 A new member of a large and archaic balaenid from the Late Miocene of Sapporo, Hokkaido, Japan partly fills a gap of right whale evolution
A new member of a large and archaic balaenid from the Late Miocene of Sapporo, Hokkaido, Japan partly fills a gap of right whale evolution
Article number: 28.2.a37
https://doi.org/10.26879/1549
Copyright Society of Vertebrate Paleontology, August 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 March 2025. Acceptance: 12 July 2025.
ABSTRACT
The family Balaenidae (right whales) includes two genera and four extant species, all of which are endangered and giant animals measuring approximately 17 to 20 m in length. The history of the Balaenidae spans about 20 million years. Several small sized extinct balaenids from the Pliocene have been identified. However, half of this history remains unknown owing to a 9-million-year gap from 15.2 to 6.1 m.y.a. in the fossil record. A well-preserved fossil balaenid skeleton, designated SMAC 2731, from the Late Miocene approximately 9 m.y.a. in Sapporo, Hokkaido, Japan, is named as Megabalaena sapporoensis gen. et sp. nov. This specimen preserves the skull, periotics in situ, tympanic bullae, right mandible, basihyal-thyrohyal, right stylohyal, sternum, seven cervical vertebrae, nine thoracic vertebrae, and 16 more posterior vertebrae, rib fragments, scapulae, and left forelimb elements. All preserved vertebral epiphyses are fused, indicating that SMAC 2731 was physically mature. Notably, M. sapporoensis can be distinguished from other balaenids by its excavated orbit in dorsal view with a large postorbital process, dorsoventrally high anterior part of the involucrum of the tympanic bulla, long compound posterior process, high coronoid process and deeper subcondylar furrow of the mandible, incipient cervical fusion (C2+C3 only), and its slender forelimb bones, including the humerus, radius and ulna. Based on a bizygomatic width of 2.2 m, the estimated total length of the holotype of M. sapporoensis is 12.7 m. Overall, M. sapporoensis indicates that balaenids diversified prior to the Late Miocene.
Yoshihiro Tanaka. Sapporo Museum Activity Center, 5-15-1-6, Hiragishi, Toyohira-ku, Sapporo, Hokkaido 062-0935 Japan; Division of Academic Resources and Specimens, Hokkaido University Museum, Kita 10, Nishi 8, Kita-ku, Sapporo, Hokkaido, 060-0810 Japan; and Numata Fossil Museumm 6-493 Nishi Machi, Numata Town, Hokkaido 078-2204, Japan (corresponding author). yoshi.tanaka.research@gmail.com
Toshiyuki Kimura. Gunma Museum of Natural History, 1674-1 Kamikuroiwa, Tomioka, Gunma, 370-2345 Japan. kimura@gmnh.pref.gunma.jp
Tatsuya Shinmura. Division of Academic Resources and Specimens, Hokkaido University Museum Kita 10, Nishi 8, Kita-ku, Sapporo, Hokkaido, 060-0810 Japan and Ashoro Museum of Paleontology, 29-25, Konan 1, Ashoro Hokkaido 089-3727, Japan. shinmura@ashoromuseum.com
Hiroto Ohira. Shimane University, Matsue, Shimane 690-8504, Japan. ohira@riko.shimane-u.ac.jp
Hitoshi Furusawa. Sapporo Museum Activity Center, 5-15-1-6, Hiragishi, Toyohira-ku, Sapporo, Hokkaido 062-0935 Japan
Keywords: Balaenidae; new genus; new species; Tortonian; gigantism; Japan
https://zoobank.org/EA2A31AC-7125-4F3A-9E47-66418F5CDDDD
Final citation: Tanaka, Yoshihiro, Kimura, Toshiyuki, Shinmura, Tatsuya, Ohira, Hiroto, and Furusawa, Hitoshi. 2025. A new member of a large and archaic balaenid from the Late Miocene of Sapporo, Hokkaido, Japan partly fills a gap of right whale evolution. Palaeontologia Electronica, 28(2): a37.
https://doi.org/10.26879/1549
palaeo-electronica.org/content/2025/5581-archaic-balaenid-from-japan
Copyright: August 2025 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons. org/licenses/by/4.0
INTRODUCTION
The family Balaenidae (right whales) includes two genera and four extant species (Committee on Taxonomy, 2022), which are endangered and large animals measuring about 17-20 m in length (Jefferson et al., 2008). Modern right whales have robust bodies and swim slowly and efficiently perform skim feeding (Woodward et al., 2006).
The history of the Balaenidae spans approximately 20 million years. The oldest known record of this family is Morenocetus parvus from Patagonia, Argentina (Cabrera, 1926; Buono et al., 2017) dating to the Early Miocene (19.8-18.2 m.y.a. [Ma]) (Marx and Fordyce, 2015). Another primitive named balaenid Peripolocetus vexillifer (16.0-15.2 Ma) from California has need for a modern redescription and reinterpretation (Kellogg, 1931; Marx and Fordyce, 2015). Another named balaenid Eubalaena shinshuensis (6.1-5.9 Ma) is known from Japan (Kimura et al., 2007). There is a about 9 million years gap of balaenid fossils, which can be identified into species level between the two records 15.2 to 6.1 Ma as above. Marx and Lambert (2021) call it as “the Miocene gap” of balaenids.
Previously, the diversity of the Balaenidae was thought to be low during the Miocene (Buono et al., 2017) and to have suddenly increased during the Early Pliocene (Kimura, 2009). Early members of balaenids from the Early to Middle Miocene are known to have been small, around 4-6 m in length (Bisconti et al., 2003, 2005, 2021). In a previous study, Bisconti et al. (2021) estimated body lengths of extinct balaenids and proposed a hypothesis regarding the evolution of body size: (1) the origin of gigantic body size in the genus Eubalaena including E. shinshuensis , occurred as early as the latest Miocene, likely coinciding with pulses of nutrients sustaining large zooplankton populations; (2) a reduction in total body length occurred in some linages independently during the Pliocene; (3) the origin of gigantic body size in Balaena mysticetus is likely related to the invasion of the Arctic Ocean in the last 3 million years.
 In summary, the history of the Bal
In summary, the history of the Bal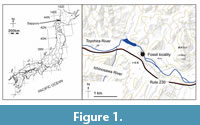 aenidae has a major gap in the Miocene, spanning about 9 million years between 15.2-6.1 Ma (Marx and Lambert, 2021). Specifically, only a few specimens are known from the Miocene specimens are known. The question remains, what occurred during this long unknown interval? A well-preserved fossil balaenid skeleton discovered from the Late Miocene (approximately 9 Ma evaluated by fission track analysis of zircon crystals, Table 1) in Sapporo, Hokkaido, Japan (Figure 1, Figure 2), has a body length as 12.7 m, including the skull, and a mandible is reported here for the first time. This skeleton has an estimated body length of 12.7 m. and preserves the skull, mandible, and postcranial elements, and partly fills the gap in right whale evolution. This is a large balaenid, which is approxima tely a size three times larger than those of early balaenids. Regarding body siz e diversity, t his new fossil enhances our understanding of balaenid diversity, which appears to have expanded earlier than the Late Miocene.
aenidae has a major gap in the Miocene, spanning about 9 million years between 15.2-6.1 Ma (Marx and Lambert, 2021). Specifically, only a few specimens are known from the Miocene specimens are known. The question remains, what occurred during this long unknown interval? A well-preserved fossil balaenid skeleton discovered from the Late Miocene (approximately 9 Ma evaluated by fission track analysis of zircon crystals, Table 1) in Sapporo, Hokkaido, Japan (Figure 1, Figure 2), has a body length as 12.7 m, including the skull, and a mandible is reported here for the first time. This skeleton has an estimated body length of 12.7 m. and preserves the skull, mandible, and postcranial elements, and partly fills the gap in right whale evolution. This is a large balaenid, which is approxima tely a size three times larger than those of early balaenids. Regarding body siz e diversity, t his new fossil enhances our understanding of balaenid diversity, which appears to have expanded earlier than the Late Miocene.
MATERIALS AND METHODS
Morphological terminology follows Mead and Fordyce (2009) mainly, Flower (1885) for postcranial skeleton, and Tanaka (2024) for the basihyal-thyrohyal. Because of the difficulty in examining heavy fossils, we used a 3D scanner (Artec Spider/Eva, Artec Group, Luxembourg) to generate 3D models. Such models were used for producing figures and life restoration.
The phylogenetic position of Megabalaena sapporoensis gen.et sp.nov. (SMAC 2731) was analyzed using the matrix of Tanaka et al. (2020), which was derived from the matrix of Buono et al. (2017). This study added codings of M. sapporoensis and recently established balaenids Antwerpibalaena liberatlas and Charadrobalaena valentinae (Duboys de Lavigerie et al., 2020; Bisconti et al., 2023). In the phylogenetic analysis, character 226 on cervical vertebrae was added a state, and was changed several codings (Appendix 4). The matrix contains 257 morphological characters and 46 taxa (Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5, Appendix 6, Appendix 7, Appendix 8, and Appendix 9). The percentage of coded characters for M. sapporoensis is 56% (including soft tissue characters) and 59% (excluding soft tissue).
The matrix was managed using the software MESQUITE 3.81 (Maddison and Maddison, 2021). Analysis was performed using TNT v.1.5 (Goloboff and Catalano, 2016). All the characters were treated as unordered, equal, and implied weights (K=1 and 3) with backbone constraint of extant taxa, based on the topology of the molecular tree by McGowen et al. (2009). The analysis used New Technology Search with recover minimum length trees equal to 1000 times. The random seed value was 1. The resulting most parsimonious trees were summarized using strict and 50% majority consensus trees.
Institutional Abbreviations
CAS, California Academy of Sciences, San Francisco, USA. FCCP, Fukagawa City Cultural Properties, Hokkaido, Japan. IRSNB, Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium. KMNH, Kitakyushu Museum of Natural History, Fukuoka, Japan. MCMV, Museo Civico, Montopoli Valdarno (Pisa), Italy. MCRE, Musei Civici di Reggio Emilia, Reggio Emilia, Italy. MLP, Museo de La Plata, La Plata, Argentina. MNHNP, Museum National d’Histoire Naturelle, Centre National d’Etude des Mammiferes Marins, Paris, France. MSNTUP, Museo di Storia Naturale e del Territorio, Università di Pisa, Pisa, Italy. MTUF, Museum of Marine Science, Tokyo University of Marine Science and Technology, Tokyo, Japan. NMB, Natuurmuseum Brabant, Tilburg, The Netherlands. NMNS, National Museum of Nature and Science, Tsukuba, Japan. NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand. SDSNH, San Diego Natural History Museum, San Diego, California, USA. SFM, Shinshushinmachi Fossil Museum, Shinshushinmachi, Japan. SMAC, Sapporo Museum Activity Center, Hokkaido, Japan. USNM, National Museum of Natural History, Smithsonian Institution, Washington D.C., USA. ZMUC, Zoological Museum of the University of Copenhagen, Denmark.
