 Taphonomy of an Eocene micromammal assemblage in a lake-margin depositional setting elucidates an ancient food web
Taphonomy of an Eocene micromammal assemblage in a lake-margin depositional setting elucidates an ancient food web
Article number: 25.2.a16
https://doi.org/10.26879/1214
Copyright Palaeontological Association, May 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 31 January 2022. Acceptance: 3 May 2022.
ABSTRACT
The taphonomy of the micromammalian assemblage from an unusually widespread lake-margin depositional context in the early Priabonian How Ledge Limestone, Totland Bay Member, Headon Hill Formation, Isle of Wight, UK, was studied in order to understand its method of accumulation, the trophic interrelationships between species and families, and their spatial relationships in the palaeoenvironment. The fossil remains studied consist of mainly dissociated bones and teeth, belonging to 28 species, which show selective anatomical representation and characteristic types of damage (fragmentation, etching, puncture marks), which are documented quantitatively. Predation and scavenging were important factors in the accumulation of the assemblage, with little subaerial weathering. The main predators are identified as the mammals Paramiacis sp. and Amphiperatherium species B, both present as fossils and themselves also predated, plus two possible owls not represented in the fauna. Scavenging is attributed to the predators, plus glirids, possibly nyctitheres and insects. The accumulation of remains in the lake environment is interpreted to result from seasonal retreat and advance of the lake margin, with minimal hydraulic transport. Comparison is made with a previous study of a similar micromammalian assemblage in the younger Priabonian Osborne Member, whose depositional environment is a floodplain pond. The similarities and differences are assessed in the context of a similar assemblage, although with a different dominant mammalian predator. Low post-mortem transport in each case is shown to have resulted in the preservation of an assemblage, which closely reflects the original community with some of its key trophic interactions.
Katerina Vasileiadou, deceased. Formerly of the Natural History Museum of Lesvos Petrified Forest, 17 8th November, Postcode 81100, Mytilene, Lesvos, Greece.
Jerry J. Hooker. Department of Earth Sciences, Natural History Museum, Cromwell Road, London, SW7 5BD. j.hooker@nhm.ac.uk (corresponding author)
Margaret E. Collinson. Department of Earth Sciences, Royal Holloway University of London, Egham, Surrey, TW20 0EX; Department of Earth Sciences, Natural History Museum, Cromwell Road, London, SW7 5BD. m.collinson@rhul.ac.uk
Keywords: bone breakage; etching; food web; passive accumulation; predation; Priabonian
Final citation: Vasileiadou, Katerina, Hooker, Jerry J., and Collinson, Margaret E. 2022. Taphonomy of an Eocene micromammal assemblage in a lake-margin depositional setting elucidates an ancient food web. Palaeontologia Electronica, 25(2):a16. https://doi.org/10.26879/1214
palaeo-electronica.org/content/2022/3615-eocene-micromammal-taphonomy
Copyright: May 2022 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
DEDICATION
Katerina Vasileiadou sadly passed away while this paper was being finalized. Her co-authors dedicate it to her memory, in recognition of her insightful taphonomic work and her important contributions to the palaeomammalogy of Greece.
INTRODUCTION
Accurate palaeoenvironmental reconstruction is an important aim in palaeobiology and is dependent on a detailed understanding of the taphonomy of a given site or area. Small mammals, in addition to providing relatively abundant fossil remains in certain continental strata, have particular relationships with their living environment through trophic interaction, both as primary consumers of diverse food types and as prey items. They are therefore ideal as subjects for taphonomic study with the aim of reconstructing ancient terrestrial communities. Earlier studies of micromammal taphonomy, both fossil and modern include Dodson and Wexlar (1979), Korth (1979), Laudet and Selva (2005), and Vasileiadou et al. (2007a, 2009; and references therein). The Hampshire Basin Solent Group of the UK is a 5 m.y. long succession of Late Eocene (Priabonian) to Early Oligocene (Rupelian) dominantly continental strata, with numerous horizons containing mammalian faunas, especially ones with micromammals (Hooker, 1987, 2021; Hooker et al., 2009). Earlier taphonomic studies in this succession concerned one such horizon in the middle Priabonian Osborne Member, whose depositional environment was a river floodplain pond (Vasileiadou et al., 2007a, 2007b, 2009).
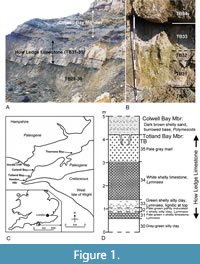 The present study concerns a micromammalian assemblage from a clay bed (TB33) in the How Ledge Limestone, Totland Bay Member, Headon Hill Formation (early Priabonian) of SW Headon Hill, Isle of Wight (see Figure 1 and Hooker, 2021, for stratigraphical details). Screen washing of bed TB33 sediment with a mesh size of 0.5 mm has allowed recovery of nearly all the disarticulated, but excellently preserved, remains of vertebrate bones and teeth, especially of mammals. The How Ledge Limestone represents a lake-margin depositional environment (Daley and Edwards, 1974). This contrasts with that of bed O3 (Hooker, 2021; referred to as “Os1” by Vasileiadou et al. 2007a, 2009), Osborne Member, Thorness Bay, Isle of Wight, previously studied, which represents a floodplain pond, and allows a detailed comparison to be made between the two levels. Despite the different depositional environments and the c.2 m.y. older age of the How Ledge Limestone (Hooker et al., 2009), there is much overlap in the mammalian taxa represented in both, which greatly facilitates distinguishing between the biological and physical factors involved in formation of the respective micromammalian assemblages.
The present study concerns a micromammalian assemblage from a clay bed (TB33) in the How Ledge Limestone, Totland Bay Member, Headon Hill Formation (early Priabonian) of SW Headon Hill, Isle of Wight (see Figure 1 and Hooker, 2021, for stratigraphical details). Screen washing of bed TB33 sediment with a mesh size of 0.5 mm has allowed recovery of nearly all the disarticulated, but excellently preserved, remains of vertebrate bones and teeth, especially of mammals. The How Ledge Limestone represents a lake-margin depositional environment (Daley and Edwards, 1974). This contrasts with that of bed O3 (Hooker, 2021; referred to as “Os1” by Vasileiadou et al. 2007a, 2009), Osborne Member, Thorness Bay, Isle of Wight, previously studied, which represents a floodplain pond, and allows a detailed comparison to be made between the two levels. Despite the different depositional environments and the c.2 m.y. older age of the How Ledge Limestone (Hooker et al., 2009), there is much overlap in the mammalian taxa represented in both, which greatly facilitates distinguishing between the biological and physical factors involved in formation of the respective micromammalian assemblages.
GEOLOGICAL CONTEXT AND DEPOSITIONAL ENVIRONMENT
The How Ledge Limestone (Blake, 1881) is an unranked lithostratigraphic unit at the top of the Totland Bay Member, Headon Hill Formation, cropping out in the west-central part of the Hampshire Basin (Figure 1; Daley, 1999; Hooker, 2021). It comprises two limestone units with intervening clays. Its lithological characteristics and fossil content remain essentially unchanged from SW Headon Hill in the southwest to Colwell Bay in the northeast via N Totland Bay (Keeping and Tawney, 1881), where it can be traced, through coastal exposures in the Isle of Wight, for a distance of 3.75 km. It has also been traced onto the mainland as far as Hordle Cliff, Hampshire, 6 km north-northwest of SW Headon Hill, where the limestones have increased mud content, forming marls (Tawney and Keeping, 1883; Edwards and Daley, 1997; beds 32-33). At Hordle Cliff, the equivalent of bed TB33, the subject of this work, is bed 32c.
The How Ledge Limestone is shelly throughout beds TB31-34 (Hooker, 2021), the biotas being dominated by the pulmonate gastropods Lymnaea and Australorbis (and other planorbids) with occasional land snails and the prosobranch Viviparus. Charophytes (Riveline, 1984), ostracods (Keen, 1977), teleost fish (Stinton, 1971), crocodilians, and turtles also occur. All indicate a very shallow freshwater lake-margin depositional environment (Daley, 1972; Daley and Edwards, 1974; Keen, 1977). Abundant micrommamals (the subject of this study), lizards, snakes, and amphibians (Rage and Ford, 1980; Klembara and Green, 2010; where TB33 is variously referred to as “HH2” or “Green Clay under Howledge Limestone”) indicate a largely terrestrial origin.
The lower limestone unit (bed TB31) has usually a sharp contact with underlying strata of the Totland Bay Member, or is locally transitional below to a sand, which itself rests sharply on underlying strata. The How Ledge Limestone has therefore been identified as representing an early transgressive systems tract, with the underlying sand unit as lowstand incised valley fill and the overlying Colwell Bay Member as late transgressive systems tract (King, 2016, p. 425). The widespread nature of the How Ledge Limestone, the absence of associated overbank deposits indicative of floodplain, and its position representing base-level rise in a stratigraphical sequence does not support its implied local setting within a river floodplain as suggested by Daley and Edwards (1974) and Plint (1984). Instead, it suggests a widespread development of the lake-margin environment. The dominantly calcareous nature of the deposit probably resulted from erosion of exposed chalk from the nearby Brixton anticline to the south, which was periodically active in later parts of the Eocene (Gale et al., 1999; Newell and Evans, 2011). Consistent with this uplift history is the more marly development at Hordle Cliff which is farthest away from the anticline. It is probable that an alluvial environment existed to the south of Headon Hill, but which has been removed by erosion following subsequent major anticlinal uplift.
The Colwell Bay Member is dominantly of normal magnetic polarity, although a reversed interval occurs at the very base in the eastern Isle of Wight (Gale et al., 2006). This reversed interval is interpreted as the top of Chron C16r (Hooker et al., 2009). The How Ledge Limestone lies immediately below the Colwell Bay Member in the west (represented by a hiatus in the east) and may therefore also belong in C16r, although no palaeomagnetic measurements have been recorded in the upper part of the Totland Bay Member. If correct, the age of the How Ledge Limestone would be c.36.5 Ma (Speijer et al., 2020).
