 Feeding strategies of circum‑Mediterranean hipparionins during the late Miocene: Exploring dietary preferences related to size through dental microwear analysis
Feeding strategies of circum‑Mediterranean hipparionins during the late Miocene: Exploring dietary preferences related to size through dental microwear analysis
Article number: 25.1.a13
https://doi.org/10.26879/990
Copyright Palaeontological Association, April 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
ABSTRACT
The adaptive radiation of hipparionins after their Old World dispersal was linked with a trend towards smaller body sizes. The appearance of the small-sized forms has usually been associated to open environments and grazing diets. A recent approach, moreover, highlights the role of life history modifications related to habitat conditions as triggers of their size shifts. Here, we test the relationship between hipparionin size and diet analyzing the dental microwear textures of different-sized hipparionins from Vallesian and Turolian circum-Mediterranean localities. Our results show that hipparionins were mainly mixed-feeders and that there was no general link between body size and diet. However, we identified broader feeding spectra in western Mediterranean smaller forms and more specialized grazing diets in larger ones, a differentiation not found in the eastern Mediterranean hipparionins. At odds with the notion of more open habitats eastward, we detected a larger browsing component in eastern hipparionin diets. The consumption by extant equids of more woody browse during the dry season leads us to propose a greater seasonality as a possible cause. Considering the arguable role of external abrasives on the microwear, another interpretation might involve the presence of more grit in the eastern opener habitats. Interestingly, we found that sympatric hipparionins tend to have similar feeding habits, which points to the fact that their diets were influenced by the local environment. Our results, then, suggest that the small size of some hipparionins resulted from different selective pressures rather than to a general adaptation to increasing habitat opening.
Guillem Orlandi-Oliveras. Institut Català de Paleontologia Miquel Crusafont (ICP), Edifici Z, carrer de les Columnes s/n., Campus de la Universitat Autònoma de Barcelona, 08193 Bellaterra (Barcelona), Spain. guillem.orlandi@icp.cat
Meike Köhler. Institut Català de Paleontologia Miquel Crusafont (ICP), Edifici Z, carrer de les Columnes s/n., Campus de la Universitat Autònoma de Barcelona, 08193 Bellaterra (Barcelona), Spain and Institució Catalana de Recerca i Estudis Avançats (ICREA), passeig Lluís Companys 23, 08010 Barcelona, Spain. meike.kohler@icp.cat
Julien Clavel. Univ Lyon, Université Claude Bernard Lyon 1, CNRS, ENTPE, UMR 5023 LEHNA, F-69622, Villeurbanne, France. julien.clavel@univ-lyon1.fr
Robert S. Scott. Department of Anthropology and Center for Human Evolutionary Studies, Rutgers, The State University of New Jersey, New Jersey 08901 New Brunswick, USA. robertsc@scarletmail.rutgers.edu
Serdar Mayda. Department of Biology, Faculty of Science, Ege University, Bornova 35100, Izmir, Turkey and Natural History Museum, Ege University, Bornova 35100, Izmir, Turkey. serdar.mayda@ege.edu.tr
Gildas Merceron. Laboratoire PALEVOPRIM, UMR 7262 CNRS and University of Poitiers, TSA 51106, 86073 Poitiers Cedex9, France. gildas.merceron@univ-poitiers.fr
Keywords: Hipparion; Equidae; diet; body size; dental microwear texture analysis
Final citation: Orlandi-Oliveras, Guillem, Köhler, Meike, Clavel, Julien, Scott, Robert S., Mayda, Serdar, Kaya, Tanju, and Merceron, Gildas. 2022. Feeding strategies of circum-Mediterranean hipparionins during the late Miocene: Exploring dietary preferences related to size through dental microwear analysis. Palaeontologia Electronica, 25(1):a13. https://doi.org/10.26879/990
palaeo-electronica.org/content/2022/3591-hipparionin-dental-microwear
Copyright: April 2022 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
The living Equidae are today represented by only a few species from the genus Equus, which chiefly graze and inhabit open habitats (Bauer et al., 1994; Moehlman, 2002). However, in the past the Equidae exhibited much greater taxonomic richness (Woodburne and MacFadden, 1982; MacFadden, 1992, 2005) and a broader spectrum of dietary adaptations (MacFadden et al., 1999; Tütken et al., 2013; Semprebon et al., 2016) more comparable to modern African bovids (Gagnon and Chew, 2000). Similarly, extinct equids occupied more habitat types than their extant relatives (MacFadden, 1992; Semprebon et al., 2016) and showed a broader range of body sizes (MacFadden, 1992; Alberdi et al., 1995; Gould and MacFadden, 2004).
Hipparionins are an equid tribe that was especially diverse during the late Miocene, when its representatives were very abundant in many of the mammal assemblages from the Holarctic realm (Woodburne, 1989; Bernor et al., 1996). These three-toed horses radiated soon after their first dispersal from North America to the Old World (Woodburne, 1989, 2009; Bernor et al., 1996, 2003; Scott et al., 2003; Cantalapiedra et al., 2017). Consequently, sympatric and morphologically diverse species are found in western and eastern Mediterranean regions already during the beginning of the Vallesian (Forsten, 1997; Scott et al., 2003; Koufos, 2016) [European Land Mammal Age, 11.2-8.9 Ma (Hilgen et al., 2012)]. Although the hipparionins from circum-Mediterranean regions underwent an important taxonomic and morphological diversification (Forsten, 1968; Koufos, 1990; Bernor et al., 1990b, 1996), in central Europe the specific diversity was much lower (Forsten, 1978a; Bernor et al., 1990a). It is during the Turolian [European Land Mammal Age, 8.9-5.3 Ma (Hilgen et al., 2012)], however, that the Old World hipparionin horses reached their highest taxonomic and ecological diversity (Bernor et al., 1990a, 1996; Scott and Maga, 2005; Merceron et al., 2016a). This diversification is marked by an increase in the range of body sizes, especially towards smaller sizes (Bernor et al., 1996; Ortiz-Jaureguizar and Alberdi, 2003). The “ Cremohipparion clade” [sensu Bernor et al. (1996)], for instance, diversified along two opposite trajectories; leading to the appearance of a lineage characterized by large-sized horses (e.g., Cremohipparion proboscideum) and to the appearance of other forms that decreased in body size (e.g., Cremohipparion matthewi) (Bernor et al., 1996, 2016). Those hipparionins that decreased in body size may have evolved the small size independently (Woodburne and MacFadden, 1982; Bernor and Tobien, 1989). Hence, contrary to the commonly described phyletic size increase in Equidae, size decrease processes took place numerous times during their evolutionary history (MacFadden, 1992; Gould and MacFadden, 2004).
The shift towards smaller size in some hipparionins, together with their diversification, has been associated with increasing climate seasonality and its repercussions on floral communities (Bernor et al., 1990a, 1996; Saarinen, 2009). Accordingly, some authors have suggested that the small-sized hipparionins dwelt in more open habitats (Bernor et al., 1996, 2021; Saarinen, 2009) and were adapted to forage on less nutritious xerophytic forage (Forsten, 1968, 1978b). In the same way, small-sized Equus of the European Pleistocene have been suggested to inhabit open environments and to consume less browse than larger forms (Saarinen et al., 2021). On the other hand, some authors have argued that the smaller hipparionin forms were adapted to more closed and forested areas (Ortiz-Jaureguizar and Alberdi, 2003), like extant “smaller zebras” (Alberdi et al., 1995, p. 363). The hipparionin size diminution trends, moreover, have been associated with shifts in adult mortality regimes in some cases, and resource availability in others (Orlandi-Oliveras et al., 2018). These two scenarios can also be indirectly linked to changes in habitat structure.
Animal body size is related to many physiological and ecological factors (Peters, 1983; Calder, 1984; Palkovacs, 2003). One of the central variables determining body size is growth rate, which in turn is strongly affected by the environmental effects on food availability and foraging risk (Dmitriew, 2011). Hence, the items consumed by an animal do not only depend on the availability and quality of the resources, but also on the competitive pressure and predation risk associated with the exploitation of these resources (Brown, 1988). In turn, these factors all depend on habitat type and structure. A general relationship can thus be described for body mass, diet, and habitat in extant African ungulates: large-sized species tend to inhabit open areas and graze on abundant low quality food, while smaller taxa generally browse on less abundant high quality items in closed habitats (Bell, 1971; Jarman, 1974; Case, 1979; Gagnon and Chew, 2000; Hopcraft et al., 2010).
It was long assumed that hipparionins were mostly open savanna grazers due to their high-crowned teeth (Matthew, 1926; Stirton, 1947; Stebbins, 1981; MacFadden and Hulbert, 1988). Because of that, their arrival to the Old World was tentatively linked to the spread of grasslands (Gabunia and Chochieva, 1982; MacFadden, 1992), a thesis no longer supported by posterior studies on hipparionin diets (Hayek et al., 1992; MacFadden et al., 1999; Kaiser, 2003; Tütken et al., 2013; Merceron et al., 2016a), by evidence of grassland presence predating their appearance (Strömberg, 2006; Strömberg et al., 2007), as well as a tree cover that may have been more dense as initially thought (Solounias, 1999). In this context, while some studies have not identified significant feeding differences between sympatric hipparionin species (Solounias et al., 2010; Clavel et al., 2012; Rey et al., 2013), others detected dietary niche-partitioning between taxa (MacFadden et al., 1999; Merceron et al., 2016a). Similarly, life history strategies have also been found to differ between hipparionins of different sizes (Orlandi-Oliveras et al., 2018, 2019). Although hipparionin dietary preferences have been extensively studied from a paleoenvironmental point of view, the relationship between their size and diet has not yet been explored. Due to 1) the long-time interval of hipparionin presence in the Old World, 2) their wide geographical distribution, and 3) their broad range of body sizes, hipparionins constitute a good model for exploring size related niche partitioning with respect to habitat and feeding preferences across closely-related ungulates.
In previous studies, the hipparionin size differences have been explored from a life history approach by means of bone and dental histology analysis (Orlandi-Oliveras et al., 2018, 2019). In this work, we first aim to evaluate the scaling relationship between ecological preferences (diet) and hipparionin body size. The present study, hence, might help to test the interpretations on hipparionin paleobiology obtained from previous life history inferences. Moreover, the obtained data will also shed light on the dietary preferences of the studied hipparionin groups as well as habitat differences between the Mediterranean areas through the late Miocene. We therefore applied Dental Microwear Texture Analysis (DMTA) on teeth of different-sized hipparionins from Vallesian and Turolian sites located on eastern and western Mediterranean basins, with special interest on sympatric taxa, to infer their diet. DMTA has proven to be a powerful tool for tracking fine grained differences in diet such as seasonal variations on extant species and has successfully been applied to fossil species (Merceron, et al., 2009, 2021; DeSantis, 2016; Berlioz et al., 2017; Martin et al., 2018; Hullot, et al., 2019). Our results will help to understand the dietary preferences of the hipparionins of diverse size classes, and to explore the changes they went through from Vallesian to Turolian in different areas of their distribution range. In this regard, we start from a series of hypothesis. First, if the hipparionin trend towards smaller body sizes represents adaptations to diet and habitat (cf. Forsten, 1978; Bernor et al., 1990a, 1996), we could expect shared dietary strategies among small hipparionins from their DMT. Second, the hipparionin dietary signal can help us to identify paleoecological differences between the western and eastern Mediterranean biomes, the latter is considered to have more open habitats (cf. Bonis et al., 1992), as we could also expect to identify changes on the floral associations from the Vallesian to the more dry and seasonal Turolian (cf. Fortelius et al., 1996). Finally, this data might help to shed more light on the dietary behavior of the different hipparionin groups analyzed, challenging the grazing specialization due to their hypsodont dentition.
MATERIAL AND METHODS
Sample and Study Area
 The fossil specimens come from 20 late Miocene fossil sites of circum-Mediterranean basins (Figure 1). We have focused on localities that yielded large hipparionin assemblages and with those exhibiting a high hipparionin size diversity. We predominantly sampled the more diverse Turolian fossil sites. Far from being homogeneous, eastern Mediterranean faunas show some dissimilarities during most part of the Turolian (Kostopoulos, 2009; Koufos and Vlachou, 2016). We divided, hence, the eastern Mediterranean Turolian sample in the western Anatolian (Samos island and continental western Anatolia) and the Balkans bioprovinces (continental Greece and Bulgaria). Vallesian hipparionins come from western Mediterranean and from the Balkans, while Turolian forms come from the three bioprovinces studied (see Appendix 1 for detailed information).
