 Seagrass and cuttlefish—an historic association
Seagrass and cuttlefish—an historic association
Article number: 22.3.79
https://doi.org/10.26879/881
Copyright Palaeontological Association, December 2019
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 22 April 2018. Acceptance: 25 November 2019.
ABSTRACT
Except for the New World and Antarctica, cuttlefish are currently found globally in seagrass and other environments. They had an origin in the Late Cretaceous in the North Atlantic. Although cuttlefish are generally rare, Belosaepiidae may locally be more common. These and Sepiidae are recorded in faunal collections that may have come from the proximity of past seagrass environments. By the middle Eocene, cuttlefish may have evolved into modern Sepia. The record of cuttlefish in the New World ceased by the end of the Eocene, possibly as a result of climate cooling. The lack of cuttlefish in the New World continues to the present day. The distribution of cuttlefish during the Late Cretaceous and Cenozoic are strongly clustered in the North Atlantic area including Tethys where seagrass had its origin and from which it later radiated globally. Cuttlefish had a relationship with seagrass, which was formed in the Late Cretaceous and this continues to the present day. This fidelity may be due to cuttlefish behaviours in seagrass including feeding, protection, reproduction and seagrass acting as nursery. Fossil cuttlefish are nearly restricted to seagrass environments, which provided the appropriate preservational conditions. Future taphonomic studies may provide reasons for their rarity and near absence from other environments.
George F. Forsey. 13 Maclean Close, Northampton, NN3 3DJ, UK. georgesue4c@virginmedia.com
Keywords: cuttlefish; seagrass; Belosaepiidae; Sepiidae; nursery; taphonomy
Forsey, George F. 2019. Seagrass and cuttlefish—an historic association. Palaeontologia Electronica 22.3.79 1–24. https://doi.org/10.26879/881
palaeo-electronica.org/content/2019/2852-seagrass-and-cuttlefish
Copyright: December 2019 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Cuttlefish and their presumed fossil relatives were investigated as part of a larger study of functional groups in faunas from possible past seagrass. Currently cuttlefish have a wide geographical coverage except for the New World (Neige, 2003a, 2003b) and can be found in a number of marine environments. They have been recorded from seagrass (Jager, 1993; Rueda et al., 2009; Cox and Scholar, 2017; O'Brien et al., 2017; Petović et al., 2017), mangrove (Pawar, 2012) and in the green alga Caulerpa prolifera from Crete (eastern Mediterranean) (Koulouri et al., 2016). Zavodnik et al. (2006) provided an exhaustive faunal list from seagrass related environments around Pag Island (Adriatic Sea, Croatia), which included Sepiidae (Table 1).
Seagrass provides an important nursery for cuttlefish, which lay their eggs on the plants (Ezzedine-Najai, 1997; Blanc et al., 1998; Blanc and Daguzan, 1998; Bloor et al., 2013a; Jackson et al., 2015). Shelter and nursery for fossil as well as extant cuttlefish may have been provided by seagrass (Bałuk and Radwański, 1977). Predatory ambush hunters like cuttlefish would find seagrass attractive.
Tracking tagged cuttlefish in the gulf of Tunis, which is a major seagrass area in the Mediterranean (Telesca et al., 2015), showed they moved towards the littoral zone during the spawning season (Ezzedine-Najai, 1997). Some species of cuttlefish are involved in commercial fisheries, which would be adversely affected by seagrass decline (Jackson et al., 2015). Cuttlefish spent about 20% of their time in seagrass with juveniles spending more time than other commercial forms (Jackson et al., 2015), presumably using seagrass as a nursery. Bloor et al. (2013b) noted that Sepia officinalis moved in and out of seagrass environments. There are ~100 living species mostly attributable to Sepia (Neige, 2003b). Košťák et al. (2016) indicates a similar fossil number, mostly belosaepiids sensu lato, with only ~20 species related to Sepia.
Extant cuttlefish are likely to be in seagrass environments, which are utilised for feeding, spawning and as a nursery. This account indicates that this may be an old relationship possibly established during the early appearances of seagrass in the late Cretaceous. Although sepiids may have had an origin in the Early Cretaceous (Kröger et al., 2011), the first fossil evidence is from the Late Cretaceous (Clements et al., 2017). A molecular age suggests a separation age of 88 Ma for sepiids (Tanner et al., 2017), just prior to the appearance of seagrass in the early Campanian (Late Cretaceous).
Fossil cuttlefish are comparatively rare, consisting of the cuttlebone or its fragments. There is a single record of cuttlefish statoliths from the middle Eocene of France (Neige et al., 2016). Part of this rarity may be due to the fibrous, aragonitic nature of the cuttlebone (Checa et al., 2015) as well as preservational conditions.
METHODS
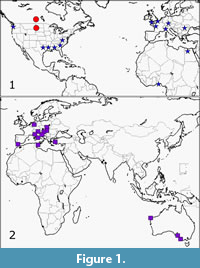 Extensive use of the Paleobiology Database was made. This involved literature searches noted for cuttlefish records and associated faunas using appropriate key words such as cuttlefish, Cephalopoda and Sepiidae. The recorded associated faunas which emerged were then searched for possible seagrass indicators. For a few results, no associated faunas could be found. These mostly compose the ~20% of records for which no past seagrass relationship could be interpreted. Online literature searches provided further information on Recent and fossil faunas related to cuttlefish sites (Figure 1).
Extensive use of the Paleobiology Database was made. This involved literature searches noted for cuttlefish records and associated faunas using appropriate key words such as cuttlefish, Cephalopoda and Sepiidae. The recorded associated faunas which emerged were then searched for possible seagrass indicators. For a few results, no associated faunas could be found. These mostly compose the ~20% of records for which no past seagrass relationship could be interpreted. Online literature searches provided further information on Recent and fossil faunas related to cuttlefish sites (Figure 1).
The Paleobiology Database is a widely used resource for inputting and extracting information on fossil taxa and fossil faunal assemblages. The Paleobiology Database can be accessed directly or through Fossilworks: Gateway to the Paleobiology Database (fossilworks.org). It can be referred to as PaleoDatabase and accession to data results in a number referring to the faunal assemblage. In this account, such numbers are prefixed by PDB. These faunal assemblages have a primary reference, but the faunal assemblage may contain information from other references. A `more details` link leads to other related faunas.
Since seagrass has low preservation potential, (only three of the results used here record seagrass), several indications for past seagrass have been used. This multiproxy approach overcomes problems where indicators may be found in other environments as well as seagrass. Seagrass often has a large fauna with extensive diversity of abundant foraminifers, especially large benthic foraminifera such as Amphistegina, Peneroplis and soritids, molluscs (bivalves, including lucinids and pinnids and gastropods such as Smaragdia, Jujubinus, Atys, Bittium, Tricolia, and rissoids), ostracods, fish (Sparidae, Sygnathus), palaeophiid snakes and presence of sirenians (Domning, 2001; Brzobohatý et al., 2007; Vélez-Juarbe, 2014; Reich, 2014; Reich et al., 2015; Forsey, 2016; Koskeridou et al., 2019). Past seagrass interpretations were accepted for the presence of seagrass and those made by earlier authors. Interpretations made here were based on the presence of indicators noted.
Available literature to find cuttlefish records have been used but there are others which have not been located, particularly to older literature. Often, although extant Sepia is recorded as associated with seagrass, other elements of the fauna are not given. An example from Pag Island (Croatia) (Zavodnik et al., 2006) provides selected details of an extant warm temperate seagrass fauna compared to a fossil fauna from the early Pleistocene (Rhodes, Greece) (Moissette et al., 2007, 2016; Koskeridou et al., 2019) (Table 1). Similar taxa have been used from the fossil record to interpret past seagrass in this account.
RESULTS
Considering that cuttlefish apparently spend a minority of their time in seagrass and some in areas in proximity to seagrass, the results indicate a closer relationship of cuttlefish to past seagrass than may have been anticipated. Around 80% of the records indicate a possible relationship between cuttlefish and seagrass. Figure 1 indicates distribution of fossil cuttlefish and other records used in this account. About 20% (seven records) of faunal records contained seagrass or had previously been interpreted as seagrass. The results have been divided into two sets; Late Cretaceous-Eocene and Oligocene-Pleistocene.
Late Cretaceous-Eocene
Hewitt and Jagt (1999) described Ceratisepia vanknippenbergi from a fine- to coarse-grained calcarenite at the base of the Gronsveld Member (late Maastrichtian) at the ENCI-Maastricht BV quarry (Maastricht, Netherlands). From the same quarry and member, Duffin and Reynders (1995) provided a list of the associated biota including the seagrass Thalassocharis bosqueti and Pinna gr. cretacea. Jagt et al. (2019) recorded seagrass accumulations including a pinnid from the Gronsveld Member. Also associated was a scaphitid ammonite, sharks and a mosasaur, the latter two possibly being cephalopod predators.
Meyer (1993) described Ceratisepia elongata from the limestones of the Calcaire de Vigny Formation (early Paleocene) of Vigny (France) from where Pacaud et al (2000) recorded an extensive molluscan fauna containing three lucinid bivalves and the seagrass associated gastropods Jujubinus and Rissoina indicative of seagrass (PDB [=Paleobiology Database accession number] 20816). The presence of potamidid and batillariid gastropods are suggestive of an input from mangrove mud flats.
Košťák et al. (2013) recorded Aegyptosaepia lugeri and incompletely preserved remains of ?Anomalosaepia from marls of the Garra Formation (late Paleocene) of the Western Desert (Egypt) (PDB 172932-172934) but without a fauna for seagrass interpretation
Košťák and Hoşgör (2012) recorded a single specimen of Belosaepia sp. from the thin-bedded argillaceous limestones and mudstones of the Kavalköy Formation (Ypresian, early Eocene) of Cilo, Turkey. They dated this on the basis of larger benthic foraminifera, including soritid and Alveolina, which are suggestive of seagrass (Košťák and Hoşgör, 2012).
Belosepia pennae was recorded from several localities from shallow subtidal, sandy claystones of the Marquez Shale Member (Reklaw Formation, Ypresian, early Eocene of Texas, USA) with extensive molluscan faunas including lucinids ?Atrina, ?Pinna sp. and the seagrass associated gastropod Bittium spp. (Garvie, 1996; PDB 7788, 7789, 83950, 84337).
From shallow subtidal, glauconitic marl from Hatchetigbee, Washington County, Alabama (USA) Hatchetigbee Bluff Member (Hatchetigbee Bluff Formation, Ypresian, lower Eocene), Toulmin (1977) recorded a molluscan fauna with a lucinid and Belosaepia indet. (PDB 80792).
A large molluscan fauna with no seagrass indicators recorded from shallow subtidal, glauconitic, calcareous sandstone from Lisbon, Choctaw County (Alabama, USA) (Lisbon Formation, Lutetian, middle Eocene) contained Belosaepia veatchi (Toulmin, 1977; PDB 81224).
From the Lower Lisbon Member (Lisbon Formation, Lutetian, middle Eocene) of Monroe County (Alabama, USA) from a glauconitic, silty, calcareous sandstone Belosaepia saccaria and Belosaepia uncinata were recorded associated with a small gastropod fauna with no seagrass indicators (Palmer and Brann, 1965; PDB 92351). From the Upper Lisbon Member Belosaepia alabamensis, Belosaepia harrisi, Belosaepia uncinata were recorded from a large molluscan fauna containing lucinids (Palmer and Brann, 1965; PDB 98355).
A large molluscan fauna from temporary exposures of sandstone of the Nummulites laevigatus Bed (Earnley Formation, Lutetian, early Eocene) of Hampshire, England (PDB 3258), was recorded by Bone et al. (1991), which included Belosepia sepioidea, four lucinids, seagrass associated gastropod Rissoina and labrid fish (Labrus) suggests a seagrass environment. Associated vertebrates included sharks as possible predators and cheloniid and palaeophiid reptiles (PDB 4187).
Williams (2002) recorded a fauna including Belosepia sp., thyasirid bivalves, decapod crustaceans and the extinct marine snake Palaeophis from silty clays of the London Clay excavation at Aveley, Essex, England (PDB 76601). Belosepia sepioidea and Belosepia blainvillei were noted from the London Clay of Sheppey (England) (Newton and Harris, 1894). Friedman et al. (2016) noted the sparid fish Sciaenurus bowerbanki, Podocephalus nitidus, Podocephalus curryi and the labrids Phyllodus toliapicus from all London Clay (Ypresian, early Eocene) divisions at Sheppey (England) (PDB 13299). Brignon (2018) indicated that a vertebra previously identified as crocodile from the London Clay of Sheppey was that of the palaeophiid snake Palaeophis toliapicus.
Tracey et al (1996) recorded an extensive molluscan fauna from a glauconitic, argillaceous, silty sandstone from the Selsey Formation (Lutetian, middle Eocene) from Selsey (West Sussex, England) containing Belosaepia sepioidea, the seagrass Posidonia sp., six lucinids, a pinnid, seagrass related gastropods (Alvania, Rissoina) and six potamidids (PDB 8124) suggestive of a seagrass community close to mangrove.
