 Bioerosion traces on the Campanian turtle remains: New data from the lagoonal deposits of the Quseir Formation, Kharga Oasis, Egypt
Bioerosion traces on the Campanian turtle remains: New data from the lagoonal deposits of the Quseir Formation, Kharga Oasis, Egypt
Article number: 26.3.a40
https://doi.org/10.26879/1315
Copyright Paleontological Society, October 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 29 June 2023. Acceptance: 19 September 2023.
ABSTRACT
The uppermost part of the Campanian Quseir Formation of Kharga Oasis, Egypt, contains a concentration of turtle skeletal remains in a lagoon setting. They appear as three successive horizons (I‒III), alternated between the variegated shales and the glauconitic mudstones and conglomeratic layers within the Hindaw Member. However, bones recovered in horizon III present a higher preservation potential than the others. Therein, turtle remains are represented by mostly complete shells, partial shells and many scattered and weathered shell fragments. The studied turtles lived in small ponds and marshes and were deposited as autochthonous to parautochthonous relics. Their bones display significant bioerosion signatures on both carapace and plastron. The morphological analysis of the bioerosion structures preserved revealed 11 ichnospecies, belonging to nine ichnogenera. Eight of these ichnotaxa are recorded for the first time in turtle bones of Egypt. The recognised bioerosional structures appear as borings, shallow chambers, grooves, and punctures produced by ticks, beetles, polychaete worms, fishes/crocodile, and gastropods. They were attributed to the ichnogenera Karethraichnus, Cubiculum, Osteocallis, Radulichnus, Osteichnus, Osedacoides, Sulculites, and Machichnus. In addition, some bite marks assigned to Nihilichnus also occur on a carapace peripheral. This grade of bioerosion was likely caused by relatively long exposure time before the final deposition or burial. In some cases, borings may be produced during the host’s lifetime. The new material considerably expands the stratigraphic and geographic distribution of this trace fossil assemblage and reveals that their producers may have been able to survive in other palaeoenvironmental conditions.
Magdy El Hedeny. Department of Geology, Faculty of Science, Alexandria University, Alexandria 21568, Egypt. magdy.elhedeny@alexu.edu.eg
Sara Mohesn. Geology Department, Faculty of Sciences, New Valley University, New Valley, Egypt. saramohsen1711@gmail.com
Abdel-aziz Tantawy. Geology Department, Faculty of Sciences, New Valley University, New Valley, Egypt. aatantawy@nv.aun.edu.eg
Ahmed El-Sabbagh. Department of Geology, Faculty of Science, Alexandria University, Alexandria 21568, Egypt. Corresponding author. ah.elsabbagh@alexu.edu.eg
Mohamed AbdelGawad. Department of Geology, Faculty of Science, Cairo University, Giza 12613, Egypt. mkabdelgawad@cu.edu.eg
Gebely Abu El-Kheir. Geology Department, Faculty of Sciences, New Valley University, New Valley, Egypt. gebely2006@sci.nvu.edu.eg
Keywords: turtles; bioerosion; trace fossils; Campanian; Kharga Oasis; Egypt
Final citation: El Hedeny, Magdy, Mohesn, Sara, Tantawy, Abdel-aziz, El-Sabbagh, Ahmed, AbdelGawad, Mohamed, and Abu El-Kheir, Gebely. 2023. Bioerosion traces on the Campanian turtle remains: New data from the lagoonal deposits of the Quseir Formation, Kharga Oasis, Egypt. Palaeontologia Electronica, 26(3):a40.
https://doi.org/10.26879/1315
palaeo-electronica.org/content/2023/3968-bioerosion-traces-on-turtles
Copyright: October 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Upper Cretaceous deposits exposed in Kharga Oasis, in the Southern part of the Western Desert of Egypt, contain abundant vertebrate fossils in near-shore marine environments. Previous studies have led to the recovery and identification of crocodyliforms (cf. Dyrosaurus), dinosaurs (cf. Spinosaurus), turtles (Podocnemis aegyptiacus Andrews, 1900), lungfish (Ceratodus and Protopterus), bony fish, and sharks from the Mut Member, the lower part of the Quseir Formation (previously called the Mut Formation) from Dakhla Oasis (e.g., Churcher and de Iuliis, 2001; Claeson et al., 2014). Turtles of the Family Bothremydidae Baur, 1891 are among the most common fossil animals found at this locality. It represents an extinct family of side-necked turtles (Pleurodira) known from the Early Cretaceous (Albian) to the Eocene (Gaffney et al., 2006; Cadena et al., 2012). They were adapted to live in freshwater and brackish-coastal environments of North and South America, Europe, Africa, and India (Gaffney et al., 2001a, 2001b, 2006, 2007, 2009a, 2009b; Laurent et al., 2002; Gaffney and Tong, 2008; Lehman and Wick, 2010; AbdelGawad et al., 2023).
In Egypt, several authors have recorded members of the Family Bothremydidae from deposits at many localities, including the Campanian Quseir Formation, Kharga Oasis (e.g., AbdelGawad et al., 2019, 2023; Abu El-Kheir, 2020), the Maastrichtian Ammonite Hill Member of the Dakhla Shale, Abu Minqar (e.g., Lapparent de Broin and Werner, 1998; Abu El-Kheir et al., 2021) and the lower Cenomanian Bahariya Formation in Gebel El Dist, Bahariya Oasis (Lapparent de Broin, 2000; Abu El-Kheir, 2020). Despite studies having examined the taxonomy of the turtle assemblages in each locality, little is known about the palaeoecology and taphonomic signatures on these faunas, with their role as agents responsible for bone accumulations not fully understood.
 Bioerosion (e.g., borings, grooves, scratch marks, tooth marks, and other evidence of feeding, reproduction, predation and scavenging on fossil material) is an important taphonomic process that has potential use as palaeoecological markers and can be significant for taphonomic studies of turtles (e.g., Behrensmeyer, 1978; Fiorillo, 1987, 1990; Tappan, 1994; Hasiotis, 2004; Tapanila et al., 2004; Zonneveld et al., 2022a, 2022b). So far, little attention has been paid to bioerosional traces on turtle bones. Owing to the fact that borings have not been described from the bones of modern turtles, the significance, origin, and frequency of borings in fossil turtles are still unclear (Zonneveld et al., 2022a, 2022b). The target of this study is to describe a suite of bioerosional traces on fossil bothremydid turtles collected from deposits of the uppermost Hindaw Member of the Quseir Formation of Kharga Oasis, Egypt (Figure 1) and to study their distribution on the plastron and carapace from palaeoecological and taphonomic perspectives. An attempt was made to distinguish if borings were produced during the host’s lifetime (in vivo) or post-mortem.
Bioerosion (e.g., borings, grooves, scratch marks, tooth marks, and other evidence of feeding, reproduction, predation and scavenging on fossil material) is an important taphonomic process that has potential use as palaeoecological markers and can be significant for taphonomic studies of turtles (e.g., Behrensmeyer, 1978; Fiorillo, 1987, 1990; Tappan, 1994; Hasiotis, 2004; Tapanila et al., 2004; Zonneveld et al., 2022a, 2022b). So far, little attention has been paid to bioerosional traces on turtle bones. Owing to the fact that borings have not been described from the bones of modern turtles, the significance, origin, and frequency of borings in fossil turtles are still unclear (Zonneveld et al., 2022a, 2022b). The target of this study is to describe a suite of bioerosional traces on fossil bothremydid turtles collected from deposits of the uppermost Hindaw Member of the Quseir Formation of Kharga Oasis, Egypt (Figure 1) and to study their distribution on the plastron and carapace from palaeoecological and taphonomic perspectives. An attempt was made to distinguish if borings were produced during the host’s lifetime (in vivo) or post-mortem.
LOCATION AND STRATIGRAPHIC CONTEXT
Excellent exposures of the Upper Cretaceous formations crop out in Kharga Oasis and its surrounding areas in the Southern parts of the Western Desert of Egypt (Ball, 1900; Beadnell, 1909; Said, 1962, 1990; Awad and Ghobrial, 1965; Hermina, 1967, 1990). Among them, the Quseir Formation (Youssef, 1957) deposited during the Campanian, represents mixed limnic and marine conditions following an intense erosion period that started after the tectonically-controlled early Turonian regression (Awad and Ghobrial, 1965; Hendriks et al., 1984; Hermina, 1990). In the Southern parts of Egypt, the Quseir Formation is also known as the Variegated Shales (Said, 1961, 1962, 1990; Awad and Ghobrial, 1965) or the Mut Formation (Barthel and Herrmann-Degen, 1981).
