 Unravelling the origin of the brown hyena (Parahyena brunnea) and its evolutionary and paleoecological implications for the Pachycrocuta lineage
Unravelling the origin of the brown hyena (Parahyena brunnea) and its evolutionary and paleoecological implications for the Pachycrocuta lineage
Article number: 27.1.a18
https://doi.org/10.26879/1372
Copyright Palaeontological Association, March 2024
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 29 January 2024. Acceptance: 1 March 2024.
ABSTRACT
The dentition of several species of hyenas within the lineages leading to Parahyaena brunnea and Pachycrocuta brevirostris was analysed from a multivariate point of view. The probable origin of these lineages can be traced back to China in the late Miocene or early Pliocene, from an initial stock that dispersed across the Old World during the Zanclean. This ancestral stock is believed to comprise Parahyaena howelli and Pliocrocuta perrieri from the Zanclean and is suggested to be grouped into Pachycrocuta pyrenaica. The subsequent Pliocrocuta perrieri arose from such a stock and is proposed to be reassigned to Pachycrocuta perrieri. The findings obtained substantiate the hypothesis that Pachycrocuta bellax represents a species distinct from Pachycrocuta brevirostris. Pachycrocuta perrieri is posited as the ancestor of Pachycrocuta brevirostris in Eurasia, and Parahyaena brunnea in Africa. The sequence Pachycrocuta pyrenaica, Pachycrocuta perrieri, and Pachycrocuta brevirostris delineates an evolutionary trend marked by greater specialization in the scavenging niche, manifested through augmented overall dentition size and development of bone-breaking part of the dentition at the expense of a reduction in its cutting region. Present-day forms of Parahyaena brunnea also exhibit such specialization, but the size of the dentition is similar to that of Pachycrocuta perrieri. ‘Hyaena’ prisca is considered a basal form of Parahyaena brunnea, akin to specimens recovered from Elandsfontein approximately 1 million years ago, which recolonized Europe following the extinction of Pachycrocuta brevirostris. The degree of divergence of the extant forms of Parahyaena brunnea from ‘Hyaena’ prisca justifies their classification as distinct species. A proposal is made to integrate both the brown hyena and ‘Hyaena’ prisca into the genus Pachycrocuta.
Juan Antonio Pérez-Claros. Departamento de Ecología y Geología, Facultad de Ciencias, Universidad de Málaga. Campus de teatinos s/n. 29071 Málaga, Spain. johnny@uma.es
Keywords: Brown hyena evolution; Pliocrocuta; Pachycrocuta; Parahyaena; Paleobiogeography; Ecomorphology
Final citation: Pérez-Claros, Juan Antonio. 2024. Unravelling the origin of the brown hyena (Parahyena brunnea) and its evolutionary and paleoecological implications for the Pachycrocuta lineage. Palaeontologia Electronica, 27(1):a18.
https://doi.org/10.26879/1372
palaeo-electronica.org/content/2024/5173-the-origin-of-the-brown-hyena
Copyright: March 2024 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
One of the central species of this paper is ‘Hyaena perrieri’ or hyena of Perrier, as originally named by Croizet and Jobert in 1828. The fossil assemblage where this hyena was described has special significance in the beginning of both Paleontology and Geology. Such an assemblage was composed of extinct species of still extant genera, which together with the fact that the materials where the remains were recovered were stratigraphically far below lava flows covering the region, was used by Lyell and Murchison (1829) as arguments to indicate that such a fossil assemblage was of immense antiquity and that the deep excavation of the valleys by the erosive action of the rivers observed in the French Auvergne region indicated current geological forces acting over an incalculable period of time. These arguments supported a uniformitarian interpretation as opposed to the catastrophist thesis maintained by Cuvier, which Buckland even tried to associate with the biblical deluge (Rudwick, 1985). More than 150 years later, Howell and Peter (1980) assigned ‘Hyaena perrieri’ to the genus Pachycrocuta and differentiated it from Pachycrocuta pyrenaica (Depéret, 1890). Subsequently, Werdelin and Solounias (1991) synomized both, reassigning them to Pliocrocuta perrieri.
Pliocrocuta perrieri is one of the most abundant and widespread hyenas during the Pliocene of the Old World. This species exhibits dental adaptations typical of the ecomorph fully developed bone-crackers of Werdelin and Solounias (1996). This type of trophic adaptation has arisen independently within the family Hyaenidae on several occasions in both, the subfamily Percrocutinae (Pérez-Claros, 2023) and the subfamily Hyaeninae, with Allohyaena sarmatica (Semenov, 1994) being the first representative of the latter subfamily at the beginning of the Tortonian (Pérez-Claros, 2022).
The durophagous ecomorph can be further subdivided into two distinct sets of adaptations that are metrically reflected in their dentition (Coca-Ortega and Pérez-Claros, 2019; Pérez-Claros and Coca-Ortega, 2020): social hunting durophages with the spotted hyena (Crocuta crocuta [Erxleben, 1777]) as its only present-day representative and solitary scavenging durophages with the striped and brown hyena (Hyaena hyaena [Linnaeus, 1758] and Parahyaena brunnea [Thunberg, 1820], respectively) as living species of the latter. Such differences may explain the sympatry of C. crocuta with the other two hyenas and the allopatry between H. hyaena and Pa. brunnea, since the similarity in body size and ecomorphological adaptations between them can be interpreted in terms of competitive exclusion (Pérez-Claros, 2022).
On the other hand, the common adaptations observed in H. hyaena and Pa. brunnea are the consequence of an evolutionary convergence, since they come from ancestors that had not developed the fully developed durophagous characteristics they share. In fact, the ecomorphological analysis by Coca-Ortega and Pérez-Claros (2019) showed that Parahyaena howelli (Werdelin, 2003), an early member of the Pa. brunnea lineage according to Werdelin and Lewis (2008), although nominally associated with the fully developed bone cracker ecomorph, shows a position in the morphospace of both the upper and lower dentition relatively close to the transitional bone cracker ecomorph of Werdelin and Solounias (1996). Since Ikelohyaena abronia (Hendey, 1974), which is an early member of the lineage that gave rise to H. hyaena, also belongs to the transitional bone cracker ecomorph, the fully durophagous characteristics observed in H. hyaena and Parahyaena brunnea must have evolved independently. On the other hand, considering that Pa. howelli is known only from the localities of Kanapoi and Laetoli (Werdelin, 2003; Werdelin and Dehghani, 2011) both of Zanclean age around 4 Ma, the separation of the lineages that gave rise to H. hyaena and Pa. brunnea must have taken place earlier, well into the early Pliocene or even the late Miocene.
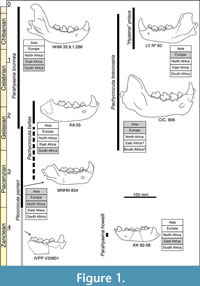 Parahyaena brunnea and Pl. perrieri show adaptations typical of the solitary scavenging durophagous ecomorph (Coca-Ortega and Pérez-Claros, 2019; Pérez-Claros and Coca-Ortega, 2020). Both forms are evolutionarily close although their exact phylogenetic relationships have been interpreted differently by different authors. Figure 1 shows some examples of mandibles, stratigraphic ranges, and geographic occurrences of those scavenging durophagous species related to the Pa. brunnea lineage (as indicated, the rest of the extant durophagous hyenas: H. hyaena and C. crocuta do not belong to such a lineage).
Parahyaena brunnea and Pl. perrieri show adaptations typical of the solitary scavenging durophagous ecomorph (Coca-Ortega and Pérez-Claros, 2019; Pérez-Claros and Coca-Ortega, 2020). Both forms are evolutionarily close although their exact phylogenetic relationships have been interpreted differently by different authors. Figure 1 shows some examples of mandibles, stratigraphic ranges, and geographic occurrences of those scavenging durophagous species related to the Pa. brunnea lineage (as indicated, the rest of the extant durophagous hyenas: H. hyaena and C. crocuta do not belong to such a lineage).
Both Kurtén (1954) and Howell and Petter (1980) considered that Pa. brunnea and Pl. perrieri came from a common ancestor in the Pliocene. Howell and Petter (1980) specified that this common ancestor was P. pyrenaica. That is, according to the scheme followed by Howell and Petter (1980), Pachycrocuta pyrenaica would be the ancestor of both P. perrieri and Pa. brunnea. On the other hand, Turner (1990) proposes that P. perrieri and Pa. brunnea would be conspecific. The similarity between these species is undeniable, and according to Turner (1990), if P. perrieri were recovered from a site in South Africa, it would be directly assigned to Pa. brunnea.
As indicated above, Werdelin and Solounias (1991) grouped Pachycrocuta pyrenaica and Pachycrocuta perrieri within Pliocrocuta perrieri on the grounds that the metric distinction made by Howell and Petter (1980) mixed specimens and individuals, which concealed variation within Pliocrocuta perrieri. However, the discrepancy between Werdelin and Solounias (1991) and Howell and Petter (1980) is more taxonomic than evolutionary in nature. Indeed, the former recognize that specimens of Pliocrocuta perrieri from the Zanclean (i.e., Pachycrocuta pyrenaica sensu Howell and Petter, 1980), considered as a whole, tend to have more primitive characteristics than those from the Piacenzian and Gelasian, which gradually change to the condition seen in Pachycrocuta brevirostris.
