 Late Pleistocene and Holocene pikas (Mammalia, Lagomorpha) from Europe and the validity of Ochotona spelaea: New insights based on mtDNA analysis
Late Pleistocene and Holocene pikas (Mammalia, Lagomorpha) from Europe and the validity of Ochotona spelaea: New insights based on mtDNA analysis
Article number: 26.1.a3
https://doi.org/10.26879/1241
Copyright Paleontological Society, February 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 1 September 2022. Acceptance: 28 December 2022.
ABSTRACT
Pikas were among small mammals that inhabited mammoth steppes during the last glacial. The evolutionary history of ochotonids in Europe is relatively well studied, although the taxonomic status of many described forms remains ambiguous, and the majority of extant species of the genus Ochotona are poorly represented in the fossil record. The present study aims to analyse the taxonomic relationships of a sample of Late Pleistocene-Holocene pikas based on mtDNA data and to clarify the status of the species described from Europe. A phylogenetic analysis has revealed that pikas form two large clades: one includes O. pusilla and the other includes the extant Asian and North American species. The study of haplotypes has shown similar results. The analysis supports the view that in the Late Pleistocene and Holocene O. pusilla was distributed throughout Europe, and its geographic range has contracted to the east until reaching its modern limits. The analysis of samples provided evidence that O. pusilla had survived in Eastern Europe until relatively recently and disappeared only about 150 years ago. The molecular data inferred from mtDNA do not support the species status of O. spelaea, despite morphological differences possibly related to the particular ecology of the Late Pleistocene.
Emilia Rabiniak. Wrocław University of Environmental and Life Sciences, Institute of Biology, Chełmonskiego 38c, Wrocław 51-630, Poland. emiliarabiniak@gmail.com
Leonid Rekovets. Wrocław University of Environmental and Life Sciences, Institute of Biology, Chełmonskiego 38c, Wrocław 51-630, Poland. leonid.rekovets@upwr.edu.pl
John R. Stewart. Faculty of Science and Technology, Bournemouth University, Christchurch House, Talbot Campus, Poole BH12 5BB, Dorset, United Kingdom. jstewart@bournemouth.ac.uk
Love Dalén. Department for Bioinformatics and Genetics, Swedish Museum of Natural History, Svante Ahrenius väg 3, Stockholm SE-11418, Sweden, and Centre for Palaeogenetics, Svante Arrhenius väg 20C, Stockholm SE-10691, Sweden and Department of Zoology, Stockholm University, Stockholm SE-10691, Sweden. love.dalen@zoologi.su.se
Nick Barton. Institute of Archaeology, Beaumont 36, Oxford OX1 2PG, United Kingdom. nick.barton@arch.ox.ac.uk
Tomasz Strzała. Wrocław University of Environmental and Life Sciences, Department of Genetics, Kożuchowska 7, Wrocław 51-631, Poland. tomasz.strzala@upwr.edu.pl
Zoltán Barkaszi. Department of Palaeontology, National Museum of Natural History, National Academy of Sciences of Ukraine, Bohdan Khmelnytsky 15, Kyiv 01054, Ukraine. zlbarkasi@ukr.net
Oleksandr Kovalchuk. Department of Palaeontology, National Museum of Natural History, National Academy of Sciences of Ukraine, Bohdan Khmelnytsky 15, Kyiv 01054, Ukraine and Department of Palaeozoology, Faculty of Biological Sciences, University of Wrocław, Sienkiewicza 21, Wrocław 50-335, Poland. oleksandr.kovalchuk@uwr.edu.pl Corresponding author: biologiest@ukr.net
Keywords: Ochotona; ancient DNA; cytochrome b; molecular phylogenetics; taxonomic relationships
Final citation: Rabiniak, Emilia, Rekovets, Leonid, Stewart, John R., Dalén, Love, Barton, Nick, Strzała, Tomasz, Barkaszi, Zoltán, and Kovalchuk, Oleksandr. 2023. Late Pleistocene and Holocene pikas (Mammalia, Lagomorpha) from Europe and the validity of Ochotona spelaea: New insights based on mtDNA analysis. Palaeontologia Electronica, 26(1):a3. https://doi.org/10.26879/1241
palaeo-electronica.org/content/2023/3748-ancient-dna-of-european-pikas
Copyright: February 2023 Paleontological Society/Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
During the last glacial, the so-called tundra steppe or mammoth steppe was the most extensive biome on Earth (Guthrie, 2001). The animal biomass and plant productivity of this hyperzone were similar to those of the modern African savannah (Zimov et al., 2012). The vegetation was mainly represented by grasses, herbs, and willow shrubs (Guthrie, 1990; Sher et al., 2005; Zimov et al., 2012), while deer, bison, horses, and mammoths dominated among animals (Guthrie, 2001; Kahlke, 2014). The fauna of the tundra steppe also included mammal species such as woolly rhinoceros, muskox, saiga antelope, arctic fox, wolf, dhole, cave lion, cave bear, cave hyena, various rodents (e.g., lemmings, voles, and marmots), and lagomorphs (Vereshchagin and Baryshnikov, 1992). This ecosystem covered a wide area of the Northern hemisphere, but by around 12,000 years ago it had become contracted to small regions.
The mammal fauna of the tundra steppe had a non-analogue taxonomic composition (species from non-adjacent geographic zones), whose populations often showed particular morphological adaptations. The latter have at time been classified as extinct taxa that differ morphologically from their extant relatives (Rekovets, 1985, 1994, 1995). The species composing the tundra steppe fauna had a high level of specialization and unique biocoenotic relationships within the ecological communities (Rekovets and Nadachowski, 2007).
Morphological studies of fossil remains and, especially, analyses of ancient DNA allow clarification of the relationships between extinct and extant mammalian species. Such studies also help track their migration routes in Europe and Asia during the Pleistocene and Holocene (e.g., Campos et al., 2010; Baca et al., 2012; Lagerholm et al., 2014; Doan et al., 2018). Data from palaeogenetic and phylogeographic studies allow for a deeper and more accurate taxonomic identification of Pleistocene mammals, which have so far been analysed using only morphological approaches (Niedziałkowska, 2017; Rekovets and Kovalchuk, 2017; Baca et al., 2019, 2020; Kovalchuk et al., 2021; Stefaniak et al., 2021, 2022; Doan et al., 2022; Marciszak et al., 2022; Plis et al., 2022; Ratajczak-Skrzatek et al., 2022 and many others).
The evolutionary history of the family Ochotonidae in Central and Eastern Europe is well studied (Argyropulo and Pidoplichka, 1939; Gureev, 1964; Topachevsky and Skorik, 1977; Lungu, 1981; Agadjanian and Erbajeva, 1983; Rekovets, 1985; Čermák, 2004, 2007, 2010; Čermák and Rekovets, 2010; Fostowicz-Frelik and Frelik, 2010; Čermák, 2016). The extinct taxa that occurred in these European regions from the Miocene to the Pleistocene have been identified as separate species within the genera Prolagus, Pliolagomys, Ochotonoma, and Ochotona. The taxonomic status of many of these forms is however still debated despite the fact that the morphology of their remains has been studied in detail. The genus Ochotona has been represented in the Palaearctic since the early Late Miocene (Čermák, 2016).
