 Diversity of culicomorphan dipterans in the Eocene Kishenehn Konservat-Lagerstätte (Montana, USA) and its palaeoecological implications
Diversity of culicomorphan dipterans in the Eocene Kishenehn Konservat-Lagerstätte (Montana, USA) and its palaeoecological implications
Article number: 25.1.a4
https://doi.org/10.26879/1165
Copyright Paleontological Society, January 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 May 2021. Acceptance: 21 December 2021.
ABSTRACT
This report highlights the eight new morphotypes of culicomorphan dipterans from 46-million-year-old Kishenehn Formation (Montana, USA). Five of these morphotypes are non-biting midges of the family Chironomidae (cf. Conchapelopia, cf. Psectrotanypus, Hintelmanniella noncatafractata sp. nov., cf. Tanytarsus, and Rheotanytarsus lacustris sp. nov.), one morphotype is a biting midge of the family Ceratopogonidae (cf. Alluaudomyia), one is a phantom midge of the family Chaoboridae (Chaoborus kishenehnicus sp. nov.), and the last one is a true mosquito, family Culicidae (Neoculex). We discuss implications of the diversity and abundance of representatives of Culicomorpha in the Formation for understanding the palaeoecology of the deposit. Abundance of Chaoboridae and presence of the seemingly surface skating Chironomidae indicate a large, lacustrine habitat.
Viktor A. Baranov. Ludwig-Maximilians-Universität München, Biocenter, Planegg, Bayern, Germany. Correspondence author. baranow@biologie.uni-muenchen.de
Joachim T. Haug. Ludwig-Maximilians-Universität München, Biocenter, Planegg, Bayern, Germany and Geobio-Center, Ludwig-Maximilians-Universität München, München, Bayern, Germany. jhaug@biologie.uni-muenchen.de
Dale E. Greenwalt. Department of Paleobiology, National Museum of Natural History MRC 121, Smithsonian Institution, 10th & Constitution Ave. NW, Washington, D.C., United States. 20013-7012, USA. GreenwaltD@si.edu
Ralph Harbach. Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, United Kingdom, R.Harbach@nhm.ac.uk
Keywords: Diptera; Kishenehn Formation; Eocene; larva; pupa; new species
Final citation: Baranov, Viktor A., Haug, Joachim T., Greenwalt, Dale E., and Harbach, Ralph. 2022. A vanished ecosystem: Diversity of culicomorphan dipterans in the Eocene Kishenehn Konservat-Lagerstätte (Montana, USA) and its palaeoecological implications. Palaeontologia Electronica, 25(1):a4. https://doi.org/10.26879/1165
palaeo-electronica.org/content/2022/3505-midges-of-kishenehn-formation
Copyright: January 2022 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/750E51F4-E7F2-4411-8F90-
INTRODUCTION
Fossil deposits with outstanding preservation of soft-bodied animals, the so-called Konservat-Lagerstätten (Grimaldi and Engel, 2005), are among the most lauded sources of data about environments of the past. Konservat-Lagerstätten often capture components of ecosystems not preserved elsewhere, such as animals without hard body parts, life-like behaviour, and interactions of organisms or traces of their activities (Arratia et al., 2015). Lagerstätten are often also very good in preserving small and fragile animals, such as representatives of the hyper-diverse group Insecta, which are otherwise rare in sedimentary rocks (Grimaldi and Engel, 2005; Greenwalt et al., 2014). Insects are excellent proxies of environmental conditions and can be used to reconstruct ecosystems of the past (Merrit and Cummins, 1996; Grund, 2006). One such exemplary indicator is the flies (Diptera). Diptera is a monophyletic group with at least 160,000 extant species comprised of gnats, midges, mosquitoes, and myriads of related flies (Marshall, 2012). Due to this diversity and abundance, flies can serve as important indicators of environmental conditions in both modern and fossil ecosystems (Grund, 2006).
A relatively recently recognized Konservat-Lagerstätte with diverse and abundant dipteran fossils is the Middle Eocene Coal Creek Member of the Kishenehn Formation in north-western Montana, USA. So far, 23 species of Diptera representing 21 large families have been reported from the Kishenehn Formation (Greenwalt et al., 2019). Here we deal with representatives of the Culicomorpha (midges and mosquitoes), which make up over 50% of the fossil insects in the formation. Several species of the culicomorphan ingroups Culicidae and Dixidae have already been described (Harbach and Greenwalt, 2012; Greenwalt et al., 2014; Greenwalt and Moulton, 2016). In total, five major culicomorphan families have been recorded in the Kishenehn Formation: Ceratopogonidae, Chaoboridae, Chironomidae, Culicidae, and Dixidae (Greenwalt et al., 2014, 2019). The Kishenehn Formation is in fact one of the richest sources of fossil mosquitoes (Culicidae) in the world (Harbach and Greenwalt, 2012). Two of the 27 known fossil species of Culicidae were described from the Kishenehn, with nearly 100 specimens collected from the formation. About 10 of these are blood-engorged, the only such specimens known to science. Particularly spectacular was an engorged mosquito that contained host heme in its blood meal (Greenwalt et al., 2013). Here we report previously undescribed representatives of Culicomorpha, including forms of Chaoboridae, Chironomidae, and Ceratopogonidae from the formation, as well as one of the very few fossil larvae of Culicidae known from the fossil record. We also discuss implications of the diversity and abundance of representatives of Culicomorpha in the Kishenehn Formation for understanding the palaeoecology of the deposit.
MATERIAL AND METHODS
Imaging
Specimens were imaged using either a Canon EF-S 18-55 mm macro lens or a Canon MP-E 65 mm f/2.8 1-5x macro lens. Exact methods follow those of Haug et al. (2011). Images were taken with additional light-spot polarized illumination, with a CanonEos Rebel Canon EOS 5D MKIII camera on a repro-stand. Some specimens were imaged using a Keyence VHX-6000 digital microscope, either with ring light illumination or cross-polarized co-axial illumination (Haug et al., 2018). All images are vertical stacks and horizontal composites done with inbuilt microscope software (in case of VHX-6000) or Photoshop Elements CS 11 panorama functionality and PICOLAY open software (www.picolay.de). Body dimensions of specimens were measured from photos with ImageJ (public domain, Schindelin et al., 2012). In total, we have examined 428 specimens of Culicomorpha from the Kishenehn Formation.
Geological Setting
The Coal Creek Member of the Kishenehn Formation, which contains well-preserved fossils of Insecta, is estimated to be 46.2 ± 0.4 MYA (Middle Eocene) based on single-crystal laser fusion Ar40/Ar39r dating of 12 biotite grains from tephra (Constenius, 1996). Climate at the location of the fossil deposition in the Middle Eocene was tropical or near tropical (Wolfe, 1995; Pierce and Constenius, 2001; Zachos et al., 2001; Huber and Caballero, 2011; Greenwalt et al., 2014). It appears that most of the smaller fossils were deposited in the littoral and profundal area of a large lake, while larger fossils (mostly representatives of Vertebrata) that occur in the formation were preserved in the profundal area of the waterbody (Constenius et al., 1989; Pierce and Constenius, 2014; Greenwalt et al., 2014).
Fossiliferous shales are made up from varves (annual layers) composed of two or three distinct components: (i) eolian siliciclastic particles; (ii) a black layer of the algae-derived carbonaceous materials; +/- (iii) a distinct layer of small amorphous particles between the latter two layers, probably formed as a result of CaCO3 precipitation (Greenwalt et al., 2014). Precipitation of CaCO3 seems to have been caused by the annual warming of the littoral area (probably during spring) prior to subsequent blooms of algae and/or cyanobacteria (Greenwalt et al., 2014). Massive annual algal blooms were a regular occurrence in the ancient lake of the Kishenehn Formation and appear to be the main factor responsible for the fine-scale preservation of the fossil insects, due to the production of a large amount of mucopolysaccharides by cyanobacteria (Greenwalt et al., 2014, 2019). Mucopolysaccharides are known to entrap and ‘stabilize’ insects’ bodies in both other fossil settings and extant aquatic habitats (Dunn et al., 1997; Martínez-Delclòs et al., 2004). The antimicrobial activity of mucopolysaccharides and the anoxic environment created by algal blooms have been shown to provide protection against the decomposition of dead insects (Martínez-Delclòs et al., 2004).
Taxonomy
Wherever possible, we decided not to use Linnean ranks (‘rankless taxonomy’). Ranks (or “categories” sensu Mayr, 1942, p.102) represent arbitrary constructs in a way that do not hold ‘comparative values’ (Mayr, 1942, p. 291, line 3) and, in our view, do not contribute to an easier understanding of the phylogenetic relations among species and higher groups (Haug et al., 2020).
Three new species are formally described herein, and a new ‘genus-level’ name is also established. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system of the ICZN. The ZooBank Life Science Identifiers (LSIDs) can be resolved, and the associated information viewed through any standard web browser, by appending the LSID to the prefix http://zoobank.org/.
The morphological terminology largely follows Marshall et al. (2017) and Borkent and Sinclair (2017), and specifically follows Harbach and Knight (1980) for mosquito anatomical terminology and abbreviations and Borkent (2012) for culicomorphan pupae. Yet, to enhance the understandability for non-experts, we amended some of the special morphological terms with more general terms. As Insecta is an accepted ingroup of Crustacea s.l., ‘crustacean’ terms given in square brackets were necessary to provide a wider frame of correspondence (Baranov et al., 2019a, 2019b, 2020). In this paper, we deal mostly with morphotypes, i.e., distinct morphological groups of organisms. We assume most if not all of them represent a single species, but representatives of some morphotypes are too poorly preserved to know for certain. We call all the remnants of the pupal life stage ‘pupa/pupae’ although many of the specimens are actually ‘pupal exuviae’, the integument left after emergence of the adult fly.
Data Availability
All fossil specimens have been deposited in the palaeobiology collection of the National Museum of Natural History, Washington, DC, USA (USNM). A complete list of all accessed specimens that were studied is provided in Appendix 1.
SYSTEMATIC PALAEONTOLOGY
Eight new morphotypes of culicomorphan dipterans from the Kishenehn Formation are recorded here. Five of the morphotypes are non-biting midges, i.e., representatives of Chironomidae, one morphotype is a biting midge, i.e., a representative of Ceratopogonidae, one is a phantom midge, i.e., a representative of Chaoboridae, and the last one is a true mosquito, i.e., a representative of Culicidae.
DIPTERA Linnaeus, 1758
CHIRONOMIDAE Newman, 1838
TANYPODINAE Thienemann and Zavřel, 1916
CONCHAPELOPIA Fittkau, 1957
cf. CONCHAPELOPIA
Figure 1A-D, Figure 2A-C, Figure 3A-C
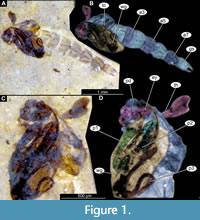 Material. This morphotype is represented by numerous specimens (see Appendix Table 1 for the full list). These specimens are representatives of Chironomidae, based on the following combination of characters: antennae extending posteriorly past the head of the pupa; maxillary palpi directed laterally; trunk end without articulated terminal paddles (Figure 1A-D) (Borkent, 2012).
Material. This morphotype is represented by numerous specimens (see Appendix Table 1 for the full list). These specimens are representatives of Chironomidae, based on the following combination of characters: antennae extending posteriorly past the head of the pupa; maxillary palpi directed laterally; trunk end without articulated terminal paddles (Figure 1A-D) (Borkent, 2012).
 Remarks. This morphotype of Chironomidae is exclusively represented by early and late pupae (pharate adults); therefore, we have refrained from a formal description of a species (Figure 1A-D, Figure 2A-C, Figure 3A-C).
Remarks. This morphotype of Chironomidae is exclusively represented by early and late pupae (pharate adults); therefore, we have refrained from a formal description of a species (Figure 1A-D, Figure 2A-C, Figure 3A-C).
Taxonomic attribution. Within Chironomidae, this morphotype most closely resembles representatives of the extant group Conchapelopia Fittkau, 1957 based on the following combination of characters: thoracic horn trumpet-shaped with plastron plate (surface for retention of the air film, providing a gas exchange interface); plastron of the thoracic horn occupying about 40% of the internal volume  of the entire structure; thoracic comb present behind the respiratory organ, row of curved cuticular hooks absent from tergite of abdominal unit two, abdominal unit one with a longitudinal, sclerotized mark present on the tergite; no lateral filaments visible on abdominal units 2-6; bifurcated spinules present on the abdominal tergite; abdominal unit 8 not extended posteriorly on each side by more than 0.2 of its median length; anal lobes (based on preserved genitals, and the shape of the imprint of the posterior abdomen) much longer than wide, tapering posteriorly (Figure 1A-D, Figure 2A-C, Figure 3A-C) (Langton, 1991). The combination of these characters indicates affinity with extant representatives of Conchapelopia, but unfortunately, many important characters, such as the shape and armament of the anal lobes, are poorly preserved. Therefore, a precise taxonomic affinity of this morphotype at the genus level cannot be provided.
of the entire structure; thoracic comb present behind the respiratory organ, row of curved cuticular hooks absent from tergite of abdominal unit two, abdominal unit one with a longitudinal, sclerotized mark present on the tergite; no lateral filaments visible on abdominal units 2-6; bifurcated spinules present on the abdominal tergite; abdominal unit 8 not extended posteriorly on each side by more than 0.2 of its median length; anal lobes (based on preserved genitals, and the shape of the imprint of the posterior abdomen) much longer than wide, tapering posteriorly (Figure 1A-D, Figure 2A-C, Figure 3A-C) (Langton, 1991). The combination of these characters indicates affinity with extant representatives of Conchapelopia, but unfortunately, many important characters, such as the shape and armament of the anal lobes, are poorly preserved. Therefore, a precise taxonomic affinity of this morphotype at the genus level cannot be provided.
