 Diversity and fossil record of larvae of three groups of lacewings with unusual ecology and functional morphology: Ithonidae, Coniopterygidae and Sisyridae
Diversity and fossil record of larvae of three groups of lacewings with unusual ecology and functional morphology: Ithonidae, Coniopterygidae and Sisyridae
Article number: 25.2.a14
https://doi.org/10.26879/1212
Copyright Palaeontological Association, May 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Appendices
Submission: 25 January 2022. Acceptance: 14 April 2022.
ABSTRACT
Neuroptera is an ingroup of Insecta with a moderate species richness compared to several other ingroups of Insecta. While not as iconic as butterflies, mosquitoes, or beetles, there are some rather well-known forms, such as green lacewings, antlions, owlflies or mantis lacewings. All these have peculiar predatory larvae with prominent paired, venom-injecting sucking stylets formed by the upper and lower jaws. Three less well-known lineages of Neuroptera have larvae that are stronger derived in their appearance. The larvae of Sisyridae (spongilla lacewings) have very long and thin stylets; they are aquatic and feed on sponges. The larvae of Coniopterygidae (dustywings) have very small stylets that are often covered by a large labrum. The larvae of Ithonidae (moth lacewings) are grub-like (scarabaeiform) and possess stout mandibles. We discuss the overall known record of extant and fossil larvae of these three lineages and report some new fossil specimens. A quantitative analysis of the head and stylet shapes reveals that two distinct groups of extant larvae of Coniopterygidae can be differentiated, one with larger stylets and one with smaller stylets. Some obscure holometabolan larvae from the Cretaceous are quite similar in head and stylet shape to larvae of Coniopterygidae with short stylets.
Joachim T. Haug. Ludwig-Maximilians-Universität München (LMU Munich), Faculty of Biology, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany and GeoBio-Center at LMU, Richard-Wagner-Str. 10, 80333 München, Germany. corresponding author. joachim.haug@palaeo-evo-devo.info
Serita van der Wal. Ludwig-Maximilians-Universität München (LMU Munich), Faculty of Biology, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany. vanderwal@biologie.uni-muenchen.de
Carsten Gröhn. Bünebüttler Weg 7, 21509 Glinde, Germany. jcgroehn@t-online.de
Christel Hoffeins. Liseistieg 10, 22149 Hamburg, Germany. chw.hoffeins@googlemail.com
Hans-Werner Hoffeins. Liseistieg 10, 22149 Hamburg, Germany. hoffeins@aol.com
Carolin Haug. Ludwig-Maximilians-Universität München (LMU Munich), Faculty of Biology, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany and GeoBio-Center at LMU, Richard-Wagner-Str. 10, 80333 München, Germany. carolin.haug@palaeo-evo-devo.info
Keywords: Ithonidae; Sisyridae; Coniopterygidae; Neuroptera; larva; quantitative morphology
Final citation: Haug, Joachim T., van der Wal, Serita, Gröhn, Carsten, Hoffeins, Christel, Hoffeins, Hans-Werner, and Haug, Carolin. 2022. Diversity and fossil record of larvae of three groups of lacewings with unusual ecology and functional morphology: Ithonidae, Coniopterygidae and Sisyridae. Palaeontologia Electronica, 25(2):a14. https://doi.org/10.26879/1212
palaeo-electronica.org/content/2022/3601-unusual-lacewing-larvae
Copyright: May 2022 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Adults of lacewings (Neuroptera) are conspicuous representatives of the group Insecta, resembling quite a variety of well-known, but not closer related forms such as moths, dragonflies or praying mantises. As larvae, lacewings are mostly highly specialised predators, with rather unique paired, venom-injecting sucking stylets formed by their upper and lower jaws (mandibles and maxillae; e.g., Aspöck and Aspöck, 2007).
In the modern fauna, Neuroptera is represented by only about 6,000 species. With this, the group is clearly much less species-rich than the groups of beetles (Coleoptera), moths (Lepidoptera), flies (Diptera), or wasps (Hymenoptera), of which each has more than 100,000 formally recognised species (Grimaldi and Engel, 2005; Beutel et al., 2013; Engel et al., 2018). Yet, it appears that Neuroptera has played a more important role in ecosystems of the past, possibly fulfilling some of the roles that in modern ecosystems are fulfilled by representatives of the four hyper-diverse groups mentioned above (e.g., Yang et al., 2014; Labandeira et al., 2016; Liu et al., 2018a).
Representatives of Neuroptera, as well as those of the four hyper-diverse lineages and some others, are all representatives of the group Holometabola. Early post-embryonic stages, the larvae, differ significantly in their morphology and also ecology from their corresponding adults. Furthermore, the larval phase often takes up a large share of the overall lifespan. The ecological impact of lacewings is therefore not only that of the adult, but to a large part (possible even the larger part) of the larvae.
Understanding the decline of lacewing diversity therefore also demands understanding of the decline of larval diversity of lacewings. Larvae and adults have a certain evolutionary independence (Scholtz, 2005), hence fossil adults should not be simply used as proxies for reconstructing larval diversity of the past. Luckily, especially in amber, lacewing larvae have astonishingly frequently been found (Pérez-de la Fuente et al., 2012, 2016, 2018, 2019, 2020; Liu et al., 2016, 2018b; Badano et al., 2018; Haug et al., 2018, 2019a-c, 2020a, b). In earlier studies, we have used quantitative aspects of the larval morphologies of lacewings and compared these over time to recognise changes of diversity in several lineages (Haug et al., 2020c, 2021a, b, c, 2022).
Hence many major lineages of Neuroptera have gathered quite some attention concerning the fossil record of their larvae, including:
1) antlion-like groups, i.e., Psychopsidae (e.g., Makarkin, 2018; Haug et al., 2020c), Nymphidae (Wang et al., 2016; Haug et al., 2022), Crocinae (Haug et al., 2021a), Nemopterinae (Haug et al., 2021b), Ascalaphidae and Myrmeleontidae (Wang et al., 2016; Badano et al., 2018);
2) lacewing larvae with straight stylets, i.e., Mantispidae, Berothidae, Rhachiberothidae (possibly an ingroup of the former), Dilaridae and Osmylidae (Haug et al., 2021c);
3) aphidlions, i.e., larvae of Chrysopidae and Hemerobiidae and closely related larvae (Wang et al., 2016; Liu et al., 2016, 2018b, 2022); and
4) Nevrorthidae (Haug et al., 2020d).
This set of studies leaves some more “obscure” lineages with highly specialised or unusual appearing larvae so far largely unconsidered for an overview of their larval diversity. We fill this gap by providing an overview of the diversity and fossil record of the larvae of the groups Ithonidae (moth lacewings), Sisyridae (“spongilla flies” or sponge lacewings), and Coniopterygidae (dustywings).
MATERIAL AND METHODS
Material
Four specimens were directly studied. One specimen (CCHH 540-1) is part of the collection of two of the authors (ChH, HWH) and will be deposited in the amber collection of Senckenberg Deutsches Entomologisches Institut (SDEI), Müncheberg, Germany. Two specimens (CCGG 1383, 7122) are part of one of the authors (CG) and will be deposited in the Leibniz Institute for the Analysis of Biodiversity Change - Hamburg site (LIB, partim formerly museum of the Geological-Palaeontological Institute of the University Hamburg (GPIH), later Centrum für Naturkunde (CeNak)). All three are Eocene Baltic amber pieces.
One specimen (PED 0596) is part of the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-Universität Mün-chen, Germany. The piece is from Cretaceous Myanmar amber (“Burmese amber”), about 99 million-years-old deposits, Hukawng Valley, Kachin State, Myanmar (Cruickshank and Ko, 2003; Shi et al., 2012; Yu et al., 2019). The specimen was legally purchased via the internet platform ebay.com, from the trader burmite-miner.
Further fossil as well as extant specimens were studied from the literature, including all available illustrations. For detailed information see Appendix 1.
Documentation Methods
Documentation was done by photographing the fossil specimens using a Keyence VHX-6000 digital microscope. Photographs of each specimen were recorded in dorsal and ventral view (if accessible) using coaxial cross-polarised light (Haug et al., 2013a) and unpolarised ring light, respectively. The built-in HDR function of the microscope was activated to optimise the photographs (cf. Haug et al., 2013b). Image stacks were recorded to ensure full-focus images throughout the depth of field. Several stacks adjacent to the specimen were recorded to achieve a complete field of view (Haug et al., 2008, 2011). In order to ensure the best contrast to the background, the specimens were documented (in both instances of illumination) on a white and a black background, respectively (Haug et al., 2018). The images providing the best contrast were included here. Adobe Photoshop CS2 was used to optimise the images (i.e., altering the histograms, saturation and sharpness) and create figure plates. The presentations of the images were further enhanced by colour-marking all visible structures of the fossils as an interpretation of the structures.
Shape Analysis
Adobe Illustrator CS2 and Inkscape were used to create digital illustrations of the head region (head capsule and mandibulo-maxillary stylets) of the examined fossil specimens and specimens from the literature. These illustrations were done by drawing the better preserved half in dorsal view and mirroring the half to create a full illustration. The software program SHAPE was used to analyse the resulting outline illustrations (exact methods follow Braig et al., 2019 and Haug et al., 2020c). To analyse the most important characters for morphological diversity in the dataset, a Principal Component Analysis (PCA) was performed.
RESULTS
Extant Larvae of Ithonidae
The group Ithonidae has been re-analysed in recent years and is now considered to include species (and specimens) formerly included into the groups Polystoechotidae and Rapismatidae (Winterton and Makarkin, 2010). These latter group names appear to no longer be in use. Hence, all reports in older literature referring to Polystoechotidae or Rapismatidae in fact refer to Ithonidae. In total, 10 extant specimens of larvae of Ithonidae were found in the literature that could be used for our analysis.
1) Welch (1914) provided several drawings of a larva (specimen 6001) of Polystoechotes punctatus. Drawings included an overview in dorsal view (figure 1 plate 1), close-ups on a trunk appendage (figure 2 plate 1), the antenna (figure 3 plate 1) and a mandible (figure 5 plate 1). The size range of the larvae studied by Welch (1914) was given as between 1.32-1.5 mm (p. 4). Some drawings were re-figured by Gepp (1984).
2) Tillyard (1922) showed that Ithone fusca does not develop through three larval stages (in contrast to most lacewings; see discussion in Haug et al., 2020d), but through five larval stages, and provided many images of the larvae. Images include: drawings of a larva hatching from the egg (figure 1b p. 209), a stage 1 larva in lateral view (figure 2 p. 211), details of a stage 1 larva, namely the mandible and antenna (figure 3a p. 212), maxilla (figure 3b p. 212), labium (figure 4 p. 213), a scheme of the chaetotaxy (figure 5 p. 215, figure 6 p. 216, figure 7 p. 217), an anterior body region of a stage 4 larva (figure 8 p. 217), details of a stage 5 larva, namely the head in lateral view, (figure 9 left p. 218), a trunk appendage (figure 9 right p. 218), and also photographs of the larval stages in lateral view (figures 1-8 plate IV). Despite the high degree of detail, no image of the head was provided in dorsal view and therefore none of these specimens could be considered for our analysis. Some drawings were re-figured by Peterson (1951), Gepp (1984), and Dorohova (1987).
3) Withycombe (1925) provided a drawing (figure 5 plate XXXIX) of a head of a stage 1 larva (specimen 6002) of Polystoechotes punctatus in dorsal view. Size was provided as a magnification factor, which was not informative in combination with the electronic versions available to the present authors. The image was re-figured by Tauber (1987) and Tauber et al. (2003).
4) Peterson (1951) re-figured (figure N3F p. 359) a drawing from Tillyard (1922). Apparently there are later editions and reprints of this work; these have not all been viewed by the current authors (the edition available was labelled 1957), but we can assume that they also contain the image. Hence, also citations of these later editions are likely to refer to this specimen. There are varying information about the exact year of different editions or reprints, we have found reference to the years 1953, 1956, 1957, 1960, 1962, 1965, 1967, and 1979.
5) MacLeod (1964) provided drawings of two specimens. The first one was a stage 3 larva (specimen 6003) of Polystoechotes punctatus. Drawings included a dorsal view of the head (figure 1 plate I), a ventral view of the head (figure 5 plate III), and a detail of the tentorium (figure 7 plate III). According to the provided scale, the head capsule of the larva was 2.4 mm long. The specimen was re-figured by Zimmermann et al. (2019).
The second was a stage 5 larva (specimen 6004) of Ithone fusca. Drawings included a dorsal view of the head (figure 2 plate I) and a ventral view of the head (figure 6 plate III). According to the provided scale, the head capsule of the larva was 1.6 mm long.
6) Riek (1970) provided a drawing (figure 29.5H p. 476) of a larva (specimen 6005) of Ithone (stage not specified) in ventral view. Images were attributed to M. Quick. No indication of size was provided. Additionally, a lateral view of the entire larva (figure 29.10A p. 486) and a head in dorsal view (figure 29.10B p. 486) was provided. The dorsal view matches quite well to the ventral view in outline and hence both are interpreted as depicting the same individual. Some drawings were re-figured by Gepp (1984).
7) Gepp (1984) re-figured several images from several sources, including Welch (1914; Gepp, 1984, figure 14a plate 7 p. 198), Tillyard (1922; Gepp, 1984, figure 7a plate 3 p. 194) and Riek (1970; Gepp, 1984, figure 7b plate 3 p. 194).
8) Dorohova (1987) re-figured (figure 4-4 p. 11) a drawing from Withycombe (1922).
