 Histology of spinosaurid dinosaur teeth from the Albian-Cenomanian of Morocco: Implications for tooth replacement and ecology
Histology of spinosaurid dinosaur teeth from the Albian-Cenomanian of Morocco: Implications for tooth replacement and ecology
Article number: 23(3):a48
https://doi.org/10.26879/1041
Copyright Palaeontological Association, October 2020
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 25 October 2019. Acceptance: 14 September 2020.
ABSTRACT
High numbers of spinosaurid teeth found in Morocco suggest that this clade was very abundant during the "Mid-"Cretaceous in northern Africa. Several reasons have been proposed to account for this abundance of spinosaur teeth, from sampling biases to ecology. However, the number of teeth in the fossil record also depends strongly on the tooth replacement rate. So far, little is known about the tooth formation time and replacement rates in spinosaurids. Here, we analysed the histology of several spinosaur teeth to estimate tooth formation time and replacement rates, using the count of lines of von Ebner, the daily formed incremental lines in the dentine of the teeth. Line counts indicated a maximum tooth formation time of 271 days, and replacement rates were predicted to be between 59 and 68 days. These rates are faster than in other large theropods for which data are known, and, together with the rather high number of teeth in a spinosaurid dentition, might thus help to explain the abundance of spinosaur teeth in the "Mid-"Cretaceous of northern Africa.
Nicola S. Heckeberg. Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstraße 43, D-10115 Berlin, Germany; Staatliche Naturwissenschaftliche Sammlungen Bayerns (SNSB), Bayerische Staatssammlung für Paläontologie und Geologie, Richard-Wagner-Str. 10, D-80333 München, Germany; Department für Geo- und Umweltwissenschaften, Ludwig-Maximilians-Universität, Richard-Wagner-Str. 10, D-80333 München, Germany. nicola.heckeberg@mfn.berlin
Oliver W. M. Rauhut. Staatliche Naturwissenschaftliche Sammlungen Bayerns (SNSB), Bayerische Staatssammlung für Paläontologie und Geologie, Richard-Wagner-Str. 10, D-80333 München, Germany; Department für Geo- und Umweltwissenschaften, Ludwig-Maximilians-Universität, Richard-Wagner-Str. 10, D-80333 München, Germany; GeoBioCenter, Ludwig-Maximilians-Universität, Richard-Wagner-Str. 10, D-80333 München, Germany. rauhut@snsb.de
Keywords: theropod teeth; tooth formation times; tooth replacement rates; Spinosauridae; histology
Final citation: Heckeberg, Nicola S. and Rauhut, Oliver W. M. 2020. Histology of spinosaurid dinosaur teeth from the Albian-Cenomanian of Morocco: Implications for tooth replacement and ecology. Palaeontologia Electronica, 23(3):a48. https://doi.org/10.26879/1041
palaeo-electronica.org/content/2020/3170-histology-of-spinosaurid-teeth
Copyright: October 2020 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Spinosaurid theropod dinosaurs included some of the largest predators during the Cretaceous, with an almost worldwide distribution (Hone and Holtz, 2017). Currently, several species are recognised across the two subfamilies Spinosaurinae and Baryonychinae (Hendrickx et al., 2016; Candeiro et al., 2017; Malafaia et al., 2019). Their fossil remains are common in Lower (Barremian) to early Upper (Cenomanian) Cretaceous sediments of Africa (Algeria, Egypt, Niger, Sudan, Tunisia), Asia (China, Laos, Thailand), Australia, Europe (England, Portugal, Spain), and South America (Brazil) (Bertin, 2010; Candeiro et al., 2017; Hone and Holtz, 2017).
The most diverse occurrences of spinosaurid remains are from northern Gondwana and European localities (Bertin, 2010; Candeiro et al., 2017). Although their phylogenetic relationships indicate that spinosaurids must have been around since at least the Middle Jurassic (e.g., Carrano et al., 2012; Rauhut et al., 2016), fossil remains referred to the clade prior to the Cretaceous are sparse and debated (see Buffetaut, 2008, 2011; Rauhut, 2011; Allain et al., 2012; Vullo et al., 2014; Hendrickx et al., 2019). Pre-Barremian Cretaceous occurrences are mainly based on isolated teeth (e.g., Sales et al., 2017), and the group might have disappeared soon after the Cenomanian; younger reports are fragmentary based on isolated teeth (e.g., Candeiro et al., 2004; Hone et al., 2010) and more material would be needed to corroborate their survival after the Cenomanian.
Spinosaurids are characterised by a highly specialised anatomy, which was originally described on the basis of a partial skeleton of the name-giving genus Spinosaurus from the Cenomanian of Egypt by Stromer (1915). The dorsal and sacral vertebrae have elongated neural spines, likely the support for a large back sail, the function of which (e.g., display, thermoregulation, swimming) has been extensively discussed (Stromer, 1915; Bailey, 1997; Holtz, 1998; Ibrahim et al., 2014; Gimsa et al., 2016; Candeiro et al., 2017). The cranium is long and low with a lateral compression and a long and narrow snout (Dal Sasso et al., 2005; Rayfield et al., 2007). Recent morphofunctional analysis of the mandibular-quadrate articulation demonstrated specialised jaw mechanics in these animals (Hendrickx et al., 2016). Spinosaurus had 15-16 teeth in each side of the lower jaw (Stromer, 1915), and the spinosaurine Irritator had at least 11 and probably not more than 13 maxillary teeth (Sues et al., 2002). Likewise, a spinosaurine snout from the Cenomnian of Morocco has six premaxillary and 12 maxillary teeth (Dal Sasso et al., 2005). Baryonychines have higher tooth counts, with 32 teeth in each mandibular ramus of Baryonyx (Charig and Milner, 1997), and for Suchomimus seven premaxillary and 22 maxillary teeth were reported (Sereno et al., 1998).
Teeth of spinosaurids are relatively easy to identify, since they are less labio-lingually compressed and often less curved than in other theropods. Furthermore, spinosaurines have no serrations on the mesial and distal carinae and the denticles are reduced in size in baryonychines. Weak longitudinal ridges (flutes) and a typical microstructural ornamentation are present in many taxa (Kellner and Mader, 1997; Rayfield et al., 2007; Hasegawa, 2010; Hendrickx et al., 2019). Although large crocodyliforms with similar tooth sizes are known from several localities in which spinosaurs occur also (e.g., De Broin and Taquet, 1966; Sereno et al., 1999; De Broin, 2002), their teeth differ from those of spinosaurs in being relatively more robust, lacking flutes, and often having a distinct medial curvature (see Sereno et al., 1999; de Broin, 2002). The diet of spinosaurids was most likely partially piscivorous and focused on aquatic prey, but also included pterosaurs and small-bodied ornithischians (Hone and Holtz, 2017).
