 The earliest fossil evidence of spiny feather (pinnate-leaved) palms from the K-Pg of Gondwana
The earliest fossil evidence of spiny feather (pinnate-leaved) palms from the K-Pg of Gondwana
Article number: 27.1.a12
https://doi.org/10.26879/1273
Copyright Paleontological Society, February 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 9 February 2023. Acceptance: 24 November 2023.
ABSTRACT
Palms, a plant family that forms a major component of lowland tropical rainforests worldwide, are represented by a large number of fossils from Cenozoic sedimentary successions and K-Pg sediments of India, but no spiny palm has been reported from there to-date. Here, we report fossilized 'feather' (well-separated leaflets that are attached to a single leaf axis, similar to a feather) palm leaf specimens that bear spines from the latest Maastrichtian (Late Cretaceous)-earliest Danian (Early Paleocene) sediments of the Deccan Intertrappean beds of Madhya Pradesh, central India. They provide the first evidence that spiny pinnately-leaved palms were present in India during Chron 29R, which spans the K-Pg transition, and when the bulk of the subcontinent was still in the Southern Hemisphere. Other reliable records of pinnate palms are from the Northern Hemisphere (Europe) and are much younger (Eocene, Oligocene, and Miocene) than the fossils reported here. Although our data are limited, the new finds suggest that Cenozoic palm dispersal may be consistent with the “Out-of-India” hypothesis seen in several other plant groups. This report also provides new information on the distribution, diversification, and evolution of spiny palms in deep time. The evidence of fossilized spiny palms dating to the latest Cretaceous may link to a defense mechanism evolved as protection against predation from large herbivores, presumably dinosaurs.
Sanchita Kumar. Palaeobotany and Palynology Laboratory, Department of Botany, Sidho-Kanho-Birsha University, Ranchi Road, Purulia-723104, India. kumarsanchita631@gmail.com
Tao Su. CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, China. sutao@xtbg.org.cn
Robert A. Spicer. School of Environment, Earth and Ecosystem Sciences, The Open University, Milton Keynes, MK7 6AA, UK; State Key Laboratory of Tibetan Plateau Earth System, Resources and Environment (TPESRE), Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing 100101, China. ras6@open.ac.uk
Mahasin Ali Khan. Palaeobotany and Palynology Laboratory, Department of Botany, Sidho-Kanho-Birsha University, Ranchi Road, Purulia-723104, India. khan.mahasinali@gmail.com
Keywords: Spiny pinnate palms; Arecaceae; K-Pg; Madhya Pradesh; new species; biodiversity
Final citation: Kumar, Sanchita, Su, Tao, Spicer, Robert A., and Khan, Mahasin Ali. 2024. The earliest fossil evidence of spiny feather (pinnate-leaved) palms from the K-Pg of Gondwana. Palaeontologia Electronica, 27(1):a12.
https://doi.org/10.26879/1273
palaeo-electronica.org/content/2024/5099-spiny-pinnate-palms-from-india
Copyright: February 2024 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
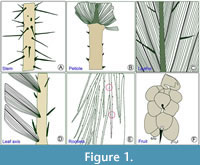 Spines in palms are restricted to certain taxonomic groups and so have systematic value (Tomlinson, 1961, 1962a; Fisher, 1981). Spines may originate from superficial structures or internal vascular tissues (vascular spines) or endogenously developed organs (root- and rootlet spines). They result from a modification of an existing aerial organ, such as in leaflet spines, rachilla spines, and petiole spines, as well as emergences from superficial tissue, that is to say not a modification of an existing organ. Moreover, different spine categories are determined by their location. Spines may occur on vegetative organs (leaflet margins, leaflet midribs, petiole margins, surfaces of the leaf axis or rachis, stem surfaces) and reproductive organs (bracts, inflorescence axes, and even fruits) (Clement and Manshardt, 2000) (Figure 1). The function of these spines does vary since in some climbing palms they serve in support, but otherwise, the function of spines in palms seems to be mainly for physical protection against large herbivores.
Spines in palms are restricted to certain taxonomic groups and so have systematic value (Tomlinson, 1961, 1962a; Fisher, 1981). Spines may originate from superficial structures or internal vascular tissues (vascular spines) or endogenously developed organs (root- and rootlet spines). They result from a modification of an existing aerial organ, such as in leaflet spines, rachilla spines, and petiole spines, as well as emergences from superficial tissue, that is to say not a modification of an existing organ. Moreover, different spine categories are determined by their location. Spines may occur on vegetative organs (leaflet margins, leaflet midribs, petiole margins, surfaces of the leaf axis or rachis, stem surfaces) and reproductive organs (bracts, inflorescence axes, and even fruits) (Clement and Manshardt, 2000) (Figure 1). The function of these spines does vary since in some climbing palms they serve in support, but otherwise, the function of spines in palms seems to be mainly for physical protection against large herbivores.
In India, fossil palm leaves are mostly in the form of impressions of palmate leaves (Sahni, 1964; Achuthan, 1968; Trivedi and Chandra, 1971; Bonde, 1986; Mathur et al., 1996; Singh and Patnaik, 2012). They are described under the fossil genera Palmacites, or Palmites or more appropriately Palmophyllum. In addition, a few costapalmate leaves have been reported and described under the fossil genus Sabalites (Srivastava et al., 2014; Roy et al., 2020; Kumar et al., 2022a). Only three reliable fossil species of pinnately compound palm leaf remains have been reported in the Indian fossil record (Guleria and Mehrotra, 1998; Guleria et al., 2005; Singh et al., 2012), but no reliable spiny pinnate palm has been reported from there to-date. By contrast, palmate palms with spines have been reported from the Eocene of Tibet (spine-like structures mainly on the costa) (Su et al., 2019), North America (Berry, 1924), New Zealand (Hartwich et al., 2010), the Oligocene of China (Wang et al., 2015), England (Chandler, 1957), and the Miocene of France (Huard, 1967).
In this study, we describe under a new fossil genus two pinnately compound spiny palm leaves from the latest Maastrichtian (Late Cretaceous)-earliest Danian (Early Paleocene) sediments of the Deccan Intertrappean beds of Madhya Pradesh, central India (Figure 2). These fossil pinnate palm leaves with well-preserved spines and spine scars represent the earliest reliable fossil record of spiny palms and also inform our understanding of their early-stage evolutionary and biogeographic histories.
MATERIAL AND METHODS
Locality
 The fossil spiny palm leaf remains described here were collected during fieldwork in 2020 and 2021. They were recovered from the latest Maastrichtian (Late Cretaceous) to earliest Danian (Early Paleocene) sediments of the Mandla Lobe, Deccan Intertrappean beds of Umariya Ryt. village (N 22°46′26”, E 80°32′19”, 490 m a.s.l.), Dindori district, Madhya Pradesh, central India (Figure 2A, B). The fossil locality was situated at a low palaeolatitude of ~18° S (Figure 2C) (http://www.odsn.de/odsn/index.html; accessed November 21, 2021) at the time the leaves were deposited.
The fossil spiny palm leaf remains described here were collected during fieldwork in 2020 and 2021. They were recovered from the latest Maastrichtian (Late Cretaceous) to earliest Danian (Early Paleocene) sediments of the Mandla Lobe, Deccan Intertrappean beds of Umariya Ryt. village (N 22°46′26”, E 80°32′19”, 490 m a.s.l.), Dindori district, Madhya Pradesh, central India (Figure 2A, B). The fossil locality was situated at a low palaeolatitude of ~18° S (Figure 2C) (http://www.odsn.de/odsn/index.html; accessed November 21, 2021) at the time the leaves were deposited.