Bed TB33 occupies the upper part of the more clastic middle part of the How Ledge Limestone. It is mainly a greenish silty clay with calcareous patches. It is shelly, although less so than the underlying bed TB32, with which it has a transitional contact. It shows a gradual upward reduction in shell content and increase in vertebrate content and a gradual change in colour from greenish to buff, terminating in a thin, black, non-shelly silty clay. Despite this gradual transition of lithologies, there is no visible bedding or bioturbation. Its upper boundary is sharp with the overlying upper limestone (TB34) (Hooker, 2021). It is probable that beds TB31-33 represent a parasequence, the clastic beds prograding over the lower limestone, the upper limestone representing a second base level rise, temporarily reducing the clastic input. The progradation involving beds TB32-33 may therefore represent a progressive lake shallowing from open waters to marginal conditions.
The sedimentation rate for the lower part of the Solent Group was calculated to be 32-37 m/m.y. (Hooker et al., 2009). Since then, the Priabonian GSSP has been established (Speijer et al., 2020), suggesting that base Solent Group is somewhat younger than base Priabonian, probably within C17n1n (Hooker, 2021). Despite this difference and with new dates (Speijer et al., 2020), choosing the maximum thickness (c.55 m) of the range of strata from base Solent Group to top C16n (Hooker et al., 2009) in the Fort Victoria Member (Hooker 2021) (formerly lower Cliff End Member: Gale et al., 2006, figure 3; King, 2016, p. 429) produces a similar rate, suggesting a maximum duration of 9.5 k.y. for deposition of bed TB33. Given the presence of two substantial limestones, which typically have slower depositional rates than clastics (e.g., Platt and Wright, 1991), and hiatuses at two sequence boundaries and at two transgressive surfaces in this segment of the Solent Group succession, the accumulation time of bed TB33 is likely to have been considerably shorter than 9.5 k.y.
MATERIALS and METHODS
At SW Headon Hill, the site chosen for the present study, TB33 is c.35 cm thick, in which sampling was restricted to the upper less shelly 20 cm, to reduce residue sorting time. Sampling was carried out over a number of years (between 1982 and 1999), resulting in the collection of 1108 kg of sediment. After screenwashing, much of the earlier collected samples were treated with 5% acetic acid, buffered with calcium orthophosphate, to remove the shell. In a later sampling, only the 0.5-1.0 mm fraction was in part so treated. Residues were size-graded and sorted using a binocular microscope. The total number of identified mammalian specimens is 2998, of which 2621 were identified to at least family level, giving the average number of specimens recovered per kg as 2.7. Some of the theridomyid teeth in one of the samples had been destroyed for an isotope study (Grimes et al., 2005) and could not be used to judge surface damage. All other specimens are stored in the Earth Sciences Department, Natural History Museum, London (NHMUK.PV).
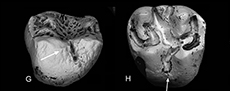
The methodology used was as for the earlier study (Vasileiadou et al., 2009; see also Vasileiadou, 2006). Thus, a range of damage effects on teeth and bones have been documented quantitatively. Recent damage caused by sampling was distinguished from original damage by sharpness of the breaks and colour of the broken surfaces. The aim was to establish cause of death and search for additional taphonomic agents that influenced the accumulation of each species. However, the difficulty in attributing many postcranial bones to species meant that treatment of bones in most cases had to be at family level. Table 1 shows the mammalian faunal list for TB33. Of the 39 species recorded, 30 were found in the samples studied here (representing all but the rarest). All except two of these (Plesiarctomys gervaisii, Magnadapis sp.) belong to Andrews et al.’s (1979) body size class AB (<1 kg body weight), the criterion used here to classify them as micromammals, the subject of the study. These two exceptions belong to size class C (1-10 kg) and are excluded from this study. 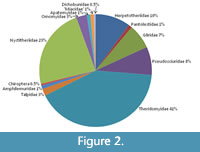 The descending order of abundance of the dominant families is Theridomyidae, Nyctitheriidae, Herpetotheriidae, and nearly coequal Gliridae and Pseudosciuridae; the remaining nine families represent only a minor proportion of the whole assemblage (Table 1; Figure 2). Species richness (especially of the rodents) is much higher than in the Osborne Member fauna studied earlier (Vasileiadou et al., 2007a, 2009). In particular, family Gliridae is represented by four rather than only two species in the Osborne Member. Family Pseudosciuridae is represented by four species, whereas the family is unknown in the Osborne Member. This has made identification of postcranial elements to species harder. Thus, many of these are only identified to family level and are reflected in the family number of individual specimens (NISP), which are often larger than those of the species in Table 1. Various levels of uncertainty are recorded in the identification of different bones of theridomyids, partly because of incompleteness, but also because of the possibility of confusion of the smaller Isoptychus sp. with pseudosciurids. However, Thalerimys fordi is distinctive because of its large size, whilst specimens of both Isoptychus and pseudosciurids are few in number, so are unlikely to influence the overall numbers attributed to Theridomyidae as a whole.
The descending order of abundance of the dominant families is Theridomyidae, Nyctitheriidae, Herpetotheriidae, and nearly coequal Gliridae and Pseudosciuridae; the remaining nine families represent only a minor proportion of the whole assemblage (Table 1; Figure 2). Species richness (especially of the rodents) is much higher than in the Osborne Member fauna studied earlier (Vasileiadou et al., 2007a, 2009). In particular, family Gliridae is represented by four rather than only two species in the Osborne Member. Family Pseudosciuridae is represented by four species, whereas the family is unknown in the Osborne Member. This has made identification of postcranial elements to species harder. Thus, many of these are only identified to family level and are reflected in the family number of individual specimens (NISP), which are often larger than those of the species in Table 1. Various levels of uncertainty are recorded in the identification of different bones of theridomyids, partly because of incompleteness, but also because of the possibility of confusion of the smaller Isoptychus sp. with pseudosciurids. However, Thalerimys fordi is distinctive because of its large size, whilst specimens of both Isoptychus and pseudosciurids are few in number, so are unlikely to influence the overall numbers attributed to Theridomyidae as a whole.
A number of bones are not identifiable to family, although they include ones that can be identified as Rodentia and Gliridae/small Nyctitheriidae. The phalanges identified as Gliridae/small Nyctitheriidae are mostly fragments. The characters of nyctithere first and second phalanges have been recognized, when complete specimens are available (Hooker, 2014), but the normal broken state of the specimens in the sample makes trying to distinguish them here impracticable. Most of the “flat” or “elongate” bones are broken, including many undiagnostic parts. The majority of the metapodials and phalanges are distal fragments, so may complement proximal fragments that have been identified. They vary in size, but do not form clusters. They cannot inform about the taphonomic history of a particular taxon, so have been excluded from the study. Fifty simple incisiform and caniniform teeth could not be identified reliably and have also been excluded from the study.
RESULTS
Bone Fragmentation
Bone fragmentation is high (Table 2), whilst a far lower percentage of skeletal elements is recorded than would be expected from the minimum number of individuals (MNI), according to the number of bones in the skeleton of each type. The family with the most easily identifiable bones and with the best known skeleton is the extinct Theridomyidae. The tally of bones is derived from a complete skeleton of the theridomyid Issiodoromys medius, documented by Schmidt-Kittler and Storch (1985) as Pseudoltinomys gaillardi and reidentified by Vianey-Liaud et al. (2015). Table 3 shows the large discrepancy between the expected and actual representation of theridomyid skeletal elements in TB33. The maximum representation of any element is 10% and most are much lower, whilst vertebrae, ribs, and pelves are not represented at all. It should be noted that vertebrae, ribs, and pelves (recorded for some of the other taxa) are the least identifiable bones of the skeleton, especially when fragmented, so there is an element of analytical absence here (Lyman, 1994).
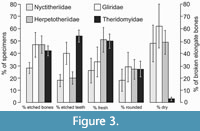 Breakage of limb bones has affected different parts. Thus, humeri, tibiae and fibulae are represented only by distal parts, radii, and femora only by proximal parts and ulnae mainly by shafts and by a few proximal parts (see Table 4, for Theridomyidae, the best represented family). Bone breakage has been classified into different types. Critically important is the difference between spiral irregular versus perpendicular smooth types (Table 5; Figure 3). Spiral irregular breakage shows that the bone was fresh when broken; perpendicular smooth breakage shows that it was dry and therefore not fresh (Andrews 1990). Elongate bones (i.e., limb bones, metapodials, and non-terminal phalanges) exemplify these types as they are some of the most easily broken and have a relatively regular symmetry, facilitating breakage shape recognition. Theridomyids show the largest proportion of irregular spirally to smooth perpendicularly broken elongate bones (50:3), Herpetotheriidae show nearly equal proportions, whilst Gliridae and Nyctitheriidae show more smooth perpendicular than irregular spiral breakages (Table 5; Figure 3). Flat bones (maxillae, dentaries, scapulae, and pelves) show even more breakage, and compact bones (carpals, tarsals, and ungual phalanges) much less (Table 6). Of the three common families with taxonomically readily identifiable bones, elongate bones of Herpetotheriidae show the greatest amount of breakage (74%), followed by Theridomyidae (63%) and Gliridae (53%) (Table 6).
Breakage of limb bones has affected different parts. Thus, humeri, tibiae and fibulae are represented only by distal parts, radii, and femora only by proximal parts and ulnae mainly by shafts and by a few proximal parts (see Table 4, for Theridomyidae, the best represented family). Bone breakage has been classified into different types. Critically important is the difference between spiral irregular versus perpendicular smooth types (Table 5; Figure 3). Spiral irregular breakage shows that the bone was fresh when broken; perpendicular smooth breakage shows that it was dry and therefore not fresh (Andrews 1990). Elongate bones (i.e., limb bones, metapodials, and non-terminal phalanges) exemplify these types as they are some of the most easily broken and have a relatively regular symmetry, facilitating breakage shape recognition. Theridomyids show the largest proportion of irregular spirally to smooth perpendicularly broken elongate bones (50:3), Herpetotheriidae show nearly equal proportions, whilst Gliridae and Nyctitheriidae show more smooth perpendicular than irregular spiral breakages (Table 5; Figure 3). Flat bones (maxillae, dentaries, scapulae, and pelves) show even more breakage, and compact bones (carpals, tarsals, and ungual phalanges) much less (Table 6). Of the three common families with taxonomically readily identifiable bones, elongate bones of Herpetotheriidae show the greatest amount of breakage (74%), followed by Theridomyidae (63%) and Gliridae (53%) (Table 6).
