 Lopingian (Late Permian) trilobites from the North Caucasus, Russia, with an overview of their distribution worldwide
Lopingian (Late Permian) trilobites from the North Caucasus, Russia, with an overview of their distribution worldwide
Article number: 28.2.a22
https://doi.org/10.26879/1399
Copyright Palaeontological Association, May 2025
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 16 April 2024. Acceptance: 5 May 2025.
ABSTRACT
Trilobites from the Upper Permian (Changhsingian) of the North Caucasus, previously described by Weber (1944), are revised. Brachymetopus (?) caucasicus Licharew in Weber, 1944, known only from its pygidium, belongs to the Brachymetopus (Acutimetopus), and not Cheiropyge, as some researchers believed, since it lacks a terminal lobe, characteristic of Cheiropyge. A new species Paraphillipsia urushtensis sp. nov. has been described. For the first time, photographs of all specimens of the type series of the new species are presented. Kathwaia capitorosa Grant, 1966, described from the Wuchiapingian of Pakistan, does not have significant morphological differences from the North Caucasian K. caucasica (Weber, 1944) and is here considered a junior subjective synonym of the latter. Other trilobite assemblages of the North Caucasus are represented by Pseudophillipsia solida Weber, 1944, Ps. (?) caucasica Weber, 1944 and Ps. (?) cf. mustafensis Tumanskaya, 1935. It is proposed not to use the subgenus Pseudophillipsia (Nodiphillipsia) based on its redundancy. The “problem of similar pygidia” of Pseudophillipsia and Ditomopyge (Carniphillipsia) is discussed. It is proposed to conditionally classify all species known exclusively from highly segmented pygidia as Pseudophillipsia. All currently known localities of Lopingian trilobites in the World are considered, and their stratigraphical occurrences are clarified. This list is supplemented by localities from Crimea, Far East, Hungary, New Zealand and Spitsbergen. The latter localities indicate that Lopingian trilobites were not limited to the Palaeo-Tethys, but were present in mid-latitudes. Trilobites of the Lopingian were not as diverse as in the Guadalupian and were represented by only nine (probably 10) genera and 36 species (and species determined in open nomenclature). This time interval is characterized by an extremely low rate of origination of new genera and a high rate of extinction.
Eduard V. Mychko. Shirshov Institute of Oceanology, Russian Academy of Sciences, Nahimovskiy prospekt 36, Moscow, 117997, Russia. Scientific and Educational Center “Environmental geology and Maritime Management”, Immanuel Kant Baltic Federal University, Nevskogo Street 14, Kaliningrad, 236016, Russia.
ORCID: 0000-0003-1601-3618
eduard.mychko@gmail.com
Keywords: Trilobita; Proetida; Brachymetopidae; Phillipsiidae; Lopingian; Changhsingian; Russia; North Caucasus.
Final citation: Mychko, Eduard V. 2025. Lopingian (Late Permian) trilobites from the North Caucasus, Russia, with an overview of their distribution worldwide. Palaeontologia Electronica, 28(2):a22.
https://doi.org/10.26879/1399
palaeo-electronica.org/content/2025/5533-latest-trilobites
Copyright: May 2025 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
https://zoobank.org/F98BEDBC-302A-405A-94FE-9856F92A817A
INTRODUCTION
Widely distributed and numerous in Early Palaeozoic, trilobites decreased in diversity from benthic communities in post-Devonian times. Trilobites survived in Carboniferous and Permian, becoming extinct during the Great Permian Extinction. Therefore, in deposits of the Permian, remains of these arthropods are relatively rare and their diversity is low. Unfortunately, due to the rarity and impossibility of using this group to solve biostratigraphical problems, Late Palaeozoic trilobites turned out to be one of the least studied groups.
Analyzing studies of Lopingian trilobites of the World, it becomes obvious that the overwhelming number of articles are episodic and regional, and comprehensive works covering all known species of this era are practically absent. In fact, research work on Lopingian trilobites can be divided into countries: Slovenia (Hahn et al., 1970), Hungary (Schréter, 1948), Iran (Hahn and Hahn, 1981; Lerosey-Aubril, 2012), Pakistan (Grant, 1966), China (Diener, 1897; Lu, 1974; Qian, 1977; Yin, 1978; Qian, 1981; Zhang, 1982, etc.), Indonesia (Beyrich, 1865; Hahn and Brauckmann, 1975; Brauckmann and Gröning, 2013), Japan (Kobayashi and Hamada, 1984a; 1984b), Thailand (Kobayashi and Sakagami, 1989), and Spitsbergen (Kobayashi, 1987; Bruton, 1999). There are very few generalized studies that provide lists and distribution of known trilobites of the Lopingian. Here I should mention the works of Owens (1983; 2003), as well as the summary article by Hahn, Hahn and Brauckmann (2001).
Major studies of Permian (and Carboniferous) trilobites in Russia were carried out over 80 years ago (Toumansky, 1930; Tumanskaya, 1935; Weber, 1932; 1933; 1937; 1944). Since then, trilobites of this age have been hardly studied by Soviet and Russian palaeontologists, except for several works by the author (Mychko, 2012; Mychko and Alekseev, 2017; Mychko and Savchuk, 2019; Mychko, 2023, etc.), as a result of which the systematic position and stratigraphic the distribution of previously described taxa requires revision in accordance with modern ideas. In addition, over the past decades, a fairly large amount of new, not yet described factual material has accumulated.
The most important and major revision of the Carboniferous-Permian trilobites of the World (including the territory of former USSR) was carried out by German palaeontologists over almost half a century (Hahn and Hahn, 1969; 1970; 1972; 1993; 1996; 2008; 2015; 2016; Hahn et al., 2019). These publications revised almost all known Carboniferous and Permian trilobites. Of course, it was quite difficult to cover such a large amount of data, and the authors of these revisions could not personally familiarize themselves with the material stored in the USSR (Russia), but, nevertheless, the cited works can be considered key for the study of modern ideas about the taxonomy and synonymy of many species of trilobites Carboniferous and Permian.
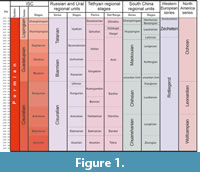 The trilobites of the North Caucasus studied in this article were first described by the Soviet palaeontologist Weber in his fundamental work on the Permian trilobites of the USSR (1944), published posthumously. Over a long period of time, the Caucasian species and species determined in open nomenclature described in this study were partially revised by Hahn and Hahn. However, neither Weber's original publication nor the Hahn’s catalogues provided photographs of all specimens of the type series of these trilobites, and many taxonomic questions require clarification in the light of new data. An equally important aspect of this publication is not only a systematic revision, but also a clarification of the age of the host deposits. The age of the discussed North Caucasian trilobites has varied among different authors from the Cisuralian, to the Guadalupian and Lopingian (Figure 1). The latest data (see the Localities section) confidently support the Lopingian age.
The trilobites of the North Caucasus studied in this article were first described by the Soviet palaeontologist Weber in his fundamental work on the Permian trilobites of the USSR (1944), published posthumously. Over a long period of time, the Caucasian species and species determined in open nomenclature described in this study were partially revised by Hahn and Hahn. However, neither Weber's original publication nor the Hahn’s catalogues provided photographs of all specimens of the type series of these trilobites, and many taxonomic questions require clarification in the light of new data. An equally important aspect of this publication is not only a systematic revision, but also a clarification of the age of the host deposits. The age of the discussed North Caucasian trilobites has varied among different authors from the Cisuralian, to the Guadalupian and Lopingian (Figure 1). The latest data (see the Localities section) confidently support the Lopingian age.
LOCALITIES
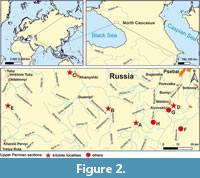 Permian trilobites of the North Caucasus are confined to the upper Changhsingian formations, exposed a number of localities in the basins of the Belaya River, Bol’shaya Laba River and Malaya Laba River in its north-western part (Figure 2). The famous Russian geologist Robinson discovered these outcrops in 1912, and later (Robinson, 1932) based on the Chernyshev’s determinations of brachiopods, considered them to be Cisuralian. With the advent of monographic descriptions of brachiopods and bivalves, Likharev determined the age of these deposits as Lopingian, more precisely post-Kungurian on the modern stratigraphic scale (Kotlyar et al., 2004).
Permian trilobites of the North Caucasus are confined to the upper Changhsingian formations, exposed a number of localities in the basins of the Belaya River, Bol’shaya Laba River and Malaya Laba River in its north-western part (Figure 2). The famous Russian geologist Robinson discovered these outcrops in 1912, and later (Robinson, 1932) based on the Chernyshev’s determinations of brachiopods, considered them to be Cisuralian. With the advent of monographic descriptions of brachiopods and bivalves, Likharev determined the age of these deposits as Lopingian, more precisely post-Kungurian on the modern stratigraphic scale (Kotlyar et al., 2004).
The stratigraphy of the Permian deposits of the Northwestern Caucasus was described by Miklouho-Maclay (1954, 1956), who, based on lithology and foraminiferal assemblages, established four formations (later Triassic ammonoids were discovered in one of these formations). The three Permian formations are: Kutanskaya (basal conglomerates and sandstones with some limestone interbeds in the upper part), Nikitino (laminated algal limestones with abundant foraminifera) and Urushten (reef limestones and shales) (Kotlyar et al., 2004).
 Studies of fossils from these deposits have yielded varying age estimates. Brachiopod assemblages were dated from the Midian-Dorashamian (Figure 1) of Tethyan scale (Grunt and Dmitriev, 1973; Kotlyar et al., 1983; Kotlyar, 1989); ammonoids of the Urushten Formation were dated to the cis-Dzhulfian interval of the Tethyan scale (Bogoslovskaya, 1984), and foraminifera to the cis-Dorashamian of the Tethyan scale (Kotlyar et al., 1983). Later Kotlyar et al. (1999a), as well as Pronina-Nestell and Nestell (2001) established that the age of these deposits is Late Changhsingian (International Stratigraphical Chart). According to Pronina-Nestell and Nestell (2001) in the Lopingian of the North Caucasus, there are small foraminifera and fusulinaceans, characteristic of the zones Palaeofusulina sinensis = Palaeofusulina nana and Colaniella parva of the Late Changhsingian of Palaeo-Tethys, and therefore these deposits can be attributed to this age.
Studies of fossils from these deposits have yielded varying age estimates. Brachiopod assemblages were dated from the Midian-Dorashamian (Figure 1) of Tethyan scale (Grunt and Dmitriev, 1973; Kotlyar et al., 1983; Kotlyar, 1989); ammonoids of the Urushten Formation were dated to the cis-Dzhulfian interval of the Tethyan scale (Bogoslovskaya, 1984), and foraminifera to the cis-Dorashamian of the Tethyan scale (Kotlyar et al., 1983). Later Kotlyar et al. (1999a), as well as Pronina-Nestell and Nestell (2001) established that the age of these deposits is Late Changhsingian (International Stratigraphical Chart). According to Pronina-Nestell and Nestell (2001) in the Lopingian of the North Caucasus, there are small foraminifera and fusulinaceans, characteristic of the zones Palaeofusulina sinensis = Palaeofusulina nana and Colaniella parva of the Late Changhsingian of Palaeo-Tethys, and therefore these deposits can be attributed to this age.
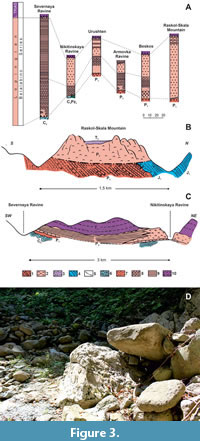
The Upper Changhsingian formations of the Northwestern Caucasus are placed in the Belalabino Group (Figure 3), which is separated from the underlying and overlying deposits by erosional unconformities. These formations contain diverse and abundant algae, foraminifera, sponges, brachiopods, bivalves and gastropods, ammonoids and trilobites (Kotlyar et al., 2004).
In total, only five localities of Lopingian trilobites are known in the North Caucasus (Figure 2). These were previously characterized in the works of Mychko and Alekseev (2017). The information below has been clarified and supplemented.
Urushten (Figure 2A, Figure 3). Krasnodar Krai, Mostovsky district, Malaya Laba River basin, Urushten tract (=paraje) and Urushten River. In the Malaya Laba River basin, south from the Urushten, in the deposits of the Upper Formation (P1b) Robinson (1932, p. 23) discovered trilobites, which Weber originally identified as Proetus postcarbonarius Gemm., Pr. ? semipustulatus Weber in Robinson, 1932 and Phillipsia tschernyschewi (Netschaew in Weber, 1932).
These findings, as well as material collected from here by Likharev, were later described by Weber as Paraphillipsia karpinskyi Tumanskaya, 1935 (Weber, 1944, p. 12, table 1, figs. 18-20, 22), Pseudophillipsia elegans var.? (Weber, 1944, p. 13, table 2, fig. 2), P. (?) solida Weber, 1944 (Weber, 1944, p. 13, table 2, figs. 8, 9) and Proetus girtyi var. caucasica Weber, 1944 (Weber, 1944, p. 15, fig. 17).
A different list for the Urushten Formation of the North Caucasus according to earlier definitions by Weber was given by Miklouho-Maclay (1956, p. 71): Proetus postcarbonarius Gemmellaro, 1892, Pr. semipustulatus Weber in Robinson, 1932, Phillipsia tschernyschewi (Netschaew in Weber, 1932), Pseudophillipsia elegans Gemmellaro, 1892. Apparently, the monograph by Weber (1944), devoted to the description of the Permian trilobites of the USSR, was unfamiliar to her. In the older Nikitino Formation, Miklouho-Maclay did not mention trilobites.
Likharev (1939, p. 200) for the Permian of the North Caucasus (the Malaya Laba River basin) cited only Proetus ? girtyi Tumanskaya, 1935.
Brachiopods Scacchinella jakovlevi, Leptodus richthofeni and Camarophoria caucasica, found together with trilobites, belong to the assemblage of the Urushten Formation (Kotlyar et al., 1983).
Mountain Gefo (Figure 2B). Republic of Adygea, Maikop district, Belaya River basin. The work of Robinson (1932, p. 24) provides a list of Likharev’s fauna in the light limestones of the Upper Formation (P1b) near Mountain Gefo.
It includes a mention of the discovery of the trilobite Pseudophillipsia elegans Gemmellaro, 1892 (definition by Weber) and foraminifera Palaeofusulina nana, characteristic only of the Urushten Formation (Miklouho-Maclay, 1954; Kotlyar et al., 1983).
Likharev found near Mount Gefo, in blocks of limestone along the Tegen’ River (outcrop No. 30) the following: Griffithides (Neogriffithides) cf. almensis Tumanskaya, 1935 (Weber, 1944, p. 11, table 1, fig. 15), Pseudophillipsia elegans var. caucasica Weber, 1944 (Weber, 1944, p. 12, table 2, fig. 4), Ps. mustafaensis Tumanskaya, 1935? (Weber, 1944, p. 13, table 2, fig. 3) and Ps. (?) solida Weber, 1944 in outcrop No. 33 (Weber, 1944, p. 14).
Khamyshki (Figure 2C, Figure 3). Republic of Adygea, Maikop district, Belaya River basin. Around this village, near Raskol Rock (mountain), in the western part of the block (outcrop No. 42c) Likharev discovered Griffithides (Neogriffithides) cf. almensis Tumanskaya, 1935 (Weber, 1944, p. 11, table 1, fig. 16) and Proetus girtyi var. caucasica Weber, 1944 (Weber, 1944, p. 15, table 2, fig. 16). In this locality, limestones of the Urushten Formation are exposed (Kotlyar et al., 1983; Kotlyar et al., 2004).
Nikitinskaya ravine (Figure 2D, Figure 3). Krasnodar Krai, Mostovsky district. Malaya Laba River basin. Likharev found the pygidium of Paraphillipsia karpinskyi Tumanskaya, 1935 at this locality, 2.25 km above its entrance (Weber, 1944, p. 12), in the same place in the scree of Pseudophillipsia elegans var.? (Weber, 1944, p. 13), and not far from this locality in a block (outcrop No. 842) Robinson discovered the pygidium of Brachymetopus (?) caucasicus Licharew in Weber, 1944 (Weber, 1944, p. table 2, fig. 15). In this locality, deposits of the Nikitino and Urushten formations are exposed (Kotlyar et al., 1983; Kotlyar et al., 2004).
Attempts to isolate conodonts from the samples collected here by Grunt and transferred to the Department of Palaeontology of Lomonosov Moscow State University (Russia), after many years of dissolution were successful – single elements of the shallow-water genus Hindeodus were found (personal commun. by Prof. A.S. Alekseev, 02.20.2024). In the same samples, an unidentified pygidium, about 1 mm in length, with about six rings in the axis, but lacking ribs on the pleural lobes as recovered; it appears to be a larval stage, perhaps a meraspis. The search for trilobites in this locality by author and M.S. Boiko in 2019 was unsuccessful: when visiting the locality (Figure 3D), it turned out that a mountain road had been built through it. The remaining outcrops contained rare fossils, in particular a few brachiopods.
Mountain Khuko (Figure 2E). Krasnodar Krai, Sochi urban district, southern slope of the Greater Caucasus. On the northeastern slope of Khuko Mountain in the axial part of the Greater Caucasus Range, in the “calcareous-terrigenous sequence” or Khuko Formation (Vyalov, 1934), and according to Miklouho-Maclay (1952, p. 12) – in the Nikitino Formation, the trilobite Pseudophillipsia sp. was found together with brachiopods (Belov, 1967, p. 89). Belov considered the age of this strata to be Cisuralian (Artinskian). These deposits near the Mountain Khuko were also noted by Miklouho-Maclay (1956, p. 61).
SYSTEMATIC PALAEONTOLOGY
About the systematics. In the Permian, trilobites of three proetid families are found: Phillipsiidae, Proetidae and Brachymetopidae. The first, Phillipsiidae, are the most numerous and diverse, the second and third are rare. To a first approximation, the morphology of these three families seems very different (for example, fused facial sutures in many Brachymetopidae), which may lead to agree with Adrain (2011) about the relationship of these families in two different orders. However, the author adheres to the opinion of Lamsdell and Selden (2014) and considers it necessary to leave the division of the order Proetida into two superfamilies Aulacopleuroidea and Proetoidea.
The Permian Phillipsiidae includes the following subfamilies: Ditomopyginae, Bollandiinae and Cummingellinae; for the Permian Proetidae, subfamilies have not been established, and Brachymetopidae in the Permian are represented by only one subfamily, Brachymetopinae.
About subspecies. In further revision, the author is of the opinion that the use of subspecies in taxonomic studies of fossil organisms is redundant (Burbrink et al., 2022). Subspecies (and varieties) previously established by other authors are considered here as independent species.
About the storage location. All studied specimens of trilobites from the Lopingian of the North Caucasus are stored in the collections of the Chernyshev Central Geological Research Museum (CNIGRmuseum) in St. Petersburg (Russia). The holotype of Kathwaia capitorosa is kept in the palaeontological collection of the Smithsonian National Museum of Natural History (USNM) in Washington (USA). One specimen of Pseudophillipsia solida is kept in the collection of the Geological and Palaeontological Institute of the University of Ljubljana (GPIUL) in Slovenia.
Abbreviations. Cc – complete exoskeleton, Cph – cephalon, Cr – cranidium, Gl – glabella, Lg – librigena (=free cheek), Py – pygidium, Hy – hypostome.
Order PROETIDA Fortey and Owens, 1975
Superfamily AULACOPLEUROIDEA Angelin, 1854
Family BRACHYMETOPIDAE Prantl and Přibyl, 1950
Subfamily BRACHYMETOPINAE Prantl and Přibyl, 1950
Genus BRACHYMETOPUS McCoy, 1847
Subgenus BRACHYMETOPUS (ACUTIMETOPUS) Hahn and Hahn, 1985
1985 Brachymetopus (Acutimetopus) – Hahn and Hahn, p. 445, 460, 461, 465, 474, 476, 477, Abb. 9.
1987 Brachymetopus (Acutimetopus) – Gandl, p. 6,10, 48, 49, 53-54.
1987 Brachymetopus (Acutimetopus) – Hahn and Hahn, p. 573, 574.
1989a Brachymetopus (Acutimetopus) – Hahn, Hahn, and Schneider, p. 650.
1989b Brachymetopus (Acutimetopus) – Hahn, Hahn, and Yuan, p. 113,119,121,123,124,126.
1993 Brachymetopus (Acutimetopus) – Owens and Hahn, p. 170,173.
1994 Brachymetopus (Acutimetopus) – Brauckmann, p. 30.
1996 Brachymetopus (Acutimetopus) – Hahn and Hahn, p. 8, 35, 38, 39, 40-42, 44, 47, 50, 52, 56, 62, 65,146,153,154.
1996 Brachymetopus (Acutimetopus) – Hammel, p. 751.
2003 Acutimetopus – Jell and Adrain, p. 337.
2011 Brachymetopus (Acutimetopus) – Gandl, p. 103-106.
2016 Brachymetopus (Acutimetopus) – Mychko, p. 34,61,136,141,152-153.
2019 Brachymetopus (Acutimetopus) – Mychko and Savchuk, p. 346, 347, 348, 349.
2021 Brachymetopus (Acutimetopus) – Flick and Shiino, p. 91, 92, 97, 99.
2023 Brachymetopus (Acutimetopus) – Brezinski, p. 3,9-11,15,16.
Type species. Cheiropyge kansasensis Weller, 1944; Upper Pennsylvanian, upper part of the Haskell Limestone (or Cass Formation, the upper part of the Kasimovian, see: Heckel, 1999; Heckel et al., 2007); USA, Kansas, Leavenworth; designated by Hahn and Hahn (1985, p. 445).
Diagnosis. Cephalon elongated, subtriangular, with an apical peak and genal angles (often rounded, but some species have short genal spines); covered with tubercles; facial sutures ankylosed; glabella cylindrical, moderate to long, tapering towards the anterior part, bears poorly developed small L1 -lobes; preglabellar field wide; eyes medium-sized, set towards back of cephalon; pygidium elongated, often with marginal spines on the extensions of pleural ribs; pygidial axis long, has 18 or more axial rings, and 6-7 pleural ribs; sometimes there is a post-axial spine.
Comparison. From other subgenera Brachymetopus (Acutimetopus) differs mainly in the subtriangular outline of the cephalon due to the presence of an apical peak in the anterior part, which makes it similar to Cheiropyge. It differs from the latter in the absence of a swollen terminal lobe in the posterior part of the pygidium.
Remarks. It is necessary to provide a list of the remaining subgenera of Brachymetopus because two of them, after their description, turned out to be homonyms, but some authors continue to use the same names. Thus, Brachymetopus includes the nominate subgenus B. (Brachymetopus) McCoy, 1847 (Upper Devonian – Upper Pennsylvanian of Eurasia, North America and Australia), B. (Acutimetopus) Hahn and Hahn, 1985, B. (Spinimetopus) Hahn and Hahn, 1985 (Mississippian of Eurasia and Australia), B. (Conimetopus) Hahn and Hahn, 1985 (Mississippian – Cisuralian of Eurasia and North America) and B. (Hahnus) Özdikmen, 2009 (Mississippian of Eurasia), which should be considered a synonym of B. (Eometopus) Hahn and Hahn, 1996 and B. (Narinia) Archbold, 1997 (Guadalupian of Asia), which is a synonym of B. (Iriania) Archbold, 1981.
