 A Miocene cetacean vertebra showing a partially healed longitudinal shear-compression fracture, possibly the result of domoic acid toxicity or failed predation
A Miocene cetacean vertebra showing a partially healed longitudinal shear-compression fracture, possibly the result of domoic acid toxicity or failed predation
Article number: 25.3.a28
https://doi.org/10.26879/1171
Copyright Society of Vertebrate Paleontology, September 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 11 June 2021. Acceptance: 25 August 2022.
ABSTRACT
CT-scans of a cetacean pathological vertebra from the Calvert Formation of the Miocene Chesapeake Group of Maryland, show features characteristic of a shear-compression fracture with comminution and significant periosteal reaction. The etiology of the injury suggests an intense hyperflexion of vertebrae in at least the lumbar region of the axial column. The trauma was sufficiently forceful to break much of the lower two-fifths of the centrum away from the anterior end of the body of the vertebra. However, the trauma was not immediately fatal as significant fusion of fragmented elements was well underway at the time of death. Much of the lateral and ventral surfaces of the centrum are covered with a thick layer of periosteal reactive bone. This reactive periosteal bone growth could be due to spondyloarthritis, infection, or from the traumatic event itself, if the direct muscle attachments on the vertebra were avulsed.
A single megatoothed shark tooth from Otodus megalodon was found with the vertebra. It is not known if the tooth came to be there serendipitously, or if it was associated because it was lost as a result of the possible originating failed predation event, or during a final successful predation or subsequent scavenging event.
The fractures are severe and unlikely to have had an endogenous origin like convulsions, seizures, or spasms. Seizures can cause vertebral fractures in humans, including elderly adults with poor bone health as well as physically fit younger individuals. Seizures causing injuries of this magnitude have not been observed in cetaceans, though domoic acid toxicity from harmful algal blooms are known to cause seizures in cetaceans, and are implicated in the deaths of neonatal skim-feeding mysticetes. It is unlikely, but possible, that a large mysticete would be affected by domoic acid toxicity to the point of a spinal fracture-causing seizure. Similarly, protozoal infections are known to cause seizures in cetaceans, though physical diagnosis of this is impossible in a fossil. Partly healed bone fractures of the face from possible collisions with the seafloor have been reported from fossil mysticetes of shallower regions of this fauna, but a spinal fracture this far back in the spinal column seems unlikely to be the result of a seafloor collision. Even though the cause of the vertebral hyperflexion and resulting trauma is unknown, a plausible cause was a crushing ambush delivered by a macropredatory shark or macroraptorial physeteroid. In spite of extant cetaceans being subjected to anthropogenically-induced trauma, which include vessel-strike blunt force injuries of many different kinds, shear-compression fractures and periosteal reactions like the ones detailed here have not yet been reported in extant cetaceans. Therefore, we consider the fracture as likely due to an impact from a predator, such as Otodus megalodon, or possibly from seizures due to a harmful algal bloom and resulting domoic acid toxicity. In either scenario, the cetacean survived.
Stephen J. Godfrey. Department of Paleontology, Calvert Marine Museum, P.O. Box 97, Solomons, Maryland, 20688, U.S.A. and Research Associate, National Museum of Natural History, The Smithsonian Institution, Washington, D.C., U.S.A. (correspondence author) Stephen.Godfrey@calvertcountymd.gov
Brian L. Beatty. Research Associate, National Museum of Natural History, The Smithsonian Institution, Washington, D.C., U.S.A. and New York College of Osteopathic Medicine, Northern Boulevard, PO Box 8000, Old Westbury, New York 11568. bbeatty@nyit.edu
Keywords: shear-compression fracture; cetacean vertebrae; seizure; harmful algal bloom; failed predation; Otodus megalodon; Calvert Cliffs; Miocene
Final citation: Godfrey, Stephen J. and Beatty, Brian L. 2022. A Miocene cetacean vertebra showing a partially healed longitudinal shear-compression fracture, possibly the result of domoic acid toxicity or failed predation. Palaeontologia Electronica, 25(3):a28. https://doi.org/10.26879/1171
palaeo-electronica.org/content/2022/3694-a-shear-compression-fracture
INTRODUCTION
Although a variety of vertebral column malformations have been documented in cetaceans (Alstrup et al., 2013; Beatty and Rothschild, 2008; Berghan and Visser, 2000; Bertulli et al., 2015; Costa et al., 2016; Hellier et al., 2011; Félix et al., 2007; Kompanje, 1999 and the references therein), reports of osteological trauma akin to shear-compression fractures are exceedingly rare (Watson et al., 2004; Godfrey and Altman, 2005; and possibly Thomas et al., 2008, fig. 2B LACM 151218). In this case study, we describe two associated Miocene cetacean vertebrae displaying extreme periosteal reactions and one of which preserves evidence of a major shear-compression fracture with comminution. Both were collected from Miocene-age siliciclastic sediments cropping out in sea bluffs known as Calvert Cliffs. These naturally eroding cliffs extend intermittently for approximately 50 km along the western shore of Chesapeake Bay, Maryland, U.S.A. and form the most complete sequence of Miocene marine sediments exposed on the East Coast of North America. These sediments preserve one of the most diverse assemblages of extinct cetaceans known. Over 35 named species of both odontocetes (toothed whales) and mysticetes (baleen whales) of this taxonomically diverse fauna were reviewed most recently by Gottfried et al. (1994). The skeletal remains of cetaceans are amongst the most common vertebrate fossils that erode from the cliffs. Although it is not unusual to recover fossilized tooth-marked cetacean bone (usually evidence of shark predation/scavenging (Godfrey et al., 2018; Godfrey and Lowry, 2021)), pathological elements are not as common (Kellogg, 1965, figs. 17 and 19; Kellogg, 1969, plates 6 and 8; Dawson and Gottfried, 2002; Godfrey and Altman, 2005; Gerholdt and Godfrey, 2009; Nance et al., 2017).
The pathological cetacean vertebrae described herein (CMM-V-10108) are sufficiently unique to warrant this case study. Damage to the vertebrae was so severe that the cause was possibly extracorporeal, like an impact from a conspecific, or a failed predatory attack by a macropredatory shark or a macroraptorial physeteroid. Alternatively, they may have come about endogenously (although caused indirectly by an external agent), the result of a massive seizure from domoic acid poisoning resulting from a harmful algal bloom.
