 A new genus and species of fossil pseudoscorpion (Arachnida: Pseudoscorpiones) from the Eocene amber of Western India
A new genus and species of fossil pseudoscorpion (Arachnida: Pseudoscorpiones) from the Eocene amber of Western India
Article number: 27.2.a26
https://doi.org/10.26879/1276
Copyright Paleontological Society, May 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 23 February 2023. Acceptance: 12 April 2024.
ABSTRACT
A new genus and species of fossil pseudoscorpion, Geogaranya valiyaensis gen. nov. sp. nov., is described from the Valia Lignite Mine, Cambay Basin, Gujarat. The new fossil taxon is exceptionally preserved in the early Eocene Cambay amber and is a member of the family Geogarypidae, with similar affinities to the modern genus Geogarypus (Chamberlin, 1930) recorded from Sri Lanka, India and New Guinea. The taxon is one of the smallest known adult fossils of pseudoscorpion in amber from the Cambay Basin and adds to the bark-dwelling arthropod biodiversity identified in the Eocene amber from Western India.
Priya Agnihotri. DST-Birbal Sahni Institute of Palaeosciences, 53-University Road, Lucknow, 226 007, India; Academy of Scientific and Innovative Research, Ghaziabad, 201 002, India. (Corresponding author) agnihotripriya18@yahoo.com
Hukam Singh. DST-Birbal Sahni Institute of Palaeosciences, 53-University Road, Lucknow, 226 007, India; Academy of Scientific and Innovative Research, Ghaziabad, 201 002, India. hukams@gmail.com
Kumarapuram A. Subramanian. Southern Regional Centre, Zoological Survey of India, Chennai, 600 028, India. subbu.ka@zsi.gov.in
Jagannadh Vishwanathan. Department of Zoology, St. Peter’s College, Kolenchery Ernakulam, Kerala, 682 311, India. drvjagan92@gmail.com
Ashok Sahni. Centre of advanced study in Geology, Panjab University, Chandigarh, 160 014, India. ashok.sahni@gmail.com
Key words: Arachnida; Cambay amber; Eocene; new genus; pseudoscorpion; Valia
Final citation: Agnihotri, Priya, Singh, Hukam, Subramanian, Kumarapuram A., Vishwanathan, Jagannadh, and Sahni, Ashok. 2024. A new genus and species of fossil pseudoscorpion (Arachnida: Pseudoscorpiones) from the Eocene amber of Western India. Palaeontologia Electronica, 27(2):a26.
https://doi.org/10.26879/1276
palaeo-electronica.org/content/2024/5207-pseudoscorpion-in-indian-amber
Copyright: May 2024 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/54B4B8D8-0E59-47F0-BD66-872B43CC1CB5
INTRODUCTION
Unique fossil resources have been found in the Cenozoic amber deposits recovered from India and many sections of the Asian Lignite Mines over recent years. The Indian amber deposits from the Cambay Basin are linked to the resinous trees of Dipterocarpaceae, classified as a class II (dammar) resin (Dutta et al., 2009, 2011; Rust et al., 2010; Shi et al., 2014; Dutta and Mallick 2017; Bansal, 2022). Unlike compression fossils, biotic inclusions in amber have resulted in an array of exceptionally preserved floral and faunal species. The Cambay Basin has been a rich palaeontological hotspot for terrestrial mammals (Rana et al., 2005, 2008; Smith et al., 2007; Rose et al., 2009; Mayr and Smith, 2019) and amber inclusions (Alimohammadian et al., 2005; Rust et al., 2010). Resinous trees, such as the Dipterocarpaceae, originated from the warm period of the Ypresian, indicating the presence of early angiosperm-based megathermal forests (Heinrichs et al., 2016; Khan et al., 2020). Cambay amber holds records of primitive flower and pollen species (Singh H. et al., 2021), arthropods including social insects (Grimaldi and Singh, 2012; Kania et al., 2015; Stebner et al., 2016, 2017), such as termites (Engel et al., 2011), beetles (Ortega-Blanco et al., 2013) and freshwater crustaceans.
 Here, we describe a new genus and species of fossil pseudoscorpion (Family Geogarypidae) (Figure 1) in Cambay amber from the Valia Lignite Mine, Gujarat. Pseudoscorpions are an ancient lineage of terrestrial arachnids (Shear et al., 1989), which are morphologically similar to true scorpions but lack a tail and stinger. Certain families possess a distinct venom apparatus in the chelal fingers of the pedipalps, which evolved independently from that of scorpions and spiders (Kramer et al., 2019). Recent studies have also supported the inclusion of pseudoscorpions as the sister group of scorpions (Ontano et al., 2021). The term Panscorpiones has been proposed for the clade uniting scorpions and pseudoscorpions, based on the data of taxonomic sampling while studying the phylogenetic placement of pseudoscorpions. The presence of venom glands on both chelal fingers is presumed to be a plesiomorphic trait, and later lost in one of the fingers (Murienne et al., 2008). A few species have a capacity to use phoresy for dispersion, while others act as commensals, living in mammal or bird nests (Turienzo et al., 2010). In comparison to other more widely preserved arachnid groups of spiders and mites, they have a limited fossil record with significant biogeographical implications. Because of their delicate bodies and small size, these fossils are mostly discovered in amber deposits across the world, rather than in sedimentary deposits. Forty-nine pseudoscorpion species have been documented from the Baltic and Rovno amber which is of Eocene in age (Harvey, 2013; Harms and Dunlop, 2017; Schwarze et al., 2021). Based on the International Chronostratigraphic Chart (Cohen et al., 2013), new pseudoscorpion taxa were listed from Cenozoic and Cretaceous ambers, including Progonatemnus (Atemnidae), Roncus and Neobisium (Neobisiidae), Electrochelifer (Cheliferidae), Cheiridium (Cheiridiidae) and Geogarypus (Geogarypidae). Quality preservations have also been mentioned from the Bitterfeld (Ahrens et al., 2019) and Burmese (Harvey et al., 2018) amber, with data of unique genera Allochthonius, Centrochthonius and Weygoldtiella respectively. From the Cenomanian Burmese deposits of Myanmar, the genera Electrobisium, Amblyolpium and Protofeaella have also been mentioned. Baltic amber preserves Eocene genera Pseudogarypus, Pychnochelifer, Cheiridium and extinct genus Chelignathus. Major Baltic contribution was by Max Beier, who described seventeen pseudoscorpion species in his articles (Beier, 1937, 1947, 1955) and by Harvey (1992), who proposed the first objective phylogeny based on morphological traits, differentiating two lineages, Epiocheirata and Iocheirata. Schawaller (1978) and Judson (2003) later added three more genera Chthonius, Microcreagrus and Neobisium to the list. Pseudoscorpion families in Dominican amber from the Neotropical regions of the State of Chiapas and Island of Hispaniola yield significant species of Pachychernes, Pseudochthonius, Lechytia, Cryptocheiridium, extant species Idiogaryops, Pseudochiridium and other recent records listed by Penney and co-workers (2008). Pseudoscorpion researches from the Indian subcontinent and Sri Lanka have also looked into indigenous families Chernetidae (genus Meghachernes), Withiidae (genus Metawithius) and Hyidae (genus Indohya) of both contemporary and Gondwanan origin (Harvey and Volschenk, 2007; Batuwita and Benjamin, 2014; Novak and Harvey, 2018; Johnson et al., 2019). However, there are no previously published records of fossil pseudoscorpions from India.
