 Insights on the evolution of the tribe Pliomyini (Arvicolinae, Rodentia): Ancient DNA from the extinct Pliomys lenki
Insights on the evolution of the tribe Pliomyini (Arvicolinae, Rodentia): Ancient DNA from the extinct Pliomys lenki
Article number: 27.3.a47
https://doi.org/10.26879/1403
Copyright Palaeontological Association, September 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 13 May 2024. Acceptance: 9 September 2024.
ABSTRACT
The partially unresolved phylogeny of the Arvicolinae subfamily has been a recurrent topic of discussion in scientific papers. Among the species belonging to this subfamily, Dinaromys bogdanovi is one of the species with an unclear phylogenetic position. Paleontologists have traditionally compared and related its molars to the extinct Pliomys, whose geologically youngest fossil representative, Pliomys lenki, persisted until the Late Pleistocene. Although both genera have been morphologically related, there has always been discussion about when these genera should have separated. In this paper, we use ancient DNA data from Pliomys lenki to demonstrate the phylogenetic relationship between Dinaromys and Pliomys, forming the tribe Pliomyini. Additionally, we propose an evolutionary model of the Pliomyini tribe, placing the separation Dinaromys/Pliomys around 3.8 Ma (2.6-4.9 Ma).
María Pilar Alfaro-Ibáñez. Aragosaurus-IUCA, Departamento Ciencias de la Tierra, Facultad de Ciencias, Universidad de Zaragoza, C/ Pedro Cerbuna, 12, 50009 Zaragoza, Spain. (Corresponding author.) alfaromp@unizar.es
Jaime Lira-Garrido. Centre d’Anthropobiologie et de Génomique de Toulouse (CAGT), CNRS UMR 5288, Université Paul Sabatier, Toulouse 31000, France and Departamento de Medicina Animal (Área de Medicina y Cirugía Animal), Facultad de Veterinaria, Universidad de Extremadura, Cáceres, Spain. jaime.e.lira@gmail.com
Gloria Cuenca-Bescós. Aragosaurus-IUCA, Departamento Ciencias de la Tierra, Facultad de Ciencias, Universidad de Zaragoza, C/ Pedro Cerbuna, 12, 50009 Zaragoza, Spain. cuencag@unizar.es
Joan Pons. Institut Mediterrani d’Estudis Avançats, IMEDEA (UIB-CSIC), C/ Miquel Marquès 21, 07190 Esporles, Illes Balears, Spain. jpons@imedea.uib-csic.es
Pere Bover. ARAID Foundation, Avenida de Ranillas, 1-D, planta 2a, Oficina B, 50018 Zaragoza, Spain and Instituto Universitario de Investigación en Ciencias Ambientales (IUCA) - Grupo Aragosaurus, Universidad de Zaragoza, C/ Pedro Cerbuna, 12, 50009 Zaragoza, Spain. (Corresponding author.) pbover@unizar.es
Keywords: Ancient DNA; Pliomys; Arvicolinae; mitochondrial genome; phylogeny
Final citation: Alfaro-Ibáñez, María Pilar, Lira-Garrido, Jaime, Cuenca-Bescós, Gloria, Pons, Joan, and Bover, Pere. 2024. Insights on the evolution of the tribe Pliomyini (Arvicolinae, Rodentia): Ancient DNA from the extinct Pliomys lenki. Palaeontologia Electronica, 27(3):a47.
https://doi.org/10.26879/1403
palaeo-electronica.org/content/2024/5329-pliomys-lenki-ancient-dna
Copyright: September 2024 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Arvicolinae is a subfamily of Cricetidae rodents comprising up to 28 extant genera and 151 extant species, with the total number of genera and species continuously increasing through paleontological studies, application of new methods in study of recent and fossil species, and even taxonomic re-analyses (e.g., identification of cryptic species, etc.). The Arvicolinae include the voles sensu lato, such as water vole, snow vole, field vole, etc, as well as the lemmings.
The presence/absence and stratigraphical distribution of Arvicolinae species in paleontological/archaeological deposits have been widely used to analyze paleobiogeographical and paleoenvironmental inferences, and biostratigraphy and paleontological correlation in Quaternary deposits (e.g., Chaline et al., 1999; Fejfar et al., 2011; Cuenca-Bescós et al., 2016; Alfaro et al., 2023). Some examples of the use of this subfamily of rodents can be found in correlation with paleontological localities containing human fossil remains, such as Atapuerca, Orce, and Aroeira, among others, in the Iberian Peninsula, Petralona in Greece, Aragó in France, etc. (e.g., Agustí et al., 2010; Cuenca-Bescós et al., 2015; Lebreton et al., 2016; López-García et al., 2018; Piskoulis et al., 2023). The presence or absence of some Arvicolinae species is also an indicator of environmental characteristics, as is the case of species related to humid environments, such as the Iberomys lineage (Cuenca-Bescós et al., 2014), or water bodies, as in the case of the Arvicola lineage (e.g., Desclaux et al., 2000; Maul et al., 2000; Cuenca-Bescós et al., 2010a; Mahmoudi et al., 2019). Another biostratigraphic marker is Pliomys, which is absent in areas with well-developed forests (Tesakov, 2005). Some species of Arvicolinae are exceptional as fossil guides, as in the case of the Mimomys lineage, that marks the biostratigraphic limit of the Early to the Middle Pleistocene (Koenigswald and Kolfschoten, 1996; Maul et al., 2007).
The geologically oldest fossil representatives of Arvicolinae (Cricetidae, Rodentia, Mammalia) are observed in Europe and Asia, and they are most ascribed to Promimomys [Miocene-Pliocene boundary, ca. 5.3 million of years (Ma) ago] (Hordijk and de Bruijn, 2009; Martin, 2010). According to Chaline et al. (1999) Promimomys gave rise to Mimomys, the ancestor of many of the extant vole species, and the ancestor of the Arvicolines present on the North America continent as well. However, some authors considered the microtoid cricetid Pannonicola as the first representative of Arvicolinae (Middle Turolian, ca. 7.3 Ma ago) (Kretzoi, 1965; Zazhigin, 1982), that according to Fejfar et al. (2011) would give rise to the Ondatrini and Dycrostonychini species. The arvicolines are characterized by the presence of a higher crown and the reduction and disappearance of roots in a great number of species, developing in this hypsodont or hypselodont molars (or mesodont in the case of early arvicolines as Promimomys) that display alternating triangles on the occlusal view.
Within Arvicolinae, Pliomys is an extinct genus generally included in the tribe Pliomyini, in which is also included the only living species of this tribe Dinaromys bogdanovi, the Balkan vole (Kretzoi, 1969; Kryštufek and Bužan, 2008), a species currently observed in Balkan regions of countries such as Bosnia and Herzegovina, Croatia, Montenegro, North Macedonia, and Serbia (Kryštufek, 2018). This Pliomys-Dinaromys relationship is based in the description of Dinaromys bogdanovi as “a cementum-bearing type of Pliomys” due to the characters shared by both species regarding the molar morphology (Chaline et al., 1999). The derived character that makes Pliomyini molars unique is the combination of having roots and a high number of triangles, formed by the morphology of the enamel in salients and re-entrants’ angles on the occlusal view, in the lower first molar (m1), a tooth widely used in Arvicolinae taxonomy (Bartolomei et al., 1975; Terzea, 1983). However, some studies abnegate the relationship of these two genera (Robovský et al., 2008). 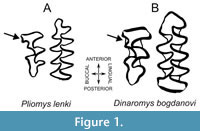 Pliomyini species also display a third upper molar (M3) with narrow incoming angles in the anterior lobe and with a ‘pliomian islet’ or ‘Pliomys structure’ in the anterior lobe (Chaline et al., 1999; Yamikova et al., 2022) (Figure 1).
Pliomyini species also display a third upper molar (M3) with narrow incoming angles in the anterior lobe and with a ‘pliomian islet’ or ‘Pliomys structure’ in the anterior lobe (Chaline et al., 1999; Yamikova et al., 2022) (Figure 1).