The fossil specimens come from 20 late Miocene fossil sites of circum-Mediterranean basins (Figure 1). We have focused on localities that yielded large hipparionin assemblages and with those exhibiting a high hipparionin size diversity. We predominantly sampled the more diverse Turolian fossil sites. Far from being homogeneous, eastern Mediterranean faunas show some dissimilarities during most part of the Turolian (Kostopoulos, 2009; Koufos and Vlachou, 2016). We divided, hence, the eastern Mediterranean Turolian sample in the western Anatolian (Samos island and continental western Anatolia) and the Balkans bioprovinces (continental Greece and Bulgaria). Vallesian hipparionins come from western Mediterranean and from the Balkans, while Turolian forms come from the three bioprovinces studied (see Appendix 1 for detailed information).
Here we decided to use the broad sense of the genus Hipparion because of the lack of cranial material available for some of the analyzed taxa, which hinders their unequivocal determination within the proposed supraspecific groupings (Bernor et al., 1996). We organized the eastern Mediterranean hipparionin taxa following the morphotypes defined by Vlachou (2013). We grouped the western Mediterranean hipparionins considering size morphotypes, which follow specific differences, due to the general lack of complete cranial material.
Microwear Data Acquisition
The sample studied comprises 372 teeth from a wide range of hipparionin populations (Table 1; Appendix 2). We discarded unworn and senile specimens with very worn molars, together with those that showed post-mortem alterations as observed on the microwear scans (see Calandra and Merceron, 2016). We preferably selected second upper or lower molars, although in some cases we also analyzed first/third molars and third/fourth premolars as variations in DMT along the tooth row are not significant (Ramdarshan et al., 2017). Following standard procedures, the occlusal surfaces of the teeth were cleaned using acetone-soaked cotton swabs to remove dust particles and glue. Afterwards, dental replicas were made using a polyvinyl siloxane silicone of medium consistency (ISO 4823, President Regular Body, Coltène-Whaledent). These molds were scanned with the “TRIDENT” Leica DCM8 white-light confocal profilometer (PALEVOPRIM lab CNRS University of Poitiers, France) with a 100× lens (numerical aperture = 0.90; working distance = 0.9 mm). We scanned preferentially the lingual facets of paracone and buccal facets of protoconid, but metacone and hypoconid were selected when the former showed alterations. The lateral resolution (x,y) of the scans was set to 0.129 μm, and the vertical spacing to less than 0.002 μm. Once scanned, the surfaces were saved as.plμ files by LeicaScan software (Leica Microsystems) and imported for processing to LeicaMap following procedures shown in Merceron et al. (2016a). A 200 × 200 μm area was extracted and saved as a.sur file. All the specimens were scanned and processed at PALEVOPRIM (CNRS and University of Poitiers, France). Photo-simulations of the surfaces analyzed are available in Appendix 3.
Dental Microwear Texture Variables and Statistical Analyses
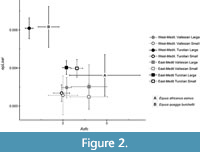 The scans obtained from DMTA can be quantified repeatably, limiting the risks for intra- and inter-observer errors compared to other microwear techniques (Ungar et al., 2003; Scott et al., 2005, 2006; DeSantis et al., 2013). The surfaces were processed using Toothfrax and Sfrax software (Surfract, http://www.surfract.com) applying Scale Sensitive Fractal Analysis (SSFA) and following the standard procedures detailed in Scott et al. (2006). SSFA generates a set of Dental Microwear Texture (DMT) parameters that quantify the enamel surface. These are described in Scott et al. (2005, 2006). In the present study, we focused on the ones that better reflect surface textural attributes correlated to diet: complexity (area scale fractal complexity, Asfc), anisotropy (exact proportion of length scale anisotropy of relief at the resolution of 1.8 µm, epLsar), heterogeneity of complexity with an 8 × 8 mesh (heterogeneity of area scale fractal complexity, HAsfc), and textural fill volume with square edge set at 2 µm (Tfv) (Scott et al., 2006). Here we consider the heterogeneity of complexity over 64 cells per scan, because this setting contributed the most to the variance in our sample (tested via Principal Component Analysis, Appendix 4). Finally, a linear combination of all four dental microwear texture parameters was performed by means of a Principal Component Analysis. The most explicative principal component (PC1) has been used as a proxy of the overall microwear texture, gathering the major variance from the four DMT variables. Unfortunately, because extant equids are mostly grazers, there is no extensive dataset to which to compare the DMT of extinct equids with that of extant forms with different diets. However, we considered a sample of wild Burchell’s zebras and semi-wild asses from Iran as comparative baseline (see Figure 2). Also, we relied on the framework provided by Scott (2012) in which the DMT has been explored in relation to diet in another group of ungulates with much diverse dietary adaptations.
The scans obtained from DMTA can be quantified repeatably, limiting the risks for intra- and inter-observer errors compared to other microwear techniques (Ungar et al., 2003; Scott et al., 2005, 2006; DeSantis et al., 2013). The surfaces were processed using Toothfrax and Sfrax software (Surfract, http://www.surfract.com) applying Scale Sensitive Fractal Analysis (SSFA) and following the standard procedures detailed in Scott et al. (2006). SSFA generates a set of Dental Microwear Texture (DMT) parameters that quantify the enamel surface. These are described in Scott et al. (2005, 2006). In the present study, we focused on the ones that better reflect surface textural attributes correlated to diet: complexity (area scale fractal complexity, Asfc), anisotropy (exact proportion of length scale anisotropy of relief at the resolution of 1.8 µm, epLsar), heterogeneity of complexity with an 8 × 8 mesh (heterogeneity of area scale fractal complexity, HAsfc), and textural fill volume with square edge set at 2 µm (Tfv) (Scott et al., 2006). Here we consider the heterogeneity of complexity over 64 cells per scan, because this setting contributed the most to the variance in our sample (tested via Principal Component Analysis, Appendix 4). Finally, a linear combination of all four dental microwear texture parameters was performed by means of a Principal Component Analysis. The most explicative principal component (PC1) has been used as a proxy of the overall microwear texture, gathering the major variance from the four DMT variables. Unfortunately, because extant equids are mostly grazers, there is no extensive dataset to which to compare the DMT of extinct equids with that of extant forms with different diets. However, we considered a sample of wild Burchell’s zebras and semi-wild asses from Iran as comparative baseline (see Figure 2). Also, we relied on the framework provided by Scott (2012) in which the DMT has been explored in relation to diet in another group of ungulates with much diverse dietary adaptations.
The DMTs of extant mammals have shown to be correlated to the mechanical properties of the food processed in their diet (hardness and toughness). This has proven to be true despite the amount of exogenous abrasives present (Merceron et al., 2016b; Schulz-Kornas, et al., 2020; Ackermans, et al., 2020b; Hua, et al., 2020). The consumption of tough food—such as mature grasses—tends to produce high anisotropic textures, whereas the processing of harder and more brittle items—such as woody browse—results in textures of lower anisotropy indices and high complexities. Generalists and mixed-feeders consume diverse types of resources with varying mechanical properties, which it appears to increase the heterogeneity of the texture complexity (see review in Calandra and Merceron, 2016). Therefore, the dietary ecology of extinct taxa can be revealed by means of their dental microwear textures.
Correlation between body size and diet. Body mass estimates have been used as a measure of body size (Damuth and MacFadden, 1990). We compiled from the literature body mass estimations based on metapodial measures (Pesquero and Alberdi, 2012; Vlachou, 2013; Orlandi-Oliveras et al., 2018), and calculated our own estimations using published data (Forsten and Kaya, 1995; Clavel et al., 2012) and own measures (Appendix 5). Calculations were performed using equations from Scott (1990) and Eisenmann and Sondaar (1998). We tested the link between the hipparionin size and their dietary preferences using linear least squares regressions: mean body mass estimates per population were correlated to our obtained dietary proxies [i.e., each DMT variable and the calculated principal component (PC1)].
Dietary differences between hipparionin populations. While considering the size differences, we have further explored the dietary diversity shown among hipparionins through time and space. In this regard, eastern and western Mediterranean hipparionins were differentiated, divided depending on the age of the fossil association (Vallesian/Turolian), and separated by their size category. Our smallest “work unit” was hipparionin population, which we defined as each group present in a specific fossil locality. The mean body masses estimates of the sampled populations range from 31 kg (periafricanum group from El Arquillo) to more than 250 kg (primigenium group from Hadjidimovo) (Table 1). We used a 100 kg threshold to establish two body size categories, dividing the large- to medium-sized hipparionins (> 100 kg), and the small-sized ones (< 100 kg), which separates quite well the forms considered by previous authors as small-sized hipparionins (Alberdi, 1989; Bernor, et al., 1996). However, despite surpassing the 100 kg threshold, we included into the small group the medium- to small-sized Hipparion sp. from the western Mediterranean early Vallesian (Forsten, 1997) (154 kg), the Hipparion macedonicum from the Axios Valley Vallesian (103 kg), and the H. cf. matthewi from Samos (108 kg), by considering the larger body masses of their sympatric taxa (Table 1), and that these groups represent the smallest forms present in their associations (Appendix 1). Thus, the body mass of the large members from the eastern Mediterranean ranges from 113 kg to 281 kg, while in the small taxa range from 62 kg to 108 kg. In the western Mediterranean, the body mass of the large hipparionins goes from 124 kg to 205 kg, and from 31 kg to 154 kg (84 kg, excluding the early Vallesian form) in the small members.
To explore the variation on the dental microwear texture related to regional, chronological, and body size factors, a first set of analyses based on three-way ANOVAs were performed for each DMT parameter. Post-hoc pairwise comparisons tests, the Tukey’s Honest Significant Differences test (HSD) and the less conservative Fisher’s Least Significant Differences test (LSD), were then used in tandem to identify the source of variation. These parametric procedures were applied to rank-transformed variables for approximating distribution free (e.g., non-parametric) tests (Conover and Iman, 1981). Facing unbalanced factorial designs, here we used Type II sums of squares (SS) because it has been proved to be more powerful than Type III SS and does not depend on the arbitrary order of the model terms (Langsrud, 2003). Because marginality principle applies to multifactorial ANOVA designs (Nelder, 1977; Langsrud, 2003), the significance of the main effects was only interpreted when the interaction term was insignificant.
Moreover, a second set of analyses consisting in three-way nested ANOVAs were performed to explore in more detail the hipparionin dietary regimes during the Turolian, when this clade achieved its higher taxonomic and size diversity. Here, we tested for differences on the dental microwear textures between the three bioprovinces surveyed (western Mediterranean, Balkans and western Anatolia) and the hipparionin groups present within these areas (Appendix 1), while also considering the localities in which these groups are present. Differences were tested on the four DMT variables and on our calculated PC1. Again, HSD and LSD were used as post-hoc tests, and all the variables were rank-transformed prior to the analyses. A significance level of α = 0.05 has been used for all tests.
RESULTS
Relationship between Hipparionin Diet and Size
The first principal component (PC1) built with the four DMT variables, and considered as a more direct dietary proxy, gathered 34.6% of the total variance (Table 2). The variance of this PC1 is chiefly explained by complexity (Asfc, 45.2%), anisotropy (epLsar; 28.5%), and heterogeneity of complexity (HAsfc; 26.3%), whereas textural filled volume (Tfv) accounts for less than the 0.1% of it. Anisotropy is positively correlated with this principal component, while Asfc, HAsfc, and Tfv are negatively correlated. Hence, higher PC1 values are linked to less complex enamel surfaces, which in turn are more anisotropic and homogeneous, and have smaller microwear features; traits which are typically shown by tough leaf browsing species, and most especially, grazing species (Hedberg and DeSantis, 2017; Merceron et al., 2021). The regressions of this PC1 and the four microwear textural parameters with the hipparionin body mass show no significant correlation (Table 3, Appendix 6). Thus, there seems to be no general relationship between the microwear signal and the hipparionin body mass when all the complete sample is considered. However, some significant correlations are detected when separating the three bioprovinces. Heterogeneity of complexity (HAsfc) is negatively correlated with hipparionin body mass in western Mediterranean (p < 0.01), while it is positively correlated in the Balkans sample (p < 0.01) (Table 3). Moreover, the PC1 is positively correlated with the hipparionin body mass in the western Mediterranean area (p = 0.03). In all cases, there is a high dispersion of the data—with R2 always below 0.05 (Table 3)—because the dependent variables (DMT parameters) show high variability as individual data are considered, whereas the independent variable (mass) is represented by mean values per each population. The low R2 values may hinder interpretations, but from these results we can elucidate a trend that can be further explored through the subsequent analyses.
