 Ethological interpretation of making the pellet designs by the bubbler crab Dotilla on the modern intertidal beaches: A study from the Bay of Bengal coast, Eastern India
Ethological interpretation of making the pellet designs by the bubbler crab Dotilla on the modern intertidal beaches: A study from the Bay of Bengal coast, Eastern India
Article number: 27.1.a11
https://doi.org/10.26879/1234
Copyright Paleontological Society, February 2024
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 5 August 2022. Acceptance: 6 November 2024.
ABSTRACT
Prolific growth of the feeding pellet designs (radial, concentric, concentric-radial, petaloid, asteroid and leaf-shaped at individual and mossy and mat at community levels) of the tiny bubbler crabs Dotilla on the intertidal beach of the Bay of Bengal coast has been attributed to burrow protection measure against their common predators (similar sized conspecific and heterospecific crabs) as a new neoichnological perception. With progressive feeding, the crabs increase the SI scores, a newly proposed security index, of their pellet designs through structural transformation, conjugation and merging of pellet designs, besides construction of pellet barricades. Four specialized substrate exploration techniques (sector of a circle, radially diverging, concentric and combined concentric-radial feeding modes) have been identified that ensure, as per prevailing conception, optimum food collection, economic substrate utilization and minimum chance of re-exploration of already explored area.
The delicate pellet structures, despite having poor preservation potential, can be preserved in the sedimentary records in special circumstances, such as, ex situ preservation as pellet-filled burrows, ripple troughs and mud cracks and in situ preservation of the pellet mats through microbial stabilization following algal blooms and storm deposition. Geologically, the preserved Dotilla pellet spread and burrows ichnozone, besides confirming shallow-marine littoral settings, delineates the positions of ancient upper intertidal flat, land and sea, palaeo-hightide level and palaeo-shoreline configuration, i.e., data important for basin analysis. Stratigraphic disposition of the microbially stabilized pellet mats relative to different coastal facies helps interpret transgression and regression events of the palaeo-sea, besides episodic storm deposition and algal blooms.
Chandreyee De. Central Ground Water Board, 419 A Kanwali Road, Balliwala Chowk, Dehradun 248001, Uttarakhand, India. chandreyee_de@yahoo.co.in
Keywords: tiny crabs; pellet structures; security index; Bengal coast; geology; hydrogeology
Final citation: De, Chandreyee. 2024. Ethological interpretation of making the pellet designs by the bubbler crab Dotilla on the modern intertidal beaches: A study from the Bay of Bengal coast, Eastern India. Palaeontologia Electronica, 27(1):a11.
https://doi.org/10.26879/1234
palaeo-electronica.org/content/2024/3891-dotilla-pellet-designs
Copyright: February 2024 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
The tiny sand bubbler crabs of the genera Dotilla and Scopimera, belonging to the family Dotillidae (Peter et al., 2008), thrive permanently in enormous number in the warm-temperate and tropical-subtropical intertidal beaches (Ansell, 1988; Chapman and Reiss, 1999; McLachlan and Brown, 2006), estuary bars (Bulcao and Hodgson, 2012) and moist sandy to silty tidal flats across the Indo-Pacific coastal regions covering parts of Asia, Australia, East Africa and even Hawaii. These crabs are surface deposit feeders and quasiterrestrial. Some specific morphological, physiological and behavioural adaptations enable them to inhabit and overcome the odds of tidal environment (Suzuki and Taiji, 1984; Gherardi and Russo, 1997, 2001; Gherardi et al., 2002) and to adopt an isospatial strategy (self-confinement within sandy coast facing alternate exposure to air and water; Vannini and Chelazzi, 1985). For maintaining quasiterrestrial and deposit-feeding life habits they have developed: 1) large membranous disks (“tympana” or gas window) on the meral segments of the legs and sometimes, as in Dotilla, on the thoracic sterna three for aerial gas exchange (Maitland, 1986); 2) tufts or rows of setae for water uptake (Hartnoll, 1973); 3) grooves on the carapace for absorbing water by pressing specialized abdominal setae into the sand, called a “sponging” behaviour (Fishelson, 1983; Dray and Paula, 1998) and 4) spoon-shaped setae on the second maxillipeds for sorting organic matters (bacteria, diatoms, blue-green algae, ciliates and nematodes) from the sand (Bigalke, 1921; Miller, 1961; Ono, 1965; Robertson and Newell, 1982; Vogel, 1984). They have evolved sediment digging mechanism to construct dwelling-feeding burrows and igloos (Gherardi and Russo, 2001).
Their presence in large number and prolific biogenic activities are marked by the production of thick population of very narrow (a few mm wide) and short (1 to 6 cm long) dwelling burrows (vertical, I and J shaped and unbranched) and globular sand balls or feeding pellets arranged systematically into spectacular designs, often referred to as “Sand Ball Galaxies” (Maitland, 1986; Chakrabarti et al., 2006; Peter et al., 2008). Supposedly, they do so to avoid searching the same sand twice for food.
From the biological point of view, the crab genus Dotilla was very well studied over the past 50 years or more. Their breeding, growth patterns, other life habits and ontogeny-based zoogeographic distribution over tropical-subtropical sandy shore were well illustrated (Hails and Yaziz, 1982). Wada (1981) revealed their burrow-mouth barricading life habit by constructing chimneys, excavated sediment heaps or domes and igloos. Koga (1998) elaborated their reproductive success and mating modes. Chapman and Reiss (1999) discussed the mechanism of forming inflated sand bubbles through egestion of indigestible wet sand grains by Dotilla. Their ability to modify food resources biochemically (Takagi et al., 2010), habitat segregation vis-à-vis cellulase activity (Kawaida et al., 2013) and visual image processing by compound eyes (Zeil and Hemmi, 2010) were well addressed. Algorithmic framework was used to generate visual art based on the collective feeding behavior and feeding pellet designs of the sand bubbler crabs on sandy beaches (Richter, 2018).
Their ichnological attributes were also equally well studied. Modern burrows, burrow mouth chimneys, surficial pellet designs (mossy, concentric-semicircular, concentric-radial, asteroid, bird-foot etc.) and bioturbation textures produced by endobenthic quasiterrestrial decapod crustaceans, including the bubbler crabs Dotilla, in the tide influenced Bay of Bengal coast of the Eastern India were addressed (Chakrabarti, 1972; Bakshi et al., 1980; De, 1998a, 1998b, 2000, 2005, 2009, 2010, 2019; Chakrabarti et al., 2006; Baucon et al., 2012) mainly to depict their palaeoenvironmental significance in rock records. Detailed mechanism of “igloo” formation by Dotilla (Takeda et al., 1996), their burrowing activities in the Kenyan mangrove swamps (Gherardi and Russo, 2001) and feeding activities in South African estuary (Bulcao and Hodgson, 2012) were well studied. Barricade-building life habit of Ilyoplax pusillus was reported (Keiji, 1984) without details of exact mechanism. Despite so much work, the ethological cause behind ubiquitous and habitual construction of spectacular pellet designs by the bubbler crabs on modern intertidal beaches is not revealed, so far. Moreover, transformation and merging of one pellet design to another; micro-zonation of different pellet designs; their diurnal pellet-making phases in relation to local sea level fluctuations, tidal processes and groundwater level fluctuations; preservation potential of these tiny sand pellets; their possible ancient analogues and detailed geological and hydrogeological significance have not been worked out.
The present work focuses on: 1) the sediment feeding-cum-pellet-making life habits and architecture of various feeding pellet designs formed by the sand bubbler crabs Dotilla spp. in the soft and sandy Bakkhali intertidal beach (the study area) of the Bay of Bengal coast, Eastern India; 2) geometric transformation and spatial distribution of various pellet designs with time across the beach profile to reveal the cause behind the formation of pellet designs; 3) relationship of the crab’s pellet-making phases with fluctuating local substrate water level and tidal actions to highlight hydrogeological significance and 4) preservation potential of the Dotilla pellets, burrows and pellet-spread zones, as a whole, in the sediment records to infer their possible geological significance including antient marine episodic events (e.g., transgression, regression, algal bloom and storm actions).
SITE DESCRIPTION
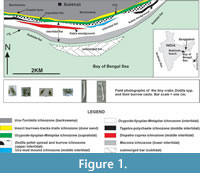 The studied crescent-shaped Bakkhali (21º 33’ 50” N and 88º 15’ 49” E) beach (E-W trending and on average 1.5 km wide and more than 8 km long) forms a small part of the world’s most colossal coastal mangrove ecosystem of the Sundarban Delta Complex in the Bay of Bengal coast, Eastern India and Bangladesh (Figure 1). The coastal landforms developed here are mangrove vegetated wide (>1 km) backswamps (greyish-black clays and silts); coastal dune ridges (10 to 70 m wide, un-oxidized, medium to fine, well sorted sands); sandy supratidal-intertidal-subtidal beaches (>1 km wide, calculated MZ 2.75 to 1.4 ϕ following Pettijohn, 1975) with increasing contents of silt and clay towards low tide level and a submerged sand bar successively from land to sea (Figure 1). Stratigraphically, these unconsolidated sediments constitute the Recent Nabadweep Formation that overlies the subRecent Kalna Formation exposed in this beach sector as inliers of rigid mudground (with root traces and upright tree trunks) and woodground (buried palaeoforest). The large Himalayan River systems (the Ganga and Brahmaputra) and other major Indian Peninsular rivers (the Mahanadi, Godavari, Krishna, Kaveri and the Irrawady), besides the Salween River from the Myanmar, contribute about 2000 million tons of sediments annually to the Bay of Bengal (Mohanty et al., 2008). The effects of tide and wave actions are very conspicuous and rigorous on this beach. The coast regionally experiences average maximum and minimum temperature of 40 ºC (May to June) and 24 ºC (December), a high salinity range (21‰ in the inner vs. 37‰ in the outer delta), moderately high annual rainfall (1518 to 2439 cm), moderate pH (7.8 to 8.2) and a mean maximum semidiurnal spring tidal range of 6.6 to7.5 m and mean minimum neap tidal range of 2.4 to 2.7 m (Port Trust of India, India Meteorological Department and Survey of India database averaged for the last 10 years:
The studied crescent-shaped Bakkhali (21º 33’ 50” N and 88º 15’ 49” E) beach (E-W trending and on average 1.5 km wide and more than 8 km long) forms a small part of the world’s most colossal coastal mangrove ecosystem of the Sundarban Delta Complex in the Bay of Bengal coast, Eastern India and Bangladesh (Figure 1). The coastal landforms developed here are mangrove vegetated wide (>1 km) backswamps (greyish-black clays and silts); coastal dune ridges (10 to 70 m wide, un-oxidized, medium to fine, well sorted sands); sandy supratidal-intertidal-subtidal beaches (>1 km wide, calculated MZ 2.75 to 1.4 ϕ following Pettijohn, 1975) with increasing contents of silt and clay towards low tide level and a submerged sand bar successively from land to sea (Figure 1). Stratigraphically, these unconsolidated sediments constitute the Recent Nabadweep Formation that overlies the subRecent Kalna Formation exposed in this beach sector as inliers of rigid mudground (with root traces and upright tree trunks) and woodground (buried palaeoforest). The large Himalayan River systems (the Ganga and Brahmaputra) and other major Indian Peninsular rivers (the Mahanadi, Godavari, Krishna, Kaveri and the Irrawady), besides the Salween River from the Myanmar, contribute about 2000 million tons of sediments annually to the Bay of Bengal (Mohanty et al., 2008). The effects of tide and wave actions are very conspicuous and rigorous on this beach. The coast regionally experiences average maximum and minimum temperature of 40 ºC (May to June) and 24 ºC (December), a high salinity range (21‰ in the inner vs. 37‰ in the outer delta), moderately high annual rainfall (1518 to 2439 cm), moderate pH (7.8 to 8.2) and a mean maximum semidiurnal spring tidal range of 6.6 to7.5 m and mean minimum neap tidal range of 2.4 to 2.7 m (Port Trust of India, India Meteorological Department and Survey of India database averaged for the last 10 years:
https://smportkolkata.shipping.gov.in/index1.php?layout=1&lang=1&level=1&sublinkid=156&lid=190; https://www.imdpune.gov.in/library/public/Climate%20of%20WestBengal.pdf; https://en.wikipedia.org/wiki/Climate_of_West_Bengal).
The prevailing wind directions are from S to SW during summer (March to June) and N to NE during winter (November to February). The entire coastal zone experiences pre-monsoon cyclonic storms, locally called “Kalbaishakhi” (southeast to northwest, 85 to 150 km/hr and eight to ten times every year between March and May; De, 2000) that thoroughly reorganize the beach and dune sediments.
Amongst the trace-making infaunal invertebrates, the quasiterrestrial decapod crustaceans play predominant role in the bioturbation of soft sandy to clayey sediments of the Bay of Bengal coast. About 86 decapod crustacean species belonging to 13 families are known from the coastal Bengal (Deb, 1998). Of these, several species of tiny sand bubbler crabs Dotilla thrive in thick population and produce conspicuous feeding pellet designs and short and thin burrow tubes during low tides on the sandy intertidal beaches. Other commonly associated burrowing and pellet-making decapod crabs are Ocypode ceratophthalmus Pallas, O. macrocera Edwards, O. cardimanus Desmarest and O. stimpsoni Ortmann, Uca marionis Alcock and Uca lactea De Haan, besides a borer crab Charybdis rostrata Edwards (De, 2005, 2009, 2010, 2019). Polychaetes (e.g., Diopatra cuprea Bosc), gastropods, bivalves, other worms and insects are also involved in the trace-making activities in the study area. A bewildering array of primary sedimentary structures of wind and aquatic origin are produced on the beach. Biogenic activities are greatly influenced by beach hydrodynamics and sedimentary structures. Dotilla pellet designs are also observed produced on highly rippled surfaces where the placement of pellet spreads and burrow openings are found controlled by ripple crest and troughs (discussed later).
The diminishing mangrove vegetation, shoreline retreat and coastal erosion/inundation at alarming rates with rising sea level during the past couple of decades are the main concerns of the Bay of Bengal coast today (De, 2019).
MATERIALS AND METHODS
The study materials include beach sediments for grain size and mineralogical analyses; feeding pellets for size and shape determination; pellet ornamentations or designs of the bubbler crabs for analyzing sequential development of different pellet designs and their transformation; extensive photographic documents of different stages of pellet-spreading activities with time and space; paraffin wax casts of burrows for measuring burrow parameters and crab samples for taxonomic identification. The work was self-sponsored and done during January-February 2015 over about 12 km² area of the Bakkhali beach (Figure 1). It was based on remapping of the already known ichnozones developed here (De, 2000, 2005) and very close field observations on the biogenic activities of Dotilla and some of their associates.
The entire coastal geomorphic profile was subdivided from land (north) towards sea (south) into backswamp, coastal dunes, supratidal, intertidal and subtidal flats for work reference. Dry sieving technique was applied for grain size measurement. Measurement of sediment moisture contents involved collection of surficial beach sediments in sealed tubes, weighing the samples before and after oven drying and calculating weight (%) of the moisture content. Major mineralogical and organic constituents of the dried beach sands were identified under petrological and stereo microscopes (Leica MZ-12). Weight (%) of the constituents was calculated from point counter data. On spot population density of the tiny Dotilla crabs was difficult to measure. This parameter is otherwise tentatively measured by counting the tiny (millimetric) burrow openings within one square meter area where the tiny crabs Dotilla predominantly thrive.
