 A large coelacanth, †Whiteia giganteus sp. nov., from the Triassic of Texas, USA, establishes a Pangean radiation of early Mesozoic actinistians
A large coelacanth, †Whiteia giganteus sp. nov., from the Triassic of Texas, USA, establishes a Pangean radiation of early Mesozoic actinistians
Article number: 26.1.a9
https://doi.org/10.26879/1254
Copyright Society for Vertebrate Paleontology, March 2023
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 18 November 2022. Acceptance: 13 March 2023.
ABSTRACT
An intriguing pattern among extant lineages of vertebrates is the existence of depauperons: clades that have remained species-poor over huge time spans (i.e., >100 million years ago). Among these are so-called living fossil fishes like gars, sturgeons, bichirs, and lungfishes. Perhaps the most famous of these are the coelacanths, which are today represented by only two species yet are known from a fossil record comprising over 400 million years of evolution. Despite the high morphological and body size disparity observable in extinct coelacanths, the total number of species belonging to the coelacanth lineage Actinistia remains very low. Here, I describe the skull of a new species of large-bodied coelacanth from the Late Triassic Dockum Group of Texas. †Whiteia giganteus sp. nov. is the most massive member of its genus and one of the largest known Triassic actinistians. Phylogenetic analysis shows that this clade is stemward of the divergence–Latimerioidei–that produced almost all coelacanth diversity after the Triassic. In turn, †W. giganteus substantiates a new episode of body size increase in the coelacanth total clade and suggests an unsampled degree of coelacanth diversity in the Triassic of the United States, which has previously produced only latimerioid fossils.
Chase D. Brownstein. Department of Ecology and Evolutionary Biology, Yale University, New Haven, Connecticut, USA and Department of Collections and Exhibitions, Stamford Museum and Nature Center, Stamford, Connecticut, USA. chasethedinosaur@gmail.com
Keywords: Coelacanths; Actinistia; Fishes; Phylogenetics; Triassic; new species
Final citation: Brownstein, Chase D. 2023. A large coelacanth, †Whiteia giganteus sp. nov., from the Triassic of Texas, USA, establishes a Pangean radiation of early Mesozoic actinistians. Palaeontologia Electronica, 26(1):a9.
https://doi.org/10.26879/1264
palaeo-electronica.org/content/2023/3804-big-dockum-group-coelacanth
Copyright: March 2023 Society for Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
https://zoobank.org/References/DA817624-1940-4AF7-9A64-42CC3428CC1D
INTRODUCTION
Coelacanths (clade Actinistia) are a depauperate clade of fishes that represent the living sister group to all other sarcopterygians (lungfishes and tetrapods; e.g., Rosen et al., 1981; Amemiya et al., 2013; Meyer et al., 2021). Although their extant and extinct species diversity is extremely low (e.g., Schaeffer, 1952; Casane and Laurenti, 2013; Cavin and Guinot, 2014; Cavin et al., 2021; Toriño et al., 2021), coelacanths show an appreciable degree of morphological and body size evolution across their more than 400-million-year evolutionary history (Friedman, 2007; Toriño et al., 2021). This high degree of phenotypic innovation produced some of the largest freshwater fishes (e.g., Cavin et al., 2021), anguilliform (Friedman and Coates, 2005), hump-backed (Cavin et al., 2017), and fork-tailed (Wendruff and Wilson, 2012) forms, as well as deepwater species like the extant genus Latimeria.
Coelacanths reached peak diversity in the Triassic Period (Schaeffer, 1952; Wendruff and Wilson, 2012; Cavin et al., 2013; Cavin et al., 2017; Toriño et al., 2021): total richness spikes to ~20 genera representing at least ten long-lived clades (Toriño et al., 2021). Although most of these are within the two major post-Paleozoic coelacanth groups Latimeriidae and Mawsoniidae, several enigmatic species postdate their closest relatives by up to 100 million years (Wendruff and Wilson, 2012; Cavin et al., 2017; Toriño et al., 2021). This poses the question of whether these genera represent the last survivors of Paleozoic coelacanth ghost lineages or are part of as-yet-undetected radiations of archaic actinistians.
