 Reaching across the ocean of time: A midge morphotype from the Cretaceous of Gondwana found in the Eocene Baltic amber
Reaching across the ocean of time: A midge morphotype from the Cretaceous of Gondwana found in the Eocene Baltic amber
Article number: 22.2.38
https://doi.org/10.26879/955
Copyright Paleontological Society, June 2019
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 27 December 2018. Acceptance: 29 May 2019
{flike id=2605}
ABSTRACT
Non-biting midges (Chironomidae) have a fossil record reaching back into the Triassic. The non-biting midge ingroup Libanochlites Brundin, 1976, was so far known from a single species. Fossil specimens of this species came from the Cretaceous of Gondwana, more precisely from Lebanese amber (130 million years). A new species, based on fossils in Eocene Baltic amber, is so similar to the so far single species of Libanochlites that it can only be attributed to this group. This is extending the known geological range of this so far unique morphology by 80 million years. This provides us with the first case of the long-term survival (“Bradytely”) of a distinct Mesozoic morphotype of a representative Diptera with aquatic larvae into the Cenozoic. Bradytely is a phenomenon describing evolutionary stasis. This represents a case of bradytely in insects with aquatic larvae and has an impact on our understanding of the Cretaceous terrestrial revolution. Primarily, this record allows us to determine conditions which are permitting the survival of the Mesozoic aquatic insects into the Palaeogene. The conditions are related to the bradytely of the insects with aquatic larvae in the temperate, subtropical forest.
Viktor Baranov. Department of Biology II, LMU Munich, Großhaderner Str. 2, 82152, Martinsried-Planegg, Germany. baranow@biologie.uni-muenchen.de
Christel Hoffeins. Liseistieg 10, D-22149 Hamburg, Germany. chw.hoffeins@googlemail.com
Hans-Werner Hoffeins. Liseistieg 10, D-22149 Hamburg, Germany. chw.hoffeins@googlemail.com
Joachim T. Haug. GeoBio-Center, LMU Munich, Großhaderner Str. 2, 82152, Martinsried-Planegg, Germany and Department of Biology II, LMU Munich, Großhaderner Str. 2, 82152, Martinsried-Planegg, Germany. jhaug@bio.lmu.de
Key words: fossil insect; Cretaceous Terrestrial Revolution; Bradytely; Diptera; Chironomidae; amber; new species
Baranov, Viktor, Hoffeins, Christel, Hoffeins, Hans-Werner, and Haug, Joachim T. 2019. Reaching across the ocean of time: A midge morphotype from the Cretaceous of Gondwana found in the Eocene Baltic amber. Palaeontologia Electronica 22.2.38A 1-17. https://doi.org/10.26879/955
palaeo-electronica.org/content/2019/2605-libanochlites-from-eocene
Copyright: June 2019 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/F2D63E3A-134A-4E32-A390-246C66990F67
INTRODUCTION
Bradytely is a phenomenon describing evolutionary stasis. In other words, bradytely is a condition under which the rate of evolutionary changes and diversification in a specific group of the organisms becomes stagnant (Simpson, 1944). Bradytely seems relatively common among terrestrial forms of Euarthropoda, especially those which live in stable conditions for longer stretches of time (Clarke and Chatzimanolis, 2009; Yamamoto et al., 2017; Lohrmann and Engel, 2017). Stable conditions in occupied habitats seem to cause long-term conservations of morphotypes, which could, in some cases persist for hundreds of millions of years (Haug et al., 2012; Yamamoto et al., 2017).
Some groups of beetles (Coleoptera) living in hygropetric (thin layer of water covering a rock surface) or other hygrophilous (very moist) habitats have shown remarkably little change in the last 100 million years. The morphological similarity to modern-day species has led to the fact that many Cretaceous fossils have been interpreted as representatives of genera with modern representatives (Clarke and Chatzimanolis, 2009; Yamamoto et. al., 2017).
The taxonomic rank 'genus' is, in principle, arbitrary. There is no clear criterion when to apply it. Still the principal concept behind the genus essentially expresses that two (or more) species are principally more similar in morphology to each other than to other clusters of species separated by morphological hiatus. A fossil species attributed to a genus with extant representatives is therefore indicative of conservation of a morphotype, which is a relatively frequent occurrence for species from the Cretaceous.
Some genera with extant representatives of caddisflies (Trichoptera) such as Ecnomus McLachlan, 1864, or Phylocentropus Banks, 1907, have ingroup species known from Early Cretaceous Lebanese amber (Wichard and Azar, 2018). Similarly genera with extant representatives of culicomorphans (Diptera), including Corethrella Coquillett, 1902, (Corethrellidae) and also Austroconops Wirth and Lee, 1959, and Leptoconops Skuse, 1889, (both Ceratopogonidae) also include species described based on fossils from Lebanese amber (Szadziewski, 1995, 2008).
Chironomidae, the group of non-biting midges, is a species-rich ingroup of Diptera. The group includes about 6,500 extant species (Pape et al., 2011) and has a rich geological record. Yet, so far no known morphotypes (≈genera) of Chironomidae appear to have survived from the Mesozoic into the Cenozoic (Kalugina, 1974; Kalugina and Kovalev, 1984; Doitteau and Nel, 2008).
The only indications for such a case are specimens reported by Boesel (1937). He identified two fossils from Late Cretaceous Canadian amber as representatives of genera with extant representatives, namely of Smittia Holmgren, 1869, and Metriocnemus van der Wulp, 1874. Yet, this material is poorly preserved and should be treated with caution.
The absence of such cases of survival of Mesozoic morphotypes of Chironomidae is in fact surprising. Many freshwater habitats (such as streams), in which such forms live, are very stable. We should therefore expect that bradytely would be relatively common among the ingroups of Chironomidae (Wagner et al., 2011; Lancaster and Downes, 2013).
Most non-biting midges (Chironomidae) preserved in Eocene amber are so similar to modern forms that they are generally interpreted as representatives of genera that include extant species (Seredszus and Wichard, 2007; Doitteau and Nel, 2008; Wichard et al., 2011). Less than 10% of the fossil non-biting midges preserved in Oise and Baltic ambers have been interpreted as representatives of genera without modern-day representatives (Seredszus and Wichard, 2007; Doitteau and Nel, 2008; Wichard and Seredszus, 2010; Baranov et al., 2015). Among others, species attributed to the genus Buchonomyia appear to have a very high degree of morphological conservation, with Buchonomyia succinea Seredszus and Wichard, 2002, from the Eocene Baltic amber being very similar morphologically to the extant species Buchonomyia thienemanni Fittkau, 1955 (Seredszus and Wichard, 2002).
This makes the absence of reliable records of Mesozoic morphotypes of non-biting midges in Cenozoic deposits even more surprising (Gründ, 2006; Seredszus and Wichard, 2010; Baranov et al., 2015). In particular, Lebanese amber, which is an amber deposit with the highest species richness of Chironomidae in the Cretaceous, has not yet yielded any fossil that is similar enough to extant or Cenozoic non-biting midges to attribute it to a genus with extant or Cenozoic representatives (Veltz et al., 2007; Azar et al., 2010).
The first fossil of Chironomidae ever described from Lebanese amber was a peculiar midge Libanochlites neocomicus Brundin, 1976, which was interpreted by Brundin (1976) as a representative of Podonominae (Brundin, 1972, 1976). Since its original description, L. neocomicus has been extensively studied (Brundin, 1976; Veltz et al., 2007; Azar et al., 2008).
Here we report a possible case of bradytely in Chironomidae, based on a new fossil interpreted as a representative of Libanochlites, from Eocene Baltic amber.