Geological Setting and Age
The Deccan Traps of western, central, and southern parts of India, one of the largest continental flood basalts in Earth’s history, were formed as a result of the outpouring of lava onto the Indian peninsula associated with the movement of the Indian Plate over the Reunion hotspot (Chatterjee et al., 2013). Geographically, the Deccan Volcanic Province is divided into three main sub-provinces, namely the Malwa lobe, the Mandla lobe, and the Central Deccan Provinces. Sediments commonly known as Deccan Intertrappean beds are composed of shales, porcellanitic shales, cherts, limestone, and clays.
The Mandla Lobe of the Deccan Volcanic Province (DVP), comprises a 900 m thick package of 29 flows dated as primarily belonging to Chron 29R (Pathak et al., 2017), with deposition lasting <1 Ma and spanning the Cretaceous-Paleogene (K-Pg) transition. In the Mandla Lobe, sediments represent lacustrine and fluviatile environments deposited during quiescent periods between episodes of volcanic activity, often lasting thousands of years. The age of these Deccan Intertrappean sediments is generally assigned to the latest Maastrichtian-earliest Danian based on radiometric (40 Ar/39 Ar) dating, planktonic foraminifera, and magnetostratigraphy (Venkatesan et al., 1997; Khosla, 1999; Hofmann et al., 2000; Chenet et al., 2009; Keller et al., 2009; Renne et al., 2015; Schoene et al., 2015; Shrivastava et al., 2015; Smith et al., 2015).
Deccan Floristic Composition
The Deccan Intertrappean Beds are highly fossiliferous and contain a rich biota (Prasad et al.,1995; Bajpai, 2009; Khosla and Verma, 2015; Kapur et al., 2019). The Deccan Intertrappean (Maastrichtian-Danian) flora hosts one of the richest fossil plant assemblages in India and offers valuable insights for understanding the diversity, evolution, and palaeobiogeography of the Indian flora during the K-Pg transition when India was still a relatively isolated landmass (Kapgate, 2005; Chatterjee et al., 2013; Smith et al., 2015). It is interesting to note that palms have been recognized as an important component of this flora, represented by numerous permineralized stems of Palmoxylon (Prakash and Ambwani, 1980; Ambwani, 1984a, b; Manchester et al., 2016; Khan et al., 2019, 2020a, b), fruits (Prakash, 1960; Bande et al., 1982; Mehrotra, 1987; Bonde, 1990; Srivastava and Srivastava, 2014; Manchester et al., 2016; Matsunaga et al., 2019) as well as several species of leaf remains (Sahni 1964; Bonde, 1986; Srivastava et al., 2014; Roy et al., 2021; Kumar et al., 2022a). In addition to palm woods, other abundant plant megafossils reported from the Deccan Traps are silicified dicot woods (Lakhanpal et al., 1979; Bande and Khatri, 1980; Bande and Prakash, 1980, 1983; Bande et al., 1986; Mehrotra, 1989; Srivastava, 2010; Baas et al., 2017), and a few dicot leaf fossils (Prasad et al., 2013: Khan et al., 2020c).
Observation
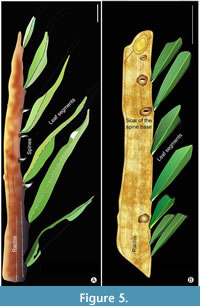
 The two studied fossil spiny palm leaf impressions (Figure 3, Figure 4) were first cleaned with a chisel and hammer and then photographed in natural low-angled light using a digital camera (NIKON D3300). Line drawings of fossil specimens (Figure 5) were made using CorelDraw ver. 20 software. To determine the reliable taxonomic affinity of recovered Deccan palm leaf fossil specimens, we compare them with vouchers of modern taxa of pinnately compound spiny palm leaf species featured in digital herbarium catalogues, specifically the Kew Herbarium catalogue, and the Global Biodiversity Information Facility. In addition, we also compare our specimens with previously reported fossil pinnate palm leaf species from India and elsewhere. Terms used to describe the K-Pg palm leaves conform to the standard terminologies used for architectural description of fossil palm leaves (Read and Hickey, 1972).
The two studied fossil spiny palm leaf impressions (Figure 3, Figure 4) were first cleaned with a chisel and hammer and then photographed in natural low-angled light using a digital camera (NIKON D3300). Line drawings of fossil specimens (Figure 5) were made using CorelDraw ver. 20 software. To determine the reliable taxonomic affinity of recovered Deccan palm leaf fossil specimens, we compare them with vouchers of modern taxa of pinnately compound spiny palm leaf species featured in digital herbarium catalogues, specifically the Kew Herbarium catalogue, and the Global Biodiversity Information Facility. In addition, we also compare our specimens with previously reported fossil pinnate palm leaf species from India and elsewhere. Terms used to describe the K-Pg palm leaves conform to the standard terminologies used for architectural description of fossil palm leaves (Read and Hickey, 1972).
Repository
The recovered specimens (SKBUH/PPL/Um/L/48, Figure 3A, B (part), Figure 3C (counterpart); SKBUH/PPL/UL28, Figure 4A) are deposited in the Museum of the Department of Botany, Sidho-Kanho-Birsha University (SKBUH), Purulia, India. The new names are registered with a unique PFN number in the Plant Fossil Names Registry, hosted and operated by the National Museum, Prague for the International Organisation of Palaeobotany.
SYSTEMATIC PALAEONTOLOGY
Family. Arecaceae Schultz Sch.
Genus. Spinopinnophyllum S. Kumar, T. Su and M.A. Khan gen. nov.
PFN003225
Type species. Spinopinnophyllum acanthorachis S. Kumar, T. Su, and M. A. Khan sp. nov.
PFN003226
Etymology. “spino” is used to symbolize the presence of the spine and “pinnophyllum” refers to the pinnately compound leaves.
Species. Spinopinnophyllum acanthorachis S. Kumar, T. Su, and M.A. Khan sp. nov.
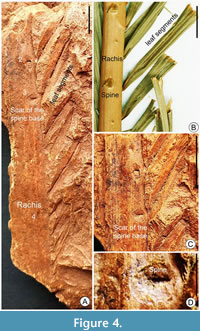 Diagnosis. Leaf pinnately compound, leaflets attached to stout rachis; prominent pointed spines (Figure 3) or scars of the base of spines (Figure 4A) present on the rachis; preserved leaflets narrow emerging straight from one side of the rachis at an acute angle (Figure 3, Figure 4); thick and prominent midvein in each leaflet; mid-vein paralleled by prominent secondary lateral veins, oblique and transverse cross veins also present on both sides of the secondary lateral vein.
Diagnosis. Leaf pinnately compound, leaflets attached to stout rachis; prominent pointed spines (Figure 3) or scars of the base of spines (Figure 4A) present on the rachis; preserved leaflets narrow emerging straight from one side of the rachis at an acute angle (Figure 3, Figure 4); thick and prominent midvein in each leaflet; mid-vein paralleled by prominent secondary lateral veins, oblique and transverse cross veins also present on both sides of the secondary lateral vein.
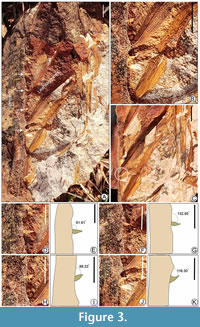
Holotype. SKBUH/PPL/Um/L/48 (Figure 3A)
Paratype. SKBUH/PPL/UL28 (Figure 4A)
Etymology. The specific epithet “acanthorachis” is used to symbolize the presence of spines on the rachis of palm leaves.
Type locality. Umaria Ryt. village (N 22°46′26”, E 80°32′19”, 490 m a.s.l.) in Dindori district, Madhya Pradesh, central India.