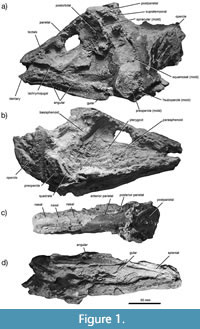 In this paper, I describe a new, large bodied (>1 m) species of coelacanth from the Upper Triassic (Carnian) Dockum Group of Texas, USA (Figure 1). The species is known from a nearly complete skull first reported by Schaeffer and Gregory (1961) from approximately 1 meter above the contact of the Dockum Formation with the Permian Quartermaster Formation in Palo Duro Canyon near Canyon, Texas, although it may be that the specimen was eroded from a younger layer in the Dockum Group higher up the canyon walls. The Dockum Group skull was preliminarily referred to the coelacanth †Chinlea sp. by Schaeffer (1967), an assignment that was agreed upon without extensive review of the specimen by Forey (1998). However, Shaeffer and Gregory (1961) noted the similarities between the Dockum Group skull and those of individuals in the genus †Whiteia. Phylogenetic analysis of the skull shows that the new coelacanth, †Whiteia giganteus sp. nov., is at or near the base of the major lineage including extant coelacanths and their closest extinct relatives (Latimerioidei). †Whiteia giganteus is exceptionally large for its geological age (Schaeffer and Gregory, 1961; Schaeffer, 1967; Cavin et al., 2017; Toriño et al., 2021; Cavin et al., 2021; Brownstein and Bissell, 2022), but possesses the mediolaterally compressed, poorly ornamented skull characteristic of †Whiteia that differentiates it from other large Mesozoic coelacanths. The body size, phylogenetic position, and provenance of †Whiteia giganteus considerably extend both the spatiotemporal range and the body size disparity of a major Triassic clade of coelacanths that radiated across Pangaea.
In this paper, I describe a new, large bodied (>1 m) species of coelacanth from the Upper Triassic (Carnian) Dockum Group of Texas, USA (Figure 1). The species is known from a nearly complete skull first reported by Schaeffer and Gregory (1961) from approximately 1 meter above the contact of the Dockum Formation with the Permian Quartermaster Formation in Palo Duro Canyon near Canyon, Texas, although it may be that the specimen was eroded from a younger layer in the Dockum Group higher up the canyon walls. The Dockum Group skull was preliminarily referred to the coelacanth †Chinlea sp. by Schaeffer (1967), an assignment that was agreed upon without extensive review of the specimen by Forey (1998). However, Shaeffer and Gregory (1961) noted the similarities between the Dockum Group skull and those of individuals in the genus †Whiteia. Phylogenetic analysis of the skull shows that the new coelacanth, †Whiteia giganteus sp. nov., is at or near the base of the major lineage including extant coelacanths and their closest extinct relatives (Latimerioidei). †Whiteia giganteus is exceptionally large for its geological age (Schaeffer and Gregory, 1961; Schaeffer, 1967; Cavin et al., 2017; Toriño et al., 2021; Cavin et al., 2021; Brownstein and Bissell, 2022), but possesses the mediolaterally compressed, poorly ornamented skull characteristic of †Whiteia that differentiates it from other large Mesozoic coelacanths. The body size, phylogenetic position, and provenance of †Whiteia giganteus considerably extend both the spatiotemporal range and the body size disparity of a major Triassic clade of coelacanths that radiated across Pangaea.
RESULTS
Systematic Paleontology
ANIMALIA Linneaus 1758
CHORDATA Haeckel 1874
ACTINISTIA Cope 1871
COELACANTHIFORMES Berg 1937
†WHITEIA Moy-Thomas 1935
†Whiteia giganteus sp. nov.
(Figure 1, Figure 2, Figure 3, Figure 4)
zoobank.org/1ED458EE-4F84-457B-8AF1-2BD3AD876605
Holotype. Yale Peabody Museum Vertebrate Paleontology (YPM VP) 3928, nearly complete eroded skull and mandibles.
Etymology. Giganteus, giant (Ancient Greek).
Locality and paleoenvironment. Lower Dockum Group (Carnian), Palo Duro Canyon, Texas Panhandle, Texas, USA. The environment represented by the Dockum Group consists of braided streams, deltaic plains, and lakes (Lucas and Anderson, 1993).
