 Scavengers and opportunists at work on whale carcasses: Decapods, echinoids and fishes
Scavengers and opportunists at work on whale carcasses: Decapods, echinoids and fishes
Article number: 28.3.a52
https://doi.org/10.26879/1561
Copyright Paleontological Society, November 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 12 April 2025. Acceptance: 28 October 2025.
ABSTRACT
The early Pleistocene whale fall events recognized at the Bargiano site (southwestern Umbria, central Italy) provided the opportunity to document and reconstruct the community of scavengers and opportunists, vertebrates and invertebrates that gathered around cetacean remains. A rich fauna of ghost shrimps and brachyuran crustaceans (the most represented ones), teleost fish and other osteichthyes (i.e., Ophidiiformes and Sciaenidae), sharks, as well as some specimens of irregular echinoids, have been analysed. The occurrence of otoliths of the rare ophidiid fish Hoplobrotula orcianensis Schwarzhans, 1994 and the irregular echinoid Ova canalifera (Lamarck, 1816) are herein reported for the first time in early Pleistocene marine deposits from Umbria. Among the decapods, Jaxea nocturna Nardo, 1847, Goneplax rhomboides (Linnaeus, 1758), and Asthenognathus alleronensis Pasini, Garassino and De Angeli, 2017 are herein recorded, together with some additional well-preserved specimens of the previously reported Albaidaplax ispalensis Garassino, Pasini and Castro, 2013 and Chlinocephalus demissifrons Ristori, 1886. A shark teeth assemblage related to whale fall events is here documented. Moreover, the common tracks of burrowing organisms, referable to activity of crustaceans and echinoids, have been documented, allowing to reconstruct the life on the shallow seabed (maximum depth of about 150m) of scavengers and opportunists, which developed around a whale carcass. The identification of a specialized community (biome) of benthonics and nectonics, contributes to best define this peculiar bioenvironment, confirming the development at Bargiano and Montemoro sites of the four Ecological stages (Scavengers, Opportunists, Sulphophilic and Reef stages) linked to the whale fall events.
Angela Baldanza (corresponding author). Department of Physics and Geology, University of Perugia, Via Pascoli, 06123 Perugia, Italy; Museo dei Cicli geologici di Allerona- Il Golfo dei Cetacei. Via Roma, 05011 Allerona (Terni, Italy). angela.baldanza@unipg.it
Giovanni Pasini. Via Alessandro Volta 16, I-22070 Appiano Gentile (CO), Italy. giovannialdopasini@gmail.com
Alessandro Garassino. Department of Earth and Biological Sciences, Loma Linda University, Loma Linda, 92350 CA, USA. alegarassino@gmail.com
Antonella Carosi. Department of Chemistry, Biology and Biotechnology, University of Perugia, St. Elce di Sotto 5, 06123, Perugia, Italy. antonella.carosi@unipg.it
Bettina Reichenbacher. Department for Earth and Environmental Sciences, Ludwig-Maximilians-University München, Richard Wagner-Str. 10, 80333 Munich, Germany. b.reichenbacher@lrz.uni-muenchen.de
Massimo Lorenzoni. Department of Chemistry, Biology and Biotechnology, University of Perugia, St. Elce di Sotto 5, 06123, Perugia, Italy. massimo.lorenzoni@unipg.it
Federico Famiani. Parco e Museo Vulcanologico, Piazza Roma 1, 05010 San Venanzo, Terni, Italy. federico.famiani@gmail.com
Roberto Bizzarri. Department of Physics and Geology, University of Perugia, Via Pascoli, 06123 Perugia, Italy; PhD freelance researcher. roberto.bizzarri@libero.it
Keywords: Whale falls, Crustacea, Echinoids, Fishes, Sharks, specialized community, southern Europe
Final citation: Baldanza, Angela, Pasini, Giovanni, Garassino, Alessandro, Carosi, Antonella, Reichenbacher, Bettina, Lorenzoni, Massimo, Famiani, Federico, and Bizzarri, Roberto. 2025. Scavengers and opportunists at work on whale carcasses: Decapods, echinoids and fishes. Palaeontologia Electronica, 28(3):a52.
https://doi.org/10.26879/1561
palaeo-electronica.org/content/2025/5709-decapods-echinoids-and-fishes
Copyright: November 2025 Paleontological Society
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
In the present as well as in the past, whale falls (“habitat island” sensu Smith et al., 2015) represent peculiar environments, with a surprising, but transitory, richness of living forms. Several taxa, indeed, proliferate around cetacean remains, regardless of depth, giving rise to specialized biocommunities (Fujioka et al., 1993; Wada et al., 1994; Smith C.R. et al., 2002, 2015; Smith and Baco, 2003; Goffredi et al., 2004; Fujiwara et al., 2007; Lundsten et al., 2010a, 2010b; Amon et al., 2013; Linse et al., 2014; Smith et al., 2014; Sumida et al., 2016). Contrarily to recent case studies, fossil whale falls do not always ensure to document such a species diversity, due to taphonomic processes (Dominici et al., 2009; Danise et al., 2010, 2014; Danise and Dominici, 2014; Baldanza et al., 2018; Zazzera et al., 2022).
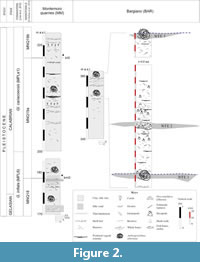
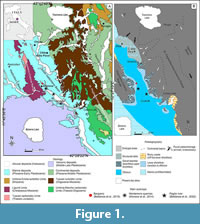 The early Pleistocene Bargiano site (Umbria, central Italy) and the surrounding badland area (Figure 1), represents a unique marine paleoenvironment in which several whale-fall events (WFEs) took place over the span of about one hundred thousand years (MNN18 p.p. - MNN19c Nannofossil zones: Monaco et al., 2014, CNPl8 Nannofossil Zones: Baldanza et al., 2018; Bizzarri and Baldanza, 2020; MNQ18-MNQ19a: this work). In marine clay/silty-clay deposits, referred to a relatively shallow sea environment, four baleen whale skeletons and more than 30 trace fossils, attributed to sperm-whales, were documented (Baldanza et al., 2013a, 2018; Monaco et al., 2014). Through the local stratigraphic section (Figure 2), at least 4-5 (or 6) separated events were identified (Monaco et al., 2014; Baldanza et al., 2018; Bizzarri and Baldanza, 2020); WFEs are not uniformly distributed in time, with a probable increase upwards in WFE frequency. The causes of these concentrations in a strict spatial and temporal range are still unclear. Nonetheless, the whole palaeoenvironment close to a whale carcass was a very peculiar place where for some decades it was possible to exploit the enrichment in organic matter for the development of a long food chain.
The early Pleistocene Bargiano site (Umbria, central Italy) and the surrounding badland area (Figure 1), represents a unique marine paleoenvironment in which several whale-fall events (WFEs) took place over the span of about one hundred thousand years (MNN18 p.p. - MNN19c Nannofossil zones: Monaco et al., 2014, CNPl8 Nannofossil Zones: Baldanza et al., 2018; Bizzarri and Baldanza, 2020; MNQ18-MNQ19a: this work). In marine clay/silty-clay deposits, referred to a relatively shallow sea environment, four baleen whale skeletons and more than 30 trace fossils, attributed to sperm-whales, were documented (Baldanza et al., 2013a, 2018; Monaco et al., 2014). Through the local stratigraphic section (Figure 2), at least 4-5 (or 6) separated events were identified (Monaco et al., 2014; Baldanza et al., 2018; Bizzarri and Baldanza, 2020); WFEs are not uniformly distributed in time, with a probable increase upwards in WFE frequency. The causes of these concentrations in a strict spatial and temporal range are still unclear. Nonetheless, the whole palaeoenvironment close to a whale carcass was a very peculiar place where for some decades it was possible to exploit the enrichment in organic matter for the development of a long food chain.
 In the study area, especially the Bargiano site revealed ideal for an investigation of the relationships among opportunistic micro- and macrofauna in WFEs. This particularly emerged during the excavation and restoration of the most recent cetacean discovery in the area. The communities of benthic foraminifera and decapods associated with these WFEs, in the relatively shallow sea environment of Bargiano, were previously reported by Baldanza et al. (2018). In this paper, the community of opportunists is enriched with the description of new taxa, never reported in conjunction with WFEs. During the recent restoration of the cetacean skeleton (attributed to a probable Balenula sp., WFE 2 in Baldanza et al., 2018), now exhibited in the Allerona museum (catalogued as MUAL 30: Museo dei Cicli Geologici di Allerona, Umbria, Italy), several well-preserved decapod specimens and echinoids were extracted from the silty clay deposits that incorporated the skull and vertebrae. Moreover, the presence of some incomplete remains and burrows referable to potential ghost shrimps was also observed from the Bargiano section (Baldanza et al., 2018) but not described due to their ill preservation (Pasini et al., 2017). In addition to some new burrow structures, related to decapods, fishes, or echinoids activity, they are herein documented and briefly discussed. Finally, particular attention has been paid to sharks, never documented close to fossil whale fall events of Bargiano and Montemoro sites, some of which identified in the past in the Allerona-Orvieto area (Bellocchio et al., 1991). Together with the valuable collection of burrows, presented and analysed here for the first time, new and old fossil findings are discussed, with the aim to reconstruct the infaunal and epifaunal organisms that developed and lived around the cetacean remains.
In the study area, especially the Bargiano site revealed ideal for an investigation of the relationships among opportunistic micro- and macrofauna in WFEs. This particularly emerged during the excavation and restoration of the most recent cetacean discovery in the area. The communities of benthic foraminifera and decapods associated with these WFEs, in the relatively shallow sea environment of Bargiano, were previously reported by Baldanza et al. (2018). In this paper, the community of opportunists is enriched with the description of new taxa, never reported in conjunction with WFEs. During the recent restoration of the cetacean skeleton (attributed to a probable Balenula sp., WFE 2 in Baldanza et al., 2018), now exhibited in the Allerona museum (catalogued as MUAL 30: Museo dei Cicli Geologici di Allerona, Umbria, Italy), several well-preserved decapod specimens and echinoids were extracted from the silty clay deposits that incorporated the skull and vertebrae. Moreover, the presence of some incomplete remains and burrows referable to potential ghost shrimps was also observed from the Bargiano section (Baldanza et al., 2018) but not described due to their ill preservation (Pasini et al., 2017). In addition to some new burrow structures, related to decapods, fishes, or echinoids activity, they are herein documented and briefly discussed. Finally, particular attention has been paid to sharks, never documented close to fossil whale fall events of Bargiano and Montemoro sites, some of which identified in the past in the Allerona-Orvieto area (Bellocchio et al., 1991). Together with the valuable collection of burrows, presented and analysed here for the first time, new and old fossil findings are discussed, with the aim to reconstruct the infaunal and epifaunal organisms that developed and lived around the cetacean remains.
GEOLOGICAL SETTING AND PALEOENVIRONMENTAL BACKGROUND
In the Bargiano section, massive to thinly laminated offshore marine deposits crop out, ranging from clay to clayey silt to silty clay (Figure 2), deposited in a shallow water palaeoenvironment during the early Pleistocene (Baldanza et al., 2013a, 2018; Monaco et al., 2014; Bizzarri and Baldanza, 2020). The site lies close to the town of Allerona and is part of the South Valdichiana Basin (Figure 1). During the early Pleistocene, the basin accommodated a relatively shallow marine environment, on the order of a maximum of 100−150m in depth, based on the comparison between sedimentological data, paleoenvironmental restoration and benthic assemblages (Monaco et al., 2014; Bizzarri et al., 2015; Bizzarri and Baldanza, 2020). The geological, sedimentological, and stratigraphic description of the Bargiano site can be found in Baldanza et al. (2013a, 2018) and Monaco et al. (2014). In addition, a specific GPR investigation on the cololites at the Bargiano site was also led (Ercoli et al., 2021).
In the area between the two sites of Bargiano and Montemoro (Figure 1, Figure 2), early Pleistocene deposits are mainly referable to the MNQ18-MNQ19a Nannofossil Zones (sensu Di Stefano et al., 2023). The two sites are at different altitudes a.s.l. (Bargiano section: 350 to 360 m, Montemoro section: 170 to 230 m), as a consequence of local tectonics (Monaco et al., 2014). In the whole area, beds have a general ~10° NE dip. A composite, ~60m-thick stratigraphic section well represents the Montemoro-Bargiano area (Figure 2). Massive to thinly stratified, clayey silt to clay deposits prevail (Figure 2), with secondary fine sands. Fossils are commonly scattered in the sediment, often found in life position or showing minimal reworking: nonetheless, almost monospecific layers, made of solitary corals or echinoid fragments, as well as accumulations of large pectinids and/or oysters, were noticed (Figure 2). These accumulations may reflect hydrodynamic conditions, changes in sedimentation rate, or local paleoenvironmental features (Bizzarri and Baldanza, 2020). The most relevant, several WFEs (at least 5, but probably more), were reported (Monaco et al., 2014; Bizzarri and Baldanza, 2020). Only considering the site of Bargiano, three events, with their particular fossil associations, have been recognized in a 6 m-section (magnification in Figure 2: Baldanza et al., 2018).