Description. The fossil specimens preserve the middle portion of the pinnate leaves, both the petiole and apical portion are not observed; length is 9.2-14.4 cm and width is 1.3-6.9 cm; leaflets are observed on one side of the prominent rachis (Figure 3A; Figure 4A); rachis is stout, 14.2 cm in length and 0.8-1.1 cm in breadth, some faint longitudinal fiber-like structures are also seen on the surface of rachis; 6-7 leaflets are preserved one side of the rachis (Figure 3A; Figure 4A), and the leaflets are attached to the rachis by the entire base, leaflets are narrower at the point of attachment, the terminal portion of the leaflets is not preserved; leaflets are 1.1-6.5 cm long, 0.4-1 cm wide, unarmed, plicate, plication induplicate, emerging at a narrow acute angle from the rachis, thick, coriaceous, and the angle of divergence of leaf segments is 30°-35°; venation is pinnate, decurrent, and each leaflet has a characteristic thick, distinct, midvein, or primary vein (0.4 mm thick) that is strong and uniform (Figure 3B, C; Figure 4C); about 4-7 distinct lateral veins run parallel on each side of the midvein (Figure 3B; Figure 4C) equidistant from each other, 2° veins are 0.1-0.2 mm apart, some lateral veins lack oblique cross-veins; four spines are clearly observed on the rachis, distinct and well-preserved on a stout rachis; spines are 0.2-0.6 mm long and pointed (Figure 3A-C; Figure 4D), and present along the margin of the rachis at an angle of ~61-116° (Figure 3D-K); In one specimen, four scars of the base of spines are observed on the rachis (Figure 4A, C), distinct spines are also well-preserved within some spine bases (Figure 4C, D).
Horizon and age. Deccan Intertrappean beds; latest Maastrichtian (Late Cretaceous)-earliest Danian (Early Paleocene), Chron 29R.
Taxonomic Determination of the New Palm Genus
 The above-mentioned diagnostic features, such as a thick coriaceous pinnate leaf, entirely separated pinnae joined to the strong spiny rachis by their entire narrow bases, pinnate venation, a strong prominent primary vein, and numerous parallel secondaries on either side of the primary vein indicate that our Deccan fossil specimens belong to parts of pinnately leaved palms, especially spiny pinnate palms (family Arecaceae). Given their fragmentary nature and lack of cuticular information, it is not possible to assign them to a particular modern tribe or genus in Arecaceae. This is because such induplicate types of pinnate leaves are found in a large number of palms, e.g., Areca L., Bentinckia Berry ex Roxb., Cocos L. (Arecoideae), Calamus L., Plectocomia Martius and Blume (Calamoideae), and Phoenix L. (Coryphoideae) (Tomlinson, 1990). In addition, Read and Hickey (1972) have pointed out that numerous similarities in the form and external macromorphological features of palm leaves make it difficult to assign fossil palm leaves to modern palm genera based only on external gross morphology. They also have suggested that no attempt should be made to place fossil palm fragments in modern palm genera unless unquestionably identifiable to them. They provided a key for assigning fragmentary true palm leaves to six fossil genera. They described unarmed palm leaf segments under the fossil genus Amesoneuron, with armed leaf segments on one side under Bactrites, a costapalmate palm leaf with a prominent costa under Sabalites, a palmate leaf segment without any costa under Palmacites, a pinnately compound leaf with pinnae reduplicate on the adaxial surface and without any spines under Phoenicites and a pinnately compound leaf with induplicate pinnae on the adaxial surface, but with lowermost pinnae on the rachis spine-like (like modern genus Phoenix), under the fossil genus Phoenix. Thus, in being different from Read and Hickey’s fossil genera, especially the pinnately compound fossil leaf genera Phoenicites and Phoenix, we propose a new fossil genus, Spinopinnophyllum S. Kumar, T. Su, and M. A. Khan gen. nov. for the fossil spiny pinnate palm leaf specimens described here.
The above-mentioned diagnostic features, such as a thick coriaceous pinnate leaf, entirely separated pinnae joined to the strong spiny rachis by their entire narrow bases, pinnate venation, a strong prominent primary vein, and numerous parallel secondaries on either side of the primary vein indicate that our Deccan fossil specimens belong to parts of pinnately leaved palms, especially spiny pinnate palms (family Arecaceae). Given their fragmentary nature and lack of cuticular information, it is not possible to assign them to a particular modern tribe or genus in Arecaceae. This is because such induplicate types of pinnate leaves are found in a large number of palms, e.g., Areca L., Bentinckia Berry ex Roxb., Cocos L. (Arecoideae), Calamus L., Plectocomia Martius and Blume (Calamoideae), and Phoenix L. (Coryphoideae) (Tomlinson, 1990). In addition, Read and Hickey (1972) have pointed out that numerous similarities in the form and external macromorphological features of palm leaves make it difficult to assign fossil palm leaves to modern palm genera based only on external gross morphology. They also have suggested that no attempt should be made to place fossil palm fragments in modern palm genera unless unquestionably identifiable to them. They provided a key for assigning fragmentary true palm leaves to six fossil genera. They described unarmed palm leaf segments under the fossil genus Amesoneuron, with armed leaf segments on one side under Bactrites, a costapalmate palm leaf with a prominent costa under Sabalites, a palmate leaf segment without any costa under Palmacites, a pinnately compound leaf with pinnae reduplicate on the adaxial surface and without any spines under Phoenicites and a pinnately compound leaf with induplicate pinnae on the adaxial surface, but with lowermost pinnae on the rachis spine-like (like modern genus Phoenix), under the fossil genus Phoenix. Thus, in being different from Read and Hickey’s fossil genera, especially the pinnately compound fossil leaf genera Phoenicites and Phoenix, we propose a new fossil genus, Spinopinnophyllum S. Kumar, T. Su, and M. A. Khan gen. nov. for the fossil spiny pinnate palm leaf specimens described here.
DISCUSSION
Spines as a Valuable Taxonomic Character in Palms
Spiny palms are unevenly distributed throughout the major tribes (Tomlinson et al., 2011). The Bactridineae and lepidocaryoid palms are highly spinous (Tomlinson, 1961; Dransfield et al., 2008). In the climbing palms of the lepidocaryoid group, spines are developed for physical support as well as defense, whilst other genera (Metroxylon, Pigatetta, Raphia, and Salacca) in this group have protective spines (Bailey, 1941; Tomlinson, 1962a).
 The subfamily Calamoideae is predominantly spiny; the spines are present generally either on the leaves, leaf sheaths, inflorescences, and leaf rachis (abaxially), or on the extended terminal cirrus and may be modified into hooks in the climbing species. Structures that are associated with climbing are facilitated by the elongation of stems, the presence of spines or reflexed leaflets, or a combination of all of these features. They are most frequent among the four subtribes of Calamoideae (Korthalsiinae, Plectocomiinae, Ancistrophyllinae, and Calaminae), and three unrelated tribes of Arecoideae (Chamaedoreeae, Cocoseae, and Areceae) (Dransfield et al., 2008). The Calamoideae exhibit a wide diversity of emergent spines on leaves, leaf sheaths, and inflorescences (Figure 6).
The subfamily Calamoideae is predominantly spiny; the spines are present generally either on the leaves, leaf sheaths, inflorescences, and leaf rachis (abaxially), or on the extended terminal cirrus and may be modified into hooks in the climbing species. Structures that are associated with climbing are facilitated by the elongation of stems, the presence of spines or reflexed leaflets, or a combination of all of these features. They are most frequent among the four subtribes of Calamoideae (Korthalsiinae, Plectocomiinae, Ancistrophyllinae, and Calaminae), and three unrelated tribes of Arecoideae (Chamaedoreeae, Cocoseae, and Areceae) (Dransfield et al., 2008). The Calamoideae exhibit a wide diversity of emergent spines on leaves, leaf sheaths, and inflorescences (Figure 6).
