 First occurrence of a †coccolepidid fish (?Chondrostei: †Coccolepididae) from the Upper Lias (Toarcian, Early Jurassic) of southern Germany
First occurrence of a †coccolepidid fish (?Chondrostei: †Coccolepididae) from the Upper Lias (Toarcian, Early Jurassic) of southern Germany
Article number: 27.1.a23
https://doi.org/10.26879/1326
Copyright Palaeontological Association, April 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 25 July 2023. Acceptance: 3 April 2024.
ABSTRACT
The non-neopterygian group †Coccolepididae, a moderately diverse predominantly freshwater family, remains an imperfectly known Mesozoic group of actinopterygians, currently classified within Chondrostei based on the presence of several acipenseriform synapomorphies. Coccolepidids first appear during the Early Jurassic in marine sediments, although their fossils are poorly known from this time, and none have yet been described from the Toarcian (Upper Lias). Here, we describe a new genus and species of coccolepidid fish, †Toarcocephalus morlok gen. et sp. nov. from the Lower Jurassic (Toarcian) Posidonienschiefer Formation of Holzmaden in southern Germany. †Toarcocephalus morlok is diagnosed by a unique combination of characters including a shallow lower jaw with a massive angular, opercle and subopercle equal in size, preopercle that only borders the subopercle but does not reach the opercle; dermal skull bones strongly punctate, with externally smooth upper and lower jaw bones. Discovery of a coccolepidid at Holzmaden represents the first occurrence of the group from a Toarcian deposit, as well as the oldest record of the family in mainland Europe. Both described specimens of †T. morlok were victims of successful predation events: one individual was likely decapitated (pabulite) and the other preserved within a regurgitalite (fossilized oral ejecta). The evolution of Coccolepididae is discussed briefly in relation to a marine/freshwater origin.
Samuel L.A. Cooper. Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1-3, 70191, Stuttgart, Germany. (Corresponding author) Samuel.cooper@smns-bw.de and Fachgebiet Paläontologie, Institute für Biologie, Universität Hohenheim, 70599, Stuttgart, Germany.
Adriana López-Arbarello. Department of Earth and Environmental Sciences, Paleontology and Geobiology, Ludwig Maximilian University, Munich, Germany; a.lopez-arbarello@lrz.uni-muenchen.de and GeoBio-Center, Ludwig-Maximilians-Universität München, Richard-Wagner-Str. 10, 80333 München, Germany
Erin E. Maxwell. Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1-3, 70191, Stuttgart, Germany. Erin.maxwell@smns-bw.de
Keywords: Coccolepididae; Chondrostei; Posidonienschiefer Formation; Early Jurassic; paleobiogeography; regurgitalite
Final citation: Cooper, Samuel L.A., López-Arbarello, Adriana, and Maxwell, Erin E. 2024. First occurrence of a †coccolepidid fish (?Chondrostei: †Coccolepididae) from the Upper Lias (Toarcian, Early Jurassic) of southern Germany. Palaeontologia Electronica, 27(1):a23.
https://doi.org/10.26879/1326
palaeo-electronica.org/content/2024/5197-new-toarcian-coccolepidid-fish
Copyright: April 2024 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
†Coccolepididae (sensu López-Arbarello et al., 2013) is an enigmatic family of early actinopterygians with still debatable phylogenetic relationships. Historically, coccolepidids were lumped within the obsolete polyphyletic ‘palaeoniscoids’ because of their supposed conservative skeletons (e.g., Woodward, 1890a, 1891; Gardiner, 1960, 1967, 1993; Kirkland, 1998). Recent revision of the family Coccolepididae has identified key anatomical synapomorphies with the monophyletic Acipenseriformes (López-Arbarello et al., 2013), thus proposing the placement of the family within the monophyletic Chondrostei, a clade comprising the living sturgeons (Acipenseridae) and paddlefishes (Polyodontidae), as well as extinct relatives, including †Peipiaostidae, †Chondrosteidae, and †Birgeriidae (Grande and Bemis, 1996; Hilton et al., 2004; Hilton and Forey, 2009). This has led many authors to propose the combination of †Coccolepididae within Chondrostei although the close relationship of Birgeriidae to Chondrostei and the assignment of other fossil actinopterygians have been drawn into question (López-Arbarello et al., 2013; Argyriou et al., 2018; Olive et al. 2019; Ebert et al., 2021; López-Arbarello and Ebert, 2021; but see Schultze and Arratia, 2015, Schultze et al., 2021, for alternative hypotheses whereby Coccolepididae is allied with the paraphyletic Palaeoniscimorpha) - however, this hypothesis for Coccolepididae being included with Chondrostei has never been subjected to a phylogenetic analysis (Olive et al., 2019).
†Coccolepididae was erected by Berg (1940) who diagnosed the family simply as including “all non-neopterygian fishes with only a single series of ossified radials supporting the dorsal fin”. Subsequent works regard Berg’s diagnosis as inadequate to differentially diagnose the family (Gardiner, 1960; Hilton et al., 2004; López-Arbarello et al., 2013; Olive et al., 2019) - in part due to the poor post-cranial ossification in these fishes; and because the number of radial supports in the dorsal fin among basal actinopterygians has been poorly surveyed (Hilton et al., 2004). Despite difficulties diagnosing members of the family, taxa currently included in †Coccolepididae range from the Sinemurian stage of the Early Jurassic to the Barremian- Albian stages of the Early Cretaceous (Forey et al., 2010; Olive et al., 2019). They are predominately found in freshwater environments, although a few rare occurrences in shallow marine deposits are documented (Woodward, 1890a; Gardiner, 1960; Hilton et al., 2004; Ebert et al., 2021; López-Arbarello and Ebert, 2021). The type taxon, †Coccolepis bucklandi Agassiz, 1843, is described from the Tithonian Solnhofen Plattenkalk in Bavaria (Germany), with numerous additional species ranging from the Early Jurassic - Early Cretaceous subsequently referred to †Coccolepis (see Hilton et al., 2004 and López-Arbarello and Ebert, 2021, for a review). However, many of these species have since been placed into their own genera (e.g., †Coccolepis macroptera = †Barbalepis macroptera: Olive et al., 2019) or are recognized as being in urgent need of taxonomic revision (e.g., †‘Coccolepis’ liassica Woodward, 1890a); only †C. bucklandi and †C. solnhofensis López-Arbarello and Ebert, 2021 are regarded as true members of Coccolepis. Therefore, †Coccolepis is now restricted to the Tithonian stage of the Late Jurassic (López-Arbarello and Ebert, 2021).
Although coccolepidids were a rare faunal component throughout their evolution, their oldest records are already widely distributed in eastern (China and Siberia) and central (England) Laurasia. During the Middle and Late Jurassic, the group expanded geographically into western Laurasia (North America) and southern Gondwana (Argentina, Australia), later being restricted in their occurrences to southern Gondwana and central Laurasia (England) during the Early Cretaceous (Olive et al., 2019). However, little is yet known of the group’s origins, early diversity and distribution during the Early Jurassic. Only two coccolepidids have been described from the Early Jurassic: †‘Coccolepis’ liassica from the Sinemurian of Lyme Regis in England (Woodward, 1890a, 1891; Gardiner, 1960; Forey et al., 2010), and †Plesiococcolepis hunanensis Wang, 1977, from Hunan Province in China (Chang and Miao, 2004). Both of these taxa remain inadequately described, and the generic affinity of †‘C.’ liassica remains ambiguous. Coccolepidids have not previously been described from the Toarcian. A privately-owned specimen from the Posidonienschiefer Formation of Schandelah (near Braunschweig), Lower Saxony (northern Germany) was figured and labelled as ‘Coccolepis sp.’ as part of a regional fossil guide (Hauff et al., 2017); however, no further records of coccolepidid-like fishes have thus far been documented from the formation.
Here we describe the first occurrences of coccolepidid fishes (†Coccolepididae) from the Posidonienschiefer Formation at Holzmaden, based on two partial specimens. The Holzmaden coccolepidid represents the first described from the Toarcian, bridging an outstanding gap in the family’s evolutionary history. The cranium of this fish is highly diagnostic, which in addition to its chronostratigraphic isolation from all other coccolepidids strongly supports assignment to a new genus and species.
GEOLOGICAL SETTING
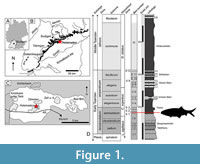 The Lower Jurassic (lower Toarcian) Posidonienschiefer Formation is a marine black shale Konservat-Lagerstätte famed for its exceptionally well-preserved marine fossils. The formation crops out in Germany from Waldshut-Tiengen in the south to Lower Saxony in the northwest, with coeval aged and similar lithostratigraphic deposits in Luxembourg, the Netherlands, France, and the United Kingdom (Riegraf et al., 1984; Röhl and Schmid-Röhl, 2005; Bour et al., 2007; Trabucho-Alexandre et al., 2012; Mönnig et al., 2018). Of particular interest is the area around Holzmaden (Baden-Württemberg; approximately 30 km SW of Stuttgart) where the majority of historic and modern collecting has taken place. The historic label of ‘Holzmaden’ is often used anecdotally to denote any number of former or active quarries within the Holzmaden area, particularly between the towns of Kirchheim unter Teck, Holzmaden, Ohmden, Schlierbach, Zell unter Aichelberg, and Bad Boll (Maxwell et al., 2022; Cooper et al., 2022, fig. 1c) (Figure 1A-C). The section at Holzmaden represents a continuous succession of the lower to lower-middle Toarcian Liassic rocks, spanning the tenuicostatum - bifrons a mmonite zones (Hauff, 1921; Riegraf et al., 1984) (Figure 1D). The palaeoenvironment represents a shallow to moderately deep, restricted silliclastic shelf setting within a sub-tropical epicontinental sea (Röhl et al., 2001; Röhl and Schmid-Röhl, 2005; Mönnig et al., 2018). Oxygen levels sporadically fluctuated throughout the Toarcian, with the onset of the global Early Toarcian Anoxic Event (E-TOAE) occurring in the latest tenuicostatum Zone (Röhl et al., 2001). The laminated beds in the lower part of the section (tenuicostatum Zone) represent a shallower environment more proximal to the palaeo-shoreline; but becoming progressively deeper and shifting basin-ward in the upper beds, influenced by a regional marine transgression (Röhl et al., 2001; Mönnig et al., 2018).
The Lower Jurassic (lower Toarcian) Posidonienschiefer Formation is a marine black shale Konservat-Lagerstätte famed for its exceptionally well-preserved marine fossils. The formation crops out in Germany from Waldshut-Tiengen in the south to Lower Saxony in the northwest, with coeval aged and similar lithostratigraphic deposits in Luxembourg, the Netherlands, France, and the United Kingdom (Riegraf et al., 1984; Röhl and Schmid-Röhl, 2005; Bour et al., 2007; Trabucho-Alexandre et al., 2012; Mönnig et al., 2018). Of particular interest is the area around Holzmaden (Baden-Württemberg; approximately 30 km SW of Stuttgart) where the majority of historic and modern collecting has taken place. The historic label of ‘Holzmaden’ is often used anecdotally to denote any number of former or active quarries within the Holzmaden area, particularly between the towns of Kirchheim unter Teck, Holzmaden, Ohmden, Schlierbach, Zell unter Aichelberg, and Bad Boll (Maxwell et al., 2022; Cooper et al., 2022, fig. 1c) (Figure 1A-C). The section at Holzmaden represents a continuous succession of the lower to lower-middle Toarcian Liassic rocks, spanning the tenuicostatum - bifrons a mmonite zones (Hauff, 1921; Riegraf et al., 1984) (Figure 1D). The palaeoenvironment represents a shallow to moderately deep, restricted silliclastic shelf setting within a sub-tropical epicontinental sea (Röhl et al., 2001; Röhl and Schmid-Röhl, 2005; Mönnig et al., 2018). Oxygen levels sporadically fluctuated throughout the Toarcian, with the onset of the global Early Toarcian Anoxic Event (E-TOAE) occurring in the latest tenuicostatum Zone (Röhl et al., 2001). The laminated beds in the lower part of the section (tenuicostatum Zone) represent a shallower environment more proximal to the palaeo-shoreline; but becoming progressively deeper and shifting basin-ward in the upper beds, influenced by a regional marine transgression (Röhl et al., 2001; Mönnig et al., 2018).
