 Neoichnology of the eastern spadefoot toad, Scaphiopus holbrookii (Anura: Scaphiopodidae): criteria for recognizing anuran burrows in the fossil record
Neoichnology of the eastern spadefoot toad, Scaphiopus holbrookii (Anura: Scaphiopodidae): criteria for recognizing anuran burrows in the fossil record
Article number: 18.2.43A
https://doi.org/10.26879/558
Copyright Palaeontological Association, August 2015
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 20 April 2015. Acceptance: 22 July 2015
{flike id=1299}
ABSTRACT
Anurans first appear in the Early Jurassic, and although terrestrial anurans are considered to be ancestral, their early record is less understood than that of aquatic forms. Many extant terrestrial anurans produce burrows to escape unfavorable environmental conditions. Fossil anuran burrows could, therefore, serve as proxies for the presence of anurans if their morphology was known. The eastern spadefoot toad, Scaphiopus holbrookii (Anura: Scaphiopodidae), belongs to one of four groups of burrowing terrestrial anurans. This study describes the burrowing behaviors of S. holbrookii as well as the qualitative and quantitative morphology of their burrows produced in laboratory experiments with varying sediment conditions. The toads used a hindlimb-first burrowing technique moving a minimal amount of sediment to the surface. Three distinct architectures were produced: isolated, ovoid chambers, vertical shafts with ovoid chambers, and subvertical shafts with ovoid chambers. The shapes and sizes of the burrow chambers were similar to the occupying toad. Impressions on the burrow walls were produced by the hindlimbs and feet of the toads. Qualitative burrow morphology was consistent between individuals and experiments. Quantitative properties of the toad burrows were compared statistically to each other and to burrows of scorpions, salamanders, and skinks. Spadefoot toad burrows were similar to each other and different from those of the other animals. The results of this study will aid in the identification of anuran burrows in the fossil record by providing an analog for comparison. Application of this data can improve the understanding of the evolution of terrestrial anurans, their behavior, and paleoenvironmental significance.
Lauren M. Johnson. Department of Geological Sciences, Ohio University, 316 Clippinger Laboratories, Athens, Ohio 45701, USA lj237209@gmail.com
Daniel I. Hembree. Department of Geological Sciences, Ohio University, 316 Clippinger Laboratories, Athens, Ohio 45701, USA hembree@ohio.edu Corresponding author
Keywords: ichnology; trace fossil; anuran; amphibian; continental; paleoecology
Final citation: Johnson, Lauren M. and Hembree, Daniel I. 2015. Neoichnology of the eastern spadefoot toad, Scaphiopus holbrookii (Anura: Scaphiopodidae): criteria for recognizing anuran burrows in the fossil record. Palaeontologia Electronica 18.2.43A: 1-29. https://doi.org/10.26879/558
palaeo-electronica.org/content/2015/1299-neoichnology-of-toads
INTRODUCTION
Trace fossils are the products of behavioral interactions between an organism and a substrate or medium (Seilacher, 1967). Interpretations of the identity and behavior of the trace-making organism can be made aided by the analysis of trace fossil morphology (Bromley, 1996). In doing so, biodiversity can be estimated from trace fossil assemblages, improving interpretations made from body fossils alone since trace fossils record the presence of organisms inhabiting environments with low preservation potential such as soils (Bromley, 1996; Hasiotis, 2007; Hasiotis et al., 2007). Trace fossil morphology is also controlled by the environmental conditions under which the trace was produced allowing for the interpretation of conditions that cannot be determined from the physical sedimentary record (Bromley, 1996; Hasiotis, 2007; MacEachern et al., 2007b). As a result, paleoenvironmental and paleoecological interpretations that incorporate trace fossils are typically more accurate (Bromley, 1996; MacEachern et al., 2007a).
Members of the modern subclass Lissamphibia appear in the fossil record in the Early Triassic and include the orders Urodela (salamanders and newts), Apoda (caecilians), and Anura (toads and frogs) (Carroll, 2009). Prosalirus bitis, the earliest known anuran, is from the Early Jurassic Kayenta Formation of Arizona (Shubin and Jenkins, 1995). Fossil anurans from the Jurassic and Early Cretaceous are primarily aquatic forms (Nevo, 1968). The fossil record of terrestrial anurans is not well documented, although these are typically considered the more ancestral forms of the group (Carroll, 2009).
Burrowing behaviors are widespread among many extant terrestrial species of Anura. Anurans construct burrows in order to avoid extreme environmental conditions (Emerson, 1976; Pinder et al., 1992; Hasiotis et al., 2007). Burrows typically serve as short-term dwelling spaces for the animals, but can also be occupied for longer periods of time when the animal aestivates (Pinder et al., 1992). Dwelling and aestivating in a burrow below the sediment surface allows terrestrial anurans to maintain body water content (Pinder et al., 1992; Stebbins and Cohen, 1995).
The long evolutionary history of terrestrial anurans and their tendency to construct burrows in continental environments suggests that their trace fossils are likely abundant in the fossil record. There is, however, a lack of documentation of fossil anuran burrows. This limited record may be due to an inability to distinguish fossil toad burrows from those of other animals. Interpretations of the burrowing behaviors, burrowing methods, and tracemakers represented by trace fossils cannot be accurately deduced without taking into consideration information obtained by neoichnological studies (Hembree, 2013).
The purpose of this paper is to document the burrowing techniques, behaviors, and burrow morphologies of a modern species of terrestrial anuran, Scaphiopus holbrookii (Amphibia: Anura) (Figure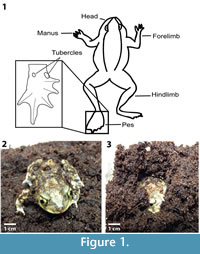 1). The morphology of the burrows constructed in different types of sediment are qualitatively and quantitatively described and compared to the burrows of other terrestrial animals produced in similar conditions. The goal of this research is to aid in the recognition of fossil anuran burrows by producing a model that defines the basic morphology of modern anuran burrows.
1). The morphology of the burrows constructed in different types of sediment are qualitatively and quantitatively described and compared to the burrows of other terrestrial animals produced in similar conditions. The goal of this research is to aid in the recognition of fossil anuran burrows by producing a model that defines the basic morphology of modern anuran burrows.
Anuran Ecology and Behavior
The Order Anura consists of approximately 6,436 species divided into the suborders Archaeobatrachia (four families, 27 species), Mesobatrachia (six families, 168 species), and Neobatrachia (21 families, > 5000 species) (Frost et al., 2006). General anuran morphology is characterized by a short body (< 1 cm to 30 cm) with only eight or nine dorsal vertebrae, a wide head with a large mouth, two extra bones in the ankle that significantly increase the length of their hindlimbs, a long rod-shaped bone, the urostyle, in the pelvis, and lack of a tail (Stebbins and Cohen, 1995).
Anurans inhabit a wide range of environments and climates in North, Central, and South America, Europe, Asia, Africa, and Australia (Stebbins and Cohen, 1995). Some anurans inhabit regions that experience strong seasonality, and a few species are capable of living in very dry areas such as grasslands and deserts (Stebbins and Cohen, 1995). The majority of anurans, however, occur in hot and humid, tropical regions (Stebbins and Cohen, 1995).
Since the Early Jurassic, the locomotion mechanism used by anurans has been a unique saltatory mechanism (Carroll, 2009). Anurans have long and extensible hindlimbs and more proximally positioned muscle masses that allow for saltatory locomotion (Emerson, 1982). The specialized morphology of anurans also enhances their ability to burrow (Hildebrand and Goslow, 1974). Four groups of terrestrial anurans are highly specialized burrowers: Breviceptidae, Bufonidae, Schaphiopodidae, and Pelobatidae (Voorhies, 1983). There are two main types of burrowing techniques used by anurans: hindlimb-first digging and headfirst digging. Hindlimb-first digging is considered to have evolved first in anurans; it is a behavior unique to anurans and is employed by more than 95% of burrowing species (Emerson, 1976). In hindlimb-first digging, the well-developed hindlimbs are used to excavate sediment from behind the anuran while the animal drops down and backwards into the developing burrow (Emerson, 1976). The forelimbs are primarily used to brace the anuran during excavation and to reposition the body (Emerson, 1976). Headfirst digging involves the use of anuran’s snout to first penetrate the soil followed by sweeping sediment out of the developing burrow with the forelimbs (Emerson, 1976). The hindlimbs are used only to reposition the body as the burrow is excavated (Emerson, 1976). Headfirst burrowers tend to be entirely fossorial, developing elongate tunnels, but include only nine species of extant anurans (Emerson, 1976). Anurans construct burrows primarily to serve as temporary shelters to maintain body water content and avoid short-term extremes in temperatures (Pinder et al., 1992).