Pellet designs were geometrically classified. Line tracings of different pellet designs were made (Figure 2, Figure 3, Figure 4) with reference to burrow openings (open and closed), runways, pellet walls or rings, pellet rows and pellet spread areas. A newly introduced conception of the Safety Index (SI) and Attack Index (AI) for the pellet designs requires an arbitrary circular field to cover 360º around the burrow opening and the pellet design. The structures being largely radial and variable in size, the openings in the pellet structure through which a predator can enter the burrow hole can be measured in terms of angular gaps for uniformity. A circle with center at the burrow opening is drawn encompassing roughly the entire pellet design to identify and measure all the angular gaps (in degrees) through which a small predator of Dotilla size could freely sneak into the burrow opening to attack the burrower. One or a few missing pellets without breaking the basic trend of the pellet rows or zones are not considered here as gaps. All such angular gaps are added to calculate the Attack Index (AI) in degrees expressed as percentage (%) of 360º for the pellet structure. 100 minus AI% is considered here as the Safety Index (SI%), as a new parameter for the pellet structures ever thought of and calculated to compare numerically the relative security status of different pellet designs. For safe pellet structure SI is considered 100%. SI ranging from 80% to 100% is considered a fairly safe structure. SI below 50% may be considered as a very unsafe structure. Pelletal design with plugged or closed burrow opening is considered having SI = 100% as the predator cannot enter the burrow tube. Clear runways or trenches favour predators’ entry and hence considered uniformly as gap in SI or AI calculation. Here, the SI is not assumed as absolute safety for the crabs in their eco-space. Other usual safety parameters are assumed to be uniform for the individuals of the concerned crab genus. For community pellet structures the same technique was adopted for each pellet design, and SI was averaged for one square meter area of the beach surface.
A man-made assessment of AI or SI scores of the pellet structures is favored because counting of predation events of competing members in the field is problematic and may be erroneous for several reasons. It is difficult to follow Dotilla and their predators (discussed later) in the field for more than a few seconds because of their millimetric size; excellent camouflaging ability; sensitivity to light, sound and vibration; fast body movement and ability to hide promptly inside the burrow tube or to bury themselves into semifluid sandy substrate. Given the above characteristics of the Dotilla, it is very difficult to distinguish the predators from the predated crabs of the same species, in particular.
Line tracings are drawn to aid simplified representation and comparison of the pellet structures. Plan outlays are drawn (Figure 5) from the line tracings with reference to openings to represent basic architectures of the pellet arrangements by lines (thick and thin) that highlight geometric relationships between the major pellet rings or walls and rows. The closures (clockwise and anticlockwise) of the pellet rings are highlighted by red colour. Arrows are placed to denote routes through which predators could sneak into the burrow opening. Schematic diagrams to represent growth and transformation of the pellet designs are added as per reviewer’s suggestion.
BURROWING AND PELLET-MAKING LIFE HABITS OF DOTILLA
The tiny sand bubbler crabs belonging to the genus Dotilla (Figure 1) form various, but conspicuous, feeding pellet designs on the upper intertidal beach of the study area during low tide hours when the substrate gets subaerially exposed and dried. These crabs were originally accommodated within the Family Ocypodidae. Subsequently, Peter et al. (2008) classified this genus within the Family Dotillidae. They are tiny (3-10 mm across the carapace, body spherical, pincers very long, slender and folded downward with the claws pointed towards itself); supersensitive to light, sound and vibration; grey to dark grey in colour camouflaging with the beach sand; quasiterrestrial; prolific burrower and sand forager in the intertidal beach emerged during low tides and architect of very meticulously constructed feeding pellet structures or designs. These crabs are well adapted to a life out of water as they possess “gas windows” on the merus of the legs and thoracic sternites to absorb air (Maitland, 1986). They absorb water from the moist sand through silky hairs on their abdomen. Unlike most of the associated Ocypodid crabs, they can run forward as well as sideways very fast.
Field observations suggest that with the onset of each low tide several physical and biological processes start on the intertidal zone. The recession of water triggers gradual subaerial exposure of the intertidal flat down slope and progressive drying up of sediments from the upper to lower flat. The tiny bubbler crab Dotilla started appearing on the surface in millions from their subsurface plugged burrow tubes. Their population density increases gradually as the substrate dries up. A majority of them immediately start fresh burrowing or modification of their earlier burrows (I and J shaped, a few millimeters wide and 5-6 cm long with a bottle neck just below opening) for dwelling purpose (Figure 1) and speedy but very systematic burrow-centric foraging of the surficial semidried beach sand layers for feeding. A group forms dense aggregation or droves, as reported, that move away from the normal distribution zone towards a low water line (Hartnoll, 1973; Gherardi and Russo, 2001). They scrap up sand particles with downward pointed pincers and bring the particles to the mouth to sift out tiny food particles (mainly microorganisms). The sifted sands are then discarded and tossed behind as little inflated balls (globular pellets; 25-30 pellets per minute as observed in field) of equal size and shape in a systematic manner to form various geometrically distinguished designs (discussed later). Stepwise and sideward movement of the crabs with simultaneous scraping of sediment produces a linear (straight as well as slightly curved) row of pellets. Sediment probing in diverging and converging directions to and fro from central burrow opening produces radial rows of pellets. Hartnoll (1973) also observed this type of Dotilla feeding pattern that progressively covers a growing sector of circle. Away from the opening there lies a larger space between two successive rows of pellets. This extra space is also probed radially forming distant sub-parallel and shorter radial rows of pellets. These shorter pellet rows often join the main radial rows forming distally branched pellet rows. Probing in concentric fashion around burrow opening produces concentric rows of pellets. With time and increased substrate dryness, the foraging and pellet making activities of the crabs intensify. Simpler design transforms into a complex one with increasing pellet density (discussed later). The upper intertidal flat, because of their early subaerial exposure and higher dryness relative to the middle and lower intertidal flats, gets covered by Dotilla pellet designs early. A burrow opening with a discrete pellet design formed around defines an individual crab’s feeding territory. The runways or feeding trenches (one or more, usually radial) formed within the feeding territory are used as a communication route between the foraging crab and its burrow opening. It was interesting to observe that the identity of individual pellet design gets lost with time with increased feeding activities and pellet formation that led to gradual expansion and merging of discrete pellet designs into what is called here as pellet mat design (see later). On the contrary, the pellet concentration and foraging activities gradually decrease towards the receding low tide level with the increase in sediment moistness. As the next high tide approaches, they start plugging their burrow mouths to trap air bubble inside the burrow tubes wherein they spend the high tide hours (Hartnoll, 1973; De, 2005). Since the crabs have only a few hours’ time during the low tide to perform all these life-saving activities, they are adapted to a very fast mode of life functions. Being so small and numerous, the bubbler crabs have a load of natural predators: from the local birds to crabs, even of their own kind (discussed later). Interestingly, these conspicuous and globally widespread physical evidence of prolific sand foraging and pellet design-making activities of tiny bubbler crabs on the upper intertidal sand flats get immediately erased by following high tide leaving very little or no chance of their preservation in sediment (or rock) records, except in a few very special circumstances (discussed later).
Dotilla, unlike other bubbler crab genera Scopimera and Ilyoplax, in wet and semi-fluid sand builds ‘igloo’ structures made up of sand pellets by rotating itself within sediment to seal an air bubble around itself (Hartnoll, 1973; Takeda et al., 1996). The igloos are often observed connected by Dotilla trackways and associated with Dotilla burrows. The crab continues to burrow downwards and scoop up sand from below and plaster the sand at the bottom of the sediment roof that covers the air bubble. Although igloos made by Dotilla in the moist substrates of the Bakkhali intertidal beach were observed, they are not included in the present study as their typical pellet spread design. Contemporaneous ichnological activities performed by other associated organisms (crabs, bivalves, gastropods, annelids, polychaetes, insects, etc.) on the studied beach sector are also kept outside the purview of present work as these are already well addressed in published literature (De, 2005, 2009, 2010, 2019).
RESULTS
Ichnozones, Sediments and Beach Dynamics
The studied Bakkhali beach includes more than 1 km wide intertidal (between high and low tide levels) flat with an average slope of 4o to 5o towards the southern sea (Figure 1). Bubbler crabs Dotilla, their burrows and pellet designs largely restricted to the upper intertidal flat, are referred here as the Dotilla pellet spread and burrow ichnozone (very narrow; 30-90 m wide, Figure 1) that discontinuously runs parallel with the coastline. The middle and lower intertidal flat is occupied by a wide Ocypode - Ilyoplax - Metaplax (young and adult burrows) ichnozone that incorporates narrow, discontinuous and coast parallel Tagelus (bivalve), Diopatra cuprea (polychaetae), Uca mud mound and Macoma ichnozones (Figure 1) successively towards the low tide mark. Towards land, the successive coast-parallel ichnozones developed are Ocypode - Ilyoplax - Metaplax (old and juvenile burrows) ichnozone in the supratidal flat, insect burrows - tracks - trails ichnozone in the coastal dune sand ridges and Uca - Turritella ichnozone in the backswamps (Figure 1). These ichnological zones fairly conform to those worked out by De (2000, 2005) for the Bakkhali and neighboring beach sectors of the Bay of Bengal coast. This work focuses on the Dotilla pellet spread and burrows ichnozone of the upper intertidal flat.
The sediments across the beach show gradual variation in grain size. The calculated MZ varies from 1.4 ϕ (medium sand) in supratidal zone to 2.5 ϕ (fine sand) in the lower intertidal and subtidal zone through 1.8 ϕ (medium sand) in the upper intertidal zone. The coastal dunes are formed of finer sands (MZ 2.75 ϕ) windblown from the intertidal flat. The backswamps are rich in clay and silt. The upper intertidal sediments sampled from eight different spots in the Dotilla pellet spread and burrow ichnozone contain well-sorted and sub-rounded quartz (79% – 86%), biotite flakes (6% – 10%), broken organic particles (4% – 6%) and heavy minerals (2% – 3%) in descending order of abundance. These analyses suggest that there is hardly any significant sedimentological variation within this ichnozone and contents of organic particles are fairly high. Sediment moistness within this ichnozone, as measured from the collected samples during the low tides, varies widely with time (of subaerial exposure) and space (geomorphic position, proximity of the tidal sea water and substrate depth) from 0.5% to 23% by weight.
The intertidal beach experiences subaerial exposure and inundation during the low and high tides, respectively, at regular time interval. On a sub-horizontal beach, the tidal action induces some sweeping effects on the sediments and burrowing organisms. The duration of subaerial exposure of the upper intertidal flat is more relative to that in the lower flat. This has a reverse effect on surficial sediment moistness. This time factor favours the bubbler crabs to perform various life functions (burrowing, feeding-cum-pellet making, locomotion and others) for a longer time on the dry to semi-moist substrate of the upper intertidal flat compared to that of the middle and lower intertidal flat. Natural selection of the upper intertidal flat by the bubbler crabs for prolific burrowing and feeding (pellet-making) activities is, thus, governed basically by the tidal dynamics, very low beach slope, nutrient-rich sediments and low sediment moistness. Controlled by tidal dynamics, within the bubbler crab ichnozone, the degree of bioturbation has a visibly falling trend towards the sea.
Pellet Designs Formed by the Bubbler Crab Dotilla (Figure 2, Figure 3, Figure 4, Figure 5)
Field observations suggest that the juvenile, young, adult and old individuals of the crab Dotilla thrive together within the Dotilla pellet spread and burrow ichnozone without showing any ontogeny based micro-zoogeographic partitioning, which is often reported for the neighboring burrowing crabs Ocypode from the study area (De, 2000). The upper intertidal zone provides a unique but constricted eco-space for all the ontogenetic variants of the crab Dotilla to perform various life functions due to presence of suitably uniform and dry to semi-wet sandy substrates with rich food contents and favorable tidal dynamics (rhythmic high and low tidal actions triggering alternate phase of substrate inundation and exposure). Eco-space shrinkage problem under the perspective of recent rising sea condition, as observed globally as well as in the Bay of Bengal coast (De, 2019), may be a reason for the development of very narrow (30-90 m wide), coast-parallel and discontinuous Dotilla pellet spread and burrow ichnozone (Figure 1).
Adult individuals of Dotilla (6-10 mm across carapace) produce larger pellets (about 1-2 mm diameter), while the juveniles (3-5 mm across the carapace) produce smaller pellets (about 0.6-1 mm diameter). The juveniles produce a few simpler pellet designs, while the adults can produce all types of pellet designs described below. As a general trend, the growth or transformation of one simpler pellet design to another more complex one depends directly on the extent and intensity of feeding activities. With continued biogenic activities, the pellet structures become more and more complex and populated with pellets. With further feeding and pellet-making activities by a community of Dotilla crab, as often permitted by tidal dynamics, some of the pellet designs ultimately merge so as to form what is termed here as pellet mat design on the upper intertidal beach surface. The identity of initial discrete pellet designs is totally lost in the pellet mat, excepting some randomly oriented runways connecting open burrow mouths. All the pellet structures are addressed below in further details.
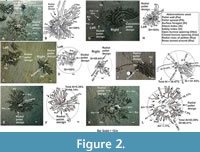 Radial pellet design. It is defined by several straight to slightly curved rows (a few in the initial stage to 50 or more in the advanced stage of feeding or pellet formation) of equidimensional and spherical pellets arranged radially from a central circular burrow opening covering a circular to semicircular area of feeding activity by individual crab (Figure 2A-L). While probing the substrate for food, making pellets and promptly arranging them in radiating rows, the crabs, irrespective of growth stages, move laterally from the burrow opening outward in close spaced radiating lines (10-90 cm long; Figure 2A-L) or narrow zones ensuring thorough and systematic foraging of substrate around the burrow opening. At places, depending on the movement of the crab, the adjacent radial pellet rows (Rrp in Figure 2) show distal turn around or rounded connection (Rta in Figure 2A, E, G, K). The pellet rows, thus, define a radial design with a central burrow opening. An ideally complete radial design covers almost 360o area around the opening leaving a very distinct, narrow and straight pellet free zone referred here as runway (Figure 2C - right, E, I- left) used for to and fro movements by the crab. The pellet rows converge towards the opening in such a manner that a dense pellet spread zone is produced around the burrow opening (Figure 2C - left). In the early stage of formation, the structure possesses fewer pellet rows and a wide pellet free circum-opening area (Figure 2K) yet to be probed. Away from the opening the interspace between the two successive pellet rows increases. This space is also probed linearly in radial fashion, thus, forming branching out pellet rows, especially in the peripheral parts of the pellet design (Figure 2A, G - left, K). The sizes of the burrow aperture and pellet diameter are proportional to the size of the crab. The aperture in most cases is flushed with the surface (Figure 2C-K) and rarely surrounded by an excavated sediment heap or dome (Figure 2A). A burrow opening with radial pellet design around is the result of feeding activity of a single crab.
Radial pellet design. It is defined by several straight to slightly curved rows (a few in the initial stage to 50 or more in the advanced stage of feeding or pellet formation) of equidimensional and spherical pellets arranged radially from a central circular burrow opening covering a circular to semicircular area of feeding activity by individual crab (Figure 2A-L). While probing the substrate for food, making pellets and promptly arranging them in radiating rows, the crabs, irrespective of growth stages, move laterally from the burrow opening outward in close spaced radiating lines (10-90 cm long; Figure 2A-L) or narrow zones ensuring thorough and systematic foraging of substrate around the burrow opening. At places, depending on the movement of the crab, the adjacent radial pellet rows (Rrp in Figure 2) show distal turn around or rounded connection (Rta in Figure 2A, E, G, K). The pellet rows, thus, define a radial design with a central burrow opening. An ideally complete radial design covers almost 360o area around the opening leaving a very distinct, narrow and straight pellet free zone referred here as runway (Figure 2C - right, E, I- left) used for to and fro movements by the crab. The pellet rows converge towards the opening in such a manner that a dense pellet spread zone is produced around the burrow opening (Figure 2C - left). In the early stage of formation, the structure possesses fewer pellet rows and a wide pellet free circum-opening area (Figure 2K) yet to be probed. Away from the opening the interspace between the two successive pellet rows increases. This space is also probed linearly in radial fashion, thus, forming branching out pellet rows, especially in the peripheral parts of the pellet design (Figure 2A, G - left, K). The sizes of the burrow aperture and pellet diameter are proportional to the size of the crab. The aperture in most cases is flushed with the surface (Figure 2C-K) and rarely surrounded by an excavated sediment heap or dome (Figure 2A). A burrow opening with radial pellet design around is the result of feeding activity of a single crab.
