 Trophic relationships in the Early Miocene Upper Marine Molasse of Baden-Württemberg, Southwest Germany, with special emphasis on the elasmobranch fauna
Trophic relationships in the Early Miocene Upper Marine Molasse of Baden-Württemberg, Southwest Germany, with special emphasis on the elasmobranch fauna
Article number: 26.3.a46
https://doi.org/10.26879/1233
Copyright Paleontological Society, November 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 5 August 2023. Acceptance: 5 October 2023.
ABSTRACT
The Early Miocene Upper Marine Molasse (OMM) in south-western Germany contains a diverse fossil ecosystem in which elasmobranch teeth are especially abundant. However, the scarcity of outcrops and sometimes poor preservation of fossils resulted in scant recent literature about the OMM. Here, we focus on the elasmobranch fauna to determine the trophic relationships within the OMM, using fossil teeth as proxies for diet and trophic levels based on functional morphology and an actualistic species- or genus-level approach. Herein we present a fresh and comprehensive palaeoecological reconstruction of the OMM ecosystem in Baden-Württemberg. All five outcrop areas available for the present analysis (Baltringen, Meßkirch-Rengetsweiler, Meßkirch-Walbertsweiler, Ulm-Ermingen, and Ursendorf) exhibit a similar faunal composition, with the apex predator being Otodus (Megaselachus) sp. Among the other elasmobranchs, there are mostly piscivorous and malacophagous species; taxa that feed on a variety of other invertebrates or amniotes (including marine mammals) are also present. The OMM sediments deposited in shallow-water settings, but there are fossils of more oceanic species that might, at times, have approached the shore. With a soft bottom, partly covered by sea grass, the OMM environment would have been like the present-day warm-waters settings of the Mediterranean.
Olaf Höltke. Staatliches Museum für Naturkunde Stuttgart, Stuttgart, Germany. laf.hoeltke@smns-bw.de
Rodrigo B. Salvador. Department of Arctic and Marine Biology, The Arctic University of Norway, Tromsø, Norway and The Arctic University Museum of Norway, The Arctic University of Norway, Tromsø, Norway. salvador.rodrigo.b@gmail.com
Michael W. Rasser. Staatliches Museum für Naturkunde, Stuttgart, Germany. michael.rasser@smns-bw.de
Keywords: dental morphology; Elasmobranchii; Obere Meeresmolasse; OMM; palaeoecology; palaeoenvironment
Final citation: Höltke, Olaf, Salvador, Rodrigo B., and Rasser, Michael W. 2023. Trophic relationships in the Early Miocene Upper Marine Molasse of Baden-Württemberg, Southwest Germany, with special emphasis on the elasmobranch fauna. Palaeontologia Electronica, 26(3):a46.
https://doi.org/10.26879/1233
palaeo-electronica.org/content/2023/4000-trophic-relationships
Copyright: November 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Trophic relationships or food chains are a common theme in the literature about modern marine ecosystems (e.g., Lindeman, 1942; Arias-Gonzalez et al., 1997; Heithaus and Vaudo, 2012; Heithaus et al., 2012; Wetherbee et al., 2012; Bănaru et al., 2013; Bornatowski et al., 2014, 2018), as well as about palaeoecosystems (e.g., Scott, 1978; Maisey, 1994; Bianucci et al., 2000; Westgate, 2001; Stanton Jr., 2006; Aguilera and de Aguilera, 2014; Perez et al., 2017, 2021; Alberti and Reich, 2018 and references therein; Collareta, 2021).
Recently, isotopes from shark teeth have been used to determine the trophic position of extant and extinct species (see Martin et al., 2015; Kast et al., 2022; and McCormack et al., 2022). In palaeoecosystems, several of the parameters observable in their extant counterparts are generally not available, including stomach content, primary productivity data, and information on dietary energy levels between trophic levels (TL). Another important difference between recent and fossil ecosystems is the time factor. In modern ecosystems, the “everyday” (or seasonal, annual, etc.) conditions are directly accessible to study. Even ‘long-term’ studies conducted in modern ecosystems represent an insignificant time interval when compared to the fossil record. Indeed, a typical fossil assemblage usually represents a broad interval of geological time (time-averaging), whose duration is often difficult to estimate. Additionally, the time intervals for analysis need to be defined in accordance with litho- and sedimentological evidence, to properly acknowledge possible environmental changes throughout the section (e.g., from shallow to deep waters). Understanding the trophic relationships is a valuable tool for getting a better and more detailed picture of the paleoenvironment.
The sediments of the Upper Marine Molasse (“Obere Meeresmolasse” in German, abbreviated as OMM) in Baden-Württemberg, southwestern Germany, contains a diverse fossil ecosystem in which sharks and rays are especially abundant, being mostly represented by isolated teeth. The OMM fossil assemblage represents a relatively short timespan in the Miocene (early to middle Ottnangian, ca. 18 to 17.6 myr). Therefore, it can be assumed that the faunal elements present in the OMM in Baden-Württemberg inhabited the same suite of palaeohabitats and were largely contemporaneous. However, many of the elasmobranch fossil teeth are poorly preserved. The amount of determinable shark and ray teeth are much lower than that of other similar deposits, such as those of northern Germany. Other macrofossils are also often poorly preserved. This may be the reason for the scant recent literature about OMM macrofossils in Baden-Württemberg (Barthelt et al., 1991; Pfeil, 1991; Baier et al., 2004; Höltke, 2009; Nebelsick et al., 2019; Höltke et al., 2020; Feichtinger and Pollerspöck, 2021; Höltke et al., 2022; see also the informative outreach website by Feichtinger et al., 2022).
In the present study, we focused on the elasmobranch fauna to determine the trophic relationships within the OMM by using fossil teeth as proxies for diet and trophic levels based on functional morphology and an actualistic genus or species-level approach (Rasser et al., 2019). We present a novel and comprehensive palaeoecological reconstruction of the OMM ecosystem in Baden-Württemberg.
GEOLOGICAL OVERVIEW
In southern Germany, the sediments within the North Alpine Foreland Basin are divided in the following units: Lower Marine Molasse, Lower Freshwater Molasse, Upper Marine Molasse, Brackish Molasse, and Upper Freshwater Molasse. The Upper Marine Molasse (OMM) in Baden-Württemberg belongs to the early Miocene and ranges from the early to the middle Ottnangian (middle Burdigalian, ca. 18 to 17.6 myr; for details, see Geyer and Gwinner, 1991; Heckeberg et al., 2010). During the deposition of the Brackish Molasse (upper Ottnangian), the so-called “Graupensandrinne” level was formed, which eroded most of the underlying OMM sediments (Geyer and Gwinner, 1991). Only parts of the OMM sediments were preserved within this Graupensandrinne; they are called “Grobsandzug”.
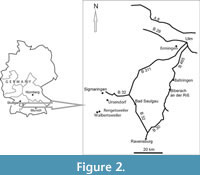
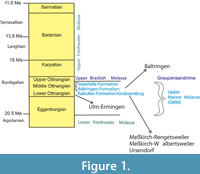 The Grobsandzug is time-equivalent to the early Ottnangian Kalkofen Formation, an OMM formation of the Molasse Basin of Southern Germany (Heckeberg et al., 2010) (Figure 1). The authors defined a new lithostratigraphic terminology for the Ottnangian deposits of the OMM in Southwest Germany. The hanging of the Kalkofen Formation is formed by the Baltringen Formation and the Steinhöfe Formation (Figure 1). For more specific details about OMM geology, we refer the reader to previous works (Schreiner, 1966; Geyer and Gwinner, 1991; Kuhlemann and Kempf, 2002; Höltke, 2009; Heckeberg et al., 2010). We focus our analysis on the following OMM fossiliferous deposits: Baltringen (middle Ottnangian), Meßkirch-Rengetsweiler (early Ottnangian), Meßkirch-Walbertsweiler (early Ottnangian), Ulm-Ermingen (early Ottnangian), and Ursendorf (early Ottnangian) (Figure 2). Meßkirch-Rengetsweiler, Meßkirch-Walbertsweiler, and Ursendorf are part of the Grobsandzug mentioned above. In addition to shark and ray teeth, fossil remains of other taxa were also recovered from these deposits, including bonyfish teeth and molluscs.
The Grobsandzug is time-equivalent to the early Ottnangian Kalkofen Formation, an OMM formation of the Molasse Basin of Southern Germany (Heckeberg et al., 2010) (Figure 1). The authors defined a new lithostratigraphic terminology for the Ottnangian deposits of the OMM in Southwest Germany. The hanging of the Kalkofen Formation is formed by the Baltringen Formation and the Steinhöfe Formation (Figure 1). For more specific details about OMM geology, we refer the reader to previous works (Schreiner, 1966; Geyer and Gwinner, 1991; Kuhlemann and Kempf, 2002; Höltke, 2009; Heckeberg et al., 2010). We focus our analysis on the following OMM fossiliferous deposits: Baltringen (middle Ottnangian), Meßkirch-Rengetsweiler (early Ottnangian), Meßkirch-Walbertsweiler (early Ottnangian), Ulm-Ermingen (early Ottnangian), and Ursendorf (early Ottnangian) (Figure 2). Meßkirch-Rengetsweiler, Meßkirch-Walbertsweiler, and Ursendorf are part of the Grobsandzug mentioned above. In addition to shark and ray teeth, fossil remains of other taxa were also recovered from these deposits, including bonyfish teeth and molluscs.
Ulm-Ermingen (early Ottnangian)
 Ermingen is famous for the so-called “Erminger Turritellenplatte”, a mass accumulation of gastropod shells belonging to the genus Turritella Lamarck, 1799 (Baier, 2008; Höltke, 2009; Nebelsick et al., 2019). A succession of 3.5 m of the Turritellenplatte was excavated in 2005 by a team from the Staatliches Museum für Naturkunde Stuttgart (SMNS; Stuttgart, Germany). The Turritella shells are firmly cemented within bioclastic, coarse grained-sandstone with a calcareous matrix that can pass into a quartz-rich limestones (Nebelsick et al., 2019). According to Nebelsick et al. (2019), silty marls with sands are also present, containing isolated specimens of Turritella and bivalves (Veneridae). Towards the top of the excavated succession, unconsolidated sands appear that are rich in Turritella specimens, followed by large blocks in situ (Nebelsick et al., 2019). The changes in the fabric of the Turritella -dominated beds were used by Nebelsick et al. (2019) to reconstruct a generally deepening environment that corresponds to an Early Miocene transgression. Despite this deepening, a shallow-water, soft-bottom community persisted, with endo- and epibenthic and pelagic organisms, like recent faunas of warm-temperate to (sub)tropical continental shelves. The mass occurrence of the semi-infaunal gastropod genus Turritella indicates a nutrient-rich palaeoenvironment, which in turn, implies a high degree of water movement within the Molasse Sea, with nutrients being readily available from local upwelling and tidal current transport (Nebelsick et al., 2019). Turritella -dominated assemblages are also known from other rich palaeoecosystems such as the Gatun Formation from the Upper Miocene of Panama (Anderson et al. 2017) as well as from the Pisco Formation (Late Miocene) of Cerro Los Quesos, Ica Desert, Peru (Di Celma et al., 2015).
Ermingen is famous for the so-called “Erminger Turritellenplatte”, a mass accumulation of gastropod shells belonging to the genus Turritella Lamarck, 1799 (Baier, 2008; Höltke, 2009; Nebelsick et al., 2019). A succession of 3.5 m of the Turritellenplatte was excavated in 2005 by a team from the Staatliches Museum für Naturkunde Stuttgart (SMNS; Stuttgart, Germany). The Turritella shells are firmly cemented within bioclastic, coarse grained-sandstone with a calcareous matrix that can pass into a quartz-rich limestones (Nebelsick et al., 2019). According to Nebelsick et al. (2019), silty marls with sands are also present, containing isolated specimens of Turritella and bivalves (Veneridae). Towards the top of the excavated succession, unconsolidated sands appear that are rich in Turritella specimens, followed by large blocks in situ (Nebelsick et al., 2019). The changes in the fabric of the Turritella -dominated beds were used by Nebelsick et al. (2019) to reconstruct a generally deepening environment that corresponds to an Early Miocene transgression. Despite this deepening, a shallow-water, soft-bottom community persisted, with endo- and epibenthic and pelagic organisms, like recent faunas of warm-temperate to (sub)tropical continental shelves. The mass occurrence of the semi-infaunal gastropod genus Turritella indicates a nutrient-rich palaeoenvironment, which in turn, implies a high degree of water movement within the Molasse Sea, with nutrients being readily available from local upwelling and tidal current transport (Nebelsick et al., 2019). Turritella -dominated assemblages are also known from other rich palaeoecosystems such as the Gatun Formation from the Upper Miocene of Panama (Anderson et al. 2017) as well as from the Pisco Formation (Late Miocene) of Cerro Los Quesos, Ica Desert, Peru (Di Celma et al., 2015).
