 Twenty-five well-justified fossil calibrations for primate divergences
Twenty-five well-justified fossil calibrations for primate divergences
Article number: 26.1.a8
https://doi.org/10.26879/1249
Copyright Society for Vertebrate Paleontology, March 2023
Fossil Calibration Issue
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 27 October 2022. Acceptance: 23 February 2023.
ABSTRACT
Phylogenies with estimates of divergence times are essential for investigating many evolutionary questions. In principle, “tip-dating” is arguably the most appropriate approach, with fossil and extant taxa analysed together in a single analysis, and topology and divergence times estimated simultaneously. However, “node-dating” (as used in many molecular clock analyses), in which fossil evidence is used to calibrate the age of particular nodes a priori, will probably remain the dominant approach, due to various issues with analysing morphological and molecular data together. Here, we provide a list of 25 well-justified node calibrations for primate divergences, following best practices: 16 within Haplorhini, four within Strepsirrhini, one for crown Primates, and four for older divergences within Euarchontoglires. In each case, we provide a hard minimum bound, and for 22 of these we also provide a soft maximum bound and a suggested prior distribution. For each calibrated node, we provide the age of the oldest fossil of each daughter lineage that descends from it, which allows use of the “CladeAge” method for specifying priors on node ages.
Dorien de Vries. Ecosystems and Environment Research Centre, School of Science, Engineering and Environment, University of Salford, Manchester, UK. (Corresponding author) d.devries@salford.ac.uk @PaleoDorien
Robin M.D. Beck. Ecosystems and Environment Research Centre, School of Science, Engineering and Environment, University of Salford, Manchester, UK. r.m.d.beck@salford.ac.uk @robinmdbeck
Keywords: phylogenetics; fossil calibrations; molecular clock; Primates; Euarchonta; Euarchontoglires
Final citation: de Vries, Dorien and Beck, Robin M.D. 2023. Twenty-five well-justified fossil calibrations for primate divergences. Palaeontologia Electronica, 26(1):a8.
https://doi.org/10.26879/1249
palaeo-electronica.org/content/2023/3777-primate-fossil-calibrations
Copyright: March 2023 Society for Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Phylogenies that provide estimates of divergence times between different lineages of organisms (“timetrees”) have the potential to be extremely useful for answering a wide range of questions relating to evolutionary patterns and processes (Blair Hedges and Kumar, 2009; Ho, 2021). There has been a long history of applying such approaches to primates, for example to identify when humans diverged from their closest living relatives (Sarich and Wilson, 1967; Hasegawa et al., 1985; Scally and Durbin, 2012; Schrago and Voloch, 2013; Püschel et al., 2021), to determine when key phenotypic changes (e.g., increases in brain size; Ni et al., 2019; Püschel et al., 2021) occurred, to clarify the timing of significant biogeographical events (e.g., the dispersal of primates to Madagascar and to South America during the Cenozoic; Poux et al., 2005, 2006; Gunnell et al., 2018; Seiffert et al., 2020), and to infer the likely impact of major environmental changes (e.g., shifts in global climate) on primate diversification (Springer et al., 2012; Herrera, 2017; Godfrey et al., 2020).
Calculation of absolute divergence times between lineages usually requires some form of calibration by incorporating known temporal information, and for “deep time” divergences this typically means evidence from the fossil record (Nguyen and Ho, 2020). In a phylogenetic context, arguably the most appropriate approach (at least in principle) is “tip-dating”, in which fossil taxa of known age are incorporated as terminals (“tips”) in the phylogenetic analysis, and this temporal information is used to estimate divergence times at the nodes (Pyron, 2011; Ronquist et al., 2012; O’Reilly et al., 2015; Zhang et al., 2016; Lee, 2020); this approach, in which fossil and extant taxa can be analysed together, has the advantage that topology and divergence times are estimated simultaneously, in the context of a single analysis, and so the relationships of the fossil taxa do not need to be assumed a priori.
However, it seems unlikely that large quantities of molecular data will ever become available for fossil taxa older than a few million years old (Allentoft et al., 2012; Millar and Lambert, 2013; Welker, 2018), and so tip-dating analyses that include fossil taxa will typically require morphological data to inform their evolutionary relationships. This poses several problems. The most widely used model for analysing discrete morphological data (the “Mk” model of Lewis, 2001) makes a number of simplifying assumptions that seem biologically unrealistic (O’Reilly et al., 2015; Wright et al., 2016; Pyron, 2017; Billet and Bardin, 2019; Wright, 2019). Another is that, even for primates (perhaps the most studied of any clade of organisms), morphological datasets are still small in comparison to equivalent molecular datasets, in terms of both taxon and character sampling (Guillerme and Cooper, 2016b). Given that scoring of morphological datasets requires anatomical expertise, considerable amounts of time, and access to widely scattered museum collections or high quality (ideally 3D) images of specimens, it is unlikely that future morphological datasets for primates will ever be sampled as densely in terms of taxa as will current and future molecular datasets. Thus, directly combining a phylogenomic dataset that samples extant primates at the population or subpopulation level with a compatible morphological dataset will result in a total evidence dataset in which most extant terminals will lack morphological data (Guillerme and Cooper, 2016a). In addition, phylogenomic datasets for primates can comprise millions of base pairs of sequence data (e.g., Jameson et al., 2011; dos Reis et al., 2018; Vanderpool et al., 2020), while primate morphological datasets typically comprise a few hundred characters (e.g., Rasmussen et al., 2019; Kay et al., 2019; Seiffert et al., 2020; Gilbert et al., 2020), and so combining such datasets will mean that fossil taxa (without molecular data) might end up with >99.99% missing data. The impact of such large amounts of missing data on phylogenetic inference is not fully understood and may not be as severe as might be expected (Wiens et al., 2005; Guillerme and Cooper, 2016a), but nevertheless can still negatively affect accurate inference of topology, support values, and (crucially for the estimation of divergence times) branch lengths (Lemmon et al., 2009; Simmons, 2012, 2014; Xia, 2014).
For these and other reasons (including, we suspect, the relative unfamiliarity to many bioinformaticians of morphological data and of appropriate methods for analysing it), it seems likely that molecular clock analyses, which use molecular data only, will remain the most common approach for inferring divergence times among lineages. In such analyses, fossil taxa for which molecular data are unavailable cannot be included directly as terminals/tips, but instead can be used to calibrate the age of one or more nodes in the phylogeny (“node-dating”; Nguyen and Ho, 2020). Because fossils are assigned a priori to particular nodes under this approach, it is crucial that each calibrating fossil is assigned appropriately, based on the most accurate and up-to-date information (Parham et al., 2012; Marjanović, 2021).
However, as discussed at length by Marjanović (2021), identifying appropriate fossil calibrations is not trivial, and even comparatively recent lists of calibrations (e.g., Benton et al., 2015) now appear to be at least partly out of date. Such issues affect molecular clock analyses of primates. For example, several studies (Perelman et al., 2011; Meredith et al., 2011; Springer et al., 2012) have used the late Eocene fossil primate Saharagalago, which was originally described as a galagid (Seiffert et al., 2003), to place a minimum bound on the age of crown Lorisiformes (= the Galagidae-Lorisidae split). However, a number of subsequent studies - notably, several tip-dating analyses (Herrera and Dávalos, 2016; Gunnell et al., 2018; Seiffert et al., 2020) - have instead placed Saharagalago as a stem lorisiform (see summaries in López-Torres and Silcox, 2020; López-Torres et al., 2020), suggesting that it is unsuitable for calibrating the Galagidae-Lorisidae split. Phillips (2016) and Phillips and Fruciano (2018) also found that use of Saharagalago for this calibration results in a seeming mismatch in molecular rates, further suggesting this fossil taxon is not a crown lorisiform. Tip-dating may therefore still play a key role in determining divergence times within primates, as part of a two-stage process: tip-dating analyses of smaller (in terms of both taxa and characters) datasets, which ideally use combined morphological and molecular (=total evidence) data and which show good overlap of characters between taxa, can be used to robustly identify fossil taxa suitable for calibrating particular nodes; these calibrations can then be applied to clock analyses of larger, molecular-only datasets.
The way that fossil calibrations are specified in a node-dating analysis is known to have a major impact on the estimated divergence times (Warnock et al., 2012, 2015). Although fossil calibrations have been used to specify normal distributions on the ages of nodes in some studies (e.g., Perelman et al., 2011), it seems more appropriate to view the minimum age of a calibrating fossil as providing a minimum bound on the age of that node (Benton and Donoghue, 2007; Benton et al., 2009; Ho and Phillips, 2009; Parham et al., 2012). Wherever possible, it is appropriate to also specify a maximum bound, otherwise there is no direct constraint on the maximum age of a calibrated node (Phillips, 2016; Marjanović, 2021). If maximum and minimum bounds are specified, then a prior probability distribution between these bounds (and, if these bounds are “soft”, outside them as well) also needs to be specified (Ho and Phillips, 2009). In principle, any distribution could be used, but from a paleontological perspective, perhaps the most defensible are: 1) a uniform distribution, in which there is an equal probability that the divergence occurred at any time between the minimum and maximum bounds, and which appears most appropriate in cases where the fossil record is known or suspected to be very incomplete, such that the oldest calibrating fossil may in fact substantially postdate the age of the divergence being calibrated; 2) an exponential distribution, in which the probability that the divergence is older than the minimum bound decreases exponentially, and which appears most appropriate in cases where the calibrating fossil is suspected to be very close in time to the actual time of divergences (explained in more detail below; Ho and Phillips, 2009).
Analytical methods for inferring maximum bounds and prior probability distributions on calibrations have been proposed (Marshall, 2008; Wilkinson et al., 2011; Nowak et al., 2013; Matschiner et al., 2017; Matschiner, 2019; Claramunt, 2022), but these often require estimates of parameters such as diversification and/or sampling rates that are not always easy to obtain, even for primates (but see Silvestro et al., 2014; Herrera, 2017; 2019). For this reason, maximum bounds are typically based on somewhat subjective interpretations of available phylogenetic and fossil evidence (as in Benton et al., 2015; Roos et al., 2019; Marjanović, 2021; and most calibrations used by dos Reis et al., 2018). However, dos Reis et al. (2018) based their prior distributions for two nodes - namely the ages of crown Primates and crown Anthropoidea - on the analytical estimates of Wilkinson et al. (2011); nevertheless, we note that the maximum bounds of both of these calibrations (88.6 Ma for crown Primates, 62.1 Ma for crown Anthropoidea) seem implausibly old based on the fossil record (see our proposed calibrations for both of these nodes below). In turn, this may explain why the Late Cretaceous divergence time for crown Primates estimated by dos Reis et al. (2018; 95% Highest Posterior Density [HPD] interval of 70.0-79.2 Ma) is also strongly incongruent with the fossil record, although their estimate for crown Anthropoidea (95% HPD: 41.8-48.3 Ma) is in better agreement with the (very limited) fossil evidence for this node (see “Fossil calibrations” below).
Here, we take these considerations into account to specify an up-to-date set of well-justified fossil calibrations for divergences within Primates, including several entirely new calibrations. In our initial survey of the literature, we identified numerous recent studies that have formally tested the affinities of various fossil primates and relatives using large morphological and total evidence datasets, many of them using tip-dating (e.g., Dembo et al., 2015, 2016; Herrera and Dávalos, 2016; Gunnell et al., 2018; Ni et al., 2019; Seiffert et al., 2020; Püschel et al., 2021; Beck et al., 2023). We combined the published evidence regarding the fossil record and phylogeny of primates and other mammals to identify calibrations for 25 nodes: four outside Primates (crown Euarchontoglires, crown Glires, crown Euarchonta and crown Primatomorpha), crown Primates itself, and 20 divergences within crown Primates. This is a >50% increase in the number of calibrations compared to other recent broadscale molecular clock analyses of primates (Perelman et al., 2011; Springer et al., 2012; dos Reis et al., 2018; Vanderpool et al., 2020). For each calibration, we follow the best practices recommended by Parham et al. (2012). We provide a minimum bound for each calibration, based on the minimum age of the calibrating specimen, and for 22 of our 25 calibrations we also provide a maximum bound and a suggested prior probability distribution (either uniform or exponential), based on our interpretation of the available phylogenetic evidence and the relative completeness of the known fossil record. To maximise the utility of our calibration list to other researchers, we also identify the age of the oldest member of the sister lineage of the calibrating fossil taxon, as required by the CladeAge method for inferring divergence times (Matschiner et al., 2017; Matschiner, 2019). Finally, we compare our proposed calibrations with those suggested in other recent studies (in particular, Benton et al., 2009, 2015; dos Reis et al., 2018; Roos et al., 2019), highlight cases in which there are major differences, and further justify our proposals.
METHODS
Identification of Fossil Calibrations
Based on published studies, we identified fossil calibrations for divergences within crown Primates, for crown Primates itself, and for four divergences outside Primates but within Euarchontoglires: crown Euarchontoglires, crown Glires, crown Euarchonta, and crown Primatomorpha. In identifying appropriate fossil calibrations, wherever possible we based our decisions on the results of formal, algorithmic phylogenetic analyses that have robustly tested the affinities of relevant fossil taxa, using the following hierarchy (from what we consider to be the most robust analyses to the least robust analyses): total evidence tip-dating analyses; undated total evidence analyses; undated analyses with a molecular scaffold; morphology-only tip-dating analyses; undated morphology-only analyses (see Beck et al., 2023). In two cases, we propose calibrations that are not based on evidence presented in formal phylogenetic analyses (specifically, crown Haplorhini and crown Papionini); for most of these, we have based our decisions on the presence of one or more morphological synapomorphies that clearly support assignment of that fossil taxon to a particular clade.
We have followed the recommendations of Parham et al. (2012) for “best practices” for fossil calibrations; these include identifying a specific fossil specimen for each calibration, providing a full phylogenetic justification for using a particular fossil taxon (following our general approach listed above), discussing (where relevant) differences between morphological and molecular phylogenetic analyses, giving detailed locality and stratigraphic information for the calibrating specimen, and explaining clearly how this translates to a particular fixed age or age range. Ages are listed according to the degree of precision present in the source publications. For each calibration, we provide a minimum bound, which we argue can reasonably be viewed as “hard” (i.e., zero probability of the divergence being younger than this). For most calibrations, we also propose a maximum bound, which should be viewed as “soft” (i.e., with a small probability that the divergence is older than this). Where we have provided a minimum and a maximum bound, we also propose a prior probability distribution. For most calibrations, we propose a uniform distribution, in which all ages between the minimum and the maximum bounds have equal probability, which we consider to be most appropriate in cases where the fossil record is obviously highly incomplete (Ho and Phillips, 2009). For a few calibrations, however, we consider that the fossil record is sufficiently complete to be relatively confident the true age of divergence is close to the minimum bound. In these cases, we suggest that this should be modelled as an offset exponential distribution, with the minimum bound as the offset, and the shape of the exponential distribution specified such that there is a 5% probability of the divergence being older than the maximum bound (Ho and Phillips, 2009). We propose an exponential distribution for seven nodes: crown Euarchontoglires, crown Glires, crown Euarchonta, crown Primates, crown Cercopithecidae, crown Hominoidea, and crown Hominidae. For each calibrated node, we explain in detail why we consider a uniform or an offset exponential prior distribution to be appropriate.
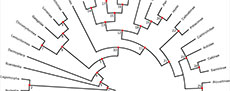
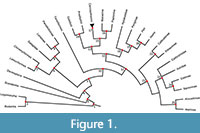 Our list of a minimum and (where relevant) maximum bound for a calibrated node reflects standard practice in node-dating analyses. However, Matschiner et al. (2017) proposed the “CladeAge” method (see also Matschiner, 2019), in which information about the oldest fossils for particular clades is combined with estimates of sampling and diversification rates to construct prior distributions on the ages of those clades. The simulation study of Matschiner (2019) suggests that the CladeAge method is more robust to model violations than are standard node dating-analyses that use the Fossilised Birth Death (FBD) model (Stadler, 2010; Heath and Huelsenbeck, 2014; Gavryushkina et al., 2014). The CladeAge method requires information on the oldest member of each clade present in a phylogeny, not just the oldest of the daughter clades descending from a particular node (as required in “standard” node-dating). Therefore, to maximise the utility of our calibration list to other researchers, and to allow use of the CladeAge method, for each calibrated node, we provide ages for the oldest member of each daughter lineage descending from that node. It should be noted the CladeAge method also requires estimates of sampling and diversification rates, which we do not give here (such rates can be estimated using programs such PyRate; Silvestro et al., 2014; 2019). Table 1 summarises our full calibration list, including minimum and (where relevant) maximum bounds, suggested prior age distributions, and calibrations in a format suitable for use in the CladeAge method. Figure 1 shows the 25 fossil calibrations in phylogenetic context.
Our list of a minimum and (where relevant) maximum bound for a calibrated node reflects standard practice in node-dating analyses. However, Matschiner et al. (2017) proposed the “CladeAge” method (see also Matschiner, 2019), in which information about the oldest fossils for particular clades is combined with estimates of sampling and diversification rates to construct prior distributions on the ages of those clades. The simulation study of Matschiner (2019) suggests that the CladeAge method is more robust to model violations than are standard node dating-analyses that use the Fossilised Birth Death (FBD) model (Stadler, 2010; Heath and Huelsenbeck, 2014; Gavryushkina et al., 2014). The CladeAge method requires information on the oldest member of each clade present in a phylogeny, not just the oldest of the daughter clades descending from a particular node (as required in “standard” node-dating). Therefore, to maximise the utility of our calibration list to other researchers, and to allow use of the CladeAge method, for each calibrated node, we provide ages for the oldest member of each daughter lineage descending from that node. It should be noted the CladeAge method also requires estimates of sampling and diversification rates, which we do not give here (such rates can be estimated using programs such PyRate; Silvestro et al., 2014; 2019). Table 1 summarises our full calibration list, including minimum and (where relevant) maximum bounds, suggested prior age distributions, and calibrations in a format suitable for use in the CladeAge method. Figure 1 shows the 25 fossil calibrations in phylogenetic context.
List of Institutional Abbreviations
AUH: Abu Dhabi Islands Archaeological Survey (Abu Dhabi, United Arab Emirates); CGM: Cairo Geological Museum (Cairo, Egypt); DPC: Duke University Division of Fossil Primates (Durham, North Carolina, USA); DU/IGM: Duke University (Durham, North Carolina, USA) and Instituto National de Investigaciones Geologico-Mineras (INGEOMINAS, Bogota, Colombia); GSP (Y) and YGSP: Yale University (New Haven, Connecticut, USA) and the Geological Survey of Pakistan (Quetta, Pakistan); GWM: National Museum of Ethiopia (Addis Ababa, Ethiopia), followed by the collecting area and ‘P’ for palaeontology; IGM-KU: Instituto National de Investigaciones Geologico-Mineras (INGEOMINAS, Bogota, Colombia) and Kyoto University (Kyoto, Japan); IVPP V: Institute of Vertebrate Paleontology and Paleoanthropology (Beijing, China), with ‘V’ being the specimen prefix of IVPP. LX, IVPP’s fossil locality number in the Linxia Basin; KNM: National Museums of Kenya (Nairobi, Kenya), with ‘FT’ being the prefix for specimens from Fort Ternan, ‘LU’ being the prefix for specimens from Lukeino, ‘SO’ being the prefix for specimens from Songhor, ‘TH’ being the prefix for specimens from Tugen Hills; LACM: Natural History Museum of Los Angeles County (Los Angeles, California, USA); MGPT-PU: Museum of Geology and Paleontology of Torino University (Torino, Italy); OCO: Orrorin Community Organisation, Kipsaraman Museum (Tugen Hills, Kenya); RRBP: Rukaw Rift Basin Project, prefix used by the Tanzania Antiquities Unit (Dar es Salaam, Tanzania); RZO: Laboratory of Geology and Paleontology (Thessaloniki, Greece), ‘RZO’ being the prefix used for specimens from locality Ravin des Zouaves 5; UCMP: University of California Museum of Paleontology, (Berkeley, California, USA); UFAC-LPP: Universidade Federal do Acre Laboratório de Pesquisas Paleontológicas (Acre, Brazil); UM: University of Michigan Museum of Paleontology (Ann Arbor, Michigan, USA).
FOSSIL CALIBRATIONS
Crown Euarchontoglires = Euarchonta-Glires split
Calibrating taxon. Purgatorius mckeeveri
Specimen. UCMP 150021, an isolated left m2, from Harley’s Point locality in the lowermost part of the Tullock Member of the Fort Union Formation in Montana, USA (Wilson Mantilla et al., 2021).
Phylogenetic justification. Purgatorius mckeeveri is the oldest known member of “Plesiadapiformes”, a likely non-monophyletic grade of fossil euarchontans (Silcox et al., 2017). Some phylogenetic analyses focused on deep relationships within Eutheria have recovered Purgatorius outside Placentalia (e.g., Wible et al., 2007, 2009; Goswami et al., 2011). However, recent phylogenetic analyses specifically intended to resolve euarchontan relationships consistently place Purgatorius and other “plesiadapiforms” within crown Euarchonta, and specifically closer to Primates and Dermoptera than to Scandentia, although the precise affinities of “plesiadapiforms” vary in these analyses (e.g., Bloch et al., 2007; Chester et al., 2015, 2017; Li and Ni, 2016; Ni et al., 2016; Silcox et al., 2017; Gunnell et al., 2018; Morse et al., 2019; Seiffert et al., 2020): different “plesiadapiform” taxa may represent stem members of Primates and/or Dermoptera, or they may fall outside crown Primatomorpha (=Primates+Dermoptera; Mason et al., 2016) entirely. Definitive stem euarchontans older than P. mckeeveri have not been identified. The oldest members of the sister-taxon of Euarchonta, namely Glires, are younger than the oldest known material of P. mckeeveri (see “Crown Glires” below). Thus, P. mckeeveri is the oldest known taxon that can be used to calibrate the Euarchonta-Glires split.
Hard minimum bound. 65.79 Ma
Soft maximum bound. 125.816 Ma
Suggested prior distribution. Offset exponential
Age justifications. High resolution geochronological data constrains the age of the oldest known material of Purgatorius mckeeveri to the early Puercan (Pu1), chron C29r, and specifically to within ~208 kyr after the K/Pg boundary (Wilson Mantilla et al., 2021). Wilson Mantilla et al. (2021) reported an 40Ar/39Ar data of a tuff 78 cm located above the Harley’s Point locality, source of our calibrating specimen UCMP 150021, of 65.844 ± 0.033/0.054 Ma (with the uncertainty shown as analytical/systematic uncertainty). We thus use a minimum age of 65.79 Ma for this node. A second date from an underlying tuff has an age of 66.052 ± 0.008/0.043 Ma, bracketing the age of UCMP 150021 to 66.095-65.79 Ma; we use the maximum age as our soft maximum bound for the age of crown Primates (see below).
Placing a maximum bound on the age of Euarchontoglires is difficult because early members of Placentalia, including stem members of Euarchontoglires, probably differed little from stem eutherians in terms of their overall morphology (Bininda-Emonds et al., 2012). This may explain why probable early placentals such as Purgatorius (generally accepted as a euarchontan; see above) and Protungulatum (often placed within Laurasiatheria, typically close to euungulates; O’Leary et al., 2013) fall outside Placentalia in some phylogenetic analyses (e.g., Wible et al., 2007, 2009; Goswami et al., 2011). We have chosen to use a conservative maximum bound based on the age of well-preserved eutherians from the Yixian Formation such as Ambolestes, Eomaia, and Sinodelphys, which have fallen outside Placentalia in all published phylogenetic analyses to date (e.g., Ji et al., 2002; Kielan-Jaworowska et al., 2004; Bi et al., 2018). The age of the Yixian Formation has now been constrained to between 125.755 ± 0.061 and 124.122 ± 0.048 Ma, based on U-Pb chemical abrasion-isotope dilution-isotope ratio mass spectrometry (CA-ID-IRMS; Zhong et al., 2021); we use the maximum age of this range (125.816 Ma) as our maximum bound here. However, this is almost certainly highly conservative, as the oldest convincing records of Placentalia are from the earliest Palaeocene (e.g., Purgatorius mckeeveri; Wilson Mantilla et al., 2021) or very slightly before (e.g., the latest Cretaceous Protungulatum coombsi; Archibald et al., 2011). The putative leptictidan Gypsonictops is known from the Late Cretaceous (Campanian-Maastrichtian; Kielan-Jaworowska et al., 2004) but has also been reported from much older, Turonian deposits (Cohen and Cifelli, 2015), although this material remains to be formally published; if Gypsonictops is indeed a leptictidan, and if leptictidans are crown clade placentals, then this would push the origin of Placentalia considerably earlier than the K-Pg boundary. However, this remains uncertain (see Springer et al., 2019; Marjanović, 2021), and the recent study by Velazco et al. (2022) found leptictidans (including Gypsonictops) to fall outside Placentalia. The preponderance of current fossil evidence appears to support an origin of Placentalia and of the major placental superorders closer to the K-Pg boundary (see also Budd and Mann, 2022); we explicitly take this into account by proposing that this calibration be modelled as an offset exponential prior distribution. Assuming a 5% probability of exceeding the soft maximum bound, this would give a mean and median prior on this divergence of 85.9 and 79.7 Ma, respectively.
Additional CladeAge calibration. As discussed above, Purgatorius mckeeveri is the oldest known member of Euarchonta. The sister-taxon of Euarchonta is Glires, and the oldest definitive members of Glires (Heomys sp., Mimotona wana, and M. lii) are from the lower part of the Upper Member of the Wanghudun Formation in Anhui Province, China (Li, 1977; see “Crown Glires” below). The lower part of the Upper Member of the Wanghudun Formation is currently interpreted as the Shanguan Stage spanning 66.0-62.278 Ma (Wang, Y. et al., 2019; Speijer et al., 2020). Anagalidans have been suggested to be stem members of Glires (López-Torres and Fostowicz-Frelik, 2018), and the earliest members of this group are from the Lower Member of the Wanghudun Formation (Marjanović, 2021: node 155), thus predating Heomys sp., Mimotona wana, and M. lii. However, the precise relationship of Anagalida to Glires remains to be clearly resolved (López-Torres and Fostowicz-Frelik, 2018), and we note that they fell outside Euarchontoglires in the recent phylogenetic analysis of Asher et al. (2019). In addition, Wang et al. (2019) did not provide separate ages for the Lower Member and the lower part of the Upper Member of the Wanghudun Formation, and so the same age range (66.0-62.22 Ma) would apply even if we elected to use anagalidans for this additional CladeAge calibration.
Comments. The material of Purgatorius mckeeveri recently described by Wilson Mantilla et al. (2021) results in a very slightly older minimum on the age of this node than assumed in some previous studies (Benton et al., 2015; Marjanović, 2021). Benton et al. (2009) followed a similar approach to that used here and based their soft maximum bound on the age of the Yixian eutherians, which were the oldest definitive eutherians known at the time. In their revised list of calibrations, Benton et al. (2015) used the maximum age of an even older eutherian discovered subsequently, namely Juramaia sinensis (Luo et al., 2011), to set a maximum bound of 164.6 Ma; however, such a liberal maximum bound is unlikely to place much constraint on the age of this node. By contrast, Marjanović (2021: node 152) proposed a hard maximum bound of 72 Ma on the age of Placentalia, which would force the maximum age of Euarchontoglires (and all other divergences within Placentalia) to be younger than this; however, this very tight maximum bound implicitly endorses an “explosive” model of placental origins, and the validity of this model remains controversial (Springer et al., 2019; but see Budd and Mann, 2022). We prefer to leave this node quite loosely calibrated (although not as loosely as Benton et al., 2015), an approach that we feel is warranted given continuing uncertainty regarding the timing of the origin of Placentalia (Bininda-Emonds et al., 2012; Foley et al., 2016; Springer et al., 2019; Álvarez-Carretero et al., 2022; Budd and Mann, 2022).
Crown Glires = Rodentia-Lagomorpha split
Calibrating taxon. Heomys sp.
Specimen. IVPP V4323, a crushed skull without cheek teeth, from the lower part of the Upper Member of the Wanghudun Formation in Anhui Province, China (Li, 1977).
Phylogenetic justification. Recent phylogenetic analyses (Asher et al., 2019; Rankin et al., 2020) support Heomys sp. as the earliest known member of Simplicidentata, which includes Rodentia. Of perhaps greatest significance, simplicidentates (including Heomys sp.) are characterised by the presence of a single pair of upper incisors, a synapomorphic feature shared by all rodents (Li et al., 2016; Fostowicz-Frelik, 2017, 2020).
Hard minimum bound. 62.278 Ma
Soft maximum bound. 66 Ma
Suggested prior distribution. Offset exponential
Age justifications. IVPP V4323 is from the lower part of the Upper Member of the Wanghudun Formation in Qianshan, Anhui Province (Li, 1977). According to Wang et al. (2019), the Lower Member and lower part of the Upper Member of the Wanghudun Formation can be correlated to the Shanghuan Stage, corresponding to the upper-middle part of chron C29r to C27n, which is 66.0 to 62.278 Ma (Wang, Y. et al., 2019; Speijer et al., 2020); the latter date therefore provides the hard minimum bound for this node.
As summarised by Marjanović (2021: node 155), crown clade Glires (rodents and other simplicidentates; lagomorphs and other duplicidentates) have not been found in the earlier, Lower Member of the Wanghudun Formation (Wang et al., 2016). However, fossil sites from the Lower Member are characterised by a diverse range of anagalidans (anagalids, the pseudictopid Cartictops, and the astigalid Astigale; Marjanović, 2021: node 155). The affinities of anagalidans are in need of detailed study, but it is widely accepted that they include stem relatives of crown Glires (Meng et al., 2003; Fostowicz-Frelik, 2017; López-Torres and Fostowicz-Frelik, 2018; but see the phylogenetic analysis of Asher et al., 2019). Evidence from their molar structure (including a tendency to hypsodonty) and tooth wear suggests that anagalidans were at least partially herbivorous (Fostowicz-Frelik, 2017), as is also the case for most living and fossil members of crown Glires, and features of the postcranial skeleton indicates that pseudictopids were cursorially adapted, similar to lagomorphs (Rose, 2006). We consider the presence in the Lower Member of the Wanghudun Formation of probable stem Glires (namely anagalidans, including some that were probably ecologically similar to lagomorphs), in combination with the apparent absence of crown Glires, to be reasonable evidence that the Rodentia-Lagomorpha split had not occurred by this time. We therefore propose the maximum age of the Shanghuan Stage (66.0 Ma; Wang, Y. et al., 2019) as the soft maximum bound on this node.