Spatial and Temporal Dietary Variations Related to Hipparionin Size
In our first model we test whether three factors—geological age, geographic region, and body size—contribute to the variance of the dental microwear texture and might have influenced the hipparionin diet. Our results confirm that these three factors explain the variations in dental microwear textures, being in many cases involved in significant interactions (Table 4). Hence, changes in the dental microwear textures due to one of these factors follow different directions depending on a second factor. We detect a significant decrease on the complexity parameter (Asfc) from the Vallesian to the Turolian 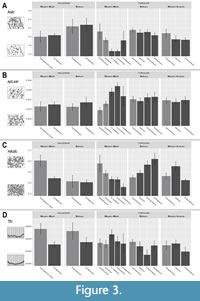 in the large hipparionins, but not for smaller ones (Table 5), which maintain high levels of complexity during the Turolian (Figure 2). Moreover, complexity is generally higher in the eastern hipparionins compared to the western groups (Figure 2). Anisotropy (epLsar), on the other hand, increases from Vallesian to Turolian in both regions (Table 4; Figure 2). In the western Mediterranean, the small-sized hipparionins show significantly lower anisotropy (epLsar) values than the larger forms, while in the east the means between both size groups are similar (Table 6; Figure 2). The western Mediterranean small hipparionins have also higher values of heterogeneity of complexity (HAsfc) than the large-sized forms from the same region. In the eastern Mediterranean, we detect the opposite trend, since large-sized species show higher HAsfc than the smaller ones (Table 6; Figure 3C). Heterogeneity of complexity also tends to increase from the Vallesian to the Turolian in hipparionins from eastern basins, while it does not vary in the western area (Table 7; Figure 3C). Finally, the textural fill volume (Tfv) of the small Vallesian forms is higher than that of the larger Vallesian ones, a trend that is not followed during the Turolian (Table 5; Figure 3D).
in the large hipparionins, but not for smaller ones (Table 5), which maintain high levels of complexity during the Turolian (Figure 2). Moreover, complexity is generally higher in the eastern hipparionins compared to the western groups (Figure 2). Anisotropy (epLsar), on the other hand, increases from Vallesian to Turolian in both regions (Table 4; Figure 2). In the western Mediterranean, the small-sized hipparionins show significantly lower anisotropy (epLsar) values than the larger forms, while in the east the means between both size groups are similar (Table 6; Figure 2). The western Mediterranean small hipparionins have also higher values of heterogeneity of complexity (HAsfc) than the large-sized forms from the same region. In the eastern Mediterranean, we detect the opposite trend, since large-sized species show higher HAsfc than the smaller ones (Table 6; Figure 3C). Heterogeneity of complexity also tends to increase from the Vallesian to the Turolian in hipparionins from eastern basins, while it does not vary in the western area (Table 7; Figure 3C). Finally, the textural fill volume (Tfv) of the small Vallesian forms is higher than that of the larger Vallesian ones, a trend that is not followed during the Turolian (Table 5; Figure 3D).
Hipparionin Diets during the Turolian
Nested ANOVAs on the more diverse Turolian sample show that the microwear patterns differ between hipparionin groups within the same bioprovince, and between the hipparionin populations within the same group (Table 8). Moreover, differences on the Asfc and HAsfc between the three bioprovinces are detected. In this regard, complexity (Asfc) values are generally higher in the Balkans than in western Anatolia, while western Mediterranean hipparionins show the lowest complexities (Appendix 7.1). Differences in heterogeneity of complexity (HAsfc) are only significant between the Balkan sample and the less heterogeneous microwear signatures of the western Mediterranean hipparionins (Appendix 7.2).
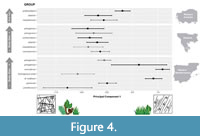 Turolian hipparionin groups significantly differ in all parameters but Tfv (Table 8). The most notable differences are found between two large-sized groups from the western Mediterranean (concudense and cf. matthewi) and the rest (Figure 3, Figure 4; Appendix 7.3). Their dental microwear textures are less complex (low Asfc) and tend to be more anisotropic (high epLsar) and homogeneous (low HAsfc) than in other hipparionins (Table 1; Figure 3; Appendix 7.3), showing, hence, higher PC1 scores (Figure 4). The general higher complexity values of the Balkans hipparionins are also detected in the inter-group comparisons, since some groups (e.g., macedonicum and proboscideum) show minor Asfc differences compared to other forms from western Anatolia and the western Mediterranean (Figure 3A; Appendix 7.3). Other significant differences are found between the large-sized proboscideum and primigenium groups from the Balkans compared to other hipparionins that have lower heterogeneity values (HAsfc) (Figure 3C; Appendix 7.3). These inter-group dissimilarities apart, the locality from which the samples came from is also an important factor affecting the variance of our sample (Table 8). Focusing on the groups present in more than one locality, pairwise comparisons between fossil sites show that there are minor differences in DMT parameters between populations (Appendix 7.4, Appendix 7.5, Appendix 7.6, Appendix 7.7). Most of these dissimilarities are caused by the high epLsar values of Nikiti-2 macedonicum and dietrichi morphotypes in comparison with other populations from the same groups; together with the higher HAsfc values from Gülpınar and Hadjidimovo hipparionins (Table 1; Appendix 7.4, Appendix 7.5, Appendix 7.6, Appendix 7.7). On the other hand, when considering the localities with sympatric taxa, no significant differences are found among forms coexisting on the same fossil site (Appendix 7.8, Appendix 7.9, Appendix 7.10, Appendix 7.11, Appendix 7.12, Appendix 7.13, Appendix 7.14, Appendix 7.15). Only some minor variations are detected inside El Arquillo locality, with higher heterogeneity (HAsfc) values in the small-sized periafricanum and gromovae hipparionins compared to that of the larger primigenium group (Figure 3C; Appendix 7.15).
Turolian hipparionin groups significantly differ in all parameters but Tfv (Table 8). The most notable differences are found between two large-sized groups from the western Mediterranean (concudense and cf. matthewi) and the rest (Figure 3, Figure 4; Appendix 7.3). Their dental microwear textures are less complex (low Asfc) and tend to be more anisotropic (high epLsar) and homogeneous (low HAsfc) than in other hipparionins (Table 1; Figure 3; Appendix 7.3), showing, hence, higher PC1 scores (Figure 4). The general higher complexity values of the Balkans hipparionins are also detected in the inter-group comparisons, since some groups (e.g., macedonicum and proboscideum) show minor Asfc differences compared to other forms from western Anatolia and the western Mediterranean (Figure 3A; Appendix 7.3). Other significant differences are found between the large-sized proboscideum and primigenium groups from the Balkans compared to other hipparionins that have lower heterogeneity values (HAsfc) (Figure 3C; Appendix 7.3). These inter-group dissimilarities apart, the locality from which the samples came from is also an important factor affecting the variance of our sample (Table 8). Focusing on the groups present in more than one locality, pairwise comparisons between fossil sites show that there are minor differences in DMT parameters between populations (Appendix 7.4, Appendix 7.5, Appendix 7.6, Appendix 7.7). Most of these dissimilarities are caused by the high epLsar values of Nikiti-2 macedonicum and dietrichi morphotypes in comparison with other populations from the same groups; together with the higher HAsfc values from Gülpınar and Hadjidimovo hipparionins (Table 1; Appendix 7.4, Appendix 7.5, Appendix 7.6, Appendix 7.7). On the other hand, when considering the localities with sympatric taxa, no significant differences are found among forms coexisting on the same fossil site (Appendix 7.8, Appendix 7.9, Appendix 7.10, Appendix 7.11, Appendix 7.12, Appendix 7.13, Appendix 7.14, Appendix 7.15). Only some minor variations are detected inside El Arquillo locality, with higher heterogeneity (HAsfc) values in the small-sized periafricanum and gromovae hipparionins compared to that of the larger primigenium group (Figure 3C; Appendix 7.15).
DISCUSSION
Paleoecology of Hipparionins in Relation to Their Size
The linkage between body size and dietary strategy is a matter still debated in extant ungulates (Codron et al., 2007; De Iongh et al., 2011). Contrary to previous interpretations (Bell, 1971; Jarman, 1974; Gagnon and Chew, 2000), more recent studies failed to detect a correlation between body mass and diet type in extant African herbivores (Codron et al., 2007), and even between their body mass and the quality of the forage they ingest (De Iongh et al., 2011). A lack of association between size and diet has also been found in Pleistocene ungulates (Saarinen et al., 2016) and in Eocene brontotheres (Mihlbachler and Solounias, 2002). Similarly, our results show that there is no evidence to support a general link between hipparionin size and their dietary preferences at a global scale. However, at a regional scale we still detect 1) an effect of size on the dental microwear texture and 2) a slight coupling between the hipparionin body size and the heterogeneity of complexity of their dental microwear textures. This later tendency, however, follows opposite directions: in the Balkans the small hipparionins’ diets were less generalized than those of the larger species, while western Mediterranean small forms fed on wider spectra of preferred items. Moreover, the small hipparionins from the western Mediterranean consumed fewer tough items than the larger taxa, a trend not found in the eastern groups. Accordingly, there is a significant decrease in the grazing behavior related to the size decrease in western Mediterranean, but not in the other bioprovinces. These biogeographical differences within size groups strengthen the idea of no general correlation between diet and hipparionin size.
Paleoenvironmental inferences should preferably be based on the dietary habits of a broad range of ungulates rather than from the diets of a reduced group of them. Nevertheless, the opportunistic feeding habits of at least some hipparionins (Tütken et al., 2013), and the fact that their dietary strategies are related to the available vegetation and, thus, to the habitat properties (Kaiser, 2003), allows us to get a broad idea of the environment in which these hipparionins might have dwelt. Considering the absence of a global relationship between size and diet, we suggest that there could be no general correspondence between hipparionin size and their habitat preferences. However, further habitat proxies might be necessary to make finer interpretations on the habitats of the different-sized hipparionin forms, especially considering the finding that many hipparionin taxa were mixed-feeders, so they could have dwelled both in more forested or open bushy habitats. As the small-sized forms analyzed seem to not strictly have similar dietary regimes (thus, probably occupying different types of landscapes) our results seem to indicate that the vegetation composition might has not directly affected their body size. Other physiological, ecological, and life history factors may have had a significant influence on the size decrease trends undergone by some hipparionins (Orlandi-Oliveras et al., 2018) and other equids (Saarinen et al., 2016, 2021). At a regional scale, however, western Mediterranean small taxa seem to be related to wider dietary spectra and, during the Turolian, to more browsing diets compared to that of their larger counterparts. Therefore, we infer that these smaller hipparionins probably preferred more heterogeneous and somewhat denser habitats. Small eastern Mediterranean hipparionins, on the other hand, have similar microwear textures to those of the larger species, suggesting that they might have occupied comparable niches.
Temporal and Biogeographical Differences in Hipparionin Diets
The study of the dietary strategies of the circum-Mediterranean hipparionins further allows us to compare their feeding preferences and potential habitats through time and space. In this regard, the eastern Mediterranean habitats are supposed to be more open than those from the western side (Bonis et al., 1992; Koufos, 2006), while a general trend of increasing seasonality and habitat opening has been suggested in all the Mediterranean area from Vallesian to Turolian (Fortelius et al., 1996), a pattern which is corroborated with ectotherms vertebrates (Böhme et al. 2008, 2011). These latter temporal changes have been linked to an increase of xerophytic vegetation and the development of open landscapes with a rich herbaceous layer that includes grasses (Agustı́ et al., 2003; Jiménez-Moreno et al., 2007). The xerophytic foliage with thicker epidermis and grasses at least available seasonally may have contributed to the observed higher anisotropy in the Turolian forms. In the eastern Mediterranean, besides, hipparionins generally differed from Vallesian to Turolian by the broadening of their diets, which were already based on mixed-feeding strategies. This dietary niche expansion suggests the prevalence of mosaic habitats that allow the exploitation of food resources with diverse physical properties. Recent isotopic studies did not detect a major climate nor habitat change during the Vallesian-Turolian transition in this area (Merceron et al., 2013; Rey et al., 2013), but only a slight decrease in the precipitation regime (Rey et al., 2013). However, Böhme et al. (2008, 2011) have shown more severe variations in environmental conditions through Europe during the late Vallesian and at the Vallesian/Turolian transition. Wider seasonal variations and more perturbations during the Turolian, could then have enhanced the presence of heterogeneous landscapes with grasses, bushes, and shrubs (Merceron et al., 2005a, 2016a; but see Solounias et al. 1999), that would have provided a high variety of resources, including both browse and graze. This diversity might have favored the coexistence of diverse hipparionin taxa, which do not strongly differ in dietary strategies (Solounias et al., 2010) as our results suggest.
Broadly, the main differences between regions lie in the different patterns observed through the hipparionin size range (see previous section), and the more complex dental microwear textures of eastern hipparionins compared to western forms. Therefore, compared to western Mediterranean hipparionins, the Balkans groups have dental microwear textures suggesting broader diets that include items of harder nature, a dietary regime compatible with a more mixed-feeding strategy. As stated, the eastern Mediterranean is typically supposed to be more arid and less wooded than the western realm during the late Miocene, based on the comparison of the fossil mammal assemblages (Bonis et al., 1992; Koufos, 2006) and to other proxies that point to the open character of the eastern habitats (Merceron et al., 2005a, 2010, 2016a; Ioakim and Koufos, 2009; Koufos et al., 2009; Strömberg et al., 2007; Rey et al., 2013). Hence, the eastern Mediterranean biotope has mainly been characterized by an open dry savanna with a rich layer of grasses and with some bushes and shrubs (Merceron et al., 2005a, 2016a; Koufos et al., 2009), although other proxies suggest the existence of more densely wooded areas (Solounias et al., 1999, 2010; Denk, 2016; Denk et al., 2018). In this context, we might have expected more grazing feeding strategies in the eastern Mediterranean taxa compared to western ones.