Scaphiopus holbrookii, or the eastern spadefoot toad, is a member of the Family Scaphiopodidae (Figure 1). Three features characterize the Family Schaphiopodidae: 1) fusion of the joint between the sacrum and the urostyle; 2) exostose frontoparietals; and 3) presence of a metatarsal spade supported by a well-ossified prehallux (Figure 1) (Stebbins and Cohen, 1995). In addition, S. holbrookii has broad, sharp-edged epidermal tubercles (“spades”) located on the inner edge of the hind feet that assist in digging (Figure 1.1) (Voorhies, 1983). Scaphiopus holbrookii inhabits arid to semiarid regions in fields, farmlands, dunes, and woodlands and is well-adapted to burrowing (Pearson, 1955). Spadefoot toads spend most of their lives in their burrows and come to the surface at night, when the air is moist, to hunt for food (Pearson, 1955). They are both active and inactive hunters that feed on a variety of invertebrates, including beetles, snails, spiders, and caterpillars (Pearson, 1955). Spadefoot toads generally use an ambush method of hunting, staying inside their burrows waiting for prey to pass by the opening (Pearson, 1955).
MATERIALS AND METHODS
Four adult individuals of Scaphiopus holbrookii were used in three separate experiments. The toads were housed individually in 20-gallon (76 L) (60l × 30w × 42h cm) terraria (one toad per terrarium) filled with sediment composed of organic matter and sand to a depth of 25 cm. The sediment surface was smoothed and leveled prior to placing specimens in the terraria. The moisture content of the sediment was set at 60%, maintained by daily spraying of the sediment surface, and checked in the center of the tank using an Aquaterr EC-300 Multimeter. The air temperature was kept at 25-30° C and air moisture was 30-50%. The laboratory was set on an 8-hour light and 16-hour dark cycle. The toads were fed live crickets once per week during and between experiments.
Three experiments were conducted (Table 1). Experiment 1 was designed as a reference to observe the burrowing behavior of the toads in a setting similar to their natural environment. The composition of the sediment was modeled after the density and organic content of soil found in a typical habitat of Scaphiopus holbrookii. The sediment composition used in Experiment 1 was 100% coconut fiber, an organic-rich, fine-grained medium (Table 1). The lower 23 cm of the sediment was firmly packed whereas the upper 2 cm was loosely packed. Seven trials of Experiment 1 were run (Table 1); five trials were 10-14 days in duration and two were 30-40 days in duration to test the effects of time of occupation on burrow morphology.
The second and third experiments were designed to observe changes, if any, in burrowing behavior and burrow morphology in varying sediment compositions. The sediment composition used in Experiment 2 consisted of alternating layers of coconut fiber and sand. The bottom 15 cm of the terrarium was filled with firmly packed coconut fiber. The top 10 cm consisted of alternating, 2 cm layers of coconut fiber and fine- to medium-grained quartz sand. Four trials of Experiment 2 were run each lasting 14 days (Table 1). The sediment composition of Experiment 3 consisted of a 1:1 (by volume), homogenous mixture of coconut fiber and sand. Three trials of Experiment 3 were run each lasting 7 days, although they were planned for 10-14 days (Table 1). The duration of Experiment 3 was shortened as a result of observed stressed behavior from the toads.
In addition to the three experiments, individual toads were placed in a narrow (10 cm wide) terrarium filled to a depth of 50 cm with 100% coconut fiber. These terrariums permitted better observation of the body movements used by the toads when burrowing and increasing the likelihood of observing subsurface burrowing.
The toads were observed daily over the course of the experiments. Using digital photography and digital video recordings, each toad’s behavior associated with constructing and occupying burrows was documented. Once an experiment concluded, the toad was removed from the terrarium. Toads not at the sediment surface upon conclusion of an experiment were removed from the terrarium through careful excavation. These excavated burrows were not cast, but their general morphology was noted. The toads remained out of experiments for a week to provide time for acclimation to background laboratory conditions.
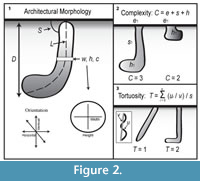 Open burrows were cast with Drystone© plaster. Once the plaster cured, casts were excavated and extracted from the terrarium. Burrows were catalogued with the code SH (Scaphiopus holbrookii) and a number based on their order of collection. The burrow casts were described using qualitative ichnotaxobases including general form, types of walls, and surface ornamentation. The ichnotaxobases were used to define distinct and consistent burrow architectures. Following the methods of Hembree et al. (2012), quantitative data was also obtained from the casts. Measurements of the depth, length, slope, angle of branching, tunnel height, width, circumference, and cross-sectional width-to-height ratio were collected (Figure 2.1). Depth, length, and circumference were determined with a tape measure; tunnel width and height were measured with a digital caliper. Two scale-independent measurements were calculated: complexity and tortuosity (Meadows, 1991; Hembree and Hasiotis, 2006). Complexity (C) is calculated by summing three variables: the number of openings at the surface of the sediment (e), the number of segments (s), and the number of chambers present (h): C = e + s + h (Figure 2.2). Tortuosity is obtained by dividing the total length of a segment (u) by the straight-line distance between the ends of a segment (v) (Figure 2.3). These scale-independent measurements allow for the comparison of burrow systems produced by animals of contrasting size (Meadows, 1991).
Open burrows were cast with Drystone© plaster. Once the plaster cured, casts were excavated and extracted from the terrarium. Burrows were catalogued with the code SH (Scaphiopus holbrookii) and a number based on their order of collection. The burrow casts were described using qualitative ichnotaxobases including general form, types of walls, and surface ornamentation. The ichnotaxobases were used to define distinct and consistent burrow architectures. Following the methods of Hembree et al. (2012), quantitative data was also obtained from the casts. Measurements of the depth, length, slope, angle of branching, tunnel height, width, circumference, and cross-sectional width-to-height ratio were collected (Figure 2.1). Depth, length, and circumference were determined with a tape measure; tunnel width and height were measured with a digital caliper. Two scale-independent measurements were calculated: complexity and tortuosity (Meadows, 1991; Hembree and Hasiotis, 2006). Complexity (C) is calculated by summing three variables: the number of openings at the surface of the sediment (e), the number of segments (s), and the number of chambers present (h): C = e + s + h (Figure 2.2). Tortuosity is obtained by dividing the total length of a segment (u) by the straight-line distance between the ends of a segment (v) (Figure 2.3). These scale-independent measurements allow for the comparison of burrow systems produced by animals of contrasting size (Meadows, 1991).
Comparative analysis of the quantitative properties of the toad burrows was performed using the Bray-Curtis similarity measure, a nonparametric statistical analysis that determines the degree of similarity between objects represented by multiple quantitative measurements (Bray and Curtis, 1957). All of the quantitative properties of each burrow were statistically analyzed simultaneously. The Bray-Curtis similarity measure ranks the burrows based on the similarity of these quantitative properties. The numerical value representing the degree of similarity ranges from 0.0 (most dissimilar) to 1.0 (identical). Burrows with a similarity value of 1.0 were considered identical whereas values between 0.9-0.8 were highly similar, 0.7-0.6 were moderately similar, and ≤ 0.5 were dissimilar (e.g., Hembree et al., 2012: Hembree, 2013; Bowen and Hembree, 2014; Catena and Hembree, 2014; Dzenowski and Hembree, 2014; Hils and Hembree, 2015). These analyses were performed to determine if the toads produced consistent, repeatable burrow morphologies. The Bray-Curtis test was then used to compare the toad burrow casts to the burrow casts of other animals obtained in previously conducted experiments including scorpions (Hembree, 2014), salamanders (Dzenowski and Hembree, 2014), and skinks (Catena and Hembree, 2014). These analyses were used to evaluate the relative uniqueness of toad burrow morphology.
Cluster analyses were performed using the Bray-Curtis similarity measure method to produce similarity dendrograms. The cluster analyses allow for better visualization of the relationships between burrow morphology and the tracemaker. Based on the analytical results of the Bray-Curtis analysis, the degree of association between burrow morphology and tracemaker can be evaluated with the cluster diagrams.
The quantitative properties driving the differentiation of burrows into discrete groups were evaluated using Mann-Whitney and Kolmogorov-Smirnov tests. The Mann- Whitney test evaluates the medians of two samples to determine whether or not they are from the same population. The Kolmogorov-Smirnov test determines if two samples are similar based on the distribution of values within the sample. Eight individual properties were compared for each test and included: 1) depth, 2) length, 3) mean width, 4) mean height, 5) mean circumference, 6) mean slope, 7) complexity, and 8) tortuosity. The tests were used to determine if statistically significant changes occurred in burrow properties produced by Scaphiopus holbrookii in the three different sediment compositions, to determine if the eight quantitative burrow properties of S. holbrookii were different from those of other burrowing animals, and to determine which burrow properties drove the major groupings to occur in the Bray-Curtis cluster diagrams.