Of the remarkable vertebrate fossils recovered from the formation - which include ichthyosaurs, plesiosaurs, crocodylomorphs, and pterosaurs - it is the ray-finned fishes that are the most diverse and abundant. Described actinopterygians include the large (ca. 3 m total length (TL)) chondrosteid †Stronglyosteus, two species of the †saurichthyid †Saurorhynchus (Maxwell and Stumpf, 2017), six genera of †pachycormiforms (Cooper et al., 2022), two ganoid-scaled halecomorphs (†Holzmadenfuro and †Ohmdenfuro: both Ebert, Thies and Hauff, 2020), the caturid, †Caturus smithwoodwardi White, 1925, several deep-bodied semionotiforms (†Dapedium spp. + †Tetragonolepis spp.) (Thies and Herzog, 1999; Thies and Hauff, 2011), the †ptycholepid, †Ptycholepis bollensis Agassiz, 1843; the lepisosteiform †Lepidotes gigas Agassiz, 1832 (López-Arbarello, 2012), an abundance of the shoaling teleost †Leptolepis spp. And several taxa currently lumped within the problematic genus †‘Pholidophorus’ (Hauff and Hauff, 1981).
MATERIAL AND METHODS
Two fossil fishes in the collection of the SMNS represent unique specimens of a hitherto undescribed Toarcian coccolepidid from the Posidonienschiefer Formation. SMNS 59978 comprises an articulated but incomplete skull preserved in right lateral aspect on a small piece of shale, collected in 1993 from bed ԑII1 (‘Koblenzer’; tenuicostatum Zone) of the G. Fischer quarry in Holzmaden (located opposite Urwelt Museum Hauff; Figure 1). The second specimen (SMNS 52044) derives from the exact horizon and bed number as SMNS 59978, and was donated to the SMNS in 1982. SMNS 52044 consists of a well-articulated skull in ventro-left-lateral view, with some disarticulated elements of the post-cranial skeleton amalgamated in a confined concentration behind the operculum. The preservation of this specimen is consistent with the typical skeletal accumulation pattern of a regurgitalite, representing the first known coccolepidid preserved as a bromalite inclusion (see Discussion). Both specimens were previously mislabelled in the collection as †pholidophoriforms however neither specimen shows any characteristic features of this group.
Anatomical nomenclature and taxonomy of †Coccolepididae follows Hilton et al. (2004) and López-Arbarello et al. (2013). Our definition of the genus †Coccolepis follows the revised diagnosis by López-Arbarello and Ebert (2021). Revised nomenclature for the actinopterygian palatoquadrate follows Schultze et al. (2021) after Nielsen (1942, figs. 35a, 36-37). As outlined in the introduction, the interrelationships of †Coccolepididae within Actinopterygii remain unresolved and are outside the scope of this contribution. For the new Holzmaden coccolepidid, we tentatively refer †Coccolepididae to Chondrostei, following the most current diagnosis of the family (López-Arbarello et al., 2013). Standard ichthyological parameters were measured using a digital calliper, and specimens were photographed with a Canon EOS 200D camera with EFS 55 mm and EF 100 mm macro-lenses. UV photography was attempted for these specimens, however, due to the low florescence feedback and high pyrite content in the matrix, the results were not informative.
Institutional Abbreviations. NHMUK = The Natural History Museum, London, U.K. (formally The British (Natural History) Museum); SMNS = Staatliches Museum für Naturkunde Stuttgart, Stuttgart, Germany.
SYSTEMATIC PALAEONTOLOGY
Class ACTINOPTERYGII Cope, 1887
Subclass ?CHONDROSTEI Müller, 1845
sensu Grande and Bemis (1996)
Family †COCCOLEPIDIDAE Berg, 1940 sensu López-Arbarello et al. (2013)
Remarks. Referral to the family †Coccolepididae is based on the following combination of characters (based on revised diagnosis by López-Arbarello et al., 2013): (1) dermal bones of the skull roof ornamented with small and regularly spaced tubercles; (2) maxilla with large postorbital plate; (3) supracleithrum massive, either as large as or larger than the cleithrum. Much of the pre-orbital region is missing in both Holzmaden specimens, creating ambiguity over the possible presence of coccolepidid features in this region of the skull, particularly those pertaining to the contacts between the nasals (Hilton et al., 2004; López-Arbarello et al., 2013). The left nasal is partially exposed in SMNS 52044 although unfortunately the bone contacts are obscured. The new Holzmaden fish is excluded from the Early Jurassic family †Centrolepididae due to the absence of both suborbital bones and complex dermal ornamentation formed by thick enameloid (Gardiner, 1960, p. 248). Likewise, the absence of a broad coronoid process, a large expanded preopercle, an upright suspensorium and variation in dermal ornamentation differentiates the new Holzmaden actinopterygian from the Lower Liassic family †Platysiagidae (Gardiner, 1960, p. 256). It is also excluded from †Ptycholepidae due to the absence of the diagnostic ridged ganoine ornamentation, a large gular plate, an opercle which is significantly larger than the subopercle, and dentition consisting of very small, close-set teeth (Woodward, 1891; Gardiner, 1960, p. 261).
†Toarcocephalus gen. nov.
zoobank.org/2BB90CAA-8FAD-45B3-A8D6-685B096F632D
Diagnosis. As for the type and so far only known species.
†Toarcocephalus morlok gen. et sp. nov.
zoobank.org/09AB0516-607C-4C73-9614-7BEC974045D1
Diagnosis. †Toarcocephalus morlok gen. et sp. nov. is diagnosed from all other coccolepidid fishes by the following unique combination of characters: upper and lower jaws smooth and unornamented; mandible well elongated and gracile, longer than maxilla and shallow posteriorly; angular large and lenticular; large postorbital expansion of maxilla strongly convex and twice as long as deep with a strongly recurved ventral margin; short premaxilla holding several recurved teeth that are slightly larger than those on the maxilla; skull roof very weakly tuberculated with pronounced striated ridges that are marginally serrated; subopercle trapezoidal and equal in size to opercle; preopercle slender, forming a posteroventral lobular expansion, only as tall as the subopercle and extending no further than the midpoint of the postorbital plate of the maxilla; operculum and supracleithrum mostly smooth with fine, regularly spaced punctae; supracleithrum massive, accounting for more than 80% of operculum height; large triangular dorsal process on the supracleithrum; nine branchiostegal rays, each thin, lacking distal expansions and confined to the posterior corner of the mandible; gular plate egg-shaped and placed roughly at the midpoint of the lower jaw length; elongated hyomandibula obliquely inclined forward, thin and weakly bow-shaped; ceratohyal well elongated but thin; hypohyals short and robust; sclerotic ring thin and delicate; scales weakly developed; vertebral column aspondylous, composed of simple arcocentral arches with proportionately short spines.
Etymology. Generic name chosen for its discovery in Toarcian-aged strata, with suffix -cephalus for head, denoting the diagnostic cranium. Species epithet morlok named after the savage subterranean antagonists in H.G. Wells’ The Time Machine (1895), due to their similarly grotesque appearance characterised by large eyes, a blunt face and pointed teeth.
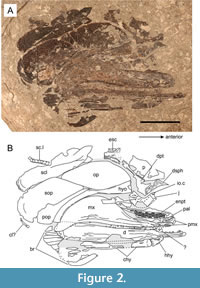 Holotype. SMNS 59978, a near-complete and articulated skull, missing the pre-orbital region (Figure 2).
Holotype. SMNS 59978, a near-complete and articulated skull, missing the pre-orbital region (Figure 2).
Paratype. SMNS 52044, an articulated skull exposed in left ventrolateral view, with associated postcranial fragments preserved as a regurgitalite (Figure 3).
Type Locality. G. Fischer Quarry, Holzmaden, Baden-Württemberg, Germany.
Type Horizon. Bed ԑII1 (‘Koblenzer’), semicelatum Subzone; tenuicostatum Zone, Posidonienschiefer Formation, Lower Toarcian, Early Jurassic.
Description
General features. The skull is 44 mm long and 30 mm deep (SMNS 59978), with a maximum known skull length of 73 mm (SMNS 52044). The postorbital region accounts for 60% of the skull length, with a mandible length approximate to 75% of the skull length. The angular measures approximately 35% of the total mandible length. Collectively the operculum is twice as deep as it is long with the massive supracleithrum accounting for 80% of the operculum depth. The skull displays the condition that is shared with both coccolepidids and many other early actinopterygians, notably an anteriorly placed orbit, large postorbital plate on the maxilla, absence of the supramaxilla, jaws extending far behind the orbit and an obliquely inclined suspensorium. Both skulls are incomplete, with the skull roof and rostral portions poorly known in this taxon. The jaws, palate, and suspensorium of the holotype specimen are exposed in internal view, whilst most of the operculum, pectoral gridle, branchiostegal rays, and skull roof are exposed in external view.
Cranial Skeleton
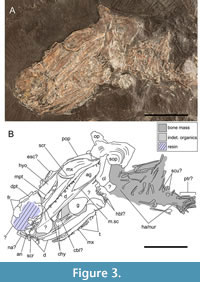 Skull roof. Only the dermopterotic, dermosphenotic and extrascapular of the right side of the skull roof are preserved in SMNS 59978 (Figure 3). Fragments of smooth dermal bone placed anterodorsally to the orbit in SMNS 52044 likely represent part of the left frontal (Figure 3). The dermosphenotic is asymmetrically semi-lunate, taller than it is wide, and significantly smaller than, but articulating anterior to, the large dermopterotic. The dermosphenotic is confined to the dorsoposterior corner of the orbit and is ornamented externally with weakly developed denticle-like protuberances. Proportionately, the dermosphenotic is smaller than that of †‘Coccolepis’ liassica.
Skull roof. Only the dermopterotic, dermosphenotic and extrascapular of the right side of the skull roof are preserved in SMNS 59978 (Figure 3). Fragments of smooth dermal bone placed anterodorsally to the orbit in SMNS 52044 likely represent part of the left frontal (Figure 3). The dermosphenotic is asymmetrically semi-lunate, taller than it is wide, and significantly smaller than, but articulating anterior to, the large dermopterotic. The dermosphenotic is confined to the dorsoposterior corner of the orbit and is ornamented externally with weakly developed denticle-like protuberances. Proportionately, the dermosphenotic is smaller than that of †‘Coccolepis’ liassica.
The dermopterotic is poorly preserved in SMNS 59978. It is more than twice as long as it is wide, is wider at the anterior where it lies just above the dorsal head of the hyomandibula, and becomes progressively narrower towards the posterior (Figure 3). Superficially, the surviving bone is axe-shaped, although the true dimensions of this element are unknown. The ventral bone margin is undamaged, and is obtusely concave such that its shape fits well with the convex dorsal margin of the opercle. The infraorbital sensory canal cannot be observed on account of the poor preservation. A wide and thin expansive bone, partially overlain (in ventral view) by the posterior region of the dermopterotic, most plausibly represents remnants of the broken right parietal. The externa is very weakly ornamented with poorly developed protuberances.