MATERIALS AND METHODS
Two associated pathological lumbar vertebrae (CMM-V-10108) from a Miocene cetacean, and one Otodus megalodon tooth (CMM-V-8522) were found together by Mike Ellwood and recovered from Shattuck-Zone (SZ) 12, 36 m north of E-Gate (in the Scientists Cliffs community) at Warrior’s Rest Sanctuary, along Calvert Cliffs, Calvert County, Maryland (Figure 1 ). GPS coordinates for where the specimens were found are N38º31’38.08”, W76º30’54.86”. Sediments within SZ 12 were deposited approximately 15 Ma (Perez et al., 2019, fig. 1). Mr. Ellwood prepared the vertebrae and tooth by removing the entombing sediment with dental scalers and brushes. Although no consolidant was applied to the bones or tooth, some PaleoBOND was used to reattach loose fragments back onto the body of the vertebra preserving the shear-compression fracture.
). GPS coordinates for where the specimens were found are N38º31’38.08”, W76º30’54.86”. Sediments within SZ 12 were deposited approximately 15 Ma (Perez et al., 2019, fig. 1). Mr. Ellwood prepared the vertebrae and tooth by removing the entombing sediment with dental scalers and brushes. Although no consolidant was applied to the bones or tooth, some PaleoBOND was used to reattach loose fragments back onto the body of the vertebra preserving the shear-compression fracture.
The vertebrae were CT-scanned on a General Electric Optima CT600 computed tomography (= CT) scanner at the Calvert Memorial Hospital, Prince Frederick, Maryland. One mm helical CT-scans in the transverse plane of the vertebrae were taken at 120 kV and 200 mA.
The two vertebrae and the Otodus megalodon tooth were photographed on black velvet under fluorescent light using a Nikon CoolPix P510 camera. The photos (including the CT-scans) were edited in Adobe Photoshop® and final images compiled in Adobe Illustrator®.
Abbreviations: CMM-V-, Calvert Marine Museum Vertebrate paleontology collection, Solomons, MD, USA; LACM, Natural History Museum of Los Angeles County, Los Angeles, CA, USA; Ma, Mega-annum; SZ, Shattuck-Zone; USNM, United Stated National Museum, the Smithsonian Institution, Washington, D.C., USA.
GEOLOGY
The two pathological vertebrae and Otodus megalodon tooth were found in SZ 12 of the Plum Point Member of the Calvert Formation (Langhian), Middle Miocene Chesapeake Group. Shattuck-Zone 12 is only on average about 50 cm thick. In terms of number of vertebrate fossils found, SZ 12 appears to preserve more shark teeth and dolphin skulls than any other bed along Calvert Cliffs (S.J.G. pers. obs.; Visaggi and Godfrey, 2010), which is why it is referred to as the “Shattuck-Zone 12 bone bed” by Kidwell et al. (2015). Although it is referred to as a bone bed, vertebrate specimens are widely scattered. Aragonitic shell material—mostly specimens of Chione parkeria and articulated, life-positioned Glossus fraterna—is poorly preserved (Kidwell et al., 2015). Much of this fossiliferous bed is heavily burrowed, with burrows filled with greenish-gray clay and/or silt from the overlying SZ 13 (Kidwell et al., 2015). Shattuck-Zone 12 is unique along Calvert Cliffs in that it is, apparently, the only bed characterized by prolonged sediment starvation with ~zero net siliciclastic accumulation. This took place on the ~outer shelf during maximum transgression, allowing marine vertebrate material to become relatively concentrated without high-energy reworking (Kidwell, 1984, 1989).
DESCRIPTION
The Pathological Vertebrae
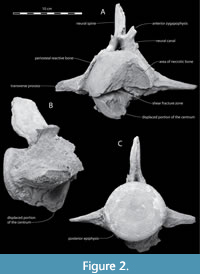 In spite of the fact that these vertebrae were found together, we do not know if they were juxtaposed in life or their positional order within the axial column. The epiphyses are firmly ankylosed to the centra; an indication that these two vertebrae were derived from a mature individual. The following features suggest that these vertebrae (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6) originated from the posterior lumbar region of the axial column: 1) the centra are not compressed anteroposteriorly as in Miocene cetacean cervical vertebrae; 2) neither is there any development of prominent ventrolateral protuberant parapophyses as would be present in the cervical series; 3) the neural arch preserved in the fractured vertebra (the neural arch is not preserved in the second vertebra) does not extend posterior to the plane formed by the posterior
In spite of the fact that these vertebrae were found together, we do not know if they were juxtaposed in life or their positional order within the axial column. The epiphyses are firmly ankylosed to the centra; an indication that these two vertebrae were derived from a mature individual. The following features suggest that these vertebrae (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6) originated from the posterior lumbar region of the axial column: 1) the centra are not compressed anteroposteriorly as in Miocene cetacean cervical vertebrae; 2) neither is there any development of prominent ventrolateral protuberant parapophyses as would be present in the cervical series; 3) the neural arch preserved in the fractured vertebra (the neural arch is not preserved in the second vertebra) does not extend posterior to the plane formed by the posterior  epiphysis; 4) as preserved, the neural arch is only about as high as the centrum is deep; 5) there are no postzygapophyses as would be present in cervical and most thoracic vertebrae; 6) the neural canal is reduced in size; 7) the transverse processes are low on the centrum (they extend laterally from approximately the midpoint in the height of the centrum) as would be present on lumbar and anterior caudal vertebrae; 8) each transverse process is as long as the neural arch is high; 9) there is no facet on the distal end of the transverse process to accommodate the head of a rib; and 10) there are no facets on the ventral side of the centra to accommodate the haemal arches as would be present on at least anterior caudal vertebrae.
epiphysis; 4) as preserved, the neural arch is only about as high as the centrum is deep; 5) there are no postzygapophyses as would be present in cervical and most thoracic vertebrae; 6) the neural canal is reduced in size; 7) the transverse processes are low on the centrum (they extend laterally from approximately the midpoint in the height of the centrum) as would be present on lumbar and anterior caudal vertebrae; 8) each transverse process is as long as the neural arch is high; 9) there is no facet on the distal end of the transverse process to accommodate the head of a rib; and 10) there are no facets on the ventral side of the centra to accommodate the haemal arches as would be present on at least anterior caudal vertebrae.
