 Diversification events of the shield morphology in shore crabs and their relatives through development and time
Diversification events of the shield morphology in shore crabs and their relatives through development and time
Article number: 26.3.a53
https://doi.org/10.26879/1305
Copyright Paleontological Society, December 2023
Proceedings of the 8th Symposium on Fossil Decapod Crustaceans
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 11 May 2023. Acceptance: 14 November 2023.
ABSTRACT
The group of shore crabs and their relatives, Carcinidae, are first known from the Eocene (56–33.9 mya). Since the group’s first appearance, its representatives have evolved a variety of lifestyles and morphologies. The exact evolutionary steps resulting in these morphologies are still a matter of debate. Here, we analyse the changes of morphology within Carcinidae over time. We also investigate the change of morphology through ontogeny to see how it relates to the evolutionary changes. We focus on the outline of the shield as a proxy for morphology, as it has a strong effect on the appearance of the crab and yields the largest sample size of all body parts. Using an elliptic Fourier transformation of the shield outline and a principal component analysis, we create a morphospace for the shields of Carcinidae. Using the morphospace as input data and a new phylogenetic tree based on a molecular and a morphological character matrix from literature, we reconstruct ancestral states for the shield. Combining data of fossil and extant specimens and reconstructed ancestral states, we analyse changes of the shield shape through time. From the morphospace, we find that the morphological diversity of the shield is strongly influenced by ontogeny, but not so much by ecology. Yet, the shields of the adults show a large diversity, corresponding to adaptations to different lifestyles. The reconstruction of ancestral states showed that the earliest representatives of Carcinidae had quasi-hexagonal shields, which could correspond to an epibenthic lifestyle. From these forms, different shapes evolved, significantly during the Oligocene (ca. 33 mya) and Pliocene (ca. 5 mya).
Florian Braig. Faculty of Biology, LMU Munich, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany. braig@bio.lmu.de (Corresponding author)
Orcid: https://orcid.org/0000-0003-0640-6012
Carolin Haug. Faculty of Biology, LMU Munich, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany. carolin.haug@palaeo-evo-devo.info
Orcid: https://orcid.org/0000-0001-9208-4229
Joachim T. Haug. Faculty of Biology, LMU Munich, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany and GeoBio-Center of the LMU Munich, Richard-Wagner-Str. 10, 80333 Munich, Germany. joachim.haug@palaeo-evo-devo.info
Orcid: https://orcid.org/0000-0001-8254-8472
Key words: Ancestral state reconstruction; Brachyura; Carcinidae; elliptic Fourier transformation; geometric morphometrics; quantitative morphology
Final citation: Braig, Florian, Haug, Carolin, and Haug, Joachim T. 2023. Diversification events of the shield morphology in shore crabs and their relatives through development and time. Palaeontologia Electronica, 26(3):a53.
https://doi.org/10.26879/1305
palaeo-electronica.org/content/2023/5037-carcinidae-diversity
Copyright: December 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Carcinization describes an evolutionary process in crustaceans, by which representatives of different lineages of crustaceans evolve into a crab-like appearance (e.g., Borradaile, 1916; McLaughlin and Lemaitre, 1997; Keiler et al., 2017; Wolfe et al., 2021), with a rather broad body and the “shrimp tail” (pleon) being folded under the body (for more aspects of the process see Scholtz, 2014; Keiler et al., 2017). This crab morphology seems of evolutionary and ecological significance as crab-like animals are quite successful concerning number of species and range of conquered habitats.
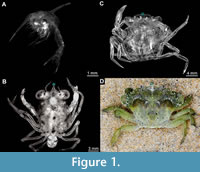 The changes towards a crab-like body shape are mostly restricted to the juvenile and adult phases of development (Martin, 2014), while the larvae (see Haug, 2020a for ambiguities of the term) retain a more ancestral morphology (Haug, 2020b). Representatives of Brachyura (i.e., true crabs) usually first go through a planktic larval phase (zoea) after hatching, characterised by locomotory exopods on the thoracic appendages (Williamson, 1969). Zoea larvae are still far from crab-looking (Figure 1A). Their bodies are more laterally compressed with spiny shields and a pleon not yet folded underneath the body. The zoea phase is then followed by a transition phase (often a single stage), the megalopa larva, characterised as the first stage with functional pleopods (Williamson, 1969). The megalopa already appears more crab-like with the typical dorso-ventrally compressed body and wider shield (Figure 1B).
The changes towards a crab-like body shape are mostly restricted to the juvenile and adult phases of development (Martin, 2014), while the larvae (see Haug, 2020a for ambiguities of the term) retain a more ancestral morphology (Haug, 2020b). Representatives of Brachyura (i.e., true crabs) usually first go through a planktic larval phase (zoea) after hatching, characterised by locomotory exopods on the thoracic appendages (Williamson, 1969). Zoea larvae are still far from crab-looking (Figure 1A). Their bodies are more laterally compressed with spiny shields and a pleon not yet folded underneath the body. The zoea phase is then followed by a transition phase (often a single stage), the megalopa larva, characterised as the first stage with functional pleopods (Williamson, 1969). The megalopa already appears more crab-like with the typical dorso-ventrally compressed body and wider shield (Figure 1B).
This pattern of development is, for example, well represented in species of Carcinidae sensu Spiridonov, 2020, the group of shore crabs. This ingroup of Portunoidea (relatives of swimming crabs; for competing taxonomic views, see Evans, 2018) has currently 48 formally described extant, exclusively marine species (Evans, 2018). Representatives of Carcinidae show a global distribution, not least due to the European shore crab Carcinus maenas (Linnaeus, 1758), which is a globally invasive species (e.g., Roman and Palumbi, 2004; Young and Elliott, 2020). The group also shows a variety of adaptations to different lifestyles, such as burrowing or swimming (Spiridonov, 2020). The earliest fossil representatives of Carcinidae are known from the Eocene (56-39 mya; e.g., Rathbun, 1926).