Palaeoecological background
The paleontological heritage of the Bargiano site, where a cetacean skeleton (Balenula sp., MUAL 30) and about 27 fossil structures classified as Ambergrisichnus alleronae ichnofossil (linked to the presence of sperm-whale carcasses) have been found, represents a window into an early Pleistocene marine paleoenvironment. The entire Allerona area (Montemoro quarries and Bargiano badlands, Figure 1, Figure 2) represents an unusual, relatively shallow water paleoenvironment where several WFEs took place over a span of about 100,000 years (Monaco et al., 2014; Baldanza et al., 2018). Table 1 shows the distribution of fossil groups (Protozoa, Molluscan, Decapods, Echinoids, Teleost and Cartilaginous Fishes, Echinoderms) found at the Bargiano and Montemoro sites.
The benthonic foraminifera communities and the decapods recovered from the site (Pasini et al., 2017; Baldanza et al., 2018), clearly revealed that a locally high nutrient flux influenced biota in the first few millimetres or centimetres of water-sediment interface. Moreover, the enrichment in nutrients on the sea bottom, related to whale-carcass biomass (evidenced by frequencies of opportunistic benthic foraminifera) confirmed the lateral dispersion, around the carcass, for a radius of at least 10 m (Baldanza et al., 2018). Some opportunists, among the Protozoa, such as the benthic foraminifera associated with these WFEs (i.e. the shallow infaunal taxa Bigenerina nodosaria, Bannerella gibbosa, Marginulinopsis costata, and Vaginulina cf. V. striatissima, along with the epifaunals Lenticulina calcar and Siphotextularia concava; Table 1) respond to short-term favourable conditions by increasing in number and maintaining stable populations, revealing that a nutrient-rich environment favoured their proliferation (Baldanza et al., 2018). The occurrences of benthic foraminifera across the three WFEs along with the presence of the brachyuran Albaidaplax ispalensis Garassino, Pasini and Castro, 2013 (Goneplacidae), Chlinocephalus demissifrons Ristori, 1886 (Euryplacidae), and Asthenognathus alleronensis Pasini, Garassino and De Angeli, 2017 (Varunidae), also offered new insights into shallow sea whale-fall fossil communities.
MATERIALS AND METHODS
Both during the 2016 field investigation and the successive restoration of baleen remains (2018-2022), several specimens of decapod crustaceans, fish bones and otoliths, shark teeth, echinoids, as well as trace fossils have been recovered and are herein described.
The fossils were extracted from the interval indicated in Figure 2 as WFE 2 (Bargiano site), in clay deposits of about 20 cm thick and in deposits 20 cm above. There is no evidence of reworking or mechanical concentration by bottom currents. The decapods and irregular echinoids were found, respectively, with the carapace and the thecae in life position, not overturned.
The decapod studied specimens are three-dimensionally preserved, embedded in small-sized blocks of grey/light blue clay recovered within the bones of the whale carcass during the restoration of the whale skeleton. The irregular echinoids are slightly compressed. These specimens were both collected in the field (during the 2016 campaign) or recovered in the laboratory phase, during the restoration of whale skeleton. The fish remains were disarticulated, and most of the skeleton pulverized during the recovery; only otoliths and vertebrae are preserved. The decapod and echinoid specimens were fixed with a film of polyvinyl acetate for study and preservation. The specimens of echinoid, decapod, fish vertebrae and otoliths, whale skeleton (Cataloguing codes MUAL 1-61), shark teeth (Subcode MM 1-12 and BAR 1) and trace fossils (temporarily not catalogued), are housed in the palaeontological collection of the Museo dei Cicli Geologici di Allerona (Umbria, Italy) and the best preserved displayed in the main room. Burrows and other trace fossils were firstly described and photographed on the site, and the best preserved were collected and photographed in detail in laboratory.
RESULTS
Decapod crustaceans
 Jaxea nocturna Nardo, 1847 (2 specimens: MUAL 35, 45), Goneplax rhomboides (Linnaeus, 1758) (1 specimen: MUAL 31) and Asthenognathus alleronensis Pasini, Garassino and De Angeli, 2017 (1 specimen: MUAL 42) are herein added to the list of the species part of the WFE Bargiano community collected around the whale bones and skull (Figure 2). We point out the discovery of an additional specimen of Chlinocephalus demissifrons Ristori, 1886 (MUAL 32-33), preserving right P1 and fragments of P2 and P3 (Figure 3E-F) (13 specimens overall) and six specimens of Albaidaplax ispalensis Garassino, Pasini and Castro, 2013 (MUAL 36, 38; 43; 44; 46; 47), both species already recorded by Baldanza et al. (2018) representing the most common decapod species in the WFEs decapod community.
Jaxea nocturna Nardo, 1847 (2 specimens: MUAL 35, 45), Goneplax rhomboides (Linnaeus, 1758) (1 specimen: MUAL 31) and Asthenognathus alleronensis Pasini, Garassino and De Angeli, 2017 (1 specimen: MUAL 42) are herein added to the list of the species part of the WFE Bargiano community collected around the whale bones and skull (Figure 2). We point out the discovery of an additional specimen of Chlinocephalus demissifrons Ristori, 1886 (MUAL 32-33), preserving right P1 and fragments of P2 and P3 (Figure 3E-F) (13 specimens overall) and six specimens of Albaidaplax ispalensis Garassino, Pasini and Castro, 2013 (MUAL 36, 38; 43; 44; 46; 47), both species already recorded by Baldanza et al. (2018) representing the most common decapod species in the WFEs decapod community.
Abbreviations - lcxp: carapace length (including rostrum); lpa: palm length (excluding index); LT: total length; MUAL: Museo dei Cicli Geologici di Allerona (TR); P1-P5: pereiopods 1 to 5; wcxp: width of carapace; wpa: palm width.
SYSTEMATIC PALAEONTOLOGY
Infraorder THALASSINIDEA Latreille, 1831
Superfamily THALASSINIDEA Latreille, 1833
Family LAOMEDIIDAE Borradaile, 1903
Genus JAXEA Nardo, 1847
Type species. Jaxea nocturna Nardo, 1847, by original designation.
Included fossil species. Jaxea kuemeli Bachmayer, 1954; J. nocturna Nardo, 1847.
Jaxea nocturna Nardo, 1847
Figure 3A-B
Material and measurements. One nearly complete carapace with chelipeds twisted laterally:
MUAL 35 - LT: 30mm; lcxp: 20mm; wcxp: 6mm; lpa: 10mm; wpa: 4mm; and one smaller, nearly complete articulated specimen in lateral view. MUAL 45 - lcxp: 8.5mm; wcxp: 4mm; lpa: 5mm; wpa: 3mm.
Description. Carapace subcylindrical, elongate, convex posteriorly; linea thalassinica well defined and straight antero-posteriorly; dorsal ridges present; distinct cervical groove; short front with triangular, short pointed rostrum; P1 chelate, equal, strongly developed; movable finger slightly longer than fixed finger; occlusal margins of both fingers with three/four large rounded teeth proximally, median triangular tooth and small round teeth in distal half; slender, incomplete P2-P5.
Discussion. As reported by Ngoc-Ho (2003), the extant Jaxea nocturna is characterized in having triangular rostrum, pointed anteriorly; linea thalassinica; cervical groove well defined, longitudinal dorsal ridges present, posterior margin convex; P1 chelate, equal, and greatly developed, nearly as long as body; movable finger slightly longer than fixed finger; occlusal margins of movable and fixed fingers with three or four large rounded teeth proximally, median triangular tooth and small rounded teeth in distal half; and P2-P5 slender and simple.
These morphological characters well fit those of the studied specimen that is assigned to this species. The extant (and fossil) species is considered an active infaunal burrower in near shore (Dworschak, 2004), widespread in SW Scotland, Ireland, SW England, English Channel, Bay of Biscay, Canyon of Cape Breton, and Marseille (France), South Spain, Atlantic coast of Morocco, and East Mediterranean Sea (Ngoc-Ho, 2003).
Jaxea nocturna is known to date in the fossil record from the Pliocene and Pleistocene of some localities of Tuscany (central Italy) (Delle Cave, 1988; Baldanza et al., 2013a, 2017; Pasini et al., 2017), and from the Pliocene of Catalonia (Spain) (Garassino et al., 2009).
Note. Several loose, incomplete ghost shrimp propodi were previously observed from the Bargiano fossiliferous site, left in open nomenclature as Jaxea sp. (Pasini et al., 2017).
Infraorder BRACHYURA Latreille, 1803
Superfamily GONEPLACOIDEA MacLeay, 1838
Family GONEPLACIDAE MacLeay, 1838
Subfamily GONEPLACINAE MacLeay, 1838
Genus GONEPLAX Leach, 1814
Type species. Ocypoda bispinosa Lamarck, 1801, by original designation.
Included fossil species. See Garassino et al. (2013).
Goneplax rhomboides (Linnaeus, 1758)
Figure 3C
Material and measurements. One nearly complete carapace with chelipeds and incomplete walking legs in dorsal view MUAL 31 - lcxp: 26mm; wcxp: 31mm.
Description. Subrectangular carapace, convex longitudinally; straight front extended beyond the orbits; well-developed orbits larger than the front; sinuous, elongate supraorbital margin; pointed outer-orbital spine; lateral margins slightly concave up to the small antero-lateral spine and convergent the posterior margin; dorsal regions not marked; smooth dorsal surface; branchial regions with a weak transverse depression; elongate P1 palm with gently curved, pointed dactylus; elongate slender P2-P5.
Discussion. Based upon Garassino et al. (2013), the morphological characters above described are consistent with those observed in the fossil and extant Goneplax rhomboides (Linnaeus, 1758) to which the studied specimen is assigned. Extant G. rhomboides is widespread in the eastern Atlantic, northern Africa, and Mediterranean Sea. Goneplax rhomboides burrows in sublittoral shallow muddy and sandy bottoms from a few to about 100 m deep (Garassino et al., 2012). The fossil record from Italy ranges from the Miocene to the Pleistocene of Piedmont, Emilia Romagna, Tuscany, Lazio, Basilicata and Sicily (Garassino et al., 2013; Girone et al., 2024).
Note. The presence of G. rhomboides at the Bargiano WFE was previously suspected only, based on some specimens not having, however, enough diagnostic characters for a confident assignment to this species (Pasini et al., 2017).
Superfamily GRAPSOIDEA Macleay, 1838
Family VARUNIDAE H. Milne-Edwards, 1853
Subfamily ASTENOGNATHINAE Stimpson, 1858
Genus ASTHENOGNATHUS Stimpson, 1858
Type species. Asthenognathus inaequipes Stimpson, 1858, by monotypy.
Included species. See Schweitzer et al. (2010).
Asthenognathus alleronensis Pasini, Garassino and De Angeli, 2017
Figure 3D
Material and measurements. One slightly compressed complete carapace, in dorsal view. MUAL 42 - lcxp: 9.5mm; wcxp: 1.1mm (as preserved).
Description. Carapace subexagonal, slightly compressed transversally, convex dorsally wider than long; front short, nearly straight with a median longitudinal groove; orbits ellipsoidal, oblique to the frontal margin; anterolateral margins diverging posteriorly, sinuous medially; posterolateral margins shorter than the anterolateral margins, tapering posteriorly; posterior margin straight, shorter than the orbitofrontal margin; dorsal regions well defined, inflated, separated by deep grooves; inner surface covered with weak granulations; brachial regions wide, inflated dorsally.
Discussion. The studied specimen shares the morphological characters of Asthenognathus Stimpson, 1858, and especially of A. alleronensis Pasini, Garassino and De Angeli, 2017 in having notably less elongate carapace, shorter front, oblique orbits to the frontal margin; nearly straight anterolateral margins, poorly defined oblique ridge facet on posterolateral margin; shorter posterior margin and more inflated rounded branchial regions. This represents the second report for the species, known from the early Pleistocene of Bargiano only.
Echinoids
About 15 specimens of echinoids have been recovered during the paleontological excavation of the whale skeleton. At least five specimens (MUAL 50-54) are in good preservation state, neither fragmented nor bioeroded. The theca is grey or dark brown in colour; unfortunately, the clayey sediments have not preserved the three dimensionality of the theca, but the morphological details (petals and plaques) are perfectly well-preserved (Figure 4).
Due to compaction, only a limited number of measurements could be made: nonetheless, the shape and morphology of the theca lead to the attribution to the genus Ova, and in particular to the species Ova canalifera.
