 Morphological insights into the lobster genus Uncina Quenstedt, 1851 based on new material from the Ya Ha Tinda Konservat-Lagerstätte, Canada (Early Jurassic)
Morphological insights into the lobster genus Uncina Quenstedt, 1851 based on new material from the Ya Ha Tinda Konservat-Lagerstätte, Canada (Early Jurassic)
Article number: 26.1.a11
https://doi.org/10.26879/1158
Copyright Paleontological Society, April 2023
Proceedings of the 8th Symposium on Fossil Decapod Crustaceans
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 28 March 2021. Acceptance: 15 February 2023.
ABSTRACT
It is rare to find Early Jurassic crustacean material outside of Europe but the discovery of the Ya Ha Tinda Konservat-Lagerstätte in Alberta, Canada, has significantly increased the number of crustacean specimens, specifically of the genus Uncina. New articulated specimens of Uncina pacifica and Uncina ollerenshawi preserve most of the cephalothorax and pleon, not just the chelae of the first pereiopod. This new material from Ya Ha Tinda permits revised morphologic descriptions as well as a quantitative analysis of the intra- and interspecific variability of Uncina claw morphology. Morphometric similarities and differences are noted between species, indicating that a notch at the distal end of the occlusal surface of the fixed finger is diagnostic for the genus. Our findings suggest that Uncina species are usually isochelous, with the exception of Uncina pacifica, which is heterochelous. The two co-occurring species of Uncina in the Ya Ha Tinda Formation, Uncina pacifica and Uncina ollerenshawi, likely occupied different niches, based upon differences in chela morphology.
Brooke A. Bogan. Department of Geological Sciences, University of Texas at Austin, 2275 Speedway Stop C9000, 1 University Station C1100, Austin, TX 78712, USA. Current Address: Department of Museum Research and Collections, Alabama Museum of Natural History, 427 Sixth Avenue, The University of Alabama, Tuscaloosa, AL 35487, USA. babogan@ua.edu.
Rowan C. Martindale. Department of Geological Sciences, University of Texas at Austin, 2275 Speedway Stop C9000, 1 University Station C1100, Austin, TX 78712, USA. (corresponding author) martindale@jsg.utexas.edu
Rodney M. Feldmann. Department of Earth Sciences, Kent State University, 221 McGilvrey Hall, Kent, Ohio 44242, USA. rfeldman@kent.edu.
Carrie E. Schweitzer. Department of Earth Sciences, Kent State University at Stark, 6000 Frank Avenue NW, North Canton, Ohio 44720, USA. cschweit@kent.edu.
A. Drew Muscente. Department of Geology, Cornell College, 600 First Street SW, Mount Vernon, Iowa, 52314, USA. a.d.muscente@gmail.com.
Key words: Pliensbachian; Toarcian; marine; crustacean; morphology; systematic paleontology
Final citation: Bogan, Brooke A., Martindale, Rowan C., Feldmann, Rodney M., Schweitzer, Carrie E., and Muscente, A. Drew. 2023. Morphological insights into the lobster genus Uncina Quenstedt, 1851 based on new material from the Ya Ha Tinda Konservat-Lagerstätte, Canada (Early Jurassic). Palaeontologia Electronica, 26(1):a11.
https://doi.org/10.26879/1158
palaeo-electronica.org/content/2023/3778-fossil-lobsters-from-canada
Copyright: April 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Crustaceans are important components of benthic marine communities in both modern and ancient oceans. Decapod crustaceans have a well-documented fossil record through the Jurassic but are mostly known from European sites, such as the Toarcian Posidonia Shale in Germany, and Upper Jurassic lithographic limestones, like the Solnhofen Limestone in Germany (Schweigert et al., 2003; Klompmaker et al., 2013; Schweitzer and Feldmann, 2014). To date, there has been very little work on Jurassic crustaceans from sites outside of Europe (i.e., the Panthalassa Ocean).
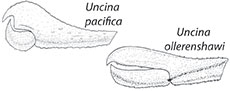
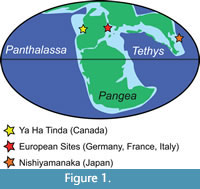 The Jurassic lobster Uncina Quenstedt, 1851, belongs to the superfamily Enoplometopoidea de Saint Laurent, 1988, and the family Uncinidae Quenstedt, 1851 (Ahyong, 2006; Karasawa et al., 2013). Globally, Uncina is represented by four extinct species from the Early Jurassic (201 Ma to 174 Ma). Although uncinid lobsters have been found in deposits from the Panthalassa Ocean, namely Japan (Karasawa, 2002) and Canada (Feldmann and Copeland, 1988; Schweigert et al., 2003), most studied and articulated specimens are from Tethyan strata in Franconia, northwest and southwest Germany, as well as eastern France, and northern Italy (Schweigert et al., 2003) (Figure 1). In addition to the paucity of material from Panthalassa, Uncina pacifica Schweigert Garassino, Hall, Hauff, and Karasawa, 2003 (Canada and Japan) and Uncina alpina Schweigert, Garassino, Hall, Hauff, and Karasawa, 2003 (Germany) are only known from a few specimens of cheliped material. Moreover, Uncina ollerenshawi Feldmann and Copeland, 1988 (Canada) has, until recently, only been known from one molted specimen with poor sclerotization (Feldmann and Copeland, 1988). The diagnosis of Uncina originally was largely based on the type species, Uncina posidoniae Quenstedt, 1851 (Germany) and emended diagnoses have not benefitted from extensive studies of complete specimens (i.e., majority of the cephalothorax, pleon, and chelae) from the three other species (Schweigert et al., 2003). Recent discoveries at the Ya Ha Tinda Konservat-Lagerstätte in Alberta, Canada (Figure 1) (Martindale et al., 2017; Muscente et al., 2019), have substantially increased the number of Uncina specimens available for study including several articulated or mostly complete body fossils yielding new morphological information on the two Panthalassan species, U. ollerenshawi and U. pacifica. These new finds include 21 previously undescribed specimens of U. pacifica (two exhibiting carapace, chelae, uropods, and pleons, hereafter referred to as a “full body” specimen) and nine new specimens of U. ollerenshawi including two full body specimens. Herein, we provide emended diagnoses of the Panthalassan Uncina species as well as the genus, with emphasis on intraspecific variation of the first pereiopods as they are the most commonly and best-preserved part of the animal (Schweigert et al., 2003). This work also discusses the stratigraphic range and ecomorphology of species of this genus, with particular attention to the information yielded from the North American specimens.