On the contrary, the large hipparionins from the Turolian western Mediterranean are the species that grazed more, while eastern hipparionins have microwear textures consistent with the consumption of harder items (higher complexities) of diverse physical properties. We might interpret this pattern as a consequence of seasonal dry conditions given the open character of the eastern Mediterranean habitats, and that the late Miocene environments were arguably subject to significant precipitation shifts (Merceron et al., 2005b, 2013). Indeed, during the dry seasons, extant African ungulates widen their dietary niches thus following less strict grazing diets (De Iongh et al., 2011). Accordingly, extant equids tend to include higher quantity of harder woody browse in their diets during periods of low food availability due to drought (Estes, 1991; Moehlman, 2002). The lower selective behavior and higher intake rates of the hindgut fermenters (Janis, 2008) can facilitate the broadening of their diets and compensate for the resource fluctuations. Moreover, studies on fossil mammals coping with instable or limited resource supplies also point towards an expansion of the dietary regimes and the inclusion of more browse material (Winkler et al., 2013; Smith and DeSantis, 2018). It is during the drought periods in seasonal climates with harsh dry seasons that the herbaceous layer and notably monocots are deeply impoverished, as their radicular net does not go deep enough to access water resources. Therefore, the increase in the browsing behavior of hipparionins during these periods may be related to the seasonal depletion of herbaceous monocotyledons due to their lower water competitive capacity compared to bushes, shrubs, and herbaceous dicots whose roots go deeper in soil (Hipondoka et al., 2003; Wang et al., 2010; Donzelli et al., 2013; February et al., 2013). Once rains are back, then, herbaceous are more reactive to gain water (Hipondoka et al., 2003; Scheiter and Higgins, 2007). Keeping in mind how monocots and dicots access to water resources, the absence of strictly grazing hipparionins in the eastern Mediterranean is not surprising. Besides, finding the most grazing hipparionins in the western Mediterranean makes sense as well, since water shortages might be less severe and thus monocots might have remained more abundant all around the year, especially at places where flooding or megaherbivores stop the growth of a bushy/tree layer. The more humid conditions near lakes and floodplains can, moreover, support a richer layer of herbaceous vegetation (Rodgers, 1982), which is more present in the diets of our large western Mediterranean hipparionins. Indeed, the populations that show a stronger grazing signal (Hipparion concudense from Concud, and Hipparion cf. matthewi from Venta del Moro) come from fossil associations developed on lacustrine environments (Montoya et al., 2006; Pesquero et al., 2013). Hipparionin obligate grazers, hence, were rather rare and probably inhabited somewhat humid environments and/or faced seasonal migrations. Similarly, the extant zebra species that graze more (Equus quagga and Equus zebra) are in turn those which undergo larger migrations and are more water-dependent (Bell, 1971; Estes, 1991; Moehlman, 2002).
Besides the physical properties of the food and its phytolith content, the dental wear of ungulate teeth has been related to the action of exogenous particles of grit and dust (Sanson et al., 2007; Lucas et al., 2014; Madden, 2014). The role of these particles on the microwear signal is an issue still debated, mostly because they are diverse in nature, hardness, dimension, shape, density, and periodicity (Merceron et al., 2016b; Schulz-Kornas et al., 2020). On one hand, some studies have highlighted the fact that their presence does not significantly alter the dietary signal of the DMT (Burgman et al., 2016; Merceron et al., 2016b; Adams et al., 2020). On the other hand, other experiments have demonstrated that grit particles of different size can have an influence on the DMTA parameters (Ackermans et al., 2020b; Schulz-Kornas et al., 2020). Actually, the inclusion of dust (simulating natural abundance with diameter below 100 µm) has shown to not increase the complexity of the microwear texture most likely because those particles actually mimic phytoliths of biosilica (Merceron et al., 2016b). However, larger sand particles of millimetric grit could have an impact on microwear through the generation of large pits (Solounias and Semprebon, 2002), so arguably increasing complexity (Hedberg and DeSantis, 2017). Indeed, recent studies have shown that the size of the grit particles have a stronger effect than their concentration (Ackermans et al., 2020b; Schulz-Kornas et al., 2020), and that the processing of large grit particles can result in higher DMT complexities (Ackermans et al., 2020b). Nevertheless, at least among ruminants, rumen acts as a washing mechanism, which allow rumination free of large grit particles as they decant by gravity at the bottom of the dietary bolus. Hipparionins, however, did not have this washing mechanism. For this reason, it could be possible to relate the higher complexity of the DMTs of the eastern Mediterranean hipparionins to the higher presence of grit in their drier opener environments compared to those of the western Mediterranean.
The dietary reconstructions proposed here potentially reflect the last weekly to monthly preferences, due to the nature of the dental microwear studies (Teaford and Oyen, 1989; Teaford et al., 2017). On the other hand, long-term feeding strategies can be reached from mesowear and isotopic analyses when serial sampling is privileged, which can provide other comparative proxies for diet and overcome possible caveats. The short-term nature of microwear studies could represent a limitation, so future paleoenvironmental studies comparing both regions might require the study of diverse ungulate groups, together with the inclusion of isotopic and/or mesowear data that complements the information obtained from the microwear texture.
Diets of Hipparionin Groups and Populations
Previous studies have suggested a mixed-feeding and opportunistic dietary strategy in the primigenium hipparionins from the central European Vallesian (Kaiser, 2003; Tütken et al., 2013). Accordingly, we show that the Vallesian forms analyzed here were intermediate feeders. Within each region, the coexisting different-sized Vallesian hipparionins did not strongly differ in dental microwear textures, supporting quite similar feeding habits, except for the broader dietary spectra of the smaller western Mediterranean forms. In this case, they followed a more generalist diet than the larger hipparionins of the same area, including a wider range of preferred items in a weekly basis, suggesting different habits between these groups. On the other hand, the primigenium and the macedonicum hipparionins from the eastern Mediterranean Vallesian show similar dietary preferences, consistent with their comparable enamel carbon isotopes (Rey et al., 2013). We may then suggest that these groups had similar preferences of feeding habits and possibly habitats preferences. Compared between regions, the dental microwear textures of large-sized primigenium hipparionins are more complex in the eastern than in the western Mediterranean Vallesian, consistent with the general pattern observed comparing both areas (see previous section). However, this contrasts with a previous mesowear study that suggests a more browsing diet in the western Mediterranean primigenium forms compared to those of the east (Scott et al., 2013). This disagreement can be related to the distinct time scales addressed with the meso- and the microwear studies (Davis and Pineda-Munoz, 2016). On one hand, the mesowear signal might illustrate the long-term feeding preferences (Ackermans et al., 2020a) of the hipparionins due to closer habitats in the west and opener habitats in the east (Koufos, 2006; Scott et al., 2013). On the other hand, our shorter term dental microwear results might reveal the seasonal addition of woodier browse due to a stronger seasonal depletion of monocot resources in the eastern Mediterranean or the higher presence of large grit particles in the more open environment eastwards. Another provocative alternative would be to consider molar mesowear relief and cusp shape are not only dependent from the proportion of silica bearing plants in diet but also from the proportion of fibrous component (lignin, cellulose) in the dietary bolus. Besides, the grit presence could also have contributed.
When we focus on the diverse Turolian hipparionin assemblages, the greatest differences are found when comparing the large-sized western hipparionins with the others. As mentioned before, western Mediterranean H. concudense and H. cf. matthewi most likely fed on grasses, while the other hipparionins were probably mixed-feeders including then more browse in their diets. The Turolian primigenium group from the western Mediterranean could also have followed a somewhat grazing strategy. However, the small sample hinders a more reliable interpretation. Further comparisons are not possible since no other dental wear studies on Turolian western Mediterranean hipparionins have hitherto been done. The smaller hipparionins from this region, on the other hand, were generalist mixed-feeders that fed on a higher percentage of browse than other western hipparionins. In the eastern Mediterranean, and especially in the Balkans bioprovince, the hipparionins show again more complex microwear textures, which we have mainly related to the higher consumption of browse during seasonal dry phases when the herbaceous layer is poor (see previous section). In this regard, the Balkans’ macedonicum group included in their diet—besides grasses—more quantity of hard browse material in comparison to other western Mediterranean and western Anatolian hipparionins. This group, however, fed likely on a narrow range of items on a daily basis (as suggested by the low HAsfc values), thus pointing to a seasonal mixed-feeding strategy rather than a “meal-by-meal” one (Solounias and Semprebon, 2002).
Previous dietary reconstructions of eastern Mediterranean Turolian hipparionins have mainly inferred grazing diets (Hayek et al., 1992; Koufos et al., 2009; Solounias et al., 2010). Our results rather point to more intermediate feeding preferences both in a daily or seasonal basis. Similar mixed-feeding diets have also been suggested within the proboscideum group (Hayek et al., 1992; Solounias et al., 2010), while other authors indicated a more grazing tendency for this group (Koufos et al., 2009; Clavel et al., 2012). In our sample, although being mixed-feeders, the proboscideum hipparionins from the western Anatolia represent the forms that grazed more of the eastern Mediterranean. On the contrary, the Balkan proboscideum group depicts a “meal-by-meal” generalist feeding behavior (sensu Solounias and Semprebon, 2002) as shown by their higher HAsfc values. The dietrichi and macedonicum hipparionins, on the other hand, do not differ in diet between the two eastern bioprovinces. In the case of the dietrichi group, our inferred mixed-feeding strategy agrees with previous interpretations based on material from Samos (Koufos et al., 2009), but contrasts with those that suggest a clear grazing diet (Hayek et al., 1992; Solounias et al., 2010). Our results also point to intermediate feeding preferences in the macedonicum hipparionins, in contrast to previously reported grazing diets (Hayek et al., 1992; Koufos et al., 2006; Solounias et al., 2010). Merceron et al. (2016a) also detected lower grass consumption preferences compared to obligate grazers (variable grazing) and even mixed-feeding within the dietrichi and macedonicum groups; a pattern that they related to the inclusion of more browse during dry seasons. This last interpretation can be supported by our results pointing to intermediate feeding preferences but with narrow daily/weekly dietary spectra.
Local populations within each hipparionin group also show some differences in their microwear textures. For example, the macedonicum and dietrichi hipparionins from Nikiti-2 grazed more than their analogues from Perivolaki. To a lesser extent, the macedonicum hipparionins from Nikiti-2 consumed higher proportion of grasses than those from the localities of Ravin des Zouaves-5 and Dytiko. Additionally, broader dietary preferences have been detected in the macedonicum group from Gülpınar, and in the proboscideum and primigenium hipparionins from Hadjidimovo in comparison to their counterparts from other sites. Comparable dietary variations within the same hipparionin group present in different localities have already been identified (Kaiser, 2003). These differences illustrate the influence of the local environments; like the possible presence of a richer herbaceous layer including monocots in Nikiti-2, as well as a more diverse habitat structure or higher intra- or inter-specific competition levels in Gülpınar and Hadjidimovo sites.
In agreement with this pattern, we find a quite homogeneous dental microwear texture within each fossil site, with no significant differences between hipparionin groups coexisting on the same locality. We therefore suggest that the taxa present in one area exploited similar resources. This does not mean there was no niche partitioning between species, but other factors than strictly diet may have contributed. The same low degree of dietary differentiation has also been found in previous studies on hipparionin diets (Koufos et al., 2006; Solounias et al., 2010; Clavel et al., 2012), in other extinct perissodactyls (Mihlbachler and Solounias, 2002), and even in extant ungulate assemblages (De Iongh et al., 2011). The presence of various hipparionin groups with no clear trophic niche differentiation can therefore be explained by: 1) the wide mixed-feeding strategies that allow them to consume a large variety of resources (diminishing the trophic competition), 2) the body size differences that might favor the exploitation of different herbaceous strata (Koufos et al., 2006) in a diverse and rich environment (Solounias et al., 2010), or 3) by differences on social and migratory behaviour. The coexistence in Hadjidimovo of two large hipparionins, Hipparion mediterraneum and Hipparion brachypus (Hristova, 2009) that show some of the broadest dietary spectra of our sample (higher HAsfc), may support the hypothesis of an expansion of the dietary preferences due to competition rather than a niche differentiation strategy. In our sample, however, we have also detected some minor differences that may suggest a case of niche differentiation within El Arquillo fossil site from the western Mediterranean area. Here, the smallest hipparionins follow a more browse dominated generalist diet compared to the larger primigenium group.