Given our assumption that the absence of crown Glires in the Lower Member of the Wanghudun Formation is not an artefact of incomplete sampling, but that it is an indication that Rodentia-Lagomorpha split had yet to occur, we consider that this calibration is most appropriately modelled using an offset exponential distribution. Assuming a 5% probability of exceeding the soft maximum bound, this would give a mean and median prior on this divergence of 63.5 and 63.1 Ma, respectively.
Additional CladeAge calibration. Our calibrating taxon, Heomys sp., is the oldest known stem rodent. The duplicidentates Mimotona wana and M. lii are from the same deposit as Heomys sp. (Li, 1977; Dashzeveg and Russell, 1988; Li et al., 2016). Mimotona and other duplicidentates differ from simplicidentates such as rodents and Heomys sp. but resemble lagomorphs in retaining two upper incisors (Li et al., 2016; Fostowicz-Frelik, 2020). However, presence of two upper incisors is plesiomorphic for crown Glires, and so does not by itself constitute evidence that duplicidentates are stem lagomorphs rather than stem Glires. Nevertheless, Mimotona does share one distinctive derived dental synapomorphy with lagomorphs that is not seen in rodents or other simplicidentates, namely a longitudinal groove on the labial surface of the anteriormost upper incisor (Li and Ting, 1993; Li et al., 2016). Mimotona was also placed as a stem lagomorph in the recent phylogenetic analyses of Asher et al. (2019) and Rankin et al. (2020). Based on this collective evidence, we consider Mimotona wana and M. lii to be the oldest known stem representatives of Lagomorpha, at 66.0-62.278 Ma, and so provide the second CladeAge calibration for this node.
Comments. Benton et al. (2009) proposed a similar minimum bound for this node as that proposed here, but they proposed a much older maximum bound of 131.5 Ma, based on the maximum age of stem eutherians from the Yixian Formation (see “Crown Euarchontoglires” above). Benton et al. (2015) proposed an even older maximum bound, 164.6 Ma, based on the maximum age of the oldest currently known stem eutherian Juramaia (see “Crown Euarchontoglires” above). However, we consider both of these maximum bounds to be unduly conservative given the distinctive craniodental apomorphies of members of crown Glires, and the failure to find such taxa in any mammal-bearing site from the Cretaceous (see also Marjanović, 2021: node 155).
Crown Euarchonta = Scandentia-Primatomorpha split
Calibrating taxon. Purgatorius mckeeveri
Specimen. UCMP 150021, an isolated lower m2, from Harley’s Point locality in the lowermost part of the Tullock Member of the Fort Union Formation in Montana, USA (Wilson Mantilla et al., 2021).
Phylogenetic justification. Retrotransposon insertions provide statistically significant support for the hypothesis that Primates and Dermoptera form a clade (= Primatomorpha) to the exclusion of Scandentia (Mason et al., 2016; Doronina et al., 2022). Some published analyses examining deep relationships within Eutheria have recovered Purgatorius outside Placentalia (e.g., Wible et al., 2007, 2009; Goswami et al., 2011), but all recent published phylogenetic analyses focused on euarchontan relationships have placed Purgatorius closer to Primates and/or Dermoptera than Scandentia (Bloch et al., 2007; Ni et al., 2013, 2016; Chester et al., 2015, 2017; Li and Ni, 2016; Gunnell et al., 2018; Morse et al., 2019; Seiffert et al., 2020; see “Crown Euarchontoglires” above).
Hard minimum bound. 65.79 Ma
Soft maximum bound. 125.816 Ma
Suggested prior distribution. Offset exponential
Age justifications. In contrast to the craniodentally distinctive early crown members of Glires such as Heomys and Mimotona (see “Crown Glires” above), the earliest crown euarchontans may have been morphologically little different from stem eutherians (Bininda-Emonds et al., 2012), which might explain why, for example, Purgatorius falls outside Placentalia in some published analyses (Wible et al., 2007, 2009; Goswami et al., 2011). For this reason, we use the same minimum and maximum bounds for this node as for Crown Euarchontoglires, and again suggest modelling this as an offset exponential prior (see “Crown Euarchontoglires” above).
Additional CladeAge calibration. The fossil record of Scandentia is sparse. Besides the questionable Eodendrogale parva from the middle Eocene (Tong, 1988; Ni and Qiu, 2012), the oldest known scandentian is Ptilocercus kylin from the early Oligocene Lijiawa locality, Yunnan Province, China, which has an age estimate of ~34 Ma (Li and Ni, 2016), and represents a second CladeAge calibration for this node. Phylogenetic analyses place P. kylin within crown Scandentia, sister to the extant P. lowii, suggesting an extensive unsampled history of earlier scandentians.
Comments. Benton et al. (2009) proposed similar minimum and maximum bounds to those used here, whilst Benton et al. (2015) instead suggested a maximum bound of 164.6 Ma based on the maximum age of the oldest currently known stem eutherian, Juramaia (see “Crown Euarchontoglires” above). Marjanović (2021) did not calibrate this node.
Crown Primatomorpha = Primates-Dermoptera split
Suggested prior distribution.
Calibrating taxon. Teilhardina brandti
Specimen. UM 99031 (holotype), an isolated m2, from UM locality SC-351 at the head of Big Sand Coulee in the Clarks Fork Basin, Wyoming (Gingerich, 1993a).
Phylogenetic justification. As summarised above (see “Crown Euarchontoglires”), the precise relationships of the various “plesiadapiforms” to the extant primatomorphan orders Primates and Dermoptera differ quite markedly between recent phylogenetic analyses; we therefore consider them unsuitable for calibrating this node. Two groups with Paleocene representatives, namely plagiomenids and mixodectids, have been proposed by some authors to be dermopteran relatives (Szalay and Lucas, 1993, 1998; Rose, 2006), but this has been questioned (MacPhee et al., 1989; Yapuncich et al., 2011), and so we have also chosen not to use these to calibrate this node. Instead, we use the oldest well-supported member of crown Primates, the omomyiform Teilhardina brandti (Gingerich, 1993a; Rose et al., 2011; Morse et al., 2019) as a necessarily conservative minimum bound.
Hard minimum bound. 55.935 Ma
Soft maximum bound. None
Suggested prior distribution. Not applicable (minimum bound only)
Age justifications. The oldest known material of Teilhardina brandti, including our calibrating specimen UM 99031, comes from the Bighorn Basin in Wyoming (Gingerich, 1993a; Smith et al., 2006; Rose et al., 2011; Morse et al., 2019). T. brandti material has been reported from various localities in the Bighorn Basin: Big Sand Coulee in the Clarks Fork Basin, a northern sub-basin in the Bighorn Basin (Gingerich, 1993a; Smith et al., 2006), the Willwood Formation (Bown and Rose, 1987), and the Sand Creek Divide and Cabin Fork sections (Rose et al., 2011; Morse et al., 2019). All these localities correlate to the second earliest biozone of the early Eocene, Wasatchian-0 (Wa-0), which follows the brief Wa-M biozone and coincides with most of the Paleocene-Eocene Thermal Maximum (PETM; Rose et al., 2011) which is marked by a global carbon isotope excursion (CIE; Yans et al., 2006). Rose et al. (2011) reported that, based on carbon isotopic stratigraphy, Teilhardina brandti appeared only 25 kyr after the onset of the PETM. Using 56.01 ± 0.05 Ma for the start of the PETM (Zeebe and Lourens, 2019), the age estimate of the appearance of Teilhardina brandti 25 kyr after this is then 55.985 ± 0.05 Ma, giving a minimum age of 55.935 Ma for UM 99031, which we use as our minimum bound here, and a maximum age of 56.035 Ma.
If the early Palaeocene Purgatorius is closer to Primates than to Dermoptera, or vice versa, then it seems likely that the Primates-Dermoptera split could predate the K-Pg boundary; conversely, if Purgatorius and other early “plesiadapiforms” are stem rather than crown primatomorphans, then the Primates-Dermoptera split could potentially be close to the Palaeocene-Eocene boundary (the age of the oldest definitive, crown, primates). For this reason, we have chosen not to propose a soft maximum bound on this node.
Additional CladeAge calibration. The oldest known definitive dermopteran material that we use for the CladeAge calibration of this node is Dermoptera indet. from the Pondaung Formation of Myanmar (Marivaux, Bocat, et al., 2006), which has been radiometrically dated to 40.31-40.22 Ma (Khin Zaw et al., 2014; Jaeger et al., 2019).
Comments. This node does not appear to have been calibrated in recent molecular clock analyses, perhaps because compelling evidence for monophyly of Primatomorpha has only become available comparatively recently (Mason et al., 2016; Doronina et al., 2022).
Crown Primates = Haplorhini-Strepsirrhini split
Calibrating taxon. Teilhardina brandti
Specimen. UM 99031 (holotype), an isolated m2, from UM locality SC-351 at the head of Big Sand Coulee in the Clarks Fork Basin, northwestern Wyoming (Gingerich, 1993a).
Phylogenetic justification. Teilhardina brandti has been identified as an omomyiform (Gingerich, 1993a; Rose et al., 2011; Morse et al., 2019). Phylogenetic analyses consistently place Omomyiformes generally, and Teilhardina specifically, within crown Primates. In these analyses, omomyiforms are usually placed within Haplorhini as stem members of the lineage leading to modern tarsiers (=Tarsiiformes), with which they share large orbit size, elongated tarsals, small body size, an anteriorly positioned foramen magnum indicating a vertical head posture, and shortened crania (Ni et al., 2013, 2016; Gunnell et al., 2018; Morse et al., 2019; Jaeger et al., 2019). Even if T. brandti and other omomyiforms are discounted as the oldest crown primates (Godinot, 2015, 2017; Gunnell and Miller, 2018), the oldest known stem strepsirrhine (the 55.8-55.12 Ma old Donrussellia provincialis; see below) is only slightly younger than the oldest material of T. brandti, and so this would have little impact on the minimum bound of this calibration.
Hard minimum bound. 55.935 Ma
Soft maximum bound. 66.095 Ma
Suggested prior distribution. Offset exponential
Age justifications. The minimum bound is based on the minimum age of the oldest specimen of the oldest crown primate, Teilhardina brandti (see “Crown Primatomorpha” above). The maximum bound is based on the maximum age of the oldest specimen of the oldest known plesiadapiform Purgatorius mckeeveri (see “Crown Euarchontoglires” above). Although the affinities of Purgatorius and other “plesiadapiforms” vary between analyses, they have not been recovered within crown Primates in any recently published study of which we are aware. A diversity of “plesiadapiforms” are known throughout the Palaeocene (Silcox et al., 2017). At least some of them were likely ecologically similar to early crown primates (Silcox et al., 2017), and they are known from fossil deposits in the same regions (particularly North America) where crown primates are known from younger sites. The presence of “plesiadapiforms” but the absence of ecologically similar crown primates in these Palaeocene sites (several of which are comparatively rich and well-sampled), and the approximately synchronous appearance of members of Haplorhini (Teilhardina spp.) and Strepsirrhini (Donrussellia spp.) in the earliest Eocene, collectively suggests to us that crown Primates probably originated close to the Palaeocene-Eocene boundary. Based on this, we suggest that this calibration is most appropriately modelled as an offset exponential prior. Assuming a 5% probability of exceeding the soft maximum bound, this would give a mean and median prior on this divergence of 59.3 and 58.3 Ma, respectively.
Additional CladeAge calibration. We recognise Teilhardina brandti as the oldest known haplorhine. Based on current evidence, the oldest known strepsirrhine appears to be the adapiform Donrussellia, with three species known from various early Eocene (MP7) sites in Europe (Donrussellia magna and D. provincialis from France: Godinot, 1978, 1998; and D. lusitanica from Portugal: Estravís, 2000). A fourth species, D. gallica, is slightly younger (MP8+9; Ramdarshan et al., 2015). Of these, Donrussellia provincialis and D. gallica have had their phylogenetic affinities formally tested in the context of large scale analyses (e.g., Ni et al., 2013; Morse et al., 2019) and are usually recovered as stem strepsirrhines. Donrussellia provincialis is also the best known species, based on multiple dental specimens and an isolated astragalus from the Rians locality (Boyer et al., 2017). We therefore use D. provincialis as our additional CladeAge calibration as the oldest known strepsirrhine, with an age range of 55.8-55.12 Ma, based on Solé et al.’s (2015) suggested age for Rians.
Comments. We differ from Benton et al. (2015) and dos Reis et al. (2018), who used Altiatlasius koulchii from the Adrar Mgorn 1 locality, Morocco (Sigé et al., 1990), as the earliest record of crown primates. Adrar Mgorn 1 can be correlated to Chron 24r (Seiffert et al. 2010), which spans the Paleocene-Eocene boundary, but based on associated fauna of invertebrates and selachians a latest Paleocene age for Adrar Mgorn 1 appears more likely (Gheerbrant 1998; Seiffert et al. 2010). This results in a minimum age of 56.0 Ma for Altiatlasius koulchii, based on the age of the end of the Thanetian (Speijer et al., 2020), which is only 0.065 Ma older than the minimum bound we set based on the appearance of Teilhardina brandti. However, Altiatlasius is of very uncertain phylogenetic relationships: it has been identified as a stem primate (Hooker et al., 1999; Morse et al., 2019), a crown primate of uncertain affinities (Silcox, 2008), a stem tarsiiform (Boyer et al., 2010), a basal haplorhine (Marivaux, 2006; Patel et al., 2012), or a stem anthropoid (Godinot, 1994; Marivaux, 2006; Bajpai et al., 2008; Seiffert et al., 2009; Tabuce et al., 2009; Patel et al., 2012) by different authors. Additionally, Seiffert et al. (2010) note that the morphological variation shown by the upper molars of Altiatlasius is problematic, although they still conclude that Altiatlasius is more likely to be an anthropoid than a plesiadapiform. Given the uncertainty surrounding its relationships, and the fact its minimum age being very close to that of Teilhardina brandti, we do not use A. koulchii to calibrate this node.
Benton et al. (2015) used a similar maximum bound to ours, but dos Reis et al. (2018) preferred a much older maximum (88.6 Ma), based on the results of statistical modelling of primate diversification by Wilkinson et al. (2011). In principle, such a quantitative approach is preferable to the admittedly subjective interpretation of the fossil record used here and in most other attempts to identify fossil calibrations for primates. However, we consider a Cretaceous origin for crown Primates to be highly unlikely. Not only is there no record of crown Primates from any Cretaceous site, including the comparatively well-sampled North American record (Kielan-Jaworowska et al., 2004; Wilson, 2014), but a Cretaceous origin for crown Primates would require that all deeper nodes within Euarchontoglires also occurred in the Cretaceous or earlier; there is, however, no record of Cretaceous “plesiadapiforms” either. Furthermore, there does not appear to be a clear explanation why the plesiomorphic “plesiadapiform” Purgatorius (a small-bodied [~100g], predominantly insectivorous, arboreal form; Chester et al., 2015; Silcox et al., 2017; Wilson Mantilla et al., 2021) should appear in the fossil record almost immediately after the K-Pg boundary (Wilson Mantilla et al., 2021), but the oldest crown primates, which appear to have been ecologically broadly similar to Purgatorius, appear ~10 Ma later (and approximately simultaneously in North America, Asia, and Europe; Smith et al., 2006; Beard, 2008; Rose et al., 2011) if the lineages leading to Purgatorius and crown primates had already diverged in the Cretaceous. Instead, we consider the late Cretaceous and Paleocene fossil record to be sufficiently well sampled to support an origin of crown Primates close to the Palaeocene-Eocene boundary.
Crown Strepsirrhini = Lorisiformes-(Lemuriformes+Chiromyiformes) split
Calibrating taxon. Saharagalago misrensis
Specimen. CGM 40266 (type), a lower first molar from the BQ-2 locality in the Fayum region, Egypt (Seiffert et al., 2003).
Phylogenetic justification. Recent phylogenetic analyses of Saharagalago misrensis consistently place it as a crown strepsirrhine, typically as a stem lorisiform (Seiffert et al., 2018, 2020; Gunnell et al., 2018). A second taxon from BQ-2, Karanisia clarki, is also usually placed as a crown strepsirrhine (Seiffert et al., 2018, 2020; Gunnell et al., 2018; López-Torres and Silcox, 2020), providing further evidence that the Lorisiformes-(Lemuriformes+Chiromyiformes) split predates the age of this locality.
Hard minimum bound. 36.573 Ma
Soft maximum bound. 55.8 Ma
Suggested prior distribution. Uniform
Age justifications. The BQ-2 locality falls in a zone of normal polarity and has been correlated with Chron 17n.1n (Seiffert, 2006; Seiffert et al., 2008), which is currently recognised as spanning 37.385-36.573 Ma (Speijer et al., 2020), resulting in a minimum bound for this node of 36.573 Ma. The maximum bound is based on the maximum age of the earliest well-known stem strepsirrhine, Donrussellia provincialis (see “Crown Primates” above), based on the assumption that the divergence of crown Strepsirrhini is unlikely to predate the oldest stem member of the clade. There is a comparatively rich record of stem strepsirrhines from the Eocene in Europe, but the African record is still poorly known, with only three definitive stem strepsirrhines (Djebelemur, Azibius and Algeripithecus) known from the middle Eocene (~48 Ma; Van Couvering and Delson, 2020) of Algeria and Libya (Tabuce et al., 2009; Marivaux et al., 2013), followed by a ~11 million year gap until the probable crown strepsirrhines Saharagalago and Karanisia from BQ-2 mentioned above. For this reason, we suggest that this calibration be implemented as a uniform prior between the minimum and maximum bounds.
Additional CladeAge calibration. We accept Saharagalago as the oldest known lorisiform. We consider the oldest well-supported member of the sister-taxon of Lorisiformes, namely the Chiromyiformes+Lemuriformes clade, to be the stem chiromyiform Plesiopithecus teras, from Quarry L-41 in the Fayum region, Egypt (Simons, 1992; Gunnell et al., 2018), which is dated to 35.102-33.9 Ma (Seiffert, 2006; Seiffert et al., 2008; see “Chiromyiformes-Lemuriformes split” below).
Comments. While we have used Saharagalago to provide the minimum bound on this node, Benton et al. (2015) instead used Karanisia clarki (Seiffert et al., 2003) to provide a minimum bound on the age of this node. In its original description, Karanisia was placed as a crown lorisid (Seiffert et al., 2003), but its position in subsequent studies has varied, having been found as a stem strepsirrhine, stem lorisiform, or stem lemuriform (see summary in López-Torres and Silcox, 2020). We therefore prefer to use Saharagalago, which has been consistently placed as a lorisiform in recent analyses, to calibrate this node, as did dos Reis et al. (2018). For the maximum bound, both Benton et al. (2015) and dos Reis (2018) used the age of Altiatlasius, which they recognised as the oldest crown primate. However, as already discussed (see “Crown Primates” above), Altiatlasius is of uncertain affinities, and we instead use the age of the early stem strepsirrhine, Donrussellia provincialis, as our maximum bound here. Nevertheless, the ages of the minimum and maximum bounds proposed here fall closely to those of Benton et al. (2015) and dos Reis et al. (2018).
Chiromyiformes-Lemuriformes split
Calibrating taxon. Plesiopithecus teras
Specimen. DPC 12393, a crushed but nearly complete cranium with maxillary dentition from Quarry L-41 in the Fayum Depression, Egypt (Simons, 1992; Simons and Rasmussen, 1994).
Phylogenetic justification. Gunnell et al. (2018) presented compelling morphological evidence that Plesiopithecus (and a second taxon, the Miocene Propotto) is a stem member of Chiromyiformes (see also comments by Godinot, 2006), which today is represented by a single species, the aye-aye Daubentonia madagascariensis. This conclusion is supported by total evidence phylogenetic analyses, with and without the use of a clock model (Gunnell et al., 2018).
Hard minimum bound. 33.9 Ma
Soft maximum bound. 55.8 Ma
Suggested prior distribution. Uniform
Age justifications. Plesiopithecus teras comes from Quarry L-41 in the Fayum Depression, Egypt. The age of L-41 has been debated (Gingerich, 1993b; Seiffert, 2006, 2010; Seiffert et al., 2008; Van Couvering and Delson, 2020), but we follow the correlation of L-41 with chron C13r proposed by Seiffert (2006), which is supported by a better statistical fit than the correlations proposed by Kappelman et al. (1992), Gingerich (1993a), and Van Couvering and Delson (2020), and which requires fewer extra, unexplained reversals in the local magnetostratigraphic record (Seiffert et al., 2008, p. 79-81). In addition to the correlation of L-41 with chron C13r, Seiffert (2006) argued that a large unconformity just above the L-41 locality was “likely due to near-coastal erosion associated with the major marine regression that occurred near the Eocene-Oligocene boundary” (see appendix S1 of Sallam and Seiffert, 2016, p. 3) and thus that the L-41 locality predates the Oligocene. Applying the maximum age of chron C13r and the age of the Eocene-Oligocene boundary following Speijer et al. (2020), this results in an age range of 35.102-33.9 Ma for L-41.
Our proposed maximum bound is the same as for crown Strepsirrhini (see above). In particular, Plesiopithecus shows a range of unusual chiromyiform specialisations (Godinot, 2006; Gunnell et al., 2018), suggesting that it probably postdates the Chiromyiformes-Lemuriformes split quite considerably, and implying an extensive unsampled ghost lineage. The Oligocene Bugtilemur mathesoni from the Bugti Hills, Pakistan, was originally described as a crown lemuriform (Marivaux et al., 2001), but was subsequently identified as an adapiform, and hence a stem strepsirrhine, following the discovery of additional specimens (Marivaux et al., 2006). The 37.385-36.573 Ma old Karanisia was placed as a stem lemuriform in the tip-dating analysis of Seiffert et al. (2018), but most other analyses place it as a stem lorisiform (see summary in López-Torres et al., 2020). Thus, no definitive stem lemuriform fossils are currently known. However, if the Chiromyiformes-Lemuriformes split occurred in mainland Africa, as concluded by Gunnell et al. (2018), then lemuriforms should be expected to be found in the African fossil record. Indeed, Gunnell et al. (2018) implied that the poorly known Notnamaia from the middle Eocene (~47 Ma; Van Couvering and Delson, 2020) of Namibia (Pickford et al., 2008) might be a stem lemuriform (but see Godinot et al., 2018), although this has not (to our knowledge) been tested via formal phylogenetic analysis. Thus it seems possible that the Chiromyiformes-Lemuriformes split might be much older than 35.102-33.9 Ma. We therefore consider that a uniform age prior is most appropriate for this node.
Additional CladeAge calibration. Plesiopithecus teras is the oldest known chiromyiform. The oldest definitive records of the sister clade of Chiromyiformes, Lemuriformes, are subfossil remains from Madagascar, the earliest of which are Hadropithecus stenognathus, dating to about 7500 years ago (Burney et al., 2008; Godfrey et al., 2010); this record provides a very young additional CladeAge calibration.
Comments. Benton et al. (2015) and dos Reis et al. (2018) did not calibrate this node.
Crown Lorisiformes = Lorisidae-Galagidae split
Calibrating taxon. Komba robustus
Specimen. KNM-SO 501 (holotype), a right mandibular fragment with p4-m2, from Songhor, Kenya (Le Gros Clark and Thomas, 1952).
Phylogenetic justification. A position for Komba within crown lorisiforms, as a galagid, receives consistently strong support in recent published phylogenetic analyses (Seiffert et al., 2018, 2020; Gunnell et al., 2018). The older Saharagalago (see “Crown Strepsirrhini” above) Karansia and Wadilemur have been recovered as stem galagids in some analyses, but are placed outside crown Lorisiformes in others (see summaries in López-Torres and Silcox, 2020; López-Torres et al., 2020). Also of note are the findings of Phillips (2016) and Phillips and Fruciano (2018) that use of Saharagalago to calibrate the lorisid-galagid split results in extremely high apparent dating error. Using the results of molecular dating analyses to assess the appropriateness of particular fossil calibrations risks circularity, but in this case the strong mismatch in molecular rates found by Phillips (2016) and Phillips and Fruciano (2018) when Saharagalago is assumed to be a crown lorisiform, together with the fact that Saharagalago falls outside crown Lorisiformes in at least some analyses (Seiffert et al., 2018, 2020; Gunnell et al., 2018), persuades us that Komba robustus is a more appropriate calibrating fossil taxon for this divergence.
Hard minimum bound. 18.5 Ma
Soft maximum bound. 55.8 Ma
Suggested prior distribution. Uniform
Age justifications. Species of Komba (as well as several other putative galagids that have not had their phylogenetic affinities robustly tested, such as Progalago spp. and Mioeuoticus spp.) are known from multiple early Miocene sites in east Africa (Harrison, 2010a: table 20.2). Although radiometric dates are available for at least some of these sites, including Songhor from where KNM-SO 501 was collected, this dating was done in the 1960s using K-Ar dating (Bishop et al., 1969), and it is in need of verification using more modern techniques (Cote et al., 2018). Songhor is currently recognised as falling within the Legetetian African Land Mammal Age (Van Couvering and Delson, 2020), and so pending new radiometric dating of this site, we use the minimum age of the Legetetian (which spans 22.5-18.5 Ma according to Van Couvering and Delson, 2020) as our minimum bound here, namely 18.5 Ma. Our maximum bound is the same as for crown Strepsirrhini and Chiromyiformes-Lemuriformes (see above).
Given that Saharagalago, Karanisia, and Wadilemur have all been recovered as stem galagids in some analyses, it is possible that the galagid-lorisid split predates considerably our proposed minimum bound. For this reason, this calibration is most appropriately modelled as a uniform distribution.
Additional CladeAge calibration. The oldest lorisid that has had its phylogenetic affinities rigorously tested is Nycticeboides simpsoni, which is dated to ~8.9 Ma and falls within crown Lorisidae in most recent analyses (see “Crown Lorisidae” below).
Comments. Benton et al. (2015) did not calibrate this node. By contrast, dos Reis et al. (2018) used a similar minimum bound to ours (18 Ma), based on the early Miocene Mioeuoticus, which they recognised as a crown lorisid, but a tighter maximum bound (38 Ma) that seems questionable given the possibility that the 37.385-36.573 Ma Saharagalago is a crown lorisiform (see above); indeed, we note that their 95% posterior credibility interval for the Lorisidae-Galagidae split (34.1-40.9 Ma) exceeds their proposed maximum bound.
Crown Lorisidae = Lorisinae-Perodicticinae split
Calibrating taxon. Nycticeboides simpsoni
Specimen. YGSP 8091 (holotype), a near complete dentition formed by mandibular and maxillary fragments, some skull fragments, and a few postcranial fragments including a distal humerus, all believed to represent a single individual, from the YGSP 363 locality in the Dhok Pathan Formation, Pakistan (Jacobs, 1981).
Phylogenetic justification. Nycticeboides simpsoni closely resembles extant Nycticebus species (Jacobs, 1981; MacPhee and Jacobs, 1986; Flynn and Morgan, 2005) and is typically found to be a crown lorisine in published phylogenetic analyses: either sister to Nycticebus (Seiffert et al., 2015, 2018; Herrera and Dávalos, 2016) or sister to Loris (Seiffert et al., 2018). In a few analyses, however, Nycticeboides is placed as a stem lorisine, outside Loris + Nycticebus (Seiffert et al., 2015), or as part of an unresolved polytomy with Loris and Nycticebus (Seiffert et al. 2010). Regardless, all of these phylogenetic placements support the use of Nycticeboides to place the minimum bound for the divergence between Lorisinae and Perodicticinae. An exception to this general pattern is seen in the total-evidence phylogenetic analyses by Seiffert et al. (2018), in which Nycticeboides was placed as a stem rather than crown lorisid. However, Gunnell et al. (2018) and Seiffert et al. (2020) used morphological matrices that were expanded from Seiffert et al. (2018), and in both of these studies Nycticeboides was placed within crown Lorisidae. Morphological synapomorphies that support Nycticeboides as a lorisine (and hence a crown lorisid) are found in its facial, dental, and postcranial morphology (Jacobs, 1981; MacPhee and Jacobs, 1986), and so we are confident in using this taxon to calibrate this node here.
Hard minimum bound. 8.9 Ma
Soft maximum bound. 37.385 Ma
Suggested prior distribution. Uniform
Age justifications. The YGSP 363 (or Y363) locality in the Dhok Pathan Formation, Pakistan, has been argued to be younger than 8 Ma based on dating of older sites in the same section (Tauxe, 1979), and Nycticeboides was assigned an approximate age of ~8-7 Ma in its original description (Jacobs, 1981). MacPhee and Jacobs (1986) listed an age of 7.5-7.0 Ma for the holotype based on tracing of the lithologic unit to a measured section dated by Tauxe and Opdyke (1982). However, Flynn and Morgan (2005) subsequently reported an age of 9.1-7.8 Ma for YGSP 363, and this locality is currently believed to be ~8.9 Ma old (L. J. Flynn, pers. comm. 21/01/2021); we use this latter date as our hard minimum bound here.
As discussed above (see “Crown Strepsirrhini” and ”Crown Lorisiformes” above), most recent published phylogenetic analyses find that Saharagalago and Karanisia are stem lorisiforms, and so it seems likely that they predate divergences within the crown lorisiform families Lorisidae and Galagidae. We therefore use the maximum age of the BQ-2 Quarry (see “Crown Strepsirrhini” above) as our maximum bound here.
There are a number of fossil putative lorisids that are older than Nycticeboides simpsoni, at least some of which may be members of crown Lorisidae. These include Mioeuoticus from the early Miocene (~19-18 Ma) of East Africa (Le Gros Le Gros Clark, 1956; Leakey, 1962), ? Nycticebus linglom from the Miocene (18.0-17.0 Ma or 14.2-12.0 Ma) of Thailand (Mein and Ginsburg, 1997), and an isolated m1 from the middle Miocene (~15.2 Ma) locality Y682 in the Kamlial Formation of Pakistan that Flynn and Morgan (2005) identified as Nycticeboides sp. We have not used these taxa to inform our proposed minimum bound on this divergence here, because their phylogenetic affinities are either controversial or have not been formally tested; nevertheless, they suggest that the Lorisinae-Perodicticinae split may predate considerably the age of Nycticeboides simpsoni, and so a uniform prior distribution on the age of this node seems appropriate.