SYSTEMATIC PALEONTOLOGY
CETACEA Brisson, 1762
NEOCETI Fordyce and de Muizon, 2001
MYSTICETI Gray, 1864
CHAEOMYSTICETI Mitchell, 1989
BALAENIDAE Gray, 1825
Megabalaena gen. nov
zoobank.org/E011831E-D47F-4A06-938A-E27A8D5330A9
Type species. Megabalaena sapporoensis sp. nov.
Etymology. The generic name, Megabalaena , is named derived from ancient Greek megas meaning great, large and mighty, and the type genus name of the family Balaenidae.
Diagnosis. As for the holotype and only species.
Megabalaena sapporoensis sp. nov.
(Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, FIgure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20, Figure 21, Figure 22, Table 2, Table 3, Table 4, and Table 5)
zoobank.org/BB19FDAE-EEA2-44A2-86DD-EC9530CF30FA
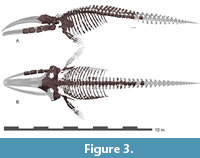
Holotype. SMAC 2731, the skull consists of the posterior half, missing all but the base of the rostrum, including the posterior ends of the maxillae, premaxillae, nasals, frontals, jugals, palatines, vomer, parietals, pterygoids, squamosals, exoccipitals, supraoccipital, exoccipitals, basioccipital, basisphenoid, periotics in situ, tympanic bullae, right posterior mandible, basihyal-thyrohyal, right stylohyal, sternum, seven cervical vertebrae including a fused axis and third cervical vertebra, nine thoracic vertebrae, and 16 more posterior vertebrae, rib fragments, scapulae, and left forelimb elements including humerus, ulna, radius, five carpals, three metacarpals, and two phalanges.
Locality and horizon. SMAC 2731 was found at a riverbed of Toyohira River in Sapporo City, Hokkaido, Japan, by Kazuhisa Mori on 10 October 2008: Latitude 42°58'1.24"N, longitude 141°13'18.01"E (Figure 1 and Figure 2). SMAC 2731 was found from the upper part of the Toyama Formation. At the fossil area, the diatomaceous siltstone Toyama Formation is distributed. Diatomaceous siltstone of the Toyama Formation is exposed at the type locality (Ozaki and Komatsubara, 2014). A 5-6 cm thick layer of tuff is deposited approximately 1 m below the type stratum of SMAC 2731. The geochronologic age of the tuff was evaluated by fission track analysis of zircon crystals. Euhedral zircon crystals were successfully extracted by magnetic and heavy liquid separation and subsequent experimental procedures followed Ohira (2004). Fission track analysis resulted in a date of 9.0 ± 0.4 Ma (Table 1). The chi-square test (P(χ2): 23.3%) passed 5% significance level, which assures the general absence of extra-Poissonian errors in the analysis, suggesting an identical grain-age population in the sample. Thus, the age of SMAC 2731 is approximately 8.6-9.4 Ma (Tortonian, early Late Miocene). About 200 m downstream from the locality of SMAC 2731, four ribs and the sternum of a sirenian Hydrodamalis sp. was discovered from the Toyama Formation too, and diatom zone and fission track analysis indicated an age of about 8 Ma (Furusawa 2007). The Toyama Formation provides molluskan fossils Lucinoma and Conchocele , which live at bathyal to deep cold waters (Suzuki, 2007).
Etymology. Named after the fossil locality, Sapporo City.
Diagnosis. Megabalaena sapporoensis is a member of the Balaenidae because it has a combination of these character states such as a posteriorly pointed anterior edge of the supraorbital process lateral to the ascending process of the maxilla with the skull in dorsal view (Character 31, state 0), laterally oriented postorbital process in dorsal view (Character 38, state 1), confluent posterior border of the zygomatic process of the squamosal and exoccipital in dorsal view (Character 67, state 1), dorsoventrally higher than long parietal in lateral view (Character 76, state 1), anterolaterally directed zygomatic process of the squamosal in dorsal view (Character 86, state 2), distinctly higher than long squamosal including the zygomatic and postglenoid processes (Character 92, state 1), short squamosal fossa (Character 96, state 1), foramen pseudovale opening posteriorly between the squamosal and pterygoid (Character 118, state 1), and posteriorly diverging basioccipital crests in ventral view (Character 125, state 0).
Among the Balaenidae, Megabalaena sapporoensis uniquely has an excavated orbit in dorsal view with a large postorbital process, dorsoventrally high anterior part of the involucrum of the tympanic bulla, long compound posterior process, high coronoid process and deeper subcondylar furrow of the mandible. M. sapporoensis uniquely has an autapomorphic combination of characters among the Balaenidae: a posterolaterally projected lateral process of the maxilla at the base of the rostrum (Character 15, state 0), overlapped jugal with the zygomatic process of the squamosal (Character 44, state 0), an anteroposteriorly compressed exposure of the frontal on the skull vertex (Character 73, state 1), more posteriorly located posterior apex of nuchal crest than the occipital condyle (Character 80, state 0), a confluent medial border of postglenoid process in ventral view with more medial portion of squamosal (Character 111, state 0), an anteroposteriorly flattened compound posterior process (Character 171, state 2), indistinct medial lobe of the tympanic bulla (Character 187, state 1), lack of the transverse crease on the dorsal surface of the involucrum of the tympanic bulla (Character 191, state 0), a subcondylar furrow extended across the posterior surface of the mandibular condyle, separating it from the angular process both medially and laterally (Character 212, state 3). M. sapporoensis also uniquely has unfused cervical vertebrae except the axis and third cervical vertebra (Character 226, state 1), and slender forelimb long bones including the humerus with a low and proximodistally short deltoid ridge, radius, and ulna (see discussion).
Compared to modern balaenids (Balaena mysticetus, Eubalaena australis, E. glacialis, and E. japonica) and some fossil species (Peripolocetus vexillifer, Balaenella brachyrhynus, Balaenula astensis, and Antwerpibalaena liberatlas), M. sapporoensis can be differentiated by having a ventrally oriented lateral furrow of the tympanic bulla in lateral view (Character 178, state 0). Compared to more earlier branching balaenids (Morenocetus parvus and P. vexillifer) and some other fossil species (B. brachyrhynus and B. astensis), M. sapporoensis can be differentiated by having a poorly exposed compound posterior process of the tympanoperiotic on lateral skull wall (Character 172, state 0). Compared to Eubalaena shinshuensis from Nagano, Japan, M. sapporoensis has a low anterolateral part of the nuchal crest. E. shinshuensis has a strong and laterally projected anterior part of the nuchal crest. Compared to Archaeobalaena dosanko from Hokkaido Japan, and some other balaenids (Morenocetus parvus, Eubalaena ianitrix, and B. astensis), M. sapporoensis can be differentiated by having the supramastoid crest of zygomatic process of the squamosal. Compared to fossil balaenids (M. parvus, B. brachyrhynus, Eubalaena ianitrix, A. dosanko, and B. astensis), M. sapporoensis can be differentiated by having strongly laterally projected pre- and postorbital processes. Compared with Balaenotus insignis and Idiocetus gucciardinii (Van Beneden 1880; Capellini 1905), M. sapporoensis can be differentiated by having a wider atlas with a dorsoventrally high transverse process. Compared with Balaenotus insignis , M. sapporoensis can be differentiated by having a dorsoventrally higher tympanic bulla in medial view, much wider fan-shaped scapula and much slenderer humerus (see more in discussion). Compared with Idiocetus gucciardinii, M. sapporoensis can be differentiated by having a wide anterior lobe of the tympanic bulla in dorsal view, and a mandible with a developed and more medially tilted coronoid process.
DESCRIPTION
Ontogenetic age. All preserved epiphyses of the vertebrae are fused. Through ontogeny, the order of vertebral fusion starts from the cervical and caudal regions to the middle (Flower, 1864; Ito and Miyazaki, 1990; Galatius and Kinze, 2003; Moran et al., 2015). Omura (1969) reported several Eubalaena glacialis including photos of physically mature males with ossified thoracic and lumbar vertebrae (IDs 61A (17.1 m long) and 61B (17.0 m long)). Megabalaena sapporoensis and the two individuals of E. glacialis have open suture of the parietal and squamosal at the temporal fossa, which might be not fused in adult balaenids. The observation and these previous studies suggest that SMAC 2731 is a physically matured adult.
Body size. The body size of the holotype of Megabalaena sapporoensis can be inferred from the Slater et al. (2017) formula for extinct mysticetes [log (TL) = 0.92 × (log (BZ) - 1.72) +2.68], which was originally established formula for stem mysticetes in Pyenson and Sponberg (2011), and the Bisconti et al. (2021) formula for the Balaenidae [log (TL) = 0.86303 × log (BZ) + 1.2125]. The bizygomatic width (BZ) of the holotype of Megabalaena sapporoensis is 2232 mm, giving reconstructed body length of 15.0 m and 12.7 m, respectively. We prefer 12.7 m, for the estimated total length of M. sapporoensis , because the formula of Bisconti et al. (2021) is specialized for the Balaenidae. We categorize balaenids by their total length as small (0-5.5 m), middle (5.6-8 m), large (10-13.5 m) and gigantic (>14 m) (Bisconti et al., 2021). Following the previous study, M. sapporoensis can be categorized as large balaenid (10-13.5 m), but it belongs to gigantic size (>14 m) if the result uses the formula from Slater et al. (2017).
