 Diverse endobiotic symbiont fauna from the late Katian (Late Ordovician) of Estonia
Diverse endobiotic symbiont fauna from the late Katian (Late Ordovician) of Estonia
Article number: 25.3.a31
https://doi.org/10.26879/1232
Copyright Paleontological Society, November 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 31 July 2022. Acceptance: 5 November 2022.
ABSTRACT
Endobiotic cornulitids formed symbiotic associations with tabulate corals and stromatoporoids in the Katian (Late Ordovician) of Estonia. The cornulitids benefited from a stable substrate and additional protection against predators offered by the skeleton of their hosts. Symbiotic lingulates and Chaetosalpinx-like bioclaustration structures are here reported from bryozoans for the first time. The endobiotic lingulates were also symbionts of tabulate corals in the Katian of Estonia. Bryozoans hosted the most diverse fauna of endobionts in the Katian of Baltica. Corals and stromatoporoids hosted just few groups of endobionts in the Katian of Baltica.
Olev Vinn. Institute of Ecology and Earth Sciences, University of Tartu, Ravila 14A, 50411 Tartu, Estonia. olev.vinn@ut.ee
Mark A. Wilson. The College of Wooster, Department of Earth Sciences, Wooster, Ohio, USA. mwilson@wooster.edu
Lars E. Holmer. Department of Earth Sciences, Palaeobiology, Uppsala University, Uppsala, Sweden. lars.holmer@pal.uu.se
Andrej Ernst. Institut für Geologie, Universität Hamburg, Bundesstr. 55, 20146 Hamburg, Germany. Andrej.Ernst@uni-hamburg.de
Oive Tinn. Institute of Ecology and Earth Sciences, University of Tartu, Ravila 14A, 50411 Tartu, Estonia. oive.tinn@ut.ee
Ursula Toom. Department of Geology, Tallinn University of Technology, Tallinn, Estonia. ursula.toom@taltech.ee
Keywords: Calcareous sponges; tabulate corals; bryozoans; endobionts; bioclaustrations; Upper Ordovician; Baltica
Final citation: Vinn, Olev, Wilson, Mark A., Holmer, Lars E., Ernst, Andrej, Tinn, Oive, and Toom, Ursula. 2022. Diverse endobiotic symbiont fauna from the late Katian (Late Ordovician) of Estonia. Palaeontologia Electronica, 25(3):a31. https://doi.org/10.26879/1232
palaeo-electronica.org/content/2022/3709-endobiotic-symbiont-fauna
Copyright: November 2022 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Endobiotic invertebrate symbionts were common and widespread in the Late Ordovician (Tapanila, 2005; Vinn et al., 2022). The macroscopic invertebrate symbionts are usually preserved as bioclaustrations or fossilized skeletons, both constituting the best examples of symbiotic interactions in the fossil record. The exact nature of these early symbiotic associations has mostly remained problematic. Many bioclaustrations may have been parasitic (Zapalski, 2007, 2011), but Taylor (2015) demonstrated that it is difficult to establish the exact type of symbiosis using the fossil material. The evolution of parasitic associations has recently been the focus of several studies (De Baets and Littlewood, 2015; Huntley and De Baets, 2015; De Baets et al., 2011, 2015, 2021a, b; Huntley et al., 2021; van Dijk and De Baets, 2021). There is growing evidence suggesting that biodiversity mediates parasite prevalence. The parasitism was likely scale-dependent and it has increased through the Phanerozoic. There is a support to the amplification of parasitism with biodiversity in the history of life (De Baets et al., 2021a, b). Symbiotic interaction in the Ordovician of Baltica has been described in detail by Vinn and Wilson (2015).
Bioclaustrations are trace fossil cavities, which resulted from embedment of symbiont within the skeleton of the host organism (Palmer and Wilson, 1988; Słowiński et al., 2020). Late Ordovician bioclaustrations left by probable parasites are common in bryozoans both in Baltica and Laurentia (Palmer and Wilson, 1988; Vinn et al., 2014, 2018). The cornulitids often formed symbiotic associations with other invertebrates in the Late Ordovician of Baltica (Vinn and Mõtus, 2012; Vinn, 2013) and Laurentia (Dixon, 2010). Chaetosalpinx is a common bioclaustration ichnogenus that has been reported from Late Ordovician corals of Laurentia (Elias, 1986; Tapanila, 2003, 2004, 2005) and Baltica (Vinn and Mõtus, 2012). The Chaetosalpinx bioclaustrations have been studied at least since Oekentorp (1969) and more recently by Tapanila (2003, 2004, 2005) and Zapalski (2008, 2009). The biological affinities of organisms that inhabited Chaetosalpinx bioclaustrations are unknown, but they likely had a worm-like body plan. The endobiotic lingulate brachiopods were symbionts of stromatoporoids in the Late Ordovician of North America (Tapanila and Holmer, 2006; Stewart et al., 2010). It has previously been assumed that almost all fossil lingulids had an active infaunal burrowing and vertically inclined life habit more or less identical to that of their living representatives (e.g., Yugan et al., 1993). However, it is now clear that some lingulids, e.g., the exceptionally preserved lingulid brachiopods in Chengjiang and Burgess Shale, had epifaunal lifestyles (e.g., Wang et al., 2014; Stolk et al., 2010).
