 The integument of pelagic crocodylomorphs (Thalattosuchia: Metriorhynchidae)
The integument of pelagic crocodylomorphs (Thalattosuchia: Metriorhynchidae)
Article number: 24.2.a25
https://doi.org/10.26879/1099
Copyright Paleontological Society, July 2021
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 3 June 2020. Acceptance: 16 June 2021.
ABSTRACT
Metriorhynchidae are the only archosaurs that show adaptations to a highly pelagic lifestyle. This morphology is paralleled by ichthyosaurs and plesiosaurs, which share a streamlined, largely scaleless skin surface. Although published metriorhynchid material indicates a similar condition, no detailed description of their integument has been carried out. New data from several specimens representing at least three genera are shown here, revealing a uniform skin type lacking any traces of scales or scutes and instead showing folded and transverse fibers. A hypocercal tail fluke comparable to that of ichthyosaurs is present in metrorhynchids. Surface anomalies on metriorhynchid skin are interpreted as potential epizootic scars, similar to those from barnacles, or bite marks. A broad comparison implies deep homologies with plesiosaur and ichthyosaur skin. We also describe skin details of early (teleosauroid) thalattosuchians for the first time. Unlike Metriorhynchidae, their integument is demonstrated to be very similar to that of extant crocodylians, which can be partially explained by their inferred onshore behavior. There is generally a high threshold for extensive skin modifications in aquatic reptiles.
Frederik Spindler. Dinosaurier Museum Altmühltal, Dinopark 1, 85095 Denkendorf, Germany. mail@frederik-spindler.de
René Lauer. Lauer Foundation for Paleontology, Science and Education, Wheaton, Illinois, USA. rene@lauerfoundationpse.org
Helmut Tischlinger. Tannenweg 16, 85134 Stammham, Germany and Jura-Museum Eichstätt, Willibaldsburg, 85072 Eichstätt, Germany. htischlinger@online.de
Matthias Mäuser. Staatliche Naturwissenschaftliche Sammlungen Bayerns, Naturkunde-Museum Bamberg, Fleischstr. 2, 96047 Bamberg, Germany. maeuser@snsb.de
Keywords: Crocodyliformes; pelagic adaptation; marine reptiles; epizoa; Jurassic; plattenkalk
Final citation: Spindler, Frederik, Lauer, René, Tischlinger, Helmut, and Mäuser, Matthias. 2021. The integument of pelagic crocodylomorphs (Thalattosuchia: Metriorhynchidae). Palaeontologia Electronica, 24(2):a25. https://doi.org/10.26879/1099
palaeo-electronica.org/content/2021/3399-metriorhynchid-skin
Copyright: July 2021 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
During the transition from terrestrial to a secondarily aquatic lifestyle, some amniote lineages underwent substantial modification of their integument, in large part to increase hydrodynamic efficiency. Cenozoic mammals, most dramatically cetaceans, lost their fur and evolved a smooth, highly streamlined skin (Themudo et al., 2020). This adaptation was somewhat paralleled by the Mesozoic ichthyosaurs (Fraas, 1888; Lingham-Soliar and Plodowski, 2007; Renesto et al., 2020) and plesiosaurus (Frey et al., 2017; Vincent et al., 2017), which lost the scaly integument of their terrestrial reptile ancestors in favor of smooth skin. Not all marine reptile groups became scaleless, however. Marine turtles retain scaly skin, although they exhibit a leathery, less keratinized skin around their major joints and scutes covering the shell have been lost in Dermochelys (Maderson, 1972). Exceptionally preserved specimens confirm that the integument of the Late Cretaceous, fully marine mosasaurs retained the typical scales of squamates (Lindgren et al., 2009, 2010), and a comparable condition is present in the Jurassic pleurosaurids, a group of highly modified aquatic rhynchocephalians (Broili, 1926). Several crocodyliform lineages became aquatic during the Mesozoic, of which the Thalattosuchia were most highly adapted for a marine lifestyle (Young et al., 2010). Thalattosuchia is composed to two major subclades, of which the Teleosauroidea was less specialized for marine life. Teleosauroids exhibit rows of osteoderms similar to those of the vast majority of other crocodyliforms (Johnson et al., 2020b), but their soft tissue anatomy is poorly known and has received only cursory review in the literature (e.g., Westphal, 1962). For the more specialized thalattosuchians, the Metriorhynchoidea, early researchers argued for a fully marine lifestyle and smooth skin (e.g., Fraas 1901, 1902), but with limited actual documentation of soft tissue preservation. Here, we describe extensive new soft tissue records for thalattosuchians from the Late Jurassic plattenkalk localities of Wattendorf and Painten (Bavaria, Germany), comparing them to other marine reptiles in order to shed light on the evolution and function of the metriorhynchid integument.
Reptilian Integumentary Terminology
Reptiles are usually associated with a ‘scaly’ appearance, which is often popularly assumed to reflect an ancestral condition. However, what are called ‘scales’ in different reptilian subclades are not identical in structure or evolutionary history. The overlapping, epidermally-defined scales of Lepidosauria (squamate lizards and snakes [including mosasaurs], as well as the tuatara and its fossil kin, see Maderson, 1968) differ fundamentally from the non-overlapping scales of crocodiles, turtles, and bird limbs. Scales in these latter taxa are covered with rigid β-keratin and interlinked by α-keratin hinges (Alibardi et al., 2009; Rutland et al., 2019) and are inferred to represent the more ancient condition. Whereas lepidosaurs perform singular ecdysis, these other reptiles shed their epidermis in fragmented flakes (Spearman and Riley, 1969). Variability in scale development can occur even within a single taxon; in crocodylians, for example, the ‘scales’ on the head are the result of rhomboidal fracturing of the skin during development (Milinkovitch et al. 2013), unlike the genetically-modeled scales elsewhere on the body.
In fossils, integumentary structures such as scales are infrequently preserved and can be difficult to discern. Their recognition in fossil material is often predicated on the presence of regular arrangements of discretely-bounded structures. However, similar structures in fossils can be produced by folding of the skin (either post mortem or as a natural condition in the living animal). Both fine squamation and detailed, regular folding occur in areas of increased skin movement in life. Differentiation of these in fossils can be problematic, but folds can generally be recognized by gradual fading of their edges, unlike the sharp boundaries present in all scales.
In the connective tissue of the integument, fibers occur, which also form the initial stages of osteoderm growth (Vickaryous and Sire, 2009). Dermal fibers are flexible, strengthening elements that would have maintained structural integrity in the skin of scale-less marine saurians (Lingham-Soliar and Plodowski, 2007; Lindgren et al., 2011), and are detectable in fossils as strong, discrete, straight-to-weakly curved elements arranged in parallel sets. Fossilized muscular fascia, which could be confused with skin fibers, appear less sharp and exhibit broader stripes instead of unidimensional fibers.
Specimen Repositories
Fossil specimens, which contributed to our detailed analysis for this study, are housed in the following paleontological repositories: LF - Lauer Foundation for Paleontology, Science and Education, Wheaton, IL USA; NKMB - Naturkunde-Museum Bamberg, Bavaria, Germany; DMA - Dinosaurier Museum Altmühltal, Bavaria, Germany. Additional specimens referenced in this work are housed at: NHMUK - Natural History Museum, London, UK; SMNS - Staatliches Museum für Naturkunde Stuttgart, Baden-Württemberg, Germany; BMMS - Bürgermeister Müller Museum Solnhofen, Bavaria, Germany; JME - Jura-Museum Eichstätt, Bavaria, Germany.
METHODS
The current study addresses macroscopic structures observed and photographed under daylight (herein visible light) and ultraviolet light (UV, or Ultraviolet Induced Fluorescence, UVIF). Frequent UV investigations during the past 20 years have shown that fossils from the Upper Jurassic limestones of southern Germany are often auto-fluorescent (e.g., Tischlinger, 2002, 2005, 2015; Tischlinger and Unwin, 2004; Frey and Tischlinger, 2012; Tischlinger and Arratia, 2013; Schwarz et al., 2019). This technique allows for more precise investigation of morphological details of both hard and mineralized soft tissue, including delicate structures that are poorly or not discernible in visible light.
UV investigations for DMA and NKMB specimens were carried out with the help of different high-performance UV-A lamps with a wavelength of 365-366 nanometers and intensities between 4000 and more than 50 000 microwatts per 10 mm², depending on the distance concerned and the number of lamps: 2 Benda UV-lamps, type N, 16 watt, UV A, 366 nanometers (nm), size of filter 200 mm x 50 mmm. 1 Labino UV-lamp, spotlight S 135, 35 watt, UV-A, 365 nm with custom-made Midlight-reflector-inset. 1 UV-inspection lamp Convoy S2+ UV 365 nm with Li-ion 1865.
The photo documentation was done with Panasonic Lumix GX80 with Lumix G-Vario 3,5-5,6 /14-140 mm, Leica DG Summilux 1,4/25 mm ASPH and Panasonic macro lens H-HSO30E Lumix G macro 30 mm F2.8 O.I.S. Applying different filters allowed a selective visualization of some fine structures. Color compensation filters (yellow, cyan, and magenta of different types and densities) made of special colored glass or gel were placed atop the camera lens. The furthermost filter was a UV-filter, which blocked UV light up to 390 nanometers.
Ultraviolet Induced Fluorescence (UVIF) Digital Photography of LF specimens was utilized to identify and document mineralization of rarely seen soft tissue preservation, such as skin and muscle; and fine morphological details and structures of hard surfaces, such as teeth and bones. The technique also helps to identify areas of repair and restoration, which impact accurate analysis of fossil specimens and their enclosing matrix. All specimens were photographed under both visible light and UV light, results of which were provided as RAW files.
UVIF photographic documentation was performed with the use of the following photographic equipment: A Nikon D4 DSLR camera (without sensor modification), tethered to a 4K monitor, and a wireless shutter release. Camera lenses include: AF Nikkor 28 mm f2.8; AF-S micro Nikkor 60 mm 2.8G ED; and Nikkor 20 mm lens. Filters include: a linear polarizing filter, used for both visible light and UVIF photography: MidOpt PRO 32-62; (+ polarizing film on the lamp for some visible light images); and a color correction balancing filter, used for UVIF photography: (orange) MidOpt LAT20-62.
Lights/lamps utilized for visible light photography include: two Fotodiox ring lights, color temperature of 5500k, and use of diffusers (one diffuser fitted with polarizing film was used in combination with a linear polarizing lens filter (cross-polarization) to reduce unwanted glare and provide definition.) Lights/lamps utilized for UVIF photography include: a 95 watt Quad High-Performance UV Lamp; equipped with 3 - 95W bulbs: UV A (320 - 400 nm), UV B (280 - 320 nm), and UV C (280 - 100 nm) wavelengths; Hoya U-325 and Komodo UV-400 filters; and a remote control. UV wavelengths were used individually, and together, to identify specific UVIF responses. In this case UV A, B, and C wavelengths were used together. All UVIF images were captured in a darkened room with the UV lamp as the sole light source. Additional UV lights/lamps include: 2 - 9 watt, triple UV lamps: equipped with UV A, B and C wavelength bulbs, used individually and together, fitted with Hoya U325C and Kokomo UV-400 filters; 2 - 9 watt custom-designed triple UV Gooseneck lamps, and equipped with filters to utilize UV A, B or C individually; and 2 UV/LED flashlights were used for investigation: Convoy S2+ 365 nm UV with LG LED.
Additional equipment includes customized stands and mounts, designed to stabilize all elements during long exposure photography of UVIF images. They are fully adjustable to easily accommodate a wide variety of sized specimens, while also providing consistent and repeatable photographic results including: a custom-built lamp stand for the 95 Watt UV Quad lamp array; a camera stand, Manfrotto #806 Salon 190; a 3-way geared pan/tilt head with quick-release plate, Manfrotto #410 and #410PL; and a table with motorized lift. In addition, customized identification tiles and cubes (1 cm) were utilized to identify data not captured within the camera’s metadata such as the UV wavelengths, the specific filters, and provide scale.
Additional macro photography of specific details of specimens in the LF, DMA, JME, and NKMB collections were captured with a Canon EOS 600D, macro lens EFS 18-55 mm, a polarizing filter, and tripod.
GEOLOGICAL BACKGROUND
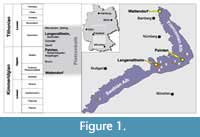 The sites of the Late Jurassic fossils discussed herein are located on the northern and southern Franconian Alb (Wattendorf and Painten, respectively; Figure 1), Bavaria, Germany. Both localities are outcrops of laminated carbonates (plattenkalks), which are typical for the famous Konservatlagerstaetten of the Solnhofen Archipelago. The timeframe for their deposition ranges from upper Kimmeridgian to lower Tithonian. The Wattendorf Plattenkalk contains the oldest preservation of material, dated as early late Kimmeridgian age (Pseudomutabilis subzone, see Schweigert, 2007). The Painten Plattenkalk was also deposited earlier than the typical Solnhofen plattenkalk environment and dates to the late Kimmeridgian (Ulmense subzone).