A small pentagonal bone located posterior to dermopterotic is identified as the left extrascapular (Figure 3). The bone is strongly ornamented with deep, longitudinally orientated bony ridges, although is incomplete along the anterior and lateral margins. In relation to the cheek, the extrascapular is situated dorsal to the midpoint of the opercle, with the serrated posterior margin of the bone representing the suture with the missing posttemporal bone.
An incomplete extrascapular from the right side of the skull roof is present posterior to the dermopterotic and parietal in external view. The bone is damaged anteriorly but is superficially pentagonal. The extrascapular is strongly ornamented with several deep well-developed ridges and grooves aligned longitudinally, as well as a slightly serrated lateral bone margin. In relation to the cheek, the extrascapular is situated roughly at the midpoint of the dorsal margin of the opercle (Figure 3). The empty gap between the extrascapular and the supracleithrum represents the void left from the missing posttemporal.
Orbital series. The orbit is placed close to the anterior end of the skull, with the jaws extending well behind it. The majority of the orbital series is mostly obscured or missing in both specimens of †Toarcocephalus morlok. The sclerotic ring is incompletely preserved, thin and delicate, lacks ornamentation, and possesses a slightly thickened external ridge (Figure 3). The sclerotic ring is disarticulated in SMNS 52044 with the displaced posterior plate lying on the top of the maxilla. The number of infraorbitals is uncertain as only the incomplete right jugal is preserved, placed posterior to the orbit in SMNS 59978. The jugal is thin and smooth, superficially semi-circular, and holds the infraorbital sensory canal, which passes longitudinally through the midline of the bone (Gardiner, 1960). The anterior surface of the jugal is arc-shaped, indicating its contribution the posteroventral margin of the orbit (Figure 3). The infraorbital sensory canal passes dorsally towards the overlying dermosphenotic (likely bridged by the missing infraorbitals), similar to †Coccolepis (e.g., Hilton et al., 2004).
Much of the orbital region is present in SMNS 52044; however, a combination of rough fossil preparation, repairs using obstructing resin, and the skull’s preservation within a regurgitalite all contribute to the difficulty of identifying the individual bones in this region of the skull. A small (1 mm width) hatchet-shaped bone is interpreted as the antorbital as it displays a prominent semi-circular notch on the anterodorsal corner, which (based on †Coccolepis) in life would have aligned with a corresponding notch on the nasal to form the narial foramen (Gardiner, 1960). A taller rectangular bone placed dorsal to the antorbital likely corresponds to either the left or right nasal (Figure 3). The naris is therefore placed anteroventral to the orbit, similar to its placement in †‘Coccolepis’ liassica (Woodward, 1890a; Gardiner, 1960) and in Late Jurassic coccolepidids (López-Arbarello et al., 2013; Skrzycka, 2014; López-Arbarello and Ebert, 2021). Suborbitals are absent. Supraorbital and rostral bones are not preserved in either specimen.
 Upper jaw. The upper jaw is composed of a large maxilla, exposed externally in SMNS 52044 (Figure 3, Figure 4A) and internally in SMNS 59978 (Figure 2, Figure 4B-C), and a short premaxilla which is only preserved in SMNS 59978. The maxilla is slender anteriorly but drastically expands and broadens behind the orbit, forming a dorsal plate-like expansion. The postorbital portion of the maxilla is massive with respect to the anterior (infraorbital) portion, accounting for more than 60% of the maxilla length. The postorbital expansion is twice as long as it is deep, is strongly convex with an asymmetrically rounded dorsal margin marked by a roughly medial peak. The dorsal margin obtusely slopes anteriorly, becoming much shallower at the junction below the jugal. From here, the maxilla becomes progressively slenderer, extending only as far forward as the anterior margin of the toothed pterygoid, but terminating short of the proximal end of the dentary. The ventroposterior corner of the maxilla is characterised by a short lobe-like expansion that partially overlies and extends further backwards than the lower jaw. The occlusal surface of this expansion holds several small teeth, which are curved anteriorly. A single row of size-graded teeth extends along the entirety of the oral surface of the maxilla. Teeth comprise of tall, recurved cone-like crowns, each separated by an interval of between 3-4 shorter and more gracile crowns. Tooth morphology and arrangement are comparable to those on the dentary. Similar to the lower jaw, the externa of the maxilla is smooth and lacks any ornamentation (Figure 4A), helping to differentiate this taxon from most other coccolepidid fishes (see discussion).
Upper jaw. The upper jaw is composed of a large maxilla, exposed externally in SMNS 52044 (Figure 3, Figure 4A) and internally in SMNS 59978 (Figure 2, Figure 4B-C), and a short premaxilla which is only preserved in SMNS 59978. The maxilla is slender anteriorly but drastically expands and broadens behind the orbit, forming a dorsal plate-like expansion. The postorbital portion of the maxilla is massive with respect to the anterior (infraorbital) portion, accounting for more than 60% of the maxilla length. The postorbital expansion is twice as long as it is deep, is strongly convex with an asymmetrically rounded dorsal margin marked by a roughly medial peak. The dorsal margin obtusely slopes anteriorly, becoming much shallower at the junction below the jugal. From here, the maxilla becomes progressively slenderer, extending only as far forward as the anterior margin of the toothed pterygoid, but terminating short of the proximal end of the dentary. The ventroposterior corner of the maxilla is characterised by a short lobe-like expansion that partially overlies and extends further backwards than the lower jaw. The occlusal surface of this expansion holds several small teeth, which are curved anteriorly. A single row of size-graded teeth extends along the entirety of the oral surface of the maxilla. Teeth comprise of tall, recurved cone-like crowns, each separated by an interval of between 3-4 shorter and more gracile crowns. Tooth morphology and arrangement are comparable to those on the dentary. Similar to the lower jaw, the externa of the maxilla is smooth and lacks any ornamentation (Figure 4A), helping to differentiate this taxon from most other coccolepidid fishes (see discussion).
The premaxilla is short, accounting for only 10% of the upper jaw length. It is well exposed in SMNS 59978 where the bone has flipped orally to expose the external surface (Figure 2, Figure 4B-C). The bone is slender posteriorly, becoming progressively deeper at the anterior to form a stout dorsal expansion. The occlusal surface is gently concave and bears five stout teeth, each with a wide base and evenly spaced with narrow diastemas. These teeth are slightly larger than those on the maxilla.
Lower jaw. The lower jaw is very elongate with a mandible length equal to approximately 94% of the postorbital length. The left lower jaw is exposed in lateral view in SMNS 52044 but is incomplete anteriorly, whereas SMNS 59978 exposes the entirety of the right lower jaw in internal view. The right ramus of the lower jaw in SMNS 52044 is partly exposed in ventromedial view where it displays an acute, inwards anterior curvature of the rami towards the mandibular symphysis. The lower jaw is shallow and gracile, with an extremely high length to depth ratio of 16:1. Compared to the maxilla, the lower jaw is longer and extends further where it terminates in a proximal gape level with the premaxilla of the upper jaw. Externally, the lower jaw is composed of an elongate dentary, which bears teeth along its entire occlusal surface, and an elliptical angular which is large and well pronounced: extending for approximately 25% of the total lower jaw length (Figure 2-Figure 3). The angular articulates with the dentary via a wedge-shaped suture, with the dentary bone extending both underneath and dorsal to the angular. Externally, the mandible is mostly smooth but becomes weakly ornamented towards the anterior with fine longitudinal striations. Foramina for the mandibular sensory canal are aligned longitudinally along the ventral margin of the lower jaw, extending below the angular bone but not crossing into it; instead, the canal terminates at the ventroposterior corner of the dentary (Figure 3). The medial surface of the lower jaw is imperfectly exposed in SMNS 59978 to reveal to a narrow tube-like Meckelian groove. Unfortunately, the posterior medial surface of the lower jaw, including the dentary contact with the prearticular and articular, and the possible presence of toothed coronoids, are poorly distinguishable due to imperfect preservation in this region.
Teeth are present along the entire length of the dentary, with two prominent size-graded tooth morphologies present, similar in morphology and arrangement pattern to those on the upper jaw. The taller crowns are slender and conical with widened bases and curved apexes. Each of the tall crowns are separated laterally by 2-4 smaller tooth crowns, which are strongly recurved and are less than 1/3 of the height of the larger teeth. All tooth crowns are smooth rather than ornate, with well-developed acrodin caps (Figure 4A).
Suspensorium. The left side of the palatoquadrate is nicely exposed in SMNS 59978. The palatoquadrate is a large, lenticular, mostly dentigerous compound structure formed of a large endopterygoid, one or two dermopalatines, and remains of a probable ectopterygoid and dermometapterygoid. The dentigerous surface is armed with a chaotic field of small denticle-like teeth, numbering more than 100; each is roughly identical in morphology and significantly smaller than the marginal dentition. The posterior-most region of the palatoquadrate and its contact with the left suspensorium is partially obscured by the postorbital plate of the right maxilla. The endopterygoid forms the majority of the palatoquadrate, is lenticular in shape, and mostly dentigerous in occlusal view. The anterior-most end of the endopterygoid pinches out proximally where it becomes abruptly edentulous.
The dermometapterygoid is mostly covered by the right maxilla, with the exposed region being confined to the posterodorsal margin of the endopterygoid where it contributes less than 1/5 of the exposed dentigerous tooth field. The posteroventral region of the palatoquadrate poorly exposes part of the dentigerous ectopterygoid; albeit the bone is damaged and mostly covered by the right maxilla.
The dermopalatine is placed anterior to the ectopterygoid where the bone is sutured along the entire ventral margin of the endopterygoid. Due to incompleteness and specimen damage, the number of dermopalatine bones in the palatoquadrate is uncertain. The ventral margin of the dermopalatine holds a single row of teeth, larger than those of the dentigerous field carried by the endopterygoid, dermometapterygoid and ectopterygoid; however, only a single dermopalatine tooth is exposed underneath the overlying premaxilla towards the front of the palatoquadrate complex. The dorsal margin of the endopterygoid expands medially to form a tall and wide edentulous flange for anchorage underneath the parasphenoid, which according to Gardiner (1960; although referred to therein as the suprapterygoid), would run the entire dorsal surface of the palatoquadrate.
 The hyomandibula is fully exposed in medial view in SMNS 59978, with the dorsal head of the hyomandibula exposed externally in SMNS 52044. We identify this element as the hyomandibula on the basis that it is exposed in internal view partially underlying the operculum in the holotype, does not hold any dermal ornamentation in SMNS 52044 where the dorsal head is externally exposed, and is overall consistent with the shape and placement of the corresponding hyomandibula in other coccolepidids (e.g. ‘Coccolepis’ liassica, pers. observ. SC). The hyomandibula is tall and obliquely inclined towards the anterior. The bone is gently arc-shaped, narrow along the midshaft and ventral margin, but gradually widening to form a slightly expanded hatchet-shaped dorsal head. The bone is placed dorsoposterior to the maxilla, extending from the dorsal border of the lower jaw to just anterodorsal of the opercle; extending further forward than the postorbital portion of the maxilla (Figure 2, Figure 5). The quadrate, which should articulate ventrally between the hyomandibula and lower jaw, is not exposed in either specimen.