Etching
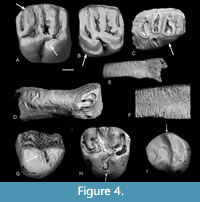 Etching is present on the teeth of nearly all the families present, even most of the poorly represented ones (Table 2, Figure 4, Figure 5, Figure 6, Figure 7). Table 7 and Figure 3 show the number and percentages of etched teeth in each of the four commonest families, the Theridomyidae having the highest percentage, followed by Gliridae, then subequal Herpetotheriidae and Nyctitheriidae.
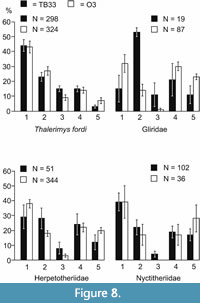
Etching is present on the teeth of nearly all the families present, even most of the poorly represented ones (Table 2, Figure 4, Figure 5, Figure 6, Figure 7). Table 7 and Figure 3 show the number and percentages of etched teeth in each of the four commonest families, the Theridomyidae having the highest percentage, followed by Gliridae, then subequal Herpetotheriidae and Nyctitheriidae. 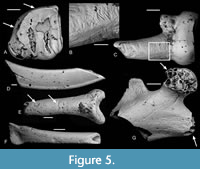 The degree of etching is categorized across 5 etching groups, 5 being the most extreme. The etching groups are those defined by Vasileiadou et al. (2007a). For Thalerimys fordi, the only abundant theridomyid in bed TB33, most cheek teeth show group 1 etching, the least severe; apart from equal representation of groups 3 and 4, there is a general reduction from group 1 to group 5 (Figure 8). In contrast, plots of etching groups for Gliridae, Herpetotheriidae, and Nyctitheriidae show bimodality, with peaks at groups 1 or 2 and 4, the lowest values being in group 3 (and also group 5 for Gliridae, although relatively small numbers of glirids in TB33 may be influencing the results) (Figure 8).
The degree of etching is categorized across 5 etching groups, 5 being the most extreme. The etching groups are those defined by Vasileiadou et al. (2007a). For Thalerimys fordi, the only abundant theridomyid in bed TB33, most cheek teeth show group 1 etching, the least severe; apart from equal representation of groups 3 and 4, there is a general reduction from group 1 to group 5 (Figure 8). In contrast, plots of etching groups for Gliridae, Herpetotheriidae, and Nyctitheriidae show bimodality, with peaks at groups 1 or 2 and 4, the lowest values being in group 3 (and also group 5 for Gliridae, although relatively small numbers of glirids in TB33 may be influencing the results) (Figure 8).
 |
 |
 |
Bones also show etching (Figure 4D, Figure 5E-G, Figure 6E). In particular, high percentages of those elongate bones in Theridomyidae, Gliridae, Herpetotheriidae, and Nyctitheriidae that have irregular spiral breakage have those breakage surfaces rounded by etching (Table 5, Figure 3, Figure 5F). Higher percentages of the teeth of rodents than of the insectivorous groups Herpetotheriidae and Nyctitheriidae show etching, although there is very little difference in the percentages of the rounding of broken bone surfaces (Table 7, Table 8).
Puncture Marks and Other Surface Features
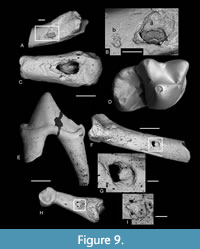 Some bones show puncture marks (Figure 9). They are oval depressed fractures and are concentrated on elongate bones. In Thalerimys and ?Thalerimys, they occur on a distal tibia, three metapodials and one second phalanx. One herpetotheriid incisor also bears two such marks on its root (Figure 6F-G). Some bones show parallel or near parallel grooves, ranging in width from 10-50 μm; some are in lines perpendicular to the long axis of the bone (Figure 4D-F, Figure 5E). In groove arrangement they resemble those of the ichnogenus Machichnus (Mikuláš et al., 2006; Ekrt et al., 2016). Other types of surface features involve splitting and flaking of bones and of the dentine of teeth (Figure 5D), chipping and cracking of teeth, and randomly orientated fine striations on bones (Figure 5B-C, Table 2). The splitting and flaking are taken as evidence of weathering and Table 9 shows the distribution of postcranial elements of families Theridomyidae, Gliridae, Herpetotheriidae, and Nyctitheriidae across the four weathering stages of Andrews (1990). Diagenesis is generally low in clays of the Solent Group (Grimes et al., 2003, 2005), and the mammal remains appear essentially unaffected (e.g., not crushed or heavily mineralized), except for some examples of pyrite crystals filling narrow cracks in bones and limonitic encrustation.
Some bones show puncture marks (Figure 9). They are oval depressed fractures and are concentrated on elongate bones. In Thalerimys and ?Thalerimys, they occur on a distal tibia, three metapodials and one second phalanx. One herpetotheriid incisor also bears two such marks on its root (Figure 6F-G). Some bones show parallel or near parallel grooves, ranging in width from 10-50 μm; some are in lines perpendicular to the long axis of the bone (Figure 4D-F, Figure 5E). In groove arrangement they resemble those of the ichnogenus Machichnus (Mikuláš et al., 2006; Ekrt et al., 2016). Other types of surface features involve splitting and flaking of bones and of the dentine of teeth (Figure 5D), chipping and cracking of teeth, and randomly orientated fine striations on bones (Figure 5B-C, Table 2). The splitting and flaking are taken as evidence of weathering and Table 9 shows the distribution of postcranial elements of families Theridomyidae, Gliridae, Herpetotheriidae, and Nyctitheriidae across the four weathering stages of Andrews (1990). Diagenesis is generally low in clays of the Solent Group (Grimes et al., 2003, 2005), and the mammal remains appear essentially unaffected (e.g., not crushed or heavily mineralized), except for some examples of pyrite crystals filling narrow cracks in bones and limonitic encrustation.
INTERPRETATION
Predation and Causal Agents
Mammalian bite marks. A pattern of bone breakage, tooth etching, etching of the broken edges of irregular spiral fractures on elongate bones, and the presence of puncture marks on bones, involving a micromammal assemblage in bed O3, Osborne Member, Thorness Bay, near identical to that documented here for bed TB33, How Ledge Limestone, was interpreted to have resulted from predation or scavenging by a mammalian predator (Vasileiadou et al., 2007a, 2009). The bone puncture marks were recognized as bite marks from the predator/scavenger. A mortality profile for one of the main groups of micromammal from that bed, the Theridomyidae, has been shown to be attritional (Vasileiadou et al., 2007b). The resulting large accumulation of small skeletal remains was considered most likely to have been mainly through predation thus reducing the rate of their normal rapid destruction (Andrews, 1990), rather than through purely random scavenging (Vasileiadou et al., 2007a). The same interpretation is therefore applied to the accumulation of the bed TB33 assemblage described here, of which the theridomyid Thalerimys fordi also has an attritional mortality signature (Vasileiadou et al., 2007b).
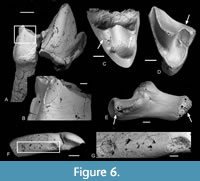
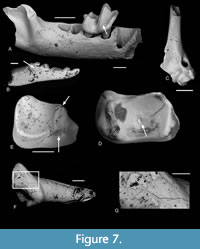
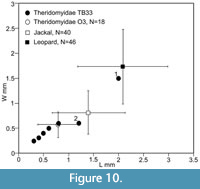 The mammalian predator identified in the Osborne Member was the amphicyonid carnivoran Cynodictis cf. lacustris (compared for size and dental morphology with the modern Arctic fox, Vulpes lagopus, weighing c.3.5 kg), because of the presence of its teeth in the bed and the size matching of the puncture marks on the bones with its teeth (Vasileiadou et al., 2007a, 2009). A different smaller mammalian predator, the “miacid” carnivoran Paramiacis sp. (about the size of a modern polecat, Mustela putorius, weighing c.800 g), is the only one to occur in bed TB33, and its teeth likewise match most of the bite marks on the theridomyid bones (Figure 9F-I), which are smaller than those in the Osborne Member, but with slight overlap (Figure 10). However, there is one bite mark that is just longer than the 95% error bar of the Osborne Member ones (M62154), plus another that is much larger than all the rest (M67679) and plots close to the mean for a leopard (Figure 10). Andrews and Fernandez-Jalvo (1997) attributed punctures that showed wide size variation (observed on human bones from the Middle Pleistocene of Cueva Mayor, Spain) to more than one carnivore species. Similarly, the large sizes of the punctures on the distal metapodial, M62154, and the distal tibia, M67679, might suggest the action of more than one carnivore species on the theridomyids. These two punctures, however, are different from the others from either level. In M67679, the piece of bone that was depressed inwards was broken and separated from the rest of the bone and the gap filled with sediment (Figure 9A-B). The tip of a cusp that initially creates a puncture is pointed, but that cusp widens closer to the crown base. A deep bite therefore more closely reflects the size of this more basal part of the tooth. In fact, M67679 bears two punctures, a small one near the main large one. The distance between them is similar to the distance between the protoconid and either the metaconid, paraconid, or hypoconid of the lower carnassial of Paramiacis, all three of which cusps are smaller and lower than the protoconid (Figure 9D). Therefore, the punctures on M67679 are also likely to have been made by Paramiacis sp., just with a deeper bite. A similar scenario is possible for M62154, but this bone appears to show weathering, which may have subsequently enlarged a smaller bite mark (Figure 9C). The two tiny (c.150 μm diameter) bite marks on the root of a herpetotheriid incisor (M67680) (Figure 6F-G) are too small to have been made by Paramiacis. The distance measured between their centres is c.900 μm. The most likely candidate is the large herpetotheriid Amphiperatherium species B (estimated weight c.300g). Modern didelphids of similar size and tooth morphology to the Solent Group herpetotheriids usually prey on small tetrapods as well as insects (e.g., Redford and Eisenberg, 1992; de Carvalho et al., 2019). The smaller nyctitheres and the talpid Eotalpa have less robust teeth with relatively slender cusps, suggestive of a more exclusively insectivorous diet, excluding them as candidates.