The sites of the Late Jurassic fossils discussed herein are located on the northern and southern Franconian Alb (Wattendorf and Painten, respectively; Figure 1), Bavaria, Germany. Both localities are outcrops of laminated carbonates (plattenkalks), which are typical for the famous Konservatlagerstaetten of the Solnhofen Archipelago. The timeframe for their deposition ranges from upper Kimmeridgian to lower Tithonian. The Wattendorf Plattenkalk contains the oldest preservation of material, dated as early late Kimmeridgian age (Pseudomutabilis subzone, see Schweigert, 2007). The Painten Plattenkalk was also deposited earlier than the typical Solnhofen plattenkalk environment and dates to the late Kimmeridgian (Ulmense subzone).
Numerous hypotheses have been presented to explain the origin of these Konservatlagerstaetten (Viohl, 2015). The current understanding of this paleoenvironment is that laminated plattenkalk formed in restricted basins, so-called “wannen” (Fesefeldt, 1962), which were embedded in a relief of microbialite-sponge bioherms and biostroms. The undisturbed lamination and preservation of articulated vertebrate skeletons suggest anoxic conditions on the seafloor and in the sediment, in stagnant water conditions - some kind of a ‘dead zone’ hostile to higher life.
During the Late Jurassic these special conditions prevailed along a wide belt following the southern edge of the Central European shelf sea in delimitation to the deeper Tethys. The resultant plattenkalk range extends from Wattendorf in the northeast across the Southern Franconian Alb and the Swabian Mountains to the French Jura in the southwest. The type of sedimentary rock and the quality of fossil preservation vary considerably along this depositional area. This variance is mostly due to local synsedimentary environmental conditions. The Wattendorf Plattenkalk was deposited within a cove of a microbialite-sponge reef, which opened towards the Wattendorf-wanne. The sediment of this cove consists of laminated plattenkalk facies, intercalated between graded turbidite beds and coarsely-grained detrital beds (Fürsich et al., 2007). The sequence measures about 7 m at its deepest point and thins toward the reef. In the immediate vicinity of the reef the plattenkalk limestone turns into a very thinly laminated dolobindstone. In the latter, Dakosaurus NKMB-P-Watt06/508 was found; whereas Cricosaurus bambergensis NKMB-P-Watt14/274 comes from a sequence of very finely-grained graded beds, microbial laminae, and ultra-thin clay laminae in the center of the cove. The latter beds of the Wattendorf section are particularly notable for their excellent preservation of vertebrates (Mäuser, 2015).
The stratigraphic horizon that yielded the herein-discussed crocodyliforms from the Painten-Quarry consists of a 5-meter-thick package of graded turbidite-beds, laminated limestone (bindstone) and coccolith-beds (Meyer and Schmidt-Kaler, 1984, Albersdörfer and Häckel, 2015). They were deposited in the northern area of the Painten-wanne. The carbonates have a considerable concentration of silica, as is common for like-aged carbonates of the Southern Franconian Alb. Both the high quality of preservation and the abundance of articulated vertebrates in these beds are outstanding.
DESCRIPTION OF NEW MATERIAL
Specific Morphology
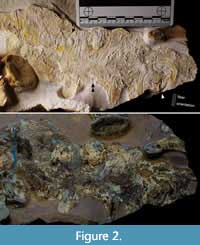 Metriorhynchidae indet. (cf. Dakosaurus), LF 3474 (Figure 2, Figure 3, Figure 4). Proposed generic assignment is based on robustness of the skeleton and known presence of this taxon in the assemblage (Young et al., 2012). A new specimen from Painten, partially disarticulated and inferred to represent a burst carcass. The trunk, hip, and hind limbs, as well as most of the tail, are preserved in separate slabs with associated soft tissue. Single vertebrae and sets of dorsal ribs are disarticulated but still closely situated. Post mortem movement resulted in some flipped-over distal phalanges. The skull was washed away in a turbidite, leaving only an impression (R. Albersdörfer, pers. comm.). Documentation was carried out under visible light and UV (directly compared in Figure 2).
Metriorhynchidae indet. (cf. Dakosaurus), LF 3474 (Figure 2, Figure 3, Figure 4). Proposed generic assignment is based on robustness of the skeleton and known presence of this taxon in the assemblage (Young et al., 2012). A new specimen from Painten, partially disarticulated and inferred to represent a burst carcass. The trunk, hip, and hind limbs, as well as most of the tail, are preserved in separate slabs with associated soft tissue. Single vertebrae and sets of dorsal ribs are disarticulated but still closely situated. Post mortem movement resulted in some flipped-over distal phalanges. The skull was washed away in a turbidite, leaving only an impression (R. Albersdörfer, pers. comm.). Documentation was carried out under visible light and UV (directly compared in Figure 2).
 Numerous skin patches are present. Some of them preserve sub-parallel folding (Figure 2, Figure 3C, D, F), some have sets of folds in crisscross arrangement (Figure 3A), or with oblique zones of repeated onset of folds (Figure 3E). The latter indicates either a major zone of movement in life or early diagenetic compression. Patches with possible musculature are present (Figure 3E). A few larger areas are smooth, possibly representing the epidermal surface when tightened (Figure 2, Figure 3C, F). Next to the knee, a single fold is sharp and deep for more than 20 mm (Figure 3D), suggesting small-scale flexibility of the outermost skin layers in life or post-mortem decay. Hinges in the form of crossing fold systems between even skin fields, usually keratinized in modern turtles and crocodylians, are not paralleled by reinforcements or scute rims (Figure 3A, B). Independent of the softly rounded folds, there are layers with long, sharp, sub-parallel fibers (Figure 2). These fibers occur in only one direction per area, transversal (perpendicular) to the long axis of the entire body.
Numerous skin patches are present. Some of them preserve sub-parallel folding (Figure 2, Figure 3C, D, F), some have sets of folds in crisscross arrangement (Figure 3A), or with oblique zones of repeated onset of folds (Figure 3E). The latter indicates either a major zone of movement in life or early diagenetic compression. Patches with possible musculature are present (Figure 3E). A few larger areas are smooth, possibly representing the epidermal surface when tightened (Figure 2, Figure 3C, F). Next to the knee, a single fold is sharp and deep for more than 20 mm (Figure 3D), suggesting small-scale flexibility of the outermost skin layers in life or post-mortem decay. Hinges in the form of crossing fold systems between even skin fields, usually keratinized in modern turtles and crocodylians, are not paralleled by reinforcements or scute rims (Figure 3A, B). Independent of the softly rounded folds, there are layers with long, sharp, sub-parallel fibers (Figure 2). These fibers occur in only one direction per area, transversal (perpendicular) to the long axis of the entire body.
 Whereas certain pieces of the fossil cannot be rearranged with certainty, three slabs can be united to show most of the tail (Figure 4). Associated soft tissue gives an impression of relative thickness in life, although diagenetic compaction might have widened the area in question. Assuming that the skin retained a relatively rigid integrity throughout a certain perimeter, the relative height of the tail in life could be estimated as about two thirds of the transverse width of soft tissue in the fossil (a factor of 0.63, where the preserved width is treated as an approximation of half of the original circumference). The tail tapers constantly towards the bending zone of the column, where the soft tissue would have tightly wrapped the vertebrae. The dorsal lobe of the fluke is incomplete but can be estimated from its discrete, darkly colored front edge. The hypocercal ventral lobe is largely complete, showing a narrow front apron and a concave, tapering rear apron, which meet in a rounded, but discrete tip.
Whereas certain pieces of the fossil cannot be rearranged with certainty, three slabs can be united to show most of the tail (Figure 4). Associated soft tissue gives an impression of relative thickness in life, although diagenetic compaction might have widened the area in question. Assuming that the skin retained a relatively rigid integrity throughout a certain perimeter, the relative height of the tail in life could be estimated as about two thirds of the transverse width of soft tissue in the fossil (a factor of 0.63, where the preserved width is treated as an approximation of half of the original circumference). The tail tapers constantly towards the bending zone of the column, where the soft tissue would have tightly wrapped the vertebrae. The dorsal lobe of the fluke is incomplete but can be estimated from its discrete, darkly colored front edge. The hypocercal ventral lobe is largely complete, showing a narrow front apron and a concave, tapering rear apron, which meet in a rounded, but discrete tip.
Irregularities are present on parts of the preserved skin of the trunk (but apparently not on the tail). Irregular skin structures include subcircular marks approximately 15 mm in diameter and incomplete ‘curl’ marks approximately 3 cm in diameter (Figure 2, Figure 3C). The surface of these areas is sometimes broken and sometimes smooth. Sharp, transversal fibers cross the smaller irregularities, but are cut or superimposed by the larger curls. Smooth skin folds bypass or stop at these irregularities, implying that the latter were stiffened, maybe representing keratinized/callused scar tissue.
Dakosaurus sp., DMA-JP-2009/001 (Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10). Nearly complete and articulated skeleton from Painten, osteology currently under description. Generic attribution based on the short, broad snout, characteristic of this genus within the Painten assemblage (Young et al., 2010). Skin preservation is present on most of the straight portion of the tail, dorsal to the trunk and around the neck. Additional soft tissue, gut contents, and diagenetic mineralization blurs detail in many regions. Observations were made under visible light and UV (Figure 5).
 |
 |
 |
The skin is preserved in layers (Figure 6A, C, Figure 8), one of which is reddish-brown and extremely brittle, covered by whitish, most likely phosphatized matter. Fine, transversal striation is visible on the best-preserved areas (Figure 5, Figure 6A, C, Figure 7). Coarser, soft folds appear short, while straight folds are long and apparently closely related to the fiber layer. Oblique and crisscross patterns are rare, with one patch observed on the neck and one on the tail (Figure 6B, C). No scutes are observed; all folds show free terminal points, not enclosed, discrete areas. Based on their positions, these patches likely represent areas of increased movement in life. On the proximal portion of the tail, sharp and discrete folds are observed, less densely parallel than in more anterior portions (Figure 8). The crisscross patch contains additional longitudinal striae (Figure 6C), which are loosely parallel and therefore likely folded, instead of indicating another layer of fibers.
 |
 |
 |
Similar to the trunk of LF 3474, the tail and probably the neck of DMA-JP-2009/001 is associated with enigmatic skin irregularities in a ‘nest-like’ concentration (Figure 7, Figure 8, Figure 9, Figure 10). The smaller, rounded marks have a diameter of about 1.2 cm, while larger, less circular structures reach up to 3.5 cm, if retaining a discrete outline at all. Fibers and discrete folds partially cross these structures (Figure 8, Figure 10) and soft-looking folds sometimes encircle the marks. The restricted compaction and shapes of these irregularities suggest a hardened structure prior to embedding.
Metriorhynchidae indet., NKMB-P-Watt06/508 (Figure 11, Figure 12, Figure 13, Figure 14, Figure 15). Complete and articulated posterior half of a medium-sized, robust skeleton from Wattendorf. Soft tissue preservation is scattered over most of the body (Figure 11), but is disrupted by extensive diagenetic alteration. Observations were made under visible light and UV documentation. Although caudal vertebrae are large up to the bending zone, the soft tissue covering tapers constantly and is confluent with the trunk. Softly folded skin is also present on the ventral belly region.
 |
 |
 |
 Fine, transversal fluting and fibers similar to the Painten specimens are present (Figure 12, Figure 14, Figure 15). However, NKMB-P-Watt06/508 also preserves many more longitudinal striae. These are poorly defined, only sub-parallel, and are therefore interpreted as folding, not distinct fibers.
Fine, transversal fluting and fibers similar to the Painten specimens are present (Figure 12, Figure 14, Figure 15). However, NKMB-P-Watt06/508 also preserves many more longitudinal striae. These are poorly defined, only sub-parallel, and are therefore interpreted as folding, not distinct fibers.
 Little to nothing can be observed of the webbing of the feet (Figure 13). The scaleless skin type is visible on a patch associated with the right metatarsal region or the webbing of the left foot. The outline of the leading edge around the first toe of the right foot is narrow and suggests that the terminal phalanx was encased by the outline of the hind flipper.
Little to nothing can be observed of the webbing of the feet (Figure 13). The scaleless skin type is visible on a patch associated with the right metatarsal region or the webbing of the left foot. The outline of the leading edge around the first toe of the right foot is narrow and suggests that the terminal phalanx was encased by the outline of the hind flipper.
There are skin irregularities on the tail (Figure 12, Figure 14, Figure 15). These imperfections are only vaguely similar to those observed in the specimens described from Painten. In NKMB-P-Watt06/508, they are smaller, covering a range from 2 to 8 mm in longest extent. Instead of larger, curl-shaped structures, these marks are round-to-oval, mostly flared, sometimes inconspicuously concentric, and questionably hollow. Those irregularities that appear raised are from the exposed right side of the body, while the flat or rimmed impressions represent the underlying left side. Compared to the surrounding skin folds, the irregularities appear rigid, sometimes smooth and inflexible, indicating greater resilience under the otherwise strong compaction of preserved soft tissue.