Additional CladeAge calibration. As summarised above, we consider Nycticeboides simpsoni to be the oldest well-supported member of crown Lorisidae. The affinities of most other fossil lorisids currently known are controversial or have not been tested via formal phylogenetic analysis. Pickford (2012) described OCO 119’10, a partial rostrum (preserving part of the upper dentition) of a lorisid from the Aragai locality in the Lukeino Formation, and tentatively referred this specimen to the extant perodicticine genus Arctocebus. Although OCO 119’10 has not been included in a published phylogenetic analysis, its close overall resemblance to Arctocebus means that we consider it the oldest definitive perodicticine. The Aragai locality is currently considered to be ~6.1 Ma (Gilbert et al., 2010).
Comments. dos Reis et al. (2018) used a considerably older minimum bound for this divergence of 14 Ma, based on an undescribed genus and species from Fort Ternan in Kenya, which Harrison (2010a) reported “is most similar to Perodicticus, and may eventually be referable to the Perodicticinae.” However, pending description of this specimen and formal testing of its affinities, we prefer a younger minimum bound here. The maximum bound of dos Reis et al. (2018) is similar to that used here.
Crown Haplorhini = Anthropoidea-Tarsiiformes split
Calibrating taxon. Tarsius eocaenus
Specimen. IVPP V14563, a left premaxillary-maxillary fragment preserving the crown of P3, alveoli for I2, C1, P2, and the mesial roots of P4, from Shanghuang fissure D, near the village of Shanghuang, southern Jiangsu Province, China (Rossie et al., 2006).
Phylogenetic justification. Tarsius eocaenus has not, to our knowledge, been included in a comprehensive phylogenetic analysis to formally test its affinities, but its preserved cranial morphology is almost identical to that seen in modern tarsiids, and includes several unusual derived traits (Rossie et al., 2006). Based on this, we are confident that Tarsius eocaenus is a definitive tarsiiform. Omomyiforms, including the oldest known member of this group Teilhardina brandti, are typically placed as stem tarsiiforms in recent phylogenetic analyses (see “Crown Primates” above). However, some doubts remain as to whether omomyiforms are indeed members of the tarsiiform lineage (Godinot, 2015; Gunnell and Miller, 2018).
Based on current evidence, the oldest anthropoids are eosimiids and amphipithecids from the Eocene of Asia (Beard et al., 1994; Beard and Wang, 2004; Marivaux et al., 2005; Seiffert, 2012; Seiffert et al., 2018; Jaeger et al., 2019, 2020). The eosimiid Eosimias was placed as a stem haplorhine, rather than an anthropoid, by López-Torres and Silcox (2018), but this study focused on the phylogeny of plesiadapiforms rather than haplorhines. In recent phylogenetic analyses that have been specifically intended to resolve the relationships of haplorhines, however, eosimiids have been consistently placed as stem anthropoids (e.g., Marivaux et al., 2005; Seiffert, 2012; Ni et al., 2013, 2016; Seiffert et al., 2018, 2020; Gunnell et al., 2018; Morse et al., 2019; Jaeger et al., 2019, 2020). The oldest known eosimiid is Eosimias sinensis, which, like Tarsius eocaenus, is from the Shanghuang fissure fills (Beard et al., 1994; Ni et al., 2020, see Age Justification below); additional eosimiid taxa may be present among the Shanghaung primate material, but they remain unnamed (Gebo et al., 2017).
Older putative records of anthropoids are based on specimens that are much more fragmentary and are correspondingly more equivocal; they include Altiatlasius koulchii from the Palaeocene-Eocene of Africa, which is of very uncertain relationships (see “Crown Primates” above), and Anthrasimias gujaratensis from the early Eocene of India (Bajpai et al., 2008), the material of which has subsequently been suggested to in fact represent the asiadapid (stem strepsirrhine) Marcgodinotius indicus (Rose et al., 2009, 2018). There is thus an ~8-15 million year gap between the oldest omomyiform (Teilhardina brandti, ~56 Ma; see “Crown Primatomorpha” above) and the oldest definitive tarsiiform (Tarsius eocaenus) and oldest widely accepted anthropoid (Eosimias sinensis), both of which are 47.8-41.0 Ma old (see Age Justification below). While the primate fossil record is obviously far from complete, the large gap between the oldest omomyiforms and the oldest tarsiiforms and anthropoids may be an indication that at least some omomyiforms are stem rather than crown haplorhines; thus, we do not use omomyiforms to calibrate this node.Although Tarsius eocaenus and Eosimias sinensis are both from the Shanghuang fissure fillings, the presence of highly distinctive tarsiiform features in T. eocaenus, together with the somewhat labile position of Eosimias within Haplorhini (López-Torres and Silcox, 2018) means that we use the former as our calibrating taxon here, and use E. sinensis for the CladeAge calibration (see below).
Hard minimum bound. 41.0 Ma
Soft maximum bound. None
Suggested prior distribution. Not applicable (minimum bound only)
Age justifications. Five fissures with fills preserving fossil mammals are known from Shanghuang, and these are referred to as fissures A-E. The cranial fragment of Tarsius eocaenus that we use as our calibrating specimen is from fissure D (Rossie et al., 2006; see above), but the holotype (IVPP V11030, an isolated right m1) and other dental specimens are known from fissures A and B (Beard et al., 1994). Eosimias sinensis is known from two partial right mandibles: the holotype (IVPP V10591) from fissure B, and a referred specimen (IVPP V10592) from fissure A. Fissures D and E have been argued to be older than A-C based on mammalian biostratigraphy (Wang and Dawson, 1994; Beard et al., 1994; Qi et al., 1996; Qi and Beard, 1996; Métais et al., 2004; Rossie et al., 2006). However, Ni et al. (2020) did not recognise different ages for the different Shanghuang fissure fills, assigning all of them to the Irdinmanhan Asian Land Mammal Age, which spans 47.8-41.0 Ma. We therefore assume an age range of 47.8-41.0 Ma for both Tarsius eocaenus and Eosimias sinensis, and thus a minimum of 41.0 Ma on this node.
Given the uncertainty regarding the affinities of omomyiforms discussed above, we find it difficult to define an appropriate maximum bound and associated prior age distribution, and so do not propose these for this node.
Additional CladeAge calibration. As discussed (see Phylogenetic Justification), we recognise Eosimias sinensis as the oldest known anthropoid, which therefore represents our additional CladeAge calibration for this node, and which has the same age range (47.8-41.0 Ma) as Tarsius eocaenus.
Comments. Although dos Reis et al. (2018) did not discuss omomyiform affinities, it is notable that they chose to specify the minimum bound on crown Haplorhini using Tarsius eocaenus (as done here), together with a second fossil tarsiid from China (Xanthorhysis), rather than using an omomyiform. Like us, they left the maximum bound on this node uncalibrated.
Crown Anthropoidea = Catarrhini-Platyrrhini split
Calibrating taxon. Catopithecus browni
Specimen. DPC 8701, a near complete skull, from Quarry L-41 in the Fayum Depression, Egypt (Simons, 1989, 1990).
Phylogenetic justification. Catopithecus has beeen proposed to be a stem catarrhine, and therefore a crown anthropoid, based on the apomorphic loss of the upper and lower second premolars, and development of a honing blade for the upper canine on a sexually dimorphic lower p3 (Simons and Rasmussen, 1996; Seiffert and Simons, 2001). A stem catarrhine position for Catopithecus has been supported by recent phylogenetic analyses focused on relationships within Haplorhini, including those of Ni et al. (2016), Morse et al. (2019), Seiffert et al. (2020), and Beck et al. (2023).
Hard minimum bound. 33.9 Ma
Soft maximum bound. 56.035 Ma
Suggested prior distribution. Uniform.
Age justifications. Catopithecus browni comes from Quarry L-41 in the Fayum Depression, Egypt, for which we assume an age range of 35.102-33.9 Ma, following Seiffert (2006; see “Chiromyiformes-Lemuriformes split” above). For the maximum bound we use the maximum age of our calibrating specimen of the oldest crown primate, Teilhardina brandti (see “Crown Primatomorpha” above).
A few stem anthropoids have been described from African sites that are slightly older than Quarry L-41 (e.g., Biretia, Talahpithecus), but as yet no definitive crown anthropoids; however, a currently undescribed taxon from the 37.385-36.573 Ma BQ-2 locality may represent a stem catarrhine (Gunnell and Miller, 2018; E.R. Seiffert, pers. comm. 24/03/2021), which would result in a slightly older minimum bound than that proposed here. In addition, some phylogenetic analyses presented by Jaeger et al. (2019) placed Aseanpithecus from the 40.31-40.22 Ma Pondaung Formation of Myanmar within crown Anthropoidea, although Jaeger et al. (2020) subsequently considered this taxon to be “of uncertain familial status”. At present we consider that this calibration is best modelled as a uniform distribution, although we suspect that this divergence is almost certainly closer to the minimum than the maximum bound.
Additional CladeAge calibration. Catopithecus browni is the oldest known stem catarrhine. Antoine et al. (2021) recently described highly fragmentary primate teeth from Shapaja, San Martín, Peruvian Amazonia in a site (TAR-21) that they dated to between 33.9 and 34.5 Ma, i.e., the latest Eocene (Antoine et al., 2021). These specimens resemble Perupithecus ucayaliensis from the early Oligocene (29.6 ± 0.08 Ma) Santa Rosa Fauna of Peru (Campbell et al., 2021), which is probably a stem platyrrhine (Bond et al., 2015; Kay et al., 2019; Seiffert et al., 2020; Beck et al., 2023), and so we tentatively recognise them as stem platyrrhines as well. However, the reported ages of the Shapaja sites were questioned by Campbell et al. (2021), with these authors concluding that an Oligocene date was more likely. Pending resolution of this issue, we prefer to use the detrital zircon date for the Santa Rosa Fauna, source of Perupithecus, as our second CladeAge calibration: this is 29.68-29.52 Ma. Talahpithecus from the Dur At-Talah escarpment, central Libya (Van Couvering and Delson, 2020), was recovered as a stem platyrrhine in the phylogenetic analysis of Bond et al. (2015), but its position as sister to Perupithecus implies a very complex biogeographical origin for Platyrrhini with multiple crossings of the Atlantic Ocean, and so we do not use Talahpithecus as the oldest record of Platyrrhini here.
Comments. Benton et al. (2015) and dos Reis et al. (2018) also used Catopithecus to provide a minimum bound on this node. However, both these studies used a more conservative maximum bound than that proposed here. Benton et al. (2015) used 66 Ma, based in part on their identification of Altiatlasius as the oldest crown primate and possible crown anthropoid; however, we consider the affinities of Altiatlasius to be uncertain (see “Crown Primates” above) and do not use it for calibration purposes. Dos Reis et al. (2018), meanwhile, used a maximum of 62.1 Ma based on the modelling of primate diversification by Wilkinson et al. (2011), about which we have concerns (see “Crown Primates” above).
Crown Catarrhini = Cercopithecoidea-Hominoidea split
Calibrating taxon. Rukwapithecus fleaglei
Specimen. RRBP 12444A (holotype), a right mandible including p4-m3 and part of ascending ramus from Nsungwe 2B, Tanzania
Phylogenetic justification. Rukwapithecus fleaglei was consistently recovered as a stem hominoid (within the clade Nyanzapithecinae) in the parsimony and Bayesian phylogenetic analyses of Stevens et al. (2013), indicating that it postdates the Cercopithecoidea-Hominoidea split. Stevens et al. (2013) noted that some nodes within their illustrated phylogeny have low support values, but there are various synapomorphies reported for four nodes leading up to Nyanzapithecinae, and for this subfamily itself. Rukwapithecus fleaglei shares two synapomorphies with Miocene and extant hominoids that are not present in cercopithecoids or stem catarrhines: a buccal position of the M2 hypoconulid, and the mesial migration of cusps on the buccal side of lower molars such that the hypoconid is positioned opposite the lingual notch between the metaconid and the entoconid (Stevens et al., 2013).
Hard minimum bound. 25.193 Ma
Soft maximum bound. 35.102 Ma
Suggested prior distribution. Uniform
Age justifications. Rukwapithecus fleaglei comes from locality Nsungwe 2B in the Oligocene Nsungwe Formation in southwestern Tanzania (Stevens et al., 2013). The age of the fossil bearing unit is constrained by two volcanic tuffs dated by U-Pb zircon CA-TIMS (U-Pb chemical abrasion thermal ionisation mass spectrometry) at 25.237 ± 0.098 and 25.214 ± 0.021 Ma (Stevens et al., 2013). Taking into account these confidence intervals, the minimum age for this specimen is 25.193 Ma and the maximum is 25.335 Ma. For the soft maximum bound, we use the maximum age of the oldest known probable stem catarrhine, Catopithecus browni, from the Quarry L-41 of the Fayum Depression, Egypt (see “Crown Strepsirrhini” above). The late Oligocene record of primates and other terrestrial mammals in Africa is notoriously poor (Kappelman et al., 2003; Wilkinson et al., 2011; Stevens et al., 2013), and for this reason we suggest that this calibration is best modelled as a uniform calibration.
Additional CladeAge calibration. Another fossil species from Nsungwe 2B is Nsungwepithecus gunnelli, currently known from a single specimen (RRBP 11178), a left partial mandible with a lower m3 (Stevens et al., 2013). Nsungwepithecus was not included in the phylogenetic analyses by Stevens et al. (2013), but the authors reported the presence of numerous lower molar synapomorphies that are shared with “victoriapithecid” cercopithecoids (“Victoriapithecidae” is a paraphyletic assemblage of stem cercopithecoids in the phylogenetic analyses of Stevens et al., 2013 and Rasmussen et al., 2019), such as deeply incised buccal clefts, a high degree of buccal flare, and the lack of a buccal cingulid. Rasmussen et al. (2019) confirmed the stem cercopithecoid position of Nsungwepithecus in their phylogenetic analysis, but they argued that the phylogenetic position of Nsungwepithecus should be regarded as tentative until more material is available. We therefore recognise Nsungwepithecus gunnelli as the oldest (stem) representative of Cercopithecoidea, with the same age estimate as Rukwapithecus fleaglei; 25.335-25.193 Ma.
Comments. Although differing in detail, Benton et al. (2015), dos Reis et al. (2018), and Roos et al. (2019) all proposed very similar minimum and maximum bounds for this node.
Crown Cercopithecidae = Cercopithecinae-Colobinae split
Calibrating taxon. Colobinae gen. et. sp. indet.
Specimen. KNM-TH 48368, an isolated right lower molar (?m3) from the Baringo Paleontological Research Project (BPRP) no. 38 site in the Kabasero type section of the Ngorora Formation, Tugen Hills succession, Kenya (Rossie et al., 2013).
Phylogenetic justification. Phylogenetic analyses by Rossie et al. (2013) consistently placed KNM-TH 48368 as an early colobine, regardless of whether it was coded as an m2 or an m3. KNM-TH 48368 displays a very small but distinct hypoconulid, which is also present in the fossil colobines Microcolobus and Mesopithecus and many extant colobines (Rossie et al., 2013). Synapomorphies that KNM-TH 48368 shares with extant colobines are: “tall and sharp transverse lophids, reduced basal flare of the crown, a wide and deep median buccal cleft, buccal cusps with a columnar profile and mesial tilt, a long talonid basin relative to overall crown length, and subequal mesial and distal crown breadths” (Rossie et al., 2013).
Hard minimum bound. 12.47 Ma
Soft maximum bound. 25.235 Ma
Suggested prior distribution. Offset exponential
Age justifications. KNM-TH 48368 comes from the Kabasero section of the Ngorora Formation in the Tugen Hills, Kenya (Rossie et al., 2013). 40Ar/39Ar dating of the fossiliferous horizon itself provides an age of 12.49 ± 0.02 Ma for this locality, resulting in a minimum and maximum age for this specimen of 12.47 and 12.51 Ma respectively. The horizon is also bracketed below and above by 40Ar/39Ar dates of 12.56 ± 0.04 Ma and 12.26 ± 0.07 Ma, respectively (Deino et al., 2002; Hill et al., 2002; Rossie et al., 2013). The maximum bound for this node is based on the maximum age of the two oldest known crown catarrhines, namely the stem cercopithecoid Nsungwepithecus and stem hominoid Rukwapithecus from Nsungwe 2B, Tanzania, with a maximum age of 25.214 ± 0.021 Ma (see “Crown Catarrhini” above).
Between the oldest known stem cercopithecoid Nsungwepithecus and KNM-TH 48368, a diverse range of fossil cercopithecoids are known from multiple early and middle Miocene (~22.5-15 Ma; Van Couvering and Delson, 2020) sites throughout Africa, comprising at least nine species-level taxa (Locke et al., 2020, table 1). Not all of these have had their phylogenetic affinities formally tested, but those that have (namely the “victoriapithecids” Prohylobates, Noropithecus, and Victoriapithecus) consistently fall outside crown Cercopithecidae (Miller et al., 2009; Stevens et al., 2013; Rasmussen et al., 2019). The African primate fossil record is sparse between 15 and 6 Ma (Rossie et al., 2013). However, the diversity of stem cercopithecids between 22.5 and 15 Ma (Locke et al., 2020, table 1) and the apparent absence of crown cercopithecids in this same time interval persuades us that this divergence is likely to be close to our minimum bound, and so we propose an offset exponential prior distribution. Assuming a 5% probability of exceeding the soft maximum bound, this would give a mean and median prior on this divergence of 16.7 and 15.4 Ma, respectively.
Additional CladeAge calibration. KNM-TH 48368 is the oldest known colobine. The oldest known record of Cercopithecinae is possible stem papionin material from the Beticha locality in the Chorora Formation, Ethiopia (Suwa et al., 2015; Katoh et al., 2016). Based on available evidence, we do not consider the Beticha material to be unequivocally papionin (see “Crown Cercopithecinae” below), but we do recognise it as cercopithecine. The Beticha fossil-bearing unit is above a pumiceous tuff that has been dated to 8.18 +/-0.15 Ma by K-Ar dating and 7.86 +/- 0.10 Ma by 40Ar-39Ar dating, and below a consolidated tuff dated to 7.67 +/- 0.17 Ma by K-Ar dating and 7.82 +/- 0.11 Ma by 40Ar-39Ar dating (Katoh et al., 2016). Taking the maximum and minimum bounds for these radiometric dates, this gives an age range of 8.33-7.5 Ma, which we suggest as our additional CladeAge calibration.
Comments. dos Reis et al. (2018) do not calibrate this node, but our maximum and minimum bounds are similar to those proposed by Roos et al. (2019).
Crown Colobinae = Colobini-Presbytini split
Calibrating taxon. Mesopithecus pentelicus delsoni (Mesopithecus delsoni according to de Bonis et al. 1990; recognised here as subspecies of Mesopithecus pentelicus following Alba et al., 2015).
Specimen. RZO 159 (holotype of Mesopithecus delsoni according to de Bonis et al., 1990; recognised as a subspecies of Mesopithecus pentelicus by Alba et al., 2014a; 2015), a nearly complete adult male mandible, from Ravin des Zouaves-5, Greece.
Phylogenetic justification. Mesopithecus has been placed as a member of Presbytini in the few published morphological phylogenetic analyses that have specifically examined this question (Jablonski, 1998; Byron, 2001). However, these analyses have not incorporated molecular data, and that of Jablonski (1998) shows important differences to the current molecular consensus view of relationships within Colobinae. In attempt to remedy this, one of us (RMDB) has undertaken preliminary total evidence analyses combining Jablonski’s (1998) morphological matrix with 55.5 kb of nuclear and mitochondrial DNA sequence data (taken from Springer et al., 2012), using both undated and tip-dating approaches, similar to those used by Beck et al. (2023); these analyses place Mesopithecus within Presbytini with strong support (Beck, in prep.). Dental metrics of Mesopithecus are more similar to modern presbytins than to colobins (Pan et al., 2004), and mandibular morphology of Mesopithecus shows particular similarities to that of the modern presbytin genera Rhinopithecus and Pygathrix (Jablonski et al., 2020), but these resemblances are only suggestive because they have not been placed in an explicit phylogenetic context.
Some researchers have cited the unreduced pollex of Mesopithecus as evidence that it falls outside crown Colobinae, all living members of which are characterised by a reduced-to-absent pollex (with a greater degree of pollicial reduction in colobins than presbytins; Frost et al., 2015; Alba et al., 2015). However, Mesopithecus has been reported to have a slightly reduced pollex (Jablonski et al., 2020; but see Frost et al. 2015), and Jablonski (1998: character 148) specifically included “thumb length” as one of the 455 morphological characters used in her phylogenetic analysis. As noted by Jablonski et al. (see also Nakatsukasa et al., 2010; 2020), pollicial reduction has occurred at least twice within Anthropoidea, as the pollex is greatly reduced or absent in the platyrrhine atelids Ateles and Brachyteles (Rosenberger et al., 2008), and we agree with those authors that undue weight should not be placed on a single morphological character, particularly when datasets based on multiple characters are available (Jablonski, 1998; Byron, 2001). We therefore recognise Mesopithecus as the earliest definitive presbytin based on the results of published phylogenetic analyses (Jablonski, 1998; Byron, 2001), and our own unpublished work (Beck, in prep.), and therefore suitable for calibrating this node. However, we acknowledge that this relationship warrants further testing, particularly with datasets that include a denser sampling of fossil taxa (Mesopithecus is the only fossil taxon included in the dataset of Jablonski, 1998, as used by Beck, in prep.).
Hard minimum bound. 8.125 Ma
Soft maximum bound. 15 Ma
Suggested prior distribution. Uniform
Age justifications. The source of our calibrating specimen, the Ravin des Zouaves-5 locality in Greece, is estimated to date to ~8.2 Ma based on magnetostratigraphic evidence and its correlation to C4r.1r (Sen et al., 2000; Koufos, 2009), which has an age range of 8.257-8.125 Ma (Raffi et al., 2020), of which we use the minimum bound. A Mesopithecus specimen from another Greek locality, Nikiti 2, may slightly pre-date this (Koufos, 2016), but its minimum age is also 8.2 Ma. Additionally, the material from Nikiti 2 comprises of one metacarpal and one metatarsal, and we prefer to use the Ravin des Zouaves-5 specimen (which is a near complete mandible) to calibrate this node. A maxillary fragment of Mesopithecus has also been reported from Grebeniki 1 (Gremyatskii, 1961), Ukraine, which was originally dated to the early Turolian, specifically zone MN11 (8.8-7.9 Ma, following collated information by Alba et al., 2015). A subsequent faunal correlation analysis by Vangengeim and Tesakov (2013) correlated Grebeniki 1 with the preceding zone MN10 (9.7-8.8 Ma) which would imply an older minimum bound on this node; however, given the current uncertainty surrounding the age of this site (see Koufos, 2019), we do not use it to calibrate this node.
The oldest stem colobine material is the ~12.5 Ma Colobinae gen. et. sp. indet. from Tugen Hills (Rossie et al., 2013; see “Crown Cercopithecidae” above). However, we have decided against using this material as the basis for our maximum bound due to the poor African record of primates between 15 and 6 Ma (Rossie et al., 2013); instead, we use 15 Ma as our maximum bound, as the better sampled Miocene record prior to this date reveals a diversity of stem cercopithecoids (“victoriapithecids”) but no crown forms (Locke et al., 2020, table 1; see “Crown Cercopithecidae” above). Based on this poor record 15-6 Ma, we suggest modelling this calibration as a uniform prior.
Additional CladeAge calibration. We recognise Mesopithecus pentelicus delsoni as the oldest known presbytin (see above). The oldest member of Colobini appears to be an astragalus from the Lukeino Formation, which is ~6.1 Ma (KNM-LU 344, Gilbert et al., 2010). The astragalus displays apparently diagnostic features of Colobini, such as the distinct groove for the flexor tibialis and the lateral suppression of the facet for the lateral calcaneonavicular ligament, making it the oldest fossil specimen identified as exhibiting specific colobin synapomorphies (Gilbert et al., 2010). As far as we are aware, the affinities of KNM-LU 344 have not been tested by formal phylogenetic analysis, but we consider the presence of features that (on current evidence) appear to be synapomorphies of Colobini (Gilbert et al., 2010) to be sufficient to use this specimen to provide the CladeAge calibration for this node. KNM-LU 344 comes from the Aragai site 2/228 near the base of the Lukeino Fm. in Tugen Hills, Kenya (Gilbert et al., 2010). The Lukeino Formation is bracketed between 6.2-5.7 Ma (Deino et al., 2002; Gilbert et al., 2010), and as the Aragai site 2/228 lies near the base of the Lukeino Formation, it was assigned a tentative date of ~6.1 Ma by Gilbert et al. (2010). However, in the absence of specific dating information for Aragai site 2/228, we instead propose the entire age range of the Lukeino Formation of 6.2-5.7 Ma as the CladeAge calibration of this node.
Comments. We refrain from using the ~7 Ma old possible colobin “Cercopithecoides” bruneti from Toros-Menalla, Chad, which has been referred to Colobini based on its gracile mandibular morphology and adaptations to at least some degree of terrestrial locomotion (Pallas et al., 2019). The assignment of “C.” bruneti to the Cercopithecoides genus needs to be further substantiated as “C” bruneti lacks the distinct mandibular features of Plio-Pleistocene Cercopithecoides (i.e., dorsoventrally short and buccolingually broad mandibles; Pallas et al., 2019). Furthermore, although Pallas et al. (2019) identified several dental features in Cercopithecoides bruneti that they considered to be “consistent with a Colobini morphology”, they did not clearly identify specific apomorphic features that would support its placement in Colobini.
The study of dos Reis et al. (2018) used the 9.8 Ma colobine Microcolobus to provide a minimum bound on this node, but to our knowledge Microcolobus has not been demonstrated to be a member of crown Colobinae, and in fact Rossie et al. (2013) found it to be more closely related the older Tugen Hills material than to extant colobines, suggesting that this taxon is more likely to be a stem form. Roos et al. (2019), meanwhile, used Mesopithecus to provide a minimum bound on this node in their “calibration set 1”, which is their more restrictive set of calibrations.
The maximum bound of 23 Ma proposed for this node by dos Reis et al. (2018) is more conservative than ours and does not appear to take into account the diverse early Miocene record of stem cercopithecoids (see above, Locke et al., 2020). In contrast, Roos et al. (2019) set their maximum bound for this node at 12.5 Ma based on the Kabasero colobine material (Rossie et al., 2013; see “Crown Cercopithecidae” above), which, as discussed above, we consider overly restrictive given the poor African primate record 15-6 Ma (Rossie et al., 2013).
Crown Cercopithecinae = Cercopithecini-Papionini split
Calibrating taxon. Cercopithecini sp. indet
Specimen. AUH 1321, a lower left molar, most likely an m1, from the SHU 2-2 locality in the Baynunah Formation, Abu Dhabi (Gilbert et al., 2014).
Phylogenetic justification. Phylogenetic analyses indicate that AUH 1321 is a crown cercopithecin (Gilbert et al., 2014; Plavcan et al., 2019; see “Crown Cercopithecini” below).
Hard minimum bound. 6.5 Ma
Soft maximum bound. 15.0 Ma
Suggested prior distribution. Uniform
Age justifications. There are no radiometric dates for the SHU 2-2 locality, and so its age estimate is based on geochronological comparisons with Asian and African faunas. These faunal correlations indicate an age between 8.0 and 6.5 Ma, with the most probable age reported as being around 7.0 Ma (Gilbert et al., 2014), but we prefer to use the minimum of this age range as our minimum bound here. As already discussed (see “Crown Cercopithecidae” and “Crown Colobinae” above), a diverse range of stem cercopithecoids, but no crown forms, are known from the early Miocene prior to ~15 Ma (Locke et al., 2020, table 1), with the African fossil record becoming scarce 15-6 Ma (Rossie et al., 2013). A few fossils are known within this interval that may be relevant for calibrating this node, in particular a possible stem papionin from the Beticha locality of the Chorora Formation at 8.33-7.5 Ma (Suwa et al., 2015; Katoh et al., 2016). However, material of this Beticha taxon is extremely fragmentary (Suwa et al., 2015), it has (not to our knowledge) been included in a formal phylogenetic analysis, and Roos et al. (2019) pointed out the difficulty in determining whether it is a stem papionin or stem cercopithecine without lower incisors that might reveal whether or not enamel was present lingually (absence of lingual enamel is the only compelling dental synapomorphy of Papionini). For this reason, we do not use the Beticha taxon to provide our minimum bound.
We use 15.0 Ma as our maximum bound, based on the same reasoning as for crown Colobinae (see “Crown Colobinae” above). Because of the poor fossil record 15-6 Ma, and the possibility of a markedly earlier divergence (based on the Beticha taxon) than specified by our minimum bound, a uniform prior on this calibration seems most appropriate.
Additional CladeAge calibration. We recognise AUH 1321 as the oldest known cercopithecin. Discounting the possible stem papionin from Beticha for the reasons discussed above, we consider the oldest record of Papionini to be the “ Parapapio ” lothagamensis material from the Nawata Formation at Lothagam, Kenya (Leakey et al., 2003). Although yet to be rigorously tested by a suitably comprehensive phylogenetic analysis, it is generally accepted that “Parapapio” lothagamensis is a stem papionin, albeit probably warranting referral to a separate genus (Leakey et al., 2003; Harrison, 2011; Gilbert, 2013; Pugh and Gilbert, 2018). The oldest known material of “Parapapio” lothagamensis material appears to be from the Lower Nawata Formation (Leakey et al., 2003), the age of which can be constrained based on dated tuffaceous horizons to between ~9.1 ±0.2 Ma (the youngest age of the underlying Nabwal Arangan Formation) and 6.54 ± 0.04 Ma (the oldest age of the overlying Upper Nawata Formation; McDougall and Feibel, 2003; Brown and McDougall, 2011), giving an age range of 9.3-6.5 Ma for our CladeAge calibration.
Comments. Our minimum bound on this node is the same as that proposed by Roos et al. (2019) in their more conservative “calibration set 2”. By contrast, dos Reis et al. (2018), used a younger minimum bound of 5.0 Ma, based on “Parapapio” lothagamensis, but this is problematic because the phylogenetic analyses of Gilbert et al. (2014) and Plavcan et al. (2019), although differing somewhat, both place the ~6.5 Ma AUH 1321 within crown Cercopithecini (see below), and so the Cercopithecini-Papionini split must predate this. For a maximum bound, Roos et al. (2019) used the ~12.5 Ma Kabasero colobine material (Rossie et al., 2013; see “Crown Cercopithecidae” above), which, as discussed, we consider overly restrictive given the poor African primate record 15-6 Ma (Rossie et al., 2013). The maximum bound of dos Reis et al. (2018) meanwhile, was 23 Ma, based on the presence of Kamoyapithecus (which dos Reis et al., 2018, considered to be hominoid) at ~25 Ma, and the appearance of the stem cercopithecid Prohylobates at 19.5 Ma onwards. Similarly to crown Colobinae (see above), we consider this overly conservative: the diversity of stem cercopithecoids but absence of crown forms in the early Miocene African record prior to ~15 Ma persuades us that the Cercopithecini-Papionini split probably postdates this.