Ochotonids appeared in Asia between the Late Eocene and Early Oligocene and continued to evolve along with the distribution of C3 grasses in previously forest-dominated areas through the ‘climatic optimum’ from the Late Oligocene to Middle Miocene (Ge et al., 2013). They thrived in Eurasia, North America, and Africa. The peak of their diversity occurred during the period from the Early to Middle Miocene. Most pikas became extinct during the transition from the Miocene to Pliocene, which was accompanied by an increase in the diversity of leporids (Erbajeva et al., 2011). This switch between ochotonids and larger leporids was probably caused by the expansion of C4 plants due to global cooling in the Late Miocene, since extant pikas show a strong preference towards C3 plants (Ge et al., 2013). Ochotona is the sole extant genus within the family, although many fossil genera have been described (Erbajeva et al., 2011). The genus Ochotona includes 34 living species, most of which are restricted to China. Only one species — the steppe pika, Ochotona pusilla Pallas, 1768 — is today represented in the fauna of Europe (in particular, in southern Russia and northern Kazakhstan), while the American pika Ochotona princeps (Richardson, 1829) and collared pika Ochotona collaris (Nelson, 1893) occur in North America (Hoffmann and Smith, 2005).
The genus Ochotona includes a number of extinct species described from Pliocene and Pleistocene deposits of Europe and Asia. At the same time, the majority of extant species of this genus (including O. pusilla) are relatively poorly documented in the fossil record, especially for the last several thousand years (Fostowicz-Frelik and Frelik, 2010).
The systematics of the genus Ochotona based on both morphological and molecular data is therefore unstable and is subject to changes (Niu et al., 2004; Čermák, 2010; Lissovsky et al., 2013). An especially noteworthy Late Pleistocene species Ochotona spelaea was erected by Richard Owen, the famous nineteenth century British anatomist. O. spelaea, originally Lagomys spelaeus, was named on the basis of material from the Late Pleistocene deposits of Kent’s Cavern, England, and described as having different cranial and dental characters.
Systematic relationships within the genus Ochotona have been recently updated due to the inclusion of a large number of extant species into morphological analyses (Hoffmann and Smith, 2005; Erbajeva et al., 2011, Reese et al., 2013; Lissovsky, 2014) as well as the use of molecular data and up-to-date statistical methods (Yu et al., 1997, 2000; Niu et al., 2004; Lissovsky et al., 2007; Lanier and Olson, 2009). At the same time, DNA sequences of the genus Ochotona derived from fossil remains of its representatives remain to be studied.
The present study sheds light on the phylogeny of Late Pleistocene and Holocene pikas based on mtDNA data. We also assess the likely taxonomic status of the extinct European species Ochotona spelaea (Owen, 1846) that has been inconsistently identified from Late Pleistocene localities in Europe. The type material of O. spelaea from Kent’s Cavern is not available to sample and validate the species so instead we investigate coeval material from the British Isles and Belgium in addition to a single specimen from Novgorod-Siverskyi in Ukraine that was identified as O. spelaea by Rekovets (1985). The material from Britain and Belgium has been identified merely as Ochotona sp. in recognition of the uncertainties over the Late Pleistocene pika identifications in Europe and because it was not analysed morphologically.
MATERIAL AND METHODS
Sampling and Sample Preparation
Cytochrome b mitochondrial DNA sequences were obtained for 24 individuals of the genus Ochotona (Table 1), ranging in length from 250 to 510 bp. These specimens were previously assigned to O. pusilla (N=9) or O. spelaea (N=1) based on morphological characters, and Ochotona sp. (N=14) for the material that was not analysed morphologically in detail. Sequences of various Ochotona species available in the GenBank database (39 specimens, 14 species) were also included in the analysis (Table 2). The sequences of Microtus pennsylvanicus and Peromyscus boylii were used for rooting the phylogenetic tree. The comparative sequences taken from the GenBank database belong to recent individuals. Eighteen of the 24 individuals whose sequences were produced in this study date back to 550-20,000 years while the other six individuals from Kazakhstan, Russia and Kozatske in Ukraine are much younger (Table 1).
The mtDNA of all specimens of the genus Ochotona was obtained using the method described in Ersmark et al. (2015). After cleaning the fossil remains, the outer surface of each bone has been removed and a sample (10-50 mg of bone powder) was obtained with a tungsten drill. Bone powder was then placed in a 1.5 ml Eppendorf tube, to which the following reagents were successively added: 630 µl EDTA (0.5 M, pH 8), 70 µl UREA, and 15 µl proteinase K (10 µg/µl). The samples were incubated for about 24 hours in a mobile incubator at 55°C and then centrifuged at 2300 rpm for 5 min. The supernatant was then transferred to Vivaspin® columns and centrifuged at 12,000 rpm for 7-10 min (until 120-100 µl or less of fluid has left in the filter). The obtained sample mix was transferred using fine tips to a 1.5 ml tube containing 600 µl of PB buffer. The entire sample was vortexed and centrifuged. The sample/PB mix was then placed into a QIAquick column (QIAGEN) and centrifuged at 13,000 rpm for 1 min. After the residual buffer was removed, 720 µl PE buffer was added to the column and centrifuged at 13,000 rpm for 1 min, then the PB buffer was removed and centrifuged again. The column was transferred to 1.5 ml Eppendorf tubes and allowed to dry for about 5 min with the lids open. Then 50 µl of EB buffer was added directly to the filter. It was left for 1 min and centrifuged at 13,000 rpm for 1 min. Another 50 µl of EB buffer was added directly to the filter. It was left for 1 min and centrifuged at 13,000 rpm for 1 min. The DNA extract was transferred to a new 1.5 ml tube with a screw cap. The extract obtained in this way was amplified within cytochrome b with seven short overlapping fragments, approximately 500 bp long. After amplification, the DNA was purified and checked on agarose gel to verify the PCR results. The positively verified samples were sequenced in both directions on an ABI 3730XL sequencer. The study of the samples (determination, purification, milling, isolation, amplification, sequencing, and bioinformatic analysis) was carried out in the Department of Bioinformatics and Genetics, Swedish Museum of Natural History in Stockholm.
Amplification of the Cytochrome b Fragment
Ancient DNA is highly fragmented thus it is recommended to use primer pairs for a few relatively short fragments (Poinar, 2003), product length ca. 80-200 bp (Table 3). Additionally, in order to verify the results, at least three replications of the PCR and sequencing reactions were performed for each reaction. The PCR reaction was prepared in a volume of 25 µl. It was performed with 2.5 μl of PCR buffer, 0.5 μl of each primer (10 μM), 0.5 μl of dNTP (10 μM), 1 μl of MgCl (2.5 mM), 2.5 μl of BSA (1 mg/ml), 0.4 μl of Hot Star polymerase (Qiagen, Germany), distilled water, and the appropriate volume of DNA depending on the quality and concentration of the template. The mixtures prepared in this way were placed in the Eppendorf Mastercycler EP thermocycler and amplified using the following program: 95°C — 10 min, then 55 cycles [94°C — 30 sec., 50-52°C (depending on the primer) — 30 sec., 72°C — 30 sec.], 72°C — 7 min, 12°C ‘for ever’. The next step was a comprehensive bioinformatic and phylogenetic analysis of the results of sequencing of the mitochondrial genome fragments obtained from fossils together with data on extant species.
