 Synchrotron-radiation computed tomography uncovers ecosystem functions of fly larvae in an Eocene forest
Synchrotron-radiation computed tomography uncovers ecosystem functions of fly larvae in an Eocene forest
Article number: 24.1.a07
https://doi.org/10.26879/1129
Copyright Palaeontological Association, February 2021
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 16 September 2020. Acceptance: 27 January 2021.
ABSTRACT
We report a hitherto unprecedented diversity of fly larvae (Diptera) from Eocene Baltic amber and the use of these to address palaeo-ecosystem functions and processes in the surrounding forests. Fly larvae have been considered exceptionally rare by the research community and have, like most insect larvae, been deemed of limited utility owing to challenges in identification. Herein, however, using synchrotron-x-ray radiation CT (SR-µCT) allowed us to detect and identify dozens of fly larvae from Baltic amber, and to infer their ecological interactions. One particular piece of amber contains 56 fly larvae and apparent mammalian feces. This fossil is of great interest for our understanding of carbon cycling in the Eocene forest. The occurrence of such a large number of fly larvae on the fecal remains indicates an important role of flies in recycling organic matter in the Eocene forest, much as some larvae do today. Analysis of the fly palaeo-communities also allowed us to hypothesize a mechanism by which massive, geologically relevant deposits of amber were formed in the Baltic region. Scanning allowed us to identify seven larvae closely related to the extant Syrphidae, whose larvae inhabit nests of eusocial Hymenoptera, or, sometimes, flows of sap dripping from trees damaged by other burrowing insect larvae.
Viktor A. Baranov. Biology II, Ludwig-Maximilians-Universität München, Planegg, Bayern, Germany. Correspondence author. baranow@biologie.uni-muenchen.de
Michael S. Engel. Natural Sciences and Mathematics - Ecology & Evolutionary Biology, KU Biodiversity Institute, Kansas, USA. msengel@ku.edu
Jörg Hammel. Institute of Materials Research, Helmholtz-Zentrum Geesthacht, Geesthacht, Germany. joerg.hammel@hzg.de
Marie K. Hörnig. University of Greifswald, Zoological Institute and Museum,Cytology and Evolutionary Biology, Greifswald, Germany. marie.hoernig@palaeo-evo-devo.info
Thomas van de Kamp. Institute for Photon Science and Synchrotron Radiation (IPS), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany and Laboratory for Applications of Synchrotron Radiation (LAS), Karlsruhe Institute of Technology (KIT), Kaiserstr. 1, 76131 Karlsruhe, Germany. thomas.vandekamp@kit.edu
Marcus Zuber. Institute for Photon Science and Synchrotron Radiation (IPS), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany and Laboratory for Applications of Synchrotron Radiation (LAS), Karlsruhe Institute of Technology (KIT), Kaiserstr. 1, 76131 Karlsruhe, Germany. marcus.zuber@kit.edu
Joachim T. Haug. Biology II, Ludwig-Maximilians-Universität München, Planegg, Bayern, Germany and Geobio-Center, Ludwig-Maximilians-Universität München, München, Bayern, Germany. jhaug@biologie.uni-muenchen.de
Keywords: Eocene; climate change; amber; insects; climatological proxy; Diptera
Final citation: Baranov, Viktor A., Engel, Michael S., Hammel, Jörg, Hörnig, Marie K., van de Kamp, Thomas, Zuber, Marcus, and Haug, Joachim T. 2021. Synchrotron-radiation computed tomography uncovers ecosystem functions of fly larvae in an Eocene forest. Palaeontologia Electronica, 24(1):a07. https://doi.org/10.26879/1129
palaeo-electronica.org/content/2021/3294-diptera-larvae-in-baltic-amber
Copyright: February 2021 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
The Eocene epoch (55-33.9 MYA) was a pivotal time span in Earth history, marked by some of the highest recorded rates of warming (Paleocene-Eocene thermal maximum; Zachos et al., 2001). This 700,000-year span of warming was followed by a longer, cooler period, the Eocene Thermal optimum (Zachos et al., 2001). Understanding major changes in past Earth climate and their impact on biodiversity is one of the critical tools permitting us to predict middle- to long-term outcomes of man-made global climate change (Shaffer et al., 2016). Our knowledge of the Eocene climate and biodiversity improved tremendously in the past 50 years due to studies of lithology (Methner et al., 2019), isotope analysis (Barnet et al. 2019), oceanic microplankton (Frieling et al., 2017), and macrofossils of plants and animals (Smith et al., 2019). Among the most lauded sources of data about the Eocene is Baltic amber. This vast deposit of fossil resin formed in the extensive forest covering modern northern Europe (Weitschat and Wichard, 2010). Studying the palaeoecology of animals and plants from the deposit answers some crucial questions about Eocene ecosystems, many of which cannot be tackled by other means. Among such crucial questions are the main avenues of matter and energy transfer in Eocene forests, as well as the nature of the whole biome beyond those commonly preserved organisms with hard external or internal skeletons. Amber provides a unique peek into these aspects of Eocene ecosystems by preserving small soft-bodied organisms, absent from other fossil deposits, but playing vital roles in the functioning of the environment (Andersen et al., 2009; Wichard, 2009; Peñalver et al. 2012, 2018, Ramirez et al., 2017; Cai et al. 2018).
Flies (Diptera) are hyper-diverse, with at least 160,000 extant species and encompassing the gnats, midges, mosquitos, and myriad other relatives (Marshall, 2012). Species of flies can be exceedingly abundant. For example, larvae of non-biting midges (Chironomidae) can occur in certain lakes at densities of more than 100,000 individuals/m2 (Einarsson et al., 2002). Beyond individuals of a single species, communities can also harbor a staggeringly high number of species themselves: a 4-hectare area of cloud forest in Costa Rica (Zurquí) was found to include at least 4332 species of flies (Brown et al., 2018). Flies have colonized diverse habitats - from the surface of the open ocean and over frozen glaciers, to 1000-m deep caves and the bodies of other organisms such as bats or birds (Andersen et al., 2015; McAlister, 2017). Fly species perform an enormous number of ecosystem functions such as pollination, carbon cycling, and water purification (Marshall, 2012). Due to their diversity and abundance, flies serve as important indicators of environmental conditions in both modern and fossil ecosystems (Baranov et al., 2019a). Owing to the specificity of the ecological requirements, flies are often used to reconstruct habitats and climatic conditions based on the occurrence of particular groups in a deposit (e.g., Walker, 2001; Brooks, 2006; Rolland et al., 2008). While adult flies are most often used for this purpose (e.g., Moe et al., 2005; Grund, 2006; Szadziewski, 2018), they are short-lived (days to weeks) and frequently do not feed, serving solely as the dispersive and reproductive stage (Marshall, 2012). Fly larvae, in contrast, are tightly associated with certain types of habitat and perform most ecosystem functions and are therefore the stage that provides the greatest direct linkage between the fly and its specific environment (Baranov et al., 2019a, 2019b). The distinctness of larval and adult fly biology is one of the reasons for their evolutionary success (Grimaldi and Engel, 2005). Given this, it stands to reason that fossil fly larvae are perhaps better proxies for the reconstruction of palaeo-environments, as larval habitat and ecological preferences can be inferred more directly from their morphology (Haug and Haug, 2019; Baranov et al., 2019a, 2020a, 2020b). Therefore, studying immature and adult flies in concert provides additional and enhanced information about palaeoecosystems (Baranov et al., 2019b). Most palaeontologists conventionally believe that fly larvae are rare in the fossil record (except for larvae and pupae of several aquatic groups) and rarer still in amber (Baranov et al., 2019a). If true, then it would be challenging to rely on fly larvae in reconstructing complex palaeo-environments. Additionally, flies and other insect larvae are considered difficult to identify as fossils owing to a perceived paucity of visible diagnostic characters. Should these difficulties be overcome, fly larvae could be crucial pieces toward understanding the puzzle of Eocene climate and biodiversity.
Herein we document a considerably high diversity of fly larvae preserved in Baltic amber (middle Eocene) and demonstrate that these preserve an amazing breadth of taxonomically, morphologically, and ecologically important characters. These new data were obtained using synchrotron-radiation computed tomography (SR-µCT). From the available data, we can identify the functional ecology of individual fly larvae to provide a more comprehensive perspective on the environment and functioning of the Baltic amber forest, highlighting previously inaccessible parts of the forest biome and its functioning, including matter and energy metabolism, and possibly, very drivers of the amber formation.
MATERIAL AND METHODS
We examined over 73 specimens of immature flies preserved in Baltic amber, mostly of larvae but also several puparia (for terminological issues see Haug, 2020), as well as several published records. Identification of these larvae to species is mostly impossible, but also unnecessary for understanding their ecological role, and we therefore defaulted our identifications to larval morphotypes. In order to observe functional morphological traits critical to linking individual larvae to particular ecosystem functions, we utilized synchrotron-radiation-based micro-computed tomography (SRµCT) to reconstruct specimens and observe features otherwise obscured or challenging to view via traditional optical microscopy.