Hewitt and Jagt (1999) noted several specimens of Belosaepia sepioidea from Barton, Hampshire, southern England, Barton Beds (Bartonian, middle Eocene). An extensive fauna from the glauconitic, sandy claystones of horizon A3 of the Barton Beds contained Belosepia sepioidea, a lucinid, potamidid and batillariid gastropods, seagrass associated gastropods (Alvania, Bittium), sparids Sparidarum Dentex and the extinct marine snake Palaeophis (Burton, 1933; PDB 42636). Burton (1933) noted rhizomes from possible aquatic plants, which may have been seagrass. Stinton (1984) recorded sparid fish otoliths from Barton and elsewhere suggesting that there might have been seagrass meadows in southern England during parts of the Eocene. The fauna suggests seagrass presence in the early part of the Barton Beds (Middle Eocene) associated with mangroves.
Belosepia proxima was recorded from glauconitic sandstone from Boekelo (Netherlands) (Bartonian, middle Eocene) in a molluscan fauna containing a lucinid (PDB 3718).
Yancey et al. (2010) described the ontogeny of Belosaepia ungula based on 160 individuals, mostly consisting of the strongly calcified posterior portion of the skeleton, from the Crockett Formation (Bartonian stage, middle Eocene) of Texas compared to similar aged material in Mississippi, Alabama and elsewhere in Texas. No information was available to judge on past seagrass presence.
From offshore, glauconitic, argillaceous sandstone of the Stone City Formation (Bartonian, middle Eocene) from Burleson County, Texas Belosaepia ungulata with diverse fauna including a large molluscan fauna but no seagrass indicators was recorded (PDB 7439).
Belosepia sepioidea fragments were recorded from carbonaceous shale and claystone associated with an estuarine molluscan fauna containing Pinna, several lucinids, the foraminifera Peneroplis and potamidids from Ameki (Nigeria, Ameki Formation, Lutetian, middle Eocene) suggestive of a seagrass environment with associated mangrove (Newton, 1922; PDB 51240-51242, 60711). Belosaepia sepioides was reported from offshore shale of the Ilaro Formation (Lutetian) overlying the Paleocene of south-western Nigeria from a diverse fauna lacking seagrass indicators (PDB 77257).
Neige et al. (2016) recorded coleoid statoliths from the middle Lutetian (middle Eocene) of Thiverval-Grignon (Paris Basin, France). They erected two new species, Sepia boletzkyi and ?Sepia pira for statoliths from beds 3b (clayey limestone) and 4a (glauconitic, calcareous sand) at the Thiverval-Grignon section. As well as the seagrass Cymodoceites (bed 3b) they also recorded the seagrass related soritid foraminifera Orbitolites (beds 3b and 4a). Bairdoppilata gliberti, Xestoleberis subglobosa and a rare loxoconchid were recorded from the same horizons (Guernet et al., 2012). These ostracods were thought to be suggestive of seagrass (Forsey, 2016). Sample PB4 of Dominici and Zuschin (2016), approximately the same level as beds 3b/4a, contained the lucinid Parvilucina turgidula. Huyghe et al. (2012) recorded the lucinid bivalve Saxolucina saxorum and mud flat related gastropod Batillaria from higher up the sequence. Hence, cuttlefish remains were reported from a possible seagrass environment.
Szôrényi (1934) recorded new species Sepia oligocaenica, Sepia harmati and Belosepia and erected the genus Archaeosepia for two species including a new species Archaeosepia naefi from the Lutetian (middle Eocene) of Tatabánya, Hungary. By not designating a type species she was in breach of the International Code of Zoological Nomenclature. Doyle et al. (1994) corrected this by erecting Hungarosepia with type species Archaeosepia naefi Szôrényi, 1934. Kordos (2002) recorded a number of sirenian remains from the middle Eocene of Tatabánya, Hungary, indicating past seagrass proximity.
Fragments of Belosaepia blainvillei and B. dufouri were recorded from the shelly quartz sands of Bois-Gouët (Loire-Atlantique, France) (Bartonian, middle Eocene) from an extensive molluscan fauna including lucinids (Lebrun et al., 2012).
The Gosport Sand (Bartonian, Middle Eocene) is highly fossiliferous with 495 molluscan species recorded from glauconitic, calcareous quartz sand, with carbonaceous shale (Pietsch et al., 2016). Several Belosaepia species have been recorded from the Claiborne Bluff locality (Monroe County, Alabama, USA) from the Gosport Sand. These include, Palmer (1937) who recorded B. uncinata, B. alabamensis and B. harrisi; Palmer and Brann (1965) who recorded an extensive molluscan fauna including nine lucinids and seven species of Belosaepia Claiborne Bluff (PDB 90600 and other collections) and Allen (1968) who recorded beak fragments of Belosaepia vokesi. CoBabe and Allmon (1994) recorded four lucinids from extensive molluscan collections and the seagrass associated gastropod Atys (PDB 5320, 5321, 5322 and others). Arata and Jackson (1965) noted a record of a sirenian rib fragment from the Gosport Sand (Monroe County, Alabama, USA). These are suggestive of seagrass at the Claiborne Bluff locality. Outside of this locality Blake (1950) described ostracods from the Little Stave Creek (Clarke County, Alabama, USA, Gosport Formation, middle Eocene), including Bairdia, Bairdoppilata, Xestoleberis and Loxoconcha (PDB 94040), considered to indicate a past seagrass environment (Forsey, 2016). CoBabe and Allmon (1994) (PDB 5318) noted the molluscan fauna from the same site, which included lucinid bivalves. Arata and Jackson (1965) recorded a sirenian rib fragment from Little Stave Creek (Gosport Formation). Haveles and Ivany (2010) indicated that the large size of some Gosport molluscs indicated a high productivity environment (such as might be found in seagrass). These records suggest that past seagrass environments may have been prevalent through the Gosport Sand Formation.
From the Cook Mountain Formation (Bartonian, Middle Eocene) of Texas and Louisiana (USA), Belosaepia uncinata, B. ungula, B. veatchi, B. stenzeli and B. jeletzkyi were recorded from small molluscan faunas without seagrass indicators (Allen, 1968; Palmer and Brann, 1965; PDB 92346, 98787, 99026). Domning et al. (1982) recorded sirenians from Cook Mountain Formation of Texas.
Fornaseiro and Vicariotto (1995) recorded Archaeosepia monticulimajoris from a shallow subtidal shelly argillaceous, calcareous marl (Marna di Priabona Formation, Priabonian, late Eocene) with a fauna including a lucinid from the Veneto Region of Italy (PDB 81043). Archaeosepia (and presumably Hungarosepia?) may represent the earliest representative of the Sepia lineage (Košťák et al., 2016).
Weaver and Ciampaglio (2003) proposed the genus Anomalosaepia with four species based on several hundred fragmentary specimens from spoil heaps from Martin Marietta quarries (New Hanover, Pender and Onslow counties, North Carolina, USA) from bryozoan-echinoid calcirudites and calcarenites from possibly the Comfort member of the Castle Hayne Limestone (Bartonian, middle Eocene). Sirenian fragments, molluscan faunas with lucinids, Strombus and schizasterid echinoids were recorded from the New Hanover and Pender localities (Kellum, 1926; Palmer and Brann, 1965; Domning et al., 1982; Powell and Baum, 1982; PDB 5436, 5437, 5366, 5386, 5367, 91736, 91737) indicative of seagrass across the Castle Hayne Limestone area in North Carolina. In addition, the palaeophiid snake Palaeophis grandis has been recorded from the Castle Hayne Formation (Anonymous, 2015, figure 11).
The belosaepiid, Mississaepia mississippiensis, was recorded from two fragments from the Town Creek, Jackson, Hinds County, Mississippi (Moodys Branch Formation, middle Eocene) (Weaver et al, 2010b). The Moodys Branch Formation is a thin transgressive unit consisting of glauconitic sands and marls. Molluscan faunas containing lucinids and pinnid have been recorded from the Town Creek locality (Dockery, 1977; PDB 164232) indicative of past seagrass. Doguzhaeva et al. (2014) noted that taphonomic condition implied that Mississaepia mississippiensis was buried were it lived in waters estimated to be 25-50 m.
Allen (1968) recorded poorly preserved Belosaepia sp. from Moodys Branch Formation at Montgomery Landing (Grant County, Louisiana, USA). From the same site from argillaceous, calcareous siltstone molluscan faunas contained lucinid and pinnid bivalves, palaeophiid snake and schizasterid echinoids (Toulmin, 1977; PDB 1413-1415).
Belosaepiids have been recorded from fossiliferous, blocky, blue-gray clay from the Miss Lite Clay Pit, Cynthia, Hinds County, Mississippi (Yazoo Clay, late Eocene) (Haasl and Hansen, 1996; Weaver et al, 2010b; PDB 6802). Amongst, the recorded molluscan fauna were lucinid and pinnid bivalves, seagrass associated gastropod Bittium and a palaeophiid snake; a basilosaurid may have been a predator (Haasl and Hansen, 1996; PDB 6802). Hence the presence of seagrass communities with cuttlefish may have continued for at least 7 million years in this area. A 90% extinction followed the Yazoo Formation (Haasl and Hansen, 1996), which may relate to the lack of cuttlefish in the New World after the Eocene.
Possibly representing the last of the lineage, together with material from the Yazoo Clay above, Squires (1988) recorded fragmentary Belosepiidae indet. from the late Eocene Hoko River Formation, northwestern Washington (USA), near Kydikabbit Point, Neah Bay area (PDB 167461). This also appears to be the last record of cuttlefish from the New World. The two specimens were situated near the separation of true Sepiidae from Belosepiidae (Squires, 1988). No further evidence was available to ascertain seagrass presence. This is the first and last record of cuttlefish in the eastern Pacific. It would be interesting to know when cuttlefish and possibly seagrass entered this region of the world.
Oligocene-Pleistocene
Szörényi (1933) recorded Sepia harmati from Kiscell (Budapest, Hungary) from the Kiscell Clay Formation (Rupelian, early Oligocene). Several records of Sirenidae indet. and cf. Manatherium delheidi were noted by Szabó and Kocsis (2016) who also recorded a diverse shark fauna, indicative of seagrass proximity.
Sepia oligocaenica was recorded from the Eger Formation (Chattian, late Oligocene) of Eger, Hungary (Szörényi, 1933) from where Nolf and Brzobohatý (1994) recorded a fish fauna, including juveniles and sparid fish, possibly indicating a fish nursery. Monostori (2008) recorded the ostracods Aurila?, Bairdia, Loxoconcha spp. and Xestoleberis spp. adding to an interpretation of past seagrass.
The Korytnica Clays Formation (Langhian, middle Miocene) was deposited in a small sheltered basin to the South of the Holy Cross Mountains, Poland. From the structureless clays a diverse and abundant fauna has been recorded. Bałuk (1977) recorded fragments of Sepia sanctacrucensis and conjectured that the cuttlefish migrated to the shallower parts of the Korytnica Clays basin for the breeding season. Bałuk (1975) recorded seagrass gastropods Smaragdia, Gibbula and Jujubinus as well as Sepia fragments. Bałuk and Radwański (1977) interpreted the Korytnica Clays as seagrass partly on the basis of presence of cuttlefish. Radwańska (1992) noted that the seagrass and algal environments of the Korytnica Clays would have provided predation possibilities for Sepia sanctacrucensis, which may have included the large diversity of sparid fish. Seagrass indicators included lucinid bivalves and the large benthic foraminifera Amphistegina (Hoffman, 1979, Kowalewski, 1990, Rögl and Brandstätter, 1993). Szczechura and Aiello (2003) recorded ostracods including Aurila, Hemicytherura, Loxoconcha, Semicytherura and Xestoleberis, which they considered indicated a low-energy near-shore environment, most probably with plants, interpreted as seagrass (Forsey, 2016). A diversity of sparid fish have been recorded including Boops, Diplodus spp., Pagellus spp., Pagrus, Dentex spp. and Spondyliosoma aff. cantharus (Śmigielska, 1979; Radwańska, 1992). Some species were represented by juveniles, suggestive of a fish nursery. Seagrass conditions were prevalent in the Korytnica Clays.
Košťák et al. (2016) recorded Sepia mikuzi from grey sandy/silty marlstones from Plesko, Slovenia (Langhian, middle Miocene). Sparid fish (Diplodus jomnitanus, Pagrus cinctus) and possible predatory shark Cosmopolitodus hastalis have been recorded from Plesko (Mikuž et al., 2013).