9) Tauber (1987) re-figured specimen 6002, i.e., the specimen from Withycombe (1925). There is a later edition from 1991 of the same work.
10) Tauber et al. (2003) re-figured specimen 6002, i.e., the specimen from Withycombe (1925), but cited Tauber (1991 ≈ Tauber, 1987) as the source.
11) Grebennikov (2004) provided images of five different larvae. The first was an older larva of Ithone fusca (specimen 6006). Images included various drawings (all on p. 411): an overview in lateral view (figure 1), the trunk end in ventral view (figure 2), trunk appendages (figures 3, 4) and the head in dorsal (figure 5), frontal (figure 6), ventral (figure 7), and lateral view (figure 8). According to the provided scale, the larva was about 22 mm long.
The second was an older larva of Oliarces clara (specimen 6007). Images included various drawings (all on p. 412): an overview in lateral view (figure 9), the trunk end in ventral view (figure 11), trunk appendages (figures 10, 12) and the head in dorsal (figure 14), frontal (figure 16), ventral (figure 15), and lateral view (figure 13), the antenna (figure 16), and the labial palps (figure 17). According to the provided scale, the larva was about 24 mm long.
The third was a stage 1 larva of Ithone fusca (specimen 6008). Images included various drawings (all on p. 413): an overview in lateral view (figure 18), the head in dorsal (figure 19), ventral (figure 20), and lateral view (figure 21), and the trunk end in lateral view (figure 22). According to the provided scale, the larva was about 3.7 mm long.
The fourth was a stage 1 larva of Oliarces clara (specimen 6009). Images included various drawings. Images included various drawings (all on p. 414): an overview in lateral view (figure 23), the head in dorsal (figure 24), ventral (figure 25), and lateral view (figure 26), thorax segments in lateral view (figure 27), the trunk end in lateral view (figure 28), and the tip of the antenna (figure 29). According to the provided scale, the larva was about 2 mm long.
The fifth was a stage 1 larva of Polystoechotidae (specimen 6010). Images included various drawings (all on p. 415): an overview in lateral view (figure 30), the head in dorsal (figure 31), ventral (figure 32), and lateral view (figure 33), thorax segments in lateral view (figure 34), the trunk end in lateral view (figure 35), and a trunk appendage (figure 36). According to the provided scale, the larva was about 5 mm long. The specimen was re-figured by Heckman (2017).
12) Heckman (2017) provided a figure (figure 2.26) that states “unidentified larva in the family Berothidae” in the figure legend. Yet, the specimen was cited as originating from Grebennikov (2004). It is a re-figure of specimen 6010.
13) Zimmermann et al. (2019) re-figured (figure 11.4f p. 365) specimen 6003, i.e., the drawing from MacLeod (1964).
Extant Larvae of Coniopterygidae
In total, 43 extant specimens of larvae of Coniopterygidae were found in the literature and databases that could be used for our analysis.
1) Curtis (1834) provided a drawing (plate 528 L) of a larva (specimen 6201) of Coniopterygidae in dorsal view. The image was re-figured in Aspöck and Aspöck (2009).
2) Westwood (1840, part 2 figure 65.8 p. 52) provided a drawing of a larva of Coniopterygidae. Yet, the drawing is rather simplified, lacking sufficient details of the head. Therefore, the specimen could not be further considered. The image was re-figured in Aspöck and Aspöck (2009).
3) Dujardin (1851) provided drawings of a larva of Coniopterygidae (specimen 6202) that he referred to as Hemerobius hirtus. Drawings (on an unnumbered figure plate) included an overview in dorsal view (figure 15) and details of the head (figures 16-19). Size was provided as a magnification factor, which was not informative in combination with the electronic version of the article available to the authors. One image was re-figured in Aspöck and Aspöck (2009).
4) Schlechtendal (1882) provided drawings of immatures of Coniopteryx psociformis. Drawings included a lateral view on a very late larva (figure 1) already close to moulting to the pupa (pre-pupa), a detail of the leg of this larva (figure 1a), an animal during this moult (figure 2), and the pupa (figure 3). Due to the orientation, no image could be considered for our analysis.
5) Löw (1885) figured two larvae of Coniopterygidae. The first one (specimen 6203) was a larva of Coniopteryx. Drawings included an overview in dorsal view (figure 1 plate XXII), head in dorsal view (figure 2 plate XXII), head in lateral view (figure 3 plate XXII), detail of labial palp (figure 4 plate XXII), detail of stylet (figure 5 plate XXII), trunk end (figure 6 plate XXII) and a detail of the trunk appendages (figure 7 plate XXII). The stage of the larva remains unclear. No indication of size was provided.
The second one (specimen 6204) was a larva of Aleuropteryx lutea. Drawings included an overview in dorsal view (figure 8 plate XXII), head in dorsal view (figure 9 plate XXII), head in ventral view (figure 10 plate XXII), and details of the trunk appendages (figures 11, 12 plate XXII). The stage of the larva remains unclear. No indication of size was provided.
One image was re-figured in Aspöck and Aspöck (2009).
6) Emerton (1906) provided drawings of immatures of Coniopteryx vicina. Drawings included a lateral view on a very late larva (p. 74 lower middle) already close to moulting to the pupa (pre-pupa) and the pupa, also in lateral view (p. 74 lower right). Due to the orientation, no image could be considered for our analysis.
7) Arrow (1917) provided a drawing (p. 254) of a larva of Conwentzia psociformis in dorsal view. Yet, the drawing is simplified in certain aspects, especially concerning details of the head region. Therefore, the specimen could not be further considered for our analysis.
8) Crampton (1921) provided a drawing of the head of a larva in ventral view, referred to as Cowenzia [sic!] hageni. Yet, the posterior border of head capsule is not accessible. Therefore, the specimen could not be further considered. The image was re-figured in Genay (1953).
9) Withycombe (1922) provided four drawings of larvae of Coniopterygidae in dorsal view (figs 1-4 plate XLIII). Yet, the details of the head are rather small and do not provide enough details for recognising the exact outline. Hence, these could not be included in our analysis (but see below, Killington, 1936). Yet, he additionally figured a detailed drawing (figure 7 plate XLIII) of a head of a larva (specimen 6205) of Conwentzia psociformis in ventral view. No indication of size was provided. Some images were re-figured in Killington (1936) and Ghilarov (1962).
10) Withycombe (1925) provided numerous sections through final instar larvae of Conwentzia psociformis (figure 21 plate XLII, figures 28-32 plate XLIII, figure 37 plate XLIV), but no entire larva or a close-up of the head.
11) Stitz (1931) provided several drawings of a larva of Conwentzia psociformis (specimen 6206). Drawings included an overview in dorsal view (figure 36 p. 35.99), body sculpture (figure 37 p. 35.99), the antenna (figure 38 p. 35.99), details of the mouthparts (figures 39, 40 p. 35.100), trunk appendage (figure 41 p. 35.101), and malphigian tubules (figure 43 p. 35.102). In addition, a detail of the trunk appendage of the larva of Semidalis aleyrodiformis was depicted (figure 42 p. 35.101), yet no overview or close-up of the head was provided. Some images were re-figured in Genay (1953).
12) Killington (1936) depicted drawings of four larvae, all stage 3 larvae in dorsal view (on plate VIII). The first was a larva of Conwentzia psociformis (figure 1). Length was stated to be 3-3.5 mm. The second was a larva of Coniopteryx pygmaea (figure 2). Length was stated to be 2-2.5 mm. The third was a larva of Coniopteryx tineiformis (figure 3). Length was stated to be 2.5 mm. The fourth was a larva of Semidalis aleyrodiformis (figure 4). Length was stated to be 2-2.5 mm.
First and fourth specimens were stated to be original, second and third to be re-figured from Withycombe (1922). In fact, first to third specimens resemble drawings in Withycombe (1922), all are depicted at a larger size and provide more details. The first specimen is potentially identical with the one of which the head was depicted in Withycombe (1922). It is, therefore, not considered further to avoid considering the same specimen twice. All other three specimens, second to fourth, are further considered for our analysis (specimens 6207-6209).
13) Collyer (1951) provided drawings of three different larvae (figure 2 p. 558; figure 3 p. 559; figure 4 p. 563). Yet, in all three cases the transition between head capsule and antenna are only very weakly indicated. Therefore, it is not possible to properly reconstruct the outline of the head capsule, and the specimens could not be further considered here. Some drawings were re-figured by Tauber (1987).
14) Peterson (1951, figures N2 I, J) provided drawings of a larva (specimen 6210) of Conwentzia. Images included overview drawings in lateral view (figure N2 I) and a ventral view (figure N2 J). According to figure legend, the larva was 2 mm long. Apparently there are later editions and reprints of this work; these have not all been viewed by the current authors (the edition available was labelled 1957), but we can assume that they also contain the image. Hence, also citations of these later editions are likely to refer to this specimen. There is varying information about the exact year of different editions or reprints. We have found reference to years 1953, 1956, 1957, 1960, 1962, 1965, 1967, and 1979.
15) Genay (1953) provided several drawings of a larva of Coniopteryx pygmaea. Drawings included the head in dorsal view (figure 10), an overview in dorsal view (figure 11), the head in dorsal view but with transparency to see ventral details (figure 12) and the labium (figure 13). We consider all images to show a single specimen (specimen 6211). No indication of size was provided. Genay (1953) also re-figured (without figure numbers) some drawings of details from Crampton (1921) and Stitz (1931).
16) Badgley et al. (1955) reported on the post-embryonic development of Spiliconis picticornis. Most remarkably, they explicitly reported that there are four larval stages before the pupa. A drawing (figure 1 plate 8) of such a (supposed?) stage 4 larva (specimen 6212) in dorsal view was provided, together with a close-up on a single specialised seta (figure 2 plate 8). No indication of size was provided.
17) Rousset (1956a) reported about the head of larvae (muscles) of Coniopterygidae, but did not figure anything.
18) Rousset (1956b) reported about the head of larvae (muscles) of Coniopterygidae, but did not figure anything.
19) Rousset (1958) reported about the head of larvae (nervous system) of Coniopterygidae, but did not figure anything.
20) Ghilarov (1962) re-figured (figure 13 p. 412) two of the overview drawings from Withycombe (1922).
21) Aspöck and Aspöck (1964) provided a drawing (figure 52 p. 266) of a stage 3 larva (specimen 6213) of Coniopteryx in dorsal view. No indication of size was provided. The drawing was re-figured by Jacobs (1998).
22) MacLeod (1964) provided drawings of two larvae of Coniopterygidae. The first one was a stage 3 larva (specimen 6214) of ? Helicoconis lutea. Images included drawings of the head in dorsal view (figure 58 plate XIX), ventral view (figure 59 plate XIX), lateral view (figure 60 plate XIX), and a detail of the ventral mouthpart arrangement (figure 61 plate XX).
The second one was a larva (specimen 6215) of Coniopteryx ? vicina images included drawings of the head in dorsal view (figure 62 plate XX), head in lateral view (figure 63 plate XXI), a detail of the posterior head region (figure 64 plate XXI), and the head in ventral view (figure 65 plate XXI).
Some drawings were re-figured by Aspöck and Aspöck (2007), Zimmermann et al. (2009, 2019) and Aspöck (2019).
23) Rousset (1966) provided many drawings of four different larvae of Coniopterygidae. Images of Coniopteryx pygmea included an overview in dorsal view (figure 1 p. 8), head in dorsal (figure 2 p. 10), ventral (figure 3 p. 10) and lateral view (figure 4 p. 11), musculature of the pharynx (figure 5 p. 11), muscle attachment of the head (figure 6 p. 13), antenna (figure 7 p. 14), sections through the mouthparts (figure 8 p. 16), mandible (figure 9 p. 20), maxilla (figure 10 p. 21), muscles in the mandible (figure 11 p. 21), muscles of the maxilla (figure 12 p. 24), muscles of the labium (figure 13 p. 27), and aspects of the nervous system (figures 14-16 p. 31). We consider all images to refer to a single specimen (specimen 6216); for our analysis we use the dorsal view on the head (figure 2 p. 10). According to the provided scale, the head capsule was 0.29 mm long.
Images of Semidalis aleyrodiformus included an overview in dorsal view (figure 17 p. 34), head in dorsal (figure 18 p. 36), ventral (figure 19 p. 36) and lateral view (figure 20 p. 37), musculature of the pharynx (figure 21 p. 37), muscle attachment of the head (figure 22 p. 38), antenna (figure 23 p. 39), mandible (figure 24 p. 43), maxilla (figure 25 p. 43), muscles in the mandible (figure 26 p. 45), muscles of the maxilla (figure 27 p. 45), muscles of the labium (figure 28 p. 48), and aspects of the nervous system (figures 29-31 p. 52). We consider all images to refer to a single specimen (specimen 6217); for our analysis we use the dorsal view of the head (figure 18 p. 36). According to the provided scale, the head capsule was 0.28 mm long.
Images of Conwentzia psociformis included an overview in dorsal view (figure 32 p. 53), head in dorsal (figure 33 p. 55), ventral (figure 34 p. 55) and lateral view (figure 35 p. 56), musculature of the pharynx (figure 36 p. 56), muscle attachment of the head (figure 37 p. 57), antenna (figure 38 p. 58), mandible (figure 39 p. 61), maxilla (figure 40 p. 61), muscles in the mandible (figure 41 p. 63), muscles of the maxilla (figure 42 p. 63), muscles of the labium (figure 43 p. 66), and aspects of the nervous system (figures 44-46 p. 69). We consider all images to refer to a single specimen (specimen 6218); for our analysis we use the dorsal view of the head (figure 33 p. 55). According to the provided scale, the head capsule was 0.33 mm long.