Here, we analyse the changes in shield morphology in Carcinidae through time and ontogeny with the use of quantitative morphology. The shield is among the characters that is most often preserved in fossil representatives of Brachyura. It also dominates the general appearance of the body and provides adaptations to different lifestyles (Spiridonov, 2020). We investigate how the typical crab-like shield diversified in the group since their appearance in the early Eocene and what it may have looked like in the earliest representatives. We also analyse how the shield changes throughout ontogeny. We hypothesize that adult ecology is the major driver behind shield diversity and that it increased in the group over time. Furthermore, megalopa shield diversity is expected to be lower than adult shield diversity, as megalopae represent a transition phase, which likely possesses comparable constraints for all representatives of the group.
MATERIAL AND METHODS
Material
Material used for this study originated from published images and reconstruction drawings in literature (Appendix 1). Furthermore open databases (GBIF.org, 2022; WoRMS Editorial Board, 2022), collections and museums (Deutsches Zentrum für Marine Biodiversitätsforschung, DZMB; Florida Museum of Natural History, FM; Göteborg Natural History Museum, GNM; Instituto Español de Oceanografía Centro Oceanográfico de Cádiz, IEO; Institut de Ciències del Mar, ICM-CSIC; Muséum national d’Histoire naturelle Paris, MNHN; Natural History Museum of Denmark, NHMD; Senckenberg am Meer, SBaM; University Museum of Bergen, UiB) and a developmental series of C. maenas provided by the Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, Helgoland. Additional images were provided by Oliver Mengedoht/Panzerwelten.de, Recklinghausen. For a detailed overview on data origin, see Appendix 2. From these sources, the outline of the shield in dorsal view was reconstructed for each specimen (as in earlier studies; Braig et al., 2023).
In total, 127 adults, 16 juveniles (i.e., crab 1 and crab 2 stages), and 28 megalopae were reconstructed from extant material. We also reconstructed 19 fossil representatives, all of which were originally identified as adults (Table 1). We did not include zoea larvae in the ontogenetic comparison for two reasons: 1) Zoea larvae appear drastically different in their shield morphology from megalopae, juveniles, and adults (e.g., Spitzner et al., 2018); 2) In the literature, zoea larvae of Carcinidae are often only depicted laterally, rendering a comparison with the dorsal depictions of megalopa and adult shields impossible (e.g., Paula, 1988).
We used vector graphic software, Adobe Illustrator CS2 and the free and open software InkScape, for the reconstruction of shield outlines. To prevent variation in the data due to left-right asymmetry, we only reconstructed the left or the right half of the shield and then duplicated and mirrored it in anterior-posterior axis and stitched it together to form an entire symmetric shield (Haug et al., 2020; Braig et al., 2023). Fluctuating asymmetry has been found in Carcinidae (Spani and Scalici, 2018), but due to its random nature we disregarded it to reduce noise in the data. Furthermore, we used material from the Zoological State Collection Munich (ZSM) to investigate phylogenetic character states (referenced in Appendix 3).
Geometric Morphometrics
Quantitative comparison of shield outlines was achieved by outline analysis, creating a morphospace. We used elliptic Fourier analysis (EFA) of outlines to decompose the two-dimensional shield outline into a mathematical object. The shape is hereby decomposed into a harmonic sum of trigonometric functions, weighted with harmonic coefficients describing the shape (Kuhl and Giardina, 1982; Bonhomme et al., 2014). The harmonic coefficients are aligned by the tip of the rostrum i.e., median point in the anterior rim of the shield (Figure 1; blue mark). The results of the outline analysis were then analysed with a principal component analysis (PCA) to extract the dimensions of largest variation (Bonhomme et al., 2014; Braig et al., 2019, 2023). The outline analysis was conducted with the R-statistics environment (ver. 4.1.0; R Core Team, 2021) using the package Momocs (ver. 1.3.2; Bonhomme et al., 2014). Shapes were registered with 2001 +/- 540 coordinates per shape. We used calibration functions of the Momocs package to calculate the optimal number of harmonics to use, resulting in 24 harmonics for the elliptic Fourier analysis. In this way, we created a morphospace of all specimens available to us.
Estimation of Ancestral States
To reconstruct the ancestral state (ASR) of the shield morphology in Carcinidae, we first had to obtain a phylogenetic tree for the group on which to base our analysis (Litsios and Salamin, 2012). We chose a total evidence approach based on two-character matrices, a morphological matrix from Karasawa et al. (2008) and a molecular matrix from Evans (2018; “163_taxa_concatenated_alignment”). Some species included in the molecular character matrix were not included in the morphological character matrix. For these specimens, we scored character states by investigating preserved material under dissection microscopes (updated character matrix in Appendix 3; character list and their states in Appendix 4).
We combined the two-character matrices in Mesquite (ver. 3.7; Maddison and Maddison, 2021), creating a matrix with one morphological and four molecular partitions (16s rRNA, CO1, H3, 28s rRNA), all characters being unweighted. Extant specimens had values for all partitions, fossil specimens had values only for the morphological partition. We then conducted a phylogenetic reconstruction in MrBayes (ver. 3.2.7a; Ronquist et al., 2012), following Aria and Caron (2017). We used a Bayesian MCMC method to create the tree (5,000,000 generations, four parallel chains, tree sample every 10,000 generations, 20% burn-in) with Carpilius convexus (Forskål, 1775) as outgroup. To account for the five fossil specimens in the tree not having molecular values we applied backbone constraint on the ingroups, following Aria and Caron (2017). We dated the tree with five fossil specimens (one representative each of Portumnus tricarinatus Lörenthey and Beurlen, 1929, Bathynectes muelleri Ossó and Stalennuy 2011, Megokkos alaskensis Rathbun, 1926, Megokkos sp., and Liocarcinus heintzi Schweitzer and Feldmann, 2010), following Zhang et al. (2016). These fossil specimens were included as individuals in the tree and assigned time ranges in the phylogenetic analysis, according to their occurrence data (Appendix 5). Clock rates were set to 0.0115 for mtDNA partitions and 0.00115 for nuclear DNA partitions as lognormal priors, following Liu et al. (2018). The code used is provided in Appendix 6 and the tree file in Appendix 7. We imported the resulting tree into the R-statistics environment, using phytools (ver. 0.7-90; Revell, 2012).