Species. 16 species and two species determined in open nomenclature (Table 1).
Occurrence. Pennsylvanian (Bashkirian) – Lopingian (Changhsingian); Eurasia, Arctic and North America.
Brachymetopus (Acutimetopus) caucasicus Licharew in Weber, 1944
Figure 4
 1944 Brachymetopus (?) caucasicus – Weber, p. 15,18, pl. II, fig. 15a-b.
1944 Brachymetopus (?) caucasicus – Weber, p. 15,18, pl. II, fig. 15a-b.
1969 Cheiropyge ? caucasica – Hahn and Hahn, p. 41-42.
1975 Cheiropyge ? caucasica – Hahn and Hahn, p. 17.
1978 Cheiropyge ? caucasica – Koizumi and Sasaki, p. 299.
1981 Brachymetopus (?) caucasicus – Archbold, 1981, p. 36,37.
1981 Cheiropyge ? caucasica – Přibyl and Vaněk, p. 187-188.
1983 Brachymetopus caucasicus – Owens, p. 34.
1984a Brachymetopus (?) caucasicus – Kobayashi and Hamada, p. 37.
1984a Cheiropyge ? caucasica – Kobayashi and Hamada, p. 25,29,33,38,39.
1985 Brachymetopus (Acutimetopus?) caucasicus – Hahn and Hahn, p. 465,468.
1986 Brachymetopus caucasicus – Owens, p. 13.
1987 Brachymetopus (Acutimetopus) caucasicus – Gandl, p. 53.
1989b Brachymetopus (Acutimetopus) caucasicus – Hahn, Hahn, and Yuan, p. 125.
1992 Brachymetopus (Acutimetopus) caucasicus – Hahn and Hahn, p. 117.
1992 Brachymetopus caucasicus – Brezinski, p. 928.
1996 Brachymetopus (Acutimetopus) caucasicus – Hahn and Hahn, p. 43-44, abb. 51.
2011 Brachymetopus (Acutimetopus) caucasicus – Gandl, p. 103.
2016 Brachymetopus (Acutimetopus) caucasicus – Mychko, p. 153, pl. I, fig. 4a-b.
2017 Brachymetopus (?) caucasicus – Mychko and Alekseev, p. 68.
2019 Brachymetopus (Acutimetopus) caucasicus – Mychko and Savchuk, p. 348, fig. 1d,e.
2021 Cheiropyge ? caucasica – Flick and Shiino, p. 92.
Holotype. CNIGRmuseum, No. 86/5217, incomplete pygidium; Urushten or Nikitino Formations, Changhsingian, Lopingian; Nikitinskaya Ravine, Malaya Laba River, Krasnodar Krai, Russia; discovered by Robinson in 1924; Weber, 1944, pl. II, fig. 15; designated by monotype.
Description. Pygidium slightly convex, subtriangular, elongated (L/W = 0.6); pygidial axis in anterior part equal in width to lateral lobes, strongly narrowing towards the posterior end of pygidium; number of axial rings is about 20 (15 anterior rings clearly visible, then rings merge, but their number >5); rings with flattened tubercles; first, third and fifth rings each have one large central tubercle; on anterior rings the number of tubercles – 8; furrows between rings deep; lateral lobes uniformly convex, with 6 pair pleural ribs, semicircular in cross-section, without pleural furrows; ribs located at an angle gradually decreasing towards posterior end of pygidium, so that last rib almost parallel with axis; interpleural furrows very deep and wide; widen towards the edge of pygidium; ribs bear numerous small tubercles; apparently, ribs ended with spines (which are not visible on the holotype due to incomplete preservation).
Dimensions (in mm). Length of pygidium ~7; width of pygidium ~13(?); width of axis in the anterior part – 3.3; ratio of length to width of pygidium ~0.5; ratio of the width of the axis in the anterior part to the width of the pygidium – 3.9.
Comparison. In terms of the number of axial rings of pygidium is similar to the species B. (A.) kansasensis and B. (A.) weberi, but differs in a different number of pairs of pleural ribs (B. (A.) kansasensis has 6, in B. (A.) weberi – 8). It also differs from B. (A.) kansasensis in the more triangular shape of the pygidium. It differs from B. (A.) acuticeps in the triangular shape of the pygidium, a larger number of axial rings (B. (A.) acuticeps has 12 axial rings), the absence of obvious pleural furrows, a narrower axis, and a less steep angle between the pleural ribs and the axis. It differs from B. (A.) edwardsi and the closely related species B. (A.) spinicauda in a larger number of axial rings (in these species there are up to 18 axial rings) and in the absence of obvious pleural furrows. It differs from B. (A.) gracilis in a larger number of axial rings (in B. (A.) gracilis there are up to 18 axial rings) and in a smaller number of pleural ribs (in B. (A.) gracilis there are seven pairs). It differs from B. (A.) kalodermatus by a more triangular shape of the pygidium, a larger number of axial rings (B. (A.) kalodermatus has about 15 axial rings), and a more pronounced angle between the pleurae and the axis. It differs from B. (A.) chamberlaini in the triangular shape of the pygidium, a larger number of axial rings (B. (A.) chamberlaini has about 12), the absence of obvious pleural furrows, a narrower axis and a larger number of pairs of pleural ribs (in B. (A.) chamberlaini there are six). Similar to the closely related species B. (A.) pseudometopina and B. (A.) macgrathensis, but differs in a larger number of axial rings.
Remarks. Unfortunately, the poor preservation of the specimen does not allow us to establish the morphology of the ends of the pleural ribs of the pygidium, which most likely terminated in spines, as in most members of Brachymetopus (Acutimetopus). However, from the available material it is noticeable that in the posterior part of the pygidium there is no swollen unpaired terminal lobe, characteristic of the genus Cheiropyge. This is important, since some researchers, not having the opportunity to familiarize themselves directly with the holotype and, having only a drawing and photograph from the work of Weber (1944), conditionally classified this species as Cheiropyge (e.g., Kobayashi and Hamada, 1984a; Flick and Shiino, 2021, etc.).
The author of the name of this species should be considered Likharev, since he is listed as such in synonymies in the original description of the species (Weber, 1944, p. 15) with the addition that this name was indicated in the collection (“nom. in coll.”).
Occurrence. Lopingian, Changhsingian; North Caucasus (Krasnodar Krai).
Material. Holotype (monotype).
Superfamily PROETOIDEA Hawle and Corda, 1847
Family PHILLIPSIIDAE Oehlert, 1886
Subfamily CUMMINGELLINAE Hahn and Hahn, 1967
Genus PARAPHILLIPSIA Toumansky, 1930
1930 Phillipsia (Paraphillipsia) – Toumansky, 1930, p. 474-476,477.
1935 Paraphillipsia – Tumanskaya, 1935, p. 19-20.
1935 Paraphillipsia – Weller, p. 31-32.
1937 Paraphillipsia – Gheyselinck, 1937, p. 4,36,58,63.
1939 Paraphillipsia – Likharev, p. 198.
1944 Paraphillipsia – Weber, p. 4,6,7,11-12,17-19.
1944 Paraphillipsia – Weller, p. 320,326-327.
1955 Paraphillipsia – Hupé, p. 208.
1959 Paraphillipsia – Weller, p. O401.
1960 Paraphillipsia – Maximova, p. 138.
1966 Paraphillipsia – Grant, p. 70.
1967 Paraphillipsia – Hahn and Hahn, p. 337,346.
1970 Paraphillipsia – Hahn and Hahn, p. 294-295.
1975 Paraphillipsia – Hahn and Hahn, p. 16,17,57-58.
1977 Paraphillipsia – Chamberlain, p. 758.
1979 Paraphillipsia – Kobayashi and Hamada, p. 3,12.
1980 Paraphillipsia – Haas, Hahn, and Hahn, p. 120.
1981 Paraphillipsia – Kobayashi and Hamada, p. 4.
1982 Paraphillipsia – Kobayashi and Hamada, p. 46,47.
1983 Paraphillipsia – Owens, p. 24,25,26,35-38.
1984 Paraphillipsia – Hahn, Hahn, and Brauckmann, p. 67.
1984a Paraphillipsia – Kobayashi and Hamada, p. 3,15,20,23,24,25,26,28,30,44,45,84.
1985 Paraphillipsia – Hahn and Hahn, p. 448.
1989b Paraphillipsia – Hahn, Hahn, and Yuan, p. 153,159.
1990 Paraphillipsia – Hahn, Hahn, and Ramovš, p. 146,154,156,158,160,161.
1990 Paraphillipsia – Hahn, S. 41.
1992 Paraphillipsia – Hahn and Hahn, p. 105.
1992 Paraphillipsia – Brezinski, p. 926.
1993 Paraphillipsia – Owens and Hahn, p. 174,175.
2003 Paraphillipsia – Jell and Adrain, p. 421,477.
2003 Paraphillipsia – Owens, p. 377,380,383,388,391.
2008 Paraphillipsia – Hahn and Hahn, p. 1,6,12,14,20,25,27,30,35,194,300-305,306,323.
2012 Paraphillipsia – Mychko, p. 575,577-580.
2016 Paraphillipsia – Mychko, p. 187-200.
2017 Paraphillipsia – Mychko and Alekseev, p. 67,68,69,70.
2019 Paraphillipsia – Schraut, p. 625-631.
2020 Paraphillipsia – Schraut, p. 217, tab. 3.
Type species. Paraphillipsia karpinskyi Tumanskaya, 1935; Roadian, Guadalupian; block of Dzhien-Sofu (=Totai-Koi), Salgir water pool, near of Simferopol City, Crimea; designated by Tumanskaya (1935, p. 19).
Diagnosis. Cephalon elongated, rounded at genal angles; glabella large, swollen, long, “cummingellid” in shape (i.e., similar to that in Cummingella); L1 -lobes well defined, elongated towards occipital ring, separated by distinct S1 -furrows; furrows S2 -S4 present, but very weakly expressed; eyes large, narrow, and bean-shaped; palpebral lobes shortened and protrude slightly to sides; facial sutures run close to glabella; thorax consists of 9 segments; pygidium semicircular, elongated in width; axis very wide, of moderate length, convex, and consists of 7-11 rings; lateral lobes convex, bearing up to nine pairs of pleural ribs (usually 5-6); no border furrow; surface of pygidium smooth.
Comparison (with Permian genera of the Cummingellinae). It differs from the closely related Bedicella Hahn and Hahn, 1990 in having smaller eyes, a longer pygidium, and the absence of a border furrow on it. It differs from Cummingella Reed, 1942 in the less pronounced S2 -S4 -furrows of the glabella, the L1-lobes more elongated towards the occipital ring, the absence of a border furrow on the pygidium and a relatively wider axis.
Remarks. In a previous work (Mychko, 2012), the author reviewed the Paraphillipsia species described by Tumanskaya (1935) from the Guadalupian olistoliths of Crimea. According to the results of this study, the species P. kussicum, P. netschaewi and the variety P. tauricum var. anfensis were synonymized with the species P. taurica, since they do not have significant morphological differences from the latter. The authors of more recent studies agree with this opinion (e.g., Schraut, 2019).
Species. Eleven species and four species determined in open nomenclature (Table 2).
Occurrence. Cisuralian (Artinskian) – Lopingian (Changhsingian); Eurasia (Slovenia, Austria, Crimea, Tajikistan, China, Laos and Japan).
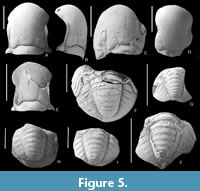
Paraphillipsia urushtensis sp. nov.
Figure 5A-J, Figure 6F, G
zoobank.org/96354C7C-395A-4287-BB25-E02084B3368C
 1944 Paraphillipsia karpinskyi – Weber, 1944, p. 12, pl. I, figs. 18-20, 22.
1944 Paraphillipsia karpinskyi – Weber, 1944, p. 12, pl. I, figs. 18-20, 22.
2003 Paraphillipsia aff. karpinskyi – Owens, 2003, Text-fig. 3 F,G.
2008 Paraphillipsia karpinskyi – Hahn and Hahn, 2008, Abb. 332-335.
2008 non Paraphillipsia karpinskyi – Hahn and Hahn, 2008, Abb. 331.
2016 nomen nudum Paraphillipsia uruschtensis – Mychko, 2016, p. 190-192, pl. III, figs. 3-6.
2017 Paraphillipsia karpinskyi – Mychko and Alekseev, p. 68.
Holotype. CNIGRmuseum, No. 62/5217, cephalon; Belalabino Group, Changhsingian, Lopingian; vicinity of the Urushten (outcrop No. 309), Malaya Laba River Basin Krasnodar Krai, Russia; selected here as the specimen with the best preservation.
Etymology. By the name of the Urushten.
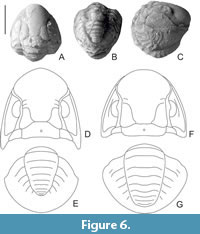 Description. Сephalon oval, laterally flattened; wide glabella occupies majority of cephalon; glabella “cummingellid” in shape (has a constriction in the middle, and is slightly wider in the anterior part than in the posterior part); slightly swollen in front, descends steeply to anterior border, overlapping it; in posterior part of glabella long; barely noticeable L 1 -lobes, quite wide, extending with their posterior edges onto occipital ring; on some specimens the second glabellar furrows (S2) barely visible; facial sutures very close to glabella; eyes large, long, bean-shaped, highly raised, occupying space from posterior end of librigena to anterior edge of glabella, where it bends towards border; palpebral lobes narrow; occipital ring long, narrow, with small median tubercle; librigenae steeply declined from glabella, with rounded genal angles; on surface of cephalon, especially glabella, very small tubercles visible, scattered in a checkerboard pattern; pygidium semicircular with broad axis bounded by distinct deep dorsal lateral furrows, gradually narrowing towards posterior edge; consists of nine clear rings; lateral lobes convex, with six pairs pleural ribs; interpleural furrows extend only to middle of lobes; pleural furrows indistinguishable; border furrow wide.
Description. Сephalon oval, laterally flattened; wide glabella occupies majority of cephalon; glabella “cummingellid” in shape (has a constriction in the middle, and is slightly wider in the anterior part than in the posterior part); slightly swollen in front, descends steeply to anterior border, overlapping it; in posterior part of glabella long; barely noticeable L 1 -lobes, quite wide, extending with their posterior edges onto occipital ring; on some specimens the second glabellar furrows (S2) barely visible; facial sutures very close to glabella; eyes large, long, bean-shaped, highly raised, occupying space from posterior end of librigena to anterior edge of glabella, where it bends towards border; palpebral lobes narrow; occipital ring long, narrow, with small median tubercle; librigenae steeply declined from glabella, with rounded genal angles; on surface of cephalon, especially glabella, very small tubercles visible, scattered in a checkerboard pattern; pygidium semicircular with broad axis bounded by distinct deep dorsal lateral furrows, gradually narrowing towards posterior edge; consists of nine clear rings; lateral lobes convex, with six pairs pleural ribs; interpleural furrows extend only to middle of lobes; pleural furrows indistinguishable; border furrow wide.
Dimensions (Table 3, Table 4).
Comparison. It is closest to Paraphillipsia karpinskyi (Figure 6A-E), but differs somewhat in the shape of the glabella: the median constriction at P. urushtensis is not as noticeable as in P. karpinskyi; L1 -lobes of the former are somewhat larger than those of the latter and extend further onto the occipital ring. The eyes of P. urushtensis are narrower and longer, and librigenae of P. karpinskyi are wider. The pygidia both species are very similar, but the axis of P. urushtensis is comparatively longer, has a constriction, and does not taper as strongly towards the posterior as P. karpinskyi. The end of the axis at P. karpinskyi is more pointed than in P. urushtensis. Moreover, the axis of P. urushtensis consists of a smaller number of segments (in P. karpinskyi 10 axial rings are visible). As far as can be seen from the holotype of P. karpinskyi, the distance from the end of the axis to the edge of the pygidium at P. urushtensis is slightly less. It differs from P. vnweberi mainly in the morphology of the pygidium: which is wider, a shorter and narrower axis, more distinct interpleural furrows and more distinct furrows between the axial rings, as well as less segmentation of the axis (P. vnweberi has 10 rings and six pleural ribs). Also, the glabella of P. vnweberi has more obvious S 2 -S3 pairs of furrows. It differs from P. taurica in less pronounced S2-S4 pairs of glabellar furrows, the absence of an S4 pair, a different shape of L1 -lobes, the absence of obvious tuberculation on the exoskeleton, a smaller number of axial rings (P. taurica has 9-11 rings), a wider pygidium, a shortened axis, a smaller number of pleural ribs (P. taurica has 5-7 pairs of ribs) and shallower pleural furrows and furrows on between the axial rings. Pygidium of P. urushtensis sp. nov. similar to P. baltensis, but differs in a large number of pleural ribs (the latter has only four pairs of noticeable ones). From P. tschernyschewi it differs a wider pygidium, an elongated axis, many axial rings (P. tschernyschewi has seven rings) and a large number of pleural ribs (P. tschernyschewi has three pairs of ribs). Comparatively P. urushtensis differs strongly from P.? sinensis in its glabella shape and less developed L1 -lobes. The pygidia are similar, but more elongated in length (the ratio of length to width of the pygidium in P.? sinensis is 0.7). Axis of P.? sinensis has fewer rings (7-8). It differs from P. pahara in having a more convex glabella, a less raised occipital ring, and also (apparently) in the presence of rudimentary of S2 -glabellar furrows, which are reduced in P. pahara. It differs greatly from P. levigata in the shape of the L1 -lobes, which are more elongated in the new species. The pygidium of P. urushtensis is distinguished by a smaller number of axial rings (in P. levigata there are 9-10) and pleural ribs (in P. levigata there are 7-8). It is similar to P. ? carnica but differs in a smaller number of axial rings (the latter has 10 rings). From P.? sp., described by Weber (1944, p. 12, pl. 1, fig. 21a,b), differs by a smaller number of axial rings and pleural ribs (Weber’s species has >7 axial rings and most likely more than five pairs of ribs) and weaker interpleural furrows. From P.? sp., aff. P.? taurica, described by Hahn and Hahn (1970), is different by a large number of axial rings (in P.? aff. taurica has seven axial rings). From another P.? sp., also described by Hahn and Hahn (1970), differs in having the absence of an obvious border furrow (which, by the way, apparently may exclude the relation of this species to Paraphillipsia). It is rather difficult to compare with P. middlemissi, since we only have a drawing (Diener, 1897, pl. I, fig. 3a-b), but the number of axial rings and pleural ribs correspond to those of P. urushtensis sp. nov.
Remarks. Despite minor differences in morphology between Paraphillipsia karpinskyi and P. urushtensis sp. nov. I cannot attribute them to the same species, since the deposits from which their type series originate represent different stratigraphic intervals (the Roadian of the Guadalupian and the Changhsingian of the Lopingian). The interval between the formation of these deposits and the existence of these species is about or more than 10 Ma. More likely, P. urushtensis sp. nov. is a close relative descended from P. karpinskyi. It is worth noting that we do not have complete exoskeletons of P. urushtensis sp. nov., and we cannot with full confidence attribute the discussed pygidia (Table 5) to this species.
Occurrence. Lopingian, Changhsingian; Russia (Krasnodar Krai, North Caucasus, Malaya Laba River Basin).
Material. Nine specimens (Table 5).
Subfamily BOLLANDIINAE Hahn and Brauckmann, 1988
Genus KATHWAIA Grant, 1966
1966 Kathwaia – Grant, p. 69-71.
1967 Kathwaia – Hahn and Hahn, p. 336, 337, 343, 345, 346.
1970 Kathwaia – Hahn and Hahn, p. 231.
1975 Kathwaia – Hahn and Hahn, p. 16,17,61.
1980 Kathwaia – Haas, Hahn, and Hahn, tab. 8.
1983 Kathwaia – Owens, p. 16,17, 36, 37.
1984 Kathwaia – Hahn, Hahn, and Brauckmann, p. 66,67.
1984a Kathwaia – Kobayashi and Hamada, p. 23, 25, 28, 84.
1985 Kathwaia – Kobayashi and Hamada, p. 282.
1988 Kathwaia – Hahn and Brauckmann, p. 121,126.
1989b Kathwaia – Hahn, Hahn, and Yuan, p. 174,175.
1992 Kathawaia [sic!] – Brezinski, p. 927.
1993 Kathwaia – Owens and Hahn, p. 174,175.
2001 Kathwaia – Hahn, Hahn, and Brauckmann, p. 271, 272, 274.
2003 Kathwaia – Jell and Adrain, p. 391,477.
2003 Kathwaia – Owens, p. 380, 386, 388, 391.
2012 Kathwaia – Lerosey-Aubril and Feist, p. 551.
2015 Kathwaia – Hahn and Hahn, p. 3, 6,11,15,18,19, 20,103-104,109.
2016 Kathwaia – Mychko, 2016, p. 38,178-181.
Types species. Kathwaia capitorosa Grant, 1966 (= K. caucasica (Weber, 1944)); Lopingian; Pakistan (Kathwai-Kushab, Salt Range) and Russia (Malaya Laba River Basin, Krasnodar Krai).
Diagnosis. Сephalon subtriangular, semi-elliptical; glabella strongly swollen, hangs vertically and overlaps anterior border; large, separate and distinct L1 -lobes; fixigenae narrow; small eyes sickle-shaped; sculpture often represented by large tubercles scattered; pygidium elongated; pygidial axis consists of 7-9 rings, lateral lobes have 6-9 pairs of pleural ribs; pleural ribs ornamented with two rows of tubercular.
Comparison. The genus is similar to Bollandia Reed, 1943 but differs in reduced S2 -S 4 pairs of glabellar furrows, more distinct L1 -lobes, smaller eyes and palpebral lobes, and the presence of tubercle ornamentation on the exoskeleton. It differs from Neoproetus Tesch, 1923 in having distinct and more distinct L1 -lobes, deeper and wider S1 -furrows, the absence of a wrinkled structure on the surface of the glabella, and the presence of numerous tubercles on the exoskeleton. It differs from Neogriffithides Toumansky, 1930 in reduced S2 -S4 -pairs of glabellar furrows, more isolated L1 -lobes, stronger and coarser tuberculation of the cephalon and pygidium, and smaller eyes. It differs from Carbonoproetus Gandl, 1987 in the shape of the glabella which is closer to conical and flatter, reduced S 2 -S4 -pairs of glabellar furrows, and more isolated L1 -lobes. It differs from Reediella Osmólska, 1970 in the shape of the glabella, which is closer to conical and less swollen, reduced S2 -S4 -pairs of glabellar furrows (in Reediella the S2 and S3 pairs are highly developed), more isolated L1 -lobes, and less segmentation of the pygidium.
Species. Four species and one species determined in open nomenclature (Table 6).
Occurrence. Cisuralian (?) – Lopingian (Changhsingian); Crimea and North Caucasus, Greece (?), India (?), China (?) and Pakistan.
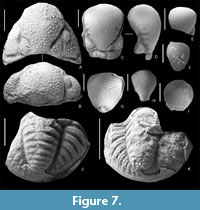
Kathwaia caucasica (Weber, 1944)
Figure 7A-K, Figure 8A-H
 1932 nomen nudum Proetus ? semipustulatus – Weber in Robinson, p. 23.