The Jurassic lobster Uncina Quenstedt, 1851, belongs to the superfamily Enoplometopoidea de Saint Laurent, 1988, and the family Uncinidae Quenstedt, 1851 (Ahyong, 2006; Karasawa et al., 2013). Globally, Uncina is represented by four extinct species from the Early Jurassic (201 Ma to 174 Ma). Although uncinid lobsters have been found in deposits from the Panthalassa Ocean, namely Japan (Karasawa, 2002) and Canada (Feldmann and Copeland, 1988; Schweigert et al., 2003), most studied and articulated specimens are from Tethyan strata in Franconia, northwest and southwest Germany, as well as eastern France, and northern Italy (Schweigert et al., 2003) (Figure 1). In addition to the paucity of material from Panthalassa, Uncina pacifica Schweigert Garassino, Hall, Hauff, and Karasawa, 2003 (Canada and Japan) and Uncina alpina Schweigert, Garassino, Hall, Hauff, and Karasawa, 2003 (Germany) are only known from a few specimens of cheliped material. Moreover, Uncina ollerenshawi Feldmann and Copeland, 1988 (Canada) has, until recently, only been known from one molted specimen with poor sclerotization (Feldmann and Copeland, 1988). The diagnosis of Uncina originally was largely based on the type species, Uncina posidoniae Quenstedt, 1851 (Germany) and emended diagnoses have not benefitted from extensive studies of complete specimens (i.e., majority of the cephalothorax, pleon, and chelae) from the three other species (Schweigert et al., 2003). Recent discoveries at the Ya Ha Tinda Konservat-Lagerstätte in Alberta, Canada (Figure 1) (Martindale et al., 2017; Muscente et al., 2019), have substantially increased the number of Uncina specimens available for study including several articulated or mostly complete body fossils yielding new morphological information on the two Panthalassan species, U. ollerenshawi and U. pacifica. These new finds include 21 previously undescribed specimens of U. pacifica (two exhibiting carapace, chelae, uropods, and pleons, hereafter referred to as a “full body” specimen) and nine new specimens of U. ollerenshawi including two full body specimens. Herein, we provide emended diagnoses of the Panthalassan Uncina species as well as the genus, with emphasis on intraspecific variation of the first pereiopods as they are the most commonly and best-preserved part of the animal (Schweigert et al., 2003). This work also discusses the stratigraphic range and ecomorphology of species of this genus, with particular attention to the information yielded from the North American specimens.
GEOLOGICAL SETTINGS
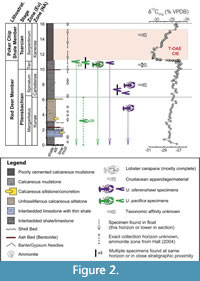 Crustaceans from Ya Ha Tinda have been recovered from the Red Deer and Poker Chip Shale members of the Lower Jurassic Fernie Formation (Figure 2, Table 1) (Martindale et al., 2017). The Pliensbachian to early Toarcian Red Deer Member is composed of dark grey to black, platy, calcareous shale interbedded with fine siltstones and thin limestone shell beds, whereas the Toarcian Poker Chip Shale Member is chiefly black, calcareous shale, and mudstone that is fine-grained, poorly cemented, and fissile (Them et al., 2017, and references therein). These members are interpreted to have been deposited in periodically dysoxic conditions with regular intervals of oxygenation (Martindale and Aberhan, 2017; Muscente et al., 2019; Sinha et al., 2021). The chronostratigraphy of the site is best constrained by ammonite biostratigraphy and stable isotope chemostratigraphy, namely the carbon isotope excursion associated with the Toarcian Oceanic Anoxic Event (Figure 2) (Them et al., 2017). Both near-complete articulated specimens of Uncina and disarticulated material are abundant in strata prior to the Toarcian Oceanic Anoxic Event (Figure 2), but during the event and in the recovery from the event, lobster material is limited (Figure 2) (Martindale and Aberhan, 2017).
Crustaceans from Ya Ha Tinda have been recovered from the Red Deer and Poker Chip Shale members of the Lower Jurassic Fernie Formation (Figure 2, Table 1) (Martindale et al., 2017). The Pliensbachian to early Toarcian Red Deer Member is composed of dark grey to black, platy, calcareous shale interbedded with fine siltstones and thin limestone shell beds, whereas the Toarcian Poker Chip Shale Member is chiefly black, calcareous shale, and mudstone that is fine-grained, poorly cemented, and fissile (Them et al., 2017, and references therein). These members are interpreted to have been deposited in periodically dysoxic conditions with regular intervals of oxygenation (Martindale and Aberhan, 2017; Muscente et al., 2019; Sinha et al., 2021). The chronostratigraphy of the site is best constrained by ammonite biostratigraphy and stable isotope chemostratigraphy, namely the carbon isotope excursion associated with the Toarcian Oceanic Anoxic Event (Figure 2) (Them et al., 2017). Both near-complete articulated specimens of Uncina and disarticulated material are abundant in strata prior to the Toarcian Oceanic Anoxic Event (Figure 2), but during the event and in the recovery from the event, lobster material is limited (Figure 2) (Martindale and Aberhan, 2017).