Although we created our own phylogenetic tree, we still could not include every species of our initial data set into said tree due to missing molecular data or inaccessibility of morphological characters for some specimens. To further progress with our analysis, we therefore had to create a subset of specimens, selecting one randomly chosen adult specimen for each species represented in our tree. This subset of our initial analysis, henceforth called “tree subset”, is representing the tip states for the ancestral state reconstruction. The tip states are a list of continuous characters for each tip in the tree, from which character states are then inferred for each node in the tree (Litsios and Salamin, 2012). To obtain a continuous character matrix for the tip states, we had to perform a second outline analysis (again 24 harmonics), repeating the methodology as for the complete data set (as detailed in the section “Geometric morphometrics”), but only including the specimens of the “tree subset”. This step was necessary because, as elliptic Fourier outline analysis has different results dependent on which specimens are included, a new outline analysis with only the specimens included in the tree contains less noise and variation. Using the continuous character matrix obtained from the PCA of this “tree subset” as tip data, we then computed the estimates for ancestral states of the shield morphology for every node in the tree. We hereby selected the first 10 principal components (PCs) of the continuous character matrix from the PCA as they represented over 95% of variation in the data, to reduce computational time and dimensionality. For these 10 dimensions, we computed the estimated values for every node in our tree. To do so, we fitted different models of character evolution to the data (i.e., matrix of first ten PCs from PCA of “tree subset” and the phylogenetic tree) using the mvMORPH package (ver. 1.1.4; Clavel et al., 2015). We fitted univariate and multivariate “Brownian-motion” and “Ornstein-Uhlenbeck” models of character evolution to this data. We then compared Akaike information criterion values of these models, tested for significant differences between models, and tested if reliable solutions could be reached by each model. Based on these tests, we decided on a univariate Brownian-motion model.
The mathematical estimates for each node in the tree from the ASR-model were then used to reconstruct shield outlines within the morphospace provided by the PCA on the shield outline analysis of the “tree subset”. However, this could only be performed for two PCs at the same time, meaning that only the morphological variation explained by two PCs can be expressed graphically with this method. Therefore, the graphical representation (and only the graphical representation) comes with some uncertainty. The code used is provided in Appendix 8.
Statistical Analysis
Statistical analysis was conducted in the R-statistics environment, using the package dispRity (ver. 1.6.0; Guillerme, 2018).
For a quantitative comparison of groups, which were defined by phylogenetic relationship or by ontogenetic phase, we used different metrics for measuring morphological diversity (also called “disparity”; Guillerme et al., 2020). We calculated the sum of variances for all principal component coordinates for the groups, retrieved from the PCA on the whole data set. These measures were used as an estimation of the portion of morphospace occupied by each group. The median distance of each group to the centre of the morphospace was used as a measure of position within the morphospace (Guillerme et al., 2020).
Using the PCA of the “tree subset”, the estimates for ancestral character states, the phylogenetic results, as well as the occurrence data of the species (Appendix 5), we then calculated the change of morphospace occupation (sum of variance) through time, following Guillerme and Cooper (2018). We defined time bins according to geological periods (Cohen et al., 2023). The code used is provided in Appendix 8.
RESULTS
Morphospace of Carcinidae
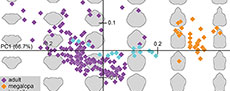
The PCA performed on the outline analysis of the shields resulted in ten effective principal components, describing 95% of the morphological variation of shield shapes in the whole data set (PC1 = 66.7%; PC2 = 10.4%; PC3 = 6.2%; PC4 = 4.6%; PC5 = 1.8%; PC6 = 1.5%; PC7 = 1.3%; PC8 = 1.2%; PC9 = 0.8%; PC10 = 0.6%). Visual interpretation was performed by plotting the first two principal components of the PCA against each other, since they covered most variation in the data (77.1%). Graphical component loadings are given in the appendix (Appendix 9).
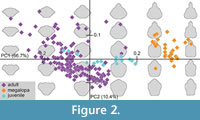 PC1 described the differences of slim and wide shields (Figure 2). Positive values described slim shields with prominent rostrums and posterior notches, while negative values described widened shields with antero-lateral extensions of the shield without a rostrum or posterior notch (Figure 2). PC2 described the differences between posteriorly wider and anteriorly wider shields (Figure 2). Positive values described shields that are anteriorly tapering with a wider posterior region, while negative values described anteriorly widened shields with extending antero-lateral regions and slimmer posterior regions.
PC1 described the differences of slim and wide shields (Figure 2). Positive values described slim shields with prominent rostrums and posterior notches, while negative values described widened shields with antero-lateral extensions of the shield without a rostrum or posterior notch (Figure 2). PC2 described the differences between posteriorly wider and anteriorly wider shields (Figure 2). Positive values described shields that are anteriorly tapering with a wider posterior region, while negative values described anteriorly widened shields with extending antero-lateral regions and slimmer posterior regions.
Initial investigation of the morphospace showed that ontogenetic grouping best explained the patterns in the morphospace. Hereby, the cluster of megalopa larvae plotted on the right side of the morphospace in a tight spread, indicating their slim shields with prominent rostrums and posterior notches. In the centre of the morphospace, in a line along the PC1 axis, plotted the group of juveniles, showing rather oval shields with shallow rostrums. The adults plotted from the bottom of the morphospace, spreading out towards the upper left. This large spread indicated a range of body forms, from slimmer shields with tapering anterior ends to widened shields with strongly extending antero-lateral regions.