MATERIAL AND METHODS
Material
The single specimen in focus of this study was selected from a bag with chironomid inclusions by C.H. in 2013, offered by Dr. Andrey Krylov, Sea Venture Bureau, Kaliningrad, Russia. The amber material was obtained from different private sources in Yantarnyj, Kaliningrad district (formerly Palmnicken, Königsberg), then sorted to higher taxonomic units (“orders” or “families”) and offered for sale.
The specimen, a non-biting midge is embedded in a clear piece of Baltic amber without syninclusions. After the description, the amber with the type specimen will be embedded in polyester resin to avoid alteration (Hoffeins, 2001). Holotype is deposited in Senckenberg Deutsches Entomologisches Institut, Münchenberg, Germany (SDEI), under the permanent number HT: Dip 00606 male.
The specimen was compared to a specimen of L. neocomicus from Hammana-Mdeyrij outcrop, Baabda District, Mount Lebanon Governorate and Central Lebanon. The specimen examined here is deposited at the Natural History Museum of the Lebanese University, Azar collection, Fanar, Lebanon. Accession number AUBL-NBS-2B.
For comparison an extant representative of Afrochlus harrisoni Freeman, 1964, was also documented. It comes from the Zoological State Collection Munich (Zoologische Staatsammlung München; Germany; no collection number). Comparative material of the Corethrella marksae Colles, 1986, is deposited in the entomology collection of West Lafayette, Purdue University, USA (PURC, no collection number).
Imaging Methods
The specimen from Baltic amber was imaged using a Keyence VHX-6000 Digital microscope, either with ring light type illumination (Haug et al., 2018) or cross-polarised co-axial illumination (Haug, J.T., et al., 2013). All photos presented are composite images (e.g., Haug et al., 2008, 2011). Each image details were recorded with a stack of images, which were then fused to a single sharp image. Resulting sharp images were stitched to a single panorama, all using the built-in software (Haug et al., 2019). We also employed the HDR function included in the Keyence microscope software (Haug C., et al., 2013; Haug and Rötzer, 2018; Haug et al., 2019), i.e., every single frame of a stack is a composite from several images taken under different exposure times.
The specimen of L. neocomicus was scanned using Laser-Scanning confocal microscope in the imaging centre of the London Natural History Museum using settings described in Baranov et al. (2018). The extant specimen of Afrochlus harrisoni Freeman, 1964, was documented using DCM 510 ocular camera and Leitz Diaplan optical microscope. The specimen of the extant species Corethrella marksae was documented with a scanning electron microscopy (SEM). Scanning was performed using a Carl Zeiss Leo 1430VP scanning electron microscope in the Zoologische Staatssammlung München (Germany). Scanning was performed with beam’s electric current 80 µA; filament electric current 2500 A; and electric potential 10-20 kV. Scanning was performed in low vacuum (<2e-005 mbar).
Image editing, i.e., optimisation of contrast, histogram, colour and sharpness, was performed in Adobe Photoshop CS2. Drawings were prepared in Inkscape 0.48 and Adobe Illustrator CS2.
SYSTEMATIC PALAEONTOLOGY
DIPTERA Linnaeus, 1758
CHIRONOMIDAE Newman, 1834
Genus of uncertain position (“incerta sedis”)
LIBANOCHLITES Brundin, 1976
Type species. Libanochlites neocomicus Brundin, 1976, by original designation.
Libanochlites eocenicus sp. nov.
Figure 1.1, Figure 2.1, Figure 3.1, Figure 4.1-4.2, Figure 5.1-5.4, Figure 6.1-6.3
zoobank.org/6430391B-C958-4AFE-A1EF-C58995990852
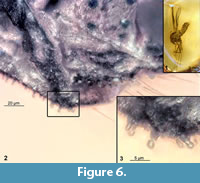
 [Differential] diagnosis. Species can be easily distinguished from the L. neocomicus based on the following combination of the characters: Medium size midge (total length 4.8 mm), with long maxillary palpi, reaching mid-length of the femur of first thoracic appendage (Figure 2.1); gonocoxite bearing strong erect setae in the groups of three on the series of the 10 elevated warts rather than four warts as in L. neocomicus (Figure 5.2); gonostylus IX without accessory lobes (Figure 5.4). Abdominal segments bearing mushroom-shaped sensilla (Figure 6.1-6.3).
[Differential] diagnosis. Species can be easily distinguished from the L. neocomicus based on the following combination of the characters: Medium size midge (total length 4.8 mm), with long maxillary palpi, reaching mid-length of the femur of first thoracic appendage (Figure 2.1); gonocoxite bearing strong erect setae in the groups of three on the series of the 10 elevated warts rather than four warts as in L. neocomicus (Figure 5.2); gonostylus IX without accessory lobes (Figure 5.4). Abdominal segments bearing mushroom-shaped sensilla (Figure 6.1-6.3).
Amended generic diagnosis. Small- to mid-sized representatives of Chironomidae with well-developed mandibles and lacinia, dome-shaped clypeus, wing with crossvein Mcu-present, radial vein 2+3 absent, with wide gap between radial veins 1 and 4+5, lyrate spur with six denticles, hypopigium with strong protruding setae on the four to 10 warts on the gonocoxite. Dome-shaped clypeus and tibial spurs with six denticles are the autapomorphies.
Etymology. Named “eocenicus” after Eocene epoch of the Palaeogene and to contrast with the name of the type species of the genus L. neocomicus.
Holotype. Dip 00606 male deposited in SDEI.
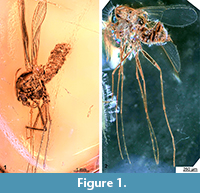
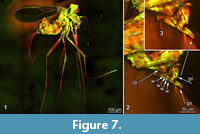
Description. Single midge preserved in a piece of Baltic amber. Male based on genitalia. Total body length is 4.8 mm. Body length/wing length ratio=1.13 (Figure 1.1). Generally well-preserved specimen with head, thorax bearing appendages and wings, and abdomen. All thoracic appendages (“legs”) miss the distal part.
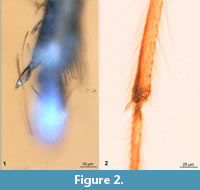 Head: Bearing compound eyes, antennae (first pair of appendages) and mouthparts. Compound eye with distinct dorsal-median extension, nine erect postorbital setae present. Clypeus large, distinctly dome-shaped, bearing >20 clypeal setae (Figure 2.1). Antenna 1.5 mm long. Pedicellus (antenna element 2) bulbous with 4-5 setae (Figure 2.1). The total number of flagellomeres (antennal elements) is impossible to count due to the dense plumage, lengths of proximal 10 flagellomeres in µm as following: 30, 41, 33, 42, 65, 34, 45, 54, 28, and 26. Remaining flagellomeres together 1.2 mm long. Mouthparts well developed, mandibles present. Maxilla with stiletto-like lacinia and maxillary palp. Maxillary palp very long, reaching the mid-length of the femore of the first throacic appendages. Maxillary palp element 3 350 µm, palp element 4 330 µm, palp element 5 550 µm.
Head: Bearing compound eyes, antennae (first pair of appendages) and mouthparts. Compound eye with distinct dorsal-median extension, nine erect postorbital setae present. Clypeus large, distinctly dome-shaped, bearing >20 clypeal setae (Figure 2.1). Antenna 1.5 mm long. Pedicellus (antenna element 2) bulbous with 4-5 setae (Figure 2.1). The total number of flagellomeres (antennal elements) is impossible to count due to the dense plumage, lengths of proximal 10 flagellomeres in µm as following: 30, 41, 33, 42, 65, 34, 45, 54, 28, and 26. Remaining flagellomeres together 1.2 mm long. Mouthparts well developed, mandibles present. Maxilla with stiletto-like lacinia and maxillary palp. Maxillary palp very long, reaching the mid-length of the femore of the first throacic appendages. Maxillary palp element 3 350 µm, palp element 4 330 µm, palp element 5 550 µm.
