 Neoichnology of the burrowing spiders Gorgyrella inermis (Mygalomorphae: Idiopidae) and Hogna lenta (Araneomorphae: Lycosidae)
Neoichnology of the burrowing spiders Gorgyrella inermis (Mygalomorphae: Idiopidae) and Hogna lenta (Araneomorphae: Lycosidae)
Article number: 18.1.7A
https://doi.org/10.26879/500
Copyright Palaeontological Association, March 2015
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 19 June 2014. Acceptance: 23 January 2015
{flike id=1057}
ABSTRACT
Burrowing arachnids are important to modern soil ecosystems, but knowledge of these animals in ancient soil ecosystems is limited. In this study, two species of burrowing spiders were studied: Gorgyrella inermis (South African trapdoor spider) and Hogna lenta (field wolf spider). Individuals of each species were studied to investigate their burrowing techniques and behaviors and to categorize the morphologies of their burrows. Experiments were run with variations in sediment density and sediment moisture to evaluate the effects of environmental conditions on burrow morphology. Seven burrow architectures were produced by the spiders: vertical shafts, vertical shafts with terminal chambers, subvertical shafts, subvertical shafts with terminal chambers, J-shaped burrows, Y-shaped burrows, and isolated chambers. All burrow architectures share common features that make them identifiable as spider burrows. Sediment density and moisture had little influence on burrow morphology, but architecture diversity was greatest in sediments of moderate density and moisture. Results from this study show that spiders produce unique biogenic structures that can be distinguished from the burrows of other soil organisms. Data collected from this study can be used to better interpret the paleoecology and evolutionary history of spiders and soil arthropods.
J. Michael Hils. Department of Geological Sciences, Ohio University, 316 Clippinger Laboratories, Athens, Ohio 45701, USA. jhils2@udayton.edu
Daniel I. Hembree. Department of Geological Sciences, Ohio University, 316 Clippinger Laboratories, Athens, Ohio 45701, USA. Corresponding author. hembree@ohio.edu
Keywords: ichnofossils; trace fossils; continental; arthropod; arachnid; paleoecology
Final citation: Hils, J. Michael and Hembree, Daniel I. 2015. Neoichnology of the burrowing spiders Gorgyrella inermis (Mygalomorphae: Idiopidae) and Hogna lenta (Araneomorphae: Lycosidae). Palaeontologia Electronica 18.1.7A: 1-62. https://doi.org/10.26879/500
palaeo-electronica.org/content/2015/1057-neoichnology-of-spiders
INTRODUCTION
Organisms produce biogenic structures by modifying a substrate in response to abiotic and biotic environmental conditions. These structures may be preserved in the geologic record as trace fossils. Traces produced in marine ecosystems have long been studied (Dapples, 1938; Moore, 1938; MacGinitie, 1945; Barnes and Powell, 1950; Frey, 1968; Ginrgras et al., 2008), but more recent studies have begun to focus on the traces found in continental ecosystems (Ahlbrandt et al., 1978; Ratcliffe and Fagerstrom, 1980; O’Green and Busacca, 2001; Gingras et al., 2002; Hembree and Hasiotis, 2006; Lawfield and Pickerill, 2006; Genise et al., 2009; Hamer and Sheldon, 2010; Buynevich et al., 2011). Trace fossils may be used to infer the presence of organisms whose bodies are rarely fossilized (Cameron, 1969; de Gibert et al., 2000; Chin et al., 2013; Fernández and Pazos, 2013) and to interpret the environmental conditions under which the trace was produced (Turner et al., 1981; Savrda and Bottjer, 1986; Maples and Archer, 1989; Kraus and Hasiotis, 2006; Dashtgard et al., 2008). These interpretations are possible because of studies of living tracemakers in the field and laboratory (Hasiotis, 2007).
Terrestrial arthropods contribute to soil formation by burrowing through and manipulating sediment as they make shelter, find food, and create places for reproduction (Smith and Hasiotis, 2008; Counts and Hasiotis, 2009; Hembree, 2009; Halfen and Hasiotis, 2010; Melchor et al., 2010; Fairchild and Hasiotis, 2011; Getty et al., 2013; Mikuś and Uchman, 2013; Hembree, 2013; Bowen and Hembree, 2014; Hembree, 2014). Ratcliffe and Fagerstrom (1980) provided some examples of the types of continental biogenic structures produced by several extant soil arthropods and the purposes of these structures. Arachnids (spiders, scorpions, mites) are common burrowing arthropods with an extensive fossil record extending to the Silurian (Dunlop, 2010). Prior neoichnological studies on arachnids such as scorpions and whip scorpions have shown that modern burrowing arachnids produce unique biogenic structures and are active agents in soil formation (Sadler, 1993; Hembree et al., 2012; Hembree, 2013; Schmerge et al., 2013; Hembree, 2014). Burrowing spiders are another important group of soil-dwelling arachnids, but few studies have documented the morphology of spider burrows or how these animals respond to environmental stresses.
The purpose of this study is to document the burrow morphology of the trapdoor spider Gorgyrella inermis and the wolf spider Hogna lenta (Arachnida: Araneae) in a controlled laboratory setting. The spiders burrowed in sediments modeled after their natural habitats as well as sediments with such stresses as varying sediment composition and moisture. The reasons for this study are to: 1) improve our understanding of the behaviors and techniques associated with burrowing by spiders; 2) aid in the interpretation of terrestrial arthropod-produced trace fossils; 3) facilitate the inference of spiders in strata through the recognition of their fossil burrows, thereby improving paleoecological interpretations; and 4) allow for improved paleoenvironmental interpretations of these strata by linking soil conditions to specific spider burrow properties.
Spider Evolution and Behavior
Araneae consists of 44,032 extant species of spiders (Platnick, 2013) and is divided into two suborders: Mesothelae and Opisthothelae. Mesothelae is considered to be the most basal of the extant spiders. Opisthothelae contains two infraorders: Mygalomorphae and Araneomorphae. Mygalomorphae contains 2,792 species in 329 genera (Platnick, 2013). Mygalomorph spiders are generally bulky, ground-dwelling spiders such as trapdoor spiders and tarantulas. Araneomorphae contains most extant spiders with 41,748 species in 3,595 genera (Platnick, 2013). These spiders are generally slender, web-building spiders such as orb-weaver spiders, black widows, and wolf spiders.
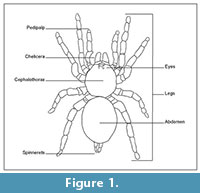 Spider bodies are divided into two sections: the anterior cephalothorax and the posterior abdomen (Figure 1) (Rupert et al., 2004). Spiders use pedipalps, or non-walking appendages near the chelicerae, to grab and kill prey as well as to assist in reproduction (Pechenik, 1991). The segments composing the cephalothorax are completely fused and those of the abdomen are fused in all derived spiders (Rupert et al., 2004). Spiders produce silk using spinnerets located at the posterior of the abdomen.
Spider bodies are divided into two sections: the anterior cephalothorax and the posterior abdomen (Figure 1) (Rupert et al., 2004). Spiders use pedipalps, or non-walking appendages near the chelicerae, to grab and kill prey as well as to assist in reproduction (Pechenik, 1991). The segments composing the cephalothorax are completely fused and those of the abdomen are fused in all derived spiders (Rupert et al., 2004). Spiders produce silk using spinnerets located at the posterior of the abdomen.
The fossil record of spiders extends to the Carboniferous and currently consists of approximately 1,185 valid species (Dunlop et al., 2013). The oldest known fossil mesothele is from the Carboniferous of France near Montceau-les-Mines (Selden, 1996; Selden, 2000). The oldest known opisthotheles appear in the Triassic consisting of a mygalomorph from the Grès à meuels Formation of France (Selden and Gall, 1992) and two contemporaneous araneomorphs from the Molteno Formation of South Africa and the Cow Branch Formation of Virginia (Selden et al., 1999).
All extant mesothele spiders construct burrows (Bristowe, 1976; King, 2004) as do most mygalomorphs (Hamilton et al., 2012). Positively identified fossil spider burrows are rare, however, and occur mainly in Cenozoic strata, including the Miocene (Pickford, 2000) and the Pleistocene (Hasiotis, 2002). Spiders construct burrows as protection against predators and changes in climatic conditions as well as for brooding and obtaining prey (Gertsch, 1949; Hamilton et al., 2012). Construction of a burrow is energetically expensive and, therefore, sites are chosen carefully (Bond and Coyle, 1995; M’Rabet et al., 2007; Hamilton et al., 2012). Burrows are excavated using both the chelicerae and pedipalps (Gertsch, 1949). The burrows are typically lined with silk, but the thickness of the silk lining varies between species and sediment types (Gertsch, 1949). Burrow size and complexity depends on the species and age of the individual (Gertsch, 1949).
MATERIALS AND METHODS
Two species of spider were chosen for this experiment: the mygalomorph spider Gorgyrella inermis and the araneomorph spider Hogna lenta. These two species were chosen because they are both known burrowers, and they exhibit different types of behaviors associated with their burrows as discussed below. Members of the two infraorders were also chosen to compare burrows made by spiders that are only distantly related.
 Gorgyrella inermis (Figure 2.1-2) is native to the grasslands of South Africa. This trapdoor spider belongs to the family Idiopidae, which is known only from the recent (Platnick, 2013). This species is an ambush predator, waiting at the opening of their burrows for prey to wander into striking range. The burrows of G. inermis have been described as long, simple shafts with an oval-shaped opening (Dippenaar-Schoeman, 2002).
Gorgyrella inermis (Figure 2.1-2) is native to the grasslands of South Africa. This trapdoor spider belongs to the family Idiopidae, which is known only from the recent (Platnick, 2013). This species is an ambush predator, waiting at the opening of their burrows for prey to wander into striking range. The burrows of G. inermis have been described as long, simple shafts with an oval-shaped opening (Dippenaar-Schoeman, 2002).
Hogna lenta (formerly Lycosa lenta) (Figure 2.3) belongs to the araneomorph family Lycosidae, which has a fossil record extending to the Cretaceous (Platnick, 2013). Hogna lenta is native to the southeastern portion of the United States, particularly Florida (Wallace, 1942). They occupy a variety of habitats although they are often found in sandy sediments (Eisman et al., 2010). Members of this family are active, nocturnal hunters that use their burrows as a daytime retreat (Wallace, 1942). The burrows produced by H. lenta are generally simple, vertical shafts that may have a turret (Wallace, 1942; Gertsch, 1949). The burrows are sealed with a trapdoor constructed of a thick layer of silk covered by sand or other material (Eisman et al., 2010).
Eight individuals of Gorgyrella inermis and 11 individuals of Hogna lenta obtained from a commercial source were used in this study. All representatives of H. lenta were adults, whereas G. inermis included five adults (red) and three juveniles (black). Adult G. inermis had a mean body length of 3.7 cm and a body width of 1.4 cm, whereas juveniles had a mean body length of 2.6 cm and body width of 0.85 cm. Hogna lenta specimens had a mean body length of 2.3 cm and body width of 0.85 cm. The spiders of both species were not sexed, but four representatives of H. lenta produced eggs multiple times during the study.
The spiders were allowed to acclimate to laboratory conditions for at least two weeks before the experiments began. During this time, they were housed individually in 10 gal (38 L) terraria filled with 100% finely shredded coconut fiber, a medium not used for the experiments. The laboratory was kept on a 12-hour light cycle with temperatures between 25-30 °C and humidity at ~35%. Burrowing media were kept moist by daily spraying of water both during the acclimation period and the experiments. In addition, individuals of Hogna lenta were provided with water dishes which were refilled when media were moistened. Both species were fed live crickets weekly; any dead crickets were removed from the terraria. Direct handling of the spiders was minimized to avoid altering their behavior and to avoid additional stress.