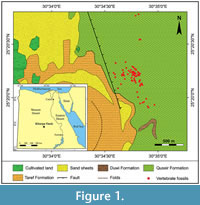
 In the Kharga area, the Quseir Formation unconformably overlies the Turonian sandstone of the Taref Formation and conformably underlies the upper Campanian/lower Maastrichtian phosphate-bearing Duwi Formation. The present work is based on the succession exposed in the Qarn Ganah area (25°20'15"N, 30°34'45"E), about 10 km south of the Kharga city (Figure 1). In the study area, deposits are mainly composed of alternating shales and mudstones, attaining a thickness of about 26 m (Figure 2). Shales are variegated and laminated. Mudstones are grey to greyish green, silty, and glauconitic. Within this outcrop, and throughout Kharga Oasis, the two members of the Quseir Formation can be recognised readily in the field (e.g., Omara et al., 1976). They are from base to top: the Mut and Hindaw members (about 5 and 21 m thick, respectively).
In the Kharga area, the Quseir Formation unconformably overlies the Turonian sandstone of the Taref Formation and conformably underlies the upper Campanian/lower Maastrichtian phosphate-bearing Duwi Formation. The present work is based on the succession exposed in the Qarn Ganah area (25°20'15"N, 30°34'45"E), about 10 km south of the Kharga city (Figure 1). In the study area, deposits are mainly composed of alternating shales and mudstones, attaining a thickness of about 26 m (Figure 2). Shales are variegated and laminated. Mudstones are grey to greyish green, silty, and glauconitic. Within this outcrop, and throughout Kharga Oasis, the two members of the Quseir Formation can be recognised readily in the field (e.g., Omara et al., 1976). They are from base to top: the Mut and Hindaw members (about 5 and 21 m thick, respectively).
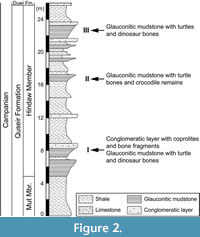
Sedimentary deposits of the Quseir Formation in this new site contain distinctive Campanian reptile fossils mainly occurring in three successive horizons. Vertebrate fossils include lungfishes, turtles, crocodile bones, and some disarticulated skeletons of dinosaurs. In addition, strata of the Hindaw Member are intercalated with four thin layers (0.3‒0.5 m thick) of ferruginous conglomeratic sandstone, containing bone fragments and coprolites (Figure 2).
MATERIAL AND METHODS
Up to 50 turtle remains have been collected from three fossiliferous horizons of the Quseir Formation exposed in the Qarn Ganah area, Kharga Oasis; the majority of them belonging to horizon III. Turtle specimens are scattered, randomly oriented, and unsorted. Most of the discovered materials are isolated plates; no cranial elements have been recovered thus far. However, two well-preserved, mostly complete shells that include both carapace and plastron were discovered.
In the laboratory, all specimens were gently cleaned under running water, scrubbing with a soft toothbrush. Then, each specimen was systematically screened for bioerosional traces using naked eye, a hand lens, and binocular microscope when necessary. Specimens were photographed with a Canon Power-Shot A3000 digital camera and the SZ61TR-LED zoom stereo microscope. Determination of bioerosion structures was mainly based on criteria given by Vialov and Nessov (1974), Thenius (1988a), Mikuláš et al. (2006), Roberts et al. (2007), Karl et al. (2012), Xing et al. (2015), and Zonneveld et al. (2016, 2022a, 2022b).
Although taxonomic identification to ichnospecies level was possible for several specimens, the highly eroded specimens meant that in most cases specimens could only be identified to ichnogeneric level. All specimens reported in this study are curated at the New Valley Vertebrate Palaeontology Center, New Valley University, New Valley, Egypt. Numbered turtle specimens are prefixed by NVP.
SYSTEMATIC ICHNOLOGY
At least 11 ichnospecies, representing nine ichnogenera, were identified, described and illustrated (Figure 3, Figure 4, Figure 5). Eight of these ichnotaxa have been recorded for the first time in Egypt (Table 1).
Ichnogenus Nihilichnus
Mikuláš et al., 2006
Type ichnospecies. Nihilichnus nihilicus Mikuláš et al., 2006
Diagnosis. Roughly triangular, circular or ovoid holes or external pits, occurring solitarily or in groups, which may show recurring patterns. Outer part of the margin of the cavity shows minute, irregular jags, resulting from a brittle deformation (Mikuláš et al., 2006).
Nihilichnus nihilicus Mikuláš et al., 2006
(Figure 3A)
 Material. Three ellipsoid borings on a carapace fragment (NVP001).
Material. Three ellipsoid borings on a carapace fragment (NVP001).
Description. Circular, subcircular to ellipsoidal holes in outline, straight and perpendicular margin towards the outer and inner shell surfaces; not penetrating the cortical compact bone with minor irregular jags, diameter of holes ranges from 2 to 4 mm, situated 2 cm apart. No bioglyphes have been recorded.
Discussion. The presence of a set of grooves with irregular outlines in the studied specimen supports the assignment to the ichnofamily Machichnidae Wisshak et al., 2019 (which includes the ichnogenera Machichnus, Nihilichnus, Linichnus, Knethichnus, and Mandaodonites). Due to subsequent erosion of the superficial layers of the studied bone that leaves the internal-most section of the exposed traces, each groove exhibits a circular outline with minor irregular jags, allowing its assignment to the ichnogenus Nihilichnus. The borings described show most of the morphological characters of the holotype described and illustrated by Mikuláš et al. (2006, p. 120, figure 6A-K). In general, Nihilichnus is known from terrestrial, palustrine, freshwater (reported herein), and marine environment (Rasser et al., 2016). Although it most commonly occurs on vertebrate bone substrate, it has also been recorded in other invertebrate skeletal substrate (molluscan shells) (Rasser et al., 2016; Ahmed et al., 2022).
Producer. The ichnogenus Nihilichnus represents putative bite marks on fossil bone. Crocodiles may be considered potential makers, as crocodylian remains are documented from the Quseir Formation (Abu El-Kheir, 2020). Similar cases have been reported previously by other authors (e.g., Milàn et al., 2011; Schwarz-Wings et al., 2014). Additionally, marine mammals, reptiles, and bony fish could be responsible for N. nihilicus (Irazoqui and Acosta Hospitaleche, 2022).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). It is also recorded from the Middle Triassic of Germany (Mujal et al., 2022), latest Triassic of Poland (Dzik et al., 2008; Niedźwiedzki et al., 2011), Kimmeridgian of Germany (Karl and Tichy, 2004), Lower Palaeocene of Denmark (Milàn et al., 2011), Eocene of Antractica (Irazoqui and Acosta Hospitaleche, 2022), Lower Miocene of Czech Republic and Egypt (Mikuláš et al., 2006; Zonneveld et al., 2022a), and Upper Miocene of Brazil (Di Gregorio et al., 2020).
Ichnogenus Karethraichnus
Zonneveld et al., 2016
Type ichnospecies. Karethraichnus lakkos Zonneveld et al., 2016
Diagnosis. Circular to subcircular and oval pits and holes bored into bony substrates. The hole may penetrate fully through the substrate or terminate within the substrate as a shallow, bowl-shaped pit or as a deeper shaft with a rounded, blunt, or pointed terminus (Zonneveld et al., 2016).
Karethraichnus lakkos Zonneveld et al., 2016
(Figure 3B, C)
Material. Four borings on a plastron (NVP002) and several bowl-shaped pits on three carapaces (NVP003‒005).
Description. Simple shallow, circular to subcircular bores, bowl-shaped pits, sometimes closely spaced, and fused (Figure 3B) unbranched shafts into bone borings. They vary in diameter from 0.6 to 6 mm, 1‒4 mm in depth. Depression sides are generally smooth, flattened to rounded.
Discussion. Karethraichnus lakkos represents the third dominant trace fossil in the studied turtle bones. The specimens described show most of the morphological characters of the holotype of K. lakkos as described and illustrated by Zonneveld et al. (2016, p. 6, figure 6.1). This ichnospecies is common on the external surfaces of the plastron and carapaces of the majority of turtle shells. In the present study, K. lakkos appeared in two forms; one of them corresponds to small, unjagged rounded holes, not completely penetrative, apart from each other with varying distances and are not occurring in sets, affecting the external surface of plastron fragments, especially at sulci between epidermal scalesplastron. The second form is slightly larger, consisting of shallow, bowl-shaped pits, closely spaced, and sometimes fused together on the external surfaces of carapace. Borings of the ichnospecies K. lakkos are only located in accessible places in living turtles (in vivo), such as external surfaces of the carapace and plastron, especially at marginal or lip areas. Although K. lakkos was first described from fossil shell bones of geoemydid turtles from lower Eocene non-marine strata of Wyoming (Zonneveld et al., 2016), it was observed also in both terrestrial tortoises and aquatic turtles from the Lower Miocene of Egypt (Zonneveld et al., 2022a).