 Cricosaurus albersdoerferi, BMMS-BK 1-2 (Figure 16, Figure 17, Figure 18). Articulated skeleton from Painten. Generic attribution based on the combination of bicarinate teeth without enamel ornamentation, well-developed surangular and angular with anterior tips anterior to the orbit, rounded prefrontals, and external nares separated by the premaxillary septum (Sachs et al., 2021). The specimen includes moderate repair along the margins of pieces of the slab and the bones that were split between these pieces. Skin preservation is interrupted by cracks in the specimen. However, there is still a wide distribution of skin preserved which was observed under visible light and UV.
Cricosaurus albersdoerferi, BMMS-BK 1-2 (Figure 16, Figure 17, Figure 18). Articulated skeleton from Painten. Generic attribution based on the combination of bicarinate teeth without enamel ornamentation, well-developed surangular and angular with anterior tips anterior to the orbit, rounded prefrontals, and external nares separated by the premaxillary septum (Sachs et al., 2021). The specimen includes moderate repair along the margins of pieces of the slab and the bones that were split between these pieces. Skin preservation is interrupted by cracks in the specimen. However, there is still a wide distribution of skin preserved which was observed under visible light and UV.
 The largest skin patch, in the throat area (Figure 16), is smooth with minimal folding. Dorsal to the neck, a string of patches clearly indicates sub-transversal to diagonally forward-tilted striae made up of sharp grooves (Figure 18A, C, D). Some of these structures are curved, possibly resulting from overlap of both sides of the body subsequent to final compressive flaring of the soft tissue. Longitudinal sets of coarser fluting indicate musculature (Figure 17, Figure 18A). Under a certain angle of light, a single dorsal patch reveals crossing sets of parallel folds, producing an acute rhomboidal pattern (Figure 18D).
The largest skin patch, in the throat area (Figure 16), is smooth with minimal folding. Dorsal to the neck, a string of patches clearly indicates sub-transversal to diagonally forward-tilted striae made up of sharp grooves (Figure 18A, C, D). Some of these structures are curved, possibly resulting from overlap of both sides of the body subsequent to final compressive flaring of the soft tissue. Longitudinal sets of coarser fluting indicate musculature (Figure 17, Figure 18A). Under a certain angle of light, a single dorsal patch reveals crossing sets of parallel folds, producing an acute rhomboidal pattern (Figure 18D).
 Between some of the posterior trunk ribs, a single skin patch with a cell-like, sub-square relief pattern is present (Figure 18B). We tentatively interpret this structure as shrinkage or compaction under lateral narrowing, reflecting a pattern of crossing folds, rather than sharply outlined scutes. A blurred set of diagonal striae might be associated with the femur (Figure 18E). Although this striation texture is somewhat coarser, it is consistent with the remaining integument. A strip of soft tissue bearing squamous-appearing structures close to the femur is very diffuse, probably the result of overlaid soft tissues from the trunk and nodule-like diagenetic mineralization rather than actual scales.
Between some of the posterior trunk ribs, a single skin patch with a cell-like, sub-square relief pattern is present (Figure 18B). We tentatively interpret this structure as shrinkage or compaction under lateral narrowing, reflecting a pattern of crossing folds, rather than sharply outlined scutes. A blurred set of diagonal striae might be associated with the femur (Figure 18E). Although this striation texture is somewhat coarser, it is consistent with the remaining integument. A strip of soft tissue bearing squamous-appearing structures close to the femur is very diffuse, probably the result of overlaid soft tissues from the trunk and nodule-like diagenetic mineralization rather than actual scales.
One of the associated patches of skin on the ventral tail is very smooth (Figure 18F). Possibly, this type of preservation is more representative of the normal surface of the body, if not representing adipocere. The distal tip of a hemapophysis served as a pressure center for radial folds during compaction.
Tiny skin irregularities are best preserved on the chest (Figure 17). Blurred, round, crater-like structures of about 2 mm in diameter appear as a random ‘nest.’ They show no observable effects on the texture of the surrounding integument. A larger scar is present on the tail (Figure 18F).
 Cricosaurus bambergensis, NKMB-P-Watt14/274 (Figure 19, Figure 20, Figure 21). Largely complete and articulated skeleton from Wattendorf. Referral based on Sachs et al. (2019). Skin was preserved as reddish, delicate layers, occurring in fragmented patches from the shoulder region to the proximal tail. Observations were made under visible light, also checked under UV.
Cricosaurus bambergensis, NKMB-P-Watt14/274 (Figure 19, Figure 20, Figure 21). Largely complete and articulated skeleton from Wattendorf. Referral based on Sachs et al. (2019). Skin was preserved as reddish, delicate layers, occurring in fragmented patches from the shoulder region to the proximal tail. Observations were made under visible light, also checked under UV.
 Fine, sub-transversal striation was observed anterodorsal and anteroventral to the shoulder (Figure 19). This striation is combined with folding that is apparently associated with certain bones, indicating a long-lasting flexible behavior during compaction.
Fine, sub-transversal striation was observed anterodorsal and anteroventral to the shoulder (Figure 19). This striation is combined with folding that is apparently associated with certain bones, indicating a long-lasting flexible behavior during compaction.
Brownish, more brittle patches with crisscrossed fluting next to some gastralia may indicate uneven diagenesis, deserving closer investigation in the future. The current study also revealed crustaceans as gut contents (Figure 20), preserved in a similar manner.
 Dorsal to the lumbar region, more oblique striation occurs in a large skin patch. Longitudinal ridges are branching and suggest some shrinkage or flaring effects (Figure 20). This pattern is also interlinked with transverse processes of the neural arches, which implies that a considerable amount of flesh was present during compaction.
Dorsal to the lumbar region, more oblique striation occurs in a large skin patch. Longitudinal ridges are branching and suggest some shrinkage or flaring effects (Figure 20). This pattern is also interlinked with transverse processes of the neural arches, which implies that a considerable amount of flesh was present during compaction.
The area of the proximal part of the tail, anterior to the gap where the distal tail is dislocated (Sachs et al., 2019), retains the sub-transversal, fibrous striation of the anterior trunk, with some diagonal and slightly sigmoidal orientation (Figure 21). In the ventral portion, crossing layers can be observed, with a backward-tilted set of wider striae that preserve musculature. The outline of soft tissue preservation gives an impression of the amount of flesh surrounding the hemapophyses, indicating the presence of a typically powerful crocodyliform tail.
 Cf. Rhacheosaurus gracilis, LF 2426 (Figure 22). Largely complete and articulated juvenile (tail is missing) from Langenaltheim. Referral made on the basis of the short temporal area and uncarinated teeth (Young and Andrade, 2009; Parrilla-Bel et al., 2013). Patchy and fragmented skin remains are preserved from what is potentially a throat pouch to the tail. Observation under several UV wavelengths revealed no discrete scutes or osteoderms.
Cf. Rhacheosaurus gracilis, LF 2426 (Figure 22). Largely complete and articulated juvenile (tail is missing) from Langenaltheim. Referral made on the basis of the short temporal area and uncarinated teeth (Young and Andrade, 2009; Parrilla-Bel et al., 2013). Patchy and fragmented skin remains are preserved from what is potentially a throat pouch to the tail. Observation under several UV wavelengths revealed no discrete scutes or osteoderms.
Cf. Dakosaurus, DMA-JP-2018/002 (not illustrated). A very young juvenile from Painten, complete skeleton currently under description. Generic attribution based on the short snout, unique to this genus among Painten metriorhynchids; although possibly related to allometry, coeval genera tend to show proportionally longer snouts even as juveniles (see cf. Rhacheosaurus specimen above). Its postcranial portion is completely surrounded by a delicate shadow of soft tissue. Observations under visible light and UV revealed no scales, implying that the metriorhynchid skin type experienced no major ontogenetic changes.
Metriorhynchid Integument: Summary
Our modern picture of metriorhynchid aquatic adaptations is largely the same as that determined by Fraas (1901, 1902), including a smooth skin surface and hypocercal tail fluke. No osteoderms were observed in any of the specimens. The metriorhynchid skin type was rather uniform, soft and scaleless, with internally fibrous tissue. It was tight and streamlined, somewhat like in dolphins. Repeated patterns of folding reflect zones of increased movement as well as common taphonomical effects. Dermal skull bones of the investigated taxa lack the osteoderm-like groove-and-ridge sculpturing (Figure 16, Figure 22) that is commonly found in anamniotes (Clack et al., 2016) and other aquatic crocodyliforms (Wu et al., 2001; Johnson et al., 2020b). This implies that skin modification in metriorhynchids includes the cranial cover.
 This skin morphology is in accordance with an impressive historical specimen of Rhacheosaurus gracilis (NHMUK R.3948; Figure 23) that contains a blurred “skin shadow” with informative outlines around the tail (the only described metriorhynchid that preserves an upper fluke lobe; Young et al., 2010). From this slab, Ammon (1906, figure 9) described a whitish, striated, phosphorite mass, which he interpreted as fossilized musculature (Reis, 1893). This is very similar to some portions of the Painten Cricosaurus BMMS-BK 1-2. (Figure 17, Figure 18A).
This skin morphology is in accordance with an impressive historical specimen of Rhacheosaurus gracilis (NHMUK R.3948; Figure 23) that contains a blurred “skin shadow” with informative outlines around the tail (the only described metriorhynchid that preserves an upper fluke lobe; Young et al., 2010). From this slab, Ammon (1906, figure 9) described a whitish, striated, phosphorite mass, which he interpreted as fossilized musculature (Reis, 1893). This is very similar to some portions of the Painten Cricosaurus BMMS-BK 1-2. (Figure 17, Figure 18A).
Within the layered skin, only one set of fibers can be identified so far, with a clear sub-transversal orientation. Terminal phalanges in the foot of Metriorhynchidae are barely curved and lack pronounced tubercles, indicating they would be of minimal use in scratching. Some are remarkably shortened and reduced to tiny discs. Only a few longer terminal phalanges suggest the vague possibility for the formation of larger rigid skin structures, maybe analogous to the claw-bearing Trionychidae (softshell turtles), but unlike Dermochelys (leatherback sea turtle).
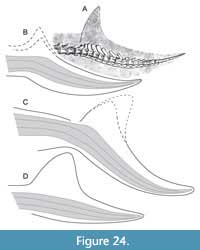 Additional spikes on the anterior edge of vertebral spines in the mid-caudals of many metriorhynchids seem to contribute to tail mobility. In the same region, some teleosauroids show elongated spines (pers. obs. of Aeolodon, JME unnumbered), possibly to support a crest immediately posterior to the dorsal caudal osteoderm shield. However, this region did not support a dorsal fin or seam in any thalattosuchian specimen, as confirmed by the new observations. Instead, the tail fluke is a constant structure (Figure 24). Elongated, forward-tilted spines in the bending zone of the caudal column are believed to attach with connective tissue fibers, similar to the anatomy of mosasaurs (Lindgren et al., 2010, figure 6C). A complete fluke is present on an unpublished “Solnhofen” metriorhynchid housed at the Wyoming Dinosaur Center (Cowan, 2010; pers. obs., HT; albeit with unknown degree of restoration). Its imperfect tail bend suggests that this is an immature individual.
Additional spikes on the anterior edge of vertebral spines in the mid-caudals of many metriorhynchids seem to contribute to tail mobility. In the same region, some teleosauroids show elongated spines (pers. obs. of Aeolodon, JME unnumbered), possibly to support a crest immediately posterior to the dorsal caudal osteoderm shield. However, this region did not support a dorsal fin or seam in any thalattosuchian specimen, as confirmed by the new observations. Instead, the tail fluke is a constant structure (Figure 24). Elongated, forward-tilted spines in the bending zone of the caudal column are believed to attach with connective tissue fibers, similar to the anatomy of mosasaurs (Lindgren et al., 2010, figure 6C). A complete fluke is present on an unpublished “Solnhofen” metriorhynchid housed at the Wyoming Dinosaur Center (Cowan, 2010; pers. obs., HT; albeit with unknown degree of restoration). Its imperfect tail bend suggests that this is an immature individual.
Metriorhynchids lack osteoderm armor, which might also be relevant for habitat selection. Although metriorhynchid gastralia are more robust than those of ichthyosaurs, they do not differ much from the gastralia of extant crocodylians that bear ventral osteoderms. Ventral armor is present in teleosauroid thalattosuchians, turtles, and sauropterygians due to specialized gastralia and ventral girdle elements. Compared to these cases, Metriorhynchidae and Mosasauridae represent an opposing, ventrally less-protected pelagic bauplan.
Although not belonging to the integument in a narrower sense, scleral rings are ectoderm derivates, formed via intramembranous ossification (Franz-Odendaal, 2005, 2008). Thalattosuchia and certain marine Mesosuchia are the only crocodyliforms that possessed scleral rings (Wu et al., 2001; Pol and Gasparini, 2009), representing a convergent feature comparable to that of ichthyosaurs, and may represent an example of deep homology within Pseudosuchia (Nesbitt et al., 2013).