Crown Papionini = Macacina-Papionina split
Calibrating taxon. cf. Macaca sp.
Specimen. MGPT-PU 130508, a partial male cranium, from the Moncucco Torinese locality, Italy (Alba et al. 2014b).
Phylogenetic justification. In a conference abstract, Alba et al. (2014a) reported that MGPT-PU 130508 is “undoubtedly papionin, as evidenced by facial and dental morphology and size”, and that its molars “display the typical generalised papionin morphology that is characteristic of Macaca, and their size fits with the upper-most range of M. sylvanus subspp.”, and they identified it as cf. Macaca sp. A full description of this significant specimen has yet to be published, and it lacks a full phylogenetic context, but based on the information provided by Alba et al. (2014a) we tentatively recognise this as a member of Macacina. In particular, we consider that it provides a more robust basis for calibrating this node than older (~7.0-5.8 Ma) but much more fragmentary remains of ? Macaca sp. from Menacer, Algeria (Arambourg, 1959; Delson, 1975), which have been used by some previous authors (see below).
Hard minimum bound. 5.33 Ma
Soft maximum bound. 12.51 Ma
Suggested prior distribution. Uniform
Age justifications. The fossil locality at Moncucco Torinese has been assigned a late Turolian (MN13, late Miocene) age based on its fossil fauna. The presence of an ostracod assemblage assigned to the Loxocorniculina djafarovi Zone allows a further refinement of the age to 5.40-5.33 Ma (Alba et al. 2014b), with the minimum age providing our minimum bound.
For a maximum bound, we propose the maximum age of the oldest crown cercopithecid, namely the Kabasero Colobinae gen. et. sp. indet. material, which is 12.51 Ma (see “Crown Cercopithecidae” above); although the African primate fossil record is poor 15-6 Ma (Rossie et al., 2013), it seems unlikely that the Macacina-Papionina split, which is nested well within Cercopithecidae, would predate the overall oldest crown cercopithecid record.
We do not use the 7.0-5.8 Ma record of ? Macaca sp. from Menacer, Algeria (Arambourg, 1959; Delson, 1975) to calibrate this node (see below), but this record raises the possibility that our minimum bound is relatively conservative; we therefore propose a uniform prior on this calibration.
Additional CladeAge calibration. We consider MGPT-PU 130508 to be the oldest robust record of Macacina. Based on available evidence, we consider the oldest robust record of Papionina to be 4.2-4.1 Ma old specimens from Kanapoi, West Turkana, and Kenya, which have been identified as Theropithecus sp. indet. (Frost et al., 2020), and which provide our additional CladeAge calibration.
Comments. Dos Reis et al. (2018) did not calibrate this node. However, Roos et al. (2019) used an older minimum bound on this node of 5.8 Ma in their “calibration set 1” based on ~7.0-5.8 Ma old remains of ? Macaca sp. from Menacer, Algeria (Arambourg, 1959; Delson, 1975). Roos et al. (2019) noted themselves that it is unclear whether ? Macaca sp. from Menacer falls on the Macacina or the Papionina lineage. More seriously, Jablonski and Frost (2010) observed that there are no features of the ? Macaca sp. material from Menacer that would distinguish it from being a stem papionin, as was also noted by Delson (1975, 1980) and Szalay and Delson (1979). We therefore refrain from using this taxon for calibrating this node and instead use the slightly younger cf. Macaca from Moncucco Torinese discussed above. Roos et al. (2019) also used a comparatively young maximum bound of 8 Ma based on the possible stem papionin from the Beticha locality; we have already discussed the uncertainty surrounding this material (see “Crown Cercopithecinae” above), and such a tight maximum bound seems unjustified given the comparatively poor record of primates in Africa between 15 and 6 Ma (Rossie et al., 2013).
Crown Cercopithecini
Calibrating taxon. Cercopithecini sp. indet.
Specimen. AUH 1321, a lower left molar (most likely the first molar), from the SHU 2-2 locality in the Baynunah Formation, Abu Dhabi (Gilbert et al., 2014).
Phylogenetic justification. As already mentioned (see “Crown Cercopithecinae” above), published phylogenetic analyses indicate that AUH 1321 is a crown cercopithecin: it was placed as sister to Chlorocebus or Cercopithecus in the analysis of Gilbert et al. (2014), but sister to Miopithecus or in a polytomous clade with all extant cercopithecin genera except Allenopithecus in the analysis of Plavcan et al. (2019). A combination of features makes AUH 1321 most similar to non- Allenopithecus cercopithecins, namely a small and narrow molar with low-to-moderately flaring, elongated basin, and a distally expanded lophid (Gilbert et al., 2014). Nevertheless, the variation in the position of AUH 1321 between these analyses means that its precise affinities are unclear. Furthermore, molecular phylogenies support a somewhat different set of relationships within Cercopithecini than do the analyses of Plavcan et al. (2019), in which the deepest split among extant cercopithecins is between Allenopithecus and the remaining genera; for example, Perelman et al. (2011) found Allenopithecus to be part of a clade that also includes Chlorocebus and Erythrocebus, whilst dos Reis et al. (2018) recovered an Allenopithecus + Miopithecus clade. These issues notwithstanding, we consider the phylogenetic analyses of Gilbert et al. (2014) and Plavcan et al. (2019) to collectively comprise sufficient evidence that AUH 1321 postdates the deepest split within Cercopithecini, and so can be used to provide a minimum bound on this node.
Hard minimum bound. 6.5 Ma
Soft maximum bound. 12.51 Ma
Suggested prior distribution. Uniform
Age justifications. The age of the SHU 2-2 locality, which informs the minimum bound of this node, is discussed above (see “Crown Cercopithecinae”). Our maximum bound and suggested prior distribution follow the same logic as for crown Papionini (see above).
Additional CladeAge calibration. Because it is uncertain exactly where AUH 1321 fits within crown Cercopithecini, and because of the incongruence between morphological (Gilbert et al., 2014; Plavcan et al., 2019) and molecular (e.g., Perelman et al., 2011; dos Reis et al., 2018) phylogenies of Cercopithecini, we refrain from suggesting an additional CladeAge calibration for this node.
Comments. This node was not calibrated by Benton et al. (2015), dos Reis et al. (2018), or Roos et al. (2019).
Crown Hominoidea = Hominidae-Hylobatidae split
Calibrating taxon. Kenyapithecus wickeri
Specimen. KNM-FT 46a-b (holotype), left maxillary fragment with C1 and P4-M2 present, from Fort Ternan, Kenya (Leakey, 1961).
Phylogenetic justification. Kenyapithecus has consistently been referred to as a crown hominoid, and specifically a hominid, by researchers (Pickford, 1985; Kelley et al., 2008; Harrison, 2010b; Alba, 2012) based in particular on the presence of the putative hominid synapomorphy of an anteriorly situated zygomatic root that is relatively high above the alveolar plane. This has been supported by formal phylogenetic analyses focused on hominoid relationships, with Kenyapithecus typically recovered as a stem hominid (e.g., Young and MacLatchy, 2004; Worthington, 2012; Begun et al., 2012; Pugh, 2022). In contrast, Nengo et al. (2017) and Gilbert et al. (2020) found it to fall within crown Hominidae as a pongine, but we note that these analyses were focused on deeper relationships within Catarrhini, and include relatively limited sampling of hominoids (in contrast to e.g., Pugh, 2022). On available evidence, Kenyapithecus is a probable stem hominid, and its position within crown Hominoidea is well supported (but see Benoit and Thackeray, 2017); we consider it the oldest definitive crown hominoid currently known.
Hard minimum bound. 13.4 Ma
Soft maximum bound. 25.235 Ma
Suggested prior distribution. Offset exponential
Age justifications. The minimum age is based on dates of the Fort Ternan fossil locality published by Pickford et al. (2006), who report on whole-rock K/Ar and single-crystal 40Ar/39Ar dates of lava flows underlying and overlying the fossil beds at Fort Ternan. The fossil beds at Fort Ternan are estimated to be 13.7 ± 0.3 Ma (Pickford et al., 2006), giving an age range of 14.0-13.4 Ma, with the minimum age as our hard minimum bound. A second species of Kenyapithecus, K. kizili, has been described from Paşalar, Turkey (Kelley et al., 2008), which may be slightly older than K. wickeri (Roos et al., 2019). However, the age of Paşalar is poorly constrained (Casanovas-Vilar et al., 2011), and we do not use K. kizili to inform our minimum bound here. The maximum bound is based on the maximum age of the oldest stem hominoid Rukwapithecus (see “Crown Catarrhini” and “Crown Cercopithecidae” above).
In a situation equivalent to that seen in cercopithecoids (see “Crown Cercopithecidae”), the early Miocene African fossil record of Hominoidea is characterised by a diversity of stem taxa (proconsuline and nyanzapithecine “proconsulids”) without any evidence of crown representatives (Harrison, 2010b; Stevens et al., 2013; Nengo et al., 2017; Almécija et al., 2021). We tentatively interpret this as evidence that the Hominidae-Hylobatidae split was probably much closer to our minimum bound than our maximum bound, and so we propose calibrating this divergence with an offset exponential prior distribution. Assuming a 5% probability of exceeding the soft maximum bound, this would give a mean and median prior on this divergence of 17.4 and 16.1 Ma, respectively.
Additional CladeAge calibration. We consider Kenyapithecus wickeri to be the oldest known hominid (stem or crown, see above). For the CladeAge calibration of this node, we conservatively propose the 8.2-7.1 Ma Yuanmoupithecus xiaoyuan from the Late Miocene of Yunnan in southern China (Pan, 2006; Gilbert et al., 2020; Ji et al., 2022). Recent published phylogenetic analyses support Yuanmoupithecus as a stem hylobatid (Gilbert et al., 2020; Ji et al., 2022). The ~13.8-12.5 Ma Kapi ramnagarensis from the Lower Siwaliks of Ramnagar, India, was identified as a stem hylobatid by Gilbert et al. (2020), an inference supported by their phylogenetic analysis. However, Kapi is currently known only from a single lower third molar, and the phylogenetic analyses of Ji et al. (2022) found it to be a pliopithecoid or a more primitive stem catarrhine. Based on evidence published to date, we consider the affinities of Kapi to be too uncertain for use as a CladeAge calibration, and we instead use the younger Yuanmoupithecus xiaoyuan, which is more robustly supported as a definitive hylobatid (Gilbert et al., 2020; Ji et al., 2022).
Comments. Our minimum and maximum bounds are broadly similar to those of Roos et al. (2019). By contrast, Benton et al. (2015) proposed the crown hominid (stem pongine) Sivapithecus as the oldest crown hominoid, with a minimum age of 11.6 Ma, and used the age of the earliest anthropoids in the Fayum Depression as their maximum bound; in light of our discussion above, we consider both minimum and maximum bounds proposed for this node by Benton et al. (2015) to be unduly conservative. Dos Reis et al. (2018) did not calibrate this node, but they stated in three separate places that they considered the ~25 Ma old Kamoyapithecus to be a “crown hominoid”, a conclusion that they themselves admitted is “controversial”. However, the paper they cited in support of this conclusion, Zalmout et al. (2010), found Kamoyapithecus to be a stem (not crown) hominoid, and more distantly related to Hylobatidae+Hominidae than is “Proconsulidae”; table 1 of dos Reis et al. (2018) also lists Kamoyapithecus as a stem hominoid, in agreement with current evidence, as summarised above.
Crown Hominidae = Homininae-Ponginae split
Calibrating taxon. Sivapithecus indicus
Specimen. (GSP) Y 16075, maxilla (Raza et al., 1983; Kappelman et al., 1991) with the connection between the maxilla and premaxilla partially preserved (Begun, 2015), from locality Y494 from the Chinji Formation, Pakistan (Pilgrim, 1910).
Phylogenetic justification. Sivapithecus has been consistently recovered as a pongine in recent phylogenetic analyses (e.g., Begun et al., 2012; Nengo et al., 2017; Gilbert et al., 2020; Pugh, 2022). Y 16075 preserves the derived subnasal anatomy characteristic of modern orangutans (Pongo spp.; Kappelman et al., 1991). Isolated teeth from slightly older sites in the Chinji Formation have been referred to Sivapithecus, but they lack diagnostic features to support this referral (Kappelman et al., 1991), and so we do not use these for calibration purposes. We note that the slightly older Kenyapithecus (see “Crown Hominoidea” above) has been recovered as a pongine in some recent phylogenetic analyses (Nengo et al., 2017; Gilbert et al., 2020), but others place it as a stem hominid (e.g., Young and MacLatchy, 2004; Worthington, 2012; Begun et al., 2012; Pugh, 2022), and so it is not suitable for calibrating this node.
Hard minimum age. 12.3 Ma
Soft maximum age. 25.235 Ma
Suggested prior distribution. Offset exponential
Age justifications. We base our minimum age on the reported age of 12.3 Ma for another site in the mid-Chinji Formation, Y647 (which also preserves Sivapithecus indicus specimens), which is stated to be at the same stratigraphic level as Y494 (Morgan et al., 2015); this age is stated to be based on magnetostratigraphy, but Morgan et al. (2015) do not provide further details, and so it should be treated as tentative.
We base the maximum bound on the maximum age of the oldest stem hominoid Rukwapithecus (see “Crown Catarrhini”, “Crown Cercopithecidae”, and “Crown Hominoidea” above). A potential alternative maximum bound would be to use the maximum age of the oldest hominoid Kenyapithecus, which is 14.0 Ma (see Crown Hominoidea above). However, given that a few published analyses have placed Kenyapithecus as a crown hominid (Nengo et al., 2017; Gilbert et al., 2020), it may be unduly restrictive to use this taxon to inform our maximum bound. We therefore choose a more conservative approach based on the age of Rukwapithecus, as this taxon has been consistently found to be a stem hominoid in recent phylogenetic analyses (see “Crown Catarrhini” and “Crown Cercopithecidae” above). We consider the offset exponential distribution to be most appropriate for this calibration, based on the same arguments given for the crown Hominoidea node (see “Crown Hominoidea” above). Assuming a 5% probability of exceeding the soft maximum bound, this would give a mean and median prior on this divergence of 16.6 and 15.3 Ma, respectively.
Additional CladeAge calibration. We consider Sivapithecus indicus to be the oldest definitive pongine. Pugh (2022) presented a series of phylogenetic analyses of Miocene hominoids based on a large (41 taxa, 274 characters) morphological character matrix, using different character coding schemes and analytical methods. Most of these analyses found Nakalipithecus (described by Kunimatsu et al., 2007) to be the oldest (stem) hominine among the taxa included (see Pugh, 2022: figure 5); total evidence tip-dating analysis of the Pugh (2022) matrix in combination with DNA and protein sequence data also strongly supports Nakalipithecus as a stem hominine (Beck, in prep.). All known specimens of Nakalipithecus are from Upper Member of the Nakali Formation of Kenya, within Chron C5n.1n (Kunimatsu et al., 2007), which is 9.937-9.786 Ma (Raffi et al., 2020), and provides our additional CladeAge calibration here.
Comments. Roos et al. (2019) used Kenyapithecus wickeri (with a maximum age of 14.9 Ma) as their maximum bound on this node, on the assumption that it is a stem hominid. The stem hominid position of Kenyapithecus has been supported by most recent phylogenetic analyses (e.g., Pugh, 2022), but it was placed as a crown hominid (pongine) by Nengo et al. (2017) and Gilbert et al. (2022), hence our decision to use the maximum age of the oldest stem hominoid Rukwapithecus fleaglei (see “Crown Hominoidea” above) for setting the soft maximum bound at 25.235 Ma. Benton et al. (2015), meanwhile, used a maximum of 33.9 Ma based on the age of the oldest known crown anthropoids from the L-41 Quarry of the Fayum Depression, Egypt (see “Crown Anthropoidea” above), which seems excessively conservative given the diversity of stem hominoids but absence of crown forms in the early Miocene African record (see “Crown Hominoidea” above).
Homo-Pan split
Calibrating taxon. Ardipithecus ramidus
Specimen. GWM5sw/P56, a mandibular ramus and partial dentition (p3-m3) from GWM-5sw locality in Gona, Ethiopia (Semaw et al., 2005; Simpson et al., 2019).
Phylogenetic justification. Notable features of A. ramidus that appear to be synapomorphies placing it as a member of the Homo lineage include the more incisiform canines, an anteriorly located foramen magnum, and a proximal ulnar morphology that is shared with Australopithecus species (White et al., 1995, 2009; Suwa, Asfaw, et al., 2009; Suwa, Kono, et al., 2009; but see Harrison, 2010c). This interpretation has been tested in formal phylogenetic analyses by Dembo et al. (2015, 2016), Mongle et al. (2019), Püschel et al. (2021), and Pugh (2022), all of whom recovered A. ramidus as a member of the Homo lineage. Although Ardipithecus kadabba is slightly older than A. ramidus (5.8-5.2 Ma, Haile-Selassie, 2001; WoldeGabriel et al., 2001; 2004), we refrain from using this species to calibrate this node as it has not been included in any of these phylogenetic analyses, most likely due to the scarcity of Ardipithecus kadabba material. The phylogenetic analyses of Dembo et al. (2015, 2016), Mongle et al. (2019), and Püschel et al. (2021) also placed Sahelanthropus (which may be older than A. ramidus) closer to Homo than to Pan, but doubts over the stratigraphic provenance of Sahelanthropus, and hence its age, mean that we do not use it as our calibrating taxon here (see “Comments” below).
Hard minimum bound. 4.631 Ma
Soft maximum bound. 15 Ma
Suggested prior distribution. Uniform
Age justifications. The oldest A. ramidus localities (GWM-1, GWM-5sw, and GWM-9) have been assigned to the C3n.2r magnetozone (Simpson et al., 2019) which corresponds to an age of 4.799-4.631 Ma (Raffi et al., 2020). Pickford and Senut (2005) reported a ~12.5 Ma isolated lower molar from the Ngorora Formation that they suggested may belong to the Pan lineage (but which Kunimatsu et al. (2007) considered resembles Gorilla), which raises the possibility that this divergence may be markedly older than our minimum bound. Based on these factors, we take a conservative approach for this node, and use the same 15 Ma maximum bound as for crown Colobinae and crown Cercopithecinae (see above); this was chosen to reflect the generally poor record of primates in Africa 15-6 Ma (Rossie et al., 2013), and the fact that stem hominoids (proconsuline and nyanzapithecine “proconsulids”; see “Hominidae-Hylobatidae split” above) were diverse but crown hominoids were apparently absent in Africa during the early Miocene prior to 15 Ma. For the same reason, we also suggest a uniform bound is the appropriate prior distribution for this node.
Additional CladeAge calibration. We recognise Ardipithecus ramidus as the oldest known member of the Homo lineage that has a well-constrained age. The fossil record of its sister-clade, the Pan lineage, is extremely limited. To date, the oldest fossils are specimens that have been referred to the modern genus Pan from the Kapthurin Formation of Kenya (McBrearty and Jablonski, 2005; although this was questioned by Harrison, 2010b, who instead argued that they may belong to Homo), the age is constrained by 40Ar/39Ar dates of 545 ± 3 kyr for deposits underlying the fossils and 284 ± 12 kyr for deposits overlying them (Deino and McBrearty, 2002; McBrearty and Jablonski, 2005). The fossils are located most closely to the underlying deposit, and McBrearty and Jablonski (2005) argued that their age is likely to be close to 0.5 Ma. However, we prefer to use the entire age range (including confidence intervals) for this record, giving an additional CladeAge calibration for this node of 0.548-0.272 Ma.
Comments. Unlike us, Benton et al. (2015), dos Reis et al. (2018), and Roos et al. (2019) all used the age of Sahelanthropus to provide a minimum bound on this divergence, although the precise date used varied between these studies: Benton et al. (2015) used 6.5 Ma, Roos et al. (2019) used 6.2 Ma, and dos Reis et al. (2018) used 7.5 Ma. The known cranial morphology of Sahelanthropus preserves several apparent synapomorphies shared with members of the Homo lineage, to the exclusion of Pan (see e.g., Brunet et al., 2002; Zollikofer et al., 2005; MacLatchy et al., 2010; Emonet et al., 2014). Although this interpretation has been questioned by some authors (Wolpoff et al., 2002; Wolpoff and Pickford, 2006), recent phylogenetic analyses by Dembo et al. (2015, 2016), Mongle et al. (2019), and Püschel et al. (2021) have consistently placed Sahelanthropus closer to Homo than to Pan, as the deepest diverging member of the Homo lineage among the taxa included in these analyses. However, questions have been raised about the stratigraphic origin of Sahelanthropus material (Beauvilain, 2008), and thereby on its reported age of 7.2-6.8 Ma (Lebatard et al., 2008). Ahern (2018) concluded that the fossil material of Sahelanthropus is most likely of late Miocene age, but suggested that its age could not be constrained more accurately than 7.5-5.0 Ma based on available data. We accept that Sahelanthropus is most likely a member of the Homo lineage, but due to the uncertainty surrounding its age we prefer to take a more conservative approach and use the more securely dated Ardipithecus ramidus for calibrating this node. However, if more robustly dated Sahelanthropus material is reported that predates the age of A. ramidus, the minimum age of this calibration will need to be updated.
Orrorin from Kenya, dating to 6.0-5.7 Ma (Senut et al., 2001; Sawada et al., 2002), is another proposed member of the Homo lineage that predates A. ramidus. Like Sahelanthropus, Orrorin shares putative Homo- lineage synapomorphies with Ardipithecus, such as small canines and features hinting at early stages of bipedalism present in the basicranium and lower limb bones (Richmond and Jungers, 2008; MacLatchy et al., 2010; Harrison, 2017). However, to our knowledge, Orrorin has not been included in suitably comprehensive, published phylogenetic analyses (such as those by Dembo et al., 2015, 2016; Mongle et al., 2019; Püschel et al., 2021; and Pugh, 2022), that would support its Homo -lineage affinity, and so we refrain from using it to calibrate this node.
Benton et al. (2015) proposed a maximum bound of 10 Ma on this node, given that “a range of ape taxa, Ankarapithecus from Turkey (10 Ma), Gigantopithecus from China (8-0.3 Ma), Lufengopithecus from China (10 Ma), Ouranopithecus from Greece (~9.3 Ma), and Sivapithecus from Pakistan (10-7 Ma) give maximum ages of 10 Ma, early in the late Miocene, and these deposits have yielded no fossils attributable to either chimps or humans.” Importantly, however, all of these taxa are Eurasian not African, and current evidence supports relatively limited dispersal of hominoids out of Africa (Gilbert et al., 2020). In particular, it seems likely that the split between the Homo and Pan lineages occurred in Africa (but see Fuss et al., 2018), with members of the Homo lineage probably not dispersing out of Africa until the early Pleistocene (Trifonov et al., 2019), and members of the Pan lineage apparently never doing so. Thus, the absence of members of the Homo and Pan lineages in ~10 Ma old Eurasian deposits would still be expected even if the split between these lineages had already occurred by this time, and so there seems no compelling reason to use this as a maximum bound on this node. Roos et al. (2019), meanwhile, assumed a maximum bound of 8 Ma based on the putative stem gorillin Chororapithecus (Suwa et al. 2007; Katoh et al. 2016). Dos Reis et al. (2018) also used Chororapithecus to provide a maximum bound on this divergence, but assumed a 10 Ma age for Chororapithecus based on the initial report by Suwa et al. (2007), whereas its age has now been revised down to 7.5-8.33 Ma (Katoh et al., 2016). Regardless, we consider the use of Chororapithecus to inform the maximum bound on this node to be inappropriate, firstly because the phylogenetic position of Chororapithecus was found to be unstable by Pugh (2022), secondly because of the ~12.5 Ma potential Pan relative described by Pickford and Senut (2005), and thirdly because of the overall poor African record of primates 15-6 Ma (Rossie et al., 2013), as already discussed.
Crown Platyrrhini = Pitheciidae-(Aotidae+Atelidae+Callitrichidae+Cebidae) split
Calibrating taxon. Stirtonia victoriae
Specimen. DU/IGM 85-400 (holotype), a right maxilla preserving erupted dP2-dP4 M1-M2, and mineralised but unerupted C1 and P2-P4, from Duke Locality 28, La Venta, Colombia (Kay et al., 1987).
Phylogenetic justification. The total evidence phylogenetic analyses of Beck et al. (2023) identified Stirtonia as the oldest well-supported member of crown Platyrrhini. Stirtonia was strongly supported as sister to Alouatta, within crown Atelidae (Beck et al., 2023). Cebupithecia and Nuciruptor were both also strongly supported as crown platyrrhines (specifically, stem pitheciines) by Beck et al. (2023), and a partial postcranial skeleton (IGM 184667) that might be referable to one or other of these genera is known from Duke Locality 79 at La Venta (Meldrum and Kay, 1990, 1997; Horovitz, 1999), which lies just below the Chunchullo Sandstone in the La Victoria Formation of the Honda Group (Flynn et al., 1997), and so is slightly older than Duke Locality 28 (Montes et al., 2021). However, in the absence of associated dental material that might clarify to which taxon IGM 184667 belongs, we have not used it for calibration purposes here.
One other candidate for the oldest crown platyrrhine is Proteropithecia neuquenensis from the Collón Curá Formation of Argentina, which has been considered by most authors to be a stem pitheciine (Kay et al., 2013; Rosenberger and Tejedor, 2013; Kay, 2015; Tejedor and Novo, 2016; Rosenberger, 2020); however, Beck et al. (2023, table 2) considered the age of this taxon to be poorly constrained to 19.76-10.4 Ma, and so it could potentially be younger than Stirtonia and the other La Venta primates (see Beck et al., 2023, table 2). In addition, several of the phylogenetic analyses of Beck et al. (2023) found Proteropithecia to be unstable, and these authors noted the possibility that this taxon may in fact be a stem platyrrhine. All older fossil platyrrhines, including Panamacebus transitus (which has been found to be a cebid in some other published analyses; Bloch et al., 2016; Kay et al., 2019), were placed outside the crown clade in analyses by Beck et al. (2023).
Hard minimum bound. 13.363 Ma
Soft maximum bound. None
Suggested prior distribution. Not applicable (minimum bound only)
Age justifications. Stirtonia victoriae is currently the oldest Stirtonia species known, with all known material from Duke Locality 28 within the Cerro Gordo Beds of the La Victoria Formation at La Venta (Guerrero, 1997), approximately 290 m below the stratigraphic level from where specimens of the younger S. tatacoensis have been collected (Kay et al., 1987). Guerrero (1993, 1997) and Flynn et al. (1997) indicated that the Cerro Gordo Beds, the overlying Chunchullo Beds, and the underlying San Alfonso Beds all lie within Chron C5ABn (see Montes et al., 2021), which spans from 13.608 to 13.363 Ma (Raffi et al., 2020), with the latter providing our minimum bound.
For less inclusive divergences within crown Platyrrhini (primarily divergences within families; see the calibrations that follow), we have proposed a maximum bound based on the maximum reported age of the oldest probable stem platyrrhine specimens, which is 34.5 Ma (Antoine et al. 2021, see “Pitheciinae” below for a detailed justification of this), although we note that this date has been questioned (Campbell et al., 2021). The ancestor of crown Platyrrhini was probably a very small (~400g), insectivore-frugivore (Lynch Alfaro, 2017; Silvestro, Tejedor, et al., 2019) that is likely to have been little different morphologically from the specimens described by Antoine et al. (2021). This, together with the overall poor record of platyrrhines, means that it is difficult to rule out an early (pre-Oligocene) origin for crown Platyrrhini on fossil grounds alone; for this reason, we do not propose a maximum bound for this calibration.
Additional CladeAge calibration. Stirtonia victoriae is the oldest known atelid (and is, in fact, a crown form, closer to the alouattine Alouatta than to atelines), and so is the oldest known member of the Aotidae+Atelidae+Callitrichidae+Cebidae clade. Ignoring Proteropithecia for the reasons already discussed, the oldest known member of its sister clade, Pitheciidae, is the crown pitheciid Cebupithecia sarmientoi, which is 13.183-13.032 Ma old (see “Crown Pitheciidae” below).
Comments. Benton et al. (2015) did not calibrate this node (or any nodes within Platyrrhini), but dos Reis et al. (2018) proposed a minimum of 15.7 Ma based on the age of Proteropithecia reported by Kay et al. (1998); as noted above, the total evidence analyses by Beck et al. (2023) do not unambiguously support Proteropithecia as a crown platyrrhine. Dos Reis et al.’s (2018) maximum bound was 33 Ma, based on the age of the oldest crown anthropoid, the stem catarrhine Catopithecus (see “Crown Anthropoidea” above). However, if the 34.5 Ma date reported by Antoine et al. (2021) is correct, then the oldest record of probable platyrrhines predates this (although we accept that these are almost certainly stem forms). Overall, we consider the platyrrhine fossil record to be too incomplete to confidently apply a maximum bound on this node.
Crown Pitheciidae = Callicebinae-Pitheciinae split
Calibrating taxon. Cebupithecia sarmientoi
Specimen. UCMP 38762 (holotype), a nearly complete skull, mandible, axial skeleton, and limb bones, from the Monkey Beds at La Venta, Colombia (Stirton and Savage, 1951).
Phylogenetic justification. Numerous synapomorphies support Cebupithecia as a pitheciine (Stirton and Savage, 1951; Orlosky, 1973; Rosenberger, 1979; Kay, 1990), and it has been consistently placed within crown Pitheciidae as a stem pitheciine in phylogenetic analyses (Kay, 2015; Bloch et al., 2016; Marivaux et al., 2016; Ni et al., 2019: fig. S1; Kay et al., 2019). Total evidence phylogenetic analyses by Beck et al. (2023) also support Cebupithecia as a stem pitheciine. A second fossil platyrrhine from La Venta, Nuciruptor rubricae, has also been consistently placed as a stem pitheciine in published phylogenetic analyses (Kay, 2015; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019), including the total evidence analyses of Beck et al. (2023). The oldest definitive material of Nuciruptor is from the El Cardon Red Beds (C5Ar.2r to C5An.2n Guerrero 1993; 1997; Flynn et al. 1997; as summarised by Montes et al. 2021, figure 3; with ages for these chrons of 12.829-12.272 Ma according to Raffi et al. 2020), which are younger than the Monkey Beds (13.183 to 13.032 Ma, see below). As already discussed (see “Crown Platyrrhini” above), IGM 184667 is a partial postcranial skeleton from Duke Locality 79 at La Venta, which may belong to either Cebupithecia or Nuciruptor and which predates the Monkey Beds; however, given the uncertainty regarding its taxonomic assignment, we do not consider this specimen further. Also as discussed (see “Crown Platyrrhini” above), the putative pitheciine Proteropithecia neuquenensis (Kay et al., 2013; Rosenberger and Tejedor, 2013; Kay, 2015; Tejedor and Novo, 2016; Rosenberger, 2020) was not consistently recovered as a member of this subfamily in the total evidence analyses of Beck et al. (2023). Thus, we have not used Proteropithecia to calibrate this node.