In some species of Calamus and Daemonorops, the interweaving adjacent whorls of spines form galleries in which ants live. The rachis of climbing palms (Desmoncus, Chamaedorea, and Ancistrophyllunm) is extended into a long cirrus along which the pairs of leaflets are widely spaced. Each leaflet develops as a rigid, backwardly directed grapnel spine or hook. Grapnel spines occur on the back of the leaf axis and are usually restricted to the long terminal cirrus of certain scandent palms. Grapnel leaflets of Desmoncus are almost offensive weapons compared with the passive nature of more orthodox spines (Tomlinson, 1962a). A second climbing organ, i.e., the flagellum identified as a sterile inflorescence, which may bear grapnel spines is found in the genus Calamus. In Salacca spines are aggregated on the petiole in a transverse arrangement to form a linear series (Tomlinson et al., 2011).
Many coryphoid groups such as Borasseae, Phoeniceae, and Rhapidinae also produce significant spines. Spines are mostly present on the petiole margin, but basal leaflets reduced to a stiff, pointed spine-like structure are usually referred to as an acanthophyll, and are diagnostic in Phoeniceae. In Rhapidinae, leaf sheath vascular spines are present in Guihaia, Maxburretia, Trachycarpus, and Rhapidophyllum. Most members of Trachycarpeae have marginal petiolar spines, and occasionally only the juvenile leaves have spines (Saribus, Washingtonia). Some members (Cocoseae, Areceae, and Iriarteeae) of the large palm group Arecoideae are spiny, and within Cocoseae members of the Bactridinae, Attaleinae, and Elaeidinae bear spines. Bactridinae are usually heavily spiny with spines on the leaf rachis and leaf blade. Leaf-sheath vascular spines are found in some genera (Butia, Syagrus) of Attaleinae. Spines are also present on the leaf sheath of genera of the Oncospermatinae within the Areceae (Tomlinson, 1962a, b; Tomlinson et al., 2011).
We compare our Deccan spiny palm leaf specimens with modern pinnate palm leaves having spines on the rachis. Spines on rachises exist in many extant palm species. This character is not taxonomically restricted and commonly occurs in a range of different genera (Astrocaryum, Bactris, Acrocomia, and Aiphanes) of Bactridinae palms. Some members of the subfamily Calamoideae (climbing lepidocaryoid palms) are also predominantly spiny and spines are present on the leaf rachis such as in Calamus acamptostachys (Becc.) Baker, 2015; Desmoncus chinantlensis Liebm. ex Martius and Plectocomia dransfieldiana Madulid, 1981 (Tomlinson, 1962a, b; Tomlinson et al., 2011). The spines are variable in morphology being long and needle-like, or short and broad. The bactroid (Tribe Cocoseae; Subtribe Bactridinae) group of palms is exclusively American and is particularly heavily spiny (Bailey, 1941; Tomlinson et al., 2011). Spines on the rachis of different extant palm species such as Acrocomia aculeata (Jacq.) Lodd. ex Martius, 1834; A. totai Martius, 1844; Aiphanes horrida (Jacq.) Burret, 1932; Astrocaryum sciophilum (Miq.) Pulle, 1906; A. murumuru Martius, 1824l Bactris gasipaes Kunth, 1816; and B. setiflora Burret, 1939 of the subtribe Bactridinae (Bailey, 1941; de Lima et al., 2018; Table 1; Figure 6) are more or less morphologically equivalent to those of our fossil spiny palm leaves where spines are located on the rachises of the pinnate leaves. Most of the Bactridinae grow today in the tropical forests of Central and South America (Dransfield et al., 2008).
Comparison with Previously Reported Fossil Pinnate Palms from India and Elsewhere
Here, we also compare the recovered fossil palm leaf specimens with earlier reported fossil pinnate leaf taxa not only from India but also more widely. Mahabale (1966) reported a fossil specimen showing a resemblance to the pinnate leaf of Phoenix as Palmophyllum mohgaonense based on the basal part of the leaf exhibiting spines on the axis or rachis. However, the fragmentary nature of Mahabale’s specimen does not permit a detailed comparison with the present fossil specimens. As far as we are aware, there are only some limited records of fossilized pinnately compound palm leaf remains from India (Guleria and Mehrotra, 1998; Singh et al., 2012; Guleria et al., 2005). Our fossil leaf specimens are different from them in the arrangement of pinnae on the rachis and the length and thickness of the rachis. In addition, incomplete pinnate leaves are also reported from the Rajahmundry Sandstones (Cretaceous) of Bommuru, Andhra Pradesh (Mahabale and Rao, 1973), and the Eocene sediments of the Garo Hills, Assam (Lakhanpal, 1964).
Pinnate palm leaves (Phoenicites jungii; P. spectabilis; P. angustifolius; P. borealis; P. pumila; P. pallavicinii; P. italica; P. danteana; P. densifolia, etc.) are also reported from the Cenozoic (Eocene, Oligocene, and Miocene) sediments of Europe (Brongniart, 1828; Arenes and Depape, 1956; Massalongo, 1858; Visiani, 1864; Friedrich, 1883; Spitzlberger, 1989; Teodoridis et al., 2015). We compared our fossil leaf specimens with them and found that the Deccan specimens differ in their arrangement of pinnae on the rachis, and the length and thickness of the rachis. Our specimens also differ in having spines on their rachises.
The Calamoideae (rattan palm) leaf species Calamoides pikopiko reported by Hartwich et al. (2010) from the Late Eocene (Beaumont Formation) sediments of Pikopiko, Southland, New Zealand, has prominent punctate prickle (?) scars along the leaf veins. Huard (1967) reported fossil species Spinophyllum from the Neogene (Miocene) Arjuzanx leaf assemblage of France, where lignite adjacent to leaf-bearing sands yielded spiny sheaths belonging to palm stems. Berry (1924) reported the fossil leaf palm species Bactrites pandanifoliolus from the Eocene sediments (Lisbon Formation) of Georgia, USA. The North American species possess spiny leaf fragments with small teeth on one margin. In a few leaves, the primary veins bore spines on one or both surfaces as shown by two specimens in which the spines are preserved. The fossil species Calamus daemonorops reported by Chandler (1957) from the Oligocene sediments of South Devon, England, exhibits spines and spine bases. The above-mentioned four non-Asian fossil spiny palm species can also be distinguished from the Deccan material described here based on spine position. They have spines on the leaflets, whereas our Deccan specimens possess spines on the rachis. Therefore, we assign these newly discovered pinnate spiny palm leaves as Spinopinnophyllum S. Kumar, T. Su, and M.A. Khan gen. nov.
Palaeobiogeographic Significance
Palms are represented by a large number of fossils from India and elsewhere (Rao and Achuthan, 1973; Harley, 2006; Dransfield et al. 2008; Kumar et al., 2022b). However, the spiny palms have a poor fossil record in deep time, making hypotheses concerning their origin and dispersal difficult to evaluate. From this point of view, our finding of well-preserved spiny pinnate palms from the latest Maastrichtian (Late Cretaceous) to early Danian (earliest Paleocene) sediments (Chron 29R, c. 66-65 Ma) of the Mandla Lobe of the Deccan Intertrappean Beds of Madhya Pradesh, Central India, before good evidence of direct India-Eurasia land contact, is important because it represents the oldest reliable fossil record of spiny pinnate palms, and supports their Gondwanan origin.