Observations from Non-metriorhynchid Thalattosuchia
 Two specimens preserving considerable integumentary anatomical detail are known in the Teleosauroidea, the sister-group of the metriorhynchoids. SMNS 10985 (Figure 25) is a fragmentary skeleton historically referred to “Steneosaurus” bollensis from the Lower Jurassic locality of Frittlingen, Germany (for revised taxonomy see Johnson et al., 2020a; “S.” bollensis is currently placed in the genus Macrospondylus). This specimen was previously mentioned by Westphal (1962, figure 4) and Godefroit (1994, figure 28). Skin is preserved along the rear apron of the femur down to the metatarsus. The soft tissue cover was obviously tight and narrow around the ankle, whereas external to the fourth metatarsal a narrow web can be assumed. The bent toes with broad terminal phalanges suggest that functional, somewhat ‘hoof-like’ claws resembling those of other crocodyliforms (Brochu, 2013) were projecting over a potential paddle. Fine skin folding posterior to the knee indicates frequent movement in life.
Two specimens preserving considerable integumentary anatomical detail are known in the Teleosauroidea, the sister-group of the metriorhynchoids. SMNS 10985 (Figure 25) is a fragmentary skeleton historically referred to “Steneosaurus” bollensis from the Lower Jurassic locality of Frittlingen, Germany (for revised taxonomy see Johnson et al., 2020a; “S.” bollensis is currently placed in the genus Macrospondylus). This specimen was previously mentioned by Westphal (1962, figure 4) and Godefroit (1994, figure 28). Skin is preserved along the rear apron of the femur down to the metatarsus. The soft tissue cover was obviously tight and narrow around the ankle, whereas external to the fourth metatarsal a narrow web can be assumed. The bent toes with broad terminal phalanges suggest that functional, somewhat ‘hoof-like’ claws resembling those of other crocodyliforms (Brochu, 2013) were projecting over a potential paddle. Fine skin folding posterior to the knee indicates frequent movement in life. Strong compaction typical of black shales likely exaggerated the broadness of the preserved skin patch. That this broadness is partially a diagenetic effect is evidenced by the fact that the scute patterns on both sides are flattened and the impression of the underlying side can be traced through the exposed side. The lower limb has a less pronounced soft tissue thickness. If compaction of the body was strictly vertical, this distribution would indicate strong musculature for femoral retraction, used in swimming, walking, or possibly nesting. The surface structure (Figure 26) consists of rectangular scutes with discrete and straight hinges, arranged primarily in chessboard (square) orientation, with locally sheared, rhomboid outlines, or an initial offset of neighboring rows. The quality of the integument is astonishingly similar to that of extant crocodiles, underlining a considerable continuity throughout crocodyliform evolution. The only difference between the morphology of SMNS 10985 and extant representatives is that the latter feature a greater variety of scute sizes, including larger scutes, particularly on the outer side (in SMNS 10985 it is blurred by the overlaying internal side), which are partially associated with osteoderms. Detailed photographs did not indicate the regular dotting patterns of sensory pits, as would be expected from comparison with extant crocodylids.
Strong compaction typical of black shales likely exaggerated the broadness of the preserved skin patch. That this broadness is partially a diagenetic effect is evidenced by the fact that the scute patterns on both sides are flattened and the impression of the underlying side can be traced through the exposed side. The lower limb has a less pronounced soft tissue thickness. If compaction of the body was strictly vertical, this distribution would indicate strong musculature for femoral retraction, used in swimming, walking, or possibly nesting. The surface structure (Figure 26) consists of rectangular scutes with discrete and straight hinges, arranged primarily in chessboard (square) orientation, with locally sheared, rhomboid outlines, or an initial offset of neighboring rows. The quality of the integument is astonishingly similar to that of extant crocodiles, underlining a considerable continuity throughout crocodyliform evolution. The only difference between the morphology of SMNS 10985 and extant representatives is that the latter feature a greater variety of scute sizes, including larger scutes, particularly on the outer side (in SMNS 10985 it is blurred by the overlaying internal side), which are partially associated with osteoderms. Detailed photographs did not indicate the regular dotting patterns of sensory pits, as would be expected from comparison with extant crocodylids.
 Another teleosauroid taxon preserving skin, but from the Late Jurassic plattenkalk of the Solnhofen Archipelago, is Aeolodon priscus (Figure 27; for validity of Aeolodon see Young and Andrade, 2009; Ösi et al., 2018). In this taxon, as indicated by an unnumbered JME specimen, stretched, rhomboid-to-random polygonal shaped scales cover the webbed foot surface. Due to some restoration to the terminal phalanges, no clear information can be determined about the inclusion of claws in the paddle. Further observations include large, sub-rectangular scutes on the tail, with a longitudinal row of large dorsal scutes that form a fin-like edge, similar to that found in modern crocodylians. The middle portion of the tail is narrowly wrapped by soft tissue. The hind limbs show rhomboid scutes, consistent with SMNS 10985.
Another teleosauroid taxon preserving skin, but from the Late Jurassic plattenkalk of the Solnhofen Archipelago, is Aeolodon priscus (Figure 27; for validity of Aeolodon see Young and Andrade, 2009; Ösi et al., 2018). In this taxon, as indicated by an unnumbered JME specimen, stretched, rhomboid-to-random polygonal shaped scales cover the webbed foot surface. Due to some restoration to the terminal phalanges, no clear information can be determined about the inclusion of claws in the paddle. Further observations include large, sub-rectangular scutes on the tail, with a longitudinal row of large dorsal scutes that form a fin-like edge, similar to that found in modern crocodylians. The middle portion of the tail is narrowly wrapped by soft tissue. The hind limbs show rhomboid scutes, consistent with SMNS 10985.
From our current knowledge, no semi-aquatic crocodyliform has secondarily achieved a smooth and naked skin type (unlike certain turtles, hippopotamids, or modern amphibians, which contrast with the ancestral semi-aquatic tetrapod condition; Witzmann, 2011). Scutes seem necessary for protection during onshore basking, but such behavior is questionable in teleosauroids. Their scutes may have been retained as a constraint for osteoderm formation (see below). This could explain why an open-sea habitat, as inferred for the teleosauroids Aeolodon and Machimosaurus (Johnson et al., 2020b), does not automatically result in the evolution of smooth skin. In contrast, metriorhynchid skin was probably at risk from persistent exposure to sunlight, underlining that the shift to a cetacean level of integumentary adaptation was not possible until after completion of the transition to a pelagic lifestyle. This assumption is in accordance with the discovery that the metriorhynchoid tail fluke evolved prior to the loss of osteoderms (Ösi et al., 2018).
Although teleosauroids show a number of initial to derived marine adaptations, for example reduced front limbs and loss of air-borne hearing (Montefeltro et al., 2016; Schwab et al., 2020; Johnson et al., 2020b, figure 64), their integument appears to have been very much like modern crocodylians, including the texture of their osteoderms, some dermal skull textures, and soft tissue architecture. Thalattosuchians and the lineage leading to modern crocodylians had diverged by the early Jurassic at the latest. Based on this phylogenetic bracketing, we can determine that the shared integumentary features of these groups would have applied to the majority of Crocodyliformes. Although the precise integumentary morphology of teleosauroids should not necessarily be used as a general plesiomorphic model for all Crocodyliformes, it is a useful example of how the ancestral crocodyliform skin type can react under semi-aquatic selection (applicable to e.g., Stomatosuchidae, Goniopholididae). A broader review of the integument across crocodile-line archosaurs would be useful to tease apart distinctions between semi-aquatic lineages and highly terrestrial lineages that also exhibit square osteoderm armor, like Aetosauria (Heckert et al., 2010) or Simosuchus (Hill, 2010).
DISCUSSION
Comparisons with Other Marine Reptiles
Smooth skin is known to occur in some other saurian clades. The arguably most highly specialized marine reptiles are the Ichthyosauria, which may have originated shortly after the Permian-Triassic transition (Huang et al., 2019). Skin impressions and macrostructures of derived ichthyosaurs include large, hypocercal tail flukes (Bardet and Fernandez, 2000). Additionally, authentic dorsal fins and flippers of Lower Jurassic Holzmaden specimens show a low-angled radial striation. According to Lingham-Soliar (1999, 2001), ichthyosaur skin was composed of a number of sets of crisscrossed fibers of different sizes, and ranged in texture from rough interior to finer exterior layers. This construction is interpreted as strong and flexible, mainly to support persistent hydrodynamic shaping and high-speed control (Lingham-Soliar and Plodowski, 2007). Tiny, polygonal scales have been documented on an ichthyosaur flipper (Fraas, 1888), though these could be artifacts of diagenetic shrinkage. Broili (1942) provided more details on these structures, which seem to be restricted to the leading edges of flippers and fins.
The next-appearing marine reptile radiation that evolved a smooth skin surface includes the Plesiosauria. One of the earliest integumentary structures recognized in this clade was a fluke-like, asymmetrical tail fin in Seeleyosaurus (Dames, 1895). Such structures might have been rather common among plesiosaurs, as reflected by a “pygostyle-like” tail tip in an elasmosaurid (Kubo et al., 2012). The skin preservation in Seeleyosaurus features a delicate, scaleless integument with interior striation similar to that of ichthyosaurs (S. Sachs, personal commun., 2020; including close-up photographs). Soft tissue around the anterior paddle of Hydrorion (= ? Microcleidus) was described by Huene (1923, plate 1, figure 1). The rear apron of the flipper has either extremely fine scales or is scaleless (according to photographs, courtesy of S. Sachs, personal commun., 2020). The best published material belongs to an indeterminate plesiosaur (Vincent et al., 2017) with crisscrossed fibers under various angles, a tail with transversal skin striation, and a hind paddle interpreted to contain rows of delicate, non-overlapping scales with fine epidermal ornamentation. The asymmetrical outline of the fluke of Seeleyosaurus suggests a vertical orientation in life. Unlike the fluke of metriorhynchids or ichthyosaurs, its small size implies a minor role for propelling (Sennikov [2019] argued for a horizontal fluke with vertical undulation, which contrasts with the results of Carpenter et al. [2010]). The general convergence of vertical flukes in marine reptiles can be explained by a predominantly horizontal undulation of the vertebral column shared with their terrestrial ancestors, unlike marine mammals with horizontal flukes (Cetacea, Sirenia), whose ancestors performed dorsoventral flexion. Given horizontal undulation in the ancestors of metriorhynchids, we predict the prevalent plane of motion for the fluke in this group to be dorsal/frontal.
Frey et al. (2017) reported a new plesiosaurian specimen (holotype of Mauriciosaurus fernandezi) with extensive soft tissue preservation including a very broad horizontal soft tissue apron around the proximal tail. Further observations included: soft tissue aprons also occurring at the rear edge of the flippers, which may not have been fully covered with scales; skin surface characterized by crossed lines of small scales (square coordination); trunk integument exhibiting longitudinal rows of fine, transversely rectangular units, interpreted as scales; hip region including some larger scales; and adipocere deposition. Altogether, plesiosaur skin seems less perfectly hydrodynamically modified than that of ichthyosaurs, but with a clear reduction of macrorelief and surface texture compared to terrestrial reptiles.
Little can be said about the integument of plesiosaur outgroups. Early Eosauropterygia have not been discovered with well-preserved skin (Wachtler, 2018, p. 55). A reinforced integument in these aquatic reptiles has been inferred from taphonomic indicators, however (Renesto, 2006; Bearmore and Furrer, 2017). Placodontia reflect a generally scaly condition, as osteoderm patterns are usually reflected by scutes (Scheyer et al., 2012; Maderson [1972] noted the extant Dermochelys as an exception; there is also a general reduction of scutes in many Trionychidae). Considerable ornamentation in placodont osteoderms confirms this assumption, along with direct evidence from impressions of scute rims like those of turtles (Westphal, 1975). It is possible that a highly modified skin type did not occur before the sauropterygian shift to pelagic forms during the Triassic-Jurassic transition (Neenan et al., 2017). As osteoderms occur in early sauropterygians (Scheyer et al., 2017) and similar armor elements are found in early ichthyosauromorphs (Carroll and Dong, 1991), this could represent a common pattern, whether due to shared ancestry (if these clades are sister-taxa; e.g., Chen et al., 2014) or similar initial adaptations to semi-aquatic life.