In a number of papers (Vinn and Mõtus, 2012; Vinn, 2013; Vinn and Wilson, 2015; Toom et al. 2019), symbiotic interactions involving corals have been described from the Katian of Estonia, but all these associations were found from the Pirgu Regional Stage, which is younger than materials from the Vormsi Regional Stage described here for the first time. In addition, all symbiotic associations involving lingulids, stromatoporoids and bryozoans that are described here are new for the late Katian of Estonia and Baltica.
This paper: (1) describes bioclaustrations of endobiotic symbionts from corals, stromatoporoids and bryozoans of the Katian of Estonia; (2) discusses the palaeoecology of these symbiotic associations; and (3) discusses symbiotic interactions in the Katian of Estonia.
GEOLOGICAL BACKGROUND
During the Katian, Baltica was located in the subtropical realm (Nestor and Einasto, 1997; Torsvik and Cocks, 2013). The area of modern Estonia was covered by a shallow epicontinental sea during the Katian (Nestor and Einasto, 1997). Climatic change resulted in an increase in carbonate production and sedimentation rate on the carbonate shelf in the Baltic Basin (Nestor and Einasto, 1997). The Katian in northern Estonia is characterized by pure limestones. In addition, it was a time when tabulate and rugose corals rapidly diversified in Baltica (Sokolov, 1951; Mõtus, 1997; Kaljo et al., 2011) and bioproduction rose in the region (Delabroye et al., 2011; Kaljo et al., 2011; L. Hints et al., 2018; Truuver et al., 2021).
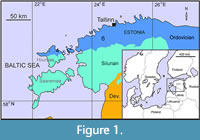 The first part of the studied material was collected from Saxby (Vormsi Island) and Sutlema, Vormsi Regional Stage, Kõrgessaare Formation, upper Katian (Figure 1-Figure 2). The Kõrgessaare Formation (up to 21 m) is represented by bioclastic limestones in northern Estonia. The association of diverse shelly fauna (about 200 species) of corals, bryozoans, brachiopods, molluscs and trilobites has been reported from the Kõrgessaare Formation (Hints and Meidla, 1997). Several fossil groups (tabulate corals, gastropods, brachiopods and bryozoans) of the Kõrgessaare Formation have oversized taxa (Toom et al., 2021). Bioerosion is common with borings on hardgrounds and different biogenic substrates (Vinn et al., 2015; Toom et al., 2019). Most borings are in tabulates, bryozoans, rugosans and stromatoporoids. The environmental conditions were different in the northern and southern part of the Saxby shore outcrop. Nautiloids and large Pseudolingula occur only in the northern part of the Saxby coastal outcrop. We suspect that the waters were more turbulent in the northern part of the outcrop than in the southern part of the locality. Specimens of laminar heliolitids are mostly broken; corals and bryozoans are commonly upside-down, and specimens are smaller (max. diameter of bryozoans is 14 cm, and in heliolitid corals 21 cm) than those from the southern part of the outcrop (max. diameter of bryozoans is 27 cm, in stromatoporoids 55 cm, in heliolitid corals 30 cm). The largest specimens of upper Katian heliolitids and bryozoans were collected from the southern part of the Saxby outcrop.
The first part of the studied material was collected from Saxby (Vormsi Island) and Sutlema, Vormsi Regional Stage, Kõrgessaare Formation, upper Katian (Figure 1-Figure 2). The Kõrgessaare Formation (up to 21 m) is represented by bioclastic limestones in northern Estonia. The association of diverse shelly fauna (about 200 species) of corals, bryozoans, brachiopods, molluscs and trilobites has been reported from the Kõrgessaare Formation (Hints and Meidla, 1997). Several fossil groups (tabulate corals, gastropods, brachiopods and bryozoans) of the Kõrgessaare Formation have oversized taxa (Toom et al., 2021). Bioerosion is common with borings on hardgrounds and different biogenic substrates (Vinn et al., 2015; Toom et al., 2019). Most borings are in tabulates, bryozoans, rugosans and stromatoporoids. The environmental conditions were different in the northern and southern part of the Saxby shore outcrop. Nautiloids and large Pseudolingula occur only in the northern part of the Saxby coastal outcrop. We suspect that the waters were more turbulent in the northern part of the outcrop than in the southern part of the locality. Specimens of laminar heliolitids are mostly broken; corals and bryozoans are commonly upside-down, and specimens are smaller (max. diameter of bryozoans is 14 cm, and in heliolitid corals 21 cm) than those from the southern part of the outcrop (max. diameter of bryozoans is 27 cm, in stromatoporoids 55 cm, in heliolitid corals 30 cm). The largest specimens of upper Katian heliolitids and bryozoans were collected from the southern part of the Saxby outcrop.