Pliomys includes several extinct species, and for this reason, the origin and evolution knowledge on the Pliomys species is based exclusively on the fossil record. There are more than 200 stratigraphic levels in European Quaternary containing Pliomys (e.g., Bartolomei et al., 1975; Carls and Rabeder, 1988; Dema and Rekovets, 2004; Cuenca-Bescós et al., 2008, 2010b). Whereas several species of the genus are known for the European Quaternary fossil record (e.g., Jeannet, 1974; Chaline et al., 1999; Tesakov, 2005), three species have been found in the Iberian Peninsula (western Europe) in the nearly 100 Early Pleistocene to the Late Pleistocene stratigraphic levels containing Pliomys: Pliomys simplicior, P. episcopalis, and P. lenki (=Pliomys coronensis). Pliomys simplicior was firstly recorded from the earlier Early Pleistocene Razvode site in Croatia (Jánossy, 1986), but the arrival at the Iberian Peninsula has been accurately dated in the Early Pleistocene levels of the Sima del Elefante (lower levels TELRU, TE7 to TE14, in Atapuerca, Spain, Cuenca-Bescós et al., 2016). Other Iberian Early Pleistocene sites with Pliomys are Bagur 2, Gran Dolina TD4 to TD6, Casablanca 3 and Chaparral (López et al., 1976; Agustí and Galobart, 1986; López-García et al., 2012; Cuenca-Bescós et al., 2016). Pliomys episcopalis was defined in the type site, Somlyó-berg (Kordos and Pazonyi, 2012). This is the type species of the genus Pliomys, both described by Mehely in 1914. The last species, P. lenki, became extinct at the end of the Quaternary (Marquet, 2001; Cuenca-Bescós et al., 2010b). The well-recorded extinction process of Pliomys started in eastern Europe, consequently disappearing westwards with the Iberian Peninsula as final refuge (Cuenca-Bescós et al., 2010b). In general, this genus has been considered of great relevance for the Quaternary biostratigraphy of the Iberian Peninsula since they are abundant in the deposits, and their morphology and size make them easy to classify at the genus level (Cuenca-Bescós and Morcillo-Amo, 2022).
The taxonomy of Arvicolinae is particularly complicated, especially in terms of generic attribution and identification of subgenera as genera or even in the phylogenetic relationships within species, genera, and tribes (e.g., Chaline, 1975; Chaline et al., 1999; Fejfar et al., 2011; Kryštufek and Shenbrot, 2022). Even recent studies using considerably large genomic datasets do not agree in the phylogenetic relationships for some arvicoline taxa (Bužan et al., 2008; Robovský et al., 2008; Abramson et al., 2021; Withnell and Scarpetta, 2024, among others). With the development of ancient DNA methods and high through-output sequencing platforms it is currently possible to obtain a vast amount of molecular information for even small species that can contribute to understand phylogenetic and phylogeographic aspects of zoological taxa such as some Arvicoline (e.g., Baca et al., 2022, 2023).
In this paper we obtained for the first time a partial mitochondrial genome of a Late Pleistocene Pliomys lenki individual from the El Mirón Cave deposit (Spain). These sequences allowed us to infer the phylogenetic relationships of the extinct species within the Arvicolinae.
MATERIAL AND METHODS
Study Site and Species
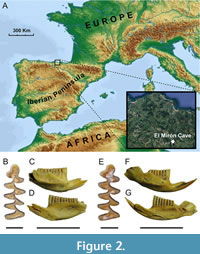 Two right mandibles identified as Pliomys lenki from the paleo-archaeological site of El Mirón Cave (Cantabria, Spain) (Figure 2A) were used for the paleogenomic analysis. The identification of the mandibles as belonging to P. lenki was based on diagnostic morphological characteristics of the molars such as larger size than those from the other vole species recorded in the Late Pleistocene of the Iberian Peninsula, presence of roots, absence of cement in the re-entrant angles, and thicker continuous enamel. The generally good preservation of the fossil remains from this site has already allowed us to obtain ancient DNA from other Arvicolinae remains (e.g., Baca et al., 2022, 2023; Alfaro-Ibáñez et al., 2023). The first mandible (laboratory number 218) displayed the complete lower molar teeth-row (molars 1 to 3, m1-m3) and one incisor, and the proximal part of the mandible was broken (Figure 2B-D). The second mandible (laboratory number 219) displayed m1, m2, and the incisor, the condylar and coronoid processes were complete but lacked most of the bottom part of the mandible, being the incisor almost fully exposed (Figure 2E-G). Mandible 218 were excavated from level 130 (Spit 27, Square w10, Subsquare B) and mandible 219 from level 129 (Spit 22, Square x10, Subsquare B), with a chronology for these levels of 50,900-39,280 and 46,890-33,160 years cal BP, respectively (Hopkins et al., 2021).
Two right mandibles identified as Pliomys lenki from the paleo-archaeological site of El Mirón Cave (Cantabria, Spain) (Figure 2A) were used for the paleogenomic analysis. The identification of the mandibles as belonging to P. lenki was based on diagnostic morphological characteristics of the molars such as larger size than those from the other vole species recorded in the Late Pleistocene of the Iberian Peninsula, presence of roots, absence of cement in the re-entrant angles, and thicker continuous enamel. The generally good preservation of the fossil remains from this site has already allowed us to obtain ancient DNA from other Arvicolinae remains (e.g., Baca et al., 2022, 2023; Alfaro-Ibáñez et al., 2023). The first mandible (laboratory number 218) displayed the complete lower molar teeth-row (molars 1 to 3, m1-m3) and one incisor, and the proximal part of the mandible was broken (Figure 2B-D). The second mandible (laboratory number 219) displayed m1, m2, and the incisor, the condylar and coronoid processes were complete but lacked most of the bottom part of the mandible, being the incisor almost fully exposed (Figure 2E-G). Mandible 218 were excavated from level 130 (Spit 27, Square w10, Subsquare B) and mandible 219 from level 129 (Spit 22, Square x10, Subsquare B), with a chronology for these levels of 50,900-39,280 and 46,890-33,160 years cal BP, respectively (Hopkins et al., 2021).
DNA Extraction, Library Construction and Sequencing
All laboratory tasks using the Pliomys lenki samples were performed in the dedicated paleogenomics laboratory of the University Institute for Research in Environmental Sciences of Aragon (IUCA) of the University of Zaragoza (Spain). Mandibles 218 and 219 were UV radiated for 15 minutes on each side. Surface of the mandibles was slightly wiped with bleach 1%, and then submerged in water for 10 minutes and subsequently in 80% ethanol for 10 min. After air drying each mandible, they were placed in 2 ml screw-cap tubes. Bone powder was mechanically obtained using sterile tweezers inside the tubes. A total amount of 113 and 116 mg, respectively, was used for the DNA extraction.
DNA from both mandibles were extracted following the silica suspended protocol of Brotherton et al. (2013) and using a modified binding buffer (Bover et al., 2019) based on Dabney et al. (2013) protocol. Shortly, an initial predigestion step using 1 ml EDTA on a rotary wheel for 1 hour at room temperature was followed by an overnight digestion/decalcification using ~1 mL of a digestion buffer (900 μL EDTA 0.5M ph8.0 and 20 μL of 20 mg/mL Proteinase K) on a rotary wheel at 55ºC. DNA extraction was performed using 13 mL of a modified binding buffer [13.6 mL PB buffer (Qiagen), 420 μL Sodium Acetate 3M and 7 μL Tween-20] and 100 μL of suspended silica particles. After three washes (first using 900 μL of remaining binding buffer and two subsequent ones using 900 μL 80% ethanol), DNA was eluted in 100 μL of TLE buffer.
Double-stranded libraries based in protocol by Meyer and Kircher (2010) with modifications described in Llamas et al. (2016) (i.e., ligation of short 7-bp P5 and P7 adapters) and using a partial uracil-DNA glycosylase treatment (Rohland et al., 2015) of sequencing libraries were constructed. Purified and quantified libraries were finally sequenced in an Illumina HiSeqX platform (2 x 150 bp).