Sepia juliebarborarum was recorded from calcareous claystone from the clay pit at Devínska Nová Ves, Bratislavia, Slovakia (Serravallian, middle Miocene) (Košťák et al., 2016). Lucinid bivalves and seagrass associated gastropods Smaragdia Alvania spp., Rissoina spp., Tricolia spp., Gibbula and Jujubinus were recorded from the vineyard locality (PDB 73138). Gregorová (2009) described an articulated specimen of the sparid fish Diplodus sp from the brickfield at Devínska Nová Ves. Domning and Pervesler (2012) noted multiple records of sirenians from nearby localities at Devínska Nová Ves. Zlinská et al. (2013) recorded foraminifera and ostracods from shallow boreholes, the composite ostracod fauna of which contained Aurila spp., Bairdoppilata, Loxoconcha, Semicytherura and Xestoleberis, which has been interpreted as indicating seagrass (Forsey, 2016). These all suggest the presence of past seagrass.
Košťák et al. (2016) recorded Sepia vindobonensis from section B1 of Zuschin et al. (2004) from Grund, Austria (Langhian, middle Miocene) from which section a diverse molluscan fauna was recorded containing lucinids, seagrass associated gastropod Alvania spp. and potamidids (Zuschin et al., 2004) suggestive of seagrass with mangrove in proximity. Daxner-Höck et al. (2004) recorded sparid fish from section B1 and possible shark predators (PDB 51002). From sections nearby a diverse composite ostracod fauna (61 species) derived from several sections and samples included Aurila spp., Hemicytherura, Loxoconcha spp., Semicytherura spp. and Xestoleberis spp. was recorded by Zorn (2004). The sediments have been interpreted as being influenced by storm events presumably explaining the mixture of marine and terrestrial fossils (Roetzel and Pervesler, 2004).
Sepia vindobonensis was recorded from grey, clayey calcareous siltstones belonging to the Planostegina facies from Retznei, Austria (Langhian, middle Miocene) (Hiden, 1995; Košťák et al., 2016). The large benthic foraminifera Planostegina is an indicator for seagrass (Kopecká et al., 2018). Riegl and Piller (2000) noted a later carbonate buildup at Retznei represented by coral carpets, seagrass meadows and coralline algal beds with interbedded small patch reefs. Domning and Pervesler (2012) recorded sirenian fragments from the carbonate suggestive of past seagrass proximity.
Sepia aff. sanctacrucensis was recorded from a conglomerate within a shallow subtidal, sandy claystone sequence from a temporary excavation from a level corresponding to bed E2 of Zuschin et al. (2007) from Gainfarn, Austria (Langhian, middle Miocene). Zuschin et al. (2007) recorded a molluscan assemblage (PDB 174159) containing a lucinid bivalve and the seagrass associated gastropods Tricolia, Bittium, Gibbula and Smaragdia and suggested seagrass for the environment. A partial sirenian skeleton was recorded from this level (Domning and Pervesler, 2012). Brzobohatý (1994) recorded a diverse fish fauna including sparids from Gainfarn.
Sepia from Poivre Formation (middle Miocene) Barrow island, Western Australia (Australia) in association with seagrass fauna including lucinid bivalves, foraminifera Marginopora vertebralis, Sorites spp., Elphidium, Amphistegina and Peneroplis spp. and the gastropod Thalotia was recorded by McNamara and Kendrick (1994). McNamara and Kendrick (1994) conjectured that the Sepia specimen must have been washed onto a shoal, reminiscent of Recent cuttlebone strandings (Jongbloed et al., 2016) and rapidly covered with sediment.
Notosepia cliftonensis was recorded from shelly silty marl of Muddy Creek Marl Member (Port Campbell Limestone Formation, Langhian, middle Miocene) from near Hamilton, Victoria, Australia, with a fish fauna including sharks (Košťák et al., 2017; PDB 46165) without seagrass interpretation.
Košťák et al. (2017) recorded Sepia sp. from a bioclastic limestone from Green Point Member, Gambier Limestone Formation (Langhian, middle Miocene), from Mount Gambier, South Australia (Australia) associated with seagrass associated foraminifera Cibicides, Elphidium spp. and Lobatula.
From the marls of Blue Clay (Tortonian, late Miocene) of Malta, Bianucci et al. (2011) recorded the presence of Sepia in the same sequence as sirenian and dolphin remains in a layer rich in fossils including bivalves and gastropods (PDB 121989). From the top of the marls of the Blue Clay sequence, Hewitt and Pedley (1978) recorded three species of Sepia and noted the prevalence of the echinoid Schizaster. They also noted the possible depth of water as 150-200 m (Hewitt and Pedley, 1978).
Košťák and Jagt (2018) recorded fragmentary Sepia fabianschwankei from the sandy clays of a temporary exposure of the Meistermann clay pit (Twistringen Beds, Langhian, middle Miocene) Lower Saxony, Germany. From Twistringen, a rich, diverse fish fauna including Cosmopolitodus hastalis and Carcharodon megalodon was recorded but did not contain sparids (PDB 161965). Janssen (1972) recorded an extensive molluscan fauna from the Twistringen Beds containing Lucinoma borealis and the seagrass associate gastropods Alvania spp. and Bittium spp.
Sepia vandervoorti was recorded from the Köselerli Formation (middle Miocene) from Mut, Turkey, without associated fauna (Košťák et al., 2019).
Gaudant et al. (2010) described articulated fish remains from the Tortonian (late Miocene) of Pecetto di Valenza, Piedmont, Italy. These included a seagrass associate fish Syngnathus and related foraminifera. A single drifted, cuttlefish shell was found with oyster attached. This is one of the few fossil records, which meets expectations of drifted cuttlebones being preserved (Jongbloed et al., 2016). A fragmentary Sepia sp. was recorded from Montaldo Roero, Italy (early Pliocene) (Košťák et al., 2019).
Mayoral and Muñíz (1994) recorded Sepia (Parasepia) melendezí from the Early Pliocene Huelva Sands of Lepe, southern Spain. The fauna from the lower Pliocene Huelva Sands Formation includes lucinid and pinnid bivalves (Mayoral and Reguant, 1995; Muñiz et al., 1999; Esperante et al., 2009), seagrass ostracods including Aurila, Semicytherura, Hiltermannicythere, Xestoleberis and Loxoconcha, sparid fish (Esperante et al., 2009; Ruiz et al, 2008, 2018) indicating proximity of seagrass. Possible predators include sharks Cosmopolitodus hastalis and Carcharocles megalodon and phocid seals (Esperante et al., 2009; García et al., 2009; Rahmat et al., 2019; PDB 15188). The fauna associated with Zostera seagrass beds currently in the Bay of Cadiz include pinnid bivalve, Sepia, sparid fish, dolphin species, but lack lucinid bivalves (Aguilar et al., 2010) and hence resembles that of the Huelva Sands.
Pasini et al. (2014) recorded Sepia sp. from clay sediments near Volterra (Pisa, Tuscany, Italy) (early Pleistocene). Since it was associated with a bathyal crustacean community, it is unlikely to have a seagrass connection.
DISCUSSION
The evidence presented here is not exhaustive. Entry into other and older literature on fossil cuttlefish can be found in Košťák et al. (2013, 2016). Several aspects can be discussed from the evidence presented. These are noted in the following sections. Firstly, there is a strong relationship between fossil cuttlefish and seagrass environments. Next the apparent general rarity of cuttlefish is noted with respect to an outline of cuttlefish taphonomy. The history of cuttlefish related to seagrass is followed by a speculation about other Late Cretaceous cephalopods and seagrass. Finally, some aspects of cuttlefish ecology in seagrass can be determined including reproductive behaviour and cuttlefish prey and cuttlefish predators.
Cuttlefish and Seagrass
Seagrass has low preservation potential. Rare remains of seagrass have been recorded from the Late Cretaceous, with the earliest from the early Campanian (van der Ham et al., 2007). The records of Late Cretaceous cuttlefish are hence relatively close to the first evidence for seagrass. About 80% of the records suggest a possible relationship between cuttlefish and seagrass, closer than may have been anticipated from extant cuttlefish. Cuttlefish utilise seagrass for reproduction, predation and shelter, which are discussed below.
The history of cuttlefish, from this account, appears to follow the development of seagrass in the North Atlantic area during the Late Cretaceous and its subsequent radiation along Tethys. In so doing cuttlefish were lost from the Old World, possibly by the end of the Eocene. Apart from Australia and India, there is a lack of records from the Indo-West Pacific, representing the greatest cuttlefish diversity currently.
There are several concentrations of records. For instance, belosaepiids have been recorded from the London Clay (Ypresian), Bracklesham Beds (Lutetian) and Barton Beds (Bartonian) of southern England, suggestive of continuous seagrass presence through the early-middle Eocene of England (Tracey, 1996; Hewitt and Jagt, 1999; Williams, 2002). Newton and Harris (1894) recorded Belosepia sepioidea, B. oweni and B. blainvillei from several early-middle Eocene localities in southern England some of which were noted in the Results. Belosaepia sepioidea was noted from the London Clay (Ypresian, early Eocene) of southern England but without localities (Hewitt and Jagt, 1999).
Cuttlefish have not been recorded from some notable seagrass localities. For instance, Monte Bolca (Italy) is a well-studied lagerstätten of late Ypresian (early Eocene) age, known particularly for fish preservation (Bannikov, 2014; Marramà et al., 2016; Friedman and Carnevale, 2018). Several sparid fish have been recorded together with seagrass fragments and a large molluscan fauna with several lucinid bivalves (Bannikov, 2014; Dominici, 2014; Marramà et al., 2016; Friedman and Carnevale, 2018), but without any cuttlefish.
Taphonomy
 Several aspects of the fossil history of cuttlefish remain enigmatic not least of which is their comparative rarity. Currently cuttlefish bones may be washed on to beaches around the UK and North Sea, sometimes in large numbers (Jongbloed et al., 2016). These may be broken, show signs of being scavenged and may have organisms growing on them (Jongbloed et al., 2016). Cuttlebones on modern beaches, all showing signs of scavenging, have been personally observed from North Norfolk, Poole Harbour, Studland Bay, Rhossili Bay (UK) and Sardinia in proximity to seagrass beds (Figure 2). There may be an expectation that cuttlefish might be as abundant as shelled cephalopods from the Palaeozoic and Mesozoic.
Several aspects of the fossil history of cuttlefish remain enigmatic not least of which is their comparative rarity. Currently cuttlefish bones may be washed on to beaches around the UK and North Sea, sometimes in large numbers (Jongbloed et al., 2016). These may be broken, show signs of being scavenged and may have organisms growing on them (Jongbloed et al., 2016). Cuttlebones on modern beaches, all showing signs of scavenging, have been personally observed from North Norfolk, Poole Harbour, Studland Bay, Rhossili Bay (UK) and Sardinia in proximity to seagrass beds (Figure 2). There may be an expectation that cuttlefish might be as abundant as shelled cephalopods from the Palaeozoic and Mesozoic.
There are few fossil records meeting this expectation. For instance, McNamara and Kendrick (1994) conjectured that Sepia from Poivre Formation (middle Miocene), Barrow Island, Western Australia (Australia) in association with a seagrass fauna, must have been washed onto a shoal, reminiscent of Recent cuttlebone strandings (Jongbloed et al., 2016) and rapidly covered with sediment. Gaudant et al. (2010) noted a single drifted, cuttlefish shell with oyster attached from the Tortonian (late Miocene) of Pecetto di Valenza (Piedmont, Italy) associated with a seagrass fauna.
Although generally rare, past cuttlefish records vary from single occurrences (McNamara and Kendrick, 1994; Gaudant et al., 2010; Lebrun et al., 2012), to many specimens (Weaver and Ciampaglio, 2003; Yancey et al., 2010; Košťák et al., 2016) and multispecies presence (Palmer, 1937; Palmer and Brann, 1965; Weaver and Ciampaglio, 2003; Košťák et al., 2016). In several cases there is evidence for cuttlefish and seagrass being present over several million years (Weaver et al., 2010b; Tracey et al., 1996).
Preservation of belosaepiids often consists of the strongly calcified posterior portion of the cuttlebone when hundreds of such fragments may be noted (Weaver and Ciampaglio, 2003; Yancey et al., 2010). Although also consisting of fragments, some Sepia are well preserved as cuttle bones (Košťák et al., 2016) with rare soft part preservation. Soft tissue preservation (ink sac) of Sepia juliebarborarum from the Late Badenian (Serravallian, middle Miocene) of Devínska Nová Ves (Slovakia) and fragmentary Sepia fabianschwankei from the Meistermann clay pit (Twistringen Beds, Langhian, middle Miocene) (Lower Saxony, Germany) suggest rapid burial with intermittent hypoxia (Košťák et al., 2018; Košťák and Jagt, 2018). Clements et al. (2017) have indicated that buoyancy mechanisms may limit cephalopod soft tissue preservation and may have implications for lack of fossilised cuttlefish. The record of Sepia statoliths from the middle Lutetian (middle Eocene) of Thiverval-Grignon (Paris Basin, France) (Neige et al., 2016) infers that the cuttlebone has gone and that soft parts have disintegrated or been eaten leaving only the aragonitic statoliths to be preserved.