TANYPODINAE Thienemann and Zavřel, 1916
PSECTROTANYPUS Kieffer, 1909
cf. PSECTROTANYPUS
Figure 4A-D, Figure 5A-B
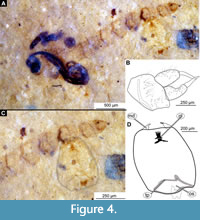 Material. This morphotype is represented by numerous specimens (see Appendix Table 1 for the full list). These specimens are representatives of Chironomidae, based on the following combination of pupal characters: antennae extending posteriorly past the head of the pupa; maxillary palpi directed laterally; strongly sclerotized arches from the anterior parts of the abdominal tergites; terminus of trunk without articulated terminal paddles (Figure 4A-D, Figure 5A-B).
Material. This morphotype is represented by numerous specimens (see Appendix Table 1 for the full list). These specimens are representatives of Chironomidae, based on the following combination of pupal characters: antennae extending posteriorly past the head of the pupa; maxillary palpi directed laterally; strongly sclerotized arches from the anterior parts of the abdominal tergites; terminus of trunk without articulated terminal paddles (Figure 4A-D, Figure 5A-B).
Remarks. This morphotype of Chironomidae is represented by early or late pupae (pharate adults) and a single larval head capsule with partially preserved abdominal cuticle. Because some crucial characters (anal lobes, male genitalia (hypopygium), wings) were not preserved, we have refrained from a formal description of a species (Figure 4A-D, Figure 5A-B).
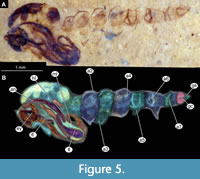 Taxonomic attribution. It was difficult to interpret the early or late pupae (pharate adults) of this morphotype within Chironomidae due to the lack of preserved wings, legs, anal lobes, and other important structures. Fortunately, it is quite common for pupae of Chironomidae to have their fourth-instar larval exuviae attached to the end of their abdomen, and one of the specimens from the Coal Creek Member (USNM 624474) has just that, a partially preserved larval exuviae still attached to the pupa (Figure 4A-D) (Armitage et al., 1995). We therefore assume that this larva belongs to the same individual as the pupa. The head capsule of this larva provided us with a means to further elucidate the identity of this morphotype. Based on the preserved larval characters, this morphotype is a representative of Tanypodinae, based on the distinct gula present behind the submentum of the head capsule (Figure 4C-D) (Cranston and Epler, 2013). This morphotype most closely resembles representatives of Psectrotanypus Kieffer, 1909, based on the following combination of characters: head capsule longer than wide but less than two times as long, mandible elongate, not curved, labial endites (ligula) with four teeth (Figure 4C-D) (Cranston and Epler, 2013). Association with this larval head capsule, as well as several features of the pupae (e.g., strongly sclerotized arches from the anterior parts of the abdominal tergites) and a similar habitus of male genitalia (hypopygium), visible through the late pupal cuticle, indicate that this morphotype is closely related to Psectrotanypus Kieffer, 1909 or being an ingroup, though it is impossible to further ascertain due to the many important characters not available (Roback, 1971; Langton, 1991).
Taxonomic attribution. It was difficult to interpret the early or late pupae (pharate adults) of this morphotype within Chironomidae due to the lack of preserved wings, legs, anal lobes, and other important structures. Fortunately, it is quite common for pupae of Chironomidae to have their fourth-instar larval exuviae attached to the end of their abdomen, and one of the specimens from the Coal Creek Member (USNM 624474) has just that, a partially preserved larval exuviae still attached to the pupa (Figure 4A-D) (Armitage et al., 1995). We therefore assume that this larva belongs to the same individual as the pupa. The head capsule of this larva provided us with a means to further elucidate the identity of this morphotype. Based on the preserved larval characters, this morphotype is a representative of Tanypodinae, based on the distinct gula present behind the submentum of the head capsule (Figure 4C-D) (Cranston and Epler, 2013). This morphotype most closely resembles representatives of Psectrotanypus Kieffer, 1909, based on the following combination of characters: head capsule longer than wide but less than two times as long, mandible elongate, not curved, labial endites (ligula) with four teeth (Figure 4C-D) (Cranston and Epler, 2013). Association with this larval head capsule, as well as several features of the pupae (e.g., strongly sclerotized arches from the anterior parts of the abdominal tergites) and a similar habitus of male genitalia (hypopygium), visible through the late pupal cuticle, indicate that this morphotype is closely related to Psectrotanypus Kieffer, 1909 or being an ingroup, though it is impossible to further ascertain due to the many important characters not available (Roback, 1971; Langton, 1991).
CHIRONOMINAE Macquart, 1838
CHIRONOMINI Macquart, 1838
Hintelmanniella Baranov and Haug, gen. nov.
Figure 6A-C, Figure 7A-D, Figure 8A-D, Figure 9A-E
zoobank.org/74C024CF-E621-4CBB-A8F2-
 Etymology. This genus is named after Mr. Robert Johann Heinrich Hintelmann (1918-1984) and Mrs. Elisabeth Hintelmann for their contribution to the development of zoological science in Germany, expressed by support provided towards early career researchers in zoological systematics through the R.J.H. Hintelmann Wissenschafts-Preis für Zoologische Systematik.
Etymology. This genus is named after Mr. Robert Johann Heinrich Hintelmann (1918-1984) and Mrs. Elisabeth Hintelmann for their contribution to the development of zoological science in Germany, expressed by support provided towards early career researchers in zoological systematics through the R.J.H. Hintelmann Wissenschafts-Preis für Zoologische Systematik.
Type species. Hintelmanniella noncatafractata Baranov and Haug, sp. nov.
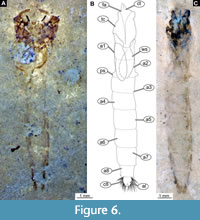
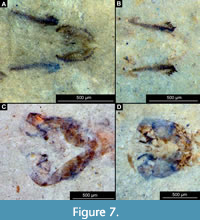 Diagnosis (based on adult males and pupal exuviae). The pupa of this species can be easily distinguished from all other known pupae of the group Chironomidae based on the following combination of characters: highly rugose cuticle of the thorax; tergite of the abdominal unit 2 lacks armament (Figure 6A-C, Figure 7A-D), most notably it has no row of hooks on the posterior margin; remaining abdominal tergites without armament/shagreen as well; abdominal unit 2 with prominent pedes spuri B (lateral protrusions of the tergite 2) (Figure 6B); abdominal unit 8 with prominent posterolateral comb comprised of 4-7 strongly sclerotized large spines and a number of smaller spines inserted between them; anal lobes with well-developed fringe of setae (Figure 7A-B).
Diagnosis (based on adult males and pupal exuviae). The pupa of this species can be easily distinguished from all other known pupae of the group Chironomidae based on the following combination of characters: highly rugose cuticle of the thorax; tergite of the abdominal unit 2 lacks armament (Figure 6A-C, Figure 7A-D), most notably it has no row of hooks on the posterior margin; remaining abdominal tergites without armament/shagreen as well; abdominal unit 2 with prominent pedes spuri B (lateral protrusions of the tergite 2) (Figure 6B); abdominal unit 8 with prominent posterolateral comb comprised of 4-7 strongly sclerotized large spines and a number of smaller spines inserted between them; anal lobes with well-developed fringe of setae (Figure 7A-B).
Pharate males can be distinguished from the other representatives of the Chinomidae based on the combination of genitalia (hypopygium), with its broad inferior volsella and gonostylus narrow proximally, broadest medially tapering distally again, resembling those of Lipiniella Shilova, 1961. Hindtibial combs also resemble those of Lipiniella (Figure 8D) (Wiederholm, 1989). The new species has relatively short antennae, short wings and extremly robust and short hindlegs.
HIINTELMANNIELLA NONCATAFRACTATA Baranov and Haug, sp. nov.
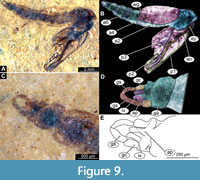
Figure 6A-C, Figure 7A-D, Figure 8A-D, Figure 9A-E
zoobank.org/6B988B7E-EFBD-4C54-B1BD-E7C7072C9317
 Material. Holotype USNM 717431 pupa and associated adult male: paratypes, adult male USNM 624424 and pupa USNM 624644 - male in a state of eclosion, and pupal exuvium (partial) USNM 717428 and additional pupae USNM 722339 and USNM 717494. Additional material - see Appendix Table 1.
Material. Holotype USNM 717431 pupa and associated adult male: paratypes, adult male USNM 624424 and pupa USNM 624644 - male in a state of eclosion, and pupal exuvium (partial) USNM 717428 and additional pupae USNM 722339 and USNM 717494. Additional material - see Appendix Table 1.
Etymology. Named ‘noncatafractata’ from Latin for ‘not armoured’, in reference to the complete lack of any armament (hooks or shagreen) on the tergites of the abdominal units.
Diagnosis. As for the genus per monotypy.
Pupa. (Figure 6A-C, Figure 7A-D). Habitus. Medium-sized pupa, with flattened, comma-shaped body (in lateral aspect). Body length 5.9-9.2 mm (n = 23); length of head and thorax combined 1.3-2.3 mm (n = 23); abdomen length 4.4-7.4 mm (n = 23). Body differentiated into presumably 20 segments, ocular segment plus 19 post-ocular segments (Figure 6A-C).
 Anterior part of the body is composed of head and thorax, visible as a single globose structure. Antenna is missing or incomplete on the available specimens (Figure 7A-D). Frons of the head bears two conical cephalic tubercles, without visible setae; ocular segment and post-ocular segments 1-5 (presumably) forming a distinct capsule (head capsule); mouthparts located ventrally, short, ending before attachment of the anterior ambulatory appendages (Figure 6A-C, Figure 7A-D). Thorax bears wings and ambulatory appendages (legs) (Figure 6A-C, Figure 7A-D). Thoracic horn (respiratory organ) not preserved. Cuticle of the thorax thick and rugose, wrinkled. No setae visible on the thorax. Developing wings reaching beyond the thorax to the abdomen. Legs not preserved in the available specimens (Figure 6A-C, Figure 7A-D, Figure 9A-B).
Anterior part of the body is composed of head and thorax, visible as a single globose structure. Antenna is missing or incomplete on the available specimens (Figure 7A-D). Frons of the head bears two conical cephalic tubercles, without visible setae; ocular segment and post-ocular segments 1-5 (presumably) forming a distinct capsule (head capsule); mouthparts located ventrally, short, ending before attachment of the anterior ambulatory appendages (Figure 6A-C, Figure 7A-D). Thorax bears wings and ambulatory appendages (legs) (Figure 6A-C, Figure 7A-D). Thoracic horn (respiratory organ) not preserved. Cuticle of the thorax thick and rugose, wrinkled. No setae visible on the thorax. Developing wings reaching beyond the thorax to the abdomen. Legs not preserved in the available specimens (Figure 6A-C, Figure 7A-D, Figure 9A-B).
Abdomen (posterior trunk). Tergites of the abdominal units 1-9 without visible armament or shagreen. Most notably, tergite 2 without any armament, including complete absence of a row of hooks on the posterior edge of the tergite. Lateral edges of the abdominal units slightly darker than the rest of the cuticle. Comb at the posterolateral edge of tergite 8 with 4-7 strong, wide spurs, and, occasionally, a number of smaller denticles nested between the spines (Figure 7A-B). Anal lobes with a dense fringe of setae with over 50 well-preserved setae present.
Adult male. Medium-sized midge with long legs and long abdomen ending with prominent external genitalia. Wings present on several specimens, relatively short in relation to the body. Paratype USNM 717431 (Figure 8A-D, Figure 9A-E). Paratype USNM 624644, pharate male, with the remnants of the pupa and partial imprint of the wing. Paratype USNM 717431 is preserved mostly in dorsal aspect. Body length 4.8 mm. Paratype USNM 624644 is preserved in lateral aspect, 6.2 mm long.