RESULTS
Specimens of Scaphiopus holbrookii produced permanent, open burrows of three distinct morphologies based on qualitative and quantitative properties independent of the sediment composition: vertical shafts with ovoid terminal chambers (VS), subvertical shafts with ovoid terminal chambers (SS), and isolated ovoid chambers (IC) (Table 1). A total of 41 complete and well-preserved burrow casts were produced from the 13 experimental trials. All of the experimental trials yielded at least one well-preserved burrow that could be cast. Though rare, some burrows were destroyed before they could be cast primarily due to gravitational collapse. In all of the experiments, however, the architectural morphology of the burrows in situ was recorded.
Behavior
Within 1-3 hours after placing specimens of Scaphiopus holbrookii in the experimental terraria, open burrows were constructed and the toads were well beneath the surface of the sediment. For the brief interval of time before burrowing, the degree of movement above the surface varied. Upon placement into the terraria, both highly active behavior and relative stillness were observed. In the experiments consisting of terraria filled with 100% coconut fiber, one to four burrows were constructed within the first 72 hours of the trial. More burrows, from four to six, were constructed within the first 72 hours when the sediment consisted of alternating layers of coconut fiber and sand or a mixture of coconut fiber and sand. There was no consistency regarding the location of burrow openings with respect to the walls of the terraria.
 Scaphiopus holbrookii burrowed using a hindlimb first burrowing technique (Appendix 1). The hindlimbs of the toad moved in a sweeping motion against the sediment. While the hindlimbs were more actively engaged in the burrowing process, the forelimbs were used to redirect minor amounts of loose sediment. During the construction of an open burrow it was observed that the entire body of the toad would rotate both clockwise and counterclockwise around the developing burrow opening while dropping backwards and downwards beneath the sediment surface. During initial construction of the burrow, loosely packed sediment was deposited above the toad so the opening of the burrow was partially sealed. The seal on the opening was removed once the toad exited the burrow, typically to feed, and thereafter the burrow remained open. Despite the temporary seal, the single opening of a burrow remained open for the majority of a trial. It was observed, however, that after 12-14 days the openings became filled with sediment, primary due to gravitational collapse. The toads came to the sediment surface to feed and hydrate only at times when the laboratory was dark.
Scaphiopus holbrookii burrowed using a hindlimb first burrowing technique (Appendix 1). The hindlimbs of the toad moved in a sweeping motion against the sediment. While the hindlimbs were more actively engaged in the burrowing process, the forelimbs were used to redirect minor amounts of loose sediment. During the construction of an open burrow it was observed that the entire body of the toad would rotate both clockwise and counterclockwise around the developing burrow opening while dropping backwards and downwards beneath the sediment surface. During initial construction of the burrow, loosely packed sediment was deposited above the toad so the opening of the burrow was partially sealed. The seal on the opening was removed once the toad exited the burrow, typically to feed, and thereafter the burrow remained open. Despite the temporary seal, the single opening of a burrow remained open for the majority of a trial. It was observed, however, that after 12-14 days the openings became filled with sediment, primary due to gravitational collapse. The toads came to the sediment surface to feed and hydrate only at times when the laboratory was dark.
Open burrows served as temporary to permanent dwellings. Permanent dwellings were defined as those that were constructed and maintained throughout the experimental period, even if they were unoccupied for short intervals. Dwellings that were abandoned and not later re-occupied during the experimental period were considered temporary. It was observed that the toads did not remain within a burrow for longer than 12-24 hours, leaving typically during dark hours to acquire food. The toads then either returned to the original burrow or excavated a new burrow. Toads were observed occupying and maintaining multiple burrows within a terrarium. Within the time interval between leaving and returning to a burrow, a varying number of new burrows were constructed.
Specimens of Scaphiopus holbrookii constructed open burrows regardless of the sediment composition (Table 1). Burrowing was more prolific in the alternating layers of coconut fiber and sand and the mixed coconut fiber and sand. This increased burrowing activity, however, was limited to the initial 1-3 days of an experimental trial.
Surface Morphology
In all of the experiments, mounds of excavated sediment surrounding the openings of the burrows altered the surface topography producing small-scale (1-3 cm) highs and lows. In addition, shallow pits excavated just below the surface of the sediment were observed regardless of sediment conditions (Figur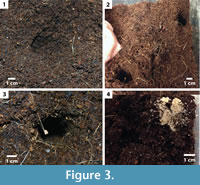 e 3.1). The behavior associated with these pits was not observed and is not clear; the spatial distribution appeared random. The surface openings of the burrows constructed by S. holbrookii were circular in shape (Figure 3.2-3). Both the size and shape of the openings were similar to those of the cross sections of the burrow shafts. A minimal amount of sediment was transported to the surface and typically deposited over or near the burrow opening; this was most apparent in experiments containing sand layers (Figure 3.4).
e 3.1). The behavior associated with these pits was not observed and is not clear; the spatial distribution appeared random. The surface openings of the burrows constructed by S. holbrookii were circular in shape (Figure 3.2-3). Both the size and shape of the openings were similar to those of the cross sections of the burrow shafts. A minimal amount of sediment was transported to the surface and typically deposited over or near the burrow opening; this was most apparent in experiments containing sand layers (Figure 3.4).
Burrow Morphology
Burrow morphologies were generally similar across sediment types. Open burrows had sharp, irregular walls and showed no evidence of constructed lining. Surficial features were abundant on casts of burrows that were constructed in sandy media, whether layered or mixed with coconut fiber. The center of the chamber floors had a raised ridge preserved as an elongate furrow on the base of the chamber cast (Figure 4.1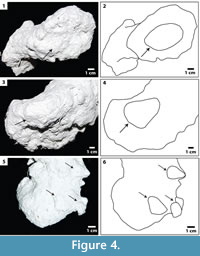 -2). Triangular protrusions made by the pes and hindlimbs during burrow construction occurred on chamber walls and floors (Figure 4.3-6). The size and shape of the protrusions were consistent with that of the limbs, with mean lengths and widths of 2.24 cm and 1.20 cm, respectively. Impressions made by the manus and forelimbs were present as well, but less abundant.
-2). Triangular protrusions made by the pes and hindlimbs during burrow construction occurred on chamber walls and floors (Figure 4.3-6). The size and shape of the protrusions were consistent with that of the limbs, with mean lengths and widths of 2.24 cm and 1.20 cm, respectively. Impressions made by the manus and forelimbs were present as well, but less abundant.
There were three basic burrow architectures including vertical shafts leading to an ovoid terminal chamber, subvertical shafts leading to an ovoid terminal chamber, and isolated ovoid chambers. All three architectures were produced in the three different experiments (Table 1). In addition, each individual toad constructed burrows of all three architectures.
Vertical shafts with ovoid chambers. This burrow architecture (n = 32) includes a single opening with a vertically oriented shaft (70-90°, mean = 89°, SD = 4°) that leads to a subvertically or vertically oriented (10-86°, mean = 55°, SD = 24°), ovoid chamber (Fi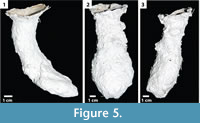 gure 5; Table 2). The shafts are circular in cross sections and have a width-to-height ratio of 0.70-1.96 (mean = 1.36, SD = 0.24). The shafts are 1.42-5.09 cm (mean = 2.94 cm, SD = 0.49 cm) wide and 1.05-3.61 cm (mean = 2.19 cm, SD = 0.47 cm) high with a circumference of 4.8-14.9 cm (mean = 8.9 cm, SD = 1.1 cm). The length of the entire burrow ranges from 6.5-11.8 cm (mean = 8.8 cm, SD = 1.5 cm). Chambers are elliptical in cross section and have a circumference of 7.3-14.9 cm (mean = 10.5 cm, SD = 1.5 cm). All vertical shafts possess a complexity value of 3; this value includes the single surface opening, a single tunnel, and a chamber. Tortuosity values of the vertical shafts range from 1.01-1.61 (mean = 1.24, SD = 0.18).
gure 5; Table 2). The shafts are circular in cross sections and have a width-to-height ratio of 0.70-1.96 (mean = 1.36, SD = 0.24). The shafts are 1.42-5.09 cm (mean = 2.94 cm, SD = 0.49 cm) wide and 1.05-3.61 cm (mean = 2.19 cm, SD = 0.47 cm) high with a circumference of 4.8-14.9 cm (mean = 8.9 cm, SD = 1.1 cm). The length of the entire burrow ranges from 6.5-11.8 cm (mean = 8.8 cm, SD = 1.5 cm). Chambers are elliptical in cross section and have a circumference of 7.3-14.9 cm (mean = 10.5 cm, SD = 1.5 cm). All vertical shafts possess a complexity value of 3; this value includes the single surface opening, a single tunnel, and a chamber. Tortuosity values of the vertical shafts range from 1.01-1.61 (mean = 1.24, SD = 0.18).