Werdelin and Solounias (1991) also grouped within Pliocrocuta perrieri another form unique to the Middle Pleistocene of Europe known as ‘Hyaena’ prisca. Such grouping creates a discontinuity within the stratigraphic range of Pliocrocuta perrieri, since this species disappears from the fossil record at the end of the Gelasian. Recently, Lannucci et al. (2021) have shown metric arguments that reject the conspecificity of Pliocrocuta perrieri and ‘Hyaena’ prisca.
Finally, there are two additional species of the genus Pachycrocuta phylogenetically close to the species previously considered: P. brevirostris (Gervais, 1850) and P. bellax (Ewer, 1954a). Pachycrocuta brevirostris is widely known from the Early Pleistocene of Eurasia, disappearing from Europe at the end of the Early Pleistocene (Lannucci et al., 2021), although it persisted in China until the Middle Pleistocene (Liu et al., 2021). There is some consensus that Pliocrocuta perrieri is the ancestor of P. brevirostris (Howell and Petter, 1980; Qiu, 1987; Werdelin and Solounias, 1991). The clearest difference between Pl. perrieri and P. brevirostris is the larger size of the latter in addition to the fact that the former has a metaconid on the first lower molar and in general P. brevirostris does not. Ecomorphological and paleoecological studies show that this species would be a highly specialized scavenger (Palmqvist et al., 2011; Pérez-Claros and Coca-Ortega, 2020).
Pachycrocuta bellax is known from few remains in South Africa. Turner (1990) suggested its conspecificity with P. brevirostris, and although its relationship to the Pliocrocuta perrieri - P. brevirostris lineage seems clear (Howell and Petter, 1980; Werdelin and Solounias, 1991), certain primitive features (e.g., a clearly differentiated metaconid) do not allow it to be clearly integrated into P. brevirostris without a re-evaluation of the variation of the latter (Werdelin and Solounias, 1991). In fact, Howell and Petter (1980) indicated that P. bellax has some characteristics that resemble P. perrieri and others resemble P. brevirostris.
Although some morphological data that have served as a basis for establishing phylogenetic relationships between the hyena species analysed here are certainly important, metric data such as those studied in the present study are indispensable. Morphometric analyses are necessary as some morphological data, such as the presence/absence of a metaconid or the number of cusps of the talonid of the lower molar, have been questioned in some cases for taxonomic classification as they are certainly variable (e.g., Kurtén, 1956). A classic and widely used method of analysing metric data for phylogenetic purposes has been the use of ratio diagrams (e.g., Kurtén, 1956; Howell and Petter, 1980; Werdelin and Solunias, 1991). Another way to analyse metric data to establish phylogenetic relationships is by coding them as discrete variables to be subsequently incorporated into cladistic analyses (e.g., Werdelin and Solunias, 1991).
In addition to the discovery of new paleontological sites, important compilations of metric data have been published in recent years (e.g., Liu et al., 2021; Lewis and Werdelin, 2022), which allow evaluation of the degree of overlap and morphological continuity of the species of this and other lineages from a multivariate statistical point of view (e.g., Pérez-Claros et al., 2021). Lannucci et al. (2021) assessed with such methodology the relationships among most species of this lineage together with other social durophages, including living C. crocuta. The results obtained in such a study, like others published previously (Coca-Ortega and Pérez-Claros, 2019; Pérez-Claros and Coca-Ortega, 2020), indicated a clear separation between the two types of durophagous ecomorphs based on the relative increase in the length of the lower carnassial in the case of social hunters (e.g., genus Crocuta). On the other hand, Lannucci et al. (2021) obtained some separation in size between the different species belonging to each type of durophages, although the degree of overlap in morphology for the species that integrate each category is remarkable.
The aim of the present work is to re-evaluate the relationships between the species that are part of or closely related to the lineage leading to Pa. brunnea and P. brevirostris from a multivariate metric perspective and its paleoecological and paleogeographical implications. For this purpose, the sample analysed has been expanded with respect to previous studies both qualitatively and quantitatively, also including the upper dentition. In addition, the samples are analysed at the individual level as well as at the fossil assemblage level by averaging the different variables in each fossil locality. This makes it possible to analyse fossiliferous localities that, although providing several remains, lack complete individuals. In cases where there are a reasonable number of remains, the mean can be considered a value close to the population mean. Finally, in all analyses, the three living durophagous species have been incorporated to evaluate the effect of individual versus population variation.
MATERIALS AND METHODS
The analysed sample consists of the mesio-distal lengths (L) and bucco-lingual widths (W) of the last two lower premolars and first molar (p3, p4, m1) and the last three upper premolars (P2, P3, P4) for the three living durophagous hyenas (H. hyaena, Pa. brunnea, and C. crocuta) as well as for the previously mentioned extinct species: Pa. howelli, Pl. perrieri, P. brevirostris, P. bellax, and ‘H.’ prisca.
For certain analyses the sample of Pl. perrieri has been divided into the Zanclean specimens and those of later age (Piacenzian and Gelasian) as well as the sample of Pa. brunnea that has been divided into its living and fossil representatives. To complement the sample with fossil representatives of the hunting durophagous ecomorph, C. spelaea (sensu Lewis and Werdelin, 2022) has been included in the analyses, since this species is known from a high number of remains and is well delimited both geographically and temporally. The sample sizes for each species are listed in Table 1.
Virtually all data come from literature sources, except for certain specimens of H. hyaena from the Western Sahara obtained from the Museum of the Estación Biológica de Doñana (Seville, Spain). The length of the trigonid of m1, if not explicitly reported by the authors, has been estimated directly from photographs of the specimens (if figured), following Werdelin and Solounias (1991). The numerical age assigned to the paleontological sites was the midpoint of the reported time interval. All measurements and approximate ages of the specimens related to the lineages under study, as well as the references where they have been extracted, are shown in the Appendix 1.
Four individuals attributed to H. hyaena by Cardoso (1993) and two to Hyaena sp. by Geib (1915) have been excluded from the analysed sample of extant species. A previous discriminant analysis showed in the first case that the upper or lower dentition (or both) of such specimens (MNHN 1877-113, MNHN 1922-301, MNHN A-1536, and MNHN A-7940) were attributed to Pa. brunnea with a probability close to 1. In the case of Geib (1915), one of the dentitions (upper or lower) of the specimens A.1.14 and 583/A. 235b was attributed to Pa. brunnea and the other to H. hyaena, with probabilities close to 1. It is not possible to ascertain whether these are nomenclatural errors of the museum or truly atypical H. hyaena individuals. Atypical individuals, although perfectly acceptable from a biological point of view, can negatively affect statistical procedures (Reyment, 1990), and for this reason have been excluded. Nevertheless, the sample size of H. hyaena analysed was comparatively high (N = 56, Table 1), so it is expected that the sample reflects the biological variability of this species.
The specimen from the Lunel-Viel site (France) LVI-4-1412 attributed to Crocuta spelaea intermedia by Bonify (1971) has been assigned to ‘Hyaena’ prisca fossils in agreement with Lewis and Werdelin (2022). Specimen CD 3218 from the Cooper’s Cave site (South Africa) assigned to Pa. brunnea by Kuhn et al. (2017) has not been incorporated into the analysis since the values fall outside the range observed for such a species (Kuhn et al., 2017, figure 2c shows that CD 3218 projects outside the convex hull of Pa. brunnea and within the region occupied by C. crocuta and C. ultra).
Specimens HMV 1201 and HMV 1202 from the Londang site (Linxia Basin, China) attributed to Pachycrocuta licenti by Qiu et al. (2004) have been initially attributed to Pl. perrieri according to Lannucci et al. (2021), although as will be discussed below they are intermediate forms between Pl. perrieri and P. brevirostris (as indicated by Qiu et al., 2004 in the diagnosis of P. licenti). Finally, specimen V 7296 from Baihaicum locality 26 (Yushe Basin, China) attributed to Crocuta honanensis by Qiu (1987) has been considered Pl. perrieri; the justification for this assignment is presented below in the results section. These three specimens have not been included within Pl. perrieri in the plots and have been represented with different symbols to facilitate identification.
The data corresponding to the fossils have been analysed at the individual level for those cases where it is possible to estimate the values for the six variables in the same individual and also at the fossil locality level by averaging the values of all the individuals. In some cases, the data for the individual and the population coincide since only a single complete individual is known. The different levels (or cave members) of the fossil localities have been considered as independent samples in case they have been differentiated. Similarly, the early and late forms of Pa. brunnea described by Hendey (1974) in Elandsfontein have been considered as independent observations. In order to have a frame of reference for intraspecific variability, the living species have not been grouped into populations.
Principal component analysis was performed independently for both lower and upper dentition variables using covariance matrices since all variables are measured on the same scale. For the test of multivariate means the non-parametric multivariate analysis of variance (PERMANOVA) of Anderson (2001) was used with Euclidean distances between scores on the retained principal components, using 9999 replicates. PERMANOVA is more robust to heterogeneity in balanced designs than other nonparametric rank-based alternatives such as Clarke’s (1993) Analysis of Similarities (ANOSIM) and is more specific for testing the differences in centroids (Anderson and Walsh, 2013). This is especially important due to the nature of the data analysed here given that there are differences in the correlations between variables as well as in the sample sizes of the groups analysed. Bonferoni’s sequential p-values have been considered for the acceptance or rejection of the null hypotheses in the case of multivariate tests. All analyses were performed using free software for scientific data analysis PAST v. 3.24 (Hammer et al., 2001).