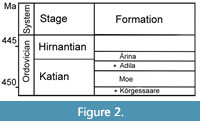 The other part of the studied material was collected from the Pirgu Regional Stage, Adila Formation of Katian (Late Ordovician) age, Hosholm (Vormsi Island) and Vohilaid localities, northwestern Estonia (Figure 1-Figure 2). The Hosholm locality represents a soft mud bottom environment with considerable influx of sediments. The Adila Formation corresponds to the topmost part of the Katian. The Adila Formation is characterized by nodular limestones with marl intercalations. These sediments formed in normal marine, shallow shelf environment (Hints et al., 2005). The fauna of the Adila Formation includes abundant tabulate and rugose corals, cephalopods, gastropods, somewhat fewer bryozoans, brachiopods and stromatoporoids. The corals of the Adila Formation are massive, with bulbous or domical shapes commonly encrusting on large cephalopods or gastropods. Almost all the corals with fine structure have Trypanites borings.
The other part of the studied material was collected from the Pirgu Regional Stage, Adila Formation of Katian (Late Ordovician) age, Hosholm (Vormsi Island) and Vohilaid localities, northwestern Estonia (Figure 1-Figure 2). The Hosholm locality represents a soft mud bottom environment with considerable influx of sediments. The Adila Formation corresponds to the topmost part of the Katian. The Adila Formation is characterized by nodular limestones with marl intercalations. These sediments formed in normal marine, shallow shelf environment (Hints et al., 2005). The fauna of the Adila Formation includes abundant tabulate and rugose corals, cephalopods, gastropods, somewhat fewer bryozoans, brachiopods and stromatoporoids. The corals of the Adila Formation are massive, with bulbous or domical shapes commonly encrusting on large cephalopods or gastropods. Almost all the corals with fine structure have Trypanites borings.
MATERIAL AND METHODS
The tabulate, stromatoporoid and bryozoan specimens were collected from the upper Katian of Estonia during a series of field projects from 1997 to 2021. The studied material consists of 442 specimens that yielded 29 specimens with endobionts (see Appendix 1 for specimens containing endobionts). The apertures of the endobionts were discovered on the surfaces of the tabulates, stromatoporoids and bryozoan colonies using a binocular light microscope Leica S8APO. All endobiont apertures were photographed using a Canon EOS 5Dsr digital camera and apochromatic zoom system Leica Z16 APO. The diameters of the endobiont apertures were measured on calibrated photos. Several specimens of each type of endobiont cavities were longitudinally sectioned using a stone saw. The polished sections of the endobiont cavities were digitally photographed using the photo equipment mentioned above. The studied specimens were deposited at the Department of Geology, Tallinn University of Technology (GIT).
RESULTS
Endobiotic Cornulitids
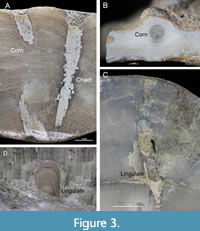 New cornulitid material was found in several tabulate corals from the Pirgu Regional Stage (Adila Formation). At maximum there is one cornulitid specimen per host coral specimen in Paleofavosites, five cornulitid specimens per host coral in Protoheliolites norvegicus (Figure 3A) and two cornulitid specimens per host coral in Stelliporella parvistella . The endobiotic cornulitids are described here for the first time from the Vormsi Regional Stage. They occur in Diploepora sp. (max. four cornulitids per host tabulate), Protaraea sp. (max. single cornulitid per host tabulate specimen), Stelliporella parvistella (max. single cornulitid per host tabulate) and an unidentified heliolitid (max. two cornulitids per host tabulate specimen) (Table 1). Cornulitids are partially to completely embedded, except their apertures, within the corals. The apertures are flush with the growth surface of the tabulates or situated within a small elevation on the tabulate’s growth surface. The cornulitids are subparallel to subperpendicular to the growth surface of the tabulate. In addition to tabulates, cornulitids are intergrown with stromatoporoids (two cornulitids per host) in the Vormsi regional Stage (Table 1). The endobiotic cornulitids were previously unknown from Ordovician stromatoporoids of Estonia and Baltica. The cornulitids are partially to completely embedded within their host and are located subparallel to the growth surface of the stromatoporoid (Figure 3B).
New cornulitid material was found in several tabulate corals from the Pirgu Regional Stage (Adila Formation). At maximum there is one cornulitid specimen per host coral specimen in Paleofavosites, five cornulitid specimens per host coral in Protoheliolites norvegicus (Figure 3A) and two cornulitid specimens per host coral in Stelliporella parvistella . The endobiotic cornulitids are described here for the first time from the Vormsi Regional Stage. They occur in Diploepora sp. (max. four cornulitids per host tabulate), Protaraea sp. (max. single cornulitid per host tabulate specimen), Stelliporella parvistella (max. single cornulitid per host tabulate) and an unidentified heliolitid (max. two cornulitids per host tabulate specimen) (Table 1). Cornulitids are partially to completely embedded, except their apertures, within the corals. The apertures are flush with the growth surface of the tabulates or situated within a small elevation on the tabulate’s growth surface. The cornulitids are subparallel to subperpendicular to the growth surface of the tabulate. In addition to tabulates, cornulitids are intergrown with stromatoporoids (two cornulitids per host) in the Vormsi regional Stage (Table 1). The endobiotic cornulitids were previously unknown from Ordovician stromatoporoids of Estonia and Baltica. The cornulitids are partially to completely embedded within their host and are located subparallel to the growth surface of the stromatoporoid (Figure 3B).