Producer. Trace makers usually associated with K. lakkos include leeches and ixodid arthropods (ticks), which are known to feed on blood sinuses within shell bone, especially at sulci between epidermal scales (e.g., Siddall and Gaffney, 2004; Zonneveld et al., 2016). However, barnacles attaching onto a bone substrate are also a suggested producer of this ichnotaxon (Zonneveld et al., 2016, 2022b; Collareta et al., 2022).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). Karethraichnus lakkos has been recorded from the Lower Eocene of USA (Zonneveld et al., 2016; Adrian et al., 2021), Miocene of Peru, Italy and Egypt (Collareta et al., 2022; Collareta et al., 2023a; Zonneveld et al., 2022a), to Recent (Zonneveld et al., 2022b). This determination extends the stratigraphic range of the taxon at minimum to the Campanian.
Ichnogenus Cubiculum
Roberts et al., 2007
Type ichnospecies. Cubiculum ornatus Roberts et al., 2007
Diagnosis. Discrete ovoid borings in bone. Hollow, oval chambers with concave flanks bored into inner spongy and outer cortical bone surfaces. Chamber length three to four times greater than diameter. May be isolated, but observed more commonly in dense, sometimes overlapping concentrations. Walls commonly roughened, composed of shallow, arcuate (apparently paired) grooves (emended after Roberts et al., 2007).
Cubiculum ornatus Roberts et al., 2007
(Figure 3D, E)
Material. Up to 35 chambers on seven carapace remains (NVP006‒012).
Description. Elongate, straight, or arcuate chambers with semi-circular ends, occurring in isolated dense and rarely overlapping concentrations. Boring long axes are parallel to the bone surfaces. Borings differ in their sizes; long axes: 1.8‒10.8 mm; borings depth: 1.0‒1.5 mm. Boring walls bear well-developed ornament (bioglyphs).
Discussion. The ichnospecies Cubiculum ornatus represents the most common ichnotaxon in our assemblage. Dimensions and overall form of the specimens closely match bone borings described from the Upper Cretaceous of Madagascar (Roberts et al., 2007). These borings represent pupation chambers made by dermestid beetles (Kitching, 1980; Martin and West, 1995; Hasiotis et al., 1999; Ozeki et al., 2020; Perea et al., 2020). The presence of these borings suggests that the turtle bones had a relatively long time of exposure, which was sufficient to allow beetles to posture, but not long enough to allow weathering to destroy them.
Producer. Generally, Cubiculum ichnospecies have been previously documented and interpreted as dermestid beetle borings in many palaeontological studies (e.g., Martin and West, 1995; Hasiotis et al., 1999; Laudet and Antoine, 2004; Roberts et al., 2007; Britt et al., 2008; Bader et al., 2009; Saneyoshi et al., 2011).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). This ichnospecies has been recorded from the Middle‒Upper Jurassic of South Africa (Britt et al., 2008; Xing et al., 2015), Upper Cretaceous of Brazil, Morocco (Ibrahim et al., 2014; Francischini et al., 2016) to Pleistocene of Brazil (West and Hasiotis, 2007; Dominato et al., 2009).
Cubiculum inornatus Xing et al., 2015
(Figure 3D, F)
Material. Four ellipsoidal chambers on a carapace remain (NVP006).
Description. Discrete, ellipsoidal borings in bone with marked concavity of flanks and bottom and marked constriction of walls in the upper area. Boring boundaries, wall and bottom are sharp and smooth. No bioglyphs or fillings were observed.
Discussion. In the present assemblage, C. inornatus is associated with other trace fossils; C. ornatus and Osteocallis mandibulus. Cubiculum inornatus resembles C. ornatus but is differentiated by having tapering ends and the absence of bioglyphs in their walls. Cubiculum levis Pirrone et al. (2014, p. 253, figure 3) differs from our specimens in having interior bowl-shaped morphology and a marked constriction of walls in the upper area.
Producer. Indeterminate osteophagous insect (Xing et al., 2015; Paes Neto et al., 2016).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). Cubiculum inornatus was recorded from the Middle Triassic of Brazil (Paes Neto et al., 2016) to Lower‒Middle Jurassic of China and South Africa (Xing et al., 2015). The present determination greatly expands the stratigraphic range of C. inornatus to the Upper Cretaceous (Campanian).
Cubiculum isp.
(Figure 3G, Figure 4F)
Material. Many boring clusters on five carapace remains (NVP0013‒017).
 Discussion. The present specimens are closely allied to Cubiculum isp. This is especially true for the general outline, dimension, and arrangement of borings. Borings are arranged in groups of three to five and parallel to each other, according to its major axis. However, due to sediment infilling and poor preservation, they could not be determined at the ichnospecific level.
Discussion. The present specimens are closely allied to Cubiculum isp. This is especially true for the general outline, dimension, and arrangement of borings. Borings are arranged in groups of three to five and parallel to each other, according to its major axis. However, due to sediment infilling and poor preservation, they could not be determined at the ichnospecific level.
Ichnogenus Osteocallis
Roberts et al., 2007
Type ichnospecies. Osteocallis mandibulus Roberts et al., 2007
Diagnosis. Shallow, meandering trail of arcuate grooves (apparently paired) bored into external (cortical) bone surfaces. May be single trail or network of randomly overlapping trails (Roberts et al., 2007).
Osteocallis mandibulus Roberts et al., 2007
(Figure 3D, H, Figure 4A, B)
Material. Small trails on four carapace remains (NVP006, 019‒021).
Description. Shallow, small trail of arcuate grooves, mostly formed of paired scratches (Figure 3H), sometimes consist of a network of randomly overlapping trails (Figure 4A, B). Ranging in width between 0.9 to 11 mm, generally less than 0.7 mm in length.
Discussion. This ichnotaxon was recorded for the first time in dinosaur bone from continental deposits of the Upper Cretaceous of Madagascar and Utah (Roberts et al., 2007). In the present study, O. mandibulus was recorded together with insect pupal chambers (Cubiculum ornatus; Figure 3D). Because similar bioglyph appeared in both ichnotaxa, the trace-making organisms are believed to be the same (Roberts et al., 2007). Palaeoecologically, O. mandibulus is found in nonmarine environmental settings (Paes Neto et al., 2016). The presence of these borings suggests that the turtle bones had a relatively long time of exposure, which was sufficient to allow the silphid and histerid beetles to leave such a significant bone modification.
Producer. Necrophagous or osteophagous insects are considered to be the main producers of O. mandibulus (Roberts et al., 2007). They usually use their robust mouthparts for feeding.
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). Osteocallis mandibulus has been recorded from the Upper Triassic of Southern Brazil (Paes Neto et al., 2016), Upper Jurassic of Western Colorado (McHugh et al., 2020), the Upper Cretaceous of Utah and Madagascar (Roberts et al., 2007), and Lower Pliocene of Italy (Collareta et al., 2023b).
Ichnogenus Radulichnus
Voigt, 1977
Type ichnospecies. Radulichnus inopinatus Voigt, 1977
Diagnosis. Minute patches or shallow grooves with parallel or subparallel striae arranged side by side in transverse rows or irregularly distributed (Voigt, 1977).
Radulichnus inopinatus Voigt, 1977
(Figure 4C, D)
Material. Many arcuate bundles on three carapace remains (NVP019‒021).
Description. Parallel to sub-parallel arcuate bundles closely spaced in groups, each group ranges from six to eight patches, covering some areas of the turtle skeletons.
Discussion. Radulichnus inopinatus occurs commonly on the external surfaces of the carapace of some turtle skeletons, uncovering the whole surfaces. In some cases, the studied ichnotaxon is associated with Osteocallis mandibulus. The latter ichnospecies differ from R. inopinatus by their small trail of arcuate grooves and sometimes consist of a network of randomly overlapping trails. Two ichnospecies of Radulichnus have been described: Radulichnus inopinatus and R. transversus. The second ichnospecies was characterised by parallel and short grooves (less than 1 mm in length) arranged in rows, concentrated in the central part of the inner surface of the valves of the clam species Anomalocardia brasiliana. Radulichnus inopinatus corresponds to the marks left by gastropods, while R. transversus to those left by polyplacophorans (Lopes and Pereira, 2019). Radulichnus inopinatus has been recognised from invertebrate shells (Lopes and Pereira, 2019), as well as from vertebrate remains (Jagt et al., 2020).
Producer. Invertebrate (chiefly molluscan) shells and polyplacophorans (chitons) (Voigt, 1977; Jagt, 2003; Gibert et al., 2007; Lopes and Pereira, 2019; Collareta et al., 2023b).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). Radulichnus inopinatus was previously recorded from the Middle Devonian of Germany (Bohatý, 2011), Maastrichtian of Netherlands and Belgium (Jagt, 2003; Mulder et al., 2005; Janssen et al., 2013), Pliocene of France and Italy (Gibert et al., 2007; Collareta et al., 2023b), and Pleistocene of Southern Brazil (Lopes and Pereira, 2019).