The similarities of metriorhynchid, plesiosaurian, and ichthyosaurian transformations might be more than just superficial convergence (Kelley and Pyenson, 2015). Possibly, there are deep-homologous genetic patterns resulting in these convergent evolutionary modifications, as paralleled by marine mammals (Zhou et al., 2015). Unlike these other Mesozoic marine reptile radiations, the Late Cretaceous Mosasauridae (Squamata) retained scaly skin. Mosasaurs exhibit varying patterns of flat square, rhomboidal, or hexagonal coordination of overlapping and possibly osteoderm-bearing scales that show sculpture for hydrodynamic optimization (Williston, 1914; Lindgren et al., 2009, 2010). Mosasaur dermal fibers show a crisscross set of orientations, with a dominant longitudinal set and others that parallel the diagonal scale rows (Lindgren et al., 2011). The unique combination of a scaly skin while being fully pelagic is probably linked to specialized features of the integument in squamates, with overlapping epidermal scales showing vertical keratin generations, unlike the horizontal alternation of α- and β-keratin in the archosaurian skin (Maderson, 1972; Rutland et al., 2019).
Secondary Loss of Osteoderms and Scutes
Osteoderms are the only direct skin feature that can commonly be traced through the fossil record. Crocodylian osteoderms are highly vascularized on their external surface to support heat absorption and radiation (Grigg and Gans, 1993; Clarac et al., 2018). The presence of such bony armor in early thalattosuchians, in particular its overall resemblance with that of living crocodylians, suggests a similar thermoregulatory use, maybe associated with prolonged onshore or surface-near basking.
Nearly all crocodylomorphs exhibit osteoderms, with rare exceptions represented by the Metriorhynchidae and some terrestrial forms (Clark et al., 2004; Godoy et al., 2016; Montefeltro, 2019). Apart from protective armor (Hill, 2010; Marinho and Carvalho, 2009), dorsal osteoderms yield attachment sites for the tendon apparatus of the neural arches (Frey, 1988; Dilkes and Brown, 2007; Buchwitz and Voigt, 2010), which is why osteoderms tend to be retained even under reduced armor in cursorial forms (Sereno and Larsson, 2009; O’Connor et al., 2010). A role for crocodylian osteoderms in acidosis buffering has also been hypothesized, with reserves of carbonates in the osteoderm serving to buffer elevated lactate levels in the blood during prolonged apnea (Clarac et al., 2018) performed during resting or ambush predation. It remains unclear whether or not transitional metriorhynchoids could have lost their scutes but retained subcutaneous osteoderms. Dermochelys, trionychid turtles, and occasional mammalian examples (McDonald, 2018) demonstrate that this morphology is possible, but it is rare.
A summary on osteoderm growth in modern crocodylians is provided by Vickaryous and Sire (2009). Prior to mineralization of an osteoderm, a knot-like aggregation of fibrous, cellular connective tissue appears in the keel of the associated epidermal scale, which is otherwise identical to the matrix of the stratum superficiale. This development is delayed as compared to the remainder of the skeleton. Juveniles lack bony skin armor until ossification spreads, beginning around the dorsal neck and shoulder. This suggests the following evolutionary scenario for the origin of the specialized metriorhynchid skin type:
1. It is assumed that the thalattosuchian armor was lost rather suddenly (on a geological timescale), not stepwise per region, and not with a continuous decrease of osteoderm size over a longer period of evolution. Teleosauroid juveniles smaller than 70 cm have not been recorded (Westphal, 1962), but larger juveniles resemble adults in their osteoderm morphology.
2. Instead of local fibrous aggregations of connective tissue, a more planar reinforcement might have been achieved in the stratum superficiale, which subsequently replaced the shaping function and tightening effect of keratin scutes in the entire dermis.
3. The inclusion of dorsal osteoderms into tendon attachment sites of the vertebral column is not an a priori feature in early ontogeny. Therefore, the tendon apparatus is modular and flexible from an evolutionary perspective.
The loss of osteoderms in Metriorhynchidae is a question of adaptive value. Possibly, bony armor simply became obsolete as an additional acidosis buffer after the transition to a fully pelagic lifestyle. It could be buffered by the common disposability of ions in the seawater, especially due to the pelagic incorporation of biocalcite common since the Jurassic (Aloisi, 2018). The relationship of this system to metriorhynchid osteoderm loss is uncertain, however, due to missing data on various aspects of metriorhynchid paleobiology (unknown diving habits, hypothetically increased oxygen absorption) and associated geochemistry (Ridgwell, 2005; Gothmann et al., 2015). Osteoderm loss could alternatively (or additionally) be a means of increasing mobility in the pelagic metriorhynchids. Increase of trunk flexibility as an explanation for metriorhynchid osteoderm loss also has problems, however, since this thalattosuchian trend was somewhat reversed by secondary stiffening in Metriorhynchidae (Molnar et al., 2015). Furthermore, the fact that armored teleosauroid thalattosuchians survived nearly as long as metriorhynchids suggests that their less specialized bauplan was not maladaptive for marine life.
Crocodylian scutes grow continually or annually, forming corneous layers underneath the latest generation (Alibardi, 2010, figure 7). This mode offers a potential model for evolutionary reduction of scutes in Metriorhynchidae, which probably occurred over the entire body and decreased periodic keratinization. The remaining soft skin cover would have retained its function as a barrier for water and electrolyte exchange, contributing to euryhaline homeostasis (Grigg, 1981; Taplin et al., 1982; Grigg and Gans, 1993). As Metriorhynchidae share a smooth skin surface with other pelagic saurians, the ultimate adaptation is seen in hydrodynamic efficiency to optimize streamlining. However, the presence of some armored teleosauroid thalattosuchians in open-water habitats suggests that this was not the only viable approach, with parallel radiations occurring simultaneously between Teleosauridae and Metriorhynchidae (Young et al., 2011).
Interpretation of Skin Irregularities
Similar skin irregularities in the two larger specimens from Painten might be attributed to integumentary strengthening structures, but differ from Cricosaurus from the same locality and an indeterminate, medium-sized metriorhynchid (NKMB-P-Watt06/508) from Wattendorf. Moreover, there is no regular repeated distribution on the body. Therefore, these irregularities are unlikely to be regular anatomical features and are more parsimoniously interpreted as pathological. We tentatively interpret these structures as epibiont scars.
Epibiota encompass a diversity of taxa, of which some can be excluded as originators for the structures in question. Algae would have little potential of preservation or even settlement on a fast-moving marine predator. The attachment of brachiopods and goose barnacles (Pedunculata) is organic (chitinous cuticle of pedicle) or cemented. Although reported as having an epiplanktonic lifestyle since the early Jurassic (Gale and Schweigert, 2015), goose barnacles would be very uncommon on higher nekton, or require deep, skin-doming peduncles (as reported from certain sharks; Rees et al., 2014). Stalked epibionts would probably have been actively scrubbed off by nektonic reptiles. Although Stramentidae lived as closely attached epizoa, these mostly Late Cretaceous barnacles left characteristic, internally segmented traces (Ifrim et al., 2011; Gale, 2016) that would be readily recognizable and are dissimilar from that observed here. Slipper limpets (Calyptraeidae) have a record reaching back only to the Cretaceous (Sohl, 1987). Oysters (Ostreoidea) are byssate and interlock with structured hardgrounds, which do not produce rounded attachment scars. Suckerfish or remoras (Echeneidae) are non-invasive and cannot be traced older than the Oligocene (Friedmann et al., 2013). Lampreys (Petromyzontidae) rip deeper wounds upon attachment, however, the scars in question appear to be very flat and superficial and sometimes do not affect the surrounding skin. On the other hand, healed wounds can show similar structures, as is reported from fossil examples (Rothschild and Depalma, 2013). Given the unordered arrangement and sometimes irregular shape of the structures in question, however, no gnathostome producer of potentially healed bitemarks is considered likely at this time.
Two possible epizoa show a closer match with the observed traces. First, limpets (Patellogastropoda) have been a common element in marine communities since the Paleozoic. They generate immense suction through the action of their muscular foot and adhering mucus. Long-term shell contact can cause subcircular troughs on intertidal rock (pers. obs.). Limpets are rarely found on whales today, but have been reported as epizoa on Cretaceous ammonites (Kase et al., 1998) reinterpreted records of mosasaur bites as limpet home scars, though such bites were later confirmed based upon other material; Tsujita and Westermann, 2001). Although referred scars (Kase et al., 1994) resemble some of the curl-shaped structures observed on Painten metriorhynchids, most limpets prefer stationary hardgrounds in surface waters. Extant limpets perform homing (Cook et al., 1969), which does not match with a regular epizoic lifestyle on agile apex predators. However, limpets cover a much greater ecological spectrum, traced back to the Mesozoic (Chen et al., 2019). Only one very questionable limpet fossil is known from the Bavarian Jurassic Solnhofen Archipelago (Nützel, 2015), likely due to rare hardgrounds in the surf zone.
Second, acorn barnacles (Sessilia) are the most abundant epibionts on extant sea turtles, as well as baleen and toothed whales (for overview see Hayashi, 2013). Integrative analyses have demonstrated their middle Jurassic origin and late Jurassic radiation (Pérez-Losada et al., 2004, 2008), but there is a lack of fossil evidence from the Jurassic (Schweigert, 2015). It appears attractive to link this hypothetical origin with a pelagic shift in turtles (new specimens from Wattendorf and Eichstätt [Joyce et al., 2021] as well as from Painten [pers. obs. of the authors] with marine adapted flippers suggest a late Jurassic occurence of pelagic turtles; compare with Hirayama, 1998), as well as speculative coevolution with Metriorhynchidae. However, known early acorn barnacles were solely epibenthic, and there is no record on older turtle fossils (for which Reolid et al., [2015] state possible taphonomic effects, such as post mortem loss of epibionts). Only a few lineages are adapted to penetrate the cutis of whales, with some evolutionary plasticity of anchorage mechanisms (Seilacher, 2005). Balanomorpha can be traced back to the Late Cretaceous (Yamaguchi and Newman, 1990; Gale and Sørensen, 2014), Neobalanomorpha and the epizootic Coronuloidea to the Eocene. Specialized whale barnacles (Coronulidae) are not recorded older than Neogene (Hayashi, 2013; Gale and Skelton, 2018). This result does not a priori exclude hidden, less specialized Mesozoic epizootic cases, but leaves this interpretation as pure speculation.
Although barnacles are usually treated as commensal on pelagic tetrapods, there might be a minor parasitic effect in terms of hydrodynamic costs (which is why ships are cleaned of barnacles). Whales are noted to actively remove barnacles during surface activity, causing characteristic scars (Scarff, 1986; Félix et al., 2006) that are admittedly more circular than the larger irregularities observed in the new metriorhynchid material. There is clear preference of host taxa (Hayashi et al., 2013), but Collareta et al., 2016 describe exceptional epibiont dispersal of barnacles from turtle to whale in the fossil record. Therefore, a flexible spectrum of hosts is conceivable, including barnacles on extant crocodiles and alligators (Cupul-Magaña et al., 2011; Nifong and Frick, 2011).
The inferred scars on metriorhynchid skin appear to be rather shallow, precluding any epizoic parasites that possessed deep anchoring adaptations. Cetacean hosts shed their skin regularly, against which barnacles have some defense. Similarly, such strong attachment prevents barnacles from being shed off during ecdysis of marine snake hosts (Key et al., 1995). If the scars on the two larger specimens from Painten are from older fouling, they might have been deformed during growth. In DMA-JP-2009/001, smaller scars look fresher due to a deeper relief and a sharp, circular outline. In LF 3474, smaller scars are less pronounced, while the largest curl structures appear to be a deeper and stronger incrustation, probably due to aging parasites. The presence of the largest scars on the biggest metriorhynchid specimens might reflect a lower frequency of skin renovation in mature animals.
 The potential traces of epizoa reported herein would be the first documentation of coevolution with Mesozoic marine reptiles. Zangerl (1948) reported on bryozoans and a questionable barnacle on a Cretaceous turtle, but likely a freshwater form, hence epibenthic on allochthonous bones (see also Brownstein, 2018). Particularly for the Late Jurassic Solnhofen Archipelago, no match with known invertebrates could be recognized, implying a new ecological component (Figure 28). A major challenge to this interpretation is the lack of a confirmed identification of the producer of these skin irregularities, which requires further understanding and investigation of metriorhynchid biostratinomy. If quick burial took place, lamprey bites could be more likely. Mesozoic lampreys are very similar to modern forms (Chang et al., 2006), although no certain Late Jurassic marine representative can be named. The fossil record of this group is overall poor, which is not surprising in the light of restricted biomineralization. However, well-sampled Konservatlagerstaetten like the Bavarian Late Jurassic plattenkalk formations raise the potential for discovery of fossil lampreys, if present.
The potential traces of epizoa reported herein would be the first documentation of coevolution with Mesozoic marine reptiles. Zangerl (1948) reported on bryozoans and a questionable barnacle on a Cretaceous turtle, but likely a freshwater form, hence epibenthic on allochthonous bones (see also Brownstein, 2018). Particularly for the Late Jurassic Solnhofen Archipelago, no match with known invertebrates could be recognized, implying a new ecological component (Figure 28). A major challenge to this interpretation is the lack of a confirmed identification of the producer of these skin irregularities, which requires further understanding and investigation of metriorhynchid biostratinomy. If quick burial took place, lamprey bites could be more likely. Mesozoic lampreys are very similar to modern forms (Chang et al., 2006), although no certain Late Jurassic marine representative can be named. The fossil record of this group is overall poor, which is not surprising in the light of restricted biomineralization. However, well-sampled Konservatlagerstaetten like the Bavarian Late Jurassic plattenkalk formations raise the potential for discovery of fossil lampreys, if present.