Three sizes of terraria were used during the experiments: 10 gal (38 L) (50 l × 25 w × 30 h cm), 20 gal (76 L) (60 l × 30 w × 42 h cm), and 30 gal (114 L) (90 l × 42 w × 30 h cm). The terrarium sizes used with each species were determined by evaluating the size of the adult individuals and the potential size of the burrows as determined during the acclimation period. The three sizes of terraria were filled with sediment to depths of 25, 26, and 35 cm, respectively. These depths were chosen to allow enough room for the spiders to burrow but also to prevent escape. The terraria were covered with screen lids to further prevent escape of the individuals. All study specimens were kept at laboratory temperatures with no additional heat input. The sediments used were different mixtures of finely shredded coconut fiber (organic material), fine- to medium-grained quartz sand, and sifted potting soil (organic rich silt and clay). Sediment mixtures were homogenized and moistened prior to filling the terraria. The density and moisture of the sediment were measured using a Field Scout SC900 Soil Compaction Meter (Spectrum Technologies Inc.) and an Aquaterr EC-300 Salinity Multimeter, respectively.
Three experiments were conducted with different sediment compositions and moisture contents to determine if burrow morphology changed in response to changes in environment (Table 1.1). The point of reference for the burrow morphology of each species was established in Experiment 1. The sediment mixtures and sediment moisture levels in Experiment 1 resembled those of each species’ natural habitat (Table 1.1). Two additional parameters were tested in Experiment 1: the time allowed for burrowing and the size of the terrarium used. Spiders were allowed to burrow over time intervals of 14 and 30 days in order to determine if the morphology of the burrows would change over time. The spiders were housed in two terrarium sizes according to the species, one small (10 or 20 gal) and one large (20 or 30 gal), to test the effect of enclosure size on burrow morphology in laboratory conditions. In Experiment 2 the sediment composition was altered to include two sediment types with an increased percentage of fine-grained material (Table 1.2). The sediment moisture content was then altered in Experiment 3 by decreasing and increasing sediment moisture content by 20% per trial (Table 1.3).
The spiders were observed, photographed, and digitally recorded during the experiment. Recorded observations included time elapsed before the spiders began a burrow, silk-production related to burrow construction, and sediment surface features resulting from burrowing. Spiders were removed from the experimental set-up at the conclusion of each trial by either capturing them on the surface or direct extraction from the burrow. The latter method was used for most individuals of Gorgyrella inermis, which rarely leave their burrows, and some individuals of Hogna lenta.
Open burrows were filled with Drystone® plaster. The trapdoors on the burrows of Gorgyrella inermis were covered with plaster and kept attached to the burrow cast to maintain orientation of the burrow. The burrows of Hogna lenta were marked to indicate where the spider generally entered and exited the burrow. In these ways, the height and width of the burrow relative to the spider in life position were known for the measurements. The relative thickness of the silk lining and how deeply it lined the burrow cast were noted. The burrow casts were washed and cleaned of silk and sediment before measurements were taken and the silk lining was discarded.
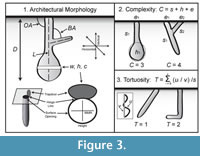 Qualitative data of the burrows were recorded including general shape, number of surface openings, number of burrow branches, and number of chambers (Figure 3.1). Quantitative measurements included the maximum depth, total length, the maximum and minimum angles of the burrow with respect to the sediment surface, and the mean angle of branching if present. Burrow width, height, and circumference were measured at 1 cm intervals along the burrow cast’s length beginning at the surface opening. The width-to-height ratio at these intervals was then calculated. Complexity and tortuosity of the burrow casts were also determined. Both are scale-independent measurements that can be used to compare burrow systems produced by animals of different sizes (Meadows, 1991; Hembree and Hasiotis, 2006). Burrow complexity is the sum of the number of segments (s), the number of openings in the sediment surface (e), and the number of chambers (h) (Figure 3.2). Tortuosity is the measure of the deviation of a shaft or tunnel from a straight line calculated by dividing the total length of a segment (u) by the straight line distance between the ends of the segment (v) (Figure 3.3). Quantitative properties were used to statistically compare the burrow casts. All statistical analyses were performed using PAST (ver. 2.17b) (Hammer et al., 2001).
Qualitative data of the burrows were recorded including general shape, number of surface openings, number of burrow branches, and number of chambers (Figure 3.1). Quantitative measurements included the maximum depth, total length, the maximum and minimum angles of the burrow with respect to the sediment surface, and the mean angle of branching if present. Burrow width, height, and circumference were measured at 1 cm intervals along the burrow cast’s length beginning at the surface opening. The width-to-height ratio at these intervals was then calculated. Complexity and tortuosity of the burrow casts were also determined. Both are scale-independent measurements that can be used to compare burrow systems produced by animals of different sizes (Meadows, 1991; Hembree and Hasiotis, 2006). Burrow complexity is the sum of the number of segments (s), the number of openings in the sediment surface (e), and the number of chambers (h) (Figure 3.2). Tortuosity is the measure of the deviation of a shaft or tunnel from a straight line calculated by dividing the total length of a segment (u) by the straight line distance between the ends of the segment (v) (Figure 3.3). Quantitative properties were used to statistically compare the burrow casts. All statistical analyses were performed using PAST (ver. 2.17b) (Hammer et al., 2001).
The Bray-Curtis similarity measure was used to determine the degree of similarity of burrow morphologies within each individual species and between the two species of spiders. The Bray-Curtis similarity measure is a nonparametric statistical analysis in which the sum of the differences for each quantitative measurement is divided by the sum of the total measurements in the sample (Bray and Curtis, 1957). This number is subtracted from 1.0 to determine similarity. Similarity is ranked from 0.0 (completely dissimilar) to 1.0 (identical). Two burrows with a rank of 1.0 were considered identical, ranks between 0.9 and 0.6 express high to moderate similarity, and ranks ≤ 0.5 are dissimilar (cf. Hembree et al., 2012; Hembree, 2013; Bowen and Hembree, 2014; Catena and Hembree, 2014; Dzenowski and Hembree, 2014; Hembree, 2014).
The scores resulting from the Bray-Curtis analysis were used to construct dendrograms of similarity. Analyses of the dendrograms reveal the amount of similarity between burrows with different qualitative architectures and those produced under different environmental conditions. The dendrograms were also used to determine if the burrows of different animals separate into specific clusters.
 The burrows produced by the spiders were compared to those produced by four other burrowing arthropods from previous studies. These burrows were measured and described in the same manner as the spider burrows. The burrows of three other arachnid species were used to determine similarity of burrows within Arachnida: the Mexican blond tarantula (Figure 4.1), Aphonopelma chalcodes (n = 2) (Araneae: Theraphosidae), the Arizona desert hairy scorpion (Figure 4.2), Hadrurus arizonensis (n = 17) (Scorpiones: Caraboctonidae) (Hembree et al., 2012), and the whip scorpion (Figure 4.3), Mastigoproctus giganteus (n = 18) (Thelyphonida: Thelyphonidae) (Hembree, 2013). It was expected that arachnid burrows would be similar as all five species share the same basic arachnid body plan. The burrows of the American giant millipede (Figure 4.4), Narceus americanus (n = 15) (Diplopoda: Spirobolida) (Bowen and Hembree, 2014), were used to determine the similarity of the spider burrows with unrelated arthropods. It was expected that the spider burrows would not be similar to the millipede burrows because the body plans and life habits of millipedes are very different than those of arachnids.
The burrows produced by the spiders were compared to those produced by four other burrowing arthropods from previous studies. These burrows were measured and described in the same manner as the spider burrows. The burrows of three other arachnid species were used to determine similarity of burrows within Arachnida: the Mexican blond tarantula (Figure 4.1), Aphonopelma chalcodes (n = 2) (Araneae: Theraphosidae), the Arizona desert hairy scorpion (Figure 4.2), Hadrurus arizonensis (n = 17) (Scorpiones: Caraboctonidae) (Hembree et al., 2012), and the whip scorpion (Figure 4.3), Mastigoproctus giganteus (n = 18) (Thelyphonida: Thelyphonidae) (Hembree, 2013). It was expected that arachnid burrows would be similar as all five species share the same basic arachnid body plan. The burrows of the American giant millipede (Figure 4.4), Narceus americanus (n = 15) (Diplopoda: Spirobolida) (Bowen and Hembree, 2014), were used to determine the similarity of the spider burrows with unrelated arthropods. It was expected that the spider burrows would not be similar to the millipede burrows because the body plans and life habits of millipedes are very different than those of arachnids.
The nonparametric Mann-Whitney and Kolmogorov-Smirnov tests were used to evaluate the differences in individual properties between groups of burrows. Properties compared for each test included: 1) total depth, 2) total length, 3) mean width, 4) mean height, 5) mean circumference, 6) mean slope, 7) complexity, and 8) tortuosity. The tests were used for two different analyses. The first was to determine if statistically significant changes occurred in burrow properties in different sediment compositions and in different sediment moisture conditions. The second was to determine if the quantitative burrow properties of Gorgyrella inermis and Hogna lenta were similar, and then to determine if the burrow properties of both spider species were similar to those of the other arthropods.
RESULTS
Behaviors
 Individuals of Gorgyrella inermis and Hogna lenta burrowed 0-15 days after the experiments began with an average of five days to begin burrowing. The smaller individuals of both species generally burrowed faster than larger ones. Some individuals, most commonly H. lenta, never produced a burrow. Burrows could be located at any place in the terraria, but the spiders often burrowed along the side of the glass wall. Both species began constructing their burrows by manipulating the sediment with their pedipalps and chelicerae.
Individuals of Gorgyrella inermis and Hogna lenta burrowed 0-15 days after the experiments began with an average of five days to begin burrowing. The smaller individuals of both species generally burrowed faster than larger ones. Some individuals, most commonly H. lenta, never produced a burrow. Burrows could be located at any place in the terraria, but the spiders often burrowed along the side of the glass wall. Both species began constructing their burrows by manipulating the sediment with their pedipalps and chelicerae.
Individuals of Hogna lenta were observed pushing the sediment against the walls of their burrows (Figure 5.1-5) (Appendix 1). Although Gertsch (1949) reported that wolf spiders removed sediment wrapped in silk from their burrows, this behavior was not observed. A thin layer of silk was deposited on the sides of the burrow as the spider moved into the sediment (Appendix 2). Hogna lenta covered their burrow openings with a thin layer of sediment stuck together using silk (Figure 5.6) (Appendix 3).
 The burrows of Hogna lenta were used for dwelling, hunting, and nesting. These spiders were often observed at the surface, but typically retreated into their burrows in response to bright lights and disturbances near their enclosures.
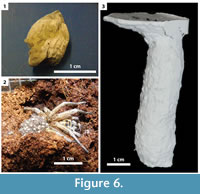
The burrows of Hogna lenta were used for dwelling, hunting, and nesting. These spiders were often observed at the surface, but typically retreated into their burrows in response to bright lights and disturbances near their enclosures.  Although H. lenta is an active surface hunter, some individuals were also observed ambushing prey from their burrow entrances. Two H. lenta individuals produced eggs during the course of the experiments, which were collected into sacks made of silk (Figure 6.1). Newly hatched spiders stayed with their mother until their first molt (Figure 6.2). The nesting burrows were large enough to house the spider and the egg sack (Figure 6.3). Hogna lenta individuals with eggs rarely left their burrows to hunt.