Ichnogenus Osteichnus
Thenius, 1988a
Type ichnospecies. Asthenopodichnium ossibiontum (Thenius, 1988a)
Diagnosis. U-shaped burrows having a spreite (weblike construction) between the two limbs of the burrow, with a tube diameter of 3-5 mm, at an average notch length of 8-15 mm. Depth of U-constructions up to 7 mm (Thenius, 1988a).
Osteichnus ossiobontum (Thenius, 1988a)
(Figure 4E)
Material. Up to 25 borings on a carapace remain (NVP022).
Description. Cylindrical non-branched parallel borings with fused U-notches, inclined to the bone surface, cross-section long-oval at deeper levels with smooth interior surface. Dimensions vary between 3-8 mm long and between 1-3 mm in width. Depth of borings is ~2-3 mm.
Discussion. The specimens resemble the type ichnospecies Asthenopodichnium ossibiontum as described and illustrated by Thenius (1988a, p. 9, pl. 3, figures 1, 2) from the Miocene of Austria. The lithic-boring Rogerella Saint-Seine (1951) has closely similar features to the present osteic skeletal substrates ichnogenus. Unlike the producer of the present ichnogenus, barnacles of the Order Acrothoracica are considered to be the trace makers of Rogerella (e.g., Mikuláš, 1992).
Producer. Asthenopodinae mayfly larvae (Thenius, 1988a, 1988b; Britt et al., 2008; Ozeki et al., 2020). In particular, the Asthenopodinae mayfly larvae, a subfamily of Polymitarcyidae, have been assigned to be the producer of the present ichnospecies (Thenius, 1979, 1988a; Jalvo and Andrews, 2016; Genise, 2017). Nowadays, species of this family live in moderate to high energy fluvial systems (Barber-James et al., 2008).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). Upper Jurassic of USA (Britt et al., 2008) to Middle‒Upper Miocene of Austria (Thenius, 1988a, 1988b).
Ichnogenus Osedacoides
Karl et al., 2012
Type ichnospecies. Osedacoides jurassicus Karl et al., 2012
Diagnosis. Simple, basally thickened to branched borings in marine vertebrate bones with a single opening to the surface (Karl et al., 2012).
Osedacoides jurassicus Karl et al., 2012
(Figure 4F)
Material. Several circular borings on four carapace remains (NVP023‒026).
Description. Simple, cylindrical borings, circular to semi-circular in cross-section, with a single entrance perpendicular to slightly inclined to the bone surface, closely spaced, bore diameter ranges between 1-4 mm.
Discussion. Osedacoides are simple, basally-branching borings in bone. They penetrate into bone as a shaft and branch at depth. Osedacoides are exclusively recorded in organic substrates (Karl, 2016).
Producer. Osteophagous polychaetes (Karl et al., 2012).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). The type ichnospecies has been recorded from the Upper Jurassic (Middle Kimmeridgian) of Germany (Karl, 2016). This determination may extend the stratigraphic range of the taxon up into the Upper Cretaceous (Campanian).
Ichnogenus Sulculites
Vialov and Nessov, 1974
Type ichnospecies. Sulculites bellus Vialov and Nessov, 1974
Diagnosis. Furrows have a flat bottom and width from 0.18-0.5 mm or a little more, but usually about 0.3‒0.4 mm. They are straight or curved; more often continuous, only rarely intermittent; sometimes single, sometimes forming chains of parallel systems. Groups of parallel furrows can be quite closely spaced to each other, being oriented in different directions. Another common type is curved and often intertwining relatively long furrows. Shorter depressions may be interspersed with them. The ends of the furrows either gradually narrow or abruptly break off (Vialov and Nessov, 1974).
Sulculites bellus Vialov and Nessov, 1974
(Figure 4G)
Material. Many narrow grooves on three carapace remains (NVP027‒029).
Description. Smooth, slender, narrow, nonbranched straight to curved grooves in the surface of bones. They occur solitarily or in clusters, sometimes crossing each other. Width ranges from 0.25-0.6 mm
Discussion. The specimens described show most of the morphological characters of the holotype of S. bellus as described and illustrated by Vialov and Nessov (1974, p. 101, figures 1‒4).
Producer. Sulculites most likely belongs to the category fodinichnia (Zonneveld, personal comm.). According to Vialov and Nessov (1974), worms are suggested to be the main producer of this trace fossil.
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). Sulculites bellus is firstly recorded from the Albian of Eastern Uzbekistan (Vialov and Nessov, 1974). This determination extends the stratigraphic range of the taxon up into the Campanian.
Ichnogenus Machichnus
Mikuláš et al.,2006
Type ichnospecies. Machichnus regularis Mikuláš et al., 2006
Diagnosis. Shallow serial parallel or subparallel grooves in turtle bone. The groove surface is smooth or longitudinally striated. Each series consists usually of dozens of grooves, which are typically uniform in shape and dimensions. The grooves are oriented perpendicular to substrate edge (Mikuláš et al., 2006).
Machichnus ?bohemicus Mikuláš et al., 2006
(Figure 4H)
Material. Three parallel scratches on a carapace remain (NVP030).
Diagnosis. Shallow, thin, discrete, parallel to subparallel, smooth-bottomed scratches. The scratches occur in small groups or series of corresponding width and length; width typically reaches 0.2-1.0 mm and usual length is up to 10 mm (Mikuláš et al., 2006).
Description. Shallow, thin, discrete, parallel to subparallel scratches. At the studied site, they occur on cortical bone of long bones or on the surfaces of antlers. The scratches occur in small groups. Within the series, they typically cover 10-50% of the surface. Each individual scrape is very short and does not overlap or approach any adjacent scrape. Length for each individual mark is typically greater (average 0.65 mm) than width. Each pair is approximately one millimetre apart.
Remarks. Scratches of the given morphology most likely occur during the feeding activity on soft tissues surrounding the bones. In the case of antlers, the scratches are interpreted as gnawing traces, as there is no digestible tissue to be consumed, except the felt on young antlers.
Discussion. The ichnofamily Machichnidae is characterised by a set of grooves, with somewhat irregular outlines on the cortical bone. This family is represented by five distinctive ichnogenera: Machichnus, Nihilichnus, Linichnus, Knethichnus, and Mandaodonites. Chumakov et al. (2013) erected three new ichnospecies, namely Machichnus normani, M. harlandi, and M. jeansi, for scratches on phosphorite nodules and pebbles from the Upper Cretaceous of England. Herein, the presence of shallow, thin, discrete, parallel to subparallel scratches that occur in small groups or series are undoubtedly assigned to the ichnogenus Machichnus. However, because of the smaller dimensions of each pair than the holotype of M. bohemicus described by Mikuláš et al. (2006), their ichnospecific determination is uncertain and needs more additional, well-preserved specimens to be confirmed.
 Producer. The ichnospecies Machichnus bohemicus is interpreted as a biting trace, possibly made by crocodylians (Mikuláš et al., 2006; Mikuláš and Dvořák, 2010). This ichnospecies is suggested to be produced by mechanical action of carnivore teeth (e.g., fishes and crocodiles) on the bones (Bieńkowska-Wasiluk et al., 2013; Irazoqui and Acosta Hospitaleche, 2022).
Producer. The ichnospecies Machichnus bohemicus is interpreted as a biting trace, possibly made by crocodylians (Mikuláš et al., 2006; Mikuláš and Dvořák, 2010). This ichnospecies is suggested to be produced by mechanical action of carnivore teeth (e.g., fishes and crocodiles) on the bones (Bieńkowska-Wasiluk et al., 2013; Irazoqui and Acosta Hospitaleche, 2022).
Stratigraphic and geographic distribution. Campanian of the Qarn Ganah, Kharga Oasis (this study). Machichnus bohemicus has been recorded from the Middle Danian (Lower Palaeocene) of Denmark (Milàn et al., 2011), Eocene of West Antarctica (Irazoqui and Acosta Hospitaleche, 2022) and Miocene of Czech Republic (Mikuláš et al., 2006). This determination extends the stratigraphic range of the ichnotaxon through at minimum the latest Maastrichtian. In addition, Mujal et al. (2022) identified bite traces on tetrapod bones from the Middle Triassic of Southwestern Germany that were identified as Machichnus -like traces.
PALAEOECOLOGY AND POST-MORTEM ALTERATIONS
The Quseir Formation was deposited in terrestrial and brackish environments that graded to a shallow shelf (Ward and McDonald, 1979; Hermina, 1990). It contains vertebrate remains of fishes, crocodiles, dinosaurs, mosasaurids, and turtles in the Campanian deposits (Abu El-Kheir, 2020). In the Qarn Ganah area, the uppermost part of the Hindaw Member (the Quseir Formation) is formed of about 35 m of variegated shale alternating with glauconitic sandstone and siltstone. Turtle concentrations appeared in this member in three successive horizons (Figure 2), alternated between the variegated shales and the glauconitic mudstones and the conglomeratic layers. The bones found in such deposits indicate a supratidal marsh environment, concordant with the occurrence of side-necked turtles (Bothremydidae) (Abu El Kheir, 2020; AbdelGawad et al., 2023).