Subvertical shafts with ovoid chambers. This burrow architecture (n = 15) includes a single surface opening leading to a shallowly sloping (40-70°, mean = 41°, SD = 14°), non-branching shaft that ends in a subvertically to vertically oriented ovoid chamber similar to those in vertical shafts (Figur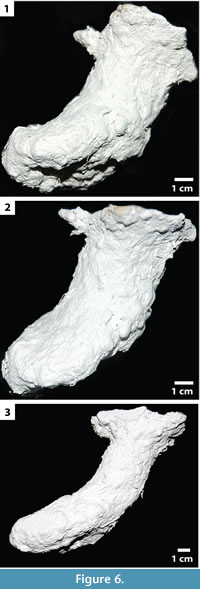 e 6; Table 3). The shafts are 1.76-3.27 cm (mean = 2.51 cm, SD = 0.49 cm) wide and 1.31-2.37 cm (mean = 1.74 cm, SD = 0.32 cm) high with a circumference of 5.9-8.6 cm (mean = 7.3 cm, SD = 0.9 cm). The width-to-height ratio ranges from 1.06-1.48 (mean = 1.36, SD = 0.14). The length of the entire burrow including the shaft and chamber varies from 4.7-12.1 cm (mean = 8.6 cm, SD = 1.9 cm). The subvertically oriented shafts are circular in cross section whereas the terminal chambers are elliptical in cross section. All subvertical shafts possess a complexity value of 3; this value includes the single surface opening, a single tunnel, and a chamber. Tortuosity values of the subvertical shafts range from 1.20-2.32 (mean = 1.51, SD = 0.44).
e 6; Table 3). The shafts are 1.76-3.27 cm (mean = 2.51 cm, SD = 0.49 cm) wide and 1.31-2.37 cm (mean = 1.74 cm, SD = 0.32 cm) high with a circumference of 5.9-8.6 cm (mean = 7.3 cm, SD = 0.9 cm). The width-to-height ratio ranges from 1.06-1.48 (mean = 1.36, SD = 0.14). The length of the entire burrow including the shaft and chamber varies from 4.7-12.1 cm (mean = 8.6 cm, SD = 1.9 cm). The subvertically oriented shafts are circular in cross section whereas the terminal chambers are elliptical in cross section. All subvertical shafts possess a complexity value of 3; this value includes the single surface opening, a single tunnel, and a chamber. Tortuosity values of the subvertical shafts range from 1.20-2.32 (mean = 1.51, SD = 0.44).
Isolated ovoid chambers. This burrow architecture (n = 27) includes a single surface opening that leads directly to a terminal chamber (Figur e 7; Table 4). Chambers are elliptical in cross section with a width-to-height ratio of 1.01-1.88 (mean = 1.38, SD = 0.21) and have a circumference ranging from 3.6-14.7 cm (mean = 10.0 cm, SD = 1.5 cm). The depth of the chambers varies from 2.5-7.9 cm (mean = 4.7 cm, SD = 1.4 cm), whereas the length of the chambers ranges from 3.3-9.4 cm (mean = 7.1 cm, SD = 2.0 cm). The chambers are 1.41-5.71 cm (mean = 3.28 cm, SD = 0.48 cm) wide and 1.13-4.32 cm (mean = 2.44 cm, SD = 0.44 cm) high. The width-to-height ratio of the chambers varies from 1.01-1.88 (mean = 1.38, SD = 0.20). The orientation of the chambers with respect to the surface of the sediment varies from 9-90° (mean = 49°, SD = 22°). The isolated chambers possess a complexity value of 2, which includes a single surface opening and a chamber. The tortuosity of the isolated chambers varies from 1.06-2.96 (mean = 1.57, SD = 0.47).
e 7; Table 4). Chambers are elliptical in cross section with a width-to-height ratio of 1.01-1.88 (mean = 1.38, SD = 0.21) and have a circumference ranging from 3.6-14.7 cm (mean = 10.0 cm, SD = 1.5 cm). The depth of the chambers varies from 2.5-7.9 cm (mean = 4.7 cm, SD = 1.4 cm), whereas the length of the chambers ranges from 3.3-9.4 cm (mean = 7.1 cm, SD = 2.0 cm). The chambers are 1.41-5.71 cm (mean = 3.28 cm, SD = 0.48 cm) wide and 1.13-4.32 cm (mean = 2.44 cm, SD = 0.44 cm) high. The width-to-height ratio of the chambers varies from 1.01-1.88 (mean = 1.38, SD = 0.20). The orientation of the chambers with respect to the surface of the sediment varies from 9-90° (mean = 49°, SD = 22°). The isolated chambers possess a complexity value of 2, which includes a single surface opening and a chamber. The tortuosity of the isolated chambers varies from 1.06-2.96 (mean = 1.57, SD = 0.47).
Burrow Analysis
Burrows produced by Scaphiopus holbrookii. Seventy-four burrows produced by Scaphiopus holbrookii were compared to each other to determine the level of similarity of the burrow morphologies within the species. All burrows produced by S. holbrookii, regardless of architecture, have a similarity of 0.36-0.99 with a mean similarity of 0.80. Similarity values below 0.50 only occur when comparing burrow SH16 to the other burrows produced by S. holbrookii. Excluding this burrow, similarity values range from 0.50-0.99 with a mean similarity of 0.80. A cluster analysis of the 74 burrows separates them into two major groups, Cluster 1 and Clusters 2, 3, and 4. These two groups have a similarity of 0.71 (Fig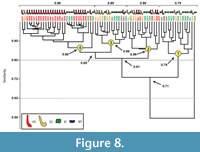 ure 8). Cluster 1 is composed of 11 isolated chambers as well as three subvertical shafts and one vertical shaft. Burrows in Cluster 1 have a similarity of 0.79. Cluster 1 differs from Clusters 2, 3, and 4 in 11 quantitative properties (Appendix 2.1). Clusters 2, 3, and 4 consist of all three burrow architectures produced by S. holbrookii (vertical shafts, subvertical shafts, and isolated chambers) and have a similarity of 0.81. Cluster 2 includes a mixture of burrow architectures (43% vertical shafts, 36% subvertical shafts, and 21% isolated chambers) which differ from burrows in Clusters 3 and 4 in terms of slope and tortuosity (Appendix 2.2). Clusters 3 and 4 consist of mostly vertical shafts (58% vertical shafts, 16% subvertical shafts, and 26% isolated chambers) with a similarity of 0.85 and differing in 12 quantitative properties (Appendix 2.3). Cluster 3 contains almost an equal amount of subvertical shafts and isolated chambers (43% subvertical shafts, 50% isolated chambers, and 7% vertical shafts) whereas Cluster 4 consists of mostly vertical shafts (81% vertical shafts, 3% subvertical shafts, and 16% isolated chambers).
ure 8). Cluster 1 is composed of 11 isolated chambers as well as three subvertical shafts and one vertical shaft. Burrows in Cluster 1 have a similarity of 0.79. Cluster 1 differs from Clusters 2, 3, and 4 in 11 quantitative properties (Appendix 2.1). Clusters 2, 3, and 4 consist of all three burrow architectures produced by S. holbrookii (vertical shafts, subvertical shafts, and isolated chambers) and have a similarity of 0.81. Cluster 2 includes a mixture of burrow architectures (43% vertical shafts, 36% subvertical shafts, and 21% isolated chambers) which differ from burrows in Clusters 3 and 4 in terms of slope and tortuosity (Appendix 2.2). Clusters 3 and 4 consist of mostly vertical shafts (58% vertical shafts, 16% subvertical shafts, and 26% isolated chambers) with a similarity of 0.85 and differing in 12 quantitative properties (Appendix 2.3). Cluster 3 contains almost an equal amount of subvertical shafts and isolated chambers (43% subvertical shafts, 50% isolated chambers, and 7% vertical shafts) whereas Cluster 4 consists of mostly vertical shafts (81% vertical shafts, 3% subvertical shafts, and 16% isolated chambers).
Comparisons with burrows of other animals. Burrows produced by Scaphiopus holbrookii were compared to burrows produced by emperor scorpions ( Pandinus imperator), tiger salamanders (Ambystoma tigrinum), and gold skinks (Mabuya multifasciata) (Figu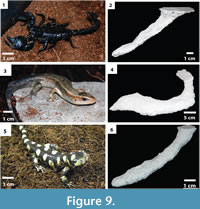 re 9). While these three species each produced a variety of different burrow architectures, only morphologies similar to the toad burrows were considered.
re 9). While these three species each produced a variety of different burrow architectures, only morphologies similar to the toad burrows were considered.