There are only a few unarmed pinnate palms in the fossil record, and these are reported from the Cenozoic (Eocene, Oligocene, and Miocene) sediments of Europe (Italy, France, and Germany) and Africa (Brongniart, 1828; Arenes and Depape, 1956; Massalongo, 1858; Visiani, 1864; Friedrich, 1883; Spitzlberger, 1989; Pan et al., 2006; Teodoridis et al., 2015). The material described here allows us to propose probable migration pathways of pinnate palms from India to Europe after the docking of the Indian subcontinent with Eurasia during the early Paleogene. The exact time at which this occurred is still debated but the earliest evidence for very close proximity between India and Eurasia is at ~ 61 Ma (An et al., 2021). However, this may represent the docking of a breakaway Indian plate fragment that now makes up the Tibetan Himalaya, with the main part of India making contact at ~ 53 Ma (Yuan et al., 2022). Our finding of spiny pinnate palm leaves at the K/Pg therefore, although preliminarily, supports an “Out-of-India” dispersal hypothesis. Such a proposal runs counter to a prevailing hypothesis that suggests a Laurasian palm origin (Baker et al., 2013a, b), but this dated molecular phylogenetic analysis lacked the benefit of our new well-dated fossils, and a re-analysis is now called for.
Some researchers have suggested that biotic exchange between India and Eurasia may not have required direct contact between the two landmasses (Chatterjee and Scotese, 2010). As an alternative, the biotic exchange may have occurred via African-Arabian connections between India and Eurasia (Greater Somalia or Oman- Kohistan-Dras Island Arc) towards the end of the Cretaceous. This hypothesis depends on the presence of pinnate palm megafossils in Africa (Pan et al., 2006). As both pinnate and palmate palm leaf fossils are reported from Africa, our data support the “Out-of-India” hypothesis and that pinnate palms entered Europe either directly from the Indian subcontinent or on the breakaway Indian plate fragment that became the Tibetan Himalaya.
Our pinnate spiny palm materials, together with the previously reported spineless palms from the same locality, indicate that palms were one of the major angiosperm components of the Madhya Pradesh Deccan flora. It is worth noting that pinnate spiny palms are absent in the fossil locality at the present day. Climate variability over evolutionary time and monsoon intensification might be key reasons for the loss of spiny palms from the present-day vegetation there.
The Relevance of Spiny Palm Fossils to the History of Palm Physiology and Palaeoecology
The role of spines on palm leaves is not completely understood and is likely multifunctional, but the main function of the spines is as a defense mechanism against larger herbivores (Tomlinson, 1962a; Zhang et al., 2022). The large animals against which the spiny defensive mechanisms of palms evolved are now extinct (Tomlinson, 1962a). Apart from signifying an age for spiny palms that goes back at least to a period when the forests were inhabited by much larger herbivores than occur today, these fossils also suggest that spininess is a primitive feature in palms. More recently-evolved palms have no defensive mechanisms because the need has reduced and there are many small spineless delicate palms (Tomlinson, 1962a). Also, it may be that ancient spineless palms originated in areas free from herbivorous predators, and the existence of so many genera of spineless arecoid palms on isolated islands in the Pacific area might support this view (Tomlinson, 1962a). So, the evidence of fossil spiny palms from the latest Maastrichtian-earliest Danian sediments of the Deccan Intertrappean beds of Madhya Pradesh may be a rough measure of the former distribution and predation intensity of large herbivores that are now extinct. We assume these large herbivores might have been dinosaurs and not mammals because Late Cretaceous mammals tend to be much smaller than those that exist today, or in the early Cenozoic (Grossnickle et al., 2019). Based on evidence from the western USA, it seems that it was only after the K-Pg that mammal body mass began to exceed 50 kg (Lyson et al., 2019).
The function of spines in several climbing palms is not for protection, but as grapnels that serve to attach the leaves of the palm to the surrounding foliage and branches of taller trees, so that the palm grows into the forest canopy with the support of adjacent vegetation, rather than by investing in its mechanical tissues. These spines are architecturally and functionally different from the ones appearing in our fossil material.
Spines on other angiosperms are most pronounced today in low productivity semi-arid open environments, where there is heavy grazing pressure from herbivores and competition for plant food is intense (Charles-Dominique et al., 2016; Ratnam et al., 2016). However, modern heavily spined palm genera (such as Bactris and Astrocaryum) generally grow in moist and shady environments (Tomlinson, 1962a).
CONCLUSIONS
Pinnately-leaved spiny palm fossils collected from the K-Pg sediments of the Deccan Intertrappean beds of Madhya Pradesh, central India, are assigned to the new fossil genus Spinopinnophyllum. Our finding indicates that such pinnate spiny palms lived in what is now Madhya Pradesh during the Late Cretaceous-early Paleocene, and suggests that palms had already diversified there at that time. As fossil pinnate spiny palms are little known, the present report provides important evidence for exploring the evolutionary history of these palms. The present fossil evidence of pinnate spiny palms supports the “Out-of-India” hypothesis, i.e., pinnate palms originated in Gondwana and were subsequently “ferried” to Europe on the “raft” of India, spreading within Eurasia following the establishment of the India-Eurasia land contact early in the Cenozoic. The presence of spiny pinnately-leaved palms at the K-Pg suggests defense mechanisms offering protection from large herbivores developed during the late Cretaceous when the only common large herbivores were dinosaurs. Further studies on fossil records of spiny palms are necessary to properly demonstrate the significance of our current hypotheses.
ACKNOWLEDGMENTS
Financial support from the Department of Science and Technology (DST), New Delhi (Ref. no. DST/INSPIRE/03/2019/001456) is gratefully acknowledged. This work was supported by an INSPIRE fellowship awarded to S.K. by The Department of Science and Technology, New Delhi, INSPIRE Code (IF190496). T. S. is supported by the National Key R & D Program of China (2022YFF0800800). M.K. and S.K. gratefully acknowledge the Department of Botany, Sidho-Kanho-Birsha University for providing infrastructural facilities to accomplish this work.
REFERENCES
Achuthan, V. 1968. Palmophyllum dakshinense sp. nov., petrified fragment of a palm leaf from the Deccan Intertrappean beds. Palaeobotanist, 16:103–107.
Ambwani, K. 1984a. Palmoxylon siltherensis sp. nov., from the Deccan Intertrappean beds of Mandla District, Madhya Pradesh. Palaeobotanist, 31:213–217.
Ambwani, K. 1984b. Palmoxylon dilacunosum sp. nov., from the Deccan Intertrappean beds of Mandla District, Madhya Pradesh. Palaeobotanist, 32:211-216.
An, W., Hu, X., Garzanti, E., Wang, J.G., and Liu, Q. 2021. New precise dating of the India-Asia collision in the Tibetan Himalaya at 61 Ma. Geophysical Research Letters, 48:e2020GL090641.
https://doi.org/10.1029/2020GL090641
Arenes, J. and Depape, G. 1956. La floreburdigalienne des îles Baléares (Majorque). Revue générale de botanique, 66:1–43.
Baas, P., Manchester, S.R., Wheeler, E.A., and Srivastava, R. 2017. Fossil wood with dimorphic fibers from the Deccan Intertrappean Beds of India – The oldest fossil Connaraceae? IAWA, 38:124–133.
https://doi.org/10.1163/ 22941932-20170162
Bailey, L.H. 1941. Acrocomia –preliminary paper. Gentes Herbarum, 4:421–476.
Bajpai, S. 2009. Biotic perspective of the Deccan volcanism and India-Asia collision: Recent advances. Current Trends in Science–Platinum Jubilee:505–517.
Baker, W.J. 2015. A revised delimitation of the rattan genus Calamus (Arecaceae). Phytotaxa, 197:139–152.