Here, we describe a new genus and species of fossil pseudoscorpion (Family Geogarypidae) (Figure 1) in Cambay amber from the Valia Lignite Mine, Gujarat. Pseudoscorpions are an ancient lineage of terrestrial arachnids (Shear et al., 1989), which are morphologically similar to true scorpions but lack a tail and stinger. Certain families possess a distinct venom apparatus in the chelal fingers of the pedipalps, which evolved independently from that of scorpions and spiders (Kramer et al., 2019). Recent studies have also supported the inclusion of pseudoscorpions as the sister group of scorpions (Ontano et al., 2021). The term Panscorpiones has been proposed for the clade uniting scorpions and pseudoscorpions, based on the data of taxonomic sampling while studying the phylogenetic placement of pseudoscorpions. The presence of venom glands on both chelal fingers is presumed to be a plesiomorphic trait, and later lost in one of the fingers (Murienne et al., 2008). A few species have a capacity to use phoresy for dispersion, while others act as commensals, living in mammal or bird nests (Turienzo et al., 2010). In comparison to other more widely preserved arachnid groups of spiders and mites, they have a limited fossil record with significant biogeographical implications. Because of their delicate bodies and small size, these fossils are mostly discovered in amber deposits across the world, rather than in sedimentary deposits. Forty-nine pseudoscorpion species have been documented from the Baltic and Rovno amber which is of Eocene in age (Harvey, 2013; Harms and Dunlop, 2017; Schwarze et al., 2021). Based on the International Chronostratigraphic Chart (Cohen et al., 2013), new pseudoscorpion taxa were listed from Cenozoic and Cretaceous ambers, including Progonatemnus (Atemnidae), Roncus and Neobisium (Neobisiidae), Electrochelifer (Cheliferidae), Cheiridium (Cheiridiidae) and Geogarypus (Geogarypidae). Quality preservations have also been mentioned from the Bitterfeld (Ahrens et al., 2019) and Burmese (Harvey et al., 2018) amber, with data of unique genera Allochthonius, Centrochthonius and Weygoldtiella respectively. From the Cenomanian Burmese deposits of Myanmar, the genera Electrobisium, Amblyolpium and Protofeaella have also been mentioned. Baltic amber preserves Eocene genera Pseudogarypus, Pychnochelifer, Cheiridium and extinct genus Chelignathus. Major Baltic contribution was by Max Beier, who described seventeen pseudoscorpion species in his articles (Beier, 1937, 1947, 1955) and by Harvey (1992), who proposed the first objective phylogeny based on morphological traits, differentiating two lineages, Epiocheirata and Iocheirata. Schawaller (1978) and Judson (2003) later added three more genera Chthonius, Microcreagrus and Neobisium to the list. Pseudoscorpion families in Dominican amber from the Neotropical regions of the State of Chiapas and Island of Hispaniola yield significant species of Pachychernes, Pseudochthonius, Lechytia, Cryptocheiridium, extant species Idiogaryops, Pseudochiridium and other recent records listed by Penney and co-workers (2008). Pseudoscorpion researches from the Indian subcontinent and Sri Lanka have also looked into indigenous families Chernetidae (genus Meghachernes), Withiidae (genus Metawithius) and Hyidae (genus Indohya) of both contemporary and Gondwanan origin (Harvey and Volschenk, 2007; Batuwita and Benjamin, 2014; Novak and Harvey, 2018; Johnson et al., 2019). However, there are no previously published records of fossil pseudoscorpions from India.
Family Geogarypidae is amongst those groups of bark-dwelling and litter-dwelling species which are similar to family Garypidae in having a characteristic subtriangular carapace and eyes near the anterior margin. The family contains more than seventy species with habitat preferences suitable in tropical and subtropical regions with a few reported from temperate biomes (Harvey, 2013; Nassirkhani, 2014; Neethling and Haddad, 2017). Geogarypidae are more common in the Baltic and Rovno amber with a few records from the Cretaceous Burmese amber (Table 1). Unlike their scarce records in fossils, their modern counterparts have been recorded from all major biogeographical regions, including Europe, Central Asia, North America and North Africa (Harvey, 2013). Their fossil records serve as a source to understand palaeoenvironment and climatic conditions during the Early Eocene Climatic Optimum (EECO) (Cramer et al., 2003; Nicolo et al., 2007; Rust et al., 2010; Zachos et al., 2010).