1932 nomen nudum Proetus ? semipustulatus – Weber in Robinson, p. 23.
1944 Proetus (?) girtyi caucasica – Weber, p. 15, pl. II, figs. 16, 17.
1944 Griffithides (Neogriffithides) cf. almensis – Weber, p. 11, pl. I, figs. 15, 16.
1966 Kathwaia capitorosa – Grant, p. 71-72, pl. 13, fig. 1 a-d.
1970 Kathwaia capitorosa – Hahn and Hahn, p. 231.
1970 Kathwaia sp – Hahn and Hahn, p. 233.
1970 Kathwaia girtyi caucasica – Hahn and Hahn, p. 232.
1970 Kathwaia sp – Hahn and Hahn, p. 233.
1975 Kathwaia capitorosa – Hahn and Hahn, p. 17, 61, pl. 12, fig. 1 a-b.
1983 Kathwaia capitorosa – Owens, p. 17, pl. 2, figs. 1-4.
1983 Proetus (?) girtyi – Owens, p. 17.
1983 Griffithides (Neogriffithides) cf. almensis – Owens, p. 17.
1984a Kathwaia capitorosa – Kobayashi and Hamada, p. 22, 29.
1984a Kathwaia girtyi caucasica – Kobayashi and Hamada, p. 25.
1987 Kathwaia capitorosa – Kobayashi and Hamada, p. 141.
 1988 Kathwaia capitorosa – Hahn and Brauckmann, pl. 2, figs. 20-21.
1988 Kathwaia capitorosa – Hahn and Brauckmann, pl. 2, figs. 20-21.
1989b Kathwaia capitorosa – Hahn, Hahn, and Yuan, 153.
2001 Kathwaia capitorosa – Hahn, Hahn, and Brauckmann, p. 275, 276, 294, pl. 2, fig. 1 a-d.
2003 Kathwaia capitorosa – Jell and Adrain, p. 391.
2003 Kathwaia capitorosa – Owens, p. 380, text-fig. 3 A-B.
2015 Kathwaia caucasica – Hahn and Hahn, p. 106-107, Abb. 110.
2015 Kathwaia capitorosa – Hahn and Hahn, p. 6, 9,103,104-106,107, Abb. 108-109.
2016 Kathwaia caucasica – Mychko, p. 180-181, pl. II, figs. 17-20.
2017 Proetus girtyi var. caucasica – Mychko and Alekseev, p. 68.
Lectotype. CNIGRmuseum, No. 88/5217, incomplete pygidium; Lopingian, Changhsingian, Urushten Formation; Russia, Krasnodar Krai, Malaya Laba River Basin, vicinity of the Urushten; designated by Hahn and Hahn (1970, p. 232).
Paralectotype. CNIGRmuseum, No. 53/5217, cephalon; Lopingian, Changhsingian, Urushten Formation; Russia, Krasnodar Krai, Malaya Laba River Basin, Gefo Mountain, blocks along the Tegen’ River, outcrop No. 30; designated here.
Hypotype. USNM PAL 145320, complete enrolled exoskeleton; Lopingian, Wuchiapingian, Wargal Formation (=Wargal Limestone or Middle Productus Limestone); Pakistan, Salt Range, Kathwai-Kushab; holotype of Kathwaia capitorosa Grant, 1966.
Description. Сephalon elongated; glabella strongly swollen, hangs vertically and overlaps narrow and convex anterior border; L 1 -lobes distinct, large, subtriangular, separated by deep S 1 -furrows; S2 -S4 -furrows not noticeable (but on casts above L1 -lobes, in middle part of glabella, three pairs of small swellings, which obviously L2 -L4 -lobes); occipital ring relatively narrow, with median tubercle; fixigenae very narrow, as facial sutures located close to dorsal furrows; palpebral lobes small and do not completely cover visual surfaces of eyes; eyes small, smaller than L 1 -lobes; librigenae convex and have deep border furrow, sharply separating lateral border; cephalon ends at rounded genal angles; surface of cephalon, except for anterior border, covered with large, closely spaced tubercles of same size; anterior border with terrace lines; thorax consists of nine segments; on dorsal side of axial rings row of small tubercles; pygidium relatively large, elongated and semi-elliptical; axis subtriangular-rounded in cross-section, strongly convex, shortened and relatively wide: the ratio of width of pygidium to width of axis in anterior part – 2:4; it tapers slightly towards posterior end, where it terminates bluntly, not reaching pygidial border; it hase nine rings separated by deep furrows; lateral lobes slightly convex; they contain 7-8 pairs of pleural, distinct ribs, separated by deep interpleural furrows; pleural ribs with slight backward bend; pleural furrows distinct, dividing pleural ribs into two parts equal in width; ribs with two rows of large tubercles (from five to 10); border furrow absent and pleural ribs merge into pygidial border, ornamented with thin terrace lines.
Dimensions (Table 7, Table 8).
Comparison. Very similar to Kathwaia girtyi (Figure 8J, K), but differs in relatively larger sizes (pygidia of K. girtyi are less than 3 mm wide), a different shape of the axis (in K. girtyi it is shorter and tapers more strongly towards the posterior end), a large number of axial rings and pleural ribs (K. girtyi has about eight rings and six pairs of pleural ribs), as well as a smaller number of larger, densely located tubercles on the pleural ribs. It differs from K. sinensis in the less pronounced tuberculation of the cranidium, smaller L1 -lobes and a more convex glabella.
Remarks. Part of the type series of the species under discussion (pygidia, specimen CNIGRmuseum, No. 87 and 88/5217) were first described by Weber (1944, p. 15) as a subspecies Proetus (?) girtyi var. caucasica. By that time, the cephala (CNIGRmuseum, No. 53-59/5217) were provisionally attributed to Weber (1944, p. 11) to another species Griffithides (Neogriffithides) cf. almensis.
Later pygidia Proetus (?) girtyi var. caucasica and cephala Griffithides (Neogriffithides) cf. almensis were described by Hahn and Hahn (1970, pp. 232-233) as Kathwaia girtyi caucasica and Kathwaia sp. respectively. And in a newer revision (Hahn and Hahn, 2015), the subspecies Kathwaia girtyi caucasica was identified as an independent species Kathwaia caucasica, and the cephala of Kathwaia sp. (= Griffithides (Neogriffithides) cf. almensis) were assigned to Kathwaia capitorosa.
Since both pygidia and cephala come from coeval deposits of the North Caucasus, I believe that they most likely belong to the same species. Moreover, the identical morphology of cephala from the Changhsingian of the North Caucasus with that of the Pakistani Kathwaia capitorosa allows us to consider the latter a junior synonym of Kathwaia caucasica. Small differences in the pygidium (North Caucasian pygidia have one more pair of pleural ribs) can be considered as intraspecific variability due to insufficient material.
It is worth noting that on some glabella moulds (Figure 7C, D, H) small, faintly defined lobes L2 -L 4 visible. However, these are not observed on specimens with a exoskeleton. The absence of lobes on the glabella (except L1) is one of the main diagnostic characters of Kathwaia. Apparently, we are observing an incompletely reduced trait inherited from ancestral forms (Hahn and Hahn, 2015, Abb. 4), such as the Mississippian genus Bollandia.
Occurrence. Lopingian, Wuchiapingian-Changhsingian; Russia (Krasnodar Krai, North Caucasus) and Pakistan.
Material. 10 specimens (Table 9).
Subfamily DITOMOPYGINAE Hupé, 1953
Genus PSEUDOPHILLIPSIA Gemmellaro, 1892
1892 Pseudophillipsia - - Gemmellaro, p. 21.
1930 Pseudophillipsia – Toumansky, 1930, p. 474,477.
1933 Griffithides (Pseudophillipsia) – Weber, 1933, p. 9,10,12-17,46-48,57.
1935 Pseudophillipsia – Tumanskaya, 1935, p. 24-25.
1935 Pseudophillipsia – Weller, p. 34.
1937 Griffithides (Pseudophillipsia) – Gheyselinck, 1937, p. 49, 50, 51, 53-55,59.
1939 Pseudophillipsia – Licharew, p. 198.
1944 Pseudophillipsia – Teichert, p. 457-458.
1944 Pseudophillipsia – Weber, p. 5-6.
1944 Pseudophillipsia – Weller, p. 324-325.
1955 Pseudophillipsia – Hupé, p. 210.
1957 Pseudophillipsia – Goldring, p. 197-201, 201-202.
1959 Pseudophillipsia – Weller, p. O402-403.
1960 Pseudophillipsia – Maximova, p. 140.
1970 Pseudophillipsia – Hahn, Hahn, and Ramovš, p. 314.
1970 Pseudophillipsia – Hahn and Hahn, p. 165, 303-304.
1974 Pseudophillipsia – Termier and Termier, p. 260.
1975 Pseudophillipsia – Hahn and Hahn, p. 15,17, 67, 83.
1975 Pseudophillipsia (Pseudophillipsia) – Hahn and Brauckmann, p. 119;
1977 Pseudophillipsia – Qian, 1977, p. 279-280.
1983 Pseudophillipsia – Owens, p. 28-29.
1984a Pseudophillipsia (Pseudophillipsia) – Kobayashi and Hamada, p. 17, 20,51, 52, 56.
1984a Pseudophillipsia (Nodiphillipsia) – Kobayashi and Hamada, p. 9,15,16, 20, 51, 52, 58, 83.
1993 Pseudophillipsia (Nodiphillipsia) – Owens and Hahn, p. 174.
1998 Pseudophillipsia (Nodiphillipsia) – Ishibashi, p. 226.
2001 Pseudophillipsia (Pseudophillipsia) – Hahn, Hahn, and Brauckmann, p. 272-273.
2001 Pseudophillipsia (Nodiphillipsia) – Hahn, Hahn, and Brauckmann, p. 273-274.
2003 Pseudophillipsia – Owens, p. 382, 385, 388.
2003 Pseudophillipsia – Jell and Adrain, p. 434,477.
2003 Nodiphillipsia – Jell and Adrain, p. 412,477.
2009 Pseudophillipsia (Nodiphillipsia) – Lerosey-Aubril and Angiolini, p. 433-438.
2011 Pseudophillipsia (Pseudophillipsia) – Gandl, p. 95-98.
2012 Pseudophillipsia – Lerosey-Aubril, p. 10.
2015 Pseudophillipsia (Nodiphillipsia) – Fortey and Heward, p. 208.
2016 Pseudophillipsia (Pseudophillipsia) – Mychko, p. 46, 253-254.
2016 Pseudophillipsia (Nodiphillipsia) – Mychko, p. 47,61, 253.
2020 Pseudophillipsia (Nodiphillipsia) – Schraut, p. 214.
2021 Pseudophillipsia (Nodiphillipsia) – Flick and Shiino, p. 117.
Type species. Phillipsia sumatrensis Roemer, 1880; Guadalupian, Wordian; Indonesia, Sumatra; designated by Hahn and Brauckmann (1975, p. 118).
Diagnosis. Exoskeleton elongated; cephalon semi-elliptical in outline, ending in medium or long genal spines; in some species latter may have a spatulate shape; eyes medium to large, bean-shaped; behind glabella lateral and unpaired (medial) preoccipital lobes; in posterior part of glabella distinctive “festoon structure” formed by three pairs of L 2 -L4, typically these convex, well separated, and semicircular; surface of glabella, apart from lobes, usually smooth; number of thoracic segments – 9; pygidium elongated, oval-triangular; pygidial axis has ~20-27 rings separated by distinct furrows and has lateral constriction; pleural ribs – 10-17.
Comparison. From the closely related subgenus Ditomopyge (Carniphillipsia) is differs by the presence of a “festoon structure” in the posterior part of the glabella, formed by isolated L2 -L4 lobes, and also, sometimes, by a larger number of axial rings at pygidium. It differs from Acropyge in the less triangular pygidium and the absence of a post-axial ridge behind the axis. Similar to Anisopyge, but differs in a different shape of the glabella, more isolated preoccipital lobes, a less triangular shape of the pygidium, and a smaller number of axial rings (in the latter their number reaches 33).
Discussion. Members of Pseudophillipsia are characterized mainly by the presence of a so-called “festoon structure” in the posterior part of the glabella, formed by the lobes L2 -L4. This character, as well as the highly segmented pygidium, have long been the main distinguishing characters of this genus from other members of the subfamily Ditomopyginae, in particular the nominative genus Ditomopyge, widespread in the Late Pennsylvanian and Cisuralian and surviving until the Lopingian.
In 1965, Gauri (1965) described several Pseudophillipsia species from the Upper Pennsylvanian of the Carnic Alps (Austria), particularly Pseudophillipsia ogivalis, which has a highly segmented pygidium (18+ axial rings and 10 pleural ribs). However, glabella of Ps. ogivalis does not have L2 -L4 -lobes, which makes it more similar to Ditomopyge. Gauri noted (1965, p. 13) that the species he identified appears to be a transitional form between the earlier genus Ditomopyge and the later Pseudophillipsia.
Later, Hahn and Brauckmann (1975) divided the genus Pseudophillipsia into two subgenera: Ps. (Pseudophillipsia) and Ps. (Carniphillipsia). Type species of the latter subgenus is Ps. ogivalis. They noted that the anterior glabellar furrows (S2 -S4) at Ps. (Carniphillipsia) weakly incised or absent, but preoccipital (lateral and medial) lobes very distinct. In their opinion, the pygidia of Ps. (Pseudophillipsia) and Ps. (Carniphillipsia) differed in the degree of segmentation: Ps. (Pseudophillipsia) – has 20-27 axial rings and 13-17 pleural ribs, at Ps. (Carniphillipsia) – 17-21 axial rings and 9-13 pleural ribs.
That Carniphillipsia can be considered a subgenus of both Pseudophillipsia and Ditomopyge has been noted previously (e.g., Owens, 1983, p. 28). Gandl (2011, p. 72) made a detailed argument for Carniphillipsia is a subgenus of Ditomopyge. Both the author of this work, and recent publications (Mychko and Alekseev, 2018), and other authors (e.g., Schraut, 2020, p. 211) agree with this opinion.
The differences between Ditomopyge (Carniphillipsia) and Pseudophillipsia can only be observed in the structure of the cephalon; the pygidia of both taxa cannot serve as a reliable element for identification. Despite the opinion that the pygidia of Ditomopyge (Carniphillipsia) are less segmented than those of Pseudophillipsia, which is given in various works (e.g., Hahn and Brauckmann, 1975; Kobayashi and Hamada, 1984a), there are species among Ditomopyge (Carniphillipsia), which may even have 22-25 axial rings and up to 15 pairs of pleural ribs. This high degree of segmentation of the pygidium is quite consistent with that of Pseudophillipsia.
This raises the fundamental and important problem of identifying Permian trilobites solely from pygidia. Previously, researchers, having no remains of cranidia, classified one or another highly segmented pygidium as Pseudophillipsia in the broad sense (sensu lato). A similar record – Pseudophillipsia (s.l.) – can be seen, for example, in the work of Lerosey-Aubril (2012), which meant that the pygidium under study can be attributed to any of the subgenera of Pseudophillipsia. However, if we adhere to the opinion that Carniphillipsia belongs to the genus Ditomopyge, such a record becomes inappropriate. Therefore, I propose to classify species and species determined in open nomenclature known only from pygidia into the genus Pseudophillipsia conditionally, with a question mark. In some ways, Pseudophillipsia becomes a “junk taxon”, which includes representatives of Ditomopyge (Carniphillipsia), for which the cephalon is unknown. But this is a temporary solution until reliable new discoveries of cephala are made.
Almost 10 years after the description of Pseudophillipsia (Carniphillipsia), Kobayashi and Hamada (1984b) described another subgenus of Pseudophillipsia – Ps. (Nodiphillipsia). Type species of this, Ps. (Nodiphillipsia) spatulifera from the Guadalupian of Japan. According to the authors, the only and most important difference between the new subgenus and Ps. (Pseudophillipsia) was what Ps. (Nodiphillipsia) L 2 -L4 -lobes of the glabella were small swollen nodules. The number of axial rings (23) and pleural ribs (17-18) at spatulifera was quite consistent with that in representatives of Pseudophillipsia. It is interesting that in Ps. (Nodiphillipsia) Kobayashi and Hamada classified a number of species, including described in this article Ps. solida.
However, Kobayashi and Hamada did not take into account that the type material of all species they classified as Pseudophillipsia (Nodiphillipsia) is represented by casts. And the lobes of the glabella, which appear to be knots or nodules, are only the result of conservation. This was noted by Hahn, Hahn and Brauckmann (2001, p. 273). It was clarified that the type species, Ps. (Nodiphillipsia) spatulifera has special (highly specialized) spatulate-shaped genal spines, which are unusual for other representatives of Pseudophillipsia.
This feature made it possible to retain Pseudophillipsia (Nodiphillipsia) in the work of Lerosey-Aubril and Angiolini (2009), where the authors clarified the diagnosis of Ps. (Nodiphillipsia), reducing it exclusively to spatulate genal spines. Ps. (Nodiphillipsia) spatulifera was assigned to this subgenus and the species described in their article – Ps. (Nodiphillipsia?) aff. obtusicauda. Moreover, the species obtusicauda was assigned to Ps. (Nodiphillipsia?) is conditional, and in some places in this publication the type species spatulifera belongs [sic!?] to the subgenus Ps. (Pseudophillipsia).
It is important to understand that neither the holotype of Pseudophillipsia (Nodiphillipsia) obtusicauda, nor on the type material of Ps. (Nodiphillipsia) aff. obtusicauda has no preserved genal spines, so it is difficult to compare their structure with that of Ps. (Nodiphillipsia) spatulifera.
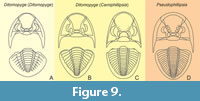 The presence of Ps. (Nodiphillipsia) is apparently redundant, and its distinctive feature in the form of spatulate genal spines is at the species level, not the generic level, since the generic taxonomy of proetids is based solely on the morphology of the cranidium and then the pygidium. All types of Ps. (Nodiphillipsia) should be classified as Pseudophillipsia, and the subgenus itself should be synonymized with the genus Pseudophillipsia, which is no longer divided into subgenera in this work (Figure 9).
The presence of Ps. (Nodiphillipsia) is apparently redundant, and its distinctive feature in the form of spatulate genal spines is at the species level, not the generic level, since the generic taxonomy of proetids is based solely on the morphology of the cranidium and then the pygidium. All types of Ps. (Nodiphillipsia) should be classified as Pseudophillipsia, and the subgenus itself should be synonymized with the genus Pseudophillipsia, which is no longer divided into subgenera in this work (Figure 9).
Undoubtedly, it is necessary to conduct a detailed revision of all known species and species determined in open nomenclature of Pseudophillipsia, of which more than 46 are already known (Table 10). Some of them are described exclusively from pygidia and may well be representatives of Ditomopyge (Carniphillipsia).
Species. 42 species and five species determined in open nomenclature (Table 10).
Occurrence. Pennsylvanian (Moscovian) – Lopingian (Changhsingian); Eurasia and Africa (Tunisia).
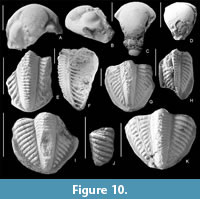
Pseudophillipsia solida Weber, 1944
Figure 10A-D
1944 Pseudophillipsia (?) solida – Weber, p. 13-14, pl. II, fig. 8,9.
1957 Delaria solida – Goldring, p. 197.
1970 Pseudophillipsia solida – Hahn and Hahn, p. 304, 314-315.
 1970 Pseudophillipsia solida – Hahn, Hahn, and Ramovš, p. 314-316, pl. 1, fig. 4, abb. 2.
1970 Pseudophillipsia solida – Hahn, Hahn, and Ramovš, p. 314-316, pl. 1, fig. 4, abb. 2.
1975 Pseudophillipsia solida – Hahn and Hahn, p. 17.
1983 Pseudophillipsia solida – Owens, p. 28.
1984a Pseudophillipsia (Nodiphillipsia) solida – Kobayashi and Hamada, p. 24,25,28.
1984a Pseudophillipsia (Pseudophillipsia) solida – Kobayashi and Hamada, p. 51.
1984a Pseudophillipsia (?) solida – Kobayashi and Hamada, p. 58.
2001 Pseudophillipsia (Pseudophillipsia) solida – Hahn, Hahn, and Brauckmann, S. 273,276.
2016 Pseudophillipsia (Pseudophillipsia) solida – Mychko, p. 263-264, pl. VI, fig. 6, 7.
2017 Pseudophillipsia (?) solida – Mychko and Alekseev, p. 68.
2020 Pseudophillipsia (sensu lato) solida – Schraut, 2020, p. 217, 218.
Lectotype. CNIGRmuseum, No. 79/5217, cephalon; Changhsingian, Lopingian; outcrop No. 127, 3,05 km from the estuary of the Urushten River, Malaya Laba River Basin, Krasnodar Krai, Russia; Weber, 1944, pl. II, fig. 8a-c; designated by Hahn and Hahn (1970, p. 314).
Description. The cephalon is semi-elliptical, elongated. Genal spines apparently existed, but are unknown. The glabella is long, pear-shaped, strongly tapering towards the border margin. The border furrow is almost invisible, so the glabella in anterior passes into a narrow anterior border, steeply descending to the ventral side. In the posterior part of the glabella there are three pairs of small swollen lobes L2 -L4. The medial preoccipital lobe is small, spherical and strongly convex; there are small teardrop-shaped lateral preoccipital lobes. The eyes are bean-shaped, large and high. The surface of the librigenae is convex, sharply defined by furrows from a broad border. The surface of the cephalon apparently contained no sculptural elements, with the exception of subtle terrace lines on the border.
Dimensions (Table 11).
Comparison. This species differs from other Lopingian representatives of Pseudophillipsia in the elongated glabella and almost complete reduction of the preglabellar furrow, causing the glabella to over hang the border furrow. However, in terms of the structure of the cranidium, the closest species (among the Lopingian) can be called Ps. hanaokensis Kobayashi et Hamada, 1984b.
Remarks. Apart from specimens of the type series Pseudophillipsia solida Weber, 1944, described from Changhsingian of the North Caucasus, the cranidium depicted in the work of Hahn et al. (1970, taf. 1, fig. 4, abb. 2) was assigned to this species. They compared the the Slovenian cranidium and found similarities not so much with the lectotype (CNIGRmuseum, No. 79/5217), but with the paratype (CNIGRmuseum, No. 80/5217). The preservation of both cranidia does not allow us to attribute them to Ps. solida.
The pygidium is unknown for this species. It is quite possible that pygidia Pseudophillipsia (?) caucasica Weber, 1944 or Ps. (?) mustafensis Tumanskaya, 1935, also known from the Changhsingian of the North Caucasus, may belong to this species. However, to test this hypothesis we need new finds, preferably complete exoskeletons, which we could confidently associate with Ps. solida Weber, 1944.
Occurrence. Changhsingian, Lopingian; Russia (Krasnodar Krai, North Caucasus) and Slovenia (vicinity of the village of Vrzdenec).
Material. Casts of the cephalon and two cranidia (Table 12).