Diagnosis. Non-latimerioid coelacanth distinguished by: large (>1 m) size, which greatly exceeds other †Whiteia species (115-240 mm; Forey, 1998; Yabumoto and Brito, 2016; Yabumoto et al., 2019; a skull potentially referable to †Whiteia sp. from British Columbia indicates an animal approaching 1 m, but still considerably smaller than the Dockum Group specimen) as well as the small-bodied Triassic coelacanth genera Ticinepomis, Foreyia, Diplurus, Heptanema, Yunnancoelacanthus, and Luopingcoelacanthus; Moy-Thomas, 1935; Schaeffer, 1952, 1967; Schaeffer and Gregory, 1961; Forey, 1998; Wen et al., 2013; Cavin et al., 2017); posterior pterygoid teeth grow to nearly twice as large as anterior crowns on tooth plate (small in other †Whiteia for which this bone is known; Forey, 1998); enlarged angular foramina row (a handful of elongated pores in other †Whiteia; Forey, 1998; Yabumoto et al., 2019); anterior terminus of lachrymojugal reaches discrete apex (squared-off in †Whiteia woodwardi and †Whiteia sp.; Schaeffer and Gregory, 1961; Forey, 1998). Differs from †Whiteia woodwardi, †W. tuberculata, and †W. africana, but shares with †W. nielseni a lachrymojugal with a pronounced ventral angle below the orbit (Forey, 1998). Differs from †Whiteia nielseni and †W. uyenoteruyai, but shares with †W. woodwardi, †W. tuberculata, and †W. africana a dentary that is not hook-shaped, a prefrontal equal to less than 15% of total skull length, and a parietonasal shield with a flat to convex lateral profile (Forey, 1998; Yabumoto et al., 2019). Differs from †Whiteia uyenoteruyai, †W. tuberculata, and †W. nielseni, but shares with †W. woodwardi a straightened angular foramina row (Yabumoto et al., 2019).
†W. giganteus is assignable to the genus †Whiteia based on the following combination of features: presence of an elongated preorbital region more than 1/3rd the length of the total skull roof, a short, widened postparietal shield and mediolaterally compressed parietonasal shield, a first anterior supraorbital that excludes the preorbital from the orbital margin, a lachrymojugal that is deflected ventroanteriorly at its anterior end, and a large, rounded opercle with a strongly pointed ventral terminus, a cheek composed of the abutting lachrymojugal, postorbital, preopercle, and squamosal, a small subopercle, a shallow mandible, gular plates that terminate anterior to the posterior terminus of the mandibles, and sparse ornamentation of the skull dermal bones (Forey, 1998).
Description
Schaeffer and Gregory (1961) provided a preliminary description of most of the osteological features of the holotype skull (Figure 1), which represents a fish easily exceeding 1 meter in length based on comparisons with specimens of †Diplurus, †Rhabdoderma, †Whiteia, †Mawsonia, and Latimeria in the YPM collections.
 Skull Roof. The skull roof (Figure 1A–D) as preserved consists of the paired postparietals, supratemporals, and parietal and nasal rows (Moy-Thomas, 1935; Forey, 1998). There are two sets of paired parietals and three sets of paired nasals (Figure 1A-D, Figure 2). The preserved surfaces of the skull roof (Figure 1C) show a very low degree of ornamentation. Ornaments on the postparietals are the best preserved of those from the skull roof and consist of ridges radiating from ossification centers (Figure 1C). As in other †Whiteia and members of †Mawsoniidae (Schaeffer, 1967; Forey, 1998), the postparietals of †W. giganteus are widened relative to the rest of the skull roof. Despite missing the anterior eighth of the rostrum, the skull of †W. giganteus is elongated (Figure 2) relative to †Diplurus longicaudatus (Schaeffer, 1948), †Diplurus newarki (Schaeffer, 1952), †Foreyia maxkhuni (Cavin et al., 2017), and †Ticinepomis peyeri (Rieppel, 1980). Instead, the elongation of the skull most closely resembles that of †Whiteia spp. (Moy-Thomas, 1935; Forey, 1998). Similar elongation of the skull is also present in †Chinlea sorenseni (Schaeffer, 1967), although the skull of this species is more heavily built and dorsoventrally flattened (Schaeffer, 1967; Elliot, 1987). Because of the preservation of the holotype skull, the morphology of the sensory canals in †W. giganteus cannot be described in detail. However, the postparietals do appear to have borne sensory canals near the lateral margins of their dorsal surfaces.