Mitochondrial Genome Assembly and Phylogenetic Analyses
Mitochondrial genome assembly. The quality of raw sequencing reads was analysed using fastQC v0.11.2 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). We demultiplexed sequences from both samples by filtering both P5 and P7 barcodes (allowing one mismatch) using Sabre v.1.0 (http://github.com/najoshi/sabre) and adapter sequences were trimmed with AdapterRemoval v.2.3.0 (Schubert et al., 2016) using parameter values as follows: mismatch rate 0.1, minimum Phred quality 4, quality base 33, trim ambiguous bases (N), and trim bases with qualities equal to or less than the given minimum quality, and collapsing (merging) paired reads overlapping by at least 11 bp. Reads shorter than 30 bp were excluded from further analysis. At the end of the process up to 10,196,653 collapsed reads (~70%) and 19,505,962 (~81%) were retained for samples 218 and 219, respectively, for downstream analysis.
We performed a preliminary mapping of collapsed reads for both samples to evaluate the quality of the samples using BWA v.0.7.17 backtrack algorithm (Li and Durbin, 2009) and parameters widely accepted for ancient data mapping (i.e., aln -n 0.01, -o 2, -l 1024), and using the mitochondrial genome of D. bogdanovi (GenBank Accession Number MT588182) as reference. Duplicate reads were filtered using FilterUniqueSAMCons.py (Kircher, 2012). The higher number of reads mapped to the reference for sample 219A than the ones for sample 218A (542 versus 55 reads) suggested the selection for this first sample for further analyses.
We constructed two draft mitochondrial genomes of Pliomys sample 219A following a “Multi-reference Iterative Mapping Approach” (MIMA, Torres-Roig et al., 2021) using BWA. In both cases, collapsed reads were mapped to three different arvicoline mitochondrial genomes as references: Dinaromys bogdanovi (GenBank Accession Number MT588182), Eolagurus luteus (GenBank Accession Number MT492448), and Microtus thomasi (GenBank Accession Number NC_057558). We used the more stringent BWA parameters for ancient data mapping (i.e., -n 0.01, -o 2) and removing reads with minimum mapping quality (-q 25) using SAMtools v.1.11 (Li et al., 2009) to construct the first draft, but more relaxed parameters (i.e., -n 0.001 and -q 20) suggested by Westbury and Lorenzen (2022) for high phylogenetic divergence mitochondrial genomes as Pliomys and the species used as references. D. bogdanovi was used as reference due to the morphological similarities that present with P. lenki, and specifically for being the only current representative of the Pliomiyni tribe. Eolagurus luteus was selected as a reference for being a genus close to Dinaromys according to DNA studies, whereas Microtus thomasi is a species more phylogenetically distant and morphologically different from Dinaromys (Abramson et al., 2021). Duplicate reads were removed as explained above (FilterUniqueSAMCons.py). Using the two different sets of parameters, we performed iterative rounds of mapping. At the end of each round of mapping for each reference, an intermediate 75% majority consensus sequences were generated using Geneious Prime v.2022.0.2 (Biomatters, http://www.geneious.com Kearse et al., 2012), retaining the reference nucleotides for sites with read-depth < 3X. The new consensus was then used as the new reference, and the process was iterated until no more reads were mapped. Final 75% majority consensus sequences for the iterative mapping against each reference were then generated in Geneious, calling nucleotides only at sites with read-depth ≥ 3X. Number of iterations and unique reads mapped are listed in Appendix 1, but a higher number of mapped reads were obtained when using D. bogdanovi as reference (i.e., 1,173 unique reads using stringent mapping parameters, covering 58% of the reference at a mean depth of 4.5X and 1,898 unique reads using relaxed parameters, covering 84% of the reference at a mean depth of 7.3X). The consensus sequences generated from this first iterative mapping were identical for regions where they overlapped. As better results were obtained using D. bogdanovi, we aligned the three consensus sequences with the mitogenome of this species using the MUSCLE algorithm implemented in Geneious, generating a new merged reference by retaining nucleotides called from our sequence data and filling any gaps with the corresponding nucleotides from D. bogdanovi. This new merged reference was then used for another round of iterative mapping (as described above, using both sets of mapping parameters). Using stringent parameters up to six rounds of mapping were needed to map 1,678 unique reads to the reference covering 74% of it at a mean depth of 6.4X, whereas using relaxed parameters 2,175 unique reads mapped covering 93% of the reference at a mean depth of 8.5X). A final consensus for each mapping strategy was generated in Geneious using a 75% majority consensus, retaining the reference nucleotide in positions with coverage depth ≥ 3X, and obtaining ~68% of the mitochondrial genome using the stringent mapping strategy and around ~89% using the relaxed mapping strategy. Ancient DNA misincorporation and fragmentation patterns were assessed using mapDamage v.2.1.1 (Jónsson et al., 2013).
Phylogenetic analyses. The two partial mitochondrial genome sequences of the fossil of Pliomys from El Mirón Cave (stringent-STR and relaxed-REL) were annotated using the mitochondrial genome of Dinaromys bogdanovi as base and adjusting changes in Protein Coding Genes (PCGs) using Geneious. Each Pliomys reference was aligned to 112 arvicoline mitochondrial genomes from 66 species available at GenBank (see Appendix 2). For those species with multiple mitochondrial genomes, we randomly selected five of them after removing incomplete mitogenomes or, in case of just partial genomes available, discarding those with more unknown (N) nucleotides. Akodon montensis, Cricetulus griseus, and C. longicaudatus were used as outgroups. The 116 mitochondrial genomes were realigned using MUSCLE implemented in Geneious, and after removing the Control Region (CR) we divided the alignment in PCGs, tRNAs, 12S_rRNA, and 16S_rRNA and were realigned again using MUSCLE. We further divided PCGs by codon positions using DAMBE v.7.0.5 (Xia, 2017), and ambiguously aligned regions of tRNAs and rRNAs were removed using default parameters using Gblocks v.091b (Castresana, 2000) in Phylogeny.fr (Dereeper et al., 2008), retaining 852 out of 986 (86%) positions for 12S_rRNA, 1301 out of 1654 (78%) for 16S_rRNA and 1401 out of 1642 (85%) positions for tRNAs. The final length of the alignment was 14,885 bp. Partitioning scheme and nucleotide substitution models were inferred using the implemented ModelFinder (Kalyaanamoorthy et al., 2017) in IQTREE2 (Minh et al., 2020) for RAxML, MrBayes and Beast2 (see Appendix 3). A partitioned Maximum Likelihood analysis was performed using RAxML v.8.2.11 (Stamatakis, 2014), with node support values estimated by performing 100 bootstrap replicates. We also performed a partitioned MrBayes v.3.2.3 analysis (Ronquist et al., 2012), comprising four separate runs of four Markov chains each using default priors. Each chain ran for 25 million generations sampling trees and parameter values every 104 generations. Topological convergence was assessed using the average standard deviation of clade (split) frequencies (< 0.02), while convergence in individual parameter values was assessed through broadly overlapping distributions and effective sample sizes > 200 in Tracer v.1.7 (Rambaut et al., 2018). All sampled trees were summarized as a majority-rule consensus tree after discarding the first 10% of trees as burn-in.
A partitioned analysis under relaxed molecular clock was performed using BEAST v1.10.4 (Drummond et al., 2012) and one individual per species (see Appendix 2). We initially followed all calibrations and priors used by Abramson et al. (2021; see table S3 in that paper), except the Arvicolinae calibration (Offset= 5.3 Ma, 95% HPD = 5.4-10.3 Ma), as Promimomys is more generally considered the first Arvicolinae in the fossil record at 5.3 Ma (Fejfar et al., 2011). However, all except Dicrostonychii calibrations showed convergence of the posterior values. We decided to exclude the Dicrostonychii constraint in further analyses since conversion of posterior values requires multiple analyses from different prior distributions. Four analyses were run for 107 generations sampling every 104 generations, discarding the first 10% of samples as burn-in. Convergence was assessed through combined analysis of four independent runs whose parameters showed effective sample sizes > 200 as calculated using Tracer v.1.7. Individual run outputs were combined using LogCombiner v.1.8.4 and a final maximum clade credibility tree was generated using TreeAnnotator v.1.8.4 implemented in BEAST.
Path Sampling (PS)/Stepping Stone sampling (SS) approaches were used to estimate marginal likelihood (MLE) values so they can be directly compared despite the number of parameters of the models, as these methods increase model selection accuracy (Baele et al., 2012). We analyzed the performance of Yule and Birth-Death process competing models using for both scenarios default parameters (Baele et al., 2012). We analyzed 100 path steps with 106 length chains and log likelihood every 1000 per path step, with a Beta (α, 1.0) distribution power posterior. Setting α=0.3 has been considered an optimal value for estimations (Xie et al., 2011). Model fit to the data was evaluated calculating Bayes Factor (BF) as a ratio of both marginal likelihoods (Baele et al., 2012).