 In the study section, more than 50 moderately to well-preserved turtle remains are recorded. They are distributed in very small areas along the three fossil-bearing horizons (Figure 2). While horizons I and II contain fragmentary and highly weathered turtle remains, samples gathered from horizon III represent the highest concentration of moderately to well-preserved turtle remains in the study section. This massive concentration of turtle skeletons may be due their habit of living in small pond and marshes (AbdelGawad et al., 2023). Such shallow, nutrient-rich ponds were exposed to sunlight with little water flowing through it. Many plant remains were recorded in the grey glauconitic mudstone of the study area (Figure 6A‒D). In general, glauconite contains many trace elements that serve as necessary micro-nutrients for the plant growth (e.g., El-Habaak et al., 2016). Accordingly, turtles may have lived at the margin of the pond, with a high density of plants (Mahmoud, 2003). However, pond turtles are omnivorous and will eat anything available, including other turtles.
In the study section, more than 50 moderately to well-preserved turtle remains are recorded. They are distributed in very small areas along the three fossil-bearing horizons (Figure 2). While horizons I and II contain fragmentary and highly weathered turtle remains, samples gathered from horizon III represent the highest concentration of moderately to well-preserved turtle remains in the study section. This massive concentration of turtle skeletons may be due their habit of living in small pond and marshes (AbdelGawad et al., 2023). Such shallow, nutrient-rich ponds were exposed to sunlight with little water flowing through it. Many plant remains were recorded in the grey glauconitic mudstone of the study area (Figure 6A‒D). In general, glauconite contains many trace elements that serve as necessary micro-nutrients for the plant growth (e.g., El-Habaak et al., 2016). Accordingly, turtles may have lived at the margin of the pond, with a high density of plants (Mahmoud, 2003). However, pond turtles are omnivorous and will eat anything available, including other turtles.
This concentration may be responsible for greater densities of turtles in horizon III (Figure 6D). The associated crocodiliforms might have lived together with turtles in this pond, with very little or without transportation of fossil elements by the currents. The study area was incised laterally by fluvial sandstone meandering channels, which may have transported the separated elements of sauropod dinosaurs into the existing pond.
In the field, the turtle fossils of the Qarn Ganah area are represented by complete shells, partial shells and many scattered and little-weathered turtle shell fragments. No significant transport or bone sorting is observed, and both complete and fragmentary bones in the assemblages show the same biostratinomic features. Vertebrate fossils with rounded or polished edges are absent. These features suggest mass mortalities of turtles, and burial before many shells disarticulated. All these observations indicate that we are dealing with little-transported, autochthonous to parautochthonous concentration of turtle remains.
In the turtle-rich layer (horizon III; Figure 6D), turtles are almost in a similar taphonomic condition, with many shells mostly intact and subjected to little weathering. No cranial turtle remains are recorded and only a few limb bones are collected from the surface of the fossil bearing layers. These elements may have been transported by currents to other sites after the exposure of the turtle skeletons (Brand et al., 2000). The majority of turtle shells are partially buried in the sediments, as the buried portion is still associated while the exposed part is disarticulated, indicating an erosional process of skeletons after removal of the sediments (Brand et al., 2000). Few complete turtle shells were collected from the third horizon. The turtle shells in the turtle-rich layer have a normal position, where the carapace is up and plastron is down, which means that these turtles were not moved along with the currents. All turtle remains display very little abrasion, confirming a small-scale transportation of fauna.
Herein, bioerosion is informative as a general indicator of exposure time of the turtle skeletons at the sediment-water interface before the final burial. It is remarked that most bioerosive structures are recorded in the carapaces, indicating little transportation and relatively prolonged period of exposure prior to deposition, while bioerosion in the plastrons are fewer, reflecting post-mortem period of colonisation. It has been noticed that bioeroders occurred both on carapace and plastron, preferring carapace surfaces.
The evidence of predators and bioerosion are quite well recorded in turtle shells of the study area. Signs of insect feeding represent the predominant bioerosional structures in the turtle bones. This reflects an increased insect activity and a high consumption of the nutrient-rich spongy tissue, causing loss of large areas of the turtle bone surface. The infestation by insect larvae is well pronounced in turtle bones of this study, which indicates longer exposure times before the final deposition or burial. This is confirmed by the work of Lopes and Ferigolo (2015) who indicated that bones protected by flesh and skin until the final burial displayed no infestation by insects. The scarcity of bioerosional traces that could be attributed to predators or scavengers (e.g., Nihilichus) indicates that carnivory was an unessential element in the bioerosion of turtle remains. On the other hand, the record of Osedacoides that were produced by Osedax worms implies that the bone was still fresh and contained enough usable organic matter to support the colonisers.
Although the presence of any bioerosive records on turtle bones almost always indicates post-mortem production, the presence of some structures (e.g., Karethraichnus and Nihilichnus) may be indicative of the opposite. The presence of these borings suggests that the turtle bones had a relatively long time of exposure, which was sufficient to allow the silphid and histerid beetles to leave such a significant effect on their bones. Karethraichnus lakkos borings are formed as a result of various activities of parasitic organisms that should be produced in vivo rather than post-mortem (Zonneveld et al., 2016). In addition, the ichnogenus Nihilichnus was recorded on a carapace fragment of a tortoise that may have been emplaced pre- or post-mortem on the host animal (Zonneveld et al., 2022a).
The bioerosive assemblages in the studied bones indicate that they were deposited in a low-energy environment, with low rates of sedimentation. It is suggested that bioeroder activities most probably occurred immediately after the death and accumulation on the substrate of the turtle remains, but before the carapace was overturned by large scavengers.
CONCLUSIONS
1. The uppermost part of the Campanian Quseir Formation (the Hindaw Member) of Kharga Oasis, Egypt, contains a high concentration of turtle skeletal remains that lived in small ponds and marshes and were deposited as autochthonous to parautochthonous relics.
2. The turtle skeletal remains display significant bioerosional structures, indicating that they were utilised by predators and scavengers.
3. Common signs of insect feeding reflect increased insect activity and a slightly prolonged time of exposure prior to final sedimentation.
4. Nine ichnogenera (Nihilichnus, Karethraichnus, Cubiculum, Osteocallis, Radulichnus, Osteichnus, Osedacoides, Sulculites, and Machichnus), representing 11 ichnospecies, were identified. Eight of these ichnotaxa are recorded for the first time in Egypt.
5. The recognised bioerosional structures appeared as borings, shallow chambers, grooves and punctures produced by ticks, beetles, polychaete worms, fishes/crocodiles and gastropods.
6. Such bioerosion traces were likely caused by relatively long exposure time before the final deposition or burial. In some cases, borings may have been produced in vivo.
7. The new material considerably expands the stratigraphic and geographic distribution of this trace fossil assemblage and reveals that their producers may have been able to survive in other palaeoenvironmental conditions.
ACKNOWLEDGEMENTS
We are grateful to the Executive Editor (M. Hyžný) and the Handling Editor (G. Sobral) for their assistance and encouragement to revise the manuscript. Insightful reviews and suggestions by two anonymous reviewers, were greatly appreciated. We are indebted to J-P. Zonneveld (University of Alberta) and D. Knaust (Equinor ASA) for their advice, fruitful comments, and suggestions in the early manuscript. The authors sincerely thank A. Farag, M. Kamel, and A. Essam, for their help during the field trips and the fossils excavations. We also thank H. Farahat and D. Gamal, New Valley Vertebrate Palaeontology Centre, New Valley University, for their great efforts in the preparation of the studied fossils in the laboratory. Special thanks go to A. El-Refaiy (Alexandria University) for his help in preparation of some illustrations.
REFERENCES
AbdelGawad, M., Kassab, W., and Abu El-Kheir, G. 2019. Reviewing of the Testudines occurrence in the Late Cretaceous from South Western Desert, Egypt. European Geoscience Union General Assembly, 21:1671.