LOCALITY AND AGE
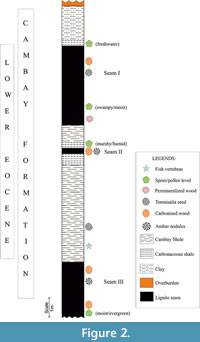 The open-cast Valia Lignite Mine (latitude 21° 30' 52' N, longitude 73° 12' 20” E) is located in the Cambay Basin, with a well-established biostratigraphic framework (Biswas, 1993; Bhandari et al., 2005; Sahni et al., 2006; Punekar and Saraswati, 2010; Rust et al., 2010; Prasad et al., 2013; Rao et al., 2013; Singh H. et al., 2015, 2021). The Cambay Shale Formation overlies the Deccan traps and is underlain by the Paleocene-lower Eocene Vagadkhol Formation (Sudhakar and Basu, 1973; Singh H. et al., 2011). It is assigned a Ypresian age with context to dinoflagellate cysts (Garg et al., 2008) and benthic foraminiferal data of Nummulites burdigalensis from the adjacent Vastan Lignite mine (Punekar and Saraswati, 2010). The rock type of the studied mine exposure comprises of carbonaceous and grey shales of varied thickness. The stratigraphic section consists of 3 lignite seams, and the upper and basal seams are thicker (4.5 m and 3 m) than the middle seam of 0.3 m (Figure 2). Amber nodules are collected from lignite seams I and III. Of significant findings, Gynocardia fossil wood (Shukla et al., 2015) and vertebrate remains and pollens (Singh V. P. et al., 2021) were documented from the respective lignite and shale beds. Geochemical data (bulk rock XRD and clay mineralogy) carried on rock samples infer the presence of kaolonite, siderite, quartz, smectite and chlorite. Kaolonite abundance indicates high degree of chemical weathering and high erosion (Singh V. P. et al., 2021).
The open-cast Valia Lignite Mine (latitude 21° 30' 52' N, longitude 73° 12' 20” E) is located in the Cambay Basin, with a well-established biostratigraphic framework (Biswas, 1993; Bhandari et al., 2005; Sahni et al., 2006; Punekar and Saraswati, 2010; Rust et al., 2010; Prasad et al., 2013; Rao et al., 2013; Singh H. et al., 2015, 2021). The Cambay Shale Formation overlies the Deccan traps and is underlain by the Paleocene-lower Eocene Vagadkhol Formation (Sudhakar and Basu, 1973; Singh H. et al., 2011). It is assigned a Ypresian age with context to dinoflagellate cysts (Garg et al., 2008) and benthic foraminiferal data of Nummulites burdigalensis from the adjacent Vastan Lignite mine (Punekar and Saraswati, 2010). The rock type of the studied mine exposure comprises of carbonaceous and grey shales of varied thickness. The stratigraphic section consists of 3 lignite seams, and the upper and basal seams are thicker (4.5 m and 3 m) than the middle seam of 0.3 m (Figure 2). Amber nodules are collected from lignite seams I and III. Of significant findings, Gynocardia fossil wood (Shukla et al., 2015) and vertebrate remains and pollens (Singh V. P. et al., 2021) were documented from the respective lignite and shale beds. Geochemical data (bulk rock XRD and clay mineralogy) carried on rock samples infer the presence of kaolonite, siderite, quartz, smectite and chlorite. Kaolonite abundance indicates high degree of chemical weathering and high erosion (Singh V. P. et al., 2021).
MATERIAL AND METHODS
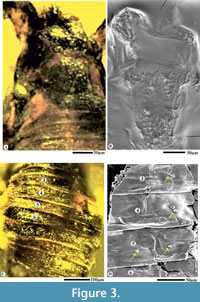
Amber nodules incorporating fossils were recovered from the open-cast Valia Lignite Mine, 30 km South East from Bharuch district, Gujarat. On a Buehler flat lap wheel, the amber slab containing the pseudoscorpion fossil was ground and polished using emery sheets. The specimen was observed and photographed under Leica stereoscope M205A. For scanning electron microscopy imaging (Figure 3), the amber slab was completely dissolved in toluene and left for the resin to totally evaporate. The fossil was then placed on a stub and scanned using a scanning electron microscope Leo 430. The holotype is deposited at the Birbal Sahni Institute of Palaeosciences, Lucknow repository, with museum number as 41982. This specimen is also included in Singh V.P. et al. (2021). Systematic terminologies follow that of Chamberlin (1931).
SYSTEMATIC PALAEONTOLOGY
GEOGARYPIDAE (Chamberlin, 1930)
GEOGARANYA gen. nov.
G. valiyaensis sp. nov. ♂
Figure 1, Figure 3, Figure 4
zoobank.org/53588E48-742C-485E-9CE3-7B23C3B93E1B
Type material. BSIP museum no. 41982; a geogarypid adult pseudoscorpion, well-preserved in amber resin.
 Locality. Valia Lignite Mine, Cambay Basin, Gujarat.
Locality. Valia Lignite Mine, Cambay Basin, Gujarat.
Age. early Eocene
Etymology. Genus name derived from the Indian Geogarypus and Aranya, meaning “wilderness” ' in sanskrit; the specific epithet named after Valia Lignite Mine.
Diagnosis. G. valiyaensis gen. nov. sp. nov. differs from the other genera of family Geogarypidae in possessing carapaceal alae, smallest size, triangular carapace, eyes on ocular tubercles, exceptionally long chelal fingers, non-clavate bristles, distinctly granulated prosoma, and articulation differences between appendages.
zoobank.org/CC948B47-5C06-
Genus description. Prosoma- chelal fingers with 5-6 pair of setae, no teeth on movable fingers, tarsal claws absent, prosoma coarsely granulated, flagellum with minute bristles, ovate chelicerae, no tubercles lining the thorax, non-clavate bristles unlike other genera; opisthosoma- male sternites without margins, pitted surface lining the Ist tergite, tergites II-VI with prominent keels, spiracles and tarsal setae absent; appendages- articulation difference between front, mid and hind legs unlike the heterofemorate articulation found in other genera of Family Geogarypidae.