Images of Aleuropteryx loewi included an overview in dorsal view (figure 47 p. 71), head in dorsal (figure 48 p. 73), ventral (figure 49 p. 73) and lateral view (figure 50 p. 71), musculature of the pharynx (figure 51 p. 71), muscle attachment of the head (figure 52 p. 77), a section through the mouthparts (figure 53 p. 78), mandible (figure 54 p. 80), maxilla (figure 55 p. 80), muscles in the mandible (figure 56 p. 83), muscles of the maxilla (figure 57 p. 83), muscles of the labium (figure 58 p. 86), sections through the labium (figure 59 p. 87), and aspects of the nervous system (figures 60-61 p. 90, figure 66 p. 91). We consider all images to refer to a single specimen (specimen 6219); for our analysis we use the dorsal view of the head (figure 48 p. 73). According to the provided scale, the head capsule was 0.30 mm long.
Some images were re-figured by Tauber (1987).
24) Muma (1967) provided two micrographs (both on unnumbered plate) of a stage 1 larva (figure 3) and a stage 3 larva (figure 4) of Coniopteryx vicina. Yet, the available details are not sufficient for considering these specimens further.
25) Riek (1970) provided several drawings of a larva of Coniopterygidae (specimen 6220). Images included an overview in dorsal view (his figure 29.9A p. 484), head in ventral view (figure 29.9B p. 484), and mouthparts (figure 29.9C p. 484). No indication of size was provided.
26) Ward (1970) provided several drawings of a final stage larva (specimen 6221) of Aleuropteryx juniperi. Images included an overview (figure 2 p. 76), left half ventral, right half dorsal, and details of the setae (figure 3 p. 76). According to the provided scale, the larva was 1.3 mm long.
27) Greve (1974) provided several drawings of a larva (specimen 6222) of Helicoconis lutea. Drawings included an overview in dorsal view (figure 1B p. 20), details of the micro-structure (figure 1A p. 20), details of the trunk appendages (figure 1C-D p. 20), the head in dorsal (figure 2A p. 21) and ventral view (figure 2B p. 21) as well as the trunk end (figure 2C p. 21). According to the provided scale, the larva was 2.7 mm long. Some images were re-figured by Makarkin (1995).
28) Meinander (1974) provided drawings of two larvae. The first was a stage 3 larva (specimen 6223) of Conwentzia baretti. Images included (all on p. 13) details of the maxilla (figure 1A), the mandible (figure 1B), the head in dorsal (figure 1C) and ventral view (figure 1D), and an overview in dorsal view (figure 1E). According to the text, the larva was 3 mm long.
The second was a stage 3 larva (specimen 6224) of Semidalis vicina. Images (all on p. 15) included an overview in dorsal view (figure 2A), head in dorsal (figure 2B) and ventral view (figure 2C), details of mandible (figure 2D) and maxilla (figure 2E). Additionally, the head of a very late stage larva (pre-pupa) was shown in lateral view (figure 2F), but could not be further considered due to the orientation. According to the text, the larva was between 1.6-1.8 mm long.
Some images were re-figured by New (1989).
29) Gepp (1984) provided drawings of a stage 3 larva of Helicoconis (specimen 6225). Images included an overview in dorsal view (figure 6a plate 3 p. 194) and the head in lateral view (figure 6b plate 3 p. 194). According to the figure legend, the larva was 4 mm long.
30) Dorohova (1987) provided a small drawing (figure 2 p. 40), a larva of Coniopterygidae. Yet, the details of the head are not well accessible due to the rather small size. Therefore, the specimen could no longer be considered.
31) Tauber (1987, on p. 131; there is a later edition of the same work from 1991) re-figured (figure 33.4) a drawing from Collyer (1951) and several drawings (figure 33.5-33.8) from Rousset (1966).
32) New (1989) re-figured (figure 135 p. 97) a larva of Conwentzia barretti from Meinander (1974).
33) Monserrat et al. (1990) figured drawings of two larvae. The first was a larva (specimen 6226) of Semidalis pluriramosa. Images (all on p. 111) included an overview in dorsal view (figure 6), setae arrangement of the dorsal surface (figure 7), details of antenna (figure 8), head in dorsal view (figure 9), and trunk end in ventral (figure 10) and dorsal view (figure 11). According to the provided scale, the head capsule was 0.27 mm long.
The second (specimen 6227) was a stage 3 larva of Semidalis pseudouncinata. Images (all on p. 113) included an overview in dorsal view (figure 12), setae arrangement of the dorsal surface (figure 13), details of antenna (figure 14), head in dorsal view (figure 15) and trunk end in ventral (figure 16) and dorsal view (figure 17). According to the provided scale, the head capsule was 0.27 mm long.
34) Makarkin (1995) re-figured (figure 17.2 p. 43) a drawing from Greve (1974).
35) Jacobs (1998) re-figured (figure C-173 p. 158) a drawing from Aspöck and Aspöck (1964).
36) Aspöck and Aspöck (1999) provided a micrograph of a larva of Coniopteryx (figure 47 p. 19). Yet, the image does not provide sufficient details of the head and is, therefore, not further considered. The image was re-figured in Aspöck and Aspöck (2009).
37) Gepp (1999) provided micrographs of two larvae of Coniopterygidae (figures 23, 24 p. 194). Yet, the images do not provide sufficient details of the head to be further considered here.
38) Stürzer and Gepp (2004) provided several drawings of four different larvae of Coniopterygidae. The first (figure 1 p. 7) was a dorsal overview of a stage 3 larva (specimen 6228) of Conwentzia pineticola. According to the figure legend, the specimen was 4 mm long.
The second (figure 2 p. 7) was a dorsal overview of a stage 3 larva (specimen 6229) of C. psociformis. According to the figure legend, the specimen was 5 mm long.
The third was a stage 3 larva (specimen 6230) of Conwentzia pineticola. Drawings (all on p. 8) included an overview in dorsal view (figure 3), details of the stylets (figure 3a), the antenna (figure 3b), and the head in dorsal view (figure 3c). According to the figure legend, the specimen was 5 mm long.
The fourth was a stage 3 larva (specimen 6231) of Conwentzia psociformis. Drawings (all on p. 9) included an overview in dorsal view (figure 4), details of the labial palp (figure 4a), and of the stylets (figure 4b). According to the figure legend, the specimen was 6 mm long.
39) Sziráki and Flint (2005; sometimes also cited as 2007, e.g., in Zimmermann et al., 2009) apparently figured a larva of Brucheiser penai, with at least a drawing of the head in dorsal view (specimen 6232). This source was not directly seen by the current authors and is cited after Zimmermann et al. (2009), where the specimen was re-figured. It was also re-figured in Aspöck (2019).
40) Aspöck and Aspöck (2007) re-figured (figure 91 p. 487) a drawing from MacLeod (1964).
41) Zizzari et al. (2008) provided a micrograph (figure 1A p. 411) of a larva of Conwentzia psociformis. Yet, in the image the head is not apparent. Therefore, the specimen could not be further considered here.
42) Aspöck and Aspöck (2009) re-figured numerous images. Images included the micrograph (figure 2 p. 73) from Aspöck and Aspöck (1999), drawings (figure 13 p. 85, figure 15 p. 86) from Curtis (1834), a drawing (figure 22 p. 93) from Westwood (1840), a drawing (figure 23 p. 94) from Dujardin (1851) and a drawing (figure 33 p. 106) from Löw (1885).
43) Zimmermann et al. (2009) re-figured (on p. 657) two drawings (figure 3A, C) from MacLeod (1964) and a drawing from Sziráki and Flint (2005).
44) Aspöck (2019) re-figured (on p. 67) two drawings (figures 10, 12) from MacLeod (1964) and a drawing (figure 11) from Sziráki and Flint (2005).
45) Zimmermann et al. (2019) re-figured (11.4b p. 365) a drawing from MacLeod (1964).
46) Websites are generally not considered to be “proper” scientific sources. Yet, given the scarceness of data on larvae of Coniopterygidae, we decided to use them here as additional data source. Especially the community BugGuide (https://bugguide.net) is very active and well sorted. It is furthermore hosted by the Department of Entomology of the Iowa State University. This website has already been used as a source for comparable studies (e.g., Haug and Haug 2019).
Image 609199 (© 2012 Charley Eiseman) was labelled “Dustywing larva” (specimen 6233). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. The size of the specimen has been stated to be 1.5 mm.
Image 169171 (© 2008 Cheryl Moorehead) was labelled “Dustywing Larva” (specimen 6234). The photograph shows the specimen in dorsal view. The size of the specimen has been stated to be 3 mm.
Image 323300 (© 2009 Scott Justis) was labelled “Life on a Mushroom” (specimen 6235). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. The size of the specimen has been stated to be 0.5 mm.
Image 1904189 (© 2020 Aaron Schusteff) was labelled “Mystery (Beetle?) Larva” (specimen 6236). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. The size of the specimen has been stated to be 2.5-2.7 mm.
Image 1101390 (© 2015 John Rosenfeld) was labelled “Dustywing” (specimen 6237). The photograph shows the specimen in dorsal view. No indication of size was provided.
Image 1005087 (© 2014 John Rosenfeld) was labelled “Tiny thing” (specimen 6238). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. No indication of size was provided.
Image 892391 (© 2014 John Rosenfeld) was labelled “Larva ?” (specimen 6239). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. No indication of size was provided.
Image 428392 (© 2010 Sean McVey) was labelled “Clueless” (specimen 6240). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. No indication of size was provided.
Image 331046 (© 2009 Charley Eiseman) was labelled “Dustywing larva?” (specimen 6241). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. The size of the specimen has been stated to be about 2 mm.
Image 142474 (© 2007 tom murray) was labelled “Dustywing” (specimen 6242). The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. The size of the specimen has been stated to be less than 1 mm.
Image 589947 (© 2011 Diane Young) was labelled “unknown live oak inhabitant - Semidalis ” (specimen 6243). The photograph shows the specimen in dorsal view. The size of the specimen has been stated to be about 1 mm.
Extant Larvae of Sisyridae
In total, 41 extant specimens of larvae of Sisyridae were found in the literature and databases that could be used for our analysis.
1) Needham and Betten (1901) provided a coloured drawing (figure 3 plate 12) of a larva (specimen 6401) of Climacia dictyona in dorsal view. Size was provided as a magnification factor, which was not informative in combination with the electronic versions available to the present authors.
2) Crampton (1921) provided a drawing (figure 50 plate VI) of a ventral view of a larva (specimen 6402) of Climacea. No indication of size was provided.
3) Lestage (1921) provided a drawing (figure 102 p. 339) of dorsal view of a larva (specimen 6403) of Sisyra fuscata. Size was provided by an unlabelled scale bar, hence the size remains unclear.
4) Withycombe (1922) provided a drawing (figure 2 plate XXXVIII) of dorsal view of a larva (specimen 6404) of Sisyra fuscata. Size was provided as a magnification factor, which was not informative in combination with the electronic versions available to the present authors. The specimen was re-figured by Killington (1936), Ghilarov (1962), Aspöck and Aspöck (1964), Makarkin (1995), and Monserrat (2014).
In addition, a hatching stage larva was provided in lateral view (figure 3 plate XXXVIII). This specimen could not be further considered due to the orientation.
5) Killington (1936) re-figured (figure 2 plate 9) specimen 6404, i.e., the specimen from Withycombe (1922).
6) Townsend (1939) provided a drawing (figure 5 p. 354) of a larva (specimen 6405) of Sisyra in dorsal view. No indication of size was provided.
7) Peterson (1951; there are later editions of this work, see above) provided drawings of larvae of Climacia areolaris. Drawings included a larva (specimen 6406) in ventral view (figure N4A) and a different larva (specimen 6407) in dorsal view (figure N4B). Length was stated to be 2 mm for the first specimen and 2.5 mm for the second specimen.
8) Brown (1952) provided drawings of several larvae of Climacia areolaris. The first was a stage 1 larva (specimen 6408) in lateral (figure 6 p. 142) and dorsal (figure 7 p. 142) view. Total length of the slightly curved specimen is around 0.5 mm based on the provided scale.
The second was a stage 2 larva (specimen 6409) in dorsal view (figure 8 p. 145). According to the provided scale, the total length was 2.38 mm.
The third was a stage 3 larva (specimen 6410) in dorsal view (figure 9 p. 146). According to the provided scale, the total length was 5.26 mm.
Furthermore, a drawing of a very late larva (pre-pupa) in ventral view was provided (figure 12 p. 150). The overall shape appears to be already partly deformed due to the formation of the pupa inside the cuticle of the larva. Therefore, this specimen was not further considered here.
Some images were re-figured in Bowles (2008).
9) Parfin and Gurney (1956) provided drawings of larvae of Climacia areolaris and Sisyra vicaria (all on p. 431). The first was a stage 1 larva (specimen 6411) of Climacia areolaris in dorsal view (figure 3A). No indication of size was provided.
The second was a stage 2 larva (specimen 6412) of Climacia areolaris in dorsal view (figure 3B). No indication of size was provided.
Additionally, some details of a stage 3 larva of Climacia areolaris were depicted including head in ventral view (figure 3E), head dissected in ventral view (figure 3F), distal part of antenna (figure 3H), head dissected in lateral view (figure 3J). The combined information of the figures allows us to reconstruct an outline for this third larva (specimen 6413). No indication of size was provided.
The fourth was a stage 3 larva (specimen 6414) of Sisyra vicaria in dorsal view (figure 3D). No indication of size was provided. Additionally, some details of this larva were depicted including the distal part of antenna (figure 3G) and a ventral view on the trunk with gills (figure 3I).