The majority of cuttlefish fossils are associated with seagrass. More particularly they may have resulted from one key time in cuttlefish ontogeny, that of spawning. Possible taphonomy includes spawning followed by death. Most dying and dead cuttlefish will be predated and scavenged releasing the buoyant cuttlebone. Carcasses drift away and apparently usually vanish from the record. A small number are buried intact and then decay away leaving pristine shell and possible soft preservation (Košťák et al., 2018; Košťák and Jagt, 2018). These are the ones which predominantly appear in this account. The scenario has a resonance with the only known spawning aggregation of cuttlefish in the world (Hall and Hanlon, 2002). Large numbers of Sepia apama congregate over hard substrate for spawning in shallow water in proximity to seagrass in South Australia (Hall and Hanlon, 2002). Some of these may have travelled up to 100 km (Payne et al., 2013). The fossil record has captured this part of the narrative, but reasons why cuttlefish are not preserved elsewhere are not clear. This needs to be determined more fully. No records of taphonomic studies on cuttlefish bone have been found.
Cuttlefish History
There is a development from Ceratisepia (Late Cretaceous-Paleocene, Košťák et al., 2017) via Belocurta (early Paleocene: Avnimelech, 1958; Košťák et al., 2013) and Aegyptosaepia (late Paleocene: Košťák et al., 2013) to belosaepiids (Eocene) (Košťák et al., 2013, figure 11). Belosaepiids constitute the majority of Eocene records.
Belosaepiids gave rise to sepiids via archaeosepiids such as Hungarosepia (= Archaeosepia invalid) during the Eocene (Hewitt and Jagt, 1999; Košťák et al., 2016). For instance, Fornaseiro and Vicariotto (1995) recorded Archaeosepia monticulimajoris (PDB 81043) from the Priabonian (late Eocene) of Europe.
Neige et al. (2016) identified Sepia from the middle Eocene. Weaver et al. (2007) considered that the middle Eocene Anomalosaepia was a direct ancestor of Sepia. Late Eocene belosaepiid fragments were close to separation of sepiids (Squires, 1988). Jeletzky (1969) noted a transition between Sepiidae and Belosepiidae in the middle Eocene beds of the Paris Basin. Hence Sepia may have evolved in the early part of the Eocene (Jeletsky, 1969; Squires, 1988; Weaver et al., 2007; Neige et al., 2016; Košťák et al., 2016).
It is likely that belosaepiids became extinct in the late Eocene possibly related to seagrass decline in the North Atlantic. Yancey (2010) commented that their extinction was an apparent casualty of the rapid cooling of climate at the end of the Eocene. Decreased sirenian diversity during the early Oligocene could have been related to the change from Greenhouse to Icehouse conditions and subsequent development of Antarctic ice sheets (Vélez-Juarbe, 2014). This would have affected seagrasses, their consumers and led to local and global extinctions (Vélez-Juarbe, 2014) amongst other seagrass inhabitants such as cuttlefish and is an explanation for the lack of New World cuttlefish currently. Possibly the last fossil cuttlefish from the New World appeared in the late Eocene (Squires, 1988).
Following the extinction of belosaepiids Sepia evolved from Oligocene archaeosepiids whose records come largely from Hungary and Italy (Košťák et al., 2017; 2019). This seems to be similar to a pattern in marine vertebrates in seagrass involving extinctions or reductions at the end of the Eocene followed by new groups developing by the late Oligocene.
The rest of cuttlefish history is concerned with Sepiidae, mostly recorded from Europe. Sepia has been recorded from India and Australia (McNamara and Kendrick, 1994; Košťák et al., 2016, 2017). There is a lack of evidence for fossil cuttlefish in the Indo-Pacific area, which now possibly represents the greatest diversity of cuttlefish.
The highest diversity and morphological disparity are seen during the middle Miocene; this is followed by a rapid decrease during the late Miocene and a renewed radiation during the Pliocene (Košťák et al., 2019).
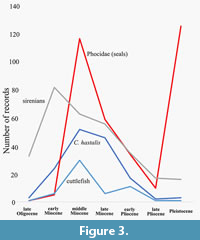 The lack of evidence of cuttlefish precludes a narrative from the late Pliocene-Recent. However, a speculative story based on evidence from elsewhere can be made. Pimiento et al. (2017) indicated a marine megafaunal extinction event, which included reduction of sirenians, near extinction of seals and extinction of the shark Cosmopolitodus hastalis (Figure 3). This suggests a reduction in seagrass and its fauna including cuttlefish which seals and possibly Cosmopolitodus hastalis used as prey. Cuttlefish may then have increased during the Pleistocene to occupy generally shallow marine areas except for the New World. The diverse cuttlefish fauna currently may hence be recent.
The lack of evidence of cuttlefish precludes a narrative from the late Pliocene-Recent. However, a speculative story based on evidence from elsewhere can be made. Pimiento et al. (2017) indicated a marine megafaunal extinction event, which included reduction of sirenians, near extinction of seals and extinction of the shark Cosmopolitodus hastalis (Figure 3). This suggests a reduction in seagrass and its fauna including cuttlefish which seals and possibly Cosmopolitodus hastalis used as prey. Cuttlefish may then have increased during the Pleistocene to occupy generally shallow marine areas except for the New World. The diverse cuttlefish fauna currently may hence be recent.
Seagrass and Late Cretaceous Cephalopods
Although this account is focused on cuttlefish, records of other cephalopods from the Late Cretaceous may indicate a wider relationship to seagrass. Doyle et al. (1994) established the cuttlefish family Actinosepiidae for Actinosepia. However, Hewitt and Jagt (1999) placed Actinosepia into Trachyteuthididae (i.e., vympyromorphid coleoids) where it remains. For instance, Actinosepiidae was not regarded by Košťák et al. (2013) in their sepiid phylogeny. Russell and Landes (1940) recorded Actinosepia canadensis from sandy shales from Manyberries (Alberta, Canada) (Bearpaw Formation, late Campanian) associated with a large molluscan fauna which contained two lucinids (PDB 82638). Larson (2010) noted Actinosepia canadensis presence in North America and noted its prevalence in the Bearpaw Shale of Alberta and Montana. Actinosepia canadensis was also recorded from argillaceous siltstone of the Hoploscaphites nicolletii ammonoid zone, Trail City Member and Timber Lake Member (Fox Hills Formation, late Maastrichtian) from the Black Hills (South Dakota, USA) associated with large molluscan faunas including a lucinid bivalve (Landman and Waage, 1993; PDB 88510, 88511). Molluscan faunas including Actinosepia also contained Scaphitidae species (PDB 82638, 88510, 88511), which Arkhipkin (2014) suggested might have coiled around seagrass stems. Actinosepia and heteromorph ammonites may represent a more general association of cephalopods with seagrass in the Late Cretaceous which is not investigated further in this account.
Ecology
There are several aspects of cuttlefish palaeoecology that may be interpreted from the evidence presented. In particular aspects of reproductive strategy and predator-prey aspects may be determined.
Cuttlefish reproduction. It may be that a number of fossil records are related to cuttlefish spawning in seagrass, particularly where large numbers of apparently adult specimens were found (e.g., Weaver and Ciampaglio, 2003; Yancey et al., 2010; Košťák et al., 2016). Extant cuttlefish also spawn in other environments such as algal (Blanc et al., 1998). Carrasco and Pérez-Matus (2016) showed that the squid Doryteuthis gahi has specific requirements for spawning. Cuttlefish may also have particular requirements, which appear to have been met by seagrass in the past.
The term nursery has wide currency but has not been closely defined (Beck et al., 2001; Heck et al., 2003). Heupel et al. (2007) provided a definition of shark nursery as including sharks more commonly encountered, remaining or returning and site used over time. Nursery site might be used by several species (Heupel et al., 2007). While these criteria are difficult to apply in the past, they could include the evidence presented here for cuttlefish and seagrass. The evidence presented here suggests that fossil cuttlefish, including possible juveniles, are more commonly encountered in interpreted past seagrass environments, and some sites have been used over time by more than one species (Palmer, 1937; Palmer and Brann, 1965; Hewitt and Pedley, 1978; Neige et al, 2016). This would fulfil the requirements of nursery noted above.
This reproductive behaviour in cuttlefish may have evolved soon after the first seagrass in the early Campanian (van der Ham et al., 2007) and continued to the present day. This not only indicates a certain longevity but also fidelity in this relationship. Cuttlefish may be part of a developing general pattern of taxa entering into and maintaining a relationship with seagrass, shown also by foraminifera (Hart et al., 2016), ostracods (Forsey, 2016), sirenians (Domning, 2001; Vélez-Juarbe, 2014) and various gastropods including the nerite Smaragdia (Reich, 2014; Reich et al, 2015).
A recent analysis of tropical reef biodiversity dynamics indicated the Mediterranean area as the global biodiversity hotspot for the Miocene (~20 Ma) (Leprieur et al., 2016). This is likely to impinge on seagrass fauna such as cuttlefish. Košťák et al. (2016) commented on the high diversity of sepiids (at least nine species) during the middle Miocene of Central Paratethys (part of the Mediterranean area), which may have been a global biodiversity hotspot in which seagrass played an important part.
Cuttlefish possibly spend a minority of their time in seagrass and visit other environments (Jackson et al., 2015) including algal (Koulouri et al., 2016). However, a critical relationship with seagrass involves spawning whereupon the adults are weakened and die or become easy prey for predators. For instance, Finn et al. (2009) and Smith and Sprogis (2016) described the hunting behaviour of the Indo-Pacific bottlenose dolphin (Tursiops aduncus) on the giant cuttlefish (Sepia apama) in Australia related to spawning in seagrass.
Predator-prey relationships. Alves et al. (2006) examined gut contents of Sepia officinalis from southern Portugal and noted the presence of fish, worms, molluscs and crustaceans. These included several sparid fish, which may have come from seagrass environments. Predators include dolphins, sharks, fish, seals and other cephalopods. Stomach contents of dolphins indicated that they preyed on a variety of fish and cephalopods including sparid fish and Sepia sp. (Barros et al., 2000; Fernandez et al, 2009). Dolphins have been observed hunting cuttlefish in seagrass environments (Finn et al., 2009; Smith and Sprogis, 2016). Sepiidae were noted as a prevalent part of the diet of shark species from the seagrass of KwaZulu-Natal, South Africa (Smale and Cliff, 1998; Bandeira and Björk, 2001). One of the sharks involved, the tiger shark (Galeocerdo cuvier) may act as a recent model for the extinct Cosmopolitodus hastalis noted in this account. Grey seal (Halichoerus grypus) scats and stomach contents indicated Sepia officinalis and the sparid fish Spondyliosoma cantharus (Ridoux et al., 2007). Spondyliosoma cantharus was noted as suggestive of past seagrass in the Miocene of Kienberg (Czech Republic) (Brzobohatý et al., 2007). Pierce et al. (2011) similarly reported that a large portion of Mediterranean monk seal (Monachus monachus) diet was from cuttlefish.
For some of the records noted in this account it is possible to outline possible predator-prey scenarios involving fossil cuttlefish and hence to suggest a degree of functional uniformity from the Campanian to the Recent. The selected records below indicate diverse, prey rich environments powered by seagrass.
As already noted Actinosepia canadensis was recorded with molluscan faunas containing lucinids and Solemya (Russell and Landnes, 1940; Tsujita, 1995; PDB 82638) from the Bearpaw Formation (late Campanian), Alberta, Canada. Possible predators in seagrass included sharks (Squalicorax) and a variety of mosasaurs (Holmes, 1996; Konishi, 2012; Konishi et al., 2011, 2014; Cullen et al., 2016; PDB 181024, 119087, 119088, 119089, 23368, 107428). Possible prey included decapods and fish (Konishi et al., 2011).
Cuttlefish (Anomalosaepia, Belosaepia) (Powell and Baum, 1982; Weaver and Ciampaglio, 2003; PDB 5366, 5367) have been recorded together with lucinids and sirenians (Palmer and Brann, 1965; Domning et al., 1982; Beatty and Geisler, 2010; PDB91736, 91737) from the Castle Hayne Limestone Formation, (Bartonian, middle Eocene) North Carolina, USA. Possible predators in seagrass included the early cetacean basilosaurids (Uhen, 2005, 2013; Beatty and Geisler, 2010; PDB 5386, 5387, 7297, 7299, 41822, 60452, 75518, 99753, 132664, 132701, 133023) and sharks (Otodus, Isurus) (PDB 5386, 5387). Possible prey included fish (PDB 5387).
Belosaepia was recorded with pinnid and lucinid bivalves (Haasl and Hansen, 1996) from the Yazoo Formation, (Priabonian, late Eocene) Mississippi, USA. Possible predators included basilosaurids, sharks and palaeophiid snakes (Uhen, 2005, 2013; PDB 6802, 45627-45634, 45639,55689, 55868,55869, 32926, 135787, 132843, 132844,132820, 13822-138225, 132666, 132667, 131912, 131913, 84058, 32925). Possible prey included decapods and fish (PDB 3295).
