age segregation in sauropod dinosaurs. Palaeogeography, Palaeoclimatology, Palaeoecology, 274:96-104.
https://doi.org/10.1016/j.palaeo.2009.01.002
Olsen, P.E. 1980. Fossil great lakes of the Newark Supergroup in New Jersey, p. 352-398. In Manspeizer, W. (ed.), Field studies of New Jersey geology and guide to field trips: New York State Geological Association, 52nd Annual Meeting, Newark, New Jersey. Rutgers University, Newark College of Arts and Sciences, Geology Department.
Olsen, P.E. 1995. A new approach for recognizing track makers, p. 27, 72. Geological Society of America Abstracts with Programs.
Olsen, P.E. and Baird, D. 1986. The ichnogenus Atreipus and its significance for Triassic biostratigraphy, p. 61-87. In Padian, K. (ed.), The Beginning of the Age of Dinosaurs - Faunal Change Across the Triassic-Jurassic Boundary. Cambridge University Press, New York.
Olsen, P.E., Smith, J.B., and McDonald, N.G. 1998. Type material of the type species of the classic theropod footprint genera Eubrontes, Anchisauripus, and Grallator (Early Jurassic, Hartford and Deerfield basins, Connecticut and Massachusetts, USA). Journal of Vertebrate Paleontology, 18:586-601.
https://doi.org/10.1080/02724634.1998.10011086
Ostrom, J.H. 1972. Were some dinosaurs gregarious? Palaeogeography, Palaeoclimatology, Palaeoecology, 11:287-301.
https://doi.org/10.1016/0031-0182(72)90049-1
Oukassou, M., Klein, H., Lagnaoui, A., Charrière, A., Saber, H., Gierliński, G.D., Lallensack, J.N., Hminna, A., Boumaalif, A., Oussou, A., and Ouarhache, D. 2019. Polyonyx -like tracks from Middle-?Upper Jurassic red beds of Morocco: Implications for sauropod communities on southern margins of Tethys. Palaeogeography, Palaeoclimatology, Palaeoecology, 536:109394.
https://doi.org/10.1016/j.palaeo.2019.109394
Oussou, A., Falkingham, P.L., Butler, R.J., Boumir, K., Ouarhache, D., Ech-charay, K., Charrière, A., and Maidment, S.C.R. 2023. New Middle to ?Late Jurassic dinosaur tracksites in the Central High Atlas Mountains, Morocco. Royal Society Open Science, 10:231091.
https://doi.org/10.1098/rsos.231091
Parker, L.R. and Rowley, Jr., R.L. 1989. Dinosaur footprints from a coal mine in east-central Utah, p. 361-366. In Gillette, D.D. and Lockley, M.G. (eds.), Dinosaur Tracks and Traces. Cambridge University Press, Cambridge, UK.
Peabody, F.E. 1948. Reptile and amphibian trackways from the Lower Triassic Moenkopi Formation of Arizona and Utah. California University, Bulletin Department of Geological Sciences, 27:295-468.
Peabody, F.E. 1959. Trackways of living and fossil salamanders. University of California, Publications in Zoology, 63:1-72.
Pérez-Lorente, F. 2015. Dinosaur Footprints and Trackways of La Rioja. Indiana University Press, Bloomington, Indiana.
Petti, F.M., Avanzini, M., Nicosia, U., Girardi, S., Bernardi, M., Ferretti, P., Schirolli, P., and Sasso, C.D. 2009. Late Triassic (early-middle Carnian) chirotherian tracks from the Val Sabbia Sandstone (eastern Lombardy, Brescian Prealps, Northern Italy). Rivista Italiana di Paleontologia e Stratigrafia, 115:277-290.