Decapod material was collected from Scalp Creek, Bighorn Creek, and the East Tributary of Bighorn Creek (referred to as “East Tributary”) sections at the Ya Ha Tinda Ranch, Alberta, Canada (Royal Tyrrell Museum of Palaeontology localities L2430, L2429, and L2428, respectively), but most of the material is from the East Tributary of Bighorn Creek. Many specimens were found in situ and thus can be assigned to a stratigraphic horizon and age; however, numerous significant finds were recovered from float material on the banks of the river (i.e., float) or were collected previously and stratigraphic information was not reported. In some cases, specific stratigraphic position is not known or was not recorded, but the ammonite zone was noted by the collector, the late Russell Hall, an ammonite biostratigrapher. In these cases, the age is assumed to be correct (denoted by dashed range in Figure 2). Recently collected specimens found in float material from East Tributary can be assigned a minimum age; the river cuts up-section, and so specimens that were not found in situ are interpreted to have originated from strata that are stratigraphically below the horizon in which they were found (denoted by the dashed arrow in Figure 2). Fernie Formation decapod material (Table 1) is predominantly preserved through secondary phosphatization and now consists of apatite minerals with some carbonaceous material, as well as minor auxiliary minerals (Muscente et al., 2019; Sinha et al., 2021).
TAXONOMIC AND MORPHOMETRIC METHODS OF ANALYSIS
Rock slabs of curated specimens were trimmed and reconsolidated with Paraloid B-72 (a thermoplastic resin) if fractured, and overlying matrix was removed. Then, each specimen was photographed in plain light and angled light with a Canon EOS Rebel SL2 Digital SLR camera with an EFS 18-55 mm lens; specimens were also photographed under UV light and polarized light with limited success. Particularly informative specimens were whitened with ammonium chloride (see Feldmann, 1989, for details on this methodology) and photographed with low-angle light using a Nikon D3100 camera with AF-S micro Nikkor 60 mm lens. Images of the fossil specimens were enhanced in Adobe Photoshop to increase contrast and assist identification of features.
The most consistently preserved elements of uncinid lobsters are the chelipeds. Since these elements are not only abundant but diagnostic for each species, they are ideal for morphological assessments of cheliped variation among and between Uncina species. Numerous measurements were taken for each cheliped (Appendix 1, Appendix 2). For specimens measured physically, digital calipers were used; measurements taken from photographed specimens figured in Schweigert et al. (2003) were calculated using the measuring tool in ImageJ (Schneider et al., 2012). All statistical and morphometric analyses were performed in R studio (R Core Team, 2008). R code used for box plots and Wilcoxon (1945) Ranked-Sum tests can be found in Appendix 3. The Wilcoxon Ranked-Sum tests were employed to test the statistical similarity of measurements taken on the chelae of each species. The null hypothesis is that the median difference between the observations is zero. The confidence interval used was 0.99 and the Alpha Value was 0.05. Images of all new specimens are included in Appendix 4.
Institutional Abbreviations
TMP, The Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta, Canada; KMNH, Kitakyushu Museum of Natural History, Kitakyushu, Japan; GSC, Geological Survey of Canada, Ottawa, Ontario, Canada.
SYSTEMATIC PALEONTOLOGY
Order DECAPODA Latreille, 1802
Infraorder ASTACIDEA Latreille, 1802
Superfamily ENOPLOMETOPOIDEA de Saint Laurent, 1988
Family UNCINIDAE Beurlen, 1928
Genus UNCINA Quenstedt, 1851
(syn.: Leptochirus Krause, 1891)
Type species. Uncina posidoniae Quenstedt, 1851, by monotypy.
Diagnosis. Astacidean with weakly sclerotized carapace, median suture present; strong, serrate rostrum; granulated carapace; first pereiopods chelate, enlarged, bearing marginal, distally-directed spines with robust, elongate merus; propodus with distally curved fingers; line of highly sclerotized nodes present across manus; manus with concave inner margin and convex outer margin; first pereiopods heterochelous or isochelous; occlusal surface of fixed finger and dactylus dentate; distal part of the fixed finger deeply incised with a notch; second and third pereiopods chelate; fourth and fifth pereiopods achelate, terminating in triangular dactylus; exopod of uropod with straight diaeresis.
Remarks. With the discovery of the two full body specimens of Uncina pacifica and two new full body specimens of U. ollerenshawi described herein, many genus-level traits are confirmed. The straight diaeresis of the exopod of the uropods is present in all specimens that exhibit pleons (TMP 2018.024.0001, species unknown, telson, uropods, and pleon only; TMP 2005.028.0004, U. ollerenshawi full body specimen; GSC 80067, U. ollerenshawi full body specimen; TMP 2014.021.0002, U. pacifica full body specimen; TMP 2017.015.0001, U. pacifica full body specimen; TMP 2014.021.0052, U. ollerenshawi full body specimen; see Figure 3, Figure 4, and Appendix 4) so we confirm this trait as a generic characteristic. The new specimen of U. pacifica with both chelipeds articulated (TMP 2017.015.0001, Figure 4B) is found to be heterochelous, a previously unknown condition for uncinids; however, the three full body U. ollerenshawi specimens studied (GSC 80067, TMP 2005.028.0004, and TMP 2014.021.0002) are isochelous and fit the original genus description. No new U. alpina or U. posidoniae specimens were collected; examinations focused mainly on published material.
Uncina ollerenshawi (Feldmann and Copeland, 1988)
Figure 3
1988 ? Eryma ollerenshawi Feldmann and Copeland, p. 93., fig. 4.2; pls. 4.1-4.2.
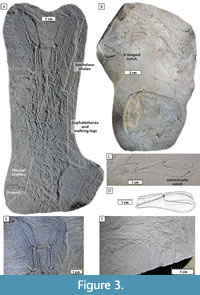 2003 Uncina ollerenshawi (Feldmann and Copeland); Schweigert et al., p. 10., pl. 11.
2003 Uncina ollerenshawi (Feldmann and Copeland); Schweigert et al., p. 10., pl. 11.
2010 Uncina ollerenshawi (Feldmann and Copeland); Schweitzer et al., p. 27.
2019 Uncina ollerenshawi (Feldmann and Copeland); Muscente et al., p. 522.
Holotype. GSC 80067.
Type locality. GSC locality 75402 on Bighorn Creek (see Frebold 1957: 85-86), SW Alberta, Canada (RTMP Locality L2429).