Especially in Barremian to Cenomanian localities in northern Africa and Europe, spinosaur teeth are found in large quantities, which has been attributed to a number of possible reasons, from sampling bias (McGowan and Dyke, 2009) to unusual ecological conditions (Läng et al., 2013). Tooth development can provide valuable insights into the feeding behaviour and palaeoecology of extinct organisms (Fiorillo and Currie, 1994; Brink et al., 2015; D’Emic et al., 2019). It is well known that reptiles have a constant tooth replacement that varies interspecifically (Erickson, 1996a). So far, no studies investigating the tooth formation and replacement rates in spinosaurs have been undertaken.
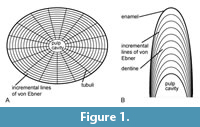 The tooth formation rate can be estimated by counting the growth lines in the dentine using histological thin sections of the teeth. Previous studies on dentine histology in extinct and extant reptiles (e.g., dinosaurs, alligators) showed that the dentine is built daily and that one incremental line of von Ebner equals one day (Erickson, 1996a, 1996b) (Figure 1). Two successive teeth of the same tooth position are necessary in order to calculate the replacement rate by subtracting the number of growth lines of the successor teeth from the number of growth lines of the active tooth (Erickson, 1996a, 1996b). However, this is of course rarely preserved in the fossil record, and only few studies have so far used von Ebner lines to estimate tooth formation and replacement rates in dinosaurs (e.g., Erickson, 1996a; Sereno et al., 2007; D'Emic et al., 2013; García and Zurriaguz, 2016; Button et al., 2017; D’Emic et al., 2019).
The tooth formation rate can be estimated by counting the growth lines in the dentine using histological thin sections of the teeth. Previous studies on dentine histology in extinct and extant reptiles (e.g., dinosaurs, alligators) showed that the dentine is built daily and that one incremental line of von Ebner equals one day (Erickson, 1996a, 1996b) (Figure 1). Two successive teeth of the same tooth position are necessary in order to calculate the replacement rate by subtracting the number of growth lines of the successor teeth from the number of growth lines of the active tooth (Erickson, 1996a, 1996b). However, this is of course rarely preserved in the fossil record, and only few studies have so far used von Ebner lines to estimate tooth formation and replacement rates in dinosaurs (e.g., Erickson, 1996a; Sereno et al., 2007; D'Emic et al., 2013; García and Zurriaguz, 2016; Button et al., 2017; D’Emic et al., 2019).
Here, we counted the tooth formation time and, based on this, estimated the replacement rates of spinosaurids for the first time, compared them to those of other archosaurs and interpreted their palaeoecological implications.
MATERIAL AND METHODS
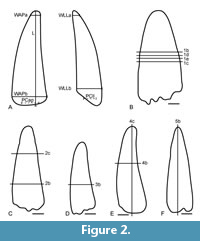 Five spinosaurid teeth from Albian to Cenomanian sediments of Morocco (the Kem Kem compound assemblage; Cavin et al., 2010) were used for preparing histological slides; as these specimens were purchased from local dealers, no exact locality information is available. The material is kept at the Bayerische Staatssammlung für Paläontologie und Geologie under the collection ID SNSB-BSPG 2008 XXXVII 1-5 (Figure 2). The resorbed roots of the teeth show that the individuals were alive when the teeth were shed. The tooth terminology follows Hendrickx et al. (2015). Naturally, not all growth lines are visible in cross sections (Figure 1). Similar to a stack of paper cups, which are piled on top of each other, a cross section would not show all cups, but a longitudinal section would do so (Hillson, 2005). A higher number of thin sections would be necessary to give a reasonable correlation between cross and longitudinal sections. The measurements of the teeth were taken with a digital calliper with a precision of 0.05 mm and are given in Table 1; the positions of the measurements and of the thin sections are shown in Figure 3.
Five spinosaurid teeth from Albian to Cenomanian sediments of Morocco (the Kem Kem compound assemblage; Cavin et al., 2010) were used for preparing histological slides; as these specimens were purchased from local dealers, no exact locality information is available. The material is kept at the Bayerische Staatssammlung für Paläontologie und Geologie under the collection ID SNSB-BSPG 2008 XXXVII 1-5 (Figure 2). The resorbed roots of the teeth show that the individuals were alive when the teeth were shed. The tooth terminology follows Hendrickx et al. (2015). Naturally, not all growth lines are visible in cross sections (Figure 1). Similar to a stack of paper cups, which are piled on top of each other, a cross section would not show all cups, but a longitudinal section would do so (Hillson, 2005). A higher number of thin sections would be necessary to give a reasonable correlation between cross and longitudinal sections. The measurements of the teeth were taken with a digital calliper with a precision of 0.05 mm and are given in Table 1; the positions of the measurements and of the thin sections are shown in Figure 3.
 Given the brittle nature of the material, preparation of the thin sections was particularly difficult and a strict protocol was followed. First, the teeth were dried and then soaked with epoxy resin. One side of the crown was then ground with carbon silicide on a dish grinding wheel to the level, where the thin section should be. Some teeth had to be cut before grinding, for example, when several thin sections of the same tooth were made. The ground side was glued onto an object slide, then cut to a thickness of 300 µm and was hardened again. Next, the teeth were glued onto another object slide, and the first object slide was ground. With an automatic lap and polish machine the thin sections were ground to a final thickness of 130 µm. After cleaning the thin section with acetone, a cover slip was glued onto it.
Given the brittle nature of the material, preparation of the thin sections was particularly difficult and a strict protocol was followed. First, the teeth were dried and then soaked with epoxy resin. One side of the crown was then ground with carbon silicide on a dish grinding wheel to the level, where the thin section should be. Some teeth had to be cut before grinding, for example, when several thin sections of the same tooth were made. The ground side was glued onto an object slide, then cut to a thickness of 300 µm and was hardened again. Next, the teeth were glued onto another object slide, and the first object slide was ground. With an automatic lap and polish machine the thin sections were ground to a final thickness of 130 µm. After cleaning the thin section with acetone, a cover slip was glued onto it.