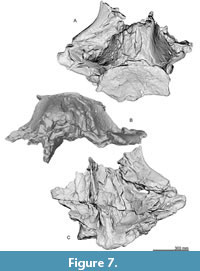
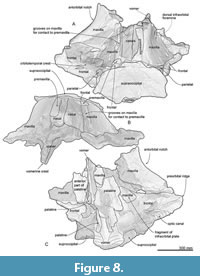
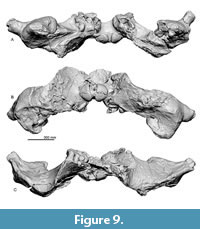
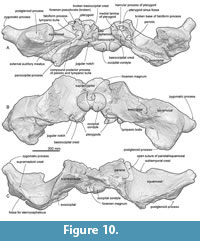
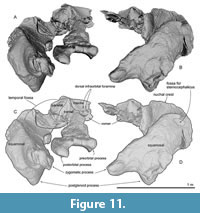
 General feature of the skull. The preserved right side of the skull (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11) has a slender supraorbital process of the frontal with laterally strongly projected pre- and postorbital processes and robust zygomatic process. It has a wider base of the rostrum than those of modern balaenids (Figure 3, Figure 4, Figure 5). The skull is separated mainly into three parts (left frontal + jugals (Figure 6), a vertex area (Figure 7, Figure 8), and a basicranial area (Figure 9, Figure 10).
General feature of the skull. The preserved right side of the skull (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11) has a slender supraorbital process of the frontal with laterally strongly projected pre- and postorbital processes and robust zygomatic process. It has a wider base of the rostrum than those of modern balaenids (Figure 3, Figure 4, Figure 5). The skull is separated mainly into three parts (left frontal + jugals (Figure 6), a vertex area (Figure 7, Figure 8), and a basicranial area (Figure 9, Figure 10).
Premaxilla. The ascending process of the right premaxilla is removed to the midline and covers the nasal dorsally. Its lateral border is straight, but its medial border is damaged. The preserved right premaxilla is about 50 mm wide and 270 mm long. The posterior end of the premaxilla contacts the frontals.
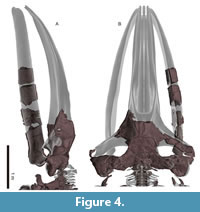 Maxilla. The left maxilla exhibits a strongly curved lateral margin at the rostrum base in dorsal and ventral view (Figure 8A and C). This area is the posterior part of the antorbital notch. The ascending process of the maxilla forms a gradual slope from the vertex to the lateral end. Three dorsal infraorbital foramina are preserved on the right maxilla (about 10 mm wide) (Figure 8A). At the vertex, a parasagittally oriented medial border of the ascending process contacts the premaxilla. In ventral view (Figure 8C), the maxilla contacts the vomer medially and frontal posteriorly. There is a triangular, smooth, and thin bony plate preserved ventral to the frontal, which is a displaced fragment of the left infraorbital plate of the maxilla.
Maxilla. The left maxilla exhibits a strongly curved lateral margin at the rostrum base in dorsal and ventral view (Figure 8A and C). This area is the posterior part of the antorbital notch. The ascending process of the maxilla forms a gradual slope from the vertex to the lateral end. Three dorsal infraorbital foramina are preserved on the right maxilla (about 10 mm wide) (Figure 8A). At the vertex, a parasagittally oriented medial border of the ascending process contacts the premaxilla. In ventral view (Figure 8C), the maxilla contacts the vomer medially and frontal posteriorly. There is a triangular, smooth, and thin bony plate preserved ventral to the frontal, which is a displaced fragment of the left infraorbital plate of the maxilla.
Nasal. Nasals are long and dorsoventrally high (left nasal is about 95 mm wide, 75 mm high, 310 mm long) and have a damaged dorsal surface. There are rounded anteriorly projected processes at the midline on the right and left nasals.
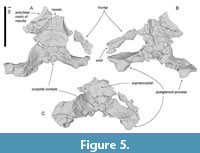 Frontal. At the vertex, the frontal exhibits a transversally wide and anteroposteriorly short exposure between the supraoccipital and maxilla. The supraorbital process is anteroposteriorly narrow with laterally well-projected pre- and postorbital processes (Figure 6A to C). The preorbital process is triangular with rounded anterior border in dorsal and ventral view. The postorbital process is robust and projects laterally well. These processes are dorsoventrally high. Between the pre- and postorbital processes, the orbital margin of the frontal is strongly concave. In lateral view, the orbit is rounded, and its anteroposterior length is 170 mm. In ventral view, the preorbital ridge is blunt, and the postorbital ridge is sharp. Between the ridges, the optic canal opens ventrally and laterally widely and continues to the lateral edge of the orbit. On the dorsal surface of the supraorbital process, the orbitotemporal crest is sharp and strongly developed medially (Figure 8B) and faint laterally on the supraorbital process (Figure 6A). The medial part of the frontal contacts with the vomer medially and is covered by the maxilla and palatine anteromedially and medially (respectively; Figure 7 and Figure 8C). At the vertex, the frontal has an anteroposteriorly short exposure, and it contacts the nasal and premaxilla anteriorly, and the parietal and supraoccipital posteriorly.
Frontal. At the vertex, the frontal exhibits a transversally wide and anteroposteriorly short exposure between the supraoccipital and maxilla. The supraorbital process is anteroposteriorly narrow with laterally well-projected pre- and postorbital processes (Figure 6A to C). The preorbital process is triangular with rounded anterior border in dorsal and ventral view. The postorbital process is robust and projects laterally well. These processes are dorsoventrally high. Between the pre- and postorbital processes, the orbital margin of the frontal is strongly concave. In lateral view, the orbit is rounded, and its anteroposterior length is 170 mm. In ventral view, the preorbital ridge is blunt, and the postorbital ridge is sharp. Between the ridges, the optic canal opens ventrally and laterally widely and continues to the lateral edge of the orbit. On the dorsal surface of the supraorbital process, the orbitotemporal crest is sharp and strongly developed medially (Figure 8B) and faint laterally on the supraorbital process (Figure 6A). The medial part of the frontal contacts with the vomer medially and is covered by the maxilla and palatine anteromedially and medially (respectively; Figure 7 and Figure 8C). At the vertex, the frontal has an anteroposteriorly short exposure, and it contacts the nasal and premaxilla anteriorly, and the parietal and supraoccipital posteriorly.
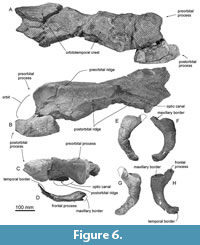 Jugal. The jugal is an anteroposteriorly long (220 mm) and dorsoventrally thin (maximum height is 47 mm) bone (Figure 6). The maxillary border has a deep and smooth excavation on the dorsal surface, which contacts the maxilla. Medial to the maxillary border, the frontal process projects anteriorly, which is dorsoventrally thin and squared in dorsal and ventral view. The jugal is weakly curved and ventrally convex and forms the ventral border of the orbit. The temporal border forms a flat surface dorsally, which contacts the zygomatic process of the squamosal.
Jugal. The jugal is an anteroposteriorly long (220 mm) and dorsoventrally thin (maximum height is 47 mm) bone (Figure 6). The maxillary border has a deep and smooth excavation on the dorsal surface, which contacts the maxilla. Medial to the maxillary border, the frontal process projects anteriorly, which is dorsoventrally thin and squared in dorsal and ventral view. The jugal is weakly curved and ventrally convex and forms the ventral border of the orbit. The temporal border forms a flat surface dorsally, which contacts the zygomatic process of the squamosal.
Parietal. The parietal is broadly exposed laterally, between the frontal and supraoccipital in lateral view (Figure 11A and C) and forms the lateral part of the vertex. The lateral exposure of the parietal forms the anteromedial surface of the temporal fossa.
Palatine. The palatine is an anteroposteriorly long and dorsoventrally thin plate just medial to the posterior end of the maxilla (Figure 8C). The shape of the maxillopalatine suture is unclear, but the anterior part of the palatine invades into the posterior border of the maxilla. The preserved anterior part of the palatine is smooth ventrally.
 Vomer. The anterior part of the vomer has a sharp vomerine crest in anterior view (Figure 8B). The preserved posterior part of the vomer has a low posterior end of the vomerine crest in ventral view (Figure 8B). There are two weak depressions on the right and left sides of the end of the vomerine crest, which correspond to the posterior internal choanae.
Vomer. The anterior part of the vomer has a sharp vomerine crest in anterior view (Figure 8B). The preserved posterior part of the vomer has a low posterior end of the vomerine crest in ventral view (Figure 8B). There are two weak depressions on the right and left sides of the end of the vomerine crest, which correspond to the posterior internal choanae.
Pterygoid. The pterygoid continues posteriorly to overlap the basioccipital crest (Figure 10A). The hamular process is a dorsoventrally thick and transversely wide bony plate forming the anterior border of the pterygoid sinus fossa.
 Squamosal. In ventral view (Figure 10A), between the squamosal and pterygoid, there is a notch, which might be a broken foramen pseudovale. The falciform is broken but thick posterior to the foramen pseudovale. Posterior to the falciform process, a sharp spiny process projects medially (Figure 12B). The posterior border of the squamosal contacts to the exoccipital laterally. Just anterior to the border, there is a long and laterally widely open external auditory meatus. Anterolateral to the external auditory meatus, a huge postglenoid process projects ventrally. The anterior surface of the postglenoid process is a weakly swollen articular surface for the mandible. The posterior surface of the postglenoid process is strongly excavated. In dorsal view, the right squamosal exhibits the open suture of the squamosal and parietal (Figure 10C). The squamosal has a slender zygomatic process projecting ventrolaterally. The anterior part of the zygomatic process is squared in dorsal and ventral view. The supramastoid crest is blunt and runs on the dorsal surface of the zygomatic process and reaches the posterior part of the nuchal crest. The sternocephalicus fossa on the posterolateral surface of the zygomatic process is shallow.
Squamosal. In ventral view (Figure 10A), between the squamosal and pterygoid, there is a notch, which might be a broken foramen pseudovale. The falciform is broken but thick posterior to the foramen pseudovale. Posterior to the falciform process, a sharp spiny process projects medially (Figure 12B). The posterior border of the squamosal contacts to the exoccipital laterally. Just anterior to the border, there is a long and laterally widely open external auditory meatus. Anterolateral to the external auditory meatus, a huge postglenoid process projects ventrally. The anterior surface of the postglenoid process is a weakly swollen articular surface for the mandible. The posterior surface of the postglenoid process is strongly excavated. In dorsal view, the right squamosal exhibits the open suture of the squamosal and parietal (Figure 10C). The squamosal has a slender zygomatic process projecting ventrolaterally. The anterior part of the zygomatic process is squared in dorsal and ventral view. The supramastoid crest is blunt and runs on the dorsal surface of the zygomatic process and reaches the posterior part of the nuchal crest. The sternocephalicus fossa on the posterolateral surface of the zygomatic process is shallow.