The line tracings (Figure 2B, D, F, H, J, L corresponding to Figure 2A, C, E, G, I, K) of some well-grown radial designs show that the converging inward and branching outward close spaced pellet rows or walls act as barricades required for safety against tiny predators and enemies. Although there is no reported evidence, the networks of the pellet rows may induce confusion or misguidance to the predators approaching the burrow tubes. Arranging feeding pellets in this manner ensures self-security, as calculated below, besides systematic foraging of substrate for collection of food particles. Calculated Security Index (SI) for majority of the well-grown radial pellet designs (Figure 2B, D, F, H) is very high (100% – 94.74%) relative to the less grown radial pellet structures (for example SI = 46.41% for Figure 2L) that are considered quite unsafe. It is a maiden observation that the safety index increases with the growth of the radial pellet designs (for example Figure 2L vs. B) that involves addition of more and more barricades in the form of an increased number of radial and branching outward pellet rows and walls, turned around pellet rows and pellets or sediment heaps around the burrow opening. These measures together with simultaneous foraging activity ensure both sufficient food and high security to the crabs Dotilla against enemies, especially of their own size and variety.
Plan outlays (Figure 5A-D) of some radial pellet designs exhibit how structural elements like dense radial rows (branching and non-branching) of pellets (Rrp), curved rings of pellets (Crp), pellet walls (Pw) and turned around pellet rows (Rta) are constructed to enhance burrow protection. It is observed that these elements are increasingly added to the structure to increase the Safety Index (SI) by closing or cutting off the probable routes of entry of predators into the burrow openings. Note details of plan outlays (Figure 5A-D) that depict a gradual increase in SI values from 46.41% to 100%.
 Concentric pellet design. This design is defined by concentric to semi-concentric, thick and curved rings of globular pellets, referred here as pellet wall (Pw in Figure 3A-X), produced around the burrow mouth (Crp in Figure 3A, C, E, G, I, K, M, O, Q, S, U, W). The number of rings depends upon the extent of feeding activity (2-3 during the early and 7-8 during the late stage). On average, the rings are about 1-1.5 cm thick for the adult (Figure 3A, C, E, G, I, M, O, Q, S, U, W) and 3-5 mm for the juvenile crabs (Figure 3K, W). The width of the ring remains constant along its length. The substrates in between the successive pellet rings are foraged by the crab. These foraged spaces are also concentric, but normally wider (1-2.5 cm, Sf in Figure 3A-X) than the pellet rings. The pellet rings hardly define a complete circle. The fully grown concentric designs characteristically show purposeful clockwise and anticlockwise closures of the pellet rings (Cpr in Figure 3A-X). Successive closures of the pellet rings produce multiple barricades and traps that ensure no entry for the predators or enemies, especially tiny ones, to the crab’s dwelling place. Here also clear runway (Figure 3A-X) is kept open for free and quick navigation in and out of the opening by the crab. At places, the burrow mouth is remarkably covered by heaps of pellets that provide additional protection measures against possible threat by the predators (Cbo in Figure 3I, M - right, O, Q - right, U - bottom).
Concentric pellet design. This design is defined by concentric to semi-concentric, thick and curved rings of globular pellets, referred here as pellet wall (Pw in Figure 3A-X), produced around the burrow mouth (Crp in Figure 3A, C, E, G, I, K, M, O, Q, S, U, W). The number of rings depends upon the extent of feeding activity (2-3 during the early and 7-8 during the late stage). On average, the rings are about 1-1.5 cm thick for the adult (Figure 3A, C, E, G, I, M, O, Q, S, U, W) and 3-5 mm for the juvenile crabs (Figure 3K, W). The width of the ring remains constant along its length. The substrates in between the successive pellet rings are foraged by the crab. These foraged spaces are also concentric, but normally wider (1-2.5 cm, Sf in Figure 3A-X) than the pellet rings. The pellet rings hardly define a complete circle. The fully grown concentric designs characteristically show purposeful clockwise and anticlockwise closures of the pellet rings (Cpr in Figure 3A-X). Successive closures of the pellet rings produce multiple barricades and traps that ensure no entry for the predators or enemies, especially tiny ones, to the crab’s dwelling place. Here also clear runway (Figure 3A-X) is kept open for free and quick navigation in and out of the opening by the crab. At places, the burrow mouth is remarkably covered by heaps of pellets that provide additional protection measures against possible threat by the predators (Cbo in Figure 3I, M - right, O, Q - right, U - bottom).
Line tracings of 18 concentric pellet designs at different growth stages (Figure 3B, D, F, H, J, L, N, P, R, T, V, X corresponding to Figure 3A, C, E, G, I, K, M, O, Q, S, U, W) are made for the calculation of Attack Index (AI) and Safety Index (SI). It is observed that all the developed structures, except six structures at the early stage of development (Figure 3B, L, R - left, middle, V - top, X), are having >90% SI values (i.e., safe). Concentric design, like radial design, is also made to ensure sufficient safety for the burrowers within their individual zone of feeding activities, but in slightly different ways. Here, unlike radial design, the barricades are purposefully made and strengthened through clockwise and anticlockwise closures of the curved pellet rings around the burrow opening in all structures (Figure 3A-X). Plan outlays drawn for six concentric pellet designs (Figure 5F, H, J, L, N, P corresponding to Figure 5E, G, I, K, M, O) at different growth stages depict how the curved rings of pellets (Crp) are constructed and oriented to form closed pellet rings (Cpr shown by red in Figure 5F, H, J, L, N, P) to enhance SI values. Note details of plan outlays (Figure 5F, H, J, L, N, P) that depict a gradual increase in SI values from 67.39% to 98.06% with an increase in numbers of Crp and Cpr.
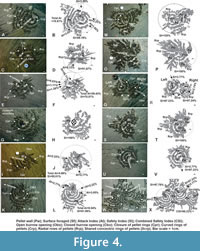 Concentric-radial pellet design. This pellet design is formed both by the adults (Figure 4A-X except G, H) and juveniles (Figure 4G, H) by involving dual modes (concentric and radial) of sediment foraging and combining inner (proximal) concentric and outer (distal) radial pellet rows. They begin initially with the formation of concentric pellet rings (three to five) around the burrow opening in the same manner as discussed above constructing repeated pellet barriers through closures of successive rings so as to ensure no entry for the predators or enemies (for example, Figure 4A, K, M, Q, S, U) from outside. From the distal concentric rows, they begin radial foraging further outward forming close spaced radial rows (2-7cm long) of pellets (Figure 4A-U). These rows are comparatively shorter than those made in the case of radial design. The radial pellet rows often connect successive concentric rows and even continue across them (Figure 4C, E, G) increasing the number of barriers against predators for better safety. For the longer period of construction of this design during low tides the burrow opening remains open (Figure 4A-X except G, M). In thickly populated areas, several such pellet designs join sharing outer concentric rings of pellets so as to form conjugate concentric-radial pellet design (Scrp in Figure 4Q, W) for enhancing safety measure over wider area. Conjugation of several concentric-radial designs is apparently a step forward towards ensuring community safety.
Concentric-radial pellet design. This pellet design is formed both by the adults (Figure 4A-X except G, H) and juveniles (Figure 4G, H) by involving dual modes (concentric and radial) of sediment foraging and combining inner (proximal) concentric and outer (distal) radial pellet rows. They begin initially with the formation of concentric pellet rings (three to five) around the burrow opening in the same manner as discussed above constructing repeated pellet barriers through closures of successive rings so as to ensure no entry for the predators or enemies (for example, Figure 4A, K, M, Q, S, U) from outside. From the distal concentric rows, they begin radial foraging further outward forming close spaced radial rows (2-7cm long) of pellets (Figure 4A-U). These rows are comparatively shorter than those made in the case of radial design. The radial pellet rows often connect successive concentric rows and even continue across them (Figure 4C, E, G) increasing the number of barriers against predators for better safety. For the longer period of construction of this design during low tides the burrow opening remains open (Figure 4A-X except G, M). In thickly populated areas, several such pellet designs join sharing outer concentric rings of pellets so as to form conjugate concentric-radial pellet design (Scrp in Figure 4Q, W) for enhancing safety measure over wider area. Conjugation of several concentric-radial designs is apparently a step forward towards ensuring community safety.
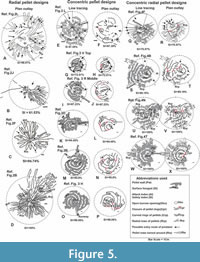 Line tracings of 10 concentric-radial (Figure 4B, D, F, H, J, L, N, P, T, V) and two conjugate concentric-radial structures (Figure 4R, X) suggest very high values of Safety Index (SI > 90%) especially for well-developed pellet design (e.g., Figure 4J, L, N, P, R, T, V, X). The pellet design, as usual, at the early stage of construction possesses low SI value (e.g., SI = 70.57% for Figure 4F). Plan outlays of four concentric-radial designs (Figure 5R, T, V, X corresponding to Figure 5Q, S, U, W) reveal how SI value improves (from 70.57% in R to 100% in X in Figure 5) with the addition of radial as well as concentric rings of pellets around the burrow openings.
Line tracings of 10 concentric-radial (Figure 4B, D, F, H, J, L, N, P, T, V) and two conjugate concentric-radial structures (Figure 4R, X) suggest very high values of Safety Index (SI > 90%) especially for well-developed pellet design (e.g., Figure 4J, L, N, P, R, T, V, X). The pellet design, as usual, at the early stage of construction possesses low SI value (e.g., SI = 70.57% for Figure 4F). Plan outlays of four concentric-radial designs (Figure 5R, T, V, X corresponding to Figure 5Q, S, U, W) reveal how SI value improves (from 70.57% in R to 100% in X in Figure 5) with the addition of radial as well as concentric rings of pellets around the burrow openings.
The above structural analyses of pellet designs upheld the proposed view that with the growth and modification of pellet design the SI values improve to desired level. In other words, the crabs grow and modify their pellet designs to full extent with an intention to create a safe environment within their zones of biogenic activities during low tide hours on the open upper intertidal flat, an obvious requirement for their survival.
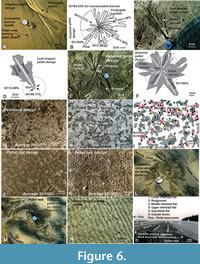 Flowery or petalloid pellet design. This pellet design (Figure 6A-B) is produced by the adult Dotilla by forming several radially diverging rows of pellets where each row turns back towards the burrow opening displaying a flower petal like appearance. Each petal has gradually widened out rounded apex and centrally closed pellet rows. Some adjacent petals share pellet rows in-between and form conjugate petals (Figure 6A-B). The length of the petals varies between 8-28 cm. The diverging petals give a flowery look for the entire pellet structure. No distinct runway is formed in this design. The burrower sometimes conceals the central burrow opening by pellet heaps (Figure 6A-B) even though the pellet design formed is in a well-advanced stage with an apparently high SI value. Concealment of the burrow mouth at any stage of pellet design formation during the low tide hours of subaerial exposure of the substrate is possible. It can be prompted by a sudden attack by the predators and can ensure instant safety (100% SI) for the burrower irrespective of how much safety is already assured by the pellet structure made.
Flowery or petalloid pellet design. This pellet design (Figure 6A-B) is produced by the adult Dotilla by forming several radially diverging rows of pellets where each row turns back towards the burrow opening displaying a flower petal like appearance. Each petal has gradually widened out rounded apex and centrally closed pellet rows. Some adjacent petals share pellet rows in-between and form conjugate petals (Figure 6A-B). The length of the petals varies between 8-28 cm. The diverging petals give a flowery look for the entire pellet structure. No distinct runway is formed in this design. The burrower sometimes conceals the central burrow opening by pellet heaps (Figure 6A-B) even though the pellet design formed is in a well-advanced stage with an apparently high SI value. Concealment of the burrow mouth at any stage of pellet design formation during the low tide hours of subaerial exposure of the substrate is possible. It can be prompted by a sudden attack by the predators and can ensure instant safety (100% SI) for the burrower irrespective of how much safety is already assured by the pellet structure made.
As in some of the previous designs, the line tracing (Figure 6B) shows a very high (100%) SI value owing to concealment of the burrow opening that ensures total blocking of all entry routes for the predators. In this case the SI would have been 83.63%, as calculated, had there been open burrow mouth (Figure 6B). Concealed burrow opening and conjugate petals, thus, independently contribute to the safety parameter of the burrower.
Leaf-shaped pellet design. This pellet design is formed by the adult Dotilla and defined by a single, widening outward, deep and linear runway and densely dispersed feeding pellets on the either side assuming a leaf-like appearance of the pellet spread design (Figure 6C) with central runway looking like a midrib. As observed in the field, the crab deeply foraged sediments along the runway only and the feeding pellets were thrown on either side to conceal, at least, lateral entry of enemies or predators into the runway. This pattern, usually having a fairly low safety index (SI about 14%; Figure 6D), is totally unsafe and represents the initial stage of formation of asteroid design.
Asteroid pellet design. The leaf-shaped pellet design, on further growth radially around a burrow mouth, transforms into an asteroid (star like) design (Figure 6E). The asteroid design is marked by several (two to eight) very distinctive and fairly wide and straight radial runways or trenches bordered on either side by densely dispersed feeding pellets that prohibit lateral entry of the predators into the runways. Interestingly, they also block the runway heads, as apparent from line tracing, with pellet spread for absolute safety (SI = 100%, Figure 6F). This design is made by adult individuals and is common in the uppermost part of the pellet spread zone where the substrate is relatively drier (0.5% to 5% moistness by weight). With the increase in feeding activities and population density of the burrows (40 to 50/m2), the asteroid design transforms into pellet mat design (Figure 6G, SI = 100%) by conjugation and merging of several asteroid pellet designs by a community of adult Dotilla.
Mossy pellet design. This design represents a community structure. It is formed collectively by densely populated (40 to 60/m2) Dotilla (both adult and juveniles) thriving in the uppermost part of the pellet spread zone. Here, the individual crab arranges feeding pellets on one side of its runway covering roughly a long triangular or quadrangular area (Figure 6H). Since the close spaced runways are randomly oriented, the pellet spread areas with growing feeding activity merge together giving a mossy look of the pellet structure (Figure 6H).
Line tracing (Figure 6I) of this community pellet design shows average SI = 85%. There are three categories of individual pellet structures that are grouped and marked by green, yellow and red circles having average SI values (>90%, 90% - 70% and <70% respectively, Figure 6I). With increased biogenic activities (i.e., increase in pellet population and enlargement of pellet spread areas) the mossy design improves SI value and transforms ultimately into pellet mat design that provides the highest safety (SI = 100%) to the burrowers.