AbdelGawad, M., Pérez-García, A., Hirayama, R., Mohesn, S., Tantawy, A.-A., and Abu El-Kheir, G. 2023. The first side-necked turtle (Pleurodira, Bothremydidae) from the Campanian (Late Cretaceous) of Egypt. Diversity, 15:284.
https://doi.org/10.3390/d15020284
Abu El-Kheir, G.A. 2020. Taphonomic conditions and assessment of the Late Cretaceous vertebrates bearing sites in the Western Desert, Egypt. Egyptian Journal of Geology, 64:471–484. https://doi.org/10.21608/EGJG.2020.216326
Abu El-Kheir, G.A., AbdelGawad, M., and Kassab, W. 2021. First known gigantic sea turtle from the Maastrichtian deposits in Egypt. Acta Palaeontologica Polonica, 66:349–355.
https://doi.org/10.4202/app.00849.2020
Adrian, B., Smith, H.F., Hutchison, J.H., and Townsend, K.E.B. 2021. Geometric morphometrics and anatomical network analyses reveal ecospace partitioning among geoemydid turtles from the Uinta Formation, Utah. The Anatomical Record, 305:1359–1393.
https://doi.org/10.1002/ar.24792
Ahmed, I.K., Salmi-Laouar, S., Veselská, M.K., Mikuláš, R., Kočí, T., Ferré, B., Naimi, M., and Váchová, L. 2022. Sclerobiont assemblages on macro-invertebrates from the Cenomanian strata of Djebel Bouarif (Aurès Range, Algeria). Historical Biology.
https://doi.org/10.1080/08912963.2022.2136032
Andrews, C.W. 1900. On a new species of chelonian (Podocnemis aegyptiaca) from the Lower Miocene of Egypt. Geological Magazine, 7:1–2.
https://doi.org/10.1017/S0016756800159801
Awad, G.H. and Ghobrial, M.G. 1965. Zonal stratigraphy of the Kharga Oasis. Geological Survey of Egypt, 34:1–77.
Bader, K.S., Hasiotis, S.T., and Martin, L.D. 2009. Application of forensic science techniques to trace fossils on dinosaur bones from a quarry in the upper Jurassic Morrison Formation, northeastern Wyoming. Palaios, 24:140–158.
https://doi.org/10.2110/palo.2008.p08-058r
Ball, J. 1900. Kharga Oasis: Its Topography and Geology. Egyptian Survey Department, Cairo.
Barber-James, H.M., Gattolliat, J.-L., Sartori, M., and Hubbard, M.D. 2008. Global diversity of mayflies (Ephemeroptera, Insecta) in freshwater. Hydrobiologia, 595:339–350.
https://doi.org/10.1007/s10750-007-9028-y
Barthel, K.W. and Herrmann-Degen, W. 1981. Late Cretaceous and early Tertiary stratigraphy in the Great Sand Sea and its SE margins (Farafra and Dakhla Oases), SW Desert, Egypt. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie, 21:141–182.
Baur, G. 1891. Notes on some little known American fossil tortoises. Proceedings of the Academy of Natural Sciences of Philadelphia, 43:411–430.
Beadnell, H.J.L. 1909. An Egyptian Oasis: An Account of the Oasis of Kharga in the Libyan Desert. John Murray, London, UK.
Behrensmeyer, A.K. 1978. Taphonomic and ecologic information from bone weathering. Paleobiology, 4:150–162.
Bieńkowska-Wasiluk, M., Bonde, N., Møller, P.R., and Gaździcki, A. 2013. Eocene relatives of cod icefishes (Perciformes: Notothenioidei) from Seymour Island, Antarctica. Geological Quarterly, 57:567–582.
https://doi.org/10.7306/gq.1112
Bohatý, J. 2011. Revision of the disparid Stylocrinus (Crinoidea) from the Devonian of Europe, Asia and Australia. Palaeontology, 54:1177–1197.
https://doi.org/10.1111/j.1475-4983.2011.01067.x
Brand, L.R., Goodwin, H.T., Ambrose, P.D., and Buchheim, H.P. 2000. Taphonomy of turtles in the Middle Eocene Bridger Formation, SW Wyoming. Palaeogeography, Palaeoclimatology, Palaeoecology, 162:171–189.
https://doi.org/10.1016/S0031-0182(00)00111-5
Britt, B.B., Scheetz, R.D., and Dangerfield, A. 2008. A suite of dermestid beetle traces on dinosaur bone from the Upper Jurassic Morrison Formation, Wyoming, USA. Ichnos, 15:59–71.
https://doi.org/10.1080/10420940701193284
Cadena, E.A., Bloch, J.I., and Jaramillo, C.A. 2012. New bothremydid turtle (Testudines, Pleurodira) from the Paleocene of northeastern Colombia. Journal of Paleontology, 86:689–699.
https://doi.org/10.1666/11-128R1.1
Chumakov, N.M., Dronov, A.V., and Mikuláš, R. 2013. New ichnospecies of scratching traces from phosphatic nodules (Cenomanian, England). Stratigraphy and Geological Correlation, 21:52–59.
https://doi.org/10.1134/S0869593813030027
Churcher, C.S. and De Iuliis, G. 2001. A new species of Protopterus and a revision of Ceratodus humei (Dipnoi: Ceratodontiformes) from the Late Cretaceous Mut Formation of eastern Dakleh Oasis, Western Desert of Egypt. Palaeontology, 44:305–323.
https://doi.org/10.1111/1475-4983.00181
Claeson, K.M., Sallam, H.M., O’Connor, P.M., and Sertich, J.J.W. 2014. A revision of the Upper Cretaceous lepidosirenid lungfishes from the Quseir Formation, Western Desert, central Egypt. Journal of Vertebrate Paleontology, 34:760–766.
https://doi.org/10.1080/02724634.2014.838574
Collareta, A., Merella, M., Bosselaers, M., Casati, S., Di Cencio, A., and Bianucci, G.A. 2022. A Karethraichnus boring on a turtle shell bone from the Miocene of Italy is assessed as the attachment scar of a platylepadid symbiont. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 303:327–337.
https://doi.org/10.1127/njgpa/2022/1052
Collareta, A., Varas-Malca, R., Bosio, G., Urbina, M., and Coletti, G. 2023a. Ghosts of the holobiont: Borings on a Miocene turtle carapace from the Pisco Formation (Peru) as witnesses of ancient symbiosis. Journal of Marine Science and Engineering, 11:45.
https://doi.org/10.3390/jmse11010045
Collareta, A., Merella, M., Casati, S., Di Cencio, A., Tinelli, C., and Bianucci, G. 2023b. Polyplacophoran feeding traces on Mediterranean Pliocene sirenian bones: Insights on the role of grazing bioeroders in shallow-marine vertebrate falls. Life, 13:327.
https://doi.org/10.3390/life13020327
Dominato, V.H., Mothe, D., Avilla, L.S., and Bertoni-Machado, C. 2009. Ação de insetos em vértebras de Stegomastodon waringi (Mammalia, Gomphotheriidae) do Pleistoceno de Águas de Araxá, Minas Gerais, Brasil. Revista Brasileira de Paleontologia, 12:77–82.
https://doi.org/10.4072/rbp.2009.1.07
Dzik, J., Sulej, T., and Niedźwiedzki, G. 2008. A dicynodont-theropod association in the latest Triassic of Poland. Acta Palaeontologica Polonica, 53:733–738.
https://doi.org/10.4202/app.2008.0415
El-Habaak, G., Askalany, M., Faraghaly, M., and Abdel-Hakeem, M. 2016. The economic potential of El-Gedida glauconite deposits, El-Bahariya Oasis, Western Desert, Egypt. Journal of African Earth Sciences, 120:186–197.
https://doi.org/10.1016/j.jafrearsci.2016.05.007
Fiorillo, A.R. 1987. Trample marks: Caution from the Cretaceous. Current Research in the Pleistocene, 4:73–75.
Fiorillo, A.R. 1990. Prey bone utilization by predatory dinosaurs. Palaeogeography, Palaeoclimatology, Palaeoecology, 88:157–166.
https://doi.org/10.1016/0031-0182(91)90062-V
Francischini, H., Paes Neto, V.D., Martinelli, A.G., Pereira, V.P., Marinho, T.S., Teixira, V.P.A., Ferraz, M.LF., Soares, M.B., and Schultz, C.L. 2016. Invertebrate traces in pseudo-coprolites from the upper Cretaceous Marília 56 Formation (Bauru Group), Minas Gerais State, Brazil. Cretaceous Research, 57:29–39.
https://doi.org/10.1016/j.cretres.2015.07.016
Gaffney, E.S. and Tong, H. 2008. Redescription of the skull of Ummulisani rutgersensis Gaffney, Tong, and Meylan, 2006, a bothremydid side-necked turtle from the Eocene of Morocco. American Museum Novitates, 3615:1–20.
https://doi.org/10.1206/607.1
Gaffney, E.S., Campos, D.D., and Hirayama, R. 2001a. Cearachelys, a new side-necked turtle (Pelomedusoides: Bothremydidae) from the early Cretaceous of Brazil. American Museum Novitates, 3319:1–25.
Gaffney, E.S., Chatterjee, S., and Rudra, D.K. 2001b. Kurmademys, a new side-necked turtle (Pelomedusoides: Bothremydidae) from the late Cretaceous of India. American Museum Novitates, 3321:1–16.
Gaffney, E.S., Hooks, G.E., and Schneider, V.P. 2009a. New material of North American side-necked turtles (Pleurodira: Bothremydidae). American Museum Novitates, 3655:1–26.