Hypsodonty and Hipparionin Diets
Surprisingly, the larger and more grazing taxon from El Arquillo has lower-crowned teeth, while the smaller and more mixed-feeders periafricanum and gromovae hipparionins show the highest hypsodonty index of the western Mediterranean Turolian (Pesquero, 2003). Despite their hypsodont teeth, most of the hipparionin groups analyzed here have shown to be intermediate feeders. The same lack of correspondence between the hipparionin high-crowned teeth and a strict grazing diet have been discussed in various studies dealing with dental wear and isotopes (Hayek et al., 1992; MacFadden et al., 1999; Solounias et al., 2010; Tütken et al., 2013). Similarly, extant ungulates show discrepancies between diet and their dental morphology, as shown by the red deer ingesting large quantity of monocots (Azorit et al., 2012; Berlioz et al., 2017) despite their mesodont teeth, or the hypsodont teeth of the mouflon considering their mixed feeding preferences (Marchand et al., 2013; Merceron et al., 2021).
When testing the general correspondence between grazing diets and hypsodonty within Equidae representatives, both Strömberg (2006) and Mihlbachler et al. (2011) identified large periods of time when the selective pressures leading to higher crowned teeth (e.g., dietary abrasives) were weak. However, the same authors found that during specific intervals there were strong selective pressures leading to a rapid change on equids’ dentition (Strömberg, 2006; Mihlbachler et al., 2011). In the case of hipparionin horses, MacFadden et al. (1999) initially proposed that the increased tooth height appeared once within the clade as an adaptation to more abrasive diets, and that this condition was retained despite the following shifts in the dietary strategies. This interpretation, however, fails to explain the observed hypsodonty increase through time in many hipparionin groups (Alberdi, 1989). Alternatively, other authors emphasized that hipparionin hypsodont teeth could represent an adaptation to flexible dietary habits (Kaiser, 2003; Tütken et al., 2013). In agreement with this interpretation, our results show that most of the hypsodont hipparionins had mixed-feeding strategies either in a daily or a seasonal basis. This feeding flexibility might have represented an advantageous trait during the climatic shifts occurred during the late Miocene (Tütken et al., 2013), since herbivores dealing with instable resource supplies tend to expand their dietary breaths (Winkler et al., 2013). Nonetheless, hypsodonty has also been related to increasing environment aridity, as indicated by the inverse correlation of the hypsodonty with the rainfall and humidity (Fortelius et al., 2002). In this sense, airborne grit has been hypothesized to favor higher crowned teeth [see Strömberg (2006)] due to its contribution to dental wear (Sanson et al., 2007; Madden, 2014). Interestingly, however, recent studies have demonstrated that despite different grit loads in the grass consumed by African buffaloes, their dental wear rate does not significantly differ (Sanson et al., 2017).
Whether by means of phytolith or grit wear, higher-crowned teeth might also contribute to the achievement of a longer life span (Carranza et al., 2004; Veiberg et al., 2007; Jordana et al., 2012), which is linked to a generally slower life history (Stearns, 1992). Indeed, as well as by the diet quality, the wear degree has shown to be influenced by the optimal timing of tooth use in relation to the schedule of growth and reproduction through life (Pérez-Barbería et al., 2015). More hypsodont teeth, then, can be related to a higher resource assignation to the soma maintenance rather than to current reproductive performance, a life history strategy which is in turn coupled to longer reproductive lifespans (Veiberg et al., 2007; Carranza et al., 2008). Enamel folding complexity on the occlusal surface, moreover, might have also played an important role in the efficiency of the hipparionin tooth durability (Famoso and Davis, 2016); while in the Equus genus dental functionality is chiefly maintained by means of increased crown heights due to the extension of the crown formation time (Nacarino-Meneses et al., 2017; Orlandi-Oliveras et al., 2019). The relationship between life history and dental development (Smith, 1991, 2000), together with the inferred slow molar formation and relatively late eruption of hipparionins’ molars (Orlandi-Oliveras et al., 2019), make us interpret the lack of linkage between hypsodonty and diet in hipparionins as possible evidence of the role of life history in the evolution of tooth hypsodonty within this group.
Size Decrease Trends
The decrease in hipparionin size has mainly been related to an adaptation to open landscapes (Bernor et al., 1996; Forsten, 1997; Saarinen, 2009) and to feeding on plants of increasing xerophytism (Forsten, 1968, 1978b). Accordingly, Saarinen et al. (2021) demonstrated that this relationship holds true in the Pleistocene Equus of Europe, as large-sized forms were more associated to forested habitats while the smaller Equus to opener ones. In the case of hipparionins, on the other hand, Ortiz-Jaureguizar and Alberdi (2003) suggested more closed-habitat affinities for the small forms, while in a recent review Bernor et al. (2021) related the smaller taxa to grazing open habitat dwellers.
If the hipparionin small size (that is, the trend towards smaller size) was the consequence of direct adaptation to specific diets or habitats (i.e., the spread of opener habitats and/or xerophytic flora), we would expect shared dietary regimes and habitat preferences among small-sized forms. Here, however, we do not find a common dietary pattern shared between the small-sized hipparionins of Vallesian and Turolian assemblages from the circum-Mediterranean regions. In consequence, we interpret that the size differences were not directly promoted by a common dietary or habitat shift but might instead represent independent adaptations that otherwise led to similar size decrease tendencies.
Indeed, the small- and large-sized groups from the eastern Mediterranean did not follow contrasting dietary regimes during the Vallesian and the Turolian. This suggests that the cause or causes triggering the small-sized hipparionin appearance were not directly related to dietary differences. Otherwise, it lends support to the idea that shifts in other factors probably underlay these changes [e.g., mortality regimes shift (Orlandi-Oliveras et al., 2018)]. During the Vallesian, the main dietary difference detected in the western Mediterranean hipparionins resides in the more generalist diets followed by the smaller hipparionins. The smaller form, hence, probably dwelt in more heterogeneous habitats as already proposed by Forsten (1997). The sharpest dietary dissimilarities between size groups, however, are found in the western Mediterranean Turolian. Nowadays, the large size of extant grazing plain zebras allows them to go through the seasonal migrations following the grasses fluxes (Moehlman, 2002). In the western Mediterranean, likewise, larger-sized hipparionins were the taxa with the stronger grazing tendencies, and they might arguably undergo seasonal migrations when the resources in monocotyledonous were depleted. The smaller and more hypsodont species, on the other hand, followed browse-dominated mixed-feeding strategies; probably exploiting the available resources in somewhat closer habitats, and broadening the spectrum of ingested items during seasons of lower resource availability. Accordingly, bone and dental paleohistological studies have pointed towards relative slower-life histories in these more hypsodont small-sized forms in comparison to larger hipparionins from the western Mediterranean Turolian (Orlandi-Oliveras et al., 2018, 2019). Similarly, extant roe deer populations of less productive environments are smaller, more hypsodont, and more longer-lived than larger-sized roe deer populations inhabiting highly productive environments (Veiberg et al., 2007).
CONCLUSIONS
The dental microwear textures of the northern circum-Mediterranean hipparionins mainly point towards mixed-feeding strategies. Some exceptions are present, since large-sized forms of the Turolian western Mediterranean grazed more than the rest. When considering all the hipparionins analyzed, we do not find a common relationship between their dietary preferences and their size. However, despite this lack of general correlation, the western Mediterranean large-sized forms tended to have narrower dietary breadths further associated to a higher percentage of grassy consumption in comparison to smaller hipparionins. On the other hand, the different-sized hipparionins from the eastern bioprovinces followed similar intermediate feeding strategies, including both browse and grasses in their diets (in some cases in a more seasonal basis and others in a more daily-weekly basis). Although we did not reconstruct the paleoenvironmental conditions from the sole diet of hipparionins, their opportunistic feeding adaptations give us clues about the habitats in which they might have dwelt. Therefore, due to the differential dietary regimes among the small-sized forms analyzed, our results question the idea that hipparionin size decrease was a direct adaptation to habitat openness.
The broad comparison between Vallesian and Turolian hipparionin assemblages from different areas also allowed us to detect some general spatiotemporal differences regarding the hipparionin feeding strategies. The inclusion of tough items in their diets tended to increase from Vallesian to Turolian; in the eastern Mediterranean, a further widening of the dietary strategies is also detected. This could reflect a general increase in plant xerophytism, and the presence of more heterogeneous environments in the eastern Mediterranean late Miocene. More solid paleoenvironmental inferences, however, should be based on dietary reconstructions of more diverse ungulate assemblages (adding other dietary proxies as isotopes and mesowear) and the study of macrobotanical and palynological remains. When comparing the eastern and western Mediterranean regions, we find significant higher complexity values in the eastern forms, specifically on the Balkans. One possible interpretation to this observation is the presence of higher levels of millimetric grit eastwards (probably linked to a more arid environment). However, considering previous studies comparing both areas (Bonis et al., 1992; Koufos, 2006), we also relate this to the presence of more dry seasonal episodes (Merceron et al., 2005a, 2016a) inducing the consumption of harder woody browse by eastern hipparionins. Similarly, extant equids (Estes, 1991; Moehlman, 2002) and other ungulates (De Iongh et al., 2011) include higher proportion of browse during the dry seasons in savanna and more arid environments.
In some cases, the diet of the hipparionin groups differs between the fossil sites surveyed, probably reflecting the season at which most of the specimens died and the influence of local conditions. Accordingly, coexisting hipparionins show no differences on the dental microwear patterns, thus suggesting no strong dietary niche partitioning over the traditional grazing-browsing couple. Alternatively, the segregation might have existed among the frame of mixed-feeders and browsers (exploiting different resources but with similar mechanical properties). The general mixed-feeding strategy inferred in most of the hypsodont hipparionin groups shows no support to the assumption of grazing diets due to high hypsodonty indices. This supports, hence, that hypsodonty within the group might have been influenced by its role on dietary flexibility (Tütken et al., 2013) and life history (Veiberg et al., 2007), although the presence of grit due to habitat openness can also be involved. Finally, the different ecologies shown by the small-sized hipparionins make us conclude that the size decrease trends were probably achieved independently rather than related to a common habitat shift.
ACKNOWLEDGEMENTS
We would like to thank M. Fernández and X. Aymerich for the molding of the material from the ICP (Institut Català de Paleontologia Miquel Crusafont). G.O-O. acknowledges the help received from F. Martin (PALEVOPRIM) during the obtainment of the DMT parameters and the posterior analyses. We further thank to D. Kostopoulos (Aristotle University of Thessaloniki) for his comments on the earlier versions of this manuscript. The editors (Y. Nakamura, A. Bush, and J.X. Samuels) and four reviewers including J. Saarinen are acknowledged for their helpful comments. Moreover, we would like to thank to the authorities of the following museum collections to give access to the related materials: Museo Nacional de Ciencias Naturales (MNCN), ICP museum, Laboratory of Geology and Palaeontology at the University of Thessaloniki, Palaeontological Museum Dimitar Kovachev at Asenovgrad, and the Ege University Natural History Museum. Funding for this study was provided by the Grant PID2020-117118GBI00 funded by MCIN/AEI/10.3039/501100011033 (M.K), the project CGL2015-63777 (M.K), and the CERCA Program, Generalitat de Catalunya. R.S.S., T.K., S.M., and G.M. were granted through a “Wenner-Gren International Collaborative Research Grant” (PIs: R.S. Scott and T. Kaya) to collect most of the Vallesian and Turolian equid dental molds from the eastern Mediterranean, and S.M. and T.K. were supported by the Ege University Research Projects TTM/001/2015 and TTM/001/2016. M.K. and G.O-O. were part of a research group recognized without funding by AGAUR (2017 SGR 960) and G.O-O. was supported by a FI-DGR 2016 grant (2016FI_B00202) awarded by AGAUR (Generalitat de Catalunya) and held an Erasmus + fellowship during his stay in the PALEVOPRIM laboratory (CNRS and University of Poitiers). The grant SYNTHESIS ES-TAF-1846 for the stay of J.C. at the MNCN of Madrid is also acknowledged.
REFERENCES
Ackermans, N.L., Martin, L.F., Codron, D., Hummel, J., Kircher, P.R., Richter, H., Kaiser, T.M., Clauss, M., and Hatt J.M. 2020a. Mesowear represents a lifetime signal in sheep (Ovis aries) within a long-term feeding experiment. Palaeogeography, Palaeoclimatology, Palaeoecology, 553:109793. https://doi.org/10.1016/j.palaeo.2020.109793
Ackermans, N.L., Winkler, D.E., Martin, L.F., Kaiser, T.M., Clauss, M., and Hatt, J.M. 2020b. Dust and grit matter: Abrasives of different size lead to opposing dental microwear textures in experimentally fed sheep (Ovis aries). Journal of Experimental Biology, 223:jeb220442. https://doi.org/10.1242/jeb.220442
Adams, N.F., Gray, T., and Purnell, M.A. 2020. Dietary signals in dental microwear of predatory small mammals appear unaffected by extremes in environmental abrasive load. Palaeogeography, Palaeoclimatology, Palaeoecology, 558:109929. https://doi.org/10.1016/j.palaeo.2020.109929
Agustı́, J., Sanz de Siria, A., and Garcés, M. 2003. Explaining the end of the hominoid experiment in Europe. Journal of Human Evolution, 45:145-153. https://doi.org/10.1016/S0047-2484(03)00091-5
Alberdi, M.T. 1989. A review of Old World hipparionine horses, p. 234-261. In Prothero, D.R. and Schoch, R.M. (eds.), The Evolution of Perissodactyls. Oxford University Press, New York.