Order SPATANGOIDA L. Agassiz, 1840
Suborder PALEOPNEUSTINA Markov and Solovjev, 2001
Family SCHIZASTERIDAE Lambert, 1905
Genus OVA Gray, 1825
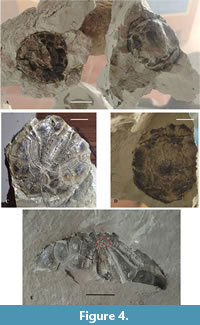 Type species. Ova canalifera (Lamark, 1816) (Figure 4).
Type species. Ova canalifera (Lamark, 1816) (Figure 4).
Material and measurement. The measurements are the total length (TL) and the width (W) of the five best preserved specimens. Specimen A, aboral side (Figure 4A) = TL 43mm, W= 41mm; Specimen B, oral side (Figure 4B) = TL 44mm, W= 42mm; Specimen C, aboral side (Figure 4C) = TL 45mm; Specimen D, oral side (Figure 4D) = TL 42mm, W=38 mm; Specimen E, aboral side half theca (Figure 4E), TL= 42mm, W (1/2) = 22 mm.
Description. The occurrence of two pores in the apical site, unfortunately not visible in all the specimens, allows to identify the species Ova canalifera. The distribution of plaques and petals is comparable with those of O. canalifera.
Distribution. Ova canalifera is reported in Piacenzian of Emilia (Sariano and Rio dei Carbonari, Piacenza) and in San Nicomede sullo Stirone (Parma); it is common in early Pleistocene deposits outcropping along the Arda creek (Castell’Arquato), Stirone creek (Salsomaggiore) and Enza creek (San Polo). Checchia Rispoli (1907, 1923) reported this species from the Pleistocene deposits of Ficarazzi (Palermo, Sicily), and from Monte Mario (Roma) and Anzio (Latium). The species is endemic of the Mediterranean Sea. In some levels along the Arda River near Castell'Arquato and the Stirone River near Salsomaggiore the presence of a good number of preserved specimens, with complete aculeation and in the living position was reported (Borghi, 2020).
Fish remains
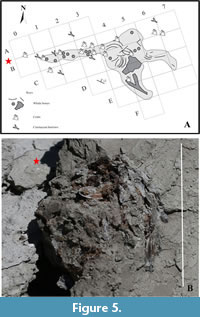 Fossil fish remains are rare, only one disarticulated skeleton was found during the 2016 Bargiano excavation, about two meters away from the whale skeleton (Figure 5, Figure 6). Unfortunately, during extraction from the enclosing clay sediment, the most delicate parts of the fragile skeleton were pulverized, only the otoliths and vertebrae are preserved.
Fossil fish remains are rare, only one disarticulated skeleton was found during the 2016 Bargiano excavation, about two meters away from the whale skeleton (Figure 5, Figure 6). Unfortunately, during extraction from the enclosing clay sediment, the most delicate parts of the fragile skeleton were pulverized, only the otoliths and vertebrae are preserved.
In addition, other four otoliths were recovered in the Montemoro site (Figure 2, Figure 7).
Order OPHIDIIFORMES Berg, 1937
Family OPHIDIIDAE Rafinesque, 1810
Subfamily NEOBYTHITINAE Radcliffe, 1913
Genus HOPLOBROTULA Gill, 1863
Type species. Hoplobrotula armata (Temminck and Schlegel, 1846)
Hoplobrotula orcianensis Schwarzhans, 1994
Figure 6
Material and measurements. Two large otoliths presumably belonging to the same specimen, anteroposterior max length: 10.3 mm; max height 5.1 mm (Figure 6E-F). One vertebral centra, in lateral and ventral view; max height 4.5 mm, max width 8.5 mm (Figure 6A-D).
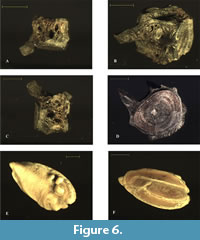 Description. Following Schwarzhans (1994), the otolith shows supposed female morphologies (Figure 6E-F). It has an elongated shape, measuring approximately twice its width. It is rounded anteriorly and distinctly pointed posteriorly, with both extremities being located at a midaxial position. The dorsal margin curves gently, displaying almost the same symmetry as the ventral margin. Sulcus and colliculi are as described in Schwarzhans (1994). It is not possible to formulate further hypotheses on the specimen size based on otolith dimension, due to the limited information available in the literature.
Description. Following Schwarzhans (1994), the otolith shows supposed female morphologies (Figure 6E-F). It has an elongated shape, measuring approximately twice its width. It is rounded anteriorly and distinctly pointed posteriorly, with both extremities being located at a midaxial position. The dorsal margin curves gently, displaying almost the same symmetry as the ventral margin. Sulcus and colliculi are as described in Schwarzhans (1994). It is not possible to formulate further hypotheses on the specimen size based on otolith dimension, due to the limited information available in the literature.
Discussion. Members of the order Ophidiiformes, to which the fossil taxon Hoplobrotula orcianensis belongs, includes mainly benthic fishes that live in close contact with the seabed, where they dig holes of mucus-coated mud or sand. The order had its greatest expansion during the Paleogene, when it was represented by a very rich neritic fauna inhabiting mainly on soft and muddy sea bottom (Nolf, 1980). There are only three extant species belonging to Hoplobrotula, which are known from the Indian Ocean and western Pacific: Hoplobrotula armata (Temminck and Schlegel, 1846), Hoplobrotula badia Machida, 1990, and Hoplobrotula gnathopus (Regan, 1921). These three species can be considered relict species of a previously much more diversified group (Nolf, 1980). Fossil otoliths of Hoplobrotula orcianensis have hitherto been recorded exclusively from the early Pliocene. In Italy, this species was described from Orciano (Tuscany) (Schwarzhans, 1994; Cigala-Fulgosi et al., 2009), from which the name of the species derives, and Monticello (Piedmont) (Nolf and Cavallo, 1994). Other fossil species of the genus Hoplobrotula are exclusively known from the Palaeomediterranean ichthyofauna (Nolf et al., 1998; Nolf, 2013; Schwarzhans and Carnevale, 2024).
Ecology, behaviour and diet. Ophidiiform fishes generally show a close association with the sea bottom, where they dig burrows into soft and movable sediments with the help of tail movements (Grzimer, 2003). They usually remain buried during the day, and they are most active at night when they go out to look for preys. Ophidiiformes have a varied diet that includes invertebrates (decapods, cephalopods and polychaetes), and small bottom dwellers fishes such as gobies. Information on the feeding habits of the extant Hoplobrotula species is scarce, but Hoplobrotula armata is known as a carnivorous species feeding on decapods (shrimps, crabs, hermit crabs) and teleost fishes (Baeck et al., 2012; Park et al., 2023).
Other Otoliths
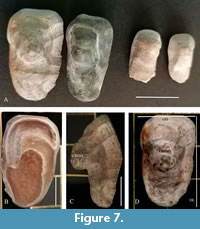 In the Montemoro site, four specimens of large otoliths (MUAL 58-61) were found in clay sediments and housed in the Museum of Allerona. The morphological characters (Figure 7) and the shape are diagnostic of Sciaenidae representatives of Argyrosomus regius (Asso, 1801) (known as meagre). The two larger otoliths possess values of anteroposterior max length of 28 mm and 26 mm, the two smaller ones of 17 mm and 16 mm. The values of otolith height are 15mm and 13mm for the two larger otoliths, and 8 mm for both smaller ones.
In the Montemoro site, four specimens of large otoliths (MUAL 58-61) were found in clay sediments and housed in the Museum of Allerona. The morphological characters (Figure 7) and the shape are diagnostic of Sciaenidae representatives of Argyrosomus regius (Asso, 1801) (known as meagre). The two larger otoliths possess values of anteroposterior max length of 28 mm and 26 mm, the two smaller ones of 17 mm and 16 mm. The values of otolith height are 15mm and 13mm for the two larger otoliths, and 8 mm for both smaller ones.
The remarkable sizes of otoliths indicate the occurrence of adult specimens of large size. The euryhaline species live on sandy bottoms close to the shore and can penetrate river mouths and brackish lagoons; it is also capable of living at depths between 15 and 300 m.
Based on Gabriel et al. (2012), it is possible to hypothesize a length and weight of fishes, based on otolith sizes. Probably, the larger sciaenid specimens of Montemoro could reach 100 cm in length, weighing 8-10 kg, and 50 cm in length weighing 3-6 kg the smaller ones.
Sharks
 Among the scavengers, sharks play a dominant role in WFEs. The cartilaginous skeleton of sharks is normally not preserved in the fossil record, making the teeth the most abundant records of fossil sharks. Some new shark teeth (Figure 8) have been found in the Bargiano site, close to the skeleton of the cetacean, and in the Montemoro area, probably related to the occurrence of three cetacean skeletons, recovered in the time span of the last 20 years. These new findings become the first report of shark activity around a whale fall event, at least for the Umbria region (central Italy). The occurrence of shark teeth is strictly related to whale fall events as demonstrated by Bianucci et al. (2019) that recovered in whale fall communities a notable biodiversity even among cartilaginous fish, in particular Carcharhiniforms sharks, opportunists par excellence who take advantage of the carcasses to feast. Ichnological studies (Merella et al., 2023) have been done on whale bones and have shown that they have tooth marks that correspond to certain species of Carcharhiniformes teeth typical of these opportunistic situations.
Among the scavengers, sharks play a dominant role in WFEs. The cartilaginous skeleton of sharks is normally not preserved in the fossil record, making the teeth the most abundant records of fossil sharks. Some new shark teeth (Figure 8) have been found in the Bargiano site, close to the skeleton of the cetacean, and in the Montemoro area, probably related to the occurrence of three cetacean skeletons, recovered in the time span of the last 20 years. These new findings become the first report of shark activity around a whale fall event, at least for the Umbria region (central Italy). The occurrence of shark teeth is strictly related to whale fall events as demonstrated by Bianucci et al. (2019) that recovered in whale fall communities a notable biodiversity even among cartilaginous fish, in particular Carcharhiniforms sharks, opportunists par excellence who take advantage of the carcasses to feast. Ichnological studies (Merella et al., 2023) have been done on whale bones and have shown that they have tooth marks that correspond to certain species of Carcharhiniformes teeth typical of these opportunistic situations.
The ichthyodontholith fauna of the Allerona area, previously studied by Bellocchio et al. (1991) and originally reported from “Piacentian” (late Pliocene) grey clays, is now stratigraphically referred to the Early Pleistocene (Calabrian). The teeth were found in the clay quarry of the Allerona brick furnace and are represented by Chondrichthyes and Osteichthyes which are mostly comparable to extant forms. Moreover, the authors identified for this fauna a circalittoral habitat, with temperate-warm waters at an average depth not exceeding -100m. Unfortunately, the authors collected specimens over the surface where they have accumulated after being carried away by runoff water that has acted on small clay gullies. Therefore, there are strong doubts about the precise source levels of specimens, and the rich and diverse assemblage could be the result of reworking processes.
The following teeth specimens were collected in the Bargiano (BAR) and Montemoro (MM) sites and now are exposed at the museum of Allerona.
Class CHONDRICHTHYES Huxley, 1880
Subclass ELASMOBRANCHII Bonaparte, 1838
Order LAMNIFORMES Berg, 1958
Family LAMNIDAE Bonaparte 1835
Genus COSMOPOLITODUS Glikman, 1964
Type species. Cosmopolitodus hastalis (Agassiz, 1838)
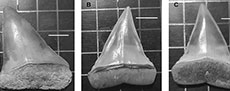
Cosmopolitodus hastalis (Agassiz, 1838)
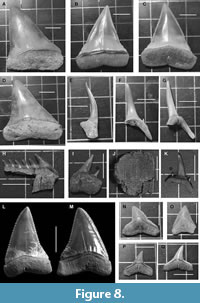
Figure 8A-D
Material and measurements. From Bargiano site 1 teeth of lower jaw (3.5 cm in length, specimen MUAL BAR1; Figure 8B-C) and from Montemoro site 4 teeth of 4.0 cm (specimen MUAL MM1; Figure 8A), 4.1cm (specimen MUAL MM2; Figure 8D), 3.8 cm, 3.2 cm and 3.0 cm (broken and without root) in length.
Description. Medium-sized teeth, erect triangular shape in the anterior teeth and slightly bent towards the commissure in the lateral and posterior teeth; extremely robust constructions with smooth and sharp edges, never serrated. Lateral denticles are absent. Bilobed root with well-defined branches.
The upper anterior teeth with regular triangular shape, external face of the crown flat or slightly concave. Near the base of the enamel surface of the external side, there are some slightly significant folds; of these folds, only the median one widens and reaches the apex. Sigmoidity of the tooth is not very accentuated, only the apex is slightly inclined towards the inside of the mouth. The root tends to widen the two branches, while the apex leans towards the commissure in a barely perceptible manner. The root has asymmetrically developed but well-defined lobes, with angles between 90° and 100°. All the specimens belong to the upper jaw.