Although H. lenta is an active surface hunter, some individuals were also observed ambushing prey from their burrow entrances. Two H. lenta individuals produced eggs during the course of the experiments, which were collected into sacks made of silk (Figure 6.1). Newly hatched spiders stayed with their mother until their first molt (Figure 6.2). The nesting burrows were large enough to house the spider and the egg sack (Figure 6.3). Hogna lenta individuals with eggs rarely left their burrows to hunt.
 Gorgyrella inermis produced burrows in two ways (Figure 7). Smaller individuals compacted sediment against the sides of their burrows with no excavation. Larger individuals wrapped sediment collected at the base of the burrow in silk (Appendix 4) and threw these packets from the burrow (Figure 7.2-5) (Appendix 5). These packets could land up to 27 cm from the burrow opening (Figure 8.1). The spiders deposited a thick layer of silk along the sides of the burrow (Figure 7.6; Appendix 6). Gorgyrella inermis made thick trapdoors constructed of organic matter and silk attached to one side of the burrow opening with a hinge (Figure 8.2).
Gorgyrella inermis produced burrows in two ways (Figure 7). Smaller individuals compacted sediment against the sides of their burrows with no excavation. Larger individuals wrapped sediment collected at the base of the burrow in silk (Appendix 4) and threw these packets from the burrow (Figure 7.2-5) (Appendix 5). These packets could land up to 27 cm from the burrow opening (Figure 8.1). The spiders deposited a thick layer of silk along the sides of the burrow (Figure 7.6; Appendix 6). Gorgyrella inermis made thick trapdoors constructed of organic matter and silk attached to one side of the burrow opening with a hinge (Figure 8.2).
 |
 |
 |
 |
 |
 |
 The burrows of Gorgyrella inermis were used for both dwelling and ambush hunting. These spiders did not leave their burrows once they were constructed so to obtain prey, G. inermis waited near the top of its burrow and grabbed prey that moved near the entrance with its pedipalps and front legs. The prey was pulled down into the burrow for consumption. Gorgyrella inermis excreted waste by climbing to the top of the burrow and forcefully expelling waste away from the burrow.
The burrows of Gorgyrella inermis were used for both dwelling and ambush hunting. These spiders did not leave their burrows once they were constructed so to obtain prey, G. inermis waited near the top of its burrow and grabbed prey that moved near the entrance with its pedipalps and front legs. The prey was pulled down into the burrow for consumption. Gorgyrella inermis excreted waste by climbing to the top of the burrow and forcefully expelling waste away from the burrow.
Surface Morphology
The surface of the sediment was smoothed prior to the introduction of a spider. Gorgyrella inermis produced obvious trapdoors at the entrances of their burrows. The trapdoors were flush with the sediment surface except when a burrow was built against the side of the terrarium and the burrow protruded ~1 cm above the sediment (Figure 8.1). Some individuals did not construct a burrow and instead produced surface silk structures. One structure was a “mat” made of silk and sediment (Figure 8.3). These mats were as thick as the lining around a burrow and were always built against the glass walls of the terraria. The second structure was similar to a cocoon; the spider first constructed a mat and then enclosed itself in a covering of silk (Figure 8.4). The cocoons were also built against the glass walls.
The burrows of Hogna lenta were usually flush with the sediment surface. In some experiments, the spiders completely closed off their burrows and did not leave during the trial. When this occurred, the sediment bulged slightly above the surrounding sediment surface, indicating the presence of a burrow (Figure 9.1). Hogna lenta also produced turrets that could be up to 2 cm above the sediment (Figure 9.2). These structures only occurred in the corners of the terraria. In addition to burrow construction, some H. lenta deposited lines of silk along the edges of their terraria when they did not produce a burrow (Figure 9.3). These lines appeared to be constructed of a few strands of a silk and ran parallel to each other. The lines were not associated with a burrow and sometimes disappeared after the spider made its burrow.
 Silk Lining of Burrow Walls
Silk Lining of Burrow Walls
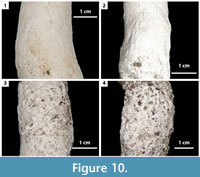
The burrows of both Gorgyrella inermis and Hogna lenta were lined with differing amounts of silk. Both species deposited silk during burrow construction, but Gorgyrella inermis produced a much thicker layer of silk than H. lenta. The silk lining of G. inermis burrows was thickest near the trapdoor and absent at the base of the burrow. Chambers had little to no silk on their walls. Scratches in the silk resulted in striations present on the surface of the burrow casts (Figure 10.1-2). These were produced by spiders bracing their legs into the silk in order to climb along the burrow’s length. The lining of H. lenta burrows had a uniform thickness along the length of the burrow, but was absent at the base. Striations were also present on H. lenta burrow casts (Figure 10.3-4); however, the striations were less well defined than those on G. inermis burrows.
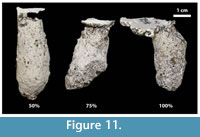 Both species altered the thickness and extent of the silk lining when environmental conditions changed. In general, sediments that were stable, either because of increased clay or moisture content, resulted in burrows with less silk than those produced in less stable sediment (Figure 11). Many Hogna lenta burrows produced in more cohesive sediment did not have any detectable silk lining and those casts have a much more irregular burrow wall than those with silk linings. Gorgyrella inermis burrows produced in these sediments had a thinner silk lining and the linings did not extend as far along the shaft. No differences in the nature of the silk lining were observed between burrows produced in 14 or 30 day trials.
Both species altered the thickness and extent of the silk lining when environmental conditions changed. In general, sediments that were stable, either because of increased clay or moisture content, resulted in burrows with less silk than those produced in less stable sediment (Figure 11). Many Hogna lenta burrows produced in more cohesive sediment did not have any detectable silk lining and those casts have a much more irregular burrow wall than those with silk linings. Gorgyrella inermis burrows produced in these sediments had a thinner silk lining and the linings did not extend as far along the shaft. No differences in the nature of the silk lining were observed between burrows produced in 14 or 30 day trials.
 Burrow Morphology
Burrow Morphology
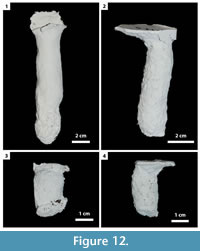
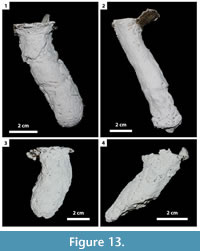
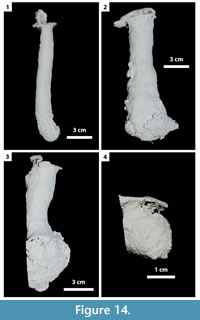
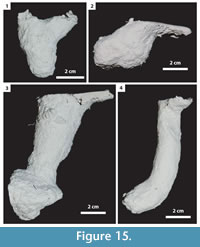
In this study, 27 burrows produced by Gorgyrella inermis and 19 burrows produced by Hogna lenta were successfully cast. Seven burrow architectures were produced during these experiments (Table 2): 1) vertical shafts (VS) (Figure 12; Table 3), 2) subvertical shafts (SS) (Figure 13; Table 4), 3) vertical shafts with terminal chambers (VC) (Figure 14; Table 5), 4) Y-shaped burrows (YS) (Figure 15.1), 5) isolated chambers (IC) (Figure 15.2), 6) subvertical shafts with terminal chambers (SC) (Figure 15.3), and J-shaped burrows (Figure 15.4).
Variation in Burrow Architecture
 Tracemaker and architecture. Gorgyrella inermis individuals were responsible for six of the seven described architectures produced during the experiments. Individuals produced 2-4 (x̄ = 2.43) different burrow architectures. The burrows produced by adult G. inermis were more diverse in architecture than those of the juvenile G. inermis, which only produced vertical shafts and the J-shaped burrow. Hogna lenta were responsible for four of the seven architectures produced during the experiments. Most individuals produced only one burrow architecture, however, and no individual produced more than two.
Tracemaker and architecture. Gorgyrella inermis individuals were responsible for six of the seven described architectures produced during the experiments. Individuals produced 2-4 (x̄ = 2.43) different burrow architectures. The burrows produced by adult G. inermis were more diverse in architecture than those of the juvenile G. inermis, which only produced vertical shafts and the J-shaped burrow. Hogna lenta were responsible for four of the seven architectures produced during the experiments. Most individuals produced only one burrow architecture, however, and no individual produced more than two.
 Environment and architecture. Gorgyrella inermis produced five burrow architectures in sediment modeled on the species’ natural habitat (Table 1.1). Increasing sediment density resulted in only two burrow architectures as well as surface mats and cocoons (Table 1.2). Decreasing sediment moisture resulted in three burrow architectures, whereas increasing sediment moisture resulted in only one burrowarchitecture and a surface mat (Table 1.3).
Environment and architecture. Gorgyrella inermis produced five burrow architectures in sediment modeled on the species’ natural habitat (Table 1.1). Increasing sediment density resulted in only two burrow architectures as well as surface mats and cocoons (Table 1.2). Decreasing sediment moisture resulted in three burrow architectures, whereas increasing sediment moisture resulted in only one burrowarchitecture and a surface mat (Table 1.3).
Hogna lenta produced three burrow architectures in sediment modeled on the species’ natural habitat (Table 1.1), but three individuals did not produce burrows during one trial. Increasing sediment density resulted in two burrow architectures (Table 1.2). Increasing sediment moisture resulted in two burrow architectures and two individuals that did not burrow, whereas decreasing sediment moisture resulted in only one burrow architecture and two individuals that did not burrow (Table 1.3).
Burrow Analyses
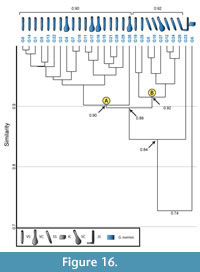
 Burrows produced by Gorgyrella inermis. All burrows produced by Gorgyrella inermis have a similarity of 1.0-0.7 with a mean similarity of 0.90. A cluster analysis of these burrows separates the burrows into two main groups (Figure 16). Cluster A includes all but two burrows that possess a vertical shaft and has a similarity of 0.90. Cluster B includes all burrows that possess a subvertical shaft as well as two vertical shafts (G19, G25), and has a similarity of 0.91. Together, these two clusters have a similarity of 0.89. The J-shaped burrow (G23) was not grouped with either cluster, and it shares a similarity of 0.84 with Clusters A and B. The isolated chamber (G6) was also not included in a cluster, and has a similarity of 0.72 with all other burrows.
Burrows produced by Gorgyrella inermis. All burrows produced by Gorgyrella inermis have a similarity of 1.0-0.7 with a mean similarity of 0.90. A cluster analysis of these burrows separates the burrows into two main groups (Figure 16). Cluster A includes all but two burrows that possess a vertical shaft and has a similarity of 0.90. Cluster B includes all burrows that possess a subvertical shaft as well as two vertical shafts (G19, G25), and has a similarity of 0.91. Together, these two clusters have a similarity of 0.89. The J-shaped burrow (G23) was not grouped with either cluster, and it shares a similarity of 0.84 with Clusters A and B. The isolated chamber (G6) was also not included in a cluster, and has a similarity of 0.72 with all other burrows.