Pfau, T., Hinton, E., Whitehead, C., Wiktorowicz-Conroy, A., and Hutchinson, J.R. 2011. Temporal gait parameters in the alpaca and the evolution of pacing and trotting locomotion in the Camelidae. Journal of Zoology, 283:193-202.
https://doi.org/10.1111/j.1469-7998.2010.00763.x
Platt, B.F. and Hasiotis, S.T. 2008. A new system for describing and classifying tetrapod tail traces with implications for interpreting the dinosaur tail trace record. PALAIOS, 23:3-13.
https://doi.org/10.2110/palo.2006.p06-111r
Platt, B.F., Suarez, C.A., Boss, S.K., Williamson, M., Cothren, J., and Kvamme, J.A.C. 2018. LIDAR-based characterization and conservation of the first theropod dinosaur trackways from Arkansas, USA. PLoS ONE, 13:e0190527.
https://doi.org/10.1371/journal.pone.0190527
Plotnick, R.E. 2012. Behavioral biology of trace fossils. Paleobiology, 38:459-473.
https://doi.org/10.1666/11008.1
Polet, D.T. and Hutchinson, J.R. 2022. Estimating gaits of an ancient crocodile-line archosaur through trajectory optimization, with comparison to fossil trackways. Frontiers in Bioengineering and Biotechnology, 9.
https://doi.org/10.3389/fbioe.2021.800311
Proctor, N.S. and Lynch, P.J. 1993. Manual of Ornithology: Avian Structure & Function. Yale University Press.
Quicke, D.L.J. 2013. Principles and Techniques of Contemporary Taxonomy. Springer Science & Business Media.
Rachal, D.M., Zeigler, K., Dello-Russo, R., and Solfisburg, C. 2021. Lake levels and trackways: An alternative model to explain the timing of human-megafauna trackway intersections, Tularosa Basin, New Mexico. Quaternary Science Advances, 3:100024.
https://doi.org/10.1016/j.qsa.2021.100024
Rainforth, E.C. 2002. Tails of saurischian dinosaurs in the Early Jurassic of the Newark Supergroup (eastern North America), p. 34, 61. Geological Society of America Abstracts with Programs.
Rainforth, E.C. 2005. Ichnotaxonomy of the fossil footprints of the Connecticut Valley (Early Jurassic, Newark Supergroup, Connecticut and Massachusetts). Ph.D. thesis, Columbia University, New York.
Rainforth, E.C. and Manzella, M. 2007. Estimating speeds of dinosaurs from trackways: a re-evaluation of assumptions, p. 41-48. Contributions to the Paleontology of New Jersey (II): Field Guide and Proceedings, Geological Association of New Jersey 24th Annual Conference and Field Trip.
Razzolini, N.L., Vila, B., Castanera, D., Falkingham, P.L., Barco, J.L., Canudo, J.I., Manning, P.L., and Galobart, À. 2014. Intra-trackway morphological variations due to substrate consistency: the El Frontal Dinosaur Tracksite (Lower Cretaceous, Spain). PLoS ONE, 9:e93708.
https://doi.org/10.1371/journal.pone.0093708
Razzolini, N.L., Vila, B., Díaz-Martínez, I., Manning, P.L., and Galobart, À. 2016. Pes shape variation in an ornithopod dinosaur trackway (Lower Cretaceous, NW Spain): new evidence of an antalgic gait in the fossil track record. Cretaceous Research, 58:125-134.
Reineck, H.-E. and Singh, I.B. 1980. Tool Marks, p. 78-83. In Reineck, H.-E. and Singh, I.B. (eds.), Depositional Sedimentary Environments: With Reference to Terrigenous Clastics. Springer Study Edition. Springer, Berlin, Heidelberg.
https://doi.org/10.1007/978-3-642-81498-3_7
Retallack, G.J. 1996. Early Triassic therapsid footprints from the Sydney basin, Australia. Alcheringa, 20:301-314.
Rey, J. and Galeotti, S. 2008. Stratigraphy: Terminology and Practice. Editions Technip, Paris.
Rich, V.I. and Maier, R.M. 2015. Chapter 6 - Aquatic Environments, p. 111-138. In Pepper, I.L., Gerba, C.P., and Gentry, T.J. (eds.), Environmental Microbiology (3rd edition). Academic Press, San Diego.