CONCLUSIONS
1. Metriorhynchidae share a uniform type of smooth and flexible skin that completely lacks scutes. Straight, mostly transversal fibers represent the only set of reinforcement structures. Evidence for this skin type, which is similar, though simpler than that of ichthyosaurs, comes from a series of metriorhynchid species. Current data suggest no dependence from size classes or metriorhynchid subclade.
2. Teleosauroid thalattosuchians had an integument very similar to that of crown crocodylians. Therefore, both living crocodiles and teleosauroids can serve as a model for the ancestral condition in Metriorhynchoidea. Coarse, square-shaped scutes could represent an adaptation to, or exaptation for, aquatic habitats. Although not unambiguous, this pattern accords with that of other aquatic archosauromorphs.
3. The skin type of Metriorhynchidae represents a convergence with Ichthyosauria and Plesiosauria. Apparently, there is a generally high threshold for extensive skin modifications in aquatic reptiles, linked to a significant transition to a pelagic lifestyle. Turtles, however, deviate from this discrimination, as there exist limnic forms with reduced keratinization and pelagic forms retaining scutes.
4. Metriorhynchidae reached a degree of aquatic adaptation that is comparable to that of Ichthyosauria and probably the similarly built Mosasauridae. This comparability underlines the fully pelagic lifestyle of Metriorhynchidae, in accordance with current research.
5. Skin formation in extant crocodylians implies an evolutionarily sudden loss of osteoderms and scutes in Metriorhynchidae, linked to their fully aquatic locomotion and potentially their limited exposure to sunlight. It is believed that Teleosauroidea performed onshore basking.
6. Frequent and irregular marks on the skin may represent traces of epizoa or scars comparable to those of lamprey bites. The trace marks are consistent with structures produced by barnacles or limpets, though this cannot be determined with certainty in the absence of preserved tracemakers. Epizoic parasites on marine tetrapods would be a new addition to our knowledge of Jurassic ecology.
ACKNOWLEDGEMENTS
We owe gratitude to the institutions which we closely worked together, the Lauer Foundation for Paleontology, Science and Education (curated by RL and B. Lauer), as well as R. Albersdörfer and M. Völker as the founders of the Dinosaurier Museum Altmühltal. For the provision of valuable information, literature aid, photographs, and helpful discussion. We want to express deep thankfulness to E. Maxwell (Staatliches Museum für Naturkunde Stuttgart), Y. Herrera (Universidad Nacional de La Plata), S. Sachs (Naturkunde-Museum Bielefeld), E. Fordyce (University of Otago), C. Kurz (Naturkundemuseum im Ottoneum Kassel), C. Ifrim (Jura-Museum Eichstätt), M. Röper (Bürgermeister-Müller-Museum Solnhofen), and M. McNamara (University College Cork). For specimen handling, support of fossil preservation and technical help, we are in debt to the RYGOL Baustoffwerk GmbH & Co. KG (Painten), Andreas Schorr GmbH & Co. KG (Baunach), the lenders of the JME crocodile, the DMA technical team (S. Segel, F. Malingriaux, and J. Reinsch), as well as the Painten excavation and preparation team (W. Häckel, S. Hahn, R. Martens, J. Geppert, S. Selzer, and D. Kümpel). We thank C. Kammerer for patient handling and a constructive editorial review.
REFERENCES
Albersdörfer, R. and Häckel, W. 2015. Die Kieselplattenkalke von Painten, p. 126-133. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Alibardi, L., Dalla Valle, L., Nardi, A., and Toni, M. 2009. Evolution of hard proteins in the sauropsid integument in relation to the cornification of skin derivatives in amniotes. Journal of Anatomy, 214:560-586. https://doi.org/10.1111/j.1469-7580.2009.01045.x
Alibardi, L. 2010. Histology, ultrastructure, and pigmentation in the horny scales of growing crocodilians. Acta Zoologica, 92(2):187-200. https://doi.org/10.1111/j.1463-6395.2010.00469.x
Aloisi, G. 2018. A pronounced fall in the CaCO3 saturation state and the total alkalinity of the surface ocean during the Mid Mesozoic. Chemical Geology, 487:39-53. https://doi.org/10.1016/j.chemgeo.2018.04.014
Ammon, L. 1905. Über jurassische Krokodile aus Bayern. Geognostische Jahreshefte, 18:55-71.
Andrews, C.W. 1913. A Descriptive Catalogue of the Marine Reptiles of the Oxford Clay, based on the Leeds Collection in the British Museum (Natural History), London. Part II. British Museum, London.
Bardet, N. and Fernandez, M. 2000. A new ichthyosaur from the Upper Jurassic lithographic limestones of Bavaria. Journal of Paleontology, 74(3):503-511. https://doi.org/10.1017/S0022336000031760
Bearmore, S.R. and Furrer, H. 2017. Land or water: using taphonomic models to determine the lifestyle of the Triassic protorosaur Tanystropheus (Diapsida, Archosauromorpha). Palaeobiodiversity and Palaeoenvironments, 98:243-258. https://doi.org/10.1007/s12549-017-0299-7
Brochu, C.A. 2013. Phylogenetic relationships of Palaeogene ziphodont eusuchians and the status of Pristichampsus Gervais, 1853. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 103:521-550. https://doi.org/10.1017/S1755691013000200
Broili, F. 1926. Ein neuer Fund von Pleurosaurus aus dem Malm Frankens. Abhandlungen der Bayerischen Akademie der Wissenschaften, 30(8):1-48.
Broili, F. 1942. Verfestigungen im Integument der Ichthyosaurier. Sitzungsberichte der mathematisch-naturwissenschaftlichen Abteilung der Bayerischen Akademie der Wissenschaften zu München, I/III:37-52.
Brownstein, C.D. 2018. Trace fossils on dinosaur bones reveal ecosystem dynamics along the coast of eastern North America during the latest Cretaceous. PeerJ, 6:e4973. https://doi.org/10.7717/peerj.4973
Buchwitz, M. and Voigt, S. 2010. Peculiar carapace structure of a Triassic chroniosuchian implies evolutionary shift in trunk flexibility. Journal of Vertebrate Paleontology, 30(6):1697-1708. https://doi.org/10.1080/02724634.2010.521685
Carroll, R.L. and Dong, Z.-M. 1991. Hupehsuchus, an enigmatic aquatic reptile from the Triassic of China, and the problem of establishing relationships. Philosophical Transactions of the Royal Society London B, 331:131-153. https://doi.org/10.1098/rstb.1991.0004
Chang, M.-M., Zhang, J., and Miao, D. 2006. A lamprey from the Cretaceous Jehol biota of China. Nature, 441:972-974. https://doi.org/10.1038/nature04730
Chen, X.-H., Motani, R., Cheng, L., Jiang, D.-Y., and Rieppel, O. 2014. The enigmatic marine reptile Nanchangosaurus from the Lower Triassic of Hubei, China and the phylogenetic affinities of Hupehsuchia. PLoS ONE, 9(7):e102361. https://doi.org/10.1371/journal.pone.0102361
Chen, C., Watanabe, H.K., Nagai, Y., Toyofuku, T., Xu, T., Sun, J., Qiu, J.-W., and Sasaki, T. 2019. Complex factors shape phenotypic variation in deep-sea limpets. Biology Letters, 15:20190504. https://doi.org/10.1098/rsbl.2019.0504
Clack, J.A., Bennett, C.E., Carpenter, D.K., Davies, S.J., Fraser, N.C., Kearsey, T.I., Marshall, J.E.A., Millward, D., Otoo, B.K.A., Reeves, E.J., Ross, A.J., Ruta, M., Smithson, K.Z., Smithson, T.R., and Walsh, S.A. 2016. Phylogenetic and environmental context of a Tournaisian tetrapod fauna. Nature Ecology and Evolution, 1:0002. https://doi.org/10.1038/s41559-016-0002
Clarac, F., de Buffrénil, V., Cubo, J., and Quilhac, A. 2018. Vascularization in ornamented osteoderms: physiological implciations in ectothermy and amphibious lifestyle in the crocodylomorphs? The Anatomical Record, 301:175-185.
Clark, J.M., Xu, X., Forster, C.A., and Wang, Y. 2004. A Middle Jurassic ‘sphenosuchian’ from China and the origin of the crocodylian skull. Nature, 430:1021-1024. https://doi.org/10.1038/nature02802
Collareta, A., Bosselaers, M., and Bianucci G. 2016. Jumping from turtles to whales: a Pliocene fossil record depicts an ancient dispersal of Chelonibia on mysticetes. Rivista Italiana di Paleontologia e Stratigrafia, 122(2):35-44.
Cook, A., Bamford, O.S., Freeman, J.D.B., and Teideman, D.J. 1969. A study of the homing habit of the limpet. Animal Behaviour, 17(2):330-339. https://doi.org/10.1016/0003-3472(69)90019-0
Cowan, S. 2010. What a croc! Houston Museum of Natural Science, Beyond Bones. Downloaded 20 March 2020. https://blog.hmns.org/2010/06/what-a-croc
Cupul-Magaña, F.G., Rubio-Delgado, A., Escobedo-Galván, A.H., and Reyes-Núñez, C. 2011. First report of the marine barnacles Lepas anatifera and Chelonibia testudinaria as epibionts on American crocodile (Crocodylus acutus). Herpetology Notes, 4:213-214.
Dames, W.B. 1895. Die Plesiosaurier der süddeutschen Liasformation. Verlag der Königlichen Akademie der Wissenschaften, Berlin.
Dilkes, D. and Brown, L.E. 2007. Biomechanics of the vertebrae and associated osteoderms of the Early Permian amphibian Cacops aspidephorus. Journal of Zoology, 271:396-407. https://doi.org/10.1111/j.1469-7998.2006.00221.x
Félix, F., Bearson, B., and Falconí, J. 2006. Epizoic barnacles removed from the skin of a humpback whale after a period of intense surface activity. Marine Mammal Science, 22(4):979-984. https://doi.org/10.1111/j.1748-7692.2006.00058.x
Fesefeldt, K. 1962. Schichtenfolge und Lagerung des oberen Weißjura zwischen Solnhofen und der Donau (Südliche Frankenalb). Erlanger Geologische Abhandlungen, 46:1-80.
Fraas, E. 1888. Ueber die Finne von Ichthyosaurus. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg, 44:280-303.
Fraas, E. 1901. Die Meerkrokodile (Thalattosuchia n.g.) eine neue Sauriergruppe der Juraformation. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg, 57:409-418.
Fraas, E. 1902. Meer-Crocodilier (Thalattosuchia) des oberen Jura unter specieller Berücksichtigung von Dacosaurus und Geosaurus. Palaeontographica, 49:1-71.
Franz-Odendaal, T.A. 2005. Intramembranous ossification of scleral ossicles in Chelydra serpentina. Zoology, 109:75-81. https://doi.org/10.1016/j.zool.2005.10.001
Franz-Odendaal, T.A. 2008. Toward understanding the development of scleral ossicles in the chicken, Gallus gallus. Developmental Dynamics, 237:3240-3251. https://doi.org/10.1002/dvdy.21754
Frey, E. 1988. Das Tragsystem der Krokodile - eine biomechanische und phylogenetische Analyse. Stuttgarter Beitrage zur Naturkunde, Serie A, 426:1-60.
Frey, E. and Tischlinger, H. 2012. The Late Jurassic pterosaur Rhamphorhynchus, a frequent victim of the ganoid fish Aspidorhynchus ? PLoS ONE, 7(3):e31945. https://doi.org/10.1371/journal.pone.0031945
Frey, E., Mulder, E.W.A., Stinnesbeck, W., Rivera-Sylva, H.E., Padilla-Gutiérrez, J.M., and González-González, A.H. 2017. A new polycotylid plesiosaur with extensive soft tissue preservation from the early Late Cretaceous of northeast Mexico. Boletín de la Sociedad Geológica Mexicana, 69(1):87-134.