Alberdi, M.T., Prado, J.L., and Ortiz-Jaureguizar, E. 1995. Patterns of body size changes in fossil and living Equini (Perissodactyla). Biological Journal of the Linnean Society, 54:349-370. https://doi.org/10.1111/j.1095-8312.1995.tb01042.x
Azorit, C., Tellado, S., Oya, A., and Moro, J. 2012. Seasonal and specific diet variations in sympatric red and fallow deer of southern Spain: a preliminary approach to feeding behaviour. Animal Production Science, 52:720-727. https://doi.org/10.1071/AN12016
Bauer, I.E., McMorrow J., and Yalden, D.W. 1994. The historic ranges of three equid species in north-east Africa: a quantitative comparison of environmental tolerances. Journal of Biogeography, 21:169-182. https://doi.org/10.2307/2845470
Bell, R.H.V. 1971. A grazing ecosystem in the Serengeti. Scientific American, 225:86-93.
Berlioz, E., Azorit, C., Blondel, C., Ruiz, M.S.T., and Merceron, G. 2017. Deer in an arid habitat: dental microwear textures track feeding adaptability. Hystrix the Italian Journal of Mammalogy, 28:222-230. https://doi.org/10.4404/hystrix-28.2-12048
Bernor, R.L. and Tobien, H. 1989. Two small species of Cremohipparion (Equidae, Mamm.) from Samos, Greece. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie, 29:207-226.
Bernor, R.L., Kovar-Eder, J., Suc, J.-P., and Tobien, H. 1990a. A contribution to the evolutionary history of European Late Miocene age hipparionines (Mammalia: Equidae). Paléobiologie Continentale, 17:291-309.
Bernor, R.L., Tobien, H., and Woodburne, M.O. 1990b. Patterns of Old World hipparionine evolutionary diversification and biogeographic extension, p. 263-319. In Lindsay, E.H., Fahlbusch, V., and Mein, P. (eds.), European Neogene Mammal Chronology. Plenum Press, New York. https://doi.org/10.1007/978-1-4899-2513-8_18
Bernor, R.L., Koufos, G.D., Woodburne, M.O., and Fortelius, M. 1996. The evolutionary history and biochronology of European and Southwest Asian Late Miocene and Pliocene hipparionine horses, p. 307-338. In Bernor, R.L., Fahlbusch, V., and Mittmann, H.-W. (eds.), The Evolution of Western Eurasian Neogene Mammal Faunas. Columbia University Press, New York.
Bernor, R.L., Scott, R.S., Fortelius, M., Kappelman J., and Sen, S. 2003. Equidae (Perissodactyla), p. 220-281. In Fortelius, M., Kappelman, J., Sen, S., and Bernor, R.L. (eds.), The Geology and Paleontology of the Miocene Sinap Formation, Turkey. Columbia University Press.
Bernor, R.L., Mirzaie Ataabadi, M., Meshida, K., and Wolf, D. 2016. The Maragheh hipparions, late Miocene of Azarbaijan, Iran. Palaeobiodiversity and Palaeoenvironments, 96:453-488. https://doi.org/10.1007/s12549-016-0235-2
Bernor, R.L., Kaya, F., Kaakinen, A., Saarinen, J., and Fortelius, M. 2021. Old world hipparion evolution, biogeography, climatology and ecology. Earth-Science Reviews, 221:103784. https://doi.org/10.1016/j.earscirev.2021.103784
Böhme, M., Ilg, A., and Winklhofer, M. 2008. Late Miocene “washhouse” climate in Europe. Earth and Planetary Science Letters, 275:39401. https://doi.org/10.1016/j.epsl.2008.09.011
Böhme, M., Winkelhofer, M., and Ilg, A. 2011. Miocene precipitation in Europe - temporal trends and spatial gradients. Palaeogeography, Palaeoclimatology, Palaeoecology, 304:212-218. https://doi.org/10.1016/j.palaeo.2010.09.028
Brown, J.S. 1988. Patch use as an indicator of habitat preference, predation risk, and competition. Behavioral Ecology and Sociobiology, 22:37-47. https://doi.org/10.1007/BF00395696
Burgman, J.H.E., Leichliter, J., Avenant, N.L., and Ungar, P.S. 2016. Dental microwear of sympatric rodent species sampled across habitats in southern Africa: Implications for environmental influence. Integrative Zoology, 11:111-127. https://doi.org/10.1111/1749-4877.12188
Calandra, I. and Merceron, G. 2016. Dental microwear texture analysis in mammalian ecology. Mammal Review, 46:215-218. https://doi.org/10.1111/mam.12063
Calder, W.A. 1984. Size, Function, and Life History. Harvard University Press, Cambridge.
Cantalapiedra, J.L., Prado, J.L., Hernández Fernández, M., and Alberdi, M.T. 2017. Decoupled ecomorphological evolution and diversification in Neogene-Quaternary horses. Science, 355:627-630. https://doi.org/10.1126/science.aag1772
Carranza, J., Alarcos, S., Sánchez-Prieto, C.B., Valencia, J., and Mateos, C. 2004. Disposable-soma senescence mediated by sexual selection in an ungulate. Nature, 432:215-218. https://doi.org/10.1038/nature03004
Carranza, J., Mateos, C., Alarcos, S., Sánchez-Prieto, C.B., and Valencia, J. 2008. Sex-specific strategies of dentine depletion in red deer. Biological Journal of the Linnean Society, 93:487-497. https://doi.org/10.1111/j.1095-8312.2007.00903.x
Case, T.J. 1979. Optimal body size and an animal’s diet. Acta Biotheoretica, 28: 54-69. https://doi.org/10.1007/BF00054680
Clavel, J., Merceron, G., Hristova, L., Spassov, N., Kovachev, D., and Escarguel, G. 2012. On Mesopithecus habitat: Insights from late Miocene fossil vertebrate localities of Bulgaria. Journal of Human Evolution, 63:162-179. https://doi.org/10.1016/j.jhevol.2012.04.007
Codron, D., Lee-Thorp, J.A., Sponheimer, M., Codron, J., De Ruiter, D., and Brink, J.S. 2007. Significance of diet type and diet quality for ecological diversity of African ungulates. Journal of Animal Ecology, 76:526-537. https://doi.org/10.1111/j.1365-2656.2007.01222.x
Conover, W.J. and Iman, R.L. 1981. Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician, 35:124-129. https://doi.org/10.2307/2683975
Damuth, J.D. and MacFadden, B.J. 1990. Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, Cambridge.
Davis, M. and Pineda‐Munoz, S. 2016. The temporal scale of diet and dietary proxies. Ecology and evolution, 6:1883-1897. https://doi.org/10.1002/ece3.2054
de Bonis, L., Bouvrain, G., Geraads, D., and Koufos, G.D. 1992. Diversity and paleoecology of Greek late Miocene mammalian faunas. Palaeogeography, Palaeoclimatology, Palaeoecology, 91:99-121. https://doi.org/10.1016/0031-0182(92)90035-4
De Iongh, H.H., De Jong, C.B., Van Goethem, J., Klop, E., Brunsting, A.M.H., Loth, P.E., and Prins, H.H.T. 2011. Resource partitioning among African savanna herbivores in North Cameroon: The importance of diet composition, food quality and body mass. Journal of Tropical Ecology, 27:503-513. https://doi.org/10.1017/S0266467411000307
Denk, T. 2016. Palaeoecological interpretation of the late Miocene landscapes and vegetation of northern Greece: A comment to Merceron et al., 2016 (Geobios 49, 135-146). Geobios, 49:423-431. https://doi.org/10.1016/j.geobios.2016.06.009
Denk, T., Zohner, C.M., Grimm, G.W., and Renner, S.S. 2018. Plant fossils reveal major biomes occupied by the late Miocene Old-World Pikermian fauna. Nature Ecology and Evolution, 2:1864-1870. https://doi.org/10.1038/s41559-018-0695-z
DeSantis, L.R.G. 2016. Dental microwear textures: Reconstructing diets of fossil mammals. Surface Topography: Metrology and Properties, 4:023002.
DeSantis, L.R.G., Scott, J.R., Schubert, B.W., Donohue, S.L., McCray, B.M., Van Stolk, C.A., Winburn, A.A., Greshko, M.A., and O’Hara, M.C. 2013. Direct comparisons of 2D and 3D dental microwear proxies in extant herbivorous and carnivorous mammals. PLoS ONE, 8:e71428. https://doi.org/10.1371/journal.pone.0071428
Dmitriew, C.M. 2011. The evolution of growth trajectories: what limits growth rate? Biological Reviews, 86:97-116. https://doi.org/10.1111/j.1469-185X.2010.00136.x
Donzelli, D., De Michele, C., and Scholes, R.J. 2013. Competition between trees and grasses for both soil water and mineral nitrogen in dry savannas. Journal of Theoretical Biology, 332:181-190. https://doi.org/10.1016/j.jtbi.2013.04.003
Eisenmann, V. and Sondaar, P.Y. 1998. Pliocene vertebrate locality of Çalta, Ankara, Turkey. 7. Hipparion. Geodiversitas, 20:409-439.
Estes, R. 1991. The Behavior Guide to African Mammals: Including Hoofed Mammals, Carnivores, Primates. University of California Press, Berkeley.
Famoso, N.A. and Davis, E.B. 2016. On the relationship between enamel band complexity and occlusal surface area in Equids (Mammalia, Perissodactyla). PeerJ, 4:e2181. https://doi.org/10.7717/peerj.2181
February, E.C., Higgins, S.I., Bond, W.J., and Swemmer, L. 2013. Influence of competition and rainfall manipulation on the growth responses of savanna trees and grasses. Ecology, 94:1155-1164. https://doi.org/10.1890/12-0540.1
Forsten, A. 1968. Revision of the palearctic Hipparion. Acta Zoologica Fennica, 119:1-134.
Forsten, A. 1978a. Hipparion primigenium (v. Meyer, 1829), an early three-toed horse. Annales Zoologici Fennici, 15:298-313.
Forsten, A. 1978b. Hipparion and possible Iberian-North African Neogene connections. Annales Zoologici Fennici, 15:294-297.
Forsten, A. 1997. Hipparion from Santiga (Spain) and its biostratigraphic significance. Paleontologia i Evolució, 30-31:77-82.
Forsten, A. and Kaya, T. 1995. The hipparions (Mammalia, Equidae) from Gülpinar (Canakkale, Turkey). Paläontologische Zeitschrift, 69:491-501. https://doi.org/10.1007/BF02987809
Fortelius, M., Werdelin, L., Andrews, P., Bernor, R.L., Gentry, A., Humphrey, L., Mittmann, H.W., and Viranta, S. 1996. Provinciality, diversity, turnover and paleoecology in land mammal faunas of the later Miocene of Western Eurasia, p. 414-448. In Bernor, R.L., Fahlbusch, V., and Mittmann, H.-W. The Evolution of Western Eurasian Neogene Mammal Faunas. Columbia University Press.
Fortelius, M., Eronen, J.T., Jernvall, J., Liu, L., Pushkina, D., Rinne, J., Tesakov, A., Vislobokova, I.A., Zhang, Z., and Zhou, L. 2002. Fossil mammals resolve regional patterns of Eurasian climate change during 20 million years. Evolutionary Ecology Research, 4:1005-1016.
Gabunia, L.K. and Chochieva, K.I. 1982. Co-evolution of the Hipparion fauna and vegetation in the Paratethys region. Evolutionary Theory, 6:1-13.
Gagnon, M. and Chew, A.E. 2000. Dietary preferences in extant African Bovidae. Journal of Mammalogy, 81:490-511. https://doi.org/10.1644/1545-1542(2000)081<0490:DPIEAB>2.0.CO;2
Gould, G.C. and MacFadden, B.J. 2004. Gigantism, dwarfism, and Cope’s Rule: “nothing in evolution makes sense without a phylogeny”. Bulletin of the American Museum of Natural History, 285:219-237. https://doi.org/10.1206/0003-0090(2004)285<0219:C>2.0.CO;2
Hayek, L.-A.C., Bernor, R.L., Solounias, N., and Steigerwald, P. 1992. Preliminary studies of hipparionine horse diet as measured by tooth microwear. Annales Zoologici Fennici, 28:187-200.