The thin sections were investigated under a light microscope with an 8-80 times magnification; where necessary, crossed polarisers were used. Photographs of the thin sections were taken with a Leica DFC 480 digital camera for microscopes. The growth lines were counted three times. For the interpretation, the maximum number of lines was used, because this represents the minimum tooth formation time.
We plotted the tooth formation rate against body mass and the tooth replacement rate against the tooth formation rate. Further, we performed a linear regression on the replacement vs. formation rate plots in order to predict the tooth replacement rate of the spinosaurine based on the maximum tooth formation rate. The body mass for the spinosaurid was taken from Henderson (2018), all other data were taken from Erickson (1996a), D’Emic et al. (2013), Schwarz et al. (2015), and D’Emic et al. (2019) (Table 2). The regression was done on the total data set and on a data set including only theropods (Table 2, Appendix 1). The software R was used for all analyses (R Core Team, 2020).
RESULTS
Description of the Teeth
As noted above, the teeth described here differ from those of large crocodyliforms known from the "Mid"-Cretaceous of Africa in being more slender, showing well-developed longitudinal striations, and lacking a marked medial curvature. The five teeth correspond to the spinosaurid tooth morphotypes MT1a and b described by Richter et al. (2013) from the Kem Kem assemblage. Within spinosaurids, they can confidently be referred to a spinosaurine spinosaurid based on the more conical and less recurved aspect of the crown and the absence of denticles (Stromer, 1915; Hendrickx et al., 2019). In contrast, in baryonichine spinosaurids weakly developed serrations are present (e.g., Charig and Milner, 1997). All five teeth are relatively well preserved, with only some wear or damage at the apices (so that the crown heights given are minimal estimates), and the enamel is mostly present (Table 1, Figure 2). Of course, nothing can be said about the ontogenetic stage of the animal the teeth came from on the basis of isolated teeth; however, as the size of these teeth is larger than that of the teeth of Baryonyx and several teeth of Suchomimus measured by Smith et al. (2005), the elements should represent at least subadult individuals.
The teeth are conidont and labiolingually flattened to different degrees. The cross section of the teeth is subcircular to elliptical. The variation in crown morphology is likely due to different positions of the teeth in the tooth row. The apices are rounded and show wear, partially in the form of spalled surfaces as described in Schubert and Ungar (2005).
Tooth SNSB-BSPG 2008 XXXVII 1a is the widest of the investigated teeth. It has a straight crown and is labiolingually flattened. The flutes on the labial and lingual sides are well-developed and there are weakly developed, unserrated carinae on the mesial and distal side (Figure 2A).
Tooth SNSB-BSPG 2008 XXXVII 2 is slender, conical, mesiolingually curved and there are few, weakly developed flutes on the labial and lingual side (Figure 2B).
Tooth SNSB-BSPG 2008 XXXVII 3 is the smallest specimen; it is conical with a rounded apex that is worn more on the distal side than on the mesial side, which probably is a wear trace from the antagonist of the tooth, potentially a spalled surface. The tooth is mesiolingually curved, there are weak flutes on the labial and lingual side, and there are weakly developed, unserrated carinae on the mesial and distal side (Figure 2C).
Tooth SNSB-BSPG 2008 XXXVII 4 is slender and conical. The tooth is mesiolingually curved, there are weak flutes on the labial and lingual side, and unserrated carinae on the mesial and distal side. The distal carina is better developed and more prominent than the mesial one (Figure 2D).
Tooth SNSB-BSPG 2008 XXXVII 5 is the highest and has the most acute tip, with little signs of wear. The tooth is slender and slightly labiolingually flattened, with a weak lingual curvature. There are weak flutes and both mesial and distal carinae are unserrated and well-developed (Figure 2E).
Thin Sections
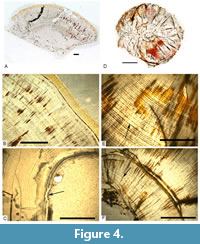 Most of the thin sections show fissures and red secondary mineralisation (Figure 4, Figure 5). While tubuli could rarely be seen, lines of von Ebner were clearly visible in all thin sections. The lines of von Ebner varied in thickness and distance to each other.
Most of the thin sections show fissures and red secondary mineralisation (Figure 4, Figure 5). While tubuli could rarely be seen, lines of von Ebner were clearly visible in all thin sections. The lines of von Ebner varied in thickness and distance to each other.
In the cross section SNSB-BSPG 2008 XXXVII 1b and 1e the growth lines are very thin and compact, especially closer to the pulp cavity (Figure 4A, 4B). We counted no more than 271 growth lines for this tooth (Table 3).
Thick growth lines sometimes alternating with thinner ones are visible in the cross sections 2008 XXXVII 2b and 2c (Figure 4D-F). The growth lines around the pulp cavity are bent towards the cavity (Figure 4F). We counted no more than 260 growth lines for this tooth (Table 3).
 Similarly, thicker and thinner growth lines, sometimes widely separated from each other, are present in the cross section 2008 XXXVII 3b. The density of the lines increases towards the pulp cavity. At one place of the pulp cavity, the first few growth lines and pulp cavity wall are discontinued and appear to be re-mineralised (Figure 4C). We counted no more than 143 growth lines for this tooth (Table 3).
Similarly, thicker and thinner growth lines, sometimes widely separated from each other, are present in the cross section 2008 XXXVII 3b. The density of the lines increases towards the pulp cavity. At one place of the pulp cavity, the first few growth lines and pulp cavity wall are discontinued and appear to be re-mineralised (Figure 4C). We counted no more than 143 growth lines for this tooth (Table 3).
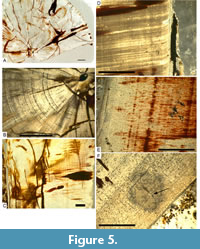
In the cross section 2008 XXXVII 4b there are thicker and thinner growth lines; the thicker growth lines are closer to the pulp cavity (Figure 5A, 5B). In the longitudinal section 2008 XXXVII 4c the growth lines are less clearly visible close to the pulp cavity (Figure 5C). We counted no more than 210 growth lines for this tooth (Table 3).
 In the longitudinal section 2008 XXXVII 5b the growth lines are clear close to the pulp cavity and fade slightly towards the enamel (Figure 5D-F). Near the pulp cavity, there is a small cuneiform splint. The growth lines show a slight diversion around the splint (Figure 5F). In Figure 5D the curvature of the dentine towards the apex of the tooth is visible at the enamel-dentine contact. We counted no more than 217 growth lines for this tooth (Table 3).
