 The Middle Devonian acanthodian Orcadacanthus n. gen. from the Orcadian Basin of Scotland
The Middle Devonian acanthodian Orcadacanthus n. gen. from the Orcadian Basin of Scotland
Article number: 26.1.a5
https://doi.org/10.26879/1240
Copyright Palaeontological Association, February 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 23 August 2022. Acceptance: 4 January 2023
ABSTRACT
Although first described more than 175 years ago and represented by many hundreds of specimens, the small (less than 60 mm long) mesacanthid acanthodiform originally named Acanthodes pusillus is the most poorly known acanthodian-grade vertebrate from the Middle Devonian of Scotland. The classification of the species received some attention in the late nineteenth century, but it is only in the last decade that more substantive interest has been shown in this little fish. Here we re-evaluate the type material for the species, and assess the validity of its putative junior synonym Mesacanthus peachi based on morphological and histological study of mesacanthid specimens from all levels of the Eifelian and Givetian from the Orcadian Basin. New information on the taxon revealed by these studies includes the shape of the jaw cartilages, ornamentation of the sclerotic plates, structure and morphology of the different fin spines, variation in scale shape and size over the body, and histological structure of the dermal elements. Our study supports synonymizing M. pusillus and M. peachi and erecting a new genus Orcadacanthus, type species O. pusillus. The depositional environment appears to have influenced the mode of preservation of the new taxon at different localities. Variability in the degree of mineralization, particularly in the head region, is linked to ontogenetic changes.
Michael J. Newman. Vine Lodge, Vine Road, Johnston, Haverfordwest, Pembrokeshire SA62 3NZ, UK. ichthyman@btinternet.com
Jan L. den Blaauwen. University of Amsterdam, Science Park 904, 1098 XH, Amsterdam, Netherlands. J.L.denBlaauwen@uva.nl
Carole J. Burrow. Geosciences, Queensland Museum, 122 Gerler Road, Hendra 4011, Queensland, Australia. carole.burrow@gmail.com
Roger Jones. 6 Burghley Road, Wimbledon, London, SW19 5BH, UK. charnaud@btinternet.com
Robert G. Davidson. University of Aberdeen, School of Geosciences, Meston Walk, Aberdeen AB24 3UE. bobgraydavidson@gmail.com
Keywords: Caithness Flagstone Group; palaeobiogeography; histology; fin spines; scales; nasal plates
Final citation: Newman, Michael J., den Blaauwen, Jan L., Burrow,Carole J., Jones, Roger, and Davidson, Robert G. 2023. The Middle Devonian acanthodian Orcadacanthus n. gen. from the Orcadian Basin of Scotland. Palaeontologia Electronica, 26(1):a5.
https://doi.org/10.26879/1070
palaeo-electronica.org/content/2023/3763-new-devonian-acanthodian
Copyright: February 2023 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/534AE920-E1DB-4A7E-9C9D-1D24517ADC1C
INTRODUCTION
The early jawed vertebrates in the Order Acanthodiformes are distinguished from other ‘acanthodians’ by having one rather than two dorsal fin spines (Denison 1979). Of the four families recognised within the Acanthodiformes, the Mesacanthidae are mostly distinguished by features that are considered ‘primitive’ because they are shared with ‘Climatiiformes’ sensu lato - having one pair of prepelvic spines, a shallowly inserted dorsal spine, an unmineralised endocranium, and a simple mandibular joint (Burrow, 2021). As noted by Burrow et al. (2022), recent cladistic analyses (e.g., Zhu et al. 2022, extended data figure 9b) retrieve mesacanthids as the sister group to most other acanthodiforms.
Specimens of a small acanthodiform fish were first collected from the Middle Devonian nodule beds in the Moray Firth area of Scotland in the 1830s. Agassiz (1844, plate 28, figures 8-10) originally assigned the species name Acanthodes pusillus to this fish and figured as syntypes three poorly preserved specimens that he noted came from Gordon Castle. Later, Egerton (1861a) briefly discussed the species and noted that the specimens came from the Old Red Sandstone near Gordon Castle, from Tynet Burn, establishing that this was the type locality. All the syntypes were subsequently considered lost, although Andrews (1982) speculated on the possible identity of counterparts of two of the specimens figured by Agassiz. At the 1859 British Association for the Advancement of Science meeting held in Aberdeen, Mr. Charles Peach (see Taylor and Anderson, 2015 and references therein for information about Peach) showed a number of fossil fish specimens he collected in Caithness in the late 1850s. One of these was a small acanthodian from Barragill (now known as East Mey), Caithness which Egerton (1861b) named Acanthodes peachi. However, he provided no description or figures. It was not until later in the same year that Egerton (1861a) formally described A. peachi and another species (Acanthodes coriaceus) collected from near Thurso (probably Pennyland), Caithness. Traquair (1888) considered A. coriaceus a synonym of A. peachi and erected a new genus Mesacanthus for A. peachi, A. pusillus, and the Lower Devonian Acanthodes mitchelli Egerton, 1861, with the latter species nominated as the type for Mesacanthus. Traquair (1888) differentiated Mesacanthus from Acanthodes based on the former having intermediate (prepelvic) fin spines. In the same work he considered that both M. pusillus and M. peachi were valid species based on morphological proportions. Traquair (1890) later differentiated Acanthodes from Mesacanthus by the location of the pelvic fin spines, as Woodward and Sherborn (1890) did not believe there were enough diagnostic characters to validate a new genus. The difficulty in differentiating between M. pusillus and M. peachi was evident to Paton (1976); in her catalogue of fossil fishes in the National Museum of Scotland she referred all Middle Devonian specimens from the Orcadian Basin to Mesacanthus sp. Trewin and Davidson (1999) were the first to publish an opinion that M. pusillus and M. peachi belonged in the same species, but provided no evidence for that assumption. Baron (2015) showed that there were no proportional morphological differences between M. pusillus and M. peachi and synonymised the two species, supporting Trewin and Davidson’s (1999) opinion, while also showing statistical differences between the older type species M. mitchelli and the Middle Devonian species. The most recent papers concerning M. pusillus are the description by Burrow et al. (2020a) of a putative dermal spiracle cover in a specimen from Cromarty, nomination of a lectotype by Baron and Seymour (2021), and description of the jaw cartilage structure by Burrow and den Blaauwen (2021). Up to date, no morphological description of the head or skeletal reconstruction of the species has been published.
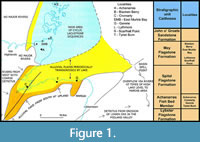 The type species for the family and genus, Mesacanthus mitchelli from the Lower Devonian (Lochkovian) Midland Valley of Scotland was recently intensively redescribed by Burrow et al. (2022). In order to further test whether the Scottish Middle Devonian mesacanthids should all be assigned to the one species, and indeed whether they should be retained in Mesacanthus, we have examined extensive material collected by MJN and JdB over the last decades from all stratigraphic levels, from numerous localities in northern Scotland (Figure 1). The ornament and distribution of dermal elements, relative dimensions and ornament of fin spines, and morphology of endoskeletal structures are described and compared. Thin sections were made of part or all of the selected specimens to show the histological structure of the spines, scales, and other dermal elements, as well as the endoskeletal jaws, scapulocoracoids, and basal fin cartilages. Scales and other small dermal elements have been imaged using scanning electron microscopy.
The type species for the family and genus, Mesacanthus mitchelli from the Lower Devonian (Lochkovian) Midland Valley of Scotland was recently intensively redescribed by Burrow et al. (2022). In order to further test whether the Scottish Middle Devonian mesacanthids should all be assigned to the one species, and indeed whether they should be retained in Mesacanthus, we have examined extensive material collected by MJN and JdB over the last decades from all stratigraphic levels, from numerous localities in northern Scotland (Figure 1). The ornament and distribution of dermal elements, relative dimensions and ornament of fin spines, and morphology of endoskeletal structures are described and compared. Thin sections were made of part or all of the selected specimens to show the histological structure of the spines, scales, and other dermal elements, as well as the endoskeletal jaws, scapulocoracoids, and basal fin cartilages. Scales and other small dermal elements have been imaged using scanning electron microscopy.
GEOLOGICAL AND STRATIGRAPHIC DISTRIBUTION
It has been demonstrated in many previous publications (e.g., Burrow et al., 2020b) that there are five fish-bearing faunal zones in the Middle Devonian Orcadian Basin. These zones equate well to the biostratigraphy of the Baltic region (Newman et al., 2017), Belarus and elsewhere in Laurussia (Plax and Newman, 2021). Here we refer to Caithness stratigraphical units in the Caithness Flagstone Group, but mesacanthid specimens occur at the same biostratigraphical levels across the whole of the Orcadian Basin (see Burrow et al., 2020b:figure 5.1 for an example of these stratigraphical units). The lowest unit, the Lybster Flagstone Formation, does contain a small acanthodian, which is probably a mesacanthid, but the specimens are for the most part disarticulated and not well preserved. In the highest faunal zone, the John o’ Groats Sandstone Formation, no acanthodian remains have been discovered so far. There are thus three faunal zones confirmed to have a mesacanthid in the Orcadian Basin (Figure 1). The lowest stratum with identifiable specimens is the Achanarras Fish Bed Member, which is at the boundary of the Lybster and Spital flagstone formations. Historically, most specimens collected from this level in the Moray Firth area have been assigned to Mesacanthus pusillus. There is then a gap in articulated remains until the base of the Mey Flagstone Formation, in fish beds with the tetrapodomorph Osteolepis panderi (Pander, 1860). Finally, articulated mesacanthid specimens are found at the top of the Mey Flagstone Formation. Most mesacanthid remains (and possibly small individuals of other acanthodians) from northern Caithness and Orkney have been assigned over the last century to Mesacanthus peachi. One mesacanthid species is present in all the strata from the Lybster Flagstone Formation up to the top of the Mey Flagstone Formation. This stratigraphical distribution is repeated in equivalent strata across the whole of the Orcadian Basin.
For this project, morphology and histology of scales and spines and other dermal elements of mesacanthid specimens from these three different zones have been investigated to determine if there are any diagnostic differences between specimens from different levels.
MATERIAL AND METHODS
Methods
Macrophotographs of NHMUK PV OR 35786, a well-preserved Tynet Burn specimen assigned by Woodward (1891) to Acanthodes pusillus, were taken using a Nikon D750. Other macrophotographs of specimens were taken using a Canon EOS 450. Microphotographs of specimens were taken using a DSC-H2 camera on a Nikon Eclipse E400. Thin sections were made by JdB using epoxy resin and carborundum grinding powder down to 4 microns.
Environmental scanning electron microscopy (ESEM) images were made using a Hitachi Tabletop TM-1000 at the Queensland Museum.
Remarks. One specimen from Blacken Berry was in the collection of Dr. Paul de Buisonjé (Amsterdam), whose collection was mostly donated in the NMS in 1996. Unfortunately, this specimen was not found when searched for and must be considered lost at present. However, at the time it was in Dr. Buisonjé’s collection it was photographed by JdB using a Canon A1 and a negative taken, which is now housed in the NMS under the registration number NMS G.2022.10.686. Other specimens show the same characters of the nasal region, but due to the particular clarity of the nasal region of this specimen the authors have made the decision to use the negative as a proxy specimen.
