 A pterosaurian connecting link from the Late Jurassic of Germany
A pterosaurian connecting link from the Late Jurassic of Germany
Article number: 27.2.a35
https://doi.org/10.26879/1366
Copyright Palaeontological Association, July 2024
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 20 December 2023. Acceptance: 4 July 2024.
ABSTRACT
Based on a unique and extraordinarily preserved complete skeleton, the “Painten pro-pterodactyloid” is formally described and named as Propterodactylus frankerlae, gen. nov., spec. nov. As previously shown, it has a nearly perfect mix of plesiomorphic rhamphorhynchoid-grade, wukongopterid, and derived pterodactyloid traits. Due to its lack of autapomorphies, Propterodactylus is a sufficient intermediate taxon that closes the greatest knowledge gap regarding the evolution of pterosaur morphology. Non-pterodactyloid features include interlocking caudal vertebrae and a functional fifth pedal toe. Derived features such as the nasoantorbital fenestra, a short tail, or initially elongated cervicals and metacarpals appear ancient within the spectrum of Pterodactyloidea. Other early Monofenestrata appear more autapomorphic. However, the late juvenile or subadult status of the described specimen suggests that the rostrum, neck, and extremities might have been even more elongated when fully grown. Despite the otherwise intermediate, transitional osteology, details of dentition types appear mosaic-like in early Monofenestrata. While the remainder of the skeleton of Propterodactylus fits into known evolutionary trends, its dentition implies a significant role of varying dietary adaptation throughout the pterodactyl transition.
Frederik Spindler. Dinosaurier Museum Altmühltal, Dinopark 1, 85095 Denkendorf, Germany. mail@frederik-spindler.de
Keywords: new genus; new species; Pterosauria; evolution; dentition
Final citation: Spindler, Frederik. 2024. A pterosaurian connecting link from the Late Jurassic of Germany. Palaeontologia Electronica, 27(2):a35.
https://doi.org/10.26879/1366
palaeo-electronica.org/content/2024/5213-pterosaurian-connecting-link
Copyright: July 2024 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
https://zoobank.org/64C24E44-2E13-42C3-A087-E07FEAA1A50F
INTRODUCTION
Pterosaurs, the oldest vertebrate lineage that performed active flight, experienced a highly successful evolution throughout the Mesozoic. For most of their long research history (Wellnhofer, 2008; Tischlinger, 2023) any specimen could be classified as belonging to one of two major types, the more ancestral long-tailed “Rhamphorhynchoidea” and the derived stubby-tailed Pterodactyloidea (Wellnhofer, 1978). The only short-faced pterosaurs, the rare Anurognathidae, are likewise short-tailed (for an exception see Jiang et al., 2015), but otherwise appear rhamphorhynchoid-grade and thus were commonly considered to nest deeply within the rhamphorhynchoid-grade (Bennett, 2007; Lü et al., 2010). A truly intermediary and thus convincingly transitional morphology between the major types remained unknown until the discovery of the Wukongopteridae (Wang, et al. 2009; Lü et al., 2010).
It is the rich faunas of the so-called Solnhofen archipelago from the Late Jurassic of the Southern Franconian Jura in Bavaria, Germany, that substantially contributed to the historical discovery, systematics, and ecological knowledge about pterosaurs (Collini, 1784; Cuvier, 1809; Wellnhofer, 1968, 1970, 1975, 1978; Vidovic and Martill, 2014, 2018). This fossil region has yielded a pterosaur diversity as rich as possible for this stratigraphic section (Tischlinger and Frey, 2015), comprising two families of rhamphorhynchoid-grade forms, an anurognathid, a broad diversity of archaeopterodactyloids, and possibly the earliest eupterodactyloids. Wukongopterids may be missing for environmental reasons (Witton, 2013), but are not ultimately ruled out (Spindler and Ifrim, 2021). Additionally, the Solnhofen region is the only one in the world that has yielded considerable complete specimens of transitional forms that are more derived than rhamphorhynchoid-grade and wukongopterid forms, but less derived than pterodactyloids (Rauhut, 2012; Tischlinger and Frey, 2013).
The most complete specimen of this transitional type, DMA-JP-2011/006, is known from the Kimmeridgian of Painten (Spindler and Albersdörfer, 2019). During its preparation, the basically Pterodactylus -like skull was cleaned first, while the virtually perfect mix of characters in the postcranium soon revealed an important missing link (R. Albersdörfer, pers. comm.) that surprisingly matched with a hypothetical sketch by Frey et al. (2003, figure 10). In the original report, Tischlinger and Frey (2013) have described the anatomy and evolutionary implications of what they colloquially referred to as the “Painten pro-pterodactyloid”, a designation that was adopted in subsequent analyses (Witton et al., 2015; Vidovic and Martill, 2018; Elgh et al., 2019; Andres, 2021).
Within the evolution of Pterosauria, the pterodactyloid transition marks the most significant change. It took place in the Jurassic, preceding the seemingly sudden occurrence of Pterodactyloidea in the Late Jurassic (Tischlinger and Frey, 2013; Andres et al., 2014). Since the discovery of the Wukongopteridae and introduction of Monofenestrata for their sister group relationship with more derived pterosaurs (Wang et al., 2009; Lü et al., 2010) it is known that the pterodactyloid transition was modular, with the derived skull pattern evolving earlier and seemingly independent from postcranial modifications. Thus, along with fragmentary mosaic forms and further discoveries (Zhou and Schoch, 2011; Martill and Etches, 2013; citations in Tischlinger and Frey, 2013; Codorniú et al., 2016; Wang et al., 2017; Zhou et al., 2021), the initial steps towards the pterodactyl transition could be clarified to a certain extent.
Nonetheless, the breakthrough in determining the very origin of pterodactyloids lies in the pro-pterodactyloid forms. One specimen informally called “Rhamphodactylus” (Rauhut, 2012) is similar to the Painten pro-pterodactyloid, although its conspecific status is unlikely, given their stratigraphic distance (Tischlinger and Frey, 2013) and proportional differences (pers. obs.). The fragmentarily known Kryptodrakon was introduced as the basal-most pterodactyl (Andres et al., 2014). Apart from controversial phylogenetic positions of Kryptodrakon relative to the Painten pro-pterodactyloid (Wang et al., 2017; Andres, 2021; but see Martin-Silverstone et al., 2022), the phylogenetic classification of the latter shows it unquestionably closing the gap between early Monofenestrata and Pterodactyloidea (Vidovic and Martill, 2018; Jiang et al., 2020). The currently ongoing formal description and detailed analysis of these intermediate forms (Rauhut, 2012; Tischlinger and Frey, 2013) begins with the present study.
MATERIAL AND METHODS
Fossils found in Upper Jurassic limestones from Bavaria are frequently auto-fluorescent. Ultraviolet induced fluorescence is used to improve contrasts in order to detect sutures or exact outlines of delicate phosphorous structures (Tischlinger, 2015; Tischlinger and Unwin, 2004; Tischlinger and Arratia, 2013; Spindler et al., 2021). The description of DMA-JP-2011/006 (Dinosaurier Museum Altmühltal: Jurassic Palaeontology) was supported with an LED flashlight (UV A, 365 nm). Photographs taken under ultraviolet light (UV) were kindly provided by Helmut Tischlinger (365 nm, see Tischlinger and Frey, 2013). Line drawings were digitally created on a WACOM tablet, using a normal light photograph in Adobe Photoshop as master template and chosen UV close-ups as additional support. The analogous pre-drawing was based particularly on intensive work using microscopy under normal light and UV, as well as digital microscopic photography (Leica Stereozoom S9i and LAS X at various low magnifications).
Two very similar partial skeletons of pro-pterodactyloids were documented in the same way (Rauhut, 2012; pers. obs.) but need closer investigation. Therefore, this material was excluded from the current study, with metric data provisionally left at the status provided by Tischlinger and Frey (2013). The discussion follows an eco-evolutionary approach to assess morphological changes, which the additional specimens do not contradict.
No phylogenetic analysis is carried out. The described specimen was coded in published tree hypotheses, repeatedly finding a sister-group relationship of the Painten pro-pterodactyloid with Pterodactyloidea (Wang et al., 2017; Vidovic and Martill, 2018) or with Lophocratia (Andres, 2021, basing on the earlier definition of Kryptodrakon as belonging to Pterodactyloidea sensu Andres et al., 2014). The description attempts to detail differences from the fragmental Kryptodrakon. Contextualisation of observed osteologic conditions uses chosen comparisons, preferably those that are representative for subclades around the pterodactyloid transition.
The revision of Late Jurassic pterosaurs from Germany has made substantial progress (Jouve, 2004; Bennett, 1996, 2006, 2013a, b; Vidovic and Martill, 2014, 2018), but is not yet underpinned by a greater number of detailed osteological documentations. In order not to confuse the reader with pending phylo-taxonomical issues, long-known pterosaurs are herein referred to as typological units, such as Pterodactylus -type forms (mainly comprising P. antiquus, Diopecephalus kochi, Aerodactylus scolopaciceps) or Germanodactylus -type pterodactyls (G. cristatus, Altmuehlopterus rhamphastinus).
SYSTEMATIC PALAEONTOLOGY
Order Pterosauria Owen, 1842 (for a rejection of Pterosaurii Kaup, 1834 see Seeley, 1870, 1891)
Clade Monofenestrata Lü, Unwin, Jin, Liu, and Ji, 2010
Propterodactylus gen. nov.
zoobank.org/AC14E4A9-B989-40EE-9CC6-2FF047B62A55
Type species. Propterodactylus frankerlae gen. nov., sp. nov.
Etymology. The genus name refers to the informal designation as a pro-pterodactyloid in the literature, meaning a supposed forerunner (ancient Greek προ- for “before”) of the iconic Pterodactylus (latinized form of Greek πτερόν plus δάκτυλος for “wing digit”) and at the same time a forerunner of the Pterodactyloidea in general.
Diagnosis. As for the type species.
Remarks. The intermediate status is underlined by a lack of apparent autapomorphies. Tischlinger and Frey (2013) listed several plesiomorphic and apomorphic traits. Similarities with the derived Pterodactyloidea comprise the skull shape and short tail. Plesiomorphies shared with e.g., Wukongopteridae, which preclude Propterodactlyus from Pterodactyloidea, are the functional fifth toe and long caudal zygapophyses. Intermediate conditions apply to the cervical elongation, metacarpal elongation, and reduced fifth toe.
Propterodactylus frankerlae gen. nov., sp. nov.
Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9
zoobank.org/7F2B8C07-B736-4830-BA20-53CD548F22E5
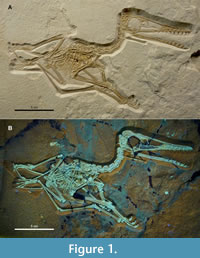 Holotype. DMA-JP-2011/006 (Figure 1, Figure 2) comprises a complete and fully articulated skeleton, exposed in right lateral view, with soft tissue remains in the trunk region and wing actinofibrils (Tischlinger and Frey, 2013, figure 8). Checked under UV, the slab contains very little repair and shows no restored areas.
Holotype. DMA-JP-2011/006 (Figure 1, Figure 2) comprises a complete and fully articulated skeleton, exposed in right lateral view, with soft tissue remains in the trunk region and wing actinofibrils (Tischlinger and Frey, 2013, figure 8). Checked under UV, the slab contains very little repair and shows no restored areas.
Etymology. The epithet honours Petra Hahn, née Frankerl (1966 - 2019), the wife of Stephan Hahn, who found the specimen in 2011 during the scientific excavation.
Horizon and locality. Lower levels of the Rygol lime works quarry near Painten, Bavaria, Germany. The outcrop contributes to the “Solnhofen archipelago“, a Jurassic reef platform complex (Viohl, 2015a, b; Wilkin, 2020). The “Paintener Wanne” (depression or basin) is among the largest areas of laminated limestones that preserved allochthoneous biota under hypoxic conditions (Albersdörfer and Häckel, 2015). In the sampled level, fine-layered to laminated, irregularly silicified limestones (Kieselplattenkalke) belong to the uppermost Ulmense Subzone (index ammonite Lithoceras ulmense), representing the end-Kimmeridgian stage (Schweigert, 2007, 2015). Therefore, the specimen described herein is a contemporary of the oldest known Archaeopteryx from a neighbouring basin (Rauhut et al., 2018, figure 1). Information about the faunal content of the Late Jurassic marine and insular environment from Painten is provided by specific studies (Rauhut et al., 2012; Bennett, 2013b; Tischlinger and Frey, 2013; Spindler, 2019; Spindler and Albersdörfer, 2019; Sachs et al., 2021; Spindler et al., 2021; Augustin et al., 2022, 2023).