RESULTS
Attribution of V 7296 to Pliocrocuta perrieri
As discussed in the previous section, specimen V 7296 described by Qiu (1987) from the Baihaicum locality has been assigned to Pl. perrieri rather than to C. honanensis based on the morphology and metrics of m1. This specimen is severely worn and difficult to identify. Paradoxically, this fact has some advantages, given that hunting-durophages are distinguished from scavengers in the type of wear. One aspect derived from the relative shortening of m1 (while maintaining its width) shown by solitary scavengers versus social hunters (i.e., genus Crocuta) is that the wear facets of m1 in the former tend to be wider and bilobed, whereas in the latter, they are more elongated and continuous.
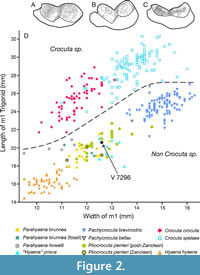 Figure 2A shows the m1 of V 7296 in lingual view, whereas Figure 2B-C show a worn m1 of Pl. perrieri and C. crocuta, respectively. As can be seen, V 7296 is more similar to Pl. perrieri than to Crocuta. The attribution of V 7296 to Pl. perrieri is further corroborated by its position on the plot of the length of the trigonid against its width (Figure 2D). This plot makes it possible to clearly discriminate the genus Crocuta from the rest of the species. Such a plot for m1 serves the same purpose as figure 2 of Kurtén (1956), which shows the metastyle length versus the total length for P4 for discrimination of Crocuta. The attribution of V 7296 to Pl. perrieri makes the fossil assemblage from Baihaicum more typical of the Pliocene, as it is relatively common to find Pl. perrieri together with the more slender hyaenid Chasmaporthetes, which has also been recovered at such a locality.
Figure 2A shows the m1 of V 7296 in lingual view, whereas Figure 2B-C show a worn m1 of Pl. perrieri and C. crocuta, respectively. As can be seen, V 7296 is more similar to Pl. perrieri than to Crocuta. The attribution of V 7296 to Pl. perrieri is further corroborated by its position on the plot of the length of the trigonid against its width (Figure 2D). This plot makes it possible to clearly discriminate the genus Crocuta from the rest of the species. Such a plot for m1 serves the same purpose as figure 2 of Kurtén (1956), which shows the metastyle length versus the total length for P4 for discrimination of Crocuta. The attribution of V 7296 to Pl. perrieri makes the fossil assemblage from Baihaicum more typical of the Pliocene, as it is relatively common to find Pl. perrieri together with the more slender hyaenid Chasmaporthetes, which has also been recovered at such a locality.
Principal Component Analysis. General Aspects
The first three principal components were retained in the four analyses performed because all of them could be biologically interpreted. The loading coefficients and explained variance are listed in Table 2. The three components encompassed more than 97% of the variance in all the four cases. The structures of the first three components were relatively similar in all the analyses. The first component reflected the dentition size and explained the largest percentage of variance. The second component is an axis of variation in shape and expresses the relative shortening/elongation of carnassials (m1 or P4) with respect to the rest of the variables, explaining between 6% and 14% of the variance depending on the case. This second axis separates the scavenging durophagous forms (shortening of the carnassials) from the hunting durophagous forms (lengthening of the carnassials). In the case of the upper dentition, the reduction in the length of the carnassial is correlated with a clear increase in the length of the second premolar. The third component is also a shape axis, in which the lengths score negatively and the widths score positively. Although this principal component explained a relatively low percentage (between 1.4% and 2.3%), it can be clearly interpreted as a robustness/sectoriality axis of the dentition.
The Mantel test for the covariance matrices between the variables obtained for individuals and localities (N = 99999) is significant for both the lower (p = 0.004) and upper dentition (p = 0.001) indicating a similar structure for both matrices. In the case of individuals, another way to support the previous result is through correlations between the scores obtained directly in the PCA and those using the principal component loadings for the localities (where they have not participated in the construction of the components). In practically all cases, the correlation values were higher than 0.9 and significant with p < 10-53, both for the upper and lower dentition. These results indicate that the information reported by the analyses of individuals and localities was very similar.
The correlations between the scores obtained in the principal component analysis and those corresponding to the between group principal components (using species as groups) are higher than 0.94 (p < 10-76) for all principal components for both the analysis of individuals and paleontological sites and for both the lower and upper dentition. The only exception was the third component of the lower dentition, whose correlations were somewhat lower, but equally significant (p < 10-13). This indicates that the number of observations per species does not affect the overall results obtained, especially for the first two components of each analysis.
In the species of the lineages analysed, no significant differences were detected among the means for the third principal component, except between Pa. brunnea and Pl. perrieri for the upper dentition (individuals and localities), indicating a greater robustness of the former (p < 0.001). Since the percentage of variance explained by this component barely exceeds 2%, the following sections are focused on the first two principal components.
Principal Component Analysis. Lower Dentition
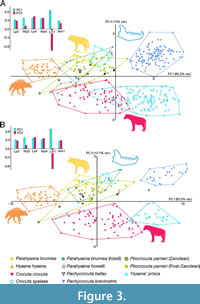 Figure 3A-B depicts the projections onto the first two principal components of the analysis for both the sample of individuals and paleontological sites, respectively. Both analyses yield similar results. The first component separates species based on size with H. hyaena at one end and P. brevirostris and C. spelaea at the other, while the second component separates hunting durophages such as C. crocuta from scavengers such as Pa. brunnea or P. brevirostris.
Figure 3A-B depicts the projections onto the first two principal components of the analysis for both the sample of individuals and paleontological sites, respectively. Both analyses yield similar results. The first component separates species based on size with H. hyaena at one end and P. brevirostris and C. spelaea at the other, while the second component separates hunting durophages such as C. crocuta from scavengers such as Pa. brunnea or P. brevirostris.
Pachycrocuta bellax is just at the limit of variation of P. brevirostris in the plot corresponding to individuals, but falls well outside the range in the fossil locality plot.
The two Longdan specimens are right on the border of Pl. perrieri, although in the region closest to P. brevirostris. Specimen V 7296 projects within the region of Pl. perrieri, which corroborates its assignment to Pl. perrieri made above.
A noteworthy aspect observed in Figure 3 is the significant correlation between size and shape (allometry) within Pl. perrieri, Pa. brunnea and P. brevirostris, both at the level of individuals and fossil locality (Table 3). The correlation in Pa. brunnea as a whole is due to the fossil sample, being absent in the extant sample. These results carry significance for taxonomy as organisms of the same species with different sizes can exhibit different morphology. A related noteworthy fact seen in Figure 3A-B is the relative continuity through the morphospace of the sequence Pl. perrieri from the Zanclean, Pl. perrieri from the Piacenzian and Gelasian and P. brevirostris, which favors the interpretation of a gradual evolution from the former to the latter.
Crocuta crocuta, C. spelaea, H. hyaena and P. brevirostris occupy distinctly separated regions in morphospace, resulting in PERMANOVA analyses showing statistically significant differences both among themselves and with the remaining species (p = 0.0001, both for individuals and localities).
As shown in Figure 3, in the case of the remaining species there is an enormous degree of overlap in the morphospace, although some increase in the value of the second component is observed from Pa. howelli to ‘H.’ prisca following the sequence: Pa. howelli, Pl. perrieri, Pa. brunnea (fossil), Pa. brunnea (living) and ‘H.’ prisca.
Table 4 summarizes the results of testing the null hypotheses of centroid equality. Similar results are obtained for both individuals and localities. Parahyaena howelli is excluded in the case of the localities since it has only been recovered in two of them (Kanapoi and Laetoli). It cannot be rejected that Pa. brunnea (fossil) is statistically different from any other species. Notably, Pl. perrieri from the Zanclean is statistically different from that from the Piacenzian and Gelasian, but its difference from Pa. howelli cannot be rejected. ‘Hyaena’ prisca is statistically different from Pa. brunnea (living), Pl. perrieri from the Zanclean and Pa. howelli. Another interesting result as discussed below is the significant difference between Pa. brunnea (living) and the Piacenzian and Gelasian Pl. perrieri.
Principal Component Analysis. Upper Dentition
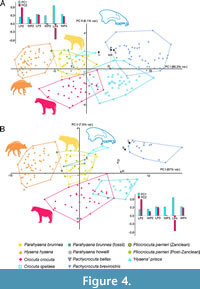 The morphospace regions occupied by the species, as illustrated in Figure 4, exhibit a general similarity to those identified in the preceding analysis. The second principal component effectively distinguishes scavenging species from hunting species. However, with the exception of P. brevirostris, there is a significant overlap between the species of the lineages analysed.
The morphospace regions occupied by the species, as illustrated in Figure 4, exhibit a general similarity to those identified in the preceding analysis. The second principal component effectively distinguishes scavenging species from hunting species. However, with the exception of P. brevirostris, there is a significant overlap between the species of the lineages analysed.