The obtained sequences were compared with the available cytochrome b sequences of selected representatives of the genus Ochotona (Yu et al., 2000; Takaki et al., 2001; Niu et al., 2004; Galbreath et al., 2009; Grigorieva et al., 2010; Kartavtseva et al., 2014; Lanier et al., 2015; Lissovsky et al., 2007, 2008; Lanier and Olson, 2009, 2013; Lissovsky, 2014; Liu et al., 2017). The sequence numbers used to compare and verify the obtained results are presented in Table 2.
Three replicates of the PCR for each specimen and each primer were aligned, and a consensus sequence was constructed on this basis. Then the sequences from both primers for the same DNA fragment were aligned, and a consensus sequence was created for a given primer pair. The final step was to align the sequences of all primers into the final consensus sequence for a given specimen. The sequences obtained in this way for all individuals, combined with homologous sequences obtained from the GenBank database, were compared using the Muscle algorithm (Edgar, 2004) implemented in the Seaview program (Gouy et al., 2010).
After alignment, the analysed sequences were truncated in order to obtain a compact block of DNA. The sets of sequences prepared in this way were used to create phylogenetic trees using the Maximum Likelihood and Bayesian Analysis methods. For analyses with the highest likelihood method, the PHYML program was used with the implemented smart model selection option for automatic selection of the substitution model (Guindon et al., 2010). The tree topology was verified with bootstrap analysis for 1000 replicates. The program MrBayes 3.2 was used to create the trees using the Bayesian method (Ronquist et al., 2012). The jModelTest 2.1.10 application was used to estimate the best-fit nucleotide substitution models used in the MrBayes program (Darriba et al., 2012). For protein coding sequences (Cytb), separate models were selected for each letter in the codon — Ochotona sp.: HKY + I + G for I, II, and III letters in the codons. During the analysis in the MrBayes program, we used the option of two independent runs each consisting of four Markov chains. The trees were sampled every hundredth generation for 20,000,000 generations (using 25% burn-in values). The analysis was completed when the standard deviation of the mean log-probability values of both robots stabilized at a level below 0.01 for all trees used to create the final tree. In many cases, standard tree-based phylogenetic analysis is unable to fully resolve close phylogenetic relationships; therefore, a haplotype network was made for individual sequences. The Median Joining method (Bandelt et al., 1999) implemented in the PopART 1.7 program (Leigh and Bryant, 2015) was used to create the network. For their implementation, the same data sets were used as in the case of trees.
RESULTS
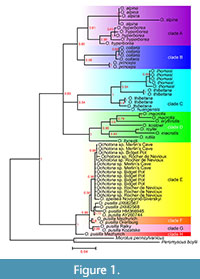 The obtained results combined with data from previous molecular analyses allowed the construction of a phylogenetic tree and haplotype network for pikas in order to study their systematic relationships. We suggest that the position of Ochotona individuals from the Pleistocene of Europe in the phylogenetic tree based on cytochrome b analysis is reliable, considering the significance level (> 0.80) of all nodes (Figure 1).
The obtained results combined with data from previous molecular analyses allowed the construction of a phylogenetic tree and haplotype network for pikas in order to study their systematic relationships. We suggest that the position of Ochotona individuals from the Pleistocene of Europe in the phylogenetic tree based on cytochrome b analysis is reliable, considering the significance level (> 0.80) of all nodes (Figure 1).
The phylogenetic tree of the genus Ochotona (Figure 1) shows two main branches. The first branch includes the clades A+B, C, and D (probability level 0.83), which represent the Asian (except for O. pusilla) and American species. Individuals of O. alpina (Pallas, 1773) and O. hyperborea (Pallas, 1811) form clade A, while O. princeps and O. collaris from North America form clade B. Clade C includes individuals belonging to O. thomasi Argyropulo, 1948 and O. thibetana (Milne-Edwards, 1871) together with O. huangensis (Matschie, 1908), which is however separated from the two above taxa. All three species of clade C almost inhabit the same area — the central part of China. The last branch (clade D) consists of South Asian species: O. macrotis (Günther, 1875), O. erythrotis (Büchner, 1890), O. koslowi (Büchner, 1890), O. roylei Ogilby, 1839, O. rutila (Severtzov, 1873), and O. forresti Thomas, 1923.
The second main branch includes the specimens analysed in this study (i.e., O. pusilla, O. spelaea, and Ochotona sp.) together with individuals of O. pusilla taken from the GenBank (clades E, F, and G). Within this second main branch, one specimen from the Late Pleistocene of Mezhyrich, Ukraine, forms a separate clade H. Two possible explanations are that either the two fossils represent a contact zone between two clades at one time or the fossils, which have not been dated, are not precisely the same age and a population turnover took place. The clades F and G within this group are represented by specimens of Holocene age (Kozatske, Raiky, and Orenburg). The specimens of O. pusilla from the GenBank along with the remaining fossil specimens form clade E. Apart from the specimen of Ochotona spelaea from Novgorod-Siverskyi, they come from the Last Glacial of England and the Younger Dryas of Belgium. Such a close connection to O. pusilla, of the studied individuals from Ukraine, Russia, and Western Europe, suggests that they represent the same species - O. pusilla (Figure 1).
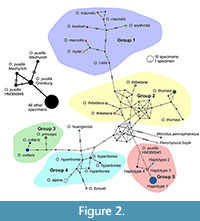 The cytochrome b sequences of the individuals analysed in this study do not differ from the respective sequence of recent O. pusilla from the GenBank. The cytochrome b sequences ranging in length from 250 to 510 bp and those from the GenBank were used to build the haplotype network of the genus Ochotona (Figure 2). The specimens of O. pusilla, O. spelaea, and Ochotona sp. created a system of four closely related haplotypes: Haplotype 1 (samples 6-7, 11-24 in Table 1), Haplotype 2 (sample 5 in Table 1), Haplotype 3 (samples 8 and 9 in Table 1), and Haplotype 4 (sample 10 in Table 1). They show a low number of substitutions compared to the modern O. pusilla haplotype, which suggests that they should be assigned to the same species (Group 5, pink). The haplotypes of O. macrotis, O. koslowi, O. erythrotis, and O. roylei together form a related group (Group 1, blue), showing little difference between particular sequences. O. rutila is also associated with the mentioned group to a greater extent than the other species. It is the node connecting this group with the rest. The haplotypes of O. thomasi and O. thibetana (both occurring in the central part of China) show a small number of substitutions (Group 2, yellow). The other haplotypes of the analysed species form two interrelated groups. Group 3 (green) represents the American species O. collaris and O. princeps, related to each other. The species O. alpina and O. hyperborea form Group 4 (azure). O. huangensis (Southeast Asia) and O. forresti (Southwest Asia) have more point changes and are therefore distant from the rest of the species. However, unlike the phylogenetic tree, in the haplotype network O. huangensis is related to O. princeps and O. hyperborea. In the tree, this species is associated with the O. thibetana/thomasi group. On the other hand, O. forresti in the tree is associated with the O. macrotis/erythrotis/koslowi/roylei/rutila group, while in the haplotype network it is associated with the O. alpina/hyperborea group (Figure 2). Nevertheless, the results of the analysis of the phylogenetic tree and the haplotype network are generally very similar.