Pseudophillipsia (?) caucasica Weber, 1944
Figure 10E-H
1944 Pseudophillipsia elegans Gemm. var.? caucasica – Weber, p. 5,6,12-13, pl. II, fig. 4.
1944 Pseudophillipsia elegans Gemm. var.? – Weber, p. 13, pl. II, fig. 2.
1957 Pseudophillipsia elegans Gemm. var.? caucasica – Goldring, p. 199.
1970 Pseudophillipsia elegans caucasica – Hahn and Hahn, p. 307.
1984a Pseudophillipsia elegans caucasica – Kobayashi and Hamada, p. 25,69.
2012 non Pseudophillipsia (s.l.) armenica – Lerosey-Aubril, 2012, p. 10, fig. 4 a.
2012 non? Pseudophillipsia (s.l.) caucasica – Lerosey-Aubril, 2012, p. 12.
2016 Pseudophillipsia (Pseudophillipsia) caucasica – Mychko, p. 62, 257-258, pl. VI, fig. 1, 2
Lectotype. CNIGRmuseum, No. 71/5217, pygidium; Changhsingian, Lopingian; blocks along the Tegen’ River, Gefo Mount, Krasnodar Krai, Russia; Weber, 1944, pl. II, fig. 4; designated by Hahn and Hahn (1970, p. 307).
Description. Pygidium semi-elliptical, slightly elongated; axis long, trapezoidal in cross-section, high, reaches the pygidial border, but not reaching it; in anterior part of pygidium it quite wide, slightly tapering posteriorly; consists of 25+ rings separated by deep distinct furrows; lateral sides of axis constricted in central part, which is why each of rings has knee-shaped bend towards anterior part of pygidium; on dorsal side of each of rings pair of swellings which resemble flattened tubercles; dorsal furrows obvious; lateral lobes slightly convex, relatively flattened; they consist of 11 pleural ribs, separated by deep interpleural furrows, widening towards pygidial border; in anterior part of pygidium, pleural ribs almost perpendicular to axis, but as they approach posterior edge they acquire longitudinal direction and sharp geniculate bend located on each rib closer to pygidial border; pleural furrows very narrow, barely noticeable; they observed on anterior ribs and located towards posterior side of each of ribs; no obvious sculpture on ribs; pygidial border wide and flattened; widest in posterior by part and decreasing towards anterior part of pygidium; terrace lines not noticeable.
Dimensions (Table 13).
Comparison. A very close species is Pseudophillipsia hanaokensis Kobayashi et Hamada, 1984b, as shown by the shape of the pygidium, and the number of axial rings and pleural ribs are equal in both species. The main difference between them is the wider pygidial border in Ps. (?) caucasica. From species determined in open nomenclature such as Ps. (?) hungarica (Schréter, 1948) and Ps. (?) cf. hungarica (Schréter, 1948), Ps. (?) aff. caucasica Weber, 1944 differs in a different number of segments, and most importantly, by the absence of single large tubercles on each of the pleural ribs. From Ps. (?) subcircularis Qian, 1977, which has a similar number of segments, differs by a narrower pygidium and a wider pygidial border.
Remarks. Lerosey-Aubril (2012, fig. 4a) shows the pygidium (holotype) of Pseudophillipsia (s.l.) armenica, described by Weber from the Wordian of Armenia, but the specimen label indicates that this specimen has the number CNIGRmuseum, No. 73/5217. This is undoubtedly an error: the specimen CNIGRmuseum, No. 73/5217 is a pygidium of Pseudophillipsia (?) caucasica (Table 14; Figure 10G), and the pygidium depicted by Lerosi-Aubril is actually numbered CNIGRmuseum, No. 75/5217.
In the same article (Lerosey-Aubril, 2012, p. 12) the Pseudophillipsia (s.l.) aff. caucasica from the Lopingian Nesen Formation of Iran is described. It is considered close to the North Caucasian species. He concludes the similarity between these species determined in open nomenclature partly from the fact that Weber (1944, p. 13 and table 2) mentions Pseudophillipsia caucasica in Armenia. However, Weber does not provide information about such a find anywhere else. The author of this article was also unable to find this specimen in the CNIGRmuseum collection No. 5217.
In Pseudophillipsia (s.l.) aff. caucasica from Iran, the smaller number of segments is striking (Pseudophillipsia (s.l.) aff. caucasica has more than 17 axial rings [apparently about 21-22] and about 10 pleural ribs, which is slightly less than in the North Caucasian species), as well as the presence in the Iranian species determined in open nomenclature large tubercles on the pleural ribs located at the geniculate bend, and then a number of small tubercles closer to the ends of the ribs. Also, the Iranian species does not have dorsal tubercles on the axial rings, similar to those of the North Caucasian. Similar morphological features are observed in the Slovenian Pseudophillipsia (?) cf. hungarica, but with some inconsistencies. For example, latter, like the North Caucasian one, has tubercles on the dorsal side of the axial rings, and single large tubercles on the pleural ribs are located closer to the dorsal furrows. Apparently, the Iranian species is either a new species, or is closely related of possibly an ontogenetic stage of another species, also found in Iran, but in another Lopingian Dalan Formation – Pseudophillipsia (?) armenica Weber, 1944, since it has similar morphological features.
The North Caucasian species Pseudophillipsia (?) caucasica Weber, 1944 is represented exclusively by pygidia, so it can most likely belong to the genus Pseudophillipsia or the subgenus Ditomopyge (Carniphillipsia). It is likely that these pygidia may even belong to Ps. solida Weber, 1944, known from the same localities. Moreover, the pygidia and cephala of Ps. hanaokensis are similar to those of Ps. (?) caucasica and Ps. solida respectively. Only the discovery of complete specimens of Ps. (?) caucasica and Ps. solida can resolve to this issue.
Occurrence. Changhsingian, Lopingian; Russia (Krasnodar Krai, North Caucasus).
Material. Four pygidia (Table 14).
Pseudophillipsia (?) cf. mustafensis Tumanskaya, 1935
Figure 10I-K
1944 Pseudophillipsia mustafensis ? – Weber, 1944, p. 13, pl. II, fig. 3.
1970 [part.] Pseudophillipsia mustafensis – Hahn and Hahn, S. 309.
2016 [part.] Pseudophillipsia (Pseudophillipsia) mustafensis – Mychko, p. 260.
Description. Large pygidium, elliptical in shape, elongated; axis convex, long, reaching pygidial border and abutting against it; consists of 25 rings separated by narrow and deep furrows; axial rings geniculate on lateral sides of axis; on dorsal side of each of rings pair of small tubercles; lateral lobes of pygidium convex and bear 12 pleural ribs, separated by deep interpleural furrows; angle between pleural ribs and dorsal furrows hardly changes from anterior to posterior and ~30 degrees; each pleural rib ornamented with one row of medium-sized flattened tubercles; pygidial border wide and flattened; greatest width of pygidial border observed on lateral parts of pygidium, but decreases in posterior part.
Dimensions (Table 15).
Comparison. This pygidium is similar to Ps. (?) mustafensis Tumanskaya, 1935 (Figure 10K) from the Roadian of Crimea, however, the North Caucasian species determined in open nomenclature has a number of small tubercles on the pleural ribs, which are absent at the Crimean species. Also, the pygidial border of Ps. (?) cf. mustafensis is much wider than that of Ps. (?) mustafensis. Ps. (?) caucasica from coeval deposits of the North Caucasus differs primarily by the location of the pleural ribs in relation to the dorsal furrows: Ps. (?) caucasica has the posterior ribs that are almost parallel to the furrows, whilst in Ps. (?) cf. mustafensis their angle is close to perpendicular.
Occurrence. Changhsingian, Lopingian; Russia (Krasnodar Krai, North Caucasus).
Material. Two pygidia (Table 16).
LOPINGIAN TRILOBITE LOCALITIES
Currently, the Lopingian is divided into two stages, the Wuchiapingian and the Changhsingian, within the framework of the International Stratigraphic Scale (Figure 1). The stratotypes for both stages are located in China. The Wuchiapingian stratotype is located in the Penglaitan Section of Guanxi Province and the Changhsingian stratotype is located in the Meishan Section of Zhejiang Province. These two stratotypes were ratified in 2004 and 2005, respectively. In 2023, the lower boundary of the Wuchiapingian was revised and reaffirmed in the same region due to flooding at the original site at the Penglaitan Section.
The boundary between the Guadalupian (Capitanian) and Lopingian (Wuchiapingian) is defined by the appearance of the conodont Clarkina postbitteri postbitteri, which correlates with the major extinction of several Guadalupian groups of invertebrates, such as corals, fusulinids, ammonoids, brachiopods (Jin et al., 2006) and trilobites. The boundary between the Lopingian and the Lower Triassic is marked by the even more extensive extinction of groups – the Great Late Permian Extinction Event or EPME. This extinction event was also accompanied by various geochemical anomalies, magmatism of varying composition (Shen et al., 2019; and others), increasing ocean temperatures (Chen et al., 2020) and others phenomena.
The radioisotopic age of the lower boundary of the Lopingian, or and of the Wuchiapingian, is currently 259.51 ± 0.21 Ma. The base of the Changhsingian is 254.14 ± 0.07 Ma. And the Changhsingian-Triassic boundary is 251.90 ± 0.03 Ma (Permophiles, 2023, p. 49). Therefore, the duration of the Lopingian Epoch was approximately 7.6 Ma.
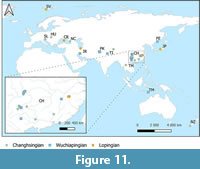 Lopingian deposits are widely distributed, occurring on all continents, and are represented by both marine and terrestrial strata. For the purposes of this article, we will be focusing on marine Lopingian deposits, in which trilobites are known. In total, there are approximately 34 known localities of this type (Table 17) located in 11 different countries (Figure 11).
Lopingian deposits are widely distributed, occurring on all continents, and are represented by both marine and terrestrial strata. For the purposes of this article, we will be focusing on marine Lopingian deposits, in which trilobites are known. In total, there are approximately 34 known localities of this type (Table 17) located in 11 different countries (Figure 11).
Slovenia. The most western Lopingian trilobites known were found to the east of Ljubljana in the area of the villages of Vrzdenec and Žažar (Hahn et al., 1970). These areas have Lopingian outcrops, from which Ramovš (1958a; 1958b) collected a rich marine fauna, associated with dark gray limestones. These deposits comprise as the Žažar Formation. Recent research suggests that this formation is identical to the Bellerophon Formation, which is widespread in the Carnic and Dolomite Alps in Austria and Italy (Kolar-Jurkovšek et al., 2018). According to their data, the presence of conodonts Hindeodus praeparvus conodonts in these formations allows us to correlate them with the uppermost part of the Changhsingian.
From outcrops of the Bellerophon Formation near the Vrzdenets Village there are two specimens of trilobites, represented by an incomplete cephalon with a pygidium of Pseudophillipsia n. sp. aff. sumatrensis (Roemer, 1880) and Pseudophillipsia solida Weber, 1944; near the Žažar Village – the pygidium of Pseudophillipsia (?) cf. hungarica (Schréter, 1948) (Hahn et al., 1970).
Unfortunately, no new trilobite finds have been reported from these localities in more than 50 years (Schraut, 2020, p. 217).
Hungary. Trilobites of Pseudophillipsia (?) hungarica (Schréter, 1948) from the Bükk Mountain in northeastern Hungary (Schréter, 1948) are found in black limestone, together with the brachiopods "Lyttonia nobilis" (Schréter, 1948). Currently this brachiopod species belongs to the genus Leptodus. For a long time, these finds were considered Guadalupian (e.g., Hahn and Hahn, 1970, p. 308; Kobayashi and Hamada, 1984a, p. 23), until Detre (1991) reported their Lopingian age, and also that that over 40 years a fairly extensive collection of trilobites (more than 100 specimens) from the deposits under discussion has accumulated. In this publication, Detre argues that the Lopingian trilobites from the Bükk Mountains represent the latest in Europe. He did not take into consideration, however, the publication by Hahn, Hahn and Ramovš (1970) describing trilobite remains from Slovenia. I note that Owens in his work (2003) indicates the Lopingian age for trilobites found in Hungary.
Indeed, Lopingian deposits are widespread in the Bükk Mountains. The Nagyvisnyó Formation, which consists of black limestone is most likely of Changhsingian (Posenato et al., 2005; Brookfield et al., 2021). The fact that trilobites occur in the upper part of the formation has also been indicated in more recent works (Brookfield et al., 2021, p. 80).
Crimea. Trilobites have been found in Late Palaeozoic blocks of Permian shallow-water organogenic limestones, up to 20-100 m in diameter. These are exposed on the northwestern side of the Crimean Mountains. The sandy-clay strata of the Eskiorda Formation (or Group), which dates back to the Upper Triassic-Lower Bajocian, are characterized by relatively shallow water facies of the Tauride Flysch Group.
Palaeozoic “rootless” limestone blocks were discovered in Crimea by Fokht (1901) and have been described in numerous publications. According to many researchers (e.g., Miklouho-Maclay and Muratov, 1958, p. 34), these blocks are parts of massifs that slid from uplifts into the immersion zone. Smaller boulders and pebbles are a result of their erosion.
The limestone of these blocks contains remains of a diverse marine invertebrate fauna, including fusulinids, ammonoids, trilobites and brachiopods; less common are bivalves and gastropods, solitary rugose corals and bryozoans (Grunt and Novikova, 2002).
Trilobites from these blocks have been known for quite a long time: these arthropods were discovered by Weber (1915), and later a number of species and species determined in open nomenclature were described by Tumanskaya (1930, 1935). There are also more recent finds (Mychko, 2012).
In total, there are three main localities in Crimea where trilobite have been found: the Kichkhi-Burnu blocks on the Marta River, the Dzhien-Sofu (=Totai-Koi) block on the Salgir River and blocks along the Alma River. Based on recent stratigraphic research (Pronina and Nestell, 1997; Kotlyar et al., 1999b), the first two block complexes, while most likely of Guadalupian, the blocks on the Alma River contain geologically younger limestones. They contain assemblages of small foraminifera and fusulinids characteristic of the upper Median-Dorashamian stage of the Tethyan scale. This roughly corresponds to the upper Capitanian-Changhsingian stages of the ICS (Leven, 2009).
However, the exact position and age of these blocks, from which trilobites were described by Tumanskaya, is now very difficult to determine due to changes in the landscape (since the time of her research, the area has been heavily forested, in 1966 the Partizansk Reservoir was constructed, and the exact locality is not known). Therefore, I consider the trilobite specimens described by Tumanskaya as very likely to be of the Lopingian.
In the boulders of the Alma River, Tumanskaya (1935, p. 10) found difficult-to-identify pygidia, which she conditionally assigned to the genus Pseudophillipsia.
North Caucasus. The stratigraphy of Lopingian (Changhsingian) trilobite localities in the North Caucasus is described in this article. The trilobite fauna of this area is relatively diverse and includes the following species: Brachymetopus (Acutimetopus) caucasicus Licharew in Weber, 1944, Paraphillipsia urushtensis sp. nov., Kathwaia caucasica (Weber, 1944), Pseudophillipsia solida Weber, 1944, Pseudophillipsia (?) caucasica Weber, 1944 and Pseudophillipsia (?) mustafensis Tumanskaya, 1935.
Far East. During a recent fieldwork (July 2024) by the author in the Russian Far East (Primorsky Krai), trilobites were discovered in Upper Permian here. The Nakhodka Reef locality is located in the Nakhodka city. It is one of the large carbonate bodies among the organogenic structures of the Guadelupian-Lopingian of Far East and has a complex structure. Trilobites were found in sandy limestones of the upper part, confined to the Lyudyanzian Substage (Lower Wuchiapingian). I have previously assigned these trilobites to genera Paraphillipsia and Neogriffithides (?). Herein, I only briefly report on them.
Iran. In this area, at least two localities for Lopingian trilobites are known, one of which is located in the north in the Alborz Mountains of Mazandaran province, near the village of Yush. Here Hahn and Hahn (1981) described three forms: Acropyge weggeni Hahn and Hahn, 1981, Acropyge ? sp. indet. and Iranaspidion sp. indet, which are represented by pygidia. In a subsequent revision of these specimens, Lerosey-Aubril (2012) assigned the Acropyge ? sp. indet. to the species Acropyge weggeni Hahn and Hahn, 1981, and identified the pygidium of Iranaspidion sp. indet as Pseudophillipsia (s.l.) aff caucasica Weber, 1944, closely related to the Norther Caucasian species.
The specimens are from the Nesen Formation, which is represented in the locality by wackestones with bivalves, brachiopods, bryozoans, crinoids, gastropods, ostracods and, in fact, trilobites (Lerosey-Aubril, 2012, p. 4). According to Lerosey-Aubril’s research, the foraminiferal assemblage belongs to the Wuchiapingian. However, later studies (Forel et al., 2015) indicate that the Nesen Formation is approximately 130 m thick and consists of marly and siliceous limestones. It is divided into lower and upper members. The first 10-15 m of the formation may belong to the upper part of the Upper Capitanian (Angiolini et al., 2010), while the rest of the lower part is characterized by the presence of Araxilevis intermedius Biozone, indicating an Early Wuchiapingian age for the formation. The presence of the conodont Hindeodus julfensis (Sweet) in this formation suggests a Late Wuchiapingian – Early Changhsingian age (Forel et al., 2015). However, it is not entirely clear which part of the formation the trilobite originates from.
From another locality in southern Iran, located on the Dena Ridge (Zagros Mountains, approximately 58 km northwest of Yasuj, Kohgilouye and Boyrahmad provinces), different species of Pseudophillipsia (?) parvizii Lerosey-Aubril, 2012 has been identified, described by well-preserved pygidia. The discovery was made in dark gray sandy wackestone along with gastropods, brachiopods, bryozoans, crinoids, ostracods and foraminifera (Lerosey-Aubril, 2012, p. 12). This wackestone belongs to the Dalan Formation, and the Wuchiapingian age of the deposits can be determined by the presence of the fusulinids, such as Codonofusiella ex gr. tenuissima (Lerosey-Aubril, 2012, p. 4).
Pakistan. In the Permian deposits, exposed on the Salt Range, two localities are known from which trilobites originate. They were discovered by Grant and Fatmi in 1963-64 and were later described by Grant (1966).
In the first locality, located between the villages of Zaluch Nala and Kala Wahan in the “Middle Productus limestone”, two enrolled exoskeletons of Ditomopyge fatmii Grant, 1966 were found. This species is classified as belonging to the subgenus Ditomopyge (Carniphillipsia) Hahn and Brauckmann, 1975 in this article.
According to recent research, the “Middle Productus limestone” is correlated with the upper part of the Wargal Formation (Sameeni, 2009, p. 69) and is estimated to be of Wuchiapingian age (Mertmann, 2003; Jin et al., 2006).
Another locality is located on the road between Kathwai and Kushab, approximately 9 km south of Kathwai. From here Grant (1966) described Kathwaia capitorosa by its well-preserved enrolled exoskeleton. In this work, it was considered to be a synonym for Kathwaia caucasica (Weber, 1944). This find also came from the Wargal Formation.
China. The largest number of localities and the number of Lopingian trilobites has been discovered in China. This is mainly due to the widespread distribution of the Lopingian sections here, their completeness, accessibility and their better study compared to other sections around the world.
Tibet. In the Himalayas, in Zanda County, Ngari Prefecture, pygidia were discovered and described by Diener (1897) as two new species: Cheiropyge himalayensis Diener, 1897 and Phillipsia middlemissi Diener, 1897. These findings come from isolated blocks Chitichun Limestone (Block No. 1).
The age of these limestone blocks is controversial. At the time of C. Diener, these formations were considered to be Permian-Carboniferous, but their ages have since been revised. Thus, Hahn, Hahn and Brauckman (2001) noted that the presence of ammonoids of the genus Cyclolobus here indicates the Wuchiapingian age of the Chitichun Limestone. However, representatives of this genus of ammonoids are also found in the Guadalupian (Leonova, 2010).
In modern stratigraphic studies (Shen and Shi, 2004), the Chitichun Limestone is considered to be a tentative Capitanian formation. However, the authors note that the collections of ammonoids, brachiopods and foraminifera from this formation include Wuchiapingian genera and species, and their stratigraphic reference requires clarification. In this work, I conditionally assign the Chitichun Limestone to the Wuchiapingian.
It is interesting that in the work of Hahn, Hahn and Brauckman (2001), only Cheiropyge himalayensis is indicated for the Lopingian of Tibet, and the second species Phillipsia middlemissi is not mentioned despite the fact that they both originate from the same locality. Moreover, in Hahn and Hahn (2008), Phillipsia middlemissi conditionally assigned the subgenus Cummingella ? (Cummingella ?), and the stratigraphic interval for it is indicated as the Cisuralian or Permian-Carboniferous. It is worth noting that Phillipsia middlemissi has been classified in various genera for a long time: Ditomopyge, Neoproetus and Paraphillipsia. However, Owens (2003, p. 380) indicates the Wuchiapingian of the Himalayas for the genus Paraphillipsia, which obviously means the definition of the species under discussion as Paraphillipsia middlemissi. I also classify this species tentatively as Paraphillipsia.
Much further north, but also from Tibet, the cranidium and pygidium of Ditomopyge (Carniphillipsia) raggyorcakaensis (Qian, 1981) are described from the Raggyorcaka Formation in Shuanghu County (Qian, 1981). The Raggyorcaka Formation has Lopingian, presumably Changhsingian age (Qiao et al., 2021).
Chongqing Municipality. There are several Lopingian trilobite localities in the vicinity of Chongqing. In the Beifengjing Section, in the Changxing Formation, layers 9-28, consisting of limestones, mudstones and wackestones, there are trilobites D. (C.) obtusicauda (Kayser, 1883), Ditomopyge (Carniphillipsia) chongqingensis (Lu, 1974) and Pseudophillipsia sp. These remains are found in association with a very diverse fauna of bivalves, brachiopods and cephalopods (Shen and He, 1991). Unfortunately, this work does not contain images of trilobites, and they are only in the list of faunas.
From the Longtan Formation of the Wuchiapingian age near Wenxing Town come finds of D. (C.) lui (Kobayashi et Hamada, 1984a) and Ditomopyge (Carniphillipsia) chongqingensis (Lu, 1974), which were found together with various corals and brachiopods (Lu, 1974; Wu and Wang, 1974; Shen and Shi, 2004).
Further north, in the lower part of the Changxing Formation of Changhsingian age, one isolated pygidium, identified as Pseudophillipsia cf. chongqingensis, was discovered near the so-called Tudiya buildup (Reinhardt, 1988, p. 258).
In the same formation, but even further north in another section of Daijiagou Beipei, trilobites have been found Pseudophillipsia sp. in limestone with brachiopods, bryozoans and conodonts Xaniognathus elongatus, Hindeodus minutus and Clarkina changxingensis (Shen et al., 1995, p. 21).
North of Daijiagou, in the Yanjingxi Section in the Changxing Formation, there are references to the presence of Pseudophillipsia sp. (Yang et al., 1987). Specimens of Pseudophillipsia sp. noted here and in oldest, Wuchiapingian deposits of the Longtan Formation of the Liziya Section (Zeng et al., 1995). Pseudophillipsia obtusicauda (Kayser, 1883) and Ditomopyge (Carniphillipsia) chongqingensis (Lu, 1974) are known from the Changxing Formation of the Huaying Section located in Linshui County (Yang et al., 1987).