 Exoccipital and supraoccipital. In dorsal views (Figure 8A), the supraoccipital has an anteriorly projected part at the center and curved lateral part of the nuchal crest. The supraoccipital apex is convex anteriorly and it covers the frontals anteriorly.
Exoccipital and supraoccipital. In dorsal views (Figure 8A), the supraoccipital has an anteriorly projected part at the center and curved lateral part of the nuchal crest. The supraoccipital apex is convex anteriorly and it covers the frontals anteriorly.
In posterior view (Figure 9B), the exoccipital and supraoccipital are fused. In ventral view (Figure 9A), the lateral end of the exoccipital is anteroposteriorly thickened. The occipital condyle is globular and projects distinctly posteriorly. A wide elliptical foramen magnum is located at a dorsal half of the occipital condyle.
 Basioccipital and basisphenoid. In ventral view (Figure 9A), basioccipital and basisphenoid are fused. A transversely thick and flat basioccipital crest projects ventrally strongly. There is a weak transverse ridge on the ventral surface of the basioccipital crest.
Basioccipital and basisphenoid. In ventral view (Figure 9A), basioccipital and basisphenoid are fused. A transversely thick and flat basioccipital crest projects ventrally strongly. There is a weak transverse ridge on the ventral surface of the basioccipital crest.
Periotic. The periotics (Figure 12 and Table 3) are in situ, and only the left one is visible (Figure 12C). The anterior process is mediolaterally wide and is still partly covered by the squamosal. The lateral tuberosity is invisible because of the matrix. Posteriorly, a transversely wide elliptical fenestra rotunda is visible. Lateral to the fenestra rotunda, there is a shallow and circular mallear fossa anteriorly and a long depression covered by the matrix including the stapedial muscle fossa, fenestra ovalis and facial sulcus posteriorly. The compound posterior process of the periotic and tympanic bulla extends posterolaterally (Figure 12A and B) and bears a shallow facial sulcus near its posterior border.
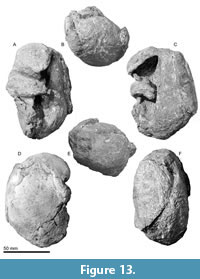
 Tympanic bulla. The tympanic bullae (Figure 12, Figure 13, Figure 14, Table 3) is broad bean shape in ventral view, which are anteroposteriorly longer than the width. The involucrum is dorsoventrally high with a weakly swollen medial lobe at the level of the conical process. The anterior lobe of the tympanic bulla is swollen medially and has a weak ridge running transversely on the anterior lobe. There is a large sigmoid process posterior to the anterior lobe, which is diagenetically displaced into the tympanic cavity. The lateral border of the sigmoid process is gradually rounded in posterior view (Figure 13B), and the medial margin of the sigmoid process is thick (approximately 9 mm long).
Tympanic bulla. The tympanic bullae (Figure 12, Figure 13, Figure 14, Table 3) is broad bean shape in ventral view, which are anteroposteriorly longer than the width. The involucrum is dorsoventrally high with a weakly swollen medial lobe at the level of the conical process. The anterior lobe of the tympanic bulla is swollen medially and has a weak ridge running transversely on the anterior lobe. There is a large sigmoid process posterior to the anterior lobe, which is diagenetically displaced into the tympanic cavity. The lateral border of the sigmoid process is gradually rounded in posterior view (Figure 13B), and the medial margin of the sigmoid process is thick (approximately 9 mm long). 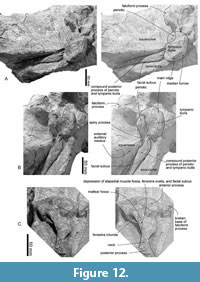 Anteriorly, the lateral furrow runs transversely and separates the sigmoid process from the anterior lobe. There is an anteroposteriorly long mallear ridge just anterior to the sigmoid process. There is an anteroposteriorly long and mediolaterally thin conical process posterior to the sigmoid process. The broken base of the posterior process is located posterior to the conical process. Posterior to the conical process, the outer posterior prominence is a larger projection compared to the inner posterior prominence. The main ridge runs anteroposteriorly on the lateral surface of the tympanic bulla from the outer posterior prominence. The inner posterior prominence is weakly projected posteriorly dorsal to the outer posterior prominence (Figure 13F). Between the prominences, a clear median furrow is not developed.
Anteriorly, the lateral furrow runs transversely and separates the sigmoid process from the anterior lobe. There is an anteroposteriorly long mallear ridge just anterior to the sigmoid process. There is an anteroposteriorly long and mediolaterally thin conical process posterior to the sigmoid process. The broken base of the posterior process is located posterior to the conical process. Posterior to the conical process, the outer posterior prominence is a larger projection compared to the inner posterior prominence. The main ridge runs anteroposteriorly on the lateral surface of the tympanic bulla from the outer posterior prominence. The inner posterior prominence is weakly projected posteriorly dorsal to the outer posterior prominence (Figure 13F). Between the prominences, a clear median furrow is not developed.
 Mandible. An incomplete right mandible is preserved as five separated fragment parts including the most posterior part (Figure 15, Table 2). The mandible in Figure 3 has been digitally re-inflated to resemble the proportions of other balaenids. The most anterior part (Figure 15D) is the body of the mandible. It is dorsoventrally high and mediolaterally narrow, but its medial surface is mediolaterally crushed during fossilization (Shinmura et al., 2024). The posterior part of the mandible has a relatively low high coronoid process as a balaenid. In dorsal view (Figure 15C), between the coronoid process and mandibular condyle, a low neck expands medially strongly. The mandibular foramen opens posterodorsally largely (80 mm in diameter) and the mandibular foramen is expanded. The mylohyoid groove is developed as a shallow longitudinal groove anterior to the mandibular foramen along the ventromedial side of the mandible. The angular process is transversely narrow and weakly projected posteriorly.
Mandible. An incomplete right mandible is preserved as five separated fragment parts including the most posterior part (Figure 15, Table 2). The mandible in Figure 3 has been digitally re-inflated to resemble the proportions of other balaenids. The most anterior part (Figure 15D) is the body of the mandible. It is dorsoventrally high and mediolaterally narrow, but its medial surface is mediolaterally crushed during fossilization (Shinmura et al., 2024). The posterior part of the mandible has a relatively low high coronoid process as a balaenid. In dorsal view (Figure 15C), between the coronoid process and mandibular condyle, a low neck expands medially strongly. The mandibular foramen opens posterodorsally largely (80 mm in diameter) and the mandibular foramen is expanded. The mylohyoid groove is developed as a shallow longitudinal groove anterior to the mandibular foramen along the ventromedial side of the mandible. The angular process is transversely narrow and weakly projected posteriorly.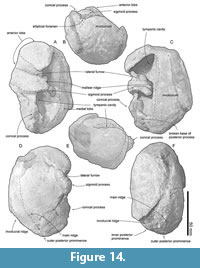 The mandibular condyle is separated from the angular process by a transversely long subcondylar furrow on the medial and posterior sides. The subcondylar furrow is deeper than those of extant balaenids. The mandibular condyle is oriented posterodorsally. The shape of the mandibular condyle is elliptical and is nearly elliptical in shape and transversely wider dorsally, forming blunt dorsolateral and dorsal corners in articular/posterior view (Figure 15A). In dorsal view, the condyle is subtriangular (Figure 15C). The lateral corner of the mandibular condyle (Tanaka and Taruno 2024) is dorsally elevated, at the same level as the dorsal border of the mandibular ramus.
The mandibular condyle is separated from the angular process by a transversely long subcondylar furrow on the medial and posterior sides. The subcondylar furrow is deeper than those of extant balaenids. The mandibular condyle is oriented posterodorsally. The shape of the mandibular condyle is elliptical and is nearly elliptical in shape and transversely wider dorsally, forming blunt dorsolateral and dorsal corners in articular/posterior view (Figure 15A). In dorsal view, the condyle is subtriangular (Figure 15C). The lateral corner of the mandibular condyle (Tanaka and Taruno 2024) is dorsally elevated, at the same level as the dorsal border of the mandibular ramus.
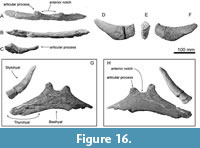
Hyoid bones. 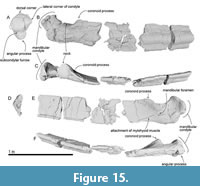 The right stylohyal and a single fused element of the basihyal-thyrohyal are preserved (Figure 16G and H). The basihyal-thyrohyal is dorsoventrally thin (about 22 mm with at the center) plate with posterolaterally projected lateral portions (preserved maximum dimensions are 410 mm wide, 145 mm long). Its ventral surface and the right end and left lateral portion are broken. There are two anteriorly well-projected articular processes for the ceratohyal on the anterior margin of the basihyal-thyrohyal. Between the articular processes, there is a widely open anterior notch. The posterior processes on the posterior margin seem absent. The right stylohyal (Figure 16D-F) is a transversely thin bar and is anteriorly slender and posteriorly thick (preserved maximum dimensions are 210 mm long, 45 mm wide, and 70 mm high). Its anterior end is broken. Its posterior end is dorsoventrally higher elliptical and flat (45 mm wide and 60 mm high) (Figure 16E).
The right stylohyal and a single fused element of the basihyal-thyrohyal are preserved (Figure 16G and H). The basihyal-thyrohyal is dorsoventrally thin (about 22 mm with at the center) plate with posterolaterally projected lateral portions (preserved maximum dimensions are 410 mm wide, 145 mm long). Its ventral surface and the right end and left lateral portion are broken. There are two anteriorly well-projected articular processes for the ceratohyal on the anterior margin of the basihyal-thyrohyal. Between the articular processes, there is a widely open anterior notch. The posterior processes on the posterior margin seem absent. The right stylohyal (Figure 16D-F) is a transversely thin bar and is anteriorly slender and posteriorly thick (preserved maximum dimensions are 210 mm long, 45 mm wide, and 70 mm high). Its anterior end is broken. Its posterior end is dorsoventrally higher elliptical and flat (45 mm wide and 60 mm high) (Figure 16E).