Material
The Baltic amber was obtained from a commercial source in Yantarnyj, Kaliningrad District (formerly Palmnicken, Königsberg); Bitterfeld amber was obtained from various sources. Tracing of the precise origin of every piece was impossible due to the nature of the amber collections. More detailed information on the deposit can be found in Weitschat and Wichard (2010) and Kasiński et al. (2020). Notes on specimen deposition: All specimens studied here are deposited in public collections (See "Results: taxonomic account” for the full list of specimens). All specimens with a “PED'' designation in the accession number are housed in the Paleo-Evo-Devo research group collection, LMU Munich, Germany; all specimens with “Dip” prefix in the number are housed at the German Entomological Institute (DEI, Münchenberg), specimens with SMF-Be prefix are deposited in the Senckenberg Research Institute, Frankfurt. Lastly, all specimens with “BI”' and “AKBS” are housed in the Centrum für Naturkunde, CeNak, Hamburg University, Hamburg.
Methods
Optical images were recorded with a Keyence VHX-6000 digital microscope, either with ring light illumination or cross-polarized coaxial illumination (Baranov et al., 2019a, 2019b). All images are composite images; each image detail was documented with a stack of images (frames) of shifting focal planes which were fused to a single sharp image with the built-in software. Several adjacent image details were stitched to a large panorama image with the built-in software, resulting in a high-resolution image.
Amber specimens have been imaged at the Imaging Beamline P05 (IBL; Greving et al., 2014; Wilde et al., 2016) operated by the Helmholtz-Zentrum-Geesthacht at the storage ring PETRA III (Deutsches Elektronen Synchrotron - DESY, Hamburg, Germany) using a photon energy of 18 keV and a sample to detector distance of 80 mm. Projections were recorded using a custom developed 20 MP CMOS camera system with an effective pixel size of 0.64 µm and 1.27 µm, respectively, depending on the selected magnification. For each tomographic scan 2401 projections at equal intervals between 0 and π have been recorded. Tomographic reconstruction was done by applying a transport of intensity phase retrieval approach and using the filtered back projection algorithm (FBP) implemented in a custom reconstruction pipeline using Matlab (Math-Works) and the Astra Toolbox (van Aarle et al., 2015, 2016). For processing, raw projections were binned two times for further processing resulting in an effective pixel size of the reconstructed volume (voxel) of 1.28 µm and 2.55 µm, respectively. SR-µCT scans of PED 230 and AKBS-0030 were performed at the imaging cluster of the KIT light source of Karlsruhe Institute of Technology using a parallel polychromatic x-ray beam produced by a 1.5 T bending magnet. The beam was spectrally filtered by 0.2 mm aluminum to remove low energy components from the beam. The resulting spectrum had a peak at about 15 keV, with a full width at half maximum bandwidth of about 10 keV. A fast-indirect detector system was employed, consisting of a 12 µm LSO: Tb scintillator (Cecilia et al., 2011), diffraction limited optical microscope (Optique Peter) coupled with a 12 bit pco.dimax high speed camera with 2016 x 2016 pixels. Scans were done by taking 3,000 projections at 70 frames per second and optical magnifications of 5X (PED-230 and AKBS-0030) and 10X (AKBS-0030), resulting in an effective pixel size of 2.44 µm and 1.22 µm, respectively. The samples were scanned in several height steps. We used the control system concert (Vogelgesang et al., 2016) for automated data acquisition and online reconstruction of tomographic slices for data quality assurance. Online and final data processing including tomographic reconstruction were performed by the UFO framework (Vogelgesang et al., 2012).
In addition to scanning all specimens using synchrotron radiation, a µCT scan of the sample AKBS-0030 was performed in the Computed Laminography / Computed Tomography (CL/CT) laboratory of the IPS. An XWT-225(X-RAY WorX) x-ray tube was employed to produce a 70 kVp conical polycromatic x-ray beam with a target power of 20W. Over an angular range of 360° with a PerkinElmer XRD1612 CN14ES flat panel detector, 2048 projections were recorded. Each frame was exposed for 4s. The distance between the source and the sample was 185 mm, and the distance between the source and the detector 1726 mm, yielding an effective pixel size of 21.5µm. The final CT slices were calculated using the cone beam reconstruction of the UFO framework (Vogelgesang et al., 2012).
In order to decrease the strain on the RAM and video card memory of the PC used for the reconstruction, all original TIFF-stacks were downscaled to 0.2 of the original size using Fiji (Schindelin et al., 2012). Afterwards, stacks were rendered in Drishti ver. 2.6.6 (Limaye, 2012). Segmentation of internal organs and surface mesh-models of the volume renders have been constructed based on the original TIFF-stacks in Drishti Paint v.2.6.4. Data for this paper is linked to the DOI of this publication and see Table 1 for locations.
RESULTS
 The most surprising result of this study is the considerable diversity of larval brachyceran species (true or short-horned flies) in Baltic amber (Figure 1 A-L, Figure 2A-I, Figure 3A-D, Figure 4A-I). Upon initial examination, most of the larvae appear to be quite similar, often obscured by a white film (“Verlumung”). This film often forms around larger amber inclusions due to decomposition. In total, we were able to identify 20 different larval morphotypes, representing diverse ingroups of flies: crane flies (Tipulidae), march flies (Bibionidae), gall-midges (Cecidomyiidae), Pachyneuridae, window gnats (Anisopodidae), non-biting midges (Chironomidae), ibis flies (Athericidae), silver flies (Chamaemyiidae), sun flies (Heleomyzidae), scuttle flies (Phoridae), as well as several morphotypes which are representative of Cyclorrhapha, but cannot be identified to further sub-groups (“families”). Eleven of these morphotypes were recorded based on new material, described in this paper. The reasoning behind the allocation of the larval morphotypes to certain lineages and short descriptions of all the morphotypes are provided below.
The most surprising result of this study is the considerable diversity of larval brachyceran species (true or short-horned flies) in Baltic amber (Figure 1 A-L, Figure 2A-I, Figure 3A-D, Figure 4A-I). Upon initial examination, most of the larvae appear to be quite similar, often obscured by a white film (“Verlumung”). This film often forms around larger amber inclusions due to decomposition. In total, we were able to identify 20 different larval morphotypes, representing diverse ingroups of flies: crane flies (Tipulidae), march flies (Bibionidae), gall-midges (Cecidomyiidae), Pachyneuridae, window gnats (Anisopodidae), non-biting midges (Chironomidae), ibis flies (Athericidae), silver flies (Chamaemyiidae), sun flies (Heleomyzidae), scuttle flies (Phoridae), as well as several morphotypes which are representative of Cyclorrhapha, but cannot be identified to further sub-groups (“families”). Eleven of these morphotypes were recorded based on new material, described in this paper. The reasoning behind the allocation of the larval morphotypes to certain lineages and short descriptions of all the morphotypes are provided below.
 Here we provide details for the identification of the observed larval morphotypes as representatives of particular ingroups of flies. We discuss both new material and already known fly larva morphotypes identified from Baltic and Bitterfeld (Saxonian) ambers.
Here we provide details for the identification of the observed larval morphotypes as representatives of particular ingroups of flies. We discuss both new material and already known fly larva morphotypes identified from Baltic and Bitterfeld (Saxonian) ambers.
Taxonomic Account
Larvae previously described in the literature from Baltic amber; diagnostic characters provided in the sources:
1. Limoniidae cf. Ormosia (Podeniene et al., 2004).
2. Anisopodidae: Mycetobia Meigen, 1818 Morphotype 1 (Baranov et al., 2019a).
3. Anisopodidae: Mycetobia Meigen, 1818 Morphotype 2 (Baranov et al., 2019a).
4. Anisopodidae: Mycetobia Meigen, 1818 Morphotype 3 (Baranov et al., 2019a).
5. Anisopodiae: Sylvicola (Baranov et al., 2019a).
6. Bibionomorpha (Dinobibio hoffeinseorum) (Baranov et al., 2019a).
7.  Pachyneuridae Schiner, 1864 (Baranov et al., 2019a).
Pachyneuridae Schiner, 1864 (Baranov et al., 2019a).
8. Cecidomyiidae (Weitschat and Wichard, 2002).
9. Chironomidae cf Bryophaenocladius (Baranov et al., 2019b).
10. Acroceridae (Kerr and Winterton, 2008).
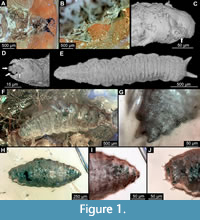
11. Athericidae. PED-230, single specimen, 11.4 mm long. The specimen is a larva of Athericidae based on the following combination of characters: the body is flattened dorsoventrally; head capsule present, but weakly sclerotized; pseudopods with small claws on each of the eight trunk segments (Figure 1A-B); posterior spiracles (openings of the respiratory system) are sitting on the trunk end (abdomen); trunk end with elongated, fringed conical fleshy lobes and two prominent ventral tubercles (Figure 1A-B, Figure 2A-B, Figure 3C) (Smith, 1989).