Head. Ocular segment and post-ocular segments 1-5 (presumably) forming distinct capsule (head capsule) (Figure 8A, Figure 9A-B). Ocular segment recognizable by a pair of large compound eyes. Eyes without macro- or microtrichia. Eyes have a short, wedge-shaped dorso-medial extension. Clypeus and labrum together 160 µm long, rectangular. Post-ocular segment 1 recognizable by its appendages, antennae (antennulae). Antenna ca. 1 mm long. It was impossible to distinguish the borders between most of the antennal elements (flagellomeres); pedicellus around 110 µm long and last flagellomere about 450 µm long (Figure 8A). Antennal ratio (AR) (length of the terminal flagellomere divided by the combined lengths of the rest of the flagellomeres) = 0.81. Each antenna bears plumage of long setae. Post-ocular segment 1 is embedded in matrix and impossible to examine. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles) (Figure 8A). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpi that consist of five elements (palpomeres), with palpomeres 1 and 2 hidden behind the labrum; lengths of elements 3 through 5: 96 µm (n = 1), 100 µm (n = 1), 110 µm (n = 1) (Figure 8A). Post-ocular segment 5 recognizable by its appendages, forming the labium (conjoined left and right maxillae). Labium mostly obscured in holotype, no details visible (Figure 8A, Figure 9A-B). Thorax (Figure 8A, Figure 9A-B) bears three pairs of the ambulatory appendages (fore-, mid- and hindlegs) on the pro-, meso-, and metathorax, respectively. Each leg consists of coxa, trochanter, femur, tibia, and tarsomeres 1-5. Wings poorly preserved on both available adult males. Paratype USNM 717431 with wings 2.4 mm long and paratype USNM 624644 with wings 3.4 mm long (Figure 8A, Figure 9A-B). Prothorax horseshoe-shaped (with arms of ‘horseshoe’ facing ventrally), narrow. Prothorax bears first pair of thorax appendages (forelegs). Foreleg element lengths (n = 2): femur 1000 µm, tibia 1005-1300 µm, tarsomere 1 960-1300 µm, tarsomere 2 520-640 µm, tarsomere 3 440-470 µm, tarsomere 4 360-370 µm, tarsomere 5 170 µm (n = 1). Claws present, non-serrate, no pulvilli visible. Mesothorax with wings poorly preserved, 2.4-3.4 mm long, venation not distinguishable. Wing length to body length ratio 0.5-0.56. Midleg element lengths: femur 1200 µm (n = 1), tibia 1300 µm (n = 1), tarsomere 1 750 µm (n = 1), tarsomere 2 500 µm (n = 1). Other elements not preserved. Claws present, non-serrate, no pulvilli visible (Figure 8A). Metathorax bears a pair of ambulatory appendages (hindlegs). Hindleg element lengths (n = 2): femur 1010-1360 µm, tibia 1060-1290 µm, tarsomere 1 530-710 µm, tarsomere 2 170-280 µm, tarsomere 3 200-440 µm, tarsomere 4 200-290 µm, and tarsomere 5 110-220 µm. Hindtibial comb separated into two parts, each part with the comb bearing a strong spur (Figure 8D). Claws present, non-serrate, no pulvilli visible (Figure 8A).
Abdomen (posterior trunk). Comprised of 10 units, only eight of which are represented as fully developed abdominal segments; units 9 and 10 incorporated into male genitalia (hypopygium). Abdominal units 1-8 roughly rectangular in shape. Only a few setae, visible in the lateral aspect, preserved on the abdomen (Figure 9A). Abdominal element 9 together with male genitalia (hypopygium) form a male copulatory apparatus (Figure 8B-C, Figure 9C-E).
Male genitalia (hypopygium) (male genitalia, appendages of abdominal unit 8 and following units). Unit 9 broadly rounded posteriorly (Figure 8B-C, Figure 9C-E). Unit 10 represented by a triangular anal point, with pointed end. Gonocoxite (2) cylindrical, 145-220 µm long (n = 4). Inferior volsella of the gonocoxite (lateral protrusion of gonocoxite) relatively long, 110-240 µm (n = 4), club-shaped, broadest distally (Figure 9C-E). Gonocoxite and gonostylus without a mobile articulation, conjoined (Figure 8B-C, Figure 9C-E). Gonostylus narrow, arched, with blunt, rounded, distal end, 200-260 µm long (n = 4) (Figure 8B-C, Figure 9C-E).
Possible adult female. Single, poorly preserved specimen, which might represent a female of H. noncatafractata (paratype USNM 72223). This female can be associated with the males of H. noncatafractata based on the similar hindtibial combs. A characteristic pupa of H. noncatafractata is present next to the female, suggesting that it may have just emerged from it. Body length of the female 4.3 mm, length of the poorly preserved wing 2.8 mm.
Remarks. The following characteristics of the adult male of this morphotype point to it being a representative of Chironomidae based on a specific combination of characters of the pupae: overall similar habitus, antennae with more than six elongate elements (flagellomeres), second element of antennae (pedicellus) conspicuously larger than the other elements, antenna with strong plumage, ocelli absent, head without functional mouthparts, thorax without distinct pre-halter lobe at the base of the true halter, scutum without distinct V-shaped suture, slender-bodied fly, tarsomere one on all (preserved) legs considerably longer than tarsomere 2, tergite of abdominal unit I without strong fringe of lateral setae (Marshall et al., 2017) (Figure 8A-D, Figure 9A-E).
Within Chironomidae, this morphotype is an ingroup of Chironominae, due to a specific combination of characters: gonostylus and gonocoxite conjoined rigidly, no articulation visible (Wiederholm, 1989). While specimens lack wings, and therefore crucial diagnostic characters are missing, we can assume that this midge is a representative of Chironomini. This assumption is based on the absence of a prominent medium volsella (lateral protrusion of gonocoxite), and an anal point without prominent spinules, arranged in longitudinal rows (Wiederholm, 1989) (Figure 8B, Figure 9C-E). Further interpretation within Chironomini is not possible, due to the insufficient preservation of the antennae and wings of the male. The new species has relatively short antennae, short wings and relatively robust and short hindlegs. These traits are exhibited by midges inhabiting the intertidal zone of the seas or living as a surface skater in hypersaline habitats (Armitage et al., 1995; Qi et al., 2018) with salinities up to 40‰ (Armitage et al., 1995; Qi et al., 2018; Shadrin et al., 2019). In the new species, these traits are less developed than in the known intertidal and marine representatives of Chironomidae, i.e., Pontomyia spp., Clunio spp., Dicrotendipes sinicus Qi and Lin, 2018 and Baeotendipes noctivagus Kieffer, 1911 (Qi et al., 2018). It is therefore probable that this combination of traits indicates that adults of H. noncatafractata sp. nov. might have led a surface-skating lifestyle on waterbodies in the Kishenehn Formation.
Association of the life stages. Pupae of the new species are associated with the adult males via paratype USNM 624644, a male, preserved in a state of eclosion from the pupa. While only the thorax of the pupa is preserved in this specimen, it exhibits characteristic strongly sclerotized, wrinkled cuticle, present in all other pupae of H. noncatafractata sp. nov. These pupae exhibit a character combination characteristic for Chironomidae: antennae extending posteriorly past the head of the pupa; maxillary palpi directed laterally; trunk end without articulated terminal paddles (Borkent, 2012). The pupa furthermore exhibits characteristic traits of pupae of Chironomini: wings without nose or a pearl row; all legs are curved under the wings; abdominal units 2-8 without strong spines; tergites of the abdominal units without paired patches of shagreen; tergite 8 with comb of strong, posterolateral spurs; abdominal units 8 and 9 do not form a disc-like structure, anal lobes with numerous simple setae, one pair of the lamellar lateral setae without posterolateral spurs; anal lobes without terminal macrosetae (Wiederholm, 1986). Remarkably, pupae of the new species are lacking armament of the abdominal tergites (Figure 6A-C). Most crucially, they lack traces of the hook row on the posterior edge of tergite 2, present in almost all other pupae of Chironomini (Wiederholm, 1986; Langton, 1991).
One notable exception from this rule is a pupa of the northern Australian group Anuncotendipes Cranston, 1999. Pupae of A. australotropicus Cranston, 1999 and A. kakadu Cranston, 1999 both lack a hook row on tergite 2 of the abdomen (Cranston, 1999). Anuncotendipes is clearly an ingroup of the Harnischia -group (Cranston, 1999). This is evident from the morphology of the known larvae of Anuncotendipes, as noted by Cranston (1999). Despite sharing the absence of the hook row, these ingroups of Chironomidae are not very similar to H. noncatafractata sp.nov.
TANYTARSINI Zavřel, 1916 [in Thienemann and Zavřel, 1916]
TANYTARSUS van der Wulp, 1874
cf. TANYTARSUS
Figure 10A-E
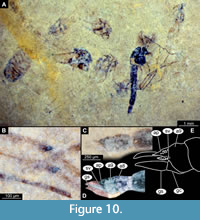 Material. This morphotype of Chironomidae is represented by a single adult male and several probably related pupae on a single slab of shale (specimen USNM 722523). As we could not ascertain the state of some important characters, most notably wing venation, we have refrained from a formal description of this morphotype.
Material. This morphotype of Chironomidae is represented by a single adult male and several probably related pupae on a single slab of shale (specimen USNM 722523). As we could not ascertain the state of some important characters, most notably wing venation, we have refrained from a formal description of this morphotype.
Remarks. Medium-sized fly with midge-shaped body, long legs and long abdomen ending with prominent external genitalia. Wings lost in the only preserved adult male, Holotype: USNM 722523. Specimen preserved mostly in dorsolateral aspect. Body length 4.8 mm.
Adult male. Ocular segment and post-ocular segments 1-5 (presumably) forming distinct capsule (head capsule) (Figure 10A-E). Ocular segment recognizable by a pair of large compound eyes. Eyes without micro- or macrotrichia. Labrum and clypeus not preserved. Post-ocular segment 1 recognizable by its appendages, antennae [antennulae]. Each antenna bears a dense plumage of long setae. Lengths of the individual elements of the antenna as follows (n = 1, USNM 722523): first and second flagellomeres only partially visible/obscured, third 50 µm, fourth 40 µm, fifth 45 µm, sixth 25 µm, seventh 40 µm, eighth 35 µm, ninth 40 µm, tenth 40 µm, eleventh 40 µm, twelfth 48 µm, and thirteenth 825 µm. Frons (frontal sclerite) of post-ocular segment 1 impossible to examine as it is embedded in matrix. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpi, which normally consist of five elements (palpomeres), with palpomeres 1 and 2 hidden behind the head; lengths of elements 3 and 4 (n = 1) 160 µm and 180 µm, respectively. Palpomere 5 not preserved. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium mostly obscured on all specimens, no details visible. Thorax (Figure 10A) bears three pairs of the ambulatory appendages (fore-, mid- and hindlegs) on the pro-, meso- and metathorax, respectively. Each leg consists of coxa, trochanter, femur, tibia and tarsomeres 1-5. Wings not preserved. Prothorax horseshoe-shaped (with arms of ‘horseshoe’ facing ventrally), narrow. Prothorax bears first thoracic appendages (forelegs). Lengths of foreleg elements: femur 870 µm (n m = 1), tibia 640 µm (n = 1), tarsomere 1 1000 µm (n = 1), tarsomere 2 530 µm (n = 1), tarsomere 3 440 µm (n = 1), tarsomere 4 280 µm (n = 1). Mesothorax with wing not preserved. Lengths of midleg elements as following: femur 670 µm (n = 1), tibia 1300 µm (n = 1); other elements not preserved. Metathorax bears a pair of ambulatory appendages (hindlegs). Lengths of hindleg elements as following: femur 1200 µm (n = 1), tibia 1400 µm (n = 1), tarsomere 1 670 µm (n = 1), tarsomere 2 400 µm (n = 1), tarsomere 3 300 µm (n = 1), tarsomere 4 320 µm (n = 1). Tibia with two separate combs (Figure 10B).
Abdomen (posterior trunk) comprised of 10 units, only 8 of which are represented as fully developed abdominal segments. Units 9 and 10 incorporated into hypopygium. Abdominal units 1-8 roughly rectangular in shape. No setae preserved on specimen (Figure 10C-E). Abdominal unit 9 together with hypopygium forms the male copulatory apparatus.
Male genitalia (hypopygium) (appendages of abdominal unit 8 and following units): unit 9 broadly rounded posteriorly, trapezoidal in shape (Figure 10C-E). Unit 10 represented by anal point, poorly preserved, seemingly parallel-sided, with two prominent rows of spinulae (eight in two rows of four). Gonocoxite (2) cylindrical, 170 µm long. Superior volsella of gonocoxite (lateral protrusion of gonocoxite) oval, elongate with a row of setal tecae. Gonocoxite and gonostylus without a flexible articulation, conjoined (Figure 10C-E). Gonostylus narrow, curved, with pointed distal end, 220 µm long (Figure 10C-E).
Taxonomic attribution. Males of this morphotype can be recognized as representatives of Chironomidae based on a specific combination of characters: overall similar habitus, body slender, antennae with more than six elongated elements (flagellomeres), second element of antenna (pedicellus) conspicuously larger than the rest of the antennal elements, antenna with strong plumage, ocelli absent, head without functional mouthparts, thorax without distinct pre-halter lobe at the base of the true halter, scutum without distinct V-shaped suture, tarsomere 1 on all (preserved) legs is considerably longer than tarsomere 2, tergite of abdominal unit 1 without fringe of strong lateral setae (Marshall et al., 2017). Within Chironomidae, this morphotype is an ingroup of Chironominae, due to a specific combination of characters: gonocoxite and gonostylus conjoined rigidly, no articulation visible (Wiederholm, 1989). Although the available specimen lacks wings, and therefore crucial diagnostic characters are missing, we can assume that this midge is a representative of Tanytarsini. This assumption is based on the combination of a prominent oval superior volsella and anal point with spinulae arranged in longitudinal rows (Wiederholm, 1989) (Figure 10E). Within the Tanytarsini, this morphotype is most similar to Tanytarsus van der Wulp, 1874, based on the following combination of characters: maxillary palpus elements are considerably longer than wide, mid- and hindtibiae with separated combs, anal point without two anteriorly directed processes and with a group of spines (Langton and Pinder, 2007). General shape of the superior volsella and characteristic spinulae of the anal point arranged into two longitudinal rows are reminiscent of the condition in extant representatives of Tanytarsus, such as T. gracilentus Holmgren, 1883 [in Homgren and Aurivillius, 1883] (Giłka, 2011a). Numerous fossil adult representatives of Tanytarsus are known from amber, but differences in amber taphonomy from that of the Kishenehn Formation makes them difficult to compare to the specimens discussed here (Giłka, 2010; 2011b; Giłka, et al. 2013; Zakrzewska and Giłka, 2015; Zakrzewska, et al. 2020). Pupae on the same slab as the adult male appear to be representatives of Tanytarsini based on the armament of the abdominal tergites and can potentially represent the same morphotype as the male. Unfortunately, these pupae are too poorly preserved to able to ascertain if they represent the same species.