In contrast to the previous section, the number of fossils is notably smaller in this analysis. The bibliographic search for the data used in the present work reveals that it is 2.6 times more likely to find a specimen with all three dental elements analysed for the lower jaw than for the upper jaw. In the case of localities, it is 1.6 times more likely to find a paleontological site with at least one tooth of each of the three analysed for the lower jaw than for the upper jaw. Although not the focus of this investigation, this substantial reduction in sample sizes may be attributed to certain taphonomic factors. This strong reduction does not have the same importance for all the species analysed; for example, the sample of Pa. howelli or Pl. perrieri from Zanclean (inherently scarcer) are notably reduced with respect to the lower dentition. Perhaps such a reduction might contribute, in part, to the absence of significant correlations between the two factors for the species of the lineages studied in contrast to those obtained for the lower dentition shown in Table 3.
Despite reduced dataset, Figure 4 facilitates additional significant inferences concerning the phylogenetic relationships within the clade. A considerable overlap persists between Pa. brunnea (both living and fossil) and Pl. perrieri. In this case ‘H.’ prisca exhibits a slight separation due to its larger size. The two specimens from Londang show different positions in Figure 4A, one falling within the convex hull of P. brevirostris but the other closer to ‘H.’ prisca.
On the other hand, specimen PEC 18 from Petralona, assigned to Pl. perrieri by Baryshnikov and Tsoukala (2010) and nominally assigned here to ‘H.’ prisca falls within P. brevirostris. The South African individuals K55 (Komdraai A) and GV 3914 (Gladysvale Cave) project outside the region occupied by P. brevirostris in both individual and locality analyses (Figure 4A-B, respectively), supporting the hypothesis that both are conspecific and belong to P. bellax.
Another noteworthy observation concerns the Ségriès-le Réservoir sample (Cornillet, France) dating back to MN17a, approximately 2.6-2.5 million years ago (Dubar, 2014). Although originally assigned to Pl. perrieri by Dubar et al. (1978), its projection indicates a higher affinity with P. brevirostris, implying a potential first occurrence of this species in Western Europe. This point will be further discussed below.
Only one or two specimens are available for Piacenzian perrieri from the Zanclean and Pa. howelli, depending on the analysis considered, which project within or very close to the convex hull of H. hyaena because of their small size. Due to the small size of the upper dentition sample only the hypotheses of equal centroid have been statistically compared for some of the analysed species (C. crocuta, C. spelaea, H. hyaena, P. brevirostris, Pl. perrieri from the Piacenzian and Gelasian, Pa. brunnea [living] and ‘H.’ prisca). In all cases, statistically significant differences between them are obtained for both individuals and localities (p = 0.0001).
Change in Principal Components Over Geological Time. Lower Dentition
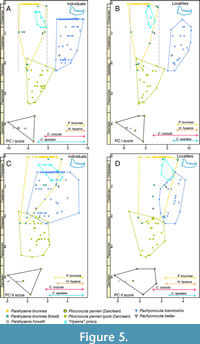 The variations through geological time of the scores on the first two principal components of the lower dentition for the lineages analysed is presented in Figure 5. As previously mentioned, there exists a certain degree of correlation between the two components for the species of the lineages under investigation, leading to a certain degree of parallelism between changes in shape and size, although the change in size is more conspicuous than the change in shape.
The variations through geological time of the scores on the first two principal components of the lower dentition for the lineages analysed is presented in Figure 5. As previously mentioned, there exists a certain degree of correlation between the two components for the species of the lineages under investigation, leading to a certain degree of parallelism between changes in shape and size, although the change in size is more conspicuous than the change in shape.
Figure 5 shows for both individuals and localities an evolution from small dentitions with relatively elongated m1 (Pa. howelli and Pl. perrieri from Zanclean) to larger forms with relatively shorter carnassials (Pl. perrieri from Piacenzian and Gelasian). Subsequently, during the first third of the Piacenzian, Pl. perrieri seems to split into three lineages: two that continue to progress in the preceding trend giving rise to P. brevirostris in Eurasia and P. bellax in Africa and another set that practically remains in stasis giving rise to Pa. brunnea.
For P. brevirostris, a significant positive correlation between the first principal component and geological age is observed for both individuals (r = 0.333, p = 2.6 10-4) and localities (r = 0.519, p = 0.011), which is evidence of a significant increase in size until its extinction (Figure 5A-B, respectively). Additionally, this species shows a significant increase of the second component over time (reflecting relative shortening of m1) for individuals (r = 0.249, p = 0.007), although it is not significant for localities (Figure 5C-D, respectively).
A noteworthy observation is the fact that virtually the entire Pa. howelli sample is included within the convex hull of Pl. perrieri from the Zanclean. On the other hand, despite the limited sample of Pa. brunnea fossils, the distinction made by Hendey (1974) between early and late morphotypes (small and large, respectively) seems appropriate.
In almost all the plots shown in Figure 5, ‘H.’ prisca is practically encompassed in the convex hull of Pa. brunnea fossil. It is further observed that ‘H.’ prisca appears to be derived from the late morphotype of Pa. brunnea as that recovered from Elandsfontein. A significant inverse correlation between both scores and geological age is observed for individuals of ‘H.’ prisca (r = -0.702, p = 0.024), though this correlation is not statistically significant for localities.
Change of Principal Components Over Geologic Time. Upper Dentition
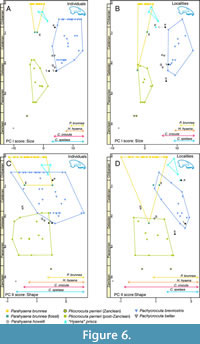 Despite the reduced number of observations for the upper dentition, Figure 6 shows that the relative positions of the species through geological age closely resemble those obtained for the lower dentition.
Despite the reduced number of observations for the upper dentition, Figure 6 shows that the relative positions of the species through geological age closely resemble those obtained for the lower dentition.
Similarly, to the previous section, P. brevirostris exhibits a direct correlation between PC I (size) and geological age for both individuals (r = 0.540, p = 0.031) and localities (r = 0.509, p = 4.9 10-4). However, unlike the lower dentition, a significant inverse correlation between the second principal component and geological age is obtained for both individuals (r = -0.644, p = 0.007) and localities (r = -0.647, p = 2.8 10-6).
This result can be interpreted as a consequence of the difference in functions within the upper carnassial. As indicated by Ewer (1954b) in her ecomorphological analysis of present-day hyenas, scavenging durophages, such as those of the lineages analysed here, show a greater development of the P4 parastyle, which has a greater role as a bone crusher than in Crocuta. In fact, this function extends to the mesial region of the P4 paracone (when the jaw is wide open), leaving the cutting function of the P4 confined to the metastyle and the distal region of the paracone (Figure 7). 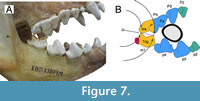 Thus, while almost the entire length of the lower carnassial corresponds to the trigonid (cutting function), the upper carnassial has a dual function (cutting and bone crusher). This is important for the functional interpretation of the second principal component of the lower and upper dentition, given that the lengths of the carnassials (m1 and P4, respectively) are the most important variables in them. To analyse how the bone-breaking part of P4 varies with respect to the total length of P4 in P. brevirostris one can appeal to the fact that the lower and upper dentition have to be functionally integrated, i.e., the trigonid (cutting part of m1) should be approximately equivalent to the cutting region of P4 (Figure 7). This implies that if the length of the trigonid is subtracted from the total length of the P4, the result should be approximately the length of the P4 involved in crushing (the mesial part of the paracone and the parastyle). There are not many fossil individuals in which m1 and P4 are preserved simultaneously, but it is possible to perform the above calculation for some fossil sites.
Thus, while almost the entire length of the lower carnassial corresponds to the trigonid (cutting function), the upper carnassial has a dual function (cutting and bone crusher). This is important for the functional interpretation of the second principal component of the lower and upper dentition, given that the lengths of the carnassials (m1 and P4, respectively) are the most important variables in them. To analyse how the bone-breaking part of P4 varies with respect to the total length of P4 in P. brevirostris one can appeal to the fact that the lower and upper dentition have to be functionally integrated, i.e., the trigonid (cutting part of m1) should be approximately equivalent to the cutting region of P4 (Figure 7). This implies that if the length of the trigonid is subtracted from the total length of the P4, the result should be approximately the length of the P4 involved in crushing (the mesial part of the paracone and the parastyle). There are not many fossil individuals in which m1 and P4 are preserved simultaneously, but it is possible to perform the above calculation for some fossil sites.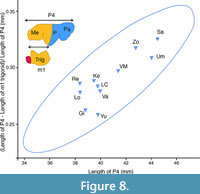 Figure 8 presents the percentage of the crushing length of P4 versus the total length of P4 for some populations of P. brevirostris, obtaining a positive and significant correlation between them (r = 0.844, p = 0.002). Such correlation evidences an increase in the length of the crushing part of P4 with increasing LP4, that is, a positive intradental allometry. In other words, in P. brevirostris, a simple increase in P4 size leads to an increase in bone-breaking specialization. Therefore, the inverse correlation of the second principal component and geological age observed in this species is a consequence of the inverse covariation of these components with LP4.
