 Systematics and dental microwear of the late Miocene Gliridae (Rodentia, Mammalia) from Hayranlı, Anatolia: Implications for paleoecology and paleobiodiversity
Systematics and dental microwear of the late Miocene Gliridae (Rodentia, Mammalia) from Hayranlı, Anatolia: Implications for paleoecology and paleobiodiversity
Article number: 16.3.21A
https://doi.org/10.26879/385
Copyright Palaeontological Association, September 2013
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 15 March 2013. Acceptance: 20 August 2013
{flike id=508}
ABSTRACT
New finds of Gliridae (Mammalia, Rodentia) from the late Miocene of Hayranlı, located in central eastern Anatolia, are described. These specimens include Microdyromys koenigswaldi De Bruijn, 1966, and Myomimus maritsensis De Bruijn et al., 1970. The morphological overlap between Myomimus and Peridyromys makes it difficult to distinguish between the two genera. The last appearance of Microdyromys was previously recorded in Ampudia 3 (MN 10, Duero Basin, Spain), but the Hayranlı collection from the middle Turolian extends its spatiotemporal occurence. Dental microwear analysis indicates that these species of dormice had a diet that involved a combination of insects, fruit, seeds, and grasses, which could point to the development of a more generalist behavior adapted to the seasonal availability of foods. Environmental changes, occurring from the middle Miocene to the late Miocene in Europe and the Eastern Mediterranean, caused a drastic decrease in the number of species of Gliridae adapted to an arboreal lifestyle and a warm and humid climate. There is a significant faunal exchange from forest dwellers to ground dwelling species, which is characterized by the increase in Myomimus finds from a number of localities during the late Miocene — probably attributable to the vegetational shift from predominating forested wetland environments to open woodland and steppe-like environments. Considering the large herbivore-omnivore mammal collection of Hayranlı the mean hypsodonty value (=1.6) depicts a relatively humid woodland and shrubby paleoenvironment.
Ferhat Kaya. Department of Geosciences and Geography, University of Helsinki, PO Box 64 (Gustaf Hällströmin katu 2a), 00014 Helsinki, Finland, ferhat.kaya@helsinki.fi
Nuretdin Kaymakçı. Department of Geological Engineering, Middle East Technical University, Ankara 06531, Turkey, kaymakci@metu.edu.tr
Keywords: Gliridae; microwear; late Miocene; Anatolia; paleoecology; paleobiodiversity
Final citation: Kaya, Ferhat and Kaymakçı, Nuretdin. 2013. Systematics and dental microwear of the late Miocene Gliridae (Rodentia, Mammalia)from Hayranlı, Anatolia: implications for paleoecology and paleobiodiversity, Palaeontologia Electronica Vol. 16, Issue 3; 21A; 22p. https://doi.org/10.26879/385
palaeo-electronica.org/content/2013/508-gliridae-from-anatolia
INTRODUCTION
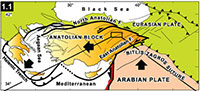
 Hayranlı is located 20 km northwest of Sivas in the central eastern part of Anatolia (Figure 1.1; 2). The localities were first discovered in 1993 by the Turkey Vertebrate Fossil Research Project (Saraç, 2003). During this field campaign Hans de Bruijn and Gerçek Saraç recovered the first set of small mammal test samples. In 1997 the researchers returned to the same site and further expanded the collection. In 2002, a new survey for large mammal fossils was conducted in the previously discovered area in collaboration with the Archaeology Museum of Sivas and Ankara University. Later, in 2009 one of us (F.K.) continued sampling for small mammal fossils to expand on the previous findings.
Hayranlı is located 20 km northwest of Sivas in the central eastern part of Anatolia (Figure 1.1; 2). The localities were first discovered in 1993 by the Turkey Vertebrate Fossil Research Project (Saraç, 2003). During this field campaign Hans de Bruijn and Gerçek Saraç recovered the first set of small mammal test samples. In 1997 the researchers returned to the same site and further expanded the collection. In 2002, a new survey for large mammal fossils was conducted in the previously discovered area in collaboration with the Archaeology Museum of Sivas and Ankara University. Later, in 2009 one of us (F.K.) continued sampling for small mammal fossils to expand on the previous findings.
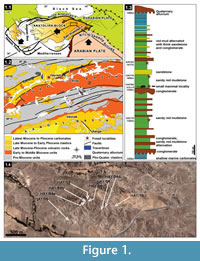
The stratigraphic sequence of the Hayranlı deposit is approximately 185 m thick from bottom to top (Figure 1.3). The fossil deposit consists of a series of large mammal bone-bearing lenses, which are dispersed vertically in different levels ranging from 1300 m to 1430 m and horizontally within an area encompassing around 50 km2. The core study area where the recent survey and excavations have been done includes Hayranlı village, an area that stretches over 10 km2 (Figure 1.4). Since 1993, a total of 92 large and small mammal fossil bearing localities have been discovered, and the richest of these (58-HAY/02-06-19-84) are exhibited on the stratigraphic sequence. Bovids from the localities 58-HAY/14, 19, 23, and 70 have been assigned to late MN11 or early MN12 (Bibi and Güleç, 2008). Additionally, lower MN11 is a suggested age for Hayranlı (58-HAY/2 and 19) based on the evolutionary stage of the suid (Microstonyx major) collection (Made et al., 2013). In 2009, the localities 58-HAY/84a, b, c, d, horizontal continuations of the 58-HAY/84, yielded new small mammal fossils. The relatively rich collection of small mammal fauna recovered from these localities includes: insectivores, murids, cricetids, squirrels, flying squirrels, and glirids. In this article the combined collection of Gliridae (Myomimus and Microdyromys) from the Hayranlı localities 58-HAY/84, 84a, b, c, and d are described. Additionally, the evolutionary and ecological history of both genera is reviewed within the framework of paleoenviromental change during the last 40 Ma.
GEOLOGICAL SETTING
The Sivas Basin is in eastern Central Anatolia and comprises two very distinct sequences extending from 1) Paleocene to Middle Miocene and 2) Late Miocene to Recent. The first configuration belongs to the closure of the Neotethys Ocean and collision of intervening continental blocks including Pontides in the north and Tauride-Anatolide platfrom in the south. While the later configuration was formed by the end of middle Miocene during the Neotectonic period out of a collision and northwards convergence of the Arabian Plate along the Bitlis-Zagros Suture Zone (Şengör and Yılmaz, 1981). The Paleocene to Eocene sequence in the basin corresponds to marine environments while much of Oligocene and lower to middle Miocene sequences relate to continental settings except for a late Burdigalian to Serravalian diachronic marine incursion developed in large areas in eastern and southeastern Anatolia (Çiner et al. 2002). These marine deposits are overlain unconformably by fluvio-lacustrine deposits mainly in the northwestern part of the Sivas Basin and are delimited mainly by ENE-WSW to NE-SW trending faults developed during the Neotectonic period (Kaymakçı et al., 2010). These continental deposits are named as İncesu Formation (Çiner et al., 2002) and are overlain conformably by lacustrine marls and carbonates intercalated with fine clastics. All these units are overlain unconformably by Plio-Quaternary alluvial deposits accumulated along the current drainage networks and adjacent to active faults.
The Hayranlı fossil locality is part of the İncesu Formation in the NW margin of the Sivas Basin (Figure 1.2). The sequence has a very distinct cyclic nature characterized by rhythmical alternation of medium to thick bedded conglomerates, thin to thick bedded red mudstones and siltstones, and medium bedded sandstone at the bottom. Towards the middle part of the sequence a very thick and widespread conglomerate level with pebble imbrications and scour-and-fill structures occur. The sequence continues upwards with similar facies above this conglomerate except for the thin to medium bedded limestone levels intercalated with similar red beds. The occurrences and thickness of the limestone levels increase upwards while the amount of coarse clastics decreases, indicating that fluvial conditions were gradually replaced by lacustrine environments. Finally, the sequence gradually grades into lacustrine limestones intercalated with gray marls, indicating that lacustrine conditions completely dominated in the basin. Although a number of horizons contain vertebrate fossils, the large mammal fossils are recovered mainly from the sandy red mudstones and our small mammal collection obtained from the green marl horizon between the conglomerate and sandy red mudstone. In the Kangal Basin the Late Miocene-Pliocene sequence comprises similar facies except for the domination of lacustrine facies and intercalated volcanics (Kaymakçı et al., 2010).
An Overview of Gliridae
Both the origin and classification of the Gliridae (Myoxidae) are still somewhat controversial. Some researchers use Gliridae while others use Myoxidae; they are synonyms for one another that both represent the same family of dormice studied by different experts over time (Holden, 2005). G.G. Simpson (1945) brought back the name Gliridae in his benchmark work "Principles of Classification and a Classification of Mammals." Wahlert et al. (1993) employ the name Myoxidae arguing that it does fill the requirements of the International Commission on Zoological Nomenclature. However, since Simpson (1945) Gliridae has been established as the most frequently used family name in scientific studies.
Wahlert et al. (1993) associate the Myoxidae (Gliridae) as a myomorph sister taxon of the Muroidae and Dipodoidae based on the size of the infraorbital foramen and positioning of the lacrimal canal in extant glirids. However, as recognized by Schaub (1958), Hartenberger (1971, 1994), and Vianey-Liaud (1985, 1989) the ancestral stock of Gliridae are protrogomorphous and Vianey-Liaud argues that an Eocene genus, Glamys, was pseudo-myomorphous due to the upward positioning of the zygomathic plate. Hartenberger (1994) and Daams and De Bruijn (1995) mentioned that the evolutionary occurrence of myomorph skull morphology in European Gliridae, Asian Muroidea, and Dipodoidea has evolved independently.