Comparison with Pandinus imperator burrows. Casts of nine subvertical tunnels produced by Pandinus imperator were compared to the 74 burrows produced by Scaphiopus holbrookii. Mann-Whitney and Kolmogorov-Smirnov tests show that 16 quantitative burrow properties are significantly different between burrows produced by S. holbrookii and burrows produced by P. imperator (Appendix 3.1). Bray-Curtis results show that the burrows of S. holbrookii and P. imperator have a similarity of 0.36-0.92 with a mean similarity of 0.62. A cluster analysis separates the burrows into two main clusters, designated A and B, with a similarity of 0.56 (Figure 10). Clusters A and B differ in 16 out of the 17 quantitative properties (Appendix 3.2). Cluster A includes eight of the nine scorpion burrows as well as the one burrow produced by S. holbrookii (SH16) that was found to be dissimilar to all of the other anuran burrows. Cluster A has a similarity of 0.66 including SH16. The eight scorpion burrows within Cluster A, however, have a similarity of 0.77. Cluster B includes 73 of 74 anuran burrows as well as one scorpion burrow and has a similarity of 0.72. Cluster B in this analysis consists of the same four clusters containing the same burrows as the dendrogram produced when comparing all burrows produced by S. holbrookii (Figure 8, Clusters 1-4). A single scorpion burrow clusters among the burrows produced by S. holbrookii (Fig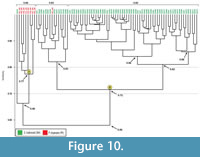 ure 10, PI2_SR). Other burrows in this cluster are the same burrows in Cluster 1 from the S. holbrookii dendrogram that is dominated by isolated chambers (Figure 8).
ure 10, PI2_SR). Other burrows in this cluster are the same burrows in Cluster 1 from the S. holbrookii dendrogram that is dominated by isolated chambers (Figure 8).
Comparison with Mabuya multifasciata burrows. Casts of 12 subvertical tunnels produced by the skink Mabuya multifasciata were compared to the anuran burrows. Burrows produced by M. multifasciata differed significantly in 13 quantitative properties from those of Scaphiopus holbrookii (Appendix 4.1). The similarity values of the burrows produced by M. multifasciata and S. holbrookii range from 0.44-0.96 with a mean similarity of 0.68. A cluster analysis results in two separate groups (Figure 11). The first group, Cluster A, consists of all but two burrows produced by S. holbrookii and has a similarity of 0.82. Cluster A corresponds to Clusters 2-4 in the Scaphiopus dendrogram (Figure 8). The two skink burrows in Cluster A have the highest slope values of all the skink burrows analyzed and higher slope values than the mean slope of all burrows in Clusters B and C. The second main group includes Clusters B and C and consists of 24 burrows, 14 produced by S. holbrookii and 10 produced by M. multifasciata. Burrows in Clusters B and C differ from those in Cluster A in terms of depth, slope, and complexity (Appendix 4.2). Burrows in Clusters B and C have a similarity of 0.77. Clusters B and C differ in nine quantitative burrow properties (Appendix 4.3). Cluster B includes only burrows produced by anurans and consists mostly isolated chambers (86%) with a similarity of 0.84 (Figur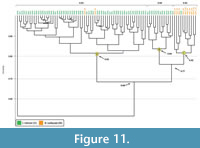 e 11). Cluster B corresponds to Cluster 1 in the Scaphiopus dendrogram. Cluster C consists of 10 of the 12 burrows produced by M. multifasciata and one burrow produced by S. holbrookii (SH42) with a similarity of 0.82. The single toad burrow in Cluster C has the lowest minimum slope of all burrows produced by S. holbrookii excluding SH16, and a lower minimum slope than the mean slope calculated for Cluster B.
e 11). Cluster B corresponds to Cluster 1 in the Scaphiopus dendrogram. Cluster C consists of 10 of the 12 burrows produced by M. multifasciata and one burrow produced by S. holbrookii (SH42) with a similarity of 0.82. The single toad burrow in Cluster C has the lowest minimum slope of all burrows produced by S. holbrookii excluding SH16, and a lower minimum slope than the mean slope calculated for Cluster B.
Comparison with Ambystoma tigrinum burrows. Casts of 42 subvertical tunnels produced by the salamander Ambystoma tigrinum were compared to the anuran burrows. Burrows produced by A. tigrinum differ from burrows produced by Scaphiopus holbrookii in 12 quantitative properties (Appendix 5.1). The similarity values of the burrows produced by A. tigrinum and S. holbrookii range from 0.33-0.97 with a mean similarity of 0.79. A cluster analysis results in five clusters with a total similarity value of 0.73 (Figure 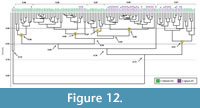 12). In general, at lower values of similarity (0.73 to 0.81) the distribution of burrows produced by S. holbrookii and burrows produced by A. tigrinum is scattered. At higher levels of similarity (≥ 0.81), however, the burrows of the two species tend to cluster among themselves. Cluster E contains an approximately even mix of anuran and salamander burrows (58% anuran burrows and 42% salamander burrows) with a similarity value of 0.81. Cluster E differs from Clusters A, B, C, and D in terms of depth, mean width, slope, complexity, and tortuosity (Appendix 5.2). Clusters A, B, C, and D have a similarity of 0.80. Cluster A includes mostly anuran burrows (85% anuran burrows and 15% salamander burrows). Cluster A differs from Clusters B, C, and D in depth, minimum tunnel width, mean height, circumference, slope, complexity, and tortuosity (Appendix 5.3). Cluster B, C, and D have a similarity of 0.84. Cluster B includes mostly anuran burrows (91% anuran burrows and 9% salamander burrows) and differs from Clusters C and D in length, maximum tunnel height, maximum circumference, slope, complexity, and tortuosity (Appendix 5.4). Clusters C and D have a similarity of 0.88 and differ in depth, length, mean tunnel width, slope, and tortuosity (Appendix 5.5). Cluster C includes only burrows produced by anurans and Cluster D includes mostly salamander burrows (78% salamander burrows and 22% anuran burrows). A single anuran (SH16) and salamander (AT5_SR) burrow did not sort into any of the five clusters and share a similarity of 0.76 with one another. The salamander burrow (AT5_SR) has the lowest maximum slope value of all burrows produced by salamanders and the anuran burrow (SH16) has the lowest maximum, minimum, and mean slope values of all burrows produced by anurans.
12). In general, at lower values of similarity (0.73 to 0.81) the distribution of burrows produced by S. holbrookii and burrows produced by A. tigrinum is scattered. At higher levels of similarity (≥ 0.81), however, the burrows of the two species tend to cluster among themselves. Cluster E contains an approximately even mix of anuran and salamander burrows (58% anuran burrows and 42% salamander burrows) with a similarity value of 0.81. Cluster E differs from Clusters A, B, C, and D in terms of depth, mean width, slope, complexity, and tortuosity (Appendix 5.2). Clusters A, B, C, and D have a similarity of 0.80. Cluster A includes mostly anuran burrows (85% anuran burrows and 15% salamander burrows). Cluster A differs from Clusters B, C, and D in depth, minimum tunnel width, mean height, circumference, slope, complexity, and tortuosity (Appendix 5.3). Cluster B, C, and D have a similarity of 0.84. Cluster B includes mostly anuran burrows (91% anuran burrows and 9% salamander burrows) and differs from Clusters C and D in length, maximum tunnel height, maximum circumference, slope, complexity, and tortuosity (Appendix 5.4). Clusters C and D have a similarity of 0.88 and differ in depth, length, mean tunnel width, slope, and tortuosity (Appendix 5.5). Cluster C includes only burrows produced by anurans and Cluster D includes mostly salamander burrows (78% salamander burrows and 22% anuran burrows). A single anuran (SH16) and salamander (AT5_SR) burrow did not sort into any of the five clusters and share a similarity of 0.76 with one another. The salamander burrow (AT5_SR) has the lowest maximum slope value of all burrows produced by salamanders and the anuran burrow (SH16) has the lowest maximum, minimum, and mean slope values of all burrows produced by anurans.
Comparison of burrows of all four species. A combined cluster analysis of the burrows of all four species results in six main clusters (Figu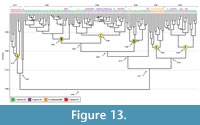 re 13; Appendix 6). Altogether the six clusters have a similarity of 0.60. Cluster A has a similarity of 0.77, consisting only of burrows produced by Pandinus imperator and is different from Clusters B, C, D, E, and F in all quantitative properties (Figure 13; Appendix 6.1). Within Cluster A, similar properties include width, height, width-to-height ratio, minimum circumference, complexity, and tortuosity (Appendix 7.1). The remaining five clusters (B-F) have a similarity of 0.71 and include burrows produced by Scaphiopus holbrookii, Ambystoma tigrinum, Mabuya multifasciata, and one burrow produced by P. imperator (Figure 13). Approximately 92% (n = 31) of the burrows in Cluster B are produced by S. holbrookii and 8% (n = 3) are burrows produced by A. tigrinum. Within Cluster B, similar quantitative burrow properties include width, height, width-to-height ratio, circumference, complexity, and tortuosity (Appendix 7.2). Cluster C is composed of 63% (n = 25) A. tigrinum burrows , 35% (n = 14) S. holbrookii burrows, and 2% (n = 1) M. multifasciata burrows. Similar properties within Cluster C include width, height, width-to-height ratio, circumference, complexity, and tortuosity (Appendix 7.3). Cluster D is composed of 88% (n = 14) S. holbrookii burrows , 6% (n = 1) A. tigrinum burrows, and 6% (n = 1) M. multifasciata burrows. Within Cluster D, similar properties include width, height, width-to-height ratio, circumference complexity, and tortuosity (Appendix 7.4). Cluster E consists of 90% (n = 10) M. multifasciata burrows and 10% (n = 1) S. holbrookii burrows . Within Cluster E, similar properties include depth, width, height, width-to-height ratio, minimum and mean circumference, complexity, and tortuosity (Appendix 7.5). Cluster F is made up of 54% (n = 13) S. holbrookii burrows , 42% (n = 10) A. tigrinum burrows, and 4% (n = 1) P. imperator burrows. Within Cluster F, similar properties include depth, width, height, width-to-height ratio, mean circumference, complexity, and tortuosity (Appendix 7.6).