Type horizon. Fernie Formation, Red Deer Member (Upper Pliensbachian, Margaritatus Zone).
Studied material. GSC 80067, TMP 2005.028.0004, TMP 2013.036.0002, TMP 2014.021.0052 (both pereiopods), TMP 2015.050.0011, TMP 2015.051.0063, TMP 2016.027.0008, TMP 2018.024.0006 and TMP 2018.024.0009 (Table 1).
Occurrence. Pliensbachian of Canada.
Diagnosis. Isochelous species of Uncina in which pleonal pleura are rectangular; fixed finger of first pereiopod almost equal in length with the manus; maximum width of fixed finger and dactylus nearly equal; semicircular to V-shaped notch in distal part of fixed finger; diagonal row of nodes from top of manus attachment to dactylus attachment; dorsal and ventral margins of chelae of first pereiopods covered with distally-directed spines.
Remarks. Previously, only the holotype of Uncina ollerenshawi was described. In this study, eight new specimens were evaluated, including two other full body specimens; these additional specimens verify traits described in Feldmann and Copeland (1988) and Schweigert et al. (2003). The holotype represents a molt of the animal (Feldmann and Copeland, 1988), so the addition of new specimens, specifically those representing corpses, were useful in confirming that the original holotype observations were not modified by the molting process. Molt specimens are recognizable by their often weaker sclerotization and wrinkled appearance. The new U. ollerenshawi specimens were used to confirm the following generic traits: serrate rostrum, ridged claws, long occlusal surface of fingers, and diaeresis on exopods of uropods.
Comparisons. The chelae of Uncina ollerenshawi appear similar to those of U. posidoniae with the key difference being the length of the fixed finger and dactylus when compared to that of the rest of the cheliped. In U. ollerenshawi, there is a nearly 1:1 ratio between the lengths of the fixed finger and the manus, whereas in U. posidoniae, the hand is much longer than the fixed finger. Uncina pacifica and U. ollerenshawi, the two species found in North America, are most notably different when considering the height of the fixed finger compared to the hand. In U. pacifica the fixed finger is much larger and more club-like when compared to the manus, whereas in U. ollerenshawi the hand and fixed finger are nearly equal in width. Uncina ollerenshawi also differs from U. alpina in this way. The pleonal pleura of U. ollerenshawi are rectangular and more pointed on their distal corners while specimens of U. posidoniae have rounded pleurae. Uncina pacifica has much more pointed corners than those of the pleurae of U. ollerenshawi. In addition, all pleurae of U. ollerenshawi are nearly equal in size, which differs strongly from U. pacifica and U. posidoniae.
Uncina pacifica (Schweigert, Garassino, Hall, Hauff, and Karasawa, 2003)
Figure 4
2002 Uncina sp., Karasawa, p. 13, pl. 2, fig. 1.
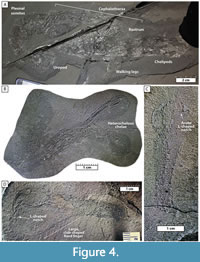 2003 Uncina pacifica Schweigert, Garassino, Hall, Hauff, and Karasawa, p. 12, figs. 4, 5b, pl. 12, figs. 1-4.
2003 Uncina pacifica Schweigert, Garassino, Hall, Hauff, and Karasawa, p. 12, figs. 4, 5b, pl. 12, figs. 1-4.
2010 Uncina pacifica Schweigert, Garassino, Hall, Hauff, and Karasawa; Schweitzer et al., p. 27.
2017 Uncina pacifica Schweigert, Garassino, Hall, Hauff, and Karasawa; Martindale et al., p. 257, fig. 3.
2019 Uncina pacifica Schweigert, Garassino, Hall, Hauff, and Karasawa; Muscente et al., p. 519, fig. 3N [unnamed] and 3O.
Holotype. TMP 2002.043.0005.
Studied material. TMP 2002.043.0002 to TMP 2002.043.0008, TMP 2002.043.0012, TMP 2002.043.0015, TMP 2014.021.0002 (note: specimen split across two samples, TMP 2013.036.0004 and TMP 2014.021.0002, hereafter specimen is referred to as TMP 2014.021.0002), TMP 2014.021.0003, TMP 2014.021.0005, TMP 2014.021.0007, TMP 2014.021.0008, TMP 2015.050.0039, TMP 2016.027.0003, TMP 2017.015.0001, TMP 2017.015.0003, TMP 2017.015.0005, and TMP 2018.024.0004 (Table 1).
Type locality. Tributary NE of Bighorn Creek (see Hall et al. 1998, fig. 2.9), Alberta, Canada; RTMP locality L2428 (East Tributary of Bighorn Creek).
Type horizon. Fernie Formation, Red Deer Member (Upper Pliensbachian, Margaritatus Zone).
Occurrence. Toarcian of SW Japan (Locality: Nishiyamanaka, Kikukawacho, Toyouragun, Yamaguchi Prefecture) and Pliensbachian to Toarcian of East Tributary of Bighorn Creek, Alberta, Canada (RTMP locality L2428).
Diagnosis. Heterochelous species of Uncina; postcervical groove arcuate, carapace of cephalothorax rounded at posterior margins, pleonal pleura rounded at proximal margins and acutely pointed at distal margins; overall shape of chelae of first pereiopod similar in both claws, but fixed finger height and notch height are greater in major claw; chelae of first pereiopod widen distally; manus almost double the length of the dactylus; diagonal row of nodes extends from the manus attachment to the dactylus attachment; strong L-shaped (right angle) notch in the distal margin of the fixed finger; dactylus narrows distally; upper and lower margins covered with forwardly-directed spines.
Remarks. The diagnosis of Schweigert et al. (2003) can be expanded. Our study of 18 new specimens of Uncina pacifica, including two mostly complete corpses and multiple specimens with both first chelipeds of a single individual intact, provides a more complete characterization of the species. Specimen TMP 2017.015.001 (Figure 4B) clearly shows the two different sizes of the claws of the first pereiopods, with the key areas that differ being the L-shaped notch and the height of the fixed finger. This specimen shows the two different shapes of the chelae of the first pereiopod. Uncina pacifica specimens also greatly differ in size and represent some of the smallest uncinid fossils known, suggesting that the initial “large” descriptor should be abandoned.