Additional details were provided (not necessarily from the specimens above). This included trunk ends (figure 4 p. 433) and lateral trunk processes (figure 5 p. 440).
One image was re-figured in New (1989).
10) Ghilarov (1962) re-figured (figure 10 p. 410) specimen 6404, i.e., the drawing from Withycombe (1922).
11) Kimmins (1962) provided a drawing (figure 7B p. 9) of a larva (specimen 6415) of Sisyra. According to the provided magnification factor, the larva was about 6 mm in total length.
12) Aspöck and Aspöck (1964) re-figured (figure 59 p. 267) specimen 6404, i.e., the drawing from Withycombe (1922).
13) MacLeod (1964) provided several drawings of a stage 3 larva (specimen 6416) of Sisyra vicaria. Drawings included the head in dorsal view (figure 26 plate VIII), the head capsule in dorsal (figure 27 plate IX) and ventral view (figure 28 plate IX), details of the cibarium (figure 29 plate IX), and the head capsule in anterior (figure 30 plate X) and lateral view (figure 31 p. X). According to the provided scale, the head capsule was 0.25 mm long.
14) Riek (1970) provided drawings of a larva of Sisyra. Drawings included the head in ventral view (figure 29.5C p. 476), the trunk in ventral view (figure 29.10C p. 486), and the larva in dorsal view (figure 29.10D p. 486). We consider all drawings to represent a single specimen (specimen 6417). No indication of size was provided.
15) Pupedis (1986) provided the drawing (figure 1 p. 55) of a stage 1 larva of Climacia areolaris in lateral view. Due to the orientation, we could not further consider this specimen.
16) New (1989) re-figured (figure 139 p. 99) a drawing from Parfin and Gurney (1956).
17) Weißmair and Waringer (1994) provided several drawings of larval stages of Sisyra. These included three larval stages each, of S. fuscata and S. terminalis.
The illustrations of the stage 1 larva of S. terminalis included the lateral view (figure 1 p. 149); the posterior trunk in lateral view (figure 3. p. 149), and the tarsus (figure 5. p. 149). The stage 2 larva (specimen 6418) was presented in dorsal view (figure 8 p. 150). The stage 3 larva was accompanied by an illustration of the antenna (figure 11 p. 150).
The drawings of the stage 1 larva of S. fuscata included the antenna (figure 2 p. 149) and the tarsus (figure 4 p. 149). Two stage 2 larvae of this species were presented, the first accompanied by illustrations of the antenna (figure 2 p. 149) and the posterior trunk in lateral view (figure 3. p. 149), the second (specimen 6419) the specimen in dorsal view (figure 9 p. 150). The illustrations of stage 3 larva of S. fuscata included the antenna (figure 10 p. 150) of one specimen and the dorsal view (figure 12 p. 150) of another specimen (specimen 6420).
Further comparative illustrations of the larval stages of S. fuscata and S. terminalis were given (all on p. 152). The stage 3 larvae were compared in terms of the gills (figures 13, 14) and setal arrangement (figures 15, 16). The stage 1 larvae were compared in terms of dorsal seta arrangement (p. 154, figures 21, 22) on the anterior trunk (thorax) and on the posterior trunk (figures 23, 24). According to the provided scales, the stage 2 larva of S. terminalis (specimen 6418) was 1.5 mm long, the stage 2 larva of S. fuscata (specimen 6419) was 1.1 mm long, and the stage 3 larva of S. fuscata (specimen 6420) was 5.2 mm long.
18) Makarkin (1995) re-figured (figure 2 p. 42) the drawing from Withycombe (1922).
19) Weißmair (1999) provided many micrographs and drawings of larvae of the group Sisyra. Three micrographs show the three larval stages of Sisyra jutlandica (all on p. 103): stage 1 (figure 6), stage 2 (figure 7), and stage 3 (figure 8). Yet, these images do not provide sufficient details to be further considered.
Several drawings of a stage 1 larva (all on p. 104) of S. jutlandica included an overview in lateral view (figure 9; not further considered due to orientation), the antenna (figure 10) and the distal part of a trunk appendage (figure 11). For comparison also the corresponding distal parts of trunk appendages of S. iridipennis (figure 12) and the prothorax sclerites (figure 13) of S. terminalis were shown.
Several drawings of a stage 2 larva (all on p. 105) of S. jutlandica included an overview in dorsal view (figure 14; specimen 6421), the antenna (figure 15), prothorax sclerite (figure 17) and the second gill (figure 18). For comparison also the corresponding antenna of S. iridipennis (figure 16) and gill (but third) of S. fuscata (figure 18) and S. iridipennis (figure 19) were shown.
Drawings of a stage 3 larva (all on p. 106) of S. jutlandica included an overview in dorsal view (figure 21; specimen 6422), the first gill (figure 22), and second gill (figure 23). For comparison also the corresponding first gill of S. iridipennis (figure 24) and S. dalii (figure 25) were shown. Further comparison was provided (on p. 107) including sclerites on thorax (figure 26) and abdomen segments 2 and 3 (figure 27) as well as the antenna of a stage 3 larva of S. jutlandica (figure 28), and the antenna of stage 3 larvae of S. dalii (figure 29) and S. jutlandica (figure 30).
Three micrographs show the three larval stages of Sisyra iridipennis (all on p. 110) in dorsal view: stage 1 (figure 36), stage 2 (figure 37), and stage 3 (figure 38). Yet, these images do not provide sufficient details to be further considered.
Drawings of a stage 2 larva (all on p. 111) of S. iridipennis included an overview in dorsal view (figure 39; specimen 6423), prothorax sclerite (figure 40), and claw of trunk appendage (figure 41). For comparison also the corresponding claw of S. dalii (figure 42) was shown.
Drawings of a stage 3 larva (all on p. 112) of S. iridipennis included an overview in dorsal view (figure 43; specimen 6424), prothorax sclerite (figure 44), and sclerites of abdomen segments 2 and 3 (figure 45).
Three micrographs show the three larval stages of Sisyra dalii (all on p. 117) in dorsal view: stage 1 (figure 61), stage 2 (figure 62), and stage 3 (figure 63). Yet, these images do not provide sufficient details to be further considered.
Drawings of a stage 2 larva (all on p. 120) of S. dalii included an overview in dorsal view (figure 64; specimen 6425) and prothorax sclerite (figure 65). For comparison also the corresponding sclerite of S. terminalis (figure 66) was shown.
Drawings of a stage 3 larva (all on p. 121) of S. dalii included an overview in dorsal view (figure 67; specimen 6426), prothorax sclerite (figure 68) and sclerites on abdomen segments 2 and 3 (figure 69). Further comparison was provided (on p. 122) including sclerites on thorax (figure 70) and abdomen segments 2 to 4 (figure 71) of a stage 3 larva of S. fuscata as well as the prothorax and abdomen segments 2 and 3 of a stage 3 larva of S. terminalis (figure 72).
According to the provided scales, specimen 6421 was 2 mm long, specimen 6422 6.0 mm, specimen 6423 2.2 mm, specimen 6424 6.2 mm, specimen 6425 2.0 mm, and specimen 6426 4.9 mm.
Some images were re-figured in Monserrat (2014).
20) Bowles (2008) provided a micrograph (figure 102 p. 3517) of Climacia chapini in dorsal view (specimen 6427). Furthermore, a life cycle (including a drawing of a larva) was depicted and stated to be based on Brown (1952) and Pupedis (1987). It appears that the depicted larva is a re-figure from Brown (1952); Pupedis (1987) deals with adults of Sisyridae; we suspect that the reference should be Pupedis (1980), where a cocoon of Climacia striata is shown (as in the life cycle by Bowles 2008). The paper by Pupedis (1980) is issue #87, which might have caused an error in the numbers.
21) Balian et al. (2008) provided a micrograph of a larva (specimen 6428) of Sisyridae in dorsal view (two times: figure 27 p. xi, figure 1B p. 410). No indication of size was provided.
22) Forteath and Osborn (2012) provided several images of larvae of Sisyra pedderensis. Images included micrographs of the trunk in ventral view of a late larva (plate 2 p. 28) and a stage 1 larva in lateral view (plate 4 p. 29), a drawing of a gill of a stage 2 larva (figure 5 p. 30), a SEM image of the trunk combined with a drawing of the gills (plate 5 p. 30). Yet, none of the images provided the necessary details that are required for our analysis, hence none of these could be considered further.
23) Hamada et al. (2014a) provided several images of stage 1 larvae of Sisyra panama. Images included a micrograph in lateral view (figure 5 p. 282) and drawings of the jumping behaviour of such a larva (figure 9 p. 282). This latter figure provides a dorsal view of the larva (specimen 6429), which can be used for our analysis (figure 9A p. 283). Comparison of the size to the specimen shown in lateral view suggests that the specimen was 0.5 mm long.
24) Hamada et al. (2014b) provided several images of larvae of Sisyridae. Images included a micrograph of a larva in lateral view (figure 3 p. 344) and drawings of a stage 3 larva (specimen 6430) in dorsal (figure 4A p. 345) and ventral view (figure 4B p. 345). No indication of size was provided.
In addition, trunk ends of the larvae of Climacia and Sisyra were shown for comparison (p. 347). Yet, no entire larva was depicted.
25) Monserrat (2014) re-figured (figure 3 p. 221) the drawing from Withycombe (1922), but cited Killington (1936). Also some details provided by Weißmair (1999) were re-figured (figure 7 p. 226).
26) Canard et al. (2015) provided a micrograph of a larva (specimen 6431) of Sisyra in dorsal view (figure 2 p. 19). No indication of size was provided.
27) Cover and Bogan (2015) re-figured (figure 41.4 p. 1064) the micrograph of specimen 6428, i.e., the specimen from Balian et al. (2008). No indication of size was provided.
28) Notteghem (2016) provided several photographs of larvae Sisyra nigra. Images included a larva (specimen 6432) in its natural habitat (photograph 1 p. 135), a larva (specimen 6433) in dorsal (photograph 2 p. 135) and ventral view (photograph 3 p. 135), as well as a specimen in lateral view (photograph 6 p. 137). No indication of size was provided.
29) Martins and Ardila-Camacho (2018) provided several images of larvae of Sisyridae. Micrographs of a first larva (specimen 6434) of Sisyra included a dorsal (figure 9.1A p. 230) and a lateral view (figure 9.1B p. 230). According to the provided scale, the larva was 3 mm long (without mouthparts and antennae).
Micrographs of a second larva (specimen 6435) of Climacia included a dorsal (figure 9.2A p. 230) and a lateral view (figure 9.12B p. 230). According to the provided scale the larva was 2.25 mm long (without mouthparts and antennae).
Additional details included a micrograph (figure 9.4A p. 232) and drawing (figure 9.4B p. 232) of a head, micrographs of the trunk in ventral view (figure 9.4C p. 232; figures 9.6, 9.7 p. 234), and a trunk appendage (figure 9.4D p. 232).
30) Jandausch et al. (2019) provided many micrographs and drawings of larvae of Sisyra nigra. Micrographs of a larva (specimen 6436) provide dorsal (figure 1a p. 3), ventral (figure 1b p. 3), and lateral view (figure 1c p. 3). According to the provided scale, the larva was 5.4 mm long.
Further details included 3D reconstructions of the inner anatomy in dorsal, lateral, and ventral view (figure 2 p. 4), SEM images of the head in dorsal, ventral, lateral, and frontal view (figure 3 p. 5), 3D-reconstructed inner details of the head (figure 4 p. 6), sections through the head (figure 5 p. 7), 3D-reconstructed muscles of the head (figure 6 p. 8, figure 7 p. 9), SEM images of the mouthparts (figure 8 p. 10), details of the anterior digestive tract (figure 9 p. 10), details of the posterior digestive tract (figure 10 p. 11), the tracheal and nervous system of the head (figure 11 p. 11), drawings of the thorax sclerites in dorsal and ventral view (figure 12a, b p. 12), a trunk appendage (figure 12c p. 12), the trunk in lateral view (figure 12d p. 12), and sections through the trunk (figure 13 p. 13).
31) Morales (2020) provided several micrographs of larvae of S. iridipennis. Images (all on p. 31) included a stage 2 larva (specimen 6437) in dorso-lateral (figure 1A) and dorsal view (figure 1B), details of its trunk appendage (figure 1C, D), a close-up on the anterior body (figure 1E) and a stage 1 larva in dorsal view (figure 1F). Yet, the image of the stage 1 larva does not provide sufficient details to be further considered. Size of specimen 6437 was provided as a magnification factor, which was not informative with the electronic version available to the authors.
32) Websites are generally not considered to be “proper” scientific sources. Yet, given the scarceness of data on larvae of Sisyridae, we decided to use them here as additional data source (see also above).
A micrograph of a larva (specimen 6438) was shown on http://www.aquatax.ca/minor.html. No indication of size was provided.
A micrograph of a larva (specimen 6439) labelled “Sisyrus sp.” was shown on the website of the Centre for Freshwater Ecosystems (the website is hosted by La Trobe University, Melbourne, Australia, available at https://www.mdfrc.org.au/bugguide/). No indication of size was provided.
A micrograph of a larva (specimen 6440) of the group Climacia was shown on Digital Key to Aquatic Insects of North Dakota (the website is hosted by Valley City State University, North Dakota, USA. Available at https://www.waterbugkey.vcsu.edu). No indication of size was provided.