The cytochrome b sequences of the individuals analysed in this study do not differ from the respective sequence of recent O. pusilla from the GenBank. The cytochrome b sequences ranging in length from 250 to 510 bp and those from the GenBank were used to build the haplotype network of the genus Ochotona (Figure 2). The specimens of O. pusilla, O. spelaea, and Ochotona sp. created a system of four closely related haplotypes: Haplotype 1 (samples 6-7, 11-24 in Table 1), Haplotype 2 (sample 5 in Table 1), Haplotype 3 (samples 8 and 9 in Table 1), and Haplotype 4 (sample 10 in Table 1). They show a low number of substitutions compared to the modern O. pusilla haplotype, which suggests that they should be assigned to the same species (Group 5, pink). The haplotypes of O. macrotis, O. koslowi, O. erythrotis, and O. roylei together form a related group (Group 1, blue), showing little difference between particular sequences. O. rutila is also associated with the mentioned group to a greater extent than the other species. It is the node connecting this group with the rest. The haplotypes of O. thomasi and O. thibetana (both occurring in the central part of China) show a small number of substitutions (Group 2, yellow). The other haplotypes of the analysed species form two interrelated groups. Group 3 (green) represents the American species O. collaris and O. princeps, related to each other. The species O. alpina and O. hyperborea form Group 4 (azure). O. huangensis (Southeast Asia) and O. forresti (Southwest Asia) have more point changes and are therefore distant from the rest of the species. However, unlike the phylogenetic tree, in the haplotype network O. huangensis is related to O. princeps and O. hyperborea. In the tree, this species is associated with the O. thibetana/thomasi group. On the other hand, O. forresti in the tree is associated with the O. macrotis/erythrotis/koslowi/roylei/rutila group, while in the haplotype network it is associated with the O. alpina/hyperborea group (Figure 2). Nevertheless, the results of the analysis of the phylogenetic tree and the haplotype network are generally very similar.
DISCUSSION
Pikas have high intraspecific morphological diversity and low interspecies diversity (Lanier and Olson, 2009). This makes it difficult to use morphological data to understand the evolutionary history of the different species. The rate of change in cytochrome b sequences within and between taxa (Irwin et al., 1991) allows its use in evolutionary studies of Ochotona (Yu et al., 2000). The topology of the phylogenetic tree, based on cytochrome b sequences, for individuals in the genus Ochotona, is similar to that in the most recent systematic analysis (Lissovsky, 2014). The division of the phylogenetic tree into two main branches — Asian and American species (A-D) and Ochotona pusilla (E-H) respectively — is in line with previous results. The position of O. pusilla in the phylogenetic tree in Lissovsky (2014), where it is classified as basal for the genus, is identical to that obtained in our study. The relationship between O. alpina and O. hyperborea within clade A is confirmed in the systematics of the genus Ochotona (Lissovsky et al., 2007; Lissovsky, 2014). These species are similar to the North American O. collaris and O. princeps, which form clade B (Lissovsky et al., 2007) previously described as the Nearctic subgroup (Yu et al., 2000; Niu et al., 2004). These relationships are consistent with the geographic range of these groups, covering the vicinity of the Bering Strait, which was a dispersal route between North America and Asia at times of low sea level.
The first representatives of the O. pusilla group, which were widely distributed in Europe, appeared in the Palearctic at the Plio-Pleistocene boundary (Erbajeva et al., 2001). The steppe pika is regarded a relict species of the Late Pliocene faunas of Eurasia based on its fossil record, molar structure, karyological traits, and mtDNA sequence data (Németh et al., 2017). Representatives of this group were characterised by a number of plesiomorphic traits, in particular their smaller body size and the large distance between the anteroconid and posteroconid of the p3 (Erbajeva et al., 2011). At present, the steppe pika is distributed in Central Asia, east of the Urals throughout southern Russia and northern Kazakhstan. During the Pleistocene, however, its range was broader and included much of Europe (Laplana et al., 2015), and later contracted to the east (Erbajeva, 2008). Ochotona pusilla survived in the Carpathian region until 5,000-4,000 years BP (Németh et al., 2017), in the central Urals until the Middle Holocene, and in the southern Urals until the Late Holocene (Kosintsev and Bachura, 2014).
Pidoplichka (1934) found 550-year-old remains of O. pusilla in Raiky, Ukraine, as well as specimens dated to the nineteeth century in Kozatske, Ukraine. He suggested that the species had existed in Europe until that time. However, on the map showing the species’ range (Pidoplichka, 1934: fig. 2), he marked individuals from Central and Western Europe as extinct O. pusilla. The age of the steppe pika remains from the localities of Raiky and Kozatske is estimated as Late Holocene (Pidoplichka, 1934). The suggested presence of O. pusilla in Ukraine can be considered correct due to the analysis of Holocene remains presented here. According to Pidoplichka (1934) and Zagorodniuk (2016), O. pusilla disappeared in Ukraine about 100-150 years ago, and the geographic range of the species continued to decrease eastward.
Rekovets (1985) had identified Ochotona remains from the Late Pleistocene of the Crimea, Kostenki-on-Don, and other Pleistocene sites as O. spelaea, which he considered an index species of periglacial faunas of Europe (together with Lepus tanaiticus, Spermophilus superciliosus, and S. severskensis). Ochotona spelaea was described as morphologically similar to O. pusilla (except for having broader frontals, more developed zygomatic arch, and simpler p3) and thought to be phylogenetically closely related (Rekovets, 1985). According to Erbajeva (1988) and Averianov (2001), small pikas from the Middle and Late Pleistocene of Europe (except for those from the Crimea) should be assigned to the subspecies Ochotona pusilla spelaea. This would signify that the species has both a large geographic and temporal range and includes a number of localities of Middle Pleistocene age, such as Varbezhnitsa in Bulgaria, Belvédére in the Netherlands, La Fage in France (Chaline, 1975; Kolfschoten, 1985; Popov, 1988). Those dated to the Late Pleistocene include: Niksloch in Austria, Gornja Bijambarska in Bosnia and Herzegovina, Veternica and Pecine u Brini in Croatia, Pillisszántó and Subalyuk in Hungary, Starye Duruitory and Brynzeny-1 in Moldova, Zalasie in Poland, Smolucka Cave in Serbia, Novgorod-Siverskyi and Mezhyrich in Ukraine (Kormos, 1916; Mottl, 1938; Lozan, 1970; Malez, 1963, 1966, 1968, 1979; Bocheński et al., 1985; Dimitrijević, 1988; Rekovets and Topachevsky, 1988; Fladerer, 1992). Other researchers such as Rekovets (1985) believed that O. spelaea was a separate species that became extinct in Europe ca. 10,000 years ago.