 The fractured vertebra (Figure 2, Figure 3, Figure 4) displays an intact and smooth nearly circular posterior epiphyseal surface that is 90 mm high by 95 mm wide. The length of the centrum is 110 mm. The height of the vertebra from the tip of the neural spine to the lower margin of the posterior epiphysis is 186 mm. The top of the neural spine is coarsely textured and may not be complete. The minimum anteroposterior length of the pedicle of the neural arch is 53 mm. The vertebra is 249.5 mm wide across its incomplete transverse processes. The anteroposterior horizontal dimension of the neural spine at the level of the top of the anterior zygapophysis is 63 mm.
The fractured vertebra (Figure 2, Figure 3, Figure 4) displays an intact and smooth nearly circular posterior epiphyseal surface that is 90 mm high by 95 mm wide. The length of the centrum is 110 mm. The height of the vertebra from the tip of the neural spine to the lower margin of the posterior epiphysis is 186 mm. The top of the neural spine is coarsely textured and may not be complete. The minimum anteroposterior length of the pedicle of the neural arch is 53 mm. The vertebra is 249.5 mm wide across its incomplete transverse processes. The anteroposterior horizontal dimension of the neural spine at the level of the top of the anterior zygapophysis is 63 mm.
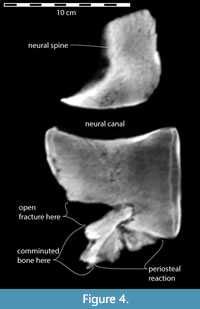
 On the anterior face of the vertebra, a deep horizontal fracture extends the full width of the centrum (Figure 2A, Figure 3A, Figure 4). The wide-open v-shaped fracture is evidence that a significant portion of the lower two-fifths of the centrum was broken away from the main body of the vertebra. A CT-scan image (Figure 4) in sagittal section shows that the lower anterior portion of the centrum, including its epiphysis, was displaced ventrally and its posterior extremity was forcefully telescoped posteriorly into the body of the centrum, forming a sequestrum. Consequently, the anterior end of the fracture gapes widely open (Figure 2A, Figure 3, and Figure 4). Figure 3 and Figure 4 also show that subsequent to the shear-compression fracture, new bone growth (proliferative periosteal reaction) on the lower and lateral surfaces of the centrum fixed the fragments in their displaced positions. This same reactive bone also ornaments the upper surface of the centrum and on both upper and lower sides of the transverse processes (Figure 3).
On the anterior face of the vertebra, a deep horizontal fracture extends the full width of the centrum (Figure 2A, Figure 3A, Figure 4). The wide-open v-shaped fracture is evidence that a significant portion of the lower two-fifths of the centrum was broken away from the main body of the vertebra. A CT-scan image (Figure 4) in sagittal section shows that the lower anterior portion of the centrum, including its epiphysis, was displaced ventrally and its posterior extremity was forcefully telescoped posteriorly into the body of the centrum, forming a sequestrum. Consequently, the anterior end of the fracture gapes widely open (Figure 2A, Figure 3, and Figure 4). Figure 3 and Figure 4 also show that subsequent to the shear-compression fracture, new bone growth (proliferative periosteal reaction) on the lower and lateral surfaces of the centrum fixed the fragments in their displaced positions. This same reactive bone also ornaments the upper surface of the centrum and on both upper and lower sides of the transverse processes (Figure 3).
 Half of the upper face of the anterior epiphysis and centrum is further marked by a conspicuous concave excavation approximately 35 mm deep (Figure 2A). The concave surface is fairly smooth, suggesting that the bone that once occupied this lesion became necrotic and was resorbed premortem. There is no evidence of new bone growth in this region of the vertebra. In life, this depression may have been filled with fibrous connective tissue that did not preserve, which at the time of the cetacean's death had not been invaded by osteocytes.
Half of the upper face of the anterior epiphysis and centrum is further marked by a conspicuous concave excavation approximately 35 mm deep (Figure 2A). The concave surface is fairly smooth, suggesting that the bone that once occupied this lesion became necrotic and was resorbed premortem. There is no evidence of new bone growth in this region of the vertebra. In life, this depression may have been filled with fibrous connective tissue that did not preserve, which at the time of the cetacean's death had not been invaded by osteocytes.
The centrum of the second associated vertebra of CMM-V-10108 is complete lengthwise (105 mm); however, its transverse processes and neural arch are not complete (Figure 5 and Figure 6). CT-scan images of this vertebra (Figure 6) show that it, too, had thick periosteal reactive bone covering most of its preserved external surface (with the exception of its epiphyses). In Figure 6A, there is a subcircular void within the periosteal reactive bone.
The Associated Otodus megalodon Tooth
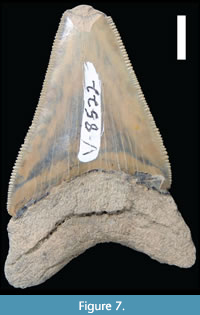 A single Otodus megalodon tooth (CMM-V-8522, Figure 7) was found touching one of the vertebrae. It was not embedded in the vertebra, and we did not observe any shark bite marks on the vertebrae. The tooth is a lower anterior that measures 57.5 mm in vertical height, although, the tip of the tooth is missing because of a conspicuous spall fracture labially. The crown height is 44 mm, and the crown width is 34.5 mm. Using the equations devised by Perez et al. (2021) to calculate the body length of O. megalodon based on the crown width of a single tooth, this tooth would have come from a shark with a body length between 6 and 7 m (Victor Perez, pers. comm.). Although this tooth is a lower tooth, from which less accurate body length estimates are made (Perez et al., 2021), nevertheless, it provides the most accurate approximation for the body length of the shark from which it came.
A single Otodus megalodon tooth (CMM-V-8522, Figure 7) was found touching one of the vertebrae. It was not embedded in the vertebra, and we did not observe any shark bite marks on the vertebrae. The tooth is a lower anterior that measures 57.5 mm in vertical height, although, the tip of the tooth is missing because of a conspicuous spall fracture labially. The crown height is 44 mm, and the crown width is 34.5 mm. Using the equations devised by Perez et al. (2021) to calculate the body length of O. megalodon based on the crown width of a single tooth, this tooth would have come from a shark with a body length between 6 and 7 m (Victor Perez, pers. comm.). Although this tooth is a lower tooth, from which less accurate body length estimates are made (Perez et al., 2021), nevertheless, it provides the most accurate approximation for the body length of the shark from which it came.