 Sternum. The sternum is shield-like and symmetrical, and the posterior part is broken (Figure 17) (preserved maximum dimensions are 260 mm long, 240 mm wide, and 70 mm high). The anterior part is wider due to the presence of the articulation facet for the first rib on the lateral margins. The articulation facet for the first rib is anteroposteriorly long elliptical and flat surface (110 mm long, 55 mm high) (Figure 17B). The ventral side of the sternum is convex and has an anteroposteriorly long ventral keel (Figure 17C).
Sternum. The sternum is shield-like and symmetrical, and the posterior part is broken (Figure 17) (preserved maximum dimensions are 260 mm long, 240 mm wide, and 70 mm high). The anterior part is wider due to the presence of the articulation facet for the first rib on the lateral margins. The articulation facet for the first rib is anteroposteriorly long elliptical and flat surface (110 mm long, 55 mm high) (Figure 17B). The ventral side of the sternum is convex and has an anteroposteriorly long ventral keel (Figure 17C).
Vertebra. 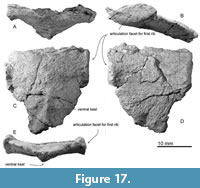 The vertebrae were found in articulation. The proposed sequence of vertebrae is based on the sequence observed during the excavation and/or preparation. All cervical vertebrae are preserved as six bones (Figure 18). The axis and third cervical vertebra are fused firmly at the bodies (Figure 18H). At least nine thoracic vertebrae have existed, and16 more posterior vertebrae cannot be identified thoracic or lumbar vertebrae because of damages.
The vertebrae were found in articulation. The proposed sequence of vertebrae is based on the sequence observed during the excavation and/or preparation. All cervical vertebrae are preserved as six bones (Figure 18). The axis and third cervical vertebra are fused firmly at the bodies (Figure 18H). At least nine thoracic vertebrae have existed, and16 more posterior vertebrae cannot be identified thoracic or lumbar vertebrae because of damages.
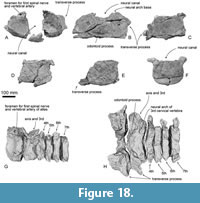 The atlas is rounded in anterior view with a concave anterior articular surface for the skull and convex posterior articular surface for the axis (Figure 18A, G, and H). The laterally projected transverse process is anteroposteriorly thick, and dorsoventrally high at its base (there is no evidence of the transverse foramen preserved). The neural canal is a dorsoventrally high foramen formed also by the socket for the odontoid process. On the broken base of the neural arch, there is a dorsoventrally high elliptical and large foramen for the first spinal nerve and vertebral artery (15-20 mm in diameter).
The atlas is rounded in anterior view with a concave anterior articular surface for the skull and convex posterior articular surface for the axis (Figure 18A, G, and H). The laterally projected transverse process is anteroposteriorly thick, and dorsoventrally high at its base (there is no evidence of the transverse foramen preserved). The neural canal is a dorsoventrally high foramen formed also by the socket for the odontoid process. On the broken base of the neural arch, there is a dorsoventrally high elliptical and large foramen for the first spinal nerve and vertebral artery (15-20 mm in diameter).
The fused axis and third cervical vertebra are very wide because of a large transverse process (490 mm wide). The anterior articular surface for the atlas is weakly concave. Between the articular surfaces, a blunt odontoid process projects anteriorly weakly. The posterior articular surface for the fourth cervical vertebra is laterally wider than high circle and is strongly excavated in the mid line (Figure 18R).
 The fourth to sixth cervical vertebrae have transversely wide and anteroposteriorly thin bodies. There are thin bodies and process ventral median ridges.
The fourth to sixth cervical vertebrae have transversely wide and anteroposteriorly thin bodies. There are thin bodies and process ventral median ridges.
The seventh cervical vertebra has slightly thicker body than the fourth to sixth cervical vertebrae. The body shape in anteroposterior view is more rounded elliptic than the other cervical vertebrae.
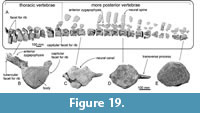
The first thoracic vertebra can be articulated to the seventh cervical vertebra. The anterior thoracic vertebrae such as the second to fourth thoracic vertebrae have anteriorly projected anterior zygapophyses (Figure 19A and B). There is a large capitular facet for the rib facing ventrolaterally at the lateral end of the transverse process. The transverse process projects dorsolaterally in more anterior thoracic vertebrae and laterally in more posterior thoracic vertebrae. There is also a smaller facet for the rib at the posterolateral edge of the body. This feature can be examined from the first to ninth thoracic vertebrae. However, none of the more posterior preserved vertebrae preserves the facet for rib.
Here, we tentatively identify the thoracic-lumbar vertebrae posterior to the ninth thoracic vertebra, because identifying the limits on boundaries between thoracic, lumbar, and caudal regions. The thoracic vertebrae are distinguishable from the lumbar vertebrae by having articular facets for the ribs on the end of the transverse process. Rib facets on vertebrae posterior to the ninth thoracic vertebra are not adequately preserved. The caudal vertebrae are distinguishable from the lumbar vertebrae by having the haemal facets. The most posterior vertebra cannot be identified as a lumbar or caudal vertebra, because it does not preserve the ventral border including the base of the haemal facets.
Vertebrae posterior to the ninth thoracic vertebra have bodies that are anteroposteriorly 1.15-2.1 times longer than more anterior vertebrae. Their transverse processes are dorsoventrally thin. All preserved neural spines are separated from the bodies. The neural arch of one of the more posterior vertebrae has anteriorly well-projected and dorsoventrally high anterior zygopophyses at the anterior edge of the neural spine in lateral view. The neural spine is about 400 mm high, and it has a squared dorsal end in lateral view.
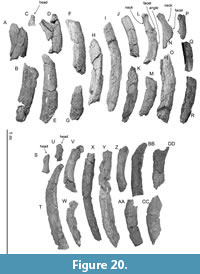 Ribs. There are over 100 rib fragments, which do not have contact with other fragments. The ribs number 1 to 18 and 19 to 30 in Figure 20 are proposed to be the left and right, respectively. The ribs are transversely wide and anteroposteriorly thin, which are probably the middle part of the ribs among the series of the thoracic. Figure 20 shows relatively well-preserved rib fragments. Rib number 1 in Figure 20 is wide, which might be the dorsal part of the most anterior rib. Its width is about 160 mm. Maximum preserved length of rib number 9 in Figure 20 is about 650 mm. Rib number 12 has a rounded top and a long neck. The distance between the head and angle of rib number 12 is about 190 mm. The longest preserved rib is number 25 in Figure 20, which is about 760 mm long.
Ribs. There are over 100 rib fragments, which do not have contact with other fragments. The ribs number 1 to 18 and 19 to 30 in Figure 20 are proposed to be the left and right, respectively. The ribs are transversely wide and anteroposteriorly thin, which are probably the middle part of the ribs among the series of the thoracic. Figure 20 shows relatively well-preserved rib fragments. Rib number 1 in Figure 20 is wide, which might be the dorsal part of the most anterior rib. Its width is about 160 mm. Maximum preserved length of rib number 9 in Figure 20 is about 650 mm. Rib number 12 has a rounded top and a long neck. The distance between the head and angle of rib number 12 is about 190 mm. The longest preserved rib is number 25 in Figure 20, which is about 760 mm long. 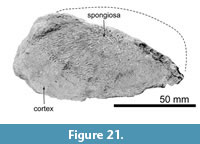 The distal end of rib number 20 in Figure 20 was cut by a saw possibly during excavation (Figure 21). It has about a 2 cm thick dense cortex and spongiosa at the medullary zone of the rib.
The distal end of rib number 20 in Figure 20 was cut by a saw possibly during excavation (Figure 21). It has about a 2 cm thick dense cortex and spongiosa at the medullary zone of the rib.
Scapula. The scapula is anteroposteriorly longer than wide and triangular, with a convex curved dorsal border (Figure 22A). On the lateral surface, there is a weak scapular spine running dorsoventrally near the anterior edge of the scapula. Ventral to the spine, there is an anteriorly well-projected and mediolaterally thin acromion process. Ventral to the acromion process, much smaller coracoid process projects anteriorly. The acromion and coracoid processes of extant balaenids are much weaker than these of Megabalaena sapporoensis. The glenoid fossa is an anteroposteriorly long ellipse in ventral view and is weakly depressed (Figure 22B).
Humerus. The humerus has a slender shaft and semi globular head (Figure 22A, D to G). The shaft is mediolaterally thinner than anteroposteriorly long. A dorsoventrally long and rugose great tuberosity projects anteriorly at the proximal end of the shaft. The deltoid ridge is low and proximodistally short. The damaged distal end is posteriorly flat, which is the articular surface for the ulna.
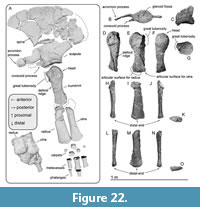 Ulna and radius. The proximodistal length of the ulna and radius are slightly longer than that of the humerus. The ulna (Figure 22A and H to K) is proximodistally shorter than the radius (Figure 22A and I to O). Both bones are mediolaterally flat and distally anteroposteriorly widened. At the distal ends, the anterior surface of the radius and the posterior surface of the ulna are transversely thin.
Ulna and radius. The proximodistal length of the ulna and radius are slightly longer than that of the humerus. The ulna (Figure 22A and H to K) is proximodistally shorter than the radius (Figure 22A and I to O). Both bones are mediolaterally flat and distally anteroposteriorly widened. At the distal ends, the anterior surface of the radius and the posterior surface of the ulna are transversely thin.
Carpal, metacarpal, and phalanges. A cast exhibits the original position of the ulna, radius, carpals, and a metacarpal (Figure 22A). The preserved metacarpals and phalanges might be belonged to the 2nd to 4th fingers based on relative position against the ulna and radius, examined in the extant species (Cooper et al., 2007). The larger preserved carpal was discovered next to the posterior end of the radius and the anterior end of the ulna, thus this might be the intermedium (90 mm long, 85 mm high, 70 mm wide). It has rugose surfaces except lateral surfaces, which are flat and smooth. The smallest carpal is about 50 mm in diameter, and all its surfaces are rugose.
The metacarpals are spool-shaped with a waist in the middle, which is an approximately cylindrical in cross-section. The metacarpals have anteroposteriorly longer and shorter ends, which possibly are the proximal and distal ends respectively. The largest preserved metacarpal is 170 mm long, 98 mm in proximal length, 64 mm in proximal width, 97 mm in distal length, 47 mm in distal width. The smallest preserved metacarpal is 132 mm long, 82 mm in proximal length, 55 mm in proximal width, 66 mm in distal length, 49 mm in distal width.