Thorax: dorsally bearing numerous prominent setae; four strong anteropronotal setae present on the upper 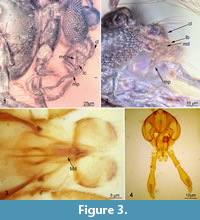 anteropronotum, at least 19 dorsocentral setae and 14 strong acrostichal setae, 12 scutelar setae, (meta) postnotum with five strong postnotal setae, (meta) postnotum without a longitudinal median groove. Thoracic appendages:femora (leg elements 3) 1700 µm on the appendage 1, 1600 on the 2nd and 1200 µm on the 3d; tibiae (leg element 4) 1300 µm on the appendage 1, 2600 on the 2nd and 2800 µm on the 3d; tibiae with prominent tibial spurs on the appendages: one on first, two on second, two on third; spur on the first simple, spur on second hard to examine, spur on third lyrate with six denticles (Figure 3.2). Tibia of the third thoracic appendage with comb comprising eight setae, occupying approximately 50% of tibia width. (Figure 3.2).
anteropronotum, at least 19 dorsocentral setae and 14 strong acrostichal setae, 12 scutelar setae, (meta) postnotum with five strong postnotal setae, (meta) postnotum without a longitudinal median groove. Thoracic appendages:femora (leg elements 3) 1700 µm on the appendage 1, 1600 on the 2nd and 1200 µm on the 3d; tibiae (leg element 4) 1300 µm on the appendage 1, 2600 on the 2nd and 2800 µm on the 3d; tibiae with prominent tibial spurs on the appendages: one on first, two on second, two on third; spur on the first simple, spur on second hard to examine, spur on third lyrate with six denticles (Figure 3.2). Tibia of the third thoracic appendage with comb comprising eight setae, occupying approximately 50% of tibia width. (Figure 3.2).
Wing: total length 4.2 mm, wings partly folded and hard to examine. Medial-cubital vein present. Radial vein 2+3 absent, the gap between radial vein 1 and radial vein 4+5 wide, 1.2 mm (Figure 1.1, Figure 4.1, 4.2).
Abdomen: abdomen relatively wide (max width/max length ratio= 0.4) and densely setose. Tergite of 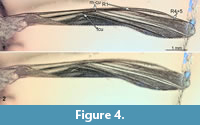 abdominal segment IX bears a number of the mushroom-like sensilla, with an apparent pore in the center (Figure 6.1-6.3). Sensilla situated on the lateral parts of the tergite IX (Figure 6.2, 6.3). Hypopygium (male genitalia, tergite IX + postgenital segments): gonocoxite (II), cylindrical, total length 170 µm, bearing 10 warts with strongly curved setae in groups of three at each wart; gonostylus curved, sickle-shaped, without basal expansion or medial spines, megaseta (terminal spine of the gonostylus) strong (Figure 5.1-5.4).
abdominal segment IX bears a number of the mushroom-like sensilla, with an apparent pore in the center (Figure 6.1-6.3). Sensilla situated on the lateral parts of the tergite IX (Figure 6.2, 6.3). Hypopygium (male genitalia, tergite IX + postgenital segments): gonocoxite (II), cylindrical, total length 170 µm, bearing 10 warts with strongly curved setae in groups of three at each wart; gonostylus curved, sickle-shaped, without basal expansion or medial spines, megaseta (terminal spine of the gonostylus) strong (Figure 5.1-5.4).
Remarks. We ascribe the new species to the genus Libanochlites based on the following combination of the characters (described in detail further below): Overall similar habitus (Figure 1.1); clypeus large, dome-shaped (Figure 2.1); third thoracic appendages (hind legs) with prominent tibial spurs, that bear 6 denticles (“lyrate”; Figure 3.1); wings without radial vein 2+3 (Figure 1.1-1.2, Figure 4.1-4.2); gonocoxite with elevated structure bearing clusters of the strong setae (Figure 5.1-5.4).
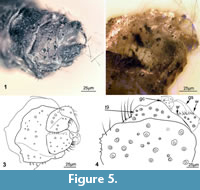 These characters establishe L. eocenicus sp. nov. as the sister species to L. neocomicus. A large, dome-shaped clypeus, translucent tibial spurs with six denticles on the third thoracic appendage, and presence of the strong setae on warts along the gonocoxite can be considered unique autapomorphies of the monophyletic group Libanochlites (Figure 5.1-5.4, Figure 7.1-7.3).
These characters establishe L. eocenicus sp. nov. as the sister species to L. neocomicus. A large, dome-shaped clypeus, translucent tibial spurs with six denticles on the third thoracic appendage, and presence of the strong setae on warts along the gonocoxite can be considered unique autapomorphies of the monophyletic group Libanochlites (Figure 5.1-5.4, Figure 7.1-7.3).
The new specimen is very similar to a species known from the Cretaceous of Lebanon - Libanochlites neocomicus Brundin, 1976. Therefore, a detailed comparison is provided in the following section.
The male specimen described here is notably larger than that of L. neocomicus (4.8 mm vs. 1.0-1.5 mm) (Figure 1.1, 1.2). Yet, this is well within known intergeneric size ranges known within Chironomidae. For example in the genus Pseudorthocladius Goetghebuer, 1943 size of adults varies between 0.7 and 3.4 mm (Andersen and Saether, 1996; Baranov et al., 2015). Hence, the differences in size will not exclude the supposed sister-species relationship between the two fossil species.
 Wings are always an important character in interpreting representatives of winged insects; hence it is unfortunate that they are partly folded in the new specimen. However, the wide gap between radial veins 1 and 4+5 seen in the new specimen is also present in L. neocomicus (Figure 1.1, 1.2, Figure 4.1, 4.2, Figure 7.1). The new specimen, as well as L. neocomicus, has a very wide abdomen (max width/max length ratio 0.55 in L. neocomicus and 0.4 in the new specimen). Both, the new specimen and L. neocomicus, have a dome-shaped clypeus with numerous setae on it (Figure 2.1, 2.2). Both possess a large, bulbous pedicellus with several setae on it (Figure 2.1, 2.2); in general, the morphology of the antennae is very similar in both specimens. The elongated, smooth mandible of the new specimen is similar to some extant male representatives of Chironomidae, more precisely those of the genus Afrochlus (Cranston and Edward, 1998; Martin et al., 2003) (Figure 2.3, 2.4). The proximal part of the maxilla is again similar in the new specimen and L. neocomicus, possessing a stiletto-like lacinia. There is a distinct difference in the morphology of the distal region of the maxillae, i.e., the palps: in L. neocomicus, the maxillary palpi are very short, they do not even reach the distal end of the mouthparts, whereas those of the new specimen are very long reaching to the middle of the femora of the first thoracic appendages. Additionally, there are numerous long setae on the palps of the new specimen (Figure 2.1), in contrast to condition in L. neocomicus.