A micrograph of a larva (specimen 6441) was shown on BugGuide (https://bugguide.net, see above). Image 201925 (© 2008 Jonas Insinga) was labelled “Spongilla fly larvae”. The photograph shows the specimen in dorsal view. Additional images of this specimen are available, but this one was most suitable for accessing details of the head. The size of the specimen has been stated to be about 1.5 mm.
Fossil Larvae in the Literature
So far, only very few fossil larvae of the groups Coniopterygidae and Sisyridae have been reported. Of the group Ithonidae, not a single fossil larva has been identified.
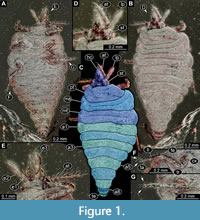 1) Weitschat and Wichard (1998; there is a later edition from 2002) provided a micrograph of a fossil larva (specimen 6301) of Coniopterygidae preserved in Baltic amber (plate 53g p. 145). The specimen was shown in dorsal view. Apparently, the image was mirrored as we reinvestigated the larva directly and re-figured it in this publication (Figure 1; it is from the collection Hoffeins, CCHH 540-1, as stated by Janzen 2002, see below).
1) Weitschat and Wichard (1998; there is a later edition from 2002) provided a micrograph of a fossil larva (specimen 6301) of Coniopterygidae preserved in Baltic amber (plate 53g p. 145). The specimen was shown in dorsal view. Apparently, the image was mirrored as we reinvestigated the larva directly and re-figured it in this publication (Figure 1; it is from the collection Hoffeins, CCHH 540-1, as stated by Janzen 2002, see below).
2) Janzen (2002) provided a drawing (figure 57 p. 60) of specimen 6301, i.e., the specimen of Weitschat and Wichard (1998, 2002). The specimen was shown in dorsal view. Length was stated to be 1.5 mm. The specimen was stated to be part of the collection Hoffeins.
3) Wichard et al. (2009) provided several images of a larva of Sisyridae preserved in Baltic amber (all on figure 07.07 p. 92) including a drawing in dorsal (a) and ventral view (b) as well as a micrograph in ventral view (c). The specimen (specimen 6501) was stated to be part of the collection Gröhn, number CCGG 1383.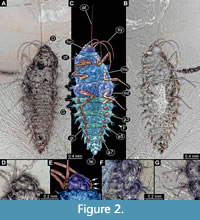 According to the provided scale, the larva was 3 mm in length (excluding mouthparts). The specimen is re-figured here (Figure 2).
According to the provided scale, the larva was 3 mm in length (excluding mouthparts). The specimen is re-figured here (Figure 2).
4) Gröhn (2015) provided a micrograph of a fossil larva of Coniopterygidae (specimen 6302) preserved in Baltic amber (figure 2703 p. 257). Length was stated to be 0.7 mm. The specimen was stated to be part of the collection Ludwig, acquired by the Museum of Natural History Mauritianum in Altenburg, Thurinigia.
5) Engel (2016) reported a specimen preserved in Myanmar amber that he suggested could represent a larva of Coniopterygyidae (figure 6 p. 11). The specimen deviated from the modern larvae of dustywings. The interpretation was supported by the presence of adult dustywings in close proximity in the same piece of amber (figure 5 p. 11). The difference in morphology was interpreted as retaining plesiomorphies.
The specimen was later interpreted as a possible larva of the group Berothidae (Pérez-de la Fuente et al., 2020; Haug et al., 2021c). Therefore, the specimen is no longer considered.
6) Haug et al. (2020b) described an unusual larva preserved in Myanmar amber, which was presented in dorsal view (figure 1 p. 6), ventral view (figure 2 p. 7), details of it under different microscopic settings (figure 3 p. 8), and as simplified restoration (figure 4 p. 13). The specimen had a combination of characters that made an interpretation in a phylogenetic frame very difficult. Some similarities were recognised with larvae of Coleoptera, Megaloptera, and Neuroptera, but some even with larvae of Mecoptera. Yet, the larva was interpreted as a representative of Neuropteriformia (= the larger group including Coleoptera, Strepsiptera, Raphidioptera, Megaloptera, and Neuroptera, see Vasilikopoulos et al., 2020 and references therein).
The mouthparts appear to form a single beak-like, forward-projecting cone, which led to the nickname “beak larva”. Together with the rather short and in dorsal view roughly triangular head, there is a slight resemblance to some larvae of Coniopterygidae. Therefore, we included the specimen into our comparison (specimen 6802).
7) Keupp (2020) reported a piece of Baltic amber with 11 tiny specimens (title figure p. 27, figure 6 lower left p. 30, figure 7 p. 31) that he interpreted as stage 1 larvae of Sisyridae. Yet, the morphology of these larvae differs in important aspects from all known larvae of Sisyridae.
First, and most prominent, stage 1 larvae usually have short, but well-apparent stylets. Such stylets are not apparent in the fossils. While in later stages stylets are flexible and can be bent, even then such stylets should be visible. It is not easy to explain when stylets are not visible in the larvae.
Second, the larvae have prominent palps. All known larvae of Sisyridae lack labial palps (and maxillary ones as all lacewing larvae).
Third, the leg elements are also very unusual. The distal leg elements (tibia and tarsus?) are quite thin and elongate. In all known larvae of Sisyridae, these elements are much shorter and more robust.
Fourth, the trunk end of the larvae is very conspicuous. It is at least three times as long as wide and widening distally. No comparable trunk ends are known from any extant larva of Sisyridae.
Additional Specimens of Fossil Larvae
1)  Specimen CCGG 7122 is a larva of Sisyridae (specimen 6502). The specimen is well accessible in dorsal (Figure 3A) and ventral view (Figure 3B, C). Only a few bubbles conceal some details of the appendages. The head has pronounced stemmata (Figure 3D, E). The ventral side provides many details as, for example, the folding pattern around the appendages (Figure 3C) and the well preserved gills (Figure 3F). The trunk end (Figure 3G) appears subdivided by distinct folds into six sub-units. While the trunk end is likely a conjoined structure of three segments (abdomen segments 9-11), the six sub-units probably do not correspond to segment borders.
Specimen CCGG 7122 is a larva of Sisyridae (specimen 6502). The specimen is well accessible in dorsal (Figure 3A) and ventral view (Figure 3B, C). Only a few bubbles conceal some details of the appendages. The head has pronounced stemmata (Figure 3D, E). The ventral side provides many details as, for example, the folding pattern around the appendages (Figure 3C) and the well preserved gills (Figure 3F). The trunk end (Figure 3G) appears subdivided by distinct folds into six sub-units. While the trunk end is likely a conjoined structure of three segments (abdomen segments 9-11), the six sub-units probably do not correspond to segment borders.
2) Specimen PED 0596 (specimen 6803) resembles specimen 6802 (the “beak larva”), i.e., the unusual larva from Haug et al. (2020b) in certain aspects.
 Small holometabolan larva, incomplete (Figure 4, Figure 5A). Body organised into head and trunk. Head small, triangular (Figure 5B) in dorsal view (presumably formed by six segments: ocular segment plus five post-ocular segments). Few setae apparent, at least two longer ones on each side, exact arrangement (chaetotaxy) not reconstructible. Anteriorly drawn out into beak-like structure, possibly formed by some of the mouthparts (some appendages of head segments). No clear indication of structures of the ocular segments (e.g., eyes) apparent. Labrum possibly contributing to beak-like protrusion. Antennae (appendages of post-ocular segment 1) shorter than beak, stout, exact subdivision unclear, possibly 3-4 elements. No external structures of post-ocular segment 2 (intercalary segment) apparent.
Small holometabolan larva, incomplete (Figure 4, Figure 5A). Body organised into head and trunk. Head small, triangular (Figure 5B) in dorsal view (presumably formed by six segments: ocular segment plus five post-ocular segments). Few setae apparent, at least two longer ones on each side, exact arrangement (chaetotaxy) not reconstructible. Anteriorly drawn out into beak-like structure, possibly formed by some of the mouthparts (some appendages of head segments). No clear indication of structures of the ocular segments (e.g., eyes) apparent. Labrum possibly contributing to beak-like protrusion. Antennae (appendages of post-ocular segment 1) shorter than beak, stout, exact subdivision unclear, possibly 3-4 elements. No external structures of post-ocular segment 2 (intercalary segment) apparent.
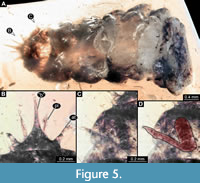 Additional mouthparts (appendages of post-ocular segments 3-5) not clearly identifiable. Parts of appendages of post-ocular segments 3 (mandible) and 4 (maxilla) possibly contributing to the beak. Distal parts presumably of appendages of post-ocular segment 5 (possibly palps of labium), slightly longer than antennae, but narrower; exact subdivision unclear, at least three elements.
Additional mouthparts (appendages of post-ocular segments 3-5) not clearly identifiable. Parts of appendages of post-ocular segments 3 (mandible) and 4 (maxilla) possibly contributing to the beak. Distal parts presumably of appendages of post-ocular segment 5 (possibly palps of labium), slightly longer than antennae, but narrower; exact subdivision unclear, at least three elements.
Trunk segment 1 (thorax segment 1, prothorax) mostly known from dorsal sclerite (pronotum). Pronotum large, without apparent subdivision into several sclerites, oval in dorsal view, longer than head width, twice as wide as head. At least six setae on each side. Very distal part of appendage of the segment protruding from under the tergite (Figure 5C, D), indicating rather short appendages in comparison to body width.
Trunk segment 2 (thorax segment 2, mesothorax) mostly known from dorsal sclerite (mesonotum). Mesonotum large, without apparent subdivision into several sclerites, oval in dorsal view, slightly longer than pronotum, also slightly wider. At least four setae apparent arising from the lateral side of the segment, below the tergite.
Trunk segment 3 (thorax segment 3, metathorax) less well accessible. Shorter than trunk segment 2, slightly wider.
Trunk segment 4 (abdomen segment 1) also not well accessible. Slightly shorter than preceding segment, about as wide.
Trunk segment 5 (abdomen segment 2) also not well accessible. About as long and wide as preceding segment. Further posterior segments not accessible.
3)  More specimens of larvae of Sisyridae seem to be present in Baltic amber. Images of five additional such specimens (specimens 6503-6507) were provided by Jonas Damzen and Marius Veta (Figure 6; for a discussion on the use of such images see Haug et al., 2020c, 2022). These specimens were not directly available for study, but the images are of high quality and clearly demonstrate that more such larvae are present in Baltic amber (see discussion in Haug et al., 2021d).
More specimens of larvae of Sisyridae seem to be present in Baltic amber. Images of five additional such specimens (specimens 6503-6507) were provided by Jonas Damzen and Marius Veta (Figure 6; for a discussion on the use of such images see Haug et al., 2020c, 2022). These specimens were not directly available for study, but the images are of high quality and clearly demonstrate that more such larvae are present in Baltic amber (see discussion in Haug et al., 2021d).
Results of the Shape Analysis
The shape analysis of the head capsule resulted in three effective principal components (PCs) (Appendix 2), summarizing to a total of 98.3% of overall variation in the data set. The first two principal components sum up to 95.6% of the overall variation of the data set. PC1 explains 92.0%, PC2 3.6%, and PC3 2.7% of the overall variation (Appendix 2).
PC1 is dominated by the overall shape of head and stylets (Appendix 3). Low values indicate a triangular head without visible stylets. High values indicate a simple head with very prominent stylets.
PC2 is dominated by the relative size of stylets and presence of a forward-projecting labrum (Appendix 3). Low values indicate rather prominent stylets and no projecting labrum, high values indicate smaller stylets and a more prominent projecting labrum.
PC3 is dominated by the position of the stylets (Appendix 3). Low values indicate stylets positioned further apart, high values indicate stylets positioned closer together.
The additional files resulting from the shape analysis are provided in Appendix 4.
DISCUSSION
Record of the Larvae of Ithonidae, Coniopterygidae, and Sisyridae
Compared to some other ingroups of Neuroptera, our knowledge of larvae of Ithonidae, Coniopterygidae, and Sisyridae is not that scarce. Presumably, the reason for that is that all these larvae are rather special in their appearance, which makes them quite easily identifiable in the field.
This recognisability should also account for the fossil record. Hence, we indeed need to ask why there are only very few fossil larvae of these groups:
Ithonidae. For Ithonidae, there are at least two factors to be considered. First, the modern larvae are rather derived, and we cannot be sure when this highly specialised morphology of the larvae has evolved. Differently expressed, it is possible that back in the Cretaceous (or even Eocene) larvae of Ithonidae still possessed many more plesiomorphic traits. A close relationship to, or even ingroup position in, Myrmeleontiformia (Badano et al., 2021) could mean that larvae in the early lineage of Ithonidae looked much more antlion-larva-like. Hence, there may have been no larvae similar to the modern ones yet. Adults of Ithonidae have already been found in the Cretaceous (Lu et al., 2017).
Second, if there would have already been modern-appearing larvae of Ithonidae, these would be less likely to be captured in amber due to their subterranean mode of life. A similar rarity has been recognised for the likewise deeper-burrowing larvae of spoon-winged lacewings, Nemopterinae (Haug et al., 2021b). Also the digging larvae of antlions (Myrmeleontidae) are quite rare in most Cretaceous ambers (Badano et al., 2018; Pérez-de la Fuente et al., 2020) and so far unknown for Eocene ambers. Interestingly, they seem more common in Miocene Dominican amber (Engel and Grimaldi, 2007; Haug et al., 2021d).
Coniopterygidae. Larvae of dustywings are quite small, and one could argue that they are easily overlooked. Yet, they are quite conspicuous in their overall morphology and not smaller than many mites, which can be regularly found in ambers of all different periods. Their rarity is, therefore, unlikely to be a simple effect of them being overlooked. Also, dustywings are well known as adults (e.g., Engel, 2016), hence we should expect to find larvae of these.