 Diagnosis. Monofenestratan pterosaur with unique combination of characters: skull moderately elongate, but longer than trunk, maxillary tooth row under nasoantorbital fenestra, nasoantorbital fenestra shorter than the massive rostrum, 11 maxillary tooth positions, nine dentary tooth positions, teeth moderately long and slightly curved conical, wide interdental spaces, cervicals slightly elongated, elongation including the eighth cervical, mid-dorsal diapophyses with offset articulation for rib read, caudal number about 18, mid-caudals moderately elongated and interlocked by needle-like prezygapophyses and hemapophyses, tail very short, distal scapula narrowed to form rounded tip, coracoid with large acrocoracoid process, pteroid slightly elongated, wing metacarpus slightly elongated, small but functional 5th toe with two phalanges.
Diagnosis. Monofenestratan pterosaur with unique combination of characters: skull moderately elongate, but longer than trunk, maxillary tooth row under nasoantorbital fenestra, nasoantorbital fenestra shorter than the massive rostrum, 11 maxillary tooth positions, nine dentary tooth positions, teeth moderately long and slightly curved conical, wide interdental spaces, cervicals slightly elongated, elongation including the eighth cervical, mid-dorsal diapophyses with offset articulation for rib read, caudal number about 18, mid-caudals moderately elongated and interlocked by needle-like prezygapophyses and hemapophyses, tail very short, distal scapula narrowed to form rounded tip, coracoid with large acrocoracoid process, pteroid slightly elongated, wing metacarpus slightly elongated, small but functional 5th toe with two phalanges.
COMPARATIVE DESCRIPTION
The following description focuses on qualitative information, as the reference of proportions requires a broader discussion regarding the newly introduced type of pterosaurs. Metric data and comparisons beyond the table of Tischlinger and Frey (2013) are subject of subsequent studies about similar specimens that are currently in preparation.
Skull
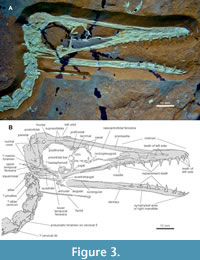 Proportions. DMA-JP-2011/006 contains an extraordinarily well-preserved skull (Figure 3), which is among the best known of any “transitional” pterosaur (see Lü et al., 2011, figure 2). The total skull length is about 93 mm long, measured along the greatest extent preserved, and 90 mm from the tip of the premaxilla to the atlas articulation. Its overall appearance is similar to Pterodactylus -type and ctenochasmatid Pterodactyloidea regarding the rostral tip (region anterior to the nasoantorbital fenestra) being longer than the nasoantorbital fenestra. In contrast, the rostral tip is shorter compared to an extended fenestra in Germanodactylus -type forms and Wukongopteridae. A rather even proportion of these zones, or slightly tip-dominated ratio, is found in Gallodactylus and Cycnorhamphus (Fabre, 1976; Bennett, 2013b). However, both the fenestra and the anterior snout tip are only moderately elongated in DMA-JP-2011/006 when compared to the skull height, making it potentially prototypic for ancient Monofenestrata. This less longirostrine condition, with the skull being only slightly longer than the trunk, bridges the morphological gap between Sordes or Jianchangopterus (Sharov, 1971; Lü and Bo, 2011) and the early pterodactyloid condition. Many proportions and specific observations, including dentition patterns, are astonishingly similar to juvenile specimens of Pterodactylus (Diopecephlaus) kochi (Vidovic and Martill, 2014, figure 3 G, H) and Kunpengopterus sinensis (Jiang et al., 2021, figure 6). In the light of DMA-JP-2011/006, the greatly enlarged heads of Wukongopteridae, exceeding 1.5 times the trunk length (Lü et al., 2010; Wang et al., 2010), seem less representative for the ancient monofenestratan condition.
Proportions. DMA-JP-2011/006 contains an extraordinarily well-preserved skull (Figure 3), which is among the best known of any “transitional” pterosaur (see Lü et al., 2011, figure 2). The total skull length is about 93 mm long, measured along the greatest extent preserved, and 90 mm from the tip of the premaxilla to the atlas articulation. Its overall appearance is similar to Pterodactylus -type and ctenochasmatid Pterodactyloidea regarding the rostral tip (region anterior to the nasoantorbital fenestra) being longer than the nasoantorbital fenestra. In contrast, the rostral tip is shorter compared to an extended fenestra in Germanodactylus -type forms and Wukongopteridae. A rather even proportion of these zones, or slightly tip-dominated ratio, is found in Gallodactylus and Cycnorhamphus (Fabre, 1976; Bennett, 2013b). However, both the fenestra and the anterior snout tip are only moderately elongated in DMA-JP-2011/006 when compared to the skull height, making it potentially prototypic for ancient Monofenestrata. This less longirostrine condition, with the skull being only slightly longer than the trunk, bridges the morphological gap between Sordes or Jianchangopterus (Sharov, 1971; Lü and Bo, 2011) and the early pterodactyloid condition. Many proportions and specific observations, including dentition patterns, are astonishingly similar to juvenile specimens of Pterodactylus (Diopecephlaus) kochi (Vidovic and Martill, 2014, figure 3 G, H) and Kunpengopterus sinensis (Jiang et al., 2021, figure 6). In the light of DMA-JP-2011/006, the greatly enlarged heads of Wukongopteridae, exceeding 1.5 times the trunk length (Lü et al., 2010; Wang et al., 2010), seem less representative for the ancient monofenestratan condition.
Premaxilla and maxilla. The very tip of the upper jaw is blunt and of moderate thickness. On the tip of the snout, no premaxillary-maxillary suture could be identified with certainty. Posterior to the sixth tooth position, a crack course may trace this contact, as the premaxilla covers the skull roof above the nasoantorbital fenestra in the same width. The same is found in Pterodactylus (Bennett, 2013a, figure 2). The rear end of the premaxilla is blunt and slightly narrower than the middle portion. If completely preserved, this posterior tip reaches the longitudinal level of the jaw joint, ending anterior to the middle of the orbit. This extent is much shorter than in the longirostrine Wukongopteridae and early Pterodactyloidea, but also cannot be assessed without including the cranial angle (see below). No trace of a striated base of an ornamental crest is present.
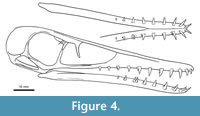 The maxilla is low, resulting in a shallow and even triangular skull profile (Figure 4). No posterodorsal process is preserved, so that the maxilla contributes only to the anterior and ventral margin of the nasoantorbital fenestra. Although this area is slightly damaged in DMA-JP-2011/006, there is no impression of a dorsal component of the fenestra margin built up by the maxilla, nor does the overlapping premaxilla allow for a greater hidden area, resulting from the articulation of the upper skull. That the anterior tip of the nasoantorbital fenestra is defined by both the premaxilla and the maxilla, opposes the condition known from wukongopterids and early pterodactyloids (Wellnhofer, 1970; Lü et al., 2010; Bennett, 2013a; Vidovic and Martill, 2018). The entire length of the maxilla covers more than 60 % of the total skull length, pending the unknown position of the anterior terminus. The posterior end of the maxilla is externally bifurcate, as the jugal interlinks on a long trough along the dorsal half of the posterior maxillary ramus. Interestingly, these maxillary characteristics match the oldest known ctenochasmatid Liaodactylus (Zhou et al., 2017). With its very end, the maxilla does not reach the level of the dorsal process of the jugal.
The maxilla is low, resulting in a shallow and even triangular skull profile (Figure 4). No posterodorsal process is preserved, so that the maxilla contributes only to the anterior and ventral margin of the nasoantorbital fenestra. Although this area is slightly damaged in DMA-JP-2011/006, there is no impression of a dorsal component of the fenestra margin built up by the maxilla, nor does the overlapping premaxilla allow for a greater hidden area, resulting from the articulation of the upper skull. That the anterior tip of the nasoantorbital fenestra is defined by both the premaxilla and the maxilla, opposes the condition known from wukongopterids and early pterodactyloids (Wellnhofer, 1970; Lü et al., 2010; Bennett, 2013a; Vidovic and Martill, 2018). The entire length of the maxilla covers more than 60 % of the total skull length, pending the unknown position of the anterior terminus. The posterior end of the maxilla is externally bifurcate, as the jugal interlinks on a long trough along the dorsal half of the posterior maxillary ramus. Interestingly, these maxillary characteristics match the oldest known ctenochasmatid Liaodactylus (Zhou et al., 2017). With its very end, the maxilla does not reach the level of the dorsal process of the jugal.
Nasal region. The nasal region of Late Jurassic monofenestratans is not documented in satisfying detail. Nonetheless, the nasoantorbital fenestra is of typical appearance in DMA-JP-2011/006. The nasal covers a broad portion of the dorsal roof about the level of the preorbital bar. A narrow and pointed nasal process points downward to end freely in the lower half of the skull depth. This condition matches with early Pterodactyloidea, but not the shortened free nasal process in most early monofenestratan forms (Martill and Etches, 2013, figure 6). In the antero-dorsal corner of the orbit, a ventral blade of the nasal appears to indicate the minimal posterior extent of this bone. This blade is laterally overlain by a short and tapering, ventral pointing prefrontal, as well as the lacrimal. The lacrimal is broad in its dorsal part, where it encloses two smaller foramina. The ventral process is about as broad as the dorsal process of the jugal, which it contacts in the upper portion of the preorbital bar. Due to minor damage, it cannot be figured out which additional element points to the lacrimal-jugal contact, but it may be the left lacrimal or a dislocated part of the palate.
Posterior skull roof. In the dorsal and posterodorsal region of the orbital rim, slender bones are set off from the frontoparietal roof. This condition is normal compared to various early monofenestratan and pterodactyloid skulls, although unclear regarding its identification (Lü et al., 2011, figure 3; Bennett, 2013a, figure 2). Following Wellnhofer (1970), these elements are labelled as supraorbital and postfrontal. This strengthened upper orbital rim is visible on both sides. No trace of sclerotic ring plates was discovered during the preparation of the right aspect, representing the only part of the skeleton that is lost due to decay.
The skull roof is constricted above the orbits to about half the width observed at the level of the preorbital bar, assessed from the dorsal part of the nasal. Of the frontal, neither the anterior (nasal) contact nor the posterior (parietal) contact can be traced by clear sutures. As compaction has exposed the posterior skull roof in dorsal aspect, no sharp sagittal crest appears behind the widening of the frontals, which corresponds with the postorbital bar. However, this area is crushed, hampering certain observations. Slightly dorsal to the rearmost portion of the skull, right in the extension of the center line of the dorsal skull roof (Figure 2, Figure 3), a strengthened, shallow V-shaped chevron could mark the concave middle of the nuchal crest (Codorniú et al., 2016, figure 1b). Alternatively, this structure belongs to the sagittal crest (Lü et al., 2011, figure 3; Witton et al., 2015, figure 1A). A likewise defined, though more delicate margin is visible deeper on the parietal, about the height of the dorsal process of the postorbital, and might indicate the base of the nuchal crest.
Jugal and postorbital. The jugal is anteriorly long, extending at least half the length of the nasoantorbital fenestra. Compared to other early monofenestratan skulls, the rostral extension seems highly variable (Lü et al., 2010, figure 2c; Wang et al., 2010, figure 2b; Vidovic and Martill, 2014, figure 3, 2018, figure 3b). Due to the large and rounded orbit, there is a wide and smooth bay between the lacrimal and the postorbital bar. This outline is in contrast with the reverse drop-shaped orbit of most Wukongopteridae and Germanodactylus -type pterosaurs but matches the condition of Pterodactylus -like forms (Lü et al., 2011; Martill and Etches, 2013; Vidovic and Martill, 2014), although an ontogenetic signal might interfere with this condition (Bennett, 2006). The lacrimal process of the jugal is inclined forward by about 70° from the tooth margin and builds up more than half the height of the preorbital bar. The postorbital process is broad at its base, while tapering in its dorsal part to allow for an oblique overlap by the postorbital.
The postorbital bears moderately elongated dorsal and ventral (jugal) processes that form a reinforced orbital rim along a circular segment. The squamosal process is short and blunt, although somewhat sharper set off from the orbital part by narrower concavities than reconstructed for most early Pterodactyloidea (Wellnhofer, 1970). The separation of the upper and lower temporal fenestra is placed in the ventral half of the skull depth.
Quadratojugal to squamosal. The region around the ventral and posterior part of the lower temporal fenestra deserves greater detail in future descriptions of Jurassic Monofenestrata. Only few reliable comparisons were possible with published documentations. The quadrate bar is broad and robust. Judged from the pattern of compaction in the whole skull, it is the right quadrate in articulation with the true jaw joint, though distorted dorsally. The outer skull surface covering the jaw joint is not set off from the ventral margin of the upper jaw by a narrow concavity. That means the quadratojugal contains a rostral portion ventral to the jugal. However, this particular region is not consistent with that documented for early pterodactyloids (Vidovic and Martill, 2014, figure 13): The quadratojugal builds up the antero-ventral corner of the lower temporal fenestra to a much larger extend, with a considerable spike leaning to the postorbital process of the jugal about half its length. Postero-ventral to the temporal fenestra, the quadratojugal forms a slender, tapering splint that shares a short contact with the ventral point of the squamosal body, in similar fashion as in Scaphognathus (Bennett, 2014, fig. 2; a long process that lacks a squamosal contact was reconstructed for the holotype of Diopecephalus, see Wellnhofer, 1968). The squamosal has a long, slender and pointed triangular process that seems to have leaned against the quadrate. As this process and the short quadratojugal contact are notched by a narrow, rounded concavity, there must have been an elongate fenestra that separated the quadrate and the longest part of the quadratojugal, as is reflected by the preserved articulation. The squamosal of DMA-JP-2011/006 is small and shallow, with its posterior margin being confluent with that of the parietal. Its postorbital process is broad and blunt. The postero-dorsal edge of the squamosal appears pointed where it is flipped to contribute to the rear wall of the upper temporal fenestra.