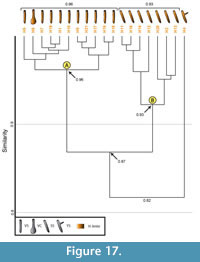
 Burrows produced by Hogna lenta. All burrows produced by Hogna lenta have a similarity of 1.0-0.8 with a mean similarity of 0.91. A cluster analysis separates these burrows into the same types of clusters as were observed in the cluster analysis of Gorgyrella inermis (Figure 17). Cluster A includes all burrows with a vertical shaft and has a similarity of 0.96. Cluster B includes all burrows with a subvertical shaft that have a similarity of 0.93. Together, these two clusters have a similarity of 0.87. The Y-shaped burrow (H4) was not grouped into a cluster, and it shares a similarity of 0.82 with Clusters A and B.
Burrows produced by Hogna lenta. All burrows produced by Hogna lenta have a similarity of 1.0-0.8 with a mean similarity of 0.91. A cluster analysis separates these burrows into the same types of clusters as were observed in the cluster analysis of Gorgyrella inermis (Figure 17). Cluster A includes all burrows with a vertical shaft and has a similarity of 0.96. Cluster B includes all burrows with a subvertical shaft that have a similarity of 0.93. Together, these two clusters have a similarity of 0.87. The Y-shaped burrow (H4) was not grouped into a cluster, and it shares a similarity of 0.82 with Clusters A and B.
 Comparison between Gorgyrella inermis and Hogna lenta. The burrows of these two species differ in depth, total length, and width-to-height ratio (Table 6.1). Bray-Curtis results show that the burrows of G. inermis and H. lenta have a similarity of 1.0-0.7 with a mean similarity of 0.91 (Table 7, Table 8). A cluster analysis of the spider burrow casts does not separate the burrows by species (Figure 18). Cluster A (vertical burrows) and Cluster B (subvertical burrows) from the previous analyses were produced with the addition of a third cluster, Cluster C, which includes the Y- and the J-shaped burrows.
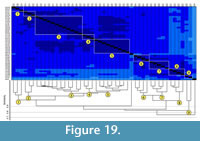
Comparison between Gorgyrella inermis and Hogna lenta. The burrows of these two species differ in depth, total length, and width-to-height ratio (Table 6.1). Bray-Curtis results show that the burrows of G. inermis and H. lenta have a similarity of 1.0-0.7 with a mean similarity of 0.91 (Table 7, Table 8). A cluster analysis of the spider burrow casts does not separate the burrows by species (Figure 18). Cluster A (vertical burrows) and Cluster B (subvertical burrows) from the previous analyses were produced with the addition of a third cluster, Cluster C, which includes the Y- and the J-shaped burrows. 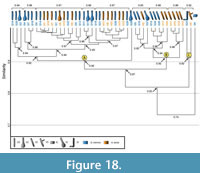 Cluster A has a similarity 0.90 whereas Cluster B has a similarity of 0.92. The similarity of both clusters is 0.87. Cluster C has a similarity of 0.92, and the similarity of all three clusters is 0.83. The isolated chamber does not fit into any of the three clusters. The overall similarity of all G. inermis and H. lenta burrows is 0.74. The burrows in Cluster A and B differ only in mean slope and complexity (Table 9.1). A comparison of these two clusters with Cluster C results in significant differences in mean width, mean height, mean circumference, and mean slope (Table 9.1). Removing the isolated chamber from the cluster analysis does not alter the composition and overall similarity of each cluster. Rearranging the Bray-Curtis test matrix to reflect the cluster analysis reveals that each cluster includes Bray-Curtis scores of 1.0-0.9 (Figure 19).
Cluster A has a similarity 0.90 whereas Cluster B has a similarity of 0.92. The similarity of both clusters is 0.87. Cluster C has a similarity of 0.92, and the similarity of all three clusters is 0.83. The isolated chamber does not fit into any of the three clusters. The overall similarity of all G. inermis and H. lenta burrows is 0.74. The burrows in Cluster A and B differ only in mean slope and complexity (Table 9.1). A comparison of these two clusters with Cluster C results in significant differences in mean width, mean height, mean circumference, and mean slope (Table 9.1). Removing the isolated chamber from the cluster analysis does not alter the composition and overall similarity of each cluster. Rearranging the Bray-Curtis test matrix to reflect the cluster analysis reveals that each cluster includes Bray-Curtis scores of 1.0-0.9 (Figure 19).
 Comparison with Aphonopelma chalcodes. Two burrow casts of Aphonopelma chalcodes with the same architecture were used in comparison (Appendix 7). The burrows of A. chalcodes and the other two spiders differ in width-to-height ratio, mean circumference, mean slope, and tortuosity (Table 6.2). Due to their difference in size, the two A. chalcodes burrows have a similarity of only 0.6. The similarity of burrows made by A. chalcodes and Gorgyrella inermis ranges from 0.8-0.5 with a mean similarity of 0.56; the similarity of A. chalcodes and Hogna lenta ranges from 0.7-0.5 with a mean similarity of 0.56 (Appendix 8)
Comparison with Aphonopelma chalcodes. Two burrow casts of Aphonopelma chalcodes with the same architecture were used in comparison (Appendix 7). The burrows of A. chalcodes and the other two spiders differ in width-to-height ratio, mean circumference, mean slope, and tortuosity (Table 6.2). Due to their difference in size, the two A. chalcodes burrows have a similarity of only 0.6. The similarity of burrows made by A. chalcodes and Gorgyrella inermis ranges from 0.8-0.5 with a mean similarity of 0.56; the similarity of A. chalcodes and Hogna lenta ranges from 0.7-0.5 with a mean similarity of 0.56 (Appendix 8)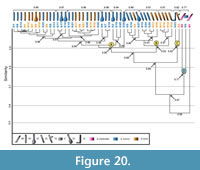 . The mean similarity of A. chalcodes with both G. inermis and H. lenta is 0.56. A cluster analysis of the three species reproduces Clusters A, B, and C (Figure 20). The isolated chamber (G6) is most similar to the smaller A. chalcodes burrow A2, and together they form Cluster D with a similarity of 0.77. Cluster D has a similarity of 0.67 with Clusters A, B, and C. Clusters A and B differ in mean slope and complexity, whereas Clusters A, B, and C differ in mean width, mean height, mean circumference, mean slope, and tortuosity (Table 9.2). Cluster D differs from A, B, and C in depth, mean height, mean slope, and complexity (Table 9.2). The larger A. chalcodes burrow A1 does not fit into any cluster, and its overall similarity with all other spider burrows is 0.56.
. The mean similarity of A. chalcodes with both G. inermis and H. lenta is 0.56. A cluster analysis of the three species reproduces Clusters A, B, and C (Figure 20). The isolated chamber (G6) is most similar to the smaller A. chalcodes burrow A2, and together they form Cluster D with a similarity of 0.77. Cluster D has a similarity of 0.67 with Clusters A, B, and C. Clusters A and B differ in mean slope and complexity, whereas Clusters A, B, and C differ in mean width, mean height, mean circumference, mean slope, and tortuosity (Table 9.2). Cluster D differs from A, B, and C in depth, mean height, mean slope, and complexity (Table 9.2). The larger A. chalcodes burrow A1 does not fit into any cluster, and its overall similarity with all other spider burrows is 0.56.
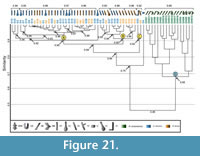 Comparison with Hadrurus arizonensis. Seventeen burrow casts comprising four different architectures, were used for comparison (Hembree et al., 2012) (Appendix 9). The burrows of Hadrurus arizonensis differ from the spider burrows in eight different properties (Table 6.3). The similarity of the H. arizonensis burrows ranges from 1.0-0.5 with a mean similarity of 0.75 (Appendix 10). The similarity of the burrows produced by H. arizonensis and Gorgyrella inermis ranges from 0.8-0.3 with a mean of 0.46; the similarity of the burrows produced by H. arizonensis and Hogna lenta ranges from 0.8-0.3 with a mean of 0.46. The comparison of H. arizonensis burrows with those of both G. inermis and H. lenta results in a mean similarity of 0.46. A cluster analysis results in a complete separation of the spider burrows and the scorpion burrows (Figure 21). All spider burrows fall into Clusters A, B, and C, with the exception of the isolated chamber. This grouping of the spider burrows has a similarity of 0.75. All H. arizonensis burrows form Cluster D, with a similarity of 0.68. Clusters A and B differ in mean slope and complexity, whereas Cluster A, B, and C differ in mean width, mean height, mean circumference, mean slope, and tortuosity (Table 9.3). Cluster D differs from Clusters A, B, and C in all properties but depth (Table 9.3). The similarity of all burrows produced by the three species is 0.46. Of particular note, no subvertical ramps produced by H. arizonensis were similar to the subvertical shafts produced by either species of spider.
Comparison with Hadrurus arizonensis. Seventeen burrow casts comprising four different architectures, were used for comparison (Hembree et al., 2012) (Appendix 9). The burrows of Hadrurus arizonensis differ from the spider burrows in eight different properties (Table 6.3). The similarity of the H. arizonensis burrows ranges from 1.0-0.5 with a mean similarity of 0.75 (Appendix 10). The similarity of the burrows produced by H. arizonensis and Gorgyrella inermis ranges from 0.8-0.3 with a mean of 0.46; the similarity of the burrows produced by H. arizonensis and Hogna lenta ranges from 0.8-0.3 with a mean of 0.46. The comparison of H. arizonensis burrows with those of both G. inermis and H. lenta results in a mean similarity of 0.46. A cluster analysis results in a complete separation of the spider burrows and the scorpion burrows (Figure 21). All spider burrows fall into Clusters A, B, and C, with the exception of the isolated chamber. This grouping of the spider burrows has a similarity of 0.75. All H. arizonensis burrows form Cluster D, with a similarity of 0.68. Clusters A and B differ in mean slope and complexity, whereas Cluster A, B, and C differ in mean width, mean height, mean circumference, mean slope, and tortuosity (Table 9.3). Cluster D differs from Clusters A, B, and C in all properties but depth (Table 9.3). The similarity of all burrows produced by the three species is 0.46. Of particular note, no subvertical ramps produced by H. arizonensis were similar to the subvertical shafts produced by either species of spider.
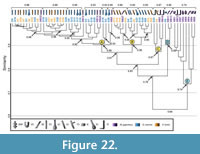 Comparison with Mastigoproctus giganteus. Eighteen burrow casts comprising five different architectures, were used for comparison (Hembree, 2013) (Appendix 11). The burrows of Mastigoproctus giganteus differ from the spider burrows in eight different properties (Table 6.4). The similarity of the M. giganteus burrows ranges from 1.0-0.5 with a mean similarity of 0.75. The similarity of the burrows produced by M. giganteus and Gorgyrella inermis ranges from 1.0-0.4 with a mean of 0.75; the similarity of the burrows produced by M. giganteus and Hogna lenta ranges from 1.0-0.5 with a mean of 0.75. The comparison M. giganteus with both G. inermis and H. lenta has a mean similarity of 0.75 (Appendix 12). A cluster analysis results in two separate groups (Figure 22). The first group (Clusters A-D) is composed of burrows produced by all three species and the second (Cluster E) consists entirely of burrows produced by M. giganteus. Five M. giganteus burrows are included in Cluster A with a similarity of 0.90, whereas one M. giganteus burrow is included in Cluster B with a similarity of 0.90. Two J-shaped burrows produced by M. giganteus are included in Cluster C with a similarity of 0.87. Clusters A and B differ in mean slope and complexity, whereas Cluster C differs from A and B in only mean slope (Table 9.4). Cluster D contains the isolated chamber (G6) of G. inermis and two subvertical shafts produced by M. giganteus with a similarity of 0.90. This new cluster differs from Clusters A, B, and C in all properties but depth (Table 9.4). The three clusters have a similarity of 0.76. Cluster E consists entirely of burrows produced by M. giganteus and includes mazeworks, U-shaped burrows, J-shaped burrows, and one subvertical shaft. The similarity of this cluster is 0.74. Cluster E shares only depth in common with the other clusters (Table 9.4). The similarity of the burrows produced by all three species is 0.64.