The hyomandibula is fully exposed in medial view in SMNS 59978, with the dorsal head of the hyomandibula exposed externally in SMNS 52044. We identify this element as the hyomandibula on the basis that it is exposed in internal view partially underlying the operculum in the holotype, does not hold any dermal ornamentation in SMNS 52044 where the dorsal head is externally exposed, and is overall consistent with the shape and placement of the corresponding hyomandibula in other coccolepidids (e.g. ‘Coccolepis’ liassica, pers. observ. SC). The hyomandibula is tall and obliquely inclined towards the anterior. The bone is gently arc-shaped, narrow along the midshaft and ventral margin, but gradually widening to form a slightly expanded hatchet-shaped dorsal head. The bone is placed dorsoposterior to the maxilla, extending from the dorsal border of the lower jaw to just anterodorsal of the opercle; extending further forward than the postorbital portion of the maxilla (Figure 2, Figure 5). The quadrate, which should articulate ventrally between the hyomandibula and lower jaw, is not exposed in either specimen.
Ventral gill skeleton and ventral hyoid arch. The ventral hyoid arch is fairly well exposed in medial view and is composed of a small robust hypohyal, and an elongate ceratohyal. The hypohyal is located close to the anterior margin of the dentary and contributes only 10% of the ventral axis of the hyoid arch. The right hypohyal is articulated with the right ceratohyal in SMNS 59978, whereas an indeterminate bone located between the dentary and upper jaw in the same specimen may be the displaced left hypohyal (Figure 4B-C). Anteriorly, the bone is narrow and robust, forming a convex head; whereas the bone gradually deepens and becomes thinner towards the posterior, forming a concave cotyle-like facet for articulation with the ceratohyal. The ceratohyal is well elongated, accounting for just over 90% of the vertical arm of the hyoid arch. The bone is narrow along the medial shaft but expands slightly at the anterior just before it articulates with the hypohyal. The ceratohyal is best exposed in SMNS 59978 except for the damaged posterior region. The anterior ends of both the left and right ceratohyal are also partially exposed in ventral view between the lower jaws in SMNS 52044 (Figure 3). A slightly slimmer bone with a rounded anterior margin is partly exposed between the paired ceratohyals, likely corresponding to the front portion of a ceratobranchial. Exposed towards the posterior margin of the cranium in SMNS 52044 are a transverse series of thin bony rods which are mostly overlain by a large mass of indeterminate bone and soft tissues (Figure 3). The identity of these bones is uncertain, although their placement and arrangement suggest that they may be remnant elements of the branchial arches.
Gular. The gular plate is present, albeit poorly preserved in SMNS 52044. The gular is small (gular length equivalent to 15% of total mandible length) weakly pentagonal to ovate, widest at the anterior, very gently convex, and is placed between the lower jaws, roughly mid-way along the dentary. It is uncertain if this position is truly anatomical or if the plate has slipped backwards during the partial decay/digestion process, similar to what has happened to the sclerotic ring in this specimen. The bone is heavily abraded, which has likely destroyed any external ornamentation.
Branchiostegal rays. Preserved branchiostegal rays number at least nine and are confined to the posterior-most corner of the ceratohyal. Each ray is very short and flat with a roughly rectangular outline, are of equal breadth and do not overlie one another. Their distal ends are sub-rounded and not expanded as per the condition observed in some Late Jurassic coccolepidid taxa (e.g., †Condorlepis; López-Arbarello et al., 2013). The ornamentation is weakly developed, consisting of a patchy leathery texture with weakly pronounced and shallow protuberances.
Operculum. The operculum, comprising the opercle, subopercle, and preopercle, is complete in SMNS 59978 (Figure 2). Only the subopercle and fragments of the opercle are preserved in SMNS 52044. As a collective, operculum height is more than twice that of its anteroposterior length, with the subopercle equal in size to the opercle (Figure 2). Similar to the hyomandibula, the operculum is obliquely inclined forward with the long axis oriented approximately 130° to the long axis of the skull. The subopercle is twice as tall as it is deep, is roughly rhombic in shape with a convexly rounded ventral margin, straight anterior margin, and a slightly narrower, albeit straightened dorsal margin where it contacts the ventral plane of the opercle.
The preopercle is slender and narrow, being only as tall as the subopercle; it does not extend dorsally past the subopercle-opercle boundary. Anteriorly, the preopercle is gently concave and narrow dorsomedially. It expands slightly into a rounded ventral fan that extends as far as the ventral margin of the subopercle.
The opercle is equal in size and of a similar shape to the subopercle; it is placed dorsoanteriorly to the two other elements of the operculum with only very brief contact with the dorsoanterior branch of the preopercle. The opercle is oval, slightly asymmetrical, with the dorsal head wider than the ventral; all margins of the bone are either strongly or weakly convex. Dorsoanteriorly, the opercle extends as far as the dorsal margin of the hyomandibula, with the obtusely curved dorsoposterior bone margin bordering the dermopterotic, extrascapular, supracleithrum, and the missing posttemporal. All bones in the operculum appear to be smooth, although under magnification are shown to be very finely punctate (perforated), lack denticles or protuberances, and show an unusual cross-hatching pattern which may be taphonomic in origin.
Postcranial Skeleton
Pectoral girdle. Only the ventral branch of the cleithrum is partially exposed underneath the damaged operculum in SMNS 52044. The bone is too incomplete to draw meaningful anatomical data, however, based on its small size relative to the subopercle, we surmise the cleithrum is proportionately smaller than the supracleithrum. The supracleithrum is massive, occupying almost the entire height of the operculum with the midpoint of the bone aligned roughly perpendicular to the suture between the opercle and subopercle (Figure 5). The supracleithrum is superficially rectangular, somewhat amorphous with equally wide proximal and distal ends. The ventral (distal) end of the bone is sub-oval with strongly convex margins similar to the bones in the operculum. The dorsal (proximal) end is expanded to form an anterodorsal plate-like ridge, terminating at a short triangular projection. The corresponding projection from the underlying left supracleithrum is partially exposed underneath the ventral margin of the right (external) supracleithrum (Figure 5). A similar process is present in the supracleithrum of Neslovicella (Åtamberg, 2007) and likely articulates underneath the missing posttemporal. Foramina for the lateral line canal pass longitudinally through the dorsal half of the supracleithrum in a somewhat obtuse trajectory (Figure 5, Figure 6).
 Vertebral column and fin support. With the exception of the pectoral girdle, the postcranial skeleton is very poorly represented. The postcranial skeleton is poorly mineralized in coccolepidids, lacking ossified ribs and centra (Hilton et al., 2004; López-Arbarello et al., 2013; Olive et al., 2019) and thereby hindering their preservation potential in the fossil record. A jumbled mass of fragmented bones from the postcranium are preserved within a regurgitated accumulation behind the skull in SMNS 52044. Due to a combination of anatomical disarticulation, rough fossil preparation, and having been churned up and partially digested in a predator’s gut, identifying most of these bones with certainty is problematic, especially in the absence of articulated comparative materials. Within this regurgitated accumulation though a few bone morphologies are identified.
Vertebral column and fin support. With the exception of the pectoral girdle, the postcranial skeleton is very poorly represented. The postcranial skeleton is poorly mineralized in coccolepidids, lacking ossified ribs and centra (Hilton et al., 2004; López-Arbarello et al., 2013; Olive et al., 2019) and thereby hindering their preservation potential in the fossil record. A jumbled mass of fragmented bones from the postcranium are preserved within a regurgitated accumulation behind the skull in SMNS 52044. Due to a combination of anatomical disarticulation, rough fossil preparation, and having been churned up and partially digested in a predator’s gut, identifying most of these bones with certainty is problematic, especially in the absence of articulated comparative materials. Within this regurgitated accumulation though a few bone morphologies are identified.
Several arcocentra in the form of arches and spines are observed, although whether they represent neural or haemal elements is unknown. An isolated element towards the ventral margin of this accumulation is complete, showing the spine to be relatively short and the arch to be a simple bifurcation with a small paired zygapophysis located just dorsal of the arch crest. The majority of the bone accumulation is composed of similar arcocentra elements; however, it is not possible to provide an estimation of their number. Two small rhombic to oval-shaped scale-like ossifications are preserved behind the arcocentra. Their origin is uncertain; however, the shape and size are reminiscent of the preanal scales of some coccolepidid fishes (Hilton et al., 2004; López-Arbarello and Ebert, 2021).
Posterior to these scales are 11 elongate bones seemingly retaining their relative anatomical arrangement, preserved in the ventral-most region of the regurgitalite. These bones are uniform in length and characterised by a narrow elongate shaft with a single hatchet-like expansion at each end. Their identity is uncertain, although they possibly correspond to either part of an articulated fin support for either for the dorsal or anal fin, or possibly preural arcocentra from the disrupted caudal region of the vertebral column.
Squamation. A partial series of articulated lateral line scales are aligned posterior to the supracleithrum in SMNS 59978 (Figure 5). The eight surviving scales are exposed in medial view indicating that these scales belong to the left lateral line scale row. The lateral line sensory canal is poorly visible due to a combination of imperfect preservation and the scales exposure in medial rather than lateral view.
Size estimations. Both specimens belong to similar sized fishes, with SMNS 52044 representing a slightly larger individual. Skull length is proportionate to four and a half times the total length in †‘Coccolepis’ liassica (Gardiner, 1960), and assuming similar proportions in the new taxon we estimate the largest individual of †Toarcocephalus morlok gen. et sp. nov. to be 315 mm TL (SMNS 52044) and the smaller specimen only 207 mm TL (SMNS 59978).
Comparative Anatomy
†Toarcocephalus morlok gen. et sp. nov. is assigned to the family †Coccolepididae based on the following shared characters: (1) dermal bones of the skull roof ornamented with small and regularly spaced tubercles; (2) maxilla with a large postorbital plate; (3) a massive supracleithrum, either as large or larger than the cleithrum (López-Arbarello et al., 2013). Absence of postorbital and suborbital bones in †T. morlok gen. et sp. nov. is a shared trait of †Coccolepididae and excludes possible referral within the morphologically similar Early Jurassic family †Centrolepididae (see Gardiner, 1960). Absence of an enlarged coronoid process, a vertical suspensorium or a plate-like preopercle placed dorsal to the maxilla additionally excludes assignment to †Platysiagidae (Gardiner, 1960). The weak dermal ornamentation is strikingly dissimilar to the well-developed ganoine ridges on the externa of the dermal bones in †Ptycholepididae. With the exception of †Ptycholepididae, these non-coccolepidid families are presently restricted to the Sinemurian stage of the Early Jurassic (e.g., Forey et al., 2010). The family †Coccolepididae is further diagnosed by a postrostral bone that separates only the posterior region of the nasal bones (López-Arbarello et al., 2013; Ebert et al., 2021). The nature of the contacts between the nasals and postrostral bone is unknown in †T. morlok gen. et sp. nov.
The genus †Coccolepis Agassiz, 1843, was until recently a speciose waste-basket genus encompassing geographically and stratigraphically distant taxa, but is now endemic to the Tithonian of Bavaria with only the type species †C. bucklandi and †C. solnhofensis considered valid members of the genus (López-Arbarello and Ebert, 2021). †Coccolepis differs from †Toarcocephalus gen. nov. based on a suite of characters, most notable being the difference in ornamentation. The skull roof and maxilla of †Coccolepis spp. are strongly ornamented with sharply pointed, posteriorly directed denticles and strongly developed tubercles (Hilton et al., 2004), whereas the maxilla is smooth and the skull roof very weakly tuberculated in the new Toarcian coccolepidid. The opercle in †Coccolepis spp. is larger than the subopercle, although both elements are of equal size in †Toarcocephalus morlok. Both taxa possess a slender lower jaw, although it is very short in Coccolepis at about half the length of the maxilla (López-Arbarello and Ebert, 2021); whereas in †Toarcocephalus morlok, the length of the lower jaw is greater than that of the maxilla. Both genera share few branchiostegal rays which are confined to the posterior region of the lower jaw, a sub-oval gular located roughly at the lower jaw midpoint, and a small trigger-shaped dermosphenotic bone which is likely not fused to the skull roof and contributes to the dorsoposterior margin of the orbit. However, †Coccolepis holds two fully developed tooth rows on the upper jaw and one on the lower jaw (Hilton et al., 2004), unlike †Toarcocephalus morlok which only holds a single tooth row on both the upper and lower jaws.