Here again, a similar aspect as in Ithonidae applies. Modern dustywing larvae are likely to be highly specialised and derived in their morphology. Larvae of dustywings in the Cretaceous, where such larvae are so far not known, might have still looked quite differently, retaining more plesiomorphies. This might have led Engel (2016) to assume that the larva later interpreted as a representative of Berothidae (Pérez-de la Fuente et al., 2020) could be a representative of Coniopterygidae. While the two lineages are not closely related, both are characterised by straight, not very long stylets. Also some larvae of Berothidae have a forward-projecting labrum (e.g., MacLeod, 1964, plate XII figure 36), although not as pronounced as in modern larvae of Coniopterygidae. The larvae of Cretaceous dustywings may still be mistaken for those of other lineages, potentially Berothidae. The question whether the unusual beak larvae (specimens 6802 + 6803; Figure 4, Figure 5; Haug et al., 2020b) could be dustywings will be elaborated further below.
Sisyridae. Larvae of Sisyridae seem to be in fact not that rare in Baltic amber, despite their aquatic lifestyle. This lifestyle can in fact also be assumed for the fossils based on the presence of distinct gill structures in at least some of the specimens (Figure 2, Figure 3). Again, the absence of such larvae in Cretaceous ambers may be due to the fact that larvae of early representatives of Sisyridae still had less specialised larvae. Potentially they may have resembled the larvae of lance lacewings (Osmylidae) (see recent review in Haug et al., 2021c).
Despite the more common occurrence in Baltic amber, it is remarkable that all seven known specimens have the morphology of stage 2 or stage 3 larvae. A stage 1 larva has not been identified so far. Of other aquatic (or possibly semi-aquatic) lacewing larvae, different stages including stage 1 larvae are known, at least two for Osmylidae (Wichard et al., 2009; Haug et al., 2021c) and quite a larger number for Nevrorthidae (Wichard et al., 2009; Haug et al., 2020d).
Quantitative Comparison
 As to be expected from a qualitative view, the three groups investigated here separate quite strictly from each other (Figure 7). Only some larvae of Coniopterygidae with quite prominent mandibles plot closer to those of Ithonidae and Sisyridae. One of these is a historical specimen that seems likely to have been misinterpreted to a certain degree (specimen 6203). Yet, in the other specimens the morphology seems to be original. It could be argued that these larvae are likely representing a more plesiomorphic (ancestral) type of morphology and that the other dustywing larvae with shorter mandibles and a prominent labrum represent a more derived morphology characterising a deeper ingroup of Coniopterygidae.
As to be expected from a qualitative view, the three groups investigated here separate quite strictly from each other (Figure 7). Only some larvae of Coniopterygidae with quite prominent mandibles plot closer to those of Ithonidae and Sisyridae. One of these is a historical specimen that seems likely to have been misinterpreted to a certain degree (specimen 6203). Yet, in the other specimens the morphology seems to be original. It could be argued that these larvae are likely representing a more plesiomorphic (ancestral) type of morphology and that the other dustywing larvae with shorter mandibles and a prominent labrum represent a more derived morphology characterising a deeper ingroup of Coniopterygidae.
The two unambiguous fossil representatives of Coniopterygidae plot among the derived representatives of Coniopterygidae. Likewise, the fossil representatives of Sisyridae plot well among the extant ones (only slightly outside the area occupied by the modern ones). This is quite different from the situation in other lacewing lineages in which fossil representatives often plot significantly outside the areas occupied by their modern representatives, indicating a certain loss of morphological diversity over time (Haug et al., 2020c, 2021a, 2022). Yet, given the rather low number of fossil specimens, this result might be heavily biased.
An important point to mention is that the two beak larvae plot well among the derived specimens of Coniopterygidae (Figure 7). This clearly indicates a strong similarity of overall head shape of dustywing larvae and the beak larvae also from a quantitative side.
The Beak Larvae
The new beak larva (PED 0596, specimen 6803) as well as the original beak larva (specimen 6802) (Haug et al., 2020b) are quite unusual. The original beak larva and the new beak larva not only appear roughly similar from a qualitative point of view, but also plot closer together in the quantitative analysis, among the more derived forms of Coniopterygidae.
Yet, from a qualitative point of view, there are also notable differences. The original beak larva has a complex sclerite system on the dorsal and ventral body side, which is unparalleled among lacewing larvae, but reminds more of that of beetle larvae (Haug et al., 2020b). The new beak larva does not clearly show such a pattern of distinct sclerites.
Even more obvious are the long processes on the posterior trunk region in the original beak larva. These are clearly absent in the new beak larva, but also in dustywing larvae. Processes, mostly for camouflaging purposes, have been reported in various lineages of extant and fossil lacewing larvae (Pérez-de la Fuente et al., 2012, 2016, 2018, 2019; Wang et al., 2016; Badano et al., 2018; Liu et al., 2022). Still, these processes are very different from those of the original beak larvae, which have at least some characteristics of gills (Haug et al., 2020b).
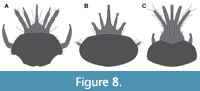 Antennae and palps of the beak larvae differ in their structure but are basically similar in number of elements. They seem to differ in the number of elements from dustywing larvae, which have fewer elements (Figure 8). All of these structures are similarly rather short, although not in all larvae of dustywings (e.g., Dujardin, 1851, figure 15; Stitz, 1931, figure 36 p. 35.99).
Antennae and palps of the beak larvae differ in their structure but are basically similar in number of elements. They seem to differ in the number of elements from dustywing larvae, which have fewer elements (Figure 8). All of these structures are similarly rather short, although not in all larvae of dustywings (e.g., Dujardin, 1851, figure 15; Stitz, 1931, figure 36 p. 35.99).
The main challenge for substantiating an interpretation of the beak larvae as dustywings or dustywing relatives is the structure of the mouthparts. Too few details are accessible in both fossils.
For the new beak larva, an interpretation as a derived type of a dustywing larva is more plausible than for the original beak larva. In any case, we have to assume a certain degree of convergence: if the new beak larva is a dustywing, but the original one is not, the similarities between the two larvae would be a result of convergence. If both are not dustywings, their similarities to dustywing larvae would be a result of convergence. If both are dustywings, the similarities of the original beak larva to beetles and certain other lacewing lineages would be a result of convergence (see also discussion in Haug et al., 2020b). At least, Coniopterygidae is (another) candidate group for interpreting these unusual larvae.
Life Style of the Fossil Larvae
As elaborated above, it is possible that the larvae of early representatives of the three lineages might still be quite different, more plesiomorphic morphologies and therefore also different ecological roles. Larvae of modern-day dustywings are predators, as is the case with most other lacewing larvae. Their specialisation is mostly manifested in the specific morphology of their upper lip, which is also known in some other lineages of Neuroptera (Haug et al., 2020c), as well as straight stylets are likewise known in some other lineages (Haug et al., 2021c).
The comparison of the beak larvae to dustywing larvae offers a new interpretation for their lifestyle. They might indeed be predators of small-sized prey rather than feeding on fungi (as discussed in Haug et al., 2020b). Still, the original beak larva might have actually been aquatic, while the new one has no indications and was most likely terrestrial.
The exact life style of larvae of Ithonidae is still not entirely clear, yet it has been assumed that they feed on plant roots (Grebennikov, 2004). As we have no fossil larvae of this group, it remains unclear when the switch from a predatory lifestyle to root-feeding might have occurred.
Larvae of Sisyridae are known to feed on sponges, hence their common name “spongilla flies” (yet the expression “sponge lacewing” would be less ambiguous). Larvae of Sisyridae have been addressed as “parasite” by MacLeod (1964 p. 217). The view has been shared by subsequent authors (Jandausch et al., 2019; Morales, 2020).
Here a conceptual ambiguity needs to be discussed. Animals with comparable lifestyles are often referred to as “micro-predators” in order to distinguish them from a parasite, emphasising that a parasite would be in longer (permanent?) contact to a host, while a micro-predator would interact only shortly, with its host (or prey?) (see discussions in van der Wal and Haug, 2019; Haug et al., 2021e).
Yet, this distinction restricts parasitism to a very narrow type of lifestyle and leads to misunderstandings in communication of lifestyles. Instead, it is often possible to make a precise add-on to the term parasite to allow proper distinction; in other words, it is necessary to distinguish between a “temporary parasite” and a “permanent parasite”. Larvae of sponge lacewings can be described as “temporary parasites” comparable to mosquitoes or ticks (see discussions in van der Wal and Haug, 2019; Haug et al., 2021e).
The morphology of the larvae of Sisyridae from Baltic amber indicates that they already were temporary parasites. It is possible that larvae in the Cretaceous were still predatory, similar to larvae of Nevrorthidae or certain larvae of Osmylidae, and therefore did not yet possess the highly specialised morphology of modern-day and Eocene larvae.
ACKNOWLEDGEMENTS
We thank two anonymous reviewers for helpful comments on the manuscript and the editorial team of PE for the swift handling. J. Damzen (amberinclusions.eu) and M. Veta (ambertreasure4u.com), both Vilnius, kindly provided images for our analysis. JTH received support from the German Research Foundation (DFG Ha 6300/6-1) and the Volkswagen Foundation (Lichtenberg Professorship). This work was partly funded by the Deutscher Akademischer Austauschdienst (DAAD; Research Grants-Doctoral Programmes in Germany, Reference no. 91693832, SvdW). We are grateful to J. Matthias Starck, Munich, for long-time support. We thank all people providing low-cost, open-access or open-source software. This is LEON publication #37.
REFERENCES
AquaTax Consulting 2022. AquaTax. Accessed 24 January 2022. http://www.aquatax.ca/minor.html
Arrow, G.J. 1917. The life-history of Conwentzia psociformis Curt. Entomologist’s Monthly Magazine, 53:254-257.
Aspöck, H. and Aspöck, U. 1964. Synopsis der Systematik, Ökologie und Biogeographie der Neuropteren Mitteleuropas im Spiegel der Neuropteren-Fauna von Linz und Oberösterreich, sowie Bestimmungsschlüssel für die mitteleuropäischen Neuropteren und Beschreibung von Coniopteryx lentiae nov. spec. Naturkundliches Jahrbuch der Stadt Linz, 1964:127-282.
Aspöck, U. 2019. Towards a homologisation of male genital sclerites in Coniopterygidae (Neuroptera) - a tightrope dance, p. 79-93. In Weihrauch, F., Frank, O., Gruppe, A., Jepson, J.E., Kirschey, L., and Ohl, M. (eds.), Proceedings of the XIII International Symposium of Neuropterology, 17-22 June 2018, Laufen, Germany. Osmylus Scientific Publishers, Wolnzach.
Aspöck, U. and Aspöck, H. 1999. Kamelhälse, Schlammfliegen, Ameisenlöwen. Wer sind sie? (Insecta: Neuropterida: Raphidioptera, Megaloptera, Neuroptera). Stapfia, 60:1-34.
Aspöck U. and Aspöck H. 2007. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia, 20:451-516.
Aspöck, U. and Aspöck, H. 2009. Raphidioptera (Snakeflies), p. 864-866. In Resh, V.H. and Cardé, R.T. (eds.), Encyclopedia of Insects, 2nd ed. Academic Press, Cambridge, Massachusetts. https://doi.org/10.1016/B978-0-12-374144-8.00226-5
Badano, D., Engel, M.S., Basso, A., Wang, B., and Cerretti, P. 2018. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nature Communications, 9:3257. https://doi.org/10.1038/s41467-018-05484-y
Badano, D., Fratini, M., Maugeri, L., Palermo, F., Pieroni, N., Cedola, A., Haug, J.T., Weiterschan, T., Velten, J., Mei, M., Di Giulio, A., and Cerretti, P. 2021. X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Systematic Entomology, 46:672-684. https://doi.org/10.1111/syen.12482
Badgley, M.E., Fleschner, C.A., and Hall, J.C. 1955. The biology of Spiloconis picticornis Banks (Neuroptera: Coniopterygidae). Psyche, 62:75-81. https://doi.org/10.1155/1955/16126
Balian, E.V., Lévéque, C., Segers, H., and Martens, K. 2008. The freshwater animal diversity assessment. Developments in Hydrobiology, 198 (Hydrobiologia, 595):1-637.
Beutel, R.G., Friedrich, F., Yang, X.-K., and Ge, S.-Q. 2013. Insect Morphology and Phylogeny: A Textbook for Students of Entomology. De Gruyter, Berlin, Boston. https://doi.org/10.1515/9783110264043
Bowles, D.E. 2008. Spongillaflies (Neuroptera: Sisyridae), p. 3516-3518. In Capinera, J.L. (ed.), Encyclopedia of Entomology. Springer, Dordrecht.