Endobiotic Lingulates
Endobiotic lingulates were previously unknown from the Ordovician of Baltica. A specimen of the tabulate Propora sp. from the Kõrgessaare Formation contains a cavity with a single lingulate shell (Figure 3C, Table 1). The lingulate is oriented with its commissure towards the aperture of the cavity on the growth surface of the tabulate. The lower part of the cavity is a bioerosional structure (Trypanites) but in the upper one-third of the cavity it is a bioclaustration. Cavities resembling the lingulate cavity in Propora occur also in Stelliporella parvistella and in an unidentified heliolitid from the Kõrgessaare Formation, but these cavities do not contain lingulates. A single boring in the bryozoan Diplotrypa densitabulata from the Kõrgessaare Formation (Vormsi Regional Stage) contains the lingulate Rowellella sp. (Figure 3D). The walls of the bryozoan show moderate reaction (i.e., bent growth lines) to the endobiont. The lingulates occur in borings with large diameters, a trend also noted by Stewart et al. (2010).
 Worm Endobionts
Worm Endobionts
Worm bioclaustrations identified as Chaetosalpinx have been previously described from tabulates of the Adila Formation (Vinn and Mõtus, 2012), but new specimens have been found from Vormsi Island and described below (Table 1). The growth layers of Protoheliolites norvegicus from the Adila Formation (Pirgu Regional Stage) are bent downwards around the shaft of Chaetosalpinx (Figure 3A). Previously unknown is a single bioclaustration resembling Chaetosalpinx? that occurs in the bryozoan Diplotrypa densitabulata (Kõrgessaare Formation, Vormsi Regional Stage). The growth layers of the host are bent downwards around the shaft at the aperture of Chaetosalpinx? The cavity is mostly bioerosional and a bioclaustration only in its apertural part (Figure 4).
DISCUSSION
The Cornulitid-tabulate Associations
The cornulitids infested living tabulates because they are fully intergrown with their hosts, having only their apertures free on the growth surface of the coral (Figure 3A). The subvertical orientation of some cornulitid specimens also indicates that they must have infested living tabulates. The infestation patterns and intergrowth morphologies of the cornulitid-Paleofavosites abstrusus, cornulitid- Protoheliolites norvegicus, cornulitid-Protaraea sp. and cornulitid-Stelliporella parvistella associations are similar. The low number of cornulitids in the above associations suggests that cornulitid larvae did not infest tabulates in large groups or had limited success in colonizing these tabulate species. The low number of symbionts may also indicate low symbiont tolerance of these tabulate species. The low number of cornulitids in these tabulates is different from common cornulitid encrustation pattern on hard substrates, where cornulitids often occur in aggregations. The cornulitid-coccoserid association differs by a larger number of cornulitids (N=4) in a single host. Large numbers of cornulitids (up to 10) have been reported from single hosts in Protoheliolites dubius and Propora speciosa (Vinn and Mõtus, 2012; Vinn, 2013). In cornulitid-tabulate associations, cornulitids benefited from the stable substrate and had additional protection against predators offered by the skeleton of the host tabulate. Based on analogy with modern symbiotic polychaete worms, Vinn and Mõtus (2012) suggested that cornulitids could have preferred colonial hosts with a good chemical defense such as cnidarians (Martin and Britayev, 1998). Cornulitid-tabulate associations have been also reported from the Hirnantian and early Silurian of Laurentia (Dixon, 2010) and Ludlow of Baltica (Vinn and Mõtus, 2008).
The Cornulitid-stromatoporoid Associations
In the case of stromatoporoid-rugose intergrowth, substrate stability was an important factor (Kershaw, 1987). Similarly, cornulitids may have colonized stromatoporoids to acquire a stable substrate for their growth and a higher tier for suspension feeding. The cornulitids may have also needed additional protection against predators offered by the thick skeleton of the stromatoporoid. The earliest cornulitid-stromatoporoid associations were previously known from the Hirnantian of Laurentia (Dixon, 2010), but it seems that these associations appeared already in the Katian of Baltica. The cornulitid-stromatoporoid associations are also widespread in the Wenlock of Baltica (Vinn and Wilson, 2010).
Lingulate-tabulate Associations
The lingulate shell occurs in Propora sp. in a bioerosional cavity, which continues as a bioclaustration in its apertural part (Figure 3C). The endobiont has influenced the growth of host tabulate, indicating that the lingulate colonized a bioerosional cavity in a living tabulate. Tapanila and Holmer (2006) suggested that lingulates did not bore into the hard substrate but they colonized existing bioerosional cavities. The lingulate larva likely settled in an abandoned bioerosional cavity made by some boring organism such as polychaete worms, for example. The lingulate managed to keep its cavity open within the living tabulate as it was not sealed off by Propora . The lingulate presumably benefited from this endobiotic life mode that protected it against predators and the high energy environment. The lingulate’s influence on the host coral is less obvious, but it likely was not a feeding competitor for the coral as corals are micro predators and brachiopods are filter feeders. Somewhat similar empty cavities in Stelliporella parvistella and in an unidentified heliolitid from the Vormsi Regional Stage may have been also inhabited by lingulates, but we lack evidence. Similar lingulate-tabulate associations occur in the Late Ordovician and early Silurian of Laurentia (Tapanila, 2005; Tapanila and Holmer, 2006; Stewart et al., 2010) and upper Silurian of Avalonia (Newell, 1970) and Baltica (Richards and Dyson-Cobb, 1976). According to Richards and Dyson-Cobb (1976), the coral-lingulate symbiotic specimens of Gotland occur in a shallow (probably less than 10 m) and quiet marine environment. Upper Ordovician sediments from North America were deposited in a shallow subtidal environment with occasional storms (Stewart et al., 2010). The Estonian specimens come from similar environments.