Košťák et al. (2016) commented on the high diversity of sepiids (at least nine species) during the Badenian (middle Miocene) of Central Paratethys. One of the sites was Devínska Nová Ves, Slovakia. As noted, seagrass was interpreted on the basis of foraminifera, ostracods, sirenians, lucinids and gastropods (Švagrovský, 1981; Domning and Pervesler, 2012: Zlinská et al., 2013). Possible predators in seagrass included sharks (such as Cosmopolitodus hastalis) and phocid seals (Koretsky and Holec, 2002; Sabol and Kováč, 2006; Koretsky and Rahmat, 2013; PDB 58983). Elsewhere in central Europe, dolphins have been recorded. For example, Czyżewska and Radwański (1991) recorded delphinid remains together with sirenians from Poland. Prey might have included fish (including sparids such as Diplodus) (Holec and Sabol, 1996; Gregorová, 2009) and decapods (Hyžný et al., 2012; Hyžný, 2016; PDB 145936).
Košťák and Jagt (2018) recorded Sepia fabianschwankei from the Meistermann clay pit (Twistringen Beds, Langhian, middle Miocene) (Lower Saxony, Germany). Seagrass interpretation was based on molluscs (Janssen, 1972). A diverse fish fauna recorded from the Twistringen Beds (PDB 161965) provided prey and included possible predators such as Cosmopolitodus hastalis.
Rahmat et al. (2019) recorded the phocid seals Homiphoca capensis and Homiphoca sp., from the Huelva Sands (early Pliocene, southern Spain) from where Mayoral and Muñíz (1994) had recorded Sepia (Parasepia) melendezí. The Huelva Sands have been interpreted as seagrass by bivalves and ostracods (Mayoral and Reguant, 1995; Muñiz et al., 1999; Ruiz et al., 2008, 2018; Esperante et al., 2009).
Future for Cuttlefish
Cuttlefish may be affected by increasing ocean acidification. Sigwart et al. (2016 and references therein) have drawn attention to the effect of elevated carbon dioxide levels, subsequent reduction in oceanic pH and the effect on cuttlefish with particular emphasis on early life history. Kaplan et al. (2013) noted that acidification affected development of statoliths in the squid Doryteuthis pealeii. The same may be true of cuttlefish whose ability to swim for instance could be impaired.
While these experimental results are of concern, those cuttlefish largely inhabiting seagrass are likely to do better than these results suggest because of the effect of seagrass removing carbon dioxide in shallow environments and hence militating against lowering of pH (Hendriks et al., 2014, 2015).
Sepia species form a relatively small part of coastal fisheries which seems to be decreasing against a background of increasing fish capture (Rodhouse et al., 2014). Doubleday et al. (2016) have suggested that the preferential removal of cuttlefish predators such as seals, sharks and other fish may result in increased abundance. Global warming may enable cuttlefish to recolonise the New World via the Arctic (Xavier et al., 2016).
The future of cuttlefish in shallow marine environments is strongly related to the success of seagrass. Unfortunately, the future remains uncertain for seagrass which is under threat from global warming and ocean acidification (Orth et al., 2006; Waycott et al., 2009) with some seagrass species facing extinction (Short et al., 2011).
CONCLUSIONS
Seagrass has provided a source of prey, protection, spawning and nursery areas for cuttlefish for possibly 80 million years. The absence of cuttlefish from the New World dates from the end of the Eocene and may be related to seagrass reduction, loss of suitable spawning and nursery areas and reduction in prey. Extant cuttlefish (Sepia spp. and related forms) may have had their origin in the middle Eocene, but their current diversity may be recent. The record of fossil cuttlefish may be explained by their relatively unique taphonomy related to seagrass. The rarity of cuttlefish fossils elsewhere is possibly related to taphonomic processes, which need to be better understood.
ACKNOWLEDGEMENTS
Valuable comments and insights from M. Košťák and an anonymous reviewer enhanced this account. The narrative was made possible by contributors to the Paleobiology Database. This is Paleobiology Database contribution number 355.
REFERENCES
Aguilar, R.P., García, E.C.M.J., and Silvia Ubero, J. 2010. Doñana and the Gulf of Cadiz, 2010: Marine Protected Area Expansion Proposal. OCEANA, Madrid.
Allen, J.E. 1968. New species of Sepiida (Mollusca, Cephalopoda) from the Eocene of the Gulf Coast. Tulane Studies in Geology and Paleontology, 6:33-37
Alroy, J. 2013. Online Paleogeographic Map Generator. http://fossilworks.org/cgi-bin/bridge.pl?action=mapForm
Alves, D.M., Cristo, M., Sendão, J., and Borges, T.C. 2006. Diet of the cuttlefish Sepia officinalis (Cephalopoda: Sepiidae) off the south coast of Portugal (eastern Algarve). Journal Marine Biological Association, 86:429-436. https://doi.org/10.1017/S0025315406013312
Anonymous. 2015. JANUS: The Newsletter of the North Carolina Fossil Club, 2015 Number 4. North Carolina Fossil Club, Raleigh.
Arata, A.A. and Jackson, C.G., Jr. 1965. Paleontological note: Cenozoic vertebrates from the Gulf Coastal Plain-I. Tulane Studies in Geology and Paleontology, 3:175-177.
Arkhipkin, A.I. 2014. Getting hooked: the role of a U-shaped body chamber in the shell of adult heteromorph ammonites. Journal of Molluscan Studies, 80:354-364. https://doi.org/10.1093/mollus/eyu019
Avnimelech, M.A. 1958. A new belemnoid genus from the Paleocene of Israel, with remarks on the classification of the Tertiary dibranchiate cephalopods. Bulletin of the Research Council of Israel, 7G:61-65.
Bałuk, W. 1975. Lower Tortonian gastropods from Korytnica, Poland. Part I. Acta Geologica Polonica, 32:1-186.
Bałuk, W. 1977. A new species of the cuttlefish from the Korytnica Clays (Middle Miocene; Holy Cross Mountains, Poland). Acta Geologica Polonica, 27:169-176.
Bałuk, W. and Radwański, A. 1977. Organic communities and facies development of the Korytnica Basin (middle Miocene; Holy Cross Mountains, central Poland). Acta Geologica Polonica, 27:85-123.
Bandeira, S.O. and Björk, M. 2001. Seagrass research in the eastern Africa region: emphasis on diversity, ecology and ecophysiology. South African Journal of Botany, 67:420-425. https://doi.org/10.1016/S0254-6299(15)31158-3
Bannikov, A.F. 2014. The systematic composition of the Eocene actinopterygian fish fauna from Monte Bolca, northern Italy, as known to date. Studi e Ricerche sui Giacimenti Terziari di Bolca, 15:23-34.
Barros, N.B., Parsons, E.C.M., and Jefferson, T.A. 2000. Prey of offshore bottlenose dolphins from the South China Sea. Aquatic Mammals, 26:2-6.
Beatty, B.L. and Geisler, J. 2010. A stratigraphically precise record of Protosiren (Protosirenidae, Sirenia) from North America. Neues Jahrbuch für Geologie und Palaeontologie - Abhandlungen, 258:185-194. https://doi.org/10.1127/0077-7749/2010/0095
Beck, M.W., Heck, K.L., Jr., Able, K.W., Childers, D.L., Eggleston, D.B., Gillanders, B.M., Halpern, B., Hays, C.G., Hoshino, K., Minello, T.J., and Orth, R.J. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience, 51:633-641. https://doi.org/10.1641/0006-3568(2001)051[0633:ticamo]2.0.co;2
Bianucci, G., Gatt, M., Catanzariti, R., Sorbi, S., Bonavia, S.G., Curmi, R., and Varola, A. 2011. Systematics, biostratigraphy and evolutionary pattern of the Oligo-Miocene marine mammals from the Maltese Islands. Geobios, 44:549-585. https://doi.org/10.1016/j.geobios.2011.02.009
Blake, D.B. 1950. Gosport Eocene Ostracoda from Little Stave Creek, Alabama. Journal of Paleontology, 24:174-184.
Blanc, A. and Daguzan, J. 1998. Artificial surfaces for cuttlefish eggs (Sepia officinalis L.) in Morbihan Bay, France. Fisheries Research, 38:225-231. https://doi.org/10.1016/S0165-7836(98)00164-7
Blanc, A., Du Sel, G.P., and Daguzan, J. 1998. Habitat and diet of early stages of Sepia officinalis L. (Cephalopoda) in Morbihan Bay, France. Journal of Molluscan Studies, 64:263-274. https://doi.org/10.1093/mollus/64.3.263
Bloor, I.S., Attrill, M.J., and Jackson, E.L. 2013a. A review of the factors influencing spawning, early life stage survival and recruitment variability in the common cuttlefish (Sepia officinalis). Advances in Marine Biol ogy, 65:1-65. https://doi.org/10.1016/B978-0-12-410498-3.00001-X
Bloor, I.S., Wearmouth, V.J., Cotterell, S.P., McHugh, M.J., Humphries, N.E., Jackson, E.L., Attrill, M.J., and Sims, D.W. 2013b. Movements and behaviour of European common cuttlefish Sepia officinalis in English Channel inshore waters: first results from acoustic telemetry. Journal of Experimental Marine Biology and Ecology, 448:19-27. https://doi.org/10.1016/j.jembe.2013.06.013
Bone, D.A., Todd, J.A., and Tracey, S. 1991. Fossils from the Bracklesham Group exposed in the M27 Motorway excavations, Southampton, Hampshire. Tertiary Research, 12:131-137.
Brignon, A. 2018. Redécouverte du “crocodile de Sheppey” de Georges Cuvier (Crocodilus delucii Gray, 1831) et signification du “monitor de Sheppey” (London Clay, Yprésien). Annales de Paléontologie, 104:329-335. https://doi.org/10.1016/j.annpal.2018.09.001
Brzobohatý, R. 1994. Die Fischotolithen des Badenien von Gainfarn, Niederösterreich (Mittelmiozän, Wiener Becken). Annalen des Naturhistorischen Museums in Wien, 96A:67-93.
Brzobohatý, R., Nolf, D., and Kroupa, O. 2007. Fish otoliths from the Middle Miocene of Kienberg at Mikulov, Czech Republic, Vienna Basin: their paleoenvironmental and paleogeographic significance. Bulletin de l’Institut royal des Sciences Naturelles de Belgique, Sciences de la Terre, 77:167-196.
Burton, E.S.J. 1933. Faunal horizons of the Barton Beds in Hampshire. Proceedings of the Geologists' Association, 44:131-167. https://doi.org/10.1016/S0016-7878(33)80015-0
Carrasco, S.A. and Pérez-Matus, A. 2016. Inshore spawning grounds of the squid Doryteuthis gahi suggest the consistent use of defoliated kelp Lessonia trabeculata in Central Chilean waters. Marine Biological Research, 12:323-328. https://doi.org/10.1080/17451000.2015.1136064
Checa, A.G., Cartwright, J.H., Sánchez-Almazo, I., Andrade, J.P., and Ruiz-Raya, F. 2015. The cuttlefish Sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor. Scientific Reports, 5:11513. https://doi.org/10.1038/srep11513
Clements, T., Colleary, C., De Baets, K., and Vinther, J. 2017. Buoyancy mechanisms limit preservation of coleoid cephalopod soft tissues in Mesozoic Lagerstätten. Palaeontology, 60:1-14. https://doi.org/10.1111/pala.12267
CoBabe, E.A. and Allmon, W.D. 1994. Effects of sampling on paleoecologic and taphonomic analyses in high-diversity fossil accumulations: an example from the Eocene Gosport Sand, Alabama. Lethaia, 27:167-178. https://doi.org/10.1111/j.1502-3931.1994.tb01572.x
Cox, K. and Scholar, H. 2017. The unique ecology of Lembeh Strait, Indonesia. Fisheries, 42:519-525. https://doi.org/10.1080/03632415.2017.1358558
Cullen, T.M., Fanti, F., Capobianco, C., Ryan, M.J., and Evans, D.C. 2016. A vertebrate microsite from a marine-terrestrial transition in the Foremost Formation (Campanian) of Alberta, Canada, and the use of faunal assemblage data as a paleoenvironmental indicator. Palaeogeography, Palaeoclimatology, Palaeoecology, 444:101-114. https://doi.org/10.1016/j.palaeo.2015.12.015
Czyżewska, T. and Radwański, A. 1991. Middle Miocene (Badenian) delphinid and phocoenid remains from the Fore-Carpathian Depression in southern Poland. Acta Geologica Polonica, 41:183-192.
Daxner-Höck, G., Miklas-Tempfer, P.M., Göhlich, U.B., Huttunen, K., Kazár, E., Nagel, D., Roessner, G.E., Schultz, O., and Ziegler, R. 2004. Marine and terrestrial vertebrates from the Middle Miocene of Grund (Lower Austria). Geologica Carpathica, 55:191-197.
Dockery, D.T. 1977. Mollusca of the Moodys Branch Formation, Mississippi. Bulletin of the Mississippi Geological, Economic and Topographical Survey, 120:1-212.