RESULTS OF MORPHOMETRIC ANALYSES OF UNCINA SPECIES CHELAE
 With the addition of multiple new articulated specimens of Uncina (Table 1), several with both first chelipeds from a single individual preserved, morphometric analyses of these elements were undertaken. Cheliped morphometric measurements were used to determine the variability between left and right claws as well as intraspecific and interspecific variability of shape (e.g., Figure 5). Plots of chosen morphometric ratios are displayed in Figure 6. To statistically compare the ratios from Figure 6 and Table 2, Wilcoxon Ranked-Sum tests were employed to distinguish significant variation between characters among the Uncina species (Table 3, Table 4, Table 5, Table 6, Table 7). It should be noted that there were often a low number of measurements for U. alpina, so statistical analyses were not always conclusive.
With the addition of multiple new articulated specimens of Uncina (Table 1), several with both first chelipeds from a single individual preserved, morphometric analyses of these elements were undertaken. Cheliped morphometric measurements were used to determine the variability between left and right claws as well as intraspecific and interspecific variability of shape (e.g., Figure 5). Plots of chosen morphometric ratios are displayed in Figure 6. To statistically compare the ratios from Figure 6 and Table 2, Wilcoxon Ranked-Sum tests were employed to distinguish significant variation between characters among the Uncina species (Table 3, Table 4, Table 5, Table 6, Table 7). It should be noted that there were often a low number of measurements for U. alpina, so statistical analyses were not always conclusive.
In the Wilcoxon Ranked-Sum tests, Uncina pacifica and U. alpina tested as statistically different only in the dactylus thickness to index height ratio (p-value = 0.006216, Table 3). The claws are morphologically very similar, except in breadth of the distal end of the claw (Figure 6); the two species are not statistically different in the ratio of the length of the fingers to the length of the manus, the notch shape, index shape, or the maximum to minimum manus heights. Uncina alpina, however, is underrepresented by measurable specimens, so results may change with the introduction of new specimens for study.
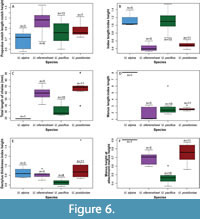 Uncina ollerenshawi and U. pacifica are statistically different in all areas except the manus length to index length (p-value = 0.3097, Table 6, Figure 6) and notch length to notch height ratios (p-value = 0.1274, Table 7, Figure 6). These ratios appear to occur consistently among all species in the genus Uncina. These two species are differentiated by their respective manus height minimum to maximum measurements (Table 5) and the index length to index height ratios (Table 4).
Uncina ollerenshawi and U. pacifica are statistically different in all areas except the manus length to index length (p-value = 0.3097, Table 6, Figure 6) and notch length to notch height ratios (p-value = 0.1274, Table 7, Figure 6). These ratios appear to occur consistently among all species in the genus Uncina. These two species are differentiated by their respective manus height minimum to maximum measurements (Table 5) and the index length to index height ratios (Table 4).
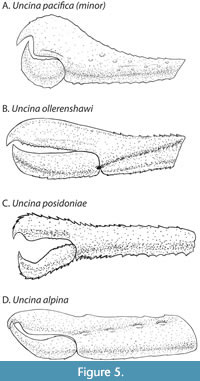
Uncina posidoniae and U. ollerenshawi exhibit no statistical differences in any of the Wilcoxon Ranked-Sum tests performed on measured variables; all morphological measurements are within the same range (Figure 6, Table 3, Table 4, Table 5, Table 6, Table 7). The claws are not statistically different in height, ratio of the length of the fingers to the length of the manus, ratio of the manus height minimum to the maximum, or the index shape. The chelae can be differentiated by the amount of ornamentation present on the outer layer of the cuticle, the shape of the dactylus, and the length of the fixed finger and dactylus when compared to that of the rest of the cheliped (Figure 5B-5C).
Uncina pacifica and U. posidoniae are statistically different in all areas except the manus length to the index length (p-value = 0.4262, Table 6) and notch length to notch height ratios (p-value = 0.588, Table 7). These ratios appear to not be statistically different when comparing all species in the genus Uncina against each other. These two species can also be differentiated by their manus height minimum to maximum measurements (p-value = 0.00001134, Table 5) and the index length to index height ratio (p-value = 0.0000008012, Table 4).
In the Wilcoxon Ranked-Sum tests, Uncina posidoniae and U. alpina were only statistically different in the index shape ratio (p-value = 0.0004579, Table 4). The claws are visually very different, but the ratios used in these tests are not statistically different. Likewise, U. ollerenshawi and U. alpina are not statistically different in any areas, even though the shapes are visually quite distinct. Herein, this is attributed to the paucity of Uncina alpina specimens available for measurements; this result may change with the discovery and inclusion of more specimens.
DISCUSSION
The Spatiotemporal Range of Uncina Species
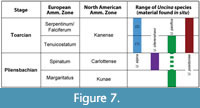 The Early Jurassic Uncina species are relatively short-lived since they are only known to occur in the late Pliensbachian and early Toarcian stages of the Early Jurassic (Figure 7). The Canadian species U. ollerenshawi has only been recovered from upper Pliensbachian strata (Spinatum Zone) or float, so it may persist to the early Toarcian, but as of now, we can only confirm that it existed during the late Pliensbachian, which is consistent with Feldmann and Copeland (1988) and Schweigert et al. (2003). Uncina pacifica specimens have been recovered from both upper Pliensbachian (Margaritatus zone) and lower Toarcian (Tenuicostatum zone) strata as well as the Toarcian of Japan (not attributed to a zone) so this lobster species may have been longer lived than the others (Figure 7). Although partial crustacean specimens have been found after the Toarcian Oceanic Anoxic Event at Ya Ha Tinda, they are not identifiable as uncinids (Martindale et al., 2017). In contrast, the European species are only recovered from lower Toarcian strata, with U. posidoniae found in the Tenuicostatum and Falciferum zones and U. alpina found in either the Tenuicostatum or Falciferum zone. Specimens from Japan are from the Toarcian Period, but no other age information has been published. All species, including those found in Europe, seem to disappear after the Toarcian Oceanic Anoxic Event (Falciferum zone) based on the specimens currently known (Hauff and Hauff, 1981; Schweigert et al., 2003).