Vianey-Liaud and Jaeger (1996) suggest that the Gliridae are paraphyletic and place the graphiurines in a separate family, the Graphiuridae. According to their hypothesis, the affinities Graphiuridae share with anomalurids qualifies them for classification in the same family. On the other hand, this hypothesis of non-monophly of the Gliridae family based on morphological similarities between Graphiurus and Anomaluridae is refuted by results from a molecular analysis of 12S RNA and cytochrcome b mitochondrial gene sequences for six glirid genera (Bentz and Montgelard, 1999; Montgelard et al., 2002; Nunome et al., 2007). On the basis of their study, on molecular systematic analysis of three nuclear fragments and a mitochondrial gene, Montgelard et al. (2003) suggest that Graphiurus probably diverged early in the evolution of the Gliridae. Presently, Graphiurus is restricted in Africa, but occurrence of Microdyromys from Miocene and Eliomys from Pliocene of Africa has been reported (Jaeger, 1977; Winkler et al., 2010). This lends to the thought that Gliridae dispersals to Africa might have first appeared by the Miocene or earlier. Nevertheless, most studies maintain that the Gliridae originated in Europe with Eogliravus and Gliravus during the Eocene (Stehlin and Schaub, 1951; Thaler, 1966; Hartenberger, 1971; Daams and De Bruijn, 1995). The fossil record shows that the Miocene constituted the heyday of the family with high numbers of species. However, many of them became extinct after the Miocene.
On the basis of dental morphology, Daams and De Bruijn (1995) distinguished 38 genera and 177 species assigned to five subfamilies: Gliravinae, Glirinae, Dryominae, Myomiminae, and Bransatoglirinae. Based on extant forms, Holden (2005) presented an alternative classification. Members of Gliridae are represented by nine genera and 28 species and divided into three subfamilies: Glirinae, Leithiinae, and Graphiurinae. According to Holden, Dryomys is a descendent of Microdyromys, and is included in the Leithiinae together with Myomimus and Eliomys in agreement with results from molecular phylogenetic analyses (Montgelard et al., 2003). In 1967, De Bruijn classified Myomimus under Dryomyinae and Peridyromys under Glirulinae. Daams (1981) suggested grouping Myomimus and Peridyromys under a new subfamily Myomiminae.
MATERIALS AND METHODS
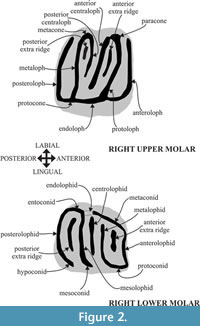 The fossil assemblages of Hayranlı consist of screen-washed material recovered during two different field campaigns—one by Hans De Bruijn and Gerçek Saraç (in 1993 and 1997) and a second one by Ferhat Kaya (in 2009). Seventy-three isolated cheek teeth were measured and dental patterns were assessed. The nomenclature of the dental features and width/length definitions follow Daams (1981) and Garcia-Paredes et al. (2010) (Figure 2). Measurements of the specimens were taken using an Olympus SZX10 stereomicroscope in mm units. Scanning electron images of the specimens were taken using the JXA-8600 Jeol electron probe (20kv, 1nA) microanalyzer in composition backscattered-electron (BSE) image mode at the University of Helsinki, Department of Geosciences and Geography (Helsinki, Finland).
The fossil assemblages of Hayranlı consist of screen-washed material recovered during two different field campaigns—one by Hans De Bruijn and Gerçek Saraç (in 1993 and 1997) and a second one by Ferhat Kaya (in 2009). Seventy-three isolated cheek teeth were measured and dental patterns were assessed. The nomenclature of the dental features and width/length definitions follow Daams (1981) and Garcia-Paredes et al. (2010) (Figure 2). Measurements of the specimens were taken using an Olympus SZX10 stereomicroscope in mm units. Scanning electron images of the specimens were taken using the JXA-8600 Jeol electron probe (20kv, 1nA) microanalyzer in composition backscattered-electron (BSE) image mode at the University of Helsinki, Department of Geosciences and Geography (Helsinki, Finland).
Data gathered (downloaded on February 15, 2013) from New and Old World (NOW) database of Neogene fossil mammal localities and species of Eurasia (Fortelius, 2013) were used to map spatial distribution of Microdyromys, Peridyromys, and Myomimus from the Oligocene to the Late Miocene using Quantum GIS Copiapo 1.6.0 software. Additionally, the data set used was derived from the NOW database (February 15, 2013) for abundance of Gliridae. The calculation method of mean hypsodonty value follows the methodology used by Fortelius et al. (2002).
Microwear analysis was conducted on 12 selected second lower molars (10 from Myomimus maritsensis and two from Microdyromys koenigswaldi). Scratches and pits were measured from scanning electron images taken at x450 magnification of a 0.01 mm2 area on the center of the hypoconid facet. The rest of the samples were unworn or did not permit the measurement of microwear features (scratches and pits) due to post-mortem alteration. The separation of microwear features follows Hautier et al. (2009) and Gomes Rodrigues et al. (2009) as adapted from Merceron et al. (2005). Four raw variables were defined: the number of fine scratches (N fs), the number of large scratches (N ls), the number of small pits (N sp), and the number of large pits (N lp). The scratches and pits were separated using the minor and major axis ratio: scratches have a ratio lower than 1/4, while that of pits is higher. If a pit's major axis is higher than 5 μm it's defined as "large" and in the case that a scratch's width is more than 5 μm it's defined as "wide." Microwear features were counted and measured using analySIS work (v. 5.0) (Olympus Soft Imagining Solutions). Statistics were calculated with the PAST (v.2.13) program. A univariate analysis of variance (ANOVA) was performed on the four microwear variables (N fs, N ws, N sp, and N lp). A probability level of 0.05 was assumed for the test.
Specimen Numbers and Abbreviations
Specimen numbers (58-HAY/84-SM5) include the province code of Sivas city (58), HAY refers to Hayranlı, locality number (84), SM refers small mammal, and specimen number (5). MN refers European Neogene land mammal units. For teeth, capital letters denote upper teeth and small letters denote lower teeth. The abbreviations (dext.) and (sin.) are used to denote a tooth in the dextral, right arcade, or the sinistral, left arcade.
SYSTEMATIC PALEONTOLOGY
Class MAMMALIA Linneaus, 1758
Order RODENTIA Bowdich, 1821
Family GLIRIDAE Thomas, 1897
MYOMIMUS Ognev, 1924
Myomimus maritsensis De Bruijn et al., 1970
(Figure 3. 1-26)
Type Locality: Maritsa 1 (Greece) Pliocene
Locality: Hayranlı (58-HAY/84)
Age: Late Miocene (middle Turolian)
Material and measurements: See Table 1.
DESCRIPTION
P4, The occlusal surface is concave and the outline is triangular or subrectangular. Three main ridges are present (protoloph, metaloph, and the posteroloph) in six out of seven specimens. In one specimen a complete anteroloph is present, which is connected to the protocone, thus creating a dental pattern that consists of four main ridges. In the remaining six specimens the anteroloph is short and reduced to one or two small cusps, with a low protocone located almost at the center of the lingual side. The protocone and metacone are prominent, and the posteroloph is connected to the protocone. There are three completely separated roots in all specimens.
M1, The M1 has a concave occlusal surface and a subquadrate shape consisting of four main ridges plus an anterior centroloph. The anterior centroloph, present in all specimens, is half the width of that of the main ridges. The anteroloph is isolated lingually and connected to the paracone labially. The trigon in all specimens is Y -shaped with the metaloph and the protoloph connected lingually. The protocone, metacone, and paracone are higher than the main ridges, and the posteroloph is connected to both the protocone and metacone. The M1 has three roots: two labially and one lingually.
M2, The dental pattern of M2 is slightly more complex than that of M1. The outline is square with rounded corners. As with the M1, the M2 has four main ridges. Some M2 specimen have an extra posterior centroloph, thus increasing the number of ridges from five to six. The anterior centroloph (present in all specimens) is always longer than the posterior centroloph (four out of 15). The anterior centroloph and the posterior centroloph are not fused, and the posterior centroloph is connected to the metacone. The metaloph and the protoloph are connected to the endoloph labially. The trigon is Y-shaped in all specimens. The M2 has three roots: two labially and one lingually.
M3, The M3 has a "fish tail" shape and has almost the same size as the M1. Its morphological pattern is relatively simple, and the occlusal surface is slightly concave. The labial border is wider than the lingual border. The M3 consists of five ridges with the four main ridges being connected by an endoloph at the lingual border. Between the protoloph and the metaloph there are three extra ridges: a relatively thin and short ridge connected to the paracone, a labially free extra ridge in the middle as well developed as a main ridge, and lastly a very tiny one that is connected to the metacone. Roots are not preserved.
p4, The outline is oval and the occlusal surface is concave. The posterior side is wider than the anterior side. There are four main ridges, with the anterolophid and metalophid not being connected to the mesolophid. The posterolophid and the mesolophid are connected at the labial side. The p4 has one relatively long root.
m1, The outline of the m1 has a trapezium-like shape, and its anterior part tends to be narrower than the posterior part. The total number of the main and extra ridges varies from five to six. The posterior extra ridge is present in all specimens except one. The centrolophid is connected to the metalophid at the labial side in all specimens. The metalophid is connected to the metaconid in four out of seven specimens. The m1 has three roots: two at the anterior and one at the posterior side.
m2,The outline of the m2 is subrectangular. The occlusal pattern of m2 is simpler than m1. The number of the ridges varies from four to six with the posterior extra ridge being present in seven out of 16 specimens. The posterior extra ridge connects to the entoconid in two out of seven specimens. There are three roots in total—two at the anterior and one at the posterior side.
m3,The outline of the m3 has a rounded triangular shape which converges to a point posteriorly. It is smaller and simpler than both m1 and m2. The number of ridges including the short centrolophid amounts to five. The m3 has three roots: two at the anterior and one at the posterior side.