In the longitudinal section 2008 XXXVII 5b the growth lines are clear close to the pulp cavity and fade slightly towards the enamel (Figure 5D-F). Near the pulp cavity, there is a small cuneiform splint. The growth lines show a slight diversion around the splint (Figure 5F). In Figure 5D the curvature of the dentine towards the apex of the tooth is visible at the enamel-dentine contact. We counted no more than 217 growth lines for this tooth (Table 3).
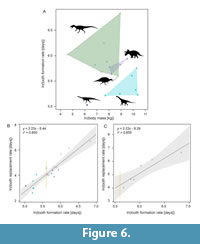
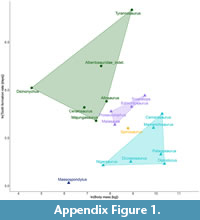
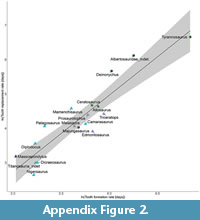
In Figure 6A the tooth formation rate vs. body mass plot is shown. Without the spinosaurine data point there would be no overlap between theropods and ornithischians (Appendix Figure 1). The linear regression on the total and the theropod data set were similar, resulting in a similar regression equation (Figure 6B-C, Appendix 1). Based on these equations the tooth replacement rate for the spinosaurine teeth was predicted to range between 59 and 68 days. The spinosaurine data point was plotted on the regression line with the standard deviation as error bars. Due to the small size of the data set, these error bars are comparatively long (Figure 6B-C, Appendix Figure 1-Appendix Figure 2).
DISCUSSION
Observations on the Thin Sections
 Investigating the thin sections under a light microscope, we discovered some interesting phenomena. The variations in the distance between the growth lines (Figure 4C) and in the thickness of the growth lines (Figure 4E) were potentially caused by irregularities in the biorhythm of the individual (e.g., incubation) or are of metabolic origin (G. Erickson pers. comm., 2008). The varying thickness of the growth lines could be linked with the quantity of food available and/or the content of minerals in the food. For example, if more calcium or phosphate was ingested, a thicker incremental line could have been deposited (Erickson, 1996a, 1996b). Environmental influences, like weather or volcanic activity, and traumatic events like diseases or starvation are potential causes for variations in depositing incremental lines. Concerning the variation in the distances between lines, the latter possibilities are more probable (G. Erickson pers. comm., 2008).
Investigating the thin sections under a light microscope, we discovered some interesting phenomena. The variations in the distance between the growth lines (Figure 4C) and in the thickness of the growth lines (Figure 4E) were potentially caused by irregularities in the biorhythm of the individual (e.g., incubation) or are of metabolic origin (G. Erickson pers. comm., 2008). The varying thickness of the growth lines could be linked with the quantity of food available and/or the content of minerals in the food. For example, if more calcium or phosphate was ingested, a thicker incremental line could have been deposited (Erickson, 1996a, 1996b). Environmental influences, like weather or volcanic activity, and traumatic events like diseases or starvation are potential causes for variations in depositing incremental lines. Concerning the variation in the distances between lines, the latter possibilities are more probable (G. Erickson pers. comm., 2008).
The more densely deposited lines of von Ebner around the pulp cavity (Figure 4C) can be explained by the younger age of the inner growth lines. Since the incremented lines of dentine are deposited on the internal side of the tooth, the older a tooth becomes the less space is left to deposit the dentine (Erickson, 1996a, 1996b).
 The mineralisation at the pulp cavity in section SNSB-BSPG 2008 XXXVII 3b (Figure 4C) may have been caused by a pathology in the root of the tooth and a successive healing before shedding the tooth. Alternatively, it could be a diagenetic re-mineralisation after shedding.
The mineralisation at the pulp cavity in section SNSB-BSPG 2008 XXXVII 3b (Figure 4C) may have been caused by a pathology in the root of the tooth and a successive healing before shedding the tooth. Alternatively, it could be a diagenetic re-mineralisation after shedding.
Curvature of the growth lines was observed at the enamel-dentine contact (Figure 4B), close to the pulp cavity (Figure 4F) as well as curvature of the dentine tubuli towards the crown apex (Figure 5D). The reasons for these curvatures likely include the formation of the longitudinal enamel ridges on the external surface of the tooth, defective growth, and possible ‘adhesion effects’ of the tubuli at the enamel-dentine boundary.
The cuneiform particle in section SNSB-BSPG 2008 XXXVII 5b possibly intruded the dentine during its development, since the growth lines seem to be affected by this particle (Figure 5F).
It is unlikely that seasonal fluctuations are observable in the incremental lines, contra Johnston (1979), who interpreted alternating line bundles in a tyrannosaur tooth as winter and summer depositions of eight years. Since it has been shown that the incremental lines represent daily growth lines and the formation time for an adult tyrannosaur is around 933 days (Erickson, 1996a), one tooth includes about 2.5 years (based on the estimation that one Cretaceous year comprised 370-373 days; Runcorn, 1968).
Temperature can be an influencing factor during tooth development, however, seasonality is not likely reflected in the incremental lines. The climate in the "Mid-"Cretaceous was not characterised by strong seasonality with cold winters and hot summers (Runcorn, 1968). Small scale variations of the temperature are likely to have had an influence on the development of the growth lines (Erickson, 1996a).
Tooth Formation Times Compared with Other Archosaurs
The differences in the number of growth lines within one tooth is most likely due to the different levels of the thin sections (Figure 3) and differing visibility of the lines. It was not possible to correlate different sections of the same tooth with each other. The growth lines are probably not continuous in thickness and distances from each other throughout the tooth. The maximum number of growth lines in these spinosaurid teeth was 271 (Table 2, Table 3) and represents the minimum tooth formation time, because not all lines of the tooth were preserved within one slide. Based on the linear regression on the tooth formation rates of other archosaurs, the tooth replacement rate for the spinosaur teeth was estimated to range from 59 days (total data set) to 68 days (theropod data set). These formation and replacement rates are most similar to those of Majungasaurus (D’Emic et al., 2019). Teeth with different volumes, i.e., broad vs narrow, probably have slightly different formation and replacement rates (slower for broader teeth, faster for narrower teeth; D’Emic et al., 2013).