Pellet mat design. With time, especially in the uppermost part of the Dotilla pellet spread and burrow ichnozone (upper 10 to 40 m of the intertidal flat), the collective pellet-making and feeding activities by a thick population of bubbler crabs (juvenile to adult) produce a densely dispersed pellet cover over the substrate, which is referred here as pellet mat design (Figure 6G, J, K). Runways and tiny burrow openings are visible within the dense gathering of globular pellets. The formation of pellet mat design is favored by certain conditions that include high population density of crabs (20 to 60/m2), long time interval (4-5 hours) of subaerial exposure of the substrate, low sediment moistness (0.5% to 5% by weight) and long duration (4-5 hours) of feeding activities, besides feeble wind action. The pellet mat is the ultimate design that provides 100% safety to a community of crab over large area because it is virtually impossible for tiny predators to break open the barricades of pellet walls or rings and sneak through the dense pellet spreads or networks to find burrow openings. Figures 6J (earlier) and 6K (later) show two different stages of pellet mat formation. Figure 6G represents a pellet mat design transformed from asteroid design (Figure 6E) through increase in crab population and their community workings. Thickly populated mossy design (Figure 6H), when allowed to grow further, transforms ultimately to pellet mat design.
Ripple crest and flank guided pellet designs. The Dotilla crabs, irrespective of ontogenetic stages, often encounter extensively rippled (symmetric wave, asymmetric current, interference, lunate, linguoid, truncated top and many other types of ripples) substrate on the upper intertidal flat while foraging sediment for food particles. A few general trends in the burrow location and pellet spread pattern on rippled substrates are noticed. The burrows are located preferably in the low laying moist ripple troughs. The feeding pellets are arranged densely over the relatively dry ripple flanks (both gentler and steeper sides) leaving the ripple crests devoid of pellets. In case of interference, lunate and linguoid ripples, the burrow protection is achieved, to some extent, through construction of concentric-radial (Figure 6L) and concentric (Figure 6M) pellet designs. In areas with sharp crested longitudinal, current and wave ripples, pellets are arranged on the steeper side (Figure 6N), while the burrow openings are located within the ripple troughs. One possible explanation for the specific positioning of the pellets and burrow openings in the ripple flanks and troughs respectively is that the ripple flank sediments are more nutritious as it gets reshuffled during ripple migration under subaqueous condition and dryer than that of ripple trough sediments. Moreover, the ripple troughs are areas of early inundation that alerts the burrowers against rushing high tide that prompts burrow plugging. If biogenic activities are permitted over a long period, the rippled substrates also get covered ultimately by pellet mat design. Figure 6O shows the field disposition of the Dotilla pellet spread and burrow zone with reference to the coastal geomorphic units.
Transformation of Pellet Designs
Pellet designs such as radial, concentric, concentric-radial, petalloid, leaf-shaped and asteroid designs are produced by crabs at individual levels. Mossy and pellet mat designs represent community structures. The first group of structures is produced at the early phase of tidal recession and subaerial exposure of the substrate when and where the population density of the crabs is low and eco-space available for an individual is sufficient to accommodate its structure and function. On the contrary, the second group of community structures is formed during the intermediate and the late stages of tidal recession and subaerial exposure of the beach when population density is high enough to suffer from eco-space problem that enforces conjugation and merging of the individual structure.
The above discourse on pellet designs suggests three basic types of transformation: 1) very conspicuous structural changes within the individual and community structures with time and increasing biogenic activities and sediment dryness; 2) subtle transformation of one individual design to another and 3) prominent changes of individual design to community design. The first type of transformation is displayed by growing numbers of pellets and radial pellet rows or rings in the radial designs (Figure 2I vs. A); growing numbers of curved pellet rings and closure of pellet rings in the concentric designs (Figure 3U - top vs. G); addition of short radial pellet rows from the distal curved pellet rings in the concentric-radial designs (Figure 4A vs. K) and sharing of curved pellet rings to form the conjugate concentric-radial designs (Figure 4Q, W); gradual construction of petal-shaped radial pellet rows around the burrow opening in case of the petalloid designs (Figure 6A) and gradual extension cum deepening of straight central trench or runway and shaping of pellet spread zone on either sides to the leaf-shaped designs (Figure 6C). The second type of transformation involves conversion of the concentric to concentric-radial design by addition of large number of long radial pellet rows to the distal curved pellet rings (Figure 3W vs. Figure 4S) and conversion of leaf-shaped design to asteroid design by adding radial runways and pellet spread zones around the burrow opening (Figure 6C, E).  The third category is represented by pellet mat design produced by conjugation and merging of many asteroid and mossy structures (Figure 6E vs. G, H vs. J, K). All types of the above-mentioned transformations aim at increasing the safety of the burrowers on the exposed beach. Transformation of pellet design over a rippled surface is also observed. If bioturbation is allowed to continue uninterruptedly, simpler concentric and concentric-radial designs (Figure 6L, M) tend to transform into the pellet mat design. Pellet mat is, hence, the ultimate design transformed from all other designs at the end of prolific biogenic activities. Some basic courses of transformation observed in the pellet designs with time is depicted pictorially and schematically in Figure 7.
The third category is represented by pellet mat design produced by conjugation and merging of many asteroid and mossy structures (Figure 6E vs. G, H vs. J, K). All types of the above-mentioned transformations aim at increasing the safety of the burrowers on the exposed beach. Transformation of pellet design over a rippled surface is also observed. If bioturbation is allowed to continue uninterruptedly, simpler concentric and concentric-radial designs (Figure 6L, M) tend to transform into the pellet mat design. Pellet mat is, hence, the ultimate design transformed from all other designs at the end of prolific biogenic activities. Some basic courses of transformation observed in the pellet designs with time is depicted pictorially and schematically in Figure 7.
Micro-zonation of the Pellet Designs
The pellet designs and burrow architecture of Dotilla take full shape minutes before inundation of the substrate during the next high tide. The discussed pellet designs, when fully grown, show clear coast-parallel micro-zones (Figure 6O) within the Dotilla pellet spread and burrow ichnozone in the upper intertidal flat. The concept of a Dotilla zone in the upper intertidal beach was also put forward by Hartnoll (1973). These micro-zones from the high tide level towards the sea are defined by pellet mats (pellet micro-zone 1), combined petalloid - mossy - asteroid - leaf shaped pellet designs (pellet micro-zone 2) and combined radial - concentric - concentric-radial pellet designs with associated igloo structures (pellet micro-zone 3). The contacts between the pellet micro-zones are gradational. Their widths are variable, and continuity is impersistent. Transformation of the pellet micro-zone 2 to pellet micro-zone 1 with time is very conspicuous. The pellet micro-zone 3 structures rarely get ample time to combine and transform into dense pellet mat design. The growth of the pellet micro-zone 1 is favored by higher population density (20–60/m2 of substrate area) of the crabs, longer time (about 4 to 5 hours) of subaerial exposure of substrate and feeding activities and lower moisture content (0.5% to 5% by weight) of sediments. Down the beach slope these factors show a gradual reversing trend. The pellet micro-zone 3 structures are favored by lower population density (5–15/m2 of substrate area), shorter time (3 to 4 hours.) of substrate exposure and feeding activities and especially more moist sediments (5% to 12% by weight). Further down the slope the sediments are too moist (16% to 18% by weight) to support formation and stability of tiny globular sand balls (pellets) and instead igloo structures are formed. Takeda et al. (1996) suggested formation of Dotilla igloos in highly moist and unstable sand. Sediment moistness, duration of subaerial exposure and population density of all other crabs than Dotilla also decrease towards the middle and lower intertidal flats. These subtle, but sensitive, variations in the biological and physical parameters are typical of tidally influenced beaches and are deciding factors, besides some others, in ichnozonation of the Bay of Bengal coast (De, 2005, 2019) and even in micro-zonation of Dotilla pellet spread and burrow ichnozone.
Immediate Fate of the Pellet Designs
The galaxy of pellet designs made by the crab Dotilla with great effort, promptness and precision on the subaerially exposing upper intertidal flat during the low tide hours are very short-lived. Sadly these spectacular structures get destroyed by the next onrushing high tide water within a few hours giving them very little or no chance for their burial and preservation within the beach sediments excepting in some rare but unique situations (discussed later in the sub-section “Preservation Potential of the Pellet Designs”). Field observations suggest that contemporaneous land and sea breezes and episodic storm activities often transport these pellets onto proximal flats where large open burrows and ripple troughs get filled with pellets. Moreover, the Bay of Bengal Sea is known for seasonal algal blooms and formation of algal mats (D’Silva et al., 2012) that stabilize sedimentary and biogenic (ichnological) structures including the pellet mats (De, 2009; De, 2015).
DISCUSSION
Intertidal Ecosystem and Adaptations of the Endobenthic Faunas
The intertidal beaches across the tropical-subtropical seas represent an extreme ecosystem wherein bewildering arrays of trace-making endobenthic organisms (invertebrates and vertebrates) thrive in large numbers. They have evolved several anatomical, physiological and behavioral adaptations (Fielder, 1970; Litulo et al., 2005; Lee, 2015) to survive the adverse and drastic environmental conditions (Barnes, 1969; Mandal and Nandi, 1989) and perform all essential life functions, especially dwelling in burrows, obtaining food, breeding, respiration and escaping from predation (De, 2019) and many others. The adverse environmental conditions include high salinity fluctuations, deep desiccation, reduced oxygen level, high temperature and shifting bottom sediments under continuous tidal currents and wave actions, besides rhythmic emergence and submergence of the intertidal substrates in tune with tidal dynamics (Banerjee et al., 2002). These make life more expensive in terms of energy. Many of these adaptations have a direct impact on their ichnological functionaries and products, including biogenic pellet formation. A few classical examples of acquiring anatomical and physiological specializations are mentioned below with reference to the Bay of Bengal coast.
The endobenthic crabs show the greatest anatomical specializations related to feeding, respiration, reproduction and vision (Fielder, 1970; Litulo et al., 2005; Lee, 2015). Nearly all the intertidal crabs have developed a quasiterrestrial or amphibious mode of life using free oxygen in the air and dissolved oxygen in water for effective respiration. The crabs Uca and Dotilla of the Bay of Bengal coast have developed weak jaws and evolved a mechanism of feeding on tiny organic particles picked up from the sediments of specific grain size, differing from species to species, to avoid interspecific competition (Mandal and Nandi, 1989). Their chelae tips are ‘spooned’ to scoop up fixed volume of sediments that form equidimensional and globular pellets. Sesarmine crabs (e.g., Sesarma, Ilyoplax and Macrophthalmus, the pumpers), when in air, circulate water in their branchial cavities through the gill chamber to re-oxygenate. The non-pumpers, such as Uca, pass on air stream through the water retained in the gill chamber for oxygenation (De, 2019). Crabs dwelling in the supratidal areas (e.g., Ocypode, Ilyoplax and Metaplax) often face respiratory problems for the lack of surficial water body, despite adapting dual modes of respiration. They overcome this problem either by extending their burrow bases to local groundwater level to assure gill moistening by capillary water (De, 2000, 2005) or migrating in droves to nearby seawater forming hundreds of meters long, continuous and cross-coast trackways (De, 2014). Adult ocypodid crabs have developed long ocular peduncle and compound eyes placed at the tip of elongated stalk. High eye position is advantageous to locate burrow mouth to escape in and to avoid predators (Barnes, 1969). Their tiny juveniles or small varieties of crabs (e.g., Ocypoda, Dotilla and Ilyoplax) with eyes very close to the ground surface face visibility problems that they overcome by constructing guided runways or excavation trenches leading to burrow mouths and sediment chimneys around burrow mouth (detailed in De, 2000, 2005, 2019). But the scientific mechanism and exact reasons behind ubiquitous construction of discrete pellet designs around burrow openings, as observed in the case of Dotilla in the study area and elsewhere, were not addressed by any previous worker.
The most conspicuous behavioral adaptation observed amongst intertidal crabs is a special burrowing life habit. They construct bottle-necked burrow tubes that get plugged and trap long tubular air bubbles with burrow base water for effective respiration during high tide hours of inundation (detailed in De, 2005). The burrows serve multiple purposes including dwelling, escaping from predators, avoiding surficial hydrodynamic forces, inhabiting regions of constant salinity to ease the strain of osmoregulation, storing food grains, mating and brooding. On the other hand, the low tide hours are spent mainly for feeding purposes on open surface, pellet making and burrow renovation or new burrow construction.
Mudskipper fish (Boleophthalmus and Periophthalmus), which are very conspicuous in mangrove-rich intertidal soft, muddy substrates of entire Sundarban, have developed fused pelvic fins to assume suctorial function to climb up trees during high tidal submergence of substrate and walk on muddy substrates during low tides producing distinct grazing marks (De, 2019).
Ethological Interpretations of Making the Pellet Designs
Predation issues. The crabs Dotilla in the upper intertidal flat spend far less time (hardly one to two hours) within their plugged burrow tubes under water during the high tides than on the open (exposed) beach surface (four to five hours) during low tides. However, the duration between the emergence and submergence of the Dotilla burrows varies round the year with tidal dynamics (rhythms), ground slope and surficial drainage patterns (Hartnoll, 1973). Field observations suggest that the subaerial exposure provides them required time and substrate to perform certain important life functions including feeding, pellet making and burrowing, but simultaneously impose a predation pressure on them, especially from the similar sized and smaller varieties of crabs of their own and associated communities (Ocypode, Uca, Ilyoplax and Metaplax) that are known to be involved in intra- and interspecific competition for space, substrate (food) and dwelling burrows (Gherardi and Russo, 2001). Some of these predators were observed to sneak successfully through the gaps in the partly grown pellet structures to reach and even enter into the burrow tubes owned by other individuals of Dotilla. Some were observed to stray here and there in a bit confused manner after being obstructed by the pellet rows or rings. Within the pellet mats the movement of the tiny crabs was observed to be restricted along the runways avoiding pellet-packed feeding territories. Because of their super sensitivity, it is difficult to follow them in the field for more than a few seconds. Associated crabs having larger body size were rarely observed within the Dotilla pellet spread and burrow ichnozone as potential attacker and invader. Because of the size factor, a small resident Dotilla crab may lose a contest for the possession of its dwelling burrow when the attacker is smaller or similar sized. For the same reason, larger individuals may be ruled out as leading predators as they can’t accommodate themselves within the tiny Dotilla burrow tubes. This perception is contrary to that (“A resident will lose a contest for the burrow when the attacker is larger”) made by Gherardi and Russo (2001) on Dotilla from the mangrove swamp of Kenya.
There are reported evidences of predation and even cannibalism practiced by some ghost (Hughes, 1966; Wolcott, 1978, 1988) and feedler crabs. Several species of Uca hunt and eat live on juveniles and just-molted soft bubbler crabs Dotilla and show cannibalistic acts on conspecific females in the mangrove fringe tidal mudflat in the Inhaca Island, Mozambique (Milner et al., 2009) and intertidal areas in Ao Tang Khen, Phuket, Thailand (Koga et al., 1995). However, similar cannibalistic act amongst the smaller individuals of Dotilla, Ocypode, Uca, Ilyoplax and Metaplax was not observed in the Bakkhali beach. Plugging of neighbor burrow mouth of Dotilla by fiddler crab Uca annulipes was reported from Japan (Wada, 1987) and Kinya (Gherardi and Russo, 2001). In the study area, some plugged Dotilla burrows were observed, but the author was unsure of their plugging by Uca or any other predators. Coastal birds are not potential predators for the small variety of crabs, as observed in the study area, because of their millimetric size; excellent camouflaging ability with the sandy substrate; super sensitivity to light, sound and vibration; very fast body movement (reflex), ability to hide promptly inside the burrow tube and ability to bury themselves directly into semifluid sandy substrate (also observed by Gherardi and Russo, 2001 in Kenyan mangrove swamp). These characteristics also constrain counting of the predation events in the Dotilla ichnozone.