 12. Chamaemyiidae. Dip-00898, SMF-Be-10616 (Figure 1C-G, Figure 2C-D, Figure 3F): two specimens, 2.2-3 mm long. Specimens are larvae of Chamaemyiidae based on the following combination of characters: head skeleton reduced and not obvious upon inspection from the outside; anterior spiracles in a form of a small lobe with four digitiform protrusions; body flattened dorsoventrally; body segments are additionally annulated with secondary furrows on trunk segments; each trunk unit subdivided into three rings; body without lateral and dorsal processes; left and right spiracles at the trunk end, widely separated; posterior spiracles on a tubular mounting, with three openings on flat, distal ends of the mountings (Figure 1 C-E).
12. Chamaemyiidae. Dip-00898, SMF-Be-10616 (Figure 1C-G, Figure 2C-D, Figure 3F): two specimens, 2.2-3 mm long. Specimens are larvae of Chamaemyiidae based on the following combination of characters: head skeleton reduced and not obvious upon inspection from the outside; anterior spiracles in a form of a small lobe with four digitiform protrusions; body flattened dorsoventrally; body segments are additionally annulated with secondary furrows on trunk segments; each trunk unit subdivided into three rings; body without lateral and dorsal processes; left and right spiracles at the trunk end, widely separated; posterior spiracles on a tubular mounting, with three openings on flat, distal ends of the mountings (Figure 1 C-E).
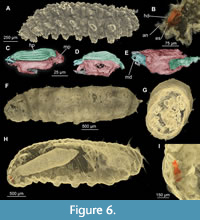
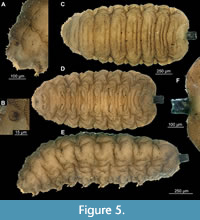 13. Syrphidae: Volucellini. Dip-00896, Dip-00897, Dip-00894, Dip-00888, Dip-00889, Dip-00895, Dip-00891. Length 1.2-6.1 mm (mean=3.4 mm, n=6) (Figure 2F, G; Figure 4E, H, I; Figure 5A-F; Figure 6A - I). Specimens are larvae of Syrphidae and more precisely Volucellini based on the following combination of characters: head skeleton reduced and not obvious upon inspection from outside; body flattened dorsoventrally, with prominent lateral processes; 2nd thoracic and 1-6 posterior trunk (abdomen) segments with prominent pseudopods, bearing crochets of claws are present; body covered with wrinkles and posterior spiracles conjoined together, sitting on the top of the short tube (Figure 6 A-I) (Ferrar, 1987; Smith, 1989; Rotheray, 1993). Seven specimens were found in the different amber pieces listed above. Since the specimens are varying considerably in size and proportion of the body parts, we hypothesize that they are representing the three larval instars that are known among representatives of this group (Ferrar, 1987). Specimens are very similar to extant representatives of Volucellini, especially Volucella bombylans (Figure 5A-F; Ferrar, 1987, figure 2.174). This similarity is also notable in the structure of the head skeleton. Two out of seven specimens have a well-preserved internal head skeleton (Figure 6B-E). Both mandibles and cephalopharyngeal skeleton, in general, are highly reminiscent of that of larvae of the group Volucella. Mandibles of both fossil larvae and those of extant representatives of Volucella are composed of two thin crescent-shaped sclerites and multiple ribs attached to them (Figure 6B-E, I) (Ferrar, 1987, figures 84.77, 84.78).
13. Syrphidae: Volucellini. Dip-00896, Dip-00897, Dip-00894, Dip-00888, Dip-00889, Dip-00895, Dip-00891. Length 1.2-6.1 mm (mean=3.4 mm, n=6) (Figure 2F, G; Figure 4E, H, I; Figure 5A-F; Figure 6A - I). Specimens are larvae of Syrphidae and more precisely Volucellini based on the following combination of characters: head skeleton reduced and not obvious upon inspection from outside; body flattened dorsoventrally, with prominent lateral processes; 2nd thoracic and 1-6 posterior trunk (abdomen) segments with prominent pseudopods, bearing crochets of claws are present; body covered with wrinkles and posterior spiracles conjoined together, sitting on the top of the short tube (Figure 6 A-I) (Ferrar, 1987; Smith, 1989; Rotheray, 1993). Seven specimens were found in the different amber pieces listed above. Since the specimens are varying considerably in size and proportion of the body parts, we hypothesize that they are representing the three larval instars that are known among representatives of this group (Ferrar, 1987). Specimens are very similar to extant representatives of Volucellini, especially Volucella bombylans (Figure 5A-F; Ferrar, 1987, figure 2.174). This similarity is also notable in the structure of the head skeleton. Two out of seven specimens have a well-preserved internal head skeleton (Figure 6B-E). Both mandibles and cephalopharyngeal skeleton, in general, are highly reminiscent of that of larvae of the group Volucella. Mandibles of both fossil larvae and those of extant representatives of Volucella are composed of two thin crescent-shaped sclerites and multiple ribs attached to them (Figure 6B-E, I) (Ferrar, 1987, figures 84.77, 84.78).
 14. Phoridae SMF-BE-10726 puparium. Length 1.9 mm (Figure 2F, G; Figure 4E, H, I; Figure 5A-F; Figure 6 A - I). This specimen is most likely a puparium of the group Phoridae. It has a flattened body, covered with strongly articulated setae, a number of strong processes at the trunk end, and small, transparent conical thoracic horns on the thorax (Ferrar, 1987). The general body shape is oval, the body is of unequal shape in lateral view, “slipper-shaped” according to Ferrar (1987) (Figure 1H-K). The specimen is similar to puparia of the group Pseudohypocera as depicted in Ferrar (1987, figure 63.104).
14. Phoridae SMF-BE-10726 puparium. Length 1.9 mm (Figure 2F, G; Figure 4E, H, I; Figure 5A-F; Figure 6 A - I). This specimen is most likely a puparium of the group Phoridae. It has a flattened body, covered with strongly articulated setae, a number of strong processes at the trunk end, and small, transparent conical thoracic horns on the thorax (Ferrar, 1987). The general body shape is oval, the body is of unequal shape in lateral view, “slipper-shaped” according to Ferrar (1987) (Figure 1H-K). The specimen is similar to puparia of the group Pseudohypocera as depicted in Ferrar (1987, figure 63.104).
15. cf. Sciomyzidae SMF-BE-10652. Poorly preserved specimen 6.2 mm long (Figure 7A, B). Preservation is not sufficient for further identification, but six long lobes surrounding the posterior spiracles, as well as general body shape are reminiscent of larvae of the group Sciomyzidae (Ferrar, 1987).
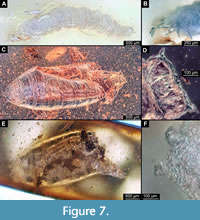 16. Heleomyzidae puparium. Dip-00890. 4.2 mm long (Figure 3D; Figure 7C-F). Specimen is a puparium of Heleomyzidae based on the following combination of characters: Barrel-shaped puparium; anterior part of puparium narrows and then expands into the straight-edged anterior end; transverse bands of locomotory spicules are present on the ventral side of abdomen segments; anterior spiracles transverse rosette shaped with 10 respiratory bulbs, resembling those of larvae of the ingroup Suillia (Figure 7C-F) (Ferrar, 1987, figure 39.34; Rotheray, 2012); posterior spiracles on short tubes, widely separated; spiracles with three straight slits, arranged radially (similar to, e.g., Suillia ustulata) (Ferrar, 1987, figure 39.49; Rotheray, 2012, figure 5B).
16. Heleomyzidae puparium. Dip-00890. 4.2 mm long (Figure 3D; Figure 7C-F). Specimen is a puparium of Heleomyzidae based on the following combination of characters: Barrel-shaped puparium; anterior part of puparium narrows and then expands into the straight-edged anterior end; transverse bands of locomotory spicules are present on the ventral side of abdomen segments; anterior spiracles transverse rosette shaped with 10 respiratory bulbs, resembling those of larvae of the ingroup Suillia (Figure 7C-F) (Ferrar, 1987, figure 39.34; Rotheray, 2012); posterior spiracles on short tubes, widely separated; spiracles with three straight slits, arranged radially (similar to, e.g., Suillia ustulata) (Ferrar, 1987, figure 39.49; Rotheray, 2012, figure 5B).
17. Cyclorrhapha morphotype 1. AKBS-0030, 56 specimens in a single piece (Figure 3A, B; Figure 4A-D; Figure 8A, B). 3.1 mm long (average length, n=46, based on the measurements of the better-preserved larvae). The absence of an external head capsule and body with massively reduced thorax units indicates that these larvae belong to the group Cyclorrhapha. Unfortunately, the larvae are too poorly preserved for any further identification (Figure 8A, B). The only way to narrow down the identity of the larvae is to use their autecology and compare it with that of extant fly larvae. Taking into account the rather small size of the larvae, and a possible fecal origin of the organic mass in amber, the larvae could be representatives of Heleomyzidae, Drosophilidae, Sepsidae, or Piophilidae (Graham Rotheray and Lain MacGowan, personal commun. 2020).
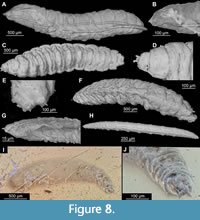 18. Cyclorrhapha morphotype 2, Dip-00892. 2.9 mm. long (Figure 8G, H). Single, poorly preserved larva. The absence of an external head capsule and a body with massively reduced thorax segments indicate this is a larva of the Cyclorrhapha. Unfortunately, the larva is too poorly preserved for any further identification. This morphotype is characterized by the most elongated body among the larval representatives of Cyclorrhapha recorded from Baltic amber so far.