Comparison with Mastigoproctus giganteus. Eighteen burrow casts comprising five different architectures, were used for comparison (Hembree, 2013) (Appendix 11). The burrows of Mastigoproctus giganteus differ from the spider burrows in eight different properties (Table 6.4). The similarity of the M. giganteus burrows ranges from 1.0-0.5 with a mean similarity of 0.75. The similarity of the burrows produced by M. giganteus and Gorgyrella inermis ranges from 1.0-0.4 with a mean of 0.75; the similarity of the burrows produced by M. giganteus and Hogna lenta ranges from 1.0-0.5 with a mean of 0.75. The comparison M. giganteus with both G. inermis and H. lenta has a mean similarity of 0.75 (Appendix 12). A cluster analysis results in two separate groups (Figure 22). The first group (Clusters A-D) is composed of burrows produced by all three species and the second (Cluster E) consists entirely of burrows produced by M. giganteus. Five M. giganteus burrows are included in Cluster A with a similarity of 0.90, whereas one M. giganteus burrow is included in Cluster B with a similarity of 0.90. Two J-shaped burrows produced by M. giganteus are included in Cluster C with a similarity of 0.87. Clusters A and B differ in mean slope and complexity, whereas Cluster C differs from A and B in only mean slope (Table 9.4). Cluster D contains the isolated chamber (G6) of G. inermis and two subvertical shafts produced by M. giganteus with a similarity of 0.90. This new cluster differs from Clusters A, B, and C in all properties but depth (Table 9.4). The three clusters have a similarity of 0.76. Cluster E consists entirely of burrows produced by M. giganteus and includes mazeworks, U-shaped burrows, J-shaped burrows, and one subvertical shaft. The similarity of this cluster is 0.74. Cluster E shares only depth in common with the other clusters (Table 9.4). The similarity of the burrows produced by all three species is 0.64.
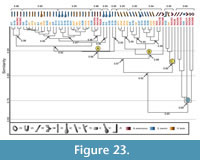 Comparison with Narceus americanus. Fifteen burrow casts comprising six different architectures were used for comparison (Bowen and Hembree, 2014) (Appendix 13). The burrows of Narceus americanus differ from the spider burrows in seven properties (Table 6.5). The similarity of the N. americanus burrows ranges from 1.0-0.5 with a mean similarity of 0.76 (Appendix 14). The similarity of the burrows produced by N. americanus and Gorgyrella inermis ranges from 1.0-0.5 with a mean of 0.78; the similarity of the burrows produced by N. americanus and Hogna lenta ranges from 1.0-0.5 with a mean of 0.78. A cluster analysis results in two separate groups (Figure 23), both of which containing burrows produced by all three species. Five N. americanus burrows are included in Cluster A with a similarity of 0.89, whereas three burrows are included in Cluster B with a similarity of 0.88. Cluster A and B differ in mean slope and complexity (Table 9.5). Cluster C includes two N. americanus burrows with a similarity of 0.85. This cluster differs from Clusters A and B in mean width, mean height, mean circumference, mean slope, complexity, and tortuosity (Table 9.5). The similarity of these three clusters is 0.82. Cluster D is composed of five burrows produced by N. americanus and one burrow (G6) produced by G. inermis. Burrow architectures included in Cluster D are subvertical shafts, O-shaped burrows, a helical burrow, and the isolated chamber produced by G. inermis. This group has a similarity of 0.69 and is distinct from the other clusters in all properties (Table 9.5). The similarity of all burrows produced by the three species is 0.65.
Comparison with Narceus americanus. Fifteen burrow casts comprising six different architectures were used for comparison (Bowen and Hembree, 2014) (Appendix 13). The burrows of Narceus americanus differ from the spider burrows in seven properties (Table 6.5). The similarity of the N. americanus burrows ranges from 1.0-0.5 with a mean similarity of 0.76 (Appendix 14). The similarity of the burrows produced by N. americanus and Gorgyrella inermis ranges from 1.0-0.5 with a mean of 0.78; the similarity of the burrows produced by N. americanus and Hogna lenta ranges from 1.0-0.5 with a mean of 0.78. A cluster analysis results in two separate groups (Figure 23), both of which containing burrows produced by all three species. Five N. americanus burrows are included in Cluster A with a similarity of 0.89, whereas three burrows are included in Cluster B with a similarity of 0.88. Cluster A and B differ in mean slope and complexity (Table 9.5). Cluster C includes two N. americanus burrows with a similarity of 0.85. This cluster differs from Clusters A and B in mean width, mean height, mean circumference, mean slope, complexity, and tortuosity (Table 9.5). The similarity of these three clusters is 0.82. Cluster D is composed of five burrows produced by N. americanus and one burrow (G6) produced by G. inermis. Burrow architectures included in Cluster D are subvertical shafts, O-shaped burrows, a helical burrow, and the isolated chamber produced by G. inermis. This group has a similarity of 0.69 and is distinct from the other clusters in all properties (Table 9.5). The similarity of all burrows produced by the three species is 0.65.
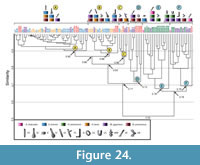 Comparison of all arthropod burrows. A cluster analysis of the six arthropod species results in two separate groups (Figure 24). The first group consists of the four distinct clusters formed by the burrows of Gorgyrella inermis and Hogna lenta. Clusters A, B, and C all include burrows produced by G. inermis, H. lenta , Mastigoproctus giganteus, and Narceus americanus. Cluster A has a similarity of 0.90, Cluster B has a similarity of 0.88, and Cluster C has a similarity of 0.86. The similarity of all three clusters is 0.83. Cluster A and Cluster B differ in mean slope and complexity, whereas Clusters A, B, and C differ in mean slope (Table 9.6). Cluster D includes the burrows produced by G. inermis, H. lenta, and N. americanus and has a similarity of 0.73. This cluster is composed of subvertical shafts, O-shaped burrows, and the isolated chamber. Cluster D differs from Clusters A, B, and C in all properties but depth and length (Table 9.6). These four clusters have a similarity of 0.71. The second group consists of two distinct clusters, Clusters E and F, and is composed of all Aphonopelma chalcodes, all Hadrurus arizonensis, eight M. giganteus, and one N. americanus burrow casts. Cluster E includes subvertical ramps and helical burrows and has a similarity of 0.78. Cluster F includes mazeworks, U-shaped burrows, J-shaped burrows, helical burrows, and subvertical ramps and has a similarity of 0.79. Clusters E and F differ in depth, width-to-height ratio, mean slope, complexity, and tortuosity (Table 9.6). Clusters A, B, C, and D differ from Clusters E and F in all properties but depth and mean height (Table 9.6). The similarity of all burrows produced by the arthropods is 0.53.
Comparison of all arthropod burrows. A cluster analysis of the six arthropod species results in two separate groups (Figure 24). The first group consists of the four distinct clusters formed by the burrows of Gorgyrella inermis and Hogna lenta. Clusters A, B, and C all include burrows produced by G. inermis, H. lenta , Mastigoproctus giganteus, and Narceus americanus. Cluster A has a similarity of 0.90, Cluster B has a similarity of 0.88, and Cluster C has a similarity of 0.86. The similarity of all three clusters is 0.83. Cluster A and Cluster B differ in mean slope and complexity, whereas Clusters A, B, and C differ in mean slope (Table 9.6). Cluster D includes the burrows produced by G. inermis, H. lenta, and N. americanus and has a similarity of 0.73. This cluster is composed of subvertical shafts, O-shaped burrows, and the isolated chamber. Cluster D differs from Clusters A, B, and C in all properties but depth and length (Table 9.6). These four clusters have a similarity of 0.71. The second group consists of two distinct clusters, Clusters E and F, and is composed of all Aphonopelma chalcodes, all Hadrurus arizonensis, eight M. giganteus, and one N. americanus burrow casts. Cluster E includes subvertical ramps and helical burrows and has a similarity of 0.78. Cluster F includes mazeworks, U-shaped burrows, J-shaped burrows, helical burrows, and subvertical ramps and has a similarity of 0.79. Clusters E and F differ in depth, width-to-height ratio, mean slope, complexity, and tortuosity (Table 9.6). Clusters A, B, C, and D differ from Clusters E and F in all properties but depth and mean height (Table 9.6). The similarity of all burrows produced by the arthropods is 0.53.
Environmental controls on burrow morphology. Eight different Mann-Whitney and Kolmogorov-Smirnov tests were run for both species, but no characteristics were found to be significantly different when conditions changed (Table 10).
DISCUSSION
Burrow Morphology and Tracemaker
Two species of burrowing spiders produced seven different burrow architectures. Gorgyrella inermis produced six of these architectures, whereas Hogna lenta produced four. Individuals of both species produced multiple burrow architectures in different experiments. Although G. inermis and H. lenta are distantly related, both species produced four of the same burrow architectures. Nearly all burrow architectures produced by G. inermis and H. lenta share common features: a single burrow opening, a nonbranching vertical to subvertical shaft with a circular-to-elliptical cross-section, a width-to-height ratio of approximately one, and surficial features related to a silk-lining. These features are reflective of the general spider body form. The bodies of G. inermis and H. lenta consist of a cephalothorax and an abdomen. The eight legs radiate from the cephalothorax, forming a generally circular shape. This allows the spiders to move up and down the length of the shafts using a chimney climbing technique (i.e., Shäfer and Craig, 1972). The burrows do not include ramps or obvious floors as the spiders can move in any direction through the shafts. Individuals of both species were observed moving along all sides of the burrows; however, all individuals did use a preferred side to enter and exit burrows.
Surficial features resulting from a silk lining are the best indicator of a spider tracemaker. Silk linings isolate the burrow void space from the surrounding sediment and provide a foothold for the spiders to climb in the burrow. These features form smooth-walled burrow casts with scratch marks running vertically along the shaft. Although silk production occurs in other arthropod classes (e.g., Insecta and Myriapoda), only spiders use silk to stabilize excavated burrows (Craig, 1997).
Comparisons of burrows produced by other arachnids, including other burrowing spiders, indicate other diagnostic characteristics of spider burrows. Although the spiders Aphonopelma chalcodes , Gorgyrella inermis, and Hogna lenta share the same spider body plan, the tarantula A. chalcodes is two to three times larger than G. inermis and H. lenta. Aphonopelma chalcodes constructed burrows by excavation of material using their pedipalps. They were not observed bundling the sediment with silk before removal. This species produced subvertical ramps with an ovoid cross section. This morphology does not allow the tarantulas to enter or exit their burrows with the chimney climbing technique used by the other two spider species. Burrow properties related to the subvertical ramp morphology and the tarantula’s locomotion technique produced statistically significant differences when compared to the other spiders (Table 6.2). Surficial features of tarantula burrows included protrusions on the burrow walls resulting from the excavation of sediment and the absence of a silk lining. The dissimilarity in burrow morphology between the tarantulas and other spiders would make linking the tarantula burrow to a large spider difficult.