REMARKS
The specimens described above share some characteristics with Myomimus dehmi from Pikermi (Greece), Eichkogel, and Kohfidisch (Austria); Myomimus maritsensis from Maritsa, Maramena, and Lefkon (Greece); and Peridyromys murinus from Ateca 3 (Spain), Paulhiac, Bouzigues, and Montaigu (France). Daams (1981) gives a detailed description of Myomimus from Spain, Greece, and Israel, and distinguishes standard morphotypes based on cheek teeth patterns. Using the same distribution of morphotypes, the Hayranlı P4 specimens show a higher frequency of morphotype b (Table 2), which has a short anteroloph reduced to a cusp, no centroloph, the protoloph, metaloph and posteroloph present, the protoloph and the metaloph always connected, while the posteroloph may be connected to the protocone. Morphotype b is found at a higher frequency in the collections of Myomimus maritsensis from Maritsa and Myomimus roachi from Givat Shaul. Peridyromys murinus, recovered from older sediments of France and Spain, also mimics the same frequency of morphtoype b. In one of the Hayranlı specimen the anteroloph is prominent and connected to the protocone, which is situated centrally at the lingual side. This specimen resembles morphotype d as found in the Çandır material (De Bruijn et al., 2003). However, the frequency of P4 morphotypes in the Çandır specimens is often represented by the complex morphotype e while the Hayranlı specimens are dominated by the simpler morphotype b.
Shape differences between M1 and M2 are more pronounced in younger than in older assemblages (Daams, 1981). The M1 is narrower anteriorly than posteriorly, while M2 is wider anteriorly than posteriorly in Hayranlı collection. The M1 specimens from Hayranlı, with a simple occlusal surface, resemble morphotype B, whereas the M2 specimens are closely related to both morphotypes B and C (Table 2). M1 has four main ridges with an anterior centroloph while M2 has five to six ridges including a posterior centroloph. Morphotype B and C are found at a higher frequency in the collections of Myomimus maritsensis from Maritsa. Using the four morphotypes for M3 distinguished by Daams (1981), the complete M3 of the Hayranlı collection best matches that of morphotype R shared by Myomimus maritsensis from Maritsa and Myomimus roachi from Qafzeh 16a-17a-19, and Ubeidyia. However, the centroloph in M3 is more developed than that of both M1 and M2, the labial end is shaped like a cusp and unconnected, and it is almost identical to a main ridge. The p4 has strongly reduced dental pattern and fits into Daams's standard morphotype b. The morphotype range for the m1 and m2 of the Hayranlı collection resembles that of Myomimus maritsensis from Maritsa in that m1 is dominated by morphotype 2. The Hayranlı m2 show an equal frequency of both morphotypes 1 and 2, more resembling the morphotype frequency of Myomimus maritsensis from Maritsa. According to Daams (1981), complex morphotype 2 and the simple type 1A in m3 occur in Spain, while all other assemblages almost exclusively have the intermediate type 1B. The m3 of Çandır are dominated by complex type 2 (De Bruijn et al., 2003). The m3 in P. murinus from Ateca3, Paulhiac, Bouzigues and Montaigu, Myomimus maritsensis from Maritsa, Myomimus roachi from Ubeidyia, Givat Shaul, Oumm Qatafa, and Hayonim (B, C, D and E levels), and Myomimus qafzensis from Qafzeh all present high ratios of morphotype 1B.
The relative frequency of standard morphotypes for the upper and lower dentition in the Hayranlı collection almost perfectly overlaps with that of Myomimus maritsensis from Maritsa. However, it is also similar to that of P. murinus from Bouzigues, Paulhiac and Ateca, though the first two lower cheek teeth of the Hayranlı specimens have three roots while P. murinus has two. However, as mentioned by De Bruijn et al. (1970; 2003), the morphological overlap between Peridyromys and Myomimus makes separation difficult. This problem includes the deviating Myomimus n.sp. from Çandır. For instance De Bruijn et al. (2003) argue that unique combination of the morphotypes and the presence of two roots in the first two lower molars of the Myomimus collection from the Middle Miocene of Çandır suggests a new species. However, two roots in the first two lower molars is also a unique characteristic of Peridyromys. Nevertheless, in sum, the dental patterns from the Hayranlı collection mentioned above resemble that of Myomimus maritsensis.
MICRODYROMYS De Bruijn, 1966
Microdyromys koenigswaldi De Bruijn, 1966
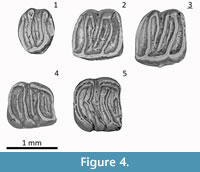
(Figure 4.1-5)
 Type Locality: Valdemoros 3B, Zaragoza Spain, Middle Miocene
Type Locality: Valdemoros 3B, Zaragoza Spain, Middle Miocene
Locality: Hayranlı (58-HAY/84)
Age: Late Miocene (middle Turolian)
Material and measurements: See Table 3
DESCRIPTION
P4, Oval outline and concave occlusal surface. Five ridges present, anteroloph reduced. Centroloph present.Two roots, one anteriorly and two (fused) posteriorly.
M1/2, The M1/2 have a subrectangular outline and concave occlusal surface. Seven ridges are present including the anterior extra ridge. The anterior centroloph is longer than posterior centroloph. The anteroloph is connected to the protocone via the complete endoloph. Lingual walls crenulated. Roots are not preserved.
m1, Outline trapezoidal or rectangular. The total number of main and extra ridges varies from five to seven. Centrolophid and posterior extra ridge present in all specimens. The anterior valley extra ridge is present in one out of two specimens. Metalophid and anterolophid connected labially. Centrolophid connected to mesolophid, which is connected to the posterolophid in one of the two specimens. There are two roots, one at the anterior and one at the posterior side.
m2, The outline of the m2 is almost rectangular, but with rounded corners. All specimens have a relatively long centrolophid, and extra ridges in the anterior and posterior valleys. There are seven ridges present. Two roots, one anteriorly and one posteriorly.
REMARKS
The Hayranlı Microdyromys specimens—although displaying a somewhat simpler dental pattern—fits Microdyromys koenigswaldi with the character combination of a concave occlusal surface with intermediate dental pattern, crenulated lingual wall of the upper cheek teeth, complete endoloph and relatively large P4 and M3 compared to other genera as described by De Bruijn (1966). It is hard to separate M1 and M2 in terms of shape (Figure 3). The anterior centroloph of M1 and M2 is longer than the posterior one. The anterior extra crest is present, whereas extra crests outside the trigone, as in Microdyromys complicatus, are absent. The accessory ridges are lower than the main ridges. The Hayranlı Microdyromys collection consists of Daams's (1981) morphotype e for P4 and H for M1/2 in the upper cheek teeth, and 3 for m1 and 3 for m2 in lower cheek teeth (Table 2). The distribution of standard morphotypes and other aspects of the dental morphology of the Hayranlı specimens are within the variation range of Microdyromys koenigswaldi — including complete endoloph, crenulated lingual wall of M1/2 and the connection of the mesolophid and the posterolophid in m1.
PHYLOGENY AND PALEOBIOGEOGRAPHY OF THE HAYRANLI GLIRIDAE
Myomimusmaritsensis and Microdyromys koenigswaldi share common dental traits in general such as relatively low crowned cheek teeth with a concave occlusal surface, which are characterized by transverse ridges and irregular extra ridges. Complexity and concavity of the occlusal pattern and size differences are significant elements employed in separating the species from one another morphologically. Additionally, the extra ridges, which are different in size and shape and are always located and directed distinctly, are used as a diagnostic characteristic in the separation of the species by many scholars. However, homology of the crests, the range of intraspecific variations, and large overlaps in the some aspects of dental patterns and stratigraphic ranges do present difficulties in distinguishing species.
Myomimus Ognev, 1924
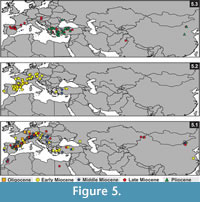 The earliest fossil record of Myomimus was reported from the early Miocene of (MN3) Kılçak in Central Anatolia (Ünay, 1994; Daams and De Bruijn, 1995) (Figure 5.3). Vianey-Liaud (2003) announced a new unnamed species from the late Oligocene of Pech du Fraysse (France). This species, represented by one specimen only, has a dental pattern close to either Peridyromys or Myomimus. These two genera have overlapping dental patterns, making it difficult to separate them morphologically at the genus level. Thus, De Bruijn's et al. (1970, 2003) suggestion to synonymize these two genera is necessary to clear and complete their evolutionary history. The diagnostic differences between these two genera are size of the P4 (Peridyromys has relatively large P4) and the root number of first two lower molars (Peridyromys has two, Myomimus has three). There is an evolutionary trend that marks that the size of P4 gets smaller from older to younger assemblages. Differences occur at the species level, but it is both difficult and risky to determine genus level based on the size of P4 only. However, the root number seems interchangeable in Myomiminae and Dryominiae subfamilies. For example, Microdyromys is identical with two roots in m1 and m2, but Microdyromys orientalis from Shuanggou (China) or Microdyromys koenigswaldi from Manchones 1 (Spain) has two or three roots. Myomimus is identical with three roots in the lower molars, but two rooted m1 and m2 recorded from Pedregueras 1A and Peralejos D. Thus, as De Bruijn et al. (2003) points out, it is not a good idea to separate two genera on the basis of root number in m1 and m2. Daams (1981) prefers to retain both genera in respect to the time gap between stratigraphic ranges in Europe. The first occurrence of Myomimus is from the late Aragonian in Western Europe (Borjas, Spain) and from the early Miocene in Eastern Europe (Antonios, Greece). Peridyromys is the oldest member of the Myomiminae subfamily known from the late Oligocene to the early middle Miocene in Europe and an early form P. murinus has a moderately complex dental pattern (Daams and De Bruijn, 1995). However, due to migration events during the middle and the late Miocene, this time gap does not exist in Anatolia (De Bruijn et al., 2003). Assuming this, De Bruijn et al. (1970; 2003) suggests that Peridyromys/Myomimus originated in Europe, migrated into Western Asia during the middle Miocene, and returned to Europe in the late Miocene. This hypothesis coincides with biogeographic distribution of Peridyromys and Myomimus from the early Miocene to the late Miocene (Figure 5.2; 3). As shown in Figure 5.2, Peridyromys is restricted mainly to Western Europe from the late Oligocene to the middle Miocene. Myomimus is known from the Late Miocene to present and has a distribution from Western Europe to Asia, with highest density in the Eastern Mediterranean (Figure 5.3). Still, if Myomimus and Peridyromys were to be combined as suggested, it would bring a more complete representation of biostratigraphical and biogeographical continuity of the species from the late Oligocene to present.