Figure 8 presents the percentage of the crushing length of P4 versus the total length of P4 for some populations of P. brevirostris, obtaining a positive and significant correlation between them (r = 0.844, p = 0.002). Such correlation evidences an increase in the length of the crushing part of P4 with increasing LP4, that is, a positive intradental allometry. In other words, in P. brevirostris, a simple increase in P4 size leads to an increase in bone-breaking specialization. Therefore, the inverse correlation of the second principal component and geological age observed in this species is a consequence of the inverse covariation of these components with LP4.
As in the previous section some observations of some importance can be made in Figure 6. In the case of Londang the two specimens analysed (HMV1200 and HMV1203) show an intermediate position between Pl. perrieri and P. brevirostris.
The Komdraai A and Gladysvale Cave fossils fall virtually outside of all convex hulls encompassing P. brevirostris, supporting the hypothesis of the validity of P. bellax as a distinct species from the former.
In the case of Petralona, Figure 5B shows the only individual with complete upper dentition from this locality (PEC 18) and the average for the paleontological site. In terms of size, the position changes from a value close to P. brevirostris for PEC 18 to one close to that of ‘H.’ prisca for the average of the locality, which points to PEC18 being P. brevirostris and the rest of the specimens being ‘H.’ prisca. That is, both taxa are present in that cave.
DISCUSSION
The results obtained are consistent with the gradual evolution of the lineage from an initial stock of populations distributed throughout the Old World at the beginning of the Zanclean, whose oldest specimen included in this study is V7289 originally attributed to Pliohyaena pyrenaica orientalis by Qiu (1987), (= Pachycrocuta pyrenaica in Qiu et al., 2004) from the Chinese locality of Wangjiagou. The site is at the base of the Nanzhuanggou Member of the Gaozhuang Formation, bounded by two unconformities dated at 4.9 and 4.2 Ma, respectively (Flynn and Qiu, 2013). Qiu (1987) also assigns specimen V7288 (an anterior skull fragment with very worn dentition) to this species. The locality where such a specimen was found (Lok. ?14, Hsingyangcun) was reassigned to Loc. 44 (Taoyangts'un) by Tedford et al. (2013: p. 32). Such a locality is included in the Taoyang Member of the aforementioned formation, which is bounded by two unconformities dated at 5.8 and 5.2 Ma, respectively. This implies that the origin of the lineage may even reach the Late Miocene.
Regarding this issue, the results obtained raise two related questions: the first is the conspecificity of Pa. howelli and Pliocrocuta perrieri from the Zanclean (= Pachycrocuta pyrenaica in Howell and Petter, 1980; Qiu, 1987 and Qiu et al., 2004) and the second whether there is reason to separate Pliocrocuta perrieri into the two taxa proposed by Howell and Petter (1980): Pachycrocuta pyrenaica and Pachycrocuta perrieri.
Howell and Petter (1980) addressed both questions. Indeed, the conspecificity of Pa. howelli and P. pyrenaica was proposed by these authors, indicating the existence of a perfectly preserved right mandible from the Kanapoi locality (south of Lake Turkana, Kenya) that has not yet been described (KNM-K. 069/6702), which corresponds to specimen KNM-KP 10033 in Werdelin (2003). Howell and Petter (1980) highlighted the good accommodation (from a morphological and metrical point of view) of this individual within the P. pyrenaica sample from Odesa Catacombs (Ukraine). On the other hand, Tseng et al. (2016) highlighted the remarkable similarity between Pa. howelli and the Zanclean Pliocrocuta perrieri specimen recovered from Zanda Basin (IVPP V20801). Although there are insufficient data to compare the upper dentition metrically, the results obtained here for the lower dentition are consistent with the conspecificity of these two species, given not only the proximity in morphospace but also that Pa. howelli also falls within the temporal range of Pliocrocuta perrieri during the Zanclean. In fact, Leaky and Werdelin (2010) indicate that, as is the case in the Miocene, during the Zanclean carnivores migrated preferentially from Eurasia to Africa than in the opposite direction. In other words, if there were no major geographic or ecological barriers to the dispersal of other carnivores to Africa during this stage, there is no reason to think that there were specific dispersal constraints for a taxon such as Pliocrocuta perrieri that ranged throughout Eurasia, from Spain to China.
The second question about the co-specificity of P. pyrenaica and P. perrieri can be rephrased as whether it is reasonable to divide the lineage into two segments: Pl. perrieri from the Zanclean and those from the Piacenzian and Gelasian. The results presented here indicate a gradual anagenetic evolution from the former to the latter. Certainly, continuity of a lineage can be an argument for not splitting it, but it should also apply to the transition between Pl. perrieri and P. brevirostris since the change is also gradual, and yet there is consensus that they are distinct species. For example, the morphological distance between the specimen of Pl. perrieri Se 312 from Senéze (France) and that of the specimen of P. brevirostris V13932.1 from Renzi Cave (China) is much smaller than that shown by many individuals of Pl. perrieri from each other (Figure 2A).
The division of any lineage into successive species segments (chronospecies) is undoubtedly arbitrary. However, the application of the typological concept of species in paleontology is not without arbitrariness. Besides being alien to the idea of evolution, sometimes the characters chosen to separate two species from a typological point of view are essential for some species and irrelevant for others, which is questionable from a biological point of view. Moreover, from a practical point of view, a specimen may resemble the holotype of one species in some characteristics and the holotype of another species in others. In addition, as features evolve, discontinuities separating paleontological species often fade as new intermediate fossils appear between them. Or put in another way, the hypodigms of two species of a lineage (ancestor and descendant) expand as new fossils appear until the two eventually touch or even overlap. In such cases, the holotypes may be markedly different, giving the false appearance of a clear separation between the species, but actually, the boundary between the two species is as arbitrary as the division of a lineage into segments.
The temporal dimension makes the biospecies concept in paleontology inapplicable and after decades of long and fruitless debates, there is no consensus on how to translate the biological species concept to paleontology (Sepkoski, 2016). However, despite these conceptual difficulties, species in paleontology has a practical value, both in biostratigraphy and paleoecology. Here, two arguments are proposed that may be useful as purely practical criteria for separating segments within the lineage studied. It is important to emphasize that such criteria are not intended to be conceptual tools but purely pragmatic.
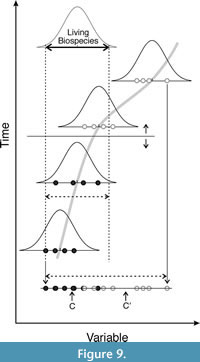 The first element that can be used as a guide is whether there are significant differences between the multivariate means of the two sets that are assumed to belong to different chronospecies. Another point that may help is whether the variability of a quantitative characteristic important for defining species within the lineage analysed exceeds that observed for related extant species. Thus, if a segment of a lineage exceeds the range observed for phylogenetically close biospecies, it could be divided into at least two arbitrary subsegments of chronospecies. Obviously, the boundaries between two chronospecies can vary if new remains recovered change such means or ranges. Figure 9 graphically illustrates both criteria.
The first element that can be used as a guide is whether there are significant differences between the multivariate means of the two sets that are assumed to belong to different chronospecies. Another point that may help is whether the variability of a quantitative characteristic important for defining species within the lineage analysed exceeds that observed for related extant species. Thus, if a segment of a lineage exceeds the range observed for phylogenetically close biospecies, it could be divided into at least two arbitrary subsegments of chronospecies. Obviously, the boundaries between two chronospecies can vary if new remains recovered change such means or ranges. Figure 9 graphically illustrates both criteria.
As presented in the results section, the first criterion is met for the lower dentition of Pl. perrieri from the Zanclean with respect to those from the Piacenzian and Gelasian, as well as for the latter with respect to P. brevirostris. Figure 5 shows that the size and shape range of Pl. perrieri taken as a whole also surpasses that exhibited by the living species, so it could be divided into two segments.
Similarly, the union of the Piacenzian and Gelasian Pl. perrieri together with P. brevirostris exceeds the range of variation observed for the extant species and it also meets the first criterion. Consequently, the proposal of Howell and Petter (1980) seems the most consistent with the two proposed guides, which implies dividing the lineage into three successive chronospecies of greater size and degree of specialization in the scavenging niche: P. pyrenaica, P. perrieri, and P. brevirostris.
This division carries certain paleoecological implications given that although the early forms of the lineage could play an ecological role similar to that of the later species, it is unlikely that they did so in exactly the same way given that they have not fully developed the morphological adaptations to do so. On the other hand, the differentiation of the lineage into three chronospecies also has some biostratigraphic value, since selection pressures maintained in the same direction have resulted in mean values of both shape and size varying in approximately the same direction over geological time.