DISCUSSION
There do not appear to be any size or morphological features that would allow definitive identification of these vertebrae as either odontocete or mysticete. For that matter, a reappraisal of the pathological vertebra, CMM-V-2194, identified by Godfrey and Altman (2005) as mysticete, reveals that that specificity was not warranted; it could also have come from an odontocete. The vertebrae preserved in CMM-V-10108 are 10% larger than the smallest Calvert Formation mysticete, Parietobalaena palmeri (Kellogg, 1968, USNM 23203) and approximately 10% smaller than lumbar vertebrae of Pelocetus calvertensis (Kellogg, 1965, USNM 23058). Although none is a precise match, the vertebrae in CMM-V-10108 are most similar in size to those of the Choptank Formation mysticete Halicetus ignotus (Kellogg, 1969, USNM 23636). Several large odontocetes are also known from the Chesapeake Group, including members of the family Squalodontidae and Physeteridae (Gottfried et al., 1994). The vertebrae of CMM-V-10108 are nearly the size of the lumbar vertebrae described by Dooley (2005) for Squalodon whitmorei, although this squalodontid is only presently known from the much older Popes Creek Sand unit in the basal Fairhaven Member of the Calvert Formation. By comparing the maximum horizontal diameter of the posterior epiphysis on the fractured vertebra with a complete and articulated skeleton of a yet unidentified St. Marys Formation mysticete (CMM-V-3988) that was approximately 7 m in total body length, we estimate that CMM-V-10108 would have come from a cetacean with a body length of about 4 m.
A forceful event imposed extreme hyperflexion on at least part of the axial column, resulting in intolerable strain on the shear-fractured vertebra. A sudden compressive or unidirectional blow to the base of the centrum from a seizure or impact most likely produced the cleavage plane for over half of the length of the bone. The gaping fracture is widely open with no intervening bone, because the force of the trauma drove the broken bottom of the vertebra back into the unbroken body of the centrum, preventing it from closing, i.e., returning to a more normal life-like position (Figure 4).
The thickness of the periosteal reaction in these two associated vertebrae is noteworthy. During the traumatic event, the muscles attached to the centrum and transverse processes could have been forcibly avulsed from the bone with their periosteal attachments; this elevation of the periosteum and accompanying hematoma resulted in the production of the new bone growth seen over most of the surface of the two associated vertebrae (Charles Howard, pers. comm.), (Figure 3 and Figure 6). The excessive bone growth on the surface of these two vertebrae appear similar in extent, if not texture, to a case of spondyloarthritis that was most likely due to infection seen in a specimen of Megaptera (Félix et al., 2007).
Unfortunately, the cause of the hyperflexion that produced the longitudinal shear-compression fracture and periosteal reactive bone growth is unknown. Seizures can cause vertebral fractures in humans, including elderly adults with poor bone health as well as physically fit younger individuals (Takahashi et al., 2002; Napier and Nolan, 2011; Stilwell et al., 2016), though these are uncommon and typically present as “burst” fractures (Robichaud et al., 2020). Seizures causing injuries of this magnitude have not been observed in cetaceans. Biotoxins, notably domoic acid, are known to cause seizures in pinnipeds (De La Riva et al., 2009; Fauquier and Landsberg, 2018), and cetaceans (Broadwater et al., 2018; Fire et al., 2010; Fire et al., 2021). Domoic acid appears to be implicated in a rise in infant mortalities in some mysticetes (Wilson et al., 2016) and deaths of adult Balaenoptera acutorostrata (Fire et al., 2010), though cetaceans typically strand already deceased from asphyxiation, and seizures resulting from domoic acid poisoning have not been directly observed in mysticetes.
The primary source of domoic acid poisoning in the oceans today is the genus of the group of algae known as Bacillariaceae, Pseudo-nitzschia (Bacillariophyceae). The historic record of Pseudo-nitzschia is challenging to discern from related taxa because of morphological diversity and overlap (Lundholm et al., 2002), and these diatoms are only lightly silicified and preserve poorly (Parsons and Dortch, 2002), making the precise origin of domoic acid poisoning from harmful algal blooms hard to date in geological time (Onyshchenko et al., 2019). It is likely, however, that Pseudo-nitzschia evolved in the Mesozoic like other harmful algae (Chacón and Gottschling, 2020).
The consumption of domoic acid from these harmful algal blooms is known to be concentrated in higher trophic levels, with concentrations being high in pinnipeds and mysticetes. Krill is particularly good at concentrating domoic acid (Bargu et al., 2002), making many mysticetes potentially more prone to domoic acid poisoning than smaller taxa (Van Dolah et al., 2002). Harmful algal blooms are suspected as a cause of death for some massive marine bonebeds in the fossil record, including seabirds (Emslie and Morgan, 1994) and the large marine Lagerstätte of the Pisco Formation of Peru (Bosio et al., 2021). A similar large faunal assemblage suspected to be the result of a harmful algal bloom is the Cerro Ballena assemblage from the Miocene of Chile (Pyenson et al., 2014). As of yet, no evidence has been reported that suggests that the Calvert Formation faunas died as a result of harmful algal blooms. The dinoflagellate record of the Calvert Formation and nearby Miocene records of Maryland and Virginia do not contain any evidence of Pseudo-nitzschia (De Verteuil and Norris, 1996).
Though domoic acid poisoning due to harmful algal blooms are known to cause the death of many marine mammals (De La Riva et al., 2009; Fauquier and Landsberg, 2018), including mysticetes (Broadwater et al., 2018; Fire et al., 2010; Fire et al., 2021; Wilson et al., 2016), there have been no reports of seizures causing vertebral fractures. Had the fractures come about as a result of domoic acid poisoning, the cetacean would have had to recover from the poisoning.
Protozoal infections are also capable of causing seizures in marine mammals, and protozoal infections are common in marine mammals, including mysticetes, today (Miller et al., 2018). Such protozoal infections do not have clear diagnostic physical characteristics, and we cannot exclude the possibility that this vertebral fracture is due to a seizure resulting from a protozoal infection.