Baker, W.J. and Couvreur, T.L.P. 2013a. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. Journal of Biogeography, 40:274–285.
https://doi.org/10.1111/j.1365-2699.2012.02795.x
Baker, W.J. and Couvreur, T.L.P. 2013b. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. II. Diversification history and origin of regional assemblages. Journal of Biogeography, 40:286–298.
https://doi.org/10.1111/j.1365-2699.2012.02794.x
Bande, M.B. and Khatri, S.K. 1980. Some more fossil woods from the Deccan Intertrappean beds near Shahpura, Mandla district, Madhya Pradesh. Geophytology, 10: 268–271.
Bande, M.B. and Prakash, U. 1980. Four new fossil dicotyledonous woods from the Deccan Intertrappean beds near Shahpura, Mandla District, Madhya Pradesh. Geophytology, 10:268–271.
Bande, M.B., Prakash, U., and Ambwani, K. 1982. A fossil palm fruit Hyphaeneocarpon indicum gen. et sp. nov. from the Deccan Intertrappean Series, India. Palaeobotanist, 30:303–309.
Bande, M.B., Mehrotra, R.C., and Prakash, U. 1986. Occurrence of Australian element in the Deccan Intertrappean flora of India. Palaeobotanist, 35:1–12.
Berry, E.W. 1924. The middle and upper Eocene floras of southeastern North America. US Geological Survey, Professional Paper, 92:1–206.
Bonde, S.D. 1986. Amesoneuron borassoides sp. nov., a borassoid palm leaf from Deccan Intertrappean beds at Mohgaonkalan, India. Biovigyanam, 12:89–91.
Bonde, S. D. 1990. Arecoidocarpon kulkarnii gen. et sp. nov. an arecoid palm fruit from Mohgaonkalan, Madhya Pradesh. Palaeobotanist, 38:212–216.
Borchsenius, F., and Balslev, H. 1989. Three new species of Aiphanes (Palmae) with notes on the genus in Ecuador. Nordic journal of botany, 9:383–392.
Borchsenius, F., Bernal, R.G. and Ruiz, M. 1989. Aiphanes tricuspidata (Palmae), a new species from Colombia and Ecuador. Brittonia, 41:156–159.
Brongniart, A. 1828. Prodrome d’une histoire des végétaux fossiles. F.G. Levrault, Paris.
Burret, M. 1932. Notizblatt des Botanischen Gartens und Museums zu Berlin-Dahlem, 11: 575.
Burret, M. 1939. Notizblatt des Botanischen Gartens und Museums zu Berlin-Dahlem, 14: 328.
Chandler, M.E.J. 1957. The Oligocene Flora of the Bovey Tracey Lake Basin, Devonshire. Bulletin of the British Museum, Natural History (Geology), 3:71–123.
Charles-Dominique, T., Davies, T.J., Hempson, G.P., Bezeng, B.S., Daru, B.H., Kabongo, R.M., Maurin, O., Muasya, A.M., Van der Bank, M., and Bond, W.J. 2016. Spiny plants, mammal browsers, and the origin of African savannas. Proceedings of the National Academy of Sciences 113:E5572–E5579.
https://doi.org/10.1073/pnas.1607493113
Chatterjee, S. and Scotese, C. 2010. The Wandering Indian Plate and Its Changing Biogeography During the Late Cretaceous-Early Tertiary Period. In: New Aspects of Mesozoic Biodiversity. Lecture Notes in Earth Sciences, vol 132. Springer, Berlin, Heidelberg.
https://doi.org/10.1007/978-3-642-10311-7_7
Chatterjee, S., Goswami, A. and Scotese, C.R. 2013. The longest voyage: Tectonic, magmatic, paleoclimatic evolution of Indian plate during its northward flight from Gondwana to Asia. Gondwana Research, 23:238–267.
https://doi.org/10.1016/j.gr.2012.07.001
Chenet, A.L., Courtillot, V., Fluteau, F., Gérard, M., and Quidelleur, X. 2009. Determination of rapid Deccan eruptions across the Cretaceous–Tertiary boundary using paleomagnetic secular variation: 2. Constraints from analysis of eight new sections and synthesis for a 3500-m-thick composite section. Journal of Geophysical Research: Solid Earth, 114:B6.
https://doi.org/10.1029/2008JB005644
Clement, C.R. and Manshardt, R.M. 2000. A review of the importance of spines for pejibaye heart-of-palm production. Scientia Horticulturae, 83:11–23.
https://doi.org/10.1016/S0304-4238(99)00066-7
de Lima, N.E., Carvalho, A.A., Meerow, A.W., and Manfrin, M.H. 2018. A review of the palm genus Acrocomia: Neotropical green gold. Organisms Diversity & Evolution, 18:151–161.
https://doi.org/10.1007/s13127-018-0362-x
de Visiani, R. 1864. Palmae pinnatae tertiariae agri veneti. Memorie dell’I. R. Istituto Veneto di scienze, lettere ed arti, 11:1–26.
Dransfield, J., Uhl, N.W., Asmussen, C.B., Baker, W.J., Harley, M.M., and Lewis, C.E. 2008. Genera Palmarum: The evolution and classification of palms. Kew, UK, Royal Botanic Gardens.
Fisher, J.B. 1981. A palm spine by another name is still a spine. Fairchild Tropical Garden Bulletin, 36:16–21.
Friedrich, P. 1883. Beiträge zur Kenntnis der Tertiär flora der Provinz Sachsen. Abhandlungen der Geologischen Spezialkarte von Preussen und den Thüringischen Staaten, 4:159–463.
Guleria, J.S. and Mehrotra R.C. 1998. On some plant remains from Deccan Intertrappean localities of Seoni and Mandla district of Madhya Pradesh, India. Palaeobotanist, 47:68–87.
Guleria, J.S., Singh, R.K., Mehrotra, R.C., Soibam, I., and Kishor, R. 2005. Palaeogene plant fossils of Manipur and their palaeoecological significance. Palaeobotanist, 54:61–77.
Grossnickle, D.M., Smith, S.M., and Wilson, G.P. 2019. Untangling the Muliple Ecological Radiations of early Mammals. Trends in Ecology and Evolution, 34:936–949.
https://doi.org/10.1016/j.tree.2019.05.008
Harley, M.M. 2006. A summary of fossil records for Arecaceae. Botanical Journal of the Linnean Society, 151:39–67.
https://doi.org/10.1111/j.1095-8339.2006.00522.x
Hartwich, S.J., Conran, J.G., Bannister, J.M., Lindqvist, J.K., and Lee, D.E. 2010. Calamoid fossil palm leaves and fruits (Arecaceae: Calamoideae) from late Eocene Southland, New Zealand. Australian Systematic Botany, 23:131–140.
https://doi.org/10.1071/SB09027
Hofmann, C., Feraund, G., and Courtillot, V. 2000. 40Ar/Ar39 dating of mineral separates and whole rocks from the Western Ghats lava pile: further constraints on duration and age of the Deccan traps. Earth and Planetary Science Letters, 180:13–27.
https://doi.org/10.1016/S0012-821X(00)00159-X
Huard, J. 1967. Restes épineux de palmier lépidocaryoïde du Néogène des Landes. Naturalia Monspeliensia, 18:319–340.
Kapgate, D.K. 2005. Megafloral analysis of Intertrappean sediments with focus on diversity and abundance of the flora of Mohgaonkalan, Mandla and adjoining areas of Madhya Pradesh. Gondwana Geological Magazine, 20:11–14.
Kapur, V.V. Khosla, A., and Tiwari, N. 2019. Paleoenvironmental and paleobiogeographical implications of the microfossil assemblage from the Late Cretaceous intertrappean beds of the Manawar area, District Dhar, Madhya Pradesh, Central India. Historical Biology, 31:1145–1160.
https://doi.org/10.1080/08912963.2018.1425408
Keith, R. 1834. Miller's Dictionary of Gardening, Botany, and Agriculture, p. 63.