Comparison of the body mass and tooth formation rates shows that the spinosaur teeth examined here plot between the ornithischian and the sauropod polygon (Figure 6A, Appendix Figure 1). It should be noted that the body mass for the spinosaur taken from Henderson (2018) probably represents slightly larger individuals than those investigated here; therefore, the estimations here, although providing some orientation, should be interpreted with caution. The tooth formation times of theropods with a similar body mass, e.g., Tyrannosaurus with 933 days (Erickson, 1996a), are considerably greater than those of spinosaurs. The smaller Ceratosaurus and Allosaurus still have replacement rates that are almost twice of those estimated here, while the only theropod, for which comparably fast replacement rates were estimated, is the abelisaurid Majungasaurus (D'Emic et al., 2019). Maiasaura and Mamenchisaurus also have comparable tooth formation rates (Table 2), however, they were herbivorous. The short formation times and even shorter replacement intervals in ornithischians and sauropods (e.g., D'Emic et al., 2013) may be explained by their predominantly herbivorous diet, the associated wear of the teeth, and the formation of dental batteries. Comparing tooth formation rates and tooth replacement rates of other theropods (Table 2, Figure 6B-C), tooth replacement rates in spinosaurids have been considerably faster than in most other taxa for which data are available.
Predatory Adaptations
Previous studies have demonstrated convergence of spinosaurs with other piscivorous animals (Rayfield et al., 2007; Rayfield, 2011); therefore, the short tooth formation time might be linked with the diet of spinosaurs. Apart from a presumed large proportion of fish, spinosaurs also included small reptiles and pterosaurs in their diet (Charig and Milner, 1986, 1997; Buffetaut et al., 2004; Holtz et al., 2004; Dyke, 2010; Hendrickx et al., 2016). Scavenging has previously been discussed as a possible mode of diet, but apart from occasionally including carrion (like other predators), a specialisation on scavenging has been ruled out (Hone and Holtz, 2017).
The elongated and laterally compressed cranial morphology of spinosaurs resembles crocodilians with slender snouts. Biomechanical analyses of the Indian gharial (Gavialis gangeticus) and the spinosaur Baryonyx walkeri showed convergence in bending resistance and torsional feeding loads, which supports piscivory (Rayfield et al., 2007; Rayfield, 2011). A recent study compared the craniodental features shared by spinosaurs and conger eels (Conger; Vullo et al., 2016); they suggested that characteristic jaw morphology is an adaptation to biting and catching quickly moving aquatic prey. Further, they assumed that integumentary mechanoreceptors were involved in prey detection in spinosaurs as they are in extant pike congers (Muraenesocidae; Vullo et al., 2016). A recent study that investigated the neuroanatomy of Irritator challengeri, more evidence for adaptations for piscivory was found (Schade et al., 2020). However, many of these features would generally be advantageous for a specialization on small, elusive prey that is being secured with the jaws before swallowing, as argued by Rauhut (2001a). Evidence from stomach contents, i.e., fish scales (e.g., Lepidotes) and remains of a juvenile iguanodontid in Baryonyx (Charig and Milner, 1997), and a spinosaur tooth in the vertebra of a pterosaur (Buffetaut et al., 2004) support the hypothesis that spinosaur diets mainly included small and elusive prey.
Isotopic data suggested a semi-aquatic lifestyle for spinosaurs (Amiot et al., 2010), although some spinosaur teeth were found with a similar isotopic signal as other terrestrial theropods. Ibrahim et al. (2014; 2020) suggested anatomical features, such as the retraction of the fleshy nostrils, modifications in the pelvic girdle and hind limbs, and a propulsive tail to also support a semi-aquatic life style and associated piscivory, although their reconstruction of Spinosaurus as a semi-aquatic animal has been criticized on anatomical (Evers et al., 2015) and biomechanical reasons (Henderson, 2018). Similarly, Gimsa et al. (2016) put forward a new hydrodynamic hypothesis concerning the function of the back sail of Spinosaurus, but this hypothesis was also made rather unlikely by the biomechanical considerations of Henderson (2018). Thus, a semi-aquatic lifestyle might be possible, but potentially was not obligatory for survival (see discussion in Hone and Holtz, 2017).
However, a specialization on small and elusive prey that had to be caught and secured in the jaws might help to explain the fast replacement rates of spinosaurs compared to other theropods. Securing live and struggling prey in the jaws would exert rather large forces on individual teeth, increasing the danger of losing teeth during feeding. Apart from the relatively high tooth count (at least in baryonichines), more robust, conical shape of the teeth and the extremely deep implantation in the jaws (e.g., Charig and Milner, 1997; Rauhut, 2001a), fast replacement rates would be of advantage to keep the number of functional teeth high.
Palaeoecology
The palaeoecology of the Kem Kem Beds (Morocco) has been difficult to interpret, one of the best known and most diverse "Mid-"Cretaceous fossil occurrences in northern Africa (Cavin et al., 2010). There is an overabundance of theropod remains, especially in the form of countless numbers of spinosaur teeth, compared to herbivorous dinosaur remains and other theropod teeth (Russel, 1996; Läng et al., 2013; Benyoucef et al., 2015). This unbalanced phenomenon may be explained by a special ecosystem unlike any present today, collection bias, taphonomic factors such as time-averaging, and stratigraphic uncertainties (McGowan and Dyke, 2009; Dyke, 2010; Läng et al., 2013; Benyoucef et al., 2015). Unknown or undiscovered behavioural aspects of the dinosaur groups may also play a role (Läng et al., 2013).
An overabundance of theropod teeth in comparison to those of herbivorous dinosaurs is also seen in other localities, and might, at least partially, be due to taphonomic factors, i.e., that herbivore teeth might be more prone to destruction due to the extensive wear suffered by most of these teeth prior to replacement (Rauhut, 2001b). The estimated fast replacement rates might furthermore be another factor to explain the overabundance of spinosaur teeth when compared to other theropods.
CONCLUSIONS
Investigations of the incremental lines in spinosaurine teeth showed relatively short tooth formation times, and the replacement rates were estimated to have been relatively fast compared to similarly large theropod dinosaurs, i.e., tyrannosaurs, but also most other theropod taxa for which data are available (see D’Emic et al., 2019) These special teeth match the overall specialised anatomy of spinosaurs well. The short formation times and predicted high replacement rates support the hypotheses of an adaptation towards small and elusive prey, such as fish.
For future studies, CT-scanning maxillary or dentary fragments with teeth that were in use at the time of death and replacement teeth in situ would be necessary in order to more reliably calculate the replacement rates of spinosaur teeth.