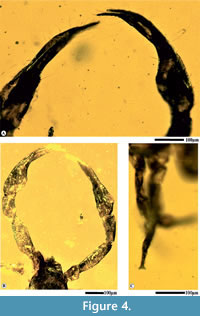 Species description. Smallest known adult (Figure 1), well preserved in Cambay amber, length from chelicerae to abdomen 0.60 mm, flattened, pear-shaped body, chelicerae rather short, distal blades with serrations, prosoma 0.16 mm, longer than broad, sub-rectangular, granular pattern over the carapace, antero-marginal position of eyes, pedipalps 0.8 mm (L and R each), twice the length as the abdomen, smooth chelae including movable fingers, no teeth over movable fingers, numerous setae on the dorsal chelal surface (Figure 4A), trochanter 0.06 mm (L and R each), femur 0.20 mm (L), 0.23 mm (R), elongate, patella 0.10 mm (L), 0.16 mm (R), chela without pedicel, 0.40 mm (L), 0.41 mm (R), swollen, trichobothria indistinguishable, carapace granular with minute setae, opisthosma 0.36 mm, ovate, marked segmentation (Figure 3C, D), twelve opisthosomal segments visible, I-XII tergites visible, median suture lines dividing each tergite longitudinally, numerous lyrifissures, no terminal stinger, spiracles absent, legs poorly preserved (Figure 4C), broken, leg 1 (L) detached from the body, coxae attached with the thorax of leg I (R), II (L, R), III (L, R) and IV (L, R).
Species description. Smallest known adult (Figure 1), well preserved in Cambay amber, length from chelicerae to abdomen 0.60 mm, flattened, pear-shaped body, chelicerae rather short, distal blades with serrations, prosoma 0.16 mm, longer than broad, sub-rectangular, granular pattern over the carapace, antero-marginal position of eyes, pedipalps 0.8 mm (L and R each), twice the length as the abdomen, smooth chelae including movable fingers, no teeth over movable fingers, numerous setae on the dorsal chelal surface (Figure 4A), trochanter 0.06 mm (L and R each), femur 0.20 mm (L), 0.23 mm (R), elongate, patella 0.10 mm (L), 0.16 mm (R), chela without pedicel, 0.40 mm (L), 0.41 mm (R), swollen, trichobothria indistinguishable, carapace granular with minute setae, opisthosma 0.36 mm, ovate, marked segmentation (Figure 3C, D), twelve opisthosomal segments visible, I-XII tergites visible, median suture lines dividing each tergite longitudinally, numerous lyrifissures, no terminal stinger, spiracles absent, legs poorly preserved (Figure 4C), broken, leg 1 (L) detached from the body, coxae attached with the thorax of leg I (R), II (L, R), III (L, R) and IV (L, R).
Remarks. G. valiyaensis gen nov. sp. nov. in amber adds major insights into the evolution of the pseudoscorpion taxa and contributes to the diverse amber arthropod community found in the Cambay Basin of Gujarat. The fossil implies a much warmer temperature, with its assimilation of distinct characteristics, akin to fauna in a warm-temperate biome. We also find similar affinities of the fossil with those recorded from the Baltic and Bitterfeld, in support of a strong tilt towards bark-dwelling taxa (Harms and Dunlop, 2017).
DISCUSSION
Pseudoscorpions represent a group of ancient lineage of the earliest arthropods which colonized the landmass of the Earth during early Devonian based on fossil evidence (Shear et al., 1989). The diversified order occupies more than three percent of the total known arachnid species (Harvey, 2007). Unlike pseudoscorpions, other arachnid taxa occur at a much younger age, with fossil records including spiders and scorpions previously recorded from the late Carboniferous and potentially originating later. Flying insects radiated much later during the Carboniferous (Dunlop, 2010; Dunlop and Penney, 2012). In addition, pseudoscorpions adapt themselves to cryptic habitats including litter and tree barks, and hence, their evidence in amber fossil record strongly supports that they also share some similarities with several clades of acariform mites from the Palaeozoic and Cretaceous (Dunlop and Penney, 2012). Due to its similarities in morphology and habitats through fossil records, it is believed that the biology and behavioral features could have been maintained between both taxa (Shear et al., 1989; Poinar et al., 1998).
With respect to the Eocene palaeoenvironment, amber-preserved biota documents an exceptional preservation of taxa living close to the amber source which are more likely to be trapped in the amber resin. In case of pseudoscorpions, the record of a bark dwelling G. valiyaensis preserved in the Cambay amber adds valuable insights into the arthropod biodiversity that survived near the resin source in a broad-leaved angiosperm dominated tropical rainforest during the early Eocene. Molecular affinities of Cambay amber are more connected to the present-day forest on peat bogs from Borneo and Sumatra, Indonesia (Naglik et al., 2018; Simoneit et al., 2020). Both show properties that are comparable to those seen in angiosperms. Shorea is the most common resin-producing genus in both regions with similar chemical composition.
The systematic description of G. valiyaensis in amber indicates a biota belonging to a much warmer temperature during the Eocene. We also state record similar affinities of the fossil with those recorded from the Baltic and Rovno, in support of a strong tilt towards bark-dwelling taxa. The occurrence of this early Eocene pseudoscorpion taxon in Cambay amber provide evidence for the presence of arachnid species in the vicinity of a warm Eocene forest, with similar diversification pattern to the Eocene spiders (Dunlop et al., 2018). Dimension of pedipalps and the structure of chelae aid in handling larger fauna, both for predation and transportation. In accordance with Judson’s (2012) theory, we have observed plesiomorphic and derived traits by interpreting Dracochela as a stem-group pseudoscorpion, clearly indicated by pedipalps (Figure 4), trichobothria and a cuticle texture implying living in moist environments like leaf litter. The ancestral forms which belong to the Geogarypidae already possessed trichobothria, pedipalps, chelicerae and galea. Presence of unusually larger pedipalps does create the possibility of phoresy, and that species from non-arboreal habitats may unintentionally be conveyed into amber, linked to their flying hosts. None of the evidences suggest that the present species had any ecological difference from other geogarypids and that it belongs to a bark-dwelling arachnid group. The fossil belongs to a clade that is not well known from the Indian Eocene amber fauna and differs from other arthropod species known from Southeast Asia. The terrestrial environment that fostered the formation of this fossil species preserved in Cambay amber, qualifies as a possible Gondwanan relict, when there was a significant transition from a temperate to tropical zone across the equator. However, based on Devonian fossils, it is possible to state that pseudoscorpions diverged into main clades earlier than certain other arachnid taxa, such as the spiders (Harms and Dunlop, 2017). Also, findings of freshwater crustaceans (ostracods) in amber from similar locality suggests that open pool systems may have existed in the region, penetrating deep into the Eocene forest, with overhanging branches of resinous trees around these water bodies.