Meßkirch-Rengetsweiler (early Ottnangian, Grobsandzug)
Not far away from the open sand pit in Walbertsweiler (see below), the active sand pit owned by the Steidle Company takes its place in Rengetsweiler. The geology of the Grobsand deposit occurring herein was documented by Bieg et al. (2007) based on an excavation by an SMNS team in September 2006. These sediments belong to the European Neogene Mammal Zones MN 2b to MN 3 (Sach, 2016). They consist of sand with muddy intercalations. The palaeoenvironment was probably shallow water like the one in Walbertsweiler, and evidence for tides is present, including flaser bedding and ripple bedding (following Bieg et al., 2007). The presence of Metaxytherium sp. (Sirenia) is an indication of the presence of sea grass meadows.
Meßkirch-Walbertsweiler (early Ottnangian, Grobsandzug)
The fossils originate from an open sand pit near Meßkirch-Walbertsweiler (Barthelt et al., 1991). Sediments consist of alternated sands and marls and were deposited in a sublittoral environment at a water depth of less than 50 m (Barthelt et al., 1991). Most fossils (especially invertebrates) are abraded and eroded, so data is scarce. Sirenian fossils have also been found at this locality, which indicates the presence of sea grass weed.
Ursendorf (early Ottnangian, Grobsandzug)
The geology of Ursendorf was described by Bieg et al. (2007) based on an excavation conducted by an SMNS team in the sand pit of the Teufel Company. The sediments consist of sands with different grain size (Bieg et al., 2007, Höltke, 2009), with fossils coming from coarse-grained and poorly sorted sands. In the late eighteenth and early nineteenth centuries, there were more (and similarly coarse-grained) sand pits in the surroundings of the present-day pit, hence some fossils in the SMNS collection probably come from these older pits. The sedimentary succession examined during the excavation in 2006 showed a cross-bedding that indicates a high-energy regime (Bieg et al., 2007) and a palaeoenvironment comprised of a soft bottom, with a rich bryozoan and sea-grass community (Höltke, 2009). However, based on the fossil content, there were also habitats below the storm wave base. The fossils of Ursendorf were the main theme of part of some publications: Molluscs (Höltke, 2009), elasmobranchs (Höltke et al., 2020), bryozoans (Miller, 1875), echinoderms, and sponges (Schütze, 1904).
Baltringen (middle Ottnangian)
The fossil fauna of Baltringen was examined by Probst (1877, 1878, 1879a), who extensively described the elasmobranchs and bony fishes. That author erected several new taxa, many of which were later put into synonymy. A more recent list of fossil fish from this locality was published by Sach (2016). Pollerspöck and Unger (2022) published a re-evaluation of the new ray taxa erected by Probst (1877). Baltringen is the type locality of the Baltringen Formation, which consist of sands with silt lenses (Heimann et al., 2009; Heckeberg et al., 2010). This formation was deposited in a tide-influenced subtidal environment (Heckeberg et al., 2010) and has been assigned to the Mammal Zone MN 4a (Sach, 2016). The presence of Sirenia (Metaxytherium sp.) is indicative of sea grass.
MATERIAL AND METHODS
The localities studied here were chosen based on the number of fossils of elasmobranchs (sharks and rays) available in the palaeontological collection of the Staatliches Museum für Naturkunde Stuttgart (SMNS, Stuttgart, Germany) as well as on the number of studies available in the literature detailing the fossil content of these deposits (Table 1 and Table 2). For reconstructing the food chain, other fossils (vertebrates and invertebrates; Table 3) were also considered, as they were the potential prey of the ancient elasmobranchs.
We also included two instances of personal communications with collectors: the finding of a dactylus of a crab in Meßkirch-Rengetsweiler (pers. comm. Member of the “Mineralien- und Fossilienfreunde Ulm / Neu-Ulm e.V” with the first author, 2007) and the presence of remnants of Cetacea and Sirenia in Meßkirch-Walbertsweiler (pers. comm. Elmar Unger with the first author, 2019). Animals comprising mainly soft tissues, such as worms, cephalopods, and even “soft-shelled” creatures like shrimps and prawns, rarely preserve in the fossil record, though their presence in the OMM can be inferred given their ubiquity in shallow marine environments globally. In Ursendorf, there had been different sand pits in the past and only remnants of them are readily visible today, apart from one active sand pit. So, it cannot be determined from which sand pit each fossil came, but all the outcrops show the same lithofacies. The same is the case for Baltringen. Probst (1871, 1877, 1878, 1879a, 1879b) collected at different sites in Baltringen, all with the same lithofacies. No reworked material from the underlying Jurassic or Lower Freshwater Molasse sediments–as evidenced by the very different faunas and preservation–were found in the present study; furthermore, there are no mentions of reworked fossils in the literature.
Tooth Morphology and Diet
We focused on the fossil teeth of elasmobranchs as a proxy for establishing the diet of these species. As a first step, we used the actualistic approach described by Rasser et al. (2019), which is based on comparisons with ecological data of living congeners. When possible, this was done on the species level, otherwise it was done on the genus-level. The data on recent species was extracted from specialized literature, mostly Ebert (2003), Compagno et al. (2005), Ebert et al. (2021), and Froese and Pauly (2019). More specific works were also used and are highlighted in the species’ entries below when pertinent. Special attention was paid to the work of Cortés (1999), which provides precise proportions of the different prey items that make up the diets of the sharks, thus enabling us to determine whether a given species has a favored food item (referred to as “staple food” herein). In extant sharks, a dietary shift during ontogeny can often be observed (e.g., of Tricas and McCosker 1984; Tricas, 1985; Powter et al., 2010; Goodman et al., 2022). Based on comparisons with modern shark teeth as well as the size and morphology of the OMM teeth used herein, they were probably all from adult specimens, so the reconstructed trophic relationships presented here pertains to adult specimens.
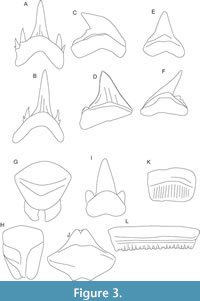 Tooth morphology provides clues to the diet of extinct taxa, as tooth shape strongly correlates with diet in extant shark species (Moss, 1977; Cappetta, 1986, 2012; Powter et al., 2010; Pollerspöck and Straube, 2019; Straube and Pollerspöck, 2020; Bazzi et al., 2021; Goodman et al., 2022). In the works of Cappetta (1986, 2012), elasmobranch teeth were classified into eight dental types according to their morphology, each corresponding to a type of trophic adaptation: 1) clutching; 2) tearing; 3) cutting (with the subtypes “sensu stricto cutting” and “cutting-clutching”); 4) crushing; 5) grinding; 6) clutching-grinding; 7) cutting-grinding; 8) crushing-grinding. Out of these eight dental types, five are known from the OMM deposits named above (1, 2, 3, 4, 5; type 3 includes both subtypes) (Figure 3). However, contrary opinions concerning the role of tooth shape can be found in the literature; e.g., the study of Whitenack and Motta (2010) concluded that shark tooth morphology is a poor predictor of trophic level. There is definitively a dietary overlap between, for example, sharks with “tearing-type” dentition (e.g., Carcharias, Mitsukurina and Pseudocarcharias) and ones with “cutting-type” dentition (e.g., Galeocerdo); both feed on bony fishes, but Galeocerdo also takes marine mammals, reptiles, and birds. Animals belonging to the ray families Myliobatidae and Rhinopteridae have teeth adapted for “grinding” hard-shelled invertebrates. According to the works of Tricas and McCosker (1984) as well as Tricas (1985), Great White sharks (Carcharodon carcharias) with a total length of < 3 m feed mostly on fishes and therefore, they have narrow tooth shape for grasping; when the sharks become larger, the teeth broaden at the base and achieve the typical triangular serrated shape, which is suitable for preying on marine mammals. Therefore, tooth morphology is not an unequivocal method for a detailed prey determination of living or fossil taxa, but it can be used to provide valuable data regarding a species’ dietary spectrum. The teeth were classified according to the definitions and descriptions provided by Cappetta (2012, p. 16-23).
Tooth morphology provides clues to the diet of extinct taxa, as tooth shape strongly correlates with diet in extant shark species (Moss, 1977; Cappetta, 1986, 2012; Powter et al., 2010; Pollerspöck and Straube, 2019; Straube and Pollerspöck, 2020; Bazzi et al., 2021; Goodman et al., 2022). In the works of Cappetta (1986, 2012), elasmobranch teeth were classified into eight dental types according to their morphology, each corresponding to a type of trophic adaptation: 1) clutching; 2) tearing; 3) cutting (with the subtypes “sensu stricto cutting” and “cutting-clutching”); 4) crushing; 5) grinding; 6) clutching-grinding; 7) cutting-grinding; 8) crushing-grinding. Out of these eight dental types, five are known from the OMM deposits named above (1, 2, 3, 4, 5; type 3 includes both subtypes) (Figure 3). However, contrary opinions concerning the role of tooth shape can be found in the literature; e.g., the study of Whitenack and Motta (2010) concluded that shark tooth morphology is a poor predictor of trophic level. There is definitively a dietary overlap between, for example, sharks with “tearing-type” dentition (e.g., Carcharias, Mitsukurina and Pseudocarcharias) and ones with “cutting-type” dentition (e.g., Galeocerdo); both feed on bony fishes, but Galeocerdo also takes marine mammals, reptiles, and birds. Animals belonging to the ray families Myliobatidae and Rhinopteridae have teeth adapted for “grinding” hard-shelled invertebrates. According to the works of Tricas and McCosker (1984) as well as Tricas (1985), Great White sharks (Carcharodon carcharias) with a total length of < 3 m feed mostly on fishes and therefore, they have narrow tooth shape for grasping; when the sharks become larger, the teeth broaden at the base and achieve the typical triangular serrated shape, which is suitable for preying on marine mammals. Therefore, tooth morphology is not an unequivocal method for a detailed prey determination of living or fossil taxa, but it can be used to provide valuable data regarding a species’ dietary spectrum. The teeth were classified according to the definitions and descriptions provided by Cappetta (2012, p. 16-23).
When available, isotope data were also considered (Kast et al., 2022; McCormack et al., 2022). McCormack et al. (2022), analysed Zn isotopes in fossil teeth of selected species (i.e., Otodus (Megaselachus) chubutensis, Carcharodon hastalis, Araloselachus cuspidatus, Carcharias contortidens, Galeocerdo aduncus, Hemipristis serra, Mitsukurina lineata, Pseudocarcharias rigida) from Walbertsweiler, one of the localities studied herein. They observed that relative δ66Zn values for tested taxa (e.g., Carcharias spp., Galeocerdo spp.) showed no statistical variation with geologic age and locality, indicating relatively stable trophic levels and ecological niches across time and space. For extinct OMM genera, where the closest living taxa are uncertain, tooth morphology alone was used as a guide to the preferred prey type. As explained by Cappetta (2012), similar dentition in recent and fossil forms–even without direct systematic relationship–is indicative of comparable feeding habits, diets and, to a lesser extent, similar modes of life. When more than one recent congener is known and dental morphology is invariant across various species (e.g., Squatina), a consensus on prey items had to be acquired. In some cases (e.g., Scyliorhinidae), the staple food of most extant species is unknown, so our interpretations are necessarily based on the few species for which the diet is known and as such, they need to be taken cautiously. For extinct genera (e.g., Physogaleus), we relied entirely on dental morphology, compared with that of allied recent species. Recent literature dealing with diet and stomach contents of sharks and rays which have fishes on their diet (e.g., Rasmussen, 2018 and references therein) suggest that these predators have no preferences for specific prey taxa, feeding on members of different families according to availability and size.
RESULTS AND DISCUSSION
A full list of species found in the studied OMM depoists and their tooth types (sensu Cappetta, 2012) are given in Table 2. Some taxa do not match exactly one type: the two taxa with tearing-type dentition, Carcharodon hastalis (Agassiz, 1843) and Isurus retroflexus (Agassiz, 1843), display a tendency toward the cutting type due to widening of the lateral of the teeth (see also Cappetta, 2012, p. 18).
In the OMM, two large-toothed Otodus species are known: O. (Megaselachus) chubutensis (Ameghino, 1901), which had teeth with lateral cusplets, and O. (Megaselachus) megalodon (Agassiz, 1843), which had teeth without lateral cusplets. Because there are transitional forms between both taxa, which have only weakly pronounced lateral cusplets, an exact determination is often problematic (Kent, 1994; Perez et al., 2019; Feichtinger and Pollerspöck, 2021; Pollerspöck et al., 2022). Concerning the diet reconstruction, the presence or absence of lateral cusplets on this tooth type is negligible. Because there is not enough well-preserved Otodus -material to separate them according to the presence/absence of lateral cusplets, the Otodus -teeth here are only determined on subgenus level. The name O. chubutensis is often used for Early Miocene “megalodon -like teeth” with lateral cusplets, whereas typical megalodon adult teeth have no lateral cusplets (Kent 1994; Applegate and Espinosa-Arrubarrena, 1996; Pollerspöck et al., 2022).
A full list of the elasmobranch species known from the OMM can be found in the Appendix, with detailed description of the palaeoecology of each species and comments on their probable diet and trophic level. The results below are a summary of that information focusing on the more important points and constructing a bigger picture for each studied site.