Skull Roof. The skull roof (Figure 1A–D) as preserved consists of the paired postparietals, supratemporals, and parietal and nasal rows (Moy-Thomas, 1935; Forey, 1998). There are two sets of paired parietals and three sets of paired nasals (Figure 1A-D, Figure 2). The preserved surfaces of the skull roof (Figure 1C) show a very low degree of ornamentation. Ornaments on the postparietals are the best preserved of those from the skull roof and consist of ridges radiating from ossification centers (Figure 1C). As in other †Whiteia and members of †Mawsoniidae (Schaeffer, 1967; Forey, 1998), the postparietals of †W. giganteus are widened relative to the rest of the skull roof. Despite missing the anterior eighth of the rostrum, the skull of †W. giganteus is elongated (Figure 2) relative to †Diplurus longicaudatus (Schaeffer, 1948), †Diplurus newarki (Schaeffer, 1952), †Foreyia maxkhuni (Cavin et al., 2017), and †Ticinepomis peyeri (Rieppel, 1980). Instead, the elongation of the skull most closely resembles that of †Whiteia spp. (Moy-Thomas, 1935; Forey, 1998). Similar elongation of the skull is also present in †Chinlea sorenseni (Schaeffer, 1967), although the skull of this species is more heavily built and dorsoventrally flattened (Schaeffer, 1967; Elliot, 1987). Because of the preservation of the holotype skull, the morphology of the sensory canals in †W. giganteus cannot be described in detail. However, the postparietals do appear to have borne sensory canals near the lateral margins of their dorsal surfaces.
Preorbital bones and cheek. The lachrymojugal, preorbital, supraorbitals, and tectals are largely unornamented as preserved (Figure 1A-B, Figure 2), differentiating †W. giganteus from the Paleozoic North American †Rhabdoderma. There are molds that indicate the presence of four or five tectals abutting the lateral margin of the preorbital portion of the skull roof (Figure 2). The preorbital appears excluded from the orbital by the first supraorbital. The lachrymojugal is massive and bent along its anteroposterior axis (Figure 2), as in †Chinlea sorenseni (Schaeffer, 1967) and †Whiteia nielseni (Forey, 1998) but unlike †Diplurus spp. and most latimeriids (Schaeffer, 1952; Clement, 2005; Dutel et al., 2013). The anterior process of the lachrymojugal gradually tapers towards its anterior apex, and dorsally contacts the first supraorbital to form the anterior margin of the orbit. Molds and small portions of the squamosal, postorbital, and supraorbitals are also present, showing they were present and formed the posterodorsal rim of the orbit. However, their surface texture is unknown since most of these bones, including the spiracular, squamosal, and preopercle, are only indicated by molds of the original bones. The cheek bones all abutted one another but do not appear to have overlapped. The postorbital appears posteriorly expanded and is positioned entirely posterior to the intracranial joint formed at the level of the junction between the parietals and postparietals.
 Jaws, palatal bones, suspensorium, and jaw articulation. Due to wear, the form of the ventral tooth-bearing portion of the upper jaw (ventral premaxilla, dermopalatine, and ectopterygoid) are missing. However, the internal palatal anatomy of †Whiteia giganteus is largely visible (Figure 3). The pterygoid is large, and the anterior pterygoid flange is inflated to comprise a larger region of the internal skull than in †Diplurus spp., †Chinlea spp., and an intermediate form without pterygoid teeth (AMNH 3201) from the Chinle Formation (Schaeffer, 1948, 1952, 1967; Schaeffer and Gregory, 1961; Brownstein and Bissell, 2022). This bone bears numerous small, rounded palatal teeth, which gradually increase in size posteroventrally along the pterygoid tooth plate. The parasphenoid is an elongated bone that sits just above the pterygoid and forms a bar traversing the buccal cavity. The quadrate is sutured to the posteroventral corner of the pterygoid and forms the hinge joint with the mandible.
Jaws, palatal bones, suspensorium, and jaw articulation. Due to wear, the form of the ventral tooth-bearing portion of the upper jaw (ventral premaxilla, dermopalatine, and ectopterygoid) are missing. However, the internal palatal anatomy of †Whiteia giganteus is largely visible (Figure 3). The pterygoid is large, and the anterior pterygoid flange is inflated to comprise a larger region of the internal skull than in †Diplurus spp., †Chinlea spp., and an intermediate form without pterygoid teeth (AMNH 3201) from the Chinle Formation (Schaeffer, 1948, 1952, 1967; Schaeffer and Gregory, 1961; Brownstein and Bissell, 2022). This bone bears numerous small, rounded palatal teeth, which gradually increase in size posteroventrally along the pterygoid tooth plate. The parasphenoid is an elongated bone that sits just above the pterygoid and forms a bar traversing the buccal cavity. The quadrate is sutured to the posteroventral corner of the pterygoid and forms the hinge joint with the mandible.