Hard minimum bound. 13.032 Ma
Soft maximum bound. 34.5 Ma
Suggested prior distribution. Uniform
Age justifications. The type specimen of Cebupithecia sarmientoi comes from the Monkey Beds at La Venta that correspond to Chron C5AAn (Flynn et al., 1997; Kay and Madden, 1997). This interval spans from 13.183 to 13.032 Ma (Raffi et al., 2020), with the latter date providing our hard minimum bound.
The total evidence tip-dating analysis of Beck et al. (2023) suggest that the most recent common ancestor of crown Platyrrhini is ~21-27 Ma old, but according to this analysis the oldest definitive crown platyrrhines (including Cebupithecia) are some 10-15 Ma younger; all fossil platyrrhines older than ~14 Ma were placed outside the crown. Thus, the early stages of the evolution of crown Platyrrhini appear to be currently unsampled, probably because they occurred in northern South America, where the fossil record for this time period remains poor (although ongoing research is starting to improve this; e.g., Antoine et al., 2012, 2017; Bond et al., 2015; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019). For this reason, we suggest a conservative maximum bound of 34.5 Ma, based on the maximum reported age of the oldest platyrrhine specimens from TAR-21 site, Shapaja, Peru (Antoine et al., 2021). The TAR-21 specimens appear to be highly plesiomorphic, and similar to the better preserved Perupithecus (Antoine et al. 2021; Bond et al. 2015), which has been dated to 29.6 ± 0.08 Ma (Campbell et al., 2021). Campbell et al. (2021) questioned the late Eocene age for TAR-21, and presented tip-dating analyses of fossil rodents suggesting an Oligocene age for this and other Shapaja sites. However, pending confident resolution of this issue, we prefer to take a conservative approach and use the maximum age for TAR-21 reported in the literature (i.e., from Antoine et al., 2021) as the maximum bound of this calibration. Given the obvious incompleteness of the fossil record, we also suggest that this should be modelled as a uniform distribution. We propose the same maximum bound and uniform prior distribution for all other divergences within Platyrrhini.
We note here that, whereas we use the age of TAR-21 reported by Antoine et al. (2021) to inform our soft maximum bound for crown Pitheciidae, we did not use it to inform our alternative CladeAge calibration for crown Anthropoidea (= age of oldest probable platyrrhines; see above), because of the question marks raised by Campbell et al. (2021). This may appear inconsistent, but in fact reflects key differences between “standard” node dating and the CladeAge method. “Standard” node dating uses bounds and associated distributions that are specified a priori as age priors; these may be deliberately chosen to be very broad/conservative to reflect uncertainty in the fossil record, and may not be tied to the ages of specific fossils (for example, a geological boundary might be specified as a maximum bound). By contrast, the CladeAge method uses first occurrence ages of specific fossil taxa and estimates of diversification and fossil sampling rates to calculate prior distributions analytically, without user-specified bounds (Matschiner et al., 2017; Matschiner, 2019). Thus, accurate, and ideally tightly constrained, estimates of the ages of specific fossil occurrences is likely to be important for successful implementation of the CladeAge method. Given the different interpretations of Antoine et al. (2021) and Campbell et al. (2021), it is not clear that the age of TAR-21 can be tightly constrained on current published evidence; thus, it can be used to inform a (deliberately conservative) maximum bound, but is of less use for the CladeAge method. Instead, we used the relatively tightly constrained (and as-yet unchallenged) detrital zircon date published by Campbell et al. (2021) for the Santa Rosa fauna (from which the holotype and only known fossil of the probable early platyrrhine Perupithecus has been recovered) for the alternative CladeAge calibration for crown Anthropoidea.
Additional CladeAge calibration. We recognise Cebupithecia sarmientoi as the oldest stem pitheciine. The fossil taxon Miocallicebus villaviejai has been described as being dentally similar to, but much larger than, Callicebus sensu lato (= the currently recognised modern callicebine genera Callicebus, Plecturocebus, and Cheracebus; Takai et al., 2001; Kay, 2015; Byrne et al., 2016). The only known specimen (IGM-KU 97001) is a partial maxilla preserving only a single fully intact tooth (M2), which is heavily worn, and its affinities have not been tested via formal phylogenetic analysis. Nevertheless, we consider the available evidence sufficient to recognise Miocallicebus as a fossil callicebine, and so we propose it as an additional CladeAge calibration here. IGM-KU 97001 comes from the Bolivia Site at La Venta, which is just above the Tatacoa Beds, towards the top of the La Victoria Formation. According to Guerrero (1993, 1997) and Flynn et al. (1997), the base of the Tatacoa Beds is within Chron C5ABr, whilst the top of the La Victoria Formation is within Chron C5AAn (as summarised by Montes et al. 2021, figure 3). Following Raffi et al. (2020), this gives an age range of 13.739-13.032 Ma for Miocallicebus villaviejai, which we use as our CladeAge calibration for the oldest record of Callicebinae.
Comments. This node was not calibrated by Benton et al. (2015) or by dos Reis et al. (2018).
Callitrichidae-Cebidae split
Calibrating taxon. Lagonimico conclucatus
Specimen. IGM 184531 (holotype), a crushed skull with partial upper dentition present and a near complete mandible with most of the mandibular dentition from Duke University/INGEOMINAS locality 90 in the Victoria Formation, La Venta, Colombia (Kay, 1994).
Phylogenetic justification. Lagonimico shares a number of dental synapomorphies with extant (crown) callitrichids (Kay, 1994: table 7), and recent phylogenetic analyses consistently place it as a stem callitrichid (Kay, 2015; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019). Beck et al. (2023) found a similar result in their tip-dating analysis, with Lagonimico being strongly supported as sister to crown callitrichids, and so it is suitable for calibrating this divergence.
Analyses by Beck et al. (2023) as well as several others (Kay, 2015; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019) suggest that two other taxa from La Venta - Mohanamico hershkovitzi (Luchterhand et al., 1986) and ‘Aotus’ dindensis (originally described as an aotid; Setoguchi and Rosenberger, 1987; see also Ni et al., 2019 fig. S1) - may also be stem callitrichids, but both are from the Monkey Beds, which are stratigraphically younger than Duke University/INGEOMINAS locality 90 (Guerrero, 1993, 1997; Flynn et al., 1997, see below).
Hard minimum bound. 13.183 Ma
Soft maximum bound. 34.5 Ma
Suggested prior distribution. Uniform
Age justifications. Duke University/INGEOMINAS locality 90, source of IGM 184531, is located stratigraphically between the overlying Tatacoa Beds and underlying Chunchullo Beds of the Victoria Formation (Guerrero, 1993, 1997; Kay, 1994, table 7; Flynn et al., 1997). According to Guerrero (1993, 1997) and Flynn et al. (1997), the base of the Tatacoa Beds is within Chron C5AAr, whilst the Chunchullo Beds are entirely within Chron C5ABn, as are the Cerro Gordo Beds that underlie them (see Montes et al., 2021). Thus, Duke University/INGEOMINAS Locality 90 must be younger than the base of Chron C5ABn, and older than the top of Chron C5AAr, which gives an age range of 13.608 to 13.183 Ma (Raffi et al., 2020), with the latter providing our minimum bound.
Given the poor fossil record of crown Platyrrhin, we once again suggest a conservative maximum bound of 34.5 Ma for this node, based on the maximum reported age of the oldest probable platyrrhine specimens described to date (Antoine et al., 2021), and a uniform prior distribution (see “Crown Pitheciidae” above).
Additional CladeAge calibration. Lagonimico conclucatus is the oldest known callitrichid. The sister taxon of Callitrichidae is either Cebidae, Aotidae, or Cebidae+Aotidae, as the precise relationships of Aotus have proven difficult to resolve (Osterholz et al., 2009; Perez et al., 2012; Valencia et al., 2018; Schrago and Seuánez 2019; Wang et al., 2019; Vanderpool et al., 2020; Beck et al., 2023). Considering the fossil record of cebids and aotids together, the oldest well-supported representative of either family is Neosaimiri, which has been consistently proposed to be closely related to the modern Saimiri from its original description by Stirton (1951) and onwards (e.g., Rosenberger et al., 1991; Takai, 1994). Congruent with this, recent phylogenetic analyses support Neosaimiri as a crown cebid, sister to Saimiri (Kay, 2015; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019; Beck et al., 2023). The oldest Neosaimiri material, including the holotype UCMP 39205, comes from the Monkey Beds in the Villavieja Formation (see “Crown Cebidae” below), which ranges from 13.183 to 13.032 Ma (see “Crown Pitheciidae” above), and this age range provides our additional CladeAge calibration.
Comments. This node was not calibrated by Benton et al. (2015) or by dos Reis et al. (2018).
Kay (2015) discussed records of two possible crown callitrichids from La Venta: an isolated upper incisor (IGM-KU 8402) and a lower fourth premolar (IGM-KU 8403) from the Monkey Beds that Setoguchi and Rosenberger (1985) tentatively referred to Micodon kiotensis (see also Rosenberger et al., 1990); and the holotype of Patasola magdalenae (IGM 184332), a partial lower jaw preserving the deciduous premolars and the molars from the stratigraphically older Duke Locality 40 (Kay and Meldrum, 1997), which is in the interval between the overlying Chunchullo Beds and underlying Cerro Gordo beds (and so appears to fall within Chron C5ABn; Guerrero, 1993; 1997; Flynn et al., 1997; as summarised by Montes et al., 2021, figure 3). However, as discussed by Setoguchi and Rosenberger (1985), Rosenberger et al. (1990), and Kay and Meldrum (1997), the evidence for referring GM-KU 8402 and 8403 to Callitrichidae (whether stem or crown) is weak, and this proposed relationship has not been tested via formal phylogenetic analysis. By contrast, Kay and Meldrum (1997) did formally test the relationships of Patasola via maximum parsimony analysis of 55 dental characters and found that it fell within crown Callitrichidae. However, the overall topology for extant callitrichids recovered by Kay and Meldrum (1997) is highly incongruent with molecular data (e.g., Garbino and Martins-Junior, 2018. Given this incongruence, which is likely to have a major impact on the polarities of the dental characters used by Kay and Meldrum (1997) to place Patasola, we do not consider the phylogenetic analysis of Kay and Meldrum (1997) to be strong evidence that Patasola is a crown callitrichid and this age range provides our additional CladeAge calibration.
Crown Cebidae = Cebinae-Saimirinae split
Calibrating taxon. Neosaimiri fieldsi
Specimen. UCMP 39205 (holotype), comprising a left hemi-mandible preserving p2-m2, and a right hemi-mandible preserving i2-m2, from UCMP locality V4517 in the Monkey Beds of the Villavieja Formation at La Venta, Colombia (Stirton, 1951).
Phylogenetic justification. As already discussed (see “Callitrichidae-Cebidae split” above), Neosaimiri has been consistently identified as a close relative of the extant saimirine genus Saimiri since its original description. Indeed, Rosenberger et al. (1991) concluded that Neosaimiri could be synonymised with Saimiri, although Takai (1994) argued that the two genera should be maintained as distinct. Regardless, a close relationship between Neosaimiri and Saimiri to the exclusion of Cebus, within crown Cebidae, has been a consistent feature of recent published phylogenetic analyses (Kay, 2015; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019; Beck et al., 2023). The ~20.9 Ma old Panamacebus transitus has also been placed within crown Cebidae in some published phylogenetic analyses (Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019), but it fell outside crown Platyrrhini in the total evidence tip-dating analysis of Beck et al. (2023), and we do not use this taxon for calibrating purposes here.
Hard minimum bound. 13.032 Ma
Soft maximum bound. 34.5 Ma
Suggested prior distribution. Uniform
Age justifications. As already discussed (see “Crown Pitheciidae” above), the age of the Monkey Beds at La Venta can be constrained to between 13.183 and 13.032 Ma. We propose the same maximum bound and uniform prior distribution as for crown Pitheciidae and the Callitrichidae-Cebidae split (see above).
Additional CladeAge calibration. As discussed, we consider Neosaimiri fieldsi to be the oldest well-supported saimirine. If Panamacebus is discounted, the oldest well-supported member of Cebinae is Acrecebus fraileyi (Kay and Cozzuol, 2006), which has been placed sister to Cebus in most recent published phylogenetic analyses (Kay, 2015; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019; Beck et al., 2023). Acrecebus fraileyi is known from a specimen, LACM 134880 (a left M2) from locality LACM 5158 (“Bandeira”), Solimoes Formation, Acre River, Acre, Brazil. The age of the Acre vertebrate fauna of the Solimoes Formation has been controversial and remains poorly constrained (Cozzuol, 2006). The Patos locality, which is near the Bandeira locality (Negri et al., 2010), has recently been proposed to be no older than 7 Ma based on palynological data (Leite et al., 2021). However, in the absence of more precise stratigraphic evidence, we follow Kay and Cozzuol (2006) in assigning the Acre vertebrate fauna to the Huayquerian SALMA. Following the age justification outlined in Beck et al. (2023) and below, we apply a conservatively wide estimate of 9.0-4.741 Ma to the Huayquerian SALMA, which is to be used as the CladeAge calibration for this node. Age estimates for the Huayquerian SALMA include 9.0-5.28 Ma (Prevosti et al., 2013; Tomassini et al., 2013) and 9.0-6.8 Ma (Flynn and Swisher, 1995). However, Prevosti et al. (2021) reported an 40Ar/39Ar date from the lower “Irenean” fauna at Quequén Salado River - which shows similarities to Huayquerian faunas - of 5.17 +/- 0.08 Ma, i.e., younger than previously proposed minimum bounds for the Huayquerian. We therefore use a more conservative minimum bound of 4.741 Ma based on the median maximum age of the Monte Hermoso fauna (Prevosti et al., 2021), which is the type fauna of the Montehermosan SALMA that follows the Huayquerian.
Comments. This node was not calibrated by Benton et al. (2015), but dos Reis et al. (2018) also used Neosaimiri to provide a minimum bound for this node. In addition, dos Reis et al. (2018) specified a maximum bound of 18 Ma based on Soriacebus, which they considered to be the oldest known atelid. It is not clear to us how the oldest record of Atelidae would directly inform the likely maximum age of crown Cebidae, and dos Reis et al. (2018) acknowledged that Cebus-Saimiri split might in fact be as old as 20-21 Ma, based in part on the age of Panamacebus, which has been found to be a crown cebid in several published analyses (Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019). However, numerous phylogenetic analyses place Soriacebus as a stem platyrrhine (Kay, 2015; Marivaux et al., 2016; Kay et al., 2019; Beck et al., 2023), and Panamacebus was also placed outside crown Platyrrhini in the total evidence tip-dating analysis of Beck et al. (2023). Thus, neither Soriacebus nor Panamacebus are of direct relevance for informing the maximum bound of this node. In any case, we consider that the very limited platyrrhine record means that a much more conservative maximum bound is appropriate for divergences within crown Platyrrhini (see “Crown Pitheciidae” above).
Crown Atelidae = Alouattinae-Atelinae split
Calibrating taxon. Stirtonia victoriae
Specimen. DU/IGM 85-400 (holotype), a right maxilla preserving erupted dP2-dP4 M1-M2, and mineralised but unerupted C and P2-P4, from Duke Locality 28, La Venta, Colombia (Kay et al., 1987).
Phylogenetic justification. Several authors have noted that Stirtonia shares numerous dental similarities (at least some of them derived) with the modern genus Alouatta (Stirton, 1951; Rosenberger, 1979; Setoguchi et al., 1981; Kay and Cozzuol, 2006), and a Stirtonia+Alouatta clade has been recovered in several recent phylogenetic analyses (Kay and Cozzuol, 2006; Bloch et al., 2016; Marivaux et al., 2016; Kay et al., 2019; Beck et al., 2023).
Hard minimum bound. 13.363 Ma
Soft maximum bound. 34.5 Ma
Suggested prior distribution. Uniform
Age justifications. See “Crown Platyrrhini” above for discussion of the age of Stirtonia victoriae.
Additional CladeAge calibration. Stirtonia victoriae is the oldest known alouattine. Kay and Cozzuol (2006) named Solimoea acrensis based on an isolated left m1 (the holotype, UFAC-LPP 5177) and an isolated right maxillary fragment preserving P3-4 (UFAC-LPP 5178) from the Patos locality (equivalent to LACM 4611) in the Solimoes Formation and identified it as an ateline. They also carried out a four different maximum parsimony analyses based on 57 dental characters (although only 25 of these were parsimony informative; Kay and Cozzuol, 2006, table 1) and using a molecular scaffold that was based on the studies of Meireles et al. (1999 a; 1999 b) but which is still in agreement with current molecular evidence (e.g., dos Reis et al., 2018): in all four analyses, Solimoea formed a clade with living atelines, with moderate-to-high (57-86%) bootstrap support depending on the analysis. Kay (2015) subsequently stated that he considered Solimoea to be specifically related to Lagothrix within crown Atelinae, although Kay and Cozzuol (2006) found that Solimoea fell outside crown Atelinae in three out of four of their phylogenetic analyses. Rosenberger et al. (2015) argued that Solimoea is more likely an alouattine, and cast doubt on whether the holotype m1 and the referred maxillary fragment represent the same taxon. However, in the absence of formal phylogenetic analysis supporting alternative relationships for Solimoea, we tentatively accept it as the oldest known ateline. Based on palynological evidence, the Patos locality is 7 Ma or younger (Leite et al., 2021); given that we accept a Huayquerian age for the Acre vertebrate fauna as a whole (see “Crown Cebidae” above) to which we apply a conservatively wide estimate of 9-4.741 Ma, we assign Solimoea acrensis an age range of 7-4.471 Ma for our additional CladeAge calibration.
Comments. This node was not calibrated by Benton et al. (2015), but dos Reis et al. (2018) used Stirtonia as the basis for a minimum bound of 12.8 Ma for this node. In addition, dos Reis et al. (2018) specified a maximum bound of 18 Ma, based on the assumption that the “divergence of atelids is unlikely to have occurred before the first appearance of the potential stem or crown atelid Soriacebus at 18 Ma” (dos Reis et al., 2018: 611). However, as already noted (see “Crown Cebidae”), several recent phylogenetic analyses have placed Soriacebus as a stem platyrrhine rather than an atelid (Kay, 2015; Marivaux et al., 2016; Kay et al., 2019; Beck et al., 2023), and so it is inappropriate to inform a maximum bound on this node. For reasons already discussed, given the extremely limited platyrrhine fossil record, we prefer a much more conservative maximum bound for divergences within Platyrrhini, based on the maximum proposed age for the oldest known platyrrhines (34.5 Ma; Antoine et al., 2021; see “Crown Pitheciidae” above for a full justification of this).
CONCLUSION
Marjanović (2021) noted that compendia of fossil calibrations quickly go out of date, due both to the discovery of new fossils and to reinterpretation and reanalysis of those already known. However, the impact of this on analyses that need to use fossil calibration information can (we hope) be lessened by careful consideration of the appropriate prior distribution for each calibrated node, to adequately reflect our current uncertainty and to take into account the likely impact of future discoveries. For example, it is certainly possible, or even probable, that crown primates that are slightly older than Teilhardina brandti will be discovered, but we think it highly unlikely that they will be found earlier than the K-Pg boundary, an assumption that is taken into account by our suggested prior distribution on the age of crown Primates. Thus, we expect that improvements in the primate fossil record will lead to tighter constraints on the ages of particular nodes (mainly due to older minimum bounds), but not ones that actively conflict with those proposed here. In turn, revisions to this list should lead to more precise, but not contradictory, estimates of divergence times in future node-dating analyses.
ACKNOWLEDGEMENTS
We thank the following people for discussion, providing references, and/or providing comments on a previous version of this manuscript: D. Alba, P.-O. Antoine, O. Bertrand, J. Boubli, L. Flynn, S. Frost, I. Goodhead, M. Janiak, R. Kay, L. Marivaux, M. Matschiner, M. Morgan, K. Pugh, C. Roos, E. Seiffert, and three anonymous reviewers. Funding for this paper was provided by NERC Standard Grant "Rise of the Continent of the Monkeys” (NE/T000341/1).
REFERENCES
Ahern, J.C.M. 2018. Sahelanthropus, p. 1-6. In Trevathan, W. (ed.), The International Encyclopedia of Biological Anthropology. John Wiley & Sons, Hoboken, New Jersey, USA. https://doi.org/10.1002/9781118584538.ieba0431
Alba, D.M. 2012. Fossil apes from the Vallès-Penedès Basin. Evolutionary Anthropology, 21:254-269. https://doi.org/10.1002/evan.21312
Alba, D.M., Delson, E., Carnevale, G., Colombero, S., Delfino, M., Giuntelli, P., Pavia, M., and Pavia, G. 2014a. New cercopithecid remains from Moncucco Torinese and the taxonomic identity of the earliest papionins from Europe, p. 4. XII EAVP Meeting. Abstract Book.
Alba, D.M., Delson, E., Carnevale, G., Colombero, S., Delfino, M., Giuntelli, P., Pavia, M., and Pavia, G. 2014b. First joint record of Mesopithecus and cf. Macaca in the Miocene of Europe. Journal of Human Evolution, 67:1-18. https://doi.org/10.1016/j.jhevol.2013.11.001
Alba, D.M., Montoya, P., Pina, M., Rook, L., Abella, J., Morales, J., and Delson, E. 2015. First record of Mesopithecus (Cercopithecidae, Colobinae) from the Miocene of the Iberian Peninsula. Journal of Human Evolution, 88:1-14. https://doi.org/10.1016/j.jhevol.2015.08.003
Allentoft, M.E., Collins, M., Harker, D., Haile, J., Oskam, C.L., Hale, M.L., Campos, P.F., Samaniego, J.A., Gilbert, M.T.P., Willerslev, E., Zhang, G., Scofield, R.P., Holdaway, R.N., and Bunce, M. 2012. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proceedings of the Royal Society B: Biological Sciences, 279:4724-4733. https://doi.org/10.1098/rspb.2012.1745
Almécija, S., Hammond, A.S., Thompson, N.E., Pugh, K.D., Moyà-Solà, S., and Alba, D.M. 2021. Fossil apes and human evolution. Science, 372:eabb4363. https://doi.org/10.1126/science.abb4363
Álvarez-Carretero, S., Tamuri, A.U., Battini, M., Nascimento, F.F., Carlisle, E., Asher, R.J., Yang, Z., Donoghue, P.C.J., and dos Reis, M. 2022. A species-level timeline of mammal evolution integrating phylogenomic data. Nature, 602:263-267.
https://doi.org/10.1038/s41586-021-04341-1
Antoine, P.-O., Marivaux, L., Croft, D.A., Billet, G., Ganerød, M., Jaramillo, C., Martin, T., Orliac, M.J., Tejada, J., Altamirano, A.J., Duranthon, F., Fanjat, G., Rousse, S., and Gismondi, R.S. 2012. Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography. Proceedings of the Royal Society B: Biological Sciences, 279:1319-1326. https://doi.org/10.1098/rspb.2011.1732
Antoine, P.-O., Salas-Gismondi, R., Pujos, F., Ganerød, M., and Marivaux, L. 2017. Western Amazonia as a hotspot of mammalian biodiversity throughout the Cenozoic. Journal of Mammalian Evolution, 24:5-17. https://doi.org/10.1007/s10914-016-9333-1
Antoine, P.-O., Yans, J., Castillo, A.A., Stutz, N., Abello, M.A., Adnet, S., Custódio, M.A., Benites-Palomino, A., Billet, G., Boivin, M., Herrera, F., Jaramillo, C., Mártinez, C., Moreno, F., Navarrete, R.E., Negri, F.R., Parra, F., Pujos, F., Rage, J.-C., Ribeiro, A.M., Robinet, C., Roddaz, M., Tejada-Lara, J.V., Varas-Malca, R., Ventura Santos, R., Salas-Gismondi, R., and Marivaux, L. 2021. Biotic community and landscape changes around the Eocene-Oligocene transition at Shapaja, Peruvian Amazonia: Regional or global drivers? Global and Planetary Change, 202:1-20. https://doi.org/10.1016/j.gloplacha.2021.103512
Arambourg, C. 1959. Vertébrés continentaux du Miocène supérieur de l’Afrique du Nord. Publications Du Service de La Carte Geologique de l’Algerie (Paleontologie Memoire), 4:5-159.
Archibald, J.D., Zhang, Y., Harper, T., and Cifelli, R.L. 2011. Protungulatum, confirmed Cretaceous occurrence of an otherwise Paleocene eutherian (placental?) mammal. Journal of Mammalian Evolution, 18:153-161. https://doi.org/10.1007/s10914-011-9162-1
Asher, R.J., Smith, M.R., Rankin, A., and Emry, R.J. 2019. Congruence, fossils and the evolutionary tree of rodents and lagomorphs. Royal Society Open Science, 6:190387. https://doi.org/10.1098/rsos.190387
Bajpai, S., Kay, R.F., Williams, B.A., Das, D.P., Kapur, V.V., and Tiwari, B.N. 2008. The oldest Asian record of Anthropoidea. Proceedings of the National Academy of Sciences of the United States of America, 105:11093-11098. https://doi.org/10.1073/pnas.0804159105
Beard, K.C. 2008. The oldest North American primate and mammalian biogeography during the Paleocene-Eocene Thermal Maximum. Proceedings of the National Academy of Sciences of the United States of America, 105:3815-3818. https://doi.org/10.1073/pnas.0710180105
Beard, K.C. and Wang, J. 2004. The eosimiid primates (Anthropoidea) of the Heti Formation, Yuanqu Basin, Shanxi and Henan Provinces, People’s Republic of China. Journal of Human Evolution, 46:401-432. https://doi.org/10.1016/j.jhevol.2004.01.002
Beard, K.C., Qi, T., Dawson, M.R., Wang, B., and Li, C. 1994. A diverse new primate fauna from middle Eocene fissure-fillings in southeastern China. Nature, 368:604-609. https://doi.org/10.1038/368604a0
Beauvilain, A. 2008. The contexts of discovery of Australopithecus bahrelghazali (Abel) and of Sahelanthropus tchadensis (Toumaï): unearthed, embedded in sandstone, or surface collected?: commentary. South African Journal of Science, 104:165-168.
Beck, R.M.D., de Vries, D., Janiak, M.C., Goodhead, I.B., and Boubli, J.P. 2023. Total evidence phylogeny of platyrrhine primates and a comparison of undated and tip-dating approaches. Journal of Human Evolution, 174:103293. https://doi.org/10.1016/j.jhevol.2022.103293
Begun, D.R. 2015. Fossil record of Miocene hominoids, p. 921-977. In Henke, W. and Tattersall, I. (eds.), Handbook of Paleoanthropology (Volume 2). Springer Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-27800-6
Begun, D.R., Nargolwalla, M.C. and Kordos, L. 2012. European Miocene hominids and the origin of the African ape and human clade. Evolutionary Anthropology, 21:10-23. https://doi.org/10.1002/evan.20329
Benoit, J. and Thackeray, F.J. 2017. A cladistic analysis of Graecopithecus. South African Journal of Science, 113:1-2. https://doi.org/10.17159/sajs.2017/a0238
Benton, M.J. and Donoghue, P.C.J. 2007. Paleontological evidence to date the tree of life. Molecular Biology and Evolution, 24:26-53. https://doi.org/10.1093/molbev/msl150
Benton, M.J., Donoghue, P.C.J., and Asher, R.J. 2009. Calibrating and constraining molecular clocks, p. 35-86. In Hedges, S.B. and Kumar, S. (eds.), The Timetree of Life. Oxford University Press, Oxford.
Benton, M.J., Donoghue, P.C.J., Asher, R.J., Friedman, M., Near, T.J., and Vinther, J. 2015. Constraints on the timescale of animal evolutionary history. Palaeontologia Electronica, 18.1.1FC:1-106. https://doi.org/10.26879/424
Bi, S., Zheng, X., Wang, X., Cignetti, N.E., Yang, S., and Wible, J.R. 2018. An Early Cretaceous eutherian and the placental-marsupial dichotomy. Nature, 558:390-395. https://doi.org/10.1038/s41586-018-0210-3
Billet, G. and Bardin, J. 2019. Serial homology and correlated characters in morphological phylogenetics: Modeling the evolution of dental crests in placentals. Systematic Biology, 68:267-280. https://doi.org/10.1093/sysbio/syy071
Bininda-Emonds, O.R., Beck, R.M.D., and MacPhee, R.D.E. 2012. Rocking clocks and clocking rocks: a critical look at divergence time estimation in mammals, p. 38-82. In Asher, R.J. and Müller, J. (eds.), From Clone to Bone: The Synergy of Morphological and Molecular Tools in Palaeobiology. Cambridge University Press, Cambridge.
Bishop, W.W., Miller, J.A., and Fitch, F.J. 1969. New potassium-argon age determinations relevant to the Miocene fossil mammal sequence in east Africa. American Journal of Science, 267:669-699. https://doi.org/10.2475/ajs.267.6.669
Blair Hedges, S. and Kumar, S. 2009. The Timetree of Life. Oxford University Press, Oxford.