The scorpion Hadrurus arizonensis and the whip scorpion Mastigoproctus giganteus are superficially very similar in morphology. Both species have large pedipalps modified into pincers, an elongate, low body, and a long tail. These arachnids, therefore, have a much different body plan than do the spiders. Hadrurus arizonensis excavated its burrows by kicking loose sediment behind it or by picking up sediment with its walking legs (Hembree et al., 2012). Mastigoproctus giganteus excavated sediment using its pedipalps and deposited the material outside of the burrow (Hembree, 2013). The scorpions and whip scorpions produced simple vertical shafts and subvertical ramps as well as more complex burrow architectures not produced by either Gorgyrella inermis or Hogna lenta , including U-shaped burrows, helical burrows, and mazeworks (Hembree et al., 2012; Hembree, 2013). The burrows of H. arizonensis and M. giganteus vary from the spider burrows in all burrow properties but depth (Table 6.3-4). The scorpions and whip scorpions produced surficial features that differ greatly from those produced by the spiders. The burrows of H. arizonensis include no bioglyphs or surface sculpture marks and the texture of the walls is controlled by the surrounding sediment (Hembree et al., 2012). The upper surface of M. giganteus burrows is irregular and the floors are smooth whereas the walls feature paired protrusions, resulting from the excavation of material by the animal’s pedipalps (Hembree, 2013).
The millipede Narceus americanus has an elongate, low body with multiple pairs of walking legs. While this species does not have any appendages specifically modified for grasping, it has been observed using its mandibles to pick up sediment grains (Bowen and Hembree, 2014). Narceus americanus produces simple vertical shafts and subvertical shafts as well as helical burrows, O-shaped burrows, and U-shaped burrows, architectures not produced by the spiders (Bowen and Hembree, 2014). The burrows of N. americanus vary from the spider burrows in all burrow properties but burrow length and width-to-height ratio (Table 6.5). The similar width-to-height ratio may result from the millipede’s anatomy. These animals are vaguely circular in cross section, and they construct their burrows by compression of the sediment with their bodies (Bowen and Hembree, 2014). The burrow walls and ceilings are typically smooth as a result of this burrowing technique, but because of absence of a silk lining the wall texture is controlled by the sediment.
Burrow Morphology and Behavior
The morphology of the burrows produced by Gorgyrella inermis and Hogna lenta is related to the behaviors exhibited by these species. Most importantly, the locomotion styles employed by the spiders controlled how the spiders constructed their burrows and how they behaved inside of the finished burrows.
The average time to beginning burrow construction was five days. All burrows began as either a vertical shaft or subvertical shaft with one surface opening. Subsequent modifications to these architectures, such as second surface opening or a chamber, occurred only in experiments lasting 30 days. Both species climbed vertically inside of their burrows by bracing their legs against the sides. This behavior produces scratch marks in the silk linings and in finer-grained sediment. The spiders also walked along the bottoms of burrows with a subvertical shaft; however, a few legs were still braced against the burrow walls.
All seven burrow architectures were used for dwelling. During the experiments, Gorgyrella inermis never left their burrows. Gorgyrella inermis individuals spent a majority of their time at the base of their burrows facing outward. The burrow shafts produced by G. inermis were not wide enough to allow individuals to turn around inside of the burrows. Gorgyrella did have to turn around, however, because G. inermis eliminates waste outside of its burrow by positioning its abdomen outside of and away from the burrow entrance. Most individuals were, therefore, forced to climb to the top of the burrow and maneuver their bodies while straddling the burrow entrance directly underneath the trapdoor. Those that constructed chambers, however, utilized them to turn their bodies and spent less time exposed at the burrow entrance. In contrast, Hogna lenta individuals left their burrows to hunt and obtain water. These excursions occurred mainly during dark hours, and the spiders utilized the entire surface area inside of the terrarium. The burrow shafts produced by H. lenta were larger than their bodies and enabled individuals to turn around inside of their burrows.
The morphology of Gorgyrella inermis burrows enables this species to ambush prey from the entrance of its burrow. Spiders waited near the top of the burrow, hidden underneath the trapdoor, and used the posterior four legs to brace against the shaft walls. Two legs held onto the rim of the burrow and the two anterior legs and pedipalps were used to grab prey that came near the burrow entrance. The spider grabbed the animal, pulled it into the burrow, and then moved to the base of the burrow to consume its prey. Individuals that did not produce burrows were still able to catch prey, but they were not as successful as evidenced by abdomens that were smaller and desiccated compared to those living in burrows. Prey was observed escaping these individuals, whereas no prey was ever seen escaping from a burrow. The burrow architectures produced by Hogna lenta did not assist in obtaining prey. Hogna lenta is an active hunter that searches for prey animals near its burrow; however, one individual was observed ambushing its prey from its burrow entrance. Most H. lenta individuals consumed their prey on the surface, but some brought their prey into the burrows.
In addition to dwelling, two Hogna lenta individuals (FWS2 and FWS5) used their burrow for brooding. Both spiders produced vertical shafts in less dense sediment and subvertical shafts in the densest sediment. The quantitative properties of these burrows were statistically similar those produced by the other H. lenta individuals that did not lay eggs (Table 11). One individual was allowed to keep its egg sac until after the young hatched (60 days). This brood burrow had no unique qualitative features, and its measurements were statistically similar to other H. lenta vertical shafts.
Burrow Morphology and Sediment Conditions
Observations of the burrows produced by Gorgyrella inermis and Hogna lenta at the conclusion of each experiment indicate that differences in sediment conditions, both sediment density and moisture, influenced the diversity of architectures produced and the possibility of burrow construction. Each species of spider was allowed to burrow in sediment of moderate density and moderate moisture. Experiments in these sediments produced the most diverse set of architectures (Table 1.1). Three of the architectures, subvertical shafts with terminal chambers, isolated chambers, and Y-shaped burrows, were only produced in this sediment type.
Increased sediment density led to the production of only two burrow architectures (Table 1.2). Only adult Gorgyrella inermis individuals produced chambers under these conditions; juvenile Gorgyrella inermis individuals and Hogna lenta individuals produced only vertical and subvertical shafts. Denser sediments limited the ability of the spiders to manipulate the sediment and construct complex burrow architectures. Comparison of adult G. inermis burrows to those of juvenile G. inermis and H. lenta suggests that smaller spiders were not physically capable of constructing more than simple burrows. One adult G. inermis individual did not construct a burrow in this sediment and instead produced a silk mat. The stability of this sediment was also shown by the lack of silk lining on the burrow walls. Burrowing animals may apply linings to the sides of the burrows to increase structural integrity (Bromley, 1996). The spiders did not expend energy shoring-up burrows that did not need the addition of a silk lining. Although architectural diversity was decreased, quantitative features of these burrows were not statistically different than those produced in more ideal sediments.
Decreasing sediment moisture limited the burrow architectures produced and even prevented the production of open burrows. Both Gorgyrella inermis and Hogna lenta produced simple burrows, although one G. inermis individual did produce a J-shaped burrow, the only experiment in which this architecture was produced. Three individuals, one G. inermis and two H. lenta, did not produce any open burrows. Neither the thickness of the silk linings nor the surface area of the burrow chamber covered in silk increased when compared to the burrows produced in sediments with moderate moisture.
Increasing sediment moisture also limited the number of burrow architectures produced and prevented the production of open burrows. Two of the four individuals of Gorgyrella inermis and two of the four individuals of Hogna lenta did not construct burrows in these conditions. Those burrows that were produced were only simple vertical shafts. As observed before, the stability of the sediment was shown by the lack of silk lining applied to the burrow walls. Again, although architectural diversity was decreased, quantitative features of these burrows were not statistically different than those produced in more ideal sediments (Table 1.3).
These observations may be useful for interpreting soil conditions in the fossil record. Spiders had difficulty burrowing in sediments that were too dense, too dry, or too moist. These conditions can be interpreted by a decrease in burrow complexity and diversity. The identification of a silk lining may be the best indicator of environmental conditions. The production of silk has physiological cost (Breed et al., 1964; Prestwich, 1977), and spiders do not use silk when it is unnecessary. Gauging the amount of silk present when a spider burrow is identified is a reliable indicator of sediment stability, although it would be difficult to distinguish if the stability is controlled by composition or moisture content by burrow analysis alone.
SIGNIFICANCE
Identification of Spider Burrows in the Fossil Record
Identification of burrows produced by a specific tracemaker is accomplished through the recognition of unique characteristics, or ichnotaxobases, present in those burrows made by living animals. Ichnotaxobases for the burrows produced by burrowing spiders include:
Architecture. Spider burrows can include vertical shafts, vertical shafts with terminal chambers, subvertical shafts, subvertical shafts with terminal chambers, J-shaped burrows, Y-shaped burrows, and isolated chambers. These burrows include one to two surface openings, shafts, tunnels, and chambers.
Overall shape. Shafts and tunnels are elliptical in cross section. There is no clear floor or roof to these burrows. Shaft and tunnel width is nearly the same as shaft and tunnel height. Shafts and tunnels tend to be straight with tortuosity values nearly equal to 1.0. Chambers may occur at the base of a shaft or off to the side along the horizontal. Chambers have the same cross-sectional shape and may be up to two times the size of shafts and tunnels. Branching occurs rarely. When present each branch has a single surface opening and leads to a primary shaft.
Orientation. Nearly all spider burrows are vertical (80°-90°) or subvertical (60°-75°) just below the burrow entrance.
Internal structure. Burrows made by spiders possess no distinguishable lining made by manipulation of sediment. Fill may be passive or active, but spiders seal their burrows with trapdoors made of silk and sediment to prevent passive fill. Burrows lacking a thick silk lining are more likely to be filled passively by gravitational collapse of the sediment.
Surficial features. Burrowing spiders line their burrows with silk. Burrows produced in sediment that is unstable have thick silk linings, whereas those produced in stable sediment have thin or no silk linings. Scratch marks are preserved in silk linings running parallel to the burrow and occur along the entire length of the burrow. Those burrows with little to no silk linings have irregular burrow walls and no surficial features.
Paleontological and Paleoecological Significance
In some circumstances, trace fossils may be more beneficial to paleoenvironmental interpretation than body fossils. Trace fossils are often preserved in the sediment in which they were formed and so are able to provide reliable indicators of the paleoenvironmental conditions (Bromley, 1996). This differs from body fossils, which may be carried away from the environment in which the organism lived. Trace fossils also provide records of organisms with low preservation potential, namely those organisms lacking hard body parts (Bromley, 1996). This is important for paleoecological interpretations as soft-bodied organisms may comprise most of the biomass in an ecosystem. As a result, the diversity of trace fossils may be used to improve estimates of biological diversity (Frey, 1975).
With a fossil record extending back to the Carboniferous, spider burrows should be commonly identified in the fossil record. Some terrestrial fossil burrows (e.g., Macanopsis and Skolithos) have been attributed to various arthropods, including spiders (Ratcliffe and Fagerstrom, 1980; Brown and Kraus, 1983; Hasiotis, 2002; and Retallack et al., 2003). A wide variety of tracemakers, however, could make burrows with similar architectures. The identification of spider burrows allows for better interpretation of this group’s evolution as well as provides a better understanding of soil ecosystems through time. Body fossils of spiders are rare in the Paleozoic, and younger fossils are often preserved in amber (Shear, 1994).