18. Cyclorrhapha morphotype 2, Dip-00892. 2.9 mm. long (Figure 8G, H). Single, poorly preserved larva. The absence of an external head capsule and a body with massively reduced thorax segments indicate this is a larva of the Cyclorrhapha. Unfortunately, the larva is too poorly preserved for any further identification. This morphotype is characterized by the most elongated body among the larval representatives of Cyclorrhapha recorded from Baltic amber so far.
19. Cyclorrhapha morphotype 3. Dip-00893, BI-2354 (Figure 8C-F). 1.3‒3 mm long (n=2). Several poorly preserved specimens. Impossible to identify with certainty. The absence of an external head capsule and a body with massively reduced thorax segments indicates that these are larvae of the Cyclorrhapha. Unfortunately, the larvae are too poorly preserved for any further identification. This morphotype is characterized by an elongated and spindle-shaped body, and the presence of short processes around the posterior spiracles.
Puparium of a fly, BI-2356, may correspond to the previous morphotype (Figure 2E), based on a general similarity of the habitus.
20. Cyclorrhapha morphotype 4, SMF-BE-10645 (Figure 8I, J). 2.6 mm. Absence of an external head capsule and a body with massively reduced thorax segments indicates that this is a larva of the Cyclorrhapha. Unfortunately, the larva is too poorly preserved for any further identification. Head and thorax are relatively well preserved, with antennae and conical anterior spiracles prominently visible.
DISCUSSION
Palaeoautoecological Remarks
We found 11 morphotypes of brachyceran larvae and puparia while SR-µCT-scanning only 16 pieces of amber (Figure 2A-K). Study of the new material revealed extensive decomposition-based trophic webs, previously unseen in Eocene ecosystems. Below, we give an overview of the possible palaeoecology of the larval morphotypes recorded.
Athericidae
Extant larvae of Athericidae are aquatic and predatory. They live in streams and rivers, either at the bottom or amid aquatic vegetation (Webb, 1977, 1994). The discovery of an ibis fly larva (Athericidae; Figure 2A, B; Figure 3C) documents interesting aspects of aquatic habits in the Eocene forest. Athericidae is a small group of flies with merolimnic species, whose larvae are aquatic predators (Webb, 1977, 1994). The fossil record of Athericidae is relatively rich, but no larvae of the group have so far been found (Mostowski et al., 2003; Zhang, 2012; Myskowiak and Nel, 2014; Nel et al., 2014; Oberprieler and Yeates, 2014). Four species of Athericidae have hitherto been described from fossil resins. Succinatherix avita Stuckenberg, 1974 and S. setifera Stuckenberg, 1974 were described from Baltic amber (Stuckenberg, 1974). Galloatherix incompletus Nel et al., 2014 was described from mid-Cretaceous French amber, while Eoatrichops jeanbernardi Myskowiak and Nel, 2014 is known from the Eocene Oise amber of France (Myskowiak and Nel, 2014). Additionally, Meunier described a fly from Baltic amber - Atherix exigua Meunier, 1910 - which he ascribed to Athericidae, but the status of this species remains uncertain.
A Jurassic ectoparasitic larva, Qiyia jurassica Jun et al., 2014 is perhaps related to Athericidae (Jun et al., 2014). The current record is the first definitive find of an immature fossil snipe fly and adds to a growing number of aquatic immatures found in Baltic amber. Immature dragonflies, mayflies, caddisflies, aquatic lacewings, and alderflies are all known from the deposit (Wichard et al., 2009), and collectively they demonstrate abundant aquatic habitat and microhabitat conditions in the broader Eocene forest producing resin (Martı́nez-Delclòs et al., 2004).
Chamaemyiidae
Extant larvae of Chamaemyiidae with a similar morphology are normally aphidivorus (Ferrar, 1987). Among the extant representatives of larval Chamaemyiidae of the group ‘leucopsis’ are most similar to the fossil specimens (Ferrar, 1987, figures 17.27, 17.28).
Syrphidae
Detailed discussion is provided below.
Heleomyzidae
Some adults of Heleomyzidae have already been described from Baltic amber (Evenhuis, 2014). Extant larvae of Heleomyzidae often develop on rotting masses of organic matter, such as dung, corpses, or fruiting bodies of fungi (Ferrar, 1987; see discussion regarding Cyclorrhapha morphotype 1, below). Therefore, multiple larval substrates would have been available on the Baltic amber forest floor.
Dung Decomposers and Decomposing Food Webs
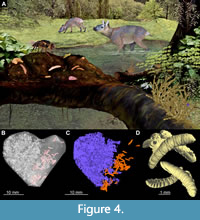
Screening revealed several unique fossils (Figure 4A-D). Most notable is a piece of amber containing 56 fly larvae in association with a possible piece of vertebrate feces (an amber coprolite). This fossil is important for understanding energy and matter transfer in decomposition food webs. This part of the food web, dealing with the transport and decomposition of dead organic matter, is crucial to the carbon cycle, and thereby to local and global climatic regulation. Unfortunately, the main actors in this interaction, such as terrestrial fly larvae or earthworms, are rarely preserved as fossils (Ulrich and Schmelz, 2001; Poinar, 2007). Therefore, understanding the decomposition of dead organic matter in the deep past has historically represented a significant gap in research.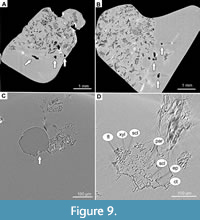 The fossil itself is a roughly heart-shaped piece of amber, 30 × 25 mm, with the left portion containing the fecal matter and the remainder including 56 larvae, heavily obscured by a white film (Figure 3A, B, Figure 4C-D). Prior to SR-µCT, it was difficult to assess the nature of the fossils inside the amber. Originally, only a small number of larvae and a compact mass of decomposed plant matter could be easily discerned. Upon closer examination, it was discovered that the larvae lie on the surface of this mass (Figure 4C, D). The compact nature of the decomposed plant material, strong maceration of plant tissues, and the presence of numerous tissue types in the mass leads us to conclude that with the mass is likely fecal material. Remnants of vascular plants, with the clearly visible epidermis, parenchyma, collenchyma, as well as phloem and xylem are abundant in the mass (Figure 9A-D). Unfortunately, identification of the plant remnants based on the available SR-µCTdata does not appear to be possible (A. Schmidt and E.-M. Sadowski, personal commun. 2020). Since the plant matter is finely macerated, it is likely that it was chewed. Maceration is evident from the presence of the many small particles of the plant tissue, with the edges of an irregular shape (Figure 9A-D). Accordingly, these droppings likely belonged to a mammal (Figure 3A; Chame, 2003). Shape of the scat is hard to discern, as it is possible that only a part of the dropping was preserved in amber (Figure 9A, B). However, it is probable, based on the rendering of the amber piece, that the feces were relatively flat with one end somewhat pointed. This shape of feces corresponds to a wide spectrum of mammalian feces, notably some smaller deer and large rodents, such as beavers (Chame, 2003). The occurrence of fly larvae in unusually high numbers strongly suggests a trophic association with the organic mass within the piece of amber. Unfortunately, the presence of large quantities of organic matter was detrimental to the preservation of the larvae as many specimens partially rotted. SR-µCTdata shows that no larvae in the piece have preserved internal mouthparts or spiracles (external openings of the tracheal system). As these characters are crucial for the identification of fly larvae, we currently can only identify the larvae as far as the group Brachycera, but not further. Such a record of a likely fly larvae and feces association in Baltic amber is important as it provides additional evidence for the presence of mammals in the Baltic amber forest (Figure 4A). These animals are naturally missing from the amber record and from our overall picture of the amber forest ecosystem (Penney, 2016). The only traces of the mammals in the deposit so far have been hair inclusions in amber and a dental impression of a hog (Kosmowska-Ceranowicz and Kulicka, 1995; Weitschat and Wichard, 2002; Sidorchuk et al., 2019). The presence of dung and saprophagous larvae thus additionally demonstrate the presence of such mammals. Unfortunately, ascertaining identity or even the size of the animal that produced the scat with any degree of precision is not possible.