One of the interesting localities of Lopingian trilobites is known in Chongqing in the volcanic ash beds of the Zhongliangshan Section (Shi et al., 2016). There complete exoskeletons and numerous remains considered Ditomopyge (Carniphillipsia) cf. chongqingensis (Lu, 1974) have been found.
Guizhou Province. From the Jiaozishan Section in the Anshun urban district, Qian (1977) described two new species Pseudophillipsia anshunensis Qian, 1977 and Pseudophillipsia subcircularis Qian, 1977. According to his data, they originate from deposits (layer 16) together with Nankinella sp., Sphaerulina sp. and Lepingoceras (?) sp., which indicate their Lopingian, most likely Wuchiapingian age. The Lopingian age of these deposits is confirmed by other researchers (Wang et al., 2011).
From the nearby Xinmin Section come trilobite pygidia and cephala identified as Pseudophillipsia sp. (Feng et al., 2011, fig. 3). They were found in carbonaceous mudstone interbeds of bentonites overlain by marl with the conodont Clarkina meishanensis and, apparently, are the youngest trilobites known to science. In this article they are considered as Ditomopyge (Carniphillipsia) cf. chongqingensis (Lu, 1974).
From the Longtan Formation of the Wuchiapingian age in the Yanbeihou K12 section in Zhijin County, the remains of Pseudophillipsia sp. are mentioned (Wang et al., 2011, p. 171).
From the Dalong Formation of the Tianshengqiao Section of Nayong County, a new species Acropyge brevica Yin, 1978 was described from the cranidium and pygidium. The Dalong Formation is Changhsingian formation (Liu et al. 2019).
At the Guiyang Mineral Exploration Factory in Guiyang City, a variety of trilobites were collected in the Wuchiapingian of the Maokou Formation, described as new species (Yuan et al., 1992): Acanthophillipsia (?) granulosa Yuan et al., 1992, Acanthophillipsia guiyangensis Yuan et al., 1992, Acanthophillipsia abnormis Yuan et al., 1992 and Acanthophillipsia abrota Yuan et al., 1992. According to the paleobiodb.org database, these deposits contain Lopingian fossils. However, according to most recent published data (Gao et al., 2020), the Maokou Formation is Guadalupian (upper part of the Roadian – Capitanian). Therefore, I do not include these species, as well as the genus Acanthophillipsia, in the review of Lopingian trilobites, but I consider it necessary to mention this locality, the age of which may require clarification.
The fossil lists of the Changxing Formation of the Wenjiangsi Section of Guiding County indicate the presence of Pseudophillipsia obtusicauda (Kayser, 1883) and Ditomopyge (Carniphillipsia) chongqingensis (Lu, 1974), found in mudstones and cherts together with a variety of bivalves, brachiopods and rare cephalopods (Shen and He, 1994).
From the Changhsingian Dalong Formation in the Zhongying Section of Qinglong County, three new trilobite species described by Qian (1977) occur, namely Pseudophillipsia qinglongensis Qian, 1977, Ditomopyge (Carniphillipsia) pyriformis (Qian, 1977) and Acropyge multisegmenta Qian, 1977.
Guangxi Zhuang autonomous region. Based on finds of a well-preserved cephalon and pygidium found in the Lopingian of the Heshan Section, Qian (1977) described a new species of Ditomopyge (Carniphillipsia) heshanensis (Qian, 1977). This section exposes limestones of the Wuchiapingian Heshan Formation and the Changhsingian Talung Formation (Shen et al., 2007). The trilobite remains in question appear to come from the Heshan Formation.
Also, in the Guangxi, but much further east, in Laibin County, the Paoshui Section, also from the Heshan Formation, has yielded of Pseudophillipsia obtusicauda (Kayser, 1883) mentioned by Yang et al. (1987) and found in siliceous limestones together with bivalves and brachiopods.
Guangdong Province. Pseudophillipsia obtusicauda (Kayser, 1883) is known from the Changxing Formation in Qujiang County (Zhou, 1977). According to Kobayashi and Hamada (1984a), this find belongs to the species Ditomopyge (Carniphillipsia) lui (Kobayashi et Hamada, 1984a).
Jiangxi Province. From here Zhang (1982) described new species: Pseudophillipsia shanggaoensis Zhang, 1982, the cranidium of which comes from the Laoshan Member (Loping Formation) in Shanggao County, and Brachymetopus gaoanensis Zhang, 1982 from similar deposits in another Gao'an County. According to modern data, the latter species is most likely a representative of Cheiropyge.
Northeast of the same province in the Mingshan Coalfield, the holotype of Pseudophillipsia obtusicauda (Kayser, 1883) comes from the Lopingian, discovered in red-gray limestone along with a rich assemblage of fossils represented by gastropods, bivalves, cephalopods, echinoderms, brachiopods and corals (Kayser, 1883). Northeast of Mingshan there is another locality with Lopingian trilobites (Zhang, 1982), from which Pseudophillipsia obtusicauda are also known (Kayser, 1883).
West Timor, Indonesia. Several fragmentary cephala come from Permian in the vicinity of the Kupang City in the Amarassi region, on the basis of which Beyrich (1865) established a new species, Phillipsia parvula Beyrich, 1865. The locality near the Kupang City is known in the literature like Ajer Mati or Kali-Mati (Mount Tabeno), beds with fossils (corals, brachiopods, bryozoans, crinoids, etc.) represented by brown marls are called Amarassi Beds. These deposits, according to modern data, are of Wuchiapingian age based on the brachiopod assemblage (Archbold in Charlton et al., 2002, p. 741; Winkler Prins, 2008, p. 390). Species of Phillipsia parvula was previously tentatively assigned to the genus Microphillipsia (Hahn and Brauckmann, 1975) and then served as the type species for the establishment of the new genus Timorcranium (Brauckmann and Gröning, 2013).
Trilobites of the genus Endops, collected in the Permian of Timor from the Artinskian to the Wuchiapingian stages and located in the private collection of Dr. J. Savill, are indicated in Owens (2003, p. 383). However, there is no further detailed information on these finds.
Japan. In the western part of the country, in Gifu Prefecture, near the Akasaka Town, outcrops of Permian “Akasaka Limestone” are well known. From there, Kobayashi and Hamada (1984a; 1984b) described many species of trilobites, most of which are Guadeloupian in age. However, from the upper part of the section belonging to the foraminiferal zone of Reichelina changhsingensis, they (1984b) described the cranidia and pygidia of a new species of Pseudophillipsia (Nodiphillipsia) hanaokensis Kobayashi and Hamada, 1984b.
The foraminiferal species Reichelina changhsingensis Sheng and Chang is the index fossil for the Lopingian (Ueno and Tsutsumi, 2009), and the deposits themselves, representing the Ichihashi Formation, are apparently Wuchiapingian (Kani et al., 2013).
In this work, I propose not to use the subgenus Pseudophillipsia (Nodiphillipsia), see above, therefore I classify all its representatives as Pseudophillipsia (more on this in the section “remarks” on the genus Pseudophillipsia).
Thailand. In the northern part of the country, in the Lampang Province, several localities of Lopingian trilobites are located nearby: a small outcrop near the Huai Mae Phlung River and on the northern ridge of the Khao Doi Pha Phlung Mountains. Here from the Huai Thak Formation, represented by thick sequences of shales and sandstones (Waterhouse, 1983, p. 114), Kobayashi and Sakagami (1989) described several pygidia, identifying them as Pseudophillipsia (Nodiphillipsia) aff. ozawai Kobayashi et Hamada, 1984b. Horizons with trilobites contain fusulinids, incl. Gallowayinella guidingensis Liu, Xiao and Dong, indicating their Changhsingian (Ueno and Sakagami, 1991). Findings of Pseudophillipsia (Nodiphillipsia) aff. ozawai in Doi Pha Phlung have been noted elsewhere (Ishibashi, 1998).
New Zealand. An interesting locality of Lopingian fauna is found in New Zealand and is associated with a thick lens of pebble conglomerate in the upper part of Countess Formation on the Oreti River near Mossburn, a town in northern Southland. From there, a rich fossil assemblage was described, including brachiopods, bryozoans, crinoids, mollusks and trilobites (Hyden et al., 1982). The latter are represented by pygidium (Hyden et al., 1982, fig. 10), which, apparently, can be attributed to the Triproetus. Re-examination of the Countess Formation by Aitchison et al. (1988) allowed it to be assigned to the Stephens Subgroup and dated to the terminal Lopingian.
Spitsbergen. The northernmost locality of Lopingian trilobites in the world is located on the Ahlstrandhalvøya Peninsula of Spitsbergen Island. Here, thick deposits of the Kapp-Starostin Formation are exposed, in which trilobites were discovered, described by Kobayashi (1987) as a new species Neoproetus borealis Kobayashi, 1987. The complete exoskeleton of Neoproetus borealis comes from the middle part of the formation (layers AP6), and two pygidia are from the upper part (layers AP9). According to Nakazawa (1999), these layers belong to its upper Hovtinden Member. According to modern data, the Kapp-Starostin Formation is of Kungurian (?) – Changhsingian age (Uchman et al., 2016) or Kungurian – Wuchiapingian (Lee et al., 2022), and the age of the Hovtinden Member is most likely Lopingian (Shen, 2018).
From the lower part of the formation of the Svenskeegga member of Akseløya Island, located north of Ahlstrandhalvøya Peninsula, numerous remains of Neoproetus borealis Kobayashi, 1987 have also been described (Bruton, 1999).
The species Neoproetus borealis Kobayashi, 1987 was assigned by Owens (2003, p. 382) to the genus Triproetus, with which subsequent researchers agree (e.g., Fortey and Heward, 2015, p. 2015).
LOPINGIAN TRILOBITE GENERA AND THEIR MORPHOLOGY
Brachymetopus (Acutimetopus) Hahn and Hahn, 1985
The latest members of the Brachymetopus genus, originating in the Early Pennsylvanian and extinct throughout the Permian. The only Lopingian species, Brachymetopus (Acutimetopus) caucasicus Licharew in Weber, 1944, is known from a pygidium from the Changhsingian of the North Caucasus (Table 17).
Brachymetopus (Acutimetopus) has a subtriangular cephalon with an apical peak in the anterior part, which distinguishes them from all other subgenera of Brachymetopus and makes them more similar to the typically Permian genus Cheiropyge. The latter is believed to be an ancestor of the subgenus discussed here. This hypothesis was proposed by Hahn and Hahn in their study on phylogeny (1996).
The pygidia of Brachymetopus (Acutimetopus) are relatively highly segmented: the axis consists of more than 18 rings, and the lateral lobes have 6-7 pairs of pleural ribs, often ending in spines. More details about the diagnosis and comparison of this subgenus can be found in the systematic part of this article.
Cheiropyge Diener, 1897
Cheiropyge species occur exclusively in the Permian and are quite rare. They have a subtriangular cephalon and are similar in morphology to Brachymetopus (Acutimetopus). Like most members of the family Brachymetopidae, the facial sutures of Cheiropyge ankylosed. The glabella is conical, relatively swollen, lacking furrows and lobes, including and L 1 -lobes. The pygidium is triangular in with a long, convex, strongly segmented axis, consisting of 13-20 rings. Six pairs of pleural ribs and an unpaired swollen terminal lobe behind the axis are present. On the surface of the pygidium, there are numerous tubercles of different sizes, the largest of which are variably located on some rings of the axis and anterior pleural ribs.
Cheiropyge differs from the closely related genus Brachymetopus in having larger eyes, the absence of genal spines and L 1 -lobes of the glabella, as well as the main difference being in the presence of an unpaired terminal lobe behind the axis.
Perhaps due to its rarity, the history of research on this genus is somewhat confusing. Cheiropyge was established by a pygidium (Diener, 1897) found in the Lopingian of the Himalayas (Table 17). Almost half a century later, Weller (1944, p. 322) described the species of Cheiropyge kansasensis from the Upper Pennsylvanian of Kansas (the upper part of the Haskell Limestone), which at that time were considered Permian. Based on the similar morphology of this pygidium to Ch. Himalayensis, Weller attributed the species of kansasensis to Cheiropyge and also noted that he was the first to describe the cephalon and thorax of this genus, but the description of the pygidium, Weller did not include an image of it in his work, noting that it was poorly preserved.
Later, Kobayashi and Hamada (1982) established a new subgenus Cheiropyge (Suturikephalion) based on Ch. koizumii, the type series of which comes from the Permian (Capitanian) of Japan. They compared the cephala of Ch. koizumii with the then known cephala Ch. kansasensis, pointed out their similar morphology, but noted that Ch. koizumii has facial sutures that are absent in the holotype of Ch. kansasensis. The presence of facial sutures was, in principle, unusual for brachymetopines, in which these structures are fused.
Owens re-examined the type material of Ch. kansasensis, provided an image of the pygidium (Owens, 1983, pl. 5, fig. 18) and attribute this species to the genus Brachymetopus. Obviously, based on the absence of a terminal lobe, the shape of the axis and the spines at the ends of the pleurae are not characteristic of Cheiropyge. A little later, Hahn and Hahn (1985) included kansasensis in the subgenus Brachymetopus (Acutimetopus).
Because of this confusion, many publications have based the diagnosis of Cheiropyge on the morphology of the cephalon of Brachymetopus (Acutimetopus) kansasensis, which is a member of a different genus. Therefore, for example, Maximova (1960, p. 140) indicated that Cheiropyge has L1 -lobes, and the pygidial ends in spines on the pleurae.
Also, Hahn and Hahn (1985) noted that Cheiropyge (Suturikephalion) is a synonym for Cheiropyge and the presence of facial sutures in Ch. koizumii, which Kobayashi and Hamada described explaining the presence of these structures as taphonomical (post-mortem) changes to the cephala.
In a later work, Kobayashi and Hamada (1984a, pl. 1, figs. 1, 2, 4) provided images of other specimens of Cheiropyge koizumii, on which the facial sutures were not visible. This has also been noted by other modern authors (Flick and Shiino, 2021), who studied the type material and new collections of Ch. koizumii. The latter pointed out that the facial sutures described by Kobayashi and Hamada (1982) look unnatural, located towards the anterior part of the glabella instead of running by the eyes. According to Flick and Shiino (2021), most specimens of Ch. koizumii do not have this feature. Accordingly, these structures can only be interpreted as postmortem structures that have no taxonomic significance.
It's interesting that another species of Cheiropyge ? gaoanensis, described by Zhang (1982) from the Lopingian of China also has structures similar to facial sutures on the cephala. However, Zhang himself does not mention this in the description. This led Hahn and Hahn (1996, p. 154) to believe that these structures were not postmortem features but a neotenic phenomenon. Which, apparently, based on the conclusion of Flick and Shiino (2021), is still incorrect.
The morphology of Cheiropyge (?) gaoanensis indicates its relation to Cheiropyge, but the pygidial pleurae end in short marginal spines, which differs from this species all other members of Cheiropyge. Therefore, following Flick and Shiino (2021), I tentatively classify gaoanensis as a member of Cheiropyge.
According to Hahn and Hahn (1996, p. 154) Cheiropyge originated from Brachymetopus (Acutimetopus) in the Early Permian. This is supported by their shared common characteristics of these taxa: the triangular shape of the cephalon with a terminal apex (apical peak), six pairs of pleural ribs on the pygidium, as well as the presence of a seventh fused pair, which forms a terminal unpaired spine behind the pygidium in B. (Acutimetopus) and a terminal swollen lobe in Cheiropyge.
Triproetus Kobayashi and Hamada, 1979
As a subgenus, Neoproetus (Triproetus) was described by Kobayashi and Hamada (1979) based on the monotypic species N. (T.) subovalis, established by them, the type series of which came from the Cisuralian (Asselian) of northern Thailand. Later, Owens (1983, p. 17) noted that the morphology of Neoproetus (Triproetus) differs from Neoproetus, and is more similar to the genera Paladin and Griffithides. It was introduced as an independent genus Triproetus by Brezinski (1992), establishing in it three new species from the Cisuralian (Wolfcampian) of Texas, and also noting the shared characters of Triproetus with Paladin and Ditomopyge.
Fortey and Heward (2015) described in detail a new species, Triproetus bonbon, from the Permian (Kungurian-Roadian) of Oman. However, Triproetus was removed from Ditomopyginae and placed in the family Proetidae (without specifying a subfamily), since it lacks the typical glabellar morphology characteristic of Ditomopyginae and has a short pygidium with a small number of segments (both axial rings and pairs of pleural ribs).
Triproetus is characterized by a pear-shaped glabella with a very swollen anterior part. The small and teardrop-shaped L 1 -lobes are separated from the glabella. The posterior part of the glabella often has L2 -L4 lobes. The pygidium in Triproetus is elongated in width, short, has a wide convex axis, usually consisting of nine rings and 5-6 pleural ribs.
Lopingian members of Triproetus are known from Spitsbergen and occur in the Kapp Starostin Formation (Table 17). They were first established there by Kobayashi (1987), however, since the anterior part of the cephalon was not preserved on the type material, he did not specify the subgenus and described the new species as Neoproetus borelais [sic!].
Later, using new and more complete material from the same formation of Spitsbergen, Bruton (1999) described these trilobites in detail, but assigned the species borealis to the subgenus Paladin (Neokaskia). This subgenus was considered by Owens (2003, p. 383) to be synonymous with the genus Triproetus.
Records of Triproetus from Spitsbergen are not the only ones of this genus in the Lopingian. The pygidium (Hyden et al., 1982, fig. 10) with a morphology similar to Triproetus comes from the Stephens Subgroup in the vicinity of Mossburn in New Zealand. Its outline and its convex axis with eight or nine rings, six pleural ribs, and distinct border allow us with some confidence to attribute this specimen to belong to this genus Triproetus.
Paraphillipsia Toumansky, 1930
This genus was established and described in detail (Toumansky 1930 and Tumanskaya 1935) based on specimens from the Guadalupian (Woardian) olistoliths of the Crimea. Paraphillipsia species occur throughout the Permian, but are most diverse in the Middle (Table 2).
Paraphillipsia has a distinctive morphology, different from most Permian genera. Its main characters include the presence of a large and very wide glabella, with a constriction in the central part. The L1 -lobes are well defined and the narrow librigenae end in rounded genal angles. An important feature of Paraphillipsia is the broad and weakly segmented pygidium, with a wide axis consisting of 11 rings and lateral lobes, which usually bear 5-6 pairs of pleural ribs, passing into a pygidial border, devoid of a border furrow.
In the Lopingian, Paraphillipsia occurs in the Changhsingian of the North Caucasus and is represented by a new species, P. urushtensis sp. nov – quite similar to P. karpinskyi Tumanskaya, 1935 from the Roadian olistoliths of Crimea. In addition to P. urushtensis sp. nov. the pygidium of P. (?) middlemissi is known, described by K. Diener (1897) from isolated blocks of Chitichun limestone in Tibet (Table 17). The latter species determined in open nomenclature is conditionally assigned to Paraphillipsia, but a more precise determination will be possible only after examination with the material, which should be stored in the Geological Survey of India (Calcutta) under number GSI 6069 (Hahn and Hahn, 2008, p 130). However, unfortunately, it was not possible to find out about its storage location.
Kathwaia Grant, 1966
Kathwaia is known from represented in the Middle, Upper and possibly Lower Series of the Permian System. It was described by Grant (1966) based on the monotypic species Kathwaia capitorosa Grant, 1966, the holotype of which was represented by a single enrolled exoskeleton discovered in the Lopingian of Pakistan (Salt Range, near the Kathwai Village).
Members of Kathwaia are characterized by a highly swollen pear-shaped glabella that hangs vertically and overlaps the anterior border. The distinct teardrop-shaped L1 -lobes are separated from the glabella by wide furrows. Kathwaia's eyes are small. The most important feature of the members of the genus is that their exoskeleton is sculptured with numerous large tubercles, clearly visible on the cephalon. The pygidium is weakly segmented: the axis consists of 7-9 rings, pleural ribs – 6-9 pairs.
In total, very few records and species of Kathwaia are known (Table 6). In the Lopingian there are two of them – K. capitorosa Grant, 1966 and K. caucasica (Weber, 1944) from the North Caucasus. In the revision of this article, I consider K. capitorosa to be a junior subjective synonym of K. caucasica. Therefore, there is only one species, Kathwaia caucasica, in the Lopingian.
Neogriffithides Toumansky, 1930
There are no reliable remains of the trilobite genus Neogriffithides known in the scientific literature. This is a fairly long-lived genus, occurring from the Middle Carboniferous (Moscovian) to the Middle Permian (Wordian). The latest representatives of this genus: N. extremorientalis Flick et Shiino, 2021 from the Wordian of Japan, N. siculus (Gemmellaro, 1892) from Wordian of Sicily, N. gemmellaroi Tumanskaya, 1935, N. almensis Tumanskaya, 1935 and N. ismailensis Tumanskaya, 1935 from the Roadian of Crimea. In July 2024, in the Russian Far East (Primorsky Krai, Nakhodka Reef Locality), scattered remains of trilobites were discovered, which I tentatively attribute to the Neogriffithides. More detailed information about this discovery should be published soon in separate articles.
Timorcranium Brauckmann and Gröning, 2013
Timorcranium is characterized by a flask-shaped (slightly pear-shaped) glabella, slightly constricted in the middle part wider in the anterior than in the posterior. The glabella overlaps the anterior border border, and has three pairs of smooth lateral furrows. The fixigenae are very wide. Timorcranium is very small (cranidium 3.2 mm long) and appears to be the smallest Permian trilobite.
The only Lopingian species, Timorcranium parvulum Beyrich, 1865, comes from the Changhsingian of West Timor (Table 17) and is represented by an incomplete cranidium. It was redescribed in detail and conditionally assigned first to Microphillipsia by Hahn and Brauckmann (1975), and later Brauckmann and Gröning (2013) established a new genus Timorcranium for it, convincingly showing the differences from Microphillipsia and the subfamily Ditomopyginae.
Acropyge Qian, 1977
Typically, Guadalupian-Lopingian trilobites. Acropyge was described by Yu. Qian (1977) based on the then monotypic species Acropyge multisegmenta Qian, 1977, the type series of which came from the Changhsingian of China (Table 17).
 Members of Acropyge have an inverted flask-shaped glabella, devoid of furrows. Behind the glabella there is a long median preoccipital lobe, and the preglabellar field is wide and depressed. The structure of the cranidium of Acropyge is close to the genus Ampulliglabella. A particular difference between Acropyge and other Permian trilobites is the long subtriangular, highly segmented pygidium, the axis of which consists of 20-28 rings and the lateral lobes bears 12-14 pairs of pleural ribs. A postaxial ridge is usually present behind the axis, so the pygidium itself is peak-shaped.
Members of Acropyge have an inverted flask-shaped glabella, devoid of furrows. Behind the glabella there is a long median preoccipital lobe, and the preglabellar field is wide and depressed. The structure of the cranidium of Acropyge is close to the genus Ampulliglabella. A particular difference between Acropyge and other Permian trilobites is the long subtriangular, highly segmented pygidium, the axis of which consists of 20-28 rings and the lateral lobes bears 12-14 pairs of pleural ribs. A postaxial ridge is usually present behind the axis, so the pygidium itself is peak-shaped.
Three species of Acropyge are known from the Lopingian: the type species Acropyge multisegmenta Qian, 1977, A. brevica Yin, 1978 from the Lopingian of China, and A. weggeni Hahn and Hahn, 1981 from the Lopingian of Iran. According to R. Lerosey-Aubril (2012, p. 9), Acropyge weggeni differs from the first two species in that it has a wider axis and a slightly different arrangement of pleural ribs, the posterior pairs of which are located almost subparallel to the axis (Figure 12).