The mitochondrial DNA sequences of the 24 Ochotona samples originating from the Pleistocene and Holocene of Europe and analysed in this study based on cytochrome b showed their considerable similarity with that of O. pusilla. The clade formed by the analysed specimens together with representatives of O. pusilla is clearly distinguished from all the other species used in the comparison. We can therefore suggest that O. pusilla was the only pika species that inhabited Europe during the Pleistocene. As a consequence, the specimens previously assigned to O. spelaea most probably belong to O. pusilla. The different morphology of the Late Pleistocene material may be a distinct set of ecomorphological traits that have no taxonomic significance. Other vertebrates have been shown to have similar morphologically distinct populations in Late Pleistocene that have no taxonomic significance and may relate to the rich ecology of the tundra steppe (e.g., Lister et al., 1987; Stewart, 1999; Meiri et al., 2013; Lagerholm et al., 2017). The possibility that they represent a separate subspecies based on morphological data (Erbajeva, 1988; Averianov, 2001) cannot be sustained by the phylogenetic analysis as they are too closely related to modern individuals of O. pusilla. Ochotona spelaea is therefore a chronospecies that has been named for the Late Pleistocene material of Europe.
It seems therefore that during the Pleistocene, O. pusilla had a wide intraspecific variability and its representatives dispersed over the vast steppe areas of Europe and Asia (Erbajeva et al., 2011). Climatic changes, the development of forests in the post-glacial era, and the disappearance of grasslands during the Holocene have significantly limited the range of this species (Shubin, 1963; Erbajeva et al., 2011). Human economic activity (in particular, the plowing of virgin lands) is also considered one of the factors that led to the destruction of natural habitats of O. pusilla and contributed to disappearance of this species in most of Eastern Europe (Sokur, 1961; Lukyanov, 2001). The results of the mtDNA analysis have also confirmed that the steppe pika occurred in the territory of modern Ukraine about 150 years ago (Pidoplichka, 1934; Pidoplichko, 1973; Zagorodniuk, 2016), although the current range of the species is mainly restricted to the steppes of Central Asia (Erbajeva et al., 2001). It is hypothesized that the steppe pika could disappear from the territory of Ukraine due to zoonoses of pestis in combination with other factors (Zagorodniuk, 2016), although such an assumption needs to be additionally verified.
ACKNOWLEDGMENTS
The senior author (ER) is sincerely thankful to A. Lissovsky (Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow) and N. Abramson (Zoological Institute, Russian Academy of Sciences, Saint Petersburg) for providing recent specimens of Ochotona pusilla that were used in the study. A. Taylor (Archaeology and Anthropology Museum at Cambridge University, UK) is acknowledged for giving access to the samples from Bridged Pot. We thank P. Lacroix who excavated the Rocher de Nevioux for access to the material recovered from that site. We further wish to thank M. Baca and D. Popović (Centre of New Technologies, University of Warsaw, Poland) for the consultations and their kind help in our research. The authors thank C. Angelone and B. Moncunill-Solé for their critical comments to the early draft and suggestions that significantly improved the original manuscript.
REFERENCES
Agadjanian, A.K. and Erbajeva, M.A. 1983. Late Cenozoic rodents and lagomorphs of the USSR. Nauka, Moscow. (In Russian)
Argyropulo, A.I. and Pidoplichka, I.G. 1939. Representatives of Ochotonidae (Duplicidentata, Mammalia) in the Pliocene of the USSR. Doklady Akademii Nauk SSSR, 24(7):723-728. (In Russian)
Averianov, A.O. 2001. Lagomorphs (Mammalia) from the Pleistocene of Eurasia. Paleontological Journal, 35(2):191-199.
Baca, M., Stankovic, A., Stefaniak, K., Marciszak, A., Hofreiter, M., Nadachowski, A., Węgleński, P., and Mackiewicz, P. 2012. Genetic analysis of cave bear specimens from Niedźwiedzia Cave, Sudetes, Poland. Palaeontologia Electronica, 15.2.21A:1-16. https://doi.org/10.26879/301
Baca, M., Popović, D., Lemanik, A., Horáček, I., and Nadachowski, A. 2019. Highly divergent lineage of narrow-headed vole from the Late Pleistocene Europe. Scientific Reports, 9:17799. https://doi.org/10.1038/s41598-019-53937-1
Baca, M., Popović, D., Baca, K., Lemanik, A., Doan, K., Horáček, I., López-García, J.M., Bañuls-Cardona, S., Pazonyi, P., Desclaux, E., Crégut-Bonnoure, E., Berto, C., Lenardić, J.M., Miękina, B., Murelaga, X., Cuenca-Bescós, G., Krajcarz, M., Marković, Z., Petculescu, A., Wilczyński, J., Knul, M.V., Stewart, J.R., and Nadachowski, A. 2020. Diverse responses of common vole (Microtus arvalis) populations to Late Glacial and Early Holocene climate changes - Evidence from ancient DNA. Quaternary Science Reviews, 233:106239. https://doi.org/10.1016/j.quascirev.2020.106239
Bandelt, H.J., Forster, P., and Röhl, A. 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16(1):37-48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Bocheński, Z., Ginter, B., Kozlowski, J.K., Mook, W.G., Muszyński, M., Nadachowski, A., Stworzewicz, E., and Szyndlar, Z. 1985. Badania osadów schronisk podskalnych w Zalasie koło Krakowa. Folia Quaternaria, 56:3-56.
Čermák, S. 2004. A new ochotonid (Lagomorpha) from the Early Pleistocene of Slovakia. Neues Jahrbuch für Geologie und Paläontologie Monatshefte, 11:662-680. https://doi.org/10.1127/njgpm/2004/2004/662
Čermák, S. 2007. New finds of Ochotonoma csarnotana (Lagomorpha, Ochotonidae) from the Pliocene of Hungary: A new look on the species. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 246(2):247-256.
https://doi.org/10.1127/0077-7749/2007/0246-0247
Čermák, S. 2010. The Late Miocene and Pliocene Ochotoninae (Lagomorpha, Mammalia) of Europe - the present state of knowledge, p. 9-28. In Nowakowski, D. (ed.), Morphology and Systematics of Fossil Vertebrates. DN Publisher, Wrocław, Poland.
Čermák, S. 2016. The Late Miocene species Ochotona kalfense (Mammalia, Lagomorpha) of Moldova: The oldest European record of the genus in the context of the earliest Ochotoninae. Comptes Rendus Palevol, 15(8):927-940. https://doi.org/10.1016/j.crpv.2016.04.010
Čermák, S. and Rekovets, L.I. 2010. Early Pliocene ochotonids (Mammalia, Lagomorpha) from Southern Ukraine. Geodiversitas, 32(1):107-120. https://doi.org/10.5252/g2010n1a3
Chaline, J. 1975. Le liévre sifleur ou Chepushka (Ochotonidae, Lagomorpha) dans le remiplissage de l’Aven I de la Fage (Corréze). Nouvelles Archives du Muséum d'Histoire Naturelle de Lyon, 13:13-15.