re 13; Appendix 6). Altogether the six clusters have a similarity of 0.60. Cluster A has a similarity of 0.77, consisting only of burrows produced by Pandinus imperator and is different from Clusters B, C, D, E, and F in all quantitative properties (Figure 13; Appendix 6.1). Within Cluster A, similar properties include width, height, width-to-height ratio, minimum circumference, complexity, and tortuosity (Appendix 7.1). The remaining five clusters (B-F) have a similarity of 0.71 and include burrows produced by Scaphiopus holbrookii, Ambystoma tigrinum, Mabuya multifasciata, and one burrow produced by P. imperator (Figure 13). Approximately 92% (n = 31) of the burrows in Cluster B are produced by S. holbrookii and 8% (n = 3) are burrows produced by A. tigrinum. Within Cluster B, similar quantitative burrow properties include width, height, width-to-height ratio, circumference, complexity, and tortuosity (Appendix 7.2). Cluster C is composed of 63% (n = 25) A. tigrinum burrows , 35% (n = 14) S. holbrookii burrows, and 2% (n = 1) M. multifasciata burrows. Similar properties within Cluster C include width, height, width-to-height ratio, circumference, complexity, and tortuosity (Appendix 7.3). Cluster D is composed of 88% (n = 14) S. holbrookii burrows , 6% (n = 1) A. tigrinum burrows, and 6% (n = 1) M. multifasciata burrows. Within Cluster D, similar properties include width, height, width-to-height ratio, circumference complexity, and tortuosity (Appendix 7.4). Cluster E consists of 90% (n = 10) M. multifasciata burrows and 10% (n = 1) S. holbrookii burrows . Within Cluster E, similar properties include depth, width, height, width-to-height ratio, minimum and mean circumference, complexity, and tortuosity (Appendix 7.5). Cluster F is made up of 54% (n = 13) S. holbrookii burrows , 42% (n = 10) A. tigrinum burrows, and 4% (n = 1) P. imperator burrows. Within Cluster F, similar properties include depth, width, height, width-to-height ratio, mean circumference, complexity, and tortuosity (Appendix 7.6).
Environmental Controls on Burrow Morphology
Mann-Whitney and Kolmogorov-Smirnov tests were used to determine if there was any variation in quantitative burrow properties due to sediment properties and experimental duration (Appendix 8). There was minimal effect on burrow properties as a result of sediment composition. When comparing the burrow properties of burrows constructed during Experiment 1 to those constructed during Experiment 2, the maximum tunnel height and mean tunnel height are significantly different (Appendix 8). Both the maximum tunnel height and mean tunnel height values are higher for burrows constructed in Experiment 1. Minimum slope is the only burrow property that is significantly different between burrows constructed in Experiment 1 and Experiment 3. Burrows constructed during Experiment 3 had higher values of minimum slope. Only values of minimum tunnel width were significantly different between burrows constructed during Experiment 2 and Experiment 3. Burrows produced during Experiment 3 had higher values of minimum tunnel width.
Experimental duration had a greater effect on quantitative burrow properties. Eight quantitative properties differed as a result of increased duration of the experiment (Appendix 9). Burrows constructed during the longer duration experiment had higher values (mean, median, standard deviation, and range) for maximum and minimum tunnel width, mean tunnel width, and circumference. The standard deviation and ranges calculated for maximum and minimum tunnel height, width-to-height ratio, and minimum circumference are higher for burrows constructed in the shorter duration experiment (Appendix 9).
DISCUSSION
Burrow Morphology and Tracemaker
Scaphiopus holbrookii produced a terminal chamber with a consistent overall shape regardless of burrow architecture, sediment composition, or experimental duration. Chambers have a consistent and unique morphology reflective of the toad body form: the chamber is asymmetrical with broad and tapered ends. These correspond to the position of the posterior and anterior ends of the toad, respectively. The dimensions of the burrow chamber correspond to the dimensions of the individual toad responsible for the construction of the burrow (Table 5). The raised ridge in the center of the chamber floor is the impression of the underside of the toad’s body, which is raised in relation to the limbs (Figure 4.1). In addition, bioglyphs that appear in several burrow casts resemble the hindlimbs and pes of S. holbrookii (Figure 4.2-3). The hind-leg burrowing technique that is used by S. holbrookii results in distinct impressions of the pes on the walls of the burrow.
Comparison of burrows produced by three other species (scorpion, skink, and salamander) results in further defining the morphology of burrows produced by anurans. Results of the cluster analyses indicate a low degree of similarity between burrows produced by Scaphiopus holbrookii and Pandinus imperator despite the similarity in architecture. Burrow properties that are significantly different between the two species can be linked to the morphology of the individual species. Properties of burrows produced by the scorpion P. imperator are an expression of the cross-sectional shape and dimensions of the animal (Hembree, 2014). Pandinus imperator is characterized by a relatively wide, but low body with an elliptical cross section that is carried close to the ground (Hjelle, 1990). In general, the body of P. imperator has a more elliptical cross-section than that of S. holbrookii, which results in significantly different tunnel widths, heights, and width-to-height ratios (Appendix 5, Appendix 6).
Burrows produced by Scaphiopus holbrookii differ in 12 quantitative properties from those produced by Mabuya multifasciata and, therefore, have low degrees of similarity. As with Pandinus imperator, the difference in burrow properties is partially a result of the difference between body morphologies. Overall, M. multifasciata has a smaller body width, height, and circumference compared to S. holbrookii. This is consistent with the relatively lower values of tunnel width, height, and circumference in burrows produced by M. multifasciata (Appendix 4). Width-to-height ratios are not significantly different between the two animals, however; both S. holbrookii and M. multifasciata have relatively circular trunk cross sections and produce burrows with similar forms.
A moderate degree of similarity exists between burrows produced by Scaphiopus holbrookii and Ambystoma tigrinum, although 11 quantitative burrow properties are significantly different between the burrows produced by the two species (Appendix 5). The lengths, heights, widths, and width-to-height ratios of burrows produced by A. tigrinum are consistently greater than the dimensions of the salamanders and so the burrows size cannot be related to the size of the tracemaker accurately (Dzenowski and Hembree, 2014). The overall shape, however, is related to the tracemaker and can further aid in distinguishing a burrow produced by a salamander from a burrow produced by an anuran. Unlike the circular cross section of burrows produced by S. holbrookii, the elliptical cross-sectional of burrows produced by A. tigrinum is a reflection of the salamanders’ elliptical body plan (Dzenowski and Hembree, 2014). Well-preserved and distinct bioglyphs produced during burrow construction by A. tigrinum are different than bioglyphs produced by S. holbrookii during burrow construction as a result of different body morphologies. Most bioglyphs produced by A. tigrinum are rounded to elongate protrusions, but some are bilobate or heart-shaped markings. Bioglyphs produced by A. tigrinum are similar to the relative shape and size of the limbs and head of the salamanders (Dzenowski and Hembree, 2014). Bioglyphs produced by S. holbrookii are similar to the shape and size of the limbs of the toads and appear as triangular protrusions.
Burrow Morphology and Behavior
The three burrow architectures produced by Scaphiopus holbrookii are constructed for dwelling purposes. Scaphiopus holbrookii produces dwelling burrows beneath the surface due to stable environmental conditions within the sediment and to avoid light, predators, and desiccation (Pearson, 1955). Burrows produced by S. holbrookii also allow for a passive, ambush hunting technique (Pearson, 1955).
Morphological similarities between the three different burrow architectures are most likely a result of the similar behavioral purpose of the burrows (i.e., Hembree, 2014). The vertical shafts and subvertical shafts were unoccupied less often during the experiments than the isolated chambers, and so served as more permanent burrows. The most dissimilar anuran burrow, the isolated chamber SH16, has been defined as a resting trace and is the only burrow with this form produced by S. holbrookii in the experiments (Figure 14). The depth, length, and slope values for burrow SH16 are lower than burrows associated with dwelling behaviors and are the lowest for all burrows produced by S. holbrookii. In contrast, values of maximum, minimum, and mean circumference are higher than values obtained for burrows associated with dwelling behaviors (Table 6).