Occiput and palate. In the ventral region of the upper temporal fenestra, a crushed depression could represent the metotic foramen. Given the highly articulated condition of the entire skeleton, there is no doubt that the articulation of the neck is ventral on the occiput (Tischlinger and Frey, 2013), as in other Monofenestrata (Lü et al., 2010, 2011; Vidovic and Martill, 2018) but not Allkaruen (Codorniú et al., 2016). The cranial angle is large, with about 160° among the largest of early Pterodactyloidea and clearly exceeding those of Wukongopteridae (Martill and Etches, 2013). Posterior to the right squamosal, a boomerang-like bone is overlapped by the occiput; this element could not be identified and is likely part of the left side of the skull.
No informative observations were possible for the palate. A pterygoid-ectopterygoid string is exposed and partially broken due to compaction.
Mandible. The mandible is nearly straight, according to the upper jaw. No clear distinction of individual bones is possible, except for the surangular that covers the posterior third of the mandible length. Its pointed anterior tip lies little more posterior than the anterior range of the jugal, as usual for early pterodactyloids (Wellnhofer, 1970). About the region of the sixth tooth position, the mandible bending indicates the posterior end of the mandibular symphysis that is flipped into a plane bedding, in contrast with the lateral exposure of the posterior mandible. This finding is in accordance with breaks around the last tooth positions. The symphysial area is not greatly widened, apparently lacking a medial shelf (compare Bennett, 2013b; Augustin et al., 2022, figure 3). No sutures of the splenial could be recognised. The articular appears flat and slightly elongated posteriorly, forming a shallow glenoid, as is also typical for Pterodactylus -type pterosaurs, but unlike the deep retroarticular process of Ctenochasma (Jouve, 2004) or the short one in Darwinopterus (Lü et al., 2011). Close to the ventral edge, a small, dislocated piece is identified as part of the angular.
Dentition. The upper jaw counts 11 tooth positions of the right side, of which the fifth, seventh, and ninth contain replacement teeth more than half as large as the exposed crown of their forerunners. Possibly also the third position wears a tiny replacement tooth, which is uncertain, but not identified as a chipped splinter from the crown base of the larger tooth, according to the dark reaction under UV. Nonetheless, a regular pattern could be assumed from the alternation of sockets that perform a replacement and those that do not at the same time. However, five of the nine positions on the mandible show ongoing replacement, namely the third and fourth as well as the sixth to eighth socket. Only the third replacement tooth is half as exposed as its forerunner, whereas the seventh had grown to the same size as its forerunner that is still attached. In total, tooth replacement went on without any convincing pattern and was highly active (compare Fastnacht 2008), including 40% of the testable sockets. Although individual tooth exposition may suffer from postmortem displacement, the tooth crowns of the rostral tip appear considerably smaller than in the following sequence, thus having proportionally more massive roots. This is indicated by a change in the UV color signal, reflecting the gum line in life. Dense dentine or enamel (the latter would frequently react with bright yellowish signals under UV light, which are missing in Figure 3) is restricted to the conical tips, whereas the broader roots and bases of the tapering crowns were situated within the gum line (covered by cementum). Color bands appear irregular, with one or two annuli indicated on each crown (compare Martill et al., 2023, figure 8)
In the upper jaw, the tooth row ends at a longitudinal level that overlaps with the anterior nasoantorbital fenestra, whereas the mandibular tooth row does not extend further back than the nasoantorbital rim. As a result, both the tooth rows of the (pre-)maxilla and the dentary span over the anterior 50 % of the upper skull and the mandible length, respectively. Among the most remarkable comparisons discussed by Tischlinger and Frey (2013) is that the widely spaced teeth point outwards in the rostral tip and by this resemble the dentition of Ornithocheiridae. Although the compaction and torsion of the right hemimandible could have biased the orientation of the forward-tilted teeth in the dentary tip, all anterior teeth show indeed a greater curvature and are lean saber-shaped. The pointed conical teeth in the middle region are more robust at their bases and bear a slender, almost offset tip. More posteriorly, this tooth type is retained, but reducing in size. The sparse arrangement, with interspaces little shorter (about 4 mm) than the largest exposed teeth (about 5 mm; maximum crown height about 4 mm), may be functionally linked to longer but still interlocking teeth, especially with such a high degree of replacement activity.
For most aspects, the dentition of DMA-JP-2011/006 matches the expected basal or least specialised condition for Pterodactyloidea, with a remarkably low number of teeth. The short conical tooth type with initially offset tips is found in Pterodactylus -type forms (Bennett, 2013a, figure 2; Augustin et al., 2022, figure 3), while the longer and more widely spaced teeth resemble certain wukongopterids (Lü et al., 2010, figure 2b). Altmuehlopterus is roughly documented but generally close to the conditions in DMA-JP-2011/006, only that the tooth row ranges farther below the nasoantorbital fenestra (Vidovic and Martill, 2018). Again, the greatest similarity is with juveniles of Pterodactylus (Diopecephlaus) kochi (Vidovic and Martill, 2014, figure 3G, H).
Visceral Skeleton
One hyoid branch (ceratobranchial) is exposed for most of its length. The posterior terminus is hidden under the third cervical, but slightly thickened, indicating that the tip is very close to the exposed part. The lotal length is thus almost twice the length of the orbit. Although the hyoid-skull-ratio is influenced by jaw elongation, Jiang et al. (2020, figure 2) could show that the longirostrine wukongopterids tend to bear long hyoids (Lü et al., 2011), while the likewise longirostrine ctenochasmatids show no significant elongation (Martill et al., 2023). Within this potential shortening trend in Jurassic pterosaur hyoids, DMA-JP-2011/006 appears intermediary between Wukongopteridae and some Pterodactylus. The hyoid is nearly straight, because a curved shape would have been preserved in plane bedding in the laminated limestone. By this condition, the hyoid resembles a specimen assigned to Pterodactylus (Bennett, 2013a, Vidovic and Martill, 2014). The anterior tip is widened to form a strengthened contact with the other hyoid. This trait is also seen in Darwinopterus (Lü et al., 2011), Changchengopterus (Zhou and Schoch, 2011), Gallodactlyus (Fabre, 1976, figure 2, if not preserving the basibranchial, see Jiang et al., 2020), as well as a specimen from Painten assigned to Pterodactylus (Augustin et al., 2022).
Axial Skeleton
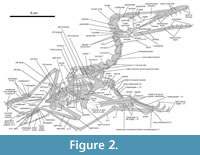
Cervical column. The vertebral column exhibits an overall close resemblance with that of early pterodactyloids. As Tischlinger and Frey (2013) remarked, the neck shows an intermediate elongation shared with wukongopterids, and less pronounced than in forms assigned to Pterodactylus. The authors expressed the elongation of individual cervical vertebrae by a length-width ratio of 5:3.5 despite the lateral exposure of the neck (see Discussion). In contrast to early pterodactyloids (Wellnhofer, 1970; Augustin et al., 2022), the vertebral elongation includes cervical eight, which isabout twice the length of mid-dorsals (Figure 2).
The atlas-axis-complex is well-preserved (Figure 3). The atlantal neural arch is bipartite, with the anterior apophyses slightly curved downwards ending in blunt tips. It is this bending that supports the interpretation as the atlas (Wellnhofer, 1970, figure 4A; 1975, figure 6a). That the left part is not the proatlas arises from the apparently mirrored morphology and parallel articulation with the right part. The rounded triangular proatlas is tentatively identified as overlying on the squamosal. Between the axis and the squamosal, the intercentrum of the axis is found as articulating with the short pleurocentrum, if the latter is correctly identified based on its postepipophysis. Alternatively, the anterior element is identified as the atlas pleurocentrum. However, no direct observation can be made on the ventral elements. The axis is shorter than all subsequent cervicals, but longer than the dorsals, and bears a long, moderately high spine.
All mid-cervicals are of typical appearance as found in Pterodactylus -type pterodactyloids (Wellnhofer, 1970; Bennett, 2013a; Augustin et al., 2022). The spines are relatively tall, in contrast with Allkaruen (Codorniù et al., 2016) and wukongopterids (Wang et al., 2009). As typical, the postzygapophyses obliquely overlay the prezygapophyses in large facets. Greatly extended postepipophyses project posteriorly beyond the zygapophysial contact, resembling an indeterminate early monofenestratan (O’Sullivan and Martill, 2018, figure 19B), Allkaruen (Codorniù et al., 2016, figure 1), and Pterodactylus (Wellnhofer, 1970, figure 4B). In the fifth cervical, a large pneumatic foramen is visible.
On cervical eight, which is the last of conspicuously lengthened cervicals, a shallow bulge on the lateral margin of the neural arch resembles a diapophyseal rib contact. As this bulge is found at the mid-length of the vertebra, it would conflict with the expected anterior position of diapophyses in early pterosaurian cervicals (Wellnhofer, 1975, figure 6b; Spindler and Ifrim, 2021, figure 4). The cervical count is in accordance with Bennett (2014), finding the last one as bearing a long cervical rib. On this nineth cervical, which is barely longer than the first dorsal vertebra, DMA-JP-2011/006 shows a clear transverse process (diapophysis) that is short, rectangular, and slender compared to those of all rib-bearing dorsals.
It cannot be precluded that also mid-cervicals had ribs, which would contrast with the ribless condition in pterodactyloids. At least, cervicals six and seven are associated with questionable fragments at a repeated anterior position (Figure 2, Figure 3). These bones could represent short but robust, pointed cervical ribs. In comparison, wukongopterids have residuals of moderately elongated cervical ribs (Wang et al., 2009; Cheng et al., 2016; Zhou et al., 2021, but see Lü et al., 2010). For anurognathids, cervical ribs are stated, but not well-documented (Dalla Vecchia, 2002; Bennett, 2007; but see Lü et al., 2018), leading Hone (2020) to evaluate them as reduced or absent. In the more primitive Changchengopterus cervical ribs are apparently absent (Zhou and Schoch, 2011).
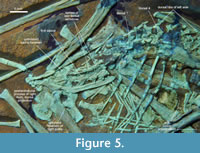 Dorsal vertebrae. Regarding the thorax, Propterodactylus is very similar to the common morphology of Jurassic Pterodactyloidea, although little comparative research has been done on the early pterosaurian torso (Tischlinger and Frey, 2013). DMA-JP-2011/006 contains at least 10 dorsals, with a greater gap near the left elbow that contains some repair, but also plane fossil material including mineralised soft tissue (Figure 5). An incomplete dorsal neural arch is located at the longitudinal level of the anterior tip of the ilia, which implies that no further vertebrae are missing and that there is a minor dislocation interrupting the dorsal column. The spines are moderately high in the anterior thorax. The subsequent column is flipped to a more dorsoventral exposure. Nonetheless, the spines are clearly defined to at least the first sacral, though shallower than in the shoulder region. The transverse processes are broadly extended starting at the first dorsal. At least in the dorsals five to seven, they bear an offset anterior knob for the articulation of the costal head (Figure 5), as similarly seen in Rhamphorhynchus (Wellnhofer, 1975, figure 6f). This condition is not observable in most early pterodactyloids due to their predominantly lateral exposure. In Gallodactylus, the diapophyses lack an anterior protrusion (Bennett, 2013b, figure 5).
Dorsal vertebrae. Regarding the thorax, Propterodactylus is very similar to the common morphology of Jurassic Pterodactyloidea, although little comparative research has been done on the early pterosaurian torso (Tischlinger and Frey, 2013). DMA-JP-2011/006 contains at least 10 dorsals, with a greater gap near the left elbow that contains some repair, but also plane fossil material including mineralised soft tissue (Figure 5). An incomplete dorsal neural arch is located at the longitudinal level of the anterior tip of the ilia, which implies that no further vertebrae are missing and that there is a minor dislocation interrupting the dorsal column. The spines are moderately high in the anterior thorax. The subsequent column is flipped to a more dorsoventral exposure. Nonetheless, the spines are clearly defined to at least the first sacral, though shallower than in the shoulder region. The transverse processes are broadly extended starting at the first dorsal. At least in the dorsals five to seven, they bear an offset anterior knob for the articulation of the costal head (Figure 5), as similarly seen in Rhamphorhynchus (Wellnhofer, 1975, figure 6f). This condition is not observable in most early pterodactyloids due to their predominantly lateral exposure. In Gallodactylus, the diapophyses lack an anterior protrusion (Bennett, 2013b, figure 5).