While Pachycrocuta perrieri in Eurasia gradually evolved into P. brevirostris, in Africa, P. perrieri gave rise to Pa. brunnea (and perhaps to P. bellax). As can be seen in Figure 5-Figure 6, the dentition of the earliest specimens ascribed to Pa. brunnea (Member 1 of Swartkrans, Kromdraai B and Malapa) is virtually indistinguishable in both size and shape from that of the smaller specimens of P. perrieri from the late Gelasian. It is relevant to note here that P. perrieri has been identified in North Africa in both the Piacenzian (L’Aïn Brimba) and the Gelasian (Ahl al Oughlam). These results corroborate Turner's (1990) claim of conspecificity at least for the earliest representatives of Pa. brunnea and P. perrieri: the main reason for ascribing the first South African specimens to Pa. brunnea rather than to P. perrieri is that they are in South Africa, as are their present-day descendants. As Geraads (2006) shows almost all ungulate genera from Ahl al Oughlam are also known in East and/or South Africa, implying that the Sahara did not act as an insurmountable geographical barrier to large mammals that prevented free faunal exchange between North Africa and the rest of the continent. In fact, even in today’s arid conditions the striped hyena is distributed from North to East Africa, reaching Tanzania (Hofer and Mills, 1998).
An inescapable question at this point is the conspecificity of present-day Pa. brunnea and the late forms of P. perrieri. Werdelin and Solunias (1991) indicated that there are certain characteristics, especially the basioccipital shape in which the two species differ. On the contrary, Vinuesa et al. (2014) show that two adult and fairly complete crania of P. perrieri (IPS36759 and IPS36758) recovered from the Spanish localities of Villaroya and La Puebla de Valverde, respectively, exhibit similar morphologies to Pa. brunnea, including the presence of a small central ridge on the basioccipital. Regardless of the shape of the basioccipital, the greater reduction of the m1 at equal size of the rest of the dentition can be assessed as an autapomorphy of living Pa. brunnea with respect to P. perrieri. As can be seen in Figure 5A-B, the size of the lower dentition of Pa. brunnea practically remains in stasis from P. perrieri, but in Figure 5C-D it is observed that Pa. brunnea shows a relative reduction of the carnassial compared with P. perrieri, converging with P. brevirostris. In this case, both the criteria for considering P. perrieri and Pa. brunnea (living) as distinct species are met, as there are significant differences between them (Table 4) and the range of values of the union of P. perrieri and Pa. brunnea (living) is greater than that of Pa. brunnea.
A question that may arise here is whether the fossil and extant forms of Pa. brunnea have diverged sufficiently to be considered distinct chronospecies. Although the range of values of living Pa. brunnea increases somewhat when including the fossil forms (Figure 5), no significant differences are obtained between them and therefore they must be considered conspecific.
In the case of ‘H.’ prisca no significant differences are obtained with the fossils of Pa. brunnea, but there are significant differences with its living representatives. Additionally, the union of ‘H.’ prisca with Pa. brunnea (living) slightly expands the range observed for the latter, and consequently, both criteria are met. Although, as discussed below, the probable ancestor of ‘H.’ prisca is Pa. brunnea, the latter may be considered to have diverged sufficiently from ‘H.’ prisca to be considered a distinct species at present. In fact, the two species are clearly separated in the morphospaces of the lower and upper dentition (Figure 3-Figure 4).
Regarding the origin of ‘H.’ prisca, in addition to its morphological similarity to Pa. brunnea, the results obtained show the high metric similarity with the lower dentition of Pa. brunnea specimens from the South African locality of Elandsfontein, especially with the late morphotype (Figure 3, Figure 4, Figure 5, Figure 6). This fact supports the hypothesis that ‘H.’ prisca originated from Pa. brunnea, which colonized Europe through the Levantine corridor during the Middle Pleistocene. One element that clearly supports this hypothesis is the fact that Pa. brunnea is detected in East Africa during the Middle Pleistocene (Werdelin and Barthelme, 1997), consequently this species could have reached the Levantine corridor and finally Europe, where it evolved in allopatry. This colonization of Europe must have occurred after the extinction of P. brevirostris since both belong to the scavenging morphotype and a competitive exclusion in sympatry would be probable.
In this regard, it is interesting to comment on the case of Petralona cave. In agreement with Lannucci et al. (2021), the specimen PEC 17 assigned to P. brevirostris by Baryshnikov and Tsoukala (2010) is probably another taxon, possibly Crocuta. Paradoxically specimen PEC 18 assigned to Pl. perrieri (i.e., ‘H.’ prisca) by Baryshnikov and Tsoukala (2010) clearly projects within the convex hull of P. brevirostris. Figure 6B shows the projection of PEC 18 and the mean for the entire Petralona locality. As can be seen, the mean change from a point close to P. brevirostris to another one close to ‘H.’ prisca, what suggests that there is a mixture of taxa in the sample. Therefore, both taxa are present at this locality. These specimens lack stratigraphic context, but it is reasonable to assume that ‘H.’ prisca and P. brevirostris were not coeval due to the principle of competitive exclusion mentioned above.
As shown, evolution from P. perrieri to P. bevirostris is a gradual process. The upper dentition specimen from the Longdan locality described by Qiu et al. (2004) is an example of this. However, the lower dentition specimens HMV 1201 and HMV 1202 clearly fall within the hypodigm of P. perrieri. Qiu et al. (2004) indicate that some fossils were purchased from local fossil collectors and perhaps these specimens come from a lower stratigraphic position. It is possible that in Linxia Basin it occurs as in the Val d'Arno Basin, where P. perrieri is detected in the lower strata and P. brevirostris in the upper ones.
Similarly, in the case of Western Europe, the sample from the French locality of Ségriès-le Réservoir can be considered as a transitional form between P. perrieri and P. brevirostris equivalent to those from the Chinese locality of Londang. Even given its greater similarity to P. brevirostris than to P. perrieri, this locality could be considered the first occurrence of P. brevirostris in Western Europe. This site is dated between 2.6-2.5 Ma (Dubar, 2014), just at the boundary between the Piacenzian and the Gelasian. It is important to note here that the fossil assemblage found at Ségriès-le Réservoir presents a typical Piacenzian fauna (e.g., Nyctereutes megamastoides, Gazella borbonica, Croizetoceros ramosus, etc.). This reinforces the idea that the evolutionary process from P. perrieri to P. brevirostris was gradual and explainable by an anagenetic transformation of the Eurasian populations of P. perrieri and independent of the evolution or dispersal of other species with which it is typically found in other Gelasian fossil assemblages. In fact, the anagenetic evolution of P. brevirostris adapting in both size and shape toward greater specialization to the scavenging niche continued in China during the Middle Pleistocene until its final extinction.
The results obtained indicate that South African specimens from Komdraai A and Gladysvale Cave are outside the range of variation of P. brevirostris, which reinforces the hypothesis that P. bellax is a distinct species that would have evolved locally in Africa. This would imply that the similarities between P. brevirostris and P. bellax are an evolutionary convergence resulting from independent adaptation as specialized scavengers.
The record of P. bellax is extraordinarily scarce and probably restricted to South Africa. Werdelin (1999) proposed the conspecificity of this taxon with P. brevirostris and its occurrence at some localities in the Turkana Basin in chronologies between 3.5 and 2.5 Ma. However, such a hypothesis is supported by few poorly preserved remains that had originally been attributed to a large Crocuta by Harris et al. (1988). In fact, Werdelin and Lewis (2008) subsequently reassigned some of such material to C. eturono (Werdelin and Lewis, 2008), a large Crocuta described from the Turkana Basin in such chronologies. Without additional evidence, the presence of a large Pachycrocuta species in East Africa must be cautiously discounted until assessing whether the remaining East African material is within the variability of C. eturono.
The situation in South Africa is no better, except for Kromdraai A and Gladysvale, the remains attributed to P. bellax (or P. brevirostris) in Member 3 from Makapansgat Limeworks and Members 4 and 5 from Sterkfontein, lack other diagnostic value except that they are large. Assuming that the remains recovered from Makapansgat belong to P. bellax, the origin of that species must predate the age of Member 3 of that cave, which has been estimated to be between 2.9 and 2.6 Ma (Herries et al., 2013). This age is significantly older than that of the earliest Eurasian P. brevirostris and corresponds approximately with that of the earliest P. perrieri. Consequently, P. bellax must have originated from the local evolution either from a basal stock of African P. perrieri such as those found in North Africa or even from a South African stock of P. pyrenaica / Pa. howelli. Without additional data it is not possible to specify its ancestor.
This result also raises the question of sympatry between early Pa. brunnea and P. bellax since competitive exclusion between them would be expected. There are two alternatives for the allopatry between these two taxa. One possibility is that Pa. brunnea was relegated to a marginal ecological refuge that was difficult to access for P. bellax. Such an ecological refuge could be the Namib Desert, which is separated by more than 1000 km and has different ecological conditions from those observed at Cradle of Humankind, where the Komdraai and Gladysvale Cave localities are located. At least at present Pa. brunnea is a species adapted to the extreme conditions of the Namib Desert, one of the oldest in the world, whose biome is maintained by the Benguela Current (Van Zinderen Bakker, 1975). Another possibility is the local extinction and subsequent recolonization of P. perrieri from North Africa after the extinction of P. bellax (similar to the colonization of Europe by ‘H.’ prisca, after the extinction of P. brevirostris), given that in this period there are no barriers to prevent it (Geraads, 2006).