Some fossil species more commonly identified as being of the genus Dinaromys, were ascribed to Pliomys by Chaline et al. (1999), as is the case of Dinaromys dalmatinus and Dinaromys pasai (Kowalski, 2001). To evaluate the position of our Pliomys lenki sequences in comparison with the genetic variability of Dinaromys bogdanovi, we aligned the available mitochondrial cytochrome b (CYTB) data for D. bogdanovi, CYTB sequences for three Ellobius species (sister taxa of D. bogdanovi according to Abramson et al., 2021) and the two CYTB sequences of P. lenki (both obtained using stringent and relaxed mapping parameters) using MUSCLE. The alignment was trimmed to the length of the sequence available for most of the Dinaromys sequences (51 out of 55). The final 555-bp CYTB alignment comprised a total of 61 individuals (55 Dinaromys, one Ellobius fuscocapillus, one E. lutescens, one E. talpinus, the two P. lenki generated here, and Rattus rattus as outgroup, see Appendix 4). In the case of P. lenki, the number of nucleotides unknown for this 555-bp fragment of each CYTB gene sequence was seven for the one generated using relaxed parameters, and 78 for the sequence obtained using stringent parameters. A Maximum Likelihood analysis was performed on this dataset using RAxML with node support values estimated by performing 100 bootstrap replicates.
It is beyond the scope of this study to deeper discuss the phylogenetic relationships within Arvicolinae as well as the genus/subgenus identification for some of the species. However, for clarity, we followed the genera and subfamily nomenclature proposed by Abramson et al. (2021), except in the case of Agricola agrestis, where we follow the most widely used classification of Microtus agrestis.
RESULTS
Pliomys Mitochondrial Genome Assembly
The final Pliomys mitochondrial sequences displayed 5,248 unknown positions (32.4% of the estimated length of the mitochondrial genome) using stringent parameters, and 1,768 (11.1%) using relaxed parameters. The alignment of both Pliomys consensus sequences showed that they were identical for those mitogenome regions obtained using the two different mapping strategies. Each sequence fragment larger than 50 bp in the relaxed-generated consensus (REL) not present in the stringent-generated consensus (STR) was BLASTn (Altschul et al., 1990) analysed. The first five BLASTn hits of the 16 fragments (ranging from 61-453 bp) were assigned to arvicoline/rodent species (see Appendix 5) indicating that the BWA using relaxed parameters apparently did not introduce random bacterial or other organism sequences in the consensus sequence generation. In both cases, the ancient DNA misincorporation and fragmentation patterns (Appendix 6) display the expected damage pattern observed in partial uracil-DNA glycosylase treated libraries (Rohland et al., 2015).
The new Pliomys mitochondrial genome sequence (using relaxed BWA mapping parameters, REL) is available in GenBank under accession number PP873348. The mitochondrial genome sequence using stringent BWA mapping parameters (STR) is provided in Appendix 7. Both partial genomes display the characteristic structure of vertebrate mitochondrial genomes containing genes for 13 proteins (Protein Coding Genes, PCGs), 22 transfer RNAs (tRNA), two ribosomal rRNAs, and the two non-coding regions Control Region (CR) and L-strand origin of replication (see Appendix 8 for full detail).
Phylogenetic Analyses
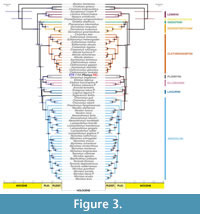 The topology of the phylogenetic trees obtained from the ML and BI analyses (Figure 3, Appendix 9, Appendix 10) widely recapitulates previously published results based on complete or near complete mitochondrial genomes (e.g., Folkertsma et al., 2018; Lamelas et al., 2020; Abramson et al., 2021). All our phylogenetic analyses unequivocally [Maximum Likelihood Bootstrap (MLB)=100, Posterior Probability (PP)=1] placed Pliomys lenki as sister taxa of Dinaromys bogdanovi, although the relationships of this clade with a clade comprising Ellobius (and Hyperacrius, depending on analyses,) is not fully supported (MLB < 75, PP < 0.95). The variable position of Hyperacrius in our phylogenetic trees agrees with that observed using both mitochondrial, nuclear, and combined data (Abramson et al., 2021; Withnell and Scarpetta, 2024).
The topology of the phylogenetic trees obtained from the ML and BI analyses (Figure 3, Appendix 9, Appendix 10) widely recapitulates previously published results based on complete or near complete mitochondrial genomes (e.g., Folkertsma et al., 2018; Lamelas et al., 2020; Abramson et al., 2021). All our phylogenetic analyses unequivocally [Maximum Likelihood Bootstrap (MLB)=100, Posterior Probability (PP)=1] placed Pliomys lenki as sister taxa of Dinaromys bogdanovi, although the relationships of this clade with a clade comprising Ellobius (and Hyperacrius, depending on analyses,) is not fully supported (MLB < 75, PP < 0.95). The variable position of Hyperacrius in our phylogenetic trees agrees with that observed using both mitochondrial, nuclear, and combined data (Abramson et al., 2021; Withnell and Scarpetta, 2024).
As obtained using a larger mitochondrial genes dataset, the phylogenetic analysis of the mitochondrial CYTB fragment of Pliomys and the available data for Dinaromys clearly places (Appendix 11) the fossil taxon as sister taxa of D. bogdanovi (MLB = 99), but clearly out of its genetic diversity (MLB = 97). The obtained supported distribution of Dinaromys lineages (Northwestern, Southwestern, and Central) according to Kryštufek et al. (2007) and the wide geographic sampling of Dinaromys individuals that these authors performed for their study unequivocally indicates that Pliomys cannot be considered as a representative of the current Dinaromys bogdanovi species.
Bayes Factor (BF) values in both Stepping Stone (SS) and Path Sampling (PS) marginal likelihood estimates (MLE) showed strong and very strong support [according to Kass and Raftery (1995) strength support values, 6 < BF < 10 and BF > 10] for Yule speciation model process, respectively (see Appendix 12). Our time-calibrated BI phylogenetic analysis (Figure 3) is also in agreement with node splits observed in calibrated analyses from Abramson et al. (2021) and Withnell and Scarpetta (2024) (Appendix 13). Our results in general are particularly similar to the node ages obtained by Abramson et al. (2021) using seven calibration ages (i.e., slightly more recent than the other analyses) and to the obtained by Withnell and Scarpetta (2024) for the corresponding nodes. The divergence time between Dinaromys and Pliomys, using both STR and REL Pliomys sequences, is inferred in around 3.8 Ma (95% HPD = 2.6-4.9 Ma approx.), roughly during the Pliocene.
Furthermore, in our analyses we observe that the Pliomyini tribe has a closer relationship with Arvicolini, Ellobiusini, and Lagurini tribes, as already observed by Abramson et al. (2021) and Withnell and Scarpetta (2024). However, these latter authors also observed a sister relationship between Pliomyini and Clethriomyini using only discrete mitochondrial data.
DISCUSSION
As mentioned, Dinaromys and Pliomys share various molar characteristics, which allows clustering them within the Pliomyini tribe of Cricetidae rodents. In general, m1 displays an increased number of triangles (T) on the occlusal surface, T5 and T6 that converge to a greater or lesser degree with the anterior complex that lacks an islet or fold. The third upper molar (M3) displays the so-called ‘Pliomys structure’, consisting of an enamel island in the anterior lobe, lacking an island in the posterior complex (Figure 1). This last structure is a synapomorphy that differentiates the Pliomyini from the other Arvicolinae genera (Kordos and Pazonyi, 2012), with also Dinaromys bogdanovi displaying the Pliomys structure in M3 and a slightly larger m1 than Pliomys. Due to these similarities, both taxa were merged in one single genus, Dolomys, considering Dinaromys and Pliomys as subgenera of it (Hinton, 1926), a view that was later rejected leaving Dinaromys and Pliomys as valid genera (Kretzoi, 1955; Bartolomei et al., 1975). Although Dinaromys was described as “a cementum-bearing type of Pliomys” by Chaline et al. (1999), and there are still discussions in the literature about the attribution of some species in Dinaromys or Pliomys (e.g., Kowalsky, 2001), our results unequivocally demonstrate that Pliomys and Dinaromys, although closely related by both morphological (Kretzoi, 1969; Chaline et al., 1999) and molecular characters (Figure 3, Appendix 9, Appendix 10), should be considered different genera (Appendix 11).