†‘Coccolepis’ liassica is the closet taxon to †Toarcocephalus morlok in regard to stratigraphy and palaeobiogeography, although it is still spatially and temporally distant, being found exclusively from the Sinemurian stage of Lyme Regis, UK (Woodward, 1891; Forey et al., 2010). The most detailed description and updated diagnosis for this taxon is provided by Gardiner (1960), however, further work on this species is needed to resolve its problematic generic affinities within †Coccolepididae: Hilton et al. (2004) and López-Arbarello and Ebert (2021) supported the assignment of †‘C.’ liassica to †Coccolepididae, but not to the genus †Coccolepis. The skull bones of †‘C.’ liassica are covered with ganoine, and the ornamentation consists of coarse tubercles that sometimes merge, producing rugae (Gardiner, 1960; López-Arbarello and Ebert, 2021; pers. observ. SC). The ornamentation pattern is much weaker in the new taxon: the maxilla is smooth, with ganoine absent on the external skull bones. The teeth are very small and numerous on the jaws of †‘C.’ liassica but are strikingly larger and fewer in number, with the largest row of teeth being more widely spaced, in †T. morlok. †‘Coccolepis’ liassica possesses two tooth rows on the lower jaw (Gardiner, 1960), unlike T. morlok. †‘Coccolepis’ liassica is further differentiated from †T. morlok by a posterior deepening of the mandible (much shallower in †T. morlok); the dorsal margin of the maxilla plate being flat rather than strongly convex; a subopercle larger than the opercle (both equal in †T. morlok); and a taller and broader preopercle, encapsulating the entire dorsal margin of the maxillary plate. Branchiostegal rays are also more numerous in †‘C.’ liassica (15 vs. 9; Gardiner, 1960). The stratigraphic isolation and contrasting anatomical landmarks between the two taxa suggest that †‘C.’ liassica most likely represents a unique genus of coccolepidid distinct from both †Coccolepis and †Toarcocephalus morlok. †‘Coccolepis’ liassica, in addition to the species †Condorlepis woodwardi (Waldman, 1971) and †Sunolepis yumenensis Liu, 1957, further differ from the two species of †Coccolepis and †T. morlok on the basis that they all possess an opercle that is smaller than the subopercle (Liu, 1957; López-Arbarello and Ebert, 2021).
†Condorlepis groeberi (López-Arbarello et al., 2013) from the Tithonian of Patagonia is similar to †Toarcocephalus morlok in the shared presence of an elongated lower jaw which is equal to- or larger than the maxilla; a mandibular sensory canal confined to the ventral margin of the dentary; and branchiostegal rays confined to the posterior half of the mandible. However, unlike in †T. morlok, the dorsal margin of the maxillary plate is flat, rather than being obtusely convex, with the slim mandible of †Co. groeberi becoming drastically deeper towards the posterior. The supracleithrum and cleithrum are roughly equal in size in †Co. groeberi, although the supracleithrum is disproportionately much larger in the new Toarcian species. The branchiostegal rays are noticeably wider and better pronounced with rounded distal margins in †Co. groeberi, whereas they are skinnier and placed more posteriorly in †T. morlok gen. et sp. nov.
The Upper Jurassic †Morrolepis aniscowitchi (Gorizdor-Kulczycka, 1926) of Kazakstan and M. schaefferi Kirkland, 1998, from Utah both differ from †Toarcocephalus morlok by the presence of a thin splint-like supracleithrum, branchiostegal rays being shorter and wider and originating close to the mandibular symphysis, and the opercle being noticeably taller than the subopercle (see Kirkland, 1998; Skrzycka, 2014).
†Plesiococcolepis hunanensis from the Early Jurassic of China is of a similar chronostratigraphic age, although is spatially distant from the new Holzmaden coccolepidid. Both †P. hunanensis and †Toarcocephalus morlok share the following characteristics (based on diagnosis in Wang, 1977): large teeth evenly spaced on the lower jaw; subopercle taller than long. †Plesiococcolepis hunanensis differs from †T. morlok by the opercle being larger than the subopercle, and the smaller teeth on the maxilla being tightly packed together (Wang, 1977). The smaller teeth are more spacious with tooth crowns rarely touching one-another in †T. morlok. The ornamentation patterns are poorly described in †P. hunanensis, although stated as possessing “projecting tuberosities” which are not seen in †T. morlok. Both species also possess a highly arched margin of the maxilla, the presence of rounded corners on the opercle, and an elongate supracleithrum that is larger than the cleithrum. Based on Wang’s (1977, fig. 1b) cranial reconstruction, the rostrum is somewhat expanded to form a considerable overbite relative to the anterior margin of the lower jaw in †P. hunanensis; a feature not shared with the new Holzmaden coccolepidid.
Although not currently included in †Coccolepididae, the enigmatic Sinemurian species †Cosmolepis ornatus (Egerton, 1853) from Dorset is somewhat comparable to the new Toarcian coccolepidid fish, and thus is relevant to compare and contrast here. Both taxa share the presence of size differentiated teeth in the external jaws with the tallest crowns being well pronounced; skull roof finely tuberculated; and the operculum and jaw bones being somewhat smooth and without tubercles (Woodward, 1890a; Forey et al., 2010). †Toarcocephalus morlok differs from †Cos. ornatus in the presence of a shallow lower jaw which is proportionately more elongate; a massive angular; teeth which project backwards as opposed to slightly forwards (Forey et al., 2010); and a subopercle in †Cos. ornatus that is disproportionate in size relative to the opercle. †Cosmolepis ornatus is salient for its well mineralized squamation comprising a full body covering of small to medium palaeoniscoid scales that are weakly ornate with ridged ganoine. Egerton (1853) describes the presence of “surface ornament [on the cheek and jaws] composed of fine vermicular plaits of enamel, arranged for the most part in a longitudinal direction”. This feature is well developed in the holotype of †Cosmolepis ornatus (NHMUK PV P. 557) but absent in †Toarcocephalus morlok. †Cosmolepis also possesses numerous branchiostegal rays, which extend along almost the entire length of the lower jaw, and according to Woodward (1890a) are strongly striated, without a gular plate. Branchiostegals number far fewer and are strictly confined to the posterior corner of the mandible in †T. morlok (Figure 2).
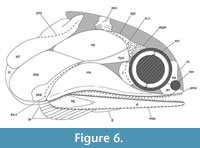
†Toarcocephalus morlok is therefore diagnosable from all other coccolepidid fishes by the following combination of characters: opercle and subopercle equal in size; maxilla shorter than the lower jaw with a strongly convex postorbital plate; single row of teeth on the upper and lower jaw composed of tall crowns which are each separated by several smaller crowns; angular massive; maxilla unornamented; lower jaw extremely narrow and elongate, shallow posteriorly; skull roof weakly tuberculated; opercular and supracleithrum bones weakly punctate; branchiostegal rays short, narrow and confined to the posterior margin of the mandible; premaxilla deeper than anterior region of the maxilla; premaxillary teeth larger than the maxillary teeth; preopercle short, extending as high as the subopercle; opercle with rounded margins; supracleithrum larger than cleithrum and externally punctate (Figure 6).
Like other coccolepidids, †Toarcocephalus morlok shares with †Birgeria, Polyodontidae and several acipenserids the very large supracleithrum, which is larger than cleithrum (López-Arbarello et al., 2013). Although less pronounced than in †Condorlepis, the subopercle of †T. morlok also forms an anteriorly directed anterodorsal process, which is similar to the process observed in acipenserids (Hilton et al., 2011: appendix III, character 27). Similarly, the absence of supraorbital bones is another feature shared between †T. morlok and other coccolepidids, which is a synapomorphy of Acipenseriformes according to Hilton et al. (2011: appendix III, character 9).
Range. Confined to the type locality of Holzmaden, tenuicostatum Zone, Toarcian, Early Jurassic.
DISCUSSION
Evidence for SMNS 59978 Being a Regurgitalite
Regurgitalites are an ethological class of bromalite traces representing fossilized vomit (oral ejecta) that has been regurgitated by a consumer (Seilacher, 2002; Hunt, 1992; Hoffman et al., 2019; Gordon et al., 2020; Cooper et al., 2021; Serafini et al., 2022). They are characterized by a tight compaction of skeletal remains usually retaining a degree of articulation, often show evidence of acid etching produced by the consumer’s stomach acids, and lack any associated coprolitic material (Myhrvold, 2012; Hoffmann et al., 2019; Qvarnström et al., 2019; Gordon et al., 2020; Freimuth et al., 2021; Serafini et al., 2021).
Regurgitalites (syn. speiballen) are scarcely documented from the Posidonienschiefer: Keller (1977) described a juvenile ichthyosaur with a tightly coiled vertebral column interpreted as the regurgitated meal of a larger ichthyosaur; a second probable regurgitalite containing ichthyosaur bones from Dotternhausen was figured by Jäger (2005, fig. 43). Thies and Hauff (2012) described a multi-taxon regurgitalite allegedly containing remains of five fishes (four †Dapedium sp. and jaw fragments of †Lepidotes sp.). Cooper et al. (2021) figured three additional fish-bearing regurgitalites with single inclusions of †Dapedium sp., †‘Pholidophorus’ sp., and a juvenile †Pachycormus macropterus. Most recently, Cooper and Maxwell (2022, fig. 3b-c) figured a large accumulation of disarticulated and brecciated bones containing the anterior portion of the pachycormid fish †Saurostomus esocinus, and remains referable to †Saurorhynchus sp. and †Leptolepis sp. interpreted to be the remains of a regurgitated slurry. Regurgitalites are therefore rare in the Posidonienschiefer despite being deposited under conditions favouring exceptional fossil preservation.
SMNS 52044 consists of a mostly articulated skull and the disrupted portion of the axial skeleton. The surviving portion of the postcranium is mostly disarticulated with the bones densely clustered together behind the skull. The central bone mass is tightly packed with individual elements accumulated together and disarticulated; thereby providing a key indicator for the presence of a regurgitalite (Serafini et al., 2022). Scavenging or abiotic disarticulating factors like water currents would cause bones to become scattered across a wide periphery, resulting in bones becoming isolated, displaced, or hydrodynamically grouped by size (Beardmore and Furrer, 2016; Serafini et al., 2022). The sclerotic ring has disarticulated; however, the displaced bones are confined to the skeletal mass rather than being displaced away from the skeleton as one would expect to see from an abiotic disarticulation event. Upon ejection from the consumer’s alimentary canal, the bones were likely held together by a mucus-like adhesive, which prevented them from dispersing and becoming scattered on the sea floor. The low energy, soupy-substrate, and oxygen-depleted sea floor of the Posidonia Shale Sea (Seilacher, 1982; Riegraf et al., 1984; Martill, 1993; Röhl et al., 2001) further assisted in the excellent preservation potential of the regurgitalite. We can therefore rule out an abiotic or scavenging event as causation for the type of bone accumulation in SMNS 52044. The fish preserved within the regurgitalite is incomplete, as some areas of the postcranium are missing. This indicates that either the fish was incompletely consumed, or that some parts of the fish were not regurgitated. Alternatively, the partially digested fish may had been fully regurgitated into several separate masses distributed apart from one another.