Considering the quantitative measurements, we found significant differences in the size of occupied morphospace between all groups (Welch Two Sample t-test; Bonferroni corrected; p-values < 0.001). Hereby, the size of the occupied area increased for each group with an increase in developmental stage, meaning that megalopae occupied the smallest area, while adults occupied the largest area (Table 2). Similarly, we found that all groups were significantly different in their position within the morphospace (Welch Two Sample t-test; Bonferroni corrected; p-values < 0.001; Table 2).
Reconstructed Ancestral States
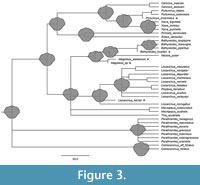 The tree based on the total evidence approach largely agrees with the molecular tree from Evans (2018; Figure 3), except for the relationship between Carcinus (Leach, 1814), Portumnus (Leach, 1814) and Xaiva (MacLeay, 1838). Our tree resolves Xaiva and Portumnus as sister groups, and Carcinus as the sister group to Xaiva and Portumnus, whereas the tree by Evans (2018) resolves Carcinus and Portumnus as sister groups and does not include Xaiva. In this aspect, our tree agrees with Karasawa et al. (2008), which also resolves Cacrinus as the sister group to Portumnus and Xaiva. However, Karasawa et al. (2008) suggest a more distant relationship between Liocarcinus (Stimpson, 1871) and Polybius (Leach, 1820); and Bacthynectes (Stimpson, 1871) and Necora (Holthuis, 1987) than our tree.
The tree based on the total evidence approach largely agrees with the molecular tree from Evans (2018; Figure 3), except for the relationship between Carcinus (Leach, 1814), Portumnus (Leach, 1814) and Xaiva (MacLeay, 1838). Our tree resolves Xaiva and Portumnus as sister groups, and Carcinus as the sister group to Xaiva and Portumnus, whereas the tree by Evans (2018) resolves Carcinus and Portumnus as sister groups and does not include Xaiva. In this aspect, our tree agrees with Karasawa et al. (2008), which also resolves Cacrinus as the sister group to Portumnus and Xaiva. However, Karasawa et al. (2008) suggest a more distant relationship between Liocarcinus (Stimpson, 1871) and Polybius (Leach, 1820); and Bacthynectes (Stimpson, 1871) and Necora (Holthuis, 1987) than our tree.
The reconstruction of ancestral state estimates resulted in a reconstructed shield shape for every node in our tree (Figure 3). Based on the first three nodes of the tree, we could roughly assume the shape of the shield in the earliest representatives of Carcinidae. It seemed to be of quasi-hexagonal shape, with a rounded shallow rostrum, and anteriorly wider than posteriorly.
Diversity Through Time Analysis
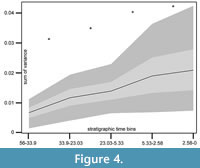 Using the ancestral state estimates for phylogenetic correction, we grouped the species according to their first and last occurrence in the fossil record, to perform a diversity through time analysis (Figure 4). The sum of variance of the first 10 PCs of the outline analysis on the “tree subset” was plotted against the stratigraphic bins in our data set. It showed a strong and significant increase of morphological diversity between every time bin (Wilcoxon rank sum test; p < 0.001).
Using the ancestral state estimates for phylogenetic correction, we grouped the species according to their first and last occurrence in the fossil record, to perform a diversity through time analysis (Figure 4). The sum of variance of the first 10 PCs of the outline analysis on the “tree subset” was plotted against the stratigraphic bins in our data set. It showed a strong and significant increase of morphological diversity between every time bin (Wilcoxon rank sum test; p < 0.001).
DISCUSSION
Limitations of the Approach
Due to a lack of suitable material in the literature, only a small number of fossils could be included into this data set. There are also no fossil megalopae included, as to date there is only one fossil crab megalopa (from the Jurassic) described in the literature (Haug et al., 2015), which is somewhat puzzling. Therefore, we cannot analyse ontogenetic changes through time yet.
As the goal of this study was not to reconstruct the phylogeny of Carcinidae, the phylogenetic reconstruction herein provided is not based on all species, fossil and extant, of the group Carcinidae sensu Spiridonov, 2020. Instead, it contains most of the species used from the two published phylogenetic reconstructions, on which we based our total evidence approach (Karasawa et al., 2008, Evans, 2018). The reconstruction of ancestral states is therefore not based on all known representatives of the group Carcinidae sensu Spiridonov, 2020, but on a large fraction (46/122 extant and fossil species).
Prominence of the Ontogenetic Signal
Against our initial expectations, the diversity of adult morphologies is not dominating the variation within the morphospace (Figure 2). Instead, the ontogenetic signal is stronger in this regard, causing large differences and discrete clustering between megalopae and adult specimens. As to be expected, juveniles plot between the two groups, forming an ontogenetic trajectory within the morphospace. This positioning indicates that, although crabs undergo their last metamorphic moult by moulting from megalopa to crab 1 stage (Haug, 2020b), they still undergo significant morphological changes afterwards.
Furthermore, within the morphospace, megalopae form a tight cluster. In contrast, but in accordance with our expectations, adults show a large variation across the morphospace, indicating different shield forms adapted to swimming, epibenthic, or burrowing lifestyles. Megalopa shields do not show such large variation in their shield form. Their shields seem to be adapted to the transition between habitats, which seems to pose a similar selective pressure across all groups. An alternative interpretation would be that, due to a lack of strong selective pressures, the megalopa shield has retained a plesiomorphic state across Carcinidae. Yet, in the latter case we would expect genetic drift leading to a higher variation in shield shapes.