Previous studies included Dinaromys in the tribe Clethrionomyini (e.g., Mckenna and Bell, 1997), Ondatrini (Kretzoi, 1955; Corbet, 1978) or Prometheomyini (Pavlinov, 2003). On the other hand, Pliomys has been considered as the ancestral clade of Clethrionomyini (e.g., Gromov and Polyakov, 1992; Martin, 2015). Our phylogenetic analyses clearly places both Dinaromys and Pliomys within the Pliomyini tribe [as observed for D. bogdanovi by Abramson et al. (2021) and Withnell and Scarpetta (2024)] as already suggested from a morphological point of view as Clethriomyini is dissimilar in its molar morphology [smaller size, continuous enamel wall, and presence of abundant cement in the reentrant angles (Fejfar et al., 2011)].
Pliomys was initially defined by Lajos Méhely at the beginning of the twentieth century in Hungary, at the Somlyó-hegy (Somlyó-berg) site. The materials had been found by the geologist Theodor Kormos who had initially classified them as Clethrionomys, another arvicoline with tooth-roots, but distinguished from it by being smaller and the abundant cement in the enamel folds. These differences encouraged Méhely to describe a new genus (Méhely, 1914). The first appearance datum (FAD) of the genus Pliomys is assigned to the species P. simplicior in the earlier Early Pleistocene. Nevertheless, some authors considered that the first representatives of Pliomys appeared during the Late Pliocene (e.g., Tesakov, 2005; Skandalos et al., 2023). However, these species are more commonly assigned to the genus Propliomys (Yamikova et al., 2022), previously described as a subgenus of Dolomys, and there is no clear correlation between these two lineages despite displaying morphological similarities. Following Fejfar et al. (2011), the genus Pannonicola could have given rise to Dolomys and Propliomys during the end of the Mammal 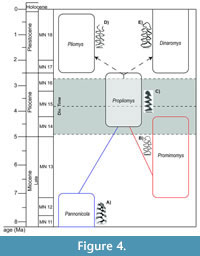 Neogene zone (MN)14 [ca. 4.2 Ma], both considered by some authors as Ondatrini (Fejfar et al., 2011). On the other hand, Yamikova et al. (2022) refer to the Propliomys as the first pliomyine rodent, thus considering it as a Pliomyini, and suggest a species with a promimomyoid morphology as the possible ancestor of the Propliomys lineage [Mimomys/Promimomys moldavicus following Tesakov (2005)]. These authors also suggest that Propliomys hungaricus (MN 15) could potentially be an ancestor of the extant genus Dinaromys. Withnell and Scarpetta (2024) proposed that the Pliomyini tribe lineage diverged around 4.2 Ma (95% HPD = 3.15-5.17 Ma), a chronology that widely agrees with the divergence time of Dinaromys - Pliomys obtained in the study presented here (3.8 Ma, 95% HPD = 2.6-4.9 Ma approx.). Despite the low number of Pliomyini species with available molecular data, this age would place the rise of this tribe during the Pliocene, probably deriving from Propliomys taking in consideration all the morphological similarities between Propliomys and Dinaromys / Pliomys that had been discussed in the scientific literature (Figure 4). In this way, our study is not in disagreement with the most recent works about the possible evolution of the tribe Pliomyini and could be another important piece of information about how the Arvicolinae evolution takes place.
Neogene zone (MN)14 [ca. 4.2 Ma], both considered by some authors as Ondatrini (Fejfar et al., 2011). On the other hand, Yamikova et al. (2022) refer to the Propliomys as the first pliomyine rodent, thus considering it as a Pliomyini, and suggest a species with a promimomyoid morphology as the possible ancestor of the Propliomys lineage [Mimomys/Promimomys moldavicus following Tesakov (2005)]. These authors also suggest that Propliomys hungaricus (MN 15) could potentially be an ancestor of the extant genus Dinaromys. Withnell and Scarpetta (2024) proposed that the Pliomyini tribe lineage diverged around 4.2 Ma (95% HPD = 3.15-5.17 Ma), a chronology that widely agrees with the divergence time of Dinaromys - Pliomys obtained in the study presented here (3.8 Ma, 95% HPD = 2.6-4.9 Ma approx.). Despite the low number of Pliomyini species with available molecular data, this age would place the rise of this tribe during the Pliocene, probably deriving from Propliomys taking in consideration all the morphological similarities between Propliomys and Dinaromys / Pliomys that had been discussed in the scientific literature (Figure 4). In this way, our study is not in disagreement with the most recent works about the possible evolution of the tribe Pliomyini and could be another important piece of information about how the Arvicolinae evolution takes place.
CONCLUSIONS
In the current study, we obtained for the first time genetic information for the fossil species Pliomys lenki, and specifically, from a right mandible obtained at the El Mirón Cave (Spain). The specimen that furnished an almost complete mitochondrial genome was excavated from a stratigraphic level with an estimated age of 46,890-33,160 years BP. This partial mitochondrial genome allowed us to confirm the close relationship of Pliomys with the current Balkan snow vole (Dinaromys bogdanovi) as previously suggested by paleontologists from a morphological perspective based on shared dental characteristics. In addition, we confirmed that Pliomys and Dinaromys should be considered as different valid genera, which diverged about 2.6-4.9 Ma., and both included in the Arvicolinae tribe Pliomyini. In addition, we show that the complex and somewhat controversial taxonomy of Arvicolinae could benefit from the genetic analysis of extinct taxa as well as ancient samples from extant species, even when small and >30 kyr old samples are considered.
ACKNOWLEDGEMENTS
Thanks are due to B. Bauluz (Universidad de Zaragoza, Spain), the Departamento de Ciencias de la Tierra and IUCA (Universidad de Zaragoza, Spain) for their support of the Paleogenomics Laboratory. The authors would like to acknowledge the Laboratory of Sequencing and Functional Genomics and the Laboratory of Proteomics of Servicios Científico-Técnicos of CIBA (IACS-Universidad de Zaragoza, Spain), and the directors of excavation [L.G. Straus (University of New Mexico, USA) and M. González Morales (Universidad de Cantabria, Spain)] and the excavation team of the Universidad de Cantabria that carry out the excavations of El Mirón Cave. The research reported in this paper is included in the project PGC2018-093925-B-C33 of the Spanish Ministerio de Ciencia, Innovación y Universidades - Generación de Conocimiento and “Aragosaurus: recursos geológicos y paleoambientes, REF: E18_23R” of the Gobierno de Aragón. María Pilar Alfaro-Ibáñez is supported by a grant from the Ministerio de Universidades of Spain (FPU20/02031).
REFERENCES
Abramson, N.I., Bodrov, S.Y., Bondareva, O.V., Genelt-Yanovskiy, E.A., and Petrova, T. 2021. A mitochondrial genome phylogeny of voles and lemmings (Rodentia: Arvicolinae): Evolutionary and taxonomic implications. PLoS ONE, 16:e0248198.
https://doi.org/10.1371/journal.pone.0248198
Agustí, J. and Galobart, A. 1986. La sucesión de micromamíferos en el complejo cárstico de Casablanca (Almenara, Castellón): problemática biogeográfica. Paleontologia i Evolució, 20:57-62.