The earliest fossil record of Myomimus was reported from the early Miocene of (MN3) Kılçak in Central Anatolia (Ünay, 1994; Daams and De Bruijn, 1995) (Figure 5.3). Vianey-Liaud (2003) announced a new unnamed species from the late Oligocene of Pech du Fraysse (France). This species, represented by one specimen only, has a dental pattern close to either Peridyromys or Myomimus. These two genera have overlapping dental patterns, making it difficult to separate them morphologically at the genus level. Thus, De Bruijn's et al. (1970, 2003) suggestion to synonymize these two genera is necessary to clear and complete their evolutionary history. The diagnostic differences between these two genera are size of the P4 (Peridyromys has relatively large P4) and the root number of first two lower molars (Peridyromys has two, Myomimus has three). There is an evolutionary trend that marks that the size of P4 gets smaller from older to younger assemblages. Differences occur at the species level, but it is both difficult and risky to determine genus level based on the size of P4 only. However, the root number seems interchangeable in Myomiminae and Dryominiae subfamilies. For example, Microdyromys is identical with two roots in m1 and m2, but Microdyromys orientalis from Shuanggou (China) or Microdyromys koenigswaldi from Manchones 1 (Spain) has two or three roots. Myomimus is identical with three roots in the lower molars, but two rooted m1 and m2 recorded from Pedregueras 1A and Peralejos D. Thus, as De Bruijn et al. (2003) points out, it is not a good idea to separate two genera on the basis of root number in m1 and m2. Daams (1981) prefers to retain both genera in respect to the time gap between stratigraphic ranges in Europe. The first occurrence of Myomimus is from the late Aragonian in Western Europe (Borjas, Spain) and from the early Miocene in Eastern Europe (Antonios, Greece). Peridyromys is the oldest member of the Myomiminae subfamily known from the late Oligocene to the early middle Miocene in Europe and an early form P. murinus has a moderately complex dental pattern (Daams and De Bruijn, 1995). However, due to migration events during the middle and the late Miocene, this time gap does not exist in Anatolia (De Bruijn et al., 2003). Assuming this, De Bruijn et al. (1970; 2003) suggests that Peridyromys/Myomimus originated in Europe, migrated into Western Asia during the middle Miocene, and returned to Europe in the late Miocene. This hypothesis coincides with biogeographic distribution of Peridyromys and Myomimus from the early Miocene to the late Miocene (Figure 5.2; 3). As shown in Figure 5.2, Peridyromys is restricted mainly to Western Europe from the late Oligocene to the middle Miocene. Myomimus is known from the Late Miocene to present and has a distribution from Western Europe to Asia, with highest density in the Eastern Mediterranean (Figure 5.3). Still, if Myomimus and Peridyromys were to be combined as suggested, it would bring a more complete representation of biostratigraphical and biogeographical continuity of the species from the late Oligocene to present.
Older Myomimus assemblages have a more complex dental pattern than younger assemblages, and there is an evolutionary trend of Myomimus dental pattern towards simplification (Daams, 1981). However, De Bruijn reported that the collection from the late Turolian of Süleymanlı (Central Anatolia) has a more complex dental pattern than Myomimus dehmi and it shows that the evolutionary trend from complexity to simplification may not be always the case (Daams and De Bruijn, 1995). Myomimus dehmi is known from Sinap (MN9-10), Bayırköy (MN12), Karaözü (MN10-12), and Düzyayla (MN12) (Ünay and De Bruijn, 1984; Saraç, 2003) in Anatolia. De Bruijn et al. (2003) reported a possible new species of Myomimus from the middle Miocene of Çandır (Central Anatolia) with two roots on the m1 and the m2, but three roots on the m3.
Myomimus sumbalenwalicus Munthe, 1980, is the first representative of the family from Chinji Formation in South Asia. This species is characterized by a relatively smaller and simpler P4 and larger M3. Myomimus maritsensis from MN14 of Greece has transitional dental patterns between the complex dental pattern of Myomimus dehmi and the simpler ones of Myomimus roachi. Myomimus maritsensis is reported from Akhisar (MN7-8), Tozaklar (MN15), Ortalıca (MN15), and Kadıözü (MN16) of Turkey (Fortelius, 2013). The Pleistocene species Myomimus qafzensis and Myomimus judaicus (synonym of Myomimus roachi) is described from Israel (Haas, 1973; Tchernov, 1968; Daams, 1981). Myomimus qafzensis is marked in having an endoloph on upper molars and ectolophid on lower molars. Additionally, Daxner-Höck and Höck (2009) describe M1/2 with complete endoloph of Myomimus dehmi from Kohfidisch and Eichkogel (MN11) in Austria. The Dryomyinae characteristic, complete endoloph, is also found in the M1/2 of Myomimus—a tendency that appears often in younger localities. It seems that some dental characteristics that are used to distinguish species, like the presence of a complete endoloph, show variation between and within groups. These variations require that we rethink the taxonomic and phylogenetic position of the species.
Microdyromys De Bruijn, 1966
Microdyromys is classified by De Bruijn (1966) as a Glirulinae, with an oldest record (Microdyromys praemurinus) dating back to the late Oligocene of Germany. Daams and De Bruijn (1995) reinterpreted the classification of the Gliridae family, and shifted Microdyromys from Glirulinae to Dryomyinae. The success of Microdyromys is evident from its wide distribution from the late Oligocene to the late Miocene of Europe, Asia, and North Africa, where apparently it filled different ecological niches (Figure 5.1). It is abundant and best known from the Miocene of Europe. Although Daams and De Bruijn (1995) suggested a Gliravine ancestor for Microdyromys, Uhlig (2001) and Freudenthal and Martin-Suarez (2007b) support a closer evolutionary relationship with Bransatoglirinae. Microdyromys has not been known from the late Eocene and early Oligocene of Europe, but Freudenthal and Martin-Suarez (2007a) suggest that this is not representing a real absence; it is a matter of taxonomical interpretation. The earliest record of Microdyromys is known from the late Eocene of Aguaton 2D of Truel Basin in Spain (Freudenthal, 1997). This species (Microdyromys sp.) has a single m1 and M2 representing a dental morphology similar to that of Microdyromys. Another early form is Bransatoglis misonnei (Vianey-Liaud, 1994) from the Early Oligocene of Hoogbutsel (Belgium) and Montalban 1D (Spain); it was transferred to genus Microdyroms (Microdyromys aff. misonnei) by Freudenthal (1997). Additionally, Freudenthal and Martin-Suarez (2007a) describe a new species of Microdyromys (Microdyromys puntarronensis) from the early Oligocene of Montalban 8, which shares a close evolutionary relationship with Microdyromys misonnei. The existence of more derived characters such anterotropid, posterotropid, prototrope, and posteroloph/metacone connection as well as more advanced features of M2 indicate an ancestral-descendant relationship between these two species. In the same study, Bransatoglisheissigi from Gröben 3 is transferred to Microdyromys. Therefore, the first occurrence of Microdyromys is from the late Eocene and early Oligocene after these taxonomic transfers of Bransatoglis species.
Freudenthal and Martin-Suarez (2007b) re-interpret the taxonomic place of the subfamily Bransatoglirinae and suggest classifying the genera Microdyromys and Bransatoglis in the same subfamily due to their identical large and rounded premolars. Thus, Microdyromys is transferred from the Dryomyinae to the Bransatoglirinae (Freudenthal and Martin-Suarez, 2007b). However, these different taxonomical interpretations of dental morphology of Microdyromys and Bransatoglis, which are mainly dependent on researcher bias, bring out controversy over their suprageneric status. The significant changes given in the emended diagnosis of Microdyromys by Freudenthal and Martin-Suarez (2007b) are the presence of extra ridges outside of the trigon, crenulated lingual wall of the upper molars, and discontinuous existence of a complete endoloph. Garcia-Paredes et al. (2010) argue that the presence of an extra ridge outside of the trigon is rare in the Oligocene species, but it is a common, almost diagnostic feature in some Miocene species. De Bruijn (1966) characterizes the Microdyromys genus by the presence of a complete endoloph and a crenulated lingual wall in upper molars. However, early Microdyromys species (for example Microdyromys preamurinus) and the species transferred from Bransatoglis to Microdyromys by Freudenthal and Martin-Suarez (2007b) show a lesser frequency of a complete endoloph and crenulated lingual wall in upper molars. According to Daams and De Bruijn (1995) Microdyromys preamurinus, with an incomplete endoloph and smooth lingual wall in upper molars, constitutes a transitional population between the Gliravinae and the Dryomyinae subfamilies. In Microdyromys misonnei, Microdyromys heissigi and Microdyromys preamurinus some shared traits like rounded premolars, incomplete endoloph and smooth lingual wall in upper molars support the likelihood of a Bransatoglirinae ancestor for the Microdyromys genus (Freudenthal and Martin-Suarez, 2007b).