Material
The Achanarras fish bed member horizon. From the Moray Firth area: Corbie Den (British Grid reference (BGR) NJ 791622), NMS G.2019.3.11; Cromarty (BGR NH 795672), NMS G.2019.3.10, NMS G.2021.7.125, NMS G.2021.7.126, NMS G.2021.7.135, NMS G.2021.7.136, NMS G.2021.7.303, NMS G.2021.7.94; Den of Findon (BGR NJ 795637; most localities referred to Gamrie come from here): NMS G.2021.7.292; Tynet Burn (BGR NJ 384121), GLAHM V3573, NHMUK PV OR 35786 (Neotype), NRM P1645. From Caithness: Achanarras Quarry (BGR ND 150545): NMS G.2021.7.27, NMS G.2021.7.284, NMS G.2021.7.285, NMS G.2021.7.29, NMS G.2021.7.45, NMS G.2021.7.51.
The lower part of the Mey Flagstone Formation (Osteolepis panderi) horizon. From Caithness: Cairnfield Quarry (BGR ND152651): NMS G.2021.7.290, NMS G.2021.7.84, NMS G.2021.7.93, NMS G.2022.10.6, NMS G.2022.10.14; Cairn of Hattel (BGR ND 191691): NMS G.2022.10.17; Castlehill (BGR ND 200694): NMS G.2022.10.13; Lythmore (also known as Overton) (BGR ND 066666): NMS G.2021.7.202, NMS G.2021.7.286, NMS G.2022.10.2, NMS G.2022.10.4, NMS G.2022.10.6; Ness of Litter (often referred to as Holborn Head) (BGR ND 080710;): NMS G.2021.7.209; Skinnet (BGR ND 123618): NMS G.2021.7.238, NMS G.2021.7.241, NMS G.2021.7.251. From Orkney: Scarfhall Point (Westray) (BGR HY 457488;): NMS G.2022.10.15.
The upper part of the Mey Flagstone Formation horizon. From Caithness: Blacken Berry (BGR ND 308749): NMS G.2018.28.15, NMS G.2019.3.8, NMS G.2021.7.202, NMS G.2021.7.265, NMS G.2021.7.269, NMS G.2021.7.64, NMS G.2022.10.1, NMS G.2022.10.12; Button Geo (Stroma) (BGR ND 345478): NMS G.1975.12.26, NMS G.2022.10.8, NMS G.2022.10.9; East Mey (BGR ND 292747): NMS G.2021.7.162; East Murkle Bay (EMB) (BGR ND 178692): NMS G.2021.7.158, NMS G.2021.7.165, NMS G.2021.7.166; Pennyland (BGR ND 110691): NMS G.1887.35, NMS G.2019.3.9, NMS G.2022.10.5. From Orkney: Surrigarth (Westray) (BGR HY 491451): NMS G.2021.7.187.
Horizon uncertain. From Caithness: Brims Ness (BGR ND 038713) NMS G.2021.7.78.
Institutional Abbreviations
ELGNM - Elgin Museum, Elgin, United Kingdom. GLAHM - Hunterian Museum, Glasgow, United Kingdom. GSM - British Geological Survey collection, Keyworth, United Kingdom. MB, Museum für Naturkunde, Berlin, Germany. MV - Museum Victoria, Melbourne, Australia. NHMUK - Natural History Museum, London, United Kingdom. NMS - National Museums of Scotland, Edinburgh, United Kingdom. ROM - Royal Ontario Museum, Toronto, Canada.
SYSTEMATIC PALAEONTOLOGY
Class CHONDRICHTHYES Huxley, 1880
Subclass ACANTHODII Owen, 1846, sensu Coates et al., 2018
Order ACANTHODIFORMES Berg, 1937
Family MESACANTHIDAE Moy-Thomas, 1937
Genus ORCADACANTHUS n. gen.
zoobank.org/857D5D7F-DA96-481F-B6AD-45803BB25622
Type and only known species. Orcadacanthus pusillus (Agassiz, 1844) n. comb.
Diagnosis. As for the type species.
Etymology. Orcad-, referring to the Orcadian Basin where the genus is found, and -acanthus, Greek for thorn.
Orcadacanthus pusillus (Agassiz, 1844) n. comb.
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20, Supplemental Figure 1
1844 Acanthodes pusillus; Agassiz, p. 35-36, pl. 28, figures 8-10.
1859 Acanthodes pusillus; Gordon, p. 41.
1860 Acanthodes pusillus; Gregory, p. 145, 147.
1861a Acanthodes peachi; Egerton, p. 57-59, pl. 6, figures 1-2.
1861a Acanthodes coriaceus; Egerton, p. 59-60, pl. 6, figures 3-5.
1861b Acanthodes peachi; Egerton, p. 78.
1868 Acanthodes pusillus Agassiz; Peach, p. 72.
1868 Acanthodes pusillus Hancock and Atthey, p. 364.
1878 Acanthodes peachii, Eg.; Geikie, p. 404, tabs. 1-2.
1878 Acanthodes coriaceus, Eg.; Geikie, tabs. 1-2.
1878 Acanthodes pusillus, Ag.; Geikie, tabs. 1-2.
1888 Mesacanthus pusillus, Ag.; Traquair, p. 512.
1888 Mesacanthus peachi, Egert.; Traquair, p. 512.
1890 Acanthodes peachi (Egerton); Woodward and Sherborn, p. 119.
1890 Acanthodes pusillus (Agassiz); Woodward and Sherborn, p. 119.
1890 Mesacanthus pusillus, Ag.; Traquair, p. 481.
1890 A. Peachii, Eg.; Traquair, p. 481.
1891 Acanthodes pusillus, Agassiz: Woodward, p. 11-12, pl. 1, figures 5-6.
1891 Acanthodes peachi, Egerton; Woodward, p. 12-13.
1894 Mesacanthus Peachi (Egert.); Traquair, p. 285.
1895 Mesacanthus pusillus, Agassiz, 1844; Traquair, p. 245.
1906 Acanthodes Pusillus; Wallace, tab. 1.
1914 Mesacanthus Peachi (Egert.); Crampton and Carruthers, p. 6.
1935 Mesacanthus peachi (Egert.); Wilson, p. 38.
1948 Mesacanthus pusillus; Read, p. 63.
1948 Mesacanthus peachi; Phemister, p. 67.
1948 Mesacanthus pusillus; Phemister, p. 68.
1964 Mesacanthus pusillus Agassiz, 1844; Novitskaya and Obruchev, p. 189.
1965 Mesacanthus pusillus; Waterston, p. 293.
1968 Mesacanthus pusillus (Agassiz); Peacock, Berridge, Harris and May, p. 135.
1970 Mesacanthus pusillus (Agassiz); Miles, p. 362.
1974 Mesacanthus peachi; Donovan, Foster and Westoll, tab. 2.
1976 Mesacanthus peachi Egerton; Mykura, p. 78.
1979 M. pusillus (Agassiz) 1844; Denison, p. 47-48.
1979 M. peachi (Egerton) 1861; Denison, p. 48.
1979 M. sp.; Denison, p. 48.
1980 Mesacanthus peachi; Donovan, p. tab. 3.
1982 Mesacanthus pusillus Ag; Andrews, p. 47, figure 19d-f.
1986 M. peachi (Egerton); Trewin, p. 37.
1997 M. peachi; Young, p. 49.
1997 M. pusillus; Young, p. 49.
1999 Mesacanthus pusillus Agassiz 1844; Trewin and Davidson, p. 543, tab. 3.
1999 Mesacanthus peachi Egerton, 1861: Dineley, p. 174, 181, 212.
1999 Mesacanthus pusillus (Agassiz, 1844); Dineley, p. 198, 212, 215, figure 6.20.
2005 Mesacanthus pusillus (Agassiz); Newman and Dean, p. 2-6.
2005 Mesacanthus pusillus Agassiz 1844; Barclay and Trewin, p. 10.
2008 Mesacanthus peachi (Agassiz, 1844)); Hanke, p. 289.
2008 Mesacanthus pusillus (Egerton, 1861); Hanke, p. 289.
2010 Mesacanthus pusillus (Agassiz, 1844); Newman, p. 13-15, figures 21-23.
2015 Mesacanthus pusillus Agassiz; Baron, p. 2-13, 15, 17-19, figures 6, 8.
2020 Mesacanthus pusillus; Burrow, Newman and den Blaauwen, 1155-6, figure 1g.
2020 Mesacanthus pusillus Agassiz, 1844; Newman, den Blaauwen and Burrow, p. 34.
2021 Mesacanthus pusillus; Baron and Seymour, p. 1-3, figures 1, 2.
2021 Mesacanthus pusillus; Burrow, p. 63, figures 26D, 27C.
2021 Mesacanthus pusillus; Burrow and den Blaauwen, p. 86, 89, figure 7d, e.
2022 Mesacanthus pusillus; Burrow, den Blaauwen, and Newman, p. 4, 12.
DIAGNOSIS AND DESCRIPTION
 Revised diagnosis. Mesacanthid acanthodian up to 60 mm long, maximum depth to length ratio about 0.2; rostrum extends about half an orbit length in front of the eye; covering of robust polygonal tesserae extending down to nares anteriorly and down to mouth post-orbitally; c. five large, wide sclerotic plates ornamented with parallel grooves that are oblique across the plates or parallel to the plate edges (autapomorphy); thin denticulated semicircular plates above anterior and posterior nares; anterior end of Meckel’s cartilage truncated (autapomorphy); main gill cover subcircular, extending below Meckel’s cartilage for about three quarters of its length; branchiostegal rays ornamented with three sharp or rounded longitudinal ridges; dorsal-most branchiostegal rays curved; scapular shaft high and circular in cross section (apomorphy); pelvic spines positioned halfway between pectoral and anal fin spines; very short (apomorphy), straight prepelvic spines just anterior to pelvic spines; pelvic spines strong and straight and at least half the length of the pectoral spines and two-thirds the length of the anal spine; large cartilages at bases of median spines; pelvic and anal fins large with long bases (apomorphy); scales up to 0.2 mm wide with a central pulp cavity that often opens out through the base (autapomorphy); scale crowns can extend more than a base length behind the posterior corner of the scale base (autapomorphy).
Revised diagnosis. Mesacanthid acanthodian up to 60 mm long, maximum depth to length ratio about 0.2; rostrum extends about half an orbit length in front of the eye; covering of robust polygonal tesserae extending down to nares anteriorly and down to mouth post-orbitally; c. five large, wide sclerotic plates ornamented with parallel grooves that are oblique across the plates or parallel to the plate edges (autapomorphy); thin denticulated semicircular plates above anterior and posterior nares; anterior end of Meckel’s cartilage truncated (autapomorphy); main gill cover subcircular, extending below Meckel’s cartilage for about three quarters of its length; branchiostegal rays ornamented with three sharp or rounded longitudinal ridges; dorsal-most branchiostegal rays curved; scapular shaft high and circular in cross section (apomorphy); pelvic spines positioned halfway between pectoral and anal fin spines; very short (apomorphy), straight prepelvic spines just anterior to pelvic spines; pelvic spines strong and straight and at least half the length of the pectoral spines and two-thirds the length of the anal spine; large cartilages at bases of median spines; pelvic and anal fins large with long bases (apomorphy); scales up to 0.2 mm wide with a central pulp cavity that often opens out through the base (autapomorphy); scale crowns can extend more than a base length behind the posterior corner of the scale base (autapomorphy).
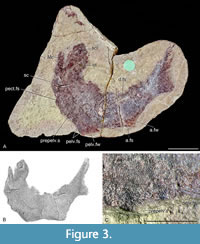 Neotype. NHMUK PV OR 35786 (Figure 3, Figure 5H), a complete specimen including the head with lower jaws from Tynet Burn, Moray, Scotland.