ACKNOWLEDGEMENTS
We thank C. Helbig for the difficult preparation of the fragile teeth and thin sections and G. Janßen for photographs of the complete teeth. We are grateful to G.M. Erickson for helpful discussions on the topic. The teeth were acquired with financial help of the Freunde der Bayerischen Staatssammlung für Paläontologie und Geologie. We thank C. Hendrickx and one anonymous reviewer for their constructive comments on the manuscript.
REFERENCES
Allain, R., Xaisanavong, T., Richir, P., and Khentavong, B. 2012. The first definitive Asian spinosaurid (Dinosauria: Theropoda) from the Early Cretaceous of Laos. Naturwissenschaften, 99:369-377. https://doi.org/10.1007/s00114-012-0911-7
Amiot, R., Buffetaut, E., Lécuyer, C., Wang, X., Boudad, L., Ding, Z., Fourel, F., Hutt, S., Martineau, F., Medeiros, M.A., and Mo, J. 2010. Oxygen isotope evidence for semi-aquatic habits among spinosaurid theropods. Geology, 38(2):139-142. https://doi.org/10.1130/g30402.1
Bailey, J.B. 1997. Neural spine elongation in dinosaurs: Sailbacks or buffalo-backs? Journal of Paleontology, 71:1124-1146. https://doi.org/10.1017/s0022336000036076
Benyoucef, M., Läng, E., Cavin, L., Mebarki, K., Adaci, M., and Bensalah, M. 2015. Overabundance of piscivorous dinosaurs (Theropoda: Spinosauridae) in the mid-Cretaceous of North Africa: The Algerian dilemma. Cretaceous Research, 55:44-55. https://doi.org/10.1016/j.cretres.2015.02.002
Bertin, T. 2010. A catalogue of material and review of the Spinosauridae. PalArch’s Journal of Vertebrate Palaeontology, 7(4):1-39.
Brink, K.S., Reisz, R.R., LeBlanc, A.R.H., Chang, R.S., Lee, Y.C., Chiang, C.C., Huang, T., and Evans, D.C. 2015. Developmental and evolutionary novelty in the serrated teeth of theropod dinosaurs. Scientific Reports, 5:12338. https://doi.org/10.1038/srep12338
Buffetaut, E. 2008. Spinosaurid teeth from the Late Jurassic of Tendaguru, Tanzania, with remarks on the evolutionary and biogeographical history of the Spinosauridae, p. 26-28. In Mazin, J.M., Pouech, J., Hantzpergue, P., and Lacombe, V. (eds.), Mid-Mesozoic Life and Environments. UFR des Sciences de la Terre, Université Claude-Bernard-Lyon, Lyon.
Buffetaut, E. 2011. An early spinosaurid dinosaur from the Late Jurassic of Tendaguru (Tanzania) and the evolution of the spinosaurid dentition. Oryctos, 10:1-8.
Buffetaut, E., Martill, D., and Escuillie, F. 2004. Pterosaurs as part of a spinosaur diet. Nature, 430:33. https://doi.org/10.1038/430033a
Button, K., You, H., Kirkland, J.I., and Zanno, L.E. 2017. Incremental growth of therizinosaurian dental tissues: implications for dietary transitions in Theropoda. PeerJ, 5:e4129. https://doi.org/10.7717/peerj.4129
Candeiro, C.R.A., Abranches, C.T., Abrantes, E.A., Avilla, L.S., Martins, V.C., Moreira, A.L., Torres, S.R., and Bergqvist, L.P. 2004. Dinosaurs remains from western São Paulo state, Brazil (Bauru Basin, Adamantina Formation, Upper Cretaceous). Journal of South American Earth Sciences, 18:1-10. https://doi.org/10.1016/j.jsames.2004.08.004
Candeiro, C.R.A., Brusatte, S.L., and de Souza, A.L. 2017. Spinosaurid dinosaurs from the Early Cretaceous of North Africa and Europe: fossil record, biogeography and extinction. Anuário do Instituto de Geociências - UFRJ, 40(3):294-302. https://doi.org/10.11137/2017_3_294_302
Carrano, M.T., Benson, R.B.J., and Sampson, S.D. 2012. The phylogeny of Tetanurae (Dinosauria: Theropoda). Journal of Systematic Palaeontology, 10:211-300. https://doi.org/10.1080/14772019.2011.630927
Cavin, L., Tong, H., Boudad, L., Meister, C., Piuz, A., Tabouelle, J., Aarab, M., Amiot, R., Buffetaut, E., Dyke, G.J., Hua, S., and Le Loeuff, J. 2010. Vertebrate assemblages from the early Late Cretaceous of southeastern Morocco: An overview. Journal of African Earth Sciences, 57:391-412. https://doi.org/10.1016/j.jafrearsci.2009.12.007
Charig, A.J. and Milner, A.C. 1986. Baryonyx, a remarkable new theropod dinosaur. Nature, 324:359-361. https://doi.org/10.1038/324359a0
Charig, A.J. and Milner, A.C. 1997. Baryonyx walkeri, a fish-eating dinosaur from the Wealden of Surrey. Bulletin of the Natural History Museum, Geology Series, 53:11-70.
Dal Sasso, C., Maganuco, S., Buffetaut, E., and Mendez, M.A. 2005. New information on the skull of the enigmatic theropod Spinosaurus, with remarks on its size and affinities. Journal of Vertebrate Paleontology, 25(4):888-896. https://doi.org/10.1671/0272-4634(2005)025[0888:niotso]2.0.co;2
De Broin, F. 2002. Elosuchus, a new genus of crocodile from the Lower Cretaceous of the North of Africa. Comptes Rendus Palevol, 1:275-285.
De Broin, F. and Taquet, P. 1966. Découverte d'un Crocodilien nouveau dans le Crétacé inférieur du Sahara. Comptes Rendus de l’Academie des Sciences de Paris D, 262:2326-2329.