The fossil itself is a roughly heart-shaped piece of amber, 30 × 25 mm, with the left portion containing the fecal matter and the remainder including 56 larvae, heavily obscured by a white film (Figure 3A, B, Figure 4C-D). Prior to SR-µCT, it was difficult to assess the nature of the fossils inside the amber. Originally, only a small number of larvae and a compact mass of decomposed plant matter could be easily discerned. Upon closer examination, it was discovered that the larvae lie on the surface of this mass (Figure 4C, D). The compact nature of the decomposed plant material, strong maceration of plant tissues, and the presence of numerous tissue types in the mass leads us to conclude that with the mass is likely fecal material. Remnants of vascular plants, with the clearly visible epidermis, parenchyma, collenchyma, as well as phloem and xylem are abundant in the mass (Figure 9A-D). Unfortunately, identification of the plant remnants based on the available SR-µCTdata does not appear to be possible (A. Schmidt and E.-M. Sadowski, personal commun. 2020). Since the plant matter is finely macerated, it is likely that it was chewed. Maceration is evident from the presence of the many small particles of the plant tissue, with the edges of an irregular shape (Figure 9A-D). Accordingly, these droppings likely belonged to a mammal (Figure 3A; Chame, 2003). Shape of the scat is hard to discern, as it is possible that only a part of the dropping was preserved in amber (Figure 9A, B). However, it is probable, based on the rendering of the amber piece, that the feces were relatively flat with one end somewhat pointed. This shape of feces corresponds to a wide spectrum of mammalian feces, notably some smaller deer and large rodents, such as beavers (Chame, 2003). The occurrence of fly larvae in unusually high numbers strongly suggests a trophic association with the organic mass within the piece of amber. Unfortunately, the presence of large quantities of organic matter was detrimental to the preservation of the larvae as many specimens partially rotted. SR-µCTdata shows that no larvae in the piece have preserved internal mouthparts or spiracles (external openings of the tracheal system). As these characters are crucial for the identification of fly larvae, we currently can only identify the larvae as far as the group Brachycera, but not further. Such a record of a likely fly larvae and feces association in Baltic amber is important as it provides additional evidence for the presence of mammals in the Baltic amber forest (Figure 4A). These animals are naturally missing from the amber record and from our overall picture of the amber forest ecosystem (Penney, 2016). The only traces of the mammals in the deposit so far have been hair inclusions in amber and a dental impression of a hog (Kosmowska-Ceranowicz and Kulicka, 1995; Weitschat and Wichard, 2002; Sidorchuk et al., 2019). The presence of dung and saprophagous larvae thus additionally demonstrate the presence of such mammals. Unfortunately, ascertaining identity or even the size of the animal that produced the scat with any degree of precision is not possible.
Saprophagy by extant species of flies is extremely important for dung decomposition and the global carbon cycle (Marshall, 2012). Housefly (Musca domestica) larvae can process up to 700 kg of pig manure per week if the manure is inoculated by 0.4-1 ml of eggs per kilogram (Čičková et al., 2012). Such densities of eggs and larvae are routine both in agriculture and in natural environments (Čičková et al., 2012; Marshall, 2012). This find indicates that fly larvae likely were an important part of the carbon cycle in the Eocene forest - just as they are today - and particularly in cycling organic carbon from fecal matter. This rare insight into the decomposition side of an Eocene food-web shows that abundant excesses of organic matter could have been quickly processed, with excess carbon likely released right back into the atmosphere. However, a single data point is insufficient, and more fossils are required in order to further elucidate this matter.
Possible Ecology of Larvae of Volucellini
The causes leading to the formation of geologically relevant volumes of amber, and the palaeobiota they preserve, remains a greatly debated topic in palaeobiology (Penney, 2016). Recently, insights into the drivers of the amber formation have begun to emerge, with mass-production of ancient resins considered to be a stress reaction on the part of the forest (McKellar et al., 2011; Seyfullah et al., 2018). In fact, much of this has lately been linked to water stress in the amber-producing trees (Seyfullah et al., 2018), potentially brought on by drought, flood, disease, or parasites. Unfortunately, in the Baltic amber forest we lack direct evidence of any such stressors. Remarkably diverse hoverfly larvae (Syrphidae: Eristalinae: Volucellini; Figure 2F, G; Figure 3E, H, I, Figure 6A-I) reveal interesting possible associations that might point to one such stressor - tree-burrowing insect larvae. We discovered seven larvae of this morphotype, all possessing characters typical for the group Volucellini (Figure 5A-D; Smith, 1989). Larvae within this morphotype exhibit significant morphological variation, which is in line with the known variation over ontogenetic sequences of extant species of Volucellini (Smith, 1989; Monfared et al., 2013). Today these larvae are mostly scavengers or predators associated with the nests of eusocial representatives of Hymenoptera, particularly social wasps (Vespidae) and corbiculate bees, such as bumble bees (Rotheray, 1993). In such cases the larvae use their prominent pseudopods and claws to navigate within the nest structures of their hosts. None of the fossils recovered, however, are in association with eusocial insects or nests, possibly indicating some other palaeobiological interaction for these larvae. It is possible that the larvae dropped from or were thrown out of the potential hosts nests, although this is unlikely as this occurs only exceptionally rarely in modern counterparts. In addition, the abundance of representatives of Volucellini trapped in the fresh resin is exceptionally unlikely if in association with eusocial insect nests where they are often in low abundance and where the insects usually complete their development, and not in aggregations outside of the nest. Interestingly, in at least one European species of Volucellini (Volucella inflata [Fabricius, 1794]), the saprophagous larvae are specialized to aggregate at seepages on trees infested by wood-boring moth larvae (Cossidae; Speight, 2010). The specificity and strength of this autecological interaction are as of yet largely unknown (Speight, 2010). The abundance of larvae of Volucellini in Baltic amber is therefore difficult to explain. We have hypothesized that such prominence of these larvae can be a result of their association with an outpouring of resin from a mass infestation of the forest by wood-boring insect larvae. Wood-boring insects disrupt water movement through the tree, leading to water stress in large portions of the plant. Larvae of Volucellini that are not linked to social insect nests therefore could be a key indicator of resin production due to infestation, possibly corroborating patterns documented for the production of significant deposits of resin available to be preserved and converted to amber (McKellar et al., 2011; Peris et al., 2016; Seyfullah et al., 2018).
In general, this hypothesis currently serves as only one possible explanation for the high abundance of larvae of Volucellini in Baltic amber. It is important to remember that the hypothesis is built on the following inferences: 1) larvae of Volucella from Baltic amber can be associated with wood-boring moth larvae, which is currently untestable; 2) that said hypothetical wood-boring moth larvae produced sap or resin exudation in the forests of the Eocene as can happen today; and 3) that a single extant species of Volucella is associated with exudate production. While sap and resin appear to have been secreted together in the Cretaceous amberiferous forests (see Lozano et al., 2020) and is common in modern forests, direct evidence of such a relationship in Baltic amber remains to be discovered. More fossil evidence will be required to elucidate the reasons for this unusually high abundance of larvae.
Fly larvae as a missing piece in Eocene ecosystems. Many biotic interactions representing important aspects of Eocene forest ecology remain obscured. Yet, we demonstrate here the potential for insect larvae - long considered too poorly preserved and difficult to identify to provide useful palaeobiological data - to illuminate specific aspects of palaeodiversity, palaeoecology, and palaeoethology. Indeed, in recent years the utility of fossilized insect larvae has been documented repeatedly, with examples from diverse periods and deposits (e.g., Wang et al., 2016; Lohrmann and Engel, 2017; Badano et al., 2018; Baranov et al., 2019; Haug and Haug, 2019; Pérez-de la Fuente et al., 2012, 2020; Haug et al., 2020). The exploration of fossil larvae has the potential to open those “dark” aspects of Eocene ecology and ecosystem functioning otherwise hidden. The advent of synchrotron-radiation based micro-computed tomography (SR-µCT) has enabled the study of fossil larvae to reach its greatest potential. This method has made it possible to access fossils otherwise impossible to study and/or to identify specimens lacking diagnostic external features. Today this powerful, albeit increasingly widespread tool, allows us to tackle aspects of palaeobiology that were once inaccessible. SR-µCT scans reveal that some of the larvae of Volucellini studied here have an unusually high degree of preservation (for amber fossils) of their internal organs (Figure 6B-E, G-I). In particular, one specimen (Figure 6B-E) has the internal mouthparts and hypopharynx completely preserved in 3D (Figure 6B-D), greatly facilitating identification. Another specimen has its esophagus and mid-gut exquisitely preserved (Figure 6G-I). This contrasts with the majority of amber insect fossils, most of which are essentially empty tubes bounded by a shadow of cuticle (Penney, 2016, but see Pohl et al., 2010). Such fidelity of preservation and character data are impossible to access without tools such as SR-µCT scans.
Understanding the Eocene carbon cycle is crucial for a more holistic vision of climatic shifts during this epoch. Carbon sources and sinks in the Eocene are relatively well constrained at the planetary scale (Zachos et al., 2001; Bowen and Zachos, 2010), but resolution of such information at the biome level is patchy at best. Data are available for matter (including carbon) production and consumption in prey-predator interactions and photosynthesis-based food webs from forests and open grasslands owing to a preponderance of fossils for producers and varied consumers, but views into the decomposition stages of these same food webs are few or lacking entirely (Weitschat and Wichard, 2010; Smith et al., 2019). Earthworms and leaf-litter and soil-associated insects are not commonly fossilized, yet they are major actors in carbon burial and release from soil. When these organisms are preserved, it is typically as inclusions in amber, yet amber mostly preserves that portion of the biota closely linked to trees or the bases of trees and therefore biases our perspective via selective trapping (Solórzano Kraemer et al., 2018). Nonetheless, there are a growing number of examples of animals not linked directly to the amber-producing trees, such as aquatic forms of Euarthropoda and parasitic roundworms (Wichard et al., 2009; Poinar, 2011). Such organisms normally appear in the amber record when parts of soil or leaf-litter are captured by the flowing resin (Beimforde et al., 2011; Speranza et al., 2015; Delclòs et al., 2020). As we demonstrate, fly larvae associated with the topsoil are also included in this list of unlikely, soft-bodied fossils from amber. The discovery of dung-decomposing larvae is especially important in building a larger picture of the Eocene Baltic amber forest. The presence of dung is also possibly indicative of the presence of larger mammals in the forest not otherwise preserved, and thereby provides additional inference regarding the palaeoecology and fauna of the European Eocene. Additionally, the discovery of fossil larvae of Volucellini suggests some clues into the potential causes of mass resin production in the Eocene forest of Europe.