CONCLUSIONS
1. Geogaranya valiyaensis gen. nov. sp. nov. is the first ever record of pseudoscorpion, extracted from an amber nodule, recovered from the amber-bearing lignite deposits of the Valia Lignite Mine of Gujarat, western India.
2. The discovery of one of the smallest known pseudoscorpion adults in amber from the Cambay Basin provides insights into the bark-dwelling arthropod taxa, similar to fossil taxa recorded in Baltic and Bitterfeld amber, which survived during the early Eocene.
3. The fossil shows strong resemblance with the modern Geogarypus (Chamberlin, 1930). The scanning electron microscopic study reveals diagnostic traits of the fossil, such as exceptionally enlarged pedipalps, which increases the idea of phoresy and suggests that species from non-arboreal habitats may have mistakenly been transported into amber and related to their flying hosts.
ACKNOWLEDGEMENT
The authors extend sincere gratitude to the Director, Birbal Sahni Institute of Palaeosciences (BSIP), Lucknow, for rendering permission to publish the manuscript (BSIP/RDCC/Publication no. 84//2021-2022) and access to the SEM laboratory of the Institute. Help rendered by Mr. Subodh Kumar, Technical Officer, BSIP, during the SEM imaging is also sincerely acknowledged. HS is grateful to the authorities of the Valia Lignite Mine for their co-operation during the field visit. KAS acknowledges the Director, ZSI (Kolkata) for encouragement and support.
REFERENCES
Ahrens, J., Harms, D., Dunlop, J.A., and Kotthoff, U. 2019. Pseudoscorpions in Bitterfeld amber-a survey. Mauritiana (Altenburg), 37:113-147.
Alimohammadian, H., Sahni, A., Patnaik, R., Rana, R.S., and Singh, H. 2005. First record of an exceptionally diverse and well-preserved amber-embedded biota from lower Eocene (~53 Ma) lignites, Vastan, Gujarat. Current Science, 89:1328-1330.
http://www.jstor.org/stable/24110837
Bansal, M., Morley, R.J., Nagaraju, Shivaprakash K., Dutta, S. Mishra, A.K., Selveraj, J. Kumar, S.,Niyolia, D., Harish, S.M., Abdelrahim, O.B., Hasan, S., Ramesh, B.R., Dayanandan, S., Morley, H.P., Ashton, P.S., and Prasad, V. 2022. Southeast Asian Dipterocarp origin and diversification driven by Africa-India floristic interchange. Science, 375:455-460.
https://doi.org/10.1126/science.abk2177
Batuwita, S. and Benjamin, S.P. 2014. An annotated checklist and a family key to the pseudoscorpion fauna (Arachnida: Pseudoscorpiones) of Sri Lanka. Zootaxa, 3814(1):37-67. https://doi.org/10.11646/zootaxa.3814.1.2
Beier, M. 1937. Pseudoscorpions from Baltic amber, Festschrift zum 60. Geburtstage von Professor Dr. Emrik Strand, Riga, 2:302-316.
Beier, M. 1947. Pseudoscorpions in Baltic amber and the investigation of amber inclusions, microscopy, Vienna, 1:188-199.
Beier, M. 1955. Pseudoscorpions in Baltic amber from the Geological State Institute in Hamburg, communication. Mining and Geology State Institute Hamburg, 25:48-54.
Bhandari, A., Singh, H. and Rana, R.S. 2005. A note on the occurrence of Ostracoda from the Vastan Lignite Mine, Gujarat. Journal Palaeontological Society of India, 50:141-146.
Biswas, S.K., Bhasin, A.L. and Jokhan, R. 1993. Classification of Indian sedimentary Basins in the framework of plate tectonics. Proceedings Second Seminar on Petroliferous Basins of India, 1:1-46.
Chamberlin, J.C. 1931. The arachnid order Chelonethida. Stanford University Publications. Biological Sciences, 7(1):1-284.
Cockerell, T.D.A. 1917. Arthropods in Burmese amber. American Journal of Science. 4(44):360- 368.
Cockerell, T.D.A. 1920. Fossil arthropods in the British Museum. Annals and Magazine of Natural History, 9(5):273-279.
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fab, J.X. 2013. The ICS International Chronostraigraphic Chart. Episodes, 36: 199-204.
https://doi.org/10.18814/epiiugs/2013/v36i3/002
Cramer, B.S., Wright, J.D., Kent, D.V., and Aubry, M.P. 2003. Orbital climate forcing of δ 13C excursions in the late Paleocene-early Eocene (chrons C24n-C25n). Paleoceanography, 18(4):1- 25.
https://doi.org/10.1029/2003pa000909
Dunlop, J.A., Kotthoff, U., Hammel, J.U., Ahrens, J., and Harms, D. 2018. Arachnids in Bitterfeld amber: a unique fauna of fossils from the heart of Europe or simply old friends? Evolutionary Systematics, 2: 31-44.
https://doi.org/10.3897/evolsyst.2.22581
Dutta, S. and Mallick, M. 2017. Chemical evidence for dammarendiol, a bioactive angiosperm metabolite, from 54 Ma old fossil resins. Review of Palaeobotany and Palynology, 237:96-99.
https://doi.org/10.1016/j.revpalbo.2016.11.004
Dutta, S., Mallick, M., Bertram, N., Greenwood, P.F., and Mathews, R.P. 2009. Terpenoid composition and class of Tertiary resins from India. International Journal of Coal Geology, 80:44-50.
https://doi.org/10.1016/j.coal.2009.07.006
Dutta, S., Mathews, R.P., Singh, B.D., Tripathi, S.K.M., Singh, A., Saraswati, P.K., Banerjee, S., and Mann, U. 2011. Petrology, palynology and organic geochemistry of Eocene lignite of Matanomadh, Kutch Basin, western India: implications to depositional environment and hydrocarbon source potential. International Journal of Coal Geology, 85:91-102.