Richter, A. and Böhme, A. 2016. Too many tracks: preliminary description and interpretation of the diverse and heavily dinoturbated Early Cretaceous ‘Chicken Yard’ ichnoassemblage (Obernkirchen Tracksite, northern Germany), p. 334-357. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press.
Richter, R. 1928. Aktuopaläontologie und Paläobiologie, eine Abgrenzung. Senckenbergiana, 19:285-292.
Richter, R. 1936. Marken und Spuren aus dem Hunsrückschiefer. II. Schichtung und Grundleben. Senckenbergiana, 18:215-244.
Robbins, L.M. 1985. Footprints: Collection, Analysis, and Interpretation. Charles C Thomas Publisher, Springfield, Illinois.
Roca-Dols, A., Losa-Iglesias, M.E., Sánchez-Gómez, R., Becerro-de-Bengoa-Vallejo, R., López-López, D., Rodríguez-Sanz, D., Martínez-Jiménez, E.M., and Calvo-Lobo, C. 2018. Effect of the cushioning running shoes in ground contact time of phases of gait. Journal of the Mechanical Behavior of Biomedical Materials, 88:196-200.
https://doi.org/10.1016/j.jmbbm.2018.08.032
Romano, M. and Whyte, M.A. 2003. Jurassic dinosaur tracks and trackways of the Cleveland Basin, Yorkshire: preservation, diversity and distribution. Proceedings of the Yorkshire Geological Society, 54:185-215.
Romano, M., Whyte, M.A., and Jackson, S.J. 2007. Trackway ratio: A new look at trackway gauge in the analysis of quadrupedal dinosaur trackways and its implications for ichnotaxonomy. Ichnos, 14:257-270.
https://doi.org/10.1080/10420940601050014
Romano, M., Citton, P., and Avanzini, M. 2020. A review of the concepts of ‘axony’ and their bearing on tetrapod ichnology. Historical Biology, 32:611-619.
https://doi.org/10.1080/08912963.2018.1516766
Romer, A.S. 1933. Vertebrate Paleontology. University of Chicago Press, Chicago.
Romilio, A. and Salisbury, S.W. 2011. A reassessment of large theropod dinosaur tracks from the mid-Cretaceous (late Albian-Cenomanian) Winton Formation of Lark Quarry, central-western Queensland, Australia: A case for mistaken identity. Cretaceous Research, 32:135-142.
https://doi.org/10.1016/j.cretres.2010.11.003
Romilio, A. and Salisbury, S.W. 2014. Large dinosaurian tracks from the Upper Cretaceous (Cenomanian-Turonian) portion of the Winton Formation, Lark Quarry, central-western Queensland, Australia: 3D photogrammetric analysis renders the ‘stampede trigger’ scenario unlikely. Cretaceous Research, 51:186-207
https://doi.org/10.1016/j.cretres.2014.06.003
Romilio, A., Tucker, R.T., and Salisbury, S.W. 2013. Reevaluation of the Lark Quarry dinosaur Tracksite (late Albian-Cenomanian Winton Formation, central-western Queensland, Australia): no longer a stampede? Journal of Vertebrate Paleontology, 33:102-120.
https://doi.org/10.1080/02724634.2012.694591
Rose, K.D. 2006. The beginning of the age of mammals. Johns Hopkins university press, Baltimore, MD.
Rubenson, J., Heliams, D.B., Lloyd, D.G., and Fournier, P.A. 2004. Gait selection in the ostrich: mechanical and metabolic characteristics of walking and running with and without an aerial phase. Proceedings of the Royal Society of London. Series B: Biological Sciences, 271:1091-1099.
https://doi.org/10.1098/rspb.2004.2702
Sadlok, G. and Pawełczyk, K. 2021. Tetrapod swim techniques interpreted from swim trace fossils from the Lower Triassic Baranów Formation, Holy Cross Mountains, central Poland. PalZ, 95:167-177.