Regurgitated bones and teeth may preserve delicate etching traces, the severity of which is generally governed by the length of time for which the prey was exposed to stomach acids whilst inside the consumer’s gut (digestion window) before being regurgitated (Keller, 1977; Hoffmann et al., 2019; Cooper et al., 2021). Unfortunately, SMNS 52044 has undergone rough mechanical preparation that has abraded large areas of the external bone surfaces; therefore, any delicate etching traces have likely been destroyed. Overall, the close compaction of the bones within a confined central bone mass, good articulation of the skull, absence of visible phosphatic material (an indicator of coprolites), or bones displaced away from the central mass, strongly supports the identification of SMNS 52044 as a regurgitalite.
The digestion window was evidently short, being halted by regurgitation within likely only a few hours of ingestion. We conclude this on the basis that the mechanical damage to the prey fish was only prolonged enough to collapse the weakly ossified vertebral column, but not enough to disarticulate the denser cranial bones. The coccolepidid was therefore likely preyed on within the Holzmaden area, implying that the fish was autochthonous to the Posidonienschiefer Formation, rather than having been preyed on elsewhere and transported for a long distance inside of the predator’s gut.
In contrast, SMNS 59978 only preserves the skull with no indication of any post-cranial elements in the surrounding matrix. The bones of the skull are mostly perfectly articulated with the exception of the pre-orbital region, which is incomplete due to specimen damage. This unusual degree of incompleteness is unlikely to result from normal decay on the sea floor, especially given the good articulation quality of the remaining skull bones. Likewise, the specimen does not show any biological evidence for having had been regurgitated. There is a possibility that the fish was decapitated in a predation event with the severed head representing the leftovers (pabulite - Klug et al., 2021) of the predator’s meal. This hypothesis would explain why the skull is almost fully articulated whilst the body of the fish is entirely absent. The incomplete but articulated lateral line scales also abruptly end within the vicinity of the pectoral girdle, thereby supporting a decapitation explanation rather than a decay-related disarticulation scenario.
 SMNS 52044 is significant because it represents the first coccolepidid fish to be preserved as a regurgitalite. This reveals that coccolepidids contributed to the diet of an unknown piscivorous predator in the Posidonia biotope. The producer of the regurgitalite is unknown, but see Thies and Hauff (2012) for a review of likely regurgitate-producing candidates in the formation. Marine crocodylomorphs (†Thalattosuchia) are identified as the most plausible candidates for the †Dapedium regurgitalite described in Thies and Hauff (2012); however, thalattosuchians are extremely rare in the tenuicostatum Zone (Figure 1D) with only a single find having been documented (Hauff, 1921). A small ichthyosaur, a hybodontiform shark, or even a large coleoid cephalopod, are more plausible candidates on account of their varied diets, moderate body size, and co-occurrence with coccolepidids in the tenuicostatum Zone of the Holzmaden area (Hauff and Hauff, 1981; Thies and Hauff, 2012) (Figure 7).
SMNS 52044 is significant because it represents the first coccolepidid fish to be preserved as a regurgitalite. This reveals that coccolepidids contributed to the diet of an unknown piscivorous predator in the Posidonia biotope. The producer of the regurgitalite is unknown, but see Thies and Hauff (2012) for a review of likely regurgitate-producing candidates in the formation. Marine crocodylomorphs (†Thalattosuchia) are identified as the most plausible candidates for the †Dapedium regurgitalite described in Thies and Hauff (2012); however, thalattosuchians are extremely rare in the tenuicostatum Zone (Figure 1D) with only a single find having been documented (Hauff, 1921). A small ichthyosaur, a hybodontiform shark, or even a large coleoid cephalopod, are more plausible candidates on account of their varied diets, moderate body size, and co-occurrence with coccolepidids in the tenuicostatum Zone of the Holzmaden area (Hauff and Hauff, 1981; Thies and Hauff, 2012) (Figure 7).
Coccolepidids in Marine Environments
The Posidonienschiefer Formation offers a rare combination of a shallow to moderately deep continental marine basin, in which oxygen became depleted numerous times during the Toarcian (Röhl et al., 2001; Klug et al., 2021). For the section at Holzmaden, sea level is interpreted as being lowest towards the base of the formation within the tenuicostatum Zone (Riegraf et al., 1984; Röhl et al., 2001; Schmid-Röhl et al., 2002). The progressive marine transgression up-sequence is associated to a progressively receding regional shoreline, with Holzmaden representing a more proximal, shallow water near-shore environment only in the lower beds (tenuicostatum Zone) where the coccolepidid specimens were found (bed ԑII1). Coccolepidid material is not observed above bed ԑII1, with their disappearance in the assemblage likely correlating with the rising sea level and shallow-water habitat loss in the area; and/or decreasing water column oxygenation.
The vast majority of coccolepidids are found exclusively in fresh water or occasionally brackish terrestrial environments (see Olive et al., 2019: table 2; Table 1), although there are a few rare exceptions to this rule. Coccolepidids found from marine environments include †‘Coccolepis’ liassica from the Lower Lias of England, and the two true species of †Coccolepis (†C. bucklandi + †C. solnhofensis - see López-Arbarello and Ebert, 2021) from the Solnhofen Plattenkalk, but are a rare faunal component of their respective faunal assemblages, with each species only being known by a handful of specimens (e.g., Gardiner, 1960; Hilton et al., 2004; Ebert et al., 2021; López-Arbarello and Ebert, 2021). Coccolepis spp. likely inhabited nearby reefs in the Solnhofen Archipelago, hence their rarity in the lagoonal deposits. A similar model is proposed for non-gyrodontid pycnodontiforms (†Pycnodontiformes), which despite their high diversity are noticeably rare in the lagoonal faunas of the Solnhofen Plattenkalks (Ebert, 2016; Capasso, 2021; Cawley et al., 2021). Described materials of †‘Coccolepis’ liassica, †Coccolepis bucklandi, and †C. solnhofensis mostly comprise of complete and fully articulated fishes, which in the absence of regular terrestrial input into the Solnhofen Plattenkalk (e.g., Wellnhofer, 2009; Arratia et al., 2015) rules out the possibility that they were transported from a freshwater terrestrial setting. These coccolepidid taxa were evidently adapted to survive in fully-marine conditions, although their distribution does appear to have been controlled by water depth, with the Lias of Lyme Regis, the lower beds of the Posidonienschiefer Formation in Holzmaden, and the Solnhofen Plattenkalk each representing fairly shallow and near-shore coastal environments (Riegraf et al., 1984; Röhl et al., 2001; Hilton et al., 2004; Lord and Davis, 2010).
Coccolepidid Evolution in the Early Jurassic
†Coccolepididae first appears in the fossil record in the lowest Jurassic, with the oldest probable species, †‘Coccolepis’ liassica, described from the Lower Lias of Lyme Regis (Woodward, 1895; Gardiner, 1960). The precise collection horizon is unspecified for †‘C.’ liassica, with the cliff exposure at Lyme Regis composed of Lower Jurassic rocks dating between the Hettangian - Pliensbachian (Lord, 2019). Forey et al. (2010) attributes all known specimens of this species to the Sinemurian-aged beds based on their similar matrix. The only other described Early Jurassic coccolepidid, †Plesiococcolepis hunanensis, was recovered from a freshwater deposit in Hunan Province (China), although precise stratigraphic data is also lacking for this species. Both †‘C.’ liassica and †P. hunanensis are considered to be morphologically plesiomorphic members of †Coccolepididae on the basis that they both retain pelvic bones - a feature which is secondarily lost in younger coccolepidids, including †Coccolepis (Hilton et al., 2004; Skrzycka, 2014). The private Schandelah coccolepidid specimen apparently lacks ossified pelvic bones (Hauff et al., 2017), indicating that the loss of these elements in coccolepidid evolution occurred prior to the Toarcian. The pelvic region is unknown in the Holzmaden material.
According to Skrzycka (2014), coccolepidids most likely evolved in Europe during the earliest Jurassic, where they underwent a rapid palaeobiogeographic radiation, reaching as far as northeast Russia and central China by the beginning of the Middle Jurassic. Precise stratigraphic data for †Plesiococcolepis is unknown, so it is uncertain as to which point during the Early Jurassic coccolepidids first appeared in southern China. If this hypothesis is correct, then it would imply that coccolepidids first evolved in the marine realm, and later colonized freshwater habitats, something that has been proposed for many other fish lineages (Cavin et al., 2008; Cavin, 2017; Cavin et al., 2020). This hypothesis also fits with the results of Betancur-R et al. (2015), which demonstrated colonization and diversification dynamics in extinct actinopterygians to be asymmetrical between habitats, with marine lineages transitioning more frequently into freshwater habitats than the reverse. The Middle - Late Jurassic saw the highest diversity and richest global distribution of Coccolepididae, with species described from Australia (†‘Coccolepis’ australis Woodward, 1895), Patagonia (†Condorlepis groeberi López-Arbarello et al., 2013), Siberia (†Iyalepis rohoni (Sytchevskaya and Yakovlev, 1985)), Kazakhstan (†Morrolepis aniscowitchi (Gorizdro-Kulczycka, 1926)), the central United States (†Morrolepis schaefferi Kirkland, 1998), southern England (†Morrolepis andrewsi [Woodward, 1891]), and SE Germany (†Coccolepis bucklandi and †Coccolepis solnhofensis López-Arbarello and Ebert, 2021) (see Table 1). Coccolepidid diversity drastically declined after the Jurassic-Cretaceous boundary, with a few freshwater species persisting into the Early Cretaceous of southern England (†‘Coccolepis’ sp., Wealden Supergroup - Forey and Sweetman, 2011), Belgium (†Barbalepis macroptera (Traquair, 1911); Olive et al., 2019), southeast Australia (†Condorlepis woodwardi (Waldman, 1971); López-Arbarello et al., 2013), and possibly Yumen (Gansu Province), China (†Sunolepis yumenensis Liu, 1957: López-Arbarello and Ebert, 2021). This tendency of freshwater environments to serve as evolutionary refugia is a common pattern that is broadly documented in the Mesozoic fish record (Cavin, 2017) and is supported by analysis of ancestral states in Actinopterygii (Betancur-R et al., 2015); it remains an actively researched hypothesis that was first proposed by Charles Darwin (Cavin et al., 2008). The cause of the extinction of †Coccolepididae is unknown; the group entirely vanished by the Albian - Cenomanian stages of the Cretaceous (Olive et al., 2019).