Wings are always an important character in interpreting representatives of winged insects; hence it is unfortunate that they are partly folded in the new specimen. However, the wide gap between radial veins 1 and 4+5 seen in the new specimen is also present in L. neocomicus (Figure 1.1, 1.2, Figure 4.1, 4.2, Figure 7.1). The new specimen, as well as L. neocomicus, has a very wide abdomen (max width/max length ratio 0.55 in L. neocomicus and 0.4 in the new specimen). Both, the new specimen and L. neocomicus, have a dome-shaped clypeus with numerous setae on it (Figure 2.1, 2.2). Both possess a large, bulbous pedicellus with several setae on it (Figure 2.1, 2.2); in general, the morphology of the antennae is very similar in both specimens. The elongated, smooth mandible of the new specimen is similar to some extant male representatives of Chironomidae, more precisely those of the genus Afrochlus (Cranston and Edward, 1998; Martin et al., 2003) (Figure 2.3, 2.4). The proximal part of the maxilla is again similar in the new specimen and L. neocomicus, possessing a stiletto-like lacinia. There is a distinct difference in the morphology of the distal region of the maxillae, i.e., the palps: in L. neocomicus, the maxillary palpi are very short, they do not even reach the distal end of the mouthparts, whereas those of the new specimen are very long reaching to the middle of the femora of the first thoracic appendages. Additionally, there are numerous long setae on the palps of the new specimen (Figure 2.1), in contrast to condition in L. neocomicus.
 The thorax of the new specimen is almost identical to the L. neocomicus. Although the new specimen lacks the distal parts of the thoracic appendages, it preserves one important feature shared with L. neocomicus, namely the characteristic lyrate tibial spurs with six denticles (Figure 3.1, 3.2).
The thorax of the new specimen is almost identical to the L. neocomicus. Although the new specimen lacks the distal parts of the thoracic appendages, it preserves one important feature shared with L. neocomicus, namely the characteristic lyrate tibial spurs with six denticles (Figure 3.1, 3.2).
Detailed examination of the genital morphology of L. neocomicus using laser-scanning confocal microscope has revealed that L. neocomicus does, in fact, bear a number of the strong setae on the four cone-shaped warts, connected by an apodeme (Figure 7.1-7.3), rather than on the single elevated pad, as figured in Veltz et al. (2007, p.183, fig. 14).
In the new specimens, the gonocoxite of abdominal segment nine bears groups of strong setae (Figure 4.1, 4.2) on a number of elevated warts. While being similar in structure those warts are much more numerous than in L. neocomicus, 10 in total (Figure 5.1-5.4, Figure 7.1-7.4).
Saether (1977) noted that while male genitalia of the Chironomidae are widely used for the species level diagnostic, other characters of males (wing venation, tibial spur structure, thorax setation) or female genitalia structure, could be used to recognise supraspecific taxonomic units in Chironomidae, such as genera. It is worth noting, however, that Chironomidae are prone to exhibit large intrageneric variation in the male genitalia structure (Brundin, 1966; Zhang et al., 2016). This phenomenon has two causes, an artificial one and a possible natural one. First this observation tells us that the tradition of forming genera (which is an artificial act) is different in Chironomidae compared to other lineages of Diptera. Second, it seems that highly differentiated genitalia represent a mechanism of the prezygotic isolation within Chironomidae (Armitage, 1995). The arrangement of the setae on the gonocoxite of the Libanochlites is quite similar to that in Trichotanypus foliaceus Wirth and Sublette, 1970 (an extant representative of Podonominae Thienemann, 1937; Wirth and Sublette, 1970: fig. 1). In T. foliaceus each seta sits on an individual wart, while in Libanochlites they are arranged in groups of three.
The gonostylus of the new specimen is generally very similar to the L. neocomicus in general shape, but lacks a lateral lobe, possessed by the latter species (Veltz et al., 2007, fig. 4D). Based on the similarities of the new specimen and L. neocomicus outlined above we interpret the new specimen as a closely related form, yet based on the significant differences as a separate species.
Occurrence. Baltic Sea coast, Samland Peninsula (Seredszus and Wichard, 2007). Ypresian to Priabonian, Eocene, 56-33.9 Ma (Seredszus and Wichard, 2007).
DISCUSSION
Broader Systematic Interpretation
The discovery of the tibial spurs in L. neocomicus by Azar et al. (2008) provided an important phylogenetic signal. Brundin (1976) initially interpreted Libanochlites as a representative of Podonominae and as a sister group to Boreochlus Edwards, 1938. While the morphology of representatives of Libanochlites seems extremely close to those of Boreochlus, no unequivocal representative of Podonominae - extant or fossil - bears lyrate tibial spurs (Brundin, 1966; Cranston et al., 2012). Hence, the discovery of lyrate tibial spurs in L. necomicus prompted reinterpretation of Libanochlites as a representative of Tanypodinae (Azar et al., 2008). This was based on the presence of the pectinate tibial spurs in combination with the presence of Medial-cubital crossvein (MCu). However, Cranston et al. (2012) have made a compelling case for the group Libanochlites being an ingroup of the Boreochlini Brundin, 1966 (Podonominae), based on the observation of Bayesian tree increased support values after inclusion of L. neocomicus as a calibration node (Ashe et al., 2018).
It is possible, however, that pectinate tibial spurs were an ancestral character state within the group Tanypodoinae Sæther, 2000, including both Tanypodinae Kieffer, 1906 and Podonominae (Silva and Ekrem, 2016; Cranston, pers. comm 01.12.2018.). It is therefore possible that all modern representatives of Podonominae have lost the serration of the tibial spurs and developed smooth, simple spurs exhibited by most representatives of Podonominae in the extant fauna (Brundin, 1966). Therefore, until more work is done on the ancestral character state reconstruction, we opine that it is impossible to provide a reliable phylogenetic interpretation of the group Libanochlites within Chironomidae. It should therefore not be shoe-horned into any existing taxonomic unit (“sub-family”) nor should a new one be created for it.
The autapomorphy on which the group Tanypodinae is recognised is only observable in the females: (i) the females have gonotergite IX - conjunction of tergite IX and gonocoxite IX, forming a strap-like structure; (ii) the female gonotergite IX has very few or no setae (Silva and Ekrem, 2016). As the abdominal structures of the females of Libanochlites neocomicus are known insufficiently (Brundin, 1976; Veltz et al., 2007), we refrain from any further speculation on the phylogenetic position of Libanochlites.
For now, we can confidently say that Libanochlites is forming a separate monophyletic group, and its close resemblance to the modern representatives of Podonominae is possibly an example of convergence (Brundin, 1966, 1976; Saether, 2000). Libanochlites is furthermore characterized by numerous symplesiomorphies, also present in representatives of Podonominae, but also three strong autapomorphies - a dome-shaped clypeus and lyrate, translucent tibial spurs, with six denticles, and strong setae on the gonocoxite sitting on the row of the warts.
Aspects of the Lifestyle
Both species of Libanochlites possess an enlarged, dome-shaped clypeus. This is indicative for the presence of strong musculature of a cibarial pump (Figure 2.1, 2.2). The enlarged clypeus is possibly housing the three major muscles: musculus frontoepipharyngalis, musculus epistomalabralis and musculus clypeopalatalis. These are contracting the cibarial pump and food channel in the other haematophagous species of Diptera (Schneeberg and Beutel, 2011). Such a large clypeus as seen in the representatives of Libanochlites hints toward a powerful cibarial pump and hypopharynx, specialised for pumping liquids (most likely blood or nectar), since muscles contracting these structures are normally housed in the clypeus in blood feeding or nectar feeding Diptera (Azar et al., 2008; Schneeberg and Beutel, 2011; Azar and Nel, 2012). It is probable that at least females of Libanochlites were haematophagous for example on smaller avian dinosaurs, mammals, squamatan reptilians, or amphibians (Azar et al., 2008). Other cretaceous representatives of Chironomidae, which were demonstrably hematophages - such as Haematotanypus libanicus Azar et al., 2008, and Wadelius libanicus Veltz et al., 2007, also have an enlarged clypeus (especially so in H. libanicus) (Azar et al., 2008). Extant representatives of Chironomidae with functional mandibles (genera Afrochlus and Archaeochlus Brundin, 1966) also potentially might be haematophagous and having a large clypeus (Figure 2.3, 2.4), but their biology is not known sufficiently for further speculations (Cranston and Edward, 1998).