Friedman, M., Johanson, Z., Harrington, R.C., Near, T.J., and Graham, M.R. 2013. An early fossil remora (Echeneoidea) reveals the evolutionary assembly of the adhesion disc. Proceedings of the Royal Society London B, 280:20131200. https://doi.org/10.1098/rspb.2013.1200
Fürsich, F.T., Werner, W., Schneider, S., and Mäuser, M. 2007. Sedimentology, taphonomy, and palaeoecology of a laminated plattenkalk from the Kimmeridgian of the northern Franconian Alb (southern Germany). Palaeogeography Palaeoclimatolology Palaeoecology, 243:92-117. https://doi.org/10.1016/j.palaeo.2006.07.007
Gale, A.S. 2016. Origin and phylogeny of the Cretaceous thoracican cirripede family Stramentidae. Journal of Systematic Palaeontology, 14(8):653-702. https://doi.org/10.1080/14772019.2015.1091149
Gale, A.S. and Schweigert G. 2015. A new phosphatic-shelled cirripede (Crustacea, Thoracica) from the Lower Jurassic (Toarcian) of Germany - the oldest epiplanktonic barnacle. Palaeontology, 59(1):59-70. https://doi.org/10.1111/pala.12207
Gale, A.S. and Skelton, P.W. 2018. The Cretaceous acorn barnacle Archaeochionelasmus nekvasilovae Kočí, Newman and Buckeridge, 2017 (Cirripedia, Neobalanomorpha) is a fragmentary rudist (Bivalvia, Mollusca). Cretaceous Research, 91:251-256. https://doi.org/10.1016/j.cretres.2018.05.017
Gale, S.A. and Sørensen, A.M. 2014. Origin of the balanomorph barnacles (Crustacea, Cirripedia, Thoracica): new evidence from the Late Cretaceous (Campanian) of Sweden. Journal of Systematic Palaeontology, 13(9):791-824. https://doi.org/10.1080/14772019.2014.954824
Godefroit, P. 1994. Les reptiles marins du Toarcien (Jurassique inférieur) belgo-luxembourgeois. Mémoires pour Servir à l'Explication des Cartes Géologiques et Minières de la Belgique, 39:1-98.
Godoy, P.L., Bronzati, M., Eltink, E., Marsola, J.C.A., Cidade, G.M., Langer, M.C., and Montefeltro, F.C. 2016. Postcranial anatomy of Pissarrachampsa sera (Crocodyliformes, Baurusuchidae) from the Late Cretaceous of Brazil: insights on lifestyle and phylogenetic significance. PeerJ, 4:e2075. https://doi.org/10.7717/peerj.2075
Gothmann, A.M., Stolarski, J., Adkins, J.F., Schoene, B., Dennis, K.J., Schrag, D.P., Mazur, M., and Bender M.L. 2015. Fossil corals as an archive of secular variations in seawater chemistry since the Mesozoic. Geochimica et Cosmochimica Acta, 160:188-208. https://doi.org/10.1016/j.gca.2015.03.018
Grigg, G.C. 1981. Plasma homeostasis and cloacal urine composition in Crocodylus porosus caught along a salinity gradient. Journal of Comparative Physiology B, 144:261-270. https://doi.org/10.1007/BF00802765
Grigg, G.C. and Gans, C. 1993. Morphology and Physiology of the Crocodylia, p. 326-336. In Glasby, C.J., Ross, G.J.B., and Beesley, P.L. (eds.), Fauna of Australia Volume 2A: Amphibia and Reptilia. Australian Government Publishing Service, Canberra.
Hayashi, R. 2013. A checklist of turtle and whale barnacles (Cirripedia: Thoracica: Coronuloidea). Journal of the Marine Biological Association of the United Kingdom, 93(1):143-182.
Hayashi, R., Chan, B.K.K., Simon-Blecher, N., Watanabe, H., Guy-Haim, T., Yonezawa, T., Levy, Y., Shuto, T., and Achituv, Y. 2013. Phylogenetic position and evolutionary history of the turtle and whale barnacles (Cirripedia: Balanomorpha: Coronuloidea). Molecular Phylogenetics and Evolution, 67:9-14. https://doi.org/10.1016/j.ympev.2012.12.018
Heckert, A.B., Lucas, S.G., Rinehart, L.F., Celeskey, M.D., Spielmann, J.A., and Hunt, A.P. 2010. Articulated skeletons of the aetosaur Typothorax coccinarum Cope (Archosauria: Stagonolepididae) from the Upper Triassic Bull Canyon Formation (Revueltian: early-mid Norian), eastern New Mexico, USA. Journal of Vertebrate Paleontology, 30(3):619-642. https://doi.org/10.1080/02724631003763524
Hill, R.V. 2010. Osteoderms of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology, 30(6, suppl.):154-176. https://doi.org/10.1080/02724634.2010.518110
Hirayama, R. 1998. Oldest known sea turtle. Nature, 392:705-708. https://doi.org/10.1038/33669
Huang, J., Motani, R., Jiang, D., Tintori, A., Rieppel, O., Zhou, M., Ren, X., and Zhang, R. 2019. The new ichthyosauriform Chaohusaurus brevifemoralis (Reptilia, Ichthyosauromorpha) from Majiashan, Chaohu, Anhui Province, China. PeerJ, 7:e7561. https://doi.org/10.7717/peerj.7561
Huene, F. 1923. Ein neuer Plesiosaurier aus dem oberen Lias Württembergs. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg, 79:3-23.
Ifrim, C., Vega, F.J., and Stinnesbeck, W. 2011. Epizoic stramentid cirripedes on ammonites from late Cretaceous Platy Limestones in Mexico. Journal of Paleontology, 85(3):524-536. https://doi.org/10.1666/10-052.1
Johnson, M.M., Young, M.T., and Brusatte, S.L. 2020a. Emptying the wastebasket: a historical and taxonomic revision of the Jurassic crocodylomorph Steneosaurus. Zoological Journal of the Linnean Society, 189:1-21. https://doi.org/10.1093/zoolinnean/zlaa027
Johnson, M.M., Young, M.T., and Brusatte, S.L. 2020b. The phylogenetics of Teleosauroidea (Crocodylomorpha, Thalattosuchia) and implications for their ecology and evolution. PeerJ, 8:e9808. https://doi.org/10.7717/peerj.9808
Joyce, W.G., Mäuser, M., and Evers, S.W. 2021. Two turtles with soft tissue preservation from the platy limestones of Germany provide evidence for marine flipper adaptations in Late Jurassic thalassochelydians. PLoS ONE, 16(6):e0252355. https://doi.org/10.1371/journal.pone.0252355
Kase, T., Shigeta, Y., and Futakami, M. 1994. Limpet home depressions in Cretaceous ammonites. Lethaia, 27:49-58. https://doi.org/10.1111/j.1502-3931.1994.tb01555.x
Kase, T., Johnston, P.A., Seilacher, A., and Boyce, J.B. 1998. Alleged mosasaur bite marks on Late Cretaceous ammonites are limpet (patellogastropod) home scars. Geology, 26(10):947-950. https://doi.org/10.1130/0091-7613(1998)026<0947:AMBMOL>2.3.CO;2
Kelley, N.P. and Pyenson, N.D. 2015. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science, 348:3716.1-3716.7. https://doi.org/10.1126/science.aaa3716
Key, M.M., Jeffries, W.B., and Voris, H.K. 1995. Epizoic bryozoans, sea snakes, and other nektonic substrates. Bulletin of Marine Science, 56(2):462-474.
Kubo, T., Mitchell, M.T., and Henderson, D.M. 2012. Albertonectes vanderveldei, a new elasmosaur (Reptilia, Sauropterygia) from the Upper Cretaceous of Alberta. Journal of Vertebrate Paleontology, 32(3):557-572. https://doi.org/10.1080/02724634.2012.658124
Lindgren, J., Alwmark, C., Caldwell, M.W., and Fiorillo, A.R. 2009. Skin of the Cretaceous mosasaur Plotosaurus: implications for aquatic adaptations in giant marine reptiles. Biology Letters, 5:528-531. https://doi.org/10.1098/rsbl.2009.0097
Lindgren, J., Caldwell, M.W., Konishi, T., and Chiappe, L.M. 2010. Convergent evolution in aquatic tetrapods: Insights from an exceptional fossil mosasaur. PLoS ONE, 5(8):e11998. https://doi.org/10.1371/journal.pone.0011998
Lindgren, J., Everhart, M.J., and Caldwell, M.W. 2011. Three-dimensionally preserved integument reveals hydrodynamic adaptations in the extinct marine lizard Ectenosaurus (Reptilia, Mosasauridae). PLoS ONE, 6(11):e27343. https://doi.org/10.1371/journal.pone.0027343
Lindgren, J., Sjövall, P., Thiel, V., Zheng, W., Ito, S., Wakamatsu, K., Hauff, R., Kear, B.P., Engdahl, A., Alwmark, C., Eriksson, M.E., Jarenmark, M., Sachs, S., Ahlberg, P.E., Marone, F., Kuriyama, T., Gustafsson, O., Malmberg, P., Thomen, A., Rodríguez-Meizoso, I., Uvdal, P., Ojika, M., and Schweitzer, M.H. 2018. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature, 564:359-365. https://doi.org/10.1038/s41586-018-0775-x
Lingham-Soliar, T. 1999. Rare soft tissue preservation showing fibrous structures in an ichthyosaur from the Lower Lias (Jurassic) of England. Proceeding of the Royal Society London B, 266:2367-2373. https://doi.org/10.1098/rspb.1999.0933
Lingham-Soliar, T. 2001. The ichthyosaur integument: skin fibers, a means for a strong, flexible and smooth skin. Lethaia, 34:287-302. https://doi.org/10.1111/j.1502-3931.2001.tb00058.x
Lingham-Soliar, T. and Plodowski, G. 2007. Taphonomic evidence for high-speed adapted fins in thunniform ichthyosaurs. Naturwissenschaften, 94:65-70. https://doi.org/10.1007/s00114-006-0160-8
Maderson, P.F.A. 1968. Observations on the epidermis of the tuatara (Sphenodon punctatus). Journal of Anatomy, 103(2):311-320.
Maderson, P.F.A. 1972. When? Why? and How?: Some speculations on the evolution of the vertebrate integument. American Zoologist, 12:159-171. https://doi.org/10.1093/icb/12.1.159
Marinho, T.S. and Carvalho, I.S. 2009. An armadillo-like sphagesaurid crocodyliform from the Late Cretaceous of Brazil. Journal of South American Earth Sciences, 27:36-41. https://doi.org/10.1016/j.jsames.2008.11.005
Mäuser, M. 2015. Die laminierten Plattenkalke von Wattendorf in Oberfranken, p. 515-535. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen – Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
McDonald, H.G. 2018. An overview of the presence of osteoderms in sloths: Implications for osteoderms as a plesiomorphic character of the Xenarthra. Journal of Mammalian Evolution, 25:485-493. https://doi.org/10.1007/s10914-017-9415-8
Meyer, R.K.F. and Schmidt-Kaler, H. 1984. Erdgeschichte sichtbar gemacht: Ein geologischer Führer durch die Altmühl-Alb. Bayerisches Geologisches Landesamt, München.
Milinkovitch, M.C., Manukyan, L., Debry, A., Di-Poï, N., Martin, S., Singh, D., Lambert, D., and Zwicker, M. 2013. Crocodile head scales are not developmental units but emerge from physical cracking. Science, 339:78-81. https://doi.org/10.1126/science.1226265
Montefeltro, F.C., Andrade, D.V., and Larsson, C.E. 2016. The evolution of the meatal chamber in crocodyliforms. Journal of Anatomy, 228:838-863. https://doi.org/10.1111/joa.12439
Montefeltro, F.C. 2019. The osteoderms of baurusuchid crocodyliforms (Mesoeucrocodylia, Notosuchia). Journal of Vertebrate Paleontology, 39:e1594242. https://doi.org/10.1080/02724634.2019.1594242
Neenan, J.M., Reich, T., Evers, S.W., Druckenmiller, P.S., Voeten, D.F.A.E., Choiniere, J.N., Barrett, P.M., Pierce, S.E., and Benson, R.B.J. 2017. Evolution of the sauropterygian labyrinh with increasingly pelagic lifestyles. Current Biology, 27:1-7. https://doi.org/10.1016/j.cub.2017.10.069
Nesbitt, S.J., Turner, A.H., and Weinbaum, J.C. 2013 (for 2012). A survey of skeletal elements in the orbit of Pseudosuchia and the origin of the crocodilian palpebral. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 103:365-381.
Nifong, J.C. and Frick, M.G. 2011. First record of the american alligator (Alligator mississippiensis) as a host to the Sea Turtle Barnacle (Chelonibia testudinaria). Southeastern Naturalist, 10(3):557-560. https://doi.org/10.1656/058.010.0316
Nützel, A. 2015. Schnecken (Gastropoda), p. 215-217. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
O’Connor, P.M., Sertich, J.J.W., Stevens, N.J., Roberts, E.M., Gottfried, M.D., Hieronymus, T.L., Jinnah, Z.A., Ridgely, R., Ngasala, S.E., and Temba, J. 2010. The evolution of mammal-like crocodyliforms in the Cretaceous Period of Gondwana. Nature, 466:748-751. https://doi.org/10.1038/nature09061
Ösi, A., Young, M.T., Galácz, A., and Rabi, M. 2018. A new large-bodied thalattosuchian crocodyliform from the Lower Jurassic (Toarcian) of Hungary, with further evidence of the mosaic acquisition of marine adaptations in Metriorhynchoidea. PeerJ, 6:e4668. https://doi.org/10.7717/peerj.4668
Parrilla-Bel, J., Young, M.T., Moreno-Azanza, M., and Canudo, J.I. 2013. The first metriorhynchid crocodylomorph from the Middle Jurassic of Spain, with implications for evolution of the subclade Rhacheosaurini. PLoS ONE, 8(1):e54275. https://doi.org/10.1371/journal.pone.0054275
Pérez-Losada, M., Høeg, J.T., and Crandall, K.A. 2004. Unraveling the evolutionary radiation of the thoracican barnacles using molecular and morphological evidence: A comparison of several divergence time estimation approaches. Systematic Biology, 53(2):244-264. https://doi.org/10.1080/10635150490423458
Pérez-Losada, M., Harp, M., Høeg, J.T., Achituv, Y., Jones, D., Watanabe, H., and Crandall, K.A. 2008. The tempo and mode of barnacle evolution. Molecular Phylogenetics and Evolution, 46:328-346. https://doi.org/10.1016/j.ympev.2007.10.004
Pol, D. and Gasparini, Z. 2009. Skull anatomy of Dakosaurus andiniensis (Thalattosuchia: Crocodylomorpha) and the phylogenetic position of Thalattosuchia. Journal of Systematic Palaeontology, 7:163-197.