D’Emic, M.D., O’Connor, P.M., Pascucci, T.R., Gavras, J.N., Mardakhayava, E., and Lund, E.K. 2019. Evolution of high tooth replacement rates in theropod dinosaurs. PLOS ONE, 14:e0224734. https://doi.org/10.1371/journal.pone.0224734
D’Emic, M.D., Whitlock, J.A., Smith, K.M., Fisher, D.C., and Wilson, J.A. 2013. Evolution of high tooth replacement rates in sauropod dinosaurs. PLoS ONE, 8(7):e69235. https://doi.org/10.1371/journal.pone.0069235
Dyke, G.J. 2010. Palaeoecology: different dinosaur ecologies in deep time? Current Biology, 20(22):R983-R985. https://doi.org/10.1016/j.cub.2010.10.001
Erickson, G.M. 1996a. Incremental lines of von Ebner in dinosaurs and the assessment of tooth replacement rates using growth line counts. Proceedings of the National Academic Sciences, 93:14623-14627. https://doi.org/10.1073/pnas.93.25.14623
Erickson, G.M. 1996b. Daily deposition of dentine in juvenile Alligator and assessment of tooth replacement rates using incremental line counts. Journal of Morphology, 228:189-194. https://doi.org/10.1002/(sici)1097-4687(199605)228:2<189::aid-jmor7>3.0.co;2-0
Evers, S.W., Rauhut, O.W.M., Milner, A.C., McFeeters, B., and Allain, R. 2015. A reappraisal of the morphology and systematic position of the theropod dinosaur Sigilmassasaurus from the “middle” Cretaceous of Morocco. PeerJ, 3:e1323. https://doi.org/10.7717/peerj.1323
Fiorillo, A.R. and Currie, P.J. 1994. Theropod teeth from the Judith River Formation (Upper Cretaceous) of south-central Montana. Journal of Vertebrate Paleontology, 14(1):74-80. https://doi.org/10.1080/02724634.1994.10011539
García, R.A. and Zurriaguz, V. 2016. Histology of teeth and tooth attachment in titanosaurs (Dinosauria; Sauropoda). Cretaceous Research, 57:248-256. https://doi.org/10.1016/j.cretres.2015.09.006
Gimsa, J., Sleigh, R., and Gimsa, U. 2016. The riddle of Spinosaurus aegytiacus’ dorsal sail. Geological Magazine, 153:544-547. https://doi.org/10.1017/s0016756815000801
Hasegawa, Y., Tanaka, G., Takakuwa, Y., and Koike, S. 2010. Fine sculptures on a tooth of Spinosaurus (Dinosauria, Theropoda) from Morocco. Bulletin of Gunma Museum of Natural History, 14:11-20.
Henderson, D.M. 2018. A buoyancy, balance and stability challenge to the hypothesis of a semi-aquatic Spinosaurus Stromer, 1915 (Dinosauria: Theropoda). PeerJ, 6:e5409. https://doi.org/10.7717/peerj.5409
Hendrickx, C., Mateus, O., and Araújo, R. 2015. A proposed terminology of theropod teeth (Dinosauria, Saurischia). Journal of Vertebrate Paleontology, 35:e982797. https://doi.org/10.1080/02724634.2015.982797
Hendrickx, C., Mateus, O., and Buffetaut, E. 2016. Morphofunctional analysis of the quadrate of Spinosauridae (Dinosauria: Theropoda) and the presence of Spinosaurus and a second spinosaurine taxon in the Cenomanian of North Africa. PLoS ONE, 11(1):e0144695. https://doi.org/10.1371/journal.pone.0144695
Hendrickx, C., Mateus, O., Araújo, R., and Choiniere, J. 2019. The distribution of dental features in non-avian theropod dinosaurs: Taxonomic potential, degree of homoplasy, and major evolutionary trends. Palaeontologia Electronica, 22.3.74:1-110. https://doi.org/10.26879/820
Hillson, S. 2005. Teeth. Cambridge University Press, Cambridge.
Holtz, T.R.J. 1998. Spinosaurs as crocodile mimics. Science, 282:1276-1277. https://doi.org/10.1126/science.282.5392.1276
Holtz, T.R.J., Molnar, R.E., and Currie, P.J. 2004. Basal Tetanurae, p. 71-110. In Weishampel, D.B., Dodson, P., and Osmólska, H. (eds.), The Dinosauria. University of California Press, Berkeley, California. https://doi.org/10.1525/california/9780520242098.003.0006
Hone, D.W.E. and Holtz, T.R. 2017. A century of spinosaurs - a review and revision of the Spinosauridae with comments on their ecology. Acta Geological Sinica, 91:1120-1132. https://doi.org/10.1111/1755-6724.13328
Hone, D.W.E., Xu, X., and Wang, D. 2010. A probable baryonychine (Theropoda: Spinosauridae) tooth from the Upper Cretaceous of Henan Province, China. Vertebrata Palasiatica, 48:19-26.
Ibrahim, N., Maganuco, S., Dal Sasso, C. Fabbri, M., Auditore, M., Bindellini, G., Martill, D.M. Zouhri, S., Mattarelli, D.A., Unwin, D.M., Wiemann, J., Bonadonna, D., Amane, A., Jakubczak, J., Joger, U., Lauder, G.V., and Pierce, S.E. 2020. Tail-propelled aquatic locomotion in a theropod dinosaur. Nature, 581:67-70. https://doi.org/10.1038/s41586-020-2190-3
Ibrahim, N., Sereno, P.C., Dal Sasso, C., Maganuco, S., Fabri, M., Martill, D.M., Zouhri, S., Myhrvold, N., and Lurino, D.A. 2014. Semiaquatic adaptations in a giant predatory dinosaur. Science, 345:1613-1616. https://doi.org/10.1126/science.1258750
Johnston, P.A. 1979. Growth rings in dinosaur teeth. Nature, 278(5705):635-636. https://doi.org/10.1038/278635a0
Kellner, A.W.A. and Mader, B.J. 1997. Archosaur teeth from the Cretaceous of Morocco. Journal of Paleontology, 71(3):525-527. https://doi.org/10.1017/s0022336000039548
Läng, E., Boudad, L., Maio, L., Samankassou, E., Tabouelle, J., Tong, H., and Cavin, L. 2013. Unbalanced food web in a Late Cretaceous dinosaur assemblage. Palaeogeography, Palaeoclimatology, Palaeoecology, 381:26-32. https://doi.org/10.1016/j.palaeo.2013.04.011
Malafaia, E., Gasulla, J.M., Escaso, F., Narváez, I., Sanz, J.L., and Ortega, F. 2019. A new spinosaurid theropod (Dinosauria: Megalosauroidea) from the upper Barremian of Vallibona, Spain: Implications for spinosaurid diversity in the Early Cretaceous of the Iberian Peninsula. Cretaceous Research, 106:104221. https://doi.org/10.1016/j.cretres.2019.104221
McGowan, A.J. and Dyke, G.J. 2009. A surfeit of theropods in the Moroccan Late Cretaceous? Comparing diversity estimates from field data and fossil shops. Geology, 37(9):843-846. https://doi.org/10.1130/g30188a.1
R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rauhut, O.W.M. 2001a. Morphology and mechanics of the jaws of spinosaurid theropods (Dinosauria): implications for predation. Ameghiniana, 38(4, Suplemento):16R.