The mammalian predator identified in the Osborne Member was the amphicyonid carnivoran Cynodictis cf. lacustris (compared for size and dental morphology with the modern Arctic fox, Vulpes lagopus, weighing c.3.5 kg), because of the presence of its teeth in the bed and the size matching of the puncture marks on the bones with its teeth (Vasileiadou et al., 2007a, 2009). A different smaller mammalian predator, the “miacid” carnivoran Paramiacis sp. (about the size of a modern polecat, Mustela putorius, weighing c.800 g), is the only one to occur in bed TB33, and its teeth likewise match most of the bite marks on the theridomyid bones (Figure 9F-I), which are smaller than those in the Osborne Member, but with slight overlap (Figure 10). However, there is one bite mark that is just longer than the 95% error bar of the Osborne Member ones (M62154), plus another that is much larger than all the rest (M67679) and plots close to the mean for a leopard (Figure 10). Andrews and Fernandez-Jalvo (1997) attributed punctures that showed wide size variation (observed on human bones from the Middle Pleistocene of Cueva Mayor, Spain) to more than one carnivore species. Similarly, the large sizes of the punctures on the distal metapodial, M62154, and the distal tibia, M67679, might suggest the action of more than one carnivore species on the theridomyids. These two punctures, however, are different from the others from either level. In M67679, the piece of bone that was depressed inwards was broken and separated from the rest of the bone and the gap filled with sediment (Figure 9A-B). The tip of a cusp that initially creates a puncture is pointed, but that cusp widens closer to the crown base. A deep bite therefore more closely reflects the size of this more basal part of the tooth. In fact, M67679 bears two punctures, a small one near the main large one. The distance between them is similar to the distance between the protoconid and either the metaconid, paraconid, or hypoconid of the lower carnassial of Paramiacis, all three of which cusps are smaller and lower than the protoconid (Figure 9D). Therefore, the punctures on M67679 are also likely to have been made by Paramiacis sp., just with a deeper bite. A similar scenario is possible for M62154, but this bone appears to show weathering, which may have subsequently enlarged a smaller bite mark (Figure 9C). The two tiny (c.150 μm diameter) bite marks on the root of a herpetotheriid incisor (M67680) (Figure 6F-G) are too small to have been made by Paramiacis. The distance measured between their centres is c.900 μm. The most likely candidate is the large herpetotheriid Amphiperatherium species B (estimated weight c.300g). Modern didelphids of similar size and tooth morphology to the Solent Group herpetotheriids usually prey on small tetrapods as well as insects (e.g., Redford and Eisenberg, 1992; de Carvalho et al., 2019). The smaller nyctitheres and the talpid Eotalpa have less robust teeth with relatively slender cusps, suggestive of a more exclusively insectivorous diet, excluding them as candidates.
Etching profiles: predators and prey. The dominance of Andrews’ (1990) etching group 1 (the mildest) with essentially gradual reduction in percentages of successive more severe etching groups (i.e., a unimodal skewed plot) affecting the cheek teeth of the theridomyids Thalerimys fordi and Isoptychus sp. in bed O3 of the Osborne Member suggested only a single mammalian predator (Vasileiadou et al., 2007a). The etching profile for the cheek teeth of T. fordi from bed TB33 is very similar to that of the Osborne Member (Figure 8) and the same conclusion can be drawn. However, the etching profiles for Gliridae, Herpetotheriidae, and Nyctitheriidae from both levels differ in that they are bimodal (Figure 8). Although numbers of glirids and herpetotheriids from TB33 and of nyctitheres from the Osborne Member are relatively low, giving rather large error bars, the profiles of all three families from both levels are consistent in showing much lower percentages for etching group 3 than for adjacent groups, producing a bimodal pattern. In contrast to the theridomyid data, this is suggestive of more than one predator of the glirids, herpetotheriids, and nyctitheres. No other strict mammalian predators have been recorded from the How Ledge Limestone, although larger carnivores belonging to the extinct order Hyaenodonta do occur rarely at other levels in the Headon Hill Formation (e.g., Cray, 1973) and might also have been responsible for predation of members of these three families. Another possibility is that the etching groups 4 and 5 peaks were caused by a raptorial bird. Fragmentary bones of birds do occur in bed TB33, but they are taxonomically indeterminate. Diurnal raptors and some owls have strong digestive juices likely to produce severe etching (Andrews, 1990), so could have been responsible. However, the amount of bone breakage in the three families is high, suggesting instead a mammalian predator. Amphiperatherium species B, approximately the size of a modern stoat (Mustela erminea), is large enough to have predated these smaller mammals. However, Amphiperatherium species B, being semiterrestial (Koenigswald and Storch, 1988; Rose et al., 2012), may have had fewer opportunities to predate upon the ground the glirids and nyctitheriids, which were scansorial (Collinson and Hooker, 2000; Hooker, 2014), but would likely have had better chances with the smaller herpetotheriids. Two additional factors also support Amphiperatherium species B being such a predator. The percentage of etched teeth is 27% in Amphiperatherium A and 31% in Amphiperatherium species C, but only 15% in Amphiperatherium species B. Perhaps Amphiperatherium species B was a more challenging prey item for Paramiacis. Although only represented by 10 specimens, the percentages of the teeth of Amphiperatherium species B in the different etching groups are 60 (etching group 1), 30 (2), 0 (3), 10 (4), and 0 (5). Therefore, group 1 is dominant as in the Theridomyidae. This supports Paramiacis sp. being its predator and suggests that Amphiperatherium species B was responsible for the second peak in the etching group profiles of the Gliridae, Nyctitheriidae, and Herpetotheriidae.
According to the etching percentages of the different families (Table 7), rodents (Theridomyidae and Gliridae) show more predation than mainly insectivorous families (Herpetotheriidae and Nyctitheriidae), suggesting a preference for the former. However, the low percentage of Herpetotheriidae in TB33 compared to O3 may be influenced somewhat by the presence of Amphiperatherium species B, which suffered less predation than the other species. The skeleton of Paramiacis is largely unknown, although the damaged skeletal remains herein do provide some evidence of locomotor adaptation. The ungual phalanges are relatively compressed laterally, and the first phalanges bear tubercles for attachment of annular ligaments, both indicative of a degree of climbing ability. It might be expected, therefore, that the scansorial glirids and nyctitheres would be preferentially targeted. However, the dental etching percentages of the scansorial Gliridae plus Nyctitheriidae are less than those of the semiterrestrial Theridomyidae plus Herpetotheriidae (Table 7), suggesting that this was not the case.
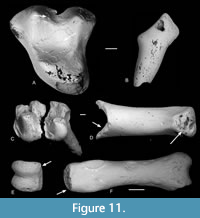 The pantolestid Cryptopithecus major is represented by 32 isolated teeth. Of these, seven show etching, of which two are in group 4 and four in group 5, whilst one is in group 1. In two of the group 5 etched teeth, there is no or almost no enamel left on the crowns (Figure 11A-B). One tooth of Vectipithex smithorum is similarly nearly enamelless (Figure 7D). Given also that pantolestids were semiaquatic (Koenigswald, 1980), it is possible that such extreme etching was caused by crocodilians, which are known to excrete enamelless teeth (Fisher, 1981) and are present in the bed.
The pantolestid Cryptopithecus major is represented by 32 isolated teeth. Of these, seven show etching, of which two are in group 4 and four in group 5, whilst one is in group 1. In two of the group 5 etched teeth, there is no or almost no enamel left on the crowns (Figure 11A-B). One tooth of Vectipithex smithorum is similarly nearly enamelless (Figure 7D). Given also that pantolestids were semiaquatic (Koenigswald, 1980), it is possible that such extreme etching was caused by crocodilians, which are known to excrete enamelless teeth (Fisher, 1981) and are present in the bed.
Evidence for avian predators. A sample of bed TB33, excavated subsequent to that used for the main part of this taphonomic study, has yielded most of an associated set of right lower teeth of the omomyid primate Microchoerus erinaceus. The teeth are first incisor, canine, P3-4, M2-3. All were found in a small subsample (covering an area of not more than 0.5 sq m and probably much less) of the sample collected. Matching interstitial facets indicate the presence of a single individual. All the crowns bear group 4 etching (Figure 12). The etching is absent from the crown bases, indicating that it occurred while the teeth were in a jaw and protected by gums. This certainly argues against the predator being Paramiacis or a similar-sized mammalian predator as the jaw would surely have been fragmented by chewing during ingestion.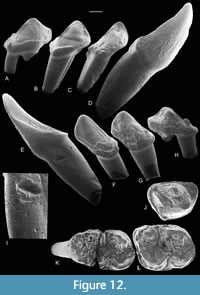 The absence of remains of the jaw in the fossil as preserved suggests that it was weakened by digestive juices, but the close association of the teeth shows that it was still present some time after being voided by the predator as a scat or pellet and only later decomposed, while lying on the sediment surface. The root of the enlarged first incisor bears the bite mark of a large theridomyid or pseudosciurid rodent and a row of fine gnaw marks perpendicular to the long axis on its medioventral edge, demonstrating scavenging by a very small mammal (?nyctithere) after decomposition of the jaw (Figure 12I). The root of the canine also bears gnaw marks (Figure 12C). This range of features is strongly suggestive of predation by a predatory bird capable of catching and eating this c.600 g nocturnal arboreal mammal (Ramdarshan and Orliac, 2016), but without breaking its dentary, thus perhaps a large owl.
The absence of remains of the jaw in the fossil as preserved suggests that it was weakened by digestive juices, but the close association of the teeth shows that it was still present some time after being voided by the predator as a scat or pellet and only later decomposed, while lying on the sediment surface. The root of the enlarged first incisor bears the bite mark of a large theridomyid or pseudosciurid rodent and a row of fine gnaw marks perpendicular to the long axis on its medioventral edge, demonstrating scavenging by a very small mammal (?nyctithere) after decomposition of the jaw (Figure 12I). The root of the canine also bears gnaw marks (Figure 12C). This range of features is strongly suggestive of predation by a predatory bird capable of catching and eating this c.600 g nocturnal arboreal mammal (Ramdarshan and Orliac, 2016), but without breaking its dentary, thus perhaps a large owl.