ACKNOWLEDGEMENTS
V.B. is grateful to M. Spies and D. Doczkal (ZSM Munich) for the invaluable help with collection of ZSM as well as help with the literature. G. Rotheray, L. MacGowan, and P. Beuk have provided valuable discussion of some aspects of the fly larvae morphology and ecology. We are grateful to R. Gromes for his help with identification of the plant remains. We are grateful to the handling editor E. Peñalver, Executive Editor A. Bush and two anonymous reviewers for their efforts in improving this manuscript. Thanks to all people providing free software. We are grateful to C. and H.-W. Hoffeins, and J. Damzen for their invaluable support and donating the specimens, which made this study possible. J.M. Starck and Carolin Haug, both LMU Munich, are thanked for long standing support. This project is supported by the Volkswagen Foundation in the frame of a Lichtenberg Professorship of Joachim T. Haug (Joachim T. Haug; Viktor Baranov). V. Baranov was supported by the LMU Excellence Initiative, via LMU Junior Researcher Fund. Scanning of the specimens was supported by the DESY Block Allocation Group project “Scanning the past - Reconstructing the diversity in million years old fossil amber specimens using SRµCT “ at PETRA III. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge the KIT light source for provision of instruments at their beamlines and we would like to thank the Institute for Beam Physics and Technology (IBPT) for the operation of the storage ring, the Karlsruhe Research Accelerator (KARA).
REFERENCES
Alekseev, V.I. 2013. The beetles (Insecta: Coleoptera) of Baltic amber: the checklist of described species and preliminary analysis of biodiversity. Zoology and Ecology, 23: 5-12. https://doi.org/10.1080/21658005.2013.769717
Alekseev, V.I. 2019. First inclusion of a trichiine beetle (Coleoptera: Scarabaeidae) from Baltic amber. Palaeoentomology, 2:425-429. https://doi.org/10.11646/palaeoentomology.2.5.4
Andersen, N.M., Weitschat, W., Brandt, A., Coleman, C.O., Myers, A.A., and Wichard, W. 2002. Taphocoenosis of an extraordinary arthropod community in Baltic amber. Mitteilungen aus dem Geologisch-Paläontologischen Institut (GPI) der Universität Hamburg, 86:189-210.
Andersen, T., Baranov, V., Hagenlund, L.K., Ivković, M., Kvifte, G.M., and Pavlek, M. 2015. Blind flight? A new troglobiotic orthoclad (Diptera, Chironomidae) from the Lukina Jama-Trojama Cave in Croatia. PLoS ONE, 11:e0152884. https://doi.org/10.1371/journal.pone.0152884
Badano, D., Engel, M.S., Basso, A., Wang, B., and Cerretti, P. 2018. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nature Communications, 9:3257. https://doi.org/10.1038/s41467-018-05484-y
Baranov, V.A., Schädel, M., and Haug, J.T. 2019a. Fly palaeo-evo-devo: immature stages of bibionomorphan dipterans in Baltic and Bitterfeld amber. PeerJ, 7:e7843. https://doi.org/10.7717/peerj.7843
Baranov, V.A., Hoffeins, C., Hoffeins, H.W., and Haug, J.T. 2019b. More than dead males: reconstructing the ontogenetic series of terrestrial non-biting midges from the Eocene amber forest. Bulletin of Geosciences, 94:187-199. https://doi.org/10.3140/bull.geosci.1739
Baranov, V.A., Wang, Y., Wedmann, S., and Haug, J. T. 2020. Eco-morphological diversity of larvae of soldier flies and their closest relatives in deep time. PeerJ, 8:e10356. https://doi.org/10.7717/peerj.10356
Barnet, J.S., Littler, K., Westerhold, T., Kroon, D., Leng, M.J., Bailey, I., and Zachos, J.C. 2019. A high-fidelity benthic stable isotope record of late Cretaceous-early Eocene climate change and carbon-cycling. Paleoceanography and Paleoclimatology, 34:672-691. https://doi.org/10.1029/2019PA003556
Beimforde, C. and Schmidt, A.R. 2011. Microbes in resinous habitats: A compilation from modern and fossil resins. Advances in Stromatolite Geobiology Lecture Notes in Earth Sciences, 131:391-407.
Bowen, G.J. and Zachos, J.C. 2010. Rapid carbon sequestration at the termination of the Palaeocene-Eocene Thermal Maximum. Nature Geoscience, 3:866-869. https://doi.org/10.1038/ngeo1014
Brooks, S.J. 2006. Fossil midges (Diptera: Chironomidae) as palaeoclimatic indicators for the Eurasian region. Quaternary Science Reviews, 25:1894-1910. https://doi.org/10.1016/j.quascirev.2005.03.021
Brown, B.V., Borkent A., Adler P.H., De Souza Amorim D., Barber, K., Bickel, D., Boucher, S., Brooks, S.E., Burger, J., Burington, Z.L., Capellari, R.S., Costa, D.N.R., Cumming, J.M., Curler, G., Dick, C.W., Epler, J.H., Fisher, E., Gaimari, S.D., Gelhaus, J., Grimaldi, D.A., Hash, J., Hauser, M., Hippa, H., Ibáñez-Bernal, S., Jaschhof, M., Kameneva, E.P., Kerr, P.H., Korneyev, V., Korytkowski, C.A., Kung, G.-A., Kvifte, G.M., Lonsdale, O., Marshall, S.A., Mathis, W., Michelsen, V., Naglis, S., Norrbom, A.L., Paiero, S., Pape, T., Pereira-Colavite, A., Pollet, M., Rochefort, S., Rung, A., Runyon, J.B., Savage, J., Silva, V.C., Sinclair, B.J., Skevington, J.H., Stireman III, J.O., Swann, J., Thompson, C.F., Vilkamaa, P., Wheeler, T., Whitworth, T., Wong, M., Wood, M.D., Woodley, N., Yau, T., Zavortink, T.J., and Zumbado, M.A. 2018. Comprehensive inventory of true flies (Diptera) at a tropical site. Communications Biology, 1:1-8. https://doi.org/10.1038/s42003-018-0022-x
Bukejs, A. and Alekseev, V. I. 2018. A new extinct species of Ataenius Harold from Baltic amber (Coleoptera: Scarabaeidae: Aphodiinae). Zootaxa, 4442:153-160. https://doi.org/10.11646/zootaxa.4442.1.8
Cai, C., Escalona, H.E., Li, L., Yin, Z., Huang, D., and Engel, M.S. 2018. Beetle pollination of cycads in the Mesozoic. Current Biology, 28:2806-2812. https://doi.org/10.1016/j.cub.2018.06.036
Cecilia, A., Rack, A., Douissard, P.A., Martin, T., dos Santos Rolo, T., Vagovič, P., Hamanna, E., van de Kamp, T., Riedel, A., Fiederle, M., and Baumbach, T. 2011. LPE grown LSO: Tb scintillator films for high resolution x-ray imaging applications at synchrotron light sources. Nuclear Instruments and Methods in Physics Research, 648 (Supplement 1):321-323. https://doi.org/10.1016/j.nima.2010.10.150
Chame, M. 2003. Terrestrial mammal feces: a morphometric summary and description. Memórias do Instituto Oswaldo Cruz, 98:71-94.
Čičková, H., Pastor, B., Kozánek, M., Martínez-Sánchez, A., Rojo, S., and Takáč, P. 2012. Biodegradation of pig manure by the housefly, Musca domestica: a viable ecological strategy for pig manure management. PLoS ONE, 7:0032798. https://doi.org/10.1371/journal.pone.0032798
Delclòs, X., Peñalver, E., Ranaivosoa, V., and Solórzano-Kraemer, M.M. 2020. Unravelling the mystery of “Madagascar copal”: Age, origin and preservation of a Recent resin. PLoS ONE, 15:e0232623. https://doi.org/10.1371/journal.pone.0232623
Einarsson, Á., Gardarsson, A., Gíslason, G.M., and Ives, A.R. 2002. Consumer-resource interactions and cyclic population dynamics of Tanytarsus gracilentus (Diptera: Chironomidae). Journal of Animal Ecology, 71:832-845. https://doi.org/10.1046/j.1365-2656.2002.00648.x
Elberling, H. and Olesen, J.M. 1999. The structure of a high latitude plant‐flower visitor system: the dominance of flies. Ecography, 22:314-323. https://doi.org/10.1111/j.1600-0587.1999.tb00507.x
Evenhuis, N.L. 2014. Heleomyzidae. Catalogue of the fossil flies of the world (Insecta: Diptera), Ver. 2.0. Data accessed 15 September 2020. http://hbs.bishopmuseum.org/fossilcat/
Ferrar, P. 1987. A guide to the breeding habits and immature stages of Diptera Cyclorrhapha. Vol. 8. EJ Brill/Scandinavian Science Press, Copenhagen.