The Megalopa as a Transitory Stage and a Possible Phylotypic Stage
The lower variation of the megalopa in comparison to the adult is not an unusual pattern for a transitory stage. The previous life phase (in this case, zoea) is highly specialised for a planktic lifestyle, while the life phase afterwards, the juvenile-adult phase, is specialised for different lifestyles (often benthic). The megalopa mediates the transition from one habitat to the others, and from one morphology to the others. It is likely that this transitory phase was ancestrally more gradual, as it is in many other lineages (e.g., Walossek, 1993; Haug & Haug, 2013, 2016; Haug et al., 2013, 2016, 2019; Haug, 2020b). However, there seems to be strong selective pressure that leads to a shortening of this phase down to a single stage, which in turn may leave fewer possibilities for variation in morphology.
In any case, a transitory stage with considerably less variation than former and later stages may be considered “phylotypic”, meaning a stage in which all representatives of a group show the largest amount of similarity (Slack et al., 1993), or the least amount of variation. Using this idea as a framework, we can draw an interesting comparison with the insectan group Holometabola, which is also characterised by a distinct transitory stage, the pupa. Like most megalopa larvae, the pupa is also a non-feeding stage. While many pupae are immobile, this is a derived condition, and in different lineages mobile pupae have been retained, making the ancestral pupa even more comparable to a megalopa. The pupa may represent a phylotypic stage as well (Haug et al., 2023). Using quantitative morphology, it has been shown that the morphological variation in the pupa is significantly smaller than in the adult. This pattern is another similarity to the pattern observed here for the megalopa.
However, for further substantiating the assumption of both pupa and megalopa representing phylotypic stages, it will be necessary to include quantitative data of the prior stages because a phylotypic stage hypothesis requires the prior stage to be more diverse or variable. As pointed out, the challenge for such a comparison is that earlier stages are often depicted in a different orientation in crabs, but also in insectan larvae (Haug et al., 2023).
Ecological Function of Fossil Representatives of Carcinidae
Based on the graphical representation of the ancestral state reconstruction, we can assume that the earliest representatives of Carcinidae had similar shield forms to modern representatives of the Liocarcinus group, an ingroup of Carcinidae (Figure 3). These quasi-hexagonal shields are often found in species with epibenthic lifestyles; representatives of the Liocarcinus group are no different in this regard (e.g., Tutman et al., 2017; Spani and Scalici, 2018). Therefore, the earliest representatives of Carcinidae may have been epibenthic as well. Correlation analyses are needed to investigate this assumption (Ricklefs and Miles, 1994), but if true, it would indicate that all further adaptations expressed by the extant representatives of Carcinidae would have evolved secondarily from this body form. Such adaptations include round shields found in species expressing burrowing lifestyles such as the extant representatives of Thia or Coelocarcinus (Schäfer, 1954; Spiridonov, 2020), long lateral spines as in Bathynectes, or elongated shields deviating slightly from the typical crab-shape as in Pirimelinae.
This evolutionary reconstruction, however, would also mean that a burrowing lifestyle with a longer-than-wide shield evolved convergently twice within Carcinidae: once in Coelocarcinus, and once in the common ancestor lineage of the groups Carcininae, Platyonichinae and Pirimelinae (Figure 3). In this second branch, Carcinus and Sirpus would have secondarily lost the burrowing lifestyle again. Nevertheless, representatives in both lineages that express burrowing lifestyles also express round shields that are rather longer than wide. This shield type therefore seems to have evolved convergently as an adaptation to the burrowing lifestyle.
Appendage morphologies specialized for burrowing lifestyles evolved convergently in many ingroups of Bracyhura as well (Luque et al., 2019). Especially in Portunoidea, the swimming crabs, of which Carcinidae is an ingroup, since the adaptation to swimming often is preceded by an adaptation to burrowing (Luque et al., 2019). It is therefore not unlikely, that the shield form evolved convergently as well. Generally, burrowing lifestyles appeared during the Cenozoic also in other ingroups of Brachyura, alongside the diversification of some of those ingroups, potentially as an adaptation to increased competition (Hartzell et al., 2022).
The Rise of the Crabs
Our study indicates a strong increase of morphological diversity within the Carcinidae since the Eocene, indicated by the significant increases in diversity (Figure 4). New body forms arise in the fossil record, first longer-than-wide shields, then shields with long spines, first in Megokkos (Schweitzer and Feldmann, 2000) during the Eocene and Oligocene. Our phylogenetic reconstruction further suggests that during the Miocene and Pliocene, representatives of Bathynectes should already include forms with long spines as well. Yet Bathynectes muelleri (Ossó and Stalennuy, 2011), the only fossil of the species group Bathynectes known so far, does not possess the long spines of extant relatives. During the Pliocene, round shields without spines as in Thia also appear in the fossil record, and more recently in Coelocarcinus (Edmondson, 1930).
This radiation into several lifestyles (indicated by different shield shapes) has been observed in other ingroups of Portunoidea as well as in Portunidae, for example, (Spiridonov, 2020). In general, an increase in diversity has been suggested during the Cenozoic for many sister groups of Portunoidea (Schweitzer and Feldmann, 2015; Hartzwell et al., 2022). The pattern of emergence of a group and consecutive increase in morphological diversity observed here is therefore not surprising. It may indicate the potential of the crab body form to be well-adaptable to a range of habitats and lifestyles.
Specific ecological factors driving this radiation have already been suggested. The development of, for example, reefs often correlates with an increase in taxonomic diversity of decapods (Ferratges et al., 2021). New reefs translate to a range of new habitats that become available to be colonized by crabs. New morphologies and lifestyles may then be better adapted to these new habitats.
CONCLUSION AND OUTLOOK
The strongest factor for morphological diversity in the shield shape of Carcinidae is ontogeny. Phylogenetic variation is prominent though, especially within the adults, where it is the cause for a large amount of morphological diversity. The early history of the group was apparently less diverse. The graphical representation of ancestral states shows a quasi-hexagonal shield shape that was most likely connected to an epibenthic lifestyle for the earliest representatives of the group. The periods between Eocene and Oligocene, as well as between Miocene and Pliocene, show strong increases in the diversity of Carcinidae, with the emergence of new fossil morphologies as well as reconstructed ancestral states. Unfortunately, the lack of more fossil material, especially of larvae, currently hinders more precise statements about the development of ontogeny through time in Carcinidae.