Dorsal ribs. Under the herein adopted counting and assuming that no dorsal vertebra is missing, ribs are present up to the eighth dorsal position, judged from the articulation. The rib cage would then typically end immediately before the ilium. In the anterior thorax, a set of imprints parallel to the preserved subsequent ribs confirms the region of the first ribs. Some parallel remains of bone sheets crossing the left forearm are of questionable identity (Figure 2). This area seems too broad to represent thickened first dorsal or last cervical ribs. Thickened anterior ribs are documented for some pterosaurs (Wellnhofer, 1987; Zhou and Schoch, 2011; Bennett, 2014; Albersdörfer and Häckel, 2015, figure 226; Wang et al., 2017; Aires et al., 2021, figure 3b). However, the thickest fragment in this enigmatic region of DMA-JP-2011/006 is most likely the upper part of the left coracoid displaced from its ventral part.
The ribs are double-headed with sharply set-off tubercles. On the first three dorsal segments, the rib heads are about twice as large as those of posterior ribs. Since the dorsal ribs of the left side are evenly oriented pointing backwards, another set of longitudinally arranged rib-like elements is identified as sternal ribs (Figure 2, Figure 5). These elements can be as thick as anterior dorsal ribs and reach more than half of their length. Apart from their simple, rod-shaped form this size is in accordance with sternal ribs reported by Claessens et al. (2009).
At least four segments of fused gastralia pairs are preserved in natural orientation. They are typically thin but wide, decreasing in width from anterior to posterior. The first is preserved close to the sternal plate and forms an almost straight angle. Judged from the evenly tapering end, an additional segment may be dislocated closer to the vertebral column.
Sacral region. No fused synsacrum is present, indicating a juvenile stage (Figure 5). Two free dorsals are laterally accompanied by the iliac preacetabular processes. They have short and narrow, but well-developed transversal processes and apparently lacked ribs. Posterior to these last dorsals, three or four vertebrae exhibit enlarged contacts with the ilium, of which the first two pairs are inclined backwards (opposing the transversal direction in e.g., Kunpengopterus, Cheng et al., 2017, figure 4C). Their identity as transversal processes or fused ribs remains unclear (Wellnhofer, 1974, 1978, 1988; Padian, 1983). The sub-circular sacral foramina lack a fused suture where the ribs meet distally. The overall morphology resembles that of Altmuehlopterus (Wellnhofer, 1970, figure 14), but is less mature than seen in Pterodactylus (Wellnhofer, 1987), Changchengopterus (Zhou and Schoch, 2011), and Wukongopteridae (Wang et al., 2009, 2010, 2017). The number of sacrals cannot be examined.
 Caudal column. Regarding its relative length and depth, the tail column is greatly reduced (about 75 % of the femoral length, ranking along with “Pterodactylus” according to Lü and Hone, 2012, table 2). It measures roughly two thirds of the trunk length, being almost as short as in early pterodactyloids (about 40 % in Pterodactylus -type forms, Wellnhofer, 1970, 1987). The exact number of caudals cannot be evaluated, as the tail tip is hidden under the left metatarsus, while the base is distorted by some damage and crushing of the region between the postacetabular processes of the ilium. An articulated series of 14 caudals preserves most of the tail but ends in an articular facet, implying at least one further caudal vertebra. At least nine vertebrae in this preserved sequence show substantially elongated prezygapophyses and hemapophyses (Figure 6), retaining the typical interlocking pattern of long-tailed “rhamphorhynchoid” pterosaurs (Tischlinger and Frey, 2013). The needle-like processes are not as super-elongate as in Rhamphorhynchus (Wellnhofer, 1975, figure 7) and still in Wukongopterids (Wang et al., 2009, 2010), but closely resemble the caudals of the long-tailed Changchangopterus (Zhou and Schoch, 2011), although the vertebral bodies are much less elongated in Propterodactylus. In Pterodactylus -type forms, the caudals are short cylindrical to disc-like, with a maximum number of 16 (Wellnhofer, 1970; Tischlinger and Frey, 2013). There is some potential for ontogenetically or functionally induced reversals (Codorniù and Chiappe, 2004; Codorniù, 2005).
Caudal column. Regarding its relative length and depth, the tail column is greatly reduced (about 75 % of the femoral length, ranking along with “Pterodactylus” according to Lü and Hone, 2012, table 2). It measures roughly two thirds of the trunk length, being almost as short as in early pterodactyloids (about 40 % in Pterodactylus -type forms, Wellnhofer, 1970, 1987). The exact number of caudals cannot be evaluated, as the tail tip is hidden under the left metatarsus, while the base is distorted by some damage and crushing of the region between the postacetabular processes of the ilium. An articulated series of 14 caudals preserves most of the tail but ends in an articular facet, implying at least one further caudal vertebra. At least nine vertebrae in this preserved sequence show substantially elongated prezygapophyses and hemapophyses (Figure 6), retaining the typical interlocking pattern of long-tailed “rhamphorhynchoid” pterosaurs (Tischlinger and Frey, 2013). The needle-like processes are not as super-elongate as in Rhamphorhynchus (Wellnhofer, 1975, figure 7) and still in Wukongopterids (Wang et al., 2009, 2010), but closely resemble the caudals of the long-tailed Changchangopterus (Zhou and Schoch, 2011), although the vertebral bodies are much less elongated in Propterodactylus. In Pterodactylus -type forms, the caudals are short cylindrical to disc-like, with a maximum number of 16 (Wellnhofer, 1970; Tischlinger and Frey, 2013). There is some potential for ontogenetically or functionally induced reversals (Codorniù and Chiappe, 2004; Codorniù, 2005).
Close to the left pedal unguals, a dislocated centrum from the tail base is visible. As it is exposed from its ventral side, it cannot be determined whether it is fused to its neural arch. If the complete vertebra was tall, it is unlikely to deposit it vertically in fine laminated limestone, thus it may have possessed an unfused neurocentral suture. Due to the declining use of the tail, it is not unambiguously indicating a juvenile feature.
Appendicular Skeleton
Pectoral girdle. Despite the advanced ossification and considerable size of DMA-JP-2011/006 compared to most Pterodactylus -type specimens, there is no fused scapulocoracoid (Figure 2). The unfused condition is seen in some species of Rhamphorhynchus or those assigned to Pterodactylus, with doubt on whether it represents a taxonomic or ontogenetic signal (Wellnhofer, 1978, 1987; Bennett, 1996, 2013a; Vidovic and Martill, 2014; Jiang et al., 2016). Still, both the scapula and the coracoid show sophisticated sculptures in their terminal regions, supporting a late co-ossification. Andres et al. (2014) argued for a fused scapulocoracoid in Kryptodrakon. Likewise, early monofenestratans imply no difference to that (Wang et al., 2017; Zhou and Schoch, 2011).
The scapula is typically broad blade-like. The antero-ventral terminus is slightly thickened and forms an oblique contact with the coracoid. Above the posteriorly protruding end of the glenoid facet, a slender buttress reinforces the shoulder joint. The distal end is best preserved on the left side. Its dorsal edge exhibits a shallow embayment, starting approximately one quarter in front of the posterior end. Beyond this point, the lower edge of the scapular blade remains almost straight, while the dorsal edge is concave and tapers towards a thickened and semi-lunar rounded terminus. This somewhat lens-shaped cap has a tiny projection over the postero-ventral edge of the scapular blade. Although this structure belongs to the numerous postcranial aspects that are not sufficiently investigated for many pterosaurs, it implies an improved coupling of the dorsal scapula to the vertebral column, maybe some early, soft-tissue-based forerunner of the notarium seen in Eupterodactyloidea and few Jurassic pterodactyls (Aires et al., 2021, figures 3, 4).
 The length of the coracoid cannot be measured due to broken pieces. The dorsal part is known from the right side, with a large glenoid facet supported by a ventral buttress. There is a large and stout acrocoracoid process, as typical for Late Jurassic pterosaurs (Wellnhofer, 1975, 1978, figure 9, 1987; Augustin et al., 2022). Close to the base of the thickened dorsal head, the anterior margin of the coracoid bears an obtuse-angled knob that is separated from the acrocoracoid process by a concave outline. Ventral to this point, the shaft tapers towards the thin ventral region. The barely thickened ventral end of the left coracoid is exposed (Figure 7). It exhibits the typical saddle-shaped articular surface for the sternal contact. On the anterior margin, a flat corner is less pronounced and more terminal than in Kryptodrakon (Andres et al., 2014, figure 1).
The length of the coracoid cannot be measured due to broken pieces. The dorsal part is known from the right side, with a large glenoid facet supported by a ventral buttress. There is a large and stout acrocoracoid process, as typical for Late Jurassic pterosaurs (Wellnhofer, 1975, 1978, figure 9, 1987; Augustin et al., 2022). Close to the base of the thickened dorsal head, the anterior margin of the coracoid bears an obtuse-angled knob that is separated from the acrocoracoid process by a concave outline. Ventral to this point, the shaft tapers towards the thin ventral region. The barely thickened ventral end of the left coracoid is exposed (Figure 7). It exhibits the typical saddle-shaped articular surface for the sternal contact. On the anterior margin, a flat corner is less pronounced and more terminal than in Kryptodrakon (Andres et al., 2014, figure 1).
Little can be said about the sternum, as the two regions exposed are covered by or interlaced with other postcrania. What is interpreted as the left side of the medial aspect of the plate contains a deep bowl-like concavity that may be diagenetically exaggerated. The right side reveals a stout lateral extension that likely served with contacting the sternal ribs. The sternal plate is puzzling in its complete outline but reveals overall convex anterior and posterior margins. This combination is seen only in some Archaeopterodactyloidea and few early Eupterodactyloidea, and may contribute to juvenile patterns (Hone, 2023, figures 9, 11, 13, 19).
Wing skeleton. In general, the front extremity (Figure 7) is very similar to that of early pterodactyloids, apart from proportional deviations. Particularly the humerus is stouter than in Diopecephalus (Wellnhofer 1987), but almost identical in the morphology of the proximal head (Vidovic and Martill, 2018, figure 1). The entire humerus is also very similar to those of Changchengopterus (Zhou and Schoch 2011) and Kunpengopterus (Cheng et al., 2017), despite that the latter two bear a slightly longer and more pointed deltopectoral crest and a taller rim of the caput. In DMA-JP-2011/006, the caput forms a shallow saddle with a low anterior bulge. The proximal head of the humerus is moderately broad, opposing the slender appearance in Darwinopterus (Wang et al., 2010). DMA-JP-2011/006 differs from rhamphorhynchids in lacking an extended, laterally constricted deltopectoral process. Instead, this crest is stout and rounded rectangular, confluently connected with the shaft by a shallow concavity (as in most early pterosaurs, O’Sullivan et al., 2013, figure 5). In comparison, the crest is more offset from the shaft in Darwinopterus and Kunpengopterus (Lü et al., 2011; Wang et al., 2015, respectively). The medial process in the proximal humerus is weakly developed, but still larger than e.g., in Douzhanopterus (Wang et al., 2017). In overall proportions, the humerus is robust and short. The proximal head is almost one third of the entire length of the bone, which contrasts with more basal monofenestratans (about one quarter in Douzhanopterus, Wang et al., 2017; intermediary between this and DMA-JP-2011/006 are Darwinopterus, see Lü et al., 2010, and Kunpengopterus, Cheng et al., 2017). However, the stout and clunky humerus of a juvenile Diopecephalus (Augustin et al., 2022) implies that ontogenesis may also overlay interspecific distinctness. That the distal condyles appear uneven in size is likely an artefact due to compaction. Unfortunately, it can thus not be tested regarding the distal width (Witton, 2015, figure 4). Also, the shaft is crushed and appears broader than in life. No certain foramina were observed.
The forearm is also robust, not as slender as the radius and ulna visible in both grown Rhamphorhynchus and Pterodactylus -type pterodactyloids (Wellnhofer, 1975; Vidovic and Martill, 2014). The shafts are straight and closely attached. Thickened ends are observed on the proximal ulna, forming a tiny olecranon-like bulge, and on the distal ulna and radius. The forearm is proportionally conservative and not as elongated as in wukongopterids (Wang et al., 2010; Lü et al., 2011)
In the articulated wrists (Figure 2, Figure 7), the ulnare and radiale are separated, not forming a fused proximal syncarpal. The ulnare is slightly larger than the radiale and contains lip-like blades to contact with the ulna and the fourth distal carpal laterally. There is a slightly curved pteroid. Measuring about one third of the forearm, it is elongated in a wukongopterid or initial pterodactyloid manner, contrasting with the shorter and bluntly ending rhamphorhynchid pteroid (Tischlinger and Frey, 2013). The distal carpals are also unfused, preserving the large fourth carpal to contact with the wing metacarpal and at least one small additional distal carpal. Antero-distal to this element, a preaxial carpal is present, if not already representing sesamoids. The condition of unfused elements is found in several small- to medium-sized specimens of a variety of pterosaurs (Wellnhofer, 1970, figure 4, 1975, 1987; Wang et al., 2010; Vidovic and Martill, 2018, figure 1; Bennett, 2018, figure 2), while the segment-wise fusion is also found throughout pterosaur evolution (Wellnhofer, 1978, figure 11; Zhou and Schoch, 2011). For DMA-JP-2011/006, this finding points to incomplete skeletal maturity.