Unresolved Interrelationships of Coccolepididae
The placement of Coccolepididae within Actinopterygii has changed several times and remains controversial (e.g., Woodward, 1891; Gardiner, 1960) with some recent authors proposing its inclusion within Chondrostei (Hilton et al. 2004; López-Arbarello et al., 2013; Olive et al., 2019) based on some shared characters with Acipenseriformes. Some authors disagree with this grouping (Schultze and Arratia, 2015), with Schultze et al. (2021) proposing possible referral of †Coccolepididae to a non-monophyletic grouping Palaeoniscimorpha on the basis that two of the unique characters of Chondrostei (symphysis between endochondral upper jaw bones, and absence of premaxilla and maxilla) are absent in coccolepidids. However, Schultze et al. (2021) does not discuss the synapomorphies shared between †Condrolepis groeberi and the Acipenseriformes: a parasphenoid with a median anterior process; an opercle smaller than the subopercle; the anterior margin of the subopercle with an anteriorly directed anterodorsal process; a single ventral caudal fulcrum; presence of a pectoral fin spine (Hilton et al., 2011: appendix III, characters 17, 22, 27, 52, 53); and the lateral line bending upwards and continuing in the body lobe following the bases of the caudal fin rays. Furthermore, †Condorlepis shares with †Birgeria and the Polyodontidae a very long and narrow supracleithrum, and along with several acipenserids, a supracleithrum which is as large or larger than the cleithrum (López-Arbarello et al., 2013). In †Condorlepis the antorbital bone is peculiarly shaped, resembling an inverted ‘Y’, as is the case with the most anterior lateral bone in the infraorbital series of Acipenser or †Peipiaosteus (López-Arbarello et al., 2013). The presence of an opercle that is smaller than the subopercle (Hilton et al. 2004) is irregularly distributed across †Coccolepididae, seen in †Condorlepis but not †Coccolepis and †Morrolepis (Skrycka, 2014; Schultze et al., 2021). Until rigorously tested with a phylogenetic analysis surveying characters across both Chondrostei and the taxa grouped in the non-monophyletic Palaeoniscimorpha, the true interrelationships of †Coccolepididae within Actinopterygii will remain debatable.
CONCLUSIONS
A new genus and species of coccolepidid fish, †Toarcocephalus morlok gen. et sp. nov., is described from the Lower Jurassic Posidonienschiefer Formation of southern Germany. Coccolepididae is reported for the first time from the Toarcian stage of the Early Jurassic, thereby bridging an outstanding gap in the fossil record. †Toarcocephalus morlok is diagnosed by the following characters: a large postorbital expansion of the maxilla that is strongly convex and unornamented; the lower jaw is longer than maxilla and is shallow posteriorly; the opercle is equal in size to the subopercle, with a short preopercle that only extends as high as the subopercle; a single row of teeth on the upper and lower jaw which are size graded (1 tall: 2-4 short: 1 tall); teeth well-spaced and recurved; few brachiostegal rays restricted to the posterior end of the lower jaw; skull roof very weakly tuberculated; operculum and pectoral girdle smooth with fine punctae. Based on its patchy fossil record, †Coccolepididae originated in the earliest Jurassic, likely in the marine setting of the Western Tethys, but quickly dispersed into terrestrial settings to become a predominately freshwater group from the Middle Jurassic onwards. The Holzmaden coccolepidid was easy prey for an unknown piscivore(s) in the assemblage, as both known specimens of this species show evidence of being predated on: one is preserved as a regurgitalite and the other as a decapitated pabulite.
ACKNOWLEDGMENTS
Emma Bernard (NHMUK) is thanked for allowing SC access to examine comparative coccolepidids and other actinopterygian specimens in the collection. R. Moreno (SMNS) assisted with specimen photography and photo stacking. S. Henderson and a second anonymous reviewer are warmly thanked for their detailed and insightful comments, which have greatly improved the scientific quality of this paper.
REFERENCES
Agassiz, L. 1832. Untersuchungen über die fossilen Fische der Lias- Formation. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde, 3:139–149.
Agassiz, L. 1833–1844. Recherches sur les Poissons Fossiles. Petitpierre, Neuchâtel.
Argyriou, T., Giles, S., Friedman, M., Romano, C., Kogan, I., and Sánchez-Villagra, M.R. 2018. Internal cranial anatomy of Early Triassic species of †Saurichthys (Actinopterygii: †Saurichthyiformes): implications for the phylogenetic placement of †saurichthyiforms. BMC Evolutionary Biology, 18:161.
https://doi.org/10.1186/s12862-018-1264-4
Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.) 2015. Solnhofen: Ein Fenster in die Jurazeit. Volume 1. Verlag Dr. Friedrich Pfeil, München.
Beardmore, S.R. and Furrer, H. 2016. Evidence of a preservational gradient in the skeletal anatomy of Ichthyopterygia (Reptilia) from Europe. Palaeogeography, Palaeoclimatology, Palaeoecology, 443, 131–144.
https://doi.org/10.1016/j.palaeo.2015.11.049
Berg, L.S. 1940. Classification of fishes both recent and fossil. Travaux de l’Institut zoologique de l’Académie des Sciences de l’URSS, 5:1–517.
Betancur-R, R., Ortí, G., and Pyron, R.A. 2015. Fossil-based comparative analysis reveal ancient marine ancestry erased by extinction in ray-finned fishes. Ecology Letters, 18:441–450.
https://doi.org/10.1111/ele.12423
Bordas, A. 1943. Peces del Cretáceo del rio Chubut (Patagonia). Physis, 19:313–318.
Bour, I., Mattiolo, E., and Pittet, B. 2017. Nannofacies analysis as a tool to reconstruct paleoenvironmental changes during the Early Toarcian anoxic event. Palaeogeography, Palaeoclimatology, Palaeoecology, 249:58–79.
https://doi.org/10.1016/j.palaeo.2007.01.013
Capasso, L. 2021. Pycnodonts: an overview and new insights in the Pycnodontomorpha Nursall, 2010. Occasional Papers of the University Museum of Chieti, Monographic Publication, 1:1–223.
Cavin, L. 2017. Freshwater fishes: 250 million years of evolutionary history. ISTE Press/Elsevier, London.
Cavin, L., Garcia, G., and Valentin, X. 2020. A minute freshwater pycnodont fish from the Late Cretaceous of southern France: palaeoecological implications. Cretaceous Research, 106:104242.
https://doi.org/10.1016/j.cretres.2019.104242
Cavin, L., Longbottom, A., and Richter, M. (eds.) 2008. Fishes and the Break-up of Pangaea. Geological Society of London Special publications, 295. The Geological Society, London.
Cawley, J.J., Marramà, G., Carnevale, G., Villafaña, J.A., López-Romero, F.A., and Kriwet, J. 2021. Rise and fall of †Pycnodontiformes: Diversity, competition and extinction of a successful fish clade. Ecology and Evolution, 11:1769–1796.
https://doi.org/10.1002/ece3.7168
Chang, M. and Miao, D. 2004. An overview of Mesozoic fishes in Asia, pp. 535–563 In Arratia, G. and Tintori, A. (eds.), Mesozoic Fishes 3 - Systematics, Paleoenvironments and Biodiversity. Dr. Friedrich Pfeil Verlag, Munich.
Cooper, S.L.A., Marson, K.J., Smith, R.E., and Martill, D.M. 2021. Contrasting preservation in pycnodont fishes reveals first record of regurgitalites from the Upper Cretaceous (Maastrichtian) Moroccan phosphate deposits. Cretaceous Research, 131:105111.
https://doi.org/10.1016/j.cretres.2021.105111
Cooper, S.L.A., Giles, S., Young, H., and Maxwell, E.E. 2022. A new large †pachycormiform (Teleosteomorpha: †Pachycormiformes) from the Lower Jurassic of Germany, with affinities to the suspension-feeding clade, and comments on the gastrointestinal anatomy of pachycormid fishes. Diversity, 14:1026.
https://doi.org/10.3390/d14121026
Cooper, S.L.A. and Maxwell, E.E. 2022. Revision of the pachycormid fish Saurostomus esocinus Agassiz from the Early Jurassic (Toarcian) of Europe with new insight into the origins of suspension-feeding in Pachycormidae. Papers in Palaeontology, 8:e1467.
https://doi.org/10.1002/spp2.1467
Cope, E.D. 1887. Zittel’s manual of palaeontology. American Naturalist, 17, 1014–1019.
Ebert, M. 2016. The Pycnodontidae (Actinopterygii) in the Late Jurassic: 2) Turboscinetes gen. nov. in the Solnhofen Archipelago (Germany) and Cerin (France). Archaeopteryx, 33:12–53.
Ebert, M., Thies, D., and Hauff, R.B. 2020. First evidence of ganoin-scaled Halecomorphi (Neopterygii) in the Lower Jurassic of Holzmaden and Ohmden, Germany. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 295:307–326.
https://doi.org/10.1127/njgpa/2020/0889
Ebert, M., Bapst, L., Kölbl-Ebert, M., Malvesy, T., and López-Arbarello, A. 2021. Coccolepis Agassiz, 1843 (Coccolepididae, Chondrostei) from the Upper Jurassic Solnhofen-Archipelago - rediscovery of the type specimen and open questions. Neues Jahrebuch für Geologie und Paläontologie Abhandlugen, 300:11–22.
https://doi.org/10.1127/njgpa/2021/0976
Egerton, P. 1853. On some new genera and species of fossil fishes. The Annals and Magazine of Natural History, London, 13:433–436.
Forey, P., Longbottom, A., and Mulley, J. 2010. Fishes–Bony Fishes, pp. 341–371. In Lord, A.L. and Davis, P.G. (eds.), Fossils from the Lower Lias of The Dorset Coast. Palaeontological Association Field Guide to Fossils, 13, London, UK.
Forey, P. and Sweetman, S.C. 2011. 18. Bony Fishes, pp. 225–235. In Batten, D.J. (ed.), English Wealden Fossils. Palaeontological Association Field Guide to Fossils. The Palaeontological Association, London.
Freimuth, W.J., Varricchio, D.J., Brannick, A.L., Weaver, N.L., and Mantilla, G.P.W. 2021. Mammal-bearing gastric pellets potentially attributable to Troodon formosus at the Cretaceous Egg Mountain locality, Two Medicine Formation, Montana, USA. Palaeontology, 65:699–725.
https://doi.org/10.1111/pala.12546
Gardiner, B.G. 1960. A revision of certain actinopterygian and coelacanth fishes, chiefly from the Lower Lias. Bulletin of The British Museum (Natural History), 4:239–384.
Gardiner, B.G. 1967. Further notes on palaeoniscid fishes with a classification of the Chondrostei. Bulletin of The British Museum (Natural History) Geology, 14:144–206.
Gardiner, B.G. 1993. Osteichthyes: basal actinopterygians, pp. 611–619. In Benton, M.J. (ed.), The Fossil Record 2. Chapman and Hall, London, UK.
Gordon, C.M., Roach, B.T., Parker, W.G., and Briggs, D.E. 2020. Distinguishing regurgitalites and coprolites: a case study using Triassic bromolite with soft tissue of the pseudosuchian archosaur Revueltosaurus. PALAIOS, 35:111–121.
https://doi.org/10.2110/palo.2019.099
Gorizdro-Kulczycka, F. 1926. Fishes of the Karatau shales. Izvestiya Sredneazitskogo Komiteta, 1:184–192. (In Russian)
Grande, L. and Bemis, W.E. 1996. Interrelationships of Acipenseriformes, with comments on “Chondrostei”, pp. 85–115. In Stiassny, M.J.L., Parenti, L.R., and Johnson, G.D. (eds.), Interrelationships of Fishes. Academic Press, San Diego, USA.
Hauff, B. 1921. Untersuchung der Fossilfundstätten von Holzmaden im Posidonienschiefer des Oberern Lias Württembergs. Palaeontolographica, 64:1–42.
Hauff, B. and Hauff, R.B. 1981. Das Holzmadenbuch. Repro-Druck GmbH, Fellbach.
Hauff, R.B., Heunisch, C., Hochsprung, U., Ilgar, J.-M., Joger, U., Klopschar, M., Kosma, R., Krüger, F.J., Thies, D., and Zellmer, H. eds. 2017. Jurameer: Niedersachsens versunkene Urwelt. Verlag Dr. Friedrich Pfeil, München, Germany.