Barricade building life habit. Increasing predation pressure on Dotilla over gradually exposing intertidal beach during the tidal recession demands safety for the burrows and burrowers against the entry of predators to perform essential life functions. For Dotilla, there seems no other means than speedy construction of protective barricades around the burrow openings by arranging pellets that they generate through feeding activity. The processes of feeding, construction of feeding pellets and their disposal on the beach surface, as observed in field, were burrow centric and ran simultaneously, possibly to save time and energy. The pellet disposal process, as observed in the field, was very systematic and fast and followed some geometric patterns (radial, concentric, combined concentric-radial, asteroid, petalloid and mossy) that eventually gave rise to discussed pellet designs. The author differentiates the pellet making from the pellet design making in terms of process (feeding mechanism vs. pellet organization) and purpose (feeding microorganisms vs. protecting burrows and burrowers from predators). This very perception is supported by the observation that majority of the pellet patterns with high SI values are equipped with inbuilt barrier components, such as complex network of radial pellet rows (Rrp in Figure 2A, E), burrow centric wide pellet spread zones or pellet walls (Pw in Figure 3A, C, G, M; 4A, C, M, O, S; Figure 6F), curved rings of pellets (Crp in Figure 3K; Figure 4A-W) and successively closed curved pellet rings (Cpr in Figure 3A, C, E, G, I, K) having similar or more heights than that of the conspecific predators. The application of geometric sense in skillful arrangement of pellets by Dotilla has been identified here as the key factor behind the origin of distinguished pellet designs. The veracity of the above statement can also be tested when one tries unsuccessfully to draw a free line from the outer periphery to the burrow mouth (try in structures represented by Figure 2A, C - right, G - left; Figure 3M, O, U - bottom; Figure 4M, O, S; Figure 6E, G, K) without crossing any one of the above said pellet barriers. There is no mention, so far in the published literature, of the acquisition and deployment of a geometric sense by Dotilla in arranging feeding pellets into distinguished designs for self and burrow protection despite their presence across the tropical - subtropical shallow marine coasts round the world (Hails and Yaziz, 1982; Maitland, 1986; Chakrabarti et al., 2006; Peter et al., 2008).
The barricade building life habit for burrow protection and territorial security, as observed in the current field or reported from elsewhere, is common amongst burrowing shallow marine crabs and other organisms. Crab-generated surficial barricade structures, as recorded from the present field, are burrow mouth sediment plugs, domes, chimneys and conical mud mounds (De, 2000, 2005, 2019; De, 2015). Burrow mouth chimneys composed of arranged pellets are made by some species of Uca for burrow defense by keeping intruders away from the burrows (Wada and Murata, 2000; Shih et al., 2005; Slatyer et al., 2008). Conical tower-building behavior of manicure Ocypodid crab Cleistostom dilatatum from the upper intertidal mudflat of the Gangwha Island, west coast of South Korea (Kim et al., 2011) was attributed to the safety of the burrowers and burrows (maintenance of suitable moistness and ventilation inside burrow tube). Earthen barricade building life habit of the crab Ilioplax pusillus (a very common associate of Dotilla in the study area) from the Aku River Estuary, Sirahama, Japan, ensures territorial defense to deter invasion by neighbor crabs (Keiji, 1984). Ohata and Keiji (2008) attributed male-biased construction of mud walls by adult Ilioplax pusillus around their burrow openings from the tidal flat in Uchinoura, Japan to barricade-building life habit that aided in courtship and mating success. Protection of burrowers and their burrows, however, may involve many other strategies than barrier construction in the case of some other organisms. Ophiomorpha burrows of shrimp origin, for example, possess densely packed mamillated or nodose fecal pellets on the outer burrow wall to secure and strengthen entire burrow system (Frey et al., 1978). Polychaetae Diopatra cuprea produces agglutinated burrows above sediment-water interface to protect burrow tube against hydrodynamic forces (Myers, 1972; De, 2002a). These examples signify that protection of burrows and burrowers is a common instinct and an important aspect of life habits of endobenthic burrowers. Being a prodigious burrower, the tiny Dotilla crabs can’t be an exception.
Substrate utilization strategies and pellet structures. A short preview of the present understanding on this topic relative to the crab Dotilla is introduced here. Dotilla, after emerging from its subsurface burrow on the beach during the low tide hours, targets a burrow mouth-centric circular to subcircular area mainly for feeding purposes. The radius of this area normally allows ample food collection and safe and rapid escape of the burrower into its own burrow hole when attacked by the predators. “When resources are distributed homogeneously or with such a complexity that animals cannot efficiently sample or interpret food gradients, a circular area that is progressively sampled, with the hole as the center and a radius that allows for a rapid escape into the hole, optimizes net resource yields” (as rightly quoted by Zimmer-Faust, 1990 in Gherardi and Russo, 2001). “Systematic deposit-feeding of this type reduces the chances that a crab will feed in areas previously explored and it is thus a commonly observed phenomenon among other invertebrate and vertebrate grazers and deposit feeders” (Gherardi and Russo, 2001; Zimmer-Faust, 1990), including many Scopimerinae, Ocypodidae, Dotillidae and Varunidae crabs (Tweedie, 1950; Altevogt, 1957; Silas and Sankarankutty, 1967; McIntyre, 1968; Fielder, 1970; Chakrabarti, 1972; Dörjes, 1972, 1978; Dörjes and Hertweck,1975; Dörjes and Howard, 1975; Bakshi et al., 1980; Zimmer-Faust, 1990; De, 1998b, 2000, 2010, 2019). Hartnoll (1973) mentioned to and fro movement of the crab from the burrow and systematic excavation of a sector of a circle. The author described feeding behaviour of Dotilla and Scopimera (five species each) in the light of substrate affinity and sorting efficiency and concluded that majority of the species of these two genera are specialized for feeding on sand rather than on mud (Tweedie, 1950). Normally, the entire (360º) circular target area is exploited within a single tide cycle. Luschi et al. (1997), however, recorded faster coverage (60% per hour) by Dotilla wichmanni. Some other aspects of the life habits of Dotilla, including ubiquitous occurrence of spectacular pellet designs, referred often as “sand ball galaxies” (Maitland, 1986; Chakrabarti et al., 2006; Peter et al., 2008), have been researched during the past 50 years (see Introduction for details), but, neither the structural details of the pellet designs nor their basic cause of formation has been addressed in depth. The present author, based on the field observations and numerical analysis of the SI values of all pellet structures, attributes formation of the distinctive Dotilla pellet designs to security measure for the burrows and burrowers against common predators through skillful construction of the distinctive pellet designs equipped with pellet barriers or barricades.
The substrate utilization strategy of Dotilla, as observed in the field within a tide cycle, involves several processes, such as, 1) site selection of a circular to subcircular area around the burrow opening to establish so called a feeding territory; 2) simultaneous substrate excavation, sifting of excavated sediments (up to 2 mm to 3 mm depth) for food particles (mostly microorganisms), formation of sand balls (globular and equidimensional feeding pellets) and their arrangement into some distinctive geometric patterns; 3) renovation or reconstruction of the burrow tubes and 4) burrow mouth plugging just before tidal inundation.
The first activity leads to the formation of a burrow opening surrounded partly or fully by a feeding territory. Field observations suggest that the size of the feeding territory varies from place to place and increases directly with the size of the crabs (for examples, Figure 3I vs. U; Figure 2A, E. K vs. G - left; Figure 4A, M, O vs. E; larger for the adults vs. smaller for the juveniles), duration of feeding activity (Figure 2A vs. I; prolong feeding activity and larger size vs. brief feeding activity and smaller size) and substrate suitability (with favourable moistness, food content etc.), while population density of the crabs and predation pressure on them exert an obvious negative effect on the size (Figure 6G, H, J, K). The shape of the feeding territory depends on the extent and modes of feeding activity (columns 1 to 7 in Figure 7).
The second set of activities involves varied feeding modes. The most common feeding mode, as observed in the field, covers an expanding (clockwise or anticlockwise) sector of a circle, which is in conformation with the observations of many workers (Tweedie, 1950; Altevogt, 1957; Silas and Sankarankutty, 1967; McIntyre, 1968; Fielder, 1970; Hartnoll, 1973; Zimmer-Faust, 1990; Gherardi and Russo, 2001) and accordingly shapes the feeding territory from a small sector (say 10º - 35º; sextants to octants) of a circle (columns 1to 4 in Figure 7) to a complete circle (360º) (Figures 4Q, U; columns 1to 4 in 7;) through a semicircle (180º, Figure 3C; columns 1 and 4 in Figure 7; half-disk). Given the pellet-making behaviour of the crabs Dotilla, these foraged or excavated sectors soon get covered by pellets either commonly arranged into some patterns (e.g., radial, concentric and concentric-radial; columns 2 to 4 in Figure 7) or rarely to homogenous pellet spread or fill (column 1 in Figure 7; comparable with Hartnoll, 1973, figure II a and c). Majority of the developed pellet structures described here (radial, concentric and concentric-radial patterns; Figure 2A-K except I; Figure 3; Figure 4) are the product of sector (of a circle) feeding strategy that ensure economic utilization of substrate, maximum food collection and no exploration of already explored substrate (Hartnoll, 1973; Gherardi and Russo, 2001). Pellet structures produced by this feeding mode typically display transitional varieties from low angle circle sector to complete circle in shapes (columns 1 to 4 in Figure 7) and a few short runways or feeding trenches.
Some pellet structures suggest a systematic radially diverging mode of growth with progressive increase both in the radius and circumference of the structure (e.g., radial and asteroid designs; column 5 in Figure 7). Hartnoll (1973) attributed some pellet structures of Scopimera proxima from the East African coast to radially diverging mode of substrate exploration. The East African (Hartnoll, 1973) and Gulf of Mannar and Palk Bay (Silas and Sankarankutty, 1967) pellet structures of Dotilla and Scopimera origin are comparable to that of the Bakkhali asteroid design as they share several long radiating trenches each backed up by its own pellet spread zone on either side (column 5 in Figure 7). Compared to the circle sector feeding mode, the radially diverging mode is less effective in terms of substrate exploration (Hartnoll, 1973). The radial pellet structures produced by radially divergent feeding mode can be distinguished from those made by the sector (of a circle) feeding mode by the presence of several diverging runways or trenches, long and branched pellet rows with turn around end, large gaps in the distribution of pellet rows and absence of sector feeding shapes in the former (compare forms of column 2 and 5 in Figure 7). However, in the absence of a sufficient number of radial forms, it may be difficult to infer the exact feeding mode involved.
Possibly a concentric feeding mode is involved in the formation of some concentric pellet designs (column 6 in Figure 7). These structures, unlike those formed by sector feeding, typically grow with the construction of more and more concentric pellet rings one closing on the other (Figure 5N, L, P). The foraging marks present all along the spaces between two successive concentric pellet rings (column 6 in Figure 7) confirm the crab’s concentric movement and feeding pattern. The concentric structures belonging to the sector feeding modes (column 3 in Figure 7) contain varied shapes based on the angular coverage of a circular feeding territory. Both the types of concentric structures on further growth acquire radially diverging rows of pellets from the distal pellet rings (Figure 4A-O, S, U; column 4 and 7 forms in Figure 7), thus confirming a dual or concentric-radial mode of feeding strategy.
The third and fourth activities may not be consecutive in sensu stricto and perhaps depend on microenvironmental and predation issues. For example, burrow mouth plugging, as observed in the field, could be due to sudden attack by predator at any stage of feeding process, sudden storm action or rainfall and routine substrate inundation.
The author emphasizes the above made observations related to the feeding strategies, extent of substrate utilization and resultant pellet structures. All the four identified feeding modes, especially the sector feeding one, are systematic and progressive and hence ensure optimum food collection, economic exploration of substrate and minimum chances of re-exploration of a substrate portion already explored. This agrees with the previous works mentioned. The feeding sectors having similar shape and size may be internally composed of different pellet designs (column 1- 4 forms in Figure 7). This suggests that making of the pellet designs is linked to some other purpose than the sector feeding strategy. Structural complexities of the pellet designs increase with expansion of feeding territory irrespective of feeding modes (Figure 7). The pellet designs scoring high SI values are generally equipped with barrier components required for the safety of the burrows and burrowers. It is also observed in Figure 7 that a pellet design (e.g., radial, concentric and concentric-radial) may have multiple feeding modes of origin (e.g., sector feeding, radially diverging, concentric and concentric-radial). These observations suggest that design building is independent of the feeding modes (or activities) and hence these two processes are genetically and objectively different. Above findings challenge the view that sand pellet designs of Dotilla are solely related to feeding activities. Ubiquitous and prolific growth of distinguished pellet structures by the crab Dotilla is a natural ethological adaptation in the tidal regime to secure burrow safety against common predators.
The present perception of the author (that the Dotilla pellet designs are armed with physical barriers that ensure burrow safety and restrict entry of common predators) supports and further elaborates the initial views expressed by Hartnoll (1973) and Gherardi and Russo (2001) that the pellet making behavior aimed to avoid re-exploration of the same sand for food. Although none of these respected authors had formally described and analyzed the formation of any one of the nine described pellet designs of Dotilla, they attributed progressive and systematic (clockwise and anticlockwise) sector (of a circle) feeding behaviour (not the pellet designs) to optimized net resource yields, economic usage of substrate and nonrepetitive exploration of the same area (Zimmer-Faust, 1990). As far as the substrate utilization strategies are concerned, the present author identifies at least four feeding modes (see above), namely circular sector feeding, radially diverging, concentric and combined concentric-radial modes based on the analysis of the pellet structures sampled from the field (columns 1-7 in Figure 7) and agrees with the propositions made by Hartnoll (1971), Zimmer-Faust (1990) and Gherardi and Russo (2001) relative to the first mode.
Safety index and security issues. The Safety Index (SI) measured forms the distinctive pellet designs (Figure 2, Figure 3, Figure 4, Figure 5) has been correlated with the security status of the Bakkhali Dotilla crabs and their burrows. Other security parameters, such as availability of food; episodic events of rainfall, storms, flooding and algal blooms; drastic changes in tidal dynamics; presence of air born predators (birds) and human activities are presumed to be constant for all the crabs thriving within the narrow Dotilla pellet spread and burrow ichnozone. Formation of similar pellet designs by Dotilla across the world in parallel environmental settings suggests a plausible common cause.
All the pellet designs, when fully grown (Figure 2, Figure 3, Figure 4, Figure 6), are observed to be equipped with various protective barriers (discussed in “Barricade building life habit” subsection above). The crab’s intention of improving safety Index, both at individual and community levels, is very clear from following observations.
1. SI values of the pellet structures improve with their growth and transformation (Figure 2K vs. B, F; Figure 3L, V - top, Q vs. B, F, H; Figure 4F vs. T, V).
2. There are conjugation (Figure 4Q, W) and merging or crowding of several pellet structures (Figure 6G, H, J, K) with increase in SI values.
3. Clockwise and anticlockwise closures of the curved pellet rings and growing numbers of radial pellet rows, especially in concentric (Figure 5E-P, SI 67.39% to 98.06%) and concentric-radial pellet designs (Figure 5Q-X, SI% 70.57 to 100%) are unique and effective architectural measures to improve SI values, as evidenced from drawn plan outlays (Figure 5).
4. Closing or plugging of burrow openings at any stage of making pellet design (Figure 3I, M - right, O - top, Q - right, U - bottom; Figure 4G, M) is another unique and emergency safety measure (SI = 100%) taken by crabs against sudden attack by the predators.
5. Transformation of asteroid and mossy designs into pellet mat design (Figure 6E-G, H-J, K) is another mechanism of increasing SI values and establishing high safety.