Chaetosalpinx-Protoheliolites norvegicus Association
The growth layers of the Protoheliolites norvegicus are bent downwards around the shaft of Chaetosalpinx, suggesting a syn vivo interaction between these two invertebrates (Figure 3A). Worms responsible for Chaetosalpinx bioclaustrations presumably benefited from protection against predators offered by the coral skeleton. The downward bending growth lamellae around the endobiont’s shaft (Figure 3A) suggest that Chaetosalpinx may have had a negative effect on the host coral, or at least it actively inhibited the heliolitid growth to protect itself against overgrowth. Chaetosalpinx-like bioclaustrations common in tabulates both in the Late Ordovician (Tapanila, 2005; Vinn and Mõtus, 2012) and Silurian (Mõtus and Vinn, 2009) of Baltica and Laurentia.
Rowellella-bryozoan Association
Growth layers of Diplotrypa densitabulata are somewhat bent around the shaft of the lingulate, suggesting a syn vivo association. After settling in a pre-existing empty boring, the lingulate managed to keep its cavity open within the living bryozoan (Figure 3D). The lingulate presumably benefited from the endobiotic life mode that protected it against predators similar to the lingulate-coral and lingulate-stromatoporoid associations. The lingulate’s influence on the host bryozoan is not obvious, but it may have been a feeding competitor for the bryozoan as bryozoans and brachiopods are both suspension feeders. There are no previous records of symbiotic lingulates in fossil or recent bryozoans.
Symbiotic Relationships in the Katian of Baltica
A major rise in the diversity of endobiotic symbionts is associated with the Darriwilian-Sandbian boundary in the Baltica (Vinn et al., 2018, 2022). However, both the Darriwilian and Sandbian endobionts preferably colonized bryozoans (Vinn et al., 2018, 2019, 2022). The Baltic Katian symbiotic faunas differ from the Middle Ordovician faunas by the lack of Tremichnus borings in the echinoderms (Rozhnov, 1989) and from the Sandbian faunas by the lack of symbiotic worm borings in the rhynchonellate brachiopods (Vinn, 2005). In Baltica, the Katian is characterized by the appearance of the first macroscopic coral and stromatoporoid symbionts. This is because the Katian was a time when corals and stromatoporoids appeared and rapidly diversified in Baltic Basin. The earliest stromatoporoids and tabulates appeared in the Oandu Regional Stage in the beginning of Katian, which is somewhat earlier than the appearance of their endobionts in the Vormsi Regional Stage (late Katian). In Baltica, the earliest symbiotic rugosans in the bryozoans appeared in the Katian (Vinn et al., 2016). Borers and endobionts preferred massive hosts that provided safer domiciles and greater availability of nutrients. Due to the warming of the climate, low sedimentation rate, coastal upwelling and input of nutrients from pyroclastic material, the bioerosion and diversification of endobionts was enhanced in the region (Toom et al., 2021) Similar to the Sandbian (Vinn et al., 2022), Katian bryozoans hosted a diverse fauna of symbiotic endobionts including rugosans, cornulitids, conulariids ( Climacoconus ) and worms responsible for various bioclaustrations (Vinn et al., 2016, 2018a, b, 2019). The bryozoans hosted the most diverse fauna of endobionts in the Katian of Baltica, but most records of bryozoan symbiosis are restricted to the early Katian. The corals and stromatoporoids hosted just a few groups of endobionts in the Katian. The numerous bryozoan-hosted associations seem to be a major characteristic of the symbiotic endobiont faunas of the Middle to Late Ordovician of Baltica. However, the high number of symbiotic associations with bryozoans does not mean that the endobiont taxa preferred bryozoans over corals and stromatoporoids, but at least partially can be explained by the dominant position of bryozoans among the potential host taxa in the Ordovician of Estonia.
CONCLUSIONS
In addition to tabulate corals, endobiotic cornulitids also colonized stromatoporoids in the Katian of Estonia. As in the Late Ordovician of Laurentia, endobiotic lingulates lived in cavities inside tabulate corals. However, they also colonized massive bryozoan colonies in the Katian of Estonia, which has no known analogue in the Ordovician elsewhere. The Katian is characterized by the appearance of the first macroscopic coral and stromatoporoid symbionts in the Baltica. This is because the Katian was a time when tabulate corals and stromatoporoids appeared and rapidly diversified in the Baltic Basin. The bryozoans hosted the most diverse fauna of endobionts in the Katian, but this can at least partially be explained by the dominant position of bryozoans among the potential host taxa in the Late Ordovician of Estonia.