 However, due to the severity of the lesions, the most tenable cause is that it came about as a result of the whale being impacted by a conspecific or a predator. To our knowledge, there are no published records of these kinds of injuries having been caused by conspecific impacts. In terms of predators, the two large otodontids, Otodus chubutensis and Otodus megalodon, were the largest and most common macropredators within the Chesapeake Group, (Kent, 2018; Perez et al., 2019). Although this claim is speculative, few others are comparable in likelihood (Figure 8). A single O. megalodon tooth was found touching one of the vertebrae (Figure 7). However, to be clear, there are several likely explanations for the association of this tooth with these vertebrae. 1) This tooth may have become associated simply by chance, unrelated in any way to the originating trauma or even the final death and presumed scavenging of the cetacean. 2) The tooth became embedded in the body of the cetacean, the result of a bite associated with the originating trauma. Then the tooth remained embedded in the body of the cetacean until it died, and both the vertebrae and tooth were subsequently preserved in very close proximity (i.e., touching). 3) The tooth was shed by the shark that killed the cetacean. Or finally, 4) the tooth was shed by a shark as it scavenged the cetacean’s carcass.
However, due to the severity of the lesions, the most tenable cause is that it came about as a result of the whale being impacted by a conspecific or a predator. To our knowledge, there are no published records of these kinds of injuries having been caused by conspecific impacts. In terms of predators, the two large otodontids, Otodus chubutensis and Otodus megalodon, were the largest and most common macropredators within the Chesapeake Group, (Kent, 2018; Perez et al., 2019). Although this claim is speculative, few others are comparable in likelihood (Figure 8). A single O. megalodon tooth was found touching one of the vertebrae (Figure 7). However, to be clear, there are several likely explanations for the association of this tooth with these vertebrae. 1) This tooth may have become associated simply by chance, unrelated in any way to the originating trauma or even the final death and presumed scavenging of the cetacean. 2) The tooth became embedded in the body of the cetacean, the result of a bite associated with the originating trauma. Then the tooth remained embedded in the body of the cetacean until it died, and both the vertebrae and tooth were subsequently preserved in very close proximity (i.e., touching). 3) The tooth was shed by the shark that killed the cetacean. Or finally, 4) the tooth was shed by a shark as it scavenged the cetacean’s carcass.
Despite their global dominance as the largest macropredators for more than 20 million years, relatively few case studies have been published on their trophic interactions with prey of any kind (Purdy, 1996; Renz, 2002; Godfrey and Altman, 2005; Aguilera et al., 2008; Carrillo-Briceño et al., 2016; Collareta et al., 2017; Godfrey et al., 2018; Mierzwiak and Godfrey, 2019; Godfrey et al., 2021). The large, serrated teeth of Otodus chubutensis and Otodus megalodon are found within the Chesapeake Group (Kent, 1994, Kent, 2018, Perez et al, 2019), confirmation that they were present within the waters of the Miocene Salisbury Embayment. From tooth-marked bone, it is now known that O. chubutensis and O. megalodon were consuming cetaceans in this area during the Miocene (Godfrey et al., 2018; Kent, 2018). Furthermore, O. megalodon -bitten whale bones have been found in the Pliocene of North Carolina (Purdy, 1996; Kallal et al., 2021), Florida (Hulbert, 2001; Renz, 2002), Venezuela (Aguilera et al., 2008) and elsewhere.
In spite of their impressive size and purported predatory preferences, one should not lose site of the fact that within the Calvert Formation, there are several other macropredatory sharks that might also have been capable of inflicting the injuries shown by CMM-V-10108. The teeth of Parotodus benedenii occur in this formation (Kent, 2018), although they are exceedingly rare. Although the teeth are not nearly as large as those of Otodus chubutensis or Otodus megalodon, their roots are relatively massive, suggesting that they may have been aggressive predators. Because the teeth of P. benedenii are unserrated, no shark tooth-marked cetacean bones have been associated with predation or scavenging by this shark. The teeth of the Miocene white shark, Carcharodon hastalis, are commonly found along Calvert Cliffs. Although they attained a body length comparable to that of the living great white shark, Carcharodon carcharias, we do not know if they were sufficiently large to have inflicted the kinds of injuries sustained by this cetacean.
A few isolated large physeteroid teeth have been found within the Chesapeake Group, although not within the Calvert Formation. Nonetheless, we cannot rule out a failed predatory encounter between the cetacean and a macroraptorial sperm whale.
The kinds of injuries described herein rank with those seen in extant cetaceans subjected to anthropogenically-induced trauma, like vessel-strike blunt force injuries to their skull and vertebral fractures, blubber and muscle contusions, and large blood clots (Knowlton and Kraus, 2020; Moore et al., 2013; Raverty et al., 2020; Sharp et al., 2019). In spite of these anthopogenically-induced traumas, shear-compression fractures have not yet been reported in extant cetaceans. Partly-healed fractures of this magnitude have rarely been reported in fossil cetaceans and were interpreted as impacts from a conspecific or with the seafloor during benthic feeding (Beatty and Dooley, 2009).
Whatever the nature of the originating trauma, this much can be said, it was not immediately fatal, and the cetacean made some headway in restoring the integrity of the vertebrae prior to the whale’s ultimate demise. Thus, assuming a correlation with human healing (on the basis that bone healing is bone healing), it is estimated that the whale lived for a minimum of 6-8 weeks (Charles Howard, pers. comm.) following the initiating trauma.
ACKNOWLEDGMENTS
We gratefully acknowledge M. Ellwood who found, prepared, and donated the specimens described herein to the Calvert Marine Museum. The CT-scans came about as a result of the generosity of the Calvert Memorial Hospital (CMH). We would like to thank B. Cherry (Vice President of Operations), K. Sweeney (Vice President, Strategy and Business Development), and B Vess (Radiology Director) for making this happen. J. Gillikin, A. Jones, and R. Mahey (Radiology Technologists) expertly CT-scanned the fossil whale vertebrae. Dr. G. Selman (CMH) provided very helpful initial comments on this specimen. Based on the size of the associated Otodus megalodon tooth, Dr. V. Perez calculated its body length, and Dr. C. Howard contributed to the interpretation of the pathologies. CMM Paleontology Collections Manager, J.R. Nance provided liberal access to the fossils in his care.