Darriba, D., Taboada, G.L., Doallo, R., and Posada, D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9(8):772. https://doi.org/10.1038/nmeth.2109
Doan, K., Mackiewicz, P., Sandoval-Castellanos, E., Stefaniak, K., Ridush, B., Dalén, L., Węgleński, P., and Stankovic, A. 2018. The history of the Crimean red deer population and Cervus phylogeography in Eurasia. Zoological Journal of the Linnean Society, 183:208-225. https://doi.org/10.1093/zoolinnean/zlx065
Doan, K., Niedziałkowska, M., Stefaniak, K., Sykut, M., Jędrzejewska, B., Ratajczak-Skrzatek, U., Piotrowska, N., Ridush, B., Zachos, F.E., Popović, D., Baca, M., Mackiewicz, P., Kosintsev, P., Makowiecki, D., Charniauski, D., Boeskorov, G., Bondarev, A.A., Danila, G., Kusak, J., Rannamäe, E., Saarma, U., Arakelyan, M., Manaseryan, N., Krasnodębski, D., Titov, V., Hulva, P, Bălășescu, A., Trantalidou, K., Dimitrijević, V., Shpansky, A., Kovalchuk, O., Klementiev, A.M., Foronova, I., Malikov, D.G., Juras, A., Nikolskiy, P., Grigoriev, S.E., Cheprasov, M.Y., Novgorodov, G.P., Sorokin, A.D., Wilczyński, J., Protopopov, A.V., Lipecki, G., and Stanković, A. 2022. Phylogenetics and phylogeography of red deer mtDNA lineages during the last 50,000 years in Eurasia. Zoological Journal of the Linnean Society, 194(2):431-456. https://doi.org/10.1093/zoolinnean/zlab025
Dimitrijević, V. 1988. The first find of Ochotona pusilla (Pallas) (Lagomorpha, Mammalia) in Serbia. Geološki anali Balkanskoga poluostrva, 51:379-389.
Edgar, R.C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5):1792-1797. https://doi.org/10.1093/nar/gkh340
Erbajeva, M.A. 1988. Cenozoic Pikas (Taxonomy, Systematics, Phylogeny). Nauka, Moscow. (In Russian)
Erbajeva, M.A., Montuire, S., and Chaline, J. 2001. New ochotonids (Lagomorpha) from the Pleistocene of France. Geodiversitas, 23(3):395-409.
Erbajeva, M.A. 2008. Late Pleistocene small ochotonids of Eurasia with emphasis to the history of Ochotona pusilla, p. 38-40. In Kremparská, Z. (ed.), 6th Meeting of the European Association of Vertebrate Paleontologists, Abstracts. Spišská Nová Ves, Slovak Republic.
Erbajeva, M.A., Mead, J.I., Alexeeva, N.V., Angelone, C., and Swift, S.L. 2011. Taxonomic diversity of Late Cenozoic Asian and North American ochotonids (an overview). Palaeontologia Electronica, 14(3.42A):1-9. https://palaeo-electronica.org/2011_3/25_erbajeva/index.html
Ersmark, E., Orlando, L., Sandoval-Castellanos, E., Barnes, I., Barnett, R., Stuart, A., Lister, A., and Dalén, L. 2015. Population demography and genetic diversity in the Pleistocene cave lion. Open Quaternary, 1(4):1-14. https://doi.org/10.5334/oq.aa
Fladerer, F.A. 1992. Neue Funde von Steppenpfeifhasen (Ochotona pusilla Pallas) und Schneehasen (Lepus timidus L.) im Spätglazial der Ostalpen. Mitteilungen der Kommission für Quartärforschung, 8:189-209.
Fostowicz-Frelik, Ł. and Frelik, G.J. 2010. The earliest occurrence of the steppe pika (Ochotona pusilla) in Europe near the Pliocene/Pleistocene boundary. Naturwissenschaften, 97(3):325-329. https://doi.org/10.1007/s00114-009-0634-6
Galbreath, K.E., Hafner, D.J., and Zamudio, K.R. 2009. When cold is better: climate-driven elevation shifts yield complex patterns of diversification and demography in an alpine specialist (American pika, Ochotona princeps). Evolution, 63(11):2848-2863. https://doi.org/10.1111/j.1558-5646.2009.00803.x
Ge, D., Wen, Z., Xia, L., Zhang, Z., Erbajeva, M., Huang, C., and Yang, Q. 2013. Evolutionary history of lagomorphs in response to global environmental change. PLoS ONE, 8(4):e59668. https://doi.org/10.1371/journal.pone.0059668
Gouy, M., Guindon, S., and Gascuel, O. 2010. SeaView Version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution, 27(2):221-224. https://doi.org/10.1093/molbev/msp259
Grigorieva, T., Surin, V., and Formozov, N. 2010. Direct submission in GenBank.
Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. 2010. A new algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59(3):307-321.
https://doi.org/10.1093/sysbio/syq010
Gureev, A.A. 1964. Fauna of the USSR (Lagomorpha). Nauka, Moscow. (In Russian)
Guthrie, R.D. 1990. Frozen Fauna of the Mammoth Steppe. The University of Chicago Press, Chicago.
Guthrie, R.D. 2001. Origin and causes of the mammoth steppe: a story of cloud cover, woolly mammal tooth pits, buckles, and inside-out Beringia. Quaternary Science Reviews, 20(1-3):549-574. https://doi.org/10.1016/S0277-3791(00)00099-8
Hoffmann, R.S. and Smith, A.T. 2005. Order Lagomorpha, p. 185-211. In Wilson, D.E. and Reeder, D.M. (eds.), Mammal Species of the World. Johns Hopkins University Press, Baltimore.
Irwin, D.M., Kocher, T.D., and Wilson, A.C. 1991. Evolution of the cytochrome b gene of mammals. Journal of Molecular Evolution, 32:128-144. https://doi.org/10.1007/BF02515385
Kahlke, R.-D. 2014. The origin of Eurasian Mammoth Faunas (Mammuthus - Coelodonta Faunal Complex). Quaternary Science Reviews, 96:32-49. https://doi.org/10.1016/j.quascirev.2013.01.012
Kartavtseva, I.V., Sheremetyeva, I.N., Gus’kov, V.Y., Vakurin, A.A., Kumaksheva, E.V., and Frisman, L.V. 2014. Taxonomic status of northern pika (Ochotona hyperborea) from the Sikhote-Alin. Bulletin of the Far East Branch of the Russian Academy of Sciences, 2:79-85. (In Russian)
Kormos, T. 1916. Die Felsnische Pilisszanto Beiträge zur Geologie, Archäologie und Fauna der Postglazialzeit. Mitteilungen aus dem Jahrbuche der königlich Ungarischen Geologischen Reichsanstalt, 23(6):333-523.
Kosintsev, P.A. and Bachura, O.P. 2014. Formation of recent ranges of mammals in the Urals during the Holocene. Biology Bulletin, 41(7):629-637.