The present study, thus, attributes 1) development of different pellet designs by Dotilla with high SI scores to a barrier building strategy mainly for the protection of burrows and burrowers against predators and 2) specialized feeding modes to ensure economic usage of substrate, optimum food collection and no exploration of already explored area. Considering the worldwide distribution of the crab Dotilla, this ethological connotation may have global application potential. The ghost and fiddler crabs, that are common associates of Dotilla, also produce similar feeding pellets on intertidal beach. Their pellet making life habits may be better understood in the light of present revelations.
Among the modern trace-making and shallow marine endobenthic organisms, possibly the ethological aspects of the above-ground biogenic structures (e.g., chimneys, hoods, pillars, domes, semidomes, ovens, igloos, mudballs, mud mounds, mud volcanoes, pellets, and rims) of fiddler crab origin were best studied (von Hagen, 1968; Crane,1975; Christy, 1988; Zimmer-Faust, 1990; Christy et al., 2001; Shih et al., 2005; Yamaguchi et al., 2005; De, 2009; De, 2015; Carvalho et al., 2018). Pardo et al., (2020) summarized above-ground bio-sedimentary structures (except pellets) built at least by 47 fiddler crab species (nine genera) and correlated them to multiple ethological functions, including burrow defense (hood, semidome, pillar, rim and chimney for example). The author recommends enrolment of Dotilla “sand ball galaxy” as a new burrow protection strategy in the neoichnological database. This also opens a new window to evaluate the reported fossilized pellet structures of crab origin (Chamberlain, 1971; Chakrabarti and Baskaran, 1989; Noda, 1990; Bromley, 1994; Mazumdar et al., 2011; Šimo and Starek, 2015) in terms of burrow defense in addition to paleoenvironments.
Preservation Potential of the Pellet Designs
Preservation potential and other taphonomic aspects of modern organo-sedimentary structures decide their chances of fossilization in ancient sedimentary rocks where they are physically recorded as trace fossils. The architecture of trace fossils depends on the morphology of the trace makers, their life habits and physiological properties, besides modes of preservation, which are largely controlled by syn-depositional and post-depositional physical and chemical parameters. No biogenic structure can be said to have zero preservation potential (Bromley, 1990, 1994) since a majority of the most delicate organo-sedimentary structures known from the modern settings have ancient analogues in rock records. From the study area a large number and variety of modern traces of crustacean origin were reported (De, 2015; De, 2019). The preservation potential and possible ancient analogues of these traces were addressed. Published data show at least two tiers of preservation: deep tier preservation (Bromley,1990) of large and thick crab burrows within beach sediments under constant high energy hydrodynamic conditions and shallow tier preservation (Ausich and Bottjer,1982) of short and thin burrows and delicate surficial traces under episodic conditions of storm deposition, algal bloom and monsoon-induced surge during high tides. The second mode is involved in the preservation of concerned pellet structures made by Dotilla in the study area.
The quasiterrestrial, endobenthic and coastal marine varieties of crabs are known as prolific burrowing agents (Frey et al., 1984) within soft sandy to clayey substrates of modern coasts. Their modern traces include bewildering array of burrows and delicate surficial traces (tracks, trackways, trails, chimneys, mounds, pod marks, anchor marks, scratch marks, pellets and pelletal ornamentations and resting – grazing – foraging – brooding traces besides many others). Many of these features, by virtue of high preservation potential, are very well represented in the fossil records as trace fossils (Chamberlain, 1971; Bromley, 1994; Buatois and Mángano, 1995; Buatois et al., 1998). Bioerosion traces in a hard lithified substrate (Santos et al., 2010; Wisshak et al., 2017) have a high fossilization potential.
Preservation potential of surficial pellets and pelletal ornamentations (the present concern), regardless of their diversity and population density, is poor as they are regularly made to be destroyed immediately by next sweeping high tidal currents and coastal wind actions. However, some of them are known (Bromley, 1994) to be occasionally preserved under special episodic conditions (e.g., sudden, but permanent burial through storm, spring tidal and aeolian deposition) and recorded rarely in trace fossil records. Bioerosion traces in a hard lithified substrate (Santos et al., 2010; Wisshak et al., 2017) have a high fossilization potential. The oldest ever known fossilized sand spherules associated with Ophiomorpha burrows from the Eocene shallow-marine deposits of western Carpathians, Slovakia, were identified as brachyuran crab feeding pellets (Noda, 1990; Šimo and Starek, 2015) and interpreted as a good indicator of the shoreline palaeoenvironment (foreshore to upper shoreface). Crustacean (extant Callianassa) fecal and spoil mounds Chomatichnus wegberensis from the Late Cretaceous Dakota Sandstone of New Mexico are known (Chamberlain, 1971). Mazumdar et al. (2011) reported fecal pellet-filled tubular decapod burrows from 46-58 k.y. old carbonate sediments of the Krishna-Godavari Basin, Bay of Bengal, India. Biogenic fecal pellet mounds formed by crabs around their burrow openings were reported from the Quaternary Miliolite Limestone deposits of Saurashtra, western India (Chakrabarti and Baskaran, 1989). In both the above Indian occurrences the pellet bearing traces were attributed to shallow marine littoral palaeoenvironment.
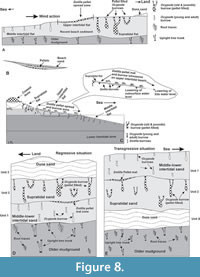 Although complete destruction of the Dotilla pellet mats by high tides is almost a regular phenomenon, occasional pre-monsoon storm (named Kalbaishakhi) events trigger transportation of large number of pellets (dry and hence light) by surface rolling from the pellet microzone-1 (Figure 6O) to the proximal supratidal flat where they fill in the larger open burrows of the crab Ocypode, Metaplax and Ilyoplax. This process provides the pellets a rare chance of ex situ preservation as burrow infill (Figure 8A) sediments. Moreover, the diurnal and seasonal land and sea breezes contribute to the air born (rolling) transportation of huge quantity of pellets into open burrows. Other proximal structural traps, such as aquatic and aeolian ripple troughs and sun cracks in the mudflats, provide ample scope in ex situ preservation of pellets (Figure 8B). Thus, wind actions, both normal and episodic, play an important role in trapping and preservation of the pellets.
Although complete destruction of the Dotilla pellet mats by high tides is almost a regular phenomenon, occasional pre-monsoon storm (named Kalbaishakhi) events trigger transportation of large number of pellets (dry and hence light) by surface rolling from the pellet microzone-1 (Figure 6O) to the proximal supratidal flat where they fill in the larger open burrows of the crab Ocypode, Metaplax and Ilyoplax. This process provides the pellets a rare chance of ex situ preservation as burrow infill (Figure 8A) sediments. Moreover, the diurnal and seasonal land and sea breezes contribute to the air born (rolling) transportation of huge quantity of pellets into open burrows. Other proximal structural traps, such as aquatic and aeolian ripple troughs and sun cracks in the mudflats, provide ample scope in ex situ preservation of pellets (Figure 8B). Thus, wind actions, both normal and episodic, play an important role in trapping and preservation of the pellets.
The Bay of Bengal Sea is known for several phases of algal blooms during withdrawal of south-west monsoon and pre-monsoon periods (D’Silva et al., 2012). These form greenish and greenish yellow layers of algal mats all over the beach, especially in the upper to middle intertidal flats, covering and stabilizing many of the surficial sedimentary and organo-sedimentary structures including Uca mud volcanoes (De, 2009; De, 2015), Tagelus burrows, pellet mats and burrow chimneys. Prodigious burrowing activity of thalassinidean shrimps in the modern intertidal margins of hypersaline lagoons (e.g., the Bahamas) results in biogenic mounds stabilized by microbial mats (Curran and Martin, 2003). Diurnal fluctuations in tide levels, light rainfall, episodic storm deposition and frequent algal blooms help in stabilization, final burial and in situ preservation of the Dotilla pellet mat layers (Figure 8D-E). These trapped layers, if lithified, are expected to be preserved differently within the beach stratigraphy under transgressive and regressive modes of sea (Figure 8E, D). Under a regressive sea condition, the preserved Dotilla pellet mat zone is expected to be trapped between overlying supratidal sands with Dotilla pellet in-filled larger burrows of associated crabs (unit 2) and underlying middle-lower intertidal sands with Ocypode burrows (unit 1 in Figure 8D). The sequence of preservation of different lithofacies in beach stratigraphy under a transgressive sea condition will be reversed, i.e., in the order dune sands with aeolian ripples, supratidal sands with pellet in-filled large burrows, trapped pellet mat layers and middle-lower intertidal sands with Ocypode burrows from bottom to top (Figure 8E).
Geological and Hydrogeological Significance of Dotilla Pellet and Burrow Structures
Since their appearance in the Jurassic Period (Schweitzer and Feldmann, 2010), decapod crustaceans had performed as very important geologic agents both in the ancient and recent coastal marine facies (Frey et al., 1984) for their selective adaptability, wide range of traces (Edwards and Frey, 1977), specific diversity (4500 out of 26000 crustacean species) and bioerosional capabilities (Letzsch and Frey, 1980; De, 1998a). Organo-sedimentary structures, especially burrows, tracks, trackways, mud mounds and sediment chimneys, of true crab origin (fossilized and unfossilized) have received considerable attention, especially in the marginal marine and quasi-marine facies, for palaeoshoreline, palaeoenvironmental, palaeosea level (transgression and regression), sedimentological, stratigraphic, palaeontological, and geotechnical interpretations, besides hydrocarbon exploration (Farrow, 1971; Howard, 1972; Curran and Frey, 1977; Frey and Seilacher, 1980; Chakraborti, 1981; Bown, 1982; Bown and Kraus, 1983; Howard and Scott, 1983; Frey and Pemberton, 1984; Hasiotis and Bown, 1992; Wilson et al., 1998; De, 1998a, 2000, 2002a, 2002b, 2009, 2019; Buatois and Mángano, 2000). On the contrary, mentions of geological application of modern as well as ancient analog of pellet structures of crabs, such as those produced by Dotilla in the study area, are too rare to refer. Some of the plausible geological importance of the discussed Dotilla pellet and burrow structures, if preserved in stratigraphy under said favorable circumstances, is addressed below.
Formation of coast-parallel Dotilla pellet spread and burrows ichnozone in association with other mentioned ichnozones is palaeoenvironmentally and palaeogeomorphologically significant. If preserved and identified in rock record, this ichnozone, besides confirming a shallow marine littoral setting, delineates position and extent of ancient upper intertidal flat, indicates the relative positions of ancient sea and land (e.g., pellet micro-zone 1 and 3 on the landward and seaward side respectively, Figure 60), palaeo-hightide level (upper level of pellet micro-zone 1) and palaeo-shoreline configuration. These data are also important in basin analysis.
Spatiotemporal disposition of the pellet mats relative to different facies or units of beach sediments may be suggestive of transgressive - regressive events of palaeosea (Figure 8D-E). The pellet mat layers superposed by lower to middle intertidal or subtidal sediments (fine sand admixed with silt and clay) with small- to moderate-sized Ocypode burrows and supratidal medium sands with large and complex network burrows of decapods and aeolian ripples are indicative of transgression and regression events. This interpretation can be reconfirmed from the analyses of order of superposition of different ichnozones as described by De (2000, 2005).
The bubbler crab-induced bioturbation of the surficial sediment layers of the upper intertidal zone helps in loosening and transportation of beach sands to coastal dunes fields by wind action. Development of coastal dunes is thus remotely linked with profuse bioturbation of the proximal intertidal beach and concomitant wind action. The role of Dotilla crabs in moving beach sediments is also acknowledged by Gherardi and Russo (2001).
Preservation modes of the pellet mats stabilized by biomats help identify episodic conditions of deposition in geologic past, such as light rainfall, storm induced flooding events, algal bloom and aeolian settlement of fine dune sands over beach surface. Presence of pellet-filled burrow tubes and ripple troughs in close association with pellet mats indicates episodic wind actions across the ancient coast.
De (2000, 2005) revealed the biophysical mechanism of the intertidal quasimarine decapod crustaceans thriving in the soft sandy beaches of the Bay of Bengal coast. It was interpreted that quasimarine crab burrow length is limited to local subsurface water level. During inundation of substrate the bottle necked crab burrow mouth gets plugged trapping a long air column or bubble inside burrow tube, the base of which touches the groundwater level. The burrower, being adapted to amphibious mode of life, uses the trapped air and burrow base water with dissolved oxygen for respiration during hours of submersion. This control of subsurface water level on crab burrow lengths is manifested in ichnozonation (Figure 1) across the Bakkhali (supratidal long, thick and compound burrows of old and juvenile Ocypode, Metaplax and Ilyoplax ichnozone vs. simple I-shaped, short and thin burrows of young and adult Ocypode, Metaplax and Ilyoplax ichnozone in lower-middle intertidal flat) and neighboring coasts (De, 2005, 2010, 2019). Like cray fish burrows, the crab burrow lengths are also considered proportional to depth of water table and tidal range in a beach profile (Hasiotis, 1990; Hasiotis and Mitchell, 1993). Haque and Choudhury (2014) studied ecology and behavior of Ocypode macrocera on the sandy beaches of the Sagar Island of Indian Sundarbans and opined that the burrows don’t penetrate the groundwater table. Paul et al. (2019) studied burrow morphology of the ocypodid crab Ocypode ceratophthalmus at Chandipur Coast, Eastern India, and concluded that burrow length and diameter sharply decrease from backshore to foreshore.
In a beach profile, the local subsurface water level coincides with that of the proximal sea water level that goes further down with receding low tide. This causes gradual, contemporaneous and equivalent lowering of the subsurface water level and downward subaerial exposure of the intertidal flat. Dotilla trying to maintain an average burrow length of five to six centimeters is forced to relocate its burrow down the exposed slope (Figure 8C). Thus, the lowering of tide level and consequent drop in subsurface water level trigger down slope expansion of the burrowing activities by Dotilla to the extent where burrow base capillary water supports aquatic respiration and sediment moistness is low enough not to cause burrow collapse. Hydrogeological implication of tidal dynamics on the growth of Dotilla pellet spread and burrow ichnozone, as revealed here, can have multiple dimensions if seasonal and diurnal variations are taken into consideration. It is concluded that availability or extent of suitable eco-space for prolific biogenic activities by Dotilla largely depends on tidal hydrodynamics. That is why these crabs and their burrows and pellet structures are very conspicuous across the intertidal zones of modern seas and oceans.
CONCLUSIONS
Prolific growth of the spectacular feeding pellet designs and burrows of the tiny bubbler crabs Dotilla on the intertidal sandy beach of Bakkhali, Bay of Bengal coast, Eastern India, is a very conspicuous phenomenon. On a global scale, this is a part of so-called Dotilla “Sand Ball Galaxy”. They have evolved specialized substrate exploration strategies, special anatomical devices to sift surface sediment for microorganisms and form equidimensional globular feeding pellets and an ability to arrange the pellets into distinguished designs. With progressive feeding activity, the pellet designs (radial, concentric, concentric-radial, petalloid, leaf-shaped and asteroid at individual and mossy and pellet mat at community levels), grow, conjugate, transform into another (e.g., concentric to concentric-radial and leaf-shaped to asteroid) and even merge into one another (e.g., mossy and asteroid to pellet mat).
They deploy progressive and burrow-centric substrate feeding techniques (circle sector, radially diverging, concentric and combined concentric-radial feeding) that ensure optimum food collection, economic use of substrate and minimum chance of re-exploration of already explored area. Of these, the last two feeding modes are new to our understanding.