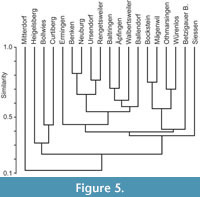
Table 2 summarizes 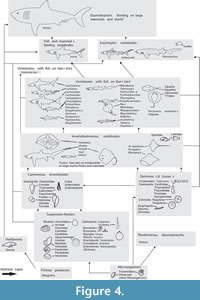 the prey items that have thus been reconstructed for each elasmobranch species including the tooth type. In Table 3, the other occurring taxa were listed. Figure 4 shows a generalized food chain with all the taxa that are known from the five OMM localities, with special emphasis on the sharks and rays. According to the analysis of Höltke et al. (2022b), all the localities studied here have a similar shark and ray fauna, although there are still a few differences in the faunal composition of the single deposits. Figure 5, reproduced from Höltke et al. (2020; 2022b), shows the cluster analysis of the similarity in elasmobranch faunas across OMM deposits in Baden-Württemberg in comparison to the ones outside of Baden-Württemberg. The reasons for the clusters were the palaeogeographic and palaeoenvironmental settings (Höltke et al. 2020), but biases due to differing collection effort might have influenced the analysis to some extent (see discussion in Höltke et al. 2020; 2022b). Each of the five deposits is discussed below on its own, and a summary is presented afterwards.
the prey items that have thus been reconstructed for each elasmobranch species including the tooth type. In Table 3, the other occurring taxa were listed. Figure 4 shows a generalized food chain with all the taxa that are known from the five OMM localities, with special emphasis on the sharks and rays. According to the analysis of Höltke et al. (2022b), all the localities studied here have a similar shark and ray fauna, although there are still a few differences in the faunal composition of the single deposits. Figure 5, reproduced from Höltke et al. (2020; 2022b), shows the cluster analysis of the similarity in elasmobranch faunas across OMM deposits in Baden-Württemberg in comparison to the ones outside of Baden-Württemberg. The reasons for the clusters were the palaeogeographic and palaeoenvironmental settings (Höltke et al. 2020), but biases due to differing collection effort might have influenced the analysis to some extent (see discussion in Höltke et al. 2020; 2022b). Each of the five deposits is discussed below on its own, and a summary is presented afterwards.
Fossil Assemblages and Food Chains
 Ulm-Ermingen (early Ottnangian). The mass-occurring Turritella is a suspension feeder (Höltke 2009), alongside bivalves (Arcidae, Chamidae, Cardiidae, Carditidae, Glycymerididae, Mytilidae, Ostreidae, Pectinidae, Pholadidae, Veneridae), other gastropods (Calyptraea Lamarck, 1799), bryozoans, barnacles, worms (Serpula Linnaeus, 1758) and sponges. Grazers and detritivores were represented by gastropods (Fissurellidae, Cerithiidae, Xenophoridae) and scaphopods. Carnivorous gastropods were represented by Fasciolariidae, Ficidae, Naticidae, Olividae, and Turridae; the Naticidae, in particular, fed on Turritella, as shown by the occurrence of typical drill holes on the shells. A remarkable aspect of this deposit is the high number of preserved molluscan shells in comparison to the other four deposits. As it can be seen in Table 3, the locality Ursendorf (discussed below) has a similar number of molluscan taxa, but these are mostly only found as steinkerns. Two families of bony fishes (Labridae, Sparidae) are observed in Ermingen, typically feeding on shelled invertebrates. Omnivorous turtles (Trionyx sp.) were also present. Among the elasmobracnhs, there are a few molluscivores, such as Aetobatus arcuatus, Aetobatus sp., Rhinoptera studeri and R. sp. (feeding on bivalves), Myliobatis sp. (gastropods and bivalves), Iago angustidens and Alopias exigua (cephalopods). Myliobatis sp. would also have fed on crustaceans, a diet shared with Dasyatis sp. and Raja sp. The latter probably also have fed on bony fishes. Most elasmobranchs were naturally piscivores, but several also preyed up on cephalopods (see Table 2). The top predators in the ancient sea of Ermingen were the two eurytrophic sharks Galeocerdo aduncus and Notorynchus primigenius, as well as Carcharodon hastalis. Lutzeier (1922) also mentioned Otodus (Megaselachus) megalodon from Ermingen, which would have been the topmost predator, as it probably fed on larger sharks and cetaceans. That author also mentioned the long-snouted dolphin Schizodelphis sp. from this locality, which would have fed on bony fishes.
Ulm-Ermingen (early Ottnangian). The mass-occurring Turritella is a suspension feeder (Höltke 2009), alongside bivalves (Arcidae, Chamidae, Cardiidae, Carditidae, Glycymerididae, Mytilidae, Ostreidae, Pectinidae, Pholadidae, Veneridae), other gastropods (Calyptraea Lamarck, 1799), bryozoans, barnacles, worms (Serpula Linnaeus, 1758) and sponges. Grazers and detritivores were represented by gastropods (Fissurellidae, Cerithiidae, Xenophoridae) and scaphopods. Carnivorous gastropods were represented by Fasciolariidae, Ficidae, Naticidae, Olividae, and Turridae; the Naticidae, in particular, fed on Turritella, as shown by the occurrence of typical drill holes on the shells. A remarkable aspect of this deposit is the high number of preserved molluscan shells in comparison to the other four deposits. As it can be seen in Table 3, the locality Ursendorf (discussed below) has a similar number of molluscan taxa, but these are mostly only found as steinkerns. Two families of bony fishes (Labridae, Sparidae) are observed in Ermingen, typically feeding on shelled invertebrates. Omnivorous turtles (Trionyx sp.) were also present. Among the elasmobracnhs, there are a few molluscivores, such as Aetobatus arcuatus, Aetobatus sp., Rhinoptera studeri and R. sp. (feeding on bivalves), Myliobatis sp. (gastropods and bivalves), Iago angustidens and Alopias exigua (cephalopods). Myliobatis sp. would also have fed on crustaceans, a diet shared with Dasyatis sp. and Raja sp. The latter probably also have fed on bony fishes. Most elasmobranchs were naturally piscivores, but several also preyed up on cephalopods (see Table 2). The top predators in the ancient sea of Ermingen were the two eurytrophic sharks Galeocerdo aduncus and Notorynchus primigenius, as well as Carcharodon hastalis. Lutzeier (1922) also mentioned Otodus (Megaselachus) megalodon from Ermingen, which would have been the topmost predator, as it probably fed on larger sharks and cetaceans. That author also mentioned the long-snouted dolphin Schizodelphis sp. from this locality, which would have fed on bony fishes.
Tooth types present (number of species in parenthesis; see also Table 2): Clutching (3); Crushing (female), Clutching (male) (2); Cutting (2); Cutting-Clutching (8, but see Table 2 and Appendix); Grinding (5); Tearing (7); Tearing tending towards cutting (1).
Meßkirch-Rengetsweiler (early Ottnangian, Grobsandzug). The lowest verified trophic level consists of foraminifera (including Elphidium de Montfort, 1808). The next level consists of different suspension-feeders: sponges (Cliona Grant, 1826), bryozoans, brachiopods (Terebratula Müller, 1776), bivalves (Anomiidae, Ostreidae, Pectinidae, Pholadidae), and balanids. The only identified herbivore was Metaxytherium sp. (Sirenia). Grazers were represented by gastropods (Trochoidea) and sea urchins (Cidaroida). Detritivores include gastropods (Cerithiidae), scaphopods, crustaceans (“Callianassa” sp.), and sea urchins (Irregularia). Other invertebrates include carnivorous gastropods (Conidae, Ficus sp.) and omnivorous crabs (Brachyura). Bony fishes (Sparidae) and elasmobranchs (Aetobatus arcuatus and cf. Myliobatis sp.) that fed on shelled molluscs were also present (the latter form may also have fed on crabs). Alopias exigua was more specialized for preying on cephalopods, and the same can also be said for Isistius triangulus, which probably also lived as an ectoparasite on larger fishes and marine mammals. Crustacean-feeding members of Dasyatidae were also present. As it can be seen in Table 2, other elasmobranchs were mostly piscivorous, with many also feeding on cephalopods and crustaceans. Piscivorous marine mammals (Odontoceti) were also present in this deposit. Sach (2016) named the following representatives of Odontoceti: Squalodontidae indet., cf. Squalodelphis sp., Odontoceti indet. Also, remnants of sea turtles (Chelonioidea) were found, which had different ways of life depending on species and genus. The top-predator in the ancient Rengetsweiler sea was Otodus (Megaselachus) sp. In addition, other top predators included Carcharodon hastalis, Galeocerdo aduncus, and Notorynchus primigenius. Many land mammal remains were found in Rengetsweiler; their carrion was probably also eaten by G. aduncus and N. primigenius.
Tooth types present (number of species in parenthesis; see also Table 2): Clutching (5); Crushing (female), Clutching (male) (2); Cutting (2); Cutting-Clutching (7); Grinding (2); Tearing (6); Tearing tending towards cutting (2).
Meßkirch-Walbertsweiler (early Ottnangian, Grobsandzug). The lowest recorded trophic level consisted of foraminifera and ostracods. Herbivores consisted of Sirenia, and suspension-feeders were represented by bryozoans and bivalves (Pectinidae and Ostreidae). Detritus-feeder included crustaceans (“Callianassa” sp. and ostracods). Remnants of echinoids were also reported, nothing more is known about their affinities, and the same applies to the fossil teeth of bony fishes. As seen in Table 3, the fossil record concerning non-elasmobranchs, especially invertebrates, is comparably low in comparison to other localities, which may be simply due to collection bias or taphonomic reasons. Planktivorous elasmobranchs were represented by Mobula sp. and Keasius parvus. Molluscivorous, durophagous elasmobranchs were represented by Aetobatus arcuatus and Rhinoptera studeri, as well as by Myliobatis sp. (which also preyed on crabs) and Rhinobatos sp. (which also preyed on crustaceans and small bony fishes). Other elasmobranchs were more specialized on cephalopods: Alopias exigua, Rolfodon bracheri, Iago angustidens, Iago sp., Paragaleus tenuis, and lsistius triangulus (the latter probably being also an ectoparasite of large fishes and marine mammals). Crustaceans were also hunted by Dasyatis cavernosa, D. probsti, D. rugosa, D. sp. and Raja sp. Several others fed on cephalopods, bony fishes, and crustaceans (Table 2). Since now, this deposit is the only one in the OMM of Germany and from which the shark species Megalolamna paradoxodon could be verified, a taxon already designated in 2016. Piscivorous marine mammals (Cetacea) were also present. Like in the previous locality, the top predator was Otodus (Megaselachus) sp., followed by Carcharodon hastalis, Notorynchus primigenius, and Galeocerdo aduncus, the latter being potentially a carrion-feeder as well, possibly including remnants of terrestrial mammals in its diet.
Tooth types present (number of species in parenthesis; see also Table 2): Clutching (13); Crushing (2); Crushing (female), Clutching (male) (4); Cutting (3); Cutting-Clutching (17); Grinding (3); Tearing (8); Tearing and grasping on anterior teeth, cutting on lateral ones (1); Tearing tending towards cutting (2).
Ursendorf (early Ottnangian, Grobsandzug). The lowest trophic level recorded for Ursendorf is occupied by foraminifera. As it can be seen in Table 3, a remarkable amount on non-elasmobranch-taxa, especially invertebrates, could be verified for this deposit. The following suspension feeders are known from Ursendorf: sponges (Cliona), solitary corals (Ballanophyllia Wood, 1844), brachiopods (Terebratula), bryozoans, balanids, crinoideans (Antedon de Fréminville, 1811), gastropods (Calyptraea) and bivalves (Anomiidae, Carditidae, Corbulidae, Glycymerididae, Limidae, Ostreidae, Pectinidae, and Pholadidae). The only strict herbivores were marine mammals (Sirenia). Detritivorous forms include crustaceans (“Callianassa” sp.), scaphopods, irregular sea urchins (Amphiope Agassiz, 1840, Fibularia Lamarck, 1816, Scutella Lamarck, 1816 and Spatangus Gray, 1825) and gastropods (Cypraeidae). Most species of Cypraeidae are herbivorous grazers, but some are carnivorous, specifically sponge eaters (Passamonti, 2015). In Figure 4 the Cypraeidae are thus mentioned both in the “Carnivore invertebrates” section and in the “Detritivores and Grazers” section, in both cases being marked by a question mark.
Grazers were represented by gastropods (Trochidae) and regular sea urchins (Cidaridae, Psammechinus L. Agassiz and Desor, 1846 and Stirechinus Desor, 1856). Carnivorous gastropods (Conidae, Epitoniidae, Fasciolariidae, Ficidae, Mitridae, and Naticidae) and crustaceans (crabs) were also present. The occurrence of members of the gastropod family Epitoniidae could also be an indication of the presence of sea anemones (Actiniaria), because these animals are their preferred prey (Kilias, 1997). Species of bony fishes (Sparidae) and elasmobranchs (Aetobatus arcuatus and Rhinoptera studeri) fed on the shelled invertebrate fauna. Alopias exigua fed on cephalopods and Raja sp. and Dasyatis rugosa on crustaceans. Taeniurops cavernosus fed on crustaceans and bony fishes. The following taxa fed next to bony fishes possibly also on small sharks, squid, and other invertebrates: Pachyscyllium dachiardii and Pachyscyllium distans. Most of the elasmobranchs were mainly piscivores, but some also featured cephalopods in their diet (Table 2). As other localities above, the top predator was Otodus (Megaselachus) sp., followed by Carcharodon hastalis, Notorynchus primigenius and Galeocerdo aduncus.