Another similar case from the main samples studied involves associated left and right teeth, without jaws, the holotype of another omomyid primate, Vectipithex smithorum (see Hooker and Harrison, 2008). This also suggests predation by a bird, likely to be an owl as V. smithorum was probably nocturnal like Microchoerus. This bird would have been sizeable as V. smithorum was of similar size to M. erinaceus. In this case, however, tooth etching is very light, suggesting a different owl from the one that predated the M. erinaceus.
Eleven teeth and 47 bones belonging to Paramiacis sp. appear to belong to a single individual, as there is no repetition of elements and all came from two subsamples totalling 119 kg dry weight (Table 10). Bone breakage for this partial skeleton is high (64%). Seven of the 30 (26 if one refits broken epiphyses and shafts of first phalanges - see below) broken elongate bones show spiral irregular breakage, four of which show rounding of the broken edges. Seven of the teeth show no etching, but there are two in etching group 1 and one each in groups 2 and 3. The four etched, spirally broken bones suggest the activity of a mammalian predator larger than Paramiacis itself as does the near absence of vertebrae and all the main limb bones. However, the local accumulation of a partial skeleton argues against a mammalian predator, which is unlikely to have excreted so much of the skeleton at the same time and place. Also, spiral breakage has affected only a small number of the elongate bones, which could possibly have happened during capture rather than during mastication. Moreover, at least one modern owl (Otus asio) does cause substantial bone breakage (Hoffman, 1988). It seems more likely that integrity of discrete parts of the skeleton, including parts of left and right feet and petrosals, would be achieved in the pellet of a large bird. Etching of the teeth ranges from groups 0-3, suggesting a different predatory bird from that which predated the Microchoerus, perhaps also an owl. Of the few teeth not associated with this partial skeleton, one shows etching of group 5, whilst another shows group 1.
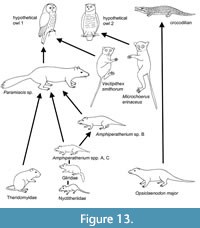 Figure 13 summarizes the inferred relationships between the predators and prey for bed TB33. Although the owls are hypothetical, as they are not represented by fossils in the bed, the food web shown is based on the damage and skeletal representation patterns documented.
Figure 13 summarizes the inferred relationships between the predators and prey for bed TB33. Although the owls are hypothetical, as they are not represented by fossils in the bed, the food web shown is based on the damage and skeletal representation patterns documented.
Weathering, scavenging and causal agents
Most remains show no signs of weathering and none greater than Andrews’ (1990) weathering stage 3 (Table 2, Table 9). The highest percentage in stage 1 (the lowest level of effect) affects the Theridomyidae with 17%, the lowest the Nyctitheriidae with 4.8%. Percentages in stages 2 and 3 are negligible. This suggests that the length of time the remains lay on a subaerial surface was relatively short and that they were soon covered by sediment. However, marks of gnawing probably by glirids on theridomyid bones and others possibly by nyctitheres and insects (Vasileiadou et al., 2009) occur (Table 2). Those shown in Figure 4E-F, Figure 5E, Figure 12I are too small to be attributed to glirids (Collinson and Hooker, 2000) and may be attributable to nyctitheres. Nyctitheres have enlarged slender pointed lower first incisors (Hooker, 2021) and might be expected to produce gnaw marks similar to those of shrews (Andrews, 1990, figure 1.3E-F; Fernández-Jalvo and Andrews, 2016, figures A174, A175, A183). Even finer more randomly orientated gnaw marks shown on a glirid tooth root (Figure 5B-C) resemble in arrangement those attributed to insects from the Oligocene site of Detan, Czech Republic (Fejfar and Kaiser, 2005). This shows that remains lay exposed long enough subaerially for a range of organisms to scavenge them. Few show fine, irregular, randomly orientated surface striations (Table 2), thought to be evidence of trampling (Vasileiadou et al., 2009), further possible evidence of a short exposure time prior to burial.
Four first phalanges belonging to the partial skeleton of Paramiacis sp. (see above) show an unusual pattern of damage (Figure 11E-F). Three fragments representing the distal articulations of three first phalanges were found to fit three first phalanges that lacked the distal end. In a fourth case, a proximal fragment was found to fit another first phalanx lacking the proximal end. The breakages are perpendicular to the long axes and therefore occurred after the bone had dried. Their edges are also slightly rounded, although this does not detract from reliable fits. The pattern of this breakage and the close association of the broken parts suggest that the phalanges were all from a single foot, which was still enclosed in flesh or skin, presumably dried out. Scavenging by a large carnivorous mammal, such as a hyaenodont, using long carnassial blades, could have sliced through or at least broken the foot with a minimum of two bites to produce this pattern, but perhaps without completely severing the skin, allowing the fragments to remain in close proximity. Although not known from the How Ledge Limestone, Hyaenodon is known from other levels in the Headon Hill Formation and could be expected to occur. Presumably the rounding of the broken edges of the bones took place subsequently, perhaps following decomposition of the remaining flesh and/or skin, shortly before burial.
Spatial relationships of the mammals and palaeoenvironmental reconstruction
Nature of the assemblage. The freshwater assemblage of the How ledge Limestone is judged autochthonous according to the ostracods, which belong to assemblage I, the Candona-Cypridopsis Assemblage (Keen, 1977), which lived alongside the pulmonate gastropods and charophytes in a very shallow lake or lake margin environment, less than 1 m deep, potentially emergent, with rooted water plants and algal mats (Keen, 1975). This freshwater biota is known from all known exposures of the How Ledge Limestone (Stinton, 1971; Edwards and Daley, 1997). The assemblage of land micromammals from bed TB33 studied here occurs with the freshwater biota throughout the bed and shows similar levels of abundance with little difference in recorded species at the different sites of SW Headon Hill, Totland Bay and Hordle Cliff (Bosma, 1974; Hooker, 1987, 2021; Hooker et al., 2005). Very limited sampling because of poor exposure in Colwell Bay suggests a yield comparable to the other sites.
There is no evidence of shape sorting in the TB33 assemblage, Voorhies assemblages 1-3 being present (Voorhies, 1969). Only pelves, vertebrae and ribs are poorly represented and normally broken so taxonomically unidentifiable, but this is judged to result from predation (Vasileiadou, 2006; Vasileiadou et al., 2007a). In particular, the poor representation of mammalian vertebrae cannot be attributed to hydraulic processes as snake, lizard and amphibian vertebrae are common. There is also no size sorting. Large mammals co-occur with the micromammals. Their relative rarity in samples can be attributed to their smaller population size expected per unit area for a large than a small mammal. There are several examples of partial associated skeletal and dental remains. Although now disarticulated, their close proximity to one another, discovered through careful subsampling, together with a very low energy depositional environment, indicates minimal hydraulic transport, between disarticulation and burial. This essentially limits post-mortem transport of the assemblage to that caused by the predators, especially the avian ones. A mortality profile for the most abundant micromammal, Thalerimys fordi, indicates an attritional assemblage (Vasileiadou et al., 2007b). These different lines of evidence categorize the assemblage as a passive accumulation (Badgley, 1986).
Behaviour of the prey. It is difficult to know if all the mammals were living around the depositional environment, although there are strong indications for some. Glamys priscus fed mainly on the seeds of the floating aquatic plant Stratiotes and probably of other plants, whilst T. fordi fed on thick-walled plant tissues, which may have included marginal aquatic plants (Collinson and Hooker, 2000; Grimes et al., 2004). This is strong evidence that G. priscus typically lived close to the margins of freshwater bodies where the plants would have grown. There is the potential that T. fordi did too. Evidence of predation and scavenging from bite marks and gnawings on bones, attributed to the predators Paramiacis sp. and Amphiperatherium species B and to the theridomyid and glirid and possible nyctitheriid scavengers, indicates that they too were living as well as dying at the site of deposition. The likelihood that the relatively rare arboreal omomyids (and possibly apatemyids) were predated by owls suggests that they were not living close to the lake but in a treed habitat some distance away and were introduced into the depositional environment only as corpses by the predators. Even these taxa plus other less well represented ones may well have needed to visit the lake edge to drink, especially in dry periods. They would then have been highly vulnerable to predation.
Water-land interface. If neither the aquatic nor terrestrial assemblages in bed TB33 display evidence of more than minimal hydraulic transport, there is conflict in understanding their co-occurrence. This conflict can be reconciled by invoking periodic changes in water level, credible for a water depth of less than 1 m (Keen, 1975). Thus, the freshwater biota would be living in the lake during a high water level, whilst the mammals would be living and dying there during a drier period when the water retreated, leaving a land area adjacent to the lake. As the bones and teeth of the mammals show only minor degrees of weathering, they must have been covered by sediment relatively rapidly. They must have lain on the exposed land surface long enough for scavengers to attack them but not long enough for them to decompose and in some cases to dissociate. Andrews (1990) recorded that subaerial disarticulation of the skeleton of a small mammal like a vole takes between 12 days (in a hot climate) and 40 days in a temperate climate. Summer freshwater temperatures at the time are estimated to have ranged from 27-32o C (Grimes et al., 2005), indicating a subtropical climate. Moreover, such remains disintegrate further within one year (Voorhies, 1969). This implies that the periodicity of dry and wet periods involving bed TB33 was seasonal.
Surface detail appears to be better preserved, with less scavenging damage, on bones from lower than higher parts of bed TB33, suggesting that, as progradation took place, the edge of the lake retreated and the land area remained for a longer period of the year. The underlying bed TB32 was probably deposited farther out in the lake and rarely dried out. In tentative support of this, an articulated skeleton of the semiaquatic Cryptopithecus major has been found in bed TB32, with no evidence of scavenging.
Such fluctuations in water level have been invoked for similar depositional settings with mixed freshwater and terrestrial biota at other sites. Thus, shallow trough-like lakes with freshwater gastropods, ostracods, and algae, in which peat, now lignite, accumulated at the Middle Eocene site of Geiseltal, Germany, have been interpreted to have dried out during the dry season, when mammals and other terrestrial vertebrates lived and died there, and became buried when the lake waters returned in the rainy season. They have been termed “Leichenfelder”, translated as corpse-strewn fields (Weigelt, 1931; Krumbiegel, 1959, 1975; Krumbiegel et al., 1983; Hellmund, 2018). Mammalian remains are scattered over the entire area as both isolated bones and as articulated skeletons. The Leichenfeld sites differ from bed TB33 in representing a swampy lake with in situ tree stumps preserved (Krumbiegel et al., 1983, figure 22), which has implications for the living situation of the mammals, different from that of TB33.