Rees, D.J., Noever, C., Høeg, J.T., Ommundsen, A., and Glenner, H. 2014. On the origin of a novel parasitic-feeding mode within suspension-feeding barnacles. Current Biology, 24(12):1-6. https://doi.org/10.1016/j.cub.2014.05.030
Reis, O.M. 1893. Untersuchungen über die Petrificirung der Muskulatur. Archiv für mikroskopische Anatomie, 41:492-584.
Renesto, S. 2006. Peculiar preservation of a juvenile pachypleurosaurid from Besano (Italy). Rivista Italiana di Paleontologia e Stratigrafia, 112(3):373-382. https://doi.org/10.13130/2039-4942/6347
Renesto, S., Dal Sasso, C., Fogliazza, F., and Ragni, C. 2020. New findings reveal that the Middle Triassic ichthyosaur Mixosaurus cornalianus is the oldest amniote with a dorsal fin. Acta Palaeontologica Polonica, 65:511-522. https://doi.org/10.4202/app.00731.2020
Reolid, M., Santos, A., and Mayoral, E. 2015. Grazing activity as taphonomic record of necrobiotic interaction: A case study of a sea turtle carapace from the Upper Jurassic of the Prebetic (south Spain). Revista Mexicana De Ciencias Geológicas, 32(1):21-28.
Ridgwell, A. 2005. A Mid Mesozoic Revolution in the regulation of ocean chemistry. Marine Geology, 217:339-357. https://doi.org/doi:10.1016/j.margeo.2004.10.036
Rothschild, B.M. and Depalma, R. 2013. Skin pathology in the Cretaceous: evidence for probable failed predation in a dinosaur. Cretaceous Research 42:44-47. https://doi.org/10.1016/j.cretres.2013.01.005
Rutland, C.S., Cigler, P., and Kubale, V. 2019. Reptilian skin and its special histological structures. In Rutland, C.S. (ed.), Veterinary Anatomy and Physiology, IntechOpen, London. https://doi.org/10.5772/intechopen.84212
Sachs, S., Young, M.T., Abel, P., and Mallison, H. 2019. A new species of the metriorhynchid crocodylomorph Cricosaurus from the Upper Jurassic of southern Germany. Acta Palaeontologica Polonica, 64(2):343-356. https://doi.org/10.4202/app.00541.2018
Sachs, S., Young, M.T., Abel, P., and Mallison, H. 2021. A new species of Cricosaurus (Thalattosuchia, Metriorhynchidae) based upon a remarkably well-preserved skeleton from the Upper Jurassic of Germany. Palaeontologia Electronica, 24(2):a24. https://doi.org/10.26879/928
Scarff, J.E. 1986. Occurrence of the barnacles Coronula diadema, C. reginae and Cetopirus complanatus (Cirripedia) on right whales. Scientific Reports of the Whales Research Institute, 37:129-153.
Scheyer, T.M., Neenan, J.M., Renesto, S., Saller, F., Hagdorn, H., Furrer, H., Rieppel, O., and Tintori, T. 2012. Revised paleoecology of placodonts - with a comment on ‘The shallow marine placodont Cyamodus of the central European Germanic Basin: its evolution, paleobiogeography and paleoecology’ by C.G. Diedrich (Historical Biology, iFirst article, 2011, 1-19, https://doi.org/10.1080/08912963.2011.575938). Historical Biology, 24(3):257-267. https://doi.org/10.1080/08912963.2011.621083
Scheyer, T.M., Neenan, J.M., Bodogan, T., Furrer, H., Obrist, C., and Plamondon, M. 2017. A new, exceptionally preserved juvenile specimen of Eusaurosphargis dalsassoi (Diapsida) and implications for Mesozoic marine diapsid phylogeny. Nature Scientific Reports, 7:4406. https://doi.org/10.1038/s41598-017-04514-x
Schwab, J.A., Young, M.T., Neenan, J.M., Walsh, S.A., Witmer, L.M., Herrera, Y., Allain, R., Brochu, C.A., Choiniere, J.N., Clark, J.M., Dollman, K.N., Etches, S., Fritsch, G., Gignac, P.M., Ruebenstahl, A., Sachs, S., Turner, A.H., Vignaud, P., Wilberg, E.W., Xu, X., Zanno, L.E., and Brusatte, S.L. 2020. Inner ear sensory system changes as extinct crocodylomorphs transitioned from land to water. Proceedings of the National Academy of Sciences of the United States of America, 117(19):10422-10428. https://doi.org/10.1073/pnas.2002146117
Schwarz, D., Kundrát, M., Tischlinger, H., Dyke, G., and Carney, R.M. 2019. Ultraviolet light illuminates the avian nature of the Berlin Archaeopteryx skeleton. Nature Scientific Reports, 9,6518:1-10. https://doi.org/10.1038/s41598-019-42823-5
Schweigert. G. 2007. Ammonite biostratigraphy as a tool for dating Upper Jurassic lithographic limestones from South Germany - first results and open questions. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 245(1):117-125. https://doi.org/10.1127/0077-7749/2007/0245-0117
Schweigert, G. 2015. Zehnfußkrebse (Decapoda) und andere Krebstiere, p. 271-291. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Seilacher, A. 2005. Whale barnacles: exaptational access to a forbidden paradise. Paleobiology, 31(2):27-35. https://doi.org/10.1666/0094-8373(2005)031[0027:WBEATA]2.0.CO;2
Sereno, P.C. and Larsson, H.C.E. 2009. Cretaceous crocodyliforms from the Sahara. ZooKeys, 28:1-143. https://doi.org/10.3897/zookeys.28.325
Sohl, N.F. 1987. Cretaceous gastropods: contrasts between Tethys and the temperate provinces. Journal of Paleontology, 61(6):1085-1111.
Spearman, R.I.C. and Riley, P.A. 1969. A comparison of the epidermis and pigment cells of the crocodile with those in two lizard species. Zoological Journal of the Linnean Society, 48:453-466.
Taplin L.E., Grigg, G.C., Harlow, P., Ellis, T.M., and Dunson, W.A. 1982. Lingual salt glands in Crocodylus acutus and C. johnstoni and their absence from Alligator mississipiensis and Caiman crocodilus. Journal of Comparative Physiology, 149:43-47. https://doi.org/10.1007/BF00735713
Themudo, G.E., Alves, L.Q., Machado, A.M., Lopes-Marques, M., da Fonseca, R.R., Fonseca, M., Ruivo, R., and Castro, L.F.C. 2020. Losing genes: The evolutionary remodeling of Cetacea skin. Frontiers in Marine Science, 7:592375. https://doi.org/10.3389/fmars.2020.592375
Tischlinger, H. 2002. Der Eichstätter Archaeopteryx im langwelligen UV-Licht. Archaeopteryx, 20:21-38.
Tischlinger, H. and Unwin, D. 2004. UV-Untersuchungen des Berliner Exemplars von Archaeopteryx lithographica H.v.Meyer 1861 und der isolierten Archaeopteryx -Feder. Archaeopteryx, 22:17-50.
Tischlinger, H. 2005. Ultraviolet Light Investigations of Fossils from the Upper Jurassic Plattenkalks of Southern Frankonia. Zitteliana B, 26:26.
Tischlinger, H. and Arratia, G. 2013. Ultraviolet light as a tool for investigating Mesozoic fishes, with a focus on the ichthyofauna of the Solnhofen archipelago, p. 549-560. In Arratia, G., Schultze, H.-P., and Wilson, H. (eds.), Mesozoic Fishes 5 - Global Diversity and Evolution. Verlag Dr. Friedrich Pfeil, München.
Tischlinger, H. 2015. Arbeiten mit ultraviolettem Licht, p. 109-113. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Tsujita, C.J. and Westermann, G.E.G. 2001. Were limpets or mosasaurs responsible for the perforations in the ammonite Placenticeras ? Palaeogeography, Palaeoclimatology, Palaeoecology, 169:245-270. https://doi.org/10.1016/S0031-0182(01)00220-6
Vickaryous, M.K. and Sire, J.-Y. 2009. The integumentary skeleton of tetrapods: origin, evolution, and development. Journal of Anatomy, 214:441-464. https://doi.org/10.1111/j.1469-7580.2008.01043.x
Vincent P., Allemand, R., Taylor, P.D., Suan, G., and Maxwell, E. 2017. New insights on the systematics, palaeoecology and palaeobiology of a plesiosaurian with soft tissue preservation from the Toarcian of Holzmaden, Germany. Science of Nature, 104(51),1-13. https://doi.org/10.1007/s00114-017-1472-6
Viohl, G. 2015. Der geologische Rahmen: die Südliche Frankenalb und ihre Entwicklung, p. 56-62. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Wachtler, M. 2018. The marine reptile Neusticosaurus from the Eastern Alps, p. 47-57. In Wachtler, M. and Perner, T. (eds.), Some new and exciting Triassic Archosauria from the Dolomites (Northern Italy). Dolomythos Museum, Innichen, South Tyrol.
Westphal, F. 1962. Die Krokodilier des deutschen und englischen oberen Lias. Palaeontographica A, 118:23-118.
Westphal, F. 1975. Bauprinzipien im Panzer der Placodonten (Reptilia triadica). Paläontologische Zeitschrift, 49:97-125.
Williston, S.W. 1914. Water Reptiles of the Past and Present. University of Chicago Press.
Witzmann, F. 2011. Morphological and histological changes of dermal scales during the fish-to-tetrapod transition. Acta Zoologica, 92:281-302. https://doi.org/10.1111/j.1463-6395.2010.00460.x
Wu, X.C., Russell, A.P., and Cumbaa, S.L. 2001. Terminonaris (Archosauria: Crocodyliformes): new material from Saskatchewan, Canada, and comments on its phylogenetic relationships. Journal of Vertebrate Paleontology, 21(3):492-514. https://doi.org/10.1671/0272-4634(2001)021[0492:TACNMF]2.0.CO;2
Yamaguchi, T. and Newman, W.A. 1990. A new and primitive barnacle (Cirripedia: Balanomorpha) from the North Fiji Basin Abyssal Hydrothermal Field, and its evolutionary implications. Pacific Science, 44(2):135-155.
Young, M.T. and Andrade, M.B. 2009. What is Geosaurus ? Redescription of Geosaurus giganteus (Thalattosuchia: Metriorhynchidae) from the Upper Jurassic of Bayern, Germany. Zoological Journal of the Linnean Society, 157:551-585. https://doi.org/10.1111/j.1096-3642.2009.00536.x
Young, M.T., Brusatte, S.L., Ruta, M., and Andrade, M.B. 2010. The evolution of Metriorhynchoidea (Mesoeucrocodylia, Thalattosuchia): an integrated approach using geometric morphometric, analysis of disparity, and biomechanics. Zoological Journal of the Linnean Society, 158:801-859. https://doi.org/10.1111/j.1096-3642.2009.00571.x
Young, M.T., Bell, M.A., Andrade, M.B., and Brusatte, S. 2011. Body size estimation and evolution in metriorhynchid crocodylomorphs: implications for species diversification and niche partitioning. Zoological Journal of the Linnean Society, 163:1199-1216. https://doi.org/10.1111/j.1096-3642.2011.00734.x
Young, M.T., Brusatte, S.L., Beatty, B.L., de Andrade, M.B., and Desojo, J.B. 2012. Tooth-on-tooth interlocking occlusion suggests macrophagy in the Mesozoic marine crocodylomorph Dakosaurus. The Anatomical Record, 295:1147-1158.
Zangerl, R. 1948. The vertebrate fauna of the Selma Formation of Alabama. Part I. Introduction. Fieldiana, 3(1):2-16.
Zhou, X., Seim, I., and Gladyshev, V.N. 2015. Convergent evolution of marine mammals is associated with distinct substitutions in common genes. Nature Scientific Reports, 5:16550. https://doi.org/10.1038/srep16550