Tooth types present (number of species in parenthesis; see also Table 2): Clutching (3); Crushing (female), Clutching (male) (4); Cutting (2); Cutting-Clutching (7); Grinding (2); Tearing (8); Tearing tending towards cutting (2).
Baltringen (middle Ottnangian). The lowest trophic level recorded is represented by a distinctive Foraminifera fauna. Several suspension feeders are known from Baltringen: Balanids (Balanus Costa, 1778), bivalves (Arcidae, Cardiidae, Ostreidae, Mytilidae, Pectinidae, Pholadidae, and Veneridae) and gastropods (Turritella). The only detritus feeder that could be observed was the scaphopod Dentalium ? sp. Other invertebrates are not known from these sediments. There are no signs of ecological reasons for the missing of other invertebrates so they may be simply not preserved in the fossil record. Herbivores were represented by Sirenia (Metaxytherium sp.). Molluscivorous animals were bony fishes (Labridae, Sparidae) and elasmobranchs (Aetobatus arcuatus, Rhinoptera studeri, Myliobatis sp., the latter also likely fed on crabs). Cephalopod-eating specialists included: Alopias exigua, Paragaleus tenuis and, to some extent, lsistius triangulus (also reconstructed as a likely ectoparasite on large fishes or marine mammals). Other elasmobranchs featured bony fishes and sometimes cephalopods and/or crustaceans in their diets (Table 2). Piscivorous cetaceans as well as omnivorous turtles (Trionyx sp.) are also known from the ancient sea of Baltringen. Sach (2016) mentioned 14 different taxa of Cetacea for this locality. As for most of the investigated localities, the top predator was Otodus (Megaselachus) sp., followed by Carcharodon hastalis, Notorynchus primigenius and Galeocerdo aduncus. The latter two were also a likely carrion-feeder that used to feeding on drifting carcasses of terrestrial mammals.
Tooth types present (number of species in parenthesis; see also Table 2): Clutching (5); Crushing (1); Crushing (female), Clutching (male) (3); Cutting (2); Cutting-Clutching (12); Grinding (3), Tearing (9); Tearing tending towards cutting (2).
CONCLUSION
The composition of the elasmobranch fauna, and hence the trophic relationships they entail, is very similar across all six investigated localities. Taxa with fish on their diet dominated the elasmobranch fauna, which could be a sign of a more diverse bony fish fauna in the ancient OMM sea that has not been preserved in the fossil record. Teeth of bony fishes are also not commonly found. That said, there are no recent research efforts focusing on the OMM Osteichthyes (the latest being Probst, 1882) despite the abundant fossil teeth.
In all localities, the apex predator was Otodus (Megaselachus) sp. The presence of this large shark was accompanied by the presence of marine mammals (Sirenia and/or Cetacea). These mammals probably were the preferred prey for those large sharks (Morgan, 1994; Purdy, 1996; Godfrey and Altman, 2005; Collareta et al., 2017a). Another aspect of note is the presence of the deep-water shark genera Echinorhinus, lsistius, Mitsukurina, and/or Squaliolus in the deposits studied herein, especially in Meßkirch-Walberstweiler. The OMM deposits mentioned in this paper were shallow water, featuring no sedimentological evidence of deep-water habitats (furthermore, all the remaining components of the palaeofauna were inhabitants of the neritic realm). The sharks may have occasionally frequented shallower waters, recalling what is known for the recent Echinorhinus cookei and Mitsukurina owstoni.
All in all, the trophic data depict a fully marine, shallow-water ecosystem with a soft-bottom and partly covered with sea grass, like recent warm oceans such as the Mediterranean Sea. Further revision of the taxonomic affinities of other faunal elements that comprise the OMM assemblages will certainly improve the present reconstruction of this palaeoenvironment.
ACKNOWLEDGEMENTS
We thank the two anonymous reviewers for their critical examination of our manuscript.
REFERENCES
Agassiz, L. 1833-1843. Recherches sur les poissons fossiles (5 volumes). Imprimerie de Petitpierre. Neuchâtel.
https://doi.org/10.5962/bhl.title.4275
Agassiz L., 1840. Catalogus systematicus Ectyporum Echinodermatum fossilium Musei Neocomiensis, secundum ordinem zoologicum dispositus; adjectis synonymis recentioribus, nec non stratis et locis in quibus reperiuntur. Sequuntur characters diagnostici generum novorum vel minus cognitorum. Petitpierre, Neuchâtel.
https://doi.org/10.5962/bhl.title.8820
Agassiz, L. 1858. Prof. Agassiz described a new species of Skate from the Sandwich Islands, for which he proposed to constitute a new genus, under the name of Goniobatis. Proceedings of the Boston Society of Natural History, 6:385.
Agassiz, L. and Desor, P. J. E. 1846. Catalogue raisonné des familles, des genres, et des espèces de la classe des échinodermes. Annales des Sciences Naturelles, Troisième Série, Zoologie, 6(3):305-374.
Aguilera, O. and de Aguilera, D.R. 2014. Giant-toothed White Sharks and Wide-toothed Mako (Lamnidae) from the Venezuela Neogene: Their Role in the Caribbean, Shallow-water Fish Assemblage. Caribbean Journal of Science, 40(3):368-382.
Alberti, M. and Reich, S. 2018. A palaeoecological review of the lower Gatun Formation (Miocene) of Panama with special emphasis on trophic relationships. Palaeobiodiversity and Palaeoenvironments, 98:571-591.
https://doi.org/10.1007/s12549-018-0326-3
Ameghino, F. 1901. L'âge des formations sédimentaires de Patagonie. Anales de la Sociedad Científica Argentina, 51:20-39, 65-91.
Anderson, B.M., Hendy, A., Johnson, E.H., and Allmon, W.D. 2017. Paleoecology and paleoenvironmental implications of turritellinegastropod-dominated assemblages from the Gatun Formation (Upper Miocene) of Panama. Palaeogeography, Palaeoclimatology, Palaeoecology, 470:132-146.
https://doi.org/10.1016/j.palaeo.2017.01.026
Andrianavalona, T.H., Ramihangihajason, T.N., Rasoamiaramanana, A., Ward, D.J., Ali, J.R., and Samonds, K.E. 2015. Miocene shark and batoid fauna from Nosy Makamby (Mahajanga Basin, northwestern Madagascar). PLoS ONE, 10(6):e0129444.
https://doi.org/10.1371/journal.pone.0129444
Antunes, M.T. and Jonet, S. 1970. Requins de l'Helvétien supérieur et du Tortonien de Lisbonne. Revista Da Faculdade de Ciênclas de Lisboa, 16(1):119-280.
Applegate, S.P. 1974. A revision of the higher taxa of Orectoloboids. Journal of the Marine Biological Association of India, 14(2):743-751.
Applegate, S.P. and Espinosa-Arrubarrena, L. 1996. The fossil history of Carcharodon and its possible ancestor, Cretolamna A study in tooth identification, p. 19-36. In Klimley, A. and Ainley, D. (eds.), Great White Sharks: the Biology of Carcharodon carcharias. Academic Press, San Diego.
https://doi.org/10.1016/B978-012415031-7/50005-7
Arias-Gonzalez, J., Delesalle, B., Salvat, B., and Galzin, R. 1997. Trophic functioning of the Tiahura reef sector, Moorea Island, French Polynesia. Coral Reef, 16:231-246.
https://doi.org/10.1007/s003380050079
Ayres, W.O. 1855. Shark of a new generic type: Notorynchus maculatus. Proceedings of the California Academy of Sciences, (Series 1), 1:72-73.
Baier, J. 2008. Ein Beitrag zur Erminger Turritellenplatte (Mittlere Schwäbische Alb, SW-Deutschland). Jahresbericht des oberrheinischen geologischen Vereins, N.F., 90: 9-17.
https://doi.org/10.1127/jmogv/90/2008/9
Baier, J., Schmitt, K.-H., and Mick, R. 2004. Notizen zur untermiozänen Hai-und Rochenfauna der Erminger Turritellenplatte (Mittlere Schwäbische Alb, SW-Deutschland). Jahresbericht des oberrheinischen geologischen Vereins, N.F., 86:361-371.
https://doi.org/10.1127/jmogv/86/2004/361
Bănaru, D., Mellon-Duval, C., Roos, D., Bigot, J-L., Souplet, A., Jadaud, A., Beaubrun, P., and Fromentin, J-M. 2013. Trophic structure in the Gulf of Lions marine ecosystem (north-western Mediterranean Sea) and fishing impacts. Journal of Marine Systems, 111-112:45-68.
https://doi.org/10.1016/j.jmarsys.2012.09.010
Barbosa du Bocage, J.V. and de Brito Capello, F. 1864. Sur quelques espèces inédites de Squalidae de la tribu Acanthiana Gray, qui fréquentent les côtes du Portugal. Proceedings of the Zoological Society of London, 1864:260-263.
Barthelt, D., Fejfar, O., Pfeil, F.H., and Unger, E. 1991. Notizen zu einem Profil der Selachier-Fundstelle Walbertsweiler im Bereich der miozänen Oberen Meeresmolasse Süddeutschlands. Münchner Geowissenschaftliche Abhandlungen, Reihe A, 19:195-208.
Bass, A.J., D'Aubrey, J.D., and Kistinasamy, N. 1975. Sharks of the east coast of Southern Africa. IV. The families Odontaspididae, Scapanorhynchidae, Isuridae, Cetorhinidae, Alopiidae, Orectolobidae and Rhiniodontidae. Investigational Report Oceanographic Research Institute, 39:1-102.
Bazzi, M., Campione, N.E., Kear, B.P., Pimiento, C., and Ahlberg, P.E. 2021. Feeding ecology has shaped the evolution of modern sharks. Current Biology, 31(23):5138-5148.
https://doi.org/10.2139/ssrn.3770097
Berg, L.S. 1937. A classification of fish-like vertebrates. Izvestiya Akademii Nauk SSSR, Seriya Biologicheskaya, 4:1277-1280.
Berg, L.S. 1940. Sistema ryboobraznykh i ryb, nyne zhivushchikh i iskopaemykh. [Classification of fishes, both recent and fossil]. Trudy Zoologicheskogo Instituta. Akademiia Nauk SSSR, 5(2):87-517.
Berg, L.S. 1958. System der Rezenten und Fossilen Fischartigen und Fische. In series: Borriss, H. and Gersch, M. (eds.), Hochschulbücher für Biologie 4. VEB Deutscher Verlag der Wissenschaften, Berlin, Germany.
Bianucci, G., Bisconti, M., Landini, W., Storai, T., Zuffa, M., Giuliani, S., and Mojetta, A. 2000.
Trophic interaction between white shark, Carcharodon carcharias, and cetaceans: a comparison between Pliocene and recent data from central Mediterranean Sea. Proceedings of the 4th European Elasmobranch Association Meeting, Livorno, Italy, 33-48.
Bigelow, H.B. and Schroeder, W.C. 1948. Sharks in Fishes of the western North Atlantic. Memoir of the Sears Foundation for Marine Research, 1 (part 1), 59-579.
Blainville, H.M.D., 1816. Prodrome d'une nouvelle distribution systématique du règne animal. Bulletin des Sciences, par la Société Philomatique de Paris, 8:113-124.
Bleeker, P. 1852. Bijdrage tot de kennis der Plagiostomen van den Indischen Archipel. Verhandelingen van het Bataviaasch Genootschap van Kunsten en Wettenschappen, 24:1-92.
Bleeker, P. 1859. Over eenige vischsoorten van de Kaap de Goede Hoop. Natuurkundig Tijdschrift voor Nederlandsch Indië, 21:49-80.
Bloch, M.E. and Schneider, J.G. 1801. M.E. Blochii Systema Ichthyologiae iconibus ex illustratum. Post obitum auctoris opus inchoatum absolvit, correxit, interpolavit. J.G. Schneider, Saxo.
https://doi.org/10.5962/bhl.title.5750
Bonaparte, C.L. 1835. Prodromus systematis ichthyologiae. Nuovi Annali delle Science Naturali Bologna, 4(2):181-196, 272-277.
Bonaparte, C.L. 1838. Selachorum tabula analytica. Nuovi Annali della Science Naturali Bologna, 1(2):195-214.
Bonnaterre 1788. Ichthyologie. Tableau encyclopédique et méthodique des trois règnes de la nature. Packoucke, Paris.
https://doi.org/10.5962/bhl.title.11660
Bor, T., Reinecke, T., and Verschueren, S. 2012. Miocene Chondrichthyes from Winterswijk-Miste, the Netherlands. Palaeontos, 21:1-136.