Ditomopyge (Carniphillipsia) Hahn and Brauckmann, 1975
This subgenus, which ranges from the Pennsylvanian until the end of the Lopingian, is one of the longest-living Carboniferous and Permian proetids. It was established by Hahn and Brauckmann (1975) as a subgenus of Pseudophillipsia (Carniphillipsia) with the type species Ps. ogivalis (Gauri, 1965) from the Upper Pennsylvanian of the Carnic Alps (Austria).
Ditomopyge (Carniphillipsia) is apparently a transitional form between the genera Ditomopyge and Pseudophillipsia, and therefore bears many of the common characteristics of them. The cephalon resembles that of Pseudophillipsia: there are medial and lateral preoccipital lobes, but the glabellar furrows are weakly expressed or, more often, absent, which makes it similar to Ditomopyge. Some species of Ditomopyge (Carniphillipsia) have laterally depressed areas on the glabella, therefore the species of the subgenus can be divided into three groups, having a glabella devoid of furrows and depressions, a glabella with depressions, and a glabella with small furrows (Figure 9).
The pygidium more closely resembles that of Ditomopyge and is somewhat less segmented than that of Pseudophillipsia, with an average of 17-21 axial rings and 9-13 pleural ribs. However, in some species the number of axial rings can reach up to 25, and pairs of pleural ribs – up to 15 (Table 18).
Among the Lopingian trilobites, five species of this subgenus are reliably known (Table 18). One of them, D. (C.) fatmii Grant, 1966, comes from the Wuchiapingian of Pakistan. The remaining species are confined to the Lopingian of China: D. (C.) chongqingensis occurs in a number of localities of Changhsingian in Southern China; D. (C.) heshanensis (Qian, 1977) is known from the Changhsingian of Guizhou; D. (C.) pyriformis from the Changhsingian of Guizhou; D. (C.) lui – from the Changhsingian of Chongqing; D. (C.) raggyorcakaensis (Qian, 1981) from the Changhsingian of Tibet.
A detailed comparison of these species is discussed in the work of Lerosey-Aubril and Angiolini (2009). Abbreviated information is provided here along with reconstructions (Table 18; Figure 9).
Pseudophillipsia Gemmellaro, 1892
 The most widespread and typical of Lopingian trilobites. Its oldest members are known from the Pennsylvanian and, apparently, separated from Ditomopyge (Carniphillipsia) in that epoch. The main feature of the morphology of Pseudophillipsia is the lateral and medial preoccipital lobes isolated from the glabella, forming a “festoon” structure, convex glabellar lobes (L2 -L4), genal spines, as well as an elongated pygidium, oval-triangular in shape, having a highly segmented axis (20-27 rings) and many pleural ribs (13-17). More about the genus diagnosis and comparison in the corresponding section.
The most widespread and typical of Lopingian trilobites. Its oldest members are known from the Pennsylvanian and, apparently, separated from Ditomopyge (Carniphillipsia) in that epoch. The main feature of the morphology of Pseudophillipsia is the lateral and medial preoccipital lobes isolated from the glabella, forming a “festoon” structure, convex glabellar lobes (L2 -L4), genal spines, as well as an elongated pygidium, oval-triangular in shape, having a highly segmented axis (20-27 rings) and many pleural ribs (13-17). More about the genus diagnosis and comparison in the corresponding section.
In the Lopingian, the species diversity of Pseudophillipsia is the highest among species of other genera (Table 19) and amounts to 47% (Figure 13). Of these, in seven species the structure of the cephalon, namely the glabella, is known, according to which they can be quite confidently attributed to the genus Pseudophillipsia. Four of them are described from China.
Thus, Pseudophillipsia anshunensis Qian, 1977, represented by a cephalon and pygidium, comes from the Wuchiapingian of the Guizhou province. Cephalon Ps. anshunensis is relatively narrow, and the glabella greatly widens towards the anterior part. In front of it, as far as can be judged from the photograph (Qian, 1977, pl. I, figs. 4), there is a wide preglabellar field, and in the posterior part of the glabella there are three pairs of distinct L2 -L4 lobes.
Another species, Pseudophillipsia qinglongensis Qian, 1977, described from Changhsingian of the same province, is represented by an almost complete exoskeleton, but the lower part of its pygidium is broken off. From Ps. anshunensis differs by a wider cephalon, larger preoccipital lobes (both lateral and medial), and, apparently, a smaller preglabellar field.
Pseudophillipsia shanggaoensis Zhang, 1982, described from its cranidium, comes from the Wuchiapingian of Jiangxi Province. Its characteristic feature is its protruding palpebral lobes. However, its preservation, represented by a cast, does not allow, in my opinion, to strongly distinguish it from another widespread species in the Lopingian of China – Pseudophillipsia obtusicauda (Kayser, 1883), recorded from a number of Lopingian (both Wuchiapingian and Changhsingian) deposits of South China.
One species, Pseudophillipsia hanaokensis Kobayashi et Hamada, 1984b, is described from the Lopingian of Japan, and its type series consists of cranidia and pygidia. It is characterized by a swollen exoskeleton, small preoccipital lobes and a narrow occipital ring.
From the Changhsingian of Slovenia comes the Pseudophillipsia n. sp., aff. sumatrensis (Roemer, 1880), represented by an incomplete cephalon with pygidium. The cephalon partially preserves the cranidium and part of the librigena. Based on similar characters, Hahn et al. (1970) considered it close to the type species Pseudophillipsia sumatrensis (Roemer, 1880), described from the Guadalupian of Indonesia. It is worth noting that a very wide occipital ring and rather large preoccipital lobes greatly distinguish the Slovenian species determined in open nomenclature from other Lopingian trilobites, however, the fragmentary of the material does not allow it to be compared in detail either with the type species or to describe a new one.
The species, Pseudophillipsia solida Weber, 1944, is described from Changhsingian of the North Caucasus and is discussed in detail in this article. The cranidium assigned to this species by Hahn et al. (1970) comes from Changhsingian of Slovenia.
Some species and species determined in open nomenclature are known only from pygidia, so in this article they are conditionally classified as Pseudophillipsia. Since we do not know the structure of their cephala, especially the glabella, we can assume that they may be members of the related Ditomopyge (Carniphillipsia). Thus, from the Changhsingian of Thailand come pygidia identified by Kobayashi (Kobayashi and Sakagami, 1989) as Pseudophillipsia (Nodiphillipsia) aff. ozawai Kobayashi et Hamada, 1984b. Indeed, the general shape of these pygidia, the border furrow, the convexity of the lateral lobes and the number of pleural ribs are consistent with those of Pseudophillipsia ozawai Kobayashi et Hamada, 1984b from the Capitanian of Japan (see: Kobayashi and Hamada, 1984a, pl. IX, figs. 4,5). However, Thai pygidia are somewhat narrower, have a narrower axis and a greater number of axial rings (up to 27). One of them (Kobayashi and Sakagami, 1989, fig. 1 a) has a somewhat peaked shape, which makes it similar to Acropyge, although the posterior part of the pygidium is not preserved, and this shape is possibly only a consequence of taphonomic deformation. I very tentatively assign these pygidia to the genus Pseudophillipsia (as well as to the species Pseudophillipsia ozawai).
The species Pseudophillipsia (?) hungarica, described by Schréter (1948) based on pygidia, is known from Changhsingian of Hungary. A peculiarity of this species is the presence of a large tubercle at the geniculate bend of each pleural rib. Unfortunately, for Ps. (?) hungarica, no cephala are described, which in all likelihood are known from there, since Detre (1991) reported significant collections of these trilobites. A similar species to Ps. (?) hungarica was described by Hahn et al. (1970) from the Changhsingian of Slovenia.
The pygidium Ps. (?) parvizii Lerosey-Aubril, 2012 comes from the Wuchiapingian of Iran and have rather unusual morphology: its wide pygidial border widens significantly towards the rear. A similar structure is characteristic of the Late Pennsylvanian and Cisuralian species of Ditomopyge. This is also reported by the author (Lerosey-Aubril, 2012, p. 13), comparing the Iranian pygidium with the Cisuralian Ditomopyge (Carniphillipsia) rotunda Hahn and Hahn in Hahn, Hahn and Ramovš, 1990. Its closeness to Ditomopyge (Carniphillipsia) is also indicated by the number of pygidial segments (Ps. (?) parvizii has 21 axial rings and 13 pairs of pleural ribs), and Lerosey-Aubril (2012) classified parvizii as belonging to the Pseudophillipsia in the broad sense (sensu lato). Following Lerosey-Aubril (2012), I also classify this species tentatively as Pseudophillipsia and hope for new finds of cephala from the Dalan Formation of the Zagros Mountains.
In the blocks on the Alma River in Crimea Tumanskaya (1935, p. 10) discovered difficult-to-identify remains of trilobites, which she described as species determined in open nomenclature of Neogriffithides sp. ind. No. 1 (block C), N. (?) sp. ind. No. 2 (block A) and Pseudophillipsia sp. ind. No. 1 (block B). The first two are represented by incomplete poorly preserved pygidia. It is worth noting that Tumanskaya (1935) described the genus Neogriffithides and its species without complete exoskeletons, and associated the highly segmented pygidia of Pseudophillipsia with them. It was later noted (Ruggieri, 1959, p. 4; Owens, 1983, p. 18; Hahn and Hahn, 2015, p. 113-114) that members of Neogriffithides actually have a weakly segmented pygidium. I associate the remains of trilobites from blocks on the Alma River, identified by Tumanskaya as Neogriffithides, rather with both Pseudophillipsia and Ditomopyge (Carniphillipsia) due to their highly segmentation.
Pseudophillipsia sp. ind. No. 1, known by its incomplete pygidium, has a large number of axial rings (probably 25-26) and 11 pleural ribs obliquely descending to the posterior end, which, according to Tumanskaya (1935, p. 28) and Hahn et al. (1970, p. 317) relate it more closely to the genus Anisopyge. However, finds of the Anisopyge are limited to the Cisuralian and Guadalupian of North America (Owens, 2003, p. 381). Therefore, based on fragmentary material, I conditionally classify this species determined in open nomenclature as Pseudophillipsia.
The species Pseudophillipsia (?) subcircularis Qian, 1977, represented by a pygidium with 23-24 axial rings and 12 pairs of pleural ribs, is described from the Wuchiapingian of Guizhou (Qian, 1977, p. 283). Other finds are also known from the Lopingian of Southern China, usually pygidia, considered in this article as Pseudophillipsia (?) sp.
Herein, details are given of the pygidia of the species Pseudophillipsia (?) cf. mustafensis Tumanskaya, 1935 and Pseudophillipsia (?) caucasica Weber, 1944, known from the Changhsingian of the North Caucasus. Pseudophillipsia (?) aff. caucasica Weber, 1944 was described from the Lopingian of Iran. Its differences from the North Caucasian species are given in the section notes on the species Pseudophillipsia (?) caucasica Weber, 1944.
DISCUSSION
Taxonomically, Lopingian trilobites were relatively poorly represented (Table 20, Figure 13D-F). At the family level (Figure 13F), the majority of the species were classified as Phillipsiidae (85%), with Brachymetopidae and Proetidae accounted for 9% and 6%, respectively. Approximately the same proportion of trilobite families was present throughout the Permian. The distribution of species (and species determined in open nomenclature) of known Lopingian trilobites (Figure 13E) suggests that most of them belonged to the two closely related Pseudophillipsia and Ditomopyge (Carniphillipsia). Almost half (47%) of the known species of Lopingian trilobites belong to the first genus; almost three times fewer (17%) belong to the second. Species of the Acropyge accounted for 9%. Other species and varieties are much less commonly found. So Brachymetopus (Acutimetopus), Cheiropyge, Triproetus and Paraphillipsia make up 6% of the total number of species, and Kathwaia and Timorcranium account for 3%, as they are represented only by only one species each.
Compared to the Guadalupian diversity, the Lopingian trilobites has lost several genera, which became extinct during the Wordian-Capitanian (Figure 13A). In particular, members of 17 genera are not known to survive into the Lopingian: Hildaphillipsia, Neogriffithides, Neoproetus, Acanthophillipsia, Ampulliglabella, Anisopyge, Delaria, Ditomopyge (Ditomopyge), Endops, Jimbokranion, Microphillipsia, Novoameura, Permoproetus, Timoraspis, Doublatia, Nipponaspis and Weania. Additionally, in the Wuchiapingian only one genus was recorded that was absent from the Guadalupian – Timorcranium. This is more likely due to the incomplete fossil record than the appearance of this genus during the Lopingian Epoch.
The distribution of Lopingian trilobites across stages (Figure 13D) shows the highest diversity in the Changhsingian, with 48%, and in the Wuchiapingian with only 37%. Lopingian trilobites, which are found in deposits that could not be reliably dated to a specific stage, account for approximately 15%. Here, it's worth noting that most of the Lopingian sections, from which trilobites originate, require more detailed stratigraphical clarification. And the figures presented here should not be interpreted as a basis for concluding that trilobite diversity increased in the Changhsingian relative to the Wuchiapingian. In fact, we observe another distortion in the sample from more studied sections.
The palaeobiogeographic distribution of Lopingian trilobites has decreased compared to the previous epochs of the Permian and the Carboniferous, but it is not limited to only a few areas. The first striking change (Figure 13G) is the disappearance of trilobites from the western edge of the Midcontinent, which lived on the Panthalassa shelf. The trilobite fauna of this region, which was widespread in the Guadalupian, was quite endemic. Their genera were not found in Tethyan regions. The palaeobiogeographic areas of the Guadalupian trilobite fauna, noted by various researchers (Owens and Hahn, 1993; Brezinski, 2023) were very endemic (genera Delaria, Novoameura, Anisopyge and Vidria) and confined to subequatorial latitudes.
However, trilobite faunas associated with Panthalassa during the Lopingian seem to have been preserved in mid-latitudes in both hemispheres. (Figure 13G). This is confirmed by the discovery of Lopingian trilobite in Spitsbergen and New Zealand. It is possible that these palaeogeographic regions were refugia for some trilobite populations, where they were able to survive. It is equally curious that they are represented only by one genus, Triproetus. In comparison with the Tethyan trilobite fauna of the Lopingian, this genus is very rare. The lack of obvious marine connections to the Palaeo-Tethys can be explained by the absence of typical Tethyan genera in the mid latitudes of Panthalassa, as well as by the lack of Triproetus in the Tethyan areas.
The Tethys and Palaeo-Tethys margins continued to be rich in trilobite faunas. The main component of these faunas was Pseudophillipsia (Figure 13G), which was found in almost all Lopingian Tethyan deposits. The closely related Ditomopyge (Carniphillipsia), which lived mainly in the eastern and northern parts of the Palaeo-Tethys and the southern Tethys oceans, but was absent from the western parts of both oceans, had a slightly less widespread distribution. Palaeo-Tethys also features the presence of a third species of the subfamily Ditomopyginae – Acropyge. This genus is restricted to southern China and northern Iran.
Similar Lopingian trilobite assemblages lived on both sides of the equator in the northwestern Palaeo-Tethys and the southern Tethys. The presence of these common assemblages is evidenced by the presence of the Kathwaia and Paraphillipsia, as well as Cheiropyge and Brachymetopus (Acutimetopus). The only genus that was not found in other Tethyan areas, Timorcranium, lived in the southeastern part of the Tethys.
Apparently, Pseudophillipsia and Ditomopyge (Carniphillipsia) were cosmopolitan and fairly successful trilobites from the Lopingian, surviving and until close to the major Permian-Triassic extinction events.
The Lopingian deposits of a pre-boreal sea basin, the Zechstein, located on the East European Platform, also lack trilobite remains. This can be attributed to hypersalinity and aridity. However, Zechstein deposits contain reef facies (Raczyński and Biernacka, 2014), as well as arthropod finds, including and cyclidans (Schweitzer et al., 2020, p. 279), which shared a similar lifestyle to trilobites.
During the Permian, the diversity of trilobites was represented by only one order (Proetida), three families (Brachymetopidae, Proetidae and Phillipsiidae) and about 38 genera (see supplements), which is extremely low compared to previous periods of the Palaeozoic. As is clearly evident from the presented graph (Figure 13A), throughout the three ages of the Cisuralian, the number of genera remained approximately constant (17-19), but in the Kungurian age it began to increase and reached 23 genera. The next surge in diversity was confined to the Wordian age of Guadalupian, when the number of genera increased to 24, and the level and rate of originations of new genera reached 25% and 9.5 (genera per Ma) respectively (Figure 13B, C). However, the second half of the Guadalupian is also characterized by a significant decrease in diversity: in the Wordian, the level of extinction of genera reached 50% and in the Capitanian – 43%. A high rate of extinction was noted during the Wordian (~19 genera per one Ma).
Apparently, the Late Guadalupian reduction in generic diversity can be linked to the Guadalupian mass extinction event, which has been noted by many researchers (e.g., Rampino and Shen, 2019). According to some researchers, this extinction event may be associated with eruptions from the Emeishan Large Igneous Province. This is evident not only through basaltic formations, but also through other geochemical anomalies (Bond et al., 2010; Ling et al., 2023). According to recent research, there may have been two mass extinction events during the Capitanian Age (Song et al., 2023). However, the largest reduction in trilobite genera (Figure 13B) is not confined to the Capitanian, but also to the Wordian.
Apparently, trilobite faunas were unable to recover after the Guadalupian extinctions and, throughout the Lopingian, lived out their last epoch of their existence. In the Lopingian, the only origination of new genera is restricted to Timorcranium in the Wuchiapingian and a reduction in diversity from nine in Wuchiapingian and seven genera in Changhsingian, and then the complete disappearance of the entire group during the Permian-Triassic extinction.
On the one hand, it seems quite obvious that trilobite the diversity declined during the biocrises of the Guadalupian mass extinction events and continued to decline throughout the Lopingian. However, Guadalupian-Lopingian trilobites may exhibit the “Signor-Lipps effect” (Signor and Lipps, 1982), in which the “alleged extinction” of trilobites before the Capitanian in Wordian events, as well as before the EPME events, may be due to sampling bias and incomplete palaeontological records.
It is possible that such a distortion may be due primarily to the fact that the geological sections of the Guadalupian are more widespread than those of the Lopingian. Thus, “The Paleobiology Database (PBDB)” contains information about 453 formations of marine origin of the Cisuralian, 265 of the Guadalupian and 206 of the Lopingian. It is also worth noting that the Guadalupian trilobites have been studied somewhat better and more fully than the Lopingian trilobites. Some works (e.g., Tumanskaya, 1935) show not so much the true diversity of Guadalupian trilobites, but rather the extensive material collected by the author during long and painstaking research. No less significant in understanding the distortion under discussion is the fact that the duration of the Guadalupian is 13.9 Ma, and the Lopingian is half as long – 7.2 Ma (Permophiles, 2023, p. 49), which accordingly can indicate that the total number of trilobite taxa in Guadalupian (29 genera), other things being equal, should be higher than in Lopingian (nine genera).
Chinese researchers (Shi et al., 2016) reported numerous finds of trilobites Ditomopyge (Carniphillipsia) cf. chongqingensis from the Upper Changhsingian deposits in the Zhongliangshan Section, located in Chongqing, China. Moreover, these remains come from beds of volcanic ash. The authors noted that the number of trilobites decreased in the section with each subsequent bed of volcanic ash. In their opinion, the temporal coincidence between volcanic eruptions and the disappearance of trilobites and other species supports the idea of a cause-and-effect relationship between these events. Trilobites in the ash bed of the Zhongliangshan Section appear before the extinction of the Clarkina yini conodonts and the culmination of a negative carbon isotope excursion, which means that the onset of the mass extinction began in Lopingian. Explosive volcanic events caused massive releases of CO2, toxic gases and volcanic ash and led to habitat loss for some species in Tethys waters. This phenomenon could lead to the sudden death of trilobites and the catastrophic disappearance of the biodiversity of other groups of marine and terrestrial fauna.
However, in this case, what about the trilobites that were recorded in the Lopingian in the mid, close to the high latitudes – in Spitsbergen and New Zealand (Figure 13G)? There is no clear evidence of volcanic activity in these sections. In general, it is difficult and speculative to talk about the unambiguous reason for the disappearance of trilobites at the Permian-Triassic boundary, since there are many different points of view on this matter.
Recently, researchers have questioned whether Permian trilobites could be considered “living fossils” in relation to before-Permian trilobites (Hopkins et al., 2023). In their opinion, the low taxonomic richness, small geographical range, and morphological dullness and other characteristics of Permian trilobites allow them to be called relicts, although not in all respects.
It is interesting to note that in the terminal part of the Changhsingian Dalong Formation in the Xinmin Section (Anshun, Guizhou, China) trilobite Pseudophillipsia cf. chongqingensis in carbonaceous mudstone bentonite beds overlain by marl with the conodont Clarkina meishanensis, indicating that these trilobites originate from the major extinction event layer (Feng et al., 2011, fig. 3). Trilobites appear to exhibit another interesting effect called “Dead Clades Walking” (Jablonski, 2002). Some relatively small populations of trilobites may have survived the major events of the EPME, and then disappeared after, perhaps even during very the Early Triassic.
ACKNOWLEDGEMENTS
The author is grateful to A.S. Alekseev (Lomonosov Moscow State University, Russia) and A.S. Biakov (Shilo North-East Interdisciplinary Scientific Research Institute RAS, Magadan, Russia) for valuable comments and recommendations to article. I am also extremely grateful to P. Müller (Germany), T. Hegna (State University of New York at Fredonia, USA), T. Liu (Paleontological Museum of Liaoning, Shenyang Normal University, China) for help with the literature. I am grateful to photographer M.T. Miller (Department of Paleobiology, National Museum of Natural History, Smithsonian Institution) and R. Feldmann (Kent State University, USA) for photos of holotype of Kathwaia capitorosa Grant, 1966. Director of the CNIGRmuseum (St. Petersburg) A.R. Sokolov and the curator of the “Paleozoic hall” N.M. Kadlec for help in selecting trilobites for study; N.K. Semenov (St. Petersburg) for help with photography. Reviewers R. Owens (National Museum Wales, UK) and D. Brezinski (Maryland Geological Survey, USA) for their careful reviews of the manuscript; and also the Palaeontologia Electronica editors Matúš Hyžný (Comenius University Bratislava, Slovakia) and Lukáš Laibl (Czech Academy of Sciences, Prague, Czech Republic) for their comprehensive assistance.
REFERENCES
Adrain, J.M. 2011. Class Trilobita Walch, 1771. In Zhang, Z.-Q. (ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148:104-109.
https://doi.org/10.11646/zootaxa.3148.1.15
Aitchison, J.C., Landis, C.A., and Turnbull, I.M. 1988. Stratigraphy of Stephens Subgroup (Maitai Group) in the Countess Range – Mararoa River area, northwestern Southland, New Zealand. Journal of the Royal Society of New Zealand, 18(3):271-284.
https://doi.org/10.1080/03036758.1988.10426470
Angelin, N.P. 1854. Palaeontologia Scandinavia. P.1. Crustacea Formationis Transitionis. Fasc II. T.O. Weigel, Lipsiae.