Kovalchuk, O., Rekovets, L., Tsvelykh, A., Yanenko, V., Manko, V., and Tajkova, S. 2021. Living in a time of change: Late Pleistocene/Holocene transitional vertebrate fauna of Grot Skeliastyi (Crimea, Ukraine). Historical Biology, 33(10):2074-2084.
https://doi.org/10.1080/08912963.2020.1769094
Lagerholm, V.K., Sandoval-Castellanos, E., Ehrich, D., Abramson, N.I., Nadachowski, A., Kalthoff, D.C., Germonpré, M., Angerbjörn, A., Stewart, J.R., and Dalén, L. 2014. On the origin of the Norwegian lemming. Molecular Ecology, 23(8):2060-2071. https://doi.org/10.1111/mec.12698
Lagerholm, V.K., Sandoval-Castellanos, E., Vaniscotte, A., Potapova, O.R., Tomek, T., Bochenski, Z.M., Shepherd, P., Barton, N., Van Dyck, M.-C., Miller, R., Höglund, J., Yoccoz, N.G., Dalén, L., and Stewart, J.R. 2017. Range shifts or extinction? Ancient DNA and distribution modelling reveal past and future responses to climate warming in cold-adapted birds. Global Change Biology, 23(4):1425-1435. https://doi.org/10.1111/gcb.13522
Lanier, H.C. and Olson, L. 2009. Inferring divergence times within pikas (Ochotona spp.) using mtDNA and relaxed molecular dating techniques. Molecular Phylogenetics and Evolution, 53(1):1-12. https://doi.org/10.1016/j.ympev.2009.05.035
Lanier, H.C., Gunderson, A.M., Weksler, M., Fedorov, V.B., and Olson, L.E. 2015. Comparative phylogeography highlights the double-edged sword of climate change faced by arctic- and alpine-adapted mammals. PLoS ONE, 10(3):e0118396.
https://doi.org/10.1371/journal.pone.0118396
Laplana, C., Sevilla, P., Arsuaga, J.L., Arriaza, M.C., Baquedano, E., Pérez-González, A., and López-Martínez, N. 2015. How far into Europe did pikas (Lagomorpha: Ochotonidae) go during the Pleistocene? New evidence from central Iberia. PLoS ONE, 10(11):e0140513. https://doi.org/10.1371/journal.pone.0140513
Leigh, J. and Bryant, D. 2015. PopART: full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6:1110-1116. https://doi.org/10.1111/2041-210X.12410
Lissovsky, A.A. 2014. Taxonomic revision of pikas Ochotona (Lagomorpha, Mammalia) at the species level. Mammalia, 78(2):199-216. https://doi.org/10.1515/mammalia-2012-0134
Lissovsky, A.A., Ivanova, N.V., and Borisenko, A.V. 2007. Molecular phylogenetics and taxonomy of the subgenus Pika (Ochotona, Lagomorpha). Journal of Mammalogy, 88(5):1195-1204. https://doi.org/10.1644/06-MAMM-A-363R.1
Lissovsky, A.A., Yang, Q., and Pil’nikov, A.E. 2008. Taxonomy and distribution of the pikas (Ochotona, Lagomorpha) of alpina-hyperborea group in North-East China and adjacent territories. Russian Journal of Theriology, 7(1):5-16.
Lister, A.M. 1987. Giant deer and giant red deer from Kent’s cavern, and the status of Strongyloceros spelaeus Owen. Transactions and Proceedings, Torquay Natural History Society, 19:189-198.
Liu, S., Jin, W., Liao, R., Sun, Z., Zeng, T., Fu, J., Liu, Y., Wang, X., Li, P., Tang, M., Chen, L., Dong, L., Han, M., and Gou, D. 2017. Phylogenetic study of Ochotona based on mitochondrial Cytb and morphology with a description of one new subgenus and five new species. Acta Theriologica Sinica, 37:1-43. https://doi.org/10.16829/j.slxb.201701001
Lozan, M.N. 1970. Rodents of Moldova. The history of the fauna development and ecology of recent species. Academy of Sciences of the Moldavian SSR, Chişinău. (In Russian)
Lukyanov, S.B. 2001. The range history, modern distribution and some biology features of the European small pika. PhD thesis. Penza. (In Russian)
Lungu, A.N. 1981. Hipparion fauna of middle Sarmatian of Moldavia (Insectivora, Lagomorpha and Rodentia). Shtiintsa Press, Chişinău. (In Russian)
Marciszak, A., Ivanoff, D.V., Semenov, Y.A., Talamo, S., Ridush, B., Stupak, A., Yanish, Ye., and Kovalchuk, O. 2022. The Quaternary lions of Ukraine and a trend of decreasing size in Panthera spelaea. Journal of Mammalian Evolution.
https://doi.org/10.1007/s10914-022-09635-3
Malez, M. 1963. Kvartarna fauna pecine Veternice u Medwednici. Palaeontologia Jugoslavica, 5:1-193.
Malez, M. 1966. Die Gattung Ochotona Link, 1795 (Lagomorpha Brandt, 1855) in Jugoslawien. Bulletin of Science Yougoslavia, Sect. A, 11(1-2):5-6.
Malez, M. 1968. Ochotona pusilla (Pallas) in the Upper Pleistocene of Central Bosnia. Bulletin of Science Yougoslavia, Sect. A, 13(1-2):2-3.
Malez, M. 1979. Prilog poznavanju rasprostranjenosti stepskezvizdare u gornjem pleistocenu Hrvatske. Rad Jugoslavenske Akademije Znanosti i Umjetnosti, Prirodne znanosti, 383:345-361.
Meiri, M., Lister A.M., Stewart, J.R., Straus, L.G., Obermaier, H., González Morales, M.R., Marín- Arroyo, A.B., Higham, T.F.G., and Barnes, I. 2013. Late glacial recolonisation and phylogeography of European red deer (Cervus elaphus L.). Molecular Ecology, 22(18): 4711-4722. https://doi.org/10.1111/mec.12420
Mottl, M. 1938. A lerakodasok Allatvilaga. A cserepfalui Mussolini-Bar-Lang (Subalyuk). Geologica Hungarica, Series Paleontologica, 14:205-308.
Németh, A., Bárány, A., Csorba, G., Magyari, E., Pazonyi, P., and Pálfy, J. 2017. Holocene mammal extinctions in the Carpathian Basin: a review. Mammal Review, 47(1):38-52. https://doi.org/10.1111/mam.12075
Niedziałkowska, M. 2017. Phylogeography of European moose (Alces alces) based on contemporary mtDNA data and archaeological records. Mammalian Biology, 84:35-43. https://doi.org/10.1016/j.mambio.2017.01.004
Niu, Y., Wei, F., Li, M., Liu, X., and Feng, Z. 2004. Phylogeny of pikas (Lagomorpha, Ochotona) inferred from mitochondrial cytochrome b sequences. Folia Zoologica, 53:141-155.
Owen, R. 1846. History of British fossil mammals and birds. J. Van Voorst, London.
Pidoplichka, I.G. 1934. Time of extinction of the steppe pika in the south of USSR. Priroda, 12:78-80. (In Russian)
Pidoplichko, I.G. 1973. On the time of disappearance of the steppe lemming on the Right Bank of Ukraine. Vestnik Zoologii, 5:35-41. (In Russian)
Plis, K., Niedziałkowska, M., Borowik, T., Lang, J., Heddergott, M., Tiainen, J., Bunevich, A., Šprem, N., Paule, L., Danilkin, A., Kholodova, M., Zvychaynaya, E., Kashinina, N., Pokorny, B., Flajšman, K., Paulauskas, A., Djan, M., Ristić, Z., Novák, L., Kusza, S., Miller, C., Tsaparis, D., Stoyanov, S., Shkvyria, M., Suchentrunk, F., Kutal, M., Lavadinović, V., Šnjegota, D., Krapal, A.-M., Dănilă, G., Veeroja, R., Dulko, E., and Jędrzejewska, B. 2022. Mitochondrial DNA diversity and the population genetic structure of contemporary roe deer (Capreolus capreolus) in Europe. Mammalian Biology.
https://doi.org/10.1007/s42991-022-00274-y
Poinar, H.N. 2003. The top 10 list: criteria of authenticity for DNA from ancient and forensic samples. International Congress Series, 1239:575-579.