ACKNOWLEDGEMENTS
We would like to thank two anonymous reviewers for their comments, which, in our opinion, greatly improved the clarity of the manuscript. We would like to thank J. Slapcinsky (Florida Museum of Natural History) for providing an image from the collection. We thank V. Bonhomme (Roquedur, France) for providing support with code implementation. We would like to thank O. Mengedoht/Panzerwelten.de, Recklinghausen for providing images. We thank all providers of free software and Open-Access tools. We would like to thank Prof. J.M. Starck (LMU) for long-standing support. This study was funded by the German Research Foundation under DFG Ha 6300/3-3 and by the Volkswagen Foundation in the frame of a Lichtenberg professorship.
REFERENCES
Aria, C. and Caron, J.B. 2017. Mandibulate convergence in an armoured Cambrian stem chelicerate. BMC Evolutionary Biology, 17:261.
https://doi.org/10.1186/s12862-017-1088-7
Bonhomme, V., Picq, S., Gaucherel, C., and Claude, J. 2014. Momocs: Outline Analysis - Using R. Journal of Statistical Software, 56(13):1–24.
https://doi.org/10.18637/jss.v056.i13
Borradaile, L.A. 1916. Crustacea. Part II. - Porcellanopagurus: An instance of carcinization. British Antarctic (“Terra Nova”) Expediton, 1910. Natural History Report, 3:111–126.
Braig, F., Haug, J.T., Schädel, M., and Haug, C. 2019. A new thylacocephalan crustacean from the Upper Jurassic lithographic limestones of southern Germany and the diversity of Thylacocephala. Palaeodiversity, 12:69–87.
https://doi.org/10.18476/pale.v12.a6
Braig, F., Haug, C., and Haug, J.T. 2023. Phenotypic variability in the shield morphology of wild- vs. lab-reared eumalacostracan larvae. Nauplius, 31:e2023004.
https://doi.org/10.1590/2358-2936e2023004
Clavel, J., Escarguel, G., and Merceron, G. 2015. mvMORPH: an R package for fitting multivariate evolutionary models to morphometric data. Methods in Ecology and Evolution, 6:1311–1319.
https://doi.org/10.1111/2041-210X.12420
Cohen, K.M., Harper, D.A.T., and Gibbard, P.L. 2023. ICS International Chronostratigraphic Chart 2023/04. International Commission on Stratigraphy, IUGS. Available from www.stratigraphy.org. Accessed 09 April 2023.
Edmondson, C.H. 1930. New Hawaiian Crustacea. Occasional papers of the Bernice P. Bishop Museum, 9(10):1–18.
Evans, N. 2018. Molecular phylogenetics of swimming crabs (Portunoidea Rafinesque, 1815) supports a revised family-level classification and suggests a single derived origin of symbiotic taxa. PeerJ, 6:e4260.
https://doi.org/10.7717/peerj.4260
Ferratges, F.A., Zamora, S., and Aurell, M. 2021. Unravelling the distribution of decapod crustaceans in the lower Eocene coral reef mounds of NE Spain (Tremp-Graus Basin, southern Pyrenees). Palaeogeography Palaeoclimatology Palaeoecology, 575:110439. https://doi.org/10.1016/j.palaeo.2021.110439
Forskål, P. 1775. Descriptiones Animalium, Avium, Amphibiorum, Piscium, Insectorum, Vermium; quae in Itinere Orientali Observavit Petrus Forskål. Post Mortem Auctoris editit Carsten Niebuhr. Adjuncta est materia Medica Kahirina. Mölleri, Hafniae, 19 + xxxiv + 164 pp.
GBIF.org, 2022. GBIF Home Page. Available from: https://www.gbif.org [Accessed at 09 April 2023]
Guillerme, T. 2018. dispRity: A modular R package for measuring disparity. Methods in Ecology and Evolution, 9:1755–1763.
https://doi.org/10.1111/2041-210X.13022
Guillerme, T. and Cooper, N. 2018. dispRity manual. figshare. Preprint. https://doi.org/10.6084/m9.figshare.6187337.v1
Guillerme, T., Puttick, M.N., Marcy, A.E., and Weisbecker, V. 2020. Shifting spaces: Which disparity or dissimilarity measurement best summarize occupancy in multidimensional spaces? Ecology and Evolution, 10:7261–7275.
https://doi.org/10.1002/ece3.6452
Hartzell, S.M., Schweitzer, C.E., and Feldmann, R.M. 2022. Extinction and survival of raninoid crabs (Decapoda: Brachyura: Raninoida) from the Early Cretaceous to the present. Journal of Crustacean Biology, 42(4): ruac053.
https://doi.org/10.1093/jcbiol/ruac053
Haug, C., Pérez-de la Fuente, R., Baranov, V., Haug, G.T., Kiesmüller, C., Zippel, A., Hörnig, M.K., and Haug, J.T. 2023. The first fossil record of a mantis lacewing pupa, and a review of pupae in Mantispidae and their evolutionary significance. Rivista Italiana di Paleontologia e Stratigrafia, 129:185–205.
https://doi.org/10.54103/2039-4942/18275
Haug, G.T., Haug, C., Pazinato, P.G., Braig, F., Perrichot, V., Gröhn, C., Müller, P., and Haug, J.T. 2020. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontologia Electronica, 23(2):a39.
https://doi.org/10.26879/1029
Haug, J.T. 2020a. Why the term “larva” is ambiguous, or what makes a larva? Acta Zoologica, 101:167–188.
https://doi.org/10.1111/azo.12283
Haug, J.T. 2020b. Metamorphosis in crustaceans, p. 254-283. In Anger, K., Harzsch, S., and Thiel, M. (eds.), Volume 7. Developmental Biology and Larval Ecology. The Natural History of the Crustacea, Oxford University Press, Oxford.