Keller, G., Adatte, T., Bajpai, S., Mohabey, D.M., Widdowson, M., Khosla, A., Sharma, R., Khosla, S.C., Gertsch, B., Fleitmann, D., and Sahni, A. 2009. K-T transition in Deccan Traps of central India marks major marine Seaway across India. Earth and Planetary Science Letters, 282:10–23.
https://doi.org/10.1016/j.epsl.2009.02.016
Khan, M., Mandal, M., and Bera, S. 2019. A new species of permineralized palm stem from the Maastrichtian Danian sediments of Central India and its palaeoclimatic signal. Botany Letters, 166:189–206.
https://doi.org/10.1080/23818107.2019.1600166
Khan, M.A., Roy, K., Hazra, T., Mahato, S., and Bera, S. 2020a. A new coryphoid palm from the Maastrichtian-Danian sediments of Madhya Pradesh and its palaeoenvironmental implications. Journal of the Geological Society of India, 95(1):75–83.
https://doi.org/10.1007/s12594-020-1388-1
Khan, M.A., Hazra, M., Mahato, S., Spicer, R.A., Roy, K., Hazra, T., Bandopadhaya, M., Spicer, T.E., and Bera, S. 2020b. A Cretaceous Gondwana origin of the wax palm subfamily (Ceroxyloideae: Arecaceae) and its paleobiogeographic context. Review of Palaeobotany Palynology, 283:104318.
https://doi.org/10.1016/j.revpalbo.2020.104318
Khan, M.A., Spicer, R.A., Spicer, T.E., Roy, K., Hazra, M., Hazra, T., Mahato, S., Kumar, S., and Bera, S. 2020c. Dipterocarpus (Dipterocarpaceae) leaves from the K-Pg of India: a Cretaceous Gondwana presence of the Dipterocarpaceae. Plant Systematics and Evolution, 306:1–18.
https://doi.org/10.1007/s00606-020-01718-z
Khosla, A. and Verma, O. 2015. Paleobiota from the Deccan volcano-sedimentary sequences of India: paleoenvironments, age and paleobiogeographic implications. Historical Biology, 27:898–914.
https://doi.org/10.1080/08912963.2014.912646
Khosla, S.C. 1999. Costabuntonia, a new genus of Ostracoda from the Intertrappean beds (Paleocene) of east coast of India. Micropaleontology, 45:319–323.
Kumar, S., Hazra, T., Spicer, R.A., Hazra, M., Spicer, T.E., Bera, S., and Khan, M.A. 2022a. Coryphoid palms from the K-Pg boundary of central India and their biogeographical implications: Evidence from megafossil remains. Plant Diversity, 45:80–97.
https://doi.org/10.1016/j.pld.2022.01.001
Kumar, S., Manchester, S., Hazra, T., and Khan, M.A. 2022b. A review of palm macrofossils from India and perspectives. Arabian Journal of Geosciences, 15:1720.
https://doi.org/10.1007/s12517-022-10989-4
Kunth, C.S. 1816. Recherches sur les plantes trouvées dans les tombeaux égyptiens par M. Passalacqua. Annales des sciences naturelles, 8:418–423.
Lakhanpal, R.N. 1964. A new record of angiospermic leaf impressions from the Garo Hills, Assam. Current Science, 33:276.
Lakhanpal, R.N., Prakash, U., and Bande, M.B. 1979. Fossil dicotyledonous woods from the Deccan Intertrappean beds of Mandla District in Madhya Pradesh. Palaeobotanist, 25:190–204.
Liebm. ex Martius, 1849. Historia Naturalis Palmarum, 3:309.
Liebm. ex Martius 1853. Historia Naturalis Palmarum, 3:321.
Lyson, T.R., Miller, I.M., Bercovici, A.D., Weissenburger, K., Fuentes, A.J., Clyde, W.C., Hagadorn, J.W., Butrim, M.J., Johnson, K.R., Fleming, R.F., Barclay, R.S., Macctacken, S.A., Lloyd, B., Wilson, G.P., Krause, D.W., and Chester, S.G.B. 2019. Exceptional Continental Record of Biotic Recovery after the Cretaceous-Paleogene Mass Extinction. Science, 366:977–983.
https://doi.org/10.1126/science. aay2268
Madulid, D.A. 1981. A monograph of Plectocomia (Palmae: Lepidocaryoideae). Kalikasan, 10:1–94.
Mahabale, T.S. 1966. Evolutionary trends in the Palmae with special reference to fossil palms. Palaeobotanist, 14:214–222.
Mahabale, T.S. and Rao, S.V. 1973. Fossil flora of Rajahmundry area. Proceedings of the Symposium on Deccan Trap Country. Indian National Science Academy, Bulletin Indian National Science Academy, 45:192–214.
Manchester, S.R., Bonde, S.D., Nipunage, D.S., Srivastava, R., Mehrotra, R.C., and Smith, S.Y. 2016. Trilocular palm fruits from the Deccan Intertrappean Beds of India. International Journal of Plant Sciences, 177:633–641.
Martius, C.F.P. von. 1853. Historia Naturalis Palmarum, 3:321.
Martius, C.F.P. von.1824. Historia Naturalis Palmarum, 2:70.
Martius, C.F.P. von.1844. A.D. d'Orbigny. Voyage dans l'Amérique Méridionale, 7:78.
Martius, C.F.P. von.1853. Historia Naturalis Palmarum, 3:323.
Martius, C.F.P. von. and Blume C.L. 1830. Systema Vegetabilium, 7:1333.
Massalongo, A. 1858. Palaeophyta rariora formationis tertiariae agri veneti. Coi tipi di G. Antonelli, 3:768–769.
Mathur, A.K., Mishra, V.P., and Mehra, S. 1996. Systematic study of plant fossils from Dagshai, Kasauli and Dharamsala formations of Himachal Pradesh. Director General, Geological Survey of India, 50:1–121.
Matsunaga, K.K., Manchester, S.R., Srivastava, R., Kapgate, D.K., and Smith, S.Y. 2019. Fossil palm fruits from India indicate a Cretaceous origin of Arecaceae tribe Borasseae. Botanical Journal of the Linnean Society, 190:260–280.
https://doi.org/10.1093/botlinnean/boz019
Mehrotra, R.C. 1987. Some new palm fruits from the Deccan Intertrappean beds of Mandla District, Madhya Pradesh. Geophytology, 17:204–208.
Mehrotra, R.C. 1989. Fossil wood of Walsura from the deccan intertrappean beds of the Mandla district, with a review on the intertrappean flora of the district. Review of Palaeobotany and Palynology, 58:205–213.
Moore, H.E. 1963. Gentes Herbarum, 9:251.
Nobel, P.S. 2000. Cacti: Biology and Uses. University of California, Berkeley, California, USA.
Pan, A.D., Jacobs, B.F., Dransfield, J., and Baker, W.J. 2006. The fossil history of palms (Arecaceae) in Africa and new records from the Late Oligocene (28-27 Mya) of north-western Ethiopia. Botanical Journal of the Linnean Society, 151:69–81.
https://doi.org/10.1111/j.1095-8339.2006.00523.x
Pathak, V., Patil, S.K., and Shrivastava, J.P. 2017. Tectonomagmatic setting of lava packages in the Mandla lobe of the eastern Deccan volcanic province, India: palaeomagnetism and magnetostratigraphic evidence. Geological Society, London, Special Publications, 445:69–94.
https://doi.org/10.1144/SP445.3
Prakash, U. 1960. On two palm fruits from the Deccan Intertrappean beds of Mohgaonkalan. Current Science, 29:20–21.
Prakash, U. and Ambwani, K. 1980. A petrified Livistona -like palm stem, Palmoxylon livistonoides sp. nov. from the Deccan Intertrappean beds of India. Palaeobotanist, 26:297–306.