Similar fluctuations in water level (partly through tectonism) have been invoked for two beds with terrestrial mammals mixed with aquatic plant seeds, charophytes, ostracods, and gastropods in organic-rich sediments in middle Priabonian strata at Zambrana, Spain (Badiola et al., 2009). Here again was a swampy lake margin environment, with no evidence of significant hydraulic transport and with little weathering, suggesting rapid burial. Although observation of breakage types was inhibited by diagenetic crushing, no tooth marks on bones were found, suggesting predation was not the main cause of death. Large mammals are more common than small ones, suggesting that the latter may have been transported in from a short distance away. The assemblage was considered to be attritional (Badiola et al., 2009), although the mortality profile for the ungulates, with more adults than either young or old individuals, is more consistent with a catastrophic accumulation.
The Spanish late Miocene site of Cerro de la Garita is also dominated by large mammals (Pesquero et al., 2013). It is also a lake margin deposit, but alkaline unlike Zambrana, thus more like TB33, and has a similar freshwater biota. All mammal remains consist of isolated teeth and bones, with no shape sorting. Bone fracture, tooth marks, and etching point to predation, although there was little rodent gnawing. Evidence of trampling is an important feature of bone surfaces, but there is little evidence of weathering. The mortality profile of Hipparion concudense indicates an attritional assemblage. Some preferential bone orientation affected the large but not the small mammals, suggesting wave action rather than transport. Thus, this is another example of fluctuating lake water levels, with rapid burial of a passive accumulation. The importance of trampling at the site may reflect the larger average size of ungulates in the Miocene than in the Eocene.
A similar passive process of accumulation was invoked for extensive attritional terrestrial mammal assemblages in tabular grey mudstones of the Early Eocene Willwood Formation, Wyoming, USA (Bown and Kraus, 1981). In this case, the depositional setting is different, consisting of floodplain rather than lake-margin. Accumulation took place subaerially on soil surfaces, which were subsequently buried when riverine flooding introduced sediment across the area.
The three non-UK lacustrine examples summarised above involve depositional environments relatively restricted in area. The areas of the excavations of Zambrana and Cerro de la Garita were no larger than 25 m2. The area of the Geiseltal Leichenfeld was 8000 m2. That of the Willwood overbank mudstones was larger at 2.5 km2. However, Bed TB33 and its lateral equivalents extend linearly for 6 km. Assuming the present coastal exposures do not represent the total outcrop, the four sites subtend an area of c. 9 km2, likely a minimum. This is an unusually large area to yield a consistent abundance of mammalian remains and shows that the fossil assemblage is not a hydraulic concentration but accumulated in a freshwater sedimentary system characterized by seasonal water-level fluctuations, resulting in alternating subaqueous and subaerial environments.
TAPHONOMIC COMPARISON OF BED TB33 WITH BED O3, OSBORNE MEMBER
Predation and Causal Agents
At each level (beds O3 and TB33), a mammal has been identified as a predator of the theridomyids, glirids, herpetotheriids, and nyctitheriids. According to the tooth etching profiles, its digestive juices were weak, producing mainly group 1 etching. A second peak in the etching profiles of the glirids, herpetotheriids, and nyctitheriids at both levels indicates a second mammalian predator with strong digestive juices (Figure 8). In addition, there is evidence of bird predation on some of the larger micromammals. Such evidence is, however, minor in bed O3, as reflected in the greater bone fragmentation compared with bed TB33.
Despite those major similarities, the identity of the main mammalian predator of all four families is different at family level between the two beds. In bed O3 it is the amphicyonid Cynodictis cf. lacustris, whereas it is the “miacid” Paramiacis sp. in bed TB33. C. cf. lacustris was larger than Paramiacis sp., the former approximating the size of an Arctic fox, the latter that of a polecat. The fact that, based on tooth etching, a slightly smaller percentage of theridomyids and nyctitheriids and less than half the percentage of herpetotheriids (glirid percentages are nearly the same) were predated in TB33 than in O3 (Table 7) may reflect the smaller size of Paramiacis sp. However, the small herpetotheriid percentage may be somewhat biased by the large stoat-sized Amphiperatherium species B, which may have been a difficult prey item for Paramiacis. Amphiperatherium species B is not recorded from O3, so a different predator would need to have been responsible for the second etching peak affecting glirids, herpetotheriids, and nyctitheriids in that bed. Perhaps Peratherium cuvieri, which is slightly larger than Amphiperatherium species A and more common there than in TB33, was this additional predator. As in O3, fewer insectivorous mammals were predated than rodents in TB33. Fewer scansorial mammals (Gliridae + Nyctitheriidae) than semiterrestrial mammals (Theridomyidae + Herpetotheriidae) were predated in both levels, but the difference is greater in TB33 than in O3 (Table 7). This is counterintuitive, given that some evidence suggests that Paramiacis had better climbing abilities than Cynodictis, which is shown to have been terrestrial (Fournier et al., 2020). There are fewer bite marks attributable to Paramiacis on bones from TB33 than to Cynodictis from O3. This might be due to a weaker bite from a smaller animal. There are also fewer bones relative to teeth preserved in TB33 than in O3. If this is due to greater predatory destruction, it may reflect the combined effects of Paramiacis and the additional predator Amphiperatherium species B in TB33.
Spatial Relationships
In both beds, land-based mammals are preserved together with an aquatic biota, in a restricted area floodplain pond in O3, in a widespread lake margin environment in TB33. In O3, evidence from a greater degree of weathering and possibly more trampling than in TB33 and higher concentration of remains, points to a longer time of exposure on a land surface, during which time scavenging took place. This exposure was followed by the mammalian remains being washed periodically by riverine flooding into the pond, rather than being buried in place as in TB33. Distance of travel is thought to have been short for the O3 assemblage, except for the pantolestid Cryptopithecus, whose polished bones suggest a more distant origin.
In both beds, arboreal mammals are interpreted as living some distance from their preferred treed habitat. There may have been trees growing on the floodplain near the O3 pond. Although no fossil wood has been found, root traces in the colour-mottled overbank deposits of the Osborne Member (Vasileiadou et al., 2009) are suggestive. For TB33, trees are likely to have been farther from the lake margin. Like TB33, it is probable that there was wet/dry seasonality during deposition of O3, perhaps more intense given the greater weathering of the bones.
Species Richness
The TB33 mammal fauna is considerably richer than that of O3: 39 species for the former versus 23 for the latter (Vasileiadou et al., 2009; Hooker, 2021). This seemed consistent with the oxygen isotope record, which suggested slightly cooler temperatures for O3 than for TB33 (Grimes et al., 2005; Hooker et al., 2009). However, the temperature difference seems less marked than the difference in species richness would suggest. There is no evidence that the difference in species richness is biased by predation or other taphonomic processes. In fact, the predators are fairly similar in the two beds and have produced similar patterns of damage to the bones and teeth in both beds, and there is low to minimal post-mortem transport. In contrast, bias is restricted to loss of particular anatomical parts, like vertebrae, scapulae, and limb bones, not to taxa. It is possible instead that the difference is habitat-related, as fewer micromammalian species might be expected in a floodplain habitat (Andrews et al., 1979; Hayward and Phillipson, 1979).
CONCLUSIONS
Etching on teeth and bones, a high level of bone fragmentation and bite and gnaw marks show that the micromammalian assemblage from bed TB33, Totland Bay Member, UK (early Priabonian) has been much influenced by predation and scavenging. Four key lines of evidence indicate minimal hydraulic transport to the site of burial: 1) the presence of some associated teeth from now missing jaws and associated parts of skeletons, disarticulated but not widely scattered; 2) lack of shape sorting (presence of Voorhies groups 1-3); 3) lack of size sorting; 4) a very low energy depositional environment. Low levels of weathering of the bones show that they were not exposed subaerially for long before burial. Some bite marks on theridomyid rodent bones compare well for size with the main mammalian predator preserved in the assemblage: the small “miacid” carnivoran Paramiacis sp. Other bite marks scale with the herpetotheriid marsupial Amphiperatherium species B. A skewed unimodal plot for cheek tooth etching in the theridomyid Thalerimys fordi, emphasizing low etching groups, suggests a single predator, which the bite marks indicate was Paramiacis sp. Bimodal plots for cheek tooth etching in Gliridae, Herpetotheriidae, and Nyctitheriidae suggest two predators: the low etching group peak from Paramiacis sp., the high etching group peak probably from Amphiperatherium species B. When treated separately from the other herpetotheriids, the proportional distribution of etching groups of Amphiperatherium species B is like that of T. fordi, suggesting the same predator. Paramiacis sp. itself and two arboreal, nocturnal primates (Microchoerus erinaceus and Vectipithex smithorum) show tooth and/or bone associations and different degrees of tooth etching, suggesting predation by two different species of large owl. Gnawing of bones attributable to theridomyids, glirids, and possibly nyctitheriids shows that, together with the predators, members of these families were living as well as dying at the site of deposition. Remains of the arboreal omomyids and apatemyids are likely to have been brought to the site by predators from their treed habitat some distance away. Several teeth of the semiaquatic pantolestid Cryptopithecus major have lost all or nearly all their enamel, suggesting predation by a crocodilian.
Comparison with an assemblage of similar composition from bed O3, Osborne Member (Vasileiadou et al., 2009) shows very little difference from that of bed TB33 in factors influencing its accumulation, despite a different dominant mammalian predator. For bed O3, greater weathering of the bones suggests longer subaerial exposure time, whilst the limited area of the depositional site, a floodplain pond, suggests slightly more hydraulic transport to the burial site.
The association of an autochthonous freshwater assemblage with a land-based one, showing evidence of minimal hydraulic transport to the depositional site, in bed TB33, points to fluctuations in water level. These fluctuations would have been seasonal, given the rapid destruction of small mammal bones when exposed subaerially. Thus, the area would have been land in the dry season and lake margin in the wet season. The taphonomic signature of the TB33 micromammals shows the presence of an attritional assemblage, passively accumulated, that closely reflects the original living community, preserving important elements of its food web.