Bornatowski, H., Braga, R.R., Abilhoa, V., and Corrêa, M.F.M. 2014. Feeding ecology and trophic comparisons of six shark species in a coastal ecosystem off southern Brazil. Journal of Fish Biology, 85:246-263.
https://doi.org/10.1111/jfb.12417
Bornatowski, H., Angelini, R., Coll, M., Barreto, R.R.P., and Amorim, A.F. 2018. Ecological role and historical trends of large pelagic predators in a subtropical marine ecosystem of the South Atlantic. Reviews in Fish Biology and Fisheries, 28(1):241-259.
https://doi.org/10.1007/s11160-017-9492-z
Budker, P. 1935. Description d'un genre nouveau de la famille des Carcharhinidés. Bulletin du Muséum National d'Histoire Naturelle, (Série 2), 7(2):107-112.
Cadenat, J. 1963. Notes d'ichtyologie ouest-africaine. XXXIX. Notes sur les requins de la famille des Carchariidae et formes apparentées de l'Ouest-africain (Avec la description d'une espèce nouvelle: Pseudocarcharias pelagicus, classée dans un sous-genre nouveau). Bulletin de l'Institut Français d'Afrique Noire(A), 25(2):526-537.
Cappetta, H. 1970. Les sélaciens du Miocène de la région de Montpellier. Palaeovertebrata 3 (ext): 1-139.
https://doi.org/10.18563/pv.3.ext.1-139
Cappetta, H. 1973. Les Sélacien du Burdigalien de lespignan (Herault). Géobios, 6(3):211-223.
https://doi.org/10.1016/s0016-6995(73)80016-6
Cappetta, H. 1980. Modification du statut générique de quelques espèces de sélaciens crétacés et tertiaires. Palaeovertebrata, 10(1):29-42.
Cappetta, H. 1986. Types dentaires adaptatifs chez les Sélaciens actuels et post-paléozoïques. Palaeovertebrata, 16(2):57-76.
Cappetta, H. 1987. Chondrichthyes II. Mesozoic and Cenozoic Elasmobranchii. In Schultz, H.P., (ed.), Handbook of Paleoichthyology. 3B. Gustav Fischer Verlag, Stuttgart.
Cappetta, H. 2012. Chondrichtyes-Mesozoic and Cenozoic Elasmobranchii: Teeth. In Schultze, H.P (ed.) Handbook of Paleoichthyology, 3E, Dr. Friedrich Pfeil Verlag, München.
Cappetta, H. and Ward, D.J. 1977. A new Eocene shark from the London Clay of Essex. Palaeontology, 20(1):195-202.
Cappetta, H., Morrison, K., and Adnet, S. 2021. A shark fauna from the Campanian of Hornby Island, British Columbia, Canada: an insight into the diversity of Cretaceous deep-water assemblages. Historical Biology: An International Journal of Paleobiology, 33(8):1121-1182. https://doi.org/10.1080/08912963.2019.1681421
Chun-xia, G., Si-quan, T., Xiao-jie, D., Feng, W., and You-wei, X. 2013. Preliminary analysis of the biology of the crocodile shark, Pseudocarcharias kamoharai the tropical Eastern-central Atlantic Ocean. Journal of Shanghai Ocean University, 2:289-294.
Cliff, G. and Dudley, S.F.J. 1991a. Sharks caught in the Protective Gill Nets off Natal, South Africa. 4. The Bull shark Carcharhinus leucas Valeciennes. South African Journal of Marine Science, 10 (1):253-270.
https://doi.org/10.2989/02577619109504636
Cliff, G. and Dudley, S.F.J. 1991b. Sharks caught in the protective gill nets off Natal, South Africa. 5. The Java shark Carcharhinus amboinensis (Müller and Henle). South African Journal of Marine Science, 11(1):443-453.
https://doi.org/10.2989/025776191784287817
Collareta, A., Lambert, O., Landini, W., Di Celma, C., Malinverno, E., Varas-Malca, R., Urbina, M., and Bianucci, G. 2017a. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology, 469:84-91.
https://doi.org/10.1016/j.palaeo.2017.01.001
Collareta, A., Landini, W., Chacaltana, C., Valdivia, W., Altamirano-Sierra, A.J., Urbina-Schmitt, M., and Bianucci, G. 2017b. A well preserved skeleton of the fossil shark Cosmopolitodus hastalis from the late Miocene of Peru, featuring fish remains as fossilized stomach contents. Rivista Italiana di Paleontologia e Stratigrafia, 123(1):11-22.
https://doi.org/10.13130/2039-4942/8005
Collareta, A., Lambert, O., Marx, F.G., de Muizon, C., Varas-Malca, R., Landini, W., Bosio, G., Malinverno, E., Gariboldi, K., Gioncada, A., Urbina, M., and Bianucci, G. 2021. Vertebrate Palaeoecology of the Pisco Formation (Miocene, Peru): Glimpses into the Ancient Humboldt Current Ecosystem. Journal of Marine Science and Engineering, 9(11):1188.
https://doi.org/10.3390/jmse9111188
Collareta, A., Merella, M., Casati, S., and Di Cencio, A. 2021. First fossils of the extant blacktip shark Carcharhinus limbatus from Europe and the Mediterranean Basin. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 301(1):109-118.
https://doi.org/10.1127/njgpa/2021/1002
Compagno, L.J.V. 1973. Interrelationships of living elasmobranchs. Zoological Journal of the Linnean Society, 53(Supplement 1):15-61.
Compagno, L.J.V. 1977. Phyletic relationships of living sharks and rays. American Zoologist, 17(2):303-322.
https://doi.org/10.1093/icb/17.2.303
Compagno, L.J.V. 1984. FAO Species Catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1 - Hexanchiformes to Lamniformes. FAO Fisheries Synopsis, 125(4/1):1-249.
Compagno, L.J.V. and Springer, S. 1971. Iago, a new genus of carcharhinid sharks, with a redescription of I. omanensis. Fishery Bulletin, 69(3):615-626.
Compagno, L.J.V., Dando, M., and Fowler, S. 2005. Sharks of the world. Princeton University Press, Oxford and Princeton.
Cope, A.D. 1867. An addition to the vertebrate fauna of the Miocene period, with a synopsis of the extinct Cetacea of the United States. Proceedings of the Academy of Natural Sciences of Philadelphia, 19:138-156.
Cortés, E. 1999. Standardized diet compositions and trophic levels of sharks. ICES Journal of Marine Science, 56(5):707-717.
https://doi.org/10.1006/jmsc.1999.0489
Cuvier, G.L.C.F.D. 1816. Le Règne Animal distribué d'après son organisation pour servir de base à l'histoire naturelle des animaux et d'introduction à l'anatomie comparée. Les reptiles, les poissons, les mollusques et les annélides. Deterville, Paris.
https://doi.org/10.5962/bhl.title.39612
da Silva Rodrigues-Filho, L.F., da Costa Nogueira, P., Sodré, D., da Silva Leal, J.R., Nunes, J.L.S., Rincon, G., Lessa, R.P.T., Sampaio, I., Vallinoto, M., Ready, J.S., and Sales, J.B.L. 2023. Evolutionary History and Taxonomic Reclassification of the Critically Endangered Daggernose Shark, a Species Endemic to the Western Atlantic. Journal of Zoological Systematics and Evolutionary Research, 2023:4798805.
https://doi.org/10.1155/2023/4798805
Daimeries, A. 1889. Notes ichthyologiques V. Annales de la Societe royale malacologique de Belgique, Bulletin des Seances, 24:39-44.
De Buen, F. 1926. Catalogo ictiologico del Mediterraneo Español y de Marruecos, recopilando lo publicado sobrepeces de las costas mediterraneas y proximas del Atlantico (Mar de España). Resultados de las ampafias Realizadas por Acuerdos Internacionales. Instituto Español de Oceanografia, 2:1-221.
De Figueiredo Petean, F. and de Carvalho, M.R. 2018. Comparative morphology and systematics of the cookiecutter sharks, genus Isistius Gill (1864) (Chondrichthyes: Squaliformes: Dalatiidae). PLoS ONE, 13(8):e0201913.
https://doi.org/10.1371/journal.pone.0201913
de Fréminville, M. 1811. Mémoire sur un nouveau genre de Zoophytes de l’ordre des Radiaires. Nouveau Bulletin des Sciences, par la Société Philomathique de Paris, 2:349-350.
de Lamarck, J.B.M. 1799. Prodrome d'une nouvelle classification des coquilles, comprenant une rédaction appropriée des caractères géneriques, et l'établissement d'un grand nombre de genres nouveaux. Mémoires de la Société d'Histoire Naturelle de Paris, 1:63-91.
de Lamarck, J.B. M. 1816. Histoire naturelle des animaux sans vertèbres, Tome troisième. Deterville/Verdière, Paris.
de Montfort, D. 1808. Conchyliologie systématique et classification méthodique des coquilles. Vol. 1. Paris, Schoell.
https://doi.org/10.5962/bhl.title.10571
Desor, E. 1856. Synopsis des échinides fossiles. Troisième livraison. Reinwald, Paris.
https://doi.org/10.5962/bhl.title.10163
Di Celma, C., Malinverno, E., Cantalamessa, G., Gioncada, A., Bosio, G., Villa, I.M., Gariboldi, K., Rustichelli, A., Pierantoni, P.P., Landini, W., Tinelli, C., and Collareta, A. 2016. Stratigraphic framework of the late Miocene Pisco Formation at Cerro Los Quesos (Ica Desert, Peru). Journal of Maps, 12(5):1020-1028.
https://doi.org/10.1080/17445647.2015.1115783
Dicken, M.L., Hussey, N.E., Christiansen, H.M., Smale, M.J., Nkabi, N., Cliff, G., and Wintner, S.P. 2017. Diet and trophic ecology of the tiger shark (Galeocerdo cuvier) from South African waters. PLoS ONE, 12(6):e0177897.
https://doi.org/10.1371/journal.pone.0177897
Dumeril, A.H.A. 1806. Zoologie analytique, ou méthode naturelle de classification des animaux. Paris: 1-344.
https://doi.org/10.5962/bhl.title.44835
Ebert, D.A. 1989. Life history of the sevengill shark, Notorynchus cepedianus Peron, in two northern California bays. California Fish and Game, 75(2):102-112.
Ebert, D.A. 2003. Sharks, Rays and Chimaeras of California. University of California Press, Berkeley and Los Angeles, California.
Ebert, D.A. and Bizzarro, J.J. 2007. Standardized diet compositions and trophic levels of skates (Chondrichthyes: Rajiformes: Rajoidei). Environmental Biology of Fishes, 80(2-3):221-237.
https://doi.org/10.1007/978-1-4020-9703-4_8
Ebert, D.A. and Compagno, L.J.V. 2009. Chlamydoselachus africana, a new species of frilled shark from southern Africa (Chondrichthyes, Hexanchiformes, Chlamydoselachidae). Zootaxa, 2173(1):1-18.
https://doi.org/10.11646/zootaxa.2173.1.1
Ebert, D.A. and Fowler, S. 2014. An Illustrated Pocket Guide to the Sharks of the World. Wild Nature Press, West Sussex, England.
Ebert, D.A. and Wilms, H.A. 2013. Pristiophorus lanae sp. nov., a new sawshark species from the Western North Pacific, with comments on the genus Pristiophorus Müller and Henle, 1837 (Chondrichthyes: Pristiophoridae). Zootaxa, 3752(1):86-100.
https://doi.org/10.11646/zootaxa.3752.1.7
Ebert, D.A., Dando, M., and Fowler, S. 2021. Sharks of the World. A Complete Guide. Princeton University Press, Princeton, New Jersey.
Estupiñán-Montaño, C., Estupiñán-Ortiz, J.F., Cedeño-Figueroa, L.G., Galván-Magaña, F., and Polo-Silva, C.J. 2017. Diet of the bull shark, Carcharhinus leucas, and the tiger shark, Galeocerdo cuvier, in the eastern Pacifc Ocean. Turkish Journal of Zoology, 41:1111-1117.
https://doi.org/10.3906/zoo-1610-31
Estupiñán-Montaño, C. and Galván-Magaña, F. 2021. First Insight into the Biological Aspects of the Crocodile Shark Pseudocarcharias kamoharai in the Eastern Pacific Ocean. Thalassas, 37:229-233.
https://doi.org/10.1007/s41208-020-00251-7
Euphrasen, B.A. 1790. Raja (Narinari). Kongliga Vetenskaps Akademiens nya Handlingar, Stockholm, 11:217-219.
Feichtinger, I. and Pollerspöck, J. 2021. Haie im Alpenvorland - Fossile Zeugen eines verschwundenen Paradieses. Verlag Anton Pustet, Salzburg.
Feichtinger, I., Bracher, H., Unger, E., Lüdi, B., and Pollerspöck, J. 2022. Haie und Rochen der Molasse, accessed 16 November 2022.
http://www.molasse-haie-rochen.de.