Burrow morphology is also influenced by the burrowing techniques employed. Scaphiopus holbrookii uses a hindlimb-first burrowing technique that was consistent across the construction of all 74 burrows. Using this method, Scaphiopus compresses sediment against the walls of the burrow as it descends backwards into the substrate. This results in distinct impressions of the hindlimbs and pes along the side of the shafts and at the base of the chambers. Less distinct impressions of the front legs also occur along the side of the shafts and at the base of the chambers. While in their burrows the toads did not turn around, but remained with their heads facing the burrow openings.
Variations in the behaviors and burrowing techniques of Scaphiopus holbrookii, Pandinus imperator, Mabuya multifasciata, and Ambystoma tigrinum result in differences between the burrow morphologies. Pandinus imperator constructs burrows for dwelling and feeding purposes (Hembree, 2014). Chambers of burrows produced by P. imperator are laterally expanded over time, which allows the scorpions to turn around and face toward the burrow opening to ambush prey (Hembree, 2014). The expansion of chambers is reflected in values of tunnel width, tunnel height, and width-to-height ratios that are higher than those of burrows produced by S. holbrookii. Pandinus imperator uses a direct-excavation burrowing technique in which the walking legs of the animal are used to excavate, lift, and carry material out of the burrow (Hembree, 2014). Elongate grooves and nodes preserved on the floors and walls of the burrow casts are a result of the burrowing technique employed by P. imperator. These surficial features record the scraping of sediment from the tunnel walls by the walking legs (Hembree, 2014). In addition, the overall elliptical cross-sectional form of the burrows is partially a result of the burrowing technique. Continuous movement of the scorpion in and out of the burrow causes some compaction of the burrow floor. The deposition of excavated sediment from deeper in the burrow along the upper tunnel floor evens and flattens the base of the burrow (Hembree, 2014).
Burrows produced by Mabuya multifasciata serve as temporary, subsurface dwelling structures (Catena and Hembree, 2014). Mabuya multifasciata uses an intrusion burrowing technique (Catena and Hembree, 2014). Burrowing by intrusion tends to produce burrows with dimensions that closely match the dimensions of the tracemaker and may also possess compression linings (Bromley 1996; Catena and Hembree, 2014). Hembree and Hasiotis (2006) and Hembree (2009) observed this relationship with other soil-burrowing animals such as amphisbaenians and millipedes, respectively. Most burrows produced by M. multifasciata have flattened elliptical cross-sections with moderately concave roofs and floors, and short, arching walls (Catena and Hembree, 2014). This morphology reflects the morphology of the skink with the exception of the width-to-height ratios; these are, on average, greater than 1.2 resulting from the undulating body movement the skinks employ (Catena and Hembree, 2014). In addition, skinks use their cone-shaped heads to aid in burrow construction producing distinct, triangular-shaped, irregularly spaced divots along the walls of the tunnels (Catena and Hembree, 2014).
Burrows produced by Ambystoma tigrinum are used for dwelling purposes and are produced by a combination of excavation and compaction burrowing techniques (Dzenowski and Hembree, 2014). Initially, individuals of A. tigrinum excavate their burrows head first by bracing their bodies against the sediment surface with their hindlimbs and thrusting their forelimbs into the sediment (Dzenowski and Hembree, 2014). After the burrow is started, the method of burrowing switches to compaction as the limbs and head are used to compress sediment against the walls of the burrow (Dzenowski and Hembree, 2014). The process of compaction results in rounded to elongate protrusions as well as some bilobate or heart-shaped markings produced by the manus and pes (Dzenowski and Hembree, 2014).
Burrow Morphology and Sediment Composition
The qualitative morphology of burrows produced by Scaphiopus holbrookii did not vary across the three different types of sediment. The three burrow architectures produced by S. holbrookii were constructed in all three experiments (Table 1). In addition, minimal differences in quantitative burrow properties resulted from variations in sediment composition (Appendix 8).
Sediment composed of alternating layers of organic matter and sand (Experiment 2) resulted burrows with lower values of maximum and mean tunnel heights compared to burrows produced in 100% organic matter (Appendix 8). Scaphiopus holbrookii produced burrows more quickly in sediment composed of alternating layers. Generally the burrows produced consisted of two to three isolated chambers with a high slope (80-90°) and one to two vertical or subvertical burrows with chambers. The depth of the initial isolated chambers was as deep as the second, sometimes only the first, layer of sand. The depth of the vertical and subvertical burrows varied, but penetrated at least two layers of sand. The deepest burrow produced during Experiment 2 was a vertical shaft. The terminal chamber of the burrow was located as deep as the second layer of sand with a depth of 9.8 cm. Several shallow pits, 2-3 cm deep, were also observed on the surface of the sediment during Experiment 2.
Sediment composed of a homogenized mixture of organic matter and sand (Experiment 3) produced burrows with a higher value of minimum slope compared to burrows produced in 100% organic matter. Vertical shafts were the most common architecture of the burrows produced in Experiment 3 (52% vertical shafts, 14% subvertical shafts, and 33% isolated chambers). Within the first 72 hours of placing the toads in the terraria, five to six burrows were produced, higher than in any trials of Experiments 1 and 2. Within the first four to five days of the first trial of Experiment 3, toads were observed at the surface more frequently than during previous experiments. The experimental duration was shortened due to the increased frequency of surface activity, since this was considered a sign of distress.
Identifying Anuran Burrows in the Fossil Record
In order to identify tracemakers of fossil burrows it is first necessary to recognize the architecture and surficial morphologies of burrow produced by modern animals (Hembree, 2014). There is a general lack of understanding of the fossil record of terrestrial anurans. Due to the fact that extant terrestrial anurans spend most of their lives in burrows, their traces should be abundant in the fossil record. The lack of documentation of anuran trace fossils is likely due to a failure to recognize observed fossil burrows as records of anuran behavior.
Ichnotaxonomic characters known as ichnotaxobases are used to classify trace fossils (Bromley, 1996). Ichnotaxobases consist of aspects of trace fossil morphology including overall shape, orientation with respect to the sediment surface, architecture, and surficial features (Bromley, 1996; Bertling et al., 2006). In order to identify and interpret the paleoecological and paleoenvironmental significance of trace fossils it is necessary to document a set of ichnotaxobases of burrows produced by modern organisms (Hasiotis and Mitchell, 1993).
Architecture. Anuran burrows include vertical and subvertical shafts that end in ovoid chambers as well as isolated ovoid chambers.
Overall shape. Shafts and tunnels are circular in cross section whereas chambers are elliptical in cross section. Tunnel and shaft widths are approximately 0.7-2.0 times larger than tunnel and shaft heights. Chambers are commonly 1.0-1.5 cm wider than tunnels and shafts.
Orientation. Most burrow shafts are subvertically (40-70°) or vertically (70-90°) oriented relative to the surface; burrow chambers may be oblique to vertical (9-90°).
Internal structure. Burrows are not lined. The burrow walls consist of compacted sediment that has an irregular surface. The burrows are not actively filled; instead passive filling occurs from overlying sediment collapse.
Surficial features. Shaft, tunnel, and chamber walls commonly possess raised, triangular bioglyphs.
These ichnotaxobases are likely representative of the burrows of many hindlimb-first digging anurans, although there may be some variation between the burrows of different genera or families. Head-first digging anurans engage in a significantly different burrowing technique that likely leads to distinct types of burrows with their own set of ichnotaxobases. These ichnotaxobases are presented as a model for anuran burrows with the understanding that other morphologies are possible.
CONCLUSIONS
Scaphiopus holbrookii produced three different burrow architectures including vertical to subvertical shafts with ovoid chambers, as well as isolated ovoid chambers. All three architectures were produced regardless of individual, sediment composition, or the duration of occupation. All burrows produced by S. holbrookii consist of a single surface opening, an ovoid terminal chamber that is elliptical in cross-section, tunnel shafts that are vertically or subvertically oriented relative to the surface, as well as bioglyphs reflective of the anuran body outline and burrowing techniques. Quantitatively, burrows produced by S. holbrookii are highly distinct from scorpion burrows and moderately similar to burrows of skinks and salamanders. Additionally, qualitative differences, such as bioglyphs and overall shape, aid in the distinction of anuran burrows from burrows produced by other species.
Establishing the existence of anurans in strata lacking body fossils can aid the understanding of the paleoenvironmental and paleoecological conditions. Interpretations concerning the moisture content can be estimated because the moisture of soils inhabited by extant anurans is fairly restricted. The abundance of burrows produced by anurans can also aid in interpreting environmental conditions. For example, in these experiments the highest abundance of anuran burrows were produced in sandy sediment. Since burrowing continental animals play a fundamental role in pedogenesis, neoichnological studies can also aid in paleosol interpretations (Hasiotis, 2007). Anuran burrows can be found in a variety of soil types from Histosols to Oxisols, although they are generally found in sandy soils. Given observation from these experiments, anurans can affect pedogenic processes by compacting, loosening, and mixing soil, contributing fecal material, denuding vegetation, and enhancing erosion. Open burrows also increase the porosity and permeability of the soil and serve as conduits for fluid flow (Lavelle and Spain, 2005). The paleoecology of units containing anuran burrows can be interpreted to have characteristics similar to modern ecosystems containing anurans. Anurans are members of the third trophic level represented by secondary consumers (Stebbins and Cohen, 1995). The second trophic level is represented by primary consumers (i.e., insects) and includes the prey of anurans (Stebbins and Cohen, 1995). On the other hand, anurans are prey of fourth trophic level which is represented by tertiary consumers (i.e., lizards, birds) (Stebbins and Cohen, 1995). Organisms similar to those that comprise the modern anuran food web can be inferred to have been present in the environment as well.