Frieling, J., Gebhardt, H., Huber, M., Adekeye, O.A., Akande, S.O., Reichart, G.J., and Sluijs, A. 2017. Extreme warmth and heat-stressed plankton in the tropics during the Paleocene-Eocene Thermal Maximum. Science Advances, 3:e1600891. https://doi.org/10.1126/sciadv.1600891
Greving, I., Wilde, F., Ogurreck, M., Herzen, J., Hammel, J.U., Hipp, A., Friedrich, F., Lottermoser, L., Dose, T., Burmester, H., Müller, M., and Beckmann, F. 2014. P05 imaging beamline at PETRA III: first results. In Developments in X-Ray Tomography IX, 9212:921200. https://doi.org/10.1117/12.2061768
Grimaldi, D. and Engel, M.S. 2005. Evolution of the Insects. Cambridge University Press, Cambridge.
Grund, M. 2006. Chironomidae (Diptera) in Dominican amber as indicators for ecosystem stability in the Caribbean. Palaeogeography, Palaeoclimatology, Palaeoecology, 241:410-416. https://doi.org/10.1016/j.palaeo.2006.04.005
Haug, J.T. 2020. Why the term “larva” is ambiguous, or what makes a larva? Acta Zoologica, 101:167-188. https://doi.org/10.1111/azo.12283
Haug, J.T. and Haug, C. 2019. Beetle larvae with unusually large terminal ends and a fossil that beats them all (Scraptiidae, Coleoptera). PeerJ, 7:e7871. https://doi.org/10.7717/peerj.7871
Haug, J.T., Schädel, M., Baranov, V.A., and Haug, C. 2020. An unusual 100-million-year-old holometabolan larva with a piercing mouth cone. PeerJ, 8:e8661. https://doi.org/10.7717/peerj.8661
Jun, C., Wang, B., Engel, M.S., Wappler, T., Jarzembowski, E.A., Zhang H., Wang, X., Zheng, X., and Rust, J. 2014. Extreme adaptations for aquatic ectoparasitism in a Jurassic fly larva. eLife, 3:e02844. https://doi.org/10.7554/eLife.02844
Kasiński, J.R., Kramarska, R., Słodkowska, B., Sivkov, V., and Piwocki, M. 2020. Paleocene and Eocene deposits on the eastern margin of the Gulf of Gdańsk (Yantarny P-1 bore hole, Kaliningrad region, Russia). Geological Quarterly, 64:20-53.
Kerr, P.H., and Winterton, S.L. 2008. Do parasitic flies attack mites? Evidence in Baltic amber. Biological Journal of the Linnean Society, 93(1): 9-13.
Kosmowska-Ceranowicz, B. and Kulicka, R. 1995. Amber molars. Amber & Fossils, 1:38-41.
Limaye, A. 2012. Drishti: a volume exploration and presentation tool. Developments in X-ray Tomography VIII, Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE), 8506:85060X. https://doi.org/10.1117/12.935640
Lohrmann, V. and Engel, M.S. 2017. The wasp larva’s last supper: 100 million years of evolutionary stasis in the larval development of rhopalosomatid wasps (Hymenoptera: Rhopalosomatidae). Fossil Records, 20:239-244. https://doi.org/10.5194/fr-20-239-2017
Lozano, R.P., Pérez-de la Fuente, R., Barrón, E., Rodrigo, A., Viejo, J.L., and Peñalver, E. 2020. Phloem sap in Cretaceous ambers as abundant double emulsions preserving organic and inorganic residues. Scientific Reports, 10:9751. https://doi.org/10.1038/s41598-020-66631-4
Marshall, S.A. 2012. Flies: the Natural History and Diversity of Diptera. Firefly Books, Richmond Hill, Ontario.
Martínez-Delclòs, X., Briggs, D.E.G., and Peñalver, E. 2004. Taphonomy of insects in carbonates and amber. Palaeogeography, Palaeoclimatology, Palaeoecology, 203:19-64. https://doi.org/10.1016/S0031-0182(03)00643-6
McAlister, E. 2017. The Secret Life of Flies. Firefly Books, Richmond Hill, Ontario.
McKellar, R.C., Wolfe, A.P., Muehlenbachs, K., Tappert, R., Engel, M.S., Cheng, T., and Sánchez-Azofeifa, A.G. 2011. Insect outbreaks produce distinctive carbon isotope signatures in defensive resins and fossiliferous ambers. Proceedings of the Royal Society B: Biological Sciences, 278:3219-3224. https://doi.org/10.1098/rspb.2011.0276
Methner, K., Lenz, O., Riegel, W., Wilde, V., and Mulch, A. 2019. Paleoenvironmental response of midlatitudinal wetlands to Paleocene-early Eocene climate change (Schöningen lignite deposits, Germany). Climate of the Past, 15:1741-1755. https://doi.org/10.5194/cp-15-1741-2019
Meunier, F. 1910. Monographie der Leptiden und der Phoriden des Bernsteins. Jahrbuch der Königlich Preussischen Geologischen Landesanstalt, 30:64-90.
Moe, A.P. and Smith, D.M. 2005. Using pre-Quaternary Diptera as indicators of paleoclimate. Palaeogeography, Palaeoclimatology, Palaeoecology, 221:203-214. https://doi.org/10.1016/j.palaeo.2005.02.012
Monfared, A., Azhari, Sh., and Gilasian, E. 2013. Volucella bombylans (Syrphidae, Diptera) recorded from a colony of Bombus mesomelas (Apidae, Hymenoptera) in Iran. Linzer Biologische Beiträge, 45:829-836.
Mostovski, M.B., Jarzembowski, E.A., and Coram, R.A. 2003. Horseflies and athericids (Diptera: Tabanidae, Athericidae) from the lower Cretaceous of England and Transbaikalia. Paleontological Journal, 37:162-169.
Myskowiak, J. and Nel, A. 2014. A new genus and species of ibis fly in the Lowermost Eocene amber of Oise (France) (Diptera: Athericidae). Zootaxa 3869:372-382. https://doi.org/10.11646/zootaxa.3869.4.2
Nel, A., De Ploeg, G., and Perrichot, V. 2014.The first ibis fly in mid-Cretaceous amber of France (Diptera: Athericidae). Zootaxa, 3768:591-595. https://doi.org/10.11646/zootaxa.3768.5.6
Oberprieler, S.K. and Yeates, D.K. 2014. Notoatherix antiqua gen. et sp. nov., first fossil water snipe fly from the Late Jurassic of Australia (Diptera: Athericidae). Zootaxa, 3866:138-144. https://doi.org/10.11646/zootaxa.3866.1.8
Peñalver, E., Labandeira, C.C., Barrón, E., Delclòs, X., Nel, P., Nel, A., Tafforeau, P., and Soriano, C. 2012. Thrips pollination of Mesozoic gymnosperms. Proceedings of the National Academy of Sciences, 109:8623-8628. https://doi.org/10.1073/pnas.1120499109
Peñalver, E., Barrón, E., Delclòs, X., Álvarez-Fernández, E., Arillo, A., López Del Valle Lozano, R.P., Murillo-Barroso, M., Pérez-de la Fuente, R., Peris, D., Rodrigo, A., Sánchez-García, A., Sarto i Monteys, V., Viejo, J.L., and Vilaça, R. 2018. Amber in Portugal: state of the art. Cuadernos del Museo Geominero, 25:279-287.
Penney, D. 2016. Amber Palaeobiology: Research Trends and Perspectives for the 21st Century. Siri Scientific Press, Manchester.
Pérez-de la Fuente, R., Delclòs, X., Peñalver, E., Speranza, M., Wierzchos, J., Ascaso, C., and Engel, M.S. 2012. Early evolution and ecology of camouflage in insects. Proceedings of the National Academy of Sciences, 109:21414-21419. https://doi.org/10.1073/pnas.1213775110
Pérez-de la Fuente, R., Engel, M.S., Delclòs, X., and Peñalver, E. 2020. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretaceous Research, 106:104200. https://doi.org/10.1016/j.cretres.2019.104200
Peris, D., Ruzzier, E., Perrichot, V., and Delclòs, X. 2016. Evolutionary and paleobiological implications of Coleoptera (Insecta) from Tethyan-influenced Cretaceous ambers. Geoscience Frontiers, 7:695-706. https://doi.org/10.1016/j.gsf.2015.12.007
Petrov, A.V. and Perkovsky, E.E. 2008. New species of bark beetles from the Rovno amber (Insecta: Coleoptera: Scolytidae). Paleontological Journal, 42:406-408. https://doi.org/10.1134/S0031030108040096
Petrov, A.V. and Perkovsky, E.E. 2018. A new genus and species of Scolytinae (Coleoptera: Curculionidae) from the Rovno amber. Paleontological Journal, 52:164-167. https://doi.org/10.1134/S0031030118020090
Podeniene, V., Podenas, S., and Gelhaus, J. K. 2004. First record of a crane fly larva (Diptera, Limoniidae: Chioneinae) from Baltic amber. Annals of the Entomological Society of America, 97:1126-1128. https://doi.org/10.1603/0013-8746(2004)097[1126:FROACF]2.0.CO;2
Pohl, H., Wipfler, B., Grimaldi, D., Beckmann, F., and Beutel, R.G. 2010. Reconstructing the anatomy of the 42-million-year-old fossil † Mengea tertiaria (Insecta, Strepsiptera). Naturwissenschaften, 97:855-859. https://doi.org/10.1007/s00114-010-0703-x
Poinar Jr., G.O. 2007. Enchytraeidae (Annelida: Oligochaeta) in amber. Megadrilogica, 11(5):53-57.