Fergusson, I.K., Graham, K.J., and Compagno, L.J.V. 2008: Distribution, abundance and biology of the smalltooth sandtiger shark Odontaspis ferox (Risso, 1810) (Lamniformes: Odontaspididae). Environmental Biology of Fishes, 81:207-228.
https://doi.org/10.1007/s10641-007-9193-x
Fourmanoir, P. 1979. Requins de Nouvelle-Calédonie - Nouvelles espèces de requins trouvées en Nouvelle-Calédonie. Nature calédonienne, 16:11-13.
Fowler, H.W. 1933. Descriptions of new fishes obtained 1907 to 1910, chiefly in the Philippine Islands and adjacent seas. Proceedings of the Academy of Natural Sciences of Philadelphia, 85:233-367.
Froese, R. and Pauly, D. (eds.). 2019. FishBase, accessed December 2019.
https://www.fishbase.se/search.php.
Garrick, J.A.F. and Springer, S. 1964. Isistius plutodus, a new squaloid shark from the Gulf of Mexico. Copeia, 1964 (4):678-682.
https://doi.org/10.2307/1441443
Garman, S. 1884. An extraordinary shark. Bulletin of the Essex Institute, 16:47-55.
Garman, S. 1906. New Plagiostoma. Bulletin of the Museum of Comparative Zoology at Harvard College, 46(11):203-208.
Garman, S. 1913. The Plagiostomia (Sharks, Skates and Rays). Memoirs of the Museum of Comparative Zoology at Harvard College, 36:1-528.
https://doi.org/10.5962/bhl.title.43732
Geyer, O.F. and Gwinner, M.P. 1991. Geologie von Baden-Württemberg. Schweizerbart´sche Verlagsbuchhandlung, Stuttgart.
Gibbes, R.W. 1849. Monograph of the fossil Squalidae of the United States. Journal of the Academy of Natural Sciences of Philadelphia, New Series, 1, part III:191-206.
Gill, T.N. 1862. Note on some genera of fishes of western North America. Proceedings of the Academy of Natural Sciences, Philadelphia, 14:7-9, 329-332.
Gill, T. 1865. Synopsis of the eastern American sharks. Proceedings of the Academy of Natural Sciences of Philadelphia, 16(5):258-265.
Gill, T.N. 1872. Arrangement of the families of fishes, or Classes Pisces, Marsupiobranchii, and Leptocardii. Smithsonian Miscellaneous Collections, 11(247):1-49.
https://doi.org/10.5962/bhl.title.18974
Gill, T.N. 1893. Families and subfamilies of fishes. Memoirs of the National Academy of Sciences, 6:125-138.
https://doi.org/10.5962/bhl.part.6303
Girard, C.F. 1854. Characteristics of some cartilaginous fishes of the Pacific coast of North America. Proceedings of the Academy of Natural Sciences of Philadelphia, 7:195-201.
Glikman, L.S. 1964. Sharks of Paleogene and their stratigraphic significance. Nauka Press, Moscow. (in Russian)
Godfrey, S.J. and Altman, J. 2005. A Miocene cetacean vertebra showing a partially healed compression fracture, the result of convulsions or failed predation by the Giant White Shark, Carcharodon megalodon. Jeffersoniana, 16:1-12.
Goodman, K., Niella, Y., Bliss-Henaghan, T., Harcourt, R., Smoothey, A.F., and Peddemors, V.M. 2022. Ontogenetic changes in the tooth morphology of bull sharks (Carcharhinus leucas). Journal of Fish Biology, 101(4):1033-1046.
https://doi.org/10.1111/jfb.15170
Goodrich, E.S. (1909). Vertebrata Craniata (First fascicle: Cyclostomes and Fishes), p. 1-518. In R. Lankester (ed.), A Treatise on Zoology. Adam and Charles Black, London.
Grant, R.E. 1826. Notice of a New Zoophyte (Cliona celata, Gr.) from the Firth of Forth. Edinburgh New Philosophical Journal, 1:78-81
Gray, J.E. 1825. An attempt to divide the Echinida, or Sea Eggs, into natural families. Annals of Philosophy, new series, 10:423-431.
Gray, J.E. 1851. List of the specimens of fish in the collection of the British Museum. Part I. Chondropterygii. British Museum (Natural History), London.
https://doi.org/10.5962/bhl.title.20819
Griffith, E. and Smith, C. 1834 in Cuvier, G.L.C.F.D., Griffith, E. and Smith, C. 1834. The class Pisces, arranged by the Baron Cuvier, with supplementary additions, by Edward Griffith, F.R.S. & c. and Lieut.-Col. Charles Hamilton Smith, F.R., L.S.S. &c. &c. The animal kingdom, Vol. 10. Whittaker and Co., London.
https://doi.org/10.5962/bhl.title.1979
Günther, A. 1870. Catalogue of the fishes in the British Museum. British Museum (Natural History), Vol. 8. Order of Trustees, London.
Guitart-Manday, D.J. 1966. Nuevo nombre para una especie de Tiburón del género Isurus (Elasmobranchii: Isuridae) de Aguas Cubanas. Poeyana, Ser. A, 15:1-9.
Gunnerus, J.E. 1765. Brugden (Squalus maximus), Beskrvenen ved J. E. Gunnerus. Det Trondhiemske Selskabs Skrifter, 3:33-49.
Hasse, C. 1879. Das natürliche System der Elasmobranchier auf Grundlage des Baues und der Entwicklung ihrer Wirbelsäule. Eine morphologische und paläontologische Studie. I. Allgemeiner Theil. Gustav Fischer Verlag, Jena.
https://doi.org/10.5962/bhl.title.8431
Heckeberg, N., Pippèrr, M., Läuchli, B., Heimann, F.U.M., and Reichenbacher, B. 2010. The Upper Marine Molasse (Burdigalian, Ottnangian) in Southwest Germany- facies interpretation and a new lithostratigraphic terminology. Zeitschrift der Deutschen Gesellschaft für Geowissenschaften, 163(3):285-302.
https://doi.org/10.1127/1860-1804/2010/0161-0285
Heimann, F., Schmid, D.U., Pippèrr, M., and Reichenbacher, B. 2009. Re-interpreting the Baltringer Horizont as a subtidal channel facies: implications for a new understanding of the Upper Marine Molasse "Cycles" (Early Miocene). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 254(1-2):135-149.
https://doi.org/10.1127/0077-7749/2009/0007
Heithaus, M.R. and Vaudo, J.J. 2012. Predator-Prey Interactions, p. 505-546. In Carrier, J.C., Musick, J.A., and Heithaus, M.R. (eds.), Biology of sharks and their relatives. CRC Press, Boca Raton, Florida.
Heithaus, M.R., Wirsing, A.J., and Dill, L.M. 2012. The ecological importance of intact top-predator populations: a synthesis of 15 years of research in a seagrass ecosystem. Marine and Freshwater Research, 63(11):1039-1050.
https://doi.org/10.1071/mf12024
Herman, J., Hovestadt-Euler, M., and Hovestadt, D.C. 1988. Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living superaspecific taxa of Chondrichthyan fishes. Part A: Selachii. No. 2a: Carcharhiniformes - Family: Triakidae. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie, 58:99-126.
Herman, J., Hovestadt-Euler, M., and Hovestadt, D.C. 1991. Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living superspecific taxa of Chondrichthyan fishes. Part A: Selachii. No. 2c: Order: Carcharhiniformes Families: Proscylliidae, Hemigaleidae, Pseudotriakidae, Leptochariidae and Carcharhinidae. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie, 61:73-120.
Herman, J., Hovestadt-Euler, M., and Hovestadt, D.C. 2004. Contributions to the odontological study of living Chondrichthyes. 1. The genus Alopias RAFINESQUE, 1810. Bulletin de l´Institut Royal des Sciences Naturelles de Belgique, Biologie, 74:5-32.
Höltke, O. 2009. Die Molluskenfauna der Oberen Meeresmolasse (Untermiozän) von Ermingen und Ursendorf (SW-Deutschland). Palaeodiversity, 2:67-95.
Höltke, O., Unger, E., Pollerspöck, J., and Rasser, M.W. 2020. The Elasmobranch Fauna from the Upper Marine Molasse (Lower Miocene, Burdigalian) of Ursendorf (District Sigmaringen, Baden-Württemberg, SW-Germany). Palaeontos, 33:1-56.
Höltke, O., Maxwell, E.E., Pollerspöck, J., and Rasser, M.W. 2022a. The shark and ray fauna of the Upper Marine Molasse (Lower Miocene) of Rengetsweiler (Baden- Württemberg, SW Germany). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 303(1):89-122.
https://doi.org/10.1127/njgpa/2022/1038
Höltke, O., Maxwell, E.E., Pollerspöck, J., and Rasser, M.W. 2022b. The shark and ray teeth of the Lower Miocene (Upper Marine Molasse) from Äpfingen, Baden-Württemberg, Southern Germany. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 305(3):323-342.
https://doi.org/10.1127/njgpa/2022/1092
Hovestadt, D.C. 2020. Taxonomic adjustments of the Oligocene and Miocene Odontaspididae and Carchariidae based on extant specimens. Cainozoic Research, 20(2):229-255.
Jacobsen, I.P. and Bennett, M.B. 2013. A Comparative Analysis of Feeding and Trophic Level Ecology in Stingrays (Rajiformes; Myliobatoidei) and Electric Rays (Rajiformes: Torpedinoidei). PLoS ONE, 8(8):e71348.
https://doi.org/10.1371/journal.pone.0071348
Jaekel, O. 1890. Über die systematische Stellung und über fossile Reste der Gattung Pristiophorus. Zeitschrift der Deutschen Geologischen Gesellschaft, 42:86-120.
https://doi.org/10.1017/s0016756800176769
Joleaud, L. 1912. Géologie et paléontologie de la Plaine du Comtat et de ses abords. Description des terrains néogènes. Montpellier: Imprimerie Montane, Sicardi et Valentin, 2:255-285.
Jordan, D.S. 1888. A manual of the vertebrate animals of the northern United States, including the district north and east of the Ozark mountains, south of the Laurentian hills, north of the southern boundary of Virginia, and east of the Missouri river, inclusive of marine species. A.C. McClurg and Co., Chicago.
https://doi.org/10.5962/bhl.title.20696
Jordan, D.S. 1898. Description of a species of fish (Mitsukurina owstoni) from Japan, the type of a distinct family of Lamnoid sharks. Proceedings of the California Academy of Sciences, (Series 3, Zoology), 1:199-201.
Jordan, D.S. and Evermann, B.W. 1896. The fishes of North and Middle America: a descriptive catalogue of the species of fish-like vertebrates found in the waters of North America, north of the Isthmus of Panama. Part I. Bulletin of the United States National Museum, 47:1-1240.
https://doi.org/10.5962/bhl.title.46755
Kast, E.R., Griffiths, M.L., Kim, S.L., Rao, Z.C., Shimada, K., Becker, M.A., Maisch, H.M., Eagle, R.A., Clarke, C.A., Neumann, A.N., Karnes, M.E., Lüdecke, T., Leichliter, J.N., Martínez-García, A., Akhtar, A.A., Wang, X.T., Haug, G.H., and Sigman, D.M. 2022. Cenozoic megatooth sharks occupied extremely high trophic positions. Science Advances, 8(25):eabl6259
https://doi.org/10.1126/sciadv.abl6529
Kent, B.W. 1994. Fossil sharks of the Chesapeake Bay region. Egan Ress and Boyer, Inc., Columbia, Maryland.
Kilias, R. 1997. Lexikon Marine Muscheln und Schnecken. Eugen Ulmer Verlag, Stuttgart.
Klunzinger, C.B. 1871. Synopsis der Fische des Rothen Meeres II. Theil. Verhandlungen der Königlischen Zoologischen-Botanischen Gesellschaft in Wien, 21:441-688.
Kuhlemann, J. and Kempf, O. 2002. Post-Eocene evolution of the North Alpine Foreland Basin and its response to Alpine tectonics. Sedimentary Geology, 152(1-2):45-78.
https://doi.org/10.1016/s0037-0738(01)00285-8
Last, P.R., Séret, B., Stehmann, M.F.W., and Weigmann, S. 2016a. Skates Family Rajidae, p. 204-263. In Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., and Naylor, G.J.P., (eds.), Rays of the World. CSIRO Publishing, Ithaca and London.
Last, P.R., White, W.T. and Jones, C.M. 2016b. Cownose Rays Family Rhinopteridae, p. 732-740. In Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., and Naylor, G.J.P., (eds.), Rays of the World. CSIRO Publishing, Ithaca and London.
Last P.R., White, W.T., and Pogonoski, J.J. 2007. Descriptions of new dogfishes of the genus Squalus (Squaloidea: Squalidae). CSIRO Marine and Atmospheric Research, 014:1-136.
https://doi.org/10.4225/08/58615c42b11ce
Latham, J. 1794. An essay on the various species of Sawfish. Transactions of the Linnean Society of London, 2(25):273-282.
https://doi.org/10.1111/j.1096-3642.1794.tb00259.x
Lawley, R. 1876. Nuovi studi sopra ai pesci ed altri vertebrati fossili delle Colline Toscane. Tipografia dell Arte della Stampa, Firenze.