RHEOTANYTARSUS Thienemann and Bause, 1913 [in Bause 1913]
RHEOTANYTARSUS LACUSTRIS Baranov and Haug, sp. nov.
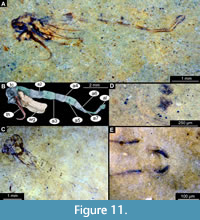
Figure 11A-E, Figure 12A-E
zoobank.org/D0CBCF87-28EF-41B5-A812-CA8D5DFAF245
 Diagnosis (based on adult males and pupal exuviae). This new species can be easily distinguished from all other species of Rheotanytarsus based on the specific combination of characters of adult males and pupae: adult male with extremely short medium volsella (lateral protrusion of gonocoxite) and blunt gonostylus. Pupae associated with the adults are well preserved with thoracic horn parallel-sided and bare; tergites 2-5 of abdominal units with paired, circular patches of spinules present; tergite 2 with shagreen split into two groups; comb of abdominal unit 8 with four strong, thorn-like, strongly sclerotized spurs.
Diagnosis (based on adult males and pupal exuviae). This new species can be easily distinguished from all other species of Rheotanytarsus based on the specific combination of characters of adult males and pupae: adult male with extremely short medium volsella (lateral protrusion of gonocoxite) and blunt gonostylus. Pupae associated with the adults are well preserved with thoracic horn parallel-sided and bare; tergites 2-5 of abdominal units with paired, circular patches of spinules present; tergite 2 with shagreen split into two groups; comb of abdominal unit 8 with four strong, thorn-like, strongly sclerotized spurs.
Etymology. Named lacustris after the lake deposits of the Kishenehn Formation the specimens were found in.
Type material. Holotype: USNM 717577 adult male and its pupal exuvium; paratypes, pupae: USNM 722460, USNM722479, USNM624824 and USNM 623669. Additional material: 76 additional pupae were examined; see Appendix Table 1 for additional material.
Pupa. Habitus. Medium-size, with flattened, comma-shaped body (in lateral aspect). Body length, 5.1-7.0 mm (n = 17); length of head and thorax combined, 0.9-1.7 mm (n = 17); abdomen length, 4.0-5.5 mm (n = 17). Body differentiated into presumably 20 segments, ocular segment plus 19 post-ocular segments (Figure 11A-C); anterior part of the body composed of head and thorax, visible as a single globose structure; thorax bears wings and ambulatory appendages (legs) (Figure 11A-C). Ocular segment and post-ocular segments 1-5 (presumably) forming a distinct capsule (head capsule); antennae missing or incomplete on the available specimens; frons of the pupae with a pair of small cephalic tubercles; mouthparts located ventrally, short, ending before attachment of the fore ambulatory appendages (Figure 11A-C). Only three specimens of Rheotanytarsus lacustris have well-preserved thoracic horns (USNM 722460, 722479 and 624824). Thoracic horn long (0.6-2.0 mm, n = 3), with parallel-sided base, tapering towards the apical end. Thoracic horns smooth, without spikes or setae. Wings reaching beyond the thorax to the abdomen. Legs not preserved in the available specimens (Figure 11A-C).
Abdomen (posterior trunk). Comprised of 10 units, only eight of which are represented as fully developed abdominal segments; units 9 and 10 incorporated into hypopygium. Tergites of abdominal units 2-5 with paired circular patches of spinules at anterior margin (Figure 11D-E). Tergite 2 with a rectangular field of shagreen on posterior margin, split by an area of smooth cuticle medially. Tergites of the units 6-9 without visible armament. Combs at the posterolateral edge of tergite 8 with four strong, wide spurs (Figure 11E). Anal lobes with a dense fringe of setae although setae not preserved per se, rather only their tecae at the edge of the lobe.
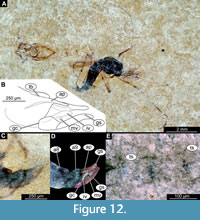 Adult male. Medium-sized midge, with long legs and long abdomen ending with prominent external genitalia. Wings are lost in the only preserved adult male, Holotype: USNM 717577 (Figure 12A-E). The specimen preserved mostly in dorsolateral aspect. Body length 4.8 mm.
Adult male. Medium-sized midge, with long legs and long abdomen ending with prominent external genitalia. Wings are lost in the only preserved adult male, Holotype: USNM 717577 (Figure 12A-E). The specimen preserved mostly in dorsolateral aspect. Body length 4.8 mm.
Ocular segment and post-ocular segments 1-5 (presumably) forming distinct capsule (head capsule) (Figure 12A-E). Ocular segment recognizable by pair of large compound eyes. Eyes without micro- or microtrichia, with well-preserved ommatidia. Frons (frontal sclerite) of post-ocular segment 1 impossible to examine as it is embedded in matrix. Labrum and clypeus 240 µm long, rectangular. Post-ocular segment 1 recognizable by its appendages, antennae [antennulae]. Antenna 1200 µm in length, impossible to distinguish the borders between the antennal elements (flagellomeres). Each antenna bears dense plumage of long setae. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles) (Figure 12A-E). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpi. Maxillary palpus consists of five elements (palpomeres) with palpomeres 1 and 2 hidden behind the labrum; lengths of elements 3 through 5: 140-150 µm (n = 2), 200 µm (n = 1) and 170 µm (n = 1), respectively. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium mostly obscured on holotype, with details not visible (Figure 12A). Thorax (Figure 12A) bears three pairs of the ambulatory appendages (fore-, mid- and hindlegs) on the pro-, meso-, and metathorax, respectively. Each leg consists of the following elements (proximal to distal): coxa, trochanter, femur, tibia, tarsomeres 1-5. Wings lost (Figure 12A). Prothorax horseshoe-shaped in frontal view (with arms of ‘horseshoe’ facing ventrally), narrow. Prothorax bears first thoracic appendages (forelegs). Lengths of foreleg elements as follow (n = 2): femur 1120-1130 µm, tibia 890-1130 µm, tarsomere 1 1120-1280 µm, tarsomere 2 760-790 µm, tarsomere 3 550-650 µm, tarsomere 4 300-490 µm, and tarsomere 5 410 µm (n = 1). Mesothorax with wings poorly preserved, 2.6 mm long, venation not distinguishable. Lengths of midleg elements as follow (n = 1): femur 1250 µm, tibia 1040 µm. Other elements have not been preserved. Metathorax bears a pair of ambulatory appendages (hindlegs). Lengths of hindleg elements as follow (n = 1): femur 770 µm, tibia 1150 µm, tarsomere 1 700 µm, tarsomere 2 480 µm, tarsomere 3 220 µm, and tarsomere 4 240 µm (Figure 12A).
Abdomen (posterior trunk). Comprised of 10 segments (abdominal units), only eight of which are represented as fully developed abdominal segments; units 9 and 10 incorporated into male genitalia (hypopygium). Abdominal units 1-8 roughly rectangular in shape. Only a few setae, visible in the lateral aspect, preserved on the abdomen (Figure 12A-D). Abdominal unit 9 together with hypopygium forms male copulatory apparatus (Figure 12B-D).
Male genitalia (hypopygium) (male genitalia, appendages of abdominal unit 8 and following units). Unit 9 broadly rounded posteriorly (Figure 12B-D). Unit 10 represented by strong, parallel-sided anal point, with two parallel crests running atop of it. Gonocoxite (2) cylindrical, 220 µm long. Inferior volsella (lateral protrusion of gonocoxite) of the gonocoxite relatively long (230 µm) and curved outwards, towards gonostylus. Medium volsella is visible as a short stump at the base of the gonocoxite. Gonocoxite and gonostylus without a flexible articulation, conjoined (Figure 12B-D). Gonostylus narrow, curved, with blunt, rounded, distal end, 230 µm long (Figure 12B-D).
Remarks. Pupa and adult of the new species are directly associated with each other on the slab with the holotype, where the adult male can be seen eclosing from the pupal exuviae. The adult male of this morphotype is a representative of Chironomidae based on the following combination of characters: overall similar habitus, body slender, antennae with > 6 elongate elements (flagellomeres), second element of antennae (pedicellus) conspicuously larger than the rest of antennal elements, antennae with strong plumage, ocelli absent, head without functional mouthparts, thorax without distinct pre-halter lobe at the base of the true halter, scutum without distinct V-shaped suture, tarsomere 1 on all (preserved) legs is considerably longer than tarsomere 2, tergite of abdominal unit 1 without fringe of strong lateral setae (Marshall et al., 2017). Within Chironomidae, this morphotype is a representative of the ingroup Chironominae, due to the following combination of characters: gonostylus and gonocoxite are conjoined rigidly, no articulation visible (Wiederholm, 1989). While the specimen lacks wings, and therefore crucial diagnostic characters are missing, we can assume that this midge is a representative of Tanytarsini. This assumption is based on prominent oval superior and medium volsellae (lateral protrusions of gonocoxite), and anal point with prominent spinules arranged in longitudinal rows (Wiederholm, 1989; Figure 12B). Within the group Tanytarsini, this morphotype is most similar too Rheotanytarsus Thienemann and Bause, 1913, based on the following combination of characters: maxillary palpal elements are considerably longer than wide, mid- and hindtibiae with double combs, anal point without rows of spines (Langton and Pinder, 2007).
The new species has a peculiar combination of characters of the male genitalia (hypopygium), such as a gonostylus with a broadly rounded apical end (similar to R. buculicaudus Kyerematen and Sæther, 2000) and a very short median volsella (similar to R. verticillus Kyerematen, Andersen and Sæther, 2000) and a relatively parallel-sided anal point (similar to R. pallidus Kyerematen, Andersen and Sæther, 2000). In general, the combination of the hypopygial characters exhibited by the new species is somewhat reminiscent of R. barrengaryensis Cranston, 1997, particularly in terms of the parallel-sided anal point, and bluntly rounded gonostylus. In contrast to R. barrengaryensis, however, the new species has an outwardly bending inferior volsella and a very short medium volsella (Cranston, 1997).
Pupae of the new species are similar to the pupa of R. barrengaryensis in the following aspects: thoracic horn bare with a medial bend; tergites of abdominal units 2-5 with circular anterior patches of spinules; shagreen of the tergite 2 medially divided by a stretch of smooth cuticle; tergite 6 without anterior patch of spinules; anal lobe with more than 20 setae in fringe (Cranston, 1997; Kyerematen and Sæther, 2000).
Numerous species of Rheotanytarsus are known from amber, but differences in amber taphonomy relative to that of the Kishenehn Formation makes them hard to compare to the specimens discussed here (Zakrzewska and Giłka, 2015; Zakrzewska et al., 2020).
DIPTERA Linnaeus, 1758
CERATOPOGONIDAE Newmann, 1834
ALLUAUDOMYIA Kieffer, 1913
cf. ALLUAUDOMYIA
Figure 13A-B, Figure 14A-F
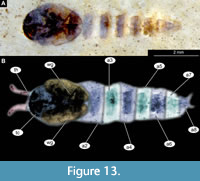 Material. This morphotype is represented by numerous early and late pupae (pharate adults) (see list in Table 1).
Material. This morphotype is represented by numerous early and late pupae (pharate adults) (see list in Table 1).
 Taxonomic attribution. This morphotype is an ingroup of Ceratopogonidae based on a specific combination of characters: antennae extended posteriorly from the head; maxillary palpus directed posteriorly; thoracic horns without transverse striation (‘undivided’), with two rows of pores; end of the abdomen without articulated terminal paddles, end of abdomen bearing two spine-like protrusions (Figure 13A-B, Figure 14A-F). It is difficult to further interpret the morphotype within Ceratopogonidae due to the poor preservation of the thorax and armament of the abdominal units. Nevertheless, a thoracic horn with two parallel rows of pores (Figure 14C-D) and a slightly bifid distal end indicates that this morphotype is like Alluaudomyia Kieffer, 1913 (Art Borkent, pers comm.; Borkent, 2014a).
Taxonomic attribution. This morphotype is an ingroup of Ceratopogonidae based on a specific combination of characters: antennae extended posteriorly from the head; maxillary palpus directed posteriorly; thoracic horns without transverse striation (‘undivided’), with two rows of pores; end of the abdomen without articulated terminal paddles, end of abdomen bearing two spine-like protrusions (Figure 13A-B, Figure 14A-F). It is difficult to further interpret the morphotype within Ceratopogonidae due to the poor preservation of the thorax and armament of the abdominal units. Nevertheless, a thoracic horn with two parallel rows of pores (Figure 14C-D) and a slightly bifid distal end indicates that this morphotype is like Alluaudomyia Kieffer, 1913 (Art Borkent, pers comm.; Borkent, 2014a).
DIPTERA Linnaeus, 1758
CHAOBORIDAE Edwards, 1912
CHAOBORUS Lichtenstein, 1800
CHAOBORUS KISHENEHNICUS Baranov and Haug sp. nov.
Figure 15A-B, Figure 16A-F, Figure 17A-D, Figure 18A-D, Figure 19A-D, Figure 20A-D, Figure 21A-B, Figure 22A-D
zoobank.org/B03B0A39-FDA2-4AF4-A5E7-EF6C15F9E9FC
 Etymology. The name ‘kishenehnicus’, meaning of or pertaining to Kishenehn, refers to the geological formation in which the species was found.