Angiolini, L., Checconi, A., Gaetani, M., and Rettori, R. 2010. The latest Permian mass extinction in the Alborz Mountains (North Iran). Geological Journal, 45:216-229.
https://doi.org/10.1002/gj.1203
Archbold, N.W. 1981. New Permian trilobite from Irian Jaya, Indonesia. Geological Research and Development Centre, Paleontology Series, 2:35-41.
Archbold, N.W. 1997. Narinia, a new name for the Permian brachymetopid trilobite genus Iriania Archbold 1981. Proceedings of the Royal Society of Victoria, 109(1):119.
Belov, A.A. 1967. O yarusnom raschlenenii permskikh otlozhenij Kavkaza. Izvestiya akademii nauk SSSR. Seriya geologicheskaya, 12:84-96. (In Russian)
Beyrich, E. 1865. Über eine Kohlenkalk-Fauna von Timor. Abhandlungen der Königlichen Akademie Wissenschaften. Berlin. (1864, Phys.):61-98. (In German)
https://archive.org/download/abhandlungenderk182272deut/abhandlungenderk182272deut.pdf
Blakey, R. 2016. Deep Time Map. Records downloaded 20 July 2023. Website.
https://deeptimemaps.com/
Bogoslovskaya, M.F. 1984. Ammonoidei: 248-256. In Kotlyar, G.V. and Stepanov, D.L. (eds.), Osnovnye cherty stratigrafii permskoj sistemy SSSR. Nedra, Leningrad. (In Russian)
Bond, D.P.G., Hilton, J., Wignall, P.B., Ali, J.R., Stevens, L.G., Sun, Y., and Lai, X. 2010. The Middle Permian (Capitanian) mass extinction on land and in the oceans. Earth-Science Reviews, 102(1-2):100-116.
https://doi.org/10.1016/j.earscirev.2010.07.004
Brauckmann, C. 1994. Zum Andenken an Henry Paul (5.7.1909 – 24.6.1944). Trilobiten aus dem oberen Ober-Devon und Unter-Karbon im Velberter Sattel. Archäologie im Ruhrgebiet, 2:19-48. (In German)
Brauckmann, C. and Gröning, E. 2013. Phillipsia ? parvula Beyrich, 1865 (Trilobites; Permian; Timor) revisited. Clausthaler Geowissenschaften, 9:45-50.
Brezinski, D.K. 1992. Permian trilobites from west Texas. Journal of Paleontology, 66(6):924-943.
Brezinski, D.K. 2023. Biogeographic patterns in Late Paleozoic trilobites. Palaeogeography, Palaeoclimatology, Palaeoecology, 609:111319.
https://doi.org/10.1016/j.palaeo.2022.111319
Brookfield, M.E., Williams, J.C., and Stebbins, A.G. 2021. Paleoenvironments and geochemistry across a continuous Permian-Triassic boundary section at Bűkk Mountains, Hungary. Geoscience Frontiers, 12(3):101092.
https://doi.org/10.1016/j.gsf.2020.09.021
Bruton, D.L. 1999. Permian trilobites from Akseløya, Svalbard. Geologica et Palaeontologica, 33:191-201.
Burbrink, F.T., Crother, B.I., Murray, C.M., Smith, B.T., Ruane, S., Myers, E.A., and Pyron, R.A. 2022. Empirical and philosophical problems with the subspecies rank. Ecology and Evolution, 12:E9069.
https://doi.org/10.1002/ece3.9069
Chamberlain, C.K. 1977. Carboniferous and Permian trilobites from Ellesmere Island and Alaska. Journal of Paleontology, 51(4):758-771.
https://www.jstor.org/stable/1303742
Charlton, T.R., Barber, A.J., Harris, R.A., Barkham, S.T., Bird, P.R., Archbold, N.W., Morris, N.J., Nicoll, R.S., Owen, H.G., Owens, R.M., Sorauf, J.E., Taylor, P.D., Webster, G.D., and Whittaker, J.E. 2002. The Permian of Timor: stratigraphy, palaeontology and palaeogeography. Joumal of Asian Earth Sciences, 20:719-774.
https://doi.org/10.1016/S1367-9120(02)00018-4
Chen, J., Shen, S., Zhang, Y., Angiolini, L., Gorgij, M.N., Crippa, G., Wang, W., Zhang, H., Yuan, D., Li, X., and Xu, Y. 2020. Abrupt warming in the latest Permian detected using high-resolution in situ oxygen isotopes of conodont apatite from Abadeh, central Iran. Palaeogeography, Palaeoclimatology, Palaeoecology, 560:109973.
https://doi.org/10.1016/j.palaeo.2020.109973
Detre, C. 1991. A Bükki felső-perm Trilobiták phylogenetikai jelentősége [The phylogenetic importance of Late Permian trilobites from the Bukk Mountains]. A Magyar Állami Földtani Intézet Évi Jelentése az 1989. Évről: 467-470. (In Hungarian with English abstract)
Diener, C. 1897. The Permocarboniferous fauna of Chitichun No. 1. Palaeontologia Indica, 15/1(3):1-105.
https://opac.geologie.ac.at/ais312/dokumente/Diener_1897_Permocarboniferous_Fauna_Chitichun.pdf
Feng, F., Hu, Q., and Feng, Q. 2011. Trilobite from Uppermost Changhsingian in Anshun, Guizhou province. Journal of stratigraphy, 35(3):295-298.
Flick, U. and Shiino, Y. 2021. A new trilobite fauna from the Middle Permian of the Kitakami Mountains/Northeast Japan. Palaeontographica, Abteilung A: Palaeozoology – Stratigraphy Article, 320(4-6):87-135.
https://doi.org/10.1127/pala/2021/0115
Fokht, K.K. 1901. O drevnejshikh osadochnykh obrazovaniyakh Kryma. Trudy Sankt-Peterburgskogo obshchestva estestvoispytatelej, 32(1):302-309. (In Russian)
Forel, M., Crasquin, S., Chitnarin, A., Angiolini, L., and Gaetani, M. 2015. Precocious sexual dimorphism and the Lilliput effect in Neo‐Tethyan Ostracoda (Crustacea) through the Permian-Triassic boundary. Palaeontology, 58(3):409-454.
https://doi.org/10.1111/pala.12151
Fortey, R.A. and Owens, R.M. 1975. Proetida – a new order of trilobites. Fossils and Strata, 4:227-239.
https://foreninger.uio.no/ngf/FOS/pdfs/F&S_04_p227.pdf
Fortey, R.A. and Heward, A.P. 2015. A new, morphologically diverse Permian trilobite fauna from Oman. Acta Palaeontologica Polonica, 60(1):201-216.
https://doi.org/10.4202/app.00106.2014
Gandl, J. 1987. Die Karbon-Trilobiten des Kantabrischen Gebirges (NW-Spanien), 4: Trilobiten aus dem höheren Namur und teiferen Westfal. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 543:1-79. (In German)
Gandl, J. 2011. Die Karbon-Trilobiten des Kantabrischen Gebirges (NW-Spanien). 5: Trilobiten des höheren Westfal. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 569:1-143. (In German)
Gao, P., He, Z., Lash, G.G., Li, S., Xiao, X., Han, Y., and Zhang, R. 2020. Mixed seawater and hydrothermal sources of nodular chert in Middle Permian limestone on the eastern Paleo-Tethys margin (South China). Palaeogeography, Palaeoclimatology, Palaeoecology, 551:109740.
https://doi.org/10.1016/j.palaeo.2020.109740
Gauri, K.L. 1965. Uralian stratigraphy. Trilobites and brachiopods of the Western Carnic Alps (Austria). Jahrbuch der Geologischen Bundesanstalt, 11:1-26.
https://www.zobodat.at/pdf/JahrbGBA-SB_11_0001-0094.pdf
Gauri, K.L. and Ramovš, A. 1964. Eolyttonia (Brach.) and Brachymetopus (Tril.) from the Upper Carboniferous (Orenburgian) of Karawanken, Yugoslavia. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 119(1):103-112.
Gemmellaro, G.G. 1892. I crostacei del calcari con Fusulina della valle del fiume Sosio nella provincia di Palermo in Sicilia. Memorie della Società Italiana delle Scienze, detta dei XL, 8(1):1-40. (In Italian)
Gheyselinck, R.F.C.R. 1937. Permian trilobites from Timor and Sicily with a revision of their nomenclature and classification. Scheltema & Holkema: Amsterdam.
Goldring, R. 1957. Pseudophillipsia (Tril.) from the Permian (or Uralian) of Oman, Arabia. Senckenbergiana lethaea, 38(3/4):195-210.
Grabau, A.W. 1936. Early Permian fossils of China. Pt. II. Fauna of the Maping Limestone of Kwangsi and Kweichow. Palaeontologia Sinica, Serie B(4):1-441.
Grant, R.E. 1966. Late Permian trilobites from the Salt Range, West Pakistan. Palaeontology, 9(1):64-73.
Greco, B. 1935. La fauna permiana del Sosio conservata nei Musei di Pisa, di Firenze e di Padova, Parte I. Palaeontographia Italica, 35:101-190. (In Italian)
Grunt, T.A. and Dmitriev, V.Yu. 1973. Permskie brakhiopody Pamira. Trudy Paleontologicheskogo Instituta RAN, 136:1-210. (In Russian)
Grunt, T.A. and Novikova, M.V. 2002. Pozdnepermskie brakhiopody Gornogo Kryma. Paleontologicheskij zhurnal, 2:32-38. (In Russian)
Haas, W., Hahn, G., and Hahn, R. 1980. Perm-Trilobiten aus Afghanistan. Palaeontographica Abteilung A, 169(4-6):73-127. (In German)
Hahn, G. 1990. Upper Carboniferous trilobites from the Carnic Alps, p. 39-42. In Venturini, C. and Krainer, K. (eds.), Field Workshop on Carboniferous to Permian sequence of the Pramollo-Nassfeld Basin (Carnic Alps). Pramollo.
Hahn, G. and Hahn, R. 1967. Zur Phylogenie der Proetidae (Trilobita) des Karbons und Perms. Zoologische Beiträge, Neue Folge 13(2/3):303-349. (In German)
Hahn, G. and Hahn, R. 1969. Trilobitae carbonici et permici I. (Brachymetopidae; Otarionidae; Proetidae: Proetinae, Dechenellinae, Drevermanniinae, Cyrtosymbolinae). In series: Fossilium Catalogus. I. Animalia, 118:1-160. (In German)
Hahn, G. and Hahn, R. 1970. Trilobitae carbonici et permici II. (Proetidae: Griffithidinae). In series: Fossilium Catalogus. I. Animalia, 119:162-331. (In German)
Hahn, G., Hahn, R., and Ramovš, A. 1970. Perm-Trilobiten aus Siowenien, NW-Jugoslawien. Senckenbergiana lethaea, 51(4):331-333.
Hahn, G. and Hahn, R. 1972. Trilobitae carbonici et permici III. In series: Fossilium Catalogus. I. Animalia, 120:332-531. (In German)
Hahn, G. and Hahn, R. 1975. Die Trilobiten des Ober-Devon, Karbon und Perm. In series: Leitfossilien, 1:127. (In German)
Hahn, G. and Brauckmann, C. 1975. Revision zweier Trilobiten-Arten aus dem Perm Asiens. Geologica et Palaeontologica, 9:117-124. (In German)
Hahn, G., Hahn, R., and Ramovš, A. 1977. Trilobiten aus dem Ober-Karbon (Gshelium) der Karawanken/Slowenien. Geologica et Palaeontologica, 11:135-160. (In German)
Hahn, G. and Hahn, R. 1981. Über Acropyge (Trilobitae; Ober-Perm). Senckenbergiana lethaea, 61:217-225. (In German)
Hahn, G., Hahn, R., and Brauckmann, C. 1984. Die Trilobiten des belgischen Kohlenkalkes (Unter-Karbon). 6. Bollandia und Parvidllmlls. Geologica et Palaeontologica, 18:65-79.
Hahn, G. and Hahn, R. 1985. Trilobiten aus dem hohen Ober-Karbon oder Unter-Perm von Alaska. Senckenbergiana lethaea, 66(6):445-485. (In German)
Hahn, G. and Hahn, R. 1987. Trilobiten aus dem Karbon von Nötsch und aus den Karnischen Alpen Österreichs. Jahrbuch der Geologischen Bundes-Anstalt, 129(3/4):567-619. (In German)
Hahn, G. and Brauckmann, C. 1988. Zur Phylogenie der Bollandiinae (Trilobita, Karbon-Perm). Jahresberichte des Naturwissenschaftlichen Vereins in Wuppertal, 41:119-131. (In German)
Hahn, G., Hahn, R., and Schneider, G. 1989a. Neue Trilobitenfunde aus der Waidegg-Formation (hohes Oberkarbon) der Karnischen Alpen (Österreich) //Jahrbuch der Geologischen Bundesanstalt, 132(4):645-664. (In German)
Hahn, G., Hahn, R., and Yuan J-L. 1989b. Trilobites from the Upper Carboniferous (Westphalian A) of S-China (N-Guangxi). Geologica et Palaeontologica, 23:113-203.
Hahn, G., Hahn, R., and Ramovš A. 1990. Trilobiten aus dem Unter-Perm (Trogkofel-Kalk, Sakmarium) der Karawanken in Slowenien. Geologica et Palaeontologica, 24:139-171. (In German)
Hahn, G. and Hahn, R. 1992. Trilobiten aus dem Karbon von SE-Alaska, Teil 2. Geologica et Palaeontologica, 26:99-133. (In German)
Hahn, G. and Hahn, R. 1993. Die Trilobiten-Taxa des Karbons und Perms. 1. Anujaspidinae, Conophillipsiinae und Cystispininae. Courier Forschungsinstitut Senckenberg, 156:1-17. (In German)
Hahn, G. and Hahn, R. 1996. Die Trilobiten-Taxa des Karbons und Perms. 2. Brachymetopidae. Courier Forschungsinstitut Senckenberg, 195:1-242. (In German)
Hahn, G. and Hahn, R. 2008. Catalogus trilobitorum cum figuris (Trilobites carbonici et permici, VI. Cummingellinae). In series: Fossilium Catalogus I: Animalia Pars 145 (Backhuys Publishers & Margraf Publishers): 1-433, Leiden – Weikersheim. (In German)
Hahn, G. and Hahn, R. 2015. Catalogus trilobitorum cum figuris (Trilobitae carbonici et permici, VII. Bollandiinae). In series: Fossilium Catalogus, I: Animalia, 153:1-192. (In German)
Hahn, G. and Hahn, R. 2016. Catalogus trilobitorum cum figuris (Thitobites carbonici et permici, VIII. Philtipsiinae et Griffithidinae). In series: Fossilium Catalogus, I: Animalia, 156: 1-373. (In German)
Hahn, G., Hahn, R., and Brauckmann, C. 2001. The last trilobites. Acta Geologica Leopoldensia, 24(52/53):271-281.
Hahn, G., Hahn, R., and Müller, P. 2019. Catalogus trilobitorum cum figuris (Trilobites devonici, carbonici et permici, IX. Cystispininae, Globusiinae, Proetinae. In series: Fossilium Catalogus Animalia, 159:1-340. (In German)
Hammel, C. 1996. Une faune nouvelle de trilobites (Brachymetopus, Namuropyge) dans le Viséen des Vosges du Sud. Conséquences stratigraphiques et paléoécologiques. Geobios, 29(6):745-755. (In French)
Hawle, I. and Corda, A.J.C. 1847. Prodrom einer Monographie der böhmischen Trilobiten. JG Calveśchen, Prague.
Heckel, P.H. 1999. Overview of Pennsylvanian (Upper Carboniferous) stratigraphy in Midcontinent region of North America. In Heckel, P.H. (ed.), Guidebook, Fieldtrip #8, XIV International Congress on the Carboniferous-Permian, 68-102. Lawrence: Kansas Geological Survey. Open File Report: 99-27.
Heckel, P.H., Alekseev, A.S., Barrick, J.E., Boardman, D.R., Goreva, N.V., Nemyrovska, T.I., Ueno, K., Villa, E., and Work, D.M. 2007. Cyclothem [“digital”] correlation and biostratigraphy across the global Moscovian-Kasimovian-Gzhelian stage boundary interval (Middle-Upper Pennsylvanian) in North America and eastern Europe. Geology, 35: 607-610.
https://doi.org/10.1130/G23564A.1
Heritsch, F. 1931. Versteinerungen aus dem Karbon der Karawanken und Karnischen Alpen. Abhandlungen der Geologischen Bundes-Anstalt, 23(3):1-56. (In German)
Hopkins, M.J., Wagner, P.J., and Jordan, K.J. 2023. Permian trilobites and the applicability of the “living fossil” concept to extinct clades. Frontiers in Ecology and Evolution, 11:1166126.
https://doi.org/10.3389/fevo.2023.1166126
Hupé, P. 1953. Classe des trilobites. Traité de paléontologie, 3:44-246. (In French)
Hupé, P. 1955. Classification des trilobites. Annales de paléontologie, 41:91-325. (In French)
Hyden, G., Begg, J.G., Campbell, H.J., and Campbell, J.D. 1982. Permian fossils from the Countess Formation, Mossburn, Southland. New Zealand Journal of Geology and Geophysics, 25:101-108.
Ishibashi, T. 1998. Dorashamian biostratigraphy of the Doi Pha Phlung area, North Thailand. Proceedings of the Royal Society of Victoria, 110:221-226.
Jablonski, D. 2002. Survival without recovery after mass extinctions. Proceedings of the National Academy of Sciences, 99(12):8139-8144.
https://doi.org/10.1073/pnas.102163299
Jell, P.A. and Adrain, J.M. 2003. Available generic names for trilobites. Memoirs of the Queensland Museum, 48(2):331-553.
Jin, Y., Shen, S., Henderson, C.M., Wang, X., Wang, W., Wang, Y., Cao, C., and Shang, Q. 2006. The Global Stratotype Section and Point (GSSP) for the boundary between the Capitanian and Wuchiapingian Stage (Permian). Episodes, 29(4):253-262.
https://doi.org/10.18814/epiiugs/2006/v29i4/003
Kani, T., Hisanabe, C., and Isozaki, Y. 2013. The Capitanian (Permian) minimum of 87Sr/86Sr ratio in the mid-Panthalassan paleo-atoll carbonates and its demise by the deglaciation and continental doming. Gondwana Research, 24:212-221.
https://doi.org/10.1016/j.gr.2012.08.025
Kayser, E. 1883. Obercarbonische fauna von Lo-Ping. In F. von Richthofen (ed.), China: Ergebnisse Eigener Reisen und Darauf Gegründeter Studien, 4:160-208. (In German)
Kobayashi, T. 1987. A Permian trilobite from Spitsbergen, Norway with a note on the biogeographic bearing of genus Neoproetus. Proceedings of the Japan Academy, Series B, 63:139-142.
Kobayashi, T. and Hamada, T. 1979. Permo-Carboniferous trilobites from Thailand and Malaysia. Geology and Palaeontology of Southeast Asia, 20:1-21.
Kobayashi, T. and Hamada, T. 1980. Three new species of Permian trilobites from West Japan. Proceedings of the Japan Academy, Ser. B, 56(3):120-124.
Kobayashi, T. and Hamada, T. 1981. Trilobites of Thailand and Malaysia. Proceedings of the Japan Academy, Ser. B, 57(1):1-6.
Kobayashi, T. and Hamada, T. 1982. Advance reports on the Permian trilobites of Japan. I. Outline of the Permian trilobite fauna. Proceedings of the Japan Academy, Ser. B, 58(3):45-48.
Kobayashi, T. and Hamada, T. 1984a. Permian trilobites of Japan in comparison with Asian, Pacific and other faunas. Palaeontological Society of Japan, Special Papers, 26:1-92.
Kobayashi, T. and Hamada, T. 1984b. The Middle and Upper Permian trilobites from the Akasaka limestone in Gifu Prefecture, West Japan. Proceedings of the Japan Academy, Series B, 60(1):1-4.
Kobayashi, T. and Hamada, T. 1985. A Late Permian trilobite from Yamaguchi Prefecture with a note on the contemporaneous trilobites in Eurasia. Proceedings of the Japan Academy, Ser. B 61(7):281-283.
Kobayashi, T. and Hamada, T. 1987. A Permian Trilobite from Spitsbergen, Norway with on the Biogeographic Bearing of Genus Neoproetus. Proceedings of the Japan Academy, Ser. B 63(5):139-142.
Kobayashi, T. and Sakagami, S. 1989. A Permian trilobite from north Thailand. Proceedings of the Japan Academy, Series B, 65:67-69.
Koizumi, H. and Sasaki, K. 1978. Occurrence of Permian trilobites at Omote-Matsukawa and Myoga-sawa, Kesenuma City, Miygai Prefecture. Chigaku-Kenkyu, 33:299-311.
Kolar-Jurkovšek, T., Jurkovšek, B., Nestell, G.P., and Aljinović, D. 2018. Biostratigraphy and sedimentology of Upper Permian and Lower Triassic strata at Masore, Western Slovenia. Palaeogeography, Palaeoclimatology, Palaeoecology, 490:38-54.
https://doi.org/10.1016/j.palaeo.2017.09.013
König, H. and Kuss, S. 1980. Neue Daten zur Biostratigraphie des permotriadischen Autochthons der Insel Kreta (Griechenland). Neues Jahrbuch für Geologie und Paläontologie – Monatshefte, 9:525-540. (In German)
Kotlyar, G.V. 1989. Brakhiopody: 117-125. In Kotlyar, G.V. and Zakharov, Yu.D. (eds.), Pozdnepermskiy etap evolyucii organicheskogo mira: Midijskij yarus SSSR. Akademia Nauk SSSR & Dalnevostochnoe otdelenie geologicheskogo instituta & Ministerstvo geologii SSSR & Vsesoyuznyj nauchno-issledovatelskij geologicheskij institut imeni A.P. Karpinskogo, Leningrad-Moscow (In Russian)
Kotlyar, G.V., Zakharov, Yu.D., and Kochirkevich B.V. 1983. Pozdnepermskij etap evolyucii organicheskogo mira. (Dzhulfinskij i dorashamskij yarusy SSSR). Nauka, Leningrad. (In Russian)
Kotlyar, G.V., Zakharov, Yu.D., and Pronina, G.P. 1999a. Chansinskie otlozheniya Rossii i sopredelnykh territorij. Doklady Mezhdunarodnogo simpoziuma «Verkhnepermskie stratotipy Povolzhya», 28:241-253. (In Russian)
Kotlyar, G.V., Baud, A., Pronina, G.P., Zakharov, Y.D., Vuks, V.Y., Nestell, M.K., Belyaeva, G.V., and Marcoux, J. 1999b. Permian and Triassic exotic limestone blocks of the Crimea. Geodiversitas, 21(3):299-323.