It is revealed that the SI (Security Index, a newly defined parameter for the pellet designs to quantify security status of the burrowers and burrows) values tend to reach 100% with the structural growth, transformation and conjugation of pellet designs, besides construction of physical pallet barriers (e.g., burrow mouth plugs, network pellet rows and curved walls, closed concentric pellet rows and conjugated and merged pellet designs) to debar entry of predators into the burrow openings. Attribution of the Dotilla pellet designs to security measure for the burrows and burrowers constitute a new ethological concept for the burrowing crabs. This opens a new window to investigate similar structures, modern and fossilized, produced by endobenthic burrowers in the light of territorial security.
The Dotilla pellet structures have a very poor chance of preservation in rock records under specialized circumstances, such as, ex situ preservation as pellet-filled burrows, aeolian ripple troughs and mud cracks by wind or storm actions and in situ preservation of the pellet mats by episodic events like stabilization by algal blooms and storm deposition. If preserved in the geological records, the Dotilla pellet spread and burrow ichnozone, besides confirming a shallow marine littoral setting, delineates position and extent of ancient upper intertidal flat, indicates the relative positions of ancient sea and land, palaeo-hightide level and palaeo-shoreline configuration. Microbially stabilized pellet mats and associated pellet-filled burrow tubes and ripple troughs help identify episodic conditions of deposition in the geologic past, such as algal blooms and storm-induced flooding and aeolian action. Stratigraphic disposition of the pellet mats relative to the associated coastal facies can suggest transgression and regression events of the palaeo-sea.
ACKNOWLEDGEMENT
The author expresses her gratitude to the Head of the Department of Geosciences, Presidency University, Kolkata for constant encouragement to bring to book the causes behind the formation of pellet designs by some tiny beach crabs and to Dr. M. Debroy, Zoologist of the Zoological Survey of India, Kolkata for helping in taxonomic identification of the crabs as Dotilla spp. collected from the Bakkhali beach (personal communication). She remains grateful to Central Groundwater Board, Department of Water Resources, River Development and Ganga Rejuvenation, for encouragement to publish this article.
REFERENCES
Altevogt, R. 1957. Beiträge zur Biologie und Ethologie von Dotilla blanfordi Alcock und Dotilla myctiroides (Milne-Edwards) (Crustacea, Decapoda). Zeitschrift für Morphologie und Ökologie der Tiere, 46:369–388. https://doi.org/10.1007/bf00409628
Ansell, A.D. 1988. Migration or shelter? Behavioural options for deposit feeding crabs on tropical sandy shores, p. 15–26. In Chelazzi, G. and Vannini, M. (eds.), Behavioral Adaptation to Intertidal Life. Plenum Press, New York.
https://doi.org/10.1007/978-1-4899-3737-7_2
Ausich, W. and Bottjer, D. 1982. Tiering in suspension-feeding communities on soft substrata throughout the Phanerozoic. Science, 216(4542):173–174.
https://doi.org/10.1126/science.216.4542.173
Bakshi, S.K., Ray, T.K., and De, C. 1980. On the workings of some crabs on the sandy beach of Western Sundarban, Bengal Delta, India. Journal of Geological Society of India, 21:184–187.
Banerjee, L.K., Rao, T.A., and Shastry, A.R.K. 2002. Diversity of coastal plant communities in India, p. 524. ENVIS-EMCBTAP, Botanical Survey of India publication, Ministry of Environment and Forests, Kolkata.
Barnes, R.D. 1969. Invertebrate Zoology. BioScience, 19(12):1131–1132.
https://doi.org/10.2307/1294886
Baucon, A., Bordy, E., Brustur, T., Buatois, L.A., Cunningham, T., De, C., Duffin, C., Felletti, F., Gaillard, C., Hu, B., Hu, L., Jensen, S., Knaust, D., Lockley, M., Lowe, P., Mayor, A., Mayoral, E., Mikuláš, R., Muttoni, G., Carvalho, C.N., Pemberton, S.G., Pollard, J., Rindsberg, A.K., Santos, A., Seike, K., Song, H., Turner, S., Uchman, A., Wang, Y., Yi-ming, G., Zhang, L. and Zhang, W. 2012. A history of ideas in ichnology, p. 3–43. In Bromley, D. and Knaust, D. (eds.), Trace Fossils as Indicators of Sedimentary Environments. Developments in Sedimentology 64.
Bigalke, R. 1921. On the habits of the crab Dotilla fenestrata Hilgendorf, with special reference to the mode of feeding. South African Journal of Natural History, 3:205–209.
Bown, T.M. 1982. Ichnofossils and rhizoliths of the nearshore fluvial Jebel Oatrani Formation (Oligocene), Fayum Province, Egypt. Palaeogeography, Palaeoclimatology, Palaeoecology, 40(4):255–309.
https://doi.org/10.1016/0031-0182%2882%2990031-1
Bown, T.M. and Kraus, M.J. 1983. Ichnofossils of the alluvial Willwood Formation (Lower Eocene), Bighorn Basin, northwest Wyoming, U.S.A. Paleogeography, Paleoclimatology, Paleoecology, 43:95–128. https://doi.org/10.1016/0031-0182(83)90050-0
Bromley, R.G. 1990. Trace fossils: Biology and Taphonomy, Unwin Hyman, London.
Bromley, R.G. 1994. The palaeoecology of bioerosion, p.134–154. In Donovan, S.K. (ed.), The palaeobiology of trace fossils. Johns Hopkins Univ. Press, Baltimore.
https://doi.org/10.1002/9780470999295.ch61
Buatois, L.A. and Mángano, M.G. 1995. The paleoenvironmental and paleoecological significance of the lacustrine Mermia ichnofacies: an archetypical subaqueous nonmarine trace fossil assemblage, Ichnos, 4:151–161.
https://doi.org/10.1080/10420949509380122
Buatois, L.A. and Mángano, M.G. 2000. Application of ichnology to hydrocarbon prospecting and reservoir characterization. Boletín de informaciones petroleras, 17:65–85.
Buatois, L.A., Mángano, M.G., Maples, C.G., and Lanier, W.P. 1998. Allostratigraphic and sedimentologic application of trace fossils to the study of incised estuarine valleys: an example from the Virgilian Tonganoxie Sandstone Member of Eastern Kansas. Bulletin Kansas Geological Survey, 241:1–27.
https://doi.org/10.17161/cres.v0i241.11782
Bulcao, C. and Hodgson, A.N. 2012. Activity and feeding of Dotilla fenestrata (Brachyura: Ocypodidae) in a warm, temperate South African estuary. African Journal of Aquatic Science, 37(3):333–338.
https://doi.org/10.2989/16085914.2012.694354
Carvalho, R.D., Pardo, J.C.F., and Costa, T.M. 2018. Construction and structure of the semidomes of the fiddler crab Minuca rapax (Brachyura: Ocypodidae) in southern Brazil. Journal Crustacean Biology, 38:241–244.
https://doi.org/10.1093/jcbiol/rux123
Chakrabarti, A. 1972. Beach structures produced by crab pellets. Sedimentology, 18:129–134.
https://doi.org/10.1111/j.1365-3091.1972.tb00008.x
Chakrabarti, A. 1981. Burrow patterns of Ocypode ceratophthalmus (Pallas) and their environmental significance. Journal of Palaeontology, 55:431–441.
https://www.jstor.org/stable/1304229
Chakrabarti, A. and Baskaran, M. 1989. Biogenic faecal pellet mounds in Quaternary Miliolites of Saurashtra, India. Palaeogeography, Palaeoclimatology, Palaeoecology, 73:311–315.
https://doi.org/10.1016/0031-0182(89)90011-4
Chakrabarti, A., Chakrabarti, R., and Hertweck, G. 2006. Surface traces and bioturbate textures from bubbler crabs: An indicator of subtropical to tropical tidal flat environments. Marine Biodiversity, 36(1):19–27.
https://doi.org/10.1007/BF03043700
Chamberlain, C.K. 1971. Biogenic mounds in the Dakota Sandstone of Northwestern New Mexico. Journal of Palaeontology, 45(4):641–644.
Chapman, J.L. and Reiss, M.J. 1999. Ecology: Principles and Applications (2nd ed.). Cambridge University Press, UK.
https://doi.org/10.1017/CBO9781107340893
Christy, J.H. 1988. Pillar function in the fiddler crab Uca beebei (I): effects on male spacing and aggression. Ethology, 78:53–71.
https://doi.org/10.1111/j.1439-0310.1988.tb00219.x
Christy, J.H., Backwell, P.R., and Goshima, S. 2001. The design and production of a sexual signal: hoods and hood building by male fiddler crabs Uca musica. Behaviour, 138:1065–1083. https://www.jstor.org/stable/4535877
Crane, J. 1975. Fiddler crabs of the world (Ocypodidae: genus Uca). Princeton University Press, Princeton.
Curran, H.A. and Frey, R.W. 1977. Pleistocene trace fossils from North Carolina (U.S.A.) and their Holocene analogues. In Crimes, T.P. and Harper, J.C. (eds.), Trace Fossils 2. Geological Journal Special Issue, 9:139–162.
Curran, H.A. and Martin, A.J. 2003. Complex decapod burrows and ecological relationships in modern and Pleistocene intertidal carbonate environments, San Salvador Island, Bahamas. Palaeogeography, Palaeoclimatology, Palaeoecology, 192(1-4):229–245.
https://doi.org/10.1016/S0031-0182(02)00687-9
De, C. 1998a. Biological reworking of sediments by crabs: A cause for erosion of the Digha beach, West Bengal. Current Science, 75(6):617–620.
De, C. 1998b. Pelletal designs by the crab Ocypode spp. News G.S.I. CHQ, 29(1-2):47.
De, C. 2000. Neoichnological activities of endobenthic invertebrates in downdrift coastal Ganges Delta Complex, India: Their significance in trace fossil interpretations and Palaeoshoreline reconstructions. Ichnos, 7:89–113.
https://doi.org/10.1080/10420940009380149
De, C. 2002a. Application of a biological tool for estimating current annual rates of erosion and deposition in modern coastal environments: A case study in the Bay of Bengal coast. Marine Georesources and Geotechnology, 20:209–220.
https://doi.org/10.1080/03608860290051903
De, C. 2002b. Continental mayfly burrows within relict-ground in inter-tidal beach profile of Bay of Bengal coast: A new ichnological evidence of Holocene marine transgression. Current Science, 83(1):64–67.
De, C. 2005. Biophysical model of intertidal beach crab burrowing: application and significance. Ichnos, 12:11–29.
https://doi.org/10.1080/10420940590914471
De, C. 2009. Uca marionis Mud Volcanoes: a unique Ichnological tool from the Bay of Bengal Coast of India for ready assessment of beach stability. Marine Georesources and Geotechnology, 27(2):1–17. https://doi.org/10.1080/10641190802625601
De, C. 2010. Sundarban Delta Complex: A special feature. Indian Journal of Geosciences, 63(4):397–428.
De, C. 2014. Longest crab trackways from the Bay of Bengal Coast, India: their geological and geotechnical applications. Palaeontologia Electronica, 17.2.31A:1–19.
https://doi.org/10.26879/325
De, C. 2015. Burrowing and mud-mound building life habits of fiddler crab Uca lactea in the Bay of Bengal coast, India and their geological and geotechnical importance. Palaeontologia Electronica, 18.2.26A:1–22.
https://doi.org/10.26879/427
De, C. 2019. Mangrove ichnology of the Bay of Bengal coast, eastern India. Series: Springer Geology. Springer. Cham, Switzerland.
https://doi.org/10.1007/978-3-319-99232-7
Deb, M. 1998. Faunas of Bengal: Part Crustacea: Decapoda; Corals. Publication of Zoological Survey of India, Kolkata. State Fauna Series, 3(10):345–403.
Dörjes, J. 1972. Georgia coastal region, Sapelo Island, USA. Sedimentology and Bioloogy, VII. Distribution and zonation of macrobenthic animals. Senckenbergiana Maritima, 4:183–216.
Dörjes, J. 1978. Sedimentologische und faunistische Untersuchungen an Watteni in Taiwan. II. Faunistische und aktuopaleontologische Studien. Senckenbergiana maritima, 10:117–143.
Dörjes, J. and Hertweck, G. 1975. Recent biocoenoses and ichnocoenoses in shallow water marine environments, p. 459–491. In Frey, R.W. (ed.), The study of Trace Fossils. Springer-Verlag, New York.
https://doi.org/10.1007/978-3-642-65923-2_20
Dörjes, J. and Howard, J.D. 1975. Estuaries of the Georgia coast, U.S.A. sedimentology and Biology. IV. Fluvial marine transition indicators in an estuarine environment, Ogeechee River Ossabaw sound. Senckenbergiana Maritima, 7:137–179.
Dray, T. and Paula, J. 1998. Ecological aspects of the populations of the crab Dotilla fenestrata (Hilgendorf, 1869) (Brachyura: Ocypodidae) in the tidal flats of Inhaca Islands (Mozambique). Journal of Natural History, 32:1525–1534.
https://doi.org/10.1080/00222939800771061
D’Silva, M.S., Anil, A.C., Naik, R.K., and D’Costa, P.M. 2012. Algal blooms: a perspective from the coasts of India. Natural Hazards, 63:1225–1253.
https://doi.org/10.1007/s11069-012-0190-9
Edwards, J.M. and Frey, R.W. 1977. Substrate characteristics within a Holocene Salt marsh, Sapelo Island, Georgia. Senckenbergiana Maritima, 9:215–259.
Farrow, G.E. 1971. Back-reef and lagoonal environments of Aldabra Atoll distinguished by their crustacean burrows. Symposia of the Zoological Society of London, 28:455–500.
Fielder, D.R. 1970. The feeding behaviour of the sand bubbler crab Scopimera inflata (Decapoda, Ocypodidae). Journal of Zoology, 160:35–49.
https://doi.org/10.1111/j.1469-7998.1970.tb02896.x
Fishelson, L. 1983. Population ecology and biology of Dotilla sulcata (Crustacea, Ocypodidae) typical for sandy beaches of the Red Sea, p. 643–654. In McLachlan, A. and Erasmus, T. (eds.), Sandy Beaches as Ecosystems. Developments in Hydrobiology, 19. Springer, Dordrecht.
Frey, R.W. and Pemberton, S.G. 1984. Trace fossil facies models, p.189–207. In Walker, R.C. (ed.), Geoscience Canada, Facies Models, Reprint Series 1. St Johns.
Frey, R.W. and Seilacher, A. 1980. Uniformity in marine invertebrate ichnology. Lethaia, 13:183–207.
https://doi.org/10.1111/j.1502-3931.1980.tb00632.x
Frey, R.W., Curran, H.A., and Pemberton, S.G. 1984. Trace making activities of crabs and their environmental significance: The ichnogenus Psilonichnus. Journal of Paleontology, 58:333–350.
Frey, R.W., Howard, J.D., and Pryor, W.A. 1978. Ophiomorpha: Its morpholgic, taxonomic, and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology, 23:199–229. https://doi.org/10.1016/0031-0182(78)90094-9
Gherardi, F. and Russo, S. 1997. Drove formation in the tropical crab, Dotilla fenestrata. Advances in Ethology, 32:257.
Gherardi, F. and Russo, S. 2001. Burrowing activity in the sand-bubbler crab, Dotilla fenestrata (Crustacea, Ocypodidae), inhabiting a mangrove swamp in Kenya. Journal of Zoology, 253(2):11–223.
https://doi.org/10.1017/S0952836901000188
Gherardi, F., Russo, S., and Lazzara, L. 2002. The Function of Wandering in the Sand Bubbler Crab Dotilla Fenestrata. Journal of Crustacean Biology, 22(3):521–531.
https://doi.org/10.1163/20021975-99990263
Hails, A.J. and Yaziz, S. 1982. Abundance, breeding and growth of the ocypodid crab Dotilla myctiroides (Milne-Edwards) on a West Malaysian beach. Estuarine, Coastal and Shelf Science, 15(2):229–239.
https://doi.org/10.1016/0272-7714(82)90030-0
Haque, H. and Choudhury, A. 2014. Ecology and behavior of the ghost crab, Ocypode Macrocera Edwards 1834 occurring in the sandy beaches of Sagar Island, Sundarbans. International Journal of Engineering Science Invention, 3(4):38–43.