Braig, F., Haug, J.T., Schädel, M., and Haug, C. 2019. A new thylacocephalan crustacean from the Upper Jurassic lithographic limestones of Southern Germany and the diversity of Thylacocephala. Palaeodiversity, 12:69-87. https://doi.org/10.18476/pale.v12.a6
Brown, H.P. 1952. The life history of Climacia areolaris (Hagen), a neuropterous 'parasite' of freshwater sponges. American Midland Naturalist, 47:130-160. https://doi.org/10.2307/2421701
BugGuide Contributors 2022. BugGuide. Accessed January 24, 2022. https://bugguide.net
Canard, M., Thierry, D., Cloupeau, R., Rausch, H., and Weißmair, W. 2015. A spongillafly new to the French fauna: Sisyra bureschi Rausch and Weißmair, 2007 (Neuropterida, Sisyridae). Bulletin de la Société entomologique de France, 120:19-24. https://doi.org/10.3406/bsef.2015.2199
Centre for Freshwater Ecosystems Editorial Board 2022. La Trobe University, Melbourne, Australia. Accessed January 24, 2022. https://www.mdfrc.org.au/bugguide/display.asp?class=17&subclass=&order=11&Couplet=0&Type=3
Collyer, E. 1951. The separation of Conwentzia pineticola End. from Conwentzia psociformis (Curt.), and notes on their biology. Bulletin of Entomological Research, 42:555-564. https://doi.org/10.1017/s0007485300028959
Cover, M.R. and Bogan, M.T. 2015. Minor insect orders, p. 1059-1072. In Thorp, J.H. and Rogers, D.C. (eds.), Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates. Academic Press, Cambridge.
Crampton, G.C. 1921. The sclerites of the head and the mouthparts of certain immature and adult insects. Annals of the Entomological Society of America, 14:65-103 + plates. https://doi.org/10.1093/aesa/14.2.65
Cruickshank, R.D. and Ko, K. 2003. Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences, 21:441-455. https://doi.org/10.1016/s1367-9120(02)00044-5
Curtis, J. 1834. British Entomology; Being Illustrations and Descriptions of the Genera of Insects Found in Great Britain and Ireland: Containing Coloured Figures from Nature of the Most Rare and Beautiful Species, and in Many Instances of the Plants upon which They Are Found. Vol. 4. Ellis and Co., London.
Digital Key to Aquatic Insects of North Dakota. 2022. Valley City State University. Accessed January 24, 2022. https://www.waterbugkey.vcsu.edu/php/orderdetails.php?idnum=12
Dorohova, G.I. 1987. Chapter 25 Neuroptera, p. 36-96. In Keys to the Insects of the European Part of the USSR Volume 4 (Part 6) Megaloptera, Raphidioptera, Neuroptera, Mecoptera and Trichoptera. Nauka, St. Petersburg.
Dujardin, F. 1851. Sur une larve qui paraît être celle de l' Hemerobius hirtus. Annales des Sciences Naturelles, Zoologie et Biologie Animale, 15:169-173.
Emerton, J.H. 1906. Cocoons and young of Coniopteryx vicina. Psyche, 13:74-75. https://doi.org/10.1155/1906/624860
Engel, M.S. 2016. Two new genera of Cretaceous dustywings in amber from northern Myanmar (Neuroptera: Coniopterygidae). Novitates Paleoentomologicae, 17:1-16. https://doi.org/10.17161/np.v0i17.6465
Engel, M.S. and Grimaldi, D.A. 2007. The neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). American Museum Novitates, 3587:1-58.
Engel, M.S., Winterton, S.L., and Breitkreuz, L.C.V. 2018. Phylogeny and evolution of Neuropterida: where have wings of lace taken us? Annual Review of Entomology, 63:531-551. https://doi.org/10.1146/annurev-ento-020117-043127
Forteath, G.N.R. and Osborn, A.W. 2012. Biology, ecology and voltinism of the Australian spongillafly Sisyra pedderensis Smithers (Neuroptera: Sisyridae). Papers and Proceedings of the Royal Society of Tasmania, 146:25-35. https://doi.org/10.26749/rstpp.146.25
Genay, A. 1953. Contribution à l'étude des Névroptères de Bourgogne. Travaux du Laboratoire de Zoologie et de la Station aquicole Grimaldi de la Faculté des Sciences de Dijon, 1953(3):1-30.
Gepp, J. 1984. Erforschungsstand der Neuropteren-Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten), p. 183-239. In Gepp, J., Aspöck, H., and Hölzel, H. (eds.), Progress in World’s Neuropterology. Proceedings of the 1st International Symposium on Neuropterology (22-26 September 1980, Graz, Austria). Privately printed, Graz, Austria.
Gepp, J. 1999. Neuropteren als Indikatoren der Naturraumbewertung. Eignung als Modellgruppe, Methodenwahl, Fallbeispiele sowie Diskussion möglicher Fragestellungen (Neuropterida). Stapfia, 138:167-208.
Ghilarov, M.S. 1962. The larva of Dilar turcicus Hag. and the position of the family Dilaridae in the order Planipennia. Entomological Review, 41:402-416. [In Russian, original = Гиляров М.С., 1962. Личинка Dilar turcicus Hag. и положение семейства Dilaridae в отряде сетчатокрылых (Planipennia). Энтомологическое Обозрение,41:402-416]
Grebennikov, V.V. 2004. Grub-like larvae of Neuroptera (Insecta): a morphological review of the families Ithonidae and Polystoechotidae and a description of Oliarces clara. European Journal of Entomology, 101:409-418. https://doi.org/10.14411/eje.2004.056
Greve, L. 1974. The larvae and pupa of Helicoconis lutea (Wallengren, 1871) (Neuroptera, Coniopterygidae). Norsk Entomologisk Tidsskrift, 21:19-23.
Grimaldi, D. and Engel, M.S. 2005. Evolution of the Insects. Cambridge University Press, New York.
Gröhn, C. 2015. Einschlüsse im Baltischen Bernstein. Wachholz Verlag - Murmann Publishers, Kiel, Hamburg.
Hamada, N., Pes, A.M.O., and Fusari, L.M. 2014a. First record of Sisyridae (Neuroptera) in Rio de Janeiro state, Brazil, with bionomic notes on Sisyra panama. Florida Entomologist, 97:281-284. https://doi.org/10.1653/024.097.0140
Hamada, N., Oliveira Pes, A.M., and Boldrini, R. 2014b. Ordem Neuroptera (neuron = veias; pteron = asas) Família Sisyridae, p. 343-348. In Hamada, N., Nessimian, J.L., and Querino, R.B. (eds.), Insetos Aquáticos na Amazônia Brasileira: Taxonomia, Biologia e Ecologia. INPA, Manaus.
Haug, C., Shannon K.R., Nyborg T., and Vega. F.J. 2013b. Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda. Bolétin de la Sociedad Geológica Mexicana, 65:273-284.
Haug, C., Herrera-Flórez, A.F., Müller, P., and Haug, J.T. 2019a. Cretaceous chimera-an unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution. Palaeodiversity, 12:1-11. https://doi.org/10.18476/pale.v12.a1
Haug, C., Haug, G.T., Baranov, V.A., Solórzano-Kraemer, M.M., and Haug, J.T. 2021d. An owlfly larva preserved in Mexican amber and the Miocene record of lacewing larvae. Boletín de la Sociedad Geológica Mexicana, 73:A271220. https://doi.org/10.18268/BSGM2021v73n3a271220
Haug, G.T., Haug, C., Pazinato, P.G., Braig, F., Perrichot, V., Gröhn, C., Müller, P., and Haug, J.T. 2020c. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontologia Electronica, 23(2):a39. https://doi.org/10.26879/1029
Haug, G.T., Baranov, V., Wizen, G., Pazinato, P.G., Müller, P., Haug, C., and Haug, J.T. 2021a. The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bulletin of Geosciences, 96:431-457. https://doi.org/10.3140/bull.geosci.1807
Haug, G.T., Haug, C., van der Wal, S., Müller, P., and Haug, J.T. 2022. Split-footed lacewings declined over time: indications from the morphological diversity of their antlion-like larvae. PalZ, 96:29-50. https://doi.org/10.1007%2Fs12542-021-00550-1
Haug, J.T. and Haug, C. 2019. Beetle larvae with unusually large terminal ends and a fossil that beats them all (Scraptiidae, Coleoptera). PeerJ, 7:e7871. https://doi.org/10.7717/peerj.7871
Haug, J.T., Haug, C., and Ehrlich, M. 2008. First fossil stomatopod larva (Arthropoda: Crustacea) and a new way of documenting Solnhofen fossils (Upper Jurassic, Southern Germany). Palaeodiversity, 1:103-109.
Haug, J.T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E.N.K., and Waloszek, D. 2011. Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy, 244:259-272.
https://doi.org/10.1111/j.1365-2818.2011.03534.x
Haug, J.T., Müller, C.H.G., and Sombke, A. 2013a. A centipede nymph in Baltic amber and a new approach to document amber fossils. Organisms Diversity and Evolution, 13:425-432. https://doi.org/10.1007/s13127-013-0129-3
Haug, J.T., Müller, P., and Haug, C. 2018. The ride of the parasite: a 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters, 4:31. https://doi.org/10.1186/s40851-018-0116-9
Haug, J.T., Müller, P., and Haug, C. 2019b. A 100-million-year old predator: a fossil neuropteran larva with unusually elongated mouthparts. Zoological Letters, 5:29. https://doi.org/10.1186/s40851-019-0144-0
Haug, J.T., Müller, P., and Haug, C. 2019c. A 100-million-year old slim insectan predator with massive venom-injecting stylets - a new type of neuropteran larva from Burmese amber. Bulletin of Geosciences, 94:431-440. https://doi.org/10.3140/bull.geosci.1753
Haug, J.T., Pazinato, P.G., Haug, G.T., and Haug, C. 2020a. Yet another unusual new type of lacewing larva preserved in 100-million-year old amber from Myanmar. Rivista Italiana di Paleontologia e Stratigrafia, 126:821-832. https://doi.org/10.13130/2039-4942/14439
Haug, J.T., Schädel, M., Baranov, V.A., and Haug, C. 2020b. An unusual 100-million-year old holometabolan larva with a piercing mouth cone. PeerJ, 8:e8661. https://doi.org/10.7717/peerj.8661
Haug, J.T., Baranov, V., Schädel, M., Müller, P., Gröhn, C., and Haug, C. 2020d. Challenges for understanding lacewings: how to deal with the incomplete data from extant and fossil larvae of Nevrorthidae? (Neuroptera). Fragmenta entomologica, 52:137-168.
https://doi.org/10.13133/2284-4880/472
Haug, J.T., Haug, G.T., Zippel, A., van der Wal, S., Müller, P., Gröhn, C., Wunderlich, J., Hoffeins, C., Hoffeins, H.-W., and Haug, C. 2021c. Changes in the morphological diversity of larvae of lance lacewings, mantis lacewings and their closer relatives over 100 million years. Insects, 12:860. https://doi.org/10.3390/ insects12100860
Haug, J.T., Haug, C., and Nagler, C. 2021e. Evolutionary history of crustaceans as parasites, p. 347-376. In De Baets, K. and Huntley, J.W. (eds), The Evolution and Fossil Record of Parasitism. Topics in Geobiology, 49. Springer, Cham.
Heckman, C.W. 2017. Polystoechotidae, p. 425-427. In Heckman, C.W. (ed.), Neuroptera (including Megaloptera). Springer, Cham.
Jacobs, W. 1998. Biologie und Ökologie der Insekten: Ein Taschenlexikon/begr. von Jacobs, W. und Renner, M., 3. Aufl. überarb. von Honomichl, K. Fischer, Stuttgart, Germany.
Jandausch, K., Beutel, R.G., and Bellstedt, R. 2019. The larval morphology of the spongefly Sisyra nigra (Retzius, 1783) (Neuroptera: Sisyridae). Journal of Morphology, 280:1742-1758. https://doi.org/10.1002/jmor.21060
Janzen, J.W. 2002. Arthropods in Baltic Amber. Ampyx Verlag, Halle.
Keupp, H. 2020. Schwammfliegen-Larven im Baltischen Bernstein. Fossilien, 37:27-31.
Killington, F.J. 1936. Monograph of the British Neuroptera. Ray Society, London.
Kimmins, D.E. 1962. Keys to the British species of aquatic Megaloptera and Neuroptera. Freshwater Biological Association, Scientific Publication, 8:1-23.
Labandeira, C.C., Yang, Q., Santiago-Blay, J.A., Hotton, C.L., Monteiro, A., Wang, Y.J., Goreva, Y., Shih, C., Siljeström, S., Rose, T.R., Dilcher, D.L., and Ren, D. 2016. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proceedings of the Royal Society B: Biological Sciences, 283:20152893. https://doi.org/10.1098/rspb.2015.2893
Lestage, J.A. 1921. Sous famille II. Sisyrinae, p. 337-342. In Rousseau, E. (ed.), Les Larves et Nymphes Aquatiques des Insects d’Europe. J. Lebègue and Co. 1, Bruxelles.
Liu, H., Luo, C., Jarzembowski, E.A., and Xiao, C. 2022. Acanthochrysa langae gen. et sp. nov., a new lacewing larva (Neuroptera: Chrysopoidea) from mid-Cretaceous Kachin amber. Cretaceous Research, 133:105146. https://doi.org/10.1016/j.cretres.2022.105146
Liu, Q., Lu, X., Zhang, Q., Chen, J., Zheng, X., Zhang, W., Liu, X., and Wang, B. 2018a. High niche diversity in Mesozoic pollinating lacewings. Nature Communications, 9:3793. https://doi.org/10.1038/s41467-018-06120-5
Liu, X., Zhang, W., Winterton, S.L., Breitkreuz, L.C., and Engel, M.S. 2016. Early morphological specialization for insect-spider associations in Mesozoic lacewings. Current Biology, 26:1590-1594. https://doi.org/10.1016/j.cub.2016.04.039
Liu, X., Shi, G., Xia, F., Lu, X., Wang, B., and Engel, M.S. 2018b. Liverwort mimesis in a Cretaceous lacewing larva. Current Biology, 28:1475-1481.
https://doi.org/10.1016/j.cub.2018.03.060
Löw, F. 1885. Beitrag zur Kenntniss der Coniopterygiden. Sitzungsberichte der Akademie der Wissenschaften in Wien, Mathematische-Naturwissenschaftliche Klasse (Abtheilung I), 91:73-89.