Poinar Jr., G.O. 2011. The Evolutionary History of Nematodes: as Revealed in Stone, Amber and Mummies. Brill, Copenhagen.
Rolland, N., Larocque, I., Francus, P., Pienitz, R., and Laperrière, L. 2008. Holocene climate inferred from biological (Diptera: Chironomidae) analyses in a Southampton Island (Nunavut, Canada) lake. The Holocene, 18:229-241. https://doi.org/10.1177/0959683607086761
Rotheray, G.E. 1993. Colour guide to hoverfly larvae (Diptera: Syrphidae). Dipterists Digest, 9:1-156.
Rotheray, G.E. 2012. Morphology of the puparium and breeding sites of eight species of Heleomyzidae (Diptera). Journal of Natural History, 46:2075-2102. https://doi.org/10.1080/00222933.2012.707241
Rozkošný, R. 1997. Diptera Stratiomyidae, Soldier Flies, p. 331-332. In Nilsson, A. (ed.), Aquatic Insects of North Europe-A Taxonomic Handbook, 2. Apollo Books, Stenstrup.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, E., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P., and Cardonaet, A. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods, 9:676-682. https://doi.org/10.1038/nmeth.2019
Seyfullah, L.J., Roghi, G., Dal Corso, J., and Schmidt, A.R. 2018. The Carnian Pluvial Episode and the first global appearance of amber. Journal of the Geological Society, 175:1012-1018. https://doi.org/10.1144/jgs2017-143
Shaffer, G., Huber, M., Rondanelli, R., and Pedersen, J.O.P. 2016. Deep time evidence for climate sensitivity increase with warming. Geophysical Research Letters, 43: 6538-6545. https://doi.org/10.1002/2016GL069243
Sidorchuk, E.A., Bochkov, A.V., Weiterschan, T., and Chernova, O.F. 2019. A case of mite-on-mammal ectoparasitism from Eocene Baltic amber (Acari: Prostigmata: Myobiidae and Mammalia: Erinaceomorpha). Journal of Systematic Palaeontology, 17:331-347. https://doi.org/10.1080/14772019.2017.1414889
Smith, K.G.V. 1989. An introduction to the immature stages of British flies: Diptera larvae, with notes on eggs, puparia and pupae. Handbooks for the Identification of British Insects 10. Royal Entomological Society, London.
Smith, K.T., Schaal, S.F., and Habersetzer, J. 2019. Messel-An Ancient Greenhouse Ecosystem. The Quarterly Review of Biology, 94:1-393.
Solórzano-Kraemer, M.M., Delclòs, X., Clapham, M.E., Arillo, A., Peris, D., Jäger, P., Stebner, F., and Peñalver, E. 2018. Arthropods in modern resins reveal if amber accurately recorded forest arthropod communities. Proceedings of the National Academy of Sciences, 115:6739-6744. https://doi.org/10.1073/pnas.1802138115
Speight, M.C.D. 2010. Species accounts of European Syrphidae (Diptera). In Speight, M.C.D., Castella, E., Sarthou, J.-P., and Monteil, C. (eds.), Syrph the Net, the database of European Syrphidae, 44, Syrph the Net Publication, Dublin. Accessed at 15 September 2020. https://www.semanticscholar.org/paper/the-syrph-the-net-database-of-european-syrphidae Speight/a9a7f071a4a8d6756fc9fcf113485c4f563fd5ed
Speranza M., Ascaso C., Delclòs X., and Peñalver E. 2015. Cretaceous mycelia preserving fungal polysaccharides: taphonomic and paleoecological potential of microorganisms preserved in fossil resins. Geologica Acta, 13:363-385.
Stuckenberg, B.R. 1974. A new genus and two new species of Athericidae (Diptera) in Baltic amber. Annals of the Natal Museum, 22: 275-288.
Szadziewski, R. 2018. Biting midges (Diptera: Ceratopogonidae) as indicators of biostratigraphy, ecological reconstructions, and identification of amber deposits. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 107: 219-230. https://doi.org/10.1017/S1755691017000378
Tamutis, V., Alekseev, V.I., and Bukejs, A. 2017. A new species of Eupariini from Eocene Baltic amber (Coleoptera: Scarabaeidae: Aphodiinae). Zootaxa, 4353:568-576. https://doi.org/10.11646/zootaxa.4353.3.10
Ulrich, H. and Schmelz, R.M. 2001. Enchytraeidae as prey of Dolichopodidae, recent and in Baltic amber (Oligochaeta; Diptera). Bonner Zoologische Beiträge, 50, 89-102.
Van Aarle, W., Palenstijn, W.J., De Beenhouwer, J., Altantzis, T., Bals, S., Batenburg, K.J., and Sijbers, J. 2015. The ASTRA Toolbox: A platform for advanced algorithm development in electron tomography. Ultramicroscopy, 157:35-47. https://doi.org/10.1016/j.ultramic.2015.05.002
Van Aarle, W., Palenstijn, W.J., Cant, J., Janssens, E., Bleichrodt, F., Dabravolski, A., De Beenhouwer, J., Batenburg, K.J., and Sijbers, J. 2016. Fast and flexible X-ray tomography using the ASTRA toolbox. Optics Express, 24:25129-25147. https://doi.org/10.1364/OE.24.025129
Vogelgesang, M., Chilingaryan, S., dos Santos Rolo, T., and Kopmann, A. 2012. UFO: A scalable GPU-based image processing framework for on-line monitoring, p. 824-829. 2012 IEEE 14th International Conference on High Performance Computing and Communication and 2012 IEEE 9th International Conference on Embedded Software and Systems, Liverpool. https://doi.org/10.1109/HPCC.2012.116
Vogelgesang, M., Farago, T., Morgeneyer, T.F., Helfen, L., dos Santos Rolo, T., Myagotin, A., and Baumbach, T. 2016. Real-time image-content-based beamline control for smart 4D X-ray imaging. Journal of Synchrotron Radiation, 23:1254-1263. https://doi.org/10.1107/S1600577516010195
Walker, I.R. 2001. Midges: Chironomidae and related Diptera, p. 43-66. In Last, W.M. and Smol, J.P. (eds.), Tracking Environmental Change Using Lake Sediments. Springer, Dordrecht.
Wang, Y.-H., Engel, M.S., Rafael, J.A., Wu, H.-Y., Rédei, D., Xie, Q., Wang, G., Liu, X.-Q., and Bu, W.-J. 2016. Fossil record of stem groups employed in evaluating the chronogram of insects (Arthropoda: Hexapoda). Scientific Reports, 6:38939. https://doi.org/10.1038/srep38939
Wappler, T., Labandeira, C.C., Engel, M.S., Zetter, R., and Grimsson, F. 2015. Specialized and generalized pollen-collection strategies in an ancient bee lineage. Current Biology, 25:3092-3098. https://doi.org/10.1016/j.cub.2015.09.021
Webb, D.W. 1977. The Nearctic Athericidae (Insecta: Diptera). Journal of the Kansas Entomological Society, 50:473-495.
Webb, D.W. 1994. The immature stages of Suragina concinna (Williston) (Diptera: Athericidae). Journal of the Kansas Entomological Society, 67:421-425.
Weitschat, W. and Wichard, W. 2002. Atlas of Plants and Animals in Baltic Amber. Pfeil Verlag, Munich.
Weitschat, W. and Wichard, W. 2010. Baltic amber, p. 80-115. In Penney, D. (ed.), Biodiversity of Fossils in Amber from the Major World Deposits, Siri Scientific Press, Manchester.
Wichard, W. 2009. Taphozönosen im baltischen Bernstein. Denisia, 26:257-266.
Wichard, W., Gröhn, C., and Seredszus, F. 2009. Aquatic Insects in Baltic Amber/Wasserinsekten im baltischen Bernstein. Verlag Kessel, Remagen.
Wilde, F., Ogurreck, M., Greving, I., Hammel, J.U., Beckmann, F., Hipp, A., Lottermoser, L., Khokhriakov, I., Lytaev, P., Dose, T., Burmester, H., Müller, M., and Schreyer, A. 2016. Micro-CT at the imaging beamline P05 at PETRA III. AIP Conference Proceedings, 1741:030035. https://doi.org/10.1063/1.4952858
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292(5517):686-693. https://doi.org/10.1126/science.1059412
Zhang, J. 2012. New horseflies and water snipe-flies (Diptera: Tabanidae and Athericidae) from the Lower Cretaceous of China. Cretaceous Research, 36:1-5. https://doi.org/10.1016/j.cretres.2012.01.004