Hilton, E.J. and Forey, P. 2009. Redescription of †Chondrosteus acipenseroides Egerton, 1858 (Acipenseriformes, †Chondrosteidae) from the Lower Lias of Lyme Regis (Dorset, England), with comments on the early evolution of sturgeons and paddlefishes. Journal of Systematic Palaeontology, 7:427–453.
https://doi.org/10.1017/S1477201909002740
Hilton, E.J., Grande, L., and Bemis, W.E. 2004. Morphology of Coccolepis bucklandi Agassiz, 1843 (Actinopterygii, Coccolepidae) from the Solnhofen Lithographic Limestone deposits (Upper Jurassic, Germany), pp. 209–238. In Arratia, G. and Tintori, A. (eds.), Mesozoic Fishes 3 - Systematics, Paleoenvironments and Biodiversity). Dr. Friedrich Pfeil Verlag, Munich, Germany.
Hilton, E.J., Grande, L., and Bemis, W.E. 2011. Skeletal anatomy of the short-nose sturgeon, Acipenser brevirostrum Lesueuer 1818, and the systematics of sturgeons (Acipenseriformes, Acipenseridae). Fieldiana (Life and Earth Sciences), 3:1–168.
Hoffman, R., Stevens, K., Keupp, H., Simonson, S., and Schweigert, G. 2019. Regurgitalites: a window into the trophic ecology of fossil cephalopods. Journal of the Geological Society, 117:82–102.
https://doi.org/10.1144/jgs2019-117
Hunt, A.P. 1992. Late Pennsylvanian coprolites from the Kinney Brick Quarry, central New Mexico, with notes on the classification and utility of coprolites. New Mexico Bureau of Mines and Mineral Resources Bulletin, 138:221–229.
Jäger, M. 2005. The museum of fossils in the Werkforum: Guidebook of the exhibition of Jurassic fossils. Holcim, Dotternhausen.
Keller, T. 1997. Fraßreste im süddeutschen Posidonienschiefer. Jahreshefte der Gesellschaft für Naturkunde in Württemberg, 132:117–134.
Kirkland, J.I. 1998. Morrison fishes. Modern Geology, 22:503–533.
Klug, C., Schweigert, G., Hoffmann, R., Weis, R., and De Baets, K. 2021. Fossilized leftover falls as sources of palaeoecological data: a ‘pabulite’ comprising a crustacean, a belemnite and a vertebrate from the Early Jurassic Posidonia Shale. Swiss Journal of Geosciences, 140:10.
https://doi.org/10.1186/s13358-021000225-z
Liu, T.S. 1957. A new Cretaceous palaeoniscid fish from Yumen of the Chiuchuan Basin, western Kansu. Vertebrata Palasiatica, 1:103–122.
López-Arbarello, A. 2012. Phylogenetic interrelationships of ginglymodian fishes (Actinopterygii: Neopterygii). PLoS ONE, 7:e39370.
https://doi.org/10.1371/journal.pone.0039370
López-Arbarello, A., Sferco, E., and Rauhu, O.W.M. 2013. A new genus of coccolepidid fishes (Actinopterygii, Chondrostei) from the continental Jurassic of Patagonia. Palaeontologia Electronica, 16.1.7A.
https://doi.org/10.26879/348
López-Arbarello, A. and Ebert, M. 2021. Diversity of chondrostean fish Coccolepis from the Late Jurassic Solnhofen Archipelago, Southern Germany. Acta Palaeontologica Polonica, 66:837–846.
https://doi.org/10.4202/app.00873.2021
Lord, R. (ed.) 2019. Fossils from the Lias of the Yorkshire Coast. Palaeontological Association Field Guide to Fossils: Number 15. The Palaeontological Association, London, UK.
Lord, A.R. and Davis, P.G. (eds.) 2010. Fossils from the Lower Lias of the Dorset Coast. Palaeontological Association Field Guide to Fossils. The Palaeontological Association, London, UK.
Martill, D.M. 1993. Soupy substrates: a medium for the exceptional preservation of ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany. Kaupia, 1993:77–97.
Maxwell, E.E. and Stumpf, S. 2017. Revision of Saurorhynchus (Actinopterygii: Saurichthyidae) from the Early Jurassic of England and Germany. European Journal of Taxonomy, 321:1–29.
https://doi.org/10.5852/ejt.2017.321
Maxwell, E.E., Cooper, S.L.A., Mujal, E., Miedema, F., Serafini, G., and Schweigert, G. 2022. Evaluating the existence of vertebrate deadfall communities from the Early Jurassic Posidonienschiefer Formation. Geosciences, 12:158.
https://doi.org/10.3390/geosciences12040158
Mönnig, E., Fraz, M., and Schweigert, G. 2018. Der Jura in der Stratigraphischen Tabelle von Deutschland (STD 2016). Zeitschrift der Deutschen Gesellschaft für Geowissenschaften, 169:225–246.
Müller, J. 1845. Über den Bau und die Grenzen Ganoiden un das natürliche System der Fusche. Abhandlungen der Akademie der Wissenschaften zu Berlin, 1845:117–216.
Myhrvold, N.P. 2012. A call to search for fossilized gastric pellets. Historical Biology, 24:505–517.
https://doi.org/10.1080/08912963.2011.631703
Nielson, E. 1942. Studies on Triassic fishes from East Greenland. 2. Glaucolepis and Boreosomus. Palaeozoologica Groenlandica, 1:1–403.
Olive, S., Taverne, L., and López-Arbarello, A. 2019. A new genus of coccolepidid actinopterygian from the Cretaceous Iguanodon -bearing locality of Bernissart, Belgium. Cretaceous Research, 95:318–335.
https://doi.org/10.1016/j.cretres.2018.11.020
Qvarnström, M., Ahlberg, P.E., and Niedzwiedzki, G. 2019. Tyrannosauroid-like osteophagy by a Triassic archosaur. Scientific Reports, 9:925.
https://doi.org/10.1038/s41598-018-37540-4
Riegraf, W., Werner, G., and Lörcher, F. 1984. Der Posidonienschiefer. Biostratigraphie, Fauna und Fazies des südwestdeutschen Untertoarciums (Lias ε). Ferdinand Enke, Stuttgart.
Röhl, H.-J., Schmid-Röhl, A., Oschmann, W., Frimmel, A., and Schwark, L. 2001. Erratum to “The Posidonia Shale (Lower Toarcian) of SW-Germany:An oxygen-depleted ecosystem controlled by sea level and palaeoclimate. Palaeogeography, Palaeoclimatology, Palaeoecology, 169:273–299.
https://doi.org/10.1016/S0031-0182(01)00201-2
Röhl, H.-J. and Schmid-Röhl, A. 2005. Lower Toarcian (Upper Liassic) Black Shales of the Central European Epicontinental Basin: a sequence stratigraphic case study from the SW German Posidonia Shale. SEPM Special Publication, 82:165–189.
Schmid-Röhl, A., Röhl, H.-J., Oschmann, W., Frimmel, A., and Schwark, L. 2002. Palaeoenvironmental reconstruction of Lower Toarcian epicontinental black shales (Posidonia Shale, SW Germany): global versus regional control. Geobios, 35:13–20.
https://doi.org/10.1016/S0016-6995(02)00005-0
Schultze, H.-P. 2016. Scales, enamel, cosmine, ganoine, and early osteichthyans. Comptes Rendus Palevol, 15:83–102.
https://doi.org/10.1016/j.crpv.2015.04.001
Schultze, H.-P. and Arratia, G. 2015. Knochenfische im weiteren Sinne (Osteichthyes oder Osteognathostomata), pp. 360–409. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.). Solnhofen. Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Schultze, H.-P., Mickle, K.E., Poplin, C., Hilton, E.J., and Grande, L. 2021. Handbook of Paleoichthyology. Volume 8A. Actinopterygii I. Palaeoniscimorpha, Stem Neopterygii, Chondrostei. Verlag Dr. Friedrich Pfeil, München.
Seilacher, D. 1982. Ammonite shells as habitats in the Posidonia Shales of of Holzmaden - floats or benthic islands? Neues Jahrbuch für Geologie und Paläontologie - Monatshefte, 82:98–114.
Seilacher, A. 2002. The strange taphonomy of coprolites and cololites, pp. 233–240. In De Renzi, M., Pardo, M., Belinchon, M., Penalver, E., Montoya, P., and Márques Aliaga, A. (eds.), Current Topics on Taphonomy and Fossilization. Ayuntamiento de Valencia, Valencia.
Serafini, G., Gordon, C.M., Foffa, D., Cobianchi, M., and Giusberti, L. 2022. Tough to digest: first record of Teleosauroidea (Thalattosuchia) in a regurgitalite from the Upper Jurassic of north-eastern Italy. Papers in Palaeontology, 8:e1474.
https://doi.org/10.1002/spp2.1474
Skrzycka, R. 2014. Revision of two relic actinopterygians from the Middle or Upper Jurassic Karabastau Formation, Karatau Range, Kazakhstan. Alcheringa, 38:1–27.
Sytchevskaya, E.K. 2006. Iyalepis nom. nov., a new replacement name for Angerichthys Sytchevskaya et Yakovlev, 1985 (Pisces, Palaeonisciformes, Coccolepididae). Palaeontological Journal, 40:339.
Sytchevskaya, E.K. and Yakovlev, V.N. 1985. Fishes, pp. 132–136. In Jurassic Continental Biocenoses of Southern Siberia and Adjacent Areas. Nauka, Moscow. (In Russian)
Thies, D. and Hauff, R.B. 2011. A new species of Dapedium Leach, 1822 (Actinopterygii, Neopterygii, Semionotiformes) from the Early Jurassic of south Germany. Palaeodiversity, 4:185–221.
Thies, D. and Hauff, R.B. 2012. A Speiballen from the Lower Jurassic Posidonia Shale of south Germany. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 267:117–124.
https://doi.org/10.1127/0077-7749/2012/0301
Thies, D. and Herzog, A. 1999. New information on †Dapedium Leach, 1822 (Actinopterygii, †Semionotiformes), pp. 143–152. In Arratia, G. and Schultze, H.-P. (eds.), Mesozoic Fishes 2 - Systematics and Fossil Record. Verlag Dr. Friedrich Pfeil, München, Germany.
Trabucho-Alexandre, J., Dirks, R., Veld, H., Klaver, G., and De Boer, P.L. 2012. Toarcian black shales in the Dutch Central Graben: Record of energetic, variable depositional conditions during an oceanic anoxic event. Journal of Sediment Research, 82:165–189.
Traquair, R.H. 1911. Les poissons wealdiens de Bernissart. Mémoires du Musée royal d’Histoire naturelle de Belgique, 21:1–65.
Waldman, M. 1971. Fish from the freshwater Lower Cretaceous of Victoria, Australia, with comments on the palaeo-environment. Special Papers in Palaeontology, 9:1–124.
Wang, N. 1977. Jurassic fish and their stratigraphic significance from the Hengyang Region of Lingling Co., Hunan Province. Vertebrata PalAsiatica, 15:233–243. (In Chinese)
Wellnhofer, P. 2009. Archaeopteryx - The Icon of Evolution. Verlag Dr. Friedrich Pfeil, München, Germany.
White, E.I. 1925. Additions to the Upper Liassic fish-fauna of Holzmaden. The Annals and Magazine of Natural History, 9:601–610.
Woodward, A.S. 1890. Notes on some ganoid fishes from the English Lower Lias. The Annals and Magazine of Natural History, 6:430–436.
Woodward, A.S. 1891. Catalogue of the Fossil Fishes in the British Museum (Natural History). Longmans and Co., London, UK.
Woodward, A.S. 1895. Catalogue of the fossil fishes in the British Museum (Natural History). British Museum (Natural History), London, UK.