Neotype. NHMUK PV OR 35786 (Figure 3, Figure 5H), a complete specimen including the head with lower jaws from Tynet Burn, Moray, Scotland.
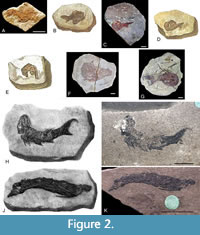
Remarks. When referring to Orcadacanthus pusillus in this section, we use the genus name that the relevant authors used at the time. Andrews (1982) stated that all of the part specimens from the syntype series were lost, but suggested that ELGNM.1978.191.1 from Tynet Burn (Figure 2A) could be the counterpart of one of Agassiz’s specimens (1844-5, plate 28, figure 8) of Acanthodes pusillus (Figure 2B). She also suggested that one of the specimens from Tynet Burn purchased by the Royal Ontario Museum from Portsoy Minerals in 1968, referred to as ROM 87 in her paper (now ROM 25872; Figure 2C), could be the counterpart of another of Agassiz’s type specimens (1844-45, plate 28, figure 9; Figure 2D). The final specimen figured by Agassiz (1844-5, plate 28, figure 10; Figure 2E) has never been compared with or referred to any specimen in a collection and the drawing is very poor. Andrews (1982) noted that the back of specimen ‘ROM 87’ had a label written by George Gordon (1801-1893; see Andrews (1982) for details on Gordon) stating the specimen was a counterpart of one of Agassiz’ figured specimens. This label has not been found, and ROM curator Kevin Seymour does not recall ever seeing one (personal commun., 2020). The typed fossil list from Portsoy Minerals held in the Royal Ontario Museum was appended in pencil by Kevin Seymour with the ROM catalogue numbers allocated to the specimens, thus for example # 87 (‘ROM 87’) was assigned catalogue number ROM 25872. However, also on this list next to specimen # 17 (also from Tynet Burn; now ROM 25859) is the typed comment that it was a reputed counterpart of an Agassiz type specimen. Neither specimen was identified taxonomically on the list. ROM 25859 (Figure 2F) is a good example of Diplacanthus crassisimus, but it clearly does not match the morphology of the Diplacanthus specimens figured by Agassiz (1844-5, plates 13-14). We can only suggest that the provenance of # 17 and # 87 on the Portsoy list were mixed up, if Andrews (1982) was correct about the label on # 87. However, Andrews (1982) stated that neither of the two Mesacanthus pusillus specimens ROM 25872 and ELGNM.1978.191.1 could be unconditionally attributed to any Agassiz’s (1844-5) figures, as the drawings were of too poor quality.
Moving forward, Baron (2015) considered the possibility that as well as ROM 25872 and ELGNM 1978.191.1, ROM 25846 (Figure 2G) could possibly be the counterpart of one of Agassiz’s type specimens. Baron (2015) also followed Andrews (1982) in concluding that none of Agassiz’s (1844-5) figures were clear enough to allow confirmation that any of the specimens mentioned above were the type counterparts.
Baron and Seymour (2021) subsequently addressed the type specimen issue and nominated ROM 25872 as the lectotype of Mesacanthus pusillus, as they thought there was enough similarity between it and the Agassiz (1844-5, plate 28, figure 9) specimen. However, there are discrepancies between ROM 25872 and that Agassiz syntype, and we are not convinced that ROM 25872 is the counterpart of this specimen. We consider that the confusion and uncertainty surrounding the provenance of ROM 25872 and ROM 25859 and the lack of specific diagnostic characters in ROM 25872 mean that under ICZN rules 74.2 and 75.5, it is not acceptable as a type specimen.
Woodward (1891, p. 11) stated that the type specimen of Acanthodes pusillus, an ‘imperfect fish’, was in Forres, Scotland, presumably in the Falconer Museum. Both S.M. Andrews and two of the authors (MJN, JdB) searched this collection for a possible type specimen, but none was found. ELGNM 1978.191.1, the other specimen identified by Andrews (1982) as a possible counterpart of a syntype (Agassiz, 1844-5, plate 28, figure 8), could be the specimen described by Woodward, as it is possible that the Elgin and Falconer museums, just 12 miles apart and closely associated, swapped material in the nineteenth century. The morphology of this specimen is similar to that illustrated by Agassiz, indicating that it might belong to the syntype series. However, it is very poorly preserved and like ROM 25872 preserves no specific diagnostic characters, also making it unsuitable as a type specimen. As Acanthodes peachi and Acanthodes coriaceus have been regarded as junior synonyms of Orcadacanthus pusillus, their type specimens GSM 21448 and GSM 28831, respectively (Figure 2H-K), were also considered as potential candidates for a neotype, but they are from localities in Caithness, not the type locality of Tynet Burn (which is in Moray).
As none of the purported surviving members of the type series precisely match the original (Agassiz, 1844) figures, no definite archival evidence links possible counterpart material to the original syntypes, and after extensive searching, we must concur with previous authors (e.g., Andrews, 1982) that considered them lost. Agassiz’s (1844) figures and description are insufficient to distinguish this species from other mesacanthids, necessitating a neotype to stabilize usage of the species name Acanthodes pusillus. After Agassiz (1844-5), Woodward (1891) provided the next detailed description of specimens of the species from the type locality of Tynet Burn. Here we designate NHMUK PV OR.35786 (Figure 3A), the best preserved of the specimens described by Woodward (1891, plate 1, figure 5; Figure 3B), as the neotype, as it exhibits the diagnostic characters for the species. Strangely, Woodward (1891) did not mention the prepelvic spines of this specimen, despite one of the spines being clearly identifiable (Figure 3A, C). Perhaps this was related to the dispute he had with Ramsay Traquair about the appropriateness of erecting the new genus, given that these spines were a defining character for Mesacanthus.
Type locality. Several exposures either side of the Tynet Burn, Moray, Scotland. British Grid reference NJ 384121. Specifically, the neotype comes from the upper nodule bed, bottom unit (Osteolepis bed) as described by Trewin and Davidson (1999).
 Remarks. Agassiz (1844-5) only stated that his specimens of Acanthodes pusillus came from Gordon Castle rather than the locality of Tynet Burn. Andrews (1982) stated that the specimens could therefore have come from elsewhere. However, we consider this unlikely as the owner of Gordon Castle (Charles Gordon-Lennox, 5th Duke of Richmond, 1791-1860) was also the owner of Tynet Burn, and Egerton (1861b, p. 58) stated that the species came from Tynet Burn.
Remarks. Agassiz (1844-5) only stated that his specimens of Acanthodes pusillus came from Gordon Castle rather than the locality of Tynet Burn. Andrews (1982) stated that the specimens could therefore have come from elsewhere. However, we consider this unlikely as the owner of Gordon Castle (Charles Gordon-Lennox, 5th Duke of Richmond, 1791-1860) was also the owner of Tynet Burn, and Egerton (1861b, p. 58) stated that the species came from Tynet Burn.
Stratigraphic and geographical occurrence. From the Middle Devonian of the Orcadian Basin of Scotland.
Description
General features. Specimens long and slender with a maximum depth to length ratio about 0.2 (Figure 3A, Figure 4A-E). The fish range in length from 15 mm to 60 mm. The smallest individuals are only partially ossified (Figure 4A), with the head being the last region to be mineralised (see discussion). The spine complement comprises paired pectoral, pelvic, and prepelvic spines, a single (posterior) dorsal spine, and an anal spine. All the spines have fin webs except the tiny prepelvic spines. The pelvic fin spines are straight and about half the length of the pectoral fin spines and two-thirds the length of the anal and dorsal fin spines. The dorsal fin spine is slightly curved, and posterior relative to the anal fin spine. Pelvic spines are halfway between the pectoral and anal spines; prepelvic spines are closer to the pelvic fin spines than the pectoral fin spines. The caudal fin has an upper lobe extended into a tapered tip and a short triangular lower lobe. The scapula shaft is relatively slender and about five times the height of the triangular area forming its base; scapula height is always less than the pectoral spine length. The ratio of scapula height to pectoral fin spine length is highly variable, possibly associated with the variable mineralisation of the suprascapular region.
 Head and branchial region, morphology. Dermal cover on the head is quite extensive, comprising large polygonal tesserae, extending down to the nares anteriorly and down to the mouth post-orbitally (Figure 5A-C). The eye is surrounded by about five (the precise number is difficult to judge precisely due to preservation) wide sclerotic plates ornamented with parallel linear grooves running obliquely (Figure 5B, bottom) or concentrically (Figure 5B, top). These grooves terminate against a narrow smooth area along the inner edge (Figure 5E-F). Two displaced sclerotic plates (Figure 5F) were misidentified as a spiracle cover in Burrow et al. (2020a, figure 1g). Behind the eye, the otic capsule is indicated by a grainy infilling (Figure 5C). This is also the case in some Tynet Burn specimens (Figure 5G), but sometimes the infilling is calcitic (Figure 5H). A preopercular canal runs just dorsal to the palatoquadrate (Figure 5H), but no other sensory lines have been identified. The tesserae covering the head (Figure 5A-C) resemble those of Mesacanthus mitchelli and other mesacanthids, being polygonal elements with a flat base, but they form a more extensive cover than in M. mitchelli.
Head and branchial region, morphology. Dermal cover on the head is quite extensive, comprising large polygonal tesserae, extending down to the nares anteriorly and down to the mouth post-orbitally (Figure 5A-C). The eye is surrounded by about five (the precise number is difficult to judge precisely due to preservation) wide sclerotic plates ornamented with parallel linear grooves running obliquely (Figure 5B, bottom) or concentrically (Figure 5B, top). These grooves terminate against a narrow smooth area along the inner edge (Figure 5E-F). Two displaced sclerotic plates (Figure 5F) were misidentified as a spiracle cover in Burrow et al. (2020a, figure 1g). Behind the eye, the otic capsule is indicated by a grainy infilling (Figure 5C). This is also the case in some Tynet Burn specimens (Figure 5G), but sometimes the infilling is calcitic (Figure 5H). A preopercular canal runs just dorsal to the palatoquadrate (Figure 5H), but no other sensory lines have been identified. The tesserae covering the head (Figure 5A-C) resemble those of Mesacanthus mitchelli and other mesacanthids, being polygonal elements with a flat base, but they form a more extensive cover than in M. mitchelli.
The main branchiostegal cover has a clearly defined sub-circular outline and comprises close-set branchiostegal plates (Figure 5I-J) that more or less curve back from the anterior angle, paralleling the dorsal and ventral edges of the cover. The branchiostegal plates extend under the posterior third of the Meckel’s cartilage (Figure 5A). The distribution of these plates is perhaps best seen in the only specimen of Acanthodes peachi figured by Egerton (1861, plate 5, figure 2; Figure 2H-I), preserved with the ventral surface of the head and branchial region exposed. These plates are ornamented with three longitudinal ridges that can be sharp crested (Figure 5I) or more rounded (Figure 5J). Despite examining hundreds of specimens, we have not identified any subsidiary gill covers above or behind the main cover, which appears to extend over all or most of the branchial region.