ACKNOWLEDGEMENTS
We are grateful to G. Baranov, Department of Geology, Tallinn University of Technology for digital photographing of the specimen. This paper is a contribution to the IGCP project 653 ‘The Onset of the Great Ordovician Biodiversification Event’. O.V. and O.T. were supported by a research grant from the Institute of Ecology and Earth Sciences, University of Tartu. U.T. was funded by the Estonian Research Council, grant number PUTJD1106. The research of LEH was supported by the Swedish Research Council (VR Project no. 2018-03390). We are grateful to two anonymous reviewers for constructive comments on the manuscript.
REFERENCES
De Baets, K. and Littlewood, D.T.J. 2015. The importance of fossils in understanding the evolution of parasites and their vectors. Advances in Parasitology, 90:1-51. https://doi.org/10.1016/bs.apar.2015.07.001
De Baets, K., Klug, C., and Korn, D. 2011. Devonian pearls and ammonoid-endoparasite co-evolution. Acta Palaeontologia Polonica, 56:159-180. https://doi.org/10.4202/app.2010.0044
De Baets, K., Keupp, H., and Klug, C. 2015. Parasites of ammonoids, p. 837-875. In Klug, C., Korn, D., De Baets, K., Kruta, I., and Mapes, R.H. (eds.), Ammonoid paleobiology: from anatomy to ecology. Springer, Berlin. https://doi.org/10.1007/978-94-017-9630-9_20
De Baets, K.D., Huntley, J.W., Klompmaker, A.A., Schiffbauer, J.D., and Muscente, A.D. 2021a. The fossil record of parasitism: Its extent and taphonomic constraints, p. 1-50. In De Baets, K. and Huntley, J.W. (eds.), The Evolution and Fossil Record of Parasitism. Springer, Cham. https://doi.org/10.1007/978-3-030-52233-9_1
De Baets, K., Huntley, J.W., Scarponi, D., Klompmaker, A.A., and Skawina, A. 2021b. Phanerozoic parasitism and marine metazoan diversity: dilution versus amplification: Philosophical Transactions of the Royal Society B, 376:20200366.
https://doi.org/10.1098/rstb.2020.0366
Delabroye, A., Vecoli, M., Hints, O., and Servais, T. 2011. Acritarchs from the Ordovician-Silurian boundary beds of the Valga-10 drill core, southern Estonia (Baltica) and their stratigraphical and palaeobiogeographical implications. Palynology, 35:4-45.
https://doi.org/10.1080/01916122.2010.491639
Dixon, O.A. 2010. Endobiotic cornulitids in Upper Ordovician tabulate corals and stromatoporoids from Anticosti Island, Quebec. Journal of Paleontology, 84:518-528. https://doi.org/10.1666/09-129.1
Elias, R.J. 1986. Symbiotic relationships between worms and solitary rugose corals in the Late Ordovician. Paleobiology, 12:32-45. https://doi.org/10.1017/S0094837300002967
Hints, L. and Meidla, T. 1997. Vormsi Stage, p. 81-82. In Raukas, A. and Teedumäe, A. (eds.), Geology and Mineral Resources of Estonia. Estonian Academy Publishers, Tallinn.
Hints, L., Oraspõld, A., and Nõlvak, J. 2005. The Pirgu Regional Stage (Upper Ordovician) in the East Baltic: lithostratigraphy, biozonation, and correlation. Proceedings of the Estonian Academy of Sciences, Geology, 54:225-259.
Hints, L., Harper, D.A.T., and Paškevičius, J. 2018. Diversity and biostratigraphic utility of Ordovician brachiopods in the East Baltic. Estonian Journal of Earth Sciences, 67:176-191. https://doi.org/10.3176/earth.2018.14
Huntley, J.W. and De Baets, K. 2015. Trace fossil evidence of trematode-bivalve parasite-host interactions in deep time. Advances in Parasitology, 90:201-231. https://doi.org/10.1016/bs.apar.2015.05.004
Huntley, J., De Baets, K., Scarponi, D., Linehan, L., Epa, Y., Jacobs, G., and Todd, J. 2021. Bivalve mollusks as hosts in the fossil record, p. 251-287. In De Baets, K. and Huntley, J.W. (eds.), The evolution and fossil record of parasitism: coevolution and paleoparasitological techniques, Topics in Geobiology 50. Springer International, Cham. https://doi.org/10.1007/978-3-030-52233-9_8
Kaljo, D., Hints, L., Hints, O., Männik, P., Martma, T., and Nõlvak, J. 2011. Katian prelude to the Hirnantian (Late Ordovician) mass extinction: a Baltic perspective. Geological Journal, 46:464-477. https://doi.org/10.1002/gj.1301
Kershaw, S. 1987. Stromatoporoid-coral intergrowths in a Silurian biostrome. Lethaia, 20:371-380. https://doi.org/10.1111/j.1502-3931.1987.tb02058.x
Martin, D. and Britayev, T.A. 1998. Symbiotic polychaetes: review of known species. Oceanography and Marine Biology, Annual Review 36:217-340.
Morris, W.R. and Rollins, H.B. 1971. The distribution and paleoecological interpretation of Cornulites in the Waynesville Formation (Upper Ordovician) of southern Ohio. The Ohio Journal of Science, 71:159-170.
Mõtus, M.-A. 1997. Tabulate corals, p. 219-223. In Raukas, A. and Teedumäe, A. (eds.), Geology and Mineral Resources of Estonia. Estonian Academy Publishers, Tallinn.