Etymology. The name ‘kishenehnicus’, meaning of or pertaining to Kishenehn, refers to the geological formation in which the species was found.
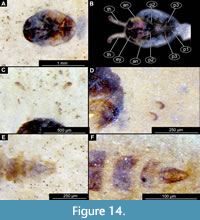
Diagnosis (based on adult males). This species is easily distinguished from all other species of Chaoborus based on a specific combination of characters (Figure 15A-B, Figure 16A-F, Figure 17A-D, Figure 18A-E, Figure 19A-D, Figure 20A-D, Figure 21A-B, Figure 22A-D): adult male without lobe or paired, strong setae on the inner surface of the apical part of the gonocoxite; pulvilli absent or very minute, and thus invisible in the fossil specimens; legs with at least the proximal part of the femur much lighter than the rest of the leg (Figure 18A-E, Figure 19A-E, Figure 20A-D).
Type material. Holotype: USNM 626053, adult male; paratypes: USNM 623065, USNM 717303; adult female USNM 624863; USNM 595142 (pupa). Additional material consisting of 140 adult or pharate males and pupae and seven females are listed in Appendix Table 1.
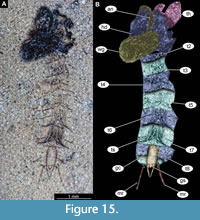
 Pupa. Habitus. Medium-sized, with flattened, comma-shaped body (in lateral aspect). Body length 2.6-3.9 mm (n = 28); abdomen length 1.9-3.1 mm (n = 28); length of thorax 0.8-1.3 mm (n = 28), body differentiated into presumably 20 segments, ocular segment plus 19 post-ocular segments (Figure 15A-B, Figure 16A-F, Figure 17A-D); anterior part of the body composed of head and thorax, visible as a single globose structure; thorax bears wings and ambulatory appendages (legs) (Figure 15A-B, Figure 16A-F, Figure 17A-D); ocular segment and post-ocular segments 1-5 (presumably) forming a distinct capsule (head capsule); mouthparts located ventrally and short, ending before attachment of the first ambulatory appendages (forelegs) (Figure 15A-B, Figure 16A-F, Figure 17A-D). Ocular segment recognizable by its appendage derivative, clypeo-labral complex, and a pair of large compound eyes. Labrum and clypeus are present, but their shape is obscured by deformation of the specimens, since most of the pupae are preserved in lateral aspect (Figure 15A-B, Figure 16A-F, Figure 17A-D). Post-ocular segment 1 recognizable by its appendages, antennae (antennulae). Antennae curved around the head, ending beneath the head, at about mid-length to 0.8 of the length of the wings. Antennae attached to the massive, rounded pedicellus (antennal element 2) (Figure 16A-B). Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles) (Figure 16A-B). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpus. Palpi are poorly preserved on the available specimens. Post-ocular segment 5 is recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium mostly obscured on all specimens, with no details visible (Figure 16A-B). Thorax bears three pairs of ambulatory appendages (fore-, mid- and hindlegs) on the pro-, meso-, and metathorax, respectively. Thoracic segments forming a single semi-globose structure, closely enveloping the head of the pupa. Ambulatory appendages of the thorax folded around and under the wings (Figure 15A-B). Prothorax bears thoracic horns (modified first spiracle). Thoracic horns spindle-shaped, widest at mid-length, tapering apically into a short tube (300-650 µm, n = 13) (Figure 16A-B, Figure 17A-B). Thoracic horn with a honeycomb-shaped surface texture and small apical opening (Figure 16A-B, Figure 17A-B). Prothorax bears first thoracic appendages (forelegs). Forelegs running posteriorly, upwards anteriorly to the upper edge of the eye and then downward to the apical edge of the wing (Figure 17B). Mesothorax bears a pair of wings and a pair of ambulatory appendages (midlegs). Midlegs situated medially to foreleg. Midlegs are also looping around the wing, distal part of the loop lying on the abdomen, beyond the distal end of the wing. Distal parts of the midlegs loop again under the wing (Figure 15A-B, Figure 16A-F). Metathorax bears a pair of ambulatory appendages (hindlegs); halteres not visible in the fossils. Hindlegs almost entirely hidden behind the coxae of the fore- and midlegs and wings (Figure 17A-B).
Pupa. Habitus. Medium-sized, with flattened, comma-shaped body (in lateral aspect). Body length 2.6-3.9 mm (n = 28); abdomen length 1.9-3.1 mm (n = 28); length of thorax 0.8-1.3 mm (n = 28), body differentiated into presumably 20 segments, ocular segment plus 19 post-ocular segments (Figure 15A-B, Figure 16A-F, Figure 17A-D); anterior part of the body composed of head and thorax, visible as a single globose structure; thorax bears wings and ambulatory appendages (legs) (Figure 15A-B, Figure 16A-F, Figure 17A-D); ocular segment and post-ocular segments 1-5 (presumably) forming a distinct capsule (head capsule); mouthparts located ventrally and short, ending before attachment of the first ambulatory appendages (forelegs) (Figure 15A-B, Figure 16A-F, Figure 17A-D). Ocular segment recognizable by its appendage derivative, clypeo-labral complex, and a pair of large compound eyes. Labrum and clypeus are present, but their shape is obscured by deformation of the specimens, since most of the pupae are preserved in lateral aspect (Figure 15A-B, Figure 16A-F, Figure 17A-D). Post-ocular segment 1 recognizable by its appendages, antennae (antennulae). Antennae curved around the head, ending beneath the head, at about mid-length to 0.8 of the length of the wings. Antennae attached to the massive, rounded pedicellus (antennal element 2) (Figure 16A-B). Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles) (Figure 16A-B). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpus. Palpi are poorly preserved on the available specimens. Post-ocular segment 5 is recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium mostly obscured on all specimens, with no details visible (Figure 16A-B). Thorax bears three pairs of ambulatory appendages (fore-, mid- and hindlegs) on the pro-, meso-, and metathorax, respectively. Thoracic segments forming a single semi-globose structure, closely enveloping the head of the pupa. Ambulatory appendages of the thorax folded around and under the wings (Figure 15A-B). Prothorax bears thoracic horns (modified first spiracle). Thoracic horns spindle-shaped, widest at mid-length, tapering apically into a short tube (300-650 µm, n = 13) (Figure 16A-B, Figure 17A-B). Thoracic horn with a honeycomb-shaped surface texture and small apical opening (Figure 16A-B, Figure 17A-B). Prothorax bears first thoracic appendages (forelegs). Forelegs running posteriorly, upwards anteriorly to the upper edge of the eye and then downward to the apical edge of the wing (Figure 17B). Mesothorax bears a pair of wings and a pair of ambulatory appendages (midlegs). Midlegs situated medially to foreleg. Midlegs are also looping around the wing, distal part of the loop lying on the abdomen, beyond the distal end of the wing. Distal parts of the midlegs loop again under the wing (Figure 15A-B, Figure 16A-F). Metathorax bears a pair of ambulatory appendages (hindlegs); halteres not visible in the fossils. Hindlegs almost entirely hidden behind the coxae of the fore- and midlegs and wings (Figure 17A-B).
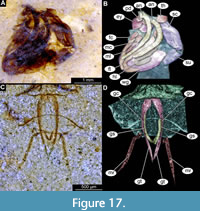 Abdomen (posterior trunk). Abdominal units 1-8 with setae of the pharate adult tergites visible through the pupal cuticle. Setae radiating from the abdominal midline, outwards, forming pointed ends, at the dorso-posterior part of each abdominal unit (Figure 15A-B, Figure 16D-E, Figure 17C-D). Trunk end (abdominal unit 9 plus remnants of abdominal unit 10) bears genitalia and remnants of two anal lobes (paddles). Anal lobes of all studied specimens consist only of a single, medial rib, membranous parts of the terminal paddles and rest of the ribs seem to have been lost. Genitalia (appendages of abdominal unit 8) present as well as a tight cluster of gonocoxal setae (Figure 16D-E, Figure 17C-D).
Abdomen (posterior trunk). Abdominal units 1-8 with setae of the pharate adult tergites visible through the pupal cuticle. Setae radiating from the abdominal midline, outwards, forming pointed ends, at the dorso-posterior part of each abdominal unit (Figure 15A-B, Figure 16D-E, Figure 17C-D). Trunk end (abdominal unit 9 plus remnants of abdominal unit 10) bears genitalia and remnants of two anal lobes (paddles). Anal lobes of all studied specimens consist only of a single, medial rib, membranous parts of the terminal paddles and rest of the ribs seem to have been lost. Genitalia (appendages of abdominal unit 8) present as well as a tight cluster of gonocoxal setae (Figure 16D-E, Figure 17C-D).
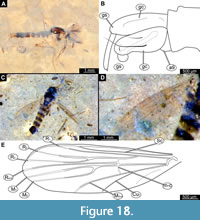 Adult male. Medium-sized fly with midge-shaped body, long legs, and long abdomen ending with prominent external genitalia. Wings are relatively well preserved in Holotype. USNM 626053 and paratype USNM 623065 males (Figure 18A-E, Figure 19A-D). Wings with poorer preservation are relatively frequent in the available material. The specimens are preserved mostly in dorsolateral aspect, but some midges are preserved in mostly lateral aspect (Figure 18A-E, Figure 19A-D). This orientation gave us the opportunity to examine different characters. Ocular segment and post-ocular segments 1-5 (presumably) forming distinct capsule (Figure 18A, C, Figure 19A). Ocular segment recognizable by a pair of large compound eyes. Eyes without micro- or macrotrichia, with long, wedge-shaped dorsomedial extension (six ommatidia long and four ommatidia across).
Adult male. Medium-sized fly with midge-shaped body, long legs, and long abdomen ending with prominent external genitalia. Wings are relatively well preserved in Holotype. USNM 626053 and paratype USNM 623065 males (Figure 18A-E, Figure 19A-D). Wings with poorer preservation are relatively frequent in the available material. The specimens are preserved mostly in dorsolateral aspect, but some midges are preserved in mostly lateral aspect (Figure 18A-E, Figure 19A-D). This orientation gave us the opportunity to examine different characters. Ocular segment and post-ocular segments 1-5 (presumably) forming distinct capsule (Figure 18A, C, Figure 19A). Ocular segment recognizable by a pair of large compound eyes. Eyes without micro- or macrotrichia, with long, wedge-shaped dorsomedial extension (six ommatidia long and four ommatidia across). 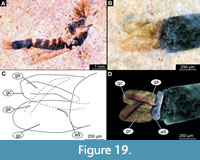 Eyes well preserved with individual ommatidia visible (Figure 18A, C), Frons (frontal sclerite) of post-ocular segment one impossible to examine as it is embedded in the matrix. Labrum rectangular, longer than wide, as evident from observation of specimens USNM 622981 and USNM 624754 (labrum 176-276 µm long). Post-ocular segment 1 recognizable by its appendages, antennae [antennulae]. Each antenna with two proximal elements (scape and pedicellus) and 13 additional elements (flagellomeres). Pedicellus donut-shaped (squashed sphere with invagination on the top), 130–240 µm in diameter (n = 5). Flagellomeres widest at mid-length, tapering apically (Figure 18A, C, Figure 19A). Each flagellomere with a whorl of setae at its ‘equator’ (Figure 18A, C, Figure 19A). Each whorl contains at least 12 setae. Lengths of individual elements of the antenna as follow (n = 3): first flagellomere 35-88 µm, second 26-68 µm, third 27-74 µm, fourth 26-70 µm, fifth 39-65 µm, sixth 29-63 µm, seventh 43-77 µm, eighth 47-77 µm, ninth 40-66 µm, tenth 45-61 µm, eleventh 56-91 µm, twelfth 57 µm (n = 1), two specimens (USNM 626083, USNM 624754) with flagellomere 13 preserved, but the border between it and flagellomere 12 is unclear, length of two flagellomeres together 192-317 µm (Figure 18A, C, Figure 19A). Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles) (Figure 18A, C, Figure 19A). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpi. Maxillary palpus consists of five elements (palpomeres), with palpomeres 1 and 2 hidden behind the labrum and clypeus; lengths of elements 3 through 5 (n = 2): 123-146 µm, 113-167 µm and 116-125 µm, respectively. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium mostly obscured on all specimens; no details visible (Figure 18A, C, Figure 19A). Thorax (Figure 18A, C, Figure 19A) bears three pairs of the ambulatory appendages (fore-, mid-, and hindlegs) on the pro-, meso-, and metathorax, respectively. Each leg consists of the following elements (proximal to distal): coxa, trochanter, femur, tibia, tarsomeres 1-5. Prothorax horseshoe-shaped in lateral view (with arms of ‘horseshoe’ facing ventrally), narrow. Prothorax bears the first pair of thoracic appendages (forelegs). Lengths of foreleg elements as follow: femur 550-860 µm (n = 3), tibia 930-1240 µm (n = 4), tarsomere 300-440 µm (n = 3), tarsomere 2 175-345 µm (n = 3), tarsomere 3 140-230 µm (n = 3), tarsomere 4 280 µm (n = 1), tarsomere 5 370 µm (n = 1) (Figure 18A, C, Figure 19A). Mesothorax of several specimens have wings relatively well preserved, but complete venation was only preserved in specimen USNM 623065. Even for this specimen, it is impossible to trace where exactly the vein reaches the edge of the wing (Figure 18D-E). Wing length (tip to arculus) 1.7-2.5 mm (n = 13). Radial 1 vein is long, terminating closer to radial 2 vein than to the subcostal vein (Figure 18D-E). R4 +5 ending at the level with M 1 and M2. Cu1 is slightly bent at the distal third. Crossveins barely visible.