The palatoquadrate and Meckel’s cartilage are the only mineralised endoskeletal elements that we have identified in the head region. Meckel’s cartilage (Figure 5K) is a single element with a truncated anterior end (Figure 5A-B, K). In no specimen is the mandibular joint well preserved. The palatoquadrate (Figure 5L) is a single element, with a short suborbital region and long curved posterodorsal edge supported by an extra-palatoquadrate ridge. The palatobasal articulation to the neurocranium consists of a small notch at the anterodorsal corner of the palatoquadrate.
 One of the distinctive characters of the species is the form of the plates surrounding the two pairs of external nares on the anteriormost rostrum, in front of the orbit (Figure 6A). The nasal plates of the left side are usually slightly separated from those of the right side, but close to the midline. The anteriormost nasal openings can be fully enclosed by four or five similar, smooth plates (Figure 6B-C), or alternatively by a robust semicircular plate and smaller plates or tesserae (Figure 6D). These variants do not appear to be related to the size of the fish. Slightly smaller plates surround (at least partially) the posterior nares, which are contiguous with the anterior nares (Figure 6D). At least some of the plates surrounding the anterodorsal nasal opening have denticles on the inner concave edge of the plate (Figure 6D). However, denticles are on the outer convex edge of some of the plates around the posterior nasal opening (Figure 6D-E). Some better preserved and more complete nasal plates (Figure 6F-G) show their complex structure with the shiny ornament layer and its sharply pointed denticles formed of thin overlapping layers.
One of the distinctive characters of the species is the form of the plates surrounding the two pairs of external nares on the anteriormost rostrum, in front of the orbit (Figure 6A). The nasal plates of the left side are usually slightly separated from those of the right side, but close to the midline. The anteriormost nasal openings can be fully enclosed by four or five similar, smooth plates (Figure 6B-C), or alternatively by a robust semicircular plate and smaller plates or tesserae (Figure 6D). These variants do not appear to be related to the size of the fish. Slightly smaller plates surround (at least partially) the posterior nares, which are contiguous with the anterior nares (Figure 6D). At least some of the plates surrounding the anterodorsal nasal opening have denticles on the inner concave edge of the plate (Figure 6D). However, denticles are on the outer convex edge of some of the plates around the posterior nasal opening (Figure 6D-E). Some better preserved and more complete nasal plates (Figure 6F-G) show their complex structure with the shiny ornament layer and its sharply pointed denticles formed of thin overlapping layers.
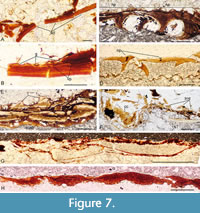 Head and branchial region, histology. Thin sections show that the sclerotic plates are composed of lamellar bone with a thin outer layer forming the parallel ornament strips visible on the surface of the elements. Each strip slightly overlaps the contiguous strip (Figure 7A-B), producing the grooves visible on the surface. Thin sections of the nasal plates show they have internal projections that are presumed to have enclosed canals (Figure 7C-D). At least in some specimens, the bone can be relatively thick and formed of thin lamellae with the paired external plates fused together (Figure 7C). In both the sclerotic and nasal plates, a vascular canal plexus extends through the base of each outer layer, delimiting the ‘ornament’ from the underlying acellular bone layers. No dentine tubules have been identified in the ornament layer, but this is most likely a preservational feature given they are often not preserved in fin spines or scales either (see below). The thin, clear zones in the outer layers forming these elements (Figure 7C), which give the shiny surface appearance in well-preserved material (Figure 6G-H), probably result from diagenetic alteration of dentine rather than being enameloid.
Head and branchial region, histology. Thin sections show that the sclerotic plates are composed of lamellar bone with a thin outer layer forming the parallel ornament strips visible on the surface of the elements. Each strip slightly overlaps the contiguous strip (Figure 7A-B), producing the grooves visible on the surface. Thin sections of the nasal plates show they have internal projections that are presumed to have enclosed canals (Figure 7C-D). At least in some specimens, the bone can be relatively thick and formed of thin lamellae with the paired external plates fused together (Figure 7C). In both the sclerotic and nasal plates, a vascular canal plexus extends through the base of each outer layer, delimiting the ‘ornament’ from the underlying acellular bone layers. No dentine tubules have been identified in the ornament layer, but this is most likely a preservational feature given they are often not preserved in fin spines or scales either (see below). The thin, clear zones in the outer layers forming these elements (Figure 7C), which give the shiny surface appearance in well-preserved material (Figure 6G-H), probably result from diagenetic alteration of dentine rather than being enameloid.
The Meckel’s cartilage is thickened along the lower edge as in M. mitchelli (Burrow et al., 2022), and lacks a separate mandibular bone. Burrow and den Blaauwen (2021, figure 7D-E) noted that the hard tissue forming the outer layer of the jaws is composed of layered blocks of amorphous mineralised tissue, and a thickened ventral edge on the Meckel’s cartilage encasing a globular calcified cartilage core (Figure 7F-G). The tissue forming the jaw surfaces shows no evidence for rings of Liesegang or bone cells and processes, and is not layered. Burrow and den Blaauwen (2021) referred to the structure as tessellated mineralised tesserae (tmt).
The crown of the dermal tesserae on the antero-dorsal part of the head can have relatively high odontodes (Figure 7E), thus differing from those of M. mitchelli, which are relatively flat. Unfortunately, their histological structure was not well preserved in any of the specimens sectioned. Preservation was also poor in sections through the branchiostegal plates (Figure 7H), only showing layering through the plates which had ‘melted’ together.
 Shoulder girdle (Figure 8A, D-E, H, Figure 9A, Figure 10A). The scapulocoracoid has a long shaft that is circular to oval in cross-section and expands ventrally to a short triangular blade. The shaft length is quite variable relative to the length of the pectoral spine, ranging from being about the same length to half the length, seemingly unrelated to stratigraphic level or the size of the fish. Both scapular shaft and ventral blade are fully perichondrally ossified laterally, but the blade is often only partly ossified medially (Figure 8A). The ventral edge of the element curves around the proximal end of the pectoral fin spine (Figure 8D-E). The spines must have been firmly attached to the scapulocoracoids by ligaments or tendons as well as articulating in the embayment of the scapulocoracoid blade (Figure 9A),
Shoulder girdle (Figure 8A, D-E, H, Figure 9A, Figure 10A). The scapulocoracoid has a long shaft that is circular to oval in cross-section and expands ventrally to a short triangular blade. The shaft length is quite variable relative to the length of the pectoral spine, ranging from being about the same length to half the length, seemingly unrelated to stratigraphic level or the size of the fish. Both scapular shaft and ventral blade are fully perichondrally ossified laterally, but the blade is often only partly ossified medially (Figure 8A). The ventral edge of the element curves around the proximal end of the pectoral fin spine (Figure 8D-E). The spines must have been firmly attached to the scapulocoracoids by ligaments or tendons as well as articulating in the embayment of the scapulocoracoid blade (Figure 9A), as they are commonly preserved in connection both in articulated and disarticulated specimens, sometimes even as isolated complexes with no other remains of the fish. As a result, this region and the head are often skewed so that both right and left pectoral girdles and spines are on the exposed surface. The scapulocoracoid is composed of lamellar perichondral bone surrounding a hollow core presumed to have been filled with uncalcified cartilage (Figure 7E, Figure 9A, Figure 10A), with the bone layers added centripetally.
as they are commonly preserved in connection both in articulated and disarticulated specimens, sometimes even as isolated complexes with no other remains of the fish. As a result, this region and the head are often skewed so that both right and left pectoral girdles and spines are on the exposed surface. The scapulocoracoid is composed of lamellar perichondral bone surrounding a hollow core presumed to have been filled with uncalcified cartilage (Figure 7E, Figure 9A, Figure 10A), with the bone layers added centripetally.
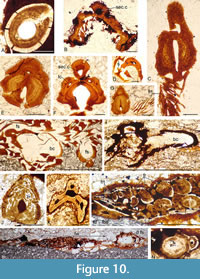 Fin spines (Figure 8, Figure 9, Figure 10B-N, Figure 11, Figure 12). The pectoral, pelvic, dorsal, and anal spines have two or three weakly developed longitudinal ridges on each side (Figure 8C, F-G, I). In cross-section, these ridges are usually symmetrical and sharp-crested. There is no groove separating the leading edge ridge and the ‘shoulders’ of the spine, just a concave slope or, rarely, an indentation. The pectoral spines are slightly asymmetric in cross-section, with a subtriangular shape. The leading edge ridge is about a third of the total width of the spine. The dorsal spines are more laterally compressed than the anal spines, with sub-parallel sides, whereas the main body of the anal spine has a more rounded cross-section
Fin spines (Figure 8, Figure 9, Figure 10B-N, Figure 11, Figure 12). The pectoral, pelvic, dorsal, and anal spines have two or three weakly developed longitudinal ridges on each side (Figure 8C, F-G, I). In cross-section, these ridges are usually symmetrical and sharp-crested. There is no groove separating the leading edge ridge and the ‘shoulders’ of the spine, just a concave slope or, rarely, an indentation. The pectoral spines are slightly asymmetric in cross-section, with a subtriangular shape. The leading edge ridge is about a third of the total width of the spine. The dorsal spines are more laterally compressed than the anal spines, with sub-parallel sides, whereas the main body of the anal spine has a more rounded cross-section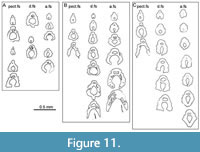 (Figure 10D-F, Figure 11, Figure 12A-B). Both of these median spine forms are deeper than wide, and the rounded leading edge ridge is about half the maximum width of the spine. The central pulp cavity in anal spines is open at the proximal end of the spines but is enclosed for most of its length (Figure 11C, Figure 12B); in dorsal spines the cavity is marginally open along most of the length (Figure 11B-C, Figure 12A-B); and in the pectoral spines the pulp cavity is open for about the proximal 20% of its length (Figure 11A). In spines of young fish, the pulp cavity is relatively wide with no inner lamellae developed (Figure 9). In older fish this main pulp cavity is gradually reduced by addition of inner layers of dentine, with dentine tubules extending through the layers
(Figure 10D-F, Figure 11, Figure 12A-B). Both of these median spine forms are deeper than wide, and the rounded leading edge ridge is about half the maximum width of the spine. The central pulp cavity in anal spines is open at the proximal end of the spines but is enclosed for most of its length (Figure 11C, Figure 12B); in dorsal spines the cavity is marginally open along most of the length (Figure 11B-C, Figure 12A-B); and in the pectoral spines the pulp cavity is open for about the proximal 20% of its length (Figure 11A). In spines of young fish, the pulp cavity is relatively wide with no inner lamellae developed (Figure 9). In older fish this main pulp cavity is gradually reduced by addition of inner layers of dentine, with dentine tubules extending through the layers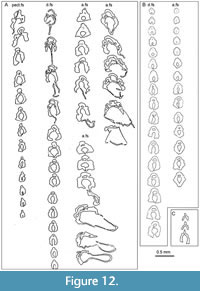 (Figure 10F-G, J). The cavity is about a third the total width of the spine proximally, with the inner lamellar layers reducing its relative width towards the distal end. All spines have a secondary longitudinal canal above the main pulp cavity, in the base of the leading edge ridge (Figure 10B-K, N), and extending the length of the spine. Smaller calibre longitudinal canals are developed in each ‘shoulder’ at the proximal end of the spine, but these only extend about two-thirds its length. The main pulp cavity on the tiny (< 2 mm long) prepelvic spines (Figure 9B-C, Figure 12C) is open for most of their length, with a very small calibre secondary canal in the base of the relatively high, narrow leading edge ridge. Both dorsal and anal spines have a large, hollow, thin-walled basal plate (here infilled, probably by calcite) extending viscerally from the open base of the inserted end of the spines (Figure 10H-I, Figure 12A). Presumably the plate was filled with uncalcified cartilage in life.