Bloch, J.I., Silcox, M.T., Boyer, D.M., and Sargis, E.J. 2007. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proceedings of the National Academy of Sciences of the United States of America, 104:1159-1164. https://doi.org/10.1073/pnas.0610579104
Bloch, J.I., Woodruff, E.D., Wood, A.R., Rincon, A.F., Harrington, A.R., Morgan, G.S., Foster, D.A., Montes, C., Jaramillo, C.A., Jud, N.A., Jones, D.S., and MacFadden, B.J. 2016. First North American fossil monkey and early Miocene tropical biotic interchange. Nature, 533:243-246. https://doi.org/10.1038/nature17415
Bond, M., Tejedor, M.F., Campbell, K.E., Jr, Chornogubsky, L., Novo, N., and Goin, F. 2015. Eocene primates of South America and the African origins of New World monkeys. Nature, 525:552. https://doi.org/10.1038/nature14955
Bown, T.M. and Rose, K.D. 1987. Patterns of dental evolution in early Eocene anaptomorphine primates (Omomyidae) from the Bighorn Basin, Wyoming. Paleontological Society Memoir, 23:1-162. https://doi.org/10.1017/S0022336000060911
Boyer, D.M., Seiffert, E.R., and Simons, E.L. 2010. Astragalar morphology of Afradapis, a large adapiform primate from the earliest late Eocene of Egypt. American Journal of Physical Anthropology, 143:383-402. https://doi.org/10.1002/ajpa.21328
Boyer, D.M., Toussaint, S., and Godinot, M. 2017. Postcrania of the most primitive euprimate and implications for primate origins. Journal of Human Evolution, 111:202-215. https://doi.org/10.1016/j.jhevol.2017.07.005
Brown, F.H. and McDougall, I. 2011. Geochronology of the Turkana depression of northern Kenya and southern Ethiopia. Evolutionary Anthropology, 20:217-227. https://doi.org/10.1002/evan.20318
Brunet, M., Guy, F., Pilbeam, D., Mackaye, H.T., Likius, A., Ahounta, D., Beauvilain, A., Blondel, C., Bocherens, H., Boisserie, J.-R., De Bonis, L., Coppens, Y., Dejax, J., Denys, C., Duringer, P., Eisenmann, V., Fanone, G., Fronty, P., Geraads, D., Lehmann, T., Lihoreau, F., Louchart, A., Mahamat, A., Merceron, G., Mouchelin, G., Otero, O., Pelaez Campomanes, P., Ponce De Leon, M., Rage, J.-C., Sapanet, M., Schuster, M., Sudre, J., Tassy, P., Valentin, X., Vignaud, P., Viriot, L., Zazzo, A., and Zollikofer, C. 2002. A new hominid from the Upper Miocene of Chad, Central Africa. Nature, 418:145-151. https://doi.org/10.1038/nature00879
Budd, G.E. and Mann, R.P. 2022. Two notorious nodes: a critical examination of MCMCTree relaxed molecular clock estimates of the bilaterian animals and placental mammals. bioRxiv preprint. https://doi.org/10.1101/2022.07.01.498494
Burney, D.A., Vasey, N., Godfrey, L.R., Ramilisonina, Jungers, W.L., Ramarolahy, M.F., and Raharivony, L.L. 2008. New findings at Andrahomana Cave, southeastern Madagascar. Journal of Cave and Karst Studies, 70:13-24.
Byrne, H., Rylands, A.B., Carneiro, J.C., Alfaro, J.W.L., Bertuol, F., da Silva, M.N.F., Messias, M., Groves, C.P., Mittermeier, R.A., Farias, I., Hrbek, T., Schneider, H., Sampaio, I., and Boubli, J.P. 2016. Phylogenetic relationships of the New World titi monkeys (Callicebus): first appraisal of taxonomy based on molecular evidence. Frontiers in Zoology 13:10.
https://doi.org/10.1186/s12983-016-0142-4
Byron, C.D. 2001. Hard tissue evidence for Asian colobine phylogeny. American Association of Physical Anthropologists Journal of Cave and Karst Studies, 70:13-24.
Campbell, K.E., Jr, O’Sullivan, P.B., Fleagle, J.G., de Vries, D., and Seiffert, E.R. 2021. An Early Oligocene age for the oldest known monkeys and rodents of South America. Proceedings of the National Academy of Sciences of the United States of America, 118:e2105956118. https://doi.org/10.1073/pnas.2105956118
Casanovas-Vilar, I., Alba, D.M., Garcés, M., Robles, J.M., and Moyà-Solà, S. 2011. Updated chronology for the Miocene hominoid radiation in Western Eurasia. Proceedings of the National Academy of Sciences of the United States of America, 108:5554-5559. https://doi.org/10.1073/pnas.1018562108
Chester, S.G.B., Bloch, J.I., Boyer, D.M., and Clemens, W.A. 2015. Oldest known euarchontan tarsals and affinities of Paleocene Purgatorius to Primates. Proceedings of the National Academy of Sciences of the United States of America, 112:1487-1492. https://doi.org/10.1073/pnas.1421707112
Chester, S.G.B., Williamson, T.E., Bloch, J.I., Silcox, M.T., and Sargis, E.J. 2017. Oldest skeleton of a plesiadapiform provides additional evidence for an exclusively arboreal radiation of stem primates in the Palaeocene. Royal Society Open Science, 4:170329. https://doi.org/10.1098/rsos.170329
Claramunt, S. 2022. CladeDate: Calibration information generator for divergence time estimation. Methods in Ecology and Evolution 13:2331-2338. https://doi.org/10.1111/2041-210x.13977
Cohen, J.E. and Cifelli, R.L. 2015. The first eutherian mammals from the early Late Cretaceous of North America, p. 108. Society of Vertebrate Palaeontology. Program and Abstracts.
Cote, S., Kingston, J., Deino, A., Winkler, A., Kityo, R., and MacLatchy, L. 2018. Evidence for rapid faunal change in the early Miocene of East Africa based on revised biostratigraphic and radiometric dating of Bukwa, Uganda. Journal of Human Evolution, 116:95-107. https://doi.org/10.1016/j.jhevol.2017.12.001
Cozzuol, M.A. 2006. The Acre vertebrate fauna: Age, diversity, and geography. Journal of South American Earth Sciences, 21:185-203. https://doi.org/10.1016/j.jsames.2006.03.005
Dashzeveg, D. and Russell, D.E. 1988. Paleocene and Eocene Mixodontia (Mammalia, Glires) of Mongolia and China. Palaeontology, 31:129-164.
de Bonis, L., Bouvrain, G., Geraads, D., and Koufos, G. 1990. New remains of Mesopithecus (Primates, Cercopithecoidea) from the late Miocene of Macedonia (Greece), with the description of a new species. Journal of Vertebrate Paleontology, 10:473-483. https://doi.org/10.1080/02724634.1990.10011830
Deino, A.L. and McBrearty, S. 2002. 40Ar/39Ar dating of the Kapthurin Formation, Baringo, Kenya. Journal of Human Evolution, 42:185-210. https://doi.org/10.1006/jhev.2001.0517
Deino, A.L., Tauxe, L., Monaghan, M., and Hill, A. 2002. 40Ar/39Ar geochronology and paleomagnetic stratigraphy of the Lukeino and lower Chemeron Formations at Tabarin and Kapcheberek, Tugen Hills, Kenya. Journal of Human Evolution, 42:117-140. https://doi.org/10.1006/jhev.2001.0521
Delson, E. 1975. Evolutionary history of the Cercopithecidae, pp. 5, 167-217. In Szalay, F.S. (ed.), Approaches to Primate Paleobiology. S. Karger, Basel.
Delson, E. 1980. Fossil macaques, phyletic relationships and a scenario of deployment, p. 10-30. In Lindburg, D.G. (ed.), The Macaques: Studies in Ecology, Behavior and Evolution. Van Nostrand Reinhold, New York.
Dembo, M., Matzke, N.J., Mooers, A.Ø., and Collard, M. 2015. Bayesian analysis of a morphological supermatrix sheds light on controversial fossil hominin relationships. Proceedings of the Royal Society B: Biological Sciences, 282:20150943. https://doi.org/10.1098/rspb.2015.0943
Dembo, M., Radovčić, D., Garvin, H.M., Laird, M.F., Schroeder, L., Scott, J.E., Brophy, J., Ackermann, R.R., Musiba, C.M., de Ruiter, D.J., Mooers, A.Ø., and Collard, M. 2016. The evolutionary relationships and age of Homo naledi: An assessment using dated Bayesian phylogenetic methods. Journal of Human Evolution, 97:17-26. https://doi.org/10.1016/j.jhevol.2016.04.008
Doronina, L., Reising, O., Clawson, H., Churakov, G., and Schmitz, J. 2022. Euarchontoglires challenged by incomplete lineage sorting. Genes, 13:1-9.
dos Reis, M., Gunnell, G.F., Barba-Montoya, J., Wilkins, A., Yang, Z., and Yoder, A.D. 2018. Using phylogenomic data to explore the effects of relaxed clocks and calibration strategies on divergence time estimation: Primates as a test case. Systematic Biology, 67:594-615. https://doi.org/10.1093/sysbio/syy001
Emonet, E.-G., Andossa, L., Taïsso Mackaye, H., and Brunet, M. 2014. Subocclusal dental morphology of Sahelanthropus tchadensis and the evolution of teeth in hominins. American Journal of Physical Anthropology, 153:116-123. https://doi.org/10.1002/ajpa.22400
Estravís, C. 2000. Nuevos mamíferos del Eoceno Inferior de Silveirinha (Baixo Mondego, Portugal). Coloquios de Paleontología, 51:281-311.
Flynn, J.J. and Swisher, C.C., III. 1995. Cenozoic South American land mammal ages: correlation to global geochronologies. Geochronology Time Scales and Global Stratigraphic Correlation, Special Publications of the Society of Sedimentary Geology (SEPM), 54:317-333.
Flynn, J.J., Guerrero, J., and Swisher, C.C. 1997. Geochronology of the Honda group, p. 44-59. In Kay, R.F., Madden, R.H., Cifelli, R.L., and Flynn, J.J. (eds.), Vertebrate Paleontology in the Neotropics: The Miocene Fauna of La Venta, Colombia. Smithsonian Press, Washington, D.C.
Flynn, L.J. and Morgan, M.E. 2005. New lower primates from the Miocene Siwaliks of Pakistan, p. 81-102. In Lieberman, D., Smith, R.W., and Kelley, J. (eds.), Interpreting the Past: Essays on Human, Primate, and Mammal Evolution in Honor of David Pilbeam. Brill Academic Publishers, Boston.
Foley, N.M., Springer, M.S., and Teeling, E.C. 2016. Mammal madness: is the mammal tree of life not yet resolved? Philosophical Transactions of the Royal Society B: Biological Sciences, 371:1-11. https://doi.org/10.1098/rstb.2015.0140
Fostowicz-Frelik, Ł. 2017. Convergent and parallel evolution in early Glires (Mammalia), p. 199- 216. In Pontarotti, P. (ed.), Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolution, Methods and Concepts. Springer, New York.
Fostowicz-Frelik, Ł. 2020. Most successful mammals in the making: A review of the Paleocene Glires, p. 99-116. In Pontarotti, P. (ed.), Evolutionary Biology – A Transdisciplinary Approach. Springer International Publishing, Cham.
Frost, S.R., Gilbert, C.C., Pugh, K.D., Guthrie, E.H., and Delson, E. 2015. The hand of Cercopithecoides williamsi (Mammalia, Primates): Earliest evidence for thumb reduction among colobine monkeys. PLoS ONE, 10:1-17. https://doi.org/10.1371/journal.pone.0125030
Frost, S.R., Ward, C.V., Manthi, F.K., and Plavcan, J.M. 2020. Cercopithecid fossils from Kanapoi, West Turkana, Kenya (2007-2015). Journal of Human Evolution, 140:1-16. https://doi.org/10.1016/j.jhevol.2019.102642
Fuss, J., Spassov, N., Böhme, M., and Begun, D. 2018. Response to Benoit and Thackeray (2017): “A cladistic analysis of Graecopithecus ”. South African Journal of Science, 114:1-2. https://doi.org/10.17159/sajs.2018/a0267
Garbino, G.S.T. and Martins-Junior, A.M.G. 2018. Phenotypic evolution in marmoset and tamarin monkeys (Cebidae, Callitrichinae) and a revised genus-level classification. Molecular Phylogenetics and Evolution, 118:156-171. https://doi.org/10.1016/j.ympev.2017.10.002
Gavryushkina, A., Welch, D., Stadler, T., and Drummond, A.J. 2014. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Computational Biology, 10:e1003919. https://doi.org/10.1371/journal.pcbi.1003919
Gebo, D.L., Dagosto, M., Beard, K.C., and Ni, X. 2017. Cuboid morphology of a basal anthropoid from the Eocene of China. Journal of Human Evolution, 102:72-74. https://doi.org/10.1016/j.jhevol.2016.10.003
Gheerbrant, E. 1998. The oldest known proboscidean and the role of Africa in the radiation of modern orders of placentals. Bulletin of the Geological Society of Denmark, 44:181-185.
Gilbert, C.C. 2013. Cladistic analysis of extant and fossil African papionins using craniodental data. Journal of Human Evolution, 64:399-433. https://doi.org/10.1016/j.jhevol.2013.01.013
Gilbert, C.C., Bibi, F., Hill, A., and Beech, M.J. 2014. Early guenon from the late Miocene Baynunah Formation, Abu Dhabi, with implications for cercopithecoid biogeography and evolution. Proceedings of the National Academy of Sciences of the United States of America, 111:10119-10124. https://doi.org/10.1073/pnas.1323888111
Gilbert, C.C., Goble, E.D., and Hill, A. 2010. Miocene Cercopithecoidea from the Tugen Hills, Kenya. Journal of Human Evolution, 59:465-483. https://doi.org/10.1016/j.jhevol.2010.05.005
Gilbert, C.C., Ortiz, A., Pugh, K.D., Campisano, C.J., Patel, B.A., Singh, N.P., Fleagle, J.G. and Patnaik, R. 2020. New Middle Miocene Ape (Primates: Hylobatidae) from Ramnagar, India fills major gaps in the hominoid fossil record. Proceedings of the Royal Society B: Biological Sciences, 287:20201655. https://doi.org/10.1098/rspb.2020.1655
Gingerich, P.D. 1993a. Early Eocene Teilhardina brandti: oldest omomyid primate from North America. Contributions from the Museum of Paleontology, the University of Michigan, 28:321-326.
Gingerich, P.D. 1993b. Oligocene age of the Gebel Qatrani Formation, Fayum, Egypt. Journal of Human Evolution, 24:207-218. https://doi.org/10.1006/jhev.1993.1015
Godfrey, L.R., Jungers, W.L., and Burney, D.A. 2010. Subfossil lemurs of Madagascar, p. 351-367. In Werdelin, L. and Sanders, W.J. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley.
Godfrey, L.R., Samonds, K.E., Baldwin, J.W., Sutherland, M.R., Kamilar, J.M., and Allfisher, K.L. 2020. Mid-Cenozoic climate change, extinction, and faunal turnover in Madagascar, and their bearing on the evolution of lemurs. BMC Evolutionary Biology, 20:97. https://doi.org/10.1186/s12862-020-01628-1
Godinot, M. 1978. Un nouvel adapidé (primate) de L’éocène inférieur de Provence. Comptes Rendus de l’Académie Des Sciences de Paris, Série D, 286:1869-1872.
Godinot, M. 1994. Early North African primates and their significance for the origin of Simiiformes (= Anthropoidea), p. 235-295. In Fleagle, J.G. and Kay, R.F. (eds.), Anthropoid Origins. Springer US, Boston. https://doi.org/10.1007/978-1-4757-9197-6_10
Godinot, M. 1998. A summary of adapiform systematics and phylogeny. Folia Primatologica, 69:218-249. https://doi.org/10.1159/000052715
Godinot, M. 2006. Lemuriform origins as viewed from the fossil record. Folia Primatologica, 77:446-464. https://doi.org/10.1159/000095391
Godinot, M. 2015. Fossil record of the primates from the Paleocene to the Oligocene, p. 1137-1259. In Henke, W. and Tattersall, I. (eds.), Handbook of Paleoanthropology. Springer, Heidelberg. https://doi.org/10.1007/978-3-642-39979-4_68
Godinot, M. 2017. Paleocene and Eocene Primates, p. 1-9. In Fuentes, A. (ed.), The International Encyclopedia of Primatology. John Wiley & Sons, Hoboken, New Jersey, USA. https://doi.org/10.1002/9781119179313.wbprim0331
Godinot, M., Senut, B., and Pickford, M. 2018. Primitive Adapidae from Namibia sheds light on the early primate radiation in Africa. Communications of the Geological Survey of Namibia, 18:140-162.
Goswami, A., Prasad, G.V.R., Upchurch, P., Boyer, D.M., Seiffert, E.R., Verma, O., Gheerbrant, E., and Flynn, J.J. 2011. A radiation of arboreal basal eutherian mammals beginning in the Late Cretaceous of India. Proceedings of the National Academy of Sciences of the United States of America, 108:16333-16338. https://doi.org/10.1073/pnas.1108723108
Gremyatskii, M.A. 1961. The main line of higher primate evolution in the Neogene. Voprosy Antropologii, 7:3-8. (In Russian)
Guerrero, J. 1993. Geology-paleomagnetism: Magnetostratigraphy of the upper part of the Honda Group and Neiva Formation. Miocene uplift of the Colombian Andes. PhD Thesis, Duke University, Durham, North Carolina, USA.
Guerrero, J. 1997. Stratigraphy, sedimentary environments, and the Miocene uplift of the Colombian Andes, p. 15-43. In Kay, R.F., Madden, R.H., Cifelli, R.L., and Flynn, J.J. (eds.), Vertebrate Paleontology in the Neotropics: The Miocene Fauna of La Venta, Colombia. Smithsonian Institution Press, Washington, D.C.
Guillerme, T. and Cooper, N. 2016a. Effects of missing data on topological inference using a Total Evidence approach. Molecular Phylogenetics and Evolution, 94:146-158. https://doi.org/10.1016/j.ympev.2015.08.023
Guillerme, T. and Cooper, N. 2016b. Assessment of available anatomical characters for linking living mammals to fossil taxa in phylogenetic analyses. Biology Letters, 12:20151003. https://doi.org/10.1098/rsbl.2015.1003
Gunnell, G.F., Boyer, D.M., Friscia, A.R., Heritage, S., Manthi, F.K., Miller, E.R., Sallam, H.M., Simmons, N.B., Stevens, N.J., and Seiffert, E.R. 2018. Fossil lemurs from Egypt and Kenya suggest an African origin for Madagascar’s aye-aye. Nature Communications, 9:3193. https://doi.org/10.1038/s41467-018-05648-w
Gunnell, G.F. and Miller, E.R. 2018. Anthropoid origins, p. 1-5. In Trevathan, W. (ed.), The International Encyclopedia of Biological Anthropology. John Wiley & Sons, Hoboken, New Jersey, USA. https://doi.org/10.1002/9781118584538.ieba0026
Haile-Selassie, Y. 2001. Late Miocene hominids from the Middle Awash, Ethiopia. Nature, 412:178-181. https://doi.org/10.1038/35084063
Haile-Selassie, Y., Suwa, G., and White, T.D. 2004. Late Miocene teeth from Middle Awash, Ethiopia, and early hominid dental evolution. Science, 303:1503-1505. https://doi.org/10.1126/science.1092978
Harrison, T. 2010a. Later Tertiary Lorisiformes (Strepsirrhini, Primates), p. 333-349. In Werdelin, L. and Sanders, W.J. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley. https://doi.org/10.1525/california/9780520257214.003.0020
Harrison, T. 2010b. Dendropithecoidea, Proconsuloidea, and Hominoidea, p. 429-470. In Werdelin, L. and Sanders, W.J. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley. https://doi.org/10.1525/california/9780520257214.003.0024
Harrison, T. 2010c. Anthropology. Apes among the tangled branches of human origins. Science, 327:532-34. https://doi.org/10.1126/science.1184703
Harrison, T. 2011. Cercopithecids (Cercopithecidae, Primates), p. 83-139. In Harrison, T. (ed.), Paleontology and Geology of Laetoli: Human Evolution in Context. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9962-4_6
Harrison, T. 2017. Miocene Primates, p. 1-5. In Fuentes, A. (ed.), The International Encyclopedia of Primatology. John Wiley & Sons, Hoboken, New Jersey, USA. https://doi.org/10.1002/9781119179313.wbprim0227
Hasegawa, M., Kishino, H., and Yano, T. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 22:160-174. https://doi.org/10.1007/BF02101694
Heath, T.A. and Huelsenbeck, J.P. 2014. The fossilized birth-death process for coherent calibration of divergence-time estimates. Proceedings of the National Academy of Sciences of the United States of America, 111:E2957-E2966. https://doi.org/10.1073/pnas.1319091111
Herrera, J.P. 2017. Primate diversification inferred from phylogenies and fossils. Evolution, 71:2845-2857. https://doi.org/10.1111/evo.13366
Herrera, J.P. and Dávalos, L.M. 2016. Phylogeny and divergence times of lemurs inferred with recent and ancient fossils in the tree. Systematic Biology, 65:772-791. https://doi.org/10.1093/sysbio/syw035
Hill, A., Leakey, M., Kingston, J.D., and Ward, S. 2002. New cercopithecoids and a hominoid from 12.5 Ma in the Tugen Hills succession, Kenya. Journal of Human Evolution, 42:75-93. https://doi.org/10.1006/jhev.2001.0518
Hooker, J.J., Russell, D.E., and Phélizon, A. 1999. A new family of Plesiadapiformes (Mammalia) from the Old World Lower Paleogene. Palaeontology, 42:377-407. https://doi.org/10.1111/1475-4983.00078
Horovitz, I. 1999. A phylogenetic study of living and fossil platyrrhines. American Museum Novitates, 3269:1-40.
Ho, S.Y.W. (ed.). 2021. The Molecular Evolutionary Clock: Theory and Practice. Springer Nature, Switzerland. https://doi.org/10.1007/978-3-030-60181-2
Ho, S.Y.W. and Phillips, M.J. 2009. Accounting for calibration uncertainty in phylogenetic estimation of evolutionary divergence times. Systematic Biology, 58:367-380. https://doi.org/10.1093/sysbio/syp035
Jablonski, N.G. 1998. The evolution of the doucs and snub-nosed monkeys and the question of the phyletic unity of the odd-nosed colobines, p. 13-52. In Jablonski, N.G. (ed.), The Natural History of the Doucs and Snub-Nosed Monkeys. World Scientific Publishing, Singapore. https://doi.org/10.1142/9789812817020_0002
Jablonski, N.G. and Frost, S.R. 2010. Cercopithecoidea, p. 393-428. In Werdelin, L. and Sanders, W.J. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley. https://doi.org/10.1525/california/9780520257214.003.0023
Jablonski, N.G., Ji, X., Kelley, J., Flynn, L.J., Deng, C., and Su, D.F. 2020. Mesopithecus pentelicus from Zhaotong, China, the easternmost representative of a widespread Miocene cercopithecoid species. Journal of Human Evolution, 146:102851. https://doi.org/10.1016/j.jhevol.2020.102851
Jacobs, L.L. 1981. Miocene lorisid primates from the Pakistan Siwaliks. Nature, 289:585-587. https://doi.org/10.1038/289585a0
Jaeger, J.-J., Chavasseau, O., Lazzari, V., Naing Soe, A., Sein, C., Le Maître, A., Shwe, H., and Chaimanee, Y. 2019. New Eocene primate from Myanmar shares dental characters with African Eocene crown anthropoids. Nature Communications, 10:3531. https://doi.org/10.1038/s41467-019-11295-6
Jaeger, J.-J., Sein, C., Gebo, D.L., Chaimanee, Y., Nyein, M.T., Oo, T.Z., Aung, M.M., Suraprasit, K., Rugbumrung, M., Lazzari, V., Soe, A.N., and Chavasseau, O. 2020. Amphipithecine primates are stem anthropoids: cranial and postcranial evidence. Proceedings of the Royal Society B: Biological Sciences, 287:20202129. https://doi.org/10.1098/rspb.2020.2129
Jameson, N.M., Hou, Z.-C., Sterner, K.N., Weckle, A., Goodman, M., Steiper, M.E., and Wildman, D.E. 2011. Genomic data reject the hypothesis of a prosimian primate clade. Journal of Human Evolution, 61:295-305. https://doi.org/10.1016/j.jhevol.2011.04.004
Ji, X., Harrison, T., Zhang, Y., Wu, Y., Zhang, C., Hu, J., Wu, D., Hou, Y., Li, S., Wang, G., and Wang, Z. 2022. The earliest hylobatid from the Late Miocene of China. Journal of Human Evolution, 171:103251. https://doi.org/10.1016/j.jhevol.2022.103251
Ji, Q., Luo, Z.-X., Yuan, C.-X., Wible, J.R., Zhang, J.-P., and Georgi, J.A. 2002. The earliest known eutherian mammal. Nature, 416:816-822. https://doi.org/10.1038/416816a
Kappelman, J., Kelley, J., Pilbeam, D., Sheikh, K.A., Ward, S., Anwar, M., Barry, J.C., Brown, B., Hake, P., Johnson, N.M., Raza, S.M., and Shah, S.M.I. 1991. The earliest occurrence of Sivapithecus from the middle Miocene Chinji Formation of Pakistan. Journal of Human Evolution, 21:61-73. https://doi.org/10.1016/0047-2484(91)90036-U
Kappelman, J., Rasmussen, D.T., Sanders, W.J., Feseha, M., Bown, T., Copeland, P., Crabaugh, J., Fleagle, J., Glantz, M., Gordon, A., Jacobs, B., Maga, M., Muldoon, K., Pan, A., Pyne, L., Richmond, B., Ryan, T., Seiffert, E.R., Sen, S., Todd, L., Wiemann, M.C., and Winkler, A. 2003. Oligocene mammals from Ethiopia and faunal exchange between Afro-Arabia and Eurasia. Nature, 426:549-552. https://doi.org/10.1038/nature02102
Kappelman, J., Simons, E.L., and Swisher, C.C. 1992. New age determinations for the Eocene-Oligocene boundary sediments in the Fayum Depression, Northern Egypt. The Journal of Geology, 100:647-667. https://doi.org/10.1086/629619
Katoh, S., Beyene, Y., Itaya, T., Hyodo, H., Hyodo, M., Yagi, K., Gouzu, C., WoldeGabriel, G., Hart, W.K., Ambrose, S.H., Nakaya, H., Bernor, R.L., Boisserie, J.-R., Bibi, F., Saegusa, H., Sasaki, T., Sano, K., Asfaw, B., and Suwa, G. 2016. New geological and palaeontological age constraint for the gorilla-human lineage split. Nature, 530:215-218. https://doi.org/10.1038/nature16510
Kay, R.F. 1990. The phyletic relationships of extant and fossil Pitheciinae (Platyrrhini, Anthropoidea), p. 175-208. In Fleagle, J.G. and Rosenberger, A.L. (eds.), The Platyrrhine Fossil Record. Academic Press, Cambridge, Massachusetts. https://doi.org/10.1016/B978-0-12-260345-7.50011-4
Kay, R.F. 1994. “Giant” tamarin from the Miocene of Colombia. American Journal of Physical Anthropology, 95:333-353. https://doi.org/10.1002/ajpa.1330950305
Kay, R.F. 2015. Biogeography in deep time - What do phylogenetics, geology, and paleoclimate tell us about early platyrrhine evolution? Molecular Phylogenetics and Evolution, 82:358-374. https://doi.org/10.1016/j.ympev.2013.12.002
Kay, R.F. and Cozzuol, M.A. 2006. New platyrrhine monkeys from the Solimões Formation (late Miocene, Acre State, Brazil). Journal of Human Evolution, 50:673-686. https://doi.org/10.1016/j.jhevol.2006.01.002
Kay, R.F. and Meldrum, D.J. 1997. A new small platyrrhine and the phyletic position of Callitrichinae, p. 435-458. In Kay, R.F., Madden, R.H., Cifelli, R.L., and Flynn, J.J. (eds.), Vertebrate Paleontology in the Neotropics: The Miocene Fauna of La Venta, Colombia. Smithsonian Institution Press, Washington, D.C.
Kay, R.F. and Madden, R.H. 1997. Mammals and rainfall: paleoecology of the middle Miocene at La Venta (Colombia, South America). Journal of Human Evolution, 32:161-199. https://doi.org/10.1006/jhev.1996.0104
Kay, R.F., Gonzales, L.A., Salenbien, W., Martinez, J.-N., Cooke, S.B., Valdivia, L.A., Rigsby, C., and Baker, P.A. 2019. Parvimico materdei gen. et sp. nov.: A new platyrrhine from the Early Miocene of the Amazon Basin, Peru. Journal of Human Evolution, 134:102628. https://doi.org/10.1016/j.jhevol.2019.05.016
Kay, R.F., Johnson, D., and Meldrum, D.J. 1998. A new pitheciine primate from the middle Miocene of Argentina. American Journal of Primatology, 45:317-336. https://doi.org/10.1002/(SICI)1098-2345(1998)45:4<317::AID-AJP1>3.0.CO;2-Z
Kay, R.F., Madden, R.H., Plavcan, J.M., Cifelli, R.L., and Díaz, J.G. 1987. Stirtonia victoriae, a new species of Miocene Colombian primate. Journal of Human Evolution, 16:173-196. Elsevier. https://doi.org/10.1016/0047-2484(87)90075-3
Kay, R.F., Meldrum, D.J., and Takai, M. 2013. Pitheciidae and other platyrrhine seed predators, p. 3-12. In Veiga, L.M., Barnett, A.A., Ferrari, S.F., and Norconk, M.A. (eds.), Evolutionary Biology and Conservation of Titis, Sakis and Uacaris. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9781139034210.005
Kelley, J., Andrews, P., and Alpagut, B. 2008. A new hominoid species from the middle Miocene site of Paşalar, Turkey. Journal of Human Evolution, 54:455-479. https://doi.org/10.1016/j.jhevol.2007.08.007
Khin Zaw, Meffre, S., Takai, M., Suzuki, H., Burrett, C., Thaung Htike, Zin Maung Maung Thein, Tsubamoto, T., Egi, N., and Maung Maung. 2014. The oldest anthropoid primates in SE Asia: Evidence from LA-ICP-MS U-Pb zircon age in the Late Middle Eocene Pondaung Formation, Myanmar. Gondwana Research, 26:122-131. https://doi.org/10.1016/j.gr.2013.04.007
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z.-X. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. Columbia University Press, New York. https://doi.org/10.7312/kiel11918
Koufos, G.D. 2009. The Neogene cercopithecids (Mammalia, Primates) of Greece. Geodiversitas, 31:817-850. https://doi.org/10.5252/g2009n4a817
Koufos, G.D. 2016. Primates. Geobios, 49:45-51. https://doi.org/10.1016/j.geobios.2016.01.008
Koufos, G.D. 2019. Late Turolian Mesopithecus (Mammalia: Cercopithecidae) from Axios Valley (Macedonia, Greece): earliest presence of M. monspessulanus in Europe. Comptes Rendus Palevol, 18:1057-1072. https://doi.org/10.1016/j.crpv.2019.07.002
Kunimatsu, Y., Nakatsukasa, M., Sawada, Y., Sakai, T., Hyodo, M., Hyodo, H., Itaya, T., Nakaya, H., Saegusa, H., Mazurier, A., Saneyoshi, M., Tsujikawa, H., Yamamoto, A., and Mbua, E. 2007. A new Late Miocene great ape from Kenya and its implications for the origins of African great apes and humans. Proceedings of the National Academy of Sciences of the United States of America, 104:19220-19225. https://doi.org/10.1073/pnas.0706190104
Leakey, L.S.B. 1961. A new Lower Pliocene fossil primate from Kenya. Annals and Magazine of Natural History, 4:689-696. https://doi.org/10.1080/00222936108651194
Leakey, L.S.B. 1962. Primates, p. 1-18. In Bishop, W.W. (ed.), The Mammalian Fauna and Geomorphological Relations of the Napak Volcanics, Karamoja: Primates. Records of the Geological Survey of Uganda, Kampala.