Hedberg, C. and DeSantis, L.R.G. 2017. Dental microwear texture analysis of extant koalas: clarifying causal agents of microwear. Journal of Zoology, 301:206-214. https://doi.org/10.1111/jzo.12413
Hilgen, F.J., Lourens, L.J., van Dam, J., Beu, A.G., Boyes, A.F., Cooper, R.A., Krijgsman, W., Ogg, J.G., Piller, W.E., and Wilson, D.S. 2012. The Neogene Period, p. 923-978. In Gradstein, F.M., Ogg, J.G., Schmitz, M., and Ogg, G. (eds.), The Geologic Time Scale 2012. https://doi.org/10.1016/B978-0-444-59425-9.00029-9
Hipondoka, M.H., Aranibar, J., Chirara, C., Lihavha, M., and Macko, S. 2003. Vertical distribution of grass and tree roots in arid ecosystems of Southern Africa: niche differentiation or competition? Journal of Arid Environments, 54:319-325. https://doi.org/10.1006/JARE.2002.1093
Hopcraft, J.G.C., Olff, H., and Sinclair, A.R.E. 2010. Herbivores, resources and risks: alternating regulation along primary environmental gradients in savannas. Trends in Ecology and Evolution, 25:119-128. https://doi.org/10.1016/j.tree.2009.08.001
Hua, L., Chen, J., and Ungar, P.S. 2020. Diet reduces the effect of exogenous grit on tooth microwear. Biosurface and Biotribology, 3:48-52. https://doi.org/10.1049/bsbt.2019.0041
Hullot, M., Antoine, P.O., Ballatore, M., and Merceron, G. 2019. Dental microwear textures and dietary preferences of extant rhinoceroses (Perissodactyla, Mammalia). Mammal Research, 64:397-409. https://doi.org/10.1007/s13364-019-00427-4
Hristova, L. 2009. Ontogeny and variability in the cheek region of hipparions from the late Miocene locality of Hadzhidimovo-1, southwest Bulgaria. Rivista Italiana di Paleontologia e Stratigrafia, 115:125-132. https://doi.org/10.13130/2039-4942/5924
Ioakim, C. and Koufos, G.D. 2009. Palynology, p. 27-35. In Koufos, G.D. and Nage, D. (eds.), The late Miocene Mammal Faunas of the Mytilinii Basin, Samos Island, Greece: New Collection. Beiträge zur Paläontologie, 31.
Janis, C.M. 2008. An evolutionary history of browsing and grazing ungulates, p. 21-45. In Gordon, I.J. and Prins, H.H.T. (eds.), The Ecology of Browsing and Grazing. Ecological Studies. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-72422-3_2
Jarman, P.J. 1974. The social organisation of antelope in relation to their ecology. Behaviour, 48:215-267. https://www.jstor.org/stable/4533573
Jiménez-Moreno, G., Popescu, S.M., Ivanov, D., and Suc, J.-P. 2007. Neogene flora, vegetation and climate dynamics in southeastern Europe and the northeastern Mediterranean, p. 503-516. In Willians, W., Haywood, A.M., Gregory, F.J., and Schmidt, D.N. (eds.), Deep-Time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies. The Micropalaeontological Society, London.
Jordana, X., Marín-Moratalla, N., DeMiguel, D., Kaiser, T.M., and Köhler, M. 2012. Evidence of correlated evolution of hypsodonty and exceptional longevity in endemic insular mammals. Proceedings of the Royal Society B: Biological Sciences, 279:3339-3346. https://doi.org/10.1098/rspb.2012.0689
Kaiser, T.M. 2003. The dietary regimes of two contemporaneous populations of Hippotherium primigenium (Perissodactyla, Equidae) from the Vallesian (Upper Miocene) of Southern Germany. Palaeogeography, Palaeoclimatology, Palaeoecology, 198:381-402. https://doi.org/10.1016/S0031-0182(03)00480-2
Kostopoulos, D.S. 2009. The Pikermian Event: Temporal and spatial resolution of the Turolian large mammal fauna in SE Europe. Palaeogeography, Palaeoclimatology, Palaeoecology, 274:82-95. https://doi.org/10.1016/j.palaeo.2008.12.020
Koufos, G.D. 1990. The hipparions of the lower Axios Valley (Macedonia, Greece) Implications for the Neogene stratigraphy and the evolution of hipparions, p. 321-338. In Lindsay, E.H., Fahlbusch, V., and Mein, P. (eds.), European Neogene Mammal Chronology. Plenum Press, New York. https://doi.org/10.1007/978-1-4899-2513-8_19
Koufos, G.D. 2006. Palaeoecology and chronology of the Vallesian (late Miocene) in the Eastern Mediterranean region. Palaeogeography, Palaeoclimatology, Palaeoecology, 234:127-145. https://doi.org/10.1016/j.palaeo.2005.01.014
Koufos, G.D. 2016. Hipparion macedonicum revisited: New data on evolution of hipparionine horses from the Late Miocene of Greece. Acta Palaeontologica Polonica, 61:519-536. https://doi.org/10.4202/app.00169.2015
Koufos, G.D., Merceron, G., Kostopoulos, D.S., Vlachou T.D., and Sylvestrou, I.A. 2006. Palaeoecology and Palaeobiogeography, p. 201-221. In Koufos, G.D. (ed.), The late Miocene Vertebrate Locality of Perivolaki, Thessaly, Greece. Palaeontographica Abteilung A, 276.
Koufos, G.D., Kostopoulos, D.S., and Merceron, G. 2009. Palaeoecology - Palaeobiogeography, p. 409-430. In Koufos, G.D. and Nagel, D. (eds.), The late Miocene Mammal Faunas of the Mytilinii Basin, Samos Island, Greece: new collection. Beiträge zur Paläontologie, 31.
Koufos, G.D. and Vlachou, T.D. 2016. Equidae, p. 85-118. In Koufos, G.D. and Kostopoulos, D.S. (eds.), Palaeontology of the Upper Miocene Vertebrate Localities of Nikiti (Chalkidiki Peninsula, Macedonia, Greece). Geobios, 49. https://doi.org/10.1016/j.geobios.2016.01.001
Langsrud, Ø. 2003. ANOVA for unbalanced data: Use Type II instead of Type III sums of squares. Statistics and Computing, 13:163-167. https://doi.org/10.1023/A:1023260610025
Lucas, P.W., Casteren, A. van, Al-Fadhalah, K., Almusallam, A.S., Henry, A.G., Michael, S., Watzke, J., Reed, D.A., Diekwisch, T.G.H., Strait, D.S., and Atkins, A.G. 2014. The role of dust, grit and phytoliths in tooth wear. Annales Zoologici Fennici, 51:143-152. https://doi.org/10.5735/086.051.0215
MacFadden, B.J. 1992. Fossil Horses: Systematics, Paleobiology, and Evolution of the Family Equidae. Cambridge University Press, New York.
MacFadden, B.J. 2005. Fossil horses—evidence for evolution. Science, 307:1728-730. https://doi.org/10.1126/science.1105458
MacFadden, B.J. and Hulbert, R.C. 1988. Explosive speciation at the base of the adaptive radiation of Miocene grazing horses. Nature, 336:466-468. https://doi.org/10.1038/336466a0
MacFadden, B.J., Solounias, N., and Cerling, T.E. 1999. Ancient diets, ecology, and extinction of 5-million-year-old horses from Florida. Science, 283:824-827. https://doi.org/10.1126/science.283.5403.824
Madden, R.H. 2014. Hypsodonty in Mammals: Evolution, Geomorphology and the Role of Earth Surface Processes, Hypsodonty in Mammals: Evolution, Geomorphology and the Role of Earth Surface Processes. Cambridge University Press, Cambridge.
Marchand, P., Redjadj, C., Garel, M., Cugnasse, J.M., Maillard, D., and Loison, A. 2013. Are mouflon Ovis gmelini musimon really grazers? A review of variation in diet composition. Mammal Review, 43: 275-291. https://doi.org/10.1111/mam.12000
Martin, F., Plastiras, C.-A., Merceron, G., Souron, A., and Boisserie, J.-R. 2018. Dietary niches of terrestrial cercopithecines from the Plio-Pleistocene Shungura Formation, Ethiopia: evidence from Dental Microwear Texture Analysis. Scientific Reports, 8:14052. https://doi.org/10.1038/s41598-018-32092-z
Matthew, W.D. 1926. The evolution of the horse: a record and its interpretation. The Quaterly Review of Biology, 1:139-185. https://www.jstor.org/stable/2808222
Merceron, G., Bonis, L. de, Viriot, L., and Blondel, C. 2005a. Dental microwear of fossil bovids from northern Greece: Paleoenvironmental conditions in the eastern Mediterranean during the Messinian. Palaeogeography, Palaeoclimatology, Palaeoecology, 217:173-185. https://doi.org/10.1016/j.palaeo.2004.11.019
Merceron, G., Bonis, L. de, Viriot, L., and Blondel, C. 2005b. Dental microwear of the late Miocene bovids of northern Greece: Vallesian/Turolian environmental changes and disappearance of Ouranopithecus macedoniensis? Bulletin de la Societe Geologique de France, 176:475-484. https://doi.org/10.2113/176.5.475
Merceron, G., Scott, J.R., Scott, R.S., Geraads, D., Spassov, N., and Ungar, P.S. 2009. Folivory or fruit/seed predation for Mesopithecus, an earliest colobine from the late Miocene of Eurasia? Journal of Human Evolution, 57:732-738. https://doi.org/10.1016/j.jhevol.2009.06.009
Merceron, G., Kaiser, T.M., Kostopoulos, D.S., and Schulz, E. 2010. Ruminant diets and the Miocene extinction of European great apes. Proceedings of the Royal Society B: Biological Sciences, 277:3105-3112. https://doi.org/10.1098/rspb.2010.0523
Merceron, G., Kostopoulos, D.S., Bonis, L. de, Fourel, F., Koufos, G.D., Lécuyer, C., and Martineau, F. 2013. Stable isotope ecology of Miocene bovids from Northern Greece and the ape/monkey turnover in the Balkans. Journal of Human Evolution, 65:185-198. https://doi.org/10.1016/j.jhevol.2013.05.003
Merceron, G., Novello, A., and Scott, R.S. 2016a. Paleoenvironments inferred from phytoliths and Dental Microwear Texture Analyses of meso-herbivores, p. 135-146. In Koufos, G.D. and Kostopoulos, D.S. (eds.), Palaeontology of the upper Miocene vertebrate localities of Nikiti (Chalkidiki Peninsula, Macedonia, Greece). Geobios, 49. https://doi.org/10.1016/j.geobios.2016.01.004
Merceron, G., Ramdarshan, A., Blondel, C., Boisserie, J.-R., Brunetière, N., Francisco, A., Gautier, D., Milhet, X., Novello, A., and Pret, D. 2016b. Untangling the environmental from the dietary: Dust does not matter. Proceedings of the Royal Society B: Biological Sciences, 283:20161032. https://doi.org/10.1098/rspb.2016.1032
Merceron, G., Berlioz, E., Vonhof, H., Green, D., Garel, M., and Tütken, T. 2021. Tooth tales told by dental diet proxies: An alpine community of sympatric ruminants as a model to decipher the ecology of fossil fauna. Palaeogeography, Palaeoclimatology, Palaeoecology, 565:110077. https://doi.org/10.1016/j.palaeo.2020.110077
Mihlbachler, M.C. and Solounias, N. 2002. Body size, dental microwear, and brontothere diets through the Eocene. Journal of Vertebrate Paleontology, 22:88A.
Mihlbachler, M.C., Rivals, F., Solounias, N., and Semprebon, G. 2011. Dietary change and evolution of horses in North America. Science, 331:1178-1181. https://doi.org/10.1126/science.1196166
Moehlman, P.D. 2002. Equids: Zebras, Asses, and Horses: Status Survey and Conservation Action Plan. IUCN/SSC Equid Specialist Group, IUCN, Gland, Switzerland and Cambridge, UK.
Montoya, P., Morales, J., Robles, F., Abella, J., Benavent, J.V, Marín, M.D., and Ruiz Sánchez, F.J. 2006. Las nuevas excavaciones (1995-2006) en el yacimiento del Mioceno final de Venta del Moro, Valencia. Estudios Geológicos, 62:313-326.