In addition to their helpful comments on this paper, H. Mallison and J.X. Samuels kept it moving through the publication process. Gracious comments by three anonymous reviewers greatly improved the final draft. This publication would not have been possible without funding from the citizens of Calvert County, MD and from the County Board of Calvert County Commissioners; thank you.
REFERENCES
Aguilera, O.A, García, L., and Cozzuol, M.A. 2008. Giant-toothed white sharks and cetacean trophic interaction from the Pliocene Caribbean Paraguaná Formation. Paläontologische Zeitschrift, 82:204-208. https://doi.org/10.1007/bf02988410
Astrup, A.K., Hedayat, A., Jensen, T.H., Hammer, A.S., Munk, O.L., and Jensen, H.E. 2013. Necropsy report of a fin whale (Balaenoptera physalus) stranded in Denmark in 2010. Aquatic Mammals, 39:385-388.
Bargu, S., Powell, C.L., Coale, S.L., Busman, M., Doucette, G.J., and Silver, M.W. 2002. Krill: a potential vector for domoic acid in marine food webs. Marine Ecology Progress Series, 237:209-216.
Beatty, B.L. and Rothschild, B.M. 2008. Decompression syndrome and the evolution of deep diving physiology in the Cetacea. Naturwissenschaften, 95:793-801. https://doi.org/10.1007/s00114-008-0385-9
Beatty, B.L. and Dooley, A.C. 2009. Injuries in a mysticete skeleton from the Miocene of Virginia, with a discussion of buoyancy and the primitive feeding mode in the Chaeomysticeti. Jeffersoniana, 20:1-28.
Berghan J. and Visser, I.N. 2000. Vertebral column malformations in New Zealand delphinids with a review of cases worldwide. Aquatic Mammals, 26:17-25.
Bertulli, C.G., Galatius, A., Kinze, C.C., Rasmussen, M.H., Deaville, R., Jepson, P., Vedder, E.J., Sánchez Contreras, G.J., Sabin, R.C., and Watson, A. 2015. Vertebral column deformities in white-beaked dolphins from the eastern North Atlantic. Diseases of Aquatic Organisms, 116:59-67. https://doi.org/10.3354/dao02904
Bosio, G., Collareta, A., Di Celma, C., Lambert, O., Marx, F.G., de Muizon, C., Gioncada, A., Gariboldi, K., Malinverno, E., Malca, R.V., and Urbina, M. 2021. Taphonomy of marine vertebrates of the Pisco Formation (Miocene, Peru): Insights into the origin of an outstanding Fossil-Lagerstätte. Plos One, 16(7):p.e0254395. https://doi.org/10.1371/journal.pone.0254395
Broadwater, M.H., Van Dolah, F.M., and Fire, S.E. 2018. Vulnerabilities of marine mammals to harmful algal blooms. Harmful Algal Blooms, 8:191-222.
Carrillo-Briceño, J.D., Aguilera, O.A., De Gracia, C., Aguirre-Fernández, G., Kindlimann, R., and Sánchez-Villagra, M.R. 2016. An early Neogene elasmobranch fauna from the southern Caribbean (Western Venezuela). Palaeontologia Electronica, 19:27A. https://doi.org/10.26879/664
Chacón, J. and Gottschling, M. 2020. Dawn of the dinophytes: A first attempt to date origin and diversification of harmful algae. Harmful Algae, 97:p.101871.
Collareta, A., Lambert, O., Landini, W., Di Celma, C., Malinverno, E., Varas-Malca, R., Urbina, M., and Bianucci, G. 2017. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology, 469:84-91. https://doi.org/10.1016/j.palaeo.2017.01.001
Costa, A.P.B., Loch, C., and Simões-Lopes, P.C. 2016. Variations and anomalies in the vertebral column of the bottlenose dolphin (Tursiops truncatus) from southern Brazil. Latin American Journal of Aquatic Mammals, 11:212-219. https://doi.org/10.5597/lajam00230
Dawson, S.D. and Gottfried, M.D. 2002. Paleopathology in a Miocene kentriodontid dolphin (Cetacea: Odontoceti). Smithsonian Contributions to Paleobiology, 93:263-270.
De La Riva, G.T., Johnson, C.K., Gulland, F.M., Langlois, G.W., Heyning, J.E., Rowles, T.K., and Mazet, J.A. 2009. Association of an unusual marine mammal mortality event with Pseudo-nitzschia spp. blooms along the southern California coastline. Journal of Wildlife Diseases, 45:109-121. https://doi.org/10.7589/0090-3558-45.1.109
De Verteuil, L. and Norris, G. 1996. Miocene dinoflagellate stratigraphy and systematics of Maryland and Virginia. Micropaleontology, 42:1-172.
Dooley, A.C., Jr. 2005. A new species of Squalodon (Mammalia, Cetacea) from the Middle Miocene of eastern North America. Virginia Museum of Natural History Memoir, 8:1-43.
Emslie, S.D. and Morgan, G.S. 1994. A catastrophic death assemblage and paleoclimatic implications of Pliocene seabirds of Florida. Science, 264(5159):684-685. https://doi.org/10.1126/science.264.5159.684
Fauquier, D. and Landsberg, J. 2018. Harmful algae and biotoxins, p. 319-328. In Dierauf, L.A., Gulland, F.M.D., and Whitman, K.L. (eds.), CRC Handbook of Marine Mammal Medicine, Third Edition. CRC Press.