Unusual Sensilla
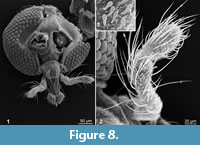 Libanochlites eocenicus sp.nov. bears, on its abdomen (Tergite IX), a number of mushroom-shaped sensilla; in L. neocomicus similar structures seem to be absent (Figure 6.1-6.3). The general shape of these sensilla is reminiscent of the clavate sensilla on the maxillary palps of the frog/biting midge Corethrella marksae (Figure 8.1-8.3). In contrast to the sensilla of C. marksae, however, the sensilla from the abdomen of the L. eocenicus sp.nov. has a pore opening on the top of the “mushroom” (Figure 6.1), which indicates that it might have an olfactory function or function as a hygroreceptor (Murphey et al., 1985). It is possible, however, that the similar shape of the sensilla in these two species indicates similar function. Since C. marksae is a haematophagous midges; it is possible that L. eocenicus sp.nov. also had a similar trophic specialization (Murphey et al., 1985).
Libanochlites eocenicus sp.nov. bears, on its abdomen (Tergite IX), a number of mushroom-shaped sensilla; in L. neocomicus similar structures seem to be absent (Figure 6.1-6.3). The general shape of these sensilla is reminiscent of the clavate sensilla on the maxillary palps of the frog/biting midge Corethrella marksae (Figure 8.1-8.3). In contrast to the sensilla of C. marksae, however, the sensilla from the abdomen of the L. eocenicus sp.nov. has a pore opening on the top of the “mushroom” (Figure 6.1), which indicates that it might have an olfactory function or function as a hygroreceptor (Murphey et al., 1985). It is possible, however, that the similar shape of the sensilla in these two species indicates similar function. Since C. marksae is a haematophagous midges; it is possible that L. eocenicus sp.nov. also had a similar trophic specialization (Murphey et al., 1985).
Morphotype Survival
While it might seem that attributing an Eocene species to a genus also known from the Cretaceous is a mere question of nomenclature conventions (“splitters versus lumpers”), it has, in fact, a deeper meaning concerning the persistence of a specific morphology. It means that the principal morphology of Libanochlites survived for 80 million years. It is especially astonishing concerning the morphotype survival and evolutionary stasis in representatives of winged insects with aquatic life phases (Kalugina, 1974a, 1974b; Brundin, 1976). Furthermore, this record is remarkable, because it is a first recorded instance of a specific Cretaceous morphotype of Chironomidae persisting into Cenozoic.
The presence of L. eocenicus sp. nov. in the Baltic amber is especially interesting in the context of the Cretaceous Terrestrial Revolution (CTR). This event evidently also had a profound effect on the diversification and extinction on pterygotan groups with aquatic life phases, including the group Chironomidae (Kalugina, 1974a, 1974b, 1976; Sinichenkova and Zherikhin, 1996; Baranov et al., 2015). The CTR seems to have been triggered by the Angiosperm revolution, a drastic rise in diversity, disparity, and species richness of the flowering plants that started in the mid-Cretaceous (Mckenna et al., 2015).
The CTR (Fastovsky et al., 2004; Weishampel et al., 2004) has manifested as a chain of fundamental changes in the matter and energy circulation in the terrestrial ecosystems (Condamine et al., 2016). These changes have resulted from the appearance of the periodic pulses of the organic matter, coming from the falling leaves of the deciduous angiosperms. This further influenced the plant-herbivore interactions, diversification of herbivores, pollinators and other organisms affected by the angiosperm revolution. Also it triggered the extinction of the animals dependant on the plant communities existing prior to the CTR (Kalugina, 1974; Sinichenkova and Zherikhin, 1996; Mckenna et al., 2015).
Freshwater ecosystems in particular were drastically transformed by the CTR due to the emergence of the organic matter pulses from deciduous plants (Kalugina, 1974a). The increased amount of particulate organic matter coming into the water bodies, in combination with the absence of developed detritus-based food webs, has led to the eutrophication of the continental freshwaters during the CTR (Kalugina, 1974a). This was in direct contrast to the mostly oligotrophic state of many continental water bodies earlier in the Mesozoic (Sinichenkova and Zherikhin, 1996). This eutrophication has led to the disappearance of oligotrophic species from lowland lakes and rivers, where an input of the external organic matter was at its highest (Kalugina, 1974a, 1974b; Sinichenkova and Zherikhin, 1996). Many species, which were common in the lowland lakes of the Jurassic and Early Cretaceous, such as stoneflies (Plecoptera) or certain midges (Podonominae), are today found only in oligotrophic boreal lakes or streams and rivers (Kalugina, 1974; Baranov et al., 2015). Evidently, these species utilised such types of oligotrophic habitats as refugia during the CTR (Kalugina, 1974; Sinichenkova and Zherikhin, 1996).
The survival of the morphology of Libanochlites into the Eocene is, therefore, surprising since the CTR appears to have been a major bottleneck for many different morphotypes within Chironomidae (Kalugina, 1974 a, 1974b; Kalugina and Kovalev, 1985). Since the morphotype of an organism is a reflection of its environmental conditions and selective pressures applied (Haug et al., 2012), it is safe to assume whatever environment L. neocomicus was specialised to, it closely matched the habitats occupied by the L. eocenicus sp. nov. Both L. neocomicus and L. eocenicus sp. nov. appear to have been haematophagous animals, with at least the females of the L. neocomicus possessing functional mandibles (Azar et al., 2008). It is impossible to tell what a potential host for the representatives Libanochlites was. Though, we assume that these hosts were small animals (birds, small squamates, mammals, or other insect species) not exterminated by the Cretaceous-Paleogene event, such as non-avian dinosaurs (Azar and Nel, 2012).
It is generally unusual to find the survival of a Mesozoic morphotype of Chironomidae over long stretches in deep time (Kalugina and Kovalev, 1985; Azar et al., 2008; Lukashevich and Przhiboro, 2011, 2012; Lukashevich, 2012). Most of the Mesozoic representatives of Chironomidae are so different from each other that the supra-specific groups are only known from their type deposit and occasionally some other deposits very close in time to the type strata (Kalugina and Kovalev, 1985; Azar et al., 2008; Lukashevich and Przhiboro, 2011, 2012; Lukashevich, 2012).
Two notable exceptions are the genera Cretaenne Azar et al., 2008 and Oryctochlus Kalugina, 1985. Cretaenne is currently known from representatives from the Jurassic of Mongolia and from the Early Cretaceous Lebanese amber (152.1-130.0 MYA) (Azar et al., 2008; Lukashevich and Przhiboro, 2012). Likewise, representatives of Jurochlus Kalugina, 1985 are known from various lacustrine deposits of Central Asia and Siberia in the range of 163.5-129.4 MYA, spanning from the Late Jurassic to Early Cretaceous (Kalugina and Kovalev, 1985; Azar et al., 2008; Lukashevich and Przhiboro, 2011, 2012; Lukashevich, 2012). Other groups such as Aenne Ansorge, 1999, and Oryctochlus are known from isolated wings as the oldest record; therefore, they do not represent reliable examples of morphotype longevity in Chironomidae (Kalugina and Kovalev, 1985; Ansorge, 1996, 1999).