Haug, J.T. and Haug, C. 2013. An unusual fossil larva, the ontogeny of achelatan lobsters, and the evolution of metamorphosis. Bulletin of Geosciences, 88:195–206.
https://doi.org/10.3140/bull.geosci.1374
Haug, J.T., and Haug, C. 2016. “Intermetamorphic” developmental stages in 150 million-year-old achelatan lobsters - The case of the species tenera Oppel, 1862. Arthropod Structure & Development, 45:108–121.
https://doi.org/10.1016/j.asd.2015.10.001
Haug, J.T., Audo, D., Charbonnier, S., and Haug, C. 2013. Diversity of developmental patterns in achelate lobsters - today and in the Mesozoic. Development Genes and Evolution, 223:363–373.
https://doi.org/10.1007/s00427-013-0452-x
Haug, J.T., Martin, J.W., and Haug, C. 2015. A 150-million-year-old crab larva and its implications for the early rise of brachyuran crabs. Nature Communications, 6:6417.
https://doi.org/10.1038/ncomms7417
Haug, J.T., Haug, C., and Garwood, R. 2016. Evolution of insect wings and development - new details from Palaeozoic nymphs. Biological Reviews, 91:53–69.
https://doi.org/10.1111/brv.12159
Haug, J.T., Haug, C., and Schweigert, G. 2019. The oldest “intermetamorphic” larva of an achelatan lobster from the Lower Jurassic Posidonia Shale, South Germany. Acta Palaeontologica Polonica, 64:685–692.
https://doi.org/10.4202/app.00627.2019
Holthuis, L.B. 1987. Necora, a new genus of European swimming crabs (Crustacea Decapoda, Portunidae) and its type species, Cancer puber L., 1767. Zoologische Mededelingen, 61(1):1–14.
Karasawa, H., Schweitzer, C.E., and Feldmann, R.M. 2008. Revision of Portunoidea (Decapoda: Brachyura) with emphasis on the fossil genera and families. Journal of Crustacean Biology, 28:82–127.
https://doi.org/10.1651/07-2882R.1
Keiler, J., Wirkner, C.S., and Richter, S. 2017. One hundred years of carcinization - the evolution of the crab-like habitus in Anomura (Arthropoda: Crustacea). Biological Journal of the Linnean Society, 121(1):200–222.
https://doi.org/10.1093/biolinnean/blw031
Kuhl, F.P., and Giardina, C.R. 1982. Elliptic Fourier features of a closed contour. Computer Graphics and Image Processsing, 18:236–258.
https://doi.org/10.1016/0146-664X(82)90034-X
Leach, W.E. 1814. Crustaceology, p. 385–437. In Brewster, D. (ed.), The Edinburgh Encyclopaedia, vol. 7(2). Balfour, Edinburgh.
https://www.biodiversitylibrary.org/page/37187640
Leach, W.E. 1820. Malacostraca Podophthalmata Britanniae; or descriptions of such British species of the Linnean genus Cancer as have their eyes elevated on footstalks. John Sowerby, Lambeth, London, 124 pp.
https://www.biodiversitylibrary.org/page/12003115#page/11/mode/1up
Linnaeus, C. 1758. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, 10th revised edition. Laurentius Salvius: Holmiae.
https://doi.org/10.5962/bhl.title.542
Litsios, G. and Salamin, N. 2012. Effects of phylogenetic signal on ancestral state reconstruction. Systematic Biology, 61(3):533–538.
https://doi.org/10.1093/sysbio/syr124
Liu, H., Li, S., Ugolini, A., Momtazi, F., and Hou, Z. 2018. Tethyan closure drove tropical marine biodiversity: Vicariant diversification of intertidal crustaceans. Journal of Biogeography, 45(4):941–951.
https://doi.org/10.1111/jbi.13183
Lőrenthey, E. and Beurlen, K. 1929. Die fossilen Dekapoden der Länder der Ungarischen Krone. Geologica Hungarica series Palaeontologica, 3:1–420.
Luque, J., Feldmann, R.M., Vernygora, O., Schweitzer, C.E., Cameron, C.B., Kerr, K.A., Vega, F.J., Duque, A., Strange, M., and Jaramillo, C. 2019. Exceptional preservation of mid-Cretaceous marine arthropods and the evolution of novel forms via heterochrony. Science Advances, 5(4):eaav3875.
https://doi.org/10.1126/sciadv.aav3875
MacLeay, W.S. 1838. Illustrations of the Annulosa of South Africa. On the brachyurous decapod Crustacea. Brought from the Cape by Dr. Smith, p. i–iv + 53–71. In Smith, A. (ed.), Illustrations of the Zoology of South Africa; consisting chiefly of Figures and Descriptions of the Objects of Natural History Collected during an Expedition into the Interior of South Africa, in the Years 1834, 1835, and 1836; fitted out by “The Cape of Good Hope Association for Exploring Central Africa”. Published under the Authority of the Lords Commissioners of Her Majesty's Treasury, London.
Maddison, W.P. and Maddison, D.R. 2021. Mesquite: a modular system for evolutionary analysis. Version 3.70.
http://www.mesquiteproject.org
Martin, J.W. 2014: Brachyura, pp. 295–310. In Martin, J.W., Olesen, J., and Høeg, J.T. (eds.) Atlas of Crustacean Larvae. Johns Hopkins University Press, Baltimore.