Agustí, J., Blain, H., Furió, M., De Marfà, R., and Santos-Cubedo, A. 2010. The early Pleistocene small vertebrate succession from the Orce region (Guadix-Baza Basin, SE Spain) and its bearing on the first human occupation of Europe. Quaternary International, 223-224:162-169.
https://doi.org/10.1016/j.quaint.2009.12.011
Alfaro-Ibáñez, M.P., Cuenca-Bescós, G., Bover, P., González-Morales, M., and Straus, L.G. 2023. Implications of population changes among the Arvicolinae (Rodentia, Mammalia) in El Mirón Cave (Cantabria, Spain) for the climate of the last c. 50,000 years. Quaternary Science Reviews, 315:108234.
https://doi.org/10.1016/j.quascirev.2023.108234
Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. 1990. Basic local alignment search tool. Journal of Molecular Biology, 215:403-410.
https://doi.org/10.1016/S0022-2836(05)80360-2
Baca, M., Popovic, D., Lemanik, A., Bañuls-Cardona, S., Conard, N.J., Cuenca-Bescós, G., Desclaux, E., Fewlass, H., Garcia, J.T., Hadravova, T., Heckel, G., Horáček, I., Knul, M.V., Lebreton, L., Manuel López-García, J.M., Luzi, E., Marković, Z., Lenardić, J.M., Murelaga, X., Noiret, P., Petculescu, A., Popov, V., Rhodes, S.E., Ridush, B., Royer, A., Stewart, J.R., Stojak, J., Talamo, S., Wang, X., Wójcik, J.M., and Nadachowski, A. 2022. Ancient DNA reveals interstadials as a driver of the common vole population dynamics during the last glacial period. Journal of Biogeography, 50:183-196.
https://doi.org/10.1111/jbi.14521
Baca, M., Popović, D., Agadzhanyan, A.K., Baca, K., Conard, N.J., Fewlass, H., Filek, T., Golubiński, M., Horáček, I. Knul, M.V., Krajcarz, M., Krokhaleva, M., Lebreton, L., Lemanik, A., Maul, L.C., Nagel, D., Noiret, P., Primault, J., Rekovets, L., Rhodes, S.E., Royer, A., Serdyuk, N.V., Soressi, M., Stewart. J.R., Strukova, T., Talamo, S., Wilczyński, J., and Nadachowski, A. 2023. Ancient DNA of narrow-headed vole reveal common features of the Late Pleistocene population dynamics in cold-adapted small mammals. Proceedings of the Royal Society B, 290:20222238.
https://doi.org/10.1098/rspb.2022.2238
Baele, G., Lemey, P., Bedford, T., Rambaut, A., Suchard, M.A., and Alekseyenko, A.V. 2012. Improving the accuracy of demographic and molecular clock model comparison while accommodating phylogenetic uncertainty. Molecular Biology and Evolution, 29:2157-2167.
https://doi.org/10.1093/molbev/mss084
Bartolomei, G., Chaline, J., Fejfar, O., Jánossy, D., Jeannet, M., Koenigswald, W., and Kowalsi, K. 1975. Pliomys lenki (Heller 1930) (Rodentia, Mammalia) en Europe. Acta Zoologica Cracoviensia, 20:393-467.
Bona, F., Zorzin, R., Accordini, M., Mazzi, R., Gatto, R., Accorsi, C.A., Bandini Mazanti, M., Bosi, G., Trevisan, G., and Torri, P. 2006. First Paleo-environmental considerations on the Pleistocene deposits of the lower cave of Covoli di Velo (VR-Italy). Scientific Annals of the School of Geology of the Aristotle University of Thessaloniki, 98:229-240.
Bover, P., Llamas, B., Mitchell, K.J., Thomson, V.A., Alcover, J.A., Lalueza-Fox, C., Cooper, A., and Pons, J. 2019. Unraveling the phylogenetic relationships of the extinct bovid Myotragus balearicus Bate 1909 from the Balearic Islands. Quaternary Science Reviews, 215:185-195.
https://doi.org/10.1016/j.quascirev.2019.05.005
Brotherton, P., Haak, W., Templeton, J., Brandt, G., Soubrier, J., Adler, C.J., Richards, S.M., Der Sarkissian, C., Ganslmeier, R., Friederich, S., Dresely, V., van Oven, M., Kenyon, R., Van der Hoek, M.B., Korlach, J., Luong, K., Ho, S.Y.W., Quintana-Murci, L., Behar, D.M., Meller, H., Alt, K.W., Cooper, A., and The Genographic Consortium. 2013. Neolithic mitochondrial haplogroup H genomes and the genetic origin of Europeans. Nature Communications, 4:1764.
https://doi.org/10.1038/ncomms2656
Bužan, E., Kryštufek, B., Hänfling, B., and Hutchinson, W.F. 2008. Mitochondrial phylogeny of Arvicolinae using comprehensive taxonomic sampling yields new insights. Biological Journal of the Linnean Society, 94:825-835.
https://doi.org/10.1111/j.1095-8312.2008.01024.x
Carls, N. and Rabeder, G. 1988. Die Arvicoliden (Rodentia, Mammalia) aus dem Altest-Pleistozan von Schernfeld (Bayern). Beiträge zur Paläontologie von Österreich, 14:123-237.
Castresana, J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17:540-552.
https://doi.org/10.1093/oxfordjournals.molbev.a026334
Chaline, J. 1975. Evolution et rapports phyletiques des Campagnols (Arvicolidae, Rodentia) apparentes a Dolomys et Pliomys dans l'hemisphere Nord. Comptes Rendus de l’Académie des Sciences Paris, 281:33-36.
Chaline, J, Brunet-Lecomte, P., Montuire, S., Viriot, L., and Courant, F. 1999. Anatomy of the arvicoline radiation (Rodentia): palaeogeographical, palaeoecological history and evolutionary data. Annales Zoologici Fennici, 36:239-267.
Corbet, G.B. 1978. The mammals of the Palaearctic region. A taxonomic review. British Museum (Natural History) and Cornell University Press, London and Ithaca (NY).
Cuenca-Bescós, G., Straus, L.G., González Morales, M.R., and García Pimienta, J.C. 2008. Paleoclima y paisaje del final del cuaternario en Cantabria: los pequeños mamíferos del Mirón (Ramales de la Victoria). Revista Española de Paleontología, 23:91-126.
https://doi.org/10.7203/sjp.23.1.20398
Cuenca-Bescós, G., Agustí, J., Lira, J., Rubio, M.M., and Rofes, J. 2010a. A new species of Water Vole from the Early Pleistocene of Southern Europe. Acta Palaeontologica Polonica, 55:565-580.
https://doi.org/10.4202/app.2009.0027
Cuenca-Bescós, G., Straus, L.G., García-Pimienta, J.C., Morales, M.G., and López-García, J.M. 2010b. Late Quaternary small mammal turnover in the Cantabrian Region: The extinction of Pliomys lenki (Rodentia, Mammalia). Quaternary International, 212:129-136.
https://doi.org/10.1016/j.quaint.2009.06.006
Cuenca-Bescós, G., López-García, J., Galindo-Pellicena, M., García‐Perea, R., Gisber, J., Rofes, J., and Ventura, J. 2014. Pleistocene history of Iberomys, an endangered endemic rodent from South Western Europe. Integrative Zoology, 9:481-497.
https://doi.org/10.1111/1749-4877.12053
Cuenca-Bescós, G., Blain, H.A., Rofes, J., Lozano-Fernández, I., López-García, J.M., Duval, M., Galan, J., and Nuñez-Lahuerta, C. 2015. Comparing two different Early Pleistocene microfaunal sequences from the caves of Atapuerca, Sima del Elefante and Gran Dolina (Spain): Biochronological implications and significance of the Jaramillo subchron. Quaternary International, 389:148-158.
https://doi.org/10.1016/j.quaint.2014.12.059
Cuenca‐Bescós, G., Blain, H., Rofes, J., López‐García, J.M., Lózano-Fernández, I., and Núñez‐Lahuerta, C. 2016. Updated Atapuerca biostratigraphy: Small-mammal distribution and its implications for the biochronology of the Quaternary in Spain. Comptes Rendus Palevol,15:621-634.
https://doi.org/10.1016/j.crpv.2015.09.006
Cuenca-Bescós, G. and Morcillo-Amo, A. 2022. Roedores, edades y paisajes en el Cuaternario de la Península Ibérica. Guías de la Naturaleza. Ed. Prames., Zaragoza, Spain.
Dabney, J., Knapp, M., Glocke, I., Gansauge, M.T., Weihmann, A., Nickel, B., Valdiosera, C., García, N., Pääbo, S., Arsuaga, J.L., and Meyer, M. 2013. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proceedings of the National Academy of Science U.S.A., 110:15758e15763.
https://doi.org/10.1073/pnas.1314445110
Dema, L. and Rekovets, L. 2004. Morphosystematic fundamentals of evolution in the genus Villanyia (Arvicolidae, Rodentia). Visnyk of Lviv University Series Biology, 38:152-156.