Kotlyar, G.V., Zakharov, Y.D., and Polubotko I.V. 2004. Late Changhsingian Fauna of the Northwestern Caucasus Mountains, Russia. Journal of Paleontology, 78(3):513-527.
https://doi.org/10.1666/0022-3360(2004)078<0513:LCFOTN>2.0.CO;2
Lamsdell, J.C. and Selden, P.A. 2014. Phylogenetic support for the monophyly of proetide trilobites. Lethaia, 48(3):375-386.
https://doi.org/10.1111/let.12113
Lee, S., Shi, G.R., Nakrem, H.A., Woo, J., and Tazawa, J.-I. 2022. Mass extinction or extirpation: Permian biotic turnovers in the northwestern margin of Pangea. GSA Bulletin, 134(9-10):2399-2414.
https://doi.org/10.1130/b36227.1
Leonova, T.B. 2010. Revision of the Permian ammonoid family Cyclolobidae. Paleontological Journal, 44(3):267-274.
https://doi.org/10.1134/s0031030110030044
Lerosey-Aubril, R. 2012. The Late Palaeozoic trilobites of Iran and Armenia and their palaeogeographical significance. Geological Magazine, 149:1023-1045.
https://doi.org/10.1017/S0016756812000179
Lerosey-Aubril, R. and Angiolini, L. 2009. Permian trilobites from Antalya Province, Turkey, and enrollment in Late Palaeozoic trilobites. Turkish Journal of Earth Sciences, 18(3):427-448.
https://doi.org/10.3906/yer-0801-5
Lerosey-Aubril, R. and Feist, R. 2012. Quantitative approach to diversity and decline in Late Palaeozoic trilobites: 535-555. In Earth and life: global biodiversity, extinction intervals and biogeographic perturbations through time.
https://doi.org/10.1007/978-90-481-3428-1_16
Leven, E.Ya. 2009. Verkhnij karbon (pensilvanij) i perm Zapadnogo Tetisa: fuzulinidy, stratigrafiya, biogeografiya. Trudy Geologicheskogo instituta RAN, 590:1-238. Izdatelstvo GEOS, Moscow. (In Russian)
Likharev, B.K. 1939. Class Trilobita. Trilobity: 196-200. In Atlas rukovodyashchikh form iskopaemykh faun SSSR. Tom 6. Permskaya sistema. Izdatelstvo GONTI NKTP SSSR, Leningrad-Moscow. (In Russian)
Ling, K., Wen, H., Grasby, S. E., Zhao, H., Deng, C., and Yin, R. 2023. The Emeishan large igneous province eruption triggered coastal perturbations and the Capitanian mass extinction: Insights from mercury in Permian bauxite beds. Chemical Geology, 617:121243.
https://doi.org/10.1016/j.chemgeo.2022.121243
Liu Sh. (ed.). 1982. Palaeontological Atlas of Hunan. Ministry of Geology and Mineral Resources, Geological Memoirs, Series 2, Stratigraphy and Palaeontology, No I. Geological Publishing House, Beijing.
Liu, W., Yao, J., Tong, J., Qiao, Y., and Chen, Y. 2019. Organic matter accumulation on the Dalong Formation (Upper Permian) in western Hubei, South China: Constraints from multiple geochemical proxies and pyrite morphology. Palaeogeography, Palaeoclimatology, Palaeoecology, 514:677-689.
https://doi.org/10.1016/j.palaeo.2018.11.015
Lu, Y.H. 1974. Permian Trilobites. Nanjing: Nanjing Institute of Geology and Palaeontology, Academia Sinica, 299.
Maximova, Z.A. 1960. Nadsemejstvo Proetoidea: 131-141. In series: Orlov, Yu. A. Osnovy paleontologii. Chlenistonogie. Trilobitoobraznye i rakoobraznye. Izdatelstvo AN SSSR. (In Russian)
Mansuy, H. 1912. Mission du Laos. Mémoires du Service Géologique de l'Indochine, 1:1-52.
McCoy, F. 1847. On the fossil botany and zoology of the rocks associated with the coal of Australia. Annals and Magazine of Natural History, Series 1(20):226-236.
https://doi.org/10.1080/037454809496037
Mertmann, D. 2003. Evolution of the marine Permian carbonate platform in the Salt Range (Pakistan). Palaeogeography, Palaeoclimatology, Palaeoecology, 191(3-4):373-384.
https://doi.org/10.1016/s0031-0182(02)00672-7
Miklouho-Maclay, A.D. and Muratov, M.V. 1958. O kamennougolnykh i permskikh porodakh Gornogo Kryma. Izvestiya VUZov. Geologiya i razvedka, 8:30-35. (In Russian)
Miklouho-Maclay, K.V. 1954. Foraminifery verkhnepermskikh otlozhenij Severnogo Kavkaza. Gosgeoltekhizdat, Moskva. (In Russian)
Miklouho-Maclay, K.V. 1956. Verkhnepermskie otlozheniya Severo-Zapadnogo Kavkaza: 60-78. In Tolstikhina, M.M. (ed.), Materialy po geologii evropejskoj territorii SSSR, 14. Gosgeoltekhizdat, Moscow. (In Russian)
Mychko, E.V. 2012. Revision of Trilobites of the Genus Paraphillipsia Tumanskaya, 1930, from the Permian Olistoliths of Crimea. Paleontological Journal, 46(6):575-582.
https://doi.org/10.1134/S003103011206007X
Mychko, E.V. 2016. Trilobites of the Middle-Upper Carboniferous and Permian of Northern Eurasia. Unpublished thesis of the candidate of geological and mineralogical sciences. Lomonosov Moscow State University, Moscow. (In Russian)
Mychko, E.V. 2023. Early Permian Trilobites and Cyclidans from the Sterlitamak Shikhans. Geologicheskii vestnik, 2:144-158.
https://doi.org/10.31084/2619-0087/2023-2-11 (In Russian with English abstract)
Mychko, E.V. and Alekseev, A.S. 2017. Locations of Middle Carboniferous-Permian trilobites in Russia and adjacent countries. Byulleten' Moskovskogo obshchestva isptytatelej prirody. Otdel geologicheskij [Bulletin of the Moscow Society of Naturalists. Geological series], 92(3):40-83. (In Russian with English abstract)
Mychko, E.V. and Alekseev, A.S. 2018. Trilobites from Gzhelian (Pennsylvanian) of Moscow Region. Paleontological Journal, 52(5):506-519
https://doi.org/10.1134/S0031030118050088
Mychko, E.V. and Savchuk, O.V. 2019. A new brachymetopid trilobite from the Early Permian Shakhtau reef complex of the southwestern Urals, Bashkortostan, Russia. Zootaxa, 4555(3):346-358.
https://doi.org/10.11646/zootaxa.4555.3.4
Nakazawa, K. 1999. Permian bivalves from West Spitsbergen, Svalbard Islands, Norway. Paleontological Research, 3(1):1-17.
Oehlert, D.V. 1886. Etude sur quelques trilobites du groupe des Proetidae. Bulletin de la Société d’Etudes Scientifique de l’Angers, N.S.,15:1-23. (In French)
Osmólska, H. 1968. Brachymetopus McCoy (Trilobita) in the Carboniferous of Poland and U.S.S.R. Acta Palaeontologica Polonica, 13(3):359-374.
Osmólska, H. 1970. Revision of non-cyrtosymbolinid trilobites from the Tounraisian-Namurian of Eurasia. Palaeontologia Polonica, 23:1-165.
Owens, R.M. 1983. A review of Permian trilobite genera. Palaeontology, 30:15-41.
Owens, R.M. 1986. The Carboniferous trilobites of Britain. Part 1. Palaeontographical Society – London, 138(570):1-26.
Owens, R.M. 2003. The stratigraphical distribution and extinctions of Permian trilobites. Special Papers in Palaeontology, 30:15-41
Owens, R.M. and Hahn, G. 1993. Biogeography of Carboniferous and Permian trilobites. Geologica et Palaeontologica, 27:165-180.
Özdikmen, H. 2009. Nomenclatural changes for twenty trilobites genera. Munis Entomology and Zoology, 4(1):155-171.
Permophiles. 2023. Newsletter of the Subcommission on Permian Stratigraphy 75.
https://permian.stratigraphy.org/publications
Posenato, R., Pelikán, P., and Hips, K. 2005. Bivalves and brachiopods near the Permian-Triassic boundary from the Bükk Mountains (Bálvány-North section, northern Hungary). Rivista Italiana di Paleontologia e Stratigrafia, 111(2):215-232.
https://doi.org/10.13130/2039-4942/6304
Prantl, F. and Přibyl, A. 1950. Revise čeledi Otarionidae R. a E. Richter z českého siluru a devonu (Trilobitae). Vydal Státní Geologický ústav Československé Republiky, Nákl. Vědecko-Technického Nakladatelství Tiskem Státní Tiskárny. Oddíl paleontogický, 17:1-160. (In Czech)
Přibyl, A. and Vaněk, J. 1981. Studie zur Morphologie und Phylogenie der familie Otarionidae R. & E. Richter (Trilobita). Palaeontographica Abteilung A, 173:160-208.
Pronina, G.P. and Nestell, M.K. 1997. Middle and late Permian Foraminifera from exotic limestone blocks of the Alma River Basin, Crimea. Late Paleozoic Foraminifera, their biostratigraphy, evolution, and paleoecology and the Mid-Carboniferous boundary: 111-114. In Ross, C.A., Ross, J.R., and Brenckle P.L. (eds.), Cushman Found. Foraminiferal Res. Spec. Publ., 36.
Pronina-Nestell, G.P. and Nestell, M.K. 2001. Late Changhsingian foraminifers of the Northwestern Caucasus. Micropaleontology, 47(4):1-30.
https://doi.org/10.2113/47.3.205
Qian, Y.Y. 1981. Some late Paleozoic trilobites from Xizang (Tibet): 335-339. In Palaeontology of Xizang, Book III, The Series of the Scientific Expedition to Qinghai-Xizang Plateau.
Qian, Yi. 1977. Upper Permian trilobites from Qinglong and Anshun of Guizhou. Acta Palaeont. Sinica, 16(2):279-286. (In Chinese)
Qiao, F., Zhang, Y., Wang, Y., Yuan, D., Ju, Q., Xu, H., Zhang, H., Zheng, Q., Cai, Y., Hou, Z., and Shen, S. 2021. An updated age of Permian strata in the Raggyorcaka and Qamdo areas, Tibet and their paleogeographic implications. Palaeogeography, Palaeoclimatology, Palaeoecology, 582:110660.
https://doi.org/10.1016/j.palaeo.2021.110660
Raczyński, P. and Biernacka, J. 2014. Zechstein in Lithuanian-Latvian Border Region. Geologija, 56/2(86):57-62.
Ramovš, A., 1958a. Razvoj zgornjega perma v Loških in Pohograjskih hribih. Razprave IV. razreda Slovenske akademije znanosti in umetnosti, 1:451-622. (In Slovenian)
Ramovš, A., 1958b. O faciesih v zgornjem wordu in zgornjem permu v Sloveniji. Geologija, 4:188-190. (In Slovenian)
Rampino, M.R. and Shen, S.-Z. 2019. The end-Guadalupian (259.8 Ma) biodiversity crisis: the sixth major mass extinction? Historical Biology, 33(5):716-722.
https://doi.org/10.1080/08912963.2019.1658096
Reed, F.R.C. 1942. Some new Carboniferous trilobites. The Annals and Magazine of Natural History, 11 series, 9(57): 649-672.
Reed, F.R.C. 1943. The genera of British Carboniferous trilobites. The Annals and Magazine of Natural History, 11 series, 10(61):54-65.
Reinhardt, J.W. 1988. Uppermost Permian reefs and Permo-Triassic sedimentary facies from the southeastern margin of Sichuan Basin, China. Facies, 18:231-288.
Robinson, V.N. 1932. Geologicheskij obzor oblasti triasa i paleozoya bassejnov rek Laby i Beloj na Severnom Kavkaze. Trudy Vsesoyuznogo geologo-razvedochnogo ob"edineniya, 226:1-55. Gosudarstvennoe nauchno-tekhnicheskoe geologo-razvedochnoe izdatelstvo, Leningrad-Moscow. (In Russian)
Roemer, F. 1880. Über eine Kohlenkalk fauna der Westküste von Sumatra. Palaeontographica Abteilung A, 27:1-11. (In German)
Ruggieri, G. 1959. Una nuova Trilobite del Permiano del Sosio (Sicilia). Memorie degli istituti di Geologia e Mineralogia dell’Università di Padova, 21:1-10. (In Italian)
Sameeni, S.J. 2009. The Salt Range: Pakistan's unique field museum of geology and paleontology=[Le Salt Range: un musée de géologie et de paléontologie à ciel ouvert au Pakistan]. In PaleoParks: the protection and conservation of fossil sites worldwide (pp. 65-73). Université de Bretagne occidentale Département des sciences de la terre.
Sarkar, S.S. 1968. Revision of trilobites from the Carboniferous and Permian of India. Records of the Geological Survey of India, 95(2):525-528.
Schraut, Von G. 2019. Paraphillipsia ? carnica n. sp – Eine neue Trilobitenart aus den roten Kalken der Zottachkopf-Formation, jüngste Rattendorf-Gruppe (jüngeres Artinskium, Unterperm) der Karnischen Alpen (Österreich). Carinthia, II(209/129):617-634. (In German)
Schraut, Von G. 2020. Trilobiten aus der Grenzland-Formation, mittlere Rattendorf-Gruppe (Sakmarium, Unterperm) der Karnischen Alpen (Österreich). Jahrbuch der Geologischen Bundesanstalt, 160(1-4):207-225. (In German)
Schréter, Z. 1948. Trilobiták a Bükk Hegységből. Földtani közlöny, 78:25-39. (In Hungarian)
Schweitzer, C.E., Mychko, E.V., and Feldmann R.M. 2020. Revision of Cyclida (Pancrustacea, Multicrustacea), with five new genera. Neues Jahrbuch für Geologie und Paläontologie – Abhandlungen, 296(3):245-303.
https://doi.org/10.1127/njgpa/2020/0905
Shen, J., Yu, J., Chen, J., Algeo, T.J., Xu, G., Feng, Q., Shi, X., Planavsky, N.J., Shu, W., and Xie, S. 2019. Mercury evidence of intense volcanic effects on land during the Permian-Triassic transition. Geology, 47:1117-1121.
https://doi.org/10.1130/G46679.1
Shen, S.-Z. 2018. Global Permian brachiopod biostratigraphy: an overview. Geological Society, London, Special Publications, 450(1):289-320.
https://doi.org/10.1144/SP450.11
Shen, S.-Z. and He, X.L. 1991. Changhsingian brachiopod assemblage sequence in Zhongliang Hill, Chongqing. Journal of Stratigraphy, 15(3):189-196.
Shen, S.-Z. and He, X.L. 1994. Changhsingian brachiopod faunas from Guiding, Guizhou. Acta Palaeontologica Sinica, 33:440-454.
Shen, S.-Z. and Shi, G.R. 2004. Capitanian (Late Guadalupian, Permian) global brachiopod palaeobiogeography and latitudinal diversity pattern. Palaeogeography, Palaeoclimatology, Palaeoecology, 208(3-4):235-262.
https://doi.org/10.1016/j.palaeo.2004.03.009
Shen, S.-Z., He, X.L., and Shi, G.R. 1995. Biostratigraphy and correlation of several Permian-Triassic boundary sections in southwestern China. Journal of Southeast Asian Earth Sciences, 12(1-2):19-30.
Shen, S.-Z., Wang, Y., Henderson, C.M., Cao, C.-Q., and Wang, W. 2007. Biostratigraphy and lithofacies of the Permian System in the Laibin-Heshan area of Guangxi, South China. Palaeoworld, 16(1-3):120-139.
https://doi.org/10.1016/j.palwor.2007.05.005
Shi, Z., Lu, L., Yin, G., Long, H., Li, W., Yang, H., Cao, R., Tian, X., Tan, Q., Wang, K., Wang, Y., and Tian, Y. 2016. Remains of trilobites and other species discovered in a volcanic ash bed of the end-Permian, Yangtze craton, South China. International Geology Review, 59(7):905-917.
https://doi.org/10.1080/00206814.2016.1244777
Signor, P.W. III and Lipps, J.H. 1982. Sampling bias, gradual extinction patterns and catastrophes in the fossil record. Geological Society of America Special Papers, 291-296.
Song, H., Algeo, T. J., Song, H., Tong, J., Wignall, P.B., Bond, D.P.G., Zheng, W., Chen, X., Romaniello, S.J., Wei, H., and Anbar, A.D. 2023. Global oceanic anoxia linked with the Capitanian (Middle Permian) marine mass extinction. Earth and Planetary Science Letters, 610:118128.
https://doi.org/10.1016/j.epsl.2023.118128
Teichert, C. 1944. Permian trilobites from Western Australia. Journal of Paleontology, 18(5):455-463.
Termier, H. and Termier, G. 1974. Pseudophillipsia azzouzi nov. sp. trilobite griffithididé du Permien du Djebel Tebaga (Tunisie). Geobios, 7:257-265.
Tesch, P. 1923. XXI. Trilobiten aus der Dyas von Timor und Letti. ln: Wanner, J. Paläontologie von Timor nebst kleineren Beiträgen zur Paläontologie einiger anderer Inseln des ostindischen Archipels. 12. Lieferung:123-132.
Toumansky, O. 1930. Permocarbonische Trilobiten der Krim. Zentralblatt der Mineralogie, Geologie, Paläontologie, Abteilung B (Geologie, Paläontologie), für 1930:473-477. (In German)
Toumanskaya, O. 1935. The Permo-Carboniferous beds of the Crimea – Part 2: The Permo-Carboniferous trilobites of the Crimea: 1-37 [rus.], 38-63 [eng.]. Glawnoe geologo-hidrogeodesitscheskoe uprawlenie. Zentralny naustschno-issledowatelskij geologo-raswedotschnyi institut (ZNIGRI), Leningrad – Moscow. (In Russian and English)
Uchman, A., Hanken, N.M., Nielsen, J.K., Grundvåg, S.A., and Piasecki, S. 2016. Depositional environment, ichnological features and oxygenation of Permian to earliest Triassic marine sediments in central Spitsbergen, Svalbard. Polar Research, 35(1):24782.
Ueno, K. and Sakagami, S. 1991. Late Permian fusulinacean fauna of Doi Pha Phlung, north Thailand. Transactions and Proceedings of the Paleontological Society of Japan, N.S., 164: 928-943.
Ueno, K. and Tsutsumi, S. 2009. Lopingian (Late Permian) foraminiferal faunal succession of a Paleo‐Tethyan mid‐oceanic carbonate buildup: Shifodong Formation in the Changning-Menglian Belt, West Yunnan, Southwest China. Island Arc, 18(1):69-93.
https://doi.org/10.1111/j.1440-1738.2008.00648.x
Vyalov, O.S. 1934. Geologicheskie issledovaniya 1931 g. v Zapadnom Kavkaze. Zapiski Vserossijskogo mineralogicheskogo obshchestva. 2/63(1):271-292. (In Russian)
Wang, H., Shao, L., Hao, L., Zhang, P., Glasspool, I.J., Wheeley, J.R., Wignall, P.B., Yi, T., Zhang, M., and Hilton, J. 2011. Sedimentology and sequence stratigraphy of the Lopingian (Late Permian) coal measures in southwestern China. International Journal of Coal Geology, 85(1):168-183.
https://doi.org/10.1016/j.coal.2010.11.003
Waterhouse, J.B. 1983. A Late Permian lyttoniid fauna from northwest Thailand. Papers -Department of Geology, University of Queensland, 10(3):111-153.
Weber, G.F. 1915. Nakhodka verkhne-kamennougolnykh trilobitov v Krymu. Izvestiya Imperatorskoj Akademii nauk, 5(14):45-49. (In Russian)
Weber, V.N. 1932. Trilobity Turkestana. Trudy Vsesoyuznogo Geologo-Razvedochnogo Ob’edineniya N.K.T.P-S.S.S.R, 178:1-157. (In Russian)
Weber, V.N. 1933. Trilobity Doneckogo bassejna [Trilobites of the Donetz Basin]. Transactions of the United Geological and Prospecting Service of USSR, 255:1-90, Leningrad – Moscow. (In Russian and English)
Weber, V.N. 1937. Trilobites of the Carboniferous and Permian deposits of the USSR. Issue. 1. Carboniferous trilobites. Monografii po paleontologii SSSR [Monographs on paleontology of the USSR]. Volume LXXI. Main edition of geological exploration and geodetic literature, Leningrad-Moscow, 160 p. (In Russian and English)
Weber, V.N. 1944. Trilobity kamennougol'nykh i permskikh otlozheniy SSSR. Vypusk 2. Permskiye trilobity. Monografii po paleontologii SSSR: 1-30. (In Russian and English)
Weller, J.M. 1935. Permian trilobites from the Central Himalayas. Memoirs of the Connecticut Academy of Arts and Sciences, 9(3):31-35.
Weller, J.M. 1944. Permian trilobite genera. Journal of Paleontology, 18:320-327.
https://www.jstor.org/stable/1299134
Weller, J.M. 1959. Superfamily Proetacea Salter, 1864. In H.J. Harrington, J.M. Weller, B.F. Howell et al. (eds.), Treatise on Inverlebrate Paleontology. Part O. Arthropoda 1. Protarthropoda. Trilobitomorpha, 382-415.
Winkler Prins, C.F. 2008. Some spiriferid brachiopods from the Permian of Timor (Indonesia). Proceedings of the Royal Society of Victoria, 120(1): 389-400.
Wu, R. and Feng Q. 1991. Carboniferous trilobites from Xinjiang, NW-China. Geologica et Palaeontologica, 25:137-145.
Wu, W.-S. and Wang, Z.-H. 1974. Permian corals: 296-299. In Nanjing Institute of Geology and Palaeontology, Academia Sinica (ed.), Handbook of the Stratigraphy and Paleontology of Southwest China. Science Press, Beijing. (In Chinese)
Yang, Z.Y., Yin, H.F., Wu, S.B., Yang, F.Q., Ding, M.H., and Xu, G.R. 1987. Permian-Triassic Boundary Stratigraphy and Fauna of South China. PRC Ministry of Geology and Mineral Resources, Geological Memoirs, Series.
Yin, G.Z. 1978. Trilobita. Paleontological Atlas of Southwest China, Guizhou, 2:440-445. (In Chinese)
Yuan, J.L., Zhao, Y.L., and Mao J.Q. 1992. On a new genus Acanthophillipsia of Ditomopyginae Hupe, 1953 from Lower Permian of Guiyang, South China. Acta Palaeontologica Sinica, 31:39-52.
Zeng, Y., He, X.L., and Zhu, M.L. 1995. Permian Brachiopods and Community Succession in the Huayin Mountains, Sichuan. China University of Mining and Technology Press, Xuzhou. (In Chinese)
Zhang, Q.Z. 1982. Trilobita: 326-329, 484-485, pl. 125. In Nanjing Institute of Geology and Palaeontology, Academia Sinica (ed.), Palaeontological Atlas of East China, 2. (In Chinese)
Zhang, T.-R. 1983. Trilobita: 534-555. In Palaeontological Atlas of Northwest China. Xinjiang-Weiwuer Autonomic Province. Part 2: Late Paleozoic. Beijing. (In Chinese)
Zhou, T.M. 1977. Trilobita. Palaeontological Atlas of South-Central China, 2:586-593. (In Chinese)
Zhou, T.M. 1987. Trilobites: 313-329 (eng.). In Late Carboniferous and Early Permian cephalopods, gastropods, bryozoans, conodonts and trilobites from Longlin, Guangxi. Bulletin of the Yichang Institute of Geology and Mineral Resources, Chinese Academy of Geological Sciences, 11:285-312. Geological Publishing House, Beijing.