Gaffney, E.S., Krause, D.W., and Zalmout, I.S. 2009b. Kinkonychelys, a new side-necked turtle (Pelomedusoides: Bothremydidae) from the late Cretaceous of Madagascar. American Museum Novitates, 3662:1–25.
Gaffney, E.S., Roberts, E., Sissoko, F., Bouare, M.L., Tapanila, L., and O’leary, M.A. 2007. Acleistochelys, a new side-necked turtle (Pelomedusoides: Bothremydidae) from the Paleocene of Mali. American Museum Novitates, 3549:1–24.
Gaffney, E.S., Tong, H.Y., and Meylan, P.A. 2006. Evolution of the side-necked turtles: the families Bothremydidae, Euraxemydidae, and Araripemydidae. Bulletin of the American Museum of Natural History, 300:1–698.
Genise, J.F. 2017. Ichnoentomology: Insect Traces in Soils and Paleosols. Springer, Switzerland.
Gibert, J.M. De, Domènech, R., and Martinell, J. 2007. Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro) bioerosion ichnofacies model. Acta Palaeontologica Polonica, 52:783–798.
Gregorio, E.B. Di and Araújo, H.I. 2020. Occurrence of the ichnospecies Nihilichnus nihilicus Mikulás, Kadlecová, Fejfar and Dvorák (2006) in vertebra of Crocodylia from the Upper Miocene Solimões Formation, Acre Basin. Anuário do Instituto de Geociências, 43:408–413.
https://doi.org/10.11137/2020_1_408_413
Hasiotis, S.T. 2004. Reconnaissance of Upper Jurassic Morrison Formation ichnofossils, Rocky Mountain Region, USA: paleoenvironmental, stratigraphic, and paleoclimatic significance of terrestrial and freshwater ichnocoenoses. Sedimentary Geology, 167:177–268.
https://doi.org/10.1016/j.sedgeo.2004.01.006
Hasiotis, S.T., Fiorillo, A.R., and Hanna, R.R. 1999. Preliminary report on borings in Jurassic dinosaur bones: Evidence for invertebrate-vertebrate interactions. Utah Geological Survey Miscellaneous Publication, 99:193–200.
Hendriks, F., Luger, P., Kallenbach, H., and Schroeder, J.H. 1984. Stratigraphical and sedimentological framework of the Kharga-Sinn El-Kaddab stretch (western and southern part of the upper Nile Basin), Western Desert, Egypt. Berliner Geowissenschaftliche Abhandlungen, Reihe A, Geologie und Paläontologie, 50:117–151.
Hermina, M. 1967. Geology of the north-western approaches of Kharga. Egyptian Geological Survey, 44:1–88.
Hermina, M. 1990. The surroundings of Kharga, Dakhla, and Farafra Oases, p. 259–292. In Said, R. (ed.), The Geology of Egypt. AA Balkema, Rotterdam, the Netherlands.
Ibrahim, N., Varricchio, D.J., Sereno, P.C., Wilson, J.A., Dutheil, D.B., Martill, D.M., Baidder, L., and Zouhri, S. 2014. Dinosaur footprints and other ichnofauna from the Cretaceous Kem Kem beds of Morocco. PLoS ONE, 9:e90751.
https://doi.org/10.1371/journal.pone.0090751
Irazoqui, F. and Acosta Hospitaleche, C. 2022. Bioerosive traces in fossil penguin bones (Aves, Sphenisciformes) from the Eocene of Marambio/Seymour Island (West Antarctica). Historical Biology, 34:2341–2349.
https://doi.org/10.1080/08912963.2021.2017915
Jagt, J.W.M. 2003. The ichnofossil genera Radulichnus and Renichnus in the Maastrichtian of the Netherlands and Belgium. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, 73:175–184.
Jagt, J.W.M., Deckers, M.J.M., Leebeeck, M. De, Donovan, S.K., and Nieuwenhuis, E. 2020. Episkeletozoans and bioerosional ichnotaxa on isolated bones of Late Cretaceous mosasaurs and cheloniid turtles from the Maastricht area, the Netherlands. Geologos, 26:39–49.
https://doi.org/10.2478/logos-2020-0003
Jalvo, Y.F. and Andrews, P. 2016. Atlas of Taphonomic Identifications: 1001+ Images of Fossil and Recent Mammal Bone Modification. Springer, the Netherlands.
Janssen, R., Van Baal, R.R., and Schulp, A.S. 2013. Bone damage in Allopleuron hofmanni (Cheloniidae, Late Cretaceous). Geologie en Mijnbouw, 92:153–157.
https://doi.org/10.1017/S0016774600000081
Karl, H.-V. 2016. First global survey of evidences the ichnogenus Osedacoides and it relates to the Rezent Zombie-Worms Osedax. Global Journal of Science Frontier Research, 16(C):1–5.
https://journalofscience.org/index.php/GJSFR/article/view/1771
Karl, H.-V. and Tichy, G. 2004. The structure of fossil teeth of chelonophagous crocodiles (Diapsida: Crocodylia). Studia Geologica Salmanticensia, 40:115–124.
Karl, H.-V., Groning, E., and Brauckmann, C. 2012. Revision of Tropidemys seebachi Portis, 1878 (Testudines: Eucryptodira) from the Kimmeridgian (Late Jurassic) of Hannover (Northwestern Germany). Studia Palaeocheloniologica, 4:11–24.
Kitching, J.W. 1980. On some fossil Arthropoda from the Limeworks, Makapansgat, Potgietersrus. Palaeontologia Africana, 23:63–68.
Lapparent de Broin, F. 2000. African chelonians from the Jurassic to the Present. A preliminary catalog of the African fossil chelonians. Palaeontologia Africana, 36:43–82.
Lapparent de Broin, F. and Werner, C. 1998. New Late Cretaceous turtles from the Western Desert, Egypt. Annales de Paléontologie, 84:131–214.
https://doi.org/10.1016/S0753-3969(98)80005-0
Laudet, F. and Antoine, P.O. 2004. Des chambres de pupation de Dermestidae (Insecta: Coleoptera) sur un os de mammifère tertiaire (phosphorites du Quercy): implications taphonomiques et paléoenvironnementales. Geobios, 37:376–381.
https://doi.org/10.1016/j.geobios.2003.04.005
Laurent, Y., Tong, H., and Claude, J. 2002. New side-necked turtle (Pleurodira: Bothremydidae) from the Upper Maastrichtian of the Petites-Pyrénées (Haute-Garonne, France). Cretaceous Research, 23:465–471.
https://doi.org/10.1006/cres.2002.1015
Lehman, T.M. and Wick, S.L. 2010. Chupacabrachelys complexus, n. gen. n. sp. (Testudines: Bothremydidae), from the Aguja Formation (Campanian) of West Texas. Journal of Vertebrate Paleontology, 30:1709–1725.
Lopes, R.P. and Ferigolo, J. 2015. Postmortem modifications (pseudopaleopathologies) in middle–late Pleistocene mammal fossils from Southern Brazil. Revista Brasileira de Paleontologia, 18:285–306.
https://doi.org/10.4072/rbp.2015.2.09
Lopes, R.P. and Pereira, J.C. 2019. Molluskan grazing traces (ichnogenus Radulichnus Voigt, 1977) on a Pleistocene bivalve from southern Brazil, with the proposal of a new ichnospecies. Ichnos, 26:141–157.
https://doi.org/10.1080/10420940.2018.1532898
Mahmoud, M.S. 2003. Palynology and palaeoenvironment of the Quseir Formation (Campanian) from central Egypt. Journal of African Earth Sciences, 36:135–148.
https://doi.org/10.1016/S0899-5362(03)00047-2
Martin, L.D. and West, D.L. 1995. The recognition and use of dermestid (Insecta, Coleoptera) pupation chambers in paleoecology. Palaeogeography, Palaeoclimatology, Palaeoecology, 113:303–310.
https://doi.org/10.1016/0031-0182(95)00058-T
McHugh, J.B., Drumheller, S.K., Riedel, A., and Kane, M. 2020. Decomposition of dinosaurian remains inferred by invertebrate traces on vertebrate bone reveal new insights into Late Jurassic ecology, decay, and climate in western Colorado. PeerJ, 8:e9510.
https://doi.org/10.7717/peerj.9510
Mikuláš, R. 1992. Early Cretaceous borings from Štramberk (Czechoslovakia). Časopis pro mineralogii a geologii, 37:297–312.
Mikuláš, R. and Dvořák, Z. 2010. Possible crocodylian bite traces, Miocene of the Most Basin (Czech Republic). New Mexico Museum of Natural History and Science Bulletin, 51:191–193.