Hartnoll, R.G. 1973. Factors affecting the distribution and behaviour of the crab Dotilla fenestrata on East African shores. Estuarine and Coastal Marine Science, 1(2):137–152.
https://doi.org/10.1016/0302-3524(73)90066-2
Hasiotis, S.T. 1990. Identification of the architectural and surficial burrow morphologies of ancient crayfish and lungfish burrows: their importance to ichnology. Proceedings to the Pacific Rim Congress, Student Competition Section, Australasian Institute of Mining and Metallurgy, Queensland, 3:529–536.
Hasiotis, S.T. and Bown, T.M. 1992. Invertebrate trace fossils: the Backbone of Continental ichnology, p. 64–104. In Maples, C.G. and West, R.R. (eds.), Short Courses in Paleontology, 5. Palaeontological Society.
https://doi.org/10.1017/s2475263000002294
Hasiotis, S.T. and Mitchell, C.E. 1993. A comparison of crayfish burrow morphologies: Triassic and Holocene fossils, paleo- and neo-ichnological evidence, and the identification of their burrowing signatures. Ichnos, 2:291–314.
https://doi.org/10.1080/10420949309380104
Howard, J.D. 1972. Trace fossils as criteria for recognizing shorelines in the stratigraphic records. In Rigby, J.K. and Hamlin, W.M.K. (eds.), Recognition of ancient sedimentary environments. Society of Economic Paleontologists and Mineralogists, Special Publication, 16:215–225.
https://doi.org/10.2110/pec.72.02.0215
Howard, J.D. and Scott, R. M. 1983. Comparison of Pleistocene and Holocene barrier island beach-to-offshore sequences, Georgia and northeast Florida coasts, U.S.A. Sedimentary Geology, 34:167–183.
https://doi.org/10.1016/0037-0738(83)90085-4
Hughes, D.A. 1966. Behavioural and ecological investigations of the crab Ocypode ceratophthalmus (Crustacea: Ocypodidae). Journal of Zoology, London, 150:129–143.
https://doi.org/10.1111/j.1469-7998.1966.tb03000.x
Kawaida, S., Kimura, T., and Toyohara, H. 2013. Habitat segregation of two dotillid crabs Scopimera globosa and Ilyoplax pusilla in relation to their cellulase activity on a marsh dominated estuarine tidal flat in central Japan. Journal of Experimental Marine Biology and Ecology, 449:93–99. https://doi.org/10.1016/j.jembe.2013.09.003
Keiji, W. 1984. Barricade building in Ilyoplax pusillus (De Haan) (Crustacea: Brachyura). Journal of Experimental Marine Biology and Ecology, 83(1):73–88.
https://doi.org/10.1016/0022-0981(84)90118-7
Kim, T.W., Ryu, H.J., Jin, H., Choi, J.B., and Cho, J.C. 2011. Tower construction by the manicure crab Cleistostoma dilatatum during dry periods on an intertidal mudflat. Journal of Ethology, 29:459–465.
https://doi.org/10.1007/s10164-011-0280-2
Koga, T. 1998. Reproductive success and two modes of mating in the sand-bubbler crab Scopimera globosa. Journal of Experimental Marine Biology and Ecology, 229(2):197–207.
https://doi.org/10.1016/S0022-0981(98)00051-3
Koga, T., Goshima, S., Murai, M., and Poovachiranon, S.1995. Predation and Cannibalism by the Male Fiddler Crab Uca tetragonon. Journal of Ethology, 13:181–183.
https://doi.org/10.1007/bf02350110
Lee, S.Y. 2015. Ecology of Brachyura. Crustacea, Treatise on Zoology - Anatomy, Taxonomy, Biology. The Crustacea, Volume 9 Part C“, published by Brill, Leiden (71-9):469–541. https://doi.org/10.1163/9789004190832_011
Letzsch, W.S. and Frey, R.W. 1980. Deposition and Erosion in a Holocene Salt Marsh, Sapelo Island, Georgia. Journal of Sedimentary Research, 50:529–542.
https://doi.org/10.1306/212f7a45-2b24-11d7-8648000102c1865d
Litulo, C., Mahanjane, Y., and Fernando, L.M M. 2005. Population biology and breeding period of the sand-bubbler crab Dotilla fenestrata (Brachyura: Ocypodidae) from Southern Mozambique. Aquatic Ecology, 39:305–313.
https://doi.org/10.1007/s10452-005-3443-9
Luschi, P., Del Seppia, C., and Crosio, E. 1997. Orientation during short-range feeding in the crab Dotilla wichmanni. Journal of Comparative Physiology, A 181:461–468.
https://doi.org/10.1007/s003590050129
Maitland, D.P. 1986. Crabs that breathe air with their legs - Scopimera and Dotilla. Nature, 319:493–495.
https://doi.org/10.1038/319493a0
Mandal, A.K. and Nandi, N.C. 1989. Faunas of Sundarban Mangrove Ecosystem, West Bengal, India, p. 1–116. In Singh, A. (ed.), Faunas of Conservation areas: 3. Doon Phototype Printing Press, Dehradun, India.
Mazumdar, A., Joshi, R.K., Peketi, A., and Kocherla, M. 2011. Occurrence of faecal pellet-filled simple and composite burrows in cold seep carbonates: A glimpse of a complex benthic ecosystem. Marine Geology, 289:117–121.
https://doi.org/10.1016/j.margeo.2011.09.003
McIntyre, A.D. 1968. The meiofauna and macrofauna of some tropical beaches. Journal of Zoology, 156:377–392.
https://doi.org/10.1111/j.1469-7998.1968.tb04360.x
McLachlan, A. and Brown, A.C. 2006. Adaptations to Sandy-beach Life, p. 91–123. In McLachlan, A. (ed.), The Ecology of Sandy Shores (Second Edition). Academic Press, Cambridge.
https://doi.org/10.1016/B978-012372569-1/50006-9
Miller, D.C. 1961. The feeding mechanism of fiddler crabs, with ecological considerations of feeding adaptations. Zoologica, 46:89–100.
https://doi.org/10.5962/p.203340
Milner, R.N.C., Detto, T., Jennions, M.D., and Backwell, P.R.Y. 2009. Hunting and predation in a fiddler crab. Journal Ethology, 28(1):171–173.
https://doi.org/10.1007/s10164-009-0156-x
Mohanty, P.K., Pradhan, Y., Nayak, S.R., Panda U.S., and Mohapatra G.N. 2008. Sediment dispersion in the Bay of Bengal (Chapter 4), p.50–78. In Mohanty, P. K. (ed.), Monitoring and Modeling Lakes and Coastal Environments, Springer, Netherlands.
https://doi.org/10.1007/978-1-4020-6646-7_5
Myers, A.C. 1972. Tube-worm-sediment relationships of Diopatra cuprea (Polychaeta: Onuphidae). Marine Biology, 17:331–334.
https://doi.org/10.1007/bf00366746
Noda, H. 1990. Crustacean sand-balls from the Middle Miocene Iriomote Formation in Iriomote-jima, Okinawa Prefecture, southwest Japan. Annual Report of the Institute of Geosciences of Tsukuba, 16:50–54.
Ohata, M. and Keiji, W. 2008. Is barricade building behavior linked to pair formation in the dotillid crab Ilyoplax pusilla ? Crustacean Research, 37:63–66.
https://doi.org/10.18353/crustacea.37.0_63
Ono, Y. 1965. On the ecological distribution of ocypod crabs in the estuary. Memoirs of the Faculty of Science of Kyushu University Series E Biology, 4:1–60.
Pardo, J.C.F., Stefanelli-Silva, G., Christy, J.H., and Costa, T.M. 2020. Fiddler crabs and their above-ground sedimentary structures: a review. Journal of Ethology, 38(2):137–154.
https://doi.org/10.1007/s10164-020-00647-1
Paul, J., Mondal, S., Kayal, R., and Sarkar, D. 2019. Burrow morphology of the ocypodid crab Ocypode ceratophthalma at Chandipur Coast, Eastern India and its implications. Current Science, 117(4):25.
https://doi.org/10.18520/cs/v117/i4/699-705
Peter, K.L.N., Danièle, G., and Peter, J.F.D. 2008. Systema Brachyurorum: Part I. An annotated checklist of extant Brachyuran crabs of the world. The Raffles Bulletin of Zoology, 17:1–286.
Richter, H. 2018. Visual Art Inspired by the Collective Feeding Behavior of Sand-Bubbler Crabs, p.1–17. In Liapis, A., Romero, C.J.J., and Ekart, A. (eds.), Computational Intelligence in Music, Sound, Art and Design. Springer-Verlag, Berlin.
https://doi.org/10.1007/978-3-319-77583-8_1
Robertson, J.R. and Newell, S.Y. 1982. Experimental studies of particle ingestion by the sand fiddler crab Uca pugilator (Bosc). Journal of Experimental Marine Biology and Ecology, 59:1–21.
https://doi.org/10.1016/0022-0981(82)90102-2
Santos, A., Mayoral, E., Marques da Silva, C., Cachão, M., and José Carlos Kullberg, J. 2010. Trypanites ichnofacies: Palaeoenvironmental and tectonic implications. A case study from the Miocene disconformity at Foz da Fonte (Lower Tagus Basin, Portugal). Palaeogeography, Palaeoclimatology, Palaeoecology, 292:35–43.
https://doi.org/10.1016/j.palaeo.2010.03.023
Schweitzer, C.E. and Feldmann, R.M. 2010. The oldest Brachyura (Decapoda: Homolodromioidea: Glaessneropsoidea) known to date (Jurassic). Journal of Crustacean Biology, 30(2):251–256.
https://doi.org/10.1651/09-3231.1
Shih, H.T., Mok, H.K., and Chang, H.W. 2005. Chimney building by male Uca formosensis Rathbun, 1921 (Crustacea: Decapoda: Ocypodidae) after pairing: a new hypothesis for chimney function. Zoological Studies, 44:242–25.
Silas, E. and Sankarankutty, C. 1967. Field investigation on the shore crabs of the gulf of Mannar and Palk Bay, with special reference to the ecology and behaviour of the pellet crab Scopimera proxima Kemp. Marine Biological Association of India, Symposium Series 2:1008–1025.
Šimo, V. and Starek, D. 2015. Sand spherules interpreted as crustacean feeding pellets from an Eocene shore environment (Western Carpathians – Slovakia). Palaeogeography, Palaeoclimatology, Palaeoecology, 438:364–378.
https://doi.org/10.1016%2Fj.palaeo.2015.08.023
Slatyer, R.A., Fok, E.S.Y., Hocking, R., and Backwell, P.R.Y. 2008. Why do fiddler crabs build chimneys? Biology Letters, 4:616–618.
https://doi.org/10.1098/rsbl.2008.0387
Suzuki, H. and Taiji, K. 1984. Studies on the life history of the sand bubbler crab, Scorpimera globosa de Haan, in Tomioka Bay, west Kyushu: II. Distribution patterns of juveniles and adults. Memoirs of Faculty of Fisheries – Kagoshima University (Japan), 7(2):173–190.
https://doi.org/10.1016/0198-0254(84)93529-5
Takagi, K.K., Cherdsukjai, P., Mimura, I., Yano, Y., Adulyanukosol, K., and Tsuchiya, M. 2010. Soldier crab (Dotilla myctiroides) distribution, food resources and subsequent role in organic matter fate in Ao Tang Khen, Phuket, Thailand. Estuarine Coastal and Shelf. Science, 87(4):611–617. https://doi.org/10.1016/j.ecss.2010.02.011
Takeda, S., Matsumasa, M., Yong, H.S., and Mura, M. 1996. “Igloo” construction by the ocypodid crab, Dotilla myctiroides (Milne-Edwards) (Crustacea; Brachyura): the role of an air chamber when burrowing in a saturated sandy substratum. Journal of Experimental Marine Biology and Ecology, 198(2):237–247.
https://doi.org/10.1016/0022-0981(96)00007-X
Tweedie, M.W.F. 1950. Notes on grapsoid crabs from Raffles Museum. II. On the habits of three ocypodid crabs. Bulletin of the Raffles Museum, 23:310–332.
Vannini, I.M. and Chelazzi, G. 1985. Adattamenti comportamentali alla vita intertidale tropicale. Oebalia, 11:23–37.
Vogel, F. 1984. Comparative and functional morphology of the spoon-tipped setae on the second maxillipeds in Dotilla Stimpson, 1858 (Decapoda, Brachyura, Ocypodidae). Crustaceana, 47:225–234.
https://doi.org/10.1163/156854084x00487
von Hagen, H.O. 1968. Studien an peruanischen Winkerkrabben (Uca). Zoologische Jahrbücher Abteilung für allgemeine Zoologie und Physiologie der Tiere, 95:395–468.
Wada, K. 1981. Growth, breeding and recruitment in Scopimera globosa and Ilyoplax pusillus (Crustacea: Ocypodidae) in the estuary of Waka River, middle Japan. Publications of the Seto Marine Biological Laboratory, 26:243–259.
https://doi.org/10.5134/176012
Wada, K. 1987. Neighbor burrow-plugging in Ilyoplax pusillus (Crustacea: Brachyura: Ocypodidae). Marine Biology, 95:299–303.
https://doi.org/10.1007/BF00409017
Wada, K. and Murata, I. 2000. Chimney building in the fiddler crab Uca arcuata. Journal of Crustacean Biology, 20:505–509.
https://doi.org/10.1163/20021975-99990066
Wilson, M.A., Curren, H.A., and White, B. 1998. Palaeontological evidence of a brief global sea-level event during the last interglacial. Lethaia, 31:241–250.
https://doi.org/10.1111/j.15023931.1998.tb00513.x
Wisshak, M., Titschack, J., Kahl, W-A., and Girod, P. 2017. Classical and new bioerosion trace fossils in Cretaceous belemnite guards characterized via micro-CT. Fossil Records, 20:173–199. https://doi.org/10.5194/fr-20-173-2017
Wolcott, T.G. 1978. Ecological role of ghost crabs, Ocypode quadrata (Fabricius) on an ocean beach: scavengers or predators? Journal of Experimental Marine Biology and Ecology, 31:647–82.
https://doi.org/10.1016/0022-0981(78)90137-5
Wolcott, T.G. 1988. Ecology, p. 55–96. In Burggren, W.W. and McMahon, B.R. (eds.), Biology of the land crabs. Cambridge University Press, Cambridge.
https://doi.org/10.1017/cbo9780511753428.004
Yamaguchi, T., Henmi, Y., and Tabata, S. 2005. Hood building and territory usage in the fiddler crab, Uca lactea (De Haan, 1835). Crustaceana, 78:1117–1141.
https://doi.org/10.1163/156854005775361007
Zeil, J. and Hemmi, J.M. 2010. Crabs and Their Visual World, p. 411–420. In Michael, D.B. and Janice, M. (eds.), Encyclopedia of Animal Behavior. Academic Press, London, UK.
https://doi.org/10.1016/B978-0-08-045337-8.00158-3
Zimmer-Faust, K. 1990. Foraging strategy of a deposit feeding crab, p. 557–568. In Hughes, R.N. (ed.), Behavioural mechanisms of food selection. NATO ASI Series. Springer-Verlag, Berlin. https://doi.org/10.1007/978-3-642-75118-9_27