ACKNOWLEDGEMENTS
This study is part of a PhD project by KV, funded by Royal Holloway University of London and the Natural History Museum, London (CASE partner). We thank: the National Trust and J. and M. Smith for site access; A.P. Currant, A.R. and the late A.C. Milner, H. Pain, N.P. Sille, and D.J. and A. Ward for help with fieldwork; S. Gibbons, S.T. Grimes, L. Heath, A.G. Lawson, E.S. Lindars, C. Plewes, P. Schreve, and D.J. Ward for help with sample processing or sorting; B. Engesser (Naturhistorisches Museum Basel), E. Heizmann (Staatliches Museum für Naturkunde, Stuttgart), and P. Tassy (Muséum National d’Histoire Naturelle, Paris) for access to collections in their care; A. Ball, I. Clatworthy, and B. Williamson (NHM) for help with use of the scanning electron microscope; and P. Andrews, J. Cooper, Y. Fernandez-Jalvo, L. Kunzmann, F. Laudet, D. Schreve, N.D. Sheldon, David Siveter, S. Walsh, J. Williams, and the late D. Yalden for helpful discussion. The comments of two anonymous referees have improved the paper.
REFERENCES
Andrews, P. 1990. Owls, Caves and Fossils: predation, preservation and accumulation of small mammal bones in caves, with analysis of the Pleistocene cave faunas from Westbury-sub-Mendip, Somerset, UK. British Museum (Natural History), London.
Andrews, P., Lord, J.M., and Evans, E.M.N. 1979. Patterns of ecological diversity in fossil and modern mammalian faunas. Biological Journal of the Linnean Society, 11:177-205. https://doi.org/10.1111/j.1095-8312.1979.tb00034.x
Andrews, P. and Fernandez-Jalvo, Y. 1997. Surface modifications of the Sima de los Huesos fossil humans. Journal of Human Evolution, 33:191-217. https://doi.org/10.1006/jhev.1997.0137
Badgley, C. 1986. Counting individuals in mammalian fossil assemblages from fluvial environments. Palaios, 1:328-338. https://doi.org/10.2307/3514695
Badiola, A., Berreteaga, A., Pereda-Superbiola, X., Elorza, J., Astibia, H., and Etxebarria, N. 2009. Taphonomy of vertebrate fossil assemblages from swampy circum-lake environments: an example from the Late Eocene of Zambrana (Iberian Peninsula). Palaios, 24:522-534. https://doi.org/10.2110/palo.2008.p08-126r
Blake, J.F. 1881. On a continuous section of the Oligocene strata from Colwell Bay to Headon Hill. Proceedings of the Geologists’ Association, 7:151-161. https://doi.org/10.1016/S0016-7878(81)80043-0
Bosma, A.A. 1974. Rodent biostratigraphy of the Eocene-Oligocene transitional strata of the Isle of Wight. Utrecht Micropaleontological Bulletins Special Publication, 1:1-127. https://dspace.library.uu.nl/handle/1874/205780
Bown, T.M. and Kraus, M.J. 1981. Vertebrate fossil-bearing paleosol units (Willwood Formation, lower Eocene, northwest Wyoming, U.S.A.): implications for taphonomy, biostratigraphy, and assemblage analysis. Palaeogeography, Palaeoclimatology, Palaeoecology, 34:31-56. https://doi.org/10.1016/0031-0182(81)90057-2
Collinson, M.E. and Hooker, J.J. 2000. Gnaw marks on Eocene seeds: evidence for early rodent behaviour. Palaeogeography, Palaeoclimatology, Palaeoecology, 157:127-149. https://doi.org/10.1016/S0031-0182(99)00158-3
Cray, P.E. 1973. Marsupialia, Insectivora, Primates, Creodonta and Carnivora from the Headon Beds (Upper Eocene) of southern England. Bulletin of the British Museum (Natural History), Geology, 23:1-102, pls 1-6.
https://www.biodiversitylibrary.org/item/113709#page/10/mode/1up
Daley, B. 1972. Macroinvertebrate assemblages from the Bembridge Marls (Oligocene) of the Isle of Wight, England, and their environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology, 11:11-31. https://doi.org/10.1016/0031-0182(72)90035-1
Daley, B. 1999. Palaeogene sections in the Isle of Wight. A revision of their description and significance in the light of research undertaken over recent decades. Tertiary Research, 19:1-69.
Daley, B. and Edwards, N. 1974. Week-end field meeting: The Upper Eocene-Lower Oligocene beds of the Isle of Wight: 6-8 October 1972. Proceedings of the Geologists’ Association, 85:281-292. https://doi.org/10.1016/S0016-7878(74)80028-3
de Carvalho, R.F., Passos, D.C., and Lessa, L.G. 2019. Diet variations in short-tailed opossum Monodelphis domestica (Didelphimorphia, Didelphidae) due to seasonal and intersexual factors. Mastozoologia Neotropical, 26:340-348.
https://www.redalyc.org/journal/457/45763089014/html/
Dodson, P. and Wexlar, D. 1979. Taphonomic investigations of owl pellets. Paleobiology, 5:275-284. https://doi.org/10.1017/S0094837300006564
Dominguez-Rodrigo, M. and Piqueras, A. 2003. The use of tooth pits to identify carnivore taxa in tooth-marked archaeofaunas and their relevance to reconstruct hominid carcass processing behaviours. Journal of Archaeological Science, 30:1385-1391.
https://doi.org/10.1016/S0305-4403(03)00027-X
Edwards, N. and Daley, B. 1997. Stratigraphy of the Totland Bay Member (Headon Hill Formation, Late Eocene) at Hordle Cliff, Hampshire, southern England. Tertiary Research, 18:35-50.
Ekrt, B., Mikuláš, R., Wagner, J., Čermák, S., Procházková, K., Kadlecová, E., and Fejfar, O. 2016. New contribution to the palaeoichnology and taphonomy of the Ahníkov fossil site, early Miocene, Most Basin (the Czech Republic). Fossil Imprint, 72:202-214. http://fi.nm.cz/en/clanek/new-contribution-to-the-palaeoichnology-and-taphonomy-of-the-ahnikov-fossil-site-early-miocene-most-basin-the-czech-republic-2/
Fejfar, O. and Kaiser, T.M. 2005. Insect bone-modification and palaeoecology of Oligocene mammal-bearing sites in the Doupov Mountains, northwestern Bohemia. Palaeontologia Electronica, 8.1.8A:11p. https://palaeo-electronica.org/2005_1/fejfar8/issue1_05.htm
Fernández-Jalvo, Y. and Andrews, P. 2016. Atlas of Taphonomic Identifications. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7432-1
Fisher, D.C. 1981. Crocodilian scatology, microvertebrate concentrations, and enamel-less teeth. Paleobiology, 7:262-275. https://www.jstor.org/stable/2400479
Fournier, M., Ladevèze, S., Le Verger, L., Fischer, V., Speijer, R.P., and Solé, F. 2020. On the morphology of the astragalus and calcaneus of the amphicyonids (Carnivora, Mammalia) from the Paleogene of Europe: implications for the ecology of the European bear-dogs. Geodiversitas, 42(18):305-325. https://doi.org/10.5252/geodiversitas2020v42a18
Gale, A.S., Jeffery, P.A., Huggett, J.M., and Connelly, P. 1999. Eocene inversion history of the Sandown Pericline, Isle of Wight, southern England. Journal of the Geological Society, London, 156:327-339. https://doi.org/10.1144/gsjgs.156.2.0327
Gale, A.S., Huggett, J.M., Pälike, H., Laurie, E., Hailwood, E.A., and Hardenbol, J. 2006. Correlation of Eocene-Oligocene marine and continental records: orbital cyclicity, magnetostratigraphy and sequence stratigraphy of the Solent Group, Isle of Wight, UK. Journal of the Geological Society, London, 163:401-415.
https://doi.org/10.1144/0016-764903-175
Grimes, S.T., Mattey, D.P., Hooker, J.J., and Collinson, M.E. 2003. Paleogene paleoclimate reconstruction using oxygen isotopes from land and freshwater organisms: the use of multiple paleoproxies. Geochimica et Cosmochimica Acta, 67:4033-4047.
https://doi.org/10.1016/S0016-7037(03)00173-X
Grimes, S.T., Collinson, M.E., Hooker, J.J., Mattey, D.P., Grassineau, N.V., and Lowry, D. 2004. Distinguishing the diets of coexisting fossil theridomyid and glirid rodents using carbon isotopes. Palaeogeography, Palaeoclimatology, Palaeoecology, 208:103-119.
https://doi.org/10.1016/j.palaeo.2004.02.031
Grimes, S.T., Hooker, J.J., Collinson, M.E., and Mattey, D.P. 2005. Summer temperatures of late Eocene to early Oligocene freshwaters. Geology, 33:189-192. https://doi.org/10.1130/G21019.1
Hayward, G.F. and Phillipson, J. 1979. Community structure and functional role of small mammals in ecosystems, p. 135-211. In Stoddart, D.M. (ed.), Ecology of small mammals. Chapman and Hall, London. https://doi.org/10.1007/978-94-009-5772-5_4
Hellmund, M. 2018. The former Geiseltal Museum (1934-2011), the Eocene Geiseltal Fossilagerstätte (Germany) and the scientific meaning of Ben Barnes as a pioneer of systematic quantitative vertebrate excavations in the Geiseltal lignites. Anuário do Instituto de Geociências, UFRJ, 41:108-119. https://doi.org/10.11137/2018_1_108_119
Hoffman, R. 1988. The contribution of raptorial birds to patterning in small mammal assemblages. Paleobiology, 14:81-90. https://doi.org/10.1017/S0094837300011817
Hooker, J.J. 1987. Mammalian faunal events in the English Hampshire Basin (late Eocene - early Oligocene) and their application to European biostratigraphy. Münchner Geowissenschaftliche Abhandlungen, A, 10:109-116.