Félix, F., Haase, B., and Aguirre, W.E. 2007. Spondylitis in a humpback whale (Megaptera novaeangliae) from the southeast Pacific. Diseases of Aquatic Organisms, 75:259-264. https://doi.org/10.3354/dao075259
Fire, S.E., Wang, Z., Berman, M., Langlois, G.W., Morton, S.L., Sekula-Wood, E., and Benitez-Nelson, C.R. 2010. Trophic transfer of the harmful algal toxin domoic acid as a cause of death in a minke whale (Balaenoptera acutorostrata) stranding in southern California. Aquatic Mammals, 36:342-350. https://doi.org/10.1578/AM.36.4.2010.342
Fire, S.E., Bogomolni, A., DiGiovanni, Jr., R.A., Early, G., Leighfield, T.A., Matassa, K., Miller, G.A., Moore, K.M., Moore, M., Niemeyer, M., and Pugliares, K. 2021. An assessment of temporal, spatial and taxonomic trends in harmful algal toxin exposure in stranded marine mammals from the US New England coast. Plos One, 16(1):p.e0243570. https://doi.org/10.1371/journal.pone.0243570
Gerholdt, J.M. and Godfrey, S J. 2010. Enigmatic osteology in Miocene odontocete rostra suggests periostitis. Marine Mammal Science, 26:381-394. https://doi.org/10.1111/j.1748-7692.2009.00342.x
Godfrey, S.J. and Altman, J. 2005. A Miocene cetacean vertebra showing a partially healed compression fracture, the result of convulsions or failed predation by the Giant White Shark, Carcharodon megalodon. Jeffersoniana, 16:1-12.
Godfrey, S.J., Ellwood, M., Groff, S., and Verdin, M.S. 2018. Carcharocles -bitten odontocete caudal vertebrae from the Coastal Eastern United States. Acta Palaeontologica Polonica, 63:463-468. https://doi.org/10.4202/app.00495.2018
Godfrey, S.J. and Lowry, A.J. 2021. The ichnospecies Linichnus bromleyi on a Miocene baleen whale radius preserving multiple shark bite-shake traces suggests scavenging. Carnets Geol., 21:391-398.
Godfrey, S.J., Nance, J.R., and Riker, N.L. 2021. Otodus -bitten sperm whale tooth from the Coastal Eastern United States. Acta Palaeontologica Polonica, 66:599-603. https://doi.org/10.4202/app.00820.2020
Gottfried, M.D., Bohaska, D.J., and Whitmore, F.C., Jr. 1994. Miocene cetaceans of the Chesapeake Group. In Berta, A. and Demere, T.A. (eds.), Contributions in Marine Mammal Paleontology Honoring Frank C. Whitmore, Jr. Proceedings of the San Diego Society of Natural History, 29:229-238.
Hellier, C.A., Hufthammer, A.K., and Lislevand, T. 2011. Osteological pathology in a Humpback (Megaptera novaeangliae) and Fin (Balaenoptera physalus) whale skeleton. International Journal of Paleopathology, 1:117-120. https://doi.org/10.1016/j.ijpp.2011.09.003
Hulbert, R.C., Jr. 2001. The Fossil Vertebrates of Florida. University Press of Florida. Gainesville, Florida. https://doi.org/10.1086/343936
Kallal, R.J., Godfrey, S.J., and Ortner, D.J. 2012. Bone reactions on a Pliocene cetacean rib indicate short- term survival of predation event. International Journal of Osteoarchaeology, 22:253-260. https://doi.org/10.1002/oa.1199
Kellogg, R. 1965. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia. Part 1. A new whalebone whale from the Miocene Calvert Formation. United States National Museum Bulletin, 247:1-45.
Kellogg, R. 1968. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia. Part 8. Supplement to description of Parietobalaena palmeri. United States National Museum Bulletin, 247:175-197.
Kellogg, R. 1969. Cetothere skeletons from the Miocene Choptank Formation of Maryland and Virginia. United States National Museum Bulletin, 294:1-40. https://doi.org/10.5479/si.03629236.294.1
Kent, B.W. 1994. Fossil Sharks of the Chesapeake Bay Region. Egan Rees and Boyer, Inc. Columbia, Maryland.
Kent, B.W. 2018. The cartilaginous fishes (chimaeras, sharks and rays) of Calvert Cliffs, Maryland, USA, p. 45-160. In Godfrey, S.J. (ed.), Geology and Vertebrate Paleontology of Calvert Cliffs. Smithsonian Contributions to Paleobiology 100. Smithsonian Institution Press, Washington, DC. https://doi.org/10.5479/si.1943-6688.100
Kidwell, S.M. 1984. Outcrop features and origin of basin margin unconformities in the lower Chesapeake Group (Miocene), Atlantic Coastal Plain, p. 37-58. In Schlee, J.S. (ed.), Interregional Unconformities and Hydrocarbon Accumulation. American Association of Petroleum Geologists Memoir 36, Tulsa, Oklahoma. https://doi.org/10.1306/m36440c3
Kidwell, S.M. 1989. Stratigraphic condensation of marine transgressive records: origin of major shell deposits in the Miocene of Maryland. Journal of Geology, 97:1-24. https://doi.org/10.1086/629278
Kidwell, S.M., Powars, D.S., Edwards, L.E., and Vogt, P.R. 2015. Miocene stratigraphy and paleoenvironments of the Calvert Cliffs, Maryland. The Geological Society of America Field Guide, 40:231-279. https://doi.org/10.1130/2015.0040(08)
Knowlton, A.R. and Kraus, S.D. 2020. Mortality and serious injury of northern right whales (Eubalaena glacialis) in the western North Atlantic Ocean. Journal of Cetacean Research and Management, 2:193-208. https://doi.org/10.47536/jcrm.vi.288
Kompanje, E.J.O. 1999. Considerations on the comparative pathology of the vertebrae in Mysticeti and Odontoceti; evidence for the occurrence of discarthrosis, zygarthrosis, infectious spondylitis and spondyloarthritis. Zoologische Mededelingen, 73:99-130.
Lundholm, N., Daugbjerg, N., and Moestrup, Ø. 2002. Phylogeny of the Bacillariaceae with emphasis on the genus Pseudo-nitzschia (Bacillariophyceae) based on partial LSU rDNA. European Journal of Phycology, 37:115-134. https://doi.org/10.1017/S096702620100347X
Mierzwiak, J.S. and Godfrey, S.J. 2019. Megalodon-bitten whale rib from South Carolina. The Ecphora, 34(2):15-20.
Miller, M., Shapiro, K., Murray, M.J., Haulena, M., and Raverty, S. 2018. Protozoan parasites of marine mammals, p. 425-470. In Dierauf, L.A., Gulland, F.M.D., and Whitman, K.L. (eds.), CRC Handbook of Marine Mammal Medicine, Third Edition. CRC Press.