Leriche, M. 1908. Sur un appareil fanonculaire de Cetorhinus trouvé à l'état fossile dans le Pliocène d'Anvers. Comptes Rendus hebdomadaires des séances de l'Academie des Sciences, 146:875-878
Leriche, M. 1927. Les poissons de la Molasse suisse. Mémoires de la Société Paléontologique Suisse, 46:1-55.
Linck, H.F. 1790. Versuch einer Eintheilung der Fische nach den Zähnen. Magazin für das Neueste aus der Physik und Naturgeschichte, 6(3):28-38.
Lindeman, RL 1942: The trophic-dynamic aspect of ecology. Ecology, 23(4):399-418.
https://doi.org/10.2307/1930126
Linnaeus, C. 1758. Systema Naturae per regna tria naturae, regnum animale, secundum classes, ordines, genera, species, cum characteribus differentiis synonymis, locis. Ed. X., 1. L. Salvius, Stockholm.
Lowe, R.T. 1841. XII.–Description of some new species of Madeiran Fishes, with additional information relating to those already described, Annals and Magazine of Natural History, 7:42, 92-94.
https://doi.org/10.1080/03745484109442671
Lucifora, L.O., García, V.B., and Escalante, A.H. 2009. How can the feeding habits of the sand tiger shark influence the success of conservation programs? Animal Conservation, 12(4):291-301.
https://doi.org/10.1111/j.1469-1795.2009.00247.x
Maisey, J. 1994. Predator-prey relationships and trophic level reconstruction in a fossil fish Community. Environmental Biology of Fishes, 40:1-22.
https://doi.org/10.1007/bf00002179
Manganelli, G. and Spadini, V. 2019: Megascyliorhinus miocaenicus (Chondrichthyes, Galeomorphii) from the Zanclean (early Pliocene) of San Quirico d'Orcia, central Italy. Bollettino della Società Paleontologica Italiana, 58(2):165-170.
https://doi.org/10.4435/bspi.2019.12
Martin, J.E., Tacail, T., Adnet, S., Girard, C., and Balter, V. 2015. Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chemical Geology, 415:118-125.
https://doi.org/10.1016/j.chemgeo.2015.09.011
Matsubara, K. 1936. A new carcharoid shark found in Japan. Dobutsugaku Zasshi, 48(7):380-382.
McCormack, J., Griffiths, M.L., Kim, S.L., Shimada, K., Karnes, M., Maisch, H., Pederzani, S., Bourgon, N., Jaouen, K., Becker, M.A., Jöns, N., Sisma-Ventura, G., Straube, N., Pollerspöck, J., Hublin, J.-J., Eagle, R.A., and Tütken, T. 2022: Trophic position of Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nature Communications, 13:2980.
https://doi.org/10.1038/s41467-022-30528-9
Miller, K. 1875. Ueber die Tiefseefacies des oberschwäbischen Miocäns und die Bryozoen von Ursendorf. Jahreshefte des Vereins für vaterländische Naturkunde, 31: 82-84.
Mitchill, S.L. 1815. The fishes of New York described and arranged. Transactions of the Literary and Philosophical Society of New York, 1:355-492.
Morgan, G.S. 1994. Miocene and Pliocene marine mammal faunas from the Bone Valley Formation of central Florida. Proceedings of the San Diego Society of Natural History, 29:239-268.
Moss, S.A. 1977. Feeding Mechanisms in Sharks. American Zoologist, 17(2):355-364.
Motta, P.J., Hueter, R.E., Tricas, T.C., Summers, A.P., Huber, D.R., Lowry, D., Mara, K.R., Matott, M.P., Whitenack, L.B., and Wintzer, A.P. 2008. Functional Morphology of the Feeding Apparatus, Feeding Constraints, and Suction Performance in the Nurse Shark Ginglymostoma cirratum. Journal of Morphology, 269(9):1041-1055.
https://doi.org/10.1002/jmor.10626
Müller, J.P. 1836. Vergleichende Anatomie der Myxinoiden, der Cyclostomen mit durchbohrtem Gaumen. Erster Theil. Osteologie und Myologie. Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin, 1834:65-340.
Müller, J.P. and Henle, F.G.J. 1837. Gattungen der Haifische und Rochen nach einer von ihm mit Hrn. Henle unternommenen gemeinschaftlichen Arbeit über die Naturgeschichte der Knorpelfische. Berichte der Königlichen Preussischen Akademie der Wissenschaften zu Berlin, 1837:111-118.
Müller, J. and Henle, F.G.J. 1841. Systematische Beschreibung der Plagiostomen. Veit und Comp., Berlin.
https://doi.org/10.5962/bhl.title.6906
Müller, O.F. 1776. Zoologiae Danicae prodromus, seu animalium Daniae et Norvegiae indigenarum characteres, nomina, et synonyma imprimis popularium. Hallageriis, Havniae (Copenhagen).
https://doi.org/10.5962/bhl.title.13268
Myers, R.A., Baum, J.K., Shepherd, T.D., Powers, S.P., and Peterson, C.H. 2007. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science, 315(5820):1846-1850.
https://doi.org/10.1126/science.113865
Nebelsick, J.H., Rasser, M.W. and Bieg, U. 2009. The North Alpine Foreland Basin: Special Volume of the 2008 Molasse Meeting. Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen 254(1-2):1-4.
https://doi.org/10.1127/0077-7749/2009/0001
Nebelsick, J.H., Rasser, M., Höltke, O., Thompson, J.R., and Bieg, U. 2019. Turritelline mass accumulations from the Lower Miocene of southern Germany: Implications for tidal currents and nutrient transport within the North Alpine Foreland Basin. Lethaia, 53(2):280-293.
https://doi.org/10.1111/let.12356
Nelson, J.S. 2006. Fishes of the World. John Wiley and Sons, Inc., Hoboken, New Jersey.
Norman, J.R. 1939. Fishes. The John Murray Expedition 1933-34. Scientific Reports, John Murray Expedition, 7(1):1-116.
Passamonti, M. 2015. The family Cypraeidae (Gastropoda Cypraeoidea) an unex-pected case of neglected animals. Biodiversity Journal, 6(1):449-466.
Perez, V.J., Pimiento, C., Hendy, A., González-Barba, G., Hubbell, G., and Macfadden, B.J. 2017. Late Miocene chondrichthyans from Lago Bayano, Panama: Functional diversity, environment and biogeography. Journal of Paleontology, 91(3):512-547.
https://doi.org/10.1017/jpa.2017.5
Perez, V.J., Godfrey, S.J., Kent, B.W., Weems, R.E., and Nance, J.R. 2019. The transition between Carcharocles chubutensis and Carcharocles megalodon (Otodontidae, Chondrichthyes): lateral cusplet loss through time. Journal of Vertebrate Paleontology, 38(6):e1546732
https://doi.org/10.1080/02724634.2018.1546732
Perez, V.J., Godfrey, S.J., and Chapman, P.F. 2021. Rare evidence of shark-on-shark trophic interactions in the fossil record. Acta Palaeontologica Polonica, 66(4):847-856.
https://doi.org/10.4202/app.00911.2021
Péron, F. 1807. Voyage de Découvertes aux Terres Australes, exécuté par ordre de sa majesté l'Empereur et Roi, sur les Corvettes la Géographe, la Naturaliste et la Goulette le Casuarina, pendant les années 1800, 1801, 1803 et 1804. Paris. Voyage de Découvertes aux Terres Australes, 1:1-496.
https://doi.org/10.5962/bhl.title.44096
Péron, F. and Lesueur, C.A. 1822 in Lesueur, C.A. 1822. Description of a Squalus, of a very large size, which was taken on the coast of New Jersey. Journal of the Academy of Natural Sciences of Philadelphia, 2:343-352.
Pfeil, F.H. 1981. Eine nektonische Fischfauna aus dem unteroligozänen Schönecker Fischschiefer des Galon-Grabens in Oberbayern. Geologica Bavarica, 82:357-388.
Pfeil, F.H. 1983. Zahnmorphologische Untersuchungen an rezenten und fossilen Haien der Ordnungen Chlamydoselachiformes and Echinorhiniformes. Palaeo Ichthyologica, 1:1-315.
Pfeil, F.H. 1984. Neoselachian teeth collected from phosporite-bearing Greensand on Chatham Rise East of New Zealand. 1984. Geologisches Jahrbuch, Reihe D, 65:107-115.
Pfeil, F.H. 1991. Haie und Rochen aus Walbertsweiler, p. 198-208 In Barthelt, D., Fejfar, O., Pfeil, F.H., and Unger, E. (eds.), Notizen zu einem Profil der Selachier-Fundstelle Walbertsweiler im Bereich der miozänen Oberen Meeresmolasse Süddeutschlands. Münchner Geowissenschaftliche Abhandlungen, Reihe A, 19.
Philippi, R.A. 1846. Tornatella abbreviata, Otodus mitis, Otodus catticus und Myliobatis testae. Palaeontographica, 1:23-26.
Pietschmann, V. 1928. Neue Fischarten aus dem Pazifischen Ozean. Anzeiger der Akademie der Wissenschaften in Wien, 65(27):297-298.
Poey, F. 1875: Enumeratio piscium cubensium. Anales de la Sociedad Española de Historia Natural, 4: 75-161.
https://doi.org/10.5962/bhl.title.12630
Poey, F. 1876. Enumeratio piscium cubensium (Parte III). Anales de la Sociedad Española de Historia Natural, 5:373-404.
Pollerspöck, J. and Beaury, B. 2014. Eine Elasmobranchierfauna (Elasmobranchii, Neoselachii) aus der Oberen Meeresmolasse (Ottnangium, Unteres Miozän) des Heigelsberger Grabens bei Teisendorf, Oberbayern. Zitteliana, A54:23-37.
https://doi.org/10.5282/ubm/epub.22321
Pollerspöck, J. and Straube, N. 2019. An identification key to elasmobranch genera based on dental morphological characters Part A: Squalomorph sharks (Superorder Squalomorphii). Bulletin of Fish Biology, 18(1/2):77-105.
Pollerspöck, J. and Unger, E. 2022. "Beiträge zur Kenntniss der fossilen Fische aus der Molasse von Baltringen"- Revision zum 200. Geburtstag von Pfarrer Josef Probst. Teil Batoidei (Probst, 1877). Jahreshefte der Gesellschaft für Naturkunde in Württemberg, 178:149-204.
https://doi.org/10.26251/jhgfn.178.2022.149-204
Pollerspöck, J. and Güthner, T., and Straube, N. 2022. Re-evaluation of the Lower Miocene (Burdigalian, Ottnangian) elasmobranch fauna (Elasmobranchii, Neoselachii) from Upper Austria (Allerding, near Schärding, Austria) with comments on the paleogeographic distribution of the recorded squaliform sharks. Annalen des Naturhistorischen Museums in Wien, Series A, 122:87-163.
Powter, D.M., Gladstone, W., and Platell, M. 2010. The influence of sex and maturity on the diet, mouth morphology and dentition of the Port Jackson shark, Heterodontus portusjacksoni. Marine and Freshwater Research, 61(1):74-85.
https://doi.org/10.1071/MF09021
Probst, J. 1871. Fossile Meeres- und Brackwasser-Conchylien aus der Gegend von Biberach. Jahreshefte des Vereins für vaterländische Naturkunde, 27:111-118.
Probst, J. 1877. Beiträge zur Kenntnis der fossilen Fische aus der Molasse von Baltringen. II. Batoidei A. Günther. Klein- und grosszahnige Rochen. Jahreshefte des Vereins für vaterländische Naturkunde, 33:69-103.
Probst, J. 1878. Beiträge zur Kenntnis der fossilen Fische aus der Molasse von Baltringen. Hayfische (Selachoidei A. Günther). Jahreshefte des Vereins für vaterländische Naturkunde, 34:113-154.
Probst, J. 1879a. Beiträge zur Kenntnis der fossilen Fische aus der Molasse von Baltringen. Hayfische (Selachoidei A. Günther) (Schluss). Jahreshefte des Vereins für vaterländische Naturkunde, 35:127-191.
Probst, J. 1879b. Verzeichnis der Flora und Fauna der Molasse im Württembergischen Oberschwaben. Jahreshefte des Vereins für vaterländische Naturkunde, 35:221-304.
Probst, J. 1882. Beiträge zur Kenntniss der fossilen Fische aus der Molasse von Baltringen: Fossile Reste von Stören und einigen andern Fischen. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg, 38: 116-136.
Purdy, R.W. 1996. Paleoecology of fossil white sharks, p. 67-78. In Klimley, A. and Ainley, D. (eds.), Great White Sharks: The Biology of Carcharodon carcharias. Academic Press, San Diego.
https://doi.org/10.1016/B978-012415031-7/50009-4
Purdy, R.W., Schneider, V.P., Applegate, S.P., Mclellan, J.H., Meyer, R.L., and Slaughter, B.H. 2001. The neogene sharks, rays, and bony fishes from Lee Creek Mine, Aurora, North Carolina, p. 71-202. In Ray, C.E. and Bohaska, D.J. (eds.), Geology and Paleontology of the Lee Creek Mine, North Carolina. Smithsonian Contributions to Paleobiology, 90.