With respect to non-metric characters, the manus is of typical appearance compared with most pterosaurs. The wing metacarpal (fourth metacarpal) is shorter and more robust than the fragmentary material of Kryptodrakon would suggest, and the distal hinge is less markedly set off from the shaft (Andres et al., 2014, figure 1H). The metacarpus is significantly longer than that of rhamphorhynchoid-grade forms (Tischlinger and Frey, 2013, citing Wellnhofer, 1975, and Lü et al., 2010). Compared to the humerus and forearm, the wing metacarpal is intermediary elongated with respect to that of pterodactyloids (Wellnhofer, 1970). It exceeds the proportional length seen in wukongopterids (Tischlinger and Frey, 2013), but not that of Douzhanopterus (Wang et al., 2017).
The remainder of the metacarpus and short digits shows no striking difference from other pterosaurs. The phalangeal formula is typically 2-3-4-4. All claws are strongly built and curved. The phalanges of the wing digit are straight, with an inconspicuous bending in the distal-most phalanx. The butt joints are posteriorly widened, while forming a straight leading edge anteriorly. The proximal joint of the first phalanx is skeletally immature in forming a flat saddle-shaped facet. This terminus is associated with isolated polygonal elements that occur in the same articulated position in both wings. The distal facet contacting the first phalanx is broadened, whereas a steep edge leans to the distal end of the wing metacarpal. Although a separated epiphysial ossification seems anomalous for pterosaurs (Steel, 2008), the elements in question resemble similar cases of other pterosaurs (Frey and Martill, 1998; Martill et al., 2023). In comparison with these examples, the sesamoid for the attachment of the extensor tendon is rounded and less mature in DMA-JP-2011/006.
Pelvic girdle. The ilium is long, overlapping about 40 % of the glenoidal-acetabular distance (Figure 5). Half of the ilium’s length is built up by the preacetabular process that is blade-like and very slightly widened close to the blunt anterior terminus. The postacetabular process bears a dorsal projection, most similar to Pterodactylus and Chanchengopterus (Wellnhofer, 1970, figure 4G; Zhou and Schoch, 2011). There is no fused puboischiadic plate, a structure that apparently co-ossified late in ontogeny (Hyder et al., 2014). The ischium is longitudinally short, deep, and rounded ventrally, with an unsharp posterior tip. The pubis is also deep and concave on its anterior margin. Behind its shaft, a broad blade-like extension reaches even deeper ventrally, where the femur hinders to trace its full size. The obturator foramen is well visible. As the femur is shifted into the foramen, it is most likely open on the rear side. The prepubis consists of a broad proximal shaft and a semilunar distal blade. A small process indicates the medial contact of the prepubes. The right prepubis is notched in its distal blade, but with rather undefined margins and not repeated on the left prepubis. Therefore, it is reconstructed as originally fully convex distally, not bifurcate as in Rhamphorhynchus (Wellnhofer, 1975). In overall shape, the pelvis of DMA-JP-2011/006 resembles that of early pterodactyloids and Dorygnathus, but not Darwinopterus (Lü et al., 2011, figure 5). It is also close to the pelvic conditions in Changchengopterus (Zhou and Schoch, 2011, figure 6, the long preacetabular ilium is visible on figure 2b).
Hindlimb. The hindlimbs are robust compared to the remainder of the skeleton and not as short and thin as in rhamphorhynchids, although not significantly elongated as in most pterodactyls. DMA-JP-2011/006 has a rather conservative femur-tibia ratio of about 85 % (Tischlinger and Frey, 2013), which is close to that of Chanchengopterus (Zhou and Schoch, 2011). Lower ratios of less than 60 % to little more than 70 % refer to Wunkongopteridae (Lü et al., 2011; Wang et al., 2017) and seem to correspond with the elongation of the skull and forelimb. The femur of DMA-JP-2011/006 is of classical pterosaur appearance, with a moderately angled head on a constricted collum. The external trochanter is as inconspicuous as in early pterosaurs to early pterodactyloids (Wellnhofer, 1978, figure 16). The femoral shaft is very slightly bent. On the thickened distal terminus, the condyles are confluent.
The tibia is little longer than the femur. As typical for pterosaurs, the tibia is straight and slightly slenderer than the femur. Both joint regions are thickened, while the shaft inconspicuously tapers towards its distal terminus. The tarsal contact is perpendicular to the shaft and formed as a simple butt joint. The fibula is reduced to a slender splint that contributes to the knee joint with an asymmetrical articular head. Distally, the fibula tapers to its pointed end, which forms a delicate onlap on the tibia at a level slightly distal to the half-length of the tibial shaft.
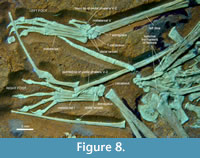 Three and four tarsals are densely packed on the right and the left foot, respectively (Figure 8). Assuming that they are located close to life articulation, the right tarsus seems to contain the associated astragalus and calcaneus, with the latter being slightly larger. Distally, a similar-sized element contacts with the lateral metatarsals and might represent fused distal tarsals. A much smaller element is found next to the distal-most wing phalanx and could be a dislocated distal tarsal. On the left foot, the largest element can only be labelled as the calcaneus, based on its size, but appears on the wrong side. It covers most of the distal terminus of the tibia. The latter might possibly show some torsion, suggested by the inclined position of the fibula and the lateral aspect of the femur. However, the distal tarsus is in perfect articulation with the metatarsus, in accordance with the enlarged (fused) distal tarsal element in the right foot. That both feet are preserved in a parallel orientation, while the knees are in opposite posture, allows for the tentative explanation of a torsion due to compaction, with a twist between the proximal and distal tarsus (joint surface in life). In the present condition of the left foot, three polygonal elements contact with the metatarsus, of which one is likely the astragalus. All tarsals exhibit certain facets, but rounded edges, so that no detailed reconstruction of the tarsus can be performed. The only specific observation that occurs on both sides is that the lateral fused distal tarsal bears an obtuse angle where the surfaces for the fourth and fifth metatarsal meet. The best assumption from the given preservation is that there are two elements ossifying in the distal tarsus. This condition matches with the spectrum of documented pterosaur ankles (Wellnhofer, 1978, figure 17; Padian, 2017).
Three and four tarsals are densely packed on the right and the left foot, respectively (Figure 8). Assuming that they are located close to life articulation, the right tarsus seems to contain the associated astragalus and calcaneus, with the latter being slightly larger. Distally, a similar-sized element contacts with the lateral metatarsals and might represent fused distal tarsals. A much smaller element is found next to the distal-most wing phalanx and could be a dislocated distal tarsal. On the left foot, the largest element can only be labelled as the calcaneus, based on its size, but appears on the wrong side. It covers most of the distal terminus of the tibia. The latter might possibly show some torsion, suggested by the inclined position of the fibula and the lateral aspect of the femur. However, the distal tarsus is in perfect articulation with the metatarsus, in accordance with the enlarged (fused) distal tarsal element in the right foot. That both feet are preserved in a parallel orientation, while the knees are in opposite posture, allows for the tentative explanation of a torsion due to compaction, with a twist between the proximal and distal tarsus (joint surface in life). In the present condition of the left foot, three polygonal elements contact with the metatarsus, of which one is likely the astragalus. All tarsals exhibit certain facets, but rounded edges, so that no detailed reconstruction of the tarsus can be performed. The only specific observation that occurs on both sides is that the lateral fused distal tarsal bears an obtuse angle where the surfaces for the fourth and fifth metatarsal meet. The best assumption from the given preservation is that there are two elements ossifying in the distal tarsus. This condition matches with the spectrum of documented pterosaur ankles (Wellnhofer, 1978, figure 17; Padian, 2017).
The metatarsals I to IV are robust and straight columns about as long as the middle toes. Only metatarsal IV is significantly shorter than I to III, which is in normal pterosaurian fashion. On the proximal end, a thickening of the articular end is visible on metatarsal IV and most conspicuously on string I. All distal ends are slightly thickened, with reduced angles of movement as in most interphalangeal joints. The toes I to IV show the common pterosaurian formula 2-3-4-5, with elongated penultimate phalanges and strongly shortened middle phalanges in the strings III and IV. The latter have gained the maximized reduction of the shaft length, which DMA-JP-2011/006 shares with Pterodactyloidea, some wukongopterids (Wang et al., 2015, figure 3) and Jianchangopterus (Lü and Bo, 2011, figure 4), but not rhamphorhynchids and Dimorphodon (Wellnhofer, 1978, figure 17). The unguals are strong and curved, somewhat larger than in most rhamphorhynchids and early pterodactyloids (Wellnhofer, 1975, figure 17, 1978, figure 17) but not as long and pointed as in wunkongopterids (Wang et al., 2015, figure 3).
DMA-JP-2011/006 shows a fifth toe that in general resembles all non-pterodactyloid pterosaurs (Figure 8). Metatarsal V is of similar width as the first, with the greatest width at about half its length where the articular region ends. Its distal half forms a constricted neck towards the phalangeal contact. As Tischlinger and Frey (2013) noted, the entire string is of typical rhamphorhynchid shape and no longer movable against the tarsus. However, the Vth metatarsal of DMA-JP-2011/006 closely resembles a splayed-out metatarsus of Rhamphorhynchus (Wellnhofer, 1975, figure 17f), which could have been controlled by the external knob, if working as a tendon scar, that is likewise present on metatarsal V of DMA-JP-2011/006. Maybe, the metatarsus of rhamphorhynchids and wukongopterids (Wellnhofer, 1975, figure 17; Wang et al., 2015, figure 3) could be fanned out in life as a specific adaptation (see Mayr et al., 2002, figure 5) or postmortem spreading occurred, while DMA-JP-2011/006 shares strictly parallel metatarsals I to IV with pterodactyloids and anurognathids. Consequently, the relative movement of string V must remain unsolved.
Two phalanges of the modified fifth toe are present on each foot. These phalanges are not as slender and elongated as in rhamphorhynchids, anurognathids, or wukongopterids (Padian, 2017; Bennett, 2007; Wang et al., 2010, 2015). On the right foot, the distal phalanx appears pointed and somewhat curved, while on the left foot this element is straight and does not taper. Instead, a joint-like thickening is present as its distal terminus (Figure 8). Although both feet are articulated, it is possible in the preserved posture - speaking about taphonomy only - that three phalanges were present before the penultimate one was dislocated from the right foot and the terminal one from the left. This unlikely reconstruction would make DMA-JP-2011/006 the only known pterosaur with more than two phalanges on the fifth toe. Triassic pterosaurs have two phalanges on toe V (Dalla Vacchia, 2003, 2009). Three phalanges were documented in the Jurassic Sordes (Sharov, 1971) but revised as counting two (Unwin and Bakhurina, 1994) according to the boomerang-shaped bending in the distal phalanx (Wellnhofer, 1975; Bennett, 2014; Wang et al., 2015; Cheng et al., 2017, figure 6). Four phalanges were reported from the holotype of Anurognathus (Wellnhofer, 1978), but this appearance is caused by bad preservation and an overlapping wing bone (pers. obs.), whereas well-preserved anurognathids exhibit two elongate phalanges on toe V (Dalla Vecchia, 2002; Bennett, 2007).
A slight widening in the phalangeal terminus of the fifth toe is visible in Wukongopterus (Wang et al., 2009, figure 3e), Campylognathoides (per. obs., SMNS 9787, SMNS 50914) or Dorygnathus (per. obs., SMNS 55886), but not in the edged, joint-like shape as in the left foot of Propterodactylus. Therefore, the asymmetrical deviation in the left foot of DMA-JP-2011/006 is explained as abnormal, maybe an acquired pathological structure. After all, the outermost toe is reduced in size but functional, representing a unique structure and implying a lesser importance as tightener of the uropatagium (Tischlinger and Frey, 2013). In the light of the ontogenetically constant fifth toes in wukongopterids and early pterodactyloids (Jiang et al., 2021; Wellnhofer 1970), the morphology of the fifth toe in DMA-JP-2011/006 is not significantly affected by its ontogenetic stage.