Dereeper, A., Guignon, V., Blanc, G., Audic, S., Buffet, S., Chevenet, F., Dufayard, J.F., Guindon, S., Lefort, V., Lescot, M., Claverie, J.M., and Gascuel, O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research, 36:W465-W469.
https://doi.org/10.1093/nar/gkn180
Desclaux, E., Abbassi, M., Marquet, J.C., Chaline, J., and Van Kolfschoten, T. 2000. Distribution and evolution of Arvicola LACÉPÈDE, 1799 (Mammalia, Rodentia) in France and Liguria (Italy) during the Middle and the Upper Pleistocene. Acta Zoologica Cracoviensia, 43:107-125.
Drummond, A.J., Suchard, M.A., Xie, D., and Rambaut, A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution, 29:1969-1973.
https://doi.org/10.1093/molbev/mss075
Fejfar, O., Heinrich, W.D., Kordos, L., and Maul, L.C. 2011. Microtoid cricetids and the early history of arvicolids (Mammalia, Rodentia). Palaeontologia Electronica, 14:27A.
Folkertsma, R., Westbury, M.V., Eccard, J.A., and Hofreiter, M. 2018. The complete mitochondrial genome of the common vole, Microtus arvalis (Rodentia: Arvicolinae). Mitochondrial DNA B Resources, 3:446-447.
https://doi.org/10.1080/23802359.2018.1457994
Gromov, V.I. and Polyakov, I.Y. 1992. Voles (Microtinae). Fauna of the USSR. Mammals. Vol III, No 8. Brill Ed.
Hinton, M.A.C. 1926. Monograph of voles and lemmings (Microtinae), living and extinct. British Museum (Natural History), London.
Hopkins, R., Straus, L.G., and González Morales, M. 2021. Assessing the chronostratigraphy of El Mirón Cave, Cantabrian Spain. Radiocarbon, 63:821-852.
https://doi.org//10.1017/RDC.2020.121
Hordijk, K. and de Bruijn, H. 2009. The succession of rodent faunas from the Mio/Pliocene lacustrine deposits of the Florina-Ptolemais-Servia Basin (Greece). Hellenic Journal of Geosciences, 44:21-103.
Jánossy, D. 1986. Pleistocene vertebrate faunas of Hungary. Developments in Palaeontology and Stratigraphy, 8. Elsevier, Amsterdam-Oxford-New York-Tokyo.
https://doi.org/10.1016/s0920-5446(08)x7001-3
Jeannet, M. 1974. Pliomys chalinei nov. sp. (Arvicolidae, Rodentia) du Pléistocène moyen d’Orgnac 3 (Ardèche). Bulletin de la Société Géologique de France, 2déc1974:1-2.
Jónsson, H., Ginolhac, A., Schubert, M., Johnson, P., and Orlando, L. 2013. mapDamage 2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics, 29:1682-1684.
https://doi.org/10.1093/bioinformatics/btt193
Kalyaanamoorthy, S., Minh, B., Wong, T., von Haeseler, A., and Jermiin, L.S. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14:587-589.
https://doi.org/10.1038/nmeth.4285
Kass, R.E. and Raftery, A.E. 1995. Bayes factors. Journal of the American Statistical Association, 90:773-795.
https://doi.org/10.2307/2291091
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., and Drummond, A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28:1647-1649.
https://doi.org/10.1093/bioinformatics/bts199
Kircher, M. 2012. Analysis of high-throughput ancient DNA sequencing data, p. 197-228. In Shapiro, B. and Hofreiter, M. (eds.), Ancient DNA: Methods and Protocols. Methods in Molecular Biology, 840. Humana Press. https://doi.org/10.1007/978-1-61779-516-9_23
Koenigswald, W.v. and Van Kolfschoten, T. 1996. The Mimomys-Arvicola boundary and the enamel thickness quotient (SDQ) of Arvicola as stratigraphic markers in the Middle Pleistocene, p. 211-226. In Turner, C. (ed.), The Early Middle Pleistocene in Europe. A.A. Balkema, Rotterdam, Netherlands.
Kordos, L. and Pazonyi, P. 2012. A magyar Arvicolinae típusok katalógusa (Catalogue of the Hungarian Arvicolinae Types). GeoLitera, Budapest, Hungary.
Kowalski, K. 2001. Pleistocene rodents of Europe. Folia Quaternaria, 72:1-389.
Kretzoi, M. 1955. Dolomys and Ondatra. Acta Geologica Hungarica, 3:347-355.
Kretzoi, M. 1965. Pannonicola brevidens n.g n.sp., ein echter Arvicolide aus dem ungarischen Unterpliozan. Vertebrata Hungarica, 7:131-139.
Kretzoi, M. 1969. Sketch of the Late Cenozoic (Pliocene and Quaternary) terrestrial stratigraphy of Hungary. Földrajzi Közlemënyek, 3:179-204.
Kryštufek, B. 2018. Dinaromys bogdanovi. The IUCN Red List of Threatened Species 2018, e.T6607A97220104.
https://doi.org/10.2305/IUCN.UK.2018-1.RLTS.T6607A97220104.en
Kryštufek, B. and Bužan, E.V. 2008. Rarity and decline in paleoendemic Martino’s vole Dinaromys bogdanovi. Mammal Review, 38:267-284.
https://doi.org/10.1111/j.1365-2907.2008.00127.x
Kryštufek, B. and Shenbrot, G.I. 2022. Voles and lemmings (Arvicolinae) of the Palaearctic Region. University of Maribor, University Press, Maribor, Slovenia.
https://doi.org/10.18690/um.fnm.2.2022
Kryštufek, B., Bužan, E.V., Hutchinson, W.F., and Hänfling, B. 2007. Phylogeography of the rare Balkan endemic Martino's vole, Dinaromys bogdanovi, reveals strong differentiation within the western Balkan Peninsula. Molecular Ecology, 16:1221-1232.
https://doi.org/10.1111/j.1365-294X.2007.03235.x
Lamelas, L., Aleix-Mata, G., Rovatsos, M., Marchal, J.A., Palomeque, T., Lorite, P., and Sánchez, A. 2020. Complete mitochondrial genome of three species of the genus Microtus (Arvicolinae, Rodentia). Animals, 10:2030.
https://doi.org/10.3390/ani10112130
Lebreton, L., Desclaux, E., Hanquet, C., Moigne, A., and Perrenoud, C. 2016. Environmental context of the Caune de l’Arago Acheulean occupations (Tautavel, France), new insights from microvertebrates in Q-R levels. Quaternary International, 411:182-192.
https://doi.org/10.1016/j.quaint.2015.12.001
Li, H. and Durbin, R. 2009. Fast and accurate short read alignment with Burrows Wheeler transform. Bioinformatics, 25:1754-1760.
https://doi.org/10.1093/bioinformatics/btp698
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., Marth, G., Abecasis, G., Durbin, R., and 1000 Genome project data processing subgroup. 2009. The sequence alignment/map (SAM) format and SAMtools. Bioinformatics, 25:2078-2079.
https://doi.org/10.1093/bioinformatics/btp352
Llamas, B., Fehren-Schmitz, L., Valverde, G., Soubrier, J., Mallick, S., Rohland, N., Nordenfelt, S., Valdiosera, C., Richards, S.M., Rohrlach, A., Barreto Romero, M.I., Flores Espinoza, I., Tomasto Cagigao, E., Watson Jiménez, L., Makowski, K., Leboreiro Reyna, I.S., Mansilla Lory, J., Ballivián Torrez, J.A., Rivera, M.A., Burger, R.L., Ceruti, M.C., Reinhard, J., Wells, R.S., Politis, G., Santoro, C.M., Standen, V.G., Smith, C., Reich, D., Ho, S.Y.W., Cooper, A., and Haak, W. 2016. Ancient mitocondrial DNA provides high-resolution time scale of the peopling of the Americas. Science Advances, 2:e1501385.
https://doi.org/10.1126/sciadv.1501385
Lopez, N., Michaux, J., and Villalta, J.F. 1976. Rongeurs et Lagomorphes de Bagur-2 (Province de Gérone, Espagne), nouveau remplissage de fissure du debut du Pléistocène Moyen. Acta Geologica Hispanica, 11:46-54.