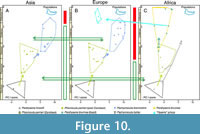 As a summary, Figure 10 shows the evolutionary hypotheses presented here from a paleogeographic perspective using the lower dentition for localities to illustrate it. Pachycrocuta pyrenaica would be a taxon with a wide distribution in the Old World that would evolve anagenetically to P. perrieri, which would later give rise to P. brevirostiris in Eurasia and perhaps also to P. bellax in Africa. These evolutionary changes determine an evolutionary trend towards an increase in size and in the bone-breaking adaptation of the dentition. In Africa P. perrieri would also give rise to Pa. brunnea. Basal forms of Pa. brunnea would colonize Europe in the Middle Pleistocene, evolving into ‘H.’ prisca. To meet the requirements of competitive exclusion, it is proposed that there must have been barriers to the dispersal of P. brevirostris to Africa during the Early Pleistocene and from Asia to Europe in the Middle Pleistocene, which allowed the isolation of P. bellax in Africa and the colonization of Europe by ‘H.’ prisca.
As a summary, Figure 10 shows the evolutionary hypotheses presented here from a paleogeographic perspective using the lower dentition for localities to illustrate it. Pachycrocuta pyrenaica would be a taxon with a wide distribution in the Old World that would evolve anagenetically to P. perrieri, which would later give rise to P. brevirostiris in Eurasia and perhaps also to P. bellax in Africa. These evolutionary changes determine an evolutionary trend towards an increase in size and in the bone-breaking adaptation of the dentition. In Africa P. perrieri would also give rise to Pa. brunnea. Basal forms of Pa. brunnea would colonize Europe in the Middle Pleistocene, evolving into ‘H.’ prisca. To meet the requirements of competitive exclusion, it is proposed that there must have been barriers to the dispersal of P. brevirostris to Africa during the Early Pleistocene and from Asia to Europe in the Middle Pleistocene, which allowed the isolation of P. bellax in Africa and the colonization of Europe by ‘H.’ prisca.
A final question that emerges from the results is whether Parahyaena brunnea should be assigned to genus Pachycrocuta. Although a more detailed study would certainly be necessary, with little taxonomic redefining it would be possible to accommodate the brown hyena within the genus Pachycrocuta. In fact, the differences between Pa. brunnea and P. perrieri are smaller than those observed between the three species of Pachycrocuta. Therefore, it seems appropriate to accept the proposal of McKenna and Bell (1997) to place Pa. brunnea within the genus Pachycrocuta, and consequently, ‘H.’ prisca as well.
CONCLUSIONS
According to Howell and Petter (1980), the synonymy of Parahyaena howelli and Pliocrocuta perrieri from the Zanclean is proposed, both of which would be integrated within Pachycrocuta pyrenaica.
Pliocrocuta perrieri from the Piacenzian and Gelasian would be reassigned to Pachycrocuta perrieri. The results obtained support the hypothesis that Pachycrocuta bellax is a different species from Pachycrocuta brevirostris.
Pachycrocuta perrieri would be the ancestor of Pachycrocuta brevirostris in Eurasia and of Parahyaena brunnea (and perhaps Pachycrocuta bellax) in Africa.
The sequence P. pyrenaica, P. perrieri, P. bellax, and P. brevirostris, implies a greater specialization in the scavenging niche by increasing size and the breaking part of the dentition.
Basal forms Parahyaena brunnea would recolonize Europe after the extinction of P. brevirostris originating ‘H.’ prisca. Parahyaena brunnea has now diverged sufficiently to be considered a distinct species from ‘H.’ prisca. A change of genus is proposed for the brown hyena, which would be integrated into Pachycrocuta, as well as ‘H.’ prisca.
ACKNOWLEDGEMENTS
This work has been supported by the Research Group RNM-146 (Plan Andaluz de Investigación, Desarrollo e Innovación) and II Plan Propio de Investigación, Transferencia y Divulgación Científica, Universidad de Málaga. I thank M.J. Salesa and an anonymous reviewer for their helpful comments and advice. In memory of Alan and Gill Turner.
REFERENCES
Anderson, M.J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26:32–46.
https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson, M.J. and Walsh, D.C. 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecological Monographs, 83:557–574.
https://doi.org/10.1890/12-2010.1
Bonifay, M.F. 1971. Carnivores quaternaires du Sud-Est de la France. Mémoires du Muséum National d'Histoire Naturelle Nouvelle Série. Série C, Sciences de la Terre, 21:1–358.
Baryshnikov, G. and Tsoukala, E. 2010. New analysis of the Pleistocene carnivores from Petralona Cave (Macedonia, Greece) based on the Collection of the Thessaloniki Aristotle University. Geobios, 43:389–402.
https://doi.org/10.1016/j.geobios.2010.01.003
Brugal, J.P., Giuliani, C., Fosse, P., Fourvel, J.B., Magniez, P., Pelletier, M., and Uzunidis, A. 2021. Preliminary Data on the Middle Pleistocene Site of Lunel Viel I (Hérault, France). Alpine and Mediterranean Quaternary, 34:75–88.
https://doi.org/10.26382/AMQ.2021.08
Cardoso, J.L. 1993. Contribuição para o conhecimento dos grandes mamíferos do Plistocénico Superior de Portugal. Câmara municipal de Oeiras, Oeiras.
Clarke, K.R. 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology, 18:117–143.
https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Coca-Ortega, C. and Pérez-Claros, J.A. 2019. Characterizing ecomorphological patterns in hyenids: a multivariate approach using postcanine dentition. PeerJ, 6:e6238.
https://doi.org/10.7717/peerj.6238
Croizet, J.B. and Jobert, A. 1828. Recherches sur les ossemens fossiles du département du Puy-de Dôme. Clermont-Ferrand, Paris, France.
Depéret, C. 1890. Les animaux Pliocènes du Roussillon. Mémoires de la Société Géologique de France, 3:1–164.
Dubar, M. 2014. Corrélation des gisements de mammifères villafranchiens de la région de Puimoisson (bassin de Valensole, Alpes de Haute-Provence, France) avec les autres sites datés (paléomagnétisme, radionucléides) d’Europe du Sud (France, Italie et Espagne). Quaternaire. Revue de l'Association française pour l'étude du Quaternaire, 25:85–89.
https://doi.org/10.4000/quaternaire.6997
Dubar, M., Guerin, C., and Heintz, E. 1978. Les nouveaux gisements villafranchiens du ravin de Cornillet (Moustiers Sainte-Marie, Alpes de Haute Provence, France) et leur contexte géologique. Geobios, 11:367–381.
https://doi.org/10.1016/S0016-6995(78)80036-9
Erxleben, J.C.P. 1777. Systema regni animalis per classes, ordines, genera, species, varietates, cum synonymia et historia animalium. Classis 1: Mammalia. Weygandianis, Lipsiae, Germany.
Ewer, R.F. 1954a. The fossil carnivores of the Transvaal caves. The Hyaenidae of Kromdraai. Proceedings of the Zoological Society of London, 124:565–585.
https://doi.org/10.1111/j.1469-7998.1954.tb07798.x
Ewer, R.F. 1954b. XXXI.–some adaptive features in the dentition of hyaenas. Annals and Magazine of Natural History, 7:188–194.
https://doi.org/10.1080/00222935408651717
Flynn, L.J. and Qiu, Z.X. 2013. Biostratigraphy and the Yushe Basin, p. 79–82. In Tedford, R.H., Qiu, Z.X., and Flynn, L.J. (eds.), Late Cenozoic Yushe Basin, Shanxi Province, China: Geology and Fossil Mammals. Springer, Heidelberg, Germany.
https://doi.org/10.1007/978-90-481-8714-0_5
Geib, K. 1915. Zwei Arten von Streifenhyänen aus dem deutschen Diluvium. Jahrbücher des Nassauischen Vereins für Naturkunde, 68:2–20.
Geraads, D. 2006. The late Pliocene locality of Ahl al Oughlam, Morocco: Vertebrate fauna and interpretation. Transactions of the Royal Society of South Africa, 61:97–101.
https://doi.org/10.1080/00359190609519958
Gervais, P. 1850. Zoologie et Paléontologie Françaises. Nouvelles Recherches sur les Animaux Vivants et Fossiles de la France. Tome I. Contenant l’Énumerération Méthodique et Descriptive des Espèces ainsi que les Principes de leur Distribution Géographique et Paléontologique. Arthus Bertrand. Paris, France
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1):a04.
https://palaeo-electronica.org/2001_1/past/issue1_01.htm
Harris, J.M., Brown, F.H., and Leakey, M.G. 1988. Stratigraphy and paleontology of Pliocene and Pleistocene localities west of Lake Turkana, Kenya. Contributions in Science, Natural History Museum of Los Angeles County, 399:1–128.
Hendey, Q.B. 1974. The late Cenozoic Carnivora of the southwestern Cape Province. Annals of the South African Museum, 63:1–369.
Herries, A.I., Pickering, R., Adams, J.W., Curnoe, D., Warr, G., Latham, A.G., and Shaw, J. 2013. A multi-disciplinary perspective on the age of Australopithecus in southern Africa, p. 21–40. In Reed, K.E., Fleagle, J.G., and Leakey, R.E. (eds.), The paleobiology of Australopithecus. Springer, Heidelberg, Germany.
https://doi.org/10.1007/978-94-007-5919-0_3
Hofer, H. and Mills, G. 1998. Worldwide distribution of hyaenas, p. 39–63. In Mills, M.G.L., and Hofer, H. (eds.), Hyaenas. Status Survey and Conservation Action Plan. IUCN/SSC Hyaena Specialist Group. IUCN, Gland, Switzerland and Cambridge, United Kingdom.