Microdyromys preamurinus from the Late Oligocene of Mirambueno and Vivel del Rio is phylogenetically situated between more primitive Microdyromys misonnei and more derived Microdyromys legidensis (Freudenberg, 1941; Freudenthal and Martin-Suarez, 2007a). Microdyromys heissigi from Gröben 3, with small sized teeth and relatively simpler upper molars, is a potential ancestor of Microdyromys monspeliensis (Aguilar, 1977; Freudenthal and Martin-Suarez, 2007a). Microdyromys monspeliensis has a very similar dental pattern to Microdyromys koenigswaldi, only relatively smaller and simpler (Daams, 1981). Microdyromys legidensis presents another example of smaller sized teeth in the genus derived from Microdyromys preamurinus and that gave rise to Microdyromys koenigswaldi and Microdyromys complicatus μmicrodyromys complicatus having a more complex dental pattern than Microdyromys koenigswaldi. Microdyromys koenigswaldi first appears the during early Miocene of Europe and survived until the late Miocene of Eurasia. The first appearance of Microdyromys koenigswaldi in Anatolia is known from the Early Miocene (Kınık 1) (Ünay and Göktaş, 2000). The last appearance of the Microdyromys was previously recorded in Ampudia 3 (MN10, Duero Basin, Spain), but the Hayranlı collection from Turolian (MN11 or MN12) replaces it as more recent.
Daams (1981) and Garcia-Peredes et al. (2010) rightfully point out that definite distinction of all specimens in the different species of Microdyromys from the Miocene is challenging due to the large overlap in dental pattern and size. Additionally, as Daams (1981) suggests, Microdyromys dental patterns reveal existence of homogenous and heterogeneous assemblages either on the basis of size or of dental pattern or both. Due to the chance of more than one species co-occurring in a locality, it is prudent to focus on possible combinations of relative frequency of dental patterns and characteristics to accurately separate species of Microdyromys.
DIETARY ADAPTATIONS and MICROWEAR PATTERNS
Diet is a significant key for understanding an animal's habitat as well as for interpreting its ecological relationships. Reconstructing diet and feeding habitat for extinct Gliridae members is not an easy task on the basis of isolated teeth only. Gliridae share a unique dental pattern characterized by a usually concave occlusal surface, transverse ridges, and relatively low crowned cheek teeth. In addition, this type of dental pattern is not very revealing when used to construct habitat requirements through the functional relationship of diet and molar shape. Despite a massive adaptive radiation during the early Miocene in Europe with further dispersal to Asia and Africa during the remainder of the Miocene—with occupation of many diverse arboreal and terrestrial habitats—their dental pattern shows relatively small variation in the number and size of main and extra ridges. Anthony and Kay (1993) suggest that, considering the occlusal pattern of arboreal forms, it is necessary to reduce food particle size for effective digestibility of a plant diet (especially cellulose) by increasing the length of shearing ridges. Therefore, the cusp pattern on the occlusal surface must adapt in order to break food up into small parts during chewing. In this sense, the higher number of transverse main and extra ridges on the occlusal surface can be seen as an indicator of adaptation to arboreal life and a plant-dominated diet in small mammals, as opposed to a more omnivore or insectivore diet (Meulen and De Bruijn, 1982). De Bruijn (1997) regards six transverse ridges as a threshold above which species could be considered as arboreal (followed by van Dam, 2006).
A second question concerns the functional morphology of the concave occlusal surface. Although there is no sharp distinction in degree of concavity (strong, moderate, and weak or absent) among the groups, it is used as a variable for separating the species (Freudenthal and Martin-Suarez, 2007c). Hautier et al. (2009) regard high occlusal concavity as an adaptation towards a more insectivorous diet. Their morphometric analysis of the mandibles of extant Eliomysquercinus ophiusae and extinct Hypnomys morpheus from the Pleistocene of Mallorca and Menorca Islands of Spain, suggest a more omnivorous diet consisting of abrasive foods like grasses for Hypnomys with its robust mandible, whereas the gracile mandible with concave cheek teeth of Eliomys indicates a more insectivorous diet. Due to the unique natural selection pressure on islands, insular glirids may not be a useful example for generalizing about the habitat specifications of Myomimus and Microdyromys, but the conclusions mentioned above provide important data for understanding functional morphological traits of the dental pattern and mandible of glirid members. Freudenthal and Martin-Suarez (2007c) argue that the relation between concavity and occlusion is unknown; some species show a combination of strongly concave upper molars with flat lower molars or vice versa. Accordingly, this unique configuration of concave and flat molars must be the result of a particular mastication processes. However, as far as we know, this combination has not yet been confirmed by a fossil sample of an articulated maxillar and mandible with cheek teeth. Additionally, Buruldağ and Kurtonur (2001) note favorite seasonal food preferences of extant Thracian (Turkey) Myomimus roachi which has relatively concave cheek teeth, trapped live in the field and reared in outdoor cages, which include: sunflower seeds, wheat, sweet fruits, insects, spiders, slugs, and lizards.
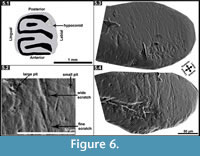 As an alternative way of inferring paleodiet, although the sample size of Microdyromys was minimal, we decided to investigate dental microwear patterns in Myomimus and Microdyromys (Figure 6; Table 4) and compare the results with available data for Hypnomys morpheus and Eliomys quercinus ophiusae as analyzed by Hautier et al. (2009). Hypnomys and Eliomys are grouped in the family Dryomyinae with Microdyromys, and they have evolutionary relationships (Daams and De Bruijn, 1995). Additionally, considering ecological behavior and diet preferences, Microdyromys is suggested to have been an arboreal form probably resembling Eliomys while Myomimus is a more terrestrial form that possibly corresponds with Hypnomys. The numbers of scratches and pits observed in all studied specimens can be seen in Appendix. ANOVA was performed on the microwear features of the hypoconid facet of the m2 of these four different glirid species (p>0.05) (Table 5). The variables for Microdyromys koenigswaldi are excluded from statistical analyses (ANOVA) due to minimal sample size. Results indicate significant differences between fine scratches, small pits, and large pits exhibited by these three species. The ANOVA test was not successful in the detection of significant differences in wide scratches on the specimens. Myomimus and Hypnomys (and Microdyromys as well) show a higher average of fine scratches than Eliomys (Table 4). The range of intraspecific variation is more significant in Myomimus and Hypnomys than in others in terms of standard deviation. Considering all the microwear patterns, it appears that Eliomys is always characterized by low averages. The high quantity of fine scratches and small pits are characteristic for feeding on grasses (particularly due to the contamination with soil particles) and soft seeds (Fortelius and Solounias, 2000; Solounias and Semprebon, 2002; Silcox and Teaford, 2002; Gomes Rodrigues et al., 2009). Myomimus maritsensis exhibits a higher number of large pits compared with Hypnomys, Microdyromys, and Eliomys. The statistical analysis indicates that Myomimus differentiates from Hypnomys,Eliomys, and Microdyromys due to differences in the number of large pits which are mainly related to either a more insectivorous diet or a diet consisting of hard nuts and seeds, or a combination of both (Silcox and Teaford, 2002). It is well known that Eliomys maintains one of the most carnivorous diets of dormice, primarily based in insectivorous consumption; therefore it is likely to observe large pits in their microwear pattern. The average number of large pits in Eliomys is, however, lower than in other species, which may be attributed to seasonal changes in food availability in its environment. Extant Eliomys quercinus in Valencia (Spain) follows the seasonal availability of the food sources; consuming more prey and insects during the summer and autumn while subsisting on primarily fruit during the spring and winter in its diet (Gil-Delgado et al., 2010). In this sense, the average number of microwear patterns strongly depends on the food type consumption of the animal before death (Fortelius and Solounias, 2000).
As an alternative way of inferring paleodiet, although the sample size of Microdyromys was minimal, we decided to investigate dental microwear patterns in Myomimus and Microdyromys (Figure 6; Table 4) and compare the results with available data for Hypnomys morpheus and Eliomys quercinus ophiusae as analyzed by Hautier et al. (2009). Hypnomys and Eliomys are grouped in the family Dryomyinae with Microdyromys, and they have evolutionary relationships (Daams and De Bruijn, 1995). Additionally, considering ecological behavior and diet preferences, Microdyromys is suggested to have been an arboreal form probably resembling Eliomys while Myomimus is a more terrestrial form that possibly corresponds with Hypnomys. The numbers of scratches and pits observed in all studied specimens can be seen in Appendix. ANOVA was performed on the microwear features of the hypoconid facet of the m2 of these four different glirid species (p>0.05) (Table 5). The variables for Microdyromys koenigswaldi are excluded from statistical analyses (ANOVA) due to minimal sample size. Results indicate significant differences between fine scratches, small pits, and large pits exhibited by these three species. The ANOVA test was not successful in the detection of significant differences in wide scratches on the specimens. Myomimus and Hypnomys (and Microdyromys as well) show a higher average of fine scratches than Eliomys (Table 4). The range of intraspecific variation is more significant in Myomimus and Hypnomys than in others in terms of standard deviation. Considering all the microwear patterns, it appears that Eliomys is always characterized by low averages. The high quantity of fine scratches and small pits are characteristic for feeding on grasses (particularly due to the contamination with soil particles) and soft seeds (Fortelius and Solounias, 2000; Solounias and Semprebon, 2002; Silcox and Teaford, 2002; Gomes Rodrigues et al., 2009). Myomimus maritsensis exhibits a higher number of large pits compared with Hypnomys, Microdyromys, and Eliomys. The statistical analysis indicates that Myomimus differentiates from Hypnomys,Eliomys, and Microdyromys due to differences in the number of large pits which are mainly related to either a more insectivorous diet or a diet consisting of hard nuts and seeds, or a combination of both (Silcox and Teaford, 2002). It is well known that Eliomys maintains one of the most carnivorous diets of dormice, primarily based in insectivorous consumption; therefore it is likely to observe large pits in their microwear pattern. The average number of large pits in Eliomys is, however, lower than in other species, which may be attributed to seasonal changes in food availability in its environment. Extant Eliomys quercinus in Valencia (Spain) follows the seasonal availability of the food sources; consuming more prey and insects during the summer and autumn while subsisting on primarily fruit during the spring and winter in its diet (Gil-Delgado et al., 2010). In this sense, the average number of microwear patterns strongly depends on the food type consumption of the animal before death (Fortelius and Solounias, 2000).