Hooker, J.J. 2014. New postcranial bones of the extinct mammalian family Nyctitheriidae (Paleogene, UK): primitive euarchontans with scansorial locomotion. Palaeontologia Electronica, 17.3.47A:1-82. https://doi.org/10.26879/482
Hooker, J.J. 2021. The mammals of the Late Eocene - Early Oligocene Solent Group. Part 1, introduction and Euarchonta: Nyctitheriidae. Monographs of the Palaeontographical Society, Publication No. 659, issued as part of Volume 175 for 2021, 1-147, pls 1-48. https://doi.org/10.1080/02693445.2021.1928440
Hooker, J.J., Cook, E., and Benton, M.J. 2005. British Tertiary fossil mammal GCR sites, p. 67-124. In Benton, M.J., Cook, E., and Hooker, J.J. (eds). Mesozoic and Tertiary Fossil Mammals and Birds of Great Britain. Geological Conservation Review Series, 32, Joint Nature Conservation Committee, Peterborough.
Hooker, J.J. and Harrison, D.L. 2008. A new clade of omomyid primates from the European Paleogene. Journal of Vertebrate Paleontology, 28:826-840. https://doi.org/10.1671/0272-4634(2008)28[826:ANCOOP]2.0.CO;2
Hooker, J.J., Grimes, S.T., Mattey, D.P., Collinson, M.E., and Sheldon, N.D. 2009. Refined correlation of the UK Late Eocene-Early Oligocene Solent Group and timing of its climate history. In Koeberl, C. and Montanari, A. (eds.), The late Eocene earth - hothouse, icehouse and impacts. Geological Society of America Special Paper, 452:179-195.
https://doi.org/10.1130/2009.2452(12)
Keen, M.C. 1975. The palaeobiology of some upper Palaeogene fresh-water ostracods. Bulletin of American Paleontology, 65:271-283.
https://www.biodiversitylibrary.org/item/92679#page/7/mode/1up
Keen, M.C. 1977. Ostracod assemblages and the depositional environments of the Headon, Osborne, and Bembridge Beds (upper Eocene) of the Hampshire Basin. Palaeontology, 20:405-445.
https://www.palass.org/publications/palaeontology-journal/archive/20/2/article_pp405-445
Keeping, H. and Tawney, E.B. 1881. On the beds at Headon Hill and Colwell Bay in the Isle of Wight. Quarterly Journal of the Geological Society of London, 37:85-127, pl. 5.
King, C. 2016. A revised correlation of Tertiary rocks in the British Isles and adjacent areas of NW Europe. Edited by Gale, A.S. and Barry, T.L. Geological Society Special Report, 27:1-719.
Klembara, J. and Green, B. 2010. Anguimorph lizards (Squamata, Anguimorpha) from the Middle and Late Eocene of the Hampshire Basin of southern England. Journal of Systematic Palaeontology, 8:97-129. https://doi.org/10.1080/14772011003603531
Koenigswald, W.v. 1980. Das Skelett eines Pantolestiden (Proteutheria, Mamm.) aus dem mittleren Eozän von Messel bei Darmstadt. Paläontologisches Zeitschrift, 54:267-287. https://doi.org/10.1007/BF02988130
Koenigswald, W.v. and Storch, G. 1988. Messeler Beuteltiere - unauffällige Beutelratten., p. 155-158. In Schaal, S. and Ziegler, W. (eds.), Messel - ein Schaufenster in die Geschichte der Erde und des Lebens. Waldemar Kramer, Frankfurt am Main.
Korth, W.W. 1979. Taphonomy of microvertebrate fossil assemblages. Annals of Carnegie Museum, 48:235-285. https://www.biodiversitylibrary.org/item/217391#page/263/mode/1up
Krumbiegel, G. 1959. Die tertiären Pflanzen- und Tierwelt der Braunkohle des Geiseltales. Die neue Brehm-Bücherei, 237, A. Ziemsen, Wittenberg.
Krumbiegel, G. 1975. Zur Palӧkologie der tertiären Fossilfundstellen des Geiseltales. Hercynia, NF, 12:400-417.
Krumbiegel, G., Rüffle, L., and Haubold, H. 1983. Das eozäne Geiseltal, ein mitteleuropäisches Braunkohlenvorkommen. Die neue Brehm-Bücherei, 237, A. Ziemsen, Wittenberg.
Laudet, F. and Selva, N. 2005. Ravens as small mammal bone accumulators: first taphonomic study on mammal remains in raven pellets. Palaeogeography, Palaeoclimatology, Palaeoecology, 226:272-286. https://doi.org/10.1016/j.palaeo.2005.05.015
Lyman, R.L. 1994. Vertebrate Taphonomy. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9781139878302
Mikuláš, R., Kadlecová, E., Fejfar, O., and Dvořák, Z. 2006. Three new ichnogenera of biting and gnawing traces on reptilian and mammalian bones: a case study from the Miocene of the Czech Republic. Ichnos, 13:113-127. https://doi.org/10.1080/10420940600850729
Newell, A.J. and Evans, D.J. 2011. Timing of basin inversion on the Isle of Wight: new evidence from geophysical log correlation, seismic sections and lateral facies change in the Palaeogene Headon Hill Formation. Proceedings of the Geologists’ Association, 122:868-882. https://doi.org/10.1016/j.pgeola.2011.04.004
Pesquero, M.D., Alcalá, L., and Fernández-Jalvo, Y. 2013. Taphonomy of the reference Miocene vertebrate mammal site of Cerro de la Garíta, Spain. Lethaia, 46:378-398. https://doi.org/10.1111/let.12016
Pickering, T.R., Dominguez-Rodrigo, M., Egerland, C.P., and Brain, C.K. 2004. Beyond leopards: tooth marks and the contribution of multiple carnivore taxa to the accumulation of the Swartkrans Member 3 fossil assemblage. Journal of Human Evolution, 46:595-604. https://doi.org/10.1016/j.jhevol.2004.03.002
Platt, N.H. and Wright, V.P. 1991. Lacustrine carbonates: facies models, facies distributions and hydrocarbon aspects. In Anadón, P., Cabrera, Ll., and Kelts, K. (eds.), Lacustrine Facies Analysis. Special Publications of the International Association of Sedimentologists, 13:57-74. https://doi.org/10.1002/9781444303919.ch3
Plint, A.G. 1984. A regressive coastal sequence from the Upper Eocene of Hampshire, southern England. Sedimentology, 31:213-225. https://doi.org/10.1111/j.1365-3091.1984.tb01960.x
Rage, J.-C. and Ford, R.L.E. 1980. Amphibians and squamates from the Upper Eocene of the Isle of Wight. Tertiary Research, 3:47-60.
Ramdarshan, A. and Orliac, M.J. 2016. Endocranial morphology of Microchoerus erinaceus (Euprimates, Tarsiiformes) and early evolution of the Euprimates brain. American Journal of Physical Anthropology, 159:5-16. https://doi.org/10.1002/ajpa.22868
Riveline, J. 1984. Les gisements à charophytes du Cénozoique (Danien au Burdigalien) d’Europe occidentale. Lithostratigraphie, biostratigraphie, chronostratigraphie. Bulletin d’Information des Géologues du Bassin de Paris, Mémoire hors série, 4:1-523.
Rose, K.D., Chew, A.E., Dunn, R.H., Kraus, M.J., Fricke, H.C., and Zack, S.P. 2012. Earliest Eocene mammalian fauna from the Paleocene-Eocene Thermal Maximum at Sand Creek Divide, southern Bighorn Basin, Wyoming. University of Michigan Papers on Paleontology, 36:i-ix,1-122. https://hdl.handle.net/2027.42/89881
Schmidt-Kittler, N. and Storch, G. 1985. Ein vollständiges Theridomyiden-Skelett (Mammalia: Rodentia) mir Rennmaus-Anpassungen aus dem Oligozän von Céreste, S-Frankreich. Senckenbergiana Lethaea, 66:89-109.
Speijer, R.P., Pälike, H., Hollis, C.J., Hooker, J.J., and Ogg, J.G. 2020. The Paleogene Period, p. 1087-1140. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. (eds.), Geologic Time Scale 2020. Elsevier, Amsterdam.
Stinton, F.C. 1971. Easter field meeting in the Isle of Wight. Proceedings of the Geologists’ Association, 82:403-410. https://doi.org/10.1016/S0016-7878(71)80018-4
Tawney, E.B. and Keeping, H. 1883. On the section at Hordwell Cliffs, from the top of the Lower Headon to the base of the Upper Bagshot Sands. Quarterly Journal of the Geological Society of London, 39:566-574, 1 fig.
Vasileiadou, K. 2006. Micromammalian assemblages from the Late Eocene of southern England: accumulation history and palaeocommunity reconstruction. Unpublished PhD thesis, University of London, UK.
Vasileiadou, K., Hooker, J.J., and Collinson, M.E. 2007a. Taphonomic evidence of a Paleogene mammalian predator-prey interaction. Palaeogeography, Palaeoclimatology, Palaeoecology, 243:1-22. https://doi.org/10.1016/j.palaeo.2006.07.001
Vasileiadou, K., Hooker, J.J., and Collinson, M.E. 2007b. Quantification and age structure of semi-hypsodont extinct rodent populations. Journal of Taphonomy, 5:15-41.
Vasileiadou, K., Hooker, J.J., and Collinson, M.E. 2009. Paleocommunity reconstruction and accumulation of micromammalian remains (Late Eocene, southern England). Palaios, 24:553-567. https://doi.org/10.2110/palo.2008.P08-035r
Vianey-Liaud, M., Hautier, L., and Marivaux, L. 2015. Morphological disparity of the postcranial skeleton in rodents and its implications for palaeobiological inferences: the case of the extinct Theridomyidae (Rodentia, Mammalia), p. 539-588. In Cox, P.G. and Hautier, L. (eds.), Evolution of the Rodents. Advances in phylogeny, functional morphology and development. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9781107360150.021
Voorhies, M.R. 1969. Taphonomy and population dynamics of an early Pliocene vertebrate fauna, Knox County, Nebraska. Contributions to Geology, University of Wyoming, 1:1-69. https://doi.org/10.2113/gsrocky.8.special_paper_1.1
Weigelt, J. 1931. Über ein Leichenfeld in der Mittelkohle der Braunkohlengrube Cecilie im Geiseltal (Mitteleozän). Palaeobiologica, 4:49-78, 1 pl. https://www.zobodat.at/search.php?q=%C3%9Cber+ein+Leichenfeld+in+der+Mittelkohle+der+Braunkohlengrube+Cecilie+im+Geiseltal+%28Mitteleoz%C3%A4n%29.+