McLaughlin, P.A. and, Lemaitre, R. 1997. Carcinization in the Anomura-fact or fiction? I. Evidence from adult morphology. Contributions to Zoology, 67(2):79–123.
https://doi.org/10.1163/18759866-06702001
Ossó, A. and Stalennuy, O. 2011. Description of the first fossil species of Bathynectes (Brachyura, Polybiidae) in the Badenian (middle Miocene) of the Medobory Hills (Ukraine, Central Parathetys), with remarks on its habitat ecology. Treballs del Museu de Geologia de Barcelona, 18:37–46.
Paula, J. 1988. The larval and post-larval development of Pennant's swimming crab, Portumnus latipes (Pennant) (Brachyura, Portunidae), reared in the laboratory. Crustaceana, 55:202–216.
https://doi.org/10.1163/156854088X00537
R Core Team 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
https://www.R-project.org/
Rathbun, M.J. 1926. The fossil stalk-eyed Crustacea of the Pacific slope of North America. Bulletin of the United States National Museum, 138:1–151.
Revell, L.J. 2012. phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3:217–223.
https://doi.org/10.1111/j.2041-210X.2011.00169.x
Ricklefs, R.E. and Miles, D.B. 1994. Ecological and evolutionary inferences from morphology: an ecological perspective, pp. 13–41. In Wainwright, P.C. and Reilly, S.M. (eds.), Ecological Morphology: Integrative Organismal Biology. University of Chicago Press, Chicago.
Roman, J. and Palumbi, S.R. 2004. A global invader at home: population structure of the green crab, Carcinus maenas, in Europe. Molecular Ecology, 13:2891–2898.
https://doi.org/10.1111/j.1365-294X.2004.02255.x
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A., and Huelsenbeck, J.P. 2012. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Systematic Biology, 61:539–542.
Schäfer, W. 1954. Form und Funktion der Brachyuren-Schere. Abhandlungen der Senckenbergischen naturforschenden Gesellschaft, 489:1–65.
Scholtz, G. 2014. Evolution of crabs-history and deconstruction of a prime example of convergence. Contributions to Zoology, 83:87–105.
https://doi.org/10.1163/18759866-08302001
Schweitzer, C.E. and Feldmann, R.M. 2000. New fossil portunids from Washington, USA, and Argentina, and a re-evaluation of generic and family relationships within the Portunoidea Rafinesque, (Decapoda: Brachyura). Journal of Paleontology, 74:636–653.
https://doi.org/10.1666/0022-3360(2000)074<0636:NFPFWU>2.0.CO;2
Schweitzer, C.E. and Feldmann, R.M. 2010. New fossil decapod crustaceans from the Remy Collection, Muséum national d'Histoire naturelle, Paris. Geodiversitas, 32(3):399–415.
https://doi.org/10.5252/g2010n3a3
Schweitzer, C.E. and Feldmann, R.M. 2015. Faunal turnover and niche stability in marine Decapoda in the Phanerozoic. Journal of Crustacean Biology, 35:1–17.
https://doi.org/10.1163/1937240X-00002359
Slack, J.M., Holland, P.W., and Graham, C.F. 1993. The zootype and the phylotypic stage. Nature, 361:490–492.
https://doi.org/10.1038/361490a0
Spani, F. and Scalici, M. 2018. Carapace asymmetries in crabs. Crustaceana, 91:1281–1290.
https://doi.org/10.1163/15685403-00003835
Spiridonov, V.A. 2020. An update of phylogenetic reconstructions, classification and morphological characters of extant Portunoidea Rafinesque, 1815 (Decapoda, Brachyura, Heterotremata), with a discussion of their relevance to fossil material. Geologija, 63(1):133–166.
https://doi.org/10.5474/geologija.2020.014
Spitzner, F., Meth, R., Krüger, C., Nischik, E., Eiler, S., Sombke, A., Torres, G., and Harzsch, S. 2018. An atlas of larval organogenesis in the European shore crab Carcinus maenas L. (Decapoda, Brachyura, Portunidae). Frontiers in Zoology, 15:27.
https://doi.org/10.1186/s12983-018-0271-z
Stimpson, W. 1871. Preliminary report on the Crustacea dredged in the Gulf Stream in the Straits of Florida, by L.F. de Pourtales, Assist. U.S. Coast Survey, Part I. Brachyura. Bulletin of the Museum of Comparative Zoology, 2(2):109–160.
https://www.biodiversitylibrary.org/page/6313618#page/125/mode/1up
Tutman, P., Kapiris, K., Kirinčić, M., and Pallaoro, A. 2017. Floating marine litter as a raft for drifting voyages for Planes minutus (Crustacea: Decapoda: Grapsidae) and Liocarcinus navigator (Crustacea: Decapoda: Polybiidae). Marine Pollution Bulletin, 120(1–2):217–221.
https://doi.org/10.1016/j.marpolbul.2017.04.063
Walossek, D. 1993. The Upper Cambrian Rehbachiella kinnekullensis and the phylogeny of Branchiopoda and Crustacea. Fossils & Strata, 32:1–202.
Williamson, D.I. 1969. Names of larvae in the Decapoda and Euphausiacea. Crustaceana, 16(2):210–213.
https://doi.org/10.1163/156854069X00510
Wolfe, J.M., Luque, J., and Bracken-Grissom, H.D. 2021. How to become a crab: Phenotypic constraints on a recurring body plan. BioEssays, 43:e2100020.
https://doi.org/10.1002/bies.202100020
WoRMS Editorial Board, 2022. World Register of Marine Species. Available from https://www.marinespecies.org at VLIZ. Accessed 09 April 2023.
https://doi.org/10.14284/170
Young, A.M. and Elliott, J.A. 2020. Life history and population dynamics of green crabs (Carcinus maenas). Fishes, 5(1):4.
https://doi.org/10.3390/fishes5010004
Zhang, C., Stadler, T., Klopfstein, S., Heath, T.A., and Ronquist, F. 2016. Total-evidence dating under the fossilized birth-death process. Systematic Biology, 65:228–249.
https://doi.org/10.1093/sysbio/syv080