Mikuláš, R., Kadlecová, E., Fejfar, O., and Dvořák, Z. 2006. Three new ichnogenera of biting and gnawing traces on reptilian and mammalian bones: A case study from the Miocene of the Czech Republic. Ichnos, 13:113–127.
https://doi.org/10.1080/10420940600850729
Milàn, J., Lindow, B.E.K., and Lauridsen, B.W. 2011. Bite traces in a turtle carapace fragment from the middle Danian (Lower Paleocene) bryozoan limestone, Faxe, Denmark. Bulletin of the Geological Society of Denmark, 59:61–67.
https://doi.org/10.37570/bgsd-2011-59-07
Mujal, E., Foth, C., Maxwell, E.E., Seegis, D., and Schoch, R.R. 2022. Feeding habits of the Middle Triassic pseudosuchian Batrachotomus kupferzellensis from Germany and palaeoecological implications for archosaurs. Palaeontology, 65:e12597.
https://doi.org/10.1111/pala.12597
Mulder, E.W.A., Jagt, J.W.M., and Schulp, A.S. 2005. Another record of a hadrosaurid dinosaur from the Maastrichtian type area (The Netherlands, Belgium): Seeley (1883) revisited. Bulletin de l’Institut Royal des Sciences Naturelles de Belqique, Sciences de la Terre, 75:201–206.
Niedźwiedzki, G., Gorzelak, P., and Sulej, T. 2011. Bite traces on dicynodont bones and the early evolution of large terrestrial predators. Lethaia, 44:87–92.
https://doi.org/10.1111/j.1502-3931.2010.00227.x
Omara, S., Philobbos, E.R., and Mansour, H.H. 1976. Contribution to the geology of the Dakhla Oasis area, Western Desert, Egypt. Bulletin of the Faculty of Science Assiut University, 5:319–339.
Ozeki, C.S., Martill, D., Smith, R., and Ibrahim, N. 2020. Biological modification of bones in the Cretaceous of North Africa. Cretaceous Research, 114:104529.
https://doi.org/10.1016/j.cretres.2020.104529
Paes Neto, V.D., Parkinson, A.H., Pretto, F.A., Soares, M.B., Schwanke, C., Schultz, C.L., and Kellner, A.W. 2016. Oldest evidence of osteophagic behavior by insects from the Triassic of Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology, 453:30–41.
https://doi.org/10.1016/j.palaeo.2016.03.026
Perea, D., Verde, M., Montenegro, F., Toriño, P., Manzuetti, A., and Roland, G. 2020. Insect trace fossils in glyptodonts from Uruguay. Ichnos, 27:70–79.
https://doi.org/10.1080/10420940.2019.1584562
Pirrone, C.A., Buatois, L.A., and Bromley, R.G. 2014. A new ichnospecies of Cubiculum from Upper Cretaceous dinosaur bones in Western Argentina. Ichnos, 21:251–260.
https://doi.org/10.1080/10420940.2014.958225
Rasser, M.W., Vallon, L.H., and Salvador, R.B. 2016. Perforations of freshwater snail shells from the Miocene of Germany: Nihilichnus covichi n. isp. Ichnos, 23:222–227.
https://doi.org/10.1080/10420940.2016.1164154
Roberts, E.M., Rogers, R.R., and Foreman, B.Z. 2007. Continental insect borings in dinosaur bone: Examples from the late Cretaceous of Madagascar and Utah. Journal of Paleontology, 81:201–208.
https://doi.org/10.1666/0022-3360(2007)81[201:CIBIDB]2.0.CO;2
Said, R. 1961. Tectonic framework of Egypt and its influence on distribution of foraminifera. American Association of Petroleum Geologists Bulletin, 45:198–218.
https://doi.org/10.1306/0BDA6335-16BD-11D7-8645000102C1865D
Said, R. 1962. The Geology of Egypt. Elsevier, Amsterdam, the Netherlands.
Said, R. 1990. Cretaceous paleogeographic maps, p. 439–449. In Said, R. (ed.), The Geology of Egypt. AA Balkema, Rotterdam, the Netherlands.
Saint-Seine, R. de. 1951. Un Cirripède acrothoracique du Crétacé: Rogerella lecointrei nov. gen., nov. sp. Comptes rendus de l’Académie des Sciences, 233:1015–1053.
Saneyoshi, M., Watabe, M., Suzuki, S., and Tsogtbaatar, K. 2011. Trace fossils on dinosaur bones from Upper Cretaceous eolian deposits in Mongolia: Taphonomic interpretation of paleoecosystems in ancient desert environments. Palaeogeography, Palaeoclimatology, Palaeoecology, 311:38–47.
https://doi.org/10.1016/j.palaeo.2011.07.024
Schwarz-Wings, D., Milàn, J., and Gravesen, P. 2014. A new eusuchian (Crocodylia) tooth from the Early or Middle Paleocene, with a description of the Early/Middle Paleocene boundary succession at Gemmas Allé, Copenhagen, Denmark. Bulletin of the Geological Society of Denmark, 62:17–26.
https://doi.org/10.37570/bgsd-2014-62-02
Siddall, M.E. and Gaffney, E.S. 2004. Observations on the leech Placobdella ornata feeding from bony tissues of turtles. Journal of Parasitology, 90:1186–1188.
https://doi.org/10.1645/GE-277R
Tapanila, L., Roberts, E.M., Bouaré, M.L., Sissoko, F., and O’Leary, M.A. 2004. Bivalve borings in phosphatic coprolites and bone, Cretaceous–Paleogene, northeastern Mali. Palaios, 19:565–573.
https://doi.org/10.1669/0883-1351(2004)019<0565:BBIPCA>2.0.CO;2
Tappan, M. 1994. Bone weathering in the tropical rain forest. Journal of Archaeological Science, 21:667–673. https://doi.org/10.1006/jasc.1994.1066
Thenius, E. 1979. Lebensspuren von Ephemeropteren-Larven aus dem Jung-Tertiär des Wiener Beckens. Annalen des Naturhistorischen Museums in Wien, 82:177–188.
Thenius, E. 1988a. Lebensspuren von aquatischen Insektenlarven aus dem Jungtertiär Niederösterreichs. Beiträge zur Paläontologie Österreich, 14:1–17.
Thenius, E. 1988b. Fossile lebensspuren aquatischer Insekten in Knochen aus dem Jungtertiär Niederösterreichs. Anzeiger der Österreichischen Akademie der Wissenschaften, 125:41–45.
Vialov, O.S. and Nessov, L.A. 1974. Post-mortem injuries of shells of some Early Cretaceous turtles by bone-damaging organisms. Paleontologicheskiy Sbornik, 11:99–103. (In Russian)
Voigt, E. 1977. On grazing traces produced by the radula of fossil and recent gastropods and chitons. Geological Journal Special Issue, 9:335–341.
Ward, W.C. and McDonald, K.C. 1979. Nubia Formation of central Eastern Desert, Egypt‒Major subdivisions and depositional setting. American Association of Petroleum Geologists Bulletin, 63:975–983.
https://doi.org/10.1306/2F9184AF-16CE-11D7-8645000102C1865D
West, D.J. and Hasiotis, S.T. 2007. Trace fossils in an archaeological context: Examples from bison skeletons, Texas, USA, p. 545–561. In Miller, W.III. (ed.), Trace Fossils–Concepts, Problems, Prospects. Elsevier, Amsterdam, the Netherlands.
Wisshak, M., Knaust, D., and Bertling, M. 2019. Bioerosion ichnotaxa: review and annotated list. Facies, 65:24.
https://doi.org/10.1007/s10347-019-0561-8
Xing, L., Parkinson, A., Ran, H., Pirrone, C.A., Roberts, E.M., Zhang, J., Burns, M., Wang, T., and Choiniere, J. 2015. The earliest fossil evidence of bone boring by terrestrial invertebrates, examples from China and South Africa. Historical Biology, 28:1108–1117.
https://doi.org/10.1080/08912963.2015.1111884
Youssef, M.I. 1957. Upper Cretaceous rocks in Kosseir Area. Bulletin of the Institute of Desert, Egypt, 7:35–54.
Zonneveld, J-P., Bartels, W.S., Guunell, G.F., and Mchugh, L.P. 2016. Borings in early Eocene turtle shell from the Wasatch Formation, South Pass, Wyoming. Journal of Paleontology, 89:802–820.
https://doi.org/10.1017/jpa.2015.61
Zonneveld, J-P., AbdelGawad, M.K., and Miller, E.R. 2022a. Ectoparasite borings, mesoparasites borings, and scavenging traces in early Miocene turtle and tortoise shell: Moghra Formation, Wadi Moghra, Egypt. Journal of Paleontology, 96:304–322.
https://doi.org/10.1017/jpa.2021.92
Zonneveld, J-P., Zonneveld, Z.E.E., Bartels, W.S., Gingras, M.K., and Head, J.J. 2022b. Bone modification features resulting from barnacle attachment on the bones of Loggerhead Sea turtles (Carretta caretta), Cumberland Island, Georgia, USA: Implications for the paleoecological and taphonomic analyses of fossil sea turtles. Palaios, 37:650–670.
https://doi.org/10.2110/palo.2022.021