https://doi.org/10.1007/s12542-019-00510-w
Salisbury, S.W., Romilio, A., Herne, M.C., Tucker, R.T., and Nair, J.P. 2016. The dinosaurian ichnofauna of the Lower Cretaceous (Valanginian-Barremian) Broome Sandstone of the Walmadany area (James Price Point), Dampier Peninsula, Western Australia. Society of Vertebrate Paleontology Memoir 16. Journal of Vertebrate Paleontology, 36 (6, Supplement):152 pp.
https://doi.org/10.1080/02724634.2016.1269539
Santi, G. and Nicosia, U. 2008. The ichnofacies concept in vertebrate ichnology. Studi Trentini di Scienze Naturali, Acta Geologica, 83:223-229.
Sarjeant, W.A.S. 1990. A name for the trace of an act: approaches to the nomenclature and classification of fossil vertebrate footprints, p. 299-314. In Carpenter, K. and Currie, P.J. (eds.), Dinosaur Systematics - Approaches and Perspectives. Cambridge University Press, Cambridge, UK.
Sarjeant, W.A.S. and Stringer, P. 1978. Triassic reptile tracks in the Lepreau Formation, southern New Brunswick, Canada. Canadian Journal of Earth Sciences, 15:594-602.
https://doi.org/10.1139/e78-064
Sarjeant, W.A.S. and Reynolds, R.E. 1999. Camel and horse footprints from the Miocene of California. San Bernardino County Museum Association Quarterly, 46:3-20.
Savrda, C.E. 2007. Taphonomy of trace fossils, p. 92-109. In Miller, W. (ed.), Trace Fossils: Concepts, Problems, Prospects. Elsevier, Amsterdam.
Schaller, N.U., D’Août, K., Villa, R., Herkner, B., and Aerts, P. 2011. Toe function and dynamic pressure distribution in ostrich locomotion. Journal of Experimental Biology, 214:1123-1130.
https://doi.org/10.1242/jeb.043596
Schanz, T., Lins, Y., Viefhaus, H., Barciaga, T., Läbe, S., Preuschoft, H., Witzel, U., and Sander, P.M. 2013. Quantitative interpretation of tracks for determination of body mass. PLoS ONE, 8:e77606.
Schanz, T., Datcheva, M., Haase, H., and Marty, D. 2016. Analysis of desiccation crack patterns for quantitative interpretation of fossil tracks, p. 366-379. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press, Bloomington and Indianapolis.
Schmitt, D. 1999. June. Compliant walking in primates. Journal of Zoology, 248:149-160.
https://doi.org/10.1111/j.1469-7998.1999.tb01191.x
Schulp, A.S. 2002. The effects of tectonic deformation on dinosaur trackway morphology. Sargetia. Acta Musei Devensis, Series Scientia Naturae, Deva, 19:e32.
Schulp, A.S. and Brokx, W.A. 1999. Maastrichtian sauropod footprints from the Fumanya site, Berguedà, Spain. Ichnos, 6:239-250.
https://doi.org/10.1080/10420949909386455
Seilacher, A. 1953. Studien zur Palichnologie. I. Über die Methoden der Palichnologie. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 96:421-452.
Seilacher, A. 1964. Biogenic sedimentary structures. Approaches to Paleoecology, 296-316.
Seilacher, A. 1967. Bathymetry of trace fossils. Marine Geology, 5:413-428.
https://doi.org/10.1016/0025-3227(67)90051-5
Seilacher, A. 1986. Evolution of behavior as expressed in marine trace fossils, p. 62-87. In Nitecki, M.H. and Kitchell, J.A. (eds.), Evolution of Animal Behavior: Paleontological and Field Approaches. Oxford University Press.
Seilacher, A. 2007. Trace Fossil Analysis. Springer, Heidelberg.