Leakey, M.G., Teaford, M.F., and Ward, C.V. 2003. Cercopithecidae from Lothagam, p. 201-248. In Leakey, M.G. and Harris, J.M. (eds.), Lothagam: The Dawn of Humanity in Eastern Africa. Columbia University Press, New York.
Lebatard, A.-E., Bourlès, D.L., Duringer, P., Jolivet, M., Braucher, R., Carcaillet, J., Schuster, M., Arnaud, N., Monié, P., Lihoreau, F., Likius, A., Mackaye, H.T., Vignaud, P., and Brunet, M. 2008. Cosmogenic nuclide dating of Sahelanthropus tchadensis and Australopithecus bahrelghazali: Mio-Pliocene hominids from Chad. Proceedings of the National Academy of Sciences of the United States of America, 105:3226-3231. https://doi.org/10.1073/pnas.0708015105
Lee, M.S.Y. 2020. Clock models for evolution of discrete phenotypic characters, p. 101-113. In Ho, S.Y.W. (ed.), The Molecular Evolutionary Clock: Theory and Practice. Springer Nature, Switzerland. https://doi.org/10.1007/978-3-030-60181-2_7
Le Gros Clark, E.W. 1956. A Miocene lemuroid skull from East Africa. Fossil Mammals of Africa, 9:1-6.
Le Gros Clark, W. and Thomas, D.P. 1952. The Miocene lemuroids of East Africa. Fossil Mammals of Africa, 5:1-20.
Leite, F.P.R., Silva-Caminha, S.A.F. da, and D’Apolito, C. 2021. New Neogene index pollen and spore taxa from the Solimões Basin (Western Amazonia), Brazil. Palynology, 45:115-141. https://doi.org/10.1080/01916122.2020.1758971
Lemmon, A.R., Brown, J.M., Stanger-Hall, K., and Lemmon, E.M. 2009. The effect of ambiguous data on phylogenetic estimates obtained by maximum likelihood and Bayesian inference. Systematic Biology, 58:130-145. https://doi.org/10.1093/sysbio/syp017
Lewis, P.O. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology, 50:913-925. https://doi.org/10.1080/106351501753462876
Li, C. 1977. Paleocene eurymyloids (Anagalida, Mammalia) of Qianshan, Anhui. Vertebrata PalAsiatica, 15:103-118.
Li, C.K. and Ting, S.Y. 1993. New cranial and postcranial evidence for the affinities of the eurymylids (Rodentia) and mimotonids (Lagomorpha), p. 151-158. In Szalay, F.S., Novacek, M.J., and McKenna, M.C. (eds.), Mammal Phylogeny. Springer, Berlin.
Li, C.K., Wang, Y.Q., Zhang, Z.Q., Mao, F.-Y., and Meng, J. 2016. A new mimotonidan Mina hui (Mammalia, Glires) from the Middle Paleocene of Qianshan, Anhui, China. Vertebrata PalAsiatica, 54:121-136.
Li, Q. and Ni, X. 2016. An early Oligocene fossil demonstrates treeshrews are slowly evolving “living fossils”. Scientific Reports, 6:18627. https://doi.org/10.1038/srep18627
Locke, E.M., Benefit, B.R., Kimock, C.M., Miller, E.R., and Nengo, I. 2020. New dentognathic fossils of Noropithecus bulukensis (Primates, Victoriapithecidae) from the late Early Miocene of Buluk, Kenya. Journal of Human Evolution, 148:102886. https://doi.org/10.1016/j.jhevol.2020.102886
López-Torres, S. and Fostowicz-Frelik, Ł. 2018. A new Eocene anagalid (Mammalia: Euarchontoglires) from Mongolia and its implications for the group’s phylogeny and dispersal. Scientific Reports, 8:13955. https://doi.org/10.1038/s41598-018-32086-x
López-Torres, S. and Silcox, M.T. 2018. New omomyoids (Euprimates, Mammalia) from the late Uintan of southern California, USA, and the question of the extinction of the Paromomyidae (Plesiadapiformes, Primates). Palaeontologia Electronica, 21.3.37A:1-28. https://doi.org/10.26879/756
López-Torres, S. and Silcox, M.T. 2020. What we know (and don’t know) about the fossil records of lorisids, p. 33-46. In Nekaris, K.A.I. and Burrows, A.M. (eds.), Evolution, Ecology and Conservation of Lorises and Pottos. Cambridge university Press, Cambridge. https://doi.org/10.1017/9781108676526.005
López-Torres, S., Selig, K.R., Burrows, A.M., and Silcox, M.T. 2020. The toothcomb of Karanisia clarki, p. 67-75. In Nekaris, K.A.I. and Burrows, A.M. (eds.), Evolution, Ecology and Conservation of Lorises and Pottos. Cambridge University Press, Cambridge. https://doi.org/10.1017/9781108676526.008
Luchterhand, K., Kay, R.F., and Madden, R.H. 1986. Mohanamico hershkovitzi, gen. et sp. nov., un primate du Miocène moyen d’Amérique du Sud. Comptes Rendus de l’Académie Des Sciences. Série 2, 303:1753-1758.
Luo, Z.-X., Yuan, C.-X., Meng, Q.-J., and Ji, Q. 2011. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature, 476:442-445. https://doi.org/10.1038/nature10291
Lynch Alfaro, J. 2017. The monkeying of the Americas: primate biogeography in the Neotropics. Annual Review of Anthropology, 46:317-336. Annual Reviews. https://doi.org/10.1146/annurev-anthro-102116-041510
MacLatchy, L.M., DeSilva, J., Sanders, W.J., and Wood, B. 2010. Hominini, p. 471-540. In Werdelin, L. and Sanders, W.J. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley. https://doi.org/10.1525/california/9780520257214.003.0025
MacPhee, R.D.E., Cartmill, M., and Rose, K.D. 1989. Craniodental morphology and relationships of the supposed Eocene dermopteran Plagiomene (Mammalia). Journal of Vertebrate Paleontology, 9:329-349. https://doi.org/10.1080/02724634.1989.10011766
MacPhee, R.D.E. and Jacobs, L.L. 1986. Nycticeboides simpsoni and the morphology, adaptations, and relationships of Miocene Siwalik Lorisidae. Rocky Mountain Geology, 24:131-161. https://doi.org/10.2113/gsrocky.24.special_paper_3.131
Marivaux, L. 2006. The eosimiid and amphipithecid primates (Anthropoidea) from the Oligocene of the Bugti Hills (Balochistan, Pakistan): new insight into early higher primate evolution in South Asia. Palaeovertebrata, 34:29-109.
Marivaux, L., Adnet, S., Altamirano-Sierra, A.J., Boivin, M., Pujos, F., Ramdarshan, A., Salas-Gismondi, R., Tejada-Lara, J.V., and Antoine, P.-O. 2016. Neotropics provide insights into the emergence of New World monkeys: New dental evidence from the late Oligocene of Peruvian Amazonia. Journal of Human Evolution, 97:159-175. https://doi.org/10.1016/j.jhevol.2016.05.011
Marivaux, L., Antoine, P.-O., Baqri, S.R.H., Benammi, M., Chaimanee, Y., Crochet, J.-Y., de Franceschi, D., Iqbal, N., Jaeger, J.-J., Métais, G., Roohi, G., and Welcomme, J.-L. 2005. Anthropoid primates from the Oligocene of Pakistan (Bugti Hills): data on early anthropoid evolution and biogeography. Proceedings of the National Academy of Sciences of the United States of America, 102:8436-8441. https://doi.org/10.1073/pnas.0503469102
Marivaux, L., Bocat, L., Chaimanee, Y., Jaeger, J.-J., Marandat, B., Srisuk, P., Tafforeau, P., Yamee, C., and Welcomme, J.-L. 2006. Cynocephalid dermopterans from the Palaeogene of South Asia (Thailand, Myanmar and Pakistan): systematic, evolutionary and palaeobiogeographic implications. Zoologica Scripta, 35:395-420. https://doi.org/10.1111/j.1463-6409.2006.00235.x
Marivaux, L., Chaimanee, Y., Tafforeau, P., and Jaeger, J.-J. 2006. New strepsirrhine primate from the late Eocene of Peninsular Thailand (Krabi Basin). American Journal of Physical Anthropology, 130:425-434. https://doi.org/10.1002/ajpa.20376
Marivaux, L., Ramdarshan, A., Essid, E.M., Marzougui, W., Khayati Ammar, H., Lebrun, R., Marandat, B., Merzeraud, G., Tabuce, R., and Vianey-Liaud, M. 2013. Djebelemur, a tiny pre- tooth-combed primate from the Eocene of Tunisia: a glimpse into the origin of crown strepsirhines. PLoS ONE, 8:e80778. https://doi.org/10.1371/journal.pone.0080778
Marivaux, L., Welcomme, J.L., Antoine, P.O., Métais, G., Baloch, I.M., Benammi, M., Chaimanee, Y., Ducrocq, S., and Jaeger, J.J. 2001. A fossil lemur from the Oligocene of Pakistan. Science, 294:587-591. https://doi.org/10.1126/science.1065257
Marjanović, D. 2021. The making of calibration sausage exemplified by recalibrating the transcriptomic timetree of jawed vertebrates. Frontiers in Genetics, 12:521693. https://doi.org/10.3389/fgene.2021.521693
Marshall, C.R. 2008. A simple method for bracketing absolute divergence times on molecular phylogenies using multiple fossil calibration points. The American Naturalist, 171:726-742. https://doi.org/10.1086/587523
Mason, V.C., Li, G., Minx, P., Schmitz, J., Churakov, G., Doronina, L., Melin, A.D., Dominy, N.J., Lim, N.T.-L., Springer, M.S., Wilson, R.K., Warren, W.C., Helgen, K.M., and Murphy, W.J. 2016. Genomic analysis reveals hidden biodiversity within colugos, the sister group to primates. Science Advances, 2:e1600633. https://doi.org/10.1126/sciadv.1600633
Matschiner, M. 2019. Selective sampling of species and fossils influences age estimates under the fossilized birth-death model. Frontiers in Genetics, 10:1064. https://doi.org/10.3389/fgene.2019.01064
Matschiner, M., Musilová, Z., Barth, J.M.I., Starostová, Z., Salzburger, W., Steel, M., and Bouckaert, R. 2017. Bayesian phylogenetic estimation of clade ages supports trans-Atlantic dispersal of cichlid fishes. Systematic Biology, 66:3-22. https://doi.org/10.1093/sysbio/syw076
McBrearty, S. and Jablonski, N.G. 2005. First fossil chimpanzee. Nature, 437:105-108. https://doi.org/10.1038/nature04008
McDougall, I. and Feibel, C.S. 2003. Numerical age control for the Miocene-Pliocene succession at Lothagam, a hominoid-bearing sequence in the northern Kenya Rift, p. 43- 64. In Leakey, M. and Harris, J. (eds.), Lothagam. Columbia University Press, New York.
Mein, P. and Ginsburg, L. 1997. Les mammifères du gisement miocène inférieur de Li Mae Long, Thaïlande: systématique, biostratigraphie et paléoenvironnement. Geodiversitas, 19:783-844.
Meireles, C.M., Czelusniak, J., Ferrari, S.F., Schneider, M.P.C., and Goodman, M. 1999. Phylogenetic relationships among Brazilian howler monkeys, genus Alouatta (Platyrrhini, Atelidae), based on γ1-globin pseudogene sequences. Genetics and Molecular Biology, 22:337-344. https://doi.org/10.1590/s1415-47571999000300009
Meireles, C.M., Czelusniak, J., Schneider, M.P.C., Muniz, J.A.P.C., Brigido, M.C., Ferreira, H.S., and Goodman, M. 1999. Molecular phylogeny of ateline New World monkeys (Platyrrhini, Atelinae) based on γ-globin gene sequences: evidence that Brachyteles is the sister group of Lagothrix. Molecular Phylogenetics and Evolution, 12:10-30.
https://doi.org/10.1006/mpev.1998.0574
Meldrum, J.D. and Kay, R.F. 1990. A new partial skeleton of Cebupithecia sarmientoi from the Miocene of Colombia. American Journal of Physical Anthropology, 81:267.
Meldrum, D.J. and Kay, R.F. 1997. Nuciruptor rubricae, a new pitheciin seed predator from the Miocene of Colombia. American Journal of Physical Anthropology, 102:407-427. https://doi.org/10.1002/(SICI)1096-8644(199703)102:3<407::AID-AJPA8>3.0.CO;2-R
Meng, J., Hu, Y., and Li, C. 2003. The osteology of Rhombomylus (Mammalia, Glires): implications for phylogeny and evolution of Glires. Bulletin of the American Museum of Natural History, 275:1-247. https://doi.org/10.1206/0003-0090(2003)275<0001:TOORMG>2.0.CO;2
Meredith, R.W., Janečka, J.E., Gatesy, J., Ryder, O.A., Fisher, C.A., Teeling, E.C., Goodbla, A., Eizirik, E., Simão, T.L.L., Stadler, T., Rabosky, D.L., Honeycutt, R.L., Flynn, J.J., Ingram, C.M., Steiner, C., Williams, T.L., Robinson, T.J., Burk-Herrick, A., Westerman, M., Ayoub, N.A., Springer, M.S., and Murphy, W.J. 2011. Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science, 334:521-524. https://doi.org/10.1126/science.1211028
Métais, G., Guo, J., and Beard, K.C. 2004. A new small dichobunid artiodactyl from Shanghuang (middle Eocene, eastern China): implications for the early evolution of proto-selenodonts in Asia. Carbon, 2004:177-197. https://doi.org/10.2992/0145-9058(2004)36[177:ANSDAF]2.0.CO;2
Millar, C.D. and Lambert, D.M. 2013. Ancient DNA: Towards a million-year-old genome. Nature, 499:34-35. https://doi.org/10.1038/nature12263
Miller, E.R., Benefit, B.R., McCrossin, M.L., Plavcan, J.M., Leakey, M.G., El-Barkooky, A.N., Hamdan, M.A., Abdel Gawad, M.K., Hassan, S.M., and Simons, E.L. 2009. Systematics of early and middle Miocene Old World monkeys. Journal of Human Evolution, 57:195-211. https://doi.org/10.1016/j.jhevol.2009.06.006
Mongle, C.S., Strait, D.S., and Grine, F.E. 2019. Expanded character sampling underscores phylogenetic stability of Ardipithecus ramidus as a basal hominin. Journal of Human Evolution, 131:28-39. https://doi.org/10.1016/j.jhevol.2019.03.006
Montes, C., Silva, C.A., Bayona, G.A., Villamil, R., Stiles, E., Rodriguez-Corcho, A.F., Beltran- Triviño, A., Lamus, F., Muñoz-Granados, M.D., Pérez-Angel, L.C., Hoyos, N., Gomez, S., Galeano, J.J., Romero, E., Baquero, M., Cardenas-Rozo, A.L., and von Quadt, A. 2021. A Middle to Late Miocene trans-Andean portal: Geologic record in the Tatacoa Desert. Frontiers in Earth Science, 8:643. https://doi.org/10.3389/feart.2020.587022
Morgan, M.E., Lewton, K.L., Kelley, J., Otárola-Castillo, E., Barry, J.C., Flynn, L.J., and Pilbeam, D. 2015. A partial hominoid innominate from the Miocene of Pakistan: description and preliminary analyses. Proceedings of the National Academy of Sciences of the United States of America, 112:82-87. https://doi.org/10.1073/pnas.1420275111
Morse, P.E., Chester, S.G.B., Boyer, D.M., Smith, T., Smith, R., Gigase, P., and Bloch, J.I. 2019. New fossils, systematics, and biogeography of the oldest known crown primate Teilhardina from the earliest Eocene of Asia, Europe, and North America. Journal of Human Evolution, 128:103-131. https://doi.org/10.1016/j.jhevol.2018.08.005
Nakatsukasa, M., Mbua, E., Sawada, Y., Sakai, T., Nakaya, H., Yano, W., and Kunimatsu, Y. 2010. Earliest colobine skeletons from Nakali, Kenya. American Journal of Physical Anthropology, 143:365-382. https://doi.org/10.1002/ajpa.21327
Negri, F.R., Bocquentin-Villanueva, J., Ferigolo, J., and Antoine, P.-O. 2010. A review of Tertiary mammal faunas and birds from western Amazonia, p. 243-258. In Hoorn, C. and Wesselingh, F.P. (eds.), Amazonia: Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell, Hoboken, New Jersey, USA.
Nengo, I., Tafforeau, P., Gilbert, C.C., Fleagle, J.G., Miller, E.R., Feibel, C., Fox, D.L., Feinberg, J., Pugh, K.D., Berruyer, C., Mana, S., Engle, Z., and Spoor, F. 2017. New infant cranium from the African Miocene sheds light on ape evolution. Nature, 548:169-174. https://doi.org/10.1038/nature23456
Nguyen, J.M.T. and Ho, S.Y.W. 2020. Calibrations from the fossil record, p. 117-133. In Ho, S.Y.W. (ed.), The Molecular Evolutionary Clock: Theory and Practice. Springer Nature, Switzerland. https://doi.org/10.1007/978-3-030-60181-2_8
Ni, X. and Qiu, Z. 2012. Tupaiine tree shrews (Scandentia, Mammalia) from the Yuanmou Lufengpithecus locality of Yunnan, China. Swiss Journal of Palaeontology, 131:51-60. https://doi.org/10.1007/s13358-011-0029-0
Ni, X., Flynn, J.J., Wyss, A.R., and Zhang, C. 2019. Cranial endocast of a stem platyrrhine primate and ancestral brain conditions in anthropoids. Science Advances, 5:eaav7913. https://doi.org/10.1126/sciadv.aav7913
Ni, X., Gebo, D.L., Dagosto, M., Meng, J., Tafforeau, P., Flynn, J.J., and Beard, K.C. 2013. The oldest known primate skeleton and early haplorhine evolution. Nature, 498:60-64. https://doi.org/10.1038/nature12200
Ni, X., Li, Q., Li, L., and Beard, K.C. 2016. Oligocene primates from China reveal divergence between African and Asian primate evolution. Science, 352:673-677. https://doi.org/10.1126/science.aaf2107
Ni, X., Li, Q., Zhang, C., Samiullah, K., Zhang, L., Yang, Y., and Cao, W. 2020. Paleogene mammalian fauna exchanges and the paleogeographic pattern in Asia. Science China Earth Sciences, 63:202-211. https://doi.org/10.1007/s11430-019-9479-1
Nowak, M.D., Smith, A.B., Simpson, C., and Zwickl, D.J. 2013. A simple method for estimating informative node age priors for the fossil calibration of molecular divergence time analyses. PLoS ONE, 8:e66245. https://doi.org/10.1371/journal.pone.0066245
O’Leary, M.A., Bloch, J.I., Flynn, J.J., Gaudin, T.J., Giallombardo, A., Giannini, N.P., Goldberg, S.L., Kraatz, B.P., Luo, Z.-X., Meng, J., Ni, X., Novacek, M.J., Perini, F.A., Randall, Z.S., Rougier, G.W., Sargis, E.J., Silcox, M.T., Simmons, N.B., Spaulding, M., Velazco, P.M., Weksler, M., Wible, J.R., and Cirranello, A.L. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science, 339:662-667. https://doi.org/10.1126/science.1229237
O’Reilly, J.E., dos Reis, M., and Donoghue, P.C.J. 2015. Dating tips for divergence-time estimation. Trends in Genetics, 31:637-650. https://doi.org/10.1016/j.tig.2015.08.001
Orlosky, F.J. 1973. Comparative dental morphology of extant and extinct Cebidae. PhD thesis, University of Washington, Washington, USA.
Osterholz, M., Walter, L., and Roos, C. 2009. Retropositional events consolidate the branching order among New World monkey genera. Molecular Phylogenetics and Evolution, 50:507-513. https://doi.org/10.1016/j.ympev.2008.12.014
Pallas, L., Daver, G., Mackaye, H.T., Likius, A., Vignaud, P., and Guy, F. 2019. A window into the early evolutionary history of Cercopithecidae: Late Miocene evidence from Chad, Central Africa. Journal of Human Evolution, 132:61-79. https://doi.org/10.1016/j.jhevol.2019.03.013
Pan, R., Groves, C., and Oxnard, C. 2004. Relationships between the fossil colobine Mesopithecus pentelicus and extant cercopithecoids, based on dental metrics. American Journal of Primatology, 62:287-299. https://doi.org/10.1002/ajp.20022
Pan, Y. 2006. Primates Linnaeus 1758, pp. 131-148, 320-322. In Qi, G. and Dong, W. (eds.), Lufengpithecus hudieensis Site. Science Press, Beijing, China.
Parham, J.F., Donoghue, P.C.J., Bell, C.J., Calway, T.D., Head, J.J., Holroyd, P.A., Inoue, J.G., Irmis, R.B., Joyce, W.G., Ksepka, D.T., Patané, J.S.L., Smith, N.D., Tarver, J.E., van Tuinen, M., Yang, Z., Angielczyk, K.D., Greenwood, J.M., Hipsley, C.A., Jacobs, L., Makovicky, P.J., Müller, J., Smith, K.T., Theodor, J.M., Warnock, R.C.M., and Benton, M.J. 2012. Best practices for justifying fossil calibrations. Systematic Biology, 61:346-359. https://doi.org/10.1093/sysbio/syr107
Patel, B.A., Seiffert, E.R., Boyer, D.M., Jacobs, R.L., St Clair, E.M., and Simons, E.L. 2012. New primate first metatarsals from the Paleogene of Egypt and the origin of the anthropoid big toe. Journal of Human Evolution, 63:99-120. https://doi.org/10.1016/j.jhevol.2012.05.002
Perelman, P., Johnson, W.E., Roos, C., Seuánez, H.N., Horvath, J.E., Moreira, M.A.M., Kessing, B., Pontius, J., Roelke, M., Rumpler, Y., Schneider, M.P.C., Silva, A., O’Brien, S.J., and Pecon-Slattery, J. 2011. A molecular phylogeny of living primates. PLoS Genetics, 7:e1001342. https://doi.org/10.1371/journal.pgen.1001342
Perez, S.I., Klaczko, J., and dos Reis, S.F. 2012. Species tree estimation for a deep phylogenetic divergence in the New World monkeys (Primates: Platyrrhini). Molecular Phylogenetics and Evolution, 65:621-630. https://doi.org/10.1016/j.ympev.2012.07.014
Phillips, M.J. 2016. Geomolecular dating and the origin of placental mammals. Systematic Biology, 65:546-557. https://doi.org/10.1093/sysbio/syv115
Phillips, M.J. and Fruciano, C. 2018. The soft explosive model of placental mammal evolution. BMC Evolutionary Biology, 18:104. https://doi.org/10.1186/s12862-018-1218-x
Pickford, M. 1985. A new look at Kenyapithecus based on recent discoveries in Western Kenya. Journal of Human Evolution, 14:113-143. https://doi.org/10.1016/S0047-2484(85)80002-6
Pickford, M. 2012. Lorisine primate from the late Miocene of Kenya. Journal of Biological Research - Bollettino Della Società Italiana Di Biologia Sperimentale, 85:47-52. https://doi.org/10.4081/jbr.2012.4063
Pickford, M. and Senut, B. 2005. Hominoid teeth with chimpanzee- and gorilla-like features from the Miocene of Kenya: implications for the chronology of ape-human divergence and biogeography of Miocene hominoids. Anthropological Science, 113:95-102. https://doi.org/10.1537/ase.04S014
Pickford, M., Sawada, Y., Tayama, R., Matsuda, Y.-K., Itaya, T., Hyodo, H., and Senut, B. 2006. Refinement of the age of the Middle Miocene Fort Ternan Beds, Western Kenya, and its implications for Old World biochronology. Comptes Rendus Geoscience, 338:545-555. https://doi.org/10.1016/j.crte.2006.02.010
Pickford, M., Senut, B., Morales, J., Mein, P., and Sanchez, I.M. 2008. Mammalia from the Lutetian of Namibia. Memoirs of the Geological Survey of Namibia, 20:465-514.
Pilgrim, E.G. 1910. Notices of new mammalian genera and species from the Tertiaries of India. Records of the Geological Survey of India, 40:63-71.
Plavcan, J.M., Ward, C.V., Kay, R.F., and Manthi, F.K. 2019. A diminutive Pliocene guenon from Kanapoi, West Turkana, Kenya. Journal of Human Evolution, 135:102623. https://doi.org/10.1016/j.jhevol.2019.05.011
Poux, C., Chevret, P., Huchon, D., de Jong, W.W., and Douzery, E.J.P. 2006. Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Systematic Biology, 55:228-244. https://doi.org/10.1080/10635150500481390
Poux, C., Madsen, O., Marquard, E., Vieites, D.R., de Jong, W.W., and Vences, M. 2005. Asynchronous colonization of Madagascar by the four endemic clades of primates, tenrecs, carnivores, and rodents as inferred from nuclear genes. Systematic Biology, 54:719-730. https://doi.org/10.1080/10635150500234534
Prevosti, F.J., Forasiepi, A., and Zimicz, N. 2013. The evolution of the Cenozoic terrestrial mammalian predator guild in South America: Competition or replacement? Journal of Mammalian Evolution, 20:3-21. https://doi.org/10.1007/s10914-011-9175-9
Prevosti, F.J., Romano, C.O., Forasiepi, A.M., Hemming, S., Bonini, R., Candela, A.M., Cerdeño, E., Madozzo Jaén, M.C., Ortiz, P.E., Pujos, F., Rasia, L., Schmidt, G.I., Taglioretti, M., MacPhee, R.D.E., and Pardiñas, U.F.J. 2021. New radiometric 40Ar-39Ar dates and faunistic analyses refine evolutionary dynamics of Neogene vertebrate assemblages in southern South America. Scientific Reports, 11:1-14. https://doi.org/10.1038/s41598-021-89135-1
Pugh, K.D. 2022. Phylogenetic analysis of Middle-Late Miocene apes. Journal of Human Evolution, 165:103140. https://doi.org/10.1016/j.jhevol.2021.103140
Pugh, K.D. and Gilbert, C.C. 2018. Phylogenetic relationships of living and fossil African papionins: Combined evidence from morphology and molecules. Journal of Human Evolution, 123:35-51. https://doi.org/10.1016/j.jhevol.2018.06.002
Püschel, H.P., Bertrand, O.C., O’Reilly, J.E., Bobe, R., and Püschel, T.A. 2021. Divergence-time estimates for hominins provide insight into encephalization and body mass trends in human evolution. Nature Ecology & Evolution, 5:808-819. https://doi.org/10.1038/s41559-021-01431-1
Pyron, R.A. 2011. Divergence time estimation using fossils as terminal taxa and the origins of Lissamphibia. Systematic Biology, 60:466-481. https://doi.org/10.1093/sysbio/syr047
Pyron, R.A. 2017. Novel approaches for phylogenetic inference from morphological data and total-evidence dating in squamate reptiles (lizards, snakes, and amphisbaenians). Systematic Biology, 66:38-56. https://doi.org/10.1093/sysbio/syw068
Qi, T. and Beard, K.C. 1996. Nanotitan shanghuangensis, gen. et sp. nov.: the smallest known brontothere (Mammalia: Perissodactyla). Journal of Vertebrate Paleontology, 16:578-581. https://doi.org/10.1080/02724634.1996.10011342
Qi, T., Beard, K.C., Wang, B., Dawson, M.R., Guo, J., and Li, C. 1996. The Shanghuang mammalian fauna, Middle Eocene of Jiangsu: history of discovery and significance. Vertebrata Palasiatica, 34:202-214.
Raffi, I., Wade, B.S., Pälike, H., Beu, A.G., Cooper, R., Crundwell, M.P., Krijgsman, W., Moore, T., Raine, I., Sardella, R., and Vernyhorova, Y.V. 2020. The Neogene period, p. 1141-1215. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. (eds.), Geologic Time Scale 2020. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-824360-2.00029-2
Ramdarshan, A., Godinot, M., Bédécarrats, S., and Tabuce, R. 2015. Holotype specimen of Donrussellia magna, an adapiform primate from the early Eocene (MP7) of Southern France. MorphoMuseuM, 1:e2. https://doi.org/10.18563/m3.1.2.e2
Rankin, A.H., Emry, R.J., and Asher, R.J. 2020. Anatomical sciuromorphy in “protrogomorph” rodents. Palaeontologia Electronica, 23(2):a25. https://doi.org/10.26879/1049
Rasmussen, D.T., Friscia, A.R., Gutierrez, M., Kappelman, J., Miller, E.R., Muteti, S., Reynoso, D., Rossie, J.B., Spell, T.L., Tabor, N.J., Gierlowski-Kordesch, E., Jacobs, B.F., Kyongo, B., Macharwas, M., and Muchemi, F. 2019. Primitive Old World monkey from the earliest Miocene of Kenya and the evolution of cercopithecoid bilophodonty. Proceedings of the National Academy of Sciences of the United States of America, 116:6051-6056. https://doi.org/10.1073/pnas.1815423116
Raza, S.M., Barry, J.C., Pilbeam, D., Rose, M.D., Shah, S.M.I., and Ward, S. 1983. New hominoid primates from the middle Miocene Chinji Formation, Potwar Plateau, Pakistan. Nature, 306:52-54. https://doi.org/10.1038/306052a0
Richmond, B.G. and Jungers, W.L. 2008. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science, 319:1662-1665. https://doi.org/10.1126/science.1154197
Ronquist, F., Klopfstein, S., Vilhelmsen, L., Schulmeister, S., Murray, D.L., and Rasnitsyn, A.P. 2012. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Systematic Biology, 61:973-999. https://doi.org/10.1093/sysbio/sys058
Roos, C., Kothe, M., Alba, D.M., Delson, E., and Zinner, D. 2019. The radiation of macaques out of Africa: Evidence from mitogenome divergence times and the fossil record. Journal of Human Evolution, 133:114-132. https://doi.org/10.1016/j.jhevol.2019.05.017
Rose, K.D. 2006. The Beginning of the Age of Mammals. Johns Hopkins University Press, Baltimore.