The Early Jurassic Uncina species are relatively short-lived since they are only known to occur in the late Pliensbachian and early Toarcian stages of the Early Jurassic (Figure 7). The Canadian species U. ollerenshawi has only been recovered from upper Pliensbachian strata (Spinatum Zone) or float, so it may persist to the early Toarcian, but as of now, we can only confirm that it existed during the late Pliensbachian, which is consistent with Feldmann and Copeland (1988) and Schweigert et al. (2003). Uncina pacifica specimens have been recovered from both upper Pliensbachian (Margaritatus zone) and lower Toarcian (Tenuicostatum zone) strata as well as the Toarcian of Japan (not attributed to a zone) so this lobster species may have been longer lived than the others (Figure 7). Although partial crustacean specimens have been found after the Toarcian Oceanic Anoxic Event at Ya Ha Tinda, they are not identifiable as uncinids (Martindale et al., 2017). In contrast, the European species are only recovered from lower Toarcian strata, with U. posidoniae found in the Tenuicostatum and Falciferum zones and U. alpina found in either the Tenuicostatum or Falciferum zone. Specimens from Japan are from the Toarcian Period, but no other age information has been published. All species, including those found in Europe, seem to disappear after the Toarcian Oceanic Anoxic Event (Falciferum zone) based on the specimens currently known (Hauff and Hauff, 1981; Schweigert et al., 2003).
It is also interesting to note the geographic ranges of the Early Jurassic Uncina species. As Schweigert et al. (2003) previously noted, of the four Uncina taxa, two are exclusively from the Panthalassa Ocean (U. ollerenshawi and U. pacifica), whereas two are exclusively from the Tethys Ocean (U. posidoniae and U. alpina). Moreover, U. alpina and U. posidoniae are recovered from different regions of the Tethys and, to our knowledge, have not been recovered from the same formations. To date, the only uncinids that occur within the same localities (and at the same horizons) are U. ollerenshawi and U. pacifica (e.g., TMP 2002.043.0003, Appendix 4).
Morphology of Uncina Species
Through the statistical analysis of the morphologies of the chelae of the first pereiopod (Figure 5), a new genus-level trait emerged. In all cases, the notch ratio (Table 7) and the ratio between the manus length and length of the fixed finger (Table 6; Appendix 2) are not statistically different between Uncina species. Our data confirms that, at the genus level, uncinids share a similar ratio between the length of the fingers to the length of the manus as well as overall notch shape ratio, which is one of the defining traits of the genus.
Uncina alpina and U. pacifica (Figure 4) appear very similar (Figure 5) but through statistical analysis, an important distinction arose: the dactylus thickness to index height ratios are different (Table 5). Although the dataset for U. alpina is small, Uncina pacifica and U. alpina share a right-angled notch (sometimes more curved, notch shape varies per specimen), which is a trait unique to these two species.
Uncina ollerenshawi and U. posidoniae are similar visually (Figure 5) so it was not surprising that their morphological measurements were often not statistically different. Nevertheless, they are distinguishable by the shape of the dactylus, which was not considered in the Wilcoxon Ranked-Sum tests. Uncina posidoniae has a very strongly hooked dactylus shape with the spine facing the notch in normal position. The spine of the dactylus of U. ollerenshawi faces the notch and is very lightly hooked in comparison to the other Uncina species (Figure 5).
Uncina pacifica and U. ollerenshawi are distinctive, which is reflected in the morphological results (Table 3, Table 4, Table 5, Table 6, Table 7, Figure 5, Figure 6). This applies to U. pacifica and U. posidoniae as well since U. posidoniae and U. ollerenshawi are so similar. The most significant morphological differences are the manus height (maximum to minimum) ratio (Table 5) and index length to index height ratio (Table 4).
Uncina alpina differs from U. ollerenshawi and U. posidoniae in much the same way. Uncina alpina is significantly similar to both taxa with the exception of the index ratio, and notch ration in Uncina alpina and U. ollerenshawi (Table 3, Table 4, Table 5, Table 6, Table 7). Uncina alpina has very little gradient between the maximum and minimum height (Table 5), an almost square-shaped index and a smaller fixed finger than hand (Figure 5). The overall shape of the claw is very different from U. ollerenshawi and U. posidoniae in several areas (Figure 5); Uncina alpina has an L-shaped notch between the hand and fixed finger (although some specimens do exhibit a more curved V), a unique trait to this species but not obvious in every specimen. Uncina alpina also has a comma-shaped (i.e., “,”) dactylus like U. pacifica (see Figure 5). There is also a limited dataset for U. alpina, so the statistical tests based on these measurements should be viewed cautiously.
Ecology of Uncina Species
As the claws of the first pereiopods of lobsters are their primary food-processing and food-capturing devices, they provide a picture of the life of the animal (Schweitzer and Feldman, 2010). Broadly, Uncina alpina, U. ollerenshawi, and U. posidoniae have similarly shaped claws, with the main difference between each of them being the shape of the dactylus. The dactyli of U. pacifica and U. alpina are both more comma-shaped while the dactyli of U. ollerenshawi and U. posidoniae are much less curved with an overall even width until the tip. Uncina ollerenshawi, U. posidoniae, and U. alpina exhibits isochelous chelae of the first pereiopod and so each hand likely had a similar crushing power as well, a trait directly correlated to claw morphology (Alexander, 1968). Therefore, it is likely that U. ollerenshawi, U. posidoniae, and possibly also U. alpina, occupied similar niches and fed on similar foods. Uncina pacifica is unique in that it is the only Uncina species that exhibits heterochelous chelae of the first pereiopod, a common trait in extant lobsters (Figure 4).