Terrestrial environments and ecosystems of the past can be better understood with the use of neoichological studies, which allow the connection of burrow morphologies to tracemakers (Hasiotis, 2007; Hasiotis et al., 2007). Documenting the burrow morphologies, burrowing techniques, and behaviors of extant species enables an accurate assessment of possible tracemakers and thus the presence of an organism without requiring body fossils. Consistent properties among the three burrow architectures produced by Scaphiopus holbrookii, the direct relationship between the size and overall shape of the terminal chambers and the toads’ bodies, and bioglyphs that reflect the size and shape of the toads’ pes and hindlimbs help to strengthen the interpretation of the tracemaker. The accurate interpretation of toad burrows will aid in understanding and reconstructing the biodiversity of terrestrial ecosystems in the fossil record.
ACKNOWLEDGMENTS
We would like to thank two anonymous reviewers for their constructive comments and suggestions which improved this manuscript. We would also like to thank J.M. Hils, M. Blair, G. Nadon, and A. Stigall for their assistance and comments on earlier drafts of the manuscript. We thank Ohio University Laboratory Animal Resources for assistance with animal care and handling. This work would not have been possible without funding from the National Science Foundation (EAR-0844256) (to DIH), the Ohio University Provost Undergraduate Research Fund (to LMJ), and the Ohio University Geological Sciences Alumni Research Grant (to LMJ).
REFERENCES
Bertling, M., Braddy S.J., Bromley, R.G., Demathieu, G.R., Genise, J., Mikul, R., Nielsen, J.K., Nielsen, K.S.S., Rindsberg, A.K., and Schlirf, M. 2006. Names for trace fossils: a uniform approach. Lethaia, 39:265-286.
Bowen, J. and Hembree, D. 2014. Neoichnology of two spirobolid millipedes: improving the understanding of the burrows of soil detritivores. Palaeontologia Electronica 17.1.18A:48pp, 21.1MB; palaeo-electronica.org/content/2014/709-neoichnology-of-spirobolids
Bray, R.J. and Curtis, J.T. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs, 27.4:325-349.
Bromley, R.G. 1996. Trace Fossils: Biology, Taxonomy and Applications. Taylor & Francis, New York.
Carroll, R. 2009. The Rise of Amphibians. The John Hopkins University Press, Baltimore.
Catena, A. and Hembree, D.I. 2014. Biogenic structures of burrowing skinks: neoichnology of Mabuya mutifasciata (Squamata: Scincidae), p. 343-369. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms. Springer, Netherlands.
Dzenowski, N. and Hembree, D.I. 2014. The neoichnology of two terrestrial Ambystomatid salamanders: quantifying amphibian burrows using modern analogs, p. 305-341. In Hembree, D.I., Platt, B.F., Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms. Springer, Netherlands.
Emerson, S.B. 1976. Burrowing in frogs. Journal of Morphology, 149:437-458.
Emerson, S.B. 1982. Frog postcranial morphology: identification of a functional complex. Copeia, 1982:603-613.
Frost, D.R., Grant, T., Faivovich, J., Bain, R.H., Haas, A., Haddad, C.F.B., De Sa, R.O., Channing, A., Wilkinson, M., Donnellan, S.C., Raxworthy, C.J., Campbell, J.A., Blotto, B.L., Moler, P., Drewes, R.C., Nussbaum, R.A., Lynch, J.D., Green, D.M., and Wheeler, W.C. 2006. The amphibian tree of life. Bulletin of the American Museum of Natural History, 297:8-370.
Hasiotis, S.T. 2007. Continental ichnology: fundamental processes and controls on trace fossil distribution, p. 268-284. In Miller III, W. (ed.), Trace Fossils: Concepts, Problems, Prospects. Elsevier, Amsterdam.
Hasiotis, S.T. and Mitchell, C.E. 1993. A comparison of crayfish burrow morphologies Triassic and Holocene fossils paleo- and neo-ichnological evidence and the identification of their burrowing signatures. Ichnos, 2:291-314.
Hasiotis, S.T., Platt, B.F., Hembree, D.I., and Everhart, M.J. 2007. The trace-fossil record of vertebrates, p.196-219. In Miller III, W. (ed.), Trace Fossils: Concepts, Problems, Prospects. Elsevier, Amsterdam.
Hembree, D.I. 2009. Neoichnology of burrowing millipedes: linking modern burrow morphology, organism behavior, and sediment properties to interpret continental ichnofossils. PALAIOS, 24:425-439.
Hembree, D.I. 2013. Neoichnology of the whip scorpion Mastigoproctus giganteus : complex burrows of predatory terrestrial arthropods. PALAIOS, 28:141-162.
Hembree, D.I. 2014. Large complex burrows of terrestrial invertebrates: neoichnology of Pandinus imperator (Scorpiones: Scorpionidae), p. 229-263. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms. Springer, Netherlands.
Hembree, D.I. and Hasiotis, S.T. 2006. The identification and interpretation of reptile ichnofossils in paleosols through modern studies. Journal of Sedimentary Research, 76:575-588.
Hembree, D.I., Johnson, L.M., and Tenwalde, R.W. 2012. Neoichnology of the desert scorpion Hadrurus arizonensis : burrows to biogenic cross lamination. Palaeontologia Electronica 15.1.10A:34pp, 14.3MB; palaeo-electronica.org/content/2012-issue-1-articles/192-neoichnology-of-scorpions
Hildebrand, M. and Goslow, G.E. 1974. Analysis of Vertebrate Structure. John Wiley & Sons, New York.
Hils, J.M. and Hembree, D.I. 2015. Neoichnology of the burrowing spiders Gorgyrella inermis (Araneae: Mygalmorphae) and Hogna lenta (Araneae: Araneomorphae). Palaeontologia Electronica 18.1.7A:62pp, 25.3MB; palaeo-electronica.org /content/2015/1057-neoichnology-of-spiders
Hjelle, J.T. 1990. Anatomy and morphology, p. 9-63. In Polis, G.A. (ed.), The Biology of Scorpions. Stanford University Press, Stanford.
Lavelle, P. and Spain. A.V. 2005. Soil Ecology. Springer, Dordrecht.
MacEachern, J.A., Bann, K.L., Pemberton, S.G., and Gingras, M.K. 2007a. The ichnofacies paradigm: high-resolution paleoenvironmental interpretation of the rock record, p. 27-64. In MacEachern, J.A., Bann, K.L., Gingras, M.K., and Pemberton, S.G. (eds.), Applied Ichnology. Society for Sedimentary Geology, Tulsa.
MacEachern, J.A., Pemberton, S.G., Bann, K.L., and Gingras, M.K. 2007b. Departures from the archetypal ichnofacies: effective recognition of physic-chemical stresses in the rock record, p. 65-93. In MacEachern, J.A., Bann, K.L., Gingras, M.K., and Pemberton, S.G. (eds.), Applied Ichnology. Society for Sedimentary Geology, Tulsa.
Meadows, P.S. 1991. The environmental impact of burrows and burrowing animals–conclusions and a model, p. 327-338. In Meadows, P.S. and Meadows, A. (eds.), The Environmental Impact of Burrowing Animals and Animal Burrows. Clarendon Press, Oxford.
Nevo, E. 1968. Pipid frogs from the early Cretaceous of Israel and pipid evolution. Bulletin of the Museum of Comparative Zoology, 136:255-318.
Pearson, P.G. 1955. Population ecology of the spadefoot toad, Scaphiopus holbrooki (Harlan). Ecological Monographs, 25:233-267.
Pinder, A.W., Storey, K.B., and Ultsch, G.R. 1992. Estivation and hibernation, p. 250-274. In Feder, M.E. and Burggren, W.W. (eds.), Environmental Physiology of the Amphibians. University of Chicago Press, Chicago.
Seilacher, A. 1967. Fossil behavior. Scientific American, 217:72-80.
Shubin, N.H. and Jenkins, F.A. 1995. An Early Jurassic jumping frog. Nature, 377:49-52.
Stebbins, R.C. and Cohen, N.W. 1995. A Natural History of Amphibians. The Princeton University Press, New Jersey.
Voorhies, M.R. 1983. Vertebrate burrows, p. 325-350. In Sarjeant, W.A.S. (ed.), Terrestrial Trace Fossils. Hutchinson Ross Publication, Stroudsburg.