Mõtus, M.-A. and Vinn, O. 2009. The worm endosymbionts in tabulate corals from the Silurian of Podolia, Ukraine. Estonian Journal of Earth Sciences, 58:185-192. https://doi.org/10.3176/earth.2009.3.03
Nestor, H. and Einasto, R. 1997. Ordovician and Silurian carbonate sedimentation basin, p. 192-204. In Raukas, A. and Teedumäe, A. (eds.), Geology and Mineral Resources of Estonia. Estonian Academy Publishers, Tallinn.
Newall, G. 1970. A symbiotic relationship between Lingula and the coral Heliolites in the Silurian. Geological Journal, Special Issue, 3:335-344.
Oekentorp, K. 1969. Kommensalismus bei Favositiden. Münstersche Forschungen zur Geologie und Paläontologie, 12:165-217.
Palmer, T.J. and Wilson, M.A. 1988. Parasitism of Ordovician bryozoans and the origin of pseudoborings. Palaeontology, 31:939-949. https://www.palass.org/publications/palaeontology-journal/archive/31/4/article_pp939-949
Richards, R.P. and Dyson-Cobb, M. 1976. A Lingula-Heliolites association from the Silurian of Gotland, Sweden. Journal of Paleontology, 50:858-864. https://www.jstor.org/stable/1303581
Rozhnov, S.V. 1989. New data about rhipidocystids (Eocrinoidea), p. 38-57. In Kaljo, D.L. (ed.), Fossil and Recent Echinoderm researches. Academy of Sciences of the Estonian SSR, Institute of Geology, Tallinn.
Słowiński, J., Surmik, D., Duda, P., and Zatoń, M. 2020. Assessment of serpulid-hydroid association through the Jurassic: A case study from the Polish Basin. PLoS ONE, 15:e0242924. https://doi.org/10.1371/journal.pone.0242924
Sokolov, B.S. 1951. Tabuliaty paleozoya Evropejskoj chasti SSSR. Chast I. Ordovik Zapadnogo Urala i Pribaltiki. Gostoptekhizdat, Leningrad-Moscow. Trudy Vsesoyuznogo Nauchno-Issledovatel’skogo Geologo-Razvedochnogo Instituta (VNIGRI), 1-132.
Stewart, L.A., Elias, R.J., and Young, G.A. 2010. Stromatoporoids and colonial corals hosting borers and linguloid brachiopods, Ordovician of Manitoba, Canada. Palaeoworld, 19:249-255. https://doi.org/10.1016/j.palwor.2010.09.013
Stolk, S.P., Holmer, L.E., and Caron, J.B. 2010. First record of the brachiopod Lingulella waptaensis with pedicle from the Middle Cambrian Burgess Shale. Acta Zoologica, 91:150-162. https://doi.org/10.1111/j.1463-6395.2009.00394.x
Tapanila, L. 2003. A new endosymbiont in Late Ordovician tabulate corals from Anticosti Island, eastern Canada. Ichnos, 9:109-116. https://doi.org/10.1080/10420940290208144
Tapanila, L. 2004. The earliest Helicosalpinx from Canada and the global expansion of commensalism in Late Ordovician sarcinulid corals (Tabulata). Palaeogeography, Palaeoclimatology, Palaeoecology, 215:99-110. https://doi.org/10.1016/j.palaeo.2004.08.006
Tapanila, L. 2005. Palaeoecology and diversity of endosymbionts in Paleozoic marine invertebrates: trace fossil evidence. Lethaia, 38:89-99. https://doi.org/10.1080/00241160510013123
Tapanila, L. and Holmer, L.E. 2006. Endosymbiosis in Ordovician-Silurian corals and stromatoporoids: a new lingulid and its trace from eastern Canada. Journal of Paleontology, 80:750-759. https://doi.org/10.1666/0022-3360(2006)80[750:EIOCAS]2.0.CO;2
Taylor, P.D. 2015. Differentiating parasitism and other interactions in fossilized colonial organisms. Advances in Parasitology, 90:329-347. https://doi.org/10.1016/bs.apar.2015.05.002
Toom, U., Vinn, O., and Hints, O. 2019. Ordovician and Silurian ichnofossils from carbonate facies in Estonia: A collection-based review. Palaeoworld, 28:123-144. https://doi.org/10.1016/j.palwor.2018.07.001
Toom, U., Isakar, M., Vinn, O., and Hints, O. 2021. Links between bioerosion and oversized benthic fossils: insights from the Upper Ordovician of Estonia, Baltica. Lille 2021, Ordovician of the World, Programme with abstracts, Lille University, Lille, p. 76-77.