(Figure 10F-G, J). The cavity is about a third the total width of the spine proximally, with the inner lamellar layers reducing its relative width towards the distal end. All spines have a secondary longitudinal canal above the main pulp cavity, in the base of the leading edge ridge (Figure 10B-K, N), and extending the length of the spine. Smaller calibre longitudinal canals are developed in each ‘shoulder’ at the proximal end of the spine, but these only extend about two-thirds its length. The main pulp cavity on the tiny (< 2 mm long) prepelvic spines (Figure 9B-C, Figure 12C) is open for most of their length, with a very small calibre secondary canal in the base of the relatively high, narrow leading edge ridge. Both dorsal and anal spines have a large, hollow, thin-walled basal plate (here infilled, probably by calcite) extending viscerally from the open base of the inserted end of the spines (Figure 10H-I, Figure 12A). Presumably the plate was filled with uncalcified cartilage in life.
In rare cases, ossified fin rays are preserved in the dorsal fin (Figure 8G). These rays are straight rods about 0.5 mm wide, extending back for about half the length of the fin from the spine insertion, and possibly segmented distally (seg?, Figure 8G). The rays are circular to oval in cross-section and are possibly formed by the fusion of two separate rays, as larger rays appear to show a median fusion and some smaller rays appear to be partly fused; growth lines parallel the outer surface (Figure 10L, M). They more or less form two layers in the fin, and are overlain by very thin, short overlapping elements that might be composed of dentine rather than bone. However, no tubules are visible so their composition cannot be determined, and we are thus uncertain as to whether these are dermal or endoskeletal structures. These short fin elements, unlike the larger fin rays, are typically preserved in all the fins (Figure 10C, G, L-M).
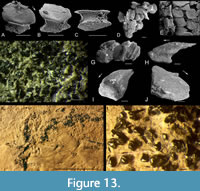 Scales. Squamation and detached scales from all three faunal zones where Orcadacanthus occurs were examined to determine any differences. Body scales of fish from Cromarty (Figure 13) in the Achanarras Fish Bed Member horizon were c. 0.2 mm wide with a slightly convex base, marked base-neck rim and deep neck (Figure 13A-E, G). The smooth crown overhangs the base all round and has a sub-hexagonal outline. The anterior edge is slightly downturned, with its two halves meeting at an angle c. 160º at the rounded median corner. The side edges are subparallel to each other, diverging from the anterior edges at c. 130º, and the posterior edges converge at the posterior corner at c. 80º. The crown surface has a low median depression just behind the anterior edge. A scattered specimen from Cromarty (Figure 13F) also yielded this type of scale, plus much larger scales (Figure 13H-J), probably from near the fin spine bases. These scales are over 0.5 mm long, with a crown that extends up to three base lengths behind the posterior corner of the base. The neck and slightly convex base are both low.
Scales. Squamation and detached scales from all three faunal zones where Orcadacanthus occurs were examined to determine any differences. Body scales of fish from Cromarty (Figure 13) in the Achanarras Fish Bed Member horizon were c. 0.2 mm wide with a slightly convex base, marked base-neck rim and deep neck (Figure 13A-E, G). The smooth crown overhangs the base all round and has a sub-hexagonal outline. The anterior edge is slightly downturned, with its two halves meeting at an angle c. 160º at the rounded median corner. The side edges are subparallel to each other, diverging from the anterior edges at c. 130º, and the posterior edges converge at the posterior corner at c. 80º. The crown surface has a low median depression just behind the anterior edge. A scattered specimen from Cromarty (Figure 13F) also yielded this type of scale, plus much larger scales (Figure 13H-J), probably from near the fin spine bases. These scales are over 0.5 mm long, with a crown that extends up to three base lengths behind the posterior corner of the base. The neck and slightly convex base are both low.
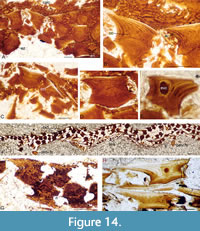 We have much more information on the internal structure of scales from all three levels, as the histological preservation is better than that of the squamation surfaces. Scales from Cromarty (Figure 14A-F) and Achanarras (Figure 14G) show a very similar structure to those of Mesacanthus mitchelli, differing mainly in having more crown growth zones, with up to seven observed. The embryonic zone has a variably developed central pulp cavity, being totally filled in most scales (Figure 14A-D). Some scales, however, retain a wide cavity, with the base formed of upwardly convex lamellae presumed to have progressively filled in the pulp canal that formerly opened out through the base (Figure 14E). Ascending canals rise up in each crown growth zone from wider circular canals (Figure 14B). A hypermineralised enameloid layer overlies a thin dentine layer in the upper horizontal area of the outer three or four growth zones (Figure 14A-D). As each hypermineralised layer merges into the underlying dentine layer rather than being clearly separated from it, the tissue is regarded as enameloid rather than enamel. Fin web scales are smaller than the body scales, usually with flatter bases and an elongate crown; short, thin fin rays appear to have formed within the web (Figure 14F). Rare scales have a pair of small protruding nodes posteriorly, low on the neck (Figure 14G). Body scales on the specimen from Corbie Den (Figure 14H) appear to have a flatter base and deeper neck.
We have much more information on the internal structure of scales from all three levels, as the histological preservation is better than that of the squamation surfaces. Scales from Cromarty (Figure 14A-F) and Achanarras (Figure 14G) show a very similar structure to those of Mesacanthus mitchelli, differing mainly in having more crown growth zones, with up to seven observed. The embryonic zone has a variably developed central pulp cavity, being totally filled in most scales (Figure 14A-D). Some scales, however, retain a wide cavity, with the base formed of upwardly convex lamellae presumed to have progressively filled in the pulp canal that formerly opened out through the base (Figure 14E). Ascending canals rise up in each crown growth zone from wider circular canals (Figure 14B). A hypermineralised enameloid layer overlies a thin dentine layer in the upper horizontal area of the outer three or four growth zones (Figure 14A-D). As each hypermineralised layer merges into the underlying dentine layer rather than being clearly separated from it, the tissue is regarded as enameloid rather than enamel. Fin web scales are smaller than the body scales, usually with flatter bases and an elongate crown; short, thin fin rays appear to have formed within the web (Figure 14F). Rare scales have a pair of small protruding nodes posteriorly, low on the neck (Figure 14G). Body scales on the specimen from Corbie Den (Figure 14H) appear to have a flatter base and deeper neck.
 Scale surface detail is very rarely preserved in squamation on specimens from the lower part of the Mey Flagstone Formation (Figure 15). The scales are tightly packed and overlapping (Figure 15C-D). Scales from this level mostly retain a pulp cavity in the embryonic crown zone (Figure 16A-D, G-I), with
Scale surface detail is very rarely preserved in squamation on specimens from the lower part of the Mey Flagstone Formation (Figure 15). The scales are tightly packed and overlapping (Figure 15C-D). Scales from this level mostly retain a pulp cavity in the embryonic crown zone (Figure 16A-D, G-I), with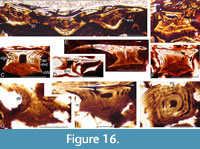 the cavity in small scales often opening out through the base (Figure 16D). Scales often have the dome-shaped lamellae in the base (Figure 16B, F) resulting from the gradual infilling of the open pulp cavity. Flat-based scales often have an elongate posterior crown extending over a base length beyond the posterior edge of the base (Figure 16E). As in the scales from the lower levels, circular canals extend round the neck region (Figure 16I), with smaller calibre ascending canals extending up from them (Figure 16F-I).
the cavity in small scales often opening out through the base (Figure 16D). Scales often have the dome-shaped lamellae in the base (Figure 16B, F) resulting from the gradual infilling of the open pulp cavity. Flat-based scales often have an elongate posterior crown extending over a base length beyond the posterior edge of the base (Figure 16E). As in the scales from the lower levels, circular canals extend round the neck region (Figure 16I), with smaller calibre ascending canals extending up from them (Figure 16F-I).
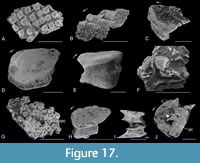 Body scales from the upper part of the Mey Flagstone Formation horizon (Figure 17) are either similar to the most common form from the Achanarras horizon, with a convex base (Figure 17A, E) and subhexagonal crown (Figure 17D, H), or, more commonly, with a deep neck (Figure 17C, F, H-J) and flat base pierced by a large pulp canal (Figure 17C, G, J). Many of the larger scales have a very short base with an elongate crown that extends well beyond the posterior edge of the base (Figure 17B-C, F). Based on our observations of scale morphology, there is no clear distinction between scales from the three horizons, other than an apparent increase in the higher levels of the proportion of flat-based scales with a pulp cavity opening out through the base. In the lowest horizon, this type of structure only seems to occur in scales from the smaller fish, presumably juveniles.
Body scales from the upper part of the Mey Flagstone Formation horizon (Figure 17) are either similar to the most common form from the Achanarras horizon, with a convex base (Figure 17A, E) and subhexagonal crown (Figure 17D, H), or, more commonly, with a deep neck (Figure 17C, F, H-J) and flat base pierced by a large pulp canal (Figure 17C, G, J). Many of the larger scales have a very short base with an elongate crown that extends well beyond the posterior edge of the base (Figure 17B-C, F). Based on our observations of scale morphology, there is no clear distinction between scales from the three horizons, other than an apparent increase in the higher levels of the proportion of flat-based scales with a pulp cavity opening out through the base. In the lowest horizon, this type of structure only seems to occur in scales from the smaller fish, presumably juveniles.
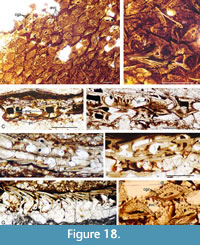 The tendency to retain the embryonic pulp cavity is even more common in the scales of fish from the upper part of the Mey Flagstone Formation horizon (Figure 9, Figure 18A-H). Small juvenile Orcadacanthus pusillus are relatively common at this level, with squamation comprising scales with just a single growth zone and a wide open pulp canal through the flat base (Figure 18E-G). We have not identified any dentine tubules or vascular canals in these scale sections. Larger scales (with the usual complement of vascular canals, dentine tubules in the multiple superposed crown growth zones) also have a relatively flat base, with just a small central convexity (Figure 9, Figure 18B, D, H). The embryonic zone in these scales remains devoid of dentine, with the outer three or four crown growth zones having regularly spaced tubules running back from the circular and ascending canals in the posterior part of the crown (Figure 18H).
The tendency to retain the embryonic pulp cavity is even more common in the scales of fish from the upper part of the Mey Flagstone Formation horizon (Figure 9, Figure 18A-H). Small juvenile Orcadacanthus pusillus are relatively common at this level, with squamation comprising scales with just a single growth zone and a wide open pulp canal through the flat base (Figure 18E-G). We have not identified any dentine tubules or vascular canals in these scale sections. Larger scales (with the usual complement of vascular canals, dentine tubules in the multiple superposed crown growth zones) also have a relatively flat base, with just a small central convexity (Figure 9, Figure 18B, D, H). The embryonic zone in these scales remains devoid of dentine, with the outer three or four crown growth zones having regularly spaced tubules running back from the circular and ascending canals in the posterior part of the crown (Figure 18H).