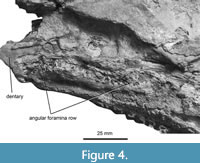 The mandible is robustly constructed (Figure 4) and as preserved includes the dentary and angular. The dentary is small, smoothly textured, and lacks any clear foramina, unlike the dentaries of †Diplurus spp. (Schaeffer, 1952; Brownstein and Bissell, 2022). The dentary forms the anterior margin of the mandible and is anteroposteriorly shortened, contrasting with the anteroposteriorly elongated dentary of †Chinlea, which runs for approximately one-third the length of the mandible and is broadly subrectangular (Schaeffer, 1967; Elliot, 1987). The angular foramina row is prominent and includes far more individual foramina than those of †Diplurus spp. (Schaeffer, 1952) and †Chinlea sorenseni (Schaeffer, 1967; Elliot, 1987). In ventral view, the dentaries anteriorly twist inward to form a firm mandibular symphysis. Between the mandibles, the elongated gulars extend to form the floor of the lower jaw.
The mandible is robustly constructed (Figure 4) and as preserved includes the dentary and angular. The dentary is small, smoothly textured, and lacks any clear foramina, unlike the dentaries of †Diplurus spp. (Schaeffer, 1952; Brownstein and Bissell, 2022). The dentary forms the anterior margin of the mandible and is anteroposteriorly shortened, contrasting with the anteroposteriorly elongated dentary of †Chinlea, which runs for approximately one-third the length of the mandible and is broadly subrectangular (Schaeffer, 1967; Elliot, 1987). The angular foramina row is prominent and includes far more individual foramina than those of †Diplurus spp. (Schaeffer, 1952) and †Chinlea sorenseni (Schaeffer, 1967; Elliot, 1987). In ventral view, the dentaries anteriorly twist inward to form a firm mandibular symphysis. Between the mandibles, the elongated gulars extend to form the floor of the lower jaw.
 Opercle and gular. The large opercle is unornamented (Figure 1A-B) and the portion of the posterior margin possesses the characteristic D-shape of this bone in †Whiteia, where the dorsal and posterior margins are rounded, the anterior margin is vertically straightened, and the ventral margin ends in a discrete apex (Figure 5; Forey, 1998). The gulars are elongated bones that are generally comparable to other †Whiteia species. Neither the gulars nor the opercle are heavily ornamented.
Opercle and gular. The large opercle is unornamented (Figure 1A-B) and the portion of the posterior margin possesses the characteristic D-shape of this bone in †Whiteia, where the dorsal and posterior margins are rounded, the anterior margin is vertically straightened, and the ventral margin ends in a discrete apex (Figure 5; Forey, 1998). The gulars are elongated bones that are generally comparable to other †Whiteia species. Neither the gulars nor the opercle are heavily ornamented.
Phylogenetic methods and results
I scored YPM 3928 for the Toriño et al. (2021) dataset, which includes 50 operational taxonomic units (OTUs) coded for 110 characters. Three wildcard taxa (†Euporosteus, †Indocoelacanthus and †Reidus ) identified by Toriño et al. (2021) were removed from the matrix used in this contribution after initial phylogenetic runs showed they continued to exert a destabilizing effect on phylogenetic resolution. I also added two OTUs based on Brownstein and Bissell (2022); these were composite codings of †Diplurus newarki and †D. enigmaticus based on specimens assigned to those species. The scorings for †Chinlea were also checked to make sure they did not include data from YPM 3928. The final matrix comprised 49 OTUs scored for 110 characters.
 I conducted parsimony analyses using the program TNT 1.5 (Goloboff and Catalano, 2016). First, I ran a Wagner search over 1000 trees with default parameters for ratchet, tfuse, drift, and sectorial search followed by a round of traditional bisection-reconnection branch swapping over 100,000 trees to fully explore possible topological space. This generated 2 most parsimonious trees of length 346 (consistency index = 0.358, retention index = 0.684), which were summarized using a strict consensus topology and a combinable components consensus with bootstrap supports sampled over 100 replicates. The strict consensus topology placed the Dockum Group skull YPM 3928 in a polytomy with †Whiteia at the base of a clade including the Mawsoniidae, Latimeriidae, and the Triassic genera †Dobrogeria and †Heptanema (Figure 6); both most parsimonious trees placed YPM 3928 one step closer to this clade than †Whiteia, albeit with no character support. No apomorphies were found for YPM 3928, whereas †Whiteia spp. was found to have three apomorphies: 5[0], 46[1], and 83[1]. The resolution of apomorphies for †Whiteia spp. is due to the fact that these characters are not scorable for YPM 3928. The combinable components consensus tree also found YPM 3928 to be the sister taxon to †Whiteia, with moderate support (bootstrap value = 44). The uncertainty surrounding the relationships of YPM 3928 are almost certainly driven by the incomplete nature of the Dockum skull. Nonetheless, these parsimony analyses provide support for the identification of YPM 3928 as a species of †Whiteia, and the Dockum skull is assignable to that genus based on numerous skeletal features (see earlier; Schaeffer et al., 1976; Forey, 1998). The input morphological dataset and output tree files are included in the Supplemental Material to this manuscript.