The phalanges have anteroposteriorly much shorter distal ends than the proximal ends. The largest preserved phalanx is 101 mm long, 88 mm in proximal length, 51 in proximal width, 54 mm in distal length, 29 mm in distal width. The smallest preserved phalanx is 124 mm long, 57 mm in proximal length, 46 mm in proximal width, 42 mm in distal length, 28 mm in distal width.
Comparisons. Compared to the oldest known balaenid M. parvus with a weaker lateral projection of the zygomatic process, the exoccipital width of M. sapporoensis measures 60% of the bizygomatic width, which is a common ratio for the Balaenidae. Between the pre- and postorbital processes, the orbital margin of the frontal is strongly concave, differing from the straight lateral margin in other balaenids (M. parvus, B. brachyrhynus, Eubalaena ianitrix, A. dosanko, B. astensis, B. mysticetus, and E. glacialis). Balaenula balaenopsis has excavated orbit in dorsal and ventral view, but its posterior orbital process does not project laterally well like that of M. sapporoensis. Regarding the cervical vertebrae fusion, M. sapporoensis has only the axis and 3rd cervical vertebra are fused, which is a minimal degree of vertebra fusion condition in the Balaenidae (see more in discussion). However, this condition can be seen in some Miocene non-balaenid baleen whales such as Thinocetus arthritus, Uranocetus gramensis and Pelocetus calvertensis (Kellogg, 1965, 1969; Steeman, 2009).
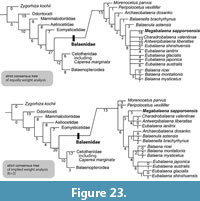 Phylogenetic results. The phylogenetic analyses present 70 shortest trees of 870 steps each for unweighted, and 10 shortest tree with a tree bisection reconnection score of 84.00321. Strict consensus trees of the equal and implied weight analyses are similar in having a clade of the Balaena, and recognizing Morenocetus parvus and Peripolocetus vexillifer as the most basal clades among the Balaenidae (Figure 23). However, only the strict consensus tree of implied weight analysis formed a clade of Megabalaena sapporoensis with Charadrobalaena valentinae + Antwerpibalaena liberatlas + Eubalaena ianitrix.
Phylogenetic results. The phylogenetic analyses present 70 shortest trees of 870 steps each for unweighted, and 10 shortest tree with a tree bisection reconnection score of 84.00321. Strict consensus trees of the equal and implied weight analyses are similar in having a clade of the Balaena, and recognizing Morenocetus parvus and Peripolocetus vexillifer as the most basal clades among the Balaenidae (Figure 23). However, only the strict consensus tree of implied weight analysis formed a clade of Megabalaena sapporoensis with Charadrobalaena valentinae + Antwerpibalaena liberatlas + Eubalaena ianitrix.
The topology of the 50% majority consensus and strict consensus trees of implied weighting analysis are the same (Appendix 5). Their branching patterns (Figure 23) are similar in forming clades of Balaena, Eubalaena and also a clade of the Balaena + Eubalaena from that of the analysis of Tanaka et al (2020), except Eubalaena ianitrix excluded from the Eubalaena clade and was placed in a clade with M. sapporoensis.
The 50% majority consensus and strict consensus trees of equally weighted analysis (Figure 23; Appendix 5) are slightly different from that of the analysis of Tanaka et al (2020) in that there is a large unresolved polytomy of Charadrobalaena valentinae + Antwerpibalaena liberatlas + Eubalaena shinshuensis + Eubalaena ianitrix and two clades of crown balaenids. M. sapporoensis was placed among the unresolved polytomy in the Balaenidae with a long branch length (Figure 23).
Our phylogenetic analysis results were different from one of the latest balaenid study by Bisconti et al. (2021) in a branching pattern of Peripolocetus vexillifer and Caperea marginata . In their study, C. marginata forms a clade with the Balaenidae, and P. vexillifer was the most basal taxon among the large group Balaenomorpha. In our analysis, C. marginata was placed in the Cetotheriidae and forms a clade with the Balaenopteridae (Figure 23).
DISCUSSION
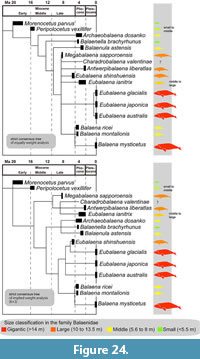 Gigantism. Body size variations among the Balaenidae are presented here (Figure 24 and Figure 25) using the result of phylogenetic analysis (Figure 23) and estimated body size of extinct species (Table 6). The estimated total lengths of balaenids excluding Megabalaena sapporoensis were obtained from Bisconti et al. (2021). Notably, the previously determined total length of Eubalaena shinshuensis based on the bizygomatic width (Buono et al., 2017; Slater et al., 2017; Bisconti et al., 2021) about 12.0 to 10.8 m is slightly underestimated, as the specimen lacks the anterior part of the zygomatic process. Thus, the estimated body length is unknown, however it is over 12.0 to 10.8 m. Modern maximum adult total length was taken from Jefferson et al. (2008).
Gigantism. Body size variations among the Balaenidae are presented here (Figure 24 and Figure 25) using the result of phylogenetic analysis (Figure 23) and estimated body size of extinct species (Table 6). The estimated total lengths of balaenids excluding Megabalaena sapporoensis were obtained from Bisconti et al. (2021). Notably, the previously determined total length of Eubalaena shinshuensis based on the bizygomatic width (Buono et al., 2017; Slater et al., 2017; Bisconti et al., 2021) about 12.0 to 10.8 m is slightly underestimated, as the specimen lacks the anterior part of the zygomatic process. Thus, the estimated body length is unknown, however it is over 12.0 to 10.8 m. Modern maximum adult total length was taken from Jefferson et al. (2008).
Our phylogenetic analyses results revealed that the earliest balaenids, such as M. parvus and Peripolocetus vexillifer known from the Early to Middle Miocene respectively are placed as earlier branching taxa. The two earliest balaenids suggest that the total lengths of Balaenidae were originally about 4 to 6 m, which are much smaller than those of modern balaenids reaching 17 to 20 m.
An approximately 9 million years long gap in the Miocene record of balaenids exists after Peripolocetus vexillifer (16.0-15.2 Ma) and prior to Eubalaena shinshuensis (6.1-5.9 Ma) (Marx and Fordyce 2015). From the late and latest Miocene (approximately 9-6 Ma), M. sapporoensis at 12.7 m and E. shinshuensis at over 12.0-10.8 m (Bisconti et al., 2021; this study) in length. Currently, M. sapporoensis partly fills the Miocene gap of balaenids, and emphasizes that the origin of gigantism is chronologically deeper than previously considered. We do not have enough numbers of balaenid fossils from the Late Miocene, thus can not justify are M. sapporoensis and E. shinshuensis represent a standard size of balaenids at that epochs or not. However, at least the first gigantism in the Balaenidae linage was happened before the Tortonian.
 From the Zanclean to Quaternary, the maximum size of balaenids increased dramatically and size disparity of the balaenids were declined (Figure 25). Our phylogenetic hypotheses showed that the extant and largest member of the family Balaena mysticetus has two middle-sized extinct relatives. It suggests that the linage leading to Balaena mysticetus became gigantic during the late Pliocene to Pleistocene or later. During the Pliocene, small balaenids were thought to have declined as a result of the onset of Northern Hemisphere glaciation and more patchy prey distribution (Marx and Fordyce, 2015; Slater et al., 2017). There are several balaenid fossils from the Pleistocene (e.g., Boessenecker, 2013). Adding named and phylogenetically examined Pleistocene balaenids will help to illuminate how extant large balaenids emerged and such small fossil balaenids disappeared.
From the Zanclean to Quaternary, the maximum size of balaenids increased dramatically and size disparity of the balaenids were declined (Figure 25). Our phylogenetic hypotheses showed that the extant and largest member of the family Balaena mysticetus has two middle-sized extinct relatives. It suggests that the linage leading to Balaena mysticetus became gigantic during the late Pliocene to Pleistocene or later. During the Pliocene, small balaenids were thought to have declined as a result of the onset of Northern Hemisphere glaciation and more patchy prey distribution (Marx and Fordyce, 2015; Slater et al., 2017). There are several balaenid fossils from the Pleistocene (e.g., Boessenecker, 2013). Adding named and phylogenetically examined Pleistocene balaenids will help to illuminate how extant large balaenids emerged and such small fossil balaenids disappeared.
Thus, we still cannot conclude that the body size of the Balaenidae increased over geological time (Duboys de Lavigerie et al., 2020). In terms of body size diversity, M. sapporoensis enhances our understanding of balaenid diversity, which was seems have expanded at least before the Late Miocene. In addition, a recent study of Nagasawa et al (2024) reported three large mandibles of mysticetes from the middle Miocene (about 13 to 12 Ma), which were revealed as cross-sections in huge concretion at the coast in Oga Peninsula, Akita, Japan. These mandibles were identified as balaenids and estimated their body sizes as 16 to 23 meters based on the cross-section shapes of the mandibles. We hope that these fossils at the site to be preserved and provided more detailed studies of these fossils to understand the origin of the first gigantism among the Balaenidae before the Late Miocene.
As fossil records of the Late Miocene balaenids are still limited, more balaenid records from this period and earlier in the Miocene are needed to develop a more detailed understanding of body size evolution including the origin(s) of gigantism.
Filling a part of morphological gap of the Balaenidae. The early balaenids are poorly known due to the scarce fossil record, M. sapporoensis helps fill a part of 9-million-year gap in the fossil record, connecting primitive conditions to specialized modern balaenids. A brief trend of the flipper shape of Miocene to modern balaenids is changed from slender to robust.