Distribution. A total of 10 specimens come from the quarry of Fornace di Allerona sampled in the surface outcrop, transported by washing from small clay gullies (Bellocchio et al., 1991), four specimens were collected in Montemoro and Bargiano sites. Cosmopolitodus hastalis was the most common predator and frequented the Gulf of Allerona until the early Pleistocene, confirming its presence within the Quaternary (Danise and Dominici, 2014).
Ecology, behavior and diet. Cosmopolitodus hastalis is closely related to modern Carcharodon carcharias, and body sizes of both taxa are also similar (more than 6 m in large adults: Ehret et al., 2013). Similar to other extinct and extant lamniform sharks, juvenile specimens of C. hastalis are thought to mainly live and forage in shallow productive marine environments (Collareta et al., 2017). For example, Collareta et al. (2017) reported a skeleton of a juvenile C. hastalis with stomach contents composed of small to medium sized fish remains, supporting the former hypothesis.
Genus CARCHARODON Smith 1838
Type species. Carcharodon carcharias (Linneus, 1758)
Material and measurement. 1 tooth (MUAL MM3: Figure 8L-M) of 3.2 cm in length.
Description. The tooth of our specimen of C. carcharias have a triangular shape with finely serrated edges. Coarse irregular serrations, and thin triangular crown. Vertical folds are visible near the contact between root and enamel. The root is bilobed with poorly developed branches. The lacking of finely serrated margin and the shape allow us to assign the tooth to the upper jaw.
The general description of teeth belonging to the maxillary arch (upper jaw) have a convex crown on the internal side, while it is flat or slightly concave on the external side. On this side, near the contact line between the root and the enamel, there are a large number of vertical folds that decrease the size going from the center of the tooth towards the edges; the only median fold crosses the entire enamel, from the apex to the root. The root is bilobed with poorly developed branches.
Lower arch: a shape that is generally slender than the upper ones. The margins are generally concave and finely serrated. The inner side has a rather accentuated convexity while the outer side is practically flat. Numerous folds of enamel are present in the teeth of the lower arch, which can be found near the crown-root attachment. The latter is bilobed and more developed on the inner side of the tooth (Bass et al., 1975; Carcharodon carcharias (Linnaeus, 1758) | Shark-References)
Distribution. Carcharodon carcharias is reported from Pliocene and early Pleistocene deposits of Sicily (Cusumano and Di Patti, 2006).
Ecology and behavior. Biology Dataset (Uhen et al., 2024) shows that in Pliocene and Pleistocene marine waters were inhabited by more than 500 shark species and among them Carcharhiniformes that represent the apex predators also of the present-day global ocean. Even if only Carcharodon carcharias is well known as a formidable active predator of marine mammals (pinnipeds and odontocetes), it may also behave as a scavenger. Carcharodon carcharias (Pliocene-Present) inhabits from temperate water seas to the oceans (Mediterranean, Pacific, Atlantic), at depths between the surface and 250 m; it is a great sea predator and is often to be spotted along the coasts (Cusumano and Di Patti, 2006). Like C. carcharias several anecdotal and scientific documentation exists for white sharks feeding on floating whale carcasses, which are rich in blubber, and consequently, highly energetic (Shark foundation database).
Family ODONTASPIDIDAE Müller and Henle, 1839
Genus CARCHARIAS Rafinesque, 1810
Type species. Carcharias taurus Rafinesque, 1810
Materials and Measurement. 11 incomplete teeth of lower jaw (4 tooth fragments shorter than 4 cm in length, with root partially preserved; 7 with length <2 cm without root) and 1complete tooth (<2 cm) of upper jaw (Figure 8E-G, K).
Description. The long teeth exemplars (Figure 8E-G; MUAL MM4-6) may belong to the lower jaw, unfortunately the roots are not complete but the lingual and groove bisect of root protuberance are present, and at least one of the two lateral denticle are preserved. The complete tooth (Figure 8K; MUAL MM7) belongs to the upper jaw.
The dentition of Carcharias taurus (Cunningham, 2000) has large narrow teeth bearing one sharp lateral denticle on each side of the base of the crowns. A distinct nutrient groove bisects the lingual root protuberance on all of the teeth. Roots are bilobed and crowns bear cutting edges which separate a strongly inflated lingual crown surface from a weakly inflated labial surface.
Distribution. Teeth similar in shape to those of the extant Carcharias taurus can be found worldwide in Neogene neritic deposits. The teeth of this morphology are the most abundant shark teeth in these deposits (Holtke et al., 2024). This species has a nearly cosmopolitan distribution with a preference for temperate to warm waters, ranging between 12.5 and 28 °C. The sand Tiger Shark occurs either alone or in small to medium-sized aggregations of 20-80 individuals (Walls and Soldo, 2016). It is present along the coasts in all oceans, except the eastern Pacific. It can be found in the Mediterranean, the Gulf of Aden and the African coast, Madagascar included, up to South Africa and Namibia (Fioravanti et al., 2020; Holtke et al., 2024).
Ecology, behavior and diet. The species is demersal and pelagic in tropical and temperate seas on the continental shelf at depths of 0-232 m, however it mainly occurs in shallow waters of 15-25 m depth (Fioravanti et al., 2020; Holtke et al., 2024). It is often observed hovering motionless just above the seabed in or near deep, sandy gutters or rocky caves, usually in the vicinity of inshore rocky reefs and islands. The species is generally coastal, usually found from the surf zone down to around 25 m depth. It may also occasionally be found in shallow bays, around coral reefs, and very rarely to depths of around 200 m on the continental shelf. It usually lives near the bottom but may also move throughout the water column (Walls and Soldo, 2016).
Order CARCHARHINIFORMES Compagno 1977
Family CARCHARINIDAE Jordan and Evermann 1896
Genus CARCHARHINUS Blainville, 1816
Type species. Carcharhinus longimanus (Poey, 1861), Figure 8N-Q
Materials and measurement. 21specimens of small size (17 lower teeth and 4 upper teeth) plus 8 medium size (2 upper teeth and 6 lower teeth).
Description. Dentition characterized by a very strong positional heterodonty and a great intraspecific variability. The tooth is of medium-small size with smooth or crenulated edges. Very developed root system with well-defined branches. The upper teeth (Figure 8N-O; MUAL MM8-9) are characterized by very broad and triangular shaped cusps, with serrated lateral cutting edges. Lateral to the cusps a distal coarse heel may be present. The anterior teeth are characterized by straight and regularly serrated lateral cutting edge. The labial face is flat, while the lingual one is convex and characterized by a well-developed neck-area between the crown-base and the root. The root is high and characterized by a weakly lingual axial groove. The basal margin of the root is straight to slightly concave.
The lower teeth (Figure 8P-Q; MUAL MM10-11) are characterized by broad, erected and nail-like cusps. The lateral cutting edges are regularly serrated, but the serrations stop at the midway along the lateral margins. Lateral to the cusps, there are smoothed mesial and distal heels. The basal margin of the root is straight to slightly concave (Marsili, 2007).
Distribution. Marsili (2007) reported that C. longimanus has been recorded in the Pliocene sediments of South-East France (Cappetta and Nolf, 1991), of Tuscany (Landini, 1977; Manganelli and Spadini, 2003) and of Alicante in South-East Spain (Mora Morote, 1996). Some voracious species of Carcharinus (i. e. leucas, longimanus and obscurus) are considered as occasional or regular predators, or scavenging of cetacean, in particular small Odontoceti (Heithaus, 2001). The occurrence of C. longimanus in the early Pleistocene of southwestern Umbria is stratigraphic information that allows to enlarge the fossil records.
Ecology, behavior and diet. C. longimanus is a solitary species, but they can be observed in groups in the presence of abundant food sources. Its diet includes cephalopods and bony fish.
The species is the most abundant in the Allerona area.
Order HEXANCHIFORMES BuenDe, 1926
Family HEXANCHIDAE Gray, 1851
Genus HEXANCHUS Rafinesque, 1810
Type species. Hexanchus griseus (Bonaterre, 1788)
Material and measurements. 3 teeth of the lower jaw and 1 tooth of the upper jaw (Figure 8H-I).
Description. Only three living genera and four species belong to the family Hexanchidae. The most conspicuous diagnostic features of these sharks are the presence of six or seven gill slits (Adnet, 2006). The tooth of lower jaw (Figure 8H; MUAL MM12) is incomplete, with best preserved the fine serrations of mesial side of 1st cusp; only 6 cusps, that get smaller toward the distal end, are visible. The root are thin, flat and rectangular. The upper anterior tooth (Figure 8I; MUAL MM13) shows a reduced number of cusps and more inflated rectangular root.
Distribution. The Hexanchus genus consists of species known to date from as far back as the Jurassic (Beaumont, 1960). Taxa are mainly based on isolated lower teeth. Fossil remains from the Late Cretaceous and Cenozoic are better known, generally more numerous and include the three extant genera. Currently only one species survives, Hexanchus griseus (Miocene-recent). This species lives mainly in deep water, beyond 200 m and as far as 2000 m. While it primarily occupies tropical and temperate waters, it may also be found in the Mediterranean (Cusumano and Di Patti, 2006).
Ecology, behavior and diet. The species spends most of the daytime in deeper areas and undertakes vertical migrations towards shallower areas during night, influenced by foraging opportunities (Andrews et al., 2009; King and Surry, 2017). The diet and feeding habits of H. griseus have been studied throughout its distribution range, including the Mediterranean Sea, using both stomach contents and stable isotopes (Ruiz-Garcia et al., 2023). The feeding activity of H. griseus upon cetaceans has been reported on multiple occasions across its distribution; foraging interactions between H. griseus and cetaceans have been determined as scavenging events using underwater cameras and analyses of carcasses and therefore, it is largely assumed that scavenging is the only cause of such interactions (Ruiz-Garcia et al., 2023). H. griseus has been observed to scavenge on carcasses at early stages of decomposition (Aguzzi et al., 2018).
Bioturbations
 Several burrows, and other biogenic structures were observed and mapped (Figure 2, Figure 5A) nearby and around the whale skeleton (Baldanza et al., 2018). However, these structures were mostly lost or destroyed during the recovery of the whale skeleton and only partially documented and collected.
Several burrows, and other biogenic structures were observed and mapped (Figure 2, Figure 5A) nearby and around the whale skeleton (Baldanza et al., 2018). However, these structures were mostly lost or destroyed during the recovery of the whale skeleton and only partially documented and collected.
 Two main morphotypes with different shape were identified in the field: 1) vertical/horizontal, mainly cylindrical burrows (Figure 9, Figure 10), in some cases bifurcated and 2) flattened, short and generally compressed traces with two rounded terminations (Figure 11). Both types are mineralized with Fe oxides or pyrite in the external coatings, as observed macroscopically. The frequency and distribution evidence a burrowing activity that generate a complex single-tier system (sensu Bromley, 1990), produced by interconnecting burrows of different origin.
Two main morphotypes with different shape were identified in the field: 1) vertical/horizontal, mainly cylindrical burrows (Figure 9, Figure 10), in some cases bifurcated and 2) flattened, short and generally compressed traces with two rounded terminations (Figure 11). Both types are mineralized with Fe oxides or pyrite in the external coatings, as observed macroscopically. The frequency and distribution evidence a burrowing activity that generate a complex single-tier system (sensu Bromley, 1990), produced by interconnecting burrows of different origin.
Shape of burrows. The first type of burrows shows vertical, inclined and horizontal structures with rugose, irregular surfaces (Figure 9, Figure 10), bifurcate or extended as mazes. The specimen of Figure 9A-B shows two planar galleries (or the casual crossing of two different galleries), respectively, with orange walls and a cream-coloured granular silt fill, with a black central part (with organic reduced materials) that runs along the entire galleries.
 A similar shape, in transverse section, is reported for the isolate burrows (Figure 9A, black arrow, and Figure 9C) that show also a delicate meniscate external wall. All these types of burrows could be referred to irregular echinoid bioturbations produced for locomotion and feeding, like Bichordites. Also, the burrow of Figure 10A-C could be a stunning specimen of Bichordites (Plaziat and Mahmoudi, 1988). The shaft of Figure 9D could be referred to Thalassinoides isp. probable produced by decapods activity (Bromley, 1990; Goldring et al., 2007).
A similar shape, in transverse section, is reported for the isolate burrows (Figure 9A, black arrow, and Figure 9C) that show also a delicate meniscate external wall. All these types of burrows could be referred to irregular echinoid bioturbations produced for locomotion and feeding, like Bichordites. Also, the burrow of Figure 10A-C could be a stunning specimen of Bichordites (Plaziat and Mahmoudi, 1988). The shaft of Figure 9D could be referred to Thalassinoides isp. probable produced by decapods activity (Bromley, 1990; Goldring et al., 2007).