I conducted parsimony analyses using the program TNT 1.5 (Goloboff and Catalano, 2016). First, I ran a Wagner search over 1000 trees with default parameters for ratchet, tfuse, drift, and sectorial search followed by a round of traditional bisection-reconnection branch swapping over 100,000 trees to fully explore possible topological space. This generated 2 most parsimonious trees of length 346 (consistency index = 0.358, retention index = 0.684), which were summarized using a strict consensus topology and a combinable components consensus with bootstrap supports sampled over 100 replicates. The strict consensus topology placed the Dockum Group skull YPM 3928 in a polytomy with †Whiteia at the base of a clade including the Mawsoniidae, Latimeriidae, and the Triassic genera †Dobrogeria and †Heptanema (Figure 6); both most parsimonious trees placed YPM 3928 one step closer to this clade than †Whiteia, albeit with no character support. No apomorphies were found for YPM 3928, whereas †Whiteia spp. was found to have three apomorphies: 5[0], 46[1], and 83[1]. The resolution of apomorphies for †Whiteia spp. is due to the fact that these characters are not scorable for YPM 3928. The combinable components consensus tree also found YPM 3928 to be the sister taxon to †Whiteia, with moderate support (bootstrap value = 44). The uncertainty surrounding the relationships of YPM 3928 are almost certainly driven by the incomplete nature of the Dockum skull. Nonetheless, these parsimony analyses provide support for the identification of YPM 3928 as a species of †Whiteia, and the Dockum skull is assignable to that genus based on numerous skeletal features (see earlier; Schaeffer et al., 1976; Forey, 1998). The input morphological dataset and output tree files are included in the Supplemental Material to this manuscript.
DISCUSSION
†Whiteia giganteus is the largest member of its genus, which is distributed throughout much of the Triassic of the northern and southern hemispheres (Moy-Thomas, 1935; Schaeffer et al., 1976; Forey, 1998), as well as the southernmost occurrence of this genus in the Americas. Previously, Schaeffer et al. (1976) described skeletons referable to †Whiteia from the Triassic of British Columbia, but these have not been examined in detail or included in a phylogenetic analysis.
More commonly found in the Triassic strata of North America are species belonging to the Latimerioidei, which includes both major clades of Mesozoic-Cenozoic coelacanths. In eastern North America, these are represented by the species †Diplurus newarki, †D. enigmaticus and †D. longicaudatus, which are known from multiple complete skulls and skeletons collected from the Atlantic Coastal Plain (Schaeffer, 1952; Forey, 1998; Brownstein and Bissell, 2022). In western North America, Triassic units have produced the species †Chinlea sorenseni and †Moenkopia wellesi (Schaefer and Gregory, 1961; Schaeffer, 1967), as well as several unnamed, probably distinct forms (Schaeffer and Gregory, 1961). The phylogenetic trees presented in this study (Figure 6) as well as previous analyses (e.g., Forey, 1998; Cavin et al., 2017; Toriño et al., 2021) confirm the placement of †Whiteia outside this clade. Thus, †Whiteia giganteus expands the diversity of Triassic American actinistians to include taxa outside the major Mesozoic-Cenozoic coelacanth lineage and expands the range of this grade of fishes several thousand kilometers southward in the Americas and into the Upper Triassic.