Seilacher, A., Reif, W.-E., and Westphal, F. 1985. Sedimentological, ecological and temporal patterns of fossil Lagerstätten. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 311:5-24.
https://doi.org/10.1098/rstb.1985.0134
Sellers, W.I., Margetts, L., Coria, R.A., and Manning, P.L. 2013. March of the titans: The locomotor capabilities of sauropod dinosaurs. PLoS ONE, 8:e78733.
https://doi.org/10.1371/journal.pone.0078733
Silva, R.C.D., Ferigolo, J., Carvalho, I.D.S., and Fernandes, A.C.S. 2008. Lacertoid footprints from the Upper Triassic (Santa Maria Formation) of Southern Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology, 262:140-156.
https://doi.org/10.1016/j.palaeo.2008.02.006
Simpson, E.L., Wizevich, M.C., Reichard-Flynn, W.R., Keebler, A.M., Evans, S., and Kuslik, I. 2022. Pustularichnus rebeccahuntfosterae, a microbially induced sedimentary structure: Early Cretaceous Cedar Mountain Formation, Mill Canyon Dinosaur Tracksite, Moab, Utah. Fossil Record 8. New Mexico Museum of Natural History and Science Bulletin, 90:371-378.
Singh, V. 2014. Arches of the Foot, p. 2nd ed., Volume II, 432-438. Textbook of Anatomy - Abdomen and Lower Limb. Reed Elsevier India Private Limited, Haryana.
Soergel, W. 1925. Die Fährten der Chirotheria: eine paläobiologische Studie. Verlag von Gustav Fischer, Jena.
Stern, Jr., J.T. and Susman, R.L. 1983. The locomotor anatomy of Australopithecus afarensis. American Journal of Physical Anthropology, 60:279-317.
https://doi.org/10.1002/ajpa.1330600302
Stettenheim, P.R. 2000. The integumentary morphology of modern birds--an overview. American Zoologist, 40:461-477.
https://doi.org/10.1093/icb/40.4.461
Stevens, K.A., Ernst, S., and Marty, D. 2016. Uncertainty and ambiguity in the interpretation of sauropod trackways, p. 226-243. In Falkingham, P.L., Marty, D., and Richter, A. (eds.), Dinosaur Tracks: The Next Steps. Indiana University Press.
Stevens, K.A., Ernst, S., and Marty, D. 2022. Coupling length: a generalized gleno-acetabular distance measurement for interpreting the size and gait of quadrupedal trackmakers. Swiss Journal of Geosciences, 115:18.
https://doi.org/10.1186/s00015-022-00418-9
Stimson, M.R., Miller, R.F., MacRae, R.A., and Hinds, S.J. 2017. An ichnotaxonomic approach to wrinkled microbially induced Sedimentary structures. Ichnos, 24:291-316.
https://doi.org/10.1080/10420940.2017.1294590
Struble, M.K. and Gibb, A.C. 2022. Do we all walk the walk? A comparison of walking behaviors across tetrapods. Integrative and Comparative Biology, icac125.
https://doi.org/10.1093/icb/icac125
Therrien, F., Zelenitsky, D.K., Quinney, A., and Tanaka, K. 2015. Dinosaur trackways from the Upper Cretaceous Oldman and Dinosaur Park formations (Belly River Group) of southern Alberta, Canada, reveal novel ichnofossil preservation style. Canadian Journal of Earth Sciences, 52:630-641.
https://doi.org/10.1139/cjes-2014-0168
Thomson, T.J. and Lovelace, D.M. 2014. Swim track morphotypes and new track localities from the Moenkopi and Red Peak formations (Lower-Middle Triassic) with preliminary interpretations of aquatic behaviors. New Mexico Museum of Natural History and Science Bulletin, 62:103-128.
Thulborn, R.A. 1982. Speeds and gaits of dinosaurs. Palaeogeography, Palaeoclimatology, Palaeoecology, 38:227-256.
https://doi.org/10.1016/0031-0182(82)90005-0
Thulborn, R.A. 1990. Dinosaur Tracks. Chapman and Hall, London, New York.