Nacarino-Meneses, C., Jordana, X., Orlandi-Oliveras, G., and Köhler, M. 2017. Reconstructing molar growth from enamel histology in extant and extinct Equus. Scientific Reports, 7:15965. https://doi.org/10.1038/s41598-017-16227-2
Nelder, J.A. 1977. A reformulation of linear models. Journal of the Royal Statistical Society. Series A (General), 140:48-77. https://doi.org/10.2307/2344517
Orlandi-Oliveras, G., Nacarino-Meneses, C., Koufos, G.D., and Köhler, M. 2018. Bone histology provides insights into the life history mechanisms underlying dwarfing in hipparionins. Scientific Reports, 8:17203. https://doi.org/10.1038/s41598-018-35347-x
Orlandi-Oliveras, G., Nacarino-Meneses, C., and Köhler, M. 2019. Dental histology of late Miocene hipparionins compared with extant Equus, and its implications for Equidae life history. Palaeogeography, Palaeoclimatology, Palaeoecology, 528:133-146. https://doi.org/10.1016/j.palaeo.2019.04.016
Ortiz-Jaureguizar, E. and Alberdi, M.T. 2003. El patrón de cambios en la masa corporal de los Hipparionini (Perissodactyla, Equidae) de la Península Ibérica durante el Mioceno superior-Plioceno superior. Coloquios de Paleontología, 1:499-509.
Palkovacs, E.P. 2003. Explaining adaptive shifts in body size on islands: A life history approach. Oikos, 103:37-44. https://doi.org/10.1034/j.1600-0706.2003.12502.x
Pérez-Barbería, F.J., Carranza, J., and Sánchez-Prieto, C. 2015. Wear fast, die young: More worn teeth and shorter lives in Iberian compared to Scottish red deer. PLoS One, 10:e0134788. https://doi.org/10.1371/journal.pone.0134788
Pesquero, M.D. 2003. Hipparion del Turoliense superior de Las Casiones (Fosa de Teruel). Coloquios de Paleontología, Volumen Ex:511-548.
Pesquero, M.D. and Alberdi, M.T. 2012. New evidence of conspecificity between Hipparion primigenium melendezi Alberdi, 1974 from Los Valles de Fuentidueña (Segovia) and Hipparion concudense concudense Pirlot, 1956 from Concud (Teruel) Spain. Estudios Geológicos, 68:247-260. https://doi.org/10.3989/egeol.40499.152
Pesquero, M.D., Alcalá, L., and Fernández-Jalvo, Y. 2013. Taphonomy of the reference Miocene vertebrate mammal site of Cerro de la Garita, Spain. Lethaia, 46:378-398. https://doi.org/10.1111/let.12016
Peters, R.H. 1983. The Ecological Implications of Body Size. Cambridge Studies in Ecology. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511608551
Ramdarshan, A., Blondel, C., Gautier, D., Surault, J., and Merceron, G. 2017. Overcoming sampling issues in dental tribology: Insights from an experimentation on sheep. Palaeontologia Electronica 19353:1-19. https://doi.org/10.26879/762
Rey, K., Amiot, R., Lécuyer, C., Koufos, G.D., Martineau, F., Fourel, F., Kostopoulos, D.S., and Merceron, G. 2013. Late Miocene climatic and environmental variations in northern Greece inferred from stable isotope compositions (δ18O, δ13C) of equid teeth apatite. Palaeogeography, Palaeoclimatology, Palaeoecology, 388:48-57. https://doi.org/10.1016/j.palaeo.2013.07.021
Rodgers, W.A. 1982. The decline of large mammal populations on the Lake Rukwa grasslands, Tanzania. African Journal of Ecology, 20:13-22. https://doi.org/10.1111/j.1365-2028.1982.tb01078.x
Saarinen, J.J. 2009. Body mass patterns of Eurasian Miocene large land mammals and their connections to environment and climate. Unpublished Master’s Thesis, University of Helsinki, Finland.
Saarinen, J.J., Eronen, J., Fortelius, M., Seppä, H., and Lister, A.M. 2016. Patterns of diet and body mass of large ungulates from the Pleistocene of Western Europe, and their relation to vegetation. Palaeontologia Electronica, 19:1-58. https://doi.org/10.26879/443
Saarinen, J.J., Cirilli, O., Strani, F., Meshida, K., and Bernor, R.L. 2021. Testing equid body mass estimate equations on modern zebras-with implications to understanding the relationship of body size, diet, and habitats of Equus in the Pleistocene of Europe. Frontiers in Ecology and Evolution, 9:6222412. https://doi.org/10.3389/fevo.2021.622412
Sanson, G.D., Kerr, S.A., and Gross, K.A. 2007. Do silica phytoliths really wear mammalian teeth? Journal of Archaeological Science, 34:526-531. https://doi.org/10.1016/j.jas.2006.06.009
Sanson, G.D., Kerr, S.A., and Read, J. 2017. Dietary exogenous and endogenous abrasives and tooth wear in African buffalo. Biosurface and Biotribology, 3:211-223. https://doi.org/10.1016/j.bsbt.2017.12.006
Scheiter, S. and Higgins, S.I. 2007. Partitioning of root and shoot competition and the stability of savannas. American Naturalist, 170:587-601. https://doi.org/10.1086/521317
Schulz-Kornas, E., Winkler, D.E., Clauss, M., Carlsson, J., Ackermans, N.L., Martin, L.F., Hummel, J., Müller, D.W.H., Hatt, J.M., and Kaiser, T.M. 2020. Everything matters: Molar microwear texture in goats (Capra aegagrus hircus) fed diets of different abrasiveness. Palaeogeography, Palaeoclimatology, Palaeoecology, 552:109783. https://doi.org/10.1016/j.palaeo.2020.109783
Scott, J.R. 2012. Dental microwear texture analysis of extant African Bovidae. Mammalia, 76:157-174. https://doi.org/10.1515/mammalia-2011-0083
Scott, K.M. 1990. Postcranial dimensions of ungulates as predictors of body mass, p. 301-335. In Damuth, J. and Macfadden, B.J. (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, Cambridge.
Scott, R.S., Fortelius, M., Huttunen, K., and Armour-Chelu, M. 2003. Abundance of “Hipparion,” p. 380-397. In Fortelius, M., Kappelman, J., Sen, S., and Bernor, R.L. (eds.), The Geology and Paleontology of the Miocene Sinap Formation, Turkey. Columbia University Press.
Scott, R.S. and Maga, M. 2005. Paleoecology of the Akkasșdağı hipparions (Mammalia, Equidae), late Miocene of Turkey, p. 809-830. In Sen, S. (ed.), Geology, mammals and environments at Akkasșdağı, late Miocene of Central Anatolia. Geodiversitas, 27.
Scott, R.S., Ungar, P.S., Bergstrom, T.S., Brown, C.A., Grine, F.E., Teaford, M.F., and Walker, A. 2005. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature, 436:693-695. https://doi.org/10.1038/nature03822
Scott, R.S., Ungar, P.S., Bergstrom, T.S., Brown, C.A., Childs, B.E., Teaford, M.F., and Walker, A. 2006. Dental microwear texture analysis: technical considerations. Journal of Human Evolution, 51:339-349. https://doi.org/10.1016/j.jhevol.2006.04.006
Scott, R.S., Clavel, J., DeMiguel, D., Kaya, T., Kostopoulos, D.S., Mayda, S., and Merceron, G. 2013. Ecology of european hipparionines and the diversity of late Miocene hominids in western Eurasia.14th RCMN Congress Neogene to Quaternary Geological Evolution of Mediterranean, Paratethys and Black Sea. Istanbul, p. 262.
Semprebon, G.M., Rivals, F., Solounias, N., and Hulbert, R.C. 2016. Paleodietary reconstruction of fossil horses from the Eocene through Pleistocene of North America Palaeogeography, Palaeoclimatology, Palaeoecology, 442:110-127. https://doi.org/10.1016/j.palaeo.2015.11.004
Smith, B.H. 1991. Dental development and the evolution of life history in Hominidae. American Journal of Physical Anthropology, 86:157-174. https://doi.org/10.1002/ajpa.1330860206
Smith, B.H. 2000. ‘Schultz’s Rule’ and the evolution of tooth emergence and replacement patterns in primates and ungulates, p. 212-228. In Teaford, M.F., Meredith Smith, M., and Ferguson, M.W.J. (eds.), Development, Function and Evolution of Teeth. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511542626.015
Smith, G.J. and DeSantis, L.R.G. 2018. Dietary ecology of Pleistocene mammoths and mastodons as inferred from dental microwear textures. Palaeogeography, Palaeoclimatology, Palaeoecology, 492:10-25. https://doi.org/10.1016/j.palaeo.2017.11.024
Solounias, N., Plavcan, M., Quade, J., and Witmer, L. 1999. The Pikermian Biome and the savanna myth, p. 427-444. In Agustı́, J., Andrews, P., and Rook, L. (eds.), Evolution of the Neogene Terrestrial Ecosystems in Europe. Cambridge University Press, Cambridge
Solounias, N. and Semprebon, G.M. 2002. Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids. American Museum Novitates, 3366:1-49. https://doi.org/10.1206/0003-0082(2002)366<0001:AITROU>2.0.CO;2
Solounias, N., Rivals, F., and Semprebon, G.M. 2010. Dietary interpretation and paleoecology of herbivores from Pikermi and Samos (late Miocene of Greece). Paleobiology, 36:113-136. https://doi.org/10.1666/0094-8373-36.1.113
Stearns, S.C. 1992. The Evolution of Life Histories. Oxford University Press, New York.
Stebbins, G.L. 1981. Coevolution of Grasses and Herbivores. Annals of the Missouri Botanical Garden, 68:75-86.
Stirton, R.A. 1947. Observations on evolutionary rates in hypsodonty. Evolution, 1:32-41. https://doi.org/10.1111/j.1558-5646.1947.tb02711.x
Strömberg, C.A.E. 2006. Evolution of hypsodonty in equids: testing a hypothesis of adaptation. Paleobiology, 32:236-258. https://doi.org/10.1666/0094-8373(2006)32[236:EOHIET]2.0.CO;2
Strömberg, C.A.E., Werdelin, L., Friis, E.M., and Saraç, G. 2007. The spread of grass-dominated habitats in Turkey and surrounding areas during the Cenozoic: Phytolith evidence. Palaeogeography, Palaeoclimatology, Palaeoecology, 250:18-49. https://doi.org/10.1016/j.palaeo.2007.02.012
Teaford, M.F. and Oyen, O.J. 1989. In vivo and in vitro turnover in dental microwear. American Journal of Physical Anthropology, 80:447-467. https://doi.org/10.1002/ajpa.1330800405
Teaford, M.F., Ungar, P.S., Taylor, A.B., Ross, C.F., and Vinyard, C.J. 2017. In vivo rates of dental microwear formation in laboratory primates fed different food items. Biosurface and Biotribology, 3:166-173. https://doi.org/10.1016/J.BSBT.2017.11.005
Tütken, T., Kaiser, T.M., Vennemann, T., and Merceron, G. 2013. Opportunistic feeding strategy for the earliest old world hypsodont equids: evidence from stable isotope and dental wear proxies. PLoS One, 8:e74463. https://doi.org/10.1371/journal.pone.0074463
Ungar, P.S., Brown, C.A., Bergstrom, T.S., and Walker, A. 2003. Quantification of dental microwear by tandem scanning confocal microscopy and scale-sensitive fractal analyses. Scanning, 25:185-193. https://doi.org/10.1002/sca.4950250405
Veiberg, V., Mysterud, A., Gaillard, J.M., Delorme, D., Van Laere, G., and Klein, F. 2007. Bigger teeth for longer life? Longevity and molar height in two roe deer populations. Biology Letters, 3:268-270. https://doi.org/10.1098/rsbl.2006.0610
Vlachou, T.D. 2013. Palaeontological, biostratigraphical and palaeoecological study of the Greek hipparions. PhD Thesis. Aristotle University of Thessaloniki, Greece. (In Greek with English summary)
Wang, L., D’Odorico, P., Ries, L., and Macko, S.A. 2010. Patterns and implications of plant-soil δ13C and δ15N values in African savanna ecosystems. Quaternary Research, 73:77-83. https://doi.org/10.1016/j.yqres.2008.11.004
Winkler, D.E., van den Hoek Ostende, L.W., Schulz, E., Calandra, I., Gailer, J.P., Landwehr, C., and Kaiser, T.M. 2013. Dietary divergence in space and time - Lessons from the dwarf-goat Myotragus balearicus (Pleisto-Holocene, Mallorca, Spain). Mammalian Biology, 78:430-437. https://doi.org/10.1016/j.mambio.2013.08.003
Woodburne, M.O. 2009. Hippotherium (Mammalia, Equidae), p. 585-604. The early Vallesian vertebrates from Atzelsdorf (Austria, Late Miocene). Annalen des Naturhistorischen Museum Wien, 111A.
Woodburne, M.O. 1989. Hipparion horses: a pattern of endemic evolution and intercontinental dispersal, p.197-233. In Prothero, D.R. and Schoch, R.M. (eds.), The Evolution of Perissodactyls. Oxford University Press, New York.
Woodburne, M.O. and MacFadden, B.J. 1982. A reappraisal of the systematics, biogeography, and evolution of fossil horses. Paleobiology, 8:315-327. https://doi.org/10.1017/S0094837300007077