DISCUSSION
Orcadacanthus pusillus has been placed in the genus Mesacanthus for many years based on the overall similar body proportions to the type species Mesacanthus mitchelli. Other valid species recognised are Mesacanthus grandis Gagnier and Goujet 1997 from the Lochkovian of Spitsbergen, based on one articulated specimen, and Mesacanthus semistriatus Woodward (1892) from the Emsian of New Brunswick, based on one articulated specimen and isolated fin spines; both are poorly known. The main characters used to assign M. grandis to the genus are the presence of a single dorsal spine (an acanthodiform character), the spine being shallowly inserted (interpreted as a mesacanthid character), and smooth crowned scales (a mesacanthid and acanthodid character). Mesacanthus semistriatus was reassigned from Acanthodes to Mesacanthus by Whiteaves (1907), but the main characters recognised for the species - a single dorsal spine and smooth crowned scales - are similarly shared by mesacanthids and acanthodids. Prepelvic spines - the main character used by Traquair (1892) to distinguish the genus from Acanthodes - have not been reported for either of these two species.
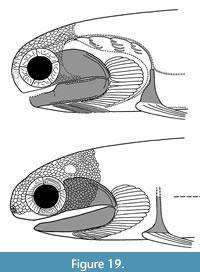 The two Scottish species Mesacanthus mitchelli and Orcadacanthus pusillus do share the character of prepelvic spines (as do other mesacanthids Promesacanthus, Lodeacanthus, and Triazeugacanthus) but are otherwise quite different from one another. Both species are known from many articulated specimens so we feel confident in erecting a new genus for O. pusillus. They differ from each other principally by the shape of the anterior part of the head (Figure 5A-C), with M. mitchelli being more snub-nosed with the eyes closer to the rostrum (Burrow et al., 2022, figure 12) and having a less extensive polygonal tesseral cover on the head. The lower jaw also differs, being narrower at the anterior end in M. mitchelli and truncated in O. pusillus (Figure 5A-B, K). Another difference is the sclerotic plate ornament, comprising low broad vermiculate ridges in M. mitchelli (Burrow et al., 2011, figure 1E; Burrow et al., 2022, figure 4a) and concentric or oblique flat overlapping layers in O. pusillus (Figure 5D-F). The scapular shaft in M. mitchelli is relatively short and broad with a semicircular cross-section, whereas in O. pusillus it is relatively longer and slender with a circular cross-section. Figure 19 shows reconstructions of the head and branchial region of O. pusillus and M. mitchelli. Spines of the two species (other than the prepelvic spines) do not differ greatly, with the main difference being the deeper groove between the leading edge ridge and lateral faces on the spines of M. mitchelli. The pelvic spines of O. pusillus are much smaller than those of M. mitchelli. Body scales of M. mitchelli are all very small, up to 0.1 mm wide with a convex base, and show little variation over the body, whereas those of O. pusillus show more variation. Typically, the crown of O. pusillus scales extends much further past the posterior corner of the base than in M. mitchelli scales. The majority of scales on fish from the higher levels have a flat base, often with a central pulp cavity opening out through the base. These scales thus appear to show extreme paedomorphism, as they are found on fish through the whole size range for the species, not just on the smaller specimens. If the scales with only a single crown growth zone from juvenile O. pusillus (Figure 18E-G) were recovered as isolated scales, they would probably be described as chondrichthyan placoid scales, or possibly even thelodont scales! Dearden et al. (2024, p. 24) noted a similar comparison in relation to the polyodontode scales of Vernicomacanthus and monodontode scales of Lupopsyrus. the two animals are similar in gross anatomy in some respects, but Lupopsyrus is much smaller and its monodontode scales look a lot like the central odontode of scales of Vernicomacanthus.
The two Scottish species Mesacanthus mitchelli and Orcadacanthus pusillus do share the character of prepelvic spines (as do other mesacanthids Promesacanthus, Lodeacanthus, and Triazeugacanthus) but are otherwise quite different from one another. Both species are known from many articulated specimens so we feel confident in erecting a new genus for O. pusillus. They differ from each other principally by the shape of the anterior part of the head (Figure 5A-C), with M. mitchelli being more snub-nosed with the eyes closer to the rostrum (Burrow et al., 2022, figure 12) and having a less extensive polygonal tesseral cover on the head. The lower jaw also differs, being narrower at the anterior end in M. mitchelli and truncated in O. pusillus (Figure 5A-B, K). Another difference is the sclerotic plate ornament, comprising low broad vermiculate ridges in M. mitchelli (Burrow et al., 2011, figure 1E; Burrow et al., 2022, figure 4a) and concentric or oblique flat overlapping layers in O. pusillus (Figure 5D-F). The scapular shaft in M. mitchelli is relatively short and broad with a semicircular cross-section, whereas in O. pusillus it is relatively longer and slender with a circular cross-section. Figure 19 shows reconstructions of the head and branchial region of O. pusillus and M. mitchelli. Spines of the two species (other than the prepelvic spines) do not differ greatly, with the main difference being the deeper groove between the leading edge ridge and lateral faces on the spines of M. mitchelli. The pelvic spines of O. pusillus are much smaller than those of M. mitchelli. Body scales of M. mitchelli are all very small, up to 0.1 mm wide with a convex base, and show little variation over the body, whereas those of O. pusillus show more variation. Typically, the crown of O. pusillus scales extends much further past the posterior corner of the base than in M. mitchelli scales. The majority of scales on fish from the higher levels have a flat base, often with a central pulp cavity opening out through the base. These scales thus appear to show extreme paedomorphism, as they are found on fish through the whole size range for the species, not just on the smaller specimens. If the scales with only a single crown growth zone from juvenile O. pusillus (Figure 18E-G) were recovered as isolated scales, they would probably be described as chondrichthyan placoid scales, or possibly even thelodont scales! Dearden et al. (2024, p. 24) noted a similar comparison in relation to the polyodontode scales of Vernicomacanthus and monodontode scales of Lupopsyrus. the two animals are similar in gross anatomy in some respects, but Lupopsyrus is much smaller and its monodontode scales look a lot like the central odontode of scales of Vernicomacanthus.
The genus with which Orcadacanthus shares most features is probably Triazeugacanthus affinis (Whiteaves, 1887) from the Frasnian Escuminac Formation, Miguasha, Canada. The latter differs in having pectoral, dorsal, and anal spines that are all about the same length, pelvic spines closer to the anal than the pectoral spines, branchiostegal plates that only extend back over half the branchial region, different ornament of irregular small pits on the sclerotic and nasal plates, and most specimens having a large median plate above the anterior nasal openings (Miles, 1966). Miles (1966:185, figure 18) noted that the large nasal plate is basically two semicircular halves fused in the middle, above the nasal openings, with the dorsal margin of the bone being strongly jagged. Gagnier (1996) described fin rays in all the fins on Triazeugacanthus, including the caudal fin, and noted that there are two pairs of nasal openings.
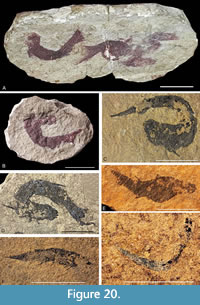
One of the notable aspects of Orcadacanthus pusillus specimens is the variation in preservation (Figure 20). Specimens from the Moray Firth are usually preserved in nodules. Whilst these are mostly solitary specimens, sometimes there can be multiple fish in one nodule (Figure 20A). Elsewhere the specimens are preserved on flagstone, often in large numbers. Egerton (1861, p. 58) erected the junior synonym Mesacanthus peachi for fish from Barragill (East Mey) flagstones, which he considered differed from O. pusillus in being “thicker and less elegant”, having proportionately longer pelvic spines, and dorsal and anal spines opposite each other. However, Baron (2015) demonstrated that any differences in spine and body dimensions within the two species are not statistically significant. When looking at large numbers of articulated specimens, it becomes clear that many specimens have suffered a high degree of rigor mortis, especially when compared to its sister taxon Mesacanthus mitchelli. This is particularly true for the nodule specimens from near the Orcadian Basin margin at Tynet Burn and other localities in the Moray Firth (Figure 20B), where fish beds resulted from rapid flooding at the lake edge by rising waters (Trewin and Davidson, 1999). The specimens here can often be doubled up on themselves. In contrast, fish in the flagstone sequences at Achanarras Quarry (Figure 4A) and other localities in Caithness and Orkney, which formed in the deeper waters in the basin centre, while often being arched back, mostly show less distortion - although there are exceptions (Figure 20C). This highly contorted preservation is also seen in higher strata (Figure 20D) and probably reflects a lack of robustness in the body of O. pusillus and the hypersalinity in the shallower lake edges mentioned by Trewin (1986).
One feature that is only seen in rare specimens from the upper part of the Mey Flagstone Formation horizon is mineralisation of the fin rays in the dorsal, and possibly anal, fins. Fin rays have previously been reported in all the fins on Triazeugacanthus (as noted earlier), unidentified fins on Homalacanthus concinnus (Whiteaves, 1887), the anterior dorsal fin of Diplacanthus crassisimus, the pelvic fin of Cheiracanthus latus Egerton, 1861, and the pectoral fins of Acanthodes (Coates, 1994, figure 3A) and Ischnacanthus gracilis (Egerton, 1861a) (Miles, 1970, figure 8), with the latter specimen showing the rays possibly becoming segmented distally. Presumably fin rays could have been present in the paired and median fins of other acanthodians but were usually unmineralised and so not fossilised. Goodrich (1909) and Watson (1937) called these elements ceratotrichia, the term used for the collagenous rays supporting fin webs in elasmobranchs. Miles (1970:358) referred to the elements as dermotrichia, and (following Jarvik, 1959) considered that they could be “...regarded as modified early generations (ontogenetically) of scale rows that have sunk down into the dermis”. Miles (1970) accepted Jarvik’s (1959) work suggesting that deep fin rays evolved separately in elasmobranchs, dipnoans, and actinopterygians, rejecting the inference from the work by Goodrich (1909) and Watson (1937) that the elements are homologous in acanthodians and chondrichthyans. Our investigations illustrate for the first time the histological structure of acanthodian fin rays. The structure of the cylindrical rays in Orcadacanthus pusillus appears to show that they formed by fusion of pairs of semicylindrical rods, resembling the structure of dorsal fin ‘spines’ in extant teleosts like tilapia and carp (Kalish-Achrai et al., 2017, figure 2), which developed from simpler actinopterygian fin rays. These are dermal structures, formed by the fusion of lepidotrichia around actinotrichia. The rays in O. pusillus are overlain by short, thin, possibly dentinous elements. One possibility is that the latter could represent the outer dentinous crown layer of a scale, and the bony ray is composed of the scale bases, equivalent to deconstructed lepidotrichia, formed around a keratinous or collagenous core as in actinopterygians. Alternatively, the fin rays could be endoskeletal elements derived from fin radials that developed around a cartilaginous core. The histological structure of acanthodian fin radials is unfortunately not known, and we have no information on the inner structure of the fins in stem chondrichthyans that are not included in the Acanthodii sensu Coates et al. (2018). The weight of evidence (e.g., Coates, 1994) seems to support Jarvik’s (1959) conclusions that fin rays in different gnathostome groups - in which we could include acanthodians - developed separately from the form of dermotrichia that occurs in basal placoderm gnathostomes.