Spiders are major predators of invertebrates, and this order consists entirely of carnivores (Clarke and Grant, 1968; Reichert and Lockley, 1984; Hodge, 1999). The density and diversity of spiders are indicative of the health of an ecosystem as they require enough animals to prey upon. Spiders are also prey items for other animals, so the presence of larger predators may be inferred as well. The number of spider individuals can be assessed by counting the number of spider burrows present in modern habitats, although the density of spider abundance varies depending on species and habitat. For example, Bradley (1996) found that the density of Australian trapdoor spiders is on average 391/ha, whereas Coyle and Incenogle (1994) found that density of a trapdoor spider in California could be up to 300 individuals per 3 m2. Species of both mygalomorphs and aranemomorphs remain faithful to their burrows for long periods of time (e.g., Suttle, 2003; Hamilton et al., 2012). Burrow fidelity was also observed in this study. Individuals of Gorgyrella inermis constructed only one burrow, whereas some individuals of Hogna lenta abandoned a burrow to construct a new one. The new burrow became the primary dwelling, and the abandoned burrow became filled with sediment or collapsed.
Importance of Spiders in Soil Formation
In this study, burrow depth for Gorgyrella inermis was restricted to 2.1-16.6 cm, whereas the burrows for Hogna lenta were restricted to 3.2-8.7 cm below the sediment surface. These depths are associated with the A and upper B soil horizons (0-20 cm). In nature, the burrows of H. lenta have been reported to be 2 m deep during the winter (Eisman et al., 2010). A major contributor to soil formation by soil animals is the mixing of sediment by transporting material from the subsurface to the surface (Lavelle et al., 2006). Animals do this when excavating burrows, but this technique is not the only way in which a burrow can be produced (Bromley, 1996). Animals can also compact sediment along the sides of a burrow as they push their way into the substrate. In this study, both species of spiders mainly constructed burrows in this way. Only adult G. inermis individuals were observed moving sediment to the surface, but not all adults engaged in this behavior. The compaction of soil, however, changes the porosity and permeability of the sediment along the burrow walls and disrupts or destroys primary sediment structures.
Open burrows provide a direct conduit for water to penetrate deeper into sediment. The presence of silken trapdoors makes this contribution unlikely while a spider inhabits a burrow. The spiders maintain burrow integrity and repair trapdoors when these are torn or removed; however, a vacated burrow will no longer be maintained. All open burrows also provide a conduit for gas exchange between the atmosphere and soil, which occurs continuously. Even burrows that have been filled with sediment will allow water and gases to move through the infill since the material will be less dense than the burrows’ walls and the surrounding matrix.
The spiders in this study were found to not contribute greatly to the introduction of organic matter into the sediment. Leftover material from prey animals was ejected from or carried out of the burrows by the spiders. Spiders that molted inside of a burrow removed the molt in a similar manner. Gorgyrella inermis excretes waste outside of its burrow, and it is assumed that Hogna lenta excretes while moving outside of the burrow. The silk linings may be the greatest contribution of organic matter to the soil. Spider silk is composed of mainly of proteins called spidroin (van Beek et al., 2002), and it is very slow to decompose. Burrows produced in holding tanks prior to the beginning of the experiments had very thick silk linings and have remained unchanged for months to years.
Although quantitative properties did not significantly change with changes in sediment properties, the diversity of burrow architectures present would enable for better interpretations of original soil composition and moisture content. The relationship between the amount of silk used to line a burrow and the cohesiveness of the sediment is also an effective means of interpreting these sediment conditions. Fossil spider burrows could be present in any soil type from Entisols to Oxisols, but it is unlikely that these burrows would be found in Gelisols or sediments in cold regions. Fossil spider burrows would be associated with a number of ichnofossils including those produced by other arachnids (scorpions, whip scorpions), other soil-dwelling arthropods (millipedes, centipedes, insects), and possibly vertebrates (amphibians, reptiles, mammals), but root traces would be most common.
Use of Cluster Analyses
A cluster analysis of six arthropod species reveals two large clusters: one composed of small arachnids and millipedes, the other composed of large arachnids and some millipedes. Within these clusters burrows produced by the same species tended to group together. If burrows produced by an unknown tracemaker are highly similar to burrows within a specific cluster, then the tracemaker is likely to either be related to those species or to engage in very similar behaviors (Table 9.6). Additional qualitative characteristics such as internal or surficial features may be used to further distinguish the identity of the tracemaker.
CONCLUSIONS
Specimens of two burrowing spiders, Gorgyrella inermis and Hogna lenta, produced seven different burrow architectures including vertical shafts, vertical shafts with terminal chambers, subvertical shafts, subvertical shafts with terminal chambers, J-shaped burrows, Y-shaped burrows, and isolated chambers. The production of these architectures was dependent on size of the individual and the sediment conditions in which the burrow was produced. Adult individuals of G. inermis produced the greatest diversity of burrow architectures whereas juvenile individuals of G. inermis and adult individuals of H. lenta produced the lowest diversity. Despite the diversity of architectures, all burrows produced by the spiders include one to two surface openings, a nearly circular cross section, and surficial features influenced by a silk lining. The presence of a silk lining is a definitive characteristic of a spider tracemaker. Increasing sediment density did not produce statistical differences in burrow measurements, but the diversity of burrow architecture was decreased and some individuals were unable to construct burrows. Sediment with low and high moisture also limited the architectural diversity and prevented some individuals from constructing burrows.
The burrows of Gorgyrellainermis and Hogna lenta are highly similar. Although these species are not closely related, their similar body morphology and life habits control the morphology and function of their burrows. This contrasts to the burrows produced by the larger spider, Aphonopelma chalcodes, which are dissimilar to those produced by G. inermis and H. lenta. The burrows of other arachnids, the scorpion Hadrurus arizonensis and the whip scorpion Mastigoproctus giganteus, are dissimilar when the burrow architecture is different from those produced by G. inermis and H. lenta, but the same architectures are highly similar. This result also occurs when the burrows of the millipede Narceus americanus are compared to the burrows produced by G. inermis and H. lenta. Qualitative data, however, can be used to differentiate those similar burrows produced by different tracemakers. Accurately identifying spider burrows and burrows produced by other arthropods such as scorpions, whip scorpions, and millipedes will aid in revealing the biodiversity of terrestrial ecosystems in the fossil record.
ACKNOWLEDGEMENTS
We thank two anonymous reviewers for their comments and suggestions. We thank the National Science Foundation (EAR-0844256), the Paleontological Society (Richard Osgood Award), the Geological Society of America, and the Ohio University Geological Sciences Alumni Association for their generous support for this study. We also thank A. Durkee, M. Ingle, and J. Bowen for their assistance in the Continental Ichnology Research Laboratory at Ohio University.
REFERENCES
Ahlbrandt, T.S., Andrews, S., and Gwynne, D.T. 1978. Bioturbation in eolian deposits. Journal of Sedimentary Petrology, 48.3:839-848.
Barnes, H. and Powell, H.T. 1950. Some observations on the effect of fibrous glass surfaces upon the settlement of certain sedentary marine organisms. The Journal of the Marine Biological Association of the United Kingdom, 29:299-302.
Bond. J.E. and Coyle, F.A. 1995. Observations on the natural history of an Ummidia trapdoor spider from Costa Rica (Araneae, Ctenizidae). Journal of Arachnology, 23:157-164.
Bowen, J. and Hembree, D. 2014. Neoichnology of two spirobolid millipedes: improving the understanding of the burrows of soil detritivores. Palaeontologia Electronica , 17.1.18A:48pp; http:// http://palaeo-electronica.org/content/2014/709-neoichnology-of-spirobolids
Bradley, R.A. 1996. Foraging activity and burrow distribution in the Sydney brown trapdoor spider (Misgolas rapax Karsch: Idiopidae). The Journal of Arachnology, 24:58-67.
Bray, R.J. and Curtis, J.T. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs, 27.4:325-349.
Breed., A.L., Levine, V.D., Peakall, D.B., and Witt, P.N. 1964. The fate of the intact orb web of the spider Araneus diadematus Cl. Behavior , 23:43-60.
Bristowe, W.S. 1976. A contribution to the knowledge of liphistiid spiders. Journal of Zoology, London, 178:1-6.
Bromley, R.G. 1996. Trace Fossils: Biology, Taphonomy and Applications. Chapman and Hall, London, United Kingdom.
Brown, T.M. and Kraus, M.J. 1983. Ichnofossils of the alluvial Willwood Formation (Lower Eocene), Bighorn Basin, Northwest Wyoming, U.S.A. Palaeogeography, Palaeoclimatology, Palaeoecology, 43:95-128.
Buynevich, I.V., Darrow, J.S., Grimes, Z.T.A., Seminack, C.T., and Griffis, N. 2011. Ungulate tracks in coastal sands: recognition and sedimentological significance. Journal of Coastal Research, 64:334-338.
Cameron, B. 1969. Paleozoic shell-boring annelids and their trace fossils. American Zoologist, 9.3:689-703.
Catena, A.M. and Hembree, D.I. 2014. Biogenic structures of burrowing skinks: neoichnology of Mabuya mutifaciata (Squamata: Scincidae), p. 343-369. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living . Topics in Geobiology, Springer Publishing.
Chin, K., Pearson, D., and Ekdale, A.A. 2013. Fossil worm burrows reveal very early terrestrial animal activity and shed light on trophic resources after the end-Cretaceous mass extinction. PLoS One, 8(8):e70920. DOI: 10.1371/journal.pone.0070920
Clarke, R.D. and Grant, P.R. 1968. An experimental study of the role of spiders as predators in a forest litter community. Part 1. Ecology. 49.6:1152-1154.
Counts, J.W. and Hasiotis, S.T. 2009. Neoichnological experiments with masked chafer beetles (Coleoptera: Scarabaeidae): implications for backfilled continental trace fossils. PALAIOS, 24:74-91.
Coyle, F.A. and Icenogle, W.R. 1994. Natural history of the Californian trapdoor spider genus Aliatypus (Araneae, Antrodiaetidae). The Journal of Arachnology, 22:225-255.
Craig, C.L. 1997. Evolution of arthropod silks. Annual Review of Entomology, 42:231-267.
Dapples, E.C. 1938. The sedimentational effects of the work of marine scavengers. American Journal of Science, 36:54-65.
Dashtgard, S.E., Gingras, M.K., and Pemberton, S.G. 2008. Grain-size controls of the occurrence of bioturbation. Palaeogeography, Palaeoclimatology, Palaeoecology, 257:224-243.
de Gibert, J.M., Fregenal-Martínez, M.A., Buatois, L.A., and Mánagano, M.G. 2000. Trace foferssils and their palaeoecological significance in Lower Cretaceous lacustrine conservation deposits, El Montsec, Spain. Palaeogeography, Palaeoclimatology, Palaeoecology, 156:89-101.
Dippenaar-Schoeman, A.S. 2002. Baboon and Trapdoor Spiders of Southern African: An Identification Manual. ARC-Plant Protection Research Institute. Agricultural Research Council, Pretoria, South Africa.
Dunlop, J.A. 2010. Geologic history and phylogeny of Chelicerata. Arthropod Structure & Development, 39:124-142.
Dunlop, J.A., Penney, D., and Jekel, D. 2013. A summary list of fossil spiders and their relatives. In Platnick, N.I. (ed.), The world spider catalog, version 14.0 American Museum of Natural History, research.amnh.org/entomology/spiders/catalog/index.html
Dzenowski, N.D. and Hembree, D.I., 2014. The neoichnology of two terrestrial salamanders: quantifying amphibian burrows using modern analogs, p. 305-341. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living. Topics in Geobiology, Springer Publishing.