The second type, shown in Figure 11A-B, is flattened and generally compressed with two rounded terminations. This peculiar structure, visible in horizontal surfaces parallel to the bedding, is comparable to that of Artichnus giberti: subhorizontal and mostly rectilinear ‘test tube’-shaped, cylindrical burrow, with a constant diameter throughout its length, a hemispherical and blind termination, and a smooth and thick laminated lining. In the most distal and lowermost part of the structure, this lining may consist of laminated, retrusive spreite (Belasteguì et al., 2014). The ichnogenus Artichnus could be referred to as holothurid burrows, for the high similarity to those produced today (Zhang et al., 2008). The structure is interpreted as having one opening for the tentacle crown to pass through during feeding. With the absence of a second opening, fecal material would be deposited around and beneath the holothurian producing a laminated halo, or spreite, around the burrow (Belasteguì et al., 2014).
Close to the second type of burrows, are visible several incomplete but very particular trace fossils (Figure11C-D), constituted by a laminated lining with subspherical or ovoidal structures protruding from trace, like little chain. These traces could be attributed to a portion of U-burrows, in particular to those produced by apodidian genus Leptosynapta (Bromley, 1996), an endobenthic holothurian funnel feeder. The U-burrow is established having a funnel at the head end, and a pile of excrement at the other. Ayranci and Dashtgard (2013) identified subsurface traces of holothuroids as diminutive-and robust- Artichnus, in assemblage with U-shaped bio-deformation structures.
DISCUSSION
The favorable concurrent factors, i.e. nutrient richness and disposable ecological niches, for various animal groups, mainly predators and scavengers, allowed for the interpretation of a particular paleoenvironment. Table 1 collects a list of protozoa, invertebrates and vertebrates, nektonic and benthonic species (some previously analyzed and described by Monaco et al., 2014 and Baldanza et al., 2018), that have frequented the seabeds of Bargiano and Montemoro for a long time, with multiple generations that succeeded for almost 100 ka. These species constitute the biome that characterized the WFEs, a set of organisms each of which with a specific ecological role. This biome represents a case study for shallow water WFEs, and it is really different from WFEs of deep-water environments. Few other examples of shallow water WFEs have recently been analyzed (lower Miocene of India: Goswami et al., 2024; upper Miocene of Peninsula de Valdes, Argentina: Farroni et al., 2024; early Pleistocene of Puglia, Southern Italy: Zazzera et al., 2022) adding important information about the communities of vertebrates and invertebrates associated.
Mollusk assemblages, echinoids, bioturbations and shark teeth represent the main comparative with the record from Bargiano.
The lower Miocene whale fall event reported by Goswami et al., (2024) was characterized by a mixed assemblages of shallow marine gastropods and bivalves, shallow infaunal detritivore echinoids as well as the characteristic Osedax boring. The mysticete of upper Miocene studied by Farroni et al. (2024), sank on a soft bottom seabed in a low-energy inner shelf environment, under normal conditions of oxygenation, and salinity. Only the scavenging stage was recorded via bite traces. The associated invertebrate assemblage essentially corresponds to the benthic community that inhabited the seafloor prior to sinking of the carcass. Deposition of the carcass had no significative ecological impact on the shallow seabed.
The whale fall event of early Pleistocene of southern Italy (Zazzera et al., 2022) seems to be the best comparable to our case study. The associated mollusks suggest a mid-shelf setting deposition, probably at 40-60 m depth. The occurrence of C. carcharias tooth and shark bite marks on a rib support an early scavenger action (Scavenger stage). The occurrence of chemosymbiotic bivalves (Anodontia cf. fragilis) near the skeleton could testify the development of the sulphophilic stage and the non chemosymbiotic molluscs such as Venus nux indicate the colonization of the skeleton in the subsequent reef stage.
 The mollusk fauna already described in Baldanza et al., (2018) concentrated around and between the whale bones (WFE 2); bivalves were found in their life position with several specimens of Ostrea lamellosa grown attached to the bones (Figure 12A, F). The Bargiano site is a large badland field where the silty-clay deposits are regularly subjected to washing with the removal of the silt-clay component and subsequent transport and fracturing of the mollusks. At first glance, their accumulations appear as shell beds, but they are concentrated near physical obstacles. Often, the fragments accumulate (Figure 12A-B) together with intact specimens in their living position. The assemblages of Bargiano and Montemoro (Table 1) are largely dominated by epifaunal taxa (10 genera) of suspension feeders, with three infaunal taxa (Glans intermedia, Glossus humanus, and Venus multilamella). Species feeding on polychaetas organic compounds (Nassarius italicus and Ringicula auriculata), carnivorous taxon (Euspira catena), and chemosynthetic taxa (Megaxinus incrassatus and Mirtea spinifera) indicative of the sulphophilic stage (Baldanza et al., 2013a, 2018).
The mollusk fauna already described in Baldanza et al., (2018) concentrated around and between the whale bones (WFE 2); bivalves were found in their life position with several specimens of Ostrea lamellosa grown attached to the bones (Figure 12A, F). The Bargiano site is a large badland field where the silty-clay deposits are regularly subjected to washing with the removal of the silt-clay component and subsequent transport and fracturing of the mollusks. At first glance, their accumulations appear as shell beds, but they are concentrated near physical obstacles. Often, the fragments accumulate (Figure 12A-B) together with intact specimens in their living position. The assemblages of Bargiano and Montemoro (Table 1) are largely dominated by epifaunal taxa (10 genera) of suspension feeders, with three infaunal taxa (Glans intermedia, Glossus humanus, and Venus multilamella). Species feeding on polychaetas organic compounds (Nassarius italicus and Ringicula auriculata), carnivorous taxon (Euspira catena), and chemosynthetic taxa (Megaxinus incrassatus and Mirtea spinifera) indicative of the sulphophilic stage (Baldanza et al., 2013a, 2018).
The data regarding benthic foraminifera communities (analyzed and described by Baldanza et al., 2018) from Bargiano and Montemoro indicate that a locally high nutrient flux significantly influenced the development of biota in the first few millimeters or centimeters of sea floor sediment. This increase in nutrients, related to whale-carcass biomass decay, is evidenced by frequencies of at least six opportunistic benthic foraminifera (i.e. Lenticulina calcar, Bigenerina nodosaria, Bannerella gibbosa, Marginulinopsis costata, Vaginulina striatissima and Siphotextularia concava) that inhabited the bottom surface and the few millimeters of the sediments. The abundance of these foraminifera decreases away from the whale skeleton, confirming lateral dispersion around the carcass of nutrients for a radius of at least 10 m (Baldanza et al., 2018).
Among the ostracods (Table 1) (analyzed and described in Baldanza et al., 2018) the most abundant species are Acanthocythereis hystrix, Carinovalva testudo, Costa edwardsii, Pterygocythereis jonesii, and Ruggeria longecarinata, and represented by valves belonging to different stages of maturity and therefore can be considered an autochthonous community (Monaco et al., 2014).
Crustacean Decapods
The crustacean decapods concentrate in the levels around and between the skeleton of the cetacean (Figure 2, Figure 5A) and are all forms that we associate with WFE. Compared to the data discussed in Pasini et al. (2017) and Baldanza et al. (2018), new species have been found that increase the association, such as Jaxea nocturna and Goneplax rhomboides.
No ecological data are available for the only fossil Asthenognatus alleronensis, closely related with the extant Asthenognathus atlanticus Monod, 1933, living in eastern Atlantic from Angola to the English Channel mainly in mud-muddy sands between 10 m to 70 m depth, and reported also from the western Mediterranean Sea (Falciai and Minervini, 1992; Jourde et al., 2012). The discovery in a paleoenvironment marine at depths of approximately 100-150 meters allows for an expansion of the species' depth range.
Two species, Jaxea nocturna and Goneplax rhomboides (extant and fossil), are opportunistic carnivorous predators active on the sea bottoms and have a burrower behaviour likely associated with their activity yet previously observed around and under the whale carcass. Chlinocephalus demissifrons, is benthonic with opportunistic behaviour, whereas there are no data for the exclusively fossil (? benthonic) Albaidaplax ispalensis.
Moreover, the new records seem to confirm that C. demissifrons with 13 specimens confidently assigned, is the predominant decapod species of the Bargiano whale-fall community (= 75% ca. of the decapod assemblage).
As pointed out and discussed by Baldanza et al. (2018: 15),“...the presence of several specimens nearby the carcass of the cetacean can be interpreted as opportunistic behaviour by these decapod crustaceans due to the presence of a great, temporary availability of food on the sea bottom.”, and “...might reveal a temporary assemblage of opportunistic scavenger crabs due to the presence of a copious source of food for a relatively long period”, moreover the specimens recorded from Bargiano stand out as “...the only confidently identified fossil brachyuran reported from a fossil whale-fall environment.” (Baldanza et al., 2018: 15), useful as reference and comparison for next possible studies on the poorly known decapod communities lied to WFE fossil ecosystems.
Echinoids
The irregular echinoid species Ova canalifera is here recorded for the first time in a whale-fall paleoenvironment, and at the moment it seems to be the only irregular echinoid occurring around the whale remains. (Figure 4) The presence of other group of echinoids, the holothurians, or sea cucumbers, has been demonstrated by the occurrence of characteristic trace fossils (Figure 11), distributed between and around the whale skeleton.
Ova canalifera currently lives in the Mediterranean between 9 and 100 m of depth in the muddy or sandy sea bottom (Mortensen, 1951), with maximum frequency between 20-30 m. According to Schinner (1993), Schizaster canaliferus, now accepted as Ova canalifera (WORMS - world register of marine species), an endemic Mediterranean spatangoid, is common in the Bay of Piran (Northern Adriatic Sea). The abundance and distribution of this burrowing species is related to sea water depth and particle size distribution of the substrate. Populations are limited to silt/clay sediments in protected sublittoral areas of the Bay, where densities range from 1.9 to 2.6 individuals/m-2. Preferred sediments range from medium to fine silt (2-20 μm). Burrowing activity was found to be functional only in fine sediments like those naturally inhabited (Bromley et al., 1997); it did not occur in sandy sediments (>63μm). Burrowing rates were positively correlated with temperature, suggesting that burrowing speeds are greater in summer than winter. Burrowing activity is limited to the upper, oxygenated sediment layers, and always just above the Redox-Potential-Discontinuity-layer (RPD). Finally, burrows were found in zones protected near the coast, sunken ca. 3-5 cm within the fine-grained, silty-clayey sediment (Bromley et al., 1997). In the Plio-Pleistocene of Emilia, this species is present both in silty sediments and silty-sandy areas of the infralittoral and upper circalittoral; moreover, the specimens contained in sediments with a higher percentage of sand are less deformed by compression (Borghi, 2020).
Teleost fishes
Ophidiiformes are benthic teleost fishes frequently found in the shallow waters of early Palaeocene, even if at present the extant species inhabit mostly bathydemersal environments (Schwarzhans and Aguilera, 2016; Stringer and Schwarzhans, 2021). As mentioned above, Hoplobrotula armata feeds on decapods and teleost fishes (Baeck et al., 2012; Park et al., 2023). Thus, it is reasonable to hypothesize that also Hoplobrotula orcianensis had carnivorous feeding habits. Considering the rich occurrence of crustaceans at the Bargiano site, it may have had the same food preference as described for the extant H. armata. However, in the paleoenvironmental context of the Bargiano site, it is also possible to hypothesize that, despite being a predator, having a whale carcass available, H. orcianensis took advantage of this food source, together with crustaceans, irregular echinoids and holothuroids. This hypothesis matches with the interpretation of the presence of many decapoda specimens nearby the whale carcass, discussed by Baldanza et al. (2018) in terms of opportunistic behaviour due to the presence of a “great, temporary availability of food on the sea bottom”.
Hoplobrotula orcianensis is first reported within a whale-fall ecosystem (WFE) of early Pleistocene.
Sharks
The assemblage of sharks, characterized by common Carcharhinus longimanus, representative of the family Carcharinidae and by the extinct Cosmopolitodus hastalis, with the extant Carcharodon carcharias, Carcharias taurus and Hexanchus griseus, already reported by Danise et al. (2010), Danise and Dominici (2014) and Zazzera et al. (2022) in concomitance with WFEs, represent for the marine early Pleistocene of western Umbria one important paleoecological record. The occurrence of epipelagic sharks typical of “oceanic” environments that prefer to live in the euphotic zone (0-200 m) as C. longimanus and C. carcharias associated with the species C. taurus typical of sublittoral zone of continental shelf, indicates the existence of an area of attraction and convergence rich in food (cetacean remains in the study case). The feast zone was attractive also for fishes, echinoids and decapods, all prey for sharks.