Thulborn, R.A. 2004. Extramorphological features of sauropod dinosaur tracks in the Uhangri Formation (Cretaceous), Korea. Ichnos, 11:295-298.
https://doi.org/10.1080/10420940490442359
Thulborn, R.A. 2013. Lark Quarry revisited: a critique of methods used to identify a large dinosaurian track-maker in the Winton Formation (Albian-Cenomanian), western Queensland, Australia. Alcheringa: An Australasian Journal of Palaeontology, 37:312-330.
https://doi.org/10.1080/03115518.2013.748482
Thulborn, R.A. 2017. Behaviour of dinosaurian track-makers in the Winton Formation (Cretaceous, Albian-Cenomanian) at Lark Quarry, Western Queensland, Australia: Running or swimming? Ichnos, 24:1-18.
https://doi.org/10.1080/10420940.2015.1129326
Thulborn, R.A. and Wade, M. 1984. Dinosaur trackways in the Winton Formation (mid-Cretaceous) of Queensland. Memoirs of the Queensland Museum, 21:413-517.
Thulborn, R.A. and Wade, M. 1989. A footprint as a history of movement, p. 51-56. In Gillette, D.D. and Lockley, G.M. (eds.), Dinosaur Tracks and Traces. Cambridge University Press, Cambridge, UK.
Thulborn, R.A. 2012. Impact of sauropod dinosaurs on lagoonal substrates in the Broome Sandstone (Lower Cretaceous), Western Australia. PLoS ONE, 7:e36208.
https://doi.org/10.1371/journal.pone.0036208
Toledo, N. and Arregui, M. 2023. Concurrent evidence from ichnology and anatomy: the scelidotheriine ground sloths (Xenarthra, Folivora) from the Pleistocene of Argentina. Historical Biology, 35:284-292.
https://doi.org/10.1080/08912963.2022.2035379
Tucker, M.E. and Burchette, T.P. 1977. Triassic dinosaur footprints from south Wales: Their context and preservation. Palaeogeography, Palaeoclimatology, Palaeoecology, 22:195-208.
https://doi.org/10.1016/0031-0182(77)90028-1
Turner, M.L., Falkingham, P.L., and Gatesy, S.M. 2020. It’s in the loop: shared sub-surface foot kinematics in birds and other dinosaurs shed light on a new dimension of fossil track diversity. Biology Letters, 16:20200309.
https://doi.org/10.1098/rsbl.2020.0309
Turner, M.L., Falkingham, P.L., and Gatesy, S.M. 2022. What is stance phase on deformable substrates? Integrative and Comparative Biology, 1-12. https://doi.org/10.1093/icb/icac009.
Vallon, L.H., Rindsberg, A.K., and Martin, A.J. 2015. The use of the terms trace, mark and structure. Annales Societatis Geologorum Poloniae, 85:527-528.
https://doi.org/10.14241/asgp.2015.014
Vallon, L.H., Rindsberg, A.K., and Bromley, R.G. 2016. An updated classification of animal behaviour preserved in substrates. Geodinamica Acta, 28:5-20.
https://doi.org/10.1080/09853111.2015.1065306
Vincelette, A.R., Renders, E., Scott, K.M., Falkingham, P.L., and Janis, C.M. 2023. Hipparion tracks and horses’ toes: the evolution of the equid single hoof. Royal Society Open Science, 10:230358.
https://doi.org/10.1098/rsos.230358
Vintaned, J.G. and Liñan, E. 1996. Revisión de la terminología icnológica en español. Revista Española de Paleontología, 11:155-176.
Voigt, S. and Haubold, H. 2000. Analyse zur Variabilität der Tetrapodenfährte Ichniotherium cottae aus dem Tambacher Sandstein (Rotliegend, U-Perm, Thüringen). Hallesches Jahrbuch Geowissenschat B, 22:17-58.
Voigt, S. and Lucas, S.G. 2018. Outline of a Permian tetrapod footprint ichnostratigraphy. Geological Society, London, Special Publications, 450:387-404.
https://doi.org/10.1144/SP450.10
Wagensommer, A., Dolch, R., Ratolojanahary, T., Donato, S., and Porchetti, S. 2021. Defining the Bemaraha megatracksite: an update on dinosaur ichnology in Madagascar. Geological Society, London, Special Publications, 522.
https://doi.org/10.1144/SP522-2021-86
Wake, M.H. 2001. Tetrapod Limbless Locomotion. Encyclopedia of Life Sciences. John Wiley & Sons, Ltd.