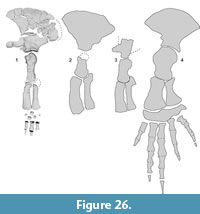 The flipper shape of modern balaenids are broad and paddle-like, aiding in low-speed maneuverability (Figure 26) (Benke, 1993; Cooper et al., 2007; DuBoys de Lavigerie et al., 2020). Previously, the Pliocene balaenid Antwerpibalaena liberatlas was documented a “previously unseen variability in balaenid flipper shape, ” with more slender forelimb long bones (Duboys de Lavigerie et al., 2020). Notably, the preserved humerus and radius of M. sapporoensis display even more slender (Table 7), resembling those of the eomysticetid Yamatocetus canaliculatus (Okazaki, 2012). Compared with Y. canaliculatus , M. sapporoensis has a lower deltoid ridge on the humerus and an anteroposteriorly longer distal end of both the ulna and radius. Compared with A. liberatlas, M. sapporoensis has a semi globular humerus head and anteroposteriorly shorter distal ends of the ulna and radius. A proportion of the humerus such as proximodistal length/anteroposterior maximum length of the humerus are 2.2 (M. sapporoensis), 1.9 (A. liberatlas), 1.3 (Eubalaena japonica), which suggests that the humerus of balaenids became robust. A proportion of the radius such as proximodistal length/anteroposterior length of the distal end of the radius are 2.8 (M. sapporoensis), 2.8 (C. valentinae), 2.6 (A. liberatlas), 1.6 (Eubalaena japonica), which also suggests that the radius of balaenids became robust. We could not use the ulna, because its proximal end is not preserved in M. sapporoensis , however most likely the ulna might had the same trend to these of the humerus and radius. In addition, a ratio of the upper and lower arms such as proximodistal length of the humerus and radius are 1.0 (M. sapporoensis), 0.8 (A. liberatlas), 1.0 (E. japonica), which suggests that the ratio of the upper and lower arm length is not so changed from Miocene to modern balaenids.
The flipper shape of modern balaenids are broad and paddle-like, aiding in low-speed maneuverability (Figure 26) (Benke, 1993; Cooper et al., 2007; DuBoys de Lavigerie et al., 2020). Previously, the Pliocene balaenid Antwerpibalaena liberatlas was documented a “previously unseen variability in balaenid flipper shape, ” with more slender forelimb long bones (Duboys de Lavigerie et al., 2020). Notably, the preserved humerus and radius of M. sapporoensis display even more slender (Table 7), resembling those of the eomysticetid Yamatocetus canaliculatus (Okazaki, 2012). Compared with Y. canaliculatus , M. sapporoensis has a lower deltoid ridge on the humerus and an anteroposteriorly longer distal end of both the ulna and radius. Compared with A. liberatlas, M. sapporoensis has a semi globular humerus head and anteroposteriorly shorter distal ends of the ulna and radius. A proportion of the humerus such as proximodistal length/anteroposterior maximum length of the humerus are 2.2 (M. sapporoensis), 1.9 (A. liberatlas), 1.3 (Eubalaena japonica), which suggests that the humerus of balaenids became robust. A proportion of the radius such as proximodistal length/anteroposterior length of the distal end of the radius are 2.8 (M. sapporoensis), 2.8 (C. valentinae), 2.6 (A. liberatlas), 1.6 (Eubalaena japonica), which also suggests that the radius of balaenids became robust. We could not use the ulna, because its proximal end is not preserved in M. sapporoensis , however most likely the ulna might had the same trend to these of the humerus and radius. In addition, a ratio of the upper and lower arms such as proximodistal length of the humerus and radius are 1.0 (M. sapporoensis), 0.8 (A. liberatlas), 1.0 (E. japonica), which suggests that the ratio of the upper and lower arm length is not so changed from Miocene to modern balaenids.
 Thus, from early members to modern balaenids, the forelimb elements were changed from longer and narrower flippers to more robust ones (Figure 27, Table 7) These morphological changes suggest different forelimb usage among these species.
Thus, from early members to modern balaenids, the forelimb elements were changed from longer and narrower flippers to more robust ones (Figure 27, Table 7) These morphological changes suggest different forelimb usage among these species.
Unlike many modern toothed whales, baleen whales generally have unfused and separated neck vertebrae. However, modern adult balaenids exhibit seven completely fused cervical vertebrae. These differences of cervical vertebra fusion had been hypothesized because of difference in feeding behavior such as the size of prey and locomotion styles that may impose varied forces on the neck (VanBuren and Evans, 2017). Here, we compare cervical vertebra fusion in the Balaenidae (Table 8). Notably, the neck vertebra fusion progresses possibly through ontogeny, as the vertebraterial epiphyses fuse through ontogeny (Moran et al., 2015). A young extant Balaena mysticetus has a fused 1st to 6th cervical vertebrae and separate 7th cervical vertebra (Nishiwaki and Kasuya 1970). Thus, Table 8 includes suggested ontogenetic stage of compared specimens.
The Late Pliocene balaenid Antwerpibalaena liberatlas also has fused 2nd to 7th cervical vertebrae, however the atlas is separated (Duboys de Lavigerie et al., 2020). Two other Early Pliocene balaenids, Balaena ricei (adult) and Charadrobalaena valentinae (older than juvenile) display completely fused cervical vertebrae (Westgate and Whitmore 2002; Bisconti et al., 2023). Descriptions of Van Beneden (1872) state that the holotype of Balaenula balaenopsis , which is a young individual has a separated atlas and seventh cervical vertebra. However, B. balaenopsis elements reported in Van Beneden (1872) are possibly not belonging to one individual (Buono et al., 2017). Balaenotus insignis has a separated atlas (with fused fifth and sixth cervical vertebrae are fused [Abel, 1941]).
The earliest known balaenid Morenocetus parvus, was initially reported to have fused second to fifth cervical vertebrae (Cabrera 1926). However, a later study noted that these vertebrae are currently missing, and a previously identified mandible attributed to M. parvus actually dates from the Pleistocene or later, making it unrelated to the original individual (Buono et al., 2017). Thus, the original description of the cervical vertebrae of M. parvus is doubtful.
Megabalaena sapporoensis provides the oldest and reliable morphological data on the cervical evolution of the Balaenidae. It has partially fused cervical vertebrae, and separated the axis and third cervical vertebra (Figure 18). There is possibility that the atlas and axis were fused via the broken and lost neural arch of the atlas and the neural spine of the axis. Therefore, in adult individuals of the Balaenidae, cervical vertebral fusion possibly began with the axis and third cervical vertebra during the Late Miocene, then vertebral fusion was followed by the fourth to sixth cervical vertebra. Finally, all cervical vertebrae were fused by the Early Pliocene, over a span of 4 million years.
CONCLUSION
Megabalaena sapporoensis gen. et sp. nov. (SMAC 2731) represents an archaic balaenid from the Late Miocene (9.0 ± 0.4 Ma), discovered in the upper part of the Toyama Formation, Sapporo, Hokkaido, Japan. It is distinguishable from other balaenids by its unfused cervical vertebrae, except the axis and third cervical vertebra, and its slender forelimb long bones, including the humerus, radius, and ulna. These features are considered primitive among the Balaenidae. M. sapporoensis partly fills a 9-million-years gap in the fossil record, bridging the transition from primitive states to specialized modern balaenids. Phylogenetic analyses place M. sapporoensis as more derived than Balaenula astensis within a large unresolved polytomy that includes Eubalaena shinshuensis , extant species of Eubalaena and others (equally weight analysis), and place M. sapporoensis in a clade with Charadrobalaena valentinae + Antwerpibalaena liberatlas + Eubalaena ianitrix (implied weight analysis). The estimated body length of M. sapporoensis is 12.7 m, which is three times longer than the oldest known balaenid, Morenocetus parvus . Overall, M. sapporoensis expands our understanding of early balaenid body size diversity during the Miocene.
AUTHORS’ CONTRIBUTIONS
Authors’ contributions. H.F. originally started the study and provided ideas of discussion. H.F. was passed away in 2023 during this research project running. T.K. carried out the first phylogenetic analysis. Y.T. wrote the first draft and carried out the phylogenetic analysis presented in this manuscript. T.K. prepared some figures, and provided some ideas on discussion. T.S. created 3D restorations. H.O. examined geologic age by fission track dating. T.K., T.S. and H.O. revised manuscript.
Funding. This work was partly supported by Japan Society for the Promotion of Science [KAKENHI Grant Numbers JP18K01110] to TK.
ACKNOWLEDGEMENTS
Thanks go to K. Mori to discover the study material. Citizens and staffs of Sapporo Museum Activity Center for digging up the specimen, and Construction management department of Hokkaido for their advice between 2008 and 2012. J. Kagemoto (Matsunoyu, Kogakeyu Onsen) provided permission to dig up the specimen. I. Sato and others for help in digging up the specimen in 2014. T. Kato and T. Oka (Earthscience Co.) helped for geological survey at the locality. H. Takanashi, Y. Tachi, T. Hiyoriyama, S. Amano, N. Morishita, M. Yoshii (all Sapporo Natural History Research Group), K. Arayama, I. Kawamata, S. Kimura, N. Nagase, T. Okano, Y. Onoe, M. Ozaki, M. Usuda (all volunteers at Hokkaido University Museum), M. Kimura, R. Sasaki, M. Takahashi, staff of SMAC and others for preparation of the specimen. We thank N. Kohno, Y. Tajima and T.K. Yamada (all NMNS) and G. Nakamura (MTUF) for their permissions to examine comparative specimens. We thank O. Lambert (Institut royal des Sciences naturelles de Belgique), R.W. Boessenecker (University of California Museum of Paleontology) and an anonymous referee for their constructive comments on this manuscript. Also, H. F. thanks to H. Ichishima (Fukui Prefectural Dinosaur Museum) for his advice on baleen whale fossils. Neutron irradiation in fission-track dating was partially supported by the Joint Usage/Research of the Institute for Integrated Radiation and Nuclear Science, Kyoto University (KURNS).
REFERENCES
Abel, O. 1941. Vorläufige mitteilungen uber die revision der fossilen Mystacoceten aus dem Tertiär Belgiens (Zweiter Bericht). Bulletin du Musée royal d’Histoire naturelle de Belgique, 17:1-29.
Allen, J. A. 1908. The North Atlantic right whale and its near allies. Bulletin of the AMNH; v. 24, article 18. New York: Published by order of the Trustees, American Museum of Natural History.
Benke, H. 1993. Investigations on the osteology and the functional morphology of the flipper of whales and dolphins (Cetacea). Investigations on Cetacea, 24:9-252.
Bisconti, M. 2003. Evolutionary history of Balaenidae. Cranium, 20:9-50.
Bisconti, M. 2005. Skull morphology and phylogenetic relationships of a new diminutive balaenid from the Lower Pliocene of Belgium. Palaeontology, 48:793-816.