Popov, V.V. 1988. Middle Pleistocene Small Mammals (Mammalia: Insectivora, Lagomorpha, Rodentia) from Varbeshnitsa (Bulgaria). Acta zoologica cracoviensia, 31(1-10):193-234.
Price, C.R. 2003. Late Pleistocene and Early Holocene Small Mammals in Southwest Britain. B.A.R. British Series, 347:1-105.
Ramsey, C.B., Higham, T.F.G., Owen, D.C., Pike, A.W.G., and Hedges R.E.M. 2002. Radiocarbon dates from the Oxford AMS System: archaeometry datelist 31. Archaeometry, 44:1-149.
Ratajczak-Skrzatek, U., Shpansky, A.V., Stefaniak, K., Orlińska, D., Cyrek, K., Sudoł-Procyk, M., and Kovalchuk, O. 2022. Upper Pleistocene remains of Bison priscus Bojanus, 1827 from Biśnik Cave (Middle Palaeolithic) and their significance for stratigraphy and palaeoecology. Quaternary International, 633:170-182. https://doi.org/10.1016/j.quaint.2022.01.001
Reese, A., Lanier, H., and Sargis, E. 2013. Skeletal indicators of ecological specialization in pika (Mammalia, Ochotonidae). Journal of Morphology, 274(5):585-602. https://doi.org/10.1002/jmor.20127
Rekovets, L.I. 1985. Small mammals of Desna-Dnieper late Palaeolithic. Naukova Dumka, Kyiv. (In Russian)
Rekovets, L.I. 1994. Small mammals of the Anthropogene of southern part of Eastern Europe. Naukova Dumka, Kyiv. (In Russian)
Rekovets, L.I. 1995. Periglacial micromammal faunas from the Late Pleistocene of Ukraine. Acta zoologica cracoviensia, 38(1):129-138.
Rekovets, L. and Kovalchuk, O. 2017. Phenomenon in the evolution of voles (Mammalia, Rodentia, Arvicolidae). Vestnik Zoologii, 51(2):99-110. https://doi.org/10.1515/vzoo-2017-0015
Rekovets, L.I. and Nadachowski, A. 2007. Evolution of biocoenoses in periglacial zone during Late Pleistocene in Eastern Europe. Vestnik Zoologii, 41(3):197-206. (In Russian)
Rekovets, L.I. and Topachevsky, I.V. 1988. Lagomorphs (Lagomorpha, Mammalia) from the Late Palaeolithic Fauna of Mezhirich. Paleontological Collection, 25:56-60. (In Russian)
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A., and Huelsenbeck, J.P. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61(3):539-542. https://doi.org/10.1093/sysbio/sys029
Sher, A.V., Kuzmina, S.A., Kuznetsova, T.V., and Sulerzhitsky, L.D. 2005. New insights into the Weichselian environment and climate of the East Siberian Arctic, derived from fossil insects, plants, and mammals. Quaternary Science Reviews, 24(5-6):533-569. https://doi.org/10.1016/j.quascirev.2004.09.007
Shubin, I.G. 1963. The range of the small pika and factors determining it. Zoogeography of land:365-366. (In Russian)
Sokur, I.T. 1961. Historical changes and the use of mammals in Ukraine. Academy of Sciences of the Ukrainian SSR Press, Kyiv. (In Ukrainian)
Stefaniak, K., Lipecki, G., Nadachowski, A., Semba, A., Ratajczak, U., Kotowski, A., Robličková, M., Wojtal, P., Shpansky, A.V., Malikov, D.G., Krakhmalnaya, T.V., Kovalchuk, O.M., Boeskorov, G.G., Nikolskiy, P.A., Baryshnikov, G.F., Ridush, B., Jakubowski, G., Pawłowska, K., Cyrek, K., Sudoł, M., Czyżewski, Ł., Krajcarz, M., Krajcarz, M.T. Żeromska, A., Gagat, P., and Mackiewicz, P. 2021. Diversity of muskox Ovibos moschatus Zimmerman, 1780 (Bovidae, Mammalia) in time and space based on cranial morphometry. Historical Biology, 33(1):62-77. https://doi.org/10.1080/08912963.2019.1666374
Stefaniak, K., Kovalchuk, O., Marciszak, A., Stepanchuk, V., Rekovets, L., van der Made, J., Yanenko, V., Tsvelykh, A., Ratajczak-Skrzatek, U., Kotowski, A., Gornig, W., and Barkaszi, Z. 2022. Middle Pleistocene faunal and palaeoenvironmental changes in the south of Eastern Europe: A case study of the Medzhybizh 1 locality (MIS 11, Ukraine). Quaternary International, 633:103-117. https://doi.org/10.1016/j.quaint.2021.07.013
Stewart, J.R. 1999. Intraspecific variation in modern and Quaternary European Lagopus. Smithsonian Contributions to Paleobiology, 89:159-168.
Takaki, M., Suzuki, H., and Yamada, F. 2001. Genetic Analyses of Ochotona hyperborea. Submitted to GenBank directly (unpublished).
Topachevsky, V.A. and Skorik, A.F. 1977. The first findings of big Ochotonoides complicidens Teil. de Chard. et Young (Lagomorpha, Lagomyidae) and the review of the Lagomyidae in Eastern Europe. Vestnik Zoologii, 6:45-52. (In Russian)
van Kolfschoten, T. 1985. The Middle Pleistocene (Saalian) and Late Pleistocene (Weichselian) Mammal Faunas from Maastricht-Belvédére (Southern Limburg, the Netherlands). Mededelingen Rijks Geologische Dienst, 39(1):45-74.
Vereshchagin, N.K. and Baryshnikov, G.F. 1992. The ecological structure of the “Mammoth Fauna” in Eurasia. Annales Zoologici Fennici, 28:253-259.
Yu, N., Zheng, C., and Shi, L. 1997. Variation in mitochondrial DNA and phylogeny of six species of pika (Ochotona). Journal of Mammalogy, 78:387-396.
Yu, N., Zheng, C., Zhang, Y., and Li, W. 2000. Molecular systematics of pikas (genus Ochotona) inferred from mitochondrial DNA sequences. Molecular Phylogenetics and Evolution, 16:85-95. https://doi.org/10.1006/mpev.2000.0776
Zagorodniuk, I. 2016. The “Ground Hare” in Eastern Europe: Ochotona or Allactaga ? Proceedings of the Theriological School, 14:16-33. (In Ukrainian) https://doi.org/10.15407/ptt2016.14.016
Zimov, S.A., Zimov, N.S., Tikhonov, A.N., and Chapin, F.S. 2012. Mammoth steppe: A high-productivity phenomenon. Quaternary Science Reviews, 57:26-45. https://doi.org/10.1016/j.quascirev.2012.10.005