The presence of paired nasal plates, and occasionally their external appearance, has previously been reported in many ‘acanthodians’ including Brochoadmones, Cassidiceps, Culmacanthus, Fallodentus, Homalacanthus, Ischnacanthus, Nerepisacanthus, probably Cheiracanthus (Burrow, 2021:14) [AUTHOR - another one ..], and all mesacanthid genera known from articulated fish. Those of Lodeacanthus Upeniece, 1996 are perhaps the best preserved of acanthodians other than Orcadacanthus pusillus, comprising two separate semicircular plates edging the nares on each side, thus indicating that the fish had close-set paired incurrent and excurrent nares (Upeniece, 2011, figures 5.5, 5.18), considered to be a general gnathostome character (e.g., Janvier, 1996:50) that is retained in the Chondrichthyes. Miles (1966:153) stated that only one pair of nares had been recognised in acanthodians, and considered that based on their small size they probably represented the fenestra exonarina anterior (anterior nares), or, less likely, shared openings for both incurrent and excurrent nasal canals. He suggested that the posterior nares “...may have been situated in the orbit as in many early actinopterygians” (Miles, 1966:154), and illustrated such an arrangement in his reconstruction of Homalacanthus concinnus (Miles, 1966, figure 14B). However, Long (1983, figure 5) identified two pairs of nares on the snout of the diplacanthiform Culmacanthus stewarti Long, 1983, and Gagnier (1996, figure 9) showed that Triazeugacanthus also has two pairs, contra Miles (1966) who only identified the anterior set. Our examination shows that Orcadacanthus pusillus, like Culmacanthus, Lodeacanthus, and Triazeugacanthus, has both anterior and posterior nares, positioned close together on the rostrum, supporting the idea that this is a general condition for the Chondrichthyes given that acanthodians are stem chondrichthyans. By comparison with extant sharks (e.g., Timm-Davis and Fish, 2015, figure 5; Rygg et al., 2013, figure 5B), the larger anterior nares would be the excurrent nasal canal openings and the smaller posterior nares the incurrent canal openings, with the latter typically smaller than the former as in Orcadacanthus. Our histological investigations reveal for the first time the inner structure of these plates, in one instance slicing through four canals presumed to represent the excurrent and incurrent nasal passages of each side (Figure 7C). Unlike extant sharks in which the nares of each side are widely separated, in Orcadacanthus and Lodeacanthus the paired nasal plates are contiguous, and sometimes fused together.
Another interesting preservational feature of Orcadacanthus pusillus is evidence of ontogenetic differences. Large and medium-sized specimens have a fully mineralised dermal head cover. Even in individuals only 25 mm long there is extensive dermal ossification over the head region (Figure 20E). However, smaller specimens (Figure 20F) show less ossification, with the smallest specimens discovered (around 15 mm long) lacking mineralised dermal and endoskeletal elements (Figure 20G). This pattern corresponds to that shown for Triazeugacanthus affinis as demonstrated by Chevrinais et al. (2015, 2016, 2017).
CONCLUSIONS
Orcadacanthus pusillus was for a long time poorly known and considered a rather uninteresting and unimportant little fish. This study has discovered many new characteristics that clearly differentiate it from the type species of Mesacanthus, Mesacanthus mitchelli, and warrant the erection of a new genus. Particularly interesting characteristics include the nasal plates, where we show their internal structure for the first time, the histological structure of acanthodian fin rays, and the apparently paedomorphic development of the body scales. The mesacanthid showing closest similarity to Orcadacanthus is Triazeugacanthus from the Frasnian of Miguasha, Canada, which also has very small prepelvic spines, fin spines with c. three longitudinal ridges on each side, a tall slender scapular shaft, and denticulated nasal plates.
ACKNOWLEDGEMENTS
 The authors would like to thank S. Johnston (Aberdeen) for donating to the NMS some of the best-preserved Cromarty specimens, D. Leather (Ilkley) for specimens from Westray, and Professor P. Wignall (University of Leeds) for the making of a thin section from NMS G.2022.10.15. We also thank E. Bernard (NHM UK) for photography of and access to the neotype, N. Clark (GLAHM) for photographing GLAHM V3573, K. Seymour (ROM) for information on the Portsoy Minerals accession, A. Wright (ELGNM) for historical collection details, T. Ziegler (MV) for photographing specimens in the MV collection, F. Witzmann (MB) for information on specimens, and P. Shepherd (GSM), S. Walsh (NMS), L. Werdelin and T. Mörs (NRM) for access to their respective collections. We would also like to thank J. Armstrong (Edinburgh) for searching the Buisonjé collection in the NMS for a missing specimen. We also thank the reviewers R. Dearden (University of Birmingham), P. Andreev (Qujing Normal University), and two anonymous reviews for their insightful comments. Finally, we would like to thank the editor H. Mallison for help in publishing this paper.
The authors would like to thank S. Johnston (Aberdeen) for donating to the NMS some of the best-preserved Cromarty specimens, D. Leather (Ilkley) for specimens from Westray, and Professor P. Wignall (University of Leeds) for the making of a thin section from NMS G.2022.10.15. We also thank E. Bernard (NHM UK) for photography of and access to the neotype, N. Clark (GLAHM) for photographing GLAHM V3573, K. Seymour (ROM) for information on the Portsoy Minerals accession, A. Wright (ELGNM) for historical collection details, T. Ziegler (MV) for photographing specimens in the MV collection, F. Witzmann (MB) for information on specimens, and P. Shepherd (GSM), S. Walsh (NMS), L. Werdelin and T. Mörs (NRM) for access to their respective collections. We would also like to thank J. Armstrong (Edinburgh) for searching the Buisonjé collection in the NMS for a missing specimen. We also thank the reviewers R. Dearden (University of Birmingham), P. Andreev (Qujing Normal University), and two anonymous reviews for their insightful comments. Finally, we would like to thank the editor H. Mallison for help in publishing this paper.
REFERENCES
Agassiz, L. 1844–1845. Monographie de poissons fossils des Vieux Grès Rouges ou Système Dévonien (Old Red Sandstone) des Îles Britanniques et de Russie. Imprimerie de Petitpierre et Prince, Neuchâtel.
Andrews, S.M. 1982. The discovery of fossil fishes in Scotland up to 1845 with checklists of Agassiz's figured specimens. Royal Scottish Museum, Edinburgh.
Barclay, W.J. and Trewin, N.H. 2005. Tynet Burn, Moray (NJ 383 618), p. 107–112. In Barclay, W.J., Browne, M.A.E., McMillan, A.A., Pickett, E.A., Stone, P., and Wilby, P.R. (eds.), The Old Red Sandstone of Great Britain, Geological Conservation Review Series No. 31. Joint Nature Conservation Committee, Peterborough.
Baron, M.G. 2015. An investigation of the genus Mesacanthus (Chordata: Acanthodii) from the Orcadian Basin and Midland Valley areas of northern and central Scotland using traditional morphometrics. PeerJ, 3:e1331. https://doi.org/10.7717/peerj.1331
Baron, M.G. and Seymour, K.L. 2021. Designating a lectotype for Mesacanthus pusillus (Gnathostomata: Acanthodii). Palaeovertebrata, 44:e2. https://doi.org/10.18563/pv.44.1.e2
Berg, L.S. 1937. A classification of fish-like vertebrates. Izvestiya Akademii Nauk USSR, Seriya Biologischeskaya, 4:1277–1280. (In English and Russian)
Burrow, C. 2021. Acanthodii, Stem Chondrichthyes. In Schultze, H.-P. (ed.), Handbook of Paleoichthyology. Vol. 5. Verlag Dr Friedrich Pfeil, München.
Burrow, C.J., Newman, M.J., Davidson, R.G., and den Blaauwen, J.L. 2011. Sclerotic plates or circumorbital bones in early jawed fishes? Palaeontology, 54:207–214. https://doi.org/10.1111/j.1475-4983.2010.01003.x
Burrow, C., den Blaauwen, J., Newman, M., and Davidson, R. 2016. The diplacanthid fishes (Acanthodii, Diplacanthiformes, Diplacanthidae) from the Middle Devonian of Scotland. Palaeontologia Electronica, 19.1.10A:1–83. https://doi.org/10.26879/601
Burrow, C.J., Newman, M.J., and den Blaauwen, J.L. 2020a. First evidence of a functional spiracle in stem chondrichthyan acanthodians, with the oldest known elastic cartilage. Journal of Anatomy, 236:1154–1159. https://doi.org/10.1111/joa.13170
Burrow, C.J., den Blaauwen, J.L., and Newman, M.J. 2020b. A redescription of the three longest-known species of the acanthodian Cheiracanthus from the Middle Devonian of Scotland. Palaeontologia Electronica, 23(1):a15. https://doi.org/10.26879/1035
Burrow, C.J., den Blaauwen, J.L., and Newman, M.J. 2022. New information on the Early Devonian acanthodian Mesacanthus mitchelli from the Midland Valley of Scotland. Scottish Journal of Geology, 58:1–17. https://doi.org/10.1144/sjg2021-004
Chevrinais, M., Cloutier, R., and Sire J-Y. 2015 The revival of a so-called rotten fish: the ontogeny of the Devonian acanthodian Triazeugacanthus. Biology Letters, 11:20140950. https://doi.org/10.1098/rsbl.2014.0950
Chevrinais, M., Balan, E., and Cloutier, R. 2016. New insights in the ontogeny and taphonomy of the Devonian acanthodian Triazeugacanthus affinis from the Miguasha Fossil-Lagerstätte, eastern Canada. Minerals, 6:1–17. https://doi.org/10.3390/min6010001
Chevrinais, M., Sire J.-Y., and Cloutier, R. 2017. From body scale ontogeny to species ontogeny: Histological and morphological assessment of the Late Devonian acanthodian Triazeugacanthus affinis from Miguasha, Canada. PLoS ONE 12:e0174655.
https://doi.org/10.1371/journal.pone.0174655
Coates, M.I. 1994. The origin of vertebrate limbs. Development, 1994 (Supplement):169–180. https://doi.org/10.1242/dev.1994.Supplement.169
Coates, M.I., Finarelli, J.A., Sansom, I.J., Andreev, P.S., Criswell, K.E., Tietjen, K., Rivers, M.L., and La Riviere, P.J. 2018. An early chondrichthyan and the evolutionary assembly of a shark body plan. Proceedings of the Royal Society B: Biological Sciences, 285:20172418. https://doi.org/10.1098/rspb.2017.2418
Crampton, C.B. and Carruthers, R.G. with contributions from Horne, J., Peach, B.N., Flett, J.S., and Anderson, E.M. 1914. The Geology of Caithness (Sheets 110, 116 with parts of 109, 115 and 117). Memoirs of the Geological Survey, Scotland, HMSO, Edinburgh.
Dearden, R.P., Blaauwen, J.L.d., Sansom, I.J., Burrow, C.J., Davidson, R., Newman, M.J., Ko, A., and Brazeau, M.D. 2021. A revision of Vernicomacanthus Miles with comments on the characters of stem-group chondrichthyans. Papers in Palaeontology, 7:1949–1976. https://doi.org/10.1002/spp2.1369
den Blaauwen, J.L., Newman, M.J., and Burrow, C.J. 2019. A new cheiracanthid acanthodian from the Middle Devonian (Givetian) Orcadian Basin of Scotland and its biostratigraphic and biogeographical significance. Scottish Journal of Geology, 55:166–177.
https://doi.org/10.1144/sjg2018-023
Denison, R.H. 1979. Acanthodii. In series: Schultze, H.-P. (ed.), Handbook of Paleoichthyology 5. Gustav Fischer Verlag, Stuttgart and New York.