Rose, K.D., Chester, S.G.B., Dunn, R.H., Boyer, D.M., and Bloch, J.I. 2011. New fossils of the oldest North American euprimate Teilhardina brandti (Omomyidae) from the Paleocene-Eocene Thermal Maximum. American Journal of Physical Anthropology, 146:281-305. https://doi.org/10.1002/ajpa.21579
Rose, K.D., Dunn, R.H., Kumar, K., Perry, J.M.G., Prufrock, K.A., Rana, R.S., and Smith, T. 2018. New fossils from Tadkeshwar Mine (Gujarat, India) increase primate diversity from the early Eocene Cambay Shale. Journal of Human Evolution, 122:93-107. https://doi.org/10.1016/j.jhevol.2018.05.006
Rose, K.D., Rana, R.S., Sahni, A., Kumar, K., Missiaen, P., Singh, L., and Smith, T. 2009. Early Eocene primates from Gujarat, India. Journal of Human Evolution, 56:366-404. https://doi.org/10.1016/j.jhevol.2009.01.008
Rosenberger, A.L. 1979. Phylogeny, evolution and classification of New World monkeys (Platyrrhini, Primates). PhD Thesis, City University of New York, New York, USA.
Rosenberger, A.L. 2020. New World Monkeys: The Evolutionary Odyssey. Princeton University Press, New Jersey, USA.
Rosenberger, A.L. and Tejedor, M.F. 2013. The misbegotten: long lineages, long branches and the interrelationships of Aotus, Callicebus and the saki-uacaris, p. 13-22. In Veiga, L.M., Barnett, A.A., Ferrari, S.F., and Norconk, M.A. (eds.), Evolutionary Biology and Conservation of Titis, Sakis and Uacaris. Cambridge University Press, Cambridge.
Rosenberger, A.L., Cooke, S.B., Halenar, L.B., Tejedor, M.F., Hartwig, W.C., Novo, N.M., and Muñoz-Saba, Y. 2015. Fossil alouattines and the origins of Alouatta: craniodental diversity and interrelationships, p. 21-54.
Rosenberger, A.L., Halenar, L., Cooke, S.B., and Hartwig, W.C. 2008. Morphology and evolution of the spider monkey, genus Ateles, p. 19-49. In Campbell, C.J. (ed.), Spider Monkeys: Behaviour, Ecology and Evolution of the Genus Ateles. Cambridge University Press, Cambridge.
Rosenberger, A.L., Hartwig, W.C., Takai, M., Setoguchi, T., and Shigehara, N. 1991. Dental variability in Saimiri and the taxonomic status of Neosaimiri fieldsi, an early squirrel monkey from La Venta, Colombia. International Journal of Primatology, 12:291-301. https://doi.org/10.1007/BF02547590
Rosenberger, A.L., Setoguchi, T., and Shigehara, N. 1990. The fossil record of callitrichine primates, p. 209-236. In Fleagle, J.G. and Rosenberger, A.L. (eds.), The Platyrrhine Fossil Record. Academic Press, Cambridge, Massachusetts. https://doi.org/10.1016/B978-0-12-260345-7.50012-6
Rossie, J.B., Gilbert, C.C., and Hill, A. 2013. Early cercopithecid monkeys from the Tugen Hills, Kenya. Proceedings of the National Academy of Sciences of the United States of America, 110:5818-5822. https://doi.org/10.1073/pnas.1213691110
Rossie, J.B., Ni, X., and Beard, K.C. 2006. Cranial remains of an Eocene tarsier. Proceedings of the National Academy of Sciences of the United States of America, 103:4381-4385. https://doi.org/10.1073/pnas.0509424103
Sallam, H.M. and Seiffert, E.R. 2016. New phiomorph rodents from the latest Eocene of Egypt, and the impact of Bayesian “clock”-based phylogenetic methods on estimates of basal hystricognath relationships and biochronology. PeerJ, 4:e1717. https://doi.org/10.7717/peerj.1717
Sarich, V.M. and Wilson, A.C. 1967. Immunological time scale for hominid evolution. Science, 158:1200-1203. https://doi.org/10.1126/science.158.3805.1200
Sawada, Y., Pickford, M., Senut, B., Itaya, T., Hyodo, M., Miura, T., Kashine, C., Chujo, T., and Fujii, H. 2002. The age of Orrorin tugenensis, an early hominid from the Tugen Hills, Kenya. Comptes Rendus Palevol, 1:293-303. https://doi.org/10.1016/S1631-0683(02)00036-2
Scally, A. and Durbin, R. 2012. Revising the human mutation rate: implications for understanding human evolution. Nature Reviews Genetics, 13:745-753. https://doi.org/10.1038/nrg3295
Schrago, C.G. and Seuánez, H.N. 2019. Large ancestral effective population size explains the difficult phylogenetic placement of owl monkeys. American Journal of Primatology, 81:e22955. https://doi.org/10.1002/ajp.22955
Schrago, C.G. and Voloch, C.M. 2013. The precision of the hominid timescale estimated by relaxed clock methods. Journal of Evolutionary Biology, 26:746-755. https://doi.org/10.1111/jeb.12076
Seiffert, E.R. 2006. Revised age estimates for the later Paleogene mammal faunas of Egypt and Oman. Proceedings of the National Academy of Sciences of the United States of America, 103:5000-5005. https://doi.org/10.1073/pnas.0600689103
Seiffert, E.R. 2010. Chronology of Paleogene mammal localities, p. 19-26. In Werdelin, L. and Sanders, W.J. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley. https://doi.org/10.1525/california/9780520257214.003.0002
Seiffert, E.R. 2012. Early primate evolution in Afro-Arabia. Evolutionary Anthropology, 21:239-253. https://doi.org/10.1002/evan.21335
Seiffert, E.R. and Simons, E.L. 2001. Astragalar morphology of late Eocene anthropoids from the Fayum Depression (Egypt) and the origin of catarrhine primates. Journal of Human Evolution, 41:577-606. https://doi.org/10.1006/jhev.2001.0508
Seiffert, E.R., Bown, T.M., Clyde, W.C., and Simons, E. 2008. Geology, paleoenvironment, and age of Birket Qarun Locality 2 (BQ-2), Fayum Depression, Egypt, p. 71-86. In Fleagle, J.G. and Gilbert, C.C. (eds.), Elwyn Simons: A Search for Origins. Springer New York, New York. https://doi.org/10.1007/978-0-387-73896-3_8
Seiffert, E.R., Boyer, D.M., Fleagle, J.G., Gunnell, G.F., Heesy, C.P., Perry, J.M.G., and Sallam, H.M. 2018. New adapiform primate fossils from the late Eocene of Egypt. Historical Biology, 30:204-226. https://doi.org/10.1080/08912963.2017.1306522
Seiffert, E.R., Costeur, L., and Boyer, D.M. 2015. Primate tarsal bones from Egerkingen, Switzerland, attributable to the middle Eocene adapiform Caenopithecus lemuroides. PeerJ, 3:e1036. https://doi.org/10.7717/peerj.1036
Seiffert, E.R., Perry, J.M.G., Simons, E.L., and Boyer, D.M. 2009. Convergent evolution of anthropoid-like adaptations in Eocene adapiform primates. Nature, 461:1118-1121. https://doi.org/10.1038/nature08429
Seiffert, E.R., Simons, E.L., and Attia, Y. 2003. Fossil evidence for an ancient divergence of lorises and galagos. Nature, 422:421-424. https://doi.org/10.1038/nature01489
Seiffert, E.R., Simons, E.L., Boyer, D.M., Perry, J.M.G., Ryan, T.M., and Sallam, H.M. 2010. A fossil primate of uncertain affinities from the earliest late Eocene of Egypt. Proceedings of the National Academy of Sciences of the United States of America, 107:9712-9717. https://doi.org/10.1073/pnas.1001393107
Seiffert, E.R., Simons, E.L., Fleagle, J.G., and Godinot, M. 2010. Paleogene anthropoids, p. 369-391. In Werdelin, L. and Sanders, W.J. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley. https://doi.org/10.1525/california/9780520257214.001.0001
Seiffert, E.R., Tejedor, M.F., Fleagle, J.G., Novo, N.M., Cornejo, F.M., Bond, M., de Vries, D., and Campbell, K.E., Jr. 2020. A parapithecid stem anthropoid of African origin in the Paleogene of South America. Science, 368:194-197. https://doi.org/10.1126/science.aba1135
Semaw, S., Simpson, S.W., Quade, J., Renne, P.R., Butler, R.F., McIntosh, W.C., Levin, N., Dominguez-Rodrigo, M., and Rogers, M.J. 2005. Early Pliocene hominids from Gona, Ethiopia. Nature, 433:301-305. https://doi.org/10.1038/nature03177
Sen, S., Koufos, G.D., Kondopoulou, D., and De Bonis, L. 2000. Magnetostratigraphy of the late Miocene continental deposits of the lower Axios valley, Macedonia, Greece. Geological Society of Greece, Special Publications, 9:197-206.
Senut, B., Pickford, M., Gommery, D., Mein, P., Cheboi, K., and Coppens, Y. 2001. First hominid from the Miocene (Lukeino Formation, Kenya). Comptes Rendus de l’Académie Des Sciences. Série 2, 332:137-144. https://doi.org/10.1016/S1251-8050(01)01529-4
Setoguchi, T., Watanabe, T., and Mouri, T. 1981. The upper dentition of Stirtonia (Ceboidea, Primates) from the Miocene of Colombia, South America and the origin of the postero- internal cusp of upper molars of howler monkeys (Alouatta). Kyoto University Overseas Research Reports of New World Monkeys, 2:51-60.
Setoguchi, T. and Rosenberger, A.L. 1985. Miocene marmosets: First fossil evidence. International Journal of Primatology, 6:615-625. https://doi.org/10.1007/BF02692292
Setoguchi, T. and Rosenberger, A.L. 1987. A fossil owl monkey from La Venta, Colombia. Nature, 326:692-694. nature.com. https://doi.org/10.1038/326692a0
Sigé, B., Jaeger, J.J., and Sudre, J. 1990. Altiatlasius koulchii n. gen., et sp., primate omomyidé du Paléocène supérieur du Maroc, et les origines des Euprimates. Palaeontographica Abteilung A, A214:31-56.
Silcox, M.T. 2008. The biogeographic origins of Primates and Euprimates: East, west, north, or south of Eden?, p. 199-231. In Sargis, E.J. and Dagosto, M. (eds.), Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6997-0_10
Silcox, M.T., Bloch, J.I., Boyer, D.M., Chester, S.G.B., and López-Torres, S. 2017. The evolutionary radiation of plesiadapiforms. Evolutionary Anthropology, 26:74-94. https://doi.org/10.1002/evan.21526
Silvestro, D., Salamin, N., Antonelli, A., and Meyer, X. 2019. Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework. Paleobiology, 45:546-570. https://doi.org/10.1017/pab.2019.23
Silvestro, D., Salamin, N., and Schnitzler, J. 2014. PyRate: a new program to estimate speciation and extinction rates from incomplete fossil data. Methods in Ecology and Evolution, 5:1126-1131. https://doi.org/10.1111/2041-210x.12263
Silvestro, D., Tejedor, M.F., Serrano-Serrano, M.L., Loiseau, O., Rossier, V., Rolland, J., Zizka, A., Höhna, S., Antonelli, A., and Salamin, N. 2019. Early arrival and climatically-linked geographic expansion of New World monkeys from tiny African ancestors. Systematic Biology, 68:78-92. https://doi.org/10.1093/sysbio/syy046
Simmons, M.P. 2012. Radical instability and spurious branch support by likelihood when applied to matrices with non-random distributions of missing data. Molecular Phylogenetics and Evolution, 62:472-484. https://doi.org/10.1016/j.ympev.2011.10.017
Simmons, M.P. 2014. A confounding effect of missing data on character conflict in maximum likelihood and Bayesian MCMC phylogenetic analyses. Molecular Phylogenetics and Evolution, 80:267-280. https://doi.org/10.1016/j.ympev.2014.08.021
Simons, E.L. 1989. Description of two genera and species of late Eocene Anthropoidea from Egypt. Proceedings of the National Academy of Sciences of the United States of America, 86:9956-9960. https://doi.org/10.1073/pnas.86.24.9956
Simons, E.L. 1990. Discovery of the oldest known anthropoidean skull from the Paleogene of Egypt. Science, 247:1567-1569. https://doi.org/10.1126/science.2108499
Simons, E.L. 1992. Diversity in the early Tertiary anthropoidean radiation in Africa. Proceedings of the National Academy of Sciences of the United States of America, 89:10743-10747. https://doi.org/10.1073/pnas.89.22.10743
Simons, E.L. and Rasmussen, D.T. 1994. A remarkable cranium of Plesiopithecus teras (Primates, Prosimii) from the Eocene of Egypt. Proceedings of the National Academy of Sciences of the United States of America, 91:9946-9950. https://doi.org/10.1073/pnas.91.21.9946
Simons, E.L. and Rasmussen, D.T. 1996. Skull of Catopithecus browni, an early Tertiary catarrhine. American Journal of Physical Anthropology, 100:261-292.
Simpson, S.W., Levin, N.E., Quade, J., Rogers, M.J., and Semaw, S. 2019. Ardipithecus ramidus postcrania from the Gona Project area, Afar Regional State, Ethiopia. Journal of Human Evolution, 129:1-45. https://doi.org/10.1016/j.jhevol.2018.12.005
Smith, T., Rose, K.D., and Gingerich, P.D. 2006. Rapid Asia-Europe-North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene-Eocene thermal maximum. Proceedings of the National Academy of Sciences of the United States of America, 103:11223-11227. https://doi.org/10.1073/pnas.0511296103
Solé, F., Falconnet, J., and Vidalenc, D. 2015. New fossil Hyaenodonta (Mammalia, Placentalia) from the Ypresian and Lutetian of France and the evolution of the Proviverrinae in southern Europe. Palaeontology, 58:1049-1072. https://doi.org/10.1111/pala.12198
Speijer, R.P., Pälike, H., Hollis, C.J., Hooker, J.J., and Ogg, J.G. 2020. The Paleogene period, p. 1087-1140. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. (eds.), Geologic Time Scale 2020. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-444-59425-9.00028-7
Springer, M.S., Foley, N.M., Brady, P.L., Gatesy, J., and Murphy, W.J. 2019. Evolutionary models for the diversification of placental mammals across the KPg Boundary. Frontiers in Genetics, 10:1241. https://doi.org/10.3389/fgene.2019.01241
Springer, M.S., Meredith, R.W., Gatesy, J., Emerling, C.A., Park, J., Rabosky, D.L., Stadler, T., Steiner, C., Ryder, O.A., Janečka, J.E., Fisher, C.A., and Murphy, W.J. 2012. Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species supermatrix. PLoS ONE, 7:e49521. https://doi.org/10.1371/journal.pone.0049521
Stadler, T. 2010. Sampling-through-time in birth-death trees. Journal of Theoretical Biology, 267:396-404. https://doi.org/10.1016/j.jtbi.2010.09.010
Stevens, N.J., Seiffert, E.R., O’Connor, P.M., Roberts, E.M., Schmitz, M.D., Krause, C., Gorscak, E., Ngasala, S., Hieronymus, T.L., and Temu, J. 2013. Palaeontological evidence for an Oligocene divergence between Old World monkeys and apes. Nature, 497:611-614. https://doi.org/10.1038/nature12161
Stirton, R.A. 1951. Ceboid monkeys from the Miocene of Colombia. University of California Publications in Geological Sciences, 28:315-356.
Stirton, R.A. and Savage, D.E. 1951. A new monkey from the La Venta Miocene of Colombia. Compilación de Los Estudios Geológicos Oficiales En Colombia, 7:345-356.
Suwa, G., Asfaw, B., Kono, R.T., Kubo, D., Lovejoy, C.O., and White, T.D. 2009. The Ardipithecus ramidus skull and its implications for hominid origins. Science, 326:68e1-7. https://doi.org/10.1126/science.1175825
Suwa, G., Beyene, Y., Nakaya, H., Bernor, R.L., Boisserie, J.-R., Bibi, F., Ambrose, S.H., Sano, K., Katoh, S., and Asfaw, B. 2015. Newly discovered cercopithecid, equid and other mammalian fossils from the Chorora Formation, Ethiopia. Anthropological Science, 123:19-39. https://doi.org/10.1537/ase.150206
Suwa, G., Kono, R.T., Katoh, S., Asfaw, B., and Beyene, Y. 2007. A new species of great ape from the late Miocene epoch in Ethiopia. Nature, 448:921-924. https://doi.org/10.1038/nature06113
Suwa, G., Kono, R.T., Simpson, S.W., Asfaw, B., Lovejoy, C.O., and White, T.D. 2009. Paleobiological implications of the Ardipithecus ramidus dentition. Science, 326:94-99. https://doi.org/10.1126/science.1175824
Szalay, F.S. and Delson, E. 1979. Evolutionary History of the Primates. Academic Press, Cambridge, Massachusetts.
Szalay, F.S. and Lucas, S.G. 1993. Cranioskeletal Morphology of Archontans, and Diagnoses of Chiroptera, Volitantia, and Archonta, p. 187-226. In MacPhee, R.D.E. (ed.), Primates and Their Relatives in Phylogenetic Perspective. Springer US, Boston, MA. https://doi.org/10.1007/978-1-4899-2388-2_6
Szalay, F.S. and Lucas, S.G. 1998. The postcranial morphology of Paleocene Chiacus and Mixodectes and the phylogenetic relationships of archontan mammals: Bulletin 7. New Mexico Museum of Natural History and Science.
Tabuce, R., Marivaux, L., Lebrun, R., Adaci, M., Bensalah, M., Fabre, P.-H., Fara, E., Gomes Rodrigues, H., Hautier, L., Jaeger, J.-J., Lazzari, V., Mebrouk, F., Peigné, S., Sudre, J., Tafforeau, P., Valentin, X., and Mahboubi, M. 2009. Anthropoid versus strepsirhine status of the African Eocene primates Algeripithecus and Azibius: craniodental evidence. Proceedings of the Royal Society B: Biological Sciences, 276:4087-4094. https://doi.org/10.1098/rspb.2009.1339
Takai, M. 1994. New specimens of Neosaimiri fieldsi from La Venta, Colombia: a middle Miocene ancestor of the living squirrel monkeys. Journal of Human Evolution, 27:329-360. https://doi.org/10.1006/jhev.1994.1049
Takai, M., Anaya, F., Suzuki, H., Shigehara, N., and Setoguchi, T. 2001. A new platyrrhine from the Middle Miocene of La Venta, Colombia, and the phyletic position of Callicebinae. Anthropological Science, 109:289-307. https://doi.org/10.1537/ase.109.289
Tauxe, L. 1979. A new date for Ramapithecus. Nature, 282:399-401. https://doi.org/10.1038/282399a0
Tauxe, L. and Opdyke, N.D. 1982. A time framework based on magnetostratigraphy for the Siwalik sediments of the Khaur area, Northern Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology, 37:43-61. https://doi.org/10.1016/0031-0182(82)90057-8
Tejedor, M.F. 2005. New specimens of Soriacebus adrianae Fleagle, 1990, with comments on pitheciin primates from the Miocene of Patagonia. Ameghiniana, 42:249-251.
Tejedor, M.F. and Novo, N.M. 2016. Evolución y paleobiogeografía de los primates platirrinos, p. 385-393. In Agnolin, F.L., Lio, G.L., Brissón Egli, F., Chimento, N.R., and Novas, F.E. (eds.), Historia Evolutiva y Paleobiogeográfica de Los Vertebrados de América Del Sur. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”.
Tomassini, R.L., Montalvo, C.I., Deschamps, C.M., and Manera, T. 2013. Biostratigraphy and biochronology of the Monte Hermoso Formation (early Pliocene) at its type locality, Buenos Aires Province, Argentina. Journal of South American Earth Sciences, 48:31-42. https://doi.org/10.1016/j.jsames.2013.08.002
Tong, Y. 1988. Fossil tree shrews from the Eocene Hetaoyuan formation of Xichuan, Henan. Vertebrata PalAsiatica, 26:4-20.
Trifonov, V.G., Tesakov, A.S., Simakova, A.N., and Bachmanov, D.M. 2019. Environmental and geodynamic settings of the earliest hominin migration to the Arabian-Caucasus region: A review. Quaternary International, 534:116-137. https://doi.org/10.1016/j.quaint.2019.03.008
Valencia, L.M., Martins, A., Ortiz, E.M., and Di Fiore, A. 2018. A RAD-sequencing approach to genome-wide marker discovery, genotyping, and phylogenetic inference in a diverse radiation of primates. PLoS ONE, 13:e0201254. https://doi.org/10.1371/journal.pone.0201254
Van Couvering, J.A. and Delson, E. 2020. African land mammal ages. Journal of Vertebrate Paleontology, 40:e1803340. https://doi.org/10.1080/02724634.2020.1803340
Vanderpool, D., Minh, B.Q., Lanfear, R., Hughes, D., Murali, S., Harris, R.A., Raveendran, M., Muzny, D.M., Hibbins, M.S., Williamson, R.J., Gibbs, R.A., Worley, K.C., Rogers, J., and Hahn, M.W. 2020. Primate phylogenomics uncovers multiple rapid radiations and ancient interspecific introgression. PLoS Biology, 18:e3000954. https://doi.org/10.1371/journal.pbio.3000954
Vangengeim, E. and Tesakov, A.S. 2013. Late Miocene Mammal Localities of Eastern Europe and Western Asia, p. 521-537. In Wang, X., Flynn, L.J., and Fortelius, M. (eds.), Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology. Columbia University Press, New York.
Velazco, P.M., Buczek, A.J., Hoffman, E., Hoffman, D.K., O’Leary, M.A., and Novacek, M.J. 2022. Combined data analysis of fossil and living mammals: a Paleogene sister taxon of Placentalia and the antiquity of Marsupialia. Cladistics: The International Journal of the Willi Hennig Society, 38:359-373. https://doi.org/10.1111/cla.12499
Wang, B.Y. and Dawson, M.R. 1994. A primitive cricetid (Mammalia: Rodentia) from the middle Eocene of Jiangsu Province, China. Annals of the Carnegie Museum, 63:239-256.
Wang, Y., Li, Q., Bai, B., Jin, X., Mao, F., and Meng, J. 2019. Paleogene integrative stratigraphy and timescale of China. Science China Earth Sciences, 62:287-309. https://doi.org/10.1007/s11430-018-9305-y
Wang, X., Lim, B.K., Ting, N., Hu, J., Liang, Y., Roos, C., and Yu, L. 2019. Reconstructing the phylogeny of New World monkeys (Platyrrhini): evidence from multiple non-coding loci. Current Zoology, 65:579-588. https://doi.org/10.1093/cz/zoy072
Wang, Y.Q., Li, C.K., Li, Q., and Li, D.S. 2016. A synopsis of Paleocene stratigraphy and vertebrate paleontology in the Qianshan Basin, Anhui, China. Vertebrata Palasiatica, 54:89-120.
Warnock, R.C.M., Parham, J.F., Joyce, W.G., Lyson, T.R., and Donoghue, P.C.J. 2015. Calibration uncertainty in molecular dating analyses: there is no substitute for the prior evaluation of time priors. Proceedings of the Royal Society B: Biological Sciences, 282:20141013. https://doi.org/10.1098/rspb.2014.1013
Warnock, R.C.M., Yang, Z., and Donoghue, P.C.J. 2012. Exploring uncertainty in the calibration of the molecular clock. Biology Letters, 8:156-159. https://doi.org/10.1098/rsbl.2011.0710
Welker, F. 2018. Palaeoproteomics for human evolution studies. Quaternary Science Reviews, 190:137-147. https://doi.org/10.1016/j.quascirev.2018.04.033
White, T.D., Suwa, G., and Asfaw, B. 1995. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature, 375:88. https://doi.org/10.1038/375088a0
White, T.D., Asfaw, B., Beyene, Y., Haile-Selassie, Y., Lovejoy, C.O., Suwa, G., and WoldeGabriel, G. 2009. Ardipithecus ramidus and the paleobiology of early hominids. Science, 326:75-86. https://doi.org/10.1126/science.1175802
Wible, J.R., Rougier, G.W., Novacek, M.J., and Asher, R.J. 2007. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature, 447:1003-1006. https://doi.org/10.1038/nature05854
Wible, J.R., Rougier, G.W., Novacek, M.J., and Asher, R.J. 2009. The eutherian mammal Maelestes gobiensis from the Late Cretaceous of Mongolia and the phylogeny of Cretaceous Eutheria. Bulletin of the American Museum of Natural History, 2009:1-123. https://doi.org/10.1206/623.1
Wiens, J.J., Fetzner, J.W., Parkinson, C.L., and Reeder, T.W. 2005. Hylid frog phylogeny and sampling strategies for speciose clades. Systematic Biology, 54:778-807. https://doi.org/10.1080/10635150500234625
Wilkinson, R.D., Steiper, M.E., Soligo, C., Martin, R.D., Yang, Z., and Tavaré, S. 2011. Dating primate divergences through an integrated analysis of palaeontological and molecular data. Systematic Biology, 60:16-31. https://doi.org/10.1093/sysbio/syq054
Wilson, G.P. 2014. Mammalian extinction, survival, and recovery dynamics across the Cretaceous-Paleogene boundary in northeastern Montana, USA. Geological Society of America Special Papers, 503:365-392. https://doi.org/10.1130/2014.2503(15)
Wilson Mantilla, G.P., Chester, S.G.B., Clemens, W.A., Moore, J.R., Sprain, C.J., Hovatter, B.T., Mitchell, W.S., Mans, W.W., Mundil, R., and Renne, P.R. 2021. Earliest Palaeocene purgatoriids and the initial radiation of stem primates. Royal Society Open Science, 8:210050. https://doi.org/10.1098/rsos.210050
WoldeGabriel, G., Haile-Selassie, Y., Renne, P.R., Hart, W.K., Ambrose, S.H., Asfaw, B., Heiken, G., and White, T. 2001. Geology and palaeontology of the Late Miocene Middle Awash valley, Afar rift, Ethiopia. Nature, 412:175-178. https://doi.org/10.1038/35084058
Wolpoff, M.H. and Pickford, M. 2006. An ape or the ape: Is the Toumaï cranium TM 266 a hominid? PaleoAnthropology, 2006:36-50.
Wolpoff, M.H., Senut, B., Pickford, M., and Hawks, J. 2002. Sahelanthropus or “ Sahelpithecus ”? Nature, 419:581-582. https://doi.org/10.1038/419581a
Worthington, S. 2012. New approaches to late Miocene hominoid systematics: Ranking morphological characters by phylogenetic signal. PhD Thesis, New York University, New York, USA.
Wright, A.M. 2019. A systematist’s guide to estimating Bayesian phylogenies from morphological data. Insect Systematics and Diversity, 3:1-14. https://doi.org/10.1093/isd/ixz006
Wright, A.M., Lloyd, G.T., and Hillis, D.M. 2016. Modeling character change heterogeneity in phylogenetic analyses of morphology through the use of priors. Systematic Biology, 65:602-611. https://doi.org/10.1093/sysbio/syv122
Xia, X. 2014. Phylogenetic bias in the likelihood method caused by missing data coupled with among-site rate variation: An analytical approach, p. 12-23. In Basu, M., Pan, Y., and Wang, J. (eds.), Bioinformatics Research and Applications. Springer International Publishing, Switzerland. https://doi.org/10.1007/978-3-319-08171-7_2
Yans, J., Strait, S.G., Smith, T., Dupuis, C., Steurbaut, E., and Gingerich, P.D. 2006. High-resolution carbon isotope stratigraphy and mammalian faunal change at the Paleocene-Eocene boundary in the Honeycombs area of the southern Bighorn Basin, Wyoming. American Journal of Science, 306:712-735. https://doi.org/10.2475/09.2006.02
Yapuncich, G., Boyer, D., Secord, R., and Bloch, J. 2011. The first dentally associated skeleton of Plagiomenidae (Mammalia, ?Dermoptera) from the Late Paleocene of Wyoming. Journal of Vertebrate Paleontology, 31:218. https://doi.org/10.13140/2.1.1302.4322
Young, N.M. and MacLatchy, L. 2004. The phylogenetic position of Morotopithecus. Journal of Human Evolution, 46:163-184. https://doi.org/10.1016/j.jhevol.2003.11.002
Zalmout, I.S., Sanders, W.J., Maclatchy, L.M., Gunnell, G.F., Al-Mufarreh, Y.A., Ali, M.A., Nasser, A.-A.H., Al-Masari, A.M., Al-Sobhi, S.A., Nadhra, A.O., Matari, A.H., Wilson, J.A., and Gingerich, P.D. 2010. New Oligocene primate from Saudi Arabia and the divergence of apes and Old World monkeys. Nature, 466:360-364. https://doi.org/10.1038/nature09094
Zeebe, R.E. and Lourens, L.J. 2019. Solar System chaos and the Paleocene-Eocene boundary age constrained by geology and astronomy. Science, 365:926-929. https://doi.org/10.1126/science.aax0612
Zhang, C., Stadler, T., Klopfstein, S., Heath, T.A., and Ronquist, F. 2016. Total-evidence dating under the fossilized birth-death process. Systematic Biology, 65:228-249. https://doi.org/10.1093/sysbio/syv080
Zhong, Y., Huyskens, M.H., Yin, Q.-Z., Wang, Y., Ma, Q., and Xu, Y.-G. 2021. High-precision geochronological constraints on the duration of “Dinosaurs Pompeii” and the Yixian Formation. National Science Review, 8:nwab063. https://doi.org/10.1093/nsr/nwab063
Zollikofer, C.P.E., Ponce de León, M.S., Lieberman, D.E., Guy, F., Pilbeam, D., Likius, A., Mackaye, H.T., Vignaud, P., and Brunet, M. 2005. Virtual cranial reconstruction of Sahelanthropus tchadensis. Nature, 434:755-759. https://doi.org/10.1038/nature03397