Biogeographical Considerations
Lebanese amber originates from the Early Cretaceous (130 MYA) coastal plains conifer forest which in the northern-eastern part of Gondwana (Poinar and Milki, 2001; Maksoud et al., 2017). So far, only a small number of the insect species from Lebanese amber were similar enough to specimens from Baltic amber to be attributed to the same genus (Poinar and Milki, 2001; Azar et al., 2010). In particular, biting midges attributed to Leptoconops and frog-biting midges attributed to Corethrella are known from both deposits (Poinar and Milki, 2001). The record of the Libanochlites from the Baltic amber indicates a further biogeographical connection between northern Gondwana and the Cretaceous Island chain which becomes Europe later in Cenozoic. Alternatively, a wider distribution of representatives of Libanochlites prior to Gondwana breakup (Azar et al., 2008) could also explain the observed distribution pattern. The first hypothesis is supported by the fact that other representatives of Chironomidae show a comparable pattern: representatives attributed to Libanodiamesa Veltz, Azar, and Nel, 2007, were found in Lebanese Cretaceous amber and the slightly younger, early Barremian amber from the Isle of Wight in the UK (Baranov et al., 2019). In fact, all cases of genera, whose distribution overlaps between Lebanese and Baltic amber are culicomorphan dipterans.
Another important biogeographic consideration pertaining to this case is the relative latitudinal position of two amber deposits. Lebanese amber paleolatitude, as was mentioned above is 3°45’ N (Azar et al., 2010), placing it next to the equator. On the other hand, paleolatitude of the Baltic amber forest was about 48°33' (Seredszus and Wichard, 2007), placing it on the shores of the North Atlantic during the Eocene. While the climate of the mid-Eocene was much milder than the climate of modern Northern Europe, it was still colder than that of the peri equatorial areas in Early Cretaceous (Poinar and Milki, 2001). The large difference in the latitude can explain the difference in the sizes of the L. neocomicus and L. eocenicus sp. nov., as congeneric representatives of chironomids are in fact known to increase in size polewards (Prat, 1985; Baranov et al., 2015). Since L. eocenicus sp. nov. lived in a much colder climate than L. neocomicus, it is not surprising that the former was substantially larger. Such a tendency was explained by the increase of biomechanic efficiency in larger flying insects in the colder climate (Baranov et al., 2015).
CONCLUSIONS
The discovery of Libanochlites eocenicus sp. nov. in Eocene Baltic amber is the first instance of a Mesozoic morphotype of Chironomidae surviving into Cenozoic. Lack of similar examples of morphological stasis in Chironomidae could be explained by the impact of the CTR on the freshwater ecosystems. After the Cretaceous-Paleogene extinction event, Chironomidae is mostly represented by forms attributed to genera with modern representatives such as Nandeva, Heterotrissocladius, Smittia, Paraphaenocladius, and others. Representatives of all of these are known from the early Eocene onwards (Wichard and Seredszus, 2007; Baranov et al., 2015; Gilka et al., 2016).
Such stability of the morphotypes in Chironomidae after the Cretaceous-Paleogene extinction event indicates that very little changes occurred to the habitats of this group in the Cenozoic. It is, however, possible, that more Cretaceous morphotypes of Chironomidae will be discovered. It would not be surprising if they survived even up to the present day in the ecosystems with stable conditions, such as streams in the subtropical evergreen forest of the Mediterranean type (Wichard et al., 2009).
ACKNOWLEDGEMENTS
VB and JTH are kindly funded by the Volkswagen Foundation (Lichtenberg professorship to JTH). VB is grateful to M. Spies (ZSM Munich) for his invaluable help with collection of ZSM as well as help with the literature. VB is grateful to M. Yavorskaya for her help with English grammar. Authors are grateful to P. Cranston (University of Canberra) for the valuble comments on the manuscript and to D. Azar (Lebanese University, Beirut) for providing the comparative material. We are grateful to the handling editor - M. Hyzny, managing editor - J. Pattison Rumford and and three anonymous reviewers for their efforts in improving this manuscript. Thanks to all people providing free software.
REFERENCES
Ansorge, J. 1996. Insekten aus dem Oberen Lias von Grimmen (Vorpommern, Norddeutschland). Neue paläontologische Abhandlungen, 2:1-132.
Ansorge, J. 1999. Aenne liasina gen. et sp. n. - the most primitive non biting midge (Diptera: Chironomidae: Aenneinae subfam. n. - from the Lower Jurassic of Germany. Polish Journal of Entomology, 68:431-443.
Ashe, P., Murray, D.A., O’Connor, J.P. 2018. Recognition of two additional fossil subfamilies in the Chironomidae (Diptera) - †Dungeyellinae subfam. nov. and †Cretodiamesinae Kalugina stat. nov. Bulletin of the Irish Biogeographical Society, 42:225-242.
Azar, D., Gèze, R., and Acra, F. 2010. Lebanese amber, p. 271-298. In Penney, D. (ed.), Biodiversity of Fossils in Amber from the Major World Deposits. Siri Scientific Press, Rochdale.
Azar, D. and Nel, A. 2012. Evolution of hematophagy in ‘non-biting midges’ (Diptera: Chironomidae). Terrestrial Arthropod Reviews, 5:15-34. https://doi.org/10.1163/187498312x620577
Azar, D., Veltz, I., and Nel, A. 2008. Mandibulate chironomids: primitive or derived? (Diptera: Chironomidae). Systematic Entomology, 33:688-699. https://doi.org/10.1111/j.1365-3113.2008.00438.x
Baranov, V., Andersen, T., and Perkovsky, E.E. 2015. Orthoclads from Eocene amber from Sakhalin (Diptera: Chironomidae, Orthocladiinae). Insect Systematic and Evolution, 46(4):359-378. https://doi.org/10.31233/osf.io/qv36y
Baranov,V., Giłka, V., Zakrzewska, M., and Jarzembowski, E. 2019. New non-biting midges (Diptera: Chironomidae) from lower Cretaceous Wealden amber of the Isle of Wight (UK). Cretaceous Research, 95:138-145. https://doi.org/10.1016/j.cretres.2018.11.012
Banks, N. 1907. Descriptions of new Trichoptera. Proceedings of the Entomological Society of Washington, 8:117-33
Boesel, M.W. 1937. Chironomidae, p. 52-55. In Carpenter, F.M., Folsom, J.W., Essig, E.O., Kinsey, A.C., Brues, C.T., Boesel, M.W., and Ewing, H.E. (eds.), Insects and Arachnids from Canadian Amber. The University of Toronto Press, Toronto.
Brundin, L. 1966. Transantarctic Relationships and Their Significance, as Evidenced by Chironomid Midges. With a Monograph of the Subfamilies Podonominae and Aphroteniinae and the Austral Heptagyiae. Kunglinga Svenska Vetenskapsakad. Handl., Fjärde.
Brundin, L. 1972. Phylogenetics and biogeography, a reply to Darlington's “practical criticism” of Hennig-Brundin. Systematic Zoology, 21:69-79.
Brundin, L. 1976. A Neocomian chironomid and Podonominae-Aphroteniinae (Diptera) in the light of phylogenetics and biogeography. Zoologica Scripta, 5:139-160. https://doi.org/10.1111/j.1463-6409.1976.tb00691.x
Clarke, D. J. and Chatzimanolis, S. 2009. Antiquity and long-term morphological stasis in a group of rove beetles (Coleoptera: Staphylinidae): Description of the oldest Octavius species from Cretaceous Burmese amber and a review of the “Euaesthetine subgroup” fossil record. Cretaceous Research, 30:1426-1434. https://doi.org/10.1016/j.cretres.2009.09.002
Colless, D.H. 1986. The Australian Chaoboridae (Diptera). Australian Journal of Zoology: Supplementary Series, 124:1-66.
Condamine, F.L., Clapham, M.E., and Kergoat, G.J. 2016. Global patterns of insect diversification: towards a reconciliation of fossil and molecular evidence? Scientific Reports, 6:19208. https://doi.org/10.1038/srep19208
Coquillett, D.W. 1902. New forms of Culicidae from North America. Journal of the New York Entomological Society, 10:191-194.