Howell, F.C. and Petter, G. 1980. The Pachycrocuta and Hyaena lineages (Plio-Pleistocene and extant species of the Hyaenidae). Their relationships with Miocene Ictitheres: Palhyaena and Hyaenictitherium. Geobios, 13:579–623.
https://doi.org/10.1016/S0016-6995(80)80004-0
Kuhn, B.F., Werdelin, L., and Steininger, C. 2017. Fossil Hyaenidae from Cooper’s Cave, South Africa, and the palaeoenvironmental implications. Palaeobiodiversity and Palaeoenvironments, 97:355–365.
https://doi.org/10.1007/s12549-016-0247-y
Kurtén, B. 1956. The status and affinities of Hyaena sinensis Owen and Hyaena ultima Matsumoto. American Museum Novitates, 1764:1–48.
Lannucci, A., Mecozzi, B., Sardella, R., and Lurino, D.A. 2021. The extinction of the giant hyena Pachycrocuta brevirostris and a reappraisal of the Epivillafranchian and Galerian Hyaenidae in Europe: Faunal turnover during the Early-Middle Pleistocene Transition. Quaternary Science Reviews, 272:107240.
https://doi.org/10.1016/j.quascirev.2021.107240
Leakey, M. and Werdelin, L. 2010. Early Pleistocene mammals of Africa: background to dispersal, p. 3–11. In Fleagle, J.G., Shea, J.J., Grine, F.E., Baden, A.L., and Leakey, R.E. (eds.), Out of Africa I: The First Hominin Colonization of Eurasia. Springer, Heidelberg, Germany.
https://doi.org/10.1007/978-90-481-9036-2
Lewis, M. and Werdelin, L. 2022. A revision of the genus Crocuta (Mammalia, Hyaenidae). Palaeontographica. Abteilung A, Palaozoologie, Stratigraphie, 322:1–115.
https://doi.org/10.1127/pala/2022/0120
Linnaeus, C. 1758. Systema Naturae. Theodorum Haak. Leyden, Netherlands.
Liu, J., Liu, J., Zhang, H., Wagner, J., Jiangzuo, Q., Song, Y., Liu, S., Wang, Y., and Jin, C. 2021. The giant short-faced hyena Pachycrocuta brevirostris (Mammalia, Carnivora, Hyaenidae) from Northeast Asia: A reinterpretation of subspecies differentiation and intercontinental dispersal. Quaternary International, 577:29–51.
https://doi.org/10.1016/j.quaint.2020.12.031
Lyell, C. and Murchison, R.I. 1829. On the Excavation of Valleys, as illustrated by the Volcanic Rocks of Central France. The Edinburgh New Philosophical Journal, 7:15–48.
McKenna, M.C. and Bell, S.K. 1997. Classification of Mammals: Above the Species Level. Columbia University Press, New York.
Palmqvist, P., Martínez-Navarro, B., Pérez-Claros, J.A., Torregrosa, V., Figueirido, B., Jiménez-Arenas, J.M., Espigares, M.P., Ros-Montoya, S., and De Renzi, M. 2011. The giant hyena Pachycrocuta brevirostris: modelling the bone-cracking behavior of an extinct carnivore. Quaternary International, 243:61–79.
https://doi.org/10.1016/j.quaint.2010.12.035
Pei, W.C. 1934. On the carnivora from locality l of Choukoutien. Palaeontologia Sinica, Series C, 8:1–166.
Pérez-Claros, J.A. 2022. When did hyenas start transporting carrion? Tracing back the origins of scavenging durophagous hyenas. Journal of Vertebrate Paleontology, 41:e2047990.
https://doi.org/10.1080/02724634.2021.2047990
Pérez-Claros, J.A. 2023. An ecomorphological characterization of the percrocutoid hyaenids: a multivariate approach using postcanine dentition. Journal of Vertebrate Paleontology, 42:e2197972.
https://doi.org/10.1080/02724634.2023.2197972
Pérez-Claros, J.A. and Coca-Ortega, C. 2020. Canines and carnassials as indicators of sociality in durophagous hyaenids: analyzing the past to understand the present. PeerJ, 8:e10541.
https://doi.org/10.7717/peerj.10541
Pérez-Claros, J.A., Coca-Ortega, C., and Werdelin, L. 2021. How many hyenas in North America? A quantitative perspective. Journal of Vertebrate Paleontology, 41:e1979988.
https://doi.org/10.1080/02724634.2021.1979988
Qiu, Z., 1987. Die Hyaeniden aus dem Ruscinium und Villafranchium Chinas. Münchner Geowissenschaftliche Abhandlungen. Reihe A. Geologie und Paläontologie, 9:1–109.
Qiu, Z., Deng, T., and Wang, B. 2004. Early Pleistocene mammalian fauna from Longdan, Dongxiang, Gansu, China. Paleontologia Sinica, New Series C, 191:1–198.
Reyment, R.A. 1990. Reification of classical multivariate statistical analysis in morphometry, p. 123–144. In Rohlf, F.J. and Bookstein, F.L. (eds.), Proceedings of the Michigan Morphometrics Workshop. The University of Michigan Museum of Zoology, Ann Arbor, Michigan.
Rudwick, M.J. 1985. The Meaning of Fossils: Episodes in the History of Palaeontology. University of Chicago Press, Chicago.
Semenov, Y.A. 1994. Allohyaena sarmatica (Carnivora, Mammalia) – a new hyaenid species from the late Miocene of the Ukraine. Acta Zoologica Cracoviensia, 37:31–38.
Sepkoski, D. 2016. The “species concept” and the beginnings of paleobiology, p. 9–27. In Allmon, W.D. and Yacobucci, M.M. (eds.), Species and Speciation in the Fossil Record. University of Chicago Press, Chicago.
https://doi.org/10.7208/chicago/9780226377582.001.0001
Tedford, R.H., Qiu, Z.X., and Flynn, L.J. 2013. Late Cenozoic Yushe Basin, Shanxi Province, China: Geology and Fossil Mammals. Volume I: History, Geology, and Magnetostratigraphy. Springer, Heidelberg, Germany.
https://doi.org/10.1007/978-90-481-8714-0
Thunberg, C.P. 1820. Beskrifning och teckning på ett nytt species, Hyaena brunnea. Kungliga Svenska Vetenskapsakademiens Handlingar, 1820:59–65.
Tseng, Z.J., Wang, X., Li, Q., and Xie, G. 2016. Pliocene bone-cracking Hyaeninae (Carnivora, Mammalia) from the Zanda Basin, Tibet Autonomous Region, China. Historical Biology, 28:69–77.
https://doi.org/10.1080/08912963.2015.1004330
Turner, A. 1990. The evolution of the guild of larger terrestrial carnivores during the Plio-Pleistocene in Africa. Geobios, 23:349–368.
https://doi.org/10.1016/0016-6995(90)80006-2
Van Zinderen Bakker, E.M. 1975. The origin and palaeoenvironment of the Namib Desert biome. Journal of Biogeography, 2:65–73.
https://doi.org/10.2307/3038074
Vinuesa, V., Madurell-Malapeira, J., Anson, M., and Alba, D.M. 2014. New cranial remains of Pliocrocuta perrieri (Carnivora, Hyaenidae) from the Villafranchian of the Iberian Peninsula. Bollettino della Società Paleontologica Italiana, 53:39–47.
https://doi.org/10.4435/BSPI.2014.04
Werdelin, L. 1999. Pachycrocuta (hyaenids) from the Pliocene of East Africa. Paläontologische Zeitschrift, 73:157–165.
https://doi.org/10.1007/BF02987989
Werdelin, L. 2003. Carnivora from the Kanapoi hominid site, Turkana Basin, northern Kenya. Contributions in Science, 498:115–132.
https://doi.org/10.5962/p.214388
Werdelin, L. and Solounias, N. 1991. The Hyaenidae: taxonomy, systematics and evolution. Fossils and Strata, 30:1–104.
https://doi.org/10.18261/8200374815-1991
Werdelin, L. and Solounias, N. 1996. The evolutionary history of hyenas in Europe and western Asia during the Miocene, p. 290–306. In Bernor, R.L., Fahlbusch, V., and Mittmann, H.W. (eds.), The Evolution of Western Eurasian Neogene Mammal Faunas. Columbia University Press, New York.
Werdelin, L. and Barthelme, J. 1997. Brown hyena (Parahyaena brunnea) from the Pleistocene of Kenya. Journal of Vertebrate Paleontology, 17:758–61.
Werdelin, L. and Lewis, M.E. 2008. New species of Crocuta from the early Pliocene of Kenya, with an overview of early Pliocene hyenas of eastern Africa. Journal of Vertebrate Paleontology, 28:1162–1170.
https://doi.org/10.1671/0272-4634-28.4.1162
Werdelin, L. and Dehghani, R. 2011. Carnivora, p. 189–232. In Harrison, T. (ed.), Paleontology and Geology of Laetoli: Human Evolution in Context. Vol. 2: Fossil Hominins and the Associated Fauna. Springer, Heidelberg, Germany.
https://doi.org/10.1007/978-90-481-9962-4_8