Bisconti, M. , Chicchi, S. , Monegatti, P. , Scacchetti, M. , Campanini, M. , Marsili, S. , and Carnevale, G. 2023. Taphonomy, osteology and functional morphology of a partially articulated skeleton of an archaic Pliocene right whale from Emilia Romagna (NW Italy). Bollettino della Società Paleontologica Italiana, 62:1-22.
Bisconti, M. , Pellegrino, L. , and Carnevale, G. 2021. Evolution of gigantism in right and bowhead whales (Cetacea: Mysticeti: Balaenidae). Biological Journal of the Linnean Society, XX:1-27.
Boessenecker, R. W. 2013. A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: pinnipeds and cetaceans. Geodiversitas, 35:815-940.
https://doi.org/10.5252/g2013n4a5
Buono, M. R. , Fernández, M. S. , Cozzuol, M. A. , Cuitiño, J. I. , and Fitzgerald, E. M. G. 2017. The early Miocene balaenid Morenocetus parvus from Patagonia (Argentina) and the evolution of right whales. PeerJ, 5:e4148.
https://doi.org/10.7717/peerj.4148
Cabrera, Á. 1926. Cetáceos fósiles del Museo de la Plata. Revista del Museo de la Plata, 29:363-411.
Capellini, G. 1905. Balene fossili toscane. III. Idiocetus guicciardinii . Memorie della Regia accademia delle Scienze dell’Istituto di Bologna, 6:71-80.
Committee on Taxonomy. 2022. Retrieved February 27, 2023, from
https://marinemammalscience.org/science-and-publications/list-marine-mammal-species-subspecies/
Cooper, L. N. , Berta, A. , Dawson, S. D. , and Reidenberg, J. S. 2007. Evolution of hyperphalangy and digit reduction in the cetacean manus. The Anatomical Record, 290:654-672.
Duboys de Lavigerie, G. D. , Bosselaers, M. , Goolaerts, S. , Park, T. , Lambert, O. , and Marx, F. G. 2020. New Pliocene right whale from Belgium informs balaenid phylogeny and function. Journal of Systematic Palaeontology, 18:1141-1166.
Flower, W. H. 1864. Notes on the skeletons of whales in the principal museums of Holland and Belgium, with descriptions of two species apparently new to science. Proceedings of the Zoological Society of London, 1864:384-420.
Flower, W. H. 1885. An Introduction to the Osteology of the Mammalia. 3 ed. Macmillan and Co. , London.
Furusawa, H. 2007. Sirenian fossils, p. 60-68. In Sapporo Museum Activity Center (ed. ), A comprehensive survey of mammal fossils from Sapporo. Sapporo Museum Activity Center, Hokkaido.
Galbraith, R. F. 1981. On statistical models for fission track counts. Journal of the International Association for Mathematical Geology, 13:471-488.
Galatius, A. and Kinze, C. C. 2003. Ankylosis patterns in the postcranial skeleton and hyoid bones of the harbour porpoise (Phocoena phocoena) in the Baltic and North Sea. Canadian Journal of Zoology, 81:1851-1861.
https://doi.org/10.1139/z03-181
Goloboff, P. A. and Catalano, S. A. 2016. TNT version 1. 5, including a full implementation of phylogenetic morphometrics. Cladistics, 32:221-238.
https://doi.org/10.1111/cla.12160
Ito, H. and Miyazaki, N. 1990. Skeletal development of the striped dolphin (Stenella coeruleoalba) in Japanese waters. Journal of the Mammalogical Society of Japan, 14:79-96.
https://doi.org/10.11238/jmammsocjapan1987.14.79
Jefferson, T. A. , Webber, M. A. , and Pitman, R. L. 2008. Marine Mammals of the World: a Comprehensive Guide to their Identification. Academic Press, Oxford, UK.
Kellogg, R. 1931. Pelagic mammals from the Temblor Formation of the Kern River region, California. Proceedings of the California Academy of Sciences, 19:217-397.
Kimura, T. 2009. Review of the fossil balaenids from Japan with a re-description of Eubalaena shinshuensis (Mammalia, Cetacea, Mysticeti). Quaderni del Museo di Storia Naturale di Livorno, 22:3-21.
Kimura, T. , Narita, K. , Fujita, T. , and Hasegawa, Y. 2007. A new species of Eubalaena (Cetacea: Mysticeti: Balaenidae) from the Gonda Formation (latest Miocene-Early Pliocene) of Japan. Bulletin of Gunma Museum of Natural History, 11:15-27.
Maddison, W. P. and Maddison, D. R. 2021. Mesquite: a modular system for evolutionary analysis. Available at
http://mesquiteproject.org
Marx, F. G. and Fordyce, R. E. 2015. Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity. Royal Society Open Science, 2:140434.
https://doi.org/10.1098/rsos.140434
Marx, F. G. and Lambert, O. 2021. Fossil record, p. 11-17. The Bowhead Whale. Elsevier.
McGowen, M. R. , Spaulding, M. , and Gatesy, J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Molecular Phylogenetics and Evolution, 53:891-906.
https://doi.org/10.1016/j.ympev.2009.08.018
Mead, J. G. and Fordyce, R. E. 2009. The therian skull: a lexicon with emphasis on the odontocetes. Smithsonian Contributions to Zoology, 627:1-248.
https://doi.org/10.5479/si.00810282.627
Moran, M. M. , Bajpai, S. , George, J. C. , Suydam, R. , Usip, S. , and Thewissen, J. G. M. 2015. Intervertebral and epiphyseal fusion in the postnatal ontogeny of cetaceans and terrestrial mammals. Journal of Mammalian Evolution, 22:93-109.
https://doi.org/10.1007/s10914-014-9256-7
Nagasawa, K. , Watanabe, A. , Sawaki, H. , Watanabe, H. , and Kawabe, T. 2024. Fossil mandibles of baleen whales in huge concretions of the Middle Miocene, Oga Peninsula, Akita Prefecture, Northeast Japan. Journal of Fossil Research, 57:15-25.
Ohira, H. 2004. Fission track ages of zircons from Miocene tuffs in the Hiki Hills and the Arakawa River, Saitama Prefecture, central Japan. Monograph of Association for the Geological Collaboration in Japan. 52:51-65.
Okazaki, Y. 2012. A new mysticete from the upper Oligocene Ashiya Group, Kyushu, Japan and its significance to mysticete evolution. Bulletin of the Kitakyushu Museum of Natural History and Human History, Series A (Natural History), 10:129-152.
Omura, H. 1958. North Pacific right whale. Scientific Reports of the Whales Research Institute, 13:1-52.
Omura, H. , Ohsumi, S. , Nemoto, T. , Nasu, K. , and Kasuya, T. 1969. Black right whales in the North Pacific. Scientific Reports of the Whales Research Institute, 21:1-78.
Ozaki, M. and Komatsubara, T. 2014. 1: 200, 000 Land geological map in the Ishikari Depression and its surrounding area with explanatory note, p. 1-28. S-4. Seamless Geoinformation of Coastal Zone “Southern Coastal Zone of the Ishikari Depression.” Geological Survey of Japan.
Pyenson, N. D. and Sponberg, S. N. 2011. Reconstructing body size in extinct crown Cetacea (Neoceti) using allometry, phylogenetic methods and tests from the fossil record. Journal of Mammalian Evolution, 18:269-288.
Shinmura, T. , Tanaka, Y. , and Furusawa, H. 2024. Using 3D CG technology for the reconstruction of whale fossils. Journal of Fossil Research, 57(1/2):44-49.
Slater, G. J. , Goldbogen, J. A. , and Pyenson, N. D. 2017. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proceedings of the Royal Society B: Biological Sciences, 284:1-8.
https://doi.org/10.1098/rspb.2017.0546
Suzuki, A. 2007. Molluskan fossils, p. 69-75. A comprehensive survey of mammal fossils from Sapporo. Sapporo Museum Activity Center.
Tanaka, Y. 2024. A feeding organ the basihyal and thyrohyal tells which size of prey do true baleen whales (Cetacea, Chaeomysticeti) eat. Palaeontologia Electronica, 27:a8.
https://doi.org/10.26879/1311
Tanaka, Y. , Furusawa, H. , and Kimura, M. 2020. A new member of fossil balaenid (Mysticeti, Cetacea) from the early Pliocene of Hokkaido, Japan. Royal Society Open Science, 7:1-20.
Tanaka, Y. and Kohno, N. 2015. A new late Miocene odobenid (Mammalia: Carnivora) from Hokkaido, Japan suggests rapid diversification of basal Miocene odobenids. PLoS ONE, 1-25.
https://doi.org/10.1371/journal.pone.0131856
Tanaka, Y. and Taruno, H. 2019. The First Cetacean Record from the Osaka Group (Middle Pleistocene, Quaternary) in Osaka, Japan. Paleontological Research, 23:166-173.
https://doi.org/10.2517/2018PR016
Tanaka, Y. and Taruno, H. 2022. First record of a right whale fossil from the pre-historic period of Japan. Aquatic Mammals, 48:255-261.
Tanaka, Y. and Taruno, H. 2024. Remains of a fin whale from Osaka, Japan, and comparison of baleen whale cases in the past and present of Osaka Bay (Seto Inland Sea). Mammal Study, 49:217-228.
https://doi.org/10.3106/ms2023-0041
Tsai, C. H. and Fordyce, R. E. 2015. The earliest gulp-feeding mysticete (Cetacea: Mysticeti) from the Oligocene of New Zealand. Journal of Mammalian Evolution, 22:1-26.
Van Beneden, P. J. 1872. Les baleines fossiles d’Anvers. Bulletins de L’Academie Royale des Sciences, des Lettres et des Beaux-arts, 2:6-20.
Van Beneden, P. J. 1880. Description des ossements fossiles des environs d’anvers: Cétacés Genre Balaenula, Balaena et Balaenotus . Annales du Musée Royal d’Histoire Naturelle de Belgique. Série Paléontologique. Hayez.
VanBuren, C. S. and Evans, D. C. 2017. Evolution and function of anterior cervical vertebral fusion in tetrapods. Biological Reviews, 92:608-626. Wiley Online Library.
Westgate, J. W. and Whitmore, F. C. 2002. Balaena ricei , a new species of bowhead whale from the Yorktown Formation (Pliocene) of Hampton, Virginia. Smithsonian Contributions to Paleobiology, 93:295-312.
Woodward, B. L. , Winn, J. P. , and Fish, F. E. 2006. Morphological specializations of baleen whales associated with hydrodynamic performance and ecological niche. Journal of Morphology, 267:1284-1294.