https://doi.org/10.1016/j.coal.2010.10.003
Engel, M.S., Grimaldi, D.A., Nascimbene, P.C., and Singh, H. 2011. The termites of Early Eocene Cambay amber, with the earliest record of the Termitidae (Isoptera). Zookeys, 148:105-123.
https://doi.org/10.3897/zookeys.148.1797
Garg, R., Ateequzzaman, K., Prasad, V., Tripathi, S.K.M., Singh, I.B., Jauhri. A.K., and Bajpai. S. 2008. Age-diagnostic dinoflagellate cysts from the lignite-bearing sediments of the Vastan lignite mine, Surat District, Gujarat, western India. Journal Palaeontological Society of India, 53:99-105
Geoffroy, E.L. 1762. Abridged history of the insects found around Paris. Durand, Paris 2
Grimaldi, D.A. 1996. Amber: Window to the Past, American Museum of Natural History, H. N. Abrams Inc., New York
Grimaldi, D.A. and Singh, H. 2012. The extinct genus Pareuthychaeta in Eocene ambers (Diptera: Schizophora: Ephydroidea). The Canadian Entomologist, 144(1): 17-28.
https://doi.org/10.4039/tce.2012.5
Harms, D. and Dunlop, J.A. 2017. The fossil history of pseudoscorpions (Arachnida: Pseudoscorpiones). Fossil Record, 20: 215-238.
https://doi.org/10.5194/fr-20-215-2017
Harvey, M.S. 2011. Order Pseudoscorpiones de Geer, 1778, in: Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148:119-120.
https://doi.org/10.11646/zootaxa.3148.1.20
Harvey, M.S. 2013. Pseudoscorpions of the World, version 3.0. Western Australian Museum, Perth.
Harvey, M.S. 2014. A review and redescription of the cosmopolitan pseudoscorpion Chelifer cancroides (Pseudoscorpiones: Cheliferidae). The Journal of Arachnology, 42: 86-104.
https://doi.org/10.1636/k13-57.1
Harvey, M.S. and Volschenk, E.S. 2007. Systematics of the Gondwanan pseudoscorpion family Hyidae (Pseudoscorpiones: Neobisioidea): new data and a revised phylogenetic hypothesis. Invertebrate Systematics, 214: 365-406.
https://doi.org/10.1071/is05030
Harvey, M.S., Cosgrove, J.G., Harms, D., Selden, P.A., Shih, C., and Wang, C.C. 2018. The oldest chthonioid pseudoscorpion Arachnida: Pseudoscorpiones: Chthonioidea: Chthoniidae: A new genus and species from mid-Cretaceous Burmese amber. Zoologischer Anzeiger, 273:102-111.
https://doi.org/10.1016/j.jcz.2017.12.009
Heinrichs, J., Scheben, A., Bechteler, J., Lee, G.E., Schafer-Verwimp, A., and Hedenas, L. 2016. Crown Group Lejeuneaceae and Pleurocarpous Mosses in Early Eocene (Ypresian) Indian Amber. PLoS ONE, 11(5):e0156301.
https://doi.org/10.1371/journal.pone.0156301
Henderickx, H. 2005. A new Geogarypus from Baltic amber (Pseudoscorpiones:Geogarypidae). Phegea, 33:87-92
Henderickx, H. and Perkovsky, E.E. 2012. The first geogarypid (Pseudoscorpiones, Geogarypidae) in Rovna Amber (Ukraine). Vestnik Zoologii, 46:33-36.
Henderickx, H. and Boone, M. 2016. The basal pseudoscorpion family Feaellidae Ellingsen, 1906 walks the Earth for 98.000.000 years: a new fossil genus has been found in Cretaceous Burmese amber (Pseudoscorpiones: Feaellidae). Entomo-Info, 27:7-12.
Henderickx, H., Perkovsky, E.E., Van Hoorebeke, L., and Boone, M. 2013. The first pseudogarypid in Rovno amber (Ukraine) (Pseudoscorpiones: Pseudogarypidae). Phegea, 41:90-92.
Hoff, C.C. 1963. Sternophorid pseudoscorpions, chiefly from Florida, American Museum Noviates, 2150:1-14.
Hong Y.C. 1983. Discovery of new fossil pseudoscorpionids in amber. Bulletin of the Tianjin Institute of Geology and Mineral Resources, 8:24-29.
Johnson, J., Romero-Ortiz, C., Mathew, A.V., Sebastian, P.A., Joseph, M.M., and Harvey, M.S. 2019. A review of the pseudoscorpion genus Metawithius (Pseudoscorpiones: Withiidae) from the Indian subcontinent. The Journal of Arachnology, 47(1):84-94.
https://doi.org/10.1636/0161-8202-47.1.84
Judson, M.L.I. 2003. Baltic amber pseudoscorpions (Arachnida: Chelonethi): a new species of Neobisium (Neobisiidae) and the status of Obisium rathkii Koch and Berendt. Geodiversitas, 25:445-450.
Judson, M.L.I. 2007. First fossil record of the pseudoscorpion family Pseudochiridiidae (Arachnida, Chelonethi, Cheirioidea) from Dominican amber. Zootaxa, 1393:45-51.
https://doi.org/10.11646/zootaxa.1393.1.5
Judson, M.L. 2012. Reinterpretation of Dracochela deprehendor (Arachnida: Pseudoscorpiones) as a stem‐group pseudoscorpion. Palaeontology, 55(2):261-283.
https://doi.org/10.1111/j.1475-4983.2012.01134.x
Kania, I., Wang, B., and Szwedo, J. 2015. Dicranoptycha Osten Sacken, 1860 (Diptera, Limoniidae) from the earliest Cenomanian Burmese amber. Cretaceous Research, (52)b:522-530.
https://doi.org/10.1016/j.cretres.2014.03.002
Khan, M.A., Spicer, R.A., Spicer, T.E., Roy, K., Hazra, M., Hazra, T., Mahato, S., Kumar, S., and Bera, S. 2020. Dipterocarpus (Dipterocarpaceae) leaves from the K-Pg of India: a Cretaceous Gondwana presence of the Dipterocarpaceae. Plant Systematics and Evolution, 306:1-18.