Identity of Tracemakers
Among invertebrates, both in modern and ancient marine environments, echinoderms have been recognized as among the most active and widespread bioturbators and bioeroders (Belaùstegui et al.,2014). The Bargiano site, with the repeated whale fall events and a considerable quantity of foods, may have been certainly the ideal environment for deposit and suspension feeders. The decapod crustaceans, very common around the WFE of Bargiano, could be excellent candidates as tracemaker for Thalassinoides burrows (Pervescer and Dworschak, 1985; Bromley, 1996); the irregular echinoids as Ova canalifera could be responsible for traces as Bichordites (among the Pascichnia; Plaziat and Mahmoudi, 1988), while the Holothuroid for peculiar examples attributable to Artichnus (as Dominichnia; Zang et al., 2008, Belasteguì et al., 2014) and U-shaped burrows (Ayranci and Dashtgard, 2013).
Echinoid burrows. The irregular echinoids as O. canalifera are indicated as burrow scavenger. This species has been studied in the Bay of Piran (Northern Adriatic Sea) by Schinner (1993) evidencing that the abundance and distribution of this burrowing sea-urchin is related to sea water depth and particle size distribution. The burrowing activities and the lifestyle of these echinoids were limited to silt/clay sediments in protected sublittoral areas of the Bay. Burrowing rates were positively correlated with temperature. O. canalifera was limited to the upper, oxygenated sediment layers and always burrowed just above the Redox-Potential-Discontinuity-layer (RPD). O. canalifera is highly substrate specific and does not appear to be as widespread in the Adriatic Sea as indicated in earlier studies (Borghi, 2020). The abundance of Ova canalifera specimens at Bargiano could support the hypothesis regarding the production of Bichordites burrows.
Holothuroid burrows. Zhang et al. (2008) pointed out the similarity of the ichnogenus Artichnus to burrows produced today by holothurians. At present, holothurians or sea cucumbers are common in both shallow- and deep-marine environments, and most of them live in mud or in fine sand, although it is also possible to find sea cucumbers in coarse sands and shell accumulations. Burrowing sea cucumbers are mainly classified in the orders Dendrochirotida, Molpadiida and Apodida (Nichols, 1969). Some of them may construct U-shaped burrows projecting the anus and the tentacles (oral part) to the surface (e.g., Frey and Howard, 1972; Bromley, 1990), or simpler burrows projecting only the tentacles or the anus (e.g., Ruppert et al., 2004). Ayranci and Dashtgard (2013) analyzed biogenic structures produced by holothurians in a moderately deep-water setting, the delta front and prodelta of the Fraser River delta (British Columbia, Canada, 30 to 330 m water depth), dominated by muddy sediments. The Fraser delta front and prodelta contain very abundant Thalassinoides with diameters larger than 1.5 cm and less than 3 cm; only in one case Molpadida intermedia was captured in an open Thalassinoides -like trace. Both Artichnus and holothurian-generated Thalassinoides occur in substrates that are greater than 55% mud and are most prevalent in sediments composed of 80% mud or higher and deposited below storm-wave base (Ayranci and Dashtgard, 2013).
Individuals belonging to three orders of infaunal holothurians were considered: (1) Molpadiida, (2) Apodida, and (3) Dendrochirotida. Infaunal holothurians are subsurface or surface deposit feeders, and ingest mainly organic detritus, while the suspension feeders filter plankton and diatoms from the water column (Rakaj and Fianchini, 2024). Any mode of nutrition would have been possible at Bargiano site, considering the large amount of nutrients and food dispersed on the seabed, in the water and within the clayey sediments (Baldanza et al., 2018). Ayranci and Dashtgard (2013) identified two distinct sizes of Artichnus: the first is “diminutive Artichnus ”, which is typically less than 3 cm in length and diameter and have an ellipsoidal morphology showing concentric to concave-downward spreite within a well-defined burrow margin, and the second form “robust Artichnus ”, is typically longer than 3 cm, and can be wider than >6,5 cm. Specimens of Bargiano are 12 and 5 cm in length and 4,5 and 1,5 cm wide respectively and therefore belong to both types.
As indicated by Rakaj and Fianchini (2024) in the Holothuroidea class, 54 species were considered in the Mediterranean basin, of which 19 (35%) were listed as endemic. They prefer well oxygenated sea bottoms rich in organic matters, as Posidonia meadows, and are present from 0 to 100 m in depth but can live also at 150 m. Long-term observations in laboratory tanks have shown that the species Holothuria poli and Holothuria tubulosa have a subsurface feeding behavior, commonly burrowing into sediments to feed on the deeper layers; emerged that H. poli is able to actively select and concentrate the organic matter and select the fine fractions of the sediment (Rakaj and Fianchini, 2024).
Decapod burrows. Among decapod crustaceans, almost two of the taxa recovered from the WFE of Bargiano, having burrower behaviour, may be suspected as the tracemakers, both with fossil and living species. Extant representatives of the brachyuran crab Goneplax rhomboides builds vertical U-shaped burrows that may be extended as mazes flattened in cross section (Bromley, 1996: 102); whereas the ghost shrimp Jaxea nocturna is known to make burrows, as shown by resin casts made in situ in the Gulf of Trieste (Northern Adriatic Sea, Italy: Pervescer and Dworschak, 1985). The common occurrence of G. rhomboides and J. nocturna increases the list of possible producers of Thalassinoides burrows.
Fish burrows. Ophidiiform fishes show a close association with the sea bottom, where they dig burrows into soft and movable sediments with the help of tail movements (Grzimer, 2003). These organisms typically remain buried during daylight hours and exhibit peak activity at night, emerging to forage for prey. No information is found about Hoplobrotula as trace maker and no fossil traces were adscript to this taxon.
The presence of diverse fossil traces has highlighted intense activity, linked not only to the presence of Thalassinids crustacea but also to Echinoderms, especially irregular sea urchins (Ova) and holothurians. The burrowing activity could generate a complex single-tier system (sensu Bromley, 1996) produced by interconnecting burrows of echinoids, fishes and decapods, representing for the Bargiano site a new window to reconstruct the relationships among marine invertebrates and small vertebrates.
CONCLUSION
The richness of macro- and microorganisms highlights the potential of the WFE ecosystem to accommodate various opportunists, both simultaneously and at different times. For about one hundred thousand years, during the early Pleistocene, a large amount of organic matter was produced on the seafloor of the Bargiano site, available for a long food chain from vertebrates to bacteria (Monaco et al., 2014; Baldanza et al., 2018). The Bargiano - Montemoro area played a role of meeting point for scavengers and opportunistic hunters, which for several thousand years provided a great abundance of food, a zone known and surely memorized from one generation to another.
The identified presence of scavengers and opportunists developed close to the seafloor and into the sediments, all around and amongst the whale skeletons evidenced that the four Ecological Stages: Scavenger stage, Opportunistic stage, Sulphophilic stage and Reef stage (sensu Smith and Baco, 2003; Smith et al., 2014), developed on the Bargiano-Montemoro Area.
1) The Scavenger Stage is evidenced by the occurrence of large size predators, mainly sharks, carnivorous fishes and decapods; predators feed on the muscles and fat of the cetacean, producing fragments that disperse in the surrounding water like bait that attracts other organisms of smaller size. Among the large size predators, C. hastalis and C. carcharias, together with C. longimanus and C. taurus have been active and constant attendees.
2) The Opportunistic stage is characterized by the arrival of other scavenging animals that feed on the remnants of meat, fat, and whale oil that has permeated the sediment on which the carcass has settled on the seabed. This second “hunger wave” included here decapod crustaceans (Chlinocephalus demissifrons, Albaidaplax ispalensis, Goneplax rhomboides), echinoderms (Ova canalifera and Holothurians), gastropods (Euspira catena), polychaetas, ghost shrimp (Jaxea nocturna) and fishes (Hoplobrotula orcianensis and Argirosomus). All these opportunists carried out their life cycle around the carcass, digging burrows and galleries in the underlying sediment and all around, actively contributing to the dispersion of nutrients. Unfortunately, we have not found traces of the worm Osedax on the bones, likely in relation to the loss of the cortical layer (Cherin, pers. comm.).
The existence of U-burrows, that in some cases were extended as mazes and the possible occurrence of a complex-tier system produced by the interconnection of burrows of fish, (Hoplobrotula) and crab (G. rhomboides), evidenced a very complex network. Bichordites, Artichnus and Thalassinoides complete the ichnoassemblage reported, for the first time, as a feature of the shallow water WFE of Bargiano.
3) The sulphophilic stage, characterized by the development of bacterial communities is indicated by the abundant presence of chemiosymbiotic bivalves (e.g. Megaxinus incrassatus and Myrthea spinifera) (Figure 12C-E). Even small crustaceans and crabs feed on bacteria and other organic substances produced by the degradation of soft part of carcasses. To note that also benthic foraminifera (e.g. Ammonia) feed on bacterial mat (Baldanza et al., 2018).
4) Oysters are the most diffused bivalves using the bones as rigid platform for the anchorage. The reef stage has been documented thanks to the diffuse occurrence of Ostrea lamellosa grown on whale bones (Figure 12 A, E); in WFE2 the ribs, portions of the skull, mandible and vertebrae provided rigid surfaces emerging from the muddy seabed.
The ten-year research at the Bargiano site, and more generally in the Allerona area, not only allowed to recognize several WFEs in a limited time interval, but also to characterize their fossil assemblages in increasing detail. The study area, full of unexpected finds, have revealed as a significant place for the knowledge and description of shallow fossil whale falls.
Although much has been documented and described so far, we do not exclude that the area may provide further findings in the next future.
ACKNOWLEDGEMENTS
We wish to thank Mr. R. Sigismondo (VA), for the additional pictures of the new decapod specimens useful to complete the iconographic section. We wish tothank A. Rakaj (University of Rome Tor Vergata) for suggestions about biology of Mediterranean Holothurians, C. Romani and M. Luciani for photos of shark teeth and echinoids and P. Goswami and N. D. Farroni for careful review and criticism.
REFERENCES
Adnet, S. 2006. Biometric analysis of the teeth of fossil and Recent hexanchid sharks and its taxonomic implications. Acta Palaeontologica Polonica, 51 (3):477-488.
Aguzzi, J., Fanelli, E., Ciuffardi, T., Schirone, A., De Leo, F.C., Doya, C., Kawato, M., Miyazaki, M., Furushima, Y., Costa, C. and Fujiwara, Y. 2018. Faunal activity rhythms influencing early community succession of an implanted whale carcass offshore Sagami Bay, Japan. Scientific reports, 8 (1):1-15.
Amon, D.J., Glover, A.G., Wiklund, H., Marsh, L., Linse, K., Rogers, A.D. and Copley, J.T. 2013. The discovery of a natural whale fall in the Antarctic deep sea. Deep-Sea Research II, 92:87-96.
https://doi.org/10.1016/j.dsr2.2013.01.028
Andrews, K.S., Williams, G.D., Farrer, D., Tolimieri, N., Harvey, C.J., Bargmann,G. and Levin, P.S. 2009. Diel activity patterns of sixgills harks, Hexanchus griseus: the ups and downs of an apexpredator. Animal Behaviour, 78 (2):525-536.
Ayranci, K. and Dashtgard, S.E. 2013. Infaunal holothurian distributions and their traces in the Fraser River delta front and prodelta, British Columbia, Canada. Palaeogeography, Palaeoclimatology, Palaeoecology, 392:232-246.
https://doi.org/10.1016/j.palaeo.2013.09.021
Backman, J., Raffi, I., Rio, D., Fornaciari, E. and Pälike, H. 2012. Biozonation and biochronology of Miocene through Pleistocene calcareous nannofossils from low and middle latitudes. Newsletters on Stratigraphy, 45: 221-244.
https://doi.org/10.1127/0078-0421/2012/0022
Baeck, G.W., Park, J.M., Ye, S.J., Jeong, J.M., andAn, Y.S. 2012.Feeding habits of Hoplobrotula armata in the Coastal Waters of Geomun-do, Korea. Korean Journal of Fisheries and Aquatic Sciences, 45(4):372-378.
Baldanza, A., Bizzarri, R., De Angeli, A., Famiani, F., Garassino, A., Pasini, G. and Pizzolato, F. 2017. A distinctive shallow marine crustacean fauna from the early Pleistocene of Poggi Gialli (Tuscany, central Italy): taxonomic inferences and paleoenvironmental reconstruction. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 286/1:35-74.
Baldanza, A., Bizzarri, R., Famiani, F., Monaco, P., Pellegrino, R., and Sassi, P. 2013a. Enigmatic, biogenically induced structures in Pleistocene marine deposits: a first record of fossil ambergris. Geology, 41:1075-1078.
https://doi.org/10.1130/g34731.1
Baldanza, A., Bizzarri, R., Famiani, F., Garassino, A., Hyžný, M. and Pasini, G. 2013b. The bathyal decapod crustacean community from the Poggio i Sodi quarries (Siena Basin, Tuscany, Italy). Boletín de la Sociedad Geológica Mexicana, 65(2):335-353.