Cranston, P.S. and Edward, D.H.D. 1998. Afrochlus Freeman: an African gondwanan midge and the phylogeny of the Podonominae (Diptera: Chironomidae). Systematic Entomology, 23:77-90. https://doi.org/10.1046/j.1365-3113.1998.00045.x
Cranston, P.S., Hardy, N.B., and Morse, G.E. 2012. A dated molecular phylogeny for the Chironomidae (Diptera). Systematic Entomology, 37:172-188.
Doitteau, G. and Nel, A. 2007. Chironomid midges from early Eocene amber of France (Diptera: Chironomidae). Zootaxa, 1404:1-66.
Edwards, F.W. 1938. A new genus of the subfamily Podonominae. p. 152-154. In Edwards, F.W. and Thienemann, A. (eds.), Neuer Beitrag zur Kenntnis der Podonominae (Dipt. Chironomidae). Chironomiden aus Lappland IV. Zoologischer Anzeiger 122(5/6).
Fastovsky, D.E., Huang, Y., Hsu, J., Martin-McNaughton, J., Sheehan, P.M., and Weishampel, D.B. 2004. Shape of Mesozoic dinosaur richness. Geology, 32:877-880. https://doi.org/10.1130/G20695.1
Fittkau, E.J. 1955. Buchonomyia thienemanni n. gen. n. sp. Chironomidenstudien IV (Diptera: Chironomidae). Beiträge zur Entomologie, 5:403-414.
Freeman, P. 1964. Notes on Chironomidae (Diptera: Nematocera). Proceedings of the Entomological Society of London (B), 33(9-10):147-150.
Gilka, W., Zakrzewska, M., Baranov, V., Wang, B., and Stebner, F. 2016. The first fossil record of Nandeva Wiedenbrug, Reiss and Fittkau (Diptera: Chironomidae) in early Eocene Fushun amber from China. Alcheringa: Australasian Journal of Palaeontology, 40:390-397. https://doi.org/10.1080/03115518.2016.1145529
Goetghebuer, M. and Lenz, F. 1943. Subfamilier Orthocladiinae. p. 65-112. In Linder, E. (ed.), Die Fliegen der Palearktische region 13. Schweizerbart, Stuttgart.
Gründ, M. 2006. Chironomidae (Diptera) in Dominican amber as indicators for ecosystem stability in the Caribbean. Palaeogeography, Palaeoclimatology, Palaeoecology, 241(3-4):410-416. https://doi.org/10.1016/j.palaeo.2006.04.005
Haug, C., Herrera Florez, A.F., Müller, P., and Haug, J. 2019. Cretaceous chimera - an unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution; Palaeodiversity, 12:1-11. https://doi.org/10.18476/pale.v12.a1
Haug, C., Nyborg, T., and Vega, F.J. 2013. An exceptionally preserved upogebiid (Decapoda: Reptantia) from the Eocene of California. Bolétin de la Sociedad Geológica Mexicana, 65:235-248.
Haug, C. and Rötzer, M.A. 2018. The ontogeny of Limulus polyphemus (Xiphosura s. str., Euchelicerata) revised: looking “under the skin”. Development Genes and Evolution, 228:49-61. https://doi.org/10.1007/s00427-018-0603-1
Haug, J.T., Haug, C., and Ehrlich, M. 2008. First fossil stomatopod larva (Arthropoda: Crustacea) and a new way of documenting Solnhofen fossils (Upper Jurassic, Southern Germany). Palaeodiversity, 1:103-109.
Haug, J.T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E.N.K., and Waloszek, D. 2011. Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy, 244:259-272. https://doi.org/10.1111/j.1365-2818.2011.03534.x
Haug, J.T., Mayer, G., Haug, C., and Briggs, D.E. 2012. A Carboniferous non-onychophoran lobopodian reveals long-term survival of a Cambrian morphotype. Current Biology, 22:1673-1675. https://doi.org/10.1016/j.cub.2012.06.066
Haug, J.T., Müller, P., and Haug, C. 2018. The ride of the parasite: A 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters, 4(31):1-8. https://doi.org/10.1186/s40851-018-0116-9
Haug, J.T., Müller, C.H.G., and Sombke, A. 2013. A centipede nymph in Baltic amber and a new approach to document amber fossils. Organisms Diversity and Evolution, 13:425-432. https://doi.org/10.1007/s13127-013-0129-3
Hoernschemeyer, T., Wedmann, S., and Poinar, G. 2010. How long can insect species exist? Evidence from extant and fossil Micromalthus beetles (Insecta: Coleoptera). Zoological Journal of the Linnean Society, 158:300-311. https://doi.org/10.1111/j.1096-3642.2009.00549.x
Hoffeins, H.W. 2001. On the preparation and conservation of amber inclusions in artificial resin. Polskie Pismo Entomologiczne, 70:215-219.
Holmgren, A.E. 1869. Bidrag til Kännedomen om Beeren Eilands och Spetsbergens Insekt-Fauna. (Contribution to the knowledge of Bear Island and Spitsbergen insect fauna.) Kunglinga Svenska Vetenskapsakademiens Handlingar, 8:1-56.
Kalugina, N.S. 1974a. Eutrophication as one possible cause for the changes in aquatic biocenoses towards the end of the Mesozoic. p. 137-139. In Antropogennoe evtrofirovanie vod [Anthropogenically Eutrophied Waters]. Abstracts of the 1 st USSR-Wide Symposium on Antgopogenic eutrophication of the Water Bodies, Borok/Chernogolovka, 16-20th September, 1974. Akademia Nauk SSSR, Chernogolov.
Kalugina, N.S. 1974b. Change in the subfamily composition of chironomids (Diptera, Chironomidae) as an indicator of possible eutrophication of bodies of water during the late Mesozoic. Journal of the Moscow Naturalist’s Society, 79:45-56.
Kalugina, N.S. and Kovalev, V.G. 1985. Jurassic Diptera of Siberia. Izdatelstvo Nauka, Moscow.
Kieffer, J.J. 1906. Diptera Fam. Chironomidae. p. 1-78. In Wytsman, P. (ed.), Genera Insectorum. Imprimeurs-Editeurs, Brussels.
Lancaster, J. and Downes, B.J. 2013. Aquatic Entomology. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199573219.001.0001
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundem classes, ordines, genera, species cum characteribus, differentis, synonymis, locis. Tomus I. Editio decima, reformata. Laurentii Salvii, Holmiae, 823 pp.
Lohrmann, V. and Engel, M.S. 2017. The wasp larva's last supper: 100 million years of evolutionary stasis in the larval development of rhopalosomatid wasps (Hymenoptera: Rhopalosomatidae). Fossil Record, 20:239-244. https://doi.org/10.5194/fr-20-239-2017
Lukashevich, E.D. and Przhiboro, A.A. 2011. New Chironomidae (Diptera) with elongate proboscises from the Late Jurassic of Mongolia. ZooKeys, 130307-322. https://doi.org/10.3897/zookeys.130.1555
Lukashevich, E.D. 2012. Pupae of Mesozoic Oryctochlus Kalugina, 1985 (Chironomidae: Podonominae), with description of two new species. In Ekrem, T., Stur, E., and Aagaard, K. (eds.), Proceedings of the 18th International Symposium on Chironomidae. Fauna Norvegica, 31:159-165. https://doi.org/10.5324/fn.v31i0.1400
Lukashevich, E.D. and Przhiboro, A.A. 2012. Pupae of Mesozoic Jurochlus Kalugina, 1985 (Diptera: Chironomidae), with description of four new species. Zootaxa, 3478:434-452.