Ontogenetic Status and Pathology
With a skull length of 9 cm and estimated wingspan of about 55 cm, DMA-JP-2011/006 is mid-sized in the spectrum of specimens from the Upper Jurassic of Bavaria. The orbit is proportionally large, but well within the range of Jurassic pterosaurs. Without any knowledge of conspecific specimens, the relative orbit size (Wellnhofer, 1970) is not indicative of a certain ontogenetic stage. Given its size and robust nature, along with fully sized carpals and tarsals, the specimen is not a young juvenile (partially opposing Wang et al., 2017). On the other hand, there are several observations that, taken together, imply skeletal immaturity or could contribute to future investigations of neotenic/paedomorphic effects in pterosaurs: the unfinished synsacrum, the unfused scapulocoracoid, the outline of the sternum, the unfused carpals, unfused proximal end of the first wing phalanx, unfused ischiopubic plate, and the possibly isolated anterior caudal centrum (see individual paragraphs). As a result, the observed relative lengths of the metacarpus and the cervicals are only minimal measurements and can be expected to have increased in adults of the same species.
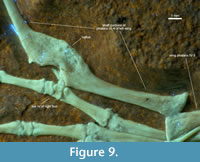 On the fourth phalanx of the left wing, a healed bone fracture resulted in a crooked callus (Figure 9). Due to the semicircular wound, Tischlinger and Frey (2013) suspected a bite injury, which the individual could survive despite impeded flight abilities, and underpinned this with a comparable case study (Tischlinger, 1993). The continuity of the rear edge speaks for a greenstick fracture, typically found in juveniles and subadults (Rothschild and Lambert, 2019; Samathi et al., 2023). The young individual age at the time of the injury is underlined by the intensively bloated callus. There is no knowledge on pterosaurian callus formation rates, but from veterinary data of birds, the pathology in DMA-JP-2011/006 does not necessarily indicate an event that occurred more than a few months before death (James et al., 1978; Richards et al., 2011), especially under the expected mechanical load (Isaksson et al., 2009).
On the fourth phalanx of the left wing, a healed bone fracture resulted in a crooked callus (Figure 9). Due to the semicircular wound, Tischlinger and Frey (2013) suspected a bite injury, which the individual could survive despite impeded flight abilities, and underpinned this with a comparable case study (Tischlinger, 1993). The continuity of the rear edge speaks for a greenstick fracture, typically found in juveniles and subadults (Rothschild and Lambert, 2019; Samathi et al., 2023). The young individual age at the time of the injury is underlined by the intensively bloated callus. There is no knowledge on pterosaurian callus formation rates, but from veterinary data of birds, the pathology in DMA-JP-2011/006 does not necessarily indicate an event that occurred more than a few months before death (James et al., 1978; Richards et al., 2011), especially under the expected mechanical load (Isaksson et al., 2009).
DISCUSSION
 The formal description of Propterodactylus confirms DMA-JP-2011/006, the “Painten pro-pterodactyloid”, as a transitional form with a mix of synapomorphies shared with Pterodactyloidea, although being clearly excluded from the monophyly of well-known members. The phylogenetic position as the sister taxon to Pterodactyloidea has been proven by several cladistic analyses (Figure 10; Wang et al., 2017; Vidovic and Martill, 2018; Jiang et al., 2020; except sensu Andres, 2021; see also Dalla Vecchia, 2022, for substantial critique on a derived position of Anurognathidae). Therefore, Propterodacylus frankerlae documents the transition from the more ancient “rhamphorhynchoid” pterosaurs (sensu Wellnhofer, 1975) to the derived pterodactyloids in a nearly predictable way. Even in the light of the transitional Wukongopteridae, it is among the best examples of an intermediate taxon.
The formal description of Propterodactylus confirms DMA-JP-2011/006, the “Painten pro-pterodactyloid”, as a transitional form with a mix of synapomorphies shared with Pterodactyloidea, although being clearly excluded from the monophyly of well-known members. The phylogenetic position as the sister taxon to Pterodactyloidea has been proven by several cladistic analyses (Figure 10; Wang et al., 2017; Vidovic and Martill, 2018; Jiang et al., 2020; except sensu Andres, 2021; see also Dalla Vecchia, 2022, for substantial critique on a derived position of Anurognathidae). Therefore, Propterodacylus frankerlae documents the transition from the more ancient “rhamphorhynchoid” pterosaurs (sensu Wellnhofer, 1975) to the derived pterodactyloids in a nearly predictable way. Even in the light of the transitional Wukongopteridae, it is among the best examples of an intermediate taxon.
Despite its transitional nature, Propterodactylus is from the same layers that also yielded the oldest Pterodactylus/Diopecephalus specimen (late Kimmeridgian; Augustin et al., 2022). The more derived Ctenochasmatidae are at least 10 Ma older (early Oxfordian; Zhou et al., 2017). Due to this stratigraphic overlap, Propterodactylus can be characterised as representing a surviving lineage. The pterodactyloid transition dates back to the Middle Jurassic. Interestingly, Propterodactylus appears to lack skeletal autapomorphies, thus is representative for this transition and suggesting a Late Jurassic stasis of at least some pterosaurs.
The main conclusion from early Monofenestrata such as Propterodacylus and wukongopterids is that major modifications of the pterodactyloid skull came first, followed by the increase of flight properties (Lü et al., 2010; Witton, 2013). Tischlinger and Frey (2013) provided a detailed list of observations on DMA-JP-2011/006 that allow for a reconstruction of pterodactyloid origins. The authors were confident to identify the final step in a gradual evolutionary pathway. However, the sometimes mosaic-like distribution of anatomical conditions raises questions on how straight the pterodactyloid transition took place, and how adaptational this central change of pterosaur evolution was (Spindler, 2023).
Some specific aspects are affected by ontogeny. The above description has found repeated similarities between juvenile specimens of Diopecephalus (Pterodactylus) kochi and the subadult holotype of Propterodactylus in terms of proportions and qualitative characters including dentition. This observation implies a greater role of ontogenetic signals like heterochrony over the pterodactyloid transition (Unwin and Lü, 2013). Future investigations should carefully test for the possibility that some incomplete juveniles assigned to pterodactyloids (Wellnhofer, 1970) could in fact be those of transitional, pro-pterodactyloid forms. A greater data set of ontogenetic paths is needed. So far, qualitative observations such as the caudal column and the outermost pedal toe serve as clear, age-independent characters in rather complete specimens.
In the preliminary description, Tischlinger and Frey (2013) tried to reconstruct the dentition patterns throughout the pterodactyl transition: DMA-JP-2011/006 is stated to appear more ancient than wukongopterids and closer to the rhamphorhynchine dentition in having large interdental spaces and rostrolaterally directed fangs. Along with the moderately long neck, the authors found a considerable resemblance with ornithocheirid pterodactyloids, despite greater differences in the proportions of long bones. In its dentition, DMA-JP-2011/006 is conspicuously different from the stout tooth crowns of early, Pterodactylus -type pterodactyloids. The resulting evolutionary history, according to Tischlinger and Frey (2013), may include independent origins of a derived dentition type in Wukongopteridae and Pterodactyloidea. Alternatively, it is stated that the less derived dentition pattern seen in Propterodactylus may have persisted up to the early Pterodactyloidea and directly retained by Ornithocheiridae. From the above cited phylogenetic relationships, no simple and straight character history can be concluded with respect to the dentition type.
Beyond these hypotheses introduced by Tischlinger and Frey (2013), even a rhamphorhynchid-ornithocheirid-type for fishing with a somewhat pasta tong-like jaw (Holgado et al., 2019, figure 4) could have evolved several times, if functionally consistent at all. The pattern in Propterodactylus, however, is barely congruent with that of ornithocheirids, although both types (non-exclusively) share enlarged interdental spaces, up to three times the basal width of teeth. In fact, the dentition of Propterodactylus is more similar to Germanodactylus -type pterodactyloids (Witton et al., 2015, figure 3), the questionable darwinopteran Pterorynchus (Czerkas and Ji, 2002), or even the less specialised non-pterodactyloids Sordes and Jianchangopterus (Sharov, 1971; Lü and Bo, 2011). Furthermore, that wukongopterids bear smaller, robust, and narrow-spaced teeth concentrated in the distal part of a greatly elongated rostrum (Wang et al., 2009, 2010) could also be rather derived or specialised. Similar dentitions are seen in a few distantly related pterodactyloids (Rodrigues and Kellner, 2013, figure 4; Albersdörfer and Häckel, 2015, figure 226; Hone et al., 2023). Interestingly, species of Darwinopterus can strikingly vary in their dentitions, including D. modularis with a superficially ornithocheirid-like, and D. robustodens with a durophageous adaptation (Lü et al., 2011, figure 6). This disparity is particularly informative since it was confirmed to be intrageneric, in an analysis that otherwise found a paraphyletic status for wukongopterids (Wang et al., 2017, figure 4). Such variability underpins a substantial component of adaptive character changes across the early evolution of Monofenestrata. Ecological signals may strongly superimpose the phylogenetic trends over the pterodactyloid transition, at least concerning diet.
CONCLUSIONS
The detailed osteological description of the Painten pro-pterodactyloid affirmed the almost perfectly intermediate mix of non-pterodactyloid and pterodactyloid traits. Its phylogenetic position around the basal-most node of Pterodactyloidea matches with its evolutionary information about the most important transition in pterosaurs. With special interest on comparative osteology, however, long-known material from the “Solnhofen archipelago” deserves a much more detailed look on detectable cranial sutures and postcranial variation.
The dentition patterns of early Monofenestrata and Pterodactyloidea do not fit into straight evolutionary trends, but appear more mosaic-like. This suggests a strong role of dietary adaptation in the pterodactyloid transition. The typology of the dentition therefore yields only a minor value for the reconstruction of relationships.
ACKNOWLEDGEMENTS
This study was possible thanks to the conscientious work of the preparators and excavators Stephan Hahn, Wolfgang Häckel and Stefan Selzer. I am also indebted to Raimund Albersdörfer and Michael Völker for various ways of support. Thankfully, I received further help from David Martill and Peter Gschwender. Along with Eberhard Frey as one of the original descriptors of the pterosaur specimen, my greatest thanks go to Helmut Tischlinger, who kindly provided his photographic documentation and is also an indispensable and highly valued supporter for a number of activities. For editing the manuscript, Victoria McCoy and Heinrich Mallison deserve thanks for their caring work, as do several anonymous reviewers of this and a preceding submission, from whom I gained further learning about pterosaurs. I would like to thank René and Bruce Lauer as well as Dave Hone for the ongoing exchange.
REFERENCES
Aires, A.S., Reichert, L.M., Müller, R.T., Pinheiro, F.L., and Andrade, M.B. 2021. Development and evolution of the notarium in Pterosauria. Journal of Anatomy, 238(2):400-415.
https://doi.org/10.1111/joa.13319
Albersdörfer, R. and Häckel, W. 2015. Die Kieselplattenkalke von Painten, p. 126-133. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Andres, B. 2021. Phylogenetic systematics of Quetzalcoatlus Lawson 1975 (Pterodactyloidea: Azhdarchoidea). Journal of Vertebrate Paleontology, 41(Supplement to No 2):203-217. https://doi.org/10.1080/02724634.2020.1801703
Andres, B., Clark, J., and Xu, X. 2014. The earliest Pterodactyloid and the origin of the group. Current Biology, 24:1011-1016.
https://doi.org/10.1016/j.cub.2014.03.030
Augustin, F.J., Kampouridis, P., Hartung, J., Albersdörfer, R., and Matzke, A.T. 2022. The geologically oldest specimen of Pterodactylus: a new exquisitely preserved skeleton from the Upper Jurassic (Kimmeridgian) Plattenkalk deposits of Painten (Bavaria, Germany). Fossil Record, 25(2):331-343.
https://doi.org/10.3897/fr.25.90692
Augustin, F.J., Rabi, M., Spindler, F., Kampouridis, P., Hartung, J., Albersdörfer, R., and Matzke, A.T. 2023. A new specimen of Solnhofia parsonsi from the Upper Jurassic (Kimmeridgian) Plattenkalk deposits of Painten (Bavaria, Germany) and comments on the relationship between limb taphonomy and habitat ecology in fossil turtles. PloS ONE, 18(7):e0287936.
https://doi.org/10.1371/journal.pone.0287936
Bennett, S.C. 1996. Year-classes of pterosaurs from the Solnhofen Limestone of Germany: taxonomic and systematic implications. Journal of Vertebrate Paleontology, 16(3):432-444.
https://doi.org/10.1080/02724634.1996.10011332
Bennett, S.C. 2006. Juvenile specimens of the pterosaur Germanodactylus cristatus, with a review of the genus. Journal of Vertebrate Paleontology, 26(4):872-878.
https://doi.org/10.1671/0272-4634(2006)26[872:JSOTPG]2.0.CO;2
Bennett, S.C. 2007. A second specimen of the pterosaur Anurognathus ammoni. Paläontologische Zeitschrift 81(4):376-398.
https://doi.org/10.1007/BF02990250
Bennett, S.C. 2013a. New information on body size and cranial display structures of Pterodactylus antiquus, with a revision of the genus. Paläontologische Zeitschrift, 87:269-289.
https://doi.org/10.1007/s12542-012-0159-8
Bennett, S.C. 2013b. The morphology and taxonomy of the pterosaur Cycnorhamphus. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 267(1):23-41.
https://doi.org/10.1127/0077-7749/2012/0295
Bennett, S.C. 2014. A new specimen of the pterosaur Scaphognathus crassirostris, with comments on constraint of cervical vertebrae number in pterosaurs. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 271(3):327-348.
https://doi.org/10.1127/0077-7749/2014/0392
Bennett, S.C. 2018. New smallest specimen of the pterosaur Pteranodon and ontogenetic niches in pterosaurs. Journal of Paleontology, 92(2):254-271.
https://doi.org/10.1017/jpa.2017.84
Cheng, X., Jiang, S., Wang, X., and Kellner, A.W. 2016. New information on the Wukongopteridae (Pterosauria) revealed by a new specimen from the Jurassic of China. PeerJ, 4:e2177.
https://doi.org/10.7717/peerj.2177
Cheng, X., Jiang, S., Wang, X., and Kellner, A.W. 2017. New anatomical information of the wukongopterid Kunpengopterus sinensis Wang et al., 2010 based on a new specimen. PeerJ, 5:e4102. https://doi.org/10.7717/peerj.4102
Claessens, L.P., O’Connor, P.M., and Unwin, D.M. 2009. Respiratory evolution facilitated the origin of pterosaur flight and aerial gigantism. PloS ONE, 4(2):e4497.
https://doi.org/10.1371/journal.pone.0004497
Codorniú, L.S. 2005. Morfología caudal de Pterodaustro guinazui (Pterosauria: ctenochasmatidae) del Cretácico de Argentina. Ameghiniana, 42(2):505-509.