Compared to the other species of uncinids, Uncina pacifica has a broad and club-like claw shape with an exaggerated index height. This height is exaggerated further in one of the chelae of the first pereiopods of the animal (Figure 5). We posit that U. pacifica had a different lifestyle than the other lobsters of this genus based on this unique trait, possibly with one claw having a greater crushing power than the other (Alexander, 1968). The chelae of U. pacifica, while heterochelous, do not differ enough for them to be considered distinct crusher and cutter (Figure 5); instead, they are referred to as major and minor. This adaptation is strongly associated with predation but also can arise by other means such as sexual selection (Schweitzer and Feldmann, 2010).
The diet of extant lobsters can be analyzed to give some clarity into the possible diets of the extinct Uncina species. The Mesozoic adaptations of the chelae of the first pereiopods in nephropid lobsters give them access to a varied diet; they are predominantly durophagous but can also consume other organisms, such as fish and other crustaceans (Schweitzer and Feldmann, 2010). Bivalves are one of the most abundant animals at Ya Ha Tinda, although the fossil record is inevitably skewed towards hard-bodied animals (Muscente et al., 2019). The abundance of bivalves at Ya Ha Tinda indicates that they were a common benthic organism during this time interval and thus were a likely food source for uncinids. That said, lobsters have a varied diet and so without physical evidence of predation from this locality, it is impossible to be certain upon what organisms they were preying.
Although U. alpina and U. posidoniae occur in the same time interval (the early Toarcian; Schweigert et al., 2003), they are found in different formations and countries and so are unlikely to have lived together in the same habitat. In contrast, U. pacifica and U. ollerenshawi occur within the same Pliensbachian-aged member of the Fernie Formation at Ya Ha Tinda and in some cases, are even found on the same bedding plane. TMP 2002.043.0003 (Appendix 4) contains 3 chelae: both chelae of a single specimen of U. pacifica and a specimen of U. ollerenshawi together suggesting that these two species likely lived in the same (or at least a similar habitat) during the late Pliensbachian. This observation does not confirm that the species interacted frequently, if at all, as the carcasses may have accumulated by other means, such as winnowing, or condensation of the section. TMP 2002.043.0003 sample supports the notion that Uncina pacifica and U. ollerenshawi were occupying different niches in the late Pliensbachian, as crustaceans of the same genus rarely occupy the same niche in the same area as they are very aggressive and competitive (Huber et al., 1997). This difference in niche may have allowed U. pacifica to survive into the Toarcian whereas U. ollerenshawi may have gone extinct at the stage boundary.
CONCLUSIONS
Through the morphometric reanalysis of specimens of Uncina species and with the addition of numerous new specimens, we confirm the presence of a diaeresis on the uropods but also observe that this genus is more morphologically variable than previously described in the following ways: a) U. pacifica is heterochelous, and b) chelae shape varies more than previously noted, both within and between species, U. ollerenshawi exhibits proportionally larger fingers when compared with other members of the genus. Understanding the paleoecology of these animals is important for reconstructions of Early Jurassic benthic ecosystems and how these communities were influenced by biotic crises, such as the Toarcian Oceanic Anoxic Event. Important new findings about the evolution and natural history of Early Jurassic astacideans, especially the superfamily Enoplometopoidea, are indicated by the observation that U. ollerenshawi and U. pacifica occupied different ecological niches in the Early Jurassic. This differentiation may have altered their ability to survive the paleoenvironmental perturbations through this interval.
ACKNOWLEDGEMENTS
We dedicate this work to the late Russell Hall who collected and determined the ages of the first Uncina pacifica specimens at Ya Ha Tinda. We thank R. and J. Smith, the Ya Ha Tinda Ranch caretakers, as well as S. Hairsine, D. Petersen, B. Perry, G. Sundbo, and D. Gummer at Parks Canada for research permits and logistical support (permit YHTR-2014-16156). The following individuals are thanks for their help with field work and logistics: T. Poulton, B. Gill, S. Marroquín, T. Them, A. Gerhardt, J. Lively, E. Tulsky, J. Visser, K. Minor and B. and S. Martindale. D. Brinkman, B. Strilisky, G. Housego, R. Russell, D. Spivak, J. McCabe, and D. Tanke at the Royal Tyrrell Museum of Palaeontology are sincerely thanked for their aid with permits, logistical support, and fossil curation (permits #13-058, #14-009, #15-019, #16063, #17-048, and #18-072) and C. Simpson is acknowledged for help with statistical analyses in R Studio. This research was supported by a grant from the National Science Foundation (NSF EAR award #1660005) and an internal UT Austin Jackson School of Geosciences seed grant to RCM.
REFERENCES
Ahyong, S.T. 2006. Phylogeny of the clawed lobsters (Crustacea: Decapoda: Homarida). Zootaxa, 1109:1-14. https://doi.org/10.11646/zootaxa.1109.1.1
Alexander, R.M. 1968. Animal Mechanics. University of Washington Press, Seattle, WA.
Beurlen, K. 1928. Die Decapoden des schwabischen Jura, mit Ausnahme der aus den oberjurassischen Plattenkalken stammenden. Palaeontographica, 70:115-278.
de Saint Laurent, M. 1988. Enoplometopoidea, nouvelle superfamille de crustacés décapodes Astacidea. Comptes Rendus hebdomadaires de l'Académie des Sciences, Paris, 3 sér. 307:59-62.
Feldmann, R.M. 1989. Whitening fossils for photographic purposes, p. 342-346. In Feldmann, R.M., Chapman, R.E., and Hannibal, J.T. (eds.), Paleotechniques. The Paleontological Society Special Publication No. 4. https://doi.org/10.1017/S2475262200005323
Feldmann, R.M. and Copeland, M.J. 1988. A new species of erymid lobster from Lower Jurassic strata (Sinemurian/Pliensbachian), Fernie Formation, southwestern Alberta. Contributions to Canadian paleontology, Geological Survey of Canada, Bulletin, 379:93-101.
https://doi.org/10.4095/126974
Frebold, H. 1957. The Jurassic Fernie Group in the Canadian Rocky Mountains and Foothills. Memoir of the Geological Survey of Canada, 287:1-197. https://doi.org/10.4095/101504
Garassino, A. and Teruzzi, G. 2001. I crostacei decapodi del Toarciano (Giurassico inferiore) di Sogno (Bergamo, N Italia). Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano, 141:187-197.