Maksoud, S., Azar, D., Granier, B., and Gèze, R. 2017. New data on the age of the Lower Cretaceous amber outcrops of Lebanon. Palaeoworld, 26:331-338. https://doi.org/10.1016/j.palwor.2016.03.003
Martin, J., Guryev, V., Macdonald, S.S., Blinov, A., and Edward, D.H.D. 2003. Phylogenetic relationships of Archaeochlus Brundin, Austrochlus Cranston and Afrochlus Freeman (Diptera: Chironomidae), basal genera with a Gondwanan connection. Cimbebasia, 19:193-203.
Mckenna, D.D., Wild, A.L., Kanda, K., Bellamy, C.L., Beutel, R.G., Caterino, M.S., Farnum, C.W., Hawks, D.C., Ivie, M.A., Jameson, M.L., Leschen, L.A.B., Marvaldi, A.E., McHugh, J.V., Newton, A.F., Robertson, J.A., Thayer, M.K., Whiting, M.F., Lawrance, J.F., Slipinski, A., Maddison, D.R., and Farrel, B.D. 2015. The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Systematic Entomology, 40:835-880. https://doi.org/10.1111/syen.12132
McLachlan, R. 1864. On the trichopterous genus Polycentropus, and the allied genera. Entomologist’s Monthly Magazine, 1:25-31.
Murphey, R.K., Bacon, J.P., and Johnson, S.E. 1985. Ectopic neurons and the organization of insect sensory systems. Journal of Comparative Physiology A, 156:381-389. https://doi.org/10.1007/bf00610730
Newman, E. 1834. Attempted division of British insects into natural order. Entomological Magazine, 2:379-431.
Pape, T., Blagoderov, V., and Mostovski, M.B. 2011. Order Diptera Linnaeus, 1758. In Zhang, Z.-Q. (ed.), Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. Zootaxa, 3148(237):222-229. https://doi.org/10.11646/zootaxa.3148.1.42
Peris, D. and Háva, J. 2016. New species from Late Cretaceous New Jersey amber and stasis in subfamily Attageninae (Insecta: Coleoptera: Dermestidae). Journal of Paleontology, 90:491-498. https://doi.org/10.1017/jpa.2016.51
Poinar, G.O. and Milki, R. 2001. Lebanese Amber. Oregon State University Press, Corvallis.
Prat, N. 1985. Variabilidad morfológica de las poblaciones de Cladotanytarsus mancus (Walker, 1856) de los embalses Es-pañoles (Diptera: Chironomidae). Graellsia, 41:65-88.
Sæther, O.A. 1977. Female genitalia in Chironomidae and other Nematocera: Morphology, phylogenies, keys. Bulletin of the Fisheries Research Board of Canada, 197:1-209.
Sæther, O.A. 2000. Phylogeny of the subfamilies of Chironomidae (Diptera). Systematic Entomology, 25:393-403. https://doi.org/10.1046/j.1365-3113.2000.00111.x
Sæther, O.A. and Andersen, T. 1996. First Afrotropical records of Doithrix and Georthocladius, with notes on the Pseudorthocladius group (Diptera: Chironomidae), Tijdschrift voor Entomologie, 139:243-256.
Sánchez-García, A. and Engel, M.S. 2017. Long-term stasis in a diverse fauna of Early Cretaceous springtails (Collembola: Symphypleona). Journal of Systematic Palaeontology, 15:513-537. https://doi.org/10.1080/14772019.2016.1194575
Schlee, D. 1975. Das Problem der Podonominae-Monophylie; Fossiliendiagnose und Chironomidae-Phylogenetik (Diptera). Entomologica Germanica, 1:316-351.
Schneeberg, K. and Beutel, R.G. 2011. The adult head structures of Tipulomorpha (Diptera, Insecta) and their phylogenetic implications. Acta Zoologica, 92:316-343. https://doi.org/10.1111/j.1463-6395.2010.00463.x
Seredszus, F. and Wichard, W. 2007. Fossil chironomids (Insecta, Diptera) in Baltic amber. Palaeontographica, Abteilung A: Paläozoolie und Stratigraphie, 279:49-91. https://doi.org/10.1127/pala/279/2007/49
Seredszus, F. and Wichard, W. 2002. Buchonomyiinae (Diptera, Chironomidae) im Baltischen Bernstein. [Buchonomyiinae (Diptera, Chironomidae) in Baltic amber]. Studia Dipterologica, 9:393-402.
Seredszus, F. and Wichard, W. 2011. Overview and description of fossil non-biting midges in Baltic amber (Diptera: Chironomidae). Studia Dipterologica, 17:121-129.
Silva, F.L. and Ekrem, T. 2016. Phylogenetic relationships of nonbiting midges in the subfamily Tanypodinae (Diptera: Chironomidae) inferred from morphology. Systematic Entomology, 41:73-92. https://doi.org/10.1111/syen.12141
Simpson, G.G. 1944. Tempo and Mode in Evolution. Columbia University Press, New York.
Sinichenkova, N.D. and Zherikhin, V.V. 1996. Mesozoic lacustrine biota: extinction and persistence of the communities. Paleontological Journal, 30:710-715.
Skuse, F.A.A. 1889. Diptera of Australia Part XI -The Chironomidae. Proceedings of the Royal Zoological Society of New South Wales, 4:215-311.
Szadziewski, R. 1995. The oldest fossil Corethrellidae (Diptera) from Lower Cretaceous Lebanese amber. Acta Zoologica Cracoviensia, 38:177-181.
Szadziewski, R. 2008. Age and recent distribution of extant genera of Ceratopogonidae (Diptera) present in the fossil record. Alavesi a, 2(8):87-99.
Thienemann, A. 1937. Podonominae, eine neue Unterfamilie der Chironomiden. (Chironomiden aus Lappland I). Mit einem Beitrag: F.W. Edwards: On the European Podonominae (Adult Stage). Internationale Revue der Gesamten Hydrobiologie und Hydrographie, 35:65-112
Veltz, I., Azar, D., and Nel, A. 2007. New chironomid flies in Early Cretaceous Lebanese amber (Diptera: Chironomidae). African Invertebrates, 48:169-191.
Wagner, R., Marxsen, J., Zwick, P., and Cox, E.J. (eds.). 2011. Central European Stream Ecosystems: The Long Term Study of the Breitenbach. John Wiley & Sons, Weinheim.
Weishampel, D.B., Dodson, P., and Osmólska, H. 2004. The Dinosauria, 2nd ed. University of California Press, Berkeley.
Wichard, W. and Azar, D. 2018. First caddisflies (Trichoptera) in Lower Cretaceous Lebanese amber. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 107:213-217. https://doi.org/10.1017/S1755691017000354
Wichard, W., Gröhn, C., and Seredszus, F. 2009. Aquatic Insects in Baltic Amber - Wasserinsekten im Baltischen Bernstein. Verlag Kessel, Remagen-Oberwinter. [In English and German]
Wirth, W.W. and Lee, D.J. 1958. Australasian Ceratopogonidae (Diptera, Nematocera). Part. 8: A new genus from Western Australian attacking man. Proceedings of the Royal Zoological Society of New South Wales, 83:337-339.
Wirth, W.W. and Sublette, J.E. 1970. A review of the Podonominae of North America with descriptions of three new species of Trichotanypus (Diptera: Chironomidae). Journal of the Kansas Entomological Society, 43:335-354
Wulp, F.M. van der. 1874. Dipterologische aanteekeningen. N°. 4. [Dipterological notes. No. 4.]. Tijdschrift voor entomologie, 17(3):109-112, (4):113-148, pl. 8.
Yamamoto, S., Takahashi, Y., and Parker, J. 2017. Evolutionary stasis in enigmatic jacobsoniid beetles. Gondwana Research, 45:275-281. https://doi.org/10.1016/j.gr.2016.12.008