Eyes well preserved with individual ommatidia visible (Figure 18A, C), Frons (frontal sclerite) of post-ocular segment one impossible to examine as it is embedded in the matrix. Labrum rectangular, longer than wide, as evident from observation of specimens USNM 622981 and USNM 624754 (labrum 176-276 µm long). Post-ocular segment 1 recognizable by its appendages, antennae [antennulae]. Each antenna with two proximal elements (scape and pedicellus) and 13 additional elements (flagellomeres). Pedicellus donut-shaped (squashed sphere with invagination on the top), 130–240 µm in diameter (n = 5). Flagellomeres widest at mid-length, tapering apically (Figure 18A, C, Figure 19A). Each flagellomere with a whorl of setae at its ‘equator’ (Figure 18A, C, Figure 19A). Each whorl contains at least 12 setae. Lengths of individual elements of the antenna as follow (n = 3): first flagellomere 35-88 µm, second 26-68 µm, third 27-74 µm, fourth 26-70 µm, fifth 39-65 µm, sixth 29-63 µm, seventh 43-77 µm, eighth 47-77 µm, ninth 40-66 µm, tenth 45-61 µm, eleventh 56-91 µm, twelfth 57 µm (n = 1), two specimens (USNM 626083, USNM 624754) with flagellomere 13 preserved, but the border between it and flagellomere 12 is unclear, length of two flagellomeres together 192-317 µm (Figure 18A, C, Figure 19A). Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles) (Figure 18A, C, Figure 19A). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpi. Maxillary palpus consists of five elements (palpomeres), with palpomeres 1 and 2 hidden behind the labrum and clypeus; lengths of elements 3 through 5 (n = 2): 123-146 µm, 113-167 µm and 116-125 µm, respectively. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium mostly obscured on all specimens; no details visible (Figure 18A, C, Figure 19A). Thorax (Figure 18A, C, Figure 19A) bears three pairs of the ambulatory appendages (fore-, mid-, and hindlegs) on the pro-, meso-, and metathorax, respectively. Each leg consists of the following elements (proximal to distal): coxa, trochanter, femur, tibia, tarsomeres 1-5. Prothorax horseshoe-shaped in lateral view (with arms of ‘horseshoe’ facing ventrally), narrow. Prothorax bears the first pair of thoracic appendages (forelegs). Lengths of foreleg elements as follow: femur 550-860 µm (n = 3), tibia 930-1240 µm (n = 4), tarsomere 300-440 µm (n = 3), tarsomere 2 175-345 µm (n = 3), tarsomere 3 140-230 µm (n = 3), tarsomere 4 280 µm (n = 1), tarsomere 5 370 µm (n = 1) (Figure 18A, C, Figure 19A). Mesothorax of several specimens have wings relatively well preserved, but complete venation was only preserved in specimen USNM 623065. Even for this specimen, it is impossible to trace where exactly the vein reaches the edge of the wing (Figure 18D-E). Wing length (tip to arculus) 1.7-2.5 mm (n = 13). Radial 1 vein is long, terminating closer to radial 2 vein than to the subcostal vein (Figure 18D-E). R4 +5 ending at the level with M 1 and M2. Cu1 is slightly bent at the distal third. Crossveins barely visible.
Lengths of midleg elements as follows: femur 740-1280 µm (n = 3), tibia 730-1220 µm (n = 3), tarsomere 1 400-460 µm (n = 2), tarsomere 2 350 µm (n = 1). Other elements have not been preserved in any of the available specimens (Figure 18A, C, Figure 19A). Metathorax bears a pair of altered wings (halteres) and a pair of ambulatory appendages (hindlegs). Lengths of hindleg elements as follow: femur 550- 860 µm (n = 3), tibia 930-1240 µm (n = 4), tarsomere 1 300-440 µm (n = 3), tarsomere 2 175-345 µm (n = 3), tarsomere 3 140-230 µm (n = 3), tarsomere 4 280 µm (n = 1), tarsomere 5 370 µm (n = 1). Posterior trunk consisted of 10 units, only eight of which are represented as fully developed abdominal segments; units 9 and 10 incorporated into hypopygium. Abdominal units 1-8 roughly rectangular in shape. No setae preserved on a specimen (Figure 18B, Figure 19B-D). Abdominal unit 9 together with hypopygial structures, forming a male copulatory apparatus.
Male genitalia (hypopygium) (appendages of abdominal unit 9 and following units). Unit 9 broadly rounded posteriorly, trapezoidal in shape (Figure 18B, Figure 19B-D). Gonocoxite (2) cylindrical 320-450 µm long. Phalapodemae can be seen impressed on unit 9 (Figure 18B, Figure 19B-D). Gonocoxite and gonostylus with a mobile articulation (Figure 18B, Figure 19B-D). No penis valve preserved. Gonostylus narrow, parallel-sided, with pointed, narrow, distal end, 230-315 µm long (Figure 18B, Figure 19B-D).
 Adult female (probable association). Medium-sized fly, with midge-shaped body, long legs and long abdomen ending with a prominent cercus. Body length 2.5-3.5 mm (n = 5). Wings with poorer preservation are relatively frequent in the available material. The specimens are preserved mostly in dorsolateral aspect, but some midges are preserved in mostly lateral aspect (Figure 20A-D).
Adult female (probable association). Medium-sized fly, with midge-shaped body, long legs and long abdomen ending with a prominent cercus. Body length 2.5-3.5 mm (n = 5). Wings with poorer preservation are relatively frequent in the available material. The specimens are preserved mostly in dorsolateral aspect, but some midges are preserved in mostly lateral aspect (Figure 20A-D).
Ocular segment and post-ocular segments 1-5 (presumably) forming distinct head capsule (Figure 20A-D). Ocular segment recognizable by pair of large compound eyes. Eyes without micro- or macrotrichia, with long, wedge-shaped dorsomedial extension (6 ommatidia long and 4 ommatidia across). Eyes well preserved with individual ommatidia structure visible (Figure 20A, C-D). Post-ocular segment 1 recognizable by its appendages, antennae [antennulae]. Each antenna with one proximal element (pedicellus) and 11 additional elements (flagellomeres) still visible. Pedicellus around 50-70 µm in diameter (n = 2), while flagellomeres are widest at mid-length, tapering apically. Lengths of individual elements of the antenna are as follow (n = 4): first flagellomere 35-70 µm, second 30-55 µm, third 45-70 µm, fourth 50-65 µm, fifth 40-50 µm, sixth 45-60 µm, seventh 40-65 µm, eighth 50-65 µm, ninth 45-86 µm, tenth 87-190 µm, eleventh 115-125 µm (n = 2). Labrum rectangular, longer than wide, 220-240 µm long (n = 2). Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 bears no recognizable appendages (mandibles) (Figure 20A). Post-ocular segment 4 recognizable by its appendage, maxilla [maxillula]. Maxilla recognizable by maxillary palpi. Maxillary palpus consists of five elements (palpomeres), with palpomeres 1 and 2 hidden behind the labrum and clypeus; lengths of elements three through five (n = 1): 145 µm, 160 µm, and 160 µm, respectively. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium mostly obscured on all specimens; no details visible.
Thorax (Figure 20A, C-D) bears three pairs of the ambulatory appendages (fore-, mid-, and hindlegs) on the pro-, meso-, and metathorax, respectively. Each leg consists of the following elements (proximal to distal): coxa, trochanter, femur, tibia, tarsomeres 1-5. None of the females have well-preserved legs fit for proper measurements. Prothorax horseshoe-shaped (with arms of ‘horseshoe’ facing ventrally), narrow, bears first pair of thoracic appendages (forelegs). Mesothorax of several specimens have wings relatively well preserved but complete venation is not visible on any of them. Wing length (tip to arculus) 2.0-2.7 mm (n = 7). Metathorax bears a pair of altered wings (halteres, both well visible) and a pair of ambulatory appendages (hindlegs).
 Posterior trunk consists of 10 segments (abdominal units), only nine of which are represented as fully developed abdominal segments. Abdominal units 1-8 roughly rectangular in shape (Figure 20A). Abdominal units 9 and 10 are reduced, forming the copulatory apparatus of the female. Paired cerci attached to the end of abdomen, but they are poorly or not at all preserved in most of the available specimens. Most complete cerci can be seen in specimen USNM 623071. A triple spermatheca, situated within abdominal unit 8, well visible in many specimens through the outer cuticle due to its strong sclerotization (Figure 20B, Figure 21A-B).
Posterior trunk consists of 10 segments (abdominal units), only nine of which are represented as fully developed abdominal segments. Abdominal units 1-8 roughly rectangular in shape (Figure 20A). Abdominal units 9 and 10 are reduced, forming the copulatory apparatus of the female. Paired cerci attached to the end of abdomen, but they are poorly or not at all preserved in most of the available specimens. Most complete cerci can be seen in specimen USNM 623071. A triple spermatheca, situated within abdominal unit 8, well visible in many specimens through the outer cuticle due to its strong sclerotization (Figure 20B, Figure 21A-B).
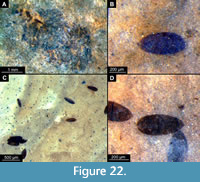 Egg clutches (probable association). We have found a number of the fossilized structures resembling euarthropodan eggs (Figure 22A-D). Large cclusters of such eggs are arranged in a spiral pattern, characteristic for the eggs of extant representatives of Chaoborus (Strickman, 1980). Therefore, we hypothesize that these fossils might represent egg clutches of C. kishenehnicus sp. nov.
Egg clutches (probable association). We have found a number of the fossilized structures resembling euarthropodan eggs (Figure 22A-D). Large cclusters of such eggs are arranged in a spiral pattern, characteristic for the eggs of extant representatives of Chaoborus (Strickman, 1980). Therefore, we hypothesize that these fossils might represent egg clutches of C. kishenehnicus sp. nov.
Remarks. We interpret this new species as an ingroup of Chaoboridae based on a specific combination of characters. Pupa: end of abdomen with a pair of large, articulated paddles, supported by a sclerotized area of cuticle, called the medial rib; mouthparts short, not reaching beyond coxae of anterior legs; bundles of the diagonally oriented setae visible on the tergites of the abdominal units (Borkent, 2012). Adult male: with radial 1 vein long, terminating closer to radial 2 vein, longer than to the subcostal vein. Pupae associated with the adults by the genitalia of the pharate specimens visible via the pupal cuticle (Figure 15, Figure 16, Figure 17) and by the direct association of the eclosing male with the pupa (Figure 21A). Pupae associated with the adults are mostly well preserved, but are always missing some crucial characters, most notably the mesal and lateral longitudinal ribs of the terminal paddle. Therefore, pupae cannot be used for a differential diagnosis. Pupae can be associated with adult males by the virtue of genitalia of pharate males' genitalia. Adult females are poorly preserved, but wing venation similar to that of males, can indicate that they are also representatives of C. kishenehnicus sp. nov. (Figure 21B).
Within Chaoboridae, the new species is an ingroup of Chaoborus, based on a specific combination of characters (listed separately for each life stage). Pupa: with spindle-shaped thoracic horns, more than three times longer than wide, terminal paddles with prominent median rib almost reaching the edge of the lobe. It is notable that pupae have their terminal paddles only partially preserved, with most of the ribs and membrane absent from all available specimens. This type of preservation is apparently common in pupae of the fossil pupae of Chaoborus, as it is also the common type of anal paddle preservation in the Oligocene Chaoborus tertiarius von Heyden, 1862 (Borkent, 1978; Figure 1A-C, Figure 2A-C). Chaoborus kishenehnicus is the second formal record of a fossil species of Chaoboridae in the United States, after the record of Chaoborus pupae from the Middle Eocene Tallahatta Formation (Johnston and Borkent, 1998; Borkent, 2014b).
This new species is highly reminiscent of many modern representatives of Chaoborus (Cook, 1956). The lack of crucial characters, such as well-preserved pulvilli or coloration, does not allow the identification of the new species as a representative of any of the ingroups of Chaoborus (‘subgenera’) (Cook, 1956; Sæther, 1970).
DIPTERA Linnaeus, 1758
CULICIDAE Meigen, 1818
CULICINAE Meigen, 1818
CULICINI Meigen, 1818
CULEX Linnaeus, 1758
NEOCULEX Dyar, 1905
Figure 23A-B
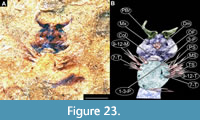 Material. This morphotype is represented by a single, partial fossil of a larva (USNM 623914) (Figure 23A-B).The fossil consists of the head, thorax, and remnants of abdominal units 1 and 2.
Material. This morphotype is represented by a single, partial fossil of a larva (USNM 623914) (Figure 23A-B).The fossil consists of the head, thorax, and remnants of abdominal units 1 and 2.
Larva. Head: broader than long; antennae and setae missing; mouthbrushes (palatal brushes) distinct, dense; dorsomentum and maxillae visible but not sharply defined; collar weakly developed, circular. Thorax: pro-, meso- and metathoracic segments clearly demarked by pleural setal groups, setae of prothorax and lateral setae of meso- and metathorax are well preserved.
Taxonomic attribution. The larva is a representative of Culicidae based on a specific combination of visible characters: head capsule well-developed, with prominent mouthbrushes (palatal brushes), maxillae borne on transverse margin of the head capsule lateral to the dorsomentum; head, thorax, and first abdominal unit not conjoined, thorax bulbous and wider than the head and abdomen, lateral clusters of the long setae present on the thorax and abdominal units (Harbach, 2007; Borkent and Sinclair, 2017).