López-García, J.M., Cuenca-Bescós, G., Blain, H.A., Cáceres, I., García, N., Van Der Made, J., Gutierrez, J.M., Santiago, A., and Pacheco, F.G. 2012. Biochronological data inferred from the early Pleistocene Arvicolinae (Mammalia, Rodentia) of the El Chaparral site (Sierra del Chaparral, Cádiz, southwestern Spain). Journal of Vertebrate Paleontology, 32:1149-1156.
https://doi.org/10.1080/02724634.2012.676584
López‐García, J.M., Blain, H., Sanz, M., Daura, J., and Zilhão, J. 2018. Refining the environmental and climatic background of the Middle Pleistocene human cranium from Gruta da Aroeira (Torres Novas, Portugal). Quaternary Science Reviews, 200:367-375.
https://doi.org/10.1016/j.quascirev.2018.10.003
Mahmoudi, A., Maul, L.C., Khoshyar, M., Darvish, J., Aliabadian, M., and Kryštufek, B. 2019. Evolutionary history of water voles revisited: confronting a new phylogenetic model from molecular data with the fossil record. Mammalia, 84:171-184.
https://doi.org/10.1515/mammalia-2018-0178
Marquet, J.C. 2001. Les rongeurs de la Grotte du Sanglier. (Reilhac, Lot). Préhistoire du Sud-Ouest, 4:175-182.
Martin, R.A. 2010. The North American Promimomys immigration event. Paludicola, 8:14-21.
Martin, R.A. 2015. A preliminary diversity-divergence model for North American arvicolid rodents. Palaeobiodiversity and Palaeoenvironments, 95:253-256.
https://doi.org/10.1007/s12549-015-0187-y
Maul, L.C., Rekovets, L., Heinrich, W., Keller, T., and Storch, G. 2000. Arvicola mosbachensis (Schmidtgen 1911) of Mosbach 2: a basic sample for the early evolution of the genus and a reference for further biostratigraphical studies. Senckenbergiana lethaea, 80:129-147.
https://doi.org/10.1007/bf03043667
Maul, L.C., Heinrich, W.D., Parfitt, S.A., and Paunescu. A.C. 2007. Comment on the correlation between magnetostratigraphy and the evolution of Microtus (Arvicolidae, Rodentia, Mammalia) during the Early and early Middle Pleistocene. Courier Forschungsinstitut Seckenberg, 259:243-263.
McKenna, M.C. and Bell, S.K. 1997. Classification of mammals above the species level. Columbia University Press, New York, NT.
Méhely, L.V. 1914. Fibrinae Hungariae. Die tertären und quartären wurzelzänigen Wühlmäuse Ungarns. Annales Historico-Naturales Musei Nationalis Hungarici, 12:155-243.
Meyer, M. and Kircher, M. 2010. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harbor Protocols, 6:1-10.
https://doi.org/10.1101/pdb.prot5448
Minh, B.Q., Schmidt, H.A., Chernomor, O., Schrempf, D., Woodhams, M.D., von Haeseler, A., and Lanfear, R. 2020. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37:1530-1534
https://doi.org/10.1093/molbev/msaa015
Pavlinov, I.Y. 2003. Systematics of recent mammals. Moscow, Russia: Moscow University Press.
Piskoulis, P., Tsoukala, Ε., and Tsiourlini, I. 2023. Late Pleistocene small mammals (Chiroptera, Rodentia, Lagomorpha) from Agios Georgios Cave (Kilkis, Central Macedonia, Greece). Geobios, 82:69-84.
https://doi.org/10.1016/j.geobios.2023.07.006
Rădulescu, C. and Samson, P. 1996. Pliocene and Early Pleistocene arvicolids (Rodentia, Mammalia) of the Dacic Basin, Romania. Acta Zoologica Cracoviensia, 39:401-406.
Rambaut, A., Drummond, A.J., Xie, D., Baele, G., and Suchard, M.A. 2018. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67:901-904.
https://doi.org/10.1093/sysbio/syy032
Robovský, J., Řičánková, V., and Zrzavý, J. 2008. Phylogeny of Arvicolinae (Mammalia, Cricetidae): utility of morphological and molecular data sets in a recently radiating clade. Zoologica Scripta, 37:571-590.
https://doi.org/10.1111/j.1463-6409.2008.00342.x
Rohland, N., Harney, E., Mallick, S., Nordenfelt, S., and Reich, D. 2015. Partial uracil-DNA-glycosylase treatment for screening of ancient DNA. Philosophical Transactions of the Royal Society B Biological Sciences, 370:20130624.
https://doi.org/10.1098/rstb.2013.0624
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A., and Huelsenbeck, J.P. 2012. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61:539-542.
https://doi.org/10.1093/sysbio/sys029
Schubert, M., Lindgreen, S., and Orlando, L. 2016. AdapterRemoval v2: rapid adapter trimming, identification, and read merging. BMC Research Notes, 12:88.
https://doi.org/10.1186/s13104-016-1900-2
Skandalos, P., Lansing, K., Demi̇rel, F.A., Alçi̇çek, M.C., Mayda, S., Dieleman, F., and van den Hoek Ostende, L.W. 2023. Early Pliocene Arvicolinae and Cricetinae from the locality of Afşar, western Turkey. Turkish Journal of Earth Sciences, 32:27-62.
https://doi.org/10.55730/1300-0985.1826
Stamatakis, A. 2014. RAxML version 8: a tool for phylogenetic and post-analysis of large phylogenies. Bioinformatics, 30:1312-1313.
https://doi.org/10.1093/bioinformatics/btu033
Storch, G. and Fejfar, O. 1990. Gundersheim-Finding, a Ruscinian rodent fauna of Asian affinities from Germany. In European Neogene mammal chronology. Springer US, Boston, MA.
Terzea, E. 1983. Pliomys "lenki" (Heller, 1930) (Rodentia, Mammalia), dans le Pléistocène de Roumanie. Travaux de l’Institut de Spéologie “Emile Racovitza”, 22:65-80.
Tesakov, A.S. 2005. Pliocene voles (Pliomys, Arvicolinae, Rodentia) from Odessa Catacombs. Russian Journal of Theriology, 4:123-135.
https://doi.org/10.15298/RUSJTHERIOL.04.2.05
Torres-Roig, E., Mitchell, K.J., Alcover, J.A., Martínez-Freiría, F., Bailón, S., Heiniger, H., Williams, M., Cooper, A., Pons, J., and Bover P. 2021. Origin, extinction and ancient DNA of a new fossil insular viper: molecular clues of overseas immigration. Zoological Journal of the Linnean Society, 192:144-168.
https://doi.org/10.1093/zoolinnean/zlaa094
Westbury, M.V. and Lorenzen, E.D. 2022. Iteratively mapping ancient DNA to reconstruct highly divergent mitochondrial genomes: An evaluation of software, parameters and bait reference. Methods in Ecology and Evolution, 13:2419-2428.
https://doi.org/10.1111/2041-210X.13990
Withnell, C.B. and Scarpetta SG. 2024. A new perspective on the taxonomy and systematics of Arvicolinae (Gray, 1821) and a new time-calibrated phylogeny for the clade. PeerJ, 12:e16693.
https://doi.org/10.7717/peerj.16693
Xia, X. 2017. DAMBE6: new tools for microbial genetics, phylogenetics and molecular evolution. Journal of Heredity, 108:431-437.
https://doi.org/10.1093/jhered/esx033
Xie, W., Lewis, P.O., Fan, Y., Kuo, L., and Chen MH. 2011. Improving marginal likelihood estimation for Bayesian phylogenetic model selection. Systematic Biology, 60:150-160.
https://doi.org/10.1093/sysbio/syq085
Yamikova, A.A., Tesakov, A.S., and Pogodina, N.V. 2022. Morphology and evolutionary position of the Early Pliocene vole Propliomys jalpugensis from Eastern Europe. Russian Journal of Theriology, 21:1-23.
https://doi.org/10.15298/rusjtheriol.21.1.02
Zazhigin, V.S. 1982. Order Rodentia-rodents, p. 294-305. In Shanzer, E.V. (ed.), Stratigraphy of the USSR: The Quaternary system. Nedra, Moscow.