Moore, M.J., van der Hoop, J., Barco, S.G., Costidis, A.M., Gulland, F., Jepson, P., Moore, K., Raverty, S., and McLellan, W. 2013. Criteria and case definitions for serious injury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Diseases of Aquatic Organisms, 103:229-264. https://doi.org/10.3354/dao02566
Nance, J.R., Kricun, M., and Godfrey, S.J. 2017. Ankylosis at the elbow joint in a baleen whale (Cetacea, Mysticeti) from the Miocene Calvert Formation of Calvert Cliffs, Maryland, USA. Marine Mammal Science, 33:376-385. https://doi.org/10.1111/mms.12363
Napier, R.J. and Nolan, P.C. 2011. Diagnosis of vertebral fractures in post-ictal patients. Emergency Medicine Journal, 28:169-170. https://doi.org/10.1136/emj.2009.088021
Onyshchenko, A., Ruck, E.C., Nakov, T., and Alverson, A.J. 2019. A single loss of photosynthesis in the diatom order Bacillariales (Bacillariophyta). American Journal of Botany, 106:560-572. https://doi.org/10.1002/ajb2.1267
Parsons, M.L. and Dortch, Q. 2002. Sedimentological evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnology and Oceanography, 47:551-558. https://doi.org/10.4319/lo.2002.47.2.0551
Perez, V.J., Godfrey, S.J., Kent, B.W., Weems, R.E., and Nance, J.R. 2019. The transition between Carcharocles chubutensis and Carcharocles megalodon (Otodontidae, Chondrichthyes): lateral cusplet loss through time. Journal of Vertebrate Paleontology, 38(6):e1546732. https://doi.org/10.1080/02724634.2018.1546732
Perez, V.J., Leder, R.M., and Badaut, T. 2021. Body length estimation of Neogene macrophagous lamniform sharks (Carcharodon and Otodus) derived from associated fossil dentitions. Palaeontologia Electronica, 24(1):a09. https://doi.org/10.26879/1140
Purdy, R.W. 1996. Paleoecology of fossil white sharks, p. 67-78. In Klimley, A.P. and Ainley, D.G. (eds.), Chapter 8. Great white sharks: the biology of Carcharodon carcharias, Academic Press, San Diego. https://doi.org/10.1016/b978-012415031-7/50009-4
Pyenson, N.D., Gutstein, C.S., Parham, J.F., Le Roux, J.P., Chavarría, C.C., Little, H., Metallo, A., Rossi, V., Valenzuela-Toro, A.M., Velez-Juarbe, J., and Santelli, C.M. 2014. Repeated mass strandings of Miocene marine mammals from Atacama Region of Chile point to sudden death at sea. Proceedings of the Royal Society B: Biological Sciences, 281(1781):20133316. https://doi.org/10.1098/rspb.2013.3316
Raverty, S., St. Leger, J., Noren, D.P., Burek Huntington, K., Rotstein, D.S., Gulland, F., Ford, J., Hanson, M.B., Lambourn, D.M., Huggins, J., Delaney, M.A., Spaven, L., Rowles, T., Barre, L., Cottrell, P., Ellis, G., Goldstein, T., Terio, K., Duffield, D., Rice, J., and Gaydos, J.K. 2020. Pathology findings and correlation with body condition index in stranded killer whales (Orcinus orca) in the northeastern Pacific and Hawaii from 2004 to 2013. Plos One, 15(12): e0242505. https://doi.org/10.1371/journal.pone.0242505
Renz, M. 2002. Megalodon, Hunting the Hunter. PaleoPress. Lehigh Acres, Florida.
Sharp, S.M., McLellan, W.A., Rotstein, D.S., Costidis, A.M., Barco, S.G., Durham, K., Pitchford, T.D., Jackson, K.A., Daoust, P.-Y., Wimmer, T., Couture, E.L., Bourque, L., Frasier, T., Frasier, B., Fauquier, D., Rowles, T.K., Hamilton, P.K., Pettis, H., and Moore, M.J. 2019. Gross and histopathologic diagnoses from North Atlantic right whale Eubalaena glacialis mortalities between 2003 and 2018. Diseases of Aquatic Organisms, 135:1-31. https://doi.org/10.3354/dao03376
Robichaud, A.S., Barry, T.K., and Barry, S.P. 2020. Seizure-induced thoracolumbar burst fractures—not to be missed. Epilepsy and Behavior Reports, 13:p.100352. https://doi.org/10.1016/j.ebr.2019.100352
Stilwell, P., Harman, K., Hsu, W., and Seaman, B. 2016. Multiple seizure-induced thoracic vertebral compression fractures: a case report. The Journal of the Canadian Chiropractic Association, 60:252.
Takahashi, T., Tominaga, T., Shamoto, H., Shimizu, H., and Yoshimoto, T. 2002. Seizure-induced thoracic spine compression fracture: case report. Surgical neurology, 58:214-216.
Thomas H.W., Barnes, L.G., Klein, J.E., and McLeod, S.A. 2008. Examples of paleopathologies in some fossil Cetacea from the North Pacific realm. Natural History Museum of Los Angeles County, Science Series, 41:153-179.
Van Dolah, F.M., Doucette, G.J., Gulland, F.M.D., Rowles, T.L., and Bossart, G.D. 2002. Impacts of algal toxins on marine mammals, p. 259-281. In Gos, J.G., Bossart, G., Fournier, M., and O´Shea, T. (eds.), Toxicology of Marine Mammals. CRC Press.
Visaggi, C.C. and Godfrey, S.J. 2010. Variation in composition and abundance of Miocene shark teeth from Calvert Cliffs, Maryland. Journal of Vertebrate Paleontology, 30:26-35. https://doi.org/10.1080/02724630903409063
Watson, A., Bahr, R.J., and Alexander, J.W. 2004. Thoracolumbar kyphoscoliosis and compression fracture of a thoracic vertebra in a captive bottlenose dolphin (Tursiops truncatus). Aquatic Mammals, 20:275-278. https://doi.org/10.1578/am.30.2.2004.275
Wilson, C., Sastre, A.V., Hoffmeyer, M., Rowntree, V.J., Fire, S.E., Santinelli, N.H., Ovejero, S.D., D’Agostino, V., Marón, C.F., Doucette, G.J., and Broadwater, M.H. 2016. Southern right whale (Eubalaena australis) calf mortality at Peninsula Valdés, Argentina: Are harmful algal blooms to blame? Marine Mammal Science, 32:423-451.