https://doi.org/10.1007/s00606-020-01718-z
Koch, C.L. and Berendt, G.C. 1854. Die im Bernstein befindlichen Crustaceen, Myriapoden, Arachniden und Apteren der Vorwelt. Die in Bernstein befindlichen organischen Reste der Vorwelt gesammelt in Verbindung mit mehreren bearbeitetet und herausgegeben, 1:1-124
Kramera, J., Pohlb, H., and Predela, R. 2019. Venom collection and analysis in the pseudoscorpion Chelifer cancroides (Pseudoscorpiones: Cheliferidae). Toxicon, 162:15-23.
https://doi.org/10.1016/j.toxicon.2019.02.009
Mayr, G. and Smith T. 2019. A diverse bird assemblage from the Ypresian of Belgium furthers knowledge of early Eocene avifaunas of the North Sea Basin. Neues Jahrbuch für Geologie und Paläontologie, 291(3):253-281.
https://doi.org/10.1127/njgpa/2019/0801
Murienne, J., Harvey, M.S., and Giribet, G. 2008. First molecular phylogeny of the major clades of Pseudoscorpiones (Arthropoda: Chelicerata). Molecular Phylogenetics and Evolution, 49 (1):179-184.
https://doi.org/10.1016/j.ympev.2008.06.002
Naglik, B., Kosmowska-Ceranowicz, B., Natkaniec-Nowak, L., Drzewicz, P., Duma’nska Slowiki, M., Matusik, J., Wagner, M., Milovsky, R., Stach, P., and Szyszka, A. 2018. Fossilization history of fossil resin from Jambi Province (Sumatra, Indonesia) based on Pchysico-Chemical Studies. Minerals, 8(95):1-13.
https://doi.org/10.3390/min8030095
Nicolo, M.J., Dickens, G.R., Hollis, C.J., and Zachos J.C. 2007. Multiple early Eocene hyperthermals: Their sedimentary expression on the New Zealand continental margin and in the deep sea. Geology, 35(8):699-702.
https://doi.org/10.1130/g23648a.1
Novak, J. and Harvey, M.S. 2018. New species and records of the pseudoscorpion genus Geogarypus (Pseudoscorpiones: Geogarypidae) from India, Sri Lanka and New Guinea. Zootaxa, 4394(3):417-427.
https://doi.org/10.11646/zootaxa.4394.3.7
Ontano, A.Z., Gainett, G., Aharon, S., Ballesteros, J.A., Benavides, L.R., Corbett, K.F., Gavish-Regev, E., Harvey, M.S., Monsma, S., Santibáñez-López, C.E., and Setton, E.V. 2021. Taxonomic sampling and rare genomic changes overcome long-branch attraction in the phylogenetic placement of pseudoscorpions. Molecular Biology and Evolution, 38(6): 2446-2467.
Ortega-Blanco, J., Chatzimanolis, S., Singh, H., and Engel, M.S. 2013. The oldest fossil of the subfamily Osoriinae (Coleoptera: Staphylinidae), from Eocene Cambay amber (India). The Coleopterists Bulletin, 67(3):304-308.
https://doi.org/10.1649/0010-065x-67.3.304
Perrichot, V. 2004. Early Cretaceous amber from south-western France: insights into the Mesozoic litter fauna. Geologica Acta, 2:9-22
Penney, D. 2008. Dominican Amber Spiders, Siri Scientific Press, Manchester, UK
Prasad, V., Singh, I.B., Bajpai, S., Garg, R., Thakur, B., Singh, A., Saravanan, N., and Kapur, V.V. 2013. Palynofacies and sedimentology-based high-resolution sequence stratigraphy of the lignite bearing muddy coastal deposits (early Eocene) in the Vastan Lignite Mine, Gulf of Cambay, India. Facies, 59:737-761.
https://doi.org/10.1007/s10347-012-0355-8
Punekar, J. and Saraswati, P.K. 2010. Age of the Vastan lignite in context of some oldest Cenozoic fossil mammals from India. Journal of the Geological Society of India, 76:63-68.
https://doi.org/10.1007/s12594-010-0076-y
Rana, R.S., Singh, H., Sahni, A., Rose, K.D., and Saraswati, P.K. 2005. Early Eocene Chiropterans from a new mammalian assemblage (Vastan Lignite mine, Gujarat, Western Peninsular margin): Oldest known bats from Asia. Journal Palaeontological Society of India, 50(1):93-100
Rana, R.S., Kumar,K., Escarguel, G., Sahni, A., Rose, K.D., Smith, T., Singh, H., and Singh, L. 2008. An ailuravine rodent from the lower Eocene Cambay Formation at Vastan, western India, and its palaeobiogeographic implications. Acta Palaeontologica Polonica, 53:1-14.
https://doi.org/10.4202/app.2008.0101
Rao, M.R., Sahni, A., Rana, R.S., and Verma, P. 2013. Palynostratigraphy and depositional environment of Vastan Lignite Mine (Early Eocene), Gujarat, western India. Journal of Earth System Science, 122(2):289-307.
https://doi.org/10.1007/s12040-013-0280-4
Rose, K.D., Rana, R.S., Sahni, A., Kumar, K., Missiaen, P., Singh, L., and Smith, T. 2009. Early Eocene Primates from Gujarat, India. Journal of Human Evolution, 56:329-438.
https://doi.org/10.1016/j.jhevol.2009.01.008
Rust, J., Singh, H., Rana, R.S., Mccann, T., Singh, U., Anderson, K., Sarkar, N., Nascimbene, P.C., Stebner, F., Thomas, J.C., Kraemer, M.S., Williams, C.J., Engel, M.S., Sahni, A., and Grimaldi, D. 2010. Biogeographic and evolutionary implications of a diverse paleobiota in amber from the early Eocene of India. Proceedings of the National Academy of Sciences, 107(43):18,360-18,365.
https://doi.org/10.1073/pnas.1007407107
Sahni, A., Saraswati, P.K., Rana, R.S., Kumar, K., Singh, H., Alimohammadian, H., and Sahni. N. 2006. Temporal constraints and depositional environments of the Vastan Lignite sequence, Gujarat: analogy for the Cambay Shale Hydrocarbon source rock. Indian Journal of Petroleum Geology, 15:1-20
Schawaller, W. 1978. New pseudoscorpions from the Baltic amber of the Stuttgart amber collection (Arachnida: Pseudoscorpionidea), Stuttgarter Beitr. Naturk. (B), 42:1-21.