Myomimus and Hypnomys have a relatively higher number of fine scratches and small pits, which are likely the result of a more terrestrial life style permitting the inclusion of more grass, soft seeds, and fruits into diet. Diets of these species of dormice are composed of insects, fruit, seed, and grass consumption, as evidenced by the occurrence of large pits, small pits, wide scratches, and fine scratches in relative average (Table 4). The combination of grass, fruit, seeds, and insect consumption in their mixed diet could point to more generalist behavior adapted to seasonal availability of the foods in Myomimus, Microdyromys, Hypnomys, and Eliomys.
PALEOECOLOGY AND PALEOBIODIVERSITY
Living examples of the dormice can be found in wooded areas, hedgerows, gardens, and rocky terrains of Europe, central and southwestern Asia, Japan, and Africa (Nowak, 1999; Holden, 2005). Graphuirus is the only genus that inhabits areas south of the Sahara in Africa. Glirids are generally nocturnal and scansorial, and have well adapted extremities for climbing trees. Their diet contains fruits, nuts, insects, eggs, and small vertebrates. Myomimus is the only genus that is not specialized for arboreal life among the extant Gliridae while Dryomys, closely related to Microdyromys, is nocturnal and arboreal. Myomimus lives in open country in clusters of trees and in nests on or under the ground in Turkmenistan, Uzbekistan, Afghanistan, Iran, Palestine, Turkey, and Bulgaria, and Dryomys inhabits dense mountain forests and thickets at higher elevations in parts of Switzerland, Germany, Latvia, Turkey, Iran, Mongolia, and northern Pakistan (Kurtonur and Özkan, 1991; Nowak, 1999; Holden, 2005).
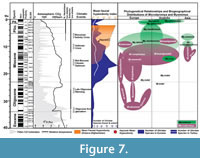 Terrestrial mammal ecosystems are mostly regional, temporally discontinuous and controlled by combination of topographic, tectonic, climatic, and vegetation dynamics (Eronen et al., 2009). The global temperature curve from the deep sea oxygen isotope constructed by Zachos et al. (2001) (Figure 7) gives a global picture of temperature change compared with the regional climatic controls of terrestrial ecosystems, but today researchers emphasize that strong temporal and geographical variations in precipitation considerably influence terrestrial ecosystems. In Figure 7, the number of Gliridae species and the phylogenetic relationships of Microdyromys and Myomimus genera are given in the context of paleo-environmental changes during the last 40 million years. The number of the Gliridae species coincides with Cenozoic terrestrial climatic events. First glirid members appeared during the late Eocene through the early Oligocene, and the number of species remained limited. According to Freudenthal and Martin-Suarez (2007a) Microdyromys misonnei and Microdyromys puntarronensis, which have an ancestor-descendent evolutionary relationship, appeared in Western Europe during this cooling event as the first representatives of the genus. At the end of the late Oligocene and beginning of the early Miocene—coincident with either with high pCO2, decreased ice volume, or the late Oligocene warming—a massive adaptive radiation of Gliridae family members occurred as they began to occupy many different ecological niches and increased in number of species. During this time period the number of Gliridae reached the highest peak of paleobiodiversity (from five to 60 species) in its history (Figure 7). The new species of Microdyromys;Microdyromys heissigi, Microdyromys praemurinus, Microdyromys monspeliensis, and Microdyromys legidensis occurred during the late Oligocene warming. Earliest representative of the genus Peridyromys murinus is known from the late Oligocene of Spain (Mirambueno 1, MP 27) (Freudenthal, 1997). Peridyromys dispersed in Western and Central Europe and Anatolia, but interestingly the fossil records of Peridyromys are very poor from Balkans (Figure 5.2). The first fossil records of the Gliridae (Microdyromys indet. and Paraglirulus indet.) are known from İnkonak M.R.6 (Kargı Section) in Anatolia and date back to the latest Oligocene and earliest Miocene (25.3-23.2 Ma) (De Bruijn and Saraç, 1991; Krijgsman et al., 1996). During the early Miocene Gliridae in Turkey were represented by seven genera; Gliridinus, Glis, Vasseuromys, Microdyromys, Paraglirulus, Miodyromys, and Bransatoglis.
Terrestrial mammal ecosystems are mostly regional, temporally discontinuous and controlled by combination of topographic, tectonic, climatic, and vegetation dynamics (Eronen et al., 2009). The global temperature curve from the deep sea oxygen isotope constructed by Zachos et al. (2001) (Figure 7) gives a global picture of temperature change compared with the regional climatic controls of terrestrial ecosystems, but today researchers emphasize that strong temporal and geographical variations in precipitation considerably influence terrestrial ecosystems. In Figure 7, the number of Gliridae species and the phylogenetic relationships of Microdyromys and Myomimus genera are given in the context of paleo-environmental changes during the last 40 million years. The number of the Gliridae species coincides with Cenozoic terrestrial climatic events. First glirid members appeared during the late Eocene through the early Oligocene, and the number of species remained limited. According to Freudenthal and Martin-Suarez (2007a) Microdyromys misonnei and Microdyromys puntarronensis, which have an ancestor-descendent evolutionary relationship, appeared in Western Europe during this cooling event as the first representatives of the genus. At the end of the late Oligocene and beginning of the early Miocene—coincident with either with high pCO2, decreased ice volume, or the late Oligocene warming—a massive adaptive radiation of Gliridae family members occurred as they began to occupy many different ecological niches and increased in number of species. During this time period the number of Gliridae reached the highest peak of paleobiodiversity (from five to 60 species) in its history (Figure 7). The new species of Microdyromys;Microdyromys heissigi, Microdyromys praemurinus, Microdyromys monspeliensis, and Microdyromys legidensis occurred during the late Oligocene warming. Earliest representative of the genus Peridyromys murinus is known from the late Oligocene of Spain (Mirambueno 1, MP 27) (Freudenthal, 1997). Peridyromys dispersed in Western and Central Europe and Anatolia, but interestingly the fossil records of Peridyromys are very poor from Balkans (Figure 5.2). The first fossil records of the Gliridae (Microdyromys indet. and Paraglirulus indet.) are known from İnkonak M.R.6 (Kargı Section) in Anatolia and date back to the latest Oligocene and earliest Miocene (25.3-23.2 Ma) (De Bruijn and Saraç, 1991; Krijgsman et al., 1996). During the early Miocene Gliridae in Turkey were represented by seven genera; Gliridinus, Glis, Vasseuromys, Microdyromys, Paraglirulus, Miodyromys, and Bransatoglis.
During the Middle Miocene Climatic Optimum, climate was predominantly humid, but some regional precipitation data shows that while some places were wet others were relatively dry (Utescher et al., 2011; van Dam, 2006). As temperatures rose, Gliridae adapted to different habitats and dispersed geographically to a wide range of locations in Europe and Asia. The dramatic decline of the glirids commenced around 16 million years ago at the beginning of the middle Miocene; attributable to environmental changes such as aridification, seasonality, and open country extension. According to Akkiraz et al. (2011), Anatolia was (suggested mean annual precipitation was between 1000 mm to 1400 mm and mean annual temperature was between 16,5°C to 20,8°C) wet, warm, and humid from the early to middle Miocene. On the other hand, paleoprecipitation estimates based on mean hypsodonty of herbivore-omnivore large mammals from Greece to Afghanistan indicates that open and grassland ecosystems appeared in the Eastern Mediterranean by the middle Miocene (Strömberg, 2011). Habitat reconstruction derived from paleosols data establishes that more open deciduous woodland and arid habitats were present at this time in Central Anatolia (Strömberg et al., 2007; Strömberg, 2011). During the middle Miocene, Gliridae in Turkey were composed of Microdyromys, Myomimus, Miodyromys, Peridyromys, Muscardinus, Vasseuromys, Glirulus, and Paraglis. Based on the simplicity of the occlusal surface, of these species only Myomimus and perhaps Peridyromys could be considered ground dwellers while the relatively higher number of fine scratches and small pits observed in Myomimus maritsensis depicts a more terrestrial lifestyle. In the late Miocene, grassland ecosystem became dominant and the number of hypsodont large mammal species adapted to open habitats increased. Bibi and Güleç (2008) suggested a shrubland to woodland environment for Hayranlı based on the percentages of grazer, browser and mixed feeders in bovid assemblages. According to the most recent excavation report, the remaining Hayranlı mammal fauna include: Carnivora (pending), Ceratotherium sp. (probably C. neumayri), Hipparion sp., Microstonyx major, Choerolophodon sp., Palaeotragus sp., Hystrix sp., Pliopetaurista cf. P.bresseana, Tamias sp., and Progonomys sp. (De Bruijn, personal commun., 2010; Made et al., 2013). The Hayranlı large mammal assemblage is typical of the Pikermian chronofauna which is characterized by the dominance of late Miocene open-terrain mammal species (Solounias et al., 2010). Considering the large herbivore-omnivore mammal collection of Hayranlı, the mean hypsodonty value (=1.6) depicts a relatively humid woodland and shrubby paleoenvironment. The mean hypsodonty value for Hayranlı resembles that of other common late Miocene localities (Garkın, Mahmutgazi, Çorakyerler, Elekçi, Düzyayla, Chomateres, Çobanpınar, etc.) from MN11 and MN12 of Eastern Mediterranean bioprovinces (Fortelius et al., 2002; Eronen et al., 2009). Drastic decrease in the number of Gliridae species adapted to an arboreal lifestyle and a warm and humid climate probably caused by the ecosystem changes, which occurred from the middle Miocene to the late Miocene in Europe and Eastern Mediterranean (Figure 7). There is significant faunal change from forest dwellers to ground dweller species, which is characterized by the augment in Myomimus findings from the number of localities during the late Miocene—probably attributable to the vegetational shift from predominating forested wetland environments to open woodland and steppe-like environments. Percentages of pollen data from the late Miocene of Central Anatolia typically indicate dry conditions (Akgün et al., 2007). In Figure 7 there are two incidences where the number of species dramatically declined over a realtively short period of time. It seems that abiotic forces were the main drivers of the decrease in number of Gliridae during the middle Miocene Climatic Optimum. However, in the second decrease during the Vallesian, abiotic forces may not have been the only driving force; biotic interactions of glirids with the other small mammal groups like Muridae must also be taken into account. The massive appearance of murids that occurred during the Vallesian (MN10) in Europe coincided with a sudden decrease in the number of Gliridae–attributable either to the arrival of Muridae or the Vallesian crisis, or both. The biodiversity of Gliridae from the late Miocene of Turkey is represented by six genera; Glirulus, Microdyromys, Miodyromys, Myomimus, Muscardinus, and Ramys. During the Pliocene and the Pleistocene the increased findings of ground dwellers, like Myomimus, can serve as evidence of the faunal change from forest dwellers to ground dwellers.