Prasad, G.V.R., Khajuria, C.K., and Manhas, B.K. 1995. Palaeobiogeographic significance of the Deccan infra‐and intertrappean biota from peninsular India. Historical Biology, 9:319–334.
https://doi.org/10.1080/10292389509380506
Prasad, M., Khare, E.G., and Singh, S.K. 2013. Plant Fossils from the Deccan Intertrappean sediments of Chhindwara District, Madhya Pradesh, India: their palaeoclimatic significance. Journal of the Palaeontological Society of India, 58:229–240.
https://doi.org/10.54991/jop.2005.69
Pulle, A.A. 1906. An enumeration of the vascular plants known from Surinam: together with their distribution and synonymy. E.J. Brill, Leiden.
Rao, A.R. and Achuthan, V. 1973. Review of fossil palm remains from India. Palaeobotanist, 20:190–202.
Ratnam, J., Tomlinson, K.W., Rasquinha, D.N., and Sankaran, M. 2016. Savannahs of Asia: antiquity, biogeography, and an uncertain future. Philosophical Transactions of the Royal Society B: Biological Sciences, 371:20150305.
https://doi.org/10.1098/rstb.2015.0305
Read, R.W. and Hickey, L.J. 1972. A revised classification of fossil palm and palm‐like leaves. Taxon, 21:129–137.
Renne, P.R., Sprain, C.J., Richards, M.A., Self, S., Vanderkluysen, L., and Pande, K. 2015. State shift in Deccan volcanism at the Cretaceous-Paleogene boundary, possibly induced by impact. Science, 3:76–78.
https://doi.org/10.1126/science.aac7549
Roy, K., Hazra, T., Hazra, M., Mahato, S., Bera, S., and Khan, M.A. 2021. A new coryphoid costapalmate palm leaf from the Maastrichtian-Danian of India. Botany Letters, 168:155–166.
https://doi.org/10.1080/23818107.2020.1845974
Sahni, B. 1964. Revision of Indian fossil plants III, Monocotyledons. BSIP, Lucknow.
Schoene, B., Samperton, K.M., Eddy, M.P., Keller, G., Adatte, T., Bowring, S.A., Khadri, S.F.R., and Gertsch, B. 2015. U-Pb geochronology of the Deccan Traps and relation to the end-Cretaceous mass extinction. Science, 347:183–184.
https://doi.org/10.1126/science.aaa0118
Shrivastava, J.P., Duncan, R.A., and Kashyap, M. 2015. Post-K/Pg younger 40Ar-39Ar ages of the Mandla lavas: implications for the duration of the Deccan volcanism. Lithos, 224: 214–224. https://doi.org/10.1016/j.lithos.2015.03.006
Singh, M.C., Kushwaha, R.A.S., Srivastava, G., and Mehrotra, R.C. 2012. Plant remains from the Laisong formation of Manipur. Journal of Geological Society of India, 79:287–294.
https://doi.org/10.1007/s12594-012-0042-y
Singh, R.R. and Patnaik, R. 2012. A fossil palm leaf impression from∼ 11.2 Ma old, Siwalik deposits of Kangra Valley, Himachal Pradesh. Journal of Geological Society of India, 79):85–88.
https://doi.org/10.1007/s12594-012-0008-0
Smith, S.Y., Manchester, S.R., Samant, B., Mohabey, D.M., Wheeler, E., Baas, P., Kapgate, D., Srivastava, R., and Sheldon, N.D. 2015. Integrating paleobotanical, paleosol, and stratigraphic data to study critical transitions: a case study from the Late Cretaceous-Paleocene of India. The Paleontological Society Papers, 21:137–166.
https://doi.org/10.1017/S1089332600002990
Spitzlberger, G. 1989. Die Miozänfundstelle Goldernbei Landshut (Niederbayern). Geologica Bavarica, 94:371–407.
Srivastava, R. 2010. Fossil dicotyledonous woods from Deccan Intertrappean sediments of Ghansor, Seoni District, Madhya Pradesh, India. Palaeobotanist, 59:129–138.
Srivastava, R. and Srivastava, G. 2014. Fossil fruit of Cocos L. (Arecaceae) from Maastrichtian-Danian Sediments of Central India and its Phytogeographical Significance. Acta Palaeobotanica, 54:67–75.
https://doi.org/10.2478/acpa-2014-0003
Srivastava, R., Srivastava, G., and Dilcher, D.L. 2014. Coryphoid palm leaf fossils from the Maastrichtian-Danian of central India with remarks on phytogeography of the Coryphoideae (Arecaceae). PLoS ONE, 9:1–10.
https://doi.org/10.1371/journal.pone.0111738
Su, T., Farnsworth, A., Spicer, R.A., Huang, J., Wu, F.X., Liu, J., Li, S.F., Xing, Y.W., Deng, Y.J., Huang, W.Y.D., and Tang, H. 2019. No high Tibetan Plateau until the Neogene. Science Advances, 5:2189.
https://doi.org/10.1126/sciadv.aav2189
Teodoridis, V., Kvaček, Z., Agostini, S., Martinetto, E., Rossi, M.A., and Cavallo, O. 2015. Feather palm foliage from the Messinian of Italy (Capo di Fiume, Palena and Pollenzo near Alba) within the framework of northern Mediterranean late Miocene flora. Historia Naturalis, 72:301–314.
Tomlinson, P.B. 1961. Essays on the morphology of palms V: the habit of palms. Principes, 5:83–89.
Tomlinson, P.B. 1962a. Essays on the morphology of palms. VII. A digression about spines. Principes, 6:44–52.
Tomlinson, P.B. 1962b. The leaf base in palms its morphology and mechanical biology. Journal of the Arnold Arboretum, 43:23–50.
Tomlinson, P.B. 1990. The Structural Biology of Palms. Oxford University Press, Oxford, UK.
Tomlinson, P.B., Horn, J.W., and Fisher, J.B. 2011. The anatomy of palms: Arecaceae-Palmae. Oxford University press, Oxford, USA.
Trivedi, B.S., Chandra, R. 1971. A palm leaf from the Deccan Intertrappean Series. Mohgaon Kalan (M.P.). India. Current Science, 40:526–527.
Venkatesan, T.R., Kumar, A., Gopalan, K., and Al'Mukhamedov, A.I. 1997. 40Ar-39 Ar age of Siberian basaltic volcanism. Chemical Geology, 138:303–310.
Wang, Q.J., Ma, F.J., Dong, J.L., Yang, Y., Jin, P.H., and Sun, B.N. 2015. Coryphoid palms from the Oligocene of China and their biogeographical implications. Comptes Rendus Palevol, 14:263–279.
https://doi.org/10.1016/j.crpv.2015.03.005
Yuan, J., Deng, C., Yang, Z., Krijgsman, W., Thubtantering, Qin, H., Shen, Z., Hou, Y., Zhang, S., Yu, Z., Zhao, P., Zhao, L., Wan, B., He, H., and Guo, Z. 2022. Triple-stage India-Asia collision involving arc-continent collision and subsequent two-stage continent-continent collision. Gobal and Planetary Change, 212:103821.
https://doi.org/10.1016/j.gloplacha.2022.103821
Zhang, X., Gélin, U., Spicer, R.A., Wu, F., Farnsworth, A., Chen, P., Del Rio, C., Li, S., Liu, J., Huang, J., Spicer, T.E.V., Tomlinson, K.W., Valdes, P.J., Xu, X., Zhang, S., Deng, T., Zhou, Z., and Su, T. 2022. Rapid Eocene diversification of spiny plants in subtropical woodlands of central Tibet. Nature Communications, 13:3787.
https://doi.org/10.1038/s41467-022-31512-z