Codorniú, L. and Chiappe, L.M. 2004. Early juvenile pterosaurs (Pterodactyloidea: Pterodaustro guinazui) from the Lower Cretaceous of central Argentina. Canadian Journal of Earth Sciences, 41(1):9-18.
https://doi.org/10.1139/e03-080
Codorniú, L., Carabajal, A.P., Pol, D., Unwin, D., and Rauhut O.W.M. 2016. A Jurassic pterosaur from Patagonia and the origin of the pterodactyloid neurocranium. PeerJ, 4:e2311.
https://doi.org/10.7717/peerj.2311
Collini, C.A. 1784. Sur quelques Zoolithes du Cabinet d’Histoire naturelle de S.A.S.E Palatine & de Bavière, à Mannheim. Acta Academiae Theodoro Palatinae, Mannheim, Pars Physica, 5:58-103.
Cuvier, G. 1809. Mémoire sur le squelette fossile d’un reptile volant des environs d’Aichstedt, que quelques naturalistes ont pris pour un oiseau, et dont nous formons un genre de Sauriens, sous le nom de Ptero-Dactyle. In Annales du Muséum national d’Histoire Naturelle, Paris, 13:424-437
Czerkas, S.A. and Ji, Q. 2002. A new rhamphorhynchoid with a headcrest and complex integumentary structures, p. 15-41. In Czerkas, S.J. (ed.), Feathered Dinosaurs and the Origin of Flight. The Dinosaur Museum journal 1, Blanding.
Dalla Vecchia, F.M. 2002. Observations on the non-pterodactyloid pterosaur Jeholopterus ningchengensis from the Early Cretaceous of northeastern China. Natura Nascosta, 24:8-27
Dalla Vecchia, F.M. 2003. New morphological observations on Triassic pterosaurs, p. 23-44. In Buffetaut, E. and Mazin, J.-M. (eds.), Evolution and Palaeobiology of Pterosaurs. Geological Society Special Publications 217, London.
Dalla Vecchia, F.M. 2009. Anatomy and systematics of the pterosaur Carniadactylus gen. n. rosenfeldi (Dalla Vecchia, 1995). Rivista Italiana di Paleontologia e stratigrafia, 115(2):159-186.
Dalla Vecchia, F.M. 2022. The Presence of an Orbitoantorbital Fenestra: Further Evidence of the Anurognathid Peculiarity within the Pterosauria. Rivista Italiana di Paleontologia e Stratigrafia, 128(1):23-42.
https://doi.org/10.54103/2039-4942/16973
Elgh, E., Pieńkowski, G., and Niedźwiedzki, G. 2019. Pterosaur track assemblages from the Upper Jurassic (lower Kimmeridgian) intertidal deposits of Poland: Linking ichnites to potential trackmakers. Palaeogeography, Palaeoclimatology, Palaeoecology, 530:32-48.
https://doi.org/10.1016/j.palaeo.2019.05.016
Fabre, J.A. 1974. Un Nouveau Pterodactylidae du Gisement de Canjures (Var) Gallodactylus canjuersensis nov. gen., nov. sp. Annales de Paléontologie (Vertébrés), 62(1):35-70.
Fastnacht, M. 2008. Tooth replacement pattern of Coloborhynchus robustus (Pterosauria) from the Lower Cretaceous of Brazil. Journal of Morphology, 269(3):332-348.
https://doi.org/10.1002/jmor.10591
Frey, E. and Martill, D.M. 1998. Late ontogenetic fusion of the processus tendinis extensoris in Cretaceous pterosaurs from Brazil. Neues Jahrbuch für Geologie und Paläontologie - Monatshefte 10:587-594.
https://doi.org/10.1127/njgpm/1998/1998/587
Frey, E., Tischlinger, H., Buchy, M.C., and Martill, D.M. 2003. New specimens of Pterosauria (Reptilia) with soft parts with implications for pterosaurian anatomy and locomotion, p. 233-266. In Buffetaut, E. and Mazin, J.-M. (eds.), Evolution and Palaeobiology of Pterosaurs. Geological Society Special Publications 217, London.
https://doi.org/10.1144/GSL.SP.2003.217.01.14
Holgado, B., Pêgas, R.V., Canudo, J.I., Fortuny, J., Rodrigues, T., Company, J., and Kellner, A.W. 2019. On a new crested pterodactyloid from the Early Cretaceous of the Iberian Peninsula and the radiation of the clade Anhangueria. Scientific Reports, 9(1):4940.
https://doi.org/10.1038/s41598-019-41280-4
Hone, D.W. 2020. A review of the taxonomy and palaeoecology of the Anurognathidae (Reptilia, Pterosauria). Acta Geologica Sinica ‐ English Edition, 94(5):1676-1692.
https://doi.org/10.1111/1755-6724.14585
Hone, D.W. 2023. The anatomy and diversity of the pterosaurian sternum. Palaeontologia Electronica, 26(1).a12:1-34.
https://doi.org/10.26879/1261
Hone, D.W., Lauer, R., Lauer, B., and Spindler, F. 2023. Petrodactyle wellnhoferi gen. et sp. nov.: A new and large ctenochasmatid pterosaur from the Late Jurassic of Germany. Palaeontologia Electronica, 26(2).a25:1-28.
https://doi.org/10.26879/1251
Hyder, E.S., Witton, M.P., and Martill, D.M. 2014. Evolution of the pterosaur pelvis. Acta Palaeontologica Polonica, 59(1):109-124.
https://doi.org/10.4202/app.2011.1109
Isaksson, H., Gröngröft, I., Wilson, W., van Donkelaar, C.C., van Rietbergen, B., Tami, A., Huiskes, R., and Ito, K. 2009. Remodeling of fracture callus in mice is consistent with mechanical loading and bone remodeling theory. Journal of Orthopaedic Research, 27(5):664-672.
https://doi.org/10.1002/jor.20725
James, A.E., Montali, R.J., Novak, G.R., and Bush, M. 1978. The use of xeroradiographic imaging to evaluate fracture repair in avian species. Skeletal Radiology, 2:161-168.
https://doi.org/10.1007/BF00347315
Jiang, S., Wang, X., Cheng, X., Costa, F.R., Huang, J., and Kellner, A.W. 2015. Short note on an anurognathid pterosaur with a long tail from the Upper Jurassic of China. Historical Biology, 27(6):718-722.
https://doi.org/10.1080/08912963.2014.954570
Jiang, S., Cheng, X., Ma, Y., and Wang, X. 2016. A new archaeopterodactyloid pterosaur from the Jiufotang Formation of western Liaoning, China, with a comparison of sterna in Pterodactylomorpha. Journal of Vertebrate Paleontology, 36(6):e1212058.
https://doi.org/10.1080/02724634.2016.1212058
Jiang, S., Li, Z., Cheng, X., and Wang, X. 2020. The first pterosaur basihyal, shedding light on the evolution and function of pterosaur hyoid apparatuses. PeerJ, 8:e8292.
http://doi.org/10.7717/peerj.8292
Jiang, S., Wang, X., Zheng, X., Cheng, X., Zhang, J., and Wang, X. 2021. An early juvenile of Kunpengopterus sinensis (Pterosauria) from the Late Jurassic in China. Anais da Academia Brasileira de Ciências, 93(Suppl. 2):e20200734.
Jouve, S. 2004. Description of the skull of a Ctenochasma (Pterosauria) from the latest Jurassic of eastern France, with a taxonomic revision of European Tithonian Pterodactyloidea. Journal of Vertebrate Paleontology, 24(3):542-554.
https://doi.org/10.1671/0272-4634(2004)024[0542:DOTSOA]2.0.CO;2
Kaup, J. 1834. Versuch einer Eintheilung der Säugethiere in 6 Stämme und der Amphibien in 6 Ordnungen. Isis, 1834(3):312-316.
Lü, J. and Bo, X. 2011. A new rhamphorhynchid pterosaur (Pterosauria) from the Middle Jurassic Tiaojishan Formation of western Liaoning, China. Acta Geologica Sinica, 85(5):977-983.
https://doi.org/10.1111/j.
Lü, J. and Hone, D.W. 2012. A new Chinese anurognathid pterosaur and the evolution of pterosaurian tail lengths. Acta Geologica Sinica, 86(6):1317-1325.
https://doi.org/10.1111/1755-
Lü, J., Unwin, D.M., Jin, X., Liu, Y., and Ji, Q. 2010. Evidence for modular evolution in a long-tailed pterosaur with a pterodactyloid skull. Proceedings of the Royal Society B: Biological Sciences, 277(1680):383-389.
https://doi.org/10.1098/rspb.
Lü, J., Xu, L., Chang, H., and Zhang, X. 2011. A new darwinopterid pterosaur from the Middle Jurassic of western Liaoning, northeastern China and its ecological implications. Acta Geologica Sinica, 85(3):507-514.
https://doi.org/10.1111/j.
Lü, J., Meng, Q., Wang, B., Liu, D., Shen, C., and Zhang, Y. 2018. Short note on a new anurognathid pterosaur with evidence of perching behaviour from Jianchang of Liaoning Province, China, p. 95-104. In Hone, D.W.E., Witton, M.P., and Martill, D.M. (eds.), New Perspectives on Pterosaur Palaeobiology. Geological Society Special Publications 455, London.
https://doi.org/10.1144/SP455.
Martill, D.M. and Etches, S. 2013. A New Monofenestratan Pterosaur from the Kimmeridge Clay Formation (Kimmeridgian, Upper Jurassic) of Dorset, England. Acta Palaeontologica Polonica 58(2):285-294.
https://doi.org/10.4202/app.2011.0071
Martill, D.M., Frey, E., Tischlinger, H., Mäuser, M., Rivera-Sylva, H.E., and Vidovic, S.U. 2023. A new pterodactyloid pterosaur with a unique filter-feeding apparatus from the Late Jurassic of Germany. PalZ, 97:383-424.
https://doi.org/10.1007/s12542-022-00644-4
Martin-Silverstone, E.G., Unwin, D.M., Cuff, A.R., Brown, E.E., Allington-Jones, L., and Barrett, P.M. 2022. A new pterosaur from Skye, Scotland and the early diversification of flying reptiles. bioRxiv, 2022-02.
https://doi.org/10.1101/2022.02.14.480264
Mayr, G., Peters, D.S., and Rietschel, S. 2002. Petrel-like birds with a peculiar foot morphology from the Oligocene of Germany and Belgium (Aves: Procellariiformes). Journal of Vertebrate Paleontology, 22(3): 667-676.
https://doi.org/10.1671/0272-4634(2002)022[0667:PLBWAP]2.0.CO;2
O’Sullivan, M. and Martill, D.M. 2018. Pterosauria of the Great Oolite Group (Bathonian, Middle Jurassic) of Oxfordshire and Gloucestershire, England. Acta Palaeontologica Polonica, 63(4):617-644.
https://doi.org/10.4202/app.00490.2018
O’Sullivan, M., Martill, D.M., and Groocock, D. 2013. A pterosaur humerus and scapulocoracoid from the Jurassic Whitby Mudstone Formation, and the evolution of large body size in early pterosaurs. Proceedings of the Geologists' Association, 124(6): 973-981.
https://doi.org/10.1016/j.pgeola.2013.03.002
Owen, R. 1842. Report on British fossil reptiles, part II. Report of the eleventh meeting of the British Association for the Advancement of Science held at Plymouth in July 1841, John Murray, London, p. 60-204.