Doguzhaeva, L.A., Weaver, P.G., and Ciampaglio, C.N. 2014. A unique late Eocene coleoid cephalopod Mississaepia from Mississippi, USA: new data on cuttlebone structure, and their phylogenetic implications. Acta Palaeontologica Polonica, 59:147-163. https://doi.org/10.4202/app.2011.0208
Dominici, S. 2014. The mollusk fauna of the Monte Postale. Rendiconti della Società Paleontologica Italiana, 4:89-94.
Dominici, S. and Zuschin, M. 2016. Palaeocommunities, diversity and sea-level change from middle Eocene shell beds of the Paris Basin. Journal of the Geological Society, 173:889-900. https://doi.org/10.1144/jgs2015-150
Domning, D.P. 2001 Sirenians, seagrasses, and Cenozoic ecological change in the Caribbean Palaeogeography, Palaeoclimatology, Palaeoecology, 166:227-250. https://doi.org/10.1016/S0031-0182(00)00200-5
Domning, D.P., Morgan, G.S., and Ray, C.E. l982. North American Eocene sea cows (Mammalia: Sirenia). Smithsonian Contributions to Paleontology, 52:1-69.
Domning, D.P. and Pervesler, P. 2012. The sirenian Metaxytherium (Mammalia: Digongidae) in the Badenian (Middle Miocene) of central Europe. Austrian Journal of Earth Science, 105:125-160.
Doubleday, Z.A., Prowse, T.A., Arkhipkin, A., Pierce, G.J., Semmens, J., Steer, M., Leporati, S.C., Lourenço, S., Quetglas, A., Sauer, W., and Gillanders, B.M. 2016. Global proliferation of cephalopods. Current Biology, 26:R406-R407. https://doi.org/10.1016/j.cub.2016.04.002
Doyle, P., Donovan, D.T., and Nixon, M. 1994. Phylogeny and systematics of the Coleoidea. The University of Kansas Paleontological Contributions, 5:1-15.
Duffin, C.J. and Reynders, J.P. 1995. A fossil Chimaeroid from the Gronsveld Member (Late Maastrichtian, Late Cretaceous) of northeast Belgium. Professional Paper Geological Survey of Belgium, 278:111-156.
Esperante, R., Guinea, F.M., and Nick, K.E. 2009. Taphonomy of a mysticeti whale in the Lower Pliocene Huelva Sands Formation (southern Spain). Geologica Acta, 7:489-505.
Ezzedine-Najai, S. 1997. Tagging of the cuttlefish, Sepia officinalis L (Cephalopoda: Decapoda), in the Gulf of Tunis. Scientia Marina, 61:59-65.
Fernandez, R., Santos, M.B., Carrillo, M., Tejedor, M., and Pierce, G.J. 2009. Stomach contents of cetaceans stranded in the Canary Islands 1996-2006. Journal of the Marine Biological Association, 89:873-883. https://doi.org/10.1017/S0025315409000290
Finn, J., Tregenza, T., and Norman, M. 2009. Preparing the perfect cuttlefish meal: complex prey handling by dolphins. PLoS ONE, 4:e4217. https://doi.org/10.1371/journal.pone.0004217
Fornaseiro, M. and Vicariotto, G. 1995. Fossil cuttlebones in the Vicentinian Priabonian (Late Eocene, Veneto Region, NE Italy). Memorie Di Scienze Geologiche, 47:173-178.
Forsey, G.F. 2016. Ostracods as proxies for past seagrass: a review. Palaeogeography, Palaeoclimatology, Palaeoecology, 447:22-28. https://doi.org/10.1016/j.palaeo.2016.01.028
Friedman, M., Beckett, H.T., Close, R.A., and Johanson, Z. 2016. The English chalk and London Clay: two remarkable British bony fish Lagerstätten. Geological Society, London, Special Publications, 430:165-200. https://doi.org/10.1144/SP430.18
Friedman, M. and Carnevale, G. 2018. The Bolca Lagerstätten: shallow marine life in the Eocene. Journal of the Geological Society, 175:569-579. https://doi.org/10.1144/jgs2017-164
García, M., Xio, E., Telles-Antunes, M., Cáceres-Balbino, A., Ruiz-Muñoz, F., and Civis-Llovera, J. 2009. Los tiburones Lamniformes (Chondrichthyes, Galeomorphii) del Plioceno inferior de la Formación Arenas de Huelva, suroeste de la cuenca del Guadalquivir, España. Revista Mexicana de Ciencias Geológicas, 26:674-686.
Garvie, G.L. 1996. The molluscan macrofauna of the Reklaw Formation, Marquez Member (Eocene: Lower Claibornian), in Texas. Bulletin American Paleontology, 111:1-177.
Gaudant, J., Courme-Rault, M.D., Fornaciari, E., and Fourtanier, E. 2010. The Upper Miocene fossil fish locality of Pecetto di Valenza (Piedmont, Italy): a multidisciplinary approach. Bollettino della Societa Paleontologica Italiana, 49:203-225.
Gregorová, R. 2009. Diplodus sp. (Sparidae, Perciformes): a new fossil record of an articulated skeleton from Devínska Nová Ves (Upper Badenian, Vienna Basin, Slovakia). Annalen des Naturhistorischen Museums in Wien, 111A:313-322.
Guernet, C., Huyghe, D., Lartaud, F., Merle, D., Emmanuel, L., Gély, J.P., Michel, F., and Pilet, O. 2012. Les Ostracodes de la falunière de Grignon (Lutétien du Bassin de Paris): implications stratigraphiques. Geodiversitas, 34:909-959. https://doi.org/10.5252/g2012n4a12
Haasl, D.M. and Hansen, T.A. 1996. Timing of latest Eocene molluscan extinction patterns in Mississippi. Palaios, 11:487-494. https://doi.org/10.2307/3515214
Hall, K. and Hanlon, R., 2002. Principal features of the mating system of a large spawning aggregation of the giant Australian cuttlefish Sepia apama (Mollusca: Cephalopoda). Marine Biology, 140:533-545. https://doi.org/10.1007/s00227-001-0718-0
Hart, M.B., FitzPatrick, M.E.J., and Smart, C.W. 2016. The Cretaceous/Paleogene boundary: foraminifera, sea grasses, sea level change and sequence stratigraphy. Palaeogeography, Palaeoclimatology, Palaeoecology, 441:420-429. https://doi.org/10.1016/j.palaeo.2015.06.046
Haveles, A.W. and Ivany, L.C. 2010. Rapid growth explains large size of mollusks in the Eocene Gosport Sand, United States Gulf Coast. Palaios, 25(9):550-564. https://doi.org/10.2110/palo.2009.p09-148r
Heck, K.L., Jr., Hays, G., and Orth, R.J. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series, 253:123-136. https://doi.org/10.3354/meps253123
Hendriks, I.E., Duarte, C.M., Olsen, Y.S., Steckbauer, A., Ramajo, L., Moore, T.S., Trotter, J.A., and McCulloch, M. 2015. Biological mechanisms supporting adaptation to ocean acidification in coastal ecosystems. Estuarine, Coastal and Shelf Science, 152:A1-A8. https://doi.org/10.1016/j.ecss.2014.07.019
Hendriks, I.E., Olsen, Y.S., Ramajo, L., Basso, L., Steckbauer, A., Moore, T.S., Howard, J., and Duarte, C.M. 2014. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences, 11:333-346. https://doi.org/10.5194/bg-11-333-2014
Heupel, M.R., Carlson, J.K., and Simpfendorfer, C.A. 2007. Shark nursery areas: concepts, definition, characterization and assumptions. Marine Ecology Progress Series, 337:287-297. https://doi.org/10.3354/meps337287
Hewitt, R.A. and Jagt, J.W.M. 1999. Maastrichtian Ceratisepia and Mesozoic cuttlebone homeomorphs. Acta Palaeontologica Polonica, 44:305-326.
Hewitt, R.A. and Pedley, H.M. 1978. The preservation of the shells of Sepia in the middle Miocene of Malta. Proceedings of the Geologists Association, 89:227-237. https://doi.org/10.1016/S0016-7878(78)80013-3
Hiden, H.R. 1995. Sepia vindobonensis (Cephalopoda, Coleoida) aus dem Mittel-Miozän von Retznei (Steiermark, Österreich). Mitteilungen der Abteilung für Geologie, Paläontologie am Landesmuseum Joanneum, 52:111-124.
Hoffman, A. 1979. A consideration upon macrobenthic assemblages of the Korytnica Clays (middle Miocene; Holy Cross Mountains, central Poland). Acta Geologica Polonica, 29:345-352.
Holec, P. and Sabol, M. 1996. Tertiary vertebrates (Vertebrata) from Devínska Kobyla. Mineralia Slovaca, 28:519-522. [In Slovak]
Holmes, R. 1996. Plioplatecarpus primaevus (Mosasauridae) from the Bearpaw Formation (Campanian, Upper Cretaceous) of the North American Western Interior Seaway. Journal of Vertebrate Paleontology, 16:673-687. https://doi.org/10.1080/02724634.1996.10011357
Huyghe, D., Merle, D., Lartaud, F., Cheype, E., and Emmanuel, L. 2012. Middle Lutetian climate in the Paris Basin: implications for a marine hotspot of paleobiodiversity. Facies, 58:587-604. https://doi.org/10.1007/s10347-012-0307-3
Hyžný, M. 2016. Diversity and distribution patterns of the Oligocene and Miocene decapod crustaceans (Crustacea: Malacostraca) of the Western and Central Paratethys. Geologica Carpathica, 67:471-494. https://doi.org/10.1515/geoca-2016-0030
Hyžný, H., Hudackova, N., Biskupic, R., Rybar, S., Fuksi, T., Halasova, E., Zagorsek, K., Jamrich, M., and Ledvak, P. 2012. Devínska Kobyla--a window into the Middle Miocene shallow-water marine environments of the Central Paratethys (Vienna Basin, Slovakia). Acta Geologica Slovaca, 4:95-111.
Jackson, E.L., Wilding, C., and Attrill, M.J. 2015. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conservation Biology, 29:899-909. https://doi.org/10.1111/cobi.12436
Jager, Z. 1993. The distribution and abundance of young fish in the Banc d'Arguin, Mauritania. Hydrobiologia, 258:185-196.
Jagt, J.W., Deckers, M., Donovan, S.K., Fraaije, R., Goolaerts, S., van der Ham, R., Hart, M.B., Jagt-Yazykova, E.A., van Konijnenburg-van Cittert, J., and Renkens, S. 2019. Latest Cretaceous storm-generated sea grass accumulations in the Maastrichtian type area, the Netherlands - preliminary observations. Proceedings of the Geologists' Association, 130:590-598. https://doi.org/10.1016/j.pgeola.2019.05.003
Jeletzky, J.A. 1969. New or poorly understood Tertiary sepiids from southeastern United States and Mexico. University of Kansas Paleontological Contributions, 41:1-39.
Jongbloed, C.A., de Gier, W., van Ruiten, D.M., and Donovan, S.K. 2016. Aktuo-paläontologie of the common cuttlefish, Sepia officinalis, an endocochleate cephalopod (Mollusca) in the North Sea. Paläontologische Zeitschrift, 90:307-313. https://doi.org/10.1007/s12542-016-0293-9
Kaplan, M.B., Mooney, T.A., McCorkle, D.C., and Cohen, A.L. 2013. Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PLoS ONE, 8(5):e63714. https://doi.org/10.1371/journal.pone.0063714
Kellum, L.B. 1926. Paleontology and stratigraphy of the Castle Hayne and Trent marls in North Carolina. United States Geological Survey Professional Paper, 143:1-41.
Konishi, T. 2012. The northernmost occurrence of Prognathodon (Squamata: Mosasauridae) from the Western Interior Seaway of North America. Canadian Journal of Earth Sciences, 49:1111-1115. https://doi.org/10.1139/e2012-038
Konishi, T., Brinkman, D., Massare, J.A., and Caldwell, M.W. 2011. New exceptional specimens of Prognathodon overtone (Squamata, Mosasauridae) from the upper Campanian of Alberta, Canada, and the systematics and ecology of the genus. Journal of Vertebrate Paleontology, 31:1026-1046. https://doi.org/10.1080/02724634.2011.601714
Konishi, T., Newbrey, M.G., and Caldwell, M.W. 2014. A small, exquisitely preserved specimen of Mosasaurus missouriensis (Squamata, Mosasauridae) from the Upper Campanian of the Bearpaw Formation, western Canada, and the first stomach contents for the genus. Journal of Vertebrate Paleontology, 34:802-819. https://doi.org/10.1080/02724634.2014.838573
Kopecká, J., Holcová, K., Nehyba, S., Hladilová, Š., Brzobohatý, R., and Bitner, M.A. 2018. The earliest Badenian Planostegina bloom deposit: reflection of an unusual environment in the westernmost Carpathian Foredeep (Czech Republic). Geological Quarterly, 62:18-37. https://doi.org/10.7306/gq.1398
Kordos, L. 2002. Eocene sea cows (Sirenia, Mammalia) from Hungary. Fragmenta Palaeontologica Hungarica, 20:43-48.