Baldanza, A., Bizzarri, R., Famiani, F., Garassino, A, Pasini, G., Cherin, M. and Rosatini, F. 2018. The early Pleistocene whale-fall community of Bargiano (Umbria, Central Italy): Paleoecological insights from benthic foraminifera and brachyuran crabs. Palaeontologia Electronica, 21.1.11A 1-27.
https://doi.org/10.26879/779
Bass, A.J., d'Aubrey, J.D., Kistnasamy, N. 1975. Sharks of the east coast of Southern Africa. IV. The families Odontaspididae, Scapanorhynchidae, Isuridae, Cetorhinidae, Alopiidae, Orectolobidae and Rhiniodontidae. Investigational Report Oceanographic Research Institute, 39, 1-102.
Beaumont, G. de 1960. Contribution à l’étude des genres Orthacodus Woodward et Notidanus Cuvier (Selachii). Mémoire Suisse de Paléontologie,77:4-36.
Belasteguì, Z., Domenech, R. and Martinell, J. 2014. Artichnus giberti isp. nov., a possible holothurian burrow from the Miocene of El Camp de Tarragona Basin (NE Spain). Spanish Journal of Palaeontology, 29 (2):143-150.
Bellocchio, G., Nami, M., Carboni, M.G. and Pallini, G. 1991. Fauna ad ittiodontoliti del Pliocene di Allerona (Terni, Umbria). Bollettino Società Naturalisti in Napoli, 100: 41-73, Napoli.
Bianucci, G., Llàcer, S., Cardona, J.Q., Collareta, A. and Florit, A.R. 2019. A new beaked whale record from the Upper Miocene of Menorca, Balearic Islands, based on CT-scan analysis of limestone slabs. Acta Palaeontologica Polonica, 64:291-302.
Bizzarri, R., Rosso, A., Famiani, F. and Baldanza, A. 2015. Lunulite bryozoans from Early Pleistocene deposits of SW Umbria (Italy): sedimentological and paleoecological inferences. Facies, 61(2015): 420.
https://doi.org/10.1007/s10347-014-0420-6
Borghi, E. 2020. Gli Schizoasteridi (Echinoidea) del Plio-Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali, (2020):20-38.
Bromley, R.G. 1990. Trace Fossils, Biology and Taphonomy. Unwin Hyman Edit., pp. 280,London.
Bromley, R.G. 1996. Trace Fossils. Biology, Taphonomy and Applications. Chapman and Hall Edit., pp.361, London.
Bromley, R.G., Asgaard, U. and Jensen, M. 1997. Experimental study of sediment structures created by a spatangoid echinoid, Echinocardium mediterraneum. Proceedings of the Geologists Association, 108:183-189.
Cappetta, H. and Nolf, D. 1991. Les Selaciens du Pliocéne Inferieur de Le Puget-sur-Argent (Sud-Est de la France). Palaeontographica Abteilung A, 218:49-67.
Checchia-Rispoli, G. 1907. Gli echinidi viventi e fossili della Sicilia - Gli Echinidi del piano Siciliano dei dintorni di Palermo. Palaeontographia Italica, 13:199-231
Checchia-Rispoli,G. 1923. Gli Echinidi del Pliocene di Anzio. Memorie della Carta Geologica d’Italia, 9 (parte I), Roma: 1-29.
Cigala-Fulgosi, F., Casati, S., Orlandini, A. and Persico, D. 2009. A small fossil fish fauna, rich in Chlamydoselachus teeth, from the Late Pliocene of Tuscany (Siena, central Italy).Cainozoic Research, 6(1-2):3-23.
Collareta, A., Landini, W., Chacaltana, C., Valdivia, W., Altamirano-Sierra, A., Urbina-Schmitt, M. and Bianucci, G. 2017. A well-preserved skeleton of the fossil shark Cosmopolitodus hastalis from the late Miocene of Peru, featuring fish remains as fossilized stomach contents. Rivista Italiana di Paleontologia e Stratigrafia 123(1):11-22.
Cunningham, S. B. 2000. A comparison of isolated teeth of early Eocene Striatolamia macrota (Chondrichthyes, Lamniformes), with those of a Recent sand shark, Carcharias taurus. Tertiary Research, 20(1-4):17-31.
Cusumano, A. and Di Patti, C. 2006. Sicilian Cenozoic sharks from the Collection of the G.G. Gemmellaro Museum. Quaderni del Museo Geologico Gemmellaro, 9:110-120.
Danise, S. and Dominici, S. 2014. A record of fossil shallow-water whale falls from Italy. Lethaia, 47:229-243.
https://doi.org/10.1111/let.12054
Danise, S., Dominici, S. and Betocchi, U. 2010. Mollusk species at a Pliocene shelf whale fall (Orciano Pisano, Tuscany). Palaios, 25:449-456.
https://doi.org/10.2110/palo.2009.p09-139r
Danise, S., Dominici, S., Glover, A.G. and Dahlgren, T.G. 2014. Molluscs from a shallow-water whale-fall and their affinities with adjacent benthic communities on the Swedish west coast. Marine Biology Research, 10:3-16.
https://doi.org/10.1080/17451000.2013.793811
Delle Cave, L. 1988. Jaxea cf. nocturna (Crustacea, Decapoda, Anomura) from the Early Pliocene of Tuscany, Italy. Bollettino della Società Paleontologica Italiana, 27 (1):3-10.
Di Stefano, A., Baldassini, N., Raffi, I., Fornaciari, E., Incarbona, A., Negri, A., Bonomo. S., Villa, G., Di Stefano, E. and Rio, D. 2023. Neogene Quaternary Mediterranean calcareous nannofossil biozonation and biochronology: A review. Stratigraphy, 20 (4):259-302.
https://doi.org/10.29041/strat.20.4.02
Dominici, S., Cioppi, E., Danise, S., Betocchi, U., Gallai, G., Tangocci, F., Valleri, G. and Monechi, S. 2009. Mediterranean fossil whale falls and the adaptation of mollusks to extreme habitats. Geology, 37:815-818.
https://doi.org/10.1130/g30073a.1
Dworcshak, P.C. 2004. Biology of Mediterranean and Caribbean Thalassinidea. Conference Proceedings of the Symposium on Ecology of Large Bioturbators in Tidal Flats and Shallow Sublittoral Sediments - From Individual Behaviour to Their Role as Ecosystem Engineers, Nagasaki University, Japan, p. 15-22.
Ercoli M., Bizzarri R., Baldanza A., Bertinelli A., Mercantili D. and Pauselli C. 2021. GPR detection of fossil structures in conductive media supported by FDTD modelling and attributes analysis: an example from early Pleistocene marine clay at Bargiano Site (central Italy). Geosciences, 11 (9): 386.
https://doi.org/10.3390/geosciences11090386
Ehret, D.J., Macfadden, B.J., Jones, D.S., Devries, T.J., Foster, D.A. and Salas-Gismondi, R. 2013. Origin of the white shark Carcharodon (Lamniformes: Lamnida) based on recalibration of the upper Neogene Pisco Formation of Peru. Palaeontology, 55 (6):1139-1153.
https://doi.org/10.1111/j.1475-4983.2012.01201.x
Falciai, L. and Minervini, R. 1992. Guida dei Crostacei Decapodi d’Europa. Franco Muzzio, Padova, pp. 282.
Farroni, N.D., Cuitino, J. I., Lazo, D.G., and Buono, M.R. 2024. Taphonomic analysis of an articulated Baleen Whale (Cetacea; Mysticeti) from upper Miocene inner shelf of Peninsula di Valdes, Patagonia, Argentina. Palaios, 39: 97-112.
https://doi.org/10.2110/palo.2022.039
Fioravanti, T., Bargnesi, F., Splendiani, A., Giovannotti, M., Renzi, F. and Caputo Barucchi, V. 2020. Historical DNA as a tool to genetically characterize the Mediterranean sand tiger shark (Carcharias taurus, Lamniformes: Odontaspididae): A species probably disappeared from this basin. Aquatic Conservation-Marine and Freshwater Ecosystems, 2020:1-11.
https://doi.org/10.1002/aqc.3294
Frey, R.W. and Howard, J.D. 1972. Georgia coastal region, Sapelo Island, USA: sedimentology and biology. VI. Radiographic study of sedimentary structures made by beach and offshore animals in aquaria. Senckenbergiana Maritima, 4:169-82.
Froese, R. and Pauly, D. Editors. 2000. FishBase 2000: concepts, design and data sources. ICLARM, Los Baños, Laguna, Philippines. 344 p.
Fujioka, K., Wada, H. and Okano, H. 1993. Torishima whale bone deep-sea animal community assemblage - new finding by Shinkai 6500. Journal of Geography, 102:507-517 (in Japanese with English abstract).
https://doi.org/10.5026/jgeography.102.507
Fujiwara, Y., Kawato, M., Yamamoto, T., Yamanaka, T., Sato-Okoshi, W., Noda, C., Tsuchida, S., Komai, T., Cubelio, S.S., Sasaki, T., Jacobsen, K., Kubokawa, K., Fujikura, K., Maruyama, T., Furushima, Y., Okoshi, K., Miyake, H., Miyazaki, M., Nogi, Y., Yatabe, A. and Okutani, T. 2007. Three-year investigations into sperm whale-fall ecosystems in Japan. Marine Ecology, 28:219-232.
https://doi.org/10.1111/j.1439-0485.2007.00150.x
Gabriel, S., Prista, N., Costa, M.J. 2012. Estimating meagre (Argyrosomus regius) size from otoliths and vertebrae. Journal of Archaeological Science, 39:2859-2865.
https://doi.org/10.1016/j.jas.2012.04.046
Garassino, A., Artal, P. and Pasini, G. 2009. Jaxea nocturna Nardo, 1847 (Crustacea, Thalassinidea, Laomediidae) from the Pliocene of Catalonia (Spain). Atti della Società italiana di Scienze naturali e del Museo civico di Storia naturale in Milano, 150 (1):69-76.
Garassino, A., Pasini, G. and Castro, P. 2013. Revision of fossil species of Goneplax Leach, 1814 (Crustacea, Decapoda, Brachyura, Goneplacidae). Boletín de la Sociedad Geologíca Mexicana, 65 (2):355-368.
Garassino, A., Pasini, G., De Angeli, A., Charbonnier, S., Famiani, F., Baldanza, A. and Bizzarri, R.2012. The decapod community from the Early Pliocene (Zanclean) of “La Serra” quarry (San Miniato, central Italy): sedimentology, systematics, and palaeoenvironmental implications. Annales de Paléontologie, 98:1-61.
https://doi.org/10.1016/j.annpal.2012.02.001
Girone, A., Garassino, A., Pasini, P., Zazzera, A., Gallicchio, S., Maiorano, P., Marino, M. and La Perna, R. 2024. New report of decapod and isopod crustaceans from the Lower-Middle Pleistocene of Montalbano Jonico, Matera (Basilicata, Southern Italy). Rivista Italiana di Paleontologia e Stratigrafia, 130 (1):129-151.
Goffredi, S.K., Paull, C.K., Fulton-Bennett, K., Hurtado, L.A. and Vrijenhoek, R.C. 2004. Unusual benthic fauna associated with a whale fall in Monterey Canyon, California. Deep-Sea Research I, 51:1295-1306.
https://doi.org/10.1016/j.dsr.2004.05.009
Goldring, R., Cadée, G.C., and Polland, J.E. 2007. Chapter 10. Climatic control of Marine trace fossil distribution. In Trace Fossils concepts, problems, prospects. Ed: William Miller III, Geology Department Humboldt State University, Arcata USA, 159-171.
Goswami, P, Das, S. S. and Panja, S. 2024. Discovery of Miocene whale fall fauna from India: Taphonomy and palaeoecology of a vertebrate invertebrate assemblage. Historical Biology.
https://doi.org/10.1080/08912963.2024.2405057
GrzimeR's Animal Life Encyclopedia, 2nd edition. 2003. Vol 4-5, Fishes I-II, edited by M. Hutchins, D.A. Thoney, P.V. Loiselle, and N. Schlager. Farnington Hills, MI: Gale Group. Italiana, 27 (1): 3-10.
Heithaus, M.R. 2001. Predator-prey and competitive interactions between sharks (order Selachii) and dolphins (Suborder Odontoceti): a review. Journal of Zoology, 253:53-68.
Holtke, O., Maxwell, E.E., Rasser, M.W. 2024. A Review of the Paleobiology of Some Neogene Shark sand the Fossil Records of Extant Shark Species. Diversity, 16, 147.
https://doi.org/10.3390/d16030147
Jourde, J., Alizier, S., Dancie, C, Drovin, J.C., Desroy, N., Dubut, S., Gentil, F., Grall, J., Hanin, C., Lanshere, J. andThiébaut, E. 2012. First and repeated records of the tropical-temperate crab Asthenognatus atlanticus Monod, 1932 (Decapoda: Brachyura) in the eastern part of the Bay of Seine (Central English Channel, France). Cahiers de Biologie Marine, 53:525-532.
Landini, W. 1977. Revisione degli “Ittiodontoliti” pliocenici della collezione Lawley. Palaeontografia Italica, 70:92-134, Pisa.