Quoy, J.R.C. and Gaimard, J.P. 1824. Description des Poissons. Chapître IX, p. 192-401. In de Freycinet, L. (ed.), Voyage autour du monde, entrepris par ordre du roi. Exécuté sur les corvettes de S.M. l'Uranie et la Physicienne, pendant les années 1817, 1818, 1819 et 1820. Pillet aîné, Paris.
https://doi.org/10.5962/bhl.title.152367
Rafinesque, C.S. 1810a. Caratteri di alcuni nuovi generi e nuove specie di animali e pinate della Sicilia, con varie osservazioni sopra I medisimi, I. partie. Sanfilippo, Palermo.
https://doi.org/10.5962/bhl.title.104418
Rafinesque, C.S. 1810b. Indice d'ittiologia siciliana ossia catalogo metodico dei nomi latini, italiani, e siciliani, e siciliani dei pesci, che si rinvengono in Sicilia disposti secondo un metodo naturale eseguito da un appendice che contiene la descrizione di alcuni nuovi pesci siciliani. Ill Opuscolo del signore C.S. Rafinesque Schmaltz. Giovanni del Nobolo, Messina.
https://doi.org/10.5962/bhl.title.6893
Rasser, M.W., Höltke, O., and Salvador, R.B. 2019. Gastropod palaeohabitats of Miocene Lake Randeck Maar and its hinterland defined by an actualistic genus-level approach. Lethaia, 53(2):229-241.
https://doi.org/10.1111/let.12353
Rasmussen, T. 2018. Does host diet govern the structure and diversity of tapeworm assemblages in sharks? Insights from the literature and a model shark species; Cephaloscyllium isabellum. Unpublished Masters Thesis in Ecology, University of Otago, Dunedin, New Zealand.
Reinecke, T., Louwye, S., Havekost, U., and Moths, H. 2011. The elasmobranch fauna of the late Burdigalian, Miocene, at Werder-Uesen, Lower Saxony, Germany, and its relationships with early Micocebe faunas in the North Atlantic, Central Paratethys and Mediterranean. Palaeontos, 20:1-170.
Reinecke, T., Moths, H., Grant, A., and Breitkreuz, H. 2005. Die Elasmobranchier des norddeutschen Chattiums, insbesondere des Sternberger Gesteins (Eochattium, Oligozän). Palaeontos, 8:1-135.
Risso, A. 1810. Ichthyologie de Nice, ou, Histoire naturelle des poissons du département des Alpes Maritimes. F. Schoell, Paris.
https://doi.org/10.5962/bhl.title.7052
Sach, V. 2016. Fossilienkatalog der Miozän-Molasse in Südwestdeutschland. Documenta naturae, Sonderband 70:1-115.
Schreiner, A. 1966. Zur Stratigraphie der Oberen Meeresmolasse zwischen der Oberen Donau und dem Überlinger See (Baden-Württemberg). Jahresbericht und Mitteilungen des oberrheinischen geologischen Vereins, Neue Folge, 48:91-104.
https://doi.org/10.1127/jmogv/48/1966/91
Schütze, E. 1904. Die Fauna der schwäbischen Meeresmolasse. I. Teil: Spongien und Echinodermen. Jahreshefte des Vereins für vaterländische Naturkunde, 60:147-188.
Schwartz, F.J. 2000. Food of tiger sharks, Galeocerdo cuvier (Carcharhinidae) from the northwest Atlantic Ocean, off North Carolina. Journal of the Elisha Mitchell Scientific Society, 116(4):351-355
Scott, R.W. 1978. Approaches to trophic analysis of paleocommunities. Lethaia, 11(1):1-14.
https://doi.org/10.1111/j.1502-3931.1978.tb01210.x
Séret, B., Last, P.R., and Naylor, G.J.P. 2016: Guitarfishes Family Rhinobatidae, p. 77-109. In Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., and Naylor, G.J.P. (eds.), Rays of the world. CSIRO Publishing, Ithaca and London.
Shimada, K., Chandler, R.E., Lam, O.L.T., Tanaka, T., and Ward, D.J. 2016. A new elusive otodontid shark (Lamniformes: Otodontidae) from the lower Miocene, and comments on the taxonomy of otodontid genera, including the ‘megatoothed’ clade. Historical Biology, 29(5):704-714.
https://doi.org/10.1080/08912963.2016.1236795
Shirai, S. and Nakaya, K. 1992. Functional morphology of feeding apparatus of the cookie-cutter shark, Isistius brasiliensis (Elasmobranchii, Dalatiinae). Zoological Science, 9(4):811-821.
Smale, M.J. 2005. The diet of the ragged-tooth shark Carcharias taurus Rafinesque 1810 in the Eastern Cape, South Africa. African Journal of Marine Science, 27(1):331-335.
https://doi.org/10.2989/18142320509504091
Smith, A. 1838. Pisces. Illustrations of the zoology of South Africa. Smith, Elder and Co, London.
https://doi.org/10.5962/bhl.title.10416
Smith, H.M. and Radcliff, L. 1912. The squaloid sharks of the Philippine Archipelago, with descriptions of new genera and species. Proceedings of the United States National Museum, 41:677-685.
Springer, S. 1940. Three new sharks of the genus Sphyrna from the Pacific coast of tropical America. Stanford Ichthyological Bulletin, 1(5):161-172.
Springer, V.G. 1964. A revision of the carcharhinid shark genera Scoliodon, Loxodon, and Rhizoprionodon. Proceedings of the United States National Museum, 115(3493):559-632.
https://doi.org/10.5479/si.00963801.115-3493.559
Stanton, R.J. Jr. 2006. Nutrient models for the development and location of ancient reefs. Geol.Alp, 3:191-206.
Strasburg, D.W. 1963. The diet and dentition of Isistius brasiliensis, with remarks on tooth replacement in other sharks. Copeia, 1963 (1):33-40.
https://doi.org/10.2307/1441272
Straube, N. and Pollerspöck, J. 2020. Intraspecific dental variations in the deep-sea shark Etmopterus spinax and their significance in the fossil record. Zoomorphology, 139(4):483-491.
https://doi.org/10.1007/s00435-020-00503-3
Taylor, L.R., Compagno, L.J.V., and Struhsaker, P.J. 1983. Megamouth - a new species, genus and family of lamnoid sharks, (Megachasma pelagios family Megachasmidae), from the Hawaiian Islands. Proceedings of the California Academy of Sciences, (Series 4), 43(8):87-110.
Teng, H.-T. 1959. Studies on the elasmobranch fishes from Formosa. Part 4. Squaliolus alii, a new species of deep sea squaloid shark from Tung-Kang, Formosa. Reports of the Laboratory of Fishery Biology of the Taiwan Fisheries Research Institute, 5:69-72.
Tillett, B.J., Meekan, M.G., and Field, I.C. 2014. Dietary overlap and partitioning among three sympatric carcharhinid sharks. Endangered Species Research, 25:283-293.
https://doi.org/10.3354/esr00615
Tricas, T.C. 1985. Feeding ethology of the white shark, Carcharodon carcharias. Memoirs of the Southern California Academy of Sciences, 9:81-91.
Tricas, T.C. and McCosker, J.E. 1984. Predatory behavior of the white shark (Carcharodon carcharias), with notes on its biology. Proceedings of the California Academy of Sciences, (Series 4), 43(14):221-238
Valenciennes, A. 1839. Spec. 16. Carcharias (Prionodon) oxyrhynchus. N., p. 41-42. In Müller, J.P. and Henle, F.G.J. (eds.), Systematische Beschreibung der Plagiostomen. Veit-Verlag, Berlin.
Valenciennes, A. 1839. Spec. 17. Carcharias (Prionodon) leucas. Val., p. 42-43. In Müller, J.P. and Henle, F.G.J. (eds.), Systematische Beschreibung der Plagiostomen. Veit-Verlag, Berlin.
Van Hasselt, J.C. 1823. Uittreksel uit een brief van Dr. J.C. van Hasselt aan den Heer C.J. Temminck. Algemeene Konst- en Letter-Bode Deel, 1(20): 315-317; (21):328-331.
Vialle, N., Adnet, S. and Cappetta, H. 2011: A new shark and ray fauna from the Middle Miocene of Mazan, Vaucluse (southern France) and its importance in interpreting the paleoenvironment of marine deposits in the southern Rhodanian Basin. Swiss Journal of Palaeontology, 130:241-258.
https://doi.org/10.1007/s13358-011-0025-4
Voigt, M. and Weber, D. 2011 Field Guide for Sharks of the Genus Carcharhinus. Friedrich Pfeil Verlag, München.
Ward, D.J. and Bonavia, C.G. 2001. Additions to and a review of the Miocene shark and ray fauna of Malta. The Central Mediterranean Naturalist, 3(3):131-146.
Welton, B.J. 2013. A new archaic basking shark (Lamniformes: Cetorhinidae) from the late Eocene of western Oregon, U.S.A., and description of the dentition, gill rakers and vertebrae of the recent basking shark Cetorhinus maximus (Gunnerus). New Mexico Museum of Natural History and Science Bulletin. 58:1-48.
Werner, J. 1966. Ergebnisse der Auswertung von Flachbohrungen im Bereich des Grobsandzuges der Oberen Meeresmolasse (Gebiet Stockach - Pfullendorf). Jahresberichte und Mitteilungen des Oberrheinischen Geologischen Vereins, N.F., 48:105-120.
https://doi.org 10.1127/jmogv/48/1966/105
Westgate, J.W. 2001. Paleoecology and biostratigraphy of marginal marine Gulf Coast Eocene vertebrate localities, p. 263-297. In Landman, N.H. and Jones, D.S. (eds.), Topics in Geobiology, Vo. 18, Eocene biodiversity. Springer, New York.
Wetherbee, B.M., Cortés, E., and Bizzarro. J.J. 2012. Food consumption and feeding habits, p. 239-264. In Carrier, J.C., Musick, J.A., and Heithaus, M.R. (eds.), Biology of sharks and their relatives. CRC Press, Boca Raton, Florida.
White, W.T. 2012. A redescription of Carcharhinus dussumieri and C. sealei, with resurrection of C. coatesi and C. tjutjot as valid species (Chondrichthyes: Carcharhinidae). Zootaxa, 3241:1-34.
https://doi.org/10.11646/zootaxa.3241.1.1
White, W.T. and Last, P.R. 2016a. Pelagic Eagle Rays Family Aetobatidae, p. 726-731. In Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., and Naylor, G.J.P. (eds.), Rays of the world. CSIRO Publishing, Ithaca and London.
White, W.T. and Last, P.R. 2016b. Devilrays Family Mobulidae, p. 741-749. In Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., and Naylor, G.J.P. (eds.), Rays of the world. CSIRO Publishing, Ithaca and London.
White, W.T. and Last, P.R. 2016c. Eagle Rays Family Myliobatidae, p. 706-725. In Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., and Naylor, G.J.P. (eds.), Rays of the world. CSIRO Publishing, Ithaca and London.
Whitenack, L.B. and Motta, P.J. 2010. Performance of shark teeth during puncture and draw: implications for the mechanics of cutting. Biological Journal of the Linnean Society, 100(2):271-286.
https://doi.org/10.1111/j.1095-8312.2010.01421.x
Whitley, G.P. 1929. Additions to the check-list of the fishes of New South Wales. No. 2. Australian Zoologist, 5(4):353-357.
Whitley, G.P. 1939. Taxonomic notes on sharks and rays. Australian Zoologist, 9(3):227-262.
Winkler, T.C. 1874. Deuxième mémoire sur des dents de poissons fossiles du terrain bruxellien. Archives du Musée Teyler, 4(1):16-48.
Wood, S. V. 1844. III.–Descriptive Catalogue of the Zoophytes from the Crag. Annals and Magazine of Natural History, 13:10-21.
Yano, K., Miya, M., Aizawa, M., and Noichi, T. 2007. Some aspects of the biology of the goblin shark, Mitsukurina owstoni, collected from the Tokyo Submarine Canyon and adjacent waters, Japan. Ichthyological Research, 54(4):388-398.
https://doi.org/10.1007/s10228-007-0414-2
Yokota, L., White, W.T., and de Carvalho, M.R. 2016. Butterfly rays, Family Gymnuridae, p. 511-521. In Last, P.R., White, W.T., Carvalho, M.R. de, Séret, B., Stehmann, M.F.W., and Naylor, G.J.P (eds.), Rays of the World. CSIRO Publishing, Melbourne.
Yano, K., Miya, M., Aizawa, M., and Noichi, T. 2007. Some aspects of the biology of the goblin shark, Mitsukurina owstoni, collected from the Tokyo Submarine Canyon and adjacent waters, Japan. Ichthyological Research, 54(4):388-398.
https://doi.org/10.1007/s10228-007-0414-2
Yokota, L., White, W.T., and de Carvalho, M.R. 2016. Butterfly rays, Family Gymnuridae, p. 511-521. In Last, P.R., White, W.T., Carvalho, M.R. de, Séret, B., Stehmann, M.F.W., and Naylor, G.J.P (eds.), Rays of the World. CSIRO Publishing, Melbourne.