Koretsky, I.A. and Holec, P. 2002. A primitive seal (Mammalia: Phocidae) from the early middle Miocene of Central Paratethys. Smithsonian Contributions to Paleobiology, 93:163-178.
Koretsky, I. A. and Rahmat, S. J. 2013. First record of fossil Cystophorinae (Carnivora, Phocidae): middle Miocene seals from the northern Paratethys. Rivista Italiana di Paleontologia e Stratigrafia, 119:325-350.
Koskeridou, E., Thivaiou, D., Giamali, C., Agiadi, K., and Mantzouka, D. 2019. Seagrass-associated molluscan and fish communities from the Early Pleistocene of the island of Rhodes (Greece). IOP Conference Series: Earth and Environmental Science, 221:1-9. https://doi.org/10.1088/1755-1315/221/1/012050
Košťák, M. and Hoşgör, I. 2012. Belosaepiid (Cephalopoda, Coleoidea) record from the Early Eocene of the Hakkari area (Southeast Turkey) and its significance. Neues Jahrbuch für Geologie und Palaeontologie - Abhandlungen, 266:59-65. https://doi.org/10.1127/0077-7749/2012/0260
Košťák, M. and Jagt, J.W. 2018. A new species of Sepia (Cephalopoda, Coleoidea) from the Miocene of northwest Germany: a contribution to sepiid palaeobiogeography. Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen, 288:273-281. https://doi.org/10.1127/njgpa/2018/0741
Košťák, M., Jagt, J.W.M., and Schlögl, J. 2019. Diversity and distribution of Miocene-Pliocene sepiids (Cephalopoda) in the Mediterranean area, with new records from Italy and Turkey. Swiss Journal Palaeontology, 138:99-108. https://doi.org/10.1007/s13358-018-0179-4
Košťák, M., Jagt, J.W.M., Speijer, R.P., Stassen, P., and Steurbaut, E. 2013. New Paleocene sepiid coleoids (Cephalopoda) from Egypt: evolutionary significance and origin of the sepiid ‘rostrum’. PLoS ONE, 8:e81180. https://doi.org/10.1371/journal.pone.0081180
Košťák, M., Ruman, A., Schlögl, J., Hudáčková, N., Fuchs, D., and Mazuch, M. 2017. Miocene sepiids (Cephalopoda, Coleoidea) from Australia. Fossil Record, 20:159-172. https://doi.org/10.5194/fr-20-159-2017
Košťák, M., Schlögl, J., Culka, A., Tomašových, A., Mazuch, M., and Hudáčková, N. 2018. The unique preservation of Sepia soft tissues in the Miocene deposits (Serravalian, Vienna Basin): implications for the origin of microbodies in the fossil record. Palaeogeography, Palaeoclimatology, Palaeoecology, 493:111-118. https://doi.org/10.1016/j.palaeo.2018.01.005
Košťák, M., Schlögl, J., Hudáčková, N., Kroh, A., Halásová, E., Gašparič, R., Hyžný, M., and Wanzenböck, G. 2016. Sepia from the Miocene of the Central Paratethys: new taxa and notes on late Cenozoic cuttlefish diversity. Journal of Systematic Palaeontology, 14:1033-1057. https://doi.org/10.1080/14772019.2015.1135194
Koulouri, P., Kalogirou, S., Maidanou, M., Koutsoubas, D., and Dounas, C. 2016. Fish and cephalopod assemblage structure of green alga Caulerpa prolifera (Chlorophyta) meadow in the eastern Mediterranean Sea (Elounda Bay, Crete Island). Regional Studies in Marine Science, 3:33-41. https://doi.org/10.1016/j.rsma.2015.12.002
Kowalewski, M. 1990. A hermeneutic analysis of the shell-drilling gastropod predation on mollusks in the Korytnica Clays (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geologica Polonica, 40:183-214.
Kröger, B., Vinther, J., and Fuchs, D. 2011. Cephalopod origin and evolution: a congruent picture emerging from fossils, development and molecules. Bioessays, 33:602-613. https://doi.org/10.1002/bies.201100001
Landman, N.H. and Waage, K.M. 1993. Scaphitid ammonites of the Upper Cretaceous (Maastrichtian) Fox Hills Formation in South Dakota and Wyoming. Bulletin of the American Museum of Natural History, 215:1-257.
Larson, N.L. 2010. Fossil coleoids from the Late Cretaceous (Campanian & Maastrichtian) of the Western Interior. Ferrantia, 59:78-113.
Lebrun, P., Courville, P., and Pacaud, J.M. 2012. Les mollusques des sables éocènes de Bois-Gouët (Loire-Atlantique). Revue Française de Paléontologie, hors Série, 3:92-97.
Leprieur, F., Descombes, P., Gaboriau, T., Cowman, P.F., Parravicini, V., Kulbicki, M., Melián, C.J., de Santana, C.N., Heine, C., Mouillot, D., Bellwood, D.R., and Pellissier, L. 2016. Plate tectonics drive tropical reef biodiversity dynamics. Nature Communications, 7:11461. https://doi.org/10.1038/ncomms11461
Mączyńska, S. 1987. A supplementary account on the echinoids from the Korytnica Basin (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geologica Polonica, 37:145-154.
Marramà, G., Bannikov, A.F., Tyler, J.C., Zorzin, R., and Carnevale, G. 2016. Controlled excavations in the Pesciara and Monte Postale sites provide new insights about the palaeoecology and taphonomy of the fish assemblages of the Eocene Bolca Konservat-Lagerstätte, Italy. Palaeogeography, Palaeoclimatology, Palaeoecology, 454:228-245. https://doi.org/10.1016/j.palaeo.2016.04.021
Mayoral, E. and Muñíz, F. 1994. Presencia de un nuevo Cefalópodo Sepioideoen el Neógeno superior de la Cuenca del Guadalquivir (Lepe, Huelva, España). Coloquios de Paleontolología, 46:161-174.
Mayoral, E. and Reguant, S. 1995. Palaeoecology and taphonomy of bivalves, mainly Glycymeris insubrica (Brocchi), and bryozoans from the Huelva Sands Fm. (lower Pliocene, SW Spain). Revista Española de Paleontologia, 7:31-47.
McNamara, K.J. and Kendrick, G.W. 1994. Cenozoic molluscs and echinoids of Barrow Island, Western Australia. Records of the Western Australian Museum, Supplement, 51:1-50.
Meyer, J.C. 1993. Un nouveau Coleoide Sepioide, Ceratisepia elongata nov. gen., nov. sp. du Paléocène inférieur (Danien) de Vigny. Implications taxinomiques et phylogénétiques. Geobios, 26:287-304. https://doi.org/10.1016/S0016-6995(06)80383-9
Mikuž, V., Šoster, A., and Ulaga, Š. 2013. Miocene fish teeth from the Plesko quarry, Slovenia. Folia Biologica et Geologica, 54:121-133.
Moissette, P., Koskeridou, E., Cornée, J.-J., Guillocheau, F., and Lécuyer, C. 2007. Spectacular preservation of seagrasses and seagrass-associated communities from the Pliocene of Rhodes, Greece. Palaios, 22:200-211. https://doi.org/10.2110/palo.2005.p05-141r
Moissette, P., Koskeridou, E., Drinia, H., and Cornee, J.J. 2016. Facies associations in warm-temperate siliciclastic deposits: Insights from early Pleistocene eastern Mediterranean (Rhodes, Greece). Geological Magazine, 153:61-83. https://doi.org/10.1017/S0016756815000230
Monostori, M. 2008 Upper Oligocene (Egerian) ostracods in Hungary - systematic description. Annales Universitatis Scientiarum Budapestinensis de Rolando Eotvos Nominatae, Sectio Geologica, 36:9-99.
Muñiz, F., Alfaro, E.M., Barrón, E., and Cachao, M.A. 1999. Nuevos datos sobre macroflora del plioceno en el Suroeste de la Península Ibérica (Lepe, Huelva, España). Geogaceta, 25:143-146.
Neige, P. 2003a. Combining disparity with diversity to study the biogeographic pattern of Sepiidae. Berliner Paläobiologischer Abhandlungen, 3:189-197.
Neige, P. 2003b. Spatial patterns of disparity and diversity of the Recent cuttlefishes (Cephalopoda) across the Old World. Journal of Biogeography, 30:1125-1137. https://doi.org/10.1046/j.1365-2699.2003.00918.x
Neige, P., Lapierre, H., and Merle, D. 2016. New Eocene coleoid (Cephalopoda) diversity from statolith remains: taxonomic assignation, fossil record analysis, and new data for calibrating molecular phylogenies. PLoS ONE, 11:e0154062. https://doi.org/10.1371/journal.pone.0154062
Newton, R.B. 1922. Eocene Mollusca from Nigeria. Geological Survey of Nigeria, Bulletin, 3:1-140.
Newton, R.B. and Harris, G.F. 1894. A revision of the British Eocene Cephalopoda. Journal of Molluscan Studies, 1:119-131.
Nolf, D. and Brzobohatý, R. 1994. Fish otoliths from the Late Oligocene (Eger and Kiscell Formations) in the Eger area (northeastern Hungary). Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre, 64:225-252.
O'Brien, C.E., Mezrai, N., Darmaillacq, A.S., and Dickel, L. 2017. Behavioral development in embryonic and early juvenile cuttlefish (Sepia officinalis). Developmental Psychobiology, 59:145-160. https://doi.org/10.1002/dev.21476
Orth, R.J., Carruthers, T.J., Dennison, W.C., Duarte, C.M., Fourqurean, J.W., Heck, K.L., Jr., Hughes, A.R., Kendrick, G.A., Kenworthy, W.J., Olyarnik, S., Short, F.T., Waycott, M., and Williams, S.L. 2006. A global crisis for seagrass ecosystems. Bioscience, 56:987-996. https://doi.org/10.1641/0006-3568(2006)56[987:agcfse]2.0.co;2
Pacaud, J.-M., Merle, D., and Meyer, J.-C. 2000. La faune danienne de Vigny (Val-d’Oise, France): importance pour l’étude de la diversification des mollusques au début du Tertiaire. Comptes Rendus de l'Académie des Sciences, Series IIA, 330:867-873. https://doi.org/10.1016/S1251-8050(00)00226-3
Palmer, K.V.W. 1937. The claibornian Scaphopoda, Gastropoda and dibranchiate Cephalopoda of the southern United States. Bulletin American Paleontology, 7(32):1-731.
Palmer, K.V.W. and Brann, D.C. 1965. Catalogue of the Paleocene and Eocene mollusca of the southern and eastern United States. Part 1. Pelecypoda, Amphineura, Peteropoda, Scaphopoda and Cephalopoda. Bulletin American Paleontology, 48:1-471.
Pasini, G., Garassino, A., Hyžný, M., Baldanza, A., Bizzarri, R., and Famiani, F. 2014. The bathyal decapod crustacean community from the early Pleistocene of Volterra (Pisa, Tuscany, central Italy). Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen, 271:243-259. https://doi.org/10.1127/0077-7749/2014/0388
Pawar, P.R. 2012. Molluscan diversity in mangrove ecosystem of Uran (Raigad), Navi Mumbai, Maharashtra, west coast of India. Bulletin of Environment, Pharmacology and Life Sciences, 1(6):55-59.
Payne, N.L., Snelling, E.P., Semmens, J.M., and Gillanders, B.M. 2013. Mechanisms of population structuring in giant Australian cuttlefish Sepia apama. PLoS ONE, 8(3):e58694. https://doi.org/10.1371/journal.pone.0058694
Petović, S., Gvozdenović, S., and Ikica, Z. 2017. An annotated checklist of the marine molluscs of the South Adriatic Sea (Montenegro) and a comparison with those of neighbouring areas. Turkish Journal of Fisheries and Aquatic Sciences, 17:921-934.
Pierce, G.J., Hernandez-Milian, G., Santos, M.B., Dendrinos, P., Psaradellis, M., Tounta, E., Androukaki, E., and Edridge, A. 2011. Diet of the monk seal (Monachus monachus) in Greek waters. Aquatic Mammals, 37:284-297. https://doi.org/10.1578/AM.37.3.2011.284
Pietsch, C., Harrison, H.C., and Allmon, W.D. 2016. Whence the Gosport Sand (Upper Middle Eocene, Alabama)? The origin of glauconitic shell beds in the Paleogene of the US Gulf Coastal Plain. Journal of Sedimentary Research, 86:1249-1268. https://doi.org/10.2110/jsr.2016.72
Pimiento, C., Griffin, J.N., Clements, C.F., Silvestro, D., Varela, S., Uhen, M.D., and Jaramillo, C. 2017. The Pliocene marine megafauna extinction and its impact on functional diversity. Nature Ecology & Evolution, 1:1100-1106. https://doi.org/10.1038/s41559-017-0223-6
Powell, R.J. and Baum, G.R. 1982. Eocene biostratigraphy of South Carolina and its relationship to Gulf Coastal Plain zonations and global changes of coastal onlap. Geological Society of America Bulletin, 93:1099-1108. https://doi.org/10.1130/0016-7606(1982)93%3C1099:ebosca%3E2.0.co;2
Radwańska, U. 1992. Fish otoliths in the Middle Miocene (Badenian) deposits of southern Poland. Acta Geologica Polonica, 42:141-328.