Schawaller, W. 1980. Fossile Chthoniidae in Dominikanischem Bernstein, mit phylogenetischen Anmerkungen (Stuttgarter Bernsteinsammlung: Arachnida, Pseudoscorpionidea). Stuttgarter Beiträge zur Naturkunde 63(B):1-19.
Schawaller, W. 1981. Cheiridiidae in Dominikanischem Bernstein, mit Anmerkungen zur morphologischen Variabilität (Stuttgarter Bernsteinsammlung: Arachnida, Pseudoscorpionidea). Stuttgarter Beiträge zur Naturkunde 75(B):1-14.
Schwarze, D., Harms, D., Hammel. J.U., and Kotthoff, U. 2021. The first fossils of the most basal pseudoscorpion family (Arachnida: Pseudoscorpiones: Pseudotyrannochthoniidae): evidence for major biogeographical shifts in the European paleofauna. PalZ, 96:11-27.
https://doi.org/10.1007/s12542-021-00565-8
Shear, W.A., Schawaller, W., and Bonamo, P.M. 1989. Record of Palaeozoic pseudoscorpions. Nature, 341:527-529.
https://doi.org/10.1038/341527a0
Shi, G., Dutta, S., Paul, S., Wang, B., and Jacques, F.M.B. 2014. Terpenoid compositions and botanical origins of late cretaceous and Miocene amber from China. PLoS ONE, 9(10):1-8.
https://doi.org/10.1371/journal.pone.0111303
Shukla, A., Singh, H., and Mehrotra, R.C. 2015. A fossil wood of Gynocardia from the Valia lignite mine, Bharuch District, Gujarat. Palaeobotanist, 64:163-168.
https://doi.org/10.54991/jop.2015.111
Simoneita, B.R.T, Orosb, D.R., Karwowskic, L., Szenderad, L., Smolarek-Lachc, J., Goryle, M., Buchac, M., Rybickic, M., and Marynowski, L. 2020. Terpenoid biomarkers of ambers from Miocene tropical paleoenvironments in Borneo and of their potential extant plant sources. International Journal of Coal Geology, 221:103430.
https://doi.org/10.1016/j.coal.2020.103430
Singh, H., Prasad, M., Kumar, K., and Singh, S.K. 2011. Paleobotanical remains from the Paleocene-lower Eocene Vagadkhol Formation, western India and their paleoclimatic and phytogeographic implications. Palaeoworld, 20(4):332-356.
https://doi.org/10.1016/j.palwor.2011.04.002
Singh, H., Prasad, M., Kumar, K., Rana, R.S., and Singh, S.K. 2015. Early Eocene macroflora and associated palynofossils from the Cambay Shale Formation, western India: phytogeographic and palaeoclimatic implications. Palaeoworld, 24:293-324.
https://doi.org/10.1016/j.palwor.2015.05.002
Singh, H., Judd, W.S, Samant, B., Agnihotri, P, Grimaldi, D. A., and Mancester, S.R. 2021. Flowers of Apocynaceae in amber from the early Eocene of India. American Journal of Botany, 108(5):1-10.
https://doi.org/10.1002/ajb2.1651
Singh, V.P., Singh, B.D., Mathews, R.P., Mendhe, V.A., Agnihotri, P., Mishra, S., Radhwani, M., Dutta, S., Subramanina, K.A., and Singh, H. 2021. Petro-geochemical characteristics and floral-faunal composition of Valia lignite deposits of Cambay Basin (western India), in relation with palaeoenvironment, palaeoecology, depositional settings and hydrocarbon generation potential. International Journal of Coal Geology, 248:103866.
https://doi.org/10.1016/j.coal.2021.103866
Smith, T., Rana, R.S., Missiaen, P., Rose, K. D., Sahni, A., Singh, U., and Singh, L. 2007. High bat (Chiroptera) diversity in the Early Eocene of India. Naturwissenschaften, 99:1003-1009.
https://doi.org/10.1007/s00114-007-0280-9
Stebner, F., Szadziewski, R., Ruhr, P.T., Singh, H., Hammel, J., Kvifte, G.M., and Rust, J. 2016. A fossil biting midge (Diptera: Ceratopogonidae) from early Eocene amber with a complex pheromone evaporator. Scientific Reports, 6(1):34352.
https://doi.org/10.1038/srep34352
Stebner, F., Szadziewski, R., Singh, H., Gunekel, S., and Rust, J. 2017. Biting midges (Diptera: Ceratopogonidae) from Cambay amber indicate that the Eocene fauna of the Indian Subcontinent was not isolated. PLoS ONE, 12(1):e0173135.
https://doi.org/10.1371/journal.pone.0169144
Sudhakar, R. and Basu, D.N. 1973. A reappraisal of the stratigraphy of southern Cambay Basin. Oil and Natural Gas Co-operation Bulletin, 10:55-76.
Whalley, P.E.S. 1980. Neuroptera (Insecta) in amber from the Lower Cretaceous of Lebanon. Bulletin of the British Museum (Natural History). Geology, 33:157-164.
Xing, L., McKellar, R.C., and Zhizhong, G. 2018. Cretaceous hitchhikers: a possible phoretic association between a pseudoscorpion and bird in Burmese amber. Acta Geologica Sinica, 92(6):2434-2435.
https://doi.org/10.1111/1755-6724.13739
Zachos, J.C., Mccarren, H., Murphy, B., Rohl, U., and Westerhold, T. 2010. Tempo and scale of late Paleocene and early Eocene carbon isotope cycles: Implications for the origin of hyperthermals. Earth and Planetary Science Letters, 299(1-2):242-249.
https://doi.org/10.1016/j.epsl.2010.09.004