Linse, K., Jackson, J.A., Malyutina, M.V. and Brandt, A. 2014. Shallow-water northern hemisphere Jaxea (Crustacea, Isopoda, Janiridae) found on whale bones in the Southern Ocean deep sea: ecology and description of Jaera tyleri sp. nov. PLoS ONE, 9: e93018.
https://doi.org/10.1371/journal.pone.0093018
Lirer, F., Foresi, L.M., Iaccarino, S.M., Salvatorini, G., Turco, E., Cosentino, C., Sierro, F. J., Caruso, A. 2019. Mediterranean Neogene planktonic foraminifer biozonation and biochronology. Earth Sciences Review, 196, 102889.
https://doi.org/10.1016/j.earscirev.2019.05.013
King, J.R. and Surry, A.M., 2017. Seasonal and daily movements of the bluntnose sixgill shark (Hexanchus griseus) in the strait of Georgia from satellite tag data. Environmental Biology of Fishes, 100 (12):1543-1559.
Lundsten, L., Paull, C.K., Kyra L. Schlining, K.L., McGann, M. and Ussler, W. III. 2010a. Biological characterization of a whale-fall near Vancouver Island, British Columbia, Canada. Deep-Sea Research I, 57:918-922.
https://doi.org/10.1016/j.dsr.2010.04.006
Lundsten, L., Schlining, K.L., Frasier, K., Johnson, S.B., Kuhnz, L.A., Harvey, J.B.J., Clague, G. and Vrijenhoek, R.C. 2010b. Time-series analysis of six whale-fall communities in Monterey Canyon, California, USA. Deep-Sea Research I, 57:1573-1584.
https://doi.org/10.1016/j.dsr.2010.09.003
Machida, Y. 1990. A new ophidiid species, Hoplobrotula badia, from Sagami Bay, central Japan. Japanese Journal of Ichthyology,37(3):209-214.
Manganelli, G. and Spadini, V. 2003. Gli squali del Pliocene Senese. Sistema Musei Senesi Quaderni scientifico- Naturalistici, 3: 80 pp. Siena
Marsili, S. 2007. Revision of the teeth of the Genus Carcharhinus (Elasmobranchii; Carcharhinidae) from the Pliocene of Tuscany. Rivista Italiana di Paleontologia e Stratigrafia,113 (1):79-95.
Medeiros, S., Oddone, M.C., Francischini, H., Diniz, D. and Dentzien-Dias, P. 2023. Quaternary fossil shark (Neoselachii: Galeomorphii and Squalomorphii) diversity from southern Brazil. Journal of South American Earth Sciences, 122 (2023) 104176.
Merella, M., Collareta, A., Casati, S., Di Cencio, A., Landini, W. and Bianucci, G. 2020. The lower Pliocene elasmobranch assemblage from Arcille (Campagnatico, Grosseto Province): palaeoecological and palaeoenvironmental significance. Fossilia, Volume 2020: 41-43.
https://doi.org/10.32774/FosRepPal.2020.0611
Merella, M., Collareta, A., Casati, A., Di Cencio, A. and Bianucci, G. 2021. An unexpected deadly meeting: deep-water (hexanchid) shark bite marks on a sirenian skeleton from Pliocene shoreface deposits of Tuscany (Italy). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 301(3):295-305.
Merella, M., Collareta, A. and Casati, S. 2023. Pliocene Geotourism: Innovative Projects for Valorizing the Paleontological Heritage of Three Different-Staged Quarries of Tuscany (Central Italy). Geoheritage, 15:82.
https://doi.org/10.1007/s12371-023-00838-5
Mercier, A., Gebruk, A., Kremenetskaia, A. and Hamel, J.F. 2024. Chapter 1. An overview of taxonomic and morphological diversity in sea cucumbers (Holothuroidea: Echinodermata). In: Mercier, A., Hamel, J.-F., Suhrbier, A., Pearce, C., (Eds.), The World of Sea Cucumbers. Academic Press, pp. 3e15.
https://doi.org/10.1016/B978-0-323-95377-1.00001-1
Monaco, P., Baldanza, A., Bizzarri, R., Famiani, F., Lezzerini, M., and Sciuto, F.2014. Ambergris cololites of Pleistocene sperm whales from central Italy and description of the new ichnogenus and ichnospecies Ambergrisichnus alleronae. Palaeontologia Electronica, 17.2.29A:20p.
https://doi.org/10.26879/464
Mortensen, T. 1951. A monograph of the Echinoidea V, 2. Spatangoida Il. C.A. Reitzel, Copenhagen: pp. 593.
Ngoc-Ho, N. 2003. European and Mediterranean Thalassinidea (Crustacea, Decapoda). Zoosystema, 25 (3): 440-555.
Nolf, D. 1980. Etude monographiques des otolithes des Ophidiiformes actuels et revision des especes fossiles (Pisces, Teleostei). Mededelingen van de werkgroep voor tertiaire en kwartaire geologie, 17(2):71-195.
Nolf, D. 2013. The diversity of fish otoliths, past and present. Brussels: Royal Belgian Institute of Natural Sciences; p. 581.
Nolf, D. and Cavallo, O. 1994. Otolithes de poissons du Pliocène Inférieur de Monticello d’Alba (Piémont, Italie). Rivista Piemontese di Storia Naturale, 15:11-40.
Nolf, D., Mane, R., and Lopez, A. 1998. Otolithes de poissons du Pliocène inférieur de Papiol, près de Barcelone. Palaeovertebrata, 27(1-2):1-17.
Park, D.Y., Jin, S., Jeong, J.M., Lee, J.H., and Baeck, G.W. 2023. Feeding habits of the Armored Cusk, Hoplobrotula armata in the south sea, Korea. Korean Journal of Ichthyology, 35(3):183-188.
Pasini, G., Garassino, A. and De Angeli A. 2017. Asthenognathus alleronensis n. sp. (Decapoda, Brachyura, Varunidae) from the early Pleistocene of Bargiano, Terni (Umbria, central Italy). Neues Jahrbuch für Geologie und Paläontologie, Abhandlugen, 283(1):69-71.
Plaziat, J.C.and Mahmoudi, M. 1988. Trace fossils attributed to burrowing Echinoids: a revision including new ichnogenus and ichnospecies. Geobios, 21 (2): 209-233.
Perveseler, P. and Dworschak, P.C. 1985. Burrows of Jaxea nocturna Nardo in the Gulf of Trieste. Senkerbergiana maritima,17(1-3):33-53.
Rakaj, A. and Fianchini, A. 2024. Chapter 48. Mediterranean sea cucumbers. Biology, ecology, and exploitation. In: Mercier, A., Hamel, J.F., Suhrbier, A., Pearce, C., (Eds.).The World of Sea Cucumbers. Academic Press, pp. 753e773.
https://doi.org/10.1016/B978-0-323-95377-1.00015-1
Regan, C.T. 1921. New fishes from deep water off the coast of Natal. Annals and Magazine of Natural History (Series 9), 7(41):412-420.
Ruppert, E.E., Fox, R.S., and Barnes, R.D. 2004. Invertebrate Zoology.A functional evolutionary approach. 7th ed. Brooks/Cole-Thomson Learn, Belmont, California, 1056 p.
Ruiz-Garcia, D., Garcia-Salinas, P., Raga, J. A., Moura, A. E., Dromby, M., and Barria, C. 2023. New record of Hexanchus griseus in the northwestern Mediterranean Sea with insights into its biology and feeding ecology: predator or scavenger? Mediterranean Marine Science, 24 (1):109-116.
https://doi.org/10.12681/mms.29940
Schäfer, W. 1972. Ecology and palaeoecology of marine environments. Oliver and Boyd, Edinburgh, pp. 568.
Shark Reference.com. Carcharodon carcharias (Linnaeus, 1758) | Shark-References.
https://shark-references.com/post/687
Schinner, G.O.1993.Burrowing behaviour, Substratum Preference, and Distribution of Schizaster canaliferus (Echinoidea: Spatangoida) in the Northern Adriatic Sea. Marine ecology, 14(2):129-145.
https://doi.org/10.1111/j.1439-0485.1993.tb00371.x
Schwarzhans, W.1994. Sexual and ontogenetic dimorphism in otoliths of the family Ophidiidae. Cybium, 18(1):71-98.
Schwarzhans, W. and Aguilera, O. 2016. Otoliths of the Ophidiiformes from the Neogene of tropical America. Palaeo Ichthyologica, 14:1-124.
Schwarzhans, W. and Carnevale, G. 2024. Fish otoliths from the upper Oligocene and lower Miocene of the Monferrato and Turin Hill, northern Italy. Rivista Italiana di Paleontologia e Stratigrafia, 130:663-709.
Schweitzer, C.E., Feldmann, R.M., Garassino, A., Karasawa, H. and Schweigert, G. 2010. Systematic list of fossil decapod crustacean species. CrustaceanaMonographs, 10:1-222.
Smilek, K.R. and Hembree, D.I. 2012. Neoichnology of Thyonella gemmata: A Case Study for Understanding Holothurian Ichnofossils. The Open Paleontology Journal, 4:1-10
Smith, C.R, and Baco, A.R. 2003. Ecology of whale falls at the deep-sea floor. Oceanography and Marine Biology: An Annual Review, 41:311-354.
Smith, C.R., Baco, A.R. and Glover, A.G. 2002. Faunal succession on replicate deep-sea whale falls: Time scales and vent-seep affinities. Cahiers de Marine Biologie, 43:293-297.
Smith, C.R., Glover, A.G., Treude, T., Higgs, N.D. and Amon, D.J. 2015. Whale-Fall ecosystems: recent insights into ecology, paleoecology, and evolution. Annual review of Marine Science, 7:571-596.
https://doi.org/10.1146/annurev-marine-010213-135144
Smith, K.E., Thatje, S., Singh, H., Amsler, M.O. Vos, S.C., McClintock, J.B., Brothers, C.J., Brown, A., Ellis, D., Anderson, J.S. and Aronson, R.B. 2014. Discovery of a recent, natural whale fall on the continental slope off Anvers Island, western Antarctic Peninsula. Deep-Sea Research I, 90:76-80.
https://doi.org/10.1016/j.dsr.2014.04.013
Stringer, G. and, Schwarzhans,W.2021. Upper Cretaceous teleostean otoliths from the Severn Formation (Maastrichtian) of Maryland, USA, with an unusual occurrence of Siluriformes and Beryciformes and the oldest Atlantic coast Gadiformes. Cretaceous Research, 125:104867.
Sumida, P.Y.G., Alfaro-Lucas, J.M., Shimabukuro, M., Kitazato, H., Perez, J.A.A., Soares-Gomes, A., Toyofuku, T., Lima, A.O.S., Ara, K. and Fujiwara,Y. 2016. Deep-sea whale fall fauna from the Atlantic resembles that of the Pacific Ocean. Scientific Reports, 6:22139.
https://doi.org/10.1038/srep22139
Temminck, C.J. and Schlegel, H. 1846. Pisces. In: Siebold, P.F. de (ed.): Fauna Japonica, sive descriptio animalium, quae in itinere per Japoniam suscepto annis 1823-1830 collegit, notis, observationibus et adumbrationibus illustravit Ph. Fr. de Siebold. Lugduni Batavorum [Leiden] (A. Arnz et soc.). Parts 10-14: 173-269.
Uhen, M.D., Allen, B., Behboudi, N., Clapham, M. E., Dunne, E., Hendy, A., Holroyd, P.A., Hopkins, M., Mannion, P., Novack-Gottshall, P., Pimiento, C., and Wagner, P. 2023. Paleobiology Database User Guide Version 1.0. PaleoBios, 40 (11): 1-56.
https://doi.org/10.5070/P9401160531
Wada, H., Naganuma, T., Fujioka, K., Kitazato, H., Kawamura, K. and Akazawa, Y. 1994. The discovery of the Torishima whale bone animal community and its meaning. Journal of Deep-Sea Research, 10:37-47.
Walls, R.H.L. and Soldo, A. 2016. Carcharias taurus (Mediterranean assessment). The IUCN Red List of Threatened Species 2016: e.T3854A16527817. Accessed on 21 February 2025.
WoRMS Editorial Board 2024. World Register of Marine Species. Available from
https://Www.Marinespecies.Org
Zazzera, A., Girone, A., La Perna, R., Marino, M., Maiorano, P., Sardella, R., Montenegr, V., Francescangeli, R., Bianucci, G. 2022. Systematics, taphonomy and palaeobiogeography of a balenopterid (Cetacea, Mysticeti) from the Early Pleistocene of southern Italy. Geobios, 71: 51-65.
Zhang, G., Uchman, A., Chodyń, R. and Bromley, R.G. 2008. Trace fossil Artichnus pholeoides igen. nov. isp. nov. in Eocene turbidites, Polish Carpathians: possible ascription to holothurians. Acta Geologica Polonica, 58:75-86.