Eisman, C., Charney, N., and Carlson, J. 2010. Tracks & Sign of Insects and Other Invertebrates. Stackpole Books, Mechanicsburg, Pennsylvania.
Fairchild, J.M. and Hasiotis, S.T. 2011. Terrestrial and aquatic neoichnological laboratory experiments with the freshwater crayfish Orconectes : trackways on media of varying grain size, moisture, and inclination. PALAIOS, 26:790-804.
Fernández, D.E. and Pazos, J.P. 2013. Xiphosurid trackways in a Lower Cretaceous tidal flat in Patagonia: palaeoecological implications and the involvement of microbial mats in trace-fossil preservation. Palaeogeography, Palaeoclimatology, Palaeoecology, 375:16-29.
Frey, R. 1968. The Lebensspuren of some common marine invertebrates near Beaufort, North Carolina. I. Pelycopod burrows. Journal of Paleontology, 42:570-574.
Frey, R.W. 1975. The realm of ichnology, its strengths and limitations, p. 13-38. In Frey, R.W. (ed.), The Study of Trace Fossils. Springer-Verlag, New York.
Genise, J.F., Melchor, R.N., Archangelsky, M., Bala, L.O., Straneck, R., and de Valais, S. 2009. Application of neoichnological studies to behavioral and taphonomic interpretation of fossil bird-like tracks from lacustrine settings: the Late Triassic-Early Jurassic? Santo Domingo Formation, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology, 272:143-161.
Gertsch, W.J. 1949. American Spiders. D. Van Nostrand Company, INC., Toronto.
Getty, P.R., Sproule, R., Wagner, D.L., and Bush, A.M. 2013. Variation in wingless insect trace fossils: insights from neoichnology and the Pennsylvanian of Massachussetts. PALAIOS , 28:243-258.
Gingras, M.K., Pickerill, R., and Pemberton, S.G. 2002. Resin cast of modern burrows provides analogs for composite trace fossils. PALAIOS, 17:206-211.
Gingras, M.K., Dashtgard, S.E., MacEachern, J.A., and Pemberton, S.G. 2008. Biology of shallow marine ichnology: a modern perspective. Aquatic Biology, 2:255-268.
Halfen, A.F. and Hasiotis, S.T. 2010. Neoichnological study of the traces and burrowing behaviors of the western harvester ant Pgonomyremex occidentalis (Insecta: Hymenoptera: Formicidae): paleopedological and paleoecological implications. PALAIOS, 25:703-720.
Hamer, J.M.M. and Sheldon, N.D. 2010. Neoichnology at lake margins: implications for paleo-lake systems. Sedimentary Geology, 228:319-327.
Hamilton, D.E., McIntyre, N.E., and Densmore, L.D. 2012. Using implanted passive integrated transponders to monitor long-term burrow fidelity in a theraphosid spider, Aphonopelma hollyi. The Southwestern Naturalist, 54:144-147.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological Statistics software packages for education and data analysis. Palaeontologia Electronica 4(1):9pp, 178kb; http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hasiotis, S.T. 2002. Continental ichnology: fundamental processes and controls on trace fossil distribution. The Continental Realm, p. 268-284. In Miller, W. (ed.), Trace Fossils: Concepts, Problems, Prospects. Elsevier Science.
Hasiotis, S.T. 2007. Continental Trace Fossils .SEPM Short Course Notes, No. 51. 113-114.
Hembree, D.I. 2009. Neoichnology of burrowing millipedes: linking modern burrow morphology, organism behavior, and sediment properties to interpret continental ichnofossils. PALAIOS, 24:425-439.
Hembree, D.I. 2013. Neoichnology of the whip scorpion Mastigoproctus giganteus : complex burrows of predatory terrestrial arthropods. PALAIOS, 28:141-162.
Hembree, D.I. 2014. Large, complex burrows of terrestrial invertebrates: neoichnology of Pandinus imperator, p. 229-263. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living. Topics in Geobiology, Springer Publishing.
Hembree, D.I. and Hasiotis, S.T. 2006. The identification and interpretation of reptile ichnofossils in paleosols through modern studies. Journal of Sedimentary Research , 76:575-588.
Hembree, D.I., Johnson, L.M., and Tenwalde, R.W. 2012. Neoichnology of the desert scorpion Hadrurus arizonensis : burrows to biogenic cross lamination. Palaeontologia Electronica. 15.1.10A:34pp; palaeo-electronica.org/content/2012-issue-1-articles/192-neoichnology-of-scorpions
Hodge, M.A. 1999. The implications of intraguild predation for the role of spiders in biological control. Journal of Arachnology, 27(1):351-362.
King, G.F. 2004. The wonderful world of spiders: preface to the special Toxicon issue on spider venoms. Toxicon, 43:471-475.
Kraus, M.J. and Hasiotis, S.T. 2006. Significance of different modes of rhizolith preservation to interpreting paleoenvironmental and paleohydrological settings: examples from Paleogene paleosols, Bighorn Basin, Wyoming, U.S.A. Journal of Sedimentary Research, 76:633-646.
Lavelle, P., Decaëns, T., Aubert, M., Barot, S., Blouin, M., Bureae, F., Margerie, P., Mora, P., and Rossi, J.-P. 2006. Soil invertebrates and ecosystem services. European Journal of Soil Biology, 42:S3-S15.
Lawfield, M.W. and Pickerill, R.K. 2006. A novel contemporary fluvial ichnocoenoses: Unionid bivalves and the Scoyenia-Mermia ichnofacies transition. PALAIOS, 21.4:391-396.
M’Rabet, S.M., Hénaut, Y., Sepúlveda, A., Rojo, R., Calmé, S., and Geissen, V. 2007. Soil preference and burrow structure of an endangered tarantula, Brachypelma vagans (Mygalomorphae: Therophosidae). Journal of Natural History , 41:1025-1033.
MacGinitie, G.E. 1945. The size of the mesh openings in mucous feeding nets of marine animals. Biological Bulletin, 88:107-111.
Maples, C.G. and Archer, A.W. 1989. The potential of Paleozoic nonmarine trace fossils for paleoecological interpretations. Palaeogeography, Palaeoclimatology, Palaeoecology, 73:185-195.
Meadows, P.S. 1991. The environmental impact of burrows and burrowing animals - conclusions and a model. p. 327-388. In Meadows, P.S. and Meadows, A. (eds.), The Environmental Impact of Burrowing Animals and Animal Burrows : The Zoological Society of London, Clarendon Press, Oxford, UK.
Melchor, R.N., Genise, J.F., Farina, J.L., Sánchez, M.V., Sarzetti, L., and Visconti, G. 2010. Large striated burrows from fluvial deposits of Neogene Vinchina Formation, La Rioja, Argentina: a crab origin suggested by neoichnology and sedimentology. Palaeogeography, Palaeoclimatology, Palaeoecology, 291:400-418.
Mikuś, P. and Uchman, A. 2013. Beetle burrows with a terminal chamber: a contribution to the knowledge of the trace fossil Macanopsis in continental sediments. PALAIOS, 28:403-413.
Moore, H.B. 1938. Faecal pellets in relation to marine deposits. Recent Marine Sediments , 516-524.
O’Green, A.T. and Busacca, A.J. 2001. Faunal burrows as indicators of paleo-vegetation in eastern Washington, USA. Palaeogeography, Palaeoclimatology, Palaeoecology, 169:23-37.
Pechenik, J.A. 1991. Biology of the Invertebrates (second edition). Wm. C. Brown Publishers, Dubuque, Iowa.
Pickford, M. 2000. Fossil spider’s webs from the Namib Desert and the antiquity of Seothyra (Araneae, Eresidae). Annales de paleontology, 86(3):147-155.
Platnick, N. I. 2013. The world spider catalog, version 14.5. American Museum of Natural History, online at research.amnh.org/entomology/spiders/catalog/index.html DOI: 10.5531/db.iz.0001.
Prestwich, K.N. 1977. The energetics of web-building in spiders. Comparative Biochemistry and Physiology Part A: Physiology, 57.3:321-326.
Ratcliffe, B.C. and Fagerstrom, J.A. 1980. Invertebrate Lebensspuren of Holocene floodplains: their morphology, origin, and paleoecological significance. Journal of Paleontology, 54.3:614-630.
Retallack, G.J., Smith, M.H., and Ward, P.D. 2003. Vertebrate extinction across Permian-Triassic boundary in Karoo Basin, South Africa. Geological Society of America Bulletin , 115.9:1133-1152.
Reichert, S.E. and Lockley, T. 1984. Spiders as biological control agents. Annual Review of Entomology, 29.1:299-320.
Rupert, E.E., Fox, R.S., and Barnes, R.D. 2004. Invertebrate Zoology: A Functional Evolutionary Approach (seventh edition). Thomson Books/Cole, Belmont, California.
Sadler, C.J. 1993. Arthropod trace fossils from the Permian De Chelly Sandstone, Northeastern Arizona. Journal of Paleontology, 67(2):240-249.
Savrda, C.E. and Bottjer, D.J. 1986. Trace-fossil model for reconstruction of paleo-oxygenation in bottom waters. Geology, 14:3-6.
Schmerge, J.D., Riese, D.J., and Hasiotis, S.T. 2013. Vinegaroon (Arachnida: Thelyphonida: Thelyphonidae) trackway production and morphology: implications for media and moisture control on trackway morphology and a proposal for a novel system of interpreting arthropod trace fossils. PALAIOS, 28:116-128.
Selden, P.A. 1996. First fossil mesothele spider, from the Carboniferous of France. Revue Suisse de Zoologie, vol. hors série : 585-596.
Selden, P.A. 2000. Paleothele, replacement name for the fossil mesothele spider Eothele Selden non Rowell. Bulletin-British Arachnological Society, 11.7:292.
Selden, P.A. and Gall, J.-C. 1992. A Triassic mygalomorph spider from the northern Vosges, France. Palaeontology, 35:211-235.
Selden, P.A., Anderson, J.M., Anderson, H.M., and Fraser, N.C. 1999. Fossil araneomorph spiders from the Triassic of South Africa and Virginia. Journal of Arachnology, 27:401-414.
Shäfer, W. and Craig, G.Y. 1972. Ecology and Palaeoecology of Marine Environments. Oliver and Boyd, Edinburgh.
Shear, W.A. 1994. Untangling the evolution of the web. American Scientist, 82:256-266.
Smith, J.J. and Hasiotis, S.T. 2008. Traces and burrowing behaviors of the cicada nymph Cicadetta callipe : neoichnology and paleoecological significance of extant soil-dwelling insects. PALAIOS, 23.8:503-513.
Suttle, K.B. 2003. Burrow use in a northern Californian population of the wolf spider Schizocosa mccooki (Araneae, Lycosidae). Journal of Arachnology , 31:433-436.
Turner, B.R., Stanistreet, L.G., and Whateley, M.K.G. 1981. Trace fossils and palaeoenvironments in the Ecca Group of the Nongoma Graben, Nothern Zululand, Southern Africa. Palaeogeography, Palaeoclimatology, Palaeoecology, 36:113-123.
Van Beek, J.D., Hess, S., Vollrath, F., and Meier, B.H. 2002. The molecular structure of spider dragline silk: folding and orientation of the protein backbone. Proceedings of the National Academy of Sciences, 99:10266-10271.
Wallace, H.K. 1942. A study of the lenta group of the genus Lycosa , with descriptions of new species (Araneae, Lycosidae). American Museum Novitates, 1185:1-21.