Within Culicidae, this morphotype is most similar to extant species of the group Neoculex Dyar, 1905. The most important character supporting this interpretation is the very short seta 3-P in the cluster of setae 1-3-P on the prothorax. Adults of the related group Culiseta are known from the Kishenehn Formation (Harbach and Greenwalt, 2012), but setae 1-3-P are equally long in extant species of that group. Short setae 3-P also occur in most extant species of the groups Aedinus Lutz, 1904 [in Lutz and Bouroull, 1904], Anoedioporpa Dyar, 1923, Belkinomyia Adames and Galindo, 1973, Carrollia Lutz, 1905, Melanoconion Theobald, 1903, Micraedes Coquillett, 1906, Microculex Theobald, 1907, and Tinolestes Coquillett, 1906. However, the representatives of those ingroups, except for a few species that extend into subtropical areas, are confined to the Neotropical Region. Neoculex is predominantly an Old-World group, but several extant species occur in the Nearctic Region.
Larvae of most extant representatives of Neoculex inhabit shallow vegetated areas of permanent and temporary bodies of groundwater, but some species have been found in rock holes, crab holes, tree holes, bamboo internodes and artificial containers. Adult females are known to feed on frogs, birds and river rats (Belkin, 1962; Becker et al., 2010).
DISCUSSION
Taxonomic Diversity of the Group Culicomorpha in the Kishenehn Formation
Culicomorphan flies represent most fossils in the Kishenehn Formation. In particular, Greenwalt et al. (2014) have stated that in the unbiased collection of the Kishenehn material, fossils initially thought to be representatives of Chironomidae make up 50.5% of all insect fossils. Upon closer examination of the available material, it became clear that about 50% of these supposed fossils of Chironomidae are representatives of a single species of phantom midges (Chaoboridae), here named Chaoborus kishenehnicus sp. nov., or pupae of a biting midge (Ceratopogonidae) of the group Alluaudomyia. In total, we here examined 428 specimens of the culicomorphans from the formation. Among these, 113 specimens (26%) were pupae of Ceratopogonidae (Alluaudomyia sp.), 140 (33%) of the specimens were representatives of Chaoboridae (C. kishenehnicus sp.nov.), 175 (41%) were representatives of Chironomidae, and one specimen (0.2%) was a larval representative of Culicidae.
This subsample of representatives of Culicomorpha from the formation is not strictly representative, as we have pre-selected the studied specimens based on the quality of their preservation. These preliminary data do, however, reflect prevalence of representatives of Ceratopogonidae, Chaoboridae and Chironomidae in the fossil fauna of the formation.
In total, there are now 14 morphotypes of the culicomorphans recorded from the formation (Harbach and Greenwalt, 2012; Greenwalt and Moulton, 2016; Greenwalt et al., 2019; data herein). Nine of these morphotypes represent formally described species. Among the latter are two species of Culicidae, four of Dixidae, two of Chironomidae and a single species of Chaoboridae (Harbach and Greenwalt, 2012; Greenwalt and Moulton, 2016; Greenwalt et al., 2019; data herein). In general, the species diversity of Culicomorpha in the formation is relatively low, which, in combination with their high abundance, provides us with additional information on the palaeoenvironment of the formation.
Palaeoecological Implications of the Culicomorphan Diversity in the Kishenehn Formation
We have attempted to use the diversity of the fossil culicomorphans in the Kishenehn Formation to better understand the palaeoecology of this deposit. We have used both insights on the taphonomy of culicomorphans and extrapolated autecology of the individual morphotypes. The formation is relatively young (Middle Eocene) and the majority of the recorded culicomorphan morphotypes closely resemble their modern relatives. Therefore, we think it is feasible to extrapolate the autecology of these morphotypes based on their extant relatives (Grund, 2006; Stebner et al., 2017).
The majority of the recorded culicomorphan morphotypes have extant relatives associated with open lake habitats. Extant species of Chaoborus often inhabit ponds and lakes, often fishless or with a low-density of fishes (Sæther, 1997). Rheotanytarsus, Tanytarsus, Conchapelopia, and Psectrotanypus all contain large numbers of extant species, with diverse autecologies, but all of them have at least some representatives that inhabit lacustrine habitats (Langton, 1991). Extant representatives of Alluaudomyia (biting midges) inhabit a wide variety of habitats, often stagnant waters (Borkent, 2014a). Likewise, extant representatives of Culicidae occupy a spectrum of aquatic environments, and most of them feed on suspended particulate matter and microorganisms, which they extract from the water with filamentous mouth brushes (Belkin, 1962).
In the case of Hintelmanniella noncatafractata sp. nov., it is impossible to extrapolate its ecology from extant relatives, as the new species seems to be rather different from all extant species of Chironomini (Wiederholm, 1989; Cranston, 1999). Nevertheless, the rather unusual combination of the morphological characters of the adult males of the species indicates that they might have been surface-skimmers, as are a number of extant representatives of Chironomidae (Qi et al., 2018). Prominent characters of H. noncatafractata that point towards a surface skimming lifestyle include shortened antennae, robust and widened legs (especially femora) and relatively short wings. These traits are conductive to skimming on the water surface, utilising surface tension, and wind to glide along the water surface (Armitage et al., 1995). Most of the surface-skimming representatives of Chironomidae inhabit marine environments, but other surface-skimming insects, such as water bugs (Gerridae), Mayflies (Ephemeroptera), stoneflies (Plecoptera) (Marden and Thomas, 2003), and possibly also the representatives of the extinct group Chresmodidae are (or were) inhabiting open freshwater habitats (Dong et al., 2010). Additionally, some of the glacier and snow dwelling non-biting midges like numerous representatives of Diamesinae (Giłka, Soszyńska-Maj and Paasivirta, 2013), Orthocladiinae (Epler, 2012) and some of Podonominae (Serra-Tosio and Brundin, 1990), do have brachypterous conditions of the wings and similar legs. However, we think that presence of this ecomorphotype in the Kishenehn Formation is unlikely due to what we know about the palaeoecology of the deposit (Greenwalt et al., 2013).
ACKNOWLEDGEMENTS
V.B. is grateful to M. Spies (ZSM Munich) for his invaluable help with collection of ZSM as well as help with the literature. V.B is also grateful to A. Borkent for his valuable comments on Chaoboridae and Ceratopogonidae taxonomy and help with the literature. D.G. would like to thank H. Dailey, who as a student at Montana State University, did preliminary organizational work on the Kishenehn Formation Culicomorphans. We are grateful to two anonymous reviewers, the Handling Editor E. Peñalver, and the Executive Editor M. Hyžný for their efforts in improving this manuscript. Thanks to all people providing free software. J.M. Starck and C. Haug, both LMU Munich, are thanked for long standing support. This project was supported by the Volkswagen Foundation in the frame of a Lichtenberg Professorship of J.T. Haug (J.T. Haug; V. Baranov). V. Baranov was supported by the LMU Excellence Initiative, via LMU Junior Researcher Fund. V.B. visit to D.C. was supported by the Smithsonian Natural History Museum.
REFERENCES
Adames, A. J. and Galindo, P. 1973. A new subgenus and species of Culex from Colombia. Contributions of the American Entomological Institute, 9(3):55-61.
Armitage, P.D., Pinder, L.C., and Cranston, P.S. 1995. The Chironomidae: biology and ecology of non-biting midges. Springer Science and Business Media, London.
Arratia, G., Schultze, H.P., Tischlinger, H., and Viohl, G. 2015. Solnhofen -Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, Munich.
Baranov, V., Hoffeins, C., Hoffeins, H.W., and Haug, J.T. 2019a. Reaching across the ocean of time: a midge morphotype from the Cretaceous of Gondwana found in the Eocene Baltic amber. Palaeontologia Electronica, 22(2):1-17. https://doi.org/10.26879/955
Baranov, V., Schädel, M., and Haug, J.T. 2019b. Fly palaeo-evo-devo: immature stages of bibionomorphan dipterans in Baltic and Bitterfeld Amber. PeerJ, 7:e7843. https://doi.org/10.7717/peerj.7843
Baranov, V., Wang, Y., Wedmann, S., and Haug, J.T. 2020. Eco-morphological diversity of larvae of soldier flies and their closest relatives in deep time. PeerJ, 8:e10356. https://doi.org/10.7717/peerj.10356
Bause, E. 1913. Die Metamorphose der Gattung Tanytarsus und einiger verwandter Tendipedidenarten. Ein Beitrag zur Systematik der Tendipediden. Archiv für Hydrobiologie - Supplement, 2(1):1-26.
Becker, N., Petrić, D., Zgomba, M., Boase, C., Madon, M., Dahl, C., and Kaiser, A. 2010. Mosquitoes and their control. Second edition. Springer-Verlag, Berlin Heidelberg.
Belkin, J.N. 1962. The mosquitoes of the South Pacific (Diptera, Culicidae). Volumes 1 and 2. University of California Press, Berkeley and Los Angeles.
Borkent, A. 1978. Upper Oligocene fossil pupae and larvae of Chaoborus tertiarius (von Heyden) (Chaoboridae, Diptera) from West Germany. Quaestiones Entomologicae, 14:491-496.
Borkent, A. 2012. The pupae of Culicomorpha-morphology and a new phylogenetic tree. Zootaxa, 3396(1):1-98.
Borkent, A. 2014a. The pupae of the biting midges of the world (Diptera: Ceratopogonidae), with a generic key and analysis of the phylogenetic relationships between genera. Zootaxa, 3879:1-327. https://doi.org/10.11646/zootaxa.3879.1.1
Borkent, A. 2014b. World catalog of extant and fossil Chaoboridae (Diptera). Zootaxa, 3796(3): 469-493. https://doi.org/10.11646/zootaxa.3796.3.4
Borkent, A. and Sinclair, B.J. 2017. Key to Diptera families-larvae, p. 357-405. In Kirk-Spriggs, A.H. and Sinclair, B.J. (eds.). Manual of afrotropical diptera. Volume 1: introductory chapters and keys to Diptera families. South African National Biodiversity Institute, Suricata, Pretoria.
Constenius, K.N., Dawson, M.R., Pierce, H.G., Walter, R.C., and Wilson, M.V. 1989. Reconnaissance paleontologic study of the Kishenehn Formation, northwestern Montana and southeastern British Columbia, p. 189-203. In Montana Geological Society (ed.), 1989 Field Conference Guidebook: Montana Centennial Edition. Geologic Resources of Montana.
Constenius, K.N. 1996. Late Paleogene extensional collapse of the Cordilleran foreland fold and thrust belt. Geological Society of America Bulletin, 108:20-39. https://doi.org/10.1130/0016-7606(1996)108<0020:LPECOT>2.3.CO;2
Coquillett, DW. 1906. A classification of the mosquitoes of North and Middle America (No. 11). US Government Printing Office, Washington, D.C.
Cook, E. F. 1956. The Nearctic Chaoborinae (Diptera: Culicidae). University of Minnesota, Agricultural Experiment Station.
Cranston, P.S. 1997. Revision of Australian Rheotanytarsus Thienemann et Bause (Diptera: Chironomidae), with emphasis on the immature stages. Invertebrate Systematics, 11:705-734.
Cranston, P.S. 1999. Two unusual Chironomini (Diptera: Chironomidae) from Australian rainforest streams: one new genus and a neotropical genus new for the region. Australian Journal of Entomology, 38(4):291-299.
Cranston, P.S. and Epler, J.H. 2013. The larvae of Tanypodinae (Diptera: Chironomidae) of the Holarctic region-Keys and diagnoses. Insect Systematics and Evolution Supplements, 66:39-136.
Dong, R., Chungkun, S., Taiping, G., Yunzhi, Y., and Yunyun, Z. 2010. Silent stories: insect fossil treasures from Dinosaur Era of the Northeastern China. Science Press, Beijing.
Dunn, K.A., Mclean, R.J.C., Upchurch, G.R., Jr. and Folk, R.L. 1997. Enhancement of leaf fossilization potential by bacterial biofilms. Geology, 25:1119-1122. https://doi.org/10.1130/0091-7613(1997)025<1119:EOLFPB>2.3.CO;2
Dyar, H.G. 1905. Remarks on genitalic genera in the Culicidae. Proceedings of the Entomological Society of Washington, 79:43-49.
Edwards, F.W. 1912. Synopsis of the species of African Culicidae, other than Anopheles. Bulletin of Entomological Research, 3:1-53.
Epler, J.H. 2012. A brachypterous Bryophaenocladius (Diptera: Chironomidae: Orthocladiinae) with hypopygium inversum from Heggie’s Rock, Georgia, USA. Zootaxa, 3355(1):51-61. https/doi.org/ 10.11646/zootaxa.3355.1.3
Fittkau, E.J. 1957. Thienemannimyia und Conchapelopia, zwei neue Gattungen innerhalb der Ablabesmyia-Costalis -Gruppe (Diptera, Chironomidae). Chironomidenstudien VII. Archiv für Hydrobiologie, 53:313-332.