ACKNOWLEDGMENTS
We thank E. Ünay, H. De Bruijn, J. van Dam, and M. Fortelius for their important comments. Thanks to P. Heikkila, R. Michallik, and E. Sahlstedt at the University of Helsinki, Department of Geosciences and Geography (University of Helsinki, Finland) for their kind help with SEM imaging. We are grateful to E. Güleç for the loan of glirid material from the fossil collection of the Hayranlı Late Miocene Excavation Project. Also to our colleagues and the students who assisted in the fieldwork that yielded the new fossil material used in this study, thank you. To A.H. Kaya, we are grateful for the English improvement. Studies of fossil material were supported by the NSF-RHOI "Anatolian Upper Miocene Project" (Grant No: 0321893) and by the Academy of Finland by grant to Mikael Fortelius.
REFERENCES
Aguilar, J.P. 1977. Les gisements continentaux de Plaissan et de la nouvelle Facultè de Mèdecine (Herault); leur position stratigraphique. Geobios, 10:81–101.
Akgün, F., Kayseri, M.S., and Akkiraz, M.S. 2007. Palaeoclimatic evolution and vegetational changes during the late Oligocene-Miocene period in Western and Central Anatolia (Turkey). Palaeogeography, Palaeoclimatology, Palaeoecology, 253:56–90.
Akkiraz, M.S., Akgün, F., Utescher, T., Bruch, A.A., and Mosbrugger, V. 2011. Precipitation gradients during the Miocene in Western and Central Turkey as quantified from pollen data. Palaeogeography, Palaeoclimatology, Palaeoecology, 304:276–290.
Anthony, M.R.L. and Kay, R.F. 1993. Tooth form and diet in Ateline and Alouattine primates: Reflections on the comparative method. American Journal of Science, 293:356–382.
Bentz, S. and Montgelard, C. 1999. Systematic position of the African dormouse Graphiurus (Rodentia, Gliridae) assessed from cytochrome b and 12S rRNA mitochondrial genes. Journal of Mammalian Evolution, 6:67–83.
Bibi, F. and Güleç, E. 2008. Bovidae (Mammalia: Artiodactyla) from the late Miocene of Sivas, Turkey. Journal of Vertebrate Paleontology, 28:501–519.
Bowdich, T.E. 1821. An Analysis of the Natural Classifications of Mammalia for the Use of Students and Travellers. J. Smith, Paris.
Buruldağ, E. and Kurtonur, C. 2001. Hibernation and postanal development of the mouse-tailed dormouse, Myomimus roachi reared outdoor's in a cage. Trakya University Journal of Scientific Research Series B, 2:179–186.
Çiner, A., Kosun, E., and Deynoux, M. 2002. Fluvial, evaporitic and shallow-marine facies architecture, depositional evolution and cyclicity in the Sivas Basin (Lower to Middle Miocene), Central Turkey. Journal of Asian Earth Sciences, 21:147–165.
Daams, R. 1981. The dental pattern of the dormice Dryomys, Myomimus, Microdyromys and Peridyromys. Utrecht Micropaleontological Bulletins, Special Publication 3:1–113.
Daams, R. and De Bruijn, H. 1995. A classification of the Gliridae (Rodentia) on the basis of dental morphology. Hystrix 6:3–50.
Daxner-Höck, G. and E. Höck. 2009. New data on Eomyidae and Gliridae (Rodentia, Mammalia) from the late Miocene of Austria. Annalen des Naturhistorischen Museums in Wien, 111:375–344.
De Bruijn, H. 1966. Some new Miocene Gliridae (Rodentia, Mammalia) from the Calatayud area (prov. Zaragoza, Spain). Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen B, 69:58–78.
De Bruijn, H. 1967. Gliridae, Sciuridae y Eomyidae (Rodentia, Mammalia) miocenos de Calatayud (provincia de Zaragoza, Espana) y su relacion con la bioestratigrafia del area. Boletin del Instituto Geologico y Minero de Espana, 78:189–373.
De Bruijn, H. 1997. Vertebrates from the early Miocene lignite deposits of the opencast mine Oberdorf (Western Styrian Basin, Austria). Annalen des Naturhistorischen Museums in Wien, 99:99–137.
De Bruijn, H. and Saraç, G. 1991. Early Miocene rodent faunas from the eastern Mediterranean area; The genus Eumyarion. Proceedings Koninklijke Nederlandse Academie van Wetenschappen B, 94:1–36.
De Bruijn, H., Dawson, M.R., and Mein, P. 1970. Upper Pliocene Rodentia, Lagomorpha and Insectivora (Mammalia) from the isle of Rhodes (Greece). Proceedings Koninklijke Nederlandse Academie van Wetenschappen B, 73:535–584.
De Bruijn, H., Hoek Ostende, L.W.v.d., Kristkoiz-Boon, E., Rummel, M., Theocharopoulas, C., and Ünay, E. 2003. Rodents, lagomorphs and insectivores, from the middle Miocene hominoid locality of Çandır (Turkey), p. 51-89. In Güleç, E., Begun, D., and Geraads D. (eds.), Geology and Vertebrate Paleontology of the Middle Miocene Hominoid Locality Çandır (Central Anatolia, Turkey). Courier Forschungsinstitut Senckenberg Volume 240.
Eronen, J.T., Puolamaki, K., Liu, L., Lintulaakso, K., and Damuth, J. 2010a. Precipitation and large herbivorous mammals I: estimates from present-day communities. Evolutionary Ecology Research, 12:217–33.
Eronen, J.T., Puolamaki, K., Liu, L., Lintulaakso, K., and Damuth, J. 2010b. Precipitation and large herbivorous mammals II: application to fossil data. Evolutionary Ecology Research, 12:235–48.
Eronen, J.T., Ataabadia, M.M., Micheelsb, A., Karme , A., Bernor, R.L., and Fortelius, M. 2009. Distribution history and climatic controls of the late Miocene Pikermian chronofauna. Proceedings of the National Academy of Sciences of the United States of America, 106:11867–11871.
Fortelius, M. (coordinator). 2013. New and Old Worlds Database of Fossil Mammals(NOW). University of Helsinki. http://www.helsinki.fi/science/now/.
Fortelius, M. and Solounias, N. 2000. Functional characterization of ungulate molars using the abrasion–attrition wear gradient: a new method for reconstructing paleodiets. American Museum Novitates, 3301:1–36.
Fortelius, M., Eronen, J.T., Jernvall, J., Liu, L., and Pushkina, D. 2002. Fossil mammals resolve regional patterns of Eurasian climate change during 20 million years. Evolutionary Ecology Research, 4:1005–1016
Freudenberg, H. 1941. Die oberoligocanen Nager von Gaimersheim bei Ingolstadt und ihre Verwandten. Palaeontographica, A92:99–164.
Freudenthal, M. 1997. Paleogene rodent faunas from the province of Teruel (Spain), p. 397–415. In Aguilar, J.P, Legendre, S., and Michaux, J. (eds.), Actes du Congres Biochro M'97. Mèmoires Travaux Ecole Pratique des Hautes Etudes, Institutè Montpellier 21.
Freudenthal, M. and Martin-Suarez, E. 2007a. Microdyromys (Gliridae, Rodentia, Mammalia) from the Early Oligocene of Montalban (Province of Teruel Basin, Spain). Scripta Geologica, 135:179–211.
Freudenthal, M. and Martin-Suarez, E. 2007b. Revision of the subfamily Bransatoglirinae (Gliridae, Rodentia, Mammalia). Scripta Geologica, 135:241–273.
Freudenthal, M. and Martin-Suarez, E. 2007c. An Index for concavity of the occlusal surface of the cheek teeth and an assessment of concavity in Gliridae (Mammalia, Rodentia). Palaeontologia Electronica 10.2.9A–24pp, 1.3MB; http://palaeo-electronica.org/2007_2/00122/index.html
Garcia-Paredes, I., Pelaez-Campomanes, P., and Alvarez-Sierra, A. 2010. Microdyromys remmerti, sp. nov., a new Gliridae (Rodentia, Mammalia) from the Aragonian type area (Miocene, Calatayud-Montalban Basin, Spain). Journal of Vertebrate Paleontology, 30:1594–1609.
Gil-Delgado, J.A., Mira, O., Vinals, A., Gómez, J., Banyuls, N., and Vives-Ferrándiz, C. 2010. Diet of the garden dormouse (Eliomys quercinus Linnaeus 1766) in orange groves: seasonal variation and use of available resources. Mammalia, 74:147–151.
Gomes Rodrigues, H.G., Merceron, G., and Viriot, L. 2009. Dental microwear patterns of extant and extinct Muridae (Rodentia, Mammalia): ecological implications. Naturwissenschaften, 96:537–542.