Padian, K. 1983. A functional analysis of flying and walking in pterosaurs. Paleobiology, 9(3):218-239.
https://doi.org/10.1017/S009483730000765X
Padian, K. 2017. Structure and evolution of the ankle bones in pterosaurs and other ornithodirans. Journal of Vertebrate Paleontology, 37(5):e1364651.
https://doi.org/10.1080/02724634.2017.1364651
Rauhut, O.W.M. 2012. Ein „ Rhamphodactylus “ aus der Mörnsheim-Formation von Mühlheim. Jahresbericht 2011 und Mitteilungen der Freunde der Bayerischen Staatssammlung für Paläontologie und Historische Geologie München e.V. 40:69-74
Rauhut, O.W.M., Foth, C., Tischlinger, H., and Norell, M.A. 2012. Exceptionally preserved juvenile megalosauroid theropod dinosaur with filamentous integument from the Late Jurassic of Germany. Proceedings of the National Academy of Sciences, 109(29):11746-11751.
https://doi.org/10.1073/pnas.1203238109
Rauhut, O.W.M., Foth, C., and Tischlinger, H. 2018. The oldest Archaeopteryx (Theropoda: Avialiae): a new specimen from the Kimmeridgian/Tithonian boundary of Schamhaupten, Bavaria. PeerJ, 6:e4191.
https://doi.org/10.7717/peerj.4191
Richards, G.J., Nasr, M.A., Brown, S.N., Szamocki, E.M.G., Murrell, J., Barr, F., and Wilkins, L.J. 2011. Use of radiography to identify keel bone fractures in laying hens and assess healing in live birds. Veterinary Record, 169(11):279-279.
https://doi.org/10.1136/vr.d4404
Rodrigues, T. and Kellner, A.W.A. 2013. Taxonomic review of the Ornithocheirus complex (Pterosauria) from the Cretaceous of England. ZooKeys, 308.5559:1-112.
https://doi.org/10.3897/zookeys.308.5559
Rothschild, B. and Lambert, H.W. 2019. First documentation of a greenstick fracture in the fossil record. Possible gout also noted in Arkansaurus fridayii. Historical Biology, 33(9):1349-1351.
https://doi.org/10.1080/08912963.2019.1693558
Sachs, S., Young, M.T., Abel, P., and Mallison, H. 2021. A new species of Cricosaurus (Thalattosuchia, Metriorhynchidae) based upon a remarkably well-preserved skeleton from the Upper Jurassic of Germany. Palaeontologia Electronica, 24(2).a24:1-28.
https://doi.org/10.26879/928
Samathi, A., Weluwanarak, J., Duanyai, P., Kaikaew, S., and Suteethorn, S. 2023. An unusual metatarsal of theropod dinosaur from the lower Cretaceous of Thailand: the first detailed study of paleopathology in Megaraptora. Historical Biology, 35:1-6.
https://doi.org/10.1080/08912963.2023.2166833
Schweigert, G. 2007. Ammonite biostratigraphy as a tool for dating Upper Jurassic lithographic limestones from South Germany first results and open questions. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 245(1):117-125.
https://doi.org/10.1127/0077-7749/2007/0245-0117
Schweigert, G. 2015. Biostratigraphie der Plattenkalke der Südlichen Frankenalb, p. 63-66. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Seeley, H.G. 1870. Remarks on Prof. Owen's monograph on Dimorphodon. Journal of Natural History, 6(32): 129-152.
https://doi.org/10.1080/00222937008696217
Seeley, H.G. 1891. On the shoulder-girdle in Cretaceous Ornithosauria. Journal of Natural History, 7(41): 438-445.
https://doi.org/10.1080/00222939109460638
Sharov, A.G. 1971. Новые летающие рептилии из мезозоя Казахстана и Киргизии (New Flying Reptiles From The Mesozoic Of Kazakhstan And Kyrgyzstan). Труды ПИН АН СССР, 130:104-113. (in Russian)
Spindler, F. 2019. Live Birth in a Jurassic Marine Crocodile. Paleo & Life - Abstracts of the 90th Annual Meeting of the Paläontologische Gesellschaft, Munich, Germany, p. 141.
Spindler, F. 2023. The missing link of Pterosauria and the wading revolution. From Early Life to the Neandertals - Abstract Volume 94. Jahrestagung Paläontologische Gesellschaft, Braunschweig, Germany, p. 21-22.
https://www.palaeontologische-gesellschaft.de/fileadmin/user_upload/PalGes/Tagungen/Abstractband_PalGes2023.pdf
Spindler, F. and Albersdörfer, R. 2019. Schatzkammer im Herzen Bayerns. Die einzigartigen Fossilfunde von Painten. Dinosaurier Museum Altmühltal, Denkendorf.
Spindler, F. and Ifrim, C. 2021. Die Spur einer Spur - ein möglicher erster Flugsaurier aus Ettling. Archaeopteryx, 37:75-83.
Spindler, F., Lauer, R., Tischlinger, H., and Mäuser, M. 2021. The integument of pelagic crocodylomorphs (Thalattosuchia: Metriorhynchidae). Palaeontologia Electronica, 24(2):1-41.
https://doi.org/10.26879/1099
Steel, L. 2008. The palaeohistology of pterosaur bone: an overview. Zitteliana, B28:109-125.
https://epub.ub.uni-muenchen.de/12008/1/zitteliana_2008_b28_06.pdf
Tischlinger, H. 1993. Überlegungen zur Lebensweise der Pterosaurier anhand eines verheilten Oberschenkelbruches bei Pterodactylus kochi (Wagner). Archaeopteryx, 11:63-71
Tischlinger, H. 2015. Arbeiten mit ultraviolettem Licht, p. 109-113. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Tischlinger, H. 2023. Eichstätter Fossilien im fürstbischöflichen Hochstiftskalender von 1759. Archaeopteryx, 38:64-72
Tischlinger, H. and Arratia, G. 2013. Ultraviolet light as a tool for investigating Mesozoic fishes, with a focus on the ichthyofauna of the Solnhofen archipelago, p. 549-560. In Arratia, G., Schultze, H.-P., and Wilson, H. (eds.), Mesozoic Fishes 5 - Global Diversity and Evolution. Verlag Dr. Friedrich Pfeil, München.
Tischlinger, H. and Frey, E. 2013. Ein neuer Pterosaurier mit Mosaikmerkmalen basaler und pterodactyloider Pterosauria aus dem Ober-Kimmeridgium von Painten (Oberpfalz, Deutschland). Archaeopteryx 31:1-13
Tischlinger, H. and Frey, E. 2015. Flugsaurier (Pterosauria), p. 459-480. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Tischlinger, H. and Unwin, D. 2004. UV-Untersuchungen des Berliner Exemplars von Archaeopteryx lithographica H.v.Meyer 1861 und der isolierten Archaeopteryx -Feder. Archaeopteryx, 22:17-50
Unwin, D.M. and Bakhurina, N.N. 1994. Sordes pilosus and the nature of the pterosaur flight apparatus. Nature, 371(6492):62-64.
https://doi.org/10.1038/371062a0
Unwin, D.M. and Lü, J. 2013. The basal monofenestratan Darwinopterus and its implications for the origin and basal radiation of pterodactyloid pterosaurs. International Symposion on Pterosaurs, Rio de Janeiro, Brazil. Universidade Federal do Rio de Janeiro, Museu National, Short communications, p. 98-101.
Vidovic, S.U. and Martill, D.M. 2014. Pterodactylus scolopaciceps Meyer, 1860 (Pterosauria, Pterodactyloidea) from the Upper Jurassic of Bavaria, Germany: the problem of cryptic pterosaur taxa in early ontogeny. PloS ONE, 9(10):e110646.
https://doi.org/10.1371/journal.pone.0110646
Vidovic, S.U. and Martill, D.M. 2018. The taxonomy and phylogeny of Diopecephalus kochi (Wagner, 1837) and ‘ Germanodactylus rhamphastinus ’ (Wagner, 1851), p. 125-147. In Hone, D.W.E., Witton, M.P., and Martill, D.M. (eds.), New Perspectives on Pterosaur Palaeobiology. Geological Society Special Publications 455, London.
https://doi.org/10.1144/SP455.12
Viohl, G. 2015a. Der geologische Rahmen: die Südliche Frankenalb und ihre Entwicklung, p. 56-62. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Viohl, G. 2015b. Die Lebensräume, p. 153-156. In Arratia, G., Schultze, H.-P., Tischlinger, H., and Viohl, G. (eds.), Solnhofen - Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München.
Wang, X., Kellner, A.W.A., Jiang, S., and Meng, X. 2009. An unusual long-tailed pterosaur with elongated neck from western Liaoning of China. Anais da Academia Brasileira de Ciências, 81(4):793-812.
Wang, X., Kellner, A.W.A., Jiang, S., Cheng, X., Meng, X., and Rodrigues, T. 2010. New long-tailed pterosaurs (Wukongopteridae) from western Liaoning, China. Anais da Academia Brasileira de Ciências, 82:1045-1062.
Wang, X., Kellner, A.W.A., Cheng, X., Jiang, S., Wang, Q., Sayão, J.M., Rodrigues, T., Costa, F.R., Li, N., Meng, X., and Zhou, Z. 2015. Eggshell and histology provide insight on the life history of a pterosaur with two functional ovaries. Anais da Academia Brasileira de Ciências, 87:1599-1609.
Wang, X., Jiang, S., Zhang, J., Cheng, X., Yu, X., Li, Y., Wie, G., and Wang, X. 2017. New evidence from China for the nature of the pterosaur evolutionary transition. Scientific Reports, 7(1):42763.
https://doi.org/10.1038/srep42763
Wellnhofer, P. 1968. Über Pterodactylus kochi (Wagner 1837). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 132(1):97-126.
Wellnhofer, P. 1970. Die Pterodactyloidea (Pterosauria) der Oberjura-Plattenkalke Süddeutschlands. Bayerische Akademie der Wissenschaften Mathematisch-Naturwissenschaftliche Klasse, Abhandlungen Neue Folge, 141:1-133.
Wellnhofer, P. 1974. Campylognathoides liasicus (Quenstedt), an Upper Liassic pterosaur from Holzmaden - The Pittsburgh Specimen. Annals of the Carnegie Museum, 45:5-34.
Wellnhofer, P. 1975. Die Rhamphorhynchoidea (Pterosauria) der Oberjura-Plattenkalke Süddeutschlands. Teil I Allgemeine Skelettmorphologie. Palaeontographica A, 148(1-3):1-33.
Wellnhofer, P. 1978. Handbuch der Paläoherpetologie: Teil 19. Pterosauria. Gustav Fischer, Stuttgart.
Wellnhofer, P. 1987. Die Flughaut von Pterodactylus (Reptilia, Pterosauria) am Beispiel des Wiener Exemplares von Pterodactylus kochi (Wagner). Annalen des Naturhistorischen Museums Wien A, 88:149-162.
https://www.jstor.org/stable/41701859
Wellnhofer, P. 1988. Terrestrial locomotion in pterosaurs. Historical Biology, 1(1):3-16.
https://doi.org/10.1080/08912968809386464
Wellnhofer, P. 2008. A short history of pterosaur research. Zitteliana, B28:7-19.
https://epub.ub.uni-muenchen.de/12004/1/zitteliana_2008_b28_02.pdf
Wilkin, J. 2020. The south German Plattenkalks. Geology Today, 36(1):27-32.
https://doi.org/10.1111/gto.12288
Witton, M.P. 2013. Pterosaurs. Princeton University Press, Oxford.
Witton, M.P. 2015. Were early pterosaurs inept terrestrial locomotors? PeerJ, 3:e1018.
https://doi.org/10.7717/peerj.1018
Witton, M.P., O’Sullivan, M., and Martill, D.M. 2015. The relationships of Cuspicephalus scarfi Martill and Etches 2013 and Normannognathus wellnhoferi Buffetaut et al. 1998 to other monofenestratan pterosaurs. Contributions to Zoology, 84(2):115-127.
https://doi.org/10.1163/18759866-08402002
Zhou, C.-F. and Schoch, R.R. 2011. New material of the non-pterodactyloid pterosaur Changchengopterus pani Lü 2009 from the Late Jurassic Tiaojishan Formation of western Liaoning. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 260(3):265-275.
http://doi.org/10.1127/0077-7749/2011/0131
Zhou, C.-F., Gao, K.-Q., Yi, H., Xue, J., Li, Q., and Fox, R.C. 2017. Earliest filter-feeding pterosaur from the Jurassic of China and ecological evolution of Pterodactyloidea. Royal Society Open Science, 4(2):160672.
https://doi.org/10.1098/rsos.160672
Zhou, X., Pêgas, R.V., Ma, W., Han, G., Jin, X., Leal, M.E.C., Bonde, N., Kobayashi, Y., Lautenschlager, S., Wei, X., Shen, C., and Ji, S. 2021. A new darwinopteran pterosaur reveals arborealism and an opposed thumb. Current Biology, 31(11):2429-2436.
https://doi.org/10.1016/j.cub.2021.03.030