Torsvik, T.H., Cocks, L.R.M., and Harper, D.A.T. 2013. New global palaeogeographical reconstructions for the Early Paleozoic and their generation, p. 5-24. In Servais, T. (ed.), Early Paleozoic Biogeography and Palaeogeography, Geological Society Memoirs. Geological Society, London. https://doi.org/10.1144/M38.2
Truuver, K., Meidla, T., and Tinn, O. 2021. End-Ordovician ostracod faunal dynamics in the Baltic Palaeobasin. Estonian Journal of Earth Sciences, 70:51-69. https://doi.org/10.3176/earth.2021.02
van Dijk, J. and De Baets, K. 2021. Biodiversity and host-parasite (co)extinction, p. 75-97. In De Baets, K. and Huntley, J.W. (eds.), The evolution and fossil record of parasitism: coevolution and paleoparasitological techniques, Topics in Geobiology 50. Springer, Cham. https://doi.org/10.1007/978-3-030-52233-9_3
Vinn, O. 2005. The distribution of worm borings in brachiopod shells from the Caradoc Oil Shale of Estonia. Carnets de Géologie, CG2005_A03. https://doi.org/10.4267/2042/2454
Vinn, O. 2013. Cornulitid tubeworms from the Ordovician of eastern Baltic. Carnets de Géologie, CG2013_L03. https://doi.org/10.4267/2042/51214
Vinn, O. and Mõtus, M.-A. 2008. The earliest endosymbiotic mineralized tubeworms from the Silurian of Podolia, Ukraine. Journal of Paleontology, 82:409-414. https://doi.org/10.1666/07-056.1
Vinn, O. and Mõtus, M.-A. 2012. Diverse early endobiotic coral symbiont assemblage from the Katian (Late Ordovician) of Baltica. Palaeogeography, Palaeoclimatology, Palaeoecology, 321-322:137-141. https://doi.org/10.1016/j.palaeo.2012.01.028
Vinn, O. and Wilson, M.A. 2010. Endosymbiotic Cornulites in the Sheinwoodian (Early Silurian) stromatoporoids of Saaremaa, Estonia. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 257:13-22. https://doi.org/10.1127/0077-7749/2010/0048
Vinn, O. and Wilson, M.A. 2015. Symbiotic interactions in the Ordovician of Baltica. Palaeogeography, Palaeoclimatology, Palaeoecology, 436:58-63. https://doi.org/10.1016/j.palaeo.2015.06.044
Vinn, O., Wilson, M.A., and Toom, U. 2015. Bioerosion of inorganic hard substrates in the Ordovician of Estonia (Baltica). PLoS ONE 10:e0134279. https://doi.org/10.1371/journal.pone.0134279
Vinn, O., Ernst, A., and Toom, U. 2016. Earliest symbiotic rugosans in cystoporate bryozoan Ceramopora intercellata Bassler, 1911 from Late Ordovician of Estonia (Baltica). Palaeogeography, Palaeoclimatology, Palaeoecology, 461:140-144.
https://doi.org/10.1016/j.palaeo.2016.08.016
Vinn, O., Ernst, A., and Toom, U. 2018a. Symbiosis of cornulitids and bryozoans in the Late Ordovician of Estonia (Baltica). Palaios, 33:290-295. https://doi.org/10.2110/palo.2018.018
Vinn, O., Ernst, A., and Toom, U. 2018b. Bioclaustrations in Upper Ordovician bryozoans from northern Estonia. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 289:113-121. https://doi.org/10.1127/njgpa/2018/0752
Vinn, O., Ernst, A., Wilson, M.A., and Toom, U. 2019. Symbiosis of conulariids with trepostome bryozoans in the Upper Ordovician of Estonia (Baltica). Palaeogeography, Palaeoclimatology, Palaeoecology, 518:89-96. https://doi.org/10.1016/j.palaeo.2019.01.018
Vinn, O., Ernst, A., Wilson, M.A., and Toom, U. 2022. Symbiosis in trepostome bryozoans from the Sandbian (Late Ordovician) of Estonia. Historical Biology, 34:1029-1038. https://doi.org/10.1080/08912963.2021.1959579
Wang, H., Zhang, Z., and Holmer, L.E. 2014. Oldest glosselline linguliform brachiopod with soft parts from the Lower Cambrian of Yunnan, Southern China. GFF, 136:539-547. https://doi.org/10.1080/11035897.2014.914969
Yugan, J., Xianguang, H., and Huayu, W. 1993. Lower Cambrian pediculate lingulids from Yunnan, China. Journal of Paleontology, 67:788-798. https://doi.org/10.1017/S0022336000037057
Zapalski, M.K. 2007. Parasitism versus commensalism–the case of tabulate endobionts. Palaeontology, 50:1375-1380. https://doi.org/10.1111/j.1475-4983.2007.00716.x
Zapalski, M.K. 2009. Parasites in Emsian-Eifelian Favosites (Anthozoa, Tabulata) from the Holy Cross Mountains (Poland): changes of distribution within colony, p. 125-129. In Königshof, P. (ed.), Devonian Change: Case Studies in Palaeogeography and Palaeoecology: Special Publications, 314. The Geological Society, London. https://doi.org/10.1144/SP314.6
Zapalski, M.K. 2011. Is absence of proof a proof of absence? Comments on commensalism. Palaeogeography, Palaeoclimatology, Palaeoecology, 302:484-488. https://doi.org/10.1016/j.palaeo.2011.01.013
Zapalski, M.K., Pinte, E., and Mistiaen, B. 2008. Late Famennian ?Chaetosalpinx in Yavorskia (Tabulata): the youngest record of tabulate endobionts. Acta Geologica Polonica, 58:321-324. https://geojournals.pgi.gov.pl/agp/article/view/10033