In addition to confirming that non-latimerioid coelacanths had a wide distribution in the Triassic of North America, †Whiteia giganteus also illuminates the genus †Whiteia as an exemplar Pangean radiation. The currently valid species of †Whiteia have a near-cosmopolitan distribution; the type species †W. woodwardi (Moy-Thomas, 1935), †W. tuberculata (Moy-Thomas, 1935), and the recently described †W. uyenoteruyai (Yabumoto et al., 2019) come from Madagascar, †W. africana comes from South Africa (Forey, 1998), and †W. nielseni (Forey, 1998) is from Greenland. Although the phylogenetic relationships of these species and †W. giganteus among †Whiteia remain unclear (Yabumoto et al., 2019), †Whiteia and its parent clade †Whiteiidae (possibly paraphyletic, see Toriño et al., 2021) constitutes one of the most widespread clades of actinistians in the fossil record (Yabumoto et al., 2019; Toriño et al., 2021). †W. giganteus extends this distribution by several thousand kilometers, confirming a trans-Pangean distribution for this genus and regional differences in †Whiteia species occurrences.
On a macroevolutionary scale, †Whiteia giganteus substantiates a novel episode of large (>1 m) body size acquisition outside Latimerioidei, which includes the vast majority of large-bodied coelacanths (Cavin et al., 2021). During the Triassic, the latimerioids began to diversify into a variety of body plans within a larger spike in coelacanth species richness and morphological disparity (Schaeffer, 1952; Cavin et al., 2017; Cavin et al., 2021). Along with a handful of other non-latimerioid large-bodied species from the Triassic (e.g., Rebellatrix, Wendruff and Wilson, 2012), †Whiteia giganteus substantiates a final stage in the evolutionary history of ‘archaic’ coelacanth clades as they began to be overtaken by the Latimerioidei in the early Mesozoic.
ACKNOWLEDGEMENTS
Thanks to Dan Brinkman and Vanessa Rhue, collections managers. I also thank the editor and reviewers for their comments, which greatly improved this manuscript.
REFERENCES
Amemiya, C.T., Alföldi, J., Lee, A.P., Fan, S., Philippe, H., MacCallum, I., Braasch, I., Manousaki, T., Schneider, I., Rohner, N., and Organ, C. 2013. The African coelacanth genome provides insights into tetrapod evolution. Nature, 496(7445):311-316. https://doi.org/10.1038/nature12027
Brownstein, C.D. and Bissell, I.C. 2022. Species delimitation and coexistence in an ancient, depauperate vertebrate clade. BMC Ecology and Evolution, 22(1):1-15. https://doi.org/10.1186/s12862-022-02043-4
Casane, D. and Laurenti, P. 2013. Why coelacanths are not 'living fossils’. A review of molecular and morphological data. Bioessays, 35(4):332-338. https://doi.org/10.1002/bies.201200145
Cavin, L. and Guinot, G. 2014. Coelacanths as “almost living fossils”. Frontiers in Ecology and Evolution, 2:49. https://doi.org/10.3389/fevo.2014.00049
Cavin, L., Mennecart, B., Obrist, C., Costeur, L., and Furrer, H. 2017. Heterochronic evolution explains novel body shape in a Triassic coelacanth from Switzerland. Scientific Reports, 7(1):1-7. https://doi.org/10.1038/s41598-017-13796-0
Cavin, L., Piuz, A., Ferrante, C., and Guinot, G. 2021. Giant Mesozoic coelacanths (Osteichthyes, Actinistia) reveal high body size disparity decoupled from taxic diversity. Scientific Reports, 11(1):1-3. https://doi.org/10.1038/s41598-021-90962-5
Cavin, L., Furrer, H., and Obrist, C. 2013. New coelacanth material from the Middle Triassic of eastern Switzerland, and comments on the taxic diversity of actinistans. Swiss Journal of Geosciences, 106(2):161-177. https://doi.org/10.1007/s00015-013-0143-7
Clement, G. 2005. A new coelacanth (Actinistia, Sarcopterygii) from the Jurassic of France, and the question of the closest relative fossil to Latimeria. Journal of Vertebrate Paleontology, 25(3):481-491. https://doi.org/10.1671/0272-4634(2005)025[0481:ANCASF]2.0.CO;2
Dutel, H., Herrel, A., Clément, G., and Herbin, M. 2013. A reevaluation of the anatomy of the jaw-closing system in the extant coelacanth Latimeria chalumnae. Naturwissenschaften, 100(11):1007-1022. https://doi.org/10.1007/s00114-013-1104-8
Elliott, D.K. 1987. A new specimen of Chinlea sorenseni from the Chinle Formation, Dolores River, Colorado. Journal of the Arizona-Nevada Academy of Science, 22(1):47-52.
Forey, P. 1998. History of the coelacanth fishes. Springer Science and Business Media, Berlin, Germany.