Lu, X., Zhang, W., Ohl, M., and Liu, X. 2017. The first moth lacewing (Insecta: Neuroptera: Ithonidae) from the mid-Cretaceous amber of Myanmar. Cretaceous Research, 78:78-83. https://doi.org/10.1016/j.cretres.2017.05.027
MacLeod, E.G. 1964. A comparative morphological study of the head capsule and cervix of larval Neuroptera (Insecta). Ph.D. dissertation, Harvard University, Cambridge, Massachusetts, USA.
Makarkin, V.N. 1995. Notes on Palearctic Hemerobiidae (Neuroptera). 1. Introduction and genus Wesmaelius Kruger, 1922. Part 1. Subgenus Wesmaelius. Far Eastern Entomologist, 24:1-13.
Makarkin, V.N. 2018. Re-description of Grammapsychops lebedevi Martynova, 1954 (Neuroptera: Psychopsidae) with notes on the Late Cretaceous psychopsoids. Zootaxa, 4524:581-594. https://doi.org/10.11646/ZOOTAXA.4524.5.5
Martins, C.C. and Ardila-Camacho, A. 2018. Order Neuroptera, p. 229-236. In Thorp, J.H. and Rogers, D.C. (eds.), Thorp and Covich’s Freshwater Invertebrates. Academic Press, Cambridge.
Meinander, M. 1974. The larvae of two North American species of Coniopterygidae (Neuroptera). Notulae Entomologicae, 54:12-16.
Monserrat, V.J. 2014. Los sisíridos de la Península Ibérica (Insecta: Neuropterida: Neuroptera: Sisyridae). Heteropterus Revista de Entomología, 14:215-239.
Monserrat, V.J., Díaz-Aranda, L.M., and Hölzel, H. 1990. Nuevos datos sobre los coniopterígidos ibéricos (Neuroptera: Coniopterygidae). Neuroptera International, 6:39-49.
Morales, J. 2020. Notas ecológicas de esponjas dulceacuícolas parasitadas por larvas de Sisyra iridipennis Costa, 1884 (Neuroptera: Sisyridae) en el río Águeda (Salamanca, España). Nova, 27:29-34.
Muma, M.H. 1967. Biological notes on Coniopteryx vicina (Neuroptera: Coniopterygidae). The Florida Entomologist, 50:285-293. https://doi.org/10.2307/3493158
Needham, J.G. and Betten, C. 1901. Aquatic insects in the Adirondacks: a study conducted at the entomologic field station, Saranac Inn NY. Bulletin of the New York State Museum, 68:383-612 + 36 plates.
New, T.R. 1989. Planipennia: Lacewings. Handbuch der Zoologie, Vol. 4, Part 30. Walter de Gruyter, Berlin.
Notteghem, P. 2016. La Sisyre noire (Sisyra nigra), Névroptère autochtone, parasite de la Pectinatelle (Pectinatella magnifica), Bryozoaire allochtone. Revue scientifique Bourgogne-Nature, 23:133-140.
Parfin, S.I. and Gurney, A.B. 1956. The spongilla-flies, with special reference to those of the western hemisphere (Sisyridae, Neuroptera). Proceedings of the United States National Museum, 105:421-529 + 3 plates. https://doi.org/10.5479/si.00963801.105-3360.421
Pérez-de la Fuente, R., Delclòs, X., Peñalver, E., Speranza, M., Wierzchos, J., Ascaso, C., and Engel, M.S. 2012. Early evolution and ecology of camouflage in insects. Proceedings of the National Academy of Sciences, 109:21414-21419. https://doi.org/10.1073/pnas.1213775110
Pérez-de la Fuente, R., Delclòs, X., Peñalver, E., and Engel, M.S. 2016. A defensive behavior and plant-insect interaction in Early Cretaceous amber-the case of the immature lacewing Hallucinochrysa diogenesi. Arthropod Structure and Development, 45:133-139. https://doi.org/10.1016/j.asd.2015.08.002
Pérez-de la Fuente, R., Peñalver, E., Azar, D., and Engel, M.S. 2018. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Scientific Reports, 8:16663. https://doi.org/10.1038/s41598-018-34870-1
Pérez-de la Fuente, R., Delclòs, X., Engel, M.S., and Peñalver, E. 2019. A new dustywing (Neuroptera: Coniopterygidae) from the Early Cretaceous amber of Spain. Palaeoentomology, 2:279-288. https://doi.org/10.11646/palaeoentomology.2.3.13
Pérez-de la Fuente, R., Engel, M.S., Delclòs, X., and Peñalver, E. 2020. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretaceous Research, 106:104200. https://doi.org/10.1016/j.cretres.2019.104200
Peterson, A. 1951. Larvae of Insects. An Introduction to Nearctic Species. Part II. Coleoptera, Diptera, Neuroptera, Siphonaptera, Mecoptera, Trichoptera. Edward Brothers, Columbus.
Pupedis, R.J. 1980. Generic differences among the New World spongilla-fly larvae and a description of the female of Climacia striata (Neuroptera: Sisyridae). Psyche, 87:305-314. https://doi.org/10.1155/1980/65827
Pupedis, R.J. 1986. Hatching behavior of sisyrid larvae (Neuroptera, Sisyridae). Neuroptera International, 4:53-55.
Pupedis, R.J. 1987. Foraging behavior and food of adult spongila-flies (Neuroptera: Sisyridae). Annals of the Entomological Society of America, 80:758-760. https://doi.org/10.1093/aesa/80.6.758
Riek, E.F. 1970. Neuroptera (Lacewings), p. 472-494. In Waterhouse, D.F. (ed.), The Insects of Australia. Melbourne University Press, Melbourne.
Rousset, A. 1956a. Sur l'anatomie céphalique des larves de Coniopterygidae (Névroptères Planipennes). Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 242:934-936.
Rousset, A. 1956b. Sur l'anatomie céphalique des larves de Coniopterygidae (Névroptères Planipennes). Les stylets et leur musculature. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 243:869-872.
Rousset, A. 1958. Sur le système nerveux central céphalique de la larve de Coniopteryx (Névropt. Planipenne). Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 246:842-845.
Rousset A. 1966. Morphologie céphalique des larves de Planipennes (Insectes Névroptèroïdes). Mémoires du Muséum national d'Histoire naturelle, Séries A - Zoologie, 42:1-199.
Scholtz, G. 2005. Homology and ontogeny: pattern and process in comparative developmental biology. Theory in Biosciences, 124:121-143. https://doi.org/10.1007/BF02814480
Shi, G., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M., Lei, W., Li, Q., and Li, X. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research, 37:155-163. https://doi.org/10.1016/j.cretres.2012.03.014
Stitz, H. 1931. Planipennia, p. 67-304. In Schulze, P. (ed.), Biologie der Tiere Deutschlands, vol. 35. Gebrüder Borntraeger, Berlin.
Stürzer, C. and Gepp, J. 2004. Beiträge zur Larvalbiologie und -morphologie von Conwentzia pineticola Enderlein, 1905 und C. psociformis (Curtis, 1834) (Neuroptera: Coniopterygidae). Entomologica Austriaca, 11:7-10.
Sziráki, G. and Flint, O.S., Jr. 2005. Larva of Brucheiser penai Riek, 1975 (Neuroptera Coniopterygidae). In Pantaleoni, R.A., Letardi, A., and Corazza, C. (eds.), Proceedings of the Ninth International Symposium on Neuropterology (20-23 June 2005, Ferrara, Italy). Annali del Museo Civico di Storia Naturale di Ferrara, 8:45-48.
Tauber, C.A. 1987. Neuroptera, p. 126-143. In Stehr, F.W. (ed.), Immature Insects, vol. 2. Kendall-Hunt, Dubuque, IA.
Tauber, C.A., Tauber, M.J., and Albuquerque, G.S. 2003. Neuroptera (lacewings, antlions), p. 785-798. In Resh, V. and Cardé, R. (eds.), Encyclopedia of Insects. Academic Press, Burlington.
Tillyard, R.J. 1922. The life-history of the Australian moth-lacewing, Ithone fusca, Newman (Order Neuroptera Planipennia). Bulletin of Entomological Research, 13:205-223.
Townsend, L.H. 1939. Lacewings and their allies. The Scientific Monthly, 48:350-357.
van der Wal, S. and Haug, J.T. 2019. Letter to the editor referencing “The apparent kleptoparasitism in fish-parasitic gnathiid isopods” 10.1007/s00436-018-6152-8. Parasitology Research, 118:1679-1682. https://doi.org/10.1007/s00436-019-06281-2
Vasilikopoulos, A., Misof, B., Meusemann, K., Lieberz, D., Flouri, T., Beutel, R.G., Niehuis, O., Wappler, T., Rust, J., Peters, R.S., Donath, A., Podsiadlowski, L., Mayer, C., Bartel, D., Böhm, A., Liu, S., Kapli, P., Greve, C., Jepson, J.E., Liu, X., Zhou, X., Aspöck, H., and Aspöck, U. 2020. An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evolutionary Biology, 20:64. https://doi.org/10.1186/s12862-020-01631-6
von Schlechtendal, D.H.R. 1882. Coniopteryx psociformis Curtis, als Schmarotzer in Spinneneiern. Jahresbericht des Vereins für Naturkunde zu Zwickau, 1881:26-31. [Note: year of publication is not the same as the number of the issue!]
Wang, B., Xia, F., Engel, M.S., Perrichot, V., Shi, G., Zhang, H., Chen, J., Jarzembowski, E.A., Wappler, T., and Rust, J. 2016. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Science Advances, 2:e1501918. https://doi.org/10.1126/sciadv.1501918
Ward, L.K. 1970. Aleuropteryx juniperi Ohm (Neur. Coniopterygidae) new to Britain feeding on Carulaspis juniperi Bouche (Hem., Diaspididae). Entomologist's Monthly Magazine, 106:74-78.
Weißmair, W. 1999. Präimaginale Stadien, Biologie und Ethologie der europäischen Sisyridae (Neuropterida: Neuroptera). Stapfia, 60:101-128.
Weißmair, W. and Waringer, J. 1994. Identification of the larvae and pupae of Sisyra fuscata (Fabricius, 1793) and Sisyra terminalis Curtis, 1854 (Insecta: Planipennia: Sisyridae), based on Austrian material. Aquatic Insects, 16:147-155. https://doi.org/10.1080/01650429409361549
Weitschat, W. and Wichard, W. 1998. Atlas der Pflanzen und Tiere im Baltischen Bernstein. Dr. Friedrich Pfeil Verlag, München.
Weitschat, W. and Wichard, W. 2002. Atlas of Plants and Animals in Baltic Amber. Dr. Friedrich Pfeil Verlag, München.
Welch, P.S. 1914. The early stages of the life history of Polystoechotes punctatus Fabr. Bulletin of the Brooklyn Entomological Society, 9:1-6.
Westwood, J.O. 1840. An Introduction to the Modern Classification of Insects; Founded on the Natural Habits and Corresponding Organisation of the Different Families, vol. 2. Longman, Orme, Brown, Green, and Longmans, London.
Wichard, W., Gröhn, C., and Seredszus, F. 2009. Aquatic Insects in Baltic Amber: Wasserinsekten im baltischen Bernstein. Kessel, Remagen.
Winterton, S.L. and Makarkin, V.N. 2010. Phylogeny of moth lacewings and giant lacewings (Neuroptera: Ithonidae, Polystoechotidae) using DNA sequence data, morphology, and fossils. Annals of the Entomological Society of America, 103:511-522. https://doi.org/10.1603/AN10026
Withycombe, C.L. 1922. Parasemidalis annae, End., a coniopterygid new to Britain, with notes on other British Coniopterygidae. Entomologist, 55:169-172.
Withycombe, C.L. 1925. XV. Some aspects of the biology and morphology of the Neuroptera. With special reference to the immature stages and their possible phylogenetic significance. Transactions of the Royal Entomological Society of London, 72(3-4):303-411. https://doi.org/10.1111/j.1365-2311.1925.tb03362.x
Yang, Q., Wang, Y., Labandeira, C.C., Shih, C., and Ren, D. 2014. Mesozoic lacewings from China provide phylogenetic insight into evolution of the Kalligrammatidae (Neuroptera). BMC Evolutionary Biology 14, 126. https://doi.org/10.1186/1471-2148-14-126
Yu, T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F., Zhang, H., Wang, B., and Dilcher, D. 2019. An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences, 116:11345-11350. https://doi.org/10.1073/pnas.1821292116
Zimmerman, D., Klepal, W., and Aspöck, U. 2009. The first holistic SEM study of Coniopterygidae (Neuroptera)--structural evidence and phylogenetic implications. European Journal of Entomology, 106:651-662. https://doi.org/10.14411/eje.2009.081
Zimmermann, D., Randolf, S., and Aspöck, U. 2019. From chewing to sucking via phylogeny--from sucking to chewing via ontogeny: mouthparts of Neuroptera, p. 361-385. In Krenn, H.W. (ed.), Insect Mouthparts. Springer, Cham. https://doi.org/10.1007/978-3-030-29654-4_11
Zizzari, Z.V., Lupetti, P., Mencarelli, C., and Dallai, R. 2008. Sperm ultrastructure and spermiogenesis of Coniopterygidae (Neuroptera, Insecta). Arthropod Structure and Development, 37:410-417. https://doi.org/10.1016/j.asd.2008.03.001