Giłka, W. 2010. A new species group in the genus Tanytarsus van der Wulp (Diptera: Chironomidae) based on a fossil record from Baltic amber. Acta Geologica Sinica (English Edition), 84:714-719. https://doi.org/10.1111/j.1755-6724.2010.00249.x
Giłka, W. 2011a. Ochotkowate - Chironomidae, plemie: Tanytarsini, postaci dorosle, samce. Klucze do oznaczania owadow Polski.Nr. 177 serii kluczy. Czesc XXVIII, Muchowki - Diptera, zeszyt 14b. Biologica Silesiae, Wroclaw. [In Polish]
Giłka, W. 2011b. A new fossil Tanytarsus from Eocene Baltic amber, with notes on systematics of the genus (Diptera: Chironomidae). Zootaxa, 3069:63-68. https://doi.org/10.11646/zootaxa.3069.1.4
Giłka, W., Soszyńska-Maj, A., and Paasivirta, L. 2013. The peculiar winter-active midge Diamesa starmachi (Diptera: Chironomidae). Polish Journal of Entomology, 82(3):201-211.
Giłka, W., Zakrzewska, M., Dominiak, P., and Urbanek, A. 2013. Non-biting midges of the tribe Tanytarsini in Eocene amber from the Rovno region (Ukraine): a pioneer systematic study with notes on the phylogeny (Diptera: Chironomidae). Zootaxa, 3736:569-586. https://doi.org/10.11646/zootaxa.3736.5.8
Greenwalt, D.E., Goreva, Y.S., Siljeström, S.M., Rose, T., and Harbach, R.E. 2013. Hemoglobin-derived porphyrins preserved in a Middle Eocene blood-engorged mosquito. Proceedings of the National Academy of Sciences, 110(46):18496-18500. https://doi.org/10.1073/pnas.1310885110
Greenwalt, D.E., Rose, T.R., Siljestrom, S.M., Goreva, Y.S., Constenius, K.N., and Wingerath, J.G. 2014. Taphonomy of the fossil insects of the middle Eocene Kishenehn Formation. Acta Palaeontologica Polonica, 60(4):931-947. https://doi.org/10.4202/app.00071.2014
Greenwalt, D.E. and Moulton, J.E. 2016. The first fossil New World Dixidae with a critical discussion of generic definitions. Palaeontologia Electronica, 19.3.55A:1-32. https://doi.org/10.26879/656
Greenwalt, D.E., Bickel, D.J., Kerr, P.H., Curler, G.R., Brown, B., De Jong, H., Fitzgerald, S. J., Dikow, T., Tkoč, M., Kehlmaier, C., and Amorim, D.S. 2019. Diptera of the middle Eocene Kishenehn formation. I. Documentation of diversity at the family level. Palaeontologia Electronica, 22.2.50A:1-56. https://doi.org/10.26879/891
Grimaldi, D. and Engel, M.S. 2005. Evolution of the Insects. Cambridge University Press, Hog Kong.
Grund, M. 2006. Chironomidae (Diptera) in Dominican amber as indicators for ecosystem stability in the Caribbean. Palaeogeography, Palaeoclimatology, Palaeoecology, 241(3-4):410-416.
Harbach, R.E. 2007. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny, p. 591-688. In Zhang, Z.-Q. and Shear, W.A. (eds.), Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa.
Harbach, R.E. and Greenwalt, D. 2012. Two Eocene species of Culiseta (Diptera: Culicidae) from the Kishenehn Formation in Montana. Zootaxa, 3530(1):25-34. https://doi.org/10.11646/zootaxa.3530.1.2
Harbach, R.E. and Knight, K.L. 1980. Taxonomists’ Gossary of Mosquito Anatomy. Plexus Publishing, Inc., Marlton, New Jersey.
Haug, J.T., Haug, C., Kutschera, V., Mayer., G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E.N.K., and Waloszek, D. 2011. Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy, 244(3):259-272.
Haug, J.T., Müller, P., and Haug, C. 2018. The ride of the parasite: a 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters, 4: 31. https:// doi.org/10.1186/s40851-018-0116-9
Haug, J.T., Schädel, M., Baranov, V.A., and Haug, C. 2020. An unusual 100-million-year old holometabolan larva with a piercing mouth cone. PeerJ, 8:e8661. https://doi.org/10.7717/peerj.8661
Holmgren, A. E. and Aurivillius, C. 1883. Insecta a viris doctissimis Nordenskiöld illum ducem sequentibus in insulis Waigatsch et Novaja Semlia anno 1875 collecta. Hymenoptera et Diptera. Entomologisk Tidskrift, 4(3-4):141-190.
Huber, M. and Caballero, R. 2011. The early Eocene equable climate problem revisited. Climate of the Past, 7(2): 603-633. https://doi.org/10.5194/cp-7-603-2011
Johnston, J.E. and Borkent, A. 1998. Chaoborus Lichtenstein (Diptera: Chaoboridae) pupae from the middle Eocene of Mississippi. Journal of Paleontology, 72(3):491-493. https://doi.org/10.1017/S0022336000024252
Kieffer, J.-J. 1909. Diagnoses de nouveaux Chironomides d’Allemagne.d’ Allemagne Bulletin de la Société d'histoire naturelle de Metz, 26:37-56.
Kieffer, J.-J. 1911. Nouvelles descriptions des Chironomides obtenus d'éclosion. Bulletin de la Société d'histoire naturelle de Metz, 27:1-60.
Kieffer, J.-J. 1913. Nouvelle étude sur les Chironomides de l'Indian Museum de Calcutta. Records of the Indian Museum, 9(3):119-197.
Kyerematen, R.A.K. and Sæther, O.A. 2000. A review of Afrotropical Rheotanytarsus Thienemann et Bause, 1913 (Diptera: Chironomidae). Tijdschrift voor Entomologie, 143:27-669. https://doi.org/10.1163/22119434-99900038
Kyerematen, R.A.K., Andersen, T., and Sæther, O.A. 2000. A review of Oriental Rheotanytarsus Thienemann and Bause, with description of some new species (Insecta, Diptera, Chironomidae). Spixiana, 23(3):225-258.
Langton, P.H. 1991. A key to pupal exuviae of West Palaearctic Chironomidae. Privately published, Huntingdon, Cambridge.
Langton, P.H. and Pinder, L.C.V. 2007. Keys to the adult male Chironomidae of Britain and Ireland; 2 vols. Freshwater Biological Association Scientific Publication 64, Ambleside.
Lichtenstein, A.A.H. 1800. Beschreibung eines neu entdeckten Wasserinsekts. Archiv für Zoologie und Zootomie, 1:168-175.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundem classes, ordines, genera, species cum characteribus, differentis, synonymis, locis. Tomus I. Editio decima, reformata. Laurentii Salvii, Holmiae.
Lutz, A. and Bourroul, I. 1904. Mosquitos do Brasil-Faculdade de Medicina da Baía (Doctoral dissertation, Tese de doutoramento).
Lutz, A. 1905. Novas especies de mosquitos do Brasil. Imprensa Medica, 13: 125-171.
Marshall, S. 2012. Flies: the natural history and diversity of Diptera. Firefly Press, Richmond Hill.
Marshall, S., Kirk-Spriggs, H.A., Mullerm B.S., Paiero, M.S., Yau, T., and Jackson, M.D. 2017. Key to Diptera families- Adults. p. 267-355. In Kirk-Spriggs, A.H. and Sinclair, B.J. (eds. Manual of Afrotropical Diptera. Volume 1: introductory chapters and keys to Diptera families. Pretoria: South African National Biodiversity Institute, Suricata 4.
Martínez-Delclòs, X., Briggs, D.E., and Peñalver, E. 2004. Taphonomy of insects in carbonates and amber. Palaeogeography, Palaeoclimatology, Palaeoecology, 203(1-2):19-64. https://doi.org/10.1016/S0031-0182(03)00643-6
Macquart, J. 1838. Diptères exotiques nouveaux ou peu connus. Memoires de la Société (Royale) des sciences, de l'agriculture et des arts à Lille, 1:9-225.
Mayr, E. 1942. Systematics and the origin of species. Columbia University Press, New York.
Marden, J.H. and Thomas, M.A. 2003. Rowing locomotion by a stonefly that possesses the ancestral pterygote condition of co-occurring wings and abdominal gills. Biological Journal of the Linnean Society, 79(2):341-349. https://doi.org/10.1046/j.1095-8312.2003.00192.x
Meigen, J. 1818. Nouvelle classification des mouches a deux ailes (Diptera L.), p. 1-40. In Fuchs, J. J. (ed.), D'apres un plan tout nouveau, Paris.
Merritt, R.W. and Cummins, K.W. 1996. An Introduction to the Aquatic Insects of North America. Kendall Hunt, Dubuque.
Newman, E. 1834. Attempted division of British insects into natural order. Entomological Magazine, 2:379-431.
Pierce, H.G. and Constenius, K.N. 2001. Late Eocene - Oligocene nonmarine mollusks of the northern Kishenehn Basin, Montana and British Columbia. Annals of the Carnegie Museum, 70:1-112.
Pierce, H.G. and Constenius, K.N. 2014. Terrestrial and aquatic mollusks of the Eocene Kishenehn Formation, Middle Fork Flathead River, Montana. Annals of Carnegie Museum, 82(4):305-329. https://doi.org/10.2992/007.082.0401
Qi, X., Lin, X. L., Ekrem, T., Beutel, R.G., Song, C., Orlov, I., Chen, C.-T., and Wang, X.H. 2018. A new surface gliding species of Chironomidae: An independent invasion of marine environments and its evolutionary implications. Zoologica Scripta, 48(1):81-92. https://doi.org/10.1111/zsc.12331
Roback, S.S. 1971. The adults of the subfamily Tanypodinae (= Pelopiinae) in North America (Diptera: Chironomidae). Monographs-Academy of Natural Sciences of Philadelphia, 17:1-410.
Sæther, O.A. 1970. Nearctic and Palaearctic. Chaoborus (Diptera: Chaoboridae). Bulletin of the Fisheries Research Board of Canada, 174:1-57.
Sæther, O.A. 1997. Diptera Chaoboridae, phantom midges, p. 149-161. In Nilson, A. (ed.), Aquatic Insects of North Europe, part 2. Apollo Books, Stenestrup.
Serra-Tosio, B. and Brundin, L. 1990. Redescription du mâle de Microzetia mirabilis Séguy, 1965, chironomide endémique des îles Crozet (Diptera: Chironomidae). Annales de la Société entomologique de France, 26(3):411-419.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P., and Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods, 9(7):676-682. https/doi.org/10.1038/nmeth.201
Shadrin, N.V., Belyakov, V.P., Bazhora, A.I., and Anufriieva, E.V. 2019. Does salinity affect body proportions and “size/mass” ratios of highly halotolerant Baeotendipes noctivagus larvae (Diptera, Chironomidae)? Oceanological and Hydrobiological Studies, 48(4):305-315. https://doi.org/10.2478/ohs-2019-0028
Shilova, A.I. 1961. Novyi rod i vid tendipedid (Diptera, Tendipedidae) Lipiniella Shilova, gen. n. Byulletin Instituta Biologii Vodokhranilishch, Akademiya Nauk SSSR, 11:19-23. [In Russian]
Stebner, F., Baranov, V., Zakrzewska, M., Singh, H., and Giłka, W. 2017. The Chironomidae diversity based on records from early Eocene Cambay amber, India, with implications on habitats of fossil Diptera. Palaeogeography, Palaeoclimatology, Palaeoecology, 475:154-161. https://doi.org/10.1016/j.palaeo.2017.03.019
Strickman, D. 1980. Observations on adults and eggs of Chaoborus flavicans (Diptera: Chaoboridae). Hydrobiologia, 74:195-197.
Theobald, F.V. 1903. Description of a new North American Culex. The Canadian Entomologist, 35(8): 211-213.
Theobald, F.V. 1907. A Monograph of the Culicidae, Or Mosquitoes: Mainly Compiled from the Collections Received at the British Museum from Various Parts of the World in Connection with the Investigation into the Cause of Malaria Conducted by the Colonial Office and the Royal Society. Royal Society, London.
Thienemann, A. and Zavřel, J. 1916. Die Metamorphose der Tanypinen. Archiv für Hydrobiologie - Supplements, 2(3):566-654.
von Heyden, C. 1862. Gliederthiere aus der Braunkohle des Niederrheins, der Wetterau und der Röhn. Palaeontographica, 10:62-82.
Wiederholm, T. 1986. Chironomidae of the Holarctic region. Keys and diagnoses. Part II. Pupae. Entomologica scandinavica Supplement, 28:1-482.
Wiederholm, T. 1989. Chironomidae of the Holarctic region. Keys and diagnoses. Part III. Adult males. Entomologica Scandinavica Supplement, 34:1-532.
Wolfe, J.A. 1995. Paleoclimatic estimates from Tertiary leaf assemblages. Annual Review of Earth and Planetary Sciences, 23(1):119-142. https://doi.org/10.1146/annurev.ea.23.050195.001003
Wulp, F.M. van der. 1874. Dipterologische aanteekeningen. N°. 4. [Dipterological notes. No. 4.]. Tijdschrift voor entomologie, 17(3):109-112.
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292(5517):686-693. https/ doi.org/ 10.1126/science/1059412
Zakrzewska, M. and Giłka, W. 2015. The Tanytarsini (Diptera: Chironomidae) in the collection of the Museum of Amber Inclusions, University of Gdansk. Zootaxa, 3946(3):347-360. https://doi.org/10.11646/zootaxa.3946.3.3
Zakrzewska, M., Singh, H., Wagner-Wysiecka, E., and Giłka, W. 2020. Minute and diverse in fossil sticky stuff: Tanytarsini (Diptera: Chironomidae) from early Eocene Indian Cambay amber. Zoological Journal of Linnean Society, 189(4):1398-1425. https://doi.org/10.1093/zoolinnean/zlz159