Hall, R.L., Poulton, T.P., and Monger, J.W.H. 1998. Field trip Al: Calgary - Vancouver, p. 29-61. In Smith, P.L. (ed.), Field Guide for the Fifth International Symposium on the Jurassic System: Vancouver, Canada, Jurassic Subcommission of the Stratigraphic Commission of the International Union of Geological Sciences.
Hauff, B. and Hauff, R.B. 1981. Das Holzmadenbuch. Holzmaden (Self-published).
Huber, R., Smith, K., Delago, A., Isaksson, K., and Kravitz, E, A. 1997. Serotonin and aggressive motivation in crustaceans: Altering the decision to retreat. Proceedings of the National Academy of Sciences, 94(11):5939-5942. https://doi.org/10.1073/pnas.94.11.5939
Jenkyns, H.C., Sarti, M., Masetti, D., and Howarth, M.K. 1985. Ammonites and stratigraphy of Lower Jurassic black shales and pelagic limestones from the Belluno Trough, Southern Alps, Italy. Eclogae Geologicae Helvetiae, 78(2):299-311.
Karasawa, H. 2002. Fossil uncinidean and anomalan Decapoda (Crustacea) in the Kitakyushu Museum and Institute of Natural History. Bulletin of the Kitakyushu Museum of Natural History, 21:13-16.
Karasawa, H., Schweitzer, C.E., and Feldmann, R.M. 2013. Phylogeny and systematics of extant and extinct lobsters. Journal of Crustacean Biology, 33(1):78-123. https://doi.org/10.1163/1937240X-00002111
Klompmaker, A.A., Schweitzer, C.E., Feldmann, R.M., and Kowalewski, M. 2013. The influence of reefs on the rise of Mesozoic marine crustaceans. Geology, 41:1179-1182. https://doi.org/10.1130/G34768.1
Krause, P.G. 1891. Die Decapoden des norddeutschen Jura. Zeitschrift der Deutschen Geologischen Gesellschaft, 43:171-225.
Latreille, P.A. 1802-1803. Histoire naturelle, générale et particulière, des Crustacés et des Insectes, 3:1-468. F. Dufart, Paris.
Martindale, R.C. and Aberhan, M. 2017. Response of microbenthic communities to the Toarcian Oceanic Anoxic Event in northeastern Panthalassa (Ya Ha Tinda, Alberta, Canada). Palaeogeography, Palaeoclimatology, Palaeoecology, 478:103-120.
https://doi.org/10.1016/j.palaeo.2017.01.009
Martindale, R.C., Them, T.R. II, Gill, B.C., Marroquín, S.M., and Knoll, A.H. 2017. A new Early Jurassic fossil Lagerstätte from Ya Ha Tinda, Canada (~183 Ma). Geology, 45:255-258. https://doi.org/10.1130/G38808.1
Muscente, A.D., Martindale, R.C., Schiffbauer, J.D., Creighton, A.L., and Bogan, B.A. 2019. Taphonomy of the Lower Jurassic Konservat-Lagerstätte at Ya Ha Tinda (Alberta, Canada) and its Significance for exceptional fossil preservation during Oceanic Anoxic Events. Palaios, 34(11):515-541. https://doi.org/10.2110/palo.2019.050
Nakada, K. and Matsuoka, A. 2009. On the Pliensbachian/Toarcian boundary in the Lower Jurassic Toyora Group in southwest Japan. Volumina Jurassica, 7(1):47-53.
Quenstedt, F.A. 1851-1852. Handbuch der Petrefaktenkunde. Laupp, Tübingen.
R Core Team, 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.Rproject.org
Schweigert, G., Garassino, A., Hall, R.L., Hauff, R.B., and Karasawa, H. 2003. The lobster genus Uncina Quenstedt, 1851 (Crustacea: Decapoda: Astacidea: Uncinidae) from the Lower Jurassic: Stuttgarter Beiträge zur Naturkunde, Serie B: Geologie und Paläontologie, 332:1-43.
Schweitzer, C.E. and Feldmann, R.M. 2010. The Decapoda (Crustacea) as predators on Mollusca through geologic time. Palaios, 25:167-182. https://doi.org/10.2110/palo.2009.p09-054r
Schweitzer, C.E., Feldmann, R.M., Garassino, A., Karasawa, H., and Schweigert, G. 2010. Systematic list of fossil decapod crustacean species. Crustaceana Monographs, 10:1-222.
Schweitzer, C.E. and Feldmann, R.M. 2014. Lobster (Decapoda) diversity and evolutionary patterns through time. Journal of Crustacean Biology, 34:820-847. https://doi.org/10.1163/1937240X-00002288
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9(7):671-675. https://doi.org/10.1038/nmeth.2089
Sinha, S., Muscente A.D., Schiffbauer, J.D., Williams, M., Schweigert, G., and Martindale, R.C. 2021. Global controls on phosphatization of fossils during the Toarcian Oceanic Anoxic Event. Scientific Reports, 11:24087. https://doi.org/10.1038/s41598-021-03482-7
Them, T.R. II, Gill, B.C., Caruthers, A.H., Gröcke, D.R., Tulsky, E.T.T., Martindale, R.C., Poulton, T.P., and Smith, P.L. 2017. High-resolution carbon isotope records of the Toarcian Oceanic Anoxic Event (Early Jurassic) from North America and implications for the global drivers of the Toarcian carbon cycle. Earth and Planetary Science Letters, 459:118-126. https://doi.org/10.1016/j.epsl.2016.11.021
Wilcoxon, F. 1945. Individual Comparisons by Ranking Methods. Biometrics Bulletin, 1(6):80-83. https://doi.org/10.2307/3001968