Wang, W.J., Crompton, R.H., Li, Y., and Gunther, M.M. 2003. Energy transformation during erect and ‘bent-hip, bent-knee’ walking by humans with implications for the evolution of bipedalism. Journal of Human Evolution, 44:563-579.
https://doi.org/10.1016/S0047-2484(03)00045-9
Weems, R.E. 1992. A re-evaluation of the taxonomy of Newark Supergroup saurischian dinosaur tracks, using extensive statistical data from a recently exposed tracksite near Culpeper, Virginia. Proceedings 26th forum on the geology of industrial minerals. Virginia Division of Mineral Resources Publication, 119:113-127.
Weems, R.E. 2021. Behavioral patterns of the Late Triassic Kayentapus minor trackmakers at the Culpeper Quarry near Stevensburg, Virginia USA. Fossil Record 7. New Mexico Museum of Natural History & Science Bulletin, 82:459-474.
Widdowson, W. 1997. The geomorphological and geological importance of palaeosurfaces. Geological Society Special Publication, Palaeosurfaces: Recognition, Reconstruction and Palaeoenvironment:1-12.
Wilkinson, M.J., Menz, H.B., and Raspovic, A. 1995. The measurement of gait parameters from footprints. The Foot, 5:84-90.
https://doi.org/10.1016/0958-2592(95)90018-7
Wiseman, A.L.A. and De Groote, I. 2022. One size fits all? Stature estimation from footprints and the effect of substrate and speed on footprint creation. The Anatomical Record, 305:1692-1700.
https://doi.org/10.1002/ar.24833.
Wunderlich, R.E. 2022. Knuckle-Walking, p. 3795-3800. In Vonk, J. and Shackelford, T.K. (eds.), Encyclopedia of Animal Cognition and Behavior. Springer International Publishing, Cham.
Xing, L., Li, D., Falkingham, P.L., Lockley, M.G., Benton, M.J., Klein, H., Zhang, J., Ran, H., Persons, W.S., and Dai, H. 2016. Digit-only sauropod pes trackways from China - evidence of swimming or a preservational phenomenon? Scientific Reports, 6:21138.
https://doi.org/10.1038/srep21138
Xing, L., Lockley, M.G., Jia, C., Klein, H., Niu, K., Zhang, L., Qi, L., Chou, C., Romilio, A., and Wang, D. 2021. Lower Cretaceous avian-dominated, theropod, thyreophoran, pterosaur and turtle track assemblages from the Tugulu Group, Xinjiang, China: ichnotaxonomy and palaeoecology. PeerJ, 9:e11476.
https://doi.org/10.7717/peerj.11476
Xing, L., Wang, M., Klein, H., and Gao, J. 2024. Life on a lake bottom: diverse horseshoe crab and fish ichnofauna from Yanchang Group (Upper Triassic) of northern Shaanxi Province, China-Implications for palaeoenvironment. Historical Biology, 36:331-349.
https://doi.org/10.1080/08912963.2022.2162398
Yang, S., Lockley, M.G., Greben, R., Erickson, B.R., and Lim, S. 1995. Flamingo and duck‐like bird tracks from the Late Cretaceous and early Tertiary: Evidence and implications. Ichnos, 4:21-34.
https://doi.org/10.1080/10420949509380111
Zonneveld, J.-P., Fiorillo, A.R., Hasiotis, S., and Gingras, M.K. 2022. Tooth marks, gnaw marks, claw-marks, bite marks, scratch marks, etc: terminology in ichnology. Ichnos, 29:93-101.
https://doi.org/10.1080/10420940.2022.2058937
Zug, G.R. 1974. Crocodilian galloping: An unique gait for reptiles. Copeia, 1974:550-552.
https://doi.org/10.2307/1442557