Rauhut, O.W.M. 2001b. Herbivorous dinosaurs from the Late Jurassic (Kimmeridgian) of Guimarota, Portugal. Proceedings of the Geologists’ Association, 112:275-283. https://doi.org/10.1016/s0016-7878(01)80007-9
Rauhut, O.W.M. 2011. Theropod dinosaurs from the Late Jurassic of Tendaguru (Tanzania). Special Papers in Palaeontology, 86:195-239.
Rauhut, O.W.M., Hübner, T.R., and Lanser, K.-P. 2016. A new megalosaurid theropod dinosaur from the late Middle Jurassic (Callovian) of north-western Germany: implications for theropod evolution and faunal turnover in the Jurassic. Palaeontologia Electronica, 19.2.26A:1-65. https://doi.org/10.26879/654
Rayfield, E.J. 2011. Structural performance of tetanuran theropod skulls, with emphasis on the Megalosauridae, Spinosauridae and Carcharodontosauridae. Special Papers in Palaeontology, 83:241-253.
Rayfield, E.J., Milner, A.C., Xuan, V.B., and Young P.G. 2007. Functional morphology of spinosaur “crocodile-mimic” dinosaurs. Journal of Vertebrate Paleontology, 27:892-901. https://doi.org/10.1671/0272-4634(2007)27[892:fmoscd]2.0.co;2
Richter, U., Mudroch, A., and Buckley, L.G. 2013. Isolated theropod teeth from the Kem Kem Beds (Early Cenomanian) near Taouz, Morocco. Paläontologische Zeitschrift, 87:291-309. https://doi.org/10.1007/s12542-012-0153-1
Runcorn, S.K. 1968. Fossil bivalve shells and the length of month and year in the Cretaceous. Nature, 218:459. https://doi.org/10.1038/218459a0
Russell, D.A. 1996. Isolated dinosaur bones from the Middle Cretaceous of the Tafilalt, Morocco. Bulletin du Muséum National d’Histoire Naturelle, 4:349-402.
Sales, M.A.F., Liparini, A., Andrade, M.B., Aragão, P.L.O.R.L., and Schultz, C.L. 2017. The oldest South American occurrence of Spinosauridae (Dinosauria, Theropoda). Journal of South American Earth Sciences, 74:83-88. https://doi.org/10.1016/j.jsames.2016.10.005
Schade, M., Rauhut, O.W.M., and Evers, S.W. 2020. Neuroanatomy of the spinosaurid Irritator challengeri (Dinosauria: Theropoda) indicates potential adaptations for piscivory. Scientific Reports, 10:9259. https://doi.org/10.1038/s41598-020-66261-w
Schubert, B.W. and Ungar, P.S. 2005. Wear facets and enamel spalling in tyrannosaurid dinosaurs. Acta Palaeontologica Polonica, 50:93-99.
Schwarz, D., Kosch, J.C.D., Fritsch, G., and Hildebrandt, T. 2015. Dentition and tooth replacement of Dicraeosaurus hansemanni (Dinosauria, Sauropoda, Diplodocoidea) from the Tendaguru Formation of Tanzania, Journal of Vertebrate Paleontology, 35:e1008134. https://doi.org/10.1080/02724634.2015.1008134
Sereno, P.C., Beck, A.L., Dutheil, D.B., Gado, B., Larsson, H.C.E., Lyon, G.H., Marcot, J.D., Rauhut, O.W.M., Sadleir, R.W., Sidor, A.C., Varricchio, D.D., Wilson, G.P., and Wilson, J.A. 1998. A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science, 282:1298-1302. https://doi.org/10.1126/science.282.5392.1298
Sereno, P.C., Larsson, H.C.E., Sidor, C.A., and Gado, B. 1999. The giant crocodyliform Sarcosuchus from the Cretaceous of Africa. Science, 294:1516-1519.
Sereno, P.C., Wilson, J.A., Witmer, L.M., Whitlock, J.A., Maga, A., Ide, O., and Rowe, T.A. 2007. Structural extremes in a Cretaceous dinosaur. PLoS ONE, 11:1-9. https://doi.org/10.1371/journal.pone.0001230
Smith, J.B., Vann, D.R., and Dodson, P. 2005. Dental morphology and variation in theropod dinosaurs: implications for the taxonomic identification of isolated teeth. Anatomical Record A, 285A:699-736. https://doi.org/10.1002/ar.a.20206
Stromer, E. 1915. Ergebnisse der Forschungsreisen Prof. E. Stromers in den Wüsten Ägyptens. II. Wirbeltierreste der Baharije-Stufe (unterstes Cenoman). 3. Das Original des Theropoden Spinosaurus aegyptiacus nov. gen. spec. Abhandlungen der Königlich Bayerischen Akademie der Wissenschaften, Mathematisch-physikalische Klasse, 28(3):1-32.
Sues, H.-D., Frey, E., Martill, D.M., and Scott, D.M. 2002. Irritator challengeri, a spinosaurid (Dinosauria: Theropoda) from the Lower Cretaceous of Brazil. Journal of Vertebrate Paleontology, 22:535-547 https://doi.org/10.1671/0272-4634(2002)022[0535:icasdt]2.0.co;2
Vullo, R., Abit, D., Ballèvre, M., Billon-Bruyat, J.-P., Bourgeais, R., Buffetaut, E., Daviero-Gomez, V., Garcia, G., Gomez, B., Mazin, J.-M., Morel, S., Néraudeau, D., Pouech, J., Rage, J.-C., Schnyder, J., and Tong, H. 2014. Palaeontology of the Purbeck-type (Tithonian, Late Jurassic) bonebeds of Chassiron (Oléron Island, western France). Comptes Rendus Palevol, 13:421-441. https://doi.org/10.1016/j.crpv.2014.03.003
Vullo, R., Allain, R., and Cavin, L. 2016. Convergent evolution of jaws between spinosaurid dinosaurs and pike conger eels. Acta Palaeontologica Polonica, 61(4):825-828. https://doi.org/10.4202/app.00284.2016