Rahmat, S., Muniz, F., Toscano, A., Esperante, R., and Koretsky, I. 2019. First European record of Homiphoca (Phocidae: Monachinae: Lobodontini) and its bearing on the paleobiogeography of the genus. Historical Biology. https://doi.org/10.1080/08912963.2018.1507030
Reich, S. 2014. Gastropod associations as a proxy for seagrass vegetation in a tropical, carbonate setting (San Salvador, Bahamas). Palaios, 29:467-482. https://doi.org/10.2110/palo.2013.071
Reich, S., Di Martino, E., Todd, J.A., Wesselingh, F.P., and Renema, W. 2015 Indirect paleo-seagrass indicators (IPSIs): a review. Earth Science Reviews, 143:161-186. http://doi.org/10.1016/j.earscirev.2015.01.009
Ridoux, V., Spitz, J., Vincent, C., and Walton, M.J. 2007. Grey seal diet at the southern limit of its European distribution: combining dietary analyses and fatty acid profiles. Journal Marine Biological Association, 87:255-264. https://doi.org/10.1017/S002531540705463X
Riegl, B. and Piller, W.E. 2000. Reefs and coral carpets in the Miocene Paratethys (Badenian, Leitha Limestone, Austria). Proceedings 9° International Coral Reef Symposium, Bali, 1:211-216.
Rodhouse, P.G., Pierce, G.J., Nichols, O.C., Sauer, W.H., Arkhipkin, A.I., Laptikhovsky, V.V., Lipiński, M.R., Ramos, J.E., Gras, M., Kidokoro, H., and Sadayasu, K. 2014. Environmental effects on cephalopod population dynamics: implications for management of fisheries. Advances in Marine Biology, 67:99-233. https://doi.org/10.1016/B978-0-12-800287-2.00002-0
Roetzel, R. and Pervesler, P. 2004. Storm-induced event deposits in the type area of the Grund Formation (Middle Miocene, lower Badenian) in the molasse zone of lower Austria. Geologica Carpathica, 55:87-102.
Rögl, F. and Brandstätter, F. 1993. The foraminifera genus Amphistegina in the Korytnica Clays (Holy Cross Mts, Central Poland) and its significance in the Miocene of the Paratethys. Acta Geologica Polonica, 43:121-146.
Rueda, J.L., Gofas, S., Urra, J., and Salas, C. 2009. A highly diverse molluscan assemblage associated with eelgrass beds (Zostera marina L.) in the Alboran Sea: micro-habitat preference, feeding guilds and biogeographical distribution. Scientia Marina, 73:679-700. https://doi.org/10.3989/scimar.2009.73n4679
Ruiz, F., González-Regalado, M.L., and Abad, M. 2018. Nueva especie de ostrácodo del Plioceno de la Depresión del Guadalquivir (SO de España). Estudios Geológicos, 74:e073. https://doi.org/10.3989/egeol.43012.468
Ruiz, F., González-Regalado, M.L., Abad, M., Civis, J., Delgado, J.A., García, E.X., Prudêncio, M.I., and Dias, M.I. 2008. Pliocene ostracods of Southwestern Europe. Geobios, 41:845-859. https://doi.org/10.1016/j.geobios.2007.07.004
Russell, L.S. and Landes, R.W. 1940. Geology of the Southern Alberta Plains. Part II. Paleontology of the marine formations of the Montana Group. Geological Survey of Canada Memoir, 221:1-223.
Sabol, M. and Kováč, M. 2006. Badenian palaeoenvironment, faunal succession and biostratigraphy: a case study from northern Vienna Basin, Devínska Nová Ves - Bonanza site (Western Carpathians, Slovakia). Beiträge zur Paläontologie, 30:415-425.
Short, F.T., Polidoro, B., Livingstone, S.R., Carpenter, K.E., Bandeira, S., Bujang, J.S., Calumpong, H.P., Carruthers, T.J.B., Coles, R.G., Dennison, W.C., Erftemeijer, P.L.A., Fortes, M.D., Freeman, A.S., Jagtap, T.G., Kamal, A.H.M., Kendrick, G.A., Kenworthy, W.J., La Nafie, Y.A., Nasution, I.M., Orth, R.J., Prathep, A., Sanciangco, J.C., van Tussenbroek, B., Vergara, S.G., Waycott, M., and Zieman, J.C. 2011. Extinction risk assessment of the world’s seagrass species. Biological Conservation, 144:1961-1971. https://doi.org/10.1016/j.biocon.2011.04.010
Sigwart, J.D., Lyons, G., Fink, A., Gutowska, M.A., Murray, D., Melzner, F., Houghton, J.D., and Hu, M.Y.A. 2016. Elevated pCO2 drives lower growth and yet increased calcification in the early life history of the cuttlefish Sepia officinalis (Mollusca: Cephalopoda). ICES Journal of Marine Science, 73:970-980. https://doi.org/10.1093/icesjms/fsv188
Smale, M.J. and Cliff, G. 1998. Cephalopods in the diets of four shark species (Galeocerdo cuvier, Sphyrna lewini, S. zygaena and S. mokarran) from KwaZulu-Natal, South Africa. South African Journal of Marine Science, 20:241-253. https://doi.org/10.2989/025776198784126610
Smith, H.C. and Sprogis, K.R. 2016. Seasonal feeding on giant cuttlefish (Sepia apama) by Indo-Pacific bottlenose dolphins (Tursiops aduncus) in south-western Australia. Australian Journal of Zoology, 64:8-13. https://doi.org/10.1071/ZO15075
Śmigielska, T. 1979. Fish otoliths from the Korytnica Clays (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geologica Polonica, 29:295-334.
Squires, R.L. 1988. Cephalopods from the late Eocene Hoko River Formation, northwestern Washington. Journal of Paleontology, 62:76-82. https://doi.org/10.1017/S0022336000018023
Švagrovský, J. 1981. Lithofazielle Entwick lung und Molluskenfauna des oberen Badeniens (Miozän M4d) in dem Gebiet Bratislava - Devínska Nová Ves. Západné Karpaty, Séria Paleontológia, 7:5-204
Szabó, M. and Kocsis, L. 2016. A preliminary report on the early Oligocene (Rupelian, Kiscellian) selachians from the Kiscell Formation (Buda Mts, Hungary), with the re-discovery of Wilhelm Weiler’s shark teeth. Fragmenta Palaeontologica Hungarica, 33:31-64. https://doi.org/10.17111/FragmPalHung.2016.33.31
Szczechura, J. and Aiello, G. 2003. The ostracod genus Nipponocythere Ishizaki, 1971 from the Middle Miocene of the Fore-Carpathian Depression, Central Paratethys; its origin and paleoenvironment. Geologica Carpathica, 54:9-20.
Szörényi, E. 1933. Neue tertiäre Sepiinae aus Ungarn nebst Bemerkungen zum zeitlichen Auftreten und zur Entwicklung der Gattung Sepia. Földtani Közlöny, 63:183-189.
Szôrényi, E. 1934. Adatuk a harmadkori Sepiafélék ismeretéhez, héhilny iy Magyarorszdgi faj alapjan. Foldtani Kozlany, 63:183-189.
Tanner, A.R., Fuchs, D., Winkelmann, I.E., Gilbert, M.T.P., Pankey, M.S., Ribeiro, Â.M., Kocot, K.M., Halanych, K.M., Oakley, T.H., da Fonseca, R.R., and Pisani, D. 2017. Molecular clocks indicate turnover and diversification of modern coleoid cephalopods during the Mesozoic Marine Revolution. Proceedings of the Royal Society B: Biological Sciences, 284:1850. https://doi.org/10.1098/rspb.2016.2818
Telesca, L., Belluscio, A., Criscoli, A., Ardizzone, G., Apostolaki, E.T., Fraschetti, S., Gristina, M., Knittweis, L., Martin, C.S., Pergent, G., and Alagna, A. 2015. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Scientific Reports, 5(1):1-14. https://doi.org/10.1038/srep12505
Toulmin, L.D. 1977. Stratigraphic distribution of Paleocene and Eocene fossils in the eastern Gulf Coast Region. Geological Survey of Alabama, Monograph, 13:1-602.
Tracey, S., Todd, J.A., Le Renard, J., King, C., and Goodchild, M. 1996. Distribution of Mollusca in Units S1 to S9 of the Selsey Formation (middle Lutetian), Selsey Peninsula, West Sussex. Tertiary Research, 16:97-139.
Tsujita, C.J. 1995. Origin of concretion-hosted shell clusters in the Late Cretaceous Bearpaw Formation, southern Alberta, Canada. Palaios, 10:408-423. https://doi.org/10.2307/3515044
Uhen, M.D. 2005. A new genus and species of archaeocete whale from Mississippi. Southeastern Geology, 43:157-172.
Uhen, M.D. 2013. A review of North American Basilosauridae. Bulletin of the Alabama Museum of Natural History, 31(2):1-45.
van der Ham, R.W.J.M., van Konijnenburg-van Cittert, J.H.A., and Indeherberge, L. 2007. Seagrass foliage from the Maastrichtian type area (Maastrichtian, Danian, NE Belgium, SE Netherlands). Review of Palaeobotany and Palynology, 44:301-321. https://doi.org/10.1016/j.revpalbo.2006.07.008
Vélez-Juarbe, J. 2014. Ghost of seagrasses past: using sirenians as a proxy for historical distribution of seagrasses. Palaeogeography, Palaeoclimatology, Palaeoecology, 400:41-49. https://doi.org/10.1016/j.palaeo.2013.05.012
Waycott, M., Duarte, C.M., Carruthers, T.J.B., Orth, R.J., Dennison, W.C., Olyarnik, S., Calladine, A., Fourqurean, J.W., Heck, K.L., Jr., Hughes, A.R., Kendrick, G.A., Kenworthy, W.J., Short, F.T., and Williams, S.L. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences, 106:12377-12381. https://doi.org/10.1073/pnas.0905620106
Weaver, P.G. and Ciampaglio, C.N. 2003. A new genus of belosaepiid (Coleoidea) from the Castle Hayne Limestone (Eocene) of southeastern North Carolina. Journal of Paleontology, 77:1103-1106. https://doi.org/10.1666/0022-3360(2003)077%3C1103:angobc%3E2.0.co;2
Weaver, P., Ciampaglio, C.N., and Chandler, R. 2007. Rarely seen coleoid phragmacone steinkerns from the Eocene Castle Hayne Limestone of southeastern North Carolina. Palaeontographica Abteilung A, 279:159-165. https://doi.org/10.1127/pala/279/2007/159
Weaver, P.G., Ciampaglio, C.N., and Chandler, R.E. 2010a. An overview of coleoid cephalopods from Paleogene and Neogene aged rocks of southern North America. Ferrantia, 59:202-214.
Weaver, P.G., Dockery, D.T., III., and Ciampaglio, C.N. 2010b. A new genus of coleoid cephalopod from the Jackson Group (Late Eocene), Hinds County, Mississippi. Palaeontographica Abteilung A, 292:53-63. https://doi.org/10.1127/pala/292/2010/53
Williams, R.J. 2002. Observations on the London Clay excavation at Aveley, Essex. Tertiary Research, 21:95-112.
Xavier, J.C., Peck, L.S., Fretwell, P., and Turner, J. 2016. Climate change and polar range expansions: could cuttlefish cross the Arctic? Marine Biology, 163:78. https://doi.org/10.1007/s00227-016-2850-x
Yancey, T.E., Garvie, C.L., and Wicksten, M. 2010. The middle Eocene Belosaepia ungula (Cephalopoda: Coleoida) from Texas: structure, ontogeny and function. Journal of Paleontology, 84:267-287. https://doi.org/10.1666/09-018R.1
Zavodnik, D., Legac, M., and Gluhak, T. 2006. An account of the marine fauna of Pag Island (Adriatic Sea, Croatia). Natura Croatica, 15:65-107.
Zlinská, A., Zorn, I., and Zágoršek, K. 2013. Foraminifery, ostrakódy a machovky z okolia devínskej Novej Vsi a Záhorskej Bystrice (slovenská časť Viedenskej panvy). Mineralia Slovaca, 45:185-200.
Zorn, I. 2004. Ostracoda from the Lower Badenian (Middle Miocene) Grund Formation (Molasse Basin, Lower Austria). Geologica Carpathica, 55:179-190.
Zuschin, M., Harzhauser, M., and Mandic, O. 2004. Taphonomy and paleoecology of the lower Badenian (Middle Miocene) molluscan assemblages at Grund (Lower Austria). Geologica Carpathica, 55:117-128.
Zuschin, M., Harzhauser, M., and Mandic, O. 2007. The stratigraphic and sedimentologic framework of fine-scale faunal replacements in the Middle Miocene of the Vienna Basin (Austria). Palaios, 22:285-295. https://doi.org/10.2110/palo.2005.p05-023r