Haas, G. 1973. The Pleistocene glirids of Israel. Verhandlungen der Naturforschenden Gesellschaft in Basel 83:76–110.
Hartenberger, J.L. 1971. Contribution l'étude des genres Gliravus et Microparamys (Rodentia) de l'Eocéne d'Europe. Palaeovertebrata, 4:97–135.
Hartenberger, J.L. 1994. The evolution of he Gliroidea. p. 19–33. In Tomida, Y., Li, C.K., and Setoguchi, T. (eds.), Rodent and lagomorph families of Asian origins and diversification. National Sicence Museum Monographs, Tokyo.
Hautier, L., Bover, P. Alcover, J., and Michaux, J. 2009. Mandible morphometrics, dental microwear pattern, and palaeobiology of the extinct Balearic Dormouse Hypnomys morpheus. Acta Palaeontologica Polonica, 54 (2):181–194.
Holden, M.E. 2005. Family Gliridae, p. 819–841. In Wilson, D.E. and Reeder, D.M. (eds.), Mammals Species of the World, a Taxonomic and Geographic Reference, 3rd Edition, Smithsonian Institution Press, Washington.
Jaeger, J.J. 1977. Les rongeurs du Miocène moyen et supérieur du Maghreb. Palaeovertebrata, 8:1–166.
Kaymakçı, N., Inceöz, M., Ertepınar, P., and Koç, A. 2010. Late Cretaceous to Recent kinematics of SE Anatolia (Turkey). Geological Society of London, 340:409–435.
Krijgsman, W., Duermeijer, C.E., Langereis, C.G., De Bruijn, H., Saraç, G., and Andriessen, P.A.M.1996. Magnetic polarity stratigraphy of the late Oligocene to middle Miocene mammal-bearing continental deposits in Central Anatolia (Turkey). Newsletters on Stratigraphy, 34:13–29.
Kurtonur, C. and Özkan, B. 1991. New Records of Myomimus roachi (Bate, 1937) from Turkish Thrace (Mammalia: Gliridae). Senckenbergiana Biologica, 71:239–244.
Linnaeus, C. 1758. Systema Naturae per regna tria naturae, secundum classis, ordines, genera, species cum characteribus, differentiis, synoymis, locis. Tenth ed. Vol. 1.Laurentii Salvii, Stockholm. 824 p.
Merceron, G., De Bonis, L., Viriot, L., and Blondel, C. 2005. Dental microwear of fossil bovids from northern Greece: paleoenvironmental conditions in eastern Mediterranean during the Messinian. Palaeogeography, Palaeoclimatology, Palaeoecology, 217:173–185.
Meulen, A.J. v. d. and De Bruijn, H. 1982. The mammals from the Lower Miocene of Aliveri (Island of Evia, Greece); The Gliridae. Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen, 85:485–524.
Montgelard, C., Matthee, C. A., and Robinson, T. J. 2003. Molecular systematics of dormice (Rodentia: Gliridae) and the radiation of Graphiurus in Africa. Proceedings of the Royal Society of London Series B–Biological Sciences, 270:1947–1955.
Montgelard, C., Bentz, S., Tirard, C., Verneau, O., and Catzeflis, F.M. 2002. Molecular systematics of Sciurognathi (Rodentia): the mitochondrial cytochrome b and 12S rRNA genes support the Anomaluroidea (Pedetidae and Anomaluridae). Molecular Phylogenetics and Evolution, 22:220–233.
Munthe, J. 1980. Rodents of the Miocene Daud Khel Local Fauna, Mianwali district, Pakistan; Sciuridae, Gliridae, Ctenodactylidae, and Rhizomyidae. Contribution in Biology and Geology, Milwaukee Public Museum, 34:1–36.
Nowak, R.M. 1999. Walker's Mammals of the World. The John Hopkins University Press. Baltimore. Sixth Edition. II:1625–1635 .
Nunome, M., Yasuda, S.P., Sato, J.J., Vogel, P., and Suzuki. H. 2007. Phylogenetic relationships and divergence times among dormice (Rodentia, Gliridae) based on three nuclear genes. Zoologica Scripta, 36:537–546.
Ognev, S.I. 1924. Nature and sport in Ukraine, Kharkov. p.1.
Royer, D.L. 2006. CO2-forced climate thresholds during the Phanerozoic. Geochimica Cosmochimica Acta, 70:566–575.
Saraç, G. 2003. Türkiye omurgalı fosil yatakları. MTA Derleme Rapor No 10609. Jeoloji Kütüphane No 637. Jeoloji Etütleri Dairesi Ekim 2003, Ankara.
Schaub, S. 1958. Simplicidentata (Rodentia) in Piveteau, Traité de Paléontologie, 2:659–818.
Şengör, A.M.C. and Yılmaz, Y. 1981. Tethyan evolution of Turkey: a plate tectonic approach. Tectonophysics, 75:181–241.
Silcox, M.T. and Teaford, M.F. 2002. The diet of worms: an analysis of mole dental microwear. Journal of Mammalogy, 83:804–814.
Simpson, G.G. 1945. The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History, 85:1–350.
Solounias, N. and Semprebon, G. 2002. Advances in the reconstruction of ungulates ecomorphology with application to early fossil equids. American Museum Novitates, 3366:1–49.
Solounias, N., Rivals, F., and Semprebon, G. 2010. Dietary interpretation and paleoecology of herbivores from Pikermi and Samos (late Miocene of Greece). Paleobiology, 36; 113–136.
Stehlin, H.G. and Schaub, S. 1951. Die Trigonodontie der Simplicidentaten Nager. Schweiz. Abhandlungen, 67:1–385.
Strömberg, C.A.E. 2011. Evolution of grasses and grassland ecosystems. Annual Review of Earth and Planetary Sciences, 39:517–544.
Strömberg, C.A.E., Werdelin, L., Friis, E.M., and Saraç, G. 2007. The spread of grass-dominated habitats in Turkey and surrounding areas during the Cenozoic: phytolith evidence. Palaeogeography, Palaeoclimatology, Palaeoecology, 250:18–49
Tchernov, E. 1968. Succession of rodent faunas during the upper Pleistocene of Israel. Series Mammalia depicta, p.156.
Thaler, L. 1966. Les rongeurs fossiles du Bas-Languedoc dans leurs rapports avec l'histoire des faunes et la stratigraphie du Tertiaire d'Europe. Mémoires du Muséum national d'histoire naturelle, série C, Sciences de la Terre, tome XVI.
Thomas, O. 1897. On the genera of rodents: an attempt to bring up to date the current arrangement of the order. Proceedings Zoological Society London, 1897:1012–1028.
Uhlig, U. 2001. The Gliridae (Mammalia) from the Oligocene (MP24) of Gröben 3 in the folded molasse of southern Germany. Palaeovertebrata, 30:151–187.
Ünay, E. 1994. Early Miocene rodent faunas from the eastern Mediterranean area, The Gliridae. Proceedings Koninklijke Nederlandse Academie van Wetenschappen 97:445–490.
Ünay, E. and De Brujin, H.1984. On some Neogene rodent assemblages from the both sides of the Dardanelles, Turkey. Newsletters on Stratigraphy, 13:119–132.
Ünay, E. and Göktaş, F. 2000. Kınık (Gördeş) çevresindeki erken Miyosen ya?l? linyitli çökellerin küçük memeli biyokronolojisi; ön sonuçlar. Türkiye Jeoloji Bülteni, 43:1–5.
Utescher, T., Böhme, M., and Mosbrugger, V. 2011. The Neogene of Eurasia: spatial gradients and temporal trends—The second synthesis of NECLIME. Palaeogeography, Palaeoclimatology, Palaeoecology, 304:196–201.
van Dam, J. A. 2006. Geographic and temporal patterns in the late Neogene (12-3 Ma) aridification of Europe; The use of small mammals as paleoprecipitation proxies. Palaeogeography, Palaeoclimatology, Palaeoecology, 238:190–218.
van der Made, J., Güleç, E., and Erkman, A.C. 2013. Microstonyx (Suidae, Artiodactyla) from the Upper Miocene of Hayranlı-Haliminhanı, Turkey. Turkish Journal of Zoology, 37:106–122.
Vianey-Liaud, M. 1985. Possible evolutionary relationships among Eocene and Lower Oligocene rodents of Asia, Europe and North America, p. 277–309. In Luckett, W.P. and Hartenberger J.L. (eds.), Evolutionary Relationships among Rodents; A Multidisciplinary Analysis. Proceedings of a NATO ASI Series A: Life Sicences 92, Paris.
Vianey-Liaud, M. 1989. Parallelism among Gliridae (Rodentia): the genus Gliravus Stehlin & Schaub. Historical Biology, 2:213–226.
Vianey-Liaud, M. 1994. La radiation des Gliridae (Rodentia) al'Eocene supèrieur en Europe Occidentale et sa descendance oligocene. Münchner Geowissenschaftliche Abhandlungen, 26:117–160.
Vianey-Liaud, M. 2003. Gliridae (Mammalia, Rodentia) de l'Oligocene europèen: origine de trois genres miocènes. Coloquios de Paleontologia Volumen Extraordinario, 1:669–698.
Vianey-Liaud, M. and Jaeger, J.J. 1996. A new hypothesis fort he origin of African Anomaluridae and Graphiuridae (Rodentia). Palaeovertebrate, 25:349–358.
Wahlert, J.H., Sawiitzke, S.L., and Holden, M.E. 1993. Cranial anatomy and relationships of dormice (Rodentia, Myoxidae). American Museum Novitates, 306:1–32.
Winkler, A.J., Denys, C., and Avery M.D. 2010. Fossil rodents of Africa, p. 263–304. In Werdelin, L. and Sanders J. (eds.), Cenozoic Mammals of Africa, University of California Press. California.
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292:686–693.