Friedman, M. and Coates, M.I. 2005. A newly recognized fossil coelacanth highlights the early morphological diversification of the clade. Proceedings of the Royal Society B: Biological Sciences, 273(1583):245-250. https://doi.org/10.1098/rspb.2005.3316
Friedman, M. 2007. Styloichthys as the oldest coelacanth: implications for early osteichthyan interrelationships. Journal of Systematic Palaeontology, 5(3):289-343. https://doi.org/10.1017/S1477201907002052
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics, 32(3):221-38. https://doi.org/10.1111/cla.12160
Lucas, S.G. and Anderson, O.J. 1993. Lithostratigraphy, sedimentation, and sequence stratigraphy of Upper Triassic Dockum Formation, West Texas, p. 357. New Mexico Geological Society, 44th Annual Fall Field Conference Guidebook.
Meyer, A., Schloissnig, S., Franchini, P., Du, K., Woltering, J.M., Irisarri, I., Wong, W.Y., Nowoshilow, S., Kneitz, S., Kawaguchi, A. and Fabrizius, A. 2021. Giant lungfish genome elucidates the conquest of land by vertebrates. Nature, 590(7845):284-289. https://doi.org/10.1038/s41586-021-03198-8
Moy-Thomas, J.A. 1935. The coelacanth fishes from Madagascar. Geological Magazine, 72:213-226.
Rieppel, O. 1980. A new coelacanth from the Middle Triassic of Monte San Giorgio, Switzerland. Eclogae Geologicae Helvetiae, 73:921-939. http://doi.org/10.5169/seals-164996
Rosen, D.E., Forey, P.L., Gardiner, B.G., and Patterson, C., 1981. Lungfishes, tetrapods, paleontology, and plesiomorphy. Bulletin of the American Museum of Natural History, 197(4):1-124.
Schaeffer, B. 1948. A study of Diplurus longicaudatus with notes on the body form and locomotion of the Coelacanthini. American Museum Novitates, 1378:1-32.
Schaeffer, B. 1952. The Triassic coelacanth fish Diplurus, with observations on the evolution of the Coelacanthini. Bulletin of the American Museum of Natural History, 99(2):1-67.
Schaeffer, B. 1967. Late Triassic fishes from the western United States. Bulletin of the American Museum of Natural History, 135(6):285-342.
Schaeffer, B. and Gregory, J.T. 1961. Coelacanth fishes from the continental Triassic of the western United States. American Museum Novitates, 2036:1-18.
Schaeffer, B., Mangus, M. and Laudon, L.R., 1976. An early Triassic fish assemblage from British Columbia. Bulletin of the American Museum of Natural History, 156(5):515-564.
Toriño, P., Soto, M., and Perea, D. 2021. A comprehensive phylogenetic analysis of coelacanth fishes (Sarcopterygii, Actinistia) with comments on the composition of the Mawsoniidae and Latimeriidae: Evaluating old and new methodological challenges and constraints. Historical Biology, 33(12):3423-3443. https://doi.org/10.1080/08912963.2020.1867982
Wen, W., Zhang, Q.-Y., Hu, S.-X., Benton, M.J., Zhou, C.-Y., Tao, X., Huang, J.-Y., and Chen, Z.-Q. 2013. Coelacanths from the Middle Triassic Luoping Biota, Yunnan, South China, with the earliest evidence of ovoviviparity. Acta Palaeontologica Polonica, 58(1):175-193. https://doi.org/10.4202/app.2011.0066
Wendruff, A.J. and Wilson, M.V. 2012. A fork-tailed coelacanth, Rebellatrix divaricerca, gen. et sp. nov. (Actinistia, Rebellatricidae, fam. nov.), from the Lower Triassic of Western Canada. Journal of Vertebrate Paleontology, 32(3):499-511. https://doi.org/10.1080/02724634.2012.657317
Yabumoto, Y. and Brito, P.M. 2016. A new Triassic coelacanth, Whiteia oishii (Sarcopterygii, Actinistia) from West Timor, Indonesia. Paleontological Research, 20(3):233-246.
Yabumoto, Y., Brito, P.M., Iwata, M., and Abe, Y. 2019. A new Triassic coelacanth, Whiteia uyenoteruyai (Sarcopterygii, Actinistia) from Madagascar and paleobiogeography of the family Whiteiidae. Bulletin of the Kitakyushu Museum of Natural History and Human History Series A (Natural History), 17:15-28.