Dineley, D.L. 1999. Mid-Devonian fossil fish sites of Scotland, p. 167–222. In Dineley, D.L. and Metcalf, S.J. (eds.), Fossil Fishes of Great Britain. Joint Nature Conservation Committee Peterborough, UK.
Donovan, R.N. 1980. Lacustrine cycles, fish ecology and stratigraphic zonation in the Middle Devonian of Caithness. Scottish Journal of Geology, 16:35–50. https://doi.org/10.1144/sjg16010035
Donovan, R.N., Foster, R.J., and Westoll, T.S. 1974. A stratigraphical revision of the Old Red Sandstone of north-eastern Caithness. Transaction of the Royal Society of Edinburgh, 69:167–201. https://doi.org/10.1017/S0080456800015118
Duff, P. 1842. Sketch of the Geology of Moray. Forsyth and Young, Elgin.
Egerton, P. de M.G. 1861a. British fossils. Memoirs of the Geological Survey of the United Kingdom (British Organic Remains), Decade 10:51–75.
Egerton, P. de M.G. 1861b. Remarks on the ichthyolites of Farnell Road. Report of the Thirtieth Meeting of the British Association for the Advancement of Science, held at Oxford in June and July 1860, p. 77–78.
Gagnier, P.-Y. 1996. Acanthodii, p. 149–164. In Schultze, H.-P. and Cloutier, R. (eds.), Devonian Fishes and Plants of Miguasha, Quebec, Canada. Pfeil, München.
Gagnier, P.-Y. and Goujet, D. 1997. Nouveaux poissons acanthodiens du Dévonien du Spitsberg. Geodiversitas, 19:505–513.
Geikie, A. 1878. On the Old Red Sandstone of Western Europe. Transactions of the Royal Society of Edinburgh, 28:345–452. https://doi.org/10.1017/S0080456800090360
Goodrich, E.S. 1909. A Treatise on Zoology. Part 9, Vertebrata Craniata (First fascicle: Cyclostomes and fishes). Adam and Charles Black, London.
Gordon, G. 1859. On the geology of the lower or northern part of the province of Moray: its history, present state of inquiry, and points for future examination. Edinburgh New Philosophical Journal, 9:14–59.
Gregory, W. 1860. Notes on fossil fish localities of the Old Red Sandstone of the east of Scotland. The Geologist, 3:142–148.
Hanke, G.F. 2008. Promesacanthus eppleri n. gen., n. sp., a mesacanthid (Acanthodii, Acanthodiformes) from the Lower Devonian of Northern Canada. Geodiversitas, 30:287–302.
Huxley, T.H. 1880. On the application of the laws of evolution to the arrangement of the Vertebrata and more particularly the Mammalia. Proceedings of the Zoological Society of London, 43:649–662.
Janvier, P. 1996. Early Vertebrates. Oxford University Press, Oxford.
Jarvik, E. 1959. Dermal fin-rays and Holmgren's principle of delamination. Kungliga Svenska Vetenskapsakademiens Handlingar, 6:1–51.
Kalish-Achrai, N., Monsonego-Ornan, E., and Shahar, R. 2017. Structure, composition, mechanics and growth of spines of the dorsal fin of blue tilapia Oreochromis aureus and common carp Cyprinus carpio. Journal of Fish Biology, 90:2073–2096.
https://doi.org/10.1111/jfb.13287
Long, J.A. 1983. A new diplacanthoid acanthodian from the Late Devonian of Victoria. Memoirs of the Association of Australasian Palaeontologists, 1:51–65.
Miles, R.S. 1966. The acanthodian fishes of the Devonian Plattenkalk of the Paffrath Trough in the Rhineland. Arkiv för Zoologi, 18:147–194.
Miles, R.S. 1970. Remarks on the vertebral column and caudal fin of acanthodian fishes. Lethaia, 3:343–362. https://doi.org/10.1111/j.1502-3931.1970.tb00828.x
Miller, H. 1858. Cruise of the Betsey. Thomas Constable & Co., Edinburgh.
Moy-Thomas, J.A. 1939. Palaeozoic Fishes. Methuen and Co. Ltd, London.
Mykura, W. 1976. British Regional Geology. Orkney and Shetland. HMSO, Edinburgh.
Newman, M.J. 2010. Middle Devonian fish from the Orcadian Basin of Scotland, International Palaeontological Congress 4 pre-conference field trip guide, 90pp.
Newman, M.J. and Dean, M.T. 2005. A biostratigraphical framework for geological correlation of the Middle Devonian strata in the Moray-Ness Basin Project area. British Geological Survey Internal Report, IR/05/160.
Newman, M.J., den Blaauwen, J.L., and Tuuling, T. 2017. Middle Devonian coccosteid (Arthrodira, Placodermi) biostratigraphy of Scotland and Estonia. Scottish Journal of Geology, 53:63–69. https://doi.org/10.1144/sjg-2016-012
Novitskaya, L.J. and Obruchev, D.V. 1967. Class Acanthodei; p. 263–291. In Obruchev, D.V. (ed.), (1964 Russian edition), Fundamentals of Palaeontology 11. Israel Program for Scientific Translation, Jerusalem. (English translation from Russian)
Owen, R. 1846. Lectures on the Comparative Anatomy and Physiology of the Vertebrate Animals Delivered at the Royal College of Surgeons, England in 1844 and 1846. Part I, Fishes. Longman, Brown, Green and Longmans, London.
Pander, C.H. 1860. Über die Saurodipterinen, Dendrodonien, Glyptolepiden und Cheirolepiden des Devonischen Systems. Buchdruckerei der Kaiserlichen Akademie der Wissenschaften, St. Petersburg.
Paton R.L. 1976. A catalogue of fossil vertebrates in the Royal Scottish Museum, Part 5, Acanthodii. Royal Scottish Museums, Information Series, Geology, 6:1–40.
Peach, C.W. 1868. On fossil fishes of the Old Red Sandstone and Sutherland with notices of some new to those counties. Report of the British Association for the Advancement of Science, meeting 37, p.72.
Peacock, J.D., Berridge, N.G., Harris, A.L., and May, F. 1968. The Geology of the Elgin District. Memoirs of the Geological Survey, Scotland, HMSO, Edinburgh.
Phemister, J. 1948. Scotland: The Northern Highlands, 2nd edition. British Regional Geology, HMSO, Edinburgh.
Plax, D.P. and Newman, M.J. 2021. Middle Devonian acanthodians from Belarus - new data and interregional biostratigraphy. Acta Geologica Polonica, 71:393–414. https://doi.org/10.24425/agp.2020.134568
Read, H.H. 1948. The Grampian Highlands, 2nd edition revised by Macgregor, A.G. British Regional Geology, HMSO, Edinburgh.
Rygg, A.D., Cox, J.P.L., Abel, R., Webb, A.G., Smith, N.B., and Craven, B.A. 2013. A computational study of the hydrodynamics in the nasal region of a hammerhead shark (Sphyrna tudes): implications for olfaction. PLoS ONE, 8:e59783. https://doi.org/10.1371/journal.pone.0059783
Taylor, M.A. and Anderson, L.I. 2015. Additional information on Charles W. Peach (1800-1886). The Geological Curator, 10:159–180.
Timm-Davis, L.L. and Fish, F.E. 2015. Flow through the nasal cavity of the spiny dogfish, Squalus acanthias. The European Physical Journal Special Topics, 224:3407–3417. https://doi.org/10.1140/epjst/e2015-50037-1
Traquair, R.H. 1888. Notes on the nomenclature of the fishes of the Old Red Sandstone of Great Britain. The Geological Magazine, Decade III, 5:507–517. https://doi.org/10.1017/S0016756800182809
Traquair, R.H. 1890. On the fossil fishes found at Achanarras Quarry, Caithness. Annals and Magazine of Natural History, 6th series, 9:479–486. https://doi.org/10.1080/00222939008694071
Traquair, R.H. 1894. Achanarras revisited. Proceedings of the Royal Physical Society of Edinburgh, 12:279–286.
Traquair, R.H. 1895. The extinct animals of the Moray Firth area, p. 235–285. In Harvie Brown, J.A. and Buckley, T.E. (eds.), A Vertebrate Fauna of the Moray Basin. Volume 2. David Douglas, Edinburgh.
Trewin, N.H. 1986. Palaeoecology and sedimentology of the Achanarras fish bed of the Middle Old Red Sandstone, Scotland. Transactions of the Royal Society of Edinburgh, 77:21–46. https://doi.org/10.1017/S0263593300010737
Trewin, N.H. and Davidson, R.G. 1999. Lake-level changes, sedimentation and faunas in a Middle Devonian basin-margin fish bed. Journal of the Geological Society, 156:535–548. https://doi.org/10.1144/gsjgs.156.3.0535
Upeniece, I. 1996. Lodeacanthus gaugicus n. g. et sp. (Acanthodii: Mesacanthidae) from the Late Devonian of Latvia. Modern Geology, 20:383–398.
Upeniece, I. 2011. Palaeoecology and juvenile individuals of the Devonian placoderm and acanthodian fishes from Lode site, Latvia. Doctoral thesis, Geology Department, University of Latvia, Riga, Latvia.
Wallace, T.D. 1906. The sandstones of the Moray Firth area. Transactions of the Inverness Scientific Society and Field Club, 6 (1899–1906):119–132.
Waterston, C.D. 1965. Old Red Sandstone, p. 269–303. In Craig, G.Y. (ed.), The Geology of Scotland. Oliver & Boyd, Edinburgh.
Watson, D.M.S. 1937. The acanthodian fishes. Philosophical Transactions of the Royal Society (B) 228:49–146. https://doi.org/10.1098/rstb.1937.0009
Whiteaves, J.F. 1887. Illustrations of the fossil fishes of the Devonian rocks of Canada. Proceedings and Transactions of the Royal Society of Canada, 4(4):101–110.
Whiteaves, J.F. 1907. Illustrations of the fossil fishes of the Devonian rocks of Canada. Part III: Supplementary notes. Transactions of the Royal Society of Canada, 3:245–274.
Wilson, G.V. 1935. The geology of the islands as a group, p. 11–43. In Wilson, G.V., Edwards, W., Knox, J., Jones, R.C.B., and Stephens, J.V. (eds.), The Geology of the Orkneys. Memoirs of the Geological Survey, Scotland, HMSO, Edinburgh.
Woodward, A.S. 1891. Catalogue of the fossil fishes in the British Museum (Natural History). Part II. British Museum (Natural History), London.
Woodward, A.S. 1892. On the Lower Devonian fish-fauna of Campbellton, New Brunswick. The Geological Magazine, new series, Decade III, 9:1–6. https://doi.org/10.1017/S0016756800188533
Woodward A.S. and Sherborn C.D. 1890. A Catalogue of British Fossil Vertebrata. Dulau, London.
Young, V.T. 1997. Early Palaeozoic acanthodians in the collection of the Natural History Museum, London. Ichthyolith Issues Special Publication, 3:46–50.
Zhu, Y.-A., Li, Q., Lu, J., Chen, Y., Wang, J., Gai, Z., Zhao, W., Wei, G., Yu, Y., Ahlberg, P. E., and Zhu, M. 2022. The oldest complete jawed vertebrates from the early Silurian of China. Nature, 609:954–958. https://doi.org/10.1038/s41586-022-05136-8

